Abstract
A participatory epidemiological study was conducted with cattle keepers in Jimma zone, Ethiopia, between October 2018 and October 2019 to identify the causes of abortion in cattle. Data collection involved 20 group discussions (each comprising 8–12 people) in 10 peasant associations. Methods used in group discussions included semi-structured interviews, pairwise ranking, matrix scoring, proportional piling, and seasonal calendar. The result of pairwise ranking identified brucellosis, leptospirosis, listeriosis, trypanosomosis, and Foot and mouth disease (FMD) in decreasing order as the most important causes of abortion in cattle. Mechanical or physical agents were also identified as less important non-infectious causes of cattle abortion in study areas. A very strong agreement (W = 0.880; P < 0.001) was observed among informant groups in pairwise ranking as to the most important cause of cattle abortion in study areas. Proportional piling showed that brucellosis was responsible for the highest proportion of abortions (39.9%) followed by leptospirosis (22.5%) and listeriosis (16.3%). A lesser proportion of abortion was attributed to trypanosomosis and FMD which comprise 11.6% and 9.7%, respectively. Matrix scoring showed strong agreement (W = 0.572 to 0.898; p < 0.001) concerning causes of abortion and its clinical signs between informant groups. According to the discussants, brucellosis and FMD tend to occur more frequently in the winter and spring seasons whereas listeriosis and trypanosomosis occurred frequently in the summer and autumn seasons, respectively. Strong agreement was observed among informant groups about the seasonal pattern of occurrence causes of abortion (W = 0.525–0.794; P < 0.001). Participants used medicinal plants and other traditional practices to manage cattle abortion in their areas. Farmers' knowledge should be incorporated to investigate health problems of unknown causes, designing, and implementing the intervention program in the areas.
Keywords: Abortion, Cattle, Participatory epidemiology, Jimma zone, Ethiopia
Abortion, Cattle, Participatory epidemiology, Jimma zone, Ethiopia.
1. Introduction
Abortion is an important cause of production losses in the dairy industry and has a significant negative impact on the reproductive efficiency of dairy cows (De Vries, 2006; Dinka, 2013). Like in many other countries, abortion is a major problem for dairy producers in Ethiopia (Regassa and Ashebir, 2016). Abortion is defined as the premature expulsion of the fetus between 42 days (the estimated time of attachment) and approximately 260 days of gestation (the age at which the fetus can survive outside of the uterus) (Peter, 2000).
Cattle abortion is caused by infectious and non-infectious agents (Hovingh, 2009; Tulu et al., 2018). These causes are global in distribution and of great concern to the dairy cattle (Pal, 2006). An infectious cause of abortion is an important reproductive disease of cattle, which may occur in sporadic as well as in epidemic form and is caused by diverse types of agents. Infectious causes of abortion in cattle include several groups of viruses, bacteria, protozoa, and fungus (Parthiban et al., 2015). Some of the infectious causes of cattle abortion such as brucellosis, leptospirosis, listeriosis, and Q fever in cattle have also public health significance (Pal et al., 2016; Tulu et al., 2018). Non-infectious causes of abortion can be classified as genetic and non-genetic factors. The most important non-genetic factors are heat stress, production stress, and seasonal changes (Hansen, 2002; Sani and Amanloo, 2007). Abortion can be also caused by non-infectious agents like chemical poisoning, drugs, hormones, nutritional disorders, and genetics disorder (Regassa and Ashebir, 2016).
In many developing countries investigating causes of abortion is a major challenge due to resource and technical limitations (Allport et al., 2005). In this kind of setting, possible causes of abortion can be investigated using Participatory epidemiology (PE) techniques (Catley, 2005; Thrusfield, 2005). Participatory epidemiology is a proven technique that overcomes many of the limitations of conventional epidemiological methods and has been used to elucidate the causes of abortion without laboratory diagnostics (Catley, 2006; Jost et al., 2007). Participatory epidemiology provides dairy practitioners with a set of tools that maximize the chance of successfully identifying and reporting the underlying cause of dairy herd abortion (Mark, 2002).
It is important to note that the causes of abortion in cattle are numerous and thus, their diagnosis often challenging (Murray, 2006; Ernest, 2009). The laboratory diagnosis of abortion is particularly difficult in Ethiopia due to the presence of most of the infectious and non-infectious causes of abortion and the lack of resources for laboratory investigation on these possible causes. Furthermore, there is an added challenge related to appropriate sample processing and also factors on the diagnostic performance of the available tests to accurately identify the causes. In the Ethiopian setting where there are very limited veterinary diagnostic facilities, the use of PE methods could improve our understanding of potential causes of abortion in cattle. Participatory epidemiologists recognize that local people have very rich and have detailed ethnoveterinary knowledge about the cattle they keep and the characteristic of health problems that affect their cattle (Jost et al., 2007).
Pregnant cattle are the most important and promising part of the herd structure both for milk production and for calving. Thus, farmers closely follow pregnant cows and sometimes separate them from the herd and keep them around the homestead for proper care. Thus, if abortion happens farmers usually have a full history of what happened before and after the abortion making this topic ideal for a PE study. Furthermore, animal health workers in the zone always appreciate and value the livestock owners' knowledge on animal health as they always help them figure out animal diseases (including outbreaks) and their drivers in the areas (field veterinarians, personal communication). Therefore, the authors believe that a cattle producer's involvement in the initial investigation of the causes of abortion could help narrow down the most probable causes of abortion and paves way for laboratory-based diagnostic work.
Several studies indicated that abortion is one of the most frequent cattle reproductive health problems in different parts of Ethiopia (Tesfaye and Shamble, 2013; Benti and Zewdie, 2014; Regassa and Ashebir, 2016). There is widespread cattle abortion in the Jimma zone that compromises health and production. Abortion commonly occurs in dairy cattle of Jimma zone: 1041 cases in 2013, 1534 cases in 2014, and 5456 cases in 2015 were reported from various districts (Tulu, 2018). These figures only include official case reports from some peasant associations which have access to animal health workers. This implies the actual figure is expected to be very high. The local veterinary authorities were alarmed by an increasing trend of abortion episodes in cows in the past three consecutive years and approached Jimma university to help identify the possible causes of abortion.
This study was done to generate evidence on the importance and causes of abortion in the cows in the areas. The evidence can inform policy and interventions aimed at reducing the impacts of abortion. Therefore, the present study was designed to assess how farmers associate different disease syndromes with abortion, describe the seasonality of these syndromes, and describe the relative incidence of these syndromes using participatory epidemiology techniques in Limu Seka and Chora Boter districts of Jimma zone.
