Abstract
Spinal cord injury remains a scientific and therapeutic challenge with great cost to individuals and society. The goal of research in this field is to find a means of restoring lost function. Recently we have seen considerable progress in understanding the injury process and the capacity of CNS neurons to regenerate, as well as innovations in stem cell biology. This presents an opportunity to develop effective transplantation strategies to provide new neural cells to promote the formation of new neuronal networks and functional connectivity. Past and ongoing clinical studies have demonstrated the safety of cell therapy, and preclinical research has used models of spinal cord injury to better elucidate the underlying mechanisms through which donor cells interact with the host and thus increase long-term efficacy. While a variety of cell therapies have been explored, we focus here on the use of neural progenitor cells obtained or derived from different sources to promote connectivity in sensory, motor and autonomic systems.
Traumatic spinal cord injury (SCI) occurs in an instant yet sets off a cascade of molecular and cellular events that evolve over days to months following the initial trauma1 (FIG. 1). Depending on the spinal level at which the injury occurs and the severity of the injury, motor, sensory and autonomic functions are disrupted, dramatically impacting quality of life and incurring major costs for effective management. Potential therapeutic approaches must therefore address diverse and multifaceted pathophysiological processes, including the haemorrhage, oxidative stress, inflammatory signalling and immune cell infiltration that are unleashed in the acute phase of injury2–6. At a later stage, major therapeutic goals include reversing demyelination, combating chronic neuroinflammation, neutralizing local growth-limiting factors, promoting regeneration or sprouting of injured axons and restoring lost neural circuitry and connectivity7–12. These are challenging tasks due to the complexity of injury progression, which varies temporally and spatially. Thus, despite the significant progress that has been achieved over decades of research in understanding SCI pathophysiology, there remain no effective therapies in the clinic and it is unclear how a single treatment will satisfactorily address these diverse challenges.
Fig. 1 |. Spinal cord injury: pathophysiological events and potential therapeutic targets.
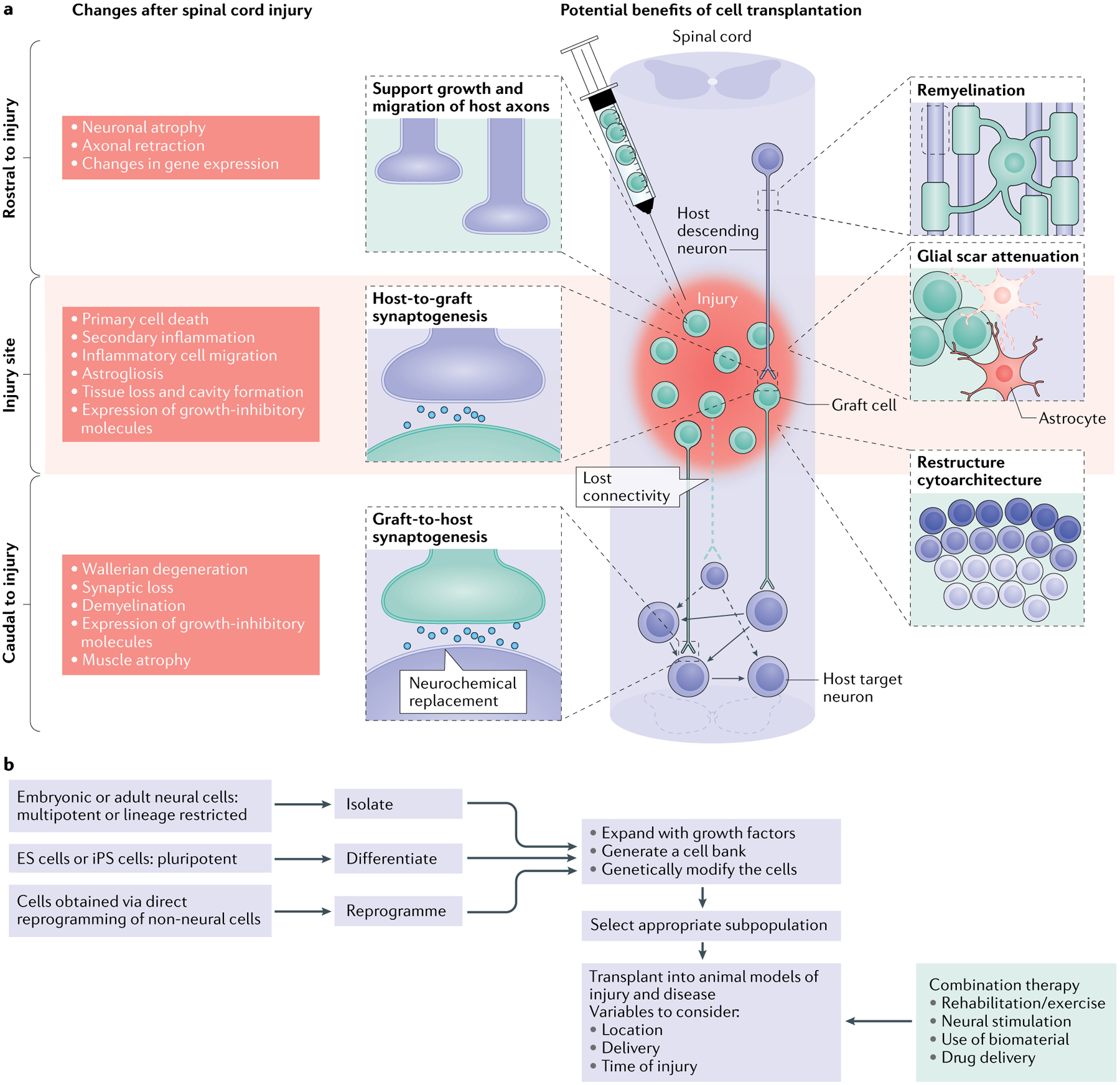
a | The left side of the schematic illustrates the complex changes that occur after spinal cord injury (SCI), which differ temporally and spatially. In a descending tract, such as that illustrated here, these changes include events rostral to the injury (including axon degeneration and changes in gene expression), at the level of the injury (encompassing acute tissue damage and cell death as well as chronic secondary injury and inflammation) and caudal to the injury (including both neural events such as demyelination and non-neural events such as muscle atrophy). Similar changes occur in ascending tracts; however, in this case the location of the events in relation to the injury will be reversed. The injured spinal cord schematic illustrates potential therapeutic targets for cell transplantation, including remyelination, support of host axon growth, glial scar attenuation, synaptogenesis and the restructuring of spinal cord cytoarchitecture. b | The flow chart depicts the decisions that must be made when a cell transplantation strategy for SCI is being developed and the processing steps involved. It shows choices of cells for transplantation in SCI, the process of their preparation, modification and selection and the parameters of their delivery alone and as part of a combination therapy. For cell choices, a wide range of neural progenitor cells can be obtained from embryonic and adult tissue, from pluripotent cells (embryonic stem cells (ES cells) and induced pluripotent stem cells (iPS cells)) and from direct reprogramming of non-neural cells. These cells can be expanded with growth factors to generate cell banks and/or can be genetically modified (to overexpress growth factors, for example). It is then possible to select a subpopulation of the resulting neurons for transplantation. The transplantation process needs to consider variables such as the location of the transplant (that is, transplantation directly into the injury site, intrathecally or systemically), the delivery method (that is, injection as a cell suspension or as part of a hydrogel scaffold) and the timing (for example, subacute transplantation versus transplantation after a 2-week delay after injury). A number of different types of combination therapy can also be initiated at different times and act synergistically with the transplant. In particular, attention should be paid to rehabilitation strategies, various neural stimulation modalities, the use of biomaterials and drug delivery.
In the past decade, the field of neural cell transplantation has made exciting progress, with advances in cellular engineering, better understanding of neural development and network formation, growing appreciation of neural plasticity and how it can be therapeutically harnessed and the application of relevant combinatorial approaches. Cell transplantation therapies are highly promising due to their ability to provide multiple benefits at the molecular, cellular and circuit levels13. Many different cell transplantation strategies are currently being investigated for use in SCI. These include the transplantation of non-neuronal cell types, such as Schwann cells, olfactory ensheathing cells, oligodendrocyte progenitor cells and mesenchymal stem cells13. These strategies are focused on providing neuroprotective benefits, promoting remyelination and modulating the immune response in the injured spinal cord. However, this Review is focused on the transplantation of neural progenitor cells (NPCs), which provide the neural building blocks of the new glial cells (astrocytes and oligodendrocytes) and neurons that are necessary to build neural networks and promote connectivity (and thus functional recovery)14. As we describe herein, recent advances in NPC transplantation research have revealed the remarkable ability of engrafted neurons to synaptically integrate into the injured nervous system, highlighting the potential for reconstruction of complete neural circuits that can support recovery of complex neurological functions.
Oligodendrocyte progenitor cells.
Cells that can differentiate into oligodendrocytes and produce myelin. They are also known as oligodendrocyte precursor cells, often described as Ng2 cells (chondroitin sulfate proteoglycan neuron/glia antigen 2) or polydendrocytes and were previously known as oligodendrocyte type 2 astrocyte (O-2A) progenitor cells.
Neural progenitor cells (NPCs).
Neural cells with less proliferative potential than neural stem cells. NPCs give rise to glial and neuronal cell types that are present in the CNS in the developing embryo, neonate and adult rodent. embryonic NPCs include neuronal-restricted precursors and glial-restricted precursors.
The new neurons generated following NPC transplantation have been shown in animal models to anatomically and functionally integrate with host neural circuits and to support the establishment of novel neuronal relays across the site of injury15–25. NPC-derived glial cells26–32 not only support the graft-derived neurons but also confer additional therapeutic benefits. These include supporting the regeneration, extension and remyelination of injured host axons that are necessary for the relay17,33–49, providing neuroprotection for both host and graft neurons50–52 and attenuating glial scar formation33,42,53. Thus, NPC transplantation can be considered a potentially powerful combinatorial therapy.
Neuronal relays.
At their simplest, three synaptically connected neurons; in the case of transplantation after spinal cord injury, these are the injured neuron, the transplant-derived neuron and the target neuron.
Glial scar.
The fibroglial cell layer surrounding the core of a lesion after spinal cord injury, composed of chondroitin sulfate proteoglycans and fibrous connective tissue.
In this Review, we describe recent advances in the preparation and characterization of NPCs from embryonic tissue and pluripotent cells and the application of transplantation strategies to improve connectivity in sensory, motor and autonomic systems, as well as the incorporation of new ideas about neural plasticity and the use of scaffolds to promote the formation of neuronal connections. For further information on the role of other neural cells (such as astrocytes and oligodendrocyte progenitor cells) and the elucidation of the mechanisms associated with their therapeutic potential, we refer the reader to a number of recent reviews13,54–60.
Brief history of neural cell therapy
The idea of transplanting neural tissues as a strategy for replacement and repair following injury and in degenerative diseases originated in the late nineteenth century and early twentieth century61,62. In 1890, one of the earliest attempts to use this approach63 transplanted brain tissue from adult cats into adult dogs; the results were encouraging but survival was limited. This was followed by experiments that transplanted neonate rat cortical tissues into the cortex of littermates, which increased survival64. At the same time, it was recognized that the limitations of regeneration in the adult mammalian CNS are, in part, due to a lack of neurotrophic support. Thus, implants of peripheral nerve were used to support the growth of severed nerves65. Many decades later, this work was followed up in studies that transplanted peripheral nervous system ‘bridges’ into the injured CNS, underscoring the potential for regeneration in a growth-permissive environment66. Another important advance was the first successful human kidney transplant, using as a donor the recipient’s identical twin67, which opened the door to autologous grafting and later allografting using immunosuppression. The 1970s and 1980s saw a rapid rise in the number of transplantation studies that demonstrated how fetal neural tissues can be used effectively to repair the CNS in neurological disorders and CNS injuries (reviewed in reF.68).
Autologous grafting.
Transplantation of cells or tissue derived from the individual’s own body, including autografts (transplants of tissue from one point to another in the same individual’s body, such as a skin or nerve graft) as well as grafts of reprogrammed autologous cells (for example, induced pluripotent stem cells).
Allografting.
Transplantation of tissue or cells from a genetically non-identical member of the same species. When cells from a different species are transplanted, they are xenografts.
During this period, two notable preclinical studies were conducted in models of Parkinson disease. These studies demonstrated as a proof of concept that transplantation of fetal brain tissue enriched with dopaminergic neurons could restore dopaminergic inputs to the denervated striatum and partially reverse functional deficits69,70. Later work demonstrated that transplantation of fetal brain tissue (taken from the ventral mesencephalon, which is enriched in dopaminergic neurons) resulted in local dopamine production and long-term functional improvements that were associated with transplant survival and integration71. This led to clinical trials72,73 which concluded that dopamine neuron grafts can survive, integrate, reinnervate the striatum and promote functional recovery in some patients. There were, however, a number of challenges, including the limited availability of embryonic tissue, variable results, immunosuppression required for allografts and persistent ethical issues74,75.
Similar advances were made in SCI, beginning with a study that reported that donor spinal cells may be able to promote axonal repair by bridging the injury and/or forming new neuronal relays76. Subsequent studies showed that transplants obtained from the appropriate tissue (such as the developing spinal cord) taken from animals of the appropriate age (for example, embryonic day 14 (E14)) could be transplanted into various models of SCI in rats, where they not only survived but also integrated with surrounding host tissue and developed identifiable neural morphology53,77. In these studies, the donor fetal spinal cord (FSC) showed a significant degree of organotypic differentiation, by forming regions with the cytological and neurotransmitter characteristics of the adult spinal cord78. Immunocytochemical and neural tracing experiments showed that host afferent axons expressing calcitonin gene-related peptide regenerated into the transplants, indicating that the FSC tissue encouraged regeneration of adult axotomized neurons35. Retrograde tracers showed that axons from the transplants extended into the host spinal cord as far as 5 mm from the host–graft interface38. From the findings taken together, the authors of these studies concluded that “intraspinal grafts of fetal spinal cord tissue can establish a short-range intersegmental circuitry in the injured, adult spinal cord” and “may contribute to the formation of a functional relay between separated segments”38.
Fetal spinal cord.
(FSC). Tissue or cells originating from animals at the fetal stage or embryonic stage of development. This cell population has been extensively characterized and widely applied to studies of animal spinal cord injury.
A key limitation faced by those conducting the pioneering FSC transplantation experiments was the difficulty of identifying the grafted cells within the host tissue. Later, with the availability of labelled neural tissue obtained from transgenic rats expressing alkaline phosphatase (and later green fluorescent protein), the long-term integration of fetal E14 spinal cord tissue transplants was confirmed, together with the projection of axons over several spinal segments29. It is remarkable that even without the use of the transgenic animals and advanced molecular tools that most studies rely on today, the early studies were able to demonstrate that fetal transplantation met the fundamental requirements of effective connectivity: the generation and survival of new neurons, the growth of axons into and out of the transplant and a modest level of functional recovery79.
Moving to NPCs
In the 1990s progress was made in defining the population of neural progenitor and neural stem cells (NSCs) that are present in the developing mammalian spinal cord. This was accompanied by the development of techniques for isolating and culturing these cells80. These studies discovered that at early stages of development the spinal cord contains mostly multipotent neuroepithelial cells, which mature into lineage-restricted progenitors, including neuronal-restricted precursors (NRPs)81, and glial-restricted precursors (GRPs)82. Both NRPs and GRPs can be isolated directly from the E13.5 rat spinal cord or can be generated from multipotent neuroepithelial cells83. Neuroepithelial cells transplanted into the adult spinal cord showed poor survival, but transplants of NRPs and GRPs showed robust and long-term survival, expressing markers of mature neurons, astrocytes and oligodendrocytes, as well as synaptic markers27. These studies indicated that progenitor cells may provide a promising cellular replacement candidate for neural cells, including neurons. It is important to note that, during development, NRPs generate the specific progenitors of ventral and dorsal lineage neurons via coordinated spatial and temporal regulation of gene expression, underscoring the complexity of the challenge of rebuilding damaged spinal circuits84,85. A comparison between the properties of FSC and NRP and/or GRP transplants, both derived from E14 spinal cord, showed that FSC cells are able to project longer axons29. This reflects the complex composition of fetal tissue, which contains non-neural cells and extracellular matrix molecules in addition to neurons. Indeed, following transplantation of dissociated FSC tissue, in which the enzyme trypsin is used to degrade extracellular matrix components, graft survival and axon growth required the addition of a cocktail of growth and matrix factors17. Another difference between the two transplant sources is that the culturing of the NRPs or GRPs results in an alteration in the composition of the donor neuronal phenotype in comparison with that of the cells present in FSC tissue22, including a downregulation of several ventral transcription factors that are developmentally expressed in motor neurons as well as some spinal interneurons. The implications of these biases in composition for spinal cord transplantation and/or repair and the possibility of selecting or engineering selective populations (such as motor neurons or specific excitatory or inhibitory interneurons) is discussed later in this Review.
