Abstract
Herpes zoster (HZ) causes considerable pain and distress, and γ-Aminobutyric acid (GABA) and its derivatives are assumed to control this, but the available data are inconsistent. This meta-analysis and systematic review aimed to assess the effectiveness of GABA derivatives in the prevention of acute herpetic pain. The meta-analysis was conducted following the PRISMA guidelines using PICO format, registered in PROSPERO number CRD42018095758. PubMed, Web of Science, Ovid, Scopus, and EMBASE databases were searched. Records were included if they were randomized controlled trials of patients undergoing HZ infection, investigating the effect of GABA derivatives versus placebo in the treatment of HZ pain. Eligible trials were evaluated for the risk of bias. Then data were extracted and analysed. The number of patients with observed presence of pain after treatment was used to calculate odds ratio in a random effect model with the DerSimonian-Laird estimator. The I2 statistic was analysed for heterogeneity. The potential risk of bias was measured using Egger’s regression test. The meta-analysis included three randomized controlled trials with a total of 297 patients. The incidence of acute HZ pain events for GABA group was significantly lower compared to placebo group,18/148 vs 44/149, respectively (OR = 0.36; 95% CI = 0.14 to 0.93; Z = 2.11; P = 0.035), Egger’s test yielded P = 0.308. In conclusion, the present meta-analysis demonstrates that GABA derivatives reduce the incidence of acute herpetic pain. However, additional, well-designed randomized clinical trials are needed to determine their dose- and time-dependency regarding this symptom.
Keywords: Acute herpes zoster pain, herpes zoster associated pain, GABA, γ-aminobutyric acid, meta-analysis, Egger’s test
1. INTRODUCTION
Reactivated Varicella-Zoster Virus (VZV) in sensory ganglia causes infection of the skin, which is termed as herpes zoster (HZ) [1, 2]. The prevalence of HZ is high. In the USA, around 500000 persons experience reactivation of HZ infection each year and the incidence of HZ in the population having normal immune response is around 1.2 to 3.4 per 1000 patient-year [2]. The clinical manifestation of HZ is defined by a painful, unilateral erythematous maculopapular rash, which normally involves 1-2 dermatomes. The rash evolves into groups of clear vesicles within 48-72 hours, then becomes pustulous, ulcerous and crusty in 7-10 days [1, 3, 4]. The disease causes generalized necrosis of nerves, nerve root and ganglion, lowering the threshold for nociceptive pain and initiating ectopic discharges, accompanied by moderate or severe pain [5, 6]. As a result, excessive peripheral nociception input induces abnormal reorganization of pain transmission system [6, 7]. At the cellular level, the disease upregulates receptors associated with pain, such as transient receptor potential vanilloid 1 (TRPV1), as well as an increasing proportion of voltage-gated sodium channels and potassium voltage-gated channels. There is also evidence of loss of γ-aminobutyric acid (GABA) inhibitory interneuron in the dorsal horn [6].
Most of the patients experience paresthesia, dysesthesia, hyperesthesia and allodynia during the course of this disease. Pain is the most common complaint in HZ infection. It tends to improve spontaneously with time or persist unpredictably [4, 8]. Thus, some patients experience HZ-associated pain after the healing of the skin lesion [8]. HZ-associated pain has been classified into acute zoster pain (acute herpetic neuralgia), subacute zoster pain (subacute herpetic neuralgia) and postherpetic neuralgia (PHN) by time frame [9]. Basically, acute pain can be derived from the initial rash, and it lasts no longer than one month; subacute pain from 1 to 4 months after the onset of the disease; PHN develops four months after the initial lesion [5, 9]. Although most investigators reported persistent pain of PHN for at least three months after the healing of the rash, others described persistent pain as early as 3-4 weeks or until six months after the healing of the rash [1, 5]. All definitions of PHN are arbitrary and range from 1 to 6 months. For clinical trials, neuralgia of 3 or more months has become the most common definition [10-13]. Determination of the point at which acute HZ pain transforms to PHN is controversial [3].
PHN will become more prevalent because of the increasing incidence of HZ and its nature to develop in the elderly, whose number is increasing [7, 14]. However, the prevalence of PHN depends on when it is measured. There is no agreement among scientists on the time point for diagnosis [15]. Pain severity during the acute phase and the extension of the lesion are risk factors of persistent pain development with relative risks 18.0 (Confidence Interval (CI) 6.6 - 48.6) and 5.3 (CI 4.2 - 17.2), respectively [16]. Advanced age, the use of immunosuppressives, and cancer were also mentioned as risk factors [1, 2, 5].
An essential treatment objective during acute HZ is to keep the patient comfortable and prevent HZ pain to become chronic [2]. Systemic and local treatments are used for pain relief and patient comfort, apart from antiviral agents. Tricyclic antidepressants, anticonvulsants, opioids, topical lidocaine and topical capsaicin, have been used for analgesic intervention in both acute and chronic forms of zoster pain [1, 2, 5, 13, 17-19]. There are indications for the usefulness of local anaesthesia, neurolytic block of sympathetic nerves, acupuncture, spinal intrathecal injection, intercostal nerve block, spinal cord stimulation, cryotherapy and botulinum toxin injection in order to treat HZ pain [20-22].
Gabapentin is a GABA analogue, binding at the α2-δ sub-unit of voltage-dependent calcium channels. It is assumed to have antineuralgic activity and anti-sensitization, modulation of GABAergic, glutaminergic and monoaminergic functions, and disruption of the neuropathic pain development process. It has been shown to inhibit ectopic discharge activities of injured peripheral nerves [1, 19, 23]. Pregabalin is also an analogue of GABA. It has a similar pharmacological profile as gabapentin [12, 24-26]. These agents are well absorbed via oral administration and excreted unchanged in the urine. The drugs do not undergo cytochrome P 450 metabolism [24, 25, 27, 28].
Previous systematic reviews and meta-analyses demonstrated no effects of steroid agents [29] and antiviral agents [30] in the prevention of PHN. Yet, there is no available clinical guideline or recommendation regarding the optimal dose of GABA derivatives preventing acute zoster pain, and PHN [4]. The results from the few available studies are contradictory. Thus, a complex approach to the effect of GABA derivatives is needed. Therefore, the purpose of this work was to investigate the effectiveness of γ-aminobutyric acid and its derivatives in the reduction of acute herpes zoster pain after herpes zoster, using the powerful tools of meta-analysis.
2. METHODS
This meta-analysis was conducted according to the Preferred Reporting Items for Systematic reviews and Meta-analysis protocol (PRISMA). To investigate the efficacy of GABA and its derivatives to reduce the acute HZ pain occurrence, the following PICO framework was applied. Population: herpes zoster; Intervention: GABA and its derivatives; Comparison: placebo treatment and Outcome: the presence of acute HZ pain. The protocol was registered in the International Prospective Register of Systematic Reviews (PROSPERO) on May 31, 2018 (registration number: CRD42018095758).
2.1. Eligibility Criteria
2.1.1. Types of Studies and Interventions
Randomized controlled trials were eligible for our review; participants of all ages with diagnosed HZ were considered. All degrees of pain severity and all areas of distribution of the disease were acceptable. We considered GABA and its derivatives such as orally administered gabapentin versus placebo treatments after HZ. Other routes of administration were excluded from this review.
2.1.2. Type of Outcome Measurement
Primary outcomes. The primary outcome was the presence of HZ-associated pain after treatments. We divided HZ-associated pain into subgroups depending on their duration from the onset of the initial rashes. We considered the pain acute if it started within a month after the onset of the initial rash, subacute pain between 1 month and four months after the initial rash, and PHN that stated four months after the initial lesion [9]. The severity of pain was measured with reliable methods such as visual analogue scale and numerical rating scale.
Secondary outcomes. Adverse events were considered as secondary outcomes. We defined these phenomena as unfavourable events, either caused by the treatment or by the disease being treated and resulted from causes that appear irrelevant to the treatment or the disease [31].
2.2. Search Methods for Identification of Studies
We performed a search for all randomized controlled trials from five different databases. The language of the literature search was restricted to English.
2.2.1. Electronic Search
The search was performed on August 8, 2018. PubMed, Web of Science, Ovid, Scopus, and EMBASE were searched for relevant studies. The following search terms were applied: herpes zoster, gamma-aminobutyric acid, Gaba, gabapentin, neurontin, and gabapentin were adapted and used in different database platforms. The searching queries are show in Appendix A. All search results were imported and pooled in EndNote® X7 (Thomson Reuter, PA, USA).
