Abstract
Objectives:
Network analysis is increasingly applied to psychopathology research. We used it to examine the core phenomenology of emerging bipolar disorder (BD I and II) and ‘at risk’ presentations (major depression with a family history of BD).
Methodology:
The study sample comprised a community cohort of 1867 twin and non-twin siblings (57% female; mean age ~26) who had completed self-report ratings of (i) depression-like, hypomanic-like and psychotic-like experiences; (ii) family history of BD; and (iii) were assessed for mood and psychotic syndromes using the Composite International Diagnostic Interview (CIDI). Symptom networks were compared for recent onset BD versus other cohort members and then for individuals at risk of BD (depression with/without a family history of BD).
Results:
The four key symptoms that differentiated recent onset BD from other cohort members were: anergia, psychomotor speed, hypersomnia and (less) loss of confidence. The four key symptoms that differentiated individuals at high risk of BD from unipolar depression were: anergia, psychomotor speed, impaired concentration, and hopelessness. However, the latter network was less stable and more error prone.
Conclusions:
We are encouraged by the overlaps between our findings and those from two recent publications reporting network analyses of BD psychopathology, especially as the studies recruited from different populations and employed different network models. However, the advantages of applying network analysis to youth mental health cohorts (which include many individuals with multi-morbidity) must be weighed against the disadvantages including basic issues such as judgements regarding the selection of items for inclusion in network models.
Keywords: bipolar disorder, sleep-wake cycle, network analysis, activation, risk factors
Introduction
In the last decade, there has been an exponential increase in the interest in network analysis in psychiatry1. A primary reason for this is the potential utility of using networks to understand phenomenology that occurs across a range of diagnostic categories (by examining so-called bridging or communicating symptoms) or to gain insights into psychopathology within diagnostic subtypes. This strategy may be useful for bipolar disorders (BD) given that its symptoms overlaps with depressive, psychotic, and other disorders and also that symptoms may differ across the BD spectrum2–4.
To briefly summarize the approach, network analysis describes a set of procedures based on the modelling of dynamic systems5. The technique provides a graphical representation of the nodes (individual factors; variables) and edges (connections; links among nodes)6,7. In psychopathology studies, the methodology has intuitive appeal because visual inspection of the network plot allows easy identification of the potential role or importance of specific symptoms and their interconnections. However, to date, network analysis has been used less frequently in research in BD compared with other mood or psychotic disorders8.
To our knowledge, there are only six BD publications. The first was by Koenders et al,9 who examined phenomenology in BD patients categorized into three subgroups that were followed over two years. They found that different symptoms were associated with each group e.g. low self-esteem and psychomotor slowness played a central role in the depression network whilst impaired concentration and suicidality were more influential in the cyclicity group. Other network analyses compared BD with another disorder: one examined cognition in BD and unipolar depression (UP)10, another examined negative symptoms in BD and schizophrenia11, whilst a small-scale study compared positive and negative affect in BD and healthy controls12. Of relevance to this article, are two recent larger-scale studies. Corponi and colleagues13 studied >2000 middle-aged adults with acute depression who were classified into UP and BD groups. A comparison of the network plots of clinician-rated symptoms did not demonstrate significant differences in overall network strength or structure between the two groups, but some ‘mixed state’ symptoms, appetite gain and hypersomnia were associated with the BD rather than UP network. Only one BD study has examined symptom networks in children and adolescents14. The sample comprised 272 participants with an age range of about 9–18 who were recruited into two randomized controlled trials (RCTs) of family interventions. About half had BD I or II whilst the remainder were individuals at high risk of BD (defined as BD NOS or depression with a family history of BD). Network analysis of observer ratings from a structured clinical interview with a parent identified that fatigue, hyperactivity, and depressed mood were prominent symptoms in BD cases whilst mood lability and irritability were important symptoms in individuals at risk of BD. These two BD studies are important, but the former addresses older adults and the latter identifies youth attending specialist clinics, further selected to participate in RCTs.
The current study examines symptom networks in a large, longitudinal community cohort of youth in the peak age range for onset of mood and psychotic disorders (i.e. about 15–25 years). It builds on the existing research in four important ways. First, it estimates networks of self-reported rather than observer-rated phenomenology in first episode and recent onset BD (allowing us to explore phenomena of importance to youth and compare these to ratings used for traditional diagnostic criteria). Second, it examines a broader range of psychopathology extending beyond depressive (DLE) and hypo/manic like experiences (HMLE) to include psychotic like experiences (PLE). Third, it compares networks between selected subgroups of youth (and used statistical tests to determine network differences). Also, we use this study as an opportunity to contribute to a wider discussion of the use of network analysis in research in youth, BD and psychopathology.
Key aims of the study are to explore self-reported phenomenology that may differentiate:
individuals with BD from ‘non-BD’ cohort members, and
individuals at high risk of BD (major depression with a positive family history BD) from those with major depression without a family history of BD.
Methods
Ethical approval was obtained from the Human Research Ethics Committee at the Queensland Berghofer Institute of Medical Research (QIMR Berghofer) for all Brisbane Longitudinal Twin Study (BLTS) research projects (reference numbers: EC00278 and P1212).
Here, we summarize key elements of the methodology. However, we also provide extensive online supplementary materials (Appendices 1–3) that provide more detailed information. For example, the current study follows the Strengthening the Reporting of Observational Studies in Epidemiology guidelines15, but the STROBE checklist is included in Appendix 1. Likewise, an overview of the BLTS protocol and a study flow chart are provided in Appendix 2 (and other detailed descriptions of the all the assessments undertaken are published elsewhere16–18). Furthermore, Appendix 2 gives an extended version of the network analysis strategy.
To briefly summarize, the BLTS is a community-based cohort study of twins and their non-twin siblings living in the greater Brisbane area. It began in 1992 and participants were recruited via media appeals and word of mouth. Written informed consent was obtained from all potential participants (parental consent was obtained for individuals aged<18 years). Individuals entered the study from age 12 onwards but potential participants were excluded if parental report indicated a history of head injuries, neurological or pre-existing psychiatric conditions, substance abuse or dependence, and/or taking medications with significant central nervous system effects. Repeated follow-ups have been undertaken at approximately three to five yearly intervals. Individuals who miss one follow-up are invited to participate again at the next wave. Below, we provide a synopsis of key information about assessments undertaken from 2009 onwards (referred to as the 19Up and 25Up follow-ups) that are relevant to this article.
Cohort for this Study
De-identified individual data were extracted from the BLTS dataset according to the following eligibility criteria: the individual had completed self-report ratings of mental health symptoms (between ~15–19 years) and that data regarding the Composite International Diagnostic Interview (CIDI)19 and FH of mental disorders were available from the 19Up or 25Up waves respectively. Due to the nesting of this data collection within a dense longitudinal framework, self-report and other assessments can be linked to data collected from earlier waves of the study16. Further, the current study design ensured that participants had undertaken key self-ratings and mental health assessments during the peak age range for onset of mood and psychotic disorders18.
Assessments
1). Demographics:
Demographics: data on age at completion of assessments, sex, zygosity, education and employment status are reported in Table 1.
