Abstract
The localization and quantification of endothelial progenitor cells (EPCs) are controversial. Circulating CD34 + cells in blood have been identified as EPCs and as biomarkers of cardiovascular disease. We discuss in this paper the current data describing differential phenotype and behavior in vitro of CD34 positive cells from the circulation and adipose tissue (AT). We also describe in brief our own findings from CD34 + cells isolated from leukopheresis cones derived from healthy platelet donors and from patients undergoing bariatric surgery. We conclude that CD34 + cells in blood and in AT are different in antigenic profile and behavior in culture. The findings described assert that CD34 + cells detected in blood previously identified as biomarkers of cardiovascular disease are predominantly HPCs rather than EPCs, and that true CD34 + EPCs can be readily identified and extracted from AT, supportive of the current evidence which suggests EPCs are resident in the tissue vasculature.
Keywords: Endothelial progenitor cells, Hematopoietic progenitor cells, Adipose tissue, Peripheral blood, Flow cytometry, Cell culture
Endothelial progenitor cells; Hematopoietic progenitor cells; Adipose tissue, Peripheral blood; Flow cytometry; Cell culture.
1. Introduction
The discovery of the endothelial progenitor cell (EPC) was a landmark in cardiovascular and regenerative medicine and is credited to Asahara et al. [1] Since that time several publications have described endothelial progenitor cells detectable in the peripheral circulation which are biomarkers of cardiovascular disease [2, 3, 4, 5, 6, 7], but controversy has existed regarding their exact phenotype [8, 9, 10]. The consensus on the identity of the true EPC has included expression of the CD34 antigen, a hematopoeitic progenitor cell (HPC) antigen which is also expressed on human vascular endothelial cells (HUVECs) and putative endothelial progenitor cells [11, 12, 13, 14, 15].
In recent years, great interest has developed in adipose tissue (AT) as a source of stem and progenitor cells [16], and there is substantial evidence for the stromal vascular fraction (SVF) from AT to yield cells capable of endothelial differentiation, and to promote angiogenesis in a hindlimb ischemia model [17, 18, 19, 20]. These findings are supportive of the hypothesis that adipose tissue may be a source of true endothelial progenitor cells.
In that regard, CD34 + cells can be isolated from adipose tissue as well as from peripheral blood, but these cells have a different phenotype, and different behavior in culture. These findings are consistent with their identity as HPCs and EPCs respectively, and the literature supporting this assertion is reviewed in detail in this paper. We also briefly describe our own experience where CD34 + cells from peripheral blood, and from adipose tissue were isolated by magnetic activated cell sorting (MACS), analyzed by flow cytometry, and cultured in endothelial and hematopoietic stem cell media. Their differential antigenic expression, and behavior in culture are described, and interpreted in the context of the current literature.
2. Literature review
Since the end of the last millennium, the existence of progenitor cells in tissues previously considered virtually devoid of any capacity for self-renewal has become apparent. The close relationship and possible common lineage of bone marrow derived progenitor cells and microvascular endothelial cells in bone marrow.is exemplified by the shared expression of a cell adhesion molecule, CD34 [21]. Further characterization of the expression of antigens on the surface of endothelial cells such as KDR (VEGFR2) and Pecam-1 (CD31) established the concept of a circulating endothelial progenitor cell that is CD34+ and shares the VEGFR2 expression of mature endothelial cells [1, 22]
Adipose tissue has been found to be an abundant source of progenitor cells [18, 20]. Pham et al suggested the existence of a distinct EPC phenotype within adipose tissue [17, 19]. Where the stromal vascular fraction is isolated from adipose tissue and placed in culture, rapid early appearance of endothelial cells has been noted by our group and by others [20, 23, 24, 25].
CD34 + positive cells have been described in adipose tissue. Eto et al described an adipose tissue derived CD34 + population which are CD31-and have a macrophage lineage and mesenchymal differentiation potential [26]. In the presence of VEGF, these cells do have the potential to differentiate into endothelial cells and form tube-like structures [27]. Elegant work by Tratuev et al identified that these CD34 + CD31-cells have a pericyte location and interact with resident endothelial cells in adipose tissue [28]. The paracrine effects of these cells has been described and the CD34 positive subfraction of ADSCs identified from the SVF express and secrete VEGF among other growth factors [29, 30]. It is noteworthy that adipose derived CD34 + cells do not express CD45 [31], consistent with a non-hematopoietic lineage.
