Abstract
Background
Nishamalaki is an Ayurvedic herbal formulation used to treat type 2 diabetes mellitus (T2DM), comprises Emblica officinalis and Curcuma longa.
Objective(s)
One of the main cause of T2DM is Insulin Resistance (IR) hence, this study was planned to evaluate IR lowering effect of a standardized Nishamalaki extract “EmbliQur” in high-fat diet (HFD) and streptozotocin (STZ) induced T2DM rats.
Materials and methods
Curcuminoids (23.89% w/w), gallic acid (5.27% w/w) and tannins (25.44% w/w) were quantified from EmbliQur. Rats were fed HFD throughout the study of 45 days and received STZ (40 mg/kg, i.p) on the 15th day of the study. Rats with more than 250 mg/dl of fasting blood glucose level (FBGL) were considered diabetic and selected for administration of EmbliQur (500 mg and 1000 mg/kg) or the standard drug metformin (120 mg/kg, p.o) from the 18th day of the study for the next 27 days. FBGL and insulin levels of all rats were measured weekly and an oral glucose tolerance test (OGTT) was done at the end of the study. The values of FBGL and insulin were used to calculate IR by the HOMA-IR, QUICKI and Matsuda methods.
Results
Rats treated with STZ/HFD had significantly higher than normal FBGL and insulin levels throughout the study and exhibited skewed IR indices in the above three methods of IR assessment. EmbliQur treatment successfully lowered the HFD/STZ-elevated BGL and insulin levels, and ameliorated IR in all models of IR evaluation.
Conclusion
EmbliQur 1000 mg/kg was noted to be more effective than EmbliQur 500 mg/kg in alleviating IR.
Keywords: Diabetes, High-fat diet, Streptozotocin, Insulin resistance, Curcuma longa, Emblica officinalis
Graphical abstract
1. Introduction
Insulin resistance (IR) causes a diminished cell response to the action of insulin in transporting glucose from the bloodstream into cells. As a result, higher amounts of insulin are required for its proper effects and thus, the pancreas produces far more insulin than usual. Blood glucose levels remain normal as long as enough insulin is produced by the pancreas to clear glucose from blood. Once the pancreas is unable to cope, the blood glucose levels start to rise, initially after meals, and eventually even in the fasting state.
Although, IR may affect many biological actions of insulin in the body, typically, it refers to a subnormal glucose response to insulin. IR often remains undetected and may lead to prediabetes, type 2 diabetes mellitus (T2DM), and the metabolic syndrome [1]. People suffering from diabetes mellitus are found to be at high risk for COVID-19, and knowing longstanding T2DM to be associated with cardiovascular diseases, such patients are at higher risk of morbidity and mortality [[2], [3], [4]].
Obesity, a major risk factor for the development of IR, is generally an outcome of a high fat diet (HFD) and physical inactivity. HFD induces alterations in carbohydrate and lipid metabolism which leads to IR and an alteration in adipokines [5].
Streptozotocin (STZ) is an anti-cancer antibiotic from the nitrosourea class of alkylating agents which is widely used to induce experimental diabetes in animals [6]. Being structurally similar to glucose, it is up-taken into the pancreatic β cells by the glucose transporter 2 (GLUT2) where it causes alkylation of DNA leading to inhibition of DNA synthesis. It releases harmful oxygen-derived products such as superoxide radicals, hydrogen peroxide, nitric oxide, and hydroxyl radicals which cause severe DNA damage leading to destruction of β cells and affecting insulin secretion, thus, causing a rise in blood glucose levels.
Today, insulin and other anti-hyperglycemic drugs such as biguanides, sulfonylureas, thiazolidinediones, α-glucosidase inhibitors, etc. are available for the treatment of T2DM but have side effects. Further, most diabetic patients require a combination of oral anti-diabetic drugs and insulin to control their blood glucose levels. Hence, more effective drugs with fewer side effects are the need of the hour. As an alternative or as an adjuvant, the use of herbs/natural products, mainly as dietary ingredients to prevent or treat IR in diabetes is advocated.
Nishamalaki, an Ayurvedic herbal formulation comprising two potent herbs viz., Emblica officinalis (Indian gooseberry) and Curcuma longa (turmeric) is a proven anti-diabetic drug [7]. Anti-hyperglycemic and insulin sensitizer effects of C. longa and its principal constituent curcumin are already reported [8]. However, more studies evaluating the effects of curcuminoids on the hyperglycemic state and IR are warranted. The E. officinalis fruit is known to improve the glycemic status and reduce oxidative stress in STZ-induced T2DM rats [9]. An E. officinalis fruit extract has been cited to be effective in preventing high fructose diet-induced IR and atherogenic dyslipidemia in ovariectomized female albino rats [10]. However, no report states its effectiveness in T2DM induced by IR.
Understanding that IR plays a significant role in the pathogenesis of T2DM and knowing Nishamalaki to be anti-diabetic, we contemplated studying whether this effect was due to a lowering of IR. This study, thus, evaluates the IR-lowering effect of Nishamalaki in HFD and STZ-induced T2DM rat model.
2. Materials and methods
2.1. Drugs and chemicals
STZ, curcumin, demethoxycurcumin, bisdemethoxycurcumin, and gallic acid were purchased from Sigma Chemical Co., St Louis, MO, USA. HPLC-grade acetonitrile, acetone, tetrahydrofuran, and sulphuric acid were purchased from Rankem, Avantor, India. Remaining chemicals were purchased from local suppliers and were of analytical grade.
2.2. Plant material
Nishamalaki extract termed as “EmbliQur” comprising of E. officinalis fruit extract and extract of C. longa rhizome in a 2:1 proportion was procured from Pharmanza Herbals Pvt. Ltd., India. It was a standardized extract containing: a) Total curcuminoids - 23.89% (by HPLC) b) Total gallic acid - 5.27% (by HPLC) and c) Total tannins - 25.44% (by Titration).
