Summary
Omega-3 fatty acid prescription drugs, Vascepa (≥96% eicosapentaenoic acid [EPA] ethyl ester) and Lovaza (46.5% EPA and 37.5% docosahexaenoic acid ethyl ester) are known therapeutic regimens to treat hypertriglyceridemia. However, their impact on glucose homeostasis, progression to type 2 diabetes, and pancreatic beta cell function are not well understood. In the present study, mice were treated with Vascepa or Lovaza for one week prior to six weeks of high-fat diet feeding. Vascepa but not Lovaza led to reduced insulin resistance, reduced fasting insulin and glucose, and improved glucose intolerance. Vascepa improved beta cell function, reduced liver triglycerides with enhanced expression of hepatic fatty acid oxidation genes, and altered microbiota composition. Vascepa has protective effects on diet-induced insulin resistance and glucose intolerance in mice.
Subject areas: Dietary supplement, Human metabolism
Graphical abstract
Highlights
-
•
Vascepa, an EPA ethyl ester prescription drug, protects from diet-induced weight gain
-
•
Acute exposure to Vascepa protects from glucose intolerance and beta cell dysfunction
-
•
Improved islet function is associated with improved insulin resistance
-
•
Vascepa upregulates hepatic fatty acid oxidation genes and alters gut microbiome
Dietary supplement; Human metabolism
Introduction
Chronic diseases including obesity, cancer, cardiovascular disease and type 2 diabetes (T2D) have increased rapidly over the past several years (World Health Organization, 2002) with T2D alone estimated to affect 510.8 million individuals by 2030 (Basu et al., 2019). T2D is characterized by chronic hyperglycemia in part caused by insulin resistance and impaired insulin secretion relative to the level of glycemia (American Diabetes Association, 2014). Obesity is highly linked to T2D risk and important factors such as diet and nutrition can reduce the risk of the incidence of diabetes. For many years, fish oil (FO) supplements have been viewed as beneficial to human health mainly due to the long-chain omega-3 fatty acids, eicosapentaenoic acid (EPA, 20:5n-3) and docosahexaenoic acid (DHA, 22:6n-3). In fact, both preclinical and clinical studies have shown that EPA and DHA exert health benefits on both cardiovascular disease and dyslipidemia (Arca et al., 2018; Khawaja et al., 2014; Skulas-Ray et al., 2019; van den Elsen et al., 2012). Thus, the World Health Organization recommends a daily intake of 0.25–2 g EPA + DHA in adults (Food and Agriculture Organization of the United Nations., 2010).
The effects of pure omega-3 fatty acid prescription drugs on glucose homeostasis and prediabetes/T2D have been previously investigated. In rodents, improvements in insulin resistance have been reported (De Castro et al., 2015; Kalupahana et al., 2010) through amelioration of oxidative stress (Molinar-Toribio et al., 2015), exertion of anti-inflammatory effects (Oh et al., 2010), reduction of hepatic fat accumulation, modulation of transcription factors involved in lipid metabolism and fatty acid oxidation (Jump, 2011; Kuda et al., 2009; Matsuura et al., 2004; Tanaka et al., 2010) and suppression of adipocytokines (Kalupahana et al., 2010). A recent randomized controlled trial in humans has also shown that omega-3 fatty acids alone reduced fasting blood glucose and HbA1c (Wang et al., 2019). However, systematic reviews and meta-analysis studies have shown that omega-3 fatty acid supplementation, seafood consumption, or circulating omega-3 biomarkers have no effect on biomarkers of glucose or insulin homeostasis including HbA1c, fasting, and postprandial plasma glucose in subjects with T2D (Brown et al., 2019; Chen et al., 2015; Montori et al., 2000; O'Mahoney et al., 2018; Wu et al., 2012; Zhou et al., 2012). In fact, omega-3 fatty acids have been reported to have different responses based on different ancestries (Li, 2015) and FO supplementation has moderately increased blood glucose and insulin resistance in a small cohort (Mostad et al., 2006). The opposing results could in part be due to dosing, composition, and purity of the FO used (Lalia and Lanza, 2016).
Recently, omega-3 fatty acid prescription drugs have been formulated and are being given to metabolic syndrome and cardiovascular patients to reduce triglyceride (TG) levels and improve cardiovascular disease outcomes (Bradberry and Hilleman, 2013). Unlike FO supplements, prescription omega-3 fatty acid drugs are FDA-approved for the treatment of hypertriglyceridemia. The two common prescription formulations available are Vascepa (Amarin), a high purity formulation of EPA ethyl ester (≥96%) (Jacobson, 2012; Kim and McCormack, 2014), and Lovaza (GlaxoSmithKline), an FO prescription drug containing EPA and DHA ethyl esters (46.5 and 37.5%, respectively) (Koski, 2008). In clinical trials, both Vascepa and Lovaza lowered TG levels of statin-treated and non-statin-treated patients with hypertriglyceridemia (Ballantyne et al., 2012; Bradberry and Hilleman, 2013; Davidson et al., 2007).
The actions of highly pure omega-3 fatty acid-enriched prescription drugs show promising effects in reducing the complication of metabolic diseases as shown in the JELIS trial, in which EPA treatment reduced the incidence of coronary artery disease in patients with impaired glucose metabolism (Oikawa et al., 2009; Saito et al., 2008; Yokoyama et al., 2007). Other clinical trials, such as the ASCEND and the ORIGIN trials, reported that omega-3 ethyl esters did not reduce cardiovascular events in patients with diabetes (Bosch, 2012; Bowman et al., 2018). Recently, the REDUCE-IT trial (Bhatt et al, 2019, 2020) reported positive results in patients with diabetes in which treatment with Vascepa reduced TG and improved cardiovascular outcomes (Bhatt et al, 2019, 2020; Tajuddin et al., 2016; Tummala et al., 2019). Given that dyslipidemia and cardiovascular disease are closely linked to T2D, the protective effects of omega-3 prescription drugs on glucose homeostasis and beta cell function need to be further studied. Here, we tested the potential protective effects of two FDA-approved omega-3 fatty acid prescription drugs, Vascepa and Lovaza, in a prediabetic mouse model. This study sought to identify the protective effects of omega-3 prescription drugs on glucose homeostasis and pancreatic beta cell function.
Results
EPA and docosapentaenoic acid are increased in the serum of type 2 diabetic patients
Owing to the current controversial effects of omega-3 fatty acids in T2D, glucose homeostasis, and pancreatic beta cell function, we first determined whether circulating fatty acids are altered in patients with T2D. Fatty acid analysis was performed in healthy vs patients with T2D. Clinical characteristics are presented in Table 1. The omega-3 fatty acid, EPA, had the highest increase in patients with T2D compared with controls. In addition, the intermediate metabolite docosapentaenoic acid (DPA), was also elevated in patients with T2D compared with controls (Figure S1A).
Table 1.
Clinical and metabolic characteristics of study population
| Characteristic | Normal (N = 28) | T2D (N = 25) | p Value |
|---|---|---|---|
| Sociodemographic characteristics | |||
| Sex | Male | Male | |
| Age (years) | 40.1 ± 1.2 | 48.5 ± 1.2 | <0.000001 |
| Height (cm) | 171.7 ± 0.9 | 170.6 ± 1.1 | 0.350 |
| Weight (kg) | 74.2 ± 1.5 | 74.8 ± 2.2 | 0.610 |
| BMI (kg/m2) | 25.2 ± 0.5 | 25.6 ± 0.6 | 0.734 |
| Clinical variables | |||
| Fasting plasma glucose (mmol/L) | 5.1 ± 0.1 | 8.9 ± 0.6 | 0.001 |
| 2 hr plasma glucose (mmol/L) | 5.8 ± 0.1 | 13.2 ± 0.9 | <0.000001 |
| HbA1c (mmol/L) | 5.4 ± 0.1 | 8.5 ± 0.4 | 0.009 |
| TG (mmol/L) | 1.7 ± 0.2 | 2.4 ± 0.4 | 0.552 |
| HDL (mmol/L) | 1.0 ± 0.05 | 1.8 ± 0.2 | 0.496 |
| LDL (mmol/L) | 3.2 ± 0.2 | 2.2 ± 0.2 | 0.395 |
Data are represented as mean ±SEM. TG, triglycerides; HDL, high-density lipoprotein; LDL, low-density lipoprotein.