2.3. Data Collection and Analysis
2.3.1. Selection of Studies
After removing duplicated records, two review authors (W.S. and T.S.) individually screened the titles and abstracts of imported articles in EndNote for relevant studies. Each author decided which trials were eligible for inclusion criteria and discussed to resolve disagreements about inclusion criteria. Disagreements between reviewers were resolved by discussion or, if necessary, by consulting a third reviewer (G.G).
2.3.2. Data Extraction and Management
Data collection was executed following the PRISMA guidelines. Study characteristics and results were extracted by two reviewers (W.S. and T.S.) independently. Discrepancies in the extracted data were resolved by discussion. The following items were extracted from the included papers: first author, year of publication, country, number of study centres, design of study, size of population, intervention and duration of intervention, demographic data, distribution and severity of lesion, number of dropout participants, reasons for dropouts, primary and secondary outcomes, other relevant information that is not specified in the study protocol.
2.3.3. Assessment of Risk of Bias in the Included Studies
Two authors (W.S. and T.S.) independently assessed the risk of bias of the included trials using the Cochrane Risk of Bias Tool [32]. All key domains, except the other biases domain, were considered to the assessment. The authors independently categorized each study into a low risk of bias if all key domains were low risk; an unclear risk of bias when there were one or more unclear risks of bias in the key domains; and a high risk of bias when there were one or more high risks of bias in the key domains [32]. The risk of bias summary table and figure were generated by the REVMan5 software.
2.3.4. Assessment of Quality of Evidence
The quality of scientific evidence was rated by the Grading of Recommendations, Assessment, Development and Evaluation (GRADE) approach using GRADEPro® (McMaster University, Hamilton, Canada). The quality of evidence is based on the risk of bias, inconsistency in results, indirectness of evidence, imprecision of results, and other considerations such as publication bias, large effect, plausible confounding and dose-response gradient, as described in the handbook for grading the quality of evidence and the strength of recommendations using the GRADE approach [33]. The quality was judged as high, moderate, low and very low.
2.3.5. Measures of Treatment Effect
The statistical analysis was conducted using Stata 11 SE (Stata Corp LLC, College Station, TX, USA). The number of patients with observed presence of pain in the test groups and control groups was used to calculate the odds ratio (OR). The OR > 1 indicates an elevated risk of acute zoster pain in the treatment group compared to the control group, while OR < 1 indicates a lower risk in the treatment group. ORs were pooled using the random-effects model with the DerSimonian-Laird estimator and displayed on forest plots as described previously [34, 35]. Summary OR estimation, and 95% confidence interval (CI) were calculated. P < 0.05 was considered as a significant difference from summary OR=1. Statistical heterogeneity was analyzed using the I2 statistic to gain probability values; p<0.05 was defined, indicating significant heterogeneity. We investigated the possible signs of a small study effect displaying the studies on a funnel plot and formally conducting the Egger’s regression test, where p < 0.05 indicates potential risks of bias.
3. RESULTS
3.1. Description of Studies
The characteristics of the included studies are listed in Table 1 and Appendix B.
Table 1.
Summary of study characteristics.
| First Author | Country | Intervention | Duration of Treatment |
Study Size
(Test/ Placebo) |
Mean Age | Sex (M/F) |
Time
Receiving Treatment |
Antiviral Treatment |
|---|---|---|---|---|---|---|---|---|
| Skvarc N. et al. 2010 | Slovenia | • 75 mg pregabalin po bid (150 mg/day) • placebo po bid • [can rise to 150 mg po bid (300 mg/day)] |
3 weeks treatment period | 29 (14/15) | Test: 67±13 Placebo: 63±13 |
10/19 | Test:12±2 (days) Placebo: 11±2 (days) |
Test: 80% Placebo: 100% |
| Wang Q. et al. 2013 | Korea | • Standard treatment • Standard treatment with gabapentin |
Not reported | 120 (60/60) | Not reported | Not reported | Not reported | Yes |
| Lee E.G. et al. 2016 | China | • 1000 mg valacyclovir tid for 7 days + 650mg acetamenophen tid + Gabapentin 300 mg tid [stepwise in 3 days (900mg/day)] • Placebo (1000 mg valacyclovir tid for 7 days + 650 mg acetamenophen tid) |
12 weeks in some cases it was extend to 24 weeks | 148 (74/74) | treatment/control (age range): 24/25(50-59), 23/24(60-69), 13/11(≥70) | 43/77 | within 96 hrs after onset of rash | Yes |
Abbreviations: RCT, randomized clinical trial.
3.1.1. Results of Search
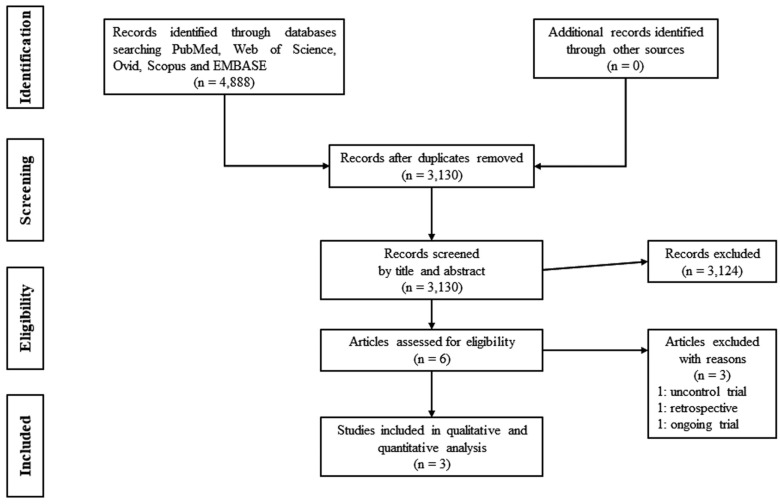
The literature search yielded a total of 3130 potentially relevant reports. After screening titles and abstracts, six records were relevant to our meta-analysis (Fig. 1). Out of these, three records were excluded because one was an uncontrolled trial; another one was a retrospective study [36, 37], while the third one was an ongoing trial [38]. We extracted the data from the remaining three suitable records [39-41]. The characteristics of these included studies are described in Table 1, and the outcomes in Table 2.
Fig. (1).
PRISMA 2009 flow diagram for identification of relevant studies.
Table 2.
Summary of study outcomes.
| First Author | Primary Outcome (Test/Placebo) | Secondary Outcome (Test/Placebo) | Statistical Methods | Note |
|---|---|---|---|---|
| Skvarc N. et al 2010 | 1-3 months (42.86%/46.67%) 6 months (14.29%/20%) |
Vertigo (50%/33.33%) Dizziness (64.29%/26.67%) Somnolence (64.29%/26.67%) Fatigue (57.14%/53.33%) Diplopia (14.29%/20%) Constipation (28.57%/13.33%) Flatulence (14.29%/13.33%) |
According to the authors data were analysed using appropriate statistical tests without further specification. P < 0.05 | PHN - after 6 months |
| Wang Q. et al. 2013 | 1 month (13%/45%) | Not reported | Statistical test were performed based on the intent-to-treat population | PHN - aftrer1 month |
| Lee E.G. et al. 2016 | ¼ month (57.5%/40.8%) ¾ month (28.8%/16.3%) 1 ¼ month (19.2%/6.1%) 2 months (9.6%/6.1%) 3 months (3.8%/6.1%) |
Dizziness (13.33%/5%) Fatigue (6.67%/5%) Constipation (8.33%/6.67%) Appetite change (5%/5%) Dyspepsia (11.67%/10%) Edema (5%/1.67%) Headache (1.67%/1.67%) Nausea (11.67%/8.33%) Sedation (1.67%/0%) |
Independent t-test, χ2, Fisher's exact, Binary logistic regression, P < 0.05 |
PHN - persisting pain more than 3 months |
3.1.2. Included Studies
Three trials with a total of 297 participants were included. Inclusion and exclusion criteria were clearly stated in two reports [39, 40]. However, an additional work, an abstract, did not state any detailed information regarding the primary outcome [41]. Thus, we could extract data only from two studies [39, 40]. These studies recruited participants at different time points of the infection. The study conducted by Lee et al., [39] involved participants of 50 years of age or more, while the other study [40] included patients aged 30-80 years. There were no significant differences in the demographic data (e.g., gender, age) of the treatment and the placebo group. The distribution of herpes rash was reported in both studies [39, 40]. Shared exclusion criteria in both trials consisted of allergy to treatment, psychiatric diagnosis, pregnancy and lactation, anticonvulsant therapies, antidepressant therapies, the use of clonidine or ketamine or local anaesthesia. None of the trials mentioned HZ vaccination of the participants, and there were no notes on special populations, such as immunocompromised patients involved in these studies.