Table 1:
Key characteristics of the study cohort
| Characteristic | N = 1867 |
|---|---|
| Mean Age in years (with 95% CI) | 26.4 (22.7, 29.5) |
| Number (%) | |
| Educational Level: Junior or Senior School only | 336 (18%) |
| Full-Time Employment | 1120 (60%) |
| Civil Status: Single | 1046 (56%) |
| Non-Twin Siblings | 578 (31%) |
| **Psychosis | 84 (4%) |
| Family History of Bipolar Disorder | 57 (3%) |
% reported to the nearest whole number; CI: confidence intervals
Odd numbers indicate only one co-twin was assessed.
CIDI: Composite International Diagnostic Interview.
CIDI does not include ratings of negative symptoms, so this diagnosis represents a psychotic syndrome with or without a mood episode (see Appendix 2 for details)
2). Diagnosis of BD I & II
We used the CIDI to determine the presence or absence of a range of DSM-IV disorders and their age at onset19. For this study, we report the presence or absence of BD (defined as BD I or II) and then examine cases of major depression (UP with/without family history of BD). Also, we provide information on the number of individuals who met criteria for a CIDI psychotic syndrome (with or without a mood episode). It should be noted that presence of any current or past lifetime comorbidities was not an exclusion criterion for this study and, as expected in epidemiological studies of youth, ~20% of BLTS cohort members met criteria for >=1 lifetime mental disorder (with ~16% reporting >=1 comorbidity).
3). Self-Reported Phenomenology
The items included in the self-report rating scales are listed in Table 2 (see Appendix 2 for descriptions of rating scales). These three subsets of symptoms represent co-occurring phenomena that are most often associated with episodes of depression, hypo/mania and psychosis; the ratings demonstrate good test-retest reliability (inter-class correlations= 0.8)20,21. The three self-rating scales were completed at the same time and include the following:
Hypomanic-Like Experiences (HMLE)- identified by five items that mirror the DSM-IV criteria and published ‘at risk’ criteria21.
Psychotic-Like Experiences (PLE)- identified by six items that assess sub-types of positive psychotic-like experiences most strongly associated with distress and poor functioning22.
Depressive-Like Experiences (DLE)- identified using the 12-item version of the Somatic and Psychological Health Report (SPHERE) which assesses the occurrence of a range of somatic and psychological symptoms of depression23.
Table 2:
Proportion of the cohort that endorse each item listed in the three self-report scales
| Item Number | Item description | N Endorsing the Item (Total N = 1867) |
Percentage (%)* |
|---|---|---|---|
| Depressive Symptoms | |||
| D1 | Nervous/Tense | 427 | 23 |
| D2 | Unhappy/Depressed | 348 | 19 |
| D3 | Feel Stressed | 591 | 32 |
| D4 | Feel Overwhelmed | 537 | 29 |
| D5 | Loss of Confidence | 395 | 21 |
| D6 | Hopelessness | 266 | 14 |
| D7 | Somatic Pain | 499 | 27 |
| D8 | Hypersomnia | 766 | 41 |
| D9 | Fatigue | 454 | 24 |
| D10 | Impaired Sleep (Quality) | 755 | 40 |
| D11 | Impaired Concentration | 493 | 26 |
| D12 | Anergia | 557 | 30 |
| Hypo/Manic Symptoms | |||
| HM1 | Feeling Elated | 872 | 47 |
| HM2 | Increased Self-Esteem | 750 | 40 |
| HM3 | Need Less Sleep | 464 | 25 |
| HM4 | Increased Psychomotor Speed (Speech) | 515 | 28 |
| HM5 | Increased Activity (Physical) | 643 | 34 |
| Psychotic Symptoms | |||
| P1 | Thoughts Not Your Own | 64 | 3 |
| P2 | Third Party Auditory Hallucinations | 24 | 1 |
| P3 | Hearing Voices (when alone) | 73 | 4 |
| P4 | Feeling Threatened by Others | 91 | 5 |
| P5 | Thinking People are Against You | 131 | 7 |
| P6 | Thought Withdrawal | 28 | 2 |
N= Number;
Percentages reported to nearest whole number
If no individual rating was available for an item, it was classed as ‘not endorsed’ (see text for details)
4). Family History of BD
Family history of major mental disorders was assessed using an online questionnaire based on the Family History Screen24. In this study, we used data from the dichotomous ratings (reporting the presence or absence of family history) and only extracted information about family history of bipolar disorders (FH of BD). If ratings were missing, responses were rated as ‘don’t know’ or were unclear, we coded the item as indicating the absence of a FH of BD.
Statistical Analyses
Statistical analysis was performed using RStudio (version 1.3) software. Descriptive statistics (means and 95% confidence intervals (CI); proportions, etc.) were used to characterize the study sample (see Table 1). We report the rates of endorsement of individual DLE, HMLE and PLE items, but these data are not analyzed separately (using univariate approaches) as the items form the core variables examined in the network analyses (Table 2). As explained in Appendix 2, the cohort members are considered singletons in the reported analyses.
Network Analysis
Several statistical applications can be used for network analysis and the optimal combination of outputs varies according to study aims25. The rational and details about the model employed here are provided in Appendix 2. Below we summarize our approach which is adapted from previous studies of complex categorical datasets4.
a). Network estimation & visualization
We estimated the connections between the HMLE, DLE and PLE items
in the entire study cohort subdivided according to the presence or absence of BD
in the subset of the cohort with major depression subdivided according to the presence or absence of FH of BD.
To harmonize the dataset for network analysis, we used a ‘+1 versus −1’ binary coding system for dichotomous (present/absent) ratings26 with sporadic missing items were coded as absent27. As we were analyzing dichotomous (binary) data, we used the eLASSO (least absolute shrinkage and selection operator)28 network estimation technique with the final model selection based on the extended Bayesian Information Criterion (eBIC)6,26. This enables the selection of simpler models and it is highly effective for estimating weighted networks from binary data28,29 as the procedure results in the removal of some edges from the network (which are most likely to be due to ‘noise’). However, it should be noted that it often leads to some variables being excluded from network comparison models (if the between group variance tends towards zero). For example, three PLE items (P1, P2, and P6) were automatically excluded from the primary network analysis (BD cases versus the rest of the BLTS cohort) and these same three items plus P3 (hearing voices) were excluded from the second analysis.
We then generated network diagrams using the Fruchterman and Reingold algorithm30 which computes the optimal layout for the plot so that HMLE, PLE and/or DLE items with stronger and/or more inter-connections are placed closer to each other and more centrally in the network31. In the figures we include in the paper, green edges represent positive associations between nodes and red edges represent negative associations, whilst the thickness of the connecting lines indicates the edge weight which reflects the strength of association. To improve interpretability, the figures focus on we connections that show moderate or strong associations (which equates to an odds ratio of >=1.5)32.
b). Centrality analysis
To help interpret the importance of individual HMLE, DLE and PLE items in the network plots, it is useful to calculate normalized scores for three key indices of node centrality33 (as these indicate the role or influence of a node within a network). The indices are called: Betweenness (the number of times that a node lies on the shortest path between two other nodes which aids identification of nodes that may be ‘hubs’); Closeness (average distance from the node to all other nodes in the network; this, so-called ‘Manhattan’ distance is a measure of how close a node is to all other nodes); and Strength, which is also referred to as Degree (absolute sum of edge weights connected to node which aids estimation of the total involvement of a node in the network). In the results section we primarily comment on findings for Degree as this is a useful marker of the overall importance of a node in the network and highlight the four variables (i.e. top 25% items) with centrality indices that most clearly differentiate between networks. We also used bootstrapping techniques so that we can comment on the stability of edges and node strength (together these provide an estimate of the accuracy of edges in the network and stability of the order of centrality. We summarize the findings in the text; additional data are reported in the supplementary material.)
c). Network Comparison Test
We compared the networks using the network comparison test (NCT) which examines differences in the structure and global strength (weighted absolute sum of all edges in the network) of the different pairs of networks34,35.