2.3. Quantification of curcuminoids and gallic acid by HPLC
Quantification of curcuminoids (curcumin, demethoxycurcumin, and bisdemethoxycurcumin) in EmbliQur was carried out on the Shimadzu LC 2030 Prominence-i integrated HPLC system consisting of a quaternary pump, vacuum degasser, auto-sampler, and UV detector. Waters Xbridge BEH C18 column (5 μm, 250 × 4.6 mm) was used for separation and the isocratic mobile phase comprised of tetrahydrofuran and 1 mg/ml citric acid in water (40:60 v/v). The flow rate was maintained at 1 ml/min and the detection was carried out at 420 nm with the column temperature maintained at 27 °C and run time of 40 min. A well-resolved and sharp peak with retention time of 10 min for 20 μl of standard curcuminoid solution (0.4 mg/ml) was obtained. The curcuminoid standard solution comprised of 5 ml curcumin, 1.25 ml of demethoxycurcumin, and 0.25 ml of bisdemethoxycurcumin in 50 ml of methanol. The calibration curve was prepared by injecting various concentrations of the standard solution of curcuminoids (0.1–1 mg/ml). The EmbliQur extract (0.2 mg/ml) was injected and eluted in the same manner. The amount of curcuminoids in EmbliQur was calculated from the calibration curve.
For gallic acid quantification in EmbliQur, the Shimadzu HPLC system was used which consisted of a binary pump (LC-20AT), auto-sampler (SIL-HTc), and a photodiode array detector (SPD-M20A). The analysis was performed on a Phenomenex C18 (5 μm, 4.6 × 250 mm) column using gradient elution with the mobile phase comprising of A (0.1% o-phosphoric acid) and B (acetonitrile) at a flow rate of 1 ml/min at 273 nm as follows: 0.01–11.00 min, 5% B; 11.00–20.0 min, 80% B; 20–21 min, 5% B; 21.0–25.00 min, 5% B stopped at 25.01min. A well-resolved peak with a retention time of 10.5 min for 20 μl of standard gallic acid (0.1 mg/ml) was obtained. The calibration curve was prepared by injecting various concentrations of standard gallic acid (0.05–0.8 mg/ml). The EmbliQur extract (4 mg/ml) was injected and eluted in the same manner. The amount of gallic acid in EmbliQur was calculated from the calibration curve.
2.4. Estimation of tannins
Quantitative estimation of tannins was carried out following the method of AOAC [11]. The accurately weighed EmbliQur powder (0.2 gm) was dissolved in 50 ml distilled water. More distilled water (250 ml) was added to dissolve the powder and the mixture was titrated against 0.02 M KMnO4 solution using 25 ml of indigo sulphonic acid solution as the indicator till a stable golden yellow color developed at the end of the titration. The volume of KMnO4 used to titrate the tannins in EmbliQur was calculated by back-titration against standard oxalic acid. The concentration of tannin was estimated using the factor 1 ml of 0.02M KMnO4 is equivalent to 0.00415 g of tannins.
2.5. Preparation of high-fat diet (HFD)
A mixture of HFD was prepared by grinding standard chow pellets (50%) with lard (38%), milk powder (5%), and butter (7%). Using this HFD mixture, pellets were prepared and fed to the animals.
2.6. Animals
Male Sprague Dawley rats (150–200 g) were procured from Bharat Serum Ltd., India. All animals were housed in clean and disinfected cages. For acclimatization, animals were put under standard conditions of humidity (50 ± 5%), temperature (25 ± 2 °C), and light (12 h light/12 h dark cycle). Animals were fed a standard diet procured from Amrut laboratory animal feed, Pranav Agro Industries, India) and drinking water ad libitum. Animals were handled with humane care. The Institutional Animal Ethics Committee (Animal House Registration no. 25/PO/ReBi/S/99/CPCSEA) had reviewed and approved experimental protocol.
2.7. Preparation of test solutions
EmbliQur and metformin solutions were prepared in distilled water and the aqueous solutions were used immediately for administration. STZ was freshly prepared in 0.1 M citrate buffer (pH 4.5) and administered intraperitoneally.
EmbliQur in 90 days subacute toxicity study (in rats) has shown no adverse effects at a dose of 1000 mg/kg. Hence, on pilot basis, we started with 1/10th of 1000 mg/kg i.e., 100 mg/kg and its two fold 200 mg/kg EmbliQur to evaluate IR-lowering activity. However, at these lower doses, we found poor activity; on the other hand, 500 mg/kg EmbliQur showed good IR-lowering activity and based upon the dose-dependence, we selected 500 mg/kg and 1000 mg/kg to evaluate IR-lowering effect of EmbliQur.
2.8. Induction of diabetes
Diabetes was induced in overnight fasted rats by injecting a single shot of freshly prepared STZ (40 mg/kg) intraperitoneally and feeding HFD regularly. Fasting blood glucose levels (FBGL) of rats were measured on the third day after STZ administration to confirm the induction of diabetes in rats. Rats more than 250 mg/dl of FBGL were considered as diabetic and selected for the experiment.
2.9. Experimental procedure
After 7 days of acclimatization, rats were randomly divided into 7 groups comprising 6 rats each. The treatment for each group was as follows:
Group I (Normal Control) - Received standard chow diet and drinking water daily for 45 days and citrate buffer (1 ml/kg, i.p) on the 15th day of the experiment.
Group II (HFD/STZ) - Received HFD throughout the experiment (45 days) and STZ (40 mg/kg, i.p) on the 15th day of the experiment.
Group III (EmbliQur 500) - Received HFD throughout the experiment (45 days), STZ (40 mg/kg, i.p) on the 15th day of the experiment, and EmbliQur (500 mg/kg,p.o) from the 18th day for the next 28 days.
