Abstract
PURPOSE:
Current FDA-approved imaging modalities are inadequate for localizing prostate cancer biochemical recurrence (BCR). 18F-DCFPyL is a highly selective, small-molecule PSMA-targeted PET radiotracer. CONDOR was a prospective study designed to determine the performance of 18F-DCFPyL-PET/CT in patients with BCR and uninformative standard imaging.
METHODS:
Men with rising PSA ≥0.2 ng/mL after prostatectomy or ≥2 ng/mL above nadir after radiation therapy were eligible. The primary endpoint was correct localization rate (CLR) defined as positive predictive value with an additional requirement of anatomic lesion co-localization between 18F-DCFPyL-PET/CT and a composite standard of truth (SOT). The SOT consisted of, in descending priority: 1) histopathology, 2) subsequent correlative imaging findings, or 3) post-radiation PSA response. The trial was considered a success if the lower bound of the 95% confidence interval for CLR exceeded 20% for 2 of 3 18F-DCFPyL-PET/CT readers. Secondary endpoints included change in intended management and safety.
RESULTS:
208 men with a median baseline PSA of 0.8 ng/mL (range: 0.2–98.4 ng/mL) underwent 18F-DCFPyL-PET/CT. The CLR was 84.8%–87.0% (lower bound of 95% CI: 77.8%–80.4%). 63.9% of evaluable patients had a change in intended management after 18F-DCFPyL-PET/CT. The disease detection rate was 59% to 66% (at least one lesion detected per patient by 18F-DCFPyL-PET/CT by central readers).
CONCLUSION:
Performance of 18F-DCFPyL-PET/CT achieved the study’s primary endpoint, demonstrating disease localization in the setting of negative standard imaging and providing clinically meaningful and actionable information. These data further support the utility of 18F-DCFPyL-PET/CT to localize disease in men with recurrent prostate cancer.
INTRODUCTION
A challenging clinical dilemma in the management of prostate cancer is the occurrence of a rising serum prostate specific antigen (PSA) after curative intent surgery or radiation therapy (RT) in the absence of informative conventional imaging.1–2 This condition, known as biochemical recurrence (BCR), indicates the presence of persistent or recurrent disease, without defining its location, and occurs in 20–50% of men within 10 years after definitive local therapy.3–6 This inability to define recurrent disease localization is an unmet need and reflects the shortcomings of both PSA and current standard-of-care imaging modalities. Conventional imaging (CT; MRI; bone scintigraphy) perform poorly in localizing sites of disease recurrence in patients with BCR, particularly when PSA values are low (<2.0 ng/mL).7–9 Novel positron emission tomography (PET) radiotracers, including FDA-approved agents (11C-choline and 18F-fluciclovine), have shown promise, but their predictive performance degrades with PSA levels <2.0 ng/mL.10–12 These limitations have stimulated the development of other agents with improved diagnostic performance, including radiotracers targeting the cell surface protein, prostate-specific membrane antigen (PSMA).13–16
18F-DCFPyL is a small molecule that binds to the extracellular domain of PSMA with high affinity17 and has shown success in studies evaluating the detection of prostate cancer across a range of disease states, including studies where histopathology served as reference standard.14,18–20
Building upon an earlier phase 2/3 multi-center trial (OSPREY) (NCT02981368)20 that evaluated the performance of 18F-DCFPyL-PET/CT in initial staging of high-risk prostate cancer and in detection of suspected recurrent/metastatic lesions seen on conventional imaging, CONDOR (NCT03739684) was designed to demonstrate the performance of 18F-DCFPyL-PET/CT in men with prostate cancer BCR.
METHODS
Trial Design
CONDOR was a phase 3, prospective, multicenter, open-label, single-arm study designed to evaluate the diagnostic performance and safety of 18F-DCFPyL-PET/CT in patients with suspected recurrent or metastatic prostate cancer with negative or equivocal conventional imaging (including 18F-fluciclovine or 11C-choline PET, CT, MRI, and/or whole-body bone scintigraphy) per institutional standard of care. The study was conducted across 13 sites in the United States and one in Canada, and was approved by the Institutional Review Board/Research Ethics Board at each participating institution. The study was conducted in accordance with the Declaration of Helsinki and the International Council on Harmonization Guidelines for Good Clinical Practice.
Study Population
Men ≥18 years of age with biochemically recurrent adenocarcinoma of the prostate treated with radical prostatectomy (RP) or RT were eligible for the study. BCR after RP was defined as a rising PSA to ≥0.2 ng/mL.21 For patients treated with RT, BCR was defined as a PSA value ≥2 ng/mL above the patient’s post-radiation nadir value.22 All enrolled patients required negative/equivocal findings for prostate cancer on standard-of-care imaging performed 60 days prior to 18F-DCFPyL injection. Before enrollment, written informed consent was obtained from all patients.
Exclusion criteria included administration of any high-energy (>300 KeV) gamma-emitting radioisotope within five physical half-lives prior to 18F-DCFPyL injection, and androgen deprivation therapy (ADT) within 3 months of imaging, or investigational therapy for prostate cancer within 60 days of imaging. Ongoing systemic therapy for prostate cancer was prohibited. Patients with medical conditions or circumstances that, in the opinion of the investigator, would compromise the safety or compliance of the patient to produce reliable data or completing the study were also excluded.
Screening
Demographic information, baseline characteristics (date of birth, race, ethnicity, height, and weight) and clinically relevant medical history were recorded. The patient’s prostate cancer medical history, including American Joint Committee on Cancer stage, Gleason score, pretreatment PSA, and all past/present therapies, were obtained. Standard-of-care imaging per local practice obtained within 60 days before 18F-DCFPyL administration was reviewed. This imaging could include CT or MRI, bone scintigraphy or PET with 18F-fluciclovine or 11C-choline. All baseline images were submitted to a central imaging core laboratory for assessment. A blood sample for total PSA obtained at screening or just before administration of 18F-DCFPyL from enrolled patients was analyzed by a central core laboratory.
Medical Management Questionnaire
The treating investigator completed a pre-18F-DCFPyL Medical Management Questionnaire (MMQ) to document the initial intended management plan for the patient based on available clinical information and local baseline imaging results, including CT, MRI, 11C-choline and 18F-fluciclovine PET. Within 60 days after 18F-DCFPyL-PET/CT, the treating Investigator completed the post-18F-DCFPyL MMQ to document whether the initial intended management plan had changed.
18F-DCFPyL dosing and PET/CT
The protocol-specified dose of 18F-DCFPyL was 9 mCi (333 MBq) administered intravenously (IV) 1–2 hours before PET/CT (Supplementary Table 1). Patients voided before imaging, and PET and non-contrast CT images were acquired from mid-thigh through skull vertex. All 18F-DCFPyL-PET/CT scans were submitted to the central imaging core laboratory for assessment. Patients with positive 18F-DCFPyL-PET/CT scans based on local interpretation were scheduled for subsequent follow-up to verify suspected lesion(s) based on a composite standard of truth (SOT). (Figure 1)
Figure 1. STARD flow diagram with Composite Standard of Truth (SOT) Validation.
