Abstract
Introduction:
Aggregation of all Black populations in US cancer mortality profiles masks remarkable heterogeneity by place of birth. Comparing U.S-born African Americans with African and Afro-Caribbean immigrants may highlight specific cancer prevention and control needs and clarify global cancer epidemiology. Such a comparison has yet to be undertaken on a population basis.
Methods:
Using 2012-2017 vital statistics data from California, Florida, Minnesota and New York, age-standardized cancer mortality rates were computed for distinct Black populations. Comparisons were made to the majority White population using mortality rate ratios (MRR) obtained from negative binomial regression.
Results:
Of the 83,460 cancer deaths analyzed among Blacks, nearly 20% were immigrants. African males and females had the lowest all-sites-combined cancer mortality rates (121 and 99 per 100,000, respectively), African Americans had the highest (232 and 163), while Afro-Caribbean were in between (140 and 106 respectively). The average Black:White MRR was significant for prostate (2.11), endometrial (2.05), stomach (2.02), multiple myeloma (1.87), premenopausal breast (1.66), liver (1.58) and cervical (1.56) cancers, (P<0.05).
Conclusion:
While, in aggregate, Blacks in the US have high cancer mortality rates, race itself is not the primary determinant of these disparities. Black immigrant populations show lower cancer mortality than both African Americans and Whites, especially for cancers where environmental factors feature more predominantly: lung, colorectal and breast. Even for cancers with high mortality among all African-descent groups, this study suggests a complex interplay between genetic and environmental factors. Endometrial cancer was unique; mortality rates were similarly high for all three analyzed Black groups.
Keywords: cancer, mortality, African Americans, Afro-Caribbeans, African, intra-racial
1. INTRODUCTION
Cancer is the second leading cause of death among non-Hispanic Blacks,1 who currently account for 13% of the total US population with over 42 million people.2 Significant racial disparities in cancer incidence, survival, and mortality have been well-documented for this population.1,3 However, nearly 10% of Blacks are immigrants,4 likely with different cancer experiences than their US-born counterparts. In 2017, there were 1.9 million African immigrants, a rapidly increasing population, and nearly 2 million Afro-Caribbeans.5 National cancer surveillance programs, while providing detailed profiles for the heterogeneity within the Asian race and Hispanic ethnicity, do not contemplate within-Black heterogeneity.
Examining cancer patterns in aggregation for all Blacks in the US misses opportunities to detect differences between distinct groups that may result from cultural, socioeconomic, and biological characteristics.6 Notwithstanding that race, as collected by self-report in general statistics, is a very crude indicator of genetic similarity, intra-racial comparisons for Blacks may suggest or refute race-specific vulnerabilities for some cancers. Understanding these patterns is important for further studies on genomics, cancer risk, and etiology, as well as to inform public health interventions. Previous studies have documented significant mortality differences between African Americans and Afro-Caribbeans in Florida7 and New York8, with African Americans showing significantly higher mortality rates in both.7, 8 Similar population-based studies to extend these comparisons for African immigrant populations are warranted. Yet, to our knowledge, only one study to date analyzed patterns for African immigrants, using incidence data from cancer registries.9 However, country of birth in incidence datasets is significantly incomplete, and the missing data follows a non-random pattern, possibly affecting the accuracy of the findings.10 Instead, when mortality data is used, birthplace is known for over 98% of the deceased subjects.10
Leveraging the quality and availability of mortality data, we present population-based cancer mortality rates for three distinct US Black groups, herein referred to as African Americans (born in the U.S), Afro-Caribbeans, and, for the first time, Africans. Additionally, given the high genetic variability of populations in Africa,11 we examine, where feasible, variation between West-Central Africans and East Africans. Cancer mortality data was assembled from four states: California, Florida, Minnesota and New York. These states provided a balance of West Africans, more common in New York, with East Africans, more common in Minnesota, and Afro-Caribbeans, largely found in Florida and New York; California’s highly diverse and populous population provided additional cases for each group.5 These four states were also selected because they provided specific country of birth upon special request.
2. METHODS
Cancer mortality data for six years, 2012- 2017, were obtained from each state’s Department of Vital Statistics; only resident cases were considered. Cancer deaths from all-sites-combined and the 18 most common cancers were analyzed.12 For female breast cancer, rates were presented overall as well as for pre- and post-menopausal, approximated by using an age cutoff of 50 years at death. For endometrial cancer, both ICD-10 codes C54 (corpus uterus) and C55 (uterus, part unspecified) were included since in US cancer registries misclassification of C55 with incident C53 (cervix) cases is negligible.13
We examined the cancer mortality burden for the majority (non-Hispanic) White population as well as three non-Hispanic populations of African descent: African American; African (Black, born in any African country), and Afro-Caribbean (Black, born in any non-Hispanic island in the Caribbean, as detailed elsewhere)7. Black decedents born in other countries (Canada, Germany, etc.) were included in the “All Blacks” category, but not analyzed as stand-alone groups. To examine differences by region of Africa, two distinct groups, East and West-Central, were created based on United Nations (UN) geographical regions14 (Figure 1). Black decedents from the five Southern and seven Northern African countries were included in the African category but not analyzed separately because of sparse data. Decedents of other races born in any African country were excluded from the African category.
Figure 1.
Map of West-Central and East African Nations based on United Nations Geographical Regions Classification.
Population denominators for all four states combined were obtained from the US Census Bureau, using 2012 to 2017 American Community Survey data5 (Table 1). Sex-stratified cancer mortality rates for the six-year period were calculated per 100,000 persons, annualized and age-standardized to the 2000 US Standard Population using 18, 5-year age-group bands, except the last, which was 85 and older (Table 2). Corresponding 95% confidence intervals were calculated using Gamma intervals modification. To directly compare rates for all analyzed populations, we computed age-adjusted site-specific mortality rate ratios (MRRs) using negative binomial regression. Models included decedents ages 35 and over, except for prostate cancer, which included ages 45 and older (Table 3 and Figure 2). To make a Black-White racial comparison that avoids the effect of over-representation of African Americans, we computed a non-weighted average of the age-adjusted mortality ratios for the three distinct Black groups, producing a ratio with Whites as reference (Table 3, last column), and corresponding 95% confidence intervals were obtained using a fixed effects meta-analysis approach.
Table 1.
