Abstract
The increase of resistant bacteria puts a huge pressure on the antimicrobials in current use. Antimicrobial resistance (AMR) results from antibiotic misuse and abuse over many years and is a global financial burden. New polices must be developed for the use of antimicrobials and to continue research efforts to mitigate AMR. It is essential to target the most harmful bacteria and concentrate on their mechanisms of resistance to develop successful antimicrobials. Essential oils (EOs) are occur naturally in plants and have long been used as antimicrobials, but most have not been researched. This review explores EOs as alternative antimicrobials, investigating their ability to decrease or inhibit biofilm formation, and assess their ability to contribute to AMR control. Low concentrations of EOs can inhibit Gram-positive and Gram-negative pathogenic bacteria. Some EOs have demonstrated strong anti-biofilm activities. If EOs are successful against biofilm formation, particularly in bacteria developing AMR, they could be incorporated into new antimicrobials. Therefore, there is a need to investigate these EOs’ potential, particularly for surface disinfection, and against bacteria from food, clinical and non-clinical environments.
Keywords: Antimicrobial resistance, Biofilm tolerance, Essential oils, Health
1. Introduction
Antimicrobials have been in use for centuries, and in the late 1920s, Alexander Fleming discovered and presented penicillin. In the 1940s, penicillin was prepared for use in treatments (Ventola, 2015), and a variety of commercially available antibiotics were rapidly used to treat infections and diseases (Debabov, 2013, Kimera et al., 2020). Fleming predicted antimicrobial resistance (AMR) by proposing that “the inappropriate use of penicillin might cause Staphylococcus aureus to mutate, resulting in more severe infections and transmission of resistant strains from host to host” (Rosenblatt-Farrell, 2009, Birgand et al., 2020). In the 1940s, resistance to penicillin was demonstrated (Ventola, 2015). Many staphylococcal strains became resistant to penicillin (Lobanovska and Pilla, 2017), and rapidly > 50% of strains were resistant (Alanis, 2005). The annual worldwide production of antibiotics is close to 100,000 tonnes (Martens and Demain, 2017), with two tonnes used every 10 min (Harbarth et al., 2015). Not only did S. aureus acquire resistance, but numerous strains became multi-drug (antibiotics and chemotherapeutic agents) resistant (MDR) (Nikaido, 2009)
MDR is a tolerance to ≥1 agent of ≥3 antimicrobial categories (Magiorakos et al., 2012). Worldwide, the leading cause of nosocomial infections are Acinetobacter baumannii, Enterococcus faecium, Enterobacter spp., Klebsiella pneumoniae, Pseudomonas aeruginosa, and S. aureus (Santajit and Indrawattana, 2016). Control of MDR bacteria requires crucial therapeutic discoveries, improved infection control, and advanced antimicrobial practices (Santajit and Indrawattana, 2016).
Increasingly, microorganisms are evading control by antimicrobials, resulting in poor ineffective management, persistence, and a spread of infection (Tanwar et al., 2014). Annually in USA, there are >63,000 hospital-acquired bacterial infections resulting in deaths (Aminov, 2010, Lakoh et al., 2020). Within the European Union, MDR infections cause ~ 25,000 patient deaths annually (DOH, 2016). MDR infections increase health care costs and decrease productivity, with a ~€ 1.5 billion annual cost each year (DOH, 2016). Worldwide, AMR may result in 10 million deaths annually by 2050, surpassing cancer as the leading cause of death (O’Neill, 2014). Accordingly, action must be taken immediately against the threat of MDR (Nikaido, 2009).
Many essential oils (EOs) possess antimicrobial activity (Millezi et al., 2016, Reda et al., 2020a, Reda et al., 2020b), but despite their widespread use for multiple purposes, only a small proportion are commercially used (Ghabraie et al., 2016, Ragno et al., 2020). It is believed that most EOs acts on the cell membrane and cell wall of bacteria (Faleiro, 2011), and investigations on evaluating a variety of EOs’ mode of action is still required. There have been very few studies of EOs to identify those with a rapid kill ability, i.e., a contact time of less than 10 min. EOs have the potential to tackle the AMR and MDR threat (Faleiro, 2011).
2. Common causes of antimicrobial resistance
The most significant cause of AMR is antibiotic misuse and overuse (Nikaido, 2009). Since the introduction and inappropriate distribution of antibiotics, their abundant consumption has played an enormous role in AMR development (Ventola, 2015). In agriculture and aquaculture, there is substantial use of antibiotics to promote growth and reduce diseases (Prestinaci et al., 2015), as there is a lack of funds to introduce new effective treatments (Levy and Bonnie, 2004). Therefore, the use of natural compounds such as probiotics (Abd El-Hack et al., 2020a, Abd El-Hack et al., 2020b, Alagawany et al., 2021a, Alagawany et al., 2021b), prebiotics (Yaqoob et al., 2021, Abd El-Hack et al., 2021), essential oils, organic acids and medicinal plants (Abou-Kassem et al., 2021, Abdelnour et al., 2020, Alagawany et al., 2021a, Alagawany et al., 2021b, Ashour et al., 2020, Reda et al., 2020a, Reda et al., 2020b, Sheiha et al., 2020, Reda et al., 2021) as antibiotic alternatives became a trend. Natural genetic resistance, biocides, and metals, contribute to increase AMR (Singer et al., 2016). Biocides from household waste, drainage water, and road/traffic emissions such as chlorhexidine, ethanol, formaldehyde, metals, quaternary ammonium compounds, and triclosan aid the co-selection of genes that promote resistance (Singer et al., 2016).
3. Over prescribing and misuse
In Europe, the UK has a low outpatient antibiotic use (Smieszek et al., 2018), however, ~20% of antibiotic prescriptions are unnecessary (Courtenay et al., 2019). Issues are caused by physicians providing an inaccurate diagnosis, or prescribe antibiotics as a precaution, or use broad-spectrum antibiotics.
Public Health England's recent report (PHE, 2018) revealed that most antibiotic prescriptions were for urinary tract or respiratory infections. However, almost 30% had no clinical reasoning. Patient non-compliance contributes to the misuse of antibiotics (Tong et al., 2018), this includes discontinuing treatment, and however, one of the main reasons for misuse is the fear of extended drugs use causing side-effects (Tong et al., 2018). Incomplete treatment primes bacteria with sub-lethal concentrations leading to acquired resistance (Niederman, 2005).
Many countries lack regulatory and legislative control, which would typically govern antimicrobial distribution (Michael et al., 2014). In developing regions, where healthcare is not provided consistently, there is less control of antibiotic use, with varying regulatory guidelines between countries (Zaman et al., 2017). Where prescriptions are not used and supply of antibiotics is not controlled, self-medication is common (Ayukekbong et al., 2017). Antiobiotic misuse creates a serious worldwide problem to public health and is considered as one of the biggest challenges to many health care systems.
4. Extensive agricultural use
Selection for antimicrobial resistance has also been exacerbated by excessive agricultural antimicrobial exposure. Most of these antimicrobials are similar, or identical, to those in clinical use. The primary transmission route for AMR organisms is the food chain (Zaman et al., 2017), as sub-lethal antibiotic doses are in constant use in agriculture, farming, and fisheries, for treating infections, preventing diseases, and growth promotion (Zaman et al., 2017). This results in gut microflora with high resistance and a cache of AMR bacteria develops (Gupta and Deka, 2018).
Although Europe banned antibiotics for growth promotion in 2006 (Prestinaci et al., 2015), the USA has only recently introduced a ban. In contrast, there is an increase in antibiotic use in animals in countries such as China, India, Pakistan, and Egypt (Anomaly, 2020).
The use of, or exposure to, antimicrobials is undeniably the most critical driver for AMR development; thus, leading to spread of resistant bacterial infections.
5. Research Issues
Bacteria generally develop resistance within ~5 years of the introduction of a new antibiotic. This rapid development of resistance, combined with few new antimicrobials being developed, often increases the threat due to a lack of research funding and incentive (Gould and Bal, 2013).
Due to antibiotic consumption being a short-course treatment, there is a lack of motivation for the drug companies due to potentially low revenue streams (Gould and Bal, 2013) and the chance of multimillion-dollar losses (Ventola, 2015).
The United States Food and Drug Administration (FDA) has regulatory strategies to fast-track novel antimicrobials to the final stages of research; however, this overlooks small companies without the funds to develop the drugs (Simpkin et al., 2017). Multidisciplinary research is needed in the agricultural, environmental and health-care sectors to develop effective antimicrobials.
6. Mechanisms of bacterial resistance
Bacterial resistance relies on the efficacy of antimicrobial products and microorganisms resistant mechanisms (Levy and Bonnie, 2004). Many resistance mechanisms have been described, but unfortunately, no antibiotic has overcome any of these mechanisms (Bonomo and Rossolini, 2008).
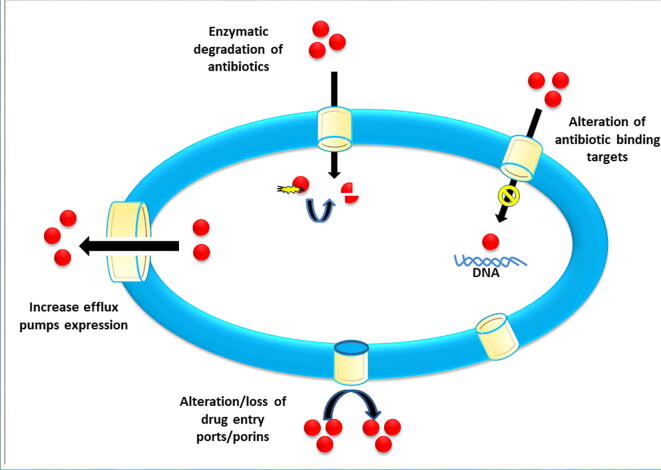
AMR is genetic or mechanistic based (Munita and Arias, 2016) and there is usually some overlap. Genetic resistance can be acquired or intrinsic (Peterson and Kaur, 2018). The genetic composition of bacteria contain intrinsic mechanisms, whereas horizontal gene transfer usually confers acquired resistance through bacteriophage, naked DNA, plasmids, integrons or transposons (Levy and Bonnie, 2004, Peterson and Kaur, 2018). Intrinsic tactics include the use of generic efflux pumps to move antimicrobials out of the cell, enzymatic inactivation the drug, and decreasing the permeability which reduces penetration (Blair et al., 2015, Birgand et al., 2020). Acquired mechanisms include enzymes to modify drugs and plasmid-encoded efflux (Peterson and Kaur, 2018). Alternative metabolic pathways can also be used by bacteria (Tenover, 2006, Lakoh et al., 2020) to modify the drugs target and prevent binding (Tenover, 2006), or the target enzyme is over-expressed to reduce the impact of the antibiotic’s inhibtion (Palmer and Kishony, 2014). The various mechanisms of bacterial antibiotic resistance are illustrated in Fig. 1.