Apart from the standard treatments of HZ, some treatment modalities were introduced as additional treatments to reduce herpetic pain and prevent PHN development. One trial [40] applied antiviral agents and 75 mg of pregabalin twice a day. In the other trial [39], participants received 300 mg of gabapentin three times per day combined with 1000 mg of Valacyclovir and 650 mg of acetaminophen three times daily. Lee et al., used stepwise doses of gabapentin every day, up to 900 mg three times a day [39]. Participants in the trial of Wang et al., [41] received standard treatments, either gabapentin or placebo. The authors did not state more details about the treatments. None of the included trials reported their methods of randomization. Follow-up was performed by phone calls [40] and physicians’ inspection [39], while Wang et al. [41] did not describe the method of follow-up. Loss of participants during the follow-ups was reported in the works of Lee et al., [39] and Skvarc et al., [40], but the former one did not give any details on the reasons for dropouts.
The outcome measurements were reported in different ways, including the presence of zoster pain after treatment. Every trial reported different cut-off times. Skvarc et al., reported the incidence of subacute herpetic neuralgia (1-3 months) and PHN (6 months) [40], while Lee et al., reported the presence of pain at weeks 1, 3, 5 and 8 and PHN incidence (3 months) [39]. Wang et al., reported the presence of zoster pain at months 1, 3, 6 and 12 [41]. The Likert’s pain scale was used in two trials [39, 40], while the third trial [41] did not explain the tools of pain measurement. Dermatology life quality index was used by Lee et al., whereas sleep disturbance was measured by Skvarc et al. [39, 40]. Two included trials [39, 40] reported adverse events during the treatment period. The most frequently reported adverse events in both studies were dizziness, somnolence, fatigue and constipation.
3.1.3. Excluded Studies
Out of the six studies remaining after screening by title and abstract, one report was excluded because it was an uncontrolled, non-randomized study [36]. Another study was also excluded because it was retrospective [37]. Our search yielded one ongoing study, in which the investigators declared a progression of 70%, but the results of this study have not been published. Therefore, we could not include this trial in our meta-analysis [38].
3.1.4. Risk of Bias in the Included Studies
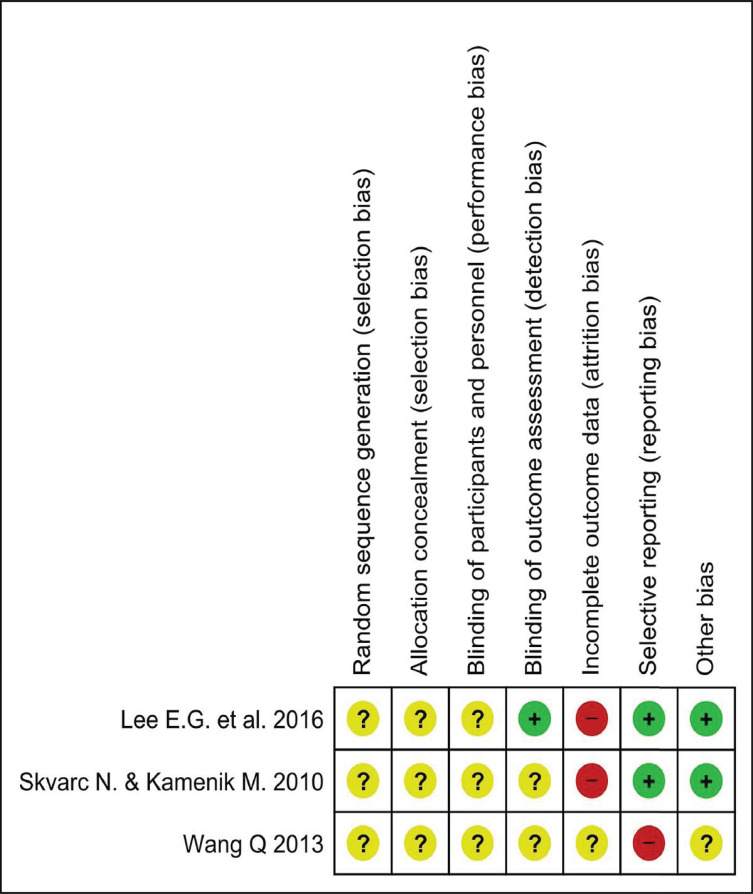
All of the included trials were rated as poor quality because there were high risks of bias and unclear risks of bias in many key domains. The risk of bias assessment graph and the summary show the judgement of the reviewers (Fig. 2 and Fig. S1 (144.9KB, pdf) ).
Fig. (2).
Risk of Bias Assessment summary: review authors’ judgements about each methodological quality item presented in different aspect from each included study. (A higher resolution / colour version of this figure is available in the electronic copy of the article).
All included trials were single-center randomized studies, but only the trial of Skvarc and coworkers was a randomized, double-blinded, placebo-controlled study [40]. The work of Lee and coinvestigators was a phased, randomized, controlled study [39]. Nevertheless, none of the studies described the method of randomization. In two studies, there was no information about hiding allocation [39, 40]. The study conducted by Wang et al., was a randomized trial, but the method used for randomization was not described [41].
3.1.5. Blinding
Blinding of participants and personnel was unclear in all studies, because of the insufficient information provided. Only one study described the method used for blinding of outcome assessment [39]. Skvarc et al., did not clearly mention how the outcome assessment was blinded [40].
3.1.6. Incomplete Outcome Data
The included studies reported different time frames and duration of follow-ups. Lee et al., followed up the patients for three months [39], Skvarc et al., for six months [40] and Wang et al., for 12 months [41]. The number of participants who completed the follow-ups and those having dropped out prior to the completion of the study was described in each of the three studies. It was the study of Skvarc et al., alone that described the reasons for dropouts [40]. The reasons for dropouts were not described in the trials conducted by Wang et al., and Lee et al., however, they had considerably lower dropouts than the study of Skvarc et al. [39, 41].
3.1.7. Selective Reporting
Two studies reported all pre-specified and expected outcomes of interest as planned protocol [39, 40]. One trial reported an incomplete outcome [41].
3.1.8. Other Potential Sources of Bias
The publication bias was assessed by visual evaluation using a funnel plot. However, the limited number of studies did not allow realistic evaluation (Fig. S2 (144.9KB, pdf) ), as described in section 10.4.3.1 of the Cochrane Handbook [32].
3.1.9. Quality of Evidence
The result of the GRADE assessment is shown in Table 3. In the beginning, all RCTs were considered as high-quality evidence; however, we had to downgrade them because of the very high risk of bias, including publication bias. Therefore, the overall quality of evidence is very low.
Table 3.
Rating the quality of evidence using GRADE approach.
| Certainty Assessment | No. of Patients | Effect | Certainty | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| No. of studies | Study Design | Risk of Bias | Inconsistency | Indirectness | Imprecision | Other Considerations | GABA | Placebo/no Treatment |
Relative
(95% CI) |
Absolute
(95% CI) |
|
| Prevalence of Acute Herpes Zoster Pain After GABA and Derivatives Use | |||||||||||
| 3 | randomised trials | very serious a | not serious | not serious | not serious | publication bias strongly suspected b | 18/148 (12.2%) | 44/149 (29.5%) | OR 0.36 (0.14 to 0.93) |
164 fewer per 1.000 (from 15 fewer to 240 fewer) |
⨁◯◯◯ VERY LOW |
Notes:a Each RCT has a high risk of bias in major domains and some unclear risk of biases.
b We can access only to abstract information.
Abbreviations: CI, Confidence interval; OR, Odds ratio.
