Abstract
Patients with one of the many chronic inflammatory disorders broadly classified as inflammatory bowel disease (IBD) now have a diverse set of immunomodulatory therapies at their disposal. Despite these recent medical advances, complete sustained remission of disease remains elusive for most patients. The full healing of the damaged intestinal mucosa is the primary goal of all therapies. Achieving this requires not just a reduction of the aberrant immunological response, but also wound healing of the epithelium. No currently approved therapy directly targets the epithelium. Epithelial repair is compromised in IBD and normally facilitates re-establishment of the homeostatic barrier between the host and the microbiome. In this review, we summarize the evidence that epithelial wound healing represents an important yet underdeveloped therapeutic modality for IBD. We highlight three general approaches that are promising for developing a new class of epithelium-targeted therapies: epithelial stem cells, cytokines, and microbiome engineering. We also provide a frank discussion of some of the challenges that must be overcome for epithelial repair to be therapeutically leveraged. A concerted approach by the field to develop new therapies targeting epithelial wound healing will offer patients a game-changing, complementary class of medications and could dramatically improve outcomes.
Introduction
If the therapies developed in the past thirty years for inflammatory bowel disease (IBD) represent the fruits of intense research into intestinal mucosal immunology, then the next thirty years may well mark the advent and profusion of therapies stemming from basic research in wound healing. The discoveries supporting this translational medicine could not be timelier. Despite access to an arsenal of medications that suppress the immune system, many IBD patients continue to experience reduced quality of life and poor outcomes that may require surgical intervention. The goal of any medical therapy for IBD, and the universally recognized gold standard that must be achieved to induce long-term remission of disease, is mucosal healing [1–3]. Central to mucosal healing is the restoration of the barrier function of the epithelium through wound healing processes. Experimental models of intestinal inflammation have highlighted important actors, including epithelial stem cells, stromal niche factors such as cytokines, and the microbiome, in the multi-scene play that restores the damaged intestinal mucosa to health. Discoveries of molecular crosstalk between these systems bring hope for a new generation of therapies that directly target epithelial wound repair. These new therapies could complement the current immune-targeting medications. Optimal outcomes in IBD patients will be achieved only after basic research and translational investments into the epithelial repair processes, and the stromal and host-microbe interactions controlling them, have yielded a new class of therapies.
With nearly 7 million people diagnosed with IBD globally [4], developing innovative approaches and interventions is an important public health matter. IBD represents a collection of many diseases that arise from the convergence of multiple factors, which by themselves are usually insufficient to cause disease. They present as two predominant phenotypes, ulcerative colitis (UC) and Crohn’s disease (CD), which have as their hallmark chronic immune activation, mucosal inflammation, and destruction. Current therapies are almost exclusively focused on reducing mucosal inflammation by acting on the immune system, although there is growing interest in modifying the gut microbiome which is typically skewed in patients with active disease. However, the importance of promoting healing of the gut epithelium and other mucosal subsystems in an injurious microenvironment has largely been neglected or understudied. Unsuccessful or inadequately treated chronic disease is often associated with a lack of mucosal healing; impaired healing can give rise to anomalous or compensatory responses. These can have serious sequelae that contributes to the chronicity of disease, treatment failure, and higher relative risk for gastrointestinal adenocarcinoma. Intestinal fibrosis can result in stricturing and fistula formation that are no longer medically manageable. In addition, the microbes comprising the intestinal microbiome must adapt to the inflammatory environment. In doing so, they change their metabolic outputs, and different taxa emerge [5, 6]. The result is a microbial dysbiosis that may sustain mucosal inflammation and further impair wound healing.
And so, the term “mucosal healing,” which refers to the restoration of normal intestinal architecture and homeostasis, has a definition that can be simultaneously narrow and broad and ambitious yet obvious. To be clear, it has not always been the endpoint of clinical treatment for IBD. For many years, it was common practice to assess a patient’s response by clinical indices based on symptomatology. However, there were often disconnects between symptom-based scoring and actual status of disease. Thus, direct endoscopic and histological criteria were developed to assess mucosal healing; these criteria are aggregated into scoring systems with defined cutoffs under which the mucosa are deemed healed (e.g., Mayo endoscopic subscore ≤ 1 [7, 8]). Endoscopic scoring systems, such as the Crohn’s Disease Endoscopic Index of Severity (CDEIS) [9] and Simple Endoscopic Score for Crohn’s Disease (SES-CD) [10], use refined criteria to qualify the depth of the lesions and approximate percentage of surface-area involvement. At the histological level, the Geboes score [11, 12], Robarts Histopathology Index [13], or Nancy Histological Index [14] are used to grade the status of mucosal healing. These systems are similar in that they consider both the status of immune cell infiltration into the mucosa and the morphology of the epithelium. To be considered healed, both the epithelial abnormalities and the immune infiltration into the mucosa must be resolved. The typical histological characteristics of inflamed mucosa and epithelial healing are shown in Figure 1. The highest grades of disease are characterized by crypt abscesses and marked attenuation of epithelium. Lower grades of disease are typified by mucosal infiltration of different types of immune cells, such as neutrophils, plasma cells, or eosinophils, into the lamina propria, and the presence of bifurcating or multifocal crypts. These scoring systems acknowledge that inflammation and epithelial damage go hand-in-hand. One notable assumption is that an epithelium exhibiting normal histological morphology exemplifies restored mucus production and tight junction assembly. Molecular mediators of wound healing have demonstrated key roles in restoring barrier function [15]. However, these aspects are not easily captured by standard hematoxylin-and-eosin staining, and no epithelium can realistically be considered fully healed without proper regulation of cell-cell junctions and the protective mucus layer.
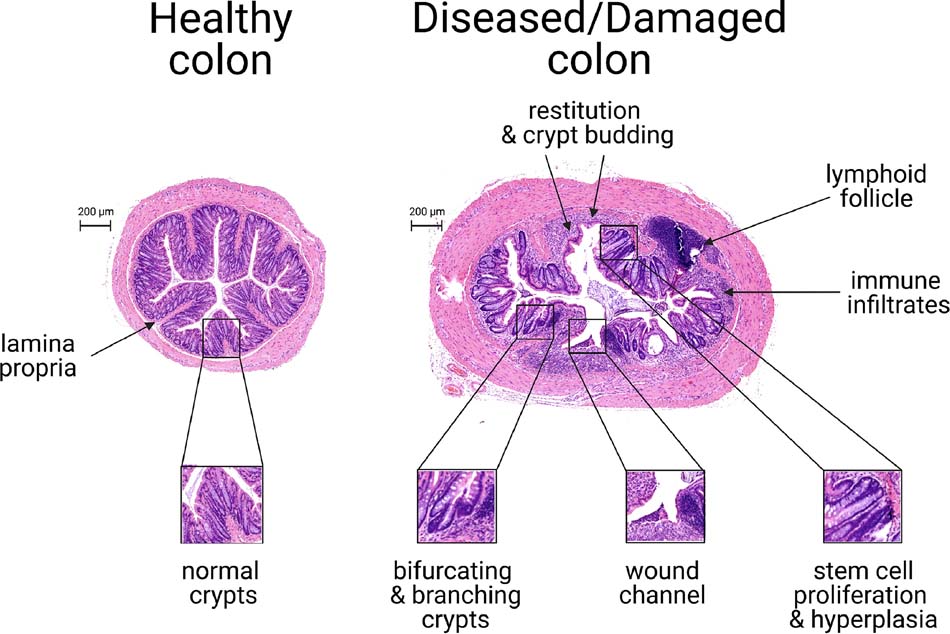
Fig 1.
Histological features of colonic epithelium during murine colitis and mucosal healing. Displayed are transverse colonic sections stained with hematoxylin and eosin. These tissues were obtained from HSP25−/− mice, which exhibit delayed wound healing responses and thereby facilitate visualization of different aspects of injury and healing within the same tissue section. The left panel shows a transverse section of uninjured mouse colon. Note that crypts are uniform and undistorted, and few immune cells are present in the lamina propria. In contrast, the right panel shows the colon 1 4 d after the induction of colitis via a 5-d treatment with 3% dextran sulfate sodium (DSS). DSS treatment caused thickening of the colon and severe epithelial damage. This colon is in various states of wound healing. Restitution, the rapid resealing of eroded mucosa by epithelial cells, is accompanied by a massive immune infiltration into the lamina propria at multiple sites. Lymphoid follicles are enlarged. Regenerative epithelial changes include the formation of 3-dimensional wound channels, the morphological distortion of crypts, including their adoption of bifurcating/branching structures, and crypt hyperplasia, which may be associated with expansion of the progenitor cell zone. Photomicrograph credit: Yun Tao, PhD.
Given the attention already paid to immunomodulation as first-line therapy, it seems that targeting the epithelium during the repair process could lead to an alternate and complementary avenue of treatments. We therefore focus this review on the epithelium-targeted mechanisms and opportunities. However, one should note that targeting other mucosal systems, for example through mesenchymal stem cells, could also indirectly promote epithelial wound healing and therefore broadly restore homeostatic function to the mucosa.
Epithelial repair is critical for breaking the vicious cycle of events underlying IBD pathology. During an active flare, a storm of cytokines and immune cells invades the intestinal mucosa. Although the exact etiology is unknown and could have idiosyncratic origins, this immune response is believed to primarily target gut luminal contents including the commensals comprising the normal microbiome. The epithelium is destroyed in concert with the immune reaction. The breakdown of the epithelial barrier results in the loss of a critical mucus layer (e.g, containing trefoil factors [16]) and ablates homeostatic regenerative functions that normally help to promote wound healing. As a result, the host immune system is further exposed to luminal contents [17], propagating the cycle of inflammation and wounding. It follows that to break this cycle, the antigenic stimulation, the immune overreaction, or the wound healing response need to be modulated. A measure of success has been achieved with immunomodulatory strategies. These include older agents such as mesalamine, corticosteroids, and antimetabolites (e.g., 6-mercaptopurine), as well as newer-generation therapies targeting TNF (e.g., infliximab), integrin subunits (e.g., vedolizumab), IL-12/23 (ustekinumab), and JAK/STAT (tofacitinib). An important limitation of these approaches is that they induce remission in only a minority of patients [18–22]. Thus, there is ample room for therapeutic innovation.
The case for wound healing
Do IBD patients really exhibit defective epithelial wound healing, and can wound healing really be therapeutically leveraged? The evidence that the intestines of IBD patients may have underlying defects associated with epithelial repair comes from a few sources.
Genetics: Genome-wide association studies [23–25] have indicated risk alleles for both CD and UC in genes involved in intercellular junctions needed for barrier maintenance (reviewed in [26]) and in intestinal cell restitution, the initial migratory step necessary for wound closure. Risk loci encoding genes with plausible roles in wound healing include: 1) PTGER4, the EP4 prostaglandin receptor that is an essential mediator of the epithelial cell-fate change required for restitution [27], 2) ERRFI1, a negative regulator of epidermal growth factor (EGF) receptor signaling [28], and 3) HNF4A, a broad transcription factor with demonstrated roles in intestinal epithelial repair and differentiation [29]. First-degree relatives of CD patients are also more likely to exhibit permeability defects after challenge than spouses of patients or the general population [30–32].
Histopathology of IBD samples: IBD represents a unique challenge to the intestinal mucosa, in which a loss of tolerance to luminal contents leads to mucosal infiltration of both lymphoid and myeloid cells. This challenge requires an epithelial repair response that can contend with high levels of cytokines and an injurious microenvironment. The inability to resolve persistent ulcers and the appearance of chronic structural changes in crypts suggests an insufficient repair response.
