Abstract
Developing hippocampal neurons undergo rapid synaptogenesis in response to neurotrophic signals to form and refine circuit connections. The adipokine leptin is a satiety factor with neurotrophic actions which potentiates both glutamatergic and GABAergic synaptogenesis in the hippocampus during neonatal development. Brief exposure to leptin enhances GABAA receptor-dependent synaptic currents in hippocampal neurons. Here, using molecular and electrophysiological techniques, we found that leptin increased the surface localization of GABAA receptors and the number of functional GABAergic synapses in hippocampal cultures from male and female rat pups. Leptin increased the interaction between GABAA receptors and the Rho guanine exchange factor β-PIX (a scaffolding protein at GABAergic postsynaptic sites) in a manner dependent on the kinase CaMKK. We also found that the leptin receptor and β-PIX formed a complex, the amount of which transiently increased upon leptin receptor activation. Furthermore, Tyr985 in the leptin receptor and the SH3 domain of β-PIX are crucial for this interaction, which was required for the developmental increase in GABAergic synaptogenesis. Our results suggest a mechanism by which leptin promotes GABAergic synaptogenesis in hippocampal neurons and reveal further complexity in leptin receptor signaling and its interactome.
Introduction
Leptin is an adipokine synthesized and released primarily from white adipose tissue, and it regulates energy homeostasis and feeding behavior in the adult by binding to its long-form isoform receptors (LepRb) in the hypothalamus, hindbrain and ventral tegmental area [1–4]. However, leptin receptors are also expressed in the hippocampus, specifically in the Cornu Ammonis 1/3 (CA1/CA3) and dentate gyrus regions [5–7]. Several behavioral studies demonstrated that mouse models that lack leptin (ob/ob) or express the long isoform of the leptin receptor (db/db) exhibit deficits in hippocampus-dependent functions, such that both young and juvenile mice lacking functional leptin receptor signaling show depressive and anxiety-like behaviors and have cognitive impairments [8–11]. Moreover, leptin administration improves the performance of wild-type mice in hippocampus-dependent behavior tests [12–14], whereas replacement of leptin in ob/ob mice alleviates their deficits in these tasks [10, 15].
Leptin stimulates glutamatergic synaptogenesis, modulates synaptic plasticity and regulates the function and surface localization of ionotropic glutamate receptors [16–20]. Moreover, it has been reported that leptin also regulates trophic actions of neurotransmitter GABA. Leptin prolongs the depolarizing actions of neurotransmitter GABA and increases the number of GABAA receptor-mediated giant depolarizing potentials during early development [21, 22], which together have been suggested to underlie the initial step of hippocampal circuitry development by facilitating synchronous neuronal firing and calcium oscillations [23, 24].
Leptin also increases GABAergic synaptic transmission and recruitment of functional GABAergic synapses in both CA1 and CA3 hippocampal neurons during development [25, 26]. ob/ob mice exhibit reduced base levels of miniature GABAA receptor mediated postsynaptic currents and lower numbers of presynaptic GABAergic terminals than the wild-type littermates during neonatal development, suggesting that endogenous leptin signaling is critical [25]. Pathways regulated by mitogen-activated protein kinase (MAPK)/extracellular signal-regulated kinase (ERK) kinase 1 and/or 2 (MEK1/2), phosphoinositide 3 kinase (PI3K) and Ca2+/calmodulin kinase kinase (CaMKK) are required for leptin-induced potentiation of GABAA receptor-mediated postsynaptic currents [25, 26]. However, much about how leptin increases GABAergic synapses is still to be elucidated.
The actin-rich cytoskeleton in dendrites and axons is crucial for the dynamic organization and stabilization of both inhibitory and excitatory synapses [27–29]. The Rho family of small G proteins are well-known regulators of the actin cytoskeleton [30]. Among various regulators of the Rho small G proteins, Rho guanine exchange factor 7 (ARHGEF7), also known as β PAK-interacting exchange factor (β-PIX), plays a role both in excitatory and inhibitory synaptogenesis [31–33]. An earlier study demonstrated that GIT1/β-PIX/Rac1/PAK1 signaling pathway recruits actin cytoskeleton and gephyrin scaffolding protein to the membrane to stabilize GABAA receptors in cortical and hippocampal neurons [33]. Furthermore, we previously showed that phosphorylation and activation of β-PIX by leptin is required for leptin-induced membrane insertion of TrpC channels [34].
In this study, we investigated whether β-PIX is involved in leptin-induced increase in GABAergic synaptogenesis. We found that acute leptin application increased GABAergic synaptogenesis by inducing an interaction between the leptin receptor and β-PIX that enhanced an interaction between β-PIX and GABAA receptors.
Results
Leptin increases GABAergic synaptogenesis
Leptin has been shown to potentiate GABAA receptor-mediated postsynaptic currents in CA1 pyramidal hippocampal neurons within 10–20 minutes of application [25, 26]. To determine if this is correlated with an increase in GABAA receptor abundance on the plasma membrane, we performed a time-course of leptin treatment to cultured rat hippocampal neurons and assessed GABAA receptor surface expression. We first live-stained the neurons for the β2/3 subunits of GABAA receptors, as these subunits are components of most GABAA receptors [35]. Surface levels of β2/3 subunit significantly increased after 2 hours of leptin treatment (Fig. 1A). We then confirmed this by measuring surface biotinylation of the β2/3 subunits. However, we did not see a change in total levels of β2/3 subunit (Fig. 1B), suggesting that leptin does not act on the transcriptional level. To verify that leptin stimulates GABAA receptor insertion through its receptor, we knocked down the expression of LepRb with a previously verified shRNA (shLepRb) [21, 34]. In the presence of shLepRb, but not a control construct that has no known target (shScramble) [36, 37], the effects of leptin were blocked, and co-transfection with shRNA-resistant LepRb rescued the effects (Fig. 1, C to F).
Figure 1: Leptin increases surface levels of GABAAR β2/3 subunit, but do not increase total protein levels.
(A) Quantification of time course of 50nM leptin treatment followed by live staining in primary rat hippocampal neurons. (B) Surface biotinylation of rat hippocampal cultures ± 50nM leptin for 2 hours. (C) Representative western blot images to show the efficiency of shRNA targeting leptin receptor (shLepRb), shScramble as a negative control, and shRNA-resistant leptin receptor for the rescue of leptin receptor knock-down in HEK293T cells. (D) Representative images for live-stained neurons for GABAAR β2/3 subunit that were transfected with empty vector (expressing plain Clover protein) along with either shLepRb or shScramble or shLepRb+shRNA-resistant leptin receptor and treated with ± 50nM leptin for 2 hours. Scale bars, 10μm. (E and F) Quantification of (E) puncta number and (F) cluster area of GABAAR β2/3 subunits. For all panels (A to F), N≥3 independent cultures. Kruskal-Wallis non-parametric ANOVA followed by Dunn’s post-hoc analysis was performed for live-staining experiments, and the student’s unpaired, two-tailed t-test was used for surface biotinylation experiments. Box plots represent first and third quartiles, whiskers show data range, and scatter plots show individual data points. Bar graphs are mean ± SEM. **P < 0.01.
To visualize GABAergic synapses, we next stained for gephyrin, a postsynaptic scaffolding protein in inhibitory synapses, and analyzed its colocalization with vesicular transferase (VGAT), a GABAergic presynaptic marker. We showed that gephyrin puncta density increased in the presence of leptin but not when the receptor knocked down by co-transfection of shLepRb (Fig. 2, A and B). In addition, the majority of gephyrin puncta were colocalized with VGAT, and leptin treatment did not change this colocalization percentage, suggesting that gephyrin staining represents functional postsynaptic terminals (Fig. 2C).
Figure 2: Leptin increases density of gephyrin staining.
(A) Representative images for gephyrin-VGAT colocalization in primary rat hippocampal neurons. Scale bars, 50μm for black and white images and 10μm for zoomed images. (B and C) Quantification of gephyrin puncta number (B) and gephyrin-VGAT colocalization (C) in the cells described in (A). N≥3 independent primary rat hippocampal cultures. Box plots represent first and third quartiles, whiskers show data range, and scatter plots show individual data points. *P < 0.05, ***P < 0.001 by Kruskal-Wallis non-parametric ANOVA followed by Dunn’s post-hoc analysis.
