Abstract
Gadolinium-based contrast agents (GBCAs) have provided much needed image enhancement in magnetic resonance imaging (MRI) important in the advancement of disease diagnosis and treatment. The paramagnetic properties of ionized gadolinium have facilitated these advancements, but ionized gadolinium carries toxicity risk. GBCAs were formulated with organic chelates designed to reduce these toxicity risks from unbound gadolinium ions. They were preferred over iodinated contrast used in computed tomography and considered safe for use. As their use expanded, the development of new diseases associated with their use (including nephrogenic systemic fibrosis) has drawn more attention and ultimately caution with their clinical administration in those with impaired renal function. Use of GBCAs in those with preserved renal function was considered to be safe. However, in this new era with emerging clinical and experimental evidence of brain gadolinium deposition in those with repeated exposure, these safety assumptions are once again brought into question. This review article aims to add new perspectives in thinking about the role of GBCA in current clinical use. The new information begs for further discussion and consideration of the risk-benefit ratio of use of GBCAs.
Keywords: Chronic Kidney Disease, Contrast Media, Fibrosis, Gadolinium, Image Enhancement, Magnetic Resonance Imaging, Nephrogenic Fibrosing Dermopathy, Risk Assessment, Tomography, X-Ray Computed
Introduction
Magnetic resonance imaging (MRI) serves as an important imaging technique necessary for disease diagnosis and treatment. Use of gadolinium-based contrast agents (GBCAs) for MRI enhancement is useful in some instances and has been considered safe in most cases. Gadolinium is currently the only heavy metal suitable for MRI enhancement.
Properties of Gadolinium and Classification of GBCAs
Gadolinium (Gd) is a rare earth metal of the lanthanide series valued for its strong paramagnetic properties important for MRI enhancement. It is a heavy metal that forms 3+ charge with seven unpaired electrons. These unpaired electrons attract nearby protons, such as in water. Gd3+ complexation reduces the relaxation time of nearby protons in a magnetic field at low concentrations to provide enhanced image quality important for tissue differentiation in disease diagnosis (1). Gadolinium is not part of the normal composition of human tissue like the trace element manganese (2), but it is preferred for MRI enhancement due to its strong paramagnetic retention. Manganese can lose its paramagnetic properties through oxidation and can be retained in the liver with toxic potential. Animal studies discussed below showed that ionized gadolinium is toxic and must be chelated by a ligand for clinical use. These observations led to the development of modern GBCAs, which contain linear or macrocyclic organic ligands to encapsulate the ion and reduce its toxicity.
GBCAs are divided into linear and macrocyclic categories on the basis of the shape of the organic ligand. Linear agents are further divided into ionic and nonionic groups and include agents such as gadopentetate dimeglumine, gadodiamide, and gadoversetamide (3). Macrocyclic agents contain organic rings complexed with Gd3+ centrally and include agents such as gadoterate meglumine, gadobutrol, and gadoteridol (3). To date, there are eight agents approved by the US Food and Drug Administration (FDA) for MRI use (4). The American College of Radiology (5) further divides them into group I, group II, and group III agents as shown in Table 1. The recommended human dose is 0.1 mmol/kg (6).
Table 1.
American College of Radiology 2018 classification of gadolinium-based contrast agents into groups I, II, and III
| Group | Classification |
| Group I | Gadodiamide, gadopentetate dimeglumine |
| Group II | Gadobenate dimeglumine, gadobutrol, gadoterate acid, gadoteridol |
| Group III | Gadoxetate |
These GBCAs were considered more stable than gadolinium chloride with lower risk of Gd3+ dissociation as reflected by a thermodynamic stability constant. This constant is determined by the rate at which ionized gadolinium dissociates from the ligand in vitro at a pH of 1. GBCAs with higher thermodynamic stability constants were considered more stable despite the constants being derived under nonphysiologic conditions.
These agents were considered to be safe for intravenous (IV) use and were preferred to iodinated IV contrast used in computed tomography, which carried risk of nephrotoxicity. The biodistribution was assumed to be extracellular (volume of distribution in intravascular and interstitial spaces) with little intracellular distribution apart from the liver in some cases (3). In addition, they have limited protein binding, enabling rapid blood elimination with a short terminal t1/2 of 1.5 hours in those with normal renal function. Therefore, elimination of GBCAs is dependent on renal function, and their retention is higher with impaired kidney function (7). GBCAs were considered safe for use in those with normal renal function, but this assumption was challenged with development of a new disease observed after GBCA exposure.
Early Experience with Gadolinium Compounds
Humans were initially exposed to gadolinium from industrial use and later through the IV administration of GBCAs for MRI in the 1980s. The free gadolinium ion is toxic and must be complexed with an organic ligand to improve its tolerance for in vivo administration. The toxicity of ionized gadolinium was demonstrated in vivo by Abel and Talbot (8) in their experiment using inhaled gadolinium oxide in male and female guinea pigs for periods of 40, 80, and 120 days. The lungs of guinea pigs exposed to inhaled gadolinium oxide “had less elastance than lungs from unexposed guinea pigs” with histologic changes including “alveolar cell hypertrophy, septal wall thickening, lymphoid hyperplasia and macrophage proliferation” (8). It was also noted that the severity of the lesions increased as exposure time increased.
