Abstract
Acinetobacter baumannii is a dangerous bacterial pathogen possessing the ability to persist on various surfaces, especially in clinical settings, and to rapidly acquire the resistance to a broad spectrum of antibiotics. Thus, the epidemiological surveillance of A. baumannii within a particular hospital, region, and across the world is an important healthcare task that currently usually includes performing whole-genome sequencing (WGS) of representative isolates. During the past years, the dissemination of A. baumannii across the world was mainly driven by the strains belonging to two major groups called the global clones or international clones (ICs) of high risk (IC1 and IC2). However, currently nine ICs are already considered. Although some clones were previously thought to spread in particular regions of the world, in recent years this is usually not the case. In this study, we determined five ICs, as well as three isolates not belonging to the major ICs, in one multidisciplinary medical center within the period 2017–2019. We performed WGS using both short- and long-read sequencing technologies of nine representative clinical A. baumannii isolates, which allowed us to determine the antibiotic resistance and virulence genomic determinants, reveal the CRISPR/Cas systems, and obtain the plasmid structures. The phenotypic and genotypic antibiotic resistance profiles are compared, and the possible ways of isolate and resistance spreading are discussed. We believe that the data obtained will provide a better understanding of the spreading and resistance acquisition of the ICs of A. baumannii and further stress the necessity for continuous genomic epidemiology surveillance of this problem-causing bacterial species.
Keywords: Acinetobacter baumannii, antibiotic resistance, virulence, whole-genome sequencing, international high-risk clones, genomic epidemiology
1. Introduction
Monitoring the spread of particular lineages of pathogenic bacteria and the associated antimicrobial resistance determinants within a particular hospital, country, or across the world represents a very important task for national and international public health institutions. Currently, such surveillance is becoming more and more dependent on next-generation sequencing (NGS) of bacterial genomes and the corresponding bioinformatics analysis pipelines [1,2,3]. Such investigations have already formed a new field called ‘genomic epidemiology’, in which methods allow to obtain huge amounts of epidemiologically and medically relevant information in a cost-, time-, and resource-efficient way [4,5,6].
During recent years, the antibiotic resistance within different species of nosocomial and community-acquired bacterial pathogens has increased to dangerous levels [7,8,9]. This problem cannot be solved without comprehensive investigations of resistance transmission mechanisms and global epidemiological surveillance.
Currently, one of the most problematic pathogens is Acinetobacter baumannii, accounting for about 2% of all healthcare-associated infections in USA and Europe [10] and up to 4% in Asia [11]. A. baumannii is a member of the ESKAPE group of bacterial pathogens, also including Enterococcus faecium, Staphylococcus aureus, Klebsiella pneumoniae, Pseudomonas aeruginosa, and Enterobacter spp., which are major causes of antibiotic-resistant infections worldwide [12]. It is a Gram-negative coccobacillus that is mainly responsible for causing pneumonia and wound infections associated with elevated morbidity and mortality in clinical settings [13]. The notable characteristics of A. baumannii include the ability to rapidly acquire multidrug-, extensive drug-, and even pandrug-resistance phenotypes [14], as well as to easily survive and transfer in the hospital environment, such as attaching to various biotic and abiotic surfaces [15]. A. baumannii evolution during the past five decades was mainly driven by two globally disseminated clones, GC1 and GC2 (also called IC1 and IC2, IC standing for ‘international clone’) [16]. However, six additional clonal lineages are currently generally accepted [17], and IC9 is on its way [18].
In Russia, A. baumannii constitutes up to 16.8% of healthcare-associated bacterial infections and exhibits a high rate of carbapenem resistance (about 77%), with the predominating clones being IC1, IC2, and IC6 [19].
Although many reports consider different clones or lineages of A. baumannii species to be associated with particular parts or regions of the world [13,20,21,22], the dramatically fast distribution of SARS-CoV-2 in 2020 has demonstrated that our knowledge regarding the spread of different pathogens is still limited.
Here we present the results of genomic epidemiology monitoring of A. baumannii in a multidisciplinary medical center in Moscow, Russia, during the period 2017–2019. Amazingly, we have revealed the isolates belonging to 5 out of 9 international clonal lineages (ICL), as well as additional isolates not clustering to any known ICL within our samples. We selected nine representative isolates for this manuscript and performed whole-genome sequencing for them using second- and third-generation (long-read) sequencing technologies. Comprehensive analyses of phenotypic and genotypic antimicrobial resistance, virulence factors, plasmids, and CRISPR arrays for the selected isolates are provided.
We believe that our data will facilitate a better understanding of A. baumannii spread across the world and the possible ways of acquiring antimicrobial resistance by this dangerous pathogen.
2. Results
2.1. Isolate Metadata and Typing
The metadata for the isolates and the results of their typing using the Pasteur MLST scheme, K-, and OCL-loci are presented in Table 1.
Table 1.
The origin and typing of the clinical A. baumannii isolates studied.
| Sample id | Patient Code |
Isolation Date | Clinical Department | Locus | MLST | OCL-Type | KL-Type | IC |
|---|---|---|---|---|---|---|---|---|
| CriePir33 | P1 | 03.05.2017 | Traumatology | Wound | ST78 | OCL1 | KL3 | IC6 (CC78) |
| CriePir87 | P2 | 04.07.2017 | Surgery | Soft tissue abscess | ST2 | OCL1 | KL33 | IC2 (CC2) |
| CriePir168 | P3 | 03.12.2017 | ICU | Urine | ST1 | OCL1 | KL17 | IC1 (CC1) |
| CriePir254 | P4 | 24.08.2018 | Surgery | Bile | ST370 | OCL1 | KL25 | CC252 |
| CriePir298 | P5 | 10.09.2019 | CNS Rehabilitation | Urine | ST25 | OCL6 | KL116 | IC7 (CC25) |
| CriePir306 | P6 | 05.08.2019 | ICU | BAL | ST15 | OCL7 | KL9 | IC4 (CC15) |
| CriePir307 | P7 | 29.06.2019 | ICU | BAL | ST1487 * | OCL2 | KL45 | CC152 |
| CriePir308 | P6 | 22.08.2019 | ICU | CVC | ST2 | OCL7 | KL9 | IC2 (CC2) |
| CriePir309 | P8 | 31.08.2019 | CNS Rehabilitation | Urine | ST911 | OCL6 | KL14 | CC132 |
ICU—intensive care unit; CVC—central venous catheter; BAL—bronchoalveolar lavage; *—novel ST identified by us.