Preclinical studies in mice: Disruption of cellular pathways regulating epithelial cell migration, proliferation, survival, barrier formation, and differentiation, key functional components of the wound healing process, exacerbates outcomes in experimental colitis models (see below). Cytokines directly modulate epithelial barrier integrity [33–36], and targeted disruption of barrier integrity and accelerates and exacerbates experimental colitis [37, 38].
Clinical outcomes: Large-scale longitudinal studies have shown that mucosal healing is a clinically significant predictor of long-term response to medical therapy. IBD patients who achieve mucosal healing after treatment with an immunomodulator have longer periods of steroid-free remission and a reduced risk of surgery [39–42]. Even among patients who are considered “healed,” with Mayo scores ≤1, additional benefits in longitudinal outcomes are observed in patients who are fully healed (score = 0) compared to those who are mostly healed (score = 1) [43]. Thus, complete healing is the ultimate goal of medical management of IBD.
Preliminary studies and small-scale clinical trials have suggested that targeting epithelial wound healing would be efficacious. Enemas containing the epidermal growth factor (EGF) peptide, when used alongside mesalamine, induced remission in >80% of UC patients [44]. The EGF receptor pathway is critical for intestinal epithelial wound healing, enhancing the migration, proliferation, and survival of epithelial cells [45–48]. Similarly, irrigation of the distal colon with butyrate, a microbial metabolite that promotes epithelial barrier function [49–52], improved the stool frequency and endoscopic scores of UC patients [53]. As promising as these clinical results are, these therapies target multiple mucosal systems related to IBD pathogenesis, and their effects are not restricted to epithelial wound healing. Thus, their efficacy cannot be taken yet as direct proof that epithelial targeting in isolation would be beneficial in IBD. Other studies of wound healing candidates have shown promise but are ultimately inconclusive due to the small size of the patient cohort [54, 55]. A better understanding of wound healing processes could strengthen the foundation for translational investment into this approach.
Below we summarize additional avenues that could lead to therapies for IBD based on activation of epithelial wound healing. The full development of these therapies and the evaluation of their potential efficacy in IBD patients will provide answers as to whether and how epithelial wound healing can be directly targeted. We have categorized these approaches as related to: 1) epithelial stem cell responses to injury and inflammation, 2) role of cytokines and immune signaling in epithelial wound healing, and 3) microbial signals to generate a favorable environment for host wound repair. A summary schematic of how these systems can work together to mediate wound healing is shown in Figure 2. Furthermore, key therapeutic approaches leveraging wound healing through these systems are listed in the Table. This review is not meant as a full treatment of the scientific principles behind each of these topics; rather, we aim to provide adequate background to contextualize some of the exciting avenues and outstanding issues.
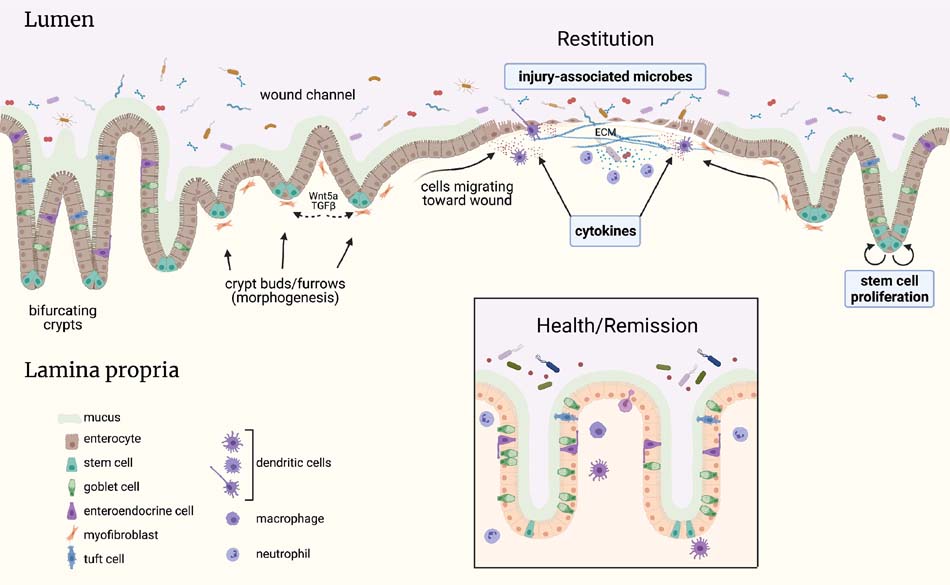
Fig 2.
Schematic of key mucosal tissue systems involved in colonic epithelial wound healing. Interactions between these systems and the epithelium could be therapeutically leveraged to restore normal mucosal architecture and barrier function. The inflammatory process of 1BD induces ulcers and epithelial erosions. At the site of the open wound, crypts adopt a “wound channel” structure, through which intestinal epithelial cells quickly migrate to re-fonn a rudimentary barrier. This process is known as restitution and does not require cell proliferation. Rapid closure of ulcers is critical to prevent gut microbes from further entry into the host. Crypts undergo morphological changes associated with stem cell proliferation to sustain wound closure and to restore the normal pattern of epithelial structure, cellular differentiation, and barrier function. Signals associated with immune cells, wound-associated mesenchyme, and the microbiome promote epithelial wound healing. For example, growth factors, cytokines, microbial metabolites and short-chain fatty acids, and microbial-associated molecular patterns modulate wound healing responses. Further understanding of these cellular and molecular interactions, and distinguishing their pathological versus beneficial effects, will advance potential therapies for mucosal healing in 1BD. Created using Biorender (biorender.com).
Table.
Candidates for epithelial wound healing-targeted therapy in IBD
| Agent | Proposed mechanism of action+ | Delivery / Formulation | Preclinical or Clinical Evidence | Example References | |
|---|---|---|---|---|---|
| Growth factors, restitutive signals, and stem cells | EGF | Epithelial migration, proliferation, and survival through EGFR signaling | Enema | Induces clinical remission in 10/12 UC patients | [44, 48, 77] |
| FGF10/KGF-2 | Epithelial restitution through FGFR2 | Systemic injection | Promotes healing of indomethacin-induced small-intestinal ulcerations in rats | [83] | |
| TFF3 | Essential for epithelial restitution, but details of molecular mechanism unknown | Enema | Mild improvement in symptoms in a minority of 8 enrolled UC patients, comparable to mesalamine | [54] | |
| IGF-1 | Epithelial cell proliferation and goblet cell regeneration through extracellular signal-related kinase (ERK) signaling | Systemic injection | Improved recovery from DSS-induced colitis in rodents | [80, 81] | |
| HGF | Epithelial proliferation | Systemic injection | Accelerated healing of inflammatory ulcers in rats | [78, 79] | |
| Mongersen | Migration of epithelial cells through inhibition of SMAD7 (i.e., potentiation of TGFbeta signaling) via antisense targeting | Oral | Clinical remission in ~50% of 83 enrolled CD patients receiving higher doses | [104] | |
| GB004 | Epithelial integrin expression and migration through inhibition of prolyl hydroxylase, resulting in stabilization of HIF-1alpha | Oral | Accelerated wound closure in TNBS-induced colitis in rodents | [88] | |
| Organoids | Direct engraftment of new epithelium onto injured area | Enema or endoscopic | Improved outcomes in mice with DSS-induced colitis | [107] | |
| Cytokine-inspired | Interleukin 10 | Epithelial proliferation through Wnt-inducible signaling protein 1 (WISP1); design of IL-10 variants with specific receptor affinities | Systemic injection | Accelerates closure of biopsy-induced wounds in mice; specific IL-10 variants decouple anti- and proinflammatory signaling | [139, 140] |
| Interleukin 2 | Activation of Tregs and epithelial restitution | Low-dose systemic injection | Ameliorates DNBS-induced and DSS-induced colitis in mice | [142, 143] | |
| Interleukin 22 | Epithelial wound closure through activation of STAT3 | Local gene delivery; treatments with variants to specifically activate STAT3 | Attenuates TCRalpha−/− colitis in mice; variants provide selective tissue healing without inflammation | [133, 134, 140] | |
| STAR2 | Selective targeting of TNF receptor 2 to promote colonic epithelial proliferation in injury | Systemic injection | Ameliorates graftvs-host disease in mice | [145, 148] | |
| IL-36R ligands | Epithelial proliferation and secretion of antimicrobial proteins | Local submucosal injection | Accelerates ulcer closure in biopsy-induced wounds in mice | [132] | |
| Rivenprost (ONO-4819CD) | Selective activation of EP4 prostaglandin receptor involved in adaptive differentiation of wound-associated epithelia | Intravenous | Improved histological score in 4 UC patients | [27, 55] | |
| Microbe-derived | Butyrate | Promotes epithelial tight junction integrity | Enema | Reduces stool frequency and endoscopic score in trial with 10 UC patients | [50, 51, 53] |
| p40 protein from Lactobacillus rhamnosus GG | Activates host EGFR signaling for wound healing | Oral delivery of p40 on hydrogel bead system | Improved preclinical outcomes and epithelial cell survival in DSS-induced and oxazolone-induced murine colitis | [173] | |
| Faecalibacterium prausnitzii | Preserves epithelial stem cell pool, proliferation, and barrier function | Intragastric delivery of F. prausnitzii strain A2–165 | Protects murine colons from radiation-induced damage | [176] | |
| Microbiome purine reconstitution | Epithelial cell metabolism, proliferation, and mucin secretion | E. coli K12 | Monocolonized mice are resistant to DSS-induced colitis compared to germ-free | [174] | |
| VSL #3 | Treatment of dysbiosis | Lactobacilli (4 strains), Bifidobacteria (3 strains), Streptococcus thermophilus | Remission in ~40% of 77 enrolled UC patients | [177, 178, 185] |
Agents can have multiple mechanisms of actions complementing their wound healing activity; only the wound healing-relevant mechanism is listed here.
Intestinal epithelial stem cells and wound healing
Mechanisms
Much of what is known about the sequence of events mediating regeneration of the intestinal epithelium comes from mouse models of biopsy punch injury or chemically-induced colitis. Damage through either of these mechanisms induces a temporary loss of epithelial barrier function, reminiscent of human IBD patients. The first stage of epithelial repair is characterized by structural rearrangements of actin filaments within differentiated cells to facilitate rapid cellular migration into the wound. This migratory response, known as restitution, occurs without requiring proliferative changes in the stem cells that normally reside at the base of the crypt. A sheet of cells, each with flattened morphology representative of what has been proposed to be a “wound-associated epithelial” (WAE) phenotype and marked by expression of claudin-4 (Cldn4), emerges from the field of surrounding crypts [56]. Over time the three dimensional shape of surviving crypts extends toward the wound bed and resembles a series of “wound channels” that are derived from horizontal elongations of wound-adjacent crypts [57]. The goal of the restitutive process is to rapidly restore a rudimentary barrier over the ulcer. Unlike wound healing in skin, intestinal epithelial restitution is not believed to involve formation of a scab.
The ”mass balance” of intestinal injury means that the epithelial cell population must eventually be renewed by proliferative activity. In biopsy injury models, upregulation of mitosis is restricted to the epithelial cell population at the base of wound channels and neighboring crypts [57]. The proliferation of epithelial cells occurs with the reshaping of crypts and wound channels: furrows near the base of these structures initiate repetitive fission events that ultimately restore the regular crypt patterning of the mucosa. The position of these furrows is, in part, specified by the location of wound-specific mesenchymal cells expressing Wnt5a [57], which in turn activates pro-repair TGFbeta signaling. Thus, neighboring mesenchymal cells supply cues (e.g., [58]) that promote epithelial repair behaviors and crypt morphogenesis after injury.