3.2. Effects of Interventions
3.2.1. Primary Outcome: The Presence of Acute Zoster Pain After Treatment
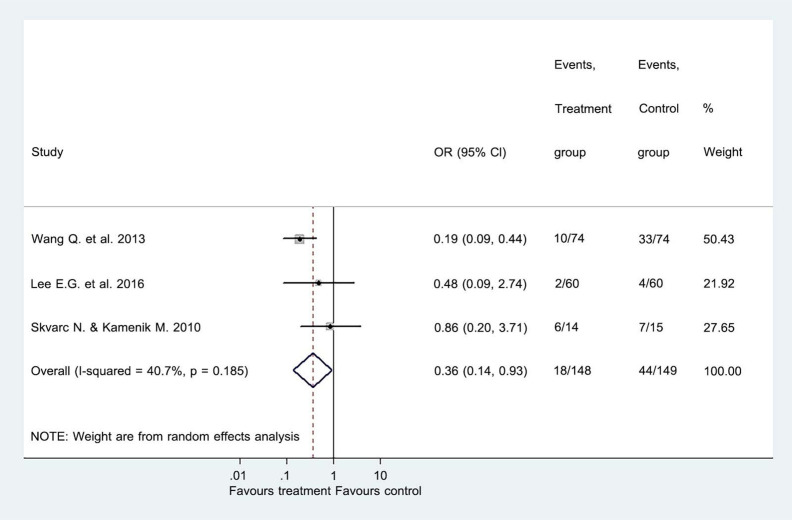
The three included trials compared the effects of GABA and its derivatives with placebo treatment involving 297 participants. Our meta-analysis showed that the incidence of acute zoster pain in the GABA and derivatives group was significantly lower compared to the placebo group, 18/148 vs 44/149, respectively (OR = 0.36; 95% CI = 0.14 to 0.93; Z = 2.11; P = 0.035; Fig. 3). Our analysis indicated that GABA and its derivatives could prevent acute zoster pain in a considerable number of patients after HZ infection. The Egger’s test suggested no sign of statistical bias (P = 0.308).
Fig. (3).
Forest plot of comparison I: GABA or its derivatives vs placebo, primary outcome measurement. The presence of PHN after treatment. . Note: Odd ratio (OR), Confident Interval (CI). (A higher resolution / colour version of this figure is available in the electronic copy of the article).
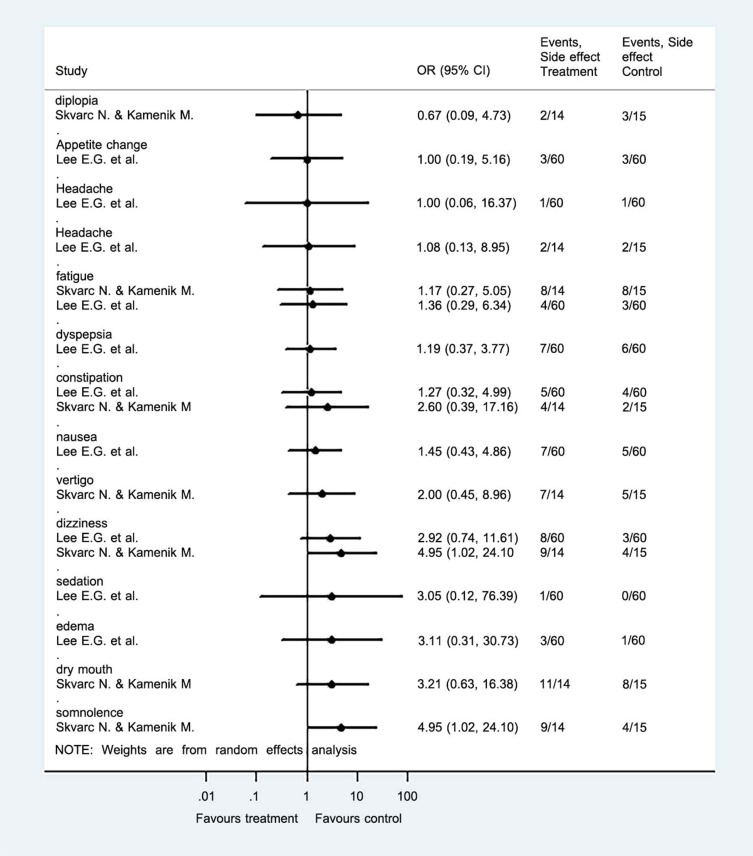
3.2.2. Secondary Outcome: The Presence of Adverse Events During Treatment
The adverse events were evaluated in many aspects such as somnolence, fatigue, diplopia, constipation, flatulence, change in appetite, dyspepsia, oedema, headache, nausea, sedation, and dry mouth. The data showed no serious side effects (Fig. 4). However, some fatigue, constipation and dizziness have been reported in the studies of Skvarc et al., and Lee et al., [39, 40].
Fig. (4).
Forest plot of comparison II: GABA or its derivatives vs placebo, secondary outcome measurement. The presence of adverse events during treatment. Note: Odd ratio (OR), Confident Interval (CI). (A higher resolution / colour version of this figure is available in the electronic copy of the article).
3.2.3. Additional Outcome Measure not Specified in the Protocol: Quality of Life
Only one trial reported results concerning the quality of life, using the Dermatology Life Quality Index (DLQI) between tested and controlled groups during the follow-up. The mean difference DLQI between the gabapentin-treated group and the placebo group was not significant [39].
4. DISCUSSION
The aim of the present study was to investigate the effectiveness of GABA and its derivatives in reducing acute pain incidence in patients having HZ. We could only include three randomized trials involving 297 participants from Slovenia, Korea and China, yielding an inhomogeneous patient population. The results showed that the treatment with GABA and its derivatives significantly reduced the number of patients with acute zoster pain. However, the absence of acute herpetic pain in one of the trials may be due to a slightly different experimental design [39], because they continued administering gabapentin beyond one month and gabapentin might mask the presence of pain.
The mechanism of action of GABA to prevent or decrease HZ-associated pain is not fully understood. Nevertheless, it is clear that the pathophysiology of the development and progress of PHN includes both central and peripheral disorders of the nervous system. Neuronal transmission is achieved by a wide range of neurotransmitters and regulatory peptides such as acetylcholine [42], noradrenaline [43], ATP [44], nitric oxide [45], GABA [46], cholecystokinin [47], gastrin [48], GRP [49], VIP [50], galanin [51], bombesin [52] and BPC 157 [53]. Among these, GABA seems to be very important in pain control, because of the descending GABA-ergic neuronal nerve fibres inhibiting peripheral nociceptive signals [46]. Acute HZ-induced pain is one of the processes which could be controlled by GABA receptor activation. During the acute HZ, the virus becomes activated and propagates via axonal transport along the affected nerves, leading to an inflammatory immune response, which then causes the damage of peripheral and central neurons [54, 55]. Thus, peripheral nerves lose their ability to suppress nociceptive pain signals, thereby decreasing the threshold of nociceptive sensory activation and producing spontaneous ectopic discharges. The consequence is peripheral sensitization, that is, the appearance of disproportionate pain in response to normally non-painful stimuli [56]. At the cellular level, PHN upregulates transient receptor potential vanilloid 1, which is typically associated with pain [57], and increases the expression of voltage-gated sodium channels and potassium voltage-gated channels [58]. Experimental data suggest that these changes can be blocked by the GABA-like compounds and also by sodium channel blockers [58]. Furthermore, it has been shown that the loss of GABA-ergic inhibitory interneurons at the dorsal horn leads to the loss of descending inhibition [57]. Therefore, the loss of GABA-ergic neurotransmission seems to play an important role in the development of acute HZ-associated pain [6, 57, 58].
The optimal dosage of GABA-like compounds is still to be determined. Case reports and clinical trials suggest the use of gabapentin from 900 to 3600 mg/day to treat PHN and other neuropathic pains [1, 2, 5, 59-64]. In response to such treatments, patients had decreased daily pain scores, improved mood and sleep quality and improved quality of life. Dizziness, somnolence, dry mouth and oedema were the most reported adverse events [1, 5, 59, 60, 62-64]. Other recorded adverse events were ataxia and weight gain [61]. These adverse events usually occurred at the beginning of the treatment and at higher dosages. Therefore, slow dose titration was suggested to reduce their occurrence [1, 2, 5, 59-64]. Alternatively, pregabalin was also applied in clinical trials in PHN patients with a dosage of 150 - 600 mg/day for 8 - 13 weeks, resulting in reduced mean pain scores and the same adverse events as in the case of gabapentin [12, 65-68]. Gabapentin and pregabalin had been shown to have higher analgesic efficacy than oxycodone and amitriptyline in comparative studies [69, 70]. The recommended dose of gabapentin for zoster pain is 1800-3600 mg/day, administered in stepwise increase, starting with 300 mg/day. Pregabalin is reported to have fewer side effects than gabapentin [25]. Its suggested initial dose is 150 mg/day, which can be increased up to 300 mg/day within a week and then increased again to 600 mg/day after 2-4 weeks. Its maximal recommended daily dose is 600 mg [3, 28, 68].
