Abstract
We have reported that testosterone-enanthate (TE) prevents the musculoskeletal decline occurring acutely after spinal cord injury (SCI), but results in a near doubling of prostate mass. Our purpose was to test the hypothesis that administration of TE plus finasteride (FIN; type II 5α-reductase inhibitor) would prevent the chronic musculoskeletal deficits in our rodent severe contusion SCI model, without inducing prostate enlargement. Forty-three 16-week-old male Sprague-Dawley rats received: 1) SHAM surgery (T9 laminectomy); 2) severe (250 kdyne) contusion SCI; 3) SCI+TE (7.0 mg/week, intramuscular); or 4) SCI+TE+FIN (5 mg/kg/day, subcutaneous). At 8 weeks post-surgery, SCI animals exhibited reduced serum testosterone and levator ani/bulbocavernosus (LABC) muscle mass, effects that were prevented by TE. Cancellous and cortical (periosteal) bone turnover (assessed by histomorphometry) were elevated post-SCI, resulting in reduced distal femur cancellous and cortical bone mass (assessed by microcomputed tomography). TE treatment normalized cancellous and cortical bone turnover and maintained cancellous bone mass at the level of SHAM animals, but produced prostate enlargement. FIN coadministration did not inhibit the TE-induced musculoskeletal effects, but prevented prostate growth. Neither drug regimen prevented SCI-induced cortical bone loss, although no differences in whole bone strength were present among groups. Our findings indicate that TE+FIN prevented the chronic cancellous bone deficits and LABC muscle loss in SCI animals without inducing prostate enlargement, which provides a rationale for the inclusion of TE+FIN in multimodal therapeutic interventions intended to alleviate the musculoskeletal decline post-SCI.
Keywords: androgen, 5α reductase, dihydrotestosterone, hypogonadism, osteoporosis
Introduction
Motor-complete spinal cord injury (SCI) produces severe bone loss below the spinal lesion,1 with individuals exhibiting a 40–70% cancellous bone loss within the first 2–3 years of injury2 and a more gradual 20–30% cortical bone loss that develops over a decade.3 These skeletal deficits combine to increase fracture risk by 20- to 100-fold, in comparison to healthy age-matched individuals,4 and result in extended post-fracture hospitalization in this population.5 The primary cause of SCI-induced bone loss is thought to be the persistent disuse that develops subsequent to the neurological insult.6 However, the skeletal decline occurring after motor-complete SCI is 4–10 times faster than that occurring in humans undergoing prolonged bed rest or microgravity exposure.7 Similarly, the bone loss that occurs after experimental SCI is more severe than in other disuse models (e.g., cast immobilization8 or sciatic neurectomy).9 These findings appear to suggest that other factors, such as SCI-induced hormonal irregularities, may contribute to the skeletal decline post-SCI.7
Hypogonadism (i.e., low testosterone), which is present in 40–60% of men with SCI,10 is one factor that may exacerbate bone loss post-injury.6 Rodents also exhibit an ~50% reduction in circulating testosterone for at least 3 weeks after severe contusion SCI, which accompanies the cancellous bone loss in this model.11 We have reported that testosterone-enanthate (TE) dose dependently prevents the cancellous bone deficits occurring acutely post-SCI in both young11 and skeletally mature animals,12 primarily through antiresorptive actions. Although these results appear promising, nandrolone, a highly potent synthetic testosterone analogue, produced only minor skeletal benefit when administered chronically after spinal cord transection.13 One possibility for the reduced skeletal efficacy of this androgen post-SCI is that nandrolone is 5α-reduced to relatively weaker androgenic metabolites,14 whereas TE undergoes dose-dependent 5α-reduction to dihydrotestosterone (DHT),15 a more potent androgen. In this regard, we are unaware of any study that has assessed the influence of 5α-reductase on androgen-mediated skeletal preservation in the unique context of SCI. Alternatively, it is possible that androgens exert reduced skeletal efficacy when administered chronically post-SCI. In this regard, only one case series has evaluated the skeletal responses to testosterone replacement therapy (TRT) in men with SCI and reported no change in femur cross-sectional area (CSA) over 16 weeks.16 As such, careful examination of the chronic cancellous and cortical bone responses to TE treatment is necessary before advancing this agent to future clinical trials.
In addition to the skeletal benefits described above, TE produces several side effects, of which prostate enlargement remains a clinical concern.17 Indeed, we have observed a near doubling of prostate mass in response to both low-dose (replacement) and high-dose (supraphysiologic) TE in our rodent SCI model,11 which precludes translation of this therapy to the SCI population. Androgen-mediated prostate enlargement primarily results from the localized prostate-specific 5α-reduction of testosterone to DHT,18 as evidenced by the ability of TE to increase prostate volume in ambulatory hypogonadal men and by that of finasteride (FIN; type II 5α-reductase inhibitor) to prevent prostate growth.19, 20 Interestingly, FIN does not interfere with the ability of TE to increase bone mineral density (BMD) in healthy ambulatory men,19,20 indicating that type II 5α-reductase activity is not required for androgen-induced skeletal protection, at least in the presence of normal musculoskeletal loading. However, the influence of FIN on androgen-mediated skeletal preservation and prostate enlargement requires further examination post-SCI, when skeletal loading is limited.
The primary purpose of this study was to determine 1) whether TE prevents the cancellous and cortical bone deficits that persist chronically post-SCI and 2) whether type II 5α-reductase activity is required for TE-induced skeletal preservation in the absence of mechanical loading. A secondary purpose was to determine whether inhibition of the type II 5α-reductase enzyme, through FIN, prevents prostate enlargement resulting from supraphysiologic TE, which addresses one barrier to the translation of higher-dose TE therapy to the SCI population. We hypothesized that TE treatment would prevent bone loss post-SCI, and that FIN coadministration would prevent TE-induced prostate enlargement without inhibiting the skeletal benefits of this therapy.
Methods
Animal care
Barrier-raised and specific pathogen-free male Sprague-Dawley rats 16 weeks of age were obtained from Charles River Laboratories (Wilmington, MA). Animals were individually housed in a temperature- and light-controlled room on a 12-h light/dark cycle. Rats were fed commercially available rodent chow (2018 Teklad Global 18% Protein Rodent Diet; Harlan Laboratories Inc., Indianapolis, IN) and tap water ad libitum. All experimental procedures conformed to the Institute of Laboratory Animal Resources Guide to the Care and Use of Experimental Animals and were approved by the Institutional Care and Use Committee at the Gainesville VA Medical Center (Gainesville, FL).
Experimental design
Rats (n = 10–12/group) were stratified by body mass into the following groups: 1) Sham surgery (T9 laminectomy) + vehicle (SHAM); 2) T9 laminectomy + severe contusion SCI + vehicle (SCI); 3) SCI+TE; and (4) SCI+TE+FIN. Animals received TE (7.0 mg/week in 0.1 mL of sesame oil, intramuscular [i.m.]) or vehicle (0.1 mL/week of sesame oil, i.m.) at time of surgery and at weekly intervals thereafter in combination with daily FIN (5.0 mg/kg/day, subcutaneous [s.c.]) or vehicle (90% safflower oil/10% ethanol) for the duration of the experiment. Blood was sampled from the tail tip at monthly intervals. Animals were assessed for open-field locomotion by two blinded observers using the Basso-Beattie-Bresnahan (BBB) locomotor rating scale21 at day 7 and every other week thereafter. Declomycin and calcein (all chemicals obtained from Sigma-Aldrich, St. Louis, MO, unless noted) were administered (15 mg/kg body weight, s.c.) at 10 and 3 days before sacrifice, respectively, for fluorochrome labeling of bone surfaces. Rats were sacrificed 8 weeks post-surgery, by thoracotomy and exsanguination while under isoflurane anesthesia, because cancellous and cortical bone loss peak within this time frame in our rodent SCI model (unpublished laboratory data). Blood was acquired by intracardiac puncture, and the left and right femurs and tibias, prostate, and levator ani/bulbocavernosus (LABC) muscle complex were excised and weighed.
Blood was centrifuged at 3000g and serum aliquots were separated and stored at −80°C until analyzed. Bones were measured with a digital micrometer (Mitutoyo, Aurora, IL). Femurs were wrapped in saline-saturated gauze to prevent dehydration and stored at −20°C for microcomputed tomography (μCT) and bone mechanical testing. Tibias were fixed in formalin for 48 h and then stored in 70% ethanol at 4°C before histomorphometry. The prostate and LABC were snap frozen in liquid nitrogen and stored at −80°C.