See also Figure S1
Vascepa mitigates high-fat diet-induced glucose intolerance and insulin resistance in vivo
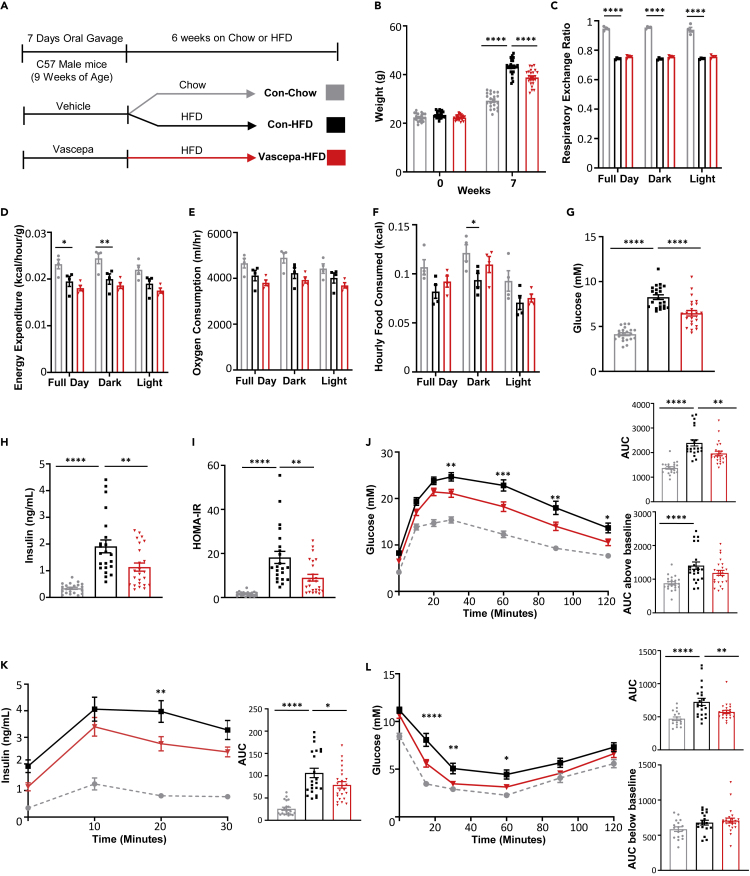
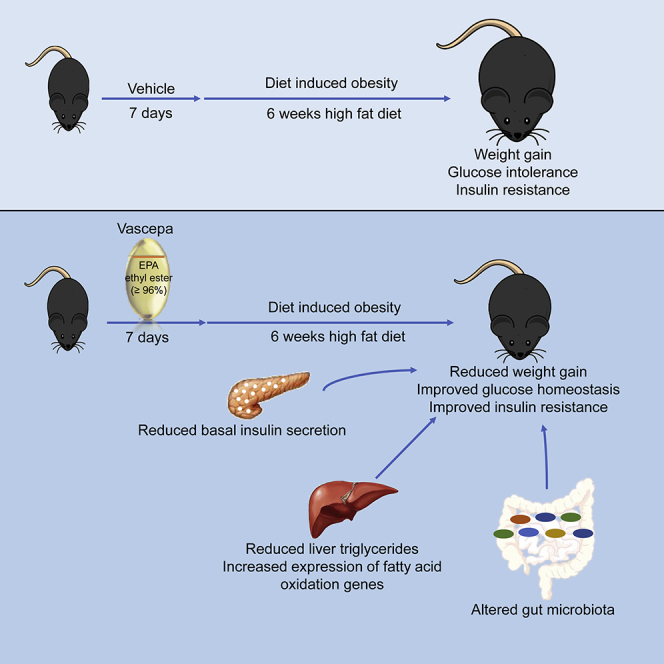
To determine the effects of omega-3 fatty acids on glucose homeostasis and insulin resistance, we have utilized Vascepa and Lovaza, two highly pure omega-3 ethyl ester prescription drugs containing primarily EPA or EPA & DHA, respectively. The composition of the omega-3 ethyl ester prescription drugs was confirmed by mass spectrometry. As anticipated, Vascepa contained only EPA ethyl ester (mw = 330.51g/mol) without the presence of DHA, whereas Lovaza contained both EPA (mw = 330.51g/mol) and DHA (mw = 356.55 g/mol) ethyl esters in the capsule (Figures S1B and S1C). These studies confirmed the high purity of Vascepa and Lovaza, indicating their suitability for further in vivo studies on glucose homeostasis. Because FO treatment is associated with improvements in circulating lipids and insulin resistance (Lalia and Lanza, 2016), we first examined the effects of the highly purified omega-3 ethyl ester prescription drugs Vascepa or Lovaza on high-fat diet (HFD)-induced glucose intolerance and insulin resistance. Since Lovaza contains only 46.5% EPA and other components such as DHA could contribute to the effects seen, the Lovaza dosage, which was given at an equivalent of 2g/day in humans, was calculated to contain the same level of EPA as Vascepa. The chow and HFD controls were the same for both the Vascepa and Lovaza group (Figures 1 and S2). Mice treated with Vascepa for 7 days, followed by HFD feeding for 6 weeks (Figure 1A), had significantly lower weight compared to control-HFD mice (Figure 1B). However, Vascepa-treated mice had no differences in food intake, respiratory exchange ratio, energy expenditure or oxygen consumption compared with control-HFD mice (Figures 1C–1F). Vascepa-treated mice also had significantly reduced fasting blood glucose and insulin (Figures 1G and 1H) and had improved glucose tolerance compared with control-HFD mice with no changes in area under curve above baseline (Figure 1J). Consistent with improved glucose tolerance, Vascepa-treated mice also had reduced insulin secretion (Figure 1K). To determine whether the reduction in insulin secretion was due to improvements in insulin resistance, an insulin tolerance test was performed. Treatment with Vascepa indeed improved HOMA-IR (Figure 1I) and insulin sensitivity with no changes in area under curve below baseline (Figure 1L) in comparison to control-HFD mice. In comparison, the effects of mice treated with Lovaza containing both omega-3 fatty acids EPA and DHA were less pronounced (Figure S2). Treatment with Lovaza (Figure S2A) had no net effect on weight (Figure S2B) or fasting insulin (Figure S2D) associated with HFD. It was also less effective than Vascepa in improving fasting glucose and correcting glucose intolerance (Figures S2C and S2F) and had no effect on HFD-induced insulin resistance, insulin secretion or HOMA-IR (Figures S2E, S2G and S2H). Interestingly, improvements in glucose homeostasis with either Vascepa or Lovaza treatment were only apparent under conditions of HFD. Both Lovaza or Vascepa did not have any remarkable effect on glucose homeostasis or insulin resistance under a standard chow diet on short-term studies (7 days oral gavage) or after a post-treatment period of 6 weeks (Figures S3 and S4).
Figure 1.
Vascepa reduces HFD-induced weight gain and protects from HFD impaired glucose intolerance and insulin resistance
(A–L) (A) Schematic diagram of study design (B) weight (n = 21,22,24) (C) respiratory exchange ratio (D) energy expenditure (E) oxygen consumption (F) food intake (n = 4/group) (G) fasting glucose levels (H) fasting insulin levels (I) calculated HOMA-IR (J) oral glucose tolerance test with area under curve and area under curve above baseline (K) insulin secretion during oral glucose tolerance test with area under curve (L) intraperitoneal insulin tolerance test with area under curve and area under curve below baseline (n = 21,22,24) ∗p < 0.05, ∗∗p < 0.01, ∗∗∗p < 0.001, ∗∗∗∗p < 0.0001 vs. con-HFD. All error bars ±SEM. See also Figures S2–S4.
Vascepa reduces basal insulin secretion ex vivo but does not appear to directly affect islet function in vitro
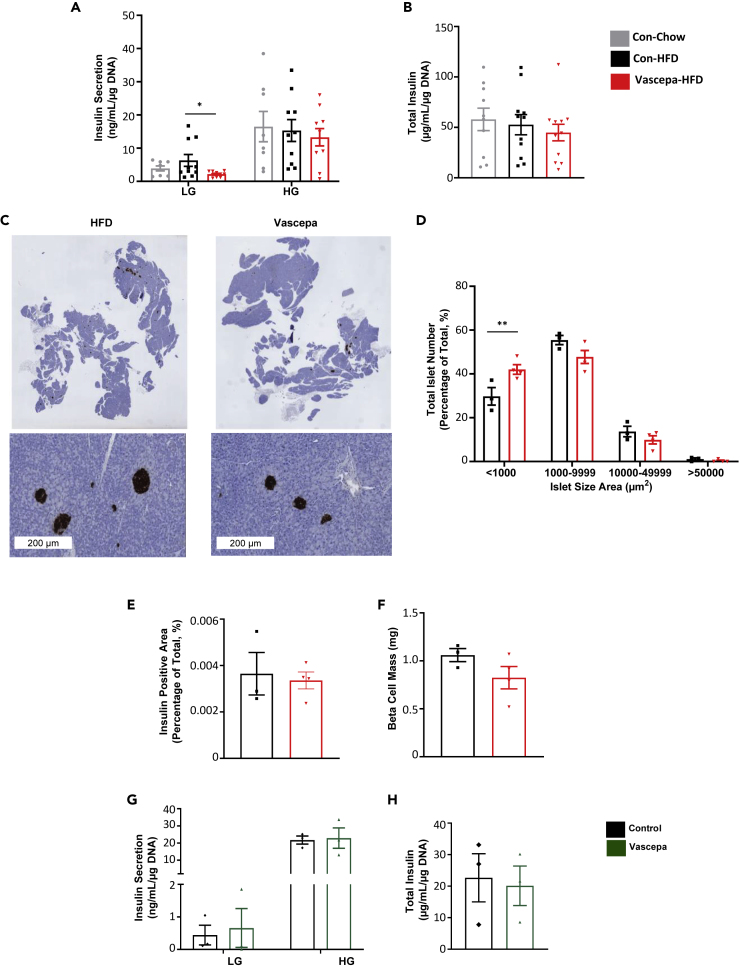
To look more closely at insulin secretion and pancreatic islet function, glucose stimulated insulin secretion (GSIS) was performed on islets isolated from mice treated with Vascepa for 7 days followed by 6 weeks of HFD feeding. Interestingly, Vascepa-treated mice had marked reductions in basal insulin secretion compared with control-HFD (Figure 2A). There was no change in high glucose stimulated secretion or total insulin content in the Vascepa-treated group compared with control-HFD (Figures 2A and 2B). Pancreatic islet morphometry analysis revealed a significant increase observed in the number of small islets in the Vascepa-treated group compared with control-HFD (Figures 2C and 2D). However, total pancreatic insulin-positive area and beta cell mass was unchanged between the groups as determined by pancreatic immunostaining (Figures 2E and 2F). In comparison, Lovaza treatment had no effect on GSIS, islet morphology analysis or beta cell mass (Figures S2I–S2M).
Figure 2.
Vascepa reduces basal insulin secretion after 6 weeks of HFD feeding
(A–H) (A) Ex vivo glucose stimulated insulin secretion and (B) total insulin content (n = 8–10) (C) pancreatic histology of insulin staining slides (Scale bar 200μm) (D) islet size distribution (E) total insulin positive area (F) beta cell mass (n = 3–4) (G) In vitro treatment of islets with Vascepa (100μM of EPA ethyl ester) for 48 hr followed by glucose stimulated insulin secretion and (H) total insulin content (n = 3). LG, low glucose; HG, high glucose. ∗p < 0.05, ∗∗p < 0.01 vs con-HFD. All error bars ±SEM. See also Figures S2 and S5.
To determine whether EPA or DHA ethyl ester, components of Vascepa or Lovaza, can acutely and directly affect islet function, islets and the glucose-responsive beta cell line MIN6K8 cells were treated with Vascepa, Lovaza, EPA ethyl ester, or DHA ethyl ester. In vitro, islets treated for 48 hr with Vascepa had no change in GSIS or total insulin content compared with the control group (Figures 2G and 2H). Treatment with Vascepa, Lovaza, EPA, or DHA ethyl ester for 24 hr did not affect GSIS in islets (Figures S5A–S5C) or MIN6K8 cells (Figures S5D–S5F).
Vascepa reduces liver TG and upregulates genes involved in the PPAR-α signaling pathway
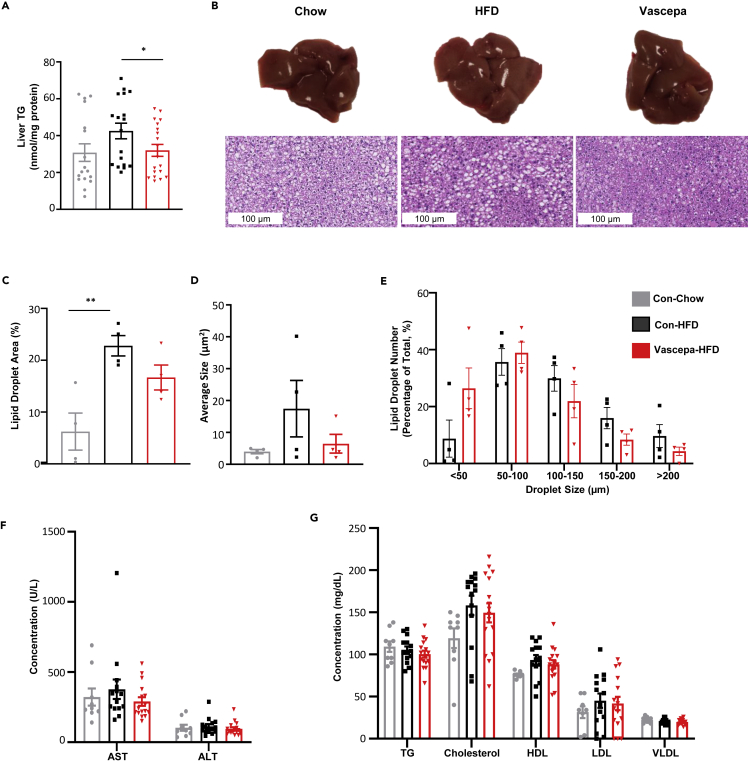
Given the improvements in diet-induced insulin resistance with Vascepa treatment and since the liver is highly susceptible to insulin resistance associated with HFD feeding, we next looked specifically at the effects of Vascepa on liver function. Mice treated with Vascepa for 7 days followed by HFD feeding for 6 weeks had significantly reduced liver TG content compared with control-HFD (Figure 3A). To determine changes in liver morphology, H&E and oil red O staining were performed on livers isolated after 6 weeks of HFD feeding. No differences were observed in the lipid droplet area and size in Vascepa-HFD compared with control-HFD mice (Figures 3B–3E). As well, no change was observed in the levels of hepatic injury markers (alanine aminotransferase (ALT) and aspartate aminotransferase (AST)), TG, cholesterol, high-density lipoprotein (HDL), low-density lipoprotein (LDL), or very low-density lipoprotein (VLDL) in the serum (Figures 3F and 3G).
