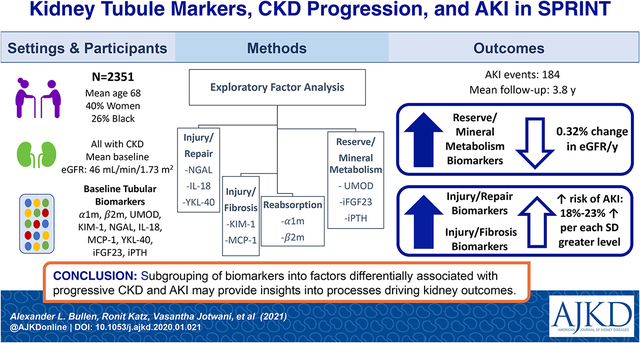
Abstract
• Rationale & Objective:
SPRINT compared the effect of intensive versus standard systolic blood pressure targets on cardiovascular morbidity and mortality. In this ancillary study, we evaluated the use of exploratory factor analysis (EFA) to combine biomarkers of kidney tubule health in urine and plasma and then study their role in longitudinal eGFR change and risk of acute kidney injury (AKI).
• Study Design:
Observational cohort nested in a clinical trial.
• Setting & Participants:
2,351 SPRINT participants with eGFR < 60 ml/min/1.73m2 at baseline.
• Exposure(s):
Levels of neutrophil gelatinase-associated lipocalin (NGAL), interleukin-18 (IL-18), chitinase-3-like protein (YKL-40), kidney injury molecule-1 (KIM-1), monocyte chemoattractant protein-1 (MCP-1), alpha-1 microglobulin (α1m) and beta-2 microglobulin (β2m), uromodulin (UMOD), fibroblast growth factor-23 (FGF23), and intact parathyroid hormone (PTH).
• Outcome(s):
Longitudinal changes in eGFR and risk of AKI.
• Analytical Approach:
We performed EFA to capture different tubule pathophysiologic processes. We used linear mixed effects models to evaluate the association of each factor with longitudinal changes in eGFR. We evaluated the association of the tubular factors scores with AKI using Cox proportional hazards regression.
• Results:
From ten biomarkers, EFA generated four factors reflecting tubule injury/repair (NGAL, IL-18 and YKL-40), tubule injury/fibrosis (KIM-1 and MCP-1), tubule reabsorption (α1m and β2m), and tubule reserve/mineral metabolism (UMOD, FGF23, and PTH). Each SD higher tubule reserve/mineral metabolism factor scores were associated with a 0.58% (0.39%, 0.67%) faster eGFR decline independent of baseline eGFR and albuminuria. Both the tubule injury/repair (HR per SD higher 1.18 [1.10, 1.37]) and tubule injury/fibrosis factors (HR 1.23 [1.02, 1.48]) were independently associated with future risk of AKI.
• Limitations:
The factors require validation in other settings.
• Conclusions:
EFA allows parsimonious subgrouping of biomarkers into factors which are differentially associated with progressive eGFR decline and AKI. These subgroups may provide insights into the pathological processes driving adverse kidney outcomes.
Graphical Abstract
INTRODUCTION
Chronic kidney disease (CKD) is a worldwide health problem that is costly and strongly associated with mortality, but its determinants remain incompletely understood.1–3 The progression of CKD varies widely across individuals, with some patients appearing not to progress over time, others progressing quickly and continuously, and yet others alternating between periods of stability and rapid decline.4, 5 Persons with CKD are also at increased risk of acute kidney injury (AKI) which is believed to contributes to the latter pattern of progression of CKD.6
Kidney tubule injury and fibrosis are dominant pathological features in both AKI and CKD.7 Both pathological findings are common on kidney biopsy, strongly predictive of progression to ESRD, and yet are poorly correlated with either GFR or albuminuria.8, 9 Thus, the clinician is often blind to the presence and extent of tubule disease except in rare instances where kidney biopsies are obtained. Several novel biomarkers have allowed non-invasive assessment of kidney tubular dysfunction and injury. These biomarkers represent proximal tubular reabsorptive capacity (alpha-1 microglobulin [α1m] and beta-2 microglobulin [β2m]), tubular injury (interleukin-18 [IL-18], kidney injury molecule-1 [KIM-1], neutrophil gelatinase-associated lipocalin [NGAL]), repair (chitinase-3-like protein [YKL-40]), fibrosis (monocyte chemoattractant protein-1 [MCP-1]), immune defense and tubular reserve (uromodulin [UMOD]), and tubular response to hormones (intact fibroblast growth factor-23 [FGF-23] and intact parathyroid hormone [PTH]). In prior work with these biomarkers in SPRINT participants with CKD, we found that several of the individual biomarkers associated with more rapid eGFR decline and risk of AKI, independent of eGFR, albuminuria, and other kidney disease risk factors.10–12 However, there is likely overlap in the underlying pathology reflected by these biomarkers, and the expanding range of available measures has created the need for data reduction methods to improve biological insights into mechanisms contributing to kidney abnormalities. Additionally, a panel of 8–10 biomarkers would not be practical in clinical practice and thus, a reduced set of markers that are useful jointly would ultimately be of value if and when the markers become available in clinical practice. Consequently, in prior work, we evaluated exploratory factor analysis (EFA) as an unsupervised approach to reduce these 10 biomarkers into 4 “factors”, based on the inter-relationships of the biomarkers with one another.13 The biomarkers that loaded onto each factor marked distinct aspects of kidney tubule biology. For example, the two urine biomarkers that signal diminished proximal tubule reabsorption created a single factor. In a recent study, we reported that several of these factors were strongly associated with cardiovascular disease (CVD) events, heart failure and all-cause mortality.13 In the present study, we evaluate whether these factors provide insights into mechanisms associated with longitudinal changes in eGFR and risk of AKI.