To further investigate the effect of leptin on functional GABAergic synaptogenesis, we used whole-cell patch clamping to record miniature inhibitory postsynaptic currents (mIPSCs) to determine whether we also see changes in the number of spontaneous GABA events. In the first set of experiments, we recorded exclusively from CA1 pyramidal neurons in acute hippocampal slices obtained from P10-P11 wild-type mouse pups. Slices were given time to recover after sectioning and then pre-treated with 50nM leptin for 2 hours and recorded in the presence leptin. Pre-treatment with leptin increased both frequency and amplitude of mIPSCs in acute slices (Fig. 3, A to C), consistent with both an increase in the number and strength of GABA synapses. Of note, no significant sex difference was observed in the frequency and amplitude of mIPSCs in both control and leptin treatment conditions. Additionally, leptin significantly increased GABAA-receptor mediated currents in response to bath application of the GABAA receptor agonist isoguvacine [21] (Fig. 3, D and E), indicating that leptin can increase the size of exogenously evoked GABAAR currents.
Figure 3: Leptin increases mIPSCs in both mouse acute slices and rat organotypic slice cultures.
(A to C) Representative images of mIPSC recordings (A) and quantification of mIPSC frequency (B) and amplitude (C) in acute slices of control or 50nM leptin treated for 2 to 4 hours N=4 female and 4 male mouse pups, assessing 13 neurons from control slices and 16 neurons from leptin-treated slices. In the control data set, male neuron mIPSCs (filled circles) were 0.50 ± 0.15Hz and 32.88 ± 3.13mA, and female neuron mIPSCs (empty circles) were 0.30 ± 0.03Hz (P=0.26) and 33.12 ± 2.01mA (P=0.72). In the leptin data set, male neuron mIPSCs (filled circles) were 0.74 ± 0.10Hz and 43.16 ± 2.36mA, and female neuron mIPSCs (empty circles) were 0.51 ± 0.07Hz (P=0.09) and 43.41 ± 3.32mA (P=0.95). **P <0.01 on pooled data and P-values within male/female groups calculated by Mann-Whitney rank sum tests. (D and E) Representative image (D) and quantification (E) of isoguvacine responses in neurons in acute slices, control or 50nM leptin treated for 2 to 4 hours. Each response was normalized to capacitance of the neuron. N=3 female and 1 male pups; 7 neurons from control slices and 9 neurons from leptin-treated slices. *P < 0.05 by Mann-Whitney rank sum tests. (F) Representative image for biolistically transfected rat hippocampal organotypic slice cultures and recording from transfected neurons. Scale bars, 200μm (top) and 20μm (middle and bottom). (G to K) Representative images for slice culture recordings (G) and quantification of frequency of mIPSCs (H), the cumulative fraction of inter-event intervals (I), amplitude of mIPSCs (J), and the cumulative fraction of amplitude of events (K) in control and LepRb-knockdown slices that were treated with control or 50nM leptin for 2 to 4 hours. All the neurons recorded for each condition were pooled from N=13 independent rat hippocampal slice cultures for control and leptin conditions (6 female and 7 male slice cultures), wherein 20 neurons were assessed for each condition; and N=4 independent hippocampal slice cultures for shLepRb and shLepRb + leptin (2 female and 2 male slice cultures), wherein 7 or 8 neurons were assessed for each respective condition). **P <0.01 and ***P < 0.001 by Kruskal-Wallis non-parametric ANOVA followed by Dunn’s post-hoc analysis. Box plots represent first and third quartiles, whiskers show data range, and scatter plots show individual data points.
Next, we measured GABA mIPSCs in organotypic hippocampal slice cultures prepared from rat pups. In these preparations, hippocampal networks are preserved (versus in dissociated hippocampal cultures) and enabling us to identify and record CA1 neurons specifically while biolistically delivering constructs to manipulate signaling pathway component expression (Fig. 3F) [17]. First, we transfected organotypic slices with Td-Tomato with or without shLepRb to visualize the transfected neurons and to knockdown LepRb. Leptin significantly increased both amplitude and frequency of mIPSCs in these preparations as well, while co-transfection of shLepRb blocked these effects (Fig. 3, G to K). Together with the above results, these findings suggest that leptin increases both the number and strength of GABA synapses through activation of LepRbs, at least in part by increasing the surface expression of GABAA receptors in hippocampal neurons.
β-PIX is required for the actions of leptin on GABAergic synaptogenesis
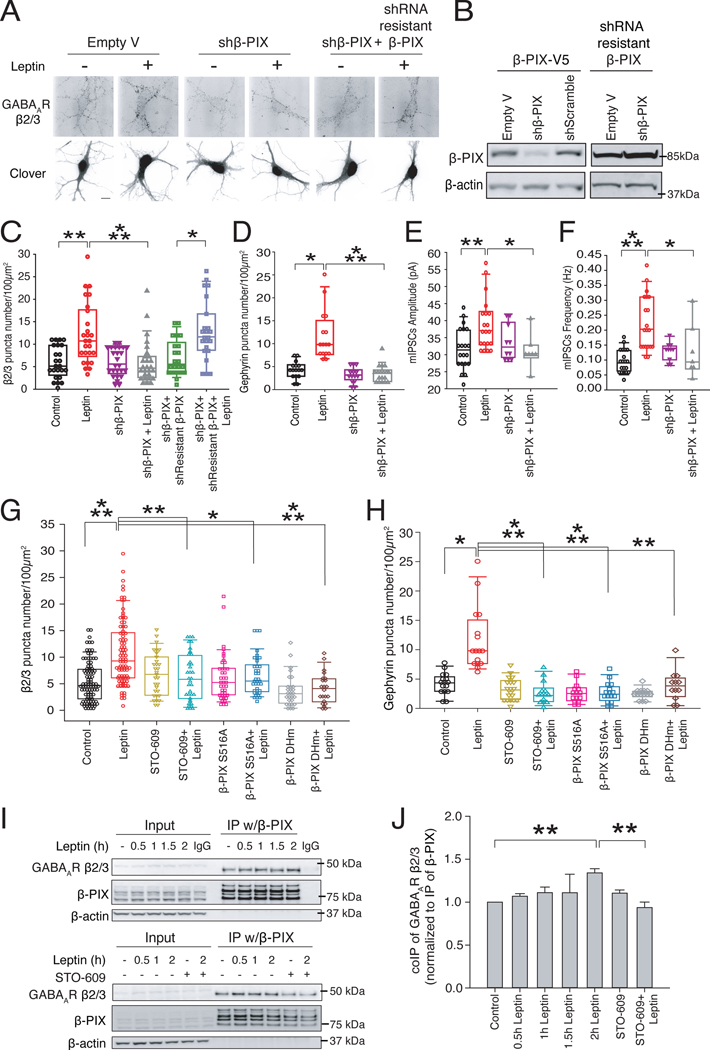
We previously reported that leptin promotes the phosphorylation of β-PIX at Ser516 leading to its activation [34]. Since β-PIX has been shown to be one of the scaffolding proteins for GABAA receptor localization, we next determined its role in leptin-induced GABAergic synaptogenesis. Knocking down the expression of β-PIX with targeted shRNA (shβ-PIX) blocked the leptin-induced increase in both β2/3 subunit surface staining and gephyrin puncta density, while the overexpression of shRNA-resistant β-PIX rescued the effects of shβ-PIX (Fig. 4, A to D). Furthermore, the leptin-stimulated increase in mIPSC frequency and amplitude were blocked also in neurons from organotypic slices transfected with shβ-PIX (Fig. 4, E and F).
Figure 4: β-PIX mediates the effects of leptin on GABAergic synaptogenesis.