Study population characteristics. California, Florida, Minnesota, New York, 2012-2017.
| Population Dataa | Cancer Mortality Data | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Total Population |
Median Ageb |
California Deaths (N) |
Florida Deaths (N) |
Minnesota Deaths (N) |
New York Deaths (N) |
Total Deaths (N) |
Higher Education |
Top countries of birth | |
| White | 41,334,862 | 50 | 220,177 | 197,566 | 54,100 | 152,121 | 623,964 | 46% | USA, Germany, Canada |
| Blackc | 9,067,576 | 43 | 26,228 | 26,771 | 1,713 | 28,748 | 83,460 | 35% | USA, Jamaica, Haiti |
| African American | 7,350,702 | 43 | 24,747 | 20,455 | 1,328 | 20,624 | 67,154 | 36% | USA |
| Afro-Caribbean | 1,227,555 | 47 | 335 | 5,791 | 15 | 6,281 | 12,422 | 31% | Jamaica, Haiti, TTOe |
| Africand | 372,082 | 39 | 547 | 121 | 359 | 631 | 1,658 | 50% | Nigeria, Ethiopia, Somalia |
| West-Central African | 206,744 | 41 | 251 | 75 | 102 | 529 | 957 | 54% | Nigeria, Ghana, Liberia |
| East African | 145,684 | 38 | 261 | 29 | 245 | 59 | 594 | 41% | Ethiopia, Somalia, Eritrea |
Annualized; Ruggles ref.#5
Median age of adults 20 years or older
Includes Black decedents not specifically studied here in a standalone group (1.3%), e.g born in Germany, Canada etc
Includes Black Africans not from West-Central, or East Africa (5.3%)
Trinidad and Tobago
Table 2.
Selected site-specific age-adjusteda cancer mortality rates per 100,000 by sex and detailed race-ethnicity. California, Florida, Minnesota, New York, 2012-2017.
| WHITE n=623,966 |
BLACKb n=83,460 |
African American n=67,154 |
Afro-Caribbean n=12,422 |
Africanc n=1,658 |
West-Central African n=957 |
East African n=594 |
|
|---|---|---|---|---|---|---|---|
| MALE | Rate (95% CI) | Rate (95% CI) | Rate (95% CI) | Rate (95% CI) | Rate (95% CI) | Rate (95% CI) | Rate (95% CI) |
| Oral | 4.1 (4.1-4.2) | 4.0 (3.7-4.2) | 4.6 (4.3-5.0) | 1.5 (1.2-2.1) | 2.7 (1.4-4.8) | 2.8 (1.2-5.7) | - |
| Esophagus | 7.3 (7.2-7.5) | 4.4 (4.1-4.7) | 5.0 (4.6-5.4) | 2.6 (2.1-3.2) | 1.6 (0.8-3.0) | - | - |
| Stomach | 3.3 (3.2-3.3) | 8.0 (7.6-8.4) | 8.1 (7.6-8.6) | 7.6 (6.7-8.6) | 3.4 (2.0-5.4) | 3.7 (1.5-7.4) | - |
| Colorectum | 15.1 (14.9-15.3) | 21.0 (20.3-21.7) | 22.8 (22.0-23.7) | 14.8 (13.6-16.1) | 9.2 (6.7-12.3) | 9.5 (5.9-14.5) | 7.8 (4.9-11.9) |
| Liver | 8.2 (8.0-8.3) | 12.6 (12.1-13.1) | 14.4 (13.8-15.0) | 5.7 (5.0-6.6) | 15.0 (11.6-19.0) | 12.3 (8.9-16.7) | 19.1 (12.6-27.3) |
| Pancreas | 12.9 (12.7-13.1) | 14.0 (13.5-14.5) | 15.4 (14.7-16.0) | 9.1 (8.2-10.2) | 9.7 (6.8-13.3) | 6.4 (3.9-10.2) | 13.1 (7.8-20.3) |
| Lung | 45.8 (45.4-46.1) | 48.2 (47.2-49.3) | 57.2 (56.0-58.6) | 20.0 (18.7-21.5) | 14.3 (11.3-18.0) | 10.3 (7.6-13.9) | 17.3 (11.6-24.6) |
| Prostate | 17.8 (17.6-18.0) | 39.7 (38.7-40.8) | 41.0 (39.9-42.2) | 33.8 (31.9-35.8) | 27.3 (21.6-33.9) | 32.7 (24.3-42.7) | 19.3 (12.1-28.7) |
| Kidney | 5.1 (5.0-5.2) | 4.7 (4.4-5.0) | 5.3 (5.0-5.7) | 2.5 (2.0-3.1) | 2.4 (1.1-4.3) | 2.8 (0.8-6.4) | - |
| Bladder | 8.3 (8.2-8.5) | 5.4 (5.1-5.8) | 6.0 (5.6-6.5) | 3.5 (2.9-4.2) | 3.2 (1.5-5.8) | - | - |
| Brain | 6.2 (6.0-6.3) | 3.3 (3.1-3.6) | 3.3 (3.1-3.6) | 3.2 (2.4-4.2) | 3.3 (1.8-5.4) | - | - |
| NHL | 7.3 (7.1-7.4) | 5.5 (5.2-5.8) | 5.2 (4.9-5.6) | 5.8 (5.1-6.7) | 5.3 (3.3-8.1) | 3.3 (1.8-6.0) | 8.0 (4.1-13.6) |
| Myeloma | 3.7 (3.6-3.8) | 7.1 (6.7-7.5) | 7.3 (6.8-7.8) | 6.5 (5.7-7.4) | 4.8 (3.0-7.2) | 5.0 (2.8-8.4) | - |
