Abstract
Auscultation has been essential part of the physical examination; this is non-invasive, real-time, and very informative. Detection of abnormal respiratory sounds with a stethoscope is important in diagnosing respiratory diseases and providing first aid. However, accurate interpretation of respiratory sounds requires clinician’s considerable expertise, so trainees such as interns and residents sometimes misidentify respiratory sounds. To overcome such limitations, we tried to develop an automated classification of breath sounds. We utilized deep learning convolutional neural network (CNN) to categorize 1918 respiratory sounds (normal, crackles, wheezes, rhonchi) recorded in the clinical setting. We developed the predictive model for respiratory sound classification combining pretrained image feature extractor of series, respiratory sound, and CNN classifier. It detected abnormal sounds with an accuracy of 86.5% and the area under the ROC curve (AUC) of 0.93. It further classified abnormal lung sounds into crackles, wheezes, or rhonchi with an overall accuracy of 85.7% and a mean AUC of 0.92. On the other hand, as a result of respiratory sound classification by different groups showed varying degree in terms of accuracy; the overall accuracies were 60.3% for medical students, 53.4% for interns, 68.8% for residents, and 80.1% for fellows. Our deep learning-based classification would be able to complement the inaccuracies of clinicians' auscultation, and it may aid in the rapid diagnosis and appropriate treatment of respiratory diseases.
Subject terms: Diagnosis, Health services
Introduction
The stethoscope has been considered as an invaluable diagnostic tool ever since it was invented in the early 1800s. Auscultation is non-invasive, real-time, inexpensive, and very informative1–3. Recent electronic stethoscopes have rendered lung sounds recordable, and it facilitated the studies of automatically analyzing lung sounds4,5. Abnormal lung sounds include crackles, wheezes, rhonchi, stridor, and pleural friction rubs (Table 1). Crackles, wheezes and rhonchi are the most commonly found among them, and detecting those sounds greatly aids the diagnosis of pulmonary diseases6,7. Crackles, which are short, explosive, and non-musical, are produced by patients with parenchymal lung diseases such as pneumonia, interstitial pulmonary fibrosis (IPF), and pulmonary edema1,8,9. Wheezes are musical high-pitched sounds associated with airway diseases such as asthma and chronic obstructive pulmonary disease (COPD). Rhonchi are musical low-pitched sounds similar to snores, usually indicating secretions in the airway, and are often cleared by coughing1.
Table 1.
Classification of abnormal lung sounds.
| Mechanism of sound production | Location | Characteristics | Acoustics | Related diseases | |
|---|---|---|---|---|---|
| Fine crackles | Unrelated to secretions | Peripheral lung |
Discontinuous High-pitched Inspiratory |
Rapidly dampened wave deflection Frequency: about 650 Hz Shorter duration (about 5 ms) |
Interstitial lung fibrosis Pneumonia Congestive heart failure |
| Coarse crackles | Intermittent airway opening, related to secretions | Peripheral lung |
Discontinuous Low-pitched Inspiratory |
Rapidly dampened wave deflection Frequency about 350 Hz Longer duration (about 15 ms) |
Same as fine crackles but usually more advanced disease |
| Wheezes |
Narrowed airway Flow limitation |
Bronchi |
Continuous High-pitched Expiratory > Inspiratory |
Sinusoid Frequency > 100–5000 Hz Duration > 80 ms |
Asthma COPD Tumor Foreign body |
| Rhonchus |
Rupture of fluid films of secretions Airway wall vibrations |
Bronchi |
Continuous Low-pitched Expiratory > Inspiratory |
Sinusoid Frequency about 150 Hz Duration > 80 ms |
Bronchitis Pneumonia |
| Stridor | Narrowed airway | Larynx, Trachea |
Continuous High-pitched Inspiratory |
Sinusoid Frequency > 500 Hz |
Epiglottitis After extubation Foreign body |
| Pleural friction rub | Pleural inflammation | Chest wall |
Continuous Low-pitched Inspiratory and expiratory |
Rhythmic succession of short sounds Frequency < 350 Hz Duration > 15 ms |
Pleurisy Pericarditis Pleural tumor |
Although auscultation has many advantages, the ability to analyze respiratory sounds among clinicians varies greatly depending on individual clinical experiences6,10. Salvatore et al. found that hospital trainees misidentified about half of all pulmonary sounds, as did medical students11. Melbye et al. reported significant inter-observer differences in terms of discriminating expiratory rhonchi and low-pitched wheezes from other sounds, potentially compromising diagnosis and treatment12. These limitations of auscultation raised the need to develop a standardized system that can classify accurately respiratory sounds using artificial intelligence (AI). AI-assisted auscultation can help a proper diagnosis of respiratory disease and identify patients in need of emergency treatment. It can be used to screen and monitor patients with various pulmonary diseases including asthma, COPD and pneumonia13,14.
Recently, deep learning is widely applied to some medical fields including a chest x-ray or electroencephalogram analysis15,16. There are several published studies on AI-assisted auscultation of heart and lung sounds13,17–20. AI was used to distinguish different murmurs and diagnose valvular and congenital heart diseases21. Auscultation of the lung is different from that of the heart in some aspects. First, the lungs are much larger than the heart; and lung sounds should be recorded at multiple sites of both lungs for an accurate analysis. Second, the quality of lung sound is easily affected by the patient’s effort to breathe.
There are several studies that tried to automatically analyze and classify respiratory sounds15,22–30. An interesting study quantified and characterized lung sounds in patients with pneumonia for generating acoustic pneumonia scores22. The sound analyzer was helpful for detecting pneumonia with 78% sensitivity and 88% specificity. In another study, crackles and wheezes in 15 children were applied to feature extraction via time–frequency/scale analysis; the positive percent agreement was 0.82 for crackle and 0.80 for wheezing23. Tomasz et al. used neural network (NN)-based analysis to differentiate four abnormal sounds (wheezes, rhonchi, and coarse and fine crackles) in 50 children18. Intriguingly, the results showed that the NN F1-score was much better than that of doctors. Gorkem et al. used a support vector machine (SVM), the k-nearest neighbor approach, and a multilayer perceptron to detect pulmonary crackles24. Gokhan et al. proposed the automatic detection of the respiratory cycle and collected synchronous auscultation sounds from COPD patients28,31,32. Interestingly, they demonstrated that deep learning is useful for diagnosing COPD and classifying the severity of COPD with significantly high-performance rates28,29.