METHODS
Study Design and Participants
SPRINT was an open-label clinical trial that randomized persons with elevated risk of CVD events to a systolic blood pressure (SBP) target of <120 mm Hg (“intensive”) vs. <140 mm Hg (“standard”). The design and primary results of SPRINT have been published elsewhere.14 Briefly, participants were recruited from 102 centers in the United States and Puerto Rico and were required to meet the following inclusion criteria: age ≥50 years, SBP between 130 and 180 mm Hg, and increased risk for CVD events (defined by prior clinical or subclinical CVD other than stroke, 10-year risk of CVD of ≥15% on the Framingham risk score, CKD defined as eGFR 20–59 ml/min/1.73m2, or age ≥ 75 years). Major exclusion criteria included diabetes mellitus and proteinuria >1 gram/day. A total of 9,361 participants were enrolled and were randomly assigned in a 1:1 ratio to the two treatment arms.15 All participants provided written informed consent and Institutional Review Boards of all participating institutions approved the study.
This ancillary study included all SPRINT participants who had CKD and available urine and blood specimens at the baseline visit. We measured serum cystatin C concentrations in all SPRINT participants at the baseline examination, and defined the subset with CKD based on an eGFR <60 ml/min/1.73m2 by the combined CKD-EPI equation for creatinine and cystatin C.16 There were 2,514 individuals meeting inclusion criteria, which differs slightly from 2,646 with eGFR<60 ml/min/1.73m2 by the four variable Modification of Diet in Renal Disease equation16 used in the SPRINT primary results manuscript. One hundred and sixty-three participants were excluded due to insufficient urine specimens at baseline, resulting in a final sample of 2,351 for this analysis.
Urine Biomarker Measurements
All urine biomarkers were measured at the Laboratory for Clinical Biochemistry Research at the University of Vermont. Urine and venous specimens were collected at the baseline visit and stored at −80°C until biomarker measurement, without prior thaw. Laboratory personnel measuring the biomarker assays were blinded to clinical information. For each urine sample, all biomarkers were measured in duplicate, and results were averaged to increase precision. Urine α1m was measured using a Siemens nephelometric assay with a detectable range from 5–480 mg/L and inter-assay CV of 3.5–8.8%. Urine β2m, uUMOD, and uNGAL measurements were performed using a multiplex assay on a MESO Scale Diagnostics (MSD) platform (Rockville, Maryland, USA). The analytic ranges were 1.2–5020 ng/ml, 0.6–2510 ng/ml, and 6–251,000 ng/mL respectively, and the inter-assay coefficients of variation (CVs) were 15–16%, 13–16%, and 11–19% respectively. Urine KIM-1, IL-18, MCP-1, and YKL-40 were measured together on multiplex assays using the MSD platform. The analytic ranges were 4–200,000 pg/ml, 2–10,000 ng/ml, 3–10,000 pg/ml, and 10–500,000 ng/ml, respectively. The inter-assay CVs were 6.1–13.0%, 4.9–13.7%, 7.1–12.0%, and 6.5–11.1%, respectively.
Serum intact PTH and intact FGF-23 were measured at the SPRINT Central Laboratory at the University of Minnesota, Minneapolis. An intact PTH immunoassay (e411 analyzer, Roche, Indianapolis, IN) was used with an analytic measurement range of 1.2–5000pg/mL and an inter-assay CV of 4.9% at 35.1 pg/mL and 2.5% at 210.4 pg/mL. A two-site ELISA (Kainos Laboratories, Tokyo, Japan) was used to measure intact FGF23, with an analytic measurement range of 2.2–800pg/mL and an inter-assay CV of 8.6% at 22.5pg/mL and 3.2% at 85.1pg/mL.
Urine creatinine and albumin were measured by an enzymatic procedure (Roche, Indianapolis, IN) and by a nephelometric method (Siemens, Tarrytown, NY), respectively.17
Outcome Ascertainment
We evaluated longitudinal change in eGFR and risk of AKI as our outcomes of interest. Estimated GFR was measured monthly for the first 3 months of the trial, and every 6 months thereafter. In companion analyses, we also evaluated the association between the factor scores and the composite kidney outcome defined in the SPRINT protocol, which consisted of 50% decrease in eGFR, incident dialysis, or kidney transplantation. We considered this outcome as secondary and exploratory in the current study, given the limited number of events and resultant lower statistical power.
AKI was captured in the course of safety monitoring for adverse events in SPRINT. Participants were considered to have a hospitalized AKI event if an AKI diagnosis was entered into the discharge summary and the central SPRINT safety committee considered it to be one of the top 3 reasons for admission or continued hospitalization.14 Additional cases of AKI that were included in our analysis occurred solely in the emergency department without subsequent hospitalization.