Figure 3.
Vascepa reduces liver TG levels with no changes in serum lipid markers
(A–G) (A) Liver TG (n = 17,18,19) (B) liver histology using H&E staining (Scale bar 100μm) (C) average lipid droplet area (%) and (D) average size using ORO staining (E) lipid droplet size distribution using H&E staining analysis (n = 4/group) (F) hepatic injury markers (AST and ALT) levels (G) serum lipid levels (TG, cholesterol, HDL, LDL, VLDL) (n = 12,14,15). AST, aspartate aminotransferase; ALT, alanine aminotransferase; TG, triglyceride; HDL, high-density lipoprotein; LDL, low-density lipoprotein; VLDL, very low-density lipoprotein. ∗p < 0.05, ∗∗p < 0.01 vs con-HFD. All error bars ±SEM.
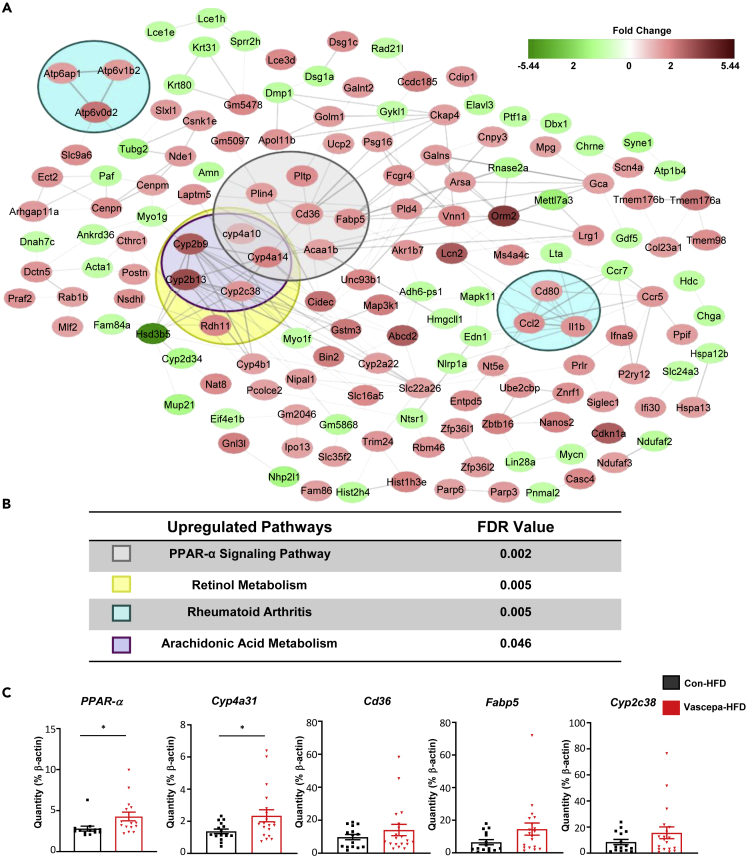
To gain an understanding of the potential mechanism through which Vascepa could reduce liver TG and change liver morphology, microarray analysis was performed (filter criteria of p < 0.05 and a fold change of 1.5). From a possible 34,472 genes that could be examined, 524 passed the filter criteria (1.52%) in which 238 genes (45.42%) were upregulated and 286 (54.58%) were downregulated. Using string network analysis to determine pathways that were differentially regulated, we found that PPAR-α signaling was the most differentially expressed pathway, followed by retinol metabolism, rheumatoid arthritis, and arachidonic acid (AA) metabolism (Figures 4A and 4B). qPCR analysis confirmed that PPAR-α expression is significantly increased in the Vascepa-HFD group compared with control-HFD along with significant upregulation in Cyp4a31, a fatty acid oxidation gene (Figure 4C). This indicates that Vascepa treatment increases expression of PPAR-α and CYP4a31 likely leading to increased fatty oxidation which reduces liver TG and improves insulin resistance.
Figure 4.
Vascepa upregulates hepatic genes involved in PPAR-α signaling pathway
(A) Hepatic upregulated (red) or downregulated genes (green) in Vascepa-HFD group compared with control-HFD.
(B) Differentially regulated pathways in Vascepa-HFD group compared with control-HFD (n = 3/group). Color boxes represent genes involved in each pathway in Figure 4A. (C) Differentially regulated gene expression levels using qPCR (n = 13–15/group). ∗p < 0.05 vs con-HFD. All error bars ±SEM.
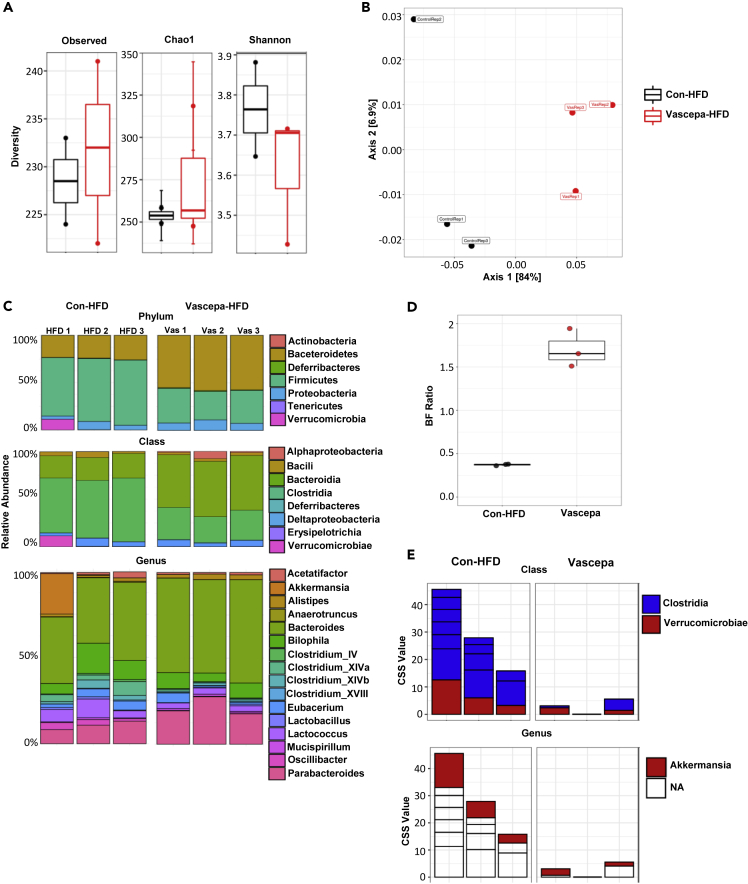
Vascepa alters fecal microbiota composition and increases the Bacteroides to Firmicutes ratio
Since the omega-3 ethyl esters were delivered orally and changes in the composition of the microbiome can impact weight gain and liver function (Machado and Cortez-Pinto, 2016), we next wanted to determine whether Vascepa treatment could affect microbiota composition. 16s rRNA sequencing was performed on fecal samples obtained after 7 days of treatment or after 6 weeks of HFD feeding. For all analysis, Operational Tax Units (OTUs), clusters which represent bacterial species, were filtered and <25% of prevalent and abundant OTUs were removed. From a total of 1033 OTUs, 687 OTUs were identified. There were no differences in the microbiota composition between Vascepa and control after 7 days of treatment in alpha diversity indicating that number of species is not different. Beta diversity which demonstrates the diversity in microbial community was also not significantly different after 7 days of treatment (Figure S6). Therefore, Vascepa treatment did not have any immediate effects on microbiota composition with the chow diet.
Although treatment with Vascepa for 7 days followed by HFD feeding for 6 weeks resulted in no change in alpha diversity (Figure 5A), it showed significant clustering in beta diversity determined by weighted UniFrac PCoA plot (Figure 5B). Looking at the Phylum level, Vascepa treatment was associated with higher levels of Bacteroides and lower levels of Firmicutes compared with control-HFD (Figures 5C and 5D). At the Class level, Vascepa treatment had lower levels of Clostridia with an increase in Bacteroidia compared to control-HFD and an increase in Bacteroides at the Genus level (Figure 5C). To look more specifically at species that were significantly changed, MetagenomeSeq revealed a significant reduction in 5 species of Clostridia and 1 species of Verrucomicrobiae (Akkermansia) in Vascepa treatment compared with control-HFD (Figure 5E). This trend was confirmed using linear discriminant analysis effect size (LEfSe) analysis. Therefore, Vascepa treatment altered the microbiota composition and increased the ratio of Bacteroidetes to Firmicutes after HFD feeding with reductions in Clostridia and Verrucomicrobiae.
Figure 5.
Vascepa treatment protects from HFD-induced changes on microbiota composition
(A–E) (A) Alpha diversity indicating species richness and evenness (B) beta diversity showing species diversity (C) relative abundance based on phylum, class, and genus (D) Bacteroides to Firmicutes (BF) ratio (E) metagenomseq analysis showing cumulative sum scaling (CSS) abundances of species (n = 3/group). See also Figure S6.
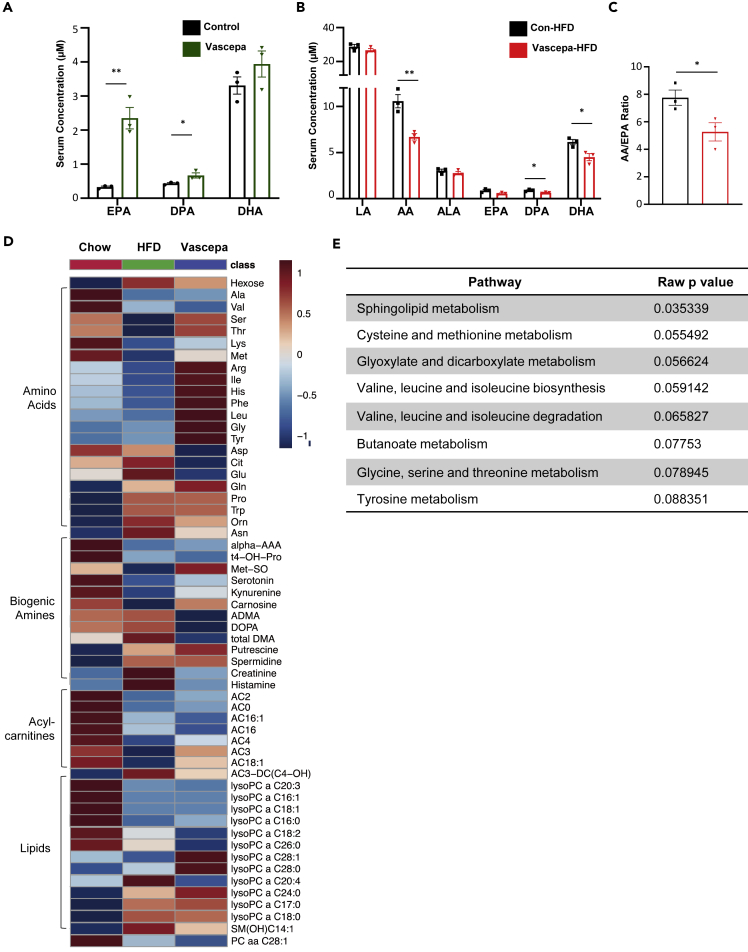
Vascepa alters serum metabolites 6 weeks after HFD feeding and reduces AA/EPA ratio
Since we have shown that omega-3 fatty acid levels are elevated in patients with T2D in Figure S1, we next determined whether Vascepa can alter the omega-3 and omega-6 fatty acid levels and circulating metabolites in mice. We first confirmed that Vascepa administration in mice for 7 days increased serum EPA and the intermediate metabolite, DPA (Figure 6A). In comparison, after 6 weeks of HFD feeding, circulating AA, DPA, and DHA metabolites were reduced in Vascepa-treated group (Figure 6B). In particular, the AA/EPA ratio, an indicator of inflammation, decreased in the Vascepa-treated group compared with control-HFD (Figure 6C). To determine whether other metabolites are changed in the Vascepa-treated group after 6 weeks of HFD feeding, high-throughput metabolomics was performed. Hexose was found to be decreased in the Vascepa-treated group. In addition, we showed a consistent trend of elevated amino acids in the Vascepa-treated group compared with HFD, such as Arg, Ile, His, Phe, Leu, Gly, and Tyr (Figure 6D). Although we did not see significant changes in specific phospholipids or sphingolipids, we found that the pathway of sphingolipid metabolism is significantly altered (Figure 6E). No significant changes in specific metabolites were observed in biogenic amines or acyl carnitines. Therefore, even though Vascepa treatment increased plasma EPA and DPA immediately after oral intake, the levels of omega-3 fatty acid and the AA/EPA ratio decreased after 6 weeks of HFD feeding. The pathways of sphingolipid and branched chain amino acid (BCAA) metabolism were affected.
