Abstract
Thrombosis is a major complication of cardiovascular disease, leading to myocardial infarction, acute ischemic stroke, or venous thromboembolism. Thrombosis occurs when a thrombus forms inside blood vessels disrupting blood flow. Developments in thrombectomy to remove thrombi from vessels have provided new opportunities to study thrombus composition which may help to understand mechanisms of disease and underpin improvements in treatments. We aimed to review thrombus compositions, roles of components in thrombus formation and stability, and methods to investigate thrombi. Also, we summarize studies on thrombus structure obtained from cardiovascular patients and animal models. Thrombi are composed of fibrin, red blood cells, platelets, leukocytes, and neutrophil extracellular traps. These components have been analyzed by several techniques, including scanning electron microscopy, laser scanning confocal microscopy, histochemistry, and immunohistochemistry; however, each technique has advantages and limitations. Thrombi are heterogenous in composition, but overall, thrombi obtained from myocardial infarction are composed of mainly fibrin and other components, including platelets, red blood cells, leukocytes, and cholesterol crystals. Thrombi from patients with acute ischemic stroke are characterized by red blood cell- and platelet-rich regions. Thrombi from patients with venous thromboembolism contain mainly red blood cells and fibrin with some platelets and leukocytes. Thrombus composition from patients with myocardial infarction is influenced by ischemic time. Animal thrombosis models are crucial to gain further mechanistic information about thrombosis and thrombus structure, with thrombi being similar in composition compared with those from patients. Further studies on thrombus composition and function are key to improve treatment and clinical outcome of thrombosis.
Keywords: blood vessels, cardiovascular diseases, myocardial infarction, thrombectomy, thrombosis
Highlights.
Thrombosis occurs when a thrombus forms inside blood vessels disrupting blood flow.
Thrombi obtained from myocardial infarction are composed of mainly fibrin and other components, including platelets, red blood cells, leukocytes, and cholesterol crystals.
Thrombi from patients with acute ischemic stroke are characterized by red blood cell- and platelet-rich regions.
Thrombi from patients with venous thromboembolism contain mainly red blood cells and fibrin with some platelets and leukocytes.
Thrombus structure and composition are important for risk of thrombosis and thrombus removal.
Changes in clot structure are of key interest due to associations with risk of myocardial infarction (MI), acute ischemic stroke (AIS), and venous thromboembolism (VTE). Most studies explored links between in vitro clot structure and thrombosis.1 Recent literature exceeds 1000 publications, with >400 in the past 5 years alone.1–3 However, studies into the structure and components of thrombi formed in vivo remain limited. With the advent of new methodologies and imaging techniques, in vivo or ex vivo thrombi obtained by thrombectomy can be studied in much greater detail than ever before. Recent studies have been taking this approach to shed light on how in vivo thrombus structures relate to thrombosis in different vascular beds. In this review, we will summarize the main findings from these studies, their technological aspects, associations with disease, insights from animal models, and highlight key areas for future research.
Major Thrombus Components
Formation of thrombi leads to vessel occlusion or the generation of emboli that block blood vessels further downstream, resulting in MI, AIS, or pulmonary embolism (PE). Principal components of thrombi include fibrin, platelets, red blood cells (RBCs), leukocytes, and neutrophil extracellular traps (NETs). However, the relative contribution of each component differs between thrombus location and disease pathology. Below we discuss each component and how they contribute, followed by differences in thrombus composition between different thrombotic diseases.
Fibrin(ogen)
Fibrinogen is a 340 kD glycoprotein that circulates in blood at 2 to 5 mg/mL.2 When coagulation is triggered, thrombin cleaves fibrinogen into fibrin that polymerizes into a network of fibers,3 stabilizing blood clots. Fibrin is a major contributor to thrombi, with changes in its structure known to affect clot formation, stability, and breakdown. High thrombin concentrations lead to dense fibrin networks that are relatively resistant to fibrinolysis.2 Previous in vitro studies have linked changes in fibrin clot structure,1 viscoelastic properties,4 and hypofibrinolysis5 to thrombosis. However, despite the consistent link between in vitro clot structure and thrombosis, it is still unclear whether comparable changes are reflected in the structure of in vivo thrombi. Early studies that explored in vivo thrombi used angioscopy to evaluate macroscopic properties. Two main types of thrombi were observed in patients with acute coronary syndromes, white and red.6 Histology indicated that white thrombi from patients with ST-segment–elevation MI (STEMI) were mainly composed of fibrin, whereas red thrombi were mainly composed of RBCs.7 Thrombi from patients with STEMI that are resistant to fibrinolysis are characterized by dense fibrin and higher contents of platelets and VWF (von Willebrand factor).8 These studies highlight how fibrin contribution to thrombi varies between disorders and may impact disease progression and outcome. In vitro studies have shown how clots with increased fibrin demonstrate greater friction,9 suggesting these thrombi are stickier. This could be an important factor in clot stability, embolization, and thrombectomy.
Recent findings presented a new structural feature of fibrin in clots. Instead of forming 3-dimensional fiber networks, fibrin molecules align into continuous films forming a protective layer across the surface of clots, providing protection against infection.10 There is evidence that fibrin also forms films within the vasculature. Images of intraluminal thrombi from patients with abdominal aortic aneurysm show signs of fibrin film both within and on the clot surface (Figure 1). Clots from murine venous thrombosis models also demonstrate the presence of film (Figure 1). In agreement with these unpublished findings, many studies have presented evidence of film in thrombi. Scanning electron microscopy (SEM) micrographs of thrombi from patients with PE,11 AIS,12 or MI13 showed fibrin film in these thrombi. A recent study showed structures surrounding thrombi removed from patients with AIS that had similar properties to fibrin films, which these authors called a shell, and which slowed thrombolysis.14 Combined with the findings that fibrin content varies in thrombi, these data indicate an important role for fibrin in thrombus characteristics, influencing stability, embolization, and breakdown. Further research is needed to understand how fibrin content in thrombi influences disease onset, progression, and outcome, and how previously described changes in in vitro fibrin clot structure relate to in vivo thrombus structure.
Figure 1.
Fibrin biofilm in thrombi.A and B, Film structures in thrombi extracted from patients with abdominal aortic aneurysm. A, Fibrin film covering the thrombus (arrows). B, Film transitioning from film to fibers (arrows) and film lining channels traversing the thrombus (asterisk). C–F, Thrombi from murine thrombosis model (FeCl3 injury of the inferior vena cava) after perfusion/fixation. C, Small thrombus showing partial film coverage (arrows). D, Film covering part of the thrombus (arrows). E and F, Film coverage localized on top of red blood cells (RBCs; arrows).
Platelets
Platelets are an attractive target for antithrombotic treatment. Activated platelets provide negatively charged membrane surfaces that are essential for assembly of the prothrombinase and tenase complexes.15 Platelets form different populations during clot formation, including procoagulant platelets which support thrombin generation and fibrin formation, and aggregating platelets involved in initial clot formation and contraction.16 Within thrombi, strongly activated platelets localize in the inner core, while discoid and quiescent platelets localize to the exterior.16 Clot contraction is mediated by aggregating platelets binding fibrin fibers via αIIbβ3.16 Procoagulant platelets show a balloon-like structure, exposing phosphatidylserine on the surface to generate thrombin in situ.17 In addition, platelets promote thrombus growth and propagation through glycoprotein VI binding to fibrin.18,19 More studies are needed to investigate the temporal and morphological contribution of platelets to the architecture of thrombi obtained ex vivo.
Leukocytes
Leukocytes contribute significantly to clot formation and are found in both arterial and venous thrombi.20,21 Leukocytes bind fibrin via integrin receptor αMβ2 (Mac-1), which supports the inflammatory response.22 Neutrophils, the most abundant leukocyte in circulation, release matrix metalloproteinases, platelet-activating factor, cathepsin G, and elastase.23,24 These molecules can impact coagulation via a number of mechanisms, including activation of coagulation factor V, factor VIII, and factor X,25–27 activation and aggregation of platelets,28 degradation of antithrombin III and proteolytic cleavage of TF (tissue factor) pathway inhibitor.29,30 Monocytes are a major source of intravascular TF expression and provide a membrane surface for coagulation initiation in a number of conditions.25,31 TF is also expressed by neutrophils in animal models.32 Some studies report TF expression in neutrophils and eosinophils,33,34 but other studies fail to detect TF expression in granulocytes.35,36 Some of these discrepancies may be attributed to direct transfer of TF from monocytes to granulocytes.37
Neutrophil Extracellular Traps
A key mechanism by which neutrophils contribute to thrombus composition involves generation of NETs. NETs are formed by neutrophils extruding DNA, histones, and granular proteins in response to microbial invasion, inflammatory stimuli, or activated platelets.38 Increasing evidence shows that NETs are associated with thrombosis.39,40 NETs are thought to trigger coagulation via the intrinsic pathway, with DNA acting as scaffold for contact assembly triggering thrombin generation.38 Heparin binds histones in NETs resulting in their breakdown and reduction of thrombosis.41,42 One study suggested that neutrophil DNA or histones can trigger coagulation, but not NETs, due to histone-histone and histone-DNA interactions.43 NETs act as scaffold for platelet aggregation, further promoting thrombus development, and there is evidence they increase resistance to thrombolysis.38,41,42 The relationship between NETs and thrombosis and the underlying mechanisms remain of key interest since NETs are present in both arterial and venous thrombi.39 Extracellular DNA has been observed in platelet-rich areas of AIS thrombi but not in RBC-rich regions.44 However, NET-like structures were found in RBC-rich regions of murine venous thrombi,39 highlighting the need for further research into the role and localization of NETs.
Red Blood Cells
Recent studies have indicated that RBCs play a more functional role in clot structure and function than previously thought. FasL/FasR (CD95) receptor ligand interactions between RBCs and activating platelets have been shown to lead to phosphatidylserine exposure on both platelets and RBCs and to platelet degranulation, contributing to thrombus formation.45,46 RBCs have been shown to support thrombin generation via the meizothrombin pathway.47 High hematocrit promotes accumulation of platelets at vascular injury sites by pushing platelets from the blood vessel center to the vessel wall.48 RBCs retention within venous thrombi is mediated by factor XIII, suggesting that targeting factor XIII to reduce RBCs contents could be a therapeutic approach in venous thrombus as less RBC content may limit thrombus mass and stability.49 RBCs are normally biconcave, however, recent studies show that RBCs adopt an alternative structure during thrombosis called polyhedrocytes.50 Forces generated by platelets pulling on fibrin fibers lead to clot contraction, compressing RBCs together, forcing them into a polyhedral structure.50 Polyhedrocytes have been detected in thrombi from patients with STEMI.50 Other studies indicate that polyhedrocytes are present in 20% to 31% of thrombi from patients with MI.51,52 Polyhedrocytes are also found in venous thrombi, pulmonary emboli, and cerebral thrombi.53,54 RBCs in clots affect fibrinolysis55 and alter clot mechanical properties.56 Furthermore, disorders such as sickle cell disease make RBCs rigid,57 reducing thrombus permeability.58 RBC-rich thrombi contain more inflammatory cells than other thrombi and associate with increased thrombus burden and impaired reperfusion in patients with STEMI.59 Altogether, 2 key mechanisms by which RBCs influence thrombosis are the formation of polyhedrocytes and the generation of additional thrombin. Better understanding of the mechanisms underpinning polyhedrocyte formation may lead to new treatments of thrombosis.