Much attention has been given in recent years to addressing whether there is a specialized epithelial stem cell population that is activated during injury. Although the homeostatic turnover of intestinal epithelial cells is sustained by the proliferation of an Lgr5+ stem cell population located at the base of the crypt [59], some studies have suggested a “reserve” or “revival” stem cell population with distinct molecular identity. These cells may be located at the +4 position (i.e., immediately above the base of the crypt) or represent “label-retaining” cells that share properties of both stem cells and Paneth cells [60–65]. In contrast, a competing hypothesis is that the broad plasticity of intestinal epithelial cell fate confers the ability of differentiated cells to revert to a stem-like state during times of physiological challenge [66–72]. This is associated with the adoption of a fetal-like state in the epithelium [73–75]. Unlike the profound epigenetic changes that accompany mitosis and differentiation in fetal development, the differentiation status of an adult intestinal epithelial cell does not appear to be associated with a specific epigenetic configuration; that is, the lack of an epigenetic signature in differentiated epithelial cell types vs. epithelial stem cells essentially confers a fluidity to cell fate specification in the intestinal epithelium [76]. One implication of these findings is that the effective size of the targetable stem cell pool for wound healing could be larger than previously anticipated, as it may include partially differentiated cells that are competent for reversion (de-differentiation).
Therapeutic opportunities
Based on the framework described above, one would predict that signals promoting the “fab five” of epithelial repair - cell survival, migration, proliferation, de-/differentiation, and barrier integrity - would have some positive impact on mucosal healing. One simple approach to enhancing wound healing therapeutically would involve directly treating IBD patients with growth factors or small-molecule regulators shown to enhance these characteristics in mouse models. A variety of bioactive agents and pathways, including EGF [48, 77], HGF [78, 79], insulin growth factor [80, 81], fibroblast growth factors [82, 83], transforming growth factor beta (TGFbeta) [84–86], HIF-1alpha [87, 88], and focal adhesion kinase (a key mediator of cell survival, migration, and barrier function) [89–92] have demonstrated key roles in epithelial wound healing. The efficacy of EGF in a small clinical trial with UC patients [44] lends substantial promise that this approach could be used to improve outcomes in IBD through the enhancement of mucosal healing.
However, the progress with this direct treatment approach has admittedly been slower than anticipated. There are three main reasons for this:
Difficulty restricting the effect on the bioactive agent to the epithelium – Receptors and intracellular targets leveraged for epithelial wound healing are found in many other mucosal cell types, especially immune cells. Signals that promote epithelial wound healing behaviors may also promote inflammatory function of immune cells, which may hinder the therapeutic benefit. For example, p38 kinase is essential for epithelial cell migration [93, 94], but it also represents a potent signal involved in the inflammatory pathophysiology of experimental colitis [95–97]. Likewise, EGFR signaling in macrophages may partially drive colitis [98], suggesting that the overall efficacy of EGF-based therapies could be improved if their activity could be skewed away from immune cells. Thus, at least conceptually, the ideal target will have expression restricted to the epithelium, or have complementary roles in immunosuppression and wound repair.
Concerns about oncogenesis – Many signaling pathways such as Wnt (APC), Ras, and EGFR that have beneficial roles in mucosal healing are implicated in the pathogenesis of colorectal cancer. However, recent preclinical studies have shown that suboptimally treated inflammation poses a higher risk for cancer than the use of mitogenic agents to aid inflammatory resolution [48, 77]. Expanded preclinical and longitudinal studies will need to be performed for medications targeting repair.
Uncertain intellectual property landscape – Growth factors were initially identified in the 1950s and are naturally occurring proteins, limiting their opportunities for intellectual property protection. However, some of these issues could be alleviated by developing novel scalable ways of production, such as using agricultural methods to produce peptides [99, 100], or devising new encapsulation strategies to target these agents to the intestinal mucosa [101, 102]. Moreover, recent approaches have turned towards using novel and patentable chemical species to “lock” enzymes within an activated state or to inhibit the activities of inhibitory proteins within the target pathway. For example, although it failed a phase 3 clinical trial for IBD, a synthetic antisense oligonucleotide to block inhibitory SMAD7 signaling, thereby potentiating reparative TGFbeta signals [103, 104], demonstrates how some creativity can be utilized to generate patentable candidates for clinical studies. Another example undergoing clinical trials is the new compound GB004, which acts as a stabilizer of the hypoxia-inducible HIF-1alpha transcription factor critical for epithelial restitution [87, 88].
The molecular identification of the intestinal epithelial stem cell population, characterization of their niche, and subsequent expansion in vitro as organoids has highlighted a new approach [105–108] to mucosal healing. Its concepts are rooted in tissue engineering. Here, patient-specific organoids are grown from a biopsy of healthy colonic tissue, then endoscopically transplanted to the ulcerated region to directly heal it. A proof of principle was demonstrated in colonic organoids grown from single Lgr5+ stem cells in mice; these fluorescently labeled donor organoids could be successfully engrafted into the colon of a recipient mice afflicted with DSS-induced colitis. The engraftment was associated with accelerated recovery from the acute colitis and provided a long-lasting, self-renewing transplant [107]. Organoids can be grown in culture indefinitely and do not appear to acquire oncogenic mutations, and new techniques have optimized their growth to reduce the number of required exogenous factors and to improve crypt patterning [109–114]. Clinical trials have been initiated using IBD patient-autologous transplants, which would minimize the risk of immunologic rejection.
A complementary source of intestinal organoids is patient-derived induced pluripotent stem cells (iPSCs). iPSCs can be isolated from non-GI tissues and subsequently differentiated to intestinal lineages through a defined and step-wise differentiation protocol that recapitulates regional cues during fetal development [115–117]. The use of iPSCs also enables the cogeneration of blood vessels and enteric neurons [118, 119], important support structures that could facilitate the engraftment and function of the organoid transplant. In organoids grown from either adult biopsied GI tissue or iPSCs, gene editing could be performed to correct genetic defects that may have contributed to the development of IBD. Whether such defects can be identified in most patients and whether the transplanted epithelium will resist future IBD-like injury remain open questions. Accumulating evidence suggests that while both iPSC-derived and adult GI-derived organoids exhibit significant plasticity enabling engraftment, the engrafted tissue may retain epigenetic hallmarks of its original tissue source [108]. In the case of iPSC-derived organoids, their transcriptional profile in culture resembles that of fetal epithelium, but their engraftment is associated with the acquisition of adult epithelial gene expression [120]. The potential long-term side effects of functional mismatches between donor organoids and target engrafted epithelium need to be studied. Moreover, in some patients the pre-existing damage to the epithelium may be too severe to establish robust organoid cultures; these patients would require a different therapeutic approach.
Cytokines and intestinal regeneration
Mechanisms
Although a hyper-inflammatory response is associated with IBD, basic studies have demonstrated the essential role of immune responses in the promotion of wound healing. Many cytokines thought to be central to the pathogenesis of IBD have, in fact, been shown to support epithelial repair in cell culture systems and mouse models. The result is a more-complex set of connections between the various cell types that secrete cytokines and the multitude of effects these cytokines can have on target tissues, including intestinal epithelium, which precludes a simple assignment of whether a particular cytokine is “friend” or “foe.”
Nearly every IBD-associated cytokine has some context in which it can boost epithelial wound healing behaviors. This has been demonstrated in both recent and classic studies of interferons [121], IL-1 [122], IL-2 [122, 123], IL-6 [124], TGFbeta [84, 86, 122], TNF [125–127], IL-15 [128], IL-17 [82, 129, 130], IL-33 [131], IL-36 [132], IL-22 [133, 134], and others, all of which act at some level by promoting epithelial cell migration, proliferation, survival, or differentiation. Common signaling intermediaries that regulate the wound healing response include members of the TGFbeta pathway [84, 86], STAT3/5 [133, 135, 136], and downstream targets of NF-kappaB [137]. Given what is known now about the importance of cytokine signals to intestinal regeneration, it never ceases to amaze that some of the modern therapies which inhibit these same pathways work at all! Indeed, the benefit of an immunomodulating therapy must be considered and balanced against its potential deleterious effects on mucosal healing. For example, inhibition of the IL-17 pathway seemed so promising from the immunologic standpoint but failed clinical trials [138], in part due to this cytokine’s pro-healing effects on the epithelium. These cautionary examples demonstrate the need for more-precise targeting of both the immunologic and the epithelial aspects of the IBD pathophysiological process.
Therapeutic opportunities
Due to the moderate clinical success achieved by anti-TNF therapies and JAK/STAT inhibitors, it seems unlikely that direct treatment with large doses of IBD-associated cytokines will become a primary treatment paradigm for patients who present with severe acute colitis, even if there are some positive effects of these cytokines on epithelial wound healing. In these patients, epithelial repair is not the immediate priority - one does not put out a forest fire by planting new trees. One exception may be administration of interleukin 10, which mediates immune tolerance and also has recently been shown to promote epithelial wound healing in cell lines and mouse models [139]. A recent study has demonstrated how the structure of interleukin 10 can be modified to improve its anti-inflammatory properties [140]. Similar perturbations to the cytokine structure-function relationship have also been recently engineered for interleukin 22 and allow specific activation of downstream STAT isoforms involved in tissue repair [141]. Some gains may also be made by administering a low dose of classically pro-inflammatory cytokines, such as interleukin 2 [142, 143]. Even so, there are additional intricacies in how overlapping immune and wound healing pathways could be activated for mucosal healing. These strategies can be roughly categorized as targeting receptor-specific signals, cell-specific signals, and time/physiology-specific signals.
Cytokine signaling can be distributed downstream across several cellular receptors. These receptors may be linked to different cellular functions which could enable discrimination of pro-inflammatory processes from epithelial wound healing. For example, TNF signaling is executed through two receptors, TNFR1 (Tnfrsf1a) and TNFR2 (Tnfrsf1b). Whereas TNFR1 can have mixed pro- and anti-inflammatory effects (e.g., [144]), selective activation of TNFR2 signaling exerts strong anti-inflammatory effects and induces epithelial repair responses in a variety of autoimmune conditions [145–148]. As another example, prostaglandin signaling through the EP4 receptor acts as a “gatekeeper” in the conversion of intestinal epithelial cells into the migratory WAE phenotype involved in restitution [27], and improves preclinical outcomes [149, 150]. Promising outcomes of UC have been obtained in a small-scale clinical trial [55] with the EP4-selective agonist rivenprost (ONO-4819CD). This strategy of selective receptor targeting could help to reduce activation of classically pro-inflammatory prostaglandin signaling [151].
Recent interrogation of mucosal cell composition using single-cell “omics” techniques has revealed a rich diversity of cytokine-secreting immune and mesenchymal cells that may each have specialized functions, including, possibly, the promotion of epithelial wound healing. As immunosuppressive strategies can have cytotoxic effects on a broad range of cells (e.g., antibody-dependent cellular cytotoxicity) [152], in regards to mucosal healing the current complement of medications may be removing some of the “good” cell types with the “bad.” The varied repertoire of stromal cells is reminiscent of the recent elaboration of different kinds of macrophages, including M1- and M2-polarized subsets, that mediate pro-inflammatory and wound healing-type responses, respectively. Recent single-cell profiling of the IBD-afflicted colon [153] has demonstrated the emergence of a specialized subpopulation of inflammation-associated mesenchymal cells. Intriguingly, this subpopulation expresses IL-33, a cytokine that promotes epithelial proliferation during wound healing via the activation of microRNAs (e.g., miR-320) [131]. Thus, further elaboration of the molecular pathways defining the different stromal cell types involved in IBD-associated inflammation may highlight new approaches to target immune or mesenchymal cells to promote wound healing.
