Abstract
The development of a high sensitivity real-time sensor for multi-site detection of dopamine (DA) with high spatial and temporal resolution is of fundamental importance to study the complex spatial and temporal pattern of DA dynamics in the brain, thus improving the understanding and treatments of neurological and neuropsychiatric disorders. In response to this need, we present here a high-surface area out-of-plane grown three-dimensional (3D) fuzzy graphene (3DFG) microelectrode arrays (MEAs) for highly selective, sensitive, and stable DA electrochemical sensing. 3DFG microelectrodes present a remarkable sensitivity to DA (2.12 ± 0.05 nA/nM, with LOD of 364.44 ± 8.65 pM), the highest reported for nanocarbon MEAs using Fast Scan Cyclic Voltammetry (FSCV). The high surface area of 3DFG allows for miniaturization of electrode down to 2 × 2 μm2, without compromising the electrochemical performance. Moreover, 3DFG MEAs are electrochemically stable under 7.2 million scans of continuous FSCV cycling, present exceptional selectivity over the most common interferents in vitro with minimum fouling by electrochemical byproducts and can discriminate DA and serotonin (5-HT) in response to the injection of their 50:50 mixture. These results highlight the potential of 3DFG MEAs as a promising platform for FSCV based multi-site detection of DA with high sensitivity, selectivity, and spatial resolution.
Keywords: 3D graphene, microelectrode arrays (MEAs), dopamine, fast scan cyclic voltammetry, electrochemical sensing
Introduction
Dopamine (DA) is involved in different neurological disorders such as schizophrenia (Howes and Kapur 2009; Seeman 1987; Swerdlow and Koob 1987), drug abuse (Di Chiara 1995; Höglinger et al. 2004; Newman et al. 2005; Salamone et al. 2003; Volkow et al. 2004), and Parkinson’s disease (Höglinger et al. 2004; Lotharius and Brundin 2002; Weintraub et al. 2006). DA functions are often controlled by separate neural circuits involving connections arising from different DA-releasing nuclei and projections to separate afferent targets (Saddoris et al. 2013). Consequently, DA release dynamic is complex and differs in different brain regions or structural subregions (Jenkins et al. 2004; Owesson‐ White et al. 2009; Puthongkham and Venton 2020; Roeper 2013; Watabe-Uchida et al. 2012), requiring high resolution multi-site measurements to understand its spatial and temporal pattern. The lack of sensors capable of accurate readings of the complex brain circuits in which DA neurons participate represents a major limitation for a more sophisticated understanding of the role of DA in neurological disorders, and for the improvement of their treatments (Saddoris et al. 2013). Thus, the development of a high sensitivity real-time sensor for a quantitative mapping of stimulus-evoked dopamine release with high spatial and temporal resolution is highly desired and has critical implications for both neuroscience and clinical communities.
For the last 3 decades, carbon fiber electrodes (CFEs) under fast scan cyclic voltammetry (FSCV) have been considered the gold standard for in-vivo DA detection (Ou et al. 2019; Puthongkham and Venton 2020). FSCV relies on the direct electron transfer reaction between redox active molecules and the electrode. Thus, by sweeping the potential window at fast scan rates (~400 V/s or higher), FSCV present sub-second temporal resolutions (hundreds of millisecond (Jacobs et al. 2014; Keithley et al. 2011; Robinson et al. 2003) that is consistent with the scale of chemical fluctuations at neuronal synapsis.(Hashemi 2013; Oh et al. 2016; Ou et al. 2019; Robinson et al. 2003; Swamy and Venton 2007a). When used in combination with behavioral and/or pharmacological paradigms, this technique has provided groundbreaking knowledge on the molecular mechanisms underlying aspects of goal-driven action and associative learning (Howe et al. 2013; Phillips et al. 2003; Roitman et al. 2004; Stouffer et al. 2015). However, FSCV measurements with CFEs are usually limited to one electrode site at a time. Additionally, CFEs present suboptimal selectivity for DA over other electrochemical species and are subjected to electrochemical fouling due to carbon degradation or electrochemical byproduct deposition when exposed to brain tissue, which results in diminished sensing capabilities over time (Harreither et al. 2016; Hensley et al. 2018; Mohebi et al. 2019; Puthongkham and Venton 2020).
To develop in-vivo robust neurotransmitter sensing paradigms, including high spatial resolution sensing, multisite measurements, simultaneous detection of different neurotransmitters, and multimodality integration, these limitations must be resolved.
To enable multi-site high spatial resolution DA sensing, CFE arrays have been fabricated and successful DA detection has been demonstrated, both in acute and chronic studies (Schwerdt et al. 2017; Schwerdt et al. 2018). However, their fabrication process is semi-manual, which is a bottleneck for batch-fabrication and scaled up production of high-density electrodes with a 3D arrangement.
On the other hand, various strategies have been explored to improve CFE sensitivity, selectivity to specific neurotransmitters, and fouling properties. For example, CFEs have been coated with different edge plane-rich, sp2-hybridized carbon nanomaterials, i.e. carbon nanotubes (CNTs)(Puthongkham and Venton 2020; Swamy and Venton 2007a), carbon nanohorns (CNHs),(Puthongkham et al. 2018), nanodiamonds (NDs) (Puthongkham and Venton 2019), and graphene oxide nanosheets alone or as composite with conducting polymers (Taylor et al. 2017). Such hybrid electrodes demonstrate different degrees of improvements in sensitivity and detection limits along with enhanced antifouling properties (Puthongkham and Venton 2020; Puthongkham et al. 2018). However, the stability of the coatings is a potential limitation towards chronic sensing of neurotransmitters. In alternative to CFEs, porous CNT fibers (Yang et al. 2017; Zestos et al. 2014) and CNT yarn microelectrodes (Jacobs et al. 2014; Shao et al. 2020) have been considered for DA detection. Their porous surface area has shown to enhance the sensitivity and the fouling resistance but, similarly to CFEs, CNT-fibers have been mainly used in a single working electrode arrangement or they would require manual assembling.
So far, only a handful of efforts have been documented in literature for batch-fabrication of carbon or nanocarbon-based microelectrode arrays (MEAs) using well-established batch-microfabrication techniques (Castagnola et al. 2020a, 2021; Castagnola et al. 2018; Fan et al. 2020; Zachek et al. 2010b). For example, 4-channel arrays of pyrolyzed glassy carbon (GC) on silicon substrate (Zachek et al. 2010a), and polyimide substrates (Castagnola et al. 2018) were used for DA sensing. Despite their success in multisite in-vivo DA detection under FSCV (Zachek et al. 2010a), and their superior DA sensitivity compared to CFEs (Zachek et al. 2010a), GC exhibits a smooth surface topography with limited electrochemical surface area, rendering further miniaturization difficult (El Merhie et al. 2018; Goshi et al. 2018; Pancrazio et al. 2017; Vomero et al. 2017). 4 channels of boron-doped diamond (BDD) microelectrodes on flexible Parylene C substrate have also been explored for neurotransmitter detection (Fan et al. 2020). BDD microelectrodes demonstrated outstanding electrochemical properties, extremely wide potential window, high chemical stability good biocompatibility and low tendency to bio-fouling (Fan et al. 2020). However, diamond is required to be doped to gain an acceptable conductivity and do not achieve the sensitivity presented by other carbon electrodes with smaller size.
Graphene-based MEAs have been fabricated for simultaneous recordings of the electrophysiology and Ca2+ signaling in electrogenic cells (El Merhie et al. 2018; Liu et al. 2019; Lu et al. 2018; Rastogi et al. 2018). However, they have not been widely investigated for FSCV detection in their basal plane configuration. The introduction of defects to graphene has been shown to enhance DA and serotonin (5-HT) adsorption and consequently increase detection sensitivity using FSCV, (Cao et al. 2019; Puthongkham and Venton 2020) making defect-rich graphene an attractive candidate for FSCV detection.
Here, we present high-surface area out-of-plane grown three-dimensional (3D) fuzzy graphene (3DFG) microelectrode arrays (MEAs). To do so, we combined a high controllable catalyst-free bottom-up growth of graphene flakes, that enable the tunability of the 3DFG flake density and size, with a standard micro- and nano-fabrication photolithographic process, that patterned the synthesized 3DFG into functional microelectrodes with sizes varying from ca. 2 × 2 μm2 to 50 × 50 μm2.
To demonstrate the significantly enhanced electrochemical properties of the 3DFG flakes for DA detection, we extensively characterized, for the first time, the DA sensitivity, selectivity, electrochemical stability, and fouling resistance of the 3DFG microelectrodes and we compared the performance with the current standard of CFEs. We demonstrated that 3DFG microelectrodes outperform the state-of-the-art materials and that the enormous 3DFG’s surface area enables the miniaturization of the electrode footprint down to ca. 2 × 2 μm2, which allow densely packed MEA configurations, offering the unique possibility of multi-channel DA mapping with high spatial and temporal resolution.
1. Material and Methods
2.1. 3DFG Microelectrode Array Fabrication
2.1.1. Outer contacts/interconnects patterning.
A (100) Si substrate with a 600 nm wet thermal oxide (p-type, ≤0.005 Ω cm, Nova Electronic Materials Ltd., catalog no. CP02 11208-OX) was cleaned by sonication for 5 min. in acetone. It was rinsed in iso-propyl alcohol (IPA) and blow dried with N2. The cleaned Si/SiO2 substrate was treated with O2 plasma. Pt outer contacts and interconnects were patterned using photolithography technique. 300 nm LOR3A (MicroChem) was spin-coated at 4000 rpm for 40 s followed by baking at 190 °C for 5 min. 500 nm Shipley S1805 (MicroChem) was then spin-coated followed by baking at 115 °C for 5 min. The resists were patterned using a mask aligner (Karl Suss MA6) followed by development for 1 min in CD26 developer (MicroChem). 5 nm Cr and 100 nm Pt were evaporated using e-beam evaporator (Kurt J. Lesker). Lift-off was performed in Remover PG (MicroChem) at 60 °C for 30 min, followed by acetone and IPA rinse.
2.1.2. 3D fuzzy graphene (3DFG) synthesis.
3DFG was synthesized through plasma-enhanced chemical vapor deposition (PECVD) process.(Garg et al. 2017) The synthesis process was carried out at 800 °C at a total pressure of 0.5 Torr for 90 min under 50 standard cubic centimeters per minute (sccm) of CH4 (5 % CH4 in Ar, Airgas). The temperature of the furnace was ramped up to 800 °C under 100 sccm of Ar (Matheson Gas). A 50 W inductively coupled plasma (ICP) was generated using a 13.56 MHz RF power supply (AG 0313 Generator and AIT-600 RF, power supply and auto tuner, respectively, T&C Power Conversion, Inc.). The plasma was turned off after the synthesis step and the 3DFG coated substrate was rapidly cooled to room temperature under the flow of 100 sccm Ar.
2.1.3. 3DFG microelectrodes patterning.
100 nm SiO2 film was deposited on 3DFG coated chips using PECVD (Trion Orion) at 375 °C, 60 W power, and 900 mTorr under 75 sccm SiH4 and 70 sccm N2O. The SiO2 coated samples were baked at 95 °C for 5 min followed by an O2 plasma treatment for 1 min at 100 W. Using standard photolithography techniques 100 nm Cr hard mask was patterned in the shape of the 50, 5 and 2 μm electrodes on the SiO2 layer. SiO2 from the regions not covered by the Cr hard-mask was etched off using reactive ion etching (RIE) (Plasma Therm 790 RIE) under 22.5 sccm CHF3 and 16 sccm O2 at 100 W power and 100 mTorr for 5 min. Post SiO2 etching, 3DFG from the non-electrode regions was also etched by RIE using 16 sccm O2 and 6 sccm Ar at 20 W power and 10 mTorr for 60 min. Post etching, Cr hard mask and the underlying SiO2 film were etched off using Cr etchant (Transene, 1020AC) and buffered oxide etchant (BOE, Transene), respectively. Finally, the Pt interconnects and the non-recording site of the 3DFG electrodes were passivated with 2 μm SU-8 (MicroChem, SU-8 2002).
2.2. Carbon fiber microelectrode fabrication.
CFEs were fabricated as previously described in (Castagnola et al. 2020b; Taylor et al. 2019). Briefly, borosilicate capillaries (0.4 mm ID, 0.6 mm OD; A- M systems Inc., Sequim, WA, USA), each containing a single carbon fiber (7μm diameter, T650; Cytec Carbon Fibers LLC., Piedmont, SC, USA), were pulled to a fine tip using a vertical puller (Narishige, Los Angeles, CA, USA). The tip was sealed with epoxy (Spurr Epoxy; Polysciences Inc., Warrington, PA, USA) and the exposed fiber was cut 400 μm from the glass seal using a scalpel under an optical microscope (Szx12, Olympus). A mercury drop was placed in the barrel for electrical contact to a hookup wire (Nichrome; Goodfellow, Oakdale, PA, USA). CFEs were soaked in isopropyl alcohol (Castagnola et al. 2020b; Taylor et al. 2019) (Fisher Chemical, USA) for 20 minutes prior to use.
2.3. Electrochemical Characterization.
EIS were performed in 1x phosphate buffered saline (PBS, composition: 11.9 mM Na2HPO4 and KH2PO4., 137mM NaCl., 2.7 mM KCl, pH 7.4) applying a sine wave (10 mV RMS amplitude) onto the open circuit potential while varying the frequency from 1 to 105 Hz. EIS was carried out using a potentiostat/galvanostat (Autolab, Metrohm, USA) connected to a three-electrode electrochemical cell with a platinum counter electrode and an Ag/AgCl reference electrode. During the CV tests, the working electrode potential was swept between 1 and −1 V (vs Ag/AgCl) with a scan rate of 100 mV/s. The charge storage capacity (CSC, mC/cm2) was calculated as CSC=(∫didt)/(geometric area) in an entire CV cycle.
2.4. Scanning electron microscopy (SEM) characterization.
SEM imaging was performed using a FEI Quanta 600 field emission gun (FEG) SEM. High-resolution images (2048 × 1768 pixels) were acquired at accelerating voltages of 5–20 kV with a working distance of 5 mm.
2.5. Raman spectroscopy characterization.
Raman spectroscopy was performed using NT-MDT NTEGRA Spectra with 532 nm excitation through a 100× objective. Raman spectra were acquired with 0.5 ND filter and an acquisition time of 30 s. Raman spectra was acquired from 30 points across 3 independent MEA chips.