Fig. 1.
The various mechanisms of bacterial antibiotic resistance.
7. Biofilms
The ability to form a biofilm is a significant concern, and a biofilm’s resistance is supported by genetic, physical, and physiological mechanisms (Ciofu and Tolker-Nielsen, 2019). Biofilms consist of bacteria in dense populations and are protected by a robust exopolymer matrix irreversibly attached to a surface. The formation of biofilms cause failure of an antimicrobial agents, and 65–80% of infections may be due to the formation of biofilms (Coenye and Nelis, 2010).
In biofilms the cells have up to 1000 times greater resistance to the antimicrobial agents (Mah and O’Toole, 2001). The formation of bacterial biofilm is induced by communication via quorum sensing (QS), the intercellular chemical signaling mechanism used for monitoring cell density (Gerdt and Blackwell, 2014). Biofilms requires a sufficient density to induce QS signal accumulation that will then result in gene expression. Many QS activated genes are beneficial, e.g., for secretion of proteases, siderophores, and toxins (Gerdt and Blackwell, 2014).
8. Formation of biofilms
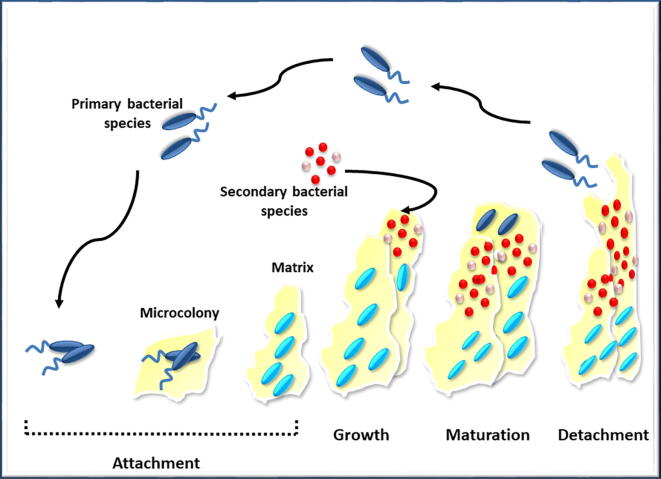
Biofilm formation and survival occurs by: attachment/detachment, growth and maturation, (O’Toole, 2003, Birgand et al., 2020), with the attachment relying on many factors for successful development (O’Toole, 2003). The requirements for biofilms include a constant flow of nutrients inside the biofilm and waste products outside the biofilm. In addition, efficient communication within the biofilm is required.
To complete the cycle, cell detachment is required to start a new cycle. Biofilms often contain a mix of species (Kommerein et al., 2018) which requires QS, metabolic cooperation, and competitive or synergistic interactions (Elias and Banin, 2012). The steps of biofilm formation are outlined in Fig. 2.
Fig. 2.
Steps of biofilm formation.
9. Attachment
Bacterial attachment to a surface relies on the ideal, hydrophobic surface with nano or micro-scale roughness to trigger the surface detection factors that control adherence (Cortes et al., 2011). With an overall negative charge, natural surfaces have a repulsive force towards the electrostatic charges used for bacterial adhesion. To adhere, the attractive forces such as Lewis acid-base, Lifshitz-van der Waals, and hydrophobic forces are used (Van-Merode et al., 2006).
Convective transport in a bulk fluid, Brownian motion, or specific gravity sedimentation transport bacteria to surfaces (Palmer et al., 2007). Biofilm formation starts with planktonic bacterial cell forming a reversible polar attachment and aligning flat to the surface to resist removal (Armbruster and Parsek, 2018). In flagellated bacteria, flagellum detachment mediated by cyclic diguanylate start after attachment as the aggregation of cells begin (Guttenplan and Kearns, 2013, Lakoh et al., 2020). Robust cell-to-cell organization is required for irreversible cell attachment, involving binding proteins, enzymic hydrolyzation of adhesion molecules, and the adsorption of protein (Pavithra and Doble, 2008). Attachment processes are regulated by carbon and oxygen levels, pH, flow, nutrient availability, and temperature (O’Toole et al., 2000, Toyofuku et al., 2016).
10. Growth and maturation
The commencement of biofilm growth requires increased QS, micro-colony development, and the formation of an extracellular polymeric covering, leading to a 3D structure of cell clusters (Toyofuku et al., 2016, Arunasri and Mohan, 2019). Micro-colonies expand as cells replicate by cell division (Toyofuku et al., 2016) and generate extracellular components, forming a glycoprotein/glycolipid coating, following the interaction with surrounding organic and inorganic materials (Dunne, 2002, Kimera et al., 2020).
The gel-like exopolysaccharide matrix (EPS) has a high water content. One of the EPS functions is to protect the microbial cells from desiccation (Carpentier and Cerf, 1993). The EPS biopolymers consists of glycoproteins, proteins, polysaccharides, extracellular DNA, and glycolipids (Flemming et al., 2007), with channels for transportation of nutrients and water, and the removal of waste (Arunasri and Mohan, 2019). A doubling of EPS mass often occurs near maturation (Jiao et al., 2010) but is dependent on bacterial strain and environmental factors (Harmsen et al., 2010, Kimera et al., 2020).
For a successful biofilm formation a suitable location, essential organisms, and ample nutrient concentration is required. A mature biofilm’s structure can constitute a homogeneous layer, dispersed micro-colonies, or protruding cell clusters (Reisner et al., 2003). At critical density, the release of chemical signals (autoinducers) recognized by the cell’s receptors occurs. These auto-inducers are considered to be released as antibiotics, siderophores, or waste products (Hense and Schuster, 2015). Autoinducing molecules found in bacteria include oligopeptide autoinducers, acyl-homoserine lactones, and autoinducer-2 (AI-2; furanosyl borate diester) (Hense and Schuster, 2015). Once autoinducers reach a critical level, the bacterial cells respond with expression or repression of the target genes (Butt and Khan, 2015).
The biofilm may benefit from gene expression by increasing virulence, promotion of genetic transfer, the upregulation of EPS production and efflux pumps, and the contribution to resistance to stressors (Cortés et al., 2011, Butt and Khan, 2015). At biofilm maturation, cell death occurs due to lack of nutrients, pH fluctuations, oxygen deprivation, or by poisoning from accumulation of waste (Dunne, 2002).
11. Detachment and dispersal
The detachment and dispersal of biofilms occurs if the biofilm matrix synthesis compounds cease, activating the biofilm matrix's degradation, and disrupting the covalent bonds between the matrix components (Solano et al., 2014). Dispersion, detachment or desorption are ways of bacteria can actively or passively leave a biofilm (Davies, 2011). Bacterial cells initiate active escape and is influenced by external forces, including predation, fluid shear, and human intervention (Kaplan, 2010), including abrasion and erosion (Petrova et al., 2016).
These types of escape mechanisms are usually induced by bacterial selection pressures and an established survival strategy (Davies, 2011, Kimera et al., 2020). When critical mass has been reached, the outermost layer of the biofilm experiences dynamic equilibrium, and planktonic cells are generated (Dunne, 2002). Cell release from outer layers of a biofilm is a standard detachment method, however, escape also occurs from the biofilm's interior by dispersion (Davies, 2011). The cells moves freely to the colony's surface, and the cycle repeats itself (Dunne, 2002).
12. Biofilm tolerance
The EPS layer secreted during maturation is the primary cause of biofilm tolerance to antimicrobials (Butt and Khan, 2015). The EPS shields the biofilm and prevents or delays the infiltration of the antimicrobials (Butt and Khan 2015). Slow growth of the biofilm can also reduce penetration of the antimicrobial agents (Mah and O’Toole, 2001). Limited nutrients results in a reduced growth rate, and this increases resistance on the approach to the stationary phase (Evans et al., 1991). Slow growth occurs in denser biofilm sections, while a faster growth rate occurred when bacteria were exposed to bulk medium (Wentland et al., 1996).
The deeper slow growing cells exist in a viable state where they become tolerant to antibiotics (Williamson et al., 2012). The biofilm’s general stress response can also increase resistance (Williamson et al., 2012). Stressors induce many physiological alterations to protect the cells from starvation, heat shock, pH and osmolarity alterations, or DNA damage (Hengge, 2014).
13. Combatting antimicrobial resistance
Researchers can avert the AMR crisis by improving education and knowledge of AMR, boosting investments, while providing support for research on novel antimicrobials while implementing strategies to combat misuse and reduce use of antibiotics worldwide (Harbarth et al., 2015, Abd El-Hack et al., 2020b). In the 1990s, some countries sought to challenge AMR's threat (Harbarth et al., 2015), recently other countries have taken up the challenge. The USA pledged to invest 1.2 billion dollars to prevent AMR, almost doubling their funding in 2015 (Obama White House, 2015). The UK’s anti-AMR strategies began in 2000 (Mayor, 2019) and employ economics expert O’Neill (2014). O’Neill (2014) suggested that ten interventions were required immediately to mitigate AMR. The suggestions focus on improving awareness of AMR worldwide by using programs and campaigns, focusing on the improvement of sanitation and hygiene, increasing surveillance of antimicrobial consumption and resistance, encouraging and supporting infectious disease researchers, investments for new drugs, improving existing drugs, and increasing non-commercial research funding (O’Neill, 2014). These efforts needs to be undertaken concurrently with a reduction of antimicrobial use in agriculture, a renewed research effort for rapid diagnostic technology, vaccines and other alternatives to antibiotics, and the generation of a global alliance (O’Neill, 2014). The UK’s latest strategy to combat AMR reported only a 7% reduction in human consumption of antibiotics but a 40% reduction of agricultural antibiotics use during 2013–2018 (HM Government, 2019). During this period, there was a 35% increase in bloodstream infections due to resistant bacteria (Courtenay et al., 2019).
Our current review focuses on research of novel antimicrobial agents, particularly those effective against biofilms. Alternative antimicrobials include the use of natural components derived from animals (lysozymes, chitosan, and lactoferrin), plants (lectins, phenolics, EOs, and polyacetylenes), fungi, algae, and bacteria (reuterin and bacteriocin) (Cowan, 1999, Gyawali and Ibrahim, 2014).