As regards CD34 + progenitor cells in the circulation, an impressive body of evidence already exists, in particular identifying their status as a biomarker of cardiovascular disease severity [2, 3, 4, 5, 6]. In these papers, circulating CD34 + cells were described as endothelial progenitor cells (EPCs), but the CD45 expression on these cells was not described. It is possible that the cells that were enumerated by these groups were in fact a combination of EPCs and hematopoetic progenitor cells (HPCs). We demonstrated similar observations to other groups in patients with early and advanced coronary artery disease [32, 33], and a canine model of heart failure [34], but application of stringent criteria regarding CD45 expression identified two distinct groups of CD34 + positive cells - a predominant CD34 + CD45dim VEGFR2-fraction (HPCs) and very rare CD34 + CD45- VEGFR2+ population (EPCs) [32, 34]. Only the HPCs were demonstrated to be altered in number in the setting of cardiovascular disease. Further supportive evidence for these rare cells has demonstrated that a CD45-fraction may exist in the circulation which has endothelial differentiation potential [35]. Indeed, current opinion suggests that both of these CD34 + cell groups may be considered EPCs, hematopoietic and non-hematopoetic [8] although the former require the existence of the CD45-fraction to form endothelial cells in vitro [36]. This is supportive of the likelihood of a paracrine effect from one or more of these cell populations [37, 38].
As such, it can be interpreted from the totality of these data that circulating HPCs have a relationship with cardiovascular disease states. It has been shown that co-culture of endothelial cells with CD34 positive selected cells is superior to culture of endothelial cells in VEGF [39]. Furthermore, a CD11b/CD133 positive subset of CD34 positive cells has been demonstrated to secrete and bind angiopoietins, growth factors which regulate the differentiation of progenitor cells into endothelial cells [40]. This strongly suggests that the role of hematopoeitic stem cells in vascular biology is probably not to differentiate into endothelial cells, but to stimulate the proliferation of existing endothelial cells in the vasculature. True endothelial progenitors may reside within the endothelium itself, and the CD34 + cells isolated from adipose tissue are probably reflective of this population [38, 41, 42].
3. Our experience
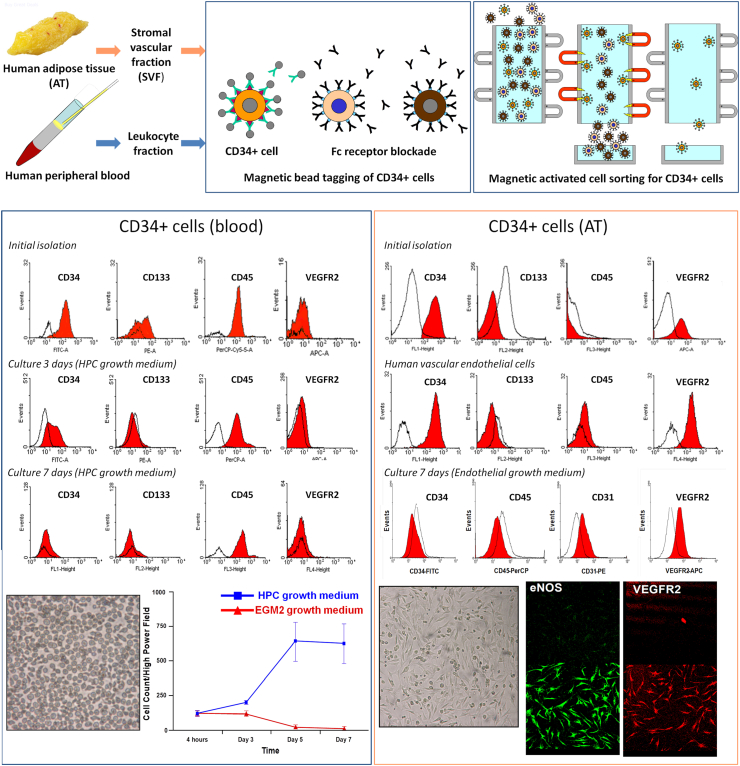
To further explore these concepts, we directly compared the phenotype of blood-derived CD34 + cells with that of adipose-derived cells. Full details of the methods and results are supplied in a supplemental document. Ethical approval was obtained from the Mayo Clinic Institutional Review Board, and informed consent was obtained from all participants. Leukopheresis cones were obtained from three healthy platelet donors, and omental adipose tissue was obtained from three human subjects undergoing bariatric surgery. Our findings were that a pure isolate of CD34 + cells from unmobilized peripheral blood is exclusively CD45 dim-positive. This is in accordance with the definition of HPCs set forth in 1996 by the ISHAGE working group [12]. These cells were also negative for expression of VEGFR2. Culture of these cells in EGM did not demonstrate attachment or formation of spindle-shaped colonies. However, when these cells were cultured in HPC growth medium, they proliferated rapidly and also demonstrated some attachment. Flow cytometric analysis of these cultured cells at 3 and 7 days revealed persistently positive CD45 expression and absent VEGFR2 expression, consistent with a hematopoietic phenotype (Figure 1, bottom left).