Surgery and post-surgical care
Animals were kept on a circulating water-heated pad to maintain body temperature and remained under isoflurane anesthesia during surgery. The T9 segment of the spinal cord was exposed by laminectomy under sterile conditions and a 250-kdyne force was applied to the spinal cord with the Infinite Horizons Impactor (Precision Systems and Instrumentation, Lexington, KY), which induces a severe mid-thoracic contusion SCI, according to our published methodology.11,12 Confirmation of T9 laminectomy/injury was verified at time of surgery using anatomical landmarks and during dissection at sacrifice. Animals received buprenorphine (0.05 mg/kg, s.c.) and ketoprofen (5.0 mg/kg, s.c.) to reduce pain and inflammation for 36 h post-SCI, and ampicillin was administered (100 mg/kg, s.c.) for 5 days post-surgery. Post-operative care included daily examinations for signs of distress, weight loss, dehydration, fecal clearance, bladder dysfunction, and for urinary tract infections (none noted). Manual bladder expression was performed at least twice-daily until spontaneous voiding returned. Ringer’s (s.c.) was provided post-surgery to promote rehydration. In order to assist with body mass recovery, animals received a daily nutritional supplement (Jell-O cube with added protein and fat) with sliced apples and Fruit Loops (Kellogg’s, Battle Creek, MI).
Drug administration
TE (Savient Pharmaceutical, East Brunswick, NJ), a slowly released testosterone ester that maintains serum testosterone in the supraphyiological range,22 was dissolved in vehicle (sesame oil) and injected into alternating quadriceps musculature once-weekly, under brief isoflurane anesthesia. The TE dose (7.0 mg/week) was chosen because we have previously reported that it completely prevents cancellous bone loss in male rodents after contusion SCI,11,12 effects that are blunted with lower TE doses,11 and because this drug regimen mimics a recent clinical trial in our laboratory assessing the musculoskeletal and prostate responses to TE treatment in non-neurologically impaired hypogonadal men.19 FIN (Sigma-Aldrich), a type II 5α-reductase inhibitor that suppresses circulating23 and intraprostatic DHT,24 was dissolved in vehicle (90% safflower oil/10% ethanol) and injected (5.0 mg/kg, s.c.) daily. The FIN dose was chosen based on findings of a pilot study in which control rats received TE, along with graded FIN doses (5.0, 7.5, or 10.0 mg/kg daily, s.c.). The 5.0-mg/kg FIN dose prevented prostate enlargement over 21 days, with no differences in prostate mass noted among doses.
Bone histomorphometry
We evaluated cancellous and cortical bone characteristics at the proximal tibial metaphysis and tibial diaphysis, respectively, using standard histomorphometric techniques, as previously described.11 Briefly, the right tibia was excised at sacrifice, cut in half cross-sectionally with a Dremel Moto Tool (Dremel, Racine, WI), placed in 10% phosphate-buffered formalin for 48-h tissue fixation, dehydrated in ethanol, and embedded undecalcified in methyl methacrylate. Proximal tibiae were sectioned longitudinally at 4- and 8-(m thicknesses with a Leica/Jung 2065 microtome. The 4-(m bone sections were stained by the Von Kossa method with a tetrachrome counterstain (Polysciences Inc., Warrington, PA) for the assessment of cancellous bone structure, and the 8-(m bone sections of the proximal tibial metaphysis remained unstained to measure fluorochrome-based indices of bone formation. The tibial diaphyses were sawed in 200-μm-thick cross-sections with an Isomet low-speed saw (Buehler, Lake Bluff, IL) at a location 1–2 mm proximal to the tibiofibular junction and ground to a thickness of 50 μm before the histomorphometric evaluation of cortical bone fluorochrome-based indices of bone formation. The sample area at the proximal tibial metaphysis began 0.5 mm distal to the growth plate and excluded the primary spongiosa and cancellous bone within 0.25 mm of the endocortical surfaces. Osteoblast (Ob.S/BS, %) and osteoclast (Oc.S/BS, %) surfaces were calculated as percentages of total cancellous perimeter, using the Osteomeasure System (Osteometrics, Atlanta, GA). Fluorochrome-based indices of cancellous (proximal metaphysis) and cortical (diaphysis) bone formation were measured under ultraviolet illumination. Mineralizing surface (MS), an index of active bone formation, was calculated as the percentage of cancellous (MS/BS), periosteal (Ps.MS/BS, %), and endocortical (Ec.MS/BS, %) bone surfaces with a double fluorochrome label. Mineral apposition rate (MAR), an index of osteoblast activity, was calculated by dividing the interlabel distance by the time interval between administration of fluorochrome labels (i.e., 7 days). Bone formation rate (BFR/BS) was calculated at each skeletal site by multiplying MS/BS by MAR.
Microcomputed tomography analysis of bone morphometry
The right femoral metaphysis and diaphysis were scanned with a Bruker Skyscan 1172 μCT (Bruker Microct, Kontich, Belgium) according to our published methods.8,11,12,25 The acquisition settings were as follows: 80 kVP/120 μA; 0.5 mm aluminum filter; 2K camera resolution; 9.86 μm voxel size; 0.7-degree rotation step; and 180-degree tomographic rotation. The cancellous region of interest (ROI) at the distal femoral metaphysis began 2 mm proximal to the growth plate and encompassed a total of 3.5 mm. The cortical ROI at the distal femoral metaphysis began at 19% of the total femur length, in order to avoid all residual growth plate in this region, and encompassed a total of 2 mm. This skeletal site was chosen because it is a primary site of bone fractures that required hospitalization in the SCI population.5 The ROI at the femoral diaphysis began at 45% of the femur length, to avoid the third trochanter, and encompassed 2 mm. Cross-sectional images were reconstructed in NRecon using a filtered back-projection algorithm and two-dimensional/three-dimensional morphometric parameters were analyzed in CTAn (Bruker Microct. Cancellous measurements at the distal femur include: cancellous bone volume (BV/TV, %); trabecular number (Tb.N, #/mm); trabecular separation (Tb.Sp, mm); trabecular thickness (Tb.Th, mm); and trabecular pattern factor (Tb.Pf, #/mm). Cortical measurements include: total cross-sectional bone (plus medullary) area (Tt.Ar, mm2); cortical bone area (Ct.Ar, mm2); medullary area (Ma.Ar, mm2); cortical area fraction (Ct.Ar/Tt.Ar, %); and cortical thickness (Ct.Th, mm). Additionally, medullary volumetric (v)BMD (cancellous bone only) was evaluated using the previously defined ROI within the distal femur and cortical volumetric tissue mineral density was assessed using the previously defined distal femur and femoral diaphysis ROI. Densities were determined following calibration with hydroxyapatite phantoms.
Bone mechanical testing
Subsequent to μCT the right distal femora underwent an anterior-posterior cantilever mechanical test using our recently described methods,8 which results in a reproducible supracondylar fracture. Briefly, femora were thawed to room temperature and cut cross-sectionally near the midshaft with a Dremel MotoTool (Dremel, Mt. Prospect, IL). The mid-shafts of the distal femora were embedded vertically in fiberglass resin (Bondo, St. Paul, MN) inside separate hard plastic cuvettes [measuring 1.25 cm (length) × 1.25 cm (width) × 4.5 cm (depth)] with the distal ~8 cm of the femora remaining unembedded. The unembedded portion of the distal femora was wrapped in saline-saturated gauze throughout this process to ensure the bone remained adequately hydrated. The cuvette was then secured in a horizontal position on a servohydraulic testing machine (MTS 858 Bionix Test System; MTX, Eden Prarie, MN) with the anterior portion of the femur facing up. A flat steel fixture attached to the MTS was placed in direct contact with the unembedded anterior portion of the femur at a distance ~5 cm from the distal end. Subsequently, 10 cycles of sinusoidal preload (from 0 to 10 N) were applied to the femur in the anterior-to-posterior direction, followed by the bending load that was applied at 1.0 mm/s until failure of the specimen, which resulted in a supracondylar fracture. The maximum load (N), displacement at maximal load (mm), and stiffness (N/mm) were determined from the load-deformation curves that were generated by the MTS software, and the distance from the distal end of the femur to the fracture was measured to assess reproducibility of the fracture location across samples.
