Abstract
The aftermath of TBI is associated with an acute stress response and the accumulation of insoluble protein aggregates. Even after the symptoms of TBI are resolved, insidious molecular processes continue to develop, which often ultimately result in the development of age-associated neurodegenerative disorders. The precise molecular cascades that drive unhealthy brain aging are still largely unknown. In this review, we discuss proteostatic dysfunction as a converging mechanism contributing to accelerated brain aging after TBI. We examine evidence from human tissue and in vivo animal models, spanning both the aging and injury contexts. We conclude that TBI has a sustained debilitating effect on the proteostatic machinery, which may contribute to the accelerated pathological and cognitive hallmarks of aging that are observed following injury.
Keywords: Unfolded protein response, heat shock response, ubiquitin-proteasome system, experimental models, cellular stress response, therapeutics
TRAUMATIC BRAIN INJURY: LINKS TO AGING
A traumatic brain injury, or TBI, is caused by a physical insult to the head. Mechanical trauma causes disruptions in normal brain function that can last years following the initial injury, resulting in millions of people worldwide who struggle with lifelong disabilities [1]. Increasing evidence suggests that there is an overlap in the molecular processes that result from TBI, and those that lead to neurodegeneration. A specific neuropathological condition termed chronic traumatic encephalopathy (CTE) has been associated with exposure to repetitive mild TBIs [2]. Multiple epidemiological studies also link TBIs of varying severities to an increased risk of developing neurodegenerative conditions such as Alzheimer’s disease (AD), Parkinson’s Disease (PD), and Amyotrophic Lateral Sclerosis (ALS) (Box 1) [3]. However, the primary risk factor for most of these diseases is aging. Loss of proteostasis that occurs early after TBI as well as during aging favors the formation of toxic insoluble protein deposits like extracellular amyloid-β (see Glossary) plaques, intracellular hyperphosphorylated tau tangles, α-synuclein aggregates, and cytosolic TAR-DNA binding protein 43 kDa (TDP-43) aggregates [1, 4]. One hypothesis that associates TBI with neurodegenerative disease suggests that TBI sets in motion a constellation of molecular events that are associated with the normal biological aging process. Common occurrence of these disease-associated aggregates underscores proteostatic dysfunction as a central theme connecting TBI with neurodegenerative disease and aging. A history of TBI appears to hasten the brain along the trajectory of aging, presenting a phenotype of a biologically “older” brain than the chronological age would suggest [4]. Functional imaging indicates that even mild TBIs can cause a reduction in cortical thickness [5] similar to aging, while moderate to severe TBIs cause tissue losses of up to 5% per year [6-8]. A predictive model of brain aging using magnetic resonance imaging found that TBI brains appear between 4.6 and 6 years older than their chronological age [9]. The study also found that cognitive decline is strongly correlated with time since injury, suggesting that the increased biological age is driven by processes that continue to occur in the brain after the initial insult. However, most in vivo animal model studies in the TBI field focus on the acute consequences of injury, with a relative paucity of studies that examine the chronic effects of TBI and its impact on aging-related processes.
BOX 1 -. TRAUMATIC BRAIN INJURY: A LINK TO NEURODEGENERATIVE DISEASE.
As the aging population increases across the world, the silent epidemic of TBI will prove to have enormous implications for public health. While the mechanistic aspects of the association between TBI and neurodegeneration are still under intense investigation, it is widely agreed that a history of head trauma can trigger pernicious processes that increase the overall risk of developing a neurodegenerative disorder. Following from clinical and pathological observations of pugilists in the early 20th century, and more recently athletes and military veterans, a specific neurological condition termed chronic traumatic encephalopathy (CTE) has been associated with exposure to repetitive mild TBIs [2]. Tentatively defined, CTE is a tauopathy that can only be diagnosed post-mortem by its pathognomonic lesions of perivascular accumulations of tau around neurons and astrocytes in the depths of cortical sulci [151]. However, around 40% of CTE cases studied to date have presented with other comorbid degenerative diseases such as AD, Lewy body disease, frontotemporal lobar degeneration (FTD) and motor neuron diseases like ALS [152]. Independent of CTE, there is evidence from multiple epidemiological studies for links between TBI of varying severities and neurodegenerative conditions [3]. Two large cohort studies conducted by the US Veterans Health Administration found that one or more instances of moderate to severe TBIs as well as mild TBIs were associated with a higher risk of developing dementia and PD [153-155]. Other large cohort studies have also found marginally increased associations between a history of a single severe or mild TBI and dementia [156, 157]. When neuropathological confirmation of AD was available, a study found that dementia onset was 3.6 years earlier when a history of TBI was involved [158]. These studies provide epidemiological weight to the hypothesis that TBI precipitates the aging process, causing an earlier advent of typically late-onset neurodegenerative processes.
Aggregation of disease proteins is the best studied link between TBI and the development of neurodegenerative disorders. TBI stimulates rapid induction of amyloid precursor protein, amyloid-β peptides, hyperphosphorylated tau, physiological TDP-43, and α-synuclein, within hours to days of the insult [4, 159]. It is unknown how these early disruptions in proteostasis eventually culminate in CTE or any other neurodegenerative disease. Therapeutic interventions aimed at halting neurodegenerative processes have been largely unsuccessful, both in the field of age-induced disorders and TBI-induced proteinopathy. This failure calls for a deeper dissection of the pathogenic mechanisms that drive neurodegeneration, to identify key therapeutically targetable processes that significantly impact progression of the disease.
In this review, we highlight the impact of proteostatic dysfunction as a mechanism contributing to accelerated brain aging after TBI. We focus on the unfolded protein response (UPR), the heat shock response (HSR), and the ubiquitin-proteasome system (UPS), which are three key proteostatic stress response pathways that become dysregulated with age (Figure 1, Key Figure). The current literature provides support for the argument that TBI mobilizes all arms of the proteostatic machinery, but has a sustained debilitating effect on protein homeostasis, as evidenced by accumulation of misfolded aggregates after injury, contributing to unhealthy brain aging. This review also discusses potential therapeutic strategies that have been successfully described in in vivo animal models to ameliorate the injury response and improve age-related signs of deterioration, such as cognition and motor function.
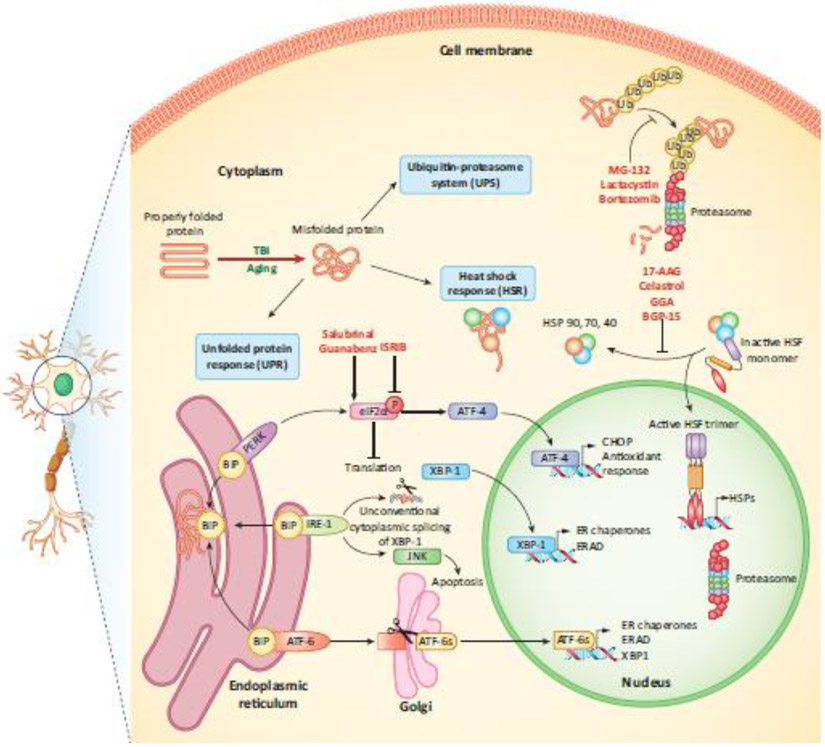
Figure 1 (Key Figure). Stress response pathways dysregulated with age and injury:
Three different stress pathways triggered by misfolded protein in neuronal and glial cells are illustrated – the heat shock response (HSR), the unfolded protein response (UPR), and the ubiquitin proteasome system (UPS). Therapeutic modulators of TBI are shown in red, indicating the pathways they affect. UPR: Misfolded proteins in the ER bind the stress-sensing chaperone, BiP, sequestering it away from PERK, IRE-1 and ATF-6 and activating them. PERK phosphorylates eIF2α leading to global repression of translation and increasing selective translation of ATF-4. IRE-1 triggers the unconventional cytoplasmic splicing of XBP-1, as well as activation of the JNK pathway. ATF-6 translocates to the Golgi apparatus and is processed to form the active transcription factor ATF-6s. Together, these mediators dictate the induction of pathways that attempt to restore normal cellular function by refolding or degrading misfolded proteins, and in the event of prolonged stress, activating apoptotic pathways. UPS: Misfolded proteins are tagged with a polyubiquitin chain and targeted to the cytoplasmic or nuclear proteasome for degradation. HSR: HSPs bind HSF and sequester it in an inactive monomeric form. During cellular stress, HSPs bind misfolded proteins, allowing HSF to trimerize, translocate to the nucleus and trigger the transcription of more HSPs. Abbreviations: BiP, Ig binding protein; PERK, PKR-like ER kinase; IRE-1, inositol requiring enzyme-1; ATF, activating transcription factor; eIF2α, eukaryotic initiation factor 2α; CHOP, C/EBP homologous protein; XBP-1, X-box binding protein-1; ER, endoplasmic reticulum; ERAD, ER associated degradation; JNK, Jun N-terminal kinase; Ub, ubiquitin; HSP, heat shock protein; HSF, heat shock factor.
