Abstract
Thanks to their biocompatibility, biodegradability, injectability and self-setting properties, calcium phosphate cements (CPCs) have been the most economical and effective biomaterials of choice for use as bone void fillers. They have also been extensively used as drug delivery carriers owing to their ability to provide for a steady release of various organic molecules aiding the regeneration of defective bone, including primarily antibiotics and growth factors. This review provides a systematic compilation of studies that reported on the controlled release of drugs from CPCs in the last 25 years. The chemical, compositional and microstructural characteristics of these systems through which the control of the release rates and mechanisms could be achieved have been discussed. In doing so, the effects of (i) the chemistry of the matrix, (ii) porosity, (iii) additives, (iv) drug types, (v) drug concentrations, (vi) drug loading methods and (vii) release media have been distinguished and discussed individually. Kinetic specificities of in vivo release of drugs from CPCs have been reviewed, too. Understanding the kinetic and mechanistic correlations between the CPC properties and the drug release is a prerequisite for the design of bone void fillers with drug release profiles precisely tailored to the application area and the clinical picture. The goal of this review has been to shed light on these fundamental correlations.
Keywords: Calcium phosphate cements, Drug release, Release rate, Antibiotics release, Bone fillers
Graphical abstract
Highlights
-
•
Drug release from calcium phosphate cements (CPCs) for bone healing and regeneration.
-
•
Release of antibiotics, anti-inflammatories, chemotherapeutics, growth factors, and metallic ions.
-
•
Influence of different CPC formulations, matrix porosity, structure, and crystallinity.
-
•
Influence of matrix additives and the release medium.
-
•
Influence of the drug type, concentration and loading method.
1. Introduction
Bony tissues count among the least accessible ones in the human body. When pathological conditions such as osteoporosis, osteomyelitis, osteosarcoma, fracture or trauma occur, invasive surgery is required to remove the pathological tissue [1] and/or insert the prostheses or regenerative scaffolds [2,3]. Bone regeneration represents an essential step on the way to full postoperative recovery [[4], [5], [6]]. During the last 30 years, the paradigm of Bone Tissue Engineering has come to embrace an approach consisting of the following four key factors: (1) a biocompatible scaffold that closely mimics the extracellular matrix niche of the natural bone; (2) morphogenic signals that help to direct the cells to the phenotypically desirable types; (3) recruitment of osteogenic cells to lay down the bone tissue matrix; and (4) sufficient vascularization to meet the growing tissue nutrient supply and clearance needs [7]. According to this paradigm, an ideal material for bone regeneration consists in a scaffold made of biocompatible, bioresorbable material(s) that deliver morphogenic molecules such as bone morphogenetic proteins (BMPs) [8], transforming growth factor β (TGF-β), and/or others [9,10]. Furthermore, the first stage of a postoperative or posttraumatic event implies the inflammation of the tissue, which begins within the first 12 h, and should be completed by the approximately 7th day after the injury [11]. Moreover, the possibility of the surgical site contamination by pathogenic bacteria must always be accounted for [[12], [13], [14], [15], [16]], from which arises the need for the local and sustained delivery of anti-inflammatory and antibiotic molecules by the biomaterial. Starting from this assumption, it comes natural that during the last 30 years there have been countless studies devoted to the development of suitable biomaterials able to meet the mechanical and biological requirements for in-vivo implantation and also to be able to host and successively release drugs or molecules aiding the regenerative process.
Bone is an organ with widely varied mechanical properties and metabolic rates across different regions of its tissues, for which reason different release profiles prove to be ideal for each of these regions [17]. Long bones, for one, display a functionally gradient nature, shifting from the soft marrow in the centre to the relatively porous cancellous bone around it and the harder and less porous cortical bone near and on the surface. With the clearance rate of the released drug consequently decreasing with the distance from the central axis of long bones, the need for the tuning of the release rate to the exact implant location arises as imperative [17]. The goal of this review has been to describe the way different structural and compositional features of a particular class of materials known as calcium phosphate cements (CPCs) affect drug release characteristics. It is assumed that with such correlations established, the field of drug delivery to the bone would be brought closer to a state where biomaterials could be in situ adjustable to produce an ideal release kinetics for the physiological niche of the bone defect.
Among all the investigated materials falling into the category of bone void fillers [18,19], CPCs have been one of the most studied due to their exceptional biocompatibility and bioresorbability, but also due to their ability to host and release a variety of atomic and molecular substances, as exhaustively reviewed by Ginebra et al. [20]. Their injectability and ability to adapt to the geometry of the bone void defect site, allowing for a minimally invasive reconstructive surgery, presents another one of the exceptional traits of CPCs [21]. CPCs are synthesized in situ by mixing a powder and a liquid phase, after which they harden within minutes.
All CPCs belong to one of the two categories depending on the composition of their powder phase: 1) single-phase ones or 2) two-phase ones [22]. In the first case, a single calcium phosphate (CP) phase is mixed with a liquid phase to trigger the setting reaction, leading to the formation of either hydroxyapatite (HAp) as the most thermodynamically stable of all CP phases under the physiological conditions or dicalcium phosphate dihydrate (DCPD), a.k.a. brushite. Precursor phases in this case are metastable under the chemical conditions provided by the liquid phase and include CPs such as amorphous CP (ACP), α- or β-tricalcium phosphate (TCP) or tetracalcium phosphate (TTCP). The following reactions apply in this scenario for the stoichiometries of ACP and TCP (Eqs. (1), (3))) and of TTCP (Eq. (2)), forming either HAp (Eqs. (1), (2))) or DCPD (Eq. (3)):
| 10Ca3 (PO4) 2 + 6H2O → 3Ca10-x (HPO4) x (PO4) 6-x (OH) 2-x + 2H2PO4− + 2H+ | (1) |
| 3Ca4 (PO4) 2O + 3H2O → Ca10-x (HPO4) x (PO4) 6-x (OH) 2-x + 2Ca(OH) 2 | (2) |
| Ca3 (PO4) 2 + H3PO4 + 6H2O → 3CaHPO4 x 2H2O | (3) |
For the second type of CPCs, two concomitant CP phases are used in the powder phase, one of which is alkaline and another one acidic. The two typical combinations of such phases are that of TTCP and brushite (Eq. (4)) or α/β-TCP and monocalcium phosphate monohydrate (MCPM) (Eq. (5)), although other combinations are possible [22]. While the former combination yields HAp as the final product of the setting reaction, the product of the latter reaction is brushite.
| 2Ca4 (PO4) 2O + 2CaHPO4 → Ca10-x (HPO4) x (PO4) 6-x (OH) 2-x | (4) |
| Ca3 (PO4) 2 + Ca(H2PO4) 2 x H2O + H2O → 4CaHPO4 x 2H2O | (5) |
Since the first pioneering studies in the early 1990s, a number of works have been focused on drug release from CPC matrices and every successive study usually led to a more in-depth comprehension of drug release mechanisms and conditions. In order to provide a proper interpretation of the obtained results, theoretical and semi-empirical models were borrowed from the pharmacokinetic field [23,24] and successfully applied to CPC-drug release systems as tools for the qualitative and quantitative analysis of drug release from CPC matrices. Once the basic characteristics of the release mechanisms were established, numerous formulations of different CPCs were proposed as host matrices for different drugs. Several combinations of powder and liquid components of CPCs, their relative quantities, the use of additives, the large repertoire of drugs, drug loading methods and many other aspects of matrix-drug systems were experimented with in search of the most satisfactory release kinetics for a specific drug under the conditions relevant for its clinical application. The optimality of the drug release has been defined by different parameters, such as the initial burst release, the minimal and maximal released amounts, the total release period, the release rate and the release curve profile, which, in turn, are all derivatives of the overall release kinetics and mechanisms. The majority of literature reports have been focused on two main aspects of the drug release from CPC scaffolds: 1) the matrix component characteristics, including the chemical composition, porosity, crystallinity, and degradability, with or without the incorporation of supplementary phases or other additives; 2) drug-related studies, where different aspects of the drug have been investigated and the release effects of parameters such as the drug type, the loaded amount and the loading method have been considered. In addition to these two main aspects, some reports have been dedicated to the study of the effect of various environmental conditions, such as the release medium or in-vivo release, on the drug release kinetics.
Within this frame, this systematic review aims to collect the contributions to the specific field of drug release from CPC matrices in the timespan of the last 25 years. Due to the methodological difficulty of comparing different matrix-drug release systems, the inclusion of different scientific contributions to the present work was accomplished by selecting only papers that meet specific requirements: 1) the included work must show quantitative release data with at least 4 experimental points (except for Sec.8, which is focused on in vivo release) in order to properly define the release profile; 2) nearly all included articles include comparative results showing changes to the release curves due to variations in one or more characteristics of the release system, such as the drug type, the structural features of the CPC matrix (e.g., porosity, microcrystallinity, the additive concentration, etc.), the drug loading method, and/or others. In the present work, a lot of effort has been made to provide a clear division between the different characteristics of CPC-drug systems. This was done in the attempt to isolate individual structural or compositional effects on the release behaviour and to find a systemic and logical rationale as to what drives the different aspects of the release process. Despite all the effort, these variables defining the structure of the CPC matrix are always interrelated. Because it is not possible to draw distinct boundaries between these different, but intrinsically interconnected variables, some doubts are bound to remain with respect to what the key features defining the release kinetics are in each discussed case. This is especially true for systems where these key parameters are multiple, working in synergy. One example with respect to the matrix composition is that of the frequent impossibility of clearly demarcating the main component of the matrix from its extra component or an additive. Endless considerations could be made in this regard, but the final decision would depend on the chemical nature of the introduced extra component (such as a secondary CP precursor powder or a biodegradable polymer), its relative quantity and the homogeneity of its distribution within the cement matrix. Even after all these considerations and rationalizations, a very thin and rather blurred line will separate these individual aspects and the decision as to which of them is dominant will be, more or less, arbitrary.
Another question that is difficult to address is that of the classification of drug release systems to tailorable and tunable depending on their physicochemical makeup and release characteristics. In a study from 2016 [17], Uskoković et al. provided an etymological and functional classification of these terms. Nevertheless, doubts still persist when it comes to classifying a complex, multicomponent system into one of these categories based on the way their drug release kinetics has been reported in the relevant literature. One example comes from the release from CPC matrices with and without the inclusion of polymeric additives, such as chitosan or a polyester. Here, a hypothetic CPC with a fixed composition can be classified as a matrix for tailorable release, but the addition of a polymer in continuously varied amounts with, say, five different concentrations in the 1–5 wt% range, would render the system tunable. In this case, however, the tunability is due to the variations pertaining to an additive component of a CPC rather than to the core matrix, producing a sense of uncertainty as to whether the system is intrinsically tailorable or tunable. Despite all the unaddressed questions and ambiguities, the structure of this work is divided to sections individuating contributions of specific compositional and structural characteristics to the drug release process. These individual characteristics stand out as the most investigated aspects of CPC-drug release systems and the in-depth understanding of their contribution to the release rates can be a way of helping the researchers engage in the rational design of release kinetics for particular medical applications. In fact, the design of a bioactive CPC for use as a ubiquitous bone void filler requires comprehensive understanding of the factors governing the drug release, both alone and in synergy. Elucidation and elaboration of these factors from a qualitative standpoint and based on a complete survey of the relevant literature presents the central topic of this article.
1.1. Kinetic equations and the semiempirical model
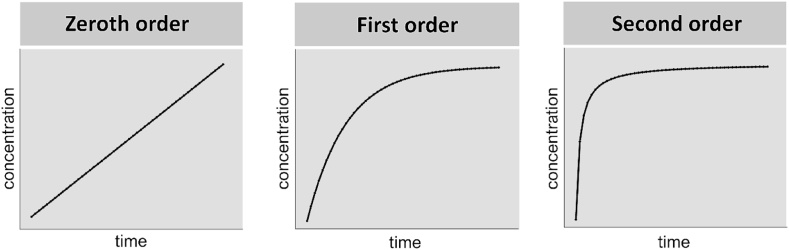
Works reported in this review make use of different equations and semiempirical mathematical models to quantitatively interpret the release outcomes. Fig. 1 schematically illustrates the three most common reaction orders of the physical release of drug molecules from the matrix, namely the zeroth, first and second.
Fig. 1.
Schematic illustration of the drug release profiles corresponding to the zeroth, the first and the second order of the reaction of release.
The zero-order kinetic equation is applicable when the dissolution of the drug is only the function of time and proceeds with irrespective of the concentration of the drug at any given time point. Mathematical representation of the zero-order equation is as follows:
where χi is the fraction of the dissolved drug at a given time t, and K0 represents the constant release rate that remains unchanged throughout the entire dissolution process.
The first-order kinetics describes the absorption/release of drugs, where the rate of the reaction linearly depends on drug concentration:
where m is the amount of adsorbed/released drug and k is the first-order rate constant. The integrated form of this equation from time t = 0 to a generic time t can be expressed as:
where m0 is the initial amount of the drug dispersed in the matrix and mt is the quantity of the drug released by time t.
The second-order kinetics implies a dependency of the drug release/adsorption rate on the squared value of the drug amount or concentration:
That could be represented in the integrated form as:
where k is a second-order rate constant and m0 and mt stand respectively for the initial amount of the drug and the amount of the drug released by time t.
Although the above-reported equations could provide accurate numerical solutions for many unidimensional and simple systems, they are of limited use for real, three-dimensional cases where the complexity and variety of environmental variables must be taken into account for the investigated systems to be modelled correctly. Moreover, there is never a straightforward and unambiguous way to extrapolate the information indicative on the release mechanism from these numerical solutions. To this end, a series of empirical and semi-empirical models and equations have been proposed for application on drug-release systems, as able to provide more realistic indications on the release kinetics and mechanism.
The Higuchi model was originally proposed in 1963 [25] to describe the rate of the drug dissolution from an ointment film. Afterwards, it was applied in a more extensive context, such as the drug release from tablets. The Higuchi equation is given as follows:
where χi is the fraction of the released drug by time t, A is the surface area of the tablet, D is the diffusion coefficient in the given medium, ε is the porosity of the matrix, τ is the tortuosity factor, C is the initial loaded amount of drug, and CS is the solubility of the drug in the release medium.
The Higuchi equation is often referred to as the “square-root” law due to the fact that the graphical representation of this relation can be linearized by reporting the fraction of the dissolved drug as a function of the square root of time (χi vs. t1/2). The Higuchi equation can be correctly applied only in systems that meet specific requirements and boundary conditions, including the following:
-
1)
The matrix contains an initial drug concentration much higher than the solubility of the drug;
-
2)
The diffusion is unidirectional because the edge effects are negligible;
-
3)
The thickness of the dosage form is much larger than the size of the drug molecules;
-
4)
The swelling or dissolution of the matrix is negligible;
-
5)
The diffusivity of the drug is constant;
-
6)
The perfect sink conditions are attained in the release environment.
Because of these abundant of requirements, this relation is often incorrectly applied in systems that do not strictly meet all of them. For this reason, all the deviations from the ideal conditions must be evaluated for a correct interpretation of the results to be reached.
A different semiempirical model was proposed by Korsmeyer et al. [26]. It is commonly called the Korsmeyer–Peppas or power law model:
The Korsmeyer–Peppas equation is a semiempirical model able to describe the drug release from polymeric or monolithic systems. In this equation, k is a parameter descriptive of all the geometrical and structural characteristics of the matrix, while n is related to the mechanism that governs the release kinetics. The value of the n factor generally indicates the release regime and for specimens with the cylindrical shape, as described in Table 1.
Table 1.
Interpretation of the Korsmeyer-Peppas exponent for cylindrical samples.
| Korsmeyer–Peppas model | ||
|---|---|---|
| n exponent value | Release regime | Release kinetic and mechanism(s) |
| 0<n < 0.45 | Hindered Fickian diffusion | Representative of systems characterized by diffusive regime with hampered release |
| n = 0.45 | Fickian diffusion (Case I) | Representative of first-order kinetic where diffusion is the main release mechanism |
| 0.45<n < 1 | Anomalous transport | Characteristic of those cases where in addition to the diffusion, other mechanisms contribute to the release of the drug |
| n = 1 | Non-Fickian transport (Case II) | Corresponds to a zeroth order kinetic and is typical of kinetics governed by phenomena of polymer degradation and relaxation or degradation/dissolution of monolithic systems (tablets, cements blocks) |
| n > 1 | Super case II | Extreme form of transport that usually occurs where severe modifications in the matrix take place |
2. General matrix features
2.1. General matrix features of pre-set CPCs
A very common approach utilized to adjust the drug release from CPC matrices consists in modifications of the powder phase, the liquid phase or both of them in the cement formulation. Several articles focused on this aspect of the control of the drug release, albeit with different objectives and results. Since the drug release rate is directly proportional to the rate of dissolution of the hardened cements, the control of this dissolution rate has been one of the methods to control the release rate, too.
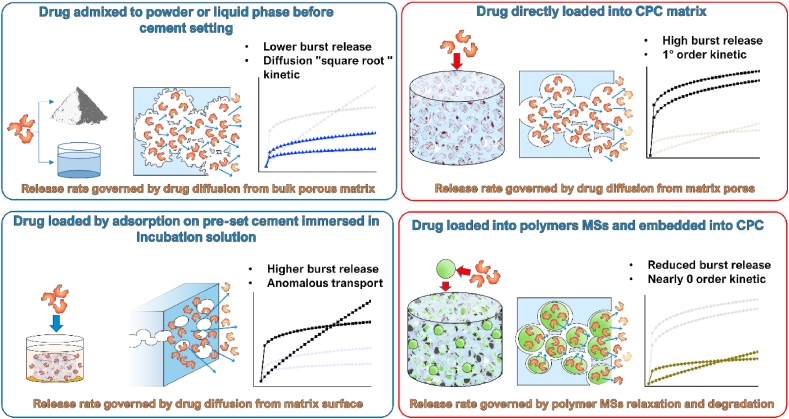
In 1998, Otsuka et al. [27] investigated the effect of the geometrical features of the matrix on release characteristics. CPC tablets of the same thickness (2 mm), but different diameter (2, 4, 15 mm) were fabricated for this purpose. In vitro and in vivo release of indomethacin at different drug loadings from HAp-forming TTCP/DCPD cements set for 1 h was examined. Here and elsewhere in the text, the term “in vitro” refers to abiotic release conditions only occasionally supplemented by biomolecular species, but without containing any cells. In the case of the release of indomethacin, the release rate followed a linear relationship with the tablet surface area, indicating a diffusion-controlled release (at least during the first stage of the experiment), as confirmed by the solid fit with the Higuchi equation.
Several works investigated the direct or the indirect effect on drug release originating from the use of the liquid phase obtained by or with the addition of components known to have a retarding effect on the CPC setting process, such as the compound class of carboxylic acids [22]. In a work from 2007 [28], Alkhraisat et al. aimed to elucidate factors that determine the superficial degradation of brushite under conditions simulating the highly blood-perfused regions in bone. In order to improve the brushite stability, different cements were prepared using different aqueous solutions of phosphoric, glycolic, tartaric, and citric acids at adequate concentrations. The specimens were immersed in the incubation solution for either 1 min (phosphoric solution) or 2 min (carboxylic acid based solutions) after the start of the setting reaction. The weight loss was measured by comparing the direct weights of the dried particles before and after the release. The experimental evidence indicated that the weight loss was dependent on the liquid phase used: phosphoric > tartaric > glycolic > citric acid. Degradation behaviour of the cement obtained with citric acid was further investigated after the addition of 0.5% hyaluronic acid and 0.5% chondroitin-4 sulphate. Superficial disintegration was noticeably reduced for the cement prepared with hyaluronic acid. An analogue approach was used in a work from 2010 by Khasaba et al. [29] where several aspects of CPC obtained with the use different liquid phases were explored. Cements were prepared using the same powder phase (a mixture of monocalcium phosphate monohydrate [MCPM], CaO and synthetic HAp), but different liquids, namely the aqueous solutions of polyacrylic acid (PA), polyalkenoic acid or 35% (w/w) polymethyl vinyl ether maleic acid. While the former two cements exhibited an intense release of calcium during the first day of incubation, the latter cement was characterized by the controlled release for the entirety of the incubation period of 8 weeks.