Another study employed a sound database of the international conference on biomedical and health informatics (ICBHI) 2017 for classifying lung sounds using a deep convolutional NN. They converted the lung sound signals to spectrogram images by using the time–frequency method, but the accuracy was relatively low (about 65%)25. There are many feature extractors with CNN classifiers including inception V3, DenseNet201, ResNet50, ResNet101, VGG16, and VGG1933–38. In this study, we tried to combine pre-trained image feature extraction from time-series, respiratory sound, and CNN classification. We also compared the performances of these feature extractors.
Although this field has been being actively studied, it is still in its infancy with significant limitations. Many studies enrolled patients of a limited age group (children only), and some studies analyzed the sounds of a small numbers of patients. The studies that used the respiratory sounds of the ICBHI 2017 or the R.A.L.E. Repository database have a limitation in types of abnormal sounds. The ICBHI database contained crackles and wheezes only, and R.A.L.E. database lacked rhonchi39.
In this study, we aimed to classify normal respiratory sounds, crackles, wheezes, and rhonchi. We made a database of 1,918 respiratory sounds from adult patients with pulmonary diseases and healthy controls. Then we used transfer learning and convolutional neural network (CNN) to classify those respiratory sounds. We tried to combine pre-trained image feature extraction from time-series, respiratory sound, and CNN classification. In addition, we measured how accurately medical students, interns, residents, and fellows categorized breathing sounds to check the accuracy of auscultation classification in real clinical practice.
Results
The general characteristics of the enrolled patients and the collected lung sounds
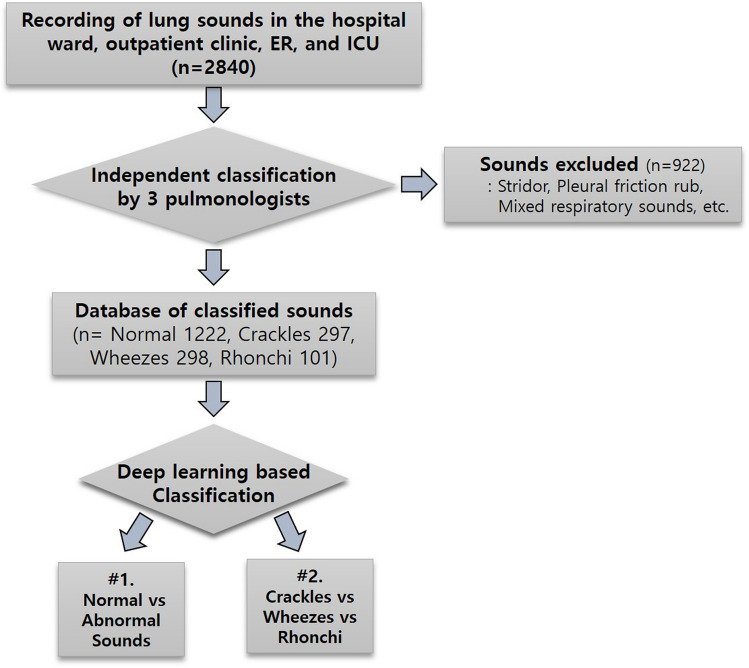
We recorded 2840 sounds and the respiratory sounds were evaluated by three pulmonologists independently and classified. Then we made a respiratory sound database contained 1222 normal sounds (63.7%) and 696 abnormal sounds (36.3%) including 297 crackles (15.5%), 298 wheezes (15.5%), and 101 rhonchi (5.3%). Our database of classified sounds was consisted of 1918 sounds from 871 patients in the clinical field. Their demographic and clinical characteristics are presented in Table 2. The mean patient age was 67.7 (± 10.9) years and 64.5% of patients were male. Sounds were collected from patients with pneumonia, IPF, COPD, asthma, lung cancer, tuberculosis, and bronchiectasis, as well as healthy controls. The proportions of COPD and asthma patients were 21% and 12.3% respectively, the pneumonia proportion 11.1%, the IPF proportion 8.0%, and the healthy control proportion 5.9%. The location of auscultation was most common in both lower lobe fields. (Table 2).
Table 2.
Characteristics of respiratory sound database.
| 871 cases (recording sounds: n = 1918) | Characteristics |
|---|---|
| Age, mean (years) | 67.7 ± 10.9 |
| Sex | Male: 562 (64.5%), Female: 309 (35.5%) |
| Diagnosis |
Pneumonia: 11.1%, IPF: 4.9%, COPD: 21%, Asthma: 12.3%, Lung cancer/mass: 13.1%, Healthy: 5.9%, Tuberculosis: 5.4%, Bronchiectasis: 4.4% ILD except IPF: 8.0%, ACO: 0.3% ETC: 13.5% |
| Respiratory sounds |
Normal: 1222 (63.7%), Crackles: 297(15.5%), Wheezes: 298 (15.5%), Rhonchi 101 (5.3%) |
| Auscultation location |
RULF: 10.4%, RMLF: 6.9%, RLLF: 34.8% LULF: 16.3%, LMLF: 9.0%, LLLF: 21.7%, Neck 0.9% |
RULF right upper lobe field, RMLF right middle lobe field, RLLF right lower lobe field, LULF left upper lobe field, LMLF left middle lobe field, LLLF left lower lobe field, COPD chronic obstructive pulmonary disease, ILD interstitial lung disease, IPF idiopathic pulmonary disease, ACO asthma-COPD overlap.
