Abstract
Key message
Studying RNAi-mediated DlP5βR1 and DlP5βR2 knockdown shoot culture lines of Digitalis lanata, we here provide direct evidence for the participation of PRISEs (progesterone 5β-reductase/iridoid synthase-like enzymes) in 5β-cardenolide formation.
Abstract
Progesterone 5β-reductases (P5βR) are assumed to catalyze the reduction of progesterone to 5β-pregnane-3,20-dione, which is a crucial step in the biosynthesis of the 5β-cardenolides. P5βRs are encoded by VEP1-like genes occurring ubiquitously in embryophytes. P5βRs are substrate-promiscuous enone-1,4-reductases recently termed PRISEs (progesterone 5β-reductase/iridoid synthase-like enzymes). Two PRISE genes, termed DlP5βR1 (AY585867.1) and DlP5βR2 (HM210089.1) were isolated from Digitalis lanata. To give experimental evidence for the participation of PRISEs in 5β-cardenolide formation, we here established several RNAi-mediated DlP5βR1 and DlP5βR2 knockdown shoot culture lines of D. lanata. Cardenolide contents were lower in D. lanata P5βR-RNAi lines than in wild-type shoots. We considered that the gene knockdowns may have had pleiotropic effects such as an increase in glutathione (GSH) which is known to inhibit cardenolide formation. GSH levels and expression of glutathione reductase (GR) were measured. Both were higher in the Dl P5βR-RNAi lines than in the wild-type shoots. Cardenolide biosynthesis was restored by buthionine sulfoximine (BSO) treatment in Dl P5βR2-RNAi lines but not in Dl P5βR1-RNAi lines. Since progesterone is a precursor of cardenolides but can also act as a reactive electrophile species (RES), we here discriminated between these by comparing the effects of progesterone and methyl vinyl ketone, a small RES but not a precursor of cardenolides. To the best of our knowledge, we here demonstrated for the first time that P5βR1 is involved in cardenolide formation. We also provide further evidence that PRISEs are also important for plants dealing with stress by detoxifying reactive electrophile species (RES).
Supplementary Information
The online version contains supplementary material available at 10.1007/s00299-021-02707-3.
Keywords: Progesterone 5β-reductases (P5βR), Cardenolide biosynthesis, Digitalis lanata, RNAi-mediated knockdown, Glutathione, Reactive electrophile species
Introduction
The successful introduction of foxglove extracts to treat congestive heart failure in the eighteenth century (Withering 1941) made plants from the genus Digitalis (Plantaginaceae) worth investigating (Luckner and Wichtl 2000). In the twentieth century cardiac glycosides, the bioactive principles of Digitalis plants replaced the use of extracts. Although other medications have superseded Digitalis in the treatment of heart failure, cardenolides came back into focus as they showed anti-viral and anti-tumoral activity (Prassas and Diamandis 2008; Bertol et al. 2011; Schneider et al. 2017). Today cardenolides such as digoxin, digitoxin, and lanatoside C are still isolated from Digitalis lanata Ehrh. leaves (Luckner and Wichtl 2000). This time-consuming process is necessary as the chemical synthesis of cardenolides is not economically feasible. Only parts of their biosynthesis in planta are known which makes it difficult to design alternative biotechnological production systems using yeast or bacteria (Rieck et al. 2019).
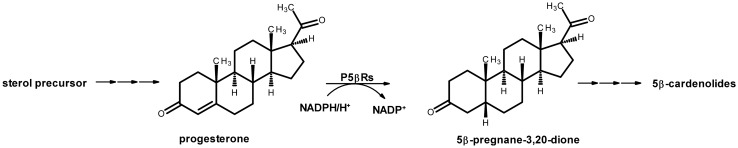
A crucial step in the biosynthesis of 5β-cardenolides is the reduction of progesterone to 5β-pregnane-3,20-dione which is assumed to be catalyzed by progesterone 5β-reductases (Gärtner et al. 1994; Herl et al. 2006; Fig. 1). However, direct proof was not provided so far. Enzymes with P5βR activity occur in many cardenolide-containing and cardenolide-free plants (Bauer et al. 2010). For example, a steroid 5β-reductase (St5βR) with a progesterone 5β-reductase activity much higher than that of D. lanata was identified in the cardenolide-free plant Arabidopsis thaliana (Herl et al. 2009). Moreover, P5βR-like enzymes (termed iridoid synthases; ISY) are involved in iridoid biosynthesis (Geu-Flores et al. 2012), and Munkert et al. (2015a, b) reported that iridoid synthase activity is common among the plant progesterone 5β-reductase family. More recently, the enzymes progesterone 5β-reductase/iridoid synthase-like enzymes were termed PRISEs (Schmidt et al. 2018) and described as a catalytic reservoir for specialized metabolism across land plants (Nguyen and O’Connor 2020). Progesterone in cardenolide biosynthesis and 8-oxogeranial in iridoid synthesis are not the only substrates for PRISEs. Various 1,4-enones, such as 2-cyclohexen-1-one, methyl vinyl ketone or citral are also accepted (e.g.,Durchschein et al. 2012; Munkert et al., 2015a, b). PRISEs seem to be involved in reactions in central plant metabolism and appeared early in plant evolution, presumably with lateral gene transfer originating from α-proteobacteria (Tarrío et al. 2011).
Fig. 1.
Putative biosynthetic pathway of 5β-cardenolides in Digitalis. Progesterone 5β-reductases catalyze the stereospecific reduction of progesterone to 5β-pregnane-3,20-dione under the consumption of NADPH/H+
Wounding or environmental stressors such as high salt concentrations or low temperatures enhanced A. thaliana St5βR (At4g24220, termed AWI 31 by Yang et al. 1997) expression (Winter et al. 2007). A. thaliana developed a phenotype with altered vein patterning, when At4g24220 (a PRISE gene termed VEP1 by Jun et al. 2002) was knocked out. The first P5βR gene described, named P5βR1 (Herl et al., 2006) in Digitalis lanata seemed to be expressed constitutively at a much higher level than P5βR2 (Perez-Bermúdez et al. 2010). On the other hand, P5βR2 expression is related to stress not only in Digitalis but in other cardenolide-containing plants like Erysimum as well (Perez-Bermúdez et al. 2010; Horn et al. 2020).
As far as engineering of the cardenolide formation is concerned, Sales et al. (2007) transformed D. minor plants with an N-truncated form of a 3-hydroxy-3-methylglutarate CoA reductase gene whose constitutive expression resulted in increased accumulation of sterols and cardenolides. More recently, Kairuz et al. (2020) reported the introduction of an A. thaliana PRISE into D. purpurea, but could not demonstrate increased cardenolide accumulation indicating that progesterone 5β-reduction is not the rate-limiting step in cardenolide biosynthesis.
The connection between progesterone 5β-reductase gene expression, plant stress response and cardenolide formation was investigated here by generating RNAi-mediated knockdown of DlP5βR1 (AY574950.1) and DlP5βR2 (HM210089.1) in shoot cultures of D. lanata. These shoot cultures provided stable, homogeneous and reliable systems to study the effects of exogenous compounds on gene expression and cardenolide formation. To eliminate the possibility that effects attributed to gene knockdown may have been provoked by stress, we investigated the influence of glutathione (GSH), buthionine sulfoximine (BSO), methyl vinyl ketone (MVK), and progesterone on cardenolide formation and progesterone 5β-reductase gene expression in wild-type and knockdown shoots. Glutathione strongly inhibited cardenolide biosynthesis in embryogenic cell cultures of D. lanata (Berglund and Ohlsson 1993) whereas buthionine sulfoximine, an inhibitor of glutathione biosynthesis, reversed this glutathione effect (Berglund and Ohlsson, 1993). Methyl vinyl ketone (MVK), a reactive electrophile species (RES) and a stress metabolite (Kai et al. 2012), is also known to induce a plant stress response (Alméras et al. 2003). Progesterone, a cardenolide precursor, could be regarded as a reactive electrophile species (RES) which can be detoxified by PRISEs. We, therefore, analyzed the effect of both progesterone and MVK on plants harboring an RNAi-mediated knockdown of DlP5βR. Finally, we here demonstrate that P5βR1 is directly involved in constitutive cardenolide biosynthesis. On the other hand, P5βR2 seems to be more important for immediate but transient cardenolide formation in stress response situations.
Materials and methods
Agrobacterium tumefaciens-meditated genetic transformation
The genetic transformation protocol commenced using axenic shoot cultures from D. lanata (line Dl1681) grown on solid MS medium (Murashige and Skoog 1962). Shoots intended for genetic transformation were transferred and sub-cultivated in half-strength liquid MS medium containing per liter: 35 g sucrose, 1.0 mg 6-benzylaminopurine (BAP) and 0.1 mg indole-3-acetic acid (IAA). The cultures were kept on gyratory shakers (80 rpm, 24 °C) in permanent light (~ 40 μmol photons m−2 s −1) and sub-cultivated as advised by Stuhlemmer et al. (1993).
Leaflets were excised and transferred onto solid MS medium containing 1.5 mg L−1 thidiazuron (TDZ) and 0.1 mg L−1 NAA (medium I). After 2 days, leaf explants were first infiltrated under vacuum for 30 s and then bathed in a suspension of Agrobacterium tumefaciens strain GV3101 containing either GUS and nptII gene on 679p935s-GusIo-rbs vector or RNAi fragments and nptII gene on pHellsgate8 vector (oD600 = 0.6 – 0.8; acetosyringone, 0.3 mg mL−1) for 30 min and transferred back onto medium I. For verification of the transformation system, we used the construct 679p935s-GusIo-rbs (provided by the Institute of Horticultural Production Systems, Section Floriculture, Gottfried-Wilhelm-Leibniz-Universität Hannover, Germany), containing a nptII gene mediating kanamycin resistance and a GUS reporter gene. Both genes were under the control of CaMV 35S promoters, for permanent gene expression. Leaf explants and adhering bacteria were co-cultivated for 2 days solid on medium I. Leaf explants were then transferred onto solid medium II containing 100 mg L−1 kanamycin and 250 mg L−1 cefotaxime for selection (medium II). Medium II was exchanged weekly until sturdy shoots developed after 4–6 weeks. These were transferred onto solid MS medium containing 30 g L−1 sucrose and 2.25 mg L−1 BAP (DDV medium) supplemented with 150 mg L−1 kanamycin and 250 mg L−1 cefotaxime (medium III). Shoots were kept under these conditions until further use with medium III exchanged weekly. To exclude the presence of the Ti-plasmids in transgenic shoots, gDNA was checked by PCR reaction against spectinomycin (SmR) and virD2 gene (Suppl. Table S1; Suppl. Fig. S1).