Hemmati et al. in a work from 2014 further evaluated the role of ascorbic acid (AA) in the release behaviour of CPCs [30]. Different CPC matrices were obtained, containing different amounts of AA: 0, 50, 100 and 200 mg/ml. Release patterns of ascorbic acid in the simulated body fluid (SBF) solution were fitted with the power law model. The calculated k parameter decreased as the AA amount increased, indicating the formation of more compact microstructures within cements obtained at 100 and 200 mg/ml of AA. On the other hand, the n factor for the cement with 50 mg/ml AA was more than twice higher than the value obtained for higher concentrations of AA (0.38 vs. 0.15). The hindered diffusional release of AA from samples with 100 and 200 mg/ml AA could be ascribed to the strong interaction of AA with CPC, being more pronounced at high concentrations of the released species.
Akashi et al. in 2001 published a work [31] where CPC based on α-TCP was selected as a carrier for antimicrobial agents. Different powders (α-TCP, α-TCP + BaSO4) and liquids (water + sodium carboxymethyl cellulose, water + citric acid + tartaric acid + polycarboxylic acid) were mixed to obtain two different cements named type I and type II, respectively. Upon mixing, the cement cylinders were kept for 1 h in a humid environment and then immersed in the release medium. The release of metronidazole, cefaclor and ciprofloxacin from these systems was investigated. Cement I consisted of needle-like crystals of HAp, characterized by a higher porosity which allowed for a faster release of the drug. On the other hand, cement II was found to have formed mainly amorphous calcium salts, with tighter particle aggregates, entailing a slower release during the early time of incubation, albeit with the propensity to disaggregate dramatically at later time points, inducing a drug release driven by matrix degradation. Results achieved in this work, along with the ones presented by Alkhraisat et al. [28], provided an interesting insight into the role played by the chemical nature of the liquid phase of the cements and how they affect the setting process and, in turn, the drug release behaviour. First, they confirmed that carboxylic acids act in general as retardants of the setting reaction. As a consequence, due to an extended setting reaction, cements retain for a longer period their state of a mouldable paste. For drug release experiments, cement pastes were immersed in incubation solutions a few minutes after the mixing. Cement pastes could be considered as highly porous systems where constituent nanoparticles are weakly entangled to each other; hence, the incubation medium can easily diffuse within the matrix, enhancing the sink and consequently allowing for a burst effect and an increased drug release as long as the setting process is retarded. Nature of the liquid components can also affect the solubility of the matrix in the release medium. Ca release, one of the markers of cement degradation, can be explained in terms of the cement solubility, which is, in turn, dependent on the inner solubility of the carboxylic acid used in the liquid phase. It could be concluded that the higher the solubility, the higher the cement degradation and, consequently, the higher amount of the drug can be released from these systems. Finally, the influence of the liquid component on the final pH of the cement can be addressed. As reported by Khashaba et al. [29], the cement pH, especially at the initial stages of the setting process, can play an important role in drug dissolution. Thus, a more acidic environment achieved with the use of the modified polyacrylic acid tends to increase the release rate, whereas cements at a more neutral pH achieved with the use of polymethyl vinyl ether maleic acid in the liquid phase are characterized by a more controlled drug release.
The group of Irbe et al. in their work from 2012 [32] followed a similar protocol by creating cements with combinations of different powders with different liquids in order to clarify the effect on the release of lidocaine. They obtained 3 different cements, namely HAp-B, HAp-A and DCP/HAp, where DCP stands for dicalcium phosphates either in the anhydrous (DCPA) or dihydrate (DCPD) form, which were mixed with two different loads of lidocaine (30 and 50 mg). The DCP/HAp cement was characterized by a strong burst desorption with 80% of the loaded drug being released within the first 10 h. In contrast, for HAp-A and HAp-B cements, a sustained linear release allowed only 40% of the drug to be released during the first 24 h, ending with a complete exhaustion that occurred by the 3rd day. A parallel experiment confirmed that pH increased over time for all the matrices except for HAp/DCP, in which case it retained a relative high acidic level. This acidic environment was responsible for the higher solubility of the cement, which explains the faster release of the drug as its corollary.
A biphasic α-TCP/HAp cement was proposed in a work of Su et al. from 2013 [33] and studied as a carrier for gentamicin. Different gentamicin loadings (4, 8, and 16%) were included in the cement. Release experiments revealed two distinct stages in the release profile. During the first stage, a burst release was observed, with the dissolution of a relatively large amount of the drug. This phase was followed by the second stage with a slower and more sustained release. The authors explained this behaviour through the influence of the drug on the structural evolution of the cement matrix during the setting stage. It was deemed that sulphate group contained in gentamicin molecules hindered the phase transformation from α-TCP to HAp, as evident from the consequent lack of formation of needle-like or plate-like crystals, which, in turn, blocked the release of gentamicin. Therefore, a comparatively large amount of the drug was released during the first hours of the reaction, before this transformation had been completed. As gentamicin and, consequently, the sulphate ion, got released, the formation of needle-like structure was boosted, slowing down the release process.
2.2. General matrix features of in-situ setting CPCs
Several articles attempted to assess the effects that different stages of the setting/hardening process of the cements have on the release behaviour. In two works by Rau et al. from 2017 to 2019 [34,35], the releases of Cu and Fe ions, respectively, were considered. In particular, in Ref. [34], the authors investigated the release of the Cu ion from the Cu-TCP precursor powder and the corresponding Cu-TCP cement forming more soluble brushite. Unexpectedly, the release of Cu from the TCP powder was higher than that from the brushite cement at all the time points except the earliest, 1 h one. When the Cu content was normalized to the amount of the actively releasing phase in the material, the released amount continued to be higher for the precursor powder than for the cement, but the difference was no longer statistically significant. Both profiles were characterized by a comparatively intense burst release and a nearly zeroth order kinetic. Two articles by the group of Ginebra et al. focused on the comparison of fresh mixed CPC with pre-set CPC in terms of the release rate. In their work from 2013 [36], doxycycline (DOXY) was loaded inside both fresh CPC and 7 days old pre-set CPC and the release behaviour was investigated. The pre-set CPC was characterized by the diffusion-controlled release. The Korsmeyer-Peppas fit provided the k parameter that remain unchanged for the duration of the release experiment, serving as the evidence that no modifications of the microstructure occurred during incubation. The non-Fickian diffusion was defined by the calculated parameter n, which ranged between 0.49 and 0.58. For the fresh CPC, a different scenario was observed, with two distinct regions of release as a function of the incubation time. The initial stage showed a burst release and was followed by a second stage with a marked rate decrease, levelling to a zeroth order kinetic with sustained release of the drug. A correlation was found between the transition time between the two different kinetic regimes and the final setting time. The authors suggested that this correlation indicated the influence of the evolution of the CPC microstructure on the release pattern. It was hypothesized that during the setting reaction, precipitation of calcium-deficient HAp (CDHA) nanocrystals occurs, filling the space previously occupied by the liquid containing the drug molecules. This resulted in an increase of tortuosity, which hampered the drug mobility, hence decreasing the release rate.
The use of the cement at different setting times can allow for modelling the release behaviour of clinically applicable injectable cements at different stages of setting and hardening. Therefore, in the follow-up work from 2015, Mestres et al. [37] focused their attention on a brushite cement matrix prepared at different times: 3 min (fresh cement) and 1 h and 15 h as pre-set cements. Simvastatin was selected as the model drug and loaded onto the abovementioned cements. The release curves were fitted by the Korsmeyer-Peppas model. The release of the drug from the 3 min and the 1h set cements showed a burst release during the first 8 h and a slower dissolution for the remaining 4 days, while the 15h cement was able to provide a sustained elution for the whole incubation period. Fitting parameters allowed to conclude that the transport mechanism was anomalous, with the corresponding n value of 0.77. By knowing the n value, it was possible to calculate the k parameter for all the examined CPCs and their evolution with time. The initial k values (k3min > k1h > k15h) were inversely related to the tortuosity factor, as could be expected from the difference between the fresh, yet-to-be-set cements and the pre-set ones. Plotting k values as a function of time highlighted the fast change occurring to the microstructure of the 3min cement with a concomitant increase of tortuosity. The 1h cement showed a slower evolution of the k value with time. Interestingly, all the k values of different cements converged at almost the same value at the end point of the release experiment, confirming the similar structural nature of the same cement investigated at different elapsed setting times.
In a work from 2016, the Uskoković group [38] showed the possibility to obtain an injectable cement with an adaptive drug release. For this study, two basic powders, HAp1 and HAp2, were taken as basic components of the cement formulations and were combined in different proportions, including HAp1:HAp2 0:100, 50:50, 85:15, and 100:0. HAp1 was prepared by the slow precipitation, while HAp2 was precipitated abruptly. The peculiarity of these two main components was that HAp2 was able to retain its amorphous state after being mixed with a liquid and before reprecipitation in a crystalline form. On the other hand, HAp1 tended to return fast to its original crystalline state. The release experiments were carried out by loading the cements with vancomycin and ciprofloxacin as model drugs. The drug dissolution patterns demonstrated that HAp2 was characterized by the high burst release and fast drug exhaustion. An opposite behaviour was observed for HAp1. Mixed component cements possessed hybrid behaviours and the release rate was directly proportional to the fraction of HAp2 used. Explanations given by the authors referred to a number of thermodynamic considerations, such as the surface energy and solubility of precursor powders with respect to their crystalline or amorphous conditions. The follow-up article from 2019 [39] focused on elucidating the characteristics of this mechanism of drug release in the same system of cements comprising HAp1 and HAp2 at different proportions. Basic considerations for the investigated cements were the same as in the previously described work [38]. This time, however, the kinetic analyses aimed to decipher the key aspects of the mechanism involved in the release process. The Korsmeyers-Peppas model was employed and the author demonstrated that by plotting the k parameter vs. HAp1/HAp2 ratio in the cements, a strong increase of the k value is observed, suggesting that the microstructure of HAp2 allows for a faster and in many scenarios more favourable release. The analogue plots of n values for the release of vancomycin demonstrated a decrease of this exponent with the HAp2 content, indicating a transition from the anomalous transport to a more common Fickian diffusion. The opposite occurred in the case of ciprofloxacin, with the n exponent increasing with the HAp2 content, suggesting that the nature of the drug can influence not only the release rate, but also the mechanism of release.
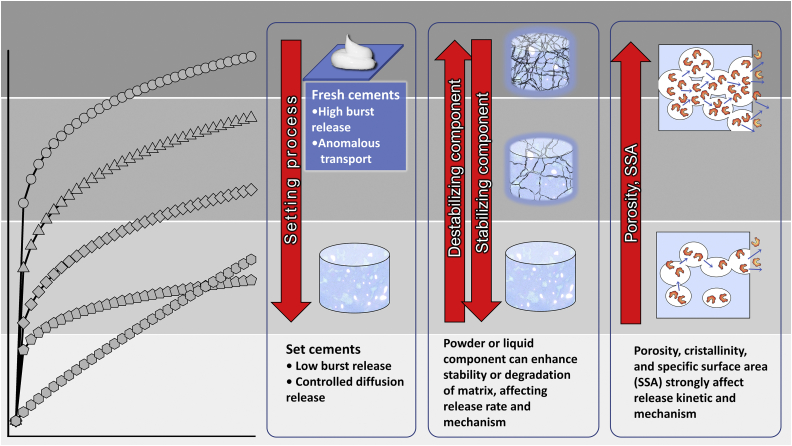
In a work from 2018 by Uchida et al. [40], a comparison between the release of vancomycin from CPC and from non-biodegradable poly(methyl methacrylate) (PMMA) was reported for both in vitro and in vivo conditions. In the in-vitro experiment, the drug release was consistently more copious from the CPC carrier than from the PMMA one and this difference in release increased constantly with time for 56 days. A comparison of the release from TCP and HAp granules was the objective of a work from 2019 by Son et al. [41]. Gentamicin sulphate was used as the model drug and its dissolution was studied using a matrix obtained from granulated TCP or a mixture of HAp and TCP particles. Three release systems were studied, where gentamicin sulphate was dispersed inside: 1) TCP granules, 2) TCP-HAp granules, and 3) the setting agent, i.e., water. The most optimal release rate was obtained when the drug was dissolved within the matrix composed of a mixture of TCP and HAp. This system showed a constant and sustained release for a period of 140 h. The authors supposed that the presence of HAp granules, which are characterized by a remarkably reduced solubility in comparison to TCP, makes the cement less prone to degradation during the setting reaction. The intrinsic stability of HAp granules helped to protect the loaded gentamicin, allowing for a more prolonged and sustained release of the drug over 144 h. Moreover, the presence of HAp granules can lead to the formation of DCP, which is characterized by an increased porosity and consequently enhanced release. In general, notwithstanding the ever-present physical effects, the drug release rates are expected to be directly proportional to the solubility of the CP phase(s) that the CPC evolves into. This conclusion can be drawn in spite of the fact that studies specifically comparing the phase-dependent kinetics of drug release from CPCs have not been conducted to date, with the exception of studies indirectly addressing this issue that were mentioned here. Considering all the effects summed in this section, Fig. 2 schematically represents the inducible trends, referring specifically to the effects of matrix degradability, freshness and microstructural properties on the kinetics and the mechanism of drug release.
Fig. 2.
Schematic description of the effect of the cement matrix features on the drug release rate and mechanism: in the first panel from the left, a fresh cement (higher release rate with possible anomalous transport) is compared with a set cement (lower rate and diffusional transport). In the second panel in the centre, a degradable matrix (higher release rate with possible anomalous transport) is compared with a non-degradable matrix (lower rate and diffusional transport). In the third panel from the left, the effects of porosity, crystallinity, and specific surface area, all of which increase the release rate, are reported.
3. Porosity and pore features
Porosity and pore features clearly represent one of the most important properties of CPC matrices, along with their chemical nature. Suitable porosity of carriers allows to host a relatively large amount of the drug within the cement, assuring at the same time the possibility of unhindered adsorption and desorption of the hosted molecules. Many authors have attempted to gain an insight into the specificity of the influence of porosity of the host matrices on the release process and several works reported possible methods for inducing, enhancing and tailoring the pore features for an optimal release. In a work from 2010 by Haghbin-Nazarpak et al. [42], the release of gentamicin sulphate from a biphasic cement (β-TCP and MCPM) was investigated. The release profile showed that the process proceeded in two different stages: in the first stage, lasting for approximately 10 days, the drug distributed along the cement surface was released at a relatively high rate. Degradation of the cement occurred in parallel with this initial release stage. Cracks and macropores with dimensions ranging in microns appeared across the cement matrix volume, causing a sudden burst release of about 15% of the drug load. From day 10 to day 15, no further release of the drug was observed, determining the end of the release.
In their work from 1997 [43], Otsuka et al. investigated the effect of sodium bicarbonate addition on the release behaviour of indomethacin from a carbonated apatite cement. Four samples were prepared by increasing the amount of NaHCO3 porogen (0%, 2%, 5%, 10%). A microporosity distribution study revealed that the pore size increased after the drug release. The amount of NaHCO3 strongly influenced the pore dimensions and, thus, the release process, with larger pore radii corresponding to the higher amounts of NaHCO3. The authors explained this effect by an augmented solubility of carbonated apatite, producing increasing amounts of pores at higher NaHCO3 contents within the matrix. However, the sublimation of the dissociated carbonates in the form of CO2 must have also been responsible for the pore formation. Variations in the pore size distribution strongly affected the release rate, which increased together with the pore size. The release curves indicated a diffusion-controlled process characterized by a sustained release with an increasing rate in direct proportion with the amount of NaHCO3 introduced. In the case of 10% NaHCO3, however, the enlargement of pores was so pronounced that it led to a burst release of nearly 100% of the loaded drug within the first 10 h. A similar approach was used in a work from 2009 by Girod-Fullana et al. [44]. In this case, the release system consisted of pectin-microspheres loaded with ibuprofen as the model drug. The microspheres were incorporated within the CPC matrix at different ratios, namely 2%, 4%, and 6% w/w, in order to explore the influence of the amount of microspheres within the composites on the release process. The total porosity and microporosity were measured for all the composite samples and it was found out that they both increased with the microsphere content, from 0% macropore volume fraction for 0% microsphere content to 29% at 6% microsphere content. Results from the release experiments showed a Fickian diffusion regime from all the composites, with the release lasting for at least 45 days. Unexpectedly, the release rates were higher for composites with the lower amounts of microspheres and, consequently, lower porosities. The authors proposed two possible explanations of this phenomenon: 1) the confinement of the drug could have induced local concentration pockets exceeding the solubility level, resulting in a slowed release; 2) the ability of pectin to form a gel, as a result of which some microspheres could have undergone swelling and filled some of the intrinsic pores of the CPC, thus reducing the real porosity.
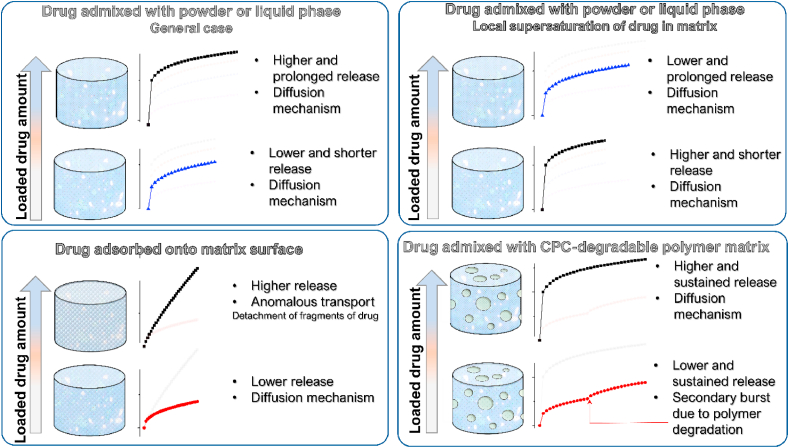
Different groups have investigated the influence of the powder-to-liquid (P/L) ratio used during the mixing process on porosity and the possibility of using this parameter to tailor the drug release rate. In their work from 2009 [45], Hofmann at el. aimed to investigate the possibility to produce a near zero porosity matrix in order to prevent the burst release of antibiotics from CPC matrices. They studied the release of vancomycin and ciprofloxacin from CPC formulations created with different P/L ratios (2.5 g/ml and 4.0 g/ml). The porosities for these two formulations were 18% and 38%, respectively. Cumulative drug release curves demonstrated that for both drugs, the release rate was greater for the P/L ratio of 4.0 than for that of 2.5, with no apparent modification of the diffusion regime. Therefore, these results conform to the notion that the release rates should increase with the total porosity of the matrix. In a paper from 2011 [46], Schnieders et al. examined the effect of the CPC porosity on drug release by varying the drug loading method, that is, by either directly loading the drug onto the CPC matrix or by encapsulating it into a biodegradable poly(lactic co-glycolic acid) (PLGA) copolymer subsequently incorporated into the CPC matrix. In order to modulate the sample porosity, different P/L ratios were employed and five different CPC formulations were created, with the P/L ratios of 2.0, 2.4, 2.7, 3.0, and 3.3 g/ml. The porosity increased almost linearly with the liquid content, i.e., reciprocally with the P/L. In the case of a system with the drug directly dispersed into the CPC, the release rate and the initial burst release increased inversely with respect to the P/L ratio. Results from the system with the drug loaded via PLGA microspheres showed a prolonged release and a decreased burst release for all the formulations as compared to those involving the direct drug loading. A more interesting insight was provided when the Higuchi model was used to fit the experimental data. While in the case of the direct loading of the drug, a good correlation was found between the Higuchi coefficient and the CPC porosity, in the case of the drug encapsulated in PLGA microspheres, no correlation could be established, suggesting that in the latter case the drug release was kinetically controlled by diffusion from the microspheres, drastically reducing the dependence of the drug release rate on the matrix porosity. Similar conclusions were drawn in the 2013 paper by the group of Ginebra [36]. In this work, a series of experiments was designed and conducted to determine how different variables affect the drug (DOXY) release, either singularly or in a synergetic manner. Focusing on the porosity factor, the authors investigated the characteristics of two CPC matrices with different liquid-to-powder (L/P) ratios: 0.35 and 0.65 ml/g. Open porosity measurements showed a greater mean pore size for samples richer in liquid (L/P of 0.65). As expected, a higher release rate was found for cements with a higher L/P ratio.
