SUMMARY
The mechanistic understanding of nascent RNAs in transcriptional control remains limited. Here, by a high sensitivity method MINT-Seq, we characterized the landscapes of N6-methyladenosine (m6A) on nascent RNAs. We uncover heavy but selective m6A deposition on nascent RNAs produced by transcription regulatory elements including promoter upstream antisense RNAs and enhancer RNAs (eRNAs), which positively correlates with their length, inclusion of m6A motif (RRACH), and RNA abundances. m6A-eRNAs mark highly active enhancers, where they recruit nuclear m6A reader YTHDC1 to phase separate into liquid-like condensates, in a manner dependent on its C-terminus intrinsically disordered region and arginine residues. The m6A-eRNA/YTHDC1 condensate co-mixes with and facilitates the formation of BRD4 coactivator condensate. Consequently, YTHDC1 depletion diminished BRD4 condensate and its recruitment to enhancers, resulting in inhibited enhancer and gene activation. We propose that chemical modifications of eRNAs together with reader proteins play broad roles in enhancer activation and gene transcriptional control. (149 words)
eTOC blurb
Lee, Wang and Xiong et al. characterized nascent RNA m6A methylome in human cells, finding a pervasive existence of m6A-marked eRNAs. These m6A-modified eRNAs recruit the nuclear m6A reader YTHDC1 to partition into liquid-like condensates, which facilitate formation of transcriptional activator condensates and therefore gene activation.
Graphical Abstract

INTRODUCTION
Since their discovery 40 years ago (Banerji et al., 1981; Moreau et al., 1981), enhancers have been the key genomic elements in dictating cell identity in metazoans by ensuring spatio-temporally regulated transcriptional programs (Furlong and Levine, 2018; Li et al., 2016). For human diseases ranging from cancer, immune disorders, cardiovascular diseases to neurodegeneration, numerous mutations or variants occur at enhancers (Chen et al., 2018; Farh et al., 2015; Hnisz et al., 2013; Khurana et al., 2016; Nott et al., 2019), some of which directly drive pathogenesis (Bahr et al., 2018; Mansour et al., 2014; Takeda et al., 2018). However, the basic mechanisms of enhancer action remain incompletely understood. Even among the causal variants, only <20% can be interpreted by altered binding between enhancer DNA and transcription factors (Farh et al., 2015; Khurana et al., 2016), raising significant challenges to fully understand the mechanisms underlying enhancer (mal)functions that may go beyond their identity as DNA elements.
Transcriptional regulatory elements are often transcription units themselves (Li et al., 2016; Li and Fu, 2019). RNAs from enhancers (i.e. eRNAs, Kim et al., 2010; De Santa et al., 2010) and promoters (i.e. upstream antisense RNAs or uaRNAs, Li and Fu, 2019) are prominent examples. That an extremely large number of eRNAs are produced in the human genome (i.e. ~60k, Arner et al., 2015) has revised our concept of enhancers: they can act not only as DNA but also potentially as regulatory RNAs (Andersson et al., 2014; Li et al., 2016; Sartorelli and Lauberth, 2020; Field and Adelman, 2020). Pathological samples generate enormous eRNAs (Hauberg et al., 2019; Zhang et al., 2019), and disease mutations highly overlap with enhancers displaying eRNA transcription (Bal et al., 2017; Chen et al., 2018; Farh et al., 2015; Vahedi et al., 2015; Dong et al., 2018), emphasizing the involvement of enhancer transcription or the eRNA transcripts in disease etiology. However, despite reported roles of individual eRNAs in gene transcriptional control, a generalizable framework to interpret the mechanisms of eRNAs and/or other nascent RNAs in transcription and chromatin control is lacking (Li et al., 2016; Li and Fu, 2019; Sartorelli and Lauberth, 2020; Field and Adelman, 2020).
In this study, we aim to examine the chemical modifications of nascent RNAs (i.e. the epitranscriptome) on chromatin, and explore the functional crosstalks between the epitranscriptome and the epigenome in transcriptional control and enhancer functions. We focus on N6-methyladenosine (i.e. m6A) because it is the most abundant internal RNA modification (Nachtergaele and He, 2018; Zaccara et al., 2019). While rapid progress in epitranscriptome studies has discovered pivotal roles of RNA modifications in development and diseases (Nachtergaele and He, 2018; Zaccara et al., 2019), the molecular roles of m6A remain largely confined to those on more abundant mRNAs, and in the process of post-transcriptional control. Their importance in transcriptional control is under-explored.
By using MINT-Seq, a high-sensitivity nascent RNA m6A profiling method (Figure S1A), here we report widespread but also selective m6A deposition on RNAs produced from transcription regulatory elements, including eRNAs. Functionally, we conducted a series of epigenomic assays, dCasRx-mediated m6A erasure, genetic and chemical perturbation of m6A writer/reader, as well as in vitro biochemical and in vivo live cell assays, by which we revealed important roles of eRNA m6A in facilitating transcriptional activation.
RESULTS
Pervasive but selective m6A deposition on enhancer RNAs
We ask a question if RNA modifications on nascent RNAs may play roles in transcription control. Previous studies have shown that m6A was deposited on some nuclear or chromosome-associated RNAs, but these work mostly used total or polyA RNA-Seq, or isolated chromatin-bound RNAs (Alarcon et al., 2015; Liu et al., 2020; Patil et al., 2016; Xiang et al., 2017; Xu et al. 2021). However, nascent RNAs are produced as the transcription process is ongoing and are known to be labile (Andersson et al., 2014; Li et al., 2016). For example, a majority of eRNAs possess half-lives of less than 10 minutes (Schwalb et al., 2016), thus they cannot be widely captured in the steady-state RNAs. To efficiently capture nascent RNAs, we took advantage of RNA metabolic labeling used in TT-Seq (Schwalb et al., 2016), and combined that with m6A-antibody MeRIP (Dominissini et al., 2012; Meyer et al., 2012) (Figure S1A). 4-Thiouridine (4SU) can be very rapidly taken in by cells, permitting short labeling time and robust nascent RNA capture. We used methanethiosulfonate (MTS) to efficiently convert 4SU-labeled nascent RNAs to biotinylated RNAs (Duffy et al., 2015). These technical improvements permit us to robustly profile nascent RNA methylome, which we refer to as methylation inscribed Nascent Transcripts Sequencing (MINT-Seq) (Xiong et al., 2021). We conducted MINT-Seq in three widely used human cell lines, MCF7, K562, and HeLa (Table S1) together with paired TT-Seq to calculate nascent RNA methylation levels (Figure S1A,B). For MCF7 cells, we conducted experiments in the presence and absence of estrogen (i.e. 17-β-estradiol, or E2), which induced acute enhancer activation (Hah et al., 2013; Li et al., 2013).
Overall, MINT-Seq often uncovered ~3–5 times more m6A peaks in the nascent transcriptome than on steady-state RNAs by MeRIP-Seq (Figure 1A). Pervasive m6A signals were detected on many nascent RNAs that cannot be detected by MeRIP-Seq, particularly for eRNAs and promoter upstream uaRNAs (Figure S1C–H). Several m6A-marked eRNAs or uaRNAs are shown as examples (Figures 1C and S1D–I). Globally, about 18.8% of p300-marked, or 21.9% of BRD4-marked, non-promoter regions (putative active enhancers) can identify at least one MINT-Seq m6A peak in its 5kb vicinity (Figure 1B). However, it is difficult to assign a m6A peak to an eRNA solely based on p300 or BRD4 peaks because the precise length of eRNAs has not been annotated (see STAR Methods). For example, m6A peaks on an eRNA can be ~10–20 kb away from p300 peaks (orange arrowheads, Figure 1C). We thus used Stringtie, a de novo transcript calling algorithm to annotate eRNA/uaRNA transcripts using TT-Seq data (see STAR Methods, Pertea et al., 2015). This permits the examination of transcript-level m6A features. In MCF7 cells, 2,207 eRNA and 1,201 uaRNA transcripts were detected, with 531 (~24%) and 576 (~48%) being m6A-marked, respectively (Figures S1C and S2A). Hundreds of m6A-marked eRNAs and uaRNAs were also found in other cells (Figure S2A). As expected for eRNAs, there is strong cell-type specificity for m6A-marked eRNAs (Figure S2B); whereas uaRNAs show moderate cell-type specificity (Figures S1C and S2B). We computationally defined a m6A modification ratio (MMR) of nascent RNAs through dividing the MINT-Seq by TT-Seq signals, which approximates the relative levels of m6A modification (Figure S1B). Intriguingly, the average MMRs of eRNAs and uaRNAs are higher than those of RefSeq gene pre-mRNAs (Figure 1D). As an example, the eRNA adjacent to the TFF1 gene (hereafter referred to as TFF1e) bears an MMR obviously higher than that of TFF1 genic region (Figure 1C), and qPCR validated that several eRNAs were methylated to levels similar or higher than that of TFF1 and GAPDH mRNAs (see below, Figure S4D).
Figure 1. A high-sensitivity map of m6A methylome on nascent eRNAs.

(A) A venn diagram showing m6A peaks detected by MINT-Seq/MeRIP-Seq in MCF7 cells.
(B) Pie charts depicting the percentage of putative enhancers marked by p300 (upper) or BRD4 (bottom) that have m6A peaks in their 5kb vicinity.
(C) A snapshot of TT-Seq, MINT-Seq, m6A methylation ratio (MMR), and ChIP-Seq at the TFF1 locus in MCF7 cells. The yellow-highlight denotes the TFF1 enhancer center, and orange arrowheads denote MINT-Seq peaks. Two arrows below show the transcription direction of the gene and the eRNA. (−): Crick strand, (+): Watson strand.
(D) Boxplots showing MMR of m6A-eRNAs, m6A-uaRNAs and m6A-pre-mRNAs. MMR was determined by the ratio of MINT-Seq/TT-Seq.
(E) Heatmap and metagene (F) plots of MINT-Seq and TT-Seq reads densities over m6A-eRNAs. Rows of heatmaps were ranked by the relative position of MINT-Seq max values. Each individual eRNA was scaled to fit the plot.
(G-K) Boxplots showing the features of non-m6A- and m6A-eRNAs: (G) transcript length, (H) RRACH motif count, (I) nascent transcripts abundance, (J) steady-state transcripts abundance, (K) relative stability.
(L) A metagene plot showing the RRACH motif density over the 10kb genomic regions downstream of the eRNA TSSs (eTSS) of non-m6A-eRNAs or m6A-eRNAs.
(M) A diagram illustrating different levels of m6A deposition to eRNAs of varied lengths. P-values in D,G-K: Mann-Whitney U test.
See also Figures S1,S2,S3.
MINT-Seq reads distribution revealed that m6A summits on eRNAs and uaRNAs take place throughout the transcripts, but appear highest in the middle of the transcripts (Figures 1E,F and S2C). Indeed, TFF1e displays fairly even transcription signals, but contains multiple distinctive m6A peaks throughout the eRNA (Figure 1C). Motif analysis of eRNA m6A peaks identified “GGACT” (Figure S2D), consistent with the canonical “RRACH” motif on mRNAs (R: A/G, H: A/C/U, Dominissini et al., 2012), suggesting m6A deposition mechanism for eRNAs is similar to that on mRNAs. Interestingly, m6A-marked eRNAs and uaRNAs are longer than non-m6A groups and contain more RRACH motifs (Figure 1G,H and Figure S2E). For example, TFF1e transcribes >20kb (Figure 1C, also see Figure S2F for other examples of long eRNAs). Functions of m6A on mRNAs were often associated with lower RNA stability in the cytoplasm (Wang et al., 2014; Zaccara and Jaffrey, 2020). We analyzed how m6A deposition may correlate with nascent RNA abundance and stability, finding that m6A-marked groups unexpectedly possess significantly higher transcript abundance at both nascent (TT-Seq) and steady-state stages (RNA-Seq) (Figures 1I,J and S2E). We used a ratio between RNA-Seq and TT-Seq to infer the relative stability of eRNAs and uaRNAs post transcription, which appeared significantly higher for m6A-marked groups (Figures 1K and S2E). Similar patterns were observed in K562 (Figure S3A,B) and HeLa cells (Figure S3C,D). These data suggested that m6A mark may promote nascent RNA transcription and/or stability, distinct from its commonly-observed negative role on mRNA stability in the cytoplasm (Wang et al., 2014; Zaccara and Jaffrey, 2020). In support of this, reanalysis of RNA half-lives estimated by a published work (Schwalb et al., 2016) confirmed that m6A-eRNAs are significantly more stable than non-m6A eRNAs (median: ~4 mins v.s. ~1 min) (Figure S3E). Hereafter, we focus on eRNAs to study the role and mechanism of nascent RNA m6A in transcription control.
Long eRNAs, stable levels and positive roles of m6A on eRNAs
While RRACH motif numbers can distinguish m6A-marked eRNAs (Figure 1H), the motif density appears comparable for eRNAs with or without m6A (eTSSs denote enhancer transcription start sites, Figure 1L,M). As m6A-marked-eRNAs transcribe longer (Figure 1G), this suggests that long eRNAs may take advantage of the RRACH motifs to permit higher levels of m6A deposition (Figure 1M).
We examined if m6A levels of eRNA are dynamically regulated. Estrogen stimulus (E2) induces rapid recruitment of ERα predominantly to enhancers, inducing eRNA transcription (Hah et al., 2013; Li et al., 2013). By TT-Seq, we detected 226 eRNA transcripts induced in MCF7 cells with a fold-change or FC>2 (Figure S4A). However, their m6A levels (MMR, Figure S1B) were not significantly altered (Figure S4B). This supports the notion that m6A modification level is largely “hard-wired” to nascent RNAs based on sequences (e.g. RRACH motif)(Darnell et al., 2018), but does not dynamically change upon rapid signaling. We confirmed this result by MeRIP-qPCR by using synthetic m6A-marked transcripts as spike-in to ensure precise m6A quantitation, which showed that eRNA levels were increased by E2 but their m6A largely unaltered (Figure S4C,D). This data also validated the MMR quantification inferred from MINT-Seq/TT-Seq (e.g. Figure 1C,D) that some eRNAs carry exceptionally high levels of m6A, comparable or higher than those on mRNAs of TFF1 or GAPDH (Figure S4D).
