Abstract
Objective
To investigate the prognostic efficacy of lymph node ratio (LNR) and log odds of positive lymph nodes (LODDS) in node-positive cardia gastric adenocarcinoma (CGA).
Design
A registry-based retrospective cohort study.
Setting
Patients diagnosed with node-positive CGA in the Surveillance, Epidemiology, and End Results database from 2010 to 2015.
Participants
A total of 1038 patients were enrolled and randomly assigned (7:3) to the training set (n=723) or validating set (n=315).
Primary outcome measure
Cancer-specific survival (CSS).
Results
The baseline characteristics of the training and validation sets were similar. Based on the optimal cut-off values, LNR was classified into low (<0.09), medium (0.09~0.33) and high (>0.33) groups; LODDS was also classified into low (<−2.09), medium (−2.09~−0.65) and high (>−0.65) groups. CSS was significantly different across LNR and LODDS subgroups. The Harrell concordance index of the N stage was lower than that of the LNR or LODDS. The Akaike information criterion of the N stage was higher than that of the LNR or LODDS. Independent predictors included race, T stage, M stage and LNR (or LODDS), and they were incorporated into nomograms for 1-year, 2-year and 5-year CSS prediction. Calibration plots showed satisfactory results for internal and external validity of the nomogram.
Conclusions
LNR and LODDS staging methods have better prognostic efficacy than the traditional N staging method in CGA with node metastasis. Moreover, the two values are promising substitutes for N staging in nomogram development when other independent prognostic factors are incorporated.
Keywords: gastrointestinal tumours, gastrointestinal tumours, surgery
Strengths and limitations of this study.
This study used the national cancer registry data for cardia gastric adenocarcinoma research.
Novel staging methods based on the number of positive lymph nodes have been established for prognostic prediction.
Nomograms based on the new staging methods were constructed and validated.
The validity of the outcomes of the study needs to be confirmed in other populations.
Introduction
Gastric cancer (GC) generally includes two topographical categories: non-cardia GC that occurs at a more distal part of the stomach and GC of the cardia that occurs at the gastro-oesophageal junction (GOJ). In contrast to the steady decline in the incidence of non-cardia GC, GC of the cardia occurs more frequently, particularly in high-income countries.1 2 This trend is associated with obesity, gastro-oesophageal reflux disease and Barrett oesophagus.2 In addition to the difference in the incidence trend, the clinic pathological features and long-term survival vary between the two GC subtypes.3 Precise staging is necessary for the accurate prediction of survival. The tumour, node, metastases (TNM) classification seventh edition of the American Joint Committee on Cancer (AJCC) recommends harvesting of at least 15 lymph nodes (LNs) for N staging.4 5 However, inadequate LN harvest is frequent because of various reasons; thus, precise staging is difficult. It has been demonstrated that the LN ratio (LNR) could provide a better estimate of the survival of patients with GC after curative gastrectomy, regardless of the number of LNs examined,6 and might be a promising aid along with the TNM staging system.7 Furthermore, in previous reports, the log odds of positive LN (LODDS) outperformed the N and LNR staging systems in predicting the survival of patients with GC.8–10 Therefore, the traditional N staging classification might be substituted with different methods with improved performance. Nevertheless, few studies have evaluated the performance of the two LN staging systems in GC of the cardia, which has distinct clinical characteristics and epidemiology than other types of GC.
Here, we used the data of a nationwide cancer registry to evaluate the prognostic value of LNR and LODDS in patients with node-positive cardia gastric adenocarcinoma (CGA) and, if possible, construct a nomogram for the prediction of survival based on the new LN staging system.
Methods
Study design and participant selection
This study was a Surveillance, Epidemiology, and End Results (SEER) registry-based retrospective cohort study, which aimed to enrol patients with node-positive CGA, review their critical clinical characteristics and observe the survival of this population. The source of the SEER data is registered cases of cancer from various locations throughout the USA. Permission for data access was obtained by sending an application form and receiving confirmation mail with a valid username (21268-Nov2019) and password.