Intravenous gadolinium chloride led to toxic histologic, hematologic, and plasma chemistry changes in male and female Sprague-Dawley rats when administered in various doses (0.07, 0.14, and 0.35 mmol/kg) by Spencer et al. (9). At 48 hours postdose, hematologic changes included elevated white cell count in both sexes, elevated monocytes in males, decreased platelet numbers in males, and prolonged activated partial thromboplastin time in both sexes. Other plasma chemistry changes included elevated alkaline phosphatase, lactate dehydrogenase, alanine aminotransferase, and aspartate aminotransferase in males. Histologic changes included hepatocellular necrosis with hemorrhage around the portal tracts and enlarged Kupffer cells containing pale basophilic material in male rats. These hepatic changes evolved into multifocal chronic inflammation in all dose groups 14 days after administration. The same pale basophilic mineral deposits seen in Kupffer cells were also observed in the capillaries throughout the kidney in all rats receiving 0.14 and 0.35 mmol/kg gadolinium chloride dosing. Multifocal necrosis of the proximal convoluted tubule was also present in female rats.
Ionized gadolinium toxicity is not unique because other rare earth metals in the lanthanide group also exhibited toxic effects in animals as summarized by Hirano and Suzuki (10).
Potential Toxic Effects of GBCAs
Nephrogenic Systemic Fibrosis
The first case of a new fibrosing dermopathy distinct from scleroderma was reported in 1997. This condition was named nephrogenic systemic fibrosis (NSF) and presents with cutaneous thickening or indurated and discolored skin affecting the limbs and trunk but sparing the face (11,12). Skin lesion histology demonstrates dermal thickening and increased collagen bundles in the superficial fascia (11). Multiorgan involvement with internal organ fibrosis has been described leading to high mortality (11). NSF was recognized as distinct from systemic sclerosis, scleromyxoedema, and eosinophilic vasculitis due to negative anticentromere and anti-scl70 antibodies. Over 400 cases of NSF have been reported since 1997. The severity of illness, time to disease manifestation, and GBCA dosing exposure vary individually.
Collidge et al. (11) in 2007 noted the association between GBCA exposure and development of NSF in those with established renal failure in their retrospective analysis of local imaging data. Gadodiamide, a nonionic linear agent, was used in most NSF cases in this study with a positive association between cumulative dose and dosing events. The association between NSF after GBCA exposure in those with renal impairment was echoed by others throughout the years as more clinical experience accumulated. NSF-like lesions were observed in rats exposed to gadodiamide in a preclinical study by Sieber et al. (13).
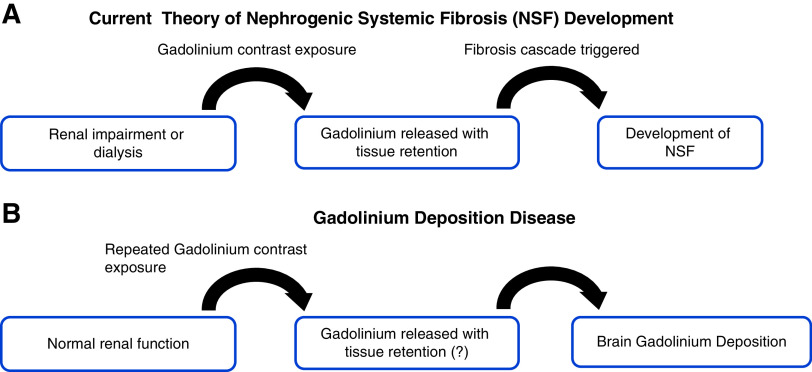
The pathogenesis of NSF development remains poorly understood. The prevailing theory is that prolonged GBCA retention due to renal impairment leads to the release of free gadolinium with tissue gadolinium deposition, ultimately triggering fibrosis (Figure 1). Kuo (14) argues that inorganic gadolinium in the body does not trigger NSF nor does it maintain disease activity. Other key risk factors, such as infection and inflammation, should be considered. The mechanism of NSF is yet to be determined.
Figure 1.
Gadolinium exposure implicated in the development of Nephrogenic Systemic Fibrosis (NSF) and Gadolinium Deposition Disease. (A) Current prevailing pathogenesis theory of nephrogenic systemic fibrosis. Patients with renal impairment or on dialysis are exposed to gadolinium-based contrast agent (GBCA) with retention of these agents due to low renal function. Gadolinium is liberated with decreased clearance leading to tissue deposition and ultimately triggering fibrosis resulting in nephrogenic systemic fibrosis. (B) Gadolinium deposition disease pathogenesis theory is less clear. Most notable are recurrent exposures to GBCA in those with normal renal function and its association with brain deposition of gadolinium, but the mechanism is unclear. MRI, magnetic resonance imaging.
NSF was associated with exposure to many brands of GBCA despite claims of low likelihood on the basis of thermodynamic stability. Gadobutrol, an agent considered with the lowest risk, was associated with NSF. Endrikat et al. (15) advised caution in use of gadobutrol in those with CKD stage 4 or 5 in their systematic review.
The association of NSF with GBCA exposure in renal impairment led to avoidance of these agents in those with low renal function or on dialysis. GBCA use in those without renal impairment was considered safe, with >300 million doses administered to date since the introduction in the 1980s. It should be noted that renal impairment was defined on the basis of eGFR as stated by the American College of Radiology (5) in ACR Manual on Contrast Media Version 10.3 ACR Committee on Drugs and Contrast Media. However, recent findings of gadolinium brain deposition have brought these assumptions under question.