Figure 1.
CD34+ cell isolation, culture and flow cytometry from human peripheral blood and adipose tissue. Top panels: Human omental adipose tissue was treated with collagenase solution and the liquid cell suspension phase (stromal vascular fraction/SVF) obtained. Buffy coat was obtained from leukopheresis cones. The cell suspensions were incubated with anti-CD34 monoclonal antibodies tagged with magnetic microbeads and the CD34 + fractions isolated by magnetic activated cell sorting. Left lower panel: CD34 + cells obtained from blood (leukopheresis cones) were analyzed by flow cytometry at baseline, and after 3 and 7 days in culture. Cells did not survive in endothelial growth medium and proliferated in hematopoietic medium only. Flow cytometry demonstrated persistent CD45 expression at isolation and at 3 and 7 days in culture, but negative for VEGFR2 expression throughout those time periods. At 7 days, CD34 expression is absent but CD45 expression persists, consistent with a more mature hematopoietic phenotype. A photomicrograph of these cells is shown (200x). Right lower panel: CD34 + cells obtained from adipose tissue were analyzed by flow cytometry at baseline, and after 7 days in culture. Cells proliferated in endothelial growth medium and when analyzed by flow cytometry were VEGFR2 positive but CD45 negative, similar to human vascular endothelial cells as shown. At 7 days in culture, CD34 + cells isolated from adipose tissue demonstrated persistent VEGFR2 expression and also expression of CD31, but no longer expressed CD34. They remained CD45 negative. A photomicrograph is shown of these AT derived cells in culture (200x magnification), demonstrating a cobblestone appearance of spindle shaped cells after 7 days. Confocal microscopy was also performed on these cells after 7 days in culture, demonstrating eNOS expression and confirming expression of VEGFR2 demonstrated by flow cytometry. Isotype control staining is in the panels immediately above and eNOS and VEGFR2 expression in the panels below.
When we performed magnetic bead extraction of CD34 + cells from adipose tissue, an abundance of CD34 + cells was identified but these were uniformly CD45 negative and positive for VEGFR2, the reverse pattern identified in CD34 + cells characterized from blood. Unlike CD34 + cell isolated from blood, these AT derived CD34 + cells demonstrated rapid attachment and spindle-shaped and cobblestone colony formation in endothelial growth medium. For comparison, human vascular endothelial cells were also analyzed by flow cytometry and demonstrated similar antigenic expression. At 7 days in culture, cells no longer expressed CD34, but were persistently VEGFR2 positive, expressed CD31, and were also positive for eNOS expression on confocal microscopy (Figure 1, bottom right). These cells did not express CD45, consistent with a non-hematopoeitic phenotype, and consistent the findings of others [31]. We concluded therefore that CD34 + cells from adipose tissue were phenotypically different to those that circulate in blood and unlike those from blood, proliferate in endothelial cell culture medium, and demonstrate some features of an endothelial cell phenotype.