(A) Representative images of live-stained rat hippocampal neurons for GABAAR β2/3 that were transfected with empty vector (expressing plain Clover protein) along with either shβ-PIX or shβ-PIX and shRNA-resistant β-PIX and treated ± 50nM leptin for 2 hours. Scale bar, 10μm. (B) Representative Western blot images to show the efficiency of shRNA targeting β-PIX, shScramble as a negative control, and shRNA resistant β-PIX for the rescue of β-PIX knock-down in HEK293T cells used in (A). (C and D) Quantification of GABAAR β2/3 puncta number (C) and gephyrin staining (D) in the rat hippocampal neurons described in (A). N≥3 independent primary rat hippocampal cultures. (E and F) Quantification of frequency (E) and amplitude (F) of mIPSCs in the presence of shβ-PIX ± 50nM leptin. N=7 independent cultures for each condition (from 3 female and 4 male rat hippocampal slice cultures), each assessing 10and 7 neurons, respectively. (G and H) The effect of leptin on GABAAR (G) and gephyrin puncta number (H) in cultured rat hippocampal neurons expressing the indicated mutant β-PIX construct. N≥3 independent primary rat hippocampal cultures. (I and J) Representative western blot images (I) and quantification (J) of co-IP of GABAAR β2/3 subunit with β-PIX in neurons treated ± 50nM leptin and ± 20μM STO-609. N≥3 independent primary rat hippocampal cultures. Box plots represent first and third quartiles, whiskers show data range, and scatter plots show individual data points. Bar graphs show mean ± SEM. *P < 0.05, **P <0.01, and ***P < 0.001 by Kruskal-Wallis non-parametric ANOVA followed by Dunn’s post-hoc analysis.
The GEF activity of β-PIX is enhanced by activation of CaMKK-CaMKI signaling pathway and the phosphorylation of Ser516 by CaMKI [31]. The CaMKK inhibitor STO-609, as well as expression of a β-PIX that cannot be phosphorylated by CaMKI (β-PIX-S516A), each blocked leptin’s effects on β2/3 surface staining and gephyrin puncta density, as did expression of a β-PIX that is deficient in GEF activity (β-PIX-DHm) (Fig. 4, G and H). Together, these data suggest that both activation of the CaMKK-CaMKI pathway and the GEF activity of β-PIX are required to mediate the leptin-induced increase in the surface levels of GABAA receptors.
Endogenous β-PIX and the β2/3 subunits of GABAA receptors form a complex [33]. To determine whether leptin facilitates this interaction, we performed co-immunoprecipitation (co-IP) assays. A time-course of leptin treatment showed that β2/3 subunits co-immunoprecipitated with β-PIX significantly after 2 hours, and that the leptin-induced increase in the interaction was blocked by pre-incubation with STO-609 (Fig. 4, I and J). Overall, these data supported the hypothesis that leptin increases GABAergic synaptic transmission by activating β-PIX.
Leptin receptor and β-PIX are in a complex
Next, we asked whether the leptin receptor is a part of the complex that is formed by β-PIX and GABAA receptors. We overexpressed tagged leptin receptor and tagged β-PIX in HEK293T cells and pulled down leptin receptor using its tag at various time points after leptin treatment (Fig. 5, A and B). We observed that the leptin receptor and β-PIX were in a complex even under control conditions, and their interaction transiently increased at 1 hour of leptin treatment, after which, it returned to control levels. We confirmed this transient increase in interaction after 1 hour of leptin treatment, with reciprocal pull-down of β-PIX (Fig. 5. C). Moreover, as a negative control for the co-IP experiments, we overexpressed an unrelated enhanced green fluorescent protein (eGFP) along with either leptin receptor or β-PIX and pulled down either leptin receptor or β-PIX; neither co-immunoprecipitated eGFP, verifying the specificity of interaction between leptin receptor and β-PIX (Fig. 5D).
Figure 5: Leptin receptor forms a transient complex with β-PIX.
(A and B) Representative Western blots of co-IP of leptin receptor with β-PIX at the indicated time points over 2 hours of leptin treatment in transfected HEK293T cells (A) and quantification of the co-IP (B), analyzed by one-way ANOVA followed by Tukey’s pairwise multiple comparison (C) Reciprocal co-IP to that shown in (A) after 1 hour of leptin treatment in HEK293T cells. (D) Negative control of co-IP with enhanced green fluorescent protein (eGFP) in transfected HEK293T cells. Experiments were repeated at least 3 different passages of HEK293T cells (passage number<30). (E) Representative western blots following co-IP of the indicated mutated leptin receptors and ΔSH3-β-PIX from transfected HEK293T cells. (F) Quantification of the co-IP in (E), *P < 0.05 by student’s t-test. (G) Representative Western blot images from HEK293T cells to show that LepR-Tyr985 and Tyr1138 were resistant to knockdown by shLepRb. (H and I) Quantification of GABAAR (H) and gephyrin (I) puncta number in the presence of mutated leptin receptor constructs. N≥3 independent primary rat hippocampal cultures. *P < 0.05, **P <0.01, and ***P < 0.001 by Kruskal-Wallis non-parametric ANOVA followed by Dunn’s post-hoc analysis. Box plots represent first and third quartiles, whiskers show data range, and scatter plots show individual data points. Bar graphs show mean ± SEM.
We next wanted to determine if one of the known phosphorylation sites of the leptin receptor are involved in the observed transient increase in interaction of the LepR and β-PIX. We used previously verified leptin receptor constructs LepRY985L and LepRY1138S, which cannot be phosphorylated at Tyr985 and Tyr1138, respectively, and consequently cannot activate the respective downstream signaling pathways [17, 38, 39]. We overexpressed each of these mutant leptin receptors along with β-PIX in HEK293T cells to determine if they affect the increased pull-down of β-PIX with leptin. Leptin treatment still increased the interaction between LepRY1138S and β-PIX. In contrast, leptin did not alter the interaction between LepRY985L and β-PIX, suggesting that phosphorylation at Tyr985 is necessary for the transient increase in interaction between β-PIX and leptin receptor (Fig. 5, E and F).
Lastly, we determined whether phosphorylation of LepR at Tyr985 is required for leptin-induced GABAergic synaptogenesis in hippocampal neurons. Because both LepRY985L and LepRY1138S are derived from mouse cDNA and resistant to shLepRb [17] (Fig. 5G), we overexpressed each of these constructs as well as shLepRb to knockdown the expression of endogenous leptin receptor. While the overexpression of LepRY1138S rescued the effects of shLepRb, overexpression of LepRY985L did not. Both the puncta density of GABAA receptor β2/3 subunits and gephyrin did not increase with leptin when LepRY985L was overexpressed in the absence of endogenous leptin receptor (Fig. 5, H and I), suggesting that phosphorylation of leptin receptor at Tyr985 is required for the leptin’s actions.
SH3 domain of β-PIX is required for leptin-induced GABAergic synaptogenesis
Next, we asked which domain in β-PIX is necessary for its interaction with the LepR. While the C-terminal of β-PIX is mainly involved in its GEF activity to activate Rac1 and other downstream effectors [40], the N-terminal of β-PIX consists of SH3 proline-rich domain where p21-activated kinase (PAK1) interacts with β-PIX [41]. When the leptin receptor is activated, phosphorylation of Tyr985 creates a docking site for SH2 domain-containing proteins [42]. The adaptor protein Grb-2 binds to this site and eventually activates the MAPK1/2-ERK1/2 pathway [43]. Grb-2 also contains two SH3 domains through which it can interact with other SH3 domain containing proteins [44]. To determine whether the SH3 domain of β-PIX was required for the actions of leptin, we generated a construct of β-PIX with its SH3 domain deleted, (ΔSH3-β-PIX) and made this construct shβ-PIX resistant by site-directed mutagenesis (Fig. 6A). Leptin had no effect on the interaction between the leptin receptor and ΔSH3-β-PIX (Fig. 5, F and G). Moreover, when we overexpressed shRNA-resistant ΔSH3-β-PIX, as well as shβ-PIX to knockdown the expression of endogenous β-PIX, in neurons, ΔSH3-β-PIX was not able to rescue the effects of shβ-PIX; as both the leptin-induced increase in β2/3 subunit surface levels and gephyrin puncta density were blocked (Fig. 6, B to D), as were leptin-induced increase in frequency and amplitude of mIPSCs (Fig. 6, E and F).
Figure 6: SH3 domain of β-PIX is required for leptin-induced GABAAR surface stabilization.
(A) Verification of shβ-PIX resistance of the construct ΔSH3-β-PIX. (B) Representative images for live staining of GABAAR β2/3 subunits in rat hippocampal neurons. Scale bar, 10μm. (C and D) Quantification of β2/3 (C) and gephyrin (D) puncta number in the presence of ΔSH3-β-PIX. N≥ 3 independent primary rat hippocampal cultures. (E and F) Quantification of frequency (E) and amplitude (F) of mIPSCs in the presence of ΔSH3-β-PIX. N=3 independent cultures for ΔSH3-β-PIX and ΔSH3-β-PIX + leptin (1 female and 2 male slice cultures), each assessing 7 neurons. Box plots represent first and third quartiles, whiskers show data range, and scatter plots show individual data points. *P < 0.05, **P <0.01, ***P < 0.001 by Kruskal-Wallis non-parametric ANOVA followed by Dunn’s post-hoc analysis.
