Abstract
Intrauterine growth retardation (IUGR) impairs immune function in children. IUGR is associated with an imbalance of oxidative stress and abnormal apoptosis. Therefore, an IUGR rats model was established to determine the antioxidant capacity and apoptosis in newborn IUGR rats and explored whether these effects were regulated after Docosahexaenoic acid (DHA) supplementation to rat pups. First, eight normal-birth-weight (NBW) and eight IUGR neonatal rats (a 10% low-protein diet) were used to obtain the antioxidant capacity and apoptosis in IUGR rat pups. Then, 32 newborn rats were randomly assigned to the normal birth weight (NBW), DHA supplementation for NBW (ND), IUGR, and DHA supplementation for IUGR (ID) groups. Starting from the 7th day after birth, DHA was given to the experimental group and the same volume of distilled water was given to the control group for 21 days. (1) DHA improved the serum and spleen CD4/CD8 ratios and IL-4 and IFN-γ mRNA expression. (2) DHA decreased the level of MDA, but increased T-AOC in serum and spleen. (3) DHA increased the protein expression of Bcl-2 while decreased Bax. (4) DHA increased protein expression of the Nrf2 signaling pathway and the downstream antioxidant genes GSH-PX and CAT. DHA may alleviate the impairment of spleen cellular immunity in IUGR rat pups by inhibiting oxidative stress and apoptosis related to the activation of Nrf2 signaling pathway.
Keywords: DHA, IUGR, Spleen cellular immunity, Oxidative stress, Apoptosis, Nrf2 signaling pathway
1. Introduction
Intrauterine growth restriction (IUGR) is defined as a fetus whose weight is less than two standard deviations of average weight for the same age or the 10th percentile of weight for the age (Black et al., 2008, Dong et al., 2015, Ferguson, 1978, Raqib et al., 2017). The morbidity and mortality of low birth weight (LBW) neonates are known to be high (Valero De et al., 2004, Yan et al., 2019), and urgently require researchers to investigate the immune function of LBW. Amarilyo et al. (2011) implied that IUGR infants with low immunity were at greater risk of infection. A study reported that impaired intestinal mucosal immune function in IUGR piglets is associated with a T lymphocyte subpopulation imbalance (Dong et al., 2015). The spleen is an important peripheral immune organ, which contains T cells and B cells to produce immune responses (Bronte and Pittet, 2013). Studies have shown that the pathogenesis of IUGR involved oxidative stress (Mert et al., 2012) and cell apoptosis (Wang et al., 2017, Zhang et al., 2020a), etc. However, the specific mechanism of spleen immune function impairment in newborns with IUGR remains to be further clarified.
A pathway, Nrf2, is that regulates oxidative stress in cells and can directly affect the oxidative stress level of the body. At present, it has been deemed that Nrf2 is a protective molecule in the pathological process of IUGR. Studies have shown that curcumin can improve the antioxidant capacity of IUGR piglets' liver by activating Nrf2 (Niu et al., 2019) and reduce jejunal damage in IUGR piglets (Yan et al., 2019). In addition, activation of Nrf2 signaling has a protective effect on immune suppression in the spleen (Wang et al., 2018). However, whether and how the Nrf2 signaling pathway is related to the immune function impairment in spleen of IUGR is unclear.
DHA is a kind of polyunsaturated fatty acid, which is mainly derived from deep-sea fish oil. DHA has immunomodulatory, antioxidant functions, inhibits apoptosis and others activities (Chaung et al., 2013, Sahin et al., 2012, Zhu et al., 2018). Therefore, an IUGR rat model was designed to elucidate the effects of DHA on IUGR rat pups. In this study, the cellular immune function of the spleen and oxidative stress markers, key proteins of apoptosis and key factors of Nrf2 in the spleen of an IUGR rat model were determined. Then, the protective mechanism of DHA on the immune function of the spleen of IUGR rats is discussed. We hope that this research will provide new insight into alleviating impairment of spleen immunity in IUGR.
2. Materials and methods
2.1. Experiment design
The experiment procedures were approved by the Animal Ethics Committee of Central South University, China (NO: 2019sydw0176), which meet the requirements of the Chinese Guidelines for Animal Experimental Protocol. Hunan SJA Laboratory Animal Co, Ltd (Changsha, China) provided the experimental Sprague-Dawley (SD) rats. And twelve female rats and six male rats were obtained from this company. The experimental rates were housed in an animal facility (24 ± 2 °C, with a 12-h/12-h light/dark cycle) in animal laboratory center, Central South University (Chang sha, China). The IUGR rats model was established. The date on the first day of pregnancy was recorded.