The group of Otsuka reported many works which share the same approach to induce the porosity in a CPC matrix. In these works, pre-set CPC blocks containing drugs were used as matrices and series of macropores were mechanically created in the blocks with the use of several stainless steels needle-like male dies arranged in a multi-cross manner in a regular 3D pattern along the blocks and then removed. In these papers [[47], [48], [49]], an identical methodology was used to investigate the effect of mechanically induced macropores (radii of 300, 500 or 600 μm) on drug release. Different cement blocks with an increasing number of cross-arranged macropores (0, 20, 40, 60) were used for the release experiments. Each block contained the same amount of the drug. The release patterns brought to light a direct relation between the number of macropores and the release rate. All the release patterns showed the diffusional trend, for which reason a Higuchi plot was used for fitting and relative Higuchi constants were calculated from these analyses. The calculated Higuchi constants were plotted as a function of the number of macropores or the corresponding specific surface area. In all cases, the experimental data were fitted well by a linear regression with a high regression coefficient, thus establishing the possibility to obtain CPC blocks loaded with different drugs with a desired release rate. In two articles from 2013 [50,51], Hesaraki et al. evaluated the possibility of inducing macroporosity in a CPC matrix by the addition of sodium dodecyl sulphate (SDS) as a surfactant. In Ref. [50], the release systems were obtained by the combination of different amounts of SDS (0, 20 and 100 mM) with different doses of cephalexin monohydrate (CMH) loaded into the cement as the drug (0, 1, 5, 10 wt%). The release was observed for over 300 h. The release curves of different systems revealed an interesting influence of the induced macroporosity on the release patterns. During the first 10 h of the release, a diffusion-controlled regime was observed and the release rate was higher when larger amounts of SDS were added to the system and more macropores were produced. Fitting these results with the Higuchi model yielded a good correlation. In the time interval of 24–300 h, deviation from this behaviour was observed, with the release being linearly dependent on time, following zeroth order kinetic profile. This deviation was attributed to the degradation of the CPC matrix, the regimen under which the release rate was no longer related to the matrix porosity. In the second work by the same group [51], the authors explored the release behaviour of demaxothasone (DEX) from porous CPCs. The experimental setup was similar to the previous one, involving the fabrication of porous CPC matrices by the addition of SDS (0 mM and 100 mM) combined with different drug dosages (0, 10, 50, 100 nM). It was observed that the addition of SDS led to an increase in macroporosity by 13–16%, with a concomitant reduction of the micropore volume relative to the SDS-free CPC. Release profiles of all the systems showed a diffusion-controlled release, with the higher rate of release corresponding to the higher amount of SDS and DEX. The release curves were fitted with both the Weibull equation and the Korsmeyer-Peppas model. The fitted parameters suggested that while macro- and micro-porous matrices shared the same release mechanism, (the differences between n values or d values were not statistically significant), a significant difference was observed for the k and t constants, which are dependent on the geometrical and physical characteristics of the matrix. The authors inferred that these differences could be attributed to the matrix modifications induced by porosity. Based on the results of this study, it could be concluded that the introduction of macropores into a microporous CPC can be a route to increasing the release rate in direct proportion with the concentration and the average size of the macropores introduced. In a work from 2013 by Vorndran et al. [52], the authors proposed an injectable, ready-to-use CPC paste as a bone filler with drug carrier properties. CPC pastes were created using an oil-based compound as the liquid phase. Characteristics of this oil-based cement were compared with its water-based analogue. The water-based CPC reference showed a total porosity of 42%, which was higher than the oil-based porosity of about 20%. The average pore diameter was 15 nm for the water-based cement vs. 25–30 nm for the oil-based one. The release profiles demonstrated a faster release for all the oil-based CPCs despite their lower total porosities, while the Korsmeyer-Peppas fit indicated that the dissolution of the drug occurred under a diffusion or diffusion/degradation regime. This was attributed to the larger pores that characterized the oil-based cements. The pore enlargement was related either to the dispersion of the aqueous solution within the cement or to the dissolution of the solid drug contained in the cement.
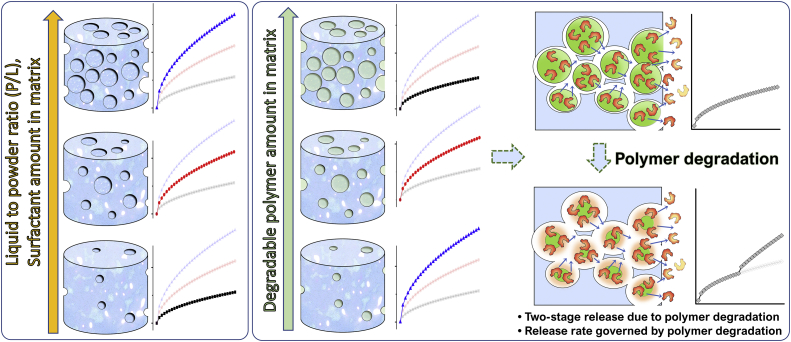
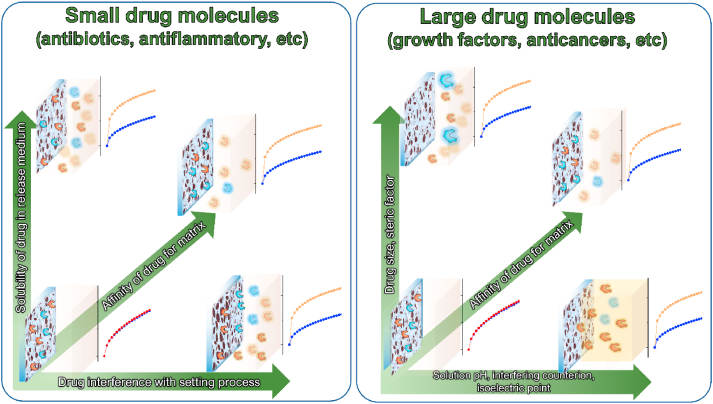
In the aforementioned article by the group of Uskoković from 2016 [38], which was focused on the preparation of a CPC matrix with a tunable release profile, several parameters were taken into account in order to discern the role of each in defining the release behaviour of two different antibiotics: vancomycin and ciprofloxacin. The investigated systems consisted of various combinations of two different HAp powders: HAp1, with higher crystallinity, relatively low porosity (42%) and a lower release rate; and HAp2, mostly amorphous, but with a higher porosity (55%) and a strong propensity for drug release. The experimental evidence revealed a parallel increase in the release rate for both drugs and the porosity, which increased with the relative amount of HAp2 in the hybrid cements. In a recent work from 2020 by Lucas-Aparicio et al. [53], a TCP-based CPC with an extensive accommodation of Si in the matrix was studied as a potential carrier of drugs able to provide a constant release with the minimal burst effect. Characterizations were conducted on the cements with different amounts of Si: 0, 40 and 80% of the stoichiometric Si substitution. It was observed that the total porosity and the average pore size decreased with the introduction of Si, while the specific surface area increased with the Si content. The increment in the Si content led to substantial changes in the release profiles: from the first-order release in the cases of 0% and 40% Si to the zeroth order release in the case of 80% Si. Furthermore, the cement with 80% Si substitution showed a negligible burst release. The authors ascribed this interesting result to the nanometric nature of the pores, which is capable of producing a greater tortuosity and becoming a key control factor for the drug diffusion. Clearly, when the dimensions of the pores are comparable to the dimensions of the drug molecules, the effect of the pore size is more intense than when the pores are significantly larger than the drug molecules, in which case the latter can freely diffuse out of the pores and the release is controlled more by diffusion than by the structural factors [54]. It could be concluded that the drug transport mechanism is strictly related to the relation existing between drug molecules and pore dimensions. Properly tuning the pore size to drug dimensions (i.e., few nanometers for small-drug molecules and tens of nanometers for large-drug molecules) makes it possible to establish a non‐Fickian diffusion driven regime at the molecular scale, where suitably sized pores allow for a single molecule release at a time, resulting in a concentration-independent, zeroth-order kinetic. Conversely, when pore sizes largely exceed the drug molecule dimensions, the stream release of the drug can occur, giving rise to the Fickian diffusion regime and the burst effect [55]. Fig. 3 schematically summarizes the different effects through which the porosity in CPCs affects the drug release rate and mechanism.
Fig. 3.
Schematic illustration of the effect of porosity on the release rate. In the left panel, the general case is represented, where increasing porosity (tunable with different approaches) corresponds to higher release rates. In the right panel, the case of porosity induced by the inclusion of a polymer in the matrix is reported. In this case, the release rate is governed by the polymer relaxation and degradation and by the subsequent drug diffusion from within the bulk of the cement, with lower release rates corresponding to the higher contents of the included polymer. When the polymer undergoes degradation, the second stage of sustained drug release takes place.
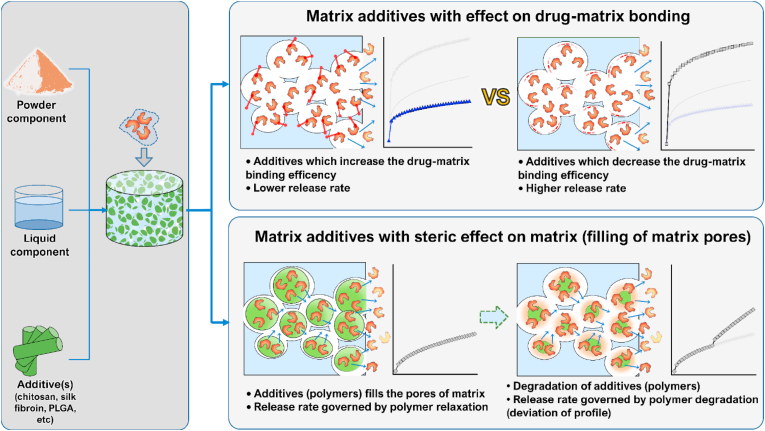
4. Matrix additive
A relatively easy method to control the drug release by the addition of polymers into the CPC matrix was reported by Wang et al. [56]. In this paper, a CPC powder was mixed with different polymers, such as mannitol crystals or salicylic-acid crystals. These additives induced macropores in the matrix and were used to enhance the release rate of growth factors. A curve showing the release of TGF-β1 from the CPC/(salicylic acid) composite was presented. Dissolution of the drug occurred with the first-order kinetic and a sustained release for over 800 h was observed.
In a work by Takechi et al. from 2002 [57], the release of an antibiotic, flomoxef sodium, from a CPC matrix modified with anti-washout chitosan was observed. Different amounts of the polymer were added to the cement to decipher its effects on the release of the drug. The release curves were obtained from cements containing 0%, 0.5% and 1% of chitosan. In the first stage, which lasted 24 h, the release was higher for the cement with the lowest amount of chitosan. After 24 h, the saline buffer was fully replenished and the release started again with diminished magnitude but inverse behaviour, specifically with a higher release rate from cements containing higher concentrations of chitosan. All profiles at both stages were characterized by a diffusion-controlled profile. The authors explained that in the first stage chitosan might have filled the pores of the cement matrix, thus promoting the retention of the drug. When the saline buffer was substituted, however, the cements with higher amounts of chitosan, now containing larger amounts of the retained drug, began to release the drug at a higher rate.
A brushite-chitosan matrix system was presented in a work from 2009 by De la Riva et al. [58] as a carrier for growth factors, specifically vascular endothelial growth factor (VEGF) and platelet-derived growth factor (PDGF). A small cylindrical chitosan sponge was first synthesized and PDGF and VEGF were included in it as components of either the liquid phase or alginate microspheres, respectively. The sponge was, in turn, incorporated inside a brushite cement, resulting in two variants of the drug-containing brushite-chitosan scaffold. Both drugs were released with a diffusion-controlled kinetic. During the first 24 h, 45 and 13% of PDGF and VEGF was released, respectively. After this initial burst release, the release of PDGF reached 70% after two consecutive weeks, while 64% of VEGF was delivered during the following 3 weeks. These results confirmed the ability of chitosan to slow down the drug release. Whereas the drug molecules adsorb mostly onto the surface binding sites on brushite grains, they get incorporated deeper into the carbonaceous network of chitosan, explaining its ability to lower the release rate by extending and complicating the escape path for the drug molecules.
The development of chitosan/HAp scaffolds was accomplished for the delivery of basic fibroblast growth factor (bFGF) and reported in an article by Tığlı et al. [59]. Two scaffolds were prepared, with 2% and 3% (w/v) of chitosan in the reaction solution and 1.25% (w/v) of HAp granules. bFGF was inserted into the scaffolds using the solvent sorption method. The release curves for both formulations indicated a hindered release rate and extended dissolution process due to the presence of HAp in the matrix, but also the faster release from the scaffold prepared at the higher concentration of chitosan. This has hinted at the possibility of controlling the release rate from CPCs through modulation of the concentration of the polymer in the reaction solution. Experimental data were fitted with the power law model. The anomalous transport characterized the release of bFGF from these HAp/chitosan scaffolds, indicating that the absorption of water and swelling-controlled diffusion were the key mechanisms of bFGF release.
In a study reported in by Lode et al. [60], the release kinetic of a CPC functionalized with VEGF was explored. The modified CPC matrices were obtained through the use of different combinations of additives. Namely, the precursor powder was mixed with 2.5% (w/w) mineralized collagen I (BioD/coll) and either 48 g/g trisodium citrate (BioD/coll/cit) or 25 mg/g O-phospho-l-serine (BioD/coll/PS). A total of three modified cement matrices were thus obtained and the release profiles for VEGF under abiotic cell culture conditions (37 °C, 5% CO2) were investigated. The profiles for each of the three different formulations showed a diffusion-controlled kinetic. Also, all the variants that included collagen (coll) showed an increased initial burst. Collagen stands for one of the most used organic additives in CP composites. Its addition into CPC precursors induces the formation of complexes with Ca2+ ions [61] and a slight delay in the setting time [62]. The tendency of collagen to increase the burst release can be explained by the ability of this macromolecule to decrease the binding efficiency of CPC for VEGF, with a consequently higher amount of unbound VEGF molecules free to diffuse out of the matrix. This stage was followed by a slower release for the remaining 7 days. The BioD/coll/PS formulation did not show a significant change with respect to the pure collagen variant. On the contrary, a decrease in the release rate was observed for the variant with trisodium citrate (BioD/coll/cit). The authors suggested that trisodium citrate could enhance the negative surface charge of the cement, thus leading to an increased electrostatic affinity for VEGF and its stronger binding to the CPC. However, for all the formulations, a markedly slowed release was registered after 7 days, with the cumulative release of 25% in the case of BioD/coll and BioD/coll/PS and 15% in the case of BioD/coll/cit.
Li et al. [63] studied the release of the salmon calcitonin (S-CT) from a CPC modified with organic phases such as chitosan oligosaccharide (CO) and collagen polypeptide (CP). Four different cements were prepared with different amounts of the CO-CP organic phase: 0%, 5%, 10% and 15% w/w. The release of S-CT from these composites was observed for 60 days. For all the formulations, two stages of the release, characterized by different rates, could be individuated. While the unmodified cement displayed a higher release rate during the first 7 days, after this time point the CO-CP modified cement showed an increased rate, where the magnitude was proportional to the CO-CP content. This is thought to be due to the filling of the matrix pores by the organic phase in the first stage of the release. During the incubation period, polysaccharides tended to dissolve, leaving open channels inside the cement matrices and allowing for a higher release of the drug. A nearly zero-order kinetic was observed, with a constant and sustained release for both stages during the whole duration of the experiment.
A CPC composite modified by the addition of 0.5% of alginate was tested as a drug carrier for gentamicin by Chen et al. [64]. The release profiles of unmodified and modified cements were compared. The addition of alginate strongly mitigated the initial burst release, which changed from 70% during the first 24 h in the unmodified cement to 51% in the modified cement. In general, a slower and more controlled release of the drug was obtained with the modified cement. According to the previous literature data, this could be explained by the selective interaction of gentamicin with the mannuronic residues of alginate. For both variants, a complete depletion of the drug was observed after one week. Finally, the addition of alginate did not modify the shape of the release profiles, which were in both cases kinetically controlled by diffusion.
Colpo et al. in their work from 2018 [65] aimed to elucidate the mechanisms governing the in-vitro release of different drugs from two different CPC matrices: 1) a cement obtained from the pure α-TCP precursor and 2) a cement obtained from the α-TCP powder with the addition of 10 wt% acrylamide polymer (α-TCP DS powder). Four different molecules were selected as model drugs for release experiments: gentamicin sulphate, lidocaine, bupivacaine, and levobupivacaine hydrochloride. A total of 2 (matrices) x 4 (drugs) = 8 experiments were carried out. Firstly, XRD results for all the investigated systems allowed to conclude that the drugs did not interfere with the setting process and that the final composition of all the cements consisted of a mixture of α-TCP, β-TCP and CDHA. The release experiments were carried out by immersion of cement cylinders in 10 ml phosphate buffer saline (PBS) solution at pH 7.0 and 37 °C for a total duration of 90 days. Similar profiles characterized all combinations of matrix/drug systems, with a burst effect occurring within the first 200 h followed by a reduced and sustained release rate. Except for the lidocaine case, all other systems showed a higher release from the matrix obtained with the 10 wt% acrylamide addition. Three different mathematical models were applied to the release experimental profiles in order to determine the release kinetics and mechanism: 1) Higuchi equation, 2) Korsmeyer–Peppas equation and 3) Peppas–Sahlin equation. The best fit with the experimental data was achieved with the use of the Peppas–Sahlin model. Comparison of n exponents of the Peppas–Sahlin equation for the same released drug and different matrices revealed that higher n values (within the 0.33–0.51 range) characterized the release from the α-TCP DS cement, indicating that the Fickian diffusion was the prevalent mechanism, with a minor contribution of the anomalous transport. The authors concluded that the latter contribution can be attributed to the chain relaxation of acrylamide and the consequent formation of hydrogel, which explains the higher release rate.
Multi-walled carbon nanotubes (MWCNTs) with carboxylic moieties were employed in a work by Lin et al. [66] to reinforce a monetite-based cement and develop a suitable carrier system for MG132, a small peptide molecule used for the inhibition of NF-κB-mediated osteoclastic resorption. Indirect measurements of the MG132 release were extrapolated from the luciferase assay conducted for a period of 28 days. It was observed that the MWCNT introduction decreased the burst release of MG132 and induced a more sustained release as compared to the unmodified CPC cement. Moreover, the pharmacological activity of MG132 was preserved for up to 28 days.
Wang et al. [67] investigated the reinforcement of an α-TCP-based cement by the introduction of silk fibroin. Semaphorin 3A (Sema3A) was loaded inside chitosan microspheres (Sema3A/CMs). Thus loaded microspheres were incorporated inside the cement/silk fibroin composite, yielding Sema3A CMs/SF/α-TCP. The release of Sema3A from this delivery system was investigated. The initial burst effect was observed during the first days of incubation. Thereafter, a continuous and sustained release of Sema3A was observed with a nearly zero-order kinetic. The authors did not provide any evidence of polymer (chitosan) degradation, for which reason the low burst effect along with a controlled zeroth order kinetic release could be considered to be in agreement with other results where the release kinetics of the drug encapsulated in polymeric microspheres is governed by the polymer relaxation.
In a work from 2016 by Zhang et al. [68], experimental orthodontic cements were prepared and characterized. To prepare these cements, the particles of ACP were incorporated at 40% in: 1) a mixture of pyromellitic glycerol dimethacrylate (PMGDM) and ethoxylated bisphenol A dimethacrylate (EBPADMA) at the mass ratio of 1:1, 2) polyethylene (PE) + 10% 2-hydroxyethyl methacrylate (HEMA) and 5% bisphenol A glycidyl dimethacrylate (PEHB), leading to the formation of two distinct composites: PE+ 40ACP and PEHB+40ACP, respectively. Two cements which did not include ACP (PE and PEHB) were also analyzed for their release of calcium and phosphate ions. Three rounds of release (recharge) and re-release were conducted. During the first round, high levels of release from PE+40ACP and particularly PEHB+40ACP were detected, indicating the importance of the resin phase formulation. The authors attributed the higher release propensity of PEHB+40ACP relative to that of PE+40ACP to the HEMA component present at 10 wt%, specifically to its hydrophilic nature, which can explain the higher water sorption by this formulation. This HEMA characteristic could promote an enhanced water diffusion within the matrix, allowing for a higher calcium and phosphate ion dissolution and release in a pH-adjusted medium. Further rounds of recharge and re-release demonstrated the capacity of PE+40ACP and PEHB+40ACP to provide a superior release compared with the cements without ACP. Every successive round was characterized by decreasing release rates for all the formulations. Further, all the release profiles for all of the cements in each of the release rounds were characterized by the diffusional regime.