Other Components
VWF, produced by megakaryocytes and endothelial cells, stabilizes factor VIII and mediates platelet adhesion, thereby supporting thrombosis.60 VWF has been detected in ex vivo thrombi from patients with MI and AIS.14,61 While the role of VWF in thrombosis through platelet activation and thrombin generation is well characterized, its presence and role(s) in thrombi require further investigation. The fibrinolysis pathway also plays a central role in thrombosis. tPA (Tissue-type plasminogen activator) converts plasminogen to plasmin, a primary fibrinolytic protease.1 tPA and its inhibitor, PAI-1 (plasminogen activator inhibitor-1), have been detected in thrombi from patients with MI.8 PAI-1 and protease nexin-1 have also been detected in AIS thrombi.14 Variation in fibrinolytic proteins and their inhibitors incorporated in thrombi may impact on resistance to therapeutic thrombolysis. Finally, cholesterol crystals are present in thrombi obtained from patients with MI which were mainly derived from plaque rupture.62 The role of cholesterol crystals in thrombi is unknown and requires further study.
Methodologies to Investigate Thrombus Structure
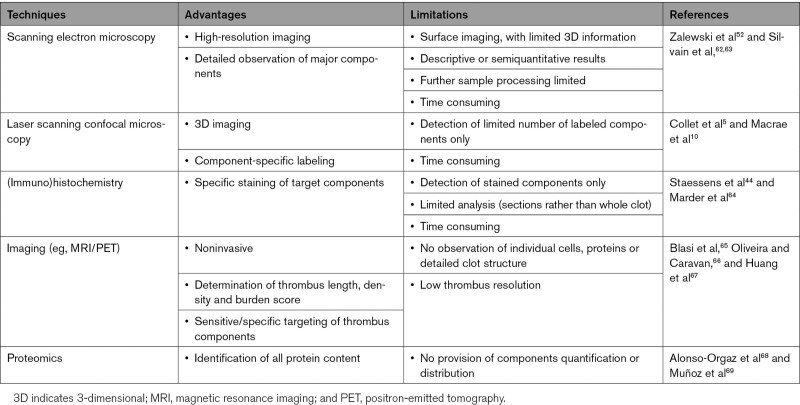
Techniques to investigate thrombus structure have their advantages and limitations (summarized in the Table). SEM has been used in several studies.52,62,63 It offers high-resolution images and visualization of major thrombus components, providing descriptive and semiquantitative data.52,62 This enables identification of thrombus components, such as polyhedrocytes50 and fibrin fibers or films.10 SEM supports the analysis of platelet morphological alterations, such as aggregation and pseudopod formation.70 In addition, SEM is very useful for the analysis of fibrin properties within a thrombus, such as fibrin coverage area and fibrin fiber diameter. However, NETs are structurally difficult to differentiate from fibrin using SEM,71 highlighting the need for combining SEM with other methods using specific labeling such as correlative light and electron microscopy, laser scanning confocal microscopy (LSCM), or immunohistochemistry to confirm the nature of some of the components analyzed.
Table.
Methods Used to Investigate Thrombus Structure
Once thrombi are prepared for SEM no further analysis (eg, histology) can be performed.62,63 Identification of components is based on morphological appearance, without specific staining. Furthermore, limited 3-dimensional information is obtained by SEM. SEM may induce artifacts due to sample processing, fixation and dehydration. Nevertheless, SEM procedures have been refined to keep artifacts to a minimum, and in most cases, negligible. In contrast, LSCM provides 3-dimensional images in fully hydrated conditions.5,10 Fluorescent antibodies can be used to label specific proteins, allowing for identification of components. However, extracted thrombi need to undergo fixation and chemical clearing before LSCM imaging.72 Resolution is lower than SEM and only labeled components are detected with a finite number of fluorophores used at once. RBCs within a thrombus may hinder optical access to the inside of the clot and thus the collection of deep, high-resolution 3-dimensional images by LSCM.72 New optical clearing methods may need to be explored to produce a suitably transparent thrombus to allow for deep imaging of thrombi at the micrometer scale.72 This method could be effective for imaging the thrombus structure of patients. LSCM and SEM can each be used sequentially on the same sample, but both are expensive and time consuming. Correlative light SEM may provide future opportunities and new developments in the field through matching confocal and electron imaging of the same thrombus area.73
Immunohistochemistry has been used to identify specific components in thrombi,44,64 via sectional analysis of thrombi with a range of resolutions (nm-µm). Recent developments allow improved imaging of thrombi and their constituents.39 However, despite analysis of sections, mostly 2- rather than 3-dimensional information has so far been obtained. Furthermore, the preparation of thin sample slices may damage the sample to be analyzed. A combination of imaging techniques is recommended to compare high-resolution methods such as SEM with methods that allow specific staining, such as immunohistochemistry and LSCM. Noninvasive, sensitive, and specific imaging techniques, including magnetic resonance imaging (MRI) and positron-emitted tomography, have also been used to study thrombus composition in patients and animal models.65,66 Imaging of AIS thrombi revealed information about clot length, clot density, and clot burden score.67 Other studies used proteomic approaches to identify thrombi constituents.68,69 Correlating thrombus proteome to clinical features could be useful for AIS cause identification, which may help selecting appropriate treatment.74
Thrombus Composition by Pathology
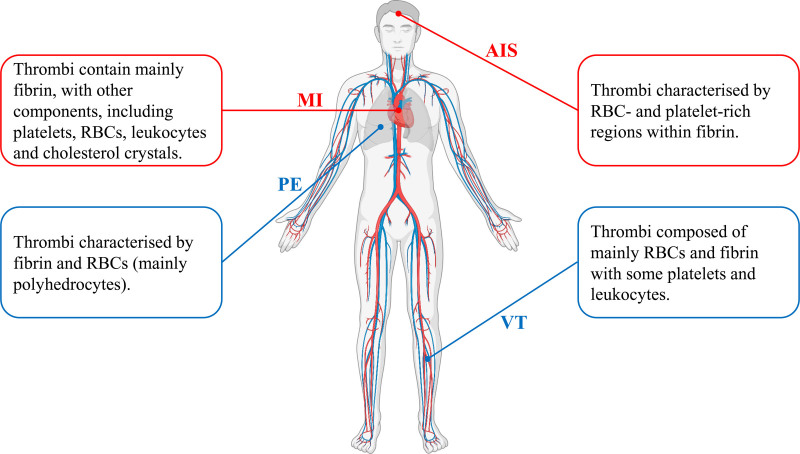
Since the development of thrombectomy and other endovascular approaches including balloon angioplasty, endovascular thrombolysis, and stenting, mortality of patients with thrombosis (particularly MI and AIS) has decreased substantially. With the advent of thrombectomy new opportunities have emerged to investigate pathological differences in thrombi extracted from patients. Several studies have assessed thrombus composition using a combination of SEM, LSCM, and immunohistochemistry (summarized in Figure 2).
Figure 2.
Thrombus composition in myocardial infarction (MI), acute ischemic stroke (AIS), venous thrombosis (VT), and pulmonary embolism (PE). RBC indicates red blood cell. Created with BioRender.com.
Venous Thromboembolism
VTE is caused by thrombi in the deep veins of the limbs, which can travel to the lungs causing PE. VTE is triggered by 3 fundamental mechanisms, endothelial dysfunction, altered blood flow, and hypercoagulability, also called Virchow’s Triad.75 Data on thrombi obtained by thrombectomy from patients with VTE is limited. A single case study showed that an embolus from a PE patient was composed of fibrin with RBCs and a small number of platelets.11 SEM indicated structures similar to fibrin film covering the embolus, although these were not commented on at the time. In another case-study of a patient with chronic venous insufficiency, thrombi were aspirated from the right atrium and pulmonary arteries. The atrial thrombus contained mostly RBCs, with platelets, a small number of leukocytes, and a random arrangement of fibrin fibers. In comparison, more fibrin and platelets aggregates were observed in pulmonary thrombi, with fibrin fibers arranged along the vessels.76 An earlier review showed a venous thrombus with densely packed RBCs resembling polyhedrocytes interspaced by fibrin.77 Furthermore, an autopsy study examined venous thrombi and pulmonary emboli from 8 patients who died from VTE, showing that all thrombi and emboli contained fibrin, RBCs, VWF, and αIIbβ3.78
A study of the composition and mechanical properties of 2 emboli obtained from a patient with PE showed that their structure was heterogeneous, with one embolus containing more RBCs but less fibrin than the other.79 Cyclic compression analysis showed that the fibrin-rich embolus exhibited a higher stress response than the RBC-rich embolus. These findings indicate that thrombus composition impacts on mechanical properties, which may affect embolization, endovascular removal by thrombectomy and thrombolysis. A recent study compared thrombi from patients with STEMI (n=45) and venous thrombi (n=25) both obtained by open thrombectomy to pulmonary emboli from autopsies (n=10).53 All arterial thrombi were composed of fibrin followed by platelets, while major components of venous thrombi and pulmonary emboli were RBCs followed by fibrin. However, pulmonary emboli showed more fibrin and fewer RBCs than venous thrombi.53 In addition, RBCs in pulmonary emboli were present in the form of polyhedrocytes. The structure of fibrin in venous thrombi was heterogenous, including fibers, sponge, and bundles, while most of the fibrin in pulmonary emboli were fibers.53 The mechanisms behind these differences in composition are not known. It is possible that thrombus areas with a particular composition embolize, or emboli may change composition after they embolize and lodge in the pulmonary circulation. In view of the relative paucity of data, further studies investigating thrombus composition in VTE are required, however, thrombectomy is not normally a treatment of choice in VTE. Future developments in imaging and in vivo models are needed to progress this area of research.
Animal models of VTE show changes in thrombus composition and susceptibility to fibrinolysis over time.80,81 However, research on thrombus maturation in humans is limited. An autopsy study has used immunohistochemistry and LSCM to examine the thrombus composition in 140 cases of subjects who died due to PE.82 Upon autopsy, thrombi were classified into phase 1 (first week), phase 2 (second to eighth week) and phase 3 (older than 2 months). Phase 1 thrombi were composed of fibrin, platelets, agglomerated RBCs, and leukocytes. There was no interaction between the thrombus and the vascular endothelium. In phase 2, thrombi showed penetration of fibroblasts, and endothelial sprouting became apparent. Furthermore, macrophages containing predominantly hemosiderin, RBCs and fibrinous transformation were observed with nuclear debris of leukocytes. In phase 3, the thrombi became hyalinized, and few leukocytes were present interspersed by fiber-rich and cell-deficient connective tissue.82
A catherization study analyzed thrombi extracted from 17 patients with deep vein thrombosis and 10 patients with PE. Thrombi were classified into stage 1 (0–1 day old; composed of fibrin, platelets, RBCs, and neutrophils), stage 2 (1–3 days old; acute thrombi containing inflammatory cells without cellular organization), stage 3 (4–7 days old; thrombi exhibiting cellular growth, including smooth muscle cells and endothelial cells), and stage 4 (>7 days old; healing thrombi characterized by layers of smooth muscle cells, proteoglycan depositions, and endothelial filtration). All thrombi contained fibrin, RBCs, platelets, and inflammatory cells, and thrombi were generally younger in PE than patients with deep vein thrombosis.83 Based on the relatively scant literature on thrombus maturation in VTE, further clinical and preclinical research is necessary to gain clearer insights into how thrombi change over time.