In this study, we aimed to capture the widest possible diversity of A. baumannii clones while keeping the number of isolates lower for the sake of presentation clarity. We selected nine isolates, six of which represented known international clones of high risk and three isolates represented singletons that could not be attributed to any ICs. Well-established ‘old’ international clones 1 and 2 [13] are presented by their general sequence types. Two isolates representing IC2 had different K- and OCL-types, so they were both included in the study to further increase the isolate diversity.
IC4 and IC6 were also represented by their major STs [17]. It is interesting that the isolates representing two different clones, CriePir306 (IC4) and CriePir308 (IC2), were isolated from the same patient, but from different sites. However, they had the same K- and OCL-types, which is rather surprising.
CriePir307 possessed a novel sequence type that was not clustered with existing international clones by either cgMLST or MLST loci. Although it possessed the blaOXA-64 gene that is known to be the characteristic of IC7 [23], it was quite different from other IC7 isolates by cgMLST profile (see Table S1 and Figure S1). Thus, it was assigned to the most frequent Pasteur ST profile clustering in the same clonal complex (CC152). CriePir298, which belonged to IC7, possessed a general MLST sequence type for this clone—ST25 [17]. However, it had a rather rare capsular type—KL116 [24].
CriePir254 and CriePir309 also did not belong to the known international clonal lineages, but could be attributed to CC252 and CC132, respectively.
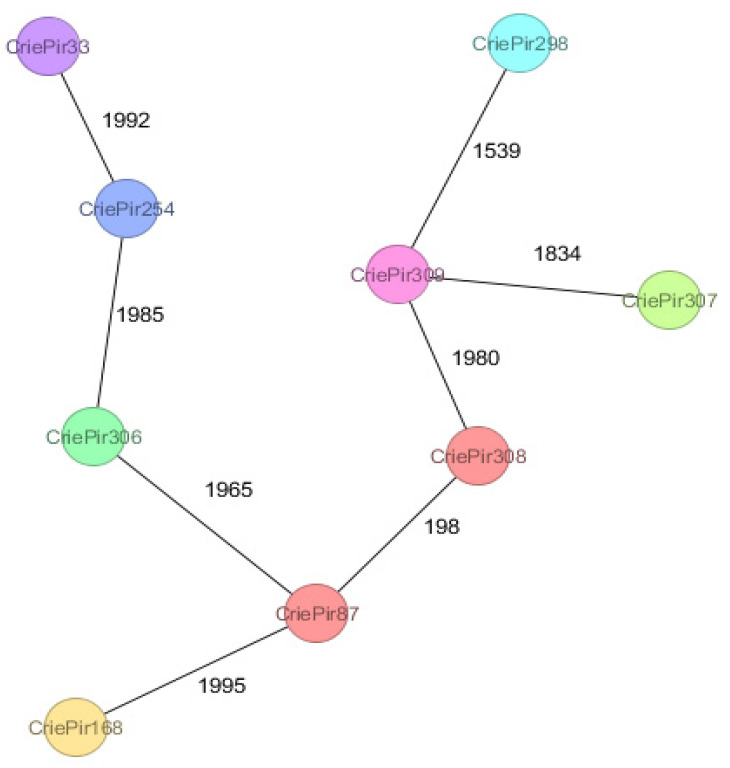
The minimum spanning tree for the isolates is presented in Figure 1. It is not surprising that the isolates are located very far from each other in terms of their cgMLST profiles since the aim of this study was to capture the maximal possible diversity. Only the isolates CriePir87 and CriePir308 belonging to IC2 were comparatively close to each other (198 allele differences).
Figure 1.
The minimum spanning tree for the isolates studied based on cgMLST profiles. The numbers indicate the amount of different alleles between the pairs of corresponding isolates. Close isolates (CriePi87 and CriePi308) are shown in the same color; other isolates are not close to each other.
We also built a cgMLST tree for our isolates and the whole set of RefSeq isolates (accessed on 20 March 2021), trying to infer the possible spreading information. A subset of this tree, including the closest matches from RefSeq to our isolates in terms of the number of cgMLST allele differences, is provided in the Figure S1.
As we can see from this tree, the isolates with unusual profiles, such as CriePir254 (rare ST, no IC) and CriePir307 (novel ST, no IC), do not have close relatives in terms of the number of allele differences. Moderately close neighbors were revealed for CriePir168 (IC1, GCA_000830055.1, Australia, distance = 187), CriePir308 (IC2, GCA_000314655.1, USA, distance = 50), CriePir306 (IC4, GCF_003583665.1, Spain, distance = 147), and CriePir33 (IC6, GCF_003948375.1, USA, distance = 87). However, this information does not allow inferring the spreading routes of the isolates, and more genomic data is required to achieve this goal.
Complete cgMLST profiles for all our isolates and their closest matches from RefSeq are presented in Table S1.
2.2. Antimicrobial Resistance
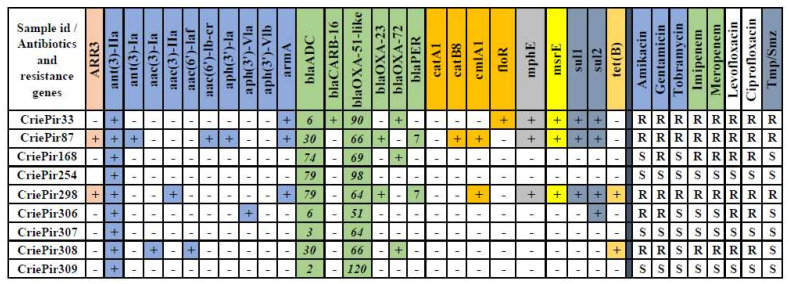
Phenotypic characterization and the genotypic resistance determinants of the isolates studied are presented in Figure 2.