Performance of AI-assisted lung sound classification
Discriminating normal sounds from abnormal sounds (crackles, wheezes, and rhonchi)
In clinical settings, distinguishing abnormal breathing sounds and normal sounds is very important in screening emergency situations and deciding whether to perform additional tests. Our sound database included 1222 normal sounds and 696 abnormal sounds. We first checked how accurately our deep-learning based algorithm can classify abnormal respiratory sounds from normal sounds (Fig. 1). The precision, recall, and F1 scores for abnormal lung sounds were 84%, 80%, and 81% respectively (Table 3). The accuracy was 86.5% and the mean AUC was 0.93 (Fig. 2).
Figure 1.
Scheme of the classification of respiratory sounds using deep learning. Lung sounds database contains normal sounds, crackles, wheezes, and rhonchi. Deep learning was used for two types of classification: The first step is the discriminating normal sounds from abnormal sounds. The second is to categorize abnormal sounds into crackles, wheezes, and rhonchi. (ER: Emergency room, ICU: intensive care unit).
Table 3.
The averages of Precision, Recall and F1 score in discriminating normal sounds from abnormal sounds.
| Precision | Recall score | F1 score | |
|---|---|---|---|
| Normal | 0.88 | 0.91 | 0.89 |
| Abnormal | 0.84 | 0.80 | 0.81 |
Figure 2.
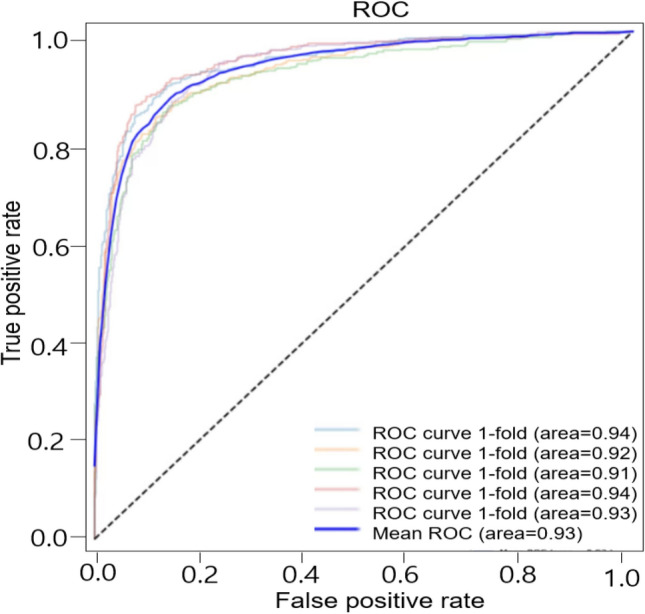
ROC of the model for discrimination of abnormal lung sounds. Each plot illustrates the ROC of the algorithm on the independent testing set for abnormal lung sounds, with AUC of 0.93.
Categorization of abnormal sounds into crackles, wheezes, and rhonchi
Next, we categorized abnormal sounds as specific types of sounds: crackles, wheezes, or rhonchi using deep learning. The sound database included 297 crackles, 298 wheezes, and 101 rhonchi that were confirmed by specialists. The precision, recall, and F1 scores for crackles were 90%, 85%, and 87% respectively. In the case of wheezes, the precision, recall, and F1 scores were 89%, 93%, and 91%. Finally, the precision, recall, and F1 scores for rhonchi were 68%, 71%, and 69% respectively (Table 4). The average accuracy was 85.7% and the mean was AUC 0.92 (Fig. 3).
Table 4.
The averages of Precision, Recall and F1 score in discriminating crackles, wheezes, and rhonchi.
| Precision | Recall score | F1 score | |
|---|---|---|---|
| Crackles | 0.90 | 0.85 | 0.87 |
| Wheezes | 0.89 | 0.93 | 0.91 |
| Rhonchi | 0.68 | 0.71 | 0.69 |
Figure 3.
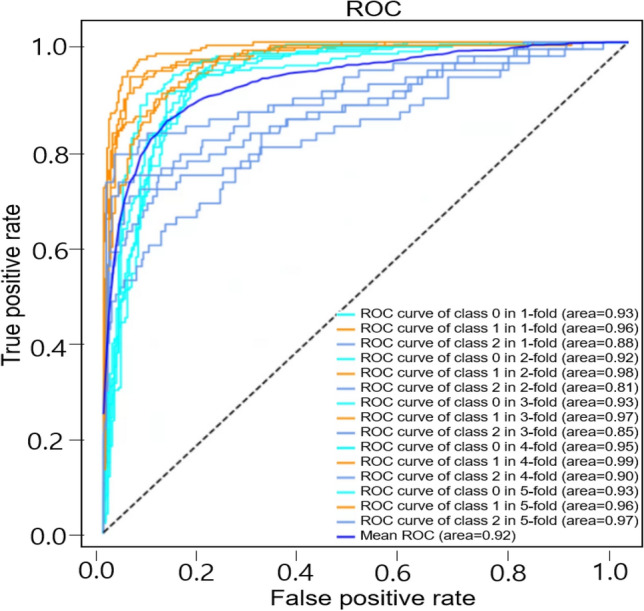
ROC of the model for classifying abnormal lung sounds into crackles, wheezes, and rhonchi. Each plot illustrates the ROC of the algorithm on the independent testing set for crackles, wheezes, and rhonchi with the mean AUC of 0.92.
Comparison of performances of different feature extractors with CNN classifier
Respiratory sounds, especially abnormal sounds, have very complicated structures with noise, and positional dependency in time. In the sound analysis, particularly mathematical point of view, its 2-D spectral-domain has more information rather than one dimensional time-series. Moreover, the deep learning structure gives an automatic feature extraction overcoming the difficulties on complicate data, especially image data. For this reason, we adopted CNN, which is a powerful method in image classification. To find out the most optimized strategy for the classification of respiratory sounds, we also compared the accuracy, precision, recall score and F1 score of each analytic method (Table 5). CNN classifier showed the best performance with VGG, especially, VGG16 rather than InceptionV3, DenseNet201, ResNet50, and ResNet101. Since VGG architecture has a better capability, especially in extracting image features for classification using transfer learning40,41, we adopted it for our AI models.
