Abstract
Purpose:
Although exercise medicine is recommended to counter treatment-related side-effects and improve health-related outcomes of patients affected by different cancers, no specific recommendations exist for patients with melanoma. As a result, we systematically examined the current evidence regarding the effects of physical activity and exercise on objectively-measured and patient-reported outcomes among patients with melanoma.
Methods:
Searches were conducted in PubMed, CINAHL, EMBASE, SPORTDiscus, and Web of Science databases. This review included published data involving physical activity or exercise and objectively-measured or patient-reported outcomes of patients with cutaneous melanoma. The quality of included studies was assessed using the McMaster University Critical Appraisal Tool for Quantitative Studies.
Results:
Six studies including 882 patients with melanoma were included. Studies presented heterogeneity of design with 2 cross-sectional surveys, 2 retrospective analyses, and 2 non-randomized intervention trials. No statistically significant change in quality of life, fatigue, physical function, cardiorespiratory fitness, body composition, psychological distress, cognitive function, or treatment-related side-effects were attributable to physical activity or exercise. Importantly, physical activity or exercise during melanoma treatment or into survivorship did not adversely impact patients/survivors.
Conclusion:
In summary, physical activity or exercise did not adversely impact quality of life, objectively-measured or patient-reported outcomes in patients with melanoma. In addition, there is a paucity of quality studies examining the effects of physical activity or exercise on patients with melanoma throughout the cancer care continuum.
Keywords: melanoma, skin cancer, physical activity, exercise, quality of life, health-related outcomes
Introduction
The worldwide incidence of melanoma has increased over the past few decades and in 2020 approximately 325 000 new cases and more than 57 000 deaths were observed.1 In Australia, cutaneous melanoma is the second and third most commonly diagnosed cancer among men and women, respectively.2 Moreover, despite a lower incidence globally compared to other solid tumors, melanoma has a high incidence among adolescents and younger adults.1 Most patients with melanoma are treated with surgery alone, whereas radiotherapy, and systemic treatment with targeted or immunotherapy, are utilized for patients with more advanced disease.3
Novel systemic therapies such as targeted therapy with BRAF inhibitors and immune checkpoint inhibitors have demonstrated a survival advantage in patients with advanced melanoma.4,5 However, these treatments are not without risk and adverse effects; dermatological, gastrointestinal, hepatic, and endocrine toxicities are frequently experienced by patients.5-7 Immune-related adverse events (irAEs) resulting from treatment with immune checkpoint inhibitors are distinctly different from the classical chemotherapy-related toxicities. However, several symptoms such as fatigue and weakness, diarrhea, arthralgia, and reductions in muscle mass are common, substantially affecting quality of life (QoL) and wellbeing of the patients during and potentially following the completion of treatment.6,8,9 Furthermore, patients with cancer also experience substantial reductions in muscle mass (ie, sarcopenia) as a result of aging and physical inactivity and this has been shown to be associated with poorer cancer treatment outcomes.8
Physical activity (eg, any bodily movement produced by skeletal muscles) and exercise (eg, physical activity that is planned, structured, repetitive, and purposive) have been suggested to mitigate and counteract primary treatment side-effects10; and have been endorsed by many professional organizations such as the American College of Sports Medicine,11-14 American Cancer Society,15,16 Spanish Society of Medical Oncology,17 and Exercise and Sports Science Australia.18,19 Existing guidelines cover exercise recommendations for various types of cancer (eg, lung, prostate, breast, lymphoma) and treatment phases (pre-treatment, treatment, survivorship, and palliation). However, a substantial gap exists for patients with melanoma. For example, it remains unclear if exercising pre- or post- melanoma diagnosis is associated with any survival benefit,20 although it has been shown that these patients are presenting with significant reductions in physical activity levels post treatment.21 The reduction in physical activity, especially in older adults, could increase the risk of sarcopenia as well as cardiovascular and metabolic diseases.22,23 As commonly observed in the aforementioned cancers, it may be that among patients with melanoma, exercise promotes significant benefits by reducing treatment-related side-effects and enhancing QoL through the course of treatment and beyond.
Given the paucity of information regarding physical activity and exercise in patients with melanoma, the present study aims to systematically review and examine the effects of physical activity and exercise on QoL as the primary outcome, and other objectively-measured (body composition, cardiorespiratory fitness, and physical function), and patient-reported (fatigue, treatment-related side-effects, cognitive function, and psychological distress) outcomes among patients with melanoma.
Methods
Study Selection Procedure
The study was undertaken in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) statement,24,25 and the method used was based on the minimum criteria established by the Cochrane Back Review Group (CBRG).26 This systematic review was not registered in any prospectively systematic review database (eg, PROSPERO).
This review included studies reporting on the impact of physical activity or exercise interventions on quality of life, or other objectively-measured and patient-reported outcomes in adult patients with cutaneous melanoma. The primary aim of this review was to examine the relationship between physical activity or exercise and QoL. The secondary aims were to evaluate the relationship between physical activity or exercise and other objectively-measured (body composition, and physical function) and patient-reported (fatigue, treatment-related side-effects, cognitive function, and psychological distress) outcomes. Studies were excluded if: (1) they involved mixed cancer patients without specific information on results from patients with melanoma; (2) they did not include or report the specific outcomes included in this review; (3) they involved pediatric patients with melanoma, and (4) were written in a language other than English. Eligibility was assessed and independently evaluated in duplicate, with differences resolved by consensus between the 2 reviewers (BC and PL), and in case of disagreement, a third reviewer (FS) was consulted.
The search was conducted up to February 2021 using the following electronic databases: PubMed, CINAHL, EMBASE, SPORTDiscus, and Web of Science. The terms used were: “Melanoma,” “Physical Activity,” and “Exercise” in association with a list of sensitive terms to search for experimental studies. We also performed a manual search of the reference lists provided in the selected papers. In addition, 2 melanoma-specific systematic reviews27,28 that did not specifically focus on patient outcomes were assessed to detect studies potentially eligible for inclusion. The search strategy used is presented in the Supplemental Digital Content Appendix 1.
Data Extraction
Titles and abstracts of all articles identified through the search strategy were independently evaluated by 2 reviewers (BC and PL). Abstracts that did not provide sufficient information regarding the inclusion criteria were selected for full-text evaluation. In the second phase, the same 2 reviewers independently evaluated these full-text articles and selected them in accordance with the eligibility criteria. Disagreements between reviewers were resolved by consensus, with a third reviewer (FS) consulted when necessary. The data extraction was performed via a standardized form. Descriptive characteristics such as cancer type, participant and treatment characteristics, and study outcomes were collected.