An IBD patient’s clinical history will change over time and is dependent on the effectiveness of the medical treatments they are offered. All of which is to say that the timing of a treatment matters. In this sense, there may be an opportunity to leverage cytokine-based treatments for mucosal healing in one disease context versus another. The colonic mucosa of a patient admitted with a flare of severe acute colitis will be in a fundamentally different biological state than one that has been treated with a powerful immunosuppressive regimen, which will in turn be different from an otherwise asymptomatic individual who is beginning to show early signs of coming out of remission or one who has chronic but mild under-treated inflammation. Animal models of intestinal inflammation have been useful for breaking down the differential roles of cytokines at different timepoints in the natural history of intestinal injury. For example, in acute chemical models such as DSS-induced or TNBS-induced colitis, there is an early phase of injury onset, followed usually by a spontaneous recovery that follows over the span of several weeks. As has been shown in regard to colitis-associated tumorigenesis [77], depending on when growth factor- or cytokine signals are administered, they may have different outcomes. In the future, one can envision that patients may be eligible for low-dose cytokine treatment after certain histological or clinical criteria have been met. This timing-based strategy respects the biological complexity of inflammation and wound healing, and takes advantage of specific windows of time in which certain immune signals could provide a big benefit towards mucosal healing.
Healing through microbial signals
Mechanisms
The intestinal epithelium resides in proximity to trillions of luminal and crypt-associated microbes that comprise the human microbiome. The dynamical nature, adaptability, and critical functions of the microbiome relative to the host mean that any meaningful mucosal healing vis-a-vis epithelial wound repair also needs be accompanied by the restoration of microbiome homeostasis. IBD is almost always associated with a microbiome state known as dysbiosis, in which the overall diversity, composition, stability, and metabolic activities of the microbiome have been perturbed. It is not known whether dysbiosis causes the initial onset of IBD, but it may contribute to delayed healing. Dysbiotic states are associated with the loss of commensals producing important homeostatic short-chain fatty acids (SCFAs) such as butyrate, propionate, and acetate. These SCFAs have shown the ability to promote intestinal epithelial restitution, proliferation, differentiation, and barrier function [49, 154, 155]. As multiple taxa can produce SCFAs, it may be more appropriate to think of microbial metabolic signatures, rather than specific taxa, that are conducive to wound healing.
Preclinical studies in mice have demonstrated that microbial signals are important for intestinal epithelial repair. Germ-free mice exhibit severe exacerbation of DSS-induced colitis [156]. Toll-like receptor signaling, which is activated upon binding by microbe-associated molecular patterns, such as endotoxin/lipopolysaccharide (TLR4), flagellin (TLR5), and unmethylated DNA (TLR9), improves outcomes in experimental colitis through the promotion of wound healing [157–162]. The microbiome can also act to promote wound healing in a localized manner. Specific microbes in proximity to an ulcer activate host epithelial proliferative signaling through a formyl peptide receptor pathway [163, 164]. The spatial topography and organization of the crypt and surrounding mucus also means that the epithelial cells are exposed to different commensal microenvironments, with implications for both host and microbial signaling [165, 166]. Differentiated cells near the top of the crypt metabolize much of the microbially derived SCFAs; as a result, the stem cells at the base of the crypt are relatively untouched by this microbe-derived signal [167]. Likewise, the presence of Paneth and deep crypt secretory cells, which secrete antimicrobial enzymes, at the crypt base changes the nature of the reciprocal signals that characterize the host-microbe relationship [168, 169]. Through symbiosis, the crypt can therefore simultaneously provide an environment facilitating disparate epithelial behaviors along its vertical axis, with proliferative stem cells at the base and differentiated cells capable of restitution at the top, matching the diversity of cell behaviors needed for wound healing.
Therapeutic opportunities
The attractiveness of the microbiome as a therapeutic target for wound healing is rivaled only by the sheer theoretical diversity of the ways it could be targeted. By now, key microbes associated with the IBD-afflicted microbiome have been identified, fueling speculation that adding back so-called “symbionts” could counteract the dysbiosis represented by the presence of “pathobionts” (e.g., [170, 171]). A simple approach could be the administration of a prebiotic or a probiotic compound. There are a few examples of this. Butyrate enemas have been shown to be effective in treating UC [53]. Even single microbial proteins can have profound effects on intestinal epithelial signaling and stromal responses. p40, a protein produced by Lactobacillus rhamnosus GG, activates host epithelial EGFR signaling and mediates wound healing [172, 173]. Restoration of microbe-sourced purines by colonization with purine-competent strains of E. coli protects the colonic epithelium against apoptosis and promotes proliferation and mucosal healing [174]. A microbe commonly depleted in IBD, Faecalibacterium prausnitzi [175], may protect epithelial stem cells during challenge [176] and may thus represent a target for restoration. Beyond single microbial species or metabolites, groups of microbes may be targeted for supplementation with probiotic mixtures. The probiotic mixture known as VSL #3, containing 4 strains of Lactobacilli, 3 strains of Bifidobacteria, and 1 strain of Streptococcus has been shown effective in preventing pouchitis and in treating flareups of UC [177–179], and may do so by partially upregulating expression of host regeneration-associated growth factors [180]. We note that while antibiotics are not classically associated with an epithelial repair response in IBD, in principle the elimination of certain sets of microbes resulting in broad shifts in the community phenotype (e.g., change in IgA status [181] or eliminating oral taxa [5, 182]) could make a more-conducive environment for wound healing.
As with any new therapeutic modality, targeting the microbiome for wound healing has some challenges. First, the details matter. Preclinical studies of the efficacy of certain microbes may apply only to certain strains. Moreover, differences in the structures of human versus mouse microbiomes may challenge the clinical translation of discoveries made primarily in mice. Second, it is not necessarily “easy” to colonize the adult colon with an exogenous microbe, as the microbial community has become adapted to the inflammatory milieu. Successful colonization likely requires pre-treatment with antibiotics to partially clear the microbial community, which may exacerbate dysbiosis. Third, and perhaps a more philosophical question, can one trust the long-term effects of an exogenously introduced microbe? Unlike a protein factor or prebiotic, a living microbe can adapt, mutate, and potentially cause unwanted side effects long after its benefits to mucosal healing have been realized. Ideally we would have some measure of control over the microbe after its introduction. One can envision that this justifies the engineering of microbes with designer molecular circuits that encode complex behaviors [183] to optimize therapeutic delivery and control.
With advances in metabolomic, lipidomic, and proteomic technologies, it should be possible to identify and develop small molecule effectors that promote mucosal healing. The advantage of this approach is that these compounds are no longer dependent on directed colonization or functional properties of probiotics or fecal microbiota transplant, all of which can be unpredictable and difficult to dose. Small molecules, on the other hand, can be administered at optimal dose-responsive levels and targeted to regions in need of mucosal healing. More study will be needed to overcome these potential hurdles and to unlock these new approaches to wound healing.
Concluding remarks
IBD is likely a collection of diseases that are more stratified than simply UC vs. CD. For example, there is growing recognition that colonic CD tends to respond to a different set of therapies than ileal-dominant CD [184]. Combined with the individuality of patient responses and the sheer number of environmental, microbiome, and genetic factors that contribute to risk of disease, it is becoming clear that personalized and precision therapies will be the future. In addition to an approved therapy to enhance wound healing, it will be important to find precise ways to assess and predict healing responses early within the treatment regimen, allowing wound healing therapies to be deployed earlier. The current practice of waiting 4–12 weeks to assess clinical response to therapy is quite hard on the patient; after all, these are real weeks, with real suffering. But with recent advances in our understanding of wound healing and a promising therapeutic pipeline, help is on the way. To be sure, the task at hand is very challenging. The dynamic and precise nature of the wound healing process means that there are many potential failure-points for newly proposed therapies. However, the reward, a generational class of therapeutics that complements emerging immunomodulatory strategies to improve patients’ lives, is well-worth the investment of scientific careers and resources to achieve it.
Acknowledgments
All authors have read the journal’s policy on disclosure of potential conflicts of interest. Eugene B. Chang (EBC) is the co-founder and Chief Medical Officer for AVnovum Therapeutics. Cambrian Y. Liu (CYL) and Candace M. Cham (CMC) declare no conflicts of interest. CMC and EBC acknowledge the following grants from the National Institute of Diabetes and Digestive and Kidney Diseases: RC2DK122394, R01DK47722, and R01DK113788; and the Center for Interdisciplinary Study of Inflammatory Intestinal Diseases (P30 DK42086). Additional support has been provided by the Gastrointestinal Research Foundation of Chicago, the David and Ellen Horing Research Fund, and the Helmsley Charitable Trust. CYL acknowledges support from a Career Development Award (#694110) granted by the Crohn’s and Colitis Foundation. All authors have read the journal’s authorship agreement. The manuscript has been reviewed and approved by all authors.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- [1].Colombel JF, D’Haens G, Lee WJ, Petersson J, Panaccione R. Outcomes and Strategies to Support a Treat-to-target Approach in Inflammatory Bowel Disease: A Systematic Review. J Crohns Colitis. 2020;14:254–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].D’Arcangelo G, Aloi M. Treat-to-Target in Pediatric Inflammatory Bowel Disease: What Does the Evidence Say? Paediatr Drugs. 2020;22:463–72. [DOI] [PubMed] [Google Scholar]