Group IV (EmbliQur 1000) - Received HFD throughout the experiment (45 days), STZ (40 mg/kg, i.p) on the 15th day of the experiment, and EmbliQur (1000 mg/kg, p.o) from the 18th day for the next 28 days.
Group V (Metformin) - Received HFD throughout the experiment (45 days), STZ (40 mg/kg, i.p) on the 15th day of the experiment, and the reference standard drug metformin (120 mg/kg, p.o) from the 18th day for the next 28 days.
For the initial period of 2 weeks, rats of all groups were provided chow/HFD and water ad libitum. After 2 weeks of diet plan, all HFDfed rats were injected a single low dose of STZ (40 mg/kg, i.p), while the Normal Control rats were given the vehicle citrate buffer (pH 4.4, 1 ml/kg, i.p). The FBGL was measured 3 days after STZ injection. The rats more than 250 mg/dl of FBGL were considered as diabetic and used for the experiment. Their dosing with EmbliQur/metformin commenced and continued for the next 28 days. The diet plan of each groups continued till the end of the study. All FBGL and insulin levels were determined on the 18th day (3rd day after STZ injection and day of commencement of treatment) and then on the 25th, 32nd, 39th, 44th and 45th days (24 h after the previous dose) by collecting blood from the tail vein/retro-orbital plexus. One day prior to the completion of study i.e., on the 44th day, Oral Glucose Tolerance Test (OGTT) was performed. To perform this test, animals of all groups were administered glucose solution (1.5 g/kg) 30 min after their respective treatments. Their blood glucose and insulin levels (as an extension of OGTT) were determined at 0, 30, 60, 90, 120 min after glucose administration. Blood glucose was estimated using a biochemical kit and insulin was assayed by using an RIA kit.
2.10. Calculation of IR
2.10.1. Homeostasis model assessment of insulin resistance (HOMA-IR)
IRHOMA = (I0 × G0)/22.5.
I0 – Fasting plasma insulin concentration (mIU/L).
G0 – Fasting plasma glucose concentration (mg/dL).
2.10.2. Quantitative insulin sensitivity check index (QUICKI)
The QUICKI was determined using the formula:
QUICKI = 1/(logI0 + logG0).
I0 – Fasting plasma insulin concentration (mIU/L).
G0 – Fasting plasma glucose concentration (mg/dL).
2.10.3. Matsuda index
Plasma glucose (mg/dl) and insulin (mIU/l) concentrations in the fasting state and during OGTT were used to calculate Matsuda index.
G0 – Fasting plasma glucose concentration (mg/dL).
I0 – Fasting plasma insulin concentration (mIU/L).
Gmean – Mean plasma glucose concentration during OGTT (mg/dl).
Imean – Mean plasma insulin concentration during OGTT (mU/l).
2.11. Glucose and insulin assays
Glucose colorimetric assay kits manufactured by Erba Mannheim were used for the study. Insulin assay in serum was performed using Radioimmunoassay (By the Board of Radiation and Isotope Technology, Govt. of India, Mumbai.)
2.12. Statistical analysis
The results are expressed as mean ± SEM from 6 animals in each group. One-way ANOVA followed by the Tukey–Kramer multiple comparison test was used to analyse the results. p < 0 05 was considered significant. To perform statistical analysis, GraphPad InStat version 4.00 of GraphPad Software Inc, San Diego, USA was used.
3. Results
3.1. Quantification of curcuminoids, gallic acid and tannins in EmbliQur
The amount of curcuminoids and gallic acid present in EmbliQur was 23.89% w/w and 5.27% w/w respectively by HPLC analysis. The amount of total tannins present in EmbliQur extract by the titration method was 25.44% w/w.
3.2. Effect of EmbliQur and metformin on FBGL
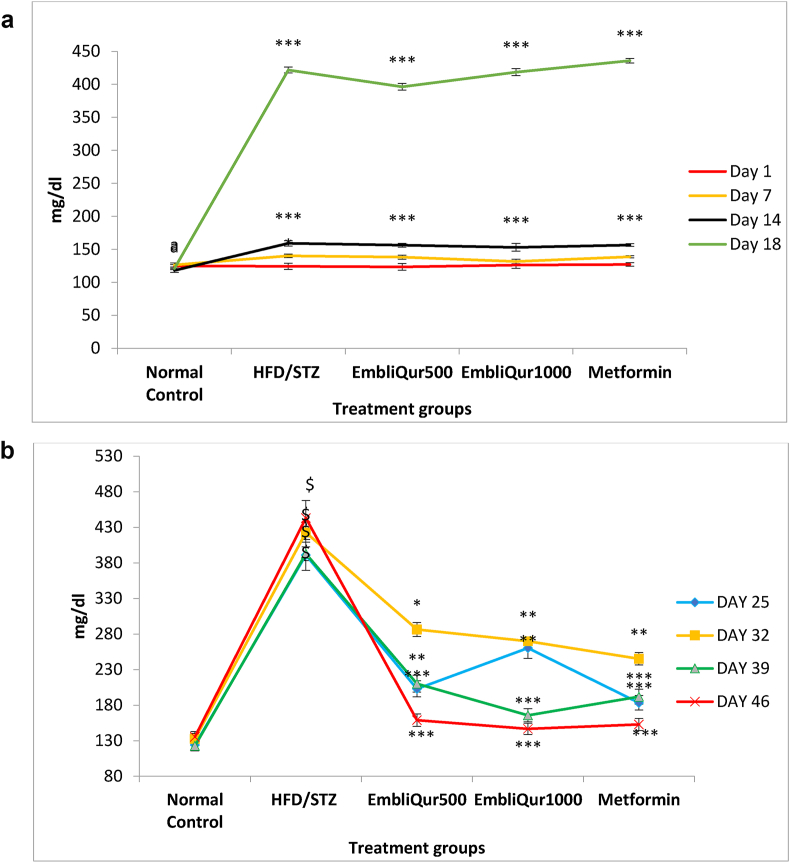
Fig. 1a and b demonstrate the effect of EmbliQur and metformin on BGL from Day-1 to the end of the study (Day 46). Fig. 1a exhibits the FBGL of all animals from Day-1 to Day-14 of HFD administration and on Day-18, 3 days after the STZ treatment. All rats started with the same FBGL and there was no significant difference between HFD fed rats and Normal Control rats till the end of the 1st week. All HFD-fed rats displayed significantly (p < 0.001) higher FBGL than Normal Control rats at the end of the 2nd week and Day-18 of the study. Fig. 1b shows BGL of rats treated with EmbliQur or metformin over 4 weeks (Day-25 through Day-46). HFD/STZ treated rats elicited significantly (p < 0.001) higher FBGL than Normal rats throughout the 4 weeks of the study after start of the treatments. At the end of the 1st week of treatment, on Day-25, all treatments significantly (p < 0.05 for EmbliQur1000 and p < 0.001 for the rest) attenuated the HFD/STZ-elevated FBGL. On Day-32, the FBGL rose in the animals of the HFD/STZ and all 3 treatment groups. However, the treatments could still significantly (p < 0.05 for EmbliQur500 and p < 0.01 for EmbliQur1000 and metformin) attenuate the HFD/STZ-elevated FBGL. The trend continued over the last 2 weeks (Day-39 to Day-46) where all 3 treatments significantly (p < 0.001) attenuated the risen FBGL due to HFD/STZ administration and brought these levels near normal.