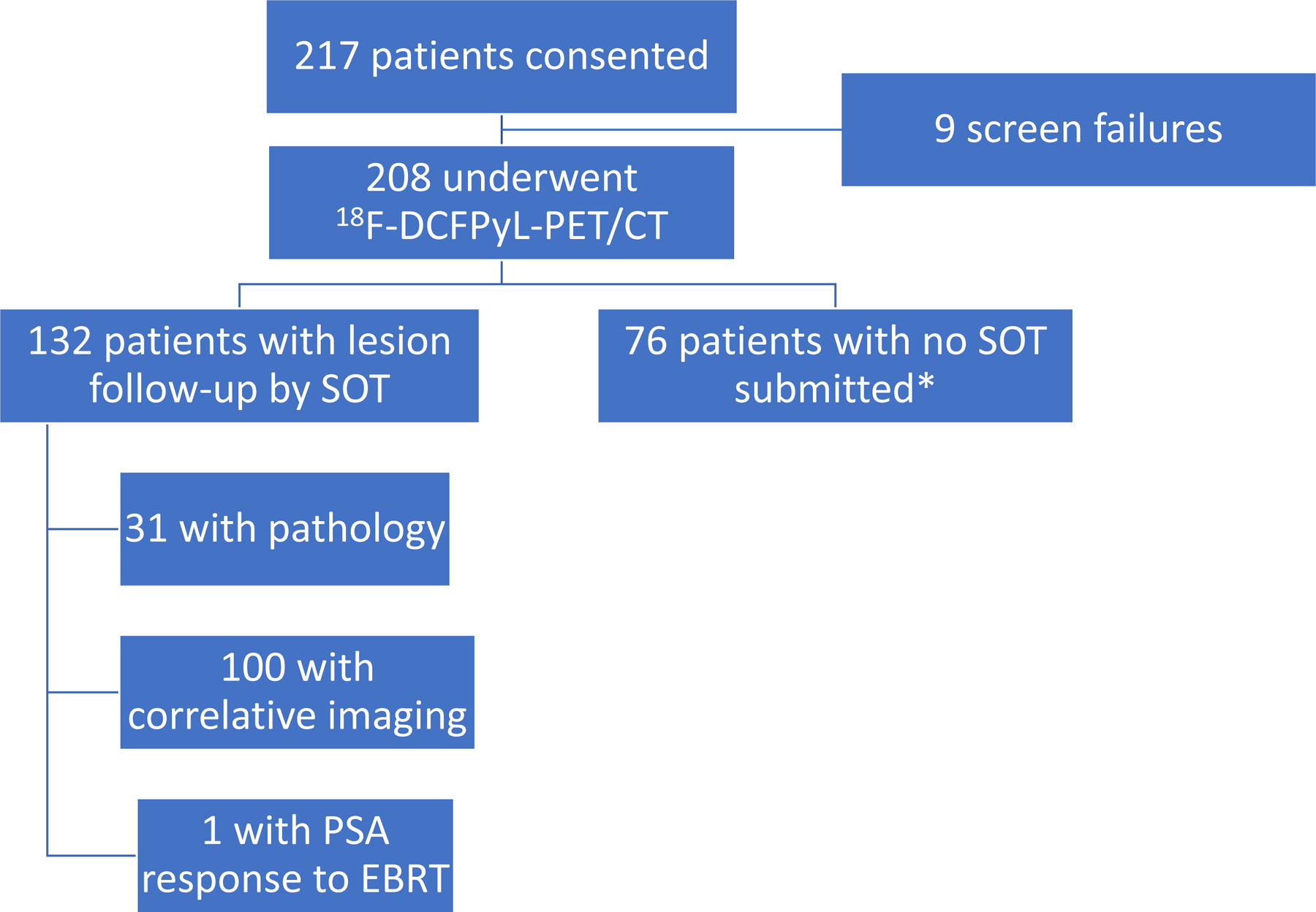
*Includes patients who withdrew from the study or did not have follow-up assessment due to negative 18F-DCFPyL-PET/CT per local read; EBRT = external beam radiation therapy
Tiered Composite SOT
Because of the expected absence of an amenable lesion for histologic verification in most patients, a composite SOT was employed following FDA discussions, based on assessment performed or initiated within 60 days following 18F-DCFPyL-PET/CT. These reference standards were defined (in order of priority) as (1) evaluable histopathology results from prostatectomy, salvage pelvic lymph node dissection or targeted biopsy; (2) correlative follow-up imaging findings using 18F-fluciclovine or 11C-choline PET, or focused MRI or CT; or (3) if neither of the above was available or informative, confirmed PSA response up to 9 months post-radiation initiation (without concomitant ADT) of all PET-positive foci. PSA response was defined as PSA decline by ≥50% from baseline that was confirmed on repeat measurement within 4 weeks, based on central laboratory results.
Central Imaging Review
A central imaging core laboratory was employed to independently manage image handling, reader training, reader sessions, and data collection. This review consisted of two discrete imaging evaluations:
18F-DCFPyL-PET/CT assessment was performed by three independent, blinded, board-certified nuclear medicine physicians trained in the interpretation of 18F-DCFPyL-PET. The readers had no access to any clinical information, including PSA values or other imaging available for a patient. Each reader independently evaluated a patient’s 18F-DCFPyL-PET/CT study without access to information from either of the other two readers, the Truth Panel, the local Investigator, or study sponsor. Each central reader also measured standardized uptake values (SUVs) on up to 25 PET-positive lesions seen on each scan.
Each image obtained as part of the SOT was assessed by two independent, board-certified nuclear medicine and radiology readers (the Truth Panel), who assessed these images in conjunction with the 18F-DCFPyL-PET/CT images for the presence or absence of prostate cancer. These readers also adjudicated the accuracy of needle placement during image-guided biopsy, if performed. The Truth Panel members were not members of the central 18F-DCFPyL-PET/CT reader group and were blinded to all data generated by the central 18F-DCFPyL-PET/CT readers.
Efficacy Outcomes
The primary endpoint of the study was the correct localization rate (CLR) of 18F-DCFPyL-PET/CT. CLR, a novel endpoint recommended by the FDA, is a measure of PPV at the patient level that employed anatomic lesion location matching (co-localization) of a lesion identified by 18F-DCFPyL-PET/CT central readers and the lesion identified by Truth Panel central readers and/or pathology. CLR was defined as the percentage of patients with a one-to-one correspondence between at least one lesion identified on 18F-DCFPyL-PET/CT by the central readers and the composite SOT.