The control group (NBW group) received routine feed (21% protein) during pregnancy until natural delivery. The IUGR group was fed a 10% low-protein diet to establish the IUGR animal model during pregnancy and was fed until natural delivery. Each newborn rat was recorded, and then, they were weighed daily. Rats were fed a routine feed (21% protein) after delivery. Thirty-two newborn rats were randomly selected and allocated to NBW, IUGR, ND (NBW + DHA supplementation) and ID (IUGR + DHA supplementation) group. Each group included 6 rats (3 males and 3 females). Starting from the 7th day after birth, the rats in ND group and ID group were given DHA 300 mg/kg every day, and the rats in NBW group and IUGR group were given distilled water at the same volume until day 28. Samples were collected on the 28th day.
2.2. Sample collection
On the 28th day after delivery, blood was collected from rat pups by cardiac puncture using 10% chloral hydrate for anesthesia. When the blood was centrifuged, serum was gained and stored at −20 °C. Then, spleen tissue samples were quickly obtained in order to work for further analysis.
2.3. Flow cytometry
Fresh anticoagulant blood samples (2 ml) were taken and slowly added into a centrifuge tube containing mononuclear cell separation solution after constant volume dilution, centrifuged for 30 min to obtain cell precipitation. Cells were washed with PBS and collected. CD4 (eBioscience, 11–0040-82) and CD8 (eBioscience, 12-0084-82) antibodies were added and incubated at 4 °C in dark for 30 min. The flow cytometry (BZ-X800, Keyence, Japan) was used to detect cells.
2.4. Immunofluorescent double-labelled staining
Spleen tissue samples were placed in 10% formaldehyde solution and paraffin-embedded. Five successive slices of spleen tissue were selected and baked for 12 h, and then dehydrated. Antigen retrieval was performed and nonspecific binding sites were blocked. After washed, the experimental slides were incubated by using primary antibodies against CD4 (1:50, Invitrogen, MA5-17390) and CD8 (1:50, Abcam, ab237709) for 1 h. The antimouse IgG (H + L) (1:50, Proteintech, USA, SA00013-4) were used to incubate the antibodies. DAPI (Wellbio, Hunan, China, NO. AR1176) was used to counterstained the nuclei. A fluorescence microscope (BA410, Motic, China) was used to get images. CD4 and CD8 in spleen tissues were calculated and assessed.
2.5. Antioxidant index analysis in spleen
Spleen tissue samples at −80 °C were homogenized in a sodium chloride buffer in order to detect Malondialdehyde (MDA) and Total antioxidant capacity (T-AOC). The supernatant was centrifuged for 10 min in order to implement further determination. MDA assay kits (A003-1) and T-AOC assay kits (A015-2-1) were offered by Nanjing Jiancheng Bioengineering Institute (Nanjing, China). The detailed method for detecting MDA and T-AOC were implemented based on the manufacturer’s instructions.
2.6. Gene expression in spleen
IL-4, IFN-γ, GSH-PX and CAT were detected by qRT- PCR. Total RNA in spleen samples was extracted using Trizol (Thermo, USA). The absorbance was measured at 260 nm and 280 nm by uv spectrophotometer, and the concentration and purity were calculated. The PrimeScript RT Reagent Kit (CoWin, Beijing, China) was made use of transcribing RNA into cDNA based on the manufacturer’s instructions. SYBR (CoWin, Beijing, China, NO. CW2601) was used to conduct PCR using the green PCR core reagent in a RT PCR system. Amplified samples were added to the reaction with actin mRNA as an internal reference to regulate the expression of protein. The target genes can be calculated based on the 2−△△Ct method (Livak and Schmittgen, 2001). Table 1 was the gene-specific primers in this study.
Table 1.
Primer sequences and amplification lengths of the destination fragments.
| Genes | Primer sequences | Product lengths (bp) |
|---|---|---|
| Actin | For ACATCCGTAAAGACCTCTATGCC | 223 |
| Rev TACTCCTGCTTGCTGATCCAC | ||
| IL-4 | For CGTGATGTACCTCCGTGCTT | 183 |
| Rev GGACTGCAAGTATTTCCCTCGT | ||
| IFN-γ | For CAACCAGGCCATCAGCAAC | 227 |
| Rev CCCAGAATCAGCACCGACT | ||
| CAT | For ATAGCCAGAAGAGAAACCCACA | 108 |
| Rev CGCTGAACAAGAAAGTAACCTG | ||
| GSH-Px | For TCATTGAGAATGTCGCGTCCCT | 240 |
| Rev TCTCACCATTCACCTCGCACT |
2.7. Protein expression determined by western blotting
The protein Bcl-2, Bax, Nrf2 in spleen was detected by western blot analysis. Protein was extracted by homogenizing spleen tissue in ice-cold RIPA buffer (Merck Milli Wave, Darmstadt, Germany), and protein of spleen was obtained by using a BCA kit (Beyotime Institute of Biotechnology, Nantong, China), which was transferred to PVDF membranes after SDS-PAGE. The primary antibodies against Keap 1 (diluted 1:3000,Proteintech, USA), Bcl-2 (diluted 1:1000, Proteintech, USA), Bax (diluted 1:5000, Proteintech, USA) and β-actin (diluted 1:5000, Proteintech, USA) were used to incubate the membranes. After incubation, the membranes were cultured using antibodies (HRP goat anti-mouse IgG, Proteintech, 1:5000 dilution, No. SA00001-1) for 2 h. ECL Chemidoc XRS (Bio-Rad, Marnesla-Coquette, France) was used to detect the blots. Photographs of the membranes were quantitative analysis by using WB imaging system (JP-K300, Jiapeng Technology, China).