Neural stem cells.
(NSCs). Multipotent neural cells with high proliferative potential that can generate both neurons and glial cells, such as the neuroepithelial cells present in the developing and adult spinal cord of rodents.
Extracellular matrix.
The non-cellular component that provides physical and chemical scaffolding for cells and signalling for tissue differentiation and homeostasis. in the context of spinal cord injury, it refers to the molecular components of the scar, such as chondroitin sulfate proteoglycans.
There have been parallel advances in identifying and characterizing NSCs and NPCs in the adult mammalian CNS. Pioneering work reported the presence of multipotent NSCs in the adult rat brain and spinal cord86,87. NPCs prepared from the adult CNS have since been extensively used for transplantation experiments in animal models of SCI60,88, and their self-renewal capacity presents advantages. In recent years, however, the emphasis in cell transplantation studies has shifted to NPCs derived from pluripotent embryonic stem cells (ES cells) and induced pluripotent stem cells (iPS cells). These NPCs can be obtained with differentiation protocols that can generate either neuronal progenitors89 or more distinct populations of cells, such as interneurons90 (BOX 1). The clear advantages of iPS cells for autologous grafting made them candidates for clinical trials91; however, the advantages of pluripotent cells come with concerns about tumour formation that may mean that additional purification steps are required to minimize the potential risk.
Box 1 |. From pluripotent cells to neural progenitor cells.
The history of pluripotent cell research began with the discovery of embryonal carcinoma cells derived from teratocarcinomas in the 1950s240. These were subsequently shown to be pluripotent and capable of continuous expansion, leading to the generation of cell lines241. The next significant step was the establishment in culture of pluripotential cells derived from mouse blastocysts and grown as embryonic stem cells (ES cells)242,243. These cells became instrumental for the study of cell differentiation and lineage analysis and enabled the production of transgenic animals244,245 and gene targeting by homologous recombination246. The intense interest in ES cells resulted in the discovery of differentiation protocols for a variety of somatic cells, including neurons104. It was not until 1996 that the first human ES cell lines were established247, as well embryonic germ lines derived from primordial human germ cells248. The discovery of a xeno-free system, which abolished the need for animal products to grow ES cells, cleared the way for future application in the clinic249.
The next breakthrough occurred in 2006 with the remarkable demonstration of the generation of induced pluripotent stem cells (iPS cells) from skin fibroblasts using four transcription factors: POU5F1, SOX2, MYC and KlF4 (REF.250). This direct cell reprogramming opened the field of regenerative medicine to unprecedented opportunities for cell replacement and repair, including the potential for autologous transplantation using patient-derived cells. In the past decade, rapid progress has improved iPS cell technology by enabling the derivation of iPS cells from a variety of cells (including human cells), modifying the reprogramming process to avoid the use of the oncogene MYC or vectors with biosafety concern and using the cells for disease modelling251. As the epigenetic background of cells affects their genetic profile and differentiation potential, researchers using iPS cell technology can face a dilemma: whether to use cells derived from less invasive procedures (such as CD34-expressing cells from the blood) or to use cells of a common ectodermal germ layer origin, such as keratinocytes (derived from skin biopsy), which have higher efficiency in producing neural progenitor cells252. It is also possible to generate neural progenitor cells directly from somatic cells without reprogramming into pluripotent cells using a combination of a small number of factors253. This strategy presents opportunities for autologous grafting without immunosuppression and with lower risk of tumorigenicity; however, it remains challenging to obtain highly efficient and consistent results from reprogramming.
There have also been continued efforts to obtain efficient and effective protocols for the differentiation of pluripotent cells into neural stem cells254. Protocols have recently moved from the preparation of a population of unspecified neural progenitors to the production of cells with specific phenotypes, such as dopaminergic neurons255, cortical neurons256, motor neurons257 and spinal interneurons90, for potential cell replacement in the CNS. There has also been progress in obtaining cells of glial lineages from pluripotent cells150.
In the transition from animal models to clinical trials, additional attention must be paid to safety and scaling. Safety concerns are focused on the preparation of clinical-grade cells and the elimination of pluripotent cells capable of producing teratomas, while the scaling issue requires the creation of government-approved cell banks containing sufficient cells for transplantation procedures. In spinal cord injury (SCI), for example, tens of millions of cells are required per patient. Finally, business models remain a challenge for SCI therapeutics, especially for biotechnology companies that need outside funding. Additional perspectives on the role of iPS cell transplants in SCI can be found in recent reviews59,60.
Improving connectivity with NPCs
In addition to the selection of particular types of cell transplants for the specific task of restoring connectivity, it is also important to target distinct neural systems in which such a strategy may be most effective. These may include those responsible for locomotion and sensory functions, those responsible for life-threatening deficits (impaired breathing, autonomic dysreflexia) and those that remain a priority to injured individuals (bladder, upper extremity function). While our Review is focused on improving connectivity with NPC transplants, it is important to note that cell transplantation strategies may have a number of additional aims — including remyelination, immunomodulation, stimulation of endogenous stem cells and attenuation of the scarring process — that may contribute to restoration of function in these systems (reviewed in REFS13,60,92). There are also other non-transplant strategies that may enhance intrinsic growth potential, such as modulating the expression of PTEN and SOCS3 (REFS93,94), or targeting extrinsic factors that limit growth (such as inhibitory molecules associated with scarring and myelin debris) to allow bridging and a generate more effective donor–host interface (reviewed in REF.95). Many studies have also focused on a variety of pharmacological strategies to modulate the inflammatory response and provide neuroprotection at the early stages of SCI to reduce the long-term damage of the secondary injury (reviewed in REFS96,97). More recently there has been promising and exciting progress in promoting neural plasticity and improved function through the use of neural interfacing such as epidural stimulation together with activity-based training98.
Transplantation of NPCs in sensory systems.
Sensory neurons of the dorsal root ganglia project both to the periphery and along the spinal cord to the brainstem. Animal models in which this spinal cord pathway (known as the dorsal column) is lesioned allow regeneration and connectivity after SCI and the recovery of sensory function to be studied99. In these models, transplants of NRPs together with GRPs have been examined for their capacity to form a functional relay between injured dorsal column sensory axons and their targets in the dorsal column nuclei (DCN) in the brainstem16 (FIG. 2). NRPs appeared to be good candidates for this role as it was known that they can generate neurons27,100, survive long term after transplantation and form synaptic connections with host neurons when grafted into the adult spinal cord29,30. Furthermore, it was shown that brain-derived neurotrophic factor (BDNF) can promote directional axon growth from NPCs transplanted into the injured spinal cord, providing a means to guide connectivity with appropriate targets101. The target for the first of these studies was the sensory axons of rat ascending dorsal column neurons, which comprise a tight bundle of fibres within the spinal cord dorsal columns. The injury model used in the study generated a complete unilateral injury of the dorsal columns, severing the tract at cervical spinal segment C1, and was followed by acute transplantation of E13.5 rat spinal cord-derived NRPs and/or GRPs16. A week later, a lentivirus expressing BDNF was injected into the DCN to generate a trophic gradient for directional axon growth. Tracing analysis showed that the host sensory axons regenerated into the transplant and made synaptic connections that were verified by immuno-electron microscopy. Graft-derived neurons extended axons into the target DCN and made synaptic connections. Functional analysis demonstrated that activation of regenerated dorsal column axons through stimulation of the sciatic nerve induced FOS expression, indicating neural activity, in graft-derived neurons. The same stimulation also evoked responses in the DCN with a delay consistent with transmission through a neuronal relay16.
Fig. 2 |. Forming a relay using neural progenitor cells.
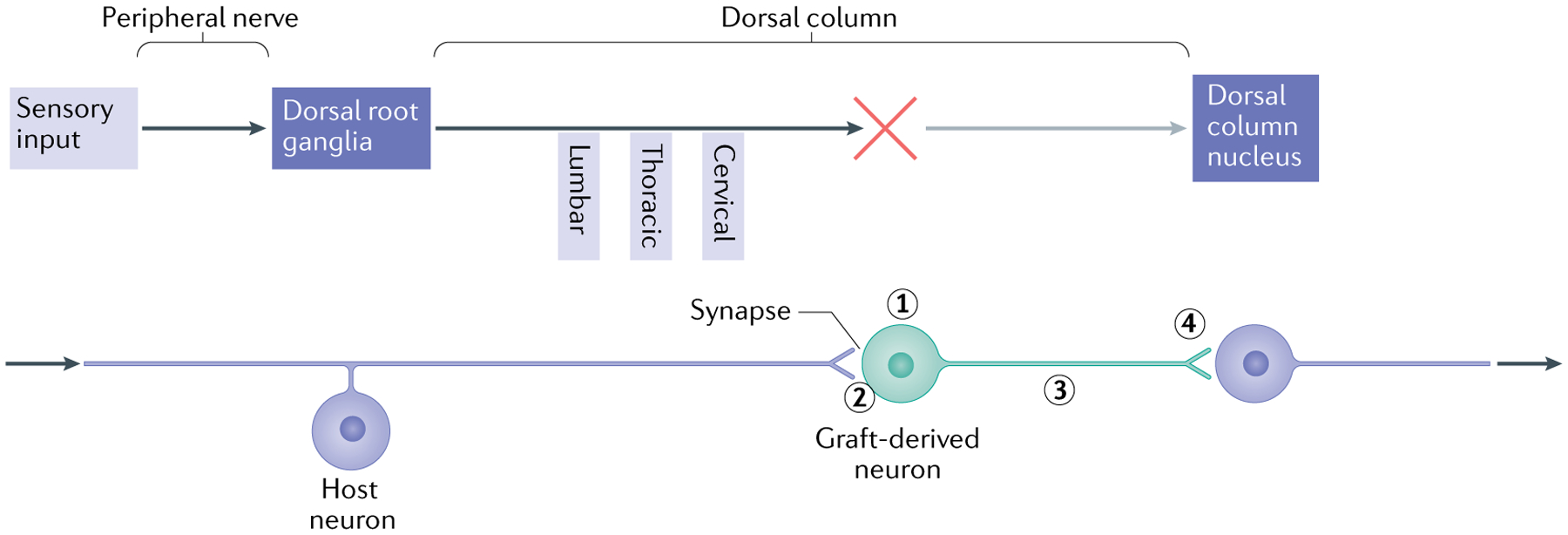
The schematic illustrates the elements required to use neural progenitor cells (NPCs) to form a relay following spinal cord injury, using the example of the sensory system in the presence of a cervical lesion of the dorsal column and transplants of neuronal-restricted precursors (NRPs) and/or glial-restricted precursors (GRPs). The upper part illustrates the elements of the ascending sensory pathway, disrupted by a cervical injury that interrupts the connectivity of sensory axons (dorsal column) with the dorsal column nucleus (DCN) in the brainstem. The lower part shows the steps required to restore the connectively using NPC transplants that form a relay. First, the transplant must survive and generate neurons with the appropriate phenotype (excitatory, for example) (1). The use of a mixture of NRPs and GRPs has been found to be effective, as the GRP-derived astrocytes generate a permissive environment for survival and differentiation of neurons16. Second, the host axons must grow into the graft and form synaptic connections (2). It appears that the presence of astrocytes in the graft attracts the sensory neurons40, but other strategies include the induction of the growth potential of host neurons through the repression of genes such as PTEN and SOCS3 (REF.238). Finally, the axons of transplanted neurons must undergo directional extension to the target (along a neurotrophic gradient to the DCN101) (3) and form synaptic connections (4). To verify the formation of a functional relay, analysis needs to be performed at different levels. Structural analysis includes the tracing of axon growth from the host and the transplant and obtaining evidence of synaptic structure by electron microscopy. Physiological analysis may involve the stimulation of axons followed by assessment of the expression of FOS in downstream neurons as well as measures of signal transmission through the transplant. Functional analysis will include behaviour tests indicative of restored connectivity of the specific tracts.
This strategy of relay formation became the basis for subsequent transplantation studies that aimed to address and resolve distinct issues, including long-term graft survival, the generation of new neurons, the growth of host axons into the graft and graft axons out of the graft, and the generation of functional synapses within the transplant and with putative targets (reviewed in REF.54). These studies emphasized the advantage of using immature neurons for transplantation, owing to their enhanced capacity to grow and overcome the inhibitory environment of the injury102, and also highlighted the essential role of donor astrocytes in generating permissive conditions for neuronal survival, differentiation, axon growth and synapse formation103. It has been shown that this relay strategy can be applied with NPCs derived from ES cells, iPS cells or cells derived from sources enriched in an appropriate population of cells (such as glutamatergic or GABAergic neurons)54,59,104,105. Selectively transplanting subpopulations of cells with particular neuronal phenotypes may also allow more directed treatments: for example, GABAergic neurons have been transplanted to treat neuropathic pain in models of SCI because they can mitigate the loss of presynaptic inhibition onto the dorsal horn neurons that are involved in gating sensory information106. These studies also illustrated the multilevel analysis of transplant success that is required at the anatomical, physiological and eventually network levels. However, it is important to realize the limitations of the relay approach and the need for additional modifications designed to increase transplant survival, increase regeneration of host neurons and maximize functional recovery without maladaptive plasticity, as well as the need to test this strategy in chronic injury.
Neuropathic pain.
Pain resulting from injury to the somatosensory nervous system. Neuropathic pain resulting from spinal cord injury typically manifests itself as sharp, shooting or burning sensations experienced in the absence of noxious stimulation or exaggerated pain responses on noxious stimulation.
Maladaptive plasticity.
Spontaneous reorganization of spared neural circuits in such a way that it produces undesired neurological outcomes such as pain or spasticity.
Transplantation of NPCs in motor systems.
Restoration of voluntary motor function has long been a central therapeutic goal of NPC transplantation after SCI. Studies have used diverse lesion models, cell sources, anatomical assessments and behavioural tests (reviewed in REFS89,107–113), producing a wide variety of results that have sometimes included negative data114 and a failure to replicate previous findings115. These studies have also highlighted the many mechanisms by which cell transplants can promote the recovery of functional connectivity and provided important lessons to be considered when potential therapeutics are being advanced from the preclinical stage to the translational stage.
One of the first studies to report enhanced motor functional recovery in SCI used FSC tissue transplanted 10 days after a contusion lesion in adult rats116. Spontaneous locomotor activity (that is, open-field locomotion) and motivated locomotor performance (gait analysis during locomotion to reach a food reward) were assessed. Despite an absence of significant improvements in some assessments of generalized locomotor performance, such as inclined plane and grid walking, rats with transplants exhibited improvement in specific aspects of gait (the base of support and stride length of the hindlimbs) during motivated locomotion116. This showed that detailed quantitative assessments of isolated aspects of motor function may be required to reveal subtle and/or targeted effects of treatment. Indeed, locomotion is a complex behaviour that requires integration of descending motor commands, sensory feedback, alternating excitation and inhibition of motor units and intersegmental coordination of motor outputs117. This study did not assess connectivity between the graft and the host, so mechanisms supporting this functional improvement remain unclear. More broadly, it is still unclear how graft–host neural relays might support the coordinated integration of the multiple neural pathways involved in motor behaviour. Progress in this area will require application of concepts learned from neuroanatomical and physiological studies of the intact and injured nervous system118–120.
Contusion lesion.
Spinal cord injury produced by a blunt force impact, typically resulting in incomplete neurological deficits with partial function remaining below the level of injury. This lesion model has been widely used in experimental studies due to its anatomical similarities to most human spinal cord injury.
Along these lines, there has been significant progress recently related to the corticospinal tract (CST), a supraspinal descending pathway implicated in skilled motor function. In a recent study focused on targeted restoration of forelimb motor function42, dissociated NPCs were transplanted into the site of a rat spinal cord dorsal column lesion that axotomized the descending axons of the CST. Following graft maturation, large numbers of CST axons were found to have grown into the NPC grafts. These axons established functional synapses onto graft-derived neurons, which in turn projected axons into the caudal host spinal cord. Performance on a skilled forelimb reaching task showed that rats that received NPC transplants performed significantly better than controls from 5 weeks after transplantation. This indicated that corticospinal axons can regenerate into the NPC transplant (exhibiting a preference for transplants with a caudalized (spinal cord) identity) and form relays through a combination of monosynaptic and polysynaptic projections42. In separate studies, other host motor system axons have been shown to project into transplants after SCI, including reticulospinal, rubrospinal and serotonergic axons17,21,45. In one of these studies, graft-derived synapse formation onto neurons in the caudal host spinal cord, as well as the presence of complete graft-mediated electrophysiological relays, was reported17. In a recent study, it was observed that some of the axons emerging from donor NPC transplants were myelinated by host oligodendrocytes, generating myelin sheaths similar to those of axons in the intact spinal cord121. This observation that graft-derived axons can be recognized and myelinated by host systems suggests that conduction of impulses through newly formed graft–host relays might be improved. Despite the focus on supraspinal tracts in these studies, it is important to consider that spinal interneurons are also strong candidates for mediating functional relays, as has been demonstrated in studies in which supraspinal control of stepping has been recovered through indirect propriospinal relay connections118.
