Abstract
Objectives
Our recent systematic review determined that remote patient monitoring (RPM) interventions can reduce acute care use. However, effectiveness varied within and between populations. Clinicians, researchers, and policymakers require more than evidence of effect; they need guidance on how best to design and implement RPM interventions. Therefore, this study aimed to explore these results further to (1) identify factors of RPM interventions that relate to increased and decreased acute care use and (2) develop recommendations for future RPM interventions.
Design
Realist review—a qualitative systematic review method which aims to identify and explain why intervention results vary in different situations. We analysed secondarily 91 studies included in our previous systematic review that reported on RPM interventions and the impact on acute care use. Online databases PubMed, EMBASE and CINAHL were searched in October 2020. Included studies were published in English during 2015–2020 and used RPM to monitor an individual’s biometric data (eg, heart rate, blood pressure) from a distance.
Primary and secondary outcome measures
Contextual factors and potential mechanisms that led to variation in acute care use (hospitalisations, length of stay or emergency department presentations).
Results
Across a range of RPM interventions 31 factors emerged that impact the effectiveness of RPM innovations on acute care use. These were synthesised into six theories of intervention success: (1) targeting populations at high risk; (2) accurately detecting a decline in health; (3) providing responsive and timely care; (4) personalising care; (5) enhancing self-management, and (6) ensuring collaborative and coordinated care.
Conclusion
While RPM interventions are complex, if they are designed with patients, providers and the implementation setting in mind and incorporate the key variables identified within this review, it is more likely that they will be effective at reducing acute hospital events.
PROSPERO registration number
CRD42020142523.
Keywords: telemedicine, public health, organisation of health services
Strengths and limitations of this study.
Our review was strengthened by a comprehensive search and inclusivity of diverse remote patient monitoring interventions across a broad spectrum of conditions and contexts.
The novel use of realist review methodology and development of theory-based constructs helped to systematically identify factors impacting implementation.
Included studies within our review had multiple study design issues. Typically, with many of these studies it is not possible (or ethical) to blind participants. Therefore, selection bias may have affected results if health professionals pragmatically selected more willing or engaged patients to participate in the trials.
While our focus was on acute care use, other aspects of care may have been overlooked that relate to care quality.
Introduction
Non-communicable diseases such as heart disease, chronic obstructive pulmonary disease (COPD), and diabetes accounts for over 70% of global deaths each year.1 Combined with the added challenge of ageing populations, health systems internationally are under enormous strain to support growing numbers of chronically unwell people.2 One of the main drivers of healthcare costs for chronically ill patients results from acute hospital admissions due to their intense resource requirements. Consequently, new models of care are being widely investigated and trialled that could extend care into the home and prevent unnecessary acute care events.
Remote patient monitoring (RPM) is a telehealth innovation that offers significant opportunities to increase the timeliness of care, enhance health outcomes, and potentially reduce hospitalisations and associated healthcare costs.3 4 RPM uses technology to observe a patient’s physiological (eg, heart rate, blood pressure) and behavioural (eg, medication adherence, physical activity) information from a distance.5 With support, many individuals could effectively self-manage chronic conditions in the community.6 Further, if alerted early, healthcare providers could intervene when a person’s health is declining, potentially preventing costly escalations to hospital. Health professionals can routinely monitor a patient’s health data and/or be alerted when measurements exceed a predetermined threshold. This allows for early intervention and ideally prevention of further exacerbation of a condition. RPM can benefit people with chronic illness as well as other population groups that benefit from continuous monitoring such as the frail and elderly, neonates or postsurgical patients.5
Despite the potential benefits of RPM, investigations into its clinical and cost-effectiveness have provided mixed results to date. For example, the impact of RPM on the heart failure population has resulted in multiple systematic reviews,7 meta-analyses8 9 and reviews of reviews.10 11 These reviews are generally positive about the potential benefits for patients and health services from RPM services,7 8 10 12 but others also report limited or no affect9 on reducing morbidity and mortality. A 2018 Cochrane review reported no difference in all-cause mortality in remotely monitored patients with heart failure and a change in hospitalisations ranging from a 64% decrease to a 60% increase.9
In our recent review,13 we provided a synthesis of the available evidence for the effect of RPM on acute care use including hospital admission events, hospital length of stay and emergency department presentations. We found that RPM was reported to reduce acute care use in approximately 45% of studies. Remaining studies largely reported no change; however, some reported an increase in acute care use. The included 91 studies covered multiple chronic conditions, countries and healthcare organisations and used various technology and models of care. While RPM can have a positive impact on reducing acute care use, certain enablers are needed. Clinicians, researchers, and policymakers require more guidance on how to design and implement RPM-facilitated models of care to achieve the greatest benefit. Consequently, further analysis is required to understand underlying mechanisms causing such variation in acute care use across RPM interventions.
We sought to understand what causes variation in outcomes from RPM interventions. Realist review methodology enables exploration of how, why and for whom interventions do and do not work. Consequently, the approach has been used across various health interventions (eg, medical education programmes,14 school feeding programmes15). The basic tenet of realist philosophy is that the effectiveness of an intervention is impacted by the context in which it is implemented which may trigger mechanisms that result in intended and unintended outcomes.16 Realist reviews are particularly helpful for complex interventions like RPM where the effectiveness is impacted by multiple interacting components such as the intervention design, users, interpersonal relationships, and institutions and settings where the intervention is delivered.
Specifically, this study aimed to (1) identify factors of RPM interventions that relate to increased and decreased acute care use, and (2) develop recommendations for future RPM intervention design and implementation.
Methods
Data extraction
We used data from our recent systematic review13 that compared acute care use between individuals who were and were not monitored using RPM. Complete details of the original systematic review have been described elsewhere.13 In brief, search terms for remote monitoring and acute care utilisation were used across three electronic databases: PubMed (MEDLINE) (1966–2020), EMBASE (OvidSP) (1974–2020) and CINAHL (EBSCOHost) (1982–2020). The search, conducted in October 2020, included articles published in the last 5 years (2015–2020). Articles were included if they used RPM to monitor an individual’s biometrics (eg, heart rate, blood pressure) from a distance while they are not in the hospital. No restrictions were placed on patient age or disease conditions; however, full-text studies had to be available in English.