2.6. Fast Scan Cyclic Voltammetry electrochemical detection.
FSCV were performed with an EI 400 (Ensman Instruments; Bloomington, IN, USA), controlled by the CV Tar Heels LabVIEW program (CV Tar Heels v4.3, University of North Carolina, Chapel Hill, NC, USA), and a 4-channel WaveNeuro FSCV Potentiostat System (Pine Research, Durham, NC 27705 USA), controlled by HDCV software (UNC at Chapel Hill, North Carolina, USA). The headstage gain was set to 1 or 5 MΩ (depending by the electrode size). Data were analyzed using HDCV software (UNC Chapel Hill, North Carolina, USA). The electrode was scanned from −0.5 to 1.3 V (vs Ag/AgCl) and back with a 400 V/s scan rate and a repetition rate of 10 Hz. DA detection was identified by inspection of background- subtracted cyclic voltammograms. In vitro DA (Dopamine hydrochloride, >98.0%, Sigma-Aldrich, St. Louis, USA) calibration were performed using freshly prepared, nitrogen-purged DA standard solutions dissolved in 1x PBS, with and without interfering agents. Electrode sensitivities were determined by the linear regression slope of the maximum faradaic current vs. DA concentration calibration plots.
3DFG selectivity has been assessed toward 200 μM L-ascorbic acid (AA, > 99.0%, BioXtra, Sigma, St. Louis, USA), 10 μM uric acid (UA, > 99.0%, Sigma Life Science, St. Louis, USA), usually presented in the brain at several order of magnitude higher than DA (Yang et al. 2017), 10 μM dihydroxyphenylacetic acid (DOPAC, 98.0%, Sigma), a DA metabolite, and 100 nM of DA, similar concentration than the absolute basal DA level measured in the rat dorsal striatum in our previous study (82 ± 6 nM).(Taylor et al. 2019). Additionally, we investigated the possible interference from epinephrine (EP, > 99.0%, Sigma, St. Louis, USA), and serotonin (5-HT, > 99.0%, Sigma, St. Louis, USA). To explore the ability of 3DFG electrodes to simultaneously detect DA and 5-HT, we also added 5-hydroxyindole-3-acetic acid (5-HIAA, 99%, Across Organics), the precursor of 5-HT, to exclude his interference with 5-HT.
2.7. FSCV Stability tests
The 3DFG and CFE microelectrodes were cycled under FSCV experiment for at least 200 h, corresponding to 7.2 million cycles at 10Hz, using a standard triangular waveform from −0.5 to 1.3 V with 400V/s scan rate, at 10Hz in PBS. The electrode sensitivity to 1μM DA and the total background current of the microelectrodes were monitored after 0.9, 2.7, 3.6, 5.4 and 7.2 million FSCV cycles, corresponding to 25, 50, 75,100, 150 and 200 hours). The CSC was calculated as CSC=(∫didt) / (geometric area) in an entire CV cycle.
2.8. FSCV Fouling characterization.
The fouling test consists in continuous FSCV cycling (triangular waveform −0.5 to 1.3 V with 400V/s scan rate, at 10Hz) of the 3DFG microelectrodes and CFEs for 24 h (corresponding to 1 million FSCV scans) in 1x PBS containing 200 μM AA, 10 μM UA, 10 μM DOPAC, and 100 nM DA. The electrode sensitivity in response to a bolus injection of 1μM DA was monitored at different timepoints (0, 0.1, 0.4, 1 million FSCV cycling).
2.9. Statistical analyses.
Statistical analyses were conducted using Origin Pro 8.1 (OriginLab Corp, Northampton, MA, USA).
One-way repeated measures ANOVA with Bonferroni post-tests was used to calculate 1) changes in the sensitivity of CFEs and 3DFG microelectrodes to DA at different time point (0, 0.9 million, 2.7, 3.6, 5.4 and 7.2 million FSCV cycles) under prolonged FSCV cycling; 2) changes in charge storage capacity (CSC, mC/cm2) of CFEs and 3DFG microelectrodes to DA at different time point under continuous FSCV scanning; and 3) changes in the sensitivity of CFEs and 3DFG microelectrodes to DA at different time point (0, 0.1, 0.4, 1 million FSCV cycles) under prolonged FSCV cycling and in presence of interferents. Significance was determined at p<0.05.
2. Results and Discussion
2.1. 3DFG MEA fabrication and characterization
3DFG was synthesized by plasma enhanced chemical vapor deposition (PECVD) onto Si/SiO2 substrates (See Materials and Methods for additional details).(Garg et al. 2017) Using standard micro- and nano-fabrication techniques, synthesized 3DFG was patterned into functional microelectrodes with sizes varying from ca. 2 × 2 μm2 to 50 × 50 μm2 (Supplementary Figure 1 and Supplementary Table 1; we note that misalginment during microfabrication of 3DFG led to slight deviation from the expected MEA dimensions).(Rastogi et al. 2020) The 3D topology of out-of-plane grown graphene flakes is well conserved after the MEA fabrication processes (Figure 1.A). The presence of the characteristic D (ca. 1348 cm−1 ), G (ca. 1588 cm−1), and symmetric 2D (ca. 2690 cm−1) peaks in the Raman spectra confirm the presence of single-few-layer graphene flakes (Figure 1.B).(Ferrari and Basko 2013; Garg et al. 2017) The emergence of a strong D peak and the D’ peak (ca. 1615 cm−1), as a shoulder to the G peak, are attributed to the presence of 3DFG edges,(Ferrari and Basko 2013; Garg et al. 2017; Torrisi et al. 2012) as evident in the SEM image in Figure 1A.
Figure 1. 3DFG morphological and electrochemical characterization.
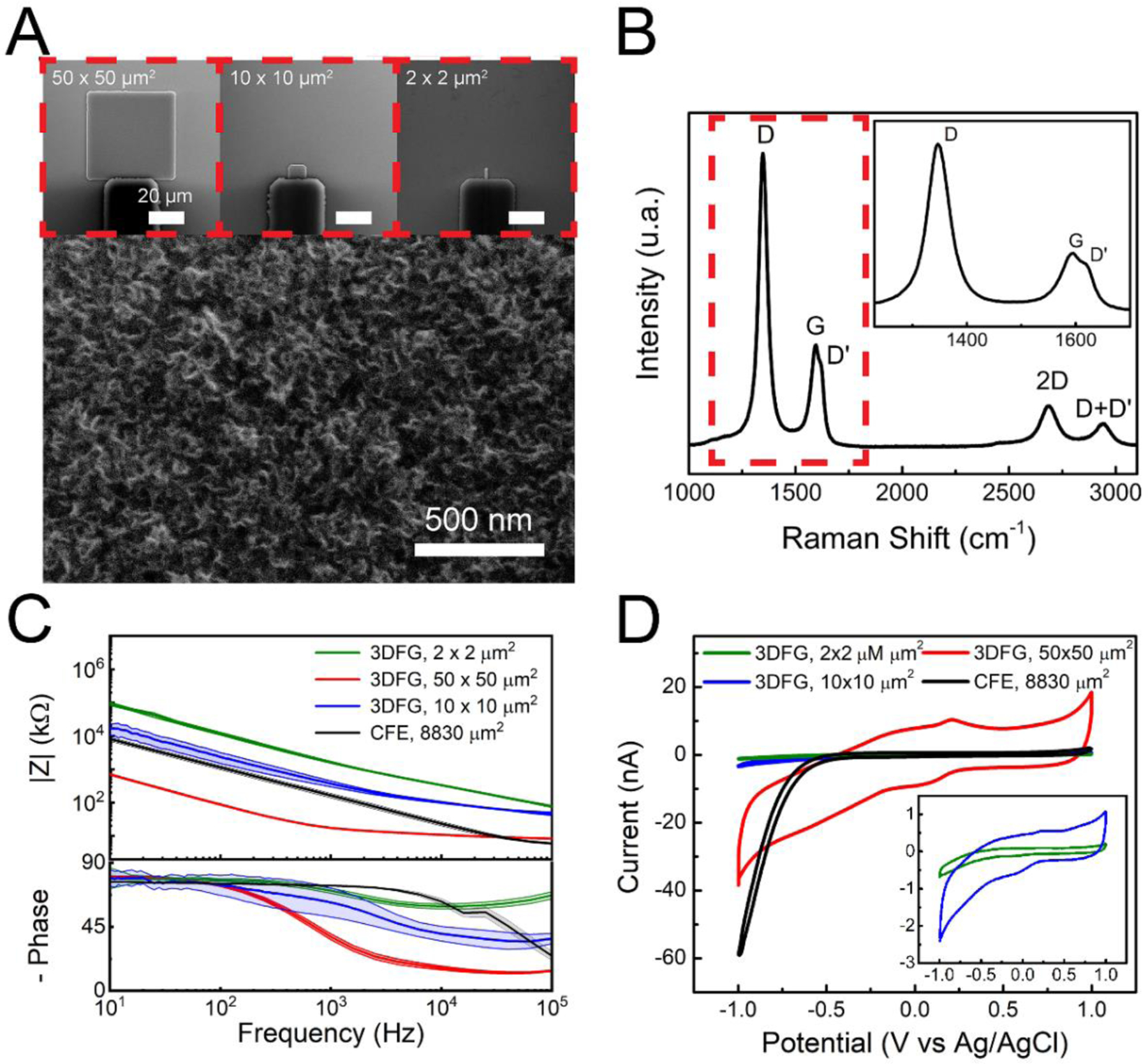
(A) Zoomed-in scanning electron microscopy (SEM) image of a 3DFG MEA. Insets present SEM images of 3DFG MEAs of varying sizes. (B) Raman spectra of a 3DFG microelectrode. Inset presnts expanded view of the Raman spectra marked by the red dashed box. (C) Impedance and negative phase as a function of frequency (mean ± sd, n =5 electrodes per size) as measured for 50 × 50 μm2 3DFG (red), 10 × 10 μm2 3DFG (blue), 2 × 2 μm2 3DFG (green) MEAs, and CFEs (black) in 1x PBS. (D) Representative cyclic voltammograms (of 50 × 50 μm2 3DFG (red), 10 × 10 μm2 3DFG (blue), 2 × 2 μm2 3DFG (green) MEAs, and CFEs (black) acquired in 1x PBS at a scan rate of 100 mV/s. Inset presents the magnified view of the representative cyclic voltammograms of 10 × 10 μm2 3DFG (blue), 2 × 2 μm2 3DFG (green) plots.
We observe that the electrochemical impedance of the 3DFG MEAs increases as the electrode size decrease in all the frequency range tested (Figure 1.C). This is expected as electrochemical impedance at the electrode-electrolyte interface is composed of double layer capacitance and solution and charge transfer resistance(Bard and Faulkner 2001; Orazem and Tribollet 2008). Capacitance increases with surface area while impedance is inversely related to the capacitance.(Orazem and Tribollet 2008) Therefore larger electrodes will have lower impedance in the frequency region where capacitance play a significant role. In the high frequency resion, solution resistance dominates. Newman derived the relationship between the solution resistance Rs and electrode radius r to be Rs= 1/(4.k.r) (k = conductivity);(Newman 1966) Larger electrodes are expected to exhibit lower solution resistance (RS) and thus lower electrode impedance. Additionally, it was shown that microelectrodes smaller than 50 μm in radius exhibits area-dependence capacitive regime even at high frequencies (~100 kHz), thus their impedance scales inversely with the area in all the frequency range tested (Newman 1966).
The electrochemical impedance of 50 × 50 μm2 3DFG MEAs is approximately an order of magnitude less than that of a carbon fiber electrode (CFE) with an area of 8830 μm2, in particular in the low frequency range (Figure 1.C). This is attributed to the highly-exposed surface area of the 3DFG MEAs.(Rastogi et al. 2020) Over the 10–105 Hz frequency domain, the 3DFG microelectrodes exhibit a near-resistive phase (approaching 0°) and an impedance modulus that is almost entirely solution resistance. At 100 Hz the impedance of the 50 × 50 μm2 3DFG is 14-times lower (87.96 ± 5.97 kΩ versus 1.14 ± 0.16 MΩ) compared to the CFEs impedance, while the impedance of the 10 × 10 μm2 3DFG microelectrodes is in the same order of magnitude (2.34 ± 0.83 MΩ) of the impedance of CFE with a ca. 88-fold greater geometric area.
Cyclic voltammetry in the presence of 1x PBS presents an approximately rectangular current response in the scanned potential window (Figure 1.D), suggesting predominantly double-layer capacitance governed response during the charging and discharging process (Cogan 2008; Nimbalkar et al. 2018), with only a small reversible faradaic reaction presenting oxidation at ca. 0.2V and reduction at ca. 0 V. Furthermore 3DFG microelectrodes show a wider water window than CFEs, with no hydrolysis reactions occurring between −1V and 1 V, while CFEs present a sharp increase of water reduction current starting at −0.5 V. Furthermore, 3DFG MEAs exhibit a15-fold greater charge stroage capacity (CSC) of 15.84 ± 0.98 mC/cm2 compared to 1.05 ± 0.09 mC/cm2 of CFEs (Figure 1.D and Supplementary Figure 2). The enhaced CSC of 3DFG MEAs is attributed to the exceptionally high surface area of the material. (Cogan 2008; Rastogi et al. 2020)
Ultrahigh surface area materials with controlled morphological structure have been shown to be promising candidates for electrode materials with excellent electrochemical performances (Peng et al. 2019; Xiao et al. 2020). Thus our results highlight the potential of the 3DFG microelectrodes to be miniaturized without compromising the electrochemical performance, opening the possibility to fabricate high-density MEAs.
2.2. FSCV detection of dopamine at 3DFG microelectrodes
DA is an electroactive compound capable of reversible oxidation to dopamine-o-quinone (DAoQ) upon application of a sufficient potential, following a two-electrons and 2 protons exchange (DA → DAoQ + 2 e− + 2 H+).(Bath et al. 2000; Taylor et al. 2019; Taylor et al. 2017)
DA is typically detected with FSCV through a triangular waveform with a scan rate of 400 V/s applied repeatedly at 10 Hz. The FSCV for DA usually starts from an holding potential of −0.4 V (vs. Ag/AgCl), applied to the working electrode to selectively preconcentrate cationic DA on the electrode surface, (Bath et al. 2000; Kim et al. 2018; Venton and Cao 2020) to a switching potential of +1 /+1.3V and back to the holding potential to oxidize DA and reduce DAoQ (Puthongkham and Venton 2020; Venton and Cao 2020). Here we use a switching potential of 1.3 V, that has shown to increase the DA sensitivity (Heien et al. 2003; Puthongkham and Venton 2020), due to activation of the carbon surface (Puthongkham and Venton 2020; Takmakov et al. 2010), while avoiding electrolysis of water and a consequent electrode degradation (Engstrom and Strasser 1984; Puthongkham and Venton 2020; Takmakov et al. 2010). The use of negative holding potentials has also shown to increase the DA sensitivity, as the electrode becomes more favorable for DA adsorption (Heien et al. 2003; Puthongkham and Venton 2020). However, below −0.5 V, oxygen reduction can occur at the CFE surface resulting in undesired radical byproducts.(Venton and Cao 2020) As previously mentioned, 3DFG microelectrodes present a wider water window that allows for the safe use of holding potential below −0.4 V. A schematic of the DA oxidation mechanism at carbon surfaces using FSCV is reported in Supplementary Figure 3.