Gadolinium Deposition Disease
Kanda et al. (16) discovered high signal intensity of both the dentate nucleus and globus pallidus on unenhanced T1-weighted magnetic resonance images in 19 patients who had received at least six doses of linear GBCAs compared with 16 patients who had MRI without GBCAs. The retrospective study revealed a dose relationship between high signal intensity with a history of GBCA administration independent of renal function. No specific GBCA was implicated as these patients had multiple exposures at different facilities, limiting comprehensive record review. Other retrospective studies confirmed these findings in those with multiple sclerosis and meningioma exposed to multiple GBCA doses in prior MRI (17). Postmortem studies confirmed that T1 shortening on MRI is probably due to gadolinium deposition (17,18). Nehra et al. (19) demonstrated gadolinium accumulation within the cerebrospinal fluid of patients who received the macrocyclic GBCA gadobutrol. McDonald et al. (20) showed gadolinium deposition in human brain tissue of adults who had received gadodiamide for MRI without intracranial abnormalities on postmortem examination. They also performed animal studies that showed gadolinium tissue deposition in rats was two- to fourfold higher following the administration of linear agents gadodiamide and gadopentate dimeglumine compared with macrocyclic agents gadobutrol and gadoteridol (21). Gadolinium has also been observed with macrocyclic agents with the extent of deposition varying between agents (22). The severity of illness and time to disease manifestation vary individually.
These new findings led to cautionary statements by world health organizations including the American College of Radiology, Health Canada, the Canadian Association of Radiologists, the Australian Department of Health, the European Medicines Agency, the New Zealand Medicines and Medical Devices Authority, and the Japanese Pharmaceuticals and Medical Devices Agency (23–29). The US FDA urged further patient education for those receiving GBCAs and for health care professionals to consider retention characteristics when choosing GBCAs for patients at higher risk for gadolinium retention (4). In addition, the FDA also required GBCA manufacturers to conduct additional human and animal studies to assess the safety of these agents (4). The National Institutes of Health urged the use of GBCAs only when clinically indicated with consideration of FDA label indications and dosing schemes, and the use of macrocyclic GBCAs in lieu of linear GBCAs if indicated. They also encouraged intra- and interdepartmental research programs to evaluate T1 shortening in the brain and other organs of those patients who received multiple doses of GBCAs (17).
Tissue gadolinium deposition was found in preclinical studies with rats exposed to gadolinium chloride preceding the above human findings. When NSF was discovered, many investigative groups replicated this condition in animal models to study it further. Our research group observed negative biologic effects of GBCA exposure in cell culture with increased expression of profibrotic proteins such as α-smooth muscle actin and fibronectin (30). We replicated NSF in both rats and mice, showing dermal histopathologic changes mirroring those observed in human NSF skin (7). We also observed gadolinium deposition in multiple organs including skin, kidney, and heart and found evidence of bone marrow–derived fibrocytes in these tissues (31,32). Myeloid C-C chemokine receptor 2 seemed to play an important role in fibrosis triggered by GBCA exposure (33). Furthermore, our animal studies uncovered metabolic disruptions caused by GBCA exposure (34).
Nephrotoxicity of GBCAs
GBCAs have been preferred for image enhancement over IV iodinated contrast because of an assumed lower potential to cause contrast-induced nephrotoxicity (CIN). This assumption was on the basis of their lower viscosity and the lower dose of use (6). Naito et al. (35) conducted a prospective, randomized human study comparing CIN between gadodiamide and gadopentetate in 694 patients with CKD stages 1–3. Using cystatin-C as a biomarker of early stages of CIN, they found that gadodiamide exposure led to cystatin-C increase in patients with CKD stage 1. In addition, gadodiamide exposure reduced creatinine clearance in patients with CKD stage 2. These changes were not observed with gadopentetate. These findings suggested “that gadodiamide has slight, but clinically unimportant, nephrotoxicity in subgroups of patients with CKD” (35). The study was limited by a single measurement of serum creatinine levels and confounders for AKI such as congestive heart failure and concomitant nephrotoxic drug administration, rendering its conclusions about the safety of gadopentetate weak. The potential for CIN remains unclear in GBCA administration, especially in those with multiple lifetime exposures.
Experimentally, we observed renal glomerular changes with the presence of lipid-like droplets and electron densities in mice exposed to GBCA (Figure 2). Proximal tubules demonstrate lipid-laden vacuoles with electron-dense nanostructures and widespread mitochondrial death (Figure 3). Gadolinium-rich precipitates rim these lipid-laden deposits in mouse renal proximal tubule (Figure 4). Di Gregorio et al. (36) showed that erythrocytes and leukocytes can retain gadolinium, challenging the previously held notion that GBCA remained mostly in the extracellular space. These results indicate that GBCAs may exert biologic effects not yet understood.
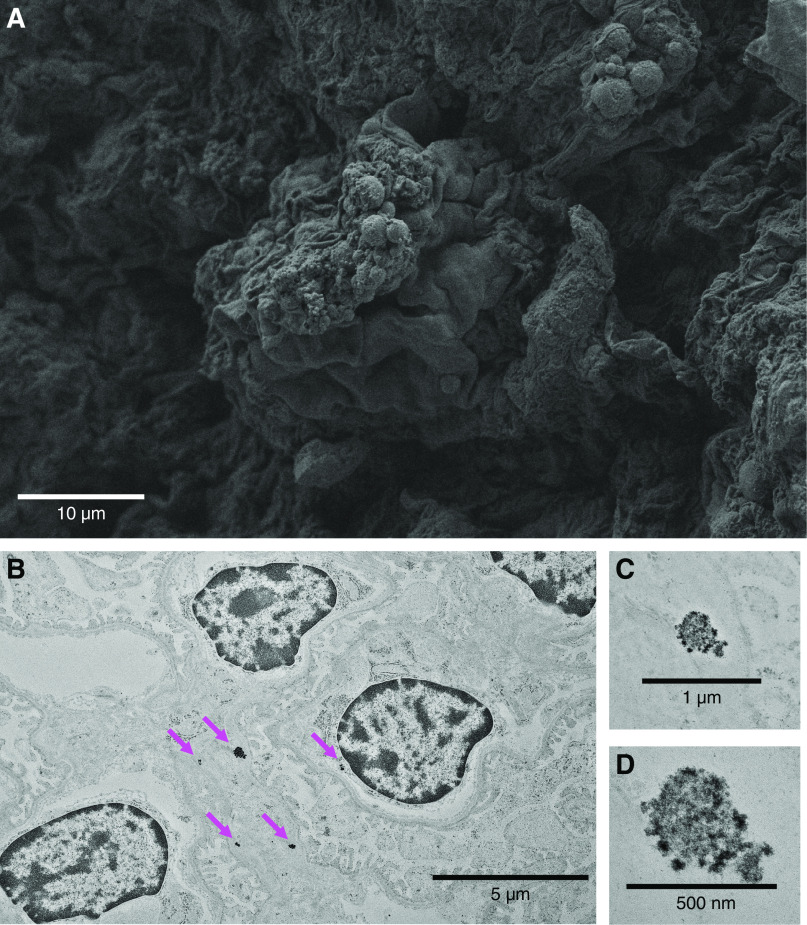
Figure 2.