MOLECULAR AGING PROCESS
A natural hallmark of the aging process is an attenuated response to stress challenges, due to a compromised ability to effectively invoke pathways that regulate stress resistance [10]. The cellular consequence of the failure of these stress response pathways is the gradual buildup of damage to proteins, lipids, and nucleic acids. Most genetic or pharmacological interventions that influence longevity impinge on various aspects of the initiation and execution of different stress response pathways [11]. Damage that accumulates in biomolecules throughout the organism can be caused by: 1) genotoxic stress brought about by mitochondrial dysfunction and the generation of reactive oxygen species, causing damage to DNA; 2) proteotoxic stress caused by a failure to refold unfolded or misfolded proteins using chaperones or to degrade them efficiently through the proteasome and lysosome functions, leading to aggregate formation [10]. Aging is associated with an imbalance between the damage caused by these stresses and the repair pathways stimulated by them, culminating in accumulations of age-related pathology and the appearance of disease. We note that stress response pathways, such as the DNA damage response system, oxidative stress response and autophagy, also contribute to the synergy between aging and TBI (see [1] and [4]), but are beyond the scope of this review.
STRESS RESPONSE: RELATIONSHIP BETWEEN TBI AND AGING
Heat shock response
The heat shock response (HSR) is an evolutionary conserved phenomenon whereby organisms produce a group of proteins, termed the heat shock proteins (HSPs) in response to a wide variety of environmental stressors such as heat, oxidative stress, and injury [12] (Fig 1). The HSR is coordinated by the family of transcription factors termed heat shock factors (HSFs) to organize the rapid induction of HSPs, which act as chaperones to aid in refolding affected proteins [13]. Along with upregulation of factors to promote recovery, there is also a large downregulation of genes regulating translation, cell cycle, and RNA processing [14]. There are five classes of HSPs – HSP100, HSP90, HSP70, HSP60, and small HSPs. Through concerted action, the chaperones broadly assist in refolding stress-denatured proteins to achieve their native state by binding to hydrophobic patches and preventing aggregation [13]. Additionally, when proteins are irreversibly misfolded or under conditions of nutritional stress, HSP70 and its cochaperones target proteins for degradation through the proteasome or autophagy [15]. Upon sufficient increase in cellular concentration, HSP70, HSP90, and HSP40 interact with HSF to repress its transcriptional activity, returning HSF to its monomeric form and attenuating the HSR [16, 17].
Aging is associated with a decline in the efficacy of the organism to maintain proteostasis and mount an effective stress response. The amount of misfolded proteins and aggregates increases with age broadly across organisms and tissues, with a concomitant increase in HSPs to counter these effects [18-20]. However, the stress-induction and chaperoning ability of HSPs also decreases with age [21-23]. Ironically, the increase in HSPs may actually serve to increase the negative regulation of HSF, decreasing the amplitude of the HSR [12, 24]. Other mechanisms of HSF inhibition with age include changes in post-translational modifications affecting its DNA-binding and activation [25-27]. An example of this is the age-dependent decrease in expression and activity of SIRT1, a histone deacetylase that targets and stabilizes HSF, promoting activation of the HSR [28, 29]. Resveratrol, a potent activator of SIRT1, is a prominent anti-aging therapeutic candidate, and one of its mechanisms of action is its ability to induce HSP production [30]. Upregulation of HSPs and HSF to prolong longevity have been demonstrated in multiple model systems like C. elegans and Drosophila [31-33]. Additionally, the insulin/IGF-1 signaling pathway, which modulates the aging process depending on extracellular growth conditions, has effects on proteostasis and HSF regulation, underscoring the intimate relationship between stress response systems and longevity [33, 34].
The induction of the HSR in response to TBI is well-documented across human studies, as well as in multiple model systems. Encouragingly, genetic, environmental, and pharmaceutical manipulations to increase the HSR have had positive effects on TBI outcome [35-40], while attempts to reduce the HSR through HSP reduction have had deleterious outcomes [35, 36]. Examination of injured human brain tissue reveals that HSP70 protein and mRNA, but not HSC70 (the constitutively expressed cognate form of the protein) is induced in response to TBI [41-44]. Elevations in HSPs have also been detected early in serum and cerebrospinal fluid (CSF) samples from patients with moderate to severe TBI. Increases in serum HSP70 are observed as early as within 30 min of injury, reaching peak values at 3d post-TBI [45, 46]. Another study demonstrated that serum HSP70 values are an effective predictor of mortality, implying that unresolved cellular stress after injury can have deleterious consequences [47]. Both HSP70 and mitochondrial HSP60 increase in CSF samples, with HSP60 levels independently associated with injury severity as defined by the Glasgow Coma Scale score (a clinical scale for assessing a patient’s level of consciousness following a brain injury) [43, 48]. These studies implicate the HSR as an immediate and early response to TBI, but it remains to be studied whether injury has any lasting effects on the ability of patients to mount an effective response to subsequent challenges.
Most of the work examining changes in HSPs following TBI in vivo in animal models has been performed in rats using the fluid percussion injury (FPI) model, which applies a brief fluid pulse to the exposed dura and deforms the brain tissue. HSP70 mRNA is increased between 2h and 6h after TBI and returns to baseline by 24h [49-52]. HSP70 protein, on the other hand is only moderately increased at 4h post-TBI and reaches a peak at 24h [50]. HSP60, a chaperone that resides in the mitochondrial matrix and responds to mitochondrial stress, is strongly upregulated at both mRNA and protein levels at 4h and 24h after injury [50]. Another commonly used TBI model is the controlled cortical impact (CCI) approach, in which a piston applies a controlled trauma to the exposed cortex. Studies using CCI indicate similar changes in HSP70 mRNA and protein [53]. The small HSPs are less well-studied, with a few studies indicating that they are induced upon TBI in young animals, but the induction is markedly lower when aged animals are injured [54, 55].
Genetic-manipulation studies in mice have underscored the importance of HSPs in TBI. Mice that lack HSP70 or HSP110 have worsened neurological and physiological outcomes following TBI, whereas transgenic mice overexpressing HSP70 have improved outcomes [35, 36]. As noted earlier, most animal models studies on TBI examine acute effects, and a relatively small number of studies have examined long-term effects. A study in rats showed that HSP70 is chronically downregulated 12 months after injury, suggesting heightened vulnerability to other stresses [56]. The study also observed that the expression levels of genes related to aging are transiently increased after injury, while the expression levels of genes associated with neuroprotection, such as the antiapoptotic factor Bcl-2 and the antioxidant glutathione peroxidase-1, are increased at 12 months in the survivors. These results suggest that TBI may have a sustained debilitating effect on some cellular stress responses such as the HSR, while other systems such as apoptosis and the antioxidant defense are upregulated for maintaining homeostasis.
