Abstract
Dysregulation of the kynurenine pathway (KP) of tryptophan catabolism has been implicated in psychotic disorders, including schizophrenia and bipolar disorder. Kynurenic acid (KYNA) is a KP metabolite synthesized by kynurenine aminotransferases (KATs) from its biological precursor kynurenine and acts as an endogenous antagonist of N-methyl-D-aspartate and α7-nicotinic acetylcholine receptors. Elevated KYNA levels found in postmortem brain tissue and cerebrospinal fluid of patients are hypothesized to play a key role in the etiology of cognitive symptoms observed in psychotic disorders. Sleep plays an important role in memory consolidation, and sleep disturbances are common among patients. Yet, little is known about the effect of altered KP metabolism on sleep–wake behavior. We presently utilized a well-established experimental paradigm of embryonic kynurenine (EKyn) exposure wherein pregnant dams are fed a diet laced with kynurenine the last week of gestation and hypothesized disrupted sleep–wake behavior in adult offspring. We examined sleep behavior in adult male and female offspring using electroencephalogram and electromyogram telemetry and determined sex differences in sleep and arousal in EKyn offspring. EKyn males displayed reduced rapid eye movement sleep, while female EKyn offspring were hyperaroused compared to controls. We determined that EKyn males maintain elevated brain KYNA levels, while KYNA levels were unchanged in EKyn females, yet the activity levels of KAT I and KAT II were reduced. Our findings indicate that elevated prenatal kynurenine exposure elicits sex-specific changes in sleep–wake behavior, arousal, and KP metabolism.
Keywords: kynurenic acid, sex differences, REM sleep, NREM sleep, tryptophan
Introduction
Disturbances in sleep are common in individuals with psychotic disorders, including schizophrenia (SZ) and bipolar disorder (BD). Dysregulation of sleep is multifaceted and, compared to healthy controls, patients have a higher incidence of sleep disorders, longer sleep onset latency, decreased sleep efficiency, more fragmented sleep, reduced sleep time, and overall poor sleep quality.1–6 Sleep disturbances often present before the hallmark clinically diagnosable symptoms and may serve as strong predictors for the manifestation of psychosis, mood, and cognitive disturbances.1,5,7,8 Cognitive dysfunction is a core domain of these disorders that remains undertreated, and unraveling molecular mechanisms linking sleep disturbances and core symptomology may lead to novel approaches to alleviate these outcomes for patients.
The kynurenine pathway (KP) of tryptophan catabolism and its neuroactive metabolite kynurenic acid (KYNA) have been implicated in the etiology of psychotic disorders afflicted by cognitive disruptions. Elevated KYNA levels, as found in the postmortem brain and cerebrospinal fluid (CSF) of individuals with SZ and BD9-16, are hypothesized to adversely impact cognitive function by inhibiting N-methyl-D-aspartate (NMDA) receptors and α7 nicotinic acetylcholine (α7nACh) receptors.17–21 KYNA is also an agonist for the aryl hydrocarbon receptor and the G protein-coupled receptor 35, both functional in the brain and periphery and highly implicated in inflammatory processes.22–26 The breadth of receptor targets of KYNA, enzymatically derived from kynurenine via kynurenine aminotransferase (KAT) enzymes (figure 1A),27,28 has various implications for the nervous system. Studies with rodents have demonstrated behavioral impairments, cognitive dysfunction, and altered sleep–wake behavior after acute KYNA elevation that are translationally relevant to understanding psychotic disorders.20,29–34 Increases in endogenous KYNA that impair memory formation, enhance arousal during quiescent time and specifically reduce rapid eye movement (REM) sleep,34 thereby disrupting a stage of sleep critical to memory processes.35
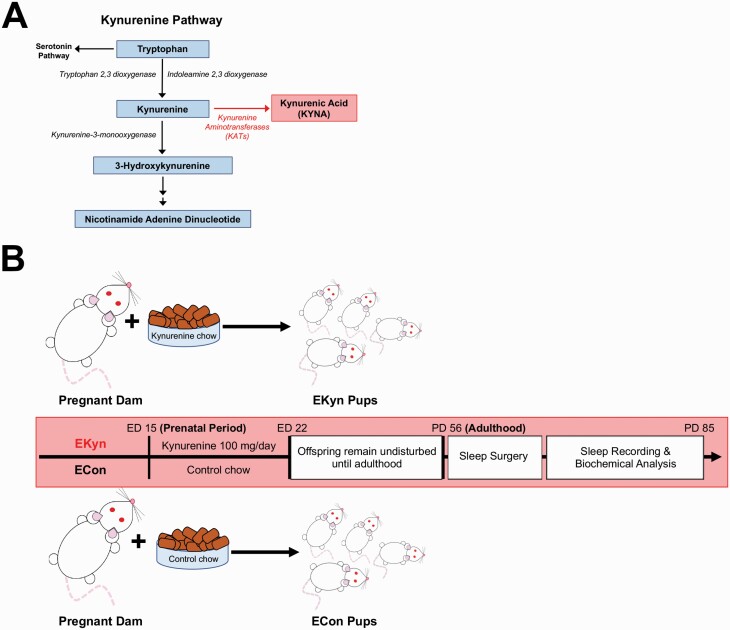
Fig. 1.
A simplified diagrammatic representation of the kynurenine pathway (KP), experimental design and timeline. (A) The KP of tryptophan catabolism produces kynurenic acid (KYNA) from kynurenine via kynurenine aminotransferases (KATs). (B) Pregnant rat dams were fed control (ECon) or kynurenine laced (EKyn) chow from embryonic day (ED) 15 until ED 22. Sleep recordings and tissue collection for biochemical analyses were carried out in adult male and female offspring after postnatal day (PD) 56.
As SZ and BD are considered neurodevelopmental disorders in which inherited or idiopathic abnormalities exist in early life yet symptoms manifest during young adulthood,36–39 we developed the embryonic kynurenine (EKyn) paradigm to experimentally recapitulate enhanced KP metabolism as seen with prenatal stress or infection.40–43 In the EKyn model (figure 1B), pregnant dams are fed chow laced with kynurenine during the last week of gestation, elevating KP metabolism and KYNA in the fetal brain.44,45 Several long-lasting, sex-specific, phenotypes have been determined with this model, including elevated brain KYNA in adult EKyn male offspring and corresponding cognitive impairments.42,44,46,47 We presently hypothesize that EKyn exposure will elicit impairments in sleep–wake behavior, specifically impacting REM sleep and wakefulness patterns, as determined with acute KYNA exposure.34
Sleep–wake behavior and sleep architecture, a quantifiable measure of sleep quality and time spent in sleep stages, were evaluated in both sexes of adult ECon and EKyn offspring. By including sex as a biological variable, we were able to capitalize our understanding of sex-dependent mechanisms, which have been significantly understudied in clinical studies.48 In rats, sleep behavior was evaluated by telemetrically recording electroencephalography (EEG) and electromyography (EMG) to quantify vigilance states, including REM sleep, non-REM (NREM) sleep, and wake. Our notable findings indicate that male EKyn offspring exhibit reduced REM sleep when KYNA levels are elevated in the brain, while female EKyn offspring display prolonged arousal. Taken together, our study highlights sleep dysfunction in EKyn offspring and emphasizes the contribution of dysfunctional KP metabolism in sleep–wake behavior.