2. Materials and methods
2.1. Ethics statement
The research protocol was approved by the Jimma University School of Veterinary medicine, college of Agriculture, and Veterinary medicine ethic committee with the AgVmVM/16/1 reference number. All activities involving human participants have followed the ethical standards of protecting the right and welfare of participants. Participants were provided with verbal information on the aim of the study. Informed consent of participants was verbally obtained before commencement of each section of participatory exercise in every community and none declined participation.
2.2. Study areas
Limu Seka district is located at an altitude of 1400–2200 m above sea level, 09°29′ North latitude, and 37°26′ East longitudes. Its agro-ecology is characterized by 13% highland and 55% mid-highland and 32% lowland. The average temperature varies from a minimum of 15 °C to a maximum of 31 °C. There are two distinct seasons in Limu Seka: the rainy season (from late March to October), and the dry season (November to early March). The rainfall is often more than 1,800 mm per annum. Limu Seka district has 295,627 cattle, 104,892 sheep, 89,079 goats and 134,370 human populations (CSA, 2017). Local cattle breeds (Horro and Guraghe breeds) are the most dominant ones followed by some crosses of Holstein-Friesian. The management systems of the study area are extensive and semi-intensive (in urban areas).
Chora Boter district is located at 9°–10°24′ North latitude and 37°56′-40° 35′ East longitude with an altitude range of 1100–2200 m above sea level. The agroecology is characterized by 25% highland, 73.5% mid-highland, and 2.3% lowland. The annual average temperature ranges from 18.3 °C to 26.7 °C. Similar to the Limu Seka district, the district has two seasons. The rainfall is often more than 1,800–2,200 mm per annum. Chora Boter district has 228,846 cattle, 47,854 sheep, 68,037 goats and 215,348 human populations (CSA, 2017). The management system of the area is extensive (crop-livestock production) and semi-intensive (urban production) systems. Local cattle breeds (Horro and Guraghe breeds) are the most dominant ones followed by some crosses of Holstein-Friesian.There is no substantial difference in cattle production between the two districts and also between the highland and the mid-highland areas (Figure 1).
Figure 1.
Map showing the study areas (Limu Seka and Chora Boter districts).
2.3. Study population
Target populations were female cattle in Limu Seka and Chora Boter districts of Jimma zone whereas the study population was breeding cows and households who keep cattle in selected peasant associations of the study districts.
2.4. Participatory epidemiology methods
Participatory epidemiological methods were applied to identify (based on cattle owner's knowledge) and prioritize the potential causes of abortion. The participatory epidemiological methods used include a semi-structured group interview, pair-wise ranking, proportional piling, matrix scoring, and seasonal calendar (Catley et al., 2001; Catley, 2005).
A semi-structured group interview was carried out by modifying the method described by Bellet et al. (2012). A prepared checklist (open-ended questions) that captures major aspects of the study objectives were used to guide the discussion. The participants (n = 234) were asked to list the most frequent cause of abortion in cattle encountered in the last year. They mentioned the local names of the diseases and described the clinical signs associated with each cause of abortion. The questions were open-ended to allow participants the opportunity to introduce topics and issues. Probing questions were used throughout the PE techniques to get detailed information on the topic of discussions and to check the consistency of information provided by other discussants. Each group interview or focus discussion (n = 20) session was run for approximately 2 h during which four activities were completed. We have ensured all participants in the discussion group had an opportunity to express their opinions, and that the discussion was open and not dominated by one or a few individuals. Participants were given enough time to discuss and reach a consensus. The facilitators followed the topic guide while being sensitive to participants’ wishes to express concerns and comments outside this frame, and ensured that the discussion was not dominated by one or a few individuals. Notes were taken on all group meetings.
2.4.1. Pairwise ranking
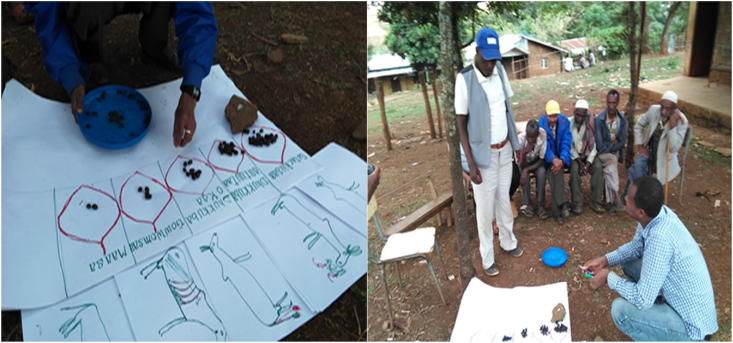
This method was used to identify the most important or frequent causes of abortion in cattle as perceived by the farmers. The diseases or causes of abortion mentioned by participants were introduced into a discussion with their local names. The local names of the disease were translated to their English equivalent with the help of the local veterinary service provider, veterinary clinical records, and the description given by the farmers of each disease. Major animal disease in the areas has an established local name given to it based on the clinical signs, its causes, or risk factors. Farmers have described how one disease differs from the other based on the clinical signs which all causes of abortion were line up with a veterinary textbook. For instance, when the local farmers mentioned that their cows are suffering from ‘Dhukkuba Hantuuta’ (literally translated as ‘disease of rats’) the veterinary service providers suspect that the pathogen associated was Leptospira species. Besides, the causes of abortion were also represented by pictures. The pictures were drawn by participants using color pens showing signs of the different causes of abortion. The pair-wise ranking was done by first listing the potential causes of abortion on cardboard vertically (y-axis) and horizontally (x-axis), and comparing two of them at a time until all the causes of abortion had been compared against each other (Catley et al., 2002). The final rank was recorded by counting the number of times a given cause of abortion was selected first over the other and ranked based on the number of times a given cause of abortion is prioritized over the others (Figure 2). The method was repeated in twenty different groups.
Figure 2.
Pairwise ranking by informant group in study areas.
2.4.2. Matrix scoring
The matrix scoring was adapted from the methods described by Catley (2005). This method was used to assess whether the participants can identify the different diseases implicated as causes of abortion based on a list of clinical signs. A matrix was drawn on a sheet of cardboard and causes of abortion were represented by pictures and placed along the top X-axis of the matrix. Each cause of abortion in the matrix scored against a list of clinical signs was illustrated along the Y-axis of the matrix. Five stones per cause of abortion were used to identify the differences easily (to count the stone easily) (Byaruhanga et al., 2015). Participants were asked to score each cause of abortion by dividing 25 piles of stone against the clinical signs. The matrix was discussed, agreed upon and the scores were recorded. The method was repeated in twenty different groups and scores were summarized using the median, minimum, and maximum scores (Figure 3).
Figure 3.
Matrix scoring by informant group in study areas.