A number of therapeutic inducers of the HSR have been tested for their potential to protect against TBI-induced deficits in rodent models. 17-AAG, Celastrol, geranylgeranylacetone (GGA), and BGP-15 are potent pharmacological inducers of HSF, either acting on HSP90 to lift its inhibition of HSF or altering the post-transcriptional modifications of HSF to allow increased activity [57]. Administration of 17-AAG either before or at the time of TBI in rodents induces an increase in HSP70 production that lasts up to 3d post-TBI and results in improved lesion size, neuronal survival, sensorimotor performance, and a reduction in pro-inflammatory cytokines [37, 38]. Celastrol and BGP-15 have protective effects comparable to those of 17-AAG when administered in two doses within 24h of injury or five doses up to one week after TBI in mice [36]. Similarly, GGA confers protection when administered either before or after TBI in mice [58]. Other compounds have been shown to increase HSP70 through other pathways, such as tert-butylhydroquinone which acts on Nrf2, and dexmedetomidine, a sedative. Both these compounds have beneficial effects on edema formation, memory loss, and apoptosis after TBI in mice [59, 60]. Environmental interventions such as exercise have also been shown to have a favorable effect in rats through stimulation of HSP70 and HSP20 production [61, 62]. An interesting environmental modulation of the HSR that was tested in rodent models is based on manipulating the environmental temperature. Specifically, rats reared for one month at a temperature slightly elevated (34°C) compared to typical rearing environments fared better than normothermic animals when subjected to TBI [39]. These heat-acclimated animals were also shown to have improved blood-brain barrier integrity, edema formation, and neurobehavior scores. The animals further displayed increased neurogenesis, anti-apoptotic and antioxidant capacity, and an immune system that was better primed to handle the TBI insult [63-66]. A key mechanism of protection achieved through heat acclimation involves the signaling pathway hypoxia-inducible factor 1 (HIF1) [67, 68]. HIF1 is activated during the adaptation response to ischemia, to increase oxygen delivery. A protective effect similar to the heat-acclimation observed in rodents was shown in a Drosophila model of TBI where pretreatment with an acute 30 min heat shock to 37°C, from normal rearing temperature of 25°C, extends lifespan and mitigates brain degeneration in injured animals [40].
Overall, it is evident that the HSR plays a key protective role in re-establishing protein homeostasis after injury as well as during the aging process. However, further work is needed for exploring the long-term effects of TBI on chaperone availability and the functionality of the HSR. Therapeutic modulations of the HSR that increase longevity in animal models are also successful in mitigating the deleterious effects of TBI, arguing that chaperone regulation is a fundamental link between the aging and injury processes. Future efforts are needed to determine whether alleviation of protein misfolding in the immediate aftermath of a TBI will have a lasting effect in preventing chronic post-traumatic neurodegenerative processes.
Unfolded Protein Response (UPR)
The UPR is instigated by endoplasmic reticulum (ER) stress when there is an accumulation of misfolded proteins. The UPR upregulates chaperones to help regulate protein folding, temporarily halts protein synthesis, targets misfolded proteins for proteasomal degradation in the event that they cannot be refolded, and finally, promotes apoptosis in cases of severe chronic stress [69]. These events are orchestrated by the three arms of the UPR: PKR-like ER kinase (PERK), inositol requiring enzyme-1 (IRE-1), and activating transcription factor-6 (ATF-6) [69] (Fig 1). The three leader proteins are normally sequestered in an inactive form in the ER membrane by the stress-sensing chaperone Ig binding protein (BiP)/glucose-regulated protein 78 (GRP78) [69]. Under ER stress, BiP/GRP78 dissociates from the three proteins, initiating the UPR. The integrated stress response (ISR) is mediated by the phosphorylation of the eukaryotic translation initiation factor 2 alpha (eIF2α) by PERK, resulting in the global repression of translation and the transcription of a few select genes, such as ATF-4 that directs the upregulation of stress response genes, including antioxidant pathways [70]. Upon severe stress, ATF-4 is also responsible for activating C/EBP homologous protein (CHOP), a transcription factor that mediates apoptosis. The second arm of the UPR, IRE-1, facilitates the splicing of X-box binding protein (XBP-1), which regulates genes related to chaperones and the ER association degradation pathway (ERAD) [71]. The IRE-1 arm also activates the Jun N-terminal kinase (JNK) pathway, leading to apoptosis. The third arm of the UPR, ATF-6, translocates to the Golgi upon activation and is processed to form a transcription factor (ATF-6s) that stimulates the transcription of several chaperones, ERAD genes, and XBP-1 [72]. Together, the three arms of the UPR serve to restore homeostasis, mobilize the protein-folding machinery, target misfolded proteins for degradation, and in the event of extreme stress, trigger cell death.
There is strong evidence that the functional components and stimulation of the UPR gradually decline with age. The levels and enzymatic activity of multiple resident ER chaperones, such as BiP/GRP78 decrease in many tissues with age throughout the body, including the brain [73-76]. Most studies show that when the aged organism is challenged by a stress, the ER fails to effectively stimulate the UPR, triggering pro-apoptotic pathways of cell death rather than survival [76-79]. Studies from nematodes and Drosophila indicate that deletion of integral UPR proteins like IRE-1 and XBP-1 decrease lifespan [80, 81], while expressing the spliced form of XBP-1 is sufficient to extend lifespan and improve stress resistance [82].
Most of what we know about the activation of the UPR after TBI comes from preclinical rodent models. The only related study in humans, to our knowledge, examined two postmortem CTE brains and reported increases in markers of all three arms of the UPR, which colocalized with hyperphosphorylated tau tangles [83], suggesting that sustained ER stress is a chronic consequence of TBI.
Signs of UPR activation in rodents, notably increases in the phosphorylated form of eIF2α, BiP/GRP78, processed XBP-1 mRNA, and CHOP have been observed as early as 4h after injury and lasting until 48-72h, accompanied by a reduction in protein synthesis up to 48h [84-86]. An in vitro model of compressive neuronal injury demonstrated a gradual increase in ER stress accompanied by a release of intracellular calcium stores from the ER [87]. Assessment of a population of dying neurons after TBI in rats showed that the neurons had increased expression of ER stress genes compared to the population of healthy neurons from the same brain area, indicating that TBI can trigger both the protective and apoptotic arms of the UPR cascade [88]. The induction of the UPR occurs not only in neurons, but also in glial cells, to promote cell survival and astrogliosis, as shown in a mouse model of TBI [89]. There is also evidence to suggest that activation of the UPR is a chronic response to TBI in mice and rats, with levels of the phosphorylated form of eIF2α and ATF-4 being increased up to a month post-injury [90, 91]. From these studies, it is evident that the UPR is triggered early in response to TBI and is sustained for months and perhaps years after the injury.
Significant effort has been put into investigating means of reducing ER stress in rodent models as a therapeutic avenue for TBI [92, 93]. In particular, compounds to modulate phosphorylation of eIF2α have been widely studied. Phosphatase inhibitors that prevent de-phosphorylation of eIF2α can enhance the protective PERK arm of the UPR, prolonging the repression of protein translation and decreasing the protein load entering the ER. The inhibitors salubrinal and guanabenz have been shown to inhibit CHOP upregulation and apoptosis, alleviate ER and oxidative stress, in addition to rescuing lesion volume, neuroinflammation, and memory deficits [94-102]. Contrarily, blocking PERK activation using drugs or a kinase dead mutant, which would prevent eIF2α phosphorylation, also seems to have beneficial effects. However, these effects are possibly mediated through the compound’s effect on cAMP response element binding protein (a transcription factor binding to the cAMP element and regulating genes related to neuronal plasticity) and may be independent of eIF2α [103, 104]. Similarly, treatment with ISRIB, an inhibitor of the ISR that blocks eIF2α phosphorylation, corrects chronic memory deficits even when administered weeks after injury in mice [90, 105]. Docosahexaenoic acid (DHA), a polyunsaturated fatty acid that is enriched in neuronal cell membranes and essential for normal brain function, has also demonstrated potential in mitigating ER stress, rescuing white matter damage and reducing accumulations of ubiquitinated material, amyloid and phosphorylated tau deposits [91, 106, 107]. Other therapeutic treatments, like dexmedetomidine (an α2-adrenoreceptor agonist), polydatin (a resveratrol precursor), hypothermia, curcumin, and tauroursodeoxycholic acid (a hydrophilic bile acid) show beneficial effects in part by impinging on the UPR and alleviating ER stress [108-112].
These studies indicate the role of the UPR in TBI pathophysiology is complex, as demonstrated by therapeutic strategies that are successful through both inhibition and promotion of translation. Thus, the type of treatment may depend on various factors that determine the prosurvival vs. the apoptotic state of the UPR after injury, which may be mediated by several factors including injury type and severity. Many studies examining UPR perturbation as a therapeutic strategy have used short-term functional studies as endpoint assays, while relatively few studies have scrutinized changes in misfolded protein accumulation. Furthermore, given the paucity of UPR studies in post-mortem human tissues, the long-term influence of TBI on ER stress in the context of human disease is still largely unknown.