Fig. 1.
a) Effect of HFD and HFD/STZ administration on FBGL in rats. All values are expressed as mean ± SEM; N = 6 in each group; One way ANOVA followed by Tukey–Kramer multiple comparison test is applied for statistical analysis; ∗∗∗p < 0.001 when experimental and HFD/STZ groups compared with Normal Control group. (b) Effect of EmbliQur and metformin on FBGL in HFD/STZ-treated rats. All values are expressed as mean ± SEM; N = 6 in each group; One way ANOVA followed by Tukey–Kramer multiple comparison test is applied for statistical analysis; $p < 0.001 when HFD/STZ group compared with Normal Control and ∗p < 0.05, ∗∗p < 0.01 and ∗∗∗p < 0.001 when experimental groups compared with HFD/STZ group.
3.3. Effect of EmbliQur and metformin on serum insulin levels
Fig. 2 shows the effect of EmbliQur and metformin treatment for 4 weeks on serum insulin levels. The HFD/STZ group rats elicited significantly higher than normal serum insulin levels from the 18th day till the end of the experiment. All treatment groups showed insulin levels similar to HFD/STZ at the start of the treatment, which declined gradually over the next 4 weeks. At the end of the study, EmbliQur (500 and 1000 mg/kg) and metformin treatments significantly (p < 0.001) attenuated the HFD/STZ-elevated serum insulin levels.
Fig. 2.
Effect of EmbliQur and metformin on serum insulin in HFD/STZ-treated rats. All values are expressed as mean ± SEM; N = 6 in each group; One way ANOVA followed by Tukey–Kramer multiple comparison test is applied for statistical analysis; $p < 0.001 when HFD/STZ group compared with Normal Control and ∗p < 0.05 and ∗∗∗p < 0.001 when experimental groups compared with HFD/STZ group.
3.4. Effect of EmbliQur and metformin on BGL and insulin levels in the OGTT
The effect of EmbliQur on the OGTT is depicted in Table 1. The HFD/STZ group of rats elicited significantly higher BGL and insulin levels than the Normal Control rats at all time points in the OGTT. At all the time intervals, EmbliQur as well as the metformin treated rats exhibited significantly lower BGL and insulin levels than the HFD + STZ group. At the end of the study EmbliQur1000 exhibited near-normal BGL and insulin levels, followed by metformin and EmbliQur 500 treatment.
Table 1.
Effect of EmbliQur and metformin on OGTT and the Matsuda index in HFD/STZ-treated rats.
| Time | Normal Control | HFD/STZ | EmbliQur 500 | EmbliQur 1000 | Metformin | |
|---|---|---|---|---|---|---|
| 0 Min | Glucose (mg/dl) | 143.67 ± 1.96 | 385.00 ± 2.43$ | 185.00 ± 1.34∗∗∗ | 141.50 ± 1.74∗∗∗ | 129.50 ± 1.17∗∗∗ |
| Insulin (mIU/l) | 14.25 ± 0.58 | 31.37 ± 1.63$ | 22.73 ± 1.14∗∗∗ | 14.13 ± 0.93∗∗∗ | 13.12 ± 0.97∗∗∗ | |
| 30 Min | Glucose (mg/dl) | 227.67 ± 20.15 | 563.00 ± 7.04$ | 336.33 ± 4.31∗∗∗ | 259.50 ± 5.10∗∗∗ | 249.50 ± 3.56∗∗∗ |
| Insulin (mIU/l) | 25.95 ± 0.68 | 45.33 ± 1.18$ | 29.37 ± 1.57∗∗∗ | 28.15 ± 1.13∗∗∗ | 27.31 ± 1.40∗∗∗ | |
| 90 Min | Glucose (mg/dl) | 183.50 ± 2.23 | 379.83 ± 3.86$ | 187.00 ± 2.2∗∗∗ | 168.17 ± 2.16∗∗∗ | 185.17 ± 2.08∗∗∗ |
| Insulin (mIU/l) | 22.43 ± 1.60 | 30.22 ± 1.46# | 22.80 ± 0.19∗∗ | 17.13 ± 0.78∗∗∗ | 22.72 ± 1.49∗∗ | |
| 120 Min | Glucose (mg/dl) | 136.17 ± 2.91 | 358.00 ± 2.39$ | 182.00 ± 4.22∗∗∗ | 137.33 ± 2.59∗∗∗ | 149.83 ± 2.81∗∗∗ |
| Insulin (mIU/l) | 13.85 ± 0.49 | 30.27 ± 0.86$ | 22.05 ± 1.31∗∗∗ | 13.83 ± 1.12∗∗∗ | 14.95 ± 1.04∗∗∗ | |
| Matsuda Index | 4.47 ± 0.15 | 2.64 ± 0.05$ | 5.84 ± 0.08∗∗∗ | 12.34 ± 0.08∗∗∗ | 12.56 ± 0.05∗∗∗ | |
All values are expressed as mean ± SEM; N = 6 in each group; One way ANOVA followed by Tukey–Kramer multiple comparison test is applied for statistical analysis; #p < 0.05 and $p< 0.001when HFD/STZ group compared with Normal Control and ∗∗p < 0.01 and ∗∗∗p < 0.001 when experimental groups compared with HFD/STZ group.
