Abstract
Metals such as iron, manganese, copper, and zinc are recognized as essential trace elements. These trace metals play critical roles in development, growth, and metabolism, participating in various metabolic processes by acting as cofactors of enzymes or providing structural support to proteins. Deficiency or toxicity of these metals can impact human and animal health, giving rise to a number of metabolic and neurological disorders. Proper breakdown, absorption, and elimination of these trace metals is a tightly regulated process that requires crosstalk between the host and these micronutrients. The gut is a complex system that serves as the interface between these components, but other factors that contribute to this delicate interaction are not well understood. The gut is home to trillions of microorganisms and microbial genes (the gut microbiome) that can regulate the metabolism and transport of micronutrients and contribute to the bioavailability of trace metals through their assimilation from food sources or by competing with the host. Furthermore, deficiency or toxicity of these metals can modulate the gut microenvironment, including microbiota, nutrient availability, stress, and immunity. Thus, understanding the role of the gut microbiota in the metabolism of manganese, iron, copper, and zinc, as well as in heavy metal deficiencies and toxicities, and vice versa, may provide insight into developing improved or alternative therapeutic strategies to address emerging health concerns. This review describes the current understanding of how the gut microbiome and trace metals interact and affect host health, particularly in pigs.
Keywords: Gut microbiota, Microbiome, Iron, Manganese, Zinc, Copper
1. Introduction
Trace metals are essential in humans and animals as they serve as critical cofactors and components of enzymes for various biological processes (Shannon and Hill, 2019). Trace elements include transition metals such as vanadium, chromium, manganese, iron, cobalt, copper, zinc, and molybdenum, as well as non-metals such as selenium, fluorine, and iodine. They are classified as micronutrients because the mammalian body requires very small quantities (generally less than 100 mg/day) of these elements. Approximately 60% of known enzymes have at least one metal as a cofactor, with zinc being the most common, followed by iron and manganese (Andreini et al., 2008). Moreover, trace metals participate in redox reactions because free radicals interact with metal-bound superoxide dismutase and promote their decomposition into less or non-toxic substances. This indicates that trace metals play critical roles in oxidative stress and numerous metabolic processes. Despite their importance in various biological activities, chronic exposure to high levels of trace metals causes health risks and toxicity. Thus, homeostasis of trace metal elements must be tightly regulated to prevent certain diseases.
The required dietary amounts of trace metal elements in the body are provided primarily by food sources. However, trace elements must be released from food and assimilated in the gastrointestinal tract to maintain an adequate supply of micronutrients and cellular stability. Several studies have identified challenges in maintaining this balance because nutrient composition, digestibility, and availability vary among food sources, in addition to the variability in the resident microbiota (Che et al., 2019). The gut comprises approximately 1012 microorganisms per gram of gut contents that form a distinct community known as the gut microbiota (Niu et al., 2015). Each bacterium contains thousands of functionally relevant genes and pathways to support essential gut functions and prevent intestinal dysbiosis (Sitkin et al., 2016; Pickard et al., 2017). Some of the gut microbiota and microbial genes (the gut microbiome) promote a healthy gut by maintaining the diversity of metabolic functions, preserving the integrity of the gut mucosa, and enhancing innate immunity as the first line of defense against foreign and toxic substances (Sitkin et al., 2016; Pickard et al., 2017). In fact, several gut bacteria and pathogens such as Campylobacter jejuni, certain Yersinia spp., and Salmonella enterica Typhimurium have shown the ability to transport, metabolize, and use trace metals for survival (Rakin et al., 2012; Diaz-Ochoa et al., 2016; Crofts et al., 2018). Interestingly, the gut microbiome can compete with the host to obtain trace metals required to persist in the gut, but the host has developed mechanisms to sequester trace metals and prevent bacterial access to them (Becker and Skaar, 2014). This dynamic interaction between the host, the gut microbiome, and metals provides an interesting field of research. However, understanding the mechanisms underlying this competition for micronutrients is still underway.
As the gut microbiome participates in maintaining normal gut functions and immunity, several factors, including host genetics and environmental conditions, can significantly modulate the diversity of the gut microbiome. For instance, genetic factors, including certain genotypes and gene mutations, have been shown to modulate the initial bacterial colonization in neonates and the gut microbiota profile in adult stages (Pajarillo et al., 2014a, Pajarillo et al., 2014b). Meanwhile, environmental factors such as diet, lifestyle choices, nutritional deficiencies, treatment interventions, and exposure to pathogens and toxicants may either promote a healthy gut or induce intestinal dysbiosis in humans. In addition, breeding method, geographical region, and feeding strategy can influence the microbial diversity of the animal gut (Pajarillo et al., 2015).
Both abnormally low and high levels of trace elements in the body can elicit health problems associated with deficiency and toxicity, respectively (Chowdhury and Chandra, 1987; Hotz et al., 2003). For example, deficiency of iron can lead to iron deficiency anemia (IDA) in humans, a serious illness characterized by low levels of iron in the body causing a reduction in the number of red blood cells supplying nutrients and oxygen throughout the body. Copper deficiency can result from a rare genetic disorder known as Menkes disease, which can impair the development of connective tissues, muscles, and the brain. Deficiency of zinc is involved in a number of gastrointestinal disorders, including Crohn's disease and irritable bowel syndrome in humans (Han et al., 2017; Higashimura et al., 2020), as well as gut dysbiosis in animals (Satessa et al., 2020). Thus, metal deficiencies present serious health concerns in the human population and cause counterproductive problems in the animal industry. Meanwhile, chronic exposure to high levels of trace metals can promote toxicities in the liver, kidneys, and brain, leading to a number of irreversible and debilitating disorders.
This review describes how trace metals such as iron, manganese, zinc, and copper are metabolized, transported, and used by members of the gut microbiota and the host (Fig. 1). We also present evidence of the impacts of mineral supplementation, deficiencies, and toxicities on animal health by modulating the gut microbiome and function, particularly in pigs. Table 1 shows a summary of the effects of iron, manganese, zinc, and copper in modulating the gut microbiota of pigs. Finally, we review the mechanisms by which the gut microbiota is modulated by trace metals and show that the gut microenvironment plays a central role in mineral bioavailability and mucosal immunity in animals.
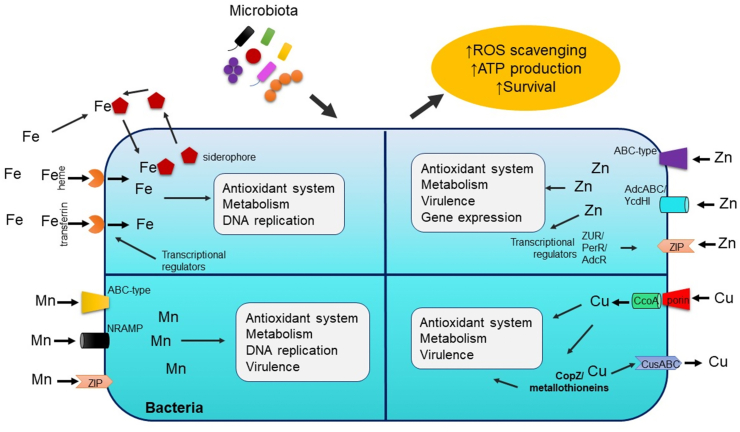
Fig. 1.
General overview of mechanisms of trace metal homeostasis and utilization by microbiota. Bacteria developed several transport systems for iron, manganese, zinc, and copper absorption. Trace metals alters bacterial survival and fitness by modulating its ability to fight oxidative stress and produce energy via metabolic processes and metal-bound enzymes. ROS = reactive oxygen species; Fe = iron; Mn = manganese; Zn = zinc; Cu = copper; ABC-type = ATP-binding protein; AdcABC/YcdHI = zinc transporter system; ZUR = zinc uptake regulator; PerR = peroxide operon regulator; AdcR = adhesin competence repressor; NRAMP = natural resistance-associated macrophage protein; ZIP = zinc import proteins; CcoA = copper uptake porter; CusABC = copper uptake system; CopZ = copper chaperone.
Table 1.
Effect of trace metals on the pig gut microbiota, diversity, composition, and other parameters.
| Trace metal | Source/Form | Pig breed1 | α-Diversity1 | Bacterial composition | Other parameters 2 | Sequencing (Sample) | Reference |
|---|---|---|---|---|---|---|---|
| Fe | Iron-deficient (2.72 mg/L Fe) | NA | NS | ↑Bifidobacterium, Dialister, Prevotella, Lactobacillus, Megasphaera ↓Bacteroides, Clostridium, Akkermansia, Clostridiaceae, Lachnospiraceae, Ruminococcaceae, |
↑VFA | 16S rRNA high-throughput sequencing (Colon and Feces) | Knight et al. (2019) |
| Lactoferrin | Duroc × Landrace × Yorkshire | ↓Shannon, Chao1 | ↑Lactobacillus, Roseburia ↓Veillonella, Escherichia-Shigella, Actinobacillus, Streptococcus, Fusobacterium |
↓IL-1β, TNF-α, Diarrhea incidence, urinary lactulose-to-mannitol ratio ↑intestinal villi height, disaccharidase activity |
16S rRNA high-throughput sequencing (Jejunum, Ileum) | Hu et al. (2019b) | |
| Lactoferrin (with probiotics) | ↑evenness, Chao1 (richness) | ↑Erysipelotrichiaceae ↓Enterobacteriaceae, Veillonellaceae |
↑ferrous ion transport genes ↓ferric ion transport genes |
16S rRNA high-throughput sequencing (Colon, Feces) | Grzywacz et al. (2019) | ||
| Mn3 | |||||||
| Zn | 100 or 200 mg/kg ZnO | Danish Landrace × Yorkshire | NA | ↑Coliforms, enterococci ↓Lactic acid bacteria, Lactobacillus spp. (2,500 mg/kg ZnO) |
↑digesta, intestinal pH, dry matter (2,500 mg/kg ZnO) ↓SCFA, lactic acid, succinic acid, urease activity |
T-RFLP (Cecum and Colon) | Hojberg et al. (2005) |
| 124 or 3,042 mg/kg ZnO | German Landrace × Pietrain | ↑Shannon, Simpson | ↑Lactobacillus spp., Weissella cibaria, W. confusa, Leuconostoc citreum, Streptococcus lutetiensis, S. equinus ↓Sarcina ventriculi |
NA | 16S rRNA pyrosequencing (Ileum) | Vahjen et al. (2011) | |
| 50 to 2,500 mg/kg ZnO | Duroc × Pietrain | ↑Total bacteria | ↓Enterobacteriaceae ↑Clostridial cluster XIVa |
↑Total SCFA ↑acetate (150 mg/kg ZnO) ↑ammonium (50 to 150 mg/kg ZnO) ↓Propionate |
DGGE (Ileum) | Pieper et al. (2012) | |
| 57 or 2,425 mg/kg ZnO | Landrace | NA | ↓Enterobacteriaceae, Escherichia group, Lactobacillus spp. | ↑acetate ↓lactate |
Quantitative real-time PCR (Stomach, Jejunun, Ileum, Colon) | Starke et al. (2014) | |
| Coated ZnO nanoparticles (100 mg/kg ZnO) | Duroc × Landrace × Yorkshire | ↑richness (ACE and Chao1) | ↑Lachnospiraceae UCG-004 ↓Ruminococcus flavefaciens |
↑occludin, zonula occludens-1 | 16S rRNA high-throughput sequencing (Feces) | Legrand et al. (2005) | |
| 2,500 mg/kg ZnO | Landrace × Yorkshire | ↑Shannon, evenness | ↑Prevotella, Clostridiaceae ↓Lactobacillus |
↑villus height, crypt depth, villi-to-crypt ratio, mucosal thickness | 16S rRNA high-throughput sequencing (Colon) | Satessa et al. (2020) | |
| 110 and 2,400 mg/kg ZnO | Topigs × Piétrain | NA | ↓Enterobacteriaceae, coliforms, | ↑claudin-1, zonula occludens-1, alkaline phosphatase, gut integrity | Plate-counting method and quantitative real-time PCR (Stomach, Small intestines) | Wang et al. (2019) | |
| 40, 110 and 2,500 ZnO, 110 mg/kg Zn-Lysinate | Landrace | ↑evenness (40, 110 ZnO) ↑Shannon (110 ZnO) |
↑Bacteroides, Parabacteroides, Collinsella, Acetivibrio, Blautia, Coprococcus, Faecalibacterium, Subdoligranulum, Holdemania (2500 mg/kg ZnO) ↓Megasphaera, Dialister, Acidaminococcus, Ruminococcus (2500 mg/kg ZnO) |
↑feed intake, average daily gain, levels of ZnT1, MT1A and MT2B (2,500 mg/kg ZnO) ↑feed intake, average daily gain (110 mg/kg Zn-Lys) ↓ileal digestibility, ZIP4 level (2,500 mg/kg ZnO) ↑colonic Zn (2,500 mg/kg ZnO) |
Shotgun metagenomics (Colon) | Pieper et al., 2020 | |
| Cu | 300 mg/kg CuSO4 (high) | Suhai suckling piglets | NS | ↓Roseburia, Acidaminococcus, Lachnospiraceae FCS020 group ↑Coprococcus, Streptococcus |
↓TNF-α, SOD, serum albumin ↑Heptanoic acid ↓carbohydrate, amino acid metabolism |
16S rRNA high-throughput sequencing (Feces) | Zhang et al. (2019a) |
| 6 mg/kg CuSO4 (low) | Suhai suckling piglets | NS | ↓Nesterenkonia, Prevotellaceae UCG-001 ↑Coprococcus, Roseburia, Acidaminococcus |
↑MDA, ↑ALT, ↑AST and ↑TBA ↑serum blood urea nitrogen ↓SOD |
|||
| 150 mg/kg CuSO4 | Landrace × Large White | NA | ↑total bacterial count, ↓Enterobacteriaceae, Streptococcus |
↑duodenal villi height, crypt depth | Plate method-counting (Cecum and Colon) | Di Giancamillo et al. (2018) | |
| 200 mg/kg CuSO4 | NA | NS | ↑Proteobacteria, Spirochaetes ↓Firmicutes, Bacteroidetes |
↑valine, leucine isoleucine, lysine biosynthesis; lipid biosynthesis ↓peptidases |
16S rRNA high-throughput sequencing (Cecum) | Zhang et al. (2019c) | |
| ↑Tenericutes, Cyanobacteria ↓Firmicutes |
↑lipoic acid metabolism, carbohydrate metabolism, carotenoid biosynthesis ↓energy metabolism, lysine biosynthesis |
16S rRNA high-throughput sequencing (Ileum) | |||||
| 160 mg/kg CuSO4 | Large White × Landrace | NS | ↑Methanosphaera, Roseburia, Enterococcus | ↑body weight, ADG, ADFE, G:F, mineral status | 16S rRNA high-throughput sequencing (Colon) | Villagomez-Estrada et al. (2020) |
Fe = iron; Mn = manganese; Zn = zinc; Cu = copper; NS = not significant; NA = not applicable/not measured; ADG = average daily gain; ADFI = average daily feed intake; G:F = gain-to-feed ratio; MDA = malondialdehyde; ALT = alanine aminotransferase; AST = aspartate transaminase; TBA = total bile acid; TNF-α = tumor necrosis factor-alpha; IL-1β = interleukin-1beta; SOD = superoxide dismutase; VFA = volatile fatty acids; SCFA = short chain fatty acids; Znt1 = zinc transporter 1; MT1A and MT2B = metallothionein 1a and 2b; DGGE = denaturing gradient gel electrophoresis; T-RFLP = terminal restriction fragment length polymorphism; ZIP = zinc import proteins.