Serum measurements
Serum measurements were performed in duplicate on a single plate. Testosterone was determined by enzyme immunoassay (EIA) that has a sensitivity of 0.02 ng/mL and an intra-assay coefficient of variation (CV) <9% (ALPCO Diagnostics, Salem, NH). Total pro-collagen type 1 N-terminal propeptide (P1NP; a circulating marker of whole-body bone formation) was determined by EIA with a sensitivity of 0.7 ng/mL, and an intra-assay CV <7.4% and tartrate resistant acid phosphatase 5b (TRAP5b; a circulating marker of whole-body bone resorption) was determined using an EIA that requires 25 μL of serum and has a sensitivity of 0.1 U/L and an intra-assay CV below 5.8% (Immunodiagnostic Systems, Fountain Hills, AZ).
Statistical analysis
Results are reported as means ± standard error of the mean. An α level of p < 0.05 was defined as the threshold of significance. Mixed-model repeated-measures analyses of variance (ANOVAs) were used to analyze variables that were assessed at multiple time points. One-way ANOVAs were used to analyze data that were assessed at a single time point. Fisher’s least significant difference post-hoc tests were performed for multiple comparisons among groups, when appropriate. Hormone values that were below the lowest detectable standard are reported as such and were assigned a value equal to the sensitivity of each individual assay for the purposes of statistical analysis. All statistical analyses were performed with the SPSS statistical software package (v15.0.0; IBM, Chicago, IL).
Results
Post-surgical recovery, body mass, and bone characteristics
Pre-surgical body mass was 496 ± 10 (SHAM), 480 ± 5 (SCI), 492 ± 9 (SCI+TE), and 476 ± 5 (SCI+TE+FIN), with no differences among groups. Post-surgery, animals that had contusion SCI exhibited persistent signs of severe SCI, including reduced appetite and thirst, temporary loss of voluntary bladder function, and persistent hindlimb locomotor deficits. BBB scores were ≤1 at week 1 for all SCI groups, indicative of only slight movement of one hindlimb joint, and gradually progressed to scores ≤7 by week 8, indicative of slight to extensive movement of two to three hindlimb joints without hindlimb stepping ability or hindlimb weight support (data not shown), with no differences among SCI groups. No locomotor deficits were observed in SHAM animals.
Body mass progressively increased in SHAM animals, with values being higher than baseline from weeks 3–8 (p < 0.01) and higher than all SCI groups at all post-surgical time points (p < 0.01, Supplementary Fig. 1) (see online supplementary material at http://www.liebertpub.com). In all SCI groups, body mass was reduced 7–10% at weeks 1–2 (p < 0.01) and progressively increased thereafter, with values returning to baseline starting at week 3. At weeks 6–8, body mass was higher in SCI animals versus SCI+TE and SCI+TE+FIN animals (p < 0.05 for week 6 and p < 0.01 for weeks 7–8) and was also higher than baseline at weeks 7–8 (p < 0.01). No differences in body mass were present between SCI+TE and SCI+TE+FIN groups at any time point. Femoral and tibial mass/length ratios were 6% lower in SCI versus SHAM groups (p < 0.01, Supplementary Table 1) (see online supplementary material at http://www.liebertpub.com), resulting from nonsignificant reductions in bone mass in SCI animals. TE administration prevented the SCI-induced reduction in tibial mass/length ratio and FIN did not interfere with this effect. In contrast, SCI+TE+FIN animals exhibited a 4% lower femoral mass/length ratio versus SHAMs (p < 0.05).
Serum testosterone and bone turnover markers
Serum testosterone was 49–55% lower in SCI versus SHAM animals at week 4 (trend, p = 0.061; Table 1) and at week 8 (not significant). In SCI+TE and SCI+TE+FIN animals, testosterone was ~2- to 3-fold higher than SHAMs (SCI+TE, p < 0.01; SCI+TE+FIN, p < 0.001) and ~4- to 7-fold higher than SCI animals (p < 0.001 both groups) at both the 4- and 8-week time points. SCI+TE+FIN also exhibited a 40–47% higher testosterone than SCI+TE at week 4 (p < 0.001) and week 8 (trend, p = 0.064). At sacrifice, serum Trap5b and P1NP were not different among SHAM and SCI animals. Trap5b was 18–30% lower in SCI+TE (p < 0.001) and SCI+TE+FIN animals versus SHAMs (p < 0.05). P1NP was 29–38% lower in SCI+TE (p < 0.01) and SCI+TE+FIN animals versus SCI (p < 0.05).
Table 1.
Serum Testosterone Concentrations and Circulating Bone Turnover Markers after Sham Surgery (T9 Laminectomy) or Severe Spinal Cord Injury (SCI) Alone or in Combination with Testosterone-Enanthate (TE) or TE Plus Finasteride (TE+FIN)
| SHAM (a) | SCI (b) | SCI+TE (c) | SCI+TE+FIN (d) | |
|---|---|---|---|---|
| Testosterone (week 4), ng/mL | 3.7 ± 0.8c*,d* | 1.9 ± 0.5c*,d* | 6.8 ± 0.5a*,b*,d* | 10.0 ± 0.9a*,b*,c* |
| Testosterone (week 8), ng/mL | 3.3 ± 0.7c*,d* | 1.5 ± 0.3c*,d* | 7.8 ± 1.5a*,b* | 10.9 ± 1.6a*,b* |
| Trap5b, U/L | 3.4 ± 0.2c*,d | 2.9 ± 0.1 | 2.4 ± 0.2a* | 2.8 ± 0.3a |
| P1NP, ng/mL | 26 ± 3 | 34 ± 4c*,d | 21 ± 2b* | 24 ± 2b |
Values are means ± standard error; n = 7–12/group.
Letters a–d indicate differences from respectively labeled groups at p < 0.05 or *p < 0.01 (a = vs. SHAM, b = vs. SCI, c = vs. SCI+TE, and d = vs. SCI+TE+FIN). Trap5b is a circulating marker of bone resorption. P1NP is a circulating marker of bone formation.
Trap5b, tartrate resistant acid phosphatase 5b; P1NP, procollagen type 1 N-terminal propeptide.
Cancellous bone histomorphometry
Histomorphometry at the proximal tibia indicated that SCI animals exhibited an 18% higher Oc.S/BS (nonsignificant) and a 319% higher Ob.S/BS (p < 0.05) in comparison to SHAM animals, with BFR/BS being 100% higher in SCI versus SHAMs (p < 0.05; Table 2). TE prevented these increases, with SCI+TE and SCI+TE+FIN groups exhibiting a 31–45% lower Oc.S/BS (nonsignificant) and a 33–70% lower Ob.S/BS in comparison to SCI (p < 0.05 for SCI+TE only). These changes normalized bone formation indices, with MS/BS and BFR/BS being 46–52% and 64–69% lower in SCI+TE and SCI+TE+FIN groups versus SCI (p < 0.01), respectively, and not different than SHAMs.
Table 2.
Cancellous Histomorphometry at the Proximal Tibial Metaphysis in Animals Receiving Sham Surgery (T9 Laminectomy) or Severe Spinal Cord Injury (SCI) Alone or in Combination with Testosterone-Enanthate (SCI+TE) or TE Plus Finasteride (SCI+TE+FIN)
| SHAM (a) | SCI (b) | SCI+TE (c) | SCI+TE+FIN (d) | |
|---|---|---|---|---|
| Oc.S/BS, % | 6.8 ± 1.4 | 8.0 ± 1.8 | 4.4 ± 1.1 | 5.5 ± 0.7 |
| Ob.S/BS, % | 1.6 ± 0.7b | 5.1 ± 1.3a,c | 1.4 ± 0.7b | 3.4 ± 1.0 |
| MS/BS, % | 17.8 ± 2.0d | 23.5 ± 3.1c*,d* | 12.7 ± 1.8b* | 11.2 ± 1.6a,b* |
| MAR, μm/day | 0.85 ± 0.06 | 1.04 ± 0.10 | 0.91 ± 0.06 | 0.93 ± 0.10 |
| BFR/BS (dL), um3/um2/day | 7.68 ± 1.72b | 15.5 ± 3.16a,c*,d* | 5.60 ± 1.73b* | 4.73 ± 1.71b* |
Values are means ± standard error; n = 912/group.
Letters a–d indicate differences from respectively labeled groups at p < 0.05 or *p < 0.01 (a = vs. SHAM, b = vs. SCI, c = vs. SCI+TE, and d = vs. SCI+TE+FIN).
Oc.S/BS, osteoclast surface; Ob.S/BS, osteoblast surface; MS/BS, mineralizing surface; MAR, mineral apposition rate; BFR/BS, bone formation rate.