The secondary endpoints were the percentage of patients with a change in intended prostate cancer treatment plans after 18F-DCFPyL-PET/CT based on the MMQ that was completed pre-and post-18F-DCFPyL-PET/CT, as well as safety of 18F-DCFPyL. Exploratory endpoints were evaluation of detection rates and PPVs of 18F-DCFPyLPET/CT at the region level (i.e., prostate/prostate bed, pelvis, and extra-pelvic regions) and detection rate of 18F-DCFPyL-PET/CT as a function of baseline PSA (<0.5, 0.5–<1.0, 1.0–<2.0, 2.0–<5.0, or ≥5.0 ng/mL).
Statistical Methods
A full description of the determination of sample size is provided in the study protocol (Supplementary Appendix). Based on a meta-analysis by Perera et al23 of 68Ga-PSMA PET/CT, approximately 76% of PSMA scans are positive in suspected prostate cancer recurrence. In this study, 60% of the imaged patients were expected to have a positive 18F-DCFPyL-PET/CT scan and of these 30% were expected to have a confirmatory SOT finding in patients with negative/equivocal baseline imaging. Therefore, 18F-DCFPyL-PET/CT was expected to detect/localize recurrent disease in approximately 18% of the study population versus ≤5% identified by conventional imaging. This required a total of 81 positive 18F-DCFPyL scans, or 134 evaluable patients. Accounting for a 30% non-evaluable/loss rate, the sample size was 192 patients.
The safety and efficacy populations for analysis consisted of all patients who received 18F-DCFPyL.
Primary Endpoint Analysis
The CLR is computed as 100×TP/(TP+FP), where TP = true positive result and FP = false positive result for each central imaging reader. A TP result is defined as a patient with both a positive lesion(s) on 18F-DCFPyL-PET/CT and a positive result on the composite SOT within the same anatomic location as defined within the Statistical Analysis Plan. The classification of the anatomic locations is provided in Supplementary Table 3.24 A FP result was defined as a patient with positive lesion(s) on 18F-DCFPyL-PET/CT identified by the central reader with negative findings for prostate cancer according to the composite SOT. The success criterion for the primary endpoint was the lower limit of the 95% CI to exceed 20% for at least 2 of the 3 readers. This criterion was based on data from the performance characteristics of other molecular imaging agents at PSA values <2 ng/mL.25 The two-sided 95% confidence interval (CI) for CLR for each reader was calculated using the normal approximation for a single binomial variable.
Secondary Endpoint Analysis
The percentage of patients with a change in intended prostate cancer treatment plan before and after 18F-DCFPyL-PET/CT was reported with the corresponding two-sided 95% CI using the normal approximation for the binomial variable.
Exploratory Endpoint Analysis
The disease detection rate was defined as the percent of positive 18F-DCFPyL PET/CT scans identified by the central imaging readers. It was calculated as the number of patients with positive scans divided by the number of patients who have evaluable scan results reported × 100%. PPV within anatomic regions without lesion-level matching was calculated for patients with positive 18F-DCFPyL-PET/CT scans as TP/(TP+FP) × 100%. Detection rate and PPV by region (i.e., prostate/prostate bed, pelvic, extra-pelvic) and as a function of baseline PSA were analyzed for each central imaging reviewer and for the local site interpretation separately using a two-sided 95% CI based on a normal approximation to the binomial distribution.
Inter-and Intra-reader Agreement
Inter-reader agreement between the central readers at the subject level was assessed using Fleiss’ generalized kappa. Agreement between each central reader and the local reader was calculated using Cohen’s pairwise kappa. For intra-reader agreement, each central reader assessed 42 subjects (20%) twice as part of their reading schedule after a washout period of at least 28 days following the initial review, without knowledge that the cases were read twice. Cohen’s kappa was calculated to assess agreement for each reader. All kappa statistics are reported with their respective 95% confidence intervals.
Safety Outcomes
Safety assessments included monitoring for the incidence of treatment-emergent adverse events (AEs) from the time of 18F-DCFPyL dosing up to 7±3 days post-dose. AEs were graded according to the National Cancer Institute Common Terminology Criteria for Adverse Events, Version 4.03.
RESULTS
The Standards for Reporting of Diagnostic Accuracy (STARD) flow diagram is depicted in Figure 1. 208 patients were enrolled between November 2018 and August 2019. The median age was 68 years (range 43–91); 67.8% were ≥65 years old. The median baseline PSA level was 0.8 ng/mL (range 0.2–98.4); 68.8% of patients had PSA levels <2.0 ng/mL. The median time from the initial prostate cancer diagnosis was 71 months (range 3–356). Prior treatment was RP in 49.5%, RP and RT in 35.6%, and radiation alone in 14.9%. Baseline characteristics, PET imaging and baseline conventional imaging details are further summarized in Table 1, Supplementary Figure 1 and Supplementary Table 3.
Table 1.
Baseline characteristics and 18F-DCFPyL dosing/uptake time
| Characteristics | n = 208 |
|---|---|
| Age (years): median (range) | 68 (43, 91) |
| Age ≥65 years, n (%) | 141 (67.8) |
| Months from prostate cancer diagnosis: median (range) | 71 (3, 356) |
| Prior prostate cancer therapies | |
| RP only, n (%) | 103 (49.5) |
| RT only, n (%) | 31 (14.9) |
| RP and RT, n (%) | 74 (35.6) |
| At least 1 prior systemic therapy, n (%) | 58 (27.9) |
| Total Gleason Score, n (%) | |
| < 8 | 153 (73.6) |
| < 6 | 3 (1.4) |
| 3 + 3 = 6 | 12 (5.8) |
| 3 + 4 = 7 | 78 (37.5) |
| 4 + 3 = 7 | 60 (28.8) |
| ≥ 8 | 55 (26.4) |
| 3 + 5 = 8 | 0 |
| 4 + 4 = 8 | 21 (10.1) |
| 5 + 3 = 8 | 0 |
| 4 + 5 = 9 | 31 (14.9) |
| 5 + 4 = 9 | 3 (1.4) |
| 5 + 5 = 10 | 0 |
| PSA (ng/mL) (n=202): Median (range) | 0.8 (0.17, 98.45) |
| PSA sample collection study day prior to administration of 18F-DCFPyl (Study Day) (n=202): Median (range) | 1 (−29, 1) |
| PSA Group (n=202), n (%) | |
| <2.0 ng/mL | 139 (68.8) |
| <0.5 ng/mL | 69 (34.2) |
| 0.5 to <1.0 ng/mL | 37 (18.3) |
| 1.0 to <2.0 ng/mL | 33 (16.3) |
| ≥2.0 ng/mL | 63 (31.2) |
| 2.0 to <5.0 ng/mL | 33 (16.3) |
| ≥5.0 ng/mL | 30 (14.9) |
| 18 F-DCFPyL dosing and uptake time | |
| Administered activity (mCi/MBq): median (range) | 9.42 (7.49–11.07)/349 (277–410) |
| Time from injection to imaging (minutes): median (range) | 79 (59–115) |
Abbreviations: PSA: Prostate-specific antigen; RP: Radical prostatectomy; RT: Radiation therapy
18F-DCFPyL-PET/CT detected ≥1 lesion in 59.1% to 65.9% patients as assessed by three independent blinded central readers. The primary endpoint of CLR was met as the lower limit of 95% CI exceeded 20% for all three readers. The CLR ranged from 84.8% to 87.0% among the three readers (the lower bound of the 95% CI ranged from 77.8% to 80.4%) (Table 2).