There is no guideline for using and dosing GABA and its derivatives to prevent acute HZ pain. One randomized controlled trial suggested the application of gabapentin from 300 mg/day initially and then an increaseddose of 1,800 mg/day [38] while Lee and his colleagues used 900mg/day of gabapentin [39] but Skvarc et al., used 150-300 mg/day of pregabalin [40]. There was no dose given in the report of Wang et al. [41]. Our pilot literature search indicated that there were very few well-designed studies to investigate GABA derivatives. Therefore, we decided to design our PICO to pool different types of GABA derivatives and compare them to the placebo. Thus, we ignored the difference between GABA compounds which certainly have differences in potency, adverse reactions, pharmacokinetics and pharmacodynamics characteristics. Also, further studies are needed to determine whether the applied doses were lower than the optimal to prevent acute HZ pain. Nevertheless, the presently available data indicate that the application of GABA-like compounds in this respect is very promising.
Our present meta-analysis indicated no serious side effects of GABA-like compounds. The most commonly reported adverse reactions of GABA compounds are somnolence, dizziness, ataxia, fatigue and peripheral oedema, which may incidentally be the cause of patient withdrawal from the treatment [71, 72]. Only the study of Skvarc et al., showed a significant difference in dizziness and somnolence between the pregabalin treated group and the placebo group [40]. Other events were not significantly different between the treatment and placebo groups. A previous analysis of adverse events from gabapentin in patients treated with PHN reported an increasing chance of peripheral oedema after a higher than 1,800 mg/day of gabapentin dose [71]. The meta-analysis on the safety of gabapentin showed that gabapentin increased the risk of adverse events significantly and nine out of eleven studies in which the applied dose was higher than 1800 mg/day [73]. Another meta-analysis on the adverse event profile of pregabalin also showed an increasing risk of adverse events in a dose-dependent manner [74]. All clinical trials included in our analysis used lower doses of gabapentin and pregabalin than the dose reported to cause adverse events.
The treatments for pain control during HZ may reduce the magnitude of the initiation phase of nociceptor-evoked central sensitivity. The application of antiviral agents limits the destruction of primary afferent nociceptor caused by the virus during acute infection and decreases pain perception as well. These procedures can reduce central sensitization [7]. There are convincing reasons for combining antiviral therapy with the effective relief of acute HZ pain as soon as possible, continuing it until the infection has completely been resolved [7]. It will decrease the risk of persistent pain below the level achieved by antiviral drugs used alone [5]. The antiviral agent should be administered within 72 hours of rash onset in order to decrease the severity of viral load [2, 3]. A currently published protocol suggests starting the administration of gabapentin at the same time as starting antiviral therapy [38]. In the included trials, the administration of gabapentin or pregabalin was not started at the same time after the onset of the disease. We have to note that the initiation time of treatment might affect the results of individual studies and the following meta-analyses.
4.1. Strengths and Limitations
The results of the present meta-analysis revealed that the administration of GABA and its derivatives is effective in preventing acute HZ pain in HZ patients. Moreover, this study is the first meta-analysis on the effect of GABA and its derivatives to reduce the incidence of acute HZ pain. It may pave the way for the prevention of long-lasting complications of HZ infection like PHN. However, there are significant limitations to our work, such as lacking high-quality evidence, low number of studies, high risk of bias, different definitions of the outcome, follow-up duration, and testing with different agents. Accordingly, the results should be interpreted considering such limitations.
4.2. Implications for Research and Clinical Practice
Our results suggest that GABA and its derivatives decrease the incidence of acute HZ pain. However, the heterogeneity of studies is high, and the quality of evidence is very low. Additionally, there is a need for evidence to verify that GABA compounds can prevent HZ-associated pain in more-extended follow-up periods. Further well-standardized, randomized clinical trials with a large number of participants are needed to clarify the beneficial effect of GABA and its derivatives on HZ-associated pain. The dosage, duration of treatment and follow-up time should be considered in these research protocols. The studies should put more emphasis on the characteristics of pain, distribution and severity of herpetic lesions, and the changes in the quality of life. Additionally, the bias of reports should be decreased to obtain more reliable data.
CONCLUSION
Our meta-analysis revealed that the administration of GABA and its derivatives reduce the incidence of HZ-associated pain in the first month after the onset of rash. However, because of the low number and moderate power of RCTs, which could be included, the presently available evidence is weak. Therefore, additional, well-designed randomized clinical trials are needed on the matter.
Acknowledgements
Declared none.
Funding Statement
The study was supported by the Hungarian Human Resources Development Operational Program (EFOP-3.6.2-16-2017-00006 and EFOP-3.6.3-VEKOP-16-2017-00009), an Economic Development and Innovation Operative Program Grant (GINOP 2.3.2-15- 2016-00048) and an Institutional Developments for Enhancing Intelligent Specialization Grant (EFOP-3.6.1-16-2016-00022) of the National Research, Development and Innovation Office. Additional support was received by the Excellence Program of the Ministry for Innovation and Technology in Hungary, within the framework of the Therapy thematic program of the Semmelweis University, Hungary.
Appendix A
Searching Queries
1. The search string used in the PubMed database was (“herpes zoster”[MeSH Terms] OR “shingles”[All Fields]) AND (“gamma-aminobutyric acid”[MeSH Terms] OR “gaba”[All Fields] OR “gabapentin”[All Fields] OR neurontin[All Fields] OR “pregabalin”[All Fields]).
2. The search string used in the Web of Science database was ((gaba) OR (gamma-aminobutyric acid) OR (gabapentin) OR (neurontin) OR (pregabalin)) AND (herpes zoster).
3. The search string used in the EMBASE database was [“herpes zoster”] AND [(“gamma-aminobutyric acid” OR “gaba” OR “gabapentin” OR neurontin OR “pregabalin”)].
4. The search string used in the Scopus database was [(ALL (gamma AND aminobutyric AND acid) OR ALL (gaba) OR ALL (gabapentin) OR ALL (neurontin) OR ALL (pregabalin))] AND [ALL (herpes AND zoster)].
5. The search string used in the Ovid database was [herpes zoster.mp. [mp=ab, bc, bo, bt, cb, cc, ds, ge, gn, mc, mi, mq, or, ps, sq, st, ti, tm, tn, tx, rn, ct, sh, ot, nm, hw, kf, px, rx, an, ui, on, sy]] AND [(gamma-aminobutyric acid or gamma aminobutyric acid or gaba or gabapentin or neurontin or pregabalin).mp. [mp=ab, bc, bo, bt, cb, cc, ds, ge, gn, mc, mi, mq, or, ps, sq, st, ti, tm, tn, tx, rn, ct, sh, ot, nm, hw, kf, px, rx, an, ui, on, sy]].