To evaluate the sensitivity of the 3DFG microelectrodes for DA detection in a range of concentration from 0.01 to 1 μM in PBS, we use a triangular waveform at a scan rate of 400 V/s and 10 Hz within a potential window of −0.5 V (to favor DA adsorption without incurring in oxygen reduction) to +1.3V (to activate the carbon surface while avoiding electrolysis of water). Figure 2 presents the CV traces corresponding to the detection of 0.01, 0.1, 0.25, 0.5, 1 and 2.5μM bolus of DA injection after subtracting the non-faradaic capacitive charging current, for 50 × 50 μm2 3DFG and (Figure 2.A) and 2 × 2 μm2 3DFG microelectrodes (Figure 2.B), respectively. The background subtracted CVs exhibit characteristic anodic DA oxidation and cathodic DAoQ reduction peaks, confirming the detection of DA.
Figure 2. Dopamine sensitivity with 3DFG.
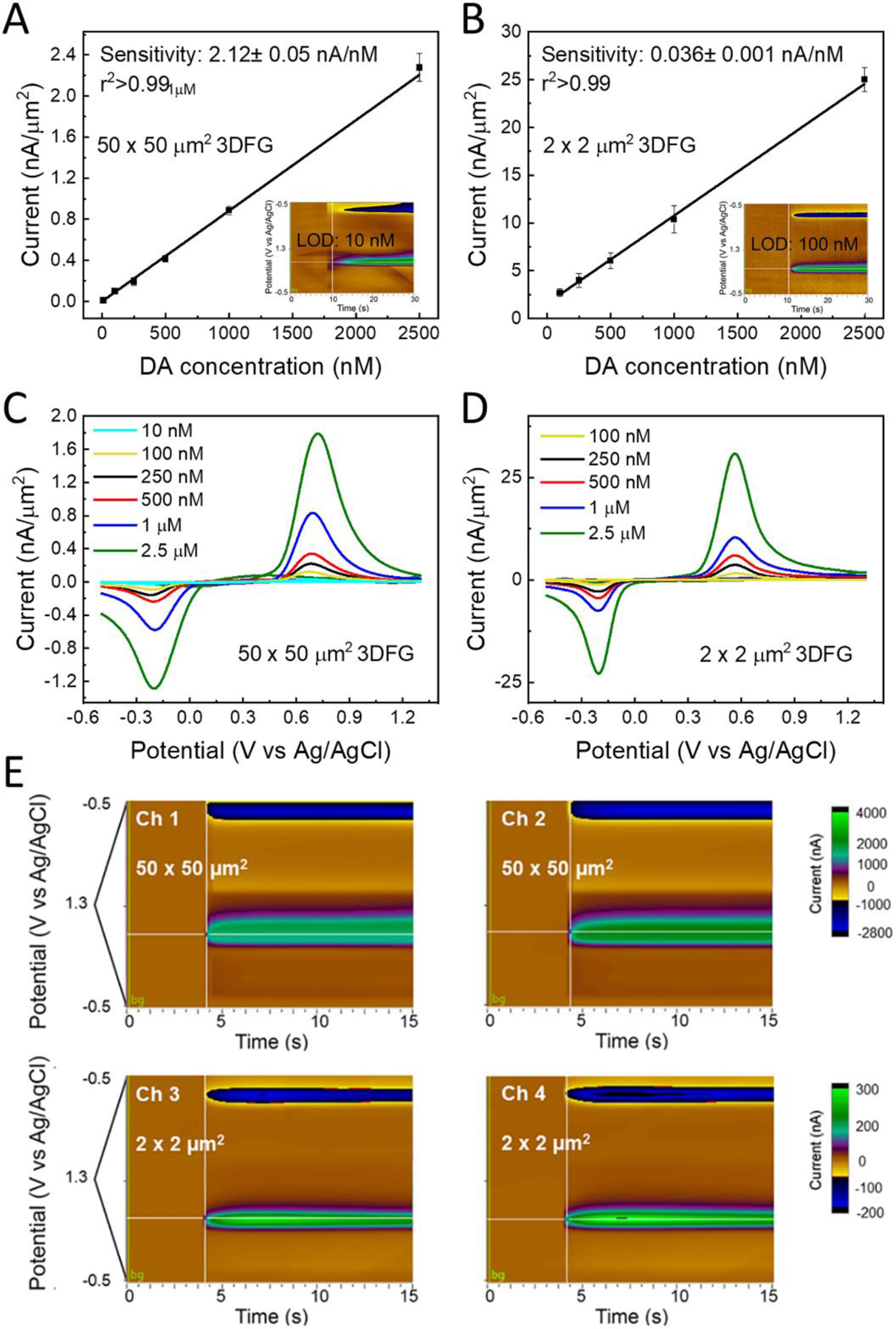
Representative background subtracted FSCVs, for 10, 100, 250, 500 nM, 1 and 2.5 μM bolus of DA injection collected using (A) 50 × 50 μm2 3DFG microelectrodes, and (B) 2 × 2 μm2 3DFG microelectrodes. Inset: representative color plots for low DA concentrations:10 nM (A) and 100 nM (B), respectively. Calibration curves – 0.01–2.5 μM concentration range- of DA in PBS using (n=6, mean±SD) (C) 50 × 50 μm2 3DFG microelectrodes and (D) 2 × 2 μm2 3DFG microelectrodes. (E) A representative example of multi-channel detection from two 2 × 2 μm2 and two 50 × 50 μm2 3DFG microelectrodes of the same MEAs in response to 1 μM bolus of DA injection.
The separation of the cathodic and anodic peaks (ΔEp) is smaller for the miniaturized 2 × 2 μm2 3DFG electrodes (ΔEp = 0.604 ± 0.003 V) in comparison to the 50 × 50 μm2 3DFG (ΔEp = 0.892 ± 0.021 V), and CFEs (ΔEp = 0.77 ± 0.02 V) indicating faster electron transfer kinetics.(Taylor et al. 2017; Yang et al. 2017) This is likely due to the small size of the miniaturized electrode that allows for a more rapid response to changes in the applied potential.(Forster 1994, 2006) Smaller RC cell time constants and significantly reduced ohmic (iR) drop have been observed for miniaturized microelectrodes, making them very attractive for investigating high speed electron-transfer reactions.
3DFG microelectrodes sensitivity towards DA, based on the linear regression slope of maximum faradaic current versus DA concentration, is determined to be 2.12 ± 0.05 nA/nM (Mean ± SD, n=10) and 0.036 ± 0.001 nA/ nM (Mean ± SD, n=10), for 50 × 50 μm2 and 2 × 2 μm2 microelectrodes, respectively (Figure 2C and D). The sensitivity of 50 × 50 μm2 3DFG microelectrodes is 163–432 fold greater than what has been reported in literature from CFEs (4.9±0.5 nA/μM,(Taylor et al. 2017) and 13±2 nA/μM (Vreeland et al. 2015)), 46–82 fold greater than PEDOT/Nafion CFEs (Vreeland et al. 2015) and 39–151 times higher than PEDOT/GO CFEs (Taylor et al. 2017) (Table 1). Since the sensitivity of CFEs can vary when using different FSCV waveforms, (Heien et al. 2003; Taylor et al. 2017) to have a direct comparison, we compared the DA sensitivity of CFE and 3DFG microelectrode using the same waveform (−0.5 to 1.3 V vs Ag/AgCl, 400 V/s) in the same DA concentration range (0.1 to 1 μM). 3DFG microelectrodes were found to be 30-fold more sensitive with respect to CFEs that exhibit approximately 4-times larger geometric area. Compared to other high surface area carbon microelectrodes, the sensitivity of 3DFG towards 1 μM DA is ca. 6-10-fold greater than CNT arrays and ca. 3-5-fold greater than CNT yarns and CNT fibers (Table 1). This outstanding sensitivity of the 3DFG material allows to miniaturize the electrodes down to 2 × 2 μm2 electrodes while maintaining a sensitivity comparable to the sensitivity of CFEs with 2200-fold greater geometric area.
Table 1:
DA sensing using CFE and nanocarbons using FSCV (* denotes current study)
| Electrode | Electrolyt e | Area | LOD | Sensitivity | Sentitivity in interferent/sel ectivity | Ref. |
|---|---|---|---|---|---|---|
| GC | PBS | 500 μm2 1500 μm2 |
≤10nM | 1.135E−9 nA/nM 164 nA/ μM 354 nA/μM– |
1 mM AA, 1.81E−10 nA/nM |
(Castagnola et al. 2021; Nimbalkar et al. 2018) |
| PEDOT/GO coated CFEs | aCSF | 7 μm diameter, 400 μm length |
0.1 μM | 14–54 nA/μM | NA | (Taylor et al. 2017) |
| CFEs | aCSF | 7 μm diameter, 400 μm length 75 μm length |
0.218 μM 20±7 nM |
4.9 ± 0.5 nA/μM 13±2 nA/μM |
NA | (Taylor et al. 2017; Vreeland et al. 2015) |
| PEDOT/Nafion coated CFEs | aCSF | 7 μm diameter, 75 μm length |
4 ± 1 nM | 26 – 46 nA/μM | DA (1.0 μM), DOPAC (20 μM), AA (200 μM) | (Vreeland et al. 2015) |
| CNT yarn microelectrodes (CNTYMEs) | PBS | 10–25 μm | 13 ± 2 nM | NA | NA | (Yang et al. 2016b) |
| CNT Grown on Metal Microelectrode s and CFEs | PBS | Currents normalized for area | CNT-Nb 11±1 nM CNT-Ta 91±27 nM CNT-CFE 46±10 nM |
CNT-Nb 197 ± 16 pA/μm2 CNT-Ta 82 ± 10 pA/μm2 CNT-CFE 100 ± 25 pA/μm2 for 1μM DA |
AA, DOPAC, 5-HT, AD, and histamine | (Yang et al. 2016a) |
| Carbon nanospike-modified microelectrodes | PBS | Wires 100 μm lenght 25 μm dia |
Ta-CNS 8 ± 2 nM Pd-CNS 27 ± 2 nM Nb-CNS 12 ± 2 nM Ni-CNS 16 ± 3 nM |
Linear from 100 nM to 100 μM | UA, AA | (Zestos et al. 2015) |
| Carbon nanopipettes electrodes (CNPEs) | PBS | ~250 nm diameter tips, and lengths ranging from 5 to 175 μm. | 25 ± 5 nM | linear response for DA in 0.1 to 10 μM range | 5-HT, and octopamine | (Rees et al. 2015) |
| Carbon Nanohorn (CNH) modified CFME and ox-CNH/CFME |
PBS | 7 μm diameter 100 μm length |
15 and 6 nM | linear response for DA in 0.05 to 5 μM range | 1μM EP, 1μM NE, 1 μM 5-HT, 200μM AA | (Puthongkha m et al. 2018) |
| 3D-Printed carbon electrode and Carbon Nanoelectrodes | PBS | diameter of 65±4μm for the spheres and the hemisphere part of the cones; 288±17nm tip size | 11±1 nM and 177 ± 21 nM | the DA current is linear with concentration up to 10 (micro electrode) μM and 50 μM (nanoelectrode) | 1μM EP, 1μM NE, 1 μM 5-HT, 200μM AA | (Cao et al. 2020; Puthongkha m et al. 2018) |
| CA/CNT Fiber | PBS | 20 μm diameter | NA | 210 ± 31 pA/μm2 for 1μM DA |
UA, AA, 5-HT | (Yang et al. 2017) |
| PEI/CNT Fiber | PBS | 20 μm diameter | NA | 122 ± 23 pA/μm2 for 1μM DA |
UA, AA, 5-HT | (Yang et al. 2017) |
| CNT Yarn | PBS | 20 μm diameter | NA | 290 ± 65 pA/μm2 for 1 μM DA |
UA, AA, 5-HT | (Yang et al. 2017) |
| 3DFG microelectrodes | PBS | 2500 μm2 | 364.44±8.65 pM | 2120±50nA/μM | UA, AA, DOPAC, EP, 5-HIAA | * |
| 3DFG miniaturized microelectrodes | PBS | 4 μm2 | 61.67±25.3 5 nM | 3.6±1 nA/ μM | UA, AA, DOPAC, EP, 5-HIAA | * |
Acronyms used: AA- ascorbic acid, UA- uric acid, DA- dopamine, DOPAC- dihydroxyphenylacetic acid, AD- adenosine, 5-H- serotonin, EP- epinephrine, NE- norepinephrine, 5-HIAA- 5-hydroxyindole-3-acetic acid, GC – glassy carbon, CFEs- carbon fiber microelectrodes, aCSF-artificial cerebrospinal fluid, PBS- Phosphate Buffered Saline, CNT- carbon nanotubes, PEDOT/GO- poly(3,4-ethylene dioxythiophene) /graphene oxide, PEI- polyethylenimine, CA- chlorosulfonic acid, PEDOT- poly(3,4-ethylene dioxythiophene)
The theoretical lower detection limit (LOD), defined as 3 times the standard deviation of the noise, (Harris 2010; Schmidt et al. 2013; Smith et al. 2018; Swamy and Venton 2007b; Taylor et al. 2017) was estimated to be 364.44 ± 8.65 pM (Mean ± SD, n = 7) for 50 × 50 μm2 3DFG microelectrodes, which is the lowest LOD value reported in literature using FSCV with carbon-based materials for DA detection (Table 1). For 2 × 2 μm2 3DFG microelectrodes the LOD is 61.67±25.35 nM (Mean ± SD, n = 6), remaining one of the lowest reported LOD value using FSCV with miniaturized carbon-based materials (Table 1). Based on visual evaluation of the color plots presented in Figures 2.C and D (insets), 10 and 100 nM of DA can be physically detected, using 50 × 50 μm2 and 2 × 2 μm2 3DFG microelectrodes, respectively.
Based on these quantifications 3DFG microelectrodes of the different size evaluated can reliably determine DA level in the physiological range, reported to be from 250 nM(Puthongkham et al. 2018) to up to 1μM (I Mitch Taylor 2012; I Mitch Taylor 2015) in striatum of rat brain (I Mitch Taylor 2012; I Mitch Taylor 2015; Swamy and Venton 2007a).
Figure 2 E reports an example of multi-channel detection from two 2 × 2 μm2 and two 50 × 50 μm2 3DFG microelectrodes of the same MEAs, demonstrating the possibility to detect DA from different channels of the same MEAs simultaneously and without cross-talks in between adjacent microelectrodes with an inter-distance of 25 to 100μm. Other examples of multi-channel FSCV collected from 2 × 2 μm2, 10 × 10 μm2 and 50 × 50 μm2 3DFG microelectrodes of the same MEAs are reported in Supplementary Figure 5.
Finally, we have characterized DA detection at 3DFG microelectrodes under varying pH of the PBS from 6 to 8 (Supplementary Figure 6). We observed that the reduction and oxidation peaks undergo a positional shift as the pH of the solution is changed from 7.4, with a peak-shift to the right under acidic conditions and to the left under basic conditions, but they do not compromise the DA detection capability of the 3DFG. These results were consistent across 3DFG microelectrode of different sizes (Supplementary Figure 6 A, B).