Quality Assessment
The quality of all included studies was assessed using the McMaster University Critical Appraisal Tool for Quantitative Studies.29 This tool includes 16 questions and covers the following components: study purpose (Was the purpose clearly stated?), literature (Was relevant background literature reviewed?), design (Is the study design appropriate to study aims?, No biases present?), sample (Was the sample described in detail?, Was sample size justified?), outcomes (Were the outcome measures reliable?, Were the outcome measures valid?), intervention (Intervention was described in detail?, Contamination was avoided?, Co-intervention was avoided?), results (Results were reported in terms of statistical significance?, Were the analysis method(s) appropriate?, Clinical importance was reported?, Drop-outs were reported?), and conclusion and implications (Conclusions were appropriate given study methods and results). Each question was rated and scored as 1 for yes, 0 for no, and NA for not applicable based on each study. A sum score was calculated for each study with higher scores indicating higher methodological quality. The risk of bias assessment for all included studies was performed independently by 2 reviewers (BC and PL). Any disagreements between reviewers were resolved by consensus through discussion with a third reviewer (FS).
Data Synthesis
A narrative/qualitative synthesis was the preferred method to provide an overview of the current literature on the topic. Given the limited number of eligible trials, heterogeneity of study designs, outcomes, and measurement tools, a meta-analysis was not undertaken. Information extracted from studies included participant demographics, health history, melanoma stage, treatment type, exercise behavior/physical activity levels, and exercise intervention information (frequency, intensity, time, type), as well as information on quality of life, fatigue, body composition/weight, physical function, treatment-related side-effects, psychological distress, cognitive function, and lymphoedema. When available, descriptive data as mean, median, and dispersion values (eg, standard deviation, 95% confidence intervals [95% CI]) from studies were reported.
Results
Study Selection
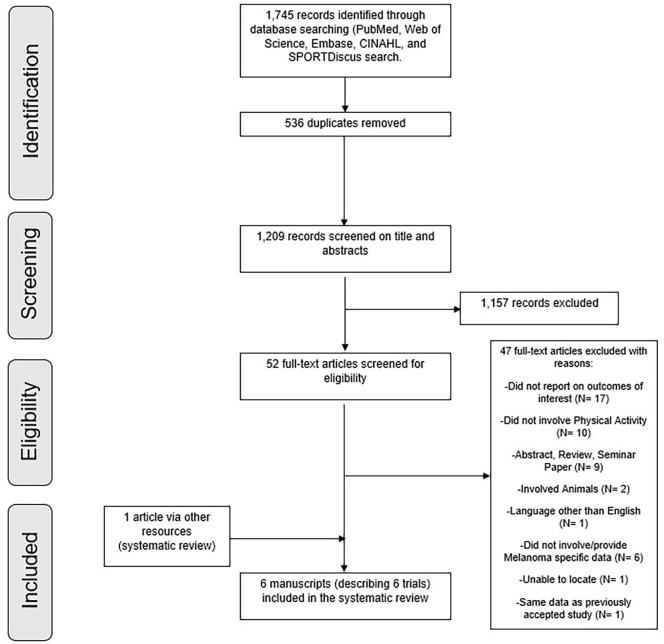
Of the 1745 retrieved studies based on the search strategy, 1209 were retained for screening after duplicate removals. Upon evaluation of titles and abstracts, 1157 articles were excluded due to their irrelevance for the research question and the remaining 52 articles were retrieved in full text for further examination. After a comprehensive assessment, 5 articles30-34 met the criteria to be included in this systematic review. In addition, 1 study35 met the inclusion criteria and was included based on the manual search. Detailed information regarding the process of study selection is shown in Figure 1.
Figure 1.
Flow chart of studies included.
Study Characteristics
A total of 882 patients with melanoma, aged 20 to 85 years, participated in the 6 included studies.30-35 There was considerable heterogeneity regarding cancer characteristics and timing of assessment, with cancer staging ranging from stage II to IV. In addition, the timing of study assessment was during treatment,32-34 post-treatment,32,35 and 1 to 10 years following diagnosis30,31 (Table 1). A variety of treatments were reported including surgery (3 of 6, 50%),30,31,35 immunotherapy (2 of 6, 33.3%),32,33 and interferon-alpha (IFN-α) (1 of 6, 16.6%).34 Of the 6 studies included in this review, 2 were cross-sectional surveys,30,32 2 were retrospective analyses,31,35 and 2 were non-randomized intervention trials.33,34. Regarding the primary outcome of this review, 3 studies included QoL assessment (50%),30,31,33 while the remaining assessed treatment-related side-effects (eg, lymphoedema) (3 of 6, 50%)31,33,35 fatigue (3 of 6, 50%),32-34 body composition (2 of 6, 33.3%),33,34 physical function (2 of 6, 33.3%),33,34 psychological distress (1 of 6, 16.6%),33 and cognitive function (1 of 6, 16.6%).34
Table 1.
Descriptive Study Data.
