Abstract
Obesity is a costly, global epidemic that is perpetuated by an unhealthy diet. A significant factor in the initial consumption and maintenance of an unhealthy diet is the abundance of highly palatable, calorically dense foods. The aim of the present study is to better understand the effects of high fat diet (HFD) consumption on food valuation and preference, and to elucidate the neurobiological mechanisms mediating these effects. By using a novel food preference assay, we found that prolonged consumption of a HFD diminishes preference for and consumption of the more calorically dense food choice when two lab diets are presented. Additionally, we demonstrated that prolonged HFD consumption dampens ventral tegmental c-fos induction during hedonic feeding, implicating the mesolimbic dopamine signaling pathway as a target of HFD. Notably, both the changes in food preference and this reduced c-fos induction were reversed during withdrawal from HFD. Further, HFD-induced alterations in food preference were attenuated by exercise. Our findings suggest that prolonged HFD consumption leads to anhedonia and altered feeding choices, and this is associated with changes in mesolimbic dopamine signaling.
Keywords: dopamine, food preference, feeding behavior, nucleus accumbens, ventral tegmental area, exercise
1. Introduction:
According to the World Health Organization, obesity is a multifaceted health crisis stemming from overconsumption of calories relative to energy expenditure, leading to excess weight gain. Obese individuals are at a dramatically increased risk for type 2 diabetes, cardiovascular disease, and some types of cancer [1]. Dieting and exercise are the most commonly prescribed treatments for obesity. However, they are often ineffective as long-term solutions due to patients’ frequent relapse into sedentary lifestyles [2,3]. Unhealthy diets are positively correlated with caloric overconsumption and are perpetuated by an abundance of readily accessible, highly-palatable, energy-dense foods [4,5]. Consumption of unhealthy diets is further promoted by prior experience with energy-dense foods [6,7]. Furthermore, the salience of palatable, high fat foods is conveyed via the mesolimbic dopamine signaling pathway, which serves as the brain’s reward circuit [8–11]. This pathway consists of dopaminergic projections from the ventral tegmental area (VTA) to the nucleus accumbens (NAc) and is activated in the presence of food, leading to elevated NAc dopamine levels [12]. Functionally, this pathway is critical for reinforcement learning, motivation, and reward processing [11]. Despite its necessity for food reward processing, the way mesolimbic dopamine signaling and its attendant feeding behaviors adapt to prolonged HFD consumption has yet to be fully characterized.
Feeding behavior involves an exceedingly complex array of processes that integrate information regarding energy homeostasis and incentive salience [12,13]. In rodents, the consumption of a HFD has been associated with remodeling of reward-related behaviors, including feeding. Prolonged consumption of a HFD has been shown to lead to a diminished preference for sucrose over water in a two-bottle choice test, and decreased preference for a location associated with food rewards in a conditioned place preference assay [14–17]. It has been shown that prolonged HFD consumption leads to devaluation of standard chow even after HFD access has been ceased [18]. In addition, long-term HFD consumption has been associated with diminished motivation to attain food reward in progressive ratio and food seeking tasks [14,16,19]. HFD-induced reward devaluation extends to drugs of abuse as well, leading to a reduced conditioned place preference for amphetamine delivery [20,21]. Integrity of the mesolimbic dopamine signaling pathway is susceptible to HFD consumption [19,22,23]. For example, prolonged HFD consumption dampens food reward or amphetamine induced dopamine release in the NAc [23,24], independent of an obesity phenotype [20]. However, despite these established HFD-mediated effects, the capability of accessible behavioral interventions (ie. dieting and exercise) to reverse them is rarely investigated.
Because the mesolimbic dopamine signaling pathway serves as a nexus for many aspects of feeding behavior, consumption of a HFD has been shown to affect both this pathway as well as the evaluation of food rewards, and dieting and exercise are the most common means to achieve weight loss in humans, we investigated the following hypotheses: (1) prolonged consumption of a HFD alters feeding behavior via perturbations of the mesolimbic dopamine signaling pathway, and (2) these alterations are reversed via dietary changes and exercise. To evaluate these hypotheses, we developed an assay that examines changes in preference between two different chows. We tested whether HFD changes food preference and amount of test food consumed. We then examined the effects of HFD on mesolimbic dopamine signaling during hedonic feeding. We discovered that HFD diminishes preference for a more calorically dense chow, and that this behavioral change can be reversed by withdrawing animals from HFD or by allowing them to engage in voluntary exercise. Additionally, we found that HFD consumption dampened c-fos induction in the VTA during palatable feeding, and that this effect was reversed in animals withdrawn from HFD. Our findings implicate a role for dopamine signaling following prolonged HFD consumption, which leads to reversible alterations in food preference.
2. Materials and Methods:
2.1. Animals
All experiments complied with NIH animal care and use guidelines, and were approved by the University of Virginia Institutional Animal Care and Use Committee. Male mice were housed on a 12-hour light/dark cycle (lights on between 7am and 7pm), in standard ventilated cages with corncob bedding, cotton nesting material (Nestlets™, Ancare, Bellmore, NY, USA) and a paper soufflé cup (product F400, Genpak, Charlotte, NC). C57BL/6 breeder mice were originally obtained from The Jackson Laboratory (Bar Harbor, ME, USA). Maintenance diets were furnished to both breeders and offspring except where acute dietary treatment is specified. All experiments took place using male mice, and all experimental mice were habituated to individual housing for at least 3 days prior to intake procedures. All behavioral tests and tissue collections took place at 14 weeks of age.
2.2. Diets used
Control mice received Envigo (Huntingdon, United Kingdom) product 8664 Teklad F6 Rodent Diet (standard diet, 3.1 kcal/g; 19% fat, 31% protein, 50% carbohydrates). Control mice in the 2 bottle choice test (Fig 1A) were provided LabDiet (St. Louis, MO) product 5053 PicoLab Rodent Diet 20 (standard diet, 3.07 kcal/g; 13% fat, 25% protein, 62% carbohydrates) due to discontinuation of Envigo product 8664. Mice on high fat diet (HFD) received: Research Diets Open Source Diets (Research Diets, New Brunswick, NJ, USA) product D12451 (OS red, 4.73 kcal/g; 45% fat, 20% protein, 35% carbohydrates).
Fig.1.