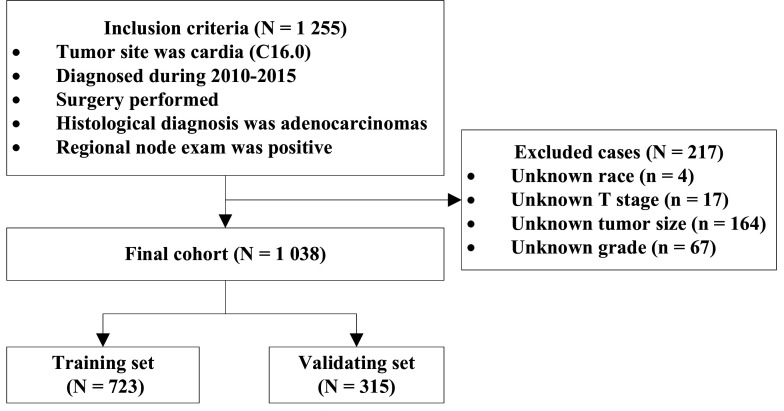
A SEER*Stat (V.8.3.8) was used to access the Incidence-SEER Research Data, 18 Registries, Nov 2019 Sub 2000–2017 (SEER 18 database)11 and to obtain data of node-positive patients with CGA. The inclusion criteria were as follows: (1) the International Classification of Disease for Oncology, Third Edition code for the primary tumour site was C16.0 (cardia); (2) broad histological recode was 8140–8389 adenomas and adenocarcinomas; (3) diagnostic confirmation was by positive histology; (4) surgery was performed; (5) diagnosis was during 2010–2015; and (6) the definite number of positive regional nodes was known and was not zero. Cases with unknown race, T stage information, tumour size or tumour grade were excluded. As shown in figure 1, the final cohort comprised 1038 patients with node-positive CGA, of whom 857 were male and 181 were female. A total of 338 (32.56%) patients were above 70 years of age. Eight hundred and ninety-six (86.32%) patients were white, 64 were black and 78 were of other races. Of the total cohort, 70% of the patients were randomly assigned to the training set (n=723), and the remaining were assigned to the validation set (n=315).
Figure 1.
Flow diagram of the patient selection and grouping.
Technical information
The main outcome was cancer-specific survival (CSS), which was defined as the period between the first diagnosis and death specifically due to CGA. In addition, we extracted the following variables for analysis: sex, race, age, AJCC seventh TNM stage information, tumour size, tumour grade, number of regional nodes examined and number of regional nodes that were positive. The information about the stage of cancer was further corrected according to the AJCC eighth criteria. LNR and LODDS were calculated as previously reported.12 Briefly, LNR was defined as the ratio of the number of positive nodes divided by the total number of examined nodes. LODDS was calculated using the formula: log(NPLN+0.50)/(NDLN−NPLN+0.50), in which 0.50 was added to both the numerator and denominator to avoid an infinite number.
The optimal thresholds for dividing LNR and LODDS into trichotomous variables were determined using the X-tile software (V.3.6.1),13 which were based on the maximal log-rank χ2 value that represented the greatest group difference in CSS probability. LNR and LODDS were classified into three levels because they are proposed as alternative indicators for N stage in node-positive GC, including N1, N2 and N3.
Statistics
The distributions of baseline characteristics between the training and validation sets were described and compared using χ2 test. Survival curves, median survival and CSS rates were generated using Kaplan-Meier method. Outcome differences between the groups were analysed using log-rank test. After testing proportional hazard assumption, a multivariable Cox regression model was used to establish a CSS prognostic model. The prognostic power was evaluated using Akaike information criterion (AIC) and Harrell concordance index (C-index). A predictive model with a lower AIC indicated a better model fit, while a higher C-index indicated a better discriminative ability. A C-index value of 0.5 indicated no predictive power, and an index of 1.0 indicated complete differentiation. Cox stepwise regression analysis was also performed to construct a nomogram for the prediction of 1--year, 2-year and 5-year CSS. Validation of the nomogram was performed using internal and external calibration plots.14 Bootstraps with 1000 resamples were used for the validation activities. Receiver operator characteristic (ROC) curves and areas under the ROC curves (AUCs) were calculated to evaluate the accuracy of CSS prediction using different models. Decision curve analysis (DCA) was performed to determine the clinical application of different models: the proportion of true positive results minus the proportion of false positive results, and the relative risks of false-positive and false-negative results were weighted to obtain the net benefits of decision making. All statistical analyses were performed using R software (V.3.5.3). A two-tailed p value of less than 0.05 was considered statistically significant.
Patient and public involvement
Due to the retrospective and observational nature of the study, the research question and outcome measures were not developed and influenced by patients’ priorities, experiences and preferences. Patients were not involved in the design, recruitment and conduct of this study. Patients were not asked to assess the burden of the intervention and time required to participate in the research. The findings of the study will be disseminated online and are freely available for public.
Results
Table 1 summarises the demographic and clinical features of the participants. In all, 628 patients (60.50%) were diagnosed with a tumour less than 5 cm. Six hundred and forty patients (61.66%) were diagnosed with grade III or IV cancer. The number of patients with T1, T2, T3 and T4 stage was 94, 125, 717 and 102, respectively. The number of patients with N1, N2 and N3 stage was 479, 330 and 229, respectively. Seventy-five patients (7.23%) had distant metastasis at presentation. The median CSS was 27 months. The rate of 1-year, 2-year and 5-year CSS was 76.8%, 53.0% and 29.2%, respectively. There was no statistical difference in the baseline characteristics between the training and validating set. The detailed information about the two sets is also presented in table 1.
Table 1.