2.3. Evaluation of the Electrochemical and DA sensing stability
To evaluate the effect of a prolonged FSCV cycling on the electrochemical stability and DA sensitivity of 3DFG, we applied the FSCV waveform (−0.5 to 1.3 V vs. Ag/AgCl, scan rate: 400 V/s) at 10 Hz on the 3DFG microelectrodes in 1x PBS for over 200 h, corresponding to 7.2 million cycles, and monitored the response to 1 μM bolus injections of DA at different time points (corresponding to 0.1, 0.9, 1.8, 2.7, 3.6, 5.4, 7.2 million). As a control, and to provide a direct comparison, the same experimental protocol was applied to bare CFEs. Over 7.2 million FSCV cycles, we observe an increase in CSC for 3DFG that became significant after 2.7 million FSCV scans (Figure 3.A and B). The generation of oxygen-functional groups at the carbon surfaces under constant electrochemical FSCV cycling with a switching potential higher than 1 V (i.e. 1.3 and 1.4 V vs. Ag/AgCl) has shown to facilitate the formation of edge planes by creating strains in the lattice (Bowling et al. 1989) and it is influenced by the duration of the electrochemical cycling. We note that the G and 2D Raman peaks of the 3DFG electrodes upshift and downshift respectively after 7.2 million cycles (Supplementary Figure 4.C and Supplementary Table 2). Similar shifts, with opposite trends in the position shift of the G and 2D peaks have been observed for strained polycrystalline graphene structures with crystallite size smaller than the size of the Raman laser spot, as in the case of 3DFG.(Bissett et al. 2012; Bissett et al. 2014) This suggests the presence of increased strain in the 3DFG lattice, due to the edge plane oxygenation as a result of the strenuous FSCV cycling. For CFEs, we observed a significant decrease in CSC over time (Figure 3.C and D). The decrease in CSC became significant after the first 0.9 million FSCV cycles and the CSC stabilized from 2.7 to 7.2 million FSCV cycles (Figure 3.C). Continuous FSCV cycling has been found to clean the electrode surface from physiosorbed impurities and alters the structure of the surface until the maximum amount of defects is formed (Bowling et al. 1989; Cao et al. 2019; Engstrom and Strasser 1984; Poon et al. 1988), at which point the electrode surface will reach a stable state. This stable surface state is likely to be reached faster at CFEs than 3DFG microelectrodes, since CFEs present lower electrochemically-active surface area. The DA sensing performance of 3DFG MEAs and CFEs did not alter as a function of FSCV cycling, since no significant difference was observed in their respective sensitivities towards DA (n=5, Repeated measurements ANOVA, F(6,30)=2.07899, P= 0.08559>0.05, Bonferroni post-hoc test ns) (Figure 4).
Figure 3. Electrochemical and DA sensing stability with 3DFG and CFEs.
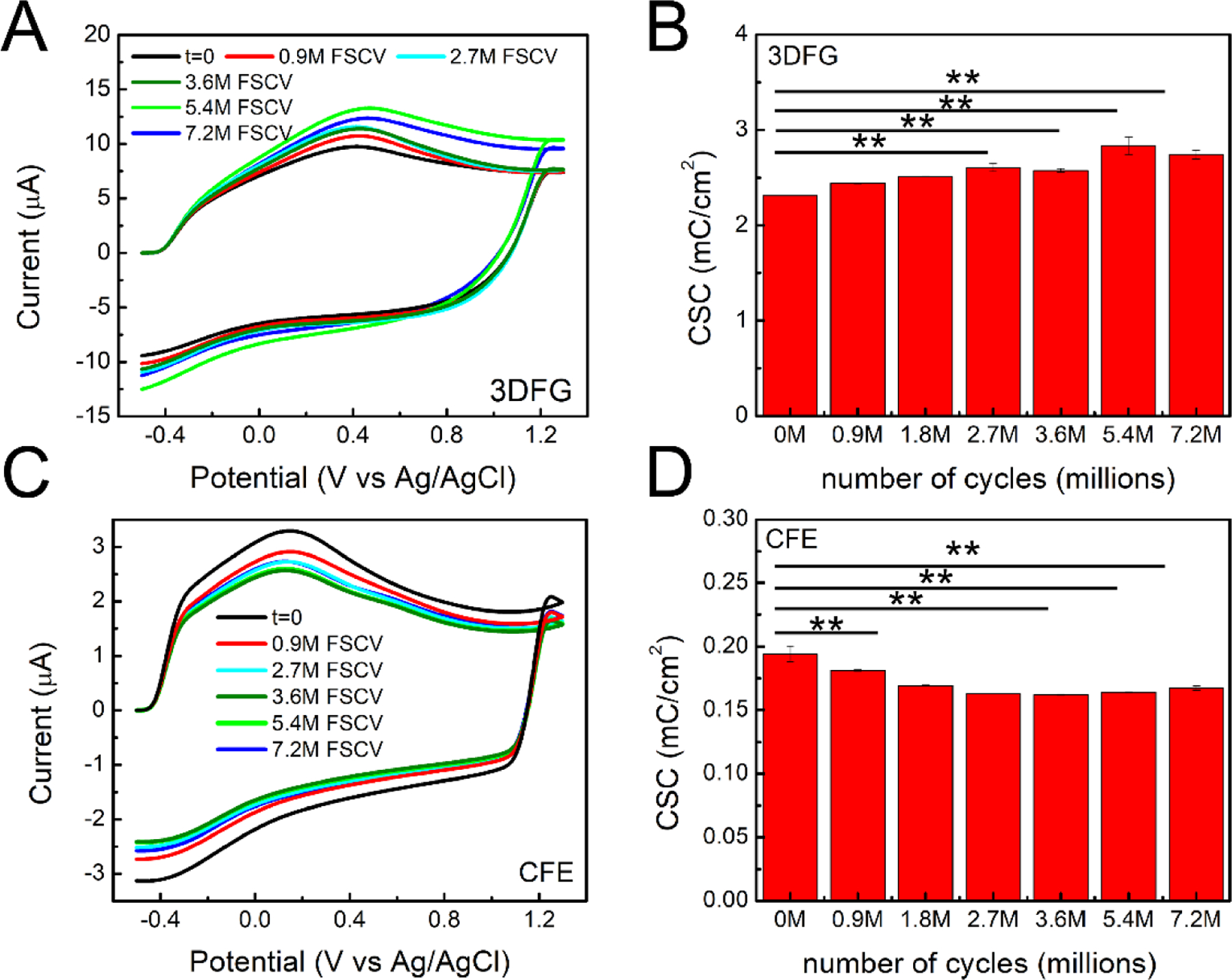
(A,B) 3DFG: (A) FSCV plots (not- background subtracted) of 50 × 50 μm2 3DFG microelectrodes at different time point (0, 0.9 million, 2.7, 3.6, 5.4 and 7.2 million FSCV cycles) (i.e. 0, 25, 75, 100, 150, 200 h) and (B) Column plot reporting the CSC at the different time points (average ± SEM, n=5 repetitions). (Repeated measurements ANOVA, F(6,24)=16.64209, P=1.72052E-7 <0.05, Bonferroni post-test** significantly different). (C,D) CFEs: (C) FSCV plots of CFE microelectrodes at different time point (0, 0.9 million, 2.7, 3.6, 5.4 and 7.2 million FSCV cycles) (i.e. 0, 25, 75, 100, 150, 200 h) and (D) Column plot reporting the CSC at the different time points (average ± SEM, n=5 repetitions). (Repeated measurements with ANOVA, F(6,24)=21.35523, P=1.57879E-8 <0.05, Bonferroni post-hoc test** significantly different).
Figure 4. DA sensing stability with 3DFG and CFEs.
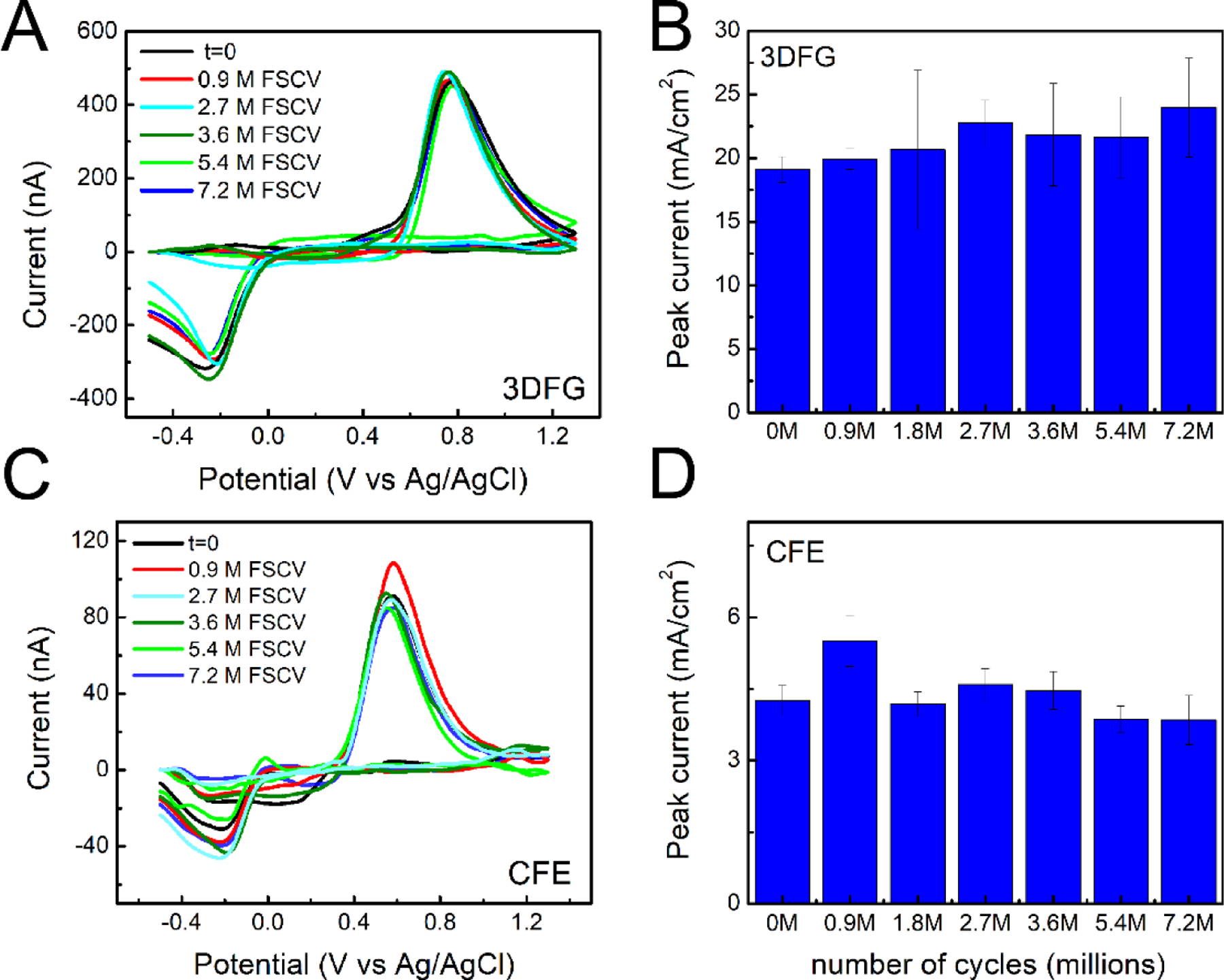
(A,B) 3DFG: (A) Representative background subtracted CVs in response to 1μM bolus injections of DA at the time 0 and after 25, 50, 75,100, 150 and 200 hours of 3DFG FSCV continuous scanning in PBS. (B) Column plot reporting the amplitude of the oxidation peaks collected in response to 1μM bolus injections of DA at time zero and after 0.9, 2.7, 3.6, 5.4 and 7.2 million FSCV cycles (corresponding to 25, 50, 75,100, 150 and 200 h) of 3DFG FSCV continuous scanning in PBS. (Repeated measurements ANOVA, F(6,30)=2.07899, P= 0.08559>0.05, Bonferroni post-hoc test ns) (C, D) CFEs: (C) Representative background subtracted CVs in response to 1μM bolus injections of DA at the time 0 and after 0.9, 2.7, 3.6, 5.4 and 7.2 million FSCV cycles of CFE FSCV continuous scanning in PBS. (D) Column plot reporting the amplitude of the oxidation peaks collected in response to 1μM bolus injections of DA at time zero and after 0.9, 2.7, 3.6, 5.4 and 7.2 million FSCV cycles (i.e. 25, 50, 75,100, 150 and 200 h) of CFE FSCV continuous scanning in PBS. (Repeated measurements with ANOVA, F(6,36)=2.04792, P=0.08432>0.05, Bonferroni post-test ns).
The ratio of the peak oxidation current (anodic, ip,a) to the peak reduction current (cathodic, ip,c) for DA provides information about the equilibrium of absorption/desorption properties of DA and DAoQ and the reversibility of the reaction (Taylor et al. 2017; Venton and Cao 2020). We observe that the ip,c/ip,a ratio is 0.76 ± 0.09 and 0.40 ± 0.09 for 50 × 50 μm2 3DFG and CFEs, respectively(Cao et al. 2019; Puthongkham and Venton 2020). The increase in the cathodic DAoQ reduction peak indicates a tighter adsorption of the oxidized DAoQ towards 3DFG than CFE surfaces.(Bard and Faulkner 2001). This is similar to what was previously observed for CNT treated CFEs (Swamy and Venton 2007a), CNT fiber microelectrodes (Yang et al. 2017), and PEDOT/GO coated CFEs (Taylor et al. 2017), which share the similarity of high surface area due to the nanocarbon morphology. On CFEs, the oxidation product of DA, DAoQ, has few sites to bind to and can easily diffuse away from the smoother electrode surface when it desorbs, resulting in much lower cathodic current ip,c due to the low concentration of DAoQ.(Bath et al. 2000; Puthongkham and Venton 2020; Venton and Cao 2020). For 3DFG, DAoQ binding is enhanced due to the high surface area (Venton and Cao 2020; Yang et al. 2017) and DAoQ’s affinity to partially charged carbon atoms near the edge (McDermott and McCreery 1994; Taylor et al. 2017). Even if DAoQ desorbs, they may remain momentarily trapped in the three dimensional nanoporous morphology and ready to adsorb again quickly, resulting in higher ip,c. This preconcentration effect can also act on DA and enhance DA detection on the next scan. Indeed, the electron transfer kinetics for DA oxidation has been demonstrated to be catalyzed by the adsorption of quinone containing species, including DAoQ, onto the carbon electrode surface (DuVall and McCreery 2000). This effect, combined with the greater exposed surface area, explains the enhancement in DA sensitivity (DuVall and McCreery 2000; Taylor et al. 2017).