Antibody-based drugs, or prebiotics and probiotics which target bacterial community communication are potential alternatives (Harbarth et al., 2015). Recent novel approaches have included sequencing prokaryote genomes (Tracanna et al., 2017), the use of metal, polymeric, and lipid-based nanoparticles (Lakshminarayanan et al., 2018) and peptide-based antibiotics (Roshan et al., 2018), nanohybrids of silica and antibiotic combination (Mosselhy et al., 2018), and revitalized phage-therapy based techniques (Kortright et al., 2019).
Other tactics to combat bacterial biofilms include the prevention of contamination, minimization of attachment, and chemical or mechanical penetration of biofilms, and eradication of cells (Donlan, 2002).
14. Essential oils
EOs are naturally occurring plant extracts of petals, seeds, leaves, stems, or roots (Butnariu and Sarac, 2018, Ragno et al., 2020, Abd El-Hack et al., 2016). The use of plant oil extracts has been documented from thousands of years ago (Baser and Buchbauer, 2015, Al-Shuneigat et al., 2020). Ancient Egyptians (~4500 BCE) used plant oil extracts for therapeutic purposes and there are records of Chinese use of herbal medicine (~3000 BCE) (Boire et al., 2013). The process of distillation of plant products (qunita essentia) was named and described by von Hohenheim, a 16th century Swiss alchemist (Guenther, 2013, Nazzaro et al., 2017). This lead the way for widespread use and the commercial production of EOs. Since the 20th century, there have been >100 countries producing EOs (Govindasamy et al., 2013, Chraibi et al., 2020).
Currently, about 300 of the 3000 known EOs are used commercially (Ghabraie et al., 2016). For the industrial sector, the most commonly used oils include lemon, peppermint, citronella, eucalyptus, mint, and orange. While for domestic use tea tree, peppermint, chamomile, lavender, rosemary, orange, lemon, rose, eucalyptus, jasmine, geranium, sandalwood, and frankincense oils are most popular (Barbieri and Borsotto, 2018).
15. Extraction and composition of EOs
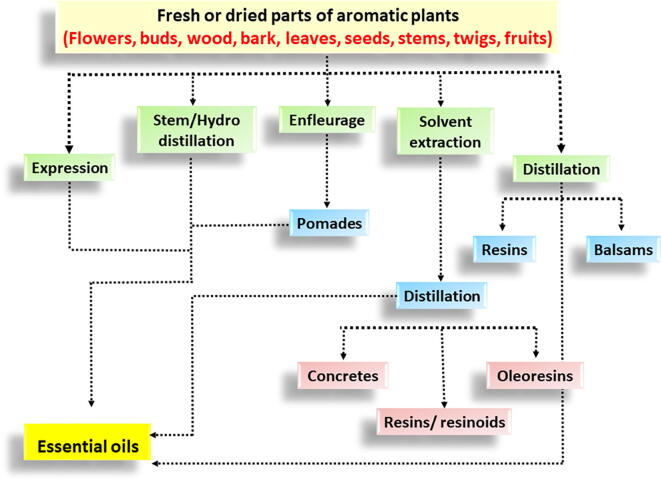
Expression, hydro-distillation (steam), and dry distillation are used to extract EOs (Baser and Buchbauer, 2015, Chraibi et al., 2020). Hydro-distillation is the most common method used in commercial EO production (Barreto and Coelho, 2015, Al-Shuneigat et al., 2020). Oil extraction has a low product return from the raw material and so is generally expensive (Butnariu and Sarac, 2018), e.g., the yield from the herbs basil, parsley, and thyme are all <0.4% oil/gram raw material (Semeniuc et al., 2017).
Nutmeg yields around 6 mL/100 g dry weight (Soni et al., 2016), lemongrass 1.8% (López et al., 2007), and lavender 6.8% oil (Zheljazkov et al., 2013). Several factors influences the extractable oil yield, including the plant species and its location, the plant tissue type being processed, drying conditions, the degree of milling of the dry matter, and distillation time (Wang et al., 2009, Zheljazkov et al., 2013, Baser and Buchbauer, 2015, Bowes and Zheljazkov, 2019). These factors also impact the oil’s chemical composition (Eslahi et al., 2017), along with seasonal variations (Zouari-Bouassida et al., 2018), the plant's maturity, and genetic factors. The factors affecting oil yield and composition are often interdependent and impact each other (Dhifi et al., 2016, Barbieri and Borsotto, 2018).
EOs are “a complex mixture of highly volatile substances” (Butnariu and Sarac, 2018), with some containing >300 different compounds. These compounds belong to numerous chemical classes, including alcohols, aldehydes, ethers, amides, amines, esters, heterocycles, ketones phenols and terpenes (Dhifi et al., 2016). In EOs, cyclic or acyclic terpenes are the most common classes of compounds, including monoterpenes, sesquiterpenes, and diterpenes (Buckle, 2015). Due to a highly complex composition, EOs are challenging to replicate synthetically (Butnariu and Sarac, 2018). The primary extraction process for Eos from aromatic plant parts is illustrated in Fig. 3.
Fig. 3.
The main extraction process for essential oils from aromatic plant parts.
16. The known uses of EOs
There are multiple roles of EOs in plants; from pest, predator and disease protection, to the attraction of pollinators (Nazzaro et al., 2017, Ragno et al., 2020). Aromatherapy is a common complementary treatment using EOs (Lee et al., 2012), and has been used for >6000 years by Chinese, Egyptians and Indians (Ali et al., 2015). More recently EOs have been used for anxiety reduction (Muzzarelli et al., 2006), cancer care (Reis and Jones, 2017), and for the improvement of sleep (Lin et al., 2019).
They have been utilized widely to treat inflammation, rheumatic joints, skin sores, bleeding, leprosy, cystitis, burns, wounds, syphilis, fungal infections, and pharyngitis (Narayanasamy et al., 2019). Commercial uses of EOs includes pharmaceuticals, perfume, fragrance, and cosmetics, products for personal care, and drinks and foods (Govindasamy et al., 2013). Recently, there is strong interest in EOs for their antimicrobial properties.
17. EOs as antimicrobials
EOs possess anti-plasmodial, antifungal, and antibacterial properties (Utchariyakiat et al., 2016). They are among the most profitable natural products known to combat fungal infections (Nazzaro et al., 2017). Some EOs antifungal activity is similar to synthetic fungicides (Zabka et al., 2014).
Whiley et al. (2018) review of EOs’ antifungal properties reported clove, thyme, tea tree, oregano, and citrus oils as the most researched agents. They have high efficacy against some viruses, and EO blends are effective against the influenza and herpes simplex 1 viruses (Brochot et al., 2017), while star anise EOs are highly effective against herpes simplex virus 1 (Astani et al., 2011). Additionally, EOs have potent action against Gram-positive and -negative bacteria in sessile and motile conditions (Millezi et al., 2016, Ragno et al., 2020). Of 53 EOs screened, all exhibited activity against pathogenic bacteria and yeasts such as Bacillus subtilis, Escherichia coli, P. aeruginosa, S. aureus and Candida albicans (Janssen et al., 1986, Swelum et al., 2021). Effective antimicrobial properties, as low as 0.02% EO, were noted against E. coli by thyme, clove, lemon myrtle, bay laurel, lemongrass, cinnamon, tea tree, oregano, and rosewood (Nazzaro et al., 2019).
In vivo evaluation after 14 days of using a mouth rinse containing EO provided significant reduction of oral bacterial pathogens including Fusobacterium nucleatum, Porphyromonas gingivalis, and Veillonella spp. (Fine et al., 2007).
Synergism of EOs combined with antibiotics can prevent AMR transmission (Mulani et al., 2019), with antibiofilm activities (Artini et al., 2018, Kuhn et al., 2019). The in vitro use of marigold EOs for cancer treatment showed no cytotoxicity in tumor cell lines (Oliveira et al., 2017).
Due to EO’s volatility, the vapor phase has potential antimicrobial properties. Early studies of 133 EOs found that the vapor of cassia, cinnamon, cherry laurel, origanum, and thyme inhibited a wide range of bacteria (Maruzzella and Sicurella, 1960). More recently, EOs vapors have been used to eradicate bacteria that cause pneumonia (Houdkova et al., 2018), inhibit molds in food products (Ji et al., 2019), and combat biofilm-forming bacteria (Benzaid et al., 2019).
EOs and their constituents are efficient antimicrobial agents against E. coli O157:H7 with contact ≥ 5 mins (Friedman et al., 2004), and EOs from Zanthoxylum limonella had high efficacy against E. coli with ≥ 3 mins contact time (Tangjitjaroenkun et al., 2012). Mayaud et al. (2008) demonstrated a contact time of ≥ 5 mins of EOs providing inhibition against various bacteria.
18. Mode of action of EOs
Antimicrobial activity of EOs can be attributed to their configuration, composition, volume, and interactions with pathogens. They affect single or multiple targets within the pathogens (Dhifi et al., 2016), with whole oil’s mode of action due to their composition. The 2–3 primary constituents of EOs making up ≤85% of the oil drive biological activity (Chouhan et al., 2017, Chraibi et al., 2020).
Often the minor components also play a role (Feyaerts et al., 2018), as the antimicrobial chemical class control the EO’s mechanism against pathogens (Swamy et al., 2016). The greatest activity occurs when there is a high proportion of phenols and aldehydes (Bassolé and Juliani, 2012) contrasting with weak or no activity from esters, ketones, or terpene hydrocarbons (Bassolé and Juliani, 2012). Hydrophobicity impacts EO’s activity by increasing cell permeability resulting in cell leakage (Dhifi et al., 2016).
Most EOs modify the bacterial cell wall or cell membrane, causing release of lipopolysaccharides (Faleiro, 2011). This changes the balance of ATP internally and externally and impacts pH fluctuation, protein synthesis, and internal cytoplasmic changes such as coagulation of cytoplasmic material, DNA disruption, and inhibition of quorum sensing (Faleiro, 2011, Lopez-Romero et al., 2015). EOs also affect growth regulation, nutritional balances and energy conversion in bacteria (Swamy et al., 2016). For example, phenolic components, such as carvacrol, eugenol, and thymol impacts the cytoplasmic membrane, electron flow, proton forces, active transportation, and cell content coagulation (Dhifi et al., 2016). EO’s components exert an additive, antagonistic, or synergistic effect on each other (Pei et al., 2009); therefore the identification of the mode of action depends on the composition of the EOs.