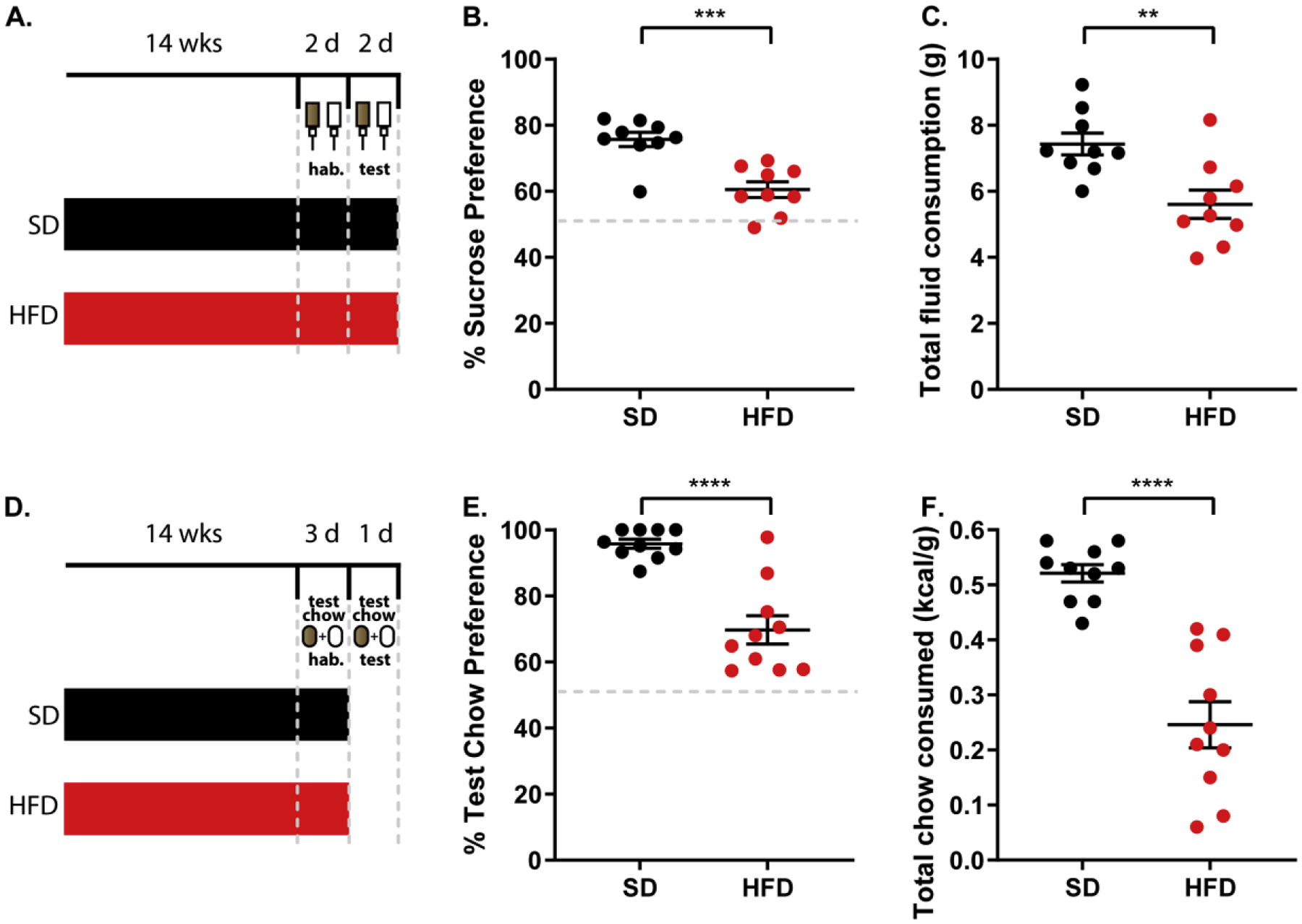
Prolonged consumption of a high fat diet diminishes preference for and consumption of a more palatable liquid or solid food choice. (A) Schematic illustrating the 2-bottle experimental setup. (B) Preference for sucrose over water in a two-bottle choice test. Student’s two-tailed t-test; n=9/group. (C) Total fluid consumed (sucrose and water) during the two-bottle choice test. Student’s two-tailed t-test; n=9/group. (D) Schematic illustrating food preference experimental setup. (E) Preference for the more energy dense test chow during the food preference test. Student’s two-tailed t-test; n=10/group. (F) Total amount of both test chows consumed during the food preference test corrected for body weight. Student’s two-tailed t-test; n=10/group. Data are represented as mean ± SEM. **p < 0.01; ***p<0.001; ****p < 0.0001. See also Supplemental Figures 1 and 2.
2.3. Food Preference Test
Mice were habituated to 2 novel test chows for 3 days prior to the preference test. During this habituation period, test chows were provided on the cage floor and maintenance diet remained in the wire cage top. At the start of the preference test, mice were weighed, the maintenance diet was withdrawn, and the test chows (~20 g of each) were weighed and added to the cage floor. Remaining test chow and animal body weights were recorded again 24 hr later. Consumption of each test chow for every animal was divided by that animal’s body weight corresponding to the start of the 24 hour test period. Preference scores were calculated as the portion of total test chow consumed corresponding to the more energy enriched test chow. Novel test chows used during 24 hr food choice tests were as follows: Bio-Serv (Flemington, NJ, USA) Product F3028 Rodent Diet Grain-Based Control (BS F3028, 3.35 kcal/g; 13% fat, 29% protein, 58% carbohydrates) versus Bio-Serv (Flemington, NJ, USA) Product F3156 Rodent Diet AIN-93G (BS F3156, 3.74 kcal/g; 17% fat, 19% protein, 64% carbohydrates). Both of these diets were selected because they are contrasting in color with respect to the animals’ bedding, and are lower in energy and fat content compared to the diet the HFD animals were raised on. Bedding was thoroughly searched for test chow crumbs when weighing test chows at the conclusion of the test.
2.4. 2-bottle choice test
Mice were habituated to our 2 bottle-choice setup for 2 days prior to the sucrose preference test. This habituation period was followed by two test days. During the habituation period, mice were housed in specialized cages (Columbus instruments DM-8 Drinking Event Monitor) with constant access to maintenance diet on the cage floor, as well as one bottle with water and a second bottle with a 1% sucrose solution. Bottle location (left vs. right side) alternated daily following each habituation and test day. Animals and bottles were weighed before and after each day. Fresh water and sucrose solutions were provided daily. Preference scores were calculated as the portion of total fluid intake attributable to the sucrose solution. In order to control for a potential side bias, the results from the two test days were averaged together for the final analysis.
2.5. Exercise
At 10 weeks of age, control mice and mice raised on HFD were provided with Bio Serv Mouse Igloos® equipped with either Fast-Trac accessory running discs or nothing. After four weeks of access to the Igloos with or without running discs, mice underwent the food preference test described previously (section 2.3). In order to quantify the amount of exercise achieved by control and HFD-raised animals, a separate cohort of animals raised on either diet were individually housed with ad libitum access to drinking water and food (control diet or HFD). Each cage was also provided with a Bio Serv Mouse Igloo® equipped with a Fast-Trac accessory running disc. A magnetic sensor was attached to the igloo and two permeant magnets were attached to the running discs to allow the recording and analysis of disc turns through the ClockLab collection and analysis system (Actimetrics, Wilmette, IL). Seven days of recoding was collected for each animal and data from days 4 and 5 were averaged for the final comparison.