Identification of transgenic shoots
Genomic DNA (gDNA) was isolated from D. lanata shoots according to Allen et al. (2006). A maximum of 100 mg plant material was ground in cold extraction buffer in a 1.5 mL reaction tube using a small pestle. The solution was rapidly heated to 65 °C and kept under these conditions for 30 min. The gDNA pellets obtained by centrifugation (20,000 × g; 20 °C) were dried at 30 °C and resuspended in sterile water.
Integration of T-DNA was verified by PCR with gDNA from shoots grown on medium I and primers against the genes nptII and GUS inserted in the T-DNA region. Additional PCRs against spectinomycin (SmR) resistance gene and virD2 gene were conducted to exclude the presence of Ti-plasmids (Suppl. Table S1; Suppl. Fig. S1). For PCR, FastGene Optima HotStart Ready Mix (Nippon Genetics Europe GmbH, Düren, Germany) and FlexCycler2 (Analytik Jena AG, Jena, Germany) were used. The PCR program consisted of an initial denaturation (95 °C for 60 s), 29 cycles (95 °C for 15 s; TA (Suppl. Table S1) for 30 s; 68 °C for 30 s) and a final amplification (68 °C for 600 s).
GUS activity was demonstrated by histochemical staining (Jefferson et al. 1987).
RNAi-mediated knockdown of P5βR1 and P5βR2 in Digitalis lanata L. shoots
For downregulating P5βR1 and P5βR2 gene expression, RNA interference (RNAi) technology and the plant binary pHellsgate8 vector system was used. Small specific fragments of around 230 bp of P5βR1 and P5βR2 were identified by sequence alignment. The Gateway cloning technique was used to yield the final vector constructs (Suppl. Fig. S2). Therefore, the specific fragments of P5βR1 and P5βR2 were amplified using the following primer pairs flanked by attB sites for homologues recombination.
P5βR1 for: GGGGACAAGTTTGTACAAAAAAGCAGGCTTCATGAGCTGGTGGGCTG.
P5βR1 rev: GGGGACTTTGTACAAGAAAGCTGGGTCTTAATTGATCGGATTATCCTCA.
P5βR2 for: GGGGACAAGTTTGTACAAAAAGCAGGCTTCATGTATACCGACACAACGACTTGG;
P5βR2 rev: GGGGACCACTTTGTACAAGAAAGCTGGGTCTTGTCGGAAAGTGGAGACAATT.
The resulting PCR products were cloned into pDONR®221 using BP-Clonase® enzyme mix (Thermo Fisher Scientific Inc., Waltham, USA). These plasmids were used as entry vectors for the Gateway cloning system mix (Thermo Fisher Scientific Inc., Waltham, USA) into pHellsgate8 (Division of Plant Physiology, University Bayreuth; Suppl. Fig. S2). The resulting plasmids were checked by sequencing (Eurofins, Ebersberg, Germany). Chemical competent A. tumefaciens strain GV3101 cells (100 µL of cell suspension) were transformed with 1 µg of each plasmid by three sequential incubation steps of 5 min each on ice, liquid nitrogen, and at 37 °C. After the addition of 750 µL SOC-medium, the cells were incubated for 4 h at 28 °C and afterward spread on solid LB medium containing 10 µg mL−1 rifampicin and 50 µg mL−1 kanamycin. Individual cell clones were checked by colony PCR and those found positive were used for the genetic transformation of D. lanata shoots.
Quantitative real-time analysis (qPCR)
D. lanata glutathione reductase (GR) and glutathione S-transferase (GST) genes were identified using the transcriptomic search tool of medicinal plant genome (http://medicinalplantgenomics.msu.edu/; Suppl. Table S2). Primers for amplifying P5βR1 and P5βR2 were identified using D. lanata P5βR1 (AY574950.1) and P5βR2 (HM210089.1) GenBank entries. The primers used did not bind to the regions used for RNAi-mediated knockdown. Primer pairs amplifying a D. lanata actin reference gene sequence were used as described by Ernst et al. 2010 (Suppl. Table S2).
To analyze the transcription rates, total RNA was isolated from shoots cultivated in vitro using Monarch® Total RNA Miniprep Kit (New England Biolabs, Ipswich, USA). cDNA was synthesized using 500 ng total RNA employing the RevertAid H Minus First Strand cDNA Synthesis Kit (Thermo Fisher Scientific Inc., Waltham, USA). For qPCR, samples were prepared using the FAST SYBR Green Mastermix Kit (Applied Biosystems, Germany) according to the user manual. qPCR was carried out with the StepOnePlus Real-Time PCR System (Life Technologies, Germany) and the relative gene expression levels were calculated using the 2−ΔΔCt method (Schmittgen and Livak 2008) with actin as the reference gene.
Quantification of 5β-cardenolides
Cardenolides were extracted as described by Wiegrebe and Wichtl (1993). Extracted cardenolides were hydrolyzed according to Schaller and Kreis (2006) to enhance sensitivity. The cardenolide aglyca released (digitoxigenin and digoxigenin) were detected and quantified by UPLC at 220 nm: digitoxigenin (RT = 4.9 min) and digoxigenin (RT = 2.9 min). Progesterone was used as the internal standard (RT = 6.8 min). Analysis was carried out in an ACQUITY Ultra Performance LC™ system (Waters, Milford, MA, USA) linked to TUV detector (Waters, Milford, MA, USA). 5–10 µL of sample solution diluted with acetonitrile 1:5 was injected into a reversed phase column (Phase: 1.7 µm Fortis C18, column dimension: 50 × 2.1 mm, Fortis Technologies Ltd, Cheshire, England, UK) kept at 40 °C. The mobile phase consisted of solvent A (H2O/0.1% HCOOH) and solvent B (acetonitrile/0.1% HCOOH). The flow-rate was 0.4 mL min−1; t = 0 min, 5% B; t = 10 min, 95% B; t = 11 min, 5% B; t = 13 min, 5% B.
Measuring progesterone 5β-reductase activity
The progesterone 5β-reductase activity assay contained: 1 mg mL−1 of the respective crude protein extract, 0.3 mM progesterone and a NADPH regenerating system consisting of 1.1 mM glucose 6-phosphate, 6.4 mM NADP+ and 4.2 nkat glucose-6-phosphate dehydrogenase. Assays were stopped after 4 h by adding 1 mL CH2Cl2. Heat inactivated samples (10 min 99 °C in thermoblock) served as control for no substrate conversion. Consumption of the substrate was determined by GC–MS as described by Ernst et al. (2010). A Shimadzu GC2010/QP-2010S was used in IE mode with helium as the carrier gas (flow: 1.2 mL min−1). A DB-5 ms column (J&W GC column) from Agilent (30 m × 0.25 mm × 0.25 μm) was used. The program commenced at 200 °C for 4 min followed by a 20 °C min−1 linear increase up to 290 °C and finished with 4 min at 300 °C. Authentic standards were from Steraloids Inc. (Newport, RI, USA).
Quantification of progesterone
The preparation of plant extracts followed the extraction method of Iino et al. (2007) with slight modifications. 5 g D. lanata shoots were frozen in liquid nitrogen, ground to a fine powder and extracted with 50 mL MeOH at room temperature (23 °C). The filtrate was evaporated and the residue dissolved in 50 mL EtOAc and washed twice with 50 mL 0.5 M K2HPO4. The organic phase was evaporated, the residue dissolved in 20 mL hexane and extracted twice with 20 mL 20% MeOH. The combined MeOH phases were diluted with 40 mL H2O and extracted twice with 60 mL hexane each. The combined hexane phases were evaporated, the residue dissolved in CH2Cl2 and filtered through 5 g activated carbon. The filtrate was evaporated again and the residue analyzed further. Progesterone was identified by GC–MS using above mentioned GC–MS program. The fidelity of progesterone detection was further verified by using samples spiked with 100 µmol progesterone. The retention time of the progesterone peak was at 10.15 min and mass spectrometric analysis indicated the separation of progesterone ions at m/z 314, 272, 124 and 43. Progesterone was quantified by UPLC (at 220 nm). The solvent gradient was set as follows: solvent A (H2O): 95%, solvent B (acetonitrile): 5%, flow-rate: 0.4 mL min−1 by t = 0 min, 5% B; t = 10 min, 95% B; t = 11 min, 5% B; t = 13 min, 5% B.
Estimation of glutathione pool
Total glutathione (t-GSH) and oxidized glutathione (GSSG) were measured according to Hajdinak et al. (2018). Fresh D. lanata shoots were homogenized with 6% metaphosphoric acid (w/v, containing 1 mM EDTA) and centrifuged at 16,000×g for 15 min at 4 °C (Sahoo et al. 2017). Removing reduced GSH for measuring oxidized glutathione (GSSG) followed the procedure described by Rahman et al. (2006). To assay t-GSH and GSSG, 85.2 µL of 50 mM sodium phosphate buffered (pH 7.5), 3 µL nicotinamide adenine dinucleotide phosphate (NADPH, 0.3 mM) and 2 µL of each supernatant were pipetted into a microtiter plate. The reaction was started by adding 5.3 µL of yeast glutathione reductase (GR, 1.6 U/ mL). The plate was incubated for 15 min at 25 °C. Finally, 4.5 µL 2-nitrobenzoic acid (DTNB, 0.225 mM) was added and the change of absorption measured spectrophotometrically at 405 nm against a blank. Results were expressed as nmol per gram fresh weight (nmol * g−1 FW). GSH content was calculated by subtracting GSSG from total glutathione (Rahman et al. 2006).