19. Safety
Some EOs, considered by the FDA as safe, have approval for food applications and consumption (Ali et al., 2015). Generally, hypersensitivity is the most common adverse reaction encountered, with risks typically controllable (Rather et al., 2016). Eye, mucous membrane, and skin irritation are the common complaints (Ali et al., 2015, Chraibi et al., 2020). More severe toxicity includes convulsions, diarrhea, epigastric pain, renal failure, vomiting, and central nervous system depression (Eisenhut, 2007). The improper storage of EOs increases the chance of toxicity which may cause, peroxidation, photoisomerization, photocyclization, “oxidation and decomposition of alcohols, ketone hydrolysis, and overall degradation” (Sarkic and Stappen, 2018). There has been limited toxicity testing, however mammalian models have been investigated. For example, lavender EOs were non-toxic orally and dermally in mice and rabbits (Mekonnen et al., 2019).
Also, there were no genotoxic effects caused by several EOs induced in human embryo lung cells (Puškárová et al., 2017). There are reports of human ingestion of EOs; citronella EO has ingestion do not cause toxicity (Vigan, 2010), and near-fatal incidents are attributed to extremely high doses (Nath et al., 2012).
To remain safe, most EO’s toxicity can be prevented by avoiding ingestion, using dilutions of EOs for topical applications, and safeguarding proper storage (Hammer et al., 2006). The antimicrobial activity of different EOs is summarized in Table 1.
Table 1.
Antimicrobial activities of essential oils.
| Essential Oils (EO) Levels/ Sources |
Pathogenic microorganisms | Most important Results | References |
|---|---|---|---|
| Arborvitae, clary sage, clove, lavender, oregano, and thyme. (10 mg each) | Alternaria alternata, Arthrobacter protophormiae, Aspergillus fumigatus, Bacillus cereus, Chaetomium globosum, Cladosporium cladosporoides, Enterococcus faecalis, Escherichia coli, Listeria monocytogenes, Penicillium chrysogenum, Pseudomonas fragi, Salmonella typhimurium, Staphylococcus aureus, and Yersinia enterocolitica. | Arborvitae, clove, oregano, and thyme: strong antibacterial activity against all strains. All essential oils: direct application and vapor resulted in different fungistatic and fungicidal activity. | Puškárová et al., 2017 |
| Two essential oil blends containing: 1: 3.52% cineol from leaf (Eucalyptus globulus), 3.52% cinnamaldehyde from bark (Cinnamomum zeylanicum), 3% cineol from leaf (Rosmarinus officinalis), 1.04% seed (Daucus carota), and 88.90% seed oil (Camelina sativa). 2: 3.53% CT cinnamaldehyde from bark (Cinnamomum zeylanicum), 3.53% CT eugenol (Syzygium aromaticum). (Synonymous: Eugenia caryophyllus Sprengel, cloves), and Origanum vulgare CT carvacol (aerial parts), 1.04% CT carotol from seed (Daucus carota), and 88.35% seed oil (Camelina sativa). |
Bacteriodes fragilis, Branhamella catarrhalis, Candida albicans, C. glabatra, C. tropicalis, Escherichia coli, Haemophilus influenza H1N1 and HSV1, Klebsiella pneumoniae, Listeria monocytogenes, Proteus mirabilis, Pseudomonas aeruginosa, Salmonella typhimurium, Staphylococcus aureus, Streptococcus pneumoniae, S. pyogenes, and Yersinia enterocolitica. | Minimal inhibitory concentrations (0.01% to 3% v/v) with minimal bactericidal concentrations from < 0.01%. EO blend of Cinnamomum zeylanicum, Daucus carota, Origanum vulgare, and Syzygium aromaticum was antifungal to Candida strains. Cinnamomum zeylanicum was effective against H1N1 and HSV1 viruses, with dual activity, against H1N1 and S. aureus and S. pneumoniae. | Brochot et al., 2017 |
| Alpinia oxymitra (64.00 μg/mL), Boesenbergia rotunda (1024.00 μg/mL), Cinnamomum cambodianum (1024.00 μg/mL), Citrus lucida (512.00 μg/mL), Limnophila aromatica (1024.00 μg/mL), Rhodamnia dumetorum (512.00 μg/mL), and Sindora siamensis (256.00 μg/mL) | Growth inhibition (in vitro): Haemophilus influenzae, Staphylococcus aureus, and Streptococcus pneumoniae | All EOs had some antibacterial efficacy. A. oxymitra rhizome oil was active against all bacteria tested, its pericarp oil had the highest efficacy against H. influenzae (in vitro). With 80% inhibitory concentration of proliferation (N512 μg/mL), this EO might be safe for human lung cell lines. | Houdkova et al., 2018 |
| Carum carvi, Citrus aurantium, C. bergamia, Coriandrum sativum, Juniperus communis, Lavandula angustifolia, Mentha arvensis, M. pulegium, Ocimum basilicum, O. citriodorum, O. majorana, O. vulgare, Pimenta racemosa, Salvia officinalis, Salvia sterea, Tanacetum vulgare, Thymus satureoides, T. vulgaris, and Zingiber cassumunar. | Alternaria alternata, Aspergillus niger, Cladosporium cladosporioides, and Stachybotrys chartarum. | High antifungal activity with up to 100% inhibition - most effective EOs were bay tree, caraway, cilantro, lemon basil, oregano, and thyme. | Zabka et al., 2014 |
| Campomanesia aurea 0.0049–10 mg/mL | Listeria monocytogenes, Pseudomonas aeruginosa, Salmonella enteritidis, and Staphylococcus aureus. | Minimal inhibitory concentration of Campomanesia aurea against Listeria monocytogenes (5.0 mg/mL) and S. aureus (0.7 mg/mL) and inhibition of L. monocytogenes biofilm formation. Campomanesia aurea inhibited biofilm formation in most pathogens tested. | Kuhn et al., 2019 |
| Red pepper (100, 200, 400 and 800 μg/ml) | Escherichia coli, Listeria monocytogenes, Salmonella enterica, and Staphylococcus aureus. | Red pepper significantly inhibited Escherichia coli, Listeria monocytogenes, Salmonella enterica, and Staphylococcus aureus. | Reda et al., 2020a, Reda et al., 2020b |
20. Conclusion
Microorganisms are robust and have many adaptations against environmental factors and antimicrobial treatments. As such, investigation and understanding of their resistance mechanism are crucial for successfully emerging novel antimicrobial agents to overcome the crisis of antibiotic resistance. Biofilm-forming microorganisms, encased within a matrix, represent a serious medical problem, as they protect themselves from antibiotics and the hosts’ immune response. These microbial communities live together on a variety of surfaces to form a barrier against sanitizers. Therefore, it is advisable to identify appropriate materials for cleaning and sanitization to reduce microbial attachment. EOs may have a significant role in controlling biofilm formation if included in cleaning products and sanitizers. They could also compliment the use of antibiotics due to the low concentration needed. This current review provides further evidence that EOs could be an essential component in the fight against antibiotic resistance due to their efficient antibiofilm activity. However, detailed investigations of the safety margin and the factors that may affect the antimicrobial potential of EOs are still needed due to a paucity of current studies. Any additional data for the properties and active compounds of EOs will add value to this field's knowledge.
Author contributions
All authors were equal contributors in the writing of this review article. All the authors have read and approved the final manuscript.
Funding
This work was supported by Abu Dhabi Award for Research Excellence-Department of Education and Knowledge [AARE 2019-ADEK-007, grant number 21S105] to Prof. Khaled El-Tarabily.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
The authors are grateful for support from their respected universities and institutes. K.A. El-Tarabily would like to thank the library at Murdoch University, Australia for the valuable online resources and comprehensive databases.
Footnotes
Peer review under responsibility of King Saud University.
References
- Abd El-Hack M.E., Alagawany M., Farag M.R., Tiwari R., Karthik K., Dhama K., Zorriehzahra J, Adel M. Beneficial impacts of thymol essential oil on health and production of animals, fish and poultry: a review. J. Essent. Oil Res. 2016;28:365–382. [Google Scholar]
- Abd El-Hack M.E., El-Saadony M.T., Shafi M.E., Alshahrani O.A., Saghir S.A., Al-Wajeeh A.S., Al-Shargi O.Y.A., Taha A.E., Mesalam N.M., Abdel-Moneim A.E. Prebiotics can restrict Salmonella populations in poultry: a review. Anim. Biotechnol. 2021;19:1–10. doi: 10.1080/10495398.2021.1883637. [DOI] [PubMed] [Google Scholar]
- Abd El-Hack M.E., El-Saadony M.T., Shafi M.E., Qattan S.Y., Batiha G.E., Khafaga A.F., Abdel-Moneim A.E., Alagawany M. Probiotics in poultry feed: A comprehensive review. J. Anim. Physiol. Anim. Nutr. 2020;104:1835–1850. doi: 10.1111/jpn.13454. [DOI] [PubMed] [Google Scholar]
- Abd El-Hack M.E., El-Saadony M.T., Shehata A.M., Arif M., Paswan V.K., Batiha G.E.S., Khafaga A.F., Elbestawy A.R. Approaches to prevent and control Campylobacter spp. colonization in broiler chickens: A review. Environ. Sci. Pollut. Res. 2020;28:4989–5004. doi: 10.1007/s11356-020-11747-3. [DOI] [PubMed] [Google Scholar]
- Abdelnour S.A., Swelum A.A., Salama A., Al-Ghadi M.Q., Qattan S.Y., Abd El-Hack M.E., Khafaga A.F., Alhimaidi A.R., Almutairi B.O., Ammari A.A., El-Saadony M.T. The beneficial impacts of dietary phycocyanin supplementation on growing rabbits under high ambient temperature. Ital. J. Anim. Sci. 2020;19:1046–1056. [Google Scholar]
- Abou-Kassem D.E.K., Mahrose M., El-Samahy R.A., Shafi M.E., El-Saadony M.T., Abd El-Hack M.E., Emam M., El-Sharnouby M., Taha A.E., Ashour E.A.A. Influences of dietary herbal blend and feed restriction on growth, carcass characteristics and gut microbiota of growing rabbits. Ital. J. Anim. Sci. 2021 (in press) [Google Scholar]
- Alagawany M., El-Saadony M.T., Elnesr S., Farahat M., Attia G., Madkour M., Reda F. Use of lemongrass essential oil as a feed additive in quail’s nutrition: Its effect on growth, carcass, blood biochemistry, antioxidant and immunological indices, digestive enzymes and intestinal microbiota. Poult. Sci. 2021;100 doi: 10.1016/j.psj.2021.101172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alagawany M., Madkour M., El-Saadony M.T., Reda F.M. Paenibacillus polymyxa (LM31) as a new feed additive: Antioxidant and antimicrobial activity and its effects on growth, blood biochemistry, and intestinal bacterial populations of growing Japanese quail. Anim. Feed Sci. Technol. 2021;276 [Google Scholar]
- Alanis A.J. Resistance to antibiotics: Are we in the post-antibiotic era? Arch. Med. Res. 2005;36:697–705. doi: 10.1016/j.arcmed.2005.06.009. [DOI] [PubMed] [Google Scholar]
- Ali B., Al-Wabel N.A., Shams S., Ahamad A., Khan S.A., Anwar F. Essential oils used in aromatherapy: A systemic review. Asian. Pac. J. Trop. Biomed. 2015;5:601–611. doi: 10.1016/j.apjtb.2015.05.007. [DOI] [Google Scholar]
- Al-Shuneigat J.M., Al-Sarayreh S.A., Al-Qudah M.A., Al-Saraireh Y.M. Antibacterial and antibiofilm activity of essential oil of Achillea biebersteinii and its mode of action. J. Pharm. Pharmacogn. Res. 2020;8:155–166. doi: 10.2478/cipms-2020-0016. [DOI] [Google Scholar]
- Aminov R.I. A brief history of the antibiotic era: Lessons learned and challenges for the future. Front. Microbiol. 2010;134:1–7. doi: 10.3389/fmicb.2010.00134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anomaly J. Antibiotics and animal agriculture: The need for global collective action. In: Selgelid M., editor. Ethics and Antimicrobial Resistance. Springer; New York: 2020. pp. 297–308. [Google Scholar]
- Armbruster C.R., Parsek M.R. New insight into the early stages of biofilm formation. Proc. Natl. Acad. Sci. 2018;115:4317–4319. doi: 10.1073/pnas.1804084115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Artini M., Patsilinakos A., Papa R., Boović M., Sabatino M., Garzoli S., Vrenna G., Tilotta M., Pepi F., Ragno R., Selan L. Antimicrobial and antibiofilm activity and machine learning classification analysis of essential oils from different mediterranean plants against Pseudomonas aeruginosa. Molecules. 2018;23:482. doi: 10.3390/molecules23020482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arunasri, K., Mohan, S.V., 2019. Biofilms: microbial life on the electrode surface. In: Mohan, S.V., Varjani, S., Pandey, A. (Eds.), Biomass, Biofuels and Biochemicals, Microbial Electrochemical Technology. Elsevier, pp. 295–313. http://dx.doi.10.1016/B978-0-444-64052-9.00011-X.