Materials and Methods
Animals
Adult, pregnant Wistar rats (gestational age: 2 days) were obtained from Charles River Laboratories and treated with special diet (details below). Offspring used in experiments were group housed in a facility fully accredited by the American Association for the Accreditation of Laboratory Animal Care. Animals were kept on a 12/12 h light–dark cycle, where lights on corresponded to zeitgeber time (ZT) 0 and lights off to ZT12. All protocols were approved by Institutional Animal Care and Use Committees and were in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals.
EKyn Treatment
From embryonic day (ED) 15 to ED 22, dams received 30 g of wet mash rodent chow laced with 100 mg kynurenine (L-kynurenine sulfate, purity of 99.4%; Sai Advantium) per day (EKyn) or control wet mash (ECon), as previously described.44,49 Upon birth, each dam received normal rodent chow ad libitum. Offspring were weaned on postnatal day (PD) 21, pair-housed by sex, and used in experiments in adulthood (PD 56–85; figure 1B). The distribution of offspring from a single ECon or EKyn litter was 1–2 rats per sex within each experimental cohort. When necessary, additional ECon or EKyn litters were added to yield a minimum of n = 6 litters per experiment.
Surgery
Under isoflurane anesthesia, animals were placed in a stereotaxic frame and implanted with transmitters for EEG/EMG telemetry (PhysiolTel HD-S02; Data Science International) according to previously published protocols.34,50 The device was intraperitoneally implanted through a dorsal incision of the abdominal area. An incision at the midline of the head was made to secure EEG leads to 2 surgical screws (Plastics One, Roanoke, VA) inserted into 0.5-mm burr holes at 2.0 mm anterior/+1.5 mm lateral and 7.0 mm posterior/−1.5 mm lateral with dental cement. Two EMG leads were inserted directly into the dorsal cervical neck muscle about 1.0 mm apart and sutured in place. The skin on top of the head was sutured and animals recovered for ≥7 days before experimentation.
Sleep Data Acquisition and Analysis
Sleep data were recorded in a quiet, designated room where rats were undisturbed, during a 2-day period for males or 4-day period for females to span the estrous cycle using Ponemah 6.10 software (DSI). Digitized signal data were scored offline with NeuroScore 3.0 (DSI) in 10-s epochs as wake, NREM, or REM (see supplementary material). Transitions between vigilance states were scored when 2 or more 10-s epochs of the new vigilance state were noted. Vigilance state parameters assessed were the total duration, number of bouts, average bout length, and the number of transitions between vigilance states. Home cage activity and body temperature were also recorded.
Biochemical Analysis
For tissue collection, cohorts of experimentally naïve adult offspring were euthanized by CO2 asphyxiation at ZT6 and ZT18. Whole trunk blood was collected in tubes containing K3-EDTA (0.15%), centrifuged to separate plasma, and the brain was promptly removed. Samples were frozen and stored at −80°C.
On the day of biochemical assays, the cortex was weighed, sonicated (1:10, w/v) in ultrapure water, and aliquoted for the determination of KYNA by high-performance liquid chromatography (HPLC).47 Plasma samples were thawed and diluted in ultrapure water (tryptophan- 1:1000, kynurenine- 1:2, KYNA- 1:10).47 Briefly, 20 μL of acidified supernatant were injected into a ReproSil-Pur C18 column (4 × 150 mm; Dr. Maisch Gmbh) using a mobile phase comprised of 50 mM sodium acetate and 5% acetonitrile at a flow rate of 0.5 mL/min. Tryptophan (excitation/emission wavelength 285/365 nm), kynurenine (365/480 nm), and KYNA (344/398 nm) were detected in the eluate fluorometrically (Waters Alliance, 2475 fluorescence detector) using 500 mM zinc acetate delivered after the column at a flow rate of 0.1 mL/min at retention times of 11, 6, and 11 min, respectively.
Enzymatic assays to determine the activity of KAT I and KAT II were adapted from previously published protocols.51 The rest of the brain, homogenized 1:5 (w/v) in ultrapure water, was diluted with homogenization buffer, 5 mM tris acetate buffer pH 8.0 containing 50 µM pyridoxal 5’-phosphate (P5P) and 10 mM β-mercaptoethanol, and dialyzed against the homogenization buffer overnight at 4°C. The next day, the homogenate (1:20 final dilution) was combined with separate reaction mixtures for KAT I (2 or 100 μM L-kynurenine sulfate, 80 μM P5P, 80 μM pyruvate, and 150 mM AMP buffer pH 9.5) or KAT II (2 or 100 μM L-kynurenine sulfate, 80 μM P5P, 80 μM pyruvate, and 150 mM tris acetate buffer pH 7.4) determination and incubated at 37°C for 2 hr. Blanks were assessed by adding 1 mM aminooxyacetate to the reaction. Addition of 50 μL of 6% perchloric acid stopped the reaction, tubes were vortexed and centrifuged, and the supernatant analyzed for KYNA via HPLC, as described above.
Statistical Analysis
All statistical analyses were performed using Prism 8.0 (GraphPad Software), and all results are shown in the supplementary statistical tables, including an overview of the assumptions met in association with the statistical tests. Normality was assessed with the Shapiro–Wilk test. Homogeneity of variance was assessed by residual and homoscedastity plots. When a parametric distribution was not achieved, visual inspection of the data was performed using Q–Q plots to confirm a relative bell-shaped distribution and the absence of outliers. Telemetry data were averaged across the recording days and 3-way repeated-measures ANOVA were conducted with prenatal treatment and sex as between-subject factors and time of day as a within-subject factor. Biochemical metabolite data were analyzed by 3-way ANOVA with prenatal treatment, sex, and phase as between-subject factors. KAT enzyme assay data were analyzed separately by sex using 3-way repeated-measures ANOVA to address prenatal treatment and ZT as between-subject factors and kynurenine concentration as a within-subject factor. Analyses were followed up with appropriate 2-way interactions; and analyses were focused on main effects of prenatal treatment group and phase when interactions were not statistically significant. Bonferroni’s correction was used for multiple comparisons with an adjusted alpha for multiple comparisons. Statistical significance was defined as P < .05.