We then re-reviewed the same 91 articles included in our original RPM systematic review, using realist review methodology to identify factors that determine intervention success and failure in various contexts. This review was guided by the work of Pawson et al17 and followed guidelines outlined by the Realist and Meta-narrative Evidence Synthesis: Evolving Standards ((online supplemental appendix A).18 17 Information was extracted that related to context (settings, populations, intervention delivery), outcomes (positive, negative or null affect on outcome of hospital use) and potential mechanisms or reasons behind the results (eg, author’s interpretation as to why the interventions did or did not work). These data were recorded in an Excel spreadsheet to facilitate a structured analysis. Two researchers (EET, MLT) independently extracted these data.
bmjopen-2021-051844supp001.pdf (237.4KB, pdf)
Evidence synthesis
The researchers then collectively examined the articles to detect patterns and developed a compendium of explanatory factors observed in the RPM studies. The researchers compared and discussed their identified factors that led to increased or decreased acute care use being reported in the studies. Findings were then combined into a table showing the number of studies proposing each mechanism and grouped by outcome (eg, increased or decreased acute care use).
The two researchers then jointly mapped recurrent patterns into explanatory context-mechanism-outcome (CMO) diagrams to illustrate how the different factors interact. Literature was also examined for opposing or conflicting viewpoints. These CMO diagrams were discussed with a third member of the research team (LJC) to confirm consistent and logical development. Key findings were synthesised into overarching themes, which are referred to as ‘theories’ in the realist review approach.17 Finally, a list of recommendations were developed from the findings and ordered by context to guide future RPM intervention design and implementation.
Patient and public involvement
Patients or the public were not involved in the design, or conduct, or reporting, or dissemination plans of our research.
Results
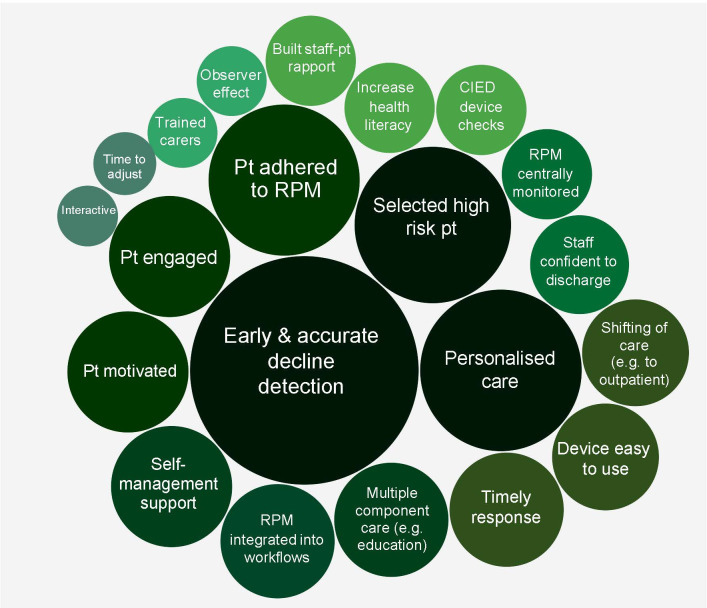
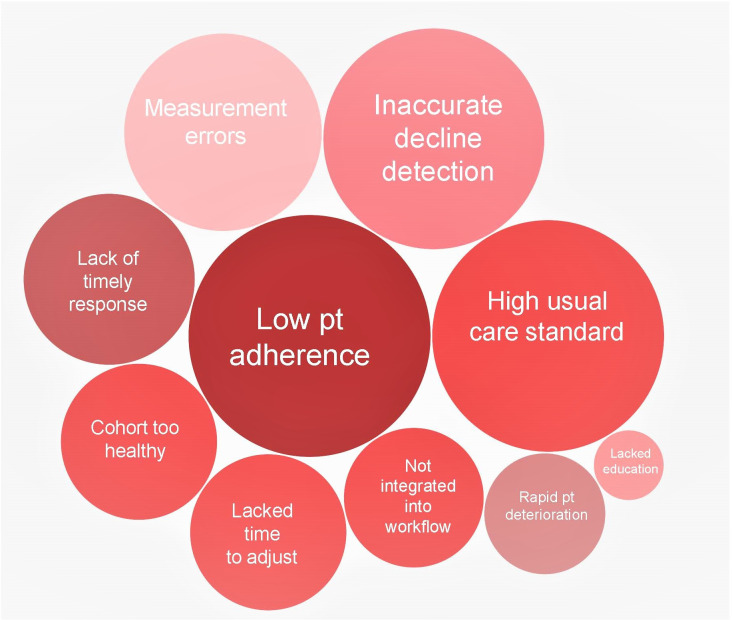
Ninety-one articles from our previous review were evaluated to determine why RPM increased, decreased or had no affect on acute care use. Thirty-one factors were identified and mapped onto two outcomes: (1) increased hospital use (21 factors), and (2) reduced hospital use (10 factors) (figures 1 and 2). Factors were also ordered by the frequency of articles that reported them as possible influences on outcomes (represented by the size of each factor in figures 1 and 2).
Figure 1.
Factors associated with RPM intervention studies that reduced acute care use. The size of each bubble relates to the number of studies that identified each factor as having an important influence on the outcome. CIED, cardiac implantable electronic devices; Pt, patient; RPM, remote patient monitoring.
Figure 2.
Factors associated with remote patient monitoring interventions studies that increased acute care use. The size of each bubble relates to the number of studies that identified each factor as having an important influence on the outcome. Pt, patient.
Theories about how RPM works
It was identified that successful RPM interventions, in this case those interventions that successfully reduced acute care use, were those that: (1) target populations at high risk; (2) accurately detect a decline in health; (3) were responsive and provided timely care; (4) provided personalised care; (5) enhanced self-management and (6) ensured collaborative and coordinated care. Each of these theories of intervention success are described later.
Target populations at high risk
Appropriate selection of patients for RPM is crucial if a change in acute care use is to be achieved. RPM interventions are likely to have more pronounced effects on acute care use when they are targeted towards populations with a high risk of hospitalisation (eg, moderate-severe disease severity, multiple comorbidities).19 Further, it is important for the intervention to be timed with periods of high-risk readmissions (eg, the first 90 days post an index event). Delaying the delivery of RPM devices to patients may reduce the effect20 (see figure 3).