In a work by Uskoković et al. from 2017 [69], a HAp-forming CPC was supplemented with silica and gelatin, which endowed the cement with thermosetting properties. The volume of the cement that would take around 45 min to set at room temperature would thus set in a matter of minutes at physiological 37 °C. These additional components also increased the surface porosity of the hardened product owing to the controlled dissolution of gelatin, which left voids in the solid material. The release of vancomycin was studied for two different cement formulations, which contained 19.4 and 26.5 wt% of the additive phases. No burst release was detected for any of these two cements and for both of them the total release was slower and more sustained than that from the additive-free CPC. The drug release kinetic was independent on the content of gelatin, suggesting that the release was controlled mainly through the affinity of vancomycin for HAp. The parallel increase in vancomycin loading efficiency and the weight percentage of HAp in the set cement supported the idea that vancomycin was not predominantly entrapped by gelatin, but that it rather got bound to the surface of HAp through electrostatic attraction and was released by overcoming this attraction with the help of the hydration force. Gelatin here, however, still played a key role by coating HAp grains and hindering the desorption of vancomycin molecules, thus promoting their slow and sustained release.
In a work from 2017 [70] by Schumacher et al., a delivery system for VEGF and lysozyme (Lyz) proteins from a matrix consisting of CPC and mesoporous bioactive glass (MBG) based on calcium silicate was proposed and investigated. Mesoporous calcium silicate was added at different ratios to the precursor α-TCP powder and the formulation containing 10 wt% MBG was selected as suitable for the drug release assessment. The cement was obtained by mixing the precursor powder with a Na2HPO4 aqueous solution. Two different loading routes were applied for the Lyz inclusion: 1) dissolving Lyz in the Na2HPO4 aqueous solution and 2) incubation of MBG particles in a Lyz-enriched buffer solution. Experiments assessing the individual release of the two proteins from the pure CPC matrix and from the CPC/MBG composite cement pre-set for 3 days were carried out. Comparison of the profiles of release from the two matrices showed a higher release rate from the CPC pure matrix than from the CPC/MBG composite. A diffusive profile characterized the release of VEGF and Lyz from the two different matrices, indicating that the diffusion from the matrices is the main involved mechanism. The lower released amount of proteins from the CPC/MBG matrix can be ascribed to the antagonistic effect induced by mesoporous silica. Namely, on one hand, MBGs are known to possess an intrinsic porosity and a high propensity for degradation in solution through the formation of hydrated silica species. This factor would theoretically induce an increase in the drug release rate. On the other hand, mesoporous silica possesses a high bioactivity, which is able to promote the deposition of a layer of CDHA on the cement surface and hinder the leaching of the drug. In this study, the dominance of the latter factor could explain the reduced release from the CPC/MBG system.
Jani et al. in their work from 2018 [71] proposed a mesoporous silica (MCM-41) and HAp composite (MCM/HA) as a delivery matrix for ibuprofen (IBU). The release behaviour of this matrix was compared with a system made of pure MCM-41. The release test was conducted under an in vitro condition in the PBS solution with a pH of 7.4. The most important conclusion drawn from the comparison of the curves of the release of IBU from MCM-41 and MCM/HA was the greater amount of the released drug from the MCM-41 matrix. The mesoporous silica-based system also displayed a higher burst release that caused the faster depletion of the contained drug during the first 10 h. An almost negligible release was observed for the remaining 60 h. On the other hand, MCM/HA showed a more contained release with a minor burst effect, followed by a decreased but sustained release. The observed burst release can be attributed to the presence of IBU molecules on the matrix surface. FTIR analysis revealed a high concentration of SiOH groups along the pore walls and OH− and COO− groups of IBU could interact with SiOH via hydrogen bonding. Furthermore, the carboxyl group of IBU could interact with the Ca2+ of HAp. The authors suggested that these factors could cause a diminished mobility of IBU within the matrix, with a consequently slower release from the MCM/HA system.
An article from 2013 by Su et al. [72] reported on calcium silicate (CS) modified β-TCP cement as a drug carrier. Formulations with different ratios of CS to β-TCP were taken into account, namely 0:100, 30:70, 50:50, 70:30, and 100:0. In vitro release of fibroblast growth factor-2 (FGF-2) from these matrices was investigated. Degradation of the matrix cement itself, when immersed in SBF solution for predetermined durations of time, was monitored for all the formulations. Inhibition of the degradation rate was found for matrices incorporating higher amounts of CS. It is well known that the accommodation of the silicate group within the CP lattice decreases the surface charge [73] due the formation of Si–OH species at the surface, which in turn enhance the dissolution rate of superficial Si [74]. Further, the formation of a SiO2-rich layer induced the precipitation of Ca2+ and PO43− from the solution, leading to the formation of a HAp layer that partially shields the matrix from the solution [75]. However, the release of FGF-2 over 12 weeks showed desorption rates that increased steadily with the CS content. Interestingly, while the curves retained a similar shape during the first stage of the release, indicating a diffusion-controlled regime, the difference between them began to accrue at longer periods of incubation and the release kinetics changed from the first order to the zeroth order in inverse proportion with the CS content. This result was in agreement with the CPC degradation evidence, suggesting that at lower amounts of CS, liberation of the drug due to a higher dissolution of the cements becomes more prominent. The more controlled drug release from the CS-rich cement is in agreement with the aforementioned process of formation of superficial HAp.
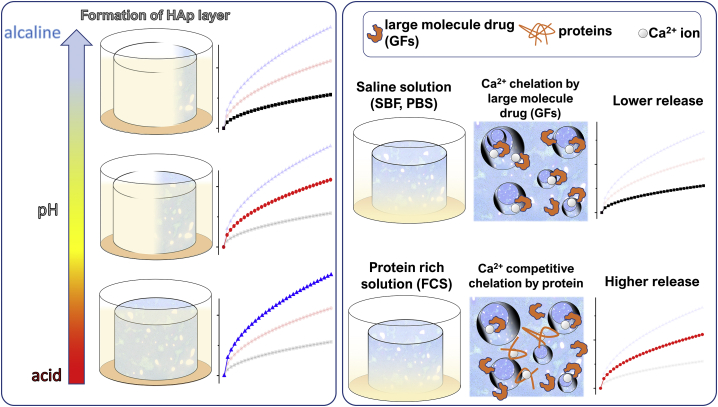
Doping the powder phase with ions presents another strategy for controlling the release rate from CPCs. Many ions capable of accommodating themselves inside the CP lattice deform it and render it more soluble, which, in theory, should make the drug load less stable and more prone to a faster release. A work from 2006 by Ito et al. [76], for example, reported on the results of the experiments aiming to explore the possibility to vary the dissolution rate of β-TCP ceramic by the addition of Zn in amounts ranging between 0 and 10 mol.%. In order to quantify the dissolution fraction of the ceramic, the Ca2+ concentration in the acetate buffer was measured. All samples showed decreased dissolution at higher contents of Zn. Dissolution flux plots suggested that the mechanism governing this process is polynucleation, whereas the equilibrium solubility decreased with the zinc content due to a decrease in the free energy of formation (equivalent to an increase in stability due to the presence of Zn). Strontium is another common cationic dopant in CPs that, like Zn, increased the solubility of TCP-based CPCs [77] and, thus, made them prone to exhibit faster drug release kinetics than pure CPCs.
The effect of strontium doping on the loading and the release of DOXY hyclate from a CPC was investigated in a work from 2009 by Alkhraisat et al. [78]. Three different CPC formulations were selected for this study: β-TCP CPC containing 0, 13.3 or 26.6% of Sr. The pore size and the specific surface area were measured for all the formulations, showing their enhancement in Sr-containing matrices as compared to the Sr-free controls. CPCs were left ageing for 24 h in different drug-containing solutions at low (5 mg/ml) and high (24 mg/ml) concentrations of DOXY. It was observed that the pore size and the specific surface area had a direct effect on drug loading, which was more efficient in Sr-containing cements due to the augmented binding sites and the accessibility of the drug molecules to them. Desorption curves confirmed the efficiency of permeation of the Sr-containing cements with the liquid medium. Saturation of the binding sites occurred for drug concentrations ≥10 mg/ml, with the excess drug molecules oversaturating the cement surface. Higher release was registered during the first 40 h of incubation for samples with 13.3% and 26.7% of Sr relative to the Sr-free control. Release curves were fitted with the power-law equation and the calculated fitting parameter suggested a Fickian transport for the low drug loadings (5 mg/ml) and the anomalous transport for the high drug loadings (24 mg/ml) of DOXY. This could be attributed to the uneven distribution of DOXY molecules across the oversaturated binding sites on the cement surface at high loadings.
The effects of strontium doping on the degradation of a α-TCP bone cement and its release of Sr ion were also the aims of a study reported by Shi et al. [79]. Different Sr-substituted cements were prepared starting from different sources of Sr: Sr-OCP powder (Sr-CPC-O), Sr-αTCP powder (Sr-CPC-S), SrCO3 powder (Sr-CPC-C), and SrCl2 solution (Sr-CPC-L). The cements had the final Ca-to-Sr substitution ratios of 0.2%, 1.0% and 1.5%. Experiments on the release of Ca2+ and Sr2+ ions from all the cements were carried out. Concentrations of the Ca ion in the ageing solution indicated a constant and slow release during the first 14 days followed by a rapid increase in the release throughout the remaining 14 days of monitoring. This was observed for all the cements, with no regard to the Sr content in the cements. The Sr release rate was primary defined by the Sr substitution degree in the CPC. Sr-CPC-C had the least concentration of Sr and it is likely that it contained SrCO3, which decomposed and got reincorporated in the matrix, contributing to the mass gain, as indicated by the experimental results. Sr-CPC-S showed a noticeable increase in the Sr release, probably due to the presence of weakly alkaline compounds in the hydrated cements containing impure β-TCP. Here, β-TCP, which is more soluble than HAp, could promote the ionic uptake and the release of Sr. Fig. 4 schematically summarizes the different effects through which the additives in CPCs affect the rate and the mechanism of drug release.
Fig. 4.
The effect of additive(s) on the release rate: the top right panel illustrates the case of an additive that affects the bonding affinity of the drug for the matrix. The enhanced binding affinity corresponds to a lower release rate, while the reduced binding affinity corresponds to a higher release rate. The bottom right panel illustrates the case of an additive that has a mainly steric effect by undergoing swelling and filling up the matrix pores. In this case, the drug release rate is governed by the polymer relaxation. Once the polymer dissolves, the drug molecules are free to diffuse out and the release rate increases.
5. Drug type
In a work from 2011 by van Staden et al. [80], an experiment was conducted to monitor the release of Enterococcus mundtii bacteriocin ST4SA from a cement made of brushite. The typical first-order kinetic with a high initial burst release was observed. This confirmed the general tendency for the fast release of antibiotics from the CPCs, especially when they are brushite-based. In an early work from 1991 by the group of Otsuka [81], the release of two different antibiotics, cephalexin and norfloxacin, both loaded at 4.8% of the loading efficiency onto a CPC, was compared. Qualitative and quantitative analyses, supplemented with the fitting of the experimental data to Higuchi's model, revealed that a typical Fickian diffusion characterized the release of both drugs, with no significant differences between them.
Monitoring the dual delivery of icariin and vancomycin from a CPC matrix was the object of research reported by Huang et al. in 2013 [82]. In this work, both drugs were loaded simultaneously in the same cement carrier. Once placed in the incubation solution, their concentrations were measured individually for a period of 30 days. Both drugs showed the sustained release for 30 days of monitoring. Although no quantitative analyses were attempted, both release profiles, albeit somewhat peculiar, could be roughly associated with a zeroth order kinetic.
Gentamicin sulphate, amoxicillin and ampicillin trihydrate were chosen to investigate the potential of Mg-substituted brushite cement as a drug carrier in a work by Saleh et al. reported in 2016 [83]. Experimental release curves signified a bimodal release, with the typical initial burst occurring within the first 24 h, followed by a sustained release of the first kinetic order. The measured release rates for the three different drugs were in the following order: gentamicin sulphate > ampicillin trihydrate > amoxicillin. In a 2001 article by Akashi et al. [31], release patterns for three different drugs, namely metronidazole, cefaclor, and ciprofloxacin were observed. Two variants of CPC matrices were used as carriers, and their role in the release behaviour was speculated on elsewhere. Release data were fitted with the Higuchi model, resulting in a diffusional mechanism with a relative high burst release within the first 24 h, followed by a markedly slowed release in later stages for all the examined drugs. Comparison of the release rates revealed that they followed this order: metronidazole > ciprofloxacin > cefaclor. The authors explained this outcome as depending on the chemical nature of the drugs and their interaction with the CPC. The highest rate of release from metronidazole was explained by the absence of the carboxylic acid group, which is present in cefaclor and ciprofloxacin. This group possesses the ability to form a stable complex with Ca ions, thus getting trapped stronger within the CPC scaffold. Moreover, ciprofloxacin dosages contain magnesium stearate as an inactive ingredient, which is able to inhibit α-TCP condensation and retard the setting reaction, therefore favouring the leak of the drug. In a work by Hofmann et al. from 2009 [45], a comparison of the release of vancomycin and ciprofloxacin from a CPC was carried out. No particular difference was found in their release mechanism, which in both cases corresponded to a typical Fickian diffusion. The initial rate was higher for vancomycin and this was explained in terms of the water solubility of vancomycin, which is 10-fold higher than that for ciprofloxacin. Another comparison between vancomycin and ciprofloxacin was performed in an experiment described in two works by the group of Uskoković [38,39]. In these works, both drugs were loaded inside different CPC matrices, indicated as HAp1, HAp2 and their combinations at different ratios, namely 85:15 HAp1:HAp2 and 50:50 HAp1:HAp2. The experimental data were fitted by the power law model, through which n and k parameters were obtained for all the drug-CPC combinations. It was observed that different drugs could have different mechanisms of release from even identical CPC matrices. For example, the mechanism of release of vancomycin gradually changed from the anomalous transport to a more regular Fickian transport as the amount of HAp2 in the CPC matrix increased. On the contrary, when shifting from a more crystalline matrix to a more amorphous matrix, the mechanism of release of ciprofloxacin evolved from the regular Fickian diffusion to a case II super-transport mechanism. By analyzing the results for both drugs released from four different matrices, it was possible to confirm that the release depended on the intimate nature of the interaction interface between the drug and the CPC. The synergetic interactions between the drugs and matrices, strongly affecting the release behaviour, were highlighted.
The work of the Gbureck group from 2013 [52] aimed to elucidate the properties of an injectable bone cement as a drug carrier. The cement was obtained by mixing a CP powder with an oil-based liquid containing a surfactant. Gentamicin and vancomycin were selected as model drugs. Both antibiotics were characterized by an initial release within the range of 7–28%. Afterwards, a square root profile defined the release rate for vancomycin, while gentamicin was released constantly for several weeks. This difference was attributed to the role played by the sulphate counterion, which interferes with the anionic hexadecyl-phosphate surfactant within the pastes, altering the drug solubility. Gentamicin sulphate is more sensitive to this effect than vancomycin because of the less positive charge density of the latter.
The influence on the drug release due to the interaction with ion and counterion species present in the release solution was assessed in a work from 2021 by Pasqual et al. [84]. In this paper, the release of gentamicin sulphate and lidocaine hydrochloride from a TCP-based cement matrix was investigated by means of electrochemical impedance spectroscopy and the results were compared with those of a more common technique, namely UV–Vis spectroscopy. The authors concluded that rate of release of drugs loaded onto the matrix in the form of salts is dependent on the rate of their dissolution in the medium and the subsequent dissociation into an ion and a counterion. Specific interactions between ions formed during drug dissolution with ionic species already present in saline buffer solutions can play a significant role in determining the release mechanisms and shaping of release profile. In a prior study by the same group [85], it was demonstrated that the addition of lidocaine hydrochloride increases the size of the needle- and plate-shaped CDHA crystals in a set TCP-based cement, which in turn accelerates the rate of the drug release. The authors explained this effect by assuming that larger particles allowed for both more copious adsorption and more copious dissolution of the drug molecules from the surface of the cement crystals.
Growth factors represent another class of biochemical compounds requiring local administration and benefiting from a sustained and controlled release. The local controlled release of VEGF and PDGF was investigated in a work reported by De la Riva et al. [58], where a composite matrix composed of brushite and chitosan was proposed as a matrix for drug release. Here, however, it must be taken into account that while PDGF was directly included within the matrix, VEGF was encapsulated in alginate microspheres, which were then introduced into the cement. As a result, VEGF exhibited a very moderate initial burst release in comparison with PDGF: 13% vs. 45%, respectively. Moreover, PDGF ended up getting fully released in 2 weeks, while the encapsulated VEGF showed a constant sustained release for over 35 days. In a work by Habraken et al. from 2008 [86], the release of three different growth factors (recombinant human TGF-b1, bFGF, and BMP-2) from a CPC was investigated by experimenting with different drug loading methods. The first-order release kinetics characterized all of the monitored release experiments. Besides, the release rates of the different growth factors were very similar, regardless of the loading method. The release curves were characterized by the following order for the release rates: bFGF > BMP-2 > TGF-b1. The authors concluded that it would be difficult to provide an explanation able to predict the release kinetics of different growth factors since a lot of factors, such as the isoelectric point, hydration and steric factor, must be taken into account and even then, this would be insufficient to provide a definitive answer. Another work from 2019 by Gunnella et al. [87] focused on the release of three growth factors belonging to the macrofamily of TGF-beta: GDF5, BB-1, and BMP-2. Different doses of each growth factor were loaded onto two different variants of a CPC scaffold, one of which was combined with PLGA fibres. Results of the cumulative release after 30 days of ageing in PBS + FCS/sheep serum showed a high rate of release for BMP-2 (43.7%) followed by GDF5 (22.6%) and BB-1 (13.2%). Diffusional kinetics were observed for all the investigated systems, characterized by a burst release followed by a gradual deceleration of the release rates. The authors ascribed the difference in the rates between the growth factors to different interactions between the matrices and the growth factors, referring in particular to BMP-2 functional groups (hydroxyl, amine, and carboxyl) with a high affinity for CPs. In addition, the steric 3D arrangement of biomolecules could play a significant role in determining the specificities of this interaction. Physicochemical, compositional and spatial organization of the cement across different scales must be taken into account as well.
Based on the results of the reported studies in this section, it is clear that the drug identity can affect the release kinetics in a more or less direct fashion. The drug can interact with CPC matrices, producing slight to noticeable repercussions on the drug dissolution. Some types of drugs possess functional groups able to interact with the cement surface via hydrogen bonding or to chelate Ca2+, as is the case with drugs containing carboxylate groups. These interactions usually delay the release of the drug in a manner that is generally proportional to the intensity of the specific chemical interaction. Another common effect concerns the variations induced to the setting properties. Small drug molecules are especially prone to induce a delay in the setting reaction and a consequent increase in the release rates during the first hours of the release. Another important effect relies on the ability of the drug to induce physicochemical changes in the final cement, such as porosity, crystallinity and specific surface area. This is especially true for the drugs containing the sulphate counterion, which can induce an increase in the final porosity and/or act as a nucleation centre for cement precipitation, thus reducing the crystallinity of the final product and speeding up the release. Fig. 5 schematically summarizes the different effects by which the molecular identity of the drug in CPCs affects the rate and the mechanism of the drug release.
Fig. 5.
The effect of the drug type on the release rate: In the left panel, parameters are reported that act as determinants for the release of small molecules: 1) solubility of the drug (the higher the solubility, the higher the release rate); 2) affinity of the drug for the matrix (the higher the affinity, the lower the release rate); 3) drug interference with the setting process (higher interference degree usually corresponds to a higher release rate). In the right panel, parameters are reported that determine the kinetics of release of large molecules: 1) drug size (the higher the molecular size, the lower the release rate); 2) affinity of the drug for the matrix; 3) boundary conditions of the matrix surface or the release medium (isoelectric point, pH, interfering counterion(s)) can also influence the release rate.