Figure 2.
Antimicrobial resistance of the A. baumannii isolates studied. Corresponding antibiotics and resistance genes are filled with the same colors. The numbers for the bla genes indicate the corresponding variants for the sake of brevity. Tmp/Smz—trimethoprim/sulfamethoxazole.
As we can see from Figure 2, the isolates form three groups containing three members each: multidrug-resistant (CriePir33, CriePir87, and CriePir298), which exhibit resistance to all antibiotics from the panel; non-resistant (CriePir254, CriePir307, and CriePir309, possessing only intrinsic oxacillinase genes); and intermediate (CriePir168, CriePir306, and CriePir308), having resistance only to some of the antibiotics tested. Five isolates were carbapenem-resistant, and the likely mechanism of such a resistance is expression of blaOXA-23 (CriePir87 and CriePir298) and blaOXA-72 (CriePir33, CriePir168, and CriePir308) carbapenemase genes. In addition, the blaOXA-23-carrying isolates also encoded PER-7 extended spectrum β-lactamase (ESBL), which was reported to demonstrate high activity against broad-spectrum cephalosporins in A. baumannii [25].
The resistant isolates also included genes and gene clusters providing resistance to aminoglycosides (for example, armA), sulfamethoxazole (sul1 and sul2), chloramphenicol (cmlA1), and tetracycline (tet(B)). However, the latter two antimicrobials were not included in the panel.
Interestingly, CriePir33 possessed the blaCARB-16 gene representing a rather rarely occurring blaCARB-5-like class A beta-lactamase gene, which was first revealed in Acinetobacter pittii [26]. The enzyme encoded by blaCARB-16 differs only by one amino acid substitution from blaCARB-5.
In general, the isolates demonstrated very good compliance between the phenotypic and genotypic characteristics of their antimicrobial resistance. The group of susceptible isolates included only intrinsic blaADC and blaOXA-51-like oxacillinases, as well as the ant(3)-IIa gene, while the isolates that exhibited the multidrug-resistant phenotype possessed the largest number of acquired resistance genes (8 for CriePir33, 13 for CriePir87, and 11 for CriePir298, respectively).
2.3. Virulence Genes
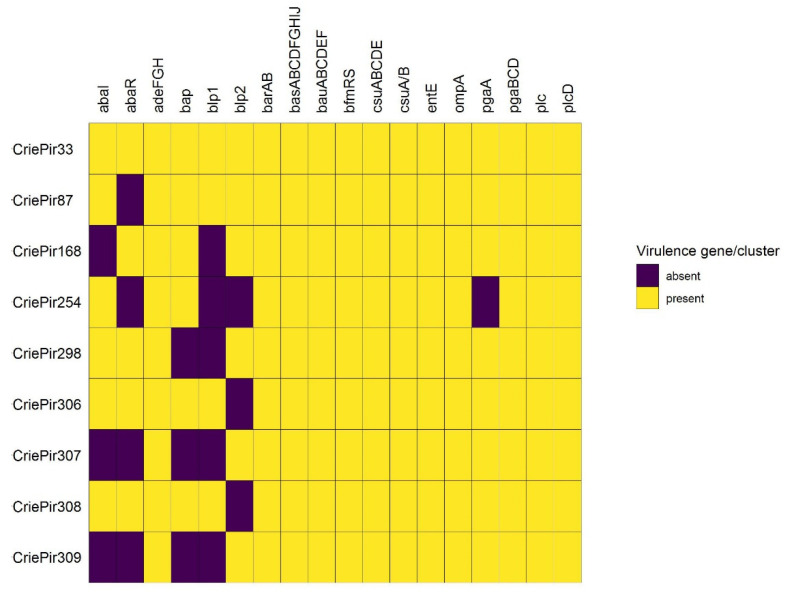
The distribution of the virulence genes in the isolates studied is shown in Figure 3.
Figure 3.
Virulence factors of the A. baumannii isolates studied. Genes constituting the same cluster presented in all isolates were combined for the sake of brevity. abaI, abaR—components of the quorum sensing system; adeFGH—efflux pump; bap—biofilm-associated protein; blp1,blp2—bap-like proteins; barAB—siderophore efflux system of the ABC superfamily; basABCDFGHIJ—proteins involved in biosynthesis of acinetobactin; bauABCDEF—receptor for ferric-acinetobactin complexes; bfmRS—two-component signal transduction system; csuA/BABCDE—Csu pili; entE—enterobactin biosynthesis; ompA—outer membrane protein; pgaABCD—biofilm formation locus; plc, plcD—phospholipase genes.
The virulence gene sets of all isolates were quite similar. They mainly included the factors involved in biofilm formation (adeFGH, csu, and pga clusters) as well as the bau and bas clusters involved in the iron acquisition system and acinetobactin functioning.
CriePir306 and CriePir308 included all 39 factors revealed, while the other isolates lacked from one to four virulence genes each. Interestingly, we have not revealed any virulence plasmids, and all virulence genes were located on chromosomes.
The list of NCBI accession numbers for the virulence genes revealed is shown in Table S2.
2.4. Plasmids
The isolates had from one to six plasmids each (Table S3). However, these plasmids usually did not carry the resistance genes, except for CriePir298, which possessed the plasmid containing all resistance determinants, excluding blaOXA-23 and the intrinsic genes, as well as CriePir33, CriePir168, and CriePir308, the latter two of which carried two copies of blaOXA-72 of plasmid origin. Interestingly, the plasmids of CriePir168 (length = 10,878 bp) and CriePir308 (length = 10,879 bp) had essentially the same sequences except for several deletions that might result from long-read assembly algorithm imperfections. These two isolates belonged to different clonal lineages (ST1 and ST2, respectively) and were isolated with an interval of 2 years. However, both of them were found in the same clinical department (ICU), and thus the persistence of the blaOXA-72-carrying plasmid within this unit could be suggested. Another interesting fact was that these plasmids had 99.7% identity with the pAB120 plasmid (Genbank accession CP031446.1) of the MDR-UNC A. baumannii isolate (ST2), which caused a fatal case of necrotizing fasciitis in a USA hospital in 2019 [27].