Myocardial Infarction
MI is caused by rupture of an atherosclerotic plaque resulting in thrombosis.84 Thrombi extracted from patients with STEMI (n=44) were analyzed by SEM and contained mainly fibrin (60%), with the remainder (40%) composed of platelets, RBCs, cholesterol crystals, and leukocytes.62 Another study of sudden cardiac death (n=23) and STEMI (n=98) showed similar results, with no difference observed between these 2 groups.63 A separate study also showed that the major component of STEMI thrombi (n=40) was fibrin (49.1%), with other components, including RBCs (24.2%), platelets (11.6%), and leukocytes (3.7%).85 Immunobiological analysis of MI thrombi showed the presence of monocytes, neutrophils, and lymphocytes.86 Acute MI thrombi (n=29) analyzed by immunohistochemistry showed that thrombi contained fibrin, platelets, RBCs, and leukocytes.87 Other immunohistochemistry analysis of occlusive MI thrombi (n=15) revealed the presence of fibrin, αIIbβ3, TF, and VWF.61 Coronary arteries from patients with MI (n=31) examined postmortem showed that thrombi associated with ruptured plaques have more fibrin (74%) than platelets (35%), while thrombi associated with eroded plaques have more platelets (70%) than fibrin (51%). Tissue factor contributed more to thrombus formation in plaque rupture than erosion.88 Antimicrobial peptides released by leukocytes have been shown to contribute to platelet activation and thromboinflammation in human and murine models of MI.89
Thrombi surfaces contain more fibrin and platelets, and fewer RBCs, than their inner parts.52 Inner parts of STEMI thrombi are rich in polyhedrocytes,50–52 providing a densely packed structure resistant to fibrinolysis. A comparison between thrombi from patients with STEMI and peripheral arterial disease indicated reduced fibrin content, fibrin fiber diameter, and fibrin/platelet ratios in the coronary thrombi.90 A retrospective study exploring fibrin films in thrombi from patients with STEMI showed that fibrin film was detected on ≈15% of thrombi.13 Film was not detected in all thrombi due to heterogeneity of thrombi and could also have been missed due to the study being retrospective.
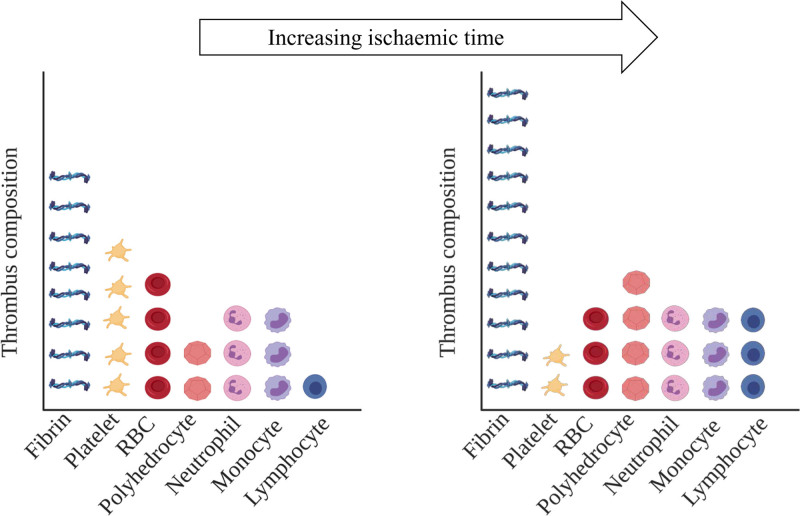
Ischemic time has been reported to affect the composition of thrombi (Figure 3). Silvain et al62,63 demonstrated that as ischemic time increased the amount of fibrin increased while platelets decreased in STEMI thrombi.62,63 STEMI thrombi (n=40) retrieved over 12 hours after the onset of symptoms showed more fibrin than thrombi retrieved within 3 hours.85 Correspondingly, RBCs decreased over time, but no associations were found for leukocyte and platelet counts.85 STEMI thrombi (n=65) retrieved >6 hours after onset of symptoms showed more compact fibrin network than thrombi retrieved <3 hours.86 With increasing time after symptom onset, platelet numbers decreased, and lymphocyte numbers increased.86 Polyhedrocyte formation increased with ischemic time in thrombi from patients with MI.51 Taken together, these findings indicate that thrombus composition may change over time after initial vessel occlusion in STEMI. Such changes in thrombus composition may have important implications for mechanical thrombectomy, thromboaspiration, and thrombolysis. Further research is required to understand the mechanisms through which thrombi change structure, for example, through thrombus component reorganization or clotting-lysis cycles.
Figure 3.
Ischemic time and thrombus composition in patients with ST-segment–elevation myocardial infarction (STEMI). The amounts of fibrin, polyhedrocytes, and lymphocytes increase with ischemic time, while platelet and red blood cell (RBC) loads decrease. Note: the ratios of components within each thrombus (individual graph), as well as the ratios of components between both thrombi (between graphs), are representative but not quantitative. Created with BioRender.com.
Acute Ischemic Stroke
AIS, the most common type of stroke, is caused by thrombosis in the cerebral circulation. AIS thrombi are due to atherosclerosis or cardiac embolism and result in disrupted blood flow in the brain and subsequent neurological disorder.91 A recent study showed that AIS thrombi (n=177) obtained by thrombectomy contain 2 distinct structural areas, platelet- and RBC-rich regions, that interspersed each other throughout the thrombi.44 Platelet-rich regions contained dense fibrin, platelets, VWF, leukocytes, and extracellular DNA. RBC-rich regions, however, were composed of RBCs and fibrin, while bordered by platelets and leukocytes.44 Another recent study showed that AIS thrombi (n=199) presented with a surface structure that resembles fibrin films.14 They referred to this as an outer shell, mainly composed of fibrin, that slowed thrombolysis. One complication in patients with AIS is thrombus migration, whereby the clot travels downstream in the cerebral vasculature, resulting in worse outcomes. Migrating thrombi contained more RBCs and less fibrin/platelets than stable thrombi.91 This may relate to previous findings that RBC-rich clots are less sticky.9 In addition, fibrin anchors clots to the site of vascular lesion, thus preventing embolism in animal models.92
Histological analyses showed that AIS emboli (n=25) were composed of platelets and fibrin with RBC-rich regions and leukocytes, including monocytes and neutrophils.64 A further histological study of AIS thrombi (n=37) showed that inflammatory T cells and monocytes were associated with RBC-rich clots, while VWF was associated with fibrin-rich clot.93 Early signs of vessel damage, including hyperdense middle cerebral artery sign and blooming artifact by computed tomography and MRI, indicate presence of thrombus.94 Thrombi from hyperdense middle cerebral artery sign and blooming artifact patients were RBC rich, while thrombi lacking these signs were fibrin rich.94 AIS thrombi (n=40) have been histologically categorized into early phase (RBC proportion dominant or equal to fibrin) and late phase (fibrin dominant and organized fibrin). Presence of hyperdense artery sign has been associated with early phase thrombus.95 Cerebral thrombi from patients with AIS (n=41) were composed of areas with many RBCs, mainly polyhedrocytes, and fibrin mixed with platelets with the presence of few leukocytes. There was no significant difference in RBC content between patients with cardioembolic and atherothrombogenic stroke. However, fibrin content was higher in patients with cardioembolic than patients with atherothrombogenic stroke.54 Similarly, previous studies showed that AIS of cardioembolic cause was associated with fibrin-rich thrombi while noncardioembolic thrombi (atherothrombogenic and cryptogenic thrombi) were associated with RBC-rich thrombi, however, the mechanisms behind these differences are unknown.96 Together, these studies indicate that thrombus composition changes by AIS cause, with RBC-rich thrombi associating with thrombus migration and fibrin-rich thrombi associating with stable and late phase thrombosis. However, one autopsy study found that the RBC content in cardioembolic thrombi was higher than in atherothrombotic stroke, indicating that the differences by cause may be more nuanced.97 The association of these types of thrombi with disease outcome and treatment such as thrombectomy and thrombolysis deserve further study, including detailed analysis of the composition of these thrombi in terms of other components using state-of-the-art methodologies.
Therapeutic Implications
Thrombosis treatments include the use of antiplatelet, anticoagulant, and fibrinolytic agents. Antiplatelets are generally used to prevent and treat arterial thrombosis,98 which is caused by atherosclerotic plaque rupture that leads to collagen exposure followed by platelet aggregation and thrombus formation.99 Treatments targeting platelets appear effective in this setting as platelets have an important role in arterial thrombus growth.99 However, venous thrombosis which occurs under low shear stress and is largely driven by a coagulation imbalance is treated with anticoagulants since venous thrombi contain an abundance of fibrin.98,99 Fibrinolytic agents may be used in both arterial and venous thrombosis settings.98
Many trials have indicated that a combination of anticoagulant and antiplatelet treatments could be an effective strategy for mitigating cardiovascular disease.100 In one study, patients with acute coronary syndrome were treated with either a combination of antiplatelets agents and rivaroxaban (an activated factor X inhibitor) or a placebo. The use of rivaroxaban reduced instances of cardiovascular death, MI, and AIS; however, it also increased the risk of major bleeding, including intracranial hemorrhage.101 In another study, coronary syndrome patients were treated with either aspirin, a combination of rivaroxaban and aspirin, or rivaroxaban without aspirin. Among these groups, the combination of rivaroxaban and aspirin yielded the best cardiovascular outcomes but was associated with a moderate increase in bleeding risk.102
Therapeutic fibrinolysis is centered around plasminogen into plasmin conversion in the thrombus using tPA or tPA analogues.103 Increasing evidence indicates that thrombolysis may be facilitated by targeting additional components in the thrombus other than fibrin. For instance, NETs that are present in venous and arterial thrombi and have been shown to delay thrombolysis by tPA could be a potential therapeutic target using DNases.104,105 Furthermore, the effectiveness of thrombectomy could be influenced by thrombus compositions. For example, in patients with AIS, it has been shown that RBC-rich thrombi are associated with successful recanalization,106,107 require fewer passes and lower procedure time than fibrin-rich thrombi.96 Thus, analysis of the thrombus structure in cardiovascular patients could aid the development of new thrombolytic or antithrombotic strategies.
Insights From Animal Models
Animals models are essential for the study of thrombosis pathophysiology or the role of drugs in thrombosis prevention.108–110 Studies discussed below provide insight in the composition of arterial or venous thrombi.
