Abstract
Sepsis is a deadly condition lacking a specific treatment despite decades of research. This has prompted the exploration of new approaches, with extracellular vesicles (EVs) emerging as a focal area. EVs are nanosized, cell-derived particles that transport bioactive components (i.e., proteins, DNA, and RNA) between cells, enabling both normal physiological functions and disease progression depending on context. In particular, EVs have been identified as critical mediators of sepsis pathophysiology. However, EVs are also thought to constitute the biologically active component of cell-based therapies and have demonstrated anti-inflammatory, anti-apoptotic, and immunomodulatory effects in sepsis models. The dual nature of EVs in sepsis is explored here, discussing their endogenous roles and highlighting their therapeutic properties and potential. Related to the latter component, prior studies involving EVs from mesenchymal stem/stromal cells (MSCs) and other sources are discussed and emerging producer cells that could play important roles in future EV-based sepsis therapies are identified. Further, how methodologies could impact therapeutic development toward sepsis treatment to enhance and control EV potency is described.
Keywords: cell priming, cell therapy, exosomes, mesenchymal stem/stromal cells, stem cells
Graphical Abstract
Extracellular vesicles (EVs) have emerged as a promising therapeutic platform in a variety of chronic and deadly disorders. However, EVs remain a relatively unexploited remedy for the complex disease known as sepsis. This review provides an in-depth look at the current exploration of EVs in sepsis and the unique possibilities EVs provide in the quest for an effective sepsis treatment.
1. Introduction
Sepsis is a dysregulated host immune response to infection that is distinguished by a heterogeneous and complex pathophysiology.[1] At point of onset, sepsis is characterized by an unstable cascade involving the excessive activation of inflammatory mediators that can prompt widespread microvascular dysfunction and systemic inflammation.[2] The resulting overcompensation by the immune system can develop into an immunosuppressant state, during which inflammation is reduced but persistent, and the patient becomes susceptible to recurrent and nosocomial secondary infections.[3] Immune dysregulation can ultimately lead to multiple-organ failure, the major cause of death in sepsis patients.[2,4] At this time, there are no drugs specifically approved for the treatment of sepsis, and while widespread adoption of sepsis management protocols including administration of antibiotics, fluid resuscitation, and hemodynamic support has significantly improved outcomes, patient recovery remains inconsistent.[5] As a result, sepsis is the leading cause of death of hospitalized patients, and with an estimated 48.9 million cases of sepsis worldwide and 11 million sepsis-related deaths reported in 2017, it persists as a major global health problem.[6] These discouraging statistics have driven numerous efforts to develop therapeutic strategies, but the failures of several clinical trials in the past decades have led for some to call for new directions in sepsis treatment.[7]
One such new direction has been the exploration of cell-based therapeutics. Cell transplantation strategies have shown potential as possible therapies for various disorders from cancer to autoimmune diseases.[8] In sepsis, cell-based research has focused mainly on mesenchymal stromal cells (MSCs);[9–12] along with other cell types including embryonic stem cells (ESCs), epithelial progenitor cells (EpPCs), and induced pluripotent stem cells (iPSCs).[13] The strong interest in MSCs as a sepsis treatment derives from seeming parallels between MSC native function and features of sepsis. While the specific mechanisms that drive sepsis are highly complex and incompletely understood, both systemic inflammation and microvascular dysfunction are common features that contribute to organ failure;[14] meanwhile, MSCs possess multifaceted features derived from their innate anti-inflammatory and endothelial-stimulatory properties, as well as their ability to repair host cell damage and initiate antimicrobial responses.[11] Indeed, MSC therapies have been shown to be effective in preclinical rodent models of sepsis.[15–19] Unfortunately, MSC-based therapies face various challenges to clinical translation that have yet to be resolved, including interdonor and intradonor cell heterogeneity, short survival times after administration, and risk of tumorigenesis, as well as several other considerations.[20,21]
On the other hand, it is now widely recognized that MSCs and many other cells exert much of their biological function through trophic mechanisms, especially the secretion of lipid-delimited nanoparticles known as extracellular vesicles (EVs).[22,23] Thus, the utilization of EVs as an alternative therapeutic to cells has become an intriguing new strategy. The term EV encompasses different subpopulations of vesicles that can be characterized on the basis of their size, biochemical composition, and/or the cell of origin.[24] These subpopulations, including exosomes, microvesicles, and others, can arise from either the outward budding of the plasma membrane or from the endosomal system as intraluminal vesicles that are secreted after the fusion of the multivesicular body with the cell membrane.[24] Overall, EVs constitute an endogenous system for paracrine and endocrine intercellular communication via cell-to-cell transfer of their bioactive cargo (i.e., DNA, RNA, lipids, and proteins) and/or the activation of target cell surface receptors.[25–27] The discovery that EVs are capable of transferring functional RNAs to recipient cells spurred an increase in EV-based research that has since implicated EVs not only as diagnostically and prognostically informative biomarkers,[28,29] but also as potential therapeutics and drug delivery vehicles in a multitude of chronic and deadly diseases, including autoimmune and cardiovascular diseases, cancer, and sepsis.[30–34]
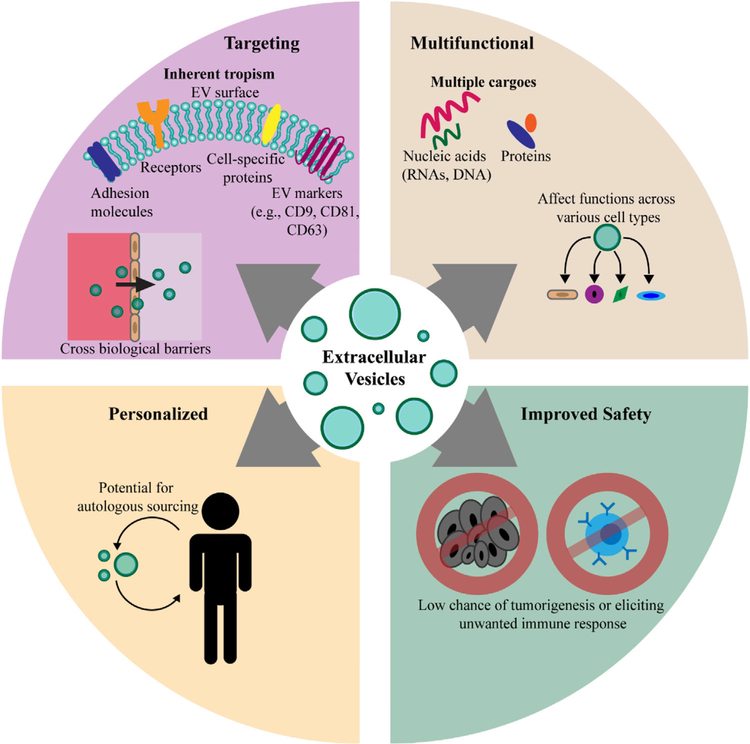
The potential of an EV therapeutic platform in sepsis partially arises from the fact that EVs mirror the composition and metabolic status of their source cells. Cells can selectively load EVs with a variety of bioactive molecules and dictate EV surface characteristics (e.g., tetraspanins and lipids), allowing EVs to behave as an extension of the original cell, targeting and modifying near or distant tissues.[35–37] Moreover, EVs are reported to cross biological barriers and efficiently traverse various tissue microenvironments.[38–40] These attributes make EVs a particularly attractive therapeutic candidate for a systemic disease like sepsis that requires a treatment with systemic effects. In terms of translational therapeutic potential, EVs possess several potential advantages compared with traditional cell therapies, including increased safety (EVs do not divide or differentiate after administration) and possibly reduced immunogenicity (studies indicate EVs can be administered across species and may not require donor-matching) (Figure 1).[41,42]
Figure 1.
Schematic representation of the reported advantages of utilizing EVs as a clinical therapy.
In this review, we first discuss the multifunctionality of EVs and its pertinence to sepsis. We then examine the endogenous roles of EVs in the progression of sepsis and subsequently detail prior studies of therapeutic EVs in preclinical sepsis models. Lastly, we discuss future opportunities for EV-based sepsis therapy, including the ongoing advancement of EV production and engineering techniques.
2. Multifunctionality of EVs
In sepsis, many RNAs and proteins have been implicated as crucial regulatory mechanisms in the pathogenesis of the disease. For example, Chen et al. showed that long noncoding RNA HOX transcript antisense RNA (HOTAIR) was significantly upregulated in the spleens of mice with experimentally induced sepsis when compared with healthy controls. HOTAIR was reported to bind miR-211, consequently up-regulating the expression of interleukin-6 receptor (IL-6R) and promoting apoptosis in monocytes.[43] Splenocyte apoptosis in murine sepsis was also shown to be correlated with the downregulation of the Toll/interleukin-1 (T1R) adaptor protein known as sterile α and HEAT/armadillo motif-containing protein (SARM).[44] In fact, the Toll-like receptors (TLRs), a family of transmembrane proteins crucial to immune signaling, as well as their adaptor proteins and antagonists, have been targeted as a therapeutic approach in the management of sepsis.[45] Moreover, noncoding RNA transcripts known as microRNAs (miRNAs), molecules capable of epigenetic reprogramming of cells under normal and pathological conditions,[46] have been associated with sepsis.[47] miRNAs are capable of post-transcriptional gene regulation via mRNA degradation and/or the inhibition of translation. Specifically, miR-223, miR-146a, and miR-150, have been identified as promising biomarkers and critical in regulating the TLR-nuclear factor kappa-light-chain-enhancer of activated B cells (TLR-NF-κB) pathway, which performs a central role in modulating sepsis.[48] With the ability to target various messenger RNAs (mRNAs), a single miRNA can affect multiple levels of a pathological process, increasing their multifaceted therapeutic potential in comparison with other singular agents.[49,50] It is known that multiple functional RNA species (e.g., miRNAs, lncRNAs, mRNAs) as well as proteins can naturally co-occur within a population of EVs,[51,52] underlining the potential of EVs as multifunctional sepsis therapeutics (Figure 1). As an example, EV-associated miR-223 has been shown to govern the inhibitory effect of MSC EVs on the inflammatory response in a murine sepsis model.[53] miR-223 enriched MSC EVs have also been implicated in having significant anti-apoptotic effects.[53] Thus, MSC EVs, either with natural levels of miR-223 or enhanced levels of miR-223 through exogenous loading techniques, could potentially be used as a therapy to ameliorate both the cytokine storm during the hyperinflammatory phase and the unregulated apoptosis characteristic of the immunosuppressive stages of sepsis.
Multifunctional potential in sepsis therapy can be further demonstrated through the capacity of EVs to modulate pleiotropic functions across various target cells (Figure 1). As an example, MSC EVs have been shown to reduce the inflammatory response in multiple cell types, including macrophages and cardiomyocytes, in a murine sepsis model.[53,54] EVs are also powerful regulators of oxidative stress, proliferation, and phenotype in various recipient cells, including endothelial cells and immune cells.[55,56] The multifunctionality of EVs may prove to be advantageous, as previous attempts to develop sepsis treatments have largely focused on using single agents to modify individual targets such as specific complement proteins, pro-coagulant factors, or inflammatory mediators.[57] However, a study by Xiao et al. in which the transcriptome of circulating leukocytes was analyzed following severe injury (i.e., blunt trauma, burn, or endotoxemia) revealed that regardless of the injury type, over 80% of the cellular functions and pathways within the leukocytes were altered.[58] While they did not study sepsis patients directly, this study indicates that identifying a single agent as a successful treatment for sepsis, which provokes imbalances across an array of cell functions, may be unlikely. Thus, the diverse effects stemming from the presence of multiple EV cargoes may be beneficial in sepsis treatment.
3. Endogenous Roles of EVs in Sepsis
Sepsis is capable of inducing systemic dysfunction that can simultaneously affect the cardiovascular, respiratory, neurological, hematologic, hepatic, and/or renal system(s).[59] As endogenous EVs are ubiquitous throughout human tissue, bodily fluids, and within the circulation,[37] there is reason to assume that EVs naturally play a role in the pathogenesis of sepsis. In fact, during the development of sepsis, endogenous EVs undergo fluctuations in both relative quantities and functional effects, making them integral in the progression of pathophysiological conditions.[60–63] Specifically, in sepsis and subsequent organ damage, endogenous EVs have been implicated as critical immunomodulatory factors that can regulate inflammation,[64–73] coagulation,[74–82] apoptosis,[83] and vascular dysfunction.[69,84–88] The ability of EVs to trigger, amplify, and sometimes suppress immune responses during disease can be attributed to the presence of distinct membranous proteins or lipids (e.g., phosphatidylserine (PS), integrins, major histocompatibility complexes) and to differential luminal cargo (e.g., miRNAs, proteins).[30,89] There is much research dissecting these attributes and the mechanisms through which they work, as it can prove not only beneficial in the development of an EV therapeutic but also in clarifying the complex pathophysiology of sepsis. For example, Xu et al. showed that circulating plasma EVs in a cecal ligation and puncture (CLP) murine model of sepsis were not only more abundant when compared with healthy mice but also contained different miRNA and had pro-inflammatory effects on bone marrow derived macrophages (BMDMs).[90] This immunomodulation was shown to be partially mediated by EV-associated miRNAs (i.e., miR-34a, miR-122, miR-146a) that signal via a TLR 7-myeloid differentiation primary response 88 (TLR7-MyD88)-dependent mechanism.[90] This research led to further investigation of the TLR7 signaling mechanism using a loss-of-function approach by Jian et al. that showed TLR7 signaling contributes to inflammation, organ injury, and mortality in murine sepsis.[91] These studies reveal important EV mechanisms as well as expose the cellular mechanisms contributing to sepsis and end-organ injury. The importance of endogenous EVs in sepsis was further highlighted in a study by Essandoh et al. in which GW4869, a neutral sphingomyelinase inhibitor that partially blocks the release of EVs, was used to successfully reduce the number of EVs and proinflammatory cytokines emitted from lipopolysaccharide-stimulated macrophages.[92] This EV reduction was correlated with decreased systemic inflammation as well as diminished cardiac dysfunction and mortality in a CLP mouse model.[92] Overall, these outcomes confirm the importance of endogenous EVs in sepsis and highlight the potential of inhibiting or exploiting this dynamic as a promising intervention.
The research discussed thus far has focused on how EVs within the body can serve to initiate and perpetuate sepsis. However, it is important to note that endogenous EVs can also lend protection during sepsis. Dalli et al. showed that neutrophil-derived EVs (nEVs) containing alpha-2-macroglobulin (A2MG), an antiprotease shown to be upregulated in nEVs in septic patients,[93] were able to mitigate bacterial titers, reduce systemic inflammation, and enhance survival in murine sepsis.[94] Additionally, a study by Gao et al. found that administration of murine septic EVs into a CLP mouse model suppressed inflammatory cytokine production (i.e., tumor necrosis factor-alpha; TNF-α), alleviated liver and lung tissue injury, and significantly prolonged survival.[95] When applied to engineered vascular constructs comprising human arterial smooth muscle cells, EVs from septic human patients were able to increase the expression of IL-10 and consequently reverse LPS-induced hyporeactivity and reduce oxidative stress.[86] Endogenous EVs can also serve as sepsis biomarkers to inform treatment strategy and timing, especially in critically ill patients.[100] As an example, in human septic patients, Dakhlallah et al. showed that circulating EVs had significantly higher loads of DNA methyltransferase (DNMT) mRNA, which regulates gene expression.[96] Specifically, more severely ill patients showed increases in the ratio of de novo methylating factors DNMT3A and DNMT3B to the maintenance methylating factor DNMT1 encapsulated within these circulating EVs.[96] Research has also shown sepsis-induced differential expression of other EV-associated molecules, including miRNAs.[69,97–99] Overall, the clear importance of endogenous EVs in sepsis demonstrates the efficacy of targeting or exploiting EVs as a potent sepsis treatment. Consequently, research on endogenous EVs and their roles in sepsis continues to grow and reveal numerous in vivo functions and diagnostic capabilities in preclinical sepsis models,[67,69,70,72,78,87,88,97,101–109] as well as in human patients.[61,64,71,77,83,98,99,107,110–128]
4. Preclinical Studies of EVs as Sepsis Therapeutics
The diverse functions of endogenous EVs in sepsis and their promising utility have spurred preclinical investigations in anticipation of EVs as a forthcoming therapeutic. The ubiquity of EVs has directed this research to span multiple cell sources and utilize both in vitro and in vivo models in search of an effective defense against the deleterious features of sepsis. In vitro analyses involve exposure to inflammatory cytokines such as interferon-gamma (IFN-γ) or, more commonly, LPS in the culture medium,[129] as it has been shown to induce similar plasma metabolites in healthy patients when compared with septic patients.[130] In vivo sepsis models are typically performed in a murine host and differ based on the mechanism of disease initiation. Sepsis-like symptoms can be induced via exogenous administration of a cecal slurry or a defined pathogen (i.e., Escherichia coli, Pseudomonas aeruginosa, or Staphylococcus aureus). Sepsis can also be initiated through surgical disruption of the endogenous protective barrier using CLP or colon ascendens stent peritonitis (CASP).[131,132] Additionally, although considered a hyperinflammatory model, endotoxemia induced by LPS or zymosan injection can potentially reveal insights into sepsis. While this relevant EV research has utilized various sepsis models that boast different attributes when attempting to recapitulate human sepsis,[131–133] these studies have laid the necessary foundation for the development of future EV therapies. The following two sections will describe pertinent work utilizing various EV sources with a primary focus on MSCs, which have intrinsic clinical value as a result of their multi-lineage differentiation capacity, natural regenerative abilities, and growth factor/cytokine secretion.
4.1. MSC EVs
MSCs are nonhematopoietic multipotent stem cells that can originate from nearly all tissues including bone marrow, umbilical cord, and adipose tissues.[134] MSC EVs are promising candidates in sepsis therapeutic studies as they can simulate the immunomodulatory, antimicrobial, antioxidant, and anti-apoptotic effects of their parental cells.[135,136] Despite this potential, relatively few studies have investigated the effects of MSC EVs in preclinical sepsis models (Table 1).
Table 1.
Preclinical research evaluating MSC EVs as a sepsis therapeutic. All EVs were administered in a phosphate-buffered saline (PBS) vehicle unless otherwise stated.
| Ref. | EV source | EV isolation method | EV quantification method for dosing | EV dose, route and time of administration | Model (s) | Sample size (n) (in vivo/in vitro) | Sampling time(s) (in vivo/in vitro) | Beneficial effects | EV mechanisms of action |
|---|---|---|---|---|---|---|---|---|---|
|
| |||||||||
| [145] | Human BM MSCs | UC | Resuspended in 10 μL per 1e6 source cells | 30 μL, jugular vein injection or IT, 0 or 12 h post-injury | E. coli endotoxin-induced ALI in mice, murine Mφ, human AECs | 4–32/4 | 48 h/6, 12, 24 h | - ↓Alveolar WBCs, neutrophils, MIP-2, edema, and BALF protein | KGF mRNA |
| [146] | Human BM MSCs | UC | Resuspended in 10 μL per 1e6 source cells | 90 μL, IV, 4 h post-injury | E. coli endotoxin-induced ALI in mice, human monocytes, AECs | 2–22/5–46 | 18, 24, 72 h/24, 48 h | - ↑Survival - ↓ Alveolar WBCs, MIP-2, bacterial load - Poly(I:C) pretreatment of MSCs enhanced effects |
KGF mRNA |
| [149] | Human BM MSCs | UC | Resuspended in 2 mL per 7.5e6 cells | Adoptive transfer of Mφ treated with EVs, intranasal, 4 h post injury | LPS-induced ALI in mice, human and murine alveolar Mφ | 3–5/3–7 | 24 h | - ↑ CD206+ human Mφ -↑Mitochondrial respiratory capacity - ↓Neutrophils, TNF-α, protein levels in BALF |
Not determined |
| [54] | Human UMC MSCs | UC | Total protein | 30 μg in 150 μL, tail vein injection, 4 h post-injury | CLP in mice, LPS-treated murine BMDMs | 5–13/3 | 48 h/24 h | - EVs from IL-1β pretreated MSCs ↑ M2 polarization of Mφs - ↓TNF-α, iNOS, ↑IL-10 in BMDMs |
miR-146a |
| [147] | Human BM MSCs | UC | Resuspended in 10 μL per 1e6 source cells | 30 μL, IT, simultaneous with injury | LPS-induced ALI in mice, murine Mφ, human LMVECs | 3–15/3–4 | 48 h/3 or 24 h | - ↓ Alveolar WBCs, neutrophils, MIP-2, albumin BALF, lung injury | Ang-1 mRNA |
| [164] | Human BM MSCs | UC | Resuspended in 10 μL per 1e6 source cells | 60 μL, in culture, simultaneous with injury | Human LMVECs exposed to cytomix (IL-1β, TNF-α, IFN-γ) | 3 | 6, 12, 24 h | - Restored F-actin, ZO-1, VE-cadherin - EV CD44 required for therapeutic effects |
Ang-1 mRNA |
| [148] | Human BM MSCs | AEX column | NTA | 5e9 EVs, IV, simultaneous with injury | LPS-induced endotoxemia in mice, murine Mφ | 5–7/3 | 2 h/4 h | -↓iNOS, IL-1β, IL-6, and TNF-α in Mφ -↓IL-6, IL-1β in spleen |
Not determined |
| [150] | Human BM MSCs | UC | Resuspended in 10 μL per 1e6 source cells | 90 μL, IV, 4 h post-injury | E. coli-induced ALI in mice, LPS-treated murine Mφ, and human monocytes | 5–13/3–9 | 12, 24 h/1.5, 4, 14, 24 h | -↑Phagocytosis of monocytes and Mφ - ↑LTB4, ↓ bacterial counts, TNF-α, MIP-2 in BALF -↑LTA4H protein levels |
Suppression of MRP1 expression via miR-145 |
| [168] | Human AD MSCs | UC | Total protein | 100 μg in 200 μL, tail vein injection, 0.5 h post-injury | LPS-induced ALI in mice, murine BMDMs | 7–12/3–8 | 24, 48 h/24 h | - EVs from young donor (25 years old) ↓ immune cell infiltration, alveolar septal thickness; ↓ Protein, neutrophils, IL-1β, and ↑ 1L-10 in BALF | ↓ miR-127-3p and miR-125b-5p in young EVs |
| [169] | Human BM MSCs | UC | Resuspended in 10 μL per 1e6 source cells | 200 or 400 μL, IV, 1 h post-injury | E. coli-induced ALI in ex vivo perfused human lung, LPS-treated human alveolar Mφ | 5–11/8 | 6 h | - ↓Lung hemorrhage, edema, neutrophil infiltration, protein permeability - EVs from poly (I:C) pretreated MSCs ↓bacterial count in BALF |
Not determined |
| [144] | Human UMC MSCs | UC | FACS | 1e8 vesicles per kg body weight, tail vein injection, 0.5 h post-injury | E. coli-induced ALI in rats, human Mφ | 3–18/3–5 | 48 h/72 h | - ↑Survival - EVs from IFN-γ pretreated MSCs ↓lung injury, alveolar-arterial oxygen gradient, alveolar protein leak, alveolar TNF-α and ↑eNOS - ↑ Mφ bacteria killing |
Not determined |
| [141] | Human AD MSCs | UC | Total protein | 100 μg in 200 μL, tail vein injection, 4 h post-injury | CLP in mice | 4–20 | 24 h, mortality monitored at 0, 6, 12, 24, 36, 48 h | - ↑Survival - ↓Serum Cr, BUN, TNF-α, IL-6, MCP-1 - ↓ Bax, cleaved caspase-3, TNF-α, NF-κB p65, HIF-1α and ↑expression of SIRT1, VEGF, and Bcl-2 |
Not determined |
| [109] | Human UMC MSCs | SDG-UC | Total protein | 120 μg in 100 μL, tail vein injection, 3 h post-injury | CLP in mice, human renal tubular epithelial cells | 6–18/3 | 24 h, mortality monitored for 3 days | - ↑Survival - ↓Serum Cr, BUN, TNF-α, and IL-1β levels - ↓Apoptosis, p-IκBα, NF-κB p-P65, TNF-α, 1L-1β, IRAK1 in kidney tissue |
miR-146b |
| [138] | Murine BM MSCs | Exo-quick | Total protein | 10 μg in 100 μL, carotid injection immediately following injury | CLP in mice, HUVECs | 4–13/3–4 | 6 h/12 h | - ↓ VCAM/ICAM and immune cells in myocardium - Rescued ejection fraction and fractional shortening |
miR-126 |
| [53] | Murine BM MSCs | UC | Total protein | 2 μg g−1 body weight in 150 μL of culture medium, tail/jugular vein injection, 1 h post-injury | CLP in mice, LPS-treated murine Mφ and CMs | 4–14/3 | 12 h, mortality monitored for 3 days/12 h | - ↑Survival, ventricular ejection fraction, and cardiac fractional shortening ↓TNF-α, IL-6, IL-1β |
miR-223 |
| [165] | Murine BM MSCs | DC | Total protein | 100 μg in 200 μL, tail vein injection, immediately prior to injury | LPS-induced ALI in mice, human type 2 AECs | 3 | 24 h/24, 48 h | - Overexpression of miR-30b-3p in EVs ↓apoptosis and ↑ proliferation in AECs - ↓ Alveolar hemorrhage, alveolar septum thickness, pulmonary edema |
↓of SAA3 via miR-30b-3p |
| [143] | Murine AD MSCs | UC | Total protein | 100 μg, IV, 3 h post-injury | CLP in rats | 6–19 | 5 Days, mortality monitored daily | - ↑Survival - ↓CD11b/c+, Ly6G+, cells; MIF+ cells; CD4+, CD8+ cells; CD68+ and CD14+ cells, - ↑Treg+ cells |
Not determined |
Abbreviations: AEC, alveolar epithelial cell; AEX, anion exchange; AD, adipose-derived; ALI, acute lung injury; Ang-1, angiopoietin 1; BALF, bronchoalveolar lavage fluid; BM, bone marrow; Bax, Bcl-2-associated X protein; Bcl-2, B-cell lymphoma-2; BMDM, bone marrow derived macrophages; BUN, blood urea nitrogen; CLP, cecal ligation and puncture; CM, cardiomyocyte; Cr, creatinine; DC, differential centrifugation; EC, endothelial cell; eNOS, endothelial nitric oxide synthase; EV, extracellular vesicle; FACS, fluorescence-activated cell sorting; HIF-1α, hypoxia-inducible factor-1 alpha; LMVEC, lung microvascular endothelial cell; IFN-γ, interferon gamma; IL-1β, interleukin-1 beta; iNOS, inducible nitric oxide synthase; IRAK1, interleukin 1 receptor-associated kinase 1; IT, intratracheal; IV, intravenous; KGF, keratinocyte growth factor; LPS, lipopolysaccharide; LTA4H, leukotriene A4 hydrolase; LTB4, leukotriene B4; Ly6G, lymphocyte antigen 6 complex locus G; Mφ, macrophage; MCP-1, monocyte chemoattractant protein-1; MIF, macrophage migration inhibitor factor; MIP-2, macrophage inflammatory protein 2; mRNA, messenger ribonucleic acid; miRNA, micro ribonucleic acid; MRP1, multidrug resistance-associated protein 1; MSC, mesenchymal stem cell; NF-κB, nuclear factor kappa-light-chain-enhancer of activated B cells; NTA, nanoparticle tracking analysis; p-IκBα, phosphorylated inhibitor of nuclear factor kappa B-alpha; Poly (I:C), polyinosinic-polycytidylic acid; SAA3, serum amyloid A3; SDG-UC, sucrose density gradient ultracentrifugation; sirt1, sirtuin 1; TNF-α, tumor necrosis factor-alpha; UC, ultracentrifugation; UMC, umbilical cord; VEGF, vascular endothelial growth factor; VE-cadherin, vascular endothelial cadherin; WBC, white blood cell; ZO-1, zonula occludens-1.