Statistical Analysis
The methodology to derive the four factors of tubular health has been previously described.13 Briefly, all urine biomarkers were indexed to urine creatinine to account for tonicity. Given skewed distributions, we log-base-2 transformed each biomarker to achieve an approximate normal distribution. To determine the number of factors to create, we initially performed principal component analysis and examined the number of eigenvalues >1 and the scree plot and determined that a model with four distinct factors was appropriate for the data. We then used factor analysis with principal components factors estimation and examined different rotations to determine the best fit to the data by Thurstone rules.18 Finally, we used regression scoring methods to derive factor scores that were standardized for each factor (i.e., mean of 0 and SD of 1). The factors were developed in an unsupervised statistical approach solely on the inter-relationship of the biomarkers themselves, without any influence of their associations with outcomes.
We stratified participants by those who were randomized to the standard versus the intensive arms and compared the distribution of demographics and risk factors for CKD progression, as well as the concentrations of all tubular markers. We also evaluated the distribution of key demographic variables, eGFR and ACR among quartiles of the factor scores. We used linear mixed effects models to evaluate the association of each factor with longitudinal change in eGFR. Covariates for multivariable models were selected a priori based on biological plausibility. An initial unadjusted model was evaluated. Model 1 adjusted for age, sex, race, randomization arm, SBP, diastolic blood pressure (DBP), number of hypertension agents at baseline, ACE-inhibitor (ACEi) or angiotensin receptor blocker (ARB) use, diuretic use, history of CVD or heart failure, smoking status (current/former/never), body mass index (BMI), low density lipoprotein (LDL), and total cholesterol. Model 2 added baseline eGFR and albuminuria. In sensitivity analyses, we excluded individuals who experienced AKI to determine the association of the factors with eGFR decline unconfounded by the effect of AKI on longitudinal eGFR. In secondary analyses, we used Cox proportional hazards regression to evaluate the associations between the tubular factor scores with the composite CKD outcome. Models were adjusted for the same covariates as summarized above. Next, we evaluated the association of the tubular factors scores with AKI using Cox proportional hazards regression, using the same sequence of adjusted models. Finally, we tested for interactions of each tubular factor by randomized treatment arm on eGFR changes and risk of AKI within Model 2 described above.
Among individuals who experienced AKI episodes during follow-up, we conducted analyses to evaluate the slope of change in eGFR before and after the AKI episodes. Associations of the baseline factor scores were compared with the eGFR slopes before and after the AKI event using multivariable adjusted linear regression.
All analyses were conducted using Stata/MP Version 15.1 (StataCorp LCC, College Station, TX). P values <0.05 were considered statistically significant for all analyses including interaction terms.
RESULTS
Among 2,351 SPRINT participants with eGFR <60 ml/min/1.73m2 at baseline, the mean age was 73 ± 9, 40% were women and 26% were Black. The mean (SD) baseline eGFR was 46 ± 11 mL/min/1.73m2. Approximately half (1149 participants) were randomized to the standard arm. Demographic variables, CKD risk factors, and baseline kidney tubule health biomarkers were similar in the two randomized treatment arms (Table 1). Table S1 depicts the distribution of key demographic variables, eGFR, and ACR across quartiles of each derived factor.
Table 1.
Baseline Characteristics of SPRINT Participants with CKD by Randomization Arm
| Randomization Arm | Standard (n=1149) | Intensive (n=1202) |
|---|---|---|
| Age, years (SD) | 73 (9) | 73 (9) |
| Female, n (%) | 463 (40) | 479 (40) |
| Race, n (%) | ||
| Non-Hispanic White | 838 (73) | 877 (73) |
| Non-Hispanic Black | 291 (25) | 310 (26) |
| Hispanic and other | 20 (2) | 15 (1) |
| Cardiovascular disease, n (%) | 295 (26) | 301 (25) |
| Heart failure, n (%) | 72 (6) | 71 (6) |
| Current smoker | 99 (9) | 109 (9) |
| Body mass index | 29.4 (5.8) | 29.5 (5.9) |
| Mean systolic BP, mm Hg (SD) | 140 (16) | 140 (16) |
| Mean diastolic BP, mm Hg (SD) | 74 (12) | 75 (12) |
| Number of BP medications | 2.2 (1.0) | 2.1 (1.0) |
| Use of ACEi or ARBs, n (%) | 726 (63) | 736 (61) |
| Use of diuretics, n (%) | 627 (55) | 643 (54) |
| Total cholesterol | 183 (41) | 184 (41) |
| HDL cholesterol | 52 (14) | 53 (15) |
| Median Triglycerides, [IQR] | 112 [82, 156] | 110 [79, 149] |
| mean eGFR, (IQR) | 46 (10) | 46 (11) |
| Median urine ACR, mg/g (IQR) | 15 [7, 50] | 14 [7, 46] |
| Median urine α1m, mg/L (IQR) | 14 [7, 26] | 13 [7, 24] |
| Median urine β2m, ng/ml (IQR) | 102 [39, 324] | 105 [38, 340] |