Results
As shown in Table 1, 1867 individuals (57% female) were eligible for inclusion in the network analysis. Their mean age was about 26 years, 56% of participants were single and 60% were in full-time employment. About one third of the sample were monozygotic twins, with similar proportions of dizygotic twins and non-twin siblings. According to the CIDI, 113 individuals (6%) met diagnostic criteria for BD I (n= 34) or BD II (n=79) and a further 484 (26%) had a major depression. About 4% of the study cohort had experienced a psychotic syndrome (N=84). The median age at onset of a mood disorder was about 19 years and for psychosis was about 23 years. Overall, 3% of the cohort (N=57) reported a FH of BD; eight of these individuals did not report a mood disorder; nine had a diagnosis of BD and 40 had a diagnosis of major depression.
The median age of completion of the three self-rating scales was about 17 years (see Scott et al, 2020b). As shown in Table 2, the number of individuals who endorsed each self-rated item ranged from one percent (P2: third party auditory hallucinations) to 47% (HM1: feeling elated). Overall, the median prevalence of positive endorsements of HMLE and DLE items was similar (25–26%), whilst each PLE items was endorsed by less than 10% of the cohort.
Comparison of symptom networks in BD cases & non-BD cohort members
Figure 1 shows the network plots for 20 nodes (self-rated phenomenology) in 113 individuals with BD (Figure 1a) compared with 1754 individuals without BD (Figure 1b).
Figure 1: Network Plots for presence or absence of BD.
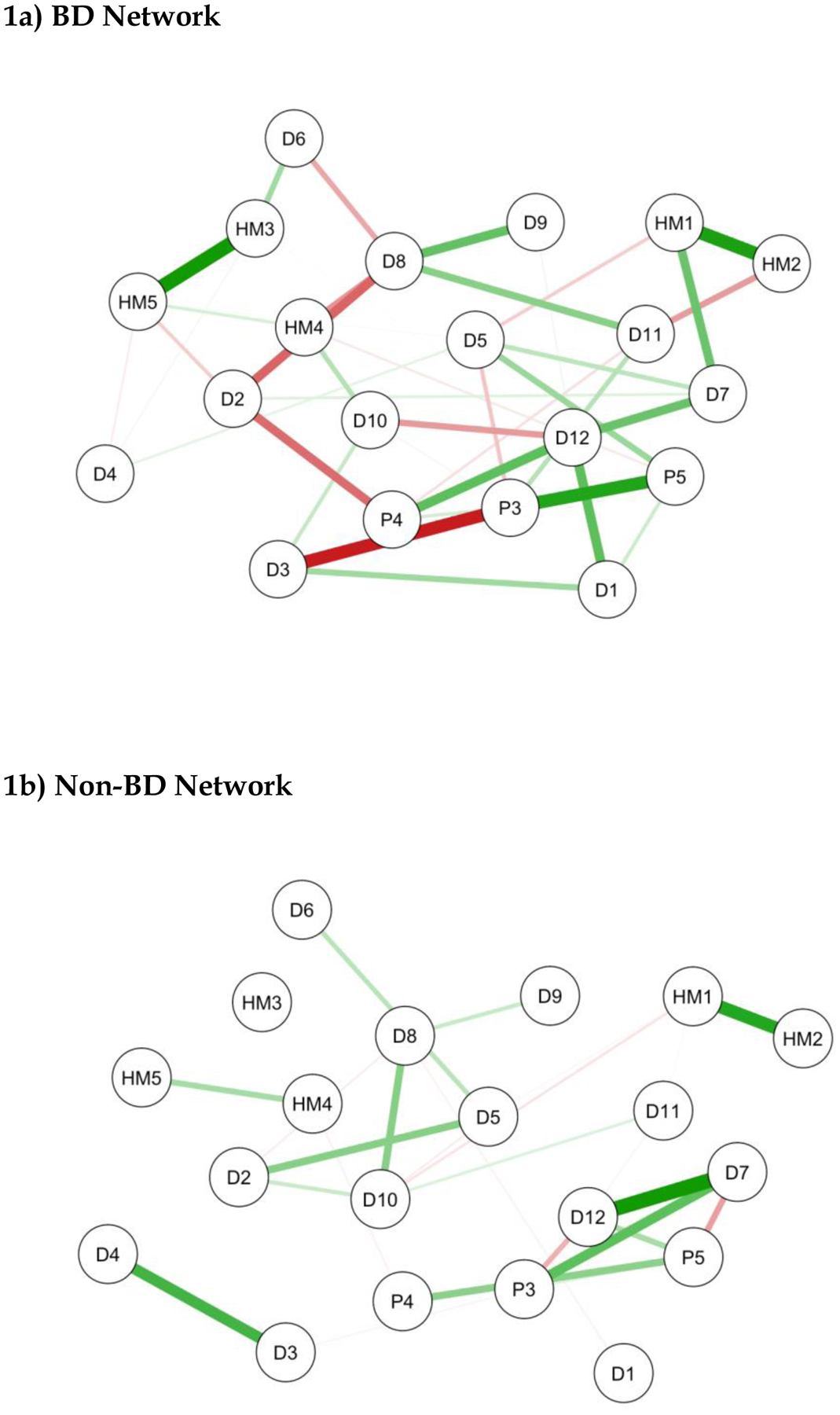
Brief note on the interpretation of network plots- HMLE, PLE and/or DLE items with stronger and/or more inter-connections are placed closer to each other and more centrally in the network. Green edges represent positive associations between nodes and red edges represent negative associations. The thickness of the connecting lines indicates the edge weight which reflects the strength of association.
KEY-
D1:Nervous/Tense; D2:Unhappy/Depressed; D3:Feel Stressed; D4:Feel Overwhelmed; D5:Lost Confidence; D6:Hopelessness; D7:Somatic Pain; D8:Hypersomnia; D9:Fatigue; D10:Poor Sleep Quality; D11:Poor Concentration; D12:Anergia; HM1: Feeling Elated; HM2: Increased Self-Confidence/Self-Esteem; HM3: Need Less Sleep; HM4: Increased Psychomotor Speed (Speech); HM5: Increased Activity (Physical); P3: Hearing Voices (when alone); P4: Feeling Threatened by Others; P5: Thinking People are Against You.
NB: Three PLE items (P1, P2, and P6) were automatically excluded from the network analysis.
As can be seen, the positive and negative connections between symptoms are greater in the BD network and centrally located nodes such as psychomotor speed (HM4) and poor sleep quality (D10) show more interconnections (betweenness) in the BD versus non-BD network plot. Interestingly, whilst elation (HM1) and increased self-esteem (HM2) are strongly interconnected, they are not a centrally located, nor are they directly linked to other hypomanic phenomenology in either network. Furthermore, there are few and/or only weak links between either of these phenomena and the other items in the ‘non-BD’ network.