- Ashour E.A., Abd El-Hack M.E., Shafi M.E., Alghamdi W.Y., Taha A.E., Swelum A.A., Tufarelli V., Mulla Z.S., El-Ghareeb W.R., El-Saadony M.T. Impacts of green coffee powder supplementation on growth performance, carcass characteristics, blood indices, meat quality and gut microbial load in broilers. Agriculture. 2020;10:457. [Google Scholar]
- Astani A., Reichling J., Schnitzler P. Screening for antiviral activities of isolated compounds from essential oils. Evid. Based. Compl. Alternat. Med. 2011;2011 doi: 10.1093/ecam/nep187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ayukekbong J.A., Ntemgwa M., Atabe A.N. The threat of antimicrobial resistance in developing countries: Causes and control strategies. Antimicrob. Resist. Infect. Control. 2017;6:47. doi: 10.1186/s13756-017-0208-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barbieri, C., Borsotto, P., 2018. Essential oils: market and legislation. In: El-Shemy, H.A. (Ed), Potential of Essential Oils. IntechOpen, pp. 107-128. http://dx.doi.10.5772/intechopen.77725.
- Barreto T.V., Coelho A.C.D. Distillation. In: Fernando S., Borém A., Caldas C., editors. Sugarcane: Agricultural Production, Bioenergy and Ethanol. Academic Press; Oxford: 2015. pp. 341–363. [DOI] [Google Scholar]
- Baser, K.H.C., Buchbauer, G., 2015. Handbook of Essential Oils: Science, Technology and Applications, second ed. Taylor & Francis. Florida. pp. 1128. http://dx.doi.10.1201/b19393.
- Bassolé I.H., Juliani H.R. Essential oils in combination and their antimicrobial properties. Molecules. 2012;17:3989–4006. doi: 10.3390/molecules17043989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benzaid C., Belmadani A., Djeribi R., Rouabhia M. The Effects of Mentha piperita essential oil on C. albicans growth, transition, biofilm formation, and the expression of secreted aspartyl proteinases genes. Antibiotics. 2019;8:10. doi: 10.3390/antibiotics8010010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birgand G., Mutters N.T., Ahmad R., Tacconelli E., Lucet J.-C., Holmes A. Risk perception of antimicrobial resistance by infection control specialists in Europe: A case-vignette study. Antimicrob. Resist. Infect. Control. 2020;9:33–42. doi: 10.1186/s13756-020-0695-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blair J.M.A., Webber M.A., Baylay A.J., Ogbolu D.O., Piddock L.J. Molecular mechanisms of antibiotic resistance. Nat. Rev. Microbiol. 2015;13:42–51. doi: 10.1038/nrmicro3380. [DOI] [PubMed] [Google Scholar]
- Boire N.A., Riedel S., Parrish N.M. Essential oils and future antibiotics: New weapons against emerging ‘superbugs’? J. Anc. Dis. Prev. Rem. 2013;1:105. doi: 10.4172/2329-8731.1000105. [DOI] [Google Scholar]
- Bonomo R.A., Rossolini G.M. Importance of antibiotic resistance and resistance mechanisms. Expert. Rev. Anti. Infect. Ther. 2008;6:549–550. doi: 10.1586/14787210.6.5.549. [DOI] [PubMed] [Google Scholar]
- Bowes K.M., Zheljazkov V.D. Factors affecting yields and essential oil quality of Ocimum sanctum L. and Ocimum basilicum L. cultivars. J. Am. Soc. Hortic. Sci. 2019;129:789–794. doi: 10.21273/JASHS.129.6.0789. [DOI] [Google Scholar]
- Brochot A., Guilbot A., Haddioui L., Roques C. Antibacterial, antifungal, and antiviral effects of three essential oil blends. Microbiol. Open. 2017;6 doi: 10.1002/mbo3.459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buckle, J., 2015. Basic plant taxonomy, basic essential oil chemistry, extraction, biosynthesis, and analysis. In: J. Buckle, (Ed.), Clinical Aromatherapy. Churchill Livingstone, Missouri, pp. 37–72. http://dx.doi.10.1016/B978-0-7020-5440-2.00003-6.
- Butnariu M., Sarac I. Essential oils from plants. J. Biotechnol. Biomed. Sci. 2018;1:35–43. doi: 10.14302/issn.2576-6694.jbbs-18-2489. [DOI] [Google Scholar]
- Butt A., Khan A. Antibiotic Resistance of Bacterial Biofilms. Middle East J. Business. 2015;10:38–45. doi: 10.5742/MEJB.2015.92718. [DOI] [Google Scholar]
- Carpentier B., Cerf O. Biofilms and their consequences, with particular reference to hygiene in food industry. J. Appl. Bacteriol. 1993;75:499–511. doi: 10.1111/j.1365-2672.1993.tb01587.x. [DOI] [PubMed] [Google Scholar]
- Chouhan S., Sharma K., Guleria S. Antimicrobial activity of some essential oils—Present status and future perspectives. Medicines. 2017;4:58. doi: 10.3390/medicines4030058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chraibi M., Farah A., Elamin O., Iraqui H.M., Fikri-Benbrahim K. Characterization, antioxidant, antimycobacterial, antimicrobial effcts of Moroccan rosemary essential oil, and its synergistic antimicrobial potential with carvacrol. J. Adv. Pharm. Technol. Res. 2020;11:25–29. doi: 10.4103/japtr.JAPTR_74_19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ciofu O., Tolker-Nielsen T. Tolerance and resistance of Pseudomonas aeruginosa biofilms to antimicrobial agents- How P. aeruginosa can escape antibiotics. Front. Microbiol. 2019;10:913. doi: 10.3389/fmicb.2019.00913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coenye T., Nelis H.J. In vitro and in vivo model systems to study microbial biofilm formation. J. Microbiol. Methods. 2010;83:89–105. doi: 10.1016/j.mimet.2010.08.018. [DOI] [PubMed] [Google Scholar]
- Cortés M., Bonilla J.C., Sinisterra R. Biofilm formation, control and novel strategies for eradication. Sci. Against Microbial. Pathog. Commun. Curr. Res. Technol. Adv. 2011;2:896–905. [Google Scholar]
- Courtenay M., Castro-Sanchez E., Fitzpatrick M., Gallagher R., Lim R., Morris G. Tackling antimicrobial resistance 2019–2024 – The UK’s five-year national action plan. J. Hospital Infect. 2019;101:426–427. doi: 10.1016/j.jhin.2019.02.019. [DOI] [PubMed] [Google Scholar]
- Cowan M.M. Plant products as antimicrobial agents. Clin. Microbiol. Rev. 1999;12:564–582. doi: 10.1128/cmr.12.4.564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davies D.G. Biofilm dispersion. In: Flemming H.C., Wingender J., Szewzyk U., editors. Biofilm Highlights. Springer; London: 2011. pp. 1–28. [Google Scholar]
- Debabov D. Antibiotic resistance: Origins, mechanisms, approaches to counter. Appl. Biochem. Microbiol. 2013;49:665–671. doi: 10.1134/S0003683813080024. [DOI] [Google Scholar]
- Department of Health, 2016. Antimicrobial resistance empirical and statistical evidence-base: A report from the Department of Health Antimicrobial Resistance Strategy Analytical Working Group. Department of Health, London, UK. https://www.gov.uk/government/uploads/system/uploads/attachment_data/file/553267/AMR_EBO_2016.pdf.
- Dhifi W., Bellili S., Jazi S., Bahloul N., Mnif W. Essential oils’ chemical characterization and investigation of some biological activities: A critical review. Medicines. 2016;3:25. doi: 10.3390/medicines3040025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donlan R.M. Biofilms: Microbial life on surfaces. Emerg. Infect. Dis. 2002;8:881–890. doi: 10.3201/eid0809.020063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunne W.M. Bacterial adhesion: seen any good biofilms lately? Clin. Microbiol. Rev. 2002;15:155–166. doi: 10.1128/CMR.15.2.155-166.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisenhut M. The toxicity of essential oils. Int. J. Infect. Dis. 2007;11:365. doi: 10.1016/j.ijid.2006.07.004. [DOI] [PubMed] [Google Scholar]
- Elias S., Banin E. Multi-species biofilms: Living with friendly neighbors. FEMS Microbiol. Rev. 2012;36:990–1004. doi: 10.1111/j.1574-6976.2012.00325.x. [DOI] [PubMed] [Google Scholar]
- Eslahi, H., Fahimi, N., Sardarian, A.R., 2017. Chemical composition of essential oils. In: Hashemi, S.M.B., Khaneghah, A.M., Sant’Ana, A. de S., (Eds.), Essential Oils in Food Processing: Chemistry, Safety and Applications. John Wiley & Sons, Ltd, pp. 119–171.
