Abstract
Objectives:
The superficial femoral artery (SFA) can be used as inflow for infra-geniculate bypass, but progressive proximal occlusive disease may affect graft durability. We sought to evaluate the effect of SFA versus common femoral artery inflow on infra-geniculate bypass patency within a large contemporary multicenter registry.
Methods:
The Vascular Quality Initiative was queried from 2013-2019 to identify patients with >30-day patency follow-up, Rutherford chronic limb ischemia stage 1-6, and an infra-geniculate bypass, excluding those with prior ipsilateral bypass. The cohort was stratified by inflow vessel, with primary, primary-assisted and secondary patency serving as the primary outcome variables. Multivariate Cox-proportional hazard models and radius-based propensity-score matching were performed to reduce treatment-selection bias due to clinical covariates.
Results:
A total of 11,190 bypass procedures were performed (8,378 CFA-, 2,812 SFA-inflow) on 10,110 patients, with a mean follow-up of 12.8 months (range 1-98). Patients receiving SFA inflow bypasses were more commonly male (p=0.002), obese (p<0.0001) and had chronic, limb threatening ischemia (p<0.0001), whereas those with CFA inflow were older (p<0.0004), and had higher baseline comorbidities including smoking (p<.0001), coronary disease (p<.0001), and pulmonary disease (p<.0001). On life-table analysis, there was no significant difference in three year estimated primary (32.1 vs 30.1%, p=0.928), primary assisted (60.5 vs 65.8%, p=0.191), or secondary patency (62.5 vs 66.7%, p=0.139) between SFA and CFA inflow groups, respectively. A multivariate Cox model found no significant association between inflow vessel and primary patency (0.96 [0.88-1.04], HR [95%CI]), primary-assisted (1.07 [0.95-1.20], HR [95%CI]), or secondary patency (1.08 [0.96-1.22]). In a propensity-matched cohort (n=11,151), there were small but statistically significant differences in primary, primary assisted, and secondary patency at latest follow-up (non-time-to-event data) between groups. The largest difference was observed when evaluating secondary patency, with CFA inflow having a marginally higher secondary patency of 88.1% compared to 85.6% for those with SFA inflow at latest follow-up (p=0.009).
Conclusions:
Within the VQI, there is no significant difference in life-table determined three-year primary, primary-assisted and secondary patency between infra-geniculate bypasses using CFA inflow compared to SFA inflow. Small, statistically significant differences exist in primary, primary-assisted and secondary patency favoring CFA inflow after propensity score matching. Long-term follow-up data is required in the VQI to better evaluate bypass graft durability as this study was limited by a mean follow-up of one year.
Keywords: infra-geniculate bypass, inflow, graft patency, superficial femoral, common femoral
Introduction
Since the early 1950s, lower extremity bypass has been routinely performed to improve perfusion to lower extremities in the setting of life-disabling claudication or chronic limb-threatening ischemia (CLTI)1,2. Traditionally, the common femoral artery (CFA) has been used as the inflow artery, as this site is easily surgically accessible and generally less often diseased. However, as bypass concepts and techniques became more refined, using a more distal arterial inflow target (superficial femoral (SFA) or above-knee-popliteal) became more common, with several single-center studies showing near equivalent patency compared to CFA inflow infra-inguinal bypasses3-5.
This concept of using the most distal arterial segment of unrestricted flow is commonly practiced, as this results in shorter conduit lengths, maximizing the availability of autologous venous conduit and graft durability. However, it is unclear how future progression of disease proximally may affect long-term patency. Studies have shown that the atherosclerotic plaque burden in the SFA is between two to six times higher than in the CFA6-8. This may affect the long-term durability of grafts originating from the SFA or above-knee popliteal artery, due to late progression of disease which may not have been hemodynamically significant and thus underappreciated at the time of the index bypass.
The Vascular Quality Initiative (VQI), started in 2010, is a nationwide registry with prospectively collected procedure-specific variables and outcomes created in partnership with the Society for Vascular Surgery (SVS)9. This allows the VQI to serve as a robust database for assessing the effect of technical factors related to vascular procedures. The purpose of this study was thus to utilize the VQI registry to investigate differences in bypass patency between CFA versus SFA for infra-geniculate bypass surgery.