CriePir298 included three plasmids, the smallest of which (length = 2343) had exactly the same sequence as pA85-1a (Genbank accession CP021784.1) from the A85 A. baumannii strain isolated in Australia in 2003. This plasmid was also found in several other isolates of IC1 [28], but its functional properties were not described. However, CriePir298 belonged to IC7. A brief description of the plasmids is provided in Table 2.
Table 2.
Plasmid characteristics of the isolates studied.
| Sample Id | Number of Plasmids | Plasmid Sizes | Virulence/Resistance Determinants in Plasmids | Related Plasmids from Other Isolates |
|---|---|---|---|---|
| CriePir33 | 1 | 17,765 | blaOXA-72 | pIBAC_oxa58_20C15, KY202458.1 |
| CriePir87 | 1 | 11,194 | - | pUnnamed1, CP035673.1 |
| CriePir168 | 3 | 1217–10,878 | blaOXA-72 | pAB120, CP031446.1 |
| CriePir254 | 2 | 10,427–90,326 | - | pGFJ6, CP016902.1 |
| CriePir298 | 3 | 2343–183,139 | mph(E), msr(E), armA, sul1, sul2, blaPER-7, ARR-3, aph(6)-Id, aph(3′′)-Ib, aac(3)-IIa, tet(B) | pA85-1a, CP021784.1 |
| CriePir306 | 4 | 2845–80,829 | - | pA1296_1, CP018333.1 |
| CriePir307 | 6 | 2278–114,430 | - | pABAY15001_6E, MK386684.1 |
| CriePir308 | 1 | 10,879 | blaOXA-72 | pAB120, CP031446.1 |
| CriePir309 | 2 | 11,195–94,551 | - | pTS134338, CP042210.1 |
2.5. CRISPR Arrays and CRISPR/Cas Systems
CRISPR arrays were revealed in seven out of nine isolates (except CriePir87 and CriePir308). However, at least five repeats with an evidence level = 4 were found only in four isolates (CriePir168, CriePir298, CriePir307, and CriePir309), and all of them except the latter possessed a full CAS-Type IF system. Interestingly, these CAS systems had different subtypes, namely, IF-1 for CriePir168 and CriePir298, and IF-2 for CriePir307. Detailed analysis of the differences between these two subtypes lies beyond the scope of this manuscript.
Interestingly, although CriePir309 had a highly confident CRISPR array with 17 repeats, it was also the only isolate carrying an anti-CRISPR element, which could prevent the development of a functional CRISPR/Cas system.
Most repeats and all cas genes were located on the chromosomes. CriePir168 had one array on the short plasmid (length = 2372), and CriePir308 had one of seven arrays on another plasmid (length = 14,128). However, both of these repeats had a low evidence level.
Detailed characteristics of the CRISPR elements, including the chromosome positions, repeat sequences, and accession numbers for the cas genes revealed, are provided in Table S4.
3. Discussion
In this manuscript, we presented the genomic epidemiology investigation of a diverse A. baumannii population within a multidisciplinary medical center in Moscow, Russia, for the period 2017–2019. We carefully selected the isolates representing the unusual international clone variability, as well as additional isolates belonging to singleton clones, and performed their long-read sequencing in order to obtain highly accurate chromosome sequences and delineate plasmids. Hybrid short- and long-read assemblies allowed us to improve the prediction of virulence and the resistance genomic determinants, as well as to retrieve additional information required for application of genomic epidemiology tools such as cgMLST analysis.
In order to perform the epidemiological surveillance and track the spreading of pathogenic bacteria within a particular healthcare facility, some geographical region, or across the world, a reliable typing scheme based on molecular or genomic characteristics of the isolates is required. Many typing schemes are already provided for pathogenic bacteria, including A. baumannii. Exemplary schemes/profiles comprise the ones based on the nucleotide frequency matrices for genomic sequences [29], CRISPR sequences [30], multilocus sequence typing (MLST) [31,32] based on seven housekeeping genes, MLST/KL loci [33], and core genome MLST (cgMLST) based on 2390 genes [34]. The latter was recently successfully applied for an outbreak investigation [35]. In addition, intrinsic carbapenemase blaOXA-51-like gene variants were also proposed as a tool for A. baumannii identification and typing [36].
However, one of the most commonly used concepts for molecular epidemiology investigations is epidemic clonal lineages, or simply ‘international clones’, which represent genetically distinct populations of A. baumannii successfully spreading in different geographic locations [37]. Eight international clones were defined earlier [17], and the ninth was introduced recently [18]. The first three clones (IC1–IC3) are distributed worldwide, with IC1 and IC2 also known as global clones (GC), while the rest were sometimes defined as regional, or even endemic clones [13,20,21,22].
In contrast to this, we revealed an isolate belonging to IC7 in our hospital, although this clone was recently reported to be prevalent in South America and not in Europe [22]. Although our initial set of isolates contained less than 50 sequenced samples, we managed to reveal five different ICs and three singleton isolates not attributed to known clonal lineages. One of these isolates belonged to IC7: CriePir298 possessed the founder sequence type ST25, the members of which were revealed in Bolivia [22]. It is interesting that the ST1487 possessed by CriePir307 is rather different from ST25 in its allelic profile, and they do not cluster according to the eBURST analysis; at the same time, these two isolates share the same intrinsic OXA-51 variant, namely, OXA-64, and thus likely belong to the same lineage IC7 [23]. However, a large number of MLST and cgMLST allele differences did not allow to assign CriePir307 to IC7, and it was closer to the members of CC152. Another intriguing fact is that the resistance characteristics of CriePir298 and CriePir307 are completely different—the former was resistant to all antibiotics from the panel, while the latter was susceptible to all antibiotics tested. However, genomic analysis revealed that most resistance genes of CriePir298 were located on the resistance plasmid, which was very similar to pMC1.1 (Genbank accession MK531536.1) revealed in Bolivia in the investigation mentioned above [22].