| Design | Study | N | Age | % of males | Disease stage | Treatment type | Timing | Behavior/intervention | Assessment tool/s | Results |
|---|---|---|---|---|---|---|---|---|---|---|
| Interventional study | Schwartz et al34 | 12* | 44 y (20-64 y) | NR | 16% stage II, 84% stage III | 67% = 20 mg sustained-release methylphenidate, 33% not recorded | During treatment | F- 4 sessions p/w for 4 mo | Schwartz cancer fatigue scale | ↔ Fatigue# |
| I- intensity limited by symptoms | 12-min walk test | ↔ Functional ability | ||||||||
| T- 15-30 min per session | NR | ↔ Body composition | ||||||||
| T- Aerobic exercise | Trail maker form A and B | ↔ Cognitive function | ||||||||
| Retrospective analysis | Hinrichs et al35 | 14 | 53 y (38-84 y) | 21 | NR | Post-surgery (multimodal therapy) | Post groin surgery | F- 2-7 d p/w | Limb circumference | ↑ (60%) limb volumes# |
| I- NR | ||||||||||
| T- 10-60 min | ||||||||||
| T- bandage exercises/self-practice physical activity | ||||||||||
| Cross-sectional survey | Blanchard et al30 | 761 | 60.2 ± 13.4 y | 50 | 93.4% Localized, 5.5% Regional, 1.1% Distant | 95.9% surgery, 15.8% chemotherapy, 9.5% radiation therapy, 6.4% hormone therapy, 6.8% IFN-α/ IL-2, 11.3% bone marrow transplant | 2, 5 and 10 y post diagnosis | Classed as physically active if >150 min of moderate or >60 min of strenuous physical activity p/w | RAND-36 health status inventory score | ↑ QoL# |
| Retrospective analysis | Carmeli and Bartoletti31 | 12 | 58 ± 7.4 y | 33 | Stage I-III | Post-surgery (multimodal therapy) | 1-4 y post-diagnosis | F- 2-7 d p/w | IDI-ILA part II | ↑ (NR) QoL# |
| I- NR | Limb circumference | ↑ (22%) limb volumes | ||||||||
| T- 10-60 min | ||||||||||
| T- bandage exercises/self-practice physical activity | ||||||||||
| Cross-sectional survey | Hyatt et al32 | 55 | 54.0 ± 10.0 y | 36 | 16% stage III, 84% stage IV | 78% currently receiving immunotherapy (72% single-agent, 26% combination, 2% unknown | During or post treatment | Classed as physically active if >150 min of moderate or >75 min of strenuous aerobic activity p/w + 2-3 resistance exercise sessions p/w | PROMIS SF7a. | ↔ Fatigue# |
| Open ended questions. | ↔ Self-reported perspectives | |||||||||
| Interventional study | Lacey et al33 | 28 | 66 y (42-85 y) | 57 | Stage IV | Pembrolizumab 2 mg/kg delivered at 3-weekly intervals | During treatment | F- 2 sessions p/w for 8-wk | FACT-G | ↔ QoL# |
| I- NR | FACT-G | ↔ Fatigue | ||||||||
| T- NRT- Aerobic, resistance, qi gong, yoga, or a combination | Modified balke treadmill test, 1RM strength (leg press, seated row) | ↔ Physical function↔ Body composition↔ Patient-reported outcomes | ||||||||
| NR | ||||||||||
| ESAS, HADS |
Abbreviations: *, Only 4 participants no longer receiving IFN-α were considered for further analyses; #, primary outcome of interest; ↔, no statistically significant change; ↑, statistically significant improvement; ↓, statistically significant deterioration; NR, not reported; FITT, frequency, intensity, time, type; IDI-ILA part II, Istituto Dermopatico Dell’immacolata Italian Lymphedema Association questionnaire part II; PROMIS SF7a, Patient-reported outcomes measurement information system fatigue–short form 7a; FACT-G, functional assessment of cancer therapy general; 1RM, one repetition maximum; ESAS, edmonton symptom assessment scale; HADS, hospital anxiety and depression scale.
Quality Assessment
The quality assessment is presented in Table 2. The overall score ranged from 8 to 13 of a total of 16 points (ranging from 50% to 81%). All studies30-35 met the criteria related to purpose, relevant background, appropriate study design, sample description, valid outcome measures, appropriate analysis methods, and clinical importance. However, issues related to design biases and sample size justification were present in all studies. In addition, 3 studies31,33,35 (50%) did not report the intervention in detail, or avoid intervention contamination or co-intervention. Two studies31,35 did not achieve the criteria in the reliability of the outcomes measure (33.3%), and 1 study did not report the results in terms of statistical significance33 (16.7%), while another did not provide a conclusion supported by the study methods and results.35 Finally, 4 criteria were deemed “not applicable” in 2 of the studies due to study design. Controlling for intervention contamination, co-intervention, and describing the intervention in detail were deemed not applicable in 2 studies30,32 (33%) while reporting results in terms of statistical significance was not applicable in 1 study32 (16.7%).
Table 2.
Assessment of Study Quality Using the McMaster University Critical Appraisal Tool for Quantitative Studies.
| Schwartz et al34 | Hinrichs et al35 | Blanchard et al30 | Carmeli and Bartoletti31 | Hyatt et al32 | Lacey et al33 | |
|---|---|---|---|---|---|---|
| Study purpose | ||||||
| Was the purpose clearly stated? | 1 | 1 | 1 | 1 | 1 | 1 |
| Literature | ||||||
| Was relevant background literature reviewed? | 1 | 1 | 1 | 1 | 1 | 1 |
| Design | ||||||
| Is the study design appropriate to study aims? | 1 | 1 | 1 | 1 | 1 | 1 |
| No biases present? | 0 | 0 | 0 | 0 | 0 | 0 |
| Sample | ||||||
| Was the sample described in detail? | 1 | 1 | 1 | 1 | 1 | 1 |
| Was sample size justified? | 0 | 0 | 0 | 0 | 0 | 0 |
| Outcomes | ||||||
| Were the outcome measures reliable? | 1 | 0 | 1 | 0 | 1 | 1 |
| Were the outcome measures valid? | 1 | 1 | 1 | 1 | 1 | 1 |
| Intervention | ||||||
| Intervention was described in detail? | 1 | 0 | NA | 0 | NA | 0 |
| Contamination was avoided? | 1 | 0 | NA | 0 | NA | 0 |
| Co-intervention was avoided? | 1 | 0 | NA | 0 | NA | 0 |
| Results | ||||||
| Results were reported in terms of statistical significance? | 1 | 1 | 1 | 1 | NA | 0 |
| Were the analysis method(s) appropriate? | 1 | 1 | 1 | 1 | 1 | 1 |
| Clinical importance was reported? | 1 | 1 | 1 | 1 | 1 | 1 |
| Drop-outs were reported? | 0 | 0 | 0 | 0 | 0 | 1 |
| Conclusion and implications | ||||||
| Conclusions were appropriate given study methods and results | 1 | 0 | 1 | 1 | 1 | 1 |
| Total | 13/16 | 8/16 | 10/13 | 9/16 | 9/12 | 10/16 |
Abbreviation: NA, not applicable.
Physical Activity Measures and Exercise Prescription
Among the included studies, both physical activity behavior and physical activity/exercise interventions were explored. In the cross-sectional studies of Blanchard et al30 and Hyatt et al,32 physical activity behavior was assessed by undertaking surveys about weekly physical activities (eg, aerobic or resistance training)32 or using the Godin Leisure-Time Exercise Questionnaire.30 Patients were categorized as physically active if they met the recommendations at the time from the American Cancer Society (ie, completing ≥150 minutes of moderate-to-strenuous or 60 minutes of strenuous physical activity per week)30 or Clinical Oncology Society Australia exercise guidelines (ie, 150 minutes of moderate-intensity or 75 minutes of vigorous-intensity aerobic exercise and 2 to 3 resistance exercise sessions each week).32 In the study by Schwartz et al,34 2 groups of patients with melanoma (one group receiving IFN-α and another composed of patients who had withdrawn from IFN-α treatment within 1 week of commencement) were instructed to follow a self-guided aerobic exercise program with sessions of 15 to 30 minutes, 4 times a week for a duration of 4 months. However, given the relevance of IFN-α treatment for current clinical practice (ie, no longer recommended for patients with melanoma), only the exercise group not on IFN-α treatment34 was considered for further analyses in this review. In the remaining 3 studies,31,33,35 exercise programs were delivered as follows: (1) 60 minute sessions, 5 to 7 days (over 1-4 weeks) of multimodal therapy involving exercise with bandages,31,35 followed by 2 to 3 days per week of 10-minutes self-practice physical activity until return of the limb to normal size, and (2) 8 weeks of multimodal supportive care intervention using any combination of aerobic, resistance, qi gong, and yoga exercises twice per week.33 No further information regarding physical activity or exercise components were reported.