Ubiquitin-proteasome system (UPS)
The UPS is the primary cellular system responsible for the selective degradation of proteins involved in a broad range of cellular activities to maintain homeostatic levels, as well as clearing misfolded or damaged proteins [113]. Proteins targeted for degradation are marked with ubiquitin, a short polypeptide, through a series of enzymatic reactions by E1, E2 and E3 enzymes to form a polyubiquitin chain [114] (Fig 1). Such tagged proteins are directed to the proteasome for proteolytic degradation. The proteasome is a massive multi-subunit assembly comprised of two main components – the 19S and 20S particles – to form the complete 26S structure [113]. The 19S is the regulatory portion of the proteasome that recognizes, unfolds, and translocates the ubiquitinated proteins to the catalytic 20S subunit for enzymatic cleavage [115].
Many studies that examine the contribution of the UPS to aging indicate that proteasome function is compromised with age, across multiple tissues and species [116-118]. Age-associated decline of proteasome activity occurs due to a loss in subunit expression levels, a decrease in the enzymatic activity of the proteases, or an overwhelming of the UPS by damaged proteins and aggregates that it is unable to resolve. Studies in rats demonstrated a reduction in proteasomal activity within cortical areas, hippocampus, and spinal cord, but not the cerebellum and brainstem [119, 120]. Other studies across various species report similar findings of proteasomal inhibition with age in rodents, nematodes, and Drosophila [121-125]. Intriguingly, genetic upregulation of different proteasomal subunits has demonstrated not only increased proteasomal activity, but also increased longevity and protection from oxidative challenges in Drosophila and human cellular senescent models [123, 126, 127]. In C. elegans, other means to extend lifespan, such as caloric or dietary restriction, have been shown to act on specific E2 and E3 ligases to offset the anticipated decline in proteostasis with age [128, 129].
In contrast to age, the number of reports studying changes in UPS activity after TBI is relatively sparse, both in humans and in animal models. A survey of human brain tissue from patients who died after a severe TBI revealed that the K48 ubiquitin linkage (the most prevalent form of polyubiquitin signal that targets proteins to the proteasome) increases within an hour of injury in both neurons and glia [130]. This is observed even in cases when the injury had happened 7 months earlier, indicating that the UPS is likely active chronically after severe TBI [130]. Elevated UPS activity is exhibited in circulating plasma following even mild injury, and the duration of this increase lasts until resolution of the symptoms of concussion [131]. Ubiquitin immunoreactivity is also detected in CSF samples after TBI, with levels becoming progressively higher until death [132]. These studies implicate the association of a dysfunctional proteasome and accumulation of degradation-targeted proteins with adverse outcomes such as death and possibly unhealthy aging.
The accumulation of ubiquitinated material acutely after TBI has been reported in multiple animal models [91, 133-135]. The increase in ubiquitin-conjugated proteins is accompanied by a depletion in free ubiquitin, suggesting a disruption in the tightly-regulated protein turnover process [136]. Similar to aged animals, injured rats exhibit a decrease in the protease activity of the proteasome as well as changes in its subunit composition [137]. Modulation of the UPS does not yield straightforward results. For instance, increase of ubiquitin carboxy-terminal hydrolase L1 (UCHL1), a brain-specific deubiquitinating enzyme that cleaves amino acids from ubiquitin to generate free monomeric ubiquitin, has a therapeutic effect in attenuating neuron death and axon injury, and reducing the ubiquitinated protein load in mice [138]. Treatment with proteasome inhibitors MG-132 and lactacystin has deleterious consequences in in vitro models of axon injury (both inhibitors) and in vivo models of TBI (only MG-132), while bortezomib, another inhibitor, yields improved physiological consequences [134, 139, 140]. Bortezomib is thought to derive its potent anti-inflammatory activity by preventing the proteasome-mediated degradation of inhibitor of NFκB, thus reducing its activation after trauma [141].
As with the other components of the proteostatic machinery, there is evidence that UPS function gradually declines with age, and promotion of UPS activity is associated with longevity. However, from studies of injured human tissue, prolonged activation of the UPS appears to be associated with detrimental outcomes, although this has not been verified in more controlled in vivo studies.
CONCLUDING REMARKS
Here we have covered key aspects of the three central proteostatic stress response pathways – the HSR, UPR, and UPS – and their integration into the study of TBI and aging, as well as some of the therapeutic approaches and opportunities they offer. There is clear evidence to demonstrate that stress sensitivity is a key phenotype of the aged organism. The pathophysiology of TBI encompasses various molecular events that mobilize many of the stress response pathways. Depending on the nature and severity of the TBI, some of the responses may become dysfunctional, as evidenced by inefficient proteasomal degradation [134] and heightened activation of the apoptotic, rather than survival, mode of the UPR [88].
The few reports that study the long-term consequences of TBI suggest that select stress pathways, such as the UPR, HSR, UPS, and autophagy are chronically affected after an injury [56, 90, 91, 130, 135]. This implies that TBI, a super-stressor in itself, may have a sustained debilitating impact on the stress response, contributing to the accelerated pathological and cognitive hallmarks of aging that are observed after injury. Furthermore, it is known that age too, has a detrimental effect on the response to TBI [142]. Aged animals that are injured have a much higher mortality rate, elicit a far less robust stress response, and have worsened pathological outcomes [55, 143-145]. The confluence of TBI and aging needs more preclinical research, to investigate and define in molecular terms both the long-term effects of TBI on the normal pathways of the aging process, as well as the response of the aged organism to TBI (see Outstanding Questions).
OUTSTANDING QUESTIONS:
How can aging be successfully incorporated into TBI studies? There are numerous studies that address cellular and molecular mechanisms affected in the acute and subacute phases of TBI. However, due to the lengthy lifespans of mammalian experimental animal models, there is a relative scarcity of in vivo studies that examine the long-term repercussions of TBI. With the advent of invertebrate TBI models in C. elegans and Drosophila, studies that focus on the interrelationship between TBI and aging become more feasible.
There are inconsistencies in TBI studies about whether augmentation or suppression of a stress response is protective. What is the source of these discrepancies? Many of these stress response systems have the ability to both restore homeostasis and trigger signaling pathways of cell death in cases of severe stress. Further research into the roles of the heat shock response, unfolded protein response, the ubiquitin-proteasome system, and autophagy are required to understand the consequences of modulating these pathways in an injury setting.
How are other pathomechanisms of the TBI-response associated with aging? The complex acute pathophysiology of TBI includes excitotoxicity, oxidative stress, DNA damage and inflammation. Most of these pathways are also known to become impacted with age, strengthening the link between a history of TBI and advanced aging. Thus, a more thorough understanding of how TBI perpetuates these measures of unhealthy brain aging, and how they can be prevented, is necessary.
Are there common genetic risk factors between negative TBI outcomes and age-related diseases? The apolipoprotein E4 allele is associated with an increased risk of poor post-traumatic outcome as well as an increased risk for Alzheimer’s disease. A deeper analysis and understanding of such risk factors can help identify individuals who may be more vulnerable to injury-induced, age-associated degenerative disorders.
Animal model studies indicate that modulation of different stress response pathways could offer a notable therapeutic strategy in overcoming the noxious effects of age, TBI, and neurodegenerative disease. Increasing chaperone availability through mechanisms that include activation of HSF extends lifespan, protects from degenerative TBI pathology, and serves a protective role in disease by inhibiting aggregation and targeting proteins for degradation through chaperone-mediated autophagy [35, 40, 146, 147]. Among the signaling pathways that merit attention in this regard is mTOR. The mTOR signaling cascade monitors environmental cues of nutrient availability to control cellular growth and metabolism through protein synthesis and autophagy, thus impinging on key processes of protein homeostasis [148]. The mTOR pathway is involved in several age-associated mechanisms, including the immune response, stem cell regulation, cellular senescence, and mitochondrial function. Inhibition of the mTOR pathway with rapamycin has been widely used to achieve longevity in animal models and is also being considered as a therapeutic in neurodegeneration, dementia and brain injury [148]. Another compound to be noted in this context is docosahexaenoic acid (DHA). DHA has been extensively studied as a neuroprotective agent that inhibits neuroinflammation, ER, and oxidative stress in the context of TBI, although a definitive mechanistic explanation for these protective effects is currently lacking. DHA also reduces age-associated cognitive decline. Lastly, manipulation of the proteasome through peptide- or small molecule-based activators, although less well-studied, remains an attractive avenue for ameliorating the toxicity associated with protein accumulation in aging, disease and injury [149].
Much work remains for elucidating the biology of TBI, particularly when taking into account the variability presented by the type and severity of injury. Addressing this variability is further complicated by the various modes for infliction of injury in experimental settings, although this variety of approaches also confers some advantages. Further research is also needed regarding the long-term repercussions of TBI: what are the molecular players involved in perpetuating the degenerative pathologies that define and characterize the injury response and its outcomes?