To understand the roles of m6A on eRNAs, we conducted dual depletion of known m6A methyltransferases, METTL3 and METTL14 (Liu et al., 2014; Zaccara et al., 2019) by siRNAs (Figure S5A). 24-hour knockdown was often sufficient to significantly reduce both proteins, but a prolonged knockdown increased protein levels of YTHDC1 and ERα, and may cause secondary effects on transcriptional programs (Figure S5A). Therefore, we used 24–36 hours of rapid knockdown in this study. The increase of YTHDC1 or coactivators (CBP/p300) after prolonged METTL3/14 loss have been observed before (Wei et al., 2021; Wang et al., 2018a). Dot blot showed a reduction of m6A on total RNAs after METTL3/14 knockdown (Figure S5B). Concomitantly, MeRIP-qPCR and -Seq revealed that the m6A levels of eRNAs were significantly reduced (Figure S5C,D). Interestingly, we found that METTL3/14 knockdown decreased eRNA abundances but did not impact the RNA stability (Figure S5E,F), suggesting that m6A regulates eRNAs possibly at the transcriptional level (see below). We further employed a recently reported chemical inhibitor of METTL3, UZH1A (Figure S5G) (Moroz-Omori et al., 2020), finding that in accord with genetic knockdown, rapid inhibition of METTL3 reduced eRNA methylation and expression level (Figure S5H,I,J), without affecting levels of YTHDC1 or ERα (Figure S5K). m6A antibodies can also immunoprecipitate N6,2-O-dimethyladenosine (m6Am), another RNA modification that takes place on the first adenosine after TSSs (Mauer et al., 2017; Sendinc et al., 2019). The often internally-distributed m6A summits on eRNAs suggested that they are unlikely m6Am (Figure 1E,F). To experimentally test it, we depleted the m6Am methyltransferase PCIF1 (Akichika et al., 2019; Sendinc et al., 2019), but did not find any effects on eRNAs (Figure S5L), indicating that m6Am was largely not involved in eRNA control.
m6A-eRNA recruits YTHDC1 to stimulate enhancer and gene transcriptional activation
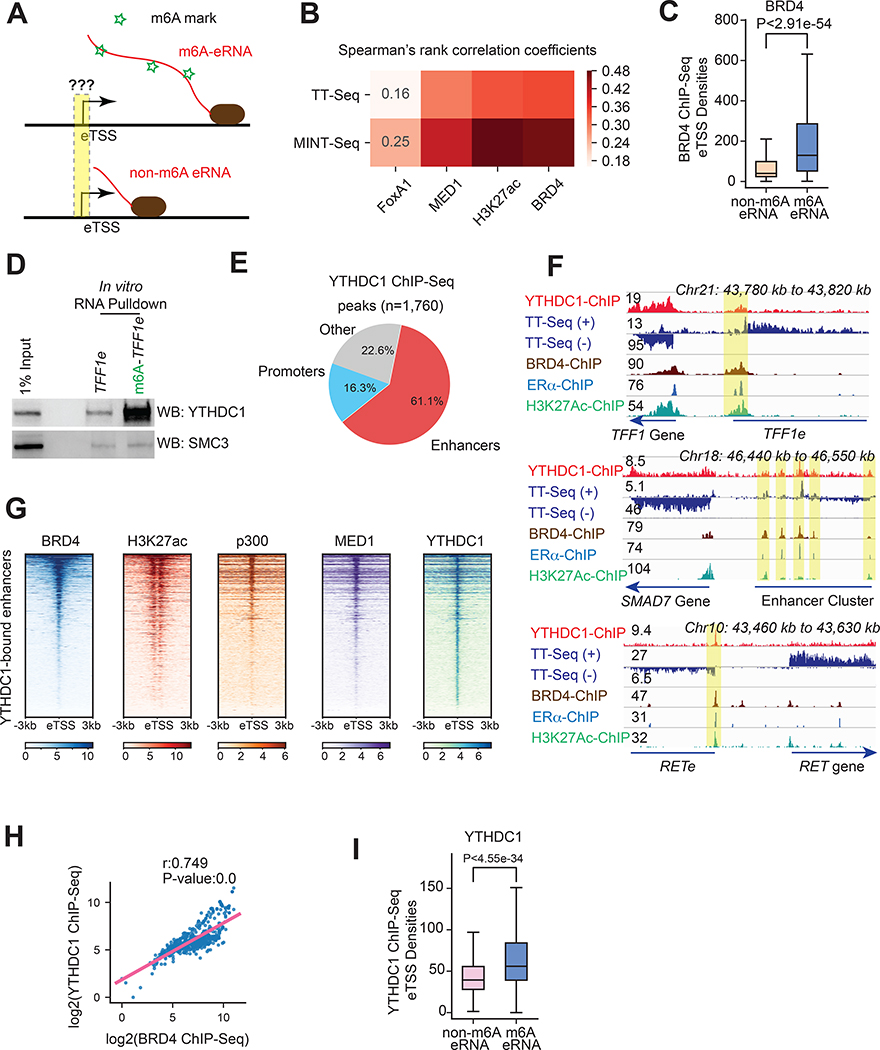
We found that m6A signals on eRNAs (i.e. MINT-Seq) are well correlated with the epigenomic marks of active enhancers, including the binding of key coactivators (e.g. BRD4 and MED1), active enhancer marker H3K27ac, and transcriptional activity (GRO-Seq). This correlation can be observed either via an eRNA-centric analysis (Figure 2A,B), or via a conventional enhancer-centric analysis (Figure S6A, see STAR Methods). In the eRNA-centric analysis, we considered the start sites of eRNAs in a way analogous to classic analysis of gene TSSs (so dubbed eTSSs, Figure 2A), and extracted the epigenomic features centered on them to correlate with the epitranscriptome features of the eRNAs (Figure 2A,B). For example, BRD4 binds the eTSSs of m6A-eRNAs with higher affinity than those of non-m6A eRNAs (Figure 2A,C).
Figure 2. YTHDC1 binds active enhancers via m6A-marked eRNAs.
(A) A diagram showing levels of m6A on eRNA transcripts versus the epigenomic features of their eTSSs. Green stars indicated m6A marks.
(B) A matrix heatmap showing Spearman’s Correlation Coefficients between TT-Seq or MINT-Seq RPKM over the entirety of eRNA transcripts and ChIP-Seq binding intensity over eTSS (+/− 3kb regions). (C) A boxplot of BRD4 ChIP-Seq densities on the eTSSs of non-m6A eRNAs (n = 1,676) or m6A-eRNAs (n = 531). P-value: Mann-Whitney U test.
(D)In vitro biotinylated m6A or non-m6A eRNA pulldown followed by Western blotting with YTHDC1 and SMC3 antibodies.
(E) A pie chart depicting genomic distribution of YTHDC1 ChIP-Seq peaks, which are largely located at enhancers.
(F) Browser tracks of YTHDC1 ChIP-Seq as aligned to TT-Seq, BRD4, ERɑ and H3K27ac at multiple loci in MCF7 cells (with E2). YTHDC1 enriched enhancers were highlighted yellow.
(G) Heatmaps showing the binding of BRD4, H3K27ac, p300, MED1 and YTHDC1 on the active enhancers with YTHDC1 binding (red group in E panel).
(H) A correlation scatter plot between BRD4 and YTHDC1 ChIP-Seq intensities on enhancers that produce m6A-eRNAs (i.e. +/− 3kb eTSSs, n = 531). The coefficient (r) and P-value are calculated by Spearman’s Correlation.
(I) A boxplot showing YTHDC1 ChIP-Seq intensity on the eTSSs of non-m6A eRNAs (n=1,676) v.s. m6A-eRNAs (n = 531). P-value: Mann-Whitney U test.
See also Figures S4,S5,S6.
We hypothesize that the m6A on eRNAs, paralleling histone modifications, may recruit reader proteins to impact enhancer activity. We conducted in vitro biotinylated eRNA pull-down experiments followed by mass spectrometry (MS) to identify such reader proteins. Using a fragment of TFF1e synthesized with or without N6-methylated-ATP as baits, MS identified 14 proteins differentially bound to m6A-marked TFF1e (Figure S6B, Table S2). This list consists of several known m6A regulators, including YTHDC1, a nuclear m6A reader (Xu et al., 2014). It also contains a series of potentially novel m6A readers on eRNAs for future investigation (Table S2). We validated the interaction between YTHDC1 and m6A-TFF1e by western blots (Figure 2D). The affinity between them is dramatically increased by the m6A presence (Figure 2D). In contrast, TFF1e can interact with SMC3 by both MS and western blots, consistent with previous reports (Li et al., 2013; Tsai et al., 2018), but this interaction was much weaker and not dependent on m6A (Figures 2D and S6B). We further validated YTHDC1 binding with multiple m6A-marked eRNAs in vivo by UV RIP-qPCR (Figure S6C).
To test if m6A-eRNAs recruit YTHDC1 to enhancers, we examined the chromatin association of YTHDC1 by ChIP-Seq, which identified 1,760 binding sites globally (MACS2, FDR<0.01). Among these, 61.1% peaks localize to putative enhancers (intergenic/intronic p300 peaks), with ~16.3% at promoters (Figure 2E). This is a strong preference for enhancers even more prominent than BRD4 (34.5% at enhancers). As examples, the binding of YTHDC1 on strong enhancers are shown, together with the binding patterns of BRD4, ERα and H3K27ac (Figure 2F). For enhancers bound by YTHDC1, heatmaps demonstrated its co-localization with BRD4/H3K27ac/p300/MED1 (Figure 2G), and there is a strong correlation between YTHDC1 and BRD4 binding on enhancers producing m6A-eRNAs (Figure 2H). The binding of YTHDC1 was much higher on eTSSs that produce m6A-eRNAs (Figure 2I), supporting that the m6A mark on eRNAs recruits YTHDC1. We knocked down TFF1e RNA by a locked nucleic acid (LNA), causing significant reduction of YTHDC1 binding to the TFF1 enhancer, without affecting other regions (Figure S6D,E). These data indicate that m6A-eRNAs recruit the m6A reader YTHDC1 to active enhancers.
We further tested if m6A-eRNAs recruit YTHDC1 via the m6A. First, ChIP-Seq in cells with METTL3/14 dual knockdown showed that YTHDC1 binding was significantly reduced (Figure S6F), and the reduction was more pronounced on eTSSs of m6A-marked eRNAs (Figure S6G). Second, we developed a m6A editor system (Figure 3A), analogous to reported methods that fuse dCas13b with m6A demethylases (Li et al., 2020; Liu et al., 2020). For this, we chose to use the recent CasRx (a.k.a., RfxCas13d) based on its high specificity and efficiency (Wessels et al., 2020). Indeed, HA-tagged CasRx together with a specific TFF1e-targeting gRNA significantly reduced the eRNA level (Figure 3B,C), validating this system for eRNA editing. MCF7 cells expressing this TFF1e-gRNA together with a catalytically-dead CasRx fused with m6A demethylase FTO (dCasRx-FTO-HA) displayed a reduced level of m6A on TFF1e (Figure 3D,E), which was accompanied by diminished interaction between YTHDC1 and TFF1e RNA (Figure 3F). As a control, an irrelevant GREB1e RNA was not affected (Figure 3E,F). Third, chemical inhibitors were recently reported to block the binding between YTHDC1 and m6A in vitro (Bedi et al., 2020). We employed one such inhibitor to test its effect (i.e., compound 11, Figure 3G). RIP-qPCR revealed that compound 11 significantly reduced YTHDC1 binding to eRNAs (Figure 3H), without altering protein expression of YTHDC1 or key coactivators (Figure 3I). These data together demonstrated that m6A mark is the main basis underlying YTHDC1 binding to eRNAs.
Figure 3. Direct roles of m6A-eRNA in recruiting YTHDC1 to active enhancers.

(A) A diagram of dCasRx mediated FTO tethering.
(B) Western blot showing the CasRx-HA protein expression by an HA antibody. GAPDH was used as a loading control.
(C) RT-qPCR showing relative expression of TFF1e in no Dox v.s. Dox (2μg/ml) treated CasRx-HA MCF7 cells expressing a specific gRNA targeting TFF1e RNA.
(D) Western blots showing the expression of HA tagged dCasRx-FTO protein.
(E) MeRIP qPCR results showing the m6A level of TFF1e RNA after dCasRx-FTO meditated m6A editing. GREB1e was used as control.
(F) UV RIP-qPCR showing the binding of YTHDC1 on TFF1e RNA after dCasRx-FTO m6A editing.
(G) A diagram showing the strategy to use an inhibitor to perturb the binding between YTHDC1 and m6A RNAs. The chemical structure of Compound 11 is shown.
(H) UV RIP-qPCR showing the binding of YTHDC1 on two eRNAs after treatment by Compound 11.
(I) Western blot using indicated antibodies after 6hrs of treatment by the YTHDC1 inhibitor.
qPCR data represents mean +/− SD of n=3 biological replicates. P values: Student’s T-tests. **, p < 0.01; ***<0.001. N.S: not significant.
See alsoFigures S5,S6, S8.
We next examined the role of YTHDC1 in regulating enhancer and transcription, first, by using siRNAs (siYTHDC1) to deplete it (Figure S7A). RT-qPCR revealed that YTHDC1 loss in MCF7 cells strongly inhibited eRNA expression (Figures S7B,C,D). YTHDC1 knockdown did not alter eRNA stability (Figure S7E), suggesting that it acts via controlling enhancer transcription. This effect was consistent by shRNA knockdown, or in another cell type (HeLa) with or without stimulus (Figure S7F,G,H). Overall, YTHDC1 depletion more consistently inhibits stimulus-induced eRNAs, such as those by E2 or epidermal growth factors (Figure S7F,G), but many other eRNAs can be inhibited as well (Figure S7F,H).
We tested if enhancer activation by YTHDC1 is based on its binding to the m6A mark on eRNAs. We performed YTHDC1 knockdown by siRNAs (Figure S7C) and then rescued by siRNA-resistant constructs expressing wild type YTHDC1 protein fused with a modified GFP tag (i.e., mClover-YTHDC1 WT), or a mutant YTHDC1 that cannot bind m6A (mClover-YTHDC1 W428A/W377A, or WA/WA; Xu et al., 2014) (Figure 4A). This experiment revealed that the WT YTHDC1 but not the WA/WA mutant conferred functional rescue of YTHDC1 loss (Figure 4A,B). We also generated WT or WA/WA mClover-YTHDC1 ChIP-Seq in these cells, which demonstrated that the m6A binding mutant (WA/WA) significantly lost binding to chromatin compared to WT (Figure S8A,B). Furthermore, we examined eRNA changes upon chemical inhibition of YTHDC1/m6A binding (Bedi et al., 2020; also see Figure 3H,I), finding that as short as 3hrs of treatment already reduced eRNA transcription (Figure S8C). These results together indicate that the m6A mark on eRNAs recruits YTHDC1 to active enhancers and they together stimulate enhancer transcription.
Figure 4. YTHDC1/m6A-eRNA stimulate enhancer activation and a broad gene transcriptional program.

(A) Western blots showing the protein levels of YTHDC1 in the rescue experiments (the same sets as in panel B). WA/WA: W377A/W428A double mutant.
(B) RT-qPCR of two eRNAs and a gene showing the knockdown effects by siYTHDC1, and their rescue by wildtype (WT) but not the WA/WA mutant.
(C,E) Snapshots showing reduced levels of two eRNAs and their neighboring genes after YTHDC1 knockdown in GRO-Seq (C) and TT-Seq (E). (+)/(−) indicate Waston/Crick strands.
(D,F) Boxplots depicting RPKM of GRO-Seq (D) and TT-Seq (F) of E2 induced eRNAs and genes in siCTL or siYTHDC1 treated MCF7 cells. P-values: Mann-Whitney U test.
(G) YTHDC1 protein levels upon rapid PROTAC3 degradation. ERα, BRD4, METTL3 are also shown.
(H) Effects after PROTAC3 degradation of YTHDC1 or conventional siYTHDC1 on the expression of TFF1e, SMAD7e and TFF1 gene in HaloTag-YTHDC1 MCF7 cells.
qPCR data represents mean +/− SD of n=3 biological replicates. P values: Student’s T-tests. **, p < 0.01; ***<0.001. N.S: not significant.
See also Figures S7,S8.
We tested the transcription changes globally by GRO-Seq (based on BrdU labeling in vitro). This experiment revealed that YTHDC1 depletion significantly dampened enhancer transcriptional activation (Figure 4C,D). The eRNAs induced by E2 (FC>2, n=368) were significantly inhibited in the absence of YTHDC1 (Figure 4D, left panel). Consistent with enhancer inhibition, E2 induction of genes (FC>2, n=1,619) were also reduced (Figure 4D, right panel). To corroborate this result, we also conducted TT-Seq (based on in vivo 4SU labeling) and validated the transcriptional defects (Figure 4E,F). Snapshots of two eRNA/gene loci were shown in Figure 4C,E. Transcriptional changes of enhancers and genes were also consistently observed by TT-Seq upon knockdown of METTL3/14 (Figure S8D). Reminiscent of our results of YTHDC1 in transcriptional activation, early work prior to realizing its identity as a m6A reader found that under microscopy, YTHDC1 often locates to transcriptionally active sub-nuclear compartments (Nayler et al., 2000).
Our data by ChIP-Seq, GRO/TT-Seq, mutant rescue, m6A editing and chemical inhibitor strongly suggested that YTHDC1 directly activates enhancers. However, as chromatin or RNA regulators may possess pleiotropic roles, we adopted a HaloTag-based rapid degradation system to further determine its direct function. We fused the endogenous YTHDC1 with an N-terminus HaloTag by CRISPR/Cas9 and microhomology-mediated end-joining (Figure S8E, see STAR Methods, Nabet et al., 2018; Sakuma et al., 2016). By the ligand PROTAC3 (Buckley et al., 2015), endogenous HaloTag-YTHDC1 showed rapid degradation in a few hours (Figure S8F), but this protein needs ~24hr to be fully depleted (Figures 4G). YTHDC1 rapid degradation confirmed the enhancer/gene transcriptional inhibition seen after siRNA/shRNA knockdown, as demonstrated by qPCR (Figure 3H) or by PRO-Seq and TT-Seq (Figure S8G). We also generated poly-A mRNA-Seq of the HaloTag-YTHDC1 cells after its degradation, finding no changes of known factors involved in transcriptional control, excluding indirect effects (Figure S8H). As soon as 8 hours of YTHDC1 depletion or 3hrs of chemical inhibition significantly inhibited eRNA transcription (Figures 3H and S8C), together with other data shown above, demonstrating a direct role of YTHDC1 in enhancer activation.