Figure 3.
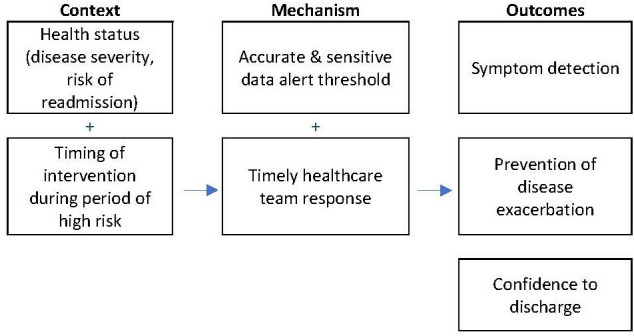
Proposed context-mechanism-outcome: target population.
Patients who are more likely to present to the hospital multiple times have a greater chance of reducing admissions due to more timely interventions. In practice, however, clinicians may have reservations about remotely assessing their most vulnerable and unwell patients. As described by Geller et al,21 ‘in clinical practice telemedicine seems to be used mainly in patients with better prognosis, probably due to the belief that those who live longer may receive more (ie, prolonged) benefit from telemonitoring than sicker patients who should be seen in the office more frequently’ (p1124). Consequently, clinicians may require additional information on how RPM can be safely delivered in high-risk cohorts.
Accurately detect a decline in health
RPM needs to accurately predict disease exacerbations by detecting a change in symptoms that relate to health deteriorations. This has been a challenge in certain populations such as COPD and heart failure patients which may have unpredictable disease progression. In the COPD population, multiple studies reported trying to determine the measurement (eg, spirometry, oximetry or a combination) which would mark the onset of an exacerbation,22–25 however, none came to a definitive conclusion.26 RPM can be used in these population groups to longitudinally track the progression of disease and develop parameters to be tested as predictors for future interventions.27
In the heart failure population, physiological signs may not provide adequate warning of decompensation. Readmission in this cohort can be a complex interplay of multiple factors and is often not solely limited to physiological variables.28 If deterioration occurs too quickly, there is limited opportunity to intervene.29 Therefore, more investigation is required to try and accurately predict health declines for individual patients and accurately pinpoint the best way for RPM to be used to support this patient population.
Implantable devices (eg, pacemakers) have an additional advantage; continuous monitoring enables undiagnosed comorbid conditions such as atrial fibrillation to be detected enabling pre-emptive intervention.30 It can also improve the efficiency of outpatient clinical care by detecting device or lead malfunctions earlier.31
Provide timely care via a responsive system
Any benefit from RPM is dependent on patients (1) using the system (eg, timely data entry) and (2) providers taking appropriate and equally timely action when out-of-range readings occur.30 Therefore, RPM systems that use automated data entry wherever possible are preferable as they can reduce errors and delays due to manual entry. As technology improves, smartphone-based programmes are likely to replace standard RPM equipment which may result in more consistent, accurate and timely data from patients.19 For innovations that rely on manual data entry, RPM innovations need to be easy to use (eg, enable efficient data entry, transportable) and useful for patients to ensure long-term use and engagement.32 Additionally, regular monitoring is required. For example, Srivastava et al19 routinely monitored data for abnormalities or lack of responses; if a patient did not submit data for 3 days, a call was initiated by nursing staff.
On the staff end, RPM alerts need to be actioned with timely and appropriate responses; the speed of decision-making and frequency of monitoring is paramount.33 A fast response often requires frequent contact with patients and effective bi-directional communication pathways between staff and patients. For instance, Trucco et al34 facilitated communication between families and the on-call team via a dedicated phone number or email address. Multiple studies report the importance of dedicated care (eg, providing an RPM nurse or dedicated case manager) in improving response time.33 35–38 This is supported by the literature with findings that patients who received either basic or intensive case management spend less time in hospital than those without.39 ‘Fast tracked’ access to primary care providers was used in the intervention reported by Pedone et al40 when abnormalities were presented, or new symptoms arose. They reported that a new model of care, rather than simply implementing a new technology, was required to obtain sizeable benefits in terms of hospitalisation outcomes.40 Where possible, RPM should be embedded into the system and provide seamless interaction between patients and the healthcare system with minimal encumbrance on both ends.19 The proposed CMO diagram is provided in figure 4.
Figure 4.
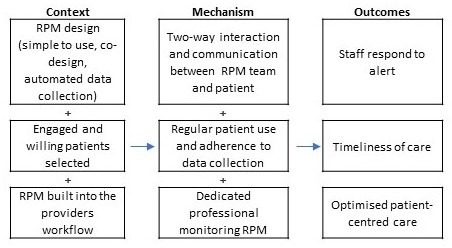
Proposed context-mechanism-outcome: timely care. RPM, remote patient monitoring.
Provide personalised care
Providing a patient-centric and personalised approach was also an important factor in determining the success of an RPM intervention in reducing acute care use.19 First, the development of the RPM innovation needs to be codesigned with patients and their families to ensure it meets their needs and maximises acceptance and uptake.41 Training patients on how to use the device will likely also need to be personalised and at times repeated. RPM alerts can also be personalised by using individual data to determine alert thresholds. Koehler et al42 recommended defining a risk category for each individual patient based on their positive results (derived from biometric data). One study author requested personalised parameters and treatment guidelines from each patient’s treating physician.43 Determining appropriate parameters for RPM applications (personalised or not) enables the treating team to be alerted to any biometric measurements that fall outside of the parameter ranges. To enable personalised parameters to be developed, physicians need to be engaged in the RPM process for their patient early. The response by the RPM monitoring team also needs to be tailored; considering the person’s medical, social and emotional needs.