2.6. C-fos immunohistochemistry
Mice were habituated to a novel HFD, D12492 (OS aqua, 5.24 kcal/g; 60% fat, 20% protein, 20% carbohydrates), on the floor of their home cages for 2 days. Additional cohorts were habituated to a novel SD, 7912 (Teklad LM-485, 3.1 kcal/g; 17% fat, 25% protein, 58% carbohydrates). During habituation, animals had 30 minutes (starting at 2pm) to freely consume the novel chow. Maintenance diet was available ad libitum in the wire cage top. A single test day followed the two habituation days and was identical in procedure to habitation. 90 minutes after cessation of the 30-minute feeding period on test day, animals were euthanized via a ketamine/xylazine cocktail (280mg/kg; 80mg/kg) and intracardially perfused with cold 0.01M phosphate buffer solution (PBS) followed by fixative solution (4% paraformaldehyde (PFA) in PBS at a pH of 7.4). Following perfusion, brains were dissected and post-fixed in PFA overnight at 4 degrees C. The next day, fixed brains were rinsed in PBS and placed in 30% sucrose in PBS for 24 hours before being frozen on dry ice. Coronal sections (30 mm) containing the VTA were collected with a cryostat (Microm HM 505 E). Sections were permeabilized with 0.5% Triton X-100 in PBS (PBS-T) and blocked with 3% normal donkey serum (Jackson ImmunoResearch) in PBS-T (PBS-T DS) for 60 min at room temperature. Sections were then incubated overnight in primary antibodies for c-Fos (rabbit, 1:1000, synaptic systems) and tyrosine hydroxylase (chicken, 1:500, Millipore AB9702) diluted in PBS-T DS. For visualization, sections were washed with PBS-T and incubated with appropriate secondary antibodies diluted in the blocking solution for 3 hours at room temperature. Secondary antibodies (Jackson Immunoresearch) were Cy-2 conjugated donkey anti-rabbit (1:250) and Cy-3 conjugated donkey anti-chicken (1:250). Sections were washed three times with PBS and mounted using DAPI Fluoromount-G (Southern Biotech). Images were captured on a Zeiss Axioplan 2 Imaging microscope equipped with an AxioCam MRm camera using AxioVision 4.6 software (Zeiss).
2.6. Food and water intake, and fat mass measurements
A separate cohort of SD and HFD-raised animals were monitored for daily consumption of water and home chow (SD or HFD) intake for four days. Each day the amount of water consumed was recorded, as was the amount of home chow. Home chow intake was corrected for body mass daily. These measures were averaged across four days for each animal. At the conclusion of the four days, animals were euthanized via cervical dislocation, and their brown (BAT), gonadal white (GWAT), and subcutaneous (SCAT) adipose tissue masses were measured.
2.7. Statistics
Statistical analysis was performed using Prism GraphPad software. Data are presented as means ± standard error of the mean (s.e.m.). All statistical analyses were performed using ANOVA with Bonferroni post-hoc for multiple comparisons. Complete statistical results are included within the text.
3. Results:
3.1. Prolonged consumption of a high fat diet diminishes preference for and consumption of a more palatable liquid or solid food choice
To confirm previous studies demonstrating that HFD consumption leads to anhedonia [14,15,17], we first tested animals in a two-bottle sucrose preference assay. Two cohorts of animals, one raised on standard lab (SD) diet and one raised on HFD [Fig. 1A], were assessed on their preference for a 1% sucrose solution over water at 14 weeks of age. We discovered that animals that had been consuming HFD demonstrated a diminished preference for sucrose compared to water [t=3.240, p<0.01, df=10; n=9/group; Fig. 1B], and diminished overall liquid consumption [t=3.593, p<0.01, df=10; n=9/group; Fig. 1C] during test days. This reduced consumption was due to dampened consumption of sucrose specifically [F(1,32)=11.44; p<0.01; n=9/group; Supplemental Fig. 1A]. These data reaffirm the development of HFD-induced anhedonia in these animals [14,15,17]. However, the two-bottle preference assay utilizes liquids, and its findings cannot necessarily be generalized to feeding behavior and food preference. To overcome this limitation, we developed a novel food preference test that assesses animals’ preference for a more palatable food choice over a less palatable one.
The two chows we used to assess food preference differed by 0.39 kcal/g (3.35 vs. 3.74 kcal/g) in caloric content. Importantly, these two chows are lower in both fat and overall energy density than the diet consumed by the HFD group (4.73 kcal/g). Animals that were raised on either SD or HFD were assessed on their preference between these two novel chows during a 24-hr period [Fig. 1D]. Similar to what we observed during the sucrose preference test, animals raised on HFD exhibited diminished preference for a more energy dense chow [t=5.802, p<0.0001, df=18; n=10/group; Fig.1E], and consumed less test chow overall [t=6.181, p<0.0001, df=18; n=10/group; Fig. 1F] during testing when compared to SD fed controls. Comparable to our observations from the sucrose preference test, the HFD-raised animals’ reduced test chow consumption is due to the decreased intake of the preferred choice specifically [F(1,36)=47.83; p<0.0001; n=10/group; Supplemental Fig. 1B]. Interestingly, HFD-raised animals were not significantly heavier than their SD-raised counterparts during both the sucrose preference test [t=2.002; p=0.0626; df=16; n=9/group; Supplemental Fig. 1C] and the food preference test [t=1.312; p=0.2060; df=18; n=10/group; Supplemental Fig. 1D]. In agreement with this finding, HFD-raised and SD-raised animals consumed similar amounts of maintenance diet [t=1.211; p=0.2459; df=14; n=8/group; Supplemental Fig. 2A] and had similar total fat mass (GWAT, BAT, SCAT) [t=0.866; p=0.4008; df=14; n=8/group; Supplemental Fig. 2B]. In addition, we observed no significant correlation between body weight and food preference [SD-raised: R2=0.001530; p=0.9146; n=10/group; HFD-raised: R2=0.3569; p=0.0682; n=10/group; Supplemental Fig. 3A] or body weight and total test chow consumption [SD-raised: R2=0.07323; p=0.4495; n=10/group; HFD-raised: R2=0.02586; p=0.6572; n=10/group; Supplemental Fig. 3B] in either group. Taken together, these findings suggest that the devaluation of the more palatable food choice occurs independent of an obese phenotype. Next, we wanted to determine if a shorter period of access to HFD could produce the same results [Fig. 2A]. Indeed, when animals consumed a HFD for only four weeks prior to testing, the same effects on preference [F(2,27)=26.40; p<0.0001; n=10/group; Fig. 2B] and consumption [F(2,27)=78.70; p<0.0001; n=10/group; Fig. 2C] were observed. This includes decreased consumption of the preferred test chow specifically [F(2,54)=72.86; p<0.0001; n=10/group; Supplemental Fig. 4A]. These findings suggest that HFD consumption can affect feeding behavior over a shorter period, and with the first exposure not occurring until later in life.
Fig.2.