Cortical bone histmorphometry
Histomorphometric analyses at the tibial diaphysis indicated the Ps.MS/BS and Ps.BFR/BS were 69% (p < 0.05) and 116% higher (p < 0.01) in SCI than SHAM animals, respectively (Table 3). TE administration prevented these effects, with SCI+TE and SCI+TE+FIN exhibiting a Ps.MS/BS that was 43–54% lower (SCI+TE, p < 0.01; SCI+TE+FIN, p < 0.05) and a Ps.BFR/BS that was 57–69% lower than SCI animals (SCI+TE, p ≤ 0.001; SCI+TE+FIN, p < 0.01) and not different than SHAMs. No differences in Ec.MS/BS were present among groups (Table 3), and there were an insufficient number of double fluorochrome labels on the endocortical surface to accurately determine Ec.MAR and Ec.BFR/BS.
Table 3.
Cortical Histomorphometry at the Tibial Diaphysis in Animals Receiving Sham Surgery (T9 Laminectomy) or Severe Spinal Cord Injury (SCI) Alone or in Combination with Testosterone-Enanthate (SCI+TE) or TE Plus Finasteride (SCI+TE+FIN)
| SHAM (a) | SCI (b) | SCI+TE (c) | SCI+TE+FIN (d) | |
|---|---|---|---|---|
| Ps.MS/BS, % | 32 ± 4b | 54 ± 4a,c*,d | 25 ± 5b* | 31 ± 8b |
| Ps.MAR, μm/day | 1.14 ± 0.11 | 1.24 ± 0.10 | 1.15 ± 0.04 | 1.35 ± 0.10 |
| Ps.BFR/BS, μm3/μm2/day | 30 ± 8b* | 64 ± 11a*,c*,d* | 20 ± 5b* | 28 ± 8b* |
| Ec.MS/BS, % | 5.3 ± 1.2 | 4.1 ± 1.2 | 1.7 ± 0.4 | 2.5 ± 0.7 |
| Ec.MAR, μm/day | N/A | N/A | N/A | N/A |
| Ec.BFR/BS, μm3/μm2/day | N/A | N/A | N/A | N/A |
Values are means ± standard error; n = 9–12/group.
Letters a–d indicate differences from respectively labeled groups at p < 0.05 or *p < 0.01 (a = vs. SHAM, b = vs. SCI, c = vs. SCI+TE, and d = vs. SCI+TE+FIN).
Ps.MS/BS, periosteal mineralizing surface; Ps.MAR, periosteal mineral apposition rate; Ps.BFR/BS, periosteal bone formation rate; Ec.MS/BS, endocortical mineralizing surface; Ec.MAR, endocortical mineral apposition rate; Ec.BFR/BS, endocortical bone formation rate; N/A, not applicable; there were no measurable double labels for the assessment of Ec.MAR or Ec.BFR/BS.
Microcomputed tomography analysis of cancellous bone
Representative μCT images of cancellous bone within the distal femur ROI are presented in Figure 1. Cancellous BMD was 39% lower in SCI compared to SHAM animals (p < 0.01; Fig. 2A). SCI-induced bone loss was characterized by a 42% lower BV/TV, a 48% lower Tb.N, 46% higher Tb.Sp, and, unexpectedly, an 11% higher Tb.Th (p ≤ 0.01 for all; Fig. 2B–E). TE completely prevented cancellous bone loss and FIN coadministration did not interfere with TE-induced skeletal protection, with SCI+TE and SCI+TE+FIN animals exhibiting a 51–53% higher BMD versus SCI (p < 0.01). TE-induced skeletal preservation was characterized by a 54–63% higher BV/TV (p < 0.05), 71–82% higher Tb.N (p < 0.05 for SCI+TE and p < 0.01 for SCI+TE+FIN), and 45–49% lower Tb.Sp (p < 0.001) for SCI+TE and SCI+TE+FIN animals in comparison to SCI. In addition, Tb.Th was 7–11% higher in SCI+TE versus SHAM (p < 0.01) and SCI+TE+FIN animals (p < 0.05). No differences in Tb.Pf were present among groups (Fig. 2F).
FIG. 1.
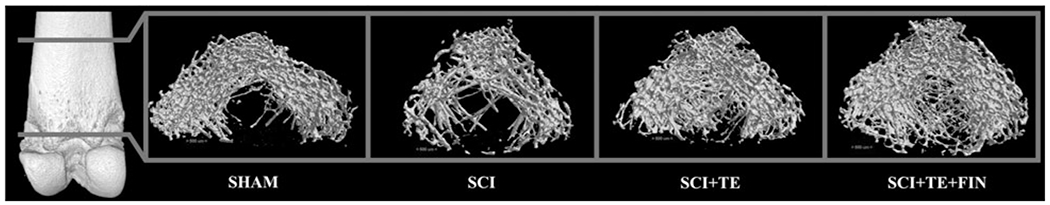
Representative three-dimensional (3D) microcomputed tomography images of cancellous bone within the region of interest at the distal femoral metaphysis after sham surgery (T9 laminectomy) or severe spinal cord injury (SCI) alone or in combination with testosterone-enanthate (SCI+TE) or TE plus finasteride (SCI+TE+FIN). Images represent 3D cross-sectional views of cancellous bone with the surrounding cortical bone removed. Accompanying morphologic data are included in Figure 2A–F.
FIG. 2.
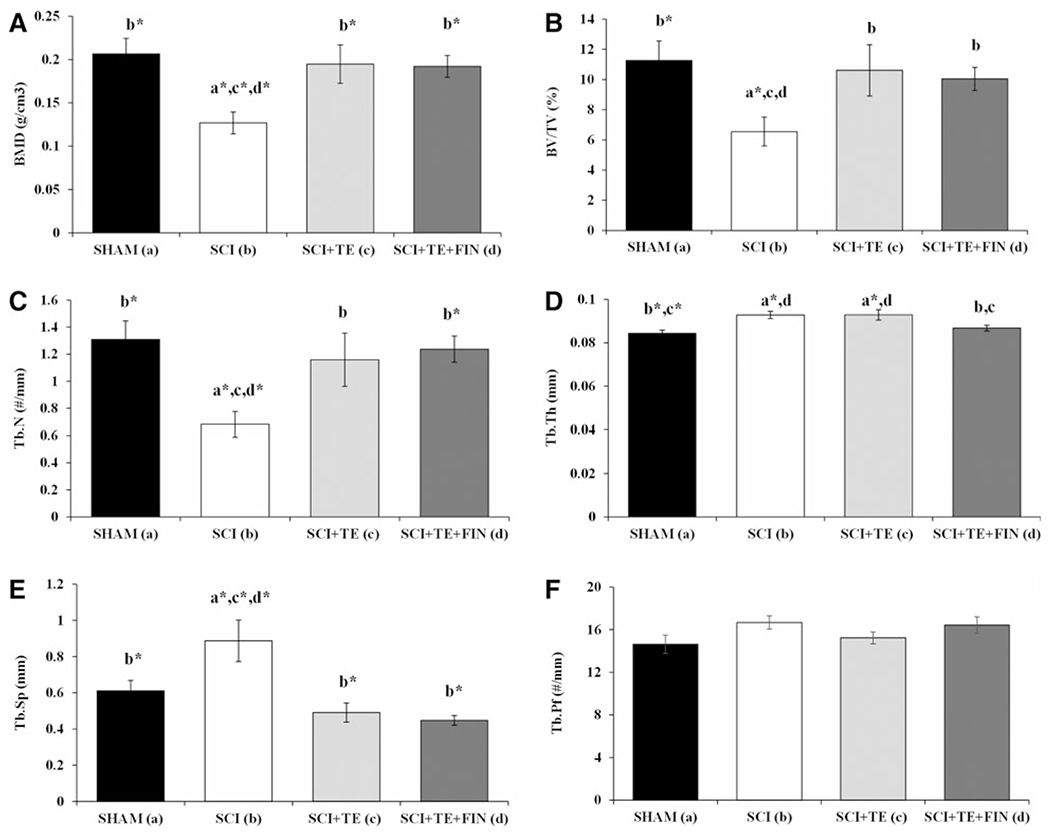
(A–F) Microcomputed tomography (μCT) based cancellous morphology at the distal femoral metaphysis region of interest after sham surgery (T9 laminectomy) or severe spinal cord injury (SCI) alone or in combination with testosterone-enanthate (SCI+TE) or TE plus finasteride (SCI+TE+FIN). Figures are BMD = bone mineral density, BV/TV = cancellous bone volume fraction, Tb.N = trabecular number, Tb.Th = trabecular thickness, Tb.Sp = trabecular separation, and Tb.Pf = trabecular pattern factor. Values are means ± standard error; n = 9–12/group. Letters a–d indicate differences from respectively labeled groups at p < 0.05 or *p < 0.01 (a = vs. SHAM, b = vs. SCI, c = vs. SCI+TE, and d = vs. SCI+TE+FIN). Accompanying μCT images of cancellous bone at this skeletal site are included in Figure 1.