Gadolinium-based contrast agents induce renal glomerular changes. It has been demonstrated that gadolinium-based contrast agent treatment induces hypercholesterolemia, hypertriglyceridemia, insulin resistance, and the Warburg effect in renal cortex (33). (A) Renal cortex from contrast-treated animals demonstrated lipid-like droplets. Scanning electron microscopy in fixed renal cortical sections (Zeiss Sigma 300 field emission scanning electron microscopy of formalin/glutaraldehyde-fixed tissue, osmicated—1 for 1 hour). (B) Electron densities in the glomeruli of contrast-treated mice. High electron densities (magenta arrows) occurred intracellularly and in the subepithelial basement membrane. (C and D) High-magnification imaging demonstrates that these nanostructures have a spiculated, sea urchin–like appearance previously reported in skin (20). Transmission electron microscopy, Hitachi HT7700 with AMT 16-megapixel digital camera. Herein, fixed sections of 80-nm thickness were stained with osmium for the transmission electron microscopy images obtained with the Hitachi HT7700.
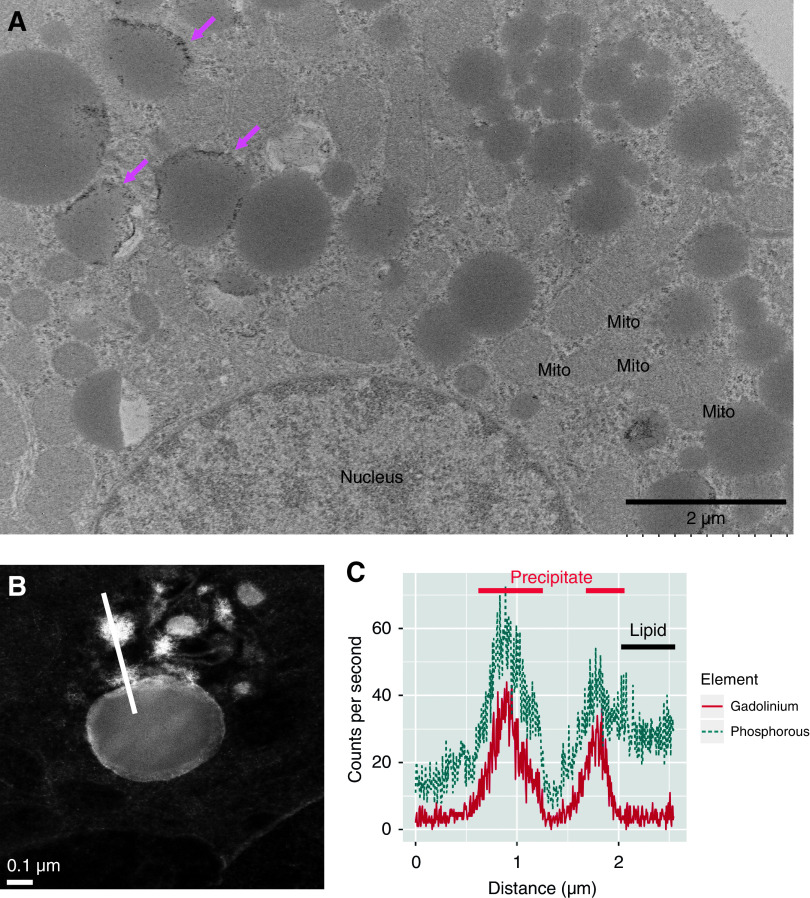
Figure 3.
Gadolinium-based contrast agents ravage the renal proximal tubule. (A) Invariably, treatment with gadolinium-based contrast agent leads to a peppering of the electron-dense nanostructures in lipid-laden vacuoles of proximal tubular cells. The low-magnification transmission electron photomicrograph demonstrates active tubular damage. (B) The magnified view highlights the lipid-laden vacuoles (cyan arrows) and widespread mitochondrial death (magenta arrows). (C) The electron-dense nanostructure precipitates contain gadolinium, phosphorus, and calcium. Sections (200-µm thick, epoxy embedded) were analyzed by scanning transmission electron microscopy equipped with energy-dispersive x-ray spectroscopy. Spectra for the electron-dense precipitates (n=13) and renal cortex from an untreated animal (n=6 measurements) were normalized. Areas under the curves were compared using William Sealy Gosset two-tailed t tests. JEOL 2010F FEGSTEM operating at 200 kV with Oxford Analytical Aztec energy-dispersive x-ray spectroscopy system equipped with an XMax 80N 80-mm2 silicon drift detector.