2.4.3. Proportional piling
Proportional piling was employed to estimate the relative frequency of abortion related to five top infectious causes of abortion that occurred in cattle during the past two years as previously identified in the pair-wise ranking. Circles were drawn on cardboard representing every cause of abortion. The most important potential causes of abortion in cattle were selected by the participants. They were provided with 100 stone counters to allocate into the circles according to the relative frequency of occurrence of abortion related to each disease (Catley et al., 2014). When placing the stones against the cause of abortion was completed, the groups were requested to thoroughly check the scores and if they want, rearrange the scores until all of them agree on the score (Figure 4). Similar exercises were conducted with twenty different focus groups and score recorded based on their final scores. At the end of each activity, stones were counted and recorded, and pictures were taken.
Figure 4.
Proportional piling by the informant group in study areas.
2.4.4. Seasonal calendar
Seasonal calendars were used to describe the seasonal occurrences of causes of abortion identified in the matrix scoring as described by Catley et al. (2002). The methodology for constructing a seasonal calendar was similar to matrix scoring. The season's local name (Afan Oromo) ‘Birraa’ (autumn), ‘Bona’ (winter), ‘Arfaasaa’ (spring), and ‘Ganna’ (summer) were listed horizontally (x-axis) on a piece of cardboard and causes of abortion were listed vertically (y-axis). Also, the season's local name and causes of abortion were represented by pictures. Five stones were used per season. For each cause of abortion, discussants were asked to score each cause of abortion by dividing 20 piles of stone against season to show the seasonal pattern of the abortion (Figure 5). This method was repeated in twenty different groups and scores were summarized using the median, minimum, and maximum scores.
Figure 5.
Seasonal calendar by informant group in study areas.
2.5. Sampling procedure and approaches
The study districts were selected purposively based on a history of abortion reported to the district veterinary departments. A simple random sampling technique was used to select the peasant associations (PA). PA also known as Kebele is the smallest administrative unit in Ethiopia. Limu Seka has 19 PAs whereas Chora Boter has 16 PAs. Six and four PAs were purposively sampled from Limu Seka and Chora Boter districts, respectively based on the history of abortion cases. A participatory epidemiological study involving a combination of group discussions, pair-wise ranking, proportional piling, matrix scoring, and the seasonal calendar was carried out in selected PAs. Two group discussions were held in each peasant associations with different people in each discussion. A total of 234 discussants was selected purposively from households who have cattle and volunteer to participate in the study. A total of 20 focus groups with 8–12 persons in each group were used in the discussion. Stones were used as counters for scoring purposes. Animal health assistants, one veterinarian, and one traditional healer (veterinary healer) were selected from each PA of the study districts as the key informants. Key informant interviews regarding the selection of group discussants and also describing local names for the common causes of abortion were held at each PA before the actual days of group discussion. Triangulation was done by probing and examining consistency in response and characteristics of the causes of abortion using all the methods used in the study. The local names of the diseases and the clinical signs mentioned by the informants were cross-checked with the district veterinarians, who are familiar with the local disease status, naming, and description of the diseases. In addition, the description provided by the informants on each cause of abortion was collated with and compared with previous clinical findings and laboratory diagnoses made in the areas and then with the available statistical records and relevant literature.
2.6. Data management and analysis
Data obtained from participatory epidemiological techniques results were recorded and stored in Microsoft® Excel for Windows 2010 and transferred to Statistical Package for the Social Sciences (SPSS) version 20.0 (IBM SPSS, 2011). The level of agreement between informant groups was assessed using Kendal's coefficient of concordance (W). Evidence of agreement between informant groups was categorized as weak (W < 0.26; P > 0.05), moderate (W = 0.26–0.38, P < 0.05), and strong (W > 0.38, P < 0.01 to 0.001) according to published guidelines on the interpretation of W (Siegel and castellan, 1998) and the P-values assigned to W.
3. Results
3.1. Demographics of participants
The age of the participants ranged between 23 and 75 years with an average mean age (mean ± SD) of 48.43 ± 16.17 years. The majority of the participants (81.2%) were males. The majority of participants (37.2%) in study areas had attended elementary school (Table 1).
Table 1.
Demographic characteristic of participants in study areas.
| Variables | Limu Seka (n = 139) | Chora Boter (n = 95) | Overall (n = 234) |
|---|---|---|---|
| Age (mean ± SD) |
49.87 ± 17.20 |
47.48 ± 15.83 |
48.43 ± 16.17 |
| Sex | |||
| Male | 115 (82.7%) | 75 (78.955%) | 190 (81.2%) |
| Female |
19 (20.0%) |
25 (26.32%) |
44 (18.8%) |
| Educational level | |||
| Illiterate | 36 (25.9%) | 31 (32.6%) | 67 (28.6%) |
| Read and write | 23 (16.6%) | 12 (12.6%) | 35 (15.0%) |
| Elementary (1–8) | 51 (36.7%) | 36 (37.9%) | 87 (37.2%) |
| Secondary school | 20 (14.4%) | 9 (9.5%) | 29 (12.4%) |
| College graduated | 6 (4.3%) | 5 (5.3%) | 11 (4.7%) |
| University graduated | 3 (2.2%) | 2 (2.1%) | 5 (2.1%) |
3.2. Focus group discussion and semi-structured interviews
The common causes of abortion in cattle were given literal meanings in local language which correspond to specific disease entities. Brucellosis was recognized by cattle keepers as ‘Gatachiisa’ (contagious abortion). The participants mentioned that brucellosis is typically characterized by retained fetal membrane and infertility. Leptospirosis, identified as another cause of abortion was known by cattle keepers as ‘Dhukkuba Hantuuta’ (rat disease), and they associated the disease with the presence of rats in stored feed. The participants characterize the disease through coffee/dark colored urine and yellowish discoloration of eyes. Listeriosis which the farmers locally called ‘Dhukkuba Hokaa’ (hay disease) was mentioned as another cause of abortion in the areas. The participants mentioned that the disease causes the animals to move in circles. Trypanosomosis was known locally as ‘Gowwoomsaa’ (deceiver) which was incriminated as one of the causes of abortion by the participants was associated with the bite of flies and characterized by loss in body weight and loss of tail hair. Foot and mouth disease (FMD) is called locally as ‘Maasa' to mean sowing disease which is characterized by salivation, lameness, and vesicles on feet and mouth. Blackleg was locally called 'Gubaa' (burn disease)/‘Abbaa Gorbaa’ which was characterized by lameness and swelling of the hind leg. Diarrhea (‘Garaa-Kaasa’), mechanical or physical (‘Rukuttaa’), seasonal change, and genetic disorder (‘Michii’/‘Umaaman’) also reported as causes of abortion in cattle (Table 2).
Table 2.