Recovery of complex motor behaviours is an immensely challenging goal of SCI research. At present, it is plausible that NPC transplantation may promote recovery of only selected, less complex aspects of motor control, rather than complete restoration of walking or hand function. Indeed, to date all studies have reported only partial recovery at best. Therefore, it is critical that preclinical efficacy studies are designed to use not only rigorous behavioural assessments but also rigorous statistical analyses designed to detect subtle but biologically meaningful effects of treatment. This is demonstrated by the findings of a recent study of human NSC transplantation into cervical SCI in non-human primates (rhesus monkeys)122. In this study, subjects were evaluated in an open-field task that sampled more 25 features of forelimb motor function. Although the authors reported that transplantation did not significantly improve any of the individual features of motor performance, principal component analysis revealed significant improvements in “an overall measure of motor function that combines all measures as compared to monkeys without surviving grafts”122. This type of multivariate statistical approach may be a particularly powerful method to reveal the effects of treatment in non-human primate models, which risk being underpowered for single variable statistics. Finally, motor behavioural outcomes in animal studies must be interpreted with regard to potential impact on quality of life. For a large fraction of the human population with SCI, a small degree of hand function may have great implications for quality of life and independence.
Principal component analysis.
An approach that uses an orthogonal transformation to convert observations that may be correlated into a set of uncorrelated variables referred to as principal components.
Transplantation of NPCs in respiratory networks.
Impaired breathing remains a leading cause of morbidity and death after SCI123. Accordingly, a range of strategies for repairing injured respiratory networks have been explored. A focus has been the phrenic motor network, which controls the diaphragm (FIG. 3). From a preclinical perspective, the phrenic network is a relatively simple neural network of readily identifiable supraspinal and spinal neuronal components which controls function of a single muscle performing a simple task and requires no training. This has enabled rigorous assessment of the reparative potential of cell therapies in this system15,18,20,22,23,124. As in most other systems, the goals of cell therapy for respiratory networks have included providing functionally relevant new neurons and/or a growth-permissive substrate to serve as a bridge to growing host axons. Building on prior work with glial progenitors in other SCI models125, recent work demonstrated in mice that transplanted GRPs promote growth of injured bulbospinal respiratory axons through the site of injury126, improving phrenic motor recovery.
Fig. 3 |. Restoring connectivity in the respiratory system.
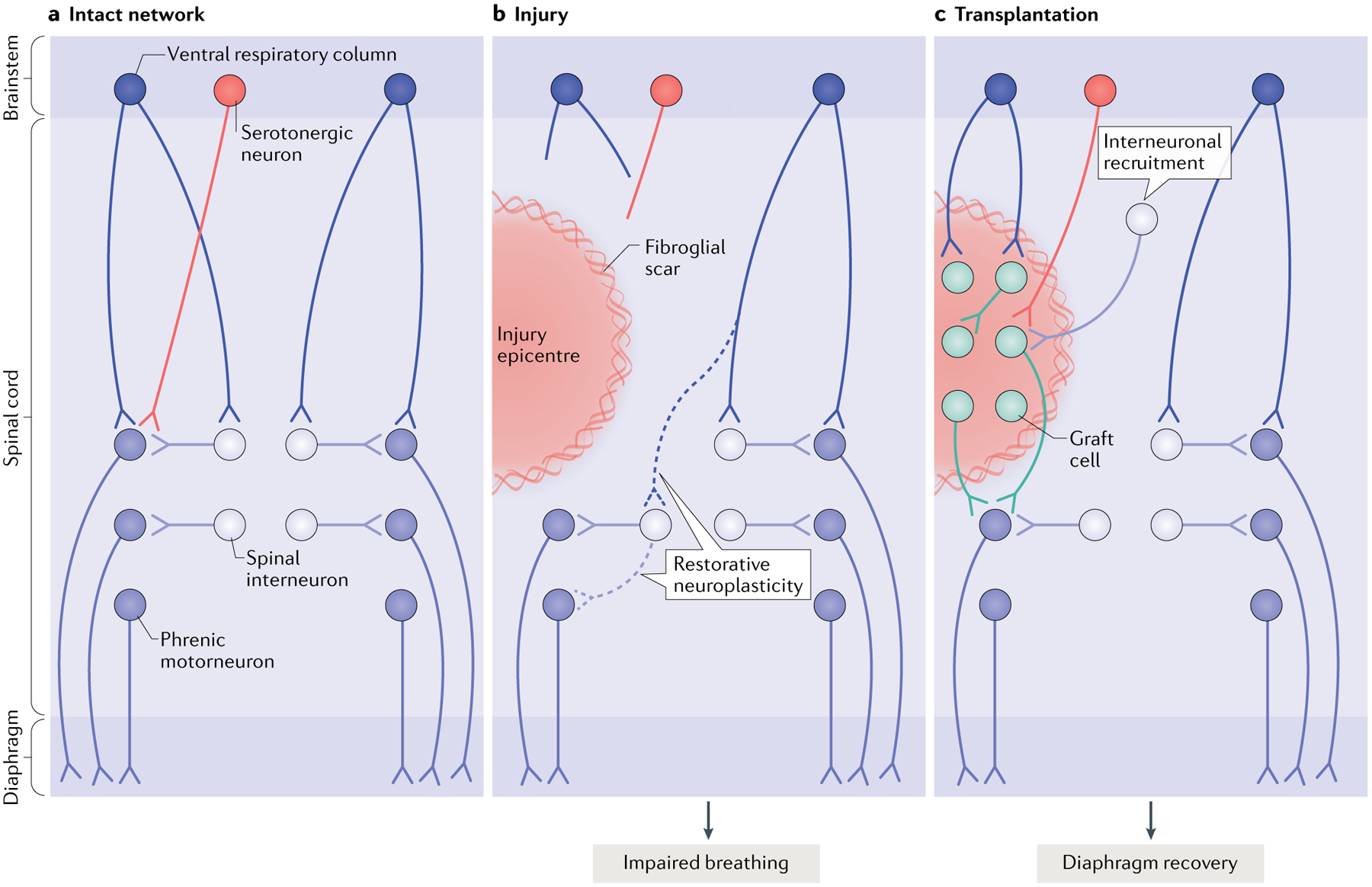
The diagram depicts the intact (part a), injured (part b) and transplant-treated (part c) spinal phrenic motor circuit within the cervical spinal cord. Respiration is driven by brainstem neurons in the ventral respiratory column that directly — or indirectly via spinal interneurons — innervate the phrenic motor neuron pool (distributed from cervical level C3 to cervical level C6). Phrenic motor neuron activity is also modulated by serotonergic pathways and populations of spinal interneurons as breathing conditions change. Phrenic motor neurons on each side of the spinal cord innervate half of the diaphragm on each side of the body via phrenic nerves. Injury (part b) can compromise descending projections, as well as phrenic spinal interneurons and motor neurons. Spared spinal neurons caudal to the injury are therefore denervated. While this is devastating, some limited recovery of diaphragm activity can occur ipsilateral to the injury via restorative neuroplasticity (dashed lines), and spared monosynaptic and polysynaptic pathways from the contralateral spinal cord (via brainstem and spinal interneurons, respectively) can facilitate plasticity in these lateralized spinal injuries. However, the extent of recovery is minimal and deficits persist. A number of cell therapies have been used to promote repair and plasticity within injured respiratory pathways. Neural progenitor cell transplants are perhaps the most often used, as they can modify glial scarring at the lesion border and provide the building blocks for tissue repair. Transplantation of neural progenitor cells into the injured phrenic network in animal models (typically directly into the lesion site as shown in part c) has resulted in extensive synaptic integration between donor neurons themselves, between host spinal and brainstem neurons and donor neurons and between donor and spinal phrenic neurons. This synaptic integration also coincides with enhanced plasticity of existing and newly formed pathways, and improved respiratory activity15,18,22,23. Without any other intervention, transplantation of cells alone is likely to lead to the formation of a vast range of new connections, which are likely to differ between treated recipients22,23, and to the recruitment of novel interneuron populations to establish novel neural networks. Research is under way to develop strategies that control this integration and connectivity. Other models of injury affecting the phrenic network and additional mechanisms of recovery are discussed elsewhere239.
NPC transplants have also been used within the phrenic motor network to provide a new population of neurons that can contribute to the formation of novel neuronal networks that relay information across the injury site (FIG. 3). Among the first of these studies were two that used FSC transplants to repair injured phrenic motor circuitry following a lateral hemisection at the C2 spinal level in adult rats15,18). In one of these studies, transneuronal tracing techniques revealed that by 4 weeks after transplantation donor cells synaptically integrated with the denervated phrenic network15. A subsequent study showed that the donor cells also received synaptic input from host neurons both rostral and caudal to the injury18. Electrophysiological recording revealed examples of spontaneous donor neuron activity that was in phase with inspiratory and expiratory phases of breathing, and was responsive to altered respiratory drive18. Combining transplantation with respiratory training127 increased this patterned respiratory activity within donor cells. Finally, it has been shown that host phrenic motor neuron output is significantly improved following transplantation128. This capacity for anatomical and functional improvement was also demonstrated following a lateral hemisection in adult rats at the C4 spinal level, in the heart of the phrenic motor neuron pool, and where the majority of human SCIs occur129.
While these hemisection-type injuries offer a reproducible proof-of-principle preclinical injury model, the neuropathological consequences of human SCI more closely resemble contusion injury or compression injury. A growing number of studies are now assessing the efficacy of neural transplantation in such models. Mechanically dissociated FSC tissue transplanted into the lesion site 1 week after a C3/4 contusion injury (subacutely) was recently shown to result in synaptic integration of donor neurons — including cholinergic interneurons — with the injured phrenic motor network and spontaneous respiratory activity23. Serotonergic host axons and putative boutons were also seen in close proximity to these donor neurons, providing further evidence of host–donor innervation. This result was replicated by the transplantation of lineage-restricted NPCs22, which were derived from the developing rodent spinal cord. The latter study also showed that enriching donor progenitors for a specific subset of spinal interneurons — CHX10-expressing, excitatory V2a neurons — further enhanced recovery. However, the extent of donor–host integration as well as the extent of functional improvement was variable. Thus, despite the functional benefits seen with FSC transplantation in these animal models, some caveats remain that need to be explored further14. The results of these recent experiments suggest not only that care should be taken in selecting the appropriate donor cell phenotype but also that transplantation without additional interventions to promote integration may not be enough to release their full therapeutic potential.
Compression injury.
A condition that puts pressure on the spinal cord, which can be achieved in animal models of spinal cord injury using calibrated clips or by placing a specific weight in the epidural space. (A mixed contusion–compression spinal cord injury model can also be generated by delivering an initial blunt impact followed by sustained pressure.)
Transplantation of NPCs in autonomic systems.
SCI at high spinal levels can affect autonomic function. For example, the loss of supraspinal control over sympathetic flow originating from sites caudal to the injury results in cardiovascular dysfunction (FIG. 4). Specifically, the altered tonic activity of sympathetic preganglionic neurons (SPNs) as a result of the loss of descending modulatory input causes abnormal haemodynamics at rest and orthostatic hypotension130,131. Autonomic dysreflexia, a serious cardiovascular disorder characterized by dangerous episodic hypertension, occurs due to bursting of massive sympathetic discharges in response to sensory or visceral stimuli below the level of injury132. Autonomic dysreflexia is another leading cause of morbidity and death in individuals with SCI and its treatment is considered to be high priority for improving quality of life133. Currently, treatments for this disorder (which is managed mostly by antihypertensive medications) have transient effects without addressing the primary sympathetic dysregulation. There is therefore an opportunity for experimental transplantation strategies using NPCs to restore the regulation of SPNs and ultimately improve long-term cardiovascular function. Autonomic dysreflexia has been modelled in multiple studies by transection of the adult rat spinal cord at spinal level T4 coupled with noxious colorectal distension134–136. Transplantation of E14 rat brainstem-derived NSCs in this injury model promoted recovery of basal cardiovascular parameters and alleviated autonomic dysreflexia136 (FIG. 4). Anatomical analysis showed survival and differentiation of the graft into catecholaminergic and serotonergic neurons, the projection of host supraspinal medullar neurons into the graft and long-distance axon growth and topographical innervation of caudal SPNs by the graft-derived neurons. Taken together, the results indicated the formation of functional relays to restore supraspinal regulation of denervated SPNs136. It is possible that this approach could be further refined through the transplantation of serotonergic neuron-enriched fetal raphe cells or serotonergic neurons prepared by specific neuronal differentiation of ES cells or iPS cells.
Fig. 4 |. Restoring connectivity in autonomic systems.
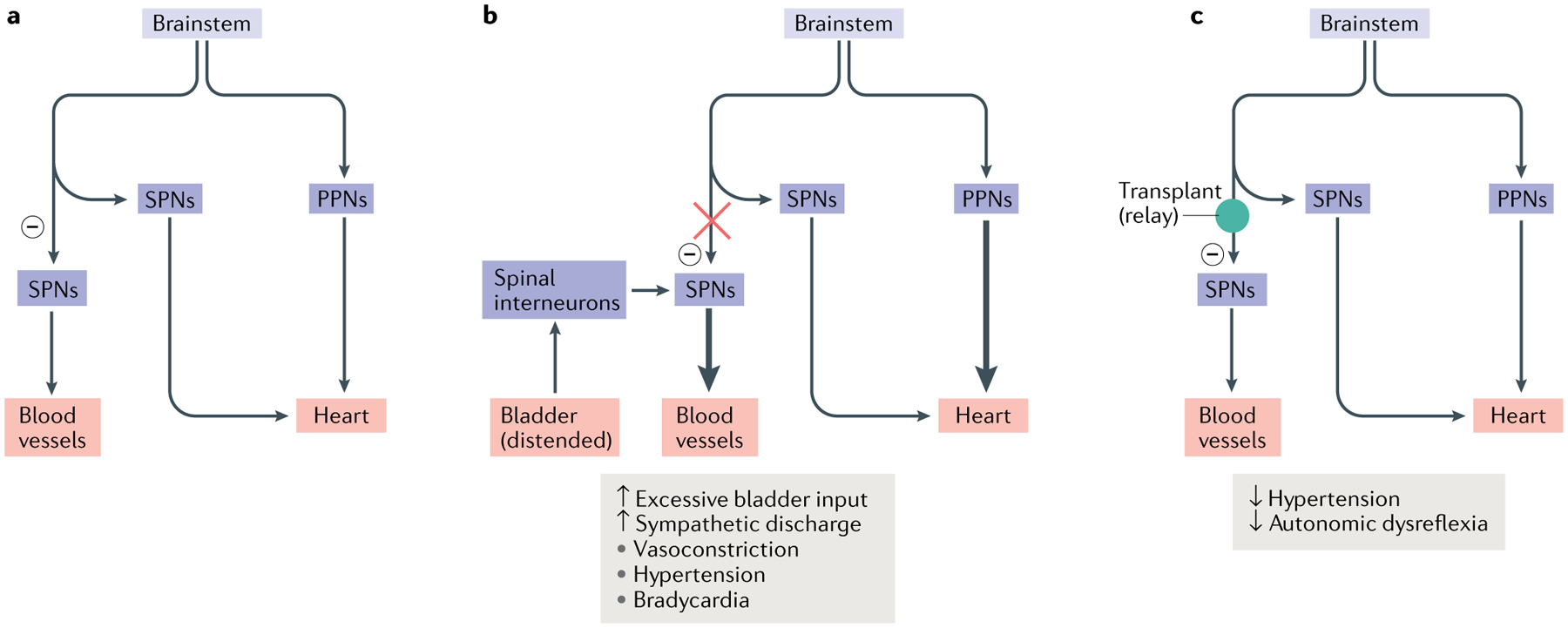
The majority of the vasculature is controlled by sympathetic activity, while the heart is regulated by both the sympathetic system and the parasympathetic system. Sympathetic preganglionic neurons (SPNs) in the spinal cord project to the periphery and synapse onto sympathetic postganglionic neurons. The latter extend axon terminals into the blood vessel and heart. a | In normal conditions, sympathetic excitation induces vasoconstriction and thus increases blood pressure. Subsequently, baroreceptor-mediated parasympathetic excitation decreases the heart rate. In addition, supraspinal vasomotor pathways provide inhibitory regulation (indicated by a minus sign) to suppress the sympathetic activity to blood vessels, leading to recovery of normal blood pressure. b | After spinal cord injury, spinal SPNs lose this descending inhibitory modulation. When excessive sensory or visceral stimulation below the level of injury (for example, bladder distension) activates SPNs via interneurons, the massive discharge of SPNs causes vasoconstriction and increases blood pressure. This causes baroreceptor-mediated bradycardia to occur. However, the absence of supraspinal inhibitory signals to caudal SPNs means that blood pressure remains high. The resulting simultaneous hypertension and bradycardia is known as autonomic dysreflexia. c | Transplantation of early-stage neurons into the lesion of the spinal cord reconstitutes supraspinal vasomotor pathways. Grafted cells relay supraspinal inhibitory signals across the lesion to target neurons in the caudal portion of the spinal cord, which can restore sympathetic regulation of cardiovascular function after spinal cord injury. PPNs, parasympathetic preganglionic neurons.