The centrality indices for these networks are shown in Figure 2. The right-hand column entitled ‘Degree’ (the measure of strength), indicates that three HMLE items (sleep, psychomotor speed, activity), four DLE items (anergia: D12; hypersomnia: D8; sad mood: D2; nervous tension: D1) and two PLE items related to paranoia (P4 and P5) have important/influential roles in the BD network. However, the four key variables that differentiate between BD and non-BD networks were: anergia, psychomotor speed, hypersomnia, and loss of confidence, with the latter being the only variable to be increased in the non-BD network (also see Table 1S in Appendix 3). The NCT confirms that the network structure for the BD and non-BD plots differed significantly (NCT test statistic 1.61; p=0.038). (For details of stability of edge and centrality indices see supplementary Figures 1S and 2S in Appendix 3).
Figure 2:
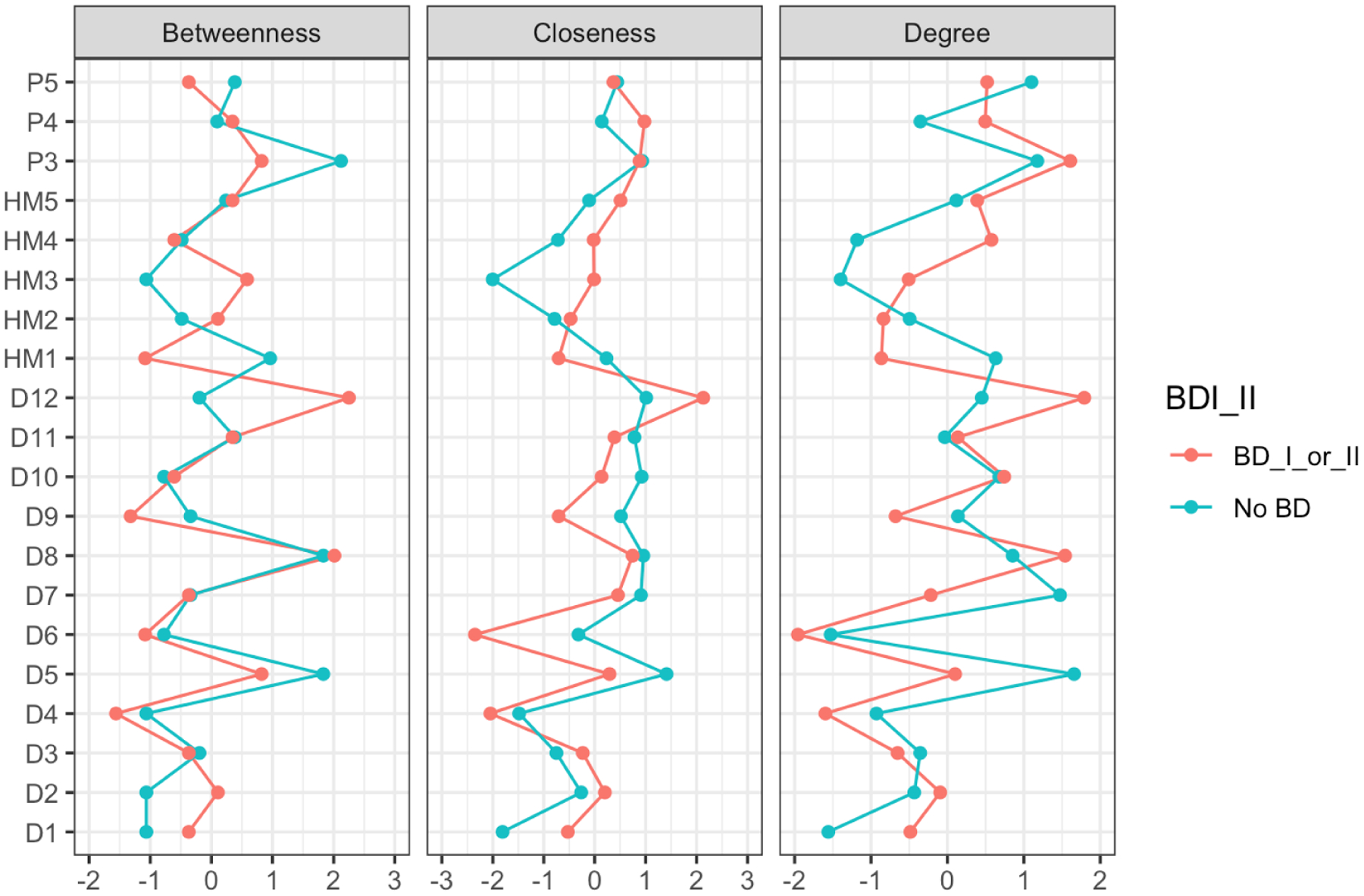
Centrality Plot for BD (BDI_II) & non-BD Networks (see text for details)
KEY-
D1:Nervous/Tense; D2:Unhappy/Depressed; D3:Feel Stressed; D4:Feel Overwhelmed; D5:Lost Confidence; D6:Hopelessness; D7:Somatic Pain; D8:Hypersomnia; D9:Fatigue; D10:Poor Sleep Quality; D11:Poor Concentration; D12:Anergia; HM1: Feeling Elated; HM2: Increased Self-Confidence/Self-Esteem; HM3: Need Less Sleep; HM4: Increased Psychomotor Speed (Speech); HM5: Increased Activity (Physical); P3: Hearing Voices (when alone); P4: Feeling Threatened by Others; P5: Thinking People are Against You.
Comparison of symptom networks in cases of major depression with or without a family history of BD
The networks plots for major depression with FH of BD (n=40) or without a FH of BD (n=444) were generated using 19 self-report items (see Figures 3a and 3b). As shown, both the betweenness and edge weights are greater in the network of major depression without FH of BD than in network of major depression with FH of BD (although this may partly be a consequence of the x10-fold difference in subsample sizes). Interestingly, anergia (D12) shows high levels of betweenness and is quite centrally located in both plots.
Figure 3: Network Plots for unipolar depression with or without a family history of bipolar disorder (FH BD).