| Leukemia | 8.9 (8.8-9.1) | 7.0 (6.6-7.4) | 7.4 (6.9-7.8) | 6.8 (5.7-8.1) | 2.8 (1.6-4.5) | 2.2 (1.1-4.5) | - |
| All-Sites-Combinedd | 183.8 (183.2-184.5) | 211.0 (208.8-213.1) | 232.0 (229.4-234.6) | 140.4 (136.5-144.5) | 120.5 (109.8-131.9) | 109.3 (96.0-123.9) | 129.9 (112.3-149.2) |
| FEMALE | |||||||
| Oral | 1.4 (1.4-1.5) | 1.3 (1.2-1.5) | 1.6 (1.4-1.8) | 0.6 (0.4-0.9) | - | - | - |
| Esophagus | 1.6 (1.5-1.6) | 1.6 (1.4-1.7) | 1.9 (1.7-2.1) | 0.9 (0.4-1.8) | - | - | - |
| Stomach | 1.7 (1.6-1.7) | 3.9 (3.6-4.1) | 3.7 (3.5-4.0) | 4.0 (3.5-4.6) | 2.0 (1.1-3.5) | - | - |
| Colorectum | 11.2 (11.1-11.4) | 14.8 (14.3-15.2) | 16.1 (15.6-16.7) | 10.7 (9.9-11.6) | 8.6 (6.2-11.5) | 6.3 (3.8-10.0) | 11.7 (7.0-18.1) |
| Liver | 3.3 (3.3-3.4) | 4.7 (4.4-4.9) | 5.0 (4.7-5.3) | 3.3 (2.7-3.9) | 7.8 (5.4-10.8) | 5.3 (2.8-9.0) | 13.6 (8.1-21.0) |
| Pancreas | 9.5 (9.4-9.7) | 11.4 (11.0-11.8) | 12.5 (12.1-13.1) | 7.5 (6.8-8.3) | 8.3 (5.9-11.4) | 10.8 (6.8-16.3) | 7.0 (3.5-12.1) |
| Lung | 35.4 (35.1-35.7) | 27.1 (26.5-27.8) | 33.5 (32.7-34.3) | 9.0 (8.2-9.8) | 7.4 (5.2-10.1) | 6.7 (4.2-10.2) | 8.6 (4.3-14.9) |
| Breaste | 20.5 (20.3-20.7) | 27.4 (26.7-28.0) | 29.5 (28.8-30.3) | 21.0 (19.8-22.2) | 15.8 (13.0-19.0) | 14.9 (11.5-19.2) | 17.8 (12.1-25.1) |
| Premenopausal | 3.0 (2.9-3.1) | 5.7 (5.4-6.0) | 6.0 (5.6-6.3) | 5.5 (4.8-6.2) | 3.7 (2.8-5.0) | 3.8 (2.6-5.9) | 4.2 (2.8-6.8) |
| Postmenopausal | 17.5 (17.3-17.7) | 21.6 (21.1-22.2) | 23.6 (22.9-24.2) | 15.5 (14.6-16.5) | 12.0 (9.4-15.1) | 11.1 (8.1-15.2) | 13.6 (8.2-20.8) |
| Cervix | 2.1 (2.0-2.1) | 3.8 (3.6-4.1) | 4.0 (3.7-4.3) | 3.0 (2.6-3.6) | 2.8 (1.7-4.5) | - | - |
| Endometrium | 4.6 (4.5-4.7) | 9.5 (9.2-9.9) | 9.3 (8.9-9.7) | 9.4 (8.7-10.3) | 11.7 (8.9-15.1) | 12.5 (8.4-17.7) | 11.6 (7.0-17.9) |
| Ovary | 7.5 (7.4-7.7) | 6.3 (6.0-6.6) | 6.3 (6.0-6.7) | 5.9 (5.3-6.6) | 4.0 (2.7-5.8) | 3.4 (1.9-5.9) | 5.0 (2.5-8.9) |
| Kidney | 2.1 (2.1-2.2) | 1.7 (1.6-1.9) | 2.1 (1.9-2.3) | 1.1 (0.5-1.9) | - | - | - |
| Bladder | 2.3 (2.2-2.4) | 2.2 (2.0-2.4) | 2.5 (2.3-2.8) | 1.2 (1.0-1.6) | - | - | - |
| Brain | 4.1 (4.0-4.2) | 2.0 (1.9-2.2) | 2.1 (1.9-2.3) | 2.3 (1.6-3.2) | - | - | - |
| NHL | 4.3 (4.2-4.4) | 3.7 (3.5-4.0) | 3.5 (3.3-3.8) | 4.2 (3.7-4.8) | 4.3 (2.6-6.7) | - | - |
| Myeloma | 2.2 (2.2-2.3) | 5.1 (4.8-5.4) | 5.3 (4.9-5.6) | 4.8 (4.1-5.8) | 2.7 (1.5-4.4) | - | - |
| Leukemia | 4.9 (4.8-5.0) | 4.3 (4.0-4.5) | 4.5 (4.2-4.8) | 3.6 (3.0-4.3) | 4.1 (2.5-6.3) | 4.4 (2.3-7.7) | - |
| All-Sites-Combinedd | 137.1 (136.6-137.7) | 148.6 (147.2-150.1) | 162.6 (160.9-164.4) | 106.0 (103.0-109.0) | 99.2 (90.8-108.1) | 91.2 (80.4-103.1) | 118.9 (102.4-137.1) |
Blank cells not reported; less than 13 deaths; Abbreviations: NHL, non-Hodgkin lymphoma
Age-adjusted to the 2000 U.S. Standard Population
Includes Black decedents not specifically studied here in a standalone group
Includes Black Africans not from West or East Africa
All sites-combined also includes those not listed here
Premenopausal approximated by using ages below 50; postmenopausal, ages 50 and above
Table 3.
Mortality Rate Ratiosa for selected cancers by race-ethnicity. California, Florida, Minnesota, New York, 2012-2017.
| WHITE | African American | Afro-Caribbean | African | Black/White Ratioc | |
|---|---|---|---|---|---|
| MALE | referent | MRR (95%CI) | MRR (95%CI) | MRR (95%CI) | Ratio (95%CI) |
| Oral | 1 | 1.15 (1.07-1.24) | 0.35 (0.28-0.45) | 0.51 (0.33-0.80) | 0.67 (0.58-0.76) |