6. Drug concentration
In a classic study by Hamanishi et al. from 1996, the release of different amounts of vancomycin from a TTCP-DCPD cement was monitored for a duration of 80 days [88]. The cement was loaded with 1%, 2% or 5% of vancomycin and its desorption rate was measured in PBS. The end point of the release was found to be related to the loaded amount of vancomycin, equalling 80 days for the 5% load, 40 days for the 2% load and 20 days for the 1% load. Next, Otsuka et al. studied the release of indomethacin at different concentrations and reported the findings in 1997 [89]. A CPC was loaded with 1%, 2% or 5% of indomethacin and the drug release was measured in SBF. In vitro release occurred in the diffusional regime, with absolute rates in mg units being proportional to the loaded drug amounts. A work from 2013 by Rabiee [90] had the study of the release of tetracycline hydrochloride (TCH) from a HAp cement as its goal. Cements loaded with 4 different TCH amounts were investigated. Both the release rate and mechanism were found to be dependent on the drug load, with the rates normalized to the initial drug load and expressed in percentages being directly proportional to the amount of the drug loaded. The kinetic transition from the first order for the high doses to the zeroth order for the low doses was observed, suggesting that for the latter, a dissolution-diffusion mechanism regulates the dissolution. A comparison between the release of vancomycin and amikacin at different drug loads was assessed by Sakamoto et al. [91]. The drugs were directly added to the powder component before the cement mixing. The release curves showed a similar behaviour: in the case of amikacin, the higher cumulative drug release was obtained due to the higher amount of the added drug, while, conversely, for vancomycin, a lower cumulative release took place due to the lower dose of the loaded drug.
The article by Zou et al. from 2007 [92] investigated the release of berberine at different concentrations from a nano-HAp – chitosan (nHAp/CS) composite delivery system. Seven nHAp/CS formulations at different berberine concentrations ranging from 0.1% to 1% were prepared. Sustained release was observed for at least 28 days. Kinetic profiles revealed two stages of the dissolution regime. During the first stage, a burst release was observed, with the release rates being directly dependent on the quantity of the loaded drug. In the second stage, an accelerated drug dissolution was observed, especially for the low doses of the drug. The Higuchi model was used to quantitatively describe the release kinetics. This second stage of the release, from day 14 onwards, showed a deviation from the square root law, especially for the low drug dosages (0.1 and 0.3%). The deviation became less prominent as the initial drug load increased. The authors explained that during the first stage, the kinetic was dominated by the dissolution of the drug adsorbed on the surface of the matrices, which was faster for the high doses of the drug. When this phase was concluded, the degradation of CS determined the kinetics of the second stage. The CS dissolution allowed for the drug molecules embedded within the bulk of the cement to dissolve, and this effect was more pronounced for samples at low drug loads.
In a study by Alkhraisat et al. [78], the uptake of the different loads of DOXY hyclate in Sr-substituted CPCs was carried out. Sr-CPC matrices at different contents of strontium (13.3% and 26.7%) were employed for this study. For these delivery systems, both rates and mechanisms were dependent on the drug load (5 and 24 mg/ml) and the Sr content in the cements. For DOXY hyclate at 5 mg/ml, the release rate and the initial burst generally increased with the Sr content, while an opposite behaviour was observed for the 24 mg/ml load of DOXY hyclate. The desorption data were fitted by the power law and the calculated n values allowed to elucidate the release mechanism. Hence, all CPCs with the low drug dose had n values < 0.45, indicating a Fickian diffusion. On the other hand, for all CPCs with the high dose of the drug, n values ranged around 0.75, suggesting an anomalous transport. This could be the result of the detachment of the fragments of DOXY hyclate adsorbed on the cement surface, which occurs to a greater extent at high concentrations of the drug. A similar approach was adopted for an article from 2020 by Lucas-Aparicio et al. [53]. A series of experiments was conducted to assess the release of vancomycin at the concentrations of 5 and 10 mg/ml from Si-substituted CPCs, with the [Si/(Si + P)] atomic ratios of 40% and 80%. Moreover, two loading methods were investigated, but the effects of the drug amount became relevant only for one of these methods, which involved the solid drug addition in the amount of 8.4 mg during the cement preparation stage. The decrease of the drug content generally led to a slower and prolonged release, along with a minor burst. The Korsmeyers-Peppas fit revealed a diffusional mechanism for low drug dosages and an anomalous transport for the high dosages. This behaviour was ascribed to the supersaturation of the drug in the high-dose case, inducing the slowing of the release. In the case of the cement with 80% of Si, a radical change of the kinetic mode was observed, passing from the first order for the cement loaded with 100 mg of vancomycin to an ideal zeroth order for the cement with 8.4 mg of vancomycin.
Different concentrations of cephalexin monohydrate (CMH), including 5 and 10 wt%, were loaded into modified CPC matrices in a work by Hesaraki et al. [50]. A TTCP-based powder was added with variable amounts of SDS as a surfactant and the CMH release was investigated. The Higuchi model fit was performed, revealing that in this specific case, the drug loading played a minor role compared to the amount of the surfactant within the cements, which strongly determined the kinetic and the mechanism of release. In a work from 2012 [93] by Montazerolghaem et al., a sustained release of simvastin (SVA) from an acidic CPC was demonstrated. Different doses of the solid drug (1, 0.5, 0.25, and 0 mg SVA/g cement) were added to a CP powder, which was mixed with a setting liquid to form a CPC drug delivery system. Cumulative release rates were inversely proportional to the drug dose. The power law was used to fit the data, and for all the SVA doses, the n value of circa 0.53 was obtained, suggesting an anomalous transport as the mechanism of release. The anomalous Fickian kinetic was attributed to changes in the microstructure that occurred during the final stage of the cement setting. Also, the k values were obtained and they showed an inverse correlation with respect to the drug load. This aspect was not discussed within this work, but the results obtained in similar experiments allow to hypothesize that the high doses of SVA could be associated with a local supersaturation and the consequent slowing down of the release. In a recent work from 2018 by van Houdt et al. [94], a high dose (5 wt%) and a low dose (0.5 wt%) of alendronate (ALN) were used to investigate the drug release from composites composed of 60 wt% CPC and 40 wt% PLGA. A sustained release was observed for more than 150 days for both the high and the low drug dose. The cumulative percent release rate was appreciably higher for the high ALN dose system. The authors concluded that the high dose of ALN could hamper the conversion of the initial TCP to the final HAp phase, retarding the hardening process, thus allowing for a higher amount of the drug to be released.
In the three works by Blom et al. [[95], [96], [97]], a similar experimental approach was adopted in order to investigate the release properties of systems consisting of rhTGF-b1 growth factor as the drug and CPC as the drug carrier. Experiments were carried out by admixing rhTGF-b1 in different loads: 5, 10, 20, 66, and 133 ng to the fixed amount of the powder component of the CPC followed by mixing with the liquid phase in order to trigger the setting reaction. Afterwards, the release of rhTGF-b1 in the cell culture medium was monitored for 48 h. It was demonstrated that the release rate of rhTGF-b1 could be directly modulated by changing the loaded amount of the growth factor. This is because a direct correlation was found between the loaded growth factor amount and the respective release rate. The experimental data also highlighted that the release took place from the surface of the CPC matrix only and but a modest quantity of rhTGF-b1 (2%) was released during the monitored 48 h. For all the growth factor loads, a burst release occurred within the first few hours of the release, which was followed by a marked slowdown of the dissolution, with the kinetics being governed by the diffusion from the matrix surface.
Habraken et al. [86] analyzed the release of different growth factors from a CPC, including TGF-b1, bFGF, and BMP-2. The BMP-2 release was also monitored for different amounts of this growth factor loaded onto a fixed amount of the cement: 50 ng, 500 ng, and 5 μg. The study confirmed that the carrier systems at different growth factor loads presented the same release profile, characterized by an initial burst release with a consequently slower release, for a total period of 45 days. Therefore, the profiles indicated that different releases of the growth factors proceeded with the same kinetic mode and mechanism and only differed in magnitude, showing a direct correlation between the growth factor load and its release rate. A recent work by Gunnella et al. [87] focused on the release of bone morphogenetic proteins GDF5, BB-1, and BMP-2 from a brushite cement, where GDF5 and BB-1 growth factors were mixed with the cement at four different concentrations: 2, 10, 200, 1000 μg/ml, considering 2 and 10 μg/ml as the low doses and 200 and 1000 μg/ml as the high doses. In this case too, the cumulative absolute release patterns indicated that the release magnitude was strictly dependent on the growth factor load. Moreover, the kinetics was similar for all the patterns, suggesting a diffusional mechanism of release from the cement surface. Nevertheless, the trends in the cumulative percentual release showed that the low growth factor dose systems were able to release higher relative quantities of the drug with a parallel increment in the initial burst release, which decreased from 30% (GDF5) and 15% (BB-1) for the 2 μg/ml dosage to 10% (GDF5) and 5% (BB-1) for the 1000 μg/ml dosage.
Cements were prepared with TCP substituted with 6.7, 20.0 or 33.3 atomic % of strontium in a study by Alkhraisat et al. from 2008 [98]. Measurements of the release of the Sr2+ ion from cements in an ageing medium were carried out. The initial burst release was observed for all the cements, ranging within 38–58 ppm depending on the Sr2+ content. At later stages, the curve became characterized by a temporary decrease at day 3 of incubation, followed by a sustained and constant, zeroth order release ranging between 18 and 30 ppm of Sr2+ for the remaining 14 days. The latter could be the consequence of the higher release rate from secondary CP phases whose formation was influenced by the Sr content. Concordant outcomes resulted from a work from 2016 by Singh et al. where the DPCD cement precursor was substituted with 5 and 10 mol.% of strontium in order to assess the release of the Sr2+ ion within the ageing medium [99]. Sr2+ concentrations were measured for 15 days during the viability assay of MC3T3-E1 cells in the culture media. A sustained release was detected for both Sr-containing cements, with the daily release of about 0.02 mM for the 5 mol.% Sr CPC and 0.05 mM for the 10 mol.% Sr CPC. Therefore, a zeroth order kinetic can be hypothesized for both cements, with release rates depending only on the substitution ratio. Another study from 2016 by Jayasree et al. [100] reported results on the release of Sr2+ from a HAp cement. The CPC with four different concentrations of Sr (3, 5, 8, 10 mol.%) along with a Sr-free cement were employed as ion-release scaffolds. Release experiments were conducted in PBS medium and the cumulative ion release curves were characterized by a very similar shape, regardless of the initial Sr concentration. Thus, after 6 weeks, the Sr2+ concentration in the culture medium had a value of 3.2–3.5 ppm for all the cement formulations. Even though no fitting to the experimental data was applied, all of the release patterns were characterized by an apparent first-order kinetic. In a work from 2016, Rau et al. [101] investigated the Ag+ ion release from Ag-substituted TCP-based cement matrices immersed for 30 days in Tris-HCl buffer solution. Matrices with two concentrations of Ag+, namely 0.6 wt% and 1.0 wt%, were selected for the release assay. The reported dissolution patterns showed a direct relationship between the release rate and the Ag+ concentration in the matrix, where the release rate of the sample with 1.0 wt% was higher than that of the sample with 0.6 wt%. Controlled release with no burst effect and with the first order kinetic characterized the Ag+ release. Another work by the same authors was focused on the dissolution rate of Zn [102]. Zn–CPCs at 0.6 and 1.2 wt% of Zn were compared and the highest dissolution rate was found for the cement with the lowest Zn concentration (0.6 wt%). This result is in accordance with the work of Ito et al. [76] where the release of Zn from CPCs decreased as the content of Zn in them increased. Clearly, the increased presence of the zinc impurity leads to a higher thermodynamic stability of the lattices forming the cements, thanks to which the release of zinc becomes hindered at its higher concentrations in the cement. Fig. 6 schematically summarizes how the concentration of the drug in the CPC carrier affects the rate and the mechanism of the drug release.
Fig. 6.
The effect of the amount or the concentration of the loaded drug on the release rate. The top left panel reports the general case with the drug directly admixed to the powder or the liquid component of the cement. In this case, the higher amount of the drug corresponds to a higher and prolonged release. The top right panel shows the case of local supersaturation of the drug within the matrix. In this case, the release rate is higher for the lower drug loads. The bottom left panel shows the case of the drug adsorbed onto the matrix surface. The higher amount of the drug corresponds to a higher and shorter release with a possible anomalous transport. The bottom right panel reports the case of inclusion of a polymeric additive in the matrix. The higher load corresponds to a higher release rate and a faster depletion, but when the polymer starts to degrade, the matrix with a lower drug load (which has retained the drug load until then) starts to release the remaining drug with a possible secondary burst.
7. Drug loading method
Habraken et al. [103] explored the effect of the drug entrapment within the microspheres of gelatin or PLGA in order to provide an enhanced control of the drug release. In this study [103], bovine serum albumin (BSA) was used as a model drug and loaded within the PLGA microspheres in two different ways: as included inside the microspheres in the double emulsion process (BSA(I)) or as adsorbed onto the microspheres in a freeze-drying process (BSA(I)). The microspheres were successively embedded within CPC matrices, creating CPC-BSA(A) and CPC-BSA(I) composites. A further sample was prepared by directly adding BSA to the CPC as a control specimen. Both CPC-BSA composites and the CPC-BSA control showed significantly decreased release rates and the initial bursts compared to the dissolution of the drug directly from PLGA microspheres. The rate of release from CPC/PLGA BSA(A) was also lower than that from the control CPC-BSA sample. The explanation for this trend could be related to the decrease in pH consequential to the degradation of PLGA microspheres, producing an acidic environment. This change in the pH could affect the surface charge of the cement, resulting in a stronger electrostatic bond between BSA molecules and the cement surface, which hindered the mobility and diffusivity of the drug within the cement matrix. Another work by the same group [86] employed a similar procedure to assess the release of different growth factors (TGF-b1, bFGF, and BMP-2) previously loaded into two different types of gelatine microspheres: GEL A (porcine) and GEL B (bovine). In addition, for each gelatine type, two loading methods were used: 1) the instant loading of growth factors into the microspheres, and 2) the prolonged loading. Once obtained, the different microspheres were mixed with the powder phase to form different CPC composites. GEL A was introduced into the CPC matrix at 5 and 10 wt%. Moreover, control samples were created by either the adsorption of the growth factors onto the CPC soaked in the solution or by the dissolution of BMP-2 into the hardening liquid prior to the cement mixing. Release patterns depicted a higher release for the former sample where BMP-2 was pre-adsorbed onto the CPC surface than for the latter sample where BMP-2 was incorporated directly within the matrix. The higher initial burst and release rate suggested that BMP-2 release was governed by diffusion from the CPC surface, while a slower diffusion from the bulk defined the kinetics of the release for BMP-2 introduced through the hardening liquid. Another experiment confirmed the difference between the instant and the prolonged loading of the growth factors inside GEL-A and GEL-B microspheres. Similar behaviour was observed for all the combinations of growth factors, GEL-types and instant vs. prolonged loading time. Specifically, for BMP-2 and TGF-beta, a burst release of about 5–6% was observed, followed by a sustained and constant release stage whose profile resembled the zeroth order kinetic. Higher release rates were observed for GEL-A in combination with the prolonged loading method. bFGF was characterized by higher release rates and, interestingly, when released from the GEL-B composites, the shapes of the release pattern were more similar to a first-order kinetic. Moreover, for the latter case, the instant loading method assured a slightly higher release rate compared to the prolonged loading method. Finally, a comprehensive investigation performed on BMP-2 demonstrated that modulating the type of gelatine and the relative wt% within the composites can be the means to tuning the release rate of growth factors, certain limitations notwithstanding.
PLGA-CPC composites and their release characteristics were investigated also by Schnieders et al. [104]. The release from PLGA microspheres and CPC-microsphere composites was investigated and gentamicin crobefate was selected as the model drug. The drug release from gelatine microspheres proceeded in three well-defined stages, consisting of a burst release, a plateau and a sustained release determined by PLGA degradation and the consequent drug leak. When the drug-loaded microspheres were included into the CPC cement, the release curves indicated a drastic change in the kinetics and the mechanism of release. The burst release strongly decreased and a zeroth order kinetic characterized the patterns, with the constant release rate being sustained for over 100 days. The authors proposed the embedment of the microspheres within the cement and the partial adsorption of the drug molecules onto the matrix surface as possible explanations for this behaviour. These systems were further investigated by the same group in Ref. [46]. Vancomycin-HCl was encapsulated in PLGA microspheres and successively incorporated inside CPCs to form a composite system for controlled drug release. For this study, various CPC matrices at different P/L ratios were prepared in order to obtain different porosities and assess their effects. A comparison between the regular cements and cements prepared by adding the PLGA microspheres containing vancomycin to the liquid phase was reported. The effects of the P/L ratio and the porosity have been discussed earlier in this review. Here, it can be stressed that for the cement prepared via the direct addition of the drug to the liquid phase, the release rate and the kinetic were strictly dependent on the P/L parameter and therefore on porosity. For the cement containing PLGA microspheres loaded with the drug, this conclusion was no longer valid, suggesting that the kinetics-determining stage of the release was the diffusion of the drug from the microspheres instead of from the matrix pores. The encapsulation of the drug inside PLGA microspheres was also proposed as a loading method by the group of Otsuka in one of their works [105]. Simvastatin (SIM) loaded into PLGA microspheres (SPLGAMs) and successively embedded in carbonated hydroxyapatite (CHAp) cement was used to obtain the SPLGAMs/CHAp composite. A control sample was prepared by adding SIM to the cement paste (SIM/CHAp). The release profiles for SIM were obtained from three different systems: SPLGAMs, SIM/CHAp, and SPLGAMs/CHAp. The release rate followed this order: SIM/CHAp > SPLGAMs > SPLGAMs/CHAp. All the formulations exhibited a nearly zeroth order kinetic. Four fitting models were employed to elucidate the release mechanisms: zeroth and first order, Higuchi and Korsmeyer-Peppas. By analysing the fits, it was inferred that SPLGAMs and SPLGAMs/CHAp systems were characterized by a case-II transport mechanism controlled by degradation and relaxation of PLGA, while SIM/CHAp release was controlled by the initial SIM diffusion from the surface of CHAp and continuous diffusion from the core to the surface of CHAp. Moreover, the zeroth order model described the SPLGAMs/CHAp kinetic as well as the Korsmeyer-Peppas model did.
A system for the controlled release of IBU from a composite comprising pectin microspheres (LMAP) in a CPC (CP-LMAP) was proposed in Ref. [44]. Composites prepared with different amounts of LMAP (0%, 2%, 4%, 6% w/w) were considered. A reference sample was also prepared by the direct incorporation of the drug inside the cement (CP/IBU). The release profiles of 2%, 4% and 6% CP-LMAP composites were compared and the fit analysis was accomplished using the Higuchi model. All the composites were characterized by the Fickian diffusion mechanism, while the release rates were inversely proportional to the amount of pectin microspheres: CP/IBU–LMAP2% > CP/IBU–LMAP4% > CP/IBU–LMAP6%. The authors proposed two possible explanations: the first consisted in the possibility of local supersaturation at high amounts of the drug, which could slow down the dissolution process. The second, backed by some literature evidence, asserted that the release rate should decrease proportionally to the amount of polysaccharide present in the cement composite because of the capacity of the latter to form a gel, which can fill up the pores of the matrix, thus blocking the drug diffusion.