3.5. Effect of EmbliQur and metformin on HOMA-IR, QUICKI, and Matsuda index
The effect of EmbliQur and metformin on IR measurement parameters HOMA-IR and QUICKI is shown in Table 2 and the effect on the Matsuda index is given in Table 1. All treatment groups including the HFD/STZ group started with the same HOMA values on the 1st day of treatment, which were significantly (p < 0.001) elevated when compared with Normal Control values, indicating the development of IR. However, gradually, while all treatment groups significantly attenuated the HFD/STZ-elevated HOMA indices, those of the HFD/STZ group remained significantly higher (p < 0.001) than the Normal Control group as well as the treatment groups. The last day of the study showed the highest HOMA-IR index in the HFD/STZ group and the lowest in the EmbliQur1000 treatment group.
Table 2.
Effect of EmbliQur and metformin on HOMA-IR and QUICKI in HFD/STZ-treated rats.
| Day of study | Index type | Normal Control | HFD + STZ | EmbliQur 500 | EmbliQur 1000 | Metformin |
|---|---|---|---|---|---|---|
| Day 18 of the study and 1st day of drug treatment | HOMA-IR | 3.78 ± 0.32 | 34.16 ± 5.17$ | 33.75 ± 4.06 | 37.86 ± 6.60 | 31.70 ± 4.75 |
| QUICKI | 0.31 ± 0.01 | 0.24 ± 0.01$ | 0.24 ± 0.01 | 0.24 ± 0.01 | 0.24 ± 0.01 | |
| Day 25 of study and 8th day of drug treatment | HOMA-IR | 3.50 ± 0.28 | 31.33 ± 5.62$ | 12.18 ± 1.20∗∗∗ | 17.70 ± 2.05∗ | 9.91 ± 0.69∗∗∗ |
| QUICKI | 0.31 ± 0.0034 | 0.24 ± 0.0054$ | 0.27 ± 0.0033∗∗∗ | 0.26 ± 0.0036∗∗∗ | 0.276 ± 0.0021∗∗∗ | |
| Day 32 of study and 15thday of drug treatment | HOMA-IR | 4.41 ± 0.41 | 35.36 ± 5.08$ | 22.66 ± 4.90 | 19.43 ± 2.46∗ | 15.80 ± 2.76∗∗ |
| QUICKI | 0.31 ± 0.0036 | 0.24 ± 0.0049$ | 0.25 ± 0.0055 | 0.25 ± 0.0033 | 0.26 ± 0.0042∗∗ | |
| Day 39 of the study and 22nd day of drug treatment | HOMA-IR | 3.78 ± 0.17 | 32.18 ± 8.49$ | 12.76 ± 0.45∗ | 6.81 ± 1.12∗∗∗ | 10.56 ± 1.70∗∗ |
| QUICKI | 0.31 ± 0.01 | 0.24 ± 0.01$ | 0.27 ± 0.00∗∗ | 0.29 ± 0.01∗∗∗ | 0.27 ± 0.01∗∗∗ | |
| Day 46 of the study and last day of drug treatment | HOMA-IR | 4.63 ± 0.25 | 38.76 ± 2.20$ | 6.23 ± 0.60∗∗∗ | 5.23 ± 0.38∗∗∗ | 5.81 ± 0.39∗∗∗ |
| QUICKI | 0.30 ± 0.01 | 0.238 ± 0.01$ | 0.29 ± 0.01∗∗∗ | 0.30 ± 0.01∗∗∗ | 0.29 ± 0.01∗∗∗ |
All values are expressed as mean ± SEM; N = 6 in each group; One way ANOVA followed by Tukey–Kramer multiple comparison test is applied for statistical analysis; $p < 0.001when HFD/STZ group compared with Normal Control and ∗∗p < 0.01 and ∗∗∗p < 0.001 when experimental groups compared with HFD/STZ group.
At the start of the administration, the HFD/STZ and the treatment groups displayed similar QUICKI values which were significantly (p < 0.001) lower than the Normal Control values indicating the development of IR. Week after week, the QUICKI values of all treatment groups increased significantly when compared with the HFD/STZ values which remained significantly (p < 0.001) lower than the Normal Control values. At the end of the treatment period, QUICKI values of all treatment groups were comparable to the Normal Control values.
For the Matsuda index, HFD/STZ treatment elicited a significantly (p < 0.001) lower value than Normal Control. All treatments could significantly (p < 0.001) elevate the HFD/STZ-lowered Matsuda index.
4. Discussion
It has been reported that short HFD regimens (2 weeks) tend to barely induce insulin resistance and/or glucose intolerance whereas 5 weeks of high-fat feeding to rats induces a higher body fat percentage and impaired glucose tolerance along with hyperinsulinemia [12]. STZ administration to HFD-fed rats elicited profound hyperglycemia; thus, the HFD/STZ rat model is an ideal one for evaluating IR [13].