2.8. Statistical analysis
The experimental data was analyzed by SPSS 24.0 (Chicago, USA). The data was expressed as the mean ± standard deviation (mean ± SD), and one-way ANOVA was used for statistical comparisons. A P < 0.05 was identified as having statistical significance.
3. Results
3.1. DHA alleviates impairment of cellular immunity in spleen of IUGR rat pups (28 d)
3.1.1. CD4/CD8 ratio of T lymphocytes
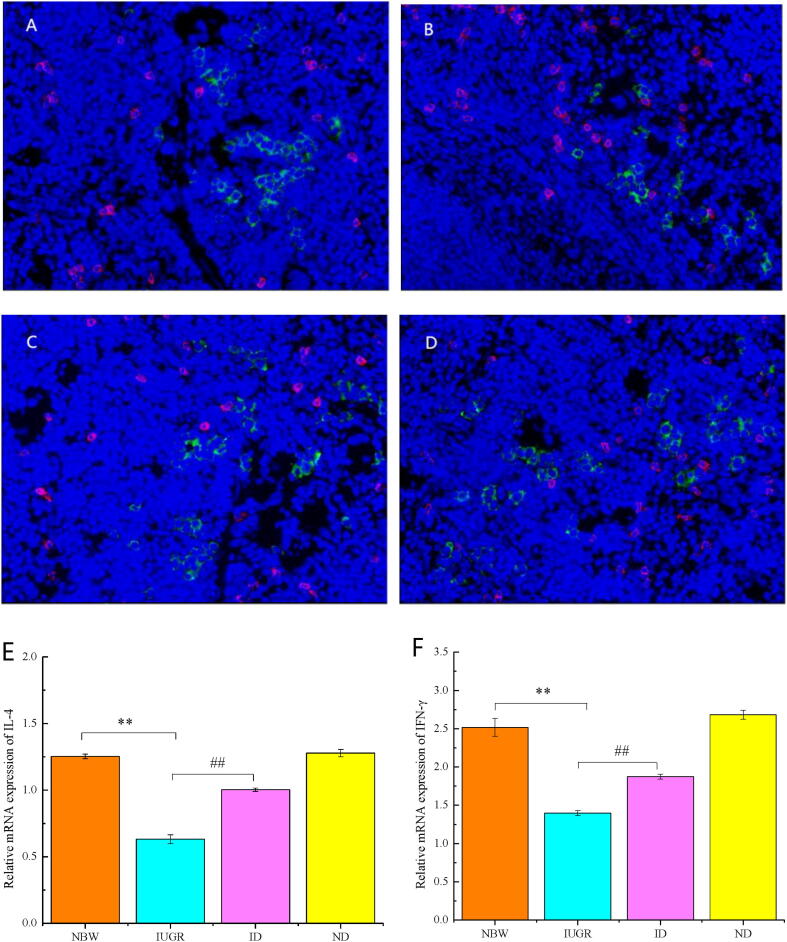
Table 2 shows that the CD4/CD8 ratios in blood and spleen decreased in IUGR (28 d) versus NBW (P < 0.01), and these ratios were obviously increased in ID compared with IUGR (P < 0.01). No significant differences have been detected between ND and NBW (P > 0.05).
Table 2.
DHA improved the CD4/CD8 ratio in IUGR rat pups (28 d).
| Items | Group |
SEM | P | |||
|---|---|---|---|---|---|---|
| NBW | IUGR | ID | ND | |||
| CD4:CD8 | ||||||
| Blood | 1.65 | 0.64* | 1.37# | 1.72 | 0.19 | 0.021 |
| Spleen | 1.91 | 0.71* | 1.65# | 1.95 | 0.56 | 0.023 |
*P < 0.05 represents a significant difference (P < 0.05) in IUGR versus NBW, and #P < 0.05 represents a significant difference (P < 0.05) in ID versus IUGR.