Dolci et al. proposed spray-congealed solid lipid microparticles as a loading method for alendronate release from a CPC matrix [106,107]. Alendronate was encapsulated within various microspheres prepared with five different lipid excipients. Results from the setting time and the compressive strength experiments led to a selection of only two suitable excipients out of the five tested: cutina and precirol. The release of alendronate was performed from CPC-microsphere composites prepared using these two excipients. In Ref. [106], the release rates of these two systems were reported and compared to the release profile of alendronate from the microspheres incubated in the release medium. Drug encapsulation in lipid microspheres greatly reduced the burst dissolution and yielded a prolonged and sustained release characterized by the Fickian diffusion for up to 21 days. Composites made with precirol microspheres showed a modestly higher release compared to that achieved with cutine microspheres. In another work of the same group [107], different amounts of cutine and precirol microspheres were embedded inside the cement. Results of the release tests allowed to conclude that both systems were suitable for the sustained release up to 21 days, with the absence on any burst stage. Moreover, the release rates could be easily modulated by the amount of microspheres loaded within the composite. Drug release from the systems loaded with one-component or two-component methods were reported in a paper from 2013 by Vorndran et al. [52]. In this work, gentamicin and vancomycin were loaded into a cement prepared by mixing a CP powder with K2PO4 dispersed in an oil-based suspension. Drugs were added by either (1) mixing solid antibiotics with the cement paste, or (2) dissolving them in aqueous solutions successively mixed with the cement paste. All formulations were characterized by the sustained release throughout a 60-day period. The Korsmeyer-Peppas analysis indicated that the one-component cement loading led to a release controlled by a diffusional process with the n value ranging within 0.20–0.39, while the two-component cement showed an anomalous transport that implied diffusion and degradation processes (n = 0.53–0.63). It was hypothesized that while the one-component release mechanism was related to the dissolution of the solid drug added to the cement paste, the two-component cement underwent the dispersion of the aqueous liquid that contained the drug, facilitating an anomalous transport mechanism. In a work from 2010 by Alkhraisat et al. [78], desorption of DOXY hyclate from a Sr-substituted CPC was analyzed. DOXY hyclate was loaded into the cement following two methods: 1) adsorption by the incubation of the cement in an antibiotic-containing solution, or 2) by using an antibiotic solution as the liquid phase of the cement. As expected, in the cement with the drug adsorbed from the solution, most of the drug was located on the surface and a fast and almost complete release of the drug occurred within few hours of the desorption. Conversely, a lower burst release and prolonged continuous release characterized the cement set with the DOXY hyclate solution. The power law model was used to assess the release mechanism. The authors discussed only n values, while the comparison of the k parameter (20–43 for adsorbed DOXY hyclate and 9–20 for DOXY hyclate in the setting solution) was left unaddressed, even though it agrees with the experimental data, confirming the more hampered dissolution of the drug from the bulk of the cements for the second loading method. A similar concept was adopted in a recent work by Lucas-Aparicio et al. [53] where vancomycin was loaded into a silicon-CPC by the following two ways: 1) adsorption of the drug from a drug-containing solution or 2) loading the drug in a solid state during the cement preparation stage. Due to the fact that this work attempted to assess the effects of multiple factors on the drug release, including the Si content within the cement matrix, the drug concentration and the loading method, it must be premised that in the frame of such an exhaustive experimental design, it was hard to isolate the effects caused by one variable at a time. Particularly, the effect of the loading method seemed to be strictly related to the drug concentration (or amount). In the case of the low drug dose, the cement loaded by adsorption displayed release profiles with a lesser burst effect and a more controlled release of the drug. However, the total amount of the released drug was considerably lower compared to the cement with the drug added in the solid state. The opposite behaviour was observed for the cement containing a high dose of the drug. In this case, the cements with the direct solid drug addition were characterized by a higher burst release and a fast depletion of the drug. The Korsmeyer-Peppas model was used to fit the release data and for the low drug dose, k values significantly changed in relation to the loading method, whereas n values did not show significant variation. Conversely, for the high drug dose, a significant variation was registered for n values, along with a more modest variation in k values. Fig. 7 schematically depicts the ways in which the drug-loading method can affect the rate and the mechanism of the drug release.
Fig. 7.
The effect of the drug loading method on the release rate. Top left and bottom left panels report the comparison of the drug directly admixed with the powder or the liquid component of the cement with the drug adsorbed onto the matrix surface by incubation in the solution. In the first case, the kinetic is governed by diffusion from the pores within the matrix bulk, leading to a moderate burst effect. In the second case, the kinetic is determined by the fast dissolution of the drug from the matrix surface, with a higher burst release and possibly anomalous transport. Right top and bottom panels report the comparison between the drug loaded directly onto the matrix surface and the drug first loaded inside polymeric microspheres and subsequently embedded inside the cement matrix. In the second case, a decremented diffusion is observed with the kinetic that can be adjusted to the zeroth order release.
8. Release Medium
The importance of the release medium for the drug kinetic profile is often underestimated, but like in the game of “scissor-rock-paper”, it is strictly connected to the matrix and the drug factor and, therefore, plays an equally pivotal role in defining the drug release behaviour. Different authors have investigated in their works the correlations between the release kinetics and the features of the release medium, ranging from its chemical composition to polarity to pH and so on. Hamanishi et al. [88] reported on a TTCP-DCPD cement as a carrier for PLGA and attempted to address the effects of multiple factors on kinetic properties. Among them, they investigated the difference in drug release from the set, poorly crystalline apatite cements in two different release media, PBS and SBF, for a period of over 7 days. It was observed that for the first 3 days, the release was faster in SBF, but after day 3, the release rate became higher in PBS and this condition remained constant for the remaining duration of the experiment. The authors hypothesized that after 3 days of incubation in SBF, CP reprecipitation occurred on the cement surface, reducing the dimensions of the pores in the cement matrix and thus hindering the drug diffusion. In a study from 1999 by Khairoun et al. [108], a CPC incorporating a glass-release system was produced and the controlled release of Na+ and Ca2+ ions from it was observed. The CPC-glass samples were immersed and aged for about 5 weeks in two different media: 0.9% NaCl solution and 0.5% CaCl2 solution. A phase composition investigation by means of XRD showed that in both cases, brushite was formed at the end of the ageing process, but its presence was higher in the case of ageing in CaCl2 solution, proving that in addition to the release of the Ca2+ ion from glass, precipitation of the same ion from the solution occurred without modifying the release rate (brushite phase was well detectable after 5 weeks in both cases). In a work from 2008 by Tamimi et al. [109], DOXY was selected for the sustained release study using a brushite cement as a carrier. For this experiment, a DOXY-loaded brushite cement was incubated in the PBS solution for over 200 h. DOXY is an antibiotic known for being highly soluble in acidic environments, hence the authors tested the effect of the solution pH on the release pattern. The study was arranged to make sure that the beginning of the incubation period coincided with the start of the setting process. During the first stage of the brushite setting reaction, pH remained at low values, inducing a burst release of DOXY. However, as the setting reaction proceeded, pH value increased and stabilized at 7.4, with the consequent slowing of the release. The cumulative release curve yielded a typical diffusion-controlled profile with a total released amount of DOXY of about 75% in the first 100 h. To test the effect of the pH of the release medium on the remaining 25% of the drug still embedded in the cement matrix, pH of the solution was adjusted to 5.9. Under this new condition, reactivation of the release process was observed until a complete depletion of the drug load was achieved, following an identical release mechanism as earlier, with no modification of the pattern shape.
The group of Otsuka conducted several experiments in order to gain an insight into the conditions that influence the release of different substances from CPC matrices. Specifically, their setup for studying the influence of the release medium was inspired by the pH conditions created by osteoclasts and osteoblasts. These two conditions were reproduced by an acetate buffer medium at pH 4.5 and SBF at pH 7.8, respectively. In all of their papers, different systems were investigated: vitamin K2 release from an apatite/collagen cement [49], simvastin dissolution from apatite cements [110], and DNA-complex release from an injectable apatite cement [111]. All these studies have had in common the same method for determination of the influence of the release medium on drug dissolution. Moreover, coherent results were achieved for all these experiments, where higher doses of the drugs were released in the acidic milieu of the acetate buffer mimicking the osteoclastic condition than in the alkaline milieu of the SBF mimicking the osteoblastic condition. This behaviour was attributed to the capacity of the SBF solution to allow CP precipitation on the cement surface with the consequent obstruction of the surface pores and hampered drug transit from the matrix surface to the solution.
A peculiar work from 2018 by Shi et al. [112] reported on the results obtained by influencing the release of gentamicin from a CPC by means of the use of a low-intensity pulsed ultrasound (LIPUS). Here, the authors explored conditions that are more related to the fluid dynamics rather than to the chemical characteristics of the release medium itself. Despite the similarity between the release patterns obtained with and without LIPUS, a higher release rate of the drug was observed under the LIPUS regime. Two possible explanations were suggested by the authors: 1) the formation of microstreaming due the alternance of high and low pressure in the release medium, which induces cavitation, 2) the possible local increase of temperature driven by the ultrasound. In a paper from 2006 [113], Ruhè et al. characterized the release of rhBMP-2 growth factor from a CPC pretreated with albumin. A comparison of the rhBMP-2 release was made using PBS or fetal calf serum (FCS) solutions as media. In vitro retention of the growth factor was found to be higher in PBS than in FCS, but except for the total released doses, both systems shared a similar behaviour typical of the diffusion-controlled regime. Although it was difficult to provide a definitive explanation for this effect, the authors cautiously suggested that when a rich protein buffer is used as a medium, the contained proteins could compete with rhBMP-2 for the formation of a calcium complex, which could improve the dissolution of rhBMP-2 from the matrix surface. No studies exploring the effects of cell culture on the release kinetics have been reported in the literature and it is uncertain whether the interaction with the cells would decelerate or accelerate the release and whether the effect would be dependent on the nature of the cells (fibroblasts, osteoblasts, osteoclasts, etc.). Fig. 8 schematically summarizes the different effects by which the release medium controls the rate and the mechanism of the drug release.
Fig. 8.
The effect of the release medium on the release rate: in the left panel, the case of a solution composed of saline buffers (SBF, PBS) is reported. In this case, alkaline solution pH can lead to precipitation and deposition of an apatitic layer on the matrix surface, creating a barrier for the drug diffusion from the cement bulk, thus decrementing the release rate. In the right panel, the case of large molecules (e.g., growth factors) with functional groups able to form complexes with Ca2+ is reported. This effect usually decreases the release rate, but when protein or nutrient enriched solutions (FCS, DMEM) are used, a competitive chelation of calcium ions can occur, thus reducing the number of binding sites for drug molecules, with a consequent increase of the release rate.
9. In vivo release
The release of rhBMP-2 loaded into a porous CPC pretreated with albumin was assessed under in vivo conditions by Ruhè et al. [113]. Experimental release curves were assessed in the rat model by scintigraphic imaging of radiolabeled scaffolds through in vivo and ex vivo gamma counting. Retention plots were reported for rhBMP-2 injected as a subcutaneous depot and for the growth factor loaded into porous CPC discs (with or without the albumin pretreatment) implanted subcutaneously in the back of the rats. In vivo kinetics of the release of rhBMP-2 from the CPC discs exhibited the one-phase exponential trend in the rat ectopic model, characterized by the retention of 20–30% after 28 days. The difference subsisted between the release from the untreated and the albumin-pretreated cements, with their final retentions being 16 ± 10% and 31 ± 5%, respectively. Nevertheless, the authors did not consider this difference to be significant, due to the assumption that the life cycle of rhBMP-2 lasts no more than four weeks and no bioactivity should be expected for longer time periods. In a work by Stallmann et al. from 2008 [114], the release of human lactoferrin 1-11 (hLF1-11) from a CPC carrier was reported. The CPC with a 50 mg/g hLF1-11 load was injected into the femoral canal of 12 rabbits. On days 1, 3 and 7, four rabbits were terminated and the femora were surgically removed for histological analyses and semiquantitative determination of the hLF1-11 concentration by means of the liquid chromatography and mass spectrometry. An initial burst release of hLF1-11 was followed by a constant decrease of the released amount. A late, slightly renewed release was observed on day 7. The crack formation and degradation of the cement, observed in the histologic analysis of specimens on day 7, could explain this release behaviour.
In a work by de la Riva et al. [58], comparative in vitro and in vivo release experiments were conducted in order to define the differences between these two drug delivery modalities. VEGF and PDGF growth factors were included in a combined brushite–chitosan delivery system. In vivo release was assessed by first injecting the cement, previously γ-irradiated with a dose of 25 kGy from a 60Co source, within the femoral bone of rabbits and then dividing the animals to different groups and removing the implants from the extracted femurs. PDGF and VEGF quantification was obtained by means of radioactivity measurements of the extracted implants. Cumulative release patterns in vivo showed a first-order kinetic, suggesting that for both growth factors, a diffusional mechanism was involved. The total released amounts for both growth factors ranged within 65–75%. However, while it took 28 days for this maximal total release to be reached for VEGF, it took only 14 days for it to be reached for PDGF. An investigation of growth factor concentrations in different areas of the implant as a function of time revealed that the maximum daily release occurred by day 3 for PDGF in the implant area, and by day 7 for VEGF. After these time points, a decrease in concentration was observed, suggesting that the growth factors diffused into the surrounding bone.
A similar approach was adopted in another study from 2012, by Delgado et al. [115]. A biodegradable CPC/polymer composite was prepared as a local in vivo delivery system for PDGF. In vivo PDGF release and tissue distribution were monitored after the implantation into femurs of rabbits using 125I-PDGF. Cumulative 125I-PDGF release profile was characterized by a sustained release for over 10 days, with an initial burst of about 40% of the initial dose of PDGF and the overall profile typified by a nearly first-order kinetic. PDGF levels were higher at the defect site and remained almost constant for about 5 days. From day 6, the 125I-PDGF concentration started to decrease for the rest of the observation period and lower levels of the growth factor were found in the surrounding tissue (around 1 ng/g of tissue in distal diaphysis).
Chitosan/CPC composite as a carrier system for in vivo conditions was also proposed by Liu et al. [116]. HAp/CS scaffolds were prepared to assess the in vivo release of the antibacterial lysostaphin enzyme. The drug-loaded scaffolds were implanted in rats subcutaneously. In vivo release of lysostaphin was measured using Qdot 625 ITK carboxyl quantum dots as the fluorescence label. The fluorescence signal was registered via an imaging system for 21 days. A control group was injected with lysostaphin and without the composite scaffold. The release of Qdot-labelled lysostaphin proceeded very fast, with a sustained dispersion within 24 h from the injection. On the contrary, for groups with implanted scaffolds loaded with the drug, a more controlled and prolonged release of Qdot-labelled lysostaphin for up to 21 days was observed.
In a work by Uchida et al. from 2018 [40], an interesting comparison between systems for the local delivery of vancomycin was proposed. The compared scaffold systems consisted either of a CPC of or PMMA. Both scaffolds were implanted inside Winstar rats in the femoral zone. The residual vancomycin was extracted from the removed test specimens on days 1, 7, 28, and 56 to determine the amount of the antibiotic released into the rat tissues. In vivo release profiles showed a significantly higher release of vancomycin from the CPC-based scaffold, with a prolonged duration of release from this scaffold too, as compared to the PMMA one. More importantly, by day 56, the CPC scaffold was still able to release 100 μg of vancomycin, whereas no detectable quantities were released from PMMA. The latter evidence is particularly relevant after taking into account that the minimum inhibitory concentration for vancomycin is 1.56 μg per g of tissue.
Some general considerations can be drawn from the comparison of in-vitro and in-vivo studies. The most important underlies the inadequacy of in-vitro models to accurately represent the complexity of in-vivo conditions. The complex conditions that characterize the in-vivo environments affect the drug release rate and mechanism in different ways. Most in-vitro experiments are characterized by a nearly steady-state or controlled evolving conditions. During in-vitro experiments, the release medium is generally kept stationary or under stirring and refreshed at established intervals. Despite the common assumption that this can be used to emulate the physiological conditions, this approach is usually not sufficient to reproduce the real hydrodynamic process within living animal models. The difference in wettability, biofluid perfusion within the matrix and continuous replacement with the fresh physiological fluid can accelerate or delay the release process. Whereas the release in stationary media commonly produces saturation effects, which hamper the release rate, such effects are mitigated under in-vivo conditions typified by a profuse fluid flow. Moreover, even the most moderate changes in environmental conditions are sufficient to prompt a faster or slower degradation of CPC matrices, with a consequent variation in the drug release kinetics and mechanisms. In order to exert an enhanced control over these environmental factors and correctly predict the drug levels within the tissue over time, different strategies have been adopted, conforming to the type of the released drug and the conditions characterizing the implantation site. They include the tuning of the matrix porosity to a suitable level, encapsulation of the drug inside polymeric microspheres and the pre-treatment of the matrix with various additives. Finally, the physiological fluid can chemically interact with the matrix-drug system and interfere with the setting process, thus shortening or prolonging the setting time, which in turn can affect the release profile. Chemical interactions of the biofluid with the drug release systems also consist in the affinity of the biological components of the blood plasma (mostly proteins) for the binding sites of the CPC matrices. In these cases, the exogenous drug and the endogenous proteins can compete for the occupation of the active site of the matrix, which usually results in an enhanced drug release. Interactions with the cells can lead to a variety of repercussions on the drug release, ranging from a hindered release as in the case of hypothetic engulfment with the fibrotic tissue to an accelerated one as in the case of the engulfment by osteoclasts or other macrophage-like cells specializing in CP matrix degradation and particle uptake.
10. Conclusions
Advances in the bone regeneration field have led to continuous innovative approaches accompanied by the use of novel formulations and combinations of materials able to improve the regenerative osteogenic process. In this frame, CPC-drug release systems play a key role as a part of the bone tissue engineering paradigm, acting as matrix scaffolds with mechanical, chemical and microstructural characteristics highly similar to those of the natural bone. Alongside this fundamental congruence, the capacity to host and release molecules necessary for the osteogenic process and the prevention of inflammatory states and/or infections is also required. Functional drug release tailored for a specific application requires a full understanding of the kinetic and mechanistic characteristics of these systems. Models describing how the individual parameters of these systems affect the release profiles are also required, especially since the CPC-drugs are complex systems where different factors can influence the release pattern, alone and/or in synergy. Most of the time these factors, such as the ones considered in this work, act synergistically, producing entangled effects, which are hard to discriminate. Still, in this work we have managed to discern the most essential factors controlling the release of drugs from CPCs and the text has been structured accordingly.
The host matrix is the most essential and complex part of these release systems and its chemical nature, degradation propensity and microstructural features represent the most important characteristics that control the drug release profiles. On the whole, the setting and the hardening of the cement influence the release rate. Generally, fresh cements, mainly composed of the amorphous phase, possess a higher rate of release governed by the anomalous transport and changeable behaviour, where the set cements with a well-defined microstructure show more stable release patterns with profiles governed by the diffusional regime. Degradable matrices allow for achieving an increased release rate, due to the drug being released together with the detached matrix fragments. For this reason, cements setting in the form of more soluble brushite tend to release their drug loads faster than the cements setting in the form of less soluble HAp, although other factors at play may reverse this trend. Stable or slowly degradable matrices further display release patterns that are kinetically and mechanistically governed by the cement microstructure. Crystallinity, crystalline grain dimensions, grain boundary properties, grain entanglements, but also porosity, pore geometry, tortuosity, interconnectedness and distribution, as well as the specific surface area strongly influence the drug release rate. Porosity can be tuned with the use of different approaches, such as acting on the P/L ratio or through the use of appropriate porogens or surfactants. Another way to enhance the porosity of the matrix involves the use of biodegradable polymers, whose dissolution is expected to leave empty cavities within the CPC matrix, thus opening up channels for the more expedient release of the drug. The use of this approach does not always lead to an increase in the release rate due to the fact that prior to dissolution, the polymer in contact with the release medium could swell and form a gel that fills up the open pores, which would slow down the release of the drug. When this process occurs, the release rate becomes governed first by the dissolution and relaxation of the polymeric gel and only then by the drug diffusion from the matrix bulk, resulting in a generally hampered release.