Compared to control group.
Growth and performance may include body weight, daily, immune status, metabolite profile and/or diarrhea incidence.
No data exists for the effects of manganese on the pig giut microbiota.
2. Iron
Iron is a critical component of hemoglobin and myoglobin which play vital roles in the metabolism of humans and animals. It is also the metal at the active site of many important enzymes, such as catalase, peroxidase, and cytochromes. The absorption of iron is carried out in the small intestines in the form of Fe2+ that binds with apoferritin to produce ferritin where Fe2+ is converted to Fe3+. Transferrin transports iron to various organs including the liver where iron is stored in the form of ferritin (Silva and Faustino, 2015). Iron is also essential for the survival of almost all aerobic organisms except Borrelia burgdorferi (Posey and Gherardini, 2000). Although iron absorption and assimilation in the body is well established, the mechanism underlying the modulation of gut microbiota is not completely understood. In fact, studies are limited in distinguishing iron's effect in the context of microbiota, focusing mostly on individual bacterial species. We discuss the current evidence showing key bacterial pathways of uptake, metabolism, and utilization of iron (Fig. 1).
Iron alters intestinal microbiota by regulating the microbial acquisition of energy from host-ingested nutrients (Dostal et al., 2015). Commensal and pathogenic bacteria acquire dietary iron in the gut in at least 2 ways including: (1) receptor-mediated transport from iron-bound proteins (i.e., transferrin and heme) and (2) iron capture by releasing siderophores. Studies have shown that iron transporters such as a ferrous uptake system (FeoAB) and a putative iron transport system (SitABCD) are expressed in several bacteria including Salmonella typhimurium (Zhou et al., 1999; Cartron et al., 2006). Moreover, some bacteria produce haemophore proteins to transport heme via receptor-mediated uptake (Krewulak and Vogel, 2008).
A wide array of Gram-positive and Gram-negative bacteria acquire luminal iron by releasing iron-chelating siderophores which exhibit a higher affinity to iron compared to a host's iron-binding proteins (Hider and Kong, 2010). Iron-bound siderophores are transported by various inner and outer membrane transporters such as enterobactin exporter S (EntS), salmochelin siderophore ATP-binding cassette (ABC) protein (IroC), and outer membrane protein (TolC), respectively (Furrer et al., 2002; Bleuel et al., 2005; Crouch et al., 2008) with the assistance of TonB-dependent outer membrane siderophore receptor (IroN) and ferric enterobactin (Fep) siderophore ABC transporter system (Hantke et al., 2003; Crouch et al., 2008). Some Gram-negative bacteria may also use ferric citrate transporters for iron uptake (Wagegg and Braun, 1981; Mahren et al., 2005). Iron is also found in regulatory proteins, which in the enteric bacterium S. enterica includes ferric uptake regulation (Fur), fumarate and nitrate reduction regulatory (Fnr) protein, redox-sensitive transcriptional activator (SoxR), HTH-type transcriptional regulator (IscR) and repressor (NsrR) (Troxell et al., 2011; Baothman et al., 2013; Vergnes et al., 2017). These findings indicate that various microorganisms developed complex transport and acquisition systems to compete for iron with the host.
2.1. Iron impacts gut microbiota
The established dietary iron requirement for young nursery pigs weighing 5 to 20 kg is 100 mg/kg per day (Shannon and Hill, 2019). Iron deficiency is a nutrient deficiency disorder that has serious effects on the host immune response and overall health. Iron deficiency anemia is a condition characterized by reduction of red blood cells or hemoglobin in the blood that can be caused by a plethora of factors, including blood loss, low dietary intake or poor absorption of iron from food (Aspuru et al., 2011). Since iron is mainly absorbed in the gut, iron deficiency can modulate mucosal immunity at least in part by changing the microbial profile and function (Knight et al., 2019). In addition, studies have shown that iron deficiency reduces brain tissue integrity (Antonides et al., 2015; Mudd et al., 2017, 2018), which may induce cognitive deficits (Antonides et al., 2015), indicating that iron is critical for normal brain development in pigs. Early-life iron deficiency alters the specific bacterial genera in the colon, promoting the growth of iron-independent bacterial communities such as Lactobacillus (Knight et al., 2019). These changes were attenuated when iron-sufficient diets were introduced at post-weaning, indicating that dietary supplementation may mitigate the effects of early-life iron deficiency. Iron deficiency induced microbial shifts in the pig gut, resulting in increased levels of volatile fatty acids (VFA), including acetate, propionate, and valerate (Knight et al., 2019). The gut microbiota affects iron metabolism and absorption throughout the gut (Cai et al., 2017), including the colon. It has been shown that VFA such as propionate, which is produced by gut microbes, regulates iron absorption, indicating that microbial propionate serves as a compensatory mechanism in response to low iron levels. These findings suggest that the gut microbiota plays a critical role in the effects of iron in both gut immunity and brain development; however, whether modulation of the gut microbiome by iron occurs directly or indirectly requires further study.
Lactoferrin is an iron-binding glycoprotein with a number of beneficial influences, including antimicrobial, antioxidant, and immunomodulatory effects (Hu et al., 2012, 2019a; Oda et al., 2014; Grigorieva et al., 2019). Studies have shown that its ability to bind iron promoted several beneficial bacteria, such as bifidobacteria and lactobacilli, under iron-restricted environments (Weinberg, 1997; Vega-Bautista et al., 2019). Increasing evidence has shown that lactoferrin modulates growth performance as well as intestinal and brain function, partly by regulating the gut microbiota in pigs (Yang et al., 2014; Berding et al., 2016; Grzywacz et al., 2019). Studies have shown that low iron levels induced better intestinal function and lower incidence of diarrhea, resulting in increased pig growth and performance (Hu et al., 2019b). This finding is attributed to an increased abundance of Lactobacillus and reduced Veillonella and opportunistic Escherichia-Shigella, resulting in a reduction of pro-inflammatory tumor necrosis factor alpha (TNF-α) levels in pigs (Hu et al., 2019b). Most bacteria in the gut need iron to grow and survive, and they employ various mechanisms to acquire iron from the diet. Many opportunistic pathogens require iron for metabolism and replication, creating a competitive environment for nutrient acquisition (Jaeggi et al., 2015; Constante et al., 2017). The inclusion of lactoferrin in the diet appears to limit the growth of bacteria with the propensity to be pathogenic such as Klebsiella and Escherichia-Shigella, resulting in enhanced metabolic activities and absorption capabilities in the pig gut (Berding et al., 2016). Another mechanism of lactoferrin is associated with the elimination of excess iron in the gut, as iron-bound lactoferrin resists breakdown and easily passes through the gut (Legrand et al., 2005), leading to reduced iron availability for pathogens.
2.2. Gut microbiota and iron metabolism
Iron regulates bacterial survival by modulating several key metabolic pathways including riboflavin biosynthesis, antioxidant enzyme function (i.e. catalase), anaerobic respiration, butyrate production as well as virulence of pathogenic bacteria (Tsolis et al., 1996; Boyer et al., 2002; Anjem and Imlay, 2012; Dostal et al., 2015; Cisternas et al., 2017), indicating that iron availability is tightly regulated in the gut and its homeostasis plays a crucial role in maintaining healthy microbiota. Iron metabolism is vital to meeting the host demand to maintain normal biological and metabolic functions, and failing to meet or exceeding the demand for iron may lead to deficiency or toxicity, respectively. Mammalian hosts have various mechanisms to regulate systemic and cellular iron levels for homeostasis (Anderson and Frazer, 2017). The gut microbiota also plays a critical role in iron metabolism and absorption in the animal gut. For instance, germ-free and antibiotic-treated mice had increased systemic iron levels (Das et al., 2020), which may have involved the repression of intestinal iron absorption pathways and enhancement of cellular iron storage (Das et al., 2020). It appears that Lactobacillus, particularly Lactobacillus johnsonii and Lactobacillus reuteri, are important in iron homeostasis as they have the ability to modulate iron homeostasis (Das et al., 2020). Microbial metabolites such as reuterin and 1,3-diaminopropane reduced intestinal hypoxia-inducible factor 2 alpha (HIF-2α) activity, resulting in reduced iron levels. HIF-2α dimerizes with aryl hydrocarbon receptor nuclear translocator, leading to reduced absorption of iron in mice (Das et al., 2020). Other mechanisms of the gut microbiota to metabolize iron may involve modulating mucin secretion in the gut, as studies have shown that mucin binds to iron, resulting in increased iron absorption (Conrad and Umbreit, 1993; Conrad et al., 1993). Lactobacilli may also modulate iron absorption in the gut by producing lactic acid, thereby lowering colonic pH. A low colonic pH seems to promote the increased conversion of ferric iron (Fe3+) into the more absorbable ferrous iron (Fe2+) in the gut microenvironment (Bering et al., 2006; Hoppe et al., 2015). Gut commensals may also increase the availability of dietary iron via ellagic acid-urolithin A conversion as urolithin A does not sequester Fe3+ compared to ellagic acid (Saha et al., 2016). Iron overload can cause gut dyshomeostasis by reducing the abundance of commensal bacteria and stimulating the growth of pathogenic bacteria (Jaeggi et al., 2015). On the other hand, the probiotic group Bifidobacteriaceae has been shown to synthesize siderophores and acquire colonic iron (Vega-Bautista et al., 2019), resulting in reduction of superoxide and lowering the risk of intestinal diseases.
Probiotics and prebiotics have been shown to exert different effects on iron absorption in vitro. For example, Bifidobacterium infantis decreased iron uptake, whereas Lactobacillus acidophilus increased iron uptake in colonic intestinal epithelial cell line (Caco-2) (Laparra et al., 2009). Interestingly, prebiotic inulin failed to influence iron uptake from beans. The mechanisms by which lactobacilli affect iron absorption may include metabolism of flavonoids and phytic acid from food sources, which plays an important role in iron bioavailability. Phytic acid binds iron, and lactobacilli facilitate the degradation of phytic acid to release bound iron for absorption in the gut (Fischer et al., 2014). These findings indicate that probiotic effects on iron absorption are species-dependent and proper selection of bacteria requires careful consideration.