Table 2.
Disease detection and CLR rate across three independent readers
| All Patients (n=208) | ||||||
|---|---|---|---|---|---|---|
| Reader 1 | 95% CI (%) | Reader 2 | 95% CI (%) | Reader 3 | 95% CI (%) | |
| Negative PyL Scan | 71 (34.1%) | (27.7, 40.6) | 84 (40.4%) | (33.7, 47.1) | 85 (40.9%) | (34.2, 47.5) |
| Positive PyL Scan | 137 (65.9%) | (59.4, 72.3) | 124 (59.6%) | (52.9, 66.3) | 123 (59.1%) | (52.5, 65.8) |
| Unevaluable * | 33 | 24 | 24 | |||
| (85.6%) | (78.8, 92.3) | (87.0%) | (80.4, 93.6) | (84.8%) | (77.8, 91.9) | |
Some patients were unevaluable for the primary endpoint, either because no SOT was submitted (between 17–25 patients, depending on the central reader), or because the 18F-DCFPyL-PET/CT scan was deemed a false negative based on lesion level co-localization (7–8 patients), which was not a component of the primary endpoint calculation (due in part to the lack of specificity of comparative conventional imaging modalities).
The performance of 18F-DCFPyL-PET/CT by CLR and PPV was maintained through all categories of the SOT: histopathology (N=31): 78.6–82.8% and 92.9–93.3% for CLR and PPV, respectively; correlative imaging (N=100): 86.1–88.6% and 87.0–89.5% for CLR and PPV, respectively; and PSA response (N=1): 100% for both CLR and PPV. Further analyses of the correlative imaging results showed CLR remained high across the different modalities used a) 18F-fluciclovine-PET/CT (N=71): (86.8–90.9%); b) MRI (N=23): (80.0–86.7%); and c) CT (n=6): (80.0–100%) (Supplementary Tables 4–6). The CLRs for each reader also were maintained across prior treatment regimens (Supplementary Table 7) and increased with the within-patient maximum standardized uptake value (SUVmax) of lesions identified on 18F-DCFPyL-PET/CT (Supplementary Table 8).
CLR by Baseline PSA and Detection Rate
In patients with baseline PSA levels <0.5 ng/mL, the median CLR was 73.3% while patients with a PSA of ≥5 ng/mL had a median CLR of 96.4 % (Figure 2A and Supplementary Table 9). The detection rate rose with increasing PSA levels ranging from 36.2% (<0.5 ng/mL) to 96.7% (≥5 ng/mL) (Figure 2B and Supplementary Table 10).
Figure 2. CLR (A) and detection rate (B) by baseline PSA levels.
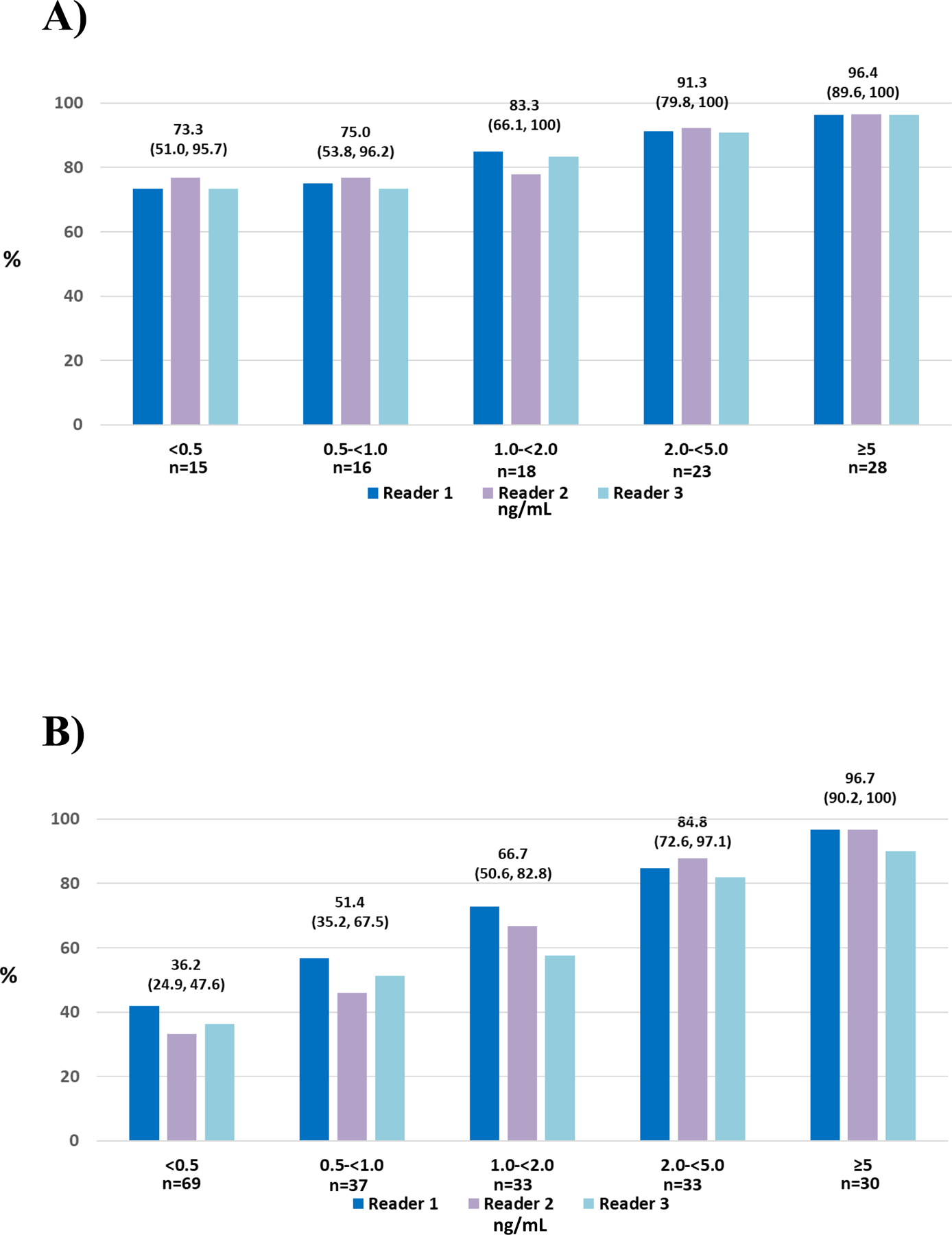
*Median (95% CI) for each group of three readers provided
Abbreviations: CLR: Correct localization rate; PSA: Prostate-specific antigen
Positive Predictive Value by Anatomic Region
PPV of 18F-DCFPyL-PET/CT was determined in detection of recurrent disease by anatomic regions (prostate/prostate bed, pelvis, and extra-pelvic regions) from the composite SOT in patients with at least one 18F-DCFPyL-positive lesion. The PPV was consistently high across all anatomic regions. The PPV in the prostatic region ranged between 75.0% and 83.3% among the three independent readers. Similarly, for pelvic lymph nodes, the PPV was between 67.2% and 72.7%, and for the extra-pelvic regions, it ranged from 67.3% to 69.8%. (Figure 3 and Supplementary Tables 11 and 12). The distribution of positive 18F-DCFPyL-PET/CT findings by anatomic region is shown in Supplementary Table 13.