| Esophagus | 1 | 0.69 (0.64-0.74) | 0.34 (0.28-0.41) | 0.27 (0.16-0.44) | 0.43 (0.38-0.49) |
| Stomach | 1 | 2.48 (2.32-2.64) | 2.30 (2.05-2.57) | 1.44 (1.03-2.02) | 2.07 (1.88-2.27) |
| Colorectum | 1 | 1.56 (1.47-1.65) | 1.01 (0.93-1.11) | 0.71 (0.57-0.89) | 1.09 (1.03-1.16) |
| Liver | 1 | 1.72 (1.21-2.45) | 0.89 (0.60-1.34) | 2.36 (1.56-3.58) | 1.66 (1.24-2.07) |
| Pancreas | 1 | 1.25 (1.17-1.34) | 0.70 (0.63-0.79) | 0.73 (0.57-0.93) | 0.89 (0.82-0.96) |
| Lung | 1 | 1.30 (1.23-1.38) | 0.45 (0.41-0.50) | 0.37 (0.31-0.45) | 0.71 (0.67-0.74) |
| Prostate | 1 | 2.59 (2.38-2.82) | 2.04 (1.84-2.25) | 1.70 (1.40-2.06) | 2.11 (1.96-2.26) |
| Kidney | 1 | 1.05 (0.97-1.13) | 0.46 (0.38-0.56) | 0.55 (0.36-0.85) | 0.69 (0.60-0.78) |
| Bladder | 1 | 0.75 (0.70-0.81) | 0.41 (0.35-0.49) | 0.47 (0.30-0.74) | 0.54 (0.46-0.62) |
| Brain | 1 | 0.52 (0.48-0.57) | 0.49 (0.41-0.58) | 0.45 (0.31-0.67) | 0.49 (0.42-0.55) |
| NHL | 1 | 0.87 (0.70-1.07) | 0.98 (0.77-1.21) | 0.87 (0.60-1.27) | 0.91 (0.76-1.05) |
| Myeloma | 1 | 1.99 (1.51-2.64) | 1.82 (1.34-2.45) | 1.60 (0.85-3.01) | 1.80 (1.36-2.25) |
| Leukemia | 1 | 0.92 (0.80-1.06) | 0.78 (0.65-0.95) | 0.50 (0.33-0.75) | 0.73 (0.64-0.83) |
| All-Sites-Combinedb | 1 | 1.30 (1.23-1.37) | 0.77 (0.72-0.82) | 0.63 (0.51-0.75) | 0.90 (0.85-0.95) |
| FEMALE | |||||
| Oral | 1 | 1.13 (1.01-1.27) | 0.37 (0.26-0.52) | - | - |
| Esophagus | 1 | 1.19 (1.07-1.33) | 0.41 (0.30-0.55) | - | - |
| Stomach | 1 | 2.18 (1.63-2.91) | 2.38 (1.75-3.23) | 1.36 (0.66-2.80) | 1.97 (1.49-2.46) |
| Colorectum | 1 | 1.48 (1.38-1.59) | 0.98 (0.89-1.08) | 0.85 (0.66-1.09) | 1.10 (1.02-1.19) |
| Liver | 1 | 1.54 (1.34-1.77) | 0.87 (0.71-1.05) | 2.08 (1.53-2.83) | 1.50 (1.26-1.73) |
| Pancreas | 1 | 1.37 (1.26-1.49) | 0.78 (0.69-0.88) | 0.93 (0.71-1.21) | 1.03 (0.93-1.12) |
| Lung | 1 | 1.02 (0.93-1.11) | 0.26 (0.23-0.30) | 0.22 (0.17-0.30) | 0.50 (0.46-0.54) |
| Breast | 1 | 1.48 (1.33-1.66) | 1.05 (0.93-1.19) | 0.88 (0.73-1.07) | 1.14 (1.05-1.23) |
| Premenopausal | 1 | 1.97 (1.83-2.12) | 1.76 (1.55-2.01) | 1.26 (0.96-1.66) | 1.66 (1.52-1.81) |
| Postmenopausal | 1 | 1.35 (1.25-1.46) | 0.90 (0.81-0.99) | 0.78 (0.64-0.97) | 1.01 (0.94-1.08) |
| Cervix | 1 | 1.98 (1.83-2.14) | 1.57 (1.36-1.82) | 1.14 (0.76-1.71) | 1.56 (1.38-1.75) |
| Endometrium | 1 | 1.89 (1.67-2.11) | 1.99 (1.74-2.27) | 2.26 (1.78-2.88) | 2.05 (1.83-2.26) |
| Ovary | 1 | 0.84 (0.80-0.89) | 0.78 (0.70-0.86) | 0.60 (0.43-0.84) | 0.74 (0.67-0.81) |
| Kidney | 1 | 0.97 (0.88-1.08) | 0.33 (0.24-0.44) | - | - |
| Bladder | 1 | 1.15 (0.81-1.63) | 0.45 (0.09-2.27) | - | - |
| Brain | 1 | 0.49 (0.44-0.54) | 0.44 (0.36-0.53) | 0.27 (0.14-0.52) | 0.40 (0.33-0.47) |
| NHL | 1 | 1.02 (0.83-1.27) | 1.20 (0.94-1.52) | 1.10 (0.72-1.68) | 1.11 (0.91-1.31) |
| Myeloma | 1 | 2.41 (2.18-2.67) | 2.03 (1.78-2.32) | 1.39 (0.88-2.18) | 1.94 (1.69-2.19) |
| Leukemia | 1 | 1.01 (0.87-1.17) | 0.80 (0.66-0.92) | 1.05 (0.72-1.53) | 0.95 (0.80-1.10) |
| All-Sites-Combinedb | 1 | 1.27 (1.17-1.37) | 0.82 (0.76-0.90) | 0.78 (0.70-0.87) | 0.96 (0.91-1.01) |
Blank cells not reported; less than 13 deaths; Abbreviations: NHL, non-Hodgkin lymphoma
MRRs derived from negative binomial regression
All sites combined includes those listed as well as those not listed here
Non-weighted average ratio between all Black populations and Whites
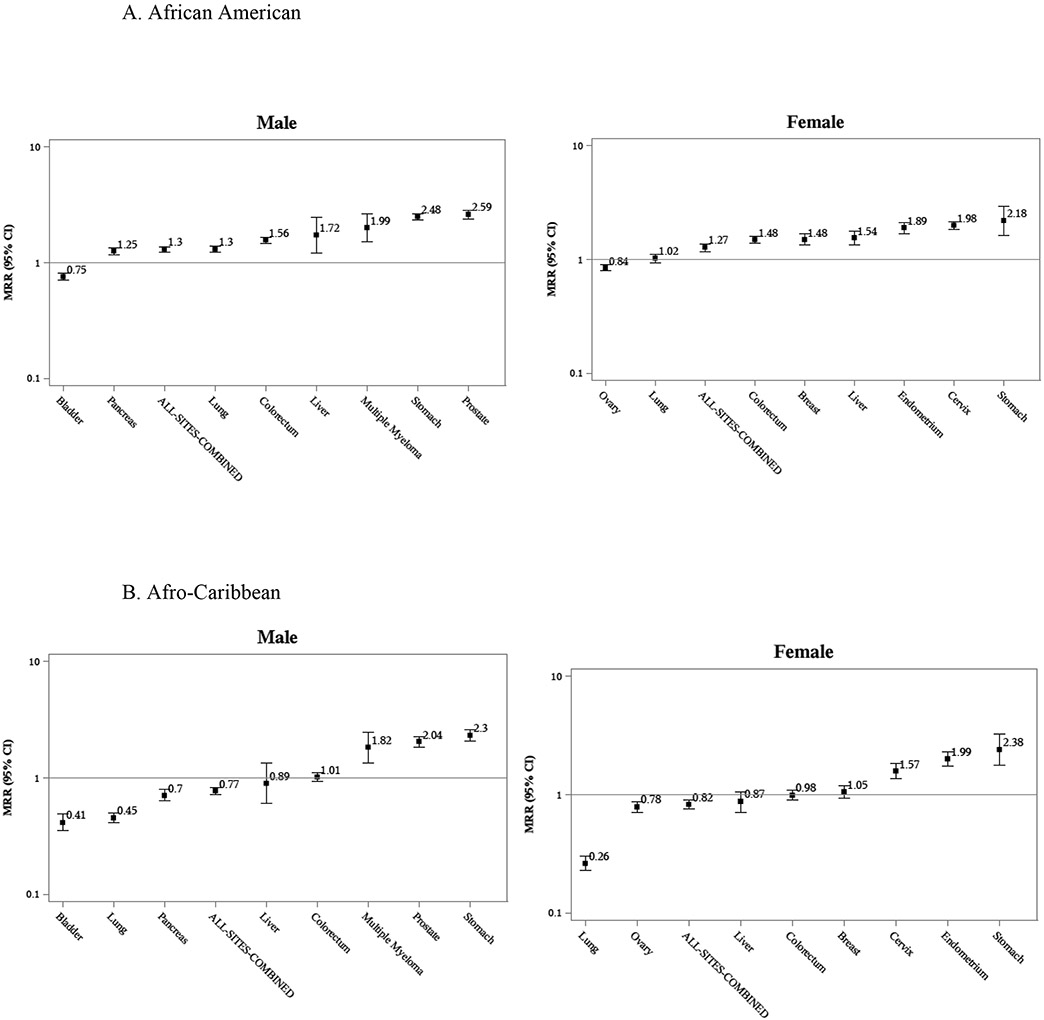
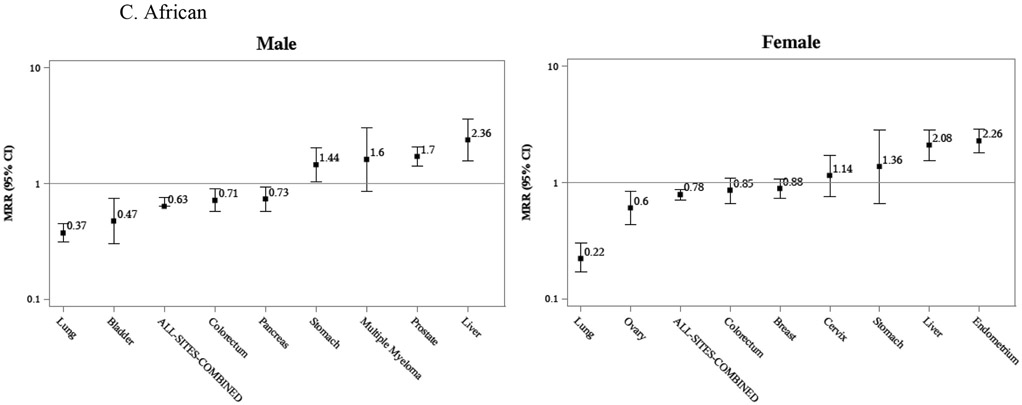
Figure 2.