Treatment with α,β-unsaturated carbonyls and 5β-pregnane-3,20-dione
WT shoots in liquid medium were fed with progesterone (PO; 0.16 mM final concentration in DMSO) to analyze the effects of progesterone, a precursor of 5β-cardenolide formation. 5β-Cardenolides were quantified by UPLC 1, 2, 3, 7 and 8 days after progesterone treatment. Expressions of the P5βR1 and P5βR2 genes were quantified by qPCR using actin as the reference gene.
WT and transgenic shoots were treated with progesterone (PO) and 5β-pregnane-3,20-dione (PR; both 0.3 mM final concentration in DMSO) and after 7 days of treatment cardenolide levels were quantified as described above.
To analyze the effects of other α,β-unsaturated carbonyls on progesterone 5β-reductase expression and cardenolide content, WT and transgenic shoots treated with 2 µmol L−1 air volume MVK (final concentration; Merck KGaA, Darmstadt, Germany) diluted in tab water for 3 h as described by Munkert et al. (2014). Water served as the control. Expressions of P5βR1, P5βR2 and GST were quantified by qPCR and cardenolide content analyzed as described above.
Glutathione (GSH) and buthionine sulfoximine (BSO) treatment
Transgenic shoots were transferred to medium III and WT shoots to DDV containing 0.3 mM BSO or 0.3 mM GSH as described by Berglund and Ohlsen (1993). In addition, WT shoots were treated with a combination of 0.04 mM BSO and 0.3 mM GSH as well as 0.3 mM BSO and 2 mM GSH as suggested by Berglund and Ohlsen (1993).
Heterologous expression of DlP5βR1 and DlP5βR2
Primer pairs for restriction-free cloning of DlP5βR2 into expression vector pDEST17 were designed using rf-cloning.org (Bond and Naus 2012).
Forward primer: 5′GCTCGAATACCCCAGAACATATGAGYTGGTGGTGGGCT′3, reverse primer: 5′TTGTACAAGAAATCTGGGTCTCAAGGAACAATCTTGTAAGC′3. The cloning procedure was realized as described by Unger et al. (2010). Amplification of “megaprimers” and cloning PCR were conducted in a FlexCycler2 thermocycler (Analytik Jena, Jena, Germany) using Phusion® High-Fidelity DNA Polymerase (New England Biolabs, Ipswich, USA). Cloning products were used for the transformation of E. coli strain DH5α. Colonies showing resistance against ampicillin were tested by colony PCR using Taq DNA Polymerase (New England Biolabs, Ipswich, USA). Plasmids of positive colonies were isolated using Roti®-Prep Plasmid MINI (Carl Roth GmbH, Karlsruhe, Germany). The correct integration of DlP5βR2 into the expression vector was verified by sequencing (Eurofins Genomics, Ebersberg, Germany). Standard primers against T7 promotor or against T7 terminator were used. E. coli SoluBL21 were transformed with positive constructs. Positively identified E. coli SoluBL21 colonies were used for heterologous expression of DlP5βR2.
For heterologous expression of DlP5βR1, a pQE30 vector (Herl et al. 2006) was used. For expression, bacteria were cultivated in 1 L LB medium containing ampicillin or ampicillin and kanamycin (pDEST17 vector: 100 mg mL−1; LBAmp; pQE30 vector: 100 mg mL−1; 25 mg mL−1; LBAmp;Kan) until an optical density of 0.5–0.7 was reached. IPTG was added to a final concentration of 0.1 mM to induce heterologous gene expression. Bacteria were cultivated at 4 °C for 96 h. Low temperatures reduced the formation of “inclusion bodies” and enhanced the amount of soluble protein (Stevens 2000) and was successfully used for the expression of P5βRs by Herl et al. (2006) and several other P5βR from different plant species (Munkert et al. 2015a, b). Cells were harvested by centrifugation (4000×g, 20 min, 4 °C) and the pellet was either used directly for protein isolation or stored at −20 °C for further use.
Pellets were thawed on ice and resuspended in 10 mL lysing buffer (50 mM NaH2PO4, 300 mM NaCl, 40 mM imidazole). 20,000 U mL−1 lysozyme (Carl Roth GmbH, Karlsruhe, Germany) was added and the solution incubated under shaking (30 min, 100 rpm). Subsequently cells were disrupted by ultrasonication (6 times for 30 s) and centrifuged (10,000×g, 40 min, 4 °C).
The supernatant was used for the purification of recombinant proteins by immobilized metal chelate affinity chromatography (IMAC). HisTrap™ HP columns (1 mL) and ÄKTA purifier chromatography system was used (GE Healthcare, Uppsala/Sweden). Protein concentration was determined according to Bradford (1976).
Proteins were separated by SDS-PAGE and semi-dry immunoblotting was carried out according to the QIAexpress Detection and Assay Handbooks (QIAgen) with slight modifications as described by Munkert et al. (2015a, b). Recombinant proteins were separated on a 12% Bis–Tris polyacrylamide gel and then transferred onto a nitrocellulose membrane by electroblotting. After blocking with 5% milk powder in Tris-buffered saline with 0.1% Tween 20, the membrane was incubated for 1 h with mouse anti-His antibodies (mixture of RGS-, Tetra-, and Penta-His antibodies; dilution 1:2000; QIAgen, Hilden, Germany). Anti-mouse IgG-peroxidase antibody (Sigma, Munich, Germany) was used as the detection antibody (dilution 1:10,000; incubation 1 h). Chemiluminescence of 3-aminophthalate released from luminol was used for detection.
Determination of kinetic constants of heterologous DlP5βR1 and DlP5βR2
To determine the kinetic constants of recombinant P5βR proteins, reductase activity was measured spectrophotometrically, as described before (Burda et al. 2009; Bauer et al. 2010; Munkert et al. 2015a, b). The conversion of NADPH/H+ (0.2 mM) (AppliChem GmbH) to NADP+ was monitored at 340 nm in the presence of the respective substrates over a time course of 5 min at 40 °C. Depending on the P5βR investigated, between 0.01 and 0.1 mg × mL−1 of recombinant protein, 0.2 mM NADPH/H+, and varying concentrations of the substrate (0.01–1.6 mM) were used in the assay. Assays without the respective substrate were used as control.
Statistical analysis
All data are expressed as the mean ± SEM. Each individual experiment was composed of n ≥ 3 biological and technical replicates. Means between the various groups were compared by one-way analysis of variance (ANOVA followed by Tukeyʼs post hoc test). In case of multiple comparisons, a post hoc Bonferroni correction was applied. P values < 0.01 and < 0.05 were considered statistically significant. Data were analyzed using GraphPad Prism 5 Software (GraphPad).
Results and discussion
Characterization of recombinant rDlP5βR1 and rDlP5βR2
Previously, various PRISEs of Digitalis and other plants have been heterologously expressed in E. coli and yeast and enzyme kinetics have been studied (Herl et al., 2006; Munkert et al. 2015a, b; Rieck et al. 2019). All available results (e.g., Herl et al. 2006; Perez-Bermúdez et al. 2010; Munkert et al. 2015a, b) show that PRISE-encoding gene P5βR1 is expressed constitutively in plants. In contrast, P5βR2 gene expression increased immediately after wounding or exposure to chemical stress resulting in elevated cardenolide levels in D. purpurea. Since recombinant DpP5βR2 has a higher substrate affinity for progesterone than recombinant DpP5βR1, it was suggested that DpP5βR2 is involved preferentially in cardenolide biosynthesis (Perez-Bermúdez et al. 2010). We here used the DpP5βR2 sequence described in the above publication to deduce primers allowing for the isolation and heterologous expression of a D. lanata homologue, termed DlP5βR2 (Suppl. Fig. S3). Recombinant forms of DlP5βR1 and DlP5βR2 (termed rDlP5βR1 and rDlP5βR2) were characterized with regard to their kinetic data for progesterone and its small mimic MVK (Table 1). rDlP5βR2 converted progesterone much faster than rDlP5βR1. On the other hand, rDlP5βR1 clearly discriminated between progesterone and MVK whereas rDlP5βR2 did not. MVK was accepted much better by rDlP5βR1 than by rDlP5βR2 indicating that DlP5βR1 and DlP5βR2 may play different roles within the plant. Differences in substrate specificity were similarly observed in other orthologue PRISEs in different plant species, such as Medicago, Catharanthus, Erysimum, and Arabidopsis (Munkert et al. 2015a, b; Nguyen and O’Connor 2020).
Table 1.
Kinetic constants of rDlP5βR1 and rDlP5βR2 for progesterone and methyl vinyl ketone (MVK)
| KM [mM] | kcat [s−1] | kcat/KM [M−1/s−1] | kcat/KM MVK:kcat/KM progesterone | |
|---|---|---|---|---|
| rDlP5βR1 | ||||
| Progesteronea | 0.36 | 0.02 | 52.2 | 97 |
| MVK | 0.27 | 1.37 | 5074 | |
| rDlP5βR2 | ||||
| Progesterone | 0.23 | 0.48 | 2115 | 0.56 |
| MVK | 0.21 | 0.26 | 1189 | |
aData from Bauer et al. (2012)
As both known PRISEs from D. lanata differ in their substrate specificity, one may assume different functions in planta. To address this question, we here generated RNAi-mediated gene knockdowns of DlP5βR1 and DlP5βR2 in D. lanata.
Stable transformation of Digitalis lanata L. shoots
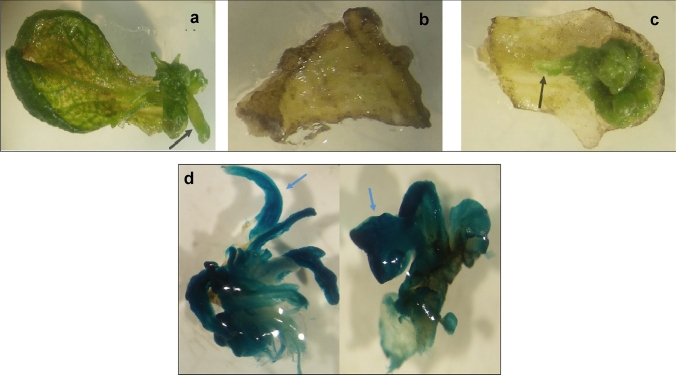
Shoots of the Digitalis lanata shoot culture line Dl1681 (Dl WT) were used for genetic transformation to ensure homogeneity in each line. Shoots were kept in liquid medium for approximately 9 days where they altered their morphology and became more susceptible to infection with Agrobacterium tumefaciens. The A. tumefaciens strain GV3101 containing the vector 679p935s-GusIo-rbs was used in the control transformation experiments (Dl VC). After 2 days of co-cultivation on solid medium I, leaf explants were transferred to selection medium II containing kanamycin and cefotaxime. Suitable selection conditions were verified by a negative control (explants transformed with Agrobacterium tumefaciens not carrying the nptII and GUS genes) and a positive control (non-transformed explants). Figure 2 shows a positive control on medium I (a), negative control after 3 weeks cultivation on medium II (b) and a transformed explant (c).