Table 5.
Comparison of Performance among feature extractors with CNN classifier.
| Feature extractor | Accuracy | Precision | Recall score | F1 score |
|---|---|---|---|---|
| InceptionV3 | 0.748 | 0.722 | 0.727 | 0.720 |
| DenseNet201 | 0.786 | 0.749 | 0.735 | 0.729 |
| ResNet50 | 0.783 | 0.747 | 0.738 | 0.738 |
| ResNet101 | 0.809 | 0.787 | 0.766 | 0.769 |
| VGG16 | 0.857 | 0.823 | 0.828 | 0.824 |
| VGG19 | 0.848 | 0.814 | 0.817 | 0.814 |
Additionally, we compared the performance between CNN and SVM classifiers in order to investigate classifier dependency of feature extractor. CNN showed better performance than SVM, and VGG16 was the best classifier for both CNN and SVM. Moreover, CNN was more efficient in computation time than SVM (Table 6).
Table 6.
Comparison of performance between CNN and SVM with feature extractors (Inception V3, DenseNet201, VGG16).
| Classifier | Feature extractor | Accuracy | Precision | Recall score | F1 score |
|---|---|---|---|---|---|
| CNN | InceptionV3 | 0.748 | 0.722 | 0.727 | 0.720 |
| DenseNet201 | 0.786 | 0.749 | 0.735 | 0.729 | |
| ResNet50 | 0.783 | 0.747 | 0.738 | 0.738 | |
| ResNet101 | 0.809 | 0.787 | 0.766 | 0.769 | |
| VGG16 | 0.857 | 0.823 | 0.828 | 0.824 | |
| VGG19 | 0.848 | 0.814 | 0.817 | 0.814 | |
| SVM | InceptionV3 | 0.746 | 0.727 | 0.622 | 0.620 |
| DenseNet201 | 0.754 | 0.733 | 0.616 | 0.604 | |
| ResNet50 | 0.747 | 0.768 | 0.599 | 0.569 | |
| ResNet101 | 0.749 | 0.757 | 0.607 | 0.589 | |
| VGG16 | 0.755 | 0.762 | 0.614 | 0.594 | |
| VGG19 | 0.750 | 0.752 | 0.617 | 0.609 |
Accuracy of auscultation analysis in real clinical practice
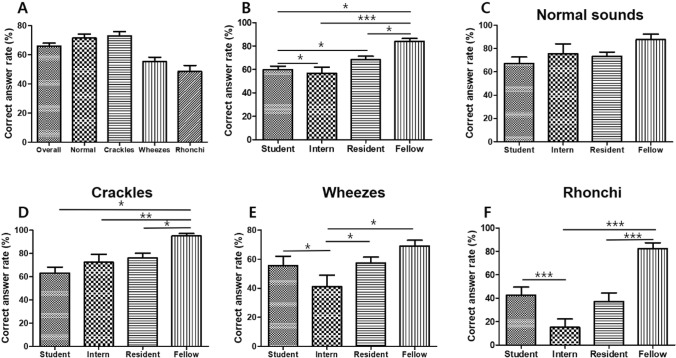
To verify the accuracy of auscultation analysis in real clinical practice and to evaluate the need for deep learning-based classification, we checked how exactly medical students, interns, residents, and fellows categorize breathing sounds (Fig. 4). We made several test sets of normal sounds and three types of abnormal lung sounds: crackle, wheezes, and rhonchi. 25 medical students, 11 interns, 23 residents, and 11 fellows of the internal medicine department of four teaching hospitals were asked to listen to the sounds and identify them. Regarding each breath sounds, the mean correct answer rates of normal sounds, crackles, wheezes, and rhonchi were 73.5%, 72.2%, 56.3%, and 41.7%, respectively. The overall correct answer rates of medical students, interns, residents, and fellows were 59.6%, 56.6%, 68.3%, and 84.0% respectively. The average correct answer rates for normal breathing were 67.1% for medical students, 75.7% for interns, 73.2% for residents, and 87.7% for fellows, while those for crackles were 62.9% for medical students, 72.3% for interns, 76.0% for residents, and 90.3% for fellows. The accuracies for wheezes were 55.6% for medical students, 41.0% for interns, 57.4% for residents, and 69.1% for fellows respectively, while those for rhonchi were 42.5% for medical students, 15.0% for interns, 37.1% for residents, and 82.2% for fellows (Fig. 4). There was no significant difference between each group in analyzing normal breathing sound, but in all three types of abnormal breathing sound, the fellows showed the highest accuracy. Among the abnormal breath sounds, interns and residents classified crackles most accurately. Rhonchi was revealed to be the most difficult sound to discriminate (Fig. 4).
Figure 4.
Accuracy of auscultation analysis in real clinical practice. (A) Mean correction answer rates for the overall sounds, normal sounds, crackles, wheezes, and rhonchi. (B) Mean correction answer rates of students, interns, residents, and fellows for overall sounds. (C) Mean correction answer rates of students, interns, residents, and fellows for normal sounds (D) Mean correction answer rates of students, interns, residents, and fellows for crackles (E) Mean correction answer rates of students, interns, residents, and fellows for wheezes. (F) Mean correction answer rates of students, interns, residents, and fellows for rhonchi. *p < 0.05, **p < 0.05 ***p < 0.001 (Student's t-test).