Figure 6.
Vascepa treatment alters serum metabolites and reduces AA/EPA ratio
(A) Quantification of EPA, DPA, and DHA levels in Vascepa-treated mice after 7 days of gavage by GC-MS.
(B) Quantification of omega-6 and omega-3 fatty acids after 6 weeks of HFD feeding.
(C) AA/EPA ratio in HFD treated group (n = 3/group).
(D and E) (D) Identification of serum metabolites after 6 weeks of HFD feeding and (E) pathway analysis (n = 10/group).
DPA, docosapentaenoic acid; LA, linoleic acid; AA, arachidonic acid; ALA, alpha-linolenic acid. ∗p < 0.05, ∗∗p < 0.01. All error bars ±SEM.
Discussion
In the present study, we sought to determine the effects of omega-3 fatty acids on glucose intolerance and pancreatic beta cell function in mice. We have utilized highly pure omega-3 prescription drugs, Vascepa and Lovaza, and reported the protective effects of Vascepa on diet-induced glucose intolerance and insulin resistance. Interestingly, using a small observational human cohort, we have shown that circulating EPA and DPA are increased in subjects with T2D, as well as in previous studies in women with gestational diabetes (Prentice et al., 2014).
It is unclear whether the increase in omega-3 fatty acids contributes to or is a consequence of T2D. Although several studies have reported no effect of omega-3 fatty acids on glucose homeostasis and T2D (Montori et al., 2000; O'Mahoney et al., 2018; Wu et al., 2012; Zhou et al., 2012), a dose response meta-analysis of prospective observational studies revealed a significant decrease of T2D incidence in Asian populations and a significant increase in T2D in US populations associated with omega-3 fatty acids with no significant change in linear dose-response meta-analyses (Neuenschwander et al., 2020). However, a recent large population-based prospective study of 392,287 individuals showed that oily fish and FO supplements are associated with a lower risk of T2D (Chen et al., 2021). Another systematic review and meta-analysis report showed that replacing carbohydrates or short chain fatty acids (SCFAs) with polyunsaturated fatty acids had more favorable effects on glycemia and insulin resistance (Imamura et al., 2016). The genetic heterogeneity in the human population, the medications used to treat diabetes or the compensatory mechanisms occurring in response to dysmetabolism are all factors that could affect the results reported in human studies and how they correlate to mouse studies. Therefore, further studies are required to address whether such changes could influence the results reported.
Vascepa and to a much lesser extent Lovaza were shown to improve underlying insulin resistance in HFD-treated mice (Figure 1L). It is well known that insulin resistance is associated with hepatic TG content and a fatty liver (Perry et al., 2014). The reduction in liver TG and reduced lipid droplet size are likely in part due to upregulation of the PPAR-α signaling triggering lipid oxidation. This is in part supported by increased expression of the fatty acid oxidation genes, PPAR-α and Cyp4a31 (Figure 4). In addition, insulin resistance is exacerbated by hepatic inflammation (Chen et al., 2017) and EPA, a key anti-inflammatory molecule, can compete with AA, a precursor to pro-inflammatory mediators, to form less inflammatory mediators/metabolites. Therefore, the AA/EPA ratio is frequently used as a marker of chronic inflammation and it is decreased by EPA intake (Nelson and Raskin, 2019). The CYP enzymes metabolize polyunsaturated fatty acids including AA, EPA, and DHA (Arnold et al., 2010). The increased CYP expression we observed in the liver could be responsible for the decreased circulating polyunsaturated fatty acids. In our study, the AA metabolism pathway was upregulated in the liver with decreased circulating levels of AA and AA/EPA ratio suggesting reduced inflammation (Figures 6B and 6C). Together, increased lipid oxidation and reduced inflammation associated with exposure to Vascepa would explain the positive effects to reduce HFD-induced insulin resistance.
It is still unknown why the positive effects of EPA persist weeks after Vascepa treatment and remains to be determined. Omega-3 fatty acids can change the composition of membrane phospholipids through displacing AA and increasing the membrane composition of EPA and DHA. Thus, they could change the production of lipid mediators, important small molecules involved in inflammatory processes. Altered fatty acid composition in cell membranes could also have multiple effects on improving lipid raft formation, membrane fluidity, and cell membrane environment important for cell signaling and protein function. The different outcomes generated from Vascepa (EPA) compared with Lovaza (EPA + DHA) could be due to the different mechanisms of action of EPA and DHA. EPA inhibits the delta-5-desaturase enzyme that produces AA which generates pro-inflammatory eicosanoids. EPA can also compete with AA to bind to phospholipase A2 enzyme which releases AA from phospholipids. Therefore, EPA can decrease AA production which reduces pro-inflammatory eicosanoids and decreases cellular inflammation. Since inflammation is closely related with insulin resistance, EPA's effects could influence insulin resistance. In contrast, DHA is an inhibitor of the delta-6-desaturase which produces gamma-linolenic acid. Gamma-linolenic acid reduction leads to reduced dihomo gamma linolenic acid which is important for generating anti-inflammatory eicosanoids. EPA and DHA have distinct effects on gene transcription in insulin sensitive tissues. In the liver, EPA moderately enhanced lipid oxidation and reduced liver lipid overload, whereas DHA reduced expression of genes involved in liver stiffness and fibrosis. In skeletal muscle, EPA upregulated cell cycle genes compared with DHA which upregulated apoptosis and cellular stress response-related genes (Kunz et al., 2019). In addition, a recent paper has also shown that EPA and DHA have distinct effects in response to LPS in ex vivo monocytes, whereas EPA is useful in balancing inflammation profiles against cytokine IL-10, DHA inhibited pro-inflammatory cytokines (So et al., 2021). Therefore, even though DHA has many reported beneficial effects and similar actions to EPA, in the context of inflammation, EPA alone may serve as a better potent omega-3 fatty acid than when it is combined with DHA.
With the significantly improved insulin resistance in liver, one would expect reduced demand on the beta cell to maintain glucose homeostasis. Indeed, we showed that Vascepa supplementation improved glucose homeostasis, reduced fasting glucose and insulin in vivo, and reduced insulin secretion under low glucose conditions in isolated islets ex vivo (Figures 1G–1K and 2A). Importantly, we did not observe any effects of EPA or DHA treatment on GSIS directly in vitro. We also observed a greater frequency of small islets in Vascepa-HFD mice compared with control-HFD (Figure 2D). Even though there is no direct evidence that determines the impact of insulin resistance on islet morphology, we believe that reduced insulin resistance alleviates stress on the beta cell and reduces insulin demand. It was previously shown that small islets actually secrete insulin more efficiently than large islets which would likely improve glucose homeostasis (Farhat et al., 2013; Lehmann et al., 2007).
Altered gut microbiota was previously linked to obesity and diabetes; changes in the microbiome lead to changes in SCFAs, affecting insulin resistance, lipogenesis, and liver function (Saad et al., 2016). A recent study has determined the beneficial effects of gut microbiome on euglycemia through the regulation of hepatic gluconeogenesis (Krisko et al., 2020). Therefore, after observing changes in weight gain and insulin resistance in the Vascepa-treated mice, we further explored the microbiota composition. Here, we show data consistent with previous publications in which there is a higher Firmicutes to Bacteroidetes ratio in the HFD-treated group (Gurung et al., 2020). However, there have been discrepancies in the Firmicutes to Bacteroidetes ratio in several studies (Magne et al., 2020). For example, an increase in Bacteroides was revealed in T1D patients (Jamshidi et al., 2019). In our study, Vascepa had higher Bacteroidetes to Firmicutes ratio compared with control-HFD. Administration of Bacteroides in diabetic mice improved glucose tolerance and insulin resistance which indicates the beneficial role of Bacteroides in glucose homeostasis and supports our findings (Gauffin Cano et al., 2012; Yang et al., 2017). It has also been reported to be reduced in patients with T2D (Zhang et al., 2013). Even though the mechanism through which gut microbiota can influence obesity is not completely understood, one mechanism through which Bacteroidetes could influence T2D is through modulating inflammation and suppressing TNF-α (Gurung et al., 2020). It has been previously suggested that an increase in Firmicutes leads to higher calorie absorption and weight gain (Kallus and Brandt, 2012). It is important to note, however, that Firmicutes are butyrate producing bacteria that are important for maintaining energy homeostasis and insulin sensitivity. Butyrate is a major source of energy for enterocytes, and it has been associated with reduced intestinal leakage, reduced intestinal inflammation and regulation of tight junctions (Canani et al., 2011). It has also been reported to attenuate fat gain in db/db mice and improve insulin sensitivity and energy expenditure (Gao et al., 2009; Oh et al., 2019). Similar to our results, it was reported that mice fed an omega-3 fatty acid-enriched diet lowered Firmicutes (Yu et al., 2014) and reduced the abundance of Clostridia species compared with an omega-6 fatty acid-enriched diet (Ghosh et al., 2013). Another study reported a decrease of Bacteroidetes in short fatty acid-rich diet compared with an omega-3 fatty acid-enriched diet (Liu et al., 2012). Although omega-3 fatty acids are beneficial in reversing the microbiota composition and restoring the Firmicutes/Bacteroidetes ratio in states of dysbiosis, the gut microbiome changes are inconclusive and remain poorly understood (Costantini et al., 2017). In our study, we reported no differences in food intake, oxygen consumption, and respiratory exchange ratio between control-HFD and Vascepa-HFD. We have also not observed a clear metabolic distinction between light and dark cycles in the respiratory exchange ratio. Since we have conducted the comprehensive lab animal monitoring system (CLAMS) experiment after 6 weeks of HFD for only 2 days, we cannot conclude that the food intake and oxygen consumption do not have an effect on the results seen. Even though the mechanisms of how microbiome alterations affect diabetes are not clear, the changes in microbiota composition may lead to changes in energy absorption and SCFAs which could overall impact liver function, induce PPAR-α signaling pathway and lead to improvements in insulin resistance and enhancement in insulin secretion.