Fig. 2.
Development of shoot tissue on leaf explants after Agrobacterium transformation. a Positive control, b negative control, and c transformed explant. Shoots developed in the positive control and the transformed explant (black arrows) while the negative control showed only small areas with callus formation. d Histochemical GUS staining was used to detect the expression of the introduced GUS gene. Blue arrows show parts of the stained areas
Shoot tufts developing on transformed explants were transferred onto medium III and cultivated further. Stable integration of GUS and nptII was verified by PCR (Suppl. Fig. S1). GUS expression was also demonstrated histochemically (Fig. 2d). To ensure the absence of the Ti-plasmid, PCR against virD2 and the spectinomycin-resistance gene (spec) was carried out (Suppl. Fig. S1). About 5% of explants subjected to the transformation protocol established here developed into transgenic shoot tufts.
Digitalis comprises several important medicinal plant species, producing biologically active 5β-cardenolides. Therefore, a plenitude of protocols for conducting biochemical and molecular biological experiments have been established (Luckner and Wichtl 2000; Kreis 2017). Several groups reported the generation of genetically modified tissues (Moldenhauer et al. 1990; Pinkwart et al. 1998) and plants (Saito et al. 1990; Lehmann et al. 1995; Thomar et al. 1998; Sales et al. 2007; Pérez-Alonso et al. 2014; Kairuz et al. 2020) using Agrobacterium tumefaciens-mediated genetic transformation of protoplasts, embryo-like cell clusters, or leaf discs. Pradel et al. (1997) used A. rhizogenes to induce hairy root formation in D. lanata and regenerated transgenic plants. Regenerating intact plants, using any of these approaches, proved to be rather inefficient. For example, Lehmann et al. (1995) described that even in the best case scenario only 5% of all leaf explants produced callus and induction of organ development was not successful. Shoots regenerated from transformed protoplast showed an exceptionally low transformation rate (Lehmann et al. 1995). We here adopted permanent shoot cultures as the starting material to establish a reliable transformation protocol using A. tumefaciens-mediated genetic transformation (Barton et al. 1983). Our protocol yielded approximately 5% transformed shoots which was similar to the recently reported rates for Digitalis purpurea (Kairuz et al. 2020).
RNAi-mediated gene knockdown of DlP5βR1 and DlP5βR2 in Digitalis lanata shoots
The shoots used for Agrobacterium-mediated transformation were propagated in vitro. The transformation protocol described above was used for RNAi-mediated gene knockdown of DlP5βR1 (described by Herl et al. 2006) and DlP5βR2. DlP5βR2 is a homologue of D. purpurea P5βR2, first reported by Pérez-Bermúdez et al. (2010) when investigating the effect of various kinds of stress in D. purpurea. They found DlP5βR2 to be only weakly expressed in various tissues of D. purpurea but induced immediately after stress. This was paralleled by a transient boost in cardenolide formation. Homology-based screening in the available gene databases indicated the presence of more than two progesterone reductases in D. purpurea and D. lanata (Schmidt et al. 2018). We here focused on the two known genes only, accepting that the knockdown of them might not silence progesterone reductase activity completely. The transgenic shoot cultures established here proved to be an excellent system to address the questions related to the physiological impact of P5βR1 and P5βR2 in D. lanata. The transformed shoots remained unchanged for an extended period of time (over 3 years), produced cardenolides and allowed for experiments in a defined environment.
Gateway cloning employing the pHellsgate8 vector yielded several P5βR knockdown lines of D. lanata shoots. Constructs containing RNAi fragments designed against DlP5βR1 or DlP5βR2 were used for the transformation experiments. Expression of nptll was demonstrated by PCR to verify genetic transformation and integration of T-DNA into the plant genome (Suppl. Fig. S1).
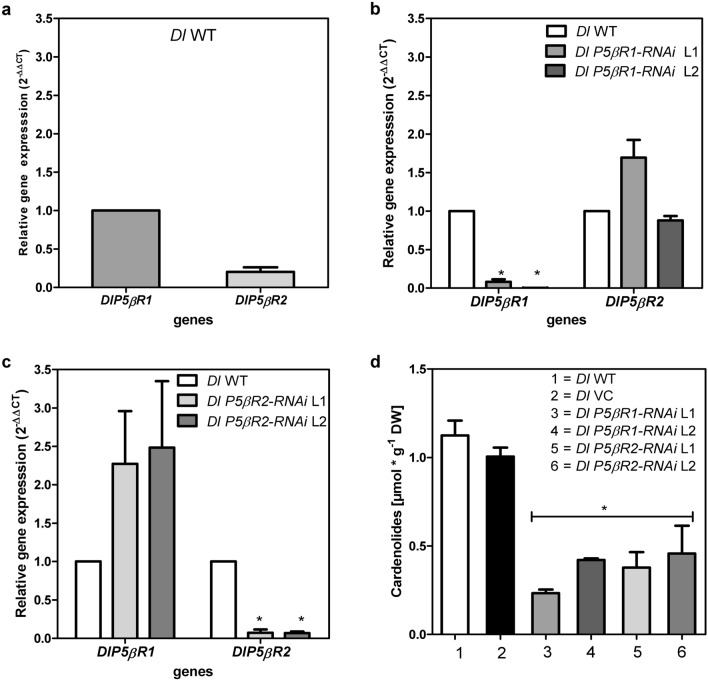
The expression of P5βR1 or P5βR2 in wild-type (WT) shoots as well as in RNAi-mediated gene knockdown lines was evaluated by qPCR (Fig. 3). Relative expression of P5βR1 in D. lanata WT shoots was around five times higher than that of P5βR2 (Fig. 3a).
Fig. 3.
Gene expression of DlP5βR1 and DlP5βR2 in Digitalis lanata WT and RNAi-mediated knockdown shoots. Relative RNA expression levels were calculated using the qPCR method by applying the 2−ΔΔCT method with actin as the reference gene. The y axis denotes the normalized relative transcript accumulation of the genes indicated in the x axis (mean ± SEM, n = 3; Tukey’s test, *P > 0.01). (a) Relative transcripts of P5βRs (DlP5βR1 and DlP5βR2) in D. lanata WT; (b) relative expression of DlP5βR1 and DlP5βR2 in a Dl P5βR1-RNAi knockdown line 1 (L1) and line 2 (L2) compared to WT shoots; c relative expression of DlP5βR1 and DlP5βR2 in a Dl P5βR2-RNAi knock down line 1 (L1) and line 2 (L2) compared to WT shoots; (d) quantification of digoxigenin and digitoxigenin in D. lanata WT shoots and transgenic shoots with reduced expression of P5βRs. Shoots transformed with 679p935s-GusIo-rbs (VC) were used as control to exclude artificial effects created by the transformation process (mean ± SEM; n = 9; Tukey’s test, *P > 0.05)
To demonstrate that the Agrobacterium transformation system itself does not influence P5βR expression, D. lanata shoots were transformed with 679p935s-GusIo-rbs (vector control) not harboring the RNAi constructs. qPCR revealed that P5βR expression was not influenced by the genetic transformation method used (Suppl. Fig. S4). On the other hand, all RNAi knockdown lines showed a strong reduction of either P5βR1 or P5βR2 expression. Exemplary gene expression is shown in two RNAi-mediated gene knockdown lines (Fig. 3b, c). In Dl P5βR1-RNAi knockdown lines P5βR1 expression was only 8–10% of that in WT shoots. P5βR2 expression in Dl P5βR2-RNAi knockdown lines was 7–13% of that in WT shoots. Knockdown of DlP5βR1 only slightly affected the expression of DlP5βR2 and vice versa (Fig. 3b, c). Gene-specific knockdown of the respective P5βR remained stable in the P5βR-RNAi lines used here for over 3 years in defined conditions.
P5βR protein activity was considerably lower in P5βR-RNAi knockdown lines than in WT shoots. Compared to heat inactivated controls WT shoots and the vector controls were able to convert 30–34%, of the fed progesterone, whereas P5βR-RNAi lines converted only 0–10%. This was indicative of the successful knockdown of the respective P5βR genes.
The influence of P5βR expression on cardenolide formation in D. lanata shoot cultures was investigated in some detail. Cardenolides were extracted in the central part of a culture cycle from WT shoots, from shoots transformed with the 679p935s-GusIo-rbs vector (vector control termed Dl VC) and from all Dl P5βR-RNAi knockdown lines. Digitoxigenin and digoxigenin, released after acidic hydrolysis of the cardenolides present in the extracts, were quantified by UPLC. Cardenolide levels of around 1 µmol g−1 DW were determined in WT shoots and vector controls, indicating that the transformation method had not influenced cardenolide levels. Cardenolide levels of WT shoots were very similar to those of earlier studies of D. lanata shoot cultures (Stuhlemmer et al. 1993; Christmann et al. 1993; Eisenbeiß et al. 1999) who reported levels of 0.6–1.0 µmol g−1 DW. Cardenolide contents were much lower in all Dl P5βR-RNAi knockdown lines. Cardenolide levels of 0.23–0.42 µmol g−1 DW and 0.38–0.46 µmol g−1 DW were detected in the Dl P5βR1-RNAi and Dl P5βR2-RNAi lines, respectively (Fig. 3d; exemplary for two Dl P5βR-RNAi knockdown lines) indicating that the lack of either of the PRISEs investigated here can impair cardenolide formation. The higher efficiency of rP5βR2 for progesterone conversion (Table 1) can maybe compensate for its lower expression abundancy (Fig. 3a). This may be taken as the first direct proof that P5βRs are involved in cardenolide biosynthesis as assumed previously (Gärtner et al. 1994; Kreis 2017).