The use of additives in a CPC matrix can influence the affinity of the drug for the internal surface of the matrix by producing local variations in the pH, surface charge, chemical affinity or other parameters. According to the type and quantity of the introduced additive, some of which have included chitosan, PLGA or carbon nanotubes, the drug/CPC binding strength could be increased or decreased, causing corresponding changes in the release rate. Furthermore, when the introduced additive consists in a biodegradable polymer, a secondary burst, causing deviations from the release profile of pure CPC, could be detected as a consequence of autocatalytic polymer degradation. The chemical nature of the drug further has a direct influence on the release rate. In general, a higher solubility of the drug in the release medium facilitates the elution, while, as stated earlier, a high affinity of the drug for the matrix surface could lower the release efficiency. The drug can also interfere with the setting and the hardening processes, usually slowing them down by sequestering the free ions and competing with the surface sites nucleating the new phase. Retention of CP in its partially amorphous, mouldable form allows for a faster dissolution of the drug and an enhanced burst effect. An excessive molecular size of the drug can hinder the diffusion from the porous matrix through the steric effects, leading to a diminished dissolution rate. Further, when releases of the same drugs loaded in identical amounts into a CPC carrier are compared, different results can be obtained if the drug loading method has been varied. When the drug is directly admixed with the powder or a liquid component, the release is governed by the diffusion from the bulk of the matrix. In this case, higher amounts of the drug result in higher and more prolonged releases. In the case of the adsorption of the drug onto the matrix surface by pre-incubation, the release is mechanistically anomalous and increases together with the amount of the drug loaded, albeit with a faster depletion of the loaded molecules. Finally, the drug can be loaded inside biodegradable polymeric microspheres, which are subsequently embedded in the cement matrix. In this case, the release kinetics is defined by the relaxation of the polymer and by the drug diffusion from the microspheres. When polymeric microspheres completely dissolve, a series of empty cavities are left inside the CPC matrix, defining a new net of interconnected pores, with a consequently faster kinetic and the release mechanism governed by the drug diffusion from the pores. A secondary burst and/or deviation from the profile characteristic of the pure CPC are often detected in such cases. Finally, the release medium can also influence the release mechanism and kinetics. Saline buffer solutions, such as PBS or SBF, can promote apatite precipitation and deposition on the cement surface, which acts as a barrier for drug diffusion from the bulk, thus decreasing the release rate. When drugs are able to form complexes with calcium ions, a slower rate characterizes the release profile. However, release media such as FCS or DMEM contain proteins and nutrients able to chelate calcium ions, in which case a competitive complexation can take place, deducting the chelation sites available to the drug and enhancing its release.
A key purpose of this article was to firstly discern the key physicochemical factors governing the drug release kinetics from CPCs and then to create a framework for their effective utilization in the rational design of bioactive bone void fillers with precisely tailorable release profiles. And now, at its end, it is worth revisiting this objective. Apparently, through a rigorous literature review, we have managed to delineate a number of factors responsible for affecting, if not solely determining the release kinetics and mechanisms. The use of a qualitative framework and the inevitable entanglement of factors into complex synergistic dependencies limit the practical application of this broad picture, but by no means do they render it ineffective. Definite principles have emerged that could be utilized in the design of CPCs with tunable release characteristics, notwithstanding that this would require a skill in the fundamental materials structure and property design. Elicitation of these basic principles would take this review into more general waters, which lie outside of its scope. Nevertheless, the pending advancements in the rational design of materials structures at ultrafine scales in combination with principles presented in this study will prove valuable in ensuring the continued reign of CPCs as the one of the most optimal small-defect, low weight-bearing bone substitutes.
CRediT authorship contribution statement
Marco Fosca: Conceptualization, Methodology, Writing – original draft, Writing – review & editing. Julietta V. Rau: Conceptualization, Supervision, Methodology, Writing – review & editing, Funding acquisition. Vuk Uskoković: Conceptualization, Supervision, Methodology, Writing – original draft, Writing – review & editing.
Declaration of competing interest
The authors declare no conflict of interest.
Acknowledgments
The assistance of Mr. Luca Imperatori in manuscript preparation is gratefully acknowledged.
Footnotes
Peer review under responsibility of KeAi Communications Co., Ltd.
Contributor Information
Julietta V. Rau, Email: giulietta.rau@ism.cnr.it.
Vuk Uskoković, Email: vuk21@yahoo.com.
References
- 1.Manabe J., Kawaguchi N., Matsumoto S., Tanizawa T. Surgical treatment of bone metastasis: indications and outcomes. Int. J. Clin. Oncol. 2004;10:103–111. doi: 10.1007/s10147-005-0478-9. [DOI] [PubMed] [Google Scholar]
- 2.Constantz B., Ison I., Fulmer M., Poser R., Smith S., VanWagoner M., Ross J., Goldstein S., Jupiter J., Rosenthal D. Skeletal repair by in situ formation of the mineral phase of bone. Science. 1995;267:1796–1799. doi: 10.1126/science.7892603. [DOI] [PubMed] [Google Scholar]
- 3.O'Keefe R.J., Mao J. Bone tissue engineering and regeneration: from discovery to the clinic—an overview. Tissue Eng. B Rev. 2011;17:389–392. doi: 10.1089/ten.teb.2011.0475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Baroli B. From natural bone grafts to tissue engineering therapeutics: brainstorming on pharmaceutical formulative requirements and challenges. J. Pharmaceut. Sci. 2009;98:1317–1375. doi: 10.1002/jps.21528. [DOI] [PubMed] [Google Scholar]
- 5.Dimitriou R., Jones E., McGonagle D., Giannoudis P.V. Bone regeneration: current concepts and future directions. BMC Med. 2011;9 doi: 10.1186/1741-7015-9-66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Soucacos P.N., Johnson E.O., Babis G. An update on recent advances in bone regeneration. Injury. 2008;39:S1–S4. doi: 10.1016/s0020-1383(08)70009-3. [DOI] [PubMed] [Google Scholar]
- 7.Amini A.R., Laurencin C.T., Nukavarapu S.P. Bone tissue engineering: recent advances and challenges. Crit. Rev. Biomed. Eng. 2012;40:363–408. doi: 10.1615/CritRevBiomedEng.v40.i5.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kanakaris N.K., Petsatodis G., Tagil M., Giannoudis P.V. Is there a role for bone morphogenetic proteins in osteoporotic fractures? Injury. 2009;40:S21–S26. doi: 10.1016/s0020-1383(09)70007-5. [DOI] [PubMed] [Google Scholar]
- 9.Kronenberg H.M. Developmental regulation of the growth plate. Nature. 2003;423:332–336. doi: 10.1038/nature01657. [DOI] [PubMed] [Google Scholar]
- 10.Sikavitsas V.I., Temenoff J.S., Mikos A.G. Biomaterials and bone mechanotransduction. Biomaterials. 2001;22:2581–2593. doi: 10.1016/s0142-9612(01)00002-3. [DOI] [PubMed] [Google Scholar]
- 11.Chaparro O., Linero I. 2016. Regenerative Medicine: A New Paradigm in Bone Regeneration, Advanced Techniques in Bone Regeneration. [Google Scholar]
- 12.Al-Mulhim F.A., Baragbah M.A., Sadat-Ali M., Alomran A.S., Azam M.Q. Prevalence of surgical site infection in orthopedic surgery: a 5-year analysis. Int. Surg. 2014;99:264–268. doi: 10.9738/intsurg-d-13-00251.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Maksimović J., Marković-Denić L., Bumbaširević M., Marinković J., Vlajinac H. Surgical site infections in orthopedic patients: prospective cohort study. Croat. Med. J. 2008;49:58–65. doi: 10.3325/cmj.2008.1.58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Thakore R.V., Greenberg S.E., Shi H., Foxx A.M., Francois E.L., Prablek M.A., Nwosu S.K., Archer K.R., Ehrenfeld J.M., Obremskey W.T., Sethi M.K. Surgical site infection in orthopedic trauma: a case–control study evaluating risk factors and cost. J. Clini. Ortho. Trauma. 2015;6:220–226. doi: 10.1016/j.jcot.2015.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Najjar Y.W., Al-Wahsh Z.M., Hamdan M., Saleh M.Y. Risk factors of orthopedic surgical site infection in Jordan: a prospective cohort study. Int. J. Surg. Open. 2018;15:1–6. doi: 10.1016/j.ijso.2018.09.003. [DOI] [Google Scholar]
- 16.Uçkay I., Hoffmeyer P., Lew D., Pittet D. Prevention of surgical site infections in orthopaedic surgery and bone trauma: state-of-the-art update. J. Hosp. Infect. 2013;84:5–12. doi: 10.1016/j.jhin.2012.12.014. [DOI] [PubMed] [Google Scholar]
- 17.Uskoković V., Ghosh S. Carriers for the tunable release of therapeutics: etymological classification and examples. Expet Opin. Drug Deliv. 2016;13:1729–1741. doi: 10.1080/17425247.2016.1200558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Vallet-Regí M., Balas F., Arcos D. Mesoporous materials for drug delivery. Angew. Chem. Int. Ed. 2007;46:7548–7558. doi: 10.1002/anie.200604488. [DOI] [PubMed] [Google Scholar]
- 19.Vallet-Regí M. Bioceramics: from bone substitutes to nanoparticles for drug delivery. Pure Appl. Chem. 2019;91:687–706. doi: 10.1515/pac-2018-0505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ginebra M.-P., Canal C., Espanol M., Pastorino D., Montufar E.B. Calcium phosphate cements as drug delivery materials. Adv. Drug Deliv. Rev. 2012;64:1090–1110. doi: 10.1016/j.addr.2012.01.008. [DOI] [PubMed] [Google Scholar]
- 21.Bohner M., Baroud G. Injectability of calcium phosphate pastes. Biomaterials. 2005;26:1553–1563. doi: 10.1016/j.biomaterials.2004.05.010. [DOI] [PubMed] [Google Scholar]
- 22.Dorozhkin S.V. Calcium orthophosphate cements for biomedical application. J. Mater. Sci. 2008;43:3028–3057. doi: 10.1007/s10853-008-2527-z. [DOI] [Google Scholar]
- 23.Strategies to Modify the Drug Release from Pharmaceutical Systems. 2015. [Google Scholar]
- 24.Mircioiu C., Voicu V., Anuta V., Tudose A., Celia C., Paolino D., Fresta M., Sandulovici R., Mircioiu I. Mathematical modeling of release kinetics from supramolecular drug delivery systems. Pharmaceutics. 2019;11 doi: 10.3390/pharmaceutics11030140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Higuchi T. Mechanism of sustained‐action medication. Theoretical analysis of rate of release of solid drugs dispersed in solid matrices. J. Pharmaceut. Sci. 1963;52:1145–1149. doi: 10.1002/jps.2600521210. [DOI] [PubMed] [Google Scholar]
- 26.Korsmeyer R.W., Gurny R., Doelker E., Buri P., Peppas N.A. Mechanisms of solute release from porous hydrophilic polymers. Int. J. Pharm. 1983;15:25–35. doi: 10.1016/0378-5173(83)90064-9. [DOI] [PubMed] [Google Scholar]
- 27.Otsuka M., Nakahigashi Y., Matsuda Y., Fox J.L., Higuchi W.I., Sugiyama Y. Effect of geometrical cement size on in vitro and in vivo indomethacin release from self-setting apatite cement. J. Contr. Release. 1998;52:281–289. doi: 10.1016/s0168-3659(97)00264-2. [DOI] [PubMed] [Google Scholar]
- 28.Alkhraisat M.H., Mariño F.T., Retama J.R., Jerez L.B., López-Cabarcos E. Beta-tricalcium phosphate release from brushite cement surface. J. Biomed. Mater. Res. 2008;84A:710–717. doi: 10.1002/jbm.a.31381. [DOI] [PubMed] [Google Scholar]
- 29.Khashaba R.M., Lockwood P.E., Lewis J.B., Messer R.L., Chutkan N.B., Borke J.L. Cytotoxicity, calcium release, and pH changes generated by novel calcium phosphate cement formulations. J. Biomed. Mater. Res. B Appl. Biomater. 2010;93B:297–303. doi: 10.1002/jbm.b.31494. [DOI] [PubMed] [Google Scholar]
- 30.Hemmati K., Hesaraki S., Nemati A. Evaluation of ascorbic acid-loaded calcium phosphate bone cements: physical properties and in vitro release behavior. Ceram. Int. 2014;40:3961–3968. doi: 10.1016/j.ceramint.2013.08.042. [DOI] [Google Scholar]
- 31.Akashi A., Matsuya Y., Unemori M., Akamine A. Release profile of antimicrobial agents from α-tricalcium phosphate cement. Biomaterials. 2001;22:2713–2717. doi: 10.1016/s0142-9612(00)00438-5. [DOI] [PubMed] [Google Scholar]
- 32.Irbe Z., Loca D., Vempere D., Berzina-Cimdina L. Controlled release of local anesthetic from calcium phosphate bone cements. Mater. Sci. Eng. C. 2012;32:1690–1694. doi: 10.1016/j.msec.2012.04.069. [DOI] [PubMed] [Google Scholar]
- 33.Su W.-Y., Chen Y.-C., Lin F.-H. A new type of biphasic calcium phosphate cement as a gentamicin carrier for osteomyelitis. Evid. base Compl. Alternative Med. 2013;2013:1–9. doi: 10.1155/2013/801374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rau J.V., Wu V.M., Graziani V., Fadeeva I.V., Fomin A.S., Fosca M., Uskoković V. The Bone Building Blues: self-hardening copper-doped calcium phosphate cement and its in vitro assessment against mammalian cells and bacteria. Mater. Sci. Eng. C. 2017;79:270–279. doi: 10.1016/j.msec.2017.05.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Uskoković V., Graziani V., Wu V.M., Fadeeva I.V., Fomin A.S., Presniakov I.A., Fosca M., Ortenzi M., Caminiti R., Rau J.V. Gold is for the mistress, silver for the maid: enhanced mechanical properties, osteoinduction and antibacterial activity due to iron doping of tricalcium phosphate bone cements. Mater. Sci. Eng. C. 2019;94:798–810. doi: 10.1016/j.msec.2018.10.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Canal C., Pastorino D., Mestres G., Schuler P., Ginebra M.-P. Relevance of microstructure for the early antibiotic release of fresh and pre-set calcium phosphate cements. Acta Biomater. 2013;9:8403–8412. doi: 10.1016/j.actbio.2013.05.016. [DOI] [PubMed] [Google Scholar]
- 37.Mestres G., Kugiejko K., Pastorino D., Unosson J., Öhman C., Karlsson Ott M., Ginebra M.-P., Persson C. Changes in the drug release pattern of fresh and set simvastatin-loaded brushite cement. Mater. Sci. Eng. C. 2016;58:88–96. doi: 10.1016/j.msec.2015.08.016. [DOI] [PubMed] [Google Scholar]
- 38.Ghosh S., Wu V., Pernal S., Uskoković V. Self-setting calcium phosphate cements with tunable antibiotic release rates for advanced antimicrobial applications. ACS Appl. Mater. Interfaces. 2016;8:7691–7708. doi: 10.1021/acsami.6b01160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Uskoković V. Mechanism of formation governs the mechanism of release of antibiotics from calcium phosphate nanopowders and cements in a drug-dependent manner. J. Mater. Chem. B. 2019;7:3982–3992. doi: 10.1039/c9tb00444k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Uchida K., Sugo K., Nakajima T., Nakawaki M., Takano S., Nagura N., Takaso M., Urabe K. In vivo release of vancomycin from calcium phosphate cement. BioMed Res. Int. 2018;2018:1–6. doi: 10.1155/2018/4560647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Son Y.-J., Lee I.-C., Jo H.-H., Chung T.-J., Oh K.-S. Setting behavior and drug release from brushite bone cement prepared with granulated hydroxyapatite and β-tricalcium phosphate. J. Kor. Chem. Soc. 2019;56:56–64. doi: 10.4191/kcers.2019.56.1.06. [DOI] [Google Scholar]
- 42.Haghbin-Nazarpak M., Moztarzadeh F., Solati-Hashjin M., Mirhabibi A.R., Tahriri M. 2010. PREPARATION , CHARACTERIZATION AND GENTAMICIN SULFATE RELEASE INVESTIGATION OF BIPHASIC INJECTABLE CALCIUM PHOSPHATE BONE CEMENT. [Google Scholar]
- 43.Otsuka M., Matsuda Y., Wang Z., Fox J.L., Higuchi W.I. Effect of sodium bicarbonate amount on in vitro indomethacin release from self-setting carbonated-apatite cement. Pharmaceut. Res. 1997;14:444–449. doi: 10.1023/a:1012039214184. [DOI] [PubMed] [Google Scholar]
- 44.Girod Fullana S., Ternet H., Freche M., Lacout J.L., Rodriguez F. Controlled release properties and final macroporosity of a pectin microspheres–calcium phosphate composite bone cement. Acta Biomater. 2010;6:2294–2300. doi: 10.1016/j.actbio.2009.11.019. [DOI] [PubMed] [Google Scholar]
- 45.Hofmann M., Mohammed A., Perrie Y., Gbureck U., Barralet J. High-strength resorbable brushite bone cement with controlled drug-releasing capabilities. Acta Biomater. 2009;5:43–49. doi: 10.1016/j.actbio.2008.08.005. [DOI] [PubMed] [Google Scholar]
- 46.Schnieders J., Gbureck U., Vorndran E., Schossig M., Kissel T. The effect of porosity on drug release kinetics from vancomycin microsphere/calcium phosphate cement composites. J. Biomed. Mater. Res. B Appl. Biomater. 2011;99B:391–398. doi: 10.1002/jbm.b.31910. [DOI] [PubMed] [Google Scholar]
- 47.Otsuka M., Nakagawa H., Ito A., Higuchi W.I. Effect of geometrical structure on drug release rate of a three-dimensionally perforated porous apatite/collagen composite cement. J. Pharmaceut. Sci. 2010;99:286–292. doi: 10.1002/jps.21835. [DOI] [PubMed] [Google Scholar]
- 48.Otsuka M., Nakagawa H., Otsuka K., Ito A., Higuchi W.I. Drug release from a three-dimensionally perforated porous apatite/collagen composite cement. Bioceram. Dev. Appl. 2012;2:1–3. doi: 10.4303/bda/D110189. [DOI] [Google Scholar]
- 49.Otsuka M., Hirano R. Bone cell activity responsive drug release from biodegradable apatite/collagen nano-composite cements—in vitro dissolution medium responsive vitamin K2 release. Colloids Surf. B Biointerfaces. 2011;85:338–342. doi: 10.1016/j.colsurfb.2011.03.006. [DOI] [PubMed] [Google Scholar]
- 50.Hesaraki S., Nemati R., Nosoudi N. Preparation and characterisation of porous calcium phosphate bone cement as antibiotic carrier. Adv. Appli. Ceramics. 2013;108:231–240. doi: 10.1179/174367608x353656. [DOI] [Google Scholar]
- 51.Forouzandeh A., Hesaraki S., Zamanian A. The releasing behavior and in vitro osteoinductive evaluations of dexamethasone-loaded porous calcium phosphate cements. Ceram. Int. 2014;40:1081–1091. doi: 10.1016/j.ceramint.2013.06.107. [DOI] [Google Scholar]