Mortality Rate Ratios for selected cancers. California, Florida, Minnesota, New York, 2012-2017.
SAS 9.4 was used for data analyses. Institutional Review Board approvals were obtained from each of the four distinct states.
3. RESULTS
From 2012 to 2017, a total of 707,426 deaths due to cancer occurred among Whites (88%) and Blacks (12%) in California, Florida, Minnesota, and New York combined. Among the Black decedents, most were African American (n=67,154), followed by 12,422 Afro-Caribbeans, and 1,658 Africans. Overall, Black decedents had a lower proportion with college education (35%) than Whites (46%). However, considerable variation was seen, with West-Central Africans having the highest proportion of college education (54%) (Table 1).
The top causes of cancer death, computed as a percent of total cancer deaths, were similar for White and African American decedents. Lung was the leading cause for both males (25.3% White; 25.1% African American) and females (26.0% and 20.7%, respectively). Prostate and breast were second for males and females, respectively; colorectal was third for both. For Afro-Caribbean and West-Central African males, prostate cancer was the leading cause of cancer death, 21.7% and 17.4%, respectively; for East African males, liver cancer was first, at 14.4%. Breast was the leading cause of cancer death among Afro-Caribbean (20.2%), West-Central African (22.2%) and East African (18.8%) females, followed by colorectal cancer for Afro-Caribbeans (10.4%) and East Africans (10.0%). Uterine (endometrial) cancer was the second cause of cancer death among West-Central Africans (12.0%), and third for Afro-Caribbean (9.7%) and East African females (9.4%).
Among all analyzed groups, African Americans had the highest all-cancers-combined rates per 100,000 (232.0; 95% CI: 229.4-234.6 in males and 162.6; 95% CI: 160.9-164.4 in females). The lowest all-cancers-combined rates per 100,000 were seen in West-Central Africans (109.3; 95% CI: 96.0-123.9 in males and 91.2; 95% CI: 80.4-103.1 in females) (Table 2). African American males showed considerably higher mortality rates than Whites and other African-descent groups for oral, stomach, colorectal, pancreatic, lung, prostate, and kidney cancers as well as multiple myeloma. African American females had highest rates of oral, esophageal, colorectal, pancreatic, breast, and cervical cancers. Afro-Caribbeans had intermediate mortality rates (140.4 in males and 106.0 per 100,000 in females) in between African Americans and African immigrants for the most common cancers, including lung, colorectum, prostate, and breast. However, they also had the lowest rates amongst the three populations for liver cancer. African immigrants (East and West central combined) had the lowest all-sites combined rates, 120.5 for males and 99.2 per 100,000 for females. By region, East African males and females had the highest rates of liver cancer mortality; West-Central African women had the highest rate of uterine cancer.
Age-adjusted mortality rate ratios showed that African American men and women had 30% and 27% greater all-cancers-combined mortality risk, respectively, than Whites. Conversely, Afro-Caribbeans had 23% (male) and 18% (female) lower all-cancers-combined mortality and Africans had 37% (male) and 22% (female) lower mortality risk than Whites. As with observed rates, variation was remarkable by cancer site and sex. For prostate cancer, all African-descent groups had higher mortality than Whites: African Americans had 2.59 times higher; Afro-Caribbeans had 2.04 times higher; and Africans had 1.70 times higher (Table 3). Comparing only West-Central and Eastern Africans, with the former as reference (data not shown), the only statistically significant difference was found for female East Africans, who had a 1.86 times (MRR 1.86 95% CI 1.04-3.32) greater mortality risk of liver cancer than West-Central African females.
4. DISCUSSION
Comparable population-based cancer mortality rates for three distinct Black groups in the US – African Americans, Africans, and Afro-Caribbeans – are presented for the first time, providing unique insights into heterogeneous intra-racial cancer patterns while also enabling comparisons of patterns common to all three Black groups that contrast with the referent White population. US-born African Americans showed the highest cancer mortality burden, not only for the most common cancers, lung, breast, prostate, and colorectal, but also for some infection-related cancers, including stomach and cervical. African immigrants generally had the lowest rates, with notable exceptions for liver and endometrial cancers.
For males, all-sites-combined cancer mortality followed a distinct descending gradient: African Americans, Afro-Caribbeans, and Africans. For females, African Americans were highest, but no significant difference in all-sites-combined cancer mortality was observed between Afro-Caribbean and African women. The higher rates observed among African Americans compared to their Afro-Caribbean immigrant counterparts have previously been explored in detail,7, 8 and can be attributed to lower smoking rates among immigrants,15, 16 higher prevalence of obesity among African Americans,17 and differences in reproductive factors.18-20 These factors impact lung, colorectal and breast cancer rates, among others. The higher mortality for Afro-Caribbeans than Africans, especially males, may be in part linked to socioeconomic factors; education levels are generally higher among Africans.21 Moreover, a longer duration of stay in the US, inferred from the much higher median age of Afro-Caribbean immigrants, is likely associated with acquisition of more cancer risk factors and/or lifestyles.22
Some cancers had mortality rates uniformly higher in Black populations e.g. prostate, premenopausal breast cancer, multiple myeloma. In general, these followed the same gradient observed for all-sites-combined: highest rates among African Americans, then Afro-Caribbeans, then Africans. While high rates across all three populations may be evidence of a possible genetic race-specific vulnerability, this gradient also suggests that modifiable environmental risk factors account for some portion of the risk. Prostate cancer, for which Blacks have long been documented with a higher burden,1,23 and for which few risk factors are known,24 serves as an interesting example. In our study, African Americans, Afro-Caribbeans and West-Central Africans all had higher rates than Whites; yet the rate for East Africans was remarkably similar to Whites. This notable distinction highlights a commonality between African Americans, Afro-Caribbeans and West-Central Africans. Given the unfortunate but well-documented demographic patterns established by the Atlantic slave trade,25 and the fact that African Americans show a very low percentage of East African genetic admixture,11 this pattern suggests a genetic vulnerability tied to the Western region of Africa. Nonetheless, significant differences were seen between the three groups of West African origin: African Americans had the highest rates, 20% higher than Afro-Caribbeans and 50% higher than West-Central Africans. Reasons for these differences are likely linked to environmental factors, thus far unknown. Therefore, focused research on both the environmental and genetic risk factors for aggressive prostate cancer is warranted.