Animal Models of Arterial Thrombosis
An early study by Randall and Wilding111 showed that thrombi from rabbits induced by electrical stimulation of the wall of carotid arteries were platelet rich.111 Clots formed in dog femoral arteries showed that tPA-induced thrombolysis of platelet-rich thrombi induced by transluminal electrode was impaired compared with that of fibrin-rich thrombi induced by intraluminal copper wire and that reocclusion under antiplatelet therapy was more frequent for fibrin-rich thrombi.112 Complementary to this, studies in rabbit femoral arteries showed that RBC-rich thrombi triggered by thrombin infusion were more prone to thrombolysis than platelet-rich thrombi induced by everted artery graft, with platelets providing a source of PAI-1.113 A later study in rat carotids showed that platelet-rich thrombi (formed by photochemical injury) were more prone to embolization causing cerebral infarcts, than fibrin-rich thrombi (formed by balloon catheter denudation).114 These studies indicate an important role for thrombus composition in the severity of thromboembolic diseases. FeCl3 injury of rat carotid arteries showed initial platelet clumping on the denuded endothelium, while occlusive thrombi consisted of RBCs and leukocytes tightly packed by a fibrin mesh, with tightly adhered platelets at the anterior side, highlighting heterogeneity of arterial thrombi.115 A pig carotid artery thrombosis model induced by balloon angioplasty showed that thrombi were heterogenous in the first 24 hours, including fibrin-rich and platelet-rich areas, and sporadic RBCs and neutrophils. Fibrin-rich areas were found at the thrombus and vessel wall interface. At day 1, thrombi consisted of granulated platelets, cellular debris, and compacted fibrin, while at 2 weeks, connective tissue was detected alongside cellular debris and unresolved fibrin. Over 3 to 9 weeks, thrombi became more fibrous, containing new blood vessels.116 This model may be relevant to human pathophysiology, particularly about thrombus composition and changes in chronic coronary occlusion.116,117
Occlusive thrombi from rabbit arteries by balloon angioplasty stained positive for αIIbβ3, fibrin, VWF, and TF,118 in agreement with human studies.119 The neointima could play a role in thrombus composition and size due to tissue factor expression on the smooth muscle cell and macrophage-rich neointima, while small, platelet-rich thrombi formed on normal intima.120 A recent study indicated that mouse carotid artery thrombi triggered by FeCl3 were similar in composition when compared with human coronary thrombi, as both were heterogeneous with compact cell-rich regions, and less dense areas with fewer cells. NETs were present in both human and murine thrombi, with similar NETs to leukocyte ratio. Inhibition of NETs resulted in decreased thrombosis and reduced infarct size.121 Staining was targeted at particular components, and RBCs were partly overlooked in this study.
Human arterial thrombi mainly result from atherosclerosis and subsequent atherosclerotic plaque rupture, which rapidly generates a clot leading to MI and AIS. However, in general, most animal models of atherosclerosis do not develop thrombosis due to plaque rupture.122 Triggering thrombus formation, for example, by FeCl3 injury only replicates the final stages of atherothrombotic disease.123 Nevertheless, FeCl3 or needles have been used to induce thrombus formation in atherosclerosis-relevant models (eg, mice deficient in apolipoprotein E).124–126 Analysis of the corresponding thrombi compositions in relevant models for atherosclerosis may provide new insights in thrombus structure in the context of cardiovascular disease. Future studies should focus on the role of RBCs in addition to other clot components, as well as address questions regarding thrombus heterogeneity, composition, and their role in outcomes and treatment.
Animal Models of Venous Thrombosis
McGuinness et al127 showed that 1-day old thrombi generated after stenosis of rat inferior vena cava consisted of platelets, RBCs, leukocytes, and fibrin, with neutrophils being the main leukocyte. Monocytes located initially to the thrombus edge but were more evenly distributed in mature thrombi.127 RBC hyperaggregability induced by pluronic F98 treated RBCs correlated with thrombosis occurrence in a rabbit venous thrombosis model.128 Venous thrombi from baboon iliac veins induced by temporary balloon occlusion contained NETs, with diffuse staining of histones and extracellular DNA colocalizing with VWF.42 Murine inferior vena cava thrombosis models showed that NETs are primarily located in RBC-rich regions, and colocalized with VWF.39 In agreement with NETosis in murine venous thrombi, NETs are also present in human venous thrombi obtained by thrombectomy.129
One-day old murine venous thrombi generated by combined reduced flow and mechanical endothelial injury were RBC-rich in the center with fibrin deposition at the periphery as demonstrated by in vivo magnetization transfer and diffusion-weighted MRI coupled with Martius scarlet blue staining. After 1 week, the central part contained RBCs encapsulated in fibrin, while after 4 weeks thrombi were mainly collagen rich.130 This suggests that magnetization transfer and diffusion-weighted MRI are promising for determining thrombus age via its composition. Young thrombi were rich in fibrin and RBCs, while collagen fibers were present after 1 week after FeCl3 injury of rat carotid arteries and femoral veins as demonstrated by histology.65 Fibrin peaked after 1 day in both venous and arterial thrombi, and venous thrombi showed more fibrin than arterial thrombi after a week, which was also detected by positron-emitted tomography imaging using the fibrin-specific 64Cu-FBP8 probe.65 Imaging techniques could provide a useful clinical noninvasive tool for detecting thrombus and assessing thrombus age. More agents targeting thrombus components, mainly fibrin and platelets, have been validated in thrombosis models in vivo.131–133 For example, contrast agents (microparticles of iron oxide and antibody targeting activated αIIbβ3) have been used to analyze platelets and to monitor thrombolysis by MRI in murine arterial thrombi induced by FeCl3.131 Contrast agent targeting fibrin (EP-2104R) allow MRI detection of fibrin content in thrombi and indicated thrombus susceptibility to thrombolysis in a murine venous thrombosis model induced by reducing blood flow and endothelial disruption.132 Another study used near-infrared fluorescence method with fibrin-targeted agent (FTP11-Cy7) in deep vein thrombosis models induced by FeCl3.133
Murine venous thrombosis models provide key data on the maturation of venous thrombus. Venous thrombi showed changes of thrombus components from 2 to 4 weeks, from fibrin-dominant to collagen-dominant thrombi with increasing infiltration of inflammatory and mesenchymal cells, and these changes were correlated with clot stiffness.80 Due to the decreasing fibrin content with age, fibrinolytic efficiency reduces with increasing thrombus age in murine venous thrombosis models.81
Furthermore, murine inferior vena cava stasis thrombi showed areas rich in RBCs, fibrin(ogen), and neutrophils, but also contained monocytes and macrophages, with leukocytes colocalizing with urokinase plasminogen activator. PAI-1 colocalized with platelets, while plasminogen and α2-antiplasmin were also present in venous thrombi.134 Taken together, these studies indicate that thrombus composition changes with age, which likely impacts on disease development and treatment.
Choice of Thrombosis Model
Overall, animal models of arterial and venous thrombosis help gain valuable information on the content and structure of thrombi, with structural characteristics similar to human thrombi, thus offering opportunities for the development of new diagnostic and therapeutic tools. However, there is no single model representing all aspects of arterial and venous thrombosis, and at all stages of disease, while different methods of thrombosis induction can impact thrombus formation timing, composition and architecture. Unlike in patients, thrombus formation in animal thrombosis models mainly occurs in healthy vessels that are acutely injured.109 Developing new models that better reflect a diseased environment (eg, inflammation and metabolic disease) could further support improved characterization of thromboembolism and thrombus structure. Small models (eg, mice) showed their value for mechanistic insights into thrombosis, particularly in view of the relative ease of genetic modifications. However, future studies in larger animal models that are anatomically more similar (including the vasculature) to humans than smaller species may be of interest.109 Therefore, with each model offering its own benefits and limitations, it is important to carefully select thrombosis models and animal species based on study-specific objectives.
Conclusions
The advent of thrombectomy to treat a growing number of diseases heralds a new era in thrombosis research. It has enabled the analysis of thrombi from patients in ever greater detail, thus learning new information about their individual make-up. Careful consideration should be given to methods of thrombus composition analysis as each has their advantages and disadvantages. Another important consideration is to complement component-specific staining-based techniques with other methods that provide structural information at high resolution so that no particular thrombus components may be overlooked. Based on the literature thus far thrombus composition clearly is heterogeneous, varying between thrombotic disorders and patients, but even within the same patient or thrombus. Areas for future research include the relationship between thrombus areas and arterial or venous thrombosis, and how thrombus composition changes over time. Other remaining questions include how thrombus composition associates with embolism, effectiveness of thrombectomy or thrombolysis, and the role of polyhedrocytes, fibrin films, or other new structures in thrombosis. The development of better techniques to investigate thrombus composition, including noninvasive imaging methods, and improved animal models that are more physiologically relevant to human disease, will further be beneficial for future improvements in prevention and treatment of this devastating disease.
Sources of Funding
R.A.S. Ariëns is supported by grants from the BHF (RG/18/11/34036) and the Wellcome Trust (204951/B/16/Z). F.L. Macrae is supported by the Wellcome Trust (215861/Z/19/Z).
Disclosures
None.
Nonstandard Abbreviations and Acronyms
- AIS
- acute ischemic stroke
- LSCM
- laser scanning confocal microscopy
- MI
- myocardial infarction
- MRI
- magnetic resonance imaging
- NETs
- neutrophil extracellular traps
- PAI-1
- plasminogen activator inhibitor-1
- PE
- pulmonary embolism
- RBCs
- red blood cells
- SEM
- scanning electron microscopy
- STEMI
- ST-segment–elevation myocardial infarction
- TF
- tissue factor
- tPA
- tissue-type plasminogen activator
- VTE
- venous thromboembolism
- VWF
- von Willebrand factor
For Sources of Funding and Disclosures, see page 2379.
Contributor Information
Ghadir Alkarithi, Email: umgaal@leeds.ac.uk.
Cédric Duval, Email: C.Duval@leeds.ac.uk.
Yu Shi, Email: umys@leeds.ac.uk.
Fraser L. Macrae, Email: medfma@leeds.ac.uk.