Discussion
The stethoscope has been considered an invaluable diagnostic tool for centuries2,3. Although many diagnostic techniques have been developed, auscultation still plays major roles1,7. For example, a pulmonologist can detect early-stage IPF or pneumonia based on inspiratory crackles even when the chest X-ray appears near-normal42. Changes in wheezes sometimes indicate the onset of asthma or COPD exacerbation. Therefore, early detection and accurate classification of abnormal breathing sounds can prevent disease progression and improve a patient’s prognosis.
Several studies have tried to automatically classify lung sounds. Chamberlain et al. classified lung sounds with a semi-supervised deep learning algorithm. The AUC were 0.86 for wheezes and 0.74 for crackles, respectively26. Guler et al. used a multilayer perceptron running a backpropagation training algorithm to predict the presence or absence of adventitious sounds27. They enrolled 56 patients and two hidden layers yielded 93.8% rated classification performance27.
In this study, we used deep learning for the classification of respiratory sounds. Compared with several lung sound classification studies that applied machine learning or deep learning for lung sounds classification27,43–47, we modified the deep learning algorithm of Bardou’s study which applied SVM46. In our study, we utilized the transfer learning method, which is easy, fast and able to use various features, but one has to be careful in connecting two deep learning networks, feature extractor and classifier. Moreover, there is a certain dependency between these two. We applied CNN instead of SVM because CNN is more efficient than a SVM for image classification.
Besides, our comparison of performances of different feature extractors demonstrated that CNN classifier showed much better performance with VGG, especially, VGG16 than InceptionV3 and Densenet201. The main contribution of this study is to develop the predictive model for respiratory sound classification combining pretrained image feature extractor of time-series, respiratory sound, and CNN classifier.
Our deep learning-based classification can detect abnormal lung sounds with an AUC of 0.93 and an accuracy of 86.5%. It has similar results in categorizing abnormal sounds into subcategorical sounds: crackles, wheezes, or rhonchi. Considering these are the result of analyzing the sounds recorded in a real clinical field with various noises, these are impressive results. We believe that these accuracies are adequate for primary screening and follow-up testing of patients with respiratory diseases.
Our test results showed that the auscultation accuracy of interns and residents were less than 80% in all four kinds of sounds and rhonchi was the most difficult sound to discriminate. The result of the test is not conclusive since the number of participants is small. However, it looks obvious that there are marked differences in the ability of each clinician to classify breathing sounds. This suggests that AI-assisted classification standardize the identification and categorization of breath sounds and greatly aid the diagnosis of pulmonary diseases.
There are several respiratory sounds in which two or more abnormal breath sounds are mixed. Such sounds are sometimes difficult even for experts and there may be disagreements between them. Few published studies have classified mixed abnormal breathing sounds, so research about these sounds is necessary. Also, since noises such as coughs, voices, heart sounds, and medical alarms are frequently recorded with breath sound, which reduces the accuracy of analysis, the technology for noise filtering is required.
Conclusion
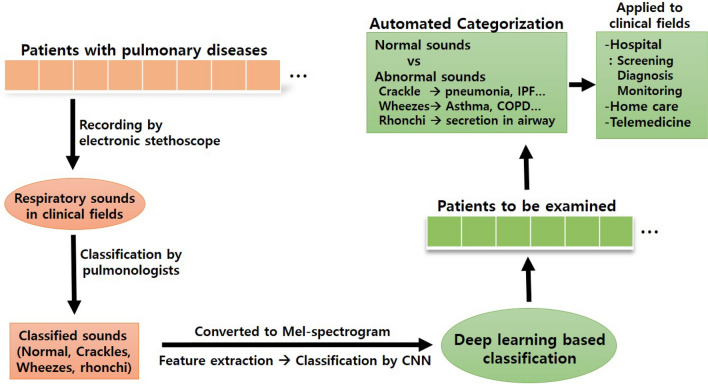
We found that our deep learning-based classification could classify the respiratory sounds accurately. Utilizing the transfer learning method, combining pre-trained image feature extraction from respiratory sound and CNN classification, worked well and was helpful for improving the classification accuracy. Though the analysis of mixed abnormal sounds and filtering noises remain challenging, recent innovations in analytic algorithm and recording technology will accelerate the advance of respiratory sound analysis more rapidly. Soon, deep learning-based automated stethoscope is expected to be used in telemedicine and home care (Fig. 5).
Figure 5.
Summary of deep learning assisted classification of respiratory sounds. Respiratory sounds were corrected from the patients with pulmonary diseases. The sounds were validated and classified by pulmonologists. The sounds were converted to Mel-spectrogram and features were extracted by VGG16 (transfer learning). Respiratory sounds were classified by CNN. Deep learning-based classification of respiratory sounds can be helpful for screening, monitoring, and diagnosis of pulmonary diseases.
Methods
Patient selection and data collection
Patients who visited an outpatient clinic or were hospitalized at Chungnam National University Hospital, regardless of the presence or type of respiratory diseases were enrolled from April 2019 to December 2020. The recording was proceeded in the actual clinical field (outpatient clinic, hospitalization room, emergency room, intensive care unit). Lung sounds were obtained from two to six sites of the posterior thorax using a Littman 3200 electronic stethoscope, downloaded to a computer, and converted into “wave” files. All sounds were carefully checked and validated by three pulmonologists. All patients gave written informed consent, and we obtained human research ethics committee approval of Chungnam National University Hospital Institutional Review Board (No. 2020-10-092). All methods were performed in accordance with the relevant guidelines and regulations. We recorded 2840 sounds and made a respiratory sound database containing 1222 normal breath sounds, 297 crackles, 298 wheezes, and 101 rhonchi.
AI models with transfer learning and CNN
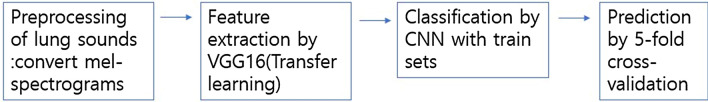
Overview of AI models
Lung sounds were converted to Mel-spectrograms and features were extracted by VGG16. CNN was applied for the classification and fivefold cross-validation was used for prediction (Fig. 6).
