Abstract
We present a 62-year-old gentleman with history of Crohn’s disease, G6PD deficiency, who presented with immune-mediated thrombotic thrombocytopenia purpura (iTTP) one week after the diagnosis of COVID-19 infection. He was admitted with worsening dyspnea, acute renal failure, and profound thrombocytopenia with marked schistocytosis on peripheral smear. ADAMTS13 level was severely deficient. He was treated with oral prednisone, plasma exchange and rituximab with complete clinical resolution. Given the temporal association of this recurrent episode of iTTP with COVID-19 infection and no other discernible cause, COVID-19 infection was the most likely trigger.
Keywords: COVID-19, thrombotic thrombocytopenic purpura, ADAMTS13
Introduction
At the end of 2019, a new coronavirus emerged from China and rapidly transmitted throughout the world causing a global pandemic which mainly causes respiratory symptoms [1]. However, it has been found to affect other organs; most notably increased risk of venous thromboembolism (VTE) [2]. It has also been proposed to trigger autoimmune diseases such as immune thrombocytopenic purpura (ITP) and Guillain-Barré [3], [4]. We describe a patient who presented after a confirmed COVID-19 infection with a relapse of iTTP. We also review the literature regarding the association between COVID-19 and TTP.
Case presentation
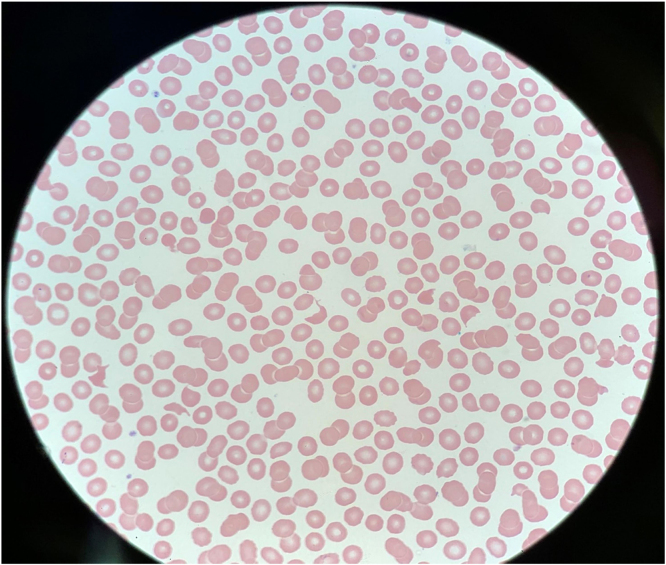
A 62-year-old male with a history of Crohn’s disease, G6PD deficiency, and iTTP who presented to our hospital one week after a positive COVID-19 infection. He had worsening shortness of breath, generalized weakness and chills. Family history was not significant for hematological disorders. Physical examination revealed tachycardia to 110, remainder of the vital signs were normal with no fever and normal oxygen saturation on room air. He had no scleral icterus or petechiae. He was alert and oriented with no neurological deficit. Chest and cardiac exam were unremarkable. Abdominal exam was negative for organomegaly. Computed tomography (CT) of his chest revealed bilateral patchy foci of ground-glass airspace opacities. His labs showed platelets 21 k/cmm, hemoglobin 16.3 g/dl, white blood cells 8 k/cmm with normal differential, urea 49 mg/dl, creatinine 3 mg/dl, total bilirubin 1.6 mg/dl, direct bilirubin 0.3 mg/dl, reticulocytes 0.57%, retic index 0.67, LDH 1392 U/l, haptoglobin was undetectable, INR 1.2, fibrinogen 688 mg/dL and D-dimer 4332 ng/ml. Peripheral blood smear notable for thrombocytopenia and significant schistocytes (Fig. 1). PLASMIC score on admission was 6. ADAMTS13 level was obtained. The patient was treated with oral prednisone 1 mg/kg daily and plasma exchange (PLEX). His labs started to improve with treatment. By day 5 post PLEX, his platelets, LDH, haptoglobin and kidney function were back to normal. His ADAMS13 panel showed severe ADAMTS13 deficiency of <5% activity with a negative ADAMTS13 inhibitor antibodies titer. The patient had an episode of TTP 6 years prior and at that time his ADAMTS13 panel showed severe deficiency of ADAMTS13 at <5% enzyme activity and positive ADAMTS13 inhibitor antibodies at 0.9 BU/ml. Given the fact that he had positive ADAMS13 inhibitors in the past, he was started on Rituximab (375 mg/m2) before discharge. He was then followed up weekly with hematology clinic and received additional 3 doses (weekly for 3 weeks). His platelet count remained stable >150 and his repeat ADAMTS13 improved to 83.3% after 4 doses of rituximab.