References
- 1.Undas A, Ariëns RA. Fibrin clot structure and function: a role in the pathophysiology of arterial and venous thromboembolic diseases. Arterioscler Thromb Vasc Biol. 2011;31:e88–e99. doi: 10.1161/ATVBAHA.111.230631 [DOI] [PubMed] [Google Scholar]
- 2.Kattula S, Byrnes JR, Wolberg AS. Fibrinogen and fibrin in hemostasis and thrombosis. Arterioscler Thromb Vasc Biol. 2017;37:e13–e21. doi: 10.1161/ATVBAHA.117.308564 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Litvinov RI, Weisel JW. What Is the Biological and Clinical Relevance of Fibrin? Semin Thromb Hemost. 2016;42:333–343. doi: 10.1055/s-0036-1571342 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Weisel JW. The mechanical properties of fibrin for basic scientists and clinicians. Biophys Chem. 2004;112:267–276. doi: 10.1016/j.bpc.2004.07.029 [DOI] [PubMed] [Google Scholar]
- 5.Collet JP, Park D, Lesty C, Soria J, Soria C, Montalescot G, Weisel JW. Influence of fibrin network conformation and fibrin fiber diameter on fibrinolysis speed: dynamic and structural approaches by confocal microscopy. Arterioscler Thromb Vasc Biol. 2000;20:1354–1361. doi: 10.1161/01.atv.20.5.1354 [DOI] [PubMed] [Google Scholar]
- 6.Abela GS, Eisenberg JD, Mittleman MA, Nesto RW, Leeman D, Zarich S, Waxman S, Prieto AR, Manzo KS. Detecting and differentiating white from red coronary thrombus by angiography in angina pectoris and in acute myocardial infarction. Am J Cardiol. 1999;83:94–7, A8. doi: 10.1016/s0002-9149(98)00786-3 [DOI] [PubMed] [Google Scholar]
- 7.Quadros AS, Cambruzzi E, Sebben J, David RB, Abelin A, Welter D, Sarmento-Leite R, Mehta RH, Gottschall CA, Lopes RD. Red versus white thrombi in patients with ST-elevation myocardial infarction undergoing primary percutaneous coronary intervention: clinical and angiographic outcomes. Am Heart J. 2012;164:553–560. doi: 10.1016/j.ahj.2012.07.022 [DOI] [PubMed] [Google Scholar]
- 8.Sambola A, García Del Blanco B, Ruiz-Meana M, Francisco J, Barrabés JA, Figueras J, Bañeras J, Otaegui I, Rojas A, Vilardosa Ú, et al. Increased von Willebrand factor, P-selectin and fibrin content in occlusive thrombus resistant to lytic therapy. Thromb Haemost. 2016;115:1129–1137. doi: 10.1160/TH15-12-0985 [DOI] [PubMed] [Google Scholar]
- 9.Gunning GM, McArdle K, Mirza M, Duffy S, Gilvarry M, Brouwer PA. Clot friction variation with fibrin content; implications for resistance to thrombectomy. J Neurointerv Surg. 2018;10:34–38. doi: 10.1136/neurintsurg-2016-012721 [DOI] [PubMed] [Google Scholar]
- 10.Macrae FL, Duval C, Papareddy P, Baker SR, Yuldasheva N, Kearney KJ, McPherson HR, Asquith N, Konings J, Casini A, et al. A fibrin biofilm covers blood clots and protects from microbial invasion. J Clin Invest. 2018;128:3356–3368. doi: 10.1172/JCI98734 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Undas A, Stepień E, Rudziński P, Sadowski J. Architecture of a pulmonary thrombus removed during embolectomy in a patient with acute pulmonary embolism. J Thorac Cardiovasc Surg. 2010;140:e40–e41. doi: 10.1016/j.jtcvs.2009.07.038 [DOI] [PubMed] [Google Scholar]
- 12.Autar ASA, Hund HM, Ramlal SA, Hansen D, Lycklama À Nijeholt GJ, Emmer BJ, de Maat MPM, Dippel DWJ, van der Lugt A, van Es ACGM, et al. ; MR CLEAN Registry Investigators. High-resolution imaging of interaction between thrombus and stent-retriever in patients with acute ischemic stroke. J Am Heart Assoc. 2018;7:e008563. doi: 10.1161/JAHA.118.008563 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ząbczyk M, Natorska J, Zalewski J, Undas A. Fibrin biofilm can be detected on intracoronary thrombi aspirated from patients with acute myocardial infarction. Cardiovasc Res. 2019;115:1026–1028. doi: 10.1093/cvr/cvz019 [DOI] [PubMed] [Google Scholar]
- 14.Di Meglio L, Desilles JP, Ollivier V, Nomenjanahary MS, Di Meglio S, Deschildre C, Loyau S, Olivot JM, Blanc R, Piotin M, et al. Acute ischemic stroke thrombi have an outer shell that impairs fibrinolysis. Neurology. 2019;93:e1686–e1698. doi: 10.1212/WNL.0000000000008395 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bevers EM, Comfurius P, van Rijn JL, Hemker HC, Zwaal RF. Generation of prothrombin-converting activity and the exposure of phosphatidylserine at the outer surface of platelets. Eur J Biochem. 1982;122:429–436. doi: 10.1111/j.1432-1033.1982.tb05898.x [DOI] [PubMed] [Google Scholar]
- 16.Heemskerk JW, Mattheij NJ, Cosemans JM. Platelet-based coagulation: different populations, different functions. J Thromb Haemost. 2013;11:2–16. doi: 10.1111/jth.12045 [DOI] [PubMed] [Google Scholar]
- 17.Agbani EO, van den Bosch MT, Brown E, Williams CM, Mattheij NJ, Cosemans JM, Collins PW, Heemskerk JW, Hers I, Poole AW. Coordinated membrane ballooning and procoagulant spreading in human platelets. Circulation. 2015;132:1414–1424. doi: 10.1161/CIRCULATIONAHA.114.015036 [DOI] [PubMed] [Google Scholar]
- 18.Mammadova-Bach E, Ollivier V, Loyau S, Schaff M, Dumont B, Favier R, Freyburger G, Latger-Cannard V, Nieswandt B, Gachet C, et al. Platelet glycoprotein VI binds to polymerized fibrin and promotes thrombin generation. Blood. 2015;126:683–691. doi: 10.1182/blood-2015-02-629717 [DOI] [PubMed] [Google Scholar]
- 19.Alshehri OM, Hughes CE, Montague S, Watson SK, Frampton J, Bender M, Watson SP. Fibrin activates GPVI in human and mouse platelets. Blood. 2015;126:1601–1608. doi: 10.1182/blood-2015-04-641654 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Vilalta N, Vazquez-Santiago M, Cuevas B, Macho R, Remacha A, Carrasco M, Mateo J, Millon J, Soria JM, Souto JC. The relationship between leukocyte counts and venous thromboembolism: results from RETROVE study. Biol Med (Aligarh). 2017;9:400. doi: 10.4172/0974-8369.1000400 [Google Scholar]
- 21.Hagberg IA, Roald HE, Lyberg T. Adhesion of leukocytes to growing arterial thrombi. Thromb Haemost. 1998;80:852–858 [PubMed] [Google Scholar]
- 22.Flick MJ, Du X, Witte DP, Jirousková M, Soloviev DA, Busuttil SJ, Plow EF, Degen JL. Leukocyte engagement of fibrin(ogen) via the integrin receptor alphaMbeta2/Mac-1 is critical for host inflammatory response in vivo. J Clin Invest. 2004;113:1596–1606. doi: 10.1172/JCI20741 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Korkmaz B, Horwitz MS, Jenne DE, Gauthier F. Neutrophil elastase, proteinase 3, and cathepsin G as therapeutic targets in human diseases. Pharmacol Rev. 2010;62:726–759. doi: 10.1124/pr.110.002733 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Seth P, Kumari R, Dikshit M, Srimal RC. Effect of platelet activating factor antagonists in different models of thrombosis. Thromb Res. 1994;76:503–512. doi: 10.1016/0049-3848(94)90279-8 [DOI] [PubMed] [Google Scholar]
- 25.Allen DH, Tracy PB. Human coagulation factor V is activated to the functional cofactor by elastase and cathepsin G expressed at the monocyte surface. J Biol Chem. 1995;270:1408–1415. doi: 10.1074/jbc.270.3.1408 [DOI] [PubMed] [Google Scholar]
- 26.Gale AJ, Rozenshteyn D. Cathepsin G, a leukocyte protease, activates coagulation factor VIII. Thromb Haemost. 2008;99:44–51. doi: 10.1160/TH07-08-0495 [DOI] [PubMed] [Google Scholar]
- 27.Plescia J, Altieri DC. Activation of Mac-1 (CD11b/CD18)-bound factor X by released cathepsin G defines an alternative pathway of leucocyte initiation of coagulation. Biochem J. 1996;319(pt 3):873–879. doi: 10.1042/bj3190873 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.LaRosa CA, Rohrer MJ, Benoit SE, Rodino LJ, Barnard MR, Michelson AD. Human neutrophil cathepsin G is a potent platelet activator. J Vasc Surg. 1994;19:306–318. doi: 10.1016/s0741-5214(94)70106-7 [DOI] [PubMed] [Google Scholar]
- 29.Jochum M, Lander S, Heimburger N, Fritz H. Effect of human granulocytic elastase on isolated human antithrombin III. Hoppe Seylers Z Physiol Chem. 1981;362:103–112. doi: 10.1515/bchm2.1981.362.1.103 [DOI] [PubMed] [Google Scholar]
- 30.Petersen LC, Bjørn SE, Nordfang O. Effect of leukocyte proteinases on tissue factor pathway inhibitor. Thromb Haemost. 1992;67:537–541 [PubMed] [Google Scholar]
- 31.Shantsila E, Lip GY. The role of monocytes in thrombotic disorders. Insights from tissue factor, monocyte-platelet aggregates and novel mechanisms. Thromb Haemost. 2009;102:916–924. doi: 10.1160/TH09-01-0023 [DOI] [PubMed] [Google Scholar]
- 32.Darbousset R, Thomas GM, Mezouar S, Frère C, Bonier R, Mackman N, Renné T, Dignat-George F, Dubois C, Panicot-Dubois L. Tissue factor-positive neutrophils bind to injured endothelial wall and initiate thrombus formation. Blood. 2012;120:2133–2143. doi: 10.1182/blood-2012-06-437772 [DOI] [PubMed] [Google Scholar]
- 33.Ritis K, Doumas M, Mastellos D, Micheli A, Giaglis S, Magotti P, Rafail S, Kartalis G, Sideras P, Lambris JD. A novel C5a receptor-tissue factor cross-talk in neutrophils links innate immunity to coagulation pathways. J Immunol. 2006;177:4794–4802. doi: 10.4049/jimmunol.177.7.4794 [DOI] [PubMed] [Google Scholar]
- 34.Moosbauer C, Morgenstern E, Cuvelier SL, Manukyan D, Bidzhekov K, Albrecht S, Lohse P, Patel KD, Engelmann B. Eosinophils are a major intravascular location for tissue factor storage and exposure. Blood. 2007;109:995–1002. doi: 10.1182/blood-2006-02-004945 [DOI] [PubMed] [Google Scholar]
- 35.Osterud B, Rao LV, Olsen JO. Induction of tissue factor expression in whole blood: lack of evidence for the presence of tissue factor expression in granulocytes. Thromb Haemost. 2000;83:861–867 [PubMed] [Google Scholar]
- 36.Sovershaev MA, Lind KF, Devold H, Jørgensen TØ, Hansen JB, Østerud B, Egorina EM. No evidence for the presence of tissue factor in high-purity preparations of immunologically isolated eosinophils. J Thromb Haemost. 2008;6:1742–1749. doi: 10.1111/j.1538-7836.2008.03105.x [DOI] [PubMed] [Google Scholar]