- [3].Gonczi L, Bessissow T, Lakatos PL. Disease monitoring strategies in inflammatory bowel diseases: What do we mean by “tight control”? World J Gastroenterol. 2019;25:6172–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Collaborators GBDIBD. The global, regional, and national burden of inflammatory bowel disease in 195 countries and territories, 1990–2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet Gastroenterol Hepatol. 2020;5:17–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Schirmer M, Denson L, Vlamakis H, Franzosa EA, Thomas S, Gotman NM, et al. Compositional and Temporal Changes in the Gut Microbiome of Pediatric Ulcerative Colitis Patients Are Linked to Disease Course. Cell Host Microbe. 2018;24:600–10 e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Gevers D, Kugathasan S, Denson LA, Vazquez-Baeza Y, Van Treuren W, Ren B, et al. The treatment-naive microbiome in new-onset Crohn’s disease. Cell Host Microbe. 2014;15:382–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Iacucci M, Fort Gasia M, Hassan C, Panaccione R, Kaplan GG, Ghosh S, et al. Complete mucosal healing defined by endoscopic Mayo subscore still demonstrates abnormalities by novel high definition colonoscopy and refined histological gradings. Endoscopy. 2015;47:726–34. [DOI] [PubMed] [Google Scholar]
- [8].Mazzuoli S, Guglielmi FW, Antonelli E, Salemme M, Bassotti G, Villanacci V. Definition and evaluation of mucosal healing in clinical practice. Dig Liver Dis. 2013;45:969–77. [DOI] [PubMed] [Google Scholar]
- [9].Mary JY, Modigliani R. Development and validation of an endoscopic index of the severity for Crohn’s disease: a prospective multicentre study. Groupe d’Etudes Therapeutiques des Affections Inflammatoires du Tube Digestif (GETAID). Gut. 1989;30:983–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Daperno M, D’Haens G, Van Assche G, Baert F, Bulois P, Maunoury V, et al. Development and validation of a new, simplified endoscopic activity score for Crohn’s disease: the SES-CD. Gastrointest Endosc. 2004;60:505–12. [DOI] [PubMed] [Google Scholar]
- [11].Jauregui-Amezaga A, Geerits A, Das Y, Lemmens B, Sagaert X, Bessissow T, et al. A Simplified Geboes Score for Ulcerative Colitis. J Crohns Colitis. 2017;11:305–13. [DOI] [PubMed] [Google Scholar]
- [12].Geboes K, Riddell R, Ost A, Jensfelt B, Persson T, Lofberg R. A reproducible grading scale for histological assessment of inflammation in ulcerative colitis. Gut. 2000;47:404–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Mosli MH, Feagan BG, Zou G, Sandborn WJ, D’Haens G, Khanna R, et al. Development and validation of a histological index for UC. Gut. 2017;66:50–8. [DOI] [PubMed] [Google Scholar]
- [14].Marchal-Bressenot A, Salleron J, Boulagnon-Rombi C, Bastien C, Cahn V, Cadiot G, et al. Development and validation of the Nancy histological index for UC. Gut. 2017;66:43–9. [DOI] [PubMed] [Google Scholar]
- [15].Luissint AC, Parkos CA, Nusrat A. Inflammation and the Intestinal Barrier: Leukocyte-Epithelial Cell Interactions, Cell Junction Remodeling, and Mucosal Repair. Gastroenterology. 2016;151:616–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Aamann L, Vestergaard EM, Gronbaek H. Trefoil factors in inflammatory bowel disease. World J Gastroenterol. 2014;20:3223–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Gitter AH, Wullstein F, Fromm M, Schulzke JD. Epithelial barrier defects in ulcerative colitis: characterization and quantification by electrophysiological imaging. Gastroenterology. 2001;121:1320–8. [DOI] [PubMed] [Google Scholar]
- [18].Sands BE, Sandborn WJ, Panaccione R, O’Brien CD, Zhang H, Johanns J, et al. Ustekinumab as Induction and Maintenance Therapy for Ulcerative Colitis. N Engl J Med. 2019;381:1201–14. [DOI] [PubMed] [Google Scholar]
- [19].Panes J, Sandborn WJ, Schreiber S, Sands BE, Vermeire S, D’Haens G, et al. Tofacitinib for induction and maintenance therapy of Crohn’s disease: results of two phase IIb randomised placebo-controlled trials. Gut. 2017;66:1049–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Sandborn WJ, Feagan BG, Rutgeerts P, Hanauer S, Colombel JF, Sands BE, et al. Vedolizumab as induction and maintenance therapy for Crohn’s disease. N Engl J Med. 2013;369:711–21. [DOI] [PubMed] [Google Scholar]
- [21].Rutgeerts P, Sandborn WJ, Feagan BG, Reinisch W, Olson A, Johanns J, et al. Infliximab for induction and maintenance therapy for ulcerative colitis. N Engl J Med. 2005;353:2462–76. [DOI] [PubMed] [Google Scholar]
- [22].Hanauer SB, Feagan BG, Lichtenstein GR, Mayer LF, Schreiber S, Colombel JF, et al. Maintenance infliximab for Crohn’s disease: the ACCENT I randomised trial. Lancet. 2002;359:1541–9. [DOI] [PubMed] [Google Scholar]
- [23].Consortium UIG, Barrett JC, Lee JC, Lees CW, Prescott NJ, Anderson CA, et al. Genome-wide association study of ulcerative colitis identifies three new susceptibility loci, including the HNF4A region. Nat Genet. 2009;41:1330–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Jostins L, Ripke S, Weersma RK, Duerr RH, McGovern DP, Hui KY, et al. Host-microbe interactions have shaped the genetic architecture of inflammatory bowel disease. Nature. 2012;491:119–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Huang H, Fang M, Jostins L, Umicevic Mirkov M, Boucher G, Anderson CA, et al. Finemapping inflammatory bowel disease loci to single-variant resolution. Nature. 2017;547:173–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].McCole DF. IBD candidate genes and intestinal barrier regulation. Inflamm Bowel Dis. 2014;20:1829–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Miyoshi H, VanDussen KL, Malvin NP, Ryu SH, Wang Y, Sonnek NM, et al. Prostaglandin E2 promotes intestinal repair through an adaptive cellular response of the epithelium. EMBO J. 2017;36:5–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Hackel PO, Gishizky M, Ullrich A. Mig-6 is a negative regulator of the epidermal growth factor receptor signal. Biol Chem. 2001;382:1649–62. [DOI] [PubMed] [Google Scholar]
- [29].Montenegro-Miranda PS, van der Meer JHM, Jones C, Meisner S, Vermeulen JLM, Koster J, et al. A Novel Organoid Model of Damage and Repair Identifies HNF4alpha as a Critical Regulator of Intestinal Epithelial Regeneration. Cell Mol Gastroenterol Hepatol. 2020;10:209–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Hollander D, Vadheim CM, Brettholz E, Petersen GM, Delahunty T, Rotter JI. Increased intestinal permeability in patients with Crohn’s disease and their relatives. A possible etiologic factor. Ann Intern Med. 1986;105:883–5. [DOI] [PubMed] [Google Scholar]
- [31].Soderholm JD, Olaison G, Lindberg E, Hannestad U, Vindels A, Tysk C, et al. Different intestinal permeability patterns in relatives and spouses of patients with Crohn’s disease: an inherited defect in mucosal defence? Gut. 1999;44:96–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Hilsden RJ, Meddings JB, Sutherland LR. Intestinal permeability changes in response to acetylsalicylic acid in relatives of patients with Crohn’s disease. Gastroenterology. 1996;110:1395–403. [DOI] [PubMed] [Google Scholar]
- [33].Su L, Nalle SC, Shen L, Turner ES, Singh G, Breskin LA, et al. TNFR2 activates MLCK-dependent tight junction dysregulation to cause apoptosis-mediated barrier loss and experimental colitis. Gastroenterology. 2013;145:407–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Wang F, Graham WV, Wang Y, Witkowski ED, Schwarz BT, Turner JR. Interferon-gamma and tumor necrosis factor-alpha synergize to induce intestinal epithelial barrier dysfunction by up-regulating myosin light chain kinase expression. Am J Pathol. 2005;166:409–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Smyth D, Phan V, Wang A, McKay DM. Interferon-gamma-induced increases in intestinal epithelial macromolecular permeability requires the Src kinase Fyn. Lab Invest. 2011;91:764–77. [DOI] [PubMed] [Google Scholar]
- [36].Madara JL, Stafford J. Interferon-gamma directly affects barrier function of cultured intestinal epithelial monolayers. J Clin Invest. 1989;83:724–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Su L, Shen L, Clayburgh DR, Nalle SC, Sullivan EA, Meddings JB, et al. Targeted epithelial tight junction dysfunction causes immune activation and contributes to development of experimental colitis. Gastroenterology. 2009;136:551–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Vetrano S, Rescigno M, Cera MR, Correale C, Rumio C, Doni A, et al. Unique role of junctional adhesion molecule-a in maintaining mucosal homeostasis in inflammatory bowel disease. Gastroenterology. 2008;135:173–84. [DOI] [PubMed] [Google Scholar]
- [39].Reinink AR, Lee TC, Higgins PD. Endoscopic Mucosal Healing Predicts Favorable Clinical Outcomes in Inflammatory Bowel Disease: A Meta-analysis. Inflamm Bowel Dis. 2016;22:1859–69. [DOI] [PubMed] [Google Scholar]
- [40].Baert F, Moortgat L, Van Assche G, Caenepeel P, Vergauwe P, De Vos M, et al. Mucosal healing predicts sustained clinical remission in patients with early-stage Crohn’s disease. Gastroenterology. 2010;138:463–8; quiz e10–1. [DOI] [PubMed] [Google Scholar]
- [41].Bryant RV, Burger DC, Delo J, Walsh AJ, Thomas S, von Herbay A, et al. Beyond endoscopic mucosal healing in UC: histological remission better predicts corticosteroid use and hospitalisation over 6 years of follow-up. Gut. 2016;65:408–14. [DOI] [PubMed] [Google Scholar]
- [42].Colombel JF, Rutgeerts P, Reinisch W, Esser D, Wang Y, Lang Y, et al. Early mucosal healing with infliximab is associated with improved long-term clinical outcomes in ulcerative colitis. Gastroenterology. 2011;141:1194–201. [DOI] [PubMed] [Google Scholar]
- [43].Barreiro-de Acosta M, Vallejo N, de la Iglesia D, Uribarri L, Baston I, Ferreiro-Iglesias R, et al. Evaluation of the Risk of Relapse in Ulcerative Colitis According to the Degree of Mucosal Healing (Mayo 0 vs 1): A Longitudinal Cohort Study. J Crohns Colitis. 2016;10:13–9. [DOI] [PubMed] [Google Scholar]
- [44].Sinha A, Nightingale J, West KP, Berlanga-Acosta J, Playford RJ. Epidermal growth factor enemas with oral mesalamine for mild-to-moderate left-sided ulcerative colitis or proctitis. N Engl J Med. 2003;349:350–7. [DOI] [PubMed] [Google Scholar]
- [45].Suzuki A, Sekiya S, Gunshima E, Fujii S, Taniguchi H. EGF signaling activates proliferation and blocks apoptosis of mouse and human intestinal stem/progenitor cells in long-term monolayer cell culture. Lab Invest. 2010;90:1425–36. [DOI] [PubMed] [Google Scholar]
- [46].Polk DB. Epidermal growth factor receptor-stimulated intestinal epithelial cell migration requires phospholipase C activity. Gastroenterology. 1998;114:493–502. [DOI] [PubMed] [Google Scholar]
- [47].Miguel JC, Maxwell AA, Hsieh JJ, Harnisch LC, Al Alam D, Polk DB, et al. Epidermal growth factor suppresses intestinal epithelial cell shedding through a MAPK-dependent pathway. J Cell Sci. 2017;130:90–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Dube PE, Yan F, Punit S, Girish N, McElroy SJ, Washington MK, et al. Epidermal growth factor receptor inhibits colitis-associated cancer in mice. J Clin Invest. 2012;122:2780–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Wang RX, Lee JS, Campbell EL, Colgan SP. Microbiota-derived butyrate dynamically regulates intestinal homeostasis through regulation of actin-associated protein synaptopodin. Proc Natl Acad Sci U S A. 2020;117:11648–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Ma X, Fan PX, Li LS, Qiao SY, Zhang GL, Li DF. Butyrate promotes the recovering of intestinal wound healing through its positive effect on the tight junctions. J Anim Sci. 2012;90 Suppl 4:266–8. [DOI] [PubMed] [Google Scholar]