Fig. 1.
Peripheral blood smear on presentation showing many schistocytes.
Discussion and conclusion
Thrombotic microangiopathy syndromes (TMA) are a group of disorders characterized clinically by microangiopathic hemolytic anemia, thrombocytopenia and microthrombi causing end organ ischemia. Thrombotic thrombocytopenic purpura (TTP) is one of the main types of TMA. TTP is further divided into either acquired or hereditary. Acquired TTP is due to ADAMTS13 inhibitor antibodies and represents more than 90% of cases. Hereditary TTP (Upshaw-Schulman syndrome) is due to congenital ADAMTS13 deficiency [5]. Multiple viral infections have been described to play a role in triggering TTP such as Influenza (including H1N1), HIV, and Chikungunya infection [6], [7], [8].
COVID-19 infection has been associated with endotheliopathy and increased risk of VTE. There is increasing evidence that it is also associated with TTP. We utilized PubMed and Google Scholar to conduct a search and identified six cases of TTP following COVID-19 infection [9], [10], [11], [12], [13], [14]. We summarized those cases in Table 1. In four of six reported cases, TTP was present at the time of the COVID-19 diagnosis or within 10 days of diagnosis. Fever was present in only one patient while neurologic manifestations were common. Four out of six COVID-19 infected patients who developed TTP had a mild course of COVID-19 infection. One patient with a prior history of TTP had a relapse associated with COVID-19 infection, similar to our patient. ADAMTS13 activity level was<10% in five cases (no level was reported in one patient), diagnostic of TTP. All patients reported had recovered after treatment, however, three patients had a prolonged and complicated recovery.
Table 1.
Clinical presentations and laboratory values of the patients described in the literature with COVID associated TTP.
| Albiol et al. | ALtowyan et al. | Beaulieu et al. | Hindilerden et al. | Marco et al. | Nicolotti et al. | ||
|---|---|---|---|---|---|---|---|
| Clinical presentation | Age (Years) | 57 | 39 | 70 | 74 | 55 | 44 |
| Gender | F | M | M | F | F | F | |
| Fever | Yes | No | No | No | No | No | |
| CNSa | No | Yes | Yes | Yes | Yes | Yes | |
| O2 requirement | No | No | No | NAb | HFNCc 15 L/min | 35% O2 | |
| Time of TTPd diagnosis since COVID-19 detection | 9 days | Same day | 19 days | Same day | 1 Month | 2 days | |
| History of TMAe | No | No | No | No | Yes | No | |
| Laboratory values at presentation | Hemoglobin | 9.9 g/dL | 6.7 g/dL | 6 g/dL | 6.6 g/dL | 7.4 g/dL | 6 g/dL |
| Platelet | 22 × 109/L | 6 × 109/L | 18 × 109/L | 48 × 109/L | 14 × 109/L | 7 × 109/L | |
| Serum Creatinine | 0.8 mg/dL | 0.87 mg/dL | Normal (Level NA) | NA | 3.18 mg/dL | 2.3 mg/dL | |
| Schistocyte count (%) | 6% | 9% | NA | NA | NA | NA | |
| PLASMIC score | NA | 6 | NA | NA | NA | NA | |
| ADAMTS13 activity | 2% | NA | <10% | 0.2% | Undetectable | <5% | |
| ADAMTS 13 inhibitor | 5.2 BU | NA | Weakly positive (1/2 titer) | >90 U/ml | 31 U/ml | 57 U/ml | |
| Treatment | Recovery | Complete | Complete but prolonged | Complete | Complete | Complete but prolonged | Complete but prolonged |
| Treatment | FFPf + PLEXg | Methylprednisolone, PLEX and Rituximab | Methylprednisolone and PLEX | Methylprednisolone and PLEX | FFP → steroids + PLEX and Caplacizumab | FFP → Methylprednisolone, PLEX and Rituximab → Caplacizumab |
Central nervous system.