Baseline information of the included patients with node positive CGA, n (%)
| Groups | Training set (n=723) |
Validating set (n=315) |
P value |
| Sex | |||
| Male | 596 (82.43) | 261 (82.86) | 0.939 |
| Female | 127 (17.57) | 54 (17.14) | |
| Age (years) | |||
| <70 | 490 (67.77) | 210 (66.67) | 0.781 |
| ≥70 | 233 (32.23) | 105 (33.33) | |
| Race | |||
| White | 628 (86.86) | 268 (85.08) | 0.437 |
| Black | 40 (5.53) | 24 (7.62) | |
| Others | 55 (7.61) | 23 (7.30) | |
| Tumour size | |||
| <5 cm | 442 (61.13) | 186 (59.05) | 0.573 |
| ≥5 cm | 281 (38.87) | 129 (40.95) | |
| Grade | |||
| I–II | 279 (38.59) | 119 (37.78) | 0.859 |
| III–IV | 444 (61.41) | 196 (62.22) | |
| T stage | |||
| T1a | 17 (2.35) | 4 (1.27) | 0.224 |
| T1b | 53 (7.33) | 20 (6.35) | |
| T2 | 83 (11.48) | 42 (13.33) | |
| T3 | 501 (69.29) | 216 (68.57) | |
| T4a | 49 (6.78) | 28 (8.89) | |
| T4b | 20 (2.77) | 5 (1.59) | |
| N stage | |||
| N1 | 332 (45.92) | 147 (46.67) | 0.921 |
| N2 | 229 (31.67) | 101 (32.06) | |
| N3 | 162 (22.41) | 67 (21.27) | |
| M stage | |||
| M0 | 678 (93.78) | 285 (90.48) | 0.079 |
| M1 | 45 (6.22) | 30 (9.52) | |
| Low nodes yield | |||
| Yes | 532 (73.58) | 243 (77.14) | 0.300 |
| No | 191 (26.42) | 72 (22.86) | |
| No. of nodes harvest | 17 (12, 25) | 16 (11, 24) | 0.400 |
| No. of positive nodes | 3 (1, 6) | 3 (1, 6) | 1.000 |
| Median survival (months) | 28 (25, 32) | 25 (21, 32) | 0.361 |
| CSS rate (%) | |||
| 1 year | 77.0 (74.0, 80.2) | 76.3 (71.6, 81.2) | |
| 2 years | 53.7 (50.1, 57.5) | 51.4 (46.0, 57.5) | |
| 5 years | 30.3 (26.7, 34.5) | 26.4 (20.9, 33.4) |
CGA, cardia gastric adenocarcinoma; CSS, cancer-specific survival.
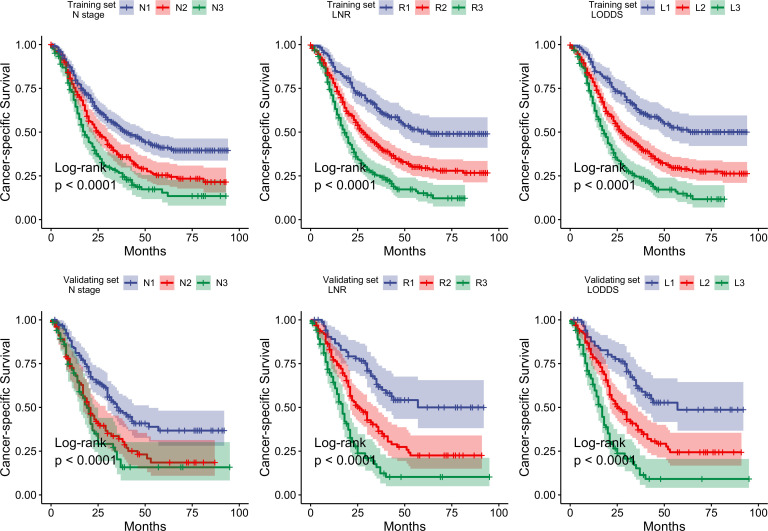
According to X-tile software results, the optimal cut-off values for LNR were 0.09 and 0.33, and for LODDS were −2.09 and −0.65. Thus, patients were classified into the low (<0.09, R1), medium (0.09~0.33, R2) or high LNR (>0.33, R3) groups, or low (<−2.09, L1), medium (−2.09~−0.65, L2) or high LODDS (>−0.65, L3) groups. For model optimisation, LNR and LODDS were also categorised into trichotomous factors using the cut-off values of P25 and P75. The discrimination ability of the model based on the interquartiles was poor (online supplemental table 1); hence, this model was not analysed further. Next, we created the survival curves of the patients according to the N staging, LNR or LODDS staging system. As shown in figure 2 in the training set, CSS was significantly different between all the three staging systems (all the log-rank p values <0.0001); however, the 95% CIs of N2 and N3 survival curve were initially divergent and partly overlapped afterward. The inferior discriminative ability of the N system was further reinforced by the AIC and C-index. As shown in table 2, the C-index of the N stage was lower than that of LNR or LODDS. Similarly, the AIC of the N stage was higher than that of the LNR or LODDS. The clinical characteristics with statistical significance for CSS were further incorporated in the Cox regression model as potential confounders (online supplemental table 2), and all the variables met the proportional hazard assumption (online supplemental figure 1, all the p values >0.05). The prognostic value of the adjusted model was generally better than that of the crude model. In addition, the prognostic value of the LNR system seemed to be poorer than that of the LODDS system; however, the difference was not significant; hence, we incorporated both the systems for nomogram construction.