- 37.Egorina EM, Sovershaev MA, Olsen JO, Østerud B. Granulocytes do not express but acquire monocyte-derived tissue factor in whole blood: evidence for a direct transfer. Blood. 2008;111:1208–1216. doi: 10.1182/blood-2007-08-107698 [DOI] [PubMed] [Google Scholar]
- 38.Martinod K, Wagner DD. Thrombosis: tangled up in NETs. Blood. 2014;123:2768–2776. doi: 10.1182/blood-2013-10-463646 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Brill A, Fuchs TA, Savchenko AS, Thomas GM, Martinod K, De Meyer SF, Bhandari AA, Wagner DD. Neutrophil extracellular traps promote deep vein thrombosis in mice. J Thromb Haemost. 2012;10:136–144. doi: 10.1111/j.1538-7836.2011.04544.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Laridan E, Martinod K, De Meyer SF. Neutrophil Extracellular Traps in Arterial and Venous Thrombosis. Semin Thromb Hemost. 2019;45:86–93. doi: 10.1055/s-0038-1677040 [DOI] [PubMed] [Google Scholar]
- 41.Longstaff C, Varjú I, Sótonyi P, Szabó L, Krumrey M, Hoell A, Bóta A, Varga Z, Komorowicz E, Kolev K. Mechanical stability and fibrinolytic resistance of clots containing fibrin, DNA, and histones. J Biol Chem. 2013;288:6946–6956. doi: 10.1074/jbc.M112.404301 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Fuchs TA, Brill A, Duerschmied D, Schatzberg D, Monestier M, Myers DD, Jr, Wrobleski SK, Wakefield TW, Hartwig JH, Wagner DD. Extracellular DNA traps promote thrombosis. Proc Natl Acad Sci U S A. 2010;107:15880–15885. doi: 10.1073/pnas.1005743107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Noubouossie DF, Whelihan MF, Yu YB, Sparkenbaugh E, Pawlinski R, Monroe DM, Key NS. In vitro activation of coagulation by human neutrophil DNA and histone proteins but not neutrophil extracellular traps. Blood. 2017;129:1021–1029. doi: 10.1182/blood-2016-06-722298 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Staessens S, Denorme F, Francois O, Desender L, Dewaele T, Vanacker P, Deckmyn H, Vanhoorelbeke K, Andersson T, De Meyer SF. Structural analysis of ischemic stroke thrombi: histological indications for therapy resistance. Haematologica. 2020;105:498–507. doi: 10.3324/haematol.2019.219881 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Reimers RC, Sutera SP, Joist JH. Potentiation by red blood cells of shear-induced platelet aggregation: relative importance of chemical and physical mechanisms. Blood. 1984;64:1200–1206 [PubMed] [Google Scholar]
- 46.Klatt C, Krüger I, Zey S, Krott KJ, Spelleken M, Gowert NS, Oberhuber A, Pfaff L, Lückstädt W, Jurk K, et al. Platelet-RBC interaction mediated by FasL/FasR induces procoagulant activity important for thrombosis. J Clin Invest. 2018;128:3906–3925. doi: 10.1172/JCI92077 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Whelihan MF, Zachary V, Orfeo T, Mann KG. Prothrombin activation in blood coagulation: the erythrocyte contribution to thrombin generation. Blood. 2012;120:3837–3845. doi: 10.1182/blood-2012-05-427856 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Walton BL, Lehmann M, Skorczewski T, Holle LA, Beckman JD, Cribb JA, Mooberry MJ, Wufsus AR, Cooley BC, Homeister JW, et al. Elevated hematocrit enhances platelet accumulation following vascular injury. Blood. 2017;129:2537–2546. doi: 10.1182/blood-2016-10-746479 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Aleman MM, Byrnes JR, Wang JG, Tran R, Lam WA, Di Paola J, Mackman N, Degen JL, Flick MJ, Wolberg AS. Factor XIII activity mediates red blood cell retention in venous thrombi. J Clin Invest. 2014;124:3590–3600. doi: 10.1172/JCI75386 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Cines DB, Lebedeva T, Nagaswami C, Hayes V, Massefski W, Litvinov RI, Rauova L, Lowery TJ, Weisel JW. Clot contraction: compression of erythrocytes into tightly packed polyhedra and redistribution of platelets and fibrin. Blood. 2014;123:1596–1603. doi: 10.1182/blood-2013-08-523860 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Zalewski J, Lewicki L, Krawczyk K, Zabczyk M, Targonski R, Molek P, Nessler J, Undas A. Polyhedral erythrocytes in intracoronary thrombus and their association with reperfusion in myocardial infarction. Clin Res Cardiol. 2019;108:950–962. doi: 10.1007/s00392-019-01425-x [DOI] [PubMed] [Google Scholar]
- 52.Zalewski J, Bogaert J, Sadowski M, Woznicka O, Doulaptsis K, Ntoumpanaki M, Ząbczyk M, Nessler J, Undas A. Plasma fibrin clot phenotype independently affects intracoronary thrombus ultrastructure in patients with acute myocardial infarction. Thromb Haemost. 2015;113:1258–1269. doi: 10.1160/TH14-09-0801 [DOI] [PubMed] [Google Scholar]
- 53.Chernysh IN, Nagaswami C, Kosolapova S, Peshkova AD, Cuker A, Cines DB, Cambor CL, Litvinov RI, Weisel JW. The distinctive structure and composition of arterial and venous thrombi and pulmonary emboli. Sci Rep. 2020;10:5112. doi: 10.1038/s41598-020-59526-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Khismatullin RR, Nagaswami C, Shakirova AZ, Vrtková A, Procházka V, Gumulec J, Mačák J, Litvinov RI, Weisel JW. Quantitative morphology of cerebral thrombi related to intravital contraction and clinical features of ischemic stroke. Stroke. 2020;51:3640–3650. doi: 10.1161/STROKEAHA.120.031559 [DOI] [PubMed] [Google Scholar]
- 55.Wohner N, Sótonyi P, Machovich R, Szabó L, Tenekedjiev K, Silva MM, Longstaff C, Kolev K. Lytic resistance of fibrin containing red blood cells. Arterioscler Thromb Vasc Biol. 2011;31:2306–2313. doi: 10.1161/ATVBAHA.111.229088 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Gersh KC, Nagaswami C, Weisel JW. Fibrin network structure and clot mechanical properties are altered by incorporation of erythrocytes. Thromb Haemost. 2009;102:1169–1175. doi: 10.1160/TH09-03-0199 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Alapan Y, Little JA, Gurkan UA. Heterogeneous red blood cell adhesion and deformability in sickle cell disease. Sci Rep. 2014;4:7173. doi: 10.1038/srep07173 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.van Gelder JM, Nair CH, Dhall DP. Erythrocyte aggregation and erythrocyte deformability modify the permeability of erythrocyte enriched fibrin network. Thromb Res. 1996;82:33–42. doi: 10.1016/0049-3848(96)00048-5 [DOI] [PubMed] [Google Scholar]
- 59.Yunoki K, Naruko T, Sugioka K, Inaba M, Iwasa Y, Komatsu R, Itoh A, Haze K, Inoue T, Yoshiyama M, et al. Erythrocyte-rich thrombus aspirated from patients with ST-elevation myocardial infarction: association with oxidative stress and its impact on myocardial reperfusion. Eur Heart J. 2012;33:1480–1490. doi: 10.1093/eurheartj/ehr486 [DOI] [PubMed] [Google Scholar]
- 60.Ruggeri ZM. The role of von Willebrand factor in thrombus formation. Thromb Res. 2007;120(suppl 1):S5–S9. doi: 10.1016/j.thromres.2007.03.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Yamashita A, Sumi T, Goto S, Hoshiba Y, Nishihira K, Kawamoto R, Hatakeyama K, Date H, Imamura T, Ogawa H, et al. Detection of von Willebrand factor and tissue factor in platelets-fibrin rich coronary thrombi in acute myocardial infarction. Am J Cardiol. 2006;97:26–28. doi: 10.1016/j.amjcard.2005.07.105 [DOI] [PubMed] [Google Scholar]
- 62.Silvain J, Collet JP, Nagaswami C, Beygui F, Edmondson KE, Bellemain-Appaix A, Cayla G, Pena A, Brugier D, Barthelemy O, et al. Composition of coronary thrombus in acute myocardial infarction. J Am Coll Cardiol. 2011;57:1359–1367. doi: 10.1016/j.jacc.2010.09.077 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Silvain J, Collet JP, Guedeney P, Varenne O, Nagaswami C, Maupain C, Empana JP, Boulanger C, Tafflet M, Manzo-Silberman S, et al. Thrombus composition in sudden cardiac death from acute myocardial infarction. Resuscitation. 2017;113:108–114. doi: 10.1016/j.resuscitation.2017.01.030 [DOI] [PubMed] [Google Scholar]
- 64.Marder VJ, Chute DJ, Starkman S, Abolian AM, Kidwell C, Liebeskind D, Ovbiagele B, Vinuela F, Duckwiler G, Jahan R, et al. Analysis of thrombi retrieved from cerebral arteries of patients with acute ischemic stroke. Stroke. 2006;37:2086–2093. doi: 10.1161/01.STR.0000230307.03438.94 [DOI] [PubMed] [Google Scholar]
- 65.Blasi F, Oliveira BL, Rietz TA, Rotile NJ, Naha PC, Cormode DP, Izquierdo-Garcia D, Catana C, Caravan P. Multisite thrombus imaging and fibrin content estimation with a single whole-body PET scan in rats. Arterioscler Thromb Vasc Biol. 2015;35:2114–2121. doi: 10.1161/ATVBAHA.115.306055 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Oliveira BL, Caravan P. Peptide-based fibrin-targeting probes for thrombus imaging. Dalton Trans. 2017;46:14488–14508. doi: 10.1039/c7dt02634j [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Huang SJ, Diao SS, Lu Y, Li T, Zhang LL, Ding YP, Fang Q, Cai XY, Xu Z, Kong Y. Value of thrombus imaging in predicting the outcomes of patients with large-vessel occlusive strokes after endovascular therapy. Neurol Sci. 2020;41:1451–1458. doi: 10.1007/s10072-020-04296-7 [DOI] [PubMed] [Google Scholar]
- 68.Alonso-Orgaz S, Moreno-Luna R, López JA, Gil-Dones F, Padial LR, Moreu J, de la Cuesta F, Barderas MG. Proteomic characterization of human coronary thrombus in patients with ST-segment elevation acute myocardial infarction. J Proteomics. 2014;109:368–381. doi: 10.1016/j.jprot.2014.07.016 [DOI] [PubMed] [Google Scholar]