Not available.
High flow nasal canula.
Thrombotic thrombocytopenic purpura.
Thrombotic microangiopathy.
Fresh frozen plasma.
Plasma exchange.
We noticed some similarities and differences in our patient compared to the published cases. Similar to Marco et al. case report [9] in which the patient had history of TTP 30 years prior to his presentation with COVID-19 and TTP relapse, our patient had history of TTP 6 years prior to his current presentation, which highlights that COVID-19 may trigger TTP relapse even years after the initial TTP presentation. Conversely to all the published cases, our patient presented with normal hemoglobin, however his severe thrombocytopenia is what pulled the trigger to evaluate his peripheral blood smear, TTP with normal Hb on presentation is rare, however it was reported in the literature [15]. The response to classic acute TTP treatment (Plasma exchange, Steroids and Rituximab) in our patient and the reported cases is reassuring, however with severe COVID-19 infection, specifically with respiratory failure the treatment might be prolonged, for example, Nicolotti, D., et al. reported 14 plasma exchange over 18 days were required until the patient completely recovered [14].
The exact etiology for COVID-19 associated TTP is not completely understood. Numerous studies described an increase in endotheial damage markers like Von Willebrand Factor Antigen (VWF:Ag), Von Willebrand activity and Factor VIII levels in COVID-19 infection [16], [17].
There is a clear correlation with the severity of COVID-19 infection; biomarker levels increase with the severity of the clinical presentation. More extensively, Mancini et al. evaluated the ADAMTS13 activity in 50 patients with COVID-19 [18]. They found ADAMTS13 activity was mildly to moderately reduced. The median values of ADAMTS13 activity correlated to the intensity of care, it was lowest in patient who required high level of care. Lastly, Fuchs et al. investigated the role of infection in exacerbating TMA [19]. Interestingly, they found the elevation in the DNA-histone complex, Myeloperoxidase from neutrophilic granules and S100A8/A9 (heterocomplex abundantly stored in neutrophils cytoplasm) correlate with acute TMA. It is plausible that in the midst of the significant inflammation which is associated with COVID-19, these inflammatory markers that are produced by neutrophils could lead to endothelial damage and expose ADAMTS13 antigen causing antibodies formation especially in patients with pre-existing risk factors. This hypothesis was also described in seasonal flu induced TTP [20]. We hypothesize that the combination of elevated VWF:Ag and activity with reduced ADAMTS13 level due to significant inflammation causes a “double hit” phenomena and predisposes a TTP flare.
There is growing evidence that COVID-19 infection may trigger TTP. This case highlights the fact that TTP may occur with milder form of COVID-19 infection. Clinicians should be aware of this association for prompt recognition and timely treatment. Further research is needed to elucidate the mechanisms of this unique TMA entity and the optimal management.
CRediT authorship contribution statement
We the authors of this paper declare that our manuscript is original and has not been published before. All named authors have read and approved the manuscript. We confirm that there are no other persons who satisfied the criteria for authorship but are not listed. We further confirm that the order of authors listed in the manuscript has been approved by all of us. We understand that the Corresponding Author is the sole contact for the Editorial process.