| Median urine UMOD, ng/ml (IQR) | 6.60 [4.45, 9.89] | 6.58 [4.29, 10.21] |
| Median urine KIM-1, pg/ml (IQR) | 841 [401, 1617] | 872 [381, 1571] |
| Median urine NGAL, ng/ml (IQR) | 27 [15, 57] | 29 [15, 61] |
| Median urine IL-18, pg/ml (IQR) | 32 [17, 59] | 29 [16, 55] |
| Median urine MCP-1, pg/ml (IQR) | 185 [96, 319] | 177 [86, 335] |
| Median urine YKL-40, pg/ml (IQR) | 554 [231, 1264] | 555 [214, 1290] |
| Median serum iPTH, pg/ml (IQR) | 48 [35, 67] | 47 [34, 66] |
| Median serum FGF23, pg/ml (IQR) | 67 [52, 88] | 65 [51, 87] |
Abbreviations: ACEi, angiotensin-converting enzyme inhibitors; ARBs, angiotensin II receptor blockers; eGFR, estimated glomerular filtration rate; ACR, albumin-creatinine ratio; α1m, alpha-1 microglobulin; β2m, beta-2 microglobulin; UMOD, uromodulin; KIM-1, kidney injury molecule-1; NGAL, neutrophil-gelatinase associated lipocalin; IL-18, interleukin 18; MCP-1, monocyte chemoattract protein-1; YKL-40, chitinase-3-like protein, iPTH, intact parathyroid hormone; FGF23, intact fibroblast growth factor-23
During a mean of 3.8 (range 0.03–4.74) years of follow-up, participants provided a mean of 5.38 (range 2–12) eGFR measurements, and the mean percentage eGFR change was −1.47% (95% CI −1.62%, −1.32%) per year. Eighty-two (3.5%) participants experienced the SPRINT kidney composite endpoint of 50% kidney function decline or ESRD or kidney transplant, and there were 184 adjudicated AKI events requiring emergency room evaluation or hospitalization.
Relationship of Kidney Tubule Factor Scores with Longitudinal Change in eGFR
In unadjusted models and in models adjusted for age, sex, race, randomization arm and CKD risk factors (Model 1), each of the four factors was associated with more rapid eGFR decline (Table 2). However, after adjusting for baseline eGFR and albuminuria, only the tubule reserve/mineral metabolism factor (comprised of UMOD, iPTH and iFGF23) remained independently associated with longitudinal eGFR change. Each standard deviation (SD) higher score on this factor associated with a 0.58% faster eGFR decline (95% confidence interval [CI] −0.76%, −0.39%). For comparison of strengths of associations, we compared this estimate with 1-SD higher log ACR at baseline, and found that the association of the tubule reserve/mineral metabolism factor with eGFR decline was approximately 1/3 as strong relative to ACR (β = −1.53%, 95% CI−1.69%, −1.36%). The association of the tubule reserve/mineral metabolism factor remained robust and essentially unaltered in sensitivity analyses excluding participants who experienced AKI episodes during follow-up (β = −0.62%, 95% CI −0.80%, −0.43%). We found no evidence that the tubular factors had differential strengths of association with eGFR change by randomized treatment arm (p for interactions all > 0.12)
Table 2.
Association of Factor Scores with Continuous eGFR Change†
| Unadjusted β (95% CI) | Model 1* β (95% CI) | Model 2** β (95% CI) | |
|---|---|---|---|
| Tubule Injury/Repair (NGAL, IL-18, and YKL-40) | −0.44 (−0.59, −0.28) | −0.38 (−0.56, −0.21) | −0.06 (−0.24, 0.12) |
| Tubule Injury/Fibrosis (KIM-1 and MCP-1) | −0.41 (−0.57, −0.25) | −0.34 (−0.50, −0.18) | −0.16 (−0.33, 0.01) |
| Tubule Reabsorption (α1m, β2m) | −0.90 (−1.06, −0.74) | −0.71 (−0.87, −0.54) | −0.07 (−0.25, 0.12) |
| Tubule Reserve/Mineral Metabolism (UMOD, iPTH, iFGF23) |
−0.94 (−1.09, −0.78) | −0.96 (−1.13, −0.80) | −0.58 (−0.76, −0.39) |
| Albumin-creatinine ratio | −1.74 (−1.89, −1.58) | −1.58 (−1.74, −1.42) | −1.53 (−1.69, −1.36) |
Percentage changein eGFRper year
model 1: age, sex, race, randomization arm, SBP, DBP, number of antihypertensive meds, ACEi or ARB use, diuretic use, history of CVD or HF, current smoker, BMI, LDL, total cholesterol.
model 2: model 1 + baseline eGFR and UACR
Each factor is modeled per SD higher. ACR was log transformed and represents a SD higher on the log scale, to provide a reference for comparison of strengths of association.
The associations of each factor with the SPRINT composite kidney endpoint across the sequence of multivariable adjusted models are shown in Table S2. No significant associations were found in the fully adjusted models.
Relationship of Kidney Tubule Factor Scores with Future Risk of Acute Kidney Injury
In unadjusted models and in models adjusted for age, sex, race, randomization arm and CKD risk factors, each of the four factors was significantly associated with risk of subsequent AKI (Table 3). With additional adjustment for eGFR and ACR (Model 2), both the tubule injury/repair and tubule injury/fibrosis factors remained significantly associated with AKI risk (HR=1.18, 95% CI 1.01, 1.37 and HR=1.23, 95% CI 1.02, 1.48, respectively), whereas the other two factors were attenuated and rendered no longer statistically significant. These associations were somewhat weaker but of similar magnitude compared to 1 SD higher log-ACR (HR=1.30, 95% CI 1.11, 1.51). Associations of the tubule injury/repair and tubule injury/fibrosis factors were similar irrespective of the randomized treatment arm (p interactions both > 0.61, Table S3).