Microcomputed tomography analysis of cortical bone and bone mechanical characteristics
Representative μCT images of cortical bone at the distal femur and femoral diaphysis ROI are presented in Figure 3. SCI animals exhibited a 14% lower Ct.Ar (p < 0.001) and 11% lower Ct.Ar/Tt.Ar (p < 0.01) at the distal femur versus SHAMs (Table 4). TE administration did not prevent these effects, with SCI+TE and SCI+TE+FIN groups exhibiting a 19–20% lower Ct.Ar (p < 0.001) and a 14–20% lower Ct.Ar/Tt.Ar at the distal femur versus SHAM animals (SCI+TE, p < 0.01; SCI+TE+FIN, p ≤ 0.001). SCI+TE+–FIN also exhibited a 9% lower Ct.Th versus SHAMs (p < 0.05), along with a 7% lower Ct.Ar and a 10% lower Ct.Ar/Tt.Ar versus SCI animals (p < 0.05 for both). In contrast, SCI+TE produced a ~2% higher vTMD at the distal femur in comparison with SHAM (p < 0.05) and SCI animals (p < 0.01). Femoral diaphysis Ct.Ar was 5–8% lower in all SCI groups versus SHAMs (SCI, p < 0.01; SCI+TE, trend, p = 0.065; SCI+TE+FIN, p < 0.01), and SCI+TE+FIN exhibited a 7% lower Ct.Th at this skeletal site in comparison to SHAM animals (p < 0.01). No differences in distal femur whole-bone mechanical characteristics were present among groups, with the supracondylar fracture site occurring at 29–32% of the bone length, as measured from the distal end (Supplementary Table 2) (see online supplementary material at http://www.liebertpub.com).
FIG. 3.
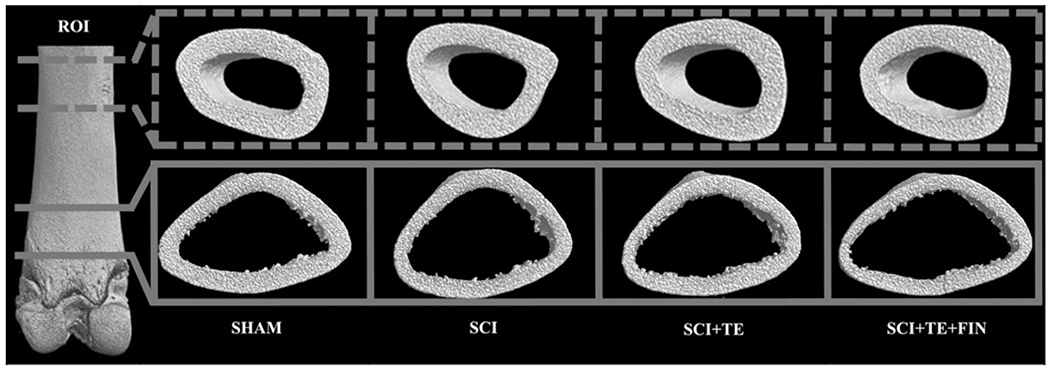
Representative three-dimensional (3D) microcomputed tomography images of cortical bone within the regions of interest (ROI) at the distal femur (solid lines) and femoral diaphysis (dashed lines) after sham surgery (T9 laminectomy) or severe spinal cord injury (SCI) alone or in combination with testosterone-enanthate (SCI+TE) or TE plus finasteride (SCI+TE+FIN). Images represent 3D cross-sectional views of cortical bone with inner cancellous bone removed (distal femur only). Accompanying morphologic data are included in Table 4.
Table 4.
μCT-Based Cortical Morphology at the Distal Femur and Femoral Diaphysis after Sham Surgery (T9 Laminectomy) or Severe Spinal Cord Injury (SCI) Alone or in Combination with Testosterone-Enanthate (SCI+TE) or TE Plus Finasteride (SCI+TE+FIN)
| SHAM (a) | SCI (b) | SCI+TE (c) | SCI+TE+FIN (d) | |
|---|---|---|---|---|
| Distal femur | ||||
| Tt.Ar, mm2 | 17.9 ± 0.53 | 17.0 ± 0.42 | 16.7 ± 0.35 | 17.6 ± 0.66 |
| Ct.Ar, mm2 | 7.36 ± 0.13b*,c*,d* | 6.30 ± 0.08a*,d | 5.95 ± 0.10a* | 5.90 ± 0.18a*,b |
| Ma.Ar, mm2 | 10.6 ± 0.46 | 10.7 ± 0.41 | 10.9 ± 0.31 | 11.7 ± 0.74 |
| Ct.Ar/Tt.Ar, % | 0.41 ± 0.01b*,c*,d* | 0.37 ± 0.01a*,d | 0.36 ± 0.01a* | 0.33 ± 0.02a*,b |
| Ct.Th, mm | 0.433 ± 0.010d | 0.404 ± 0.007 | 0.416 ± 0.013 | 0.396 ± 0.014a |
| TMD, g/mm2 | 1.215 ± 0.006c | 1.209 ± 0.004c* | 1.236 ± 0.006a,b* | 1.223 ± 0.007 |
| Femoral diaphysis | ||||
| Tt.Ar, mm2 | 12.4 ± 0.37 | 11.8 ± 0.20 | 11.9 ± 0.27 | 12.1 ± 0.47 |
| Ct.Ar, mm2 | 7.86 ± 0.17b*,d* | 7.28 ± 0.13a* | 7.46 ± 0.08 | 7.28 ± 0.15a* |
| Ma.Ar, mm2 | 4.53 ± 0.14 | 4.52 ± 0.11 | 4.54 ± 0.20 | 4.79 ± 0.37 |
| Ct.Ar/Tt.Ar, % | 0.64 ± 0.01 | 0.62 ± 0.01 | 0.62 ± 0.01 | 0.61 ± 0.02 |
| Ct.Th, mm | 0.726 ± 0.011d* | 0.693 ± 0.010 | 0.689 ± 0.006 | 0.678 ± 0.017a* |
| TMD, g/mm2 | 1.341 ± 0.005 | 1.342 ± 0.005 | 1.340 ± 0.005 | 1.331 ± 0.006 |
Values are means ± standard error; n = 9–12/group.
Letters a–d indicate differences from respectively labeled groups at p < 0.05 or *p < 0.01 (a = vs. SHAM, b = vs. SCI, c = vs. SCI+TE, and d = vs. SCI+TE+FIN).
μCT, microcomputed tomography; Tt.Ar, total area inside the periosteal envelope; Ct.Ar, cortical bone area; Ma.Ar, medullary area; Ct.Ar/Tt.Ar, cortical area fraction; Ct.Th, cortical thickness; TMD, tissue mineral density.
Androgen sensitive tissue mass
No difference in prostate mass was present among SHAM and SCI animals (Fig. 4A). In contrast, prostate mass was 76–100% higher in SCI+TE compared with SHAM (p < 0.01) and SCI animals (p < 0.001). Coadministration of FIN prevented TE induced prostate enlargement, with SCI+TE+FIN animals exhibiting a prostate mass that was 27% lower than SCI+TE (p < 0.05) and not different than SHAM or SCI animals. LABC mass was 13% lower in SCI versus SHAM animals (p < 0.05; Fig. 4B). TE administration prevented LABC muscle loss and FIN did not interfere with this effect, with SCI+TE and SCI+TE+FIN animals exhibiting an LABC mass that was 20% higher than SCI (p < 0.01) and not different than SHAM.
FIG. 4.