3. Manganese
A wide array of microorganisms requires manganese to maintain their microbial physiology and viability as well as the virulence of several pathogenic bacteria including Salmonella and Escherichia coli (Kehres and Maguire, 2003; Zaharik and Finlay, 2004; Papp-Wallace and Maguire, 2006). Bacteria developed several manganese acquisition systems such as (1) ABC-type transporters (Claverys, 2001; Papp-Wallace and Maguire, 2006), (2) natural resistance-associated macrophage protein (NRAMP) transporters (i.e. divalent metal cation transporter [MntH]) (Kehres et al., 2000) and zinc importer proteins (ZIP) (Karlinsey et al., 2010). Manganese is involved in bacterial survival and fitness by regulating the function of proteins involved in detoxification of reactive oxygen species, metabolism of carbohydrates, lipids and proteins, and DNA replication (Kehres and Maguire, 2003; Zaharik and Finlay, 2004). Manganese is utilized by several bacterial enzymes which include phosphoglyceromutase, enolase, pyruvate kinase, phosphoenolpyruvate carboxylase and carboxykinase, type I protein phosphatases, certain phosphodiesteres and guanosine pentaphosphate or tetraphosphate (ppGpp) synthetases (Zaharik and Finlay, 2004; Papp-Wallace and Maguire, 2006). Growing evidence reveals that manganese can alter DNA replication activities in bacteria by serving as a cofactor of ribonucleotide reductase which is involved in synthesis of DNA from RNA (Zaharik and Finlay, 2004), suggesting its increasing importance to bacterial fitness and survival. Besides its function as an enzyme cofactor, studies have shown that manganese promotes an antioxidative effect in bacteria independent of protein activity.
Reactive oxygen species are highly reactive oxygen-containing molecules (i.e., superoxide) to maintain cell survival, but in excess can cause severe cellular damage and injury. As a cofactor, manganese is required by superoxide dismutase to break down the toxic superoxide into water and hydrogen peroxide. Manganese also protects bacteria against oxidative stress by modulation of Dps protein, resulting in DNA binding, iron sequestration and inhibition of ferroxidase activity (Anjem and Imlay, 2012; Ardini et al., 2013). This prevents the accumulation of hydroxide radicals and subsequent damage of DNA-enriched regions (Nguyen and Grove, 2012). These indicate that manganese plays a crucial role in bacterial survival and virulence of Gram-positive and Gram-negative bacterial pathogens and understanding the mechanisms that a host employs to tightly regulate and sequester its bioavailability may provide the means to control and prevent pathogenic outgrowth.
3.1. Manganese alters gut microbiota
Manganese is required for proper enzymatic functions involved in stress response and development (Fridovich, 1974; Aschner and Aschner, 2005). Manganese functions as a cofactor for enzyme activity. Manganese absorption is influenced by iron as both metals use the same transporters, including divalent metal transporter-1 (Shannon and Hill, 2019). In fact, low iron induces abnormal accumulation of manganese, whereas excess or lack of manganese alters iron levels in pigs (Hansen et al., 2009), suggesting that manganese metabolism and absorption is influenced by iron. Manganese is complexed to albumin or an albumin-like protein when it leaves the intestine. Studies have shown that the liver and not the pancreas is the main source of endogenous gut losses of manganese (Davis et al., 1993) and several factors affect manganese absorption in the gut, including delivery, source of manganese, and diet (Lee and Johnson, 1988). Studies have also shown that manganese supplementation improved meat quality of pigs (Sawyer et al., 2007). Manganese may also delay the decomposition of pork by reducing oxidative damage (Apple et al., 2004, 2007; Sawyer et al., 2007). These findings indicate that further investigation is warranted on the role of manganese supplementation and the mechanism of its effect in improving pig growth and performance.
Adequate supplementation of manganese is required in swine farming since over- or under-supplementation of manganese causes problems in pig growth and performance. In one study, 16 mg/kg of dietary manganese was necessary for optimum metabolic activity (Pallauf et al., 2012), whereas supplementation with the National Research Council recommendation of 4 mg/kg did not fulfill piglet requirements (Pallauf et al., 2012). Low levels of manganese (0.5 to 6 mg/kg) were reported to support growth and reproduction in pigs. However, deficiency of manganese results in a variety of bone abnormalities in poultry and cattle (Spears, 2019). Manganese in feedstuffs can be released by supplementation with feed enzymes such as xylanase, β-glucanase, and phytase from wheat, wheat middlings, barley, soybean meal, corn, and wheat bran (Yu et al., 2018), indicating that digestive enzymes, whether artificially supplemented or produced by the gut microbiota, may promote the release of manganese and other minerals in pigs. Higher levels of manganese (>50 mg/kg) appear to be toxic to pigs, presenting harmful effects on their growth (Grummer et al., 1950); for instance, excessive manganese induced oxidative stress and adverse immune responses by increasing lipid peroxidation, and decreasing neutrophil burst and levels of glutathione and adenosine triphosphate (ATP) in the blood and liver of pigs (Tsebrzhinskii, 1998).
Given the significance of manganese in mammalian health, it is surprising that there are no studies on the effects of manganese supplementation, deficiency, and toxicity in the gut microbiota of pigs, which presents an exciting avenue for future research. However, several studies have reported that manganese supplementation alters the gut microbiota of poultry (i.e., broilers) and dairy cows (Faulkner et al., 2017; Bortoluzzi et al., 2020). In addition, several reports showed that manganese deficiency exacerbated dextran sulfate sodium-induced colitis in mice, indicating that adequate amounts of manganese are required for proper intestinal function and overall health (Choi et al., 2020). The effect of dietary manganese on the gut microbiota and diversity requires further study.
High levels of manganese elicit toxic effects in humans and animals (Avila et al., 2013). Manganese exposure can occur ubiquitously in the environment as manganese is found in various sources, including industrial emissions, mining sites, fertilizers, gasoline additives, and contaminated water and soil (de Tollenaer et al., 2006; Hassani et al., 2012; Harrison Brody et al., 2013; Wang et al., 2018; Okereafor et al., 2020). Thus, the role of manganese in the development of metabolic and neurological disorders must be understood. Recent studies have reported that manganese modulates the gut microbiota of mice (Chi et al., 2017; Wang et al., 2020a, 2020b). Manganese intoxication may also occur in pigs and alter their gut microbiota, as studies have detected high levels of metal pollutants, including manganese, and microbial shifts in pig feces across commercial farms (Peng et al., 2019), which may lead to severe problems in swine farming and human health.
4. Zinc
Zinc is the 29th most abundant metal in the Earth's crust. It is a necessary component of more than 300 enzymes and 1,000 transcription factors (McCall et al., 2000), indicating zinc's critical role in a wide range of biological functions and activities for immunity and cell survival. Zinc deficiency is a serious problem that can cause growth retardation, pathogenic infection, immune dysfunction, and impaired cognition in the human population, and nearly 2 billion people are affected in developing countries, particularly children and the elderly (Ibs and Rink, 2003; Prasad, 2003). Although zinc is critically involved in promoting good health, excessive zinc exposure can lead to toxic effects including metabolic dysfunction and oxidative damage (Becker and Skaar, 2014), which indicates that zinc levels in the body must be tightly regulated and maintained to prevent severe damage and health complications.
In bacteria, zinc-binding proteins range from 5% to 6% of total proteins in procaryotes (Andreini et al., 2006), which are involved in a range of cellular activities including bacterial gene expression, general metabolism, antioxidant system, and cofactor of virulence factors (Andreini et al., 2006). The role of zinc to protect against bacterial infection appears to be associated with its ability to inhibit pathogenic bacteria to import and utilize manganese (Counago et al., 2014). These further highlights the critical role that zinc plays to promote bacterial persistence and the mechanisms by which bacteria capture the required zinc to maintain its cellular processes.
4.1. Zinc impacts the gut microbiota
Zinc in the form of zinc oxide (ZnO) is commonly used in swine feeds at various levels (zinc: 125 to 3,000 mg/kg feed) that greatly exceed the dietary requirements of 50 to 125 mg/kg feed (Lopez-Alonso, 2012; Debski, 2016). Zinc ameliorates diarrhea incidence at postweaning by reducing intestinal damage and enhancing anti-inflammatory factors as well as mucosal integrity through modulating the gut microbiota in pigs. Several studies have reported that higher concentrations of zinc added to feed increase animal growth performance and control pathogens. For instance, zinc decreased enterobacteria and clostridial cluster XIV, increased acetate and butyrate levels, and enhanced gut functionality in pigs (Pieper et al., 2012). Zinc also improved growth performance of pigs by promoting intestinal health and mucosal integrity concomitant with reducing coliforms and E. coli (Kociova et al., 2020; Oh et al., 2020). In addition, zinc can inhibit pathogenic E. coli by repressing the expression of alpha-hemolysin (Velasco et al., 2018), an exotoxin produced in pathogenic strains of E. coli. It attenuated alpha-hemolysin-induced barrier dysfunction and leaky gut in a mouse model (Weigand and Egenolf, 2017). Other studies have shown that reduction of enterobacteria such as E. coli might be linked to the regulation of metabolic activity along with changes in the overall gut microbiota in pigs. However, this reduction of E. coli may not be associated with increased abundance of beneficial bacteria as zinc reduced lactobacilli and bifidobacteria in the pig gut, indicating that other mechanisms or bacterial groups play a role in diminishing pathogenic bacteria. Furthermore, zinc can directly modulate the host immune response to infection by regulating the innate immune response and cytokine production in the host (Subramanian Vignesh and Deepe, 2016; Eijkelkamp et al., 2019).
Although the overall health effects of zinc appear to be beneficial in pigs, different sources of zinc may have varying degrees of effects to the host. Several studies explored the effects of zinc nanoparticles and other zinc compounds in modulating the gut microbiota and pig performance (Kociova et al., 2020; Liu et al., 2020; Oh et al., 2020). Coated ZnO nanoparticles protected the intestinal mucosal barrier and enhanced proteins associated with barrier function such as occludin and zonula occludens in the small intestines (Liu et al., 2020). Coated ZnO nanoparticles increased microbial richness and diversity as well as Ruminococcus flavefaciens abundance compared to uncoated ZnO (Liu et al., 2020). Zinc phosphate-based nanoparticles also increased piglet growth performance and decreased the prevalence of E. coli and its virulence factors (Kociova et al., 2020). Another report showed that hot-melt extruded ZnO nanoparticles significantly decreased Clostridium spp. and coliforms concomitant with improved gut integrity compared to pigs in the control and ZnO-only groups (Oh et al., 2020). These findings indicate that compared to ZnO, other forms of zinc supplementation may provide additional benefits to gut immunity and overall health of pigs and should thus be explored further. Alternatives to ZnO supplementation in piglet diets are also being explored to offer diverse options to manage postweaning disorders, as ZnO will be phased out after 2022 in Europe.
Probiotics and prebiotics appear to be promising candidates for ZnO and in-feed antibiotic alternatives; however, the mechanisms involved in probiotic- and prebiotic-induced protection in the pig gut remain to be understood. Supplementation of lactic acid bacteria and fermented rapeseed meal induced similar or significant improvement of pig growth performance compared with zinc supplementation by improving mucosal development, inhibiting intestinal inflammation, and modulating the gut microbiota (Satessa et al., 2020). Similar to ZnO supplementation, probiotic and prebiotic supplementation increased the abundance of Prevotella (Satessa et al., 2020), which has been characterized as a resident gut commensal and a major contributor to polysaccharide breakdown in pigs (Pajarillo et al., 2014a, Pajarillo et al., 2014b). In addition, all treatments including ZnO reduced Lactobacillus spp. in the pig gut (Satessa et al., 2020), suggesting that other beneficial bacteria may promote gut immunity in pigs. Other studies have reported that ZnO increased Lactobacillus spp. (Oh et al., 2020), and some showed no effect in the abundance of lactic acid bacteria (Pieper et al., 2012) or the gut microbiota (Broom et al., 2006) of pigs.
Although zinc has been shown to reduce pathogenic infection and improve growth performance and survival of piglets at postweaning, excessive use of zinc increased the abundance of antibiotic-resistance genes and antibiotic-resistant bacteria in the pig gut (Holzel et al., 2012; Bednorz et al., 2013; Pieper et al., 2020). Extensive use of zinc in feeds may explain the increased antibiotic-resistant bacteria, as zinc usage was positively correlated with the isolation of methicillin-resistant Staphylococcus aureus in pigs (Amachawadi et al., 2015; Slifierz et al., 2015a, 2015b).