Figure 2.
Survival curves of the training and validating sets by different staging systems.
Table 2.
Prognostic values of variables for patients with node positive CGA (n=1 038)
| Variables | Crude model | Adjusted model | ||||
| HR (95% CI) | C-index | AIC | HR (95% CI) | C-index | AIC | |
| Training set (n=723) | ||||||
| N stage | 0.582 | 5403 | 0.632 | 5365 | ||
| N1 | 1 (ref) | 1 (ref) | ||||
| N2 | 1.53 (1.24 to 1.91) | 1.42 (1.14 to 1.77) | ||||
| N3 | 2.15 (1.70 to 2.71) | 2.03 (1.60 to 2.59) | ||||
| LNR | 0.607 | 5376 | 0.643 | 5350 | ||
| R1 | 1 (ref) | 1 (ref) | ||||
| R2 | 1.88 (1.44 to 2.44) | 1.74 (1.33 to 2.29) | ||||
| R3 | 3.02 (2.30 to 3.97) | 2.63 (1.97 to 3.50) | ||||
| LODDS | 0.609 | 5373 | 0.644 | 5346 | ||
| L1 | 1 (ref) | 1 (ref) | ||||
| L2 | 1.93 (1.48 to 2.51) | 1.80 (1.36 to 2.37) | ||||
| L3 | 3.13 (2.38 to 4.13) | 2.77 (2.07 to 3.70) | ||||
| Validating set (n=315) | ||||||
| N stage | 0.596 | 1957 | 0.675 | 1931 | ||
| N1 | 1 (ref) | 1 (ref) | ||||
| N2 | 1.81 (1.31 to 2.51) | 1.75 (1.25 to 2.46) | ||||
| N3 | 2.18 (1.51 to 3.15) | 2.23 (1.50 to 3.30) | ||||
| LNR | 0.646 | 1927 | 0.691 | 1913 | ||
| R1 | 1 (ref) | 1 (ref) | ||||
| R2 | 2.20 (1.47 to 3.30) | 1.91 (1.26 to 2.90) | ||||
| R3 | 4.16 (2.76 to 6.28) | 3.58 (2.30 to 5.56) | ||||
| LODDS | 0.647 | 1927 | 0.789 | 1914 | ||
| L1 | 1 (ref) | 1 (ref) | ||||
| L2 | 2.07 (1.39 to 3.09) | 2.08 (1.38 to 3.14) | ||||
| L3 | 4.22 (2.79 to 6.39) | 4.10 (2.65 to 6.34) | ||||
Adjusted model considered race, tumour size, grade, T stage and M stage.
AIC, Akaike information criterion; CGA, cardia gastric adenocarcinoma; LNR, lymph node ratio; LODDS, log odds of positive lymph nodes.
bmjopen-2021-050378supp001.pdf (44.7KB, pdf)
bmjopen-2021-050378supp002.pdf (24.9KB, pdf)
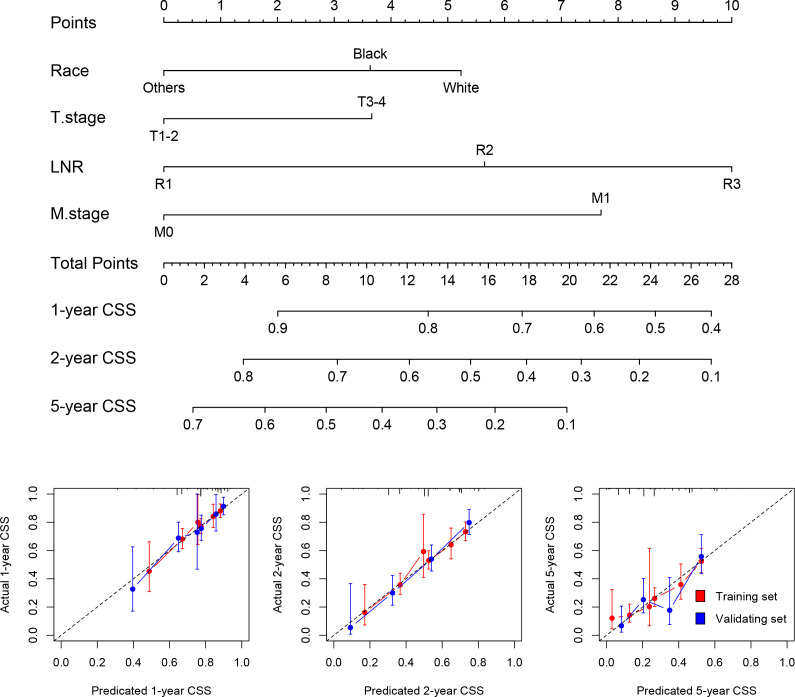
Stepwise Cox regression analysis showed race, T stage, M stage and LNR (or LODDS) were independent predictors; hence, these factors were included in the nomograms. For both LNR and LODDS, the total score was 40, and a higher score suggested lower survival (figure 3 and online supplemental figure 2). Next, the calibration plot was used to assess the internal and external validity of the nomogram (figure 3 and online supplemental figure 2). Since the cross-spot line was generally close to the grey reference line, we concluded that the predicted CSS was well correlated with the actual state. In addition, ROC curves indicated that the AUC of the model based on N stage was lower than that of the model based on the nomogram of LNR or LODDS (online supplemental figure 3). However, the DCA plot does not show advantage of the nomogram (online supplemental figure 3).