Appendix B
Characteristics of included studies
Lee et al., 2016
| Methods | Prospective Randomized Controlled Study |
|---|---|
| Participants | 120 participants (60 intervention, 60 control) who were diagnosed with acute herpes zoster, aged 50 and over and complaining about moderate to severe pain |
| Interventions | Intervention: 1000 mg valacyclovir HCL tid for 7 days + 650 mg acetaminophen tid + Gabapentin 300mg tid [stepwise in 3 days (900 mg/day)] Placebo: (1000 mg valacyclovir HCL tid for 7 days + 650mg acetaminophen tid) |
| Outcomes | PHN incidence Adverse events |
| Notes | No conflict of interest |
Risk of Bias Table
| Bias | Authors’ Judgement | Support for Judgement |
|---|---|---|
| Random sequence generation (selection bias) |
Unclear risk | Comment: There is no information about randomization. |
| Allocation concealment (selection bias) |
Unclear risk | Comment: There is no information about concealment. |
| Blinding of participants and personnel (performance bias) |
Unclear risk | Comment: There is no information about blinding. |
| Blinding of outcome assessment (detection bias) |
Low risk | Quote: “The evaluators were different from the physician who prescribed, and they had no information to which group the patient was assigned” |
| Incomplete outcome data (attrition bias) |
High risk | Quote: “Among them, 52 and 49 patients had completed follow-up period, respectively” Note: The authors did not mention any dropout reasons. |
| Selective reporting (reporting bias) |
Low risk | Quote: “The incidences of PHN in the gabapentin group was 3.8% (2/52) which was lower than that in the control group [6.1%, (3/49)]. But the difference did not reach statistical significance (p50<0.672).” Note: The trial has a protocol summary and explain statistical analysis method. The outcome was reported as a plan and it had detail in statistics. |
| Other bias | Low risk | Note: Study appears to be free of other sources of risk |
Skvarc et al., 2010
| Methods | Prospective Randomized Double-blind Placebo-controlled Study |
|---|---|
| Participants | 29 participants (14 intervention, 15 control), out patients aged 30 - 80 years who had herpes zoster pain, and assessed equal or more than 4 on a 0 - 10 point Likert scale during the period between day 7 and day 14 of acute disease |
| Interventions | Intervention: 75 mg pregabalin po bid (150mg/day) Placebo: placebo drug po bid Can rise to 150 mg po bid (300 mg/day) |
| Outcomes | PHN incidence Adverse events |
| Notes | - |
Risk of Bias Table
| Bias | Authors’ Judgement | Support for Judgement |
|---|---|---|
| Random sequence generation (selection bias) |
Unclear risk | Comment: There is no information about randomization |
| Allocation concealment (selection bias) |
Unclear risk | Comment: There is no information about concealment. |
| Blinding of participants and personnel (performance bias) |
Unclear risk | Comment: There is no information about blinding. |
| Blinding of outcome assessment (detection bias) |
Unclear risk | Quote: “patients assessed their intensity of pain, allodynia, hyperalgesia, and burning, prickling, tingling and electric shock-like sensations” |
| Incomplete outcome data (attrition bias) |
High risk | Quote: “In the pregabalin group, nine patients withdrew from the study prematurely: five because of adverse effects and four because of disappearance of pain; in the placebo group, six patients withdrew prematurely: three because of adverse effects and three because of disappearance of pain” Note: high proportion of withdrawal from study |
| Selective reporting (reporting bias) |
Low risk | Quote: “All pre-specified and expected outcomes of interest are reported” |
| Other bias | Low risk | Note: Study appears to be free of other sources of risk |
Wang et al., 2013
| Methods | Randomized Controlled Study |
|---|---|
| Participants | 148 participants |
| Interventions | Intervention: standard therapy with gabapentin Placebo: standard therapy |
| Outcomes | Incidence of PHN |
| Notes | Only abstract access |
Risk of Bias Table
| Bias | Authors’ Judgement | Support for Judgement |
|---|---|---|
| Random sequence generation (selection bias) |
Unclear risk | Note: The author mentioned that participants were randomly assigned to receive treatment, but we do not know how it was done, because we had access only the abstract. |
| Allocation concealment (selection bias) |
Unclear risk | Note: The authors indicated only that participants were randomly assigned to receive treatment, but we do not know how it was done, because we had access only the abstract. It was not described in the abstract. |
| Blinding of participants and personnel (performance bias) |
Unclear risk | Note: The authors did not mention any details about the blinding of participants and personnel in the abstract. |
| Blinding of outcome assessment (detection bias) |
Unclear risk | Note: The authors did not mention any details about the blinding of outcome assessment in the abstract. |
| Incomplete outcome data (attrition bias) |
Unclear risk | Quote: “123 patients completed 1-year follow up.” Note: 16.89% did not completed 1-year follow up. However, the proportion of dropout is low, the authors did not state any dropout reason. |
| Selective reporting (reporting bias) |
High risk | Quote: “At 3, 6, 12 months post - therapy, the incidence of PHN was still significantly lower in the gabapentin group than in the standard group” Note: The authors reported incomplete outcome results. |
| Other bias | Unclear risk | Note: We cannot assess other bias because we did not have enough data from the abstract. |
Consent for Publication
Not applicable.
Funding
The study was supported by the Hungarian Human Resources Development Operational Program (EFOP-3.6.2-16-2017-00006 and EFOP-3.6.3-VEKOP-16-2017-00009), an Economic Development and Innovation Operative Program Grant (GINOP 2.3.2-15- 2016-00048) and an Institutional Developments for Enhancing Intelligent Specialization Grant (EFOP-3.6.1-16-2016-00022) of the National Research, Development and Innovation Office. Additional support was received by the Excellence Program of the Ministry for Innovation and Technology in Hungary, within the framework of the Therapy thematic program of the Semmelweis University, Hungary.
Conflict of Interest
The authors declare no conflict of interest, financial or otherwise.
SUPPLEMENTARY MATERIAL
Supplementary material is available on the publisher's website along with the published article.
References
- 1.Beydoun A. Postherpetic neuralgia: role of gabapentin and other treatment modalities. Epilepsia. 1999;40(Suppl. 6):S51–S56. doi: 10.1111/j.1528-1157.1999.tb00933.x. [DOI] [PubMed] [Google Scholar]
- 2.Baron R. Post-herpetic neuralgia case study: optimizing pain control. Eur. J. Neurol. 2004;11(Suppl. 1):3–11. doi: 10.1111/j.1471-0552.2004.00794.x. [DOI] [PubMed] [Google Scholar]
- 3.Gan E.Y., Tian E.A., Tey H.L. Management of herpes zoster and post-herpetic neuralgia. Am. J. Clin. Dermatol. 2013;14(2):77–85. doi: 10.1007/s40257-013-0011-2. [DOI] [PubMed] [Google Scholar]
- 4.Kost R.G., Straus S.E. Postherpetic neuralgia-pathogenesis, treatment, and prevention. N. Engl. J. Med. 1996;335(1):32–42. doi: 10.1056/NEJM199607043350107. [DOI] [PubMed] [Google Scholar]
- 5.Dworkin R.H., Schmader K.E. Treatment and prevention of postherpetic neuralgia. Clin. Infect. Dis. 2003;36(7):877–882. doi: 10.1086/368196. [DOI] [PubMed] [Google Scholar]
- 6.Hadley G.R., Gayle J.A., Ripoll J., et al. Post-herpetic Neuralgia: a Review. Curr. Pain Headache Rep. 2016;20(3):17. doi: 10.1007/s11916-016-0548-x. [DOI] [PubMed] [Google Scholar]
- 7.Dworkin R.H., Perkins F.M., Nagasako E.M. Prospects for the prevention of postherpetic neuralgia in herpes zoster patients. Clin. J. Pain. 2000;16(2) Suppl.:S90–S100. doi: 10.1097/00002508-200006001-00016. [DOI] [PubMed] [Google Scholar]
- 8.Mallick-Searle T., Snodgrass B., Brant J.M. Postherpetic neuralgia: epidemiology, pathophysiology, and pain management pharmacology. J. Multidiscip. Healthc. 2016;9:447–454. doi: 10.2147/JMDH.S106340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dworkin R.H., Portenoy R.K. Pain and its persistence in herpes zoster. Pain. 1996;67(2-3):241–251. doi: 10.1016/0304-3959(96)03122-3. [DOI] [PubMed] [Google Scholar]
- 10.Weinberg J.M. Herpes zoster: epidemiology, natural history, and common complications. J. Am. Acad. Dermatol. 2007;57(6) Suppl.:S130–S135. doi: 10.1016/j.jaad.2007.08.046. [DOI] [PubMed] [Google Scholar]
- 11.Watson P.N. Postherpetic neuralgia. BMJ Clin Evid; 2010. p. 0905. [PMC free article] [PubMed] [Google Scholar]
- 12.Cappuzzo K.A. Treatment of postherpetic neuralgia: focus on pregabalin. Clin. Interv. Aging. 2009;4:17–23. [PMC free article] [PubMed] [Google Scholar]
- 13.Nicholson B.D. Diagnosis and management of neuropathic pain: a balanced approach to treatment. J. Am. Acad. Nurse Pract. 2003;15(12) Suppl.:3–9. [PubMed] [Google Scholar]
- 14.Singh D., Kennedy D.H. The use of gabapentin for the treatment of postherpetic neuralgia. Clin. Ther. 2003;25(3):852–889. doi: 10.1016/S0149-2918(03)80111-X. [DOI] [PubMed] [Google Scholar]
- 15.Wareham D.W. Postherpetic neuralgia. BMJ Clin Evid; 2007. p. 0905. [PMC free article] [PubMed] [Google Scholar]
- 16.Whitley R.J., Gnann J.W. Herpes zoster: focus on treatment in older adults. Antiviral Res. 1999;44(3):145–154. doi: 10.1016/S0166-3542(99)00064-9. [DOI] [PubMed] [Google Scholar]
- 17.Meng F.Y., Zhang L.C., Liu Y., et al. Efficacy and safety of gabapentin for treatment of postherpetic neuralgia: a meta-analysis of randomized controlled trials. Minerva Anestesiol. 2014;80(5):556–567. [PubMed] [Google Scholar]
- 18.Derry S., Sven-Rice A., Cole P., Tan T., Moore R.A. Topical capsaicin (high concentration) for chronic neuropathic pain in adults. Cochrane Database Syst. Rev. 2013;2013(2):CD007393. doi: 10.1002/14651858.CD007393.pub3. [DOI] [PubMed] [Google Scholar]
- 19.Backonja M-MMD, Serra JMD. 2004.