Results
Prenatal Kynurenine Treatment Leads to Reduced REM Duration and REM Bout Number in Adult Males
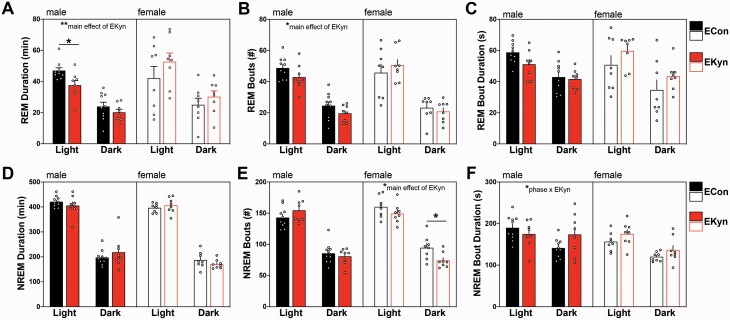
Rats cycle through sleep (REM and NREM) and wake bouts in both the light phase (ZT0-12) and dark phase (ZT12-24). To test our hypothesis that prenatal kynurenine elevation impairs sleep, we analyzed durations for each vigilance state in the light or dark phase of male and female ECon and EKyn offspring. For REM duration, we determined a significant sex × prenatal treatment interaction (F1,30=4.30, P = .047). In males, REM duration was significantly influenced by prenatal treatment (F1,16 = 12.13, P = .003). EKyn males had a −20% change in REM duration during the light phase (P = .02) compared to ECon males (figure 2A).
Fig. 2.
Prenatal kynurenine treatment alters rapid eye movement (REM) and non-REM (NREM) sleep in a sex-specific manner. Data are represented by phase: (A) REM duration; (B) number of REM bouts; (C) average REM bout duration; (D) NREM duration; (E) number of NREM bouts; (F) average NREM bout duration. Data are mean ± SEM. Repeated-measures 2-way ANOVA analyses followed by Bonferroni’s post hoc test: *P < .05; Data are mean ± SEM; n = 6–11 litters per group.
We evaluated how prenatal kynurenine elevation influences REM architecture by assessing the number of bouts and bout duration. EKyn males exhibited a significant decrease in the number of REM bouts (F1,16 = 5.11, P = .038; figure 2B). In females, the number of REM bouts was not impacted by EKyn treatment.
The average bout duration of a sleep state provides information about the ability to remain within a given phase. Analysis of the average duration of each REM bout determined only a significant main effect of phase (F1,30 = 57.5, P < .0001), with longer average durations, independent of EKyn treatment, during the light phase than the dark phase (figure 2C).
Prenatal Kynurenine Treatment Alters NREM Sleep Architecture in Adult Females
Next, we sought to determine the effects of prenatal kynurenine treatment on NREM sleep. NREM duration was significantly phase dependent (males: F1,16 = 221.1, P < .0001; females: F1,14 = 761.6, P < .0001); however, NREM duration was not different between EKyn and ECon offspring (figure 2D).
Analysis of the number of NREM bouts revealed a prenatal treatment × phase interaction (F1,30 = 4.60, P = .04; figure 2E). In males we found an approaching trend in the interaction between prenatal treatment × phase (F1,16 = 4.43, P = .051) for NREM bout number. NREM bout number (F1,14 = 5.59, P = .03) was significantly impacted by prenatal treatment and reduced by 22% in EKyn compared to ECon females (P = .04) during the dark phase.
The average duration of each NREM bout was significantly impacted by a phase × sex × prenatal treatment interaction (F1,30 = 4.72, P = .038) in addition to the main effects of sex (F1,30 = 6.86, P = .014) and phase (F1,30 = 29.16, P < .0001; figure 2F). In males, we determined a phase × prenatal treatment interaction (F1,16 = 5.76, P = .029) that was not significant in females (F1,14 = 0.06, P = .81).
Prenatal Kynurenine Treatment Prolongs Bouts of Wakefulness During the Dark Phase in Females
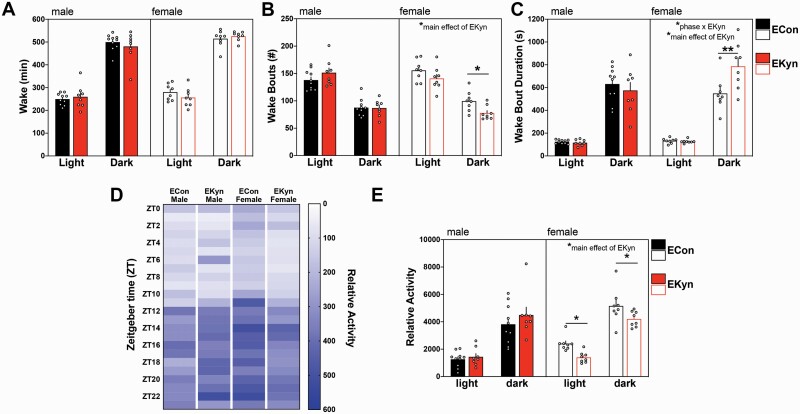
We next assessed the impact of prenatal kynurenine treatment on wake parameters. Aside from a predicted main effect of phase (F1,30 = 769.8, P < .0001), no significant interactions or main effects were observed for total wake duration during either phase (figure 3A).
Fig. 3.
Prenatal kynurenine treatment prolongs wakefulness and reduces activity in females. Data are represented by phase: (A) wake duration; (B) number of wake bouts; (C) average wake bout duration; (D) a heat map depicting relative cage activity levels per 1-h bin; (E) relative cage activity by phase. Data are mean ± SEM. Repeated-measures 2-way ANOVA analyses followed by Bonferroni’s post hoc test: *P < .05, **P < .001; Data are mean ± SEM; n = 6–11 litters per group.
Prenatal kynurenine exposure changed the architecture of wakefulness. Specifically, we found a significant interaction between sex × prenatal treatment (F1,30 = 4.69, P = .038) when we assessed the number of wake bouts (figure 3B). EKyn treatment did not impact the number of wake bouts in males (F1,16 = 0.65, P = .43); however, EKyn females had significantly less wake bouts across both phases (F1,14 = 4.92, P = .043) and a 22% reduction in wake bouts compared to ECon females during the dark phase (P = .048).
The average wake bout duration was significantly impacted by a phase × sex × prenatal treatment interaction (F1,30 = 6.01, P = .020), as well as a sex × prenatal treatment interaction (F1,30 = 5.64, P = .024; figure 3C). Sex-specific analysis determined a phase × prenatal treatment interaction in females (F1,14 = 7.18, P = .018), that was not observed in the males (F1,16 = 0.38, P = .55). Strikingly, EKyn treatment increased the duration of each wake bout in females (F1,14 = 6.93, P = .02) but not males (F1,16 = 0.55, P = .47). EKyn females had 1.4-fold longer average wake bout duration compared to ECon females (P = .0016) during the dark phase, suggesting consolidated wakefulness.
Prenatal Kynurenine Treatment Alters Activity Levels and Temperature in a Sex-Dependent Manner
To complement sleep–wake behavioral analyses, activity and core body temperature were collected during telemetric recordings. Relative cage activity was significantly impacted by several interactions, including sex × prenatal treatment (F1,30 = 5.44, P = .027), phase × sex (F23,670 = 1.87, P = .008) and phase × sex × prenatal treatment (F23,670 = 1.85, P = .009; figure 3D). When relative cage activity was assessed per phase (figure 3E), we determined that EKyn treatment significantly impacted the cage activity of females (F1,14 = 8.40, P = .01) but not males (F1,16 = 0.88, P = .36). EKyn females were 42% less active during the light phase (P = 0.03) and 18% less active during the dark phase (P = 0.04) compared to controls.