Overall, these results suggest that transient increase of the interaction between leptin receptor and β-PIX mediates the effects of leptin on GABAergic synaptogenesis and that this increase in interaction requires the SH3 domain of β-PIX.
Discussion
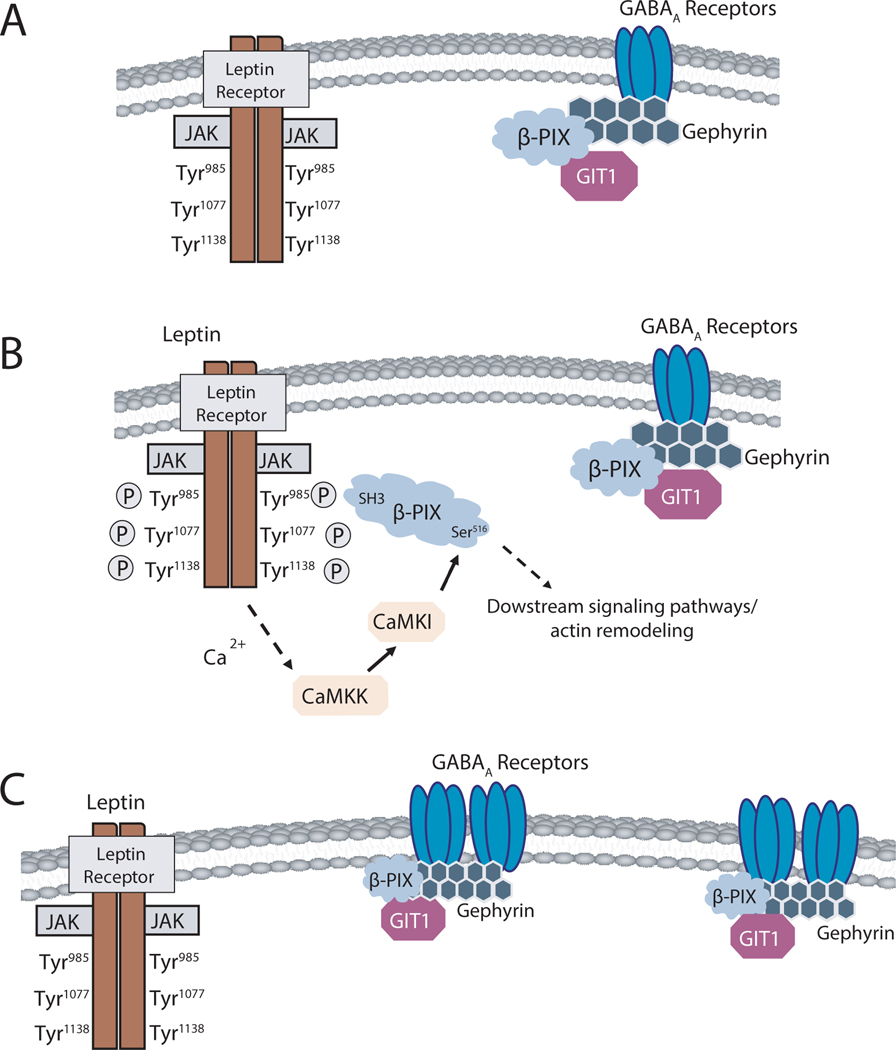
Leptin levels peak during the first postnatal week in rodents when hippocampal neurons are undergoing rapid synaptogenesis to refine network formation [45]. Leptin has also been shown to be a crucial neurotrophic factor for the development of both glutamatergic and GABAergic synaptic connections during this period [16, 19, 21, 22, 26, 46]. Here, we found: that leptin increases the number of functional GABAergic synapses in the hippocampus by enhancing a complex between its receptor and the GEF β-PIX that promotes membrane localization of GABAA receptors (Fig. 7). Together with a previous report that ob/ob mice that lack leptin synthesis have a lower number of GABAergic presynaptic terminals during neonatal development (P10) [25], our data support a critical role for leptin in promoting GABAergic synapse formation during a critical stage in development.
Figure 7: Model of the current hypothesis.
(A) In unstimulated, control conditions, leptin receptor is not active. (B) Upon leptin addition, leptin receptors are activated, and this activates downstream signaling molecules. In an hour after activation of leptin receptor, more β-PIX gets recruited to leptin receptors. (C) Later, more GABAA receptors are stabilized on the plasma membrane in the presence of β-PIX and gephyrin scaffolding.
Previously, leptin was shown to activate β-PIX through a CaM kinase-dependent pathway and that this is required for the formation of new spines, the primary sites for glutamatergic synaptogenesis [34]. Consistently, the β-PIX-Rac1-PAK1 signaling cascade was shown to induce activity-dependent glutamatergic synaptogenesis [31, 32]. β-PIX has also been identified as a scaffolding protein to stabilize GABAA receptors along with G-protein-coupled receptor interacting protein 1 (GIT1) and gephyrin [33]. However, the impact of knocking down β-PIX on the basal number of inhibitory synaptic currents at the control level is more controversial [31, 33]., potentially due to differences in the developmental stage of the neurons studied. GABAergic synapses undergo rapid maturation in cultured from DIV7 to DIV14 [47, 48]. Given that many scaffolding proteins can stabilize GABAA receptors at the plasma membrane [49, 50], it is possible that at some time points additional scaffolding proteins may compensate for a lack of β-PIX.
There is growing evidence that leptin receptor signaling is multifaceted and that the leptin receptor is capable of interacting with a number of different receptors and signaling molecules to form a complex interactome, suggesting that multiple signaling cascades can be activated in a coordinated manner [16, 17, 34, 51–54]. Our finding that β-PIX is part of the leptin receptor interactome and that the association transiently increases with leptin—more specifically upon phosphorylation of Tyr985 residue—suggests that scaffolding proteins can be activated and recruited to the plasma membrane locally to stabilize GABAA receptors in response to leptin, potentially by remodeling and stabilizing the actin cytoskeleton. The transient nature of the interaction is not surprising, given that leptin receptor signaling is terminated rapidly by numerous phosphatases [55]. Furthermore, we did not observe substantial colocalization between leptin receptors and GABAergic presynaptic terminals (less than 2%), suggesting that the duration of the interaction with β-PIX is not long enough to promote association of leptin receptors with GABAA receptors.
It is well established that the Tyr985 residue of the leptin receptor is phosphorylated by the kinase JAK2 upon leptin stimulation and that this phosphorylated receptor recruits the protein tyrosine phosphatase SHP-2 and, subsequently, the adaptor protein Grb-2 [42, 56]. SHP-2 protein interacts with Grb-2 through its SH2 domain, and Grb-2, in turn, can interact with SH3 domain-containing proteins [44]. Since we found that the deletion of the SH3 domain of β-PIX blocks the transient increase in interaction with the leptin receptor, it is possible that β-PIX directly binds to Grb-2 to form a complex with the leptin receptor through this association with Grb-2 and SHP-2.
β-PIX is a GEF that activates the small GTPase Rac1, which in turn activates the kinase PAK1, which interacts with β-PIX through its SH3 domain [41]. PAK1 has been shown to interact directly with Grb-2 [57], suggesting that PAK1 could be a part of leptin receptor interactome along with β-PIX. PAK1 is phosphorylated and activated by JAK2 [58], suggesting that leptin receptor activation might also facilitate the β-PIX–PAK1 interaction, and this signaling cascade might regulate downstream signaling pathways, including those leading to stabilization of the GABAA receptor at the plasma membrane.
Additionally, early developmental upregulation of K+-Cl- cotransporter 2 (KCC2) decreases the intracellular Cl- levels in mature neurons, and this causes GABA neurotransmitter to be a hyperpolarizing and inhibitory neurotransmitter [59–61]. During this period, leptin downregulates KCC2 expression, which underlies leptin’s actions to prolong the depolarizing actions of GABA [21]. β-PIX has been shown to interact with KCC2 [32]. It is therefore possible that KCC2 is also a part of leptin receptor interactome through its interaction with β-PIX, which could be one mechanism by which expression of KCC2 can be inhibited by leptin receptor during this critical developmental time.