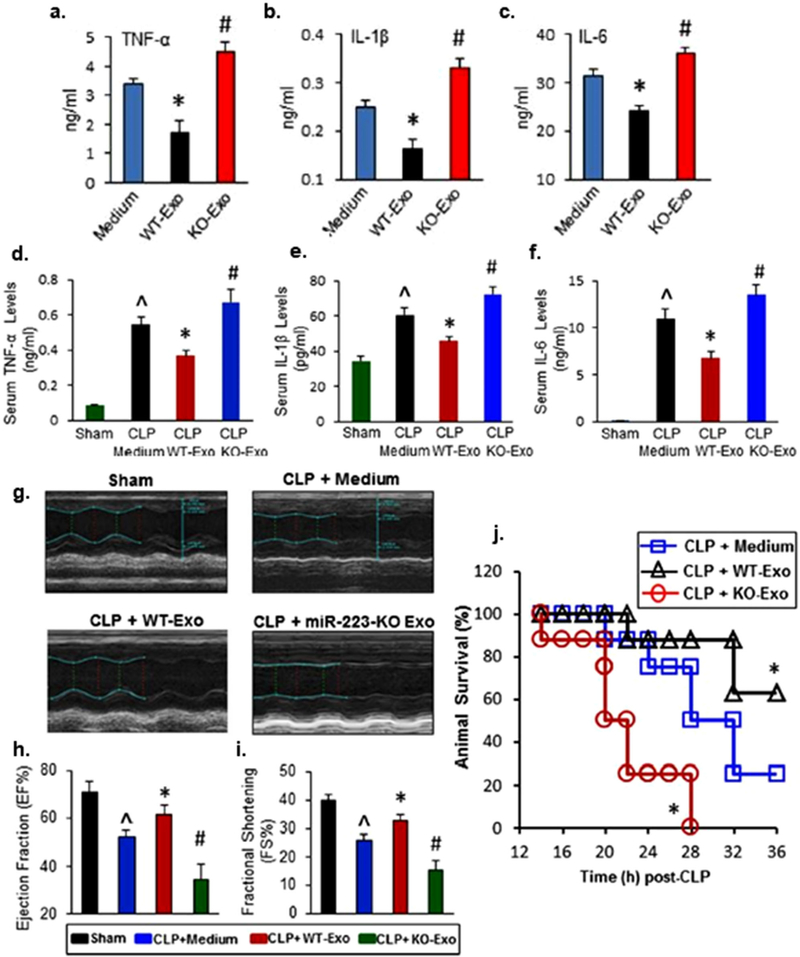
Several of these studies utilized a CLP model, perhaps the most common animal model in preclinical sepsis research.[137] Wang et al. induced sepsis in mice via CLP and observed the effects of mouse bone marrow derived MSC EVs obtained from wild type and miR-223 KO mice.[53] Wild type MSC EVs were shown to significantly increase animal survival (≈60%) compared to mice treated with the control medium (≈20%) in groups of 10–11 animals (Figure 2). MSC EV treatment decreased TNF-α, interleukin-6 (IL-6), and interleukin-1beta (IL-1β) in mouse serum and in culture supernatants of LPS-stimulated murine macrophages (Figure 2). Additional in vitro experiments demonstrated a significant reduction in apoptosis of LPS-stimulated cardiomyocytes when treated with MSC EVs. These effects were translated into the in vivo environment where cardiac function improvements were associated with MSC EVs as determined by echocardiography (Figure 2). Moreover, it was demonstrated that these MSC EV benefits were partially dependent on miR-223, possibly via the inhibition of two inflammatory mediators semaphorin 3a (sema3a) and signal transducer and activator of transcription 3 (stat3).[53] In addition to miR-223, Zhang et al. found that miR-126 was essential in suppressing the LPS-induced increase in adhesion molecule expression in human umbilical vein endothelial cells (HUVECs).[138] Delivery of EV-associated miR-126 into the right carotid artery of CLP mice was able to significantly reduce the expression of vascular cell adhesion molecule 1 (VCAM-1) and intercellular adhesion molecule 1 (ICAM-1) as well as rescue ejection fraction and fractional shortening in the myocardium (groups of 6–9 animals).[138] Another study by Song et al. utilized EVs from human umbilical cord-derived MSCs in a CLP-induced murine sepsis model.[54] By the end of the 7-day observation period in a high-grade model (100% lethality), all mice within the control group had died while ≈33% of mice receiving the MSC EV treatment survived. Priming of MSCs by IL-1β improved this survival rate to ≈70% (groups of 10–13 animals). Altered macrophage polarization as measured by surface protein expression (flow cytometry) and cytokine secretion (ELISA) was implicated as part of the mechanism of MSC EV effects, and the immunomodulatory benefits of IL-1β priming were found to be due at least partially to increased expression of miR-146a, a known mediator of innate immunity and inflammation,[139] within MSC EVs.[54] While investigating the effects of human umbilical cord-derived MSC EVs on sepsis-induced acute kidney injury (AKI), Zhang et al. found a 28% survival rate in mice that underwent CLP surgery which increased to 45% following treatment with EVs (groups of 12 animals).[109] In addition to reductions in inflammatory cytokines, they found that serum levels of creatinine and urea nitrogen, two common markers of renal dysfunction,[140] were significantly decreased in the MSC EV treatment group. MSC EVs were able to reduce renal tubular cell apoptosis as well as inhibit NF-κB activity of septic mice. Similar to Song et al., the research showed that these benefits were partly contingent on EV-associated miR-146b.[109] Gao et al. showed that another source of MSC EVs, human adipose tissue, also significantly increased survival in a CLP-induced AKI mouse model (70% vs 35% survival of control mice; groups of 20 animals).[141] Again, it was demonstrated that inflammatory cytokines and serum levels of urea nitrogen and creatinine were reduced in mice exposed to the MSC EV treatments. In opposite fashion to NF-κB, Gao et al. also showed that the protein levels of sirtuin 1 (SIRT1) in kidney tissues were increased following EV administration.[141] SIRT1 is a highly conserved mammalian protein known to inhibit the transactivation potential of NF-κB by deacetylating its subunit, RelA/p65.[142] Thus, this study provided further insight into the protective mechanisms of MSC EVs in sepsis. Finally, in a study by Chang et al., following treatment with rat adipose tissue-derived MSC EVs, rats with CLP-induced sepsis experienced an increase in 5-day survival (≈80% vs ≈50% survival of control rats, groups of 16–19 animals).[143] When MSCs were made apoptotic via oxygen deprivation and serum starvation prior to EV collection, the EVs produced exhibited less benefit (≈60% survival). The mechanism of MSC EV effects was again found to be immunomodulatory as assessed by circulating cytokine levels and other assays.[143]
Figure 2.
Protective effects of MSC EVs and miR-223-KO MSC EVs on the inflammatory response, cardiac dysfunction, and survival in a CLP-induced sepsis model. Wild-type (WT) EVs (20 μg mL−1) significantly reduced the secretion of TNF-α, IL-1β, and IL-6 in LPS-stimulated RAW264.7 cells. A–C) (n = 3 wells, *p < 0.05, vs medium controls; #p < 0.05, vs medium controls) and in the serum of CLP mice. D–F) (n = 11, ˆp < 0.05 vs sham; *p < 0.05 vs CLP; #p < 0.05 vs CLP). G–I) WT EVs rescued the left ventricular ejection fraction and fractional shortening in the myocardium of CLP mice (n = 11, ˆp < 0.05 vs sham; *p < 0.05 vs CLP; #p < 0.05). J) Injection of WT EVs significantly improved survival following CLP surgery (n = 8, *p < 0.05 vs CLP). Adapted under a Creative Commons Attribution 4.0 International License (CC BY 4.0).[53] Copyright 2015, The Authors, published by Springer Nature.
Beyond these studies, there are also several reports of the effects of MSC EVs in animals exposed to LPS or bacterial infection/instillation. Varkouhi et al. reported that EVs from human umbilical cord MSCs increased bacterial killing in human macrophages. When administered in an E. coli-induced acute lung injury (ALI) rat model, these EVs were shown to produce significant 48 h survival benefits observed in groups of 8–18 animals.[144] However, only EVs from INF-γ-primed MSCs were able to increase macrophage phagocytosis and attenuate lung injury severity.[144] In bacterial endotoxin-induced ALI models of mice, human bone marrow derived MSC EVs were able to significantly mitigate the influx of white blood cells and neutrophils, reduce the total protein and monocyte inflammatory protein-2 (MIP-2) levels in bronchoalveolar lavage fluid (BALF), and decrease edema in lung tissues.[145–147] When applied in vitro, these EVs significantly reduced the secretion of inflammatory mediators from both murine macrophages and human monocytes and decreased the monolayer permeability in human lung microvascular endothelial cells (LMVECs).[145–148] In addition to a considerable increase in survival (88% vs 40% survival of control mice; groups of 14–35 animals), Monsel et al. found that the MSC EVs had an antimicrobial effect both in vivo and in vitro. This effect may be partially mediated by an EV-induced increase in macrophage phagocytosis, a phenomenon that was observed in vitro.[146,149] In a study by Morrison et al., the increased phagocytosis observed in cultured human alveolar macrophages following treatment with MSC EVs was found to be partially dependent on the EV-mediated transfer of mitochondria.[149] Significant antimicrobial activity was also observed in a study by Hao et al. while observing the effects of human bone marrow derived MSC EVs in an E. coli-induced ALI mouse model.[150] In this study, it was established that the increase in antimicrobial activity in vivo was partially regulated by EV-associated miR-145.[150] miR-145 was shown to suppress the expression of multidrug resistance-associated protein 1 (MRP1), a highly conserved transporter protein,[151] which consequently resulted in an augmented production of leukotriene B4 (LTB4), a lipid mediator of the inflammatory response that can induce the release of antimicrobial peptides.[150,152] Moreover, the antimicrobial effect of EVs that was observed by Monsel et al. was enhanced following treatment of MSCs with the TLR3 agonist polyinosinic-polycytidylic acid (poly (I:C)).[146] Both Monsel et al. and Zhu et al. found that many of the observed benefits of MSC EVs were somewhat controlled by the presence of keratinocyte growth factor (KGF) mRNA.[145,146] KGF is an epithelial mitogen and differentiation factor that plays a pivotal role in the repair of a variety of tissues and organs.[153–155] KGF-based treatments have been reported to protect lungs from a variety of insults (e.g., LPS instillation, bacterial challenge, and ventilator placement) and are thought to work through various mechanisms such as maintaining epithelial barrier function and reducing inflammation.[156–161]
As MSC EV effects were only diminished and not abolished by the knockdown of EV-associated KGF and miR-145, it can be assumed that other factors are vital to obtain the maximum benefits from MSC EVs during sepsis. For example, Tang et al. investigated the role of MSC EV-associated angiopoieten-1 (Ang-1) mRNA in murine ALI as it has been reported to be an important factor in endothelial survival and restoration of vascular integrity.[147,162,163] Similar to previous studies exploring KGF, the observed MSC EV therapeutic effects were partly reliant on the presence of Ang-1 mRNA.[147] Furthermore, in a cytokine-induced ALI in vitro model, it was shown that the ability of human bone marrow derived MSC EVs to reduce protein permeability in human LMVECs was significantly diminished after pretreatment with Ang-1 short interfering RNA (siRNA).[164] In a different approach, Yi et al. engineered murine bone marrow derived MSC EVs to overexpress miR-30b-3p.[165] MSC EVs are known to transfer miR-30b, known for its contribution in angiogenesis,[166] to recipient cells.[167] The study showed that when overexpressing miR-30-b-3p, the ability of MSC EVs to reduce apoptosis and increase proliferation in human alveolar endothelial cells was enhanced.[165] Improvements in MSC EV abilities were also demonstrated in an LPS-induced ALI mouse model where, when compared with unmodified MSC EVs, altered MSC EVs showed a greater ability to decrease neutrophil counts and edema in lung tissues as well as an increased ability to modulate inflammatory mediators.[165] Interestingly, Huang et al. showed that donor variability, specifically among young and aged donors, may affect MSC EV therapeutic benefits.[168] Using an LPS-induced ALI mouse model, Huang et al. demonstrated that, despite having comparable physical and phenotypical traits, MSC EVs derived from the adipose tissue of a 25-year-old donor, and not those derived from a 72-year-old donor, were able to significantly decrease inflammatory cell infiltration and injury severity in lung tissue as well as control the production of immunomodulatory cytokines from cultured murine macrophages.[168] Further investigation revealed that MSC EVs derived from the younger donor were more readily internalized by murine macrophages in vitro and promoted an M2 macrophage phenotype in vivo. Additionally, the MSC EVs from the younger donor exhibited differential expression in miRNA, with a significant increase in miR-223–5p and a downregulation of both miR-127–3p and miR-125b-5p. Inhibition of miR-127–3p and −125b-5p was shown to decrease M1 macrophage polarization, elucidating a probable mechanism for the observed differences in EV-induced macrophage phenotype.[168] While this particular study highlights a significant and unavoidable obstacle in the development of an MSC EV therapeutic for sepsis (i.e., donor variability), the results must be interpreted with a degree of skepticism as donor sample size was minimal (i.e., n = 1) and other donor characteristics were not discussed.
In an attempt to utilize a more clinically relevant model, Park et al. observed the effects of human bone marrow derived MSC EVs on E. coli-induced ALI in an ex vivo perfused human lung.[169] MSC EVs administered 1 h following injury were shown to increase alveolar fluid clearance as well as reduce the level of hemorrhage, edema, and protein permeability (groups of 5–11). Although not significant, neutrophil counts and TNF-α levels within BALF were also diminished. Importantly, when MSCs were primed with poly (I:C) the subsequently secreted EVs had a significantly greater antimicrobial effect in the injured alveolus,[169] echoing previous results demonstrated in a murine model of ALI.[146] This particular study is of significance as it demonstrates the ability to replicate the effects of MSC EVs originally observed in a murine model in a more human-like model, further justifying continued investigation of an MSC EV sepsis intervention.
Despite the few peer-reviewed articles examining MSC EVs in sepsis-relevant preclinical models, the research generated has demonstrated the potential. It is important to note that although all MSCs are defined using a common stem cell phenotype,[170] the tissue source can refine certain mechanisms of action.[171] Following from this, research comparing the efficacy of MSCs from various sources (e.g., bone marrow, adipose, umbilical cord) will be required to move forward with an optimal MSC EV therapy. Overall, the results of these preclinical studies to date suggest that MSC EVs are a promising sepsis therapeutic with an extremely diverse and dynamic network of protective mechanisms in need of further exploration.
4.2. Non-MSC EVs
There is also evidence for the beneficial impact of EVs from a variety of non-MSC sources in sepsis. One of the earliest examples involves immature dendritic cell (iDC)-derived EVs, which were shown to diminish disease severity in CLP mice.[172,173] Specifically, iDC-derived EVs contain milk fat globule-epidermal growth factor 8 (MFG-E8), an opsonin, which promoted healthy phagocytosis of apoptotic immune cells in murine sepsis.[172] Septic mice had reduced inflammation and improved survival when treated with EVs derived from iDCs, but not EVs from mature DCs (which do not contain MFG-E8).[173] Other studies show that EV functionality can depend on EV subpopulation and context. As an example, only when stimulated with opsonized particles, did neutrophils produce EVs with significant antibacterial properties.[174,175]
Since maintaining endothelial integrity is critical in sepsis patients, the therapeutic impact of EVs derived from vascular cell sources has been studied. In particular, several studies have found that endothelial progenitor cell (EPC)-derived EVs are a promising avenue to explore. EPC EVs demonstrated the ability to significantly mitigate the inflammatory response,[56,176] pulmonary edema,[56,176,177] and/or mortality in mouse models of both CLP and LPS-induced ALI.[56] These effects were attributed to enriched miR-126 content in the EVs, as miR-126 is a pivotal regulator of endothelial function.[56,176,177] EVs from fully differentiated endothelial cells have also been engineered for treatment in sepsis models. Syndecan-1, an important component of the endothelial glycocalyx, was overexpressed in mouse pulmonary microvascular endothelial cells and found to co-purify with their EVs. Treatment of LPS-induced ALI in mice with these engineered EVs preserved microvascular integrity and alleviated edema and lung damage.[178] Additionally, when heat shock protein A12B (HSPA12B), which is predominantly expressed in the endothelium and upregulates miR-126, was loaded into HUVEC EVs via adenovirus-mediated overexpression, the EVs suppressed LPS-induced inflammatory cytokine secretion in murine macrophages via the downregulation of NF-κB.[179]
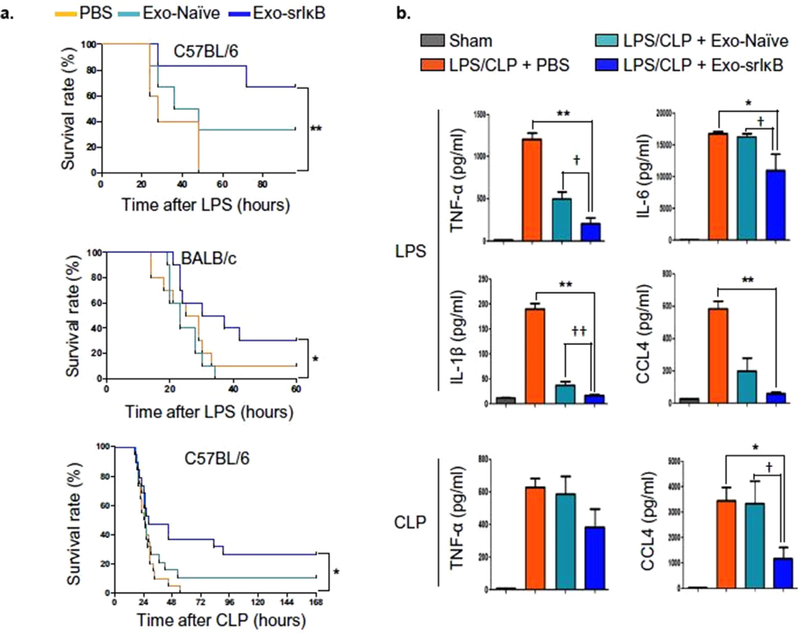
Finally, additional cell types have been investigated due to their established utility in biotechnological processes, increasing translational potential. Notably, Choi et al. recently reported the development of human embryonic kidney 293T (HEK293T) EV-based delivery of super-repressor I(kappa)B (IκB) to reduce sepsis-associated organ damage and mortality.[180] Their approach emanated from the mechanistic knowledge that NF-κB plays a critical role in pathogen-induced host gene expression modification; IκB the dominant active form of IκBα, a NF-κB inhibitor. Utilizing an optogenetically engineered system for loading IκB into EVs, they observed significant survival improvements in LPS-induced sepsis models in C57BL/6 (≈65% vs 0% for controls) and BALB/c mice (≈30% vs 0% for controls) as well as in a CLP model in C57BL/6 mice (≈30% vs 0% for controls), in addition to a reduced inflammatory response (Figure 3). Interestingly, in each study, unloaded HEK293T EVs also showed some substantial ability to increase survival, suggesting a more generic EV effect. Overall, these results suggest that non-MSC EVs with appropriate cargo may be a potential therapeutic solution for sepsis.
Figure 3.