Another functional deficit after SCI that is associated with the autonomic nervous system is the loss of voluntary micturition control, which is also rated as an important therapeutic goal for quality of life in people with SCI133. Importantly, bladder dysfunction is a cause of urinary tract infection, which is a major cause of death in people with SCI and remains a concern given the increase in antibiotic-resistant bacteria137. Lower urinary tract function is controlled by a combination of supraspinal, spinal and peripheral neurons138. Following SCI, interruption of afferent neuronal pathways initially produces bladder areflexia. Over time, new spinal neuronal circuits are established that enable the emergence of a spontaneous bladder reflex to facilitate involuntary urine voiding139. However, there is a frequent occurrence of bladder hyperactivity and detrusor–sphincter dyssynergia causing inefficient voiding140.
One of the first studies to test the efficacy of NPC transplantation on restoration of bladder function used a midthoracic contusion injury in rats followed by a subacute grafting of E14-derived NRPs and/or GRPs141. Rats with transplants exhibited an accelerated recovery from bladder areflexia, with a decrease in the high micturition pressure and amelioration of dyssynergia between the bladder and the urethral sphincter141. As dyssynergia is associated with the loss of brainstem projections into the lumbosacral spinal cord, it appears likely that the transplant reduced the secondary injury, providing protection to these modulatory systems. The fewer episodes of detrusor hyperreflexia suggested an attenuation of the hyperactive bladder reflexes because of diminished sprouting from bladder afferents. This is therefore an example of NPC transplants modulating the host environment and providing local protection and axonal sparing for descending pathways, rather than bridging connectivity. Indeed, transplantation of human GRPs in an animal model of a similar spinal cord contusion also showed improvement of bladder function, which may again reflect local beneficial effects of the transplant with respect to neuroprotection and axon sparing125. A recent study showed that transplanted NPCs derived from human ES cells differentiated to resemble GABAergic inhibitory medial ganglionic eminence neurons, received synaptic inputs and improved bladder function142. As GABA is crucial to coordinated bladder function, these improvements were hypothesized to be due to the influence of human ES cell-derived medial ganglionic eminence-like neurons on the function of the inhibitory interneurons that modulate parasympathetic preganglionic neurons and the motor neurons that innervate the detrusor muscle and external urethral sphincter, respectively.
An example of improved bladder function that did result from transplant-mediated improvement of connectivity was provided by studies that used peripheral nerve autografts, acidic fibroblast growth factor and chondroitinase ABC to treat a complete T8 spinal cord transection in adult rats and mice, showing restoration of supraspinal control of bladder function143. Urodynamic analysis revealed that the treatment was associated with regeneration of serotonergic neurons across the lesion and into the distal portion of the spinal cord.
Chondroitinase ABC.
A bacterial enzyme that degrades polysaccharide chains on chondroitin sulfate proteoglycans. This enzyme has been used as a potential therapeutic treatment for spinal cord injury due to its degradation of axon growth-inhibiting chondroitin sulfate proteoglycans that are present in the extracellular matrix of the injured spinal cord.
Cell therapy in clinical trials
The data described so far demonstrate the potential of NPC transplants to improve connectivity through a combination of host regeneration and formation of neuronal relays. However, as noted already, there are a number of additional mechanisms through which NPC transplants have been shown to provide therapeutic benefit in preclinical models, including remyelination, immunomodulation, neuroprotection and stimulation of axon growth60.
Several efforts have been made to translate neural cell therapies into the setting of human injuries as described in this section and Supplementary Table 1, which includes all the registered clinical trials evaluating NSC transplantation in SCI regardless of their therapeutic targets. Building on the early work with FSC tissue, a small-scale clinical study was initiated to test the feasibility of transplanting FSC-derived cells into people with post-traumatic syringomyelia144–147. This suggested that spinal tissue could be safely transplanted147. The next evolution of this work was a trial148 in which a mixture of human NSCs and NPCs derived from the fetal telencephalon was transplanted into patients with cervical SCI and which confirmed the safety of NPC transplantation. More recently, key clinical trials were conducted with oligodendrocyte progenitor cells149,150, olfactory ensheathing cells151, autologous Schwann cells152,153 and human NSCs (HuCNS-SC)154.
In 2009, the biopharmaceutical company Geron gained FDA approval to take human ES cell-derived oligodendrocyte progenitor cells into phase 1/2 clinical trials in people with subacute thoracic SCI150. This was a turning point not only for treatment of SCI but also for the stem cell field more generally as the cells were the first pluripotent stem cell-derived cells to be approved for clinical trials. After five people had been enrolled, the trial ended in 2011; however, in 2014, Asterias Biotherapeutics received FDA approval to continue and expand the trial, now including individuals with cervical-level injuries and incorporating an increased dose of cells for increased efficacy150 and approval to begin a phase 2 trial155. Another trial — the StemCells ‘Pathway’ trial (phase 1/2) — was a single-blind, randomized controlled trial that was initiated in 2011 and ended in 2016 after 17 people had been treated156. Despite reports of some functional gain154, the company lacked the financial support to continue the trial. In 2014, Neuralstem (now Seneca BioPharma) initiated an open-label phase 1 trial on the use of human spinal cord-derived NSCs in people with chronic thoracic or cervical SCI157. Eight patients have been enrolled to date and recruitment continues158.
These clinical trials led to the engineering and development of hardware specifically for transplanting cells into the human spinal cord159,160. It has also become clear that it is important to evaluate cells derived from the same sources in preclinical studies and subsequent clinical studies to reduce variability in outcomes: the donor cells used in the StemCells trial were a different, clinical-grade cell line than the research-grade cell line used in preclinical studies; however, only the latter cell line showed positive effects in animals114. As new strategies for derivation of donor cells are developed, it is likely that more rapid progression to clinical trials will be possible. For example, with the rapid advances in iPS cell technology and preclinical testing of the efficacy161–163, there are now plans to test human iPS cells in people with SCI91.
Despite these advances, hurdles for translation persist, and neural cell transplantation has yet to become an approved therapy. Because of the relatively small population of people with SCI, the cost–benefit ratio for developing and testing treatments can be prohibitively high and the competition for ‘qualified’ patients at the subacute stage of injury can be fierce. However, the significant socio-economic burden and the growing incidence and prevalence of SCI should stimulate continued innovation and the push for translation. With consistent improvements in communication between academic scientists, clinical professionals, industry representatives, funding agencies, governments, people with SCI and advocates, there is hope that, as a unified network, greater support can be attained to drive clinical translation forward and overcome these hurdles.
Challenges
The advances in understanding the complex process of SCI together with the exciting progress in NSC biology have positioned NPC transplants as a promising therapeutic tool and as a focus of research to elucidate strategies for cell replacement in the CNS. Nevertheless, there are still many challenges to be faced at the cell biological and neuroscience systems levels.
Improve graft survival.
The challenge of graft survival was identified in the early studies using fetal transplants: these showed an initial loss of graft cells followed by pro-liferation of the NPC population, which eventually integrated with the host tissue29. Initial strategies to improve the survival and efficacy of transplants included a delay between injury and transplantation and the addition of neurotrophins164. Later, when FSC tissue treated with trypsin was used, these measures were supplemented by a cocktail of growth factors and a fibrin matrix165. The challenges of NPC survival in the injury environment166 have further been addressed by inclusion of GRP-derived astrocytes to generate a permissive microenvironment29; however, this strategy was ineffective in a severe injury such as a complete transection31. Other strategies that have been used to promote survival of grafted NPCs include chondroitinase treatment167, genetic modifications168 and cell preconditioning169. A promising direction to address both survival and efficacy has been the use of biomaterials, as discussed in recent reviews60,170 and BOX 2.
Box 2 |. Scaffolds to the rescue.
The intersection between material engineering and neuroscience has produced a rich and productive area of research that leverages the ingenuity of engineering design to address the complexities of CNS injury and therapeutics. For the design of appropriate scaffolds to support transplantation of neural progenitor cells, engineers have considered parameters such biocompatibility, biodegradability, permeability, biomechanical properties, the possible addition of extracellular proteins, the controlled release of growth factors and surface topography (such as alignment of the scaffolds for directional axon growth)60,170. Important studies have been performed using natural polymers as scaffolds — including collagen, laminin, fibrin, hyaluronic acid, alginate, chitosan and self-assembled peptides used as hydrogels — which in general are biocompatible and biodegradable, with low immunogenicity170. These materials also have beneficial biological activities which can be matched with the transplanted cells for improved survival and differentiation60. By contrast, synthetic biomaterials such as polyglycolic acid, polylactic acid and poly(lactic-glycolic acid) as well as synthetically fabricated scaffolds such as polycaprolactone and nanotubes allow consistent production and control over a wide range of physical properties but often present challenges with respect to compatibility and potential toxicity184,185.
Hydrogels have often been used as a supportive matrix for the delivery of cells into the injury cavity to improve transplant survival258 and can be modified with molecules (such as platelet-derived growth factor to promote oligodendrocyte differentiation259) or constructed with polymers that allow local delivery of cellular transplants into the injured spinal cord. Injectable scaffolds are viscous liquid-forming space-filling hydrogels260 that can be designed to form channels that guide axon growth261 and can be prepared with aligned endothelial cells that provide both vascular structures and directional axon growth262. Recent technology allows the printing of 3D scaffolds and the generation of microstructure to promote and guide axon growth25. Furthermore, 3D cell cultures have been used for the fabrication of tissue-like constructs that can foster human neural stem cell maturation and regeneration in the injured spinal cord263.
Improve host regeneration.
There is good evidence that transplants of NPCs initiate robust long-distance donor axon growth in the host17,29. Research is therefore now focused on the ability of host descending and ascending axons, which have variable capacity to regenerate and/or sprout45,171, to grow into the transplant and overcome the inhibitory environment of the injury. A growing understanding of the intrinsic mechanisms of CNS axon growth and regeneration172,173 has allowed molecular, pharmacological and rehabilitative strategies to target and improve plasticity of distinct axon populations, including the CST174. Similarly, a growing understanding of the composition of the molecules and the cells in the injury environment175,176 has allowed strategies to neutralize or modulate their effects177. In particular, we have gained a better understanding of the various cellular and molecular components of the lesion environment95, challenging long-held assumptions about the reasons underlying regeneration failure178. Combined intrinsic and extrinsic growth-promoting manipulations have been successfully used to promote extensive regeneration of propriospinal axons after SCI; this suggests that combining intrinsic or extrinsic manipulations with NPC transplantation might also support greater regeneration compared with transplantation alone179. Following SCI and NPC transplantation, the process of axonal growth and regeneration has to be carefully defined and assessed with respect to the CST, the serotonergic system and a variety of brainstem tracts (as discussed in REF.108). While host regeneration and improved axon growth are essential to building a functional relay and direct connectivity, it is also necessary to pay attention to the age-related decline in regeneration capacity180 and the process of remyelination (BOX 3). It is also important to minimize maladaptive plasticity associated with spasticity or pain181 and to be aware of situations in which regeneration can suppress function or interfere with functional recovery182,183. Finally, it is important to consider the mechanistic differences between host axon regeneration into grafts and synaptogenesis with graft neurons (relay formation) versus long-distance host axon regeneration through a graft to extend into caudal regions of host spinal cord. Although non-neural cell grafts and biomaterial scaffolds have historically been used to promote long-distance regeneration through sites of SCI184,185, neural grafts can also act as permissive scaffolds. For example, in preliminary work, the expression of the transcription factor KLF6 in corticospinal neurons was shown to promote long-distance growth through NPC transplants, allowing synaptogenesis with caudal spinal cord neurons186.
Box 3 |. The remyelination enigma.
Demyelination following spinal cord injury (SCI) has been documented in animal models264 and humans265 as a result of loss of oligodendrocytes and degeneration events at the secondary stages of injury. Importantly, this loss can occur directly as a result of injury even without axonal compromise (primary demyelination) or indirectly as a result of axonal degeneration (secondary demyelination). effective remyelination may fail because of deficiency in the host progenitor cells and their recruitment, or incompetence of differentiation and maturation266. As a result, injured host neurons or newly growing axons may remain dysfunctional. What remains contentious is whether chronic demyelination makes a significant contribution to the deficits observed following SCI and is therefore an important therapeutic target267–272. Reaching a definite conclusion about the importance of the remyelination process to the success of neural progenitor cell (NPC) transplantation therapies is complicated by the variations between the transplantation studies with respect to the model of injury and its level and the type of functional analysis as well as by the other potential benefits of NPC transplantation besides myelination.
When examining the remyelination process following SCI, one has to consider the endogenous glial progenitors expressing neural/glial antigen 2 (NG2), which proliferate following injury and differentiate into remyelinating cells273. This response is lasts for months after SCI274. To enhance remyelination through NPC transplantation, preclinical studies have been focused on the use of oligodendrocyte progenitor cells (OPCs), which can be isolated from the CNS or derived from embryonic stem cells and induced pluripotent stem cells. One important study269 examined transplantation of human embryonic stem cell-derived OPCs into a thoracic contusion in rats. The transplanted cells survived, redistributed themselves over short distances and differentiated into oligodendrocytes. Rats that received OPCs 7 days after injury exhibited enhanced remyelination and improved locomotor ability. By contrast, when OPCs were transplanted 10 months after injury, there was no enhanced remyelination or locomotor recovery. However, it is important to note that the role of remyelination in promoting functional improvements was not directly tested in these studies. A second study was conducted to support the clinical use of OPC therapy for cervical injuries150, testing OPCs in a nude rat model of cervical SCI. The OPCs were found to significantly improve locomotor performance when administered directly into the cervical spinal cord 1 week after injury, and the functional improvement was associated with reduced cavitation and increased sparing of myelinated axons within the injury site. The study also showed that OPC migration is limited to the spinal cord and brainstem and did not cause any adverse clinical observations.
Establish and maintain functional synaptic connections.
To provide compelling evidence of connectivity through functional synapses, a multilevel approach is required. Immunohistochemistry is often used to show expression of presynaptic and postsynaptic proteins. Electron microscopy provides ultrastructural evidence for the formation of the postsynaptic density and can be combined with immuno-electron microscopy to identify the transplant-derived cell through the expression of reporters. However, synapse assembly and maturation are complex multistep processes that ultimately result in stable and functional synapses capable of transporting synaptic vesicles and expressing proper receptors and transporter proteins187. An indirect method to assess the presence of stable and mature synapses is to measure the transneuronal spread of neurotropic viruses188,189. However, the most direct evidence of functional connectivity is generated by electrophysiological methods to show the transmission of signals through the putative relay, which can provide information on the formation and strength of monosynaptic and multisynaptic connections16,17,25,42. Improved knowledge of synaptic plasticity during development and adulthood suggests new strategies for transforming the initial connection mediated by axon regeneration and relay formation into stable functional synaptic connections175. Thus, targeted efforts may be needed to promote synaptic maturation and facilitate stability and function in an activity-dependent manner so as to train new circuits to generate meaningful function.
Identify the best neurons for improving connectivity.
There are a variety of spinal interneurons with different physiological and functional properties, which are often identified by unique developmental transcription factor expression profiles190,191. These cells modulate motor neuron activity, relay information along spinal cord segments and to the contralateral spinal cord and play an important role in neuroplasticity following SCI118,192. However, the spinal cord-derived NPCs used in most transplantation studies contain diverse populations of interneuron progenitors whose composition may change during culturing and expansion relative to the original fetal tissue22. It is now possible to direct the acquisition of specific phenotypic fates, either through directed differentiation of pluripotent cells193–196 or by isolating NPCs from appropriate regions of the developing nervous system15,42,44,136. Building on the knowledge of interneuron diversity and evidence for plasticity within spinal networks, several studies have begun to explore which of these neurons contribute to plasticity and might show therapeutic advantages. In the respiratory network, excitatory premotor V2a spinal interneurons have been shown to contribute to anatomical phrenic plasticity after cervical SCI197 and transplants enriched with these cells improve functional recovery22. In parallel, transplantation of inhibitory (GABAergic) interneurons has been shown to attenuate pain-associated behaviours following SCI142,198–200. Aside from transplantation of interneurons, there is growing interest in spinal cord motor neuron replacement strategies (reviewed in REF.58). However, survival of transplanted postmitotic motor neurons remains poor, and promoting differentiation of NPCs into motor neurons is difficult in the harsh lesion environment58.