Table 3.
Association of Factor Scores with Future Risk of Acute Kidney Injury
| # AKI Events = 184 | Unadjusted HR (95% CI) | Model 1* HR (95% CI) | Model 2** HR (95% CI) |
|---|---|---|---|
| Tubule Injury/Repair (NGAL, IL-18, and YKL-40) | 1.19 (1.04, 1.36) | 1.28 (1.11, 1.49) | 1.18 (1.01, 1.37) |
| Tubule Injury/Fibrosis (KIM-1 and MCP-1) | 1.33 (1.12, 1.58) | 1.33 (1.11, 1.60) | 1.23 (1.02, 1.48) |
| Tubule Reabsorption (α1m, β2m) | 1.33 (1.15, 1.54) | 1.29 (1.11, 1.50) | 0.96 (0.81, 1.14) |
| Tubule Reserve / Mineral Metabolism (UMOD, iPTH, iFGF23) | 1.59 (1.39, 1.82) | 1.54 (1.33, 1.78) | 1.19 (0.99, 1.42) |
| Albumin-creatinine ratio | 1.58 (1.39, 1.80) | 1.50 (1.31, 1.73) | 1.30 (1.11,1.51) |
model 1: age, sex, race, randomization arm, SBP, DBP, number of antihypertensive meds, ACEi or ARB use, diuretic use, history of CVD or HF, current smoker, BMI, LDL, total cholesterol.
model 2: model 1 + baseline eGFR and UACR
Each factor is modeled per SD higher. ACR was log transformed and represents a SD higher on the log scale.
Finally, we were interested in whether the relationships of these two factors were more strongly associated with the trajectory of eGFR change before versus after the AKI event. Among 122 participants who experienced AKI during follow-up with at least two eGFR measurements to define slopes in each time period, we created pre- and post-AKI eGFR slopes. While neither of the factors appeared to be associated with eGFR trajectory before the AKI episode, both factors were associated with faster eGFR decline after the AKI event in fully adjusted models. Each SD higher tubule injury/repair score was associated with −2.92% (−5.59%, −0.25%) faster decline in eGFR and each SD higher tubule injury/fibrosis score was associated with a −4.93% (−7.59%, −2.27%) faster eGFR decline following the AKI event (Figure).
FIGURE 1. Associations of Factor Scores and ACR with eGFR Trajectory Before and After AKI.
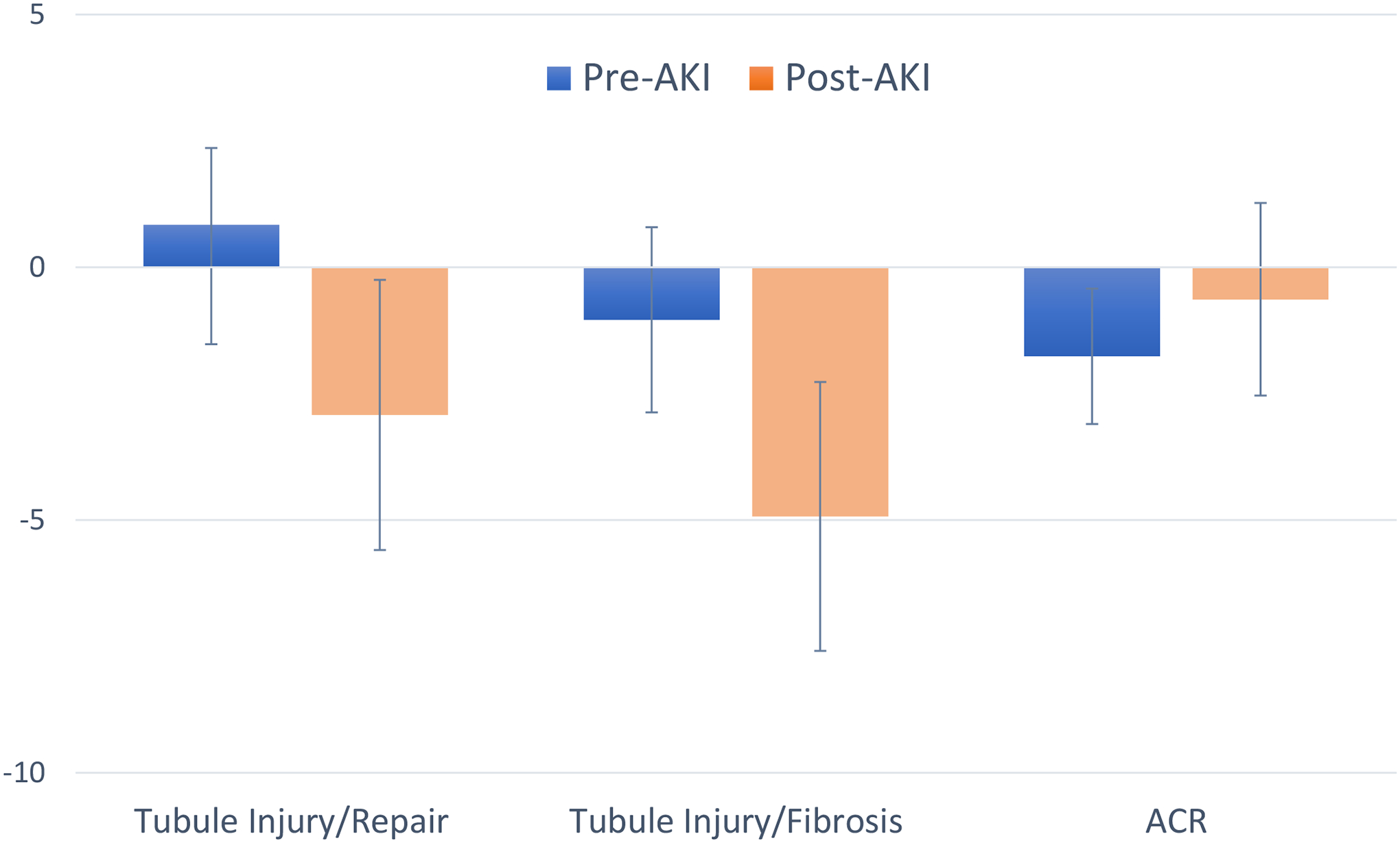
Adjusted for age, sex, race, randomization arm, SBP, DBP, number of antihypertensive meds, ACEi or ARB use, diuretic use, history of CVD or HF, current smoker, BMI, LDL, total cholesterol, baseline eGFR and UACR
DISCUSSION
In this study among SPRINT participants with CKD, we explored EFA as an unsupervised method to reduce 10 kidney tubule health biomarkers into four unique factors. Among them, the tubule reserve/mineral metabolism factor was uniquely associated with eGFR decline but was not associated with AKI. In contrast, the tubule injury/fibrosis and tubule injury/repair factors were independently associated with future risk of AKI. In sensitivity analyses, these factors associated with more rapid declines in eGFR after the AKI episode rather than before it. These findings may have implications for distilling information across multiple kidney tubule health biomarkers into factors that represent unique kidney pathological processes.
EFA provides a method to cull information from a broad biomarker panel and studied longitudinal changes in eGFR and risk of AKI. We have previously demonstrated that these same factors improved CVD risk discrimination beyond contemporary kidney measures in SPRINT.13 We have also reported that individual biomarkers of tubule cell function were associated with AKI in SPRINT.10 Specifically, when evaluated as individual biomarkers, we found strong associations of baseline α1m and UMOD with subsequent hospitalized AKI risk, even after adjusting for baseline eGFR and albuminuria; associations that were stronger than the tubule cell injury markers.10 In the present study shows that by combining individual biomarkers using EFA, we could define factors representing distinct pathological processes in the kidney and could identify new relationships with eGFR decline and AKI that were not evident when evaluating individual biomarkers alone. As novel biomarkers are discovered and added to existing panels, EFA provides a promising approach to understand the overlaps in biology provided by each, provides a method to evaluate a more manageable list of tubule biomarkers, and to describe how unique aspects of kidney pathology relate to individual outcomes.