3.1.2. Effect of DHA on gene expression
Fig. 1 (E-F) showed that IL-4 and IFN-γ obviously decreased (P < 0.01) in IUGR (28 d), but greatly increased (P < 0.01) in ID. No significant differences have been detected between ND and NBW (P > 0.05).
Fig. 1.
CD4 and CD8 in spleen of IUGR rat pups (28 d) in four groups: NBW (A), IUGR (B), ID (C) and ND (D). CD4 is shown as green fluorescence, and CD8 is shown as red fluorescence. IL-4 (E) and IFN-γ (F) in IUGR spleens (28 d) was showed. ** represents a significance difference (P < 0.01) versus NBW. ## represents a significance difference (P < 0.01) versus IUGR.
3.2. DHA alleviates oxidative stress in IUGR rat pups
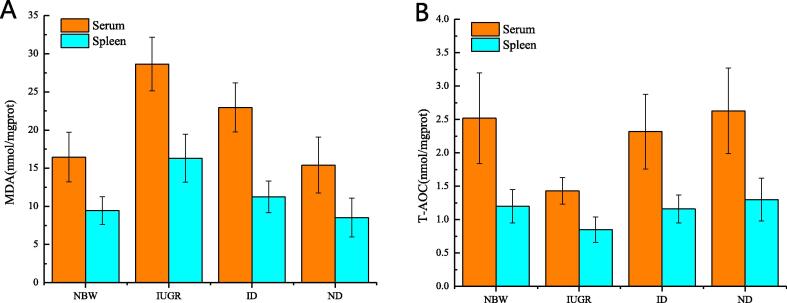
The expression of MDA and T-AOC was investigated so as to detect the oxidation in serum and spleen. In Fig. 2(A-B), Compared with NBW group, MDA was greatly increased (P < 0.01) (Fig. 2A), while T-AOC was decreased in serum and spleen of IUGR group (P < 0.01) (Fig. 2B). MDA was obviously decreased (P < 0.01) while T-AOC was greatly increased (P < 0.01) in the ID versus IUGR. No significant differences have been detected between ND and NBW (P > 0.05).
Fig. 2.
DHA-attenuated oxidative stress in IUGR rat pups (28 d). (A) MDA and (B) T-AOC.
3.3. DHA activates the Nrf2 signaling pathway in IUGR rat pups in spleen (28 d)
The proteinsNrf2 were obviously decreased in IUGR versus NBW (28 d) (P < 0.01), and they were obvious increase (P < 0.01) in ID versus IUGR (Fig. 3B). No significant differences have been detected between ND and the NBW (P > 0.05).
Fig. 3.
(A) Representative images of Nrf2 and Keap 1; Protein expression of Nrf2 (B) in spleen; (C) and (D) was the gene expression of GSH-Px and CAT. **P denotes an obvious difference versus NBW. ## denotes an obvious difference versus IUGR (28 d).
The Nrf2 downstream target genes GSH-PX and CAT were obviously reduced (P < 0.01) in IUGR versus NBW (28 d) (Fig. 3D-E). GSH-PX and CAT were greatly increased (P < 0.01) in ID versus IUGR. No significant differences have been detected between ND and NBW (P > 0.05).
3.4. DHA attenuates IUGR apoptosis in the spleen
The protein expression of Bax significantly increased (28 d) (P < 0.01), while Bcl2 greatly reduced in IUGR versus NBW (P < 0.01) (Fig. 4B-C). The protein Bax greatly reduced in ID versus IUGR (P < 0.01), while Bcl2 greatly increased in ID (P < 0.01). No significant differences have been detected between ND and NBW (P > 0.05).
Fig. 4.
Effects of DHA on the apoptosis pathways in spleen mitochondria in IUGR rat pups. (A) Representative images of Bax and Bcl-2; The protein expression of Bax (B) and Bcl-2(C). **P < 0.01 denotes a significant difference versus the NBW. ## denotes a significant difference versus IUGR (28 d).
4. Discussion
This study revealed that DHA alleviates impairment of spleen cellular immunity by activating Nrf2 signaling pathway in IUGR rat pups. The detailed findings were as follows: (1) DHA alleviated cellular immune impairment of rat pups by improving the serum and spleen CD4/CD8 ratios and IL-4 and IFN-γ mRNA expression in the spleen; (2) DHA alleviated oxidative stress in IUGR rat pups. Specifically, DHA promoted the expression of T-AOC and reduced the expression of MDA in ID group. (3) DHA attenuated apoptosis through activating of Nrf2 and downstream target genes (GSH-PX and CAT). These findings may provide substantial evidence that indicates that DHA can alleviate the impairment of spleen cellular immunity in IUGR rat pups, which may be relevant to activate Nrf2 signaling.