It is very likely that many more proteins are associated with the leptin receptor and/or critical for the leptin receptor signaling. Clearly, future studies are necessary to further elucidate the complete leptin-receptor interactome and confirm its roles in vivo.
While leptin also increases excitatory glutamate synapse numbers, this occurs a few days later in development (P11–15) [16, 17, 53]. The effect of leptin to increase GABAergic synapses at a time when GABA is thought to be excitatory could be a mechanism by which leptin increases excitatory inputs at a slightly earlier stage (P7–11). As leptin also prolongs the depolarizing actions of GABA [21], it is possible that these two effects of leptin combine to increase GABA inputs contribute to excitatory balance slightly later in development, which could alter and refine synaptic connections during this critical dynamic developmental time point. Notably, altering leptin signaling changes the number of glutamate synaptic connections in both development and in adulthood [16, 17, 62, 63]. Future studies are required to address leptin’s role on GABAergic synapses in adults. It is possible that leptin only acts as an excitatory boost during development when GABA is a depolarizing neurotransmitter; however, understanding leptin’s action in adults are critical to understand its roles in excitation and inhibition imbalance.
Both enhanced and reduced levels of inhibitory synaptic transmission have been linked to neurodevelopmental disorders [64, 65]. It is possible that alterations in leptin levels could be one of the underlying causes for the development of the imbalance in GABAergic synaptic transmission. Increased leptin levels have been reported in several neurodevelopment disorders such as autism spectrum disorder, attention deficit hyperactivity disorder, and Rett syndrome that mainly manifest as impairments in hippocampal-dependent cognitive functioning [66–70]. Therefore, a better understanding of how leptin facilitates GABAergic synaptic connections and overall, the development of the hippocampus, may provide insights into neurodevelopmental disorders.
Materials and Methods
Drugs and DNA constructs.
Constructs expressing tagged proteins were constructed by amplifying β-PIX and long isoform of leptin receptor from rat cDNA and cloned into pCAGGS destination vectors containing the designated tags using Gateway cloning (ThermoFisher). The short hairpin RNAs (shRNA) targeting β-PIX (5’-GTTCGATACGACTGCCATCAA-3’) and leptin receptor (5’-GCTCACTGTCTGTTCAGTGAC-3’) were used as previously described [31, 34]. Full-length rat recombinant leptin (50nM, Peprotech #400-21) and STO-609 (20μM, Tocris #1551) were used as described in the text and figure legends. Mouse LepRb construct was used as shRNA-resistant LepRb, as shLepRb only targets rat LepRb mRNA. The mutant LepRb constructs LepRY985L and LepRY1138S were generous gifts from Martin Myers (University of Michigan). shRNA-resistant β-PIX was achieved by mutating 3 nucleotides using Q5 site-directed mutagenesis kit (NEB) according to manufacturer’s protocol. SH3 domain of β-PIX was deleted using Q5 DNA Polymerase (NEB) following manufacturer’s protocol.
Primary cell culture.
Animals used for hippocampal cultures were carried out in compliance with Washington State University IACUC approved protocols 03717-019 and 04409-006. Hippocampal neuronal cultures were prepared from equal numbers of P1 female and male Sprague-Dawley rat pups. Briefly, dissected hippocampi were collected in cold Hibernate A (Brain Bits). Then, the hippocampi were coarsely chopped and collected in the plating media (1% B27, 1% Glutamax, 10% horse serum, 2% HEPES, pH 7.5 in Neurobasal A medium) containing 0.25% Papain (Sigma #P3125) and 0.2% DNase (Sigma Aldrich #D5025) and incubated at 37°C for 20 to 25 min with mild shaking. After settling the tissue down, the media containing papain and DNase was removed and warm plating media was added, then the tissue were triturated 6 to 10 times with fire-polished glass pipette in this media to achieve suspension of single cells. The cells were counted and plated at the density of 3 × 104 cells/cm2 for 24-well plates, used for live staining experiments, and 4.7 × 104 cells/cm2 for 6-well plates, used for biochemistry experiments, on plates that were coated previously with Poly-L-Lysine. After 2 to 3 hours of plating, the plating media was changed with growth medium (1% B27 and 1% Glutamax in Neurobasal A medium) and maintained at 37°C and 5% CO2 [34]. On day in vitro (DIV) 4, the feeding media (1% B27, 1% Glutamax and 5μM cytosine-d-arabinofuranoside (AraC) in Neurobasal A medium) was added to neurons to constitute one third of the total media. Hippocampal neurons were transfected with various constructs on DIV5–6 and treated with leptin (50nM) on DIV8–9. All control conditions received the same amount of media at the time of reagent stimulation.
Hippocampal slice culture preparation and transfection.
Organotypic hippocampal slices were prepared from post-natal day 4–6, only female or male Sprague-Dawley rat pups. The hippocampi were dissected in cold, oxygenated low sodium ACSF (1mM CaCl2, 10mM D-glucose, 4mM KCl, 5mM MgCl2, 26mM NaHCO3, 234mM sucrose and 0.1% v/v phenol red). Excess liquid was drained from the hippocampi and the tissues were placed on the tissue chopper side by side. The hippocampi were cut into 400μm thick slices. Well defined and undamaged slices were selected under light microscope and placed onto 0.4μm pore sized polycarbonate membrane inserts which were placed in the 6-well plates with Slice Culture Media (SCM; 8.4g/L MEM Eagle medium, 20% Horse serum heat inactivated, 1mM L-glutamine, 1mM CaCl2, 2mM MgSO4, 1mg/l insulin, 0.00125% ascorbic acid solution, 13mM D-glucose, 5.2mM NaHCO3, 30mM HEPES, adjusted pH to 7.27–7.28, and osmolality to 320mOsm) [71]. The slice cultures were maintained for 3–4 days at 37°C and 5% CO2 and they were transfected with pCAGGS-tdTomato (a red fluorescent protein to identify transfected neurons) along with desired cDNA constructs, using a Helios Gene Gun (BioRad) at 170ppm. Following transfection, slices were maintained until day of recording by changing their media every other day. On the recording day, either vehicle or 50nM leptin was added both on top of the slice and in the media and incubated for at least 2 hours.
HEK293T cell culture.
HEK 293T cells were maintained in DMEM supplemented with 10% FBS and 1% Pen/Strep in 37°C incubator with a humidified atmosphere of 5% CO2 in air.
Transfection.
Primary rat hippocampal cultures were transfected with Lipofectamine 2000 (Life Technologies). Native media was collected before transfection and replaced with warm growth media. Lipofectamine 2000 and experimental DNA plasmids (0.5μg/well for 24-well plates, 2μg/well for 6-well plates) were added to cells and incubated for 30 min. The media was then aspirated and replaced back with native media. Hippocampal neurons were transfected with various constructs on DIV5–6. This protocol produces a transfection efficiency of 3% - 5% of total neurons transfected. HEK293T cells were transfected in 6-well plates (2μg/well) using Opti-MEM and Lipofectamine 2000 according to the manufacturer’s protocol.
Immuno- and live staining.
On DIV8–9, rat hippocampal neurons were treated with different drugs and incubated for indicated durations. Meanwhile, a humidity chamber was prepared with parafilm inside. Anti-GABAAR β2/3 antibody (Millipore #MAB341, 1:150) was diluted in the media taken from the cells and 30μl of droplets were placed on parafilm in the humidity chamber, and coverslips were placed onto the droplets up-side down. Neurons were incubated in the incubator for 15 minutes, and then they were washed in warm neurobasal media by dipping three times and they were placed in fixing solution (4% paraformaldehyde in PHEMS buffer [60mM PIPES, 25mM HEPES, 1mM MgCl2, 5mM EGTA], 87.6mM sucrose, pH 7.4) for 20 minutes at room temperature. In co-localization staining experiments, cells were permeabilized with 0.25% Tween-20 in PBS for 15 min with mild shaking and incubated with anti-Gephyrin (Synaptic Systems #147011, 1:750) and anti-VGAT (Synaptic Systems #131002, 1:750) for 2 hours at room temperature. If no intracellular protein staining was required, permeabilization step was skipped. After primary antibody incubation, coverslips were washed with PBS and incubated with different Alexa Fluor-tagged secondary antibodies (Thermo Fisher #A31571 and #A11035, 1:1000) for 1 hour at room temperature. After that, coverslips were mounted and imaged.