- 69.Muñoz R, Santamaría E, Rubio I, Ausín K, Ostolaza A, Labarga A, Roldán M, Zandio B, Mayor S, Bermejo R, et al. Mass spectrometry-based proteomic profiling of thrombotic material obtained by endovascular thrombectomy in patients with ischemic stroke. Int J Mol Sci. 2018;19:498. doi: 10.3390/ijms19020498 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.van Rooy MJ, Duim W, Ehlers R, Buys AV, Pretorius E. Platelet hyperactivity and fibrin clot structure in transient ischemic attack individuals in the presence of metabolic syndrome: a microscopy and thromboelastography study. Cardiovasc Diabetol. 2015;14:86. doi: 10.1186/s12933-015-0249-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Krautgartner WD, Klappacher M, Hannig M, Obermayer A, Hartl D, Marcos V, Vitkov L. Fibrin mimics neutrophil extracellular traps in SEM. Ultrastruct Pathol. 2010;34:226–231. doi: 10.3109/01913121003725721 [DOI] [PubMed] [Google Scholar]
- 72.Höök P, Brito-Robinson T, Kim O, Narciso C, Goodson HV, Weisel JW, Alber MS, Zartman JJ. Whole blood clot optical clearing for nondestructive 3D imaging and quantitative analysis. Biomed Opt Express. 2017;8:3671–3686. doi: 10.1364/BOE.8.003671 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Page MJ, Thomson GJA, Nunes JM, Engelbrecht AM, Nell TA, de Villiers WJS, de Beer MC, Engelbrecht L, Kell DB, Pretorius E. Serum amyloid A binds to fibrin(ogen), promoting fibrin amyloid formation. Sci Rep. 2019;9:3102. doi: 10.1038/s41598-019-39056-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Rao NM, Capri J, Cohn W, Abdaljaleel M, Restrepo L, Gornbein JA, Yong WH, Liebeskind DS, Whitelegge JP. Peptide composition of stroke causing emboli correlate with serum markers of atherosclerosis and inflammation. Front Neurol. 2017;8:427. doi: 10.3389/fneur.2017.00427 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Wolberg AS, Rosendaal FR, Weitz JI, Jaffer IH, Agnelli G, Baglin T, Mackman N. Venous thrombosis. Nat Rev Dis Primers. 2015;1:15006. doi: 10.1038/nrdp.2015.6 [DOI] [PubMed] [Google Scholar]
- 76.Mazur P, Sobczyński R, Ząbczyk M, Babiarczyk P, Sadowski J, Undas A. Architecture of fibrin network inside thrombotic material obtained from the right atrium and pulmonary arteries: flow and location matter. J Thromb Thrombolysis. 2013;35:127–129. doi: 10.1007/s11239-012-0806-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Walton BL, Byrnes JR, Wolberg AS. Fibrinogen, red blood cells, and factor XIII in venous thrombosis. J Thromb Haemost. 2015;13(suppl 1):S208–S215. doi: 10.1111/jth.12918 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Takahashi M, Yamashita A, Moriguchi-Goto S, Marutsuka K, Sato Y, Yamamoto H, Koshimoto C, Asada Y. Critical role of von Willebrand factor and platelet interaction in venous thromboembolism. Histol Histopathol. 2009;24:1391–1398. doi: 10.14670/HH-24.1391 [DOI] [PubMed] [Google Scholar]
- 79.Chernysh IN, Spiewak R, Cambor CL, Purohit PK, Weisel JW. Structure, mechanical properties, and modeling of cyclically compressed pulmonary emboli. J Mech Behav Biomed Mater. 2020;105:103699. doi: 10.1016/j.jmbbm.2020.103699 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Lee YU, Lee AY, Humphrey JD, Rausch MK. Histological and biomechanical changes in a mouse model of venous thrombus remodeling. Biorheology. 2015;52:235–245. doi: 10.3233/BIR-15058 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Stein-Merlob AF, Kessinger CW, Erdem SS, Zelada H, Hilderbrand SA, Lin CP, Tearney GJ, Jaff MR, Reed GL, Henke PK, et al. Blood accessibility to fibrin in venous thrombosis is thrombus age-dependent and predicts fibrinolytic efficacy: an in vivo fibrin molecular imaging study. Theranostics. 2015;5:1317–1327. doi: 10.7150/thno.12494 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Fineschi V, Turillazzi E, Neri M, Pomara C, Riezzo I. Histological age determination of venous thrombosis: a neglected forensic task in fatal pulmonary thrombo-embolism. Forensic Sci Int. 2009;186:22–28. doi: 10.1016/j.forsciint.2009.01.006 [DOI] [PubMed] [Google Scholar]
- 83.Silver MJ, Kawakami R, Jolly MA, Huff CM, Phillips JA, Sakamoto A, Kawai K, Kutys B, Guo L, Cornelissen A, et al. Histopathologic analysis of extracted thrombi from deep venous thrombosis and pulmonary embolism: mechanisms and timing. Catheter Cardiovasc Interv. 2021;97:1422–1429. doi: 10.1002/ccd.29500 [DOI] [PubMed] [Google Scholar]
- 84.Mackman N. Triggers, targets and treatments for thrombosis. Nature. 2008;451:914–918. doi: 10.1038/nature06797 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Sadowski M, Ząbczyk M, Undas A. Coronary thrombus composition: links with inflammation, platelet and endothelial markers. Atherosclerosis. 2014;237:555–561. doi: 10.1016/j.atherosclerosis.2014.10.020 [DOI] [PubMed] [Google Scholar]
- 86.Ramaiola I, Padró T, Peña E, Juan-Babot O, Cubedo J, Martin-Yuste V, Sabate M, Badimon L. Changes in thrombus composition and profilin-1 release in acute myocardial infarction. Eur Heart J. 2015;36:965–975. doi: 10.1093/eurheartj/ehu356 [DOI] [PubMed] [Google Scholar]
- 87.Nishihira K, Yamashita A, Ishikawa T, Hatakeyama K, Shibata Y, Asada Y. Composition of thrombi in late drug-eluting stent thrombosis versus de novo acute myocardial infarction. Thromb Res. 2010;126:254–257. doi: 10.1016/j.thromres.2009.11.010 [DOI] [PubMed] [Google Scholar]
- 88.Sato Y, Hatakeyama K, Yamashita A, Marutsuka K, Sumiyoshi A, Asada Y. Proportion of fibrin and platelets differs in thrombi on ruptured and eroded coronary atherosclerotic plaques in humans. Heart. 2005;91:526–530. doi: 10.1136/hrt.2004.034058 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Pircher J, Czermak T, Ehrlich A, Eberle C, Gaitzsch E, Margraf A, Grommes J, Saha P, Titova A, Ishikawa-Ankerhold H, et al. Cathelicidins prime platelets to mediate arterial thrombosis and tissue inflammation. Nat Commun. 2018;9:1523. doi: 10.1038/s41467-018-03925-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Kovács A, Sótonyi P, Nagy AI, Tenekedjiev K, Wohner N, Komorowicz E, Kovács E, Nikolova N, Szabó L, Kovalszky I, et al. Ultrastructure and composition of thrombi in coronary and peripheral artery disease: correlations with clinical and laboratory findings. Thromb Res. 2015;135:760–766. doi: 10.1016/j.thromres.2015.02.004 [DOI] [PubMed] [Google Scholar]
- 91.Maegerlein C, Friedrich B, Berndt M, Lucia KE, Schirmer L, Poppert H, Zimmer C, Pelisek J, Boeckh-Behrens T, Kaesmacher J. Impact of histological thrombus composition on preinterventional thrombus migration in patients with acute occlusions of the middle cerebral artery. Interv Neuroradiol. 2018;24:70–75. doi: 10.1177/1591019917733733 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Ni H, Denis CV, Subbarao S, Degen JL, Sato TN, Hynes RO, Wagner DD. Persistence of platelet thrombus formation in arterioles of mice lacking both von Willebrand factor and fibrinogen. J Clin Invest. 2000;106:385–392. doi: 10.1172/JCI9896 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Schuhmann MK, Gunreben I, Kleinschnitz C, Kraft P. Immunohistochemical analysis of cerebral thrombi retrieved by mechanical thrombectomy from patients with acute ischemic stroke. Int J Mol Sci. 2016;17:298. doi: 10.3390/ijms17030298 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Liebeskind DS, Sanossian N, Yong WH, Starkman S, Tsang MP, Moya AL, Zheng DD, Abolian AM, Kim D, Ali LK, et al. CT and MRI early vessel signs reflect clot composition in acute stroke. Stroke. 2011;42:1237–1243. doi: 10.1161/STROKEAHA.110.605576 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Simons N, Mitchell P, Dowling R, Gonzales M, Yan B. Thrombus composition in acute ischemic stroke: a histopathological study of thrombus extracted by endovascular retrieval. J Neuroradiol. 2015;42:86–92. doi: 10.1016/j.neurad.2014.01.124 [DOI] [PubMed] [Google Scholar]
- 96.Maekawa K, Shibata M, Nakajima H, Mizutani A, Kitano Y, Seguchi M, Yamasaki M, Kobayashi K, Sano T, Mori G, et al. Erythrocyte-rich thrombus is associated with reduced number of maneuvers and procedure time in patients with acute ischemic stroke undergoing mechanical thrombectomy. Cerebrovasc Dis Extra. 2018;8:39–49. doi: 10.1159/000486042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Sato Y, Ishibashi-Ueda H, Iwakiri T, Ikeda Y, Matsuyama T, Hatakeyama K, Asada Y. Thrombus components in cardioembolic and atherothrombotic strokes. Thromb Res. 2012;130:278–280. doi: 10.1016/j.thromres.2012.04.008 [DOI] [PubMed] [Google Scholar]
- 98.Mackman N, Bergmeier W, Stouffer GA, Weitz JI. Therapeutic strategies for thrombosis: new targets and approaches. Nat Rev Drug Discov. 2020;19:333–352. doi: 10.1038/s41573-020-0061-0 [DOI] [PubMed] [Google Scholar]
- 99.Koupenova M, Kehrel BE, Corkrey HA, Freedman JE. Thrombosis and platelets: an update. Eur Heart J. 2017;38:785–791. doi: 10.1093/eurheartj/ehw550 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Barnes GD. Combining antiplatelet and anticoagulant therapy in cardiovascular disease. Hematology Am Soc Hematol Educ Program. 2020;2020:642–648. doi: 10.1182/hematology.2020000151 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Mega JL, Braunwald E, Wiviott SD, Bassand JP, Bhatt DL, Bode C, Burton P, Cohen M, Cook-Bruns N, Fox KA, et al. ; ATLAS ACS 2–TIMI 51 Investigators. Rivaroxaban in patients with a recent acute coronary syndrome. N Engl J Med. 2012;366:9–19. doi: 10.1056/NEJMoa1112277 [DOI] [PubMed] [Google Scholar]
- 102.Eikelboom JW, Connolly SJ, Bosch J, Dagenais GR, Hart RG, Shestakovska O, Diaz R, Alings M, Lonn EM, Anand SS, et al. ; COMPASS Investigators. Rivaroxaban with or without aspirin in stable cardiovascular disease. N Engl J Med. 2017;377:1319–1330. doi: 10.1056/NEJMoa1709118 [DOI] [PubMed] [Google Scholar]
- 103.Undas A, Natorska J. Improving fibrinolysis in venous thromboembolism: impact of fibrin structure. Expert Rev Hematol. 2019;12:597–607. doi: 10.1080/17474086.2019.1627193 [DOI] [PubMed] [Google Scholar]