Historic global clones were represented in our set by IC1 (CriePir168) and IC2 (CriePir87 and CriePir308). IC4, possessing the blaOXA-51 intrinsic gene, was revealed in CriePir306 (ST15). This clone was previously found in South America [38], but it is also present in Europe [39]. IC6, which was once considered to be a Russian endemic [20] and constituted about 25% of clinical isolates in Russia in 2015–2016 [19], was presented by CriePir33, a carbapenem-resistant isolate carrying, among others, blaCARB-16 and blaOXA-72 resistance determinants. This complies with the observation that IC6 isolates from Russia usually obtain carbapenem resistance by acquiring class D carbapenemase genes and not by other mechanisms such as intrinsic gene mutations [19]. Unfortunately, the cgMLST comparison with the reference isolates did not reveal any clues regarding the routes of their spreading across the world due to the ubiquitous presence of widespread ICs in various geographical regions.
Finally, two isolates, CriePir254 and CriePir309, could not be attributed to known international clones. CriePir309, carrying blaOXA-120 intrinsic beta-lactamase, can be assigned to the known complex CC132, although it is also rather close to CC33 [40], while CriePir254 is close to CC252. CriePir309 has a very rare ST911; currently, no full genomic sequences for the isolates of this sequence type are available in Genbank, and there is no information, except a definition, for this ST in the PubMLST database (https://pubmlst.org, accessed in 23 April 2021). This isolate was obtained from the only patient involved in the study living outside the Moscow region, and it possessed various interesting properties. For example, it did not carry resistance genes except intrinsic variants of the blaADC and blaOXA-51-like genes, and it was the only isolate having anti-CRISPR proteins encoded (AcrIF11). At the same time, it carried several virulence gene clusters (adeFGH, basABCDFGHIJ, csuABCD, but not bap), which made it more similar to the strains causing community-acquired infections. CriePir254 and CriePir307 were similar to CriePir309 in this sense, since they also did not have resistance determinants. Thus, we can conclude that the isolates from our dataset that appeared to be rather distant from the known international high-risk clones were in fact less dangerous in terms of their antibiotic resistance.
In summary, our results confirm that in the era of globalization and rapid pathogen spread across the world, the concept of endemic clones becomes obsolete. For example, we revealed IC7 in Russia, and IC6, which was previously attributed to Europe, was recently found in Brazil [41]. In addition, a recent publication of the spatio-temporal distribution of A. baumannii in Germany showed that the isolates belonging to IC1, 2, 4, 6, and 7 were revealed during 2000–2018 [39], which corresponds to our dataset. However, the remarkable feature of our study is the discovery of such an IC variety within one hospital during a limited period of time, and for the patients living in the Moscow region and without a history of international travelling shortly before their hospital admission. This finding will allow developing updated prevention strategies and epidemiological measures to limit further high-risk clone spreading.
While epidemiological data is vitally important for studying the spread of any pathogenic bacteria, the information regarding the presence of antibiotic resistance and virulence factors in the isolates studied, as well as the possible mechanisms of their transfer, is no less important for developing the prevention measures. Sequencing on MinION and hybrid short-long read assembly allowed us to identify the locations of genes encoding oxacillinases and other resistance genes. The blaOXA-23 genes of two isolates (CriePir87 and CriePir298) were located on the chromosome, while blaOXA-72 for each of the three isolates (CriePir33, CriePir168, and CriePir308) were revealed on plasmids. Such a distribution complies well with previous studies [36,42].
In general, the antibiotic resistance genes of our isolates were not located on plasmids, except for CriePir298 and the three isolates mentioned in the previous paragraph. However, plasmid investigation can contribute not only to antimicrobial resistance studies but also to tracing the spread of the pathogens across the world, although the plasmid complement is not a reliable measure of relatedness [28]. For example, in our isolates we revealed the plasmids identical to the ones of clinical isolates from the USA and Australia. However, these isolates belonged to different clonal lineages than ours, so the possible ways of plasmid transferring between them cannot be easily reconstructed.
In contrast, we have not revealed any virulence plasmids in our isolates, and all the virulence genes were located on the chromosomes.
Virulence factors were represented in all of the isolates by the members of csu and pga clusters involved in biofilm formation [43,44], as well as the members of bau and bas clusters taking part in the iron acquisition system and acinetobactin transport and biosynthesis, respectively [45,46]. In addition, all isolates included the genes of the adeFGH efflux pump, the overexpression of which was also found to be associated with biofilm formation [47]. These genes are rather common for clinical A. baumannii isolates and, together with the other genes revealed (e.g., bap for CriePir87 and CriePir306), provide the environmental persistence for them [14]. In addition, all isolates contained the ompA gene, encoding a major component of outer membrane vesicles, which was considered to be a crucial virulence factor of A. baumannii [48]. Interestingly, the bap gene was revealed only in the isolates lacking the CRISPR/Cas system; this could be the result of preventing horizontal gene transfer by this system.
The sets of virulence factors were similar for all isolates, except for abaR, which was not revealed in the fully susceptible isolates (CriePir254, CriePir307, and CriePir309) and CriePir87, and abaI, which was not found in CriePir168, CriePir307, and CriePir309. These genes are involved in quorum sensing and may contribute to motility and host–pathogen interaction [45]. The presence of abaI/abaR was positively correlated with bacterial resistance rates [49], and thus their absence in susceptible isolates complies with this finding. However, additional investigations are needed to elucidate the possible mechanisms since such a correlation was not perfect for our isolates.
Finally, we can conclude that obtaining extensive data on the spreading of particular strains, high-risk clones, antimicrobial resistance, and virulence factors across a particular hospital, country, and region greatly facilitates developing the epidemiological measures for preventing an exponential increase in MDR A. baumannii strains, as well as other pathogenic bacterial species. Unfortunately, these measures are not sufficient for fighting the resistant bacteria in clinical settings. Possible holistic approaches to cope with this problem could include antibiotic stewardship [50], developing novel antibiotics [51], using bacteriophages, and other antibacterial moieties, such as antibodies, synthetic membrane-active agents, or antimicrobial peptides [52,53,54,55].