- 20.Johnson R.W., Rice A.S.C. Clinical practice. Postherpetic neuralgia. N. Engl. J. Med. 2014;371(16):1526–1533. doi: 10.1056/NEJMcp1403062. [DOI] [PubMed] [Google Scholar]
- 21.Thakur R., Philip A.G. Chronic pain perspectives: Treating herpes zoster and postherpetic neuralgia: an evidence-based approach. J. Fam. Pract. 2012;61(9) Suppl.:S9–S15. [PubMed] [Google Scholar]
- 22.Makharita M.Y., Amr Y.M., El-Bayoumy Y. Single paravertebral injection for acute thoracic herpes zoster: a randomized controlled trial. Pain Pract. 2015;15(3):229–235. doi: 10.1111/papr.12179. [DOI] [PubMed] [Google Scholar]
- 23.Rose M.A., Kam P.C. Gabapentin: pharmacology and its use in pain management. Anaesthesia. 2002;57(5):451–462. doi: 10.1046/j.0003-2409.2001.02399.x. [DOI] [PubMed] [Google Scholar]
- 24.Frampton J.E., Foster R.H. Pregabalin: in the treatment of postherpetic neuralgia. Drugs. 2005;65(1):111–118. doi: 10.2165/00003495-200565010-00011. [DOI] [PubMed] [Google Scholar]
- 25.Bockbrader H.N., Burger P., Knapp L., Corrigan B.W. Population pharmacokinetics of pregabalin in healthy subjects and patients with chronic pain or partial seizures. Epilepsia. 2011;52(2):248–257. doi: 10.1111/j.1528-1167.2010.02933.x. [DOI] [PubMed] [Google Scholar]
- 26.Scheinfeld N. The role of gabapentin in treating diseases with cutaneous manifestations and pain. Int. J. Dermatol. 2003;42(6):491–495. doi: 10.1046/j.1365-4362.2003.01831.x. [DOI] [PubMed] [Google Scholar]
- 27.Dakin H., Nuijten M., Liedgens H., Nautrup B.P. Cost-effectiveness of a lidocaine 5% medicated plaster relative to gabapentin for postherpetic neuralgia in the United Kingdom. Clin. Ther. 2007;29(7):1491–1507. doi: 10.1016/j.clinthera.2007.07.006. [DOI] [PubMed] [Google Scholar]
- 28.Attal N., Cruccu G., Haanpää M., et al. EFNS Task Force. EFNS guidelines on pharmacological treatment of neuropathic pain. Eur. J. Neurol. 2006;13(11):1153–1169. doi: 10.1111/j.1468-1331.2006.01511.x. [DOI] [PubMed] [Google Scholar]
- 29.Han Y., Zhang J., Chen N., He L., Zhou M., Zhu C. Corticosteroids for preventing postherpetic neuralgia. Cochrane Database Syst. Rev. 2013;(3) doi: 10.1002/14651858.CD005582.pub4. [DOI] [PubMed] [Google Scholar]
- 30.Chen N., Li Q., Yang J., Zhou M., Zhou D., He L. Antiviral treatment for preventing postherpetic neuralgia. Cochrane Database Syst. Rev. 2014;(2) doi: 10.1002/14651858.CD006866.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Brody T. Drug Safety.Clinical Trials. Boston: Academic Press; 2012. pp. 415–469. [Google Scholar]
- 32.Higgins J, Green S.
- 33.Schünemann H., Brożek J., Guyatt G., Oxman A. Handbook for grading the quality of evidence and the strength of recommendations using the GRADE approach. 2013 https://gdt.gradepro.org/app/handbook/handbook.html
- 34.Czumbel L.M., Kerémi B., Gede N., et al. Sandblasting reduces dental implant failure rate but not marginal bone level loss: A systematic review and meta-analysis. PLoS One. 2019;14(5):e0216428. doi: 10.1371/journal.pone.0216428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Tóth B., Hegyi P., Lantos T., et al. The Efficacy of Saffron in the Treatment of Mild to Moderate Depression: A Meta-analysis. Planta Med. 2019;85(1):24–31. doi: 10.1055/a-0660-9565. [DOI] [PubMed] [Google Scholar]
- 36.Lapolla W., Digiorgio C., Haitz K., et al. Incidence of postherpetic neuralgia after combination treatment with gabapentin and valacyclovir in patients with acute herpes zoster: open-label study. Arch. Dermatol. 2011;147(8):901–907. doi: 10.1001/archdermatol.2011.81. [DOI] [PubMed] [Google Scholar]
- 37.Migita T., Maekawa T., Okada K., Kobayashi M., Egi A. Can early administration of pregabalin reduce the incidence of postherpetic neuralgia? Eur. J. Anaesthesiol. 2013;30:207. doi: 10.1097/00003643-201306001-00646. [DOI] [PubMed] [Google Scholar]
- 38.Rullán M., Bulilete O., Leiva A., et al. PHN group Efficacy of gabapentin for prevention of postherpetic neuralgia: study protocol for a randomized controlled clinical trial. Trials. 2017;18(1):24. doi: 10.1186/s13063-016-1729-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lee E.G., Lee H.J., Hyun D.J., Min K., Kim D.H., Yoon M.S. Efficacy of low dose gabapentin in acute herpes zoster for preventing postherpetic neuralgia: a prospective controlled study. Dermatol. Ther. (Heidelb.) 2016;29(3):184–190. doi: 10.1111/dth.12331. [DOI] [PubMed] [Google Scholar]
- 40.Krcevski Skvarc N., Kamenik M. Effects of pregabalin on acute herpetic pain and postherpetic neuralgia incidence. Wien. Klin. Wochenschr. 2010;122(Suppl. 2):49–53. doi: 10.1007/s00508-010-1345-x. [DOI] [PubMed] [Google Scholar]
- 41.Wang Q., Cui W., Song T. The Effectiveness of Gabapentin for the Prevention of Postherpetic Neuralgia in Patients with Acute Herpes Zoster. J. Pharmacol. Sci. 2013;121:194. [Google Scholar]
- 42.Varga G., Papp M., Hársing L.G., Jr, et al. Neuroeffector transmission of the hepatic and pancreatico-duodenal isolated arteries of the dog. Gastroenterology. 1984;87(5):1056–1063. doi: 10.1016/S0016-5085(84)80065-7. [DOI] [PubMed] [Google Scholar]
- 43.Borbély Z., Csomó B.K., Kittel Á., Gerber G., Varga G., Vizi E.S. Effect of rat spinal cord injury (hemisection) on the ex vivo uptake and release of [3H]noradrenaline from a slice preparation. Brain Res. Bull. 2017;131:150–155. doi: 10.1016/j.brainresbull.2017.04.007. [DOI] [PubMed] [Google Scholar]
- 44.Kordás K.S., Sperlágh B., Tihanyi T., et al. ATP and ATPase secretion by exocrine pancreas in rat, guinea pig, and human. Pancreas. 2004;29(1):53–60. doi: 10.1097/00006676-200407000-00056. [DOI] [PubMed] [Google Scholar]
- 45.Lohinai Z., Burghardt B., Zelles T., Varga G. Nitric oxide modulates salivary amylase and fluid, but not epidermal growth factor secretion in conscious rats. Life Sci. 1999;64(11):953–963. doi: 10.1016/S0024-3205(99)00021-1. [DOI] [PubMed] [Google Scholar]
- 46.Lau B.K., Vaughan C.W. Descending modulation of pain: the GABA disinhibition hypothesis of analgesia. Curr. Opin. Neurobiol. 2014;29:159–164. doi: 10.1016/j.conb.2014.07.010. [DOI] [PubMed] [Google Scholar]
- 47.Varga G., Kisfalvi K., Pelosini I., D’Amato M., Scarpignato C. Different actions of CCK on pancreatic and gastric growth in the rat: effect of CCK(A) receptor blockade. Br. J. Pharmacol. 1998;124(3):435–440. doi: 10.1038/sj.bjp.0701811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Varga G., Campbell D.R., Bussjaeger L.J., Solomon T.E. Role of gastrin and cholecystokinin receptors in regulation of peptone-stimulated gastric acid secretion in conscious rats. Eur. J. Pharmacol. 1993;250(1):37–42. doi: 10.1016/0014-2999(93)90618-R. [DOI] [PubMed] [Google Scholar]
- 49.Burghardt B., Wenger C., Barabás K., et al. GRP-receptor-mediated signal transduction, gene expression and DNA synthesis in the human pancreatic adenocarcinoma cell line HPAF. Peptides. 2001;22(7):1119–1128. doi: 10.1016/S0196-9781(01)00433-8. [DOI] [PubMed] [Google Scholar]