In the present study, a significant rise in FBGL was observed in all groups treated with two weeks of HFD compared to the Normal Control group. The rise in FBGL at the end of the 2nd week of HFD feeding was significantly higher than in the first week indicating that HFD-induced hyperglycemia had set in. On the 18th day, i.e., 3 days after administration of STZ, FBGL in all animals treated with STZ/HFD rose tremendously compared to the Normal Control animals and remained elevated through the next 4 weeks of the study. There was a gradual decrease in FBGL over 4 weeks of treatment with metformin or EmbliQur which began on the 18th day of the study. At the end of the study, FBGL of the HFD/STZ group were significantly elevated when compared with the Normal Control group. In contrast, FBGL of EmbliQur and metformin treatment groups were significantly lower than the HFD/STZ group and as good as the Normal Control group. Though not significantly different from each other, EmbliQur1000 was better than metformin and slightly better than the EmbliQur500 treatment. Thus, EmbliQur was seen to elicit a potent hypoglycemic effect.
For insulin levels, the same trend as that for BGL was seen with minor differences in intermediate readings. At the end of the study, insulin levels remained significantly elevated in the HFD/STZ group when compared with the Normal Control group indicating the development of hyperinsulinemia. The treatment groups exhibited significantly lower insulin levels when compared with the HFD/STZ treatment and were comparable to the Normal Control insulin levels. Also, there was no significant difference between EmbliQur 500, EmbliQur1000, and metformin treatments. Thus, EmbliQur exhibited a profound anti-hyperinsulinemic effect.
In the OGTT study, at the time points for glucose determination, insulin concentration was determined too, which was used in the Matsuda index calculation. In the OGTT, both EmbliQur and metformin could suppress the HFD/STZ-induced increase in BGL and insulin at all study intervals, indicating their ability to alleviate diabetes and IR.
IR is a reliable, independent and early predictor of several disorders such as T2DM, the metabolic syndrome, hypertension and cardiovascular disease, renal dysfunction, Alzheimer's disease, and PCOS among others [14]. Hence, an accurate method for efficiently evaluating insulin sensitivity is required. Many methods to measure IR are available, which include the hyperinsulinemic-euglycemic glucose clamp, OGTT, glucose/insulin ratio, insulinogenic index, HOMA and QUICKI, Fasting Insulin Resistance Index, GI product and log (HOMA-IR), etc. [15]. Other more practical markers of IR include the Matsuda, Gutt, Stumvoll, and Avignon indices [15]. In the present study IR was evaluated by two primary (HOMA and QUICKI) markers and one derived marker (Matsuda index) to get a comprehensive view about its alleviation by the EmbliQur extract.
Matthews et al. developed HOMA-IR in 1985. This method is used to quantify IR and β-cell function by assessing fasting glucose and insulin levels [16]. Insulin secretion depends on the response of the pancreatic β-cells to glucose concentrations in blood while, glucose concentrations are regulated by insulin-mediated glucose production in the liver. Thus, the deficient β-cell function will result in feeble glucose-stimulated insulin secretion.
In the formula HOMA-IR = (glucose × insulin)/22.5, the constant of 22.5 is a normalizing factor; i.e., the product of normal fasting plasma insulin of 5 μU/ml, and the normal fasting plasma glucose of 4.5 mmol/l, representative of a “normal” healthy individual. The HOMA-IR is a simple and easily performed method but may have a limitation in insulin-sensitive subjects.
In the present study, the HOMA-IR index was found to be significantly elevated in the HFD/STZ group of animals when compared with Normal Control animals, indicating the development of IR. Over the 4 weeks of EmbliQur/metformin administration IR was noted to be gradually attenuated. At the end of the study, all three treatments were comparable in reducing IR significantly and showed HOMA values comparable with the Normal Control group. EmbliQur 1000 was the nearest to Normal, followed by metformin and EmbliQur 500 treatments. Thus, EmbliQur displayed a strong IR alleviating effect.
QUICKI is a mathematical calculation of FBG and plasma insulin concentrations which provides a consistent and precise insulin sensitivity index (ISI) with a better positive predictive power [17]. QUICKI shows excellent linear correlation with insulin sensitivity, especially in obese and diabetic subjects. Values range between 0.45 in healthy individuals and 0.30 in diabetics. Lower values reflect greater resistance with values below 0.339 indicating IR.
For the QUICKI measure, the HFD/STZ treatment elicited a significantly lower value than the Normal Control indicating the development of IR. All treatment groups elicited values significantly lower than Normal and similar to HFD/STZ treatment at the start of the experiment, indicating the induction of IR. On weekly observation, it was noted that all treatment groups gradually, week-by-week restored the HFD/STZ-depleted QUICKI values indicating alleviation of IR. At the end of the study, all three treatment groups significantly restored to normal the HFD/STZ-depleted QUICKI index, indicating a reversal of IR.
Matsuda index is a novel index of insulin sensitivity developed by Matsuda and Defronzo. It is obtained from the simple calculations of OGTT, that provides a reasonable approximation of whole-body insulin sensitivity [18]. Here, the OGTT ISI (composite) is calculated using both, the data of the entire 3 h OGTT and the first 2 h of the test. The composite whole-body insulin sensitivity index of Matsuda and DeFronzo combines both hepatic and peripheral tissue sensitivity to insulin. It is calculated from plasma glucose (mg/dl) and insulin (mIU/l) concentrations in the fasting state and during OGTT. However, its correlation is very weak in diabetic patients.
A Matsuda value of less than 4.3 indicates the setting in of IR. In the present study, the HFD/STZ group of animals exhibited a value of 2.644 which clearly indicates presence of IR against the Normal Control value of 4.47. All treatment groups elicited significantly higher Matsuda values than the HFD/STZ treatment group indicating mitigation of IR. EmbliQur 1000 was comparable to metformin treatment in reversing IR.