- [51].Zheng L, Kelly CJ, Battista KD, Schaefer R, Lanis JM, Alexeev EE, et al. Microbial-Derived Butyrate Promotes Epithelial Barrier Function through IL-10 Receptor-Dependent Repression of Claudin-2. J Immunol. 2017;199:2976–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].Liu J, Zhu H, Li B, Lee C, Alganabi M, Zheng S, et al. Beneficial effects of butyrate in intestinal injury. J Pediatr Surg. 2020;55:1088–93. [DOI] [PubMed] [Google Scholar]
- [53].Scheppach W, Sommer H, Kirchner T, Paganelli GM, Bartram P, Christl S, et al. Effect of butyrate enemas on the colonic mucosa in distal ulcerative colitis. Gastroenterology. 1992;103:51–6. [DOI] [PubMed] [Google Scholar]
- [54].Mahmood A, Melley L, Fitzgerald AJ, Ghosh S, Playford RJ. Trial of trefoil factor 3 enemas, in combination with oral 5-aminosalicylic acid, for the treatment of mild-to-moderate left-sided ulcerative colitis. Aliment Pharmacol Ther. 2005;21:1357–64. [DOI] [PubMed] [Google Scholar]
- [55].Nakase H, Fujiyama Y, Oshitani N, Oga T, Nonomura K, Matsuoka T, et al. Effect of EP4 agonist (ONO-4819CD) for patients with mild to moderate ulcerative colitis refractory to 5-aminosalicylates: a randomized phase II, placebo-controlled trial. Inflamm Bowel Dis. 2010;16:731–3. [DOI] [PubMed] [Google Scholar]
- [56].Seno H, Miyoshi H, Brown SL, Geske MJ, Colonna M, Stappenbeck TS. Efficient colonic mucosal wound repair requires Trem2 signaling. Proc Natl Acad Sci U S A. 2009;106:256–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [57].Miyoshi H, Ajima R, Luo CT, Yamaguchi TP, Stappenbeck TS. Wnt5a potentiates TGF-beta signaling to promote colonic crypt regeneration after tissue injury. Science. 2012;338:108–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [58].Chivukula RR, Shi G, Acharya A, Mills EW, Zeitels LR, Anandam JL, et al. An essential mesenchymal function for miR-143/145 in intestinal epithelial regeneration. Cell. 2014;157:1104–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [59].Barker N, van Es JH, Kuipers J, Kujala P, van den Born M, Cozijnsen M, et al. Identification of stem cells in small intestine and colon by marker gene Lgr5. Nature. 2007;449:1003–7. [DOI] [PubMed] [Google Scholar]
- [60].Ayyaz A, Kumar S, Sangiorgi B, Ghoshal B, Gosio J, Ouladan S, et al. Single-cell transcriptomes of the regenerating intestine reveal a revival stem cell. Nature. 2019;569:121–5. [DOI] [PubMed] [Google Scholar]
- [61].Buczacki SJ, Zecchini HI, Nicholson AM, Russell R, Vermeulen L, Kemp R, et al. Intestinal label-retaining cells are secretory precursors expressing Lgr5. Nature. 2013;495:65–9. [DOI] [PubMed] [Google Scholar]
- [62].Sangiorgi E, Capecchi MR. Bmi1 is expressed in vivo in intestinal stem cells. Nat Genet. 2008;40:915–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [63].Tian H, Biehs B, Warming S, Leong KG, Rangell L, Klein OD, et al. A reserve stem cell population in small intestine renders Lgr5-positive cells dispensable. Nature. 2011;478:255–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [64].Yan KS, Chia LA, Li X, Ootani A, Su J, Lee JY, et al. The intestinal stem cell markers Bmi1 and Lgr5 identify two functionally distinct populations. Proc Natl Acad Sci U S A. 2012;109:466–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [65].Wang Y, Chiang IL, Ohara TE, Fujii S, Cheng J, Muegge BD, et al. Long-Term Culture Captures Injury-Repair Cycles of Colonic Stem Cells. Cell. 2019;179:1144–59 e15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [66].Murata K, Jadhav U, Madha S, van Es J, Dean J, Cavazza A, et al. Ascl2-Dependent Cell Dedifferentiation Drives Regeneration of Ablated Intestinal Stem Cells. Cell Stem Cell. 2020;26:377–90 e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [67].Ritsma L, Ellenbroek SIJ, Zomer A, Snippert HJ, de Sauvage FJ, Simons BD, et al. Intestinal crypt homeostasis revealed at single-stem-cell level by in vivo live imaging. Nature. 2014;507:362–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [68].Schmitt M, Schewe M, Sacchetti A, Feijtel D, van de Geer WS, Teeuwssen M, et al. Paneth Cells Respond to Inflammation and Contribute to Tissue Regeneration by Acquiring Stem-like Features through SCF/c-Kit Signaling. Cell Rep. 2018;24:2312–28 e7. [DOI] [PubMed] [Google Scholar]
- [69].Tetteh PW, Basak O, Farin HF, Wiebrands K, Kretzschmar K, Begthel H, et al. Replacement of Lost Lgr5-Positive Stem Cells through Plasticity of Their Enterocyte-Lineage Daughters. Cell Stem Cell. 2016;18:203–13. [DOI] [PubMed] [Google Scholar]
- [70].Yan KS, Gevaert O, Zheng GXY, Anchang B, Probert CS, Larkin KA, et al. Intestinal Enteroendocrine Lineage Cells Possess Homeostatic and Injury-Inducible Stem Cell Activity. Cell Stem Cell. 2017;21:78–90 e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [71].Westphalen CB, Asfaha S, Hayakawa Y, Takemoto Y, Lukin DJ, Nuber AH, et al. Long-lived intestinal tuft cells serve as colon cancer-initiating cells. J Clin Invest. 2014;124:1283–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [72].Asfaha S, Hayakawa Y, Muley A, Stokes S, Graham TA, Ericksen RE, et al. Krt19(+)/Lgr5(−) Cells Are Radioresistant Cancer-Initiating Stem Cells in the Colon and Intestine. Cell Stem Cell. 2015;16:627–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [73].Gregorieff A, Liu Y, Inanlou MR, Khomchuk Y, Wrana JL. Yap-dependent reprogramming of Lgr5(+) stem cells drives intestinal regeneration and cancer. Nature. 2015;526:715–8. [DOI] [PubMed] [Google Scholar]
- [74].Nusse YM, Savage AK, Marangoni P, Rosendahl-Huber AKM, Landman TA, de Sauvage FJ, et al. Parasitic helminths induce fetal-like reversion in the intestinal stem cell niche. Nature. 2018;559:109–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [75].Yui S, Azzolin L, Maimets M, Pedersen MT, Fordham RP, Hansen SL, et al. YAP/TAZ-Dependent Reprogramming of Colonic Epithelium Links ECM Remodeling to Tissue Regeneration. Cell Stem Cell. 2018;22:35–49 e7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [76].Kim TH, Li F, Ferreiro-Neira I, Ho LL, Luyten A, Nalapareddy K, et al. Broadly permissive intestinal chromatin underlies lateral inhibition and cell plasticity. Nature. 2014;506:511–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [77].Dube PE, Liu CY, Girish N, Washington MK, Polk DB. Pharmacological activation of epidermal growth factor receptor signaling inhibits colitis-associated cancer in mice. Sci Rep. 2018;8:9119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [78].Tahara Y, Ido A, Yamamoto S, Miyata Y, Uto H, Hori T, et al. Hepatocyte growth factor facilitates colonic mucosal repair in experimental ulcerative colitis in rats. J Pharmacol Exp Ther. 2003;307:146–51. [DOI] [PubMed] [Google Scholar]
- [79].Numata M, Ido A, Moriuchi A, Kim I, Tahara Y, Yamamoto S, et al. Hepatocyte growth factor facilitates the repair of large colonic ulcers in 2,4,6-trinitrobenzene sulfonic acid-induced colitis in rats. Inflamm Bowel Dis. 2005;11:551–8. [DOI] [PubMed] [Google Scholar]
- [80].Howarth GS, Xian CJ, Read LC. Insulin-like growth factor-I partially attenuates colonic damage in rats with experimental colitis induced by oral dextran sulphate sodium. Scand J Gastroenterol. 1998;33:180–90. [DOI] [PubMed] [Google Scholar]
- [81].Chen T, Zheng F, Tao J, Tan S, Zeng L, Peng X, et al. Insulin-Like Growth Factor-1 Contributes to Mucosal Repair by beta-Arrestin2-Mediated Extracellular Signal-Related Kinase Signaling in Experimental Colitis. Am J Pathol. 2015;185:2441–53. [DOI] [PubMed] [Google Scholar]
- [82].Song X, Dai D, He X, Zhu S, Yao Y, Gao H, et al. Growth Factor FGF2 Cooperates with Interleukin-17 to Repair Intestinal Epithelial Damage. Immunity. 2015;43:488–501. [DOI] [PubMed] [Google Scholar]
- [83].Han DS, Li F, Holt L, Connolly K, Hubert M, Miceli R, et al. Keratinocyte growth factor-2 (FGF-10) promotes healing of experimental small intestinal ulceration in rats. Am J Physiol Gastrointest Liver Physiol. 2000;279:G1011–22. [DOI] [PubMed] [Google Scholar]
- [84].Beck PL, Rosenberg IM, Xavier RJ, Koh T, Wong JF, Podolsky DK. Transforming growth factor-beta mediates intestinal healing and susceptibility to injury in vitro and in vivo through epithelial cells. Am J Pathol. 2003;162:597–608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [85].Hwang JH, Kim TH, Kim YH, Noh JR, Choi DH, Kim KS, et al. Gadd45beta promotes regeneration after injury through TGFbeta-dependent restitution in experimental colitis. Exp Mol Med. 2019;51:1–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [86].Owen CR, Yuan L, Basson MD. Smad3 knockout mice exhibit impaired intestinal mucosal healing. Lab Invest. 2008;88:1101–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [87].Karhausen J, Furuta GT, Tomaszewski JE, Johnson RS, Colgan SP, Haase VH. Epithelial hypoxia-inducible factor-1 is protective in murine experimental colitis. J Clin Invest. 2004;114:1098–106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [88].Goggins BJ, Minahan K, Sherwin S, Soh WS, Pryor J, Bruce J, et al. Pharmacological HIF-1 stabilization promotes intestinal epithelial healing through regulation of alpha-integrin expression and function. Am J Physiol Gastrointest Liver Physiol. 2021. [DOI] [PubMed] [Google Scholar]
- [89].Owen KA, Abshire MY, Tilghman RW, Casanova JE, Bouton AH. FAK regulates intestinal epithelial cell survival and proliferation during mucosal wound healing. PLoS One. 2011;6:e23123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [90].Wang Q, More SK, Vomhof-DeKrey EE, Golovko MY, Basson MD. Small molecule FAK activator promotes human intestinal epithelial monolayer wound closure and mouse ulcer healing. Sci Rep. 2019;9:14669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [91].Ma Y, Semba S, Khan RI, Bochimoto H, Watanabe T, Fujiya M, et al. Focal adhesion kinase regulates intestinal epithelial barrier function via redistribution of tight junction. Biochim Biophys Acta. 2013;1832:151–9. [DOI] [PubMed] [Google Scholar]
- [92].Thomas KS, Owen KA, Conger K, Llewellyn RA, Bouton AH, Casanova JE. Non-redundant functions of FAK and Pyk2 in intestinal epithelial repair. Sci Rep. 2019;9:4497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [93].Frey MR, Golovin A, Polk DB. Epidermal growth factor-stimulated intestinal epithelial cell migration requires Src family kinase-dependent p38 MAPK signaling. J Biol Chem. 2004;279:44513–21. [DOI] [PubMed] [Google Scholar]
- [94].Kondo Y, Higa-Nakamine S, Maeda N, Toku S, Kakinohana M, Sugahara K, et al. Stimulation of Cell Migration by Flagellin Through the p38 MAP Kinase Pathway in Cultured Intestinal Epithelial Cells. J Cell Biochem. 2016;117:247–58. [DOI] [PubMed] [Google Scholar]
- [95].Waetzig GH, Seegert D, Rosenstiel P, Nikolaus S, Schreiber S. p38 mitogen-activated protein kinase is activated and linked to TNF-alpha signaling in inflammatory bowel disease. J Immunol. 2002;168:5342–51. [DOI] [PubMed] [Google Scholar]