Effects of glutathione and buthionine sulfoximine on P5βR gene expression as well as on cardenolide content
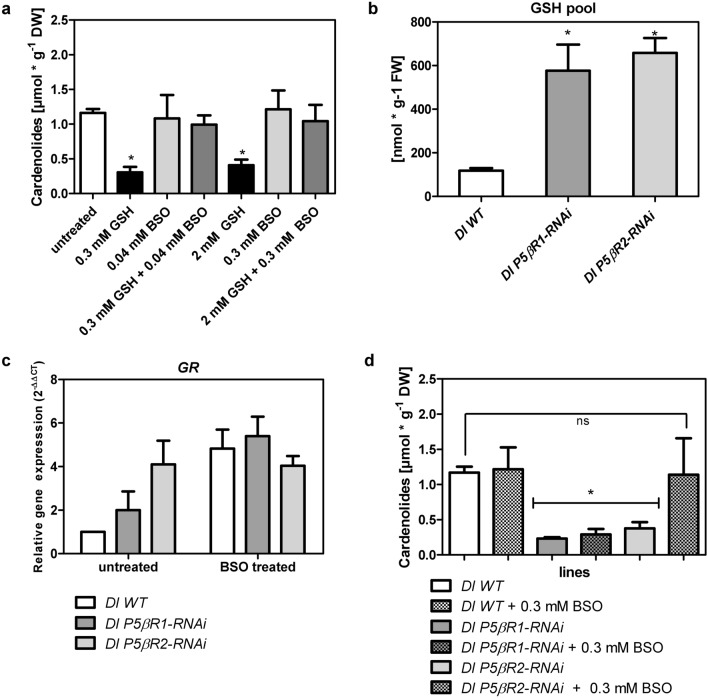
The knockdown of either DlP5βR1 or DlP5βR2 in D. lanata led to a decrease of the 5β-cardenolide content in shoot cultures (Fig. 3d). This can be taken as evidence that PRISEs are indeed involved in cardenolide formation. Berglund and Ohlsson (1993) reported that glutathione (GSH) impairs cardenolide biosynthesis. Therefore, elevated glutathione levels could also be responsible for the reduced cardenolide levels observed in the Dl P5βR-RNAi lines. Berglund and Ohlsson (1993) further reported that buthionine sulfoximine (BSO), described as an inhibitor of glutamylcysteine synthetase and stimulated cardenolide biosynthesis in D. lanata tissue cultures. They also demonstrated that the stimulating effect of BSO on digitoxin accumulation was diminished by the simultaneous addition of GSH. In our experiment with Dl WT shoots, we detected no stimulating effect of BSO on cardenolide level. This can be explained by the fact that the shoot cultures used here already contained considerable amounts of cardenolides which was not the case in the tissues used by Berglund and Ohlsson (1993). We did, however, determine the compensating effect after treating the shoots with GSH (Fig. 4a). As GSH levels can influence cardenolide level, we continued in our experiments to estimate GSH pools in WT and Dl P5βR-RNAi lines. In addition, we measured the expression of glutathione reductase (GR), and took it as a surrogate for imbalances in glutathione metabolism. GR reduces glutathione disulfide to glutathione and contributes to maintain the reducing environment in the cell in this way. Compared to WT shoots all RNAi-mediated P5βR knockdown lines showed increased levels of GSH and a higher gene expression of GR (Fig. 4b, c). Treatment with BSO amplified GR expression in WT shoots 5 times compared to non-treated controls but not in RNAi-mediated P5βR knockdown lines (Fig. 4c).
Fig. 4.
Effects of glutathione (GSH) and buthionine sulfoximine (BSO) treatment. a Effects of GSH and BSO alone or a combination on cardenolide level in D. lanata WT shoots; b estimated GSH pool in Dl WT and Dl P5βR-RNAi knockdown lines calculated by measuring t-GSH and GSSG. c Relative expression of glutathione reductase (GR) in D. lanata WT and Dl P5βR-RNAi knockdown shoots either untreated or BSO treated. RNA expression levels were calculated using the qPCR method by applying the 2−ΔΔCT method with actin as the reference gene. The y axis denotes the normalized relative transcript accumulation of the GR in the individual lines indicated in the figure legend. Mean ± SEM are shown (n = 3). d Quantification of digoxigenin and digitoxigenin in Dl WT and Dl P5βR-RNAi knockdown lines treated with 0.3 mM BSO (Mean ± SEM are shown n = 3). Tukey’s test, *P > 0.05
After BSO treatment cardenolide formation could be restored in Dl P5βR2-RNAi lines but not in DlP5βR1-RNAi lines indicating that P5βR1 is more important for constitutive cardenolide biosynthesis than P5βR2 (Fig. 4d). However, P5βR2 expression can be increased considerably by various types of stress. In the Dl P5βR1-RNAi lines, cardenolide biosynthesis could not be restored by adding BSO, indicating that the reduced cardenolide level was not only caused by altered GSH levels, but correlated directly with reduced P5βR1 expression (Fig. 4d). To the best of our knowledge, this is the first proof that P5βR1 is indeed directly involved in β-cardenolide formation. For example, VEP1, a P5βR1 homologue from Arabidopsis thaliana expressed in D. purpurea, did not enhance 5β-cardenolide accumulation (Kairuz et al. 2020).
Effects of 5β-pregnane-3,20-dione, progesterone and methyl vinyl ketone
Knowing that the successful knockdown of P5βR in D. lanata shoots caused a decrease in cardenolide level, we tried to restore cardenolide content by feeding 5β-pregnane-3,20-dione to WT and Dl P5βR1-RNAi lines. Interestingly, we observed a recovery of cardenolide content in Dl P5βR1-RNAi lines along with a reduction of glutathione levels. Cardenolide content in Dl P5βR1-RNAi lines reached around 2/3 (0.8 µmol × g−1 DW) of the cardenolide level of WT shoots. Still high GSH levels in Dl P5βR2-RNAi lines seem to continue disturbing cardenolide formation. Cardenolide levels increased here from around 0.3 µmol × g−1 DW to 0.5 µmol × g−1 DW. In WT shoots, feeding 5β-pregnane-3,20-dione did not influence cardenolide level (Suppl. Fig. S5 a, b).
Haussmann et al. (1997) administered various pregnanes to photomixotrophic, cardenolide-producing shoot cultures of Digitalis lanata as well as to cardenolide-free tissue cultures of the same plant. Depending on the pregnane precursor added the cardenolide content increased, but only in the mixotrophic shoot cultures. Studying effects of different pregnanes not only on WT shoots but also on RNAi knockdown lines similar to the study conducted by Haussmann et al. (1997) can give more detailed information on cardenolide formation in Digitalis lanata and will be part of future studies.
Since progesterone 5β-reductase activity was reduced in Dl P5βR-RNAi lines, progesterone may accumulate. Progesterone, a mammalian sex hormone, has been considered as a plant hormone (Iino et al. 2007). Assuming no feedback inhibition of progesterone biosynthesis or alternative metabolization, gene knockdown of progesterone-converting enzymes should lead to enhanced progesterone levels. We investigated the possibility of physiologically active progesterone being present by estimating progesterone levels using GC–MS and UPLC. We found that all RNAi lines contained less progesterone than the WT shoots and that cardenolide levels could not be restored by feeding exogenous progesterone to Dl P5βR-RNAi shoots (Suppl. Fig. S5c). Progesterone can also enhance the activity of antioxidant enzymes and increases GSH levels (Genisel et al. 2013). In our experiments, glutathione reductase (GR) expression was increased by a factor of 3 in Dl WT shoots and even by a factor 8 in all Dl P5βR-RNAi lines after 7 days of treatment with 0.3 mM progesterone (Suppl. Figure 5d), indicating again an imbalance of the redox state (endogenous GSH pool). This resulted in a disturbance of the progesterone metabolism and that might constitute a feedback regulation of progesterone biosynthesis. Feedback loops in pathways of specialized metabolism are known. For example, AOP2, a protein involved in aliphatic glucosinolate biosynthesis, mediated a feedback regulation in A. thaliana (Burow et al. 2015).
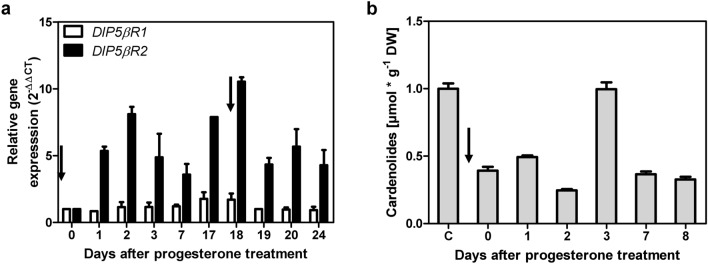
When progesterone (0.16 mM) was fed to WT shoots, it was consumed completely after 7 days of cultivation. When progesterone was fed repeatedly in identical batches, it was consumed faster with every new batch indicating an increase in progesterone-converting processes within the cell (data not shown). This can be explained by the sixfold increased expression of DlP5βR2 after the addition of progesterone, reaching a similar expression level as DlP5βR1 but having a much higher activity for converting progesterone (Table 1). DlP5βR2 expression was boosted after adding a new batch of progesterone (Fig. 5a). This explained the accelerated conversion of exogenous progesterone in the fed-batch experiment (kcat value for progesterone, Table 1). The expression of DlP5βR1 was only slightly affected by the progesterone treatment (Fig. 5a). Cardenolide formation peaked 3 days after the addition of progesterone (Fig. 5b). This increase was paralleled by a transient increase of DlP5βR2 expression. Similar effects were reported by Pérez-Bermúdez et al. (2010) when investigating stress provoked by wounding, hydroperoxide and 1-aminocyclopropane-1-carboxylic acid (ACC), a precursor of the phytohormone ethylene in plants. Progesterone was reported to occur in many plant species (Janeczko and Skoczowski 2005; Pauli et al. 2010) and exogenous progesterone influenced plant growth (Bhattacharya and Gupta 1981). It has a positive impact on abiotic stress resistance in a broad range of crops (Janeczko et al. 2013; Genisel et al. 2013; Hao et al. 2019). We, therefore, considered the cardenolide formation observed in a developmental context as “constitutive” biosynthesis (connected to the constitutive expression of DlP5βR1) and “stress-induced” biosynthesis (connected to the inducible expression of DlP5βR2 (Fig. 5a)).