2.4. Evaluation of the Selectivity and fouling properties
To assess the selectivity of the 3DFG microelectrodes towards the most common neurochemicals found throughout the complex brain environment, we characterized the DA sensing capabilities of the electrodes in presence of 200 μM ascorbic acid (AA), 10 μM uric acid (UA), 10 μM dihydroxyphenylacetic acid (DOPAC). The concentration of DA was kept constant at 100 nM since similar DA concentrations were measured in the rat’s dorsal striatum using square wave voltammetry (82 ± 6 nM) (Taylor et al. 2019) and multiple cyclic square wave voltammetry (120 ± 18 nM) (Oh et al. 2018); and in the nucleus accumbens of both mice and rats, using fast scan controlled adsorption voltammetry (90 ± 9 nM) (Atcherley et al. 2015) and convolution-based FSCV(41 ± 13 nM) (Johnson et al. 2018), respectively. Our results show that 3DFG microelectrodes of 50 × 50 μm2 (Supplementary Figure 7.A–B), 2 × 2 μm2 (Supplementary Figure 7. C), 10 × 10 μm2 (Supplementary Figure 7.D) geometric area can successfully detect DA in presence of interferents (Supplementary Figure 7 A–D). The sensitivity towards DA was well conserved compared to that observed for DA in 1x PBS, as observed by the background subtracted CVs of a 50 × 50 μm2 3DFG microelectrode in response to 1 μM bolus injections of DA in 1x PBS without and with interferents (Supplementary Figure 7.A), and from the DA calibration curves of 10 × 10 μm2 3DFG microelectrodes in 1x PBS, without and with interferents (Supplementary Figure 7.D). CFE demonstrated similar performance under interferents (Supplementary Figure 7.E and F). However, when subjected to fouling test, 3DFG significantly outperform CFEs in preserving the DA sensitivity over time (Figure 5). The sensitivity to a bolus injection of 1 μM DA was monitored at different timepoints (0, 0.1, 0.4, and 1 million FSCV cycling). We observed that the amplitudes of DA oxidation peak did not change significantly using 3DFG microelectrodes over continuous cycling in presence of only DA (Supplementary Figure 8), and DA plus interferents (Figure 5.A and B) while were significantly diminished using CFEs (Figure 5.C and D).
Figure 5. DA sensing stability and fouling properties with 3DFG and CFEs in presence of contaminant.
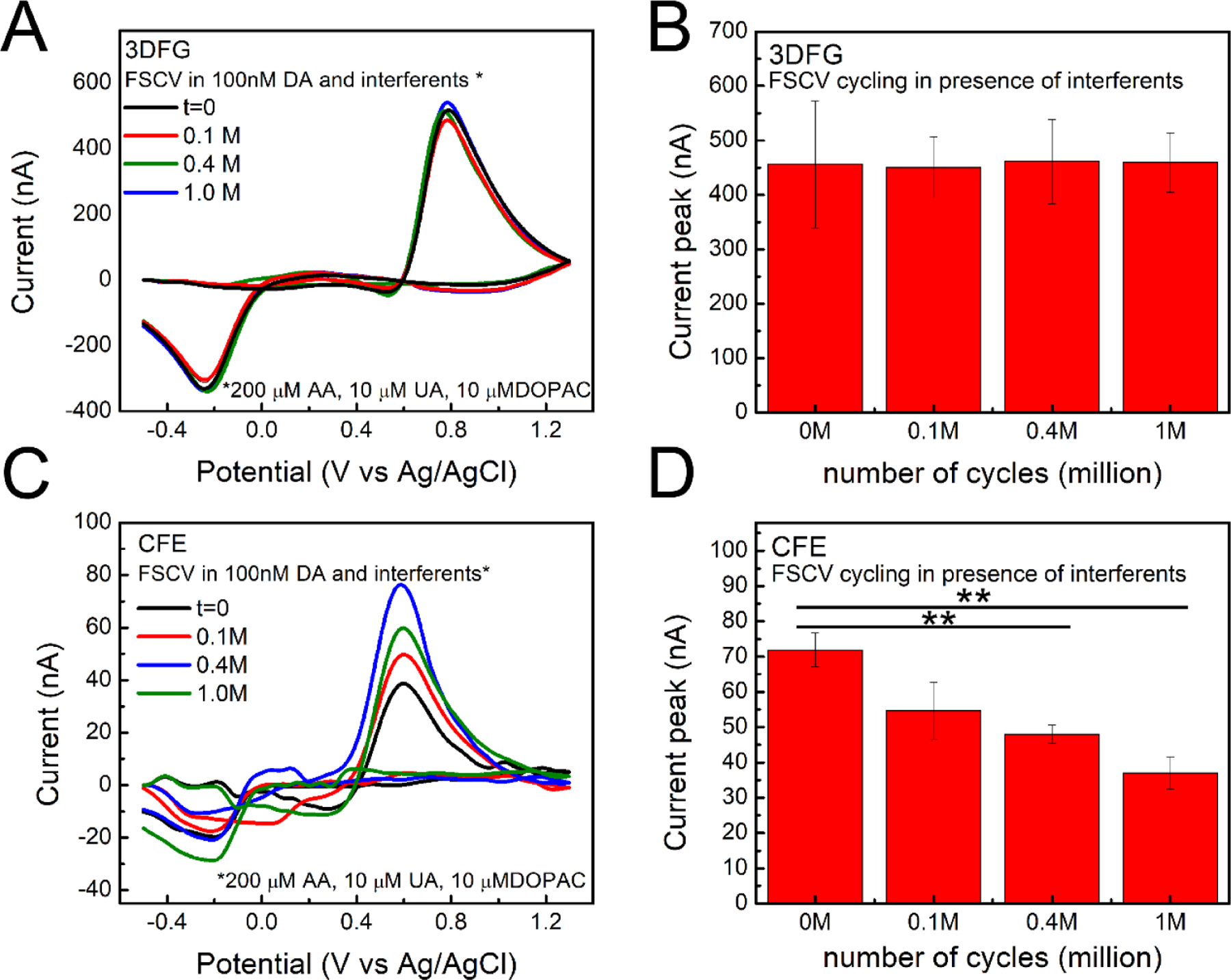
(A, B) 3DFG: (A) Representative background subtracted CVs in response to 1μM bolus injections of DA at the time 0 and after 0.1, 0.4 and 1 million (corresponding to 3, 8, and 24 hours) of 3DFG FSCV continuous scanning in PBS containing 100 nM DA and contaminants (200 μM AA, 10 μM UA, 10 μM DOPAC). (B) Column plot reporting the amplitude of the oxidation peaks collected in response to 1μM bolus injections of DA at time zero and after 0.1, 0.4 and 0.9 million of 3DFG FSCV continuous scanning in PBS containing 100 nM DA and contaminants (200 μM AA, 10 μM UA, 10 μM DOPAC), (Repeated measurements ANOVA, F(3,12)=0.05014, P=0.98443>0.05, Bonferroni post-test not stat different, n=3, 5 repetitions) (C, D) CFEs: (C) Representative background subtracted CVs in response to 1μM bolus injections of DA at the time 0 and after 0.1, 0.4 and 0.9 million of CFE FSCV continuous scanning in PBS containing 100 nM DA and contaminants (200 μM AA, 10 μM UA, 10 μM DOPAC). (D) Column plot reporting the amplitude of the oxidation peaks collected in response to 1μM bolus injections of DA at time zero and after 0.1, 0.4 and 0.9 million of CFE FSCV continuous scanning in PBS containing 100 nM DA and contaminants (200 μM AA, 10 μM UA, 10 μM DOPAC). (Repeated measurements ANOVA, F(3,33)=9.16405, P=1.4835E-4<0.05, Bonferroni post-test ** stat different, n=3, 5 repetitions).
The antifouling properties of the 3DFG surface may be due to the presence of various defects at their edge and electrocatalytic-active functional groups,(Hanssen et al. 2016a; McCreery 2008) similarly to what previously observed for other carbon nanomaterials, such as CNT (Hanssen et al. 2016a; McCreery 2008; Swamy and Venton 2007a), CNHs (Puthongkham et al. 2018), and CNT yarn microelectrode (Yang et al. 2017), when using FSCV. During the continuous cycling, the edge planes undergo oxidation that induce the formation of surface oxygen-containing groups and new defect at the graphene edge plane, that can increase the surface hydrophilicity (Garg et al. 2017) and reduce fouling (Hanssen et al. 2016a; Puthongkham and Venton 2020; Yang et al. 2010). It has been reported that fouling involving hydrophilic interactions is more reversible than that involving hydrophobic interactions (Grinnell and Feld 1981; Hanssen et al. 2016b; Hess and Vogel 2001). This can be attributed to the fact that, in aqueous electrolytes containing a strong polar solvent, hydrophilic surfaces present a strong interaction with water, that can form a stable hydration layer at the electrode surface through dipole-dipole interactions or hydrogen bonding of the charged terminal groups (Chen et al. 2005; Hanssen et al. 2016a).
Additionally, we investigate the possible interference of DA with epinephrine (EP), a monoamine neurotransmitter with very similar molecular structure. Not surprisingly, we found that EP and DA have very similar reduction and oxidation peak (Supplementary Figure 9). However, the sensitivity of 3DFG microelectrodes (n=6) to 1μM EP is about 5 times lower compared to the sensitivity of 1μM DA (Supplementary Figure 10). This result is in agreement with a previous study that observed that DA presents stronger adsorption and higher peak current than EP using FSCV at CNT yarn microelectrodes (Shao et al. 2020). Additionally, EP presents a secondary oxidation peak at 0.03 V, due to the intramolecular cyclization reaction of the ortho-quinone (Supplementary Figure 9 A). This secondary oxidation peak is observed at −0.05 V and 0.15 V for 2 × 2 μm2 and 50 × 50 μm2 3DFG microelectrodes, respectively (Supplementary Figure 9 B), showing the same peak shift observed for the primary oxidation peaks, indicating faster kinetics for smaller electrodes.(Taylor et al. 2017; Yang et al. 2017). Among the catecholamines, EP has the highest tendency for the cyclization, because the methyl group on the secondary amine of EP makes the nitrogen more nucleophilic and attack the ring structure of the quinone more easily to form leucoepinephrine, which oxidizes at 0.15V at larger electrodes (Pihel et al. 1994; Shao et al. 2020). Because these secondary peaks are more observable for epinephrine, at the interface with porous carbon surfaces (Puthongkham et al. 2018), they can be utilized to differentiate DA from EP(Heien et al. 2004; Puthongkham et al. 2018). Furthermore, most of DA studies are performed in the striatum of the basal ganglia, extensively innervated by the dopaminergic projection, where EP is not usually present at high level(Chen et al. 2020; Shu et al. 2013). Considering the poor sensitivity of 3DFG for EP compared to DA, the secondary peak identification, and the extensive dopaminergic innervation in the striatum, (Chen et al. 2020; I Mitch Taylor 2012; Mitch Taylor et al. 2012; Taylor et al. 2017), EP should not compromise future in vivo studies involving DA phasic release.
Finally, we investigated the selectivity of 3DFG in detection of DA over serotonin (5-HT). 5-HT is a cationic indolamine neurotransmitter, and like DA, can be electrochemically oxidized within the physiological pH solvent window (Wrona and Dryhurst 1990) with an oxidation reaction mechanism that involves a multi-step two-electron, two-proton transfer process (Jackson et al. 1995; Patel et al. 2013; Verbiese-Genard et al. 1984).
The detection of 5-HT using FSCV at CFEs is challenging since the by-products formed during the reaction, such as reactive carbocation intermediate and dimers, (Jackson et al. 1995; Patel et al. 2013; Wrona and Dryhurst 1990) are highly reactive and adsorb irreversibly on the electrode surface resulting in electrode fouling. This limitation has been partially solved by the use of optimized FSCV waveforms and/or electrode surface treatments (Swamy and Venton 2007a; Yang et al. 2017; Zestos et al. 2014). The Jackson waveform, (Hashemi et al. 2009; Jackson et al. 1995; Puthongkham and Venton 2020) a N-shaped waveform that holds the potential at +0.2 V to limit 5-HT by-product adsorption and scans quickly at 1000 V/s to 1.0 V and switch down to −0.1 V, allows for the detection of the 5-HT reduction peak (Hashemi et al. 2009; Jackson et al. 1995; Puthongkham and Venton 2020), while accelerating the electrode response times and limiting the fouling. However, such N-shaped FSCV waveforms cannot be effectively used to detect DA since DA sensing requires a more negative holding potential to facilitate the cationic adsorption on the electrode surface and to detect the DA reduction peak (Swamy and Venton 2007a; Zestos et al. 2014). On the other hand, different high surface area carbon materials(Swamy and Venton 2007a; Yang et al. 2017; Zestos et al. 2014), have been investigated and shown promising fouling resistance to 5-HT reaction byproduct (Mendoza et al. 2020; Swamy and Venton 2007a; Weese et al. 2019; Zestos et al. 2014), which has been mainly attributed to the presence of defect sites in high density (Mendoza et al. 2020; Zestos et al. 2014).
There is a great interest in understanding the interplay between DA and 5-HT release in reward and learning (Balasubramani et al. 2015; Fischer and Ullsperger 2017) and in the progression of neurological disease, such as Parkinson’s disease (Boileau et al. 2008; Carta et al. 2008; Politis et al. 2012; Wong et al. 1995), schizophrenia (Kapur and Remington 1996; Niederkofler et al. 2015), and depression (Boileau et al. 2008; Dremencov et al. 2004; Zangen et al. 2001). However, to the best of our knowledge, few studies have reported simultaneous detection of DA and 5-HT via FSCV (Swamy and Venton 2007a; Zestos et al. 2014; Zhou et al. 2005), using bare CFEs (Zhou et al. 2005), CNT coated CFEs (Swamy and Venton 2007a), or CNT fibers (Zestos et al. 2014). In all these cases, DA and 5-HT presented similar oxidation potentials (around 0.6 V) and the microelectrodes were only able to discriminate the reduction peaks of DA and 5-TH, at 200 mV ’ (Swamy and Venton 2007a; Zhou et al. 2005) and 400mV respectively (Zestos et al. 2014).
We explored the ability of 3DFG electrodes to simultaneously detect DA and 5-HT using FSCV and we compared their performance (Figure 6 A, B) with bare CFEs (Figure 6 C, D). Using 3DFG microelectrodes, the 5-TH background subtracted CV plot shows two clear oxidation peaks, respectively at ca. 0.51 V and ca. 0.78 V and reduction peak at ca. 0.08 V (Figure 6.B black). Well separated reduction and oxidation peaks of DA and 5-HT in response to the injection of their 50:50 mixture can be identified via color plots (Figure 6 A) and background subtracted CV plots (Figure 6.B). 3DFG detected reduction peaks of DA and 5-HT at −0.22 V and 0.08 V, respectively; and the oxidation peak of 5-HT at 0.51 V, while the second 5-HT oxidation peak at 0.78 V converges with the DA oxidation peak, resulting in a 2-fold greater peak amplitude. In the case of CFEs, only the reduction peaks of DA (−0.18 V) and 5-HT (0.08V) can be distinguished, while the oxidation peaks converged into a single peak at ca. 0.6 V vs. Ag/AgCl (Figure 6.C and D), consistent with the results from previously reported studies (Zhou et al. 2005). The unique capability of 3DFG to distinguish the peak at 0.51 V (likely assigned to the intermediate step of the 5-HT oxidation reaction)(Wrona and Dryhurst 1987), is mainly attributable to its higher sensitivity afforded by the high surface area.