Figure 3.
Construction of nomogram based on tumour–lymph node ratio–metastasis staging system and calibration plots for the nomogram.
bmjopen-2021-050378supp003.pdf (162.4KB, pdf)
bmjopen-2021-050378supp004.pdf (570.5KB, pdf)
Discussion
The present study analysed the databases of the national cancer registry and demonstrated that survival of patients with node-positive CGA could be well predicted when the traditional N staging method is substituted with an LNR or LODDS system. This outcome was seen both in the training and validation set. In the training set, the survival curves clearly separated when the patient grouping was implemented following the LNR or LODDS method, which was not achieved by the traditional N staging system. An adjusted model that simultaneously considered the staging, clinical and demographic features outperformed the crude model that only considers staging. Therefore, multiple independent survival factors were incorporated in the nomogram construction, which suggested white, deeper infiltration of the tumour, higher proportion of positive LN and metastasis as risk factors. The nomograms performed consistently across the 1-year, 2-year and 5-year prediction of the CSS as seen in the validation plots.
Previous studies have demonstrated the superiority of LNR or LODDS for prognostic prediction in GC after surgical resection.8–10 15–17 However, the patients were not further classified according to the primary tumour site; this is a critical limitation since there is a significant difference between cardia and non-cardia GC in terms of tumour features, aetiological factors and biological behaviours.3 In the AJCC cancer staging seventh edition, tumours involving GOJ were categorised as oesophageal cancer.5 This was debatable because the GC staging system has a better ability to predict survival of a GOJ tumour.18 19 In the latest eighth edition,20 a tumour that has its epicentre within 2 cm of the GOJ and involves the GOJ (Siewert type I/II) is classified as oesophageal cancer. Other types of GCs, including a tumour with an epicentre more than 2 cm from the GOJ or a tumour located with 2 cm of the GOJ but not involving the GOJ, are classified as GC. The superiority of the new system was confirmed by a retrospective observational study from two institutions in China that have a high volume of cases of GC, regardless of the Siewert type.21 In terms of the Siewert type II junctional adenocarcinoma, a marginal superiority of the oesophageal cancer was found in discriminating survival rates after 3 and 5 years. However, the advantage of the GC system lies in the division of the N3 category into N3a and N3b. Hence, the authors concluded that neither the oesophageal nor the stomach staging system is accurate in predicting survival in Siewert type II junctional cancer.22 Moreover, CGA is probably a special entity that has different biological characteristics compared with distinct gastric or oesophageal cancer. To the best of our knowledge, the present study is the first to demonstrate a superior prognostic prediction based on LNR or LODDS in patients with node-positive CGA. Unfortunately, we were unable to consider the Siewert type due to a lack of information in the SEER database; hence, further studies are necessary with a special focus on tumour location.
LNR and LODDS have been proven to be the strongest indicators of survival in gastric adenocarcinoma when LN harvest is inadequate.16 17 It has been demonstrated that, in general, more extensive LN resection is associated with better survival, which might be due to either improved N classification or a therapeutic effect of lymphadenectomy. For oesophageal cancer, the worldwide data show that harvesting 10 nodes for pT1, 20 for pT2 and 30 or more for pT3/T4 is desirable for reaching maximum 5-year survival.23 For GC, a higher LN harvest also shows improved survival.24 It is suggested that at least 16 nodes be evaluated pathologically and evaluation of more than 30 nodes is desirable.25 Overall, it is encouraged to harvest as many LNs as possible, balancing the extent of LN resection necessary for accurate N staging and maximum survival without unnecessarily increasing the morbidity caused by radical lymphadenectomy. Nevertheless, many conditions can lead to inadequate LN harvest. It is estimated that only one-fifth of the patients with GC have an adequate number of LN examined in Iran,26 while more than 15 LNs are examined in 64% of the patients in the USA.25 The LNR and LODDS staging methods do not require an adequate number of LNs to be evaluated. In the present study, a low LN yield was found to be a risk factor for poor survival in univariate analysis; however, it was not significant in the LNR or LODDS based multivariate model, which indicates that LN harvest has little impact on prediction of survival based on LNR or LODDS. In fact, the new node category method is consistent when nodal assessment is inadequate during surgery for GC8 15–17 and for colorectal cancer,27 oesophageal cancer,28 oral squamous cell carcinoma,29 gallbladder cancer30 and others.