YTHDC1 and m6A-eRNA form liquid-like condensates to facilitate BRD4 condensate formation
Many chromatin and RNA binding proteins (RBPs) that contain intrinsically disordered regions (IDRs) can phase transition to form biomolecular condensates, which are dynamically modulated by RNAs (Banani et al., 2017; Roden and Gladfelter, 2020; Lin et al., 2015). Recently, m6A-marked mRNAs were found to promote stress granules in the cytosol via enhancing phase separation of YTHDF proteins that are cytosolic m6A readers (Gao et al., 2019; Ries et al., 2019; Fu and Zhuang, 2020). As transcriptional condensate is hypothesized to underlie enhancer/gene activation (Hnisz et al., 2017), and received support by several studies (Boija et al., 2018; Cho et al., 2018; Nair et al., 2019; Sabari et al., 2018), we interrogated if m6A-eRNAs and YTHDC1 may act via phase separation to impact enhancer activity. We first tested the molecular dynamics of YTHDC1 using an OptoDroplet assay (Shin et al., 2017). Structurally, YTHDC1 contains two predicted IDRs, spanning a well-structured m6A binding motif, the YTH domain (350–500aa) (Xu et al., 2014) (Figure 5A). We cloned full-length YTHDC1 (FL-YTHDC1), its N-terminal IDR (IDR1), YTH domain (YTH), C-terminal IDR (IDR2), as well as a partial protein consisting of YTH domain and IDR2 (YTH-IDR2) into the blue-light inducible pHR-mCherry-CRY2 construct (Figure S9A). Upon blue light, mCherry tagged YTHDC1 formed dynamic droplets, displaying rapid fusion events that are characteristics of liquid-like phase separation (Figures 5B, S9B,C,D and Supplementary movies 1 and 2). While predicted to be disordered, IDR1 did not show features of dynamic droplets (Figures 5A,B and S9B,C), suggesting its dispensability for YTHDC1 droplet formation. As expected, the highly structured YTH domain (the m6A binding module, Xu et al., 2014) did not form droplets (Figures 5A,B and S9B,C). Both YTH-IDR2 and IDR2 alone exhibited droplet formation comparable to the FL-YTHDC1, but IDR2 displayed more pre-existing droplets before light stimulation and also slower dynamics (Figures 5B and S9B,C). These results indicated that YTHDC1 can form liquid-like droplets.
Figure 5. YTHDC1/m6A-eRNAs form liquid-like nuclear condensates.

(A) (Left) Numbers of amino acids (aa) of YTHDC1 domains, including the two predicted intrinsic disordered regions (IDR) flanking the YTH domain. Lower part shows disordered propensity by the PONDR VSL2 tool (1.0 indicates highly disordered). To the right, a diagram depicts the YTHDC1 binding to a m6A mark (green star).
(B) Representative images from Optodroplet assay of full length (FL) YTHDC1, its IDR1, YTH domain (YTH), IDR2 and its partial protein YTH-IDR2 in 293T cells, scale bars denote 5μm. Orange circles indicate light induced optodroplets, or lack of response for the IDR1 and YTH domain.
(C) FRAP of endogenous mClover-YTHDC1 in MCF7 cells. 10 condensates were subjected to FRAP, and their mean +/− SD were plotted. A representative mClover-YTHDC1 condensate during FRAP is shown below. Scale bar: 5μm. Pre: pre-bleaching.
(D)In vitro phase separation assay of 1μM mClover-YTH-IDR2, mClover-YTH domain or mClover-YTH-IDR2-R to A mutant, without RNA, with 1nM non-m6A-TFF1e, or with m6A-TFF1e RNAs. Scale bar: 10μm.
(E) Quantification of the particle areas for each condition in panel D.
(F, G) Representative images and quantitation of In vitro condensates of 0.5μM mClover-YTH-IDR2 without or with the addition of m6A-TFF1e at the indicated concentrations. Scale bar indicates 10 μm. P values in E and F panel: Student’s T tests. *: p <0.05, **: p <0.01, ***: p < 0.001, N.S: Not significant Data are representative of two independent experiments.
See also Figures S9,S10.
To validate this at the endogenous protein levels, we generated another knock-in (KI) cell line, in which a mClover tag was fused to the N-terminus of YTHDC1 (Figure S9E,F). Consistent with the OptoDroplet results, live-cell imaging of endogenous mClover-YTHDC1 revealed its behavior of forming local nuclear foci (Figure S9G,H). We subjected these foci to Fluorescence Recovery After Photobleaching (FRAP) experiments and found that 50% of bleaching was recovered in 9.8 sec (Average T1/2= 9.8 sec, average immobile fraction = 51.4%) (Figure 5C). This rapid recovery supports a conclusion that natively expressed YTHDC1 forms liquid-like condensates in vivo.
We next studied YTHDC1/m6A-eRNA condensate in vitro. While we failed to express a large quantity of the full-length YTHDC1 from E.Coli, we generated recombinant YTH-IDR2 with mClover tag (i.e. mClover-YTH-IDR2) (Figure S10A,B). YTH-IDR2 showed similar properties as FL-YTHDC1 in optodroplet assays (Figure 5A,B), therefore we used recombinant mClover-YTH-IDR2 to study YTHDC1 condensates. YTH-IDR2 formed condensates in a concentration-dependent manner (Figures S10C,D). In contrast, YTH domain alone (350–500aa) showed little condensate formation even at a high concentration (Figures S10C,D). When we supplemented these proteins with in vitro synthesized eRNAs, we found that m6A-TFF1e significantly increased the particle sizes of mClover-YTH-IDR2 condensates, whereas non-m6A-TFF1e caused little change (Figure 5D,E). This further supports that YTHDC1 function is dependent on the m6A mark on eRNAs, rather than merely the transcripts. By comparison, the YTH domain did not respond to addition of eRNAs (Figure 5D,E). Remarkably, this enhancing effect of m6A-TFF1e on YTHDC1 condensate is dose-dependent (Figure 5F,G), consistent with our (epi)genomic data that high levels of m6A-eRNAs correlated with stronger YTHDC1 recruitment to enhancers (Figure 2I).
To initially examine the biochemical basis of YTHDC1 condensate formation, we analyzed the amino acid composition of YTHDC1, finding that the IDR2 region is rich in arginine (i.e, R residue, Figure S10E). R residues are important for phase separation of other RNA binding proteins such as FUS or epigenetic factor CBX2 (Wang et al., 2018b; Lau et al., 2017). We therefore mutated all the 55 arginines (R) to alanines (A) in IDR2, generating a mClover-YTH-IDR2-RtoA mutant in E.Coli. Interestingly, this mutation inhibited the condensate formation of YTHDC1 (Figure S10C,D), a phenomenon similar to the R mutations of FUS (Wang et al., 2018b). The mutation also abolished the response of YTD-IDR2 to the supplement of m6A-TFF1e (Figure 5D,E). These results together indicated that, 1), YTHDC1 can form biomolecular condensates and its interaction with m6A-marked eRNAs promotes condensate assembly; 2) the IDR2 is important for YTHDC1 condensate formation, in a manner dependent on the arginine residues; and 3), after the addition of m6A-eRNA, the lack of response of YTH domain or RtoA mutant contrasting the increased condensate formation of YTH-IDR2 indicated that the binding between YTH domain and m6A-eRNA augmented the IDR2 region to form condensates (Figures 5A and S11A).
Active enhancers were bound by multiple factors with features of phase-separated condensates (Hnsiz et al., 2017). Our results lead us to investigate if the YTHDC1/m6A-eRNA condensate may crosstalk to other coactivators on active enhancers, and may, therefore, impact enhancer activity (Figure S11B). We reasoned that the multivalent interactions between YTH-domain/m6A-eRNAs and between the IDR2 and other IDRs in its nuclear neighborhood may promote the formation of enhancer-associated condensates (Figure S11B). We tested this by focusing on BRD4, a coactivator colocalizes with YTHDC1 on YTHDC1-bound enhancers (Figure 2F–H), and which forms transcriptional condensates (Sabari et al., 2018). We found that YTHDC1 knockdown weakened BRD4 recruitment to enhancers by ChIP-Seq (Figures 6A and S11C). There was no alteration of H3K27ac on these enhancers (Figure 6A), supporting that the defective BRD4 recruitment was directly caused by YTHDC1 depletion, rather than its deregulated binding to histone. An example was shown in Figure S11D. Using an orthogonal approach, we characterized BRD4 condensate in vivo. We generated a MCF7 line with a mCherry knocked into the N-terminus of endogenous BRD4 (Figures S11E and 6B, clone#7 was used). Live-cell imaging confirmed condensate-forming features of endogenous BRD4, which was significantly inhibited when YTHDC1 was depleted (Figure 6C) or when METTL3/14 was knocked down (Figure S11F). As a control, a BET inhibitor JQ1 also reduced BRD4 condensate formation (Figure S11G). We further tested this hypothesis in vitro. We mixed mClover-YTH-IDR2 protein and m6A-TFF1e RNA together with BRD4 protein. This experiment revealed that YTHDC1 co-assembled into condensates with BRD4, with the sizes of condensate increased by m6A-TFF1e supplement (Figure 6D). This enhancing effect on BRD4 condensate was not caused by the m6A-TFF1e RNA alone, and the condensate-defective YTH domain or YTH-IDR2-RtoA mutant failed to do so regardless of eRNA presence (Figure 6E,F).
Figure 6. YTHDC1/m6A-eRNAs interaction facilitates BRD4 condensate formation and its enhancer binding.
(A) Heatmaps showing BRD4 or H3K27ac ChIP-Seq signals on putative enhancers with or without YTHDC1. BRD4 antibody: Bethyl A301–985A100.
(B) Western blots showing the expression levels of the knock-in mCherry-BRD4 (upper) and non-tagged BRD4 (bottom) in parental and knockin (KI) cells. GAPDH is a loading control.
(C) Representative live cell images showing the endogenous mCherry-BRD4 condensates, and their reduction by siYTHDC1. Scale bars: 5μm. (Right) quantification of the foci number per cell. Data are representative of three independent experiments; n= cell numbers.
(D)In vitro condensate assay showing the effect of m6A-TFF1e RNA supplement to the condensate co-assembly between mClover-YTH-IDR2 (0.5μM, green) and Alexa647-labeled BRD4 (1μM, purple). Scale bars: 5μm. m6A-TFF1e was labeled with Cy3-UTP (red).
(E)In vitro phase separation of BRD4 in the absence or presence of mClover-YTH-IDR2-WT, -R to A mutant, the YTH domain only with or without m6A-marked TFF1e. For these, 1μM of BRD4, 1μM mClover-YTHDC1 proteins and 1nM m6A-marked TFF1e were used. Scale bar is 10μm. BRD4 was labeled by Alexa647 (purple color). Data are representative of two independent experiments.
(F) Quantification of condensate sizes of BRD4 in panel E.
See also Figures S10,S11.
Together, our in vivo and in vitro results indicate a mechanism that m6A-marked eRNAs recruit YTHDC1, and they form condensates, which facilitate the formation of BRD4 coactivator condensate and enhancer activation (Figure 7).
Figure 7. A model for m6A-eRNAs and YTHDC1 that function in facilitating the formation of transcriptional condensates and enhancer/gene activation.

A diagram summarizing our findings in this work. These results provide a conceptual advance to understand the roles of nascent eRNAs and their chemical modifications in transcriptional control, paving ways for further studies of epitranscriptome-epigenome crosstalks. IDR: intrinsically disordered region.
DISCUSSION
Enhancer RNA functions: act of transcription and transcripts
Despite that eRNAs have been long found to mark active enhancers, the functional mechanisms of these transcripts are still poorly understood (Li et al., 2016; Li and Fu, 2019; Field and Adelman, 2020; Sartorelli and Lauberth, 2020). Debates exist as to if any functions are based on the act of transcription or the eRNA transcripts (similar for many nuclear lncRNAs as well) (Li et al., 2016; Li and Fu, 2019; Rinn and Chang, 2020; Field and Adelman, 2020). Arguments against the role of nascent transcripts include: 1), the evolutionary sequence conservation of eRNAs and many lncRNAs is low (Derrien et al., 2012), and, 2), deletion of partial transcripts may not impact function (Engreitz et al., 2016). Also, despite a dozen proteins (many are coactivators) have been reported to bind individual eRNAs (Lee et al., 2020), a potentially common sequence feature underlying such interactions has not been identified. Our current results suggest that important sequence features of nascent RNAs may lie in short and degenerate motifs (e.g. RRACH) that are often not considered when evolutionary conservation is calculated, or when genomic deletion experiments were designed. Here, our data by both global approaches and by single loci perturbation together demonstrated critical roles of m6A-marked eRNA transcripts and their protein partners in enhancer activation. We propose that m6A and perhaps other chemical modifications that depend on short sequence motifs can act as fundamental elements in nascent RNAs to recruit reader proteins to chromatin, and to modulate the transcription process. Our work thus provides unequivocal evidence that both eRNA transcripts and the act of transcription are required for full enhancer activity: i), eRNA transcripts with m6A reader proteins nucleate condensates to augment coactivator condensates; ii), the act of enhancer transcription generates eRNAs, and the length of transcription may influence their m6A levels. While our results demonstrated the critical importance of m6A-marked eRNAs, these data do not exclude that some eRNAs with low or no m6A methylation can also be functional, particularly if the specific RNA is of relatively high abundance.
High m6A levels, long eRNAs and more active enhancers
An intriguing finding from our high sensitivity nascent RNA methylome is that m6A methylation was highly deposited to long eRNAs (Figures 1 and S2,S3), and that m6A-eRNAs carry higher m6A levels than pre-mRNAs or some mRNAs (Figures 1C,D and S4C,D). We found that the median length of m6A-marked-eRNAs is ~1.7kb in MCF7 cells (Figure 1G), which is largely consistent with their average length estimated by total RNA-Seq (i.e. ~0.5–2kb for most, but some can be >3–4kb, Natoli and Andrau, 2012). Indeed, long eRNAs have been previously observed (Koch et al., 2011; Hah et al., 2013). Among the m6A-eRNAs we identified, ~25% are longer than 5.9kb (Figure 1G), including the few prominent cases that we focused on for functional characterization, e.g. TFF1e and SMAD7e are >20kb long. The longer length of m6A-eRNAs can explain their high m6A deposition because the RRACH motifs are rather wide-spread in the enhancer regions (Figure 1L,M). These features of m6A-eRNAs (length, m6A level and YTHDC1 binding) are together highly consistent with our in vitro data that YTHDC1 condensates can be augmented by increasing amounts of m6A-marked eRNAs. Their functional connection is further corroborated by the enhancer defects seen after YTHDC1 depletion. It is noteworthy that as an entire category and/or by other methods, the length of eRNAs can be short (e.g. median ~346nt in human cells by the FANTOM project, Andersson et al. 2014; or typically <150nt in Drosophila by PRO-Seq/START-Seq, Henriques et al., 2018), indicating that m6A may be deposited to only a fraction of the ~60,000 human eRNAs (Arner et al., 2015). It is reasonable to speculate that a large portion of short eRNAs may drop off the chromatin quickly and/or degrade without m6A methylation (Figure 1M).
The full mechanisms that determine eRNA length and m6A levels are important future questions. Early work found that a specific set of strong eRNAs possessed relatively higher levels of serine2 phosphorylation of RNA PolII, which was otherwise negligible on most enhancers (Koch et al., 2011; Natoli and Andrau, 2012). It is possible that the m6A on eRNAs was deposited in a process related to transcription elongation. In addition, the length of a specific group of eRNAs can be suppressed by the Integrator complex in HeLa cells (Lai et al., 2015). It will be important to further investigate the relationship between eRNA transcriptional length, their regulators, the RRACH motif usage and the m6A levels of eRNAs, and how these impact enhancers and transcription programs.
m6A regulation of RNA expression in the nucleus: post-transcriptional or transcriptional
The functions of m6A on mRNAs were largely suggested to act via post-transcriptionally controlling mRNA stability and translation (Nachtergaele and He, 2018; Zaccara et al., 2019). However, recent work showed that several m6A readers in the cytosol play redundant roles (Zaccara and Jaffrey, 2020). A recent report showed that permanent depletion of METTL3 or YTHDC1 in mouse ESCs increased stability of ‘chromosome-associated RNAs’ including some RNAs overlapping enhancers or super-enhancers (Liu et al., 2020). Different from this, we found that YTHDC1 depletion did not alter eRNA stability, but inhibited enhancer and gene transcription. The basis for the discrepancy is not entirely clear at this stage. Besides possible cell type variations (undifferentiated mESCs versus differentiated human epithelial/cancer cells), technical approaches are important distinctions. We used transient knockdown, rapid degron as well as chemical inhibition, permitting robust characterization of transcriptional changes largely excluded from being indirect consequences. In our system, >40hours of knockdown of METTL3/14 increased protein levels of ERα or YTHDC1 (Figure S5A), consistent with others’ observation in mESCs (Wei et al., 2021). Future systematic work using rapid degradation of m6A regulators/readers in both mouse and human cells is important to clarify the direct roles of m6A/YTHDC1 on eRNAs (Chelmicki et al., 2021; Wei et al., 2021). Regardless, our work provides important insights as to how methylation on nascent RNAs can play important roles in transcriptional control.