Core body temperature was significantly impacted by phase (F23,650 = 6.75, P < .0001) and sex (F1,30 = 4.67, P = .039; supplementary figure 1A). Body temperature was significantly higher during the dark phase when the rats were also more active (supplementary figure 1B).
Prenatal Kynurenine Exposure Influences Sleep–Wake Transitions
Prenatal kynurenine treatment differentially impacted vigilance state transitions in males and females. For males, a main effect of prenatal condition was found for NREM–REM transitions (F1,16 = 7.09, P = .017) and REM–wake transitions (F1,16 = 7.21, P = .016). For females, a main effect of prenatal condition was observed for wake–NREM transitions (F1,14 = 5.22, P = .039). For REM–NREM transitions, a phase × prenatal treatment interaction was observed for females (F1,14 = 5.61, P = .033) as well as a main effect of prenatal condition (F1,14 = 7.13, P = .018). Post hoc analyses revealed a 10% decrease in wake–NREM transitions (P = .03) during the dark phase and a 226% increase in REM–NREM transitions (P = .003) during the light phase in EKyn females compared to controls (table 1).
Table 1.
Impact of EKyn treatment on number of transitions to and from each vigilance state. The number of transitions to and from each vigilance state during ZT 0–12 (light phase, white) and ZT 12–24 (dark phase, gray) are shown. Data are represented as mean ± SEM. NREM: non-rapid eye movement. REM: rapid eye movement. Data are mean ± SEM. Repeated-measures 2-way ANOVA. Main effects for EKyn treatment are shown with a red box and Bonferroni’s post hoc test differences between ECon and EKyn are shown as *P < .05, **P < .01; n = 6–11 per group
| ECon Male | EKyn Male | ECon Female | EKyn Female | |
|---|---|---|---|---|
| Wake to NREM | 132.0 ± 5.7 | 138.2 ± 4.8 | 149.2 ± 7.1 | 134.2 ± 7.2* |
| 82.1 ± 5.3 | 82.0 ± 7.4 | 91.3 ± 6.4 | 68.1 ± 4.9 | |
| REM to Wake | 38.9 ± 3.9 | 37.4 ± 3.0 | 41.3 ± 5.4 | 43.3 ± 2.7 |
| 28.3 ± 3.8 | 21.3 ± 1.8 | 17.3 ± 2.2 | 27.7 ± 2.7 | |
| REM to NREM | 1.8 ± 0.7 | 2.6 ± 1.7 | 4.1 ± 1.4 | 13.2 ± 3.2** |
| 1.9 ± 0.5 | 1.2 ± 0.5 | 0.9 ± 0.4 | 4.5 ± 1.3 | |
| NREM to Wake | 86.1 ± 6.1 | 101.3 ± 4.3 | 154.2 ± 16.3 | 142.6 ± 15.2 |
| 61.0 ± 5.1 | 60.2 ± 6.7 | 75.1 ± 6.3 | 50.4 ± 6.0 | |
| NREM to REM | 49.4 ± 3.1 | 39.9 ± 2.8 | 46.2 ± 6.4 | 48.2 ± 3.9 |
| 26.0 ± 2.0 | 22.4 ± 2.1 | 16.7 ± 2.3 | 19.7 ± 2.2 |
Basal sex differences were observed for the following transitions: wake–NREM (sex × prenatal treatment: F1,30 = 4.58, P = .04), REM–wake (phase × sex: F1,30 = 4.24, P = .048), REM–NREM (phase × sex: F1,30 = 15.73, P < .004; sex: F1,30 = 10.84, P = .0025), and NREM–wake (phase × sex: F1,30 = 28.19, P < .0001; sex: F1,30 = 14.05, P = .0008).
Sex, Not Prenatal Kynurenine Treatment, Influences REM and NREM Spectral Power
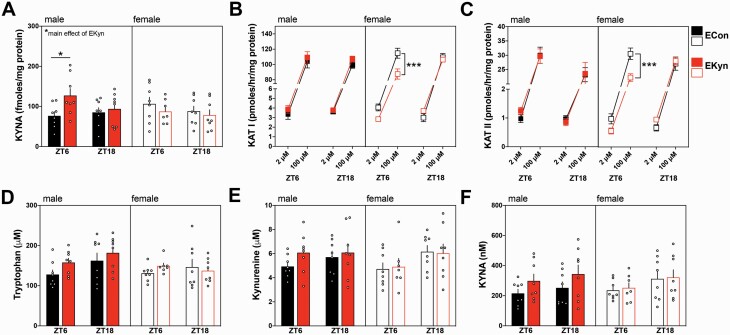
We determined that prenatal kynurenine exposure did not adversely impact spectral power during REM or NREM sleep. However, sex- and phase-dependent alterations are shown in supplementary figure 2. Brain KYNA is elevated in EKyn males during the light phase, while EKyn females have reduced activity of KAT I and II.
To pinpoint possible biochemical changes contributing to the manifestation of sleep behavior changes in EKyn offspring, tissues from male and female offspring were collected in the middle of the light phase, ZT6, and in the middle of the dark phase, ZT18, to assess KP metabolism. KYNA in the cortex was significantly impacted by a sex × prenatal treatment interaction (F1,55 = 5.102, P = .03). Specifically, during the light phase, EKyn males had 36% increase in levels of brain KYNA (F1,28 = 4.61, P = .04). Of note, EKyn females did not exhibit significant alterations in brain KYNA (figure 4A).
Fig. 4.
Brain KYNA is elevated in male EKyn offspring and KAT activity is reduced in female EKyn offspring. (A) Brain KYNA; (B) Brain KAT I activity; (C) Brain KAT II activity; (D) plasma tryptophan; (E) Plasma kynurenine; (F) Plasma KYNA were assessed at ZT 6 and ZT18. Data are mean ± SEM. Repeated-measures ANOVA with Bonferroni’s post hoc test: *P < .05, ***P < .001; n = 7–8 per group.
In males, activity of KAT I (figure 4B) and KAT II (figure 4C) were not influenced by prenatal treatment. However, in females, we determined a significant interaction between the time at which the tissue was collected and prenatal treatment for both KAT I (F1,24 = 5.27, P = .03) and KAT II (F1,24 = 7.29, P = .01) activity in the brain. Specifically, at ZT 6, post hoc analyses revealed significantly lower activity of KAT I (P = .0001) and KAT II (P = .0003) in EKyn females. Tryptophan (figure 4D), kynurenine (figure 4E), and KYNA (figure 4F) in the plasma were not significantly impacted by EKyn treatment or time of tissue collection.
Discussion
The etiology of SZ and BD points to the prominence of KP dysregulation9,14,15,52 and sleep disturbances,4,8,53,54 which often precede the onset of psychosis and are associated with symptom severity.1,55–57 Elevated KYNA has been implicated in cognitive disturbances; thus, we presently used the KYNA hypothesis-derived EKyn model in rats to investigate the role of KP dysregulation on sleep–wake behavior. We determined aberrant KP metabolism and identified sex-specific changes in vigilance state parameters in EKyn offspring. Increased brain KYNA levels and reduced REM durations were found in male EKyn offspring, while reduced brain KAT activity and a hyperaroused phenotype were determined in EKyn females compared to controls.