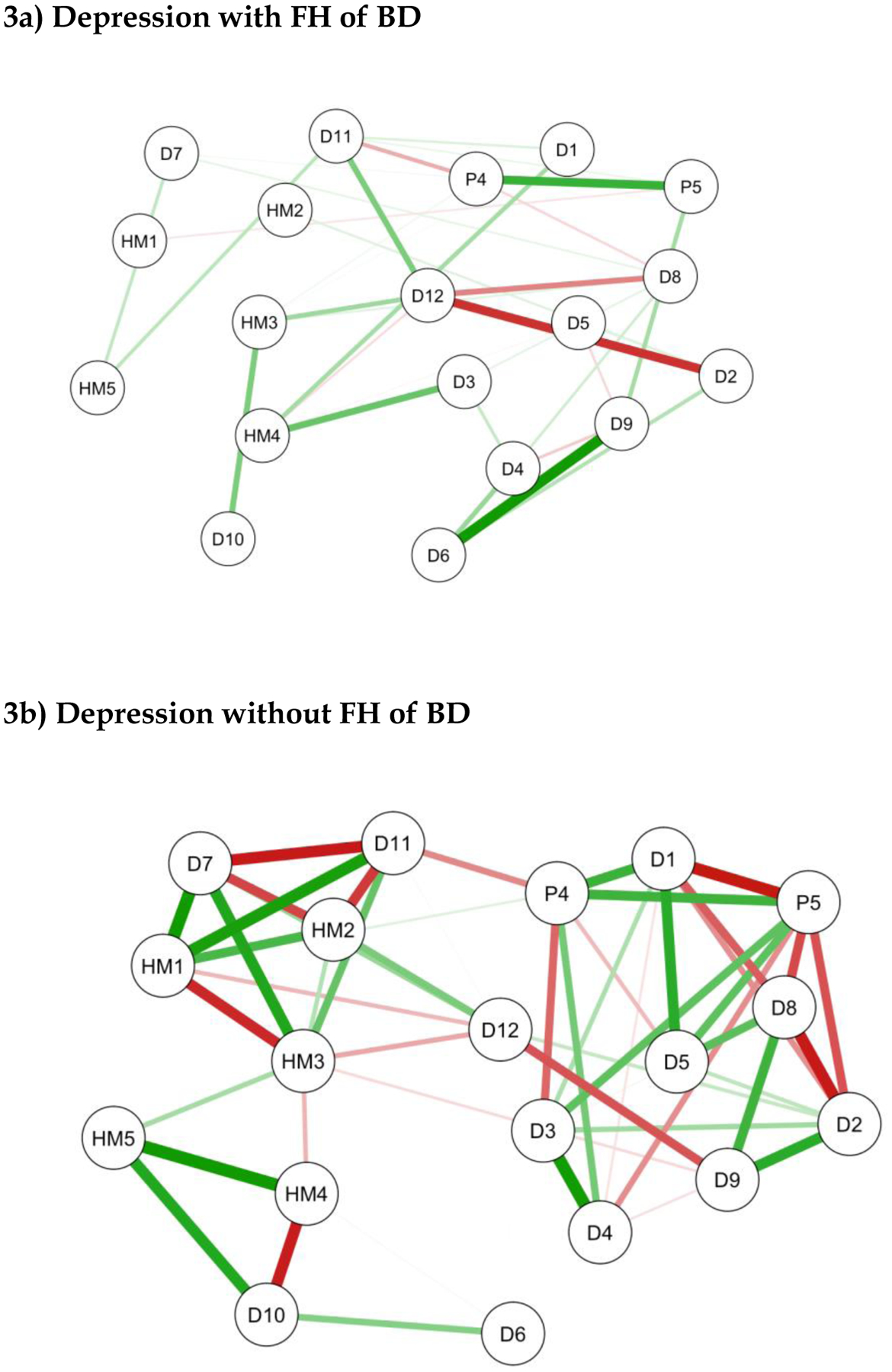
Brief note on the interpretation of network plots- items with stronger and/or more inter-connections are placed closer to each other and more centrally in the network. Green edges represent positive associations between nodes and red edges represent negative associations. The thickness of the connecting lines indicates the edge weight which reflects the strength of association.
KEY-
D1:Nervous/Tense; D2:Unhappy/Depressed; D3:Feel Stressed; D4:Feel Overwhelmed; D5:Lost Confidence; D6:Hopelessness; D7:Somatic Pain; D8:Hypersomnia; D9:Fatigue; D10:Poor Sleep Quality; D11:Poor Concentration; D12:Anergia; HM1: Feeling Elated; HM2: Increased Self-Confidence/Self-Esteem; HM3: Need Less Sleep; HM4: Increased Psychomotor Speed (Speech); HM5: Increased Activity (Physical); P4: Feeling Threatened by Others; P5: Thinking People are Against You.
Four PLE items (P1, P2, P3 and P6) were automatically excluded from the network analysis.
Other comparisons of these two networks are easier to interpret by inspecting the centrality plot shown in Figure 4. The right-hand column on Degree indicates that five DLE and two HMLE symptoms differentiate the ‘FH of BD (positive)’ network from the ‘no FH of BD’ network, with the four key variables being: anergia, psychomotor speed, impaired concentration, and hopelessness (for centrality measures for each variable see Table 2S in Appendix 3). Although there was a trend towards statistical differences in network structure, the network comparison test was not significant. Furthermore, the centrality indices for this analysis are less stable (see Figures 3S and 4S in Appendix 3). Given these indicators, the findings of this network analysis are less robust than the first model.
Figure 4:
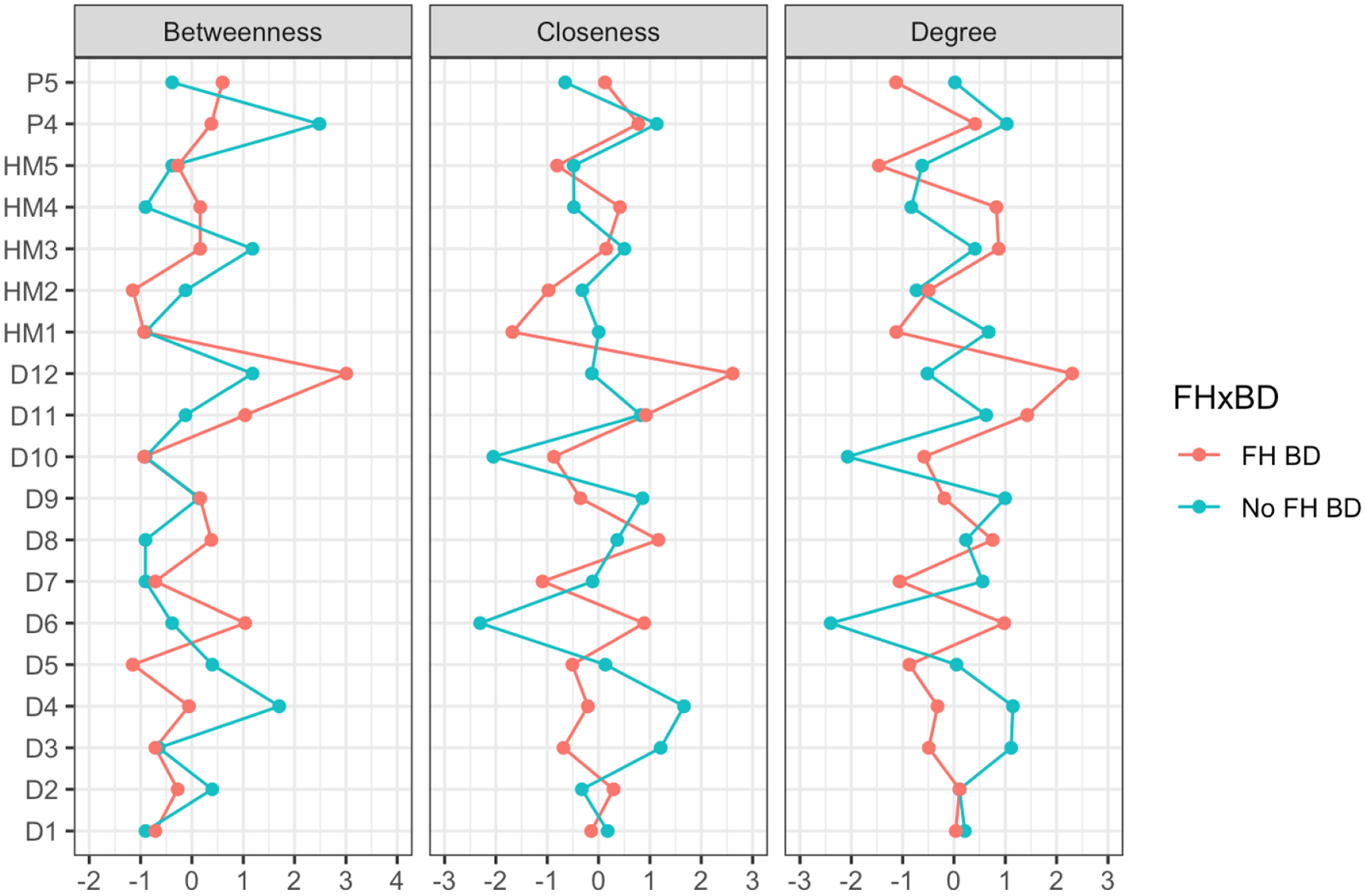
Centrality Plot for unipolar depression with or without a family history of bipolar disorder (FH BD/No FH BD)
KEY-
D1:Nervous/Tense; D2:Unhappy/Depressed; D3:Feel Stressed; D4:Feel Overwhelmed; D5:Lost Confidence; D6:Hopelessness; D7:Somatic Pain; D8:Hypersomnia; D9:Fatigue; D10:Poor Sleep Quality; D11:Poor Concentration; D12:Anergia; HM1: Feeling Elated; HM2: Increased Self-Confidence/Self-Esteem; HM3: Need Less Sleep; HM4: Increased Psychomotor Speed (Speech); HM5: Increased Activity (Physical); P4: Feeling Threatened by Others; P5: Thinking People are Against You.