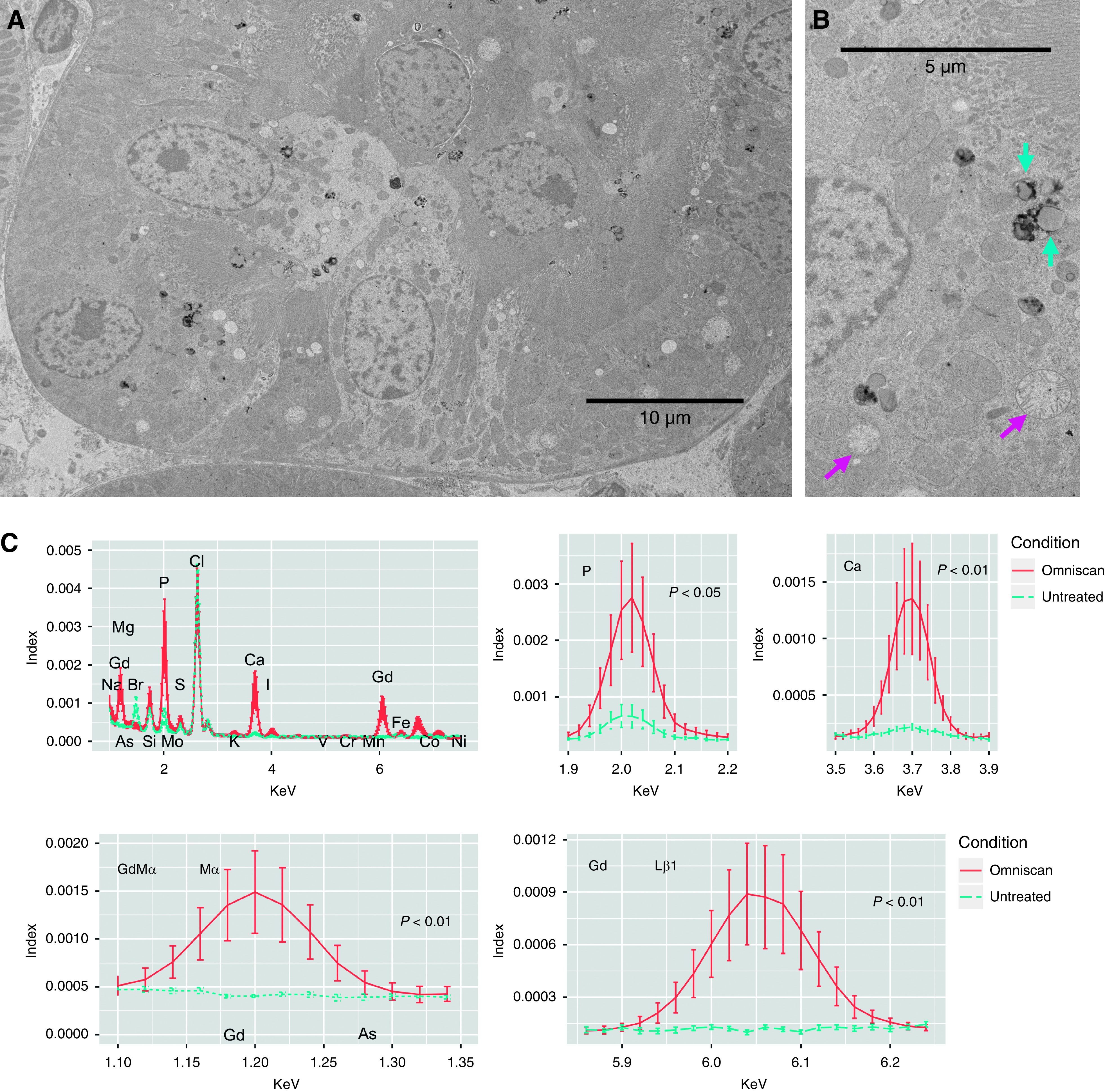
Figure 4.
Gadolinium-rich precipitates rim the vacuolized lipid-laden deposits in renal proximal tubule. (A) Fixed (formalin and glutaraldehyde-based transmission electron microscopy fixative) renal cortex from the mouse in Figure 3 was osmicated and examined with transmission electron microscopy. Lipid-filled vacuoles often were rimmed with electron-dense precipitate (magenta arrows). Mitochondria (Mito) were often swollen or disintegrating. Hitachi HT7700 with AMT 16-megapixel digital camera. (B) Energy-dispersive x-ray spectroscopy line scanning was performed through cytoplasm, the electron-dense precipitates, and the lipid-like body with scanning transmission electron microscopy (high-angle annular dark-field image). (C) The electron-dense material (bright high-Z regions in [B]) demonstrated high amounts of both gadolinium and phosphorus as well as the border of the lipid rim. JEOL 2010F FEGSTEM operating at 200 kV with Oxford Analytical AZtec energy-dispersive x-ray spectroscopy system equipped with an XMax 80N 80-mm2 silicon drift detector.
Renal Function and the Safety of GBCAs
Renal function determines the clearance rate of GBCAs and ultimately, their safety. It is reflected by GFR as measured by the urinary clearance rate of a nonprotein-bound, exogenously administered agent not secreted or metabolized by the kidneys. These GFR measurements are often laborious and difficult, leading to the use of endogenous biomarkers such as creatinine to derive an eGFR. Creatinine-based eGFR calculations use equations that include variables such as age, sex, and race. Creatinine is secreted by the renal tubules, and therefore, its clearance may overestimate true GFR. Creatinine-based equations were developed using a small number of study patients, and it is well known that they do not apply to all patients generally (37–39). eGFR is an artificial approximation of actual renal function, which may not correlate well with true renal function if the patient characteristics are not the same as the study population. If these assumptions are false, erroneous conclusions of safety will be drawn. It is also fallacious to assume that rapid clearance of GBCA (i.e., rapid removal from the body) ensures safety because the biodistribution of these agents appears to be more complex than previously believed. The use of dialysis to remove GBCA after exposure to prevent NSF was not always effective, demonstrating that removal alone is insufficient to prevent disease. Administration recommendations on the basis of eGFR should take into consideration these inherent limitations.