Local, English, and conventional names of infectious causes of cattle abortion with its clinical signs in study areas.
| Local name (Afan Oromo) | English name | Conventional medical name | Clinical signs | Number of respondent (%) |
|---|---|---|---|---|
| Gatachiisa | Contagious abortion | Brucellosis | Retained fetal membrane, infertility | 113 (48.29) |
| Dhukkuba Hantuuta | Rat disease | Leptospirosis | Dark/coffee color urine, yellowish discoloration of the eye | 40 (17.09) |
| Dhukkuba Hokaa | Hay disease | Listeriosis | Moving in circle | 31 (13.25) |
| Gowwoomsaa | Deceiver | Trypanosomosis | Loss of body weight, loss of tail hair | 20 (8.55) |
| Maasa | Sowing disease | Foot and mouth disease | Salivation, lameness, vesicle on feet and mouth | 11 (4.70) |
| Gubaa/Abbaa Gorbaa | Burn disease | Blackleg | Lameness, swelling of the hind leg | 9 (3.85) |
| Garaa-Kaasa | Diarrhea | Diarrhea, fever | 4 (1.71) | |
| Rukuttaa | Mechanical/physical | Mechanical/physical | Wound on abdomen | 4 (1.71) |
| Michii/Umaaman | Seasonal change/genetic | Seasonal change/genetic | Aborted without any signs | 2 (0.85) |
3.3. Management of causes of abortion in cattle
All participants indicated that they were treating the cause of abortion in cattle. Oxytetracycline was the drug most frequently used and it was used for different infectious causes of abortion. All participants in two districts showed that they used a 20% concentration of oxytetracycline and some of them (n = 62) also used 10% concentration. From our observation, oxytetracycline was more accessible and low-cost than other drugs and used by the cattle keepers to have a broad spectrum of activity against the various cause of cattle abortion. Some participants (n = 53) were used penicillin-streptomycin formulation for treating infectious causes of abortion in cattle. The participants (n = 68) also indicated that diminazene aceturate was used to treat trypanosomosis in their areas. The majority of participants have also used oxytetracycline and diminazene aceturate as prophylactic drugs. The participants differentiated the drugs by the color of the bottle and the price of the drugs. Identify of the drugs was obtained through probing, combined with the veterinarian and key informants. The cattle keepers did not necessarily take into account the weight of the cattle and the dose regimen was not properly followed. Medicinal plants were also reported as a treatment of the cause of abortion in cattle. These included Salvadora persica for brucellosis, Vernonia amygdaline for foot and mouth disease, Ricinus communis and Allium sativum for blackleg, and Ocimum lamiifolium for trypanosomosis. The medicinal plants were also used for prevention of abortion in cattle. Moreover, the burning of the swelling part of the cattle with a hot iron was also another method used by some participants (n = 31) to treat the blackleg.
3.4. Participatory epidemiology methods
3.4.1. Pair-wise ranking
Pair-wise ranking indicated that brucellosis is the most important cause of abortion and leptospirosis and listeriosis is the second and third most important cause of abortion in study areas, respectively (Table 3).
Table 3.
Pairwise ranking causes of abortion in cattle in study areas.
| Cause of abortion | |||||
|---|---|---|---|---|---|
| Scientific name | Local name | Number of groups | Total score | Average score | Overall rank |
| Brucellosis | Gatachiisa | 20 | 106 | 5.3 | 1 |
| Leptospirosis | Dhukkuba Hantuuta | 20 | 77 | 3.9 | 2 |
| Listeriosis | Dhukkuba Hokaa | 20 | 73 | 3.7 | 3 |
| Trypanosomosis | Gowwoomsaa | 20 | 50 | 2.5 | 4 |
| FMD | Maasa | 20 | 28 | 1.4 | 5 |
| Blackleg | Gubaa | 10 | 12 | 1.2 | 6 |
Kendall's coefficient of concordance (W) for informant groups for causes of abortion indicated strong agreement in districts (W = 0.880, P < 0.001, n = 20).
3.4.2. Proportional piling
A hundred stone counters were given to each informant group to prioritize the important causes of cattle abortion. Accordingly, brucellosis (39.9%) and leptospirosis (22.5%) were mentioned as the most frequent causes of abortion in study areas (Table 4). There was a strong agreement between the 20 informant groups (W = 0.942; P < 0.001).
Table 4.
Median scores of incidence of cattle abortion estimates for five top causes of abortion in study areas.
| Cause of abortion |
Median scores for abortion |
|||
|---|---|---|---|---|
| Scientific name | Local name | Limu Seka | Chora Boter | Overall incidence (%) |
| Brucellosis | Gatachiisa | 40.5 (34, 47) | 39.3 (30, 45) | 39.9 |
| Leptospirosis | Dhukkuba Hantuuta | 22.5 (16, 29) | 22.5 (19, 26) | 22.5 |
| Listeriosis | Dhukkuba Hokaa | 15 (11, 19) | 17.5 (16, 19) | 16.3 |
| Trypanosomosis | Gowwoomsaa | 11.9 (5, 15) | 11.1 (5,14) | 11.6 |
| FMD | Maasa | 8.5 (2, 13) | 11.5 (7, 16) | 9.7 |
Results obtained by incidence scoring technique (number of informant groups = 20), the number outside the bracket represents medians, and minimum and maximum values are in the bracket.
3.4.3. Matrix scoring
The result of the matrix scoring indicates that there was strong agreement (W = 0.572 to 0.898; P < 0.001) among the informant groups for all the causes of abortion and their clinical signs. Brucellosis received the highest score for retained fetal membrane and infertility signs whereas leptospirosis received the highest score for coffee/dark colored urine and yellowish discoloration of eyes. Listeriosis received the highest score for circling sign. FMD got the highest score for salivation, lameness, and vesicles on feet and mouth (Table 5).
Table 5.
Matrix score of abortion-causing diseases and clinical signs in 20 different focus groups.