The spleen contains a large number of lymphocytes, of which T-lymphocytes account for 35%-50% and are involved in the cellular immune response (Tarantino et al., 2011). Different helper T lymphocyte (Th) cell subsets play different functions in the immune response (Mukhopadhyay et al., 2014, Zhu and Paul, 2008). CD4 is the main surface marker of Th and participates in killing, clearing, secretion of cytokines and other functions. CD8 is the surface marker of CTLs, and these cells have a negative regulatory effect on humoral and cellular immunity while inhibiting the immune regulatory function of other immune cells (Huang and August 2015). The ratio of CD4+/CD8 + can reflect the immune state of body, and a decrease in this ratio indicates that the immune function of the body is inhibited (Zhu and Paul, 2008). The key cytokines IL-4 and IFN-γ regulate the immune response. Dong et al. (2015) found that when the serum IL-2 and IL-10 levels were low in IUGR piglets, the percentage of CD8 was increased in blood and spleen, and intestinal mucosal immunity related to the imbalance of T lymphocyte subsets was reduced. Sorensen et al. (2009) found that immunomodulatory nutrition (IMN) increased CD4/CD8 ratio with perioperative cancer patients and alleviated immunosuppression in these patients. Talvas et al. (2015) found that immune nutrients containing DHA could enhance cellular immune function and antioxidant defense in cancer patients. Our study showed that DHA can increase the serum and spleen CD4/CD8 ratio of IUGR offspring rats, increase the spleen cytokines IL-4 and IFN-γ, and enhance cellular immune function, suggesting that DHA can effectively relieve the immune impairment of IUGR rat pups, which is consistent with previous studies. Thus, further discussion will be carried out to analysis the effects of DHA on oxidation and apoptosis in spleen of IUGR rat pups.
DHA is a polyunsaturated fatty acid, and is susceptible to oxidation (Song et al., 2000). Previous studies have found that dietary DHA regulates immunity, thereby improving abnormal immune function. DHA supplementation can reduce the incidence of the cold and affect the duration of disease symptoms for children (Imhoff-Kunsch et al., 2014). It revealed that DHA protected cells away from oxidative stress (Che et al., 2018, Molinar-Toribio et al., 2015, Tatsumi et al., 2020). Additionally, DHA can activate the Nrf2 pathway and has an antiapoptotic effect (Clementi et al., 2019, Zhu et al., 2018). Saw et al. (2013) showed that DHA can induce antioxidant ability through Nrf2 in HepG2-C8 cells. Geng et al. (2018) found that DHA significantly increased the GSH-PX and CAT in breast cancer tissues and reduced the MDA concentration. Appropriate supplementation of DHA in the diet increases the activities of SOD and CAT (Yu et al., 2016). In this study, IUGR rats added DHA have obviously decreased expression level of MDA and increased expression levels of T-AOC in the spleen of IUGR rat pups, which indicated that DHA inhibited oxidative stress in spleen of IUGR rat pups. Additionally, DHA increased GSH-PX and CAT in spleen of ID rat pups compared with IUGR rats (P < 0.05), indicating that DHA inhibited oxidative stress in the spleen of IUGR rat pups, which is similar to previous research results.
Oxidative stress mediates apoptosis through death receptors, endoplasmic reticulum stress, mitochondria and other pathways and may also induce apoptosis through activation the pathways, such as mitogen-activated protein kinase, and caspases. Oxidative stress can produce some oxidative intermediates, such as MDA and T-AOC. MDA is an effective indicator that reflects the levels of oxidative damage, as well as the production of oxygen free radicals (Song et al., 2000). T-AOC is an important index that reflects the antioxidant capacity, and reflects free radicals in body. Therefore, the levels of MDA and T-AOC were used to reflect the oxidative damage and antioxidant capacity of the body. Su et al. (2017) found that the IUGR piglet jejunum had an increased MDA concentration and apoptosis index compared to the normal one. Zhang et al. (2020) found that the increase in hepatocyte apoptosis in IUGR piglets related to the accumulation of oxidative damage products. In the current study, similar to previous results, apoptosis increased in the spleen of IUGR rat pups, and DHA significantly reduced the apoptotic gene Bax protein and increased the antiapoptotic gene Bcl2 protein in the spleen of IUGR rat pups (P < 0.01), indicating that DHA has an negative effect on apoptosis in spleen of IUGR rat pups.