Zinc regulates bacterial survival via multiple mechanisms such as modulating host defense, antioxidant system, gene expression, and virulence factors as well as inhibiting transport of growth-promoting factors (Velasco et al., 2018; Eijkelkamp et al., 2019). Since zinc is required by important proteins such as superoxide dismutase, proteases, DNA polymerases, and ribosomal proteins, the microbiota require efficient zinc transport systems to compete with the host and other bacteria (Hantke, 2005). In fact, several studies revealed that bacteria developed 2 transport systems for zinc import: (1) high-affinity zinc uptake system proteins (ZnuABC) (2) zinc transport systems (AdcABC, YcdHI-YceA or YciABC, which is a collection of proteins that make up a transporter system) (Hantke, 2005). The expression of zinc transporters are dependent on zinc uptake regulator ZUR (in E. coli) (Patzer and Hantke, 2000) and peroxide operon regulator (PerR) (in Bacillus subtilis) (Gaballa and Helmann, 2002). In Streptococcus spp., zinc transporter genes are regulated by a transcription factor, adhesin competence repressor (AdcR) (Dintilhac et al., 1997). Some species and strains of Lactobacillus spp. exhibited a varying affinity to import zinc from the external environment (Xie et al., 2011), suggesting that species- and strain-specific mechanisms of bacteria regulate zinc uptake differently. Zinc also regulates the expression of manganese ABC transporter substrate-binding lipoprotein (PsaA), a substrate-binding protein that facilitates nutrient transport for the growth of Streptococcus pneumonia (Counago et al., 2014). These findings indicate that zinc plays a critical role in regulating pathogen survival in the gut.
Once zinc enters bacteria, it disrupts key processes for survival including cell stress response, carbon metabolism, motility, pathogenesis, and toxin production (Panina et al., 2003; Xie et al., 2011; Ong et al., 2015). In C. jejuni, zinc dysregulates stress response genes such as catalase (katA), alkyl hydroperoxide reductase (ahpC) and the 70 kDa heat shock proteins (DnaK) chaperone concomitant with the disruption of cell membrane (Xie et al., 2011). In Group A Streptococcus, zinc reduced glucose metabolism by inhibiting key glycolytic enzymes such as phosphofructokinase and glyceraldehyde-3-phosphate dehydrogenase, resulting in decreased capsule biosynthesis and virulence (Ong et al., 2015). Studies have also shown that zinc regulates the expression of ribosomal proteins including L36, L33, L31, and S14, indicating that zinc plays a crucial role in global protein expression and function by dysregulating ribosomal biogenesis (Panina et al., 2003). These findings reveal that zinc's effects upon its uptake by gut microbiota vary between species.
5. Copper
Copper is an essential element that acts as a cofactor to several enzymes involved in antioxidant response (i.e., superoxide dismutase), membrane and DNA integrity, and ATP production (Nargund et al., 2015; Dalecki et al., 2017). Microorganisms employ several mechanisms for transporting copper across membranes, trafficking copper between organelles, copper sequestration by metallothioneins, and oxidation of copper ions to reduce toxicity and maintain copper homeostasis (Robinson and Winge, 2010; Hernandez-Montes et al., 2012). Copper can cross the bacterial membrane via porins and major-facilitator superfamily members such as copper uptake porter (CcoA) that can transport copper from the extracellular region (Andrei et al., 2020). Once copper enters the cells, metallothioneins and chaperones (i.e., copper chaperone [CopZ]) bind copper to maintain adequate supply for the bacteria, leading to proper metabolic and enzymatic activities (Andrei et al., 2020). Excessive amounts of intracellular copper are removed by copper export systems such as copper uptake system (CusABC) proteins (Andrei et al., 2020). These represent the general mechanisms of copper homeostasis in bacteria.
Although copper is important to maintain physiological functions, it also exhibits antimicrobial activity against bacterial infection as intracellular accumulation of copper may cause protein damage and cell injury in bacteria (Dalecki et al., 2017; Pontin et al., 2021). But, excessive use of copper regulates copper tolerance of pathogenic bacteria against its toxicity, enhancing the virulence of these pathogens (Singh et al., 1986; Medardus et al., 2014; Parsons et al., 2017, 2020; Arai et al., 2019).
5.1. Copper modulates gut microbiota
High levels of copper exposure may induce toxicity and the development of copper resistance in various pathogenic bacteria (Altimira et al., 2012; Agga et al., 2014; Zhang et al., 2019b, 2019c). Extensive use of copper in industry and mining and the widespread use of pesticides in agricultural production are major sources of copper pollution of soils and water (Altimira et al., 2012; Glibota et al., 2019). Thus, the precise and tight regulation of copper levels in the body is required to prevent unwanted detrimental effects or dysbiosis in the host.
For many years, copper was widely used in the pig production industries of United States and China as a feed additive to promote the growth and postweaning survival of pigs at least in part by enhancing feed intake and metabolic activity (Shelton et al., 2011; Carpenter et al., 2019). Copper has been frequently used for its antimicrobial properties in swine feeds at various levels (copper: 10 to 250 mg/kg feed) that exceed dietary requirements (5 to 10 mg/kg) (Lopez-Alonso, 2012; Debski, 2016). Although the precise mechanism of dietary copper remains to be elucidated, studies have shown that copper supplementation improves growth performance, feed intake, and mineral absorption of pigs by decreasing the relative abundance of potential pathogens, including Enterobacter, Escherichia, and Streptococcus (Villagomez-Estrada et al., 2020). Several studies have shown that copper altered energy and protein metabolism as well as amino acid biosynthesis concomitant with modulating the gut microbiota (Zhang et al., 2019a, 2019c), indicating that copper modulates the bacterial abundance and diversity in the gut, partly by reducing pathogenic bacteria and improving gut functionality. However, the effects of copper in modulating the gut microbiota and immunity of pigs may depend highly on experimental conditions, including pig breed, copper source, and dosage. In fact, failure to metabolize copper in the gut, which can lead to its accumulation in the gut, may induce toxic buildup of pro-inflammatory TNF-α associated with changes in microbial composition and function in the gut (Zhang et al., 2017).
Certain bacteria can maintain copper homeostasis by regulating copper transport and trafficking, preventing its toxic effects (Robinson and Winge, 2010; Arguello et al., 2012). However, increasing evidence suggests that increased copper-supplemented in-feed antibiotics may promote the development of antimicrobial resistance of opportunistic and pathogenic bacteria in pigs (Amachawadi et al., 2015). It has been shown that copper can modulate the microbial community and metabolic functions in the gut of pigs (Zhang et al., 2019a). The same study also indicated that copper may exert changes in the gut microbiota and antimicrobial resistance profiles of pathogenic bacteria (Zhang et al., 2019a), suggesting that copper may promote and increase the risk of infection with multidrug-resistant bacteria. Bacteria may be conferred resistance to copper via copper-contaminated soil and water caused by extensive fungicide spraying and mining (Andreazza et al., 2011; Glibota et al., 2019). Copper also increased antibiotic resistance genes and mobile genetic elements in pig feces (Zhang et al., 2019b). Although copper can prevent bacterial growth, some bacterial species can tolerate high levels of copper by acquiring copper-resistance genes via horizontal gene transfer (Davison, 1999). However, the link between antimicrobial resistance development and increased copper supplementation has been inconsistent, as other studies reported that copper did not increase copper-resistant bacteria in pigs (Agga et al., 2014; Capps et al., 2020).
Recent observations suggest that high cholesterol-induced copper deficiency may modulate the gut microbiome of Ossabaw pigs by increasing Enterobacteriaceae and Succinivibrionaceae and decreasing Bacteroidaceae and Ruminococcaceae (Klevay, 2019; Panasevich and Rector, 2019). Additional investigation is necessary to elucidate the effects of copper deficiency on the gut microbiota and health of pigs.
6. Concluding remarks
Dysregulation of the gut microbiota has been strongly linked to pathogenesis of various immune disorders. Although host genetics and environmental factors such as dietary trace metals support the diversity and pleiotropic functions of the animal gut microbiome, both deficiency and excess of trace metals such as iron, manganese, zinc, and copper contribute to the onset and progression of metabolic disorders, pathogenic overgrowth, and immunological dysfunction (Fig. 2). Accordingly, delineating the molecular mechanisms involved in the regulation of the gut microbiota profile and function is critical to furthering our understanding of diseases associated with aberrant metal metabolism, deficiency, and toxicity. Although rapid advancement in DNA sequencing technology has been significant, researchers still need to overcome various limitations in these studies, such as sequencing accuracy and primer bias, sample preparation, and appropriate animal models, etc. In addition, bioinformatics tools, analysis software and pipelines should be standardized and streamlined in order to minimize misinterpretation of data (Poussin et al., 2018). It is also critical to eliminate potential error and bias in the data by unifying and standardizing sequence databases (Poussin et al., 2018), and accelerate our understanding of the characteristics of novel gut bacteria. Moreover, there is a lack of development and interface for in vitro studies mimicking the complexity of the gut microenvironment as well as the dynamic nature of the gut microbiota. Several methods have the potential to overcome these limitations such as the use of gnotobiotic or germ-free animals (Al-Asmakh and Zadjali, 2015; Martin et al., 2016), often limited to rodent studies; use of human and animal gut simulators (Molly et al., 1993); and microbiota-intestinal cell co-cultures (Pearce et al., 2018). Likewise, modulation of the gut microbiota by supplementation with probiotics and prebiotics constitutes an exciting direction for exploration that would extend our collective comprehension of metabolic and immune disorders and aid in the identification of potential therapeutic targets.
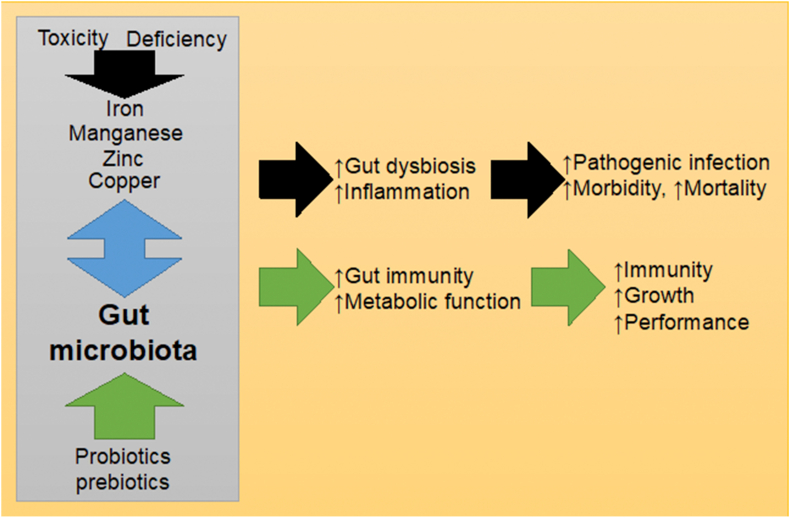
Fig. 2.
A summary of the potential role of trace metals such as iron, manganese, zinc and copper, their interaction with the gut microbiota and putative benefits of probiotic and prebiotic supplementation via gut microbiota modulation.
Author contributions
Dae-Kyung Kang initiated the idea. Edward Alain B. Pajarillo wrote the manuscript. Dae-Kyung Kang and Eunsook Lee provided suggestions and editing. All authors read and approved the final manuscript.
Conflict of interest
We declare that we have no financial and personal relationships with other people or organizations that can inappropriately influence our work, and there is no professional or other personal interest of any nature or kind in any product, service and/or company that could be construed as influencing the content of this paper.
Acknowledgements
This work was supported by a grant from National Institute of Environmental Health Sciences (R01 ES024756).
Footnotes
Peer review under responsibility of Chinese Association of Animal Science and Veterinary Medicine.