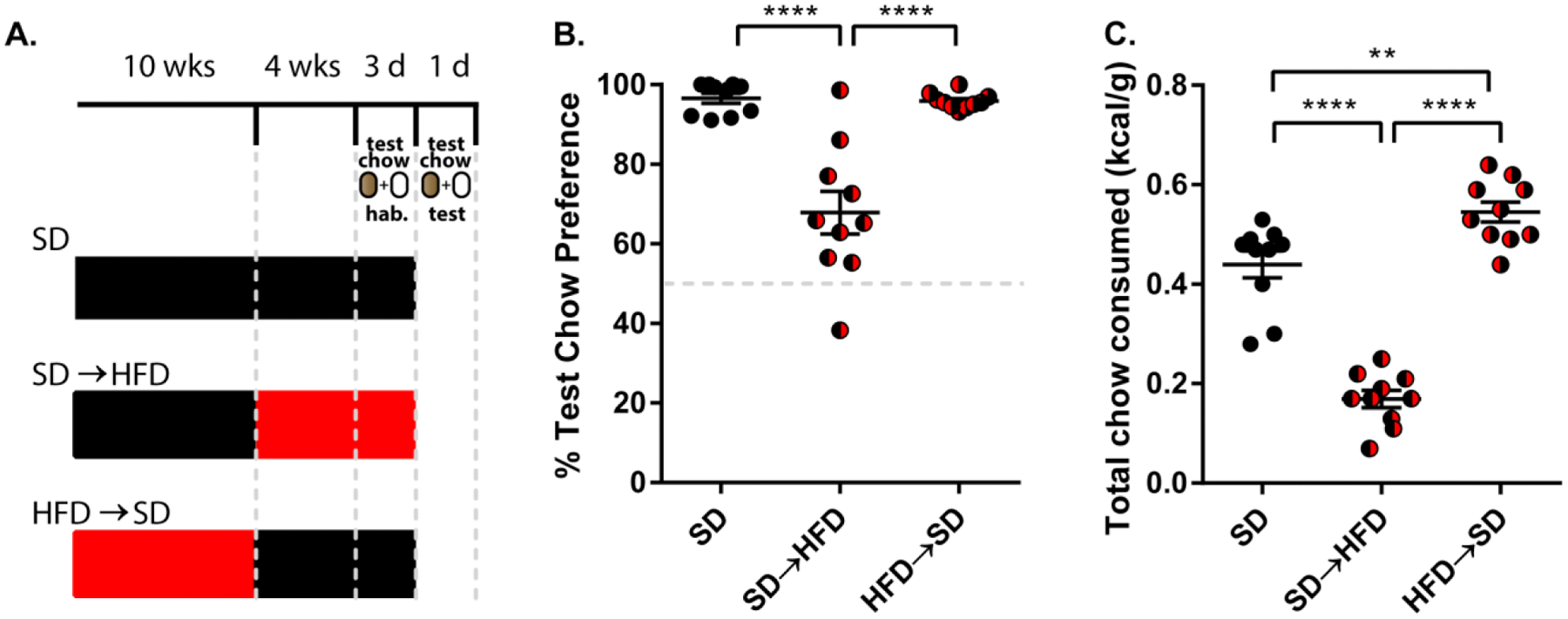
HFD-induced alterations in food preference are reversed by dieting. (A) Schematic illustrating the experimental setup. (B) Preference for the more energy dense test chow during the food preference test. One-way ANOVA with Bonferroni post-hoc comparison; n=10/group; F(2,27)=26.40; p<0.0001. (C) Total amount of both test chows consumed during the food preference test corrected for body weight. One-way ANOVA with Bonferroni post-hoc comparison; n=10/group; F(2,27)=78.70; p<0.0001. Data are represented as mean ± SEM. **p < 0.01; ****p < 0.0001. See also Supplemental Figure 4.
3.2. HFD-induced alterations in food preference are reversed by dieting or exercise
We next sought to determine if behavioral interventions could reverse the alterations in feeding behavior that are produced by prolonged HFD consumption. We first chose to examine if withdrawing HFD-raised animals from HFD prior to the preference test could reverse their reduced preference and intake during the assay. To test this, we switched HFD-raised animals to SD at 10 weeks of age and assessed their preference at 14 weeks of age [Fig. 2A]. Following this dietary switch, we found that these animals no longer exhibited dampened preference for the more energy dense test chow [F(2,27)=26.40; p<0.0001; n=10/group; Fig. 2B] or attenuated test chow consumption [F(2,27)=78.70; p<0.0001; n=10/group; Fig. 2C]. Animals withdrawn from HFD weighed the same as animals raised on SD for 14 weeks [SD=27.57±0.4292 vs. HFD->SD=27.52±1.113; p=0.9991, Bonferroni multiple comparisons test; n=10/group; Supplemental Fig. 4B]. This was unsurprising considering that our HFD-raised mice failed to gain significant weight compared to SD-raised control mice [t=1.312, p=0.2060, df=18; n=10/group; Supplemental Fig. 1C]. Interestingly, these animals consumed more test chow than their SD raised counterparts [SD=0.44±0.02708 vs. HFD->SD=0.5450±0.02029; p<0.01, Bonferroni multiple comparisons test; n=10/group; Fig. 2C]. This heightened consumption is specifically due to an increased intake of the preferred food choice [F(2,54)=72.86; p<0.0001; n=10/group; Supplemental Fig. 4A]. One possible explanation for this is that the animals withdrawn from HFD are exhibiting a sensitized behavioral response to the more palatable test chow, much like what is observed in animal models of drug abstinence and relapse [25]. Taken together, these findings demonstrate that switching to a less calorically enriched diet is sufficient to reverse HFD-induced alterations in food preference. These data demonstrate that the influence of HFD over food preference and consumption is not permanent.
We next assessed whether exercise could reverse the HFD-induced behavioral changes in preference and intake. To this end, we raised additional cohorts of animals on either SD or HFD. All groups were provided Bio Serv Mouse Igloos® for 4 weeks prior to the preference test [Fig. 3A]. One group of each diet received these igloos with Fast-Trac accessory running discs attached to them [Supplemental Fig. 5A]. When given these running discs, both SD and HFD raised animals run on them in equivalent amounts daily [t=1.782, p= 0.0900, df=20; n=11/group; Supplemental Fig. 5B]. Access to a running disc in HFD-raised animals reversed HFD-induced alterations in food preference [F(1,35)=7.401; p<0.05; n=8–14/group; Fig. 3B] without restoring total test chow consumption amount [F(1,35)=1.516; p=0.2264; n=8–14/group; Fig. 3C] or consumption of the preferred test chow specifically [F(1,70]=1.455; p=0.2318; n=8–14/group; Supplemental Fig. 5C]. This behavioral response is strikingly different than what we observed in response to dietary intervention (Fig. 2C) and suggests that food preference and consumption are independently modulated components of feeding behavior. Additionally, we were surprised to observe that HFD-raised control animals with access only to the igloo weighed more than the SD-raised igloo controls [F(1,35)=20.11; p<0.0001; n=8–14/group; Supplemental Fig. 5D] in contrast to the lack of body weight gain in HFD-raised animals [t=1.312, p=0.2060, df=18; n=10/group; Supplemental Fig. 1C]. We suspect this to be due to the potential extra warmth provided by the igloo, allowing those HFD-raised animals to conserve more energy [26]. Taken together, our findings implicate relatively simple and accessible means by which HFD-induced behavioral modifications can be reversed.
Fig.3.