Outcomes
Quality of life
Three studies reported on QoL using different questionnaires including the RAND-36 Health Status Inventory Score,30 Istituto Dermopatico Dell’immacolata Italian Lymphedema Association questionnaire part II,31 and the Functional Assessment of Cancer Therapy General (FACT-G).33 Based on these reports, localized (stage I and/or II), regional (stage III), and metastatic (stage IV) melanoma survivors in the survey study of Blanchard et al30 meeting the recommendations36 for a healthy lifestyle (ie, physical activity, fruit and vegetable consumption, and non-smoking) reported higher QoL compared to those not following the recommendation (53.7 ± 8.9 vs 50.6 ± 9.5; P < .001). Similar results were observed in the retrospective study of Carmeli and Bartoletti,31 reporting a statistically significant association between higher levels of physical activity and increased QoL (P < .05) in both localized and regional melanoma survivors. However, changes in QoL were not observed in patients with regional and metastatic melanoma following 8 weeks of multimodal supportive care in the study by Lacey et al.33
Fatigue
Fatigue was assessed using the FACT-G33 and the Schwartz Cancer Fatigue Scale34 in the intervention studies, while one cross-sectional study32 assessed fatigue with the Patient-Reported Outcomes Measurement Information System Fatigue–Short Form 7a (PROMIS SF7a) and recorded participants’ perspectives with open-ended questions. Hyatt et al32 reported that among patients with melanoma with regional and metastatic disease, fatigue scores were slightly higher (54.8 ± 9.0) on the PROMIS SF7a than the standardized mean of 50. Additionally, in open-ended questions from the survey, multiple participants attributed experiencing fewer fatigue symptoms during treatment to their physical activity/exercise. Comparatively, no difference was observed between the intervention and control group in the study of Lacey et al33 (39.7 ± 8.4 to 40.4 ± 8.8 in the intervention group; 42.8 ± 7.0 to 43.8 ± 8.8 in the control group) or in the study of Schwartz et al34 (11-18 pts in the intervention group), although the effect of exercise was above the minimum clinically important difference of 5 points.37
Body composition and body weight
Lacey et al33 reported no change in body fat percentage (from 32.0% ± 11.5% to 32.6% ± 11.3%), and a reduction in fat-free mass (from 56.7 ± 19.7 kg to 54.1 ± 18.6 kg) after undertaking a tailored multimodal 8-week supportive care intervention, although the assessment method was not reported. Both intervention studies33,34 indicated a change in body weight; however, neither reported within-group statistical significance. Lacey et al33 observed a reduction in body weight of 1 kg over 8 weeks, while Schwartz et al34 reported a reduction of 8.2 kg (85 ± 17.8 kg to 76.8 ± 17.7 kg) after the 4-month self-guided exercise intervention.
Physical function
Both intervention studies33,34 evaluated physical function before and after the intervention programs. In addition, Lacey et al33 reported upper and lower body muscle strength pre-and post-training of 32.1 ± 9.4 kg and 37.9 ± 12.3 kg for seated row and 67.1 ± 17.2 kg and 57.9 ± 19.7 kg for leg press, although no statistical comparison was undertaken. To assess cardiorespiratory capacity (cardiovascular fitness), Lacey et al33 utilized a modified Balke treadmill test with determination of peak oxygen uptake (VO2 peak) and described the results for the intervention group as stable (from 56 ± 30 ml.kg.min−1 to 51 ± 34 ml.kg.min−1) throughout the study. Schwartz et al34 evaluated aerobic capacity with a 12-minute walk test and concluded that on average participants improved by 6% over the 4-month exercise intervention period.
Treatment-related side-effects, psychological distress, cognitive function, and lymphoedema
Lacey et al33 examined treatment-related side-effects and psychological distress in patients with melanoma with regional and metastatic disease during treatment. No changes were observed after the intervention regarding the Edmonton Symptom Assessment Scale (ESAS) and Hospital Anxiety and Depression Scale (HADS) over 8 weeks of study duration. Schwartz et al34 reported cognitive function scores relative to the Trail Maker Form A and B. Over the 4-month study duration, a reduction of 5 seconds for Form A and 10 seconds for Form B was observed in exercising patients with melanoma.34 A significant reduction in post-surgery lymphoedema was observed in the retrospective studies of Carmeli & Bartoletti31 and Hinrichs et al.35 In both studies, patients who undertook the multimodal intervention presented significant reductions in lymphoedema assessed by limb volume (average reduction of 41%, ranging from −6035 to −22%31).
Discussion
In this review, we examined the effectiveness of physical activity and exercise on QoL, as well as objectively-measured and patient-reported outcomes in patients with melanoma. The main finding of this review was that physical activity/exercise did not adversely impact QoL, or objectively-measured (body composition, physical function, and cardiorespiratory fitness) and patient-reported (fatigue, treatment-related side-effects, cognitive function, and psychological distress) outcomes in patients with melanoma. In addition, major methodological and reporting issues are present within many of the currently available studies, highlighting a lack of quality research examining the relationship between physical activity/exercise and patient outcomes and current treatment side-effects.
Reductions in QoL are common as a result of treatment toxicities and are more pronounced in those patients with regional and metastatic disease.38-41 While this review did not demonstrate that physical activity and exercise have an additional effect on QoL, they likely help to maintain QoL levels in patients with melanoma during or after treatment. Although the previous studies by Blanchard et al30 and Carmeli and Bartoletti31 both suggest that in patients with melanoma confined to the primary site or with regional disease physical activity/exercise is significantly associated with improved QoL, no significant change in QoL levels was observed following 8 weeks of exercise in the intervention study of Lacey et al.33 This result may be related to the lack of exercise control as well as having prescribed vastly different exercise modalities without supervision. In addition, this study33 did not present further information regarding the desired or achieved intensities undertaken during the exercise program. In previous studies, for example, unsupervised exercise programs tend to produce modest changes when compared to supervised exercise programs on QoL,42 and this may have attenuated the exercise effects in patients with melanoma. Furthermore, the poor exercise-related reporting and lack of supervision also preclude us from determining if the minimal exercise stimulus to elicit benefits in QoL was achieved in this group of patients.43 In this way, despite several studies demonstrating the benefits of exercise medicine on QoL in different cancer populations,37,42,44,45 including metastatic disease,46 methodologically sound trials with exercise interventions of appropriate mode and dosage are needed to determine exercise efficacy on QoL in patients with melanoma.