4. Summary and conclusions
To conclude and summarize, CD34 + cells exist in both circulating blood and adipose tissue but they have different antigenic expression and behavior in culture. CD34 + cells from peripheral blood express CD45 which is testimony to their predominantly hematopoeitic origin and differentiation potential. Indeed, these data and others demonstrate that the majority do not express VEGFR2, an endothelial surface marker or have endothelial differentiation potential in culture. In contrast, CD34 + cells from adipose tissue express VEGFR2 consistent with an endothelial phenotype, but not the hematopoeitic marker CD45. These cells differentiate into endothelial-like cells in culture. This work, interpreted in the light of other evidence to date, suggests that CD34 + cells with endothelial proliferation potential in culture are predominantly resident in the tissue vasculature and represent the minority of detectable CD34 + cells in circulating blood. HPCs, which circulate in blood and represent the majority of detectable CD34 + cells, do not differentiate into endothelial cells but very likely have an important supporting role.
Declarations
Author contribution statement
All authors listed have significantly contributed to the development and the writing of this article.
Funding statement
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Data availability statement
Data included in article/supplementary material/referenced in article.
Declaration of interests statement
The authors declare no conflict of interest.
Additional information
No additional information is available for this paper.
Appendix A. Supplementary data
The following is the supplementary data related to this article:
References
- 1.Asahara T. Isolation of putative progenitor endothelial cells for angiogenesis. Science. 1997;275(5302):964–967. doi: 10.1126/science.275.5302.964. [DOI] [PubMed] [Google Scholar]
- 2.Friedrich E.B. CD34-/CD133+/VEGFR-2+ endothelial progenitor cell subpopulation with potent vasoregenerative capacities. Circ. Res. 2006;98(3):e20–e25. doi: 10.1161/01.RES.0000205765.28940.93. [DOI] [PubMed] [Google Scholar]
- 3.Heiss C. Impaired progenitor cell activity in age-related endothelial dysfunction. J. Am. Coll. Cardiol. 2005;45(9):1441–1448. doi: 10.1016/j.jacc.2004.12.074. [DOI] [PubMed] [Google Scholar]
- 4.Schmidt-Lucke C. Reduced number of circulating endothelial progenitor cells predicts future cardiovascular events: proof of concept for the clinical importance of endogenous vascular repair. Circulation. 2005;111(22):2981–2987. doi: 10.1161/CIRCULATIONAHA.104.504340. [DOI] [PubMed] [Google Scholar]
- 5.Vasa M. Number and migratory activity of circulating endothelial progenitor cells inversely correlate with risk factors for coronary artery disease. Circ. Res. 2001;89(1):E1–7. doi: 10.1161/hh1301.093953. [DOI] [PubMed] [Google Scholar]
- 6.Werner N. Circulating endothelial progenitor cells and cardiovascular outcomes. N. Engl. J. Med. 2005;353(10):999–1007. doi: 10.1056/NEJMoa043814. [DOI] [PubMed] [Google Scholar]
- 7.Peichev M. Expression of VEGFR-2 and AC133 by circulating human CD34(+) cells identifies a population of functional endothelial precursors. Blood. 2000;95(3):952–958. [PubMed] [Google Scholar]
- 8.Chong M.S., Ng W.K., Chan J.K. Concise Review: endothelial progenitor cells in regenerative medicine: applications and challenges. Stem Cells Transl. Med. 2016;5(4):530–538. doi: 10.5966/sctm.2015-0227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Shantsila E. New insights on endothelial progenitor cell subpopulations and their angiogenic properties. J. Am. Coll. Cardiol. 2008;51(6):669–671. doi: 10.1016/j.jacc.2007.09.057. [DOI] [PubMed] [Google Scholar]