References
- 1.Cucinotta D., Vanelli M. WHO declares COVID-19 a pandemic. Acta Biomed. 2020;91(1):157–160. doi: 10.23750/abm.v91i1.9397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Franchini M., Marano G., Cruciani M., Mengoli C., Pati I., Masiello F. COVID-19-associated coagulopathy. Diagnosis. 2020;7(4):357–363. doi: 10.1515/dx-2020-0078. [DOI] [PubMed] [Google Scholar]
- 3.Bomhof G., Mutsaers P., Leebeek F., Te Boekhorst P., Hofland J., Croles F.N. COVID-19-associated immune thrombocytopenia. Br J Haematol. 2020;190(2):e61–e64. doi: 10.1111/bjh.16850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Caress J.B., Castoro R.J., Simmons Z., Scelsa S.N., Lewis R.A., Ahlawat A. COVID-19-associated Guillain-Barré syndrome: the early pandemic experience. Muscle Nerve. 2020;62(4):485–491. doi: 10.1002/mus.27024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.George J.N., Nester C.M. Syndromes of thrombotic microangiopathy. N Engl J Med. 2014;371(7):654–666. doi: 10.1056/NEJMra1312353. [DOI] [PubMed] [Google Scholar]
- 6.Bitzan M., Zieg J. Influenza-associated thrombotic microangiopathies. Pedia Nephrol. 2018;33(11):2009–2025. doi: 10.1007/s00467-017-3783-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Louw S., Gounden R., Mayne E.S. Thrombotic thrombocytopenic purpura (TTP)-like syndrome in the HIV era. Thromb J. 2018;16:35. doi: 10.1186/s12959-018-0189-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kumar V., Jain R., Kumar A., Nischal N., Jorwal P., Soneja M. Chikungunya fever presenting as life threatening thrombotic thrombocytopenic purpura. J Assoc Physicians India. 2017;65(7):96–100. [PubMed] [Google Scholar]
- 9.Marco Capecchi C., Cristina Mocellin, Chiara Abbruzzese, Ilaria Mancini, Daniele Prati, Flora Peyvandi Dramatic presentation of acquired thombotic thrombocytopenic purpura associated with COVID-19. Haematologica. 2020;105(10) doi: 10.3324/haematol.2020.262345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hindilerden F., Yonal-Hindilerden I., Akar E., Kart-Yasar K. Covid-19 associated autoimmune thrombotic thrombocytopenic purpura: report of a case. Thromb Res. 2020;195:136–138. doi: 10.1016/j.thromres.2020.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Beaulieu, M.C., et al., Thrombotic thrombocytopenic purpura as the initial Presentation of COVID-19. J Thromb Haemost, 2020. [DOI] [PMC free article] [PubMed]
- 12.Altowyan E., Alnujeidi O., Alhujilan A., Alkathlan M. COVID-19 presenting as thrombotic thrombocytopenic purpura (TTP) BMJ Case Rep. 2020;13(12) doi: 10.1136/bcr-2020-238026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Albiol N., Awol R., Martino R. Autoimmune thrombotic thrombocytopenic purpura (TTP) associated with COVID-19. Ann Hematol. 2020;99(7):1673–1674. doi: 10.1007/s00277-020-04097-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nicolotti D. A case of thrombotic thrombocytopenic purpura associated with COVID-19. J Thromb Thrombolysis. 2021:1–3. doi: 10.1007/s11239-020-02362-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yarlagadda M., LePar F. Thrombotic thrombocytopenic purpura with normal initial hemoglobin. Blood. 2007;110(11):3924. -3924. [Google Scholar]
- 16.Goshua G., Pine A.B., Meizlish M.L., Chang C.H., Zhang H., Bahel P. Endotheliopathy in COVID-19-associated coagulopathy: evidence from a single-centre, cross-sectional study. Lancet Haematol. 2020;7(8):e575–e582. doi: 10.1016/S2352-3026(20)30216-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rauch A., Labreuche J., Lassalle F., Goutay J., Caplan M., Charbonnier L. Coagulation biomarkers are independent predictors of increased oxygen requirements in COVID-19. J Thromb Haemost. 2020;18(11):2942–2953. doi: 10.1111/jth.15067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mancini, I., et al., The ADAMTS13-von Willebrand factor axis in COVID-19 patients. J Thromb Haemost, 2020. [DOI] [PMC free article] [PubMed]
- 19.Fuchs T.A., Kremer Hovinga J.A., Schatzberg D., Wagner D.D., Lämmle B. Circulating DNA and myeloperoxidase indicate disease activity in patients with thrombotic microangiopathies. Blood. 2012;120(6):1157–1164. doi: 10.1182/blood-2012-02-412197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Joseph A., P F. Seasonal flu as a triggering factor for acquired thrombotic thrombocytopenic purpura. J Hematol Thromboembolic Dis. 2015:4. [Google Scholar]