Enhance self-management
To successfully reduce acute care use, RPM interventions should include support and education to increase self-management skills. Through developing knowledge, skills and positive behaviours (eg, medication adherence), patients are more likely to be able to effectively manage their condition with the aid of RPM (see figure 5).44 Additionally, increased awareness of signs and symptoms of disease progression that often occurs when patients use RPM can prompt them to contact their healthcare provider for timely management.44 Providing feedback from RPM data in a way that empowers patients to take control of their own health is important. Koehler et al42 reported that this needs to be a comprehensive approach including education and patient involvement when developing management strategies. In some instances, RPM interventions were discontinued once patients were able to correctly correlate their personal symptoms and seek help when required.43 Conversely, some RPM interventions that were unsuccessful in reducing hospitalisation events reported patients becoming overly reliant on the RPM team, for instance, alerting the team when an issue arose rather than developing autonomous self-management skills for their condition. Additionally, some known important factors such as medication adherence were not always measured and present a lost opportunity in many RPM innovations. Medication adherence and timely changes to medications are reported to confer substantial benefits for patients.42
Figure 5.
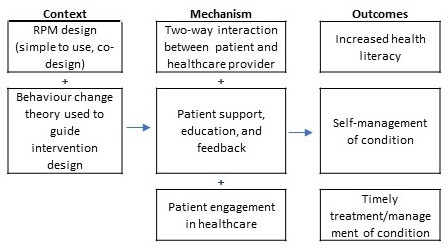
Proposed context-mechanism-outcome: self-management. RPM, remote patient monitoring.
Ensure collaborative and coordinated care
Successful RPM studies demonstrated increased connection and communication between healthcare staff and patients.31 Multidisciplinary team-based interventions that combine feedback (automated and/or provider-initiated) with other approaches (eg, coaching, motivational interviews and shared decision-making) are more likely to result in improvement in adherence.45 Involvement of primary care is crucial. As high-risk patients are often managed by primary and specialty care, both hospital and primary care settings should be involved in RPM interventions.46 Involvement of key stakeholders is required to improve continuity of care.47 Beyond healthcare professionals, the RPM intervention should also aim to include families and carers as key stakeholders in the long-term management of the person’s condition. To increase primary carers’ acceptance of and adherence to RPM, they must be involved very early on. To institute an initial change of role, staff incentives (eg, financial payments) may be required.27 Additionally, nursing staff should be considered as having leading roles in RPM interventions.48 Further, institutional support is required for these initiatives and reorganisation of care processes should be carefully planned and implemented.48
Factors that resulted in increased acute care use
A range of factors was identified as having a negative influence on hospital use (increasing admissions) (figure 2). Many of the identified factors are the reverse of what has been described earlier. For example, not targeting populations at high risk, not integrating RPM into the workflow or using systems that have measurement errors. For example, multiple study authors reported slow alert response times (N=6)32 49–53 and low patient or clinician adherence (N=11)19 20 28 32 45 54–59 as important factors resulting in no change or an increase in acute care use in the RPM group. There also appears to be a delicate balance between providing a supportive environment that empowers patients to self-manage versus having patients become reliant on the RPM device and/or the monitoring team.
Recommendations for RPM
We synthesised multiple recommendations to assist in the design and implementation of RPM interventions (figure 6).
Figure 6.
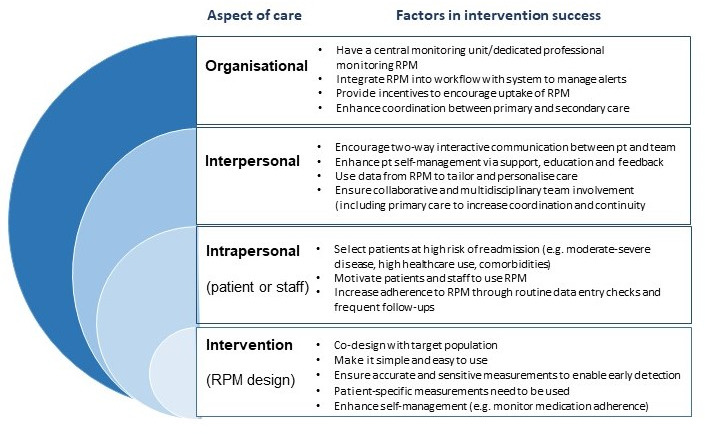
Recommendations to enhance RPM and reduce acute care use. RPM, remote patient monitoring.
When designing RPM devices, it is crucial that the measured biometrics accurately predict disease exacerbations. Alert thresholds need to be carefully determined to ensure they are sensitive to physiological changes without being too high, and where possible tailored to the patient and disease state. Further, the transmission of data needs to be reliable, and if possible, automatic.
It is essential that RPM devices are codesigned with consumers and providers to improve usability and engagement with the RPM system. It is likely that making the device interactive and building in feedback loops between the patient and clinician will enhance engagement. However, if this increases the provider’s workload it may discourage provider engagement. Multidisciplinary team interventions that combine feedback with other approaches like patient education, motivational interviewing, coaching or shared decision-making are likely to be more effective long-term.60
At the organisation level, having dedicated professionals responsible for monitoring data and communicating with patients and the healthcare team can improve the timeliness and coordination of care. Studies with nursing staff in these leading and case-management roles appeared to be more effective.48 RPM also needs to be embedded into the health system to provide seamless interaction between patients and the healthcare system. This may require reorganisation of care and additional resources (physical and personnel) to support the intervention.
Discussion
We found that RPM interventions were successful at reducing acute care use when they incorporated a number of elements including: accurately predicting a decline in health or disease exacerbation, timely response to alerts, personalised patient parameters and a focus on enhancing patient self-management. Additionally, collaboration between specialists and primary care providers was required to improve the continuity of care. To the best of our knowledge, this is the first review to elucidate why some RPM interventions are more successful than others in reducing acute care use.
RPM interventions are complex because they typically involve multiple components (eg, data collection, education, feedback) and various stakeholders across different settings (eg, community, primary and tertiary care). Given the complexity of RPM interventions, it is perhaps unsurprising that RPM studies have resulted in so much variation in the effects demonstrated regarding changes in acute care use. To date, much of the focus of RPM innovations has been on the design and development of the technology.61 62 While functioning technology that accurately detects a decline in health is important, to deliver significant benefits RPM alerts must also lead to actionable and timely responses. To achieve positive results at the healthcare system level, RPM interventions require a change to the model of care rather than simple technology implementation.63
To be successful, the right patients need to be recruited at the right time. Patients with greater disease severity and at high risk of readmissions appear to confer the greatest benefit of RPM interventions in terms of reduced acute care use.64 For instance, a recent consensus statement from the Heart Failure Society of America65 broadly concluded that heart failure RPM had the most impact when patients were most at risk (eg, recent hospitalisation, prone to fluid overload and struggles with medication adherence). Additionally, RPM should target patients who are willing and likely to adhere with RPM regimes.