Fig. 5.
Expression of progesterone 5β-reductases and cardenolide in shoot cultures of D. lanata after progesterone treatment. a Relative RNA expression levels were calculated by qPCR method by applying the 2−ΔΔCT method using actin as reference gene. The y axis denotes the normalized relative transcript accumulation of DlP5βR1 and DlP5βR2 genes after progesterone treatment indicated by black arrows. (Mean ± SEM are shown; n = 3). b Quantification of digoxigenin and digitoxigenin in D. lanata WT shoots after progesterone treatment indicated by black arrow. (Mean ± SEM are shown; n = 3)
Exogenous [14C]-progesterone was demonstrated to be incorporated into cardenolides. Low incorporation rates of less than 1% were observed with most of the radioactivity detected in a complex, unidentified mixture of products (Eisenbeiß et al. 1999). It is possible that the excess of progesterone not used for cardenolide biosynthesis influenced other metabolic processes.
Being an active α,β-unsaturated carbonyl, progesterone is a reactive electrophile substance (RES). In order to separate stress effects from precursor effects, we tested the effect of methyl vinyl ketone (MVK) on DlP5βR gene expression.
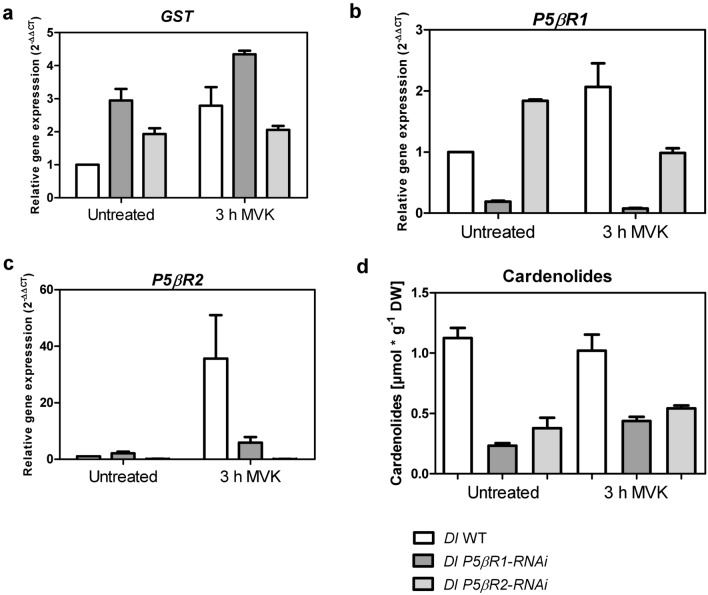
Methyl vinyl ketone (MVK) is the simplest form of such an α,β-unsaturated carbonyl and is a peroxidation product of trienoic fatty acids, that is known to accumulate in plants in stress situations (Vollenweider et al. 2000; Alméras et al. 2003; Kai et al. 2012; Jardine et al. 2013). It is not influencing “constitutive” cardenolide biosynthesis (Munkert et al. 2014; Horn et al. 2020). Furthermore, MVK is a substrate for P5βRs and can be detoxified by them (Table 1; Suppl. Fig. S6 a; Munkert et al. 2015a, b). Investigating the physiological impact of PRISEs in general, Dl WT and Dl P5βR-RNAi lines were treated with MVK for 3 h (Munkert et al. 2014). Glutathione S-transferase (GST) was used as positive control for an MVK related effect, as it is a stress-related gene (Alméras et al. 2003) and is known to be able to detoxify MVK (Horiyama et al. 2014; Suppl. Figure 6 b). We here found that after MVK treatment GST gene was upregulated in Dl WT but remained almost unchanged in Dl P5βR-RNAi lines (Fig. 6a).
Fig. 6.
Effects of MVK on gene expression, stress response and cardenolide content in Digitalis lanata WT and Dl P5βR-RNAi knockdown shoots. a–c The relative RNA expression levels were calculated using the qPCR method by applying the 2−ΔΔCT method with actin as the reference gene. The y axis denotes the normalized relative transcript accumulation of genes indicated at the top. X axis describes the MVK treatment conditions. Means ± SEM are shown (n = 3). Relative gene expression of glutathione S-transferase (GST) (a), P5βR1 (b) and P5βR2 (c), in D. lanata WT and Dl P5βR-RNAi knockdown shoots. d Quantification of digoxigenin and digitoxigenin in D. lanata WT and Dl P5βR-RNAi knockdown shoots treated with 2 µmol/L air volume MVK. Results are shown as mean ± SEM (n = 3)
In WT shoots, the expression of P5βR1 was only moderately enhanced (2.5 times) by MVK, whereas P5βR2 expression increased 35-fold (Fig. 6b, c). It is assumed that MVK is detoxified rapidly to methyl ethyl ketone (MEK), a metabolite which does not influence P5βR gene expression (Suppl. Figure 7). In Dl P5βR2-RNAi lines, P5βR1 expression was reduced to WT levels after MVK treatment, whereas P5βR2 expression was only moderately enhanced by MVK in Dl P5βR1-RNAi lines. Most obvious was the dramatic increase of P5βR2 expression in WT shoots but not in DlP5βR1-RNAi, which still contained the active P5βR2 enzyme. This indicates an altered stress response in Dl P5βR1-RNAi lines.
Similar to other studies (Munkert et al. 2014), cardenolide levels remained almost unchanged after MVK treatment in WT shoots (about 1 µmol g−1 DW) as well as in Dl P5βR-RNAi knockdown lines (0.3–0.5 µmol g−1) (Fig. 6d). Independently to cardenolide formation all in all, especially P5βR2 expression is related to plant stress response and P5βR2 enzyme might favorable be involved in detoxification reaction.
Conclusion
Our data led to the assumption that 5β-cardenolides are formed in a developmental process in which constitutively expressed P5βRs (progesterone 5β-reductases), such as P5βR1 are involved. Stress-induced P5βRs, such as P5βR2 efficiently, yet transiently boosting cardenolide biosynthesis, may be beneficial in an immediate chemical defence scenario.
Despite possibly being involved in other physiological processes, the stress-inducible P5βR2 has an affinity to progesterone much higher than that of P5βR1 in this way channeling more progesterone into the cardenolide pathway. Using RNAi-mediated P5βR1 and P5βR2 knockdown shoot culture lines of D. lanata, we here provide solid evidence that P5βRs are not only involved in cardenolide biosynthesis but are also important for plants dealing with stress by detoxifying reactive electrophile species, such as MVK.
However, detailed studies of the expression of stress-related genes and cardenolide formation over longer periods of time after treating WT shoots and Dl P5βR-RNAi lines with, e.g., progesterone, 5β-pregnane-3,20-dione (its biosynthetic follow-up product) or additional RES are necessary to further assess the impact of P5βR knockdown on stress metabolism.
Supplemental material: supplemental material includes SDS-page and western blot analysis of heterologous expressed DlP5βR2 in E. coli, verification and influence of stable transformation of and on D. lanata shoots as well as quantification and influence of progesterone and 5β-pregnane-3,20-dione in WT and RNAi lines. Tables listed primer pairs for qPCR analysis as well as verification of T-DNA integration in D. lanata genome. All sequences including accession numbers are also provided as Supporting Information.
Supplementary Information
Below is the link to the electronic supplementary material.
Acknowledgements
We are very thankful for the financial support of this study by a grant from the Dr. Hertha and Helmuth Schmauser-Stiftung (J.M.) and FAU Unibund Anschubfinanzierung (J.M.). We further thank Dr. Alexander Christmann for helpful advice regarding the manuscript editing, Barbara White for linguistic advice and Gabriele Fischer for lab assistance.
Abbreviations
- BSO
Buthionine sulfoximine
- GSH
Glutathione
- GR
Glutathione reductase
- GST
Glutathione S-transferase
- ISY
Iridoid synthases
- MVK
Methyl vinyl ketone
- PRISE
Progesterone 5β-reductase/iridoid synthase-like enzyme
- P5βR
Progesterone 5β-reductases
- PO
Progesterone
- PR
5β-Pregnane-3,20-dione
- RES
Reactive electrophile species
- St5βR
Steroid 5β-reductase
Author contribution statement
All the authors contributed to the study. WK and JM designed the study. Material preparation, data collection and analysis were performed mainly by JK with help from EH, ME, TL, AI, LW, MD and TL. The first draft of the manuscript was written by JK, WK and JM. Further, all the authors commented on previous versions of the manuscript. All the authors read and approved the final manuscript.
Funding
Open Access funding enabled and organized by Projekt DEAL.