With advances in cellular engineering, and a better understanding of the neuronal and glial components that contribute to plasticity, future work can tailor cell therapies to progenitors that can be most beneficial for survival, specificity of connectivity, network function and recovery. Matching of neuronal phenotypes in the relay is important to ensure the fidelity of the transmitted signal, including the timing and pattern of the activity, particularly for skilled voluntary motor abilities and discriminative touch54. It is likely that tailored cell therapies will eventually be combined with other treatments such as gene therapy, neural stimulation or activity-based therapies that can better direct growth and strengthen synaptic connectivity for lasting functional recovery.
Guidance of transplanted cells to appropriate targets.
Early studies were focused on host axonal growth into the graft and non-specific synaptic formation101. More recent studies have explored the use of transplants with regional specificity. For example, homologous reconstitution of the lesioned adult spinal cord with caudalized NSCs or primary spinal cord-derived NPCs supports robust regeneration of corticospinal axons, which form functional excitatory synapses with the neural replacement graft42. Similarly, it was demonstrated that sensory axons regenerate into appropriate organotypic domains of neural progenitor grafts (dorsal horn-like domains) and that these domains are avoided by regenerating corticospinal axons44. This indicates that injured adult axons retain the ability to recognize appropriate and inappropriate targets, which is conducive to restoration of circuitry. On the distal side of the relay, robust growth of axons from NPC transplants into the host spinal cord has been identified to originate from neurons located in caudal regions of the transplant201. Thus, in the absence of specific directional guidance, these neurons are most likely to extend axons caudally201, which raises the possibility that they might make maladaptive connections and highlights the need for directional and guided growth. Indeed, one study showed that intraspinal NSC grafts caused aberrant sprouting, resulting in allodynia181. To address this issue, some studies have used a neurotrophic gradient to promote and guide graft-derived axons towards the putative target16. The use of NPCs, which are composed of both neuronal and glial progenitors, may also allow guided glial progenitor cell migration from the injury and/or transplant site, creating a permissive environment for axon growth202. However, it is also possible that transplants with regional specificity may retain their ability to project towards the appropriate targets and establish area-specific circuits (as has been shown in brain transplants203–205).
Integrating with adaptive plasticity to restore mapping and function.
Assuming that connectivity can be restored sufficiently to provide a relay that forms stable synapses, two major issues remain. The first is the requirement to form faithful maps and the other is the need to retrain the new circuits to support meaningful function. In the example of relay formation in the sensory system following the axotomy of the dorsal column tract, there is good anatomical and physiological evidence for restored somatotopic connectivity with the DCN16. However, even if a sufficient number of axons reach the putative target, it may be realistically impossible to restore the original specificity of the spatial connectivity generated during development206. This is likely to degrade the quality of the sensory information from the skin unless regenerating sensory axons can retain the ability to recognize appropriate postsynaptic partners. Furthermore, SCI initiates a reorganization process within the brain that can involve substantial cortical remapping as a result of sensory or motor deficits207–210. NPC transplants that promote connectivity must therefore restore some of the ‘lost’ cortical mapping. Given that cortical reorganization is a dynamic process, it is hoped that it can be refined and reshaped by retraining the new circuits during activity. Inducing neuronal activity could also be used to promote the remyelination and functionality of the axons211.
Spontaneous plasticity within the adult nervous system following injury suggests that transplantation strategies may also rely on the ability of grafts to support functional integration with spared and reorganized host circuits. For example, plasticity in the propriospinal system allows recovery of some locomotor function after injury212, and synaptic integration of NPC grafts with spared host propriospinal neurons was shown to be necessary for improved locomotor outcomes19. Likewise, injury to the corticospinal system induces sprouting of spared as well as injured CST axons210. With use of a selective chemogenetic silencing approach, it was demonstrated that a small number of spared (dorsolateral) corticospinal axons can mediate spontaneous functional recovery of skilled locomotion after axotomy of the dorsal CST213. In light of the findings that NPC grafts support recovery of CST-mediated motor function following axotomy of dorsal and dorsolateral CST fibres42, it is possible that graft integration with spared as well as regenerating fibres may be a potent therapeutic target for relay formation in transplantation studies.
Avoiding maladaptive connectivity.
Plasticity within the injured nervous system can be adaptive, but growth and reorganization of other pathways can lead to maladaptive outcomes. For example, plasticity within nociceptive systems early after SCI has been shown to contribute to long-term functional deficits such as hypersensitivity of nociceptive signalling and inhibition of locomotor recovery214–216. A question of high clinical relevance, therefore, is whether NPC grafts pose the risk of enhancing maladaptive plasticity in the injured spinal cord. One concern is that NPC grafts can promote sprouting of nociceptive afferent fibres into both the host spinal cord and the graft tissue following transplantation44,171,217. In one study, transplanted cells failed to promote locomotor improvement, and instead caused thermal hyperalgesia and mechanical allodynia of the forepaws that was associated with sprouting of calcitonin gene-related peptide-expressing fibres into the spinal cord dorsal horn rostral to the site of injury218. Notably, the grafted cells differentiated primarily into astrocytes, suggesting that if they are transplanted into an inappropriate injury or at the wrong time after injury, graft-derived astroglia may inadvertently promote pain-associated outcomes. Similarly, another study showed that naive primary FSC cells differentiated mostly to astrocytes after transplantation and produced allodynia181. In this study, primary FSC cells transduced with the neurogenic transcription factor neurogenin 2 gave rise to grafts containing significantly greater numbers of neurons and fewer astrocytes. Neurogenin 2-expressing grafts reduced sprouting of nociceptive fibres, attenuated allodynia and promoted enhanced motor recovery181. Together, these findings suggest a potential role of transplanted astrocytes in the development of pain-like states through plasticity of nociceptive systems, potentially occurring through the secretion of astrocyte-derived growth factors that promote sprouting of nociceptive axons217,218. However, not all neural grafts have been shown to have maladaptive effects, and graft-derived astrocytes are also known to have critical roles in producing a permissive microenvironment conducive to graft survival and host regeneration16,41 and attenuating sensory dysfunction219 as well as spasticity219,220. This highlights a critical need to gain more mechanistic understanding of how specific graft components and distinct types of astrocytes influence the growth of host systems.
Efficacy in large-animal models.
Although most experimental SCI studies have used rodents, large-animal models are key in the translation to human trials221 because they are able to better match body size, neuroanatomy, immunology and complexity of neurological functions222. Moreover, differences in spinal cord lesion size and anatomy pose a special consideration in the ‘scaling up’ of cell transplantation therapies. Whereas the rodent spinal cord lesion site spans a few millimetres, human injuries are typically centimetres in length, requiring longer-distance axon growth for neural relay formation. A recent study reported the successful transplantation of allogeneic iPS cells into a porcine SCI model223 and demonstrated long-term survival of grafts in 2–3-cm-long lesion sites. The transplanted iPS cells differentiated into glial cells and neurons and produced a modest improvement in motor function. Non-human-primate SCI models offer a further advantage to examine functional efficacy due to their high neuroanatomical and functional similarity to humans. Important studies have used marmoset models of SCI224–226 and demonstrated that transplantation of allogeneic ES cell-derived NPCs resulted in remyelination of host axons by graft-derived oligodendrocytes, synaptic connectivity between host and graft-derived neurons and recovery of both locomotion and forelimb function226. Marmoset spinal cord anatomy, however, is much smaller than that of humans, underscoring the importance of recent studies showing functional efficacy with human FSC-derived NPCs transplanted into sites of cervical spinal cord hemisection in macaque primates spanning approximately 5 mm (REF.122). These grafts extended hundreds of thousands of axons rostrally and caudally from the lesion site, formed synapses with host neurons, supported regeneration of host corticospinal axons and promoted recovery of forelimb motor function. While these findings lend hope to the potential clinical efficacy of transplanted NPCs, large-animal and primate studies are costly and time-intensive and are likely to be used mostly for verification of data obtained with rodents.
Future perspectives
A haunting issue, which is rarely addressed, is that preclinical experiments are designed to use precise injury models with precise matching of animals and protocols between studies to obtain reproducible data. By contrast, the clinical reality is that there is considerable variability in injury location and severity, the time of treatment after injury and the types of treatment provided at early stages. While the study of variability between males and females, now mandated by the NIH227, is being addressed preclinically, getting robust data across all of the sources of variability found in the clinic remains a challenge. Part of the solution may be to demonstrate the efficacy of any potential therapeutic approach in a range of injuries and animal models. One potential approach may be to evaluate transplantation efficacy in canines with naturally occurring canine SCI. This large-animal model offers unique parallels to the human SCI population with diverse genetic backgrounds, heterogeneous location and severity of SCI, and anatomical similarity to the human contusion–compression lesion228. The high prevalence of naturally occurring SCI in dogs also allows the long-term evaluation of clinically relevant outcomes in large cohorts, using surgical and imaging techniques identical to those used in the clinic228. Furthermore, there is an urgent need to align the injury assessment criteria for therapeutic efficacy in animal behaviour tests with the neurological examinations following SCI that will be acceptable across clinical trials. There has been promising progress in improved clinical evaluation over the American Spinal Injury Association impairment scale229,230 and innovative assessment related to quality of life231.
The limitations of the rodent model and the need for additional and appropriate animal models to address both the gap in the size between rodents and humans and differences in the neuroanatomy (particularly for the CST) has been recognized with significant advances in porcine and primate work, which need to continue. Another encouraging development is the emphasis of patient priorities for therapies with growing research using NPC transplants to address autonomic function, bladder control and respiration. This is likely to expand to consider the consequences of SCI on multiple organs of the body.
As most clinical translation involves allogeneic transplants, patients will have to be immunosuppressed, resulting in increased risks of infection, malignancy and other side effects. Allografts can be tested in animals only with cells from their own species, and testing human cell transplants therefore presents the conundrum of a xenograft model, a gap that might be minimized with use of primates. Use of iPS cells allows autologous grafting, but the complexity of the procedures, their cost and the need for screening of genetic stability are still major obstacles. Nevertheless, clinical trials with pluripotent cells are in the pipeline, and creative solutions include banking of iPS cell lines that are HLA-matched to large sections of the population and therefore will be less likely to require immunosuppression91.
Concern has been raised about the migration of transplanted cells into other areas of the CNS forming ectopic aggregates that can induce abnormal activities232. It is possible that these concerns might be mitigated by the use of scaffolds to minimize migration and provide a stable local matrix as demonstrated in primate transplantation122. Similarly, the development of pluripotent cell lines and differentiation protocols may provide better control and greater efficiency in deriving cells with specific phenotypes but presents risks of tumour formation.
Although many NPC transplants are designed for cell replacement and restoration of connectivity in SCI, it is recognized that functional improvement may be the result of other mechanisms, such as neuromodulation, neuroprotection, synaptic reorganization, improved angiogenesis or remyelination (or a combination of these factors), which need to be considered in the design and interpretation of clinical trials92. The advantages of multifunctional NPC transplants pose a challenge to our mechanistic understanding, but as our understanding improves, we will be able to use more effectively advances in engineering to direct donor cells to treat specific systems in a targeted fashion.
Combination therapy may still be required with NPC transplants. This might include, for example, exercise or activity protocols to strengthen synaptic connections and promote plasticity, drug therapy at the acute phase to reduce secondary damage and the inclusion of scaffolds.
Despite representing the majority of patients, chronic SCI is still understudied and remains more of a challenge than the acute/subacute injury. Nevertheless, clinical trials — including those initiated by Neuralstem, NeuroRegen and Novagenesis — are moving towards treatments for chronic SCI233–236 (see Supplementary Table 1).
Several other practical difficulties face cell therapy, including the complex and expensive production of cells, their cryopreservation, delivery protocols and the relatively small population of eligible patients. The FDA and other regulatory bodies have until recently had limited experience with cell therapy; however, the growing number of approved clinical trials with strong evidence of safety and the recent guidelines from the International Campaign for Cures of Spinal Cord Injury Paralysis237 are paving the way for a more clearly defined process for future therapies.
Supplementary Material
Acknowledgements
The authors thank J. Houle for helpful suggestions and reviewing the manuscript, S. Hou for help with the autonomic function section, A. Lepore for reading the respiratory section, J. Bouyer for help in preparation of figures and E. Wirth III for comments on clinical trials. The authors’ work has been supported by NIH grant 2PO1 NS055976, the Craig H. Neilsen Foundation and a Louis and Bessie Stein Family grant (I.F.); Mission Connect (a project of the TIRR Foundation), the Craig H. Neilsen Foundation and the Paralyzed Veterans of America Research Foundation (J.N.D.); and the Lisa Dean Moseley Foundation, Wings for Life Spinal Cord Research Foundation, and NIH grant R01 NS104291(M.A.L.).
Footnotes
Competing interests
The authors declare no competing interests.
Supplementary information
Supplementary information is available for this paper at https://doi.org/10.1038/s41583-020-0314-2.