While our study focuses on acute hospital use, other authors have investigated patient-related factors that may support long-term monitoring of conditions. For example, Huygens et al suggest there is a relationship between perceived disease controllability and patients’ willingness to self-monitor.66 Patients with diabetes, asthma and hypertension were most willing to self-monitor. In contrast, patients with rheumatism, migraines and other neurological disorders were less willing. The intervention design can facilitate engagement and use. Hong and Lee64 determined that interventions with an educational component such as self-management programmes have greater effects. Another consideration is the patient’s social circumstance. One study found that RPM significantly improved outcomes for socially isolated patients,67 potentially due to the delay in care access that these patients may face. Conversely, for socially connected patients, outcomes appear to be enhanced by training caregivers.29 51
Interventions based on health behaviour models and personalised coaching were most successful.68 The findings of this review parallel some of the themes in a review of patient experiences of RPM by Walker et al.69 Similarly, self-management and early identification of clinical exacerbations were key to preventing hospitalisation. From the patient perspective, self-management was achieved by increasing confidence and providing a sense of safety. Shared decision making was identified as a key mechanism to preventing hospitalisation. Conversely, interventions that provided information but did not equip patients to self-manage were potentially at greater risk of having patients become overly reliant on the RPM team.
Patients have previously reported concerns about being lost in the data or losing interpersonal connections with health professionals and a reluctance to try something new, especially if unfamiliar with technology.69 Our findings substantiate the importance of codesigning RPM interventions with consumers to ensure they are easy to use and provide useful feedback to maintain adherence and engagement. Building rapport, providing training (sometimes multiple times) and having a two-way interactive relationship between the patient and the RPM team is crucial. Alternatively, a lack of education and timely response were identified as factors that increased acute care use.
Included studies within our review had multiple study design issues. Typically, with many of these studies it is not possible (or ethical) to blind participants. Therefore, selection bias may have affected results if health professionals pragmatically selected more willing or engaged patients to participate in the trials. However, in real-world clinical settings it is likely (and appropriate) that participants are provided with options regarding their follow-up care. The observer or Hawthorne effect70 may be at play with participants potentially acting differently due to a belief that they are being watched. Such an effect may reduce with time, and some trial lengths may have been too short for this effect to wear off. Potentially the higher number of studies reporting positive outcomes may be due to a reporting bias within the literature; consequently, there were a higher number of factors discussed in relation to reducing (n=21) rather than increasing acute care use (n=10).
Our review was strengthened by a comprehensive search and inclusivity of diverse RPM interventions across a broad spectrum of conditions and contexts. The novel use of realist review methodology and development of theory-based constructs helped to systematically identify factors impacting on implementation. However, while our focus was on acute care use, other aspects of care may have been overlooked that relate to care quality. Further, it is possible that reducing hospital admissions may shift care and associated costs to the primary care setting and potentially result in additional pressure and stress on different aspects of the system. Additionally, the theories that have been developed are based on both our and the primary study authors’ interpretation of findings in many instances and not experimental evidence.
Conclusion
RPM interventions have the potential to reduce acute care use when they are targeted to appropriate populations and disease states, designed well, and implemented with patients and providers in mind. This review has highlighted important considerations for developing effective RPM devices, systems and telehealth models of care. To achieve significant changes in acute care use, RPM data need to be routinely entered and checked, automated where possible, alerts need to accurately highlight when a person’s data are beyond an acceptable range (for that person), and healthcare staff need to respond in a timely and appropriate manner. Further, information and feedback needs be provided to patients in a way that empowers them to self-manage their condition. If designed with these considerations in mind, RPM interventions are more likely to be effective at reducing acute care use. Future studies should investigate any unintended consequences of RPM and cost implications resulting from the shifting of care.
Supplementary Material
Footnotes
Twitter: @_emma_thomas, @csnoswell, @HelenHaydon, @morocovic, @DrLiamCaffery
Contributors: This research was conceptualised by EET. EET, LJC, CLS, MLT contributed to the study design. Searches and data extraction were carried out by EET and MLT with support from LJC. Data analysis was performed by EET, MLT and LJC. Manuscript was drafted by EET, MLT, AB and LJC. Critical review of manuscript was undertaken by HMH, CLS, ACS, VMGR. All authors approved the final manuscript.
Funding: We thank Clinical Excellence Queensland for providing the financial support to enable this research. EET is supported by a Postdoctoral Fellowship (#105215) from the National Heart Foundation of Australia.
Competing interests: None declared.
Patient and public involvement: Patients and/or the public were not involved in the design, or conduct, or reporting, or dissemination plans of this research.
Provenance and peer review: Not commissioned; externally peer reviewed.
Data availability statement
Data are available in a public, open access repository. All included articles are available publically.
Ethics statements
Patient consent for publication
Not required.