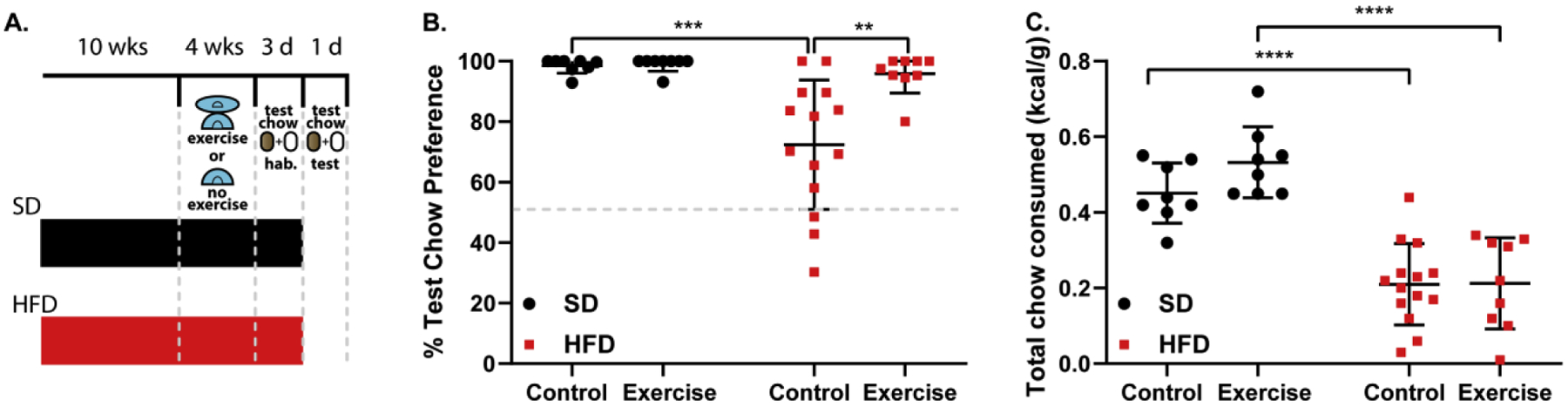
HFD-induced alterations in food preference are reversed by exercise. (A) Schematic illustrating the experimental setup. (B) Preference for the more energy dense test chow during the food preference test. Two-way ANOVA with Bonferroni post-hoc comparison; n=8–14/group; Fexercise(1,35)=7.401, p=0.0101; Fdiet(1,35)=11.01, p=0.0021. (C) Total amount of both test chows consumed during the food preference test corrected for body weight. Two-way ANOVA with Bonferroni post-hoc comparison; n=8–14/group; Fexercise(1,35)=1.516, p=0.2264; Fdiet(1,35)=68.63, p<0.0001. Data are represented as mean ± SEM. **p < 0.01; ***p < 0.001; ****p < 0.0001. See also Supplemental Figure 5.
3.3. Prolonged HFD consumption leads to a failure of the VTA to respond to a novel food reward.
We next investigated the potential neurobiological impact of prolonged HFD consumption. We focused on the mesolimbic dopamine signaling pathway because of its established role in hedonic feeding and used c-fos immunohistochemistry to assess changes in activity of this pathway. Following stimulation in VTA neurons, the immediate early gene c-fos is rapidly expressed, allowing for examination of neural activity in this region [27,28]. To assess diet-induced changes in VTA activity during hedonic feeding, SD and HFD raised mice were allowed to consume a novel HFD for 30 minutes. This diet is more energy dense (5.24 vs. 4.73 kcal/g) and higher in fat content (60% kcal vs. 45% kcal) than the diet the HFD-raised animals consumed throughout their life. After consuming the novel 60% kcal HFD diet for 30 minutes, we examined differences in c-fos and tyrosine hydroxylase (TH) expression in the VTA of these animals. TH is the rate-limiting enzyme in the synthesis of dopamine and allows for the demarcation of dopaminergic cells. HFD raised animals exhibited dampened c-fos induction in the VTA in response to the novel 60% kcal HFD when compared to the standard chow raised controls [SD=61.56±3.705 vs. HFD=33.67±5.651; p<0.05, Bonferroni multiple comparisons test; n=9–12/group; Fig. 4A]. When compared to animals given a novel SD, SD raised animals given the 60% HFD exhibit marked VTA c-fos induction [SD given SD=16.90±4.868 vs. SD given HFD=61.56±3.705; p<0.0001, Bonferroni multiple comparisons test; n=9–10/group; Supplemental Fig. 6A], whereas HFD raised animals do not differ in VTA c-fos activity when given either novel SD or HFD [HFD given SD=21.40±7.115 vs. HFD given HFD=33.67±5.651; p=0.3922, Bonferroni multiple comparisons test; n=10–12/group; Supplemental Fig. 6A]. These findings suggest that prolonged HFD consumption leads to a failure of the VTA to respond during hedonic feeding. We observed no effect of diet on the number of tyrosine hydroxylase (TH) positive cells in the VTA, suggesting that prolonged HFD exposure does not alter the number of dopaminergic cells in the VTA [F(2,28)= 1.892; p= 0.1696; n=9–12/group; Fig. 4B]. Interestingly, the observed HFD-induced reduction in VTA activity during palatable feeding is reversed by withdrawing animals from HFD for 4 weeks prior to testing [HFD=33.67±5.651 vs. HFD->SD= 61.70± 10.53; p<0.05, Bonferroni multiple comparisons test; n=10–12/group; Fig. 4A]. Additionally, this reversal appears to be associated with the hypersensitization of VTA dopaminergic cells specifically [F(2,28)=14.18; p <0.0001; n=9–12/group; Fig. 4C]. These findings show that prolonged HFD consumption blunts the response of the mesolimbic dopamine signaling pathway to rewarding food, but that dieting can restore the response via sensitization of dopamine neurons.
Fig.4.