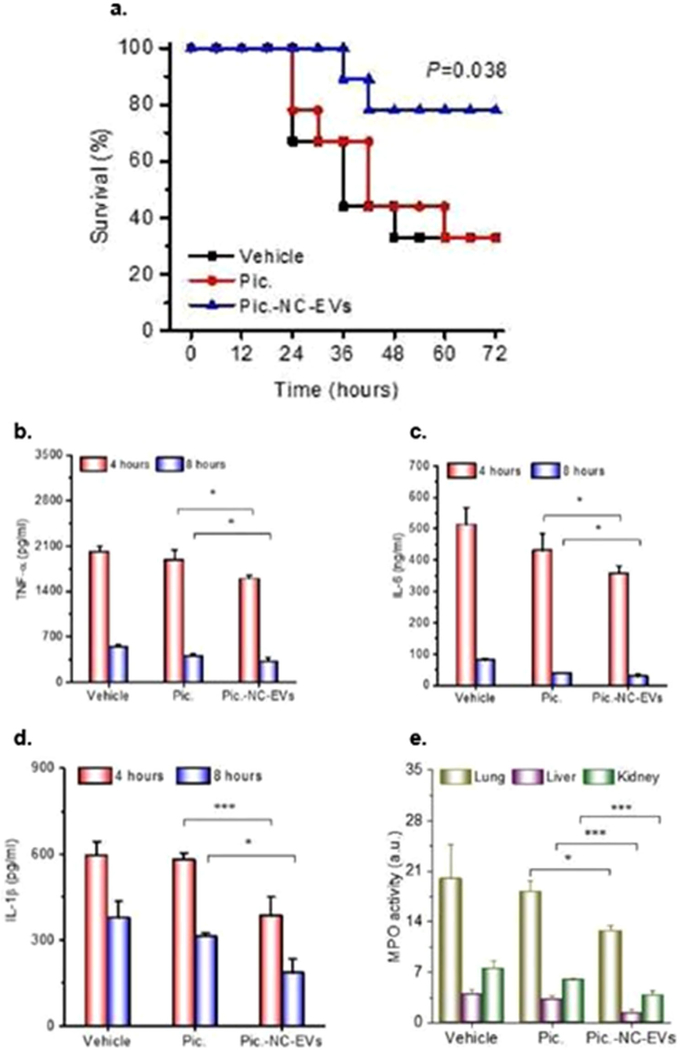
Impact of HEK293T EVs loaded with super-repressor IκB (Exo-srIκB) in LPS- and CLP-induced sepsis. A) Exo-srIκB significantly increased survival in LPS C57BL/6 mice (n = 5–6), LPS BALB/c mice (n = 10), and CLP C57BL/6 mice (n = 14–15) (**p < 0.01 and *p < 0.05 compared with the phosphate-buffered saline (PBS)-treated group). B) Levels of TNF-α, IL-6, IL-1β, and CCL4/macrophage inflammatory protein-1β in the plasma of EV-treated mice were reduced in LPS and/or CLP mice 24 h after treatment with Exo-srIκB (**p < 0.01 and *p < 0.05 compared with the PBS-treated sepsis group, †p < 0.05 compared with the Exo-naive-treated sepsis group). Adapted with permission.[180] Copyright 2020, The Authors, some rights reserved; exclusive licensee AAAS. Distributed under a Creative Commons Attribution NonCommercial License 4.0 (CC BY-NC).
5. Lessons Learned and Future Opportunities
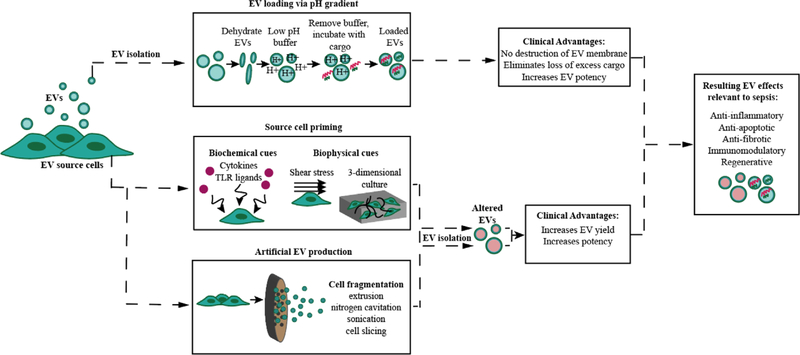
Research involving EV platforms for the treatment of diseases like sepsis is an emergent field with considerable therapeutic potential. Yet, there are still many challenges that limit efficacy and translation of EV therapies, including a lack of scalable production methods and low therapeutic efficiency. Research has shown that advancements in the EV biomanufacturing process, such as the selection of an optimal cellular source as well as manipulation of EV production conditions and EV cargo, can potentially overcome these obstacles. These, along with simultaneous advancements in the mechanistic understanding of sepsis, present ample opportunities for the development of a powerful and clinically translatable EV treatment for sepsis.
5.1. EV Cargo Loading
As natural intercellular communicators, EVs have been targeted as vehicles for the delivery of both native and non-native molecules (i.e., RNAs, DNA, proteins, and non-biologic small molecule drugs) in numerous therapeutic applications.[181–184] In comparison with current synthetic drug delivery systems such as liposomes, lipid nanoparticles, and polymeric nanoparticles, EVs may provide a platform that offers several advantages including increased biocompatibility, minimal toxicity issues, and intrinsic targeting abilities.[185] However, therapeutic molecules within EVs, such as miRNAs, may only be present at relatively low levels (e.g., 1 miRNA per ≈100 EVs).[186] Thus, to manipulate or enhance EV potency, molecules can be loaded into EVs either endogenously or exogenously. Endogenous methods involve manipulation of the loading machinery of the parental cells (e.g., via transduction or transfection). However, in addition to being expensive, reagent residues from chemical transfection methods may affect cargo functions.[187] Exogenous loading methods entail physically disrupting the EV membrane (e.g., via sonication, electroporation, heat shock, or freeze-thaw cycles),[184,188] which can produce high loading efficiencies (e.g., 1000–3000 siRNA copies per EV),[189] but often induces degradation and aggregation leading to reduced cargo functionality.[190]
Recently, a less disruptive loading method involving a transmembrane pH gradient, originally used for loading liposomes,[191] was modified for use in EVs (Figure 4).[192] Using this technique, Jeyaram et al. demonstrated the ability to load siRNA, single-stranded DNA (ssDNA), and miRNA into EVs derived from HEK293T cells. Similar to other exogenous loading methods, the pH modification method allowed the loading of thousands of RNA molecules per EV.[192] Additionally, due to the lack of introduction of high energy into the loading process, the pH method preserved the integrity of the unloaded cargo and allowed the molecules to be recycled for successive loading procedures.[192] This eliminates the loss of excess cargo resulting in a more practical loading process. Furthermore, as dendritic cell EVs naturally contain miR-146a that is capable of inhibiting endotoxin-induced inflammation in mice,[193] HEK EVs were loaded with double-stranded (ds)-miR-146a and applied to BMDMs. Consequently, the BMDMs exhibited a significant decrease in the expression of the proinflammatory enzyme interleukin-1 receptor-associated kinase 1 (IRAK1), a key adaptor molecule in the TLR4/MD-2/MyD88/NF-κB pathway,[194] as well as a reduction in the secretion of the inflammatory cytokine IL-6.[192] This study demonstrated an improved EV loading technique as well as the successful embedding of a desired, but previously nonexistent, function (i.e., anti-inflammatory) into HEK EVs using miR-146a.
Figure 4.
Upstream modifications and their potential in advancing clinical translation of an EV sepsis therapy.
miR-146a is an important EV cargo to investigate as it is a prominent mediator of innate immunity and is dysregulated in a number of diseases.[195] As of late, miR-146a is of great interest in sepsis research because while it reduces inflammation, which is crucial in ameliorating the cytokine storm during the initial stages of sepsis, it also protects against apoptosis, a characteristic of the late immunosuppressant phase of sepsis.[196,197] Actually, the use of miR-146a has been shown to mitigate excessive inflammation and cardiomyocyte apoptosis to prevent myocardial dysfunction during LPS-induced cardiac injury in mice.[197] However, a major obstacle is the lack of a practical delivery vehicle. In 2019, Funahashi et al. were able to deliver miR-146a via a plasmid/polyethyleneimine (PEI) nanoparticle complex into the tail veins of CLP mice and effectively reduce inflammation and sepsis-induced multiple organ injury.[198] Yet, they were only able to achieve a prophylactic effect, most likely due to the fact that the miRNA was not able to act instantly as time was required for transcription and maturation.[198] On the other hand, substantial experimental evidence suggests that EVs are able to deliver fully mature and functional miRNAs into recipient cells.[193,199] In fact, EV-associated miR-146a was deemed a crucial mechanism that increased survival in CLP mice.[54] Overall, future preclinical research should investigate EVs loaded using a pH transmembrane gradient or other approaches that are not deleterious to labile biological cargo such as miR-146a.
As many miRNAs are highly conserved and implicated in numerous diseases,[47,50] they are often the focus of EV cargo manipulation. However, EVs are a versatile vehicle and alternative cargo molecules may prove to be equally effective as a sepsis intervention. In fact, other types of TLR4 signaling pathway modulators have been investigated as potential therapeutics in sepsis, including TLR4 antagonists derived from natural sources (e.g., curcumin).[200] Specifically, curcumin loaded into EVs was reported to exhibit greater solubility, stability, and bioavailability.[201] Indeed, when compared with curcumin alone, curcumin-loaded EVs proved to significantly reduce LPS-induced cytokine production (i.e., IL-6 and TNF-α) and mortality in mice.[202] The stability provided by an EV delivery vehicle may prove generally useful for enhancing sepsis treatments. For instance, melatonin is an endogenous hormone that acts as a liaison between the neuroendocrine and immune systems through modulation of processes such as inflammation and redox homeostasis.[203] Recently, the innate anti-inflammatory, anti-apoptotic, and antioxidant properties of orally administered melatonin have been found to be effective in preventing sepsis-induced hepatic injury, cardiomyopathy, and brain dysfunction.[204] However, the bioavailability of oral melatonin is low (3–33%) due to metabolism in the liver.[204] With high membrane permeability (≈1.7 μm s−1) and an abundant presence in select endogenous EVs,[205,206] it is reasonable to speculate that exogenous melatonin could be loaded into EVs as a means of increasing the bioavailability and potency of this potential sepsis therapeutic.
The use of singular agents capable of multidimensional functions, such as miR-146a or melatonin, is an attractive therapeutic approach for any disease. However, the diversity and complexity of the physiological environment makes it unlikely that a singular agent will halt the progression of a complex and systemic disease such as sepsis. In fact, knockdown studies of a single cargo (e.g., a singular miRNA) to affirm the protective EV mechanism never fully abolish the beneficial effects,[54] implicating the role of additional cargoes. In addition, new insights into the pathobiology of sepsis emphasize the highly heterogeneous nature of the disease. The outcome of sepsis depends on numerous risk factors including age, comorbidities, genetics, causative organisms, as well as timing and suitability of the treatment.[207,208] These risk factors can initiate multiple distinct biological pathways that induce differential signatures of metabolites, mRNA and proteins, and cellular function leading to several possible subtypes of patients and thus necessitating different types of intervention, possibly combinatorial in nature.[208] Given their natural propensity to shuttle multiple molecules, EVs can theoretically be simultaneously loaded with several cargoes to accommodate combinatorial therapies and to recapitulate any synergistic effect or to overcome any antagonistic effects that may naturally occur in vivo. Future sepsis studies should investigate the benefits of loading multiple molecules into EVs, such as a collective of anti-inflammatory miRNAs or a combination of several types of molecules that can influence the same pathway through different mechanisms. For example, Marik et al. uncovered a promising treatment protocol involving the intravenous infusion of hydrocortisone, vitamin C, and thiamine that was able to prevent organ dysfunction and significantly reduce mortality of severely septic human patients.[209] While all three compounds exhibit anti-inflammatory and antioxidant properties, they act through different mechanisms.[210] This multi-drug protocol could be critical for informing the development of a future successful EV therapy for sepsis.
5.2. EV Producer Cell Priming
EV activity can be impacted by stress-induced adaptive responses of parental cells to their environment, whether in a physiological setting or in a controlled EV production environment.[211] This phenomenon provides an opportunity during the biopharmaceutical development process to control EV efficacy through the manipulation of upstream parameters (i.e., cell culture parameters) (Figure 4), which would potentially lessen the need to load synthetic drugs or non-native biological components (e.g., nucleic acids or proteins) into EVs during downstream procedures. This is significant as many loading methods, as mentioned previously, require disrupting the EV membrane, potentially damaging native structures and targeting features of EVs.[190] Furthermore, various loading techniques require the addition of process-related impurities (i.e., unencapsulated cargoes) to the previously purified EV preparation, demanding the implementation of supplementary purification procedures which can be costly, time-consuming, and risk even further loss and possible deformation of target EV material.[212]
Importantly, modification of cell culture parameters has previously been shown to significantly reshape EV intraluminal cargo composition,[213–238] augment EV production (e.g., number of EVs produced per cell),[213,216,219,220,229,232,237,239–247] and increase EV potency in a diverse array of applications including neurological disorders and injuries, wound healing, cartilage repair, kidney disease, ischemia/reperfusion (I/R) injury, myocardial infarction, and sepsis.[53,146,215,220,222,224,229,230,232,240,241,243,247–252] These changes can be produced through the adjustment of numerous upstream variables including cell seeding density,[240] media composition (i.e., serum-free media),[213,228,231] collection frequency of EVs,[240] architecture of the culture vessel (i.e., 2D vs 3D; 2D vs 3D),[220,222,227,229,230,239,242,243,247,248,250,253,254] fluid shear stress (i.e., static vs dynamic flow),[250,255] mechanical stimulation (e.g., cyclic stretch),[256–258] exposure to hypoxia,[143,217,219,224,228,231,232,241,245,246,251,259–265] heat,[266] oxidative stress,[225,266] electrical stimulation,[267] or coincubation with soluble signals (e.g., cytokines, growth factors).[54,144,214,215,218,221,233,234,236,237,249,250,264,268–276] While the list of upstream factors affecting EV therapeutic potential is extensive, each can be described as either biochemical or biophysical in nature. The burgeoning research investigating these cues and their effects on EVs demonstrates the integral need to understand the complexity of the cellular microenvironment when developing future EV therapies for both sepsis and other disease applications.
5.2.1. Biochemical Priming
Biochemical priming consists of exposing cells to specific molecules (e.g., growth factors, cytokines, hormones, soluble gases, and pharmacological compounds) that are capable of modulating particular intracellular signaling pathways and ultimately directing cell phenotype and behavior. As the primary tool in many cell-based therapies, MSCs have become a principal focal point of studies investigating the effects of biochemical priming. The immunosuppressive capabilities of MSCs are highly relevant to sepsis, and studies show that these are regulated by both the release of pro-inflammatory cytokines[277] as well as the engagement of TLRs during pathological processes.[278–280] Analogous to the classic macrophage polarization paradigm, MSCs have been shown to adopt either a pro-inflammatory or anti-inflammatory phenotype depending upon the stimulus.[281–285] When applied to sepsis-related models (i.e., CLP or E. coli-induced ALI), EVs from MSCs primed with pro-inflammatory cytokines (i.e., IFN-γ or IL1-β) were able to induce significant therapeutic effects not seen when using EVs from naïve MSCs, such as the tempering of organ injury,[144] and further improved the beneficial effects seen with naïve MSC EVs, such as survival.[54] Priming human umbilical cord derived MSCs with IL-6 produced EVs with significantly upregulated miRNAs such as miR-455–3p, which is involved in the control of IL-6-related signaling pathways.[268] Using a miR-455–3p agomir, the researchers were able to show that this particular miRNA played a critical role in inhibiting macrophage activation (i.e., IL-6 production) by regulating phosphoinositide-3-kinase regulatory subunit 1 (PIK3r1) in an LPS-induced endotoxemia model.[268] In other applications, EVs derived from cytokine-primed MSCs were able to skew macrophage polarization to the anti-inflammatory M2 phenotype,[234,286] alter EV cargo, and increase EV uptake into specific recipient cells.[221]
It is critical to mention that the studies utilizing a singular or even a dual cytokine combination may not be inducing the full immunosuppressive capacity of MSCs or MSC EVs.[277,287] Demonstrating this is a study by Zhang et al. in which human umbilical cord derived MSCs were primed with TGF-β, IFN-γ, or a TGF-β/IFN-γ mixture.[233]After incubating the EVs from the primed MSCs with PBMCs, it was shown that the TGF-β/IFN-γ cocktail had a greater ability to induce regulatory T-cell differentiation and secretion of anti-inflammatory cytokines (i.e., IL-10 and IDO) when compared with EVs derived from naïve MSCs or MSCs stimulated with a singular cytokine.[233] It is likely that the most effective combinatorial cytokine priming approach for the enhancement of an EV therapy has not yet been tested. In 2014, Han et al. revealed that, compared with priming MSCs with a TNF-α/IFN-γ mixture, priming MSCs with a triple cytokine blend (TNF-α, IFN-γ, and IL-17) significantly enhanced the immunosuppressive effects of the cells both in vitro and in a concanavalin A-induced hepatitis mouse model.[288] IL-17 is essential for host defense against infections and has become a known immune mediator in sepsis pathophysiology.[289,290] Thus, the TNF-α/IFN-γ/IL-17 blend may be a more accurate recapitulation of the sepsis inflammatory milieu and may produce more potent EVs if used as a MSC priming cocktail.
Similar to the effects of cytokine priming, increasing evidence has shown that priming MSCs with TLR ligands enables the secretion of EVs with an amplified anti-inflammatory effect, often due to the ability to alter macrophage plasticity. Notably, reprogramming of macrophages is suggested to govern the therapeutic effects of MSCs in sepsis.[15] Priming MSCs with the TLR4 ligand LPS has been shown to provoke the secretion of EVs with an improved ability to alter the cytokine profile of stimulated macrophages (i.e., increase IL-10 while decreasing IL-6, IL-1β, and TNF-α) and converting them to an anti-inflammatory M2 phenotype in vitro.[214,291] Ti et al. found that the M2 phenotype directive was largely mediated by EV-associated let-7b miRNA, a well-known immune and inflammatory regulator,[292–295] that operates via the TLR4/NF-κB/STAT3/AKT pathway.[214] This EV-induced enhancement on M2 macrophage polarization was also observed in vivo in a diabetic cutaneous wound model,[214] a myocardial infarction model,[291] and in an acute radiation syndrome model.[296] Priming MSCs with the TLR3 agonist poly (I:C) produced EVs that were able to direct LPS-stimulated monocytes to a more anti-inflammatory phenotype, as evidenced by a significant decrease in TNF-α and an increase in IL-10 secretion.[146] These same primed MSC EVs were shown to significantly reduce alveolar bacterial load in an E. coli-induced pneumonia model to a much greater extent than EVs from naïve MSCs.[146] In these studies it is interesting to note that when primed with poly (I:C) the MSCs themselves adopted an anti-inflammatory phenotype as indicated by an increase in IL-10 mRNA,[146] while priming with LPS induced the inflammatory response as demonstrated by an increased MSC expression of IL-6 and TNF-α.[291] However, in both cases the EVs derived from the MSCs were more potent immunosuppressors.[146,291] As there are conflicting reports as to which types of biochemical stimuli leads to which MSC phenotype,[278,280,281] detailed analyses and consistent reporting of the effects of priming on MSCs will be critical in not only understanding the immunomodulatory mechanisms of MSCs but also in informing the most efficient way to produce therapeutic EVs.
While most relevant priming investigations boast the production of EVs with enhanced anti-inflammatory, anti-apoptotic, or endothelial reparative effects, all promising characteristics for therapeutic efficacy in the acute phases of sepsis, it is important to remember the complexity of sepsis pathophysiology. As discussed previously, during sepsis the immune system over-compensates and eventually enters into a long-term state of immune paralysis, allowing secondary infections to form. Thus, for survivors, the physiological burden of the disease carries well beyond the point of hospital discharge during what is termed “post-sepsis syndrome.”[297] In addition to physical ailments, psychological and emotional sequelae (e.g., depression, anxiety, and cognitive decline) plague sepsis survivors for years after acute sepsis symptoms are resolved.[298] As an example, 25–50% of sepsis survivors experience significant cognitive decline.[298] In a murine model of Alzheimer’s disease, hypoxia priming of murine bone marrow derived MSCs created superior EVs that were capable of significantly improving spatial learning and memory impairments, increasing synaptic protein expression, while simultaneously alleviating the inflammatory response (i.e., decreasing pro-inflammatory cytokines while increasing anti-inflammatory cytokines and inhibiting NF-κB activation).[260] In another study, sodium hydrosulfide (NaHS), a precursor to the prevalent physiological signaling molecule hydrogen sulfide, was used as an MSC priming reagent.[299] When compared with naïve MSC EVs, NaHS priming resulted in EVs with a greater ability to blunt the gene expression of pro-inflammatory cytokines, reduce microglia/macrophage activation, and restore cognitive impairments in mice subjected to hypoxic-ischemic brain injury.[299] These studies demonstrate the potential for biochemical priming in the effective development of an EV therapy capable of spanning the entire sepsis timeline, from disease onset to secondary illness. With the pleiotropic effects of EVs, it will be important to look beyond the typical inflammatory models to help guide treatments for both sepsis patients and survivors.
By defining optimal molecular priming strategies for tailored and potent MSC-derived therapeutic EVs, biochemical priming research can potentially contribute to the development of an effective EV sepsis therapy. Nevertheless, disadvantages hinder the biochemical priming method and should be considered going forward. First, ligand characteristics (e.g., type, concentration, duration of exposure), cell origin (i.e., human or mouse), cell source (e.g., bone marrow, adipose, or umbilical cord derived MSCs), and culture conditions (e.g., medium) all vary considerably among studies which makes any beneficial effects difficult to characterize and reproduce.[278] Secondly, as the elicited effects of priming on the bioactivity of MSCs varies between donors,[300,301] it is highly likely this variability would be perceptible in the secreted EVs. Finally, but perhaps most importantly, these priming reagents can be expensive and may persist at low levels in the final EV product even after purification,[286] hampering adherence to good manufacturing practices (GMPs) and ultimately slowing movement into the clinic. Thus, the significance of this research may simply be a deeper understanding of the mechanisms that trigger the secretion of desired EVs and the intricate relationship between cell phenotype and EV function.
5.2.2. Biophysical Priming
In addition to the biochemical milieu, the aggregate structural and mechanical properties of the surrounding microenvironment and adjacent cells comprises a wide range of biophysical cues originating both endogenously (e.g., substrate dimensionality, topography, stiffness) and exogenously (e.g., shear stress, electromagnetic fields, tensile and compressive strain).[302,303] Research investigating the impact of these cues has contributed immensely to the fields of mechanotransduction and cell behavior and has the potential to bolster the translation of clinical therapies.[304] With such an overwhelming effect on cells, it should come as no surprise that biophysical cues have been shown to alter EV secretion dynamics and EV molecular cargoes.[305] Thus, in comparison to biochemical cues, biophysical cues demonstrate the capacity to modify downstream EV therapeutic efficacy without the addition of external molecules. Moreover, biophysical cues are often practical to implement, are clearly defined, and can be reproduced with consistent results at a large scale.[306]
One of the most noticeable advantages of implementing biophysical cues such as 3D into cell culture is the ability to more closely mimic the in vivo niche, permitting more physiologically relevant cell-to-cell and cell-to-extracellular matrix interactions.[307,308] In addition, a 3D context allows for the recapitulation of more natural gradients and diffusive transport of soluble factors.[302] Importantly, the implementation of 3D culture systems (e.g., bioreactors, microcarrier-based systems, spheroid culture) has been proven to markedly increase EV production when compared with cells cultured in a traditional 2D system (i.e., flask culture).[216,227,243,250] Strikingly, in one study it was estimated that 53 T175 culture flasks producing 800 mL of media would be needed to match the amount of EVs produced in a single collection from a hollow-fiber bioreactor system.[216] Furthermore, 3D culture has exhibited the ability to substantially boost the purity of EVs (i.e., ratio of number of EVs to μg of protein) when compared with their 2D counterparts.[229,250] As large doses of EVs are typically needed to achieve biological outcomes in mouse models (i.e., 109–1011 EVs per mouse) and purity can affect EV bioactivity,[243,309] 3D culture may prove integral in the clinical translation of an EV therapy in many disease applications, including sepsis.