Figure 6.
Overview of our AI models.
Preprocessing of lung sounds
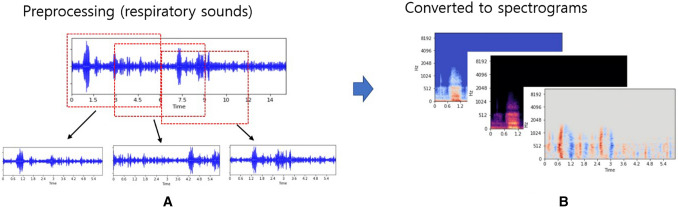
Recorded sounds were ranged from a few seconds to several tens of seconds. We divided them into 6 s each with 50% overlapping. For example, the audio file is a 14.5-s audio file of wheezing, which is divided into 3 cycles according to the start and end times (Fig. 7). And, to process the feature extraction and use the 3-dimensional input data, we used Mel-spectrogram, average of harmonic and percussive Mel-spectrogram, and the derivative of Mel-spectrogram using the Python library librosa47.
Figure 7.
Process to obtain spectrograms. (A) Given lung sound, dividing lung sound files with overlapping during 3 s (B) Obtaining three types of Mel-spectrograms with log-scale.
Feature extractor and classification
We thought at least two or three cycles of respiratory sounds are needed for accurate analysis of lung sounds. Approximately, normal respiratory rate is 15–20 per one minute (three–four seconds per one respiratory cycle) and it tends to be more rapid at pathologic conditions. So, after testing several options, we finally have decided six seconds as the length of the respiratory sound.
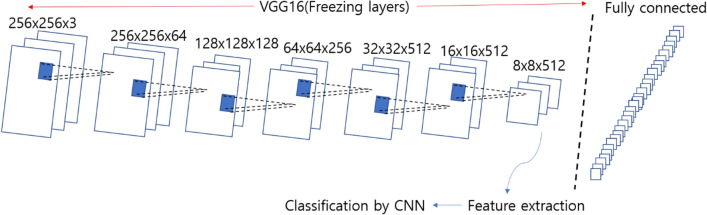
We used pre-trained models VGG16 as feature extractors in transfer learning, which was built by Karen Simonyan48,49. VGG16 is a model with 16 layers trained on fixed-size images and the input is processed through a set of convolution layers that use small-size kernels with a receptive field 3 × 3. The default input size of VGG16 is 224 × 224, but the input size for our model is 256 × 256 (Fig. 8). We used weights pre-trained on ImageNet by freezing all the five convolution blocks without fully-connected layer, and predicted the test sets with simple CNN with only one-layer.
Figure 8.
VGG16 architecture for our model. The input size for our model is 256 × 256. We freeze all layers in VGG16 without fully-connected layer, and with extracting features, we classified the respiratory sounds.
Evaluation of our models
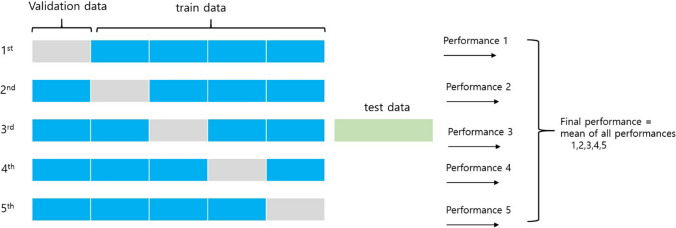
To avoid overfitting, we utilized the fivefold cross-validation method34 (Fig. 9). The dataset has been chosen randomly to split into 80% training set and 20% test set, and 20% of training set is used for validation. The main idea of the fivefold cross validation is to split the training set into 5 partitions. Each time one of the 5 partitions are used for validating the model and the other 4 partitions are used for training the model. So, each instance in the data set is used once in testing and 4 times in training. All results of the different metrics are then averaged to return the result. From results by our models, we obtained accuracy, precision, recall score and ROC curve.
Figure 9.
5-folds cross validation. Trying 5 iterations and getting final results with mean of all performances.
Statistical analysis
All values are presented as means ± standard deviation (SD). Significant differences were determined using GraphPad 5 software. The Student’s t-test was used to determine statistical differences between two groups. The receiver operating characteristic curve was plotted and the area under the curve was calculated with the 95% confidence intervals.
Acknowledgements
This work was supported by the National Research Foundation of Korea (NRF) grant funded by the Korean Government (MSIT) (No. NRF-2017R1A5A2015385). T.H, Y.H. and S.L. was supported by National Institute for Mathematical Sciences (NIMS) grant funded by the Korean government (No. B21910000). Authors appreciate Y.P, I.O, S.Y and I.J for collecting the data related to the accuracy of auscultation analysis in real clinical practice.
Author contributions
Y.K., C.C., T.H., and Y.H made substantial contributions to conception and design. Y.K., C.C., and S.S.J. performed data acquisition and validation for this study. T.H., Y.H. and S.L. implemented deep learning with lung sounds. G.Y. edited the manuscript. All authors contributed to the elaboration and redaction of the final manuscript.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
These authors contributed equally: Yoonjoo Kim and YunKyong Hyon.
Contributor Information
Chaeuk Chung, Email: universe7903@gmail.com.
Taeyoung Ha, Email: tha@nims.re.kr.