Methods
The VQI infra-inguinal bypass registry was queried for procedures performed between January 2013 and January 2018. The initial registry included over 50,000 procedures from more than 300 participating centers, and tabulates over 100 demographic, pre-operative, post-operative and procedure specific variables through 30-days from the index procedure. The sub-registry long-term follow-up module was also examined, which tracks procedure-related long-term follow-up with a focus on bypass patency, mortality, reintervention and medication regimen adherence. Graft patency in the follow-up registry was determined by use of duplex ultrasonography, ABI increase ≥0.15 compared to baseline, presence of a palpable graft or pedal pulse, or bedside doppler exam. Use of the VQI registry is deidentified and deemed exempt from our institutional review board approval process, and patient consent was not required.
Cohort Selection
Procedures in the index procedure and long-term follow-up sub-registries were cross-matched. Only patients with >30 days of follow-up and available patency data were included for analysis. In order to reduce treatment bias, only index bypasses were included, with subsequent revision bypasses performed on the index limb excluded from analysis. We included all patients treated for claudication or CLTI (defined as ischemic rest pain or tissue loss). Bypasses performed for popliteal aneurysms were excluded. Procedures were stratified into two cohorts—bypasses originating from the CFA (CFA group), and those originating from the SFA (SFA group). Bypasses from the proximal popliteal artery were excluded.
Outcome Measures
The primary outcome measures were graft patency during follow-up. Primary, primary-assisted, and secondary graft patency were calculated according to SVS reporting standards. Secondary outcome measures included all-cause mortality and major amputation, defined as an amputation above the level of the ankle.
Statistics
Each patient limb was treated independently and stratified by cohort group. Baseline demographics and procedural details were analyzed as appropriate using t-tests and χ2 tests for continuous and categorical variables, respectively. Kaplan-Meier survival estimates were performed to determine patency curves and amputation-free survival. Univariate and multivariate cox-proportional hazard models were generated to assess predictors of graft patency, reintervention, and amputation-free survival. For multivariate models, covariates were chosen if a difference (p<0.1) was detected between cohort groups or well-known factors which affect graft patency (e.g. diabetes, smoking status, etc). Hazard ratios (HRs) and corresponding 95% confidence intervals (CIs) were reported. To better control for treatment-selection bias, we performed a radius-based propensity score-matched analysis with a caliper of 0.01 using all available input covariates. A P value of ≤.05 was considered statistically significant. The analysis was completed using Stata 14.0 software (StataCorp LP, College Station, TX).
Results
Of the 58,651 procedures within the VQI infrainguinal bypass registry, 11,190 met inclusion criteria for this study. 8,378 (74.9%) bypasses utilized CFA inflow and 2,812 (25.1%) utilized SFA inflow. Mean follow-up was 12.8 months and similar between groups (p=0.214). Relevant patient limb demographics, comorbidities and medication regimens are demonstrated in Table 1. There were significant differences in baseline clinical variables between CFA versus SFA groups. Patients with limbs in the CFA group were slightly older (67.1 vs 66.3 years, p=0.0004), and had a higher prevalence of smoking use (42.2 vs 35.6%, p<0.0001), coronary disease (31.8 vs. 27.6%, p<0.0001), and pulmonary disease (25,8 vs 19.9%, p<0.0001). Patients with limbs in the SFA group were more commonly male (70 vs 66.8%, p=0.002) and on average more obese (40.4 vs 34.8% with BMI ≥ 30, p<0.0001). Procedures in the CFA group were commonly performed for claudication (24.4% vs 20.3%, p<0.0001) with a higher prevalence of a previous ipsilateral inflow procedure (19.8 vs 12.5%, p<0.0001) or infrainguinal endovascular intervention (27.5 vs 24.5%, p=0.002). The CFA group also had a higher use of dual-anti-platelet regimen (25.9 vs 20.6%,p<0.001), whereas the SFA group had higher oral anticoagulation (27.2 vs 25.2, p=0.02) and statin use (30.4 vs 25.2, p<0.0001).
Table 1 -.