Significantly, the effects of EVs can be conserved and translated from 2D to 3D culture systems where the EV therapeutic efficacy is often enhanced. EVs produced from 3D conditions have often demonstrated the ability to strengthen the angiogenic,[250] anti-inflammatory,[230] anti-apoptotic,[229] reparative, and regenerative (e.g., cognitive and osteochondral)[229,230,253] effects observed with EVs generated from 2D culture. As an example, Lamichhane et al. found that exposing human dermal microvascular endothelial cells (HDMECs) and human umbilical vein endothelial cells (HUVECs) to ethanol significantly enhanced EC-derived EV vascularization bioactivity (Figure 5). Moreover, they found that in HUVECs this effect was largely mediated by the upregulation of lncRNAs HOTAIR and MALAT1 (Figure 5).[249] The same research group was then able to recreate the ethanol-induced lncRNA EV cargo changes while enhancing pro-angiogenic abilities and yield of EVs by culturing HDMECs in a perfusion bioreactor (Figure 6).[250] Even so, it is critical to note that 3D culture can also transform the therapeutic profile of EVs. For instance, EVs from MSCs derived from human exfoliated deciduous teeth cultured on laminin-coated 3D alginate micro-carriers in a bioreactor were able to alleviate oxidative stress-induced apoptosis in human dopaminergic neurons, an effect that was not elicited by EVs isolated from the same cells grown under standard 2D conditions.[248] An increased anti-apoptotic effect could be important as sepsis-induced organ failure is associated with excess oxidative stress resulting in mitochondrial damage and ultimately cell death.[310] The observed shift in therapeutic effect may be due to the enhanced delivery of alternate molecular cargo, as 3D cell culture has been recognized to considerably modify the intraluminal contents of EVs as well as augment cellular uptake.[243] However, any change of this caliber during research and development would certainly hinder progress in the manufacturing pipeline. The study also portends the potential to overlook specific contributions of EVs to an efficient sepsis therapy during traditional preclinical research and emphasizes the importance of considering how the inevitable scale-up process (i.e., the shift in culture systems) may affect the therapeutic quality of EVs.
Figure 5.
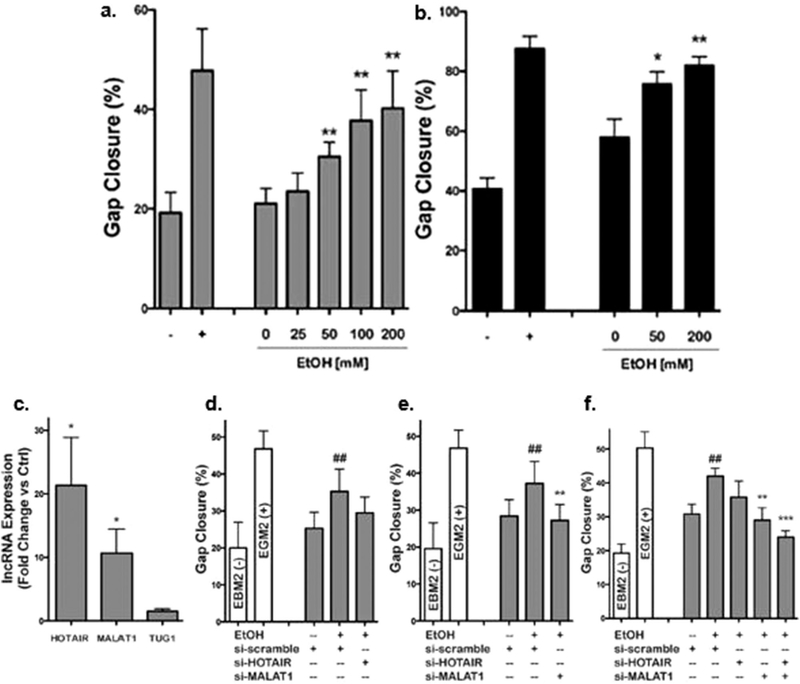
Priming of endothelial cells with ethanol (EtOH) augments EV-induced bioactivity through the regulation of EV-associated lncRNA. EVs derived from A) HUVECs and B) HDMECs stimulated with > 50 × 10−3 m EtOH significantly enhanced gap closure ability of their respective parental cells (n = 3, *p < 0.05, **p < 0.01 vs 0 × 10−3 m EtOH). C) HUVEC EVs from cells cultured in the presence or absence of 100 × 10−3 m EtOH displayed increased levels of the lncRNAs HOTAIR and MALAT1 (n = 3, *p < 0.05). The increased vascularization bioactivity of HUVEC EVs was reduced when D) HOTAIR, E) MALAT1, or F) both HOTAIR and MALAT1 were knocked down using siRNA (n = 4, ##p < 0.01 vs EtOH + scramble siRNA, **p < 0.01, ***p < 0.001 vs + EtOH + scramble siRNA). HUVECs maintained in basal medium (EBM2, without growth factors) were the negative controls and HUVECs maintained in growth medium (EGM2, with growth factors) were used as positive controls. Adapted under a Creative Commons Attribution 4.0 International License (CC BY 4.0).[249] Copyright 2017, The Authors, published by Springer Nature.
Figure 6.
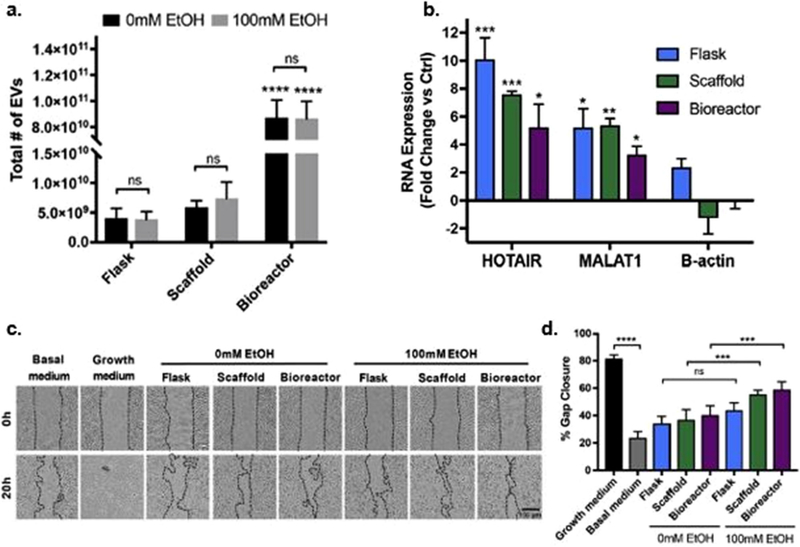
EV potency-enhancing mechanisms can be translated from 2D tissue culture to a scalable 3D culture system. A) Culture of HDMECs in a 3D scaffold with 4 mL min−1 media flow (i.e., bioreactor) significantly increased the total number of EVs generated. B) Priming HDMECs with 100 × 10−3 m EtOH for 24 h significantly increased HOTAIR and MALAT1 content in EV from HDMECs maintained in flasks, scaffolds, and the bioreactor. C,D) However, ethanol priming only enhanced vascularization bioactivity of the EVs derived from HDMECs cultured in the bioreactor. (ns, p > 0.05, *p < 0.05, **p < 0.01, ***p < 0.001, ****p < 0.0001). Adapted with permission.[250] Copyright 2018, Elsevier Ltd.
The 3D culture systems employed thus far have come equipped with tunable characteristics (e.g., stiffness, specific architecture) enabled by engineering advancements (e.g., 3D printing) that has allowed a reductionist approach in determining how biophysical cues are ultimately converted into intracellular signaling cascades. Although helpful, the adjustable components of these systems introduce a plethora of additional variables that need to be explored. For instance, some 3D culture techniques (e.g., perfusion bioreactor set-ups) involve possible effectors such as flow-induced shear stress. Mechanical stimulation instigated by flow and the subsequent effect on cells has been well-studied in a variety of contexts.[311,312] In one such study, Liu et al. revealed that by applying intermittent fluid shear stress to human bone marrow derived MSCs seeded in 3D poly lactic-co-glycolic acid (PLGA) scaffolds they were able to upregulate the activity of focal adhesion kinase (FAK).[313] In 2017, Lee et al. showed that FAK is a primary regulator of cyclooxygenase 2 (COX2) expression and the mechanical stimulation of FAK is crucial in triggering the immunosuppressive abilities of MSCs.[314] With the ability to provoke the immunomodulatory effects of MSCs, it will be critical to observe the effects of fluid shear stress on subsequently secreted EVs. As other properties of biomaterials (e.g., fiber alignment, roughness, surface microstructure) can enhance the immunomodulatory potential of cells, particularly MSCs,[315] the systematic dissection of the effects of biophysical cues on EVs will be pivotal in the translation of an EV-based sepsis therapy.
Beyond the direct enhancement of EV bioactivity, further exploration of the effects of biophysical cues on other issues in EV translation such as donor variability and storage will be imperative. Transcriptomic analysis has revealed that 3D culture of MSCs has the ability to reduce interdonor variability,[316] an avenue that has been left largely unexplored in EV research. Additionally, as the effects of primed MSCs have been shown to extend through cryopreservation,[317] it would be beneficial to evaluate the effects of storage on EVs from primed cell sources to thoroughly understand the translatability of these approaches. Notably, adjusting the biophysical cues experienced in cell culture can solve both EV potency and production issues. As cues do not occur in isolation in vivo, it is most likely going to be a collaboration of multiple biochemical and biophysical cues that guide cells to produce the most clinically relevant EV populations. However, continued careful and meticulous exploration of both biochemical and biophysical cues will ultimately inform a refined path to a potent and manufacturing friendly EV sepsis treatment.
5.3. Alternative Cell Sources
In order to transcend from the bench to the bedside, EVs must not only demonstrate a potent biological effect but also be conducive to reproducible and GMP-compliant protocols. Importantly, a major obstacle to this translation of EV therapeutics emerges from cell source constraints during ex vivo expansion.[318] For example, MSCs possess limited expansion capabilities before beginning to exhibit decreased proliferation and senescence.[319] Consequently, the number of EVs produced by a singular MSC donor source is finite and multiple derivations of MSCs from several donors are required to obtain relevant amounts of therapeutic EVs.[320] However, donor-to-donor variability (e.g., sex, age, health) can significantly alter EV therapeutic effects resulting in the inability to reliably reproduce desired effects.[321] Further confounding these issues are reports that MSC EVs can lose therapeutic potency when derived from higher passage cells.[240] These limitations are critical concerns when considering scale up and quality control for clinical translation and underline the need to consider alternative cell sources.
5.3.1. Pluripotent Stem Cells
Due to their augmented differentiation potential, pluripotent stem cells are an established and powerful platform in the fields of gene therapy, drug discovery, and disease modeling.[322,323] In its initial stages this research was primarily centered on human ESCs.[324] Recently, EVs derived from ESCs have been implicated in restorative processes during disease or injury. For instance, during pathological processes, ESC EVs have been shown to promote angiogenesis,[325,326] ameliorate the inflammatory response,[327] and provide antioxidant effects.[328] Along with their ability to contribute therapeutic benefits that may be beneficial for sepsis treatment, ESCs also possess indefinite ex vivo expansion capacity (i.e., unlimited self-renewal),[329] making them an attractive candidate as the optimal EV production factory. However, ESCs are derived from the inner cell mass of a blastocyst and thus isolation traditionally requires the destruction of an early-stage embryo or the use of human oocytes, placing the utilization of ESCs at the center of an on-going ethical debate.[330,331] In a ground-breaking study in 2007 by Takahashi et al., iPSCs were generated from human dermal fibroblasts and were demonstrated to effectively mimic ESCs in proliferation rate, molecular markers, and pluripotency status.[332] As they can be derived from easily accessible cell types (e.g., blood cells, keratinocytes, fibroblasts), iPSCs bypass the ethical dilemma of ESCs.[333] Moreover, iPSCs can be generated from a patient’s own cells and thus overcome the immunological limitations of ESCs.[333] When compared with the transplantation of other therapeutic stem cells, particularly tissue derived MSCs, iPSCs alleviate the issues of restricted expandability and the considerable effects of donor variation.[334] Additionally, with the ability to generate ≈16-fold more EVs in culture than various types of MSCs, iPSCs may be considered a superior EV source.[335]
In addition to being potential powerhouses for EV production, iPSCs hold promise as a source for an EV-sepsis therapy as demonstrated by their innate anti-apoptotic, antioxidant, and anti-inflammatory activity as well as their ability to attenuate endothelial leakage.[336–339] As EVs are often considered the main paracrine mechanism for cell activity, these characteristics of iPSCs has spurred important, albeit sparse, research on the therapeutic effects of iPSC derived EVs. For instance, iPSC EVs were able to reduce the development of fibrosis and hepatic stellate cell activation in the livers of two murine models of liver injury (i.e., carbon tetrachloride and bile duct ligation).[340] This is significant as hepatic dysfunction can occur early in sepsis and has a significant effect on sepsis severity due to the liver’s critical role in diverse physiological functions including detoxification, coagulation, energy production, and immunological homeostasis.[341] In another study, it was shown that iPSC EVs enter endothelial cells (i.e., HUVECs) more efficiently than their MSC derived counterparts.[335] Endothelial barrier repair strategies are innovative approaches to the treatment of sepsis and, with their improved access to endothelial cells, iPSC EVs may prove to be effective in these attempts to reestablish endothelial barrier function. In the same study, iPSC EVs were found to be enriched with two members of the peroxiredoxin family (i.e., PRDX1 and PRDX2).[335] PRDXs are enzymatic scavengers of intracellular H2O2, a powerful oxidant that, when excessively produced, leads to oxidative stress, a hallmark of sepsis pathogenesis. In particular, PRDX2 has been shown to reduce NF-κB activation in LPS-stimulated macrophages in addition to rescuing mice from LPS-induced lethal shock.[342]
Other cargoes specific to iPSC EVs have a potentially powerful value in sepsis therapies. In particular, global miRNA profiling by Adamiak et al. revealed that iPSC EVs were enriched in numerous miRNAs including let-7b-5p, miR-145, and miR-302a-5p,[343] all of which have significant mitigating effects on the inflammatory response and could prove useful in an EV-sepsis treatment. Specifically, it has been shown that let-7b-5p has the ability to downregulate the production of IL-6 in stimulated macrophages and decrease the expression of TLR4 and NF-κB.[293,344] Additionally, in a murine model of sepsis-induced lung injury, Cao et al. demonstrated that miR-145 agomir treatment significantly prolonged survival.[345] And, interestingly, miR-302a has been shown to suppress the expression of interferon regulatory factor-5 (IRF-5), a master transcription factor for the activation of genes for inflammatory cytokines, and was consequently able to temper the cytokine storm instigated by influenza A virus infection.[346] The downregulation of IRF-5 has been shown to inhibit the expression of inducible nitric oxide synthase (iNOS), IL-6, and TNF-α while significantly increasing survivorship in a bacterial septic peritonitis mouse model.[347] The fact that iPSC EVs are innately enriched with these immunomodulatory cargoes lends support to their use as a powerful tool in sepsis treatment regimens.
Although EVs from undifferentiated iPSCs show the capacity to become a potent sepsis treatment, their capacity to be differentiated into all varieties of somatic cells, a source of their value in cell-based therapies may also be the key to their success in producing a translatable EV-based therapy. Indeed, EVs from iPSC derived MSCs (iMSC EVs) were able to significantly reduce renal injury scores in a rat I/R model.[348] In the same study, iMSC EVs inhibited hypoxia-induced necroptosis in renal tubular cells via the delivery of specificity protein 1 (SP1).[348] Necroptosis is a regulated form of necrosis that is mediated by inflammation, cellular stress, and infection and consequently plays a pivotal role in numerous human diseases including sepsis.[349] Notably, in a neonatal rat sepsis model, inhibiting necroptosis decreased expression of inflammatory chemokines as well as attenuated lung injury and improved survival.[350] In a separate study, reducing necroptosis using the upstream effector miR-425–5p mitigated serum levels of pro-inflammatory cytokines (i.e., IL-1β and TNF-α) as well as significantly alleviated liver injury in an LPS-induced murine sepsis model.[351]
Additionally, the functions that EVs are able to provoke within their target cells generally depends on their cell of origin,[352,353] emphasizing the potential importance of differentiating iPSCs prior to use as an EV source. Compared with their tissue derived equivalents, EVs from differentiated iPSCs can display augmented therapeutic effects. For instance, when compared with synovial membrane MSCs, iMSC EVs were more effective at reducing disease severity in a collagen-induced osteoarthritis mouse model and increased both chondrocyte proliferation and motility in vitro.[41] Specifying the lineage of iPSCs prior to EV isolation may also be beneficial as several types of EVs can be isolated from a singular donor. Utilizing a murine thromboembolic stroke model, Webb et al. compared EVs derived from neural stem cells (NSCs) and MSCs that were differentiated from a single iPSC line.[354] While MSC EVs had no significant effects, NSC EV treatments were able to rescue neurological deficit scores as well as promote anti-inflammatory macrophage polarization and increase the population of circulating immunosuppressive T-regulatory cells.[354] This unique approach allowed the direct comparison of two EV sources while eliminating the effect of donor variability. Consequently, with their complex biological nature, iPSCs may allow better control and accuracy when screening EVs for translatable therapeutic potential in sepsis.
5.3.2. Platelets
Platelets are circulating cells derived from megakaryocytes that, despite lacking a nucleus, are capable of RNA processing as well as de novo protein synthesis.[355–357] They are highly responsive to vascular trauma, inflammation, and microbial colonization and therefore play critical roles in wound healing, hemostasis, and antimicrobial host defense.[358,359] As platelets are ubiquitous in peripheral blood, they compose a particularly accessible source of EVs. Moreover, the EVs derived from platelets or platelet-rich plasma (pEVs) have demonstrated significance in numerous physiological processes such as coagulation, angiogenesis, inflammation, and immunoregulation and are consequently critical in many disease pathologies, including sepsis.[80,360–365]
More specific than accessibility and pervasive presence in biological processes are the molecular targets of pEVs which make them appealing as a possible sepsis therapeutic. For instance, during sepsis, disturbances in redox regulation and inflammation can cause unfolded or misfolded proteins to accumulate, effectively disrupting the homeostasis maintained by the endoplasmic reticulum (ER) and thereby inducing ER stress.[366] When this imbalance is perceived, the ER prompts an adaptive response known as the unfolded protein response (UPR) to reestablish equilibrium. However, ER stress during sepsis is often protracted, rendering the compensatory effects of the UPR ineffective leading to the initiation of apoptosis. Consequently, the inhibition of ER stress has been recognized as a viable therapeutic target for sepsis with the demonstrated ability to attenuate apoptosis as well as inflammation in both LPS and CLP murine sepsis models.[367–370] In a study by Tao et al., pEVs were able to prevent apoptosis during in vitro and in vivo glucocorticoid-associated ER stress by activating the protein kinase B pathway and upregulating the expression of the anti-apoptotic protein B-cell lymphoma 2 (Bcl-2).[363] As widespread lymphocyte apoptosis is known to induce the immunosuppressive phase of sepsis,[371] the anti-apoptotic effects displayed by pEVs may prove valuable. Furthermore, the prolonged activation of endothelial cells during sepsis enables the excessive infiltration of neutrophils which contributes to hyperinflammation, tissue damage, and organ dysfunction. Recently, Lv et al. showed that a transcriptional coactivator known as the yes-associated protein (YAP) is essential in limiting endothelial activation during sepsis.[372] Utilizing two murine models of sepsis (i.e., LPS and CLP) it was shown that knocking YAP out in endothelial cells significantly increased mortality and the inflammatory response.[372] Importantly, treatment with pEVs has been shown to significantly increase YAP activity in human fibroblasts.[373] As YAP functions by degrading the TLR signaling adaptor TNF receptor-associated factor 6 (TRAF6) and thus constraining NF-κB activation,[372] augmenting YAP via pEVs may help regulate the inflammatory response during sepsis.
Additionally, considering the innate cargoes of pEVs may prove advantageous during the design of a sepsis intervention. It is known that platelets themselves are highly enriched in various miRNAs including miR-126 and miR-223,[374] both of which have been implicated in the progression of sepsis. For instance, in septic patients, the downregulation of serum miR-126–3p was shown to be correlated with the severity of sepsis symptoms in addition to markers of systemic inflammation, bacterial infection, as well as kidney and liver dysfunction.[375] miR-126 is known to regulate vascular integrity with genomic knockout of miR-126 leading to increased vascular permeability and a decrease in endothelial recovery following vascular injury.[376–378] In addition, miR-223 has been observed to be significantly reduced in septic patients when compared with healthy controls.[103] Upon sepsis challenge via CLP, miR-223 knockout mice experienced exacerbated myocardial inflammation and dysfunction as well as increased mortality when compared with wild type mice.[379] Importantly, it has been shown that both miR-126 and −223 are packaged into pEVs and can be delivered to recipient cells.[380–382] While investigating endothelial damage in atherosclerosis, Li et al. were able to significantly reduce the expression of ICAM-1 in TNF-α-stimulated HUVECs by pretreating the cells with EVs derived from thrombin-activated platelets.[381] Following the addition of a miR-223 inhibitor during treatment with pEVs, the effect of decreased ICAM-1 expression in HUVECs was lost, suggesting an essential role for pEV-associated miR-223.[381] Similarly, miR-126 has exhibited the ability to suppress LPS-stimulated expression of both VCAM-1 and ICAM-1 in HUVECs.[109] Notably, the expression of miR-126 within HUVECs can be enhanced via treatment with pEVs.[382] As elevated levels of ICAM-1 expression have been shown to cause endothelial cell barrier dysfunction,[383] the downregulation of adhesion molecules via pEVs may be helpful in a sepsis treatment. This is further reinforced by a study by Zhao et al. in which blocking the expression of ICAM-1 significantly increased survival and bacterial clearance while reducing apoptosis in both the spleen and thymus of a murine sepsis model.[384] More recently, Zhang et al. demonstrated that EVs loaded with miR-126 were able to increase cardiac function in septic mice by inhibiting VCAM-1 and ICAM-1 expression.[109] Collectively, these studies emphasize the potential mechanisms through which pEVs could be used to help mitigate and possibly resolve the symptoms of sepsis.