References
- 1.Ahuja CS et al. Traumatic spinal cord injury. Nat. Rev. Dis. Prim 3, 17018 (2017). [DOI] [PubMed] [Google Scholar]
- 2.Mautes AE, Weinzierl MR, Donovan F & Noble LJ Vascular events after spinal cord injury: contribution to secondary pathogenesis. Phys. Ther 80, 673–687 (2000). [PubMed] [Google Scholar]
- 3.Beattie MS Inflammation and apoptosis: linked therapeutic targets in spinal cord injury. Trends Mol. Med 10, 580–583 (2004). [DOI] [PubMed] [Google Scholar]
- 4.Donnelly DJ & Popovich PG Inflammation and its role in neuroprotection, axonal regeneration and functional recovery after spinal cord injury. Exp. Neurol 209, 378–388 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jia Z et al. Oxidative stress in spinal cord injury and antioxidant-based intervention. Spinal Cord 50, 264–274 (2012). [DOI] [PubMed] [Google Scholar]
- 6.Hilton BJ, Moulson AJ & Tetzlaff W Neuroprotection and secondary damage following spinal cord injury: concepts and methods. Neurosci. Lett 652, 3–10 (2017). [DOI] [PubMed] [Google Scholar]
- 7.Fitch MT & Silver J CNS injury, glial scars, and inflammation: Inhibitory extracellular matrices and regeneration failure. Exp. Neurol 209, 294–301 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Schwab JM, Zhang Y, Kopp MA, Brommer B & Popovich PG The paradox of chronic neuroinflammation, systemic immune suppression, autoimmunity after traumatic chronic spinal cord injury. Exp. Neurol 258, 121–129 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dulin JN & Lu P Bridging the injured spinal cord with neural stem cells. Neural Regen. Res 9, 229–231 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Papastefanaki F & Matsas R From demyelination to remyelination: the road toward therapies for spinal cord injury. Glia 63, 1101–1125 (2015). [DOI] [PubMed] [Google Scholar]
- 11.Hollis ER 2nd. Axon guidance molecules and neural circuit remodeling after spinal cord injury. Neurotherapeutics 13, 360–369 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hilton BJ & Bradke F Can injured adult CNS axons regenerate by recapitulating development? Development 144, 3417–3429 (2017). [DOI] [PubMed] [Google Scholar]
- 13.Assinck P, Duncan GJ, Hilton BJ, Plemel JR & Tetzlaff W Cell transplantation therapy for spinal cord injury. Nat. Neurosci 20, 637–647 (2017). [DOI] [PubMed] [Google Scholar]
- 14.Lane MA, Lepore AC & Fischer I Improving the therapeutic efficacy of neural progenitor cell transplantation following spinal cord injury. Expert Rev. Neurother 17, 433–440 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.White TE et al. Neuronal progenitor transplantation and respiratory outcomes following upper cervical spinal cord injury in adult rats. Exp. Neurol 225, 231–236 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bonner JF et al. Grafted neural progenitors integrate and restore synaptic connectivity across the injured spinal cord. J. Neurosci 31, 4675–4686 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lu P et al. Long-distance growth and connectivity of neural stem cells after severe spinal cord injury. Cell 150, 1264–1273 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lee KZ et al. Intraspinal transplantation and modulation of donor neuron electrophysiological activity. Exp. Neurol 251, 47–57 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yokota K et al. Engrafted neural stem/progenitor cells promote functional recovery through synapse reorganization with spared host neurons after spinal cord injury. Stem Cell Rep. 5, 264–277 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dougherty BJ et al. Respiratory outcomes after mid-cervical transplantation of embryonic medullary cells in rats with cervical spinal cord injury. Exp. Neurol 278, 22–26 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Adler AF, Lee-Kubli C, Kumamaru H, Kadoya K & Tuszynski MH Comprehensive monosynaptic rabies virus mapping of host connectivity with neural progenitor grafts after spinal cord injury. Stem Cell Rep. 8, 1525–1533 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zholudeva LV et al. Transplantation of neural progenitors and V2a Interneurons after spinal cord injury. J. Neurotrauma 35, 2883–2903 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Spruance VM et al. Integration of transplanted neural precursors with the injured cervical spinal cord. J. Neurotrauma 35, 1781–1799 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ceto S, Sekiguchi KJ, Takashima Y, Nimmerjahn A & Tuszynski MH Calcium imaging reveals host-graft synaptic network formation in spinal cord injury. Preprint at 10.1101/795583 (2019). [DOI] [Google Scholar]
- 25.Koffler J et al. Biomimetic 3D-printed scaffolds for spinal cord injury repair. Nat. Med 25, 263–269 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.McDonald JW et al. Transplanted embryonic stem cells survive, differentiate and promote recovery in injured rat spinal cord. Nat. Med 5, 1410–1412 (1999). [DOI] [PubMed] [Google Scholar]
- 27.Lepore AC et al. Differential fate of multipotent and lineage-restricted neural precursors following transplantation into the adult CNS. Neuron Glia Biol. 1, 113–126 (2004). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Watanabe K et al. Comparison between fetal spinal-cord- and forebrain-derived neural stem/progenitor cells as a source of transplantation for spinal cord injury. Dev. Neurosci 26, 275–287 (2004). [DOI] [PubMed] [Google Scholar]
- 29.Lepore AC & Fischer I Lineage-restricted neural precursors survive, migrate, and differentiate following transplantation into the injured adult spinal cord. Exp. Neurol 194, 230–242 (2005). [DOI] [PubMed] [Google Scholar]
- 30.Lepore AC et al. Long-term fate of neural precursor cells following transplantation into developing and adult CNS. Neuroscience 139, 513–530 (2006). [DOI] [PubMed] [Google Scholar]
- 31.Medalha CC, Jin Y, Yamagami T, Haas C & Fischer I Transplanting neural progenitors into a complete transection model of spinal cord injury. J. Neurosci. Res 92, 607–618 (2014). [DOI] [PubMed] [Google Scholar]
- 32.Lien BV, Tuszynski MH & Lu P Astrocytes migrate from human neural stem cell grafts and functionally integrate into the injured rat spinal cord. Exp. Neurol 314, 46–57 (2019). [DOI] [PubMed] [Google Scholar]
- 33.Reier PJ, Bregman BS & Wujek JR Intraspinal transplantation of embryonic spinal cord tissue in neonatal and adult rats. J. Comp. Neurol 247, 275–296 (1986). [DOI] [PubMed] [Google Scholar]
- 34.Bregman BS Spinal cord transplants permit the growth of serotonergic axons across the site of neonatal spinal cord transection. Brain Res. 431, 265–279 (1987). [DOI] [PubMed] [Google Scholar]
- 35.Tessler A, Himes BT, Houle J & Reier PJ Regeneration of adult dorsal root axons into transplants of embryonic spinal cord. J. Comp. Neurol 270, 537–548 (1988). [DOI] [PubMed] [Google Scholar]
- 36.Houle JD & Reier PJ Regrowth of calcitonin gene-related peptide (CGRP) immunoreactive axons from the chronically injured rat spinal cord into fetal spinal cord tissue transplants. Neurosci. Lett 103, 253–258 (1989). [DOI] [PubMed] [Google Scholar]
- 37.Itoh Y & Tessler A Regeneration of adult dorsal root axons into transplants of fetal spinal cord and brain: a comparison of growth and synapse formation in appropriate and inappropriate targets. J. Comp. Neurol 302, 272–293 (1990). [DOI] [PubMed] [Google Scholar]
- 38.Jakeman LB & Reier PJ Axonal projections between fetal spinal cord transplants and the adult rat spinal cord: a neuroanatomical tracing study of local interactions. J. Comp. Neurol 307, 311–334 (1991). [DOI] [PubMed] [Google Scholar]
- 39.Itoh Y, Sugawara T, Kowada M & Tessler A Time course of dorsal root axon regeneration into transplants of fetal spinal cord: I. A light microscopic study. J. Comp. Neurol 323, 198–208 (1992). [DOI] [PubMed] [Google Scholar]
- 40.Haas C, Neuhuber B, Yamagami T, Rao M & Fischer I Phenotypic analysis of astrocytes derived from glial restricted precursors and their impact on axon regeneration. Exp. Neurol 233, 717–732 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Haas C & Fischer I Human astrocytes derived from glial restricted progenitors support regeneration of the injured spinal cord. J. Neurotrauma 30, 1035–1052 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kadoya K et al. Spinal cord reconstitution with homologous neural grafts enables robust corticospinal regeneration. Nat. Med 22, 479–487 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Merianda TT et al. Neural progenitor cells promote axonal growth and alter axonal mRNA localization in adult neurons. eNeuro 10.1523/ENEURO.0171-16.2017 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Dulin JN et al. Injured adult motor and sensory axons regenerate into appropriate organotypic domains of neural progenitor grafts. Nat. Commun 9, 84 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Jin Y, Shumsky JS & Fischer I Axonal regeneration of different tracts following transplants of human glial restricted progenitors into the injured spinal cord in rats. Brain Res. 1686, 101–112 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Cao Q et al. Functional recovery in traumatic spinal cord injury after transplantation of multineurotrophin-expressing glial-restricted precursor cells. J. Neurosci 25, 6947–6957 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Hwang DH et al. Transplantation of human neural stem cells transduced with Olig2 transcription factor improves locomotor recovery and enhances myelination in the white matter of rat spinal cord following contusive injury. BMC Neurosci. 10, 117 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Yasuda A et al. Significance of remyelination by neural stem/progenitor cells transplanted into the injured spinal cord. Stem Cells 29, 1983–1994 (2011). [DOI] [PubMed] [Google Scholar]
- 49.Hawryluk GW et al. An examination of the mechanisms by which neural precursors augment recovery following spinal cord injury: a key role for remyelination. Cell Transpl. 23, 365–380 (2014). [DOI] [PubMed] [Google Scholar]
- 50.Park KI et al. Neural stem cells may be uniquely suited for combined gene therapy and cell replacement: Evidence from engraftment of neurotrophin-3-expressing stem cells in hypoxic-ischemic brain injury. Exp. Neurol 199, 179–190 (2006). [DOI] [PubMed] [Google Scholar]
- 51.Cusimano M et al. Transplanted neural stem/precursor cells instruct phagocytes and reduce secondary tissue damage in the injured spinal cord. Brain 135, 447–460 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Karova K et al. Transplantation of neural precursors generated from spinal progenitor cells reduces inflammation in spinal cord injury via NF-kappaB pathway inhibition. J. Neuroinflammation 16, 12 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Houle JD & Reier PJ Transplantation of fetal spinal cord tissue into the chronically injured adult rat spinal cord. J. Comp. Neurol 269, 535–547 (1988). [DOI] [PubMed] [Google Scholar]
- 54.Bonner JF & Steward O Repair of spinal cord injury with neuronal relays: from fetal grafts to neural stem cells. Brain Res. 1619, 115–123 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Falnikar A, Li K & Lepore AC Therapeutically targeting astrocytes with stem and progenitor cell transplantation following traumatic spinal cord injury. Brain Res. 1619, 91–103 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Yousefifard M et al. Neural stem/progenitor cell transplantation for spinal cord injury treatment; A systematic review and meta-analysis. Neuroscience 322, 377–397 (2016). [DOI] [PubMed] [Google Scholar]
- 57.Zhu Y, Uezono N, Yasui T & Nakashima K Neural stem cell therapy aiming at better functional recovery after spinal cord injury. Dev. Dyn 247, 75–84 (2018). [DOI] [PubMed] [Google Scholar]
- 58.Trawczynski M, Liu G, David BT & Fessler RG Restoring motor neurons in spinal cord injury with induced pluripotent stem cells. Front. Cell Neurosci 13, 369 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Nagoshi N, Tsuji O, Nakamura M & Okano H Cell therapy for spinal cord injury using induced pluripotent stem cells. Regen. Ther 11, 75–80 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Katoh H, Yokota K & Fehlings MG Regeneration of spinal cord connectivity through stem cell transplantation and biomaterial scaffolds. Front. Cell Neurosci 13, 248 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Gash DM in Neural Transplants: Development and Function (eds Sladek JR Jr. & Gash DM) Ch. 1, 1–12 (Plenum Press, 1984). [Google Scholar]
- 62.Stein DG Fetal brain tissue grafting as therapy for brain dysfunctions: unanswered questions, unknown factors, and practical concerns. J. Neurosurg. Anesthesiol 3, 170–189 (1991). [DOI] [PubMed] [Google Scholar]
- 63.Thompson WG Successful brain grafting. Science 16, 78–79 (1890). [DOI] [PubMed] [Google Scholar]
- 64.Dunn EH Primary and secondary findings in a series of attempts to transplant cerebral cortex in the albino rat. J. Comp. Neurol 27, 565–582 (1917). [Google Scholar]
- 65.Tello JF La influencia del neurotropismo en la generacion de los centros nervioso. Trab. Lab. Invest. Biol 9, 123–159 (1911). [Google Scholar]
- 66.David S & Aguayo AJ Axonal elongation into peripheral nervous system “bridges” after central nervous system injury in adult rats. Science 214, 931–933 (1981). [DOI] [PubMed] [Google Scholar]
- 67.Hodges CV, Pickering DE, Murray JE & Goodwin WE Kidney transplant between identical twins. J. Urol 89, 115–121 (1963). [DOI] [PubMed] [Google Scholar]
- 68.Ishii T & Eto K Fetal stem cell transplantation: past, present, and future. World J. Stem Cells 6, 404–420 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Bjorklund A & Stenevi U Reconstruction of the nigrostriatal dopamine pathway by intracerebral nigral transplants. Brain Res. 177, 555–560 (1979). [DOI] [PubMed] [Google Scholar]
- 70.Perlow MJ et al. Brain grafts reduce motor abnormalities produced by destruction of nigrostriatal dopamine system. Science 204, 643–647 (1979). [DOI] [PubMed] [Google Scholar]
- 71.Brundin P et al. Human fetal dopamine neurons grafted in a rat model of Parkinson’s disease: immunological aspects, spontaneous and drug-induced behaviour, and dopamine release. Exp. Brain Res 70, 192–208 (1988). [DOI] [PubMed] [Google Scholar]
- 72.Lindvall O et al. Human fetal dopamine neurons grafted into the striatum in two patients with severe Parkinson’s disease. A detailed account of methodology and a 6-month follow-up. Arch. Neurol 46, 615–631 (1989). [DOI] [PubMed] [Google Scholar]
- 73.Lindvall O Update on fetal transplantation: the Swedish experience. Mov. Disord 13(Suppl 1), 83–87 (1998). [PubMed] [Google Scholar]
- 74.Barker RA, Barrett J, Mason SL & Bjorklund A Fetal dopaminergic transplantation trials and the future of neural grafting in Parkinson’s disease. Lancet Neurol. 12, 84–91 (2013). [DOI] [PubMed] [Google Scholar]
- 75.Gonzalez C, Bonilla S, Flores AI, Cano E & Liste I An update on human stem cell-based therapy in Parkinson’s disease. Curr. Stem Cell Res. Ther 11, 561–568 (2016). [DOI] [PubMed] [Google Scholar]
- 76.Reier PJ Neural tissue grafts and repair of the injured spinal cord. Neuropathol. Appl. Neurobiol 11, 81–104 (1985). [DOI] [PubMed] [Google Scholar]
- 77.Reier PJ, Houle JD, Jakeman L, Winialski D & Tessler A Transplantation of fetal spinal cord tissue into acute and chronic hemisection and contusion lesions of the adult rat spinal cord. Prog. Brain Res 78, 173–179 (1988). [DOI] [PubMed] [Google Scholar]
- 78.Jakeman LB et al. Differentiation of substantia gelatinosa-like regions in intraspinal and intracerebral transplants of embryonic spinal cord tissue in the rat. Exp. Neurol 103, 17–33 (1989). [DOI] [PubMed] [Google Scholar]
- 79.Bregman BS et al. Recovery of function after spinal cord injury: mechanisms underlying transplant-mediated recovery of function differ after spinal cord injury in newborn and adult rats. Exp. Neurol 123, 3–16 (1993). [DOI] [PubMed] [Google Scholar]
- 80.Mayer-Proschel M, Kalyani AJ, Mujtaba T & Rao MS Isolation of lineage-restricted neuronal precursors from multipotent neuroepithelial stem cells. Neuron 19, 773–785 (1997). [DOI] [PubMed] [Google Scholar]
- 81.Kalyani AJ, Piper D, Mujtaba T, Lucero MT & Rao MS Spinal cord neuronal precursors generate multiple neuronal phenotypes in culture. J. Neurosci 18, 7856–7868 (1998). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Rao MS & Mayer-Proschel M Glial-restricted precursors are derived from multipotent neuroepithelial stem cells. Dev. Biol 188, 48–63 (1997). [DOI] [PubMed] [Google Scholar]
- 83.Bonner JF, Haas CJ & Fischer I Preparation of neural stem cells and progenitors: neuronal production and grafting applications. Methods Mol. Biol 1078, 65–88 (2013). [DOI] [PubMed] [Google Scholar]
- 84.Jessell TM Neuronal specification in the spinal cord: inductive signals and transcriptional codes. Nat. Rev. Genet 1, 20–29 (2000). [DOI] [PubMed] [Google Scholar]
- 85.Lu DC, Niu T & Alaynick WA Molecular and cellular development of spinal cord locomotor circuitry. Front. Mol. Neurosci 8, 25 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Weiss S et al. Multipotent CNS stem cells are present in the adult mammalian spinal cord and ventricular neuroaxis. J. Neurosci 16, 7599–7609 (1996). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Stenudd M, Sabelstrom H & Frisen J Role of endogenous neural stem cells in spinal cord injury and repair. JAMA Neurol. 72, 235–237 (2015). [DOI] [PubMed] [Google Scholar]
- 88.Mothe AJ, Zahir T, Santaguida C, Cook D & Tator CH Neural stem/progenitor cells from the adult human spinal cord are multipotent and self-renewing and differentiate after transplantation. PLoS One 6, e27079 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Goulao M & Lepore AC iPS cell transplantation for traumatic spinal cord injury. Curr. Stem Cell Res. Ther 11, 321–328 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.White N & Sakiyama-Elbert SE Derivation of specific neural populations from pluripotent cells for understanding and treatment of spinal cord injury. Dev. Dyn 248, 78–87 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Tsuji O et al. Concise review: laying the groundwork for a first-in-human study of an induced pluripotent stem cell-based intervention for spinal cord injury. Stem Cells 37, 6–13 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Ulndreaj A, Badner A & Fehlings MG Promising neuroprotective strategies for traumatic spinal cord injury with a focus on the differential effects among anatomical levels of injury. F1000Res 6, 1907 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Zukor K et al. Short hairpin RNA against PTEN enhances regenerative growth of corticospinal tract axons after spinal cord injury. J. Neurosci 33, 15350–15361 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Liu X, Williams PR & He Z SOCS3: a common target for neuronal protection and axon regeneration after spinal cord injury. Exp. Neurol 263, 364–367 (2015). [DOI] [PubMed] [Google Scholar]