References
- Agga G.E., Scott H.M., Amachawadi R.G., Nagaraja T.G., Vinasco J., Bai J., Norby B., Renter D.G., Dritz S.S., Nelssen J.L., Tokach M.D. Effects of chlortetracycline and copper supplementation on antimicrobial resistance of fecal Escherichia coli from weaned pigs. Prev Vet Med. 2014;114(3–4):231–246. doi: 10.1016/j.prevetmed.2014.02.010. [DOI] [PubMed] [Google Scholar]
- Al-Asmakh M., Zadjali F. Use of germ-free animal models in microbiota-related research. J Microbiol Biotechnol. 2015;25(10):1583–1588. doi: 10.4014/jmb.1501.01039. [DOI] [PubMed] [Google Scholar]
- Altimira F., Yanez C., Bravo G., Gonzalez M., Rojas L.A., Seeger M. Characterization of copper-resistant bacteria and bacterial communities from copper-polluted agricultural soils of central Chile. BMC Microbiol. 2012;12:193. doi: 10.1186/1471-2180-12-193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amachawadi R.G., Scott H.M., Nitikanchana S., Vinasco J., Tokach M.D., Dritz S.S., Nelssen J.L., Goodband R.D., Nagaraja T.G. Nasal carriage of mecA-positive methicillin-resistant Staphylococcus aureus in pigs exhibits dose-response to zinc supplementation. Foodb Pathog Dis. 2015;12(2):159–163. doi: 10.1089/fpd.2014.1851. [DOI] [PubMed] [Google Scholar]
- Anderson G.J., Frazer D.M. Current understanding of iron homeostasis. Am J Clin Nutr. 2017;106(Suppl 6):1559S–1566S. doi: 10.3945/ajcn.117.155804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andreazza R., Pieniz S., Okeke B.C., Camargo F.A. Evaluation of copper resistant bacteria from vineyard soils and mining waste for copper biosorption. Braz J Microbiol. 2011;42(1):66–74. doi: 10.1590/S1517-83822011000100009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andrei A., Ozturk Y., Khalfaoui-Hassani B., Rauch J., Marckmann D., Trasnea P.I., Daldal F., Koch H.G. Cu homeostasis in bacteria: the ins and outs. Membranes (Basel) 2020;10(9) doi: 10.3390/membranes10090242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andreini C., Banci L., Bertini I., Rosato A. Zinc through the three domains of life. J Proteome Res. 2006;5(11):3173–3178. doi: 10.1021/pr0603699. [DOI] [PubMed] [Google Scholar]
- Andreini C., Bertini I., Cavallaro G., Holliday G.L., Thornton J.M. Metal ions in biological catalysis: from enzyme databases to general principles. J Biol Inorg Chem. 2008;13(8):1205–1218. doi: 10.1007/s00775-008-0404-5. [DOI] [PubMed] [Google Scholar]
- Anjem A., Imlay J.A. Mononuclear iron enzymes are primary targets of hydrogen peroxide stress. J Biol Chem. 2012;287(19):15544–15556. doi: 10.1074/jbc.M111.330365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Antonides A., Schoonderwoerd A.C., Scholz G., Berg B.M., Nordquist R.E., van der Staay F.J. Pre-weaning dietary iron deficiency impairs spatial learning and memory in the cognitive holeboard task in piglets. Front Behav Neurosci. 2015;9:291. doi: 10.3389/fnbeh.2015.00291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Apple J.K., Roberts W.J., Maxwell C.V., Boger C.B., Fakler T.M., Friesen K.G., Johnson Z.B. Effect of supplemental manganese on performance and carcass characteristics of growing-finishing swine. J Anim Sci. 2004;82(11):3267–3276. doi: 10.2527/2004.82113267x. [DOI] [PubMed] [Google Scholar]
- Apple J.K., Roberts W.J., Maxwell C.V., Jr., Rakes L.K., Friesen K.G., Fakler T.M. Influence of dietary inclusion level of manganese on pork quality during retail display. Meat Sci. 2007;75(4):640–647. doi: 10.1016/j.meatsci.2006.10.006. [DOI] [PubMed] [Google Scholar]
- Arai N., Sekizuka T., Tamamura Y., Kusumoto M., Hinenoya A., Yamasaki S., Iwata T., Watanabe-Yanai A., Kuroda M., Akiba M. Salmonella genomic island 3 is an integrative and conjugative element and contributes to copper and arsenic tolerance of Salmonella enterica. Antimicrob Agents Chemother. 2019;63(9) doi: 10.1128/AAC.00429-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ardini M., Fiorillo A., Fittipaldi M., Stefanini S., Gatteschi D., Ilari A., Chiancone E. Kineococcus radiotolerans Dps forms a heteronuclear Mn-Fe ferroxidase center that may explain the Mn-dependent protection against oxidative stress. Biochim Biophys Acta. 2013;1830(6):3745–3755. doi: 10.1016/j.bbagen.2013.02.003. [DOI] [PubMed] [Google Scholar]
- Arguello J.M., Raimunda D., Gonzalez-Guerrero M. Metal transport across biomembranes: emerging models for a distinct chemistry. J Biol Chem. 2012;287(17):13510–13517. doi: 10.1074/jbc.R111.319343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aschner J.L., Aschner M. Nutritional aspects of manganese homeostasis. Mol Aspect Med. 2005;26(4–5):353–362. doi: 10.1016/j.mam.2005.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aspuru K., Villa C., Bermejo F., Herrero P., Lopez S.G. Optimal management of iron deficiency anemia due to poor dietary intake. Int J Gen Med. 2011;4:741–750. doi: 10.2147/IJGM.S17788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Avila D.S., Puntel R.L., Aschner M. Manganese in health and disease. Met Ions Life Sci. 2013;13:199–227. doi: 10.1007/978-94-007-7500-8_7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baothman O.A.S., Rolfe M.D., Green J. Characterization of Salmonella enterica serovar Typhimurium aconitase A. Microbiology (Read) 2013;159(Pt 6):1209–1216. doi: 10.1099/mic.0.067934-0. [DOI] [PubMed] [Google Scholar]
- Becker K.W., Skaar E.P. Metal limitation and toxicity at the interface between host and pathogen. FEMS Microbiol Rev. 2014;38(6):1235–1249. doi: 10.1111/1574-6976.12087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bednorz C., Oelgeschlager K., Kinnemann B., Hartmann S., Neumann K., Pieper R., Bethe A., Semmler T., Tedin K., Schierack P., Wieler L.H., Guenther S. The broader context of antibiotic resistance: zinc feed supplementation of piglets increases the proportion of multi-resistant Escherichia coli in vivo. Int J Med Microbiol. 2013;303(6–7):396–403. doi: 10.1016/j.ijmm.2013.06.004. [DOI] [PubMed] [Google Scholar]
- Berding K., Wang M., Monaco M.H., Alexander L.S., Mudd A.T., Chichlowski M., Waworuntu R.V., Berg B.M., Miller M.J., Dilger R.N., Donovan S.M. Prebiotics and bioactive milk fractions affect gut development, microbiota, and neurotransmitter expression in piglets. J Pediatr Gastroenterol Nutr. 2016;63(6):688–697. doi: 10.1097/MPG.0000000000001200. [DOI] [PubMed] [Google Scholar]
- Bering S., Suchdev S., Sjoltov L., Berggren A., Tetens I., Bukhave K. A lactic acid-fermented oat gruel increases non-haem iron absorption from a phytate-rich meal in healthy women of childbearing age. Br J Nutr. 2006;96(1):80–85. doi: 10.1079/bjn20061683. [DOI] [PubMed] [Google Scholar]
- Bleuel C., Grosse C., Taudte N., Scherer J., Wesenberg D., Krauss G.J., Nies D.H., Grass G. TolC is involved in enterobactin efflux across the outer membrane of Escherichia coli. J Bacteriol. 2005;187(19):6701–6707. doi: 10.1128/JB.187.19.6701-6707.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bortoluzzi C., Vieira B.S., Applegate T.J. Influence of dietary zinc, copper, and manganese on the intestinal health of broilers under eimeria challenge. Front Vet Sci. 2020;7:13. doi: 10.3389/fvets.2020.00013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyer E., Bergevin I., Malo D., Gros P., Cellier M.F. Acquisition of Mn(II) in addition to Fe(II) is required for full virulence of Salmonella enterica serovar Typhimurium. Infect Immun. 2002;70(11):6032–6042. doi: 10.1128/IAI.70.11.6032-6042.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Broom L.J., Miller H.M., Kerr K.G., Knapp J.S. Effects of zinc oxide and Enterococcus faecium SF68 dietary supplementation on the performance, intestinal microbiota and immune status of weaned piglets. Res Vet Sci. 2006;80(1):45–54. doi: 10.1016/j.rvsc.2005.04.004. [DOI] [PubMed] [Google Scholar]
- Cai X., Chen X., Yin N., Du H., Sun G., Wang L., Xu Y., Chen Y., Cui Y. Estimation of the bioaccessibility and bioavailability of Fe, Mn, Cu, and Zn in Chinese vegetables using the in vitro digestion/Caco-2 cell model: the influence of gut microbiota. Food Funct. 2017;8(12):4592–4600. doi: 10.1039/c7fo01348e. [DOI] [PubMed] [Google Scholar]
- Capps K.M., Amachawadi R.G., Menegat M.B., Woodworth J.C., Perryman K., Tokach M.D., Dritz S.S., DeRouchey J.M., Goodband R.D., Bai J., Apley M.D., Lubbers B.V., Nagaraja T.G. Impact of added copper, alone or in combination with chlortetracycline, on growth performance and antimicrobial resistance of fecal enterococci of weaned piglets. J Anim Sci. 2020;98(3) doi: 10.1093/jas/skaa003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carpenter C.B., Woodworth J.C., Derouchey J.M., Tokach M.D., Goodband R.D., Dritz S.S., Wu F., Rambo Z.J. Effects of increasing copper from either copper sulfate or combinations of copper sulfate and a copper-amino acid complex on finishing pig growth performance and carcass characteristics. Transl Anim Sci. 2019;3(4):1263–1269. doi: 10.1093/tas/txz112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cartron M.L., Maddocks S., Gillingham P., Craven C.J., Andrews S.C. Feo-transport of ferrous iron into bacteria. Biometals. 2006;19(2):143–157. doi: 10.1007/s10534-006-0003-2. [DOI] [PubMed] [Google Scholar]
- Che D., Adams S., Wei C., Gui-Xin Q., Atiba E.M., Hailong J. Effects of Astragalus membranaceus fiber on growth performance, nutrient digestibility, microbial composition, VFA production, gut pH, and immunity of weaned pigs. Microbiologyopen. 2019;8(5) doi: 10.1002/mbo3.712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chi L., Gao B., Bian X., Tu P., Ru H., Lu K. Manganese-induced sex-specific gut microbiome perturbations in C57BL/6 mice. Toxicol Appl Pharmacol. 2017;331:142–153. doi: 10.1016/j.taap.2017.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi E.K., Aring L., Das N.K., Solanki S., Inohara N., Iwase S., Samuelson L.C., Shah Y.M., Seo Y.A. Impact of dietary manganese on experimental colitis in mice. FASEB J. 2020;34(2):2929–2943. doi: 10.1096/fj.201902396R. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chowdhury B.A., Chandra R.K. Biological and health implications of toxic heavy metal and essential trace element interactions. Prog Food Nutr Sci. 1987;11(1):55–113. [PubMed] [Google Scholar]
- Cisternas I.S., Torres A., Flores A.F., Angulo V.A.G. Differential regulation of riboflavin supply genes in Vibrio cholerae. Gut Pathog. 2017;9:10. doi: 10.1186/s13099-017-0159-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Claverys J.P. A new family of high-affinity ABC manganese and zinc permeases. Res Microbiol. 2001;152(3–4):231–243. doi: 10.1016/s0923-2508(01)01195-0. [DOI] [PubMed] [Google Scholar]
- Conrad M.E., Umbreit J.N. A concise review: iron absorption-the mucin-mobilferrin-integrin pathway. A competitive pathway for metal absorption. Am J Hematol. 1993;42(1):67–73. doi: 10.1002/ajh.2830420114. [DOI] [PubMed] [Google Scholar]
- Conrad M.E., Umbreit J.N., Moore E.G. Regulation of iron absorption: proteins involved in duodenal mucosal uptake and transport. J Am Coll Nutr. 1993;12(6):720–728. doi: 10.1080/07315724.1993.10718365. [DOI] [PubMed] [Google Scholar]
- Constante M., Fragoso G., Lupien-Meilleur J., Calve A., Santos M.M. Iron supplements modulate colon microbiota composition and potentiate the protective effects of probiotics in dextran sodium sulfate-induced colitis. Inflamm Bowel Dis. 2017;23(5):753–766. doi: 10.1097/MIB.0000000000001089. [DOI] [PubMed] [Google Scholar]