The association between LNR and survival is a promising aspect of cardia GC that is currently emerging and might be clinically relevant. A higher ratio of positive LN indicates a worse outcome in cardia GC. Patients are at two to three times higher risk of cancer-specific death if the ratio is over 33%. The ratio of 9%–33% also indicates a twofold risk. This effect is independent of other crucial clinical characteristics; thus, it is a useful tool for surgeons to predict the prognosis. This is also evidence supporting truly radical surgery, that is, complete lymph node resection rather than limited resection.31 In addition, LNR minimises the ‘stage migration’ phenomenon that occurs with the current N staging system.32
One limitation of this study is that some important factors associated with survival have not been considered in the model due to unavailable data. For example, the Eastern Cooperative Oncology Group or Karnofsky Performance Status score is commonly considered in the survival analysis due to its remarkable relationship with the general status and prognosis. Unfortunately, the SEER 18 database does not record the score at diagnosis; hence, its impact is not considered in this analysis. The treatment modality is also associated with clinical outcomes. This study enrolled patients who underwent gastric resection; however, other information about chemotherapy or radiotherapy is not available in the SEER 18 database. A previous randomised clinical trial demonstrated that compared with surgery alone, preoperative administration of carboplatin and paclitaxel with concurrent radiotherapy significantly improved the overall survival among patients with oesophageal or GOJ cancer (HR=0.657).33 The National Comprehensive Cancer Network clinical practice guidelines for GOJ cancer recommend preoperative chemoradiation or perioperative chemotherapy due to substantial survival benefits compared with surgery alone.34 To overcome this limitation, a database that provides fully detailed medical records is necessary for analysis. Moreover, the inclusion of these factors would greatly improve the prognostic power of the survival prediction model. Another limitation is that our results are based on the training set and confirmed by the validation set; however, the baseline characteristics of the two groups are similar. Hence, these results need to be validated among populations with different characteristics. The third limitation is clinical usability. The DCA result is proposed for assessing the potential clinical impact of risk models for recommending treatment or intervention, and the suggested clinical usability of the nomogram may be poorer than that of other models. In this regard, although this model may have some merits regarding outcome prediction, its use for guiding clinical decisions should be further studied.
In conclusion, staging methods based on LNR and LODDS have better prognostic ability than the traditional N staging method in patients of CGA with regional lymph node metastasis. Moreover, the two values are promising substitutes for N staging in nomogram development when other independent prognostic factors are incorporated.
Supplementary Material
Acknowledgments
We would like to thank all patient advisers for their special contribution.
Footnotes
Contributors: X-QW: work conception, data interpretation, critical review for important content, final approval of the manuscript and agreement to be accountable for all aspects of the work; MB: administrative work, funding, critical review for important content, final approval of the manuscript and agreement to be accountable for all aspects of the work; CZ: acquisition, analysis of data, drafting the work, final approval of the manuscript and agreement to be accountable for all aspects of the work.
Funding: This work was supported by Domestic Visiting Study Project for Outstanding Young Talents in Colleges and Universities in Anhui Province (grant number: gxgnfx2021183) and Major Project of Natural Science of Anhui Provincial Department of Education (grant number: KJ2019ZD73).
Competing interests: None declared.
Provenance and peer review: Not commissioned; externally peer reviewed.
Supplemental material: This content has been supplied by the author(s). It has not been vetted by BMJ Publishing Group Limited (BMJ) and may not have been peer-reviewed. Any opinions or recommendations discussed are solely those of the author(s) and are not endorsed by BMJ. BMJ disclaims all liability and responsibility arising from any reliance placed on the content. Where the content includes any translated material, BMJ does not warrant the accuracy and reliability of the translations (including but not limited to local regulations, clinical guidelines, terminology, drug names and drug dosages), and is not responsible for any error and/or omissions arising from translation and adaptation or otherwise.
Data availability statement
Data are available in a public, open access repository. Dataset available from the SEER website (https://seer.cancer.gov/data-software/).
Ethics statements
Patient consent for publication
Not required.
Ethics approval
The Ethics Committee of Anhui Medical College exempted the requirement for ethics approval because of the observational nature of the study.