5.4. Artificial EVs
The barriers to the clinical translation of EV therapeutics (e.g., low EV production, inefficient isolation and purification procedures, and heterogeneous EV subpopulations) have triggered attempts to develop therapeutically relevant artificial EVs. Artificial EVs can be classified as “semi-synthetic” (i.e., the modification of naturally released EVs) or “fully synthetic” (i.e., the generation of plasma membrane fragments or decoration of artificial membranes).[385] While natural EVs have been extensively engineered (i.e., via cargo loading, alteration of membrane composition), the potential of fully synthetic EVs in sepsis treatment, specifically those generated by plasma membrane fragments, deserves consideration (Figure 4). Current methods of membrane fragmentation include serial extrusion through nanosized polycarbonate membrane filters or hydrophilic microchannels, sonication, nitrogen cavitation, or cell slicing using silicon nitride blades.[385–387] When compared with artificial materials (i.e., liposomes, nanoparticles), the production of EVs via cell fragmentation does not require the addition of organic solvents and synthetic materials and has a relatively lower propensity to induce immunogenicity.[388,389] Artificial EVs can also be produced via chemically induced membrane blebbing.[390] These methods are often quick and simple, making them superior to semisynthetic production techniques as the generation of a therapeutically relevant number of naturally derived EVs is often time-consuming and laborious.[243] Moreover, cell fragment derived EVs more closely resemble parental cells than their natural counterparts,[391] allowing for the possibility of tighter control over the final product during biomanufacturing. Importantly, cell fragmentation techniques can increase the particle yield by more than 200-fold,[386,392] demonstrating the scalability of the technique, while maintaining the biophysical and biochemical composition (i.e., size, membrane markers, and surface charge) of naturally derived EVs.[386,393,394] This allows the vesicles to retain the ability to target specific tissues and replicate complex intercellular interactions, a nearly impossible feat for liposomes and nanoparticles. Finally, these artificial EVs often harbor a reduced amount of undesirable luminal cargoes, increasing their compatibility for drug development protocols while also lessening any off-target effects.[386,394]
Research involving artificial EVs sourced from cell fragments has recently grown, with a majority of the studies being performed for anti-cancer applications.[395–399] However, similar to their natural equivalents, these artificial EVs also possess therapeutic promise in inflammatory-based diseases such as sepsis. In a study by Go et al., EVs were derived from the membrane fragments of human monocytes (U937 cells) using an alkaline solution partnered with sonication. The vesicles were then loaded with the anti-inflammatory drug dexamethasone which resulted in the significant reduction of IL-8 secretion in stimulated HUVECs in vitro and reduced symptoms (i.e., hypothermia, weight loss) as well as increased survival in an E. coli-induced lethal systemic inflammatory response syndrome (SIRS) mouse model.[386] These effects were shown to be more significant and less toxic than those elicited by the application of free dexamethasone.[386] Notably, the dexamethasone-loading capacity was 2.1 times greater than that of extrusion-based artificial EVs, possibly due to the relatively lower amount of unwanted cytosolic cargoes.[386] Another study by Gao et al. utilized nitrogen cavitation to obtain artificial EVs from neutrophils and subsequently loaded them with piceatannol, a polyphenolic compound known for its anti-inflammatory properties.[394] In comparison with free piceatannol, the loaded EVs were able to significantly decrease TNF-α, IL-6, and IL-1β levels in lung lavage fluid, reduce neutrophil infiltration in the lungs, kidneys, and liver, and increase survival in an LPS-induced model of ALI by reducing the activation of the NF-κB pathway (Figure 7).[394] In 2019, Park et al. derived bone marrow MSC artificial EVs using serial extrusion and also demonstrated significant immunomodulatory effects (i.e., reduced leukocyte infiltration and levels of inflammatory cytokines) in an E. coli-induced murine sepsis model.[400] Superior production efficiency coupled with verified immunomodulatory capabilities warrants continued exploration of artificial EVs as a viable and translatable therapeutic alternative for sepsis.
Figure 7.
Piceatannol-loaded neutrophil EVs artificially generated using nitrogen cavitation (pic-NC-EVs) are more potent than free piceatannol (pic) in an LPS-induced sepsis model. A) Septic mouse survival after the intravenous administration of piceatannol and pic-NC-EVs. Statistical analysis was done using Kaplan–Meier method (n = 9). B) TNF-α, C) IL-6, and D) IL-1β in the blood plasma 4 and 8 h after administration of LPS (22 mg kg−1). E) Neutrophil infiltration in mouse lung, liver, and kidney as evidenced by the presence of myeloperoxidase (MPO). All data are expressed as means ± SD (n = 3). *p < 0.05, **p < 0.01, and ***p < 0.001 compared to control (Student’s t-test) except when indicated. Adapted with permission.[394] Copyright 2017, Elsevier Ltd.
6. Clinical Connection
The study of EVs during sepsis has revealed their dual potential as both an interventional therapy and diagnostic means for sepsis. However, the necessary tools and knowledge needed for implementation of an EV sepsis therapy at the bedside remain underdeveloped. This lack of information is preceded by the nature of EV clinical research on sepsis in general. So far, EV-related clinical studies in sepsis are diagnostic or observational in nature and are performed in patient populations encompassing numerous infectious etiologies (e.g., urinary tract, wound, intraabdominal).[32] However, when patients are stratified according to site of infection, EV profiles have been shown to vary considerably. Lashin et al. demonstrated that EVs carrying the previously discussed A2MG were significantly upregulated in the plasma of patients with sepsis due to community acquired pneumonia when compared with those suffering from fecal peritonitis-induced sepsis or healthy volunteers.[122] This upregulation is significant as A2MG has been shown to increase neutrophil antibacterial activity and modulate inflammatory events through the sequestering of select cytokines (TNF-α, IL-6). In addition to its anti-inflammatory effect, A2MG may pose as a powerful sepsis therapeutic due to its ability to increase IFN-γ levels; an attribute that could help reverse the immunosuppressive state that plagues sepsis patients. Consequently, A2MG+ EVs have been associated with improved survival in human sepsis patients.[94] These results lend to the diagnostic power of EVs but also highlight the vastly different EV profiles of sepsis patients. Similar to what has been observed with distinct infection etiologies, EV characteristics (cargo, cell of origin, surface markers) can also fluctuate with age, gender, as well as underlying illnesses and injuries (e.g., trauma, cancer),[401,402] all of which are highly variable factors among septic patients. The value of clinical trials utilizing a diverse patient base lies in the ability to generalize to the typical heterogeneous ICU septic patient population. Nevertheless, given the significant differences among septic patient sub-groups, using this approach in an EV interventional study may conceal the EV mechanisms at work, as well as any benefits or detriments the EV therapy may generate. EV clinical studies utilizing more robust stratification strategies will be needed to accurately determine the patient population most responsive to a specific EV therapy. Additionally, just as the nature of sepsis oscillates (i.e., between inflammatory and immunosuppressive) throughout the course of the disease, the cargo and function of EVs can change as well.[96] Specifically, the profiles of EV-associated cytokines, chemokines, and growth factors display various dynamics throughout sepsis. For instance, in a mouse model, EV-associated pro-inflammatory cytokines (e.g., IL-6, TNF-α, IL-1β) were shown to be elevated in the early phase (2 h post-induction), while EV-associated anti-inflammatory cytokines (e.g., IL-4 and IL-10) were upregulated at 24–48 h,[95] suggesting a powerful relationship between the course of the disease and EV make-up. Interestingly, Raeven et al. found that most clinical trials evaluating EVs in human patients sample EVs at only one or two time points and these time points often vary considerably across trials.[32] For example, some studies collect EVs at a single unspecified time point while others will sample on the day of admission, within 72 h of admission, or at the diagnosis of septic shock.[32] With the highly fluctuating nature of both sepsis and EV profiles, these studies are most likely capturing different snapshots of the disease, hindering the ability to compare findings. The designation of these time points is further complicated by the continuously evolving definitions of sepsis and septic shock across the medical community.[403] Overall, more investigation into changing EV cargo and subsequent function throughout the course of human sepsis as well as the associated clinical outcomes is urgently needed.
While clinical studies utilizing EVs as an interventional therapy have yet to be conducted, the research investigating EVs as endogenous effectors and biomarkers has facilitated the discovery of new pathways of injury (i.e., RNA vs protein pathways) as well as potential therapeutic strategies (e.g., alteration of endogenous EVs or the use of exogenous EVs).[404] Notably, as sepsis biomarkers, EVs could be used to dictate the initiation of different disease management strategies (e.g., appropriate antibiotic regimens or discontinuance of invasive or extracorporeal treatments).[32] As an example, the upregulation of circulating EVs carrying PS and tissue factor (TF) has been identified as an early biomarker for the onset of disseminated intravascular coagulation (DIC),[80,405] a common and deadly complication of sepsis.[406] This information could indicate that, for a patient presenting with significantly increased TF- and PS-bearing EVs, an aggressive treatment with anti-coagulants may be the most appropriate treatment option. Additionally, EV cargo can determine disease severity, with septic shock patients displaying an increased number of EVs shuttling de novo DNA methylating factors compared with those derived from sepsis patients.[96] The ability to accurately delineate sepsis from septic shock could help restrict the use of more intrusive tissue perfusion strategies (i.e., massive fluid resuscitation) to the more severely ill. This is essential as the indiscriminate use of fluid resuscitation can cause widespread tissue edema resulting in significant harm to end-organ function.[407]
As a rapid diagnosis and treatment implementation is critical to therapeutic success in sepsis, these EV biomarkers would have to be analyzed quickly. Thus, weaknesses in EV isolation and characterization technologies need to be addressed before clinical use is feasible. The current gold-standard method of isolation (i.e., ultracentrifugation) requires expensive equipment and long processing times and is not conducive to a hospital setting. While emerging techniques are becoming available, further development is still needed. For example, the method known as the aqueous two-phase system (ATPS) is able to isolate EVs from biofluids within 30 min at yields comparable to ultracentrifugation,[408,409] but the resulting EV sample is co-isolated with dextran, which could affect downstream analytical techniques.[409] As for characterization, high-throughput methods currently rely on flow cytometry set ups that are expensive, difficult-to-operate, and possess a detection limit below which a majority of EVs reside (i.e., 0.3 μm).[32] The characterization of EVs is further complicated due to the fact that many EVs within the same subpopulation do not carry the same amount or type of cargo.[186] To truly harness the diagnostic power of EVs, a low-cost and easy-to-use device capable of correlating surface markers of singular EVs with encapsulated cargo would need to be developed. EV characterization would also greatly benefit from the development of methods aimed at differentiating EV-associated and Argonaute protein-associated miRNA, a demarcation that is often not addressed in EV cargo studies.[32]
With regard to an actual EV-based sepsis intervention, research conducted thus far suggests that a combinatorial therapy will likely be the most effective strategy. For example, multiple cargoes found within MSC EVs (i.e., KGF and Ang-1 mRNA; miR-146a, −145, −223) (Table 1) have been individually implicated as the partial mechanisms underlying the observed protective effects in sepsis models. Moreover, the knockdown of these individual cargoes does not fully inhibit the beneficial effects instigated by the EVs. Notably, miRNAs are known to work together as a regulatory system to ensure precise physiological responses.[410,411] For instance, Mann et al. demonstrated a combined positive and negative feedback network governed by the interplay between miR-146a and miR-155 that effectively controlled the activity of the NF-κB pathway in murine macrophages.[410] It was demonstrated that this miRNA interaction was essential in enabling an effective yet controlled inflammatory response crucial for functional immunity. Thus, the loading of synergistic cargoes into EVs would enable a more dynamic effect that does not simply counteract a singular phenomenon (e.g., hyperinflammation) but may be able to change with the progression of the disease and allow the restoration of homeostasis. An attempt to create this network of protective mechanisms within an EV vehicle could use either multiple types of cargoes (e.g., mRNA and miRNA) or those with various functions (e.g., anti-inflammatory and anti-apoptotic miRNAs), both of which could prove to be an effective means to a formidable sepsis therapy.
Similar to EV biomarkers, isolation methods for EVs therapeutics will need to be optimized prior to use in human septic patients. Variable isolation methods have been shown to result in EV populations with significantly different membrane markers, RNA cargo,[412,413] purity,[414] and bioactivity.[415] Recently, it was shown that isolation artifacts can even lead to misinterpretation of observed bioactivity as being exclusively dependent on EVs.[416] Other variable factors that present during clinical trials will need to be meticulously investigated. For instance, from the preclinical research discussed extensively in this review (Table 1), it is clear that dose effects and the schedule and route of administration have not been standardized or optimized to maximize therapeutic effects. Emphasizing the critical need for this information is research demonstrating that the route of administration (e.g., intraperitoneal, subcutaneous, intravenous injection) and dose of EVs can significantly affect EV biodistribution, and therefore therapeutic effectiveness.[417] Thus, while isolation and delivery are downstream procedures in the development process, it may be critically beneficial to consider the possible effects of the chosen method much earlier in the course of therapeutic development.
7. Conclusions
From the relevant prior research discussed in this review it is evident that EVs, from a multitude of different sources, whether artificial or natural, are poised for development into a powerful remedy for sepsis and the chronic diseases it initiates. As with all complex biologics, clinical translation of an EV sepsis therapy faces multiple barriers due to issues involving low potency, low yield, and complex heterogeneity that is compounded by the current processing techniques available (i.e., isolation and characterization methods). The research discussed presently has not only demonstrated the potential of EVs in sepsis treatment but has also laid the foundation for emerging techniques required to control and standardize the EV biophysical and biochemical profile to ultimately overcome these obstacles. Advances in the ability to finely tune EV populations also create promise in tailoring EV therapies to the specific features of sepsis that often present differently in each human patient. While the idea of personalized medicine has been around for decades,[418] this approach, with the revelation that septic patients are much more heterogeneous than previously thought, has just recently been recognized as needed in sepsis care.[419,420] EVs, with their malleable characteristics and option for autologous sourcing, may be the focal point for an effective and personalized sepsis care regimen in the near future. Regardless of the promising outlook, there is still much research needed to push a successful EV sepsis treatment into the clinic and, ultimately, onto the market. Overall, the information presented in this review indicates that the development of a future EV sepsis therapy will require a balance between unraveling the intricacies of EV biology in regard to the septic pathological environment and the careful refinement of process engineering and clinical trial development.
Acknowledgements
This work was supported by the National Institutes of Health (HL141611, NS110637, GM130923, HL141922 to S.M.J.; AI089621 to S.M.K. and A.E.P.; GM117233, GM122908, NS110567 to W.C) and the National Science Foundation (1750542 to S.M.J.). Additionally, D.L. was supported by a Clark Doctoral Fellowship from the University of Maryland.
Biography

Stephanie M. Kronstadt received her B.S. and M.S. in Marine Biology at the Florida Institute of Technology. In 2018, she joined Dr. Steven Jay’s Biotherapeutic Development and Delivery Laboratory as a Ph.D. student at the University of Maryland in College Park. She is currently working to harness the effects of biophysical cues in the cellular microenvironment in order to enhance the production, therapeutic effects, and translation of extracellular vesicle therapies.
Footnotes
Conflict of Interest
The authors declare no conflict of interest.
Contributor Information
Stephanie M. Kronstadt, Fischell Department of Bioengineering, University of Maryland, 3102 A. James Clark Hall, College Park, MD 20742, USA
Alex E. Pottash, Fischell Department of Bioengineering, University of Maryland, 3102 A. James Clark Hall, College Park, MD 20742, USA
Daniel Levy, Fischell Department of Bioengineering, University of Maryland, 3102 A. James Clark Hall, College Park, MD 20742, USA.
Sheng Wang, Translational Research Program, Department of Anesthesiology and Center for Shock Trauma and Anesthesiology Research, University of Maryland School of Medicine, Baltimore, MD 21201, USA.
Wei Chao, Translational Research Program, Department of Anesthesiology and Center for Shock Trauma and Anesthesiology Research, University of Maryland School of Medicine, Baltimore, MD 21201, USA.
Steven M. Jay, Fischell Department of Bioengineering and Program in Molecular and, Cell Biology, University of Maryland, 3102 A. James Clark Hall, College Park, MD 20742, USA
References
- [1].van der Poll T, van de Veerdonk FL, Scicluna BP, Netea MG, Nat. Rev. Immunol. 2017, 17, 407. [DOI] [PubMed] [Google Scholar]
- [2].Opal SM, van der Poll T, J. Intern. Med. 2015, 277, 277. [DOI] [PubMed] [Google Scholar]
- [3].Mira JC, Gentile LF, Mathias BJ, Efron PA, Brakenridge SC, Mohr AM, Moore FA, Moldawer LL, Crit. Care Med. 2017, 45, 253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Boomer JS, Green JM, Hotchkiss RS, Virulence 2014, 5, 45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Rhodes A, Evans LE, Alhazzani W, Levy MM, Antonelli M, Ferrer R, Kumar A, Sevransky JE, Sprung CL, Nunnally ME, Rochwerg B, Rubenfeld GD, Angus DC, Annane D, Beale RJ, Bellinghan GJ, Bernard GR, Chiche JD, Coopersmith C, De Backer DP, French CJ, Fujishima S, Gerlach H, Hidalgo JL, Hollenberg SM, Jones AE, Karnad DR, Kleinpell RM, Koh Y, Lisboa TC, et al. , Intensive Care Med. 2017, 43, 304. [DOI] [PubMed] [Google Scholar]
- [6].Rudd KE, Johnson SC, Agesa KM, Shackelford KA, Tsoi D, Kievlan DR, Colombara DV, Ikuta KS, Kissoon N, Finfer S, Fleischmann-Struzek C, Machado FR, Reinhart KK, Rowan K, Seymour CW, Watson RS, West TE, Marinho F, Hay SI, Lozano R, Lopez AD, Angus DC, Murray CJL, Naghavi M, Lancet 2020, 395, 200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Cohen J, Opal S, Calandra T, Lancet Infect. Dis. 2012, 12, 503. [DOI] [PubMed] [Google Scholar]
- [8].Buzhor E, Leshansky L, Blumenthal J, Barash H, Warshawsky D, Mazor Y, Shtrichman R, Regener. Med. 2014, 9, 649. [DOI] [PubMed] [Google Scholar]
- [9].Lombardo E, van der Poll T, DelaRosa O, Dalemans W, World J Stem Cells 2015, 7, 368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Matthay MA, Pati S, Lee JW, Stem Cells 2017, 35, 316. [DOI] [PubMed] [Google Scholar]
- [11].Horak J, Nalos L, Martinkova V, Benes J, Stengl M, Matejovic M, Stem Cells Int. 2017, 2017, 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Laroye C, Gibot S, Reppel L, Bensoussan D, Stem Cells 2017, 35, 2331. [DOI] [PubMed] [Google Scholar]