- Counago R.M., Ween M.P., Begg S.L., Bajaj M., Zuegg J., O'Mara M.L., Cooper M.A., McEwan A.G., Paton J.C., Kobe B., McDevitt C.A. Imperfect coordination chemistry facilitates metal ion release in the Psa permease. Nat Chem Biol. 2014;10(1):35–41. doi: 10.1038/nchembio.1382. [DOI] [PubMed] [Google Scholar]
- Crofts A.A., Poly F.M., Ewing C.P., Kuroiwa J.M., Rimmer J.E., Harro C., Sack D., Talaat K.R., Porter C.K., Gutierrez R.L., DeNearing B., Brubaker J., Laird R.M., Maue A.C., Jaep K., Alcala A., Tribble D.R., Riddle M.S., Ramakrishnan A., McCoy A.J., Davies B.W., Guerry P., Trent M.S. Campylobacter jejuni transcriptional and genetic adaptation during human infection. Nat Microbiol. 2018;3(4):494–502. doi: 10.1038/s41564-018-0133-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crouch M.L., Castor M., Karlinsey J.E., Kalhorn T., Fang F.C. Biosynthesis and IroC-dependent export of the siderophore salmochelin are essential for virulence of Salmonella enterica serovar Typhimurium. Mol Microbiol. 2008;67(5):971–983. doi: 10.1111/j.1365-2958.2007.06089.x. [DOI] [PubMed] [Google Scholar]
- Dalecki A.G., Crawford C.L., Wolschendorf F. Copper and antibiotics: discovery, modes of action, and opportunities for medicinal applications. Adv Microb Physiol. 2017;70:193–260. doi: 10.1016/bs.ampbs.2017.01.007. [DOI] [PubMed] [Google Scholar]
- Das N.K., Schwartz A.J., Barthel G., Inohara N., Liu Q., Sankar A., Hill D.R., Ma X., Lamberg O., Schnizlein M.K., Arques J.L., Spence J.R., Nunez G., Patterson A.D., Sun D., Young V.B., Shah Y.M. Microbial metabolite signaling is required for systemic iron homeostasis. Cell Metab. 2020;31(1):115–130 e6. doi: 10.1016/j.cmet.2019.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis C.D., Zech L., Greger J.L. Manganese metabolism in rats: an improved methodology for assessing gut endogenous losses. Proc Soc Exp Biol Med. 1993;202(1):103–108. doi: 10.3181/00379727-202-43518. [DOI] [PubMed] [Google Scholar]
- Davison J. Genetic exchange between bacteria in the environment. Plasmid. 1999;42(2):73–91. doi: 10.1006/plas.1999.1421. [DOI] [PubMed] [Google Scholar]
- de Tollenaer S.M., Buysse C., van den Anker J.N., Touw D.J., de Hoog M. Life threatening central nervous system manifestations and hypothermia due to maneb intoxication in a child: a case report. Ther Drug Monit. 2006;28(6):813–815. doi: 10.1097/01.ftd.0000243964.90340.cc. [DOI] [PubMed] [Google Scholar]
- Debski B. Supplementation of pigs diet with zinc and copper as alternative to conventional antimicrobials. Pol J Vet Sci. 2016;19(4):917–924. doi: 10.1515/pjvs-2016-0113. [DOI] [PubMed] [Google Scholar]
- Di Giancamillo A., Rossi R., Martino P.A., Aidos L., Maghin F., Domeneghini C., Corino C. Copper sulphate forms in piglet diets: microbiota, intestinal morphology and enteric nervous system glial cells. Anim Sci J. 2018;89(3):616–624. doi: 10.1111/asj.12948. [DOI] [PubMed] [Google Scholar]
- Diaz-Ochoa V.E., Lam D., Lee C.S., Klaus S., Behnsen J., Liu J.Z., Chim N., Nuccio S.P., Rathi S.G., Mastroianni J.R., Edwards R.A., Jacobo C.M., Cerasi M., Battistoni A., Ouellette A.J., Goulding C.W., Chazin W.J., Skaar E.P., Raffatellu M. Salmonella mitigates oxidative stress and thrives in the inflamed gut by evading calprotectin-mediated manganese sequestration. Cell Host Microbe. 2016;19(6):814–825. doi: 10.1016/j.chom.2016.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dintilhac A., Alloing G., Granadel C., Claverys J.P. Competence and virulence of Streptococcus pneumoniae: adc and PsaA mutants exhibit a requirement for Zn and Mn resulting from inactivation of putative ABC metal permeases. Mol Microbiol. 1997;25(4):727–739. doi: 10.1046/j.1365-2958.1997.5111879.x. [DOI] [PubMed] [Google Scholar]
- Dostal A., Lacroix C., Bircher L., Pham V.T., Follador R., Zimmermann M.B., Chassard C. Iron modulates butyrate production by a child gut microbiota in vitro. mBio. 2015;6(6) doi: 10.1128/mBio.01453-15. e01453-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eijkelkamp B.A., Morey J.R., Neville S.L., Tan A., Pederick V.G., Cole N., Singh P.P., Ong C.Y., Gonzalez de Vega R., Clases D., Cunningham B.A., Hughes C.E., Comerford I., Brazel E.B., Whittall J.J., Plumptre C.D., McColl S.R., Paton J.C., McEwan A.G., Doble P.A., McDevitt C.A. Dietary zinc and the control of Streptococcus pneumoniae infection. PLoS Pathog. 2019;15(8) doi: 10.1371/journal.ppat.1007957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faulkner M.J., Wenner B.A., Solden L.M., Weiss W.P. Source of supplemental dietary copper, zinc, and manganese affects fecal microbial relative abundance in lactating dairy cows. J Dairy Sci. 2017;100(2):1037–1044. doi: 10.3168/jds.2016-11680. [DOI] [PubMed] [Google Scholar]
- Fischer M.M., Egli I.M., Aeberli I., Hurrell R.F., Meile L. Phytic acid degrading lactic acid bacteria in tef-injera fermentation. Int J Food Microbiol. 2014;190:54–60. doi: 10.1016/j.ijfoodmicro.2014.08.018. [DOI] [PubMed] [Google Scholar]
- Fridovich I. Superoxide dismutases. Adv Enzymol Relat Area Mol Biol. 1974;41:35–97. doi: 10.1002/9780470122860.ch2. 0. [DOI] [PubMed] [Google Scholar]
- Furrer J.L., Sanders D.N., Hook-Barnard I.G., McIntosh M.A. Export of the siderophore enterobactin in Escherichia coli: involvement of a 43 kDa membrane exporter. Mol Microbiol. 2002;44(5):1225–1234. doi: 10.1046/j.1365-2958.2002.02885.x. [DOI] [PubMed] [Google Scholar]
- Gaballa A., Helmann J.D. A peroxide-induced zinc uptake system plays an important role in protection against oxidative stress in Bacillus subtilis. Mol Microbiol. 2002;45(4):997–1005. doi: 10.1046/j.1365-2958.2002.03068.x. [DOI] [PubMed] [Google Scholar]
- Glibota N., Grande Burgos M.J., Galvez A., Ortega E. Copper tolerance and antibiotic resistance in soil bacteria from olive tree agricultural fields routinely treated with copper compounds. J Sci Food Agric. 2019;99(10):4677–4685. doi: 10.1002/jsfa.9708. [DOI] [PubMed] [Google Scholar]
- Grigorieva D.V., Gorudko I.V., Shamova E.V., Terekhova M.S., Maliushkova E.V., Semak I.V., Cherenkevich S.N., Sokolov A.V., Timoshenko A.V. Effects of recombinant human lactoferrin on calcium signaling and functional responses of human neutrophils. Arch Biochem Biophys. 2019;675:108122. doi: 10.1016/j.abb.2019.108122. [DOI] [PubMed] [Google Scholar]
- Grummer R.H., Bentley O.G., Phillips P.H., Bohstedt The role of manganese in growth, reproduction, and lactation of swine. J Anim Sci. 1950;9(2):170–175. doi: 10.2527/jas1950.92170x. [DOI] [PubMed] [Google Scholar]
- Grzywacz K., Butcher J., Romain G., Li J., Stintzi A. The impact of probiotics and lactoferrin supplementation on piglet gastrointestinal microbial communities. Biometals. 2019;32(3):533–543. doi: 10.1007/s10534-019-00195-3. [DOI] [PubMed] [Google Scholar]
- Han Y.M., Yoon H., Lim S., Sung M.K., Shin C.M., Park Y.S., Kim N., Lee D.H., Kim J.S. Risk factors for Vitamin D, zinc, and selenium deficiencies in Korean patients with inflammatory bowel disease. Gut Liver. 2017;11(3):363–369. doi: 10.5009/gnl16333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansen S.L., Trakooljul N., Liu H.C., Moeser A.J., Spears J.W. Iron transporters are differentially regulated by dietary iron, and modifications are associated with changes in manganese metabolism in young pigs. J Nutr. 2009;139(8):1474–1479. doi: 10.3945/jn.109.105866. [DOI] [PubMed] [Google Scholar]
- Hantke K. Bacterial zinc uptake and regulators. Curr Opin Microbiol. 2005;8(2):196–202. doi: 10.1016/j.mib.2005.02.001. [DOI] [PubMed] [Google Scholar]
- Hantke K., Nicholson G., Rabsch W., Winkelmann G. Salmochelins, siderophores of Salmonella enterica and uropathogenic Escherichia coli strains, are recognized by the outer membrane receptor IroN. Proc Natl Acad Sci U S A. 2003;100(7):3677–3682. doi: 10.1073/pnas.0737682100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrison Brody A., Chou E., Gray J.M., Pokyrwka N.J., Raley-Susman K.M. Mancozeb-induced behavioral deficits precede structural neural degeneration. Neurotoxicology. 2013;34:74–81. doi: 10.1016/j.neuro.2012.10.007. [DOI] [PubMed] [Google Scholar]
- Hassani H., Golbabaei F., Ghahri A., Hosseini M., Shirkhanloo H., Dinari B., Eskandari D., Fallahi M. Occupational exposure to manganese-containing welding fumes and pulmonary function indices among natural gas transmission pipeline welders. J Occup Health. 2012;54(4):316–322. doi: 10.1539/joh.11-0269-fs. [DOI] [PubMed] [Google Scholar]
- Hernandez-Montes G., Arguello J.M., Valderrama B. Evolution and diversity of periplasmic proteins involved in copper homeostasis in gamma proteobacteria. BMC Microbiol. 2012;12:249. doi: 10.1186/1471-2180-12-249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hider R.C., Kong X. Chemistry and biology of siderophores. Nat Prod Rep. 2010;27(5):637–657. doi: 10.1039/b906679a. [DOI] [PubMed] [Google Scholar]
- Higashimura Y., Takagi T., Naito Y., Uchiyama K., Mizushima K., Tanaka M., Hamaguchi M., Itoh Y. Zinc deficiency activates the IL-23/Th17 Axis to aggravate experimental colitis in mice. J Crohns Colitis. 2020;14(6):856–866. doi: 10.1093/ecco-jcc/jjz193. [DOI] [PubMed] [Google Scholar]
- Hojberg O., Canibe N., Poulsen H.D., Hedemann M.S., Jensen B.B. Influence of dietary zinc oxide and copper sulfate on the gastrointestinal ecosystem in newly weaned piglets. Appl Environ Microbiol. 2005;71(5):2267–2277. doi: 10.1128/AEM.71.5.2267-2277.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holzel C.S., Muller C., Harms K.S., Mikolajewski S., Schafer S., Schwaiger K., Bauer J. Heavy metals in liquid pig manure in light of bacterial antimicrobial resistance. Environ Res. 2012;113:21–27. doi: 10.1016/j.envres.2012.01.002. [DOI] [PubMed] [Google Scholar]
- Hoppe M., Onning G., Berggren A., Hulthen L. Probiotic strain Lactobacillus plantarum 299v increases iron absorption from an iron-supplemented fruit drink: a double-isotope cross-over single-blind study in women of reproductive age. Br J Nutr. 2015;114(8):1195–1202. doi: 10.1017/S000711451500241X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hotz C., Lowe N.M., Araya M., Brown K.H. Assessment of the trace element status of individuals and populations: the example of zinc and copper. J Nutr. 2003;133(5 Suppl 1):1563S–1568S. doi: 10.1093/jn/133.5.1563S. [DOI] [PubMed] [Google Scholar]
- Hu P., Zhao D., Zhao F., Wang J., Zhu W. The effects of the combination of oral lactoferrin and iron injection on iron homestasis, antioxidative abilities and cytokines activities of suckling piglets. Animals (Basel) 2019;9(7) doi: 10.3390/ani9070438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu P., Zhao F., Zhu W., Wang J. Effects of early-life lactoferrin intervention on growth performance, small intestinal function and gut microbiota in suckling piglets. Food Funct. 2019;10(9):5361–5373. doi: 10.1039/c9fo00676a. [DOI] [PubMed] [Google Scholar]
- Hu W., Zhao J., Wang J., Yu T., Wang J., Li N. Transgenic milk containing recombinant human lactoferrin modulates the intestinal flora in piglets. Biochem Cell Biol. 2012;90(3):485–496. doi: 10.1139/o2012-003. [DOI] [PubMed] [Google Scholar]
- Ibs K.H., Rink L. Zinc-altered immune function. J Nutr. 2003;133(5 Suppl 1):1452S–1456S. doi: 10.1093/jn/133.5.1452S. [DOI] [PubMed] [Google Scholar]