4. Materials and Methods
4.1. Determination of Antibiotic Susceptibility
Species identification for all isolates was performed by time-of-flight mass spectrometry (MALDI-TOF MS) using the VITEK MS system (bioMerieux, Marcy-l’Étoile, France), and the susceptibility to antimicrobials was determined by the disc diffusion method using the Mueller–Hinton medium (bioMerieux, Marcy-l’Étoile, France) and disks with antibiotics (BioRad, Marnes-la-Coquette, France), and by the Minimum Inhibitory Concentration (MIC) method on a VITEK 2 Compact 30 analyzer (bioMerieux, Marcy-l’Étoile, France). The antibiotics panel included the following drugs: amikacin, gentamicin, tobramycin, imipenem, meropenem, levofloxacin, ciprofloxacin, and trimethoprim/sulfomethoxazole. These antimicrobial compounds reflected those agents used for human therapy in the Russian Federation. We used the EUCAST clinical breakpoints, version 11.0 (https://www.eucast.org/clinical_breakpoints/, accessed on 20 December 2020), to interpret the results obtained.
4.2. DNA Isolation, Sequencing, and Genome Assembly
Nine samples were obtained from eight patients (5 males and 3 females) in various sources and hospital departments (Table 1) of a multidisciplinary federal medical center in Moscow, Russia, during the period 2017–2019. The age of the patients involved in this study ranged from 27 to 62 years with a median equal to 56.
The total number of isolates involved in the initial screening was 145, and 49 of them were sequenced. Earlier we have investigated the properties of the CRISPR/Cas arrays and systems for some of these isolates and a set of reference isolates from RefSeq [56]. Then we carefully selected nine isolates from the initial set, which represented the diversity of the A. baumannii international clones revealed in the hospital, and performed long-read sequencing for them. Our aim was not to capture the diversity of all strains found in the hospital during the study period, but rather to investigate the spread of international high-risk clones and to check the hypothesis of their endemicity for a particular region or country. Long-read sequencing allowed us to obtain the precise genome and plasmid structures, as well as to verify the locations of antibiotic resistance and virulence determinants, and to obtain complete cgMLST profiles for the selected representative isolates.
Genomic DNA was isolated with the DNeasy Blood and Tissue kit (Qiagen, Hilden, Germany). A Nextera™ DNA Sample Prep Kit (Illumina®, San Diego, CA, USA) was used for paired-end library preparation, and whole-genome sequencing (WGS) of the isolates on Illumina® Miseq and Hiseq platforms (Illumina®, San Diego, CA, USA).
Additional WGS was performed using the Oxford Nanopore MinION sequencing system (Oxford Nanopore Technologies, Oxford, UK). The same genomic DNA was used to prepare the MinION library with the Rapid Barcoding Sequencing kit SQK-RBK004 (Oxford Nanopore Technologies, Oxford, UK). The amount of initial DNA used for barcoding kit was 400 ng for each sample. The libraries were prepared according to the manufacturer’s protocols, and were sequenced on R9 SpotON flow cell by initiating the standard 24 h sequencing protocol using the MinKNOW software (Oxford Nanopore Technologies, Oxford, UK).
Base calling of the raw MinION data was performed using Guppy Basecalling Software version 4.4.1 (Oxford Nanopore Technologies, Oxford, UK), and demultiplexing was made using Guppy barcoding software version 4.4.1 (Oxford Nanopore Technologies, Oxford, UK). Hybrid assemblies were obtained using short- and long-reads by Unicycler version 0.4.9-beta [57].
Genome assemblies were uploaded to NCBI Genbank under the following accession numbers: JAEPWJ000000000 (CriePir33), JAEPWI000000000 (CriePir87), JAEPWG000000000 (CriePir168), JAHHIS000000000 (CriePir254), JAEPWB000000000 (CriePir298), JAEPVY000000000 (CriePir306), JAEPVX000000000 (CriePir307), JAEPVW000000000 (CriePir308), and JAEPVV000000000 (CriePir309).
4.3. Data Processing
The genomes assembled were processed using a custom software pipeline described earlier [33]. We used the Resfinder 4.0 database for antimicrobial gene identification (https://cge.cbs.dtu.dk/services/ResFinder/, accessed on 20 April 2021). VFDB [58] was used to search for the virulence factors http://www.mgc.ac.cn/VFs/main.htm (accessed on 20 April 2021).
Isolate typing was first performed by MLST using the Pasteur scheme. It was chosen for typing since, according to the Oxford scheme, five isolates possessed undetectable sequence types (STs) with a duplicated gdhB locus. Additional classification was made by using capsule synthesis loci (K-loci) [59] and lipooligosaccharide outer core loci (OCL) [60]. Detection of cgMLST profiles was performed using MentaList software (https://github.com/WGS-TB/MentaLiST, version 0.2.4, accessed on 10 June 2021), and the minimum spanning tree was build using PHYLOViz online (http://online.phyloviz.net, accessed on 10 June 2021).
CRISPRCasFinder [61] was used to identify the presence of CRISPR/Cas systems and spacers in the genomes studied. Anti-CRISPR elements were searched in AcrBank http://cefg.uestc.cn/anti-CRISPRdb (accessed on 20 April 2021).
5. Conclusions
In this study, we performed third-generation sequencing-based genomic epidemiology surveillance of clinical A. baumannii isolates from a multidisciplinary medical center in Moscow, Russia, obtained during 2017–2019. Surprisingly, we revealed that our isolates included 5 of the 9 commonly defined international clones of this important nosocomial pathogen. In addition, three isolates possessed singleton sequence types not clustered with the known lineages, including ST911, for which whole-genome data are not available yet. We presented a detailed analysis of the phenotypic antimicrobial resistance and genomic resistance determinants for all the isolates studies, as well as additional data for virulence factors, plasmids, and CRISPR arrays. We believe that these data will facilitate a better understanding of the clonal spreading and resistance acquisition of A. baumannii and further highlight the necessity of continuous genomic epidemiology surveillance for this problematic pathogen.