Invertebrate model organisms, such as Drosophila and nematodes, with their short lifespan compared to mammals, have been extensively used in longevity studies, and have helped inform about basic cellular pathways of the aging process. Recent adaptations of such model systems to TBI research [40, 135, 143, 150] carry significant potential for identifying fundamental aspects of injury pathways and risk factors. These models also offer opportunities for efficient large-scale screens of genetic and therapeutic interventions, as a basis for subsequent testing in more expensive and complex animal model systems of TBI. Integrating detailed understanding of impacts on various cellular pathways of age and disease promises to further define the molecular players and pathways that confer the biological and pathological effects of TBI. Hopefully, this understanding will lead to approaches for interfering with the detrimental actions of these cellular pathways or even preventing them to achieve better outcomes after TBI, and perhaps also for promoting healthy brain aging.
HIGHLIGHTS.
The aging process represents a gradual deterioration of the various arms of the proteostatic network, with decreased capacity to maintain homeostasis and compromised ability to respond to insults. A similar cellular state of proteostatic disruption is also reflected in the post-injury setting of traumatic brain injury (TBI).
The accumulation of misfolded, degenerative proteins observed years after TBI indicates that disruption of protein homeostasis is a long-term consequence of injury. However, further research in both human tissue and animal models is warranted to examine the molecular cascades that are chronically active after TBI.
Genetic and pharmacological modulators of the proteostasis machinery can extend lifespan in animal models, as well as rescue the negative age-associated consequences of TBI, indicating shared mechanisms of therapeutic opportunities.
ACKNOWLEDGEMENTS
This work was supported by a predoctoral Howard Hughes Medical Institute fellowship (to J.S.); and the NIH (R35-NS097275, to N.M.B.)
Glossary:
- Autophagy
A highly regulated process of degradation of damaged or unnecessary cytosolic contents that is necessary to maintain cellular homeostasis.
- Amyloid-β
The main component of extracellular plaques found in AD. It is formed from amyloid precursor protein through cleavage to yield the peptide that can aggregate and form oligomers that can misfold and be toxic.
- Tau
A microtubule-binding protein whose physiological function is to bind and stabilize microtubules. Hyperphosphorylated forms of the protein aggregate and form the intracellular neurofibrillary tangles characteristic of AD and other tauopathies. Intracellular tau tangles are the predominant feature in CTE brains and used to diagnose the disease, while the localization pattern of the tangles is used to stage CTE severity.
- TAR-DNA binding protein 43 kDa (TDP-43)
A DNA/RNA-binding protein that regulates RNA splicing, mRNA stabilization, and transcriptional regulation. Although a predominantly nuclear protein, it becomes enriched in the cytoplasm in diseases like AD, ALS, CTE and FTD. Under these conditions, it is abnormally phosphorylated and ubiquitinated, and forms aggregates that can label with stress granule components.
- α-synuclein
A presynaptic neuronal protein that regulates synaptic vesicle trafficking, neurotransmitter release, and fatty acid uptake. In its pathological state, it is misfolded and aggregates into oligomers that are the main constituent of Lewy bodies, a pathological hallmark of PD. Lewy body disease appears as a common co-morbidity in CTE patients.
Footnotes
DECLARATION OF INTERESTS
The authors declare no competing interests.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Blennow K et al. (2016) Traumatic brain injuries. Nat Rev Dis Primers 2, 16084. [DOI] [PubMed] [Google Scholar]
- 2.McKee AC et al. (2009) Chronic traumatic encephalopathy in athletes: progressive tauopathy after repetitive head injury. J Neuropathol Exp Neurol 68 (7), 709–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Graham NS and Sharp DJ (2019) Understanding neurodegeneration after traumatic brain injury: from mechanisms to clinical trials in dementia. J Neurol Neurosurg Psychiatry 90 (11), 1221–1233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Smith DH et al. (2013) Chronic neuropathologies of single and repetitive TBI: substrates of dementia? Nat Rev Neurol 9 (4), 211–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Santhanam P et al. (2019) Accelerated age-related cortical thinning in mild traumatic brain injury. Brain Behav 9 (1), e01161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Harris TC et al. (2019) The Shrinking Brain: Cerebral Atrophy Following Traumatic Brain Injury. Ann Biomed Eng 47 (9), 1941–1959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cole JH et al. (2018) Spatial patterns of progressive brain volume loss after moderate-severe traumatic brain injury. Brain 141 (3), 822–836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sidaros A et al. (2009) Long-term global and regional brain volume changes following severe traumatic brain injury: a longitudinal study with clinical correlates. Neuroimage 44 (1), 1–8. [DOI] [PubMed] [Google Scholar]
- 9.Cole JH et al. (2015) Prediction of brain age suggests accelerated atrophy after traumatic brain injury. Ann Neurol 77 (4), 571–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lopez-Otin C et al. (2013) The hallmarks of aging. Cell 153 (6), 1194–217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Castillo-Quan JI et al. (2015) Genetics and pharmacology of longevity: the road to therapeutics for healthy aging. Adv Genet 90, 1–101. [DOI] [PubMed] [Google Scholar]
- 12.Lindquist SL and Kelly JW (2011) Chemical and biological approaches for adapting proteostasis to ameliorate protein misfolding and aggregation diseases: progress and prognosis. Cold Spring Harb Perspect Biol 3 (12). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hartl FU et al. (2011) Molecular chaperones in protein folding and proteostasis. Nature 475 (7356), 324–32. [DOI] [PubMed] [Google Scholar]
- 14.Vihervaara A et al. (2018) Molecular mechanisms driving transcriptional stress responses. Nat Rev Genet 19 (6), 385–397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Majeski AE and Dice JF (2004) Mechanisms of chaperone-mediated autophagy. Int J Biochem Cell Biol 36 (12), 2435–44. [DOI] [PubMed] [Google Scholar]
- 16.Shi Y et al. (1998) Molecular chaperones as HSF1-specific transcriptional repressors. Genes Dev 12 (5), 654–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Neef DW et al. (2014) A direct regulatory interaction between chaperonin TRiC and stress-responsive transcription factor HSF1. Cell Rep 9 (3), 955–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Charmpilas N et al. (2017) Small heat shock proteins in ageing and age-related diseases. Cell Stress Chaperones 22 (4), 481–492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.David DC et al. (2010) Widespread protein aggregation as an inherent part of aging in C. elegans. PLoS Biol 8 (8), e1000450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Morrow G and Tanguay RM (2003) Heat shock proteins and aging in Drosophila melanogaster. Semin Cell Dev Biol 14 (5), 291–9. [DOI] [PubMed] [Google Scholar]
- 21.Fargnoli J et al. (1990) Decreased expression of heat shock protein 70 mRNA and protein after heat treatment in cells of aged rats. Proc Natl Acad Sci U S A 87 (2), 846–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Pahlavani MA et al. (1995) The expression of heat shock protein 70 decreases with age in lymphocytes from rats and rhesus monkeys. Exp Cell Res 218 (1), 310–8. [DOI] [PubMed] [Google Scholar]
- 23.Niedzwiecki A et al. (1991) Aging affects expression of 70-kDa heat shock proteins in Drosophila. J Biol Chem 266 (14), 9332–8. [PubMed] [Google Scholar]
- 24.Heydari AR et al. (1993) Expression of heat shock protein 70 is altered by age and diet at the level of transcription. Mol Cell Biol 13 (5), 2909–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Heydari AR et al. (2000) Age-related alterations in the activation of heat shock transcription factor 1 in rat hepatocytes. Exp Cell Res 256 (1), 83–93. [DOI] [PubMed] [Google Scholar]
- 26.Locke M and Tanguay RM (1996) Diminished heat shock response in the aged myocardium. Cell Stress Chaperones 1 (4), 251–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Heydari AR et al. (1996) Effect of caloric restriction on the expression of heat shock protein 70 and the activation of heat shock transcription factor 1. Dev Genet 18 (2), 114–24. [DOI] [PubMed] [Google Scholar]
- 28.Sasaki T et al. (2006) Progressive loss of SIRT1 with cell cycle withdrawal. Aging Cell 5 (5), 413–22. [DOI] [PubMed] [Google Scholar]