Discussion
The current study is important for several reasons, not least because of the relative paucity of research on symptom networks in BD. First, we will consider the main clinical and research implications of this study for other BD projects. Then we note the study limitations and lastly, we consider whether there is any added value in employing network analysis in general psychopathology studies of youth.
Clinically, there are three findings reported here that we think shed light on the phenomenology of emerging BD. First, network analysis revealed that anergia and psychomotor speed are influential nodes for the symptom structure reported by groups targeted in this study, namely individuals with recent onset BD and those at above average risk of BD onset (i.e. those with major depression and a positive FH of BD). Further, it is worthwhile highlighting that our cohort were all recruited from the community rather than secondary care or specialist clinical settings (and so these findings are less influenced by so-called Berkson’s bias that may affect clinical studies). Second, when we compared recent onset BD to all cohort members who do not have a diagnosis of BD (i.e. >1300 youth, some of whom met diagnostic criteria for other mental disorders), we again found that anergia and psychomotor speed plus hypersomnia, (and probably less impairment in confidence) were the nodes in the symptom network that best differentiated between these subgroups. Additionally, the NCT showed that these network plots differed significantly from the one generated. Third, elation and increased self-esteem show a strong positive interconnection in this cohort of community-residing youth, but these two nodes do not appear to be any more influential in the symptom network for BD compared with non-BD network. We interpret this as meaning that, in this age group, these two symptoms do not specifically identify individuals with BD versus those without BD. This finding about elation and increased self-esteem contrasts markedly with the finding that activity/energy and sleep profile have a central role in symptom networks for BD in youth. However, it is not without precedent as it is supported by some of the results of the two previous studies of psychopathology in BD13,14. Furthermore, the agreement between studies is especially noteworthy given there were obvious differences in sampling frames, assessment tools and network models/metrics selected. Also, our findings about the importance of sleep-wake phenomena concur with results reported in community and offspring studies that use different analytic strategies36–39. If these findings are replicated, they will add support to the view that activation rather than mood state alone may be the core feature of BD and/or particularly important in adolescents and young adults. We believe this indicates these phenomena warrant greater consideration as treatment targets40. Lastly, we also note a notable difference in findings between our study and Weintraub et al14 was that the latter also found evidence that mood and irritability were important network symptoms. This may actually shed light on differences in network structures across BD subtypes as Weintraub and colleagues included a broader range of BD subtypes in their study (including spectrum disorders and BD NOS), whereas we included cases of BD I and II only.
Despite the encouraging agreement between the studies highlighted above, it is important to sound a note of caution about network analysis. This is a rapidly evolving field1, and whilst several experts have emphasized the enormous potential of this approach8, other respected researchers have emphasised that there are unresolved problems ranging from circularity of arguments about symptom connections, concerns about model selection, handling missing data, and uncertainties regarding reliability and replicability of models6,41–43. Many of these issues apply to other multivariate analytic models, but we acknowledge they may influence our and other studies. Research-wise, these concerns may be addressed by further studies, but also by greater understanding of the advantages and disadvantages of applying network analysis. For example, there is no consensus currently on which symptoms to examine in networks for BD and different approaches to these basic issues have been employed by the three psychopathology studies. In the network analysis undertaken by Corponi et al13, they included all the items representing the DSM IV criteria for depression in the network model plus a clinician rating of ‘mixed symptoms’ using a researcher-designed scale with unknown psychometric properties. Weintraub et al14 used a well-established observer rating of the presence and severity of core phenomenology of mania and depression, with symptoms assessed in a clinical sample selected for inclusion in two RCTs. In contrast, we included data about a similar total number of items but focused on self-ratings of symptoms but also included psychotic phenomena. Like Weintraub et al14, we assessed the potential influence of FH of BD, but we used a simpler and less reliable rating. Another potential limitation of the current study is the reliance on a cohort that included twins and non-twin siblings. Although we undertook some preliminary work to determine if the cohort data could be analyzed as singletons (see statistical section in Appendix 2), we of course recognize that our findings will require replication in a non-twin sample. Furthermore, some of the findings in the ‘at risk of BD’ subgroup must be treated with caution as some of the network analysis metrics suggest that the findings are less reliable. These and other limitations of the current study along with the other concerns previously outlined must be considered when comparing similarities and differences between published network analyses and warrant consideration when undertaking network analyses in the future.
The next question is whether there are any additional benefits in applying network analysis in studies of psychopathology in youth. Our view, after undertaking this study, is that this is a useful option for statistical, clinical, and conceptual reasons. For example, network analysis offers a more nuanced approach to other well-regarded non-parametric statistical procedures4, and the model does not rely on assumptions that hinder other statistical approaches (such as normality or sample size requirements, etc.)25. Clinically, it is especially helpful in exploring populations with high levels of psychiatric44,45 as network analysis focuses more on exploration of co-occurring symptoms and their relationships (rather than underlying causes of symptoms)46. We would also argue that, by using self-ratings, our study also recognizes the importance of what Hens et al46 refer to as ‘intentional information’ (about mental states) as conveyed by those with or without the disorder and that this better reflects the lived experience of the symptoms. Also, self-ratings avoid the risk that clinicians (or the assessment tools they use) impose a priori criteria for determining the presumed importance of selected symptoms47,48.
Conclusions
Recent decades have seen the emergence of robust evidence that the peak age range for onset of adult-pattern BD is about 15–25 years and furthermore that individuals with a major depressive episode and a FH of BD are at high risk of early transition BD. We selected subgroups representing recent onset BD and individuals at high risk of BD and employed network analyses to estimate the role and importance of self-rated symptoms in these subgroups as compared with other adolescents and young adults included the same study cohort. We identified that influential nodes that are common to networks for recent onset BD and at-risk individuals include anergia and psychomotor speed; results that are similar to those reported in two broadly comparable studies. These findings indicate that activity/energy (and possibly sleep-wake cycle) symptoms warrant further exploration as central features of emerging BD and deserve more attention as treatment targets.