- Evans D.J., Allison D.G., Brown M.R.W., Gilbert P. Susceptibility of Pseudomonas aeruginosa and Escherichia coli biofilms towards ciprofloxacin: Effect of specific growth rate. J. Antimicrob. Chemother. 1991;27:177–184. doi: 10.1093/jac/27.2.177. [DOI] [PubMed] [Google Scholar]
- Faleiro M. The mode of antibacterial action of essential oils. In: Méndez-Vilas A., editor. Science Against Microbial Pathogens: Communicating Current Research and Technological Advances. Formatex Research Center; Badajoz: 2011. pp. 1143–1156. [Google Scholar]
- Feyaerts A.F., Mathé L., Luyten W., De-Graeve S., Van Dyck K., Broekx L., Van Dijck P. Essential oils and their components are a class of antifungals with potent vapour-phase-mediated anti-Candida activity. Sci Rep. 2018;8:3958. doi: 10.1038/s41598-018-22395-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fine D.H., Markowitz K., Furgang D., Goldsmith D., Ricci-Nittel D., Charles C.H., Peng P., Lynch M.C. Effect of rinsing with an essential oil–containing mouthrinse on subgingival periodontopathogens. J. Periodontol. 2007;78:1935–1942. doi: 10.1902/jop.2007.070120. [DOI] [PubMed] [Google Scholar]
- Flemming H.C., Neu T.R., Wozniak D.J. The EPS matrix: the ‘house of biofilm cells’. J. Bacteriol. 2007;189:7945–7947. doi: 10.1128/JB.00858-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedman M., Henika P.R., Levin C.E., Mandrell R.E. Antibacterial activities of plant essential oils and their components against Escherichia coli O157:H7 and Salmonella enterica in apple juice. J. Agric. Food. Chem. 2004;52:6042–6048. doi: 10.1021/jf0495340. [DOI] [PubMed] [Google Scholar]
- Gerdt J.P., Blackwell H.E. Competition studies confirm two major barriers that can preclude the spread of resistance to quorum-sensing inhibitors in bacteria. ACS Chem. Biol. 2014;9:2291–2299. doi: 10.1021/cb5004288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghabraie M., Vu K.D., Tata L., Salmieri S., Lacroix M. Antimicrobial effect of essential oils in combinations against five bacteria and their effect on sensorial quality of ground meat. LWT Food Sci. Technol. 2016;66:332–339. doi: 10.1016/j.lwt.2015.10.055. [DOI] [Google Scholar]
- Gould I.M., Bal A.M. New antibiotic agents in the pipeline and how they can help overcome microbial resistance. Virulence. 2013;4:185–191. doi: 10.4161/viru.22507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Govindasamy, R., Arumugam, S. Simon, J.E., 2013. An assessment of the essential oil and aromatic plant industry with a focus on Africa. In: Juliani, H.R., Simon, J.E., Ho, C.-T., (Eds.), African Natural Plant Products. American Chemical Society, Washington, DC, pp. 289–321. http://dx.doi.10.1021/bk-2013-1127.ch018.
- Guenther E. The Essential Oils-Vol 1: History, Origin in Plants, Production and Analysis. Read Books Ltd. 2013:456. [Google Scholar]
- Gupta P., Deka S. The menace of antimicrobial resistance. Indian J. Comm. Health. 2018;30:317–322. [Google Scholar]
- Guttenplan S.B., Kearns D.B. Regulation of flagellar motility during biofilm formation. FEMS Microbiol. Rev. 2013;37:849–871. doi: 10.1111/1574-6976.12018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gyawali R., Ibrahim S.A. Natural products as antimicrobial agents. Food Control. 2014;46:412–429. doi: 10.1016/j.foodcont.2014.05.047. [DOI] [Google Scholar]
- Hammer K.A., Carson C.F., Riley T.V., Nielsen J.B. A review of the toxicity of Melaleuca alternifolia (tea tree) oil. Food Chem. Toxicol. 2006;44:616–625. doi: 10.1016/j.fct.2005.09.001. [DOI] [PubMed] [Google Scholar]
- Harbarth S., Balkhy H.H., Goossen H., Jarlier V., Kluytmans J., Laxminarayan R., Saam M., Van-Belkum A., Pittet D. Antimicrobial resistance: One world, one fight! Antimicrob. Resist. Infect. Control. 2015;4:49. doi: 10.1186/s13756-015-0091-2. [DOI] [Google Scholar]
- Harmsen M., Yang L., Pamp S.J., Tolker-Nielsen T. An update on Pseudomonas aeruginosa biofilm formation, tolerance, and dispersal. FEMS Immunol. Med. Microbiol. 2010;59:253–268. doi: 10.1111/j.1574-695X.2010.00690.x. [DOI] [PubMed] [Google Scholar]
- Hengge R. The general stress response in Gram-negative bacteria. In: Storz G., Hengge R., editors. Vol. 2. American Society for Microbiology; Washington, DC: 2014. pp. 251–289. (Bacterial Stress Responses). [DOI] [Google Scholar]
- Hense B.A., Schuster M. Core principles of bacterial autoinducer systems. Microbiol. Mol. Biol. Rev. 2015;79:153–169. doi: 10.1128/MMBR.00024-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HM Government, 2019. Tackling antimicrobial resistance 2019–2024: The UK’s five-year national action plan (online). Available from: https://assets.publishing.service.gov.uk/government/uploads/system/uploads/attachment_data/file/784894/UK_AMR_5_year_national_action_pl an.pdf. [DOI] [PubMed]
- Houdkova M., Urbanova K., Doskocil I., Rondevaldova J., Novy P., Nguon S., Chrun R., Kokoska L. In vitro growth-inhibitory effect of Cambodian essential oils against pneumonia causing bacteria in liquid and vapour phase and their toxicity to lung fibroblasts. S. Afr. J. Bot. 2018;118:85–97. doi: 10.1016/j.sajb.2018.06.005. [DOI] [Google Scholar]
- Janssen A.M., Chin N.L.J., Scheffer J.J.C., Svendsen A.B. Screening for antimicrobial activity of some essential oils by the agar overlay technique. Pharm. Weekbl. Sci. 1986;8:289–292. doi: 10.1007/BF02280052. [DOI] [PubMed] [Google Scholar]
- Ji H., Kim H., Beuchat L.R., Ryu J.H.H. Synergistic antimicrobial activities of essential oil vapours against Penicillium corylophilum on a laboratory medium and beef jerky. Int. J. Food Microbiol. 2019;291:104–110. doi: 10.1016/j.ijfoodmicro.2018.11.023. [DOI] [PubMed] [Google Scholar]
- Jiao Y., Cody G.D., Harding A.K., Wilmes P., Schrenk M., Wheeler K.E., Banfield J.F., Thelen M.P. Characterization of extracellular polymeric substances from acidophilic microbial biofilms. Appl. Environ. Microbiol. 2010;76:2916–2922. doi: 10.1128/AEM.02289-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaplan J.B. Biofilm dispersal: Mechanisms, clinical implications, and potential therapeutic uses. J. Den. Res. 2010;89:205–218. doi: 10.1177/0022034509359403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimera I.Z., Mshana S.E., Rweyemamu M.M., Mboera L.E.G., Matee M.I.N. Antimicrobial use and resistance in food-producing animals and the environment: An African perspective. Antimicrob. Resist. Infect. Control. 2020;9:37–48. doi: 10.1186/s13756-020-0697-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kommerein N., Doll K., Stumpp N.S., Stiesch M. Development and characterization of an oral multispecies biofilm implant flow chamber model. PLoS ONE. 2018;13:1–15. doi: 10.1371/journal.pone.0196967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kortright K.E., Chan B.K., Koff J.L., Turner P.E. Phage therapy: A renewed approach to combat antibiotic-resistant bacteria. Cell Host Microbe. 2019;25:219–232. doi: 10.1016/j.chom.2019.01.014. [DOI] [PubMed] [Google Scholar]
- Kuhn D., Ziem R., Scheibel T., Buhl B., Vettorello G., Pacheco L.A., Heidrich D., de Kauffmann C., Freitas E.M., Ethur E.M., Hoehne L. Antibiofilm activity of the essential oil of Campomanesia aurea O. Berg against microorganisms causing food borne diseases. LWT Food Sci. Technol. 2019;108:247–252. doi: 10.1016/j.lwt.2019.03.079. [DOI] [Google Scholar]
- Lakoh S., Li L., Sevalie S., Guo X., Adekanmbi O., Yang G., Adebayo O., Yi L., Coker J.M., Wang S., Wang T., Sun W., Habib A.G., Klein E.Y. Antibiotic resistance in patients with clinical features of healthcare-associated infections in an urban tertiary hospital in Sierra Leone: a cross-sectional study. Antimicrob. Resist. Infect. Control. 2020;9:38–47. doi: 10.1186/s13756-020-0701-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lakshminarayanan R., Ye E., Young D.J., Li Z., Loh X.J. Recent advances in the development of antimicrobial nanoparticles for combating resistant pathogens. Adv. Healthc. Mater. 2018;7:1701400. doi: 10.1002/adhm.201701400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee M.S., Choi J., Posadzki P., Ernst E. Aromatherapy for health care: An overview of systematic reviews. Maturitas. 2012;71:257–260. doi: 10.1016/j.maturitas.2011.12.018. [DOI] [PubMed] [Google Scholar]
- Levy S.B., Bonnie M. Antibacterial resistance worldwide: Causes, challenges and responses. Nat. Med. 2004;10:S122–S129. doi: 10.1038/nm1145. [DOI] [PubMed] [Google Scholar]
- Lin P.C., Lee P.H., Tseng S.J., Lin Y.M., Chen S.R., Hou W.H. Effects of aromatherapy on sleep quality: A systematic review and meta-analysis. Complement. Ther. Med. 2019;45:156–166. doi: 10.1016/j.ctim.2019.06.006. [DOI] [PubMed] [Google Scholar]