- 10.Yoder M.C. Human endothelial progenitor cells. Cold Spring Harb. Perspect. Med. 2012;2(7) doi: 10.1101/cshperspect.a006692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Duda D.G. A protocol for phenotypic detection and enumeration of circulating endothelial cells and circulating progenitor cells in human blood. Nat. Protoc. 2007;2(4):805–810. doi: 10.1038/nprot.2007.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sutherland D.R. The ISHAGE guidelines for CD34+ cell determination by flow cytometry. International Society of Hematotherapy and Graft Engineering. J. Hematother. 1996;5(3):213–226. doi: 10.1089/scd.1.1996.5.213. [DOI] [PubMed] [Google Scholar]
- 13.Barber C.L., Iruela-Arispe M.L. The ever-elusive endothelial progenitor cell: identities, functions and clinical implications. Pediatr. Res. 2006;59(4 Pt 2):26R–32R. doi: 10.1203/01.pdr.0000203553.46471.18. [DOI] [PubMed] [Google Scholar]
- 14.Siemerink M.J. CD34 marks angiogenic tip cells in human vascular endothelial cell cultures. Angiogenesis. 2014;17(3) doi: 10.1007/s10456-011-9251-z. 755-755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yang J. CD34+ cells represent highly functional endothelial progenitor cells in murine bone marrow. PloS One. 2011;6(5) doi: 10.1371/journal.pone.0020219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fraser J.K. Fat tissue: an underappreciated source of stem cells for biotechnology. Trends Biotechnol. 2006;24(4):150–154. doi: 10.1016/j.tibtech.2006.01.010. [DOI] [PubMed] [Google Scholar]
- 17.Iba T. Isolation of tissue-resident endothelial stem cells and their use in regenerative medicine. Inflamm. Regen. 2019;39:9. doi: 10.1186/s41232-019-0098-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Miranville A. Improvement of postnatal neovascularization by human adipose tissue-derived stem cells. Circulation. 2004;110(3):349–355. doi: 10.1161/01.CIR.0000135466.16823.D0. [DOI] [PubMed] [Google Scholar]
- 19.Pham P.V. Isolation of endothelial progenitor cells from human adipose tissue. Biomed. Res. Ther. 2016;3(5):645–652. [Google Scholar]
- 20.Planat-Benard V. Plasticity of human adipose lineage cells toward endothelial cells: physiological and therapeutic perspectives. Circulation. 2004;109(5):656–663. doi: 10.1161/01.CIR.0000114522.38265.61. [DOI] [PubMed] [Google Scholar]
- 21.Rafii S. Isolation and characterization of human bone marrow microvascular endothelial cells: hematopoietic progenitor cell adhesion. Blood. 1994;84(1):10–19. [PubMed] [Google Scholar]
- 22.Shi Q. Evidence for circulating bone marrow-derived endothelial cells. Blood. 1998;92(2):362–367. [PubMed] [Google Scholar]
- 23.Martinez-Estrada O.M. Human adipose tissue as a source of Flk-1+ cells: new method of differentiation and expansion. Cardiovasc. Res. 2005;65(2):328–333. doi: 10.1016/j.cardiores.2004.11.015. [DOI] [PubMed] [Google Scholar]
- 24.Wosnitza M. Plasticity of human adipose stem cells to perform adipogenic and endothelial differentiation. Differentiation. 2007;75(1):12–23. doi: 10.1111/j.1432-0436.2006.00110.x. [DOI] [PubMed] [Google Scholar]
- 25.Froehlich H. Carotid repair using autologous adipose-derived endothelial cells. Stroke. 2009;40(5):1886–1891. doi: 10.1161/STROKEAHA.108.539932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Eto H. Characterization of human adipose tissue-resident hematopoietic cell populations reveals a novel macrophage subpopulation with CD34 expression and mesenchymal multipotency. Stem Cell. Dev. 2013;22(6):985–997. doi: 10.1089/scd.2012.0442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Forghani A.K., Srinivas, Chen Cong, Leberfinger Ashley, Ravnic Dino, Hayes Daniel. Differentiation of adipose tissue–derived CD34+/CD31− Cells into endothelial cells in vitro regenerative engineering and translational. Medicine. 2020;6:101–110. doi: 10.1007/s40883-019-00093-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Traktuev D.O. A population of multipotent CD34-positive adipose stromal cells share pericyte and mesenchymal surface markers, reside in a periendothelial location, and stabilize endothelial networks. Circ. Res. 2008;102(1):77–85. doi: 10.1161/CIRCRESAHA.107.159475. [DOI] [PubMed] [Google Scholar]