From a translational perspective, paradigms that recapitulate elevated prenatal KYNA41,44,58–61 and behavioral endophenotypes that are relevant for psychotic disorders are imperative. Our study provides translational perspectives around sleep and arousal systems, specific research domain criteria, and furthers our ability to uncover mechanisms contributing to psychopathology, with the added benefit of homogeneity within the rodent subjects.62 Clinical studies in patients with SZ show an array of sleep dysfunction across the spectrum of first-episode, unmedicated, and medicated patients.3,5,8 In line with our present findings, modest decreases in REM duration have been reported in unmedicated patients, while NREM disruptions, pinpointing to architecture changes, have been more consistently noted.63–67 Poor sleep quality, reduced activity, and insomnia are common to patients with SZ and BD, and female patients endorsed more sleep disturbances.48,68,69 Within clinical studies, inconsistencies among vigilance state parameters may be attributable to antipsychotic treatments and differences in subjective and objective analysis of sleep in individuals with psychiatric illness.69,70
The conspicuous sex differences in the EKyn model may elucidate neurobiological underpinnings that present clinically in cognitive domains in male and female patients.20,71–74 We determined that EKyn males have reduced REM duration, recapitulating findings wherein REM duration is reduced with acute kynurenine challenge.34 EKyn males also transitioned less frequently from NREM to REM, suggesting a difficulty in initiating REM. As REM theta oscillations play an important role in procedural and contextual memory consolidation,35 the evidence of reduced REM duration supports cognitive dysfunction characterized in EKyn males.47 As the activation of cholinergic receptors can promote the generation of theta oscillations in the septohippocampal formation,75,76 the transient increase in KYNA during the light phase in EKyn males may inhibit cholinergic activation,18 thereby disrupting REM sleep.77 Future studies will further our understanding of aberrant sleep and investigate intricacies of sleep spindles that are critical for memory consolidation and reduced in patients with SZ67,78–81, yet acknowledged as a technical limitation presently.
EKyn females display distinct changes in sleep and arousal; however, they do not present sustained elevation in KYNA in the brain.33 Sex differences and the role of sex hormones in sleep have been well characterized in rodents, with studies demonstrating that estrogen modulates female rodent sleep homeostasis and increases wakefulness during the dark phase.50,82–84 Presently, female EKyn offspring exhibited enhanced arousal by having fewer NREM and wake bouts and longer wake bout durations than ECon offspring, indicative of an insomnia-like phenotype, which is common among women suffering from psychiatric illness.85,86 Interestingly, EKyn females also exhibited less activity during the dark phase, perhaps indicating psychomotor slowing, which could be classified as a depressive symptom87 or alternatively a sign of avolition, a core negative symptom of SZ.88 While the impact of prenatal kynurenine elevation in females remains elusive, we recently demonstrated that female EKyn offspring also do not have the same cognitive dysfunction as male EKyn offspring.47 As female patients with SZ often present with symptoms at a later age than males,89,90 future studies will investigate the trajectory of sleep dysfunction in our model.
To explore the mechanistic phenomena in brain KYNA, we assessed the activity of KYNA synthesizing enzymes KAT I and KAT II in the brain and determined decreases in both enzymes in the female EKyn. The estradiol derivative estradiol disulfate has been characterized as a potent inhibitor of both KAT I and KAT II,91 and perhaps circulating estrogen in the EKyn female are providing a protective effect on KYNA dynamics throughout early adulthood. We presently did not control for stages of the estrous cycle, an important consideration for future studies that target KAT II to inhibit KYNA formation.49,91–93 We must also thoroughly consider the contribution of the kynurenine-3-monooxygenase arm of the KP, which generates 3-hydroxykynurenine and quinolinic acid (QUIN) when interpreting future results.94 Given that female rats show conspicuous arousal patterns, without brain KYNA alterations, evaluating brain contents of QUIN may provide mechanistic insight as alterations in KP have notably been implicated in mood disorders.52
In summary, prenatal kynurenine treatment in rats during the last week of gestation elicited distinct sex-dependent changes in sleep–wake behavior and architecture and brain-specific changes in KYNA. Our findings further support the role of the KP with respect to the neurodevelopmental hypothesis of psychotic illnesses60,95 and highlight important translationally relevant sex differences. As previously shown, peripheral KP metabolism does not readily translate to metabolic activity observed in the brain,44,46,47 and, so, to get an accurate understanding clinically, direct central nervous system measurements of KP metabolites should be assessed.96,97 Continuing studies are aimed at identifying therapeutic avenues for reducing KYNA synthesis to improve sleep and cognition in addition to investigating how sex differences in KP metabolism may influence therapeutic trajectories.
Supplementary Material
Acknowledgments
This work was funded by the National Institutes of Health (Grant No. NIH R01 NS102209 and P50 MH103222) and support from the University of South Carolina (Magellan Scholar and Science Undergraduate Research Fellowships).
Conflict of interest: none.