A cross-talk between the epitranscriptome and epigenome via condensates
Transcriptional condensate has provided a conceptual framework to understand gene regulation and can explain some key observations, i.e. the presence of high concentrations of transcription factors, cofactors and RNA PolII at specific genomic regions (e.g. super-enhancers)(Hnisz et al., 2017). Our results showed that the m6A signals on eRNAs correlate with features of active enhancers (Figure 2B), suggesting that m6A contributed additional roles than eRNAs per se. Indeed, a series of experiments by using YTHDC1 mutant rescue, genetic perturbation of METTL3/14, dCasRx-FTO editing or chemical inhibition of YTHDC1/m6A binding supported this notion. In vitro condensate assays also support that it is the m6A-marked eRNAs that promote YTHDC1 condensate formation (Figure 5D,E). Given the importance of multivalent interactions in biomolecular condensates (Banani et al., 2017; Hnisz et al., 2017), we are tempted to speculate that the m6A marks on eRNAs offer a ‘valency’ to modulate the formation of transcriptional condensates.
Our results uncovered an unappreciated epitranscriptome-epigenome ‘crosstalk’ through condensates, in which the condensates formed by methylated-eRNA-reader (YTHDC1) co-mix with and promote the condensates formed by acetylated-histone-reader (i.e. BRD4). We found that the arginine residues in YTHDC1 IDR2 are important for its condensate formation and for augmenting BRD4 condensates. This is reminiscent of the ‘molecular grammar’ underlying condensate formation of other RNA binding proteins (e.g. FUS, Wang et al., 2018b) or transcriptional regulators (e.g. CBX2, Lau et al., 2017). However, the complete mechanisms of amino acids ‘grammar’ in mediating such condensate interactions still require in-depth studies. Interestingly, while the IDR2 of YTHDC1 is rich in arginine (R), the IDRs of cytosolic YTHDF proteins are depleted of R but rich in tyrosine (Y) and other residues (Fu and Zhuang., 2020). This suggests that despite sharing a common YTH domain, m6A readers may display distinctive behaviors and mechanisms of phase separation in the nucleus versus the cytoplasm.
Many RNA binding proteins (RBPs) directly associate with chromatin and can modulate transcription (Spector, 2003; Xiao et al., 2019). Our work provided a helpful, and likely generalizable principle to interpret the roles of RBPs in transcriptional control. It may be predicted that many chromatin-associated RBPs act together with nascent RNAs to form condensates to impact transcriptional activity (Li and Fu, 2019). In this sense, the growing list of m6A reader proteins in the nucleus (Zaccara et al., 2019), including the potentially unappreciated ones by our current work (Figure S6B), demand further studies of their collective action in modulating transcription and chromatin-associated condensates.
Limitations of the study
In this work, we have used multiple orthogonal methods to study m6A and YTHDC1, and our results strongly supported their causal and direct role in enhancer activation, however, it remains possible that for specific enhancers there could be indirect effects caused by the depletion of m6A enzyme complexes or YTHDC1. Caution is particularly needed for distinguishing direct or indirect effects after depleting enzymes METTL3/14, as we observed changes of key transcriptional regulators after a longer time of knockdown. Also, while YTHDC1 is enriched at sites with m6A-modified eRNAs, it cannot be fully excluded that at specific genomic sites YTHDC1 may be recruited via means other than m6A. Globally, promoter binding of YTHDC1 is infrequent as compared to enhancer binding, but it should be noted that for specific genes this protein could regulate transcription via binding promoters or the RNAs therein rather than solely acting via eRNAs.
Collectively, our current work has provided a high-resolution map of eRNA methylome and uncovered their unappreciated functions in enhancer activation. These results stimulated many questions regarding the process of nascent RNA m6A deposition and how that connects to the multi-step regulation of RNA polymerases. The resources and insights here lay a foundation for future characterization of potentially widespread epitranscriptome-epigenome crosstalks on chromatin.
STAR METHODS:
RESOURCE AVAILABILITY
Lead contact
Further information and requests for resources and reagents can be directed to and will be fulfilled by the Lead Contact, Wenbo Li (Wenbo.li@uth.tmc.edu).
Materials availability
Constructs and cell lines generated in this work can be available upon request.
Data and code availability
Data generated in this study have been deposited to NCBI GEO (GSE143441).
Codes/scripts used for the analyses in this study are based on standard algorithms, are mentioned in the specific sections in Methods below, and are available upon request.
Additional information required to reanalyze the data reported in this paper is available from the lead contact upon request.
EXPERIMENTAL MODEL AND SUBJECT DETAILS
Cell Culture
MCF7, 293T and HeLa cells were originally purchased from ATCC, and were incubated in DMEM media supplemented with 10% Fetal Bovine Serum (FBS, GenDEPOT) in a 37°C CO2 incubator. K562 cells were a gift from Dr. Yun (Nancy) Huang at Texas A&M University Health Science Center at Houston and were originally from ATCC. K562 was cultured in RPMI-1640 with 10% FBS in a 37°C CO2 incubator. In order to induce hormone signaling in MCF7 cells, the cells were incubated in phenol red-free DMEM with 10% Charcoal-stripped fetal bovine serum (CS-FBS, Omega Scientific Inc) for 36–72 hours. To induce the estrogen target enhancer and gene activation program, 100 nM 17-β-estradiol (E2) were added to the cells for an hour (used in almost all work in this paper unless otherwise stated). Cells were examined for mycoplasma contamination every 6 months. For HaloTag/PROTAC3-mediated rapid degradation, PROTAC3 (Promega, Cat# CS2072A01) were given to the cells for several to 24 hours with described concentrations in the paper. In order to achieve serum starvation and EGF signaling, HeLa cells were incubated in DMEM without serum for 16 hours. After that, 100 ng/ml of EGF (Fisher, Cat # 50–813-058) was treated to the cells for 30mins. For pharmacological inhibition of YTHDC1 or METTL3, we treated YTHDC1 inhibitor (compound 11, Cat# Z287226108, Enamine Ltd) or METTL3 inhibitor (UZH1A, generated by Dr. Amedeo Caflisch’s lab in University of Zurich) for 3 or 6 hours.
Transfection
siRNAs, LNAs or plasmids were transfected using Lipofectamine 2000 or 3000 (Thermofisher) following the manufacturer’s instructions. Often, 500,000 cells cultured in 6-well plates were transfected with 40 nM siRNA, LNA or 0.5 μg of plasmids, and two rounds of transfection were often conducted to increase the knockdown efficiency. For most of the experiments, 72 hours after transfection, cells were harvested and used for the experiments. For METTL3 and METTL14 double knockdown, Lipofectamine RNAiMAX reagent (Thermofisher) was used to transfect siRNAs, and cells were often harvested 24–36 hours after transfection to avoid secondary effects. The information for siRNAs can be found in Table S3. For rescue experiments with mClover-YTHDC1-WT or W428A/W377A mutant, we co-transfected 40nM YTHDC1 siRNA #1 and 1 μg of plasmids into MCF7 cells using Lipofectamine 3000 (Thermofisher).
Lentiviral infection
In order to generate lentiviral particle containing media, a mixture of lentiviral packaging plasmids and lentiviral transfer plasmid vector (3 μg of psPAX2, 1 μg of pMD2.G, and 4 μg of transfer plasmids) was transfected into 293T cells in 10cm dish using Lipofectamine 2000 (Life Technologies). After 16 h, we freshly changed the culturing media, and the supernatants were collected at 48 and 72 h post-transfection for two independent infections. The harvested supernatants were filtered using 0.45 μm PES syringe filter (Fisher) and used to infect target cells after being mixed with polybrene (final concentration of 8 μg/ml, Sigma). After 24hour post-infection, the cells were selected in the media with selection markers for up to a week (often Blasticidin 5μg/ml for 7 days, Hygromycin 250μg/ml for 7 days, Zeocin 100μg/ml for 7 days, Puromycin 1μg/ml for 2–3 days). For shRNA knockdown, we purchased lentiviral shRNA plasmids from sigma (sh-YTHDC1 clone# TRCN0000243987). All of the lentiviral plasmid information can be found in Table S3.
Generation of knock-In cell lines at the endogenous locus
In order to knock-in mCherry at the N-terminus of BRD4, and the HaloTag or mClover at N-terminus of YTHDC1, we used CRISPR/Cas9 and a recently developed PITCHv2 system (also see Figures S8, S9 and S11) (Nabet et al., 2018). For N terminal knock-in of mCherry tag to BRD4, a pCRIS-PITChv2-BSD-P2A-2xHA-mCherry (BRD4) vector was created by replacing the dTAG with mCherry in the pCRIS-PITChv2-BSD-dTAG(BRD4) plasmid (Addgene, Cat#91792) Clone #7 of BRD4-KI cells were used mostly in this study. Similarly, the HaloTag or mClover knock-in repair donor plasmids for the YTHDC1 locus were engineered on top of the donors for BRD4 knockin, but replaced with respective short donor arm sequences flanking the HaloTag or the mClover tag. For gRNAs, pX330A-nBRD4/PITCh (Addgene, Cat#91794) for BRD4 knock-in was used as described in Nabet et al (Nabet et al., 2018). pX330A-nYTHDC1/PITCh was generated by first inserting annealed YTHDC1-specific sgRNA oligos into vector pSpCas9(BB)-2A-Puro (PX459) V2.0 (Addgene #62988) digested with BbsI (NEB), then this plasmid was inserted with a second, donor-specific, gRNA by ligation with XbaI digested plasmid #91794 (PITCh U6-donor specific gRNA-XbaI) (Sakuma et al., 2016). For generating the knock-in, MCF7 cells were plated in 6-well plates and co-transfected with 1.2 μg of donor plasmids (e.g. pCRIS-PITChv2-BSD-mCherry (BRD4) or pCRIS-PITChv2-BSD-mClover (YTHDC1)), together with 0.8 μg of gRNA plasmid by using Lipofectamine 3000 (ThermoFisher Scientific), according to manufacturer’s instructions. After 12 hours of transfection the medium was added with 7.5 μM RS-1 (Sigma Cat# R9782) to inhibit non-homologous end joining (NHEJ). After 24 hours, the transfection was repeated to increase knock-in efficiency, which was then followed by an antibiotic selection process (often 10 μg/mL blasticidin). The transfected cells were cultivated under antibiotic medium for one week and red (mCherry) or green (mClover) fluorescent protein positive cells were sorted by FACS. The cells were then cultured to generate single cell clones, and then genotyped using PCR and Western blots to select for single homozygous knock-in clones. Knock-in of HaloTag at the N-terminus of YTHDC1 was done without sorting. Primers and sgRNA information can be found in Table S3.
Quantitative RT-PCR (qRT-PCR)
RNAs were extracted by TRIzol (Thermo Fisher) or Zymo RNA miniprep kit (Zymo Research). The extracted RNA was subjected to reverse transcription by SuperScript IV (Thermo Fisher) first strand cDNA synthesis system. For qPCR, we used the SsoAdvanced Universal SYBR green Supermix (Bio-Rad) with the following primers (Table S3). The PCR parameters follow the recommendation by the manufacturer. All of the qPCR was triplicated and each value was marked with black dot.
Western Blot Analysis
Proteins were solubilized from cells by RIPA buffer (50 mM Tris, pH 7.4, 150 mM NaCl, 1 mM EDTA, 0.1% SDS, 1% NP-40, 0.5% sodium deoxycholate, 0.5 mM DTT) supplemented with protease inhibitor cocktail (Roche) for 10-min. The lysates were sonicated for 30 sec and centrifuged at 4 °C for 10 minutes. The supernatants were harvested and used to measure protein concentration with Pierce® BCA Protein Assay Kit (Thermo Scientific). 20 μg of proteins were resolved by SDS-PAGE and transferred to PVDF membrane (BioRad). Antibody information can be found in STAR Methods, the Resource Table.
RESOURCE TABLE.