Fatigue is one of the most prevalent symptoms across different cancers and treatments,47,48 and although the cause is multifactorial there is substantial evidence demonstrating the role of exercise medicine in reducing this symptom across several cancer types.49 In the studies included in our review, although physically active patients experienced less fatigue as a result of self-guided exercise in the cross-sectional study by Hyatt et al,32 neither of the 2 included intervention trials33,34 produced meaningful changes in this outcome. This is possibly related to the generally low levels of fatigue presented by the included patients with melanoma at baseline. The moderation of this outcome by the baseline levels is such that patients respond better to the exercise programs if they present with higher levels of fatigue.49 Furthermore, another reason may be related to the potential mediators of fatigue in cancer patients.50,51 Researchers have suggested that both muscle mass and inflammatory markers play a role in the fatigue reduction observed in prostate50 and breast cancer patients51 following exercise interventions. However, only one of the intervention studies included in this review assessed fat mass and lean mass,33 presenting no substantial change in these outcomes, while neither measured inflammatory markers.33,34 This may be considered an important outcome for patients with melanoma given the benefits of resistance training programs on fatigue, muscle mass and metabolic health already demonstrated in other cancer populations.37,43
In addition to the contribution of body composition to attenuate cancer-related fatigue; muscle and fat mass are also associated with physical independence, hospitalization, treatment toxicities, and survival outcomes in cancer patients.8,52-57 Of the 2 intervention studies included in this review, neither reported exercise benefits on muscle mass, fat mass or body weight among patients with melanoma,33,34 which is a concern for this specific patient population, given the prevalence of sarcopenia. The prevalence of sarcopenia increases ~15% in patients receiving checkpoint inhibitor therapy (ipilimumab/PD-1)8 and this represents a major issue in terms of overall survival as well as treatment tolerance in this group of patients. The maintenance of body mass and in particular muscle mass, may be important to patients with melanoma with regional or metastatic disease who are receiving checkpoint inhibitor therapy given improved survival outcomes compared to those presenting with drastic reductions during treatment.58 Therefore, treatment-related reductions in body weight could be harmful in melanoma patients receiving either targeted therapy or immune checkpoint inhibitor therapy which contradicts previous reports concerning prostate or breast cancer,59-61 where a higher body mass negatively impacts treatment and survival outcomes. Thus, more research is needed to determine the effects of exercise on this specific outcome in patients with melanoma. Moreover, no meaningful effect attributed to physical activity/exercise was observed on cognitive function,34 psychological distress,33 physical function,34 or even cardiorespiratory fitness33 in the studies included in our review. This relates to the likely suboptimal exercise programs implemented with these patients (such as volume, intensity, and mode of exercise), as such benefits in psychological distress, physical function, and cardiorespiratory fitness are consistently demonstrated within other cancer types.11,18 With regard to cognitive function, there is evidence of aerobic exercise having a positive effect on older adults’ cognition, although such effects in patients with cancer remains unclear.11
Treatment-related side-effects can impact patients with melanoma receiving a variety of treatment modalities. As previously mentioned, the addition of checkpoint inhibitor therapy to standard care has given rise to a range of irAEs, unlike those seen in more traditional treatments.9 In the study by Lacey et al,33 immune checkpoint inhibitor treatment-related side-effects and symptoms within the exercise group were described as both stable and comparable to the standard care control group. This may be related to participant characteristics; many had previously been treated with immunotherapy (82%) and were long-term regional and metastatic melanoma survivors with a low symptom load at baseline.33 Nevertheless, it appears that over this 8-week intervention, exercise did not negatively influence participants’ irAEs.
Although systemic treatment with immunotherapy or targeted therapy is being used increasingly for both regional and metastatic melanoma, regional surgery for lymph node clearance (whether sentinel or complete lymph node dissection) is still commonly used. Complete lymph node dissection has been largely abandoned62,63; however, lymphoedema remains a potential side-effect of lymph node dissection. Lymphoedema is often treated via a multimodal approach, including massage, compression bandages/stockings, and specific elastic band exercises.64 Both Carmeli & Bartoletti31 and Hinrichs et al35 utilized this multimodal approach, reporting significant reductions in lymphoedema. However, it is important to note that exercise comprised only one part of a comprehensive therapy. Contrary to earlier suggestions, the International Society of Lymphology65 describes both vigorous exercise and resistance exercise under controlled conditions (supervised) as safe for peripheral lymphoedema patients. In breast cancer patients with secondary lymphoedema, both aerobic and resistance exercise are safe, while resistance exercise has been suggested as being more effective at reducing lymphoedema symptoms than usual care.13,66,67 Although comparable lymphoedema symptoms are experienced by patients with melanoma, similar trials have not been undertaken in this patient population.
To our knowledge, this is the first systematic review focusing on the effects of physical activity and exercise in patients with melanoma. Given the paucity of the published research in this area, a strength of this review is the broad inclusion criteria that enabled a comprehensive evaluation of relevant publications in this underdeveloped field as well as a number of suggestions for future research in this patient group. However, some limitations are worthy of comment. The small number of included studies with various study designs produced significant heterogeneity in the present report. For example, the limited evidence from physical activity/exercise trials in melanoma patient precludes observing trends in this current data, such as direction of results indicating benefits derived from physical activity or exercise programs. In addition, restricted outcome reporting and methodological issues also limited our conclusions about the efficacy of physical activity/exercise in patients with melanoma. Therefore, additional research is required to examine the effects of exercise medicine in this group of patients. Future well-designed single group studies with more rigorous exercise interventions and robust reporting are warranted, balancing patients’ needs and goals given the range of side-effects experienced by patients with melanoma throughout the cancer care continuum. Finally, only articles published in English were included in this review and this may be considered a limitation.