- 104.Ducroux C, Di Meglio L, Loyau S, Delbosc S, Boisseau W, Deschildre C, Ben Maacha M, Blanc R, Redjem H, Ciccio G, et al. Thrombus neutrophil extracellular traps content impair tPA-induced thrombolysis in acute ischemic stroke. Stroke. 2018;49:754–757. doi: 10.1161/STROKEAHA.117.019896 [DOI] [PubMed] [Google Scholar]
- 105.Mangold A, Alias S, Scherz T, Hofbauer M, Jakowitsch J, Panzenböck A, Simon D, Laimer D, Bangert C, Kammerlander A, et al. Coronary neutrophil extracellular trap burden and deoxyribonuclease activity in ST-elevation acute coronary syndrome are predictors of ST-segment resolution and infarct size. Circ Res. 2015;116:1182–1192. doi: 10.1161/CIRCRESAHA.116.304944 [DOI] [PubMed] [Google Scholar]
- 106.Shin JW, Jeong HS, Kwon HJ, Song KS, Kim J. High red blood cell composition in clots is associated with successful recanalization during intra-arterial thrombectomy. PLoS One. 2018;13:e0197492. doi: 10.1371/journal.pone.0197492 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Hashimoto T, Hayakawa M, Funatsu N, Yamagami H, Satow T, Takahashi JC, Nagatsuka K, Ishibashi-Ueda H, Kira JI, Toyoda K. Histopathologic analysis of retrieved thrombi associated with successful reperfusion after acute stroke thrombectomy. Stroke. 2016;47:3035–3037. doi: 10.1161/STROKEAHA.116.015228 [DOI] [PubMed] [Google Scholar]
- 108.Spaet TH, Stemerman MB, Veith FJ, Lejnieks I. Intimal injury and regrowth in the rabbit aorta; medial smooth muscle cells as a source of neointima. Circ Res. 1975;36:58–70. doi: 10.1161/01.res.36.1.58 [DOI] [PubMed] [Google Scholar]
- 109.Albadawi H, Witting AA, Pershad Y, Wallace A, Fleck AR, Hoang P, Khademhosseini A, Oklu R. Animal models of venous thrombosis. Cardiovasc Diagn Ther. 2017;7(suppl 3):S197–S206. doi: 10.21037/cdt.2017.08.10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Diaz JA, Saha P, Cooley B, Palmer OR, Grover SP, Mackman N, Wakefield TW, Henke PK, Smith A, Lal BK. Choosing a mouse model of venous thrombosis: a consensus assessment of utility and application. J Thromb Haemost. 2019;17:699–707. doi: 10.1111/jth.14413 [DOI] [PubMed] [Google Scholar]
- 111.Randall MJ, Wilding RI. Acute arterial thrombosis in rabbits: reduced platelet accumulation after treatment with dazoxiben hydrochloride (UK 37,248-01). Br J Clin Pharmacol. 1983;15(suppl 1):49s–55s. doi: 10.1111/j.1365-2125 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Haskel EJ, Adams SP, Feigen LP, Saffitz JE, Gorczynski RJ, Sobel BE, Abendschein DR. Prevention of reoccluding platelet-rich thrombi in canine femoral arteries with a novel peptide antagonist of platelet glycoprotein IIb/IIIa receptors. Circulation. 1989;80:1775–1782. doi: 10.1161/01.cir.80.6.1775 [DOI] [PubMed] [Google Scholar]
- 113.Jang IK, Gold HK, Ziskind AA, Fallon JT, Holt RE, Leinbach RC, May JW, Collen D. Differential sensitivity of erythrocyte-rich and platelet-rich arterial thrombi to lysis with recombinant tissue-type plasminogen activator. A possible explanation for resistance to coronary thrombolysis. Circulation. 1989;79:920–928. doi: 10.1161/01.cir.79.4.920 [DOI] [PubMed] [Google Scholar]
- 114.Halvorsen AM, Futrell N, Wang LC. Fibrin content of carotid thrombi alters the production of embolic stroke in the rat. Stroke. 1994;25:1632–1636. doi: 10.1161/01.str.25.8.1632 [DOI] [PubMed] [Google Scholar]
- 115.Kurz KD, Main BW, Sandusky GE. Rat model of arterial thrombosis induced by ferric chloride. Thromb Res. 1990;60:269–280. doi: 10.1016/0049-3848(90)90106-m [DOI] [PubMed] [Google Scholar]
- 116.Corti R, Osende JI, Fayad ZA, Fallon JT, Fuster V, Mizsei G, Dickstein E, Drayer B, Badimon JJ. In vivo noninvasive detection and age definition of arterial thrombus by MRI. J Am Coll Cardiol. 2002;39:1366–1373. doi: 10.1016/s0735-1097(02)01754-0 [DOI] [PubMed] [Google Scholar]
- 117.Srivatsa SS, Edwards WD, Boos CM, Grill DE, Sangiorgi GM, Garratt KN, Schwartz RS, Holmes DR., Jr.Histologic correlates of angiographic chronic total coronary artery occlusions: influence of occlusion duration on neovascular channel patterns and intimal plaque composition. J Am Coll Cardiol. 1997;29:955–963. doi: 10.1016/s0735-1097(97)00035-1 [DOI] [PubMed] [Google Scholar]
- 118.Yamashita A, Furukoji E, Marutsuka K, Hatakeyama K, Yamamoto H, Tamura S, Ikeda Y, Sumiyoshi A, Asada Y. Increased vascular wall thrombogenicity combined with reduced blood flow promotes occlusive thrombus formation in rabbit femoral artery. Arterioscler Thromb Vasc Biol. 2004;24:2420–2424. doi: 10.1161/01.ATV.0000147767.61336.de [DOI] [PubMed] [Google Scholar]
- 119.Hoshiba Y, Hatakeyama K, Tanabe T, Asada Y, Goto S. Co-localization of von Willebrand factor with platelet thrombi, tissue factor and platelets with fibrin, and consistent presence of inflammatory cells in coronary thrombi obtained by an aspiration device from patients with acute myocardial infarction. J Thromb Haemost. 2006;4:114–120. doi: 10.1111/j.1538-7836.2005.01701.x [DOI] [PubMed] [Google Scholar]
- 120.Yamashita A, Matsuda S, Matsumoto T, Moriguchi-Goto S, Takahashi M, Sugita C, Sumi T, Imamura T, Shima M, Kitamura K, et al. Thrombin generation by intimal tissue factor contributes to thrombus formation on macrophage-rich neointima but not normal intima of hyperlipidemic rabbits. Atherosclerosis. 2009;206:418–426. doi: 10.1016/j.atherosclerosis.2009.03.014 [DOI] [PubMed] [Google Scholar]
- 121.Novotny J, Chandraratne S, Weinberger T, Philippi V, Stark K, Ehrlich A, Pircher J, Konrad I, Oberdieck P, Titova A, et al. Histological comparison of arterial thrombi in mice and men and the influence of Cl-amidine on thrombus formation. PLoS One. 2018;13:e0190728. doi: 10.1371/journal.pone.0190728 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Emini Veseli B, Perrotta P, De Meyer GRA, Roth L, Van der Donckt C, Martinet W, De Meyer GRY. Animal models of atherosclerosis. Eur J Pharmacol. 2017;816:3–13. doi: 10.1016/j.ejphar.2017.05.010 [DOI] [PubMed] [Google Scholar]
- 123.Grover S, Mackman N. How useful are ferric chloride models of arterial thrombosis? Platelets. 31:432–2020. doi: 10.1080/09537104.2019.1678119 [DOI] [PubMed] [Google Scholar]
- 124.Schafer K, Müller K, Hecke A, Mounier E, Goebel J, Loskutoff DJ, Konstantinides S. Enhanced thrombosis in atherosclerosis-prone mice is associated with increased arterial expression of plasminogen activator inhibitor-1. Arterioscler Thromb Vasc Biol. 2003;23:2097–2103. doi: 10.1161/01.ATV.0000097766.36623.DF [DOI] [PubMed] [Google Scholar]
- 125.King SM, McNamee RA, Houng AK, Patel R, Brands M, Reed GL. Platelet dense-granule secretion plays a critical role in thrombosis and subsequent vascular remodeling in atherosclerotic mice. Circulation. 2009;120:785–791. doi: 10.1161/CIRCULATIONAHA.108.845461 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Hechler B, Gachet C. Comparison of two murine models of thrombosis induced by atherosclerotic plaque injury. Thromb Haemost. 2011;105(suppl 1):S3–12. doi: 10.1160/THS10-11-0730 [DOI] [PubMed] [Google Scholar]
- 127.McGuinness CL, Humphries J, Waltham M, Burnand KG, Collins M, Smith A. Recruitment of labelled monocytes by experimental venous thrombi. Thromb Haemost. 2001;85:1018–1024 [PubMed] [Google Scholar]
- 128.Yu FT, Armstrong JK, Tripette J, Meiselman HJ, Cloutier G. A local increase in red blood cell aggregation can trigger deep vein thrombosis: evidence based on quantitative cellular ultrasound imaging. J Thromb Haemost. 2011;9:481–488. doi: 10.1111/j.1538-7836.2010.04164.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Savchenko AS, Martinod K, Seidman MA, Wong SL, Borissoff JI, Piazza G, Libby P, Goldhaber SZ, Mitchell RN, Wagner DD. Neutrophil extracellular traps form predominantly during the organizing stage of human venous thromboembolism development. J Thromb Haemost. 2014;12:860–870. doi: 10.1111/jth.12571 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Phinikaridou A, Andia ME, Saha P, Modarai B, Smith A, Botnar RM. In vivo magnetization transfer and diffusion-weighted magnetic resonance imaging detects thrombus composition in a mouse model of deep vein thrombosis. Circ Cardiovasc Imaging. 2013;6:433–440. doi: 10.1161/CIRCIMAGING.112.000077 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.von zur Muhlen C, von Elverfeldt D, Moeller JA, Choudhury RP, Paul D, Hagemeyer CE, Olschewski M, Becker A, Neudorfer I, Bassler N, et al. Magnetic resonance imaging contrast agent targeted toward activated platelets allows in vivo detection of thrombosis and monitoring of thrombolysis. Circulation. 2008;118:258–267. doi: 10.1161/CIRCULATIONAHA.107.753657 [DOI] [PubMed] [Google Scholar]
- 132.Andia ME, Saha P, Jenkins J, Modarai B, Wiethoff AJ, Phinikaridou A, Grover SP, Patel AS, Schaeffter T, Smith A, et al. Fibrin-targeted magnetic resonance imaging allows in vivo quantification of thrombus fibrin content and identifies thrombi amenable for thrombolysis. Arterioscler Thromb Vasc Biol. 2014;34:1193–1198. doi: 10.1161/ATVBAHA.113.302931 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Hara T, Bhayana B, Thompson B, Kessinger CW, Khatri A, McCarthy JR, Weissleder R, Lin CP, Tearney GJ, Jaffer FA. Molecular imaging of fibrin deposition in deep vein thrombosis using fibrin-targeted near-infrared fluorescence. JACC Cardiovasc Imaging. 2012;5:607–615. doi: 10.1016/j.jcmg.2012.01.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Singh S, Houng AK, Reed GL. Venous stasis-induced fibrinolysis prevents thrombosis in mice: role of α2-antiplasmin. Blood. 2019;134:970–978. doi: 10.1182/blood.2019000049 [DOI] [PMC free article] [PubMed] [Google Scholar]