| REAGENT or RESOURCE | SOURCE | IDENTIFIER |
|---|---|---|
| Antibodies | ||
| Anti-GAPDH | Protein tech | Cat# 60004-1, RRID:AB_2107436 |
| Anti-YTHDC1 | Bethyl | Cat# A305-096A, RRID:AB_2631491 |
| Anti-YTHDC1 | AbCam | Cat# Ab122340, RRID:AB_11128253 |
| Anti-YTHDC1 | Cell Signaling | Cat# 77422, RRID:AB_2799899 |
| Anti-m6A | Synaptic Systems | Cat# 202-003, RRID:AB_2279214 |
| Anti-BRD4 | Bethyl | Cat# A301-985A100, RRID:AB_2620184 |
| Anti-BRD4 | Active Motif | Cat# #91302, RRID:AB_2813829 |
| Anti-BRD4 | Active Motif | Cat# #39010 |
| Anti-ERα | Santa Cruz | Cat# sc-543, RRID:AB_631471 |
| Anti-ERα | Diagenode | Cat# AC-066-100, RRID:AB_2716575 |
| Anti-SMC3 | Bethyl | Cat# A302-068, RRID:AB_1604224 |
| Anti-H3K27ac | AbCam | Cat# Ab4729, RRID:AB_2118291 |
| Control IgG | Santa Cruz | Cat# SC-66931, RRID:AB_1125055 |
| Anti-HA | Sigma | Cat# H9658, RRID:AB_260092 |
| Anti-GFP | AbCam | Cat# Ab290, RRID:AB_303395 |
| Anti-Tubulin | Sigma | Cat# T5168, RRID:AB_477579 |
| Anti-BrdU | Santa Cruz | Cat# sc-32323 ac, RRID:AB_626766 |
| Anti-METTL3 | Synaptic system | Cat# 417-003, RRID:AB_2782981 |
| Anti-METTL14 | Bethyl | Cat# A305-847A-M, RRID:AB_2891741 |
| Chemicals, Peptides, and Recombinant Proteins | ||
| 17-β-estradiol | Sigma | Cat# E2758 |
| PROTAC3 | Promega | Cat# CS2072A01 |
| UZH1A | Dr. Amedeo Caflisch | N/A |
| Compound #11 | Enamine Ltd | Cat# Z287226108 |
| EGF | Fisher | Cat# 50-813-058 |
| Lipofectamine 2000 | Thermo Fisher | Cat# 11668019 |
| Lipofectamine 3000 | Thermo Fisher | Cat# L3000150 |
| Lipofectamine RNAiMAX | Thermo Fisher | Cat# 13778150 |
| RS-1 | Sigma | Cat# R9782 |
| SsoAdvanced Universal SYBR Green Supermix | Biorad | Cat# 172-5274 |
| cOmplete™, Mini Protease Inhibitor Cocktail | Roche | Cat# 11836153001 |
| TRIzol™ reagent | Thermo Fisher | Cat# 15596018 |
| TRIzol™ LS reagent | Thermo Fisher | Cat# 10296028 |
| Amicon® Ultra-4 Centrifugal Filter Unit | Millipore | Cat# UFC803008 |
| N6-Methyladenosine-5’-Triphosphate (m6ATP) | Trilink | Cat# N-1013-1 |
| Biotin-11-UTP | Ambion | Cat# AM8450 |
| Cyanine 3-UTP | Enzo Life Science | Cat# ENZ-42505 |
| Dynabeads™ MyOne™ Streptavidin C1 | Thermo Fisher | Cat# 65002 |
| SUPERase• In™ RNase Inhibitor | Thermo Fisher | Cat# AM2694 |
| Flavopiridol | Sigma | Cat# F3055 |
| slip-embedded 35mm dish | MatTek | Cat# P35G-1.5-10-C |
| 4-Thiouridine (4SU) | Sigma | Cat# T4509 |
| MTSEA Biotin-XX | Biotium | Cat# 90066-1 |
| Dynabeads™ Protein G | Thermo Fisher | Cat# 10004D |
| BRD4 protein (Full length) | Reaction Biology | Cat# RD-21-153 |
| Turbo™ DNase | Thermo Fisher | Cat# AM2238 |
| Protease K | Thermo Fisher | Cat# 25530049 |
| RNAse A | Thermo Scientific | Cat# EN0531 |
| Acid-Phenol:Chloroform, pH 4.5 (with IAA, 125:24:1) | Thermo Fisher | Cat# AM9722 |
| KAPA pure beads | Roche / Kapa | Cat# KK8001 |
| DNA polymerase I | New England Biolabs | Cat# M0210 |
| T4 DNA ligase | New England Biolabs | Cat# M0202M |
| ERCC spike in | Thermo Fisher | Cat# 4456740 |
| GlycoBlue™ Coprecipitant | Thermo Fisher | Cat# AM9516 |
| RIPA Lysis and Extraction Buffer | Thermo Fisher | Cat# 89901 |
| 2x Laemmli Sample Buffer | Biorad | Cat# 1610737 |
| Critical Commercial Assays | ||
| NEBNext® Ultra™ Directional RNA Library Prep Kit for Illumina® | New England Biolabs | Cat# E7760 |
| NEBNext rRNA Depletion Kit | New England Biolabs | Cat# E6301 |
| NEBNext® Poly(A) mRNA Magnetic Isolation Module | New England Biolabs | Cat# E7490 |
| KAPA HyperPrep NGS library kit | Roche | Cat# KK8504 |
| NEB Ultra II DNA library kit | New England Biolabs | Cat# E7645 |
| 4–20% Mini-PROTEAN® TGX™ Precast Gels | Biorad | Cat# 4561094 |
| Trans-Blot® Turbo™ Mini PVDF Transfer Packs | Biorad | Cat# 1704156 |
| MEGAscript™ T7 Transcription Kit | Invitrogen | Cat# AM1334 |
| Quick-RNA MiniPrep Kit | Zymo Research | Cat# 11-328 |
| Quick-DNA Miniprep Kit | Zymo Research | Cat# 11-317C |
| Superscript™ IV First-Strand Synthesis System | Thermo Fisher | Cat# 18091050 |
| Pierce™ BCA Protein Assay Kit | Thermo Scientific | Cat# 23225 |
| NuPAGE 10% Bis-Tris gel | Life Technologies | Cat# WG1201BX1 |
| Softwares | ||
| STAR | Dobin et al., 2013 | RRID:SCR_004463 |
| bcl2fastq | https://support.illumina.com/sequencing/sequencing_software/bcl2fastq-conversion-software.html | RRID:SCR_015058 |
| MACS2 | Zhang et al., 2008 | RRID:SCR_013291 |
| Bedtools | Quinlan and Hall, 2010 | RRID:SCR_006646 |
| HOMER | Heinz et al., 2010 | RRID:SCR_010881 |
| Samtools | Li et al., 2009 | RRID:SCR_005227 |
| Stringtie | Pertea et al., 2015 | RRID:SCR_016323 |
| IGV | Robinson et al., 2011 | RRID:SCR_011793 |
| Fiji Image J | Schindelin et al., 2012 | RRID:SCR_003070 |
| Deposited Data | ||
| Illumina sequencing data (RNA-Seq, MeRIP-Seq, MINT-Seq, TT-Seq, PRO-Seq, GRO-Seq and ChIP-Seq) | This study | GEO: GSE143441 |
| Experimental Models: Cell Lines | ||
| K562 | ATCC (originally; was a gift from Yun Nancy Huang, Texas A&M U | CRL-3343 |
| HeLa | ATCC | CCL-2 |
| 293T | ATCC | CRL-3216 |
| MCF7 | ATCC | HTB-22 |
| MCF7 mCherry-BRD4 | This study | N/A |
| MCF7 HaloTag-YTHDC1 | This study | N/A |
| MCF7 mClover-YTHDC1 | This study | N/A |
| Oligonucleotides | ||
| See Table S3 | ||
| Recombinant DNA | ||
| pCRIS-PITChv2-BSD-dTAG (BRD4) | Addgene | Cat# 91792 |
| pX330A-nBRD4/PITCh | Addgene | Cat# 91794 |
| pSpCas9(BB)-2A-Puro (PX459) V2.0 | Addgene | Cat# 62988 |
| pTXB1-Tn5 | Addgene | Cat# 60240 |
| lenti sgRNA(MS2)_zeo backbone | Addgene | Cat# 61427 |
| TetO-NLS-RfxCas13d-NLS-WPRE-EFS-rtTA3-2A-Blast | Addgene | Cat# 138149 |
| pLentiRNAGuide_002-hU6-RfxCas 13d-DR-BsmBI-EFS-Puro-WPRE | Addgene | Cat# 138151 |
| pHR-mCh-CRY 2WT | Addgene | Cat# 101221 |
Dot Blot of RNA
RNA sample (1μg in 4μl) were loaded on BrightStar™-Plus Positively Charged Nylon Membrane (Invitrogen, Cat# AM10102). After 5-min, RNAs on the membrane were UV-crosslinked at 254 nm (1200uJ/cm2) twice. Then, the membrane was incbated in 5% skim milk in PBST for an hour. After an hour of incubation, the membrane was washed in PBST once and incubated in a primary antibody (m6A antibody, Synaptic Systems, Cat# 202–003, 1:5,000) solution in 5% skim milk in PBST overnight. After 5 times of washing in PBST, the membrane was incubated in a secondary antibody solution for an hour. The blots were washed 5 times in PBST and developed in a Bio-Rad ChemiDoc Touch imaging system. In order to examine total RNA amounts in the blot, the membrane was incubated in 10ml of Methylene Blue staining buffer (0.2% Methylene Blue, 0.4M Acetic acid, 0.4M Sodium Acetate) for 30 min. After brief washing in dH2O, the membrane was used for imaging in a BioRad ChemiDoc Touch imaging system.
Recombinant Protein Expression and Purification
mClover-YTH and mClover-YTH-IDR2 were cloned into pTXB1-Tn5 (Addgene Cat#60240) using Gibson cloning method and transformed into C3013 E.Coli strain (NEB). For mClover-YTH-IDR2 R to A mutant cloning, all 55 Arginines in IDR2 were mutated to alanines. The synthesized backbone of R to A mutant (Twist Bioscience) was used for gibson assembly. Protein expression was induced with 0.25 mM Isopropyl β-D-1-thiogalactopyranoside (IPTG) at 22°C for 4 hours. After centrifugation at 4,000 g for 15 minutes, the pelleted bacteria cells were washed once with HEGX buffer (20 mM HEPES-KOH pH 7.2, 0.8 M NaCl, 1 mM EDTA, 10% glycerol, 0.2% Tx-100) and resuspended with HEGX buffer supplemented with protease inhibitor (Roche). The cells were sonicated (Branson sonicator 30 sec on/30 sec off interval, 15 cycles) and centrifuged at 15,000 rpm for 30 minutes at 4 °C. The supernatant, which was reserved as a crude extract for coomassie blue gel staining, was transferred to the new tube and incubated with pre-washed chitin beads (NEB) for an hour at 4 °C with rotation. Then, the beads were washed with 10 ml HEGX buffer for 3 times and reserved as bead purified for coomassie blue gel staining. To cleave the peptides out, the beads were incubated in a 10 ml HEGX buffer containing 130 mM DTT and protease inhibitor (Roche) for 24 hours. After 24-hour rotation, the mixture was centrifuged at 1,000g for 10 min, and the supernatant was concentrated with Amicon Ultracel 30 centrifugal filters (Millipore). The proteins were resuspended in the elution buffer (20 mM HEPES pH 7.4, 300 mM KCl, 6 mM MgCl2, 0.02% NP-40). The protein concentration was measured by comparing serial diluted BSA standards and the proteins in coomassie blue stained SDS-PAGE gel.
Prediction of Intrinsic Disordered Domain (IDR)
Prediction of IDR was carried out using the VSL2 predictor in Predictor of Natural Disordered Regions (www.pondr.com).
In vitro RNA transcription
We synthesized TFF1e with or without m6A using MEGAscript Kit (Thermo Fisher) by manufacturer’s protocol. In brief, we amplified the first 500 bp of TFF1 enhancer RNA region that overlaps a strong m6A peak in MINT-Seq by PCR using two primers (TFF1e Forward-CTAACTAGGCTCTTCCGGCCAAGTGAGTGAGGGGATGTGT, TFF1e Reverse-GCGGCCCTCTAGACTCGAGAAAGCAGCAGCAGATGAGATC). About 100 ng of amplified DNA fragments were in vitro transcribed to RNAs using the MEGAscript kit. For m6A labeling of TFF1e, we used 1:1 of m6ATP (Trilink) and ATP during the in vitro transcription. For visualization of TFF1e eRNA under the microscope, 20% of UTP were labeled with Biotin-UTP (Ambion) or Cy3-UTP (Enzo Life science).
RfxCas13d-mediated eRNA knockdown (or CasRx) and dCasRX-FTO tethering experiment
In order to conduct the Cas13d-mediated RNA degradation, we generated stable cell line by infecting them with lentivirus expressing Dox-inducible Cas13d (TetO-NLS-RfxCas13d-NLS-WPRE-EFS-rtTA3–2A-Blast, Addgene #138149) and appropriate gRNA cloned in to a Cas13d gRNA backbone (pLentiRNAGuide_002-hU6-RfxCas13d-DR-BsmBI-EFS-Puro-WPRE, Addgene #138151), as previously described. gRNA sequence was designed by a algorithm described previously (Wessels et al., 2020), and primer sequences for cloning gRNA into the lentiviral backbone (Addgene #138151) for TFF1e-gRNA are the following (5’ to 3’), forward: AAACGACTGTTGCAACAAACGACCCCA, reverse: AAAATGGGGTCGTTTGTTGCAACAGTC. Cells were treated with 2μg/ml doxycycline in the culturing medium for four to seven days before harvest. To generate enzymatically dead CasRx fused with FTO and HA-tag (dCasRx-FTO-HA) expressing stable cell line, we generated a pLenti-dCasRx-FTO-HA-Blast based on the backbone of dCAS-VP64_Blast (Addgene #61425). In the dCasRX-FTO-HA expressing MCF7 stable cell line, TFF1e-gRNA for the CasRx system was introduced using lentiviral infection.
In vitro biotinylated RNA pull down and Mass-Spectrometry
We isolated the nuclear extracts from 5 million MCF7 cells using a nuclear extraction buffer (10 mM PIPES pH 6.8, 100 mM NaCl, 3 mM MgCl2, 0.3 M sucrose, 0.5% TX-100, 1 mM PMSF). After centrifugation at 3,000g, the pellets were resuspended in 1ml RIP-EXT buffer (20 mM Tris pH 7.4, 300 mM NaCl, 1 mM EDTA, 0.2% TX-100, 5% glycerol, freshly added 0.5 mM DTT) with protease inhibitor cocktail. The lysates were sonicated in Qsonica 800R (25% amplitude, 5 min, 10 sec on/20 sec off interval) and pre-cleared with 10 μl of pre-washed 10 μl Dynabeads MyOne Streptavidin C1 beads (Thermo Fisher) at 4°C rotator for an hour. After pre-clearing, the beads were removed by a magnetic rack. The lysates were centrifuged at 14,000g for 10 min. The supernatants were mixed with an equal amount of RIP-low salt buffer (20 mM Tris pH 7.4, 1 mM EDTA, 5% glycerol, freshly added 0.5 mM DTT) with protease inhibitor. The mixture was then rotated with pre-conjugated Streptavidin beads-biotinylated-RNA complex at 4 °C for 4 hours. For the pre-conjugation of the biotin-RNA-streptavidin beads complex, the RNA was heated at 90°C for 3 min and immediately cooled down on ice for 2 min. Then, the RNA was incubated in 60 μl of RNA structure structure buffer (10 mM Tris pH7.2, 0.1M KCl, 10 mM MgCl2) with 1μl Superase-IN (Thermofisher) for 20 min at room temperature. The RNA was then mixed with pre-washed streptavidin C1 magnetic beads (ThermoFisher) and incubated at room temperature for 40 min. After 4 hour incubation, the beads were washed twice in high salt buffer (20 mM Tris pH 7.4, 600 mM NaCl, 0.05% Triton X-100, 10 units per ml Superase-In) and twice in low salt buffer (20 mM Tris pH 7.4, 450 mM NaCl, 0.05% Triton X-100, 10 units per ml Superase-In). The beads were subjected to mass spectrometry or western blot analysis. Mass Spectrometry Proteomics Core of Baylor College of Medicine provided LC/MS/MS service for analyzing the proteome of in vitro biotinylated m6A or non-m6A eRNA pulldown samples in Figure S6B and Table S2. For mass spectrometry analysis, the beads were boiled with the sample loading buffer and were resolved on a NuPAGE 10% Bis-Tris gel (Life Technologies, WG1201BX10) in 1 x MOPS running buffer. The proteins were visualized with Coomassie Brilliant Blue and the gel was cut into 4 pieces based on the molecular weight. Each gel piece was in-gel digested overnight with 100 ng of trypsin (MS grade, GenDepot) in 20 μl of 50 mM NH4HCO3 at 37°C. Tryptic peptides were extracted by 100% acetonitrile, vacuumed and dried. Then they were dissolved in 10 μl 5% methanol containing 0.1% formic acid and subjected to nanoHPLC-MS/MS assay using a nLC120 (Thermo Scientific) coupled to a Q Exactive Plus (Thermo Scientific) mass spectrometer. An in-housed 2cm x 100 μm i.d. trap column with 3 μm Reprosil-Pur Basic C18 beads (Dr. Maisch HPLC GmbH, Germany) and an in housed 5 cm X 150 μm capillary column packed with 1.9 μm Reprosil-Pur Basic C18 beads was used. A 75-min discontinuous gradient of 4–26% acetonitrile, 0.1% formic acid at a flow rate of 800 nl/min was applied to column then electro-sprayed into the mass spectrometer. The instrument was operated under the control of Xcalibur software ver. 2.2 (Thermo Fisher Scientific) in data-dependent mode, acquiring fragmentation spectra of the top 35 strongest ions. Parent MS spectrum was acquired in the Orbitrap with a full MS range of 375–1300 m/z in the resolution of 140,000 and AGC target of 3 ×106. HCD fragmented MS/MS spectrum was acquired with AGC target of 2 ×104. Dynamic exclusion was applied for 18 second of exclusion duration. The MS/MS spectra were searched against target-decoy Human refseq database (release 2015_06, containing 73,637 entries) in Proteome Discoverer 1.4 interface (Thermo Fisher) with Mascot algorithm (Mascot 2.4, Matrix Science). Dynamic modifications of Acetylation of N-term and Oxidation of methionine were allowed. The precursor mass tolerance was confined within 20 ppm with fragment mass tolerance of 0.02 Dalton and a maximum of two missed cleavages was allowed. Assigned peptides were filtered with 1% false discovery rate (FDR) using Percolator validation based on q-value. Calculated area under curve of peptides was used to calculate iBAQ for protein abundance based on the previous publication (Saltzman et al., 2018).
RNA Stability Test
MCF7 cells were transfected as indicated in each paper figure by Lipofectamine 2000 or RNAiMax (Themo Fisher). After 10min treatment of 2 μM Flavopiridol (Sigma), we started to collect the cells for RNA extraction. The cells with 10 mins of Flavopiridol treatment is considered the “initial” timepoint (i.e. the “0 min” point that enhancer transcription is largely shut down), and then samples at 30 mins and 3 hrs after this time were respectively harvested. RT-qPCR was used to determine the levels of RNAs at each time point and the ratios to the initial time point were plotted.
Light-induced Optodroplet Assay
We slightly modified the protocol from Shin et al (Shin et al., 2017). YTHDC1-full length, -IDR1, -YTH domain, -IDR2, YTH-IDR2 were cloned into pHR-mCh-CRY2WT plasmid (Addgene, Cat# 101221) using Gibson cloning (primers can be found in Table S3). Cells were transiently transfected with optodroplet plasmids. The transfected cells were seeded on a poly-D-lysine coated slip-embedded 35 mm dish (MaTek). All images were taken by Nikon A1R laser scanning confocal microscope with a temperature stage at 37 °C (Tokai). For global activation intervals, we used a repetitive on/off cycle with two laser wavelengths (488 nm for CRY2 activation for one minute/560 nm for mCherry imaging). We used 0.1% of 488 nm laser for global activation. Images were obtained by Nikon A1R confocal microscope (Nikon). The intensity and condensate size were measured and quantified by ImageJ.
In vitro Phase Separation Assay
Purified recombinant proteins and in vitro synthesized eRNA were mixed in the in vitro phase separation buffer (10 mM HEPES pH 7.4, 150 mM KCl, 3 mM MgCl2, 0.01% NP-40 and 10% PEG-8000). 1 μM of mClover-YTH or mClover-YTH-IDR2 protein was mixed with 1nM of in vitro transcribed Cy3-labeled TFF1e RNA in this buffer. For co-phase separation assay using YTH-IDR2, its R-to-A mutant or YTH domain alone together with BRD4 and RNA, we mixed 0.5 or 1 μM mClover-YTH-IDR2, 1 nM Cy3 labeled 25% m6A-TFF1e and 1 μM mixed BRD4 (Full length recombinant protein, Reaction Biology, Cat# RD-21–153) and Alexa647 (Alexa Fluor™ 647 NHS Ester (Succinimidyl Ester), Invitrogen, A20006) labeled BRD4 at 1:300 ratio. The mixture of proteins and RNA was placed on a slip-embedded 35 mm dish (MaTek). After incubation in a 37 °C humidified chamber (Tokai) for 30 min, the images were obtained by Nikon A1R confocal microscope or Leica SP-8 microscope. The particle sizes of the condensates (Square Pixel) were quantified by ImageJ.