To further explore the spleen immune regulation mechanism of DHA in IUGR rat pups, the Nrf2 signaling pathway was measured. Nrf2 is an important transcription factor that regulates cell’s redox state (Motohashi and Yamamoto, 2004, Yu et al., 2019). Nrf2 not only mediates antioxidant stress but also effectively improves immune suppression (Farombi et al., 2008, Kaspar et al., 2009). In this study, DHA reduced MDA and increased Nrf2 expression, suggesting that DHA activates Nrf2 to add the antioxidant capacity of MDA and enhance immune function. Additionally, the Nrf2 signaling pathway is closely related to apoptosis. In this study, DHA promoted protein expression of Nrf2 in IUGR rat pups, indicating Nrf2 was activated. DHA enhanced the expression of Nrf2 and downstream genes (GSH-PX and CAT), indicating that DHA improves antioxidant capacity in spleen through Nrf2 signaling pathway. Nevertheless, the limitations of our study lie in the lack of Nrf2 inhibitor experiments. The mechanism of Nrf2 pathway in spleen in IUGR rat pups still requires further study. And clinical applications of DHA will have a further work.
5. Conclusion
In summary, this study verified that IUGR impaired cellular immune function and antioxidant capacity of the spleen in rat pups and increased apoptosis in the spleen. Dietary supplementation with DHA effectively improved cellular immune function, enhanced Nrf2 and the antioxidant enzyme GSH-PX in the IUGR spleen of rat pups, and inhibited apoptosis in the spleen. Our findings provide a promising strategy for the early intervention of IUGR children with nutritional therapy.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
This study was partially funded by Hunan Provincial Natural Science Foundation (NO. 2020JJ4781). We thank the help from stuff in the neonatology department of Second Xiangya hospital.
Footnotes
Peer review under responsibility of King Saud University.
References
- Amarilyo G., Oren A., Mimouni F.B., Ochshorn Y., Deutsch V., Mandel D. Increased cord serum inflammatory markers in small-for-gestational-age neonates. J. Perinatol. 2011;31:30–32. doi: 10.1038/jp.2010.53. [DOI] [PubMed] [Google Scholar]
- Black R.E., Allen L.H., Bhutta Z.A., Bhutta Z.A., Caulfield C., Onis M., Mathers C., Rivera J. Maternal and child undernutrition: global and regional exposures and health consequences. Lancet. 2008;371(9608):243–260. doi: 10.1016/S0140-6736(07)61690-0. [DOI] [PubMed] [Google Scholar]
- Bronte V., Pittet M.J. The spleen in local and systemic regulation of immunity. Immunity. 2013;39(14):806–818. doi: 10.1016/j.immuni.2013.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaung H., Chang C., Chen P., Chang C., Liu S., Chen C. Docosahexaenoicacid and phosphatidylserine improves the antioxidant activities in vitro and in vivo and cognitive functions of the developing brain. Food Chem. 2013;138:342–347. doi: 10.1016/j.foodchem.2012.10.082. [DOI] [PubMed] [Google Scholar]
- Che H.X., Fu X.Y., Zhang L.Y., Gao X., Wen M., Du L., Xue C.H., Xu J., Wang Y.M. Neuroprotective effects of n-3 polyunsaturated fatty acid-enriched phosphatidylserine against oxidative damage in PC12 cells. Cell. Mol. Neurobiol. 2018;38(3):657–668. doi: 10.1007/s10571-017-0516-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clementi M.E., Lazzarino G., Sampaolese B., Brancato A., Tringali G. DHA protects PC12 cells against oxidative stress and apoptotic signals through the activation of the NFE2L2/HO-1 axis. Int. J. Mol. Med. 2019;43(6):2523–2531. doi: 10.3892/ijmm.2019.4170. [DOI] [PubMed] [Google Scholar]
- Dong L., Zhong X., Zhang L.L., Kong L.R., Kong Y.L., Kou T., Wang T. Impaired intestinal mucosal immunity is associated with the imbalance of T lymphocyte sub-populations in intrauterine growth-restricted neonatal piglets. Immunobiology. 2015;220:775–781. doi: 10.1016/j.imbio.2014.12.017. [DOI] [PubMed] [Google Scholar]
- Farombi E.O., Shrotriya S., Na H.K., Kim S.H., Surh Y.J. Curcumin attenuates dimethylnitrosamine-induced liver injury in rats through Nrf2-mediated induction of heme oxygenase-1. Food Chem. Toxicol. 2008;46:1279–1287. doi: 10.1016/j.fct.2007.09.095. [DOI] [PubMed] [Google Scholar]