- 29.Westerheide SD et al. (2009) Stress-inducible regulation of heat shock factor 1 by the deacetylase SIRT1. Science 323 (5917), 1063–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Park EJ and Pezzuto JM (2015) The pharmacology of resveratrol in animals and humans. Biochim Biophys Acta 1852 (6), 1071–113. [DOI] [PubMed] [Google Scholar]
- 31.Walker GA and Lithgow GJ (2003) Lifespan extension in C. elegans by a molecular chaperone dependent upon insulin-like signals. Aging Cell 2 (2), 131–9. [DOI] [PubMed] [Google Scholar]
- 32.Morrow G et al. (2004) Overexpression of the small mitochondrial Hsp22 extends Drosophila life span and increases resistance to oxidative stress. FASEB J 18 (3), 598–9. [DOI] [PubMed] [Google Scholar]
- 33.Hsu AL et al. (2003) Regulation of aging and age-related disease by DAF-16 and heat-shock factor. Science 300 (5622), 1142–5. [DOI] [PubMed] [Google Scholar]
- 34.Chiang WC et al. (2012) HSF-1 regulators DDL-1/2 link insulin-like signaling to heat-shock responses and modulation of longevity. Cell 148 (1-2), 322–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kim JY et al. (2013) The 70 kDa heat shock protein protects against experimental traumatic brain injury. Neurobiol Dis 58, 289–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Eroglu B et al. (2014) Therapeutic inducers of the HSP70/HSP110 protect mice against traumatic brain injury. J Neurochem 130 (5), 626–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kim N et al. (2015) Pharmacological induction of the 70-kDa heat shock protein protects against brain injury. Neuroscience 284, 912–919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Gu Y et al. (2016) Hsp70 inducer, 17-allylamino-demethoxygeldanamycin, provides neuroprotection via anti-inflammatory effects in a rat model of traumatic brain injury. Exp Ther Med 12 (6), 3767–3772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Shohami E et al. (1994) Long term exposure to heat reduces edema formation after closed head injury in the rat. Acta Neurochir Suppl (Wien) 60, 443–5. [DOI] [PubMed] [Google Scholar]
- 40.Saikumar J et al. (2020) Dynamic neural and glial responses of a head-specific model for traumatic brain injury in Drosophila. Proc Natl Acad Sci U S A 117 (29), 17269–17277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Michael DB et al. (2005) Gene expression following traumatic brain injury in humans: analysis by microarray. J Clin Neurosci 12 (3), 284–90. [DOI] [PubMed] [Google Scholar]
- 42.Seidberg NA et al. (2003) Alterations in inducible 72-kDa heat shock protein and the chaperone cofactor BAG-1 in human brain after head injury. J Neurochem 84 (3), 514–21. [DOI] [PubMed] [Google Scholar]
- 43.Lai Y et al. (2004) Induction of the stress response after inflicted and non-inflicted traumatic brain injury in infants and children. J Neurotrauma 21 (3), 229–37. [DOI] [PubMed] [Google Scholar]
- 44.Dutcher SA et al. (1998) Patterns of heat-shock protein 70 biosynthesis following human traumatic brain injury. J Neurotrauma 15 (6), 411–20. [DOI] [PubMed] [Google Scholar]
- 45.Pittet JF et al. (2002) Serum levels of Hsp 72 measured early after trauma correlate with survival. J Trauma 52 (4), 611–7; discussion 617. [DOI] [PubMed] [Google Scholar]
- 46.Zhao J et al. (2020) Expression of heat shock protein 70 and Annexin A1 in serum of patients with acutely severe traumatic brain injury. Exp Ther Med 19 (3), 1896–1902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.da Rocha AB et al. (2005) Serum Hsp70 as an early predictor of fatal outcome after severe traumatic brain injury in males. J Neurotrauma 22 (9), 966–77. [DOI] [PubMed] [Google Scholar]
- 48.Lai Y et al. (2006) Mitochondrial heat shock protein 60 is increased in cerebrospinal fluid following pediatric traumatic brain injury. Dev Neurosci 28 (4-5), 336–41. [DOI] [PubMed] [Google Scholar]
- 49.Hayes RL et al. (1995) Changes in gene expression following traumatic brain injury in the rat. J Neurotrauma 12 (5), 779–90. [DOI] [PubMed] [Google Scholar]
- 50.Truettner JS et al. (2007) Subcellular stress response after traumatic brain injury. J Neurotrauma 24 (4), 599–612. [DOI] [PubMed] [Google Scholar]
- 51.Truettner J et al. (1999) Expression of brain-derived neurotrophic factor, nerve growth factor, and heat shock protein HSP70 following fluid percussion brain injury in rats. J Neurotrauma 16 (6), 471–86. [DOI] [PubMed] [Google Scholar]
- 52.Raghupathi R et al. (1995) Regional induction of c-fos and heat shock protein-72 mRNA following fluid-percussion brain injury in the rat. J Cereb Blood Flow Metab 15 (3), 467–73. [DOI] [PubMed] [Google Scholar]
- 53.Chen M et al. (1998) 72-kDa heat shock protein and mRNA expression after controlled cortical impact injury with hypoxemia in rats. J Neurotrauma 15 (3), 171–81. [DOI] [PubMed] [Google Scholar]
- 54.Sanz O et al. (2001) Expression of 27 kDa heat shock protein (Hsp27) in immature rat brain after a cortical aspiration lesion. Glia 36 (3), 259–70. [DOI] [PubMed] [Google Scholar]
- 55.Sun D et al. (2013) Aging- and injury-related differential apoptotic response in the dentate gyrus of the hippocampus in rats following brain trauma. Front Aging Neurosci 5, 95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Shimamura M et al. (2005) Analysis of long-term gene expression in neurons of the hippocampal subfields following traumatic brain injury in rats. Neuroscience 131 (1), 87–97. [DOI] [PubMed] [Google Scholar]
- 57.Neef DW et al. (2011) Heat shock transcription factor 1 as a therapeutic target in neurodegenerative diseases. Nat Rev Drug Discov 10 (12), 930–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Zhao Z et al. (2013) Neuroprotective effects of geranylgeranylacetone in experimental traumatic brain injury. J Cereb Blood Flow Metab 33 (12), 1897–908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Saykally JN et al. (2012) The nuclear factor erythroid 2-like 2 activator, tertbutylhydroquinone, improves cognitive performance in mice after mild traumatic brain injury. Neuroscience 223, 305–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Zhang MH et al. (2018) Neuroprotective effects of dexmedetomidine on traumatic brain injury: Involvement of neuronal apoptosis and HSP70 expression. Mol Med Rep 17 (6), 8079–8086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Chio CC et al. (2017) Exercise attenuates neurological deficits by stimulating a critical HSP70/NF-kappaB/IL-6/synapsin I axis in traumatic brain injury rats. J Neuroinflammation 14 (1), 90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Chou W et al. (2018) Exercise Rehabilitation Attenuates Cognitive Deficits in Rats with Traumatic Brain Injury by Stimulating the Cerebral HSP20/BDNF/TrkB Signalling Axis. Mol Neurobiol 55 (11), 8602–8611. [DOI] [PubMed] [Google Scholar]
- 63.Umscheif G et al. (2010) Heat acclimation provides sustained improvement in functional recovery and attenuates apoptosis after traumatic brain injury. J Cereb Blood Flow Metab 30 (3), 616–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Shein NA et al. (2008) Microglial involvement in neuroprotection following experimental traumatic brain injury in heat-acclimated mice. Brain Res 1244, 132–41. [DOI] [PubMed] [Google Scholar]
- 65.Shein NA et al. (2007) Altered cytokine expression and sustained hypothermia following traumatic brain injury in heat acclimated mice. Brain Res 1185, 313–20. [DOI] [PubMed] [Google Scholar]
- 66.Umschweif G et al. (2014) Neuroprotection after traumatic brain injury in heat-acclimated mice involves induced neurogenesis and activation of angiotensin receptor type 2 signaling. J Cereb Blood Flow Metab 34 (8), 1381–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Umschweif G et al. (2013) Hypoxia-inducible factor 1 is essential for spontaneous recovery from traumatic brain injury and is a key mediator of heat acclimation induced neuroprotection. J Cereb Blood Flow Metab 33 (4), 524–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Shein NA et al. (2007) Akt phosphorylation is required for heat acclimation-induced neuroprotection. J Neurochem 103 (4), 1523–9. [DOI] [PubMed] [Google Scholar]
- 69.Hetz C (2012) The unfolded protein response: controlling cell fate decisions under ER stress and beyond. Nat Rev Mol Cell Biol 13 (2), 89–102. [DOI] [PubMed] [Google Scholar]
- 70.Pakos-Zebrucka K et al. (2016) The integrated stress response. EMBO Rep 17 (10), 1374–1395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Adams CJ et al. (2019) Structure and Molecular Mechanism of ER Stress Signaling by the Unfolded Protein Response Signal Activator IRE1. Front Mol Biosci 6, 11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Shen J et al. (2002) ER stress regulation of ATF6 localization by dissociation of BiP/GRP78 binding and unmasking of Golgi localization signals. Dev Cell 3 (1), 99–111. [DOI] [PubMed] [Google Scholar]
- 73.Hussain SG and Ramaiah KV (2007) Reduced eIF2alpha phosphorylation and increased proapoptotic proteins in aging. Biochem Biophys Res Commun 355 (2), 365–70. [DOI] [PubMed] [Google Scholar]