To summarize, we find that Vascepa administration could improve HFD-induced glucose intolerance. This effect could be due to the upregulation of fatty acid oxidation leading to a reduced hepatic TG, changed metabolism of BCAA and sphingolipids and mitigated inflammation. Importantly, Vascepa did not have negative impact on pancreatic beta cell. On the other hand, due to the reduced insulin demand, a consequence of improved insulin resistance, the beta cell was preserved and, in some degree, improved. Overall, our study provides novel insight into the effects of omega-3 prescription drugs on glucose homeostasis and beta cell function in mice. Yet, more studies need to be done on omega-3 fatty acids in humans with proper measures of glucose homeostasis.
Limitations of the study
In this study, we have only utilized males in our human study and male mice. However, we acknowledge that sex-based differences can contribute to the effects reported. In the human study, we have used a small observational cohort with a significant difference in the age between individuals with T2D compared with controls which could have confounding effects on our findings and reduce our ability to draw firm conclusions. The lack of dietary intake in the human study makes it difficult to conclude the cause of the elevation in circulating omega-3 fatty acids. In addition, patients with T2D have comorbidities and are on medications such as Metformin; therefore the differences in the lipid profiles could in part be due to medications or pre-existing conditions. The mice selected for microbiome analysis were housed together which could be a confounding effect to the results observed.
STAR★Methods
Key resources table
| REAGENT or RESOURCE | SOURCE | IDENTIFIER |
|---|---|---|
| Biological samples | ||
| Human Plasma | Shanghai 6th People's Hospital in China | N/A |
| Chemicals, peptides, and recombinant proteins | ||
| Vascepa | Amarin Pharma | N/A |
| Lovaza | GlaxoSmithKline | N/A |
| EPA ethyl ester | Cayman | Cat#86227-47-6 |
| DHA ethyl ester | Cayman | Cat#81926-94-5 |
| Chow Diet | Research Diets Inc | D12450J |
| High Fat Diet | Research Diets Inc | D12492 |
| Deposited data | ||
| Microarray Data | This paper | GEO: GSE171107 |
| 16s rRNA sequencing Microbiome Data | This paper | BioProject: PRJNA720966 |
| Experimental models: cell lines | ||
| Mouse: MIN6 K8 | Prof. S. Seino (Kobe University, Japan) and Prof. J. Miyazaki (Osaka University, Japan) | N/A |
| Experimental models: organisms/strains | ||
| Mouse: C57BL/6 | Charles River Laboratories | 027 |
| Oligonucleotides | ||
| Primers for qPCR, see Table S1 | This paper | N/A |
| Software and algorithms | ||
| CalR | (Mina et al., 2018) | https://calrapp.org/ |
| Aperio ImageScope | Leica Biosystems | https://www.leicabiosystems.com/digital-pathology/manage/aperio-imagescope/ |
| NDP.view | Hamamatsu Photonics K.K. | https://www.hamamatsu.com/us/en/product/type/U12388-01/index.html |
| ImageJ | (Schneider et al., 2012) | https://imagej.nih.gov/ij/ |
| Transcriptome analysis console | Thermofisher Scientific | https://www.thermofisher.com/ca/en/home/global/forms/life-science/download-tac-software.html |
| Cytoscape | (Shannon et al., 2003) | https://cytoscape.org/ |
| MetaboAnalyst 4.0 | (Xia et al., 2009) | https://www.metaboanalyst.ca/ |
Resource availability
Lead contact
Further information and requests for resources and reagents should be directed to and will be fulfilled by the lead contact, Michael B. Wheeler (michael.wheeler@utoronto.ca).
Materials availability
This study did not generate new unique reagents.
Data and code availability
-
•
The microarray data generated during this study are available at GEO database under accession number GSE171107. The 16s rRNA sequencing data are available at SRA database under BioProject accession number PRJNA720966.
-
•
This paper does not report original code.
-
•
Any additional information required to reanalyze the data reported in this paper is available from the lead contact upon request.
Experimental model and subject details
Human study design
Plasma samples were obtained from male normal glucose tolerant (NGT) control and individuals with T2D after overnight fasting at the Sixth People's Hospital in Shanghai, China and stored at −80°C. The human study was approved by the Ethics Committee of Shanghai Sixth People's Hospital East Campus. Patients who do not have previous serious medical conditions (i.e., autoimmune disease such as Crohn's, Lupus, Type 1 diabetes, or other) were enrolled in this study. Each participant underwent 2-hr 75-g oral glucose tolerance test (OGTT), and they were classified by glucose tolerance as follows: diabetes based on the American Diabetes Association diagnostic criteria for the 75-g OGTT (fasting ≥ 126 mg/dL and/or 2 hr ≥ 200 mg/dL). Written informed consent was received from participants prior to inclusion in the study. All the participants enrolled in this study were included in the analysis. We have only included male individuals, however we acknowledge that sex and gender could have an influence on the results reported.
Animal study design
Male C57BL/6 mice (8 weeks old) were obtained (Charles River Laboratories, USA) and acclimatized for one week. Mice were housed in temperature controlled 12-hr light/dark cycle in groups of four per cage and had access to a standard chow diet and water ad libitum. No randomization or blinding procedures were carried out. The University of Toronto animal care committee approved all experiments and methods, and the Canadian Council of Animal Care guidelines and standard were followed. The doses of Vascepa (Amarin Pharma, Inc. Bedminster, NJ, USA) or Lovaza (GlaxoSmithKline, Research Triangle Park, NC, USA) were chosen and calculated based on clinical trial regimens administering these doses in humans (1-4g/day). The mouse dosage was calculated using surface area conversion. Mice were treated with either Vascepa (190.65mg/kg/day (∼2ul)) or Lovaza (410mg/kg/day (∼4ul)) for 7 days through oral gavage. 50 μL of olive oil (O1514, Sigma Aldrich) was used for each of the control-chow, control-HFD, Vascepa or Lovaza treated group. At the end of the treatment period, mice were fed either a 60 kcal % fat HFD (D12492; Research Diets Inc., USA) or 10 kcal% fat chow diet (D12450J, Research Diets Inc., USA) for 6 weeks. Only male mice were utilized, however the effect of sex hormones on glucose metabolism and insulin secretion could influence the results reported.
Cell lines
MIN6K8 cells were incubated at 37°C in 5% CO2 in Dulbecco's Modified Eagles Medium 57 (D-MEM) media (D5796, Sigma Aldrich) with 1.75uL beta-Mercaptoethanol, 1% penicillin/streptomycin (Pen/Strep) and 10% Fetal Bovine Serum (FBS).
Method details
Oral glucose tolerance test and intraperitoneal insulin tolerance test
An OGTT and an intraperitoneal insulin tolerance test (IPITT) were performed as described previously (Liu et al., 2016; Mohan et al., 2019; Prentice et al., 2014). Following a 15 hr overnight fast, an OGTT was performed in which mice were orally gavaged with glucose (Cat# 50-99-7, Sigma Aldrich) at 2g/kg and glucose levels were measured for 2 hr at time points 0, 10, 20, 30, 60, 90 and 120 min using a glucometer (Bayer Contour NEXT ONE Glucose meter, Ascensia Diabetes Care, Canada). 20μL of blood were collected using EDTA coated blood collection tubes (Microvette CB 300 K2E, STARSTEDT, Germany) at time points 0, 10, 20 and 30 min. Insulin levels were measured from serum samples using mouse ultrasensitive insulin ELISA kit (80-INSMSU-E01, ALPCO, USA). Following a 5 hr fast, an IPITT was performed in which mice were injected with either 1IU/kg insulin (Humulin R U-500, Eli Lilly, Indianapolis, IN, USA) for HFD-fed mice or 0.5IU/kg for Chow-fed mice. Blood glucose was measured for 2 hr at time points 0, 15, 30, 60, 90 and 120 min. HOMA-IR was calculated using the equation (Matthews et al., 1985).
Comprehensive lab animal monitoring system
Mice were housed in CLAMS chambers (Oxymax, Columbus Instruments, USA) as described previously (Mohan et al., 2019; Prentice et al., 2018). Briefly, mice were placed individually in metabolic chambers and monitored for food intake, water intake, activity, oxygen consumption, and carbon dioxide production in a room with standard 12-hr light-dark cycle. The mice were continued on the same diet provided before they were placed in the chambers. Data from the first 24 hr in which the mice were acclimatized was removed and the average was calculated from a total of 48 hr. CalR (https://calrapp.org/) software was used for calorimetry analysis (Mina et al., 2018).
Islet isolation
Mouse islets were isolated from C57BL/6 male mice as described previously (Batchuluun et al., 2018; Liu et al., 2016; Luu et al., 2013; Mohan et al., 2019; Prentice et al., 2014). Pancreas were perfused via the common bile duct with 0.6mg/mL collagenase, the pancreas were extracted, submerged in a tube containing collagenase and incubated at 37°C for 10 min. Digestion is stopped using RPMI-1640 media (R8758, Sigma Aldrich) with 10% FBS. Islets were picked three times in media (RPMI-1640, Sigma Aldrich with 10% FBS, 1% PenStrep, 1% L-glutamine), incubated at 37°C overnight and treated the following day.
In vitro preparation and treatment
Lovaza, Vascepa, EPA ethyl ester (Cat# 86,227-47-6, Cayman) or DHA ethyl ester (Cat# 81,926-94-5, Cayman) were conjugated with free fatty acid BSA (Cat# A8806, Sigma Aldrich) at a ratio of 3:1 for 4 hr in RPMI-1640 media at 37°C. Islets isolated from male C57BL/6 mice (aged 8–12 weeks) or MIN6K8 cells were treated with Lovaza, Vascepa, EPA ethyl ester (100μM of EPA) or DHA ethyl ester (70μM of DHA) for 24 hr or 48 hr.
Glucose stimulated insulin secretion assay
GSIS was performed as previously described (Batchuluun et al., 2018; Liu et al., 2016; Mohan et al., 2019; Prentice et al., 2014). Briefly, islets or MIN6K8 cells were incubated at Low Glucose (LG) (2.8mM) for 1 hr. Islets or MIN6K8 cells were incubated for 20 min in each of LG Krebs-Ringer Bicarbonate buffer (KRB) (2.8mM), High Glucose (HG) KRB (16.7mM) and HG KRB (16.7mM) with KCl (30mM) successively. The supernatant was collected, islets were placed in acid ethanol and stored at 4°C for 24 hr. To measure DNA concentration and total insulin content, islets were speed vacuumed to remove the acid ethanol and resuspended in 30μL ultrapure water. For MIN6K8 cells, the cells were frozen in −80°C and thawed at room temperature three consecutive times to measure DNA concentration and total insulin content. To read insulin measurements, homogeneous time resolved fluorescence (HTRF) assay (Insulin Ultrasensitive kit, Cat# 62IN2PEH, Cisbio, Bedford, MA) was used and the plate was read on a BMG PheraStar plate reader (BMG Labtech, Cary, NC).
Tissue histology and analysis
Histological analysis of pancreas and liver were performed as described previously (Mohan et al., 2019; Prentice et al., 2018). Briefly, pancreas and liver samples were fixed in 4% paraformaldehyde and embedded in paraffin. Thick sections (4μm) were used for H&E staining. A liver section was embedded in Optimal Cutting Temperature (OCT) compound and flash frozen for staining with Oil Red O. Samples were sent to Toronto Center for Phenogenomics (Mount Sinai Hospital, Toronto, Canada) for processing. Pancreatic and liver images were analyzed and quantified using ImageJ (Schneider et al., 2012), Aperio ImageScope and NDP.view software.