References
- 1.Bray F, Ferlay J, Soerjomataram I, et al. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 2018;68:394–424. 10.3322/caac.21492 [DOI] [PubMed] [Google Scholar]
- 2.Abdi E, Latifi-Navid S, Zahri S, et al. Risk factors predisposing to cardia gastric adenocarcinoma: insights and new perspectives. Cancer Med 2019;8:6114–26. 10.1002/cam4.2497 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Amini N, Spolverato G, Kim Y, et al. Clinicopathological features and prognosis of gastric cardia adenocarcinoma: a multi-institutional US study. J Surg Oncol 2015;111:285–92. 10.1002/jso.23799 [DOI] [PubMed] [Google Scholar]
- 4.Sobin L, Gospodarowicz M, Wittekind C. TNM classification of malignant tumours. 7th edn. Wiley-Blackwell, 2009. [Google Scholar]
- 5.Edge S, Byrd D, Compton C. AJCC cancer staging manual. 7th edn. Springer, 2009. [Google Scholar]
- 6.Hou Y, Wang X, Chen J. Prognostic significance of metastatic lymph node ratio: the lymph node ratio could be a prognostic indicator for patients with gastric cancer. World J Surg Oncol 2018;16:198. 10.1186/s12957-018-1504-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zhu J, Xue Z, Zhang S, et al. Integrated analysis of the prognostic role of the lymph node ratio in node-positive gastric cancer: a meta-analysis. Int J Surg 2018;57:76–83. 10.1016/j.ijsu.2018.08.002 [DOI] [PubMed] [Google Scholar]
- 8.Sun Z, Xu Y, Li DM, et al. Log odds of positive lymph nodes: a novel prognostic indicator superior to the number-based and the ratio-based N category for gastric cancer patients with R0 resection. Cancer 2010;116:2571–80. 10.1002/cncr.24989 [DOI] [PubMed] [Google Scholar]
- 9.Zhao E, Zhou C, Chen S. Prognostic nomogram based on log odds of positive lymph nodes for gastric carcinoma patients after surgical resection. Future Oncol 2019;15:4207–22. 10.2217/fon-2019-0473 [DOI] [PubMed] [Google Scholar]
- 10.Gu P, Deng J, Sun Z, et al. Superiority of log odds of positive lymph nodes (LODDS) for prognostic prediction after gastric cancer surgery: a multi-institutional analysis of 7620 patients in China. Surg Today 2021;51:101–10. 10.1007/s00595-020-02091-7 [DOI] [PubMed] [Google Scholar]
- 11.Surveillance, Epidemiology, and End Results (SEER) Program (www.seer.cancer.gov) SEER*Stat Database: Incidence - SEER Research Data, 18 Registries, Nov 2019 Sub (2000-2017) - Linked To County Attributes - Time Dependent (1990-2017) Income/Rurality, 1969-2018 Counties, National Cancer Institute, DCCPS, Surveillance Research Program, released April 2020, based on the November 2019 submission.
- 12.Yu Y, Zhang P, Yao R, et al. Prognostic value of log odds of positive lymph nodes in node-positive lung squamous cell carcinoma patients after surgery: a SEER population-based study. Transl Lung Cancer Res 2020;9:1285–301. 10.21037/tlcr-20-193 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Camp RL, Dolled-Filhart M, Rimm DL. X-tile: a new bio-informatics tool for biomarker assessment and outcome-based cut-point optimization. Clin Cancer Res 2004;10:7252–9. 10.1158/1078-0432.CCR-04-0713 [DOI] [PubMed] [Google Scholar]
- 14.Iasonos A, Schrag D, Raj GV, et al. How to build and interpret a nomogram for cancer prognosis. J Clin Oncol 2008;26:1364–70. 10.1200/JCO.2007.12.9791 [DOI] [PubMed] [Google Scholar]
- 15.Jian-Hui C, Shi-Rong C, Hui W, et al. Prognostic value of three different lymph node staging systems in the survival of patients with gastric cancer following D2 lymphadenectomy. Tumour Biol 2016;37:11105–13. 10.1007/s13277-015-4191-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Aurello P, Petrucciani N, Nigri GR, et al. Log odds of positive lymph nodes (LODDS): what are their role in the prognostic assessment of gastric adenocarcinoma? J Gastrointest Surg 2014;18:1254–60. 10.1007/s11605-014-2539-8 [DOI] [PubMed] [Google Scholar]
- 17.Kılıç Murat Özgür, Gündoğdu SB, Özden S, et al. The prognostic value of different node staging systems in patients with ≤15 lymph nodes following surgery for gastric adenocarcinoma. Acta Chir Belg 2018;118:1–6. 10.1080/00015458.2017.1346036 [DOI] [PubMed] [Google Scholar]
- 18.Suh Y-S, Han D-S, Kong S-H, et al. Should adenocarcinoma of the esophagogastric junction be classified as esophageal cancer? A comparative analysis according to the seventh AJCC TNM classification. Ann Surg 2012;255:908–15. 10.1097/SLA.0b013e31824beb95 [DOI] [PubMed] [Google Scholar]