References
- 1.Afonso P, Figueira ML, Paiva T. Sleep-wake patterns in schizophrenia patients compared to healthy controls. World J Biol Psychiatry. 2014;15(7):517–524. [DOI] [PubMed] [Google Scholar]
- 2.Harvey AG, Schmidt DA, Scarnà A, Semler CN, Goodwin GM. Sleep-related functioning in euthymic patients with bipolar disorder, patients with insomnia, and subjects without sleep problems. Am J Psychiatry. 2005;162(1):50–57. [DOI] [PubMed] [Google Scholar]
- 3.Klingaman EA, Palmer-Bacon J, Bennett ME, Rowland LM. Sleep disorders among people with schizophrenia: emerging research. Curr Psychiatry Rep. 2015;17(10):79. [DOI] [PubMed] [Google Scholar]
- 4.Palmese LB, DeGeorge PC, Ratliff JC, et al. Insomnia is frequent in schizophrenia and associated with night eating and obesity. Schizophr Res. 2011;133(1–3):238–243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rowland LM, Wickwire EM. A wake-up call: assess and treat sleep disorders in early psychosis. Schizophr Bull 2019;45(2):265–266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Steardo L Jr, de Filippis R, Carbone EA, Segura-Garcia C, Verkhratsky A, De Fazio P. Sleep disturbance in bipolar disorder: neuroglia and circadian rhythms. Front Psychiatry. 2019;10:501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Boland EM, Alloy LB. Sleep disturbance and cognitive deficits in bipolar disorder: toward an integrated examination of disorder maintenance and functional impairment. Clin Psychol Rev. 2013;33(1):33–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pocivavsek A, Rowland LM. Basic neuroscience illuminates causal relationship between sleep and memory: translating to schizophrenia. Schizophr Bull. 2018;44(1):7–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Erhardt S, Blennow K, Nordin C, Skogh E, Lindström LH, Engberg G. Kynurenic acid levels are elevated in the cerebrospinal fluid of patients with schizophrenia. Neurosci Lett. 2001;313(1–2):96–98. [DOI] [PubMed] [Google Scholar]
- 10.Linderholm KR, Skogh E, Olsson SK, et al. Increased levels of kynurenine and kynurenic acid in the CSF of patients with schizophrenia. Schizophrenia Bull. 2012;38(3):426–432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Miller CL, Llenos IC, Dulay JR, Weis S. Upregulation of the initiating step of the kynurenine pathway in postmortem anterior cingulate cortex from individuals with schizophrenia and bipolar disorder. Brain Res. 2006;1073–1074:25–37. [DOI] [PubMed] [Google Scholar]
- 12.Nilsson LK, Linderholm KR, Engberg G, et al. Elevated levels of kynurenic acid in the cerebrospinal fluid of male patients with schizophrenia. Schizophr Res. 2005;80(2–3):315–322. [DOI] [PubMed] [Google Scholar]
- 13.Sathyasaikumar KV, Stachowski EK, Wonodi I, et al. Impaired kynurenine pathway metabolism in the prefrontal cortex of individuals with schizophrenia. Schizophrenia Bull. 2011;37(6):1147–1156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Schwarcz R, Rassoulpour A, Wu HQ, Medoff D, Tamminga CA, Roberts RC. Increased cortical kynurenate content in schizophrenia. Biol Psychiatry. 2001;50(7):521–530. [DOI] [PubMed] [Google Scholar]
- 15.Sellgren CM, Gracias J, Jungholm O, et al. Peripheral and central levels of kynurenic acid in bipolar disorder subjects and healthy controls. Transl Psychiatry. 2019;9(1):37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sellgren CM, Kegel ME, Bergen SE, et al. A genome-wide association study of kynurenic acid in cerebrospinal fluid: implications for psychosis and cognitive impairment in bipolar disorder. Mol Psychiatry. 2016;21(10):1342–1350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Birch PJ, Grossman CJ, Hayes AG. Kynurenic acid antagonises responses to NMDA via an action at the strychnine-insensitive glycine receptor. Eur J Pharmacol. 1988;154(1):85–87. [DOI] [PubMed] [Google Scholar]
- 18.Hilmas C, Pereira EF, Alkondon M, Rassoulpour A, Schwarcz R, Albuquerque EX. The brain metabolite kynurenic acid inhibits alpha7 nicotinic receptor activity and increases non-alpha7 nicotinic receptor expression: physiopathological implications. J Neurosci. 2001;21(19):7463–7473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Konradsson-Geuken A, Wu HQ, Gash CR, et al. Cortical kynurenic acid bi-directionally modulates prefrontal glutamate levels as assessed by microdialysis and rapid electrochemistry. Neuroscience. 2010;169(4):1848–1859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pocivavsek A, Wu HQ, Potter MC, Elmer GI, Pellicciari R, Schwarcz R. Fluctuations in endogenous kynurenic acid control hippocampal glutamate and memory. Neuropsychopharmacology. 2011;36(11):2357–2367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zmarowski A, Wu HQ, Brooks JM, et al. Astrocyte-derived kynurenic acid modulates basal and evoked cortical acetylcholine release. Eur J Neurosci. 2009;29(3):529–538. [DOI] [PubMed] [Google Scholar]
- 22.DiNatale BC, Murray IA, Schroeder JC, et al. Kynurenic acid is a potent endogenous aryl hydrocarbon receptor ligand that synergistically induces interleukin-6 in the presence of inflammatory signaling. Toxicol Sci. 2010;115(1):89–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.García-Lara L, Pérez-Severiano F, González-Esquivel D, Elizondo G, Segovia J. Absence of aryl hydrocarbon receptors increases endogenous kynurenic acid levels and protects mouse brain against excitotoxic insult and oxidative stress. J Neurosci Res. 2015;93(9):1423–1433. [DOI] [PubMed] [Google Scholar]
- 24.Moroni F, Cozzi A, Sili M, Mannaioni G. Kynurenic acid: a metabolite with multiple actions and multiple targets in brain and periphery. J Neural Transm (Vienna). 2012;119(2):133–139. [DOI] [PubMed] [Google Scholar]
- 25.Oxenkrug G, Cornicelli J, van der Hart M, Roeser J, Summergrad P. Kynurenic acid, an aryl hydrocarbon receptor ligand, is elevated in serum of Zucker fatty rats. Integr Mol Med. 2016;3(4):761–763. [PMC free article] [PubMed] [Google Scholar]
- 26.Agudelo LZ, Ferreira DMS, Cervenka I, et al. Kynurenic acid and Gpr35 regulate adipose tissue energy homeostasis and inflammation. Cell Metab. 2018;27(2):378–392; e375. [DOI] [PubMed] [Google Scholar]
- 27.Guidetti P, Hoffman GE, Melendez-Ferro M, Albuquerque EX, Schwarcz R. Astrocytic localization of kynurenine aminotransferase II in the rat brain visualized by immunocytochemistry. Glia. 2007;55(1):78–92. [DOI] [PubMed] [Google Scholar]
- 28.Song C, Clark SM, Vaughn CN, et al. Quantitative analysis of kynurenine aminotransferase II in the adult rat brain reveals high expression in proliferative zones and corpus callosum. Neuroscience. 2018;369:1–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chess AC, Landers AM, Bucci DJ. L-kynurenine treatment alters contextual fear conditioning and context discrimination but not cue-specific fear conditioning. Behav Brain Res. 2009;201(2):325–331. [DOI] [PubMed] [Google Scholar]
- 30.Chess AC, Simoni MK, Alling TE, Bucci DJ. Elevations of endogenous kynurenic acid produce spatial working memory deficits. Schizophr Bull. 2007;33(3):797–804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Erhardt S, Schwieler L, Emanuelsson C, Geyer M. Endogenous kynurenic acid disrupts prepulse inhibition. Biol Psychiatry. 2004;56(4):255–260. [DOI] [PubMed] [Google Scholar]