Image acquisition and analysis.
Confocal fluorescent images were obtained using Metamorph software and a Leica DMI6000 SD confocal microscope equipped with a Yokogawa CSU-X1 spinning disk, SD EMCCD. Images were acquired with a 20x air and a 60x oil immersion lens (NA: 1.4). For each neuron, 0.15μm step of z-stacks (total 3μm) were acquired. For live-staining experiments, all the conditions were imaged in <12 hours to minimize the loss of signal among treatment groups. In each independent culture, no primary antibody condition was imaged to obtain background staining and to determine thresholding for the analysis. For analysis of puncta staining, Fiji software was used. Transfection of pCAGGS-Clover was used as a mask (Region of Interest) and the puncta staining that were above thresholding intensity and the size of 4 pixels was considered as positive staining [33, 72]. For colocalization analysis, Measure Colocalization plug-in in MetaMorph software was used to obtain Pearson’s coefficients, which is based on the relative intensity of the signal in each channel for a given pixel [73, 74]. Analysis of at least one independent experiment in every condition was blinded to the experimenter.
Co-immunoprecipitation.
For co-immunoprecipitation (co-IP) done with HEK293T cells, the cells were plated to reach 60–70% confluency for the day of transfection and after 24 hours of transfection, the cells were collected. For co-IP done with neurons, DIV8–9 cultures were used. For both cell lines and primary neurons, the samples were treated either with 50nM leptin or vehicle for indicated durations. The samples were lysed with cold TNE buffer (1% Nonidet P-40, 140 mM NaCl, 5 mM EDTA, and 50 mM Tris-HCl, pH 8.0) that was supplemented with cOmplete protease inhibitor cocktail and phosphatase inhibitor cocktail 2 and 3 (Sigma Aldrich) on ice for 20–30 min and the lysates were centrifuged for 10 min at 16,000g [32]. 10% of the lysate was separated as input and the rest of the lysate was incubated with c-myc (Sigma-Aldrich #4439, 1:100) and V5 (Cell Signaling #13202, 1:100) antibodies for HEK293T cells, and β-PIX (Millipore #07–1450-I, 1:100) antibody for neurons for 2 hours at 4°C, rotating. Then, they were incubated with Protein A/G magnetic beads for another 2 hours at 4°C, rotating. Supernatant was discarded and the beads were washed four times, each for 10 min, with TNE buffer. The samples were eluted with 50mM DTT (Fisher Scientific) and NuPage LDS Sample Buffer (Life Technologies), by heating at 75°C for 10 min and analyzed by western blotting.
Surface biotinylation.
DIV8–9 cultures were used for surface biotinylation as described previously [75, 76]. Briefly, rat hippocampal neurons were incubated on ice with biotin solution (Sulpho-NHS-biotin (Pierce) at 0.5 mg/ml in PBS containing 2.5mM Ca2+/ 1mM Mg2+ for 30 min and the reaction was stopped with quenching buffer (PBS/Ca2+/Mg2+ containing 50mM Glycine) for 15min with mild shaking. Neurons were lysed for 15–20 min in cold TNE buffer, and the lysates were centrifuged to pellet cell debris. 15% of the supernatant was taken to use as input sample and the remainder was incubated for 2 hours with 50 μl Ultralink immobilized NeutrAvidin (Pierce) at 4°C to precipitate biotin labeled membrane proteins. Beads were washed four times with TNE buffer and analyzed by western blotting.
Western blotting.
Equal amounts of samples measured with BCA assay (Thermo Scientific #23225), were loaded into Bolt 4–12% Bis-Tris gels (Life Technologies). Proteins were transferred to a PVDF membrane (Life Technologies) overnight, blocked with 5% milk (or 5% BSA for surface biotinylation), and incubated with primary antibodies against GABAAR β2/3 (Millipore #MAB341, 1:500), β-PIX (Millipore #07–1450-I, 1:1000), β-actin (Cell Signaling #4970, 1:1000), c-myc (Sigma-Aldrich #4439, 1:1000) and V5 (Cell Signaling #13202, 1:1000) either for 2 hour at room temperature or overnight at 4°C. For western blot experiments after Co-IP experiments that were done using primary neurons, secondary antibodies that were light chain specific were used to avoid imaging heavy chain of IgG (Jackson Immunoresearch Laboratories, #115–605-174; 1:800 and #211–602-171; 1:800). For western blot experiments after surface biotinylation, HRP-linked secondary antibody was used (Cell signaling, #7076, 1:2000). For the rest of the western blot experiments, Alexa Fluor-647 secondary IgG F(ab’)2 fragment antibodies (Cell Signaling, #4414 and #4410; 1:5000) were used. Secondary antibodies were incubated with membrane for 1 hour at room temperature. Blots were imaged using a Chemidoc MP imaging system (Bio Rad) and analyzed using the ImageJ 1.48 gel analyzer tool [53].
Whole-cell and slice recordings.
For acute slice recordings, transverse hippocampal slices (230μm thick) were taken from P10-P11 mice in ACSF (126mM NaCl, 3.5mM KCl, 2mM CaCl2, 1.3mM MgCl2, 1.2mM NaH2PO4, 25mM NaHCO3, and 11mM glucose, pH 7.4 equilibrated with 95% O2 and 5% CO2, 306mOsm) supplemented with 1mM kynurenic acid to reduce neurotoxicity. The slices were let to recover in ACSF at 33°C with bubbling for an hour. After recovery period, for leptin condition, 50nM leptin was added and the slices were incubated for another 2 hours and recorded in the presence leptin. Slices were then transferred to a submerged recording chamber perfused with ACSF and recorded at 33.5°C. The following internal solutions was used; 110mM CsCl, 30mM K-gluconate, 10mM N-2-hydroxyethylpiperazine-N’−2-ethanesulfonic acid (HEPES), 1.1mM ethylene glycol-bis (β-aminoethyl ether)-N,N,N’,N’-tetra-acetic acid (EGTA), 0.1mM CaCl2, 4mM MgATP, and 0.3mM NaGTP (pH adjusted 7.3 with CsOH and 280mOsm).
For organotypic slice culture recordings, transfected rat hippocampal slice cultures were preincubated with vehicle or 50nM leptin for 2–3 hours at DIV7–9. The culture medium was exchanged by an extracellular solution (ACSF) containing 126mM NaCl, 2.5mM KCl, 4mM MgCl2, 4mM CaCl2, 1mM NaH2PO4, 26mM NaHCO3, 11mM glucose (pH 7.4 and 316mOsM). Cultures were allowed to equilibrate in a recording chamber at least for 30min and recorded at 33.5°C. Transfected cells were visualized with epifluorescence unit (Olympus Optical). The following internal solution was used: 110mM CsCl, 30mM K-gluconate, 10mM HEPES, 0.6mM EGTA, 2.5mM MgCl2, 10mM Naphosphocreatine, 4mM MgATP, and 0.4mM NaGTP (pH adjusted 7.3 with CsOH, and 306mOsm). For all recordings, miniature IPSCs (mIPSCs) were isolated pharmacologically by blocking NMDA and AMPA receptors with AP-5 (40μM; Cayman Chemical) and CNQX (5μM; Cayman Chemical), respectively, and action potential generation with tetrodotoxin (1μM; Tocris Bioscience).
Recording pipettes were pulled (P-97 Flaming/Brown micropipette puller; Sutter Instrument Company, Novato, CA) from standard-wall borosilicate glass without filament (o.d. = 1.5 mm; Sutter Instrument Company). The pipette-to-bath d.c. resistance of patch electrodes ranged from 2.5 to 3.5 MΩ. Recordings were obtained using a Multiclamp 700B amplifier (Molecular Devices, Sunnyvale, CA). Analog signals were low-pass Bessel-filtered at 10kHz, digitized at 10kHz through a Digidata 1440A interface (Molecular Devices), and stored in a computer using Clampex 10.7 software (Molecular Devices). The membrane potential was held at −70 mV. Data analysis was performed using Clampfit 10.7 software (Molecular Devices) and Mini-Analysis 6.0 software (Synaptosoft, Decatur, GA). The criteria for a successful recording included an electrical resistance of the seal between the outside surface of the recording pipette and the attached cell >1 GΩ and neuron input resistance >200 MΩ at whole-cell configuration and series resistance <20 MΩ. Series resistance was assessed repetitively in response to a 5mV pulse and cells exhibiting more than 20% change in series resistance and capacitance were excluded from analysis. The recording of the mIPSCs for analyses started 5 min after membrane rupture and lasted at least 5 min.