Live Cell Imaging and FRAP
mClover-YTHDC1-knock-in cells were plated on a slip-embedded 35mm dish (MatTek). 3D images were reconstructed from 16 planes over 18 μm height using Nikon NIS-elements software (Nikon). For FRAP, a single pulse of the 405 laser for 500 ms was applied to the cell, and recovery was imaged every 2.21 seconds for 2 minutes on a Leica SP-8 microscope or Nikon A1R confocal microscope (Nikon). The images were quantified by ImageJ. For quantification of condensate foci per cell, we used “Find Maxima” in ImageJ.
MeRIP-qPCR (i.e. m6A RIP-qPCR)
For MeRIP, Trizol (Invitrogen) was used to extract RNA by manufacturer’s protocol. 2.5 μg of RNA was diluted with 1,250 μl IP buffer (10 mM Tris-HCl, pH 7.4, 150 mM NaCl, 0.1% NP-40 and Protease Inhibitor Cocktail (Roche)). 250 μl of diluted RNA was transferred to a new tube as 25% input. 5ng of equal amount m6A- and non-m6A-labeled synthetic non-human RNA mixture (see sequences in Table S3) was added to both IP and input samples as the m6A level spike-in controls. 20 μl of Dynabeads protein (Thermo Fisher / Life Technologies) was washed 3 times with 1ml IP buffer and incubated with 2 μg of m6A antibody (Synaptic Systems, Cat 202–003) at room temperature for 30min. The conjugated Dynabeads-m6A-antibody was washed in 1ml IP buffer 3 times. Then, the RNA sample was mixed with antibody-proteinG beads and rotated at room temperature for 3 hours. After 3hour rotation, the beads were washed in the IP buffer 5 times. Both immunoprecipitated and input RNAs were extracted from the beads by TRIzol extraction. The extracted RNA was subjected to cDNA synthesis and qPCR to quantify the m6A levels (for primers information see Table S3).
UV RNA Immunoprecipitation (RIP)
RNA immunoprecipitation (RIP) for proteins such as YTHDC1 was carried out according to Jeon et al (Jeon and Lee, 2011) with modifications. Briefly, 5 millions of cells were UV-crosslinked at 254 nm (400 mJ/cm2) on ice and collected by scraping. Cells were lysed with 1ml cold lysis buffer (0.5% NP-40, 0.5% sodium deoxycholate, 20U/ml Superase-In, 1x Protease Inhibitor in PBS) at 4 °C for 25 minutes with rotation. Lysate was sonicated (Qsonica 800R, 25%, 5 minutes, 10 sec on/20 sec off interval), followed by 10U/ml Turbo DNase treatment for 15 minutes at 37°C. After centrifugation at 20,000g for 10 minutes, the supernatant was incubated with 1 μg Rabbit IgG or 1μg anti-YTHDC1 antibody (Abcam, Ab122340) immobilized on 20 μl Dynabeads™ Protein G beads overnight at 4°C. The next day, the beads were washed three times with 1ml ice-cold wash buffer (1% NP-40, 150mM NaCl, 0.5% sodium deoxycholate, 20U/ml Superase-In, Protease inhibitor in PBS), and treated with 10U Turbo DNase for 30min at 37°C. After three more washes with wash buffer supplemented with 10 mM EDTA, beads were digested with 200 μl Proteinase K buffer (100 mM Tris-HCl, 50 mM NaCl, 10 mM EDTA, 0.5% SDS and 500 μg/ml Proteinase K) for 30 minutes at 65°C. RNA was recovered by TRIzol reagent and examined by RT-qPCR.
Chromatin Immunoprecipitation (ChIP) and ChIP-Seq
Cells were cross-linked by 1% Formaldehyde for 10min for ERα, BRD4 or H3K27ac ChIP, or 2% Formaldehyde) for 15 min for YTHDC1 ChIP. About 10 million cells were used for the YTHDC1 ChIP experiment, and 2–5million cells were used for other ChIP experiments. The pelleted cells were lysed in 1 ml buffer LB1 (50 mM Hepes-KOH, pH 7.5, 140 mM NaCl, 1 mM EDTA, 10% glycerol, 0.5% NP-40, 0.25% TX-100) supplemented with protease inhibitor cocktail (Roche) and 10U/ml Superase-In (Thermofisher) at 4°C for 10 min with rotation. The incubated lysates were centrifuged at 2,000g for 5min at 4 °C. Then, the pellets were resuspended in 1ml buffer LB2 (10 mM Tris-HCl, pH 8.0, 200 mM NaCl, 1 mM EDTA, 0.5 mM EGTA) with protease inhibitor and 10U/ml Superase-In (Thermofisher) and rotated for 5 min at 4 °C. After centrifugation at 2,000g for 5 min, the pellets were resuspended in 300 μl buffer LB3 (10 mM Tris-HCl, pH 8.0, 100 mM NaCl, 1 mM EDTA, 0.5 mM EGTA, 0.1% Na-Deoxycholate, 0.5% N-lauroylsarcosine) with protease inhibitor and 10U/ml Superase-In (Thermofisher) and sonicated in Qsonica 800R (20% amplitude, 10 min, 10 sec on 20 sec off interval). After the sonication, 30 μl of 10% TX-100 was supplemented into the lysates. The mixture was centrifuged at 20,000g for 10 minutes. The supernatants were transferred to the new tube, and 30 μl of supernatants were reserved for input. Into the 300 μl of supernatants, we added 700 μl of LB3, 70 μl of 10% TX-100 and 2–3μg of specific antibodies. After overnight incubation on a 4 °C rotator, 40ul of Dyna G beads were added into the tube. The mixtures were rotated at 4 °C for 3 hours. After the incubation, the beads were washed in 1 ml RIPA buffer (50 mM Hepes, pH 7.6, 1 mM EDTA, 0.7% Na-Deoxycholate, 1% NP-40, 0.5M LiCl) 6 times. The beads were washed again in 1ml TE buffer (10 mM Tris-HCl, pH 7.4, 1mM EDTA). After discarding TE buffer on the magnetic stand, DNA was incubated in 200 μl elution buffer (1% SDS, 0.1 M NaHCO3) at 65 °C. After 6 hours incubation, RNAse A treatment was carried out in the sample at 37 °C for 1 hour. Then, proteinase K was administered into the sample and incubated in 65 °C for 2 hours. The DNA was purified by phenol-chloroform extraction methods.
GRO-Seq and PRO-Seq
The run-on step of GRO-Seq largely follows a previous protocol (Li et al., 2013). Briefly, about 10million nuclei were extracted on ice. They were incubated in the nuclei run-on buffer at 30 °C for 5minutes in the presence of 500 μMof ATP, GTP, and Br-UTP together with 2 μM of CTP. For PRO-Seq, the run-on step largely follows a previous protocol using biotin-11-UTP and biotin-11-CTP (Mahat et al., 2016). Total RNAs were extracted using TRIzol LS and were fragmented using NaOH hydrolysis on ice for 30 minutes (GRO-Seq) or 15mins (PRO-Seq). The fragmented BrU or biotin labeled RNAs were purified using an anti-BrdU antibody from Santa Cruz (sc-32323 ac), or by Streptavidin C1 beads (Thermo Fisher), respectively. For library making, we largely followed a protocol used in eCLIP-Seq (Van Nostrand et al., 2016). The fragmented nuclei run-on RNAs were end-repaired by a fast Alkaline Phosphatase (Thermo Fisher) and T4 PNK (Thermo Fisher), cleaned up using Dynabeads MyOne Silane, and were then ligated to a 3’end RNA linker: RiL19 /5phos/rArGrArUrCrGrGrArArGrArGrCrGrUrCrGrUrG/3SpC3/. The ligated RNA was reverse transcribed using AffinityScript kit (Agilent) and using AR17 primer (5’- ACACGACGCTCTTCCGA - 3’). The cDNA was then cleaned up and ligated with a DNA linker carrying 10 nucleotides unique molecular index (UMI), rand10–3Tr3 (/5Phos/NNNNNNNNNNAGATCGGAAGAGCACACGTCTG/3SpC3/). The ligated cDNA was cleaned up and PCR was conducted to test the library abundance. Final PCR primers for amplification were based on a pair of PCR_F_D501 together with a barcoded PCR_Rev_p7x. For example, PCR_F_D501: 5’- AATGATACGGCGACCACCGAGATCTACACTATAGCCTACACTCTTTCCCTACACGACGCTCTTCCGATCT −3’ and one of the indexed 3’end primers PCR_Rev_p7x: 5’- CAAGCAGAAGACGGCATACGAGAT xref GTGACTGGAGTTCAGACGTGTGCTCTTCCGATC −3’ (where xref indicates 6-nucleotide index sequence), and the library was made using NEB Q5 2x PCR master mix by often around 9–13 cycles. Final library was sized-selected using TBE 10% PAGE gels with a range of ~170–400nt for GRO-Seq, or alternatively by KAPA pure beads (Roche) using 0.55X and 0.9X double size selection.
TT-Seq, MINT-Seq, total RNA-Seq and MeRIP-Seq
For TT-seq and MINT-seq, nascent RNA was labeled and captured according to Duffy et al. (Duffy et al., 2015), with modifications. In brief, 1×108 cells were cultured in normal medium supplemented with 700 μM 4SU for 15 minutes at 37 °C and then immediately lysed by TRIzol (Invitrogen). Extracted total RNA was dissolved in biotinylation buffer (10mM HEPES-KOH pH7.5, 1mM EDTA) to a concentration of 0.4 μg/ul. 1/4 volume of MTSEA biotin-XX (166μg/ml, dissolved in DMF) was added. After rotation in the dark for 2 hours, RNA was extracted with phenol/chloroform (pH 4.5) with standard protocol. Purified RNA was dissolved in the fragmentation buffer (10 mM ZnCl2, 10 mM Tris-HCl pH 7.4) and fragmented at 70 °C for 15 minutes. Fragmentation was quenched by adding 1/10 volume of 0.5 M EDTA on ice. Fragmented total RNA could be subjected to RNA-Seq and MeRIP-Seq if desired. Fragmented RNA was diluted with the high-salt buffer (50 mM Tris-HCl pH 7.4, 1 M NaCl, 10 mM EDTA, 0.05% Tween-20, 20U/ml Superase-In) by at least 5 times. Biotinylated RNA was captured with Dynabeads™ MyOne™ Streptavidin C1 beads (Invitrogen) with rotation for 30 minutes at room temperature. The beads were washed three times with high-salt buffer, twice with TET buffer (10 mM Tris-HCl pH 7.4, 1 mM EDTA, 0.05% Tween-20, 20U/ml Superase-In), once with TE buffer (10 mM Tris-HCl pH 7.4, 1 mM EDTA, 20U/ml Superase-In). Nascent RNA was eluted by 150 μl fresh-made 5% beta-mercaptoethanol (β-ME) with rotation for 15 minutes at dark and precipitated with iso-propanol according to standard protocol. Extracted nascent RNA was dissolved with 1,010 μl ice-cold IP buffer (10 mM Tris-HCl pH 7.4, 150 mM NaCl, 0.1% NP-40, 20U/ml Superase-In, 1x Protease Inhibitor). 10 μl of RNA was transferred to a new tube for TT-Seq (also serves as 1% input of MINT-seq). Equal amount of synthesized non-mammal m6A spike-in RNAs was added to both TT-Seq and MINT-Seq groups (Sequences and m6A ratios see Xiong et al., 2021). Anti-m6A antibody conjugated Dynabeads™ Protein G beads (Invitrogen) were washed 3 times with the IP buffer and added to the remaining 1000 μl RNA sample. The RNA-beads mixture was rotated at 4 °C for 4 hours. The beads were washed 3 times with ice-cold IP buffer, twice with ice-cold low-salt wash buffer (10 mM Tris-HCl pH 7.4, 50 mM NaCl, 0.1% NP-40, 20U/ml Superase-In, 1x Protease Inhibitor), twice with ice-cold high-salt wash buffer (10 mM Tris-HCl pH 7.4, 500 mM NaCl, 0.1% NP-40, 20U/ml Superase-In, 1x Protease Inhibitor), once with ice-cold TE buffer. Immunoprecipitated RNAs and previously prepared input RNAs were extracted by TRIzol-LS (Invitrogen). Both immunoprecipitated and input RNAs were mixed with equal amounts of ERCC spike-in RNAs and then subject to directional RNA library preparation (New England Biolabs, 7760) after ribosomal RNA depletion (New England Biolabs, E6310), which are MINT-seq and TT-seq, respectively.
For RNA-seq and MeRIP-seq, fragmented total RNA (see MTS-TT-seq and MINT-seq) was diluted with 1010 μl ice-cold IP buffer (10 mM Tris-HCl pH 7.4, 150mM NaCl, 0.1% NP-40, 20U/ml Superase-In, 1x Protease Inhibitor) to a final volume of 1010 μl. 10 μl of RNA was transferred to a new tube for RNA-seq (also serves as 1% input of MeRIP-seq). Equal amount of synthesized m6A spike-in RNAs was added to both TT-Seq and MINT-Seq groups (Sequences and m6A ratios for the spike-in RNAs see Xiong et al., 2021). Anti-m6A antibody conjugated Dynabeads™ Protein G beads (Invitrogen) were washed 3 times with the IP buffer and added to the remaining 1000 μl RNA sample. The RNA-beads mixture was rotated at 4 °C for 4 hours. The beads were washed 3 times with ice-cold IP buffer, twice with ice-cold low-salt wash buffer (10 mM Tris-HCl pH 7.4, 50 mM NaCl, 0.1% NP-40, 20U/ml Superase-In, 1x Protease Inhibitor), twice with ice-cold high-salt wash buffer (10 mM Tris-HCl pH 7.4, 500 mM NaCl, 0.1% NP-40, 20U/ml Superase-In, 1x Protease Inhibitor), once with ice-cold TE buffer. Immunoprecipitated RNAs and previously prepared input RNAs were extracted by TRIzol-LS (Invitrogen). Both immunoprecipitated and input RNAs were mixed with equal amounts of ERCC spike-in RNAs and then subject to directional RNA library preparation (New England Biolabs, 7760) after ribosomal RNA depletion (New England Biolabs, E6310), which are MeRIP-seq and RNA-seq, respectively.
For polyA selected mRNA sequencing after YTHDC1 degradation, polyA RNAs were first enriched by NEBNext® Poly(A) mRNA Magnetic Isolation Module (New England Biolabs, E7490) and subjected to directional RNA sequencing library preparation (New England Biolabs, E7760).
Data analyses of MINT-Seq, MeRIP-Seq, RNA-Seq, TT-Seq, GRO-Seq/PRO-Seq and ChIP-Seq
Raw data were de-multiplexed by bcl2fastq (v2.20), and quality- controlled with fastqc. All clean-reads were mapped to human reference genome hg19 by STAR v2.7.0 (Dobin et al., 2013), with parameters of –-genomeSAindexNbases 14 –outFilterMultimapNmax 10 – outFilterMismatchNmax 10. Duplicated reads were further removed, and only unique aligned reads will be considered for later visualization and quantification. For gene/mRNA quantification, hg19 RefSeq gene annotation coordinates were used. And HOMER tool-sets were used to calculate the RPKM value of individual transcripts (Heinz et al., 2010). The unique aligned reads were further converted to bigwig format with a fragment length of 75 on IGV browser. The m6A peak-calling of MINT-Seq/MeRIP-Seq/ChIP-Seq were performed with the parameters of -f BAM -q 0.01 -n in MACS2 (Zhang et al., 2008). For MINT-Seq peak calling, we considered TT-Seq as input, and for MeRIP-Seq peak calling, we treated RNA-Seq as input. The motif analyses of m6A peaks were performed by HOMER (Heinz et al., 2010). The half-lives of eRNAs in K562 were estimated by Schwalb 2016, and obtained eRNA coordinates there were converted from hg38 to hg19 using UCSC liftOver tool. m6A-eRNAs were defined as any eRNAs that overlapped a MINT-Seq peak.
de novo transcript assembly, eRNA and uaRNA annotation
To identify and characterize the transcript features of eRNAs and uaRNAs, we performed de novo assembly of nascent transcriptome by the Stringtie algorithm (Pertea et al., 2015) on TT-Seq data, and we merged TT-Seq from two sets of biological replicates (Table S1). Both EtOH and E2 TT-Seq reads were merged as the input, and the Stringite was run with the parameter of -f 0.1,-c 2, -g 200, --rf and without reference guide. The de novo assembled transcriptome annotation files were first filtered by removing those overlapping any tRNA, snoRNA, miRNA, pseudo-gene and protein-coding genes annotated in GENCODE v19 (in the same strand). The uaRNAs were defined as any transcripts whose de novo called transcript start sites are overlapped with protein-coding genes’ TSS regions (−300 to +1000) on the reverse strand. eRNAs were defined as any transcripts whose transcript initiation sites fall in +/− 3kb window of intronic or intergenic p300 ChIP-Seq peaks (the sense strands of genes were already removed in the previous steps, and removed any identified uaRNAs). The length of eRNA and uaRNA was determined by Stringtie de novo assembly. So far, there have not been experimental methods to globally define the exact transcript lengths of eRNAs, de novo transcript assembly provided currently available eRNA transcript coordinates to characterize m6A methylome at the level of eRNA transcripts. The start and end coordinates of each de novo transcript were used to calculate the length of each transcript.