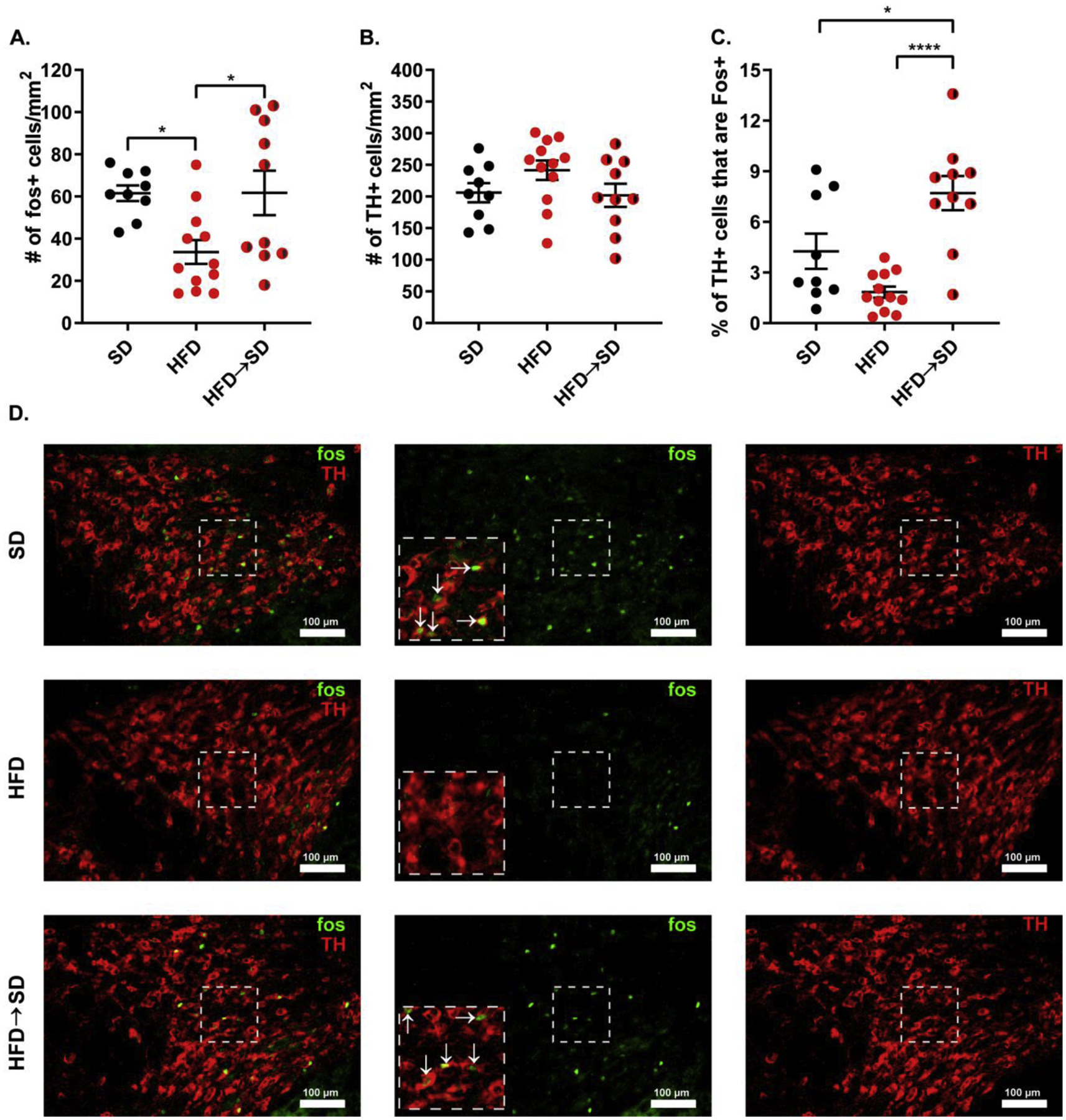
Prolonged HFD consumption leads to a failure of the VTA to respond to a novel food reward. (A) Total number of VTA c-Fos positive cells. One-way ANOVA with Bonferroni post-hoc comparison; n=9–12/group; F(2,28)=5.302; p=0.0111. (B) Total number of VTA TH positive cells. One-way ANOVA with Bonferroni post-hoc comparison; n=9–12/group; F(2,28)=1.892; p= 0.1696. (C) Percentage of VTA TH positive cells that are also c-Fos positive. One-way ANOVA with Bonferroni post-hoc comparison; n=9–12/group; F(2,28)=14.18; p <0.0001. (D) Representative images of VTA sections stained for c-Fos and TH. Dashed boxes represent corresponding areas of the same section. Arrows indicate c-Fos immunoreactivity. Data are represented as mean ± SEM. *p<0.05; ****p < 0.0001. See also Supplemental Figure 6.
4. Discussion:
Using a novel food preference assay, we have discovered that prolonged consumption of a HFD leads to alterations in feeding behavior and devaluation of a more palatable food choice. Furthermore, we investigated the neurobiological effects of HFD exposure and demonstrated that animals raised on HFD exhibit dampened VTA activity during hedonic feeding. Moreover, we also found that diet-induced behavioral alterations could be reversed via both exercise and dietary change.
Previous work has demonstrated that diet affects feeding behavior, but effects on food preference specifically are poorly understood. Two-bottle choice tests, where animals can freely consume two different solutions (alcohol, water, sucrose, sucralose, etc.), are the most frequently used measure of preference, and are a staple behavioral assessment for anhedonia in rodents [29,30]. Rodents consistently prefer sucrose solutions over water in these tests, and diminished preference for sucrose is widely accepted as a manifestation of anhedonia. HFD consumption has specifically been shown to reduce this preference [14,15,17]. Our study has yielded the same finding (Fig. 1B), however, two-bottle tests do not directly address food preference because they utilize liquids. We designed a food preference assay that would serve to similarly address preference and anhedonia but using solid foods. Animals raised on a HFD exhibited both a reduction in preference for the more calorically dense test chow, as well as decreased consumption of the test chows. These findings echo what has been shown in studies examining the effects of HFD consumption on sucrose preference. Additionally, a recent study demonstrated that prolonged HFD-consumption leads to devaluation of standard chow [18]. Specifically, this study found that when HFD-raised animals were withdrawn from HFD, their intake of the now available SD plummeted compared to SD raised controls. Taken together, these data suggest that prolonged consumption of a HFD leads to anhedonia and subsequent alterations in feeding behavior.
We were surprised to observe that all of our HFD raised cohorts failed to achieve significant weight gain when compared to their SD raised counterparts. This finding appears to contradict what has been demonstrated by other labs using the same HFD [31–34]. However, these labs use SD-fed breeders, and withhold HFD from the offspring until a predetermined time post-weaning. Our mice have access to HFD immediately after birth, and they are born from HFD-fed breeders. We suspect this distinction may be at least partially responsible for the lack of statistically significant weight gain in our animals, and that perhaps our animals are conferred some degree of protection against weight gain during prenatal development. Our lack of significant differences in maintenance diet consumption and fat mass also help explain the lack of significant gain in body weight among our HFD raised cohorts. These findings suggest that the behavioral and neurobiological differences we observe in HFD raised mice can occur independent of an obese phenotype.
Studying mice after 14 weeks of HFD consumption does not address the possibility that shorter periods of exposure to HFD could produce the same behavioral effects. Interestingly, when we restricted the time of access to HFD to just weeks 10–14, the animals exhibited the same behavioral changes during the food preference assay. This suggests that the behavioral effects of HFD can manifest after a shorter period of exposure later in life. Additionally, the effects of HFD on food preference are not permanent and can be reversed via behavioral interventions. We found that switching mice to SD reversed HFD-induced alterations in food preference and test chow intake, while exercise reversed changes in food preference, but not intake. Previous studies have suggested that exercising regularly produces changes in the mesolimbic dopamine signaling pathway [35–37]. One such study showed that 8 weeks of treadmill running in mice was sufficient to restore dampened VTA tyrosine hydroxylase and NAc dopamine levels following prolonged HFD consumption [35]. We suspect that similar neurological alterations are responsible for our observed behavioral findings. The ability of exercise to reverse changes in food preference independent of intake is noteworthy because it suggests that the two behavioral phenotypes, diminished food preference and food intake, are separable. Specifically, HFD-induced dampening of preference for the more calorically dense test chow may not simply be due to reduced intake of the test chows.