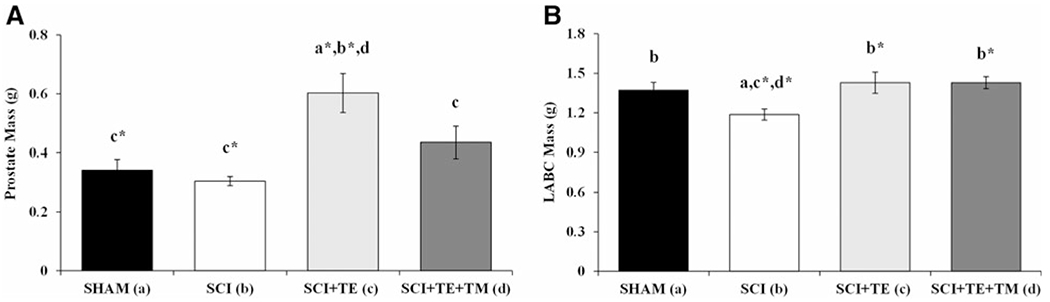
(A and B) Prostate and levator ani/bulbocavernosus (LABC) mass after sham surgery (T9 laminectomy) or severe spinal cord injury (SCI) alone or in combination with testosterone-enanthate (SCI+TE) or TE plus finasteride (SCI+TE+FIN). Values are means ± standard error; n = 10–12/group. Letters a–d indicate differences from respectively labeled groups at p < 0.05 or *p < 0.01 (a = vs. SHAM, b = vs. SCI, c = vs. SCI+TE, and d = vs. SCI+TE+FIN).
Discussion
A relatively high percentage of men with SCI exhibit persistent hypogonadism,10 suggesting that an SCI-induced testosterone deficiency may be one factor exacerbating bone loss post-injury. However, the influence of hypogonadism on SCI-induced bone loss remains controversial,26 and the effectiveness of TRT in preventing bone loss in men with SCI is unknown. We have previously reported that young and skeletally mature male rodents exhibit a testosterone deficit for at least 3 weeks post-SCI and that TE dose dependently prevented cancellous bone loss within this time frame,11,12 indicating that TE acutely influences skeletal maintenance post-SCI. However, we also observed that TE produced a near doubling of prostate mass in our rodent SCI model,11 an effect that may preclude the translation of this therapy. Herein, we expand upon these findings by reporting that a testosterone deficit persists for at least 2 months post-injury in our rodent SCI model, a time frame in which both cancellous and cortical bone loss are observable, and that a combinatory drug regimen involving TE plus FIN (an U.S. Food and Drug Administration—approved type II 5α-reductase inhibitor) completely prevented cancellous bone loss, through antiresorptive mechanisms, without producing prostate enlargement. Interestingly, TE and TE+FIN both prevented the SCI-induced elevation in periosteal bone turnover, but did not prevent cortical bone loss, with SCI+TE+FIN animals exhibiting the lowest cortical bone area and cortical thickness. Our results suggest that TE exerts somewhat divergent effects on cancellous and cortical bone maintenance and that inhibition of type II 5α-reductase activity prevents prostate enlargement, but resulted in greater deterioration of cortical bone structure in our rodent SCI model.
Cancellous bone loss and testosterone after spinal cord injury
The skeletal decline occurring post-SCI is influenced by the neurological insult and subsequent disuse.6 However, bone loss post-SCI is temporally and characteristically different than in other disuse conditions.7 As evidence, the skeletal decline in rodent SCI models occurs at nearly twice the rate7 of that following hindlimb immobilization8,27 or sciatic neurectomy.9 Similarly, humans with motor-complete SCI exhibit bone loss at the rate of ~1% per week in the initial 6–12 months post-injury, which is 4–10 times faster than that resulting from prolonged bed rest or microgravity exposure.7 This exceedingly rapid bone loss results, in part, from the uncoupling of bone turnover (i.e., highly elevated bone resorption rate without a coupled increase in bone formation) that occurs in humans28 and rodents29–31 acutely post-SCI, but that does not exist in other disuse models, suggesting that factors beyond disuse may worsen the SCI-induced skeletal decline.7 Our results support the contention that a testosterone deficit exacerbates cancellous bone loss post-SCI, given that 1) skeletally mature male rats exhibit an ~50% testosterone deficit for at least 2 months post-SCI, in a manner that temporally parallels bone loss, and 2) TE completely prevented cancellous bone loss at the distal femur, in a manner independent from loading. Specifically, we observed that SCI reduced distal femur cancellous bone volume, in a similar magnitude to previous reports.11,12 This skeletal decline appeared to result from elevated bone turnover, given that osteoblast surface and bone formation rate were elevated and that osteoclast surface was increased, albeit nonsignificantly, in SCI versus SHAM animals. In contrast, 2 months of TE reduced circulating and histomorphometric measures of bone resorption and formation, which produced a complete preservation of cancellous bone, supporting the ability of TE to exert antiresorptive effects.32 Interestingly, FIN coadministration did not interfere with the TE-mediated effects on cancellous bone, which provides the first evidence that TE produces long-term maintenance of the cancellous microarchitectural network after SCI, through antiresorptive mechanisms, and that type II 5α-reductase activity is not essential for these effects, even in the relative absence of musculoskeletal loading.
Cortical bone loss and testosterone after spinal cord injury
In comparison with the rapid cancellous bone deficits, cortical bone loss occurs slower in humans3 and rodents post-SCI.29 In our previous studies, we did not observe cortical bone loss in young11 or skeletally mature male rats12 within 3 weeks of injury, despite the presence of reduced periosteal and endocortical bone formation. In contrast, the more chronic study duration used herein produced lower cortical bone area at the femoral diaphysis and the distal femur post-SCI, with the latter being a primary site of fracture in the SCI population.5 This cortical bone loss appears to have resulted from increased bone turnover, evidenced by lower cortical bone volume despite elevated periosteal bone formation, which suggests a delayed reflexive increase in bone formation in response to an earlier elevation in bone resorption. Interestingly, these histomorphometric changes conflict with the reduced periosteal and endocortical bone formation that we observed in SCI animals acutely post-injury,11 suggesting temporal and directional differences in cortical bone turnover may exist in the acute and chronic periods post-SCI. In the current study, TE prevented the SCI-induced elevation in periosteal bone turnover at the tibial diaphysis, evidenced by reduced periosteal bone formation in comparison with SCI animals, but failed to prevent cortical bone loss at either femoral site. These incongruent cortical histomorphometric and morphological changes are difficult to explain, especially given the well-established ability of testosterone to promote cortical bone expansion in normally ambulating animals.33 Regardless, distal femur bone strength was not reduced in SCI+TE animals, likely because TE increased cortical tissue mineral density and completely preserved cancellous bone at this skeletal site, factors that both contribute to whole-bone integrity in a manner independent of cortical geometry.34 The observation that SCI+TE+FIN animals exhibited the lowest cortical bone area is also difficult to explain given that bone exhibits a relatively low type II 5α-reductase expression32 and that ambulatory men with congenital type II 5α-reductase deficiency35 or undergoing pharmacological 5α-reductase inhibition36 exhibit normal BMD. One potential explanation is that testosterone and mechanical loading may work in concert to promote cortical bone maintenance. Indeed, Laurent and colleagues recently reported that testosterone and DHT both prevented cancellous, but not cortical, bone loss in orchiectomized mice that received botulinum toxin A to induce local muscle paralysis.37 However, the clinical relevance of these findings should be interpreted with caution given that rodents lack Haversian remodeling in cortical bone, which predominates intracortical remodeling in humans.38 In this regard, only one small case series has evaluated the skeletal responses to TRT in humans with SCI and observed no changes in femur CSA or in the area of heterotopic ossification after 16 weeks of treatment.16
Testosterone and 5α-reductase in nonskeletal tissues
Testosterone induces anabolic and androgenic effects in bone, prostate, and muscle directly by androgen receptor—mediated actions and indirectly following 5α-reduction to DHT.18,32 The androgenic influence of testosterone depends largely on the tissue-specific expression of the 5α-reductase isozymes, which exhibit considerable intertissue variation.18 For example, type I 5α-reductase is the predominant isozyme in bone.32 As evidence, male 5α-reductase type I knockout mice exhibit reduced cancellous and cortical bone mass in comparison to wild-type animals.39 In contrast, type II 5α-reductase primarily influences prostate growth in adulthood,17 attributed to the high intraprostatic expression of this isozyme. In this regard, our rodent SCI model exhibited a near doubling of prostate mass in response to high-dose TE treatment, an effect that was prevented by FIN, despite a 40% higher circulating testosterone in SCI+TE+FIN animals. These results provide direct evidence that TE-induced prostate enlargement is primarily mediated by type II 5α-reductase activity and not by testosterone. Interestingly, Bauman and colleagues reported that men (n = 11) with motor-complete SCI treated with moderate-dose TRT exhibited no change in prostate-specific antigen over 12 months,40 suggesting that the risk of prostate enlargement may only occur with higher TRT doses in this population. However, Bauman and colleagues did not report skeletal outcomes, so it remains unclear whether the testosterone dose they utilized was sufficient to improve BMD or whether higher doses are required for skeletal efficacy in the SCI population, as has been observed in other non-neurologically impaired hypogonadal populations.41 In addition, SCI produces rapid muscle loss below the level of the lesion.42 Herein, we report that TE preserved mass of the androgen-sensitive LABC muscle post-SCI, supporting our previous findings.11 We expand upon this observation by reporting that FIN did not inhibit the TE-induced preservation of LABC mass post-SCI, which supports the contention that type II 5α-reductase activity is not essential for androgen-mediated muscle maintenance.19,43 This finding is provocative because the sublesional LABC muscle complex is non-weight-bearing, suggesting that TE produces myotrophic actions in a manner independent from loading. Similarly, testosterone has been shown to increase thigh muscle CSA16 and basal metabolic rate in men with motor-complete SCI,40,44 indicating that some functional benefit accompanies increased skeletal muscle mass in paralyzed limbs. We also observed that TE reduced total body mass gain in comparison with SCI animals, suggesting that an attenuation of fat gain may have accompanied the androgen-mediated preservation of musculoskeletal tissue, perhaps through mobilization of nonesterified fatty acids in response to TE administration45 or through an increased metabolic rate,40,44 although these possibilities remain to be determined in our rodent SCI model.