- [13].Guillamat-Prats R, Camprubi-Rimblas M, Bringue J, Tantinya N, Artigas A, Ann. Transl. Med. 2017, 5, 446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Vincent JL, De Backer D, Crit. Care 2005, 9, S9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Nemeth K, Leelahavanichkul A, Yuen PS, Mayer B, Parmelee A, Doi K, Robey PG, Leelahavanichkul K, Koller BH, Brown JM, Hu X, Jelinek I, Star RA, Mezey E, Nat. Med. 2009, 15, 42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Condor JM, Rodrigues CE, Sousa Moreira R, Canale D, Volpini RA, Shimizu MH, Camara NO, Noronha Ide L, Andrade L, Stem Cells Transl. Med. 2016, 5, 1048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Huang W, Fan W, Wang Y, Han D, Li X, Li S, Li C, Xu B, Huang Y, Fu X, Cao F, Cell Death Discovery 2017, 3, 16097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Saeedi P, Halabian R, Fooladi AAI, Cytotherapy 2019, 21, 41. [DOI] [PubMed] [Google Scholar]
- [19].Ahn SY, Maeng YS, Kim YR, Choe YH, Hwang HS, Hyun YM, Stem Cell Res. Ther. 2020, 11, 44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Galipeau J, Sensebe L, Cell Stem Cell 2018, 22, 824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Musial-Wysocka A, Kot M, Majka M, Cell Transplant. 2019, 28, 801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Ferreira JR, Teixeira GQ, Santos SG, Barbosa MA, Almeida-Porada G, Goncalves RM, Front. Immunol. 2018, 9, 2837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Wysoczynski M, Khan A, Bolli R, Circ. Res. 2018, 123, 138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Thery C, Witwer KW, Aikawa E, Alcaraz MJ, Anderson JD, Andriantsitohaina R, Antoniou A, Arab T, Archer F, Atkin-Smith GK, Ayre DC, Bach JM, Bachurski D, Baharvand H, Balaj L, Baldacchino S, Bauer NN, Baxter AA, Bebawy M, Beckham C, Bedina Zavec A, Benmoussa A, Berardi AC, Bergese P, Bielska E, Blenkiron C, Bobis-Wozowicz S, Boilard E, Boireau W, Bongiovanni A, et al. , J. Extracell. Vesicles 2018, 7, 1535750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].El Andaloussi S, Mäger I, Breakefield XO, Wood MJA, Nat. Rev. Drug Discovery 2013, 12, 347. [DOI] [PubMed] [Google Scholar]
- [26].Huang-Doran I, Zhang CY, Vidal-Puig A, Trends Endocrinol. Metab. 2017, 28, 3. [DOI] [PubMed] [Google Scholar]
- [27].van Niel G, D’Angelo G, Raposo G, Nat. Rev. Mol. Cell Biol. 2018, 19, 213. [DOI] [PubMed] [Google Scholar]
- [28].Valadi H, Ekstrom K, Bossios A, Sjostrand M, Lee JJ, Lotvall JO, Nat. Cell Biol. 2007, 9, 654. [DOI] [PubMed] [Google Scholar]
- [29].Shah R, Patel T, Freedman JE, Engl N. J. Med. 2018, 379, 958. [DOI] [PubMed] [Google Scholar]
- [30].Robbins PD, Morelli AE, Nat. Rev. Immunol. 2014, 14, 195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Chulpanova DS, Kitaeva KV, James V, Rizvanov AA, Solovyeva VV, Front. Immunol. 2018, 9, 1534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Raeven P, Zipperle J, Drechsler S, Theranostics 2018, 8, 3348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Chong SY, Lee CK, Huang C, Ou YH, Charles CJ, Richards AM, Neupane YR, Pavon MV, Zharkova O, Pastorin G, Wang JW, Int. J. Mol. Sci. 2019, 20, 3272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Xu H, Jia S, Xu H, Clin. Immunol. 2019, 205, 116. [DOI] [PubMed] [Google Scholar]
- [35].Belov L, Matic KJ, Hallal S, Best OG, Mulligan SP, Christopherson RI, Extracell J. Vesicles 2016, 5, 25355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Hough KP, Wilson LS, Trevor JL, Strenkowski JG, Maina N, Kim YI, Spell ML, Wang Y, Chanda D, Dager JR, Sharma NS, Curtiss M, Antony VB, Dransfield MT, Chaplin DD, Steele C, Barnes S, Duncan SR, Prasain JK, Thannickal VJ, Deshane JS, Sci. Rep. 2018, 8, 10340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Margolis L, Sadovsky Y, PLoS Biol. 2019, 17, e3000363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Alvarez-Erviti L, Seow Y, Yin H, Betts C, Lakhal S, Wood MJ, Nat. Biotechnol. 2011, 29, 341. [DOI] [PubMed] [Google Scholar]
- [39].Liu Y, Li D, Liu Z, Zhou Y, Chu D, Li X, Jiang X, Hou D, Chen X, Chen Y, Yang Z, Jin L, Jiang W, Tian C, Zhou G, Zen K, Zhang J, Zhang Y, Li J, Zhang CY, Sci. Rep. 2015, 5, 17543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Gupta R, Radicioni G, Abdelwahab S, Dang H, Carpenter J, Chua M, Mieczkowski PA, Sheridan JT, Randell SH, Kesimer M, Am. J. Respir. Cell Mol. Biol. 2019, 60, 209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Zhu Y, Wang Y, Zhao B, Niu X, Hu B, Li Q, Zhang J, Ding J, Chen Y, Wang Y, Stem Cell Res. Ther. 2017, 8, 64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Elsharkasy OM, Nordin JZ, Hagey DW, de Jong OG, Schiffelers RM, Andaloussi SE, Vader P, Adv. Drug Delivery Rev. 2020, 159, 332. [DOI] [PubMed] [Google Scholar]
- [43].Chen J, Gu X, Zhou L, Wang S, Zhu L, Huang Y, Cao F, Exp. Ther. Med. 2019, 18, 3959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Gong Y, Zou L, Cen D, Chao W, Chen D, Inflammation 2016, 39, 1930. [DOI] [PubMed] [Google Scholar]
- [45].Foley NM, Wang J, Redmond HP, Wang JH, Mil. Med. Res. 2015, 2, 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Friedman RC, Farh KK, Burge CB, Bartel DP, Genome Res. 2009, 19, 92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Liu Z, Sall A, Yang D, Int. J. Mol. Sci. 2008, 9, 978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Essandoh K, Fan GC, Biochim. Biophys. Acta 2014, 1842, 2155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Olson EN, Sci. Transl. Med. 2014, 6, 239ps3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Wronska A, Kurkowska-Jastrzebska I, Santulli G, Acta Physiol. 2015, 213, 60. [DOI] [PubMed] [Google Scholar]
- [51].Yanez-Mo M, Siljander PR, Andreu Z, Zavec AB, Borras FE, Buzas EI, Buzas K, Casal E, Cappello F, Carvalho J, Colas E, Cordeiro-da Silva A, Fais S, Falcon-Perez JM, Ghobrial IM, Giebel B, Gimona M, Graner M, Gursel I, Gursel M, Heegaard NH, Hendrix A, Kierulf P, Kokubun K, Kosanovic M, KraljIglic V, Kramer-Albers EM, Laitinen S, Lasser C, Lener T, et al. , J. Extracell. Vesicles 2015, 4, 27066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].Turchinovich A, Drapkina O, Tonevitsky A, Front. Immunol. 2019, 10, 202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Wang X, Gu H, Qin D, Yang L, Huang W, Essandoh K, Wang Y, Caldwell CC, Peng T, Zingarelli B, Fan GC, Sci. Rep. 2015, 5, 13721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54].Song Y, Dou H, Li X, Zhao X, Li Y, Liu D, Ji J, Liu F, Ding L, Ni Y, Hou Y, Stem Cells 2017, 35, 1208. [DOI] [PubMed] [Google Scholar]
- [55].Zheng G, Huang R, Qiu G, Ge M, Wang J, Shu Q, Xu J, Cell Tissue Res. 2018, 374, 1. [DOI] [PubMed] [Google Scholar]
- [56].Zhou Y, Li P, Goodwin AJ, Cook JA, Halushka PV, Chang E, Fan H, Mol. Ther. 2018, 26, 1375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [57].Cavaillon JM, Singer M, Skirecki T, EMBO Mol. Med. 2020, 12, e10128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [58].Xiao W, Mindrinos MN, Seok J, Cuschieri J, Cuenca AG, Gao H, Hayden DL, Hennessy L, Moore EE, Minei JP, Bankey PE, Johnson JL, Sperry J, Nathens AB, Billiar TR, West MA, Brownstein BH, Mason PH, Baker HV, Finnerty CC, Jeschke MG, Lopez MC, Klein MB, Gamelli RL, Gibran NS, Arnoldo B, Xu W, Zhang Y, Calvano SE, McDonald-Smith GP, et al. , J. Exp. Med. 2011, 208, 2581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [59].Hsu WT, Galm BP, Schrank G, Hsu TC, Lee SH, Park JY, Lee CC, Hypertension 2020, 75, 483. [DOI] [PubMed] [Google Scholar]
- [60].Meziani F, Delabranche X, Asfar P, Toti F, Crit. Care 2010, 14, 236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [61].Woth G, Tokes-Fuzesi M, Magyarlaki T, Kovacs GL, Vermes I, Muhl D, Ann. Clin. Biochem. 2012, 49, 554. [DOI] [PubMed] [Google Scholar]
- [62].Dickhout A, Koenen RR, Front. Cardiovasc. Med. 2018, 5, 113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [63].Woith E, Fuhrmann G, Melzig MF, Int. J. Mol. Sci. 2019, 20, 5695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [64].Mastronardi ML, Mostefai HA, Meziani F, Martinez MC, Asfar P, Andriantsitohaina R, Crit. Care Med. 2011, 39, 1739. [DOI] [PubMed] [Google Scholar]
- [65].Prakash PS, Caldwell CC, Lentsch AB, Pritts TA, Robinson BR, Trauma J Acute Care Surg. 2012, 73, 401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [66].Balusu S, Van Wonterghem E, De Rycke R, Raemdonck K, Stremersch S, Gevaert K, Brkic M, Demeestere D, Vanhooren V, Hendrix A, Libert C, Vandenbroucke RE, EMBO Mol. Med. 2016, 8, 1162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [67].Johnson BL 3rd, Midura EF, Prakash PS, Rice TC, Kunz N, Kalies K, Caldwell CC, Biochim. Biophys. Acta Mol. Basis Dis. 2017, 1863, 2554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [68].Kojima M, Gimenes-Junior JA, Chan TW, Eliceiri BP, Baird A, Costantini TW, Coimbra R, FASEB J. 2018, 32, 97. [DOI] [PubMed] [Google Scholar]
- [69].Yuan Z, Bedi B, Sadikot RT, J. Vis. Exp. 2018, 135, 57737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [70].Jiang K, Yang J, Guo S, Zhao G, Wu H, Deng G, Mol. Ther. 2019, 27, 1758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [71].Wisler JR, Singh K, McCarty AR, Abouhashem ASE, Christman JW, Sen CK, Surg. Infect. 2020, 21, 101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [72].Li JJ, Wang B, Kodali MC, Chen C, Kim E, Patters BJ, Lan L, Kumar S, Wang X, Yue J, Liao FF, J. Neuroinflammation 2018, 15, 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [73].Li ZG, Scott MJ, Brzoska T, Sundd P, Li YH, Billiar TR, Wilson MA, Wang P, Fan J, Mil. Med. Res. 2018, 5, 24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [74].Rienk Nieuwland RJB, McGregor S, Boing AN, Romijn F. P. H. T.h. M., Westendorp RGJ, Erik Hack C, Sturk A, Blood 2000, 95, 930. [PubMed] [Google Scholar]
- [75].Delabranche X, Boisrame-Helms J, Asfar P, Berger A, Mootien Y, Lavigne T, Grunebaum L, Lanza F, Gachet C, Freyssinet JM, Toti F, Meziani F, Intensive Care Med. 2013, 39, 1695. [DOI] [PubMed] [Google Scholar]
- [76].Matsumoto H, Yamakawa K, Ogura H, Koh T, Matsumoto N, Shimazu T, Shock 2015, 43, 443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [77].Mooberry MJ, Bradford R, Hobl EL, Lin FC, Jilma B, Key NS, Thromb J. Haemostasis 2016, 14, 1031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [78].Wang Y, Luo L, Morgelin M, Thorlacius H, Biochem. Biophys. Res. Commun. 2017, 487, 887. [DOI] [PubMed] [Google Scholar]
- [79].Tripisciano C, Weiss R, Eichhorn T, Spittler A, Heuser T, Fischer MB, Weber V, Sci. Rep. 2017, 7, 6522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [80].Iba T, Ogura H, J. Intensive Care 2018, 6, 68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [81].Wang Y, Zhang S, Luo L, Norstrom E, Braun OO, Morgelin M, Thorlacius H, J. Cell. Physiol. 2018, 233, 1051. [DOI] [PubMed] [Google Scholar]
- [82].Tripisciano C, Weiss R, Karuthedom George S, Fischer MB, Weber V, Front. Cell Dev. Biol. 2020, 8, 298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [83].Deng JN, Li YQ, Liu Y, Li Q, Hu Y, Xu JQ, Sun TY, Xie LX, Biochem. Biophys. Res. Commun. 2019, 513, 958. [DOI] [PubMed] [Google Scholar]
- [84].Janiszewski M, Do Carmo AO, Pedro MA, Silva E, Knobel E, Laurindo FR, Crit. Care Med. 2004, 32, 818. [DOI] [PubMed] [Google Scholar]
- [85].Gambim MH, do Carmo Ade O, Marti L, Verissimo-Filho S, Lopes LR, Janiszewski M, Crit. Care 2007, 11, R107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [86].Mostefai HA, Bourget JM, Meziani F, Martinez MC, Leonetti D, Mercat A, Asfar P, Germain L, Andriantsitohaina R, Clin. Sci. 2013, 125, 77. [DOI] [PubMed] [Google Scholar]
- [87].Mu X, Wang X, Huang W, Wang RT, Essandoh K, Li Y, Pugh AM, Peng J, Deng S, Wang Y, Caldwell CC, Peng T, Yu KJ, Fan GC, Shock 2018, 49, 429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [88].Chatterjee V, Yang X, Ma Y, Cha B, Meegan JE, Wu M, Yuan SY, Cardiovasc. Res. 2020, 116, 1525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [89].Greening DW, Gopal SK, Xu R, Simpson RJ, Chen W, Semin. Cell Dev. Biol. 2015, 40, 72. [DOI] [PubMed] [Google Scholar]
- [90].Xu J, Feng Y, Jeyaram A, Jay SM, Zou L, Chao W, J. Immunol. 2018, 201, 3392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [91].Jian W, Gu L, Williams B, Feng Y, Chao W, Zou L, Anesthesiology 2019, 131, 105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [92].Essandoh K, Yang L, Wang X, Huang W, Qin D, Hao J, Wang Y, Zingarelli B, Peng T, Fan GC, Biochim. Biophys. Acta 2015, 1852, 2362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [93].Dalli J, Montero-Melendez T, Norling LV, YIn X, Haskard D, Mayr M, Perretti M, Mol. Cell. Proteomics 2013, 12, 2205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [94].Dalli J, Norling LV, Montero-Melendez T, Federici Canova D, Lashin H, Pavlov AM, Sukhorukov GB, Hinds CJ, Perretti M, EMBO Mol. Med. 2014, 6, 27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [95].Gao K, Jin J, Huang C, Li J, Luo H, Li L, Huang Y, Jiang Y, Front. Immunol. 2019, 10, 1560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [96].Dakhlallah DA, Wisler J, Gencheva M, Brown CM, Leatherman ER, Singh K, Brundage K, Karsies T, Dakhlallah A, Witwer KW, Sen CK, Eubank TD, Marsh CB, Extracell J. Vesicles 2019, 8, 1669881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [97].Wu SC, Yang JC, Rau CS, Chen YC, Lu TH, Lin MW, Tzeng SL, Wu YC, Wu CJ, Hsieh CH, PLoS One 2013, 8, e77936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [98].Goodwin AJ, Guo C, Cook JA, Wolf B, Halushka PV, Fan H, Crit. Care 2015, 19, 440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [99].Reithmair M, Buschmann D, Marte M, Kirchner B, Hagl D, Kaufmann I, Pfob M, Chouker A, Steinlein OK, Pfaffl MW, Schelling G, J. Cell. Mol. Med. 2017, 21, 2403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [100].Ticlea M, Bratu LM, Bodog F, Bedreag OH, Rogobete AF, Crainiceanu ZP, Biochem. Genet. 2017, 55, 1. [DOI] [PubMed] [Google Scholar]
- [101].Eriksson M, Nelson D, Nordgren A, Larsson A, Acta Anaesthesiol. Scand. 1998, 42, 551. [DOI] [PubMed] [Google Scholar]
- [102].Mortaza S, Martinez MC, Baron-Menguy C, Burban M, de la Bourdonnaye M, Fizanne L, Pierrot M, Cales P, Henrion D, Andriantsitohaina R, Mercat A, Asfar P, Meziani F, Crit. Care Med. 2009, 37, 2045. [DOI] [PubMed] [Google Scholar]
- [103].Wang JF, Yu ML, Yu G, Bian JJ, Deng XM, Wan XJ, Zhu KM, Biochem. Biophys. Res. Commun. 2010, 394, 184. [DOI] [PubMed] [Google Scholar]
- [104].Panich T, Chancharoenthana W, Somparn P, Issara-Amphorn J, Hirankarn N, Leelahavanichkul A, BMC Nephrol. 2017, 18, 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [105].Nair RR, Mazza D, Brambilla F, Gorzanelli A, Agresti A, Bianchi ME, Front. Immunol. 2018, 9, 1463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [106].Subramani K, Raju SP, Chu X, Warren M, Pandya CD, Hoda N, Fulzele S, Raju R, Int. Immunopharmacol. 2018, 65, 244. [DOI] [PubMed] [Google Scholar]
- [107].Xu Y, Ku X, Wu C, Cai C, Tang J, Yan W, Biochem. Biophys. Res. Commun. 2018, 499, 856. [DOI] [PubMed] [Google Scholar]
- [108].Li W, Deng M, Loughran PA, Yang M, Lin M, Yang C, Gao W, Jin S, Li S, Cai J, Lu B, Billiar TR, Scott MJ, Front. Immunol. 2020, 11, 229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [109].Zhang R, Zhu Y, Li Y, Liu W, Yin L, Yin S, Ji C, Hu Y, Wang Q, Zhou X, Chen J, Xu W, Qian H, Biotechnol. Lett. 2020, 42, 669. [DOI] [PubMed] [Google Scholar]
- [110].Joop K, Berckmans RJ, Nieuwland R, Berkhout J, Romijn FP, Hack CE, Sturk A, J. Thromb. Haemostasis 2001, 85, 810. [PubMed] [Google Scholar]
- [111].Soriano AO, Jy W, Chirinos JA, Valdivia MA, Velasquez HS, Jimenez JJ, Horstman LL, Kett DH, Schein RM, Ahn YS, Crit. Care Med. 2005, 33, 2540. [DOI] [PubMed] [Google Scholar]
- [112].Azevedo LC, Janiszewski M, Pontieri V, Pedro Mde A, Bassi E, Tucci PJ, Laurindo FR, Crit. Care 2007, 11, R120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [113].Mostefai HA, Meziani F, Mastronardi ML, Agouni A, Heymes C, Sargentini C, Asfar P, Martinez MC, Andriantsitohaina R, Am. J. Respir. Crit. Care Med. 2008, 178, 1148. [DOI] [PubMed] [Google Scholar]
- [114].Perez-Casal M, Thompson V, Downey C, Welters I, Wyncoll D, Thachil J, Toh CH, Crit. Care 2011, 15, R195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [115].Tokes-Fuzesi M, Woth G, Ernyey B, Vermes I, Muhl D, Bogar L, Kovacs GL, Crit J. Care 2013, 28, 141. [DOI] [PubMed] [Google Scholar]
- [116].Exline MC, Justiniano S, Hollyfield JL, Berhe F, Besecker BY, Das S, Wewers MD, Sarkar A, PLoS One 2014, 9, e90968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [117].Hellum M, Ovstebo R, Brusletto BS, Berg JP, Brandtzaeg P, Henriksson CE, Thromb. Res. 2014, 133, 507. [DOI] [PubMed] [Google Scholar]
- [118].Lehner GF, Harler U, Haller VM, Feistritzer C, Hasslacher J, Dunzendorfer S, Bellmann R, Joannidis M, Shock 2016, 46, 373. [DOI] [PubMed] [Google Scholar]
- [119].Jaurila H, Koivukangas V, Koskela M, Gaddnas F, Salo S, Korvala J, Risteli M, Karhu T, Herzig KH, Salo T, Ala-Kokko TI, J. Transl. Med. 2017, 15, 11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [120].Matsumoto H, Yamakawa K, Ogura H, Koh T, Matsumoto N, Shimazu T, Shock 2017, 47, 409. [DOI] [PubMed] [Google Scholar]
- [121].Shaver CM, Woods J, Clune JK, Grove BS, Wickersham NE, McNeil JB, Shemancik G, Ware LB, Bastarache JA, Crit. Care 2017, 21, 120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [122].Lashin HMS, Nadkarni S, Oggero S, Jones HR, Knight JC, Hinds CJ, Perretti M, Shock 2018, 49, 393. [DOI] [PubMed] [Google Scholar]
- [123].Puskarich MA, Cornelius DC, Bandyopadhyay S, McCalmon M, Tramel R, Dale WD, Jones AE, Thromb. Res. 2018, 168, 138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [124].Real JM, Ferreira LRP, Esteves GH, Koyama FC, Dias MVS, Bezerra-Neto JE, Cunha-Neto E, Machado FR, Salomao R, Azevedo LCP, Crit. Care 2018, 22, 68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [125].Boscolo A, Campello E, Bertini D, Spiezia L, Lucchetta V, Piasentini E, Radu CM, Manesso L, Ori C, Simioni P, Minerva Anestesiol. 2019, 85, 625. [DOI] [PubMed] [Google Scholar]
- [126].Homsy E, Das S, Consiglio P, McAtee C, Zachman A, Nagaraja H, Wewers MD, Exline MC, Mallampalli RK, Sarkar A, Crit. Care Explor. 2019, 1, e0039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [127].Kawamoto E, Masui-Ito A, Eguchi A, Soe ZY, Prajuabjinda O, Darkwah S, Park EJ, Imai H, Shimaoka M, Shock 2019, 52, 13. [DOI] [PubMed] [Google Scholar]
- [128].Ogura H, Kawasaki T, Tanaka H, Koh T, Tanaka R, Ozeki Y, Hosotsubo H, Kuwagata Y, Shimazu T, Sugimoto H, J. Trauma 2001, 50, 801. [DOI] [PubMed] [Google Scholar]
- [129].McGarrity S, Anuforo O, Halldorsson H, Bergmann A, Halldorsson S, Palsson S, Henriksen HH, Johansson PI, Rolfsson O, Sci. Rep. 2018, 8, 6811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [130].Kamisoglu K, Haimovich B, Calvano SE, Coyle SM, Corbett SA, Langley RJ, Kingsmore SF, Androulakis IP, Crit. Care 2015, 19, 71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [131].Doi K, Leelahavanichkul A, Yuen PS, Star RA, J. Clin. Invest. 2009, 119, 2868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [132].Stortz JA, Raymond SL, Mira JC, Moldawer LL, Mohr AM, Efron PA, ILAR J. 2017, 58, 90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [133].Lewis AJ, Seymour CW, Rosengart MR, Surg. Infect. 2016, 17, 385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [134].Hass R, Kasper C, Böhm S, Jacobs R, Cell. Commun. Signaling 2011, 9, 12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [135].Borger V, Bremer M, Ferrer-Tur R, Gockeln L, Stambouli O, Becic A, Giebel B, Int. J. Mol. Sci. 2017, 18, 1450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [136].Keshtkar S, Azarpira N, Ghahremani MH, Stem Cell Res. Ther. 2018, 9, 63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [137].Dejager L, Pinheiro I, Dejonckheere E, Libert C, Trends Microbiol. 2011, 19, 198. [DOI] [PubMed] [Google Scholar]
- [138].Zhang X, Wang X, Fan M, Tu F, Yang K, Ha T, Liu L, Kalbfleisch J, Williams D, Li C, Front. Immunol. 2020, 11, 566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [139].Lee HM, Kim TS, Jo EK, BMB Rep. 2016, 49, 311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [140].van Veldhuisen DJ, Ruilope LM, Maisel AS, Damman K, Eur. Heart J. 2016, 37, 2577. [DOI] [PubMed] [Google Scholar]
- [141].Gao F, Zuo B, Wang Y, Li S, Yang J, Sun D, Life Sci. 2020, 255, 117719. [DOI] [PubMed] [Google Scholar]
- [142].Michan S, Sinclair D, Biochem. J. 2007, 404, 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [143].Chang CL, Sung PH, Chen KH, Shao PL, Yang CC, Cheng BC, Lin KC, Chen CH, Chai HT, Chang HW, Yip HK, Chen HH, Am. J. Transl. Res. 2018, 10, 1053. [PMC free article] [PubMed] [Google Scholar]
- [144].Varkouhi AK, Jerkic M, Ormesher L, Gagnon S, Goyal S, Rabani R, Masterson C, Spring C, Chen PZ, Gu FX, dos Santos CC, Curley GF, Laffey JG, Anesthesiology 2019, 130, 778. [DOI] [PubMed] [Google Scholar]
- [145].Zhu YG, Feng XM, Abbott J, Fang XH, Hao Q, Monsel A, Qu JM, Matthay MA, Lee JW, Stem Cells 2014, 32, 116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [146].Monsel A, Zhu YG, Gennai S, Hao Q, Hu S, Rouby JJ, Rosenzwajg M, Matthay MA, Lee JW, Am. J. Respir. Crit. Care Med. 2015, 192, 324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [147].Tang XD, Shi L, Monsel A, Li XY, Zhu HL, Zhu YG, Qu JM, Stem Cells 2017, 35, 1849. [DOI] [PubMed] [Google Scholar]