References
- 1.Bohadana A, Izbicki G, Kraman SS. Fundamentals of lung auscultation. N. Engl. J. Med. 2014;370:744–751. doi: 10.1056/NEJMra1302901. [DOI] [PubMed] [Google Scholar]
- 2.Bloch H. The inventor of the stethoscope: René Laennec. J. Fam. Pract. 1993;37:191. [PubMed] [Google Scholar]
- 3.Roguin A. Rene Theophile Hyacinthe Laennec (1781–1826): The man behind the stethoscope. Clin. Med. Res. 2006;4:230–235. doi: 10.3121/cmr.4.3.230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Swarup S, Makaryus AN. Digital stethoscope: Technology update. Med. Dev. (Auckland N. Z.) 2018;11:29–36. doi: 10.2147/mder.s135882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Leng S, et al. The electronic stethoscope. Biomed. Eng. Online. 2015;14:66. doi: 10.1186/s12938-015-0056-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Arts L, Lim EHT, van de Ven PM, Heunks L, Tuinman PR. The diagnostic accuracy of lung auscultation in adult patients with acute pulmonary pathologies: A meta-analysis. Sci. Rep. 2020;10:7347. doi: 10.1038/s41598-020-64405-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sarkar M, Madabhavi I, Niranjan N, Dogra M. Auscultation of the respiratory system. Ann. Thoracic Med. 2015;10:158–168. doi: 10.4103/1817-1737.160831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Vyshedskiy A, et al. Mechanism of inspiratory and expiratory crackles. Chest. 2009;135:156–164. doi: 10.1378/chest.07-1562. [DOI] [PubMed] [Google Scholar]
- 9.Fukumitsu T, Obase Y, Ishimatsu Y. The acoustic characteristics of fine crackles predict honeycombing on high-resolution computed tomography. BMC Pulm. Med. 2019;19:153. doi: 10.1186/s12890-019-0916-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hafke-Dys H, Bręborowicz A, Kleka P. The accuracy of lung auscultation in the practice of physicians and medical students. PLoS ONE. 2019;14:e0220606. doi: 10.1371/journal.pone.0220606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mangione S, Nieman LZ. Pulmonary auscultatory skills during training in internal medicine and family practice. Am. J. Respir. Crit. Care Med. 1999;159:1119–1124. doi: 10.1164/ajrccm.159.4.9806083. [DOI] [PubMed] [Google Scholar]
- 12.Melbye H, et al. Wheezes, crackles and rhonchi: Simplifying description of lung sounds increases the agreement on their classification: a study of 12 physicians' classification of lung sounds from video recordings. BMJ Open Respir. Res. 2016;3:e000136. doi: 10.1136/bmjresp-2016-000136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Andrès E, Gass R, Charloux A, Brandt C, Hentzler A. Respiratory sound analysis in the era of evidence-based medicine and the world of medicine 2.0. J. Med. Life. 2018;11:89–106. [PMC free article] [PubMed] [Google Scholar]
- 14.Ohshimo S, Sadamori T, Tanigawa K. Innovation in analysis of respiratory sounds. Ann. Intern. Med. 2016;164:638–639. doi: 10.7326/L15-0350. [DOI] [PubMed] [Google Scholar]
- 15.Altan G, Yayık A, Kutlu Y. Deep learning with ConvNet predicts imagery tasks through EEG. Neural Process. Lett. 2021;53:2917–2932. doi: 10.1007/s11063-021-10533-7. [DOI] [Google Scholar]
- 16.Tang YX, Tang YB, Peng Y. Automated abnormality classification of chest radiographs using deep convolutional neural networks. NPJ Dig. Med. 2020;3:70. doi: 10.1038/s41746-020-0273-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Coucke PA. Laennec versus Forbes : Tied for the score ! How technology helps us interpret auscultation. Rev. Med. Liege. 2019;74:543–551. [PubMed] [Google Scholar]
- 18.Grzywalski T, et al. Practical implementation of artificial intelligence algorithms in pulmonary auscultation examination. Eur. J. Pediatr. 2019;178:883–890. doi: 10.1007/s00431-019-03363-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Palaniappan R, Sundaraj K, Sundaraj S. Artificial intelligence techniques used in respiratory sound analysis: A systematic review. Biomedizinische Technik. Biomed. Eng. 2014;59:7–18. doi: 10.1515/bmt-2013-0074. [DOI] [PubMed] [Google Scholar]
- 20.Ono H, et al. Evaluation of the usefulness of spectral analysis of inspiratory lung sounds recorded with phonopneumography in patients with interstitial pneumonia. J. Nippon Med. School Nippon Ika Daigaku Zasshi. 2009;76:67–75. doi: 10.1272/jnms.76.67. [DOI] [PubMed] [Google Scholar]
- 21.Thompson WR, Reinisch AJ, Unterberger MJ, Schriefl AJ. Artificial intelligence-assisted auscultation of heart murmurs: Validation by virtual clinical trial. Pediatr. Cardiol. 2019;40:623–629. doi: 10.1007/s00246-018-2036-z. [DOI] [PubMed] [Google Scholar]
- 22.Murphy RL, et al. Automated lung sound analysis in patients with pneumonia. Respir. Care. 2004;49:1490–1497. [PubMed] [Google Scholar]
- 23.Kevat A, Kalirajah A, Roseby R. Artificial intelligence accuracy in detecting pathological breath sounds in children using digital stethoscopes. Respir. Res. 2020;21:253. doi: 10.1186/s12931-020-01523-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Serbes, G., Sakar, C. O., Kahya, Y. P. & Aydin, N. Feature extraction using time-frequency/scale analysis and ensemble of feature sets for crackle detection. In Annual International Conference of the IEEE Engineering in Medicine and Biology Society. IEEE Engineering in Medicine and Biology Society. Annual International Conference2011, 3314–3317. 10.1109/IEMBS.2011.6090899 (2011). [DOI] [PubMed]