New Guidelines: Limitations, Misinterpretations, and Our Perspective
Collective clinical experience with GBCA in MRI spans >30 years since their introduction. MRI use occurs more in the diagnosis, treatment, and monitoring of central nervous system diseases and cancer. However, not all MRI studies require enhancement and unnecessary GBCA exposure may occur. When GBCA use is needed for diagnosis and treatment, the risk of adverse outcomes should be seriously weighed against potential benefits. The American College of Radiology guidelines for GBCA administration advise against administration of group I and group III agents in those on dialysis or with CKD stage 4 or 5 to avoid potential NSF development. However, the same guidelines also state that the risk of NSF with group II agents “is sufficiently low or possibly nonexistent such that assessment of renal function with a questionnaire or laboratory testing is optional prior to intravenous administration.” Clinicians should be aware that, although NSF has not been associated with group II agents in CKD stage 4 or 5 and ESKD, the studies that support this conclusion are limited by their retrospective nature, underpowering, and limited dose exposure. Practitioners may erroneously misinterpret these guidelines to mean absolute safety with group II GBCA.
The recommendations on the basis of eGFR carry intrinsic limitations because these estimates are calculated using endogenous biomarkers such as creatinine and cystatin-C, with numerous factors limiting their accuracy (37). The use of eGFR may lead to overestimation of true renal function, mistaken conclusions about GBCA safety, and unnecessary use without true clinical benefit, endangering patient safety.
Clinicians who rely on estimations to approximate renal function may not realize that their patients lack the same characteristics as the study population from which these equations are derived, rendering these estimates invalid. We suggest the following considerations. (1) When using any equations to estimate GFR, the clinician must know and understand the patient characteristics and clinical conditions from which these equations are derived. (2) The clinician must determine whether his patient is similar to the study population to determine the validity and applicability of these calculations. If the patient does not fit the study population, then these estimates are invalid, and the clinician should seek more accurate measurements. We should not harbor illusions about eGFR safety thresholds because our clinical data have shown these assumptions to be unreliable.
The discovery of NSF and newer findings of brain gadolinium deposition reflect ongoing experience with GBCAs, and scientific theories should evolve as new evidence emerges challenging long-held assumptions. Our clinical thought process should also become more sophisticated as new information becomes available. Although brain gadolinium deposition has no objective adverse clinical effects so far, its long-term clinical significance remains uncertain, and it is premature to assume lack of harm. Observed brain gadolinium retention with repeated exposure to GBCA administration in those with normal renal function should spur caution and re-examination of current practice patterns. In particular, informed consent should be standard for all stages of CKD when administering these agents because patients should know the long-term risk to their health in their decision making. Deposition disease may be an unanticipated outcome; therefore, its risk should be fully disclosed and treated no differently than disclosure of potential adverse effects of other medical treatments. Although we currently cannot state a numerical value of risk, it does not mean that we can ignore the patient’s right to know. The use of dialysis to remove GBCAs to mitigate harm has not proven effective in NSF and should not be used to prevent deposition disease. Dialysis, an invasive procedure with its own morbidity and risks, should not be gratuitously applied to remedy a problem preventable with careful thought and consideration.
We advise clinicians to do the following to improve patient safety: (1) limit the volume of administered GBCA when used; (2) increase patient education and incorporate informed consent and shared decision making when GBCA administration occurs; (3) maintain careful institutional records of administered agents along with the volume and frequency; and (4) use institutional outcome review and follow-up for gadolinium deposition in those receiving multiple doses.
GBCA use has grown since their introduction >30 years ago. Although GBCAs have played an important role in MRI enhancement necessary for disease detection, their use is not without risk, and the long-term biologic and clinical effects remain to be seen. Their safety record has been challenged with the finding of NSF and deposition disease; therefore, caution and careful deliberation are essential when considering their use. The clinical evidence so far suggests that there is no renal function at which GBCA use is absolutely safe and risk free. For those patients needing repeat MRI with potential for multiple GBCA exposures, their risk of deposition disease and NSF may increase with cumulative dose. Clinicians should understand the limitations of eGFR in approximating true renal function before assuming low risk of gadolinium-related diseases. Informed consent should become standard with GBCA administration along with detailed records of type of GBCA, dose, and frequency of administration. Careful maintenance of records and clinical outcomes should be used to improve patient safety. Clinicians should exert discretion when ordering MRI with gadolinium enhancement because it may not confer greater clinical utility but may expose patients to unnecessary risk. As GBCA gatekeepers, they bear the ultimate responsibility for iatrogenic complications.
Disclosures
All authors have nothing to disclose.
Funding
This work was supported by the National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases grant DK-102085; US Department of Veterans Affairs grant I01 BX001958 (to B. Wagner); Dialysis Clinic, Inc. (to B. Wagner); New Mexico Brain & Behavioral Health Institute grant 2018-1008 (to B. Wagner); Center for Integrative Nanotechnology grant 2019AU0120 (to B. Wagner). Dialysis Clinic, Inc. provides financial support for the Kidney Institute of New Mexico and the work in this publication.
Author Contributions
B. Wagner conceptualized the study; A. Brearley, G. P. Escobar, J. DeAguero, T. Howard, and B. Wagner were responsible for data curation; J. DeAguero, G. P. Escobar, X. Trejo, and B. Wagner were responsible for investigation; G. P. Escobar and B. Wagner were responsible for methodology; B. Wagner was responsible for formal analysis, funding acquisition, project administration, resources, validation, and visualization; B. Wagner provided supervision; B. Wagner wrote the original draft; and J. DeAguero and C. Do reviewed and edited the manuscript.