In summary, we report, for the first time, that a combinatory drug regimen involving TE plus FIN normalized cancellous and cortical (periosteal) bone turnover and completely prevented the chronic cancellous bone deficits in our rodent severe contusion SCI model, without producing prostate enlargement, a common androgenic side effect associated with TE treatment. In addition, FIN did not inhibit TE-mediated maintenance of LABC muscle mass. However, neither TE nor TE+FIN treatment prevented cortical bone loss post-SCI. These findings indicate that 1) type II 5α-reductase activity is not essential for TE-mediated preservation of cancellous bone or androgen-sensitive muscle mass post-SCI, even in the relative absence of musculoskeletal loading, 2) actions of the type II 5α-reductase isozyme largely mediate TE-induced prostate enlargement, and 3) TE exerts somewhat divergent effects in cancellous and cortical bone compartments post-SCI. As such, inclusion of TE+FIN in future multi-modal therapeutic interventions that address both the disuse and the molecular dysregulation underlying the SCI-induced musculoskeletal decline are warranted, especially given the high prevalence of hypogonadism in the SCI population.10
Supplementary Material
Acknowledgments
This work was supported by Paralyzed Veterans of America (PVA) fellowship #2939 to Fan Ye and, in part, by resources provided by the North Florida/South Georgia Veterans Health System and by work supported by the Office of Research and Development, Rehabilitation Research and Development (RR&D) Service, Department of Veterans Affairs (VA RR&D SPiRE 1I21RX001273-01) to J.F.Y.. The work reported herein does not represent the views of the U.S. Department of Veterans Affairs or the U.S. government.
Footnotes
Author Disclosure Statement
No competing financial interests exist.
References
- 1.Bauman WA, and Cardozo CP (2015). Osteoporosis in individuals with spinal cord injury. PM R. 7, 188–201. [DOI] [PubMed] [Google Scholar]
- 2.Dauty M, Perrouin Verbe B, Maugars Y, Dubois C, and Mathe JF (2000). Supralesional and sublesional bone mineral density in spinal cord-injured patients. Bone 27, 305–309. [DOI] [PubMed] [Google Scholar]
- 3.Eser P, Frotzler A, Zehnder Y, Wick L, Knecht H, Denoth J, and Schiessl H (2004). Relationship between the duration of paralysis and bone structure: a pQCT study of spinal cord injured individuals. Bone 34, 869–880. [DOI] [PubMed] [Google Scholar]
- 4.Frisbie JH (1997). Fractures after myelopathy: the risk quantified. J. Spinal Cord Med 20, 66–69. [DOI] [PubMed] [Google Scholar]
- 5.Morse LR, Battaglino RA, Stolzmann KL, Hallett LD, Waddimba A, Gagnon D, Lazzari AA, and Garshick E (2009). Osteoporotic fractures and hospitalization risk in chronic spinal cord injury. Osteoporos. Int 20, 385–392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jiang SD, Jiang LS, and Dai LY (2006). Mechanisms of osteoporosis in spinal cord injury. Clin. Endocrinol. (Oxf.) 65, 555–565. [DOI] [PubMed] [Google Scholar]
- 7.Qin W, Bauman WA, and Cardozo C (2010). Bone and muscle loss after spinal cord injury: organ interactions. Ann. N. Y. Acad. Sci 1211, 66–84. [DOI] [PubMed] [Google Scholar]
- 8.Yarrow JF, Ye F, Balaez A, Mantione JM, Otzel DM, Chen C, Beggs LA, Baligand C, Keener JE, Lim W, Vohra RS, Batra A, Borst SE, Bose PK, Thompson FJ, and Vandenborne K (2014). Bone loss in a new rodent model combining spinal cord injury and cast immobilization. J. Musculoskelet. Neuronal Interact 14, 255–266. [PMC free article] [PubMed] [Google Scholar]
- 9.Jiang SD, Jiang LS, and Dai LY (2006). Spinal cord injury causes more damage to bone mass, bone structure, biomechanical properties and bone metabolism than sciatic neurectomy in young rats. Osteoporos. Int 17, 1552–1561. [DOI] [PubMed] [Google Scholar]
- 10.Bauman WA, La Fountaine MF, and Spungen AM (2014). Age-related prevalence of low testosterone in men with spinal cord injury. J. Spinal Cord Med 37, 32–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yarrow JF, Conover CF, Beggs LA, Beck DT, Otzel DM, Balaez A, Combs SM, Miller JR, Ye F, Aguirre JI, Neuville KG, Williams AA, Conrad BP, Gregory CM, Wronski TJ, Bose PK, and Borst SE (2014). Testosterone dose dependently prevents bone and muscle loss in rodents after spinal cord injury. J. Neurotrauma 31, 834–845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Beggs LA, Ye F, Ghosh P, Beck DT, Conover CF, Balaez A, Miller JR, Phillips EG, Zheng N, Williams AA, Aguirre JI, Wronski TJ, Bose PK, Borst SE, and Yarrow JF (2015). Sclerostin inhibition prevents spinal cord injury-induced cancellous bone loss. J. Bone Miner. Res 30, 681–689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sun L, Pan J, Peng Y, Wu Y, Li J, Liu X, Qin Y, Bauman WA, Cardozo C, Zaidi M, and Qin W (2013). Anabolic steroids reduce spinal cord injury-related bone loss in rats associated with increased Wnt signaling. J. Spinal Cord Med 36, 616–622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bergink EW, Janssen PS, Turpijn EW, and van der Vies J (1985). Comparison of the receptor binding properties of nandrolone and testosterone under in vitro and in vivo conditions. J. Steroid Biochem 22, 831–836. [DOI] [PubMed] [Google Scholar]
- 15.Lakshman KM, Kaplan B, Travison TG, Basaria S, Knapp PE, Singh AB, LaValley MP, Mazer NA, and Bhasin S (2010). The effects of injected testosterone dose and age on the conversion of testosterone to estradiol and dihydrotestosterone in young and older men. J. Clin. Endocrinol. Metab 95, 3955–3964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Moore PD, Gorgey AS, Wade RC, Khalil RE, Lavis TD, Khan R, and Adler RA (2016). Neuromuscular electrical stimulation and testosterone did not influence heterotopic ossification size after spinal cord injury: a case series. World J. Clin. Cases 4, 172–176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cui Y, Zong H, Yang C, Yan H, and Zhang Y (2013). The effect of 5alpha-reductase inhibitors on prostate growth in men receiving testosterone replacement therapy: a systematic review and meta-analysis. Int. Urol. Nephrol 45, 979–987. [DOI] [PubMed] [Google Scholar]
- 18.Yarrow JF, McCoy SC, and Borst SE (2012). Intracrine and myotrophic roles of 5alpha-reductase and androgens: a review. Med. Sci. Sports Exerc 44, 818–826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Borst SE, Yarrow JF, Conover CF, Nseyo U, Meuleman JR, Lipinska JA, Braith RW, Beck DT, Martin JS, Morrow M, Roessner S, Beggs LA, McCoy SC, Cannady DF 2nd, and Shuster JJ (2014). Musculoskeletal and prostate effects of combined testosterone and finasteride administration in older hypogonadal men: a randomized, controlled trial. Am. J. Physiol. Endocrinol. Metab 306, E433–E442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Amory JK, Watts NB, Easley KA, Sutton PR, Anawalt BD, Matsumoto AM, Bremner WJ, and Tenover JL (2004). Exogenous testosterone or testosterone with finasteride increases bone mineral density in older men with low serum testosterone. J. Clin. Endocrinol. Metab 89, 503–510. [DOI] [PubMed] [Google Scholar]