- Ferguson A.C. Prolonged impairment of cellular immunity in children with intrauterine growth retardation. J. Pediatr. 1978;93(1):52–56. doi: 10.1016/s0022-3476(78)80599-x. [DOI] [PubMed] [Google Scholar]
- Geng L., Zhou W., Liu B., Wang X., Chen B. DHA induces apoptosis of human malignant breast cancer tissues by the TLR-4/PPAR-α pathways. Oncol Lett. 2018;15:2967–2977. doi: 10.3892/ol.2017.7702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang W., August A. The signaling symphony: T cell receptor tunes cytokine-mediated T cell differentiation. J. Leukoc. Biol. 2015;97(3):477–485. doi: 10.1189/jlb.1RI0614-293R. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imhoff-Kunsch B., Stein A.D., Martorell R., Parra-Cabrera S., Romieu I., Ramakrishnan U. Prenatal docosahexaenoic acid supplementation and infant morbidity: Randomized controlled trial. Pediatrics. 2014;7:14. doi: 10.1542/peds.2010-1386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaspar J.W., Niture S.K., Jaiswal A.K. Nrf 2: INrf2 (Keap1) signaling in oxidative stress. Free Radical Biol. Med. 2009;47(9):1304–1309. doi: 10.1016/j.freeradbiomed.2009.07.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Livak K.J., Schmittgen T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta CT) method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- Mert I., Oruc A.S., Yuksel S., Cakar E.S., Buyukkagnici U., Karaer A., Danisman N. Role of oxidative stress in preeclampsia and intrauterine growth restriction. J. Obstet. Gynaecol. Res. 2012;38:658–664. doi: 10.1111/j.1447-0756.2011.01771.x. [DOI] [PubMed] [Google Scholar]
- Molinar-Toribio, Perez-Jimenez J., Ramos-Romero S., Romeu M., Giralt M., Taltavull N., Munoz-Cortes M., Jauregui O., Mendez L., Medina I., Torres J.L. Effect of n-3 PUFA supplementation at different EPA: DHA ratios on the spontaneously hypertensive obese rat model of the metabolic syndrome. Br. J. Nutr. 2015;113(6):878–887. doi: 10.1017/S0007114514004437. [DOI] [PubMed] [Google Scholar]
- Motohashi H., Yamamoto M. Nrf2-Keap1 defines a physiologically important stress response mechanism. Trends Mol. Med. 2004;10:54–557. doi: 10.1016/j.molmed.2004.09.003. [DOI] [PubMed] [Google Scholar]
- Mukhopadhyay D., Weaver L., Tobin R., Henderson S., Beeram M., Newell-Rogers M.K., Perger L. Intrauterine growth restriction and prematurity influence regulatory T cell development in newborns. J. Pediatr. Surg. 2014;49(5):727–732. doi: 10.1016/j.jpedsurg.2014.02.055. [DOI] [PubMed] [Google Scholar]
- Niu Y., He J., Ahmad H., Shen M., Zhao Y., Gan Z., Zhang L., Zhong X., Wang C., Wang T. Dietary curcumin supplementation increases antioxidant capacity, upregulates Nrf2 and Hmox1 levels in the liver of piglet model with intrauterine growth retardation. Nutrients. 2019;11:2978. doi: 10.3390/nu11122978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raqib R., Alam D.S., Sarker P., Ahmad S.M., Ara G., Yunus M., Moore S.E., Fuchs G. Low birth weight is associated with altered immune function in rural Bangladeshi children: A birth cohort study. Am. J. Clin. Nutr. 2017;3:845–852. doi: 10.1093/ajcn/85.3.845. [DOI] [PubMed] [Google Scholar]
- Sahin N., Akdemir F., Orhan C., Aslan A., Agca C., Gencoglu H., Ulas M., Tuzcu M., Viyaja J., James R. A novel nutritional supplement containing chromium picolinate, phosphatidylserine, docosahexaenoic acid, and boron activates the antioxidant pathway Nrf2/HO-1 and protects the brain against oxidative stress in high-fat-fed rats. Nutr. Neurosci. 2012;15(5):42–47. doi: 10.1179/1476830512Y.0000000018. [DOI] [PubMed] [Google Scholar]
- Saw C.L., Yang A.Y., Guo Y., Kong A.N. Astaxanthin and omega-3 fatty acids individually and in combination protect against oxidative stress via the Nrf2-ARE pathway. Food Chem. Toxicol. 2013;62:869–875. doi: 10.1016/j.fct.2013.10.023. [DOI] [PubMed] [Google Scholar]
- Song J.H., Fujimoto K., Miyazawa T. Polyunsaturated (n-3) fatty acids susceptible to peroxidation are increased in plasma and tissue lipids of rats fed docosahexaenoic acid-containing oils. J. Nutr. 2000;130(12):3028–3033. doi: 10.1093/jn/130.12.3028. [DOI] [PubMed] [Google Scholar]