We found that the tubule reserve/mineral metabolism factor was the only factor associated with longitudinal eGFR decline. This tubule factor was comprised of urine uromodulin, and serum iFGF23, and iPTH concentrations. Intact-FGF23 is a hormone that promotes phosphaturia and inhibits conversion of calcidiol to the active hormone calcitriol by its actions in kidney tubule cells.19 Intact-PTH has similar effects on kidney tubule cells in regard to promoting phosphaturia, but unlike FGF23, it activates conversion of calcidiol to calcitriol. Thus, these two hormones have similar and intertwined biology that was captured by EFA loading both onto the same factor. Why urine UMOD loaded on this factor is less clear. UMOD is exclusively produced by tubule cells in the thick ascending limb of Henle’s loop and connecting tubule, and lower levels have been associated with CKD progression and CVD risk in a variety of settings, including in our prior work in SPRINT.20, 21 UMOD is therefore negatively weighted in this factor. UMOD has been postulated to prevent development of kidney stones and may therefore be linked with mineral metabolism, and with iFGF23 and iPTH.22 Of note, in our previous study, the tubule reserve/mineral metabolism score was also strongly associated with CVD and heart failure.13 Given the consistent independent relationships with outcomes, and the fact that all three markers loaded onto one factor, future studies are warranted to understand the shared relationships of these three biomarkers to kidney tubule disease. Finally, sensitivity analyses excluding participants with AKI provided very similar results, and this factor was found not to be associated with future risk of AKI, suggesting that the tubule reserve/ mineral metabolism factor captures biology driving loss of kidney function through pathways that are likely independent of AKI.
We observed that all biomarkers related to tubule injury, repair, and fibrosis loaded on two factors. These two factors were uniquely associated with AKI risk, independent of eGFR and albuminuria and other risk factors. The strengths of these associations were independent of ACR and were fairly strong, although not quite as strong as ACR. Among a subset of participants who experienced AKI during follow-up, these two factors were also associated with more rapid decline in eGFR after rather than before the AKI event. Since the injury, fibrosis, and repair biomarkers were measured at the baseline visit in SPRINT when participants were stable and before the AKI episodes, this finding suggests that subclinical kidney tubule injury may identify individuals at higher risk of subsequent AKI events. The eGFR trajectory data imply that such sub-clinical tubule injury may identify individuals with less recovery after the AKI event. These findings require confirmation but indicate that non-invasive biomarkers incorporated into factors hold promise for potentially identify persons who are at higher risk of AKI, and the subset less likely to recover kidney function after AKI if and when they become available in clinical practice.