References
- 1.Bennett JE, Stevens GA, Mathers CD, et al. Ncd countdown 2030: worldwide trends in non-communicable disease mortality and progress towards sustainable development goal target 3.4. Lancet 2018;392:1072–88. 10.1016/S0140-6736(18)31992-5 [DOI] [PubMed] [Google Scholar]
- 2.Atun R, Jaffar S, Nishtar S, et al. Improving responsiveness of health systems to non-communicable diseases. Lancet 2013;381:690–7. 10.1016/S0140-6736(13)60063-X [DOI] [PubMed] [Google Scholar]
- 3.Snoswell CL, Smith AC, Thomas EE. Video and phone consultations only scratch the surface of what telehealth has to offer. The Conversation, 2020. Available: https://theconversation.com/video-and-phone-consultations-only-scratch-the-surface-of-what-telehealth-has-to-offer-146580
- 4.Bradford NK, Caffery LJ, Smith AC. Telehealth services in rural and remote Australia: a systematic review of models of care and factors influencing success and sustainability. Rural Remote Health 2016;16:245. [PubMed] [Google Scholar]
- 5.Malasinghe LP, Ramzan N, Dahal K. Remote patient monitoring: a comprehensive study. J Ambient Intell Humaniz Comput 2019;10:57–76. 10.1007/s12652-017-0598-x [DOI] [Google Scholar]
- 6.Hanlon P, Daines L, Campbell C, et al. Telehealth interventions to support self-management of long-term conditions: a systematic metareview of diabetes, heart failure, asthma, chronic obstructive pulmonary disease, and cancer. J Med Internet Res 2017;19:e172. 10.2196/jmir.6688 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pandor A, Gomersall T, Stevens JW, et al. Remote monitoring after recent hospital discharge in patients with heart failure: a systematic review and network meta-analysis. Heart 2013;99:1717–26. 10.1136/heartjnl-2013-303811 [DOI] [PubMed] [Google Scholar]
- 8.Clark RA, Inglis SC, McAlister FA, et al. Telemonitoring or structured telephone support programmes for patients with chronic heart failure: systematic review and meta-analysis. BMJ 2007;334:942. 10.1136/bmj.39156.536968.55 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Flodgren G, Rachas A, Farmer AJ, et al. Interactive telemedicine: effects on professional practice and health care outcomes. Cochrane Database Syst Rev 2015:CD002098. 10.1002/14651858.CD002098.pub2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bashi N, Karunanithi M, Fatehi F, et al. Remote monitoring of patients with heart failure: an overview of systematic reviews. J Med Internet Res 2017;19:e18. 10.2196/jmir.6571 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Greenhalgh T, A'Court C, Shaw S. Understanding heart failure; explaining telehealth - a hermeneutic systematic review. BMC Cardiovasc Disord 2017;17:156. 10.1186/s12872-017-0594-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ono M, Varma N. Remote monitoring to improve long-term prognosis in heart failure patients with implantable cardioverter-defibrillators. Expert Rev Med Devices 2017;14:335–42. 10.1080/17434440.2017.1306438 [DOI] [PubMed] [Google Scholar]
- 13.Taylor ML, Thomas EE, Snoswell CL, et al. Does remote patient monitoring reduce acute care use? A systematic review. BMJ Open 2021;11:e040232. 10.1136/bmjopen-2020-040232 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wong G, Greenhalgh T, Pawson R. Internet-based medical education: a realist review of what works, for whom and in what circumstances. BMC Med Educ 2010;10:1–10. 10.1186/1472-6920-10-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Greenhalgh T, Kristjansson E, Robinson V. Realist review to understand the efficacy of school feeding programmes. BMJ 2007;335:858–61. 10.1136/bmj.39359.525174.AD [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pawson R, Greenhalgh T, Harvey G. Realist synthesis: an introduction. Manchester: ESRC Research Methods Programme, University of Manchester, 2004. [Google Scholar]
- 17.Pawson R, Greenhalgh T, Harvey G, et al. Realist review-a new method of systematic review designed for complex policy interventions. J Health Serv Res Policy 2005;10 Suppl 1:21–34. 10.1258/1355819054308530 [DOI] [PubMed] [Google Scholar]
- 18.Wong G, Greenhalgh T, Westhorp G, et al. RAMESES publication standards: realist syntheses. BMC Med 2013;11:21. 10.1186/1741-7015-11-21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Srivastava A, Do J-M, Sales VL, et al. Impact of patient-centred home telehealth programme on outcomes in heart failure. J Telemed Telecare 2019;25:425–30. 10.1177/1357633X18775852 [DOI] [PubMed] [Google Scholar]
- 20.Olivari Z, Giacomelli S, Gubian L, et al. The effectiveness of remote monitoring of elderly patients after hospitalisation for heart failure: the renewing health European project. Int J Cardiol 2018;257:137–42. 10.1016/j.ijcard.2017.10.099 [DOI] [PubMed] [Google Scholar]
- 21.Geller JC, Lewalter T, Bruun NE, et al. Implant-based multi-parameter telemonitoring of patients with heart failure and a defibrillator with vs. without cardiac resynchronization therapy option: a subanalysis of the IN-TIME trial. Clin Res Cardiol 2019;108:1117–27. 10.1007/s00392-019-01447-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Steer J, Gibson GJ, Bourke SC. Predicting outcomes following hospitalization for acute exacerbations of COPD. QJM 2010;103:817–29. 10.1093/qjmed/hcq126 [DOI] [PubMed] [Google Scholar]
- 23.Seemungal TA, Donaldson GC, Bhowmik A, et al. Time course and recovery of exacerbations in patients with chronic obstructive pulmonary disease. Am J Respir Crit Care Med 2000;161:1608–13. 10.1164/ajrccm.161.5.9908022 [DOI] [PubMed] [Google Scholar]
- 24.Pinnock H, Hanley J, McCloughan L, et al. Effectiveness of telemonitoring integrated into existing clinical services on hospital admission for exacerbation of chronic obstructive pulmonary disease: researcher blind, multicentre, randomised controlled trial. BMJ 2013;347:f6070. 10.1136/bmj.f6070 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mohktar MS, Basilakis J, Redmond SJ, et al. A guideline-based decision support system for generating referral recommendations from routinely recorded home telehealth measurement data. Annu Int Conf IEEE Eng Med Biol Soc 2010;2010:6166–9. 10.1109/IEMBS.2010.5627766 [DOI] [PubMed] [Google Scholar]