- 32.Shepard PD, Joy B, Clerkin L, Schwarcz R. Micromolar brain levels of kynurenic acid are associated with a disruption of auditory sensory gating in the rat. Neuropsychopharmacology. 2003;28(8):1454–1462. [DOI] [PubMed] [Google Scholar]
- 33.Baratta AM, Buck SA, Buchla AD, et al. Sex differences in hippocampal memory and kynurenic acid formation following acute sleep deprivation in rats. Sci Rep. 2018;8(1):6963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Pocivavsek A, Baratta AM, Mong JA, Viechweg SS. Acute kynurenine challenge disrupts sleep-wake architecture and impairs contextual memory in adult rats. Sleep 2017;40(11):pii: zsx141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Boyce R, Glasgow SD, Williams S, Adamantidis A. Causal evidence for the role of REM sleep theta rhythm in contextual memory consolidation. Science. 2016;352(6287):812–816. [DOI] [PubMed] [Google Scholar]
- 36.Howes OD, McDonald C, Cannon M, Arseneault L, Boydell J, Murray RM. Pathways to schizophrenia: the impact of environmental factors. Int J Neuropsychopharmacol. 2004;7(suppl 1):S7–S13. [DOI] [PubMed] [Google Scholar]
- 37.Meyer U, Yee BK, Feldon J. The neurodevelopmental impact of prenatal infections at different times of pregnancy: the earlier the worse? Neuroscientist. 2007;13(3):241–256. [DOI] [PubMed] [Google Scholar]
- 38.Murray RM, Sham P, Van Os J, Zanelli J, Cannon M, McDonald C. A developmental model for similarities and dissimilarities between schizophrenia and bipolar disorder. Schizophr Res. 2004;71(2–3):405–416. [DOI] [PubMed] [Google Scholar]
- 39.Kloiber S, Rosenblat JD, Husain MI, et al. Neurodevelopmental pathways in bipolar disorder. Neurosci Biobehav Rev. 2020;112:213–226. [DOI] [PubMed] [Google Scholar]
- 40.van Os J, Selten JP. Prenatal exposure to maternal stress and subsequent schizophrenia. The May 1940 invasion of The Netherlands. Br J Psychiatry. 1998;172:324–326. [DOI] [PubMed] [Google Scholar]
- 41.Notarangelo FM, Schwarcz R. Restraint stress during pregnancy rapidly raises kynurenic acid levels in mouse placenta and fetal brain. Dev Neurosci. 2016;38(6):458–468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Notarangelo FM, Pocivavsek A. Elevated kynurenine pathway metabolism during neurodevelopment: implications for brain and behavior. Neuropharmacology. 2017;112(Pt B):275–285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Groer M, Fuchs D, Duffy A, Louis-Jacques A, D’Agata A, Postolache TT. Associations among obesity, inflammation, and tryptophan catabolism in pregnancy. Biol Res Nurs. 2018;20(3):284–291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Pocivavsek A, Thomas MA, Elmer GI, Bruno JP, Schwarcz R. Continuous kynurenine administration during the prenatal period, but not during adolescence, causes learning and memory deficits in adult rats. Psychopharmacology (Berl). 2014;231(14):2799–2809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Beggiato S, Notarangelo FM, Sathyasaikumar KV, Giorgini F, Schwarcz R. Maternal genotype determines kynurenic acid levels in the fetal brain: implications for the pathophysiology of schizophrenia. J Psychopharmacol. 2018;32(11):1223–1232. [DOI] [PubMed] [Google Scholar]
- 46.Pershing ML, Bortz DM, Pocivavsek A, et al. Elevated levels of kynurenic acid during gestation produce neurochemical, morphological, and cognitive deficits in adulthood: implications for schizophrenia. Neuropharmacology. 2015;90:33–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Buck SA, Baratta AM, Pocivavsek A. Exposure to elevated embryonic kynurenine in rats: sex-dependent learning and memory impairments in adult offspring. Neurobiol Learn Mem. 2020;174:107282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Chen MH, Korenic SA, Wickwire EM, Wijtenburg SA, Hong LE, Rowland LM. Sex differences in subjective sleep quality patterns in schizophrenia. Behav Sleep Med. 2020;18(5):668–679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Pocivavsek A, Elmer GI, Schwarcz R. Inhibition of kynurenine aminotransferase II attenuates hippocampus-dependent memory deficit in adult rats treated prenatally with kynurenine. Hippocampus. 2019;29(2):73–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Schwartz MD, Mong JA. Estradiol modulates recovery of REM sleep in a time-of-day-dependent manner. Am J Physiol Regul Integr Comp Physiol. 2013;305(3):R271–R280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ceresoli-Borroni G, Schwarcz R. Perinatal kynurenine pathway metabolism in the normal and asphyctic rat brain. Amino Acids. 2000;19(1):311–323. [DOI] [PubMed] [Google Scholar]
- 52.Marx W, McGuinness AJ, Rocks T, et al. The kynurenine pathway in major depressive disorder, bipolar disorder, and schizophrenia: a meta-analysis of 101 studies. Mol Psychiatry. 2020.doi: 10.1038/s41380-020-00951-9. [DOI] [PubMed] [Google Scholar]
- 53.Kaskie RE, Graziano B, Ferrarelli F. Schizophrenia and sleep disorders: links, risks, and management challenges. Nat Sci Sleep. 2017;9:227–239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Mukherjee D, Krishnamurthy VB, Millett CE, et al. Total sleep time and kynurenine metabolism associated with mood symptom severity in bipolar disorder. Bipolar Disord. 2018;20(1):27–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Bromundt V, Koster M, Georgiev-Kill A, et al. Sleep-wake cycles and cognitive functioning in schizophrenia. Br J Psychiatry. 2011;198(4):269–276. [DOI] [PubMed] [Google Scholar]
- 56.Keshavan MS, Montrose DM, Miewald JM, Jindal RD. Sleep correlates of cognition in early course psychotic disorders. Schizophr Res. 2011;131(1-3):231–234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Reeve S, Sheaves B, Freeman D. The role of sleep dysfunction in the occurrence of delusions and hallucinations: a systematic review. Clin Psychol Rev. 2015;42:96–115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Baratta AM, Kanyuch NR, Cole CA, Valafar H, Deslauriers J, Pocivavsek A. Acute sleep deprivation during pregnancy in rats: rapid elevation of placental and fetal inflammation and kynurenic acid. Neurobiol Stress. 2020;12:100204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Clark SM, Notarangelo FM, Li X, Chen S, Schwarcz R, Tonelli LH. Maternal immune activation in rats blunts brain cytokine and kynurenine pathway responses to a second immune challenge in early adulthood. Prog Neuropsychopharmacol Biol Psychiatry. 2019;89:286–294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Forrest CM, Khalil OS, Pisar M, Darlington LG, Stone TW. Prenatal inhibition of the tryptophan-kynurenine pathway alters synaptic plasticity and protein expression in the rat hippocampus. Brain Res. 2013;1504:1–15. [DOI] [PubMed] [Google Scholar]
- 61.Forrest CM, McNair K, Pisar M, Khalil OS, Darlington LG, Stone TW. Altered hippocampal plasticity by prenatal kynurenine administration, kynurenine-3-monoxygenase (KMO) deletion or galantamine. Neuroscience. 2015;310:91–105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Elmer GI, Brown PL, Shepard PD. Engaging research domain criteria (RDoC): neurocircuitry in search of meaning. Schizophr Bull. 2016;42(5):1090–1095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Monti JM, BaHammam AS, Pandi-Perumal SR, Bromundt V, Spence DW, Cardinali DP, Brown GM. Sleep and circadian rhythm dysregulation in schizophrenia. Prog Neuropsychopharmacol Biol Psychiatry. 2013;43:209–216. [DOI] [PubMed] [Google Scholar]