Endometrial cancer (EC) results were striking. All three Black populations had rates approximately twice as high as the referent White population, yet the aforementioned gradient was not observed. With EC rates approximately equivalent between the three African-descent groups, a race-specific vulnerability is suggested. EC rates in the US have been increasing among all women, although the largest increase, 2.4% annually, is seen among Black women.3 Recently, racial disparities in EC incidence,26 survival,27,28 and mortality1 have received considerable attention in the US. EC type may explain some of the observed disparities. EC type I is the most common overall and associated with a more favorable prognosis; EC type II (including carcinosarcomas and serous ECs), is less common but responsible for more cancer deaths.28 As an example, EC type II accounts for approximately 38% of all incident cases in Florida but 75% of all deaths.29 While EC type I is strongly estrogen-dependent and linked to obesity, risk factors for EC type II are much less clear.30, 31 Notably, unlike all other racial/ethnic groups, EC type II is the predominant type among Black women in the US.29 Given our findings of elevated EC mortality among all Black females, further studies into the specific risk factors for EC type II, particularly among populations of African descent, are required for better understanding and addressing this racial disparity.
Liver cancer results were intriguing. Mortality was high not only for African Americans, but also for African immigrants, while Afro-Caribbeans showed mortality similar to Whites. Moreover, within Africa, liver cancer mortality was much higher for East Africans than West-Central Africans. However, drawing conclusions from these liver cancer comparisons is difficult because of the variation in etiology by sex, geography and race/ethnicity of hepatocellular carcinoma (HCC, the main form of liver cancer32). In the US, the main cause of HCC is chronic infection with the hepatitis C virus (HCV); among Africans, chronic infection with the hepatitis B virus (HBV) is suggested as the main driver of HCC patterns.33-35 Additionally, the sex difference between HCV-related HCC in the US, with much higher rates among males than females,36 in contrast with HBV-related HCC among Africans (little difference by sex), may explain the observed relative differences between African Americans and Africans by sex. African women had much higher rates than African American women, but rates were similar between African American and African males.
Cervical cancer is linked directly to the human papilloma virus and recently vaccine-preventable; its relative burden has been linked to the effectiveness and availability of cancer prevention and control programs, including the use of screening tests. Lacking such access, women in Africa have been documented with very high rates.37 However, our results show an intriguing picture: among Black women in the US, the risk of cervical cancer mortality is higher among African Americans than their immigrant counterparts (i.e. Afro-Caribbeans). Notwithstanding possible differences in socio-economic status between the groups, Black immigrant women likely reap the benefits of the US health care system, including cervical cancer screening and treatment, which translates into drastically lower cervical cancer mortality in the US than in their countries of origin. This same pattern has been documented for other immigrant populations, including Hispanics.38
Our presentation of specific cancer rates by site for African immigrants to the US is novel; only one previous study examined the cancer burden among African immigrants on a population basis, and they used SEER incidence data.9 However, the findings were limited because they were based on proportional incidence ratios (PIRs) – relative differences in the distribution of the cases– instead of incidence rates. Also, birthplace was missing for a substantial proportion of the cases, and this missingness has been shown to be non-random, impeding valid imputation attempts.10, 39 Our use of mortality data overcomes these limitations.
We purposely avoided comparisons between our US-based mortality rates and the rates in Africa or the Caribbean because the impact of access to quality health care, and thus survival, would be magnified. For example, mortality rates for prostate and cervical cancer are very high in the Caribbean,40, 41 but substantially lower for these immigrant populations in the US.7, 8 Moreover, comparisons of our rates with African rates, even if pooled to encompass similar categories, would be biased by immigration selection factors, including higher education among immigrants 4 and the healthy immigrant effect.42,43 In addition, population-based cancer indicators for African countries, e.g. Globocan, often obtained by modelling the experience of neighboring countries,44 are impacted by the quality of data sources. In much of Sub-Saharan Africa, incompleteness is a particularly prevalent concern.45 Also, specifically for endometrial cancer it is intriguing that although we found a high burden among African-born women in the US, their second leading cause of cancer death, EC is rarely featured in studies of cancer in Africa.46 When rates have been reported (rarely), they are invariably low.47 Misclassification between endometrial and cervical cancer in African mortality statistics may explain this discrepancy; an assessment of this phenomenon is warranted.
A few studies, albeit none recent, examined the cancer experience of Black immigrants in other developed countries, mostly the United Kingdom.48,49 To further explore the dynamic between environmental and genetic risk for cancers among populations of African descent, we suggest additional, up-to date studies in countries with sizable Caribbean and/or Sub-Saharan African immigrant populations for comparison with the current analysis. Another interesting comparison would be among Black Hispanics in the US, including those from the Caribbean, Central America and South America. However, since race is self-reported, and only 1 in 4 Afro-Hispanics self-identify as Black,50 such a comparison is currently methodologically impossible.
Naturally, our study is subject to some limitations. First, mortality data does not provide information on individual-level risk factors, screening compliance or cancer treatments; all are known to impact mortality. Secondly, some rates, especially for the two distinct African populations, are relatively unstable due to calculations based on low numbers. Thirdly, the immigrants from Africa are most likely not representative of their counterparts in Africa. As shown in this study, West-Central African men, in particular, have proportionately higher advanced education level, which likely translates into greater access to quality healthcare and thus, higher survival; this potentially results in lower mortality rates.
In conclusion, among Black populations in the US, African Americans have the highest mortality for every cancer analyzed with two exceptions: endometrial cancer, which is similar across all three Black populations, and liver cancer with rates similar to Africans, although possibly for known epidemiological reasons. Endometrial, prostate, premenopausal breast, and stomach cancers as well as multiple myeloma show higher mortality for Black groups compared to the majority population in the US, Whites. More studies focusing on these particular malignancies are warranted both to reduce disparities and help stem the burden of cancer in these burgeoning populations as well as to increase our global understanding of the epidemiology of these cancers.
Highlights:
Aggregating Black populations in the US overlooks important cancer differences.
US Blacks had the highest mortality rates for common and infection-related cancers.
Caribbean Blacks had intermediate rates; African Blacks had the lowest rates.
Prostate cancer rates were higher in West Africans; East Africans had high liver rates.
Research on genetic and environmental cancer risk within Black populations is needed.
Funding Statement:
The author had no financial support for this manuscript.
List of Abbreviations
- ICD
International Classification of Diseases
- UN
United Nations
- CI
Confidence interval
- MRR
Mortality rate ratios
- EC
Endometrial cancer
- HCC
Hepatocellular carcinoma
- HCV
Hepatitis C virus
- HBV
Hepatitis B virus
- PIR
Proportional incidence ratios
- SEER
Surveillance, Epidemiology, and End Results Program
Footnotes
Conflict of Interest Statement: The authors declare no conflicts of interest.