- Jaeggi T., Kortman G.A., Moretti D., Chassard C., Holding P., Dostal A., Boekhorst J., Timmerman H.M., Swinkels D.W., Tjalsma H., Njenga J., Mwangi A., Kvalsvig J., Lacroix C., Zimmermann M.B. Iron fortification adversely affects the gut microbiome, increases pathogen abundance and induces intestinal inflammation in Kenyan infants. Gut. 2015;64(5):731–742. doi: 10.1136/gutjnl-2014-307720. [DOI] [PubMed] [Google Scholar]
- Karlinsey J.E., Maguire M.E., Becker L.A., Crouch M.L., Fang F.C. The phage shock protein PspA facilitates divalent metal transport and is required for virulence of Salmonella enterica sv. Typhimurium. Mol Microbiol. 2010;78(3):669–685. doi: 10.1111/j.1365-2958.2010.07357.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kehres D.G., Maguire M.E. Emerging themes in manganese transport, biochemistry and pathogenesis in bacteria. FEMS Microbiol Rev. 2003;27(2–3):263–290. doi: 10.1016/S0168-6445(03)00052-4. [DOI] [PubMed] [Google Scholar]
- Kehres D.G., Zaharik M.L., Finlay B.B., Maguire M.E. The NRAMP proteins of Salmonella typhimurium and Escherichia coli are selective manganese transporters involved in the response to reactive oxygen. Mol Microbiol. 2000;36(5):1085–1100. doi: 10.1046/j.1365-2958.2000.01922.x. [DOI] [PubMed] [Google Scholar]
- Klevay L.M. Copper deficiency can change the microbiome of swine. Am J Physiol Endocrinol Metab. 2019;317(1):E183. doi: 10.1152/ajpendo.00148.2019. [DOI] [PubMed] [Google Scholar]
- Knight L.C., Wang M., Donovan S.M., Dilger R.N. Early-life iron deficiency and subsequent repletion alters development of the colonic microbiota in the pig. Front Nutr. 2019;6:120. doi: 10.3389/fnut.2019.00120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kociova S., Dolezelikova K., Horky P., Skalickova S., Baholet D., Bozdechova L., Vaclavkova E., Belkova J., Nevrkla P., Skladanka J., Do T., Zitka O., Haddad Y., Kopel P., Zurek L., Adam V., Smerkova K. Zinc phosphate-based nanoparticles as alternatives to zinc oxide in diet of weaned piglets. J Anim Sci Biotechnol. 2020;11:59. doi: 10.1186/s40104-020-00458-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krewulak K.D., Vogel H.J. Structural biology of bacterial iron uptake. Biochim Biophys Acta. 2008;1778(9):1781–1804. doi: 10.1016/j.bbamem.2007.07.026. [DOI] [PubMed] [Google Scholar]
- Laparra J.M., Glahn R.P., Miller D.D. Assessing potential effects of inulin and probiotic bacteria on Fe availability from common beans (Phaseolus vulgaris L.) to Caco-2 cells. J Food Sci. 2009;74(2):H40–H46. doi: 10.1111/j.1750-3841.2008.01027.x. [DOI] [PubMed] [Google Scholar]
- Lee D.Y., Johnson P.E. Factors affecting absorption and excretion of 54Mn in rats. J Nutr. 1988;118(12):1509–1516. doi: 10.1093/jn/118.12.1509. [DOI] [PubMed] [Google Scholar]
- Legrand D., Elass E., Carpentier M., Mazurier J. Lactoferrin: a modulator of immune and inflammatory responses. Cell Mol Life Sci. 2005;62(22):2549–2559. doi: 10.1007/s00018-005-5370-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu H., Bai M., Xu K., Zhou J., Zhang X., Yu R. Effects of different concentrations of coated nano zinc oxide material on fecal bacterial composition and intestinal barrier in weaned piglets. J Sci Food Agric. 2020;101(2):735–745. doi: 10.1002/jsfa.10686. [DOI] [PubMed] [Google Scholar]
- Lopez-Alonso M. Trace minerals and livestock: not too much not too little. ISRN Vet Sci. 2012;2012:704825. doi: 10.5402/2012/704825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahren S., Schnell H., Braun V. Occurrence and regulation of the ferric citrate transport system in Escherichia coli B, Klebsiella pneumoniae, Enterobacter aerogenes, and Photorhabdus luminescens. Arch Microbiol. 2005;184(3):175–186. doi: 10.1007/s00203-005-0035-y. [DOI] [PubMed] [Google Scholar]
- Martin R., Bermudez-Humaran L.G., Langella P. Gnotobiotic rodents: an in vivo model for the study of microbe-microbe interactions. Front Microbiol. 2016;7:409. doi: 10.3389/fmicb.2016.00409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCall K.A., Huang C., Fierke C.A. Function and mechanism of zinc metalloenzymes. J Nutr. 2000;130(5S Suppl):1437S–1446S. doi: 10.1093/jn/130.5.1437S. [DOI] [PubMed] [Google Scholar]
- Medardus J.J., Molla B.Z., Nicol M., Morrow W.M., Rajala-Schultz P.J., Kazwala R., Gebreyes W.A. In-feed use of heavy metal micronutrients in U.S. swine production systems and its role in persistence of multidrug-resistant salmonellae. Appl Environ Microbiol. 2014;80(7):2317–2325. doi: 10.1128/AEM.04283-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molly K., Vande Woestyne M., Verstraete W. Development of a 5-step multi-chamber reactor as a simulation of the human intestinal microbial ecosystem. Appl Microbiol Biotechnol. 1993;39(2):254–258. doi: 10.1007/BF00228615. [DOI] [PubMed] [Google Scholar]
- Mudd A.T., Fil J.E., Knight L.C., Dilger R.N. Dietary iron repletion following early-life dietary iron deficiency does not correct regional volumetric or diffusion tensor changes in the developing pig brain. Front Neurol. 2017;8:735. doi: 10.3389/fneur.2017.00735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mudd A.T., Fil J.E., Knight L.C., Lam F., Liang Z.P., Dilger R.N. Early-life iron deficiency reduces brain iron content and alters brain tissue composition despite iron repletion: a neuroimaging assessment. Nutrients. 2018;10(2) doi: 10.3390/nu10020135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nargund S., Qiu J., Goudar C.T. Elucidating the role of copper in CHO cell energy metabolism using (13)C metabolic flux analysis. Biotechnol Prog. 2015;31(5):1179–1186. doi: 10.1002/btpr.2131. [DOI] [PubMed] [Google Scholar]
- Nguyen K.H., Grove A. Metal binding at the Deinococcus radiodurans Dps-1 N-terminal metal site controls dodecameric assembly and DNA binding. Biochemistry. 2012;51(33):6679–6689. doi: 10.1021/bi300703x. [DOI] [PubMed] [Google Scholar]
- Niu Q., Li P., Hao S., Zhang Y., Kim S.W., Li H., Ma X., Gao S., He L., Wu W., Huang X., Hua J., Zhou B., Huang R. Dynamic distribution of the gut microbiota and the relationship with apparent crude fiber digestibility and growth stages in pigs. Sci Rep. 2015;5:9938. doi: 10.1038/srep09938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oda H., Wakabayashi H., Yamauchi K., Abe F. Lactoferrin and bifidobacteria. Biometals. 2014;27(5):915–922. doi: 10.1007/s10534-014-9741-8. [DOI] [PubMed] [Google Scholar]
- Oh S.M., Kim M.J., Hosseindoust A., Kim K.Y., Choi Y.H., Ham H.B., Hwang S.J., Lee J.H., Cho H.J., Kang W.S., Chae B.J. Hot melt extruded-based nano zinc as an alternative to the pharmacological dose of ZnO in weanling piglets. Asian-Australas J Anim Sci. 2020;33(6):992–1001. doi: 10.5713/ajas.19.0140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okereafor U., Makhatha M., Mekuto L., Uche-Okereafor N., Sebola T., Mavumengwana V. Toxic metal implications on agricultural soils, plants, animals, aquatic life and human health. Int J Environ Res Publ Health. 2020;17(7) doi: 10.3390/ijerph17072204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ong C.L., Walker M.J., McEwan A.G. Zinc disrupts central carbon metabolism and capsule biosynthesis in Streptococcus pyogenes. Sci Rep. 2015;5:10799. doi: 10.1038/srep10799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pajarillo E.A., Chae J.P., Balolong M.P., Bum Kim H., Kang D.-K. Assessment of fecal bacterial diversity among healthy piglets during the weaning transition. J Gen Appl Microbiol. 2014;60(4):140–146. doi: 10.2323/jgam.60.140. [DOI] [PubMed] [Google Scholar]
- Pajarillo E.A., Chae J.P., Balolong M.P., Kim H.B., Seo K.S., Kang D.-K. Pyrosequencing-based analysis of fecal microbial communities in three purebred pig lines. J Microbiol. 2014;52(8):646–651. doi: 10.1007/s12275-014-4270-2. [DOI] [PubMed] [Google Scholar]
- Pajarillo E.A., Chae J.P., Kim H.B., Kim I.H., Kang D.-K. Barcoded pyrosequencing-based metagenomic analysis of the faecal microbiome of three purebred pig lines after cohabitation. Appl Microbiol Biotechnol. 2015;99(13):5647–5656. doi: 10.1007/s00253-015-6408-5. [DOI] [PubMed] [Google Scholar]
- Pallauf J., Kauer C., Most E., Habicht S.D., Moch J. Impact of dietary manganese concentration on status criteria to determine manganese requirement in piglets. J Anim Physiol Anim Nutr (Berl) 2012;96(6):993–1002. doi: 10.1111/j.1439-0396.2011.01213.x. [DOI] [PubMed] [Google Scholar]
- Panasevich M.R., Rector R.S. Reply to Letter to the Editor: "Copper deficiency can change the microbiome of swine". Am J Physiol Endocrinol Metab. 2019;317(1):E184. doi: 10.1152/ajpendo.00182.2019. [DOI] [PubMed] [Google Scholar]
- Panina E.M., Mironov A.A., Gelfand M.S. Comparative genomics of bacterial zinc regulons: enhanced ion transport, pathogenesis, and rearrangement of ribosomal proteins. Proc Natl Acad Sci U S A. 2003;100(17):9912–9917. doi: 10.1073/pnas.1733691100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papp-Wallace K.M., Maguire M.E. Manganese transport and the role of manganese in virulence. Annu Rev Microbiol. 2006;60:187–209. doi: 10.1146/annurev.micro.60.080805.142149. [DOI] [PubMed] [Google Scholar]
- Parsons C., Costolo B., Brown P., Kathariou S. Penicillin-binding protein encoded by pbp4 is involved in mediating copper stress in Listeria monocytogenes. FEMS Microbiol Lett. 2017;364(20) doi: 10.1093/femsle/fnx207. [DOI] [PubMed] [Google Scholar]
- Parsons C., Lee S., Kathariou S. Dissemination and conservation of cadmium and arsenic resistance determinants in Listeria and other Gram-positive bacteria. Mol Microbiol. 2020;113(3):560–569. doi: 10.1111/mmi.14470. [DOI] [PubMed] [Google Scholar]
- Patzer S.I., Hantke K. The zinc-responsive regulator Zur and its control of the znu gene cluster encoding the ZnuABC zinc uptake system in Escherichia coli. J Biol Chem. 2000;275(32):24321–24332. doi: 10.1074/jbc.M001775200. [DOI] [PubMed] [Google Scholar]
- Pearce S.C., Coia H.G., Karl J.P., Pantoja-Feliciano I.G., Zachos N.C., Racicot K. Intestinal in vitro and ex vivo models to study host-microbiome interactions and acute stressors. Front Physiol. 2018;9:1584. doi: 10.3389/fphys.2018.01584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peng Z., Zhang J., Fanning S., Wang L., Li M., Maheshwari N., Sun J., Li F. Effects of metal and metalloid pollutants on the microbiota composition of feces obtained from twelve commercial pig farms across China. Sci Total Environ. 2019;647:577–586. doi: 10.1016/j.scitotenv.2018.08.026. [DOI] [PubMed] [Google Scholar]
- Pickard J.M., Zeng M.Y., Caruso R., Nunez G. Gut microbiota: role in pathogen colonization, immune responses, and inflammatory disease. Immunol Rev. 2017;279(1):70–89. doi: 10.1111/imr.12567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pieper R., Dadi T.H., Pieper L., Vahjen W., Franke A., Reinert K. Concentration and chemical form of dietary zinc shape the porcine colon microbiome, its functional capacity and antibiotic resistance gene repertoire. ISME J. 2020;14(11):2783–2793. doi: 10.1038/s41396-020-0730-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pieper R., Vahjen W., Neumann K., Van Kessel A.G., Zentek J. Dose-dependent effects of dietary zinc oxide on bacterial communities and metabolic profiles in the ileum of weaned pigs. J Anim Physiol Anim Nutr (Berl) 2012;96(5):825–833. doi: 10.1111/j.1439-0396.2011.01231.x. [DOI] [PubMed] [Google Scholar]