Supplementary Materials
The following are available online at https://www.mdpi.com/article/10.3390/antibiotics10081009/s1, Table S1. cgMLST profiles for clinical A. baumannii isolates included in the study, Table S2. Accession numbers for the virulence genes revealed in the isolates studied, Table S3. Plasmid description for the isolates studied, Table S4. Description and statistics for the CRISPR arrays revealed in clinical A. baumannii isolates included in the study, Figure S1. cgMLST tree for the isolates under study and their closest matches from RefSeq database.
Author Contributions
All authors contributed to this study; L.P. and M.Z. performed the clinical part of the experiment; Y.M. performed the sequencing part; A.S. analyzed the data and wrote the manuscript; V.A. supervised the project and obtained funding. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by the Ministry of Science and Higher Education of the Russian Federation within the framework of a grant in the form of a subsidy for the creation and development of the «World-class Genomic Research Center for Ensuring Biological Safety and Technological Independence under the Federal Scientific and Technical Program for the Development of Genetic Technologies», agreement No. 075-15-2019-1666.
Institutional Review Board Statement
Ethical review and approval were waived for this study since the human samples were routinely collected and patients’ data remained anonymous.
Informed Consent Statement
Patient consent was waived since the human samples were routinely collected, and patients’ data remained anonymous. Patients codes reported in the manuscript are used for description purposes only and do not correspond to patient records within the hospital.
Data Availability Statement
The bacterial genomes presented in this study are openly available in NCBI Genbank under the following accession numbers: JAEPWJ000000000 (CriePir33), JAEPWI000000000 (CriePir87), JAEPWG000000000 (CriePir168), JAHHIS000000000 (CriePir254), JAEPWB000000000 (CriePir298), JAEPVY000000000 (CriePir306), JAEPVX000000000 (CriePir307), JAEPVW000000000 (CriePir308), and JAEPVV000000000 (CriePir309).
Conflicts of Interest
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Armstrong G.L., MacCannell D.R., Taylor J., Carleton H.A., Neuhaus E.B., Bradbury R.S., Posey J.E., Gwinn M. Pathogen Genomics in Public Health. N. Engl. J. Med. 2019;381:2569–2580. doi: 10.1056/NEJMsr1813907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gilchrist C.A., Turner S.D., Riley M.F., Petri W.A., Jr., Hewlett E.L. Whole-genome sequencing in outbreak analysis. Clin. Microbiol. Rev. 2015;28:541–563. doi: 10.1128/CMR.00075-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Maljkovic Berry I., Melendrez M.C., Bishop-Lilly K.A., Rutvisuttinunt W., Pollett S., Talundzic E., Morton L., Jarman R.G. Next Generation Sequencing and Bioinformatics Methodologies for Infectious Disease Research and Public Health: Approaches, Applications, and Considerations for Development of Laboratory Capacity. J. Infect. Dis. 2020;221:S292–S307. doi: 10.1093/infdis/jiz286. [DOI] [PubMed] [Google Scholar]
- 4.Hawken S.E., Snitkin E.S. Genomic epidemiology of multidrug-resistant Gram-negative organisms. Ann. N. Y. Acad. Sci. 2019;1435:39–56. doi: 10.1111/nyas.13672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Palmieri M., D’Andrea M.M., Pelegrin A.C., Mirande C., Brkic S., Cirkovic I., Goossens H., Rossolini G.M., van Belkum A. Genomic Epidemiology of Carbapenem- and Colistin-Resistant Klebsiella pneumoniae Isolates From Serbia: Predominance of ST101 Strains Carrying a Novel OXA-48 Plasmid. Front. Microbiol. 2020;11:294. doi: 10.3389/fmicb.2020.00294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Osei Sekyere J., Reta M.A. Genomic and Resistance Epidemiology of Gram-Negative Bacteria in Africa: A Systematic Review and Phylogenomic Analyses from a One Health Perspective. mSystems. 2020;5:e00897-20. doi: 10.1128/mSystems.00897-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Talebi Bezmin Abadi A., Rizvanov A.A., Haertle T., Blatt N.L. World Health Organization Report: Current Crisis of Antibiotic Resistance. BioNanoScience. 2019;9:778–788. doi: 10.1007/s12668-019-00658-4. [DOI] [Google Scholar]
- 8.Fair R.J., Tor Y. Antibiotics and bacterial resistance in the 21st century. Perspect. Medicin. Chem. 2014;6:25–64. doi: 10.4137/PMC.S14459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kuzmenkov A.Y., Trushin I.V., Vinogradova A.G., Avramenko A.A., Sukhorukova M.V., Malhotra-Kumar S., Dekhnich A.V., Edelstein M.V., Kozlov R.S. AMRmap: An Interactive Web Platform for Analysis of Antimicrobial Resistance Surveillance Data in Russia. Front. Microbiol. 2021;12:620002. doi: 10.3389/fmicb.2021.620002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Magill S.S., Edwards J.R., Bamberg W., Beldavs Z.G., Dumyati G., Kainer M.A., Lynfield R., Maloney M., McAllister-Hollod L., Nadle J., et al. Multistate point-prevalence survey of health care-associated infections. N. Engl. J. Med. 2014;370:1198–1208. doi: 10.1056/NEJMoa1306801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lob S.H., Hoban D.J., Sahm D.F., Badal R.E. Regional differences and trends in antimicrobial susceptibility of Acinetobacter baumannii. Int. J. Antimicrob. Agents. 2016;47:317–323. doi: 10.1016/j.ijantimicag.2016.01.015. [DOI] [PubMed] [Google Scholar]