In summary, there is some evidence that physical activity/exercise might present potential benefits in patients with melanoma, although major methodological and reporting limitations were present in the included studies. Thus, the main finding of this systematic review is that physical activity/exercise did not adversely impact the objectively-measured or patient-reported outcomes of patients with melanoma. This is important to support future research in this field examining the exercise effects on QoL, fatigue, body composition, physical function, cardiorespiratory fitness, treatment-related side-effects, cognitive function, and psychological distress. As a result, future well-designed studies examining the role of exercise medicine in patients with melanoma are warranted and may potentially enhance patient outcomes.
Supplemental Material
Supplemental material, sj-docx-1-ict-10.1177_15347354211040757 for Associations of Physical Activity and Exercise with Health-related Outcomes in Patients with Melanoma During and After Treatment: A Systematic Review by Brendan J. Crosby, Pedro Lopez, Daniel A. Galvão, Robert U. Newton, Dennis R. Taaffe, Tarek M. Meniawy, Lydia Warburton, Muhammad A. Khattak, Elin S. Gray and Favil Singh in Integrative Cancer Therapies
Footnotes
Author Contributions: Brendan J. Crosby and Favil Singh conceptualized and designed the study. Brendan J. Crosby and Pedro Lopez reviewed the literature and extracted data. Brendan J. Crosby drafted the manuscript. Pedro Lopez, Daniel A. Galvão, Robert U. Newton, Dennis R. Taaffe, Elin S. Gray, and Favil Singh reviewed the results and provided critical feedback. Tarek M. Meniawy, Lydia Warburton, Muhammad A. Khattak reviewed the clinical context and provided critical clinical feedback. Brendan J. Crosby and Favil Singh ensured the accuracy and integrity of the work. Favil Singh supervised the overall study. All authors reviewed and approved the final version of the manuscript.
Declaration of Conflicting Interests: The author(s) declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: The following authors have received travel support from: M.A.K. (Merck Sharp and Dohme [MSD], Bristol-Myers Squibb [BMS] and Merck Serono), T.M.M. [BMS, Novartis, AstraZeneca [AZ]) and E.S.G. (MSD). The following authors sit on advisory boards for: T.M.M. (BMS, MSD, Novartis, AZ). All remaining authors have declared no conflicts of interest.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: Brendan J. Crosby is supported by the Cancer Council WA through the Paul Katris Honours and Masters Scholarship. Pedro Lopez is supported by the National Health and Medical Research Council (NHMRC) Centre of Research Excellence (CRE) in Prostate Cancer Survivorship Scholarship. Daniel A. Galvão and Robert U. Newton are funded by an NHMRC CRE in Prostate Cancer Survivorship. Elin S Gray is supported by a fellowship from the Cancer Council WA. Lydia Warburton is supported by an NHMRC Postgraduate Scholarship (1190643). The sponsors had no involvement in the study design, analysis, or interpretation of data, manuscript writing, and decision to submit the manuscript for publication.
ORCID iDs: Brendan J. Crosby  https://orcid.org/0000-0003-1564-0388
https://orcid.org/0000-0003-1564-0388
Robert U. Newton  https://orcid.org/0000-0003-0302-6129
https://orcid.org/0000-0003-0302-6129
Dennis R. Taaffe  https://orcid.org/0000-0001-6381-1597
https://orcid.org/0000-0001-6381-1597
Supplemental Material: Supplemental material for this article is available online.
References
- 1.Sung H, Ferlay J, Siegel RL, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021;71:209-249. [DOI] [PubMed] [Google Scholar]
- 2.Welfare AIoHa. Cancer data in Australia. AIHW. 2021. Accessed January 21, 2021. Available at: https://www.aihw.gov.au/reports/cancer/cancer-data-in-australia/data
- 3.Coit DG, Thompson JA, Albertini MR, et al. Cutaneous melanoma, version 2.2019, NCCN clinical practice guidelines in oncology. JNCCN. 2019;17:367-402. [DOI] [PubMed] [Google Scholar]
- 4.Robert C, Thomas L, Bondarenko I, et al. Ipilimumab plus dacarbazine for previously untreated metastatic melanoma. N Engl J Med. 2011;364:2517-2526. [DOI] [PubMed] [Google Scholar]
- 5.Larkin J, Chiarion-Sileni V, Gonzalez R, et al. Five-year survival with combined nivolumab and ipilimumab in advanced melanoma. N Engl J Med. 2019;381:1535-1546. [DOI] [PubMed] [Google Scholar]
- 6.Bajwa R, Cheema A, Khan T, et al. Adverse effects of immune checkpoint inhibitors (programmed death-1 inhibitors and cytotoxic T-lymphocyte-associated protein-4 inhibitors): results of a retrospective study. J Clin Med Res. 2019;11:225-236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Welsh SJ, Corrie PG.Management of BRAF and MEK inhibitor toxicities in patients with metastatic melanoma. Ther Adv Med Oncol. 2015;7:122-136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Daly LE, Power DG, O’Reilly A, et al. The impact of body composition parameters on ipilimumab toxicity and survival in patients with metastatic melanoma. Br J Cancer. 2017;116:310-317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Martins F, Sofiya L, Sykiotis GP, et al. Adverse effects of immune-checkpoint inhibitors: epidemiology, management and surveillance. Nat Rev Clin Oncol. 2019;16:563-580. [DOI] [PubMed] [Google Scholar]
- 10.Caspersen CJ, Powell KE, Christenson GM.Physical activity, exercise, and physical fitness: definitions and distinctions for health-related research. Public Health Rep. 1985;100:126-131. [PMC free article] [PubMed] [Google Scholar]
- 11.Campbell KL, Winters-Stone KM, Wiskemann J, et al. Exercise guidelines for cancer survivors: consensus statement from international multidisciplinary roundtable. Med Sci Sports Exerc. 2019;51:2375-2390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Patel AV, Friedenreich CM, Moore SC, et al. American College of Sports Medicine roundtable report on physical activity, sedentary behavior, and cancer prevention and control. Med Sci Sports Exerc. 2019;51:2391-2402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Schmitz KH, Campbell AM, Stuiver MM, et al. Exercise is medicine in oncology: engaging clinicians to help patients move through cancer. CA Cancer J Clin. 2019;69:468-484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Schmitz KH, Courneya KS, Matthews C, et al. American College of Sports Medicine roundtable on exercise guidelines for cancer survivors. Med Sci Sports Exerc. 2010;42:1409-1426. [DOI] [PubMed] [Google Scholar]