- 50.Kortezova N., Mizhorkova Z., Milusheva E., Varga G., Vizi E.S., Papasova M. Non-adrenergic non-cholinergic neuron stimulation in the cat lower esophageal sphincter. Eur. J. Pharmacol. 1996;304(1-3):109–115. doi: 10.1016/0014-2999(96)00093-3. [DOI] [PubMed] [Google Scholar]
- 51.Kisfalvi I., Jr, Burghardt B., Bálint A., Zelles T., Vizi E.S., Varga G. Antisecretory effects of galanin and its putative antagonists M15, M35 and C7 in the rat stomach. J. Physiol. Paris. 2000;94(1):37–42. doi: 10.1016/S0928-4257(99)00105-9. [DOI] [PubMed] [Google Scholar]
- 52.Milusheva E.A., Kortezova N.I., Mizhorkova Z.N., et al. Role of different bombesin receptor subtypes mediating contractile activity in cat upper gastrointestinal tract. Peptides. 1998;19(3):549–556. doi: 10.1016/S0196-9781(97)00467-1. [DOI] [PubMed] [Google Scholar]
- 53.Keremi B., Lohinai Z., Komora P., et al. Antiinflammatory effect of BPC 157 on experimental periodontitis in rats. J. Physiol. Pharmacol. 2009;60(Suppl. 7):115–122. [PubMed] [Google Scholar]
- 54.Argoff C.E., Katz N., Backonja M. Treatment of postherpetic neuralgia: a review of therapeutic options. J. Pain Symptom Manage. 2004;28(4):396–411. doi: 10.1016/j.jpainsymman.2004.01.014. [DOI] [PubMed] [Google Scholar]
- 55.Head H., Cambell A.W. The pathology of herpes zoster and its bearing on sensory localisation. Brain. 1900;23(3):353–362. doi: 10.1093/brain/23.3.353. [DOI] [PubMed] [Google Scholar]
- 56.Woolf C.J., Max M.B. Mechanism-based pain diagnosis: issues for analgesic drug development. Anesthesiology. 2001;95(1):241–249. doi: 10.1097/00000542-200107000-00034. [DOI] [PubMed] [Google Scholar]
- 57.Baron R., Binder A., Wasner G. Neuropathic pain: diagnosis, pathophysiological mechanisms, and treatment. Lancet Neurol. 2010;9(8):807–819. doi: 10.1016/S1474-4422(10)70143-5. [DOI] [PubMed] [Google Scholar]
- 58.Garry E.M., Delaney A., Anderson H.A., et al. Varicella zoster virus induces neuropathic changes in rat dorsal root ganglia and behavioral reflex sensitisation that is attenuated by gabapentin or sodium channel blocking drugs. Pain. 2005;118(1-2):97–111. doi: 10.1016/j.pain.2005.08.003. [DOI] [PubMed] [Google Scholar]
- 59.Irving G., Jensen M., Cramer M., et al. Efficacy and tolerability of gastric-retentive gabapentin for the treatment of postherpetic neuralgia: results of a double-blind, randomized, placebo-controlled clinical trial. Clin. J. Pain. 2009;25(3):185–192. doi: 10.1097/AJP.0b013e3181934276. [DOI] [PubMed] [Google Scholar]
- 60.Wallace M.S., Irving G., Cowles V.E. Gabapentin extended-release tablets for the treatment of patients with postherpetic neuralgia: a randomized, double-blind, placebo-controlled, multicentre study. Clin. Drug Investig. 2010;30(11):765–776. doi: 10.2165/11539520-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 61.Rauck R.L., Irving G.A., Wallace M.S., Vanhove G.F., Sweeney M. Once-daily gastroretentive gabapentin for postherpetic neuralgia: integrated efficacy, time to onset of pain relief and safety analyses of data from two phase 3, multicenter, randomized, double-blind, placebo-controlled studies. J. Pain Symptom Manage. 2013;46(2):219–228. doi: 10.1016/j.jpainsymman.2012.07.011. [DOI] [PubMed] [Google Scholar]
- 62.Rowbotham M., Harden N., Stacey B., Bernstein P., Magnus-Miller L. Gabapentin for the treatment of postherpetic neuralgia: a randomized controlled trial. JAMA. 1998;280(21):1837–1842. doi: 10.1001/jama.280.21.1837. [DOI] [PubMed] [Google Scholar]
- 63.Rice A.S., Maton S., Postherpetic Neuralgia Study Group Gabapentin in postherpetic neuralgia: a randomised, double blind, placebo controlled study. Pain. 2001;94(2):215–224. doi: 10.1016/S0304-3959(01)00407-9. [DOI] [PubMed] [Google Scholar]
- 64.Backonja M., Glanzman R.L. Gabapentin dosing for neuropathic pain: evidence from randomized, placebo-controlled clinical trials. Clin. Ther. 2003;25(1):81–104. doi: 10.1016/S0149-2918(03)90011-7. [DOI] [PubMed] [Google Scholar]
- 65.Freynhagen R., Strojek K., Griesing T., Whalen E., Balkenohl M. Efficacy of pregabalin in neuropathic pain evaluated in a 12-week, randomised, double-blind, multicentre, placebo-controlled trial of flexible- and fixed-dose regimens. Pain. 2005;115(3):254–263. doi: 10.1016/j.pain.2005.02.032. [DOI] [PubMed] [Google Scholar]
- 66.van Seventer R., Feister H.A., Young J.P., Jr, Stoker M., Versavel M., Rigaudy L. Efficacy and tolerability of twice-daily pregabalin for treating pain and related sleep interference in postherpetic neuralgia: a 13-week, randomized trial. Curr. Med. Res. Opin. 2006;22(2):375–384. doi: 10.1185/030079906X80404. [DOI] [PubMed] [Google Scholar]
- 67.Sabatowski R., Gálvez R., Cherry D.A., et al. 1008-045 Study Group. Pregabalin reduces pain and improves sleep and mood disturbances in patients with post-herpetic neuralgia: results of a randomised, placebo-controlled clinical trial. Pain. 2004;109(1-2):26–35. doi: 10.1016/j.pain.2004.01.001. [DOI] [PubMed] [Google Scholar]
- 68.Jensen-Dahm C., Rowbotham M.C., Reda H., Petersen K.L. Effect of a single dose of pregabalin on herpes zoster pain. Trials. 2011;12:55. doi: 10.1186/1745-6215-12-55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Achar A., Chakraborty P.P., Bisai S., Biswas A., Guharay T. Comparative study of clinical efficacy of amitriptyline and pregabalin in postherpetic neuralgia. Acta Dermatovenerol. Croat. 2012;20(2):89–94. [PubMed] [Google Scholar]
- 70.Dworkin R.H., Barbano R.L., Tyring S.K., et al. A randomized, placebo-controlled trial of oxycodone and of gabapentin for acute pain in herpes zoster. Pain. 2009;142(3):209–217. doi: 10.1016/j.pain.2008.12.022. [DOI] [PubMed] [Google Scholar]
- 71.Parsons B., Tive L., Huang S. Gabapentin: a pooled analysis of adverse events from three clinical trials in patients with postherpetic neuralgia. Am. J. Geriatr. Pharmacother. 2004;2(3):157–162. doi: 10.1016/j.amjopharm.2004.09.004. [DOI] [PubMed] [Google Scholar]
- 72.Quintero G.C. Review about gabapentin misuse, interactions, contraindications and side effects. J. Exp. Pharmacol. 2017;9:13–21. doi: 10.2147/JEP.S124391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Zhang M., Gao C-X., Ma K-T., et al. A Meta-Analysis of Therapeutic Efficacy and Safety of Gabapentin in the Treatment of Postherpetic Neuralgia from Randomized Controlled Trials. BioMed Res. Int. 2018;20187474207 doi: 10.1155/2018/7474207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Zaccara G., Gangemi P., Perucca P., Specchio L. The adverse event profile of pregabalin: a systematic review and meta-analysis of randomized controlled trials. Epilepsia. 2011;52(4):826–836. doi: 10.1111/j.1528-1167.2010.02966.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material is available on the publisher's website along with the published article.