- Pontin K.P., Borges K.A., Furian T.Q., Carvalho D., Wilsmann D.E., Cardoso H.R.P., Alves A.K., Chitolina G.Z., Salle C.T.P., Moraes H.L.S., do Nascimento V.P. Antimicrobial activity of copper surfaces against biofilm formation by Salmonella Enteritidis and its potential application in the poultry industry. Food Microbiol. 2021;94:103645. doi: 10.1016/j.fm.2020.103645. [DOI] [PubMed] [Google Scholar]
- Posey J.E., Gherardini F.C. Lack of a role for iron in the Lyme disease pathogen. Science. 2000;288(5471):1651–1653. doi: 10.1126/science.288.5471.1651. [DOI] [PubMed] [Google Scholar]
- Poussin C., Sierro N., Boue S., Battey J., Scotti E., Belcastro V., Peitsch M.C., Ivanov N.V., Hoeng J. Interrogating the microbiome: experimental and computational considerations in support of study reproducibility. Drug Discov Today. 2018;23(9):1644–1657. doi: 10.1016/j.drudis.2018.06.005. [DOI] [PubMed] [Google Scholar]
- Prasad A.S. Zinc deficiency. BMJ. 2003;326(7386):409–410. doi: 10.1136/bmj.326.7386.409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rakin A., Schneider L., Podladchikova O. Hunger for iron: the alternative siderophore iron scavenging systems in highly virulent Yersinia. Front Cell Infect Microbiol. 2012;2:151. doi: 10.3389/fcimb.2012.00151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson N.J., Winge D.R. Copper metallochaperones. Annu Rev Biochem. 2010;79:537–562. doi: 10.1146/annurev-biochem-030409-143539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saha P., Yeoh B.S., Singh R., Chandrasekar B., Vemula P.K., Haribabu B., Vijay-Kumar M., Jala V.R. Gut microbiota conversion of dietary ellagic acid into bioactive phytoceutical urolithin A inhibits heme peroxidases. PloS One. 2016;11(6) doi: 10.1371/journal.pone.0156811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Satessa G.D., Kjeldsen N.J., Mansouryar M., Hansen H.H., Bache J.K., Nielsen M.O. Effects of alternative feed additives to medicinal zinc oxide on productivity, diarrhoea incidence and gut development in weaned piglets. Animal. 2020:1–9. doi: 10.1017/S1751731120000154. [DOI] [PubMed] [Google Scholar]
- Sawyer J.T., Tittor A.W., Apple J.K., Morgan J.B., Maxwell C.V., Rakes L.K., Fakler T.M. Effects of supplemental manganese on performance of growing-finishing pigs and pork quality during retail display. J Anim Sci. 2007;85(4):1046–1053. doi: 10.2527/jas.2006-262. [DOI] [PubMed] [Google Scholar]
- Shannon M.C., Hill G.M. Trace mineral supplementation for the intestinal health of young monogastric animals. Front Vet Sci. 2019;6:73. doi: 10.3389/fvets.2019.00073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shelton N.W., Tokach M.D., Nelssen J.L., Goodband R.D., Dritz S.S., DeRouchey J.M., Hill G.M. Effects of copper sulfate, tri-basic copper chloride, and zinc oxide on weanling pig performance. J Anim Sci. 2011;89(8):2440–2451. doi: 10.2527/jas.2010-3432. [DOI] [PubMed] [Google Scholar]
- Silva B., Faustino P. An overview of molecular basis of iron metabolism regulation and the associated pathologies. Biochim Biophys Acta. 2015;1852(7):1347–1359. doi: 10.1016/j.bbadis.2015.03.011. [DOI] [PubMed] [Google Scholar]
- Singh A., Yeager R., McFeters G.A. Assessment of in vivo revival, growth, and pathogenicity of Escherichia coli strains after copper- and chlorine-induced injury. Appl Environ Microbiol. 1986;52(4):832–837. doi: 10.1128/aem.52.4.832-837.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sitkin S.I., Tkachenko E.I., Vakhitov T.Y. Metabolic dysbiosis of the gut microbiota and its biomarkers. Eksp Klin Gastroenterol. 2016;12(12):6–29. [PubMed] [Google Scholar]
- Slifierz M.J., Friendship R., Weese J.S. Zinc oxide therapy increases prevalence and persistence of methicillin-resistant Staphylococcus aureus in pigs: a randomized controlled trial. Zoonoses Public Health. 2015;62(4):301–308. doi: 10.1111/zph.12150. [DOI] [PubMed] [Google Scholar]
- Slifierz M.J., Friendship R.M., Weese J.S. Methicillin-resistant Staphylococcus aureus in commercial swine herds is associated with disinfectant and zinc usage. Appl Environ Microbiol. 2015;81(8):2690–2695. doi: 10.1128/AEM.00036-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spears J.W. Boron, chromium, manganese, and nickel in agricultural animal production. Biol Trace Elem Res. 2019;188(1):35–44. doi: 10.1007/s12011-018-1529-1. [DOI] [PubMed] [Google Scholar]
- Starke I.C., Pieper R., Neumann K., Zentek J., Vahjen W. The impact of high dietary zinc oxide on the development of the intestinal microbiota in weaned piglets. FEMS Microbiol Ecol. 2014;87(2):416–427. doi: 10.1111/1574-6941.12233. [DOI] [PubMed] [Google Scholar]
- Subramanian Vignesh K., Deepe G.S., Jr. Immunological orchestration of zinc homeostasis: the battle between host mechanisms and pathogen defenses. Arch Biochem Biophys. 2016;611:66–78. doi: 10.1016/j.abb.2016.02.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Troxell B., Fink R.C., Porwollik S., McClelland M., Hassan H.M. The Fur regulon in anaerobically grown Salmonella enterica sv. Typhimurium: identification of new Fur targets. BMC Microbiol. 2011;11:236. doi: 10.1186/1471-2180-11-236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsebrzhinskii O.I. Antioxidant status in manganese poisoning. Ukr Biokhim Zh (1978) 1998;70(4):79–84. [PubMed] [Google Scholar]
- Tsolis R.M., Baumler A.J., Heffron F., Stojiljkovic I. Contribution of TonB- and Feo-mediated iron uptake to growth of Salmonella typhimurium in the mouse. Infect Immun. 1996;64(11):4549–4556. doi: 10.1128/iai.64.11.4549-4556.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vahjen W., Pieper R., Zentek J. Increased dietary zinc oxide changes the bacterial core and enterobacterial composition in the ileum of piglets. J Anim Sci. 2011;89(8):2430–2439. doi: 10.2527/jas.2010-3270. [DOI] [PubMed] [Google Scholar]
- Vega-Bautista A., de la Garza M., Carrero J.C., Campos-Rodriguez R., Godinez-Victoria M., Drago-Serrano M.E. The impact of lactoferrin on the growth of intestinal inhabitant bacteria. Int J Mol Sci. 2019;20(19) doi: 10.3390/ijms20194707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Velasco E., Wang S., Sanet M., Fernandez-Vazquez J., Jove D., Glaria E., Valledor A.F., O'Halloran T.V., Balsalobre C. A new role for Zinc limitation in bacterial pathogenicity: modulation of alpha-hemolysin from uropathogenic Escherichia coli. Sci Rep. 2018;8(1):6535. doi: 10.1038/s41598-018-24964-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vergnes A., Viala J.P., Ouadah-Tsabet R., Pocachard B., Loiseau L., Meresse S., Barras F., Aussel L. The iron-sulfur cluster sensor IscR is a negative regulator of Spi1 type III secretion system in Salmonella enterica. Cell Microbiol. 2017;19(4) doi: 10.1111/cmi.12680. [DOI] [PubMed] [Google Scholar]
- Villagomez-Estrada S., Perez J.F., Darwich L., Vidal A., van Kuijk S., Melo-Duran D., Sola-Oriol D. Effects of copper and zinc sources and inclusion levels of copper on weanling pig performance and intestinal microbiota. J Anim Sci. 2020;98(5) doi: 10.1093/jas/skaa117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagegg W., Braun V. Ferric citrate transport in Escherichia coli requires outer membrane receptor protein fecA. J Bacteriol. 1981;145(1):156–163. doi: 10.1128/jb.145.1.156-163.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang H., Yang F., Xin R., Cui D., He J., Zhang S., Sun Y. The gut microbiota attenuate neuroinflammation in manganese exposure by inhibiting cerebral NLRP3 inflammasome. Biomed Pharmacother. 2020;129:110449. doi: 10.1016/j.biopha.2020.110449. [DOI] [PubMed] [Google Scholar]
- Wang H., Zhang S., Yang F., Xin R., Wang S., Cui D., Sun Y. The gut microbiota confers protection in the CNS against neurodegeneration induced by manganism. Biomed Pharmacother. 2020;127:110150. doi: 10.1016/j.biopha.2020.110150. [DOI] [PubMed] [Google Scholar]
- Wang J., Luo X., Zhang Y., Huang Y., Rajendran M., Xue S. Plant species diversity for vegetation restoration in manganese tailing wasteland. Environ Sci Pollut Res Int. 2018;25(24):24101–24110. doi: 10.1007/s11356-018-2275-9. [DOI] [PubMed] [Google Scholar]
- Wang W., Van Noten N., Degroote J., Romeo A., Vermeir P., Michiels J. Effect of zinc oxide sources and dosages on gut microbiota and integrity of weaned piglets. J Anim Physiol Anim Nutr (Berl) 2019;103(1):231–241. doi: 10.1111/jpn.12999. [DOI] [PubMed] [Google Scholar]
- Weigand E., Egenolf J. A moderate zinc deficiency does not alter lipid and fatty acid composition in the liver of weanling rats fed diets rich in cocoa butter or safflower oil. J Nutr Metab. 2017;2017:4798963. doi: 10.1155/2017/4798963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinberg E.D. The Lactobacillus anomaly: total iron abstinence. Perspect Biol Med. 1997;40(4):578–583. doi: 10.1353/pbm.1997.0072. [DOI] [PubMed] [Google Scholar]
- Xie Y., He Y., Irwin P.L., Jin T., Shi X. Antibacterial activity and mechanism of action of zinc oxide nanoparticles against Campylobacter jejuni. Appl Environ Microbiol. 2011;77(7):2325–2331. doi: 10.1128/AEM.02149-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang C., Zhu X., Liu N., Chen Y., Gan H., Troy F.A., 2nd, Wang B. Lactoferrin up-regulates intestinal gene expression of brain-derived neurotrophic factors BDNF, UCHL1 and alkaline phosphatase activity to alleviate early weaning diarrhea in postnatal piglets. J Nutr Biochem. 2014;25(8):834–842. doi: 10.1016/j.jnutbio.2014.03.015. [DOI] [PubMed] [Google Scholar]
- Yu X., Han J., Li H., Zhang Y., Feng J. The effect of enzymes on release of trace elements in feedstuffs based on in vitro digestion model for monogastric livestock. J Anim Sci Biotechnol. 2018;9:73. doi: 10.1186/s40104-018-0289-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zaharik M.L., Finlay B.B. Mn2+ and bacterial pathogenesis. Front Biosci. 2004;9:1035–1042. doi: 10.2741/1317. [DOI] [PubMed] [Google Scholar]
- Zhang F., Zheng W., Guo R., Yao W. Effect of dietary copper level on the gut microbiota and its correlation with serum inflammatory cytokines in Sprague-Dawley rats. J Microbiol. 2017;55(9):694–702. doi: 10.1007/s12275-017-6627-9. [DOI] [PubMed] [Google Scholar]
- Zhang F., Zheng W., Xue Y., Yao W. Suhuai suckling piglet hindgut microbiome-metabolome responses to different dietary copper levels. Appl Microbiol Biotechnol. 2019;103(2):853–868. doi: 10.1007/s00253-018-9533-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang R., Gu J., Wang X., Li Y., Liu J., Lu C., Qiu L. Response of antibiotic resistance genes abundance by graphene oxide during the anaerobic digestion of swine manure with copper pollution. Sci Total Environ. 2019;654:292–299. doi: 10.1016/j.scitotenv.2018.11.094. [DOI] [PubMed] [Google Scholar]
- Zhang Y., Zhou J., Dong Z., Li G., Wang J., Li Y., Wan D., Yang H., Yin Y. Effect of dietary copper on intestinal microbiota and antimicrobial resistance profiles of Escherichia coli in weaned piglets. Front Microbiol. 2019;10:2808. doi: 10.3389/fmicb.2019.02808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou D., Hardt W.D., Galan J.E. Salmonella typhimurium encodes a putative iron transport system within the centisome 63 pathogenicity island. Infect Immun. 1999;67(4):1974–1981. doi: 10.1128/iai.67.4.1974-1981.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]