- 74.Nuss JE et al. (2008) Decreased enzyme activities of chaperones PDI and BiP in aged mouse livers. Biochem Biophys Res Commun 365 (2), 355–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Erickson RR et al. (2006) The effect of aging on the chaperone concentrations in the hepatic, endoplasmic reticulum of male rats: the possible role of protein misfolding due to the loss of chaperones in the decline in physiological function seen with age. J Gerontol A Biol Sci Med Sci 61 (5), 435–43. [DOI] [PubMed] [Google Scholar]
- 76.Paz Gavilan M et al. (2006) Cellular environment facilitates protein accumulation in aged rat hippocampus. Neurobiol Aging 27 (7), 973–82. [DOI] [PubMed] [Google Scholar]
- 77.Gavilan MP et al. (2009) Dysfunction of the unfolded protein response increases neurodegeneration in aged rat hippocampus following proteasome inhibition. Aging Cell 8 (6), 654–65. [DOI] [PubMed] [Google Scholar]
- 78.Naidoo N et al. (2008) Aging impairs the unfolded protein response to sleep deprivation and leads to proapoptotic signaling. J Neurosci 28 (26), 6539–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Li J and Holbrook NJ (2004) Elevated gadd153/chop expression and enhanced c-Jun N-terminal protein kinase activation sensitizes aged cells to ER stress. Exp Gerontol 39 (5), 735–44. [DOI] [PubMed] [Google Scholar]
- 80.Henis-Korenblit S et al. (2010) Insulin/IGF-1 signaling mutants reprogram ER stress response regulators to promote longevity. Proc Natl Acad Sci U S A 107 (21), 9730–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Luis NM et al. (2016) Intestinal IRE1 Is Required for Increased Triglyceride Metabolism and Longer Lifespan under Dietary Restriction. Cell Rep 17 (5), 1207–1216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Taylor RC and Dillin A (2013) XBP-1 is a cell-nonautonomous regulator of stress resistance and longevity. Cell 153 (7), 1435–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Lucke-Wold BP et al. (2016) Endoplasmic reticulum stress implicated in chronic traumatic encephalopathy. J Neurosurg 124 (3), 687–702. [DOI] [PubMed] [Google Scholar]
- 84.Petrov T et al. (2001) Upregulation of iNOS expression and phosphorylation of eIF-2alpha are paralleled by suppression of protein synthesis in rat hypothalamus in a closed head trauma model. J Neurotrauma 18 (8), 799–812. [DOI] [PubMed] [Google Scholar]
- 85.Hylin MJ et al. (2018) Juvenile Traumatic Brain Injury Results in Cognitive Deficits Associated with Impaired Endoplasmic Reticulum Stress and Early Tauopathy. Dev Neurosci 40 (2), 175–188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Paschen W et al. (2004) Brain trauma induces X-box protein 1 processing indicative of activation of the endoplasmic reticulum unfolded protein response. J Neurochem 88 (4), 983–92. [DOI] [PubMed] [Google Scholar]
- 87.Chen T et al. (2019) ROS-Mediated Mitochondrial Dysfunction and ER Stress Contribute to Compression-Induced Neuronal Injury. Neuroscience 416, 268–280. [DOI] [PubMed] [Google Scholar]
- 88.Boone DR et al. (2015) Pathway-focused PCR array profiling of enriched populations of laser capture microdissected hippocampal cells after traumatic brain injury. PLoS One 10 (5), e0127287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Chen Y et al. (2016) Calcineurin beta protects brain after injury by activating the unfolded protein response. Neurobiol Dis 94, 139–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Krukowski K et al. (2020) Integrated Stress Response Inhibitor Reverses Sex-Dependent Behavioral and Cell-Specific Deficits after Mild Repetitive Head Trauma. J Neurotrauma 37 (11), 1370–1380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Begum G et al. (2014) Docosahexaenoic acid reduces ER stress and abnormal protein accumulation and improves neuronal function following traumatic brain injury. J Neurosci 34 (10), 3743–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Begum G et al. (2013) ER stress and effects of DHA as an ER stress inhibitor. Transl Stroke Res 4 (6), 635–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Romero-Ramirez L et al. (2017) Integrated Stress Response as a Therapeutic Target for CNS Injuries. Biomed Res Int 2017, 6953156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Logsdon AF et al. (2014) Altering endoplasmic reticulum stress in a model of blast-induced traumatic brain injury controls cellular fate and ameliorates neuropsychiatric symptoms. Front Cell Neurosci 8, 421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Wang ZF et al. (2019) Salubrinal offers neuroprotection through suppressing endoplasmic reticulum stress, autophagy and apoptosis in a mouse traumatic brain injury model. Neurobiol Learn Mem 161, 12–25. [DOI] [PubMed] [Google Scholar]
- 96.Lucke-Wold BP et al. (2017) Endoplasmic Reticulum Stress Modulation as a Target for Ameliorating Effects of Blast Induced Traumatic Brain Injury. J Neurotrauma 34 (S1), S62–S70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Logsdon AF et al. (2016) Salubrinal reduces oxidative stress, neuroinflammation and impulsive-like behavior in a rodent model of traumatic brain injury. Brain Res 1643, 140–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Rubovitch V et al. (2015) The neuroprotective effect of salubrinal in a mouse model of traumatic brain injury. Neuromolecular Med 17 (1), 58–70. [DOI] [PubMed] [Google Scholar]
- 99.Gao Y et al. (2018) IL-33/ST2L Signaling Provides Neuroprotection Through Inhibiting Autophagy, Endoplasmic Reticulum Stress, and Apoptosis in a Mouse Model of Traumatic Brain Injury. Front Cell Neurosci 12, 95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Tan HP et al. (2018) Inhibition of endoplasmic reticulum stress alleviates secondary injury after traumatic brain injury. Neural Regen Res 13 (5), 827–836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Dash PK et al. (2015) Inhibition of Eukaryotic Initiation Factor 2 Alpha Phosphatase Reduces Tissue Damage and Improves Learning and Memory after Experimental Traumatic Brain Injury. J Neurotrauma 32 (20), 1608–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Hood KN et al. (2018) Endoplasmic Reticulum Stress Contributes to the Loss of Newborn Hippocampal Neurons after Traumatic Brain Injury. J Neurosci 38 (9), 2372–2384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Sen T et al. (2017) Activation of PERK Elicits Memory Impairment through Inactivation of CREB and Downregulation of PSD95 After Traumatic Brain Injury. J Neurosci 37 (24), 5900–5911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Sen T et al. (2020) Aberrant ER Stress Induced Neuronal-IFNbeta Elicits White Matter Injury Due to Microglial Activation and T-Cell Infiltration after TBI. J Neurosci 40 (2), 424–446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Chou A et al. (2017) Inhibition of the integrated stress response reverses cognitive deficits after traumatic brain injury. Proc Natl Acad Sci U S A 114 (31), E6420–E6426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Yin Y et al. (2018) Effects of DHA on Hippocampal Autophagy and Lysosome Function After Traumatic Brain Injury. Mol Neurobiol 55 (3), 2454–2470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Harvey LD et al. (2015) Administration of DHA Reduces Endoplasmic Reticulum Stress-Associated Inflammation and Alters Microglial or Macrophage Activation in Traumatic Brain Injury. ASN Neuro 7 (6). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Sun D et al. (2020) Dexmedetomidine attenuates endoplasmic reticulum stress-induced apoptosis and improves neuronal function after traumatic brain injury in mice. Brain Res 1732, 146682. [DOI] [PubMed] [Google Scholar]
- 109.Li L et al. (2019) Polydatin prevents the induction of secondary brain injury after traumatic brain injury by protecting neuronal mitochondria. Neural Regen Res 14 (9), 1573–1582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Wang CF et al. (2019) Mild hypothermia reduces endoplasmic reticulum stress-induced apoptosis and improves neuronal functions after severe traumatic brain injury. Brain Behav 9 (4), e01248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Huang T et al. (2018) Curcumin mitigates axonal injury and neuronal cell apoptosis through the PERK/Nrf2 signaling pathway following diffuse axonal injury. Neuroreport 29 (8), 661–677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Sun D et al. (2017) Administration of Tauroursodeoxycholic Acid Attenuates Early Brain Injury via Akt Pathway Activation. Front Cell Neurosci 11, 193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Tanaka K (2009) The proteasome: overview of structure and functions. Proc Jpn Acad Ser B Phys Biol Sci 85 (1), 12–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Komander D and Rape M (2012) The ubiquitin code. Annu Rev Biochem 81, 203–29. [DOI] [PubMed] [Google Scholar]