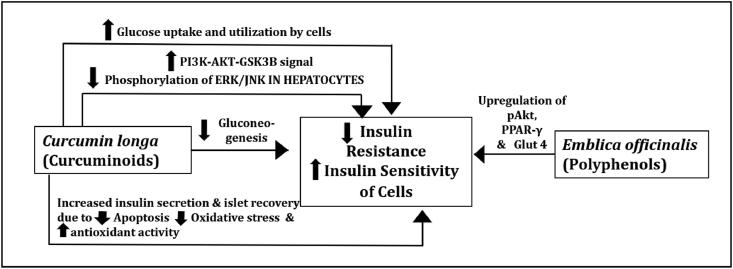
The probable mechanisms of actions involved in alleviation of IR by EmbliQur are depicted in Fig. 3. The curcuminoids of C. longa viz., curcumin, demethoxycurcumin, and bisdemethoxycurcumin have been reported to improve the sensitivity of cells to the metabolic effects of insulin [8]. Metabolites of curcumin viz., hexahydrocurcumin and octahydrocurcumin have shown improved insulin sensitivity by strengthening the PI3K-AKT-GSK3B signal and suppressing the phosphorylation of ERK/JNK in insulin-resistant HepG2 cells in recent studies of Li Pan et al. [19]. Studies of Kanitkar et al. and Balmurugan et al. report treatment of pancreatic islets with curcumin and curcuminoids in vitro to result in increased insulin secretion and islet recovery probably due to reduced apoptosis and oxidative stress to the islets, in conjunction with increased antioxidant enzyme activities by the curcuminoids [20,21]. Moreover, in vitro studies examining the effects of curcumin indicate increased glucose uptake and utilization by skeletal muscle cells and adipocytes, and inhibition of gluconeogenesis [22]. Pancreatic beta cell function may thus be improved by curcumin treatment with consequent insulin secretion, alleviating IR. Hence, curcumin and curcuminoids were quantified in EmbliQur. Gallic acid from E. officinalis is proven to improve insulin sensitivity by up-regulation of pAkt, PPAR-γ and Glut4 [23]. Gallic acid is reported to up-regulate the expression of hepatic insulin signalling proteins, including insulin receptor, insulin receptor substrate 1, phosphatidylinositol-3 kinase, Akt/protein kinase B, and Glut 2 in HFD-fed rats [24]. Gallic acid also down-regulates the expression of hepatic gluconeogenesis-related proteins, such as fructose-1,6-bisphosphatase, and up-regulates expression of hepatic glycogen synthase and glycolysis-related proteins, including hexokinase, phosphofructokinase, and aldolase, in HFD-fed rats [24]. Hence, it was quantified by HPLC in EmbliQur.
Fig. 3.
Probable mechanisms of action of EmbliQur for alleviating insulin resistance.
The combination of E. officinalis and C. longa has been proven to possess potent immunomodulatory activity due to its curcuminoids and polyphenolic contents, especially gallic acid [25]. The immunomodulatory activity of curcuminoids of C. longa is attributed to their interaction with various immunomodulatory cells such as dendritic cells, macrophages, B and T lymphocytes, and from inflammatory mediators such as cytokines and various transcription factors like NF-κB with their signal transduction pathways [26]. This immunomodulatory activity may help in boosting the immune system to protect against viral infections such as COVID-19 when taken regularly through diet or as a prophylactic in the form of a formulation such as EmbliQur.
5. Conclusion
EmbliQur (500 and 1000 mg/kg) successfully attenuated the HFD/STZ-elevated BGL and insulin levels, and ameliorated IR in 3 different models of IR assessment. EmbliQur was comparable to metformin (120 mg/kg) for the above effects. EmbliQur1000 mg/kg was insignificantly better than EmbliQur 500 mg/kg in alleviating IR. The alleviation of IR may also lead to EmbliQur's benefits in other disease conditions associated with IR. It may prove useful for its anti-diabetic and immunomodulatory properties in COVID-19.
Source(s) of funding
This research received funding from Pharmanza Herbals Pvt. Ltd., India.
Conflict of interest
Dr Lal Hingorani and Dr Amol Deshmukh are full time employees of Pharmanza Herbal Pvt Ltd. All other authors declare no competing interests.
Footnotes
Peer review under responsibility of Transdisciplinary University, Bangalore.