Liver and serum lipid analytes
TG levels in liver and serum samples were measured as described previously (Mohan et al., 2019; Prentice et al., 2018). Serum and liver samples were obtained from mice sacrificed after overnight fasting and stored at −80°C. Frozen mouse liver samples were flash frozen using liquid nitrogen, immediately ground and then homogenized in 5% NP-40. TG levels were measured from the liver and serum samples using BioVision Incorporated Triglyceride Quantification Colorimetric/Fluorometric Kit (K622-100, BioVision, USA) as per the manufacturer's instructions. PHERAstar FSX microplate reader was used to measure the absorbance of the plate at 570nm. Piccolo Lipid Panel Reagent disc (Cat# A400-0030, Piccolo Lipid Panel Plus) was loaded into the Piccolo blood chemistry analyzer (Piccolo Xpress, Abaxis, USA) to obtain the following data: cholesterol, TG, LDL, HDL, VLDL, AST and ALT levels.
Microarray and quantitative PCR
RNA extraction and microarray analysis were performed on ground frozen liver samples as previously described (Mohan et al., 2019). RNA was extracted using RNeasy Mini Plus kit (Qiagen, Hilden, Germany). The microarray was performed at The Center for Applied Genomics (The Hospital for Sick Children, Toronto, ON, Canada) using Mouse Gene 2.0 ST Array Format. 100ng of total RNA was used in a Affymetrix WT Plus assay kit and 5.5 μg cDNA was used for Mouse Gene 2.0 ST Array. Biotin Allonamid Triphosphate was used for labeling, GeneChips were washed and stained using Affymetrix Fluidics Station 450 and hybridization was performed for 16hr at 45°C. GeneChips were scanned using the Affymetrix GeneChip Scanner 3000. Transcriptome analysis console and Cytoscape (Shannon et al., 2003) were used for analysis of microarray data.
qPCR were performed as described previously (Gyulkhandanyan et al., 2006; Hardy et al., 2009) for PPAR-α, Cyp4a31, Cd36, Fabp5 and Cyp2c38 genes. RNA was extracted from frozen liver samples using RNeasy Mini Plus kit (Qiagen, Hilden, Germany). Reverse transcription was performed using total RNA, dNTP cocktail (Cat# 10,297-018, Invitrogen), Oligo dT (Cat# 18418012, Invitrogen) and M-MLV reverse transcriptase (Cat# M1302-40KU, Sigma Aldrich) as per the manufacturer's instructions. qPCR was performed using SYBR green (Cat# 4385612, ThermoFisher Scientific). 30 ng of cDNA (4μL/well) was added to a qPCR mixture (6μL/well) of SYBR green and primers for amplification. Primers were designed using Primer BLAST software (NCBI, Bethesda, ML, USA) and PrimerQuest (IDTDNA, USA). Primer sequences are listed in Table S1. A standard curve was created from Mouse XpressRef Universal Total RNA (Cat# 338114, Qiagen) and data was normalized to mouse β-actin mRNA (Wijesekara et al., 2010). QuantStudio 7 Flex Real-Time PCR system (Cat# 4485701, ThermoFisher Scientific) was used to read the plate.
Microbiota composition and 16S rRNA gene sequencing
Fecal samples were obtained from mice treated with Vascepa at the 7-day time point and after being fed with HFD for 6 weeks. Each mouse was placed in a sterile box individually and allowed to defecate, fecal pellets were collected and stored at −80°C. DNA was extracted using NucleoSpin Soil (Cat# 740780.50, MACHEREY-NAGEL, Düren, Germany). The samples were sent to the Center for the Analysis of Genome Evolution and Function (University of Toronto, Toronto, ON, Canada) for 16S rRNA sequencing and analysis.
Mass spectrometry analysis
All mass spectrometric fatty acid analysis and metabolomics were performed in The Analytical Facility for Bioactive Molecules (The Hospital for Sick Children, Toronto, ON, Canada). Quantification of long-chain fatty acids was performed by GC-MS (Biocrates Life Sciences, Innsbruck, Austria) using the AbsoluteIDQ p180 plate covering a total of 188 metabolites as described previously (Lai et al., 2020). Briefly, the AbsoluteIDQ p180 Kit was used and the samples were prepared as per the manufacturer's specifications. Analytes with >40% of measurements below limit of detection (LOD) were excluded from the analysis and the remaining “<LOD” values were imputed using the 1/2 minimum value for each specific analyte. Pathway analysis was performed using the Kyoto Encyclopedia of Genes and Genomes (KEGG; Kanehisa Laboratories, Kyoto, Japan) Mus musculus database with Metabolite Set Enrichment Analysis (MSEA) method on platform MetaboAnalyst 4.0 (Xia et al., 2009).
Human T2D metabolome
NGT control and T2D plasma samples were obtained from individuals fasted overnight at the Sixth People's Hospital in Shanghai, China and stored at −80°C. Targeted metabolomics platform was performed by Metabolon Inc which assessed over 400 metabolites from different classes of analytes including polyunsaturated fatty acids.
Quantification and statistical analysis
Statistical significance was assessed using Student's t-test, one-way ANOVA or two-way ANOVA for repeated measures, and the data were checked for normality. Post-test comparisons were performed using Bonferroni, Dunnett and Tukey. P < 0.05 was considered significant. All data are presented as mean ±SEM.
Acknowledgments
This study was funded by a Canadian Institutes of Health Research operating grant (FDN-143219) to M.B.W. F.J.S. was supported by National Institutes of Health (R01-GM125944 and R01-DK112854). D.A.R. was supported by Banting & Best Diabetes Center (BBDC) Studentship and Ontario Graduate Scholarship. A.W. was supported by BBDC Studentship and Ontario Graduate Scholarship. Y.S. was supported by BBDC Charles Hollenberg Summer Studentship. M.L. and H.M. were supported by BBDC Postdoctoral Fellowship.
Author contributions
D.A.R. conducted experiments, acquired and analyzed data, wrote and edited the manuscript. D.A.R., Y.L., and M.B.W. designed the study. Y.L., M.L., Y.S., Z.D., A.W., H.M., L.W., F.D. researched the data. M.L., A.W., and F.D. reviewed/edited the manuscript. F.J.S. contributed to the study design and reviewed/edited the manuscript. M.B.W. contributed to the study design, interpretation of the findings, and discussion and reviewed/edited the manuscript. D.A.R. is the guarantor of this work and has full access to all the data in the study.
Declaration of interests
The authors declare that they have no competing interests.
Published: August 20, 2021
Footnotes
Supplemental information can be found online at https://doi.org/10.1016/j.isci.2021.102909.
Supplemental information
References
- American Diabetes Association Diagnosis and classification of diabetes mellitus. Diabetes Care. 2014;37:S81–S90. doi: 10.2337/dc14-S081. [DOI] [PubMed] [Google Scholar]
- Arca M., Borghi C., Pontremoli R., De Ferrari G.M., Colivicchi F., Desideri G., Temporelli P.L. Hypertriglyceridemia and omega-3 fatty acids: their often overlooked role in cardiovascular disease prevention. Nutr. Metab. Cardiovasc. Dis. 2018 doi: 10.1016/j.numecd.2017.11.001. [DOI] [PubMed] [Google Scholar]
- Arnold C., Konkel A., Fischer R., Schunck W.H. Cytochrome p450-dependent metabolism of ω-6 and ω-3 long-chain polyunsaturated fatty acids. Pharmacol. Rep. 2010 doi: 10.1016/S1734-1140(10)70311-X. [DOI] [PubMed] [Google Scholar]
- Ballantyne C.M., Bays H.E., Kastelein J.J., Stein E., Isaacsohn J.L., Braeckman R.A., Soni P.N. Efficacy and safety of eicosapentaenoic acid ethyl ester (AMR101) therapy in statin-treated patients with persistent high triglycerides (from the ANCHOR study) Am. J. Cardiol. 2012;110:984–992. doi: 10.1016/j.amjcard.2012.05.031. [DOI] [PubMed] [Google Scholar]
- Basu S., Yudkin J.S., Kehlenbrink S., Davies J.I., Wild S.H., Lipska K.J., Sussman J.B., Beran D. Estimation of global insulin use for type 2 diabetes, 2018–30: a microsimulation analysis. Lancet Diabetes Endocrinol. 2019;7:25–33. doi: 10.1016/S2213-8587(18)30303-6. [DOI] [PubMed] [Google Scholar]
- Batchuluun B., Al Rijjal D., Prentice K.J., Eversley J.A., Burdett E., Mohan H., Bhattacharjee A., Gunderson E.P., Liu Y., Wheeler M.B. Elevated medium-chain acylcarnitines are associated with gestational diabetes mellitus and early progression to type 2 diabetes and induce pancreatic β-cell dysfunction. Diabetes. 2018;67:885–897. doi: 10.2337/db17-1150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhatt D.L., Miller M., Brinton E.A., Jacobson T.A., Steg P.G., Ketchum S.B., Doyle R.T., Juliano R.A., Jiao L., Granowitz C. REDUCE-IT USA: results from the 3146 patients randomized in the United States. Circulation. 2020;141:367–375. doi: 10.1161/CIRCULATIONAHA.119.044440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhatt D.L., Steg P.G., Miller M., Brinton E.A., Jacobson T.A., Ketchum S.B., Doyle R.T., Juliano R.A., Jiao L., Granowitz C. Cardiovascular risk reduction with icosapent ethyl for hypertriglyceridemia. N. Engl. J. Med. 2019;380:11–22. doi: 10.1056/NEJMoa1812792. [DOI] [PubMed] [Google Scholar]
- Bosch J. n–3 fatty acids and cardiovascular outcomes in patients with dysglycemia. N. Engl. J. Med. 2012;367:309–318. doi: 10.1056/nejmoa1203859. [DOI] [PubMed] [Google Scholar]
- Bowman L., Mafham M., Stevens W., Haynes R., Aung T., Chen F., Buck G., Collins R., Armitage J. ASCEND: a Study of Cardiovascular Events iN Diabetes: characteristics of a randomized trial of aspirin and of omega-3 fatty acid supplementation in 15,480 people with diabetes. Am. Heart J. 2018;198:135–144. doi: 10.1016/j.ahj.2017.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradberry J.C., Hilleman D.E. Overview of omega-3 fatty acid therapies. P T. 2013;38:681–691. [PMC free article] [PubMed] [Google Scholar]