- 29.Kim J.H. Transplantation of immortalized CD34+ and CD34- adipose-derived stem cells improve cardiac function and mitigate systemic pro-inflammatory responses. PloS One. 2016;11(2) doi: 10.1371/journal.pone.0147853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Suga H. Functional implications of CD34 expression in human adipose-derived stem/progenitor cells. Stem Cell. Dev. 2009;18(8):1201–1210. doi: 10.1089/scd.2009.0003. [DOI] [PubMed] [Google Scholar]
- 31.Navarro A. Fibroblast-negative CD34-negative cells from human adipose tissue contain mesodermal precursors for endothelial and mesenchymal cells. Stem Cell. Dev. 2015;24(19):2280–2296. doi: 10.1089/scd.2015.0013. [DOI] [PubMed] [Google Scholar]
- 32.Boilson B.A. Circulating CD34(+) cell subsets in patients with coronary endothelial dysfunction. Nat. Clin. Pract. Cardiovasc. Med. 2008;5(8):489–496. doi: 10.1038/ncpcardio1277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kiernan T.J. Effect of enhanced external counterpulsation on circulating CD34+ progenitor cell subsets. Int. J. Cardiol. 2011;153(2):202–206. doi: 10.1016/j.ijcard.2010.08.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Boilson B.A. Regulation of circulating progenitor cells in left ventricular dysfunction. Circ. Heart Fail. 2010;3(5):635–642. doi: 10.1161/CIRCHEARTFAILURE.109.879437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Case J. Human CD34+AC133+VEGFR-2+ cells are not endothelial progenitor cells but distinct, primitive hematopoietic progenitors. Exp. Hematol. 2007;35(7):1109–1118. doi: 10.1016/j.exphem.2007.04.002. [DOI] [PubMed] [Google Scholar]
- 36.Lee J.H. CD34 hybrid cells promote endothelial colony-forming cell bioactivity and therapeutic potential for ischemic diseases. Arterioscler. Thromb. Vasc. Biol. 2013;33(7):1622–1634. doi: 10.1161/ATVBAHA.112.301052. [DOI] [PubMed] [Google Scholar]
- 37.Heil M. A different outlook on the role of bone marrow stem cells in vascular growth: bone marrow delivers software not hardware. Circ. Res. 2004;94(5):573–574. doi: 10.1161/01.RES.0000124603.46777.EB. [DOI] [PubMed] [Google Scholar]
- 38.Rehman J. Peripheral blood "endothelial progenitor cells" are derived from monocyte/macrophages and secrete angiogenic growth factors. Circulation. 2003;107(8):1164–1169. doi: 10.1161/01.cir.0000058702.69484.a0. [DOI] [PubMed] [Google Scholar]
- 39.Yildirim S. Expansion of cord blood CD34+ hematopoietic progenitor cells in coculture with autologous umbilical vein endothelial cells (HUVEC) is superior to cytokine-supplemented liquid culture. Bone Marrow Transplant. 2005;36(1):71–79. doi: 10.1038/sj.bmt.1705001. [DOI] [PubMed] [Google Scholar]
- 40.Hildbrand P. The role of angiopoietins in the development of endothelial cells from cord blood CD34+ progenitors. Blood. 2004;104(7):2010–2019. doi: 10.1182/blood-2003-12-4219. [DOI] [PubMed] [Google Scholar]
- 41.Ingram D.A. Vessel wall-derived endothelial cells rapidly proliferate because they contain a complete hierarchy of endothelial progenitor cells. Blood. 2005;105(7):2783–2786. doi: 10.1182/blood-2004-08-3057. [DOI] [PubMed] [Google Scholar]
- 42.Zengin E. Vascular wall resident progenitor cells: a source for postnatal vasculogenesis. Development. 2006;133(8):1543–1551. doi: 10.1242/dev.02315. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data included in article/supplementary material/referenced in article.