Statistical analysis.
All data were analyzed, and all graphs were generated with SigmaPlot 14.0. First, data sets were tested for normal distribution of the population. When the data sets have normal distribution, student’s t-test were used to compare two groups. Multiple groups were compared using one-way ANOVA followed by Tukey’s pairwise comparison. When the population was not large or did not have normal distribution; to analyze more than two groups, Kruskal-Wallis non-parametric ANOVA followed by Dunn’s post-hoc analysis was used and p-values were further adjusted by Holm FWER method; or to compare two groups, Mann-Whitney test were used. All experiments done on rat hippocampal primary and slice cultures were repeated at least 3 independent culture preparations. All immunostaining data are illustrated as individual points and/or as boxplots. For the boxplots, the box extends from the first (Q1) to third (Q3) quartiles. The line inside the box represents the median. The whiskers define the outermost data point that falls within upper inner and lower inner quartile fences [Q1 – 1.5(IQR)] and [Q3 – 1.5(IQR)], respectively. Statistical significance was set to a minimum of P<0.05.
Acknowledgements:
We thank M. Myers (University of Michigan) for the leptin receptor constructs LepRY985L and LepRY1138S.
Funding: This work was funded by the National Institutes of Health grant numbers MH086032 and HD092369 to G.A.W. and DK083452 to S.M.A.
Footnotes
Competing interests: The authors declare that they have no conflicting interests.
Data and materials availability: All data needed to evaluate the conclusions in the paper are present in the paper or the Supplementary Materials.
References and Notes
- 1.Pan WW and Myers MG Jr., Leptin and the maintenance of elevated body weight. Nat Rev Neurosci, 2018. 19(2): p. 95–105. [DOI] [PubMed] [Google Scholar]
- 2.Myers MG Jr., Leptin receptor signaling and the regulation of mammalian physiology. Recent Prog Horm Res, 2004. 59: p. 287–304. [DOI] [PubMed] [Google Scholar]
- 3.Domingos AI, et al. , Leptin regulates the reward value of nutrient. Nat Neurosci, 2011. 14(12): p. 1562–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zhao S, et al. , Hindbrain leptin and glucagon-like-peptide-1 receptor signaling interact to suppress food intake in an additive manner. Int J Obes (Lond), 2012. 36(12): p. 1522–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Huang XF, et al. , Localization of leptin receptor mRNA expression in mouse brain. Neuroreport, 1996. 7(15–17): p. 2635–8. [DOI] [PubMed] [Google Scholar]
- 6.Elmquist JK, et al. , Distributions of leptin receptor mRNA isoforms in the rat brain. J Comp Neurol, 1998. 395(4): p. 535–47. [PubMed] [Google Scholar]
- 7.Caron E, et al. , Distribution of leptin-sensitive cells in the postnatal and adult mouse brain. J Comp Neurol, 2010. 518(4): p. 459–76. [DOI] [PubMed] [Google Scholar]
- 8.Li XL, et al. , Impairment of long-term potentiation and spatial memory in leptin receptor-deficient rodents. Neuroscience, 2002. 113(3): p. 607–15. [DOI] [PubMed] [Google Scholar]
- 9.Sharma AN, et al. , Neurobehavioral deficits in db/db diabetic mice. Physiol Behav, 2010. 101(3): p. 381–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yamada N, et al. , Impaired CNS leptin action is implicated in depression associated with obesity. Endocrinology, 2011. 152(7): p. 2634–43. [DOI] [PubMed] [Google Scholar]
- 11.Winocur G, et al. , Memory impairment in obese Zucker rats: an investigation of cognitive function in an animal model of insulin resistance and obesity. Behav Neurosci, 2005. 119(5): p. 1389–95. [DOI] [PubMed] [Google Scholar]
- 12.Farr SA, Banks WA, and Morley JE, Effects of leptin on memory processing. Peptides, 2006. 27(6): p. 1420–5. [DOI] [PubMed] [Google Scholar]
- 13.Oomura Y, et al. , Leptin facilitates learning and memory performance and enhances hippocampal CA1 long-term potentiation and CaMK II phosphorylation in rats. Peptides, 2006. 27(11): p. 2738–49. [DOI] [PubMed] [Google Scholar]
- 14.Liu J, et al. , Acute administration of leptin produces anxiolytic-like effects: a comparison with fluoxetine. Psychopharmacology (Berl), 2010. 207(4): p. 535–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yook JS, et al. , Leptin in hippocampus mediates benefits of mild exercise by an antioxidant on neurogenesis and memory. Proc Natl Acad Sci U S A, 2019. 116(22): p. 10988–10993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bland T, et al. , Leptin controls glutamatergic synaptogenesis and NMDA-receptor trafficking via Fyn kinase regulation of NR2B. Endocrinology, 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dhar M, et al. , Leptin induces hippocampal synaptogenesis via CREB-regulated microRNA-132 suppression of p250GAP. Mol Endocrinol, 2014. 28(7): p. 1073–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Harvey J, Leptin regulation of neuronal morphology and hippocampal synaptic function. Front Synaptic Neurosci, 2013. 5: p. 3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Harvey J, Solovyova N, and Irving A, Leptin and its role in hippocampal synaptic plasticity. Prog Lipid Res, 2006. 45(5): p. 369–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Moult PR, Milojkovic B, and Harvey J, Leptin reverses long-term potentiation at hippocampal CA1 synapses. J Neurochem, 2009. 108(3): p. 685–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dumon C, et al. , The adipocyte hormone leptin sets the emergence of hippocampal inhibition in mice. Elife, 2018. 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Dumon C, et al. , Developmental Switch of Leptin Action on Network Driven Activity in the Neonatal Rat Hippocampus. Front Cell Neurosci, 2019. 13: p. 254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Blankenship AG and Feller MB, Mechanisms underlying spontaneous patterned activity in developing neural circuits. Nat Rev Neurosci, 2010. 11(1): p. 18–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ben-Ari Y, et al. , Giant synaptic potentials in immature rat CA3 hippocampal neurones. J Physiol, 1989. 416: p. 303–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Guimond D, et al. , Leptin potentiates GABAergic synaptic transmission in the developing rodent hippocampus. Front Cell Neurosci, 2014. 8: p. 235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Solovyova N, et al. , Bi-directional modulation of fast inhibitory synaptic transmission by leptin. J Neurochem, 2009. 108(1): p. 190–201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Matus A, Actin-based plasticity in dendritic spines. Science, 2000. 290(5492): p. 754–8. [DOI] [PubMed] [Google Scholar]
- 28.Honkura N, et al. , The subspine organization of actin fibers regulates the structure and plasticity of dendritic spines. Neuron, 2008. 57(5): p. 719–29. [DOI] [PubMed] [Google Scholar]
- 29.Szabo EC, Manguinhas R, and Fonseca R, The interplay between neuronal activity and actin dynamics mimic the setting of an LTD synaptic tag. Sci Rep, 2016. 6: p. 33685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sit ST and Manser E, Rho GTPases and their role in organizing the actin cytoskeleton. J Cell Sci, 2011. 124(Pt 5): p. 679–83. [DOI] [PubMed] [Google Scholar]
- 31.Saneyoshi T, et al. , Activity-dependent synaptogenesis: regulation by a CaM-kinase kinase/CaM-kinase I/betaPIX signaling complex. Neuron, 2008. 57(1): p. 94–107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Llano O, et al. , KCC2 regulates actin dynamics in dendritic spines via interaction with beta-PIX. J Cell Biol, 2015. 209(5): p. 671–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Smith KR, et al. , GIT1 and betaPIX are essential for GABA(A) receptor synaptic stability and inhibitory neurotransmission. Cell Rep, 2014. 9(1): p. 298–310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Dhar M, et al. , Leptin-induced spine formation requires TrpC channels and the CaM kinase cascade in the hippocampus. J Neurosci, 2014. 34(30): p. 10022–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Moss SJ and Smart TG, Constructing inhibitory synapses. Nat Rev Neurosci, 2001. 2(4): p. 240–50. [DOI] [PubMed] [Google Scholar]