Strengths of this study include the use of a broad panel of serum and urine markers capturing diverse tubular physiological processes, and use of EFA to extract the information across markers into 4 unique factors. Instead of attempting to find any single biomarker that identifies loss of eGFR or AKI, we sought to integrate information across markers to maximize pathological insights. Second, the study benefited from its setting in a well-characterized multicenter clinical trial with a large sample of persons with CKD. The clinical trial setting provided a uniform protocol to follow eGFR at prespecified time-points and to capture AKI episodes. Third, we evaluated AKI and eGFR decline concomitantly to understand their overlap in propagation of kidney disease, and to provide insights into mechanisms leading to one endpoint distinct from the other.
This study also has important limitations. First, while the individual biomarkers have been studied in other settings, the factors evaluated here have not yet been validated in other cohorts. Inclusion of additional biomarkers, and/or measurement on different platforms may influence the inter-relationships of the biomarkers with one another and could therefore have resulted in different factor loading. Second, all the tubule health biomarkers were measured at baseline. Whether longitudinal changes in the biomarkers themselves or in the factor scores they comprise, may be more or less strongly associated with eGFR decline and AKI risk remains unclear. This important question requires further study. Third, AKI was captured by the SPRINT safety monitoring committee, and represents hospital admissions and emergency room visits where the diagnosis of AKI was clinically evident. While the AKI definition benefits from the fact that the events were severe enough to come to clinical recognition, subclinical AKI events may have been missed.23 The great majority of AKI events in SPRINT (84.3%) were deemed secondary to volume depletion.24 Whether or not the factors would function similarly for AKI episodes from other causes is unknown. Finally, SPRINT excluded individuals with diabetes mellitus, polycystic kidney disease, and proteinuria >1 g/day and all participants in this ancillary study had CKD at baseline. Further research is required to determine if the results generalize to other populations.
In summary, among SPRINT participants with CKD, exploratory factor analysis condensed 10 biomarkers of kidney tubule health into 4 factors representing unique aspects of tubule pathology. One marking mineral metabolism and tubule reserve independently associated with more rapid eGFR decline, while two other factors reflecting tubule injury, repair and fibrosis were independently associated with future AKI risk. Factors analysis appears to be a promising tool to reduce multiple biomarkers into fewer factors representing unique pathological processes, and to garner biological insight into CKD progression and AKI risk.
Supplementary Material
Table S1. Summary of Key Variables by Quartiles of Factor Scores
Table S2. Association of Factor Scores with 50% Kidney Function Decline or ESKD or Transplantation
Table S3. Interactions of Factor Scores with Randomization Arm on Risk of AKI
Support:
Dr. Alexander L. Bullen was supported by U.S. Department of Veterans Affairs Office of Research and Development award (IK2 BX004986-01A1), a Ruth L. Kirschstein training grant from the National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK; T32DK104717), pilot grant from the University of Alabama at Birmingham and UC San Diego - O’Brien Center for Acute Kidney Injury Research (P30 DK079337) and a grant from the NIDDK R01DK119528 (PI: Dr. Joachim H. Ix). Dr. Joachim H. Ix was supported by a mid-career mentoring award from the NIDDK (K24DK110427). The ancillary study measurements and data analysis were supported by an R01 award from the NIDDK to Drs. Ix and Shlipak (2R01DK098234). The funders did not have a role in the study design, data collection, analysis, reporting, or the decision to submit for publication.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Financial Disclosure: The authors declare that they have no relevant financial interests.
Publisher's Disclaimer: Disclaimer: The contents do not represent the views of the U.S. Department of Veterans Affairs or the United States Government.