Footnotes
See related editorial, “Use of Gadolinium-Based Contrast Agents in Patients with Severe Renal Impairment. Absence of Risk Versus Caution: A Nephrologist's Perspective” on pages 433–435
References
- 1.Weinmann HJ, Brasch RC, Press WR, Wesbey GE: Characteristics of gadolinium-DTPA complex: A potential NMR contrast agent. AJR Am J Roentgenol 142: 619–624, 1984 [DOI] [PubMed] [Google Scholar]
- 2.Mertz W: The essential trace elements. Science 213: 1332–1338, 1981 [DOI] [PubMed] [Google Scholar]
- 3.Aime S, Caravan P: Biodistribution of gadolinium-based contrast agents, including gadolinium deposition. J Magn Reson Imaging 30: 1259–1267, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.FDA.gov : FDA Drug Safety Communication: FDA warns that gadolinium-based contrast agents (GBCAs) are retained in the body; requires new class warnings, 2018. Available at: https://www.fda.gov/Drugs/DrugSafety/ucm589213.htm. Accessed November 4, 2018
- 5.American College of Radiology : ACR Manual on Contrast Media Version 10.3 ACR Committee on Drugs and Contrast Media, Reston, VA, American College of Radiology, 2018, pp 22–28 [Google Scholar]
- 6.Perazella MA: Current status of gadolinium toxicity in patients with kidney disease. Clin J Am Soc Nephrol 4: 461–469, 2009 [DOI] [PubMed] [Google Scholar]
- 7.Wagner B, Drel V, Gorin Y: Pathophysiology of gadolinium-associated systemic fibrosis. Am J Physiol Renal Physiol 311: F1–F11, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Abel M, Talbot RB: Gadolinium oxide inhalation by Guinea pigs: A correlative functional and histopathologic study. J Pharmacol Exp Ther 157: 207–213, 1967 [PubMed] [Google Scholar]
- 9.Spencer AJ, Wilson SA, Batchelor J, Reid A, Rees J, Harpur E: Gadolinium chloride toxicity in the rat. Toxicol Pathol 25: 245–255, 1997 [DOI] [PubMed] [Google Scholar]
- 10.Hirano S, Suzuki KT: Exposure, metabolism, and toxicity of rare earths and related compounds. Environ Health Perspect 104[Suppl 1]: 85–95, 1996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Collidge TA, Thomson PC, Mark PB, Traynor JP, Jardine AG, Morris ST, Simpson K, Roditi GH: Gadolinium-enhanced MR imaging and nephrogenic systemic fibrosis: Retrospective study of a renal replacement therapy cohort [published correction appears in Radiology 255: 308, 2010]. Radiology 245: 168–175, 2007 [DOI] [PubMed] [Google Scholar]
- 12.Marckmann P, Skov L, Rossen K, Dupont A, Damholt MB, Heaf JG, Thomsen HS: Nephrogenic systemic fibrosis: Suspected causative role of gadodiamide used for contrast-enhanced magnetic resonance imaging. J Am Soc Nephrol 17: 2359–2362, 2006 [DOI] [PubMed] [Google Scholar]
- 13.Sieber MA, Pietsch H, Walter J, Haider W, Frenzel T, Weinmann HJ: A preclinical study to investigate the development of nephrogenic systemic fibrosis: A possible role for gadolinium-based contrast media. Invest Radiol 43: 65–75, 2008 [DOI] [PubMed] [Google Scholar]
- 14.Kuo PH: NSF-active and NSF-inert species of gadolinium: Mechanistic and clinical implications. AJR Am J Roentgenol 191: 1861–1863, 2008 [DOI] [PubMed] [Google Scholar]
- 15.Endrikat J, Vogtlaender K, Dohanish S, Balzer T, Breuer J: Safety of gadobutrol: Results from 42 clinical phase II to IV studies and postmarketing surveillance after 29 million applications. Invest Radiol 51: 537–543, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kanda T, Ishii K, Kawaguchi H, Kitajima K, Takenaka D: High signal intensity in the dentate nucleus and globus pallidus on unenhanced T1-weighted MR images: Relationship with increasing cumulative dose of a gadolinium-based contrast material. Radiology 270: 834–841, 2014 [DOI] [PubMed] [Google Scholar]
- 17.Malayeri AA, Brooks KM, Bryant LH, Evers R, Kumar P, Reich DS, Bluemke DA: National Institutes of health perspective on reports of gadolinium deposition in the brain. J Am Coll Radiol 13: 237–241, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.McDonald RJ, McDonald JS, Kallmes DF, Jentoft ME, Murray DL, Thielen KR, Williamson EE, Eckel LJ: Intracranial gadolinium deposition after contrast-enhanced MR imaging. Radiology 275: 772–782, 2015 [DOI] [PubMed] [Google Scholar]
- 19.Nehra AK, McDonald RJ, Bluhm AM, Gunderson TM, Murray DL, Jannetto PJ, Kallmes DF, Eckel LJ, McDonald JS: Accumulation of gadolinium in human cerebrospinal fluid after gadobutrol-enhanced MR imaging: A prospective observational cohort study. Radiology 288: 416–423, 2018 [DOI] [PubMed] [Google Scholar]
- 20.McDonald RJ, McDonald JS, Kallmes DF, Jentoft ME, Paolini MA, Murray DL, Williamson EE, Eckel LJ: Gadolinium deposition in human brain tissues after contrast-enhanced MR imaging in adult patients without intracranial abnormalities. Radiology 285: 546–554, 2017 [DOI] [PubMed] [Google Scholar]