Figure 3. PPV by anatomic region (A) and extra-pelvic region (B).
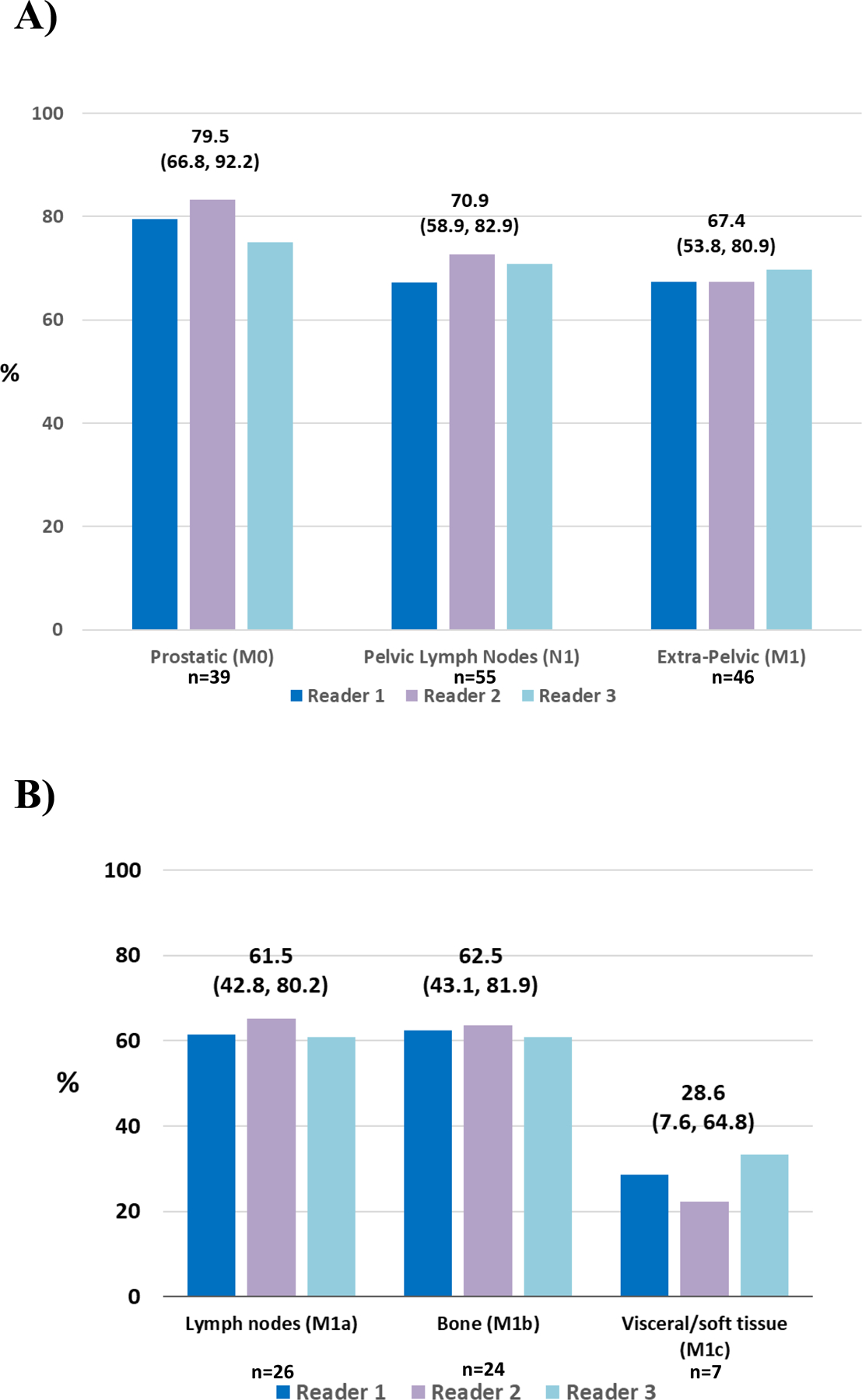
*Median (95% CI) for each group of three readers provided; PPV: Positive predictive value
Inter-and Intra-reader Agreement
Reader agreement results are summarized in Supplementary Table 14. Inter-reader agreement had a concordance of 75% and Fleiss’ kappa of 0.65 (95%CI: 0.58, 0.73). Agreement between the central and local readers had concordances of 83.2 % to 83.7% and kappas of 0.62 (95%CI: 0.50, 0.73), 0.65 (95%CI: 0.54, 0.75) and 0.64 (95%CI: 0.53.0.74) for the three readers. Intra-reader agreement had kappas of 0.94 (95% CI: 0.82,1.0), 1.0 and 0.81 (95% CI 0.64, 0.98) for the three readers.
Change in Planned Medical Management
The treating physicians completed pre- and post-18F-DCFPyL-PET/CT MMQs for 205 patients. Nearly two-thirds (63.9%; n = 131) of these patients had a change in intended disease management plan. Of these 131 patients, 103 (78.6%) were associated with positive 18F-DCFPyL-PET/CT findings, and 28 (21.4%) were associated with negative findings. Of the 144 patients that had a positive 18F-DCFPyL scan, 103 (72.5%) had a recommended change in management. The most frequent changes to treatment management plans after the 18F-DCFPyL-PET/CT imaging results included salvage local therapy that was either supplemented or replaced by systemic therapy (n = 58; 28.3%), observation to initiating therapy (n = 49; 23.9%), systemic therapy to salvage local therapy (n = 43; 21.0%), and planned treatment to observation (n = 9; 4.4%). (Figure 4 and Supplementary Table 15)
Figure 4.