Declarations
Conflict of interest
The authors have no conflict of interest.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- Allen GC, Flores-Vergara MA, Krasynanski S, Kumar S, Thompson WF. A modified protocol for rapid DNA isolation from plant tissues using cetyltrimethylammonium bromide. Nat Protoc. 2006;1:2320–2325. doi: 10.1038/nprot.2006.384. [DOI] [PubMed] [Google Scholar]
- Alméras E, Stolz S, Vollenweider S, Reymond P, Mène-Saffrané L, Farmer EE. Reactive electrophile species activate defense gene expression in Arabidopsis. Plant J. 2003;34:205–216. doi: 10.1046/j.1365-313x.2003.01718.x. [DOI] [PubMed] [Google Scholar]
- Barton KA, Binns AN, Matzke AJ, Chilton M-D. Regeneration of intact tobacco plants containing full length copies of genetically engineered T-DNA, and transmission of T-DNA to R1 progeny. Cell. 1983;32:1033–1043. doi: 10.1016/0092-8674(83)90288-X. [DOI] [PubMed] [Google Scholar]
- Bauer P, Munkert J, Brydziun M, Burda E, Müller-Uri F, Gröger H, Muller YA, Kreis W. Highly conserved progesterone 5 beta-reductase genes (P5 beta R) from 5 beta-cardenolide-free and 5 beta-cardenolide-producing angiosperms. Phytochemistry. 2010;71:1495–1505. doi: 10.1016/j.phytochem.2010.06.004. [DOI] [PubMed] [Google Scholar]
- Bauer P, Rudolph K, Müller-Uri F, Kreis W. Vein Patterning 1-encoded progesterone 5β-reductase: activity-guided improvement of catalytic efficiency. Phytochemistry. 2012;77:53–59. doi: 10.1016/j.phytochem.2012.01.022. [DOI] [PubMed] [Google Scholar]
- Berglund T, Ohlsson AB. The glutathione biosynthesis inhibitor buthionine-sulfoximine (BSO) induces cardenolide accumulation in Digitalis lanata tissue culture. J Plant Physiol. 1993;142:248–250. doi: 10.1016/S0176-1617(11)80973-9. [DOI] [Google Scholar]
- Bertol JW, Rigotto C, de Pádua RM, Kreis W, Barardi CRM, Braga FC, Simões CMO. Antiherpes activity of glucoevatromonoside, a cardenolide isolated from a Brazilian cultivar of Digitalis lanata. Antiviral Res. 2011;92:73–80. doi: 10.1016/j.antiviral.2011.06.015. [DOI] [PubMed] [Google Scholar]
- Bhattacharya B, Gupta K. Steroid hormone effects on growth and apical dominance of sunflower. Phytochemistry. 1981;20:989–991. doi: 10.1016/0031-9422(81)83014-2. [DOI] [Google Scholar]
- Bond SR, Naus CC. RF-Cloning.org: an online tool for the design of restriction-free cloning projects. Nucleic Acids Res. 2012;40:W209–W213. doi: 10.1093/nar/gks396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Burda E, Kraußer M, Fischer G, Hummel W, Müller-Uri F, Kreis W, Gröger H. Recombinant Δ4,5 -steroid 5β-reductases as biocatalysts for the reduction of activated C=C-double bonds in monocyclic and acyclic molecules. Adv Synth Catal. 2009;351:2787–2790. doi: 10.1002/adsc.200900024. [DOI] [Google Scholar]
- Burow M, Atwell S, Francisco M, Kerwin RE, Halkier BA, Kliebenstein DJ. The glucosinolate biosynthetic gene AOP2 mediates feed-back regulation of jasmonic acid signaling in Arabidopsis. Mol Plant. 2015;8:1201–1212. doi: 10.1016/j.molp.2015.03.001. [DOI] [PubMed] [Google Scholar]
- Christmann J, Kreis W, Reinhard E. Uptake, transport and storage of cardenolides in foxglove. Cardenolide sinks and occurrence of Cardenolides in the sieve tubes of Digitalis lanata. Botanica Acta. 1993;106:419–427. doi: 10.1111/j.1438-8677.1993.tb00769.x. [DOI] [Google Scholar]
- Durchschein K, Wallner S, Macheroux P, Schwab W, Winkler T, Kreis W, Faber K. Nicotinamide-dependent Ene reductases as alternative biocatalysts for the reduction of activated alkenes. Eur J Org Chem. 2012 doi: 10.1002/ejoc.201200776. [DOI] [Google Scholar]
- Eisenbeiß M, Kreis W, Reinhard E. Cardenolide biosynthesis in light- and dark-grown Digitalis lanata shoot cultures. Plant Physiol Biochem. 1999;37:13–23. doi: 10.1016/S0981-9428(99)80062-X. [DOI] [Google Scholar]
- Ernst M, de Padua RM, Herl V, Müller-Uri F, Kreis W. Expression of 3beta-HSD and P5betaR, genes respectively coding for Delta5-3beta-hydroxysteroid dehydrogenase and progesterone 5beta-reductase, in leaves and cell cultures of Digitalis lanata EHRH. Planta Med. 2010;76:923–927. doi: 10.1055/s-0030-1250007. [DOI] [PubMed] [Google Scholar]
- Gärtner DE, Keilholz W, Seitz HU. Purification, characterization and partial peptide microsequencing of progesterone 5 beta-reductase from shoot cultures of Digitalis purpurea. Eur J Biochem. 1994;225:1125–1132. doi: 10.1111/j.1432-1033.1994.1125b.x. [DOI] [PubMed] [Google Scholar]
- Genisel M, Turk H, Erdal S. Exogenous progesterone application protects chickpea seedlings against chilling-induced oxidative stress. Acta Physiol Plant. 2013;35:241–251. doi: 10.1007/s11738-012-1070-3. [DOI] [Google Scholar]
- Geu-Flores F, Sherden NH, Courdavault V, Burlat V, Glenn WS, Wu C, Nims E, Cui Y, O'Connor SE. An alternative route to cyclic terpenes by reductive cyclization in iridoid biosynthesis. Nature. 2012;492:138–142. doi: 10.1038/nature11692. [DOI] [PubMed] [Google Scholar]
- Hajdinák P, Czobor Á, Lőrincz T, Szarka A. The problem of glutathione determination: a comparative study on the measurement of glutathione from plant cells. Period Polytech Chem Eng. 2018;63:1–10. doi: 10.3311/PPch.11785. [DOI] [Google Scholar]
- Hao J, Li X, Xu G, Huo Y, Yang H. Exogenous progesterone treatment alleviates chilling injury in postharvest banana fruit associated with induction of alternative oxidase and antioxidant defense. Food Chem. 2019;286:329–337. doi: 10.1016/j.foodchem.2019.02.027. [DOI] [PubMed] [Google Scholar]
- Haussmann W, Kreis W, Stuhlemmer U, Reinhard E. Effects of various pregnanes and two 23-nor-5-cholenic acids on cardenolide accumulation in cell and organ cultures of Digitalis lanata. Planta Med. 1997;63(5):446–453. doi: 10.1055/s-2006-957731. [DOI] [PubMed] [Google Scholar]
- Herl V, Fischer G, Müller-Uri F, Kreis W. Molecular cloning and heterologous expression of progesterone 5beta-reductase from Digitalis lanata Ehrh. Phytochemistry. 2006;67:225–231. doi: 10.1016/j.phytochem.2005.11.013. [DOI] [PubMed] [Google Scholar]
- Herl V, Fischer G, Reva VA, Stiebritz M, Muller YA, Müller-Uri F, Kreis W. The VEP1 gene (At4g24220) encodes a short-chain dehydrogenase/reductase with 3-oxo-Delta 4,5-steroid 5beta-reductase activity in Arabidopsis thaliana L. Biochimie. 2009;91:517–525. doi: 10.1016/j.biochi.2008.12.005. [DOI] [PubMed] [Google Scholar]
- Horiyama S, Takahashi Y, Hatai M, Honda C, Suwa K, Ichikawa A, Yoshikawa N, Nakamura K, Kunitomo M, Date S, Masujima T, Takayama M. Methyl vinyl ketone, a toxic ingredient in cigarette smoke extract, modifies glutathione in mouse melanoma cells. Chem Pharm Bull. 2014;62:772–778. doi: 10.1248/cpb.c14-00109. [DOI] [PubMed] [Google Scholar]
- Horn E, Kemmler Y, Kreis W, Munkert J. Cardenolide and glucosinolate accumulation in shoot cultures of Erysimum crepidifolium Rchb. In Vitro Cell Dev Biol Plant. 2020 doi: 10.1007/s11627-020-10135-3. [DOI] [Google Scholar]
- Iino M, Nomura T, Tamaki Y, Yamada Y, Yoneyama K, Takeuchi Y, Mori M, Asami T, Nakano T, Yokota T. Progesterone: its occurrence in plants and involvement in plant growth. Phytochemistry. 2007;68:1664–1673. doi: 10.1016/j.phytochem.2007.04.002. [DOI] [PubMed] [Google Scholar]
- Janeczko A, Skoczowski A. Mammalian sex hormones in plants. Folia Histochem Cytobiol. 2005;43:71–79. [PubMed] [Google Scholar]
- Janeczko A, Oklešťková J, Siwek A, Dziurka M, Pociecha E, Kocurek M, Novák O. Endogenous progesterone and its cellular binding sites in wheat exposed to drought stress. J Steroid Biochem Mol Biol. 2013;138:384–394. doi: 10.1016/j.jsbmb.2013.07.014. [DOI] [PubMed] [Google Scholar]
- Jardine KJ, Meyers K, Abrell L, Alves EG, Yanez Serrano AM, Kesselmeier J, Karl T, Guenther A, Chambers JQ, Vickers C. Emissions of putative isoprene oxidation products from mango branches under abiotic stress. J Exp Bot. 2013;64:3697–3708. doi: 10.1093/jxb/ert202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jefferson RA, Kavanagh TA, Bevan MW. GUS fusions: beta-glucuronidase as a sensitive and versatile gene fusion marker in higher plants. EMBO J. 1987;6:3901–3907. doi: 10.1002/j.1460-2075.1987.tb02730.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jun JH, Ha CM, Nam HG. Involvement of the VEP1 gene in vascular strand development in Arabidopsis thaliana. Plant Cell Physiol. 2002;43:323–330. doi: 10.1093/pcp/pcf042. [DOI] [PubMed] [Google Scholar]
- Kai H, Hirashima K, Matsuda O, Ikegami H, Winkelmann T, Nakahara T, Iba K. Thermotolerant cyclamen with reduced acrolein and methyl vinyl ketone. J Exp Bot. 2012;63:4143–4150. doi: 10.1093/jxb/ers110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kairuz E, Pérez-Alonso N, Capote-Pérez A, Pérez-Pérez A, Espinosa-Antón AA, Angenon G, Jiménez E, Chong-Pérez B. Enhancement of cardenolide production in transgenic Digitalis purpurea L. by expressing a progesterone-5β-reductase from Arabidopsis thaliana L. Industrial Crops Products. 2020;146:112166. doi: 10.1016/j.indcrop.2020.112166. [DOI] [Google Scholar]