- 21.McDonald RJ, McDonald JS, Dai D, Schroeder D, Jentoft ME, Murray DL, Kadirvel R, Eckel LJ, Kallmes DF: Comparison of gadolinium concentrations within multiple rat organs after intravenous administration of linear versus macrocyclic gadolinium chelates. Radiology 285: 536–545, 2017 [DOI] [PubMed] [Google Scholar]
- 22.Gulani V, Calamante F, Shellock FG, Kanal E, Reeder SB; International Society for Magnetic Resonance in Medicine: Gadolinium deposition in the brain: Summary of evidence and recommendations. Lancet Neurol 16: 564–570, 2017 [DOI] [PubMed] [Google Scholar]
- 23.ACR-ASNR : ACR–ASNR Position Statement on the Use of Gadolinium Contrast Agents, 2016. Available at: https://www.asnr.org/wp-content/uploads/2017/03/ACR_ASNR_Position_Statement_on_the_Use_of_Gadolinium_Contrast_Agents.pdf. Accessed November 4, 2018
- 24.Canada.gc.ca : Information Update—New safety information on injectable gadolinium-based contrast agents used in MRI scans, 2017. Available at: http://healthycanadians.gc.ca/recall-alert-rappel-avis/hc-sc/2017/61676a-eng.php. Accessed November 4, 2018
- 25.TGA–Therapeutic Goods Administration : Gadolinium-based contrast agents for MRI scans, 2017. Available at: https://www.tga.gov.au/alert/gadolinium-based-contrast-agents-mri-scans. Accessed November 4, 2018
- 26.European Medicines Agency : PRAC confirms restrictions on the use of linear gadolinium agents: Benefit-risk balance of certain linear gadolinium agents no longer favourable, 2017. Available at: https://www.ema.europa.eu/documents/referral/gadolinium-article-31-referral-prac-confirms-restrictions-use-linear-gadolinium-agents_en.pdf. Accessed November 4, 2018
- 27.Medsafe.govt.nz : Trans-Tasman Early Warning System—Alert Communication, 2017. Available at: http://www.medsafe.govt.nz/safety/EWS/2017/GadoliniumContrastAgents.asp. Accessed November 4, 2018
- 28.PMDA.go.jp : Report on the Investigation Results, 2017. Available at: https://www.pmda.go.jp/files/000221379.pdf. Accessed November 4, 2018
- 29.Costa AF, van der Pol CB, Maralani PJ, McInnes MDF, Shewchuk JR, Verma R, Hurrell C, Schieda N: Gadolinium deposition in the brain: A systematic review of existing guidelines and policy statement issued by the Canadian association of Radiologists. Can Assoc Radiol J 69: 373–382, 2018 [DOI] [PubMed] [Google Scholar]
- 30.Do C, Barnes JL, Tan C, Wagner B: Type of MRI contrast, tissue gadolinium, and fibrosis. Am J Physiol Renal Physiol 307: F844–F855, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wagner B, Tan C, Barnes JL, Ahuja S, Davis TL, Gorin Y, Jimenez F: Nephrogenic systemic fibrosis: Evidence for oxidative stress and bone marrow-derived fibrocytes in skin, liver, and heart lesions using a 5/6 nephrectomy rodent model. Am J Pathol 181: 1941–1952, 2012 [DOI] [PubMed] [Google Scholar]
- 32.Drel VR, Tan C, Barnes JL, Gorin Y, Lee DY, Wagner B: Centrality of bone marrow in the severity of gadolinium-based contrast-induced systemic fibrosis. FASEB J 30: 3026–3038, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Do C, Drel V, Tan C, Lee D, Wagner B: Nephrogenic systemic fibrosis is mediated by myeloid C-C Chemokine Receptor 2. J Invest Dermatol 139: 2134–2143 e2, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Do C, Ford B, Lee DY, Tan C, Escobar P, Wagner B: Gadolinium-based contrast agents: Stimulators of myeloid-induced renal fibrosis and major metabolic disruptors. Toxicol Appl Pharmacol 375: 32–45, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Naito S, Tazaki H, Okamoto T, Takeuchi K, Kan S, Takeuchi Y, Kamata K: Comparison of nephrotoxicity between two gadolinium-contrasts, gadodiamide and gadopentetate in patients with mildly diminished renal failure. J Toxicol Sci 42: 379–384, 2017 [DOI] [PubMed] [Google Scholar]
- 36.Di Gregorio E, Furlan C, Atlante S, Stefania R, Gianolio E, Aime S: Gadolinium retention in erythrocytes and leukocytes from human and murine blood upon treatment with gadolinium-based contrast agents for magnetic resonance imaging. Invest Radiol 55: 30–37, 2020 [DOI] [PubMed] [Google Scholar]
- 37.Inker LA, Levey AS, Coresh J: Estimated glomerular filtration rate from a panel of filtration markers-hope for increased accuracy beyond measured glomerular filtration rate? Adv Chronic Kidney Dis 25: 67–75, 2018 [DOI] [PubMed] [Google Scholar]
- 38.Poggio ED, Nef PC, Wang X, Greene T, Van Lente F, Dennis VW, Hall PM: Performance of the Cockcroft-Gault and modification of diet in renal disease equations in estimating GFR in ill hospitalized patients. Am J Kidney Dis 46: 242–252, 2005 [DOI] [PubMed] [Google Scholar]
- 39.Skluzacek PA, Szewc RG, Nolan CR 3rd, Riley DJ, Lee S, Pergola PE: Prediction of GFR in liver transplant candidates. Am J Kidney Dis 42: 1169–1176, 2003 [DOI] [PubMed] [Google Scholar]