Procedure limb demographics, comorbidities and pre-operative medication regimens.
| CFA (n=8,378) |
SFA (n=2,812) |
P value | |
|---|---|---|---|
| Age | 67.1±10.5 | 66.3±12.1 | 0.0004 |
| Male | 5,599 (66.8) | 1,969 (70.0) | 0.002 |
| Caucasian | 6,885 (82.2) | 2,324 (82.6) | 0.575 |
| Follow-Up Months | 12.8±5.5 | 12.8±5.4 | 0.214 |
| Comorbidities | |||
| BMI | 27.2±6.2 | 28.2±6.1 | <0.0001 |
| Obese (BMI ≥30) | 2,913 (34.8) | 1,136 (40.4) | <0.0001 |
| Smoking History | 2,533 (42.2) | 1,000 (35.6) | <0.0001 |
| Diabetes | 4,276 (51.0) | 1,434 (51.0) | 0.969 |
| Coronary artery disease | 2,663 (31.8) | 776 (27.6) | <0.0001 |
| Chronic obstructive pulmonary disorder | 2,162 (25.8) | 559 (19.9) | <0.0001 |
| Congestive heart failure | 175 (2.1) | 55 (1.9) | 0.667 |
| End-stage renal disease | 397 (4.7) | 141 (5.0) | 0.554 |
| Non-ambulatory status | 437 (5.2) | 132 (4.7) | 0.276 |
| Indication | <0.0001 | ||
| Claudication | 2,047 (24.4) | 571 (20.3) | |
| Rest pain | 2,056 (24.6) | 660 (23.5) | |
| Tissue loss | 4,275 (51.0) | 1,581 (56.2) | |
| Prior inflow procedure | 1,658 (19.8) | 350 (12.5) | <0.0001 |
| Prior ipsilateral infrainguinal endovascular intervention | 2,302 (27.5) | 688 (24.5) | 0.002 |
| Medications | |||
| Aspirin | 6,183 (73.8) | 2,002 (71.2) | 0.007 |
| P2Y12 inhibitor | 2,878 (34.4) | 759 (27.0) | <0.0001 |
| Dual anti-platelet | 2,167 (25.9) | 579 (20.6) | <0.0001 |
| Oral anticoagulation | 2,946 (35.2) | 1,057 (37.6) | 0.020 |
| Statin-use | 2,108 (25.2) | 854 (30.4) | <0.0001 |
| Values reported as mean ± standard deviation; or no. (%). | |||
Procedural characteristics are listed in Table 2. Bypasses in the SFA group more commonly utilized a tibial vessel as the distal target (64.6 vs 36.2%, p<0.0001), whereas bypasses in the CFA group had higher use of concurrent endarterectomy (36.2 vs 16.3%,p<0.0001), ipsilateral endovascular intervention (7.8 vs 4.2%, p<.0001), and prosthetic graft (31.9 vs 12.5%, p<0.0001). Procedures in the SFA group had a higher risk of reintervention (12.7 vs 11.0%, p=0.018). There was no significant difference in length of stay between groups.
Table 2 -.
Procedural characteristics and selected outcomes
| CFA (n=8,378) |
SFA (n=2,812) |
P value | |
|---|---|---|---|
| Procedural Characteristics | |||
| Distal Target | <0.0001 | ||
| Below-Knee Popliteal | 4,658 (55.6) | 996 (35.4) | |
| Tibial Vessel | 3,720 (44.4) | 1,816 (64.6) | |
| Concurrent proximal endarterectomy | 3,027 (36.2) | 458 (16.3) | <0.0001 |
| Concurrent ipsilateral infrainguinal endovascular intervention | 651 (7.8) | 118 (4.2) | <0.0001 |
| Use of prosthetic graft | 2,671 (31.9) | 351 (12.5) | <0.0001 |
| Peri-Procedural Outcomes | |||
| Length of stay (days) | 5.8±6.3 | 5.9±5.8 | 0.252 |
| 30-day reintervention | 922 (11.0) | 355 (12.7) | 0.018 |
| Bleeding | 93 (10.1) | 44 (12.4) | 0.237 |
| Thrombosis | 225 (24.5) | 103 (29.3) | 0.087 |
| Infection | 96 (10.5) | 25 (7.1) | 0.068 |
| Unspecified | 508 (55.1) | 183 (51.5) | - |
| Post-op surgical site infection | 273 (3.2) | 90 (3.2) | .878 |
| Latest Follow-Up | |||
| Primary patency | 5,768 (68.9) | 1,963 (69.8) | 0.340 |
| Primary-assisted patency | 6,895 (82.3) | 2,319 (82.5) | 0.839 |
| Secondary patency | 7,242 (86.4) | 2,408 (85.6) | 0.282 |
| All-cause mortality | 1,363 (16.3) | 1,781 (15.9) | 0.082 |
| Major amputation | 675 (8.1) | 220 (7.8) | 0.693 |
| Values reported as mean ± standard deviation; or no. (%). | |||
At latest follow-up, there was no observed difference in primary (68.9 vs 69.8, p=0.340), primary-assisted (82.3 vs 82.5%, p=0.839), or secondary patency (86.4 vs 85.6%, p=0.282) between the CFA and SFA groups, respectively. Further, there was no significant difference in post-operative surgical site infection (p=.878), major amputation (p=0.694) or all-cause mortality (p=0.082) (Table 2).