Contributor Information
Paulo S. Pinheiro, Sylvester Comprehensive Cancer Center, Department of Public Health Sciences, Division of Epidemiology & Population Health Sciences, University of Miami School of Medicine, Clinical Research Building, 1120 N.W. 14th Street, Miami, FL 33136.
Heidy Medina, Department of Public Health Sciences, University of Miami School of Medicine.
Karen E. Callahan, School of Public Health, University of Nevada Las Vegas.
Deukwoo Kwon, Department of Public Health Sciences (Biostatistics), University of Miami School of Medicine.
Camille Ragin, Cancer Prevention and Control Program, Fox Chase Cancer Center-Temple Health.
Recinda Sherman, Program Manager, Data Use & Research, North American Association of Central Cancer Registries, Springfield, IL.
Erin N. Kobetz, Sylvester Comprehensive Cancer Center, University of Miami School of Medicine.
Ahmedin Jemal, Surveillance and Health Services Research, American Cancer Society.
References
- 1.DeSantis CE, Miller KD, Goding Sauer A, Jemal A, Siegel RL. Cancer statistics for African Americans, 2019. CA Cancer J Clin 2019;69: 211–33. [DOI] [PubMed] [Google Scholar]
- 2.Bialik K 5 facts about blacks in the US Secondary 5 facts about blacks in the US Washington, DC: Pew Research Center; 2018. https://www.pewresearch.org/fact-tank/2018/02/22/5-facts-about-blacks-in-the-u-s/.Accessed December 1, 2019 [Google Scholar]
- 3.Ward E, Sherman RL, Henley SJ, Jemal A, Siegel DA, Feuer EJ, Firth AU, Kohler BA, Scott S, Ma J, Anderson RN, Benard V, et al. Annual Report to the Nation on the Status of Cancer, 1999-2015, Featuring Cancer in Men and Women ages 20-49. J Natl Cancer Inst 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Anderson M, Lopez G. Key facts about black immigrants in the US Secondary Key facts about black immigrants in the US Washington, DC: Pew Research Center; 2018. https://www.pewresearch.org/fact-tank/2018/01/24/key-facts-about-black-immigrants-in-the-u-s/.Accessed December 1, 2019 [Google Scholar]
- 5.Ruggles S, Flood S, Goeken R, Grover J, Meyer E, Pacas J, Sobek M. IPUMS USA: Version 9.0 [dataset]. In: IPUMS, ed. Minneapolis, MN, 2019. [Google Scholar]
- 6.Arthur CM, Katkin ES. Making a case for the examination of ethnicity of Blacks in United States Health Research. J Health Care Poor Underserved 2006;17: 25–36. [DOI] [PubMed] [Google Scholar]
- 7.Pinheiro PS, Callahan KE, Ragin C, Hage RW, Hylton T, Kobetz EN. Black Heterogeneity in Cancer Mortality: US-Blacks, Haitians, and Jamaicans. Cancer Control 2016;23: 347–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pinheiro PS, Callahan KE, Boscoe FP, Balise RR, Cobb TR, Lee DJ, Kobetz E. Cancer Site-Specific Disparities in New York, Including the 1945-1965 Birth Cohort's Impact on Liver Cancer Patterns. Cancer Epidemiol Biomarkers Prev 2018;27: 917–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Medhanie GA, Fedewa SA, Adissu H, DeSantis CE, Siegel RL, Jemal A. Cancer incidence profile in sub-Saharan African-born blacks in the United States: Similarities and differences with US-born non-Hispanic blacks. Cancer 2017;123: 3116–24. [DOI] [PubMed] [Google Scholar]
- 10.Pinheiro PS, Callahan KE, Kobetz EN. Disaggregated Hispanic Groups and Cancer: Importance, Methodology, and Current Knowledge. In: Ramirez AG, Trapido EJ, eds. Advancing the Science of Cancer in Latinos. Cham: Springer International Publishing, 2020:17–34. [PubMed] [Google Scholar]
- 11.Tishkoff SA, Reed FA, Friedlaender FR, Ehret C, Ranciaro A, Froment A, Hirbo JB, Awomoyi AA, Bodo JM, Doumbo O, Ibrahim M, Juma AT, et al. The genetic structure and history of Africans and African Americans. Science 2009;324: 1035–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2019. CA Cancer J Clin 2019;69: 7–34. [DOI] [PubMed] [Google Scholar]
- 13.Florida Cancer Data System (www.fcds.miami.edu) FCDS Database: Incidence - Statewide Research Data, Nov 2017 Sub (2005-2016) - Linked To State Mortality Vital Statistics and National Death Index , data request released January 2019, based on the November 2017 submission. Accessed July 13, 2019.
- 14.The United Nations Statistics Division. Methodology:Standard country or area codes for statistical use (M49). https://unstats.un.org/unsd/methodology/m49/.Accessed November 13, 2019.