- 26.Shany T, Hession M, Pryce D, et al. A small-scale randomised controlled trial of home telemonitoring in patients with severe chronic obstructive pulmonary disease. J Telemed Telecare 2017;23:650–6. 10.1177/1357633X16659410 [DOI] [PubMed] [Google Scholar]
- 27.Shany T, Hession M, Pryce D, et al. A small-scale randomised controlled trial of home telemonitoring in patients with severe chronic obstructive pulmonary disease. J Telemed Telecare 2017;23:650–6. 10.1177/1357633X16659410 [DOI] [PubMed] [Google Scholar]
- 28.Ong MK, Romano PS, Edgington S, et al. Effectiveness of remote patient monitoring after discharge of hospitalized patients with heart failure: the better effectiveness after transition -- heart failure (BEAT-HF) randomized clinical trial. JAMA Intern Med 2016;176:310–8. 10.1001/jamainternmed.2015.7712 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Nouryan CN, Morahan S, Pecinka K, et al. Home Telemonitoring of community-dwelling heart failure patients after home care discharge. Telemedicine and e-Health 2019;25:447–54. 10.1089/tmj.2018.0099 [DOI] [PubMed] [Google Scholar]
- 30.Piccini JP, Mittal S, Snell J, et al. Impact of remote monitoring on clinical events and associated health care utilization: a nationwide assessment. Heart Rhythm 2016;13:2279–86. 10.1016/j.hrthm.2016.08.024 [DOI] [PubMed] [Google Scholar]
- 31.Akar JG, Bao H, Jones PW, et al. Use of remote monitoring is associated with lower risk of adverse outcomes among patients with implanted cardiac defibrillators. Circ Arrhythm Electrophysiol 2015;8:1173–80. 10.1161/CIRCEP.114.003030 [DOI] [PubMed] [Google Scholar]
- 32.Pekmezaris R, Nouryan CN, Schwartz R, et al. A randomized controlled trial comparing telehealth self-management to standard outpatient management in underserved black and Hispanic patients living with heart failure. Telemed J E Health 2019;25:917–25. 10.1089/tmj.2018.0219 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lyth J, Lind L, Persson HL, et al. Can a telemonitoring system lead to decreased hospitalization in elderly patients? J Telemed Telecare 2021;27:19858178. 10.1177/1357633X19858178 [DOI] [PubMed] [Google Scholar]
- 34.Trucco F, Pedemonte M, Racca F, et al. Tele-monitoring in paediatric and young home-ventilated neuromuscular patients: a multicentre case-control trial. J Telemed Telecare 2019;25:414–24. 10.1177/1357633X18778479 [DOI] [PubMed] [Google Scholar]
- 35.Böhm M, Drexler H, Oswald H, et al. Fluid status telemedicine alerts for heart failure: a randomized controlled trial. Eur Heart J 2016;37:3154–63. 10.1093/eurheartj/ehw099 [DOI] [PubMed] [Google Scholar]
- 36.De Simone V, Zanotto G, Guarise P, et al. Effects of remote monitoring of cardiac implantable electronic devices after stroke or transient ischemic attack. J Cardiovasc Med 2019;20:551–6. 10.2459/JCM.0000000000000822 [DOI] [PubMed] [Google Scholar]
- 37.Hale TM, Jethwani K, Kandola MS, et al. A remote medication monitoring system for chronic heart failure patients to reduce readmissions: a two-arm randomized pilot study. J Med Internet Res 2016;18:e91. 10.2196/jmir.5256 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kurek A, Tajstra M, Gadula-Gacek E, et al. Impact of remote monitoring on long-term prognosis in heart failure patients in a real-world cohort: results from all-comers COMMIT-HF trial. J Cardiovasc Electrophysiol 2017;28:425–31. 10.1111/jce.13174 [DOI] [PubMed] [Google Scholar]
- 39.Jaarsma T, van der Wal MHL, Lesman-Leegte I, et al. Effect of moderate or intensive disease management program on outcome in patients with heart failure: coordinating study evaluating outcomes of advising and counseling in heart failure (coach). Arch Intern Med 2008;168:316–24. 10.1001/archinternmed.2007.83 [DOI] [PubMed] [Google Scholar]
- 40.Pedone C, Rossi FF, Cecere A, et al. Efficacy of a physician-led multiparametric telemonitoring system in very old adults with heart failure. J Am Geriatr Soc 2015;63:1175–80. 10.1111/jgs.13432 [DOI] [PubMed] [Google Scholar]
- 41.Clemensen J, Rothmann MJ, Smith AC, et al. Participatory design methods in telemedicine research. J Telemed Telecare 2017;23:780–5. 10.1177/1357633X16686747 [DOI] [PubMed] [Google Scholar]
- 42.Koehler F, Koehler K, Deckwart O, et al. Efficacy of telemedical interventional management in patients with heart failure (TIM-HF2): a randomised, controlled, parallel-group, unmasked trial. Lancet 2018;392:1047–57. 10.1016/S0140-6736(18)31880-4 [DOI] [PubMed] [Google Scholar]
- 43.Thomason TR, Hawkins SY, Perkins KE, et al. Home telehealth and hospital readmissions: a retrospective OASIS-C data analysis. Home Healthc Now 2015;33:20–6. 10.1097/NHH.0000000000000167 [DOI] [PubMed] [Google Scholar]
- 44.Achelrod D, Schreyögg J, Stargardt T. Health-economic evaluation of home telemonitoring for COPD in Germany: evidence from a large population-based cohort. Eur J Health Econ 2017;18:869–82. 10.1007/s10198-016-0834-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Alshabani K, Attaway AA, Smith MJ, et al. Electronic inhaler monitoring and healthcare utilization in chronic obstructive pulmonary disease. J Telemed Telecare 2020;26:495–503. 10.1177/1357633X19850404 [DOI] [PubMed] [Google Scholar]
- 46.Orozco-Beltran D, Sánchez-Molla M, Sanchez JJ, et al. Telemedicine in primary care for patients with chronic conditions: the ValCrònic quasi-experimental study. J Med Internet Res 2017;19:e400. 10.2196/jmir.7677 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.McElroy I, Sareh S, Zhu A, et al. Use of digital health kits to reduce readmission after cardiac surgery. J Surg Res 2016;204:1–7. 10.1016/j.jss.2016.04.028 [DOI] [PubMed] [Google Scholar]