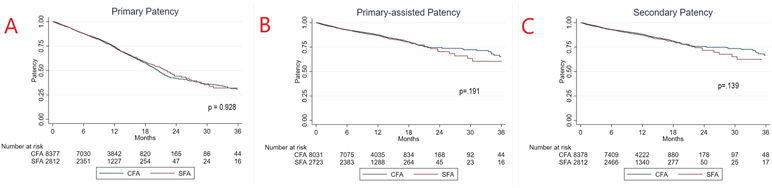
Using Kaplan-Meier methods, estimated primary, primary-assisted, and secondary patency curves were calculated (Figure 1A). There was no significant difference in primary patency between groups (p=0.928), with a three-year primary patency of only 30.8% [25.7-34.6% (95% CI)] and 31.2% [24.3-40.2%] for the CFA and SFA groups, respectively. Procedures with CFA inflow had similar primary-assisted patency from zero to two years but trended toward having a higher patency at three years (65.2% CFA [58.5-71.0] vs 58.5% [49.1-70.0%] SFA) (Figure 1B). This trend was also observed for secondary patency, with the two groups exhibiting similar rates between zero to two years, with a divergence in curves at the three-year interval (66.7% [60.3-72.2%] CFA vs 59.2% [51.8-71.4%] SFA) (Figure 1C). These relationships did not reach statistical significance on log-rank testing (p=0.139-0.191).
Figure 1:
36-month estimated graft patency curves demonstrating (A) primary patency (B) primary assisted patency (C) secondary patency.
On multivariate cox proportional hazard analysis (Table 3), female gender, critical limb ischemia indication and a bypass to a tibial target were consistently associated with primary, primary-assisted, and secondary patency loss (p<.001). A tibial-level bypass was the most significant predictor, with a hazard ratio between 1.30-1.71 for reported patency rates. Use of prosthetic graft was associated with worse primary-assisted (HR 1.68 (1.50-1.87)) and secondary patency (1.62 (1.44-1.81)) but not primary patency (1.03 (0.95-1.11). Older age (HR 0.98-0.99, p<0.001) and obesity (HR 0.88-0.89, p<0.001) were found to be protective against patency loss. Notably, the site of inflow was not associated with either loss of primary, primary-assisted or secondary patency (p=.212-.369).
Table 3 -.
Multivariate predictors of primary, primary-assisted, and secondary patency loss.
| Primary Patency | Primary-Assisted Patency |
Secondary Patency | ||||
|---|---|---|---|---|---|---|
| HR (95% CI) | p value |
HR (95% CI) | p value |
HR (95% CI) | p value | |
| SFA Inflow | 0.96 (0.88-1.04) | .359 | 1.06 (0.94-1.20) | .275 | 1.07 (0.95-1.21) | .212 |
| Age | 0.99 (0.98-0.99) | <.001 | 0.98 (0.98-0.99) | <.001 | 0.99 (0.98-0.99) | <.001 |
| Female | 1.14 (1.06-1.22) | <.001 | 1.26 (1.14-1.41) | <.001 | 1.26 (1.13-1.40) | <.001 |
| Obese | 0.89 (0.83-0.96) | .004 | 0.88 (0.79-0.98) | .027 | 0.88 (0.79-0.98) | .030 |
| Smoking history | 0.96 (0.89-1.03) | .313 | 1.03 (0.92-1.15) | .547 | 1.02 (0.91-1.15) | .629 |
| Diabetes | 1.03 (0.96-1.11) | .300 | 1.09 (0.98-1.22) | .089 | 1.11 (1.00-1.24) | .047 |
| Coronary disease | 1.00 (0.93-1.08) | .931 | 0.97 (0.86-1.08) | .586 | 0.96 (0.86-1.08) | .536 |
| End-stage renal disease | 0.97 (0.83-1.14) | .765 | 0.96 (0.76-1.21) | .740 | 0.96 (0.87-1.21) | .767 |
| COPD | 0.98 (0.94-1.02) | .492 | 0.99 (0.93-1.06) | .832 | 1.00 (0.93-1.07) | .933 |
| Prior inflow procedure | 1.06 (0.97-1.15) | .190 | 1.06 (0.92-1.21) | .396 | 1.04 (0.91-1.19) | .511 |
| Prior endovascular intervention | 1.19 (1.11-1.29) | <.001 | 1.08 (0.96-1.21) | .207 | 1.04 (0.93-1.17) | .442 |
| Critical limb ischemia indication | 1.21 (1.11-1.32) | <.001 | 1.28 (1.12-1.47) | <.001 | 1.26 (1.10-1.45) | .001 |