| Clinical signs | W | Median score (range) |
||||
|---|---|---|---|---|---|---|
| Brucellosis | Leptospirosis | Listerosis | Trypanosomosis | FMD | ||
| Bodyweight loss | 0.895∗∗∗ | 3 (0–7) | 2 (0–5) | 2 (1–4) | 17.5 (14–21) | 5 (3–7) |
| Loss of tail hair | 0.898∗∗∗ | 4 (0–7) | 3 (0–5) | 4 (1–5) | 17 (14–18) | 2 (3–6) |
| Vesicle on foot and mouth | 0.627∗∗∗ | 2 (1–3) | 7 (5–9) | 6 (1–7) | 1 (0–6) | 10 (7–11) |
| Salivation | 0.789∗∗∗ | 0 (0–0) | 0 (0–0) | 0 (0–1) | 0 (0–2) | 25 (22–25) |
| Lameness | 0.576∗∗∗ | 0 (0–2) | 0 (0–3) | 0 (0–3) | 0 (0–2) | 25 (19–25) |
| Circling | 0.727∗∗∗ | 0 (0–0) | 0 (0–2) | 25 (20–25) | 0 (0–3) | 0 (0–3) |
| Coffee colored urine | 0.821∗∗∗ | 0 (3–8) | 16 (14–17) | 2 (0–3) | 2 (0–5) | 1 (0–3) |
| Retained Placenta | 0.572∗∗∗ | 19 (15–22) | 2 (1–4) | 1 (0–3) | 3 (1–5) | 0 (0–2) |
| Infertility | 0.576∗∗∗ | 16 (13–21) | 3 (1–4) | 2 (0–4) | 2 (1–6) | 2 (0–4) |
| Yellowish color of eyes | 0.637∗∗∗ | 0.5 (0–3) | 19 (16–23) | 1 (0–3) | 3 (1–5) | 1 (0–2) |
W Kendall's coefficient of concordance for median (W < 0.26 = weak, 0.26 < W < 0.38 = moderate, W > 0.38 = strong) ∗P < 0.05, ∗∗P < 0.01, ∗∗∗P < 0.001, the number outside the bracket represents medians, and minimum and maximum values are in the bracket (Tables 5 and 6).
3.4.4. Seasonal calendar
The informant groups divided a year into autumn (Birraa), winter (Bona), spring (Arfaasaa), and summer (Ganna) seasons. Strong agreement was seen among 20 informant groups about the seasonal occurrence of brucellosis, leptospirosis, listeriosis, trypanosomosis, and FMD (W = 0.525–0.794; P < 0.001) diseases (Table 6).
Table 6.
Seasonal occurrence of different causes of abortion in cattle in study areas (Spring and Summer are wet seasons whereas Autumn and Winter are dry seasons in Ethiopia).
| Seasons |
W | Median score (range) |
|||||
|---|---|---|---|---|---|---|---|
| English names | Local names | Brucellosis | Leptospirosis | Listerosis | Trypanosomosis | FMD | |
| Autumn | Birraa | 0.525∗∗∗ | 3 (1–8) | 2.5 (0–5) | 2 (0–9) | 7 (5–9) | 4 (0–14) |
| Winter | Bona | 0.794∗∗∗ | 9 (5–12) | 8 (6–13) | 4 (1–6) | 5.5 (4–9) | 7.5 (0–12) |
| Spring | Arfaasaa | 0.683∗∗∗ | 6.5 (2–9) | 6 (4–7) | 5 (2–8) | 4 (3–6) | 5.5 (0–18) |
| Summer | Ganna | 0.752∗∗∗ | 2 (0–3) | 3 (0–4) | 9 (3–16) | 3 (1–3) | 2 (0–6) |
4. Discussion
Abortion is the most frequent cattle reproduction problem in Ethiopia. For instance, it has been reported as widespread in the Jimma zone, compromising cattle health and production (Regassa and Ashebir, 2016; Deresa et al., 2020). However, the investigation of the causes of abortion has always been challenging in developing countries like Ethiopia mainly due to resource limitations for laboratory diagnostic. The present study used a participatory epidemiological tool to identify and prioritize the causes of cattle abortion in the Jimma zone. Thus, the use of participatory epidemiology helps narrow-down to the most likely causes involved in abortion in cattle in the study areas.
In the present study, the five major diseases incriminated as causes of abortion by participants were brucellosis, leptospirosis, listeriosis, trypanosomosis, and FMD. Strong agreement was observed among focus groups for all causes of abortion and clinical signs (W = 0.572 to 0.898; P < 0.001). This indicates that discussants were knowledgeable and able to describe the diseases commonly resulting in abortion based on their clinical characterization in study areas. The clinical signs listed for each cause of abortion were consistent with the standard veterinary literature (Radostits et al., 2007).
Brucellosis was indicated as the most important cause of abortion in cattle in two districts by pair-wise ranking. The participants mentioned retained fetal membrane and infertility as a typical clinical feature for the disease which concurs with veterinary textbooks (Radostits et al., 2007). This finding is in line with some previous studies that have reported brucellosis as an endemic and widespread cause of abortion in Ethiopia (Pal et al., 2016) and Nigeria (Elelu et al., 2016). However, previous studies conducted on the seroprevalence of brucellosis in the Jimma zone reported a low prevalence (Tolosa et al., 2008; Ibrahim et al., 2010; Bashahun et al., 2015).
The participants ranked leptospirosis as the second most important cause of abortion in the study areas. They characterized the disease with coffee/dark-colored urine (hemoglobinuria) and yellowish discoloration of the eyes which is consistent with clinical signs reported in the textbook (Radostits et al., 2007). Similarly, leptospirosis was stated as the second important cause of abortion in cattle in Algeria (Derdour et al., 2017). Leptospirosis has been reported in Ethiopian cows (Moch et al., 1975) as cited in Yadeta et al. (2016) and is also one of the five priority zoonotic diseases identified in the country (Pieracci et al., 2016). Elelu et al. (2016) from Nigeria also reported leptospirosis as one of the common causes of abortion in cattle using participatory tools. The third important disease mentioned as a cause of abortion was listeriosis. According to the participants, the main clinical sign of the disease is circling which is consistent with veterinary literature (Radostits et al., 2007) as the bacteria that cause listeriosis damages the central nervous system.
Participants ranked trypanosomosis as the fourth important cause of abortion in study districts. The common clinical signs mentioned by participants (bodyweight loss and loss of tail hair) are consistent with the disease (Radostits et al., 2007). The seasonal flare-up of trypanosomosis maybe leads to abortion in pregnant cows due to fever induced by the parasite. This concurs with a previous study carried out by Jittapalapong et al. (2009) that reported protozoan pathogens such as trypanosomosis could cause abortion in cows. In addition, our results also agree with previous studies conducted in Ethiopia (Shimelis et al., 2005; Tesfaye et al., 2011; Seyoum et al., 2013), Kenya (Catley and Irungu, 2000; Machila et al., 2003), Tanzania (Catley et al., 2004) and Nigeria (Elelu et al., 2016).
FMD was the least frequent disease associated with abortion in the study areas. Salivation, lameness, and vesicle on foot and mouth were the clinical presentation of the diseases reported by participants which are consistent with veterinary literature (Radostits et al., 2007).
A strong agreement was seen among participants groups about the seasonal occurrence of abortion (W = 0.525–0.794; P < 0.001). According to the discussants, brucellosis was reported to occur during the winter and spring seasons. This could be due to the free movement of animals in search of feed and water that potentially increases the chance of contact between infected and susceptible animals. This in turn creates a favorable condition for the transmission of Brucella organism among animals. A similar observation on the seasonal pattern of brucellosis occurrence was reported in Sudan by Catley et al. (2002) and in Nigeria by Elelu et al. (2016).