- 48.Martín-Lesende I, Orruño E, Mateos M, et al. Telemonitoring in-home complex chronic patients from primary care in routine clinical practice: impact on healthcare resources use. Eur J Gen Pract 2017;23:136–43. 10.1080/13814788.2017.1306516 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Chatwin M, Hawkins G, Panicchia L, et al. Randomised crossover trial of telemonitoring in chronic respiratory patients (TeleCRAFT trial). Thorax 2016;71:305–11. 10.1136/thoraxjnl-2015-207045 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Lüthje L, Vollmann D, Seegers J, et al. A randomized study of remote monitoring and fluid monitoring for the management of patients with implanted cardiac arrhythmia devices. Europace 2015;17:1276–81. 10.1093/europace/euv039 [DOI] [PubMed] [Google Scholar]
- 51.Mirón Rubio M, Ceballos Fernández R, Parras Pastor I, et al. Telemonitoring and home hospitalization in patients with chronic obstructive pulmonary disease: study TELEPOC. Expert Rev Respir Med 2018;12:335–43. 10.1080/17476348.2018.1442214 [DOI] [PubMed] [Google Scholar]
- 52.Ringbæk T, Green A, Laursen LC, et al. Effect of tele health care on exacerbations and hospital admissions in patients with chronic obstructive pulmonary disease: a randomized clinical trial. Int J Chron Obstruct Pulmon Dis 2015;10:10. 10.2147/COPD.S85596 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Sink E, Patel K, Groenendyk J, et al. Effectiveness of a novel, automated telephone intervention on time to hospitalisation in patients with COPD: a randomised controlled trial. J Telemed Telecare 2020;26:132–9. 10.1177/1357633X18800211 [DOI] [PubMed] [Google Scholar]
- 54.D'Ancona G, Safak E, Senges J, et al. Activation of remote monitoring for cardiac implantable electronic devices: small dog for tall weeds. Clin Res Cardiol 2017;106:833–9. 10.1007/s00392-017-1127-9 [DOI] [PubMed] [Google Scholar]
- 55.Gingele AJ, Brunner-la Rocca H, Ramaekers B, et al. Telemonitoring in patients with heart failure: is there a long-term effect? J Telemed Telecare 2019;25:158–66. 10.1177/1357633X17747641 [DOI] [PubMed] [Google Scholar]
- 56.Ishani A, Christopher J, Palmer D, et al. Telehealth by an interprofessional team in patients with CKD: a randomized controlled trial. Am J Kidney Dis 2016;68:41–9. 10.1053/j.ajkd.2016.01.018 [DOI] [PubMed] [Google Scholar]
- 57.Kenealy TW, Parsons MJG, Rouse APB, et al. Telecare for diabetes, CHF or COPD: effect on quality of life, hospital use and costs. A randomised controlled trial and qualitative evaluation. PLoS One 2015;10:e0116188. 10.1371/journal.pone.0116188 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Nouryan CN, Morahan S, Pecinka K, et al. Home telemonitoring of community-dwelling heart failure patients after home care discharge. Telemed J E Health 2019;25:447–54. 10.1089/tmj.2018.0099 [DOI] [PubMed] [Google Scholar]
- 59.Wagenaar KP, Broekhuizen BDL, Jaarsma T, et al. Effectiveness of the European Society of Cardiology/Heart Failure Association website 'heartfailurematters.org' and an e-health adjusted care pathway in patients with stable heart failure: results of the 'e-Vita HF' randomized controlled trial. Eur J Heart Fail 2019;21:238–46. 10.1002/ejhf.1354 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Banbury A, Nancarrow S, Dart J, et al. Adding value to remote monitoring: Co-design of a health literacy intervention for older people with chronic disease delivered by telehealth - The telehealth literacy project. Patient Educ Couns 2020;103:597–606. 10.1016/j.pec.2019.10.005 [DOI] [PubMed] [Google Scholar]
- 61.Vegesna A, Tran M, Angelaccio M, et al. Remote patient monitoring via non-invasive digital technologies: a systematic review. Telemed J E Health 2017;23:3–17. 10.1089/tmj.2016.0051 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Farias FACde, Dagostini CM, Bicca YdeA, FACd F, YdA B, et al. Remote patient monitoring: a systematic review. Telemed J E Health 2020;26:576–83. 10.1089/tmj.2019.0066 [DOI] [PubMed] [Google Scholar]
- 63.Thomas EE, Haydon HM, Mehrotra A, et al. Building on the momentum: sustaining telehealth beyond COVID-19. J Telemed Telecare 2020;1357633X:20960638. 10.1177/1357633X20960638 [DOI] [PubMed] [Google Scholar]
- 64.Hong Y, Lee SH. Effectiveness of tele-monitoring by patient severity and intervention type in chronic obstructive pulmonary disease patients: a systematic review and meta-analysis. Int J Nurs Stud 2019;92:1–15. 10.1016/j.ijnurstu.2018.12.006 [DOI] [PubMed] [Google Scholar]
- 65.Dickinson MG, Allen LA, Albert NA, et al. Remote monitoring of patients with heart failure: a white paper from the heart failure Society of America scientific statements Committee. J Card Fail 2018;24:682–94. 10.1016/j.cardfail.2018.08.011 [DOI] [PubMed] [Google Scholar]
- 66.Huygens MWJ, Swinkels ICS, de Jong JD, et al. Self-monitoring of health data by patients with a chronic disease: does disease controllability matter? BMC Fam Pract 2017;18:40. 10.1186/s12875-017-0615-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Galinier M, Roubille F, Berdague P, et al. Telemonitoring versus standard care in heart failure: a randomised multicentre trial. Eur J Heart Fail 2020;22:985–94. 10.1002/ejhf.1906 [DOI] [PubMed] [Google Scholar]
- 68.Noah B, Keller MS, Mosadeghi S. Impact of remote patient monitoring on clinical outcomes: an updated meta-analysis of randomized controlled trials. NPJ Digital Medicine 2018;1:1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Walker RC, Tong A, Howard K, et al. Patient expectations and experiences of remote monitoring for chronic diseases: systematic review and thematic synthesis of qualitative studies. Int J Med Inform 2019;124:78–85. 10.1016/j.ijmedinf.2019.01.013 [DOI] [PubMed] [Google Scholar]
- 70.Adair JG. The Hawthorne effect: a reconsideration of the methodological artifact. J Appl Psychol 1984;69:334–45. 10.1037/0021-9010.69.2.334 [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
bmjopen-2021-051844supp001.pdf (237.4KB, pdf)
Data Availability Statement
Data are available in a public, open access repository. All included articles are available publically.