| Tibial distal target | 1.30 (1.21-1.40) | <.001 | 1.71 (1.53-1.90) | <.001 | 1.70 (1.52-1.89) | <.001 |
| Concurrent proximal endarterectomy | 1.01 (0.97-1.24) | .675 | 0.94 (0.84-1.06) | .320 | 0.94 (0.84-1.06) | .370 |
| Concurrent infrainguinal endovascular intervention | 1.10 (0.95-1.11) | .126 | 1.06 (0.87-1.30) | .515 | 1.06 (1.45-1.81) | .535 |
| Use of prosthetic graft | 1.03 (0.95-1.11) | .446 | 1.68 (1.50-1.87) | <.001 | 1.62 (1.44-1.81) | <.001 |
| DAPT | 0.98 (0.90-1.06) | .666 | 0.96 (0.85-1.09) | .544 | 0.96 (0.85-1.09) | .556 |
| Oral anti-coagulation | 1.01 (0.94-1.09) | .694 | 1.07 (1.02-1.14) | .465 | 1.12 (1.04-1.19) | .678 |
| Statin use | 0.97 (0.90-1.05) | .501 | 1.04 (0.92-1.16) | .531 | 1.03 (0.92-1.16) | .556 |
A radius-based propensity score matching algorithm was employed and incorporated 10,878 procedures, with 289 procedures discarded due to lack of support after score matching was performed. Matched covariates are displayed in Table 4. Percent bias and t-testing revealed no significant differences between covariate inputs between inflow groups. Table 5 demonstrates primary and secondary outcomes at latest follow-up after matching for propensity scores. There were small but statistically significant differences in primary, primary assisted, and secondary patency between groups, favoring CFA. The largest difference was observed when evaluating secondary patency, with the CFA group having a higher secondary patency of 88.1% compared to 85.6% in the SFA group at latest follow-up (p=0.009). Patients undergoing bypass with CFA inflow also had higher overall mortality (16.7% vs 14.9%, p=0.010) and marginally higher major amputation (7.9% vs 7.6%, p=0.007) compared to SFA inflow.
Table 4 -.
Propensity-score matched covariates using radius-based matching
| CFA (n=8,358) |
SFA (n=2,520) |
%Bias | P value | |
|---|---|---|---|---|
| Age | 66.711 | 66.758 | −0.4 | 0.884 |
| Female Gender | 29.8% | 30.7% | −2.1 | 0.460 |
| Caucasian | 82.0% | 82.6% | −1.6 | 0.573 |
| Comorbidities | ||||
| Obese (BMI ≥30) | 38.3% | 38.5% | −0.4 | 0.874 |
| Smoking History | 37.6% | 37.4% | 0.4 | 0.877 |
| Diabetes | 51.0% | 51.4% | −0.8 | 0.771 |
| Coronary artery disease | 28.8% | 28.7% | 0.4 | 0.878 |
| Chronic obstructive pulmonary disorder | 35.1% | 35.5% | −0.5 | 0.864 |
| Congestive heart failure | 2.2% | 2.0% | 1.2 | 0.677 |
| End-stage renal disease | 5.2% | 4.7% | 2.5 | 0.373 |
| Non-ambulatory status | 95.3% | 95.1% | 1.2 | 0.668 |
| Pre-operative limb characteristics | ||||
| Critical limb Ischemia indication | 79.4% | 79.2% | 0.5 | 0.847 |
| Prior inflow procedure | 13.5% | 13.5% | 0.1 | 0.960 |
| Prior infrainguinal endovascular intervention | 24.6% | 25.1% | −1.3 | 0.644 |
| Medications | ||||
| Dual anti-platelet | 21.1% | 21.2% | −0.4 | 0.896 |
| Oral anticoagulation | 37.2% | 37.4% | −0.4 | 0.694 |
| Statin-use | 28.1% | 28.6% | −1.1 | 0.914 |
| Procedural Characteristics | ||||
| Tibial Target | 61.8% | 61.8% | 0.3 | 0.914 |
| Concurrent proximal endarterectomy | 17.7% | 17.8% | −0.3 | 0.919 |
| Concurrent infrainguinal endovascular intervention |
5.1% | 4.3% | 3.3 | 0.193 |
| Use of prosthetic graft | 13.8% | 13.7% | 0.3 | 0.896 |
Table 5 -.