- [96].ten Hove T, van den Blink B, Pronk I, Drillenburg P, Peppelenbosch MP, van Deventer SJ. Dichotomal role of inhibition of p38 MAPK with SB 203580 in experimental colitis. Gut. 2002;50:507–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [97].Zheng T, Zhang B, Chen C, Ma J, Meng D, Huang J, et al. Protein kinase p38alpha signaling in dendritic cells regulates colon inflammation and tumorigenesis. Proc Natl Acad Sci U S A. 2018;115:E12313–E22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [98].Lu N, Wang L, Cao H, Liu L, Van Kaer L, Washington MK, et al. Activation of the epidermal growth factor receptor in macrophages regulates cytokine production and experimental colitis. J Immunol. 2014;192:1013–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [99].Isani M, Illingworth L, Herman E, Schmidt M, Barron L, Bowling J, et al. Soybean-derived recombinant human epidermal growth factor protects against experimental necrotizing enterocolitis. J Pediatr Surg. 2018;53:1203–7. [DOI] [PubMed] [Google Scholar]
- [100].He Y, Schmidt MA, Erwin C, Guo J, Sun R, Pendarvis K, et al. Transgenic Soybean Production of Bioactive Human Epidermal Growth Factor (EGF). PLoS One. 2016;11:e0157034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [101].Laroui H, Geem D, Xiao B, Viennois E, Rakhya P, Denning T, et al. Targeting intestinal inflammation with CD98 siRNA/PEI-loaded nanoparticles. Mol Ther. 2014;22:69–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [102].Xiao B, Laroui H, Viennois E, Ayyadurai S, Charania MA, Zhang Y, et al. Nanoparticles with surface antibody against CD98 and carrying CD98 small interfering RNA reduce colitis in mice. Gastroenterology. 2014;146:1289–300 e1–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [103].Boirivant M, Pallone F, Di Giacinto C, Fina D, Monteleone I, Marinaro M, et al. Inhibition of Smad7 with a specific antisense oligonucleotide facilitates TGF-beta1-mediated suppression of colitis. Gastroenterology. 2006;131:1786–98. [DOI] [PubMed] [Google Scholar]
- [104].Monteleone G, Neurath MF, Ardizzone S, Di Sabatino A, Fantini MC, Castiglione F, et al. Mongersen, an oral SMAD7 antisense oligonucleotide, and Crohn’s disease. N Engl J Med. 2015;372:1104–13. [DOI] [PubMed] [Google Scholar]
- [105].Khalil HA, Hong SN, Rouch JD, Scott A, Cho Y, Wang J, et al. Intestinal epithelial replacement by transplantation of cultured murine and human cells into the small intestine. PLoS One. 2019;14:e0216326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [106].Fordham RP, Yui S, Hannan NR, Soendergaard C, Madgwick A, Schweiger PJ, et al. Transplantation of expanded fetal intestinal progenitors contributes to colon regeneration after injury. Cell Stem Cell. 2013;13:734–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [107].Yui S, Nakamura T, Sato T, Nemoto Y, Mizutani T, Zheng X, et al. Functional engraftment of colon epithelium expanded in vitro from a single adult Lgr5(+) stem cell. Nat Med. 2012;18:618–23. [DOI] [PubMed] [Google Scholar]
- [108].Fukuda M, Mizutani T, Mochizuki W, Matsumoto T, Nozaki K, Sakamaki Y, et al. Small intestinal stem cell identity is maintained with functional Paneth cells in heterotopically grafted epithelium onto the colon. Genes Dev. 2014;28:1752–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [109].Altay G, Larranaga E, Tosi S, Barriga FM, Batlle E, Fernandez-Majada V, et al. Self-organized intestinal epithelial monolayers in crypt and villus-like domains show effective barrier function. Sci Rep. 2019;9:10140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [110].Wang Y, Gunasekara DB, Reed MI, DiSalvo M, Bultman SJ, Sims CE, et al. A microengineered collagen scaffold for generating a polarized crypt-villus architecture of human small intestinal epithelium. Biomaterials. 2017;128:44–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [111].Wang Y, Kim R, Gunasekara DB, Reed MI, DiSalvo M, Nguyen DL, et al. Formation of Human Colonic Crypt Array by Application of Chemical Gradients Across a Shaped Epithelial Monolayer. Cell Mol Gastroenterol Hepatol. 2018;5:113–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [112].Wang Y, DiSalvo M, Gunasekara DB, Dutton J, Proctor A, Lebhar MS, et al. Self-renewing Monolayer of Primary Colonic or Rectal Epithelial Cells. Cell Mol Gastroenterol Hepatol. 2017;4:165–82 e7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [113].Sachs N, Tsukamoto Y, Kujala P, Peters PJ, Clevers H. Intestinal epithelial organoids fuse to form self-organizing tubes in floating collagen gels. Development. 2017;144:1107–12. [DOI] [PubMed] [Google Scholar]
- [114].Gjorevski N, Sachs N, Manfrin A, Giger S, Bragina ME, Ordonez-Moran P, et al. Designer matrices for intestinal stem cell and organoid culture. Nature. 2016;539:560–4. [DOI] [PubMed] [Google Scholar]
- [115].Mithal A, Capilla A, Heinze D, Berical A, Villacorta-Martin C, Vedaie M, et al. Generation of mesenchyme free intestinal organoids from human induced pluripotent stem cells. Nat Commun. 2020;11:215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [116].Takahashi Y, Sato S, Kurashima Y, Yamamoto T, Kurokawa S, Yuki Y, et al. A Refined Culture System for Human Induced Pluripotent Stem Cell-Derived Intestinal Epithelial Organoids. Stem Cell Reports. 2018;10:314–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [117].Spence JR, Mayhew CN, Rankin SA, Kuhar MF, Vallance JE, Tolle K, et al. Directed differentiation of human pluripotent stem cells into intestinal tissue in vitro. Nature. 2011;470:105–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [118].Park CS, Nguyen LP, Yong D. Development of Colonic Organoids Containing Enteric Nerves or Blood Vessels from Human Embryonic Stem Cells. Cells. 2020;9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [119].Holloway EM, Wu JH, Czerwinski M, Sweet CW, Wu A, Tsai YH, et al. Differentiation of Human Intestinal Organoids with Endogenous Vascular Endothelial Cells. Dev Cell. 2020;54:516–28 e7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [120].Finkbeiner SR, Hill DR, Altheim CH, Dedhia PH, Taylor MJ, Tsai YH, et al. Transcriptome-wide Analysis Reveals Hallmarks of Human Intestine Development and Maturation In Vitro and In Vivo. Stem Cell Reports. 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [121].Sun L, Miyoshi H, Origanti S, Nice TJ, Barger AC, Manieri NA, et al. Type I interferons link viral infection to enhanced epithelial turnover and repair. Cell Host Microbe. 2015;17:85–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [122].Dignass AU, Podolsky DK. Cytokine modulation of intestinal epithelial cell restitution: central role of transforming growth factor beta. Gastroenterology. 1993;105:1323–32. [DOI] [PubMed] [Google Scholar]
- [123].Dignass AU, Podolsky DK. Interleukin 2 modulates intestinal epithelial cell function in vitro. Exp Cell Res. 1996;225:422–9. [DOI] [PubMed] [Google Scholar]
- [124].Kuhn KA, Manieri NA, Liu TC, Stappenbeck TS. IL-6 stimulates intestinal epithelial proliferation and repair after injury. PLoS One. 2014;9:e114195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [125].Corredor J, Yan F, Shen CC, Tong W, John SK, Wilson G, et al. Tumor necrosis factor regulates intestinal epithelial cell migration by receptor-dependent mechanisms. Am J Physiol Cell Physiol. 2003;284:C953–61. [DOI] [PubMed] [Google Scholar]
- [126].Birkl D, Quiros M, Garcia-Hernandez V, Zhou DW, Brazil JC, Hilgarth R, et al. TNFalpha promotes mucosal wound repair through enhanced platelet activating factor receptor signaling in the epithelium. Mucosal Immunol. 2019;12:909–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [127].Bradford EM, Ryu SH, Singh AP, Lee G, Goretsky T, Sinh P, et al. Epithelial TNF Receptor Signaling Promotes Mucosal Repair in Inflammatory Bowel Disease. J Immunol. 2017;199:1886–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [128].Reinecker HC, MacDermott RP, Mirau S, Dignass A, Podolsky DK. Intestinal epithelial cells both express and respond to interleukin 15. Gastroenterology. 1996;111:1706–13. [DOI] [PubMed] [Google Scholar]
- [129].Ogawa A, Andoh A, Araki Y, Bamba T, Fujiyama Y. Neutralization of interleukin-17 aggravates dextran sulfate sodium-induced colitis in mice. Clin Immunol. 2004;110:55–62. [DOI] [PubMed] [Google Scholar]
- [130].Lee JS, Tato CM, Joyce-Shaikh B, Gulen MF, Cayatte C, Chen Y, et al. Interleukin-23-Independent IL-17 Production Regulates Intestinal Epithelial Permeability. Immunity. 2015;43:727–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [131].Lopetuso LR, De Salvo C, Pastorelli L, Rana N, Senkfor HN, Petito V, et al. IL-33 promotes recovery from acute colitis by inducing miR-320 to stimulate epithelial restitution and repair. Proc Natl Acad Sci U S A. 2018;115:E9362–E70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [132].Scheibe K, Backert I, Wirtz S, Hueber A, Schett G, Vieth M, et al. IL-36R signalling activates intestinal epithelial cells and fibroblasts and promotes mucosal healing in vivo. Gut. 2017;66:823–38. [DOI] [PubMed] [Google Scholar]
- [133].Pickert G, Neufert C, Leppkes M, Zheng Y, Wittkopf N, Warntjen M, et al. STAT3 links IL-22 signaling in intestinal epithelial cells to mucosal wound healing. J Exp Med. 2009;206:1465–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [134].Sugimoto K, Ogawa A, Mizoguchi E, Shimomura Y, Andoh A, Bhan AK, et al. IL-22 ameliorates intestinal inflammation in a mouse model of ulcerative colitis. J Clin Invest. 2008;118:534–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [135].Gilbert S, Nivarthi H, Mayhew CN, Lo YH, Noah TK, Vallance J, et al. Activated STAT5 confers resistance to intestinal injury by increasing intestinal stem cell proliferation and regeneration. Stem Cell Reports. 2015;4:209–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [136].Gilbert S, Zhang R, Denson L, Moriggl R, Steinbrecher K, Shroyer N, et al. Enterocyte STAT5 promotes mucosal wound healing via suppression of myosin light chain kinase-mediated loss of barrier function and inflammation. EMBO Mol Med. 2012;4:109–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [137].Nenci A, Becker C, Wullaert A, Gareus R, van Loo G, Danese S, et al. Epithelial NEMO links innate immunity to chronic intestinal inflammation. Nature. 2007;446:557–61. [DOI] [PubMed] [Google Scholar]