- Brown T.J., Brainard J., Song F., Wang X., Abdelhamid A., Hooper L. Omega-3, omega-6, and total dietary polyunsaturated fat for prevention and treatment of type 2 diabetes mellitus: systematic review and meta-analysis of randomised controlled trials. BMJ. 2019;366 doi: 10.1136/bmj.l4697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Canani R.B., Costanzo M.D., Leone L., Pedata M., Meli R., Calignano A. Potential beneficial effects of butyrate in intestinal and extraintestinal diseases. World J. Gastroenterol. 2011;17:1519–1528. doi: 10.3748/wjg.v17.i12.1519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen C., Yu X., Shao S. effects of omega-3 fatty acid supplementation on glucose control and lipid levels in type 2 diabetes: a meta-analysis. PLoS One. 2015;10:e0139565. doi: 10.1371/journal.pone.0139565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen G.-C., Arthur R., Qin L.-Q., Chen L.-H., Mei Z., Zheng Y., Li Y., Wang T., Rohan T.E., Qi Q. Association of oily and nonoily fish consumption and fish oil supplements with incident type 2 diabetes: a large population-based prospective study. Diabetes Care. 2021 doi: 10.2337/dc20-2328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Z., Yu R., Xiong Y., Du F., Zhu S. A vicious circle between insulin resistance and inflammation in nonalcoholic fatty liver disease. Lipids Health Dis. 2017 doi: 10.1186/s12944-017-0572-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costantini L., Molinari R., Farinon B., Merendino N. Impact of omega-3 fatty acids on the gut microbiota. Int. J. Mol. Sci. 2017 doi: 10.3390/ijms18122645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davidson M.H., Stein E.A., Bays H.E., Maki K.C., Doyle R.T., Shalwitz R.A., Ballantyne C.M., Ginsberg H.N., COMBination of prescription Omega-3 with Simvastatin (COMBOS) Investigators Efficacy and tolerability of adding prescription omega-3 fatty acids 4 g/d to simvastatin 40 mg/d in hypertriglyceridemic patients: an 8-week, randomized, double-blind, placebo-controlled study. Clin. Ther. 2007;29:1354–1367. doi: 10.1016/j.clinthera.2007.07.018. [DOI] [PubMed] [Google Scholar]
- De Castro G.S., Deminice R., Simões-Ambrosio L.M.C., Calder P.C., Jordão A.A., Vannucchi H. Dietary docosahexaenoic acid and eicosapentaenoic acid influence liver triacylglycerol and insulin resistance in rats fed a high-fructose diet. Mar. Drugs. 2015;13:1864–1881. doi: 10.3390/md13041864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farhat B., Almelkar A., Ramachandran K., Williams S.J., Huang H.H., Zamierowski D., Novikova L., Stehno-Bittel L. Small human islets comprised of more β-cells with higher insulin content than large islets. Islets. 2013;5:87–94. doi: 10.4161/isl.24780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Food and Agriculture Organization of the United Nations . Food and Agriculture Organization of the United Nations; 2010. Fats and Fatty Acids in Human Nutrition : Report of an Expert Consultation; pp. 10–14. [Google Scholar]
- Gao Z., Yin J., Zhang J., Ward R.E., Martin R.J., Lefevre M., Cefalu W.T., Ye J. Butyrate improves insulin sensitivity and increases energy expenditure in mice. Diabetes. 2009;58:1509–1517. doi: 10.2337/db08-1637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gauffin Cano P., Santacruz A., Moya Á., Sanz Y. Bacteroides uniformis CECT 7771 ameliorates metabolic and immunological dysfunction in mice with high-fat-diet induced obesity. PLoS One. 2012;7:e41079. doi: 10.1371/journal.pone.0041079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghosh S., DeCoffe D., Brown K., Rajendiran E., Estaki M., Dai C., Yip A., Gibson D.L. Fish oil attenuates omega-6 polyunsaturated fatty acid-induced dysbiosis and infectious colitis but impairs LPS dephosphorylation activity causing sepsis. PLoS One. 2013;8 doi: 10.1371/journal.pone.0055468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gurung M., Li Z., You H., Rodrigues R., Jump D.B., Morgun A., Shulzhenko N. Role of gut microbiota in type 2 diabetes pathophysiology. EBioMedicine. 2020 doi: 10.1016/j.ebiom.2019.11.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gyulkhandanyan A.V., Lee S.C., Bikopoulos G., Dai F., Wheeler M.B. The Zn2+-transporting pathways in pancreatic beta-cells: a role for the L-type voltage-gated Ca2+ channel. J. Biol. Chem. 2006;281:9361–9372. doi: 10.1074/jbc.M508542200. [DOI] [PubMed] [Google Scholar]
- Hardy A.B., Fox J.E.M., Giglou P.R., Wijesekara N., Bhattacharjee A., Sultan S., Gyulkhandanyan A.V., Gaisano H.Y., MacDonald P.E., Wheeler M.B. Characterization of Erg K+ channels in alpha- and beta-cells of mouse and human islets. J. Biol. Chem. 2009;284:30441–30452. doi: 10.1074/jbc.M109.040659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imamura F., Micha R., Wu J.H.Y., de Oliveira Otto M.C., Otite F.O., Abioye A.I., Mozaffarian D. Effects of saturated fat, polyunsaturated fat, monounsaturated fat, and carbohydrate on glucose-insulin homeostasis: a systematic review and meta-analysis of randomised controlled feeding trials. PLoS Med. 2016;13 doi: 10.1371/journal.pmed.1002087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobson T.A. A new pure ω-3 eicosapentaenoic acid ethyl ester (AMR101) for the management of hypertriglyceridemia: the MARINE trial. Expert Rev. Cardiovasc. Ther. 2012;10:687–695. doi: 10.1586/erc.12.56. [DOI] [PubMed] [Google Scholar]
- Jamshidi P., Hasanzadeh S., Tahvildari A., Farsi Y., Arbabi M., Mota J.F., Sechi L.A., Nasiri M.J. Is there any association between gut microbiota and type 1 diabetes? A systematic review. Gut Pathog. 2019 doi: 10.1186/s13099-019-0332-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jump D.B. Fatty acid regulation of hepatic lipid metabolism. Curr. Opin. Clin. Nutr. Metab. Care. 2011;14:115–120. doi: 10.1097/MCO.0b013e328342991c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kallus S.J., Brandt L.J. The intestinal microbiota and obesity. J. Clin. Gastroenterol. 2012;46:16–24. doi: 10.1097/MCG.0b013e31823711fd. [DOI] [PubMed] [Google Scholar]
- Kalupahana N.S., Claycombe K., Newman S.J., Stewart T., Siriwardhana N., Matthan N., Lichtenstein A.H., Moustaid-Moussa N. Eicosapentaenoic acid prevents and reverses insulin resistance in high-fat diet-induced obese mice via modulation of adipose tissue inflammation. J. Nutr. 2010;140:1915–1922. doi: 10.3945/jn.110.125732. [DOI] [PubMed] [Google Scholar]
- Khawaja O.A., Gaziano J.M., Djoussé L. N-3 fatty acids for prevention of cardiovascular disease. Curr. Atheroscler. Rep. 2014;16:450. doi: 10.1007/s11883-014-0450-0. [DOI] [PubMed] [Google Scholar]
- Kim E.S., McCormack P.L. Icosapent ethyl: a review of its use in severe hypertriglyceridemia. Am. J. Cardiovasc. Drugs. 2014;14:471–478. doi: 10.1007/s40256-014-0099-7. [DOI] [PubMed] [Google Scholar]
- Koski R.R. Omega-3-acid ethyl esters (Lovaza) for severe hypertriglyceridemia. Pharmacol. Ther. 2008;33:271. [Google Scholar]
- Krisko T.I., Nicholls H.T., Bare C.J., Holman C.D., Putzel G.G., Jansen R.S., Sun N., Rhee K.Y., Banks A.S., Cohen D.E. Dissociation of adaptive thermogenesis from glucose homeostasis in microbiome-deficient mice. Cell Metab. 2020 doi: 10.1016/j.cmet.2020.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuda O., Jelenik T., Jilkova Z., Flachs P., Rossmeisl M., Hensler M., Kazdova L., Ogston N., Baranowski M., Gorski J. N-3 Fatty acids and rosiglitazone improve insulin sensitivity through additive stimulatory effects on muscle glycogen synthesis in mice fed a high-fat diet. Diabetologia. 2009;52:941–951. doi: 10.1007/s00125-009-1305-z. [DOI] [PubMed] [Google Scholar]
- Kunz H.E., Dasari S., Lanza I.R. EPA and DHA elicit distinct transcriptional responses to high-fat feeding in skeletal muscle and liver. Am. J. Physiol. Endocrinol. Metab. 2019;317:E460–E472. doi: 10.1152/AJPENDO.00083.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lai M., Liu Y., Ronnett G.V., Wu A., Cox B.J., Dai F.F., Röst H.L., Gunderson E.P., Wheeler M.B. Amino acid and lipid metabolism in post-gestational diabetes and progression to type 2 diabetes: a metabolic profiling study. PLoS Med. 2020;17:e1003112. doi: 10.1371/journal.pmed.1003112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lalia A.Z., Lanza I.R. Insulin-sensitizing effects of omega-3 fatty acids: lost in translation? Nutrients 8. 2016. [DOI] [PMC free article] [PubMed]
- Lehmann R., Zuellig R.A., Kugelmeier P., Baenninger P.B., Moritz W., Perren A., Clavien P.A., Weber M., Spinas G.A. Superiority of small islets in human islet transplantation. Diabetes. 2007;56:594–603. doi: 10.2337/db06-0779. [DOI] [PubMed] [Google Scholar]
- Li D. Omega-3 polyunsaturated fatty acids and non-communicable diseases: meta-analysis based systematic review. Asia Pac. J. Clin. Nutr. 2015 doi: 10.6133/apjcn.2015.24.1.21. [DOI] [PubMed] [Google Scholar]
- Liu T., Hougen H., Vollmer A.C., Hiebert S.M. Gut bacteria profiles of Mus musculus at the phylum and family levels are influenced by saturation of dietary fatty acids. Anaerobe. 2012;18:331–337. doi: 10.1016/j.anaerobe.2012.02.004. [DOI] [PubMed] [Google Scholar]
- Liu Y., Prentice K.J., Eversley J.A., Hu C., Batchuluun B., Leavey K., Hansen J.B., Wei D.W., Cox B., Dai F.F. Rapid elevation in CMPF may act as a tipping point in diabetes development. Cell Rep. 2016;14:2889–2900. doi: 10.1016/j.celrep.2016.02.079. [DOI] [PubMed] [Google Scholar]
- Luu L., Dai F.F., Prentice K.J., Huang X., Hardy A.B., Hansen J.B., Liu Y., Joseph J.W., Wheeler M.B. The loss of Sirt1 in mouse pancreatic beta cells impairs insulin secretion by disrupting glucose sensing. Diabetologia. 2013;56:2010–2020. doi: 10.1007/s00125-013-2946-5. [DOI] [PubMed] [Google Scholar]
- Machado M.V., Cortez-Pinto H. Diet, microbiota, obesity, and NAFLD: a dangerous quartet. Int. J. Mol. Sci. 2016 doi: 10.3390/ijms17040481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magne F., Gotteland M., Gauthier L., Zazueta A., Pesoa S., Navarrete P., Balamurugan R. The firmicutes/bacteroidetes ratio: a relevant marker of gut dysbiosis in obese patients? Nutrients. 2020 doi: 10.3390/nu12051474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsuura B., Kanno S., Minami H., Tsubouchi E., Iwai M., Matsui H., Horiike N., Onji M. Effects of antihyperlipidemic agents on hepatic insulin sensitivity in perfused Goto-Kakizaki rat liver. J. Gastroenterol. 2004;39:339–345. doi: 10.1007/s00535-003-1300-y. [DOI] [PubMed] [Google Scholar]
- Matthews D.R., Hosker J.P., Rudenski A.S., Naylor B.A., Treacher D.F., Turner R.C. Homeostasis model assessment: insulin resistance and β-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia. 1985;28:412–419. doi: 10.1007/BF00280883. [DOI] [PubMed] [Google Scholar]
- Mina A.I., Leclair R.A., Leclair K.B., Cohen D.E., Lantier L., Banks Correspondence A.S., Banks A.S. CalR: a web-based analysis tool for indirect calorimetry experiments cell metabolism resource CalR: a web-based analysis tool for indirect calorimetry experiments. Cell Metab. 2018;28:656–666. doi: 10.1016/j.cmet.2018.06.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohan H., Brandt S.L., Kim J.H., Wong F., Lai M., Prentice K.J., Al Rijjal D., Magomedova L., Batchuluun B., Burdett E. 3-carboxy-4-methyl-5-propyl-2-furanpropanoic acid (CMPF) prevents high fat diet-induced insulin resistance via maintenance of hepatic lipid homeostasis. Diabetes Obes. Metab. 2019;21:61–72. doi: 10.1111/dom.13483. [DOI] [PubMed] [Google Scholar]