Propensity-score matched patency outcomes between CFA versus SFA inflow groups
| Outcome at latest follow-up | CFA (n=8,358) |
SFA (n=2,520) |
% difference |
P value |
|---|---|---|---|---|
| Primary patency | 70.20% | 68.89% | 1.31% | 0.013 |
| Primary-assisted patency | 82.60% | 82.31% | 0.03% | 0.010 |
| Secondary patency | 88.14% | 85.56% | 2.58% | 0.009 |
| All-cause mortality | 16.7% | 14.9% | 1.70% | 0.010 |
| Major amputation | 7.92% | 7.65% | 0.26% | 0.007 |
Discussion
This study sought to answer the question of whether utilizing the SFA as the inflow artery for infra-geniculate bypass sacrifices graft durability compared to using the CFA. In this study of a contemporary multicenter registry of over 10,000 infra-geniculate bypasses, we found no significant differences in bypass patency on crude analysis, and a non-significant trend toward lower estimated primary-assisted and secondary patency in the SFA group at three years on life table analysis. Multivariate testing on time-to-event data was unable to detect an independent association between inflow source and patency outcomes after controlling for other clinical covariates. However, propensity-score matching was able to detect statistically significant, though likely clinically inconsequential, differences in primary, primary-assisted, and secondary patency, with the SFA group exhibiting lower patency (0.03-2.58%) at all measured endpoints.
The CFA is considered the traditional site of proximal anastomosis for infra-inguinal bypasses, as the SFA is well known to exhibit the highest severity of stenosis and subsequent occlusion for patients with peripheral vascular disease6-8. The first known study of the segmental distribution of lower extremity atherosclerotic disease was conducted in 1962 by Singer et al, who in a series of over 200 patient limbs demonstrated that the common femoral artery had the highest freedom from disease (68% normal) with the SFA suffering a higher burden of disease (8-34% normal through its length)6. A subsequent study in over 800 limbs with angiographically proven PAD found that disease was 6.25 times more prevalent in the SFA compared to the CFA8. Walsh et al further found that most progressing stenotic lesions (93%) in the SFA actually arose in areas of initially mild disease (<50% stenosis) despite more severe initial lesions elsewhere10
Despite this, several single-center studies have demonstrated the utility and durability of bypasses originating distal to the CFA3-5,11,12. Veith et al in 1981 were the first to describe a larger series of infra-popliteal bypasses with varying in-flow sites. They found that bypasses originating from the CFA (n=129) had a life-table patency rate of 50% at 5 years compared to 58% for those originating in the SFA (n=79) (p>0.25). Only 1 of 32 bypass failures were attributed to progressive proximal disease, lending overall support for bypasses originating distal to the CFA. Other smaller single center studies have reported similar findings, with infra-geniculate bypasses originating from the SFA or popliteal artery exhibiting a 5-year estimated patency between 41-61%11,12. To date, only one randomized prospective clinical trial conducted by Ballotta et al. has been performed to specifically evaluate CFA versus more distal arterial sites for inflow in below-knee bypasses5. In a cohort of 160 patients equally randomized to CFA versus SFA or popliteal artery for in-flow, they found a trend toward improved primary patency at 5 years in the SFA/popliteal group (57% vs 73%, p=0.08) with no difference in primary assisted patency (78% vs 71%, p=0.45). However, they noted that the crude rates of graft failure or graft revisions were 2-3 times more likely in the bypasses utilizing CFA inflow, concluding that more distal arterial inflow may be preferred even when there is sufficient saphenous vein conduit available to reach the CFA. Another clinical indication for using more distal inflow may be the benefit of reduced post-operative wound complications, particularly in obese patients. However, we did not find any differences in surgical site infections between groups.