- 115.Bard JAM et al. (2018) Structure and Function of the 26S Proteasome. Annu Rev Biochem 87, 697–724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Chondrogianni N et al. (2014) Protein damage, repair and proteolysis. Mol Aspects Med 35, 1–71. [DOI] [PubMed] [Google Scholar]
- 117.Rodriguez KA et al. (2010) Molecular mechanisms of proteasome plasticity in aging. Mech Ageing Dev 131 (2), 144–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Gaczynska M et al. (2001) Caretaker or undertaker? The role of the proteasome in aging. Mech Ageing Dev 122 (3), 235–54. [DOI] [PubMed] [Google Scholar]
- 119.Keller JN et al. (2000) Decreased levels of proteasome activity and proteasome expression in aging spinal cord. Neuroscience 98 (1), 149–56. [DOI] [PubMed] [Google Scholar]
- 120.Keller JN et al. (2000) Possible involvement of proteasome inhibition in aging: implications for oxidative stress. Mech Ageing Dev 113 (1), 61–70. [DOI] [PubMed] [Google Scholar]
- 121.Zeng BY et al. (2005) Proteasomal activity in brain differs between species and brain regions and changes with age. Mech Ageing Dev 126 (6-7), 760–6. [DOI] [PubMed] [Google Scholar]
- 122.Vernace VA et al. (2007) Aging perturbs 26S proteasome assembly in Drosophila melanogaster. FASEB J 21 (11), 2672–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Tonoki A et al. (2009) Genetic evidence linking age-dependent attenuation of the 26S proteasome with the aging process. Mol Cell Biol 29 (4), 1095–106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Hamer G et al. (2010) A photoconvertible reporter of the ubiquitin-proteasome system in vivo. Nat Methods 7 (6), 473–8. [DOI] [PubMed] [Google Scholar]
- 125.Kelmer Sacramento E et al. (2020) Reduced proteasome activity in the aging brain results in ribosome stoichiometry loss and aggregation. Mol Syst Biol 16 (6), e9596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Chondrogianni N et al. (2005) Overexpression of proteasome beta5 assembled subunit increases the amount of proteasome and confers ameliorated response to oxidative stress and higher survival rates. J Biol Chem 280 (12), 11840–50. [DOI] [PubMed] [Google Scholar]
- 127.Chondrogianni N et al. (2003) Central role of the proteasome in senescence and survival of human fibroblasts: induction of a senescence-like phenotype upon its inhibition and resistance to stress upon its activation. J Biol Chem 278 (30), 28026–37. [DOI] [PubMed] [Google Scholar]
- 128.Depuydt G et al. (2013) Reduced insulin/insulin-like growth factor-1 signaling and dietary restriction inhibit translation but preserve muscle mass in Caenorhabditis elegans. Mol Cell Proteomics 12 (12), 3624–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Carrano AC et al. (2009) A conserved ubiquitination pathway determines longevity in response to diet restriction. Nature 460 (7253), 396–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Sakai K et al. (2014) Immunohistochemical analysis of the ubiquitin proteasome system and autophagy lysosome system induced after traumatic intracranial injury: association with time between the injury and death. Am J Forensic Med Pathol 35 (1), 38–44. [DOI] [PubMed] [Google Scholar]
- 131.Tylicka M et al. (2014) Circulating proteasome activity following mild head injury in children. Childs Nerv Syst 30 (7), 1191–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Majetschak M et al. (2005) Ubiquitin immunoreactivity in cerebrospinal fluid after traumatic brain injury: clinical and experimental findings. Crit Care Med 33 (7), 1589–94. [DOI] [PubMed] [Google Scholar]
- 133.Anderson EN et al. (2018) Traumatic injury induces stress granule formation and enhances motor dysfunctions in ALS/FTD models. Hum Mol Genet 27 (8), 1366–1381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Staal JA et al. (2009) Disruption of the ubiquitin proteasome system following axonal stretch injury accelerates progression to secondary axotomy. J Neurotrauma 26 (5), 781–8. [DOI] [PubMed] [Google Scholar]
- 135.Barekat A et al. (2016) Using Drosophila as an integrated model to study mild repetitive traumatic brain injury. Sci Rep 6, 25252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Yao X et al. (2007) Ubiquitin and ubiquitin-conjugated protein expression in the rat cerebral cortex and hippocampus following traumatic brain injury (TBI). Brain Res 1182, 116–22. [DOI] [PubMed] [Google Scholar]
- 137.Yao X et al. (2008) Alterations of cerebral cortex and hippocampal proteasome subunit expression and function in a traumatic brain injury rat model. J Neurochem 104 (2), 353–63. [DOI] [PubMed] [Google Scholar]
- 138.Liu H et al. (2017) In vivo transduction of neurons with TAT-UCH-L1 protects brain against controlled cortical impact injury. PLoS One 12 (5), e0178049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Ding H et al. (2017) Nrf2-ARE signaling provides neuroprotection in traumatic brain injury via modulation of the ubiquitin proteasome system. Neurochem Int 111, 32–44. [DOI] [PubMed] [Google Scholar]
- 140.Qu C et al. (2010) The treatment of traumatic brain injury with velcade. J Neurotrauma 27 (9), 1625–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Paramore A and Frantz S (2003) Bortezomib. Nat Rev Drug Discov 2 (8), 611–2. [DOI] [PubMed] [Google Scholar]
- 142.Thompson HJ et al. (2006) Traumatic brain injury in older adults: epidemiology, outcomes, and future implications. J Am Geriatr Soc 54 (10), 1590–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Katzenberger RJ et al. (2013) A Drosophila model of closed head traumatic brain injury. Proc Natl Acad Sci U S A 110 (44), E4152–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Kumar A et al. (2013) Traumatic brain injury in aged animals increases lesion size and chronically alters microglial/macrophage classical and alternative activation states. Neurobiol Aging 34 (5), 1397–411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Timaru-Kast R et al. (2012) Influence of age on brain edema formation, secondary brain damage and inflammatory response after brain trauma in mice. PLoS One 7 (8), e43829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Fuhrmann-Stroissnigg H et al. (2017) Identification of HSP90 inhibitors as a novel class of senolytics. Nat Commun 8 (1), 422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Warrick JM et al. (1999) Suppression of polyglutamine-mediated neurodegeneration in Drosophila by the molecular chaperone HSP70. Nat Genet 23 (4), 425–8. [DOI] [PubMed] [Google Scholar]
- 148.Crino PB (2016) The mTOR signalling cascade: paving new roads to cure neurological disease. Nat Rev Neurol 12 (7), 379–92. [DOI] [PubMed] [Google Scholar]
- 149.Njomen E and Tepe JJ (2019) Proteasome Activation as a New Therapeutic Approach To Target Proteotoxic Disorders. J Med Chem 62 (14), 6469–6481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Miansari M et al. (2019) Inducing Mild Traumatic Brain Injury in C. elegans via Cavitation-Free Surface Acoustic Wave-Driven Ultrasonic Irradiation. Sci Rep 9 (1), 12775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.McKee AC et al. (2016) The first NINDS/NIBIB consensus meeting to define neuropathological criteria for the diagnosis of chronic traumatic encephalopathy. Acta Neuropathol 131 (1), 75–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Stein TD et al. (2014) Chronic traumatic encephalopathy: a spectrum of neuropathological changes following repetitive brain trauma in athletes and military personnel. Alzheimers Res Ther 6 (1), 4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Barnes DE et al. (2018) Association of Mild Traumatic Brain Injury With and Without Loss of Consciousness With Dementia in US Military Veterans. JAMA Neurol 75 (9), 1055–1061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Gardner RC et al. (2018) Mild TBI and risk of Parkinson disease: A Chronic Effects of Neurotrauma Consortium Study. Neurology 90 (20), e1771–e1779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Crane PK et al. (2016) Association of Traumatic Brain Injury With Late-Life Neurodegenerative Conditions and Neuropathologic Findings. JAMA Neurol 73 (9), 1062–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Fann JR et al. (2018) Long-term risk of dementia among people with traumatic brain injury in Denmark: a population-based observational cohort study. Lancet Psychiatry 5 (5), 424–431. [DOI] [PubMed] [Google Scholar]
- 157.Nordstrom A and Nordstrom P (2018) Traumatic brain injury and the risk of dementia diagnosis: A nationwide cohort study. PLoS Med 15 (1), e1002496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Schaffert J et al. (2018) Traumatic brain injury history is associated with an earlier age of dementia onset in autopsy-confirmed Alzheimer's disease. Neuropsychology 32 (4), 410–416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Uryu K et al. (2007) Multiple proteins implicated in neurodegenerative diseases accumulate in axons after brain trauma in humans. Exp Neurol 208 (2), 185–92. [DOI] [PMC free article] [PubMed] [Google Scholar]