- Molinar-Toribio E., Pérez-Jiménez J., Ramos-Romero S., Romeu M., Giralt M., Taltavull N., Muñoz-Cortes M., Jáuregui O., Méndez L., Medina I. Effect of n-3 PUFA supplementation at different EPA: DHA ratios on the spontaneously hypertensive obese rat model of the metabolic syndrome. Br. J. Nutr. 2015;113:878–887. doi: 10.1017/S0007114514004437. [DOI] [PubMed] [Google Scholar]
- Montori V.M., Farmer A., Wollan P.C., Dinneen S.F. Fish oil supplementation in type 2 diabetes: a quantitative systematic review. Diabetes Care. 2000;23:1407–1415. doi: 10.2337/DIACARE.23.9.1407. [DOI] [PubMed] [Google Scholar]
- Mostad I.L., Bjerve K.S., Bjorgaas M.R., Lydersen S., Grill V. Effects of n−3 fatty acids in subjects with type 2 diabetes: reduction of insulin sensitivity and time-dependent alteration from carbohydrate to fat oxidation. Am. J. Clin. Nutr. 2006;84:540–550. doi: 10.1093/ajcn/84.3.540. [DOI] [PubMed] [Google Scholar]
- Nelson J.R., Raskin S. The eicosapentaenoic acid:arachidonic acid ratio and its clinical utility in cardiovascular disease. Postgrad. Med. 2019 doi: 10.1080/00325481.2019.1607414. [DOI] [PubMed] [Google Scholar]
- Neuenschwander M., Barbaresko J., Pischke C.R., Iser N., Beckhaus J., Schwingshackl L., Schlesinger S. Intake of dietary fats and fatty acids and the incidence of type 2 diabetes: a systematic review and dose-response meta-analysis of prospective observational studies. PLoS Med. 2020 doi: 10.1371/journal.pmed.1003347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Mahoney L.L., Matu J., Price O.J., Birch K.M., Ajjan R.A., Farrar D., Tapp R., West D.J., Deighton K., Campbell M.D. Omega-3 polyunsaturated fatty acids favourably modulate cardiometabolic biomarkers in type 2 diabetes: a meta-analysis and meta-regression of randomized controlled trials. Cardiovasc. Diabetol. 2018 doi: 10.1186/s12933-018-0740-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oh D.Y., Talukdar S., Bae E.J., Imamura T., Morinaga H., Fan W.Q., Li P., Lu W.J., Watkins S.M., Olefsky J.M. GPR120 is an omega-3 fatty acid receptor mediating potent anti-inflammatory and insulin-sensitizing effects. Cell. 2010;142:687–698. doi: 10.1016/j.cell.2010.07.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oh T.J., Sul W.J., Oh H.N., Lee Y.K., Lim H.L., Choi S.H., Park K.S., Jang H.C. Butyrate attenuated fat gain through gut microbiota modulation in db/db mice following dapagliflozin treatment. Sci. Rep. 2019;9:1–11. doi: 10.1038/s41598-019-56684-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oikawa S., Yokoyama M., Origasa H., Matsuzaki M., Matsuzawa Y., Saito Y., Ishikawa Y., Sasaki J., Hishida H., Itakura H. Suppressive effect of EPA on the incidence of coronary events in hypercholesterolemia with impaired glucose metabolism: sub-analysis of the Japan EPA Lipid Intervention Study (JELIS) Atherosclerosis. 2009;206:535–539. doi: 10.1016/J.ATHEROSCLEROSIS.2009.03.029. [DOI] [PubMed] [Google Scholar]
- Perry R.J., Samuel V.T., Petersen K.F., Shulman G.I. The role of hepatic lipids in hepatic insulin resistance and type 2 diabetes. Nature. 2014 doi: 10.1038/nature13478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prentice K.J., Luu L., Allister E.M., Liu Y., Jun L.S., Sloop K.W., Hardy A.B., Wei L., Jia W., Fantus I.G. The furan fatty acid metabolite CMPF is elevated in diabetes and induces β cell dysfunction. Cell Metab. 2014;19:653–666. doi: 10.1016/j.cmet.2014.03.008. [DOI] [PubMed] [Google Scholar]
- Prentice K.J., Wendell S.G., Liu Y., Eversley J.A., Salvatore S.R., Mohan H., Brandt S.L., Adams A.C., Serena Wang X., Wei D. CMPF, a metabolite formed upon prescription omega-3-acid ethyl ester supplementation, prevents and reverses steatosis. EBioMedicine. 2018;27:200–213. doi: 10.1016/J.EBIOM.2017.12.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saad M.J.A., Santos A., Prada P.O. Linking gut microbiota and inflammation to obesity and insulin resistance. Physiology. 2016 doi: 10.1152/physiol.00041.2015. [DOI] [PubMed] [Google Scholar]
- Saito Y., Yokoyama M., Origasa H., Matsuzaki M., Matsuzawa Y., Ishikawa Y., Oikawa S., Sasaki J., Hishida H., Itakura H. Effects of EPA on coronary artery disease in hypercholesterolemic patients with multiple risk factors: sub-analysis of primary prevention cases from the Japan EPA Lipid Intervention Study (JELIS) Atherosclerosis. 2008;200:135–140. doi: 10.1016/J.ATHEROSCLEROSIS.2008.06.003. [DOI] [PubMed] [Google Scholar]
- Schneider C.A., Rasband W.S., Eliceiri K.W. NIH Image to ImageJ: 25 years of image analysis. Nat. Methods. 2012 doi: 10.1038/nmeth.2089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shannon P., Markiel A., Ozier O., Baliga N.S., Wang J.T., Ramage D., Amin N., Schwikowski B., Ideker T. Cytoscape: a software Environment for integrated models of biomolecular interaction networks. Genome Res. 2003;13:2498–2504. doi: 10.1101/gr.1239303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skulas-Ray A.C., Wilson P.W.F., Harris W.S., Brinton E.A., Kris-Etherton P.M., Richter C.K., Jacobson T.A., Engler M.B., Miller M., Robinson J.G. Omega-3 fatty acids for the management of hypertriglyceridemia: a science advisory from the American heart association. Circulation. 2019. [DOI] [PubMed]
- So J., Wu D., Lichtenstein A.H., Tai A.K., Matthan N.R., Maddipati K.R., Lamon-Fava S. EPA and DHA differentially modulate monocyte inflammatory response in subjects with chronic inflammation in part via plasma specialized pro-resolving lipid mediators: a randomized, double-blind, crossover study. Atherosclerosis. 2021;316:90–98. doi: 10.1016/j.atherosclerosis.2020.11.018. [DOI] [PubMed] [Google Scholar]
- Tajuddin N., Shaikh A., Hassan A. Prescription omega-3 fatty acid products: considerations for patients with diabetes mellitus. Diabetes Metab. Syndr. Obes. Targets Ther. 2016 doi: 10.2147/DMSO.S97036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaka N., Zhang X., Sugiyama E., Kono H., Horiuchi A., Nakajima T., Kanbe H., Tanaka E., Gonzalez F.J., Aoyama T. Eicosapentaenoic acid improves hepatic steatosis independent of PPARα activation through inhibition of SREBP-1 maturation in mice. Biochem. Pharmacol. 2010;80:1601–1612. doi: 10.1016/j.bcp.2010.07.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tummala R., Ghosh R.K., Jain V., Devanabanda A.R., Bandyopadhyay D., Deedwania P., Aronow W.S. Fish oil and cardiometabolic diseases: recent updates and controversies. Am. J. Med. 2019. [DOI] [PubMed]
- van den Elsen L., Garssen J., Willemsen L. Long chain N-3 polyunsaturated fatty acids in the prevention of allergic and cardiovascular disease. Curr. Pharm. Des. 2012;18:2375–2392. doi: 10.2174/138161212800165960. [DOI] [PubMed] [Google Scholar]
- Wang J.F., Zhang H.M., Li Y.Y., Xia S., Wei Y., Yang L., Wang D., Ye J.J., Li H.X., Yuan J. A combination of omega-3 and plant sterols regulate glucose and lipid metabolism in individuals with impaired glucose regulation: a randomized and controlled clinical trial. Lipids Health Dis. 2019;18 doi: 10.1186/s12944-019-1048-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wijesekara N., Krishnamurthy M., Bhattacharjee A., Suhail A., Sweeney G., Wheeler M.B. Adiponectin-induced ERK and Akt phosphorylation protects against pancreatic beta cell apoptosis and increases insulin gene expression and secretion. J. Biol. Chem. 2010;285:33623–33631. doi: 10.1074/jbc.M109.085084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- World Health Organization . World Health Organization; 2002. The World Health Report 2002 : Reducing risks, promoting healthy life. [Google Scholar]
- Wu J.H.Y., Micha R., Imamura F., Pan A., Biggs M.L., Ajaz O., Djousse L., Hu F.B., Mozaffarian D. Omega-3 fatty acids and incident type 2 diabetes: a systematic review and meta-analysis. Br. J. Nutr. 2012;107:S214–S227. doi: 10.1017/S0007114512001602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xia J., Psychogios N., Young N., Wishart D.S. MetaboAnalyst: A web server for metabolomic data analysis and interpretation. Nucleic Acids Res. 2009;37:W652–W660. doi: 10.1093/nar/gkp356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang J.Y., Lee Y.S., Kim Y., Lee S.H., Ryu S., Fukuda S., Hase K., Yang C.S., Lim H.S., Kim M.S. Gut commensal Bacteroides acidifaciens prevents obesity and improves insulin sensitivity in mice. Mucosal Immunol. 2017;10:104–116. doi: 10.1038/mi.2016.42. [DOI] [PubMed] [Google Scholar]
- Yokoyama M., Origasa H., Matsuzaki M., Matsuzawa Y., Saito Y., Ishikawa Y., Oikawa S., Sasaki J., Hishida H., Itakura H. Effects of eicosapentaenoic acid on major coronary events in hypercholesterolaemic patients (JELIS): a randomised open-label, blinded endpoint analysis. Lancet (London, England) 2007;369:1090–1098. doi: 10.1016/S0140-6736(07)60527-3. [DOI] [PubMed] [Google Scholar]
- Yu H.N., Zhu J., Pan W., Pan W.S., Shen S.R., Shan W.G., Das U.N. Effects fish oil a high content N-3 polyunsaturated fatty acids mouse gut microbiota. Arch. Med. Res. 2014;45:195–202. doi: 10.1016/j.arcmed.2014.03.008. [DOI] [PubMed] [Google Scholar]
- Zhang X., Shen D., Fang Z., Jie Z., Qiu X., Zhang C., Chen Y., Ji L. Human gut microbiota changes reveal the progression of glucose intolerance. PLoS One. 2013;8:71108. doi: 10.1371/journal.pone.0071108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou Y., Tian C., Jia C. Association of fish and n-3 fatty acid intake with the risk of type 2 diabetes: a meta-analysis of prospective studies. Br. J. Nutr. 2012 doi: 10.1017/S0007114512002036. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
-
•
The microarray data generated during this study are available at GEO database under accession number GSE171107. The 16s rRNA sequencing data are available at SRA database under BioProject accession number PRJNA720966.
-
•
This paper does not report original code.
-
•
Any additional information required to reanalyze the data reported in this paper is available from the lead contact upon request.