According to the participants, leptospirosis tends to occur in all seasons with slightly less frequency in autumn and more frequency in the rainy season. This could be due to the availability of stored feed (hay) in all seasons, which may allow rodents reservoirs of leptospirosis to breed and contaminate cattle (Tilahun et al., 2013; Tulu, 2020). This result is in line with the finding of Elelu et al. (2016), who reported leptospirosis occurred year-round and high during the rainy season in Nigeria.
In this study, most of the participants agreed that listeriosis was more common in the summer season. The participants' observation concurs with Radostits et al. (2007), who stated the seasonal pattern occurrence of listeriosis is related to stored forage and silage feeding to cattle. The farmers in the study area also mentioned that during the rainy season (summer) they feed stored grass to their animals. Thus, listeriosis frequently occurs in this season as the farmers may provide inadequately fermented feed (pH above 5.0 to 5.5) to their cattle that allows the multiplication of the pathogen (Husu et al., 1990).
The participants stated that abortion caused by trypanosomosis was more frequent during the autumn season. This could be due to the increase in tsetse fly density and the parasite challenge during the late rainy season in the study areas. Several authors in Ethiopia (Chernet et al., 2004; Shimelis et al., 2005; Tesfaye et al., 2011; Rundassa et al., 2013; Seyoum et al., 2013) and Kenya (Catley and Irungu, 2000) also reported the increase in tsetse fly density and trypanosomes challenges in the late rainy season.
Abortion caused by FMD was reported to occur more commonly during the winter and spring seasons. This result is consistent with the finding of Rafael et al. (2008) and Molla et al. (2013), who reported that the incidence of FMD was high during the dry season in Borana and South Omo, respectively. This could be related to the high movement of animals during those seasons that facilitate the chance of close-contact between infected and susceptible animals.
The highest proportion of abortion (39.9%) was caused by brucellosis and followed by leptospirosis (22.5%) using proportional piling. This is in line with the finding of Ndengu et al. (2017) who reported that the proportion of abortion caused by brucellosis was higher (21.6%) than that caused by leptospirosis (3.7%) in Zimbabwe. In the present study, the proportion of abortions caused by listeriosis was 16.3%. According to the participants, trypanosomosis was responsible for 11.6% of the proportion of abortion in study areas. This finding is consistent with the finding of Seyoum et al. (2013), who reported 12.1% of abortions to be caused by trypanosomosis in Southwestern Ethiopia. FMD was accountable for the 9.7% proportion of abortion in our study areas. This was in line with the finding of Rafael et al. (2008) in Borana, Southern Ethiopia.
The participants mentioned mechanical or physical agents as causes of cattle abortion which lines up with a standard veterinary textbook and literature (Peter, 2000; Hovingth, 2009; Givens, 2006; Radostits et al., 2007) that reported the occurrence of abortion in cattle could be due to nutritional deficiencies, trauma, and toxicities. Non-infectious causes of cattle abortion such as seasonal change and genetic disorder have also been reported by some participants which concur with previous findings (Hansen, 2002; Regassa and Ashebir, 2016). This might be due to seasonal change that may reflect changing exposure to disease agents, a changing pattern of endocrine function, the presence of a seasonal vector, or various seasonal feeding regimes (Hafez and Hafez, 2000; Ghorboni and Asadi-Alamoti, 2004). Moreover, cattle abortion also occurs due to genetic disorders such as chromosomal and single gene disorder (Thurmond et al., 2005).
5. Conclusion
Brucellosis and leptospirosis were the most important causes of abortion in cattle mentioned by the farmers. The strong agreement between different focus group discussants and the consistencies of clinical signs mentioned for the five top causes of abortion with veterinary literature shows that farmers were knowledgeable and able to diagnose and characterize different diseases causing abortion. The seasonality of causes of abortion occurrence was important for proper planning for intervention. In this study, mechanical or physical agents were also stated as non-infectious causes of cattle abortion. Participants mentioned using medicinal plants and traditional practices to prevent and treat cattle abortion in their areas. Thus, farmers' knowledge should be incorporated to investigate health problems of unknown causes, designing, and implementing the intervention program in the areas. This finding also suggests the need for further laboratory-based study to identify the precise causes of abortion and devise a control method in the study areas.
Declarations
Author contribution statement
Benti Deresa Gelalcha: Conceived and designed the experiments.
Dereje Tulu Robi: Performed the experiments; Analyzed and interpreted the data; Wrote the paper.
Feyissa Begna Deressa: Contributed reagents, materials, analysis tools or data.
Funding statement
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Data availability statement
Data will be made available on request.
Declaration of interests statement
The authors declare no conflict of interest.
Additional information
No additional information is available for this paper.
Acknowledgements
The authors would like to thank Jimma University College of Agriculture and Veterinary Medicine and the Ethiopian Institute of Agricultural Research for their assistance and cooperation during this study. They also thank the farmers who participate in this study. Moreover, the authors are grateful to the Jimma zone office for the kind help and facilitation during the fieldwork.