REFERENCES
- 1.Eggers PW. Has the incidence of end-stage renal disease in the USA and other countries stabilized? Curr Opin Nephrol Hypertens. May2011;20(3):241–5. doi: 10.1097/MNH.0b013e3283454319 [DOI] [PubMed] [Google Scholar]
- 2.Golestaneh L, Alvarez PJ, Reaven NL, et al. All-cause costs increase exponentially with increased chronic kidney disease stage. Am J Manag Care. June2017;23(10 Suppl):S163–s172. [PubMed] [Google Scholar]
- 3.Tonelli M, Wiebe N, Culleton B, et al. Chronic kidney disease and mortality risk: a systematic review. J Am Soc Nephrol. July2006;17(7):2034–47. doi: 10.1681/asn.2005101085 [DOI] [PubMed] [Google Scholar]
- 4.Zhang P, Song PX, Qu A, Greene T. Efficient estimation for patient-specific rates of disease progression using nonnormal linear mixed models. Biometrics. March2008;64(1):29–38. doi: 10.1111/j.1541-0420.2007.00824.x [DOI] [PubMed] [Google Scholar]
- 5.Walser M Progression of chronic renal failure in man. Kidney Int. May1990;37(5):1195–210. doi: 10.1038/ki.1990.103 [DOI] [PubMed] [Google Scholar]
- 6.Hsu CY, Ordonez JD, Chertow GM, Fan D, McCulloch CE, Go AS. The risk of acute renal failure in patients with chronic kidney disease. Kidney Int. July2008;74(1):101–7. doi: 10.1038/ki.2008.107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bohle A, Muller GA, Wehrmann M, Mackensen-Haen S, Xiao JC. Pathogenesis of chronic renal failure in the primary glomerulopathies, renal vasculopathies, and chronic interstitial nephritides. Kidney Int Suppl. May1996;54:S2–9. [PubMed] [Google Scholar]
- 8.Risdon RA, Sloper JC, De Wardener HE. Relationship between renal function and histological changes found in renal-biopsy specimens from patients with persistent glomerular nephritis. Lancet. August171968;2(7564):363–6. doi: 10.1016/s0140-6736(68)90589-8 [DOI] [PubMed] [Google Scholar]
- 9.Striker GE, Schainuck LI, Cutler RE, Benditt EP. Structural-functional correlations in renal disease. I. A method for assaying and classifying histopathologic changes in renal disease. Hum Pathol. December1970;1(4):615–30. doi: 10.1016/s0046-8177(70)80060-0 [DOI] [PubMed] [Google Scholar]
- 10.Bullen AL, Katz R, Lee AK, et al. The SPRINT trial suggests that markers of tubule cell function in the urine associate with risk of subsequent acute kidney injury while injury markers elevate after the injury. Kidney Int. August2019;96(2):470–479. doi: 10.1016/j.kint.2019.03.024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Patidar KR, Garimella PS, Macedo E, et al. Admission plasma uromodulin and the risk of acute kidney injury in hospitalized patients with cirrhosis: a pilot study. Am J Physiol Gastrointest Liver Physiol. October12019;317(4):G447–g452. doi: 10.1152/ajpgi.00158.2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fliser D, Kollerits B, Neyer U, et al. Fibroblast growth factor 23 (FGF23) predicts progression of chronic kidney disease: the Mild to Moderate Kidney Disease (MMKD) Study. J Am Soc Nephrol. September2007;18(9):2600–8. doi: 10.1681/asn.2006080936 [DOI] [PubMed] [Google Scholar]
- 13.Lee AK, Katz R, Jotwani V, et al. Distinct Dimensions of Kidney Health and Risk of Cardiovascular Disease, Heart Failure, and Mortality. Hypertension. October2019;74(4):872–879. doi: 10.1161/hypertensionaha.119.13339 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wright JT Jr., Williamson JD, Whelton PK, et al. A Randomized Trial of Intensive versus Standard Blood-Pressure Control. N Engl J Med. November262015;373(22):2103–16. doi: 10.1056/NEJMoa1511939 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ambrosius WT, Sink KM, Foy CG, et al. The design and rationale of a multicenter clinical trial comparing two strategies for control of systolic blood pressure: the Systolic Blood Pressure Intervention Trial (SPRINT). Clin Trials. October2014;11(5):532–46. doi: 10.1177/1740774514537404 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Inker LA, Schmid CH, Tighiouart H, et al. Estimating glomerular filtration rate from serum creatinine and cystatin C. N Engl J Med. July52012;367(1):20–9. doi: 10.1056/NEJMoa1114248 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cheung AK, Rahman M, Reboussin DM, et al. Effects of Intensive BP Control in CKD. J Am Soc Nephrol. September2017;28(9):2812–2823. doi: 10.1681/asn.2017020148 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Thurstone L Multiple Factor Analysis: A Development and expansion of vectors of the mind. Chicago, IL: University of Chicago; 1947. [Google Scholar]
- 19.Liu S, Quarles LD. How fibroblast growth factor 23 works. J Am Soc Nephrol. June2007;18(6):1637–47. doi: 10.1681/asn.2007010068 [DOI] [PubMed] [Google Scholar]
- 20.Jotwani V, Garimella PS, Katz R, et al. Tubular Biomarkers and Chronic Kidney Disease Progression in SPRINT Participants. Am J Nephrol. 2020;51(10):797–805. doi: 10.1159/000509978 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Garimella PS, Lee AK, Ambrosius WT, et al. Markers of kidney tubule function and risk of cardiovascular disease events and mortality in the SPRINT trial. Eur Heart J. 112019;40(42):3486–3493. doi: 10.1093/eurheartj/ehz392 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gudbjartsson DF, Holm H, Indridason OS, et al. Association of variants at UMOD with chronic kidney disease and kidney stones-role of age and comorbid diseases. PLoS Genet. July292010;6(7):e1001039. doi: 10.1371/journal.pgen.1001039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Grams ME, Waikar SS, MacMahon B, Whelton S, Ballew SH, Coresh J. Performance and limitations of administrative data in the identification of AKI. Clin J Am Soc Nephrol. April2014;9(4):682–9. doi: 10.2215/cjn.07650713 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rocco MV, Sink KM, Lovato LC, et al. Effects of Intensive Blood Pressure Treatment on Acute Kidney Injury Events in the Systolic Blood Pressure Intervention Trial (SPRINT). Am J Kidney Dis. March2018;71(3):352–361. doi: 10.1053/j.ajkd.2017.08.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. Summary of Key Variables by Quartiles of Factor Scores
Table S2. Association of Factor Scores with 50% Kidney Function Decline or ESKD or Transplantation
Table S3. Interactions of Factor Scores with Randomization Arm on Risk of AKI