- 36.Sahin GS, et al. , Leptin stimulates synaptogenesis in hippocampal neurons via KLF4 and SOCS3 inhibition of STAT3 signaling. Mol Cell Neurosci, 2020. 106: p. 103500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sarbassov DD, et al. , Phosphorylation and regulation of Akt/PKB by the rictor-mTOR complex. Science, 2005. 307(5712): p. 1098–101. [DOI] [PubMed] [Google Scholar]
- 38.Bjornholm M, et al. , Mice lacking inhibitory leptin receptor signals are lean with normal endocrine function. J Clin Invest, 2007. 117(5): p. 1354–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Bates SH, et al. , STAT3 signalling is required for leptin regulation of energy balance but not reproduction. Nature, 2003. 421(6925): p. 856–9. [DOI] [PubMed] [Google Scholar]
- 40.ten Klooster JP, et al. , Targeting and activation of Rac1 are mediated by the exchange factor beta-Pix. J Cell Biol, 2006. 172(5): p. 759–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Manser E, et al. , PAK kinases are directly coupled to the PIX family of nucleotide exchange factors. Mol Cell, 1998. 1(2): p. 183–92. [DOI] [PubMed] [Google Scholar]
- 42.Carpenter LR, et al. , Enhancing leptin response by preventing SH2-containing phosphatase 2 interaction with Ob receptor. Proc Natl Acad Sci U S A, 1998. 95(11): p. 6061–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Rahmouni K, et al. , Hypothalamic ERK mediates the anorectic and thermogenic sympathetic effects of leptin. Diabetes, 2009. 58(3): p. 536–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lowenstein EJ, et al. , The SH2 and SH3 domain-containing protein GRB2 links receptor tyrosine kinases to ras signaling. Cell, 1992. 70(3): p. 431–42. [DOI] [PubMed] [Google Scholar]
- 45.Lohmann C. and Kessels HW, The developmental stages of synaptic plasticity. J Physiol, 2014. 592(1): p. 13–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Bouret SG, Neurodevelopmental actions of leptin. Brain Res, 2010. 1350: p. 2–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Swanwick CC, et al. , Development of gamma-aminobutyric acidergic synapses in cultured hippocampal neurons. J Comp Neurol, 2006. 495(5): p. 497–510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Basarsky TA, Parpura V, and Haydon PG, Hippocampal synaptogenesis in cell culture: developmental time course of synapse formation, calcium influx, and synaptic protein distribution. J Neurosci, 1994. 14(11 Pt 1): p. 6402–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Khayenko V. and Maric HM, Targeting GABAAR-Associated Proteins: New Modulators, Labels and Concepts. Front Mol Neurosci, 2019. 12: p. 162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Luscher B, Fuchs T, and Kilpatrick CL, GABAA receptor trafficking-mediated plasticity of inhibitory synapses. Neuron, 2011. 70(3): p. 385–409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Jiang L, et al. , Tyrosine-dependent and -independent actions of leptin receptor in control of energy balance and glucose homeostasis. Proc Natl Acad Sci U S A, 2008. 105(47): p. 18619–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Huang H, et al. , Rho-kinase regulates energy balance by targeting hypothalamic leptin receptor signaling. Nat Neurosci, 2012. 15(10): p. 1391–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Bland T, et al. , USP8 Deubiquitinates the Leptin Receptor and Is Necessary for Leptin-Mediated Synapse Formation. Endocrinology, 2019. 160(8): p. 1982–1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Liu Q, et al. , Lipoprotein receptor LRP1 regulates leptin signaling and energy homeostasis in the adult central nervous system. PLoS Biol, 2011. 9(1): p. e1000575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.St-Pierre J. and Tremblay ML, Modulation of leptin resistance by protein tyrosine phosphatases. Cell Metab, 2012. 15(3): p. 292–7. [DOI] [PubMed] [Google Scholar]
- 56.Li C. and Friedman JM, Leptin receptor activation of SH2 domain containing protein tyrosine phosphatase 2 modulates Ob receptor signal transduction. Proc Natl Acad Sci U S A, 1999. 96(17): p. 9677–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Puto LA, et al. , p21-activated kinase 1 (PAK1) interacts with the Grb2 adapter protein to couple to growth factor signaling. J Biol Chem, 2003. 278(11): p. 9388–93. [DOI] [PubMed] [Google Scholar]
- 58.Rider L, et al. , JAK2 tyrosine kinase phosphorylates PAK1 and regulates PAK1 activity and functions. J Biol Chem, 2007. 282(42): p. 30985–96. [DOI] [PubMed] [Google Scholar]
- 59.Owens DF and Kriegstein AR, Is there more to GABA than synaptic inhibition? Nat Rev Neurosci, 2002. 3(9): p. 715–27. [DOI] [PubMed] [Google Scholar]
- 60.Sulis Sato S, et al. , Simultaneous two-photon imaging of intracellular chloride concentration and pH in mouse pyramidal neurons in vivo. Proc Natl Acad Sci U S A, 2017. 114(41): p. E8770–E8779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Ben-Ari Y, Excitatory actions of gaba during development: the nature of the nurture. Nat Rev Neurosci, 2002. 3(9): p. 728–39. [DOI] [PubMed] [Google Scholar]
- 62.McGregor G. and Harvey J, Regulation of Hippocampal Synaptic Function by the Metabolic Hormone, Leptin: Implications for Health and Neurodegenerative Disease. Front Cell Neurosci, 2018. 12: p. 340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.McGregor G, et al. , Age-dependent regulation of excitatory synaptic transmission at hippocampal temporoammonic-CA1 synapses by leptin. Neurobiol Aging, 2018. 69: p. 76–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Tabuchi K, et al. , A neuroligin-3 mutation implicated in autism increases inhibitory synaptic transmission in mice. Science, 2007. 318(5847): p. 71–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Chao HT, et al. , Dysfunction in GABA signalling mediates autism-like stereotypies and Rett syndrome phenotypes. Nature, 2010. 468(7321): p. 263–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Blardi P, et al. , Long-term plasma levels of leptin and adiponectin in Rett syndrome. Clin Endocrinol (Oxf), 2009. 70(5): p. 706–9. [DOI] [PubMed] [Google Scholar]
- 67.Blardi P, et al. , Rett syndrome and plasma leptin levels. J Pediatr, 2007. 150(1): p. 37–9. [DOI] [PubMed] [Google Scholar]
- 68.Pizzarelli R. and Cherubini E, Alterations of GABAergic signaling in autism spectrum disorders. Neural Plast, 2011. 2011: p. 297153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Banerjee A, et al. , Jointly reduced inhibition and excitation underlies circuit-wide changes in cortical processing in Rett syndrome. Proc Natl Acad Sci U S A, 2016. 113(46): p. E7287–E7296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Lozovaya N, et al. , Early alterations in a mouse model of Rett syndrome: the GABA developmental shift is abolished at birth. Sci Rep, 2019. 9(1): p. 9276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Opitz-Araya X. and Barria A, Organotypic hippocampal slice cultures. J Vis Exp, 2011(48). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Ippolito DM and Eroglu C, Quantifying synapses: an immunocytochemistry-based assay to quantify synapse number. J Vis Exp, 2010(45). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Adler J. and Parmryd I, Quantifying colocalization by correlation: the Pearson correlation coefficient is superior to the Mander’s overlap coefficient. Cytometry A, 2010. 77(8): p. 733–42. [DOI] [PubMed] [Google Scholar]
- 74.Dunn KW, Kamocka MM, and McDonald JH, A practical guide to evaluating colocalization in biological microscopy. Am J Physiol Cell Physiol, 2011. 300(4): p. C723–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Smith KR, et al. , Identification and characterisation of a Maf1/Macoco protein complex that interacts with GABAA receptors in neurons. Mol Cell Neurosci, 2010. 44(4): p. 330–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Twelvetrees AE, et al. , Delivery of GABAARs to synapses is mediated by HAP1-KIF5 and disrupted by mutant huntingtin. Neuron, 2010. 65(1): p. 53–65. [DOI] [PMC free article] [PubMed] [Google Scholar]