(Workflow to annotate eRNAs and uaRNAs from TT-Seq).
Calling of m6A-marked eRNAs
The exact eRNA length in a global scale is not known and cannot be fully determined at this stage, because there is not a golden standard to experimentally define eRNA transcripts from the start to end. Many past and current work (e.g. Kim et al., 2010; Li et al., 2013) used enhancer core and the 2–3kb regions spanning the core to define eRNAs (enhancer core is often defined by the summits of transcription factors or p300 ChIP-Seq, see the diagram below, “enhancer core based identification”). This is an acceptable strategy if one studies the transcriptional activity of enhancers, as GRO-Seq or other nascent RNA-Seq reads in these 2–3kb regions spanning the enhancer core are often correlated with the enhancer activity. Essentially, the enhancer core is a “promoter”, except that it drives noncoding eRNAs rather than pre-mRNAs (see diagram below). However, this strategy will be a problem when it comes to correctly defining m6A peaks on eRNA transcripts because the length of these transcripts can be much longer than 2 or 3 kb (as mentioned in our text, and the TFF1 eRNA is a good example). We have tested a strategy to use enhancer core and the 2–3 kb regions spanning the core to identify m6A peaks on eRNAs. However, this strategy will only identify m6A peaks close to the core, but will exclude any m6A on the distal regions of an eRNA transcript (a symbolic example shown in the diagram below). Indeed, as shown in many cases by MINT-Seq in this current paper (Figure 1C,E,F, Figures S1 and S2F), m6A peaks are often not located close to the 5’ ends of the transcripts (our Figure 1E,F show the global pattern). The peaks very close to the 5’ends actually have a chance to be m6Am, another RNA methylation mark known to mark the first Adenosine from 5’ends (Mauer et al., 2017; Sendinc et al., 2019). We extended efforts to functionally exclude the involvement of m6Am on eRNAs (see Figure S5L).
We used TT-Seq and a de novo transcript calling algorithm, Stringtie (Pertea et al., 2015) to define eRNAs, and then MINT-Seq for determining m6A sites on eRNAs (diagram below, the upper panel). This algorithm was also adopted by several recent studies in RNA transcripts identification categorization (Davidson et al., 2019; Chiu et al., 2018).

Above is a diagram illustrating two different computational strategies to annotate eRNAs and m6A marked eRNAs: 1). De novo nascent transcriptome assembly (Upper panel, which is used in the current study); the light blue boxes below the diagram show annotated eRNAs, and purple box indicates an m6A-eRNA. 2). Enhancer-core based methods: by an arbitrary extension of several kb from enhancer-core regions (Lower panel). The light blue boxes below the diagram show annotated eRNAs using this method. Blue peak was used to denote a m6A peak. Created with biorender.com
As discussed above, ways to define eRNAs based on arbitrary size cutoff of an enhancer (2kb or 3kb surround an enhancer core, e.g. p300 peaks) are acceptable if one needs to study enhancer transcription, but it is inaccurate if one needs to correctly define m6A peaks on eRNA transcripts (diagram above). Similarly, overlapping m6A peaks with super-enhancers to define so called super-enhancer RNAs shall be done with caution as super-enhancers are defined by clusters of histone H3K27ac peaks, which are often very large (~3–20kb), and they very often harbor gene promoters or exons/UTRs. Simply by overlapping m6A peaks to large Super-enhancers will incorrectly define many m6A peaks on gene promoters or exons/UTRs as enhancer RNA m6A sites. Improvement of experimental strategies in future will be needed to further increase the precision to define eRNA lengths and to fully annotate m6A-marked eRNAs.
Sequencing Library Preparation and Sequencing
ChIP-Seq libraries were made with KAPA hyper prep library generation kit (Roche, KK8504) or with NEB Ultra II DNA library kit (NEB, E7645). Libraries for TT-Seq, MINT-Seq, RNA-Seq and MeRIP-Seq were made with NEBNext Ultra Directional Library Prep Kit for Illumina (NEB, E7760) following the manufacturer’s instructions. Ribosome RNA was depleted with NEBNext rRNA Depletion Kit (NEB, E6301S). Generated libraries were quantified using the Qubit-3 system and/or qPCR, which sometimes also were examined by TBE PAGE gel and Bioanalyzer 2100. They were mostly sequenced using the NextSeq 550 Sequencing system following the manufacturer’s instruction using a pair-ended 40bp mode.
Quantification and Statistical Analysis
All bar graphs of qPCR data and microscopy data were presented as mean±SD as indicated in figure legend using Prism 6.0. Two-tailed Student’s t-test was used to compare means between groups as indicated; p < 0.05 was considered significant, and p values with asterisks are indicated in each figure panel. *, p < 0.05; **, p < 0.01; ***: p < 0.001. All other statistical analyses for genomics data were performed with Python script. Key software used in the analysis of high-throughput sequencing data are listed above in each section. Most statistical tests here were non-parametric tests because data distributions failed to conform with the assumption of normality and equal variance.
Supplementary Material
Supplementary Movie.1
Time lapse imaging of optodroplet assay of YTHDC1-FL-mCherry-CRY2 in MCF7 cells (related to Figures 5 and S9).
Supplementary Movie.2
Time lapse imaging of optodroplet assay of YTHDC1-FL-mCherry-CRY2 for a cluster of 293T cells (related to Figures 5 and S9).
HIGHLIGHTS:
MINT-Seq is of high sensitivity to characterize m6A methylome on nascent RNAs
There is a pervasive but also selective m6A deposition to long and stable eRNAs
m6A-eRNAs recruit YTHDC1 to enhancers to stimulate enhancer and gene activation
m6A-eRNA/YTHDC1 phase separate to facilitate transcriptional condensate formation
Acknowledgements:
W.L. is a Cancer Prevention and Research Institute of Texas (CPRIT) Scholar in Cancer Research. This work is supported by funding to W.L. from the University of Texas McGovern Medical School, NIH (K22CA204468, R21GM132778, R01GM136922, 4D Nucleome program U01HL156059), NCATS (UL1TR003167), CPRIT (RR160083, RP180734), Welch foundation (AU-2000-20190330) and the Gulf Coast Consortium John S. Dunn foundation. J.-H.L. is partially supported by a UTHealth Innovation for Cancer Prevention Research Training Program Post-doctoral Fellowship (RP160015). The chemical inhibitor work is supported by the Swiss National Science Foundation grants to A.C. (31003A_169007) and to E.V.M.O. (CRSK-3_190825). Most sequencing was conducted in UTHealth Cancer Genomics Core supported by CPRIT (RP180734). Imaging was supported partially by the UTHealth Nikon Center of Excellence and Center for Advanced Microscopy, partially by the Integrated Microscopy Core at Baylor College of Medicine and the Center for Advanced Microscopy and Image Informatics (CAMII) (NIH P30DK056338, P30CA125123, and P30ES030285 and CPRIT RP150578 and RP170719). The mass spectrometry and analysis were supported by NIH (P30CA125123) and CPRIT (RP170005). We thank Yun (Nancy) Huang at Texas A&M University Health Science Center in Houston for sharing the K562 cells.
Footnotes
Declaration of Interests: The authors declare no competing interests.
Disclosure:
The content is solely the responsibility of the authors and does not necessarily represent the official views of CPRIT or NIH.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Akichika S, Hirano S, Shichino Y, Suzuki T, Nishimasu H, Ishitani R, Sugita A, Hirose Y, Iwasaki S, Nureki O, et al. (2019). Cap-specific terminal N (6)-methylation of RNA by an RNA polymerase II-associated methyltransferase. Science 363. [DOI] [PubMed] [Google Scholar]
- Alarcon CR, Goodarzi H, Lee H, Liu X, Tavazoie S, and Tavazoie SF (2015). HNRNPA2B1 Is a Mediator of m(6)A-Dependent Nuclear RNA Processing Events. Cell 162, 1299–1308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andersson R, Gebhard C, Miguel-Escalada I, Hoof I, Bornholdt J, Boyd M, Chen Y, Zhao X, Schmidl C, Suzuki T, et al. (2014). An atlas of active enhancers across human cell types and tissues. Nature 507, 455–461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arner E, Daub CO, Vitting-Seerup K, Andersson R, Lilje B, Drablos F, Lennartsson A, Ronnerblad M, Hrydziuszko O, Vitezic M, et al. (2015). Transcribed enhancers lead waves of coordinated transcription in transitioning mammalian cells. Science 347, 1010–1014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bahr C, von Paleske L, Uslu VV, Remeseiro S, Takayama N, Ng SW, Murison A, Langenfeld K, Petretich M, Scognamiglio R, et al. (2018). A Myc enhancer cluster regulates normal and leukaemic haematopoietic stem cell hierarchies. Nature 553, 515–520. [DOI] [PubMed] [Google Scholar]
- Bal E, Park HS, Belaid-Choucair Z, Kayserili H, Naville M, Madrange M, Chiticariu E, Hadj-Rabia S, Cagnard N, Kuonen F, et al. (2017). Mutations in ACTRT1 and its enhancer RNA elements lead to aberrant activation of Hedgehog signaling in inherited and sporadic basal cell carcinomas. Nature medicine 23, 1226–1233. [DOI] [PubMed] [Google Scholar]
- Banani SF, Lee HO, Hyman AA, and Rosen MK (2017). Biomolecular condensates: organizers of cellular biochemistry. Nature reviews Molecular cell biology 18, 285–298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banerji J, Rusconi S, and Schaffner W (1981). Expression of a beta-globin gene is enhanced by remote SV40 DNA sequences. Cell 27, 299–308. [DOI] [PubMed] [Google Scholar]
- Bedi R, Huang D, Wiedmer L, Li Y, Dolbois A, Wojdyla J, Sharpe M, Caflisch A, and Sledz P (2020). Selectively Disrupting m6A-Dependent Protein–RNA Interactions with Fragments. ACS Chemical Biology 15, 618–625. [DOI] [PubMed] [Google Scholar]
- Boija A, Klein IA, Sabari BR, Dall’Agnese A, Coffey EL, Zamudio AV, Li CH, Shrinivas K, Manteiga JC, Hannett NM, et al. (2018). Transcription Factors Activate Genes through the Phase-Separation Capacity of Their Activation Domains. Cell 175, 1842–1855 e1816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buckley DL, Raina K, Darricarrere N, Hines J, Gustafson JL, Smith IE, Miah AH, Harling JD, and Crews CM (2015). HaloPROTACS: Use of Small Molecule PROTACs to Induce Degradation of HaloTag Fusion Proteins. ACS chemical biology 10, 1831–1837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chelmicki T, Roger E, Teissandier A, Rucli S, Dossin F, Dura M, Fouassier C, Lameiras S, and Bourc’his D (2021). m6A RNA methylation regulates the fate of endogenous retroviruses. Nature. DOI: 10.1038/s41586-020-03135-1 [DOI] [PubMed] [Google Scholar]
- Chen H, Li C, Peng X, Zhou Z, Weinstein JN, Cancer Genome Atlas Research, N., and Liang H (2018). A Pan-Cancer Analysis of Enhancer Expression in Nearly 9000 Patient Samples. Cell 173, 386–399 e312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiu A, Suzuki H, Wu X, Mahat D, Kriz A, and Sharp P (2018). Transcriptional Pause Sites Delineate Stable Nucleosome-Associated Premature Polyadenylation Suppressed by U1 snRNP. Molecular Cell 69, 648–663.e7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cho WK, Spille JH, Hecht M, Lee C, Li C, Grube V, and Cisse II (2018). Mediator and RNA polymerase II clusters associate in transcription-dependent condensates. Science 361, 412–415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Darnell RB, Ke S, and Darnell JE Jr. (2018). Pre-mRNA processing includes N(6) methylation of adenosine residues that are retained in mRNA exons and the fallacy of “RNA epigenetics”. Rna 24, 262–267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davidson S, Efremova M, Riedel A, Mahata B, Pramanik J, Huuhtanen J, Kar G, Vento-Tormo R, Hagai T, and Chen X et al. (2020). Single-Cell RNA Sequencing Reveals a Dynamic Stromal Niche That Supports Tumor Growth. Cell Reports 31, 107628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Santa F, Barozzi I, Mietton F, Ghisletti S, Polletti S, Tusi BK, Muller H, Ragoussis J, Wei CL, and Natoli G (2010). A large fraction of extragenic RNA pol II transcription sites overlap enhancers. PLoS biology 8, e1000384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Derrien T, Johnson R, Bussotti G, Tanzer A, Djebali S, Tilgner H, Guernec G, Martin D, Merkel A, Knowles DG, et al. (2012). The GENCODE v7 catalog of human long noncoding RNAs: analysis of their gene structure, evolution, and expression. Genome research 22, 1775–1789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dobin A, Davis CA, Schlesinger F, Drenkow J, Zaleski C, Jha S, Batut P, Chaisson M, and Gingeras TR (2013). STAR: ultrafast universal RNA-seq aligner. Bioinformatics 29, 15–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dominissini D, Moshitch-Moshkovitz S, Schwartz S, Salmon-Divon M, Ungar L, Osenberg S, Cesarkas K, Jacob-Hirsch J, Amariglio N, Kupiec M, et al. (2012). Topology of the human and mouse m6A RNA methylomes revealed by m6A-seq. Nature 485, 201–206. [DOI] [PubMed] [Google Scholar]
- Dong X, Liao Z, Gritsch D, Hadzhiev Y, Bai Y, Locascio JJ, Guennewig B, Liu G, Blauwendraat C, Wang T, et al. (2018). Enhancers active in dopamine neurons are a primary link between genetic variation and neuropsychiatric disease. Nature neuroscience 21, 1482–1492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duffy EE, Rutenberg-Schoenberg M, Stark CD, Kitchen RR, Gerstein MB, and Simon MD (2015). Tracking Distinct RNA Populations Using Efficient and Reversible Covalent Chemistry. Molecular cell 59, 858–866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engreitz JM, Haines JE, Perez EM, Munson G, Chen J, Kane M, McDonel PE, Guttman M, and Lander ES (2016). Local regulation of gene expression by lncRNA promoters, transcription and splicing. Nature 539, 452–455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farh KK, Marson A, Zhu J, Kleinewietfeld M, Housley WJ, Beik S, Shoresh N, Whitton H, Ryan RJ, Shishkin AA, et al. (2015). Genetic and epigenetic fine mapping of causal autoimmune disease variants. Nature 518, 337–343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Field A, and Adelman K (2020). Evaluating Enhancer Function and Transcription. Annual review of biochemistry 89, 213–234. [DOI] [PubMed] [Google Scholar]
- Fu Y, and Zhuang X (2020). m6A-binding YTHDF proteins promote stress granule formation. Nature Chemical Biology 16(9), 955–963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furlong EEM, and Levine M (2018). Developmental enhancers and chromosome topology. Science 361, 1341–1345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao Y, Pei G, Li D, Li R, Shao Y, Zhang QC, and Li P (2019). Multivalent m(6)A motifs promote phase separation of YTHDF proteins. Cell research 29, 767–769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hah N, Murakami S, Nagari A, Danko CG, and Kraus WL (2013). Enhancer transcripts mark active estrogen receptor binding sites. Genome research 23, 1210–1223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hauberg ME, Fullard JF, Zhu L, Cohain AT, Giambartolomei C, Misir R, Reach S, Johnson JS, Wang M, Mattheisen M, et al. (2019). Differential activity of transcribed enhancers in the prefrontal cortex of 537 cases with schizophrenia and controls. Molecular psychiatry 24, 1685–1695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heinz S, Benner C, Spann N, Bertolino E, Lin YC, Laslo P, Cheng JX, Murre C, Singh H, and Glass CK (2010). Simple combinations of lineage-determining transcription factors prime cis-regulatory elements required for macrophage and B cell identities. Molecular cell 38, 576–589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henriques T, Scruggs BS, Inouye MO, Muse GW, Williams LH, Burkholder AB, Lavender CA, Fargo DC, and Adelman K (2018). Widespread transcriptional pausing and elongation control at enhancers. Genes & development 32, 26–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hnisz D, Shrinivas K, Young RA, Chakraborty AK, and Sharp PA (2017). A Phase Separation Model for Transcriptional Control. Cell 169, 13–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hnisz D, Abraham BJ, Lee TI, Lau A, Saint-Andre V, Sigova AA, Hoke HA, and Young RA (2013). Super-enhancers in the control of cell identity and disease. Cell 155, 934–947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeon Y, and Lee JT (2011). YY1 tethers Xist RNA to the inactive X nucleation center. Cell 146, 119–133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khurana E, Fu Y, Chakravarty D, Demichelis F, Rubin MA, and Gerstein M (2016). Role of non-coding sequence variants in cancer. Nature reviews Genetics 17, 93–108. [DOI] [PubMed] [Google Scholar]