In our efforts to elucidate the potential neurobiological mechanism underlying the effects of HFD consumption on food choice, we found that the neural response of the VTA of HFD-raised animals was dampened during consumption of the novel HFD chow compared to the VTA of animals raised on standard lab chow. Previous work has shown that HFD-raised animals exhibit blunted dopamine release into the NAc during exposure to a novel palatable food, which parallels these findings [23]. Interestingly, prolonged HFD consumption did not appear to influence the activity of the VTA dopaminergic neurons specifically. However, the VTA also contains non-dopaminergic glutamatergic and GABAergic neuronal subpopulations [38]. These subpopulations are likely where we are observing the bulk of the changes in VTA activity. Interestingly, we found that dieting resulted in a sensitized VTA response to the novel HFD, and this response occurred specifically in the dopaminergic neurons. Such a finding mirrors sensitization and relapse responses to drugs of abuse in the same neural circuit [25]. In summary, our results suggest that prolonged HFD consumption dampens the activity of the mesolimbic dopamine signaling pathway, but dieting can reverse this through sensitization of VTA dopaminergic neurons.
In conclusion, our research indicates that prolonged consumption of a HFD leads to alterations in food preference that are associated with HFD-induced changes in reward circuitry. These alterations in food preference are reversible via dieting and exercise. Further study is needed to elucidate the neurobiological mechanisms by which dieting, and exercise reverse HFD-induced changes in food preference. Based on the dampened neuronal activity in the VTA of HFD-raised mice, we suspect remodeling of mesolimbic dopamine signaling is the responsible mechanism. Additionally, our data suggest HFD-induced changes in food preference and consumption are separable. Specifically, exercise restored food preference but not the dampened test chow consumption. This disparity suggests that prolonged HFD consumption could be exerting its effects on food preference through one mechanism, while altering intake through another. This distinction demonstrates how multifaceted the influence of HFD over feeding behavior is, but also suggests there may be multiple avenues to target therapeutically.
Supplementary Material
Highlights:
Prolonged high fat diet consumption leads to decreased preference for a more palatable food choice.
Dieting or exercise restores food preference in high fat diet raised mice.
High fat diet consumption leads to an attenuated ventral tegmental area response to palatable food.
Acknowledgements:
We are thankful for technical assistance from Elena Tenore, Laila Al Rawi, Qi Zhang, Sean Chadwick, and Ryan Grippo. We are also very grateful for manuscript feedback from Brandon Podyma, Christopher Deppmann, Ignacio Provencio, Wendy Lynch, Michael Scott, and Savannah Altherr. This work was supported by the NIH National Institute of General Medicinal Sciences [R01GM121937] (A.D.G.).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Declarations of interest: none
References
- [1].Reilly JJ, Methven E, McDowell ZC, Hacking B, Alexander D, Stewart L, Kelnar CJH, Health consequences of obesity, Arch. Dis. Child 88 (2003) 748–752. 10.1136/adc.88.9.748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Bray GA, Wadden TA, Improving long-term weight loss maintenance: Can we do it?, Obesity. 23 (2015) 2–3. 10.1002/oby.20964. [DOI] [PubMed] [Google Scholar]
- [3].Cox CE, Role of physical activity for weight loss and weight maintenance, Diabetes Spectr. 30 (2017) 157–160. 10.2337/ds17-0013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Cox DN, Perry L, Moore PB, Vallis L, Mela DJ, Sensory and hedonic associations with macronutrient and energy intakes of lean and obese consumers, Int. J. Obes 23 (1999) 403–410. 10.1038/sj.ijo.0800836. [DOI] [PubMed] [Google Scholar]
- [5].McCrory MA, Suen VMM, Roberts SB, Biobehavioral influences on energy intake and adult weight gain, in: J. Nutr, American Institute of Nutrition, 2002. 10.1093/jn/132.12.3830s. [DOI] [PubMed] [Google Scholar]
- [6].Crespi EJ, Unkefer MK, Development of food intake controls: Neuroendocrine and environmental regulation of food intake during early life, Horm. Behav 66 (2014) 74–85. 10.1016/j.yhbeh.2014.04.004. [DOI] [PubMed] [Google Scholar]
- [7].Spencer SJ, Perinatal programming of neuroendocrine mechanisms connecting feeding behavior and stress, Front. Neurosci 7 (2013). 10.3389/fnins.2013.00109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Brown HD, Mccutcheon JE, Cone JJ, Ragozzino ME, Roitman MF, Primary food reward and reward-predictive stimuli evoke different patterns of phasic dopamine signaling throughout the striatum, Eur. J. Neurosci 34 (2011) 1997–2006. 10.1111/j.1460-9568.2011.07914.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Hajnal A, Smith GP, Norgren R, Oral sucrose stimulation increases accumbens dopamine in the rat, Am. J. Physiol. - Regul. Integr. Comp. Physiol 286 (2004). 10.1152/ajpregu.00282.2003. [DOI] [PubMed] [Google Scholar]
- [10].Salamone JD, Wisniecki A, Carlson BB, Correa M, Nucleus accumbens dopamine depletions make animals highly sensitive to high fixed ratio requirements but do not impair primary food reinforcement, Neuroscience. 105 (2001) 863–870. 10.1016/S0306-4522(01)00249-4. [DOI] [PubMed] [Google Scholar]
- [11].Yang H, de Jong JW, Tak YE, Peck J, Bateup HS, Lammel S, Nucleus Accumbens Subnuclei Regulate Motivated Behavior via Direct Inhibition and Disinhibition of VTA Dopamine Subpopulations, Neuron. 97 (2018) 434–449.e4. 10.1016/j.neuron.2017.12.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Rossi MA, Stuber GD, Overlapping Brain Circuits for Homeostatic and Hedonic Feeding, Cell Metab. 27 (2018) 42–56. 10.1016/j.cmet.2017.09.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Tulloch AJ, Murray S, Vaicekonyte R, Avena NM, Neural responses to macronutrients: Hedonic and homeostatic mechanisms, Gastroenterology. 148 (2015) 1205–1218. 10.1053/j.gastro.2014.12.058. [DOI] [PubMed] [Google Scholar]
- [14].Íbias J, Miguéns M, del Rio D, Valladolid-Acebes I, Stucchi P, Ambrosio E, Martín M, Morales L, Ruiz-Gayo M, Del Olmo N, Decreased rates of operant food self-administration are associated with reward deficits in high-fat feeding mice, Eur. J. Nutr 55 (2016) 1615–1622. 10.1007/s00394-015-0980-4. [DOI] [PubMed] [Google Scholar]