Figure 6. DA and 5-HT simultaneous detection using 3DFG and CFEs.
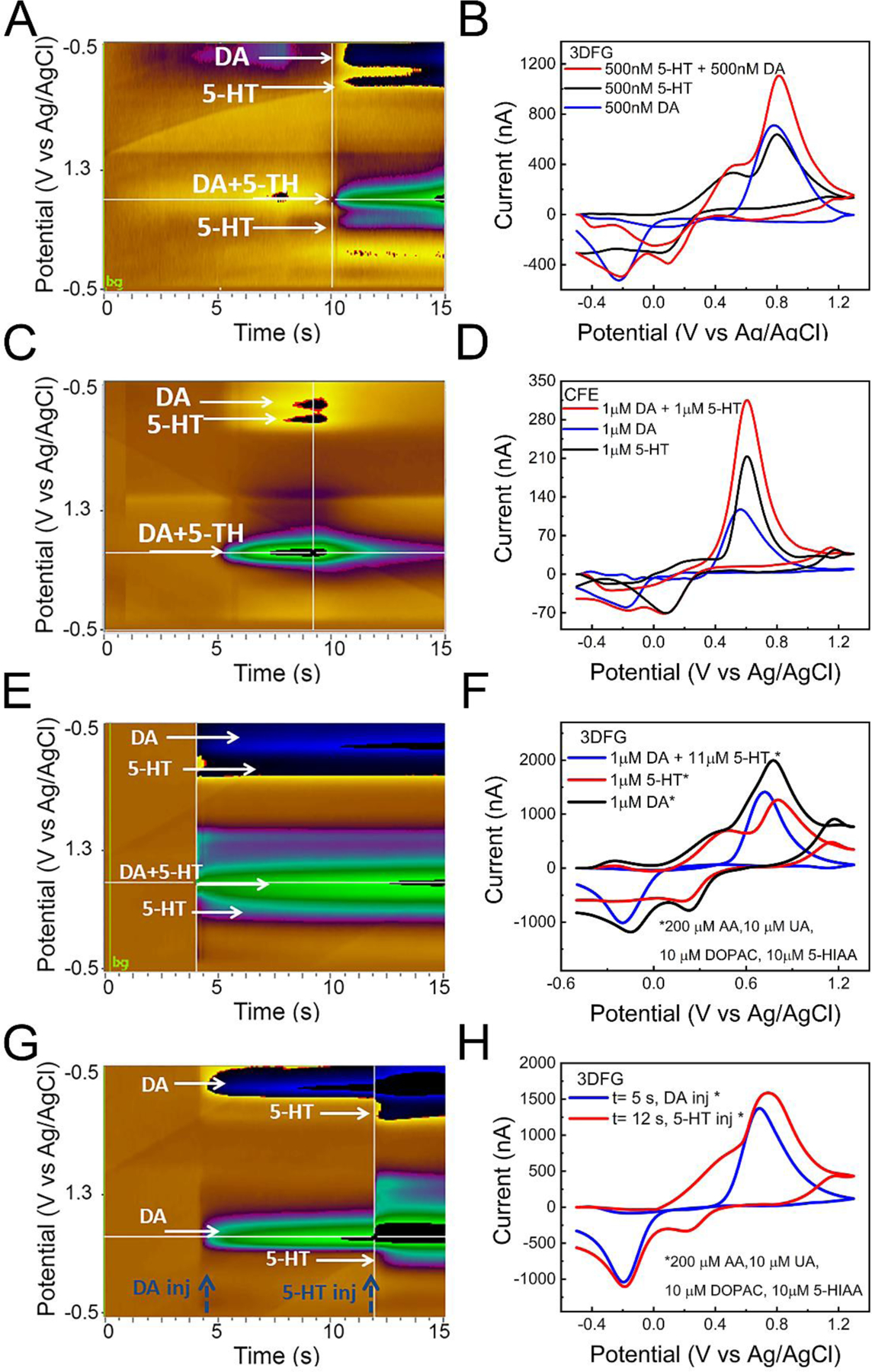
(A,B) 3DFG (A) A representative color plot showing clear separation peaks (reduction and oxidation) of DA and 5-HT and (B) Background subtracted CVs showing reduction and oxidation peaks of dopamine (DA), serotonin (5-HT) and their simultaneous detection (50:50% DA and 5-HT) using a standard pyramidal FSCV waveform in which the applied voltage was ramped from the holding potential of −0.5 V to the switching potential of +1.3V and then back to −0.5V at 400 V/s, at 10 Hz. 3DFG can discriminate both reduction and oxisation peaks. (C,D) CFEs. (A) Color plot for simultaneous DA and 5-HT detection showing showing clear separation only for the reduction peaks. (D) Background subtracted CV showing reduction and oxidation peaks of dopamine (DA), serotonin (5-HT) and their simultaneous detection using a standard pyramidal FSCV waveform in which the applied voltage was ramped from the holding potential of −0.5 V to the switching potential of +1.3V and then back to −0.5V at 400 V/s, at 10 Hz. (E, F) Representative color plot and background subtracted CVs showing reduction and oxidation peaks of dopamine (DA), serotonin (5-HT) and their simultaneous detection concentration in the presence of 200 μM AA, 10 μM UA, 10 μM DOPAC and 10 μM5-HIAA. DA and 5-HT were injected in a 50:50% mixture and signals were measured using a standard pyramidal FSCV waveform in which the applied voltage was ramped from the holding potential of −0.5 V to the switching potential of +1.3V and then back to −0.5V at 400 V/s, at 10 Hz. 3DFG can discriminate both reduction and oxidation of DA and 5-HT in simultaneous detection also in presence of contaminants. (G, H) Color plot and background subtracted CVs showing reduction and oxidation peaks of dopamine (DA, Injected at 5 s), and their simultaneous detection concentration in the presence of contaminants after a subsequent injection of serotonin (5-HT) at 12 s.
The 3DFG electrode miniaturization do not compromise the possibility of simultaneous detection of DA and 5-HT. Different example of FSCV co-detection of DA and 5-HT using 2 × 2 μm2 and 10 × 10 μm2 3DFG microelectrodes are reported in the Supplementary Figure 11 and 12.
Additionally, we demonstrated the 3DFG ability to simultaneously detect DA and 5-HT using FSCV in the presence of 200 μM AA, 10 μM UA, 10 μM DOPAC and 10 μM 5-hydroxyindole acetic acid (5-HIAA), 5-HT metabolite (Figure 6 E, F). Figure 6.G and H present the color plot and background-subtracted CVs showing reduction and oxidation peaks of DA, injected at 5 s, in the presence of interferents, and the simultaneous detection of DA and 5-HT after subsequent injection of 5-HT at 12 s, showing both oxidation and reduction 5-HT peaks.
However in the brain DA and 5-HT do not exist in fixed ratio, and concentrations of electrically stimulated 5-HT are usually lower (Dankoski and Wightman 2013; Swamy and Venton 2007a) compared to DA concentration in vivo. In particular, stimulated (phasic) 5-HT concentrations are expected to be drastically lower than DA concentrations in the dorsal striatum (Stamford et al. 1990; Swamy and Venton 2007a). To simultaneously detect DA and 5-HT, Swamy et al. (Swamy and Venton 2007a) increased the 5-HT level by administrating a synthetic precursor, 5-hydroxytryptophan (5-HTP), which has been shown to drastically increase 5-HT release (Stamford et al. 1990; Swamy and Venton 2007a)
Thus, we also evaluated the capability of 3DFG in distinguishing between DA and 5-HT at different ratios in presence of interferents (200 μM AA, 10 μM UA, 10 μM DOPAC and 10 μM 5-HIAA), i.e., 60% DA and 40% 5-HT, 70% DA and 30% 5-HT and 80% DA and 20% 5-HT. These data are presented in Supplementary Figure 13. We observed that 3DFG microelectrodes can discriminate both reduction and oxidation of DA and 5-HT in simultaneous detection using 60:40%, 70:30% DA and 5-HT mixtures and they can discriminate the reduction peaks of DA and 5-HT also in the 80:20 % mixture.
These results indicate that 3DFG microelectrodes can facilitate the co-detection of DA and 5-HT and can be employed towards quantifying the concentrations of DA and 5-HT simultaneously.
Conclusions
In this study, we present a high-density MEA platform, with 3DFG microelectrodes, synthetized using a catalyst-free process that enables high control over the materials structure. Using standard micro- and nano-fabrication techniques, the 3DFG can be pattenered into functional microelectrodes with sizes varying from 2 × 2 μm2 to 50 × 50 μm2. Electrochemical characterization and FSCV detection results highlight the ability of the 3DFG to provide exceptional electrochemical performance and DA sensitivity at ultrasmall size due to the material’s high surface area, with defect-rich, graphene based nanostructure, opening the possibility to fabricate high-density subcellular sized microelectrode arrays.
3DFG MEAs demonstrated the possibility of multi-channel FSCV DA detection from different microelectrodes of the same MEAs, simultaneously and without cross-talks in between adjacent microelectrodes with an inter-distance of 25 to 100μm.
3DFG microelectrodes also presented (i) great electrochemical and sensing stability under prolonged FSCV cycling, (ii) exceptional selectivity and (iii) fouling resistance, and (iv) the capability to discriminate reduction and oxidation peaks of DA and 5-HT, suggesting that 3DFG microelectrodes can be further investigated for the co-detection of DA and 5-HT.
Overall, these results demonstrate that 3DFG MEAs present a breakthrough platform for DA sensing using FSCV with high sensitivity, selectivity, and spatial resolution. This study represents an important first step toward the multi-site neurotransmitter mapping with high spatial and temporal resolution, opening the possibility for a deeper understanding of the complex spatial and temporal DA dynamic.
Future efforts will include (1) the transferring of the 3DFG MEAs on flexible substrate, (2) the design of the electronics with an appropriate circuit to allow for higher-channel count FSCV measurements, and (3) the implementation of a software platform for data acquisition, real-time visualization, and analysis of the large amount of FSCV data generated from high density microelectrode arrays.
Supplementary Material
Highlights:
3D fuzzy graphene (3DFG) microelectrode arrays (MEAs) demonstrate FSCV detection of dopamine with the highest sensitivity reported for nanocarbons.
3DFG MEAs exhibit high electrochemical stability, selectivity, and ability to simultaneously detect dopamine and serotonin.
The miniaturization of the 3DFG electrode sites down to 2 × 2 μm2 will enable the fabrication of densely packed MEA and dopamine multi-channel detection.
Acknowledgements
The authors thank Dr. Adrian Michael for the use of his laboratory and FSCV instrumentation. The authors acknowledge the support from the Carnegie Mellon University’s Department of Materials Science and Engineering Materials Characterization Facility (MCF-677785).
Funding
This work was supported by the National Institutes of Health [grant numbers R01NS062019, R01NS089688, R21DA043817, and R21 DA049592] from Dr. X. Tracy Cui; the National Science Foundation [Award No. CBET1552833], and the Defense Advanced Research Projects Agency [Award No. AWD00001593 (416052-5)] from Dr. Tzahi Cohen-Karni.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Supporting Information. The Supporting Information is provided as a separate file.
Conflict of Interest Contributions
All authors have no conflict of interest to declare.