- 19.Huang Q, Shi J, Feng A, et al. Gastric cardiac carcinomas involving the esophagus are more adequately staged as gastric cancers by the 7th edition of the American joint Commission on cancer staging system. Mod Pathol 2011;24:138–46. 10.1038/modpathol.2010.183 [DOI] [PubMed] [Google Scholar]
- 20.Amin M, Edge S, Greene F. AJCC cancer staging manual. 8th edn. Springer, 2016. [Google Scholar]
- 21.Liu K, Feng F, Chen X-Z, et al. Comparison between gastric and esophageal classification system among adenocarcinomas of esophagogastric junction according to AJCC 8th edition: a retrospective observational study from two high-volume institutions in China. Gastric Cancer 2019;22:506–17. 10.1007/s10120-018-0890-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Karstens K-F, Ghadban T, Sawez S, et al. Comparison of the 8th UICC staging system for esophageal and gastric cancers in Siewert type II junctional adenocarcinomas. Eur J Surg Oncol 2020;46:638–43. 10.1016/j.ejso.2019.12.011 [DOI] [PubMed] [Google Scholar]
- 23.Rizk NP, Ishwaran H, Rice TW, et al. Optimum lymphadenectomy for esophageal cancer. Ann Surg 2010;251:46–50. 10.1097/SLA.0b013e3181b2f6ee [DOI] [PubMed] [Google Scholar]
- 24.Macalindong SS, Kim KH, Nam B-H, et al. Effect of total number of harvested lymph nodes on survival outcomes after curative resection for gastric adenocarcinoma: findings from an eastern high-volume gastric cancer center. BMC Cancer 2018;18:73. 10.1186/s12885-017-3872-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Schoenleber SJ, Schnelldorfer T, Wood CM, et al. Factors influencing lymph node recovery from the operative specimen after gastrectomy for gastric adenocarcinoma. J Gastrointest Surg 2009;13:1233–7. 10.1007/s11605-009-0886-7 [DOI] [PubMed] [Google Scholar]
- 26.Khanjani N, Mirzaei S, Nasrolahi H, et al. Insufficient lymph node assessment in gastric adenocarcinoma. J Egypt Natl Canc Inst 2019;31:2. 10.1186/s43046-019-0004-1 [DOI] [PubMed] [Google Scholar]
- 27.Pei J-P, Zhang C-D, Fan Y-C, et al. Comparison of different lymph node staging systems in patients with resectable colorectal cancer. Front Oncol 2018;8:671. 10.3389/fonc.2018.00671 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cao J, Yuan P, Ma H, et al. Log odds of positive lymph nodes predicts survival in patients after resection for esophageal cancer. Ann Thorac Surg 2016;102:424–32. 10.1016/j.athoracsur.2016.03.030 [DOI] [PubMed] [Google Scholar]
- 29.Jin W, Zhu Z, Wu Y, et al. Prognostic value of log odds of positive lymph nodes in patients with resectable oral squamous cell carcinoma. Oral Oncol 2020;108:104709. 10.1016/j.oraloncology.2020.104709 [DOI] [PubMed] [Google Scholar]
- 30.Li J, Sun Y, Zhao B, et al. Lymph node Ratio-Based staging system for gallbladder cancer with fewer than six lymph nodes examined. Front Oncol 2020;10:542005. 10.3389/fonc.2020.542005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Spillane AJ, Cheung BLH, Winstanley J, et al. Lymph node ratio provides prognostic information in addition to American joint Committee on cancer N stage in patients with melanoma, even if quality of surgery is standardized. Ann Surg 2011;253:109–15. 10.1097/SLA.0b013e3181f9b8b6 [DOI] [PubMed] [Google Scholar]
- 32.Rosa F, Tortorelli AP, Alfieri S, et al. Lymph node ratio for gastric cancer: useful instrument or just an expedient to retrieve fewer lymph nodes? Ann Surg 2014;259:e65. 10.1097/SLA.0000000000000316 [DOI] [PubMed] [Google Scholar]
- 33.van Hagen P, Hulshof MCCM, van Lanschot JJB, et al. Preoperative chemoradiotherapy for esophageal or junctional cancer. N Engl J Med 2012;366:2074–84. 10.1056/NEJMoa1112088 [DOI] [PubMed] [Google Scholar]
- 34.Ajani JA, D'Amico TA, Bentrem DJ, et al. Esophageal and esophagogastric junction cancers, version 2.2019, NCCN clinical practice guidelines in oncology. J Natl Compr Canc Netw 2019;17:855–83. 10.6004/jnccn.2019.0033 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
bmjopen-2021-050378supp001.pdf (44.7KB, pdf)
bmjopen-2021-050378supp002.pdf (24.9KB, pdf)
bmjopen-2021-050378supp003.pdf (162.4KB, pdf)
bmjopen-2021-050378supp004.pdf (570.5KB, pdf)
Data Availability Statement
Data are available in a public, open access repository. Dataset available from the SEER website (https://seer.cancer.gov/data-software/).