- Kreis W. The foxgloves (digitalis) revisited. Planta Med. 2017;83:962–976. doi: 10.1055/s-0043-111240. [DOI] [PubMed] [Google Scholar]
- Lehmann U, Moldenhauer D, Thomar S, Diettrich B, Luckner M. Regeneration of plants from digitalis lanata cells transformed with agrobacterium tumefaciens carrying bacterial genes encoding neomycin phosphotransferase II and β-glucuronidase. J Plant Physiol. 1995;147:53–57. doi: 10.1016/S0176-1617(11)81412-4. [DOI] [Google Scholar]
- Luckner M, Wichtl M. Digitalis: Geschichte, Biologie, Biochemie, Chemie, Physiologie, Molekularbiologie, Pharmakologie. Medizinische Anwendung: Wissenschaftliche Verlagsgeschellschaft mbH, Stuttgart; 2000. [Google Scholar]
- Moldenhauer D, Fürst B, Diettrich B, Luckner M. Cardenolides in Digitalis lanata cells transformed with Ti-plasmids. Planta Med. 1990;56:435–438. doi: 10.1055/s-2006-961005. [DOI] [PubMed] [Google Scholar]
- Munkert J, Ernst M, Müller-Uri F, Kreis W. Identification and stress-induced expression of three 3β-hydroxysteroid dehydrogenases from Erysimum crepidifolium Rchb. and their putative role in cardenolide biosynthesis. Phytochemistry. 2014;100:26–33. doi: 10.1016/j.phytochem.2014.01.006. [DOI] [PubMed] [Google Scholar]
- Munkert J, Costa C, Budeanu O, Petersen J, Bertolucci S, Fischer G, Müller-Uri F, Kreis W. Progesterone 5β-reductase genes of the Brassicaceae family as function-associated molecular markers. Plant Biol (stuttg) 2015;17:1113–1122. doi: 10.1111/plb.12361. [DOI] [PubMed] [Google Scholar]
- Munkert J, Pollier J, Miettinen K, van Moerkercke A, Payne R, Müller-Uri F, Burlat V, O'Connor SE, Memelink J, Kreis W, Goossens A. Iridoid synthase activity is common among the plant progesterone 5β-reductase family. Mol Plant. 2015;8:136–152. doi: 10.1016/j.molp.2014.11.005. [DOI] [PubMed] [Google Scholar]
- Murashige T, Skoog F. A revised medium for rapid growth and bio assays with tobacco tissue cultures. Physiol Plant. 1962;15:473–497. doi: 10.1111/j.1399-3054.1962.tb08052.x. [DOI] [Google Scholar]
- Nguyen T-D, O'Connor SE. The progesterone 5β-Reductase/Iridoid synthase family: a catalytic reservoir for specialized metabolism across land plants. ACS Chem Biol. 2020;15:1780–1787. doi: 10.1021/acschembio.0c00220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pauli GF, Friesen JB, Gödecke T, Farnsworth NR, Glodny B. Occurrence of progesterone and related animal steroids in two higher plants. J Nat Prod. 2010;73:338–345. doi: 10.1021/np9007415. [DOI] [PubMed] [Google Scholar]
- Pérez-Alonso N, Chong-Pérez B, Capote A, Pérez A, Izquierdo Y, Angenon G, Jiménez E. Agrobacterium tumefaciens-mediated genetic transformation of Digitalis purpurea L. Plant Biotechnol Rep. 2014;8:387–397. doi: 10.1007/s11816-014-0329-0. [DOI] [Google Scholar]
- Pérez-Bermúdez P, García AAM, Tuñón I, Gavidia I. Digitalis purpurea P5betaR2, encoding steroid 5 beta-reductase, is a novel defense-related gene involved in cardenolide biosynthesis. New Phytol. 2010;185:687–700. doi: 10.1111/j.1469-8137.2009.03080.x. [DOI] [PubMed] [Google Scholar]
- Pinkwart W, Diettrich B, Luckner M. Uptake of Cardenolides from phloem sap into crown galls of Digitalis lanata. Phytochemistry. 1998;49:71–77. doi: 10.1016/S0031-9422(97)01010-8. [DOI] [Google Scholar]
- Pradel H, Dumke-Lehmann U, Diettrich B, Luckner M. Hairy root cultures of Digitalis lanata. Secondary metabolism and plant regeneration. J Plant Physiol. 1997;151:209–215. doi: 10.1016/S0176-1617(97)80154-X. [DOI] [Google Scholar]
- Prassas I, Diamandis EP. Novel therapeutic applications of cardiac glycosides. Nat Rev Drug Discov. 2008;7:926–935. doi: 10.1038/nrd2682. [DOI] [PubMed] [Google Scholar]
- Rahman I, Kode A, Biswas SK. Assay for quantitative determination of glutathione and glutathione disulfide levels using enzymatic recycling method. Nat Protoc. 2006;1:3159–3165. doi: 10.1038/nprot.2006.378. [DOI] [PubMed] [Google Scholar]
- Rieck C, Geiger D, Munkert J, Messerschmidt K, Petersen J, Strasser J, Meitinger N, Kreis W. Biosynthetic approach to combine the first steps of cardenolide formation in Saccharomyces cerevisiae. Microbiologyopen. 2019;8:e925. doi: 10.1002/mbo3.925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sahoo S, Awasthi JP, Sunkar R, Panda SK. Determining glutathione levels in plants. Methods Mol Biol. 2017;1631:273–277. doi: 10.1007/978-1-4939-7136-7_16. [DOI] [PubMed] [Google Scholar]
- Saito K, Yamazaki M, Shimomura K, Yoshimatsu K, Murakoshi I. Genetic transformation of foxglove (Digitalis purpurea) by chimeric foreign genes and production of cardioactive glycosides. Plant Cell Rep. 1990;9:121–124. doi: 10.1007/BF00232085. [DOI] [PubMed] [Google Scholar]
- Sales E, Muñoz-Bertomeu J, Arrillaga I, Segura J. Enhancement of cardenolide and phytosterol levels by expression of an N-terminally truncated 3-hydroxy-3-methylglutaryl CoA reductase in Transgenic Digitalis minor. Planta Med. 2007;73:605–610. doi: 10.1055/s-2007-967199. [DOI] [PubMed] [Google Scholar]
- Schaller F, Kreis W. Cardenolide genin pattern in Isoplexis plants and shoot cultures. Planta Med. 2006;72:1149–1156. doi: 10.1055/s-2006-947194. [DOI] [PubMed] [Google Scholar]
- Schmidt K, Petersen J, Munkert J, Egerer-Sieber C, Hornig M, Muller YA, Kreis W. PRISEs (progesterone 5β-reductase and/or iridoid synthase-like 1,4-enone reductases): catalytic and substrate promiscuity allows for realization of multiple pathways in plant metabolism. Phytochemistry. 2018;156:9–19. doi: 10.1016/j.phytochem.2018.08.012. [DOI] [PubMed] [Google Scholar]
- Schmittgen TD, Livak KJ. Analyzing real-time PCR data by the comparative C(T) method. Nat Protoc. 2008;3:1101–1108. doi: 10.1038/nprot.2008.73. [DOI] [PubMed] [Google Scholar]
- Schneider NFZ, Cerella C, Simões CMO, Diederich M. Anticancer and immunogenic properties of cardiac glycosides. Molecules. 2017 doi: 10.3390/molecules22111932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stevens RC. Design of high-throughput methods of protein production for structural biology. Structure. 2000;8(9):R177–R185. doi: 10.1016/s0969-2126(00)00193-3. [DOI] [PubMed] [Google Scholar]
- Stuhlemmer U, Kreis W, Eisenbeiss M, Reinhard E. Cardiac glycosides in partly submerged shoots of Digitalis lanata. Planta Med. 1993;59:539–545. doi: 10.1055/s-2006-959757. [DOI] [PubMed] [Google Scholar]
- Tarrío R, Ayala FJ, Rodríguez-Trelles F. The Vein Patterning 1 (VEP1) gene family laterally spread through an ecological network. PLoS ONE. 2011;6:e22279. doi: 10.1371/journal.pone.0022279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomar S, Dumke-Lehmann U, Diettrich B, Luckner M. Gene control in somatic embryos of Digitalis lanata : expression of the β-Glucuronidase gene fused to a plastocyanin promoter. Botanica Acta. 1998;111:22–27. doi: 10.1111/j.1438-8677.1998.tb00672.x. [DOI] [Google Scholar]
- Unger T, Jacobovitch Y, Dantes A, Bernheim R, Peleg Y. Applications of the restriction free (RF) cloning procedure for molecular manipulations and protein expression. J Struct Biol. 2010;172:34–44. doi: 10.1016/j.jsb.2010.06.016. [DOI] [PubMed] [Google Scholar]
- Vollenweider S, Weber H, Stolz S, Chételat A, Farmer EE. Fatty acid ketodienes and fatty acid ketotrienes: Michael addition acceptors that accumulate in wounded and diseased Arabidopsis leaves. Plant J. 2000;24:467–476. doi: 10.1046/j.1365-313x.2000.00897.x. [DOI] [PubMed] [Google Scholar]
- Wiegrebe H, Wichtl M. High-performance liquid chromatographic determination of cardenolides in Digitalis leaves after solid-phase extraction. J Chromatogr A. 1993;630:402–407. doi: 10.1016/0021-9673(93)80478-q. [DOI] [PubMed] [Google Scholar]
- Winter D, Vinegar B, Nahal H, Ammar R, Wilson GV, Provart NJ. An "electronic fluorescent pictograph" browser for exploring and analyzing large-scale biological data sets. PLoS ONE. 2007;2:e718. doi: 10.1371/journal.pone.0000718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Withering W. An account of the foxglove and some of its medical uses, with practical remarks on dropsy, and other diseases. In: Willins FA, Keys TE, editors. Classics of cardiology. New York: Henry Schuman Dover Publications; 1941. pp. 231–252. [Google Scholar]
- Yang KY, Moon YH, Choi KH, Kim YH, Eun MY, Guh JO, Kim KC, Cho BH. Structure and expression of the AWI 31 gene specifically induced by wounding in Arabidopsis thaliana. Mol Cells. 1997;7:131–135. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.