- 64.Tandon R, Shipley JE, Taylor S, et al. Electroencephalographic sleep abnormalities in schizophrenia. Relationship to positive/negative symptoms and prior neuroleptic treatment. Arch Gen Psychiatry. 1992;49(3):185–194. [DOI] [PubMed] [Google Scholar]
- 65.Yetkin S, Aydin H, Ozgen F, Sutcigil L, Bozkurt A. [Sleep architecture in schizophrenia patients]. Turk Psikiyatri Derg. 2011;22(1):1–9. [PubMed] [Google Scholar]
- 66.Sasidharan A, Kumar S, Nair AK, et al. Further evidences for sleep instability and impaired spindle-delta dynamics in schizophrenia: a whole-night polysomnography study with neuroloop-gain and sleep-cycle analysis. Sleep Med. 2017;38:1–13. [DOI] [PubMed] [Google Scholar]
- 67.Ferrarelli F, Huber R, Peterson MJ, et al. Reduced sleep spindle activity in schizophrenia patients. Am J Psychiatry. 2007;164(3):483–492. [DOI] [PubMed] [Google Scholar]
- 68.Wulff K, Dijk DJ, Middleton B, Foster RG, Joyce EM. Sleep and circadian rhythm disruption in schizophrenia. Br J Psychiatry. 2012;200(4):308–316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Lee EE, Ancoli-Israel S, Eyler LT, et al. Sleep disturbances and inflammatory biomarkers in schizophrenia: focus on sex differences. Am J Geriatr Psychiatry. 2019;27(1):21–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Chouinard S, Poulin J, Stip E, Godbout R. Sleep in untreated patients with schizophrenia: a meta-analysis. Schizophr Bull. 2004;30(4):957–967. [DOI] [PubMed] [Google Scholar]
- 71.Aleman A, Kahn RS, Selten JP. Sex differences in the risk of schizophrenia: evidence from meta-analysis. Arch Gen Psychiatry. 2003;60(6):565–571. [DOI] [PubMed] [Google Scholar]
- 72.Leger M, Neill JC. A systematic review comparing sex differences in cognitive function in schizophrenia and in rodent models for schizophrenia, implications for improved therapeutic strategies. Neurosci Biobehav Rev. 2016;68:979–1000. [DOI] [PubMed] [Google Scholar]
- 73.Mendrek A, Mancini-Marïe A. Sex/gender differences in the brain and cognition in schizophrenia. Neurosci Biobehav Rev. 2016;67:57–78. [DOI] [PubMed] [Google Scholar]
- 74.Wickens MM, Bangasser DA, Briand LA. Sex differences in psychiatric disease: a focus on the glutamate system. Front Mol Neurosci. 2018;11:197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Lu CB, Henderson Z. Nicotine induction of theta frequency oscillations in rodent hippocampus in vitro. Neuroscience. 2010;166(1):84–93. [DOI] [PubMed] [Google Scholar]
- 76.Siok CJ, Rogers JA, Kocsis B, Hajós M. Activation of alpha7 acetylcholine receptors augments stimulation-induced hippocampal theta oscillation. Eur J Neurosci. 2006;23(2):570–574. [DOI] [PubMed] [Google Scholar]
- 77.Záborszky L, Gombkoto P, Varsanyi P, et al. Specific basal forebrain-cortical cholinergic circuits coordinate cognitive operations. J Neurosci. 2018;38(44):9446–9458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Ferrarelli F, Peterson MJ, Sarasso S, et al. Thalamic dysfunction in schizophrenia suggested by whole-night deficits in slow and fast spindles. Am J Psychiatry. 2010;167(11):1339–1348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Goder R, Graf A, Ballhausen F, et al. Impairment of sleep-related memory consolidation in schizophrenia: relevance of sleep spindles? Sleep Med. 2015;16(5):564–569. [DOI] [PubMed] [Google Scholar]
- 80.Wamsley EJ, Tucker MA, Shinn AK, et al. Reduced sleep spindles and spindle coherence in schizophrenia: mechanisms of impaired memory consolidation? Biol Psychiatry. 2012;71(2):154–161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Aguilar DD, Strecker RE, Basheer R, McNally JM. Alterations in sleep, sleep spindle, and EEG power in mGluR5 knockout mice. J Neurophysiol. 2020;123(1):22–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Gervais NJ, Mong JA, Lacreuse A. Ovarian hormones, sleep and cognition across the adult female lifespan: an integrated perspective. Front Neuroendocrinol. 2017;47:134–153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Mong JA, Baker FC, Mahoney MM, et al. Sleep, rhythms, and the endocrine brain: influence of sex and gonadal hormones. J Neurosci. 2011;31(45):16107–16116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Swift KM, Keus K, Echeverria CG, et al. Sex differences within sleep in gonadally intact rats. Sleep. 2020;43(5):zsz289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Zanini MA, Castro J, Cunha GR, et al. Abnormalities in sleep patterns in individuals at risk for psychosis and bipolar disorder. Schizophr Res. 2015;169(1–3):262–267. [DOI] [PubMed] [Google Scholar]
- 86.Saunders EF, Fernandez-Mendoza J, Kamali M, Assari S, McInnis MG. The effect of poor sleep quality on mood outcome differs between men and women: A longitudinal study of bipolar disorder. J Affect Disord. 2015;180:90–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Morrens M, Hulstijn W, Sabbe B. Psychomotor slowing in schizophrenia. Schizophr Bull. 2007;33(4):1038–1053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Strauss GP, Esfahlani FZ, Kirkpatrick B, et al. Network analysis reveals which negative symptom domains are most central in schizophrenia vs bipolar disorder. Schizophr Bull. 2019;45(6):1319–1330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Grossman LS, Harrow M, Rosen C, Faull R, Strauss GP. Sex differences in schizophrenia and other psychotic disorders: a 20-year longitudinal study of psychosis and recovery. Compr Psychiatry. 2008;49(6):523–529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Ochoa S, Usall J, Cobo J, Labad X, Kulkarni J. Gender differences in schizophrenia and first-episode psychosis: a comprehensive literature review. Schizophr Res Treatment. 2012;2012:916198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Jayawickrama GS, Nematollahi A, Sun G, Gorrell MD, Church WB. Inhibition of human kynurenine aminotransferase isozymes by estrogen and its derivatives. Sci Rep. 2017;7(1):17559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Blanco-Ayala T, Sathyasaikumar KV, Uys JD, Pérez-de-la-Cruz V, Pidugu LS, Schwarcz R. N-Acetylcysteine inhibits kynurenine aminotransferase II. Neuroscience. 2020;444:160–169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Wu HQ, Okuyama M, Kajii Y, Pocivavsek A, Bruno JP, Schwarcz R. Targeting kynurenine aminotransferase II in psychiatric diseases: promising effects of an orally active enzyme inhibitor. Schizophr Bull. 2014;40(suppl 2):S152–S158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Schwarcz R, Bruno JP, Muchowski PJ, Wu HQ. Kynurenines in the mammalian brain: when physiology meets pathology. Nat Rev Neurosci. 2012;13(7):465–477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Stone TW. Neuropharmacology of quinolinic and kynurenic acids. Pharmacol Rev. 1993;45(3):309–379. [PubMed] [Google Scholar]
- 96.Plitman E, Iwata Y, Caravaggio F, et al. Kynurenic acid in schizophrenia: a systematic review and meta-analysis. Schizophr Bull. 2017;43(4):764–777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Sellgren CM, Gracias J, Watmuff B, et al. Increased synapse elimination by microglia in schizophrenia patient-derived models of synaptic pruning. Nat Neurosci. 2019;22(3):374–385. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.