- Sorensen D., McCarthy M., Baumgartner B., Demars S. Perioperative immunonutrition in head and neck cancer. Laryngoscope. 2009;119:1358–1364. doi: 10.1002/lary.20494. [DOI] [PubMed] [Google Scholar]
- Su W., Zhang H., Ying Z. Effects of dietary L-methionine supplementation on intestinal intestinal integrity and oxidative status in intrauterine growth-retarded weanling piglets intrauterine. Eur. J. Nutr. 2017;9:21. doi: 10.1007/s00394-017-1539-3. [DOI] [PubMed] [Google Scholar]
- Talvas J., Garrait G., Goncalves-Mendes N., Rouanet J., Vergnaud-Gauduchon J., Kwiatkowski F., Bachmann P., Bouteloup C., Bienvenu J., Vasson M.P. Immunonutrition stimulates immune functions and antioxidant defense capacities of leukocytes in radiochemotherapy-treated head & neck and esophageal cancer patients: A double-blind randomized clinical trial. Clin Nutr. 2015;34(5):810–817. doi: 10.1016/j.clnu.2014.12.002. [DOI] [PubMed] [Google Scholar]
- Tarantino G., Savastano S., Capone D., Colao A. Spleen: A new role for an old player? World J. Gastroenterol. 2011;17:3776–3784. doi: 10.3748/wjg.v17.i33.3776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tatsumi Y., Kato A., Banno T., Niimi N., Sango K., Himeno T., Kondo M., Tsunekawa S., Kato Y., Kamiya H., Nakamura J., Kato K. Docosahexaenoic acid attenuates oxidative stress-induced autophagy and cell death in immortalized adult rat Schwann (IFRS1) cells. Diabetes. 2020;69(supp1):551. [Google Scholar]
- Valero De B.J., Soriano T., Albaladejo R., Juarranz M., Calle M.E., Martinez D., Domınguez-Rojas V. Risk factors for low birth weight: A review. Eur. J. Obstet. Gynecol. Reprod. Biol. 2004;116:3–15. doi: 10.1016/j.ejogrb.2004.03.007. [DOI] [PubMed] [Google Scholar]
- Wang C., Zhang R., Zhou L., He J., Huang Q., Siyal F.A., Zhang L. Intrauterine growth retardation promotes fetal intestinal autophagy in rats via the mechanistic target of rapamycin pathway. J Reprod Dev. 2017;63(6):547–554. doi: 10.1262/jrd.2017-050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X., Wang Z., Wu H., Jia W., Teng L., Song J., Yang X., Wang D. Sarcodon imbricatus polysaccharides protect against cyclophosphamide-induced immunosuppression via regulating Nrf2-mediated oxidative stress. Int. J. Biol. Macromol. 2018;120:736–744. doi: 10.1016/j.ijbiomac.2018.08.157. [DOI] [PubMed] [Google Scholar]
- Yan E., Zhang J., Han H., Wu J., Gan Z., Wei C., Zhang L., Wang C., Wang T. Curcumin alleviates IUGR jejunum damage by increasing antioxidant capacity through Nrf2/Keap1 pathway in growing pigs. Animals. 2019;10(1):41. doi: 10.3390/ani10010041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu H., Gao Q., Dong S., Zhou J., Ye Z., Lan Y. Effects of dietary n-3 highly unsaturated fatty acids (HUFAs) on growth, fatty acid profiles, antioxidant capacity and immunity of sea cucumber Apostichopus japonicas (Selenka) Fish Shellfish Immunol. 2016;54:211–219. doi: 10.1016/j.fsi.2016.04.013. [DOI] [PubMed] [Google Scholar]
- Yu H., Zhang J., Ji Q., Yu K., Wang P., Song M., Cao Z., Zhang X., Li Y. Melatonin alleviates aluminium chloride-induced immunotoxicity by inhibiting oxidative stress and apoptosis associated with the activation of Nrf2 signaling pathway. Ecotoxicol. Environ. Saf. 2019;173:131–141. doi: 10.1016/j.ecoenv.2019.01.095. [DOI] [PubMed] [Google Scholar]
- Zhang H., Chen Y., Chen Y. Pterostilbene attenuates liver injury and oxidative stress in intrauterine growth-retarded weanling piglets. Nutrition. 2020;81 doi: 10.1016/j.nut.2020.110940. [DOI] [PubMed] [Google Scholar]
- Zhang H., Fan Y., Elsabagh M., Guo S., Wang M., Jiang H. Dietary supplementation of L-Arginine and N-Carbamylglutamate attenuated the hepatic inflammatory response and apoptosis in suckling lambs with intrauterine growth retardation. Mediators Inflamm. 2020;1:1–10. doi: 10.1155/2020/2453537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu J., Paul W.E. CD4 T cells: Fates, functions, and faults. Blood. 2008;112:1557–1569. doi: 10.1182/blood-2008-05-078154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu W., Ding Y., Kong W., Li T., Chen H. Docosahexaenoic acid (DHA) provides neuroprotection in traumatic brain injury models via activating Nrf2-ARE signaling. Inflammation. 2018;41:1182–1193. doi: 10.1007/s10753-018-0765-z. [DOI] [PubMed] [Google Scholar]