- Kim TK, Hemberg M, Gray JM, Costa AM, Bear DM, Wu J, Harmin DA, Laptewicz M, Barbara-Haley K, Kuersten S, et al. (2010). Widespread transcription at neuronal activity-regulated enhancers. Nature 465, 182–187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koch F, Fenouil R, Gut M, Cauchy P, Albert TK, Zacarias-Cabeza J, Spicuglia S, de la Chapelle AL, Heidemann M, Hintermair C, et al. (2011). Transcription initiation platforms and GTF recruitment at tissue-specific enhancers and promoters. Nature structural & molecular biology 18, 956–963 [DOI] [PubMed] [Google Scholar]
- Lai F, Gardini A, Zhang A, and Shiekhattar R (2015). Integrator mediates the biogenesis of enhancer RNAs. Nature 525, 399–403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lau MS, Schwartz MG, Kundu S, Savol AJ, Wang PI, Marr SK, Grau DJ, Schorderet P, Sadreyev RI, Tabin CJ, et al. (2017). Mutation of a nucleosome compaction region disrupts Polycomb-mediated axial patterning. Science 355, 1081–1084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee JH, Xiong F, and Li W (2020). Enhancer RNAs in cancer: regulation, mechanisms and therapeutic potential. RNA biology, 1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li H, Handsaker B, Wysoker A, Fennell T, Ruan J, Homer N, Marth G, Abecasis G, Durbin R, and Genome Project Data Processing, S. (2009). The Sequence Alignment/Map format and SAMtools. Bioinformatics 25, 2078–2079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li J, et al. , Nucleic Acids Res. (2020). Targeted mRNA demethylation using an engineered dCas13b-ALKBH5 fusion protein. 48(10):5684–5694. doi: 10.1093/nar/gkaa269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li W, Notani D, Ma Q, Tanasa B, Nunez E, Chen AY, Merkurjev D, Zhang J, Ohgi K, Song X, et al. (2013). Functional roles of enhancer RNAs for oestrogen-dependent transcriptional activation. Nature 498, 516–520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li W, Notani D, and Rosenfeld MG (2016). Enhancers as non-coding RNA transcription units: recent insights and future perspectives. Nature reviews Genetics 17, 207–223. [DOI] [PubMed] [Google Scholar]
- Li X, and Fu XD (2019). Chromatin-associated RNAs as facilitators of functional genomic interactions. Nature reviews Genetics 20, 503–519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin Y, Protter DS, Rosen MK, and Parker R (2015). Formation and Maturation of Phase-Separated Liquid Droplets by RNA-Binding Proteins. Molecular cell 60, 208–219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu J, Dou X, Chen C, Chen C, Liu C, Xu MM, Zhao S, Shen B, Gao Y, Han D, et al. (2020). N (6)-methyladenosine of chromosome-associated regulatory RNA regulates chromatin state and transcription. Science 367, 580–586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu J, Yue Y, Han D, Wang X, Fu Y, Zhang L, Jia G, Yu M, Lu Z, Deng X, et al. (2014). A METTL3-METTL14 complex mediates mammalian nuclear RNA N6-adenosine methylation. Nature chemical biology 10, 93–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mansour MR, Abraham BJ, Anders L, Berezovskaya A, Gutierrez A, Durbin AD, Etchin J, Lawton L, Sallan SE, Silverman LB, et al. (2014). Oncogene regulation. An oncogenic super-enhancer formed through somatic mutation of a noncoding intergenic element. Science 346, 1373–1377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahat DB, Kwak H, Booth GT, Jonkers IH, Danko CG, Patel RK, Waters CT, Munson K, Core LJ, and Lis JT (2016). Base-pair-resolution genome-wide mapping of active RNA polymerases using precision nuclear run-on (PRO-seq). Nat Protoc 11, 1455–1476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mauer J, Luo X, Blanjoie A, Jiao X, Grozhik AV, Patil DP, Linder B, Pickering BF, Vasseur JJ, Chen Q, et al. (2017). Reversible methylation of m(6)Am in the 5’ cap controls mRNA stability. Nature 541, 371–375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer KD, Saletore Y, Zumbo P, Elemento O, Mason CE, and Jaffrey SR (2012). Comprehensive analysis of mRNA methylation reveals enrichment in 3’ UTRs and near stop codons. Cell 149, 1635–1646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moreau P, Hen R, Wasylyk B, Everett R, Gaub MP, and Chambon P (1981). The SV40 72 base repair repeat has a striking effect on gene expression both in SV40 and other chimeric recombinants. Nucleic acids research 9, 6047–6068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moroz-Omori E, Danzhi H, Kumar B, Cheriyamkunnel S, Elena B, Aymeric D, Rzeczkowski M, Lars W, Paweł Ś, and Amedeo C (2020). METTL3 inhibitors for epitranscriptomic modulation of cellular processes. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nabet B, Roberts JM, Buckley DL, Paulk J, Dastjerdi S, Yang A, Leggett AL, Erb MA, Lawlor MA, Souza A, et al. (2018). The dTAG system for immediate and target-specific protein degradation. Nature chemical biology 14, 431–441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nachtergaele S, and He C (2018). Chemical Modifications in the Life of an mRNA Transcript. Annual review of genetics 52, 349–372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nair SJ, Yang L, Meluzzi D, Oh S, Yang F, Friedman MJ, Wang S, Suter T, Alshareedah I, Gamliel A, et al. (2019). Phase separation of ligand-activated enhancers licenses cooperative chromosomal enhancer assembly. Nature structural & molecular biology 26, 193–203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Natoli G, and Andrau JC (2012). Noncoding transcription at enhancers: general principles and functional models. Annual review of genetics 46, 1–19. [DOI] [PubMed] [Google Scholar]
- Nayler O, Hartmann AM, and Stamm S (2000). The ER repeat protein YT521-B localizes to a novel subnuclear compartment. The Journal of cell biology 150, 949–962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nott A, Holtman IR, Coufal NG, Schlachetzki JCM, Yu M, Hu R, Han CZ, Pena M, Xiao J, Wu Y, et al. (2019). Brain cell type-specific enhancer-promoter interactome maps and disease-risk association. Science 366, 1134–1139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patil DP, Chen CK, Pickering BF, Chow A, Jackson C, Guttman M, and Jaffrey SR (2016). m(6)A RNA methylation promotes XIST-mediated transcriptional repression. Nature 537, 369–373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pertea M, Pertea GM, Antonescu CM, Chang TC, Mendell JT, and Salzberg SL (2015). StringTie enables improved reconstruction of a transcriptome from RNA-seq reads. Nature biotechnology 33, 290–295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quinlan AR, and Hall IM (2010). BEDTools: a flexible suite of utilities for comparing genomic features. Bioinformatics 26, 841–842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ries RJ, Zaccara S, Klein P, Olarerin-George A, Namkoong S, Pickering BF, Patil DP, Kwak H, Lee JH, and Jaffrey SR (2019). m(6)A enhances the phase separation potential of mRNA. Nature 571, 424–428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rinn JL, and Chang HY (2020). Long Noncoding RNAs: Molecular Modalities to Organismal Functions. Annual review of biochemistry 89, 283–308. [DOI] [PubMed] [Google Scholar]
- Robinson JT, Thorvaldsdottir H, Winckler W, Guttman M, Lander ES, Getz G, and Mesirov JP (2011). Integrative genomics viewer. Nature biotechnology 29, 24–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roden C, and Gladfelter AS (2020). RNA contributions to the form and function of biomolecular condensates. Nature reviews Molecular cell biology. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sabari BR, Dall’Agnese A, Boija A, Klein IA, Coffey EL, Shrinivas K, Abraham BJ, Hannett NM, Zamudio AV, Manteiga JC, et al. (2018). Coactivator condensation at super-enhancers links phase separation and gene control. Science 361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakuma T, Nakade S, Sakane Y, Suzuki KT, and Yamamoto T (2016). MMEJ-assisted gene knock-in using TALENs and CRISPR-Cas9 with the PITCh systems. Nature protocols 11, 118–133. [DOI] [PubMed] [Google Scholar]
- Saltzman AB, Leng M, Bhatt B, Singh P, Chan DW, Dobrolecki L, Chandrasekaran H, Choi JM, Jain A, Jung SY, et al. (2018). gpGrouper: A Peptide Grouping Algorithm for Gene-Centric Inference and Quantitation of Bottom-Up Proteomics Data. Molecular & cellular proteomics : MCP 17, 2270–2283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sartorelli V, and Lauberth SM (2020). Enhancer RNAs are an important regulatory layer of the epigenome. Nature structural & molecular biology 27, 521–528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schindelin J, Arganda-Carreras I, Frise E, Kaynig V, Longair M, Pietzsch T, Preibisch S, Rueden C, Saalfeld S, Schmid B, et al. (2012). Fiji: an open-source platform for biological-image analysis. Nature methods 9, 676–682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwalb B, Michel M, Zacher B, Fruhauf K, Demel C, Tresch A, Gagneur J, and Cramer P (2016). TT-seq maps the human transient transcriptome. Science 352, 1225–1228. [DOI] [PubMed] [Google Scholar]
- Sendinc E, Valle-Garcia D, Dhall A, Chen H, Henriques T, Navarrete-Perea J, Sheng W, Gygi SP, Adelman K, and Shi Y (2019). PCIF1 Catalyzes m6Am mRNA Methylation to Regulate Gene Expression. Molecular cell 75, 620–630 e629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shin Y, Berry J, Pannucci N, Haataja MP, Toettcher JE, and Brangwynne CP (2017). Spatiotemporal Control of Intracellular Phase Transitions Using Light-Activated optoDroplets. Cell 168, 159–171 e114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spector DL (2003). The dynamics of chromosome organization and gene regulation. Annual review of biochemistry 72, 573–608. [DOI] [PubMed] [Google Scholar]
- Takeda DY, Spisak S, Seo JH, Bell C, O’Connor E, Korthauer K, Ribli D, Csabai I, Solymosi N, Szallasi Z, et al. (2018). A Somatically Acquired Enhancer of the Androgen Receptor Is a Noncoding Driver in Advanced Prostate Cancer. Cell 174, 422–432 e413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsai PF, Dell’Orso S, Rodriguez J, Vivanco KO, Ko KD, Jiang K, Juan AH, Sarshad AA, Vian L, Tran M, et al. (2018). A Muscle-Specific Enhancer RNA Mediates Cohesin Recruitment and Regulates Transcription In trans. Molecular cell 71, 129–141 e128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vahedi G, Kanno Y, Furumoto Y, Jiang K, Parker SC, Erdos MR, Davis SR, Roychoudhuri R, Restifo NP, Gadina M, et al. (2015). Super-enhancers delineate disease-associated regulatory nodes in T cells. Nature 520, 558–562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Nostrand EL, Pratt GA, Shishkin AA, Gelboin-Burkhart C, Fang MY, Sundararaman B, Blue SM, Nguyen TB, Surka C, Elkins K, et al. (2016). Robust transcriptome-wide discovery of RNA-binding protein binding sites with enhanced CLIP (eCLIP). Nature methods 13, 508–514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J, Choi JM, Holehouse AS, Lee HO, Zhang X, Jahnel M, Maharana S, Lemaitre R, Pozniakovsky A, Drechsel D, et al. (2018b). A Molecular Grammar Governing the Driving Forces for Phase Separation of Prion-like RNA Binding Proteins. Cell 174, 688–699 e616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X, Lu Z, Gomez A, Hon GC, Yue Y, Han D, Fu Y, Parisien M, Dai Q, Jia G, et al. (2014). N6-methyladenosine-dependent regulation of messenger RNA stability. Nature 505, 117–120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y,Li Y, Yue M, Wang J, Kumar S, Wechsler-Reya RJ., Zhang Z, Ogawa Y, Kellis M, Duester G, et al. (2018a). N6-methyladenosine RNA modification regulates embryonic neural stem cell self-renewal through histone modifications. Nature Neuroscience 21, 195–206 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei G, Almeida M, Pintacuda G, Coker H, Bowness J, Ule J, and Brockdorff N (2021). Acute depletion of METTL3 implicates N6-methyladenosine in alternative intron/exon inclusion in the nascent transcriptome. Genome Research gr.271635.120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wessels HH, Mendez-Mancilla A, Guo X, Legut M, Daniloski Z, and Sanjana NE (2020). Massively parallel Cas13 screens reveal principles for guide RNA design. Nature biotechnology 38, 722–727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiang Y, Laurent B, Hsu CH, Nachtergaele S, Lu Z, Sheng W, Xu C, Chen H, Ouyang J, Wang S, et al. (2017). RNA m(6)A methylation regulates the ultraviolet-induced DNA damage response. Nature 543, 573–576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiao R, Chen JY, Liang Z, Luo D, Chen G, Lu ZJ, Chen Y, Zhou B, Li H, Du X, et al. (2019). Pervasive Chromatin-RNA Binding Protein Interactions Enable RNA-Based Regulation of Transcription. Cell 178, 107–121 e118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiong F, Wang R, Lee JH, Li S, Chen SF, Liao Z, Hasani LA, Nguyen PT, Zhu X, Krakowiak J, et al. (2021). RNA m6A modification orchestrates a LINE-1/host interaction that facilitates retrotransposition and contributes to long gene vulnerability. Cell Research. June 9th 10.1038/s41422-021-00515-8 Online ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu C, Wang X, Liu K, Roundtree IA, Tempel W, Li Y, Lu Z, He C, and Min J (2014). Structural basis for selective binding of m6A RNA by the YTHDC1 YTH domain. Nature chemical biology 10, 927–929. [DOI] [PubMed] [Google Scholar]
- Xu W, Li J, He C, Wen J, Ma H, Rong B, Diao J, Wang L, Wang J, and Wu F et al. (2021). METTL3 regulates heterochromatin in mouse embryonic stem cells. Nature 591, 317–321. [DOI] [PubMed] [Google Scholar]
- Zaccara S, and Jaffrey SR (2020). A Unified Model for the Function of YTHDF Proteins in Regulating m(6)A-Modified mRNA. Cell 181, 1582–1595 e1518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zaccara S, Ries RJ, and Jaffrey SR (2019). Reading, writing and erasing mRNA methylation. Nature reviews Molecular cell biology 20, 608–624. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Liu T, Meyer CA, Eeckhoute J, Johnson DS, Bernstein BE, Nusbaum C, Myers RM, Brown M, Li W, et al. (2008). Model-based analysis of ChIP-Seq (MACS). Genome biology 9, R137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Z, Lee JH, Ruan H, Ye Y, Krakowiak J, Hu Q, Xiang Y, Gong J, Zhou B, Wang L, et al. (2019). Transcriptional landscape and clinical utility of enhancer RNAs for eRNA-targeted therapy in cancer. Nature communications 10, 4562. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Movie.1
Time lapse imaging of optodroplet assay of YTHDC1-FL-mCherry-CRY2 in MCF7 cells (related to Figures 5 and S9).
Supplementary Movie.2
Time lapse imaging of optodroplet assay of YTHDC1-FL-mCherry-CRY2 for a cluster of 293T cells (related to Figures 5 and S9).
Data Availability Statement
Data generated in this study have been deposited to NCBI GEO (GSE143441).
Codes/scripts used for the analyses in this study are based on standard algorithms, are mentioned in the specific sections in Methods below, and are available upon request.
Additional information required to reanalyze the data reported in this paper is available from the lead contact upon request.