- 95.Tran AP, Warren PM & Silver J The biology of regeneration failure and success after spinal cord injury. Physiol. Rev 98, 881–917 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Jorge A, Taylor T, Agarwal N & Hamilton DK Current agents and related therapeutic targets for inflammation after acute traumatic spinal cord injury. World Neurosurg. 132, 138–147 (2019). [DOI] [PubMed] [Google Scholar]
- 97.Wang S, Smith GM, Selzer ME & Li S Emerging molecular therapeutic targets for spinal cord injury. Expert. Opin. Ther. Targets 23, 787–803 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Rejc E, Angeli CA, Atkinson D & Harkema SJ Motor recovery after activity-based training with spinal cord epidural stimulation in a chronic motor complete paraplegic. Sci. Rep 7, 13476 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Attwell CL, van Zwieten M, Verhaagen J & Mason MRJ The dorsal column lesion model of spinal cord injury and its use in deciphering the neuron-intrinsic injury response. Dev. Neurobiol 78, 926–951 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Han SS, Kang DY, Mujtaba T, Rao MS & Fischer I Grafted lineage-restricted precursors differentiate exclusively into neurons in the adult spinal cord. Exp. Neurol 177, 360–375 (2002). [DOI] [PubMed] [Google Scholar]
- 101.Bonner JF, Blesch A, Neuhuber B & Fischer I Promoting directional axon growth from neural progenitors grafted into the injured spinal cord. J. Neurosci. Res 88, 1182–1192 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Ketschek AR, Haas C, Gallo G & Fischer I The roles of neuronal and glial precursors in overcoming chondroitin sulfate proteoglycan inhibition. Exp. Neurol 235, 627–637 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Hayakawa K, Haas C & Fischer I Examining the properties and therapeutic potential of glial restricted precursors in spinal cord injury. Neural Regen. Res 11, 529–533 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.McComish SF & Caldwell MA Generation of defined neural populations from pluripotent stem cells. Philos. Trans. R. Soc. Lond. B Biol. Sci 373, 20170214 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Khazaei M, Ahuja CS, Rodgers CE, Chan P & Fehlings MG Generation of definitive neural progenitor cells from human pluripotent stem cells for transplantation into spinal cord injury. Methods Mol. Biol 1919, 25–41 (2019). [DOI] [PubMed] [Google Scholar]
- 106.Dugan EA, Jergova S & Sagen J Mutually beneficial effects of intensive exercise and GABAergic neural progenitor cell transplants in reducing neuropathic pain and spinal pathology in rats with spinal cord injury. Exp. Neurol 327, 113208 (2020). [DOI] [PubMed] [Google Scholar]
- 107.Mothe AJ & Tator CH Advances in stem cell therapy for spinal cord injury. J. Clin. Invest 122, 3824–3834 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Tuszynski MH & Steward O Concepts and methods for the study of axonal regeneration in the CNS. Neuron 74, 777–791 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Antonic A et al. Stem cell transplantation in traumatic spinal cord injury: a systematic review and meta-analysis of animal studies. PLoS Biol. 11, e1001738 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Mothe AJ & Tator CH Review of transplantation of neural stem/progenitor cells for spinal cord injury. Int. J. Dev. Neurosci 31, 701–713 (2013). [DOI] [PubMed] [Google Scholar]
- 111.Deep A et al. Mouse models of spinal cord injury and stem cell transplantation. Transl. Res. Anat 1, 2–10 (2015). [Google Scholar]
- 112.Zholudeva LV & Lane MA Choosing the right cell for spinal cord repair. J. Neurosci. Res 97, 109–111 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Zholudeva LV & Lane MA Transplanting cells for spinal cord repair: who, what, when, where and why? Cell Transplant 28, 388–399 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Anderson AJ, Piltti KM, Hooshmand MJ, Nishi RA & Cummings BJ Preclinical efficacy failure of human CNS-derived stem cells for use in the pathway study of cervical spinal cord injury. Stem Cell Rep. 8, 249–263 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Sharp KG, Yee KM & Steward O A re-assessment of long distance growth and connectivity of neural stem cells after severe spinal cord injury. Exp. Neurol 257, 186–204 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Stokes BT & Reier PJ Fetal grafts alter chronic behavioral outcome after contusion damage to the adult rat spinal cord. Exp. Neurol 116, 1–12 (1992). [DOI] [PubMed] [Google Scholar]
- 117.Kiehn O Locomotor circuits in the mammalian spinal cord. Annu. Rev. Neurosci 29, 279–306 (2006). [DOI] [PubMed] [Google Scholar]
- 118.Courtine G et al. Recovery of supraspinal control of stepping via indirect propriospinal relay connections after spinal cord injury. Nat. Med 14, 69–74 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Wang X et al. Deconstruction of corticospinal circuits for goal-directed motor skills. Cell 171, 440–455 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Hayashi M et al. Graded arrays of spinal and supraspinal V2a interneuron subtypes underlie forelimb and hindlimb motor control. Neuron 97, 869–884 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Hunt M, Lu P & Tuszynski MH Myelination of axons emerging from neural progenitor grafts after spinal cord injury. Exp. Neurol 296, 69–73 (2017). [DOI] [PubMed] [Google Scholar]
- 122.Rosenzweig ES et al. Restorative effects of human neural stem cell grafts on the primate spinal cord. Nat. Med 24, 484–490 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Brown R, DiMarco AF, Hoit JD & Garshick E Respiratory dysfunction and management in spinal cord injury. Respir. Care 51, 853–868 (2006). [PMC free article] [PubMed] [Google Scholar]
- 124.Goulao M et al. Astrocyte progenitor transplantation promotes regeneration of bulbospinal respiratory axons, recovery of diaphragm function, and a reduced macrophage response following cervical spinal cord injury. Glia 67, 452–466 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Jin Y et al. Transplantation of human glial restricted progenitors and derived astrocytes into a contusion model of spinal cord injury. J. Neurotrauma 28, 579–594 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Li K et al. Human iPS cell-derived astrocyte transplants preserve respiratory function after spinal cord injury. Exp. Neurol 271, 479–492 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Gonzalez-Rothi EJ et al. Intermittent hypoxia and neurorehabilitation. J. Appl. Physiol 119, 1455–1465 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Reier PJ, Thompson FJ, Fessler R, Anderson DK & Wirth Iii ED in Axonal Regeneration in the Central Nervous System (eds Ingoglia NA & Murray M) Ch. 23, 603–648 (Marcel Dekker, 2001). [Google Scholar]
- 129.Lin CC, Lai SR, Shao YH, Chen CL & Lee KZ The therapeutic effectiveness of delayed fetal spinal cord tissue transplantation on respiratory function following mid-cervical spinal cord injury. Neurotherapeutics 14, 792–809 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Teasell RW, Arnold JM, Krassioukov A & Delaney GA Cardiovascular consequences of loss of supraspinal control of the sympathetic nervous system after spinal cord injury. Arch. Phys. Med. Rehabil 81, 506–516 (2000). [DOI] [PubMed] [Google Scholar]
- 131.Furlan JC, Fehlings MG, Shannon P, Norenberg MD & Krassioukov AV Descending vasomotor pathways in humans: correlation between axonal preservation and cardiovascular dysfunction after spinal cord injury. J. Neurotrauma 20, 1351–1363 (2003). [DOI] [PubMed] [Google Scholar]
- 132.Krassioukov A & Claydon VE The clinical problems in cardiovascular control following spinal cord injury: an overview. Prog. Brain Res 152, 223–229 (2006). [DOI] [PubMed] [Google Scholar]
- 133.Anderson KD Targeting recovery: priorities of the spinal cord-injured population. J. Neurotrauma 21, 1371–1383 (2004). [DOI] [PubMed] [Google Scholar]
- 134.Maiorov DN, Weaver LC & Krassioukov AV Relationship between sympathetic activity and arterial pressure in conscious spinal rats. Am. J. Physiol 272, H625–H631 (1997). [DOI] [PubMed] [Google Scholar]
- 135.Hou S, Lu P & Blesch A Characterization of supraspinal vasomotor pathways and autonomic dysreflexia after spinal cord injury in F344 rats. Auton. Neurosci 176, 54–63 (2013). [DOI] [PubMed] [Google Scholar]
- 136.Hou S, Tom VJ, Graham L, Lu P & Blesch A Partial restoration of cardiovascular function by embryonic neural stem cell grafts after complete spinal cord transection. J. Neurosci 33, 17138–17149 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Bader MS, Loeb M & Brooks AA An update on the management of urinary tract infections in the era of antimicrobial resistance. Postgrad. Med 129, 242–258 (2017). [DOI] [PubMed] [Google Scholar]
- 138.de Groat WC, Griffiths D & Yoshimura N Neural control of the lower urinary tract. Compr. Physiol 5, 327–396 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.de Groat WC Mechanisms underlying the recovery of lower urinary tract function following spinal cord injury. Paraplegia 33, 493–505 (1995). [DOI] [PubMed] [Google Scholar]
- 140.Zinck ND & Downie JW Plasticity in the injured spinal cord: can we use it to advantage to reestablish effective bladder voiding and continence? Prog. Brain Res 152, 147–162 (2006). [DOI] [PubMed] [Google Scholar]
- 141.Mitsui T, Shumsky JS, Lepore AC, Murray M & Fischer I Transplantation of neuronal and glial restricted precursors into contused spinal cord improves bladder and motor functions, decreases thermal hypersensitivity, and modifies intraspinal circuitry. J. Neurosci 25, 9624–9636 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Fandel TM et al. Transplanted human stem cell-derived interneuron precursors mitigate mouse bladder dysfunction and central neuropathic pain after spinal cord injury. Cell Stem Cell 19, 544–557 (2016). [DOI] [PubMed] [Google Scholar]
- 143.Lee YS et al. Nerve regeneration restores supraspinal control of bladder function after complete spinal cord injury. J. Neurosci 33, 10591–10606 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Falci S et al. Obliteration of a posttraumatic spinal cord cyst with solid human embryonic spinal cord grafts: first clinical attempt. J. Neurotrauma 14, 875–884 (1997). [DOI] [PubMed] [Google Scholar]
- 145.Thompson FJ et al. Neurophysiological assessment of the feasibility and safety of neural tissue transplantation in patients with syringomyelia. J. Neurotrauma 18, 931–945 (2001). [DOI] [PubMed] [Google Scholar]
- 146.Wirth ED 3rd et al. Feasibility and safety of neural tissue transplantation in patients with syringomyelia. J. Neurotrauma 18, 911–929 (2001). [DOI] [PubMed] [Google Scholar]
- 147.Anderson DK Neural tissue transplantation in syringomyelia: feasibility and safety. Ann. N. Y. Acad. Sci 961, 263–264 (2002). [DOI] [PubMed] [Google Scholar]
- 148.Shin JC et al. Clinical trial of human fetal brain-derived neural stem/progenitor cell transplantation in patients with traumatic cervical spinal cord injury. Neural Plast. 2015, 630932 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Priest CA, Manley NC, Denham J, Wirth ED 3rd & Lebkowski JS Preclinical safety of human embryonic stem cell-derived oligodendrocyte progenitors supporting clinical trials in spinal cord injury. Regen. Med 10, 939–958 (2015). [DOI] [PubMed] [Google Scholar]
- 150.Manley NC, Priest CA, Denham J, Wirth ED 3rd & Lebkowski JS Human embryonic stem cell-derived oligodendrocyte progenitor cells: preclinical efficacy and safety in cervical spinal cord injury. Stem Cell Transl. Med 6, 1917–1929 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Watzlawick R et al. Olfactory ensheathing cell transplantation in experimental spinal cord injury: effect size and reporting bias of 62 experimental treatments: a systematic review and meta-analysis. PLoS Biol. 14, e1002468 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Guest J, Santamaria AJ & Benavides FD Clinical translation of autologous Schwann cell transplantation for the treatment of spinal cord injury. Curr. Opin. Organ. Transpl 18, 682–689 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Anderson KD et al. Safety of autologous human schwann cell transplantation in subacute thoracic spinal cord injury. J. Neurotrauma 34, 2950–2963 (2017). [DOI] [PubMed] [Google Scholar]
- 154.Levi AD et al. Clinical outcomes from a multi-center study of human neural stem cell transplantation in chronic cervical spinal cord injury. J. Neurotrauma 36, 891–902 (2019). [DOI] [PubMed] [Google Scholar]
- 155.US National Library of Medicine. ClinicalTrials.gov, https://clinicaltrials.gov/ct2/show/NCT02302157 (2014) [DOI] [PubMed]
- 156.US National Library of Medicine. ClinicalTrials.gov, https://clinicaltrials.gov/ct2/show/NCT02163876 (2014) [DOI] [PubMed]
- 157.Goutman SA et al. Long-term Phase 1/2 intraspinal stem cell transplantation outcomes in ALS. Ann. Clin. Transl. Neurol 5, 730–740 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.US National Library of Medicine. ClinicalTrials.gov, https://clinicaltrials.gov/ct2/show/NCT01772810 (2013) [DOI] [PubMed]
- 159.Riley J et al. Cervical spinal cord therapeutics delivery: preclinical safety validation of a stabilized microinjection platform. Neurosurgery 65, 754–761 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Boulis N & Federici T Surgical approach and safety of spinal cord stem cell transplantation. Neurosurgery 68, E599–E600 (2011). [DOI] [PubMed] [Google Scholar]
- 161.Cefalo MG et al. Human iPSC for therapeutic approaches to the nervous system: present and future applications. Stem Cell Int. 2016, 4869071 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Doulames VM & Plant GW Induced pluripotent stem cell therapies for cervical spinal cord injury. Int. J. Mol. Sci 17, 530 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Khazaei M, Ahuja CS & Fehlings MG Generation of oligodendrogenic spinal neural progenitor cells from human induced pluripotent stem cells. Curr. Protoc. Stem Cell Biol 42, 2D.20.1–2D.20.14 (2017). [DOI] [PubMed] [Google Scholar]
- 164.Coumans JV et al. Axonal regeneration and functional recovery after complete spinal cord transection in rats by delayed treatment with transplants and neurotrophins. J. Neurosci 21, 9334–9344 (2001). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Lu P, Graham L, Wang Y, Wu D & Tuszynski M Promotion of survival and differentiation of neural stem cells with fibrin and growth factor cocktails after severe spinal cord injury. J. Vis. Exp 10.3791/50641 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Cao QL et al. Pluripotent stem cells engrafted into the normal or lesioned adult rat spinal cord are restricted to a glial lineage. Exp. Neurol 167, 48–58 (2001). [DOI] [PubMed] [Google Scholar]
- 167.Suzuki H et al. Neural stem cell mediated recovery is enhanced by chondroitinase ABC pretreatment in chronic cervical spinal cord injury. PLoS One 12, e0182339 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Chen J, Bernreuther C, Dihne M & Schachner M Cell adhesion molecule L1-transfected embryonic stem cells with enhanced survival support regrowth of corticospinal tract axons in mice after spinal cord injury. J. Neurotrauma 22, 896–906 (2005). [DOI] [PubMed] [Google Scholar]
- 169.Fan WL et al. Transplantation of hypoxic preconditioned neural stem cells benefits functional recovery via enhancing neurotrophic secretion after spinal cord injury in rats. J. Cell Biochem 119, 4339–4351 (2018). [DOI] [PubMed] [Google Scholar]
- 170.Wang ZZ & Sakiyama-Elbert SE Matrices, scaffolds & carriers for cell delivery in nerve regeneration. Exp. Neurol 319, 112837 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Kumamaru H, Lu P, Rosenzweig ES & Tuszynski MH Activation of intrinsic growth state enhances host axonal regeneration into neural progenitor cell grafts. Stem Cell Rep. 11, 861–868 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Steward O & Willenberg R Rodent spinal cord injury models for studies of axon regeneration. Exp. Neurol 287, 374–383 (2017). [DOI] [PubMed] [Google Scholar]
- 173.Mahar M & Cavalli V Intrinsic mechanisms of neuronal axon regeneration. Nat. Rev. Neurosci 19, 323–337 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Brown AR & Martinez M From cortex to cord: motor circuit plasticity after spinal cord injury. Neural Regen. Res 14, 2054–2062 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.O’Shea TM, Burda JE & Sofroniew MV Cell biology of spinal cord injury and repair. J. Clin. Invest 127, 3259–3270 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Bradbury EJ & Burnside ER Moving beyond the glial scar for spinal cord repair. Nat. Commun 10, 3879 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Dell’Anno MT & Strittmatter SM Rewiring the spinal cord: direct and indirect strategies. Neurosci. Lett 652, 25–34 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Sofroniew MV Dissecting spinal cord regeneration. Nature 557, 343–350 (2018). [DOI] [PubMed] [Google Scholar]
- 179.Anderson MA et al. Required growth facilitators propel axon regeneration across complete spinal cord injury. Nature 561, 396–400 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Geoffroy CG, Hilton BJ, Tetzlaff W & Zheng B Evidence for an age-dependent decline in axon regeneration in the adult mammalian central nervous system. Cell Rep. 15, 238–246 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Hofstetter CP et al. Allodynia limits the usefulness of intraspinal neural stem cell grafts; directed differentiation improves outcome. Nat. Neurosci 8, 346–353 (2005). [DOI] [PubMed] [Google Scholar]
- 182.Takeoka A et al. Axon regeneration can facilitate or suppress hindlimb function after olfactory ensheathing glia transplantation. J. Neurosci 31, 4298–4310 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Wang Z, Reynolds A, Kirry A, Nienhaus C & Blackmore MG Overexpression of sox11 promotes corticospinal tract regeneration after spinal injury while interfering with functional recovery. J. Neurosci 35, 3139–3145 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Liu S, Schackel T, Weidner N & Puttagunta R Biomaterial-supported cell transplantation treatments for spinal cord injury: challenges and perspectives. Front. Cell Neurosci 11, 430 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Liu S, Xie YY & Wang B Role and prospects of regenerative biomaterials in the repair of spinal cord injury. Neural Regen. Res 14, 1352–1363 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Jayaprakash N et al. Restoration of direct corticospinal communication across sites of spinal injury. Preprint at 10.1101/546374 (2019). [DOI] [Google Scholar]