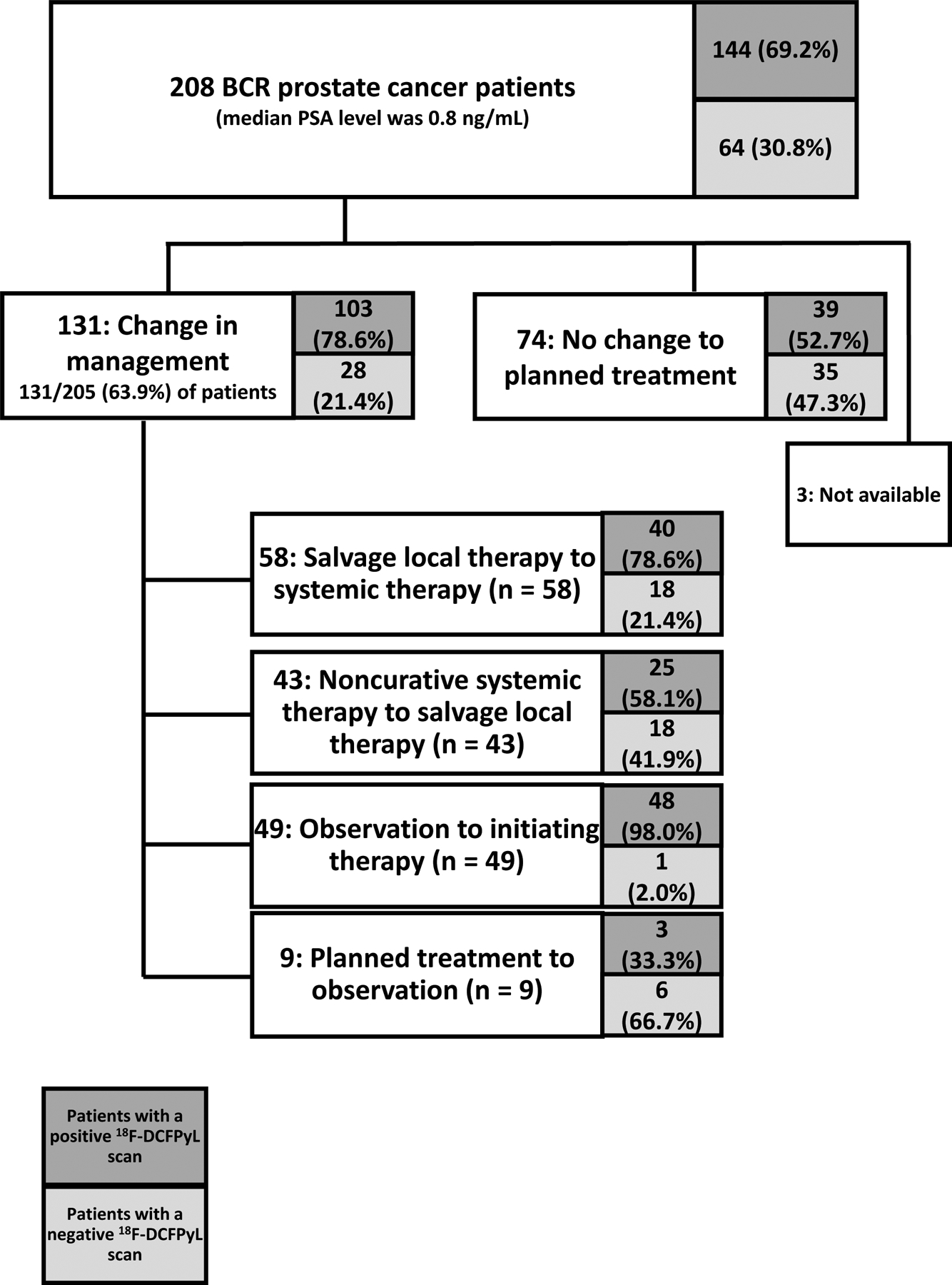
Change in Planned Medical Management
Safety Results
Fourteen (6.7%) patients experienced 21 AEs; headache (1.9%), fatigue (1.0%), and hypertension (1.0%) were most frequent. Only one patient (0.5%), with a significant history of allergic reactions, experienced serious, Grade 3 AEs (hypersensitivity, headache, paresthesia), all of which resolved. There were no Grade 4 AEs or deaths.
DISCUSSION
This study was designed to evaluate the performance of 18F-DCFPyL-PET/CT in prostate cancer patients with BCR and non-informative standard-of-care imaging. CONDOR used a central reader paradigm, and a novel primary efficacy endpoint with a composite SOT. The study demonstrated a high CLR that suggests 18F-DCFPyL-PET/CT is a superior and reliable tool for the detection and localization of sites of disease in men with BCR relative to conventional imaging.
The CONDOR population generally represents BCR patients with low PSA values (median PSA 0.8 ng/mL), when crucial clinical decisions are made as to whether the patient warrants salvage local or metastasis-directed therapy with curative intent, or systemic therapy without curative intent, or some combination of local and systemic treatments. An accurate understanding of the location and burden of disease is key to well-informed treatment planning. In our study population, the PSA was <2.0 ng/mL in 68.8% of patients, <1.0 ng/mL in 52.5% and <0.5 ng/mL in 34.2%. As such, the study provides prospective evidence of diagnostic accuracy to reliably detect prostate cancer recurrence or metastases in patients in whom currently available conventional imaging and approved molecular imaging modalities are suboptimal. Notably, a total of 59.6% to 65.9% of patients had at least one occult lesion detected among the three readers and the CLR was consistently high across all SOT determinations, anatomic regions, and in patients with PSA 0.2 to <2.0 ng/mL (>73%). This performance of 18F-DCFPyL-PET/CT is substantially better than the reported detection rates and PPVs of 18F-fluciclovine- and 11C-choline-PET in patients with similar PSA ranges, although each tracer may behave differently depending on risk factors beyond absolute PSA value, such as Gleason grade, growth rate, histologic subtype, and other measures.11,12,15
Another PSMA-targeted PET radiopharmaceutical, 68Ga-PSMA-11, is widely used in clinical practice outside of the United States and recently received FDA approval.26 In a prospective study by Fendler et al., this imaging agent had similar results, with PPV of 0.84 (95% CI, 0.75–0.90) by histopathologic comparator (primary endpoint, n = 87) and 0.92 (95% CI, 0.88–0.95) by a composite reference standard, in men with BCR; of note, unlike CONDOR, in Fendler’s study, patients were eligible irrespective of prior conventional imaging findings.27 Eiber et al. was one of the first to report positive findings with 68Ga-PSMA-11 in post-RP BCR patients with low PSA values (<0.5 ng/mL).28 However, retrospective studies with 68Ga-PSMA-11, while including prespecified endpoints and statistical designs, did not require negative standard imaging.29, 30
This study furnishes direct evidence that clinicians intend to utilize the additional information provided by the 18F-DCFPyL scan to revise their treatment plans and goals of care relative to their original plans based on clinical presentation and non-informative standard imaging. The ability of 18F-DCFPyL-PET/CT to localize and detect the extent of recurrent disease offers physicians the opportunity to adjust and tailor their treatment planning and potentially improve treatment outcomes in men with recurrent prostate cancer. Although change in patient management after PET occurred frequently, this study was not designed to determine whether such changes were implemented or ultimately benefited patients. Further, this study, which was designed to establish the relationship between a positive imaging finding and a positive composite standard of truth, does not establish that a given PSMA-avid lesion represents the only lesion that is producing PSA, or that a specifically identified lesion represents the clone of the disease likely to be lethal for the patient. However, a recent multicenter prospective study of PET/CT using 68Ga-PSMA-11 did show that negative or prostate-bed-only positive findings were highly predictive of response to salvage RT after RP. These results show that PSMA-PET can help to select patients most likely to benefit from a particular therapy.31 However, future studies will be necessary to demonstrate whether 18F-DCFPyL-PET/CT-directed changes in management lead to improved outcomes for prostate cancer patients with BCR.