- 15.Rock CL, Doyle C, Demark-Wahnefried W, et al. Nutrition and physical activity guidelines for cancer survivors. CA Cancer J Clin. 2012;62:243-274. [DOI] [PubMed] [Google Scholar]
- 16.Rock CL, Thomson C, Gansler T, et al. American Cancer Society guideline for diet and physical activity for cancer prevention. CA Cancer J Clin. 2020;70:245-271. [DOI] [PubMed] [Google Scholar]
- 17.Pollan M, Casla-Barrio S, Alfaro J, et al. Exercise and cancer: a position statement from the Spanish society of medical oncology. Clin Transl Oncol. 2020;22:1710-1729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hayes SC, Newton RU, Spence RR, Galvao DA.The exercise and sports science Australia position statement: exercise medicine in cancer management. J Sci Med Sport. 2019;22:1175-1199. [DOI] [PubMed] [Google Scholar]
- 19.Hayes SC, Spence RR, Galvao DA, Newton RU.Australian association for exercise and sport science position stand: optimising cancer outcomes through exercise. J Sci Med Sport. 2009;12:428-434. [DOI] [PubMed] [Google Scholar]
- 20.Schwitzer E, Orlow I, Zabor EC, et al. No association between prediagnosis exercise and survival in patients with high-risk primary melanoma: a population-based study. Pigment Cell Melanoma Res. 2017;30:424-427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Robinson JK.Physical activity of early stage melanoma survivors. Int J Womens Dermatol. 2019;5:14-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sanchez-Sanchez JL, Manas A, Garcia-Garcia FJ, et al. Sedentary behaviour, physical activity, and sarcopenia among older adults in the TSHA: isotemporal substitution model. J Cachexia Sarcopenia Muscle. 2019;10:188-198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Li M, Xu Y, Wan Q, et al. Individual and combined associations of modifiable lifestyle and metabolic health status with new-onset diabetes and major cardiovascular events: the China cardiometabolic disease and cancer cohort (4C) study. Diabetes Care. 2020;43:1929-1936. [DOI] [PubMed] [Google Scholar]
- 24.Liberati A, Altman DG, Tetzlaff J, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaboration. J Clin Epidemiol. 2009;62:e1-e34. [DOI] [PubMed] [Google Scholar]
- 25.Page MJ, McKenzie JE, Bossuyt PM, et al. Updating guidance for reporting systematic reviews: development of the PRISMA 2020 statement. J Clin Epidemiol. 2021;134:103-112. [DOI] [PubMed] [Google Scholar]
- 26.Furlan AD, Pennick V, Bombardier C, van Tulder M, Revi EBCB. 2009 updated method guidelines for systematic reviews in the cochrane back review group. Spine (Phila Pa 1976). 2009;34:1929-1941. [DOI] [PubMed] [Google Scholar]
- 27.Behrens G, Niedermaier T, Berneburg M, Schmid D, Leitzmann MF.Physical activity, cardiorespiratory fitness and risk of cutaneous malignant melanoma: systematic review and meta-analysis. PLoS One. 2018;13:e0206087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Jiang AJ, Rambhatla PV, Eide MJ.Socioeconomic and lifestyle factors and melanoma: a systematic review. Br J Dermatol. 2015;172:885-915. [DOI] [PubMed] [Google Scholar]
- 29.Law M, Stewart D, Letts L, Pollock N, Bosch J, Westmorland M.Guidelines for Critical Review of Qualitative Studies. McMaster University occupational therapy evidence-based practice research Group 1998:1-9. [Google Scholar]
- 30.Blanchard CM, Courneya KS, Stein K.Cancer survivors’ adherence to lifestyle behavior recommendations and associations with health-related quality of life: Results from the American Cancer Society’s SCS-II. J Clin Oncol. 2008;26:2198-2204. [DOI] [PubMed] [Google Scholar]
- 31.Carmeli E, Bartoletti R.Retrospective trial of complete decongestive physical therapy for lower extremity secondary lymphedema in melanoma patients. Support Care Cancer. 2011;19:141-147. [DOI] [PubMed] [Google Scholar]
- 32.Hyatt A, Drosdowsky A, Williams N, et al. Exercise behaviors and fatigue in patients receiving immunotherapy for advanced melanoma: a cross-sectional survey via social media. Integr Cancer Ther. 2019;18:1-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lacey J, Lomax AJ, McNeil C, et al. A supportive care intervention for people with metastatic melanoma being treated with immunotherapy: a pilot study assessing feasibility, perceived benefit, and acceptability. Support Care Cancer. 2019;27:1497-1507. [DOI] [PubMed] [Google Scholar]
- 34.Schwartz AL, Thompson JA, Masood N.Interferon-induced fatigue in patients with melanoma: a pilot study of exercise and methylphenidate. Oncol Nurs Forum. 2002;29:E85-E90. [DOI] [PubMed] [Google Scholar]
- 35.Hinrichs CS, Gibbs JF, Driscoll D, et al. The effectiveness of complete decongestive physiotherapy for the treatment of lymphedema following groin dissection for melanoma. J Surg Oncol. 2004;85:187-192. [DOI] [PubMed] [Google Scholar]
- 36.Doyle C, Kushi LH, Byers T, et al. Nutrition and physical activity during and after cancer treatment: an American Cancer Society guide for informed choices. CA Cancer J Clin. 2006;56:323-353. [DOI] [PubMed] [Google Scholar]
- 37.Lopez P, Taaffe DR, Newton RU, Buffart LM, Galvao DA.What is the minimal dose for resistance exercise effectiveness in prostate cancer patients? Systematic review and meta-analysis on patient-reported outcomes. Prostate Cancer Prostatic Dis. 2021;24:465-481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Beijer S, Kempen GIJM, Pijls-Johannesma MCG, de Graeff A, Dagnelie PC.Determinants of overall quality of life in preterminal cancer patients. Int J Cancer. 2008;123:232-235. [DOI] [PubMed] [Google Scholar]
- 39.Joseph RW, Liu FX, Shillington AC, et al. Health-related quality of life (QoL) in patients with advanced melanoma receiving immunotherapies in real-world clinical practice settings. Qual Life Res. 2020;29:2651-2660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lisy K, Lai-Kwon J, Ward A, et al. Patient-reported outcomes in melanoma survivors at 1, 3 and 5 years post-diagnosis: a population-based cross-sectional study. Qual Life Res. 2020;29:2021-2027. [DOI] [PubMed] [Google Scholar]