By comparison, we found that SFA inflow trended toward lower primary-assisted patency (58.5% vs 65.2%, p=0.191) and secondary patency (59.2% vs 66.7%, p=0.139) at 3 years on life-table analysis. However, these results were not statistically significant. Interestingly, primary-assisted and secondary patency curves appeared identical between zero to two years of follow-up, with divergence of the curves after two years. Statistical significance after this point was significantly limited by lack of samples within groups. However, this does raise the question of whether there is a sustained difference in patency at mid and late term follow-up and is an impetus for future study once longer term data is available within the VQI.
Although we did not observe a statistically significant difference in outcomes at latest follow-up between unmatched groups, we were able to detect small but statistically significant differences in primary (1.31%), primary-assisted (0.03%), and secondary patency (2.58%) which favored CFA inflow bypasses with radius-based propensity score weighted sample matching. Propensity-score matching in this study was able to further reduce treatment-selection bias, given the wide discrepancy in baseline comorbidities and limb characteristics in the raw cohorts. Nonetheless, these observed differences are small and likely clinically inconsequential, especially given that this statistical method cannot be applied to time-to-event data and thus are not applicable to traditionally understood patency rates in the literature.
Regarding overall risk factors for patency loss, we found that bypasses involving limbs with critical limb ischemia, tibial distal targets use of prosthetic grafts and female gender were associated with higher rates of graft failure, which is similar to existing literature13-15. We also found that obesity and advanced age were independently associated with less risk of patency loss, factors are not typically associated with graft protection. Focused studies on the effect of age and body habitus are thus avenues for future research within the Vascular Quality Initiative.
Multiple limitations exist with this analysis and should be taken into consideration when interpreting our results. First, there is a significant bias toward patients with shorter term follow-up, as reflected in our mean follow-up of only 12.8 months. Low amount of mid and long-term follow-up within the VQI registry is a common issue and limits the ability for analysis of three- to five-year patency rates, which is important when evaluating bypass graft durability. There was also significant treatment-selection bias between inflow groups, as noted by differences in the majority of tabulated clinical covariates. Propensity score matching and multivariate cox modelling reduces, but does not completely eliminate, this bias. For example, observed differences in propensity-matched patency at latest follow-up may be heavily influenced by differences in popliteal versus more distal targets which may not be completely accounted for in the matching algorithm. Finally, due to the VQI being a centrally managed multicenter retrospective registry, numerous assumptions exist regarding the collection and reporting of data. Specific to this study is the assumption that all patients receiving a bypass with SFA inflow did not have pre-existing treatable proximal disease. The quality of CFA inflow (e.g. extent of mild disease, presence of prior prosthetic material) and the exact location of the inflow vessel (e.g. proximal vs distal SFA) could not be ascertained within the limits of the provided data. Graft patency within the VQI is determined with multiple different modalities (duplex ultrasound, ABIs alone, clinical exam alone) and are thus not uniform, lending a certain amount of uncertainty for patency data. In addition, there is a lack of granularity in specific data variables which precludes more thorough analysis, including the lack of information regarding the specific type of previous inflow procedures (bypass vs stent) as well as the type and location of concurrent peripheral endovascular interventions or endarterectomies, and type or location of bypass re-intervention. Critically important to this study is the inability to conduct more detailed analysis regarding the quality of the conduit utilized (e.g. vein diameter) and quality of the outflow vessel. Review of pre-operative angiographic data is not possible within the limitations of the VQI, and as such are better suited toward smaller single center studies.
Conclusions
Within the VQI, there is no significant difference in life-table determined three-year primary, primary-assisted and secondary patency between infra-geniculate bypasses using CFA inflow versus SFA inflow. Small, statistically significant differences exist in primary, primary-assisted and secondary patency favoring CFA inflow after propensity score matching. Long-term follow-up data is required in the VQI to better evaluate bypass graft durability as this study was limited by a mean follow-up of one year.