- 25.Demir F, Sengur A, Bajaj V. Convolutional neural networks based efficient approach for classification of lung diseases. Health Inform. Sci. Syst. 2020;8:4. doi: 10.1007/s13755-019-0091-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chamberlain, D., Kodgule, R., Ganelin, D., Miglani, V. & Fletcher, R. R. Application of semi-supervised deep learning to lung sound analysis. In Annual International Conference of the IEEE Engineering in Medicine and Biology Society. IEEE Engineering in Medicine and Biology Society. Annual International Conference2016, 804–807. 10.1109/EMBC.2016.7590823 (2016). [DOI] [PubMed]
- 27.Guler I, Polat H, Ergun U. Combining neural network and genetic algorithm for prediction of lung sounds. J. Med. Syst. 2005;29:217–231. doi: 10.1007/s10916-005-5182-9. [DOI] [PubMed] [Google Scholar]
- 28.Altan G, Kutlu Y, Allahverdi N. Deep learning on computerized analysis of chronic obstructive pulmonary disease. IEEE J. Biomed. Health Inform. 2019 doi: 10.1109/JBHI.2019.2931395. [DOI] [PubMed] [Google Scholar]
- 29.Altan G, Kutlu Y, Pekmezci AÖ, Nural S. Deep learning with 3D-second order difference plot on respiratory sounds. Biomed. Signal Process. Control. 2018;45:58–69. doi: 10.1016/j.bspc.2018.05.014. [DOI] [Google Scholar]
- 30.Altan G, Kutlu Y, Gökçen A. Chronic obstructive pulmonary disease severity analysis using deep learning on multi-channel lung sounds. Turk. J. Electr. Eng. Comput. Sci. 2020;28:2979–2996. doi: 10.3906/elk-2004-68. [DOI] [Google Scholar]
- 31.Altan G, Kutlu Y, Garbi Y, Pekmezci AÖ, Nural S. Multimedia respiratory database (RespiratoryDatabase@ TR): Auscultation sounds and chest X-rays. Nat. Eng. Sci. 2017;2:59–72. [Google Scholar]
- 32.Aras S, Öztürk M, Gangal A. Automatic detection of the respiratory cycle from recorded, single-channel sounds from lungs. Turk. J. Electr. Eng. Comput. Sci. 2018;26:11–22. doi: 10.3906/elk-1705-16. [DOI] [Google Scholar]
- 33.Zheng L, et al. Artificial intelligence in digital cariology: A new tool for the diagnosis of deep caries and pulpitis using convolutional neural networks. Ann. Transl. Med. 2021;9:763. doi: 10.21037/atm-21-119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rezaeijo SM, Ghorvei M, Mofid B. Predicting breast cancer response to neoadjuvant chemotherapy using ensemble deep transfer learning based on CT images. J. Xray Sci. Technol. 2021 doi: 10.3233/xst-210910. [DOI] [PubMed] [Google Scholar]
- 35.Arora V, Ng EY, Leekha RS, Darshan M, Singh A. Transfer learning-based approach for detecting COVID-19 ailment in lung CT scan. Comput. Biol. Med. 2021;135:104575. doi: 10.1016/j.compbiomed.2021.104575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Jin W, Dong S, Dong C, Ye X. Hybrid ensemble model for differential diagnosis between COVID-19 and common viral pneumonia by chest X-ray radiograph. Comput. Biol. Med. 2021;131:104252. doi: 10.1016/j.compbiomed.2021.104252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wang Q, et al. Realistic lung nodule synthesis with multi-target co-guided adversarial mechanism. IEEE Trans. Med. Imag. 2021 doi: 10.1109/tmi.2021.3077089. [DOI] [PubMed] [Google Scholar]
- 38.Pu J, Sechrist J, Meng X, Leader JK, Sciurba FC. A pilot study: Quantify lung volume and emphysema extent directly from two-dimensional scout images. Med. Phys. 2021 doi: 10.1002/mp.15019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Gharehbaghi A, Linden M. A deep machine learning method for classifying cyclic time series of biological signals using time-growing neural network. IEEE Trans. Neural Netw. Learn. Syst. 2018;29:4102–4115. doi: 10.1109/TNNLS.2017.2754294. [DOI] [PubMed] [Google Scholar]
- 40.Raghu, M., Chiyuan, Z., Jon, K. & Samy, B. Transfusion: Understanding Transfer learning for medical imaging. In 33rd Conference on Neural Information Processing Systems (2019).
- 41.Park S, Kim J, Kim D. A study on classification performance analysis of convolutional neural network using ensemble learning algorithm. J. Korea Multimedia Soc. 2019;22:665–675. [Google Scholar]
- 42.Epler GR, Carrington CB, Gaensler EA. Crackles (rales) in the interstitial pulmonary diseases. Chest. 1978;73:333–339. doi: 10.1378/chest.73.3.333. [DOI] [PubMed] [Google Scholar]
- 43.Horimasu Y, et al. A machine-learning based approach to quantify fine crackles in the diagnosis of interstitial pneumonia: A proof-of-concept study. Medicine. 2021;100:e24738. doi: 10.1097/md.0000000000024738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Naves R, Barbosa BH, Ferreira DD. Classification of lung sounds using higher-order statistics: A divide-and-conquer approach. Comput. Methods Programs Biomed. 2016;129:12–20. doi: 10.1016/j.cmpb.2016.02.013. [DOI] [PubMed] [Google Scholar]
- 45.Aykanat M, Kılıç Ö, Kurt B, Saryal S. Classification of lung sounds using convolutional neural networks. J. Image Video Proc. 2017 doi: 10.1186/s13640-017-0213-2. [DOI] [Google Scholar]
- 46.McFee, B. librosa. librosa 0.8.0. 10.5281/zenodo.3955228 (2020).
- 47.Bardou D, Zhang K, Ahmad SM. Lung sounds classification using convolutional neural networks. Artif. Intell. Med. 2018;88:58–59. doi: 10.1016/j.artmed.2018.04.008(2018). [DOI] [PubMed] [Google Scholar]
- 48.Krizhevsky A, Sutskever I, Hinton GE. Imagenet classification with deep convolutional neural networks. Adv. Neural Inform. Process. Syst. 2012;25:1097–1105. [Google Scholar]
- 49.Simonyan, K. & Zisserman, A. Very deep convolutional networks for large-scale image recognition. CoRR abs1409, 1556 (2014).