- Lobanovska M., Pilla G. Penicillin’s discovery and antibiotic resistance: Lessons for the future? Yale J. Biol. Medv. 2017;90:135–145. [PMC free article] [PubMed] [Google Scholar]
- López P., Sánchez C., Batlle R., Nerín C. Vapor-phase activities of cinnamon, thyme, and oregano essential oils and key constituents against foodborne microorganisms. J. Agric. Food Chem. 2007;55:4348–4356. doi: 10.1021/jf063295u. [DOI] [PubMed] [Google Scholar]
- Lopez-Romero J.C., González-Ríos H., Borges A., Simões M. Antibacterial effects and mode of action of selected essential oils components against Escherichia coli and Staphylococcus aureus. Evid. Based Complementary Altern. Med. 2015;795435 doi: 10.1155/2015/795435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magiorakos A.P., Srinivasan A., Carey R.B., Carmeli Y., Falagas M.E., Giske C.G., Harbarth S., Hindler J.F., Kahlmeter G., Olsson-Liljequist B., Paterson D.L., Rice L.B., Stelling J., Struelens M.J., Vatopoulos A., Weber J.T., Monnet D.L. Multidrug-resistant, extensively drug-resistant and pandrug-resistant bacteria: An international expert proposal for interim standard definitions for acquired resistance. Clin. Microbiol. Infect. 2012;18:268–281. doi: 10.1111/j.1469-0691.2011.03570.x. [DOI] [PubMed] [Google Scholar]
- Mah T.F.C., O’Toole G.A. Mechanisms of biofilm resistance to antimicrobial agents. Trends Microbiol. 2001;9:34–39. doi: 10.1016/s0966-842x(00)01913-2. [DOI] [PubMed] [Google Scholar]
- Martens E., Demain A.L. An overview of the industrial aspects of antibiotic discovery. In: Kurtböke I., editor. Microbial Resources: From Functional Existence in Nature to Applications. Academic Press; 2017. pp. 149–168. [DOI] [Google Scholar]
- Maruzzella J.C., Sicurella N.A. Antibacterial activity of essential oil vapors. J. Am. Pharm. Assoc. 1960;49:692–694. doi: 10.1002/jps.3030491103. [DOI] [Google Scholar]
- Mayaud L., Carricajo A., Zhiri A., Aubert G. Comparison of bacteriostatic and bactericidal activity of 13 essential oils against strains with varying sensitivity to antibiotics. Lett. Appl. Microbiol. 2008;47:167–173. doi: 10.1111/j.1472-765X.2008.02406.x. [DOI] [PubMed] [Google Scholar]
- Mayor S. Doctors to get real time data to support antibiotic prescribing and reduce resistance. BMJ. 2019;364:1406. doi: 10.1136/bmj.l406. [DOI] [PubMed] [Google Scholar]
- Mekonnen A., Tesfaye S., Christos S.G., Dires K., Zenebe T., Zegeye N., Shiferaw Y., Lulekal E. Evaluation of skin irritation and acute and subacute oral toxicity of Lavandula angustifolia essential oils in rabbit and mice. J. Toxicol. 2019;2019:1–8. doi: 10.1155/2019/5979546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michael C.A., Dominey-Howes D., Labbate M. The antimicrobial resistance crisis: causes, consequences, and management. Front Public Health. 2014;2:145. doi: 10.3389/fpubh.2014.00145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Millezi A.F., Piccoli R.H., Oliveira J.M., Pereira M.O. Anti-biofim and antibacterial effect of essential oils and their major compounds. J. Essent. Oil Bear Plants. 2016;19:624–631. doi: 10.1080/0972060X.2014.960262. [DOI] [Google Scholar]
- Mosselhy D.A., He W., Hynönen U., Meng Y., Mohammadi P., Palva A., Feng Q., Hannula S.P., Nordström K., Linder M.B. Silica– gentamicin nanohybrids: Combating antibiotic resistance, bacterial biofilms, and in vivo toxicity. Int. J. Nanomedicine. 2018;13:7939–7957. doi: 10.2147/IJN.S182611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mulani M.S., Kamble E.E., Kumkar S.N., Tawre M.S., Pardesi K.R. Emerging strategies to combat ESKAPE pathogens in the era of antimicrobial resistance: A review. Front. Microbiol. 2019;10:539. doi: 10.3389/fmicb.2019.00539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munita, J.M., Arias, C.A., 2016. Mechanisms of antibiotic resistance. Microbiol. Spectr. 4, 1–24. http://dx.doi.10.1128/microbiolspec.VMBF-0016-2015. [DOI] [PMC free article] [PubMed]
- Muzzarelli L., Force M., Sebold M. Aromatherapy and reducing preprocedural anxiety: A controlled prospective study. Gastroenterol. Nurs. 2006;29:466–471. doi: 10.1097/00001610-200611000-00005. [DOI] [PubMed] [Google Scholar]
- Narayanasamy, K., Elangovan, E., Keerthi, D., Jagadeeswari, S., Krithiga, B., Padmanabhan, V., Periyasamy, S., 2019. Antimicrobial activity of selected essential oils against antibiotic resistant organisms. Asian. J. Pharm. Pharmacol. 5, 503–512. 10.31024/ajpp.2019.5.3.11. [DOI]
- Nath S.S., Pandey C., Roy D. A near fatal case of high dose peppermint oil ingestion- Lessons learnt. Indian J Anaesth. 2012;56:582–584. doi: 10.4103/0019-5049.104585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nazzaro F., Fratianni F., Coppola R., De Feo V. Essential oils and antifungal activity. Pharmaceuticals. 2017;10:86. doi: 10.3390/ph10040086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nazzaro, F., Fratianni, F., D’Acierno, A., Coppola, R., Ayala-Zavala, F.J., da Cruz, A.G., De Feo, V., 2019. Essential oils and microbial communication. In: El-Shemy, H., (Ed). Essential Oils - Oils of Nature. IntechOpen. http://dx.doi.10.5772/intechopen.85638.
- Niederman M.S. Principles of appropriate antibiotic use. Int. J. Antimicrob. Agents. 2005;26:170–175. doi: 10.1016/s0924-8579(05)80324-3. [DOI] [PubMed] [Google Scholar]
- Nikaido H. Multidrug resistance in bacteria. Ann. Rev. Biochem. 2009;78:119–146. doi: 10.1146/annurev.biochem.78.082907.145923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Neill J.I.M. Antimicrobial resistance: tackling a crisis for the health and wealth of nations. Rev. Antimicrob. Resist. 2014;20:1–16. [Google Scholar]
- O’Toole G., Kaplan H.B., Kolter R. Biofilm formation as microbial development. Ann. Rev. Microbiol. 2000;54:49–79. doi: 10.1146/annurev.micro.54.1.49. [DOI] [PubMed] [Google Scholar]
- O’Toole G.A. To Build a Biofilm. J. Bacteriol. 2003;185:2687–2689. doi: 10.1128/JB.185.9.2687-2689.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Obama White House, 2015. President’s 2016 budget proposes historic investment to combat antibiotic-resistant bacteria to protect public health (online). Available from: https://obamawhitehouse.archives.gov/thepress-office/2015/01/27/fact-sheet-president-s-2016-budget-proposeshistoric-investment-combat-a (Accessed 9 Jun 2019).
- Oliveira F.S., Freitas T.S., Cruz R.P., Costa M.S., Pereira R.L.S., Quintans-Júnior L.J., Andrade T.A., Menezes P.D.P., Sousa B.M.H., Nunes P.S., Serafini M.R., Menezes I.R.A., Araújo A.A.S., Coutinho H.D.M. Evaluation of the antibacterial and modulatory potential of α-bisabolol, β-cyclodextrin and α-bisabolol/β-cyclodextrin complex. Biomed Pharmacother. 2017;92:1111–1118. doi: 10.1016/j.biopha.2017.06.020. [DOI] [PubMed] [Google Scholar]
- Palmer A.C., Kishony R. Opposing effects of target overexpression reveal drug mechanisms. Nature Commun. 2014;5:1–8. doi: 10.1038/ncomms5296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palmer J., Flint S., Brooks J. Bacterial cell attachment, the beginning of a biofilm. J. Ind. Microbiol. Biotechnol. 2007;34:577–588. doi: 10.1007/s10295-007-0234-4. [DOI] [PubMed] [Google Scholar]
- Pavithra D., Doble M. Biofilm formation, bacterial adhesion and host response on polymeric implants - Issues and prevention. Biomed. Mater. 2008;3:34003. doi: 10.1088/1748-6041/3/3/034003. [DOI] [PubMed] [Google Scholar]
- Pei R., Zhou F., Ji B., Xu J. Evaluation of combined antibacterial effects of eugenol, cinnamaldehyde, thymol, and carvacrol against E. coli with an improved method. J. Food Sci. 2009;74:379–383. doi: 10.1111/j.1750-3841.2009.01287.x. [DOI] [PubMed] [Google Scholar]
- Peterson E., Kaur P. Antibiotic resistance mechanisms in bacteria: Relationships between resistance determinants of antibiotic producers, environmental bacteria, and clinical pathogens. Front. Microbiol. 2018;9:1–21. doi: 10.3389/fmicb.2018.02928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petrova O.E., Sauer K., Opin C., Author M. Escaping the biofilm in more than one way: Desorption, detachment or dispersion. Curr. Opin. Microbiol. 2016;30:67–78. doi: 10.1016/j.mib.2016.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prestinaci F., Pezzotti P., Pantosti A. Antimicrobial resistance: a global multifaceted phenomenon. Pathog. Glob. Health. 2015;109:309–318. doi: 10.1179/2047773215Y.0000000030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Public Health England, 2018. Research reveals levels of inappropriate prescriptions in England (online). Available from: https://www.gov.uk/government/news/research-reveals-levels-ofinappropriate-prescriptions-in-england (Accessed 10 Jun 2019).