- [15].Rabasa C, Winsa-Jörnulf J, Vogel H, Babaei CS, Askevik K, Dickson SL, Behavioral consequences of exposure to a high fat diet during the post-weaning period in rats, Horm. Behav 85 (2016) 56–66. 10.1016/j.yhbeh.2016.07.008. [DOI] [PubMed] [Google Scholar]
- [16].Tracy AL, Wee CJM, Hazeltine GE, Carter RA, Characterization of attenuated food motivation in high-fat diet-induced obesity: Critical roles for time on diet and reinforcer familiarity, Physiol. Behav 141 (2015) 69–77. 10.1016/j.physbeh.2015.01.008. [DOI] [PubMed] [Google Scholar]
- [17].Vagena E, Ryu JK, Baeza-Raja B, Walsh NM, Syme C, Day JP, Houslay MD, Baillie GS, A high-fat diet promotes depression-like behavior in mice by suppressing hypothalamic PKA signaling, Transl. Psychiatry 9 (2019). 10.1038/s41398-019-0470-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Mazzone CM, Liang-Guallpa J, Li C, Wolcott NS, Boone MH, Southern M, Kobzar NP, de A. Salgado I , Reddy DM, Sun F, Zhang Y, Li Y, Cui G, Krashes MJ, High-fat food biases hypothalamic and mesolimbic expression of consummatory drives, Nat. Neurosci 23 (2020) 1253–1266. 10.1038/s41593-020-0684-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Arcego DM, Krolow R, Lampert C, Toniazzo AP, Garcia E. dos S. , Lazzaretti C, Costa G, Scorza C, Dalmaz C, Chronic high-fat diet affects food-motivated behavior and hedonic systems in the nucleus accumbens of male rats, Appetite. 153 (2020). 10.1016/j.appet.2020.104739. [DOI] [PubMed] [Google Scholar]
- [20].Davis JF, Tracy AL, Schurdak JD, Tschöp MH, Lipton JW, Clegg DJ, Benoit SC, Exposure to Elevated Levels of Dietary Fat Attenuates Psychostimulant Reward and Mesolimbic Dopamine Turnover in the Rat, Behav. Neurosci 122 (2008) 1257–1263. 10.1037/a0013111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Hryhorczuk C, Florea M, Rodaros D, Poirier I, Daneault C, Des Rosiers C, Arvanitogiannis A, Alquier T, Fulton S, Dampened mesolimbic dopamine function and signaling by saturated but not monounsaturated dietary lipids, Neuropsychopharmacology. 41 (2016) 811–821. 10.1038/npp.2015.207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Alsiö J, Olszewski PK, Norbäck AH, Gunnarsson ZEA, Levine AS, Pickering C, Schiöth HB, Dopamine D1 receptor gene expression decreases in the nucleus accumbens upon long-term exposure to palatable food and differs depending on diet-induced obesity phenotype in rats, Neuroscience. 171 (2010) 779–787. 10.1016/j.neuroscience.2010.09.046. [DOI] [PubMed] [Google Scholar]
- [23].Romaní-Pérez M, Lépinay AL, Alonso L, Rincel M, Xia L, Fanet H, Caillé S, Cador M, Layé S, Vancassel S, Darnaudéry M, Impact of perinatal exposure to high-fat diet and stress on responses to nutritional challenges, food-motivated behaviour and mesolimbic dopamine function, Int. J. Obes 41 (2017) 502–509. 10.1038/ijo.2016.236. [DOI] [PubMed] [Google Scholar]
- [24].Naef L, Moquin L, Dal Bo G, Giros B, Gratton A, Walker CD, Maternal high-fat intake alters presynaptic regulation of dopamine in the nucleus accumbens and increases motivation for fat rewards in the offspring, Neuroscience. 176 (2011) 225–236. 10.1016/j.neuroscience.2010.12.037. [DOI] [PubMed] [Google Scholar]
- [25].Steketee JD, Kalivas PW, Drug wanting: Behavioral sensitization and relapse to drug-seeking behavior, Pharmacol. Rev 63 (2011) 348–365. 10.1124/pr.109.001933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Small L, Gong H, Yassmin C, Cooney GJ, Brandon AE, Thermoneutral housing does not influence fat mass or glucose homeostasis in C57BL/6 mice, J. Endocrinol 239 (2018) 313–324. 10.1530/JOE-18-0279. [DOI] [PubMed] [Google Scholar]
- [27].Herrera DG, Robertson HA, Activation of c-fos in the brain, Prog. Neurobiol 50 (1996) 83–107. 10.1016/S0301-0082(96)00021-4. [DOI] [PubMed] [Google Scholar]
- [28].Sun H-X, Wang D-R, Ye C-B, Hu Z-Z, Wang C-Y, Huang Z-L, Yang S-R, Activation of the ventral tegmental area increased wakefulness in mice, Sleep Biol. Rhythms 15 (2017) 107–115. 10.1007/s41105-017-0094-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Frie JA, Khokhar JY, An open source automated two-bottle choice test apparatus for rats, HardwareX. 5 (2019). 10.1016/j.ohx.2019.e00061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Liu MY, Yin CY, Zhu LJ, Zhu XH, Xu C, Luo CX, Chen H, Zhu DY, Zhou QG, Sucrose preference test for measurement of stress-induced anhedonia in mice, Nat. Protoc 13 (2018) 1686–1698. 10.1038/s41596-018-0011-z. [DOI] [PubMed] [Google Scholar]
- [31].Boitard C, Etchamendy N, Sauvant J, Aubert A, Tronel S, Marighetto A, Layé S, Ferreira G, Juvenile, but not adult exposure to high-fat diet impairs relational memory and hippocampal neurogenesis in mice, Hippocampus. 22 (2012) 2095–2100. 10.1002/hipo.22032. [DOI] [PubMed] [Google Scholar]
- [32].Cui L, Liu M, Chang X, Sun K, The inhibiting effect of the Coptis chinensis polysaccharide on the type II diabetic mice, Biomed. Pharmacother 81 (2016) 111–119. 10.1016/j.biopha.2016.03.038. [DOI] [PubMed] [Google Scholar]
- [33].Lang P, Hasselwander S, Li H, Xia N, Effects of different diets used in diet-induced obesity models on insulin resistance and vascular dysfunction in C57BL/6 mice, Sci. Rep 9 (2019). 10.1038/s41598-019-55987-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Hyun Jeong J, Ran Lee Y, Geun Park H, Lok Lee W, The effects of either resveratrol or exercise on macrophage infiltration and switching from M1 to M2 in high fat diet mice, J. Exerc. Nutr. Biochem 19 (2015) 65–72. 10.5717/jenb.2015.15060203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Chen W, Wang HJ, Shang NN, Liu J, Li J, Tang DH, Li Q, Moderate intensity treadmill exercise alters food preference via dopaminergic plasticity of ventral tegmental area-nucleus accumbens in obese mice, Neurosci. Lett 641 (2017) 56–61. 10.1016/j.neulet.2017.01.055. [DOI] [PubMed] [Google Scholar]
- [36].Greenwood BN, Foley TE, Le TV, Strong PV, Loughridge AB, Day HEW, Fleshner M, Long-term voluntary wheel running is rewarding and produces plasticity in the mesolimbic reward pathway, Behav. Brain Res 217 (2011) 354–362. 10.1016/j.bbr.2010.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Tümer N, Demirel HA, Serova L, Sabban EL, Broxson CS, Powers SK, Gene expression of catecholamine biosynthetic enzymes following exercise: Modulation by age, Neuroscience. 103 (2001) 703–711. 10.1016/S0306-4522(01)00020-3. [DOI] [PubMed] [Google Scholar]
- [38].Morales M, Margolis EB, Ventral tegmental area: Cellular heterogeneity, connectivity and behaviour, Nat. Rev. Neurosci 18 (2017) 73–85. 10.1038/nrn.2016.165. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