Supplementary Material
Acknowledgements
We thank the participants and their families for engagement with this longitudinal study.
Funding
Funding information BLTS: National Health and Medical Research Council, Grant/Award Numbers (CI: Martin): 1031119, 1049911, APP10499110; National Institute of Health, Grant/Award Number: K99R00, R00DA02354 (CI: Gillespie).
This work was partially supported by grants from the National Health & Medical Research Council including: Centre of Research Excellence (No. 1061043) and Australia Fellowship (No. 511921) awarded to Prof Hickie.
BDC is supported by a CJ Martin Fellowship, funded by the NHMRC (1161356).
Footnotes
Data availability statement
The data that support the findings of this study were made available to authors via the BLTS research committee (that approved the cohort study). The authors confirm that the summary data for all variables supporting the findings of this study are included within the article and its supplementary materials. The raw data are being used at the lead research centres and form part of an ongoing programme of research and data are only made available upon written request to the BLTS research committee. Data are not publicly available due to confidentiality restrictions and because research participants did not give permission for dissemination beyond the BLTS research team.
Ethical statement
The authors assert that all procedures contributing to this work comply with the ethical standards of the relevant national and institutional committees on human experimentation and with the Helsinki Declaration of 1975, as revised in 2008.
SUPPLEMENTARY MATERIALS:
Appendix 1: STROBE checklist
Appendix 2: Overview of BLTS & further details of assessment tools & network analysis
Appendix 3: Additional network estimates: Supplementary Tables (n=2) & Figures (n=4)
Declarations/Conflicts of Interest
JS is a visiting professor at the Brain and Mind Centre and at Diderot University (Paris), the Norwegian University of Science and Technology (Trondheim) and is a “Science without Borders” fellow (Brazil). She has received grant funding from the UK Medical Research Council and from the UK NIHR Research for Patient Benefit programme; she declares no financial or other conflict of interests in relation to the topics addressed in this article.
KM reports serving as the leader of the collaborative network called mMARCH, which is coordinated by a work group of the National Institute of Mental Health.
IBH was a Commissioner in Australia’s National Mental Health Commission from 2012 to 2018. He is the Co-Director, Health and Policy at the Brain and Mind Centre (BMC) University of Sydney. IBH has previously led community-based and pharmaceutical industry supported (Wyeth, Eli Lily, Servier, Pfizer, AstraZeneca) projects focused on the identification and better management of anxiety and depression. He is a Board Member of Psychosis Australia Trust and a member of Veterans Mental Health Clinical Reference group. He is the Chief Scientific Advisor to, and an equity shareholder in, InnoWell. InnoWell has been formed by the University of Sydney and PwC to administer the $30M Australian Government Funded Project Synergy. Project Synergy is a 3-year program for the transformation of mental health services using innovative technologies.
Other authors declare no conflicts.
References
- 1.Barabasi A The network takeover. Nat. Phys 2011.8:14–16. [Google Scholar]
- 2.Blanken T, Deserno M, Dalege J, Borsboom D, Blanken P, Kerkhof G, Cramer A. The role of stabilizing and communicating symptoms given overlapping communities in psychopathology networks. Sci Rep. 2018April11;8(1):5854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Isvoranu AM, Guloksuz S, Epskamp S, van Os J, Borsboom D; GROUP Investigators. Toward incorporating genetic risk scores into symptom networks of psychosis. Psychol Med. 2020;50(4):636–643 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Scott J, Bellivier F, Manchia M, Schulze T, Alda M, Etain B; investigators involved in the ConLiGen collaboration. Can network analysis shed light on predictors of lithium response in bipolar I disorder? Acta Psychiatr Scand. 2020;141(6):522–533. [DOI] [PubMed] [Google Scholar]
- 5.Barrat A, Barthelemy M, Pastor-Satorras R, Vespignani A. The architecture of complex weighted networks. Proc Natl Acad Sci USA. 2004; 101:3747–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Epskamp S, Joost K, Maarten M. Estimating Psychopathological Networks: Be Careful What You Wish for. PLoS One 2017, 12 (6): E0179891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Borsboom D Psychometric perspectives on diagnostic systems. J Clin Psychol. 2008; 64:1089–108. [DOI] [PubMed] [Google Scholar]
- 8.Borsboom D A network theory of mental disorders. World Psychiatry. 2017February;16(1):5–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Koenders M, de Kleijn R, Giltay E, Elzinga B, Spinhoven P, Spijker A. A network approach to bipolar symptomatology in patients with different course types. PLoS One. 2015October27;10(10):e0141420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Galimberti C, Bosi M, Caricasole V, Zanello R, Dell Osso B, Vigano C. Using network analysis to explore cognitive domains in patients with unipolar versus bipolar depression: a prospective naturalistic study. CNS Spectr. 2020June;25(3):380–391. [DOI] [PubMed] [Google Scholar]
- 11.Strauss G, Esfahlani F, Kirkpatrick B, Allen D, Gold J, Visser K, Sayama H. Network Analysis Reveals Which Negative Symptom Domains Are Most Central in Schizophrenia vs Bipolar Disorder. Schizophr Bull. 2019October24;45(6):1319–1330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Curtiss J, Fulford D, Hofmann S, Gershon A. Network dynamics of positive and negative affect in bipolar disorder. J Affect Disord. 2019April15; 249:270–277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Corponi F, Anmella G, Verdolini N, Pacchiarotti I, Samalin L, Popovic D, et al. Symptom networks in acute depression across bipolar and major depressive disorders: A network analysis on a large, international, observational study. Eur Neuropsychopharmacol. 2020June; 35:49–60. [DOI] [PubMed] [Google Scholar]
- 14.Weintraub MJ, Schneck CD, Miklowitz DJ. Network analysis of mood symptoms in adolescents with or at high risk for bipolar disorder. Bipolar Disord. 2020March; 22(2):128–138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.von Elm E, Altman D, Egger M, Pocock S, Gotzsche P, Vandenbroucke J; STROBE Initiative. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies. Lancet. 2007October20; 370(9596):1453–7. [DOI] [PubMed] [Google Scholar]
- 16.Couvy-Duchesne B, O’Callaghan V, Parker R, Mills N, Kirk K, Scott J, et al. Nineteen and Up study (19Up): understanding pathways to mental health disorders in young Australian twins. BMJ Open. 2018. March17; 8(3): e018959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mitchell B, Campos A, Renteria M, Parker R, Sullivan L, McAloney K, et al. Twenty-Five and Up (25Up) Study: A New Wave of the Brisbane Longitudinal Twin Study. Twin Res Hum Genet. 2019June; 22(3):154–163. [DOI] [PubMed] [Google Scholar]