- [148].Pacienza N, Lee RH, Bae EH, Kim DK, Liu Q, Prockop DJ, Yannarelli G, Mol. Ther. Methods Clin. Dev. 2019, 13, 67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [149].Morrison TJ, Jackson MV, Cunningham EK, Kissenpfennig A, McAuley DF, O’Kane CM, Krasnodembskaya AD, Am. J. Respir. Crit. Care Med. 2017, 196, 1275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [150].Hao Q, Gudapati V, Monsel A, Park JH, Hu S, Kato H, Lee JH, Zhou L, He H, Lee JW, J. Immunol. 2019, 203, 1961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [151].Cole SP, J. Biol. Chem. 2014, 289, 30880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [152].e Bel ML, Brunet A, Gosselin J, J. Innate Immun. 2014, 6, 159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [153].Jeschke MG, Richter G, Hofstadter F, Herndon DN, Perez-Polo JR, Jauch KW, Gene Ther. 2002, 9, 1065. [DOI] [PubMed] [Google Scholar]
- [154].Zhang Z, Pu Y, Pan Q, Xu X, Yan X, Artif. Cells, Nanomed., Biotechnol. 2016, 44, 1810. [DOI] [PubMed] [Google Scholar]
- [155].Jia K, Wang Y, Tong X, Wang R, Drug Des., Dev. Ther. 2020, 14, 217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [156].Viget NB, Guery BPH, Ader F, Nevie’Re R, Alfandari S, Creuzy C, Roussel-Delvallez M, Foucher C, Mason CM, Geaucaire G, Pittet JF, J. Physiol.: Lung Cell. Mol. Physiol. 2000, 279, L1199. [DOI] [PubMed] [Google Scholar]
- [157].Welsh DA, Summer WR, Dobard EP, Nelson S, Mason CM, Am. J. Respir. Crit. Care Med. 2000, 162, 1081. [DOI] [PubMed] [Google Scholar]
- [158].Chen J, Li C, Gao X, Li C, Liang Z, Yu L, Li Y, Xiao X, Chen L, PLoS One 2013, 8, e83303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [159].Shyamsundar M, McAuley DF, Ingram RJ, Gibson DS, O’Kane D, McKeown ST, Edwards A, Taggart C, Elborn JS, Calfee CS, Matthay MA, O’Kane CM, Am. J. Respir. Crit. Care Med. 2014, 189, 1520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [160].Braun S, Hanselmann C, Gassmann MG, auf dem Keller U, Born-Berclaz C, Chan K, Kan YW, Werner S, Mol. Cell. Biol. 2002, 22, 5492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [161].Widera A, Beloussow K, Kim KJ, Crandall ED, Shen WC, Cell Tissue Res. 2003, 312, 313. [DOI] [PubMed] [Google Scholar]
- [162].Kim I, Kim HG, June-No S, Kim JH, Kwak HJ, Koh GY, Circ. Res. 2000, 86, 24. [DOI] [PubMed] [Google Scholar]
- [163].Yan M, Hu Y, Yao M, Bao S, Fang Y, Wound Repair Regen. 2017, 25, 933. [DOI] [PubMed] [Google Scholar]
- [164].Hu S, Park J, Liu A, Lee J, Zhang X, Hao Q, Lee JW, Stem Cells Transl. Med. 2018, 7, 615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [165].Yi X, Wei X, Lv H, An Y, Li L, Lu P, Yang Y, Zhang Q, Yi H, Chen G, Exp. Cell Res. 2019, 383, 111454. [DOI] [PubMed] [Google Scholar]
- [166].Bridge G, Monteiro R, Henderson S, Emuss V, Lagos D, Georgopoulou D, Patient R, Boshoff C, Blood 2012, 120, 5063. [DOI] [PubMed] [Google Scholar]
- [167].Gong M, Yu B, Wang J, Wang Y, Liu M, Paul C, Millard RW, Xiao DS, Ashraf M, Xu M, Oncotarget 2017, 8, 45200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [168].Huang R, C. Q, Wang J, Hu Y, Zheng G, Qiu G, Ge M, Tao H, Shu Q, Xu J, Aging 2019, 11, 7996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [169].Park J, Kim S, Lim H, Liu A, Hu S, Lee J, Zhuo H, Hao Q, Matthay MA, Lee JW, Thorax 2019, 74, 43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [170].Dominici M, e Blanc KL, Mueller I, Slaper-Cortenbach I, Marini F, Krause D, Deans R, Keating A, Prockop D, Horwitz E, Cytotherapy 2006, 8, 315. [DOI] [PubMed] [Google Scholar]
- [171].Laroye C, Boufenzer A, Jolly L, Cunat L, Alauzet C, Merlin JL, Yguel C, Bensoussan D, Reppel L, Gibot S, Stem Cell Res. Ther. 2019, 10, 192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [172].Miksa M, Wu R, Dong W, Das P, Yang D, Wang P, Shock 2006, 25, 586. [DOI] [PubMed] [Google Scholar]
- [173].Miksa M, Wu R, Dong W, Komura H, Amin D, Ji Y, Wang Z, Wang H, Ravikumar TS, Tracey KJ, Wang P, J. Immunol. 2009, 183, 5983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [174].Timar CI, Lorincz AM, Csepanyi-Komi R, Valyi-Nagy A, Nagy G, Buzas EI, Ivanyi Z, Kittel A, Powell DW, McLeish KR, Ligeti E, Blood 2013, 121, 510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [175].Lorincz AM, Schutte M, Timar CI, Veres DS, Kittel A, McLeish KR, Merchant ML, Ligeti E, J. Leukocyte Biol. 2015, 98, 583. [DOI] [PubMed] [Google Scholar]
- [176].Zhou Y, Li P, Goodwin AJ, Cook JA, Halushka PV, Chang E, Zingarelli B, Fan H, Crit. Care 2019, 23, 44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [177].Wu X, Liu Z, Hu L, Gu W, Zhu L, Exp. Cell Res. 2018, 370, 13. [DOI] [PubMed] [Google Scholar]
- [178].Zhang C, Guo F, Chang M, Zhou Z, Yi L, Gao C, Huang X, Huan J, Exp. Cell Res. 2019, 384, 111596. [DOI] [PubMed] [Google Scholar]
- [179].Tu F, Wang X, Zhang X, Ha T, Wang Y, Fan M, Yang K, Gill PS, Ozment TR, Dai Y, Liu L, Williams DL, Li C, Front. Immunol. 2020, 11, 825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [180].Choi H, Kim Y, Mirzaaghasi A, Heo J, Kim YN, Shin JH, Kim S, Kim NH, Cho ES, n Yook JI, Yoo TH, Song E, Kim P, Shin EC, Chung K, Choi K, Choi C, Sci. Adv. 2020, 6, eaaz6980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [181].Lamichhane TN, Raiker RS, Jay SM, Mol. Pharmaceutics 2015, 12, 3650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [182].Lamichhane TN, Sokic S, Schardt JS, Raiker RS, Lin JW, Jay SM, Tissue Eng., Part B 2015, 21, 45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [183].Hall J, Prabhakar S, Balaj L, Lai CP, Cerione RA, Breakefield XO, Cell. Mol. Neurobiol. 2016, 36, 417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [184].Vader P, Mol EA, Pasterkamp G, Schiffelers RM, Adv. Drug Delivery Rev. 2016, 106, 148. [DOI] [PubMed] [Google Scholar]
- [185].Ha D, Yang N, Nadithe V, Acta Pharm. Sin B 2016, 6, 287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [186].Chevillet JR, Kang Q, Ruf IK, Briggs HA, Vojtech LN, Hughes SM, Cheng HH, Arroyo JD, Meredith EK, Gallichotte EN, Pogosova-Agadjanyan EL, Morrissey C, Stirewalt DL, Hladik F, Yu EY, Higano CS, Tewari M, Proc. Natl. Acad. Sci. USA 2014, 111, 14888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [187].Gangadaran P, Ahn BC, Pharmaceutics 2020, 12, 442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [188].Zhang D, Lee H, Zhu Z, Minhas JK, Jin Y, Am. J. Physiol.: Lung Cell. Mol. Physiol. 2017, 312, L110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [189].Didiot MC, Hall LM, Coles AH, Haraszti RA, Godinho BM, Chase K, Sapp E, Ly S, Alterman JF, Hassler MR, Echeverria D, Raj L, Morrissey DV, DiFiglia M, Aronin N, Khvorova A, Mol. Ther. 2016, 24, 1836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [190].Kooijmans SAA, Stremersch S, Braeckmans K, de Smedt SC, Hendrix A, Wood MJA, Schiffelers RM, Raemdonck K, Vader P, J. Controlled Release 2013, 172, 229. [DOI] [PubMed] [Google Scholar]
- [191].Cullis MBBPR, Madden TD, Mayer LD, Hope MJ, Trends Biotechnol. 1991, 9, 268. [DOI] [PubMed] [Google Scholar]
- [192].Jeyaram A, Lamichhane TN, Wang S, Zou L, Dahal E, Kronstadt SM, Levy D, Parajuli B, Knudsen DR, Chao W, Jay SM, Mol. Ther. 2020, 28, 975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [193].Alexander M, Hu R, Runtsch MC, Kagele DA, Mosbruger TL, Tolmachova T, Seabra MC, Round JL, Ward DM, O’Connell RM, Nat. Commun. 2015, 6, 7321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [194].Park SH, Choi H-J, Lee SY, Han J-S, Eur. J. Inflammation 2015, 13, 183. [Google Scholar]
- [195].Saba R, Sorensen DL, Booth SA, Front. Immunol. 2014, 5, 578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [196].Hotchkiss RS, Monneret G, Payen D, Lancet Infect. Dis. 2013, 13, 260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [197].An R, Feng J, Xi C, Xu J, Sun L, Oxid. Med. Cell. Longevity 2018, 2018, 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [198].Funahashi Y, Kato N, Masuda T, Nishio F, Kitai H, Ishimoto T, Kosugi T, Tsuboi N, Matsuda N, Maruyama S, Kadomatsu K, Lab. Invest. 2019, 99, 1130. [DOI] [PubMed] [Google Scholar]
- [199].Tkach M, Thery C, Cell 2016, 164, 1226. [DOI] [PubMed] [Google Scholar]
- [200].Kuzmich NN, Sivak KV, Chubarev VN, Porozov YB, Savateeva-Lyubimova TN, Peri F, Vaccines 2017, 5, 34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [201].Wu J, Wang Y, Li L, Biochim. Biophys. Acta, Mol. Basis Dis. 2017, 1863, 292. [DOI] [PubMed] [Google Scholar]
- [202].Sun D, Zhuang X, Xiang X, Liu Y, Zhang S, Liu C, Barnes S, Grizzle W, Miller D, Zhang HG, Mol. Ther. 2010, 18, 1606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [203].Carrillo-Vico A, Lardone PJ, Alvarez-Sanchez N, RodriguezRodriguez A, Guerrero JM, Int. J. Mol. Sci. 2013, 14, 8638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [204].Colunga Biancatelli RML, Berrill M, Mohammed YH, Marik PE, J. Thorac. Dis. 2020, 12, S54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [205].Yu H, Dickson EJ, Jung SR, Koh DS, Hille B, J. Gen. Physiol. 2016, 147, 63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [206].Qu P, Luo S, Du Y, Zhang Y, Song X, Yuan X, Lin Z, Li Y, Liu E, J. Pineal Res. 2020, 68, e12635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [207].Davenport EE, Burnham KL, Radhakrishnan J, Humburg P, Hutton P, Mills TC, Rautanen A, Gordon AC, Garrard C, Hill AVS, Hinds CJ, Knight JC, Lancet Respir. Med. 2016, 4, 259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [208].Leligdowicz A, Matthay MA, Crit. Care 2019, 23, 80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [209].Marik PE, Khangoora V, Rivera R, Hooper MH, Catravas J, Chest 2017, 151, 1229. [DOI] [PubMed] [Google Scholar]
- [210].Marik PE, Nutrients 2018, 10, 1762. [Google Scholar]
- [211].Yuana Y, Sturk A, Nieuwland R, Blood Rev. 2013, 27, 31. [DOI] [PubMed] [Google Scholar]
- [212].Konoshenko MY, Lekchnov EA, Vlassov AV, Laktionov PP, BioMed. Res. Int. 2018, 2018, 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [213].Li J, Lee Y, Johansson HJ, Mager I, Vader P, Nordin JZ, Wiklander OP, Lehtio J, Wood MJ, Andaloussi SE, Extracell J. Vesicles 2015, 4, 26883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [214].Ti D, Hao H, Tong C, Liu J, Dong L, Zheng J, Zhao Y, Liu H, Fu X, Han W, J. Transl. Med. 2015, 13, 308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [215].Wang Y, Lu X, He J, Zhao W, Stem Cell Res. Ther. 2015, 6, 100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [216].Watson DC, Bayik D, Srivatsan A, Bergamaschi C, Valentin A, Niu G, Bear J, Monninger M, Sun M, Morales-Kastresana A, Jones JC, Felber BK, Chen X, Gursel I, Pavlakis GN, Biomaterials 2016, 105, 195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [217].Lo Sicco C, Reverberi D, Balbi C, Ulivi V, Principi E, Pascucci L, Becherini P, Bosco MC, Varesio L, Franzin C, Pozzobon M, Cancedda R, Tasso R, Stem Cells Transl. Med. 2017, 6, 1018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [218].Lu Z, Chen Y, Dunstan C, Roohani-Esfahani S, Zreiqat H, Tissue Eng., Part A 2017, 23, 1212. [DOI] [PubMed] [Google Scholar]
- [219].Namazi H, Mohit E, Namazi I, Rajabi S, Samadian A, Hajizadeh-Saffar E, Aghdami N, Baharvand H, J. Cell. Biochem. 2018, 119, 4150. [DOI] [PubMed] [Google Scholar]
- [220].Cha JM, Shin EK, Sung JH, Moon GJ, Kim EH, Cho YH, Park HD, Bae H, Kim J, Bang OY, Sci. Rep. 2018, 8, 1171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [221].Harting MT, Srivastava AK, Zhaorigetu S, Bair H, Prabhakara KS, Toledano Furman NE, Vykoukal JV, Ruppert KA, Cox CS Jr., Olson SD, Stem Cells 2018, 36, 79. [DOI] [PubMed] [Google Scholar]
- [222].Kim MH, Wu WH, Choi JH, Kim JH, Hong SH, Jun JH, Ko Y, Lee JH, J. Biomater. Sci., Polym. Ed. 2018, 29, 1066. [DOI] [PubMed] [Google Scholar]
- [223].Minghua W, Zhijian G, Chahua H, Qiang L, Minxuan X, Luqiao W, Weifang Z, Peng L, Biming Z, Lingling Y, Zhenzhen W, Jianqing X, Huihui B, Xiaozhong W, Xiaoshu C, Cell Death Dis. 2018, 9, 320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [224].Park H, Park H, Mun D, Kang J, Kim H, Kim M, Cui S, Lee SH, Joung B, Yonsei Med. J. 2018, 59, 736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [225].Mayo JS, Kurata WE, O’Connor KM, Pierce LM, Plast. Reconstr. Surg. Glob. Open 2019, 7, e2588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [226].Palviainen M, Saari H, Karkkainen O, Pekkinen J, Auriola S, Yliperttula M, Puhka M, Hanhineva K, Siljander PR, J. Extracell. Vesicles 2019, 8, 1596669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [227].Rocha S, Carvalho J, Oliveira P, Voglstaetter M, Schvartz D, Thomsen AR, Walter N, Khanduri R, Sanchez JC, Keller A, Oliveira C, Nazarenko I, Adv. Sci. 2019, 6, 1800948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [228].Showalter MR, Wancewicz B, Fiehn O, Archard JA, Clayton S, Wagner J, Deng P, Halmai J, Fink KD, Bauer G, Fury B, Perotti NH, Apperson M, Butters J, Belafsky P, Farwell G, Kuhn M, Nolta JA, Anderson JD, Biochem. Biophys. Res. Commun. 2019, 512, 729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [229].Yan L, Wu X, Cell Biol. Toxicol. 2020, 36, 165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [230].Yang L, Zhai Y, Hao Y, Zhu Z, Cheng G, Small 2020, 16, 1906273. [DOI] [PubMed] [Google Scholar]
- [231].Yuan O, Lin C, Wagner J, Archard JA, Deng P, Halmai J, Bauer G, Fink KD, Fury B, Perotti NH, Walker JE, Pollock K, Apperson M, Butters J, Belafsky P, Farwell DG, Kuhn M, Nolta J, Anderson JD, Stem Cells Dev. 2019, 28, 398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [232].Zhang L, Liu H, Xu K, Ling Z, Huang Y, Hu Q, Lu K, Liu C, Wang Y, Liu N, Zhang X, Xu B, Wu J, Chen S, Zhang G, Chen M, Int. J. Biol. Sci. 2019, 15, 1161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [233].Zhang Q, Fu L, Liang Y, Guo Z, Wang L, Ma C, Wang H, J. Cell. Physiol. 2018, 233, 6832. [DOI] [PubMed] [Google Scholar]
- [234].An JH, Li Q, Bhang DH, Song WJ, Youn HY, Sci. Rep. 2020, 10, 2115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [235].Jing H, Zhang X, Luo K, Luo Q, Yin M, Wang W, Zhu Z, Zheng J, He X, Biomaterials 2020, 231, 119682. [DOI] [PubMed] [Google Scholar]
- [236].Mead B, Chamling X, Zack DJ, Ahmed Z, Tomarev S, Invest. Ophthalmol. Visual Sci. 2020, 61, 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [237].Ragni E, Perucca Orfei C, De Luca P, Mondadori C, Vigano M, Colombini A, de Girolamo L, Stem Cell Res. Ther. 2020, 11, 165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [238].Anderson JD, Johansson HJ, Graham CS, Vesterlund M, Pham MT, Bramlett CS, Montgomery EN, Mellema MS, Bardini RL, Contreras Z, Hoon M, Bauer G, Fink KD, Fury B, Hendrix KJ, Chedin F, El-Andaloussi S, Hwang B, Mulligan MS, Lehtio J, Nolta JA, Stem Cells 2016, 34, 601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [239].Mitchell JP, Court J, Mason MD, Tabi Z, Clayton A, J. Immunol. Methods 2008, 335, 98. [DOI] [PubMed] [Google Scholar]
- [240].Patel DB, Gray KM, Santharam Y, Lamichhane TN, Stroka KM, Jay SM, Bioeng. Transl. Med. 2017, 2, 170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [241].Zhang G, Yang Y, Huang Y, Zhang L, Ling Z, Zhu Y, Wang F, Zou X, Chen M, Biomed. Pharmacother. 2017, 90, 473. [DOI] [PubMed] [Google Scholar]
- [242].Guerreiro EM, Vestad B, Steffensen LA, Aass HCD, Saeed M, Ovstebo R, Costea DE, Galtung HK, Soland TM, PLoS One 2018, 13, e0204276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [243].Haraszti RA, Miller R, Stoppato M, Sere YY, Coles A, Didiot MC, Wollacott R, Sapp E, Dubuke ML, Li X, Shaffer SA, DiFiglia M, Wang Y, Aronin N, Khvorova A, Mol. Ther. 2018, 26, 2838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [244].Kim S, Lee SK, Kim H, Kim TM, Int. J. Mol. Sci. 2018, 19, 3119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [245].Collino F, Lopes JA, Correa S, Abdelhay E, Takiya CM, Wendt CHC, de Miranda KR, Vieyra A, Lindoso RS, Cell. Physiol. Biochem. 2019, 52, 1463. [DOI] [PubMed] [Google Scholar]
- [246].Liu H, Sun X, Gong X, Wang G, J. Cell. Biochem. 2019, 120, 14455. [DOI] [PubMed] [Google Scholar]
- [247].Yang Y, Knight R, Stephens P, Zhang Y, J. Oral Pathol. Med. 2020, 49, 342. [DOI] [PubMed] [Google Scholar]
- [248].Jarmalaviciute A, Tunaitis V, Pivoraite U, Venalis A, Pivoriunas A, Cytotherapy 2015, 17, 932. [DOI] [PubMed] [Google Scholar]
- [249].Lamichhane TN, Leung CA, Douti LY, Jay SM, Sci. Rep. 2017, 7, 13794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [250].Patel DB, Luthers CR, Lerman MJ, Fisher JP, Jay SM, Acta Biomater. 2019, 95, 236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [251].Zhu J, Lu K, Zhang N, Zhao Y, Ma Q, Shen J, Lin Y, Xiang P, Tang Y, Hu X, Chen J, Zhu W, Webster KA, Wang J, Yu H, Artif. Cells, Nanomed., Biotechnol. 2018, 46, 1282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [252].Varkouhi AK, Jerkic M, Ormesher L, Gagnon S, Goyal S, Rabani R, Masterson C, Spring C, Chen PZ, Gu FX, dos Santos CC, Curley GF, Laffey JG, Anesthesiology 2019, 130, 778. [DOI] [PubMed] [Google Scholar]
- [253].Zhang Y, Chopp M, Zhang ZG, Katakowski M, Xin H, Qu C, Ali M, Mahmood A, Xiong Y, Neurochem. Int. 2017, 111, 69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [254].Kim H, Lee MJ, Bae EH, Ryu JS, Kaur G, Kim HJ, Kim JY, Barreda H, Jung SY, Choi JM, Shigemoto-Kuroda T, Oh JY, Lee RH, Mol. Ther. 2020, 28, 1628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [255].Eichholz KF, Woods I, Riffault M, Johnson GP, Corrigan M, Lowry MC, Shen N, Labour MN, Wynne K, O’Driscoll L, Hoey DA, Stem Cells Transl. Med. 2020, 9, 1431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [256].Najrana T, Mahadeo A, Abu-Eid R, Kreienberg E, Schulte V, Uzun A, Schorl C, Goldberg L, Quesenberry P, Sanchez-Esteban J, J. Cell. Physiol. 2020, 235, 8210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [257].Xia B, Gao J, Li S, Huang L, Zhu L, Ma T, Zhao L, Yang Y, Luo K, Shi X, Mei L, Zhang H, Zheng Y, Lu L, Luo Z, Huang J, Theranostics 2020, 10, 8974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [258].Zhuang F, Shi Q, Wang WB, Bao H, Yan J, Gao S, Liu Z, Jiang ZL, Qi YX, Exp. Cell Res. 2020, 386, 111710. [DOI] [PubMed] [Google Scholar]
- [259].Feng Y, Huang W, Wani M, Yu X, Ashraf M, PLoS One 2014, 9, e88685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [260].Cui GH, Wu J, Mou FF, Xie WH, Wang FB, Wang QL, Fang J, Xu YW, Dong YR, Liu JR, Guo HD, FASEB J. 2018, 32, 654. [DOI] [PubMed] [Google Scholar]
- [261].Han YD, Bai Y, Yan XL, Ren J, Zeng Q, Li XD, Pei XT, Han Y, Biochem. Biophys. Res. Commun. 2018, 497, 305. [DOI] [PubMed] [Google Scholar]
- [262].Han Y, Ren J, Bai Y, Pei X, Han Y, Int. J. Biochem. Cell Biol. 2019, 109, 59. [DOI] [PubMed] [Google Scholar]
- [263].Almeria C, Weiss R, Roy M, Tripisciano C, Kasper C, Weber V, Egger D, Front. Bioeng. Biotechnol. 2019, 7, 292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [264].Liang B, Liang JM, Ding JN, Xu J, Xu JG, Chai YM, Stem Cell Res. Ther. 2019, 10, 335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [265].Ma J, Tao X, Huang Y, Cerebrovasc. Dis. 2020, 49, 462. [DOI] [PubMed] [Google Scholar]
- [266].Harmati M, Gyukity-Sebestyen E, Dobra G, Janovak L, Dekany I, Saydam O, Hunyadi-Gulyas E, Nagy I, Farkas A, Pankotai T, Ujfaludi Z, Horvath P, Piccinini F, Kovacs M, Biro T, Buzas K, Sci. Rep. 2019, 9, 15329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [267].Fukuta T, Nishikawa A, Kogure K, Biochem. Biophys. Rep. 2020, 21, 100713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [268].Shao M, Xu Q, Wu Z, Chen Y, Shu Y, Cao X, Chen M, Zhang B, Zhou Y, Yao R, Shi Y, Bu H, Stem Cell Res. Ther. 2020, 11, 37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [269].Kalani A, Kamat PK, Chaturvedi P, Tyagi SC, Tyagi N, Life Sci. 2014, 107, 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [270].Lopatina T, Bruno S, Tetta C, Kalinina N, Porta M, Camussi G, Cell Commun. Signaling 2014, 12, 26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [271].Di Trapani M, Bassi G, Midolo M, Gatti A, Kamga PT, Cassaro A, Carusone R, Adamo A, Krampera M, Sci. Rep. 2016, 6, 24120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [272].Zeuner MT, Patel K, Denecke B, Giebel B, Widera D, J. Transl. Med. 2016, 14, 34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [273].Du W, Zhang K, Zhang S, Wang R, Nie Y, Tao H, Han Z, Liang L, Wang D, Liu J, Liu N, Han Z, Kong D, Zhao Q, Li Z, Biomaterials 2017, 133, 70. [DOI] [PubMed] [Google Scholar]
- [274].Bai Y, Han YD, Yan XL, Ren J, Zeng Q, Li XD, Pei XT, Han Y, Biochem. Biophys. Res. Commun. 2018, 500, 310. [DOI] [PubMed] [Google Scholar]
- [275].Ding J, Wang X, Chen B, Zhang J, Xu J, BioMed. Res. Int. 2019, 2019, 9742765. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- [276].Shen H, Yoneda S, Abu-Amer Y, Guilak F, Gelberman RH, J. Orthop. Res. 2020, 38, 117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [277].Ren G, Zhang L, Zhao X, Xu G, Zhang Y, Roberts AI, Zhao RC, Shi Y, Cell Stem Cell 2008, 2, 141. [DOI] [PubMed] [Google Scholar]
- [278].Najar M, Krayem M, Meuleman N, Bron D, Lagneaux L, Immune Network 2017, 17, 89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [279].Shirjang S, Mansoori B, Solali S, Hagh MF, Shamsasenjan K, Cell. Immunol. 2017, 315, 1. [DOI] [PubMed] [Google Scholar]