References
- Allport R., Mosha R., Bahari M., Swai E., Catley A. The use community-based animal health workers to strengthen surveillance systems in Tanzania. Rev. Sci. Tech. Off. Int. des Epizooties. 2005;24:921–931. [PubMed] [Google Scholar]
- Bashahun G.D., George W.N., Benti D.G. Seroprevalence and risk factors for brucellosis in cattle in selected districts of Jimma zone, Ethiopia. Trop. Anim. Health Prod. 2015;47(7):1–7. doi: 10.1007/s11250-015-0910-8. [DOI] [PubMed] [Google Scholar]
- Bellet C., Vergne T., Grosbois V., Holl D., Roger F., Godard F. Evaluating the efficiency of participatory epidemiology to estimate the incidence and impacts of foot-and-mouth disease among livestock owners in Cambodia. Acta Trop. 2012;123(1):31–38. doi: 10.1016/j.actatropica.2012.03.010. [DOI] [PubMed] [Google Scholar]
- Benti A.D., Zewdie W. Major reproductive health problems of indigenous Borena cows in Ethiopia. J. Adv. Veterin. Anim. Res. 2014;1(4):182–188. [Google Scholar]
- Byaruhanga C., Oosthuizen M.C., Collins N.E., Knobela D. Using participatory epidemiology to investigate management options and relative importance of tick-borne diseases amongst transhumant zebu cattle in Karamoja Region, Uganda. Prev. Vet. Med. 2015;122:287–297. doi: 10.1016/j.prevetmed.2015.10.011. [DOI] [PubMed] [Google Scholar]
- Catley A. African Union/ Inter. African Bureau for Animal Resources; Nairobi: 2005. Participatory Epidemiology: A Guide for Trainers; pp. 1–42. [Google Scholar]
- Catley A. Use of participatory epidemiology to compare the clinical veterinary knowledge of pastoralists and veterinarians in East africa. Trop. Anim. Health Prod. 2006;38(3):171–184. doi: 10.1007/s11250-006-4365-9. [DOI] [PubMed] [Google Scholar]
- Catley A., Admassu B., Bekele G., Abebe D. Livestock mortality in pastoralist herds in Ethiopia and implications for drought response. Disasters. 2014;38:500–516. doi: 10.1111/disa.12060. [DOI] [PubMed] [Google Scholar]
- Catley A., Irungu P. Internation Institute for Environment and Development; 2000. Participatory Research on Bovine Trypanosomosis in Orma Cattle, Tana River District, Kenya; pp. 20–30. [Google Scholar]
- Catley A., Chibunda R.T., Ranga E., Makungu S., Magazine F.T., Magoma G., Madge M.J., Vosloo W. Participatory diagnosis of heat intolerance syndrome in Cattle in Tanzania and association with foot and mouth disease. Prev. Vet. Med. 2004;65:17–30. doi: 10.1016/j.prevetmed.2004.06.007. [DOI] [PubMed] [Google Scholar]
- Catley A., Okoth S., Osman J., Fison T., Njiru Z., Mwangi J., Jones B.,A., Leyland T.,J. Participatory diagnosis of a chronic wasting disease in cattle in southern Sudan. Prev. Vet. Med. 2001;51(3/4):161–181. doi: 10.1016/s0167-5877(01)00240-9. [DOI] [PubMed] [Google Scholar]
- Catley A., Osman J., Mawien C., Jones B.A., Leyland T.J. Participatory analysis of seasonal incidences of diseases of cattle, disease vectors, and rainfall in southern Sudan. Prev. Vet. Med. 2002;53:275–284. doi: 10.1016/s0167-5877(01)00289-6. [DOI] [PubMed] [Google Scholar]
- Chernet T., Sani R.A., Panandam J.M., Nadzr S., Speybroeck N., Van den Bossche P. Seasonal prevalence of bovine trypanosomosis in a tsetse-infested zone and a tsetse-free zone of the Amhara Region, Northwest Ethiopia. Onderstepoort J. Vet. Res. 2004;71(4):307–312. doi: 10.4102/ojvr.v71i4.250. [DOI] [PubMed] [Google Scholar]
- CSA Livestock and livestock characteristics, agricultural sample survey. Addis ababa, Ethiopia. Stat. Bull. 2017;2(583):9–13. [Google Scholar]
- De Vries A. The economic value of pregnancy in dairy cattle. J. Dairy Sci. 2006;89:3876–3885. doi: 10.3168/jds.S0022-0302(06)72430-4. [DOI] [PubMed] [Google Scholar]
- Derdour S.Y., Hafsi F., Azzag N., Tennah S., Laamari A., China B., Ghalmi F. Prevalence of the main infectious causes of abortion in dairy cattle in Algeria. J. Veterin. Res. 2017;61:337–343. doi: 10.1515/jvetres-2017-0044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deresa B., Tulu D., Deressa F.B. Epidemiological investigation of cattle abortion and its association with brucellosis in Jimma zone, Ethiopia. Vet. Med. 2020;11:87–98. doi: 10.2147/VMRR.S266350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dinka H. Reproductive performance of crossbred dairy cows under smallholder conditions in Ethiopia. J. Veterin. Med. Anim. Healt. 2013;1(5):101–103. [Google Scholar]
- Ernest H. Virginia cooperative extension publication; 2009. Common Causes of Abortions; pp. 404–488. [Google Scholar]
- Elelu N., Lawal A., Bolu S.A., Jaji Z., Eisler M.C. Participatory epidemiology of cattle diseases among the fulani pastoralists in bacita market, edu local government area, Kwara State, North-central Nigeria. EC Veterin. Sci. J. 2016;2(3):133–144. [Google Scholar]
- Ghorbani G., Asadi-Alamoti A. first ed. Industrial Unit of Jahad; Isfahan, Iran: 2004. Developed Management of Dairy Cattle; pp. 92–100. [Google Scholar]
- Givens M.D. A clinical evidence-based approach to infectious causes of infertility in beef cattle. Theriogenology. 2006;66:648–654. doi: 10.1016/j.theriogenology.2006.04.021. [DOI] [PubMed] [Google Scholar]
- Hafez E., Hafez B. seventh ed. Lippincot Williams and Wilkins; Philadelphia: 2000. Reproduction in Farm Animals; pp. 269–271. [Google Scholar]
- Hansen P.J. Embryonic mortality in cattle from the embryo’s perspective. J. Anim. Sci. 2002;80(2):33–44. [Google Scholar]
- Hovingh E. Virginia Polytechnic Institute and State University; Blacksburg: 2009. Abortions in Dairy Cattle. Common Causes of Abortions. Virginia Coop; pp. 404–488. Extension publication. [Google Scholar]
- Husu J.R., Sivela S.K., Rauramaa A.L. Prevalence of Listeria species as related to the chemical quality of farm-ensiled grass. Grass Forage Sci. 1990:309–314. [Google Scholar]
- Ibrahim N., Belihu K., Lobago F., Bekana M. Sero-prevalence of bovine brucellosis and its risk factors in Jimma zone of Oromia Region, South-western Ethiopia. Trop. Anim. Health Prod. 2010;42:35–40. doi: 10.1007/s11250-009-9382-z. [DOI] [PubMed] [Google Scholar]
- Jittapalapong S., Pinyopanuwat N., Inpankaew T., Sangvaranond A., Phasuk C., Chimnoi W., Kengradomkij C., Kamyingkird K., Sarataphan N., Desquesnes M., Arunvipas P. Prevalence of trypanosoma evansi infection causing abortion in dairy cows in Central Thailand kasetsart. J. Nat. Sci. 2009;43:53–57. [Google Scholar]