References
- 1.Menzoian JO, Koshar AL, Rodrigues N. Alexis Carrel, Rene Leriche, Jean Kunlin, and the history of bypass surgery. J Vasc Surg. 2011;54(2):571–574. doi: 10.1016/j.jvs.2011.04.028 [DOI] [PubMed] [Google Scholar]
- 2.Conte MS, Bradbur6y AW, Kolh P, White JV., Dick F, Fitridge R, et al. Global Vascular Guidelines on the Management of Chronic Limb-Threatening Ischemia. Eur J Vasc Endovasc Surg. 2019. doi: 10.1016/j.ejvs.2019.05.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Veith FJ, Gupta SK, Samson RH, Flores SW, Janko G, Scher LA. Superficial femoral and popliteal arteries as inflow sites for distal bypasses. Surgery. 1981. doi: 10.5555/uri:pii:0039606081903949 [DOI] [PubMed] [Google Scholar]
- 4.Wengerter KR, Yang PM, Veith FJ, Gupta SK, Panetta TF. A twelve-year experience with the popliteal-to-distal artery bypass: The significance and management of proximal disease. J Vasc Surg. 1992. doi: 10.1016/0741-5214(92)70022-D [DOI] [PubMed] [Google Scholar]
- 5.Ballotta E, Renon L, De Rossi A, Barbon B, Terranova O, Da Giau G. Prospective randomized study on reversed saphenous vein infrapopliteal bypass to treat limb-threatening ischemia: Common femoral artery versus superficial femoral or popliteal and tibial arteries as inflow. J Vasc Surg. 2004;40(4):732–740. doi: 10.1016/j.jvs.2004.07.023 [DOI] [PubMed] [Google Scholar]
- 6.Singer A Segmental Distribution of Peripheral Atherosclerosis. J Am Med Assoc. 1963;87(3):384–390. [DOI] [PubMed] [Google Scholar]
- 7.Ozkan U, Oguzkurt L, Tercan F. Atherosclerotic Risk Factors and Segmental Distribution in Symptomatic Peripheral Artery Disease. J Vasc Interv Radiol. 2009;20(4):437–441. doi: 10.1016/j.jvir.2009.01.010 [DOI] [PubMed] [Google Scholar]
- 8.Morris-Stiff G, Ogunbiyi S, Rees J, Davies CJ, Hicks E, Lewis MH. Variations in the anatomical distribution of peripheral vascular disease according to gender. Ann R Coll Surg Engl. 2011;93(4):306–309. doi: 10.1308/003588411X571999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cronenwett JL, Kraiss LW, Cambria RP. The society for vascular surgery vascular quality initiative. J Vasc Surg. 2012. doi: 10.1016/j.jvs.2012.03.016 [DOI] [PubMed] [Google Scholar]
- 10.Walsh DB, Powell RJ, Stukel TA, Henderson EL, Cronenwett JL. Superficial femoral artery stenoses: Characteristics of progressing lesions. J Vasc Surg. 1997;25(3):512–521. doi: 10.1016/S0741-5214(97)70262-3 [DOI] [PubMed] [Google Scholar]
- 11.Rosenbloom MS, Walsh JJ, Schuler JJ, Meyer JP, Schwarcz TH, Eldrup-Jorgensen J, et al. Long-term results of infragenicular bypasses with autogenous vein originating from the distal superficial femoral and popliteal arteries. J Vasc Surg. 1988;7(5):691–696. doi: 10.1067/mva.1988.avs0070691 [DOI] [PubMed] [Google Scholar]
- 12.Reed AB, Conte MS, Belkin M, Mannick JA, Whittemore AD, Donaldson MC. Usefulness of autogenous bypass grafts originating distal to the groin. J Vasc Surg. 2002;35(1):48–54; discussion, 54-55. doi: 10.1067/mva.2002.120380 [DOI] [PubMed] [Google Scholar]
- 13.Arhuidese I, Kernodle A, Nejim B, Locham S, Hicks C, Malas MB. Sex-based outcomes of lower extremity bypass surgery in hemodialysis patients. J Vasc Surg. 2018;68(1):153–160. doi: 10.1016/j.jvs.2017.10.063 [DOI] [PubMed] [Google Scholar]
- 14.Siracuse JJ, Huang ZS, Gill HL, Parrack I, Schneider DB, Connolly PH, et al. Defining risks and predicting adverse events after lower extremity bypass for critical limb ischemia. Vasc Health Risk Manag. 2014;10:367–374. doi: 10.2147/VHRM.S54350 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Egorova N, Vouyouka AG, Quin J, Guillerme S, Moskowitz A, Marin M, et al. Analysis of gender-related differences in lower extremity peripheral arterial disease. J Vasc Surg. 2010. doi: 10.1016/j.jvs.2009.09.006 [DOI] [PubMed] [Google Scholar]