- [280].Noronha NC, Mizukami A, Caliari-Oliveira C, Cominal JG, Rocha JLM, Covas DT, Swiech K, Malmegrim KCR, Stem Cell Res. Ther. 2019, 10, 131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [281].Waterman RS, Tomchuck SL, Henkle SL, Betancourt AM, PLoS One 2010, 5, e10088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [282].Krampera M, Leukemia 2011, 25, 1408. [DOI] [PubMed] [Google Scholar]
- [283].Wang Y, Chen X, Cao W, Shi Y, Nat. Immunol. 2014, 15, 1009. [DOI] [PubMed] [Google Scholar]
- [284].Martin-Rufino JD, Espinosa-Lara N, Osugui L, Sanchez-Guijo F, Front. Bioeng. Biotechnol. 2019, 7, 308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [285].Arango Duque G, Descoteaux A, Front. Immunol. 2014, 5, 491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [286].Domenis R, Cifu A, Quaglia S, Pistis C, Moretti M, Vicario A, Parodi PC, Fabris M, Niazi KR, Soon-Shiong P, Curcio F, Sci. Rep. 2018, 8, 13325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [287].Cosenza S, Toupet K, Maumus M, Luz-Crawford P, BlancBrude O, Jorgensen C, Noel D, Theranostics 2018, 8, 1399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [288].Han X, Yang Q, Lin L, Xu C, Zheng C, Chen X, Han Y, Li M, Cao W, Cao K, Chen Q, Xu G, Zhang Y, Zhang J, Schneider RJ, Qian Y, Wang Y, Brewer G, Shi Y, Cell Death Differ. 2014, 21, 1758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [289].Cho JS, Pietras EM, Garcia NC, Ramos RI, Farzam DM, Monroe HR, Magorien JE, Blauvelt A, Kolls JK, Cheung AL, Cheng G, Modlin RL, Miller LS, J. Clin. Invest. 2010, 120, 1762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [290].Morrow KN, Coopersmith CM, Ford ML, Front. Immunol. 2019, 10, 1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [291].Xu R, Zhang F, Chai R, Zhou W, Hu M, Liu B, Chen X, Liu M, Xu Q, Liu N, Liu S, J. Cell. Mol. Med. 2019, 23, 7617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [292].Liu G, Abraham E, Arterioscler., Thromb., Vasc. Biol. 2013, 33, 170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [293].Teng GG, Wang WH, Dai Y, Wang SJ, Chu YX, Li J, PLoS One 2013, 8, e56709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [294].He X, Jing Z, Cheng G, BioMed. Res. Int. 2014, 2014, 945169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [295].Marques-Rocha JL, Garcia-Lacarte M, Samblas M, Bressan J, Martinez JA, Milagro FI, J. Physiol. Biochem. 2018, 74, 579. [DOI] [PubMed] [Google Scholar]
- [296].Kink JA, Forsberg MH, Reshetylo S, Besharat S, Childs CJ, Pederson JD, Gendron-Fitzpatrick A, Graham M, Bates PD, Schmuck EG, Raval A, Hematti P, Capitini CM, Biol. Blood Marrow Transplant. 2019, 25, 2124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [297].Tiru B, DiNino EK, Orenstein A, Mailloux PT, Pesaturo A, Gupta A, McGee WT, Pharmacoeconomics 2015, 33, 925. [DOI] [PubMed] [Google Scholar]
- [298].Mostel Z, Perl A, Marck M, Mehdi SF, Lowell B, Bathija S, Santosh R, Pavlov VA, Chavan SS, Roth J, Mol. Med. 2019, 26, 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [299].Chu X, Liu D, Li T, Ke H, Xin D, Wang S, Cao Y, Xue H, Wang Z, J. Controlled Release 2020, 328, 13. [DOI] [PubMed] [Google Scholar]
- [300].Francois M, Romieu-Mourez R, Li M, Galipeau J, Mol. Ther. 2012, 20, 187. [DOI] [PubMed] [Google Scholar]
- [301].Gray A, Schloss RS, Yarmush M, Technology 2016, 4, 201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [302].Keung AJ, Kumar S, Schaffer DV, Annu. Rev. Cell Dev. Biol. 2010, 26, 533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [303].Maziarz A, Kocan B, Bester M, Budzik S, Cholewa M, Ochiya T, Banas A, Stem Cell Res. Ther. 2016, 7, 54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [304].Gasiorowski JZ, Murphy CJ, Nealey PF, Annu. Rev. Biomed. Eng. 2013, 15, 155. [DOI] [PubMed] [Google Scholar]
- [305].Thippabhotla S, Zhong C, He M, Sci. Rep. 2019, 9, 13012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [306].Ding S, Kingshott P, Thissen H, Pera M, Wang PY, Biotechnol. Bioeng. 2017, 114, 260. [DOI] [PubMed] [Google Scholar]
- [307].Francesco Pampaloni E. G. R. a. E. H. K. S., Nat. Rev. Mol. Cell Biol. 2007, 8, 839. [DOI] [PubMed] [Google Scholar]
- [308].Ravi M, Paramesh V, Kaviya SR, Anuradha E, Solomon FD, J. Cell. Physiol. 2015, 230, 16. [DOI] [PubMed] [Google Scholar]
- [309].Monguio-Tortajada M, Roura S, Galvez-Monton C, Pujal JM, Aran G, Sanjurjo L, Franquesa M, Sarrias MR, Bayes-Genis A, Borras FE, Theranostics 2017, 7, 270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [310].Galley HF, Br. J. Anaesth. 2011, 107, 57. [DOI] [PubMed] [Google Scholar]
- [311].Santoro M, Lamhamedi-Cherradi SE, Menegaz BA, Ludwig JA, Mikos AG, Proc. Natl. Acad. Sci. USA 2015, 112, 10304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [312].Ohashi T, Sato M, Fluid Dyn. Res. 2005, 37, 40. [Google Scholar]
- [313].Liu L, Yu B, Chen J, Tang Z, Zong C, Shen D, Zheng Q, Tong X, Gao C, Wang J, Biomech. Model. Mechanobiol. 2012, 11, 391. [DOI] [PubMed] [Google Scholar]
- [314].Lee HJ, Diaz MF, Ewere A, Olson SD, Cox CS Jr., Wenzel PL, Cell. Signaling 2017, 38, 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [315].Chen Y, Shu Z, Qian K, Wang J, Zhu H, Tissue Eng., Part B 2019, 25, 492. [DOI] [PubMed] [Google Scholar]
- [316].Papadimitropoulos A, Piccinini E, Brachat S, Braccini A, Wendt D, Barbero A, Jacobi C, Martin I, PLoS One 2014, 9, e102359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [317].Chinnadurai R, Copland IB, Garcia MA, Petersen CT, Lewis CN, Waller EK, Kirk AD, Galipeau J, Stem Cells 2016, 34, 2429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [318].Gowen A, Shahjin F, Chand S, Odegaard KE, Yelamanchili SV, Front. Cell Dev. Biol. 2020, 8, 149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [319].Yang YK, Ogando CR, See CW, Chang TY, Barabino GA, Stem Cell Res. Ther. 2018, 9, 131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [320].Rani S, Ryan AE, Griffin MD, Ritter T, Mol. Ther. 2015, 23, 812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [321].Batsali AK, Georgopoulou A, Mavroudi I, Matheakakis A, Pontikoglou CG, Papadaki HA, J. Clin. Med. 2020, 9, 856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [322].Chen IY, Matsa E, Wu JC, Nat. Rev. Cardiol. 2016, 13, 333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [323].Sayed N, Liu C, Wu JC, J. Am. Coll. Cardiol. 2016, 67, 2161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [324].Solter D, Nat. Rev. Genet. 2006, 7, 319. [DOI] [PubMed] [Google Scholar]
- [325].Khan M, Nickoloff E, Abramova T, Johnson J, Verma SK, Krishnamurthy P, Mackie AR, Vaughan E, Garikipati VN, Benedict C, Ramirez V, Lambers E, Ito A, Gao E, Misener S, Luongo T, Elrod J, Qin G, Houser SR, Koch WJ, Kishore R, Circ. Res. 2015, 117, 52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [326].Wu Q, Wang J, Tan WLW, Jiang Y, Wang S, Li Q, Yu X, Tan J, Liu S, Zhang P, Tiang Z, Chen Z, Foo RS, Yang HT, Cell Death Dis. 2020, 11, 354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [327].Mardpour S, Hassani SN, Mardpour S, Sayahpour F, Vosough M, Ai J, Aghdami N, Hamidieh AA, Baharvand H, J. Cell. Physiol. 2018, 233, 9330. [DOI] [PubMed] [Google Scholar]
- [328].Kalani A, Chaturvedi P, Kamat PK, Maldonado C, Bauer P, Joshua IG, Tyagi SC, Tyagi N, Int. J. Biochem. Cell Biol. 2016, 79, 360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [329].Zhang B, Yeo RW, Tan KH, Lim SK, Int. J. Mol. Sci. 2016, 17, 174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [330].de Wert G, Mummery C, Hum. Reprod. 2003, 18, 672. [DOI] [PubMed] [Google Scholar]
- [331].Kastenberg ZJ, Odorico JS, Transplant. Rev. 2008, 22, 215. [DOI] [PubMed] [Google Scholar]
- [332].Takahashi K, Tanabe K, Ohnuki M, Narita M, Ichisaka T, Tomoda K, Yamanaka S, Cell 2007, 131, 861. [DOI] [PubMed] [Google Scholar]
- [333].Singh VK, Kalsan M, Kumar N, Saini A, Chandra R, Front. Cell Dev. Biol. 2015, 3, 2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [334].Hai B, Shigemoto-Kuroda T, Zhao Q, Lee RH, Liu F, Stem Cells Int. 2018, 2018, 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [335].Liu S, Mahairaki V, Bai H, Ding Z, Li J, Witwer KW, Cheng L, Stem Cells 2019, 37, 779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [336].Chen SJ, Chang CM, Tsai SK, Chang YL, Chou SJ, Huang SS, Tai LK, Chen YC, Ku HH, Li HY, Chiou SH, Stem Cells Dev. 2010, 19, 1757. [DOI] [PubMed] [Google Scholar]
- [337].Lee PY, Chien Y, Chiou GY, Lin CH, Chiou CH, Tarng DC, Cell Transplant. 2012, 21, 2569. [DOI] [PubMed] [Google Scholar]
- [338].Huang WC, Ke MW, Cheng CC, Chiou SH, Wann SR, Shu CW, Chiou KR, Tseng CJ, Pan HW, Mar GY, Liu CP, PLoS One 2016, 11, e0142476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [339].Su VY, Chiou SH, Lin CS, Mo MH, Yang KY, Stem Cells 2019, 37, 1516. [DOI] [PubMed] [Google Scholar]
- [340].Povero D, Pinatel EM, Leszczynska A, Goyal NP, Nishio T, Kim J, Kneiber D, de Araujo Horcel L, Eguchi A, Ordonez PM, Kisseleva T, Feldstein AE, JCI Insight 2019, 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [341].Yan J, Li S, Li S, Int. Rev. Immunol. 2014, 33, 498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [342].Yang CS, Lee DS, Song CH, An SJ, Li S, Kim JM, Kim CS, Yoo DG, Jeon BH, Yang HY, Lee TH, Lee ZW, El-Benna J, Yu DY, Jo EK, J. Exp. Med. 2007, 204, 583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [343].Adamiak M, Cheng G, Bobis-Wozowicz S, Zhao L, KedrackaKrok S, Samanta A, Karnas E, Xuan YT, Skupien-Rabian B, Chen X, Jankowska U, Girgis M, Sekula M, Davani A, Lasota S, Vincent RJ, Sarna M, Newell KL, Wang OL, Dudley N, Madeja Z, Dawn B, Zuba-Surma EK, Circ. Res. 2018, 122, 296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [344].Li D, Jia H, Zhang H, Lv M, Liu J, Zhang Y, Huang T, Huang B, Oncoimmunology 2012, 1, 687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [345].Cao X, Zhang C, Zhang X, Chen Y, Zhang H, Biomed. Pharmacother. 2019, 111, 852. [DOI] [PubMed] [Google Scholar]
- [346].Chen X, Zhou L, Peng N, Yu H, Li M, Cao Z, Lin Y, Wang X, Li Q, Wang J, She Y, Zhu C, Lu M, Zhu Y, Liu S, J. Biol. Chem. 2017, 292, 21291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [347].Gao S, Li X, Nie S, Yang L, Tu L, Dong B, Zhao P, Wang Y, Yu Y, Wang L, Hua S, Int. J. Mol. Sci. 2017, 18, 1034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [348].Yuan X, Li D, Chen X, Han C, Xu L, Huang T, Dong Z, Zhang M, Cell Death Dis. 2017, 8, 3200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [349].Pasparakis M, Vandenabeele P, Nature 2015, 517, 311. [DOI] [PubMed] [Google Scholar]
- [350].Bolognese AC, Yang WL, Hansen LW, Denning NL, Nicastro JM, Coppa GF, Wang P, Surgery 2018, 164, 110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [351].Gu C, Hou C, Zhang S, Inflammation Res. 2020, 69, 299. [DOI] [PubMed] [Google Scholar]
- [352].Cai J, Wu J, Wang J, Li Y, Hu X, Luo S, Xiang D, Cell Biosci. 2020, 10, 69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [353].Huang CC, Kang M, Narayanan R, DiPietro LA, Cooper LF, Gajendrareddy P, Ravindran S, Front. Pharmacol. 2020, 11, 163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [354].Webb RL, Kaiser EE, Scoville SL, Thompson TA, Fatima S, Pandya C, Sriram K, Swetenburg RL, Vaibhav K, Arbab AS, Baban B, Dhandapani KM, Hess DC, Hoda MN, Stice SL, Transl. Stroke Res. 2018, 9, 530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [355].Ruggeri ZM, Nat. Med. 2002, 8, 1227. [DOI] [PubMed] [Google Scholar]
- [356].Rowley JW, Schwertz H, Weyrich AS, Curr. Opin. Hematol. 2012, 19, 385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [357].Mussano F, Genova T, Munaron L, Petrillo S, Erovigni F, Carossa S, Platelets 2016, 27, 467. [DOI] [PubMed] [Google Scholar]
- [358].Yeaman MR, Clin. Infect. Dis. 1997, 255, 951. [DOI] [PubMed] [Google Scholar]
- [359].Smyth SS, McEver RP, Weyrich AS, Morrell CN, Hoffman MR, Arepally GM, French PA, Dauerman HL, Becker RC, Colloquium PP, Thromb J. Haemostasis 2009, 7, 1759. [DOI] [PubMed] [Google Scholar]
- [360].Aatonen MT, Ohman T, Nyman TA, Laitinen S, Gronholm M, Siljander PR, J. Extracell. Vesicles 2014, 3, 24692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [361].Badimon L, Suades R, Fuentes E, Palomo I, Padro T, Front. Pharmacol. 2016, 7, 293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [362].Todorova D, Simoncini S, Lacroix R, Sabatier F, Dignat-George F, Circ. Res. 2017, 120, 1658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [363].Tao SC, Yuan T, Rui BY, Zhu ZZ, Guo SC, Zhang CQ, Theranostics 2017, 7, 733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [364].Vajen T, Mause SF, Koenen RR, Thromb J. Haemostasis 2015, 114, 228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [365].Zaldivia MTK, McFadyen JD, Lim B, Wang X, Peter K, Front. Cardiovasc. Med. 2017, 4, 74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [366].Khan MM, Yang WL, Wang P, Shock 2015, 44, 294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [367].Kim HJ, Jeong JS, Kim SR, Park SY, Chae HJ, Lee YC, Sci. Rep. 2013, 3, 1142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [368].Chen G, Li X, Huang M, Li M, Zhou X, Li Y, Bai J, Shock 2016, 46, 67. [DOI] [PubMed] [Google Scholar]
- [369].Jia Y, Li Z, Feng Y, Cui R, Dong Y, Zhang X, Xiang X, Qu K, Liu C, Zhang J, Oxid. Med. Cell. Longevity 2018, 2018, 4756846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [370].Wang QL, Yang L, Peng Y, Gao M, Yang MS, Xing W, Xiao XZ, Mediators Inflammation 2019, 2019, 6453296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [371].Hotchkiss RSS, Paul E, Freeman BD, Tinsley KW, Cobb JP, Matuschak GM, Buchman TG, Karl IE, Crit. Care Med. 1999, 27, 1230. [DOI] [PubMed] [Google Scholar]
- [372].Lv Y, Kim K, Sheng Y, Cho J, Qian Z, Zhao YY, Hu G, Pan D, Malik AB, Hu G, Circ. Res. 2018, 123, 43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [373].Guo SC, Tao SC, Yin WJ, Qi X, Yuan T, Zhang CQ, Theranostics 2017, 7, 81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [374].Pordzik J, Pisarz K, De Rosa S, Jones AD, Eyileten C, Indolfi C, Malek L, Postula M, Front. Endocrinol. 2018, 9, 74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [375].Chao Chen LZ, Huang H, Liu S, Liang Y, Xu L, Li S, Cheng Y, Tang W, Int. J. Clin. Exp. Pathol. 2018, 11, 2605. [PMC free article] [PubMed] [Google Scholar]
- [376].Fish JE, Santoro MM, Morton SU, Yu S, Yeh RF, Wythe JD, Ivey KN, Bruneau BG, Stainier DY, Srivastava D, Dev. Cell 2008, 15, 272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [377].Wang S, Aurora AB, Johnson BA, Qi X, McAnally J, Hill JA, Richardson JA, Bassel-Duby R, Olson EN, Dev. Cell 2008, 15, 261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [378].Schober A, Nazari-Jahantigh M, Wei Y, Bidzhekov K, Gremse F, Grommes J, Megens RT, Heyll K, Noels H, Hristov M, Wang S, Kiessling F, Olson EN, Weber C, Nat. Med. 2014, 20, 368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [379].Wang X, Huang W, Yang Y, Wang Y, Peng T, Chang J, Caldwell CC, Zingarelli B, Fan GC, Biochim. Biophys. Acta 2014, 1842, 701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [380].Tan M, Yan HB, Li JN, Li WK, Fu YY, Chen W, Zhou Z, Cell. Physiol. Biochem. 2016, 38, 2348. [DOI] [PubMed] [Google Scholar]
- [381].Li J, Tan M, Xiang Q, Zhou Z, Yan H, Thromb. Res. 2017, 154, 96. [DOI] [PubMed] [Google Scholar]
- [382].Sun Y, Liu XL, Zhang D, Liu F, Cheng YJ, Ma Y, Zhou YJ, Zhao YX, Curr. Vasc. Pharmacol. 2019, 17, 379. [DOI] [PubMed] [Google Scholar]
- [383].Clark PR, Manes TD, Pober JS, Kluger MS, J. Invest. Dermatol. 2007, 127, 762. [DOI] [PubMed] [Google Scholar]
- [384].Zhao YJ, Yi WJ, Wan XJ, Wang J, Tao TZ, Li JB, Wang JF, Deng XM, Mediators Inflammation 2014, 2014, 195290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [385].Garcia-Manrique P, Matos M, Gutierrez G, Pazos C, BlancoLopez MC, J. Extracell. Vesicles 2018, 7, 1422676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [386].Go G, Lee J, Choi DS, Kim SS, Gho YS, Adv. Healthcare Mater. 2019, 8, e1801082. [DOI] [PubMed] [Google Scholar]
- [387].Wang L, Abhange KK, Wen Y, Chen Y, Xue F, Wang G, Tong J, Zhu C, He X, Wan Y, ACS Omega 2019, 4, 22638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [388].Sharma A, Sharma US, Int. J. Pharm. 1997, 154, 123. [Google Scholar]
- [389].Li SP, Lin ZX, Jiang XY, Yu XY, Acta Pharmacol. Sin. 2018, 39, 542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [390].Thone MN, Kwon YJ, Methods 2020, 177, 135. [DOI] [PubMed] [Google Scholar]
- [391].Nasiri Kenari A, Kastaniegaard K, Greening DW, Shambrook M, Stensballe A, Cheng L, Hill AF, Proteomics 2019, 19, 1800161. [DOI] [PubMed] [Google Scholar]
- [392].Jo W, Kim J, Yoon J, Jeong D, Cho S, Jeong H, Yoon YJ, Kim SC, Gho YS, Park J, Nanoscale 2014, 6, 12056. [DOI] [PubMed] [Google Scholar]
- [393].Jang SC, Kim OY, Yoon CM, Choi DS, Roh TY, Park J, Nilsson J, Lötvall J, Kim YK, Gho YS, ACS Nano 2013, 7, 7698. [DOI] [PubMed] [Google Scholar]
- [394].Gao J, Wang S, Wang Z, Biomaterials 2017, 135, 62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [395].Lunavat TR, Jang SC, Nilsson L, Park HT, Repiska G, Lasser C, Nilsson JA, Gho YS, Lotvall J, Biomaterials 2016, 102, 231. [DOI] [PubMed] [Google Scholar]
- [396].Wu JY, Ji AL, Wang ZX, Qiang GH, Qu Z, Wu JH, Jiang CP, Sci. Rep. 2018, 8, 2471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [397].Zhu L, Gangadaran P, Kalimuthu S, Oh JM, Baek SH, Jeong SY, Lee SW, Lee J, Ahn BC, Artif. Cells Nanomed., Biotechnol. 2018, 46, S166. [DOI] [PubMed] [Google Scholar]
- [398].Rayamajhi S, Nguyen TDT, Marasini R, Aryal S, Acta Biomater. 2019, 94, 482. [DOI] [PubMed] [Google Scholar]
- [399].Vazquez-Rios AJ, Molina-Crespo A, Bouzo BL, Lopez-Lopez R, Moreno-Bueno G, de la Fuente M, J. Nanobiotechnol. 2019, 17, 85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [400].Park KS, Svennerholm K, Shelke GV, Bandeira E, Lasser C, Jang SC, Chandode R, Gribonika I, Lotvall J, Stem Cell Res. Ther. 2019, 10, 231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [401].Gustafson CM, Shepherd AJ, Miller VM, Jayachandran M, Biol. Sex Differ. 2015, 6, 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [402].Kuravi SJ, Yates CM, Foster M, Harrison P, Hazeldine J, Hampson P, Watson C, Belli A, Midwinter M, Nash GB, PLoS One 2017, 12, e0183640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [403].Singer M, Deutschman CS, Seymour CW, Shankar-Hari M, Annane D, Bauer M, Bellomo R, Bernard GR, Chiche JD, Coopersmith CM, Hotchkiss RS, Levy MM, Marshall JC, Martin GS, Opal SM, Rubenfeld GD, van der Poll T, Vincent JL, Angus DC, JAMA, J. Am. Med. Assoc. 2016, 315, 801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [404].Mahida RY, Matsumoto S, Matthay MA, in Annual Update in Intensive Care and Emergency Medicine (Ed: Vincent J-L), Springer Nature, Switzerland: 2020. [Google Scholar]
- [405].Schmedes CM, Grover SP, Hisada YM, Goeijenbier M, Hultdin J, Nilsson S, Thunberg T, Ahlm C, Mackman N, Fors Connolly AM, J. Infect. Dis. 2020, 222, 1392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [406].Iba T, Watanabe E, Umemura Y, Wada T, Hayashida K, Kushimoto S, J. Intensive Care 2019, 7, 32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [407].Marik PE, Byrne L, van Haren F, J. Thorac. Dis. 2020, 12, S37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [408].Shin H, Park YH, Kim YG, Lee JY, Park J, PLoS One 2018, 13, e0194818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [409].Kirbas OK, Bozkurt BT, Asutay AB, Mat B, Ozdemir B, Ozturkoglu D, Olmez H, Islek Z, Sahin F, Tasli PN, Sci. Rep. 2019, 9, 19159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [410].Mann M, Mehta A, Zhao JL, Lee K, Marinov GK, GarciaFlores Y, Lu LF, Rudensky AY, Baltimore D, Nat. Commun. 2017, 8, 851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [411].Hill M, Tran N, Trends Cell Biol. 2021, 31, 3. [DOI] [PubMed] [Google Scholar]
- [412].Taylor DD, Shah S, Methods 2015, 87, 3. [DOI] [PubMed] [Google Scholar]
- [413].Buschmann D, Kirchner B, Hermann S, Marte M, Wurmser C, Brandes F, Kotschote S, Bonin M, Steinlein OK, Pfaffl MW, Schelling G, Reithmair M, Extracell J. Vesicles 2018, 7, 1481321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [414].Gamez-Valero A, Monguio-Tortajada M, Carreras-Planella L, Franquesa M, Beyer K, Borras FE, Sci. Rep. 2016, 6, 33641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [415].Mol EA, Goumans MJ, Doevendans PA, Sluijter JPG, Vader P, Nanomedicine 2017, 13, 2061. [DOI] [PubMed] [Google Scholar]
- [416].Whittaker TE, Nagelkerke A, Nele V, Kauscher U, Stevens MM, J. Extracell. Vesicles 2020, 9, 1807674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [417].Wiklander OP, Nordin JZ, O’Loughlin A, Gustafsson Y, Corso G, Mager I, Vader P, Lee Y, Sork H, Seow Y, Heldring N, Alvarez-Erviti L, Smith CI, e Blanc KL, Macchiarini P, Jungebluth P, Wood MJ, Andaloussi SE, J. Extracell. Vesicles 2015, 4, 26316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [418].Barrera-Saldana HA, Genomics 2020, 112, 721. [DOI] [PubMed] [Google Scholar]
- [419].Christaki E, Giamarellos-Bourboulis EJ, Clin. Genet. 2014, 86, 56. [DOI] [PubMed] [Google Scholar]
- [420].Piffoux M, Nicolas-Boluda A, Mulens-Arias V, Richard S, Rahmi G, Gazeau F, Wilhelm C, Silva AKA, Adv. Drug Delivery Rev. 2019, 138, 247. [DOI] [PubMed] [Google Scholar]