References
- Atcherley CW, Wood KM, Parent KL, Hashemi P, Heien ML, 2015. The coaction of tonic and phasic dopamine dynamics. Chemical Communications 51(12), 2235–2238 DOI: 2210.1039/C2234CC06165A [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balasubramani PP, Chakravarthy VS, Ravindran B, Moustafa AA, 2015. A network model of basal ganglia for understanding the roles of dopamine and serotonin in reward-punishment-risk based decision making. Frontiers in computational neuroscience 9, 76. 10.3389/fncom.2015.00076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bard AJ, Faulkner LR, 2001. Fundamentals and applications. Electrochemical Methods 2(482), 580–632. [Google Scholar]
- Bath BD, Michael DJ, Trafton BJ, Joseph JD, Runnels PL, Wightman RM, 2000. Subsecond adsorption and desorption of dopamine at carbon-fiber microelectrodes. Analytical chemistry 72(24), 5994–6002 [DOI] [PubMed] [Google Scholar]
- Bissett MA, Izumida W, Saito R, Ago H, 2012. Effect of domain boundaries on the Raman spectra of mechanically strained graphene. ACS nano 6(11), 10229–10238 [DOI] [PubMed] [Google Scholar]
- Bissett MA, Tsuji M, Ago H, 2014. Strain engineering the properties of graphene and other two-dimensional crystals. Physical Chemistry Chemical Physics 16(23), 11124–11138 DOI: 11110.11039/C11123CP55443K [DOI] [PubMed] [Google Scholar]
- Boileau I, Warsh JJ, Guttman M, Saint- Cyr JA, McCluskey T, Rusjan P, Houle S, Wilson AA, Meyer JH, Kish SJ, 2008. Elevated serotonin transporter binding in depressed patients with Parkinson’s disease: a preliminary PET study with [11C] DASB. Movement disorders: official journal of the Movement Disorder Society 23(12), 1776–1780 [DOI] [PubMed] [Google Scholar]
- Bowling R, Packard RT, McCreery RL, 1989. Mechanism of electrochemical activation of carbon electrodes: role of graphite lattice defects. Langmuir 5(3), 683–688 [Google Scholar]
- Cao Q, Puthongkham P, Venton BJ, 2019. new insights into optimizing chemical and 3D surface structures of carbon electrodes for neurotransmitter detection. Analytical Methods 11(3), 247–261 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao Q, Shin M, Lavrik NV, Venton BJ, 2020. 3D-Printed Carbon Nanoelectrodes for In Vivo Neurotransmitter Sensing. Nano Letters 20(9), 6831–6836 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carta M, Carlsson T, Muñoz A, Kirik D, Björklund A, 2008. Serotonin–dopamine interaction in the induction and maintenance of L-DOPA-induced dyskinesias. Progress in brain research 172, 465–478 DOI: 410.1016/S0079–6123(1008)00922–00929. [DOI] [PubMed] [Google Scholar]
- Castagnola E, Thongpang S, Hirabayashi M, Nava G, Nimbalkar S, Nguyen T, Lara S, Oyawale A, Bunnell J, Moritz C, 2020a. Glassy Carbon Microelectrode Arrays Enable Voltage-Peak Separated Simultaneous Detection of Dopamine and Serotonin Using Fast Scan Cyclic Voltammetry. arXiv preprint arXiv:2011.13024. [DOI] [PubMed] [Google Scholar]
- Castagnola E, Thongpang S, Hirabayashi M, Nava G, Nimbalkar S, Nguyen T, Lara S, Oyawale A, Bunnell J, Moritz C, 2021. Glassy carbon microelectrode arrays enable voltage-peak separated simultaneous detection of dopamine and serotonin using fast scan cyclic voltammetry. Analyst. [DOI] [PubMed] [Google Scholar]
- Castagnola E, Vahidi NW, Nimbalkar S, Rudraraju S, Thielk M, Zucchini E, Cea C, Carli S, Gentner TQ, Ricci D, 2018. In vivo dopamine detection and single unit recordings using intracortical glassy carbon microelectrode arrays. MRS advances 3(29), 1629 DOI: 1610.1557/adv.2018.1698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castagnola E, Woeppel K, Golabchi A, McGuier M, Chodapaneedi N, Metro J, Taylor IM, Cui XT, 2020b. Electrochemical detection of exogenously administered melatonin in the brain. Analyst 145(7), 2612–2620 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen S-Y, Lu K-M, Ko H-A, Huang T-H, Hao JH-J, Yan Y-T, Chang SL-Y, Evans SM, Liu F-C, 2020. Parcellation of the striatal complex into dorsal and ventral districts. Proceedings of the National Academy of Sciences 117(13), 7418–7429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen S, Zheng J, Li L, Jiang S, 2005. Strong resistance of phosphorylcholine self-assembled monolayers to protein adsorption: insights into nonfouling properties of zwitterionic materials. Journal of the American Chemical Society 127(41), 14473–14478. [DOI] [PubMed] [Google Scholar]
- Cogan SF, 2008. Neural stimulation and recording electrodes. Annu. Rev. Biomed. Eng. 10, 275–309 DOI: 210.1146/annurev.bioeng.1110.061807.160518. [DOI] [PubMed] [Google Scholar]
- Dankoski EC, Wightman RM, 2013. Monitoring serotonin signaling on a subsecond time scale. Frontiers in integrative neuroscience 7, 44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Chiara G, 1995. The role of dopamine in drug abuse viewed from the perspective of its role in motivation. Drug and alcohol dependence 38(2), 95–137. [DOI] [PubMed] [Google Scholar]
- Dremencov E, Gispan-Herman I, Rosenstein M, Mendelman A, Overstreet DH, Zohar J, Yadid G, 2004. The serotonin–dopamine interaction is critical for fast-onset action of antidepressant treatment: in vivo studies in an animal model of depression. Progress in Neuro-Psychopharmacology and Biological Psychiatry 28(1), 141–147 DOI: 110.1016/j.pnpbp.2003.1009.1030 [DOI] [PubMed] [Google Scholar]
- DuVall SH, McCreery RL, 2000. Self-catalysis by catechols and quinones during heterogeneous electron transfer at carbon electrodes. Journal of the American Chemical Society 122(28), 6759–6764 [Google Scholar]
- El Merhie A, Ito D, Colombi I, Keshavan S, Mishra N, Miseikis V, Diaspro A, Coletti C, Chiappalone M, Dante S, 2018. Single layer graphene functionalized MEA for enhanced detection of neuronal network development. Sensors and Actuators B: Chemical 277, 224–233 [Google Scholar]
- Engstrom RC, Strasser VA, 1984. Characterization of electrochemically pretreated glassy carbon electrodes. Analytical Chemistry 56(2), 136–141 [Google Scholar]
- Fan B, Rusinek CA, Thompson CH, Setien M, Guo Y, Rechenberg R, Gong Y, Weber AJ, Becker MF, Purcell E, 2020. Flexible, diamond-based microelectrodes fabricated using the diamond growth side for neural sensing. Microsystems & nanoengineering 6(1), 1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrari AC, Basko DM, 2013. Raman Spectroscopy as a Versatile Tool for Studying the Properties of Graphene. Nature Nanotechnology 8(4), 235. [DOI] [PubMed] [Google Scholar]
- Fischer AG, Ullsperger M, 2017. An update on the role of serotonin and its interplay with dopamine for reward. Frontiers in human neuroscience 11, 484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forster RJ, 1994. Microelectrodes: new dimensions in electrochemistry. Chemical Society Reviews 23(4), 289–297 [Google Scholar]
- Forster RJ, 2006. Ultrafast electrochemical techniques. Encyclopedia of Analytical Chemistry: Applications, Theory and Instrumentation, 10.1002/9780470027318.a9780470025319.pub9780470027312. [DOI] [Google Scholar]
- Garg R, Rastogi SK, Lamparski M, de la Barrera SC, Pace GT, Nuhfer NT, Hunt BM, Meunier V, Cohen-Karni T, 2017. Nanowire-mesh-templated growth of out-of-plane three-dimensional fuzzy graphene. ACS nano 11(6), 6301–6311 [DOI] [PubMed] [Google Scholar]
- Goshi N, Castagnola E, Vomero M, Gueli C, Cea C, Zucchini E, Bjanes D, Maggiolini E, Moritz C, Kassegne S, 2018. Glassy carbon MEMS for novel origami-styled 3D integrated intracortical and epicortical neural probes. Journal of Micromechanics and Microengineering 28(6), 065009 [Google Scholar]
- Grinnell F, Feld MK, 1981. Adsorption characteristics of plasma fibronectin in relationship to biological activity. Journal of biomedical materials research 15(3), 363–381. [DOI] [PubMed] [Google Scholar]
- Hanssen BL, Siraj S, Wong DK, 2016a. Recent strategies to minimise fouling in electrochemical detection systems. Reviews in Analytical Chemistry 35(1), 1–28 DOI 10.1515/revac-2015-0008. [DOI] [Google Scholar]
- Hanssen BL, Siraj S, Wong DK, 2016b. Recent strategies to minimise fouling in electrochemical detection systems. Reviews in Analytical Chemistry 35(1), 1–28. [Google Scholar]
- Harreither W, Trouillon R, Poulin P, Neri W, Ewing AG, Safina G, 2016. Cysteine residues reduce the severity of dopamine electrochemical fouling. Electrochimica Acta 210, 622–629610.1016/j.electacta.2016.1005.1124. [Google Scholar]
- Harris DC, 2010. Quantitative chemical analysis. Macmillan. [Google Scholar]
- Hashemi K.M.W.a.P., 2013. Fast-Scan Cyclic Voltammetry Analysis of Dynamic Serotonin Reponses to Acute Escitalopram. ACS Chem Neurosci. 4(5), 715–720 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hashemi P, Dankoski EC, Petrovic J, Keithley RB, Wightman R, 2009. Voltammetric detection of 5-hydroxytryptamine release in the rat brain. Analytical chemistry 81(22), 9462–9471 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heien ML, Johnson MA, Wightman RM, 2004. Resolving neurotransmitters detected by fast-scan cyclic voltammetry. Analytical chemistry 76(19), 5697–5704. [DOI] [PubMed] [Google Scholar]
- Heien ML, Phillips PE, Stuber GD, Seipel AT, Wightman RM, 2003. Overoxidation of carbon-fiber microelectrodes enhances dopamine adsorption and increases sensitivity. Analyst 128(12), 1413–1419 [DOI] [PubMed] [Google Scholar]
- Hensley AL, Colley AR, Ross AE, 2018. Real-Time Detection of Melatonin Using Fast-Scan Cyclic Voltammetry. Analytical chemistry 90(14), 8642–8650 DOI: 8610.1021/acs.analchem.8648b01976. [DOI] [PubMed] [Google Scholar]
- Hess H, Vogel V, 2001. Molecular shuttles based on motor proteins: active transport in synthetic environments. Reviews in Molecular Biotechnology 82(1), 67–85. [DOI] [PubMed] [Google Scholar]
- Höglinger GU, Rizk P, Muriel MP, Duyckaerts C, Oertel WH, Caille I, Hirsch EC, 2004. Dopamine depletion impairs precursor cell proliferation in Parkinson disease. Nature neuroscience 7(7), 726–735. [DOI] [PubMed] [Google Scholar]
- Howe MW, Tierney PL, Sandberg SG, Phillips PE, Graybiel AM, 2013. Prolonged dopamine signalling in striatum signals proximity and value of distant rewards. nature 500(7464), 575–579510.1038/nature12475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howes OD, Kapur S, 2009. The dopamine hypothesis of schizophrenia: version III—the final common pathway. Schizophrenia bulletin 35(3), 549–562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor I Mitch, A.J.G., Sesack SR, Michael AC, 2012. Domain- dependent effects of DAT inhibition in the rat dorsal striatum. Journal of neurochemistry 122(2), 283–294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor I Mitch, K.M.N., Walters Seth H, Varner Erika L, Shu Zhan, Bartlow Kathleen M, Jaquins- Gerstl Andrea S, Michael Adrian C, 2015. Kinetic diversity of dopamine transmission in the dorsal striatum. Journal of neurochemistry 133(4), 522–531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson BP, Dietz SM, Wightman RM, 1995. Fast-scan cyclic voltammetry of 5-hydroxytryptamine. Analytical chemistry 67(6), 1115–1120 [DOI] [PubMed] [Google Scholar]
- Jacobs CB, Ivanov IN, Nguyen MD, Zestos AG, Venton BJ, 2014. High temporal resolution measurements of dopamine with carbon nanotube yarn microelectrodes. Analytical chemistry 86(12), 5721–5727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jenkins BG, Sanchez-Pernaute R, Brownell A-L, Chen Y-CI, Isacson O, 2004. Mapping dopamine function in primates using pharmacologic magnetic resonance imaging. Journal of Neuroscience 24(43), 9553–9560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson JA, Rodeberg NT, Wightman RM, 2018. Measurement of basal neurotransmitter levels using convolution-based nonfaradaic current removal. Analytical chemistry 90(12), 7181–7189 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kapur S, Remington G, 1996. Serotonin-dopamine interaction and its relevance to schizophrenia. American Journal of Psychiatry 153(4), 466–476 DOI: 410.1176/ajp.1153.1174.1466. [DOI] [PubMed] [Google Scholar]
- Keithley RB, Takmakov P, Bucher ES, Belle AM, Owesson-White CA, Park J, Wightman RM, 2011. Higher sensitivity dopamine measurements with faster-scan cyclic voltammetry. Analytical chemistry 83(9), 3563–3571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim DH, Oh Y, Shin H, Park C, Blaha CD, Bennet KE, Kim IY, Lee KH, Jang DP, 2018. Multi-waveform fast-scan cyclic voltammetry mapping of adsorption/desorption kinetics of biogenic amines and their metabolites. Analytical Methods 10(24), 2834–2843 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu X, Ren C, Lu Y, Hattori R, Shi Y, Zhao R, Ding D, Komiyama T, Kuzum D, 2019. Decoding ECoG High Gamma Power from Cellular Calcium Response using Transparent Graphene Microelectrodes. 2019 9th International IEEE/EMBS Conference on Neural Engineering (NER), pp. 710–713 DOI: 710.1109/NER.2019.8717147. IEEE. [Google Scholar]
- Lotharius J, Brundin P, 2002. Pathogenesis of Parkinson’s disease: dopamine, vesicles and α-synuclein. Nature reviews neuroscience 3(12), 932–942. [DOI] [PubMed] [Google Scholar]
- Lu Y, Liu X, Hattori R, Ren C, Zhang X, Komiyama T, Kuzum D, 2018. Ultralow Impedance Graphene Microelectrodes with High Optical Transparency for Simultaneous Deep Two- Photon Imaging in Transgenic Mice. Advanced Functional Materials 28(31), 1800002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCreery RL, 2008. Advanced carbon electrode materials for molecular electrochemistry. Chemical reviews 108(7), 2646–2687 [DOI] [PubMed] [Google Scholar]
- McDermott MT, McCreery RL, 1994. Scanning tunneling microscopy of ordered graphite and glassy carbon surfaces: electronic control of quinone adsorption. Langmuir 10(11), 4307–4314 [Google Scholar]
- Mendoza A, Asrat T, Liu F, Wonnenberg P, Zestos AG, 2020. Carbon Nanotube Yarn Microelectrodes Promote High Temporal Measurements of Serotonin Using Fast Scan Cyclic Voltammetry. Sensors 20(4), 1173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitch Taylor I, Jaquins- Gerstl A, Sesack SR, Michael AC, 2012. Domain- dependent effects of DAT inhibition in the rat dorsal striatum. Journal of neurochemistry 122(2), 283–294 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohebi A, Pettibone JR, Hamid AA, Wong J-MT, Vinson LT, Patriarchi T, Tian L, Kennedy RT, Berke JD, 2019. Dissociable dopamine dynamics for learning and motivation. Nature 570(7759), 65–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newman AH, Grundt P, Nader MA, 2005. Dopamine D3 receptor partial agonists and antagonists as potential drug abuse therapeutic agents. Journal of medicinal chemistry 48(11), 3663–3679. [DOI] [PubMed] [Google Scholar]
- Newman J, 1966. Resistance for flow of current to a disk. J. electrochem. Soc 113(5), 501–502. [Google Scholar]