- Jost C.C., Mariner J.C., Roeder P.L., Sawitri E., Macgregor-Skinner G.J. 2007. Participatory epidemiology in disease surveillance and research Review in Scientific and Technical of the Office International des Epizooties; pp. 537–549. 26. [PubMed] [Google Scholar]
- Machila N., Wanyangu S.W., McDermott J., Welburn S.C., Maudlin I., Eisler M.C. Cattle owners’ perceptions of African bovine trypanosomosis and its control in Busia and Kwale districts of Kenya. Acta Trop. 2003;86:25–34. doi: 10.1016/s0001-706x(02)00288-7. [DOI] [PubMed] [Google Scholar]
- Mark L.K. An epidemiologic approach to investigating abortion problems in dairy herds. Refereed Peer Review. 2002;24(4):34–39. [Google Scholar]
- Moch R.W., Ebner E.E., Barsoum L.S., Botros B.A. Leptospirosis in Ethiopia: a serological survey in domestic and wild animals. J. Trop. Med. Hyg. 1975;78(2):38–42. [PubMed] [Google Scholar]
- Molla B., Ayelet G., Asfaw Y., Jibril Y., Gelaye E. Participatory epidemiology and associated risk factors of foot-and-mouth disease in cattle in South Omo zone, South-Western Ethiopia. J. Veterin. Med. Anim. Healt. 2013;5(11):322–328. [Google Scholar]
- Murray R.D. Proceedings of the 24th World Buiatrics Conference, Nice, France. 2006. A practical approach to infectious bovine abortion diagnosis. [Google Scholar]
- Ndengu M., Garine-wichatitsky M.D., Pfukenyi D.M., Tivapasi M., Mukamuri B., Matope G. Assessment of community awareness and risk perceptions of zoonotic causes of abortion in cattle at three selected livestock-wildlife interface areas of Zimbabwe. Epidemiol. Infect. 2017:1–16. doi: 10.1017/S0950268817000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pal M. Etiology and management of infectious abortion in animals. Indian J. Field Vet. 2006;2:70–72. [Google Scholar]
- Pal M., Lemu D., Worku S., Desta G. Sero-prevalence study of bovine brucellosis and reproductive problems in small-scale dairy farms of North shewa, Ethiopia. Int. J. Livestock Res. 2016;6(9):1–10. [Google Scholar]
- Parthiban S., Malmarugan S., Murugan M.S., Johnson Rajeswar J., Pothiappan P. Review on emerging and reemerging microbial causes in bovine abortion. Int. J. Nutr. Food Sci. 2015;4(4-1):1–6. [Google Scholar]
- Peter A.T. Abortions in dairy cows: new insights and economic impact. Adv. Dairy Technol. 2000;12:233. [Google Scholar]
- Pieracci E.G., Aron J.H., Radhika G., Abraham H., Elias W., Asefa D., Getahun B., Meron K., Henry W., Ermias B. Prioritizing zoonotic diseases in Ethiopia using a one health approach. One Health. 2016;2:131–135. doi: 10.1016/j.onehlt.2016.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radostits O.M., Gay C.C., Hinchcliff K.W., Constable P.D. tenth ed. W.B., Saunders; London: 2007. Veterinary Medicine. A Textbook of Diseases of Cattle, Sheep, Pigs, Goats, and Horses; pp. 963–985. [Google Scholar]
- Rafael T., Catley A., Bogale A., Sahle M., Shiferaw Y. Foot and mouth disease in the Borana pastoral system, Southern Ethiopia, and implications for livelihoods and international trade. Trop. Anim. Health Prod. 2008;40:29–38. doi: 10.1007/s11250-007-9049-6. [DOI] [PubMed] [Google Scholar]
- Regassa T., Ashebir G. Major factors influencing the reproductive performance of dairy farms in mekelle city, tigray, Ethiopia. Journal of Dairy, Veterinary and Animal Research. 2016;3(4):88. [Google Scholar]
- Rundassa M.D., Menkir S., Kebede A. Prevalence and seasonal incidence of bovine trypanosomosis in Birbir valley, Baro Akobo River system, Western Ethiopia. J. Veterin. Med. Anim. Healt. 2013;5(5):138–143. [Google Scholar]
- Sani M.B., Amanloo H. 3rd Cong of Animal Science; Mashhad, Iran: 2007. Heat Stress Effect on Open Days in Holstein Dairy Cattle in Yazd Province, Iran; p. 85. [Google Scholar]
- Seyoum Z., Terefe G., Ashenafi H. Farmers’ perception of impacts of bovine trypanosomosis and tsetse fly in selected districts in Baro-Akobo and Gojeb river basins, Southwestern Ethiopia. BMC Vet. Res. 2013;9:214. doi: 10.1186/1746-6148-9-214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimelis D., Sangwan A.K., Getachew A. Epidemiology of bovine trypanosomosis in the Abay (Blue Nile) basin areas of northwest Ethiopia. Rev. Med. Vet. 2005;58(3):151–157. [Google Scholar]
- Siegel S., Castellan N.J. second ed. McGraw-Hill; New York: 1998. Non-Parametric Statistics for Behavioural Science. [Google Scholar]
- Tesfaye D., Shamble A. Reproductive health problems of cows under different management systems in Kombolcha, Northeast Ethiopia. Adv. Biol. Res. 2013;7(3):104–108. [Google Scholar]
- Tesfaye D., Speybroeck N., Deken R.D., Thys E. Economic burden of bovine trypanosomosis in three villages of Metekel zone, Northwest Ethiopia. Trop. Anim. Health Prod. 2011;44(4):873–879. doi: 10.1007/s11250-011-9981-3. [DOI] [PubMed] [Google Scholar]
- Thrusfield M. third ed. Blackwell Publishing; England: 2005. Veterinary Epidemiology; pp. 345–543. [Google Scholar]
- Thurmond M.C., Branscum A.J., Johnson W.O., Bedrick E.J., Hanson T.E. Predicting the probability of abortion in dairy cows: a hierarchical Bayesian logistic-survival model using sequential pregnancy data. Prev. Vet. Med. 2005;68:223–239. doi: 10.1016/j.prevetmed.2005.01.008. [DOI] [PubMed] [Google Scholar]
- Tilahun Z., Reta D., Simenew K. Global epidemiological overview of leptospirosis. Int. J. Microbiol. Res. 2013;4(1):9–15. [Google Scholar]
- Tolosa T., Regassa F., Belihu K. Seroprevalence study of bovine brucellosis in extensive management system in selected sites of Jimma zone, western Ethiopia. Bull. Anim. Health Prod. Afr. 2008;56:25–37. [Google Scholar]
- Tulu D. Jimma University, College of Veterinary Medicine and Agriculture; Jimma, Ethiopia: 2018. Epidemiological Investigation of the Cause of Abortion in Cattle at Limu Seka and Chora Boter Districts of Jimma Zone, Southwestern Ethiopia; pp. 58–71. M.Sc. Thesis. [Google Scholar]
- Tulu D. Epidemiology and zoonotic implication of leptospirosis in domestic animals in Ethiopia. Acad. J. Anim. Dis. 2020;9(1):19–32. [Google Scholar]
- Tulu D., Deresa B., Begna F., Gojam A. Review of common causes of abortion in dairy cattle in Ethiopia. J. Veterin. Med. Anim. Healt. 2018;10(1):1–13. [Google Scholar]
- Yadeta W., Bashahun G.M., Abdela N. Leptospirosis in animal and its public health implications: a review. World Appl. Sci. J. 2016;34(6):845–853. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data will be made available on request.