While prospective single-center trials with 18F-DCFPyL-PET imaging in BCR have been reported recently, only Mena et al. reported PPV verified by a composite SOT.6,14,32,33 CONDOR represents the first multicenter prospective trial of 18F-DCFPyL-PET for the BCR population. By design, the study focused on CLR, which fundamentally is a patient-level PPV with the added criterion of anatomic co-localization. Consequently, a limitation of this study is that the “truth” in men with negative 18F-DCFPyL-PET/CT per local radiology assessment is unknown, because verification of 18F-DCFPyL-PET/CT results was not required in such patients. Trying to find occult disease not detected by PET would have required following these patients without treatment to see if the disease became evident over time; this was not a practical or ethical option. Thus, we cannot determine whether these false-negative cases reflect PSMA-negative disease (which occurs in 5–10% of prostate cancers),34 inexperience of local readers, or small-volume disease (similar to the poor detection of small nodal deposits in OSPREY Cohort A),20 or obscuration of lesions in or adjacent to organs with high uptake of 18F-DCFPyL (e.g., liver) or structures containing excreted tracer (ureters, bladder, urethra). Accordingly, the negative predictive value of 18F-DCFPyL-PET/CT in this patient population with non-informative standard imaging could not be assessed.
PSMA-targeted PET radiopharmaceuticals labeled with 18F can offer an alternative to 68Ga agents. There are few direct comparisons of the two tracers,35,36 but these suggest that the performance of18F-DCFPyl is similar to that of the 68Ga agents. In summary, this study met its primary endpoint of high CLR and demonstrated that the additional information provided by 18F-DCFPyL-PET/CT was associated with frequent changes in disease management plans. These data support 18F-DCFPyL-PET/CT as a safe and robust imaging tool to reliably detect recurrent prostate cancer, even at low PSA levels, thus providing new actionable information by the localization of otherwise occult disease.
Supplementary Material
Translational Relevance.
In men with biochemically recurrent prostate cancer (BCR), there is an unmet medical need for accurate disease localization such that treatment planning will be appropriate to the distribution of disease. These clinical decisions are generally made at low PSA values, when standard imaging performs poorly at demonstrating disease. PSMA-targeted PET has been a promising candidate to detect disease otherwise not demonstrated by standard techniques. CONDOR is a prospective, multicenter study designed to demonstrate the diagnostic performance of the PSMA-targeted PET radiotracer 18F-DCFPyL for regulatory approval. The trial demonstrated that the radiotracer correctly localized disease in approximately 85% of men with BCR, all of whom had uninformative conventional imaging. These findings changed planned management in 64% of men. These data support the use of 18F-DCFPyL-PET/CT for disease localization in men with BCR, as it is significantly superior to standard imaging.
Acknowledgements
We thank the participants who volunteered to take part in this trial, as well as blinded readers, truth panel members, and all members of the trial teams at each participating institution. This study was funded by Progenics Pharmaceuticals, Inc.
Footnotes
See Appendix
Conflict of interest statement: COI’s have been submitted electronically.
References
- 1.Amling CL, Blute ML, Bergstralh EJ, Seay TM, Slezak J, Zincke H. Long-term hazard of progression after radical prostatectomy for clinically localized prostate cancer: continued risk of biochemical failure after 5 years. J Urol 2000; 164: 101–105. [PubMed] [Google Scholar]
- 2.Han M, Partin AW, Zahurak M, Piantadosi S, Epstein JI, Walsh PC. Biochemical (prostate specific antigen) recurrence probability following radical prostatectomy for clinically localized prostate cancer. J Urol 2003; 169: 517–523. [DOI] [PubMed] [Google Scholar]
- 3.Freedland SJ, Humphreys EB, Mangold LA, et al. Risk of prostate cancer-specific mortality following biochemical recurrence after radical prostatectomy. JAMA 2005; 294: 433–439. [DOI] [PubMed] [Google Scholar]
- 4.Kestin LL, Vicini FA, Ziaja EL, Stromberg JS, Frazier RC, Martinez AA. Defining biochemical cure for prostate carcinoma patients treated with external beam radiation therapy. Cancer 1999; 86: 1557–1566. [DOI] [PubMed] [Google Scholar]
- 5.Paller CJ, Antonarakis ES. Management of biochemically recurrent prostate cancer after local therapy: evolving standards of care and new directions. Clin Adv Hematol Oncol 2013; 11: 14–23. [PMC free article] [PubMed] [Google Scholar]
- 6.Song H, Harrison C, Duan H, et al. Prospective evaluation in an academic center of 18FDCFPyL-PET/CT in biochemically recurrent prostate cancer: a focus on localizing disease and changes in management. J Nucl Med 2020; 61:546–551. [DOI] [PubMed] [Google Scholar]
- 7.Shinohara K, Wheeler TM, Scardino PT. The appearance of prostate cancer on transrectal ultrasonography: correlation of imaging and pathological examinations. J Urol 1989; 142: 76–82. [DOI] [PubMed] [Google Scholar]
- 8.Beresford MJ, Gillatt D, Benson RJ, Ajithkumar T. A systematic review of the role of imaging before salvage radiotherapy for post-prostatectomy biochemical recurrence. Clin Oncol (R Coll Radiol) 2010; 22: 46–55. [DOI] [PubMed] [Google Scholar]
- 9.De Visschere PJL, Standaert C, Fütterer JJ, et al. A systematic review on the role of imaging in early recurrent prostate cancer. Eur Urol Oncol 2019; 2: 47–76. [DOI] [PubMed] [Google Scholar]
- 10.Schuster DM, Nieh PT, Jani AB, et al. Anti-3-[18F]FACBC positron emission tomography-computerized tomography and 111In-capromab pendetide single photon emission computerized tomography-computerized tomography for recurrent prostate carcinoma: results of a prospective clinical trial. J Urol 2014;191: 1446–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Calais J, Ceci F, Eiber M, et al. 18F-fluciclovine PET-CT and 68Ga-PSMA-11 PET-CT in patients with early biochemical recurrence after prostatectomy: a prospective, single centre, single-arm, comparative imaging trial. Lancet Oncol 2019; 20: 1286–1294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nanni C, Zanoni L, Pultrone C, et al. 18F-FACBC (anti1-amino-3-18F-fluorocyclobutane-1-carboxylic acid) versus 11C-choline PET/CT in prostate cancer relapse: results of a prospective trial. Eur J Nucl Med Mol Imaging 2016; 43: 1601–1610. [DOI] [PubMed] [Google Scholar]