- 21.Basso DM, Beattie MS, and Bresnahan JC (1996). Graded histological and locomotor outcomes after spinal cord contusion using the NYU weight-drop device versus transection. Exp. Neurol 139, 244–256. [DOI] [PubMed] [Google Scholar]
- 22.Yarrow JF, Conover CF, Purandare AV, Bhakta AM, Zheng N, Conrad B, Altman MK, Franz SE, Wronski TJ, and Borst SE (2008). Supraphysiological testosterone enanthate administration prevents bone loss and augments bone strength in gonadectomized male and female rats. Am. J. Physiol. Endocrinol. Metab 295, E1213–E1222. [DOI] [PubMed] [Google Scholar]
- 23.Amory JK, Wang C, Swerdloff RS, Anawalt BD, Matsumoto AM, Bremner WJ, Walker SE, Haberer LJ, and Clark RV (2007). The effect of 5alpha-reductase inhibition with dutasteride and finasteride on semen parameters and serum hormones in healthy men. J. Clin. Endocrinol. Metab 92, 1659–1665. [DOI] [PubMed] [Google Scholar]
- 24.di Salle E, Giudici D, Biagini L, Cominato C, Briatico G, and Panzeri A (1995). Effects of 5 alpha-reductase inhibitors on intraprostatic androgens in the rat. J. Steroid Biochem. Mol. Biol 53, 381–385. [DOI] [PubMed] [Google Scholar]
- 25.Beck DT, Yarrow JF, Beggs LA, Otzel DM, Ye F, Conover CF, Miller JR, Balaez A, Combs SM, Leeper AM, Williams AA, Lachacz SA, Zheng N, Wronski TJ, and Borst SE (2014). Influence of aromatase inhibition on the bone-protective effects of testosterone. J. Bone Miner. Res 29, 2405–2413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gaspar AP, Brandao CM, and Lazaretti-Castro M (2014). Bone mass and hormone analysis in patients with spinal cord injury: evidence for a gonadal axis disruption. J. Clin. Endocrinol. Metab 99, 4649–4655. [DOI] [PubMed] [Google Scholar]
- 27.Liu D, Zhao CQ, Li H, Jiang SD, Jiang LS, and Dai LY (2008). Effects of spinal cord injury and hindlimb immobilization on sublesional and supralesional bones in young growing rats. Bone 43, 119–125. [DOI] [PubMed] [Google Scholar]
- 28.Battaglino RA, Lazzari AA, Garshick E, and Morse LR (2012). Spinal cord injury-induced osteoporosis: pathogenesis and emerging therapies. Curr. Osteoporos. Rep 10, 278–285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lin T, Tong W, Chandra A, Hsu SY, Jia H, Zhu J, Tseng WJ, Levine MA, Zhang Y, Yan SG, Liu XS, Sun D, Young W, and Qin L (2015). A comprehensive study of long-term skeletal changes after spinal cord injury in adult rats. Bone Res. 3, 15028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Morse LR, Xu Y, Solomon B, Boyle L, Yoganathan S, Stashenko P, and Battaglino RA (2011). Severe spinal cord injury causes immediate multi-cellular dysfunction at the chondro-osseous junction. Transl. Stroke Res 2, 643–650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Morse L, Teng YD, Pham L, Newton K, Yu D, Liao WL, Kohler T, Muller R, Graves D, Stashenko P, and Battaglino R (2008). Spinal cord injury causes rapid osteoclastic resorption and growth plate abnormalities in growing rats (SCI-induced bone loss in growing rats). Osteoporos. Int 19, 645–652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Yarrow JF, Wronski TJ, and Borst SE (2015). Testosterone and adult male bone: actions independent of 5alpha-reductase and aromatase. Exerc. Sport Sci. Rev 43, 222–230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Callewaert F, Sinnesael M, Gielen E, Boonen S, and Van-derschueren D (2010). Skeletal sexual dimorphism: relative contribution of sex steroids, GH-IGF1, and mechanical loading. J. Endocrinol 207, 127–134. [DOI] [PubMed] [Google Scholar]
- 34.Fonseca H, Moreira-Goncalves D, Coriolano HJ, and Duarte JA (2014). Bone quality: the determinants of bone strength and fragility. Sports Med. 44, 37–53. [DOI] [PubMed] [Google Scholar]
- 35.Sobel V, Schwartz B, Zhu YS, Cordero JJ, and Imperato-McGinley J (2006). Bone mineral density in the complete androgen insensitivity and 5alpha-reductase-2 deficiency syndromes. J. Clin. Endocrinol. Metab 91, 3017–3023. [DOI] [PubMed] [Google Scholar]
- 36.Amory JK, Anawalt BD, Matsumoto AM, Page ST, Bremner WJ, Wang C, Swerdloff RS, and Clark RV (2008). The effect of 5alpha-reductase inhibition with dutasteride and finasteride on bone mineral density, serum lipoproteins, hemoglobin, prostate specific antigen and sexual function in healthy young men. J. Urol 179, 2333–2338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Laurent MR, Jardi F, Dubois V, Schollaert D, Khalil R, Gielen E, Carmeliet G, Claessens F, and Vanderschueren D (2016). Androgens have antiresorptive effects on trabecular disuse osteopenia independent from muscle atrophy. Bone 93, 33–42. [DOI] [PubMed] [Google Scholar]
- 38.Lelovas PP, Xanthos TT, Thoma SE, Lyritis GP, and Dontas IA (2008). The laboratory rat as an animal model for osteoporosis research. Comp. Med 58, 424–430. [PMC free article] [PubMed] [Google Scholar]
- 39.Windahl SH, Andersson N, Borjesson AE, Swanson C, Svensson J, Moverare-Skrtic S, Sjogren K, Shao R, Lagerquist MK, and Ohlsson C (2011). Reduced bone mass and muscle strength in male 5alpha-reductase type 1 inactivated mice. PLoS One 6, e21402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Bauman WA, Cirnigliaro CM, La Fountaine MF, Jensen AM, Wecht JM, Kirshblum SC, and Spungen AM (2011). A small-scale clinical trial to determine the safety and efficacy of testosterone replacement therapy in hypogonadal men with spinal cord injury. Horm. Metab. Res 43, 574–579. [DOI] [PubMed] [Google Scholar]
- 41.Borst SE, and Yarrow JF (2015). Injection of testosterone may be safer and more effective than transdermal administration for combating loss of muscle and bone in older men. Am. J. Physiol. Endocrinol. Metab 308, E1035–E1042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ye F, Baligand C, Keener JE, Vohra R, Lim W, Ruhella A, Bose P, Daniels M, Walter GA, Thompson F, and Vandenborne K (2013). Hindlimb muscle morphology and function in a new atrophy model combining spinal cord injury and cast immobilization. J. Neurotrauma 30, 227–235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Yarrow JF, Conover CF, McCoy SC, Lipinska JA, Santillana CA, Hance JM, Cannady DF, VanPelt TD, Sanchez J, Conrad BP, Pingel JE, Wronski TJ, and Borst SE (2011). 17beta-Hydroxyestra-4,9,11-trien-3-one (trenbolone) exhibits tissue selective anabolic activity: effects on muscle, bone, adiposity, hemoglobin, and prostate. Am. J. Physiol. Endocrinol. Metab 300, E650–E660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Bauman WA, La Fountaine MF, Cirnigliaro CM, Kirshblum SC, and Spungen AM (2015). Lean tissue mass and energy expenditure are retained in hypogonadal men with spinal cord injury after discontinuation of testosterone replacement therapy. J. Spinal Cord Med 38, 38–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Holland AM, Roberts MD, Mumford PW, Mobley CB, Kephart WC, Conover CF, Beggs LA, Balaez A, Otzel DM, Yarrow JF, Borst SE, and Beck DT (2016). Testosterone inhibits expression of lipogenic genes in visceral fat by an estrogen-dependent mechanism. J. Appl. Physiol. (1985) 121, 792–805. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