- Niederkofler V, Asher TE, Dymecki SM, 2015. Functional interplay between dopaminergic and serotonergic neuronal systems during development and adulthood. ACS chemical neuroscience 6(7), 1055–1070 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nimbalkar S, Castagnola E, Balasubramani A, Scarpellini A, Samejima S, Khorasani A, Boissenin A, Thongpang S, Moritz C, Kassegne S, 2018. Ultra-capacitive carbon neural probe allows simultaneous long-term electrical stimulations and high-resolution neurotransmitter detection. Scientific reports 8(1), 1–14 10.1038/s41598-41018-25198-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oh Y, Heien ML, Park C, Kang YM, Kim J, Boschen SL, Shin H, Cho HU, Blaha CD, Bennet KE, 2018. Tracking tonic dopamine levels in vivo using multiple cyclic square wave voltammetry. Biosensors and Bioelectronics 121, 174–182 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oh Y, Park C, Kim DH, Shin H, Kang YM, DeWaele M, Lee J, Min H-K, Blaha CD, Bennet KE, 2016. Monitoring in vivo changes in tonic extracellular dopamine level by charge-balancing multiple waveform fast-scan cyclic voltammetry. Analytical chemistry 88(22), 10962–10970 [DOI] [PubMed] [Google Scholar]
- Orazem ME, Tribollet B, 2008. Electrochemical impedance spectroscopy. New Jersey, 383–389. [Google Scholar]
- Ou Y, Buchanan AM, Witt CE, Hashemi P, 2019. Frontiers in electrochemical sensors for neurotransmitter detection: towards measuring neurotransmitters as chemical diagnostics for brain disorders. Analytical Methods 11(21), 2738–2755 DOI: 2710.1039/C2739AY00055K. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Owesson- White CA, Ariansen J, Stuber GD, Cleaveland NA, Cheer JF, Mark Wightman R, Carelli RM, 2009. Neural encoding of cocaine- seeking behavior is coincident with phasic dopamine release in the accumbens core and shell. European Journal of Neuroscience 30(6), 1117–1127 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pancrazio JJ, Deku F, Ghazavi A, Stiller AM, Rihani R, Frewin CL, Varner VD, Gardner TJ, Cogan SF, 2017. Thinking small: Progress on microscale neurostimulation technology. Neuromodulation: Technology at the Neural Interface 20(8), 745–752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel AN, Unwin PR, Macpherson JV, 2013. Investigation of film formation properties during electrochemical oxidation of serotonin (5-HT) at polycrystalline boron doped diamond. Physical Chemistry Chemical Physics 15(41), 18085–18092 DOI: 18010.11039/C18083CP53513D [DOI] [PubMed] [Google Scholar]
- Peng J, Huang Q, Liu Y, Liu P, Zhang C, 2019. Photoelectrochemical sensor based on composite of CdTe and nickel tetra-amined phthalocyanine covalently linked with graphene oxide for ultrasensitive detection of curcumin. Sensors and Actuators B: Chemical 294, 157–165. [Google Scholar]
- Phillips PE, Stuber GD, Heien ML, Wightman RM, Carelli RM, 2003. Subsecond dopamine release promotes cocaine seeking. Nature 422(6932), 614–618 [DOI] [PubMed] [Google Scholar]
- Pihel K, Schroeder TJ, Wightman RM, 1994. Rapid and selective cyclic voltammetric measurements of epinephrine and norepinephrine as a method to measure secretion from single bovine adrenal medullary cells. Analytical Chemistry 66(24), 4532–4537. [Google Scholar]
- Politis M, Wu K, Loane C, Quinn NP, Brooks DJ, Oertel WH, Björklund A, Lindvall O, Piccini P, 2012. Serotonin neuron loss and nonmotor symptoms continue in Parkinson’s patients treated with dopamine grafts. Science translational medicine 4(128), 128ra141–128ra141 DOI: 110.1126/scitranslmed.3003391. [DOI] [PubMed] [Google Scholar]
- Poon M, McCreery RL, Engstrom R, 1988. Laser activation of carbon electrodes. Relationship between laser-induced surface effects and electron transfer activation. Analytical Chemistry 60(17), 1725–1730 [Google Scholar]
- Puthongkham P, Venton BJ, 2019. Nanodiamond coating improves the sensitivity and antifouling properties of carbon fiber microelectrodes. ACS sensors 4(9), 2403–2411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puthongkham P, Venton BJ, 2020. Recent Advances in Fast-Scan Cyclic Voltammetry. Analyst 145, 1087–1102 DOI: 1010.1039/C1089AN01925A. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puthongkham P, Yang C, Venton BJ, 2018. Carbon Nanohorn- modified Carbon Fiber Microelectrodes for Dopamine Detection. Electroanalysis 30(6), 1073–1081 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rastogi SK, Bliley J, Matino L, Garg R, Santoro F, Feinberg AW, Cohen-Karni T, 2020. Three-dimensional fuzzy graphene ultra-microelectrodes for subcellular electrical recordings. Nano Research 13(5), 1444–1452 [Google Scholar]
- Rastogi SK, Bliley J, Shiwarski DJ, Raghavan G, Feinberg AW, Cohen-Karni T, 2018. Graphene microelectrode arrays for electrical and optical measurements of human stem cell-derived cardiomyocytes. Cellular and Molecular Bioengineering 11(5), 407–418 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rees HR, Anderson SE, Privman E, Bau HH, Venton BJ, 2015. Carbon nanopipette electrodes for dopamine detection in Drosophila. Analytical chemistry 87(7), 3849–3855 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson DL, Venton BJ, Heien ML, Wightman RM, 2003. Detecting subsecond dopamine release with fast-scan cyclic voltammetry in vivo. Clinical chemistry 49(10), 1763–1773 [DOI] [PubMed] [Google Scholar]
- Roeper J, 2013. Dissecting the diversity of midbrain dopamine neurons. Trends in neurosciences 36(6), 336–342. [DOI] [PubMed] [Google Scholar]
- Roitman MF, Stuber GD, Phillips PE, Wightman RM, Carelli RM, 2004. Dopamine operates as a subsecond modulator of food seeking. Journal of Neuroscience 24(6), 1265–1271 DOI: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saddoris MP, Sugam JA, Cacciapaglia F, Carelli RM, 2013. Rapid dopamine dynamics in the accumbens core and shell: learning and action. Frontiers in bioscience (Elite edition) 5, 273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salamone JD, Correa M, Mingote S, Weber SM, 2003. Nucleus accumbens dopamine and the regulation of effort in food-seeking behavior: implications for studies of natural motivation, psychiatry, and drug abuse. Journal of Pharmacology and Experimental Therapeutics 305(1), 1–8. [DOI] [PubMed] [Google Scholar]
- Schmidt AC, Wang X, Zhu Y, Sombers LA, 2013. Carbon nanotube yarn electrodes for enhanced detection of neurotransmitter dynamics in live brain tissue. ACS nano 7(9), 7864–7873 [DOI] [PubMed] [Google Scholar]
- Schwerdt HN, Shimazu H, Amemori K. i., Amemori S, Tierney PL, Gibson DJ, Hong S, Yoshida T, Langer R, Cima MJ, 2017. Long-term dopamine neurochemical monitoring in primates. Proceedings of the National Academy of Sciences 114(50), 13260–13265 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwerdt HN, Zhang E, Kim MJ, Yoshida T, Stanwicks L, Amemori S, Dagdeviren HE, Langer R, Cima MJ, Graybiel AM, 2018. Cellular-scale probes enable stable chronic subsecond monitoring of dopamine neurochemicals in a rodent model. Communications biology 1(1), 1–11 10.1038/s42003-42018-40147-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seeman P, 1987. Dopamine receptors and the dopamine hypothesis of schizophrenia. Synapse 1(2), 133–152. [DOI] [PubMed] [Google Scholar]
- Shao Z, Puthongkham P, Hu K, Jia R, Mirkin MV, Venton BJ, 2020. Thin layer cell behavior of CNT yarn and cavity carbon nanopipette electrodes: Effect on catecholamine detection. Electrochimica Acta 361, 137032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shu Z, Taylor IM, Michael AC, 2013. The dopamine patchwork of the rat nucleus accumbens core. European Journal of Neuroscience 38(8), 3221–3229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith SK, Gosrani SP, Lee CA, McCarty GS, Sombers LA, 2018. Carbon-fiber microbiosensor for monitoring rapid lactate fluctuations in brain tissue using fast-scan cyclic voltammetry. Analytical chemistry 90(21), 12994–12999 [DOI] [PubMed] [Google Scholar]
- Stamford JA, Kruk ZL, Millar J, 1990. Striatal dopamine terminals release serotonin after 5-HTP pretreatment: in vivo voltammetric data. Brain research 515(1–2), 173–180. [DOI] [PubMed] [Google Scholar]
- Stouffer MA, Woods CA, Patel JC, Lee CR, Witkovsky P, Bao L, Machold RP, Jones KT, De Vaca SC, Reith ME, 2015. Insulin enhances striatal dopamine release by activating cholinergic interneurons and thereby signals reward. Nature communications 6, 8543 DOI: 8510.1038/ncomms9543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swamy BK, Venton BJ, 2007a. Carbon nanotube-modified microelectrodes for simultaneous detection of dopamine and serotonin in vivo. Analyst 132(9), 876–884 [DOI] [PubMed] [Google Scholar]
- Swamy BK, Venton BJ, 2007b. Subsecond detection of physiological adenosine concentrations using fast-scan cyclic voltammetry. Analytical chemistry 79(2), 744–750 DOI: 710.1021/ac061820i. [DOI] [PubMed] [Google Scholar]
- Swerdlow NR, Koob GF, 1987. Dopamine, schizophrenia, mania, and depression: toward a unified hypothesis of cortico-striatopallido-thalamic function. Behavioral and brain sciences 10(2), 197–208. [Google Scholar]
- Takmakov P, Zachek MK, Keithley RB, Walsh PL, Donley C, McCarty GS, Wightman RM, 2010. Carbon microelectrodes with a renewable surface. Analytical chemistry 82(5), 2020–2028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor IM, Patel NA, Freedman NC, Castagnola E, Cui XT, 2019. Direct in Vivo Electrochemical Detection of Resting Dopamine Using Poly (3, 4-ethylenedioxythiophene)/Carbon Nanotube Functionalized Microelectrodes. Analytical chemistry 91(20), 12917–12927 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor IM, Robbins EM, Catt KA, Cody PA, Happe CL, Cui XT, 2017. Enhanced dopamine detection sensitivity by PEDOT/graphene oxide coating on in vivo carbon fiber electrodes. Biosensors and Bioelectronics 89, 400–410 doi: 410.1016/j.bios.2016.1005.1084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torrisi F, Hasan T, Wu W, Sun Z, Lombardo A, Kulmala TS, Hsieh G-W, Jung S, Bonaccorso F, Paul PJ, 2012. Inkjet-printed graphene electronics. ACS Nano 6(4), 2992–3006 [DOI] [PubMed] [Google Scholar]
- Venton BJ, Cao Q, 2020. Fundamentals of fast-scan cyclic voltammetry for dopamine detection. Analyst 145(4), 1158–1168 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verbiese-Genard N, Kauffmann J, Hanocq M, Molle L, 1984. Study of the electrooxidative behaviour of 5-hydroxyindole-3-acetic acid, 5-hydroxytryptophan and serotonine in the presence of sodium ethylenediaminetetraacetic acid. Journal of electroanalytical chemistry and interfacial electrochemistry 170(1–2), 243–254 [Google Scholar]
- Volkow ND, Fowler JS, Wang G-J, Swanson JM, 2004. Dopamine in drug abuse and addiction: results from imaging studies and treatment implications. Molecular psychiatry 9(6), 557–569. [DOI] [PubMed] [Google Scholar]
- Vomero M, Castagnola E, Ciarpella F, Maggiolini E, Goshi N, Zucchini E, Carli S, Fadiga L, Kassegne S, Ricci D, 2017. Highly stable glassy carbon interfaces for long-term neural stimulation and low-noise recording of brain activity. Scientific reports 7(1), 1–14 doi: 10.1038/srep40332(42017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vreeland RF, Atcherley CW, Russell WS, Xie JY, Lu D, Laude ND, Porreca F, Heien ML, 2015. Biocompatible PEDOT: Nafion composite electrode coatings for selective detection of neurotransmitters in vivo. Analytical chemistry 87(5), 2600–2607 DOI: 2610.1021/ac502165f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watabe-Uchida M, Zhu L, Ogawa SK, Vamanrao A, Uchida N, 2012. Whole-brain mapping of direct inputs to midbrain dopamine neurons. Neuron 74(5), 858–873. [DOI] [PubMed] [Google Scholar]
- Weese ME, Krevh RA, Li Y, Alvarez NT, Ross AE, 2019. Defect sites modulate fouling resistance on carbon-nanotube fiber electrodes. ACS sensors 4(4), 1001–1007 [DOI] [PubMed] [Google Scholar]
- Weintraub D, Siderowf AD, Potenza MN, Goveas J, Morales KH, Duda JE, Moberg PJ, Stern MB, 2006. Association of dopamine agonist use with impulse control disorders in Parkinson disease. Archives of neurology 63(7), 969–973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong P-H, Feng H, Teo W, 1995. Interaction of the dopaminergic and serotonergic systems in the rat striatum: effects of selective antagonists and uptake inhibitors. Neuroscience research 23(1), 115–119 [DOI] [PubMed] [Google Scholar]
- Wrona MZ, Dryhurst G, 1987. Oxidation chemistry of 5-hydroxytryptamine. 1. Mechanism and products formed at micromolar concentrations. The Journal of Organic Chemistry 52(13), 2817–2825 [Google Scholar]
- Wrona MZ, Dryhurst G, 1990. Electrochemical oxidation of 5-hydroxytryptamine in aqueous solution at physiological pH. Bioorganic Chemistry 18(3), 291–317 [Google Scholar]
- Xiao X, Zou L, Pang H, Xu Q, 2020. Synthesis of micro/nanoscaled metal–organic frameworks and their direct electrochemical applications. Chemical Society Reviews 49(1), 301–331. [DOI] [PubMed] [Google Scholar]
- Yang C, Jacobs CB, Nguyen MD, Ganesana M, Zestos AG, Ivanov IN, Puretzky AA, Rouleau CM, Geohegan DB, Venton BJ, 2016a. Carbon nanotubes grown on metal microelectrodes for the detection of dopamine. Analytical chemistry 88(1), 645–652 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang C, Trikantzopoulos E, Jacobs CB, Venton BJ, 2017. Evaluation of carbon nanotube fiber microelectrodes for neurotransmitter detection: Correlation of electrochemical performance and surface properties. Analytica chimica acta 965, 1–8 doi: 10.1016/j.aca.2017.1001.1039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang C, Trikantzopoulos E, Nguyen MD, Jacobs CB, Wang Y, Mahjouri-Samani M, Ivanov IN, Venton BJ, 2016b. Laser treated carbon nanotube yarn microelectrodes for rapid and sensitive detection of dopamine in vivo. ACS sensors 1(5), 508–515 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang W, Ratinac KR, Ringer SP, Thordarson P, Gooding JJ, Braet F, 2010. Carbon nanomaterials in biosensors: should you use nanotubes or graphene? Angewandte Chemie International Edition 49(12), 2114–2138 DOI: 2110.1002/anie.200903463. [DOI] [PubMed] [Google Scholar]
- Zachek MK, Park J, Takmakov P, Wightman RM, McCarty GS, 2010a. Microfabricated FSCV-compatible microelectrode array for real-time monitoring of heterogeneous dopamine release. Analyst 135(7), 1556–1563 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zachek MK, Park J, Takmakov P, Wightman RM, McCarty GS, 2010b. Microfabricated FSCV-compatible microelectrode array for real-time monitoring of heterogeneous dopamine release. Analyst 135(7), 1556–1563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zangen A, Nakash R, Overstreet DH, Yadid G, 2001. Association between depressive behavior and absence of serotonin–dopamine interaction in the nucleus accumbens. Psychopharmacology 155(4), 434–439 [DOI] [PubMed] [Google Scholar]
- Zestos AG, Jacobs CB, Trikantzopoulos E, Ross AE, Venton BJ, 2014. Polyethylenimine carbon nanotube fiber electrodes for enhanced detection of neurotransmitters. Analytical chemistry 86(17), 8568–8575 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zestos AG, Yang C, Jacobs CB, Hensley D, Venton BJ, 2015. Carbon nanospikes grown on metal wires as microelectrode sensors for dopamine. Analyst 140(21), 7283–7292 DOI: 7210.1039/C7285AN01467K. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou F-M, Liang Y, Salas R, Zhang L, De Biasi M, Dani JA, 2005. Corelease of dopamine and serotonin from striatal dopamine terminals. Neuron 46(1), 65–74 10.1016/j.neuron.2005.1002.1010. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