- 15.King G, Polednak AP, Bendel R, Hovey D. Cigarette smoking among native and foreign-born African Americans. Ann Epidemiol 1999;9: 236–44. [DOI] [PubMed] [Google Scholar]
- 16.Taylor KL, Kerner JF, Gold KF, Mandelblatt JS. Ever vs never smoking among an urban, multiethnic sample of Haitian-, Caribbean-, and U.S.-born blacks. Prev Med 1997;26: 855–65. [DOI] [PubMed] [Google Scholar]
- 17.Hales CM, Carroll MD, Fryar CD, Ogden CL. Prevalence of Obesity Among Adults and Youth: United States, 2015-2016. NCHS Data Brief 2017: 1–8. [PubMed] [Google Scholar]
- 18.American Cancer Society. Breast Cancer Facts & Figures 2019-2020 . Atlanta: American Cancer Society, Inc. 2019. [Google Scholar]
- 19.Porter P "Westernizing" women's risks? Breast cancer in lower-income countries. N Engl J Med 2008;358: 213–6. [DOI] [PubMed] [Google Scholar]
- 20.Ford K Duration of residence in the United States and the fertility of U.S. immigrants. Int Migr Rev 1990;24: 34–68. [PubMed] [Google Scholar]
- 21.Thomas KAJ. A Demographic Profile of Black Caribbean Immigrants in the United States . Washington,DC:MigrationPolicyInstitute;2012. http://www.migrationpolicy.org/research/CBI-demographic-profile-black-caribbean-immigrants. Accessed November 18, 2019. [Google Scholar]
- 22.Lara M, Gamboa C, Kahramanian MI, Morales LS, Bautista DE. Acculturation and Latino health in the United States: a review of the literature and its sociopolitical context. Annu Rev Public Health 2005;26: 367–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Altekruse SF , Kosary CL , Krapcho M , et al. SEER Cancer Statistics Review, 1975–2007 , National Cancer Institute; . Bethesda, MD: . http://seer.cancer.gov/csr/1975_2007/, based on November 2009 SEER data submission, posted to the SEER web site, 2010. Accessed December 2, 2019 . [Google Scholar]
- 24.Gann PH. Risk factors for prostate cancer. Rev Urol 2002;4Suppl 5: S3–S10. [PMC free article] [PubMed] [Google Scholar]
- 25.Salas A, Richards M, Lareu M-V, Scozzari R, Coppa A, Torroni A, Macaulay V, Carracedo A. The African diaspora: mitochondrial DNA and the Atlantic slave trade. Am J Hum Genet 2004;74: 454–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jamison PM, Noone AM, Ries LA, Lee NC, Edwards BK. Trends in endometrial cancer incidence by race and histology with a correction for the prevalence of hysterectomy, SEER 1992 to 2008. Cancer Epidemiol Biomarkers Prev 2013;22: 233–41. [DOI] [PubMed] [Google Scholar]
- 27.Collins Y, Holcomb K, Chapman-Davis E, Khabele D, Farley JH. Gynecologic cancer disparities: a report from the Health Disparities Taskforce of the Society of Gynecologic Oncology. Gynecol Oncol 2014;133: 353–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cote ML, Ruterbusch JJ, Olson SH, Lu K, Ali-Fehmi R. The Growing Burden of Endometrial Cancer: A Major Racial Disparity Affecting Black Women. Cancer Epidemiol Biomarkers Prev 2015;24: 1407–15. [DOI] [PubMed] [Google Scholar]
- 29.Johnson AL, Medina HN, Schlumbrecht MP, Reis I, Kobetz EN, Pinheiro PS. The Role of Histology on Endometrial Cancer Survival Disparities in Diverse Florida. In Press. 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Setiawan VW, Yang HP, Pike MC, McCann SE, Yu H, Xiang YB, Wolk A, Wentzensen N, Weiss NS, Webb PM, van den Brandt PA, van de Vijver K, et al. Type I and II endometrial cancers: have they different risk factors? J Clin Oncol 2013;31: 2607–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.McCullough ML, Patel AV, Patel R, Rodriguez C, Feigelson HS, Bandera EV, Gansler T, Thun MJ, Calle EE. Body mass and endometrial cancer risk by hormone replacement therapy and cancer subtype. Cancer Epidemiol Biomarkers Prev 2008;17: 73–9. [DOI] [PubMed] [Google Scholar]
- 32.Surveillance E, and End Results (SEER) Program (www.seer.cancer.gov). SEER*Stat Database: Incidence - SEER 21 Regs Limited-Field Research Data + Hurricane Katrina Impacted Louisiana Cases, Nov 2018 Sub (2000-2016) <Katrina/Rita Population Adjustment> - Linked To County Attributes - Total U.S., 1969-2017 Counties, National Cancer Institute, DCCPS, Surveillance Research Program, released April 2019, based on the November 2018 submission.
- 33.Parkin DM, Sitas F, Chirenje M, Stein L, Abratt R, Wabinga H. Part I: Cancer in Indigenous Africans--burden, distribution, and trends. Lancet Oncol 2008;9: 683–92. [DOI] [PubMed] [Google Scholar]
- 34.Pinheiro PS, Callahan K, Jones P, Morris C, Ransdell J, Kwon D, Brown C, et al. Liver Cancer: A Leading Cause of Cancer Death in the United States and the Role of the 1945-1965 Birth Cohort by Ethnicity. JHEP Reports 2019. doi: 10.1016/j.jhepr.2019.05.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Forde KA, Tanapanpanit O, Reddy KR. Hepatitis B and C in African Americans: current status and continued challenges. Clin Gastroenterol Hepatol 2014;12: 738–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Pinheiro PS, Medina H, Callahan K, Jones P, Brown CP, Altekruse SF, McGlynn K, Kobetz EN. The Association Between Etiology of Hepatocellular Carcinoma and Race-Ethnicity in the United States. In Press. 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.International Agency for Research on Cancer. Cancer Today:Population Fact Sheets. https://gco.iarc.fr/today/fact-sheets-populations.Accessed December 1, 2019.
- 38.Pinheiro PS, Callahan KE, Stern MC, de Vries E. Migration from Mexico to the United States: A high-speed cancer transition. Int J Cancer 2018;142: 477–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Pinheiro PS. The influence of Hispanic ethnicity on nonsmall cell lung cancer histology and patient survival : an analysis of the Survival, epidemiology, and end results database. Cancer 2013;119: 1285–6. [DOI] [PubMed] [Google Scholar]
- 40.Cattin LM, Pinheiro PS, Callahan KE, Hage R. Twenty-first century cancer patterns in small island nations: Grenada and the English-speaking Caribbean. Cancer Causes Control 2017;28: 1241–9. [DOI] [PubMed] [Google Scholar]
- 41.Razzaghi H, Quesnel-Crooks S, Sherman R, et al. Leading Causes of Cancer Mortality — Caribbean Region, 2003–2013. MMWR Morb Mortal Wkly Rep 2016. [DOI] [PubMed] [Google Scholar]
- 42.Singh GK, Hiatt RA. Trends and disparities in socioeconomic and behavioural characteristics, life expectancy, and cause-specific mortality of native-born and foreign-born populations in the United States, 1979-2003. Int J Epidemiol 2006;35: 903–19. [DOI] [PubMed] [Google Scholar]
- 43.Argeseanu S, Ruben JD, Narayan KM. Health of foreign-born people in the United States: a review. Health Place 2008;14: 623–35. [DOI] [PubMed] [Google Scholar]
- 44.International Agency for Research on Cancer. Cancer Today:Data and Methods. https://gco.iarc.fr/today/data-sources-methods#title-mort.Accessed December 1, 2019.
- 45.Crocker-Buque T, Pollock AM. Appraising the quality of sub-Saharan African cancer registration systems that contributed to GLOBOCAN 2008: a review of the literature and critical appraisal. J R Soc Med 2015;108: 57–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Parkin DM, Bray F, Ferlay J, Jemal A. Cancer in Africa 2012. Cancer Epidemiol Biomarkers Prev 2014;23:953–66. [DOI] [PubMed] [Google Scholar]
- 47.Jemal A, Bray F, Forman D, O'Brien M, Ferlay J, Center M, Parkin DM. Cancer burden in Africa and opportunities for prevention. Cancer 2012;118: 4372–84. [DOI] [PubMed] [Google Scholar]
- 48.Maruthappu M, Barnes I, Sayeed S, Ali R. Incidence of prostate and urological cancers in England by ethnic group, 2001-2007: a descriptive study. BMC Cancer 2015;15: 753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Grulich AE, Swerdlow AJ, Head J, Marmot MG. Cancer mortality in African and Caribbean migrants to England and Wales. Br J Cancer 1992;66: 905–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Lopez G, Gonzalez-Barrera A. Afro-Latino: A deeply rooted identity among US Hispanics. Washington, DC: Pew Research Center; 2016. https://www.pewresearch.org/fact-tank/2016/03/01/afro-latino-a-deeply-rooted-identity-among-u-s-hispanics/.Accessed December 1, 2019 [Google Scholar]