- Puškárová A., Bučková M., Kraková L., Pangallo D., Kozics K. The antibacterial and antifungal activity of six essential oils and their cyto/genotoxicity to human HEL 12469 cells. Sci. Rep. 2017;7:1–11. doi: 10.1038/s41598-017-08673-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ragno R., Papa R., Patsilinakos A., Vrenna G., Garzoli S., Tuccio V., Fiscarelli E., Selan L., Artini M. Essential oils against bacterial isolates from cystic fbrosis patients by means of antimicrobial and unsupervised machine learning approaches. Sci. Rep. 2020;10:2653–2663. doi: 10.1038/s41598-020-59553-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rather M.A., Dar B.A., Sofi S.N., Bhat B.A., Qurishi M.A. Foeniculum vulgare: A comprehensive review of its traditional use, phytochemistry, pharmacology, and safety. Arab J. Chem. 2016;9:S1574–S1583. doi: 10.1016/j.arabjc.2012.04.011. [DOI] [Google Scholar]
- Reda F.M., El-Saadony M.T., Elnesr S.S., Alagawany M., Tufarelli V. Effect of dietary supplementation of biological curcumin nanoparticles on growth and carcass traits, antioxidant status, immunity and caecal microbiota of Japanese quails. Animals. 2020;10:754. doi: 10.3390/ani10050754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reda F.M., El-Saadony M.T., El-Rayes T.K., Attia A.I., El-Sayed S.A., Ahmed S.Y., Madkour M., Alagawany M. Use of biological nano zinc as a feed additive in quail nutrition: biosynthesis, antimicrobial activity and its effect on growth, feed utilization, blood metabolites and intestinal microbiota. Ital. J. Anim. Sci. 2021;20:324–335. [Google Scholar]
- Reda F.M., Alagawany M., Mahmoud H.K., Mahgoub S.A., Elnesr S.S. Use of red pepper oil in quail diets and its effect on performance, carcass measurements, intestinal microbiota, antioxidant indices, immunity and blood constituents. Animals. 2020;14:1025–1033. doi: 10.1017/S1751731119002891. [DOI] [PubMed] [Google Scholar]
- Reis D., Jones T. Aromatherapy: Using essential oils as a supportive therapy. Clin. J. Oncol. Nurs. 2017;21:16–19. doi: 10.1188/17.CJON.16-19. [DOI] [PubMed] [Google Scholar]
- Reisner A., Haagensen J.A.J., Schembri M.A., Zechner E.L., Molin S. Development and maturation of Escherichia coli K-12 biofilms. Mol. Microbiol. 2003;48:933–946. doi: 10.1046/j.1365-2958.2003.03490.x. [DOI] [PubMed] [Google Scholar]
- Rosenblatt-Farrell N. The landscape of antibiotic resistance. Environ Health Perspect. 2009;117:A244–A250. doi: 10.1289/ehp.117-a244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roshan N., Hammer K.A., Riley T.V. Non-conventional antimicrobial and alternative therapies for the treatment of Clostridium difficile infection. Anaerobe. 2018;49:103–111. doi: 10.1016/j.anaerobe.2018.01.003. [DOI] [PubMed] [Google Scholar]
- Santajit S., Indrawattana N. Mechanisms of antimicrobial resistance in ESKAPE pathogens. BioMed. Res. Int. 2016;2016:1–8. doi: 10.1155/2016/2475067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarkic A., Stappen I. Essential oils and their single compounds in cosmetics—A critical review. Cosmetics. 2018;5:11. doi: 10.3390/cosmetics5010011. [DOI] [Google Scholar]
- Semeniuc C.A., Pop C.R., Rotar A.M. Antibacterial activity and interactions of plant essential oil combinations against Gram-positive and Gram-negative bacteria. J. Food Drug Anal. 2017;25:403–408. doi: 10.1016/j.jfda.2016.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheiha A.M., Abdelnour S.A., Abd El-Hack M.E., Khafaga A.F., Metwally K.A., Ajarem J.S., Maodaa S.N., Allam A.A., El-Saadony M.T. Effects of dietary biological or chemical-synthesized nano-selenium supplementation on growing rabbits exposed to thermal stress. Animals. 2020;10:430. doi: 10.3390/ani10030430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simpkin V.L., Renwick M.J., Kelly R., Mossialos E. Incentivizing innovation in antibiotic drug discovery and development: progress, challenges and next steps. J. Antibiot. 2017;70:1087–1096. doi: 10.1038/ja.2017.124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singer A.C., Shaw H., Rhodes V., Hart A. Review of antimicrobial resistance in the environment and its relevance to environmental regulators. Front. Microbiol. 2016;7:1728. doi: 10.3389/fmicb.2016.01728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smieszek T., Pouwels K.B., Christiaan F., Dolk K., Smith D.R.M., Hopkins S., Sharland M., Hay A.D., Moore M.V., Robotham J.V. Potential for reducing inappropriate antibiotic prescribing in English primary care. J. Antimicrob. Chemother. 2018;73:36–43. doi: 10.1093/jac/dkx500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solano C., Echeverz M., Lasa I. Biofilm dispersion and quorum sensing. Curr. Opin. Microbiol. 2014;18:96–104. doi: 10.1016/j.mib.2014.02.008. [DOI] [PubMed] [Google Scholar]
- Soni R., Sharma G., Jasuja N.D. Essential oil yield pattern and antibacterial and insecticidal activities of Trachyspermum ammi and Myristica fragrans. Scientifica. 2016;2016:1428194. doi: 10.1155/2016/1428194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swamy M.K., Akhtar M.S., Sinniah U.R. Antimicrobial properties of plant essential oils against human pathogens and their mode of action: An updated review. Evid. Based Complem. Altern Med. 2016;2016:3012462. doi: 10.1155/2016/3012462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swelum A.A., Elbestawy A.R., El-Saadony M.T., Hussein E.O., Alhotan R., Suliman G.M., Taha A.E., Ba-Awadh H., El-Tarabily K.A., Abd El-Hack M.E. Ways to minimize bacterial infections, with special reference to Escherichia coli, to cope with the first-week mortality in chicks: an updated overview. Poult. Sci. 2021;100:101039. doi: 10.1016/j.psj.2021.101039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tangjitjaroenkun J., Chavasiri W., Thunyaharn S., Yompakdee C. Bactericidal effects and time-kill studies of the essential oil from the fruits of Zanthoxylum limonella on multi-drug resistant bacteria. J. Essent. Oil Res. 2012;24:363–370. doi: 10.1080/10412905.2012.692907. [DOI] [Google Scholar]
- Tanwar J., Das S., Fatima Z., Hameed S. Multidrug resistance: An emerging crisis. Interdiscip. Perspect. Infect. Dis. 2014;2014 doi: 10.1155/2014/541340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tenover F.C. Mechanisms of antimicrobial resistance in bacteria. Am. J. Infect. Control. 2006;119:S3–S10. doi: 10.1016/j.ajic.2006.05.219. [DOI] [PubMed] [Google Scholar]
- Tong S., Pan J., Lu S., Tang J. Patient compliance with antimicrobial drugs: A Chinese survey. Am. J. Infect. Control. 2018;46:e25–e29. doi: 10.1016/j.ajic.2018.01.008. [DOI] [PubMed] [Google Scholar]
- Toyofuku M., Inaba T., Kiyokawa T., Obana N., Yawata Y., Nomura N. Environmental factors that shape biofilm formation. Biosci. Biotechnol. Biochem. 2016;80:7–12. doi: 10.1080/09168451.2015.1058701. [DOI] [PubMed] [Google Scholar]
- Tracanna V., De Jong A., Medema M.H., Kuipers O.P. Mining prokaryotes for antimicrobial compounds: from diversity to function. FEMS Microbiol Rev. 2017;41:417–429. doi: 10.1093/femsre/fux014. [DOI] [PubMed] [Google Scholar]
- Utchariyakiat I., Surassmo S., Jaturanpinyo M., Khuntayaporn P., Chomnawang M.T. Efficacy of cinnamon bark oil and cinnamaldehyde on anti-multidrug resistant Pseudomonas aeruginosa and the synergistic effects in combination with other antimicrobial agents. BMC Complement. Altern. Med. 2016;16:158. doi: 10.1186/s12906-016-1134-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van-Merode A.E.J., Van-Der Mei H.C., Busscher H.J., Krom B.P. Influence of culture heterogeneity in cell surface charge on adhesion and biofilm formation by Enterococcus faecalis. J. Bacteriol. 2006;188:2421–2426. doi: 10.1128/JB.188.7.2421-2426.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ventola C.L. The antibiotic resistance crisis: causes and threats. Pharm. Therapeut. 2015;40:277–283. [PMC free article] [PubMed] [Google Scholar]
- Vigan M. Essential oils: Renewal of interest and toxicity. Eur. J. Dermatol. 2010;20:685–692. doi: 10.1684/ejd.2010.1066. [DOI] [PubMed] [Google Scholar]
- Wang R., Wang R., Yang B. Extraction of essential oils from five cinnamon leaves and identification of their volatile compound compositions. Innov. Food Sci. Emerg. Technol. 2009;10:289–292. doi: 10.1016/j.ifset.2008.12.002. [DOI] [Google Scholar]
- Wentland E.J., Stewart P.S., Huang C.T., McFeters G.A. Spatial variations in growth rate within Klebsiella pneumoniae colonies and biofilm. Biotechnol. Prog. 1996;12:316–321. doi: 10.1021/bp9600243. [DOI] [PubMed] [Google Scholar]
- Whiley H., Gaskin S., Schroder T., Ross K. Antifungal properties of essential oils for improvement of indoor air quality: A review. Rev. Environ. Health. 2018;33:63–76. doi: 10.1515/reveh-2017-0023. [DOI] [PubMed] [Google Scholar]
- Williamson K.S., Richards L.A., Perez-Osorio A.C., Pitts B., McInnerney K., Stewart P.S., Franklin M.J. Heterogeneity in Pseudomonas aeruginosa biofilms includes expression of ribosome hibernation factors in the antibiotic-tolerant subpopulation and hypoxia-induced stress response in the metabolically active population. J. Bacteriol. 2012;194:2062–2073. doi: 10.1128/JB.00022-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yaqoob M.U., Abd El-Hack M.E., Hassan F., El-Saadony M.T., Khafaga A.F., Batiha G.E., Yehia N., Elnesr S.S., Alagawany M., El-Tarabily K.A., Wang M. The potential mechanistic insights and future implications for the effect of prebiotics on poultry performance, gut microbiome, and intestinal morphology. Poult. Sci. 2021;101143 doi: 10.1016/j.psj.2021.101143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zabka M., Pavela R., Prokinova E. Antifungal activity and chemical composition of twenty essential oils against significant indoor and outdoor toxigenic and aeroallergenic fungi. Chemosphere. 2014;112:443–448. doi: 10.1016/j.chemosphere.2014.05.014. [DOI] [PubMed] [Google Scholar]
- Zaman S.B., Hussain A., Nye R., Mehta V., Taib M.K., Hossain N. A Review on antibiotic resistance: Alarm bells are ringing. Cureus. 2017;9:e1403–e1411. doi: 10.7759/cureus.1403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheljazkov V.D., Cantrell C.L., Astatkie T., Jeliazkova E. Distillation time effect on lavender essential oil yield and composition. J. Oleo. Sci. 2013;62:195–199. doi: 10.5650/jos.62.195. [DOI] [PubMed] [Google Scholar]
- Zouari-Bouassida K., Trigui M., Makni S., Jlaiel L., Tounsi S. Seasonal variation in essential oils composition and the biological and pharmaceutical protective effects of Mentha longifolia leaves grown in Tunisia. BioMed. Res. Int. 2018;2018:7856517. doi: 10.1155/2018/7856517. [DOI] [PMC free article] [PubMed] [Google Scholar]