- 18.Scott J, Martin N, Parker R, Couvy-Duchesne B, Medland S, Hickie I. Prevalence of self-reported subthreshold phenotypes of major mental disorders and their association with functional impairment, treatment and full-threshold syndromes in a community-residing cohort of young adults. Early Interv Psychiatry. 2020. February12. [Epub ahead of print]. [DOI] [PubMed] [Google Scholar]
- 19.Kessler R, Abelson J, Demler O, Escobar J, Gibbon M, Guyer M, et al. Clinical calibration of DSM-IV diagnoses in the World Mental Health (WMH) version of the World Health Organization (WHO) Composite International Diagnostic Interview (WMHCIDI) Int J Methods Psychiatr Res. 2004; 13(2):122–139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Scott J, Davenport T, Parker R, Hermens D, Lind P, Medland S, et al. Pathways to depression by age 16 years: Examining trajectories for self-reported psychological and somatic phenotypes across adolescence. J Affect Disord. 2018April1; 230:1–6. [DOI] [PubMed] [Google Scholar]
- 21.Carpenter JS, Iorfino F, Cross S, Nichles A, Zmicerevska N, Crouse JJ, Palmer JR, Whitton AE, White D, Naismith SL, Guastella AJ, Hermens DF, Scott J, Scott EM, Hickie IB. Cohort profile: the Brain and Mind Centre Optymise cohort: tracking multidimensional outcomes in young people presenting for mental healthcare. BMJ Open. 2020March29;10(3):e030985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yung AR, Nelson B, Baker K, Buckby JA, Baksheev G, Cosgrave EM. Psychotic-like experiences in a community sample of adolescents: implications for the continuum model of psychosis and prediction of schizophrenia. Aust N Z J Psychiatry. 2009February;43(2):118–28. [DOI] [PubMed] [Google Scholar]
- 23.Hickie IB, Davenport TA, Hadzi-Pavlovic D. Development of a simple screening tool for common mental disorders in general practice. Med J Aust 2001;175(Suppl): S10–17. [DOI] [PubMed] [Google Scholar]
- 24.Milne B, Caspi A, Crump R, Poulton R, Rutter M, Sears M, Moffitt T. The validity of the family history screen for assessing family history of mental disorders. Am J Med Genet. 2009January5;150(1):41–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Epskamp S, Borsboom D, Fried EI. Estimating psychological networks and their accuracy: A tutorial paper. Behav Res Methods. 2018; 50(1):195–212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Epskamp S Dissertation: Network Psychometrics. Amsterdam: University of Amsterdam. 2016. Available at http://dare.uva.nl. [Google Scholar]
- 27.Cramer A, Waldorp L, van der Maas H, Borsboom D. Comorbidity: a network perspective. Behav. Brain Sci 2010, 33, 137–150. [DOI] [PubMed] [Google Scholar]
- 28.van Borkulo CD, Borsboom D, Epskamp S, Blanken TF, Boschloo L, Schoevers RA, Waldorp LJ. A new method for constructing networks from binary data. Sci Rep. 2014August1; 4:5918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Friedman J, Hastie T, Tibshirani R. Sparse inverse covariance estimation with the graphical lasso. Biostatistics. 2008;9(3):432–441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Csardi G, Nepusz T. The igraph software package for complex network research. Inter Journal Complex Syst. 2006;1695(5):1–9. [Google Scholar]
- 31.Fruchterman TM, Reingold EM. Graph drawing by force-directed placement. Softw: Pract Exper. 1991;21(11):1129–1164. [Google Scholar]
- 32.Boschloo L, van Borkulo CD, Borsboom D, Schoevers RA. A Prospective Study on How Symptoms in a Network Predict the Onset of Depression. Psychother Psychosom. 2016;85(3):183–4. [DOI] [PubMed] [Google Scholar]
- 33.Opsahl T, 2013. Triadic closure in two-mode networks: Redefining the global and local clustering coefficients. Social Networks 35, 32, 245–251. [Google Scholar]
- 34.van Borkulo C, Waldorp LJ, Boschloo L, Schoevers RA, Borsboom D. Statistical comparison of two networks with respect to global strength. 2015. Available from: https://github.com/cvborkulo/NetworkComparisonTest.
- 35.van Borkulo C, Boschloo L, Kossakowski JJ, Tio P, Schoevers R, Borsboom D. Comparing network structures on three aspects: a permutation test. 2016. Available from: https://www.researchgate.net/Claudia_Van_Borkulo/publication/314750838. [DOI] [PubMed]
- 36.Merikangas KR, Cui L, Kattan G, Carlson GA, Youngstrom EA, Angst J. Mania with and without depression in a community sample of US adolescents. Arch Gen Psychiatry. 2012September;69(9):943–51. [DOI] [PubMed] [Google Scholar]
- 37.Mesman E, Nolen W, Keijsers L, Hillegers M. Baseline dimensional psychopathology and future mood disorder onset: findings from the Dutch Bipolar Offspring Study. Acta Psychiatr Scand. 2017August;136(2):201–209. [DOI] [PubMed] [Google Scholar]
- 38.Shou H, Cui L, Hickie I, Lameira D, Lamers F, Zhang J, et al. Dysregulation of objectively assessed 24-hour motor activity patterns as a potential marker for bipolar I disorder: results of a community-based family study. Transl Psychiatry. 2017August22;7(8): e1211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Duffy A, Goodday S, Keown-Stoneman C, Grof P. The Emergent Course of Bipolar Disorder: Observations Over Two Decades from the Canadian High-Risk Offspring Cohort. Am J Psychiatry. 2019September1;176(9):720–729. [DOI] [PubMed] [Google Scholar]
- 40.Scott J, Murray G, Henry C, Morken G, Scott E, Angst J, Merikangas KR, Hickie IB. Activation in Bipolar Disorders: A Systematic Review. JAMA Psychiatry. 2017February1;74(2):189–196. [DOI] [PubMed] [Google Scholar]
- 41.Contreras A, Nieto I, Valiente C, Espinosa R, & Vazquez C The Study of Psychopathology from the Network Analysis Perspective: A Systematic Review. Psychother Psychosom, 2019. 1–13. [DOI] [PubMed] [Google Scholar]
- 42.Forbes M, Wright A, Markon K, Krueger R. Evidence that psychopathology symptom networks have limited replicability. Journal of Abnormal Psychology, 2017. 126(7), 969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.McNally RJ. Can network analysis transform psychopathology? Behav Res Ther. 2016November; 86:95–104. [DOI] [PubMed] [Google Scholar]
- 44.Krueger R, Markon K. Reinterpreting comorbidity: a model-based approach to understanding and classifying psychopathology. Annu Rev Clin Psychol. 2006. 2:111–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Iorfino F, Scott EM, Carpenter JS, Cross SP, Hermens DF, Killedar M, et al. Clinical Stage Transitions in Persons Aged 12 to 25 Years Presenting to Early Intervention Mental Health Services with Anxiety, Mood, and Psychotic Disorders. JAMA Psychiatry. 2019August28. 76(11):1167–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Hens K, Evers K, Wagemans J. Conceptualizing neurodevelopmental disorders as networks: Promises and challenges. Behav Brain Sci. 2019January; 42: e10. [DOI] [PubMed] [Google Scholar]
- 47.Costello E, Copeland W, Angold A. Trends in psychopathology across the adolescent years: what changes when children become adolescents, and when adolescents become adults? J Child Psychol Psychiatry. 2011. October;52(10):1015–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Wigman J, van Nierop M, Vollebergh WA, Lieb R, Beesdo-Baum K, Wittchen HU, van Os J. Evidence that psychotic symptoms are prevalent in disorders of anxiety and depression, impacting on illness onset, risk, and severity-implications for diagnosis and ultra-high-risk research. Schizophr Bull. 2012. March; 38(2):247–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