- 187.Sudhof TC Towards an understanding of synapse formation. Neuron 100, 276–293 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Card JP & Enquist LW Transneuronal circuit analysis with pseudorabies viruses. Curr. Protoc. Neurosci 68, 1.5.1–1.5.39 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Adler AF, Bjorklund A & Parmar M Transsynaptic tracing and its emerging use to assess graft-reconstructed neural circuits. Stem Cells 10.1002/stem.3166 (2020). [DOI] [PubMed] [Google Scholar]
- 190.Pierani A et al. Control of interneuron fate in the developing spinal cord by the progenitor homeodomain protein Dbx1. Neuron 29, 367–384 (2001). [DOI] [PubMed] [Google Scholar]
- 191.Shirasaki R & Pfaff SL Transcriptional codes and the control of neuronal identity. Annu. Rev. Neurosci 25, 251–281 (2002). [DOI] [PubMed] [Google Scholar]
- 192.Gonzalez-Rothi EJ et al. Spinal interneurons and forelimb plasticity after incomplete cervical spinal cord injury in adult rats. J. Neurotrauma 32, 893–907 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Kirkeby A et al. Generation of regionally specified neural progenitors and functional neurons from human embryonic stem cells under defined conditions. Cell Rep. 1, 703–714 (2012). [DOI] [PubMed] [Google Scholar]
- 194.Lippmann ES et al. Deterministic HOX patterning in human pluripotent stem cell-derived neuroectoderm. Stem Cell Rep. 4, 632–644 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Tao Y & Zhang SC Neural subtype specification from human pluripotent stem cells. Cell Stem Cell 19, 573–586 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Hoang PT et al. Subtype diversification and synaptic specificity of stem cell-derived spinal interneurons. Neuron 100, 135–149 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Zholudeva LV, Karliner JS, Dougherty KJ & Lane MA Anatomical recruitment of spinal V2a interneurons into phrenic motor circuitry after high cervical spinal cord injury. J. Neurotrauma 34, 3058–3065 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Eaton MJ et al. Subarachnoid transplant of a human neuronal cell line attenuates chronic allodynia and hyperalgesia after excitotoxic spinal cord injury in the rat. J. Pain 8, 33–50 (2007). [DOI] [PubMed] [Google Scholar]
- 199.Kim DS et al. Transplantation of GABAergic neurons from ESCs attenuates tactile hypersensitivity following spinal cord injury. Stem Cell 28, 2099–2108 (2010). [DOI] [PubMed] [Google Scholar]
- 200.Hwang I et al. Intrathecal transplantation of embryonic stem cell-derived spinal GABAergic neural precursor cells attenuates neuropathic pain in a spinal cord injury rat model. Cell Transpl. 25, 593–607 (2016). [DOI] [PubMed] [Google Scholar]
- 201.Lu P et al. Origins of neural progenitor cell-derived axons projecting caudally after spinal cord injury. Stem Cell Rep. 13, 105–114 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Yuan XB et al. Guiding migration of transplanted glial progenitor cells in the injured spinal cord. Sci. Rep 6, 22576 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Michelsen KA et al. Area-specific reestablishment of damaged circuits in the adult cerebral cortex by cortical neurons derived from mouse embryonic stem cells. Neuron 85, 982–997 (2015). [DOI] [PubMed] [Google Scholar]
- 204.Cardoso T et al. Target-specific forebrain projections and appropriate synaptic inputs of hESC-derived dopamine neurons grafted to the midbrain of parkinsonian rats. J. Comp. Neurol 526, 2133–2146 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Adler AF et al. hESC-derived dopaminergic transplants integrate into basal ganglia circuitry in a preclinical model of Parkinson’s disease. Cell Rep. 28, 3462–3473 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Kennedy TE & Tessier-Lavigne M Guidance and induction of branch formation in developing axons by target-derived diffusible factors. Curr. Opin. Neurobiol 5, 83–90 (1995). [DOI] [PubMed] [Google Scholar]
- 207.Moxon KA, Oliviero A, Aguilar J & Foffani G Cortical reorganization after spinal cord injury: always for good? Neuroscience 283, 78–94 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Oza CS & Giszter SF Trunk robot rehabilitation training with active stepping reorganizes and enriches trunk motor cortex representations in spinal transected rats. J. Neurosci 35, 7174–7189 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Martin JH Harnessing neural activity to promote repair of the damaged corticospinal system after spinal cord injury. Neural Regen. Res 11, 1389–1391 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Serradj N, Agger SF & Hollis ER 2nd. Corticospinal circuit plasticity in motor rehabilitation from spinal cord injury. Neurosci. Lett 652, 94–104 (2017). [DOI] [PubMed] [Google Scholar]
- 211.Li Q, Houdayer T, Liu S & Belegu V Induced neural activity promotes an oligodendroglia regenerative response in the injured spinal cord and improves motor function after spinal cord injury. J. Neurotrauma 34, 3351–3361 (2017). [DOI] [PubMed] [Google Scholar]
- 212.Filli L & Schwab ME Structural and functional reorganization of propriospinal connections promotes functional recovery after spinal cord injury. Neural Regen. Res 10, 509–513 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Hilton BJ et al. Re-establishment of cortical motor output maps and spontaneous functional recovery via spared dorsolaterally projecting corticospinal neurons after dorsal column spinal cord injury in adult mice. J. Neurosci 36, 4080–4092 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Ferguson AR et al. Maladaptive spinal plasticity opposes spinal learning and recovery in spinal cord injury. Front. Physiol 3, 399 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Grau JW et al. When pain hurts: nociceptive stimulation induces a state of maladaptive plasticity and impairs recovery after spinal cord injury. J. Neurotrauma 34, 1873–1890 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Turtle JD et al. Pain input impairs recovery after spinal cord injury: treatment with lidocaine. J. Neurotrauma 34, 1200–1208 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Lu P, Jones LL, Snyder EY & Tuszynski MH Neural stem cells constitutively secrete neurotrophic factors and promote extensive host axonal growth after spinal cord injury. Exp. Neurol 181, 115–129 (2003). [DOI] [PubMed] [Google Scholar]
- 218.Macias MY et al. Pain with no gain: allodynia following neural stem cell transplantation in spinal cord injury. Exp. Neurol 201, 335–348 (2006). [DOI] [PubMed] [Google Scholar]
- 219.van Gorp S et al. Amelioration of motor/sensory dysfunction and spasticity in a rat model of acute lumbar spinal cord injury by human neural stem cell transplantation. Stem Cell Res. Ther 4, 57 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.Cizkova D et al. Functional recovery in rats with ischemic paraplegia after spinal grafting of human spinal stem cells. Neuroscience 147, 546–560 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221.Kwon BK et al. Large animal and primate models of spinal cord injury for the testing of novel therapies. Exp. Neurol 269, 154–168 (2015). [DOI] [PubMed] [Google Scholar]
- 222.Lemon RN & Griffiths J Comparing the function of the corticospinal system in different species: organizational differences for motor specialization? Muscle Nerve 32, 261–279 (2005). [DOI] [PubMed] [Google Scholar]
- 223.Strnadel J et al. Survival of syngeneic and allogeneic iPSC-derived neural precursors after spinal grafting in minipigs. Sci. Transl. Med 10, eaam6651 (2018). [DOI] [PubMed] [Google Scholar]
- 224.Yamane J et al. Transplantation of galectin-1-expressing human neural stem cells into the injured spinal cord of adult common marmosets. J. Neurosci. Res 88, 1394–1405 (2010). [DOI] [PubMed] [Google Scholar]
- 225.Kobayashi Y et al. Pre-evaluated safe human iPSC-derived neural stem cells promote functional recovery after spinal cord injury in common marmoset without tumorigenicity. PLoS One 7, e52787 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226.Iwai H et al. Allogeneic neural stem/progenitor cells derived from embryonic stem cells promote functional recovery after transplantation into injured spinal cord of nonhuman primates. Stem Cell Transl. Med 4, 708–719 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227.NIH. Consideration of Sex as a Biological Variable in NIH-Funded Research https://grants.nih.gov/grants/guide/notice-files/not-od-15-102.html (2015).
- 228.Moore SA et al. Targeting translational successes through CANSORT-SCI: using pet dogs to identify effective treatments for spinal cord injury. J. Neurotrauma 34, 2007–2018 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229.Roberts TT, Leonard GR & Cepela DJ Classifications in brief: American Spinal Injury Association (ASIA) impairment scale. Clin. Orthop. Relat. Res 475, 1499–1504 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230.Jones LAT et al. Considerations and recommendations for selection and utilization of upper extremity clinical outcome assessments in human spinal cord injury trials. Spinal Cord. 56, 414–425 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 231.Beaudoin M et al. Usability of the participation and quality of life (PAR-QoL) outcomes toolkit website for spinal cord injury. Top. Spinal Cord. Inj. Rehabil 26, 64–77 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 232.Steward O, Sharp KG, Yee KM, Hatch MN & Bonner JF Characterization of ectopic colonies that form in widespread areas of the nervous system with neural stem cell transplants into the site of a severe spinal cord injury. J. Neurosci 34, 14013–14021 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 233.Levi AD et al. Emerging safety of intramedullary transplantation of human neural stem cells in chronic cervical and thoracic spinal cord injury. Neurosurgery 82, 562–575 (2018). [DOI] [PubMed] [Google Scholar]
- 234.Dalamagkas K, Tsintou M, Seifalian A & Seifalian AM Translational regenerative therapies for chronic spinal cord injury. Int. J. Mol. Sci 19, E1776 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 235.Pereira IM, Marote A, Salgado AJ & Silva NA Filling the gap: neural stem cells as a promising therapy for spinal cord injury. Pharmaceuticals 12, E65 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 236.Chhabra HS et al. Stem cell/cellular interventions in human spinal cord injury: is it time to move from guidelines to regulations and legislations? Literature review and spinal cord society position statement. Eur. Spine J 28, 1837–1845 (2019). [DOI] [PubMed] [Google Scholar]
- 237.Fawcett JW et al. Guidelines for the conduct of clinical trials for spinal cord injury as developed by the ICCP panel: spontaneous recovery after spinal cord injury and statistical power needed for therapeutic clinical trials. Spinal Cord. 45, 190–205 (2007). [DOI] [PubMed] [Google Scholar]
- 238.Jin D et al. Restoration of skilled locomotion by sprouting corticospinal axons induced by co-deletion of PTEN and SOCS3. Nat. Commun 6, 8074 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 239.Charsar BA, Urban MW & Lepore AC Harnessing the power of cell transplantation to target respiratory dysfunction following spinal cord injury. Exp. Neurol 287, 268–275 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 240.Stevens LC & Little CC Spontaneous testicular teratomas in an inbred strain of mice. Proc. Natl Acad. Sci. USA 40, 1080–1087 (1954). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 241.Solter D From teratocarcinomas to embryonic stem cells and beyond: a history of embryonic stem cell research. Nat. Rev. Genet 7, 319–327 (2006). [DOI] [PubMed] [Google Scholar]
- 242.Evans MJ & Kaufman MH Establishment in culture of pluripotential cells from mouse embryos. Nature 292, 154–156 (1981). [DOI] [PubMed] [Google Scholar]
- 243.Martin GR Isolation of a pluripotent cell line from early mouse embryos cultured in medium conditioned by teratocarcinoma stem cells. Proc. Natl Acad. Sci. USA 78, 7634–7638 (1981). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 244.Gossler A, Doetschman T, Korn R, Serfling E & Kemler R Transgenesis by means of blastocyst-derived embryonic stem cell lines. Proc. Natl Acad. Sci. USA 83, 9065–9069 (1986). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 245.Robertson E, Bradley A, Kuehn M & Evans M Germ-line transmission of genes introduced into cultured pluripotential cells by retroviral vector. Nature 323, 445–448 (1986). [DOI] [PubMed] [Google Scholar]
- 246.Thomas KR & Capecchi MR Introduction of homologous DNA sequences into mammalian cells induces mutations in the cognate gene. Nature 324, 34–38 (1986). [DOI] [PubMed] [Google Scholar]
- 247.Thomson JA et al. Embryonic stem cell lines derived from human blastocysts. Science 282, 1145–1147 (1998). [DOI] [PubMed] [Google Scholar]
- 248.Shamblott MJ et al. Derivation of pluripotent stem cells from cultured human primordial germ cells. Proc. Natl Acad. Sci. USA 95, 13726–13731 (1998). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 249.Richards M, Fong CY, Chan WK, Wong PC & Bongso A Human feeders support prolonged undifferentiated growth of human inner cell masses and embryonic stem cells. Nat. Biotechnol 20, 933–936 (2002). [DOI] [PubMed] [Google Scholar]
- 250.Takahashi K & Yamanaka S Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell 126, 663–676 (2006). [DOI] [PubMed] [Google Scholar]
- 251.Soldner F & Jaenisch R Medicine. iPSC disease modeling. Science 338, 1155–1156 (2012). [DOI] [PubMed] [Google Scholar]
- 252.Kim K et al. Donor cell type can influence the epigenome and differentiation potential of human induced pluripotent stem cells. Nat. Biotechnol 29, 1117–1119 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 253.Lujan E, Chanda S, Ahlenius H, Sudhof TC & Wernig M Direct conversion of mouse fibroblasts to self-renewing, tripotent neural precursor cells. Proc. Natl Acad. Sci. USA 109, 2527–2532 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 254.Banda E & Grabel L Directed differentiation of human embryonic stem cells into neural progenitors. Methods Mol. Biol 1307, 289–298 (2016). [DOI] [PubMed] [Google Scholar]
- 255.Daadi MM Differentiation of neural stem cells derived from induced pluripotent stem cells into dopaminergic neurons. Methods Mol. Biol 1919, 89–96 (2019). [DOI] [PubMed] [Google Scholar]
- 256.Anderson S & Vanderhaeghen P Cortical neurogenesis from pluripotent stem cells: complexity emerging from simplicity. Curr. Opin. Neurobiol 27, 151–157 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 257.Hu BY & Zhang SC Differentiation of spinal motor neurons from pluripotent human stem cells. Nat. Protoc 4, 1295–1304 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 258.Pritchard CD et al. Establishing a model spinal cord injury in the African green monkey for the preclinical evaluation of biodegradable polymer scaffolds seeded with human neural stem cells. J. Neurosci. Methods 188, 258–269 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 259.Mothe AJ, Tam RY, Zahir T, Tator CH & Shoichet MS Repair of the injured spinal cord by transplantation of neural stem cells in a hyaluronan-based hydrogel. Biomaterials 34, 3775–3783 (2013). [DOI] [PubMed] [Google Scholar]
- 260.Conova L et al. A pilot study of poly (N-isopropylacrylamide)-g-polyethylene glycol and poly(N-isopropylacrylamide)-g-methylcellulose branched copolymers as injectable scaffolds for local delivery of neurotrophins and cellular transplants into the injured spinal cord. J. Neurosurg. Spine 15, 594–604 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 261.Günther MI, Weidner N, Müller R & Blesch A Cell-seeded alginate hydrogel scaffolds promote directed linear axonal regeneration in the injured rat spinal cord. Acta Biomater. 27, 140–150 (2015). [DOI] [PubMed] [Google Scholar]
- 262.Partyka PP et al. Harnessing neurovascular interaction to guide axon growth. Sci. Rep 9, 2190 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 263.Marchini A et al. Multifunctionalized hydrogels foster hNSC maturation in 3D cultures and neural regeneration in spinal cord injuries. Proc. Natl Acad. Sci. USA 116, 7483–7492 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 264.Totoiu MO & Keirstead HS Spinal cord injury is accompanied by chronic progressive demyelination. J. Comp. Neurol 486, 373–383 (2005). [DOI] [PubMed] [Google Scholar]
- 265.Guest JD, Hiester ED & Bunge RP Demyelination and Schwann cell responses adjacent to injury epicenter cavities following chronic human spinal cord injury. Exp. Neurol 192, 384–393 (2005). [DOI] [PubMed] [Google Scholar]
- 266.Franklin RJ & Ffrench-Constant C Remyelination in the CNS: from biology to therapy. Nat. Rev. Neurosci 9, 839–855 (2008). [DOI] [PubMed] [Google Scholar]
- 267.Plemel JR et al. Remyelination after spinal cord injury: is it a target for repair? Prog. Neurobiol 117, 54–72 (2014). [DOI] [PubMed] [Google Scholar]
- 268.Myers SA, Bankston AN, Burke DA, Ohri SS & Whittemore SR Does the preclinical evidence for functional remyelination following myelinating cell engraftment into the injured spinal cord support progression to clinical trials? Exp. Neurol 283, 560–572 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 269.Keirstead HS et al. Human embryonic stem Cell-derived oligodendrocyte progenitor cell transplants remyelinate and restore locomotion after spinal cord injury. J. Neurosci 25, 4694–4705 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 270.Powers BE et al. Axonal thinning and extensive remyelination without chronic demyelination in spinal injured rats. J. Neurosci 32, 5120–5125 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 271.Duncan GJ et al. Locomotor recovery following contusive spinal cord injury does not require oligodendrocyte remyelination. Nat. Commun 9, 3066 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 272.Duncan GJ et al. The fate and function of oligodendrocyte progenitor cells after traumatic spinal cord injury. Glia 68, 227–245 (2020). [DOI] [PubMed] [Google Scholar]
- 273.Tripathi R & McTigue DM Prominent oligodendrocyte genesis along the border of spinal contusion lesions. Glia 55, 698–711 (2007). [DOI] [PubMed] [Google Scholar]
- 274.Pukos N, Goodus MT, Sahinkaya FR & McTigue DM Myelin status and oligodendrocyte lineage cells over time after spinal cord injury: what do we know and what still needs to be unwrapped? Glia 67, 2178–2202 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