- [138].Hueber W, Sands BE, Lewitzky S, Vandemeulebroecke M, Reinisch W, Higgins PD, et al. Secukinumab, a human anti-IL-17A monoclonal antibody, for moderate to severe Crohn’s disease: unexpected results of a randomised, double-blind placebo-controlled trial. Gut. 2012;61:1693–700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [139].Quiros M, Nishio H, Neumann PA, Siuda D, Brazil JC, Azcutia V, et al. Macrophage-derived IL-10 mediates mucosal repair by epithelial WISP-1 signaling. J Clin Invest. 2017;127:3510–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [140].Saxton RA, Tsutsumi N, Su LL, Abhiraman GC, Mohan K, Henneberg LT, et al. Structure-based decoupling of the pro- and anti-inflammatory functions of interleukin-10. Science. 2021;371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [141].Saxton RA, Henneberg LT, Calafiore M, Su L, Jude KM, Hanash AM, et al. The tissue protective functions of interleukin-22 can be decoupled from pro-inflammatory actions through structure-based design. Immunity. 2021;54:660–72 e9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [142].Goettel JA, Kotlarz D, Emani R, Canavan JB, Konnikova L, Illig D, et al. Low-Dose Interleukin-2 Ameliorates Colitis in a Preclinical Humanized Mouse Model. Cell Mol Gastroenterol Hepatol. 2019;8:193–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [143].Lee H, Son YS, Lee MO, Ryu JW, Park K, Kwon O, et al. Low-dose interleukin-2 alleviates dextran sodium sulfate-induced colitis in mice by recovering intestinal integrity and inhibiting AKT-dependent pathways. Theranostics. 2020;10:5048–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [144].Liu CY, Tam SS, Huang Y, Dube PE, Alhosh R, Girish N, et al. TNF Receptor 1 Promotes Early-Life Immunity and Protects against Colitis in Mice. Cell Rep. 2020;33:108275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [145].Chopra M, Biehl M, Steinfatt T, Brandl A, Kums J, Amich J, et al. Exogenous TNFR2 activation protects from acute GvHD via host T reg cell expansion. J Exp Med. 2016;213:1881–900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [146].Punit S, Dube PE, Liu CY, Girish N, Washington MK, Polk DB. Tumor Necrosis Factor Receptor 2 Restricts the Pathogenicity of CD8(+) T Cells in Mice With Colitis. Gastroenterology. 2015;149:993–1005 e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [147].Faustman DL, Davis M. TNF Receptor 2 and Disease: Autoimmunity and Regenerative Medicine. Front Immunol. 2013;4:478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [148].Mizoguchi E, Mizoguchi A, Takedatsu H, Cario E, de Jong YP, Ooi CJ, et al. Role of tumor necrosis factor receptor 2 (TNFR2) in colonic epithelial hyperplasia and chronic intestinal inflammation in mice. Gastroenterology. 2002;122:134–44. [DOI] [PubMed] [Google Scholar]
- [149].Kabashima K, Saji T, Murata T, Nagamachi M, Matsuoka T, Segi E, et al. The prostaglandin receptor EP4 suppresses colitis, mucosal damage and CD4 cell activation in the gut. J Clin Invest. 2002;109:883–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [150].Peng X, Li J, Tan S, Xu M, Tao J, Jiang J, et al. COX-1/PGE2/EP4 alleviates mucosal injury by upregulating beta-arr1-mediated Akt signaling in colitis. Sci Rep. 2017;7:1055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [151].Sheibanie AF, Yen JH, Khayrullina T, Emig F, Zhang M, Tuma R, et al. The proinflammatory effect of prostaglandin E2 in experimental inflammatory bowel disease is mediated through the IL-23-->IL-17 axis. J Immunol. 2007;178:8138–47. [DOI] [PubMed] [Google Scholar]
- [152].Levin AD, Wildenberg ME, van den Brink GR. Mechanism of Action of Anti-TNF Therapy in Inflammatory Bowel Disease. J Crohns Colitis. 2016;10:989–97. [DOI] [PubMed] [Google Scholar]
- [153].Kinchen J, Chen HH, Parikh K, Antanaviciute A, Jagielowicz M, Fawkner-Corbett D, et al. Structural Remodeling of the Human Colonic Mesenchyme in Inflammatory Bowel Disease. Cell. 2018;175:372–86 e17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [154].Pearce SC, Weber GJ, van Sambeek DM, Soares JW, Racicot K, Breault DT. Intestinal enteroids recapitulate the effects of short-chain fatty acids on the intestinal epithelium. PLoS One. 2020;15:e0230231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [155].Parada Venegas D, De la Fuente MK, Landskron G, Gonzalez MJ, Quera R, Dijkstra G, et al. Short Chain Fatty Acids (SCFAs)-Mediated Gut Epithelial and Immune Regulation and Its Relevance for Inflammatory Bowel Diseases. Front Immunol. 2019;10:277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [156].Hernandez-Chirlaque C, Aranda CJ, Ocon B, Capitan-Canadas F, Ortega-Gonzalez M, Carrero JJ, et al. Germ-free and Antibiotic-treated Mice are Highly Susceptible to Epithelial Injury in DSS Colitis. J Crohns Colitis. 2016;10:1324–35. [DOI] [PubMed] [Google Scholar]
- [157].Katakura K, Lee J, Rachmilewitz D, Li G, Eckmann L, Raz E. Toll-like receptor 9-induced type I IFN protects mice from experimental colitis. J Clin Invest. 2005;115:695–702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [158].Rose WA 2nd, Sakamoto K, Leifer CA. TLR9 is important for protection against intestinal damage and for intestinal repair. Sci Rep. 2012;2:574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [159].Vijay-Kumar M, Sanders CJ, Taylor RT, Kumar A, Aitken JD, Sitaraman SV, et al. Deletion of TLR5 results in spontaneous colitis in mice. J Clin Invest. 2007;117:3909–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [160].Shi YJ, Gong HF, Zhao QQ, Liu XS, Liu C, Wang H. Critical role of toll-like receptor 4 (TLR4) in dextran sulfate sodium (DSS)-Induced intestinal injury and repair. Toxicol Lett. 2019;315:23–30. [DOI] [PubMed] [Google Scholar]
- [161].Singh JC, Cruickshank SM, Newton DJ, Wakenshaw L, Graham A, Lan J, et al. Toll-like receptor-mediated responses of primary intestinal epithelial cells during the development of colitis. Am J Physiol Gastrointest Liver Physiol. 2005;288:G514–24. [DOI] [PubMed] [Google Scholar]
- [162].Rakoff-Nahoum S, Paglino J, Eslami-Varzaneh F, Edberg S, Medzhitov R. Recognition of commensal microflora by toll-like receptors is required for intestinal homeostasis. Cell. 2004;118:229–41. [DOI] [PubMed] [Google Scholar]
- [163].Alam A, Leoni G, Quiros M, Wu H, Desai C, Nishio H, et al. The microenvironment of injured murine gut elicits a local pro-restitutive microbiota. Nat Microbiol. 2016;1:15021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [164].Alam A, Leoni G, Wentworth CC, Kwal JM, Wu H, Ardita CS, et al. Redox signaling regulates commensal-mediated mucosal homeostasis and restitution and requires formyl peptide receptor 1. Mucosal Immunol. 2014;7:645–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [165].Donaldson GP, Chou WC, Manson AL, Rogov P, Abeel T, Bochicchio J, et al. Spatially distinct physiology of Bacteroides fragilis within the proximal colon of gnotobiotic mice. Nat Microbiol. 2020;5:746–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [166].Bergstrom K, Shan X, Casero D, Batushansky A, Lagishetty V, Jacobs JP, et al. Proximal colon-derived O-glycosylated mucus encapsulates and modulates the microbiota. Science. 2020;370:467–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [167].Kaiko GE, Ryu SH, Koues OI, Collins PL, Solnica-Krezel L, Pearce EJ, et al. The Colonic Crypt Protects Stem Cells from Microbiota-Derived Metabolites. Cell. 2016;165:1708–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [168].Moor AE, Harnik Y, Ben-Moshe S, Massasa EE, Rozenberg M, Eilam R, et al. Spatial Reconstruction of Single Enterocytes Uncovers Broad Zonation along the Intestinal Villus Axis. Cell. 2018;175:1156–67 e15. [DOI] [PubMed] [Google Scholar]
- [169].Sommer F, Nookaew I, Sommer N, Fogelstrand P, Backhed F. Site-specific programming of the host epithelial transcriptome by the gut microbiota. Genome Biol. 2015;16:62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [170].Jain U, Ver Heul AM, Xiong S, Gregory MH, Demers EG, Kern JT, et al. Debaryomyces is enriched in Crohn’s disease intestinal tissue and impairs healing in mice. Science. 2021;371:1154–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [171].Mazmanian SK, Round JL, Kasper DL. A microbial symbiosis factor prevents intestinal inflammatory disease. Nature. 2008;453:620–5. [DOI] [PubMed] [Google Scholar]
- [172].Yan F, Liu L, Dempsey PJ, Tsai YH, Raines EW, Wilson CL, et al. A Lactobacillus rhamnosus GG-derived soluble protein, p40, stimulates ligand release from intestinal epithelial cells to transactivate epidermal growth factor receptor. J Biol Chem. 2013;288:30742–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [173].Yan F, Cao H, Cover TL, Washington MK, Shi Y, Liu L, et al. Colon-specific delivery of a probiotic-derived soluble protein ameliorates intestinal inflammation in mice through an EGFR-dependent mechanism. J Clin Invest. 2011;121:2242–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [174].Lee JS, Wang RX, Goldberg MS, Clifford GP, Kao DJ, Colgan SP. Microbiota-Sourced Purines Support Wound Healing and Mucous Barrier Function. iScience. 2020;23:101226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [175].Sokol H, Pigneur B, Watterlot L, Lakhdari O, Bermudez-Humaran LG, Gratadoux JJ, et al. Faecalibacterium prausnitzii is an anti-inflammatory commensal bacterium identified by gut microbiota analysis of Crohn disease patients. Proc Natl Acad Sci U S A. 2008;105:16731–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [176].Lapiere A, Geiger M, Robert V, Demarquay C, Auger S, Chadi S, et al. Prophylactic Faecalibacterium prausnitzii treatment prevents the acute breakdown of colonic epithelial barrier in a preclinical model of pelvic radiation disease. Gut Microbes. 2020;12:1–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [177].Bibiloni R, Fedorak RN, Tannock GW, Madsen KL, Gionchetti P, Campieri M, et al. VSL#3 probiotic-mixture induces remission in patients with active ulcerative colitis. Am J Gastroenterol. 2005;100:1539–46. [DOI] [PubMed] [Google Scholar]
- [178].Rohatgi S, Ahuja V, Makharia GK, Rai T, Das P, Dattagupta S, et al. VSL#3 induces and maintains short-term clinical response in patients with active microscopic colitis: a two-phase randomised clinical trial. BMJ Open Gastroenterol. 2015;2:e000018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [179].Gionchetti P, Rizzello F, Morselli C, Poggioli G, Tambasco R, Calabrese C, et al. High-dose probiotics for the treatment of active pouchitis. Dis Colon Rectum. 2007;50:2075–82; discussion 82–4. [DOI] [PubMed] [Google Scholar]
- [180].Kumar M, Kissoon-Singh V, Coria AL, Moreau F, Chadee K. Probiotic mixture VSL#3 reduces colonic inflammation and improves intestinal barrier function in Muc2 mucin-deficient mice. Am J Physiol Gastrointest Liver Physiol. 2017;312:G34–G45. [DOI] [PubMed] [Google Scholar]
- [181].Moon C, Baldridge MT, Wallace MA, D CA, Burnham, Virgin HW, et al. Vertically transmitted faecal IgA levels determine extra-chromosomal phenotypic variation. Nature. 2015;521:90–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [182].Atarashi K, Suda W, Luo C, Kawaguchi T, Motoo I, Narushima S, et al. Ectopic colonization of oral bacteria in the intestine drives TH1 cell induction and inflammation. Science. 2017;358:359–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [183].Bober JR, Beisel CL, Nair NU. Synthetic Biology Approaches to Engineer Probiotics and Members of the Human Microbiota for Biomedical Applications. Annu Rev Biomed Eng. 2018;20:277–300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [184].Dulai PS, Singh S, Vande Casteele N, Boland BS, Rivera-Nieves J, Ernst PB, et al. Should We Divide Crohn’s Disease Into Ileum-Dominant and Isolated Colonic Diseases? Clin Gastroenterol Hepatol. 2019;17:2634–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [185].Sood A, Midha V, Makharia GK, Ahuja V, Singal D, Goswami P, et al. The probiotic preparation, VSL#3 induces remission in patients with mild-to-moderately active ulcerative colitis. Clin Gastroenterol Hepatol. 2009;7:1202–9, 9 e1. [DOI] [PubMed] [Google Scholar]