- 41.Schadendorf D, Di Giacomo AM, Demidov L, et al. Health-related quality of life in patients with fully resected BRAF(V600) mutation-positive melanoma receiving adjuvant vemurafenib. Eur J Cancer. 2019;123:155-161. [DOI] [PubMed] [Google Scholar]
- 42.Buffart LM, Kalter J, Sweegers MG, et al. Effects and moderators of exercise on quality of life and physical function in patients with cancer: an individual patient data meta-analysis of 34 RCTs. Cancer Treat Rev. 2017;52:91-104. [DOI] [PubMed] [Google Scholar]
- 43.Lopez P, Taaffe DR, Newton RU, Galvao DA.Resistance exercise dosage in men with prostate cancer: systematic review, meta-analysis, and meta-regression. Med Sci Sports Exerc. 2021;53:459-469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lahart IM, Metsios GS, Nevill AM, Carmichael AR.Physical activity for women with breast cancer after adjuvant therapy. Cochrane Database Syst Rev. 2018;1:CD011292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.McGettigan M, Cardwell CR, Cantwell MM, Tully MA.Physical activity interventions for disease-related physical and mental health during and following treatment in people with non-advanced colorectal cancer. Cochrane Database Syst Rev. 2020;5:CD012864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Dittus KL, Gramling RE, Ades PA.Exercise interventions for individuals with advanced cancer: a systematic review. Prev Med. 2017;104:124-132. [DOI] [PubMed] [Google Scholar]
- 47.Bower JE.Cancer-related fatigue–mechanisms, risk factors, and treatments. Nat Rev Clin Oncol. 2014;11:597-609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.O’Higgins CM, Brady B, O’Connor B, Walsh D, Reilly RB.The pathophysiology of cancer-related fatigue: current controversies. Support Care Cancer. 2018;26:3353-3364. [DOI] [PubMed] [Google Scholar]
- 49.Buffart LM, Sweegers MG, May AM, et al. Targeting exercise interventions to patients with cancer in need: an individual patient data meta-analysis. J Natl Cancer Inst. 2018;110:1190-1200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Newton RU, Jeffery E, Galvao DA, et al. Body composition, fatigue and exercise in patients with prostate cancer undergoing androgen-deprivation therapy. BJU Int. 2018;122:986-993. [DOI] [PubMed] [Google Scholar]
- 51.Hiensch AE, Mijwel S, Bargiela D, Wengstrom Y, May AM, Rundqvist H. Inflammation mediates exercise effects on fatigue in patients with breast cancer. Med Sci Sports Exerc. 2021;53:496-504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Heidelberger V, Goldwasser F, Kramkimel N, et al. Sarcopenic overweight is associated with early acute limiting toxicity of anti-PD1 checkpoint inhibitors in melanoma patients. Invest New Drugs. 2017;35:537. [DOI] [PubMed] [Google Scholar]
- 53.Norman K, Otten L.Financial impact of sarcopenia or low muscle mass – a short review. Clin Nutr. 2019;38:1489-1495. [DOI] [PubMed] [Google Scholar]
- 54.Sabel MS, Lee J, Cai SJ, Englesbe MJ, Holcombe S, Wang S.Sarcopenia as a prognostic factor among patients with stage III melanoma. Ann Surg Oncol. 2011;18:3579-3585. [DOI] [PubMed] [Google Scholar]
- 55.Shachar SS, Williams GR, Muss HB, Nishijima TF.Prognostic value of sarcopenia in adults with solid tumours: a meta-analysis and systematic review. Eur J Cancer. 2016;57:58-67. [DOI] [PubMed] [Google Scholar]
- 56.Spirduso WW, Cronin DL.Exercise dose-response effects on quality of life and independent living in older adults. Med Sci Sports Exerc. 2001;33:S598-S608. [DOI] [PubMed] [Google Scholar]
- 57.Versteeg KS, Blauwhoff-Buskermolen S, Buffart LM, et al. Higher muscle strength is associated with prolonged survival in older patients with advanced cancer. Oncologist. 2018;23:580-585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.McQuade JL, Daniel CR, Hess KR, et al. Association of body-mass index and outcomes in patients with metastatic melanoma treated with targeted therapy, immunotherapy, or chemotherapy: a retrospective, multicohort analysis. Lancet Oncol. 2018;19:310-322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Cao Y, Ma J.Body mass index, prostate cancer-specific mortality, and biochemical recurrence: a systematic review and meta-analysis. Cancer Prev Res (Phila). 2011;4:486-501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Gallo M, Adinolfi V, Barucca V, et al. Expected and paradoxical effects of obesity on cancer treatment response. Rev Endocr Metab Disord. Published online October 6, 2020. doi: 10.1007/s11154-020-09597-y [DOI] [PubMed] [Google Scholar]
- 61.Griggs JJ, Sorbero ME, Lyman GH.Undertreatment of obese women receiving breast cancer chemotherapy. Arch Intern Med. 2005;165:1267-1273. [DOI] [PubMed] [Google Scholar]
- 62.Faries MB, Thompson JF, Cochran AJ, et al. Completion dissection or observation for sentinel-node metastasis in melanoma. N Engl J Med. 2017;376:2211-2222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Leiter U, Stadler R, Mauch C, et al. Complete lymph node dissection versus no dissection in patients with sentinel lymph node biopsy positive melanoma (DeCOG-SLT): a multicentre, randomised, phase 3 trial. Lancet Oncol. 2016;17:757-767. [DOI] [PubMed] [Google Scholar]
- 64.Rockson SG, Keeley V, Kilbreath S, Szuba A, Towers A.Cancer-associated secondary lymphoedema. Nat Rev Dis Primers. 2019;5:22. [DOI] [PubMed] [Google Scholar]
- 65.Executive Committee. The diagnosis and treatment of peripheral lymphedema: 2016 consensus document of the international society of lymphology. Lymphology. 2016;49:170-184. [PubMed] [Google Scholar]
- 66.Kilbreath SL, Ward LC, Davis GM, et al. Reduction of breast lymphoedema secondary to breast cancer: a randomised controlled exercise trial. Breast Cancer Res Treat. 2020;184:459-467. [DOI] [PubMed] [Google Scholar]
- 67.Baumann FT, Reike A, Reimer V, et al. Effects of physical exercise on breast cancer-related secondary lymphedema: a systematic review. Breast Cancer Res Treat. 2018;170:1-13. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, sj-docx-1-ict-10.1177_15347354211040757 for Associations of Physical Activity and Exercise with Health-related Outcomes in Patients with Melanoma During and After Treatment: A Systematic Review by Brendan J. Crosby, Pedro Lopez, Daniel A. Galvão, Robert U. Newton, Dennis R. Taaffe, Tarek M. Meniawy, Lydia Warburton, Muhammad A. Khattak, Elin S. Gray and Favil Singh in Integrative Cancer Therapies