- 52.Vorndran E., Geffers M., Ewald A., Lemm M., Nies B., Gbureck U. Ready-to-use injectable calcium phosphate bone cement paste as drug carrier. Acta Biomater. 2013;9:9558–9567. doi: 10.1016/j.actbio.2013.08.009. [DOI] [PubMed] [Google Scholar]
- 53.Lucas-Aparicio J., Manchón Á., Rueda C., Pintado C., Torres J., Alkhraisat M.H., López-Cabarcos E. Silicon-calcium phosphate ceramics and silicon-calcium phosphate cements: substrates to customize the release of antibiotics according to the idiosyncrasies of the patient. Mater. Sci. Eng. C. 2020;106 doi: 10.1016/j.msec.2019.110173. [DOI] [PubMed] [Google Scholar]
- 54.Uskokovíc V. Entering the era of nanoscience: time to Be so small Vuk uskokovíc. J. Biomed. Nanotechnol. 2013;9:1441–1470. doi: 10.1166/jbn.2013.1642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Bernards D.A., Desai T.A. Nanotemplating of biodegradable polymer membranes for constant-rate drug delivery. Adv. Mater. 2010;22:2358–2362. doi: 10.1002/adma.200903439. [DOI] [PubMed] [Google Scholar]
- 56.Wang F.W., Khatri C.A., Hsii J.F. Polymer-filled calcium phosphate cement: mechanical properties and controlled release of growth factor. Adv. Bioeng. 2003:401–402. [Google Scholar]
- 57.Takechi M., Miyamoto Y., Momota Y., Yuasa T., Tatehara S., Nagayama M., Ishikawa K., Suzuki K. The in vitro antibiotic release from anti-washout apatite cement using chitosa. J. Mater. Sci. Mater. Med. 2002;13:973–978. doi: 10.1023/a:1019816830793. [DOI] [PubMed] [Google Scholar]
- 58.De la Riva B., Sánchez E., Hernández A., Reyes R., Tamimi F., López-Cabarcos E., Delgado A., Évora C. Local controlled release of VEGF and PDGF from a combined brushite–chitosan system enhances bone regeneration. J. Contr. Release. 2010;143:45–52. doi: 10.1016/j.jconrel.2009.11.026. [DOI] [PubMed] [Google Scholar]
- 59.Tığlı R.S., Akman A.C., Gümüşderelıoğlu M., Nohutçu R.M. In vitro release of dexamethasone or bFGF from chitosan/hydroxyapatite scaffolds. J. Biomater. Sci. Polym. Ed. 2012;20:1899–1914. doi: 10.1163/156856208x399945. [DOI] [PubMed] [Google Scholar]
- 60.Lode A., Wolf-Brandstetter C., Reinstorf A., Bernhardt A., König U., Pompe W., Gelinsky M. Calcium phosphate bone cements, functionalized with VEGF: release kinetics and biological activity. J. Biomed. Mater. Res. 2007;81A:474–483. doi: 10.1002/jbm.a.31024. [DOI] [PubMed] [Google Scholar]
- 61.Salemi H., Behnamghader A., Eslaminejad M.B., Ataei M. Effect of collagen on the morphology and structure of calcium phosphate nanoparticles. Biomed. Eng.: Appl. Basis and Commun. 2014;26 doi: 10.4015/s1016237214500616. [DOI] [Google Scholar]
- 62.Perez R.A., Kim H.-W., Ginebra M.-P. Polymeric additives to enhance the functional properties of calcium phosphate cements. J. Tissue Eng. 2012;3 doi: 10.1177/2041731412439555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Li D.X., Fan H.S., Zhu X.D., Tan Y.F., Xiao W.Q., Lu J., Xiao Y.M., Chen J.Y., Zhang X.D. Controllable release of salmon-calcitonin in injectable calcium phosphate cement modified by chitosan oligosaccharide and collagen polypeptide. J. Mater. Sci. Mater. Med. 2007;18:2225–2231. doi: 10.1007/s10856-007-3084-8. [DOI] [PubMed] [Google Scholar]
- 64.David Chen C.-H., Chen C.-C., Shie M.-Y., Huang C.-H., Ding S.-J. Controlled release of gentamicin from calcium phosphate/alginate bone cement. Mater. Sci. Eng. C. 2011;31:334–341. doi: 10.1016/j.msec.2010.10.002. [DOI] [Google Scholar]
- 65.Colpo J.C., Pigatto C., Brizuela N., Aragón J., dos Santos L.A.L. Antibiotic and anesthetic drug release from double-setting α-TCP cements. J. Mater. Sci. 2018;53:7112–7124. doi: 10.1007/s10853-018-2071-4. [DOI] [Google Scholar]
- 66.Lin B., Zhou H., Leaman D.W., Goel V.K., Agarwal A.K., Bhaduri S.B. Sustained release of small molecules from carbon nanotube-reinforced monetite calcium phosphate cement. Mater. Sci. Eng. C. 2014;43:92–96. doi: 10.1016/j.msec.2014.06.027. [DOI] [PubMed] [Google Scholar]
- 67.Wang J.-N., Pi B., Wang P., Li X.-F., Yang H.-L., Zhu X.-S. Sustained release of Semaphorin 3A from α-tricalcium phosphate based cement composite contributes to osteoblastic differentiation of MC3T3-E1 cells. Front. Mater. Sci. 2015;9:282–292. doi: 10.1007/s11706-015-0293-9. [DOI] [Google Scholar]
- 68.Zhang L., Weir M.D., Chow L.C., Reynolds M.A., Xu H.H.K. Rechargeable calcium phosphate orthodontic cement with sustained ion release and re-release. Sci. Rep. 2016;6 doi: 10.1038/srep36476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Uskoković V., Ghosh S., Wu V.M. Antimicrobial hydroxyapatite–gelatin–silica composite pastes with tunable setting properties. J. Mater. Chem. B. 2017;5:6065–6080. doi: 10.1039/c7tb01794d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Schumacher M., Reither L., Thomas J., Kampschulte M., Gbureck U., Lode A., Gelinsky M. Calcium phosphate bone cement/mesoporous bioactive glass composites for controlled growth factor delivery. Biomater. Sci. 2017;5:578–588. doi: 10.1039/c6bm00903d. [DOI] [PubMed] [Google Scholar]
- 71.Jani A.T., Haghighi N.B., Sheikh Hossein Pour M., Aminian M., Molzemi S. Hydroxyapatite incorporation into MCM-41 and study of ibuprofen drug release. J. Austria. Ceram. Soc. 2019;56:653–661. doi: 10.1007/s41779-019-00384-w. [DOI] [Google Scholar]
- 72.Su C.-C., Kao C.-T., Hung C., Jr., Chen Y.-J., Huang T.-H., Shie M.-Y. Regulation of physicochemical properties, osteogenesis activity, and fibroblast growth factor-2 release ability of β-tricalcium phosphate for bone cement by calcium silicate. Mater. Sci. Eng. C. 2014;37:156–163. doi: 10.1016/j.msec.2014.01.010. [DOI] [PubMed] [Google Scholar]
- 73.Vallet-Regí M., Arcos D. Silicon substituted hydroxyapatites. A method to upgrade calcium phosphate based implants. J. Mater. Chem. 2005;15:1509–1516. doi: 10.1039/b414143a. [DOI] [Google Scholar]
- 74.Arcos D., Greenspan D.C., Vallet-Regí M. A new quantitative method to evaluate the in vitrobio activity of melt and sol-gel-derived silicate glasses. J. Biomed. Mater. Res. 2003;65A:344–351. doi: 10.1002/jbm.a.10503. [DOI] [PubMed] [Google Scholar]
- 75.Arcos D., Greenspan D.C., Vallet-Regí M. Influence of the stabilization temperature on textural and structural features and ion release in SiO2−CaO−P2O5Sol−Gel glasses. Chem. Mater. 2002;14:1515–1522. doi: 10.1021/cm011119p. [DOI] [Google Scholar]
- 76.Ito A., Senda K., Sogo Y., Oyane A., Yamazaki A., LeGeros R.Z. Dissolution rate of zinc-containing β-tricalcium phosphate ceramics. Biomed. Mater. 2006;1:134–139. doi: 10.1088/1748-6041/1/3/007. [DOI] [PubMed] [Google Scholar]
- 77.Fujihara E., Kon M., Asaoka K. Strontium-substituted calcium phosphate cements prepared with strontium-containing solutions. Key Eng. Mater. 2007;330–332:795–798. doi: 10.4028/www.scientific.net/KEM.330-332.795. [DOI] [Google Scholar]
- 78.Alkhraisat M.H., Rueda C., Cabrejos-Azama J., Lucas-Aparicio J., Mariño F.T., Torres García-Denche J., Jerez L.B., Gbureck U., Cabarcos E.L. Loading and release of doxycycline hyclate from strontium-substituted calcium phosphate cement. Acta Biomater. 2010;6:1522–1528. doi: 10.1016/j.actbio.2009.10.043. [DOI] [PubMed] [Google Scholar]
- 79.Shi H., Zeng S., Liu X., Yu T., Zhou C. Effects of strontium doping on the degradation and Sr ion release behaviors of α-tricalcium phosphate bone cement. J. Am. Ceram. Soc. 2018;101:502–508. doi: 10.1111/jace.15220. [DOI] [Google Scholar]
- 80.van Staden A.D., Heunis T.D.J., Dicks L.M.T. Release of Enterococcus mundtii bacteriocin ST4SA from self-setting brushite bone cement. Probio. Antimicrob. Proteins. 2011;3:119–124. doi: 10.1007/s12602-011-9074-7. [DOI] [PubMed] [Google Scholar]
- 81.Yu D., Wong J., Matsuda Y., Fox J.L., Higuchi W.I., Otsuka M. Self‐setting hydroxyapatite cement: a novel skeletal drug‐delivery system for antibiotics. J. Pharmaceut. Sci. 1992;81:529–531. doi: 10.1002/jps.2600810611. [DOI] [PubMed] [Google Scholar]
- 82.Huang J.-G., Pang L., Chen Z.-R., Tan X.-P. Dual-delivery of vancomycin and icariin from an injectable calcium phosphate cement-release system for controlling infection and improving bone healing. Mol. Med. Rep. 2013;8:1221–1227. doi: 10.3892/mmr.2013.1624. [DOI] [PubMed] [Google Scholar]
- 83.Saleh A.T., Ling L.S., Hussain R. Injectable magnesium-doped brushite cement for controlled drug release application. J. Mater. Sci. 2016;51:7427–7439. doi: 10.1007/s10853-016-0017-2. [DOI] [Google Scholar]
- 84.Pasqual J.A.R., Freisleben L.C., Colpo J.C., Egea J.R.J., dos Santos L.A.L., de Sousa V.C. In situ drug release measuring in α-TCP cement by electrochemical impedance spectroscopy. J. Mater. Sci. Mater. Med. 2021;32 doi: 10.1007/s10856-021-06507-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Pasqual J.A.R., Pereira B.L.R., Colpo J.C., Egea J.R.J., dos Santos L.A.L., de Sousa V.C. Monitoring of the interaction of calcium phosphate cement and lidocaine hydrochloride by electrochemical impedance spectroscopy during the drug release process. J. Appl. Electrochem. 2021;51:463–471. doi: 10.1007/s10800-020-01520-2. [DOI] [Google Scholar]
- 86.Habraken W.J.E.M., Boerman O.C., Wolke J.G.C., Mikos A.G., Jansen J.A. In vitro growth factor release from injectable calcium phosphate cements containing gelatin microspheres. J. Biomed. Mater. Res. 2009;91A:614–622. doi: 10.1002/jbm.a.32263. [DOI] [PubMed] [Google Scholar]
- 87.Gunnella F., Kunisch E., Horbert V., Maenz S., Bossert J., Jandt K.D., Plöger F., Kinne R.W. In vitro release of bioactive bone morphogenetic proteins (GDF5, BB-1, and BMP-2) from a PLGA fiber-reinforced, brushite-forming calcium phosphate cement. Pharmaceutics. 2019;11 doi: 10.3390/pharmaceutics11090455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Hamanishi C., Kitamoto K., Tanaka S., Otsuka M., Doi Y., Kitahashi T. A self-setting TTCP-DCPD apatite cement for release of vancomycin. J. Biomed. Mater. Res. 1996;33:139–143. doi: 10.1002/(sici)1097-4636(199623)33:3<139::Aid-jbm3>3.0.Co;2-r. [DOI] [PubMed] [Google Scholar]
- 89.Otsuka M., Nakahigashi Y., Matsuda Y., Fox J.L., Higuchi W.I., Sugiyama Y. A novel skeletal drug delivery system using self-setting calcium phosphate cement VIII: the relationship between in vitro and in vivo drug release from indomethacin-containing cement. J. Contr. Release. 1997;43:115–122. doi: 10.1016/s0168-3659(96)01493-9. [DOI] [Google Scholar]
- 90.Rabiee S.M. Development of hydroxyapatite bone cement for controlled drug release via tetracycline hydrochloride. Bull. Mater. Sci. 2013;36:171–174. doi: 10.1007/s12034-013-0424-9. [DOI] [Google Scholar]
- 91.Sakamoto Y., Ochiai H., Ohsugi I., Inoue Y., Yoshimura Y., Kishi K. Mechanical strength and in vitro antibiotic release profile of antibiotic-loaded calcium phosphate bone cement. J. Craniofac. Surg. 2013;24:1447–1450. doi: 10.1097/SCS.0b013e31829972de. [DOI] [PubMed] [Google Scholar]
- 92.Zou Q., Li Y., Zhang L., Zuo Y., Li J., Li J. Antibiotic delivery system using nano-hydroxyapatite/chitosan bone cement consisting of berberine. J. Biomed. Mater. Res. 2009;89A:1108–1117. doi: 10.1002/jbm.a.32199. [DOI] [PubMed] [Google Scholar]
- 93.Montazerolghaem M., Engqvist H., Karlsson Ott M. Sustained release of simvastatin from premixed injectable calcium phosphate cement. J. Biomed. Mater. Res. 2014;102:340–347. doi: 10.1002/jbm.a.34702. [DOI] [PubMed] [Google Scholar]
- 94.van Houdt C.I.A., Gabbai-Armelin P.R., Lopez-Perez P.M., Ulrich D.J.O., Jansen J.A., Renno A.C.M., van den Beucken J.J.J.P. Alendronate release from calcium phosphate cement for bone regeneration in osteoporotic conditions. Sci. Rep. 2018;8 doi: 10.1038/s41598-018-33692-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Blom E.J., Burger E.H., Klein-Nulend J., van Waas M.A.J., Wolke J.G.C., Driessens F.C.M. Physicochemical properties and release characteristics of growth factor-modified calcium phosphate bone cement. Mater. Werkst. 2001;32:962–969. doi: 10.1002/1521-4052(200112)32:12<962::Aid-mawe962>3.0.Co;2-1. [DOI] [Google Scholar]
- 96.Blom E.J., Klein-Nulend J., Wolke J.G.C., van Waas M.A.J., Driessens F.C.M., Burger E.H. Transforming growth factor-?1 incorporation in a calcium phosphate bone cement: material properties and release characteristics. J. Biomed. Mater. Res. 2002;59:265–272. doi: 10.1002/jbm.1241. [DOI] [PubMed] [Google Scholar]
- 97.Blom E.J., Klein-Nulend J., Wolke J.G.C., Kurashina K., van Waas M.A.J., Burger E.H. Transforming growth factor-β1 incorporation in an α-tricalcium phosphate/dicalcium phosphate dihydrate/tetracalcium phosphate monoxide cement: release characteristics and physicochemical properties. Biomaterials. 2002;23:1261–1268. doi: 10.1016/s0142-9612(01)00246-0. [DOI] [PubMed] [Google Scholar]
- 98.Hamdan Alkhraisat M., Moseke C., Blanco L., Barralet J.E., Lopez-Carbacos E., Gbureck U. Strontium modified bio cements with zero order release kinetics. Biomaterials. 2008;29:4691–4697. doi: 10.1016/j.biomaterials.2008.08.026. [DOI] [PubMed] [Google Scholar]
- 99.Singh S.S., Roy A., Lee B., Parekh S., Kumta P.N. Murine osteoblastic and osteoclastic differentiation on strontium releasing hydroxyapatite forming cements. Mater. Sci. Eng. C. 2016;63:429–438. doi: 10.1016/j.msec.2016.02.059. [DOI] [PubMed] [Google Scholar]
- 100.Jayasree R., Kumar T.S.S., Mahalaxmi S., Abburi S., Rubaiya Y., Doble M. Dentin remineralizing ability and enhanced antibacterial activity of strontium and hydroxyl ion co-releasing radiopaque hydroxyapatite cement. J. Mater. Sci. Mater. Med. 2017;28 doi: 10.1007/s10856-017-5903-x. [DOI] [PubMed] [Google Scholar]
- 101.Rau J., Fosca M., Graziani V., Egorov A., Zobkov Y., Fedotov A., Ortenzi M., Caminiti R., Baranchikov A., Komlev V. Silver-Doped calcium phosphate bone cements with antibacterial properties. J. Funct. Biomater. 2016;7 doi: 10.3390/jfb7020010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Graziani V., Fosca M., Egorov A.A., Zobkov Y.V., Fedotov A.Y., Baranchikov A.E., Ortenzi M., Caminiti R., Komlev V.S., Rau J.V. Zinc-releasing calcium phosphate cements for bone substitute materials. Ceram. Int. 2016;42:17310–17316. doi: 10.1016/j.ceramint.2016.08.027. [DOI] [Google Scholar]
- 103.Habraken W.J.E.M., Wolke J.G.C., Mikos A.G., Jansen J.A. PLGA microsphere/calcium phosphate cement composites for tissue engineering: in vitro release and degradation characteristics. J. Biomater. Sci. Polym. Ed. 2012;19:1171–1188. doi: 10.1163/156856208785540136. [DOI] [PubMed] [Google Scholar]
- 104.Schnieders J., Gbureck U., Thull R., Kissel T. Controlled release of gentamicin from calcium phosphate—poly(lactic acid-co-glycolic acid) composite bone cement. Biomaterials. 2006;27:4239–4249. doi: 10.1016/j.biomaterials.2006.03.032. [DOI] [PubMed] [Google Scholar]
- 105.Terukina T., Saito H., Tomita Y., Hattori Y., Otsuka M. Development and effect of a sustainable and controllable simvastatin-releasing device based on PLGA microspheres/carbonate apatite cement composite: in vitro evaluation for use as a drug delivery system from bone-like biomaterial. J. Drug Deliv. Sci. Technol. 2017;37:74–80. doi: 10.1016/j.jddst.2016.10.007. [DOI] [Google Scholar]
- 106.Dolci L.S., Panzavolta S., Albertini B., Campisi B., Gandolfi M., Bigi A., Passerini N. Spray-congealed solid lipid microparticles as a new tool for the controlled release of bisphosphonates from a calcium phosphate bone cement. Eur. J. Pharm. Biopharm. 2018;122:6–16. doi: 10.1016/j.ejpb.2017.10.002. [DOI] [PubMed] [Google Scholar]
- 107.Dolci L.S., Panzavolta S., Torricelli P., Albertini B., Sicuro L., Fini M., Bigi A., Passerini N. Modulation of Alendronate release from a calcium phosphate bone cement: an in vitro osteoblast-osteoclast co-culture study. Int. J. Pharm. 2019;554:245–255. doi: 10.1016/j.ijpharm.2018.11.023. [DOI] [PubMed] [Google Scholar]
- 108.Khairoun I., Boltong M.G., Gil F.J., Driessens F.C.M., Planell J.A., Seijas M.M., Martínez S. Incorporation of a controlled-release glass into a calcium phosphate cement. J. Mater. Sci. Mater. Med. 1999;10:215–218. doi: 10.1023/a:1008954011349. [DOI] [PubMed] [Google Scholar]
- 109.Tamimi F., Torres J., Bettini R., Ruggera F., Rueda C., López-Ponce M., Lopez-Cabarcos E. Doxycycline sustained release from brushite cements for the treatment of periodontal diseases. J. Biomed. Mater. Res. 2008;85A:707–714. doi: 10.1002/jbm.a.31610. [DOI] [PubMed] [Google Scholar]
- 110.Hamada H., Ohshima H., Otsuka M. Dissolution medium responsive simvastatin release from biodegradable apatite cements and the therapeutic effect in osteoporosis rats. J. Appl. Biomater. Funct. Mater. 2012;10:22–28. doi: 10.5301/jabfm.2012.9272. [DOI] [PubMed] [Google Scholar]
- 111.Ito T., Koyama Y., Otsuka M. DNA complex-releasing system by injectable self-setting apatite cement. J. Gene Med. 2012;14:251–261. doi: 10.1002/jgm.2613. [DOI] [PubMed] [Google Scholar]
- 112.Shi M., Chen L., Wang Y., Yan S. Low-intensity pulsed ultrasound enhances antibiotic release of gentamicin-loaded, self-setting calcium phosphate cement. J. Int. Med. Res. 2018;46:2803–2809. doi: 10.1177/0300060518773023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Ruhé P.Q., Boerman O.C., Russel F.G.M., Mikos A.G., Spauwen P.H.M., Jansen J.A. In vivo release of rhBMP-2 loaded porous calcium phosphate cement pretreated with albumin. J. Mater. Sci. Mater. Med. 2006;17:919–927. doi: 10.1007/s10856-006-0181-z. [DOI] [PubMed] [Google Scholar]
- 114.Stallmann H.P., Roo R.d., Faber C., Amerongen A.V.N., Wuisman P.I.J.M. In vivo release of the antimicrobial peptide hLF1-11 from calcium phosphate cement. J. Orthop. Res. 2008;26:531–538. doi: 10.1002/jor.20511. [DOI] [PubMed] [Google Scholar]
- 115.Delgado J.J., Sánchez E., Baro M., Reyes R., Évora C., Delgado A. A platelet derived growth factor delivery system for bone regeneration. J. Mater. Sci. Mater. Med. 2012;23:1903–1912. doi: 10.1007/s10856-012-4661-z. [DOI] [PubMed] [Google Scholar]
- 116.Liu X., Xue B., Zhang C., Wang Y., Wang J., Zhang J., Lu M., Li G., Cao Z., Huang Q. A novel controlled-release system for antibacterial enzyme lysostaphin delivery using hydroxyapatite/chitosan composite bone cement. PloS One. 2014;9 doi: 10.1371/journal.pone.0113797. [DOI] [PMC free article] [PubMed] [Google Scholar]