References
- 1.Haffner S.M., Valdez R.A., Hazuda H.P., Mitchell B.D., Morales P.A., Stern M.P. Prospective analysis of the insulin-resistance syndrome (syndrome X) Diabetes. 1992;41:715–722. doi: 10.2337/diab.41.6.715. [DOI] [PubMed] [Google Scholar]
- 2.Hussain A., Bhowmik B., do Vale Moreira N.C. 2020. COVID-19 and diabetes: knowledge in progress. Diabetes research and clinical practice; p. 108142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Halpin D.M., Faner R., Sibila O., Badia J.R., Agusti A. Do chronic respiratory diseases or their treatment affect the risk of SARS-CoV-2 infection? The Lancet Respiratory Medicine. 2020;8:436–438. doi: 10.1016/S2213-2600(20)30167-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hussain A., do Vale, Moreira N.C. Clinical considerations for patients with diabetes in times of COVID-19 pandemic. Diabetes Metab Syndr. 2020;14:451. doi: 10.1016/j.dsx.2020.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.World Health Organization . World Health Organization; Geneva: 2016. World health organization global report on diabetes. [Google Scholar]
- 6.Szkudelski T. The mechanism of alloxan and streptozotocin action in B cells of the rat pancreas. Physiol Res. 2001;50:537–546. [PubMed] [Google Scholar]
- 7.Dawane J.S., Pandit V.A., Deshpande S.S., Mandpe A.S. Preventive and protective effect of Nishamalaki in STZ induced diabetic complications in wistar rats. J Clin Diagn Res. 2016;10:FF01. doi: 10.7860/JCDR/2016/17940.7912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ghorbani Z., Hekmatdoost A., Mirmiran P. Anti-hyperglycemic and insulin sensitizer effects of turmeric and its principle constituent curcumin. Int J Endocrinol Metabol. 2014;12 doi: 10.5812/ijem.18081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ansari A., Shahriar M.S., Hassan M.M., Das S.R., Rokeya B., Haque M.A. Emblica officinalis improves glycemic status and oxidative stress in STZ induced type 2 diabetic model rats. Asian Pac J Trop Med. 2014;7:21–25. doi: 10.1016/S1995-7645(13)60185-6. [DOI] [PubMed] [Google Scholar]
- 10.Koshy S.M., Bobby Z., Hariharan A.P., Gopalakrishna S.M. Amla (Emblica officinalis) extract is effective in preventing high fructose diet-induced insulin resistance and atherogenic dyslipidemic profile in ovariectomized female albino rats. Menopause. 2012;19:1146–1155. doi: 10.1097/gme.0b013e31824e5bf7. [DOI] [PubMed] [Google Scholar]
- 11.Khasabnis J., Rai C., Roy A. Determination of tannin content by titrimetric method from different types of tea. J Chem Pharmaceut Res. 2015;7:238–241. [Google Scholar]
- 12.Skovso S. Modeling type 2 diabetes in rats using high fat diet and streptozotocin. J Diabetes Invest. 2014;5:349–358. doi: 10.1111/jdi.12235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Reed M.J., Meszaros K., Entes L.J., Claypool M.D., Pinkett J.G., Gadbois T.M. A new rat model of type 2 diabetes: the fat-fed, streptozotocin-treated rat. Metab Clin Exp. 2000;49:1390–1394. doi: 10.1053/meta.2000.17721. [DOI] [PubMed] [Google Scholar]
- 14.Thomas D.D., Corkey B.E., Istfan N.W., Apovian C.M. Hyperinsulinemia: an early indicator of metabolic dysfunction. JES (J Environ Sci) 2019;3:1727–1747. doi: 10.1210/js.2019-00065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Singh B., Saxena A. Surrogate markers of insulin resistance: a review. World J Diabetes. 2010;1:36–47. doi: 10.4239/wjd.v1.i2.36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Matthews D.R., Hosker J.P., Rudenski A.S., Naylor B.A., Treacher D.F., Turner R.C. Homeostasis model assessment: insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia. 1985;28:412–419. doi: 10.1007/BF00280883. [DOI] [PubMed] [Google Scholar]
- 17.Katz A., Nambi S.S., Mather K., Baron A.D., Follmann D.A., Sullivan G. Quantitative insulin sensitivity check index: a simple, accurate method for assessing insulin sensitivity in humans. J Clin Endocrinol Metab. 2000;85:2402–2410. doi: 10.1210/jcem.85.7.6661. [DOI] [PubMed] [Google Scholar]
- 18.Matsuda M., Defronzo R.A. Insulin sensitivity indices obtained from oral glucose tolerance test: comparison with the euglycemic insulin clamp. Diabetes Care. 1999;22(9):1462–1470. doi: 10.2337/diacare.22.9.1462. [DOI] [PubMed] [Google Scholar]
- 19.Li P., Ding L., Cao S., Feng X., Zhang Q., Chen Y. Curcumin metabolites contribute to the effect of curcumin on ameliorating insulin sensitivity in high-glucose-induced insulin-resistant HepG2 cells. J Ethnopharmacol. 2020 May 25:113015. doi: 10.1016/j.jep.2020.113015. [DOI] [PubMed] [Google Scholar]
- 20.Kanitkar M., Gokhale K., Galande S., Bhonde R.R. Novel role of curcumin in the prevention of cytokine-induced islet death in vitro and diabetogenesis in vivo. Br J Pharmacol. 2008;155:702–713. doi: 10.1038/bjp.2008.311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Balamurugan A.N., Akhov L., Selvaraj G., Pugazhenthi S. Induction of antioxidant enzymes by curcumin and its analogues in human islets: implications in transplantation. Pancreas. 2009;38:454–460. doi: 10.1097/MPA.0b013e318196c3e7. [DOI] [PubMed] [Google Scholar]
- 22.Den Hartogh D.J., Gabriel A., Tsiani E. Antidiabetic properties of curcumin I: evidence from in vitro studies. Nutrients. 2020;12:118. doi: 10.3390/nu12010118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Variya B.C., Bakrania A.K., Patel S.S. Antidiabetic potential of gallic acid from Emblica officinalis: improved glucose transporters and insulin sensitivity through PPAR-γ and Akt signaling. Phytomedicine. 2020 Jul 15;73:152906. doi: 10.1016/j.phymed.2019.152906. [DOI] [PubMed] [Google Scholar]
- 24.Huang D.W., Chang W.C., Wu J.S., Shih R.W., Shen S.C. Gallic acid ameliorates hyperglycemia and improves hepatic carbohydrate metabolism in rats fed a high-fructose diet. Nutr Res. 2016;36:150–160. doi: 10.1016/j.nutres.2015.10.001. [DOI] [PubMed] [Google Scholar]
- 25.Bhandari P.R., Kamdod M.A. Emblica officinalis (Amla): a review of potential therapeutic applications. Int J Green Pharm. 2012;6(4) [Google Scholar]
- 26.Catanzaro M., Corsini E., Rosini M., Racchi M., Lanni C. Immunomodulators inspired by nature: a review on curcumin and echinacea. Molecules. 2018 Nov;23(11):2778. doi: 10.3390/molecules23112778. [DOI] [PMC free article] [PubMed] [Google Scholar]