- 13.Afshar-Oromieh A, Malcher A, Eder M, et al. PET imaging with a [68Ga]gallium-labelled PSMA ligand for the diagnosis of prostate cancer: biodistribution in humans and first evaluation of tumour lesions. Eur J Nucl Med Mol Imaging 2013; 40: 486–495. [DOI] [PubMed] [Google Scholar]
- 14.Rowe SP, Macura KJ, Mena E, et al. PSMA-based [18F]DCFPyL PET/CT is superior to conventional imaging for lesion detection in patients with metastatic prostate cancer. Mol Imaging Biol 2016; 18: 411–419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Herrmann K, Bluemel C, Weineisen M, et al. Biodistribution and radiation dosimetry for a probe targeting prostate-specific membrane antigen for imaging and therapy. J Nucl Med 2015; 56: 855–861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Afshar-Oromieh A, Hetzheim H, Kratochwil C, et al. The theranostic PSMA ligand PSMA-617 in the diagnosis of prostate cancer by PET/CT: biodistribution in humans, radiation dosimetry, and first evaluation of tumor lesions. J Nucl Med 2015; 56: 1697–1705. [DOI] [PubMed] [Google Scholar]
- 17.Chen Y, Pullambhatla M, Foss CA, et al. 2-(3-{1-Carboxy-5-[(6-[18F] fluoro-pyridine-3-carbonyl)-amino]-pentyl}-ureido)-pentanedioic acid, [18F] DCFPyL, a PSMA-based PET imaging agent for prostate cancer. Clin Cancer Res 201117: 7645–7653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Szabo Z, Mena E, Rowe SP, et al. Initial evaluation of [18F]DCFPyL for prostate-specific membrane antigen (PSMA)-targeted PET imaging of prostate cancer. Mol Imaging Biol 2015; 17: 565–574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gorin MA, Rowe SP, Patel HD, et al. Prostate specific membrane antigen targeted 18F-DCFPyL positron emission tomography/computerized tomography for the preoperative staging of high risk prostate cancer: results of a prospective, phase II, single center study. J Urol 2018; 199: 126–132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pienta KJ, Gorin MA, Rowe SP, et al. A phase 2/3 prospective multicentre study of the diagnostic accuracy of prostate-specific membrane antigen (PSMA) PET/CT with 18F-DCFPyL in patients with prostate cancer (OSPREY). Submitted
- 21.Cookson MS, Aus G, Burnett AL, et al. Variation in the definition of biochemical recurrence in patients treated with localized prostate cancer: the American Urological Association (AUA) prostate guidelines for localized prostate cancer update panel report and recommendation for a standard in the reporting of surgical outcomes. J Urol 2007; 177: 540–545. [DOI] [PubMed] [Google Scholar]
- 22.Roach M 3rd, Hanks G, Thames H Jr, et al. Defining biochemical failure following radiotherapy with or without hormonal therapy in men with clinically localized prostate cancer: recommendations of the RTOG-ASTRO Phoenix Consensus Conference. Int J Radiat Oncol Biol Phys 2006; 65: 965–974. [DOI] [PubMed] [Google Scholar]
- 23.Perera M, Papa N, Roberts M et al. Gallium-68 prostate-specific membrane antigen positron emission tomography in advanced prostate cancer-updated diagnostic utility, sensitivity, specificity, and distribution of prostate-specific membrane antigen-avid lesions: a systematic review and meta-analysis. Eur Urol 2020; 77: 403–417. [DOI] [PubMed] [Google Scholar]
- 24.Eiber M, Hermann K, Calais J, et al. Prostate cancer molecular imaging standardized evaluation (PROMISE). Proposed miTNM classification for the interpretation of PSMA-ligand PET/CT. J Nuc Med 2018; 59: 470–478. [DOI] [PubMed] [Google Scholar]
- 25.AXUMIN (fluciclovine F 18) injection, for intravenous use [prescribing information]. Oxford, UK: Blue Earth Diagnostics Ltd.; 2016. [Google Scholar]
- 26.“FDA Approves First PSMA-Targeted PET Imaging Drug for Men with Prostate Cancer” Food and Drug Administration press release, December 1, 2020. https://www.fda.gov/news-events/press-announcements/fda-approves-first-psma-targeted-pet-imaging-drug-men-prostate-cancer [Google Scholar]
- 27.Fendler WP, Calais J, Eiber M, et al. Assessment of 68Ga-PSMA-11 PET accuracy in localizing recurrent prostate cancer: a prospective single-arm clinical trial. JAMA Oncol 2019; 5: 856–863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Eiber M, Maurer T, Souvatzoglou M, et al. Evaluation of hybrid 68Ga-PSMA ligand PET/CT in 248 patients with biochemical recurrence after radical prostatectomy. J Nucl Med 2015; 56: 668–674. [DOI] [PubMed] [Google Scholar]
- 29.Afshar-Oromieh A, Avtzi E, Giesel FL, et al. The diagnostic value of PET/CT imaging with the 68Ga-labelled PSMA ligand HBED-CC in the diagnosis of recurrent prostate cancer. Eur J Nucl Med Mol Imaging 2015; 42: 197–209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Afshar-Oromieh A, Holland-Letz T, Giesel FL, et al. Diagnostic performance of 68Ga-PSMA-11 (HBED-CC) PET/CT in patients with recurrent prostate cancer: evaluation in 1007 patients. Eur J Nucl Med Mol Imaging 2017; 44: 1258–1268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Emmett L, Metser U, Bauman G, et al. Prospective, multisite, international comparison of 18F-fluoromethylcholine PET/CT, multiparametric MRI, and 68Ga-HBED-CC PSMA-11 PET/CT in men with high-risk features and biochemical failure after radical prostatectomy: clinical performance and patient outcomes. J Nucl Med 2019; 60: 794–800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rowe SP, Campbell SP, Mana-Ay M, et al. Prospective evaluation of PSMA-targeted 18F-DCFPyL PET/CT in men with biochemical failure after radical prostatectomy for prostate cancer. J Nucl Med 2020; 61: 58–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mena E, Lindenberg ML, Turkbey IB, et al. 18F-DCFPyL PET/CT imaging in patients with biochemical recurrence prostate cancer after primary local therapy. J Nucl Med 2020; 61: 881–889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wright GL, Haley C, Beckett ML, Schellhammer PF. Expression of prostate-specific membrane antigen in normal, benign, and malignant prostate tissues. Urol Oncol 1995; 1: 18–28 [DOI] [PubMed] [Google Scholar]
- 35.Kuten J, Fahoum I, Savin Z, et al. Head-to-head comparison of 68Ga-PSMA-11 with 18F-PSMA-1007 PET/CT in staging prostate cancer using histopathology and immunohistochemical analysis as a reference standard. J Nucl Med 2020; 61: 527–532. [DOI] [PubMed] [Google Scholar]
- 36.Dietlein M, Kobe C, Kuhnert G, et al. Comparison of [18F]DCFPyL and [68Ga]Ga-PSMA-HBED-CC for PSMA-PET imaging in patients with relapsed prostate cancer. Mol Imaging Biol 2015; 17: 575–584. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