- 12.Zhen X., Lundborg C.S., Sun X., Hu X., Dong H. Economic burden of antibiotic resistance in ESKAPE organisms: A systematic review. Antimicrob. Resist. Infect. Control. 2019;8:137. doi: 10.1186/s13756-019-0590-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hamidian M., Nigro S.J. Emergence, molecular mechanisms and global spread of carbapenem-resistant Acinetobacter baumannii. Microb. Genom. 2019;5:e000306. doi: 10.1099/mgen.0.000306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Harding C.M., Hennon S.W., Feldman M.F. Uncovering the mechanisms of Acinetobacter baumannii virulence. Nat. Rev. Microbiol. 2018;16:91–102. doi: 10.1038/nrmicro.2017.148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yang C.H., Su P.W., Moi S.H., Chuang L.Y. Biofilm Formation in Acinetobacter Baumannii: Genotype-Phenotype Correlation. Molecules. 2019;24:1849. doi: 10.3390/molecules24101849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Holt K., Kenyon J.J., Hamidian M., Schultz M.B., Pickard D.J., Dougan G., Hall R. Five decades of genome evolution in the globally distributed, extensively antibiotic-resistant Acinetobacter baumannii global clone 1. Microb. Genom. 2016;2:e000052. doi: 10.1099/mgen.0.000052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zarrilli R., Pournaras S., Giannouli M., Tsakris A. Global evolution of multidrug-resistant Acinetobacter baumannii clonal lineages. Int. J. Antimicrob. Agents. 2013;41:11–19. doi: 10.1016/j.ijantimicag.2012.09.008. [DOI] [PubMed] [Google Scholar]
- 18.Al-Hassan L., Elbadawi H., Osman E., Ali S., Elhag K., Cantillon D., Wille J., Seifert H., Higgins P.G. Molecular Epidemiology of Carbapenem-Resistant Acinetobacter baumannii From Khartoum State, Sudan. Front. Microbiol. 2021;12:628736. doi: 10.3389/fmicb.2021.628736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Shek E.A., Sukhorukova M.V., Edelstein M.V., Skleenova E.Y., Ivanchik N.V., Shajdullina E.R., Kuzmenkov A.Y., Dekhnich A.V., Kozlov R.S., Semyonova N.V., et al. Antimicrobial resistance, carbapenemase production, and genotypes of nosocomial Acinetobacter spp. isolates in Russia: Results of multicenter epidemiological study “MARATHON 2015–2016”. Clin. Microbiol. Antimicrob. Chemother. 2019;21:171–180. doi: 10.36488/cmac.2019.2.171-180. [DOI] [Google Scholar]
- 20.Mayanskiy N., Chebotar I., Alyabieva N., Kryzhanovskaya O., Savinova T., Turenok A., Bocharova Y., Lazareva A., Polikarpova S., Karaseva O. Emergence of the Uncommon Clone ST944/ST78 Carrying blaOXA-40-like and blaCTX-M-like Genes Among Carbapenem-Nonsusceptible Acinetobacter baumannii in Moscow, Russia. Microb. Drug Resist. 2017;23:864–870. doi: 10.1089/mdr.2016.0302. [DOI] [PubMed] [Google Scholar]
- 21.Nodari C.S., Cayo R., Streling A.P., Lei F., Wille J., Almeida M.S., de Paula A.I., Pignatari A.C.C., Seifert H., Higgins P.G., et al. Genomic Analysis of Carbapenem-Resistant Acinetobacter baumannii Isolates Belonging to Major Endemic Clones in South America. Front. Microbiol. 2020;11:584603. doi: 10.3389/fmicb.2020.584603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cerezales M., Xanthopoulou K., Wille J., Bustamante Z., Seifert H., Gallego L., Higgins P.G. Acinetobacter baumannii analysis by core genome multi-locus sequence typing in two hospitals in Bolivia: Endemicity of international clone 7 isolates (CC25) Int. J. Antimicrob. Agents. 2019;53:844–849. doi: 10.1016/j.ijantimicag.2019.03.019. [DOI] [PubMed] [Google Scholar]
- 23.Higgins P.G., Hagen R.M., Kreikemeyer B., Warnke P., Podbielski A., Frickmann H., Loderstadt U. Molecular Epidemiology of Carbapenem-Resistant Acinetobacter baumannii Isolates from Northern Africa and the Middle East. Antibiotics. 2021;10:291. doi: 10.3390/antibiotics10030291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Shashkov A.S., Cahill S.M., Arbatsky N.P., Westacott A.C., Kasimova A.A., Shneider M.M., Popova A.V., Shagin D.A., Shelenkov A.A., Mikhailova Y.V., et al. Acinetobacter baumannii K116 capsular polysaccharide structure is a hybrid of the K14 and revised K37 structures. Carbohydr. Res. 2019;484:107774. doi: 10.1016/j.carres.2019.107774. [DOI] [PubMed] [Google Scholar]
- 25.Bonnin R.A., Potron A., Poirel L., Lecuyer H., Neri R., Nordmann P. PER-7, an extended-spectrum beta-lactamase with increased activity toward broad-spectrum cephalosporins in Acinetobacter baumannii. Antimicrob. Agents Chemother. 2011;55:2424–2427. doi: 10.1128/AAC.01795-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Naas T., Oueslati S., Bonnin R.A., Dabos M.L., Zavala A., Dortet L., Retailleau P., Iorga B.I. Beta-lactamase database (BLDB)—structure and function. J. Enzyme Inhib. Med. Chem. 2017;32:917–919. doi: 10.1080/14756366.2017.1344235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Matthews L., Goodrich J.S., Weber D.J., Bergman N.H., Miller M.B. The Brief Case: A Fatal Case of Necrotizing Fasciitis Due to Multidrug-Resistant Acinetobacter baumannii. J. Clin. Microbiol. 2019;57:e01751-18. doi: 10.1128/JCM.01751-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hamidian M., Hawkey J., Wick R., Holt K.E., Hall R.M. Evolution of a clade of Acinetobacter baumannii global clone 1, lineage 1 via acquisition of carbapenem- and aminoglycoside-resistance genes and dispersion of ISAba1. Microb. Genom. 2019;5:e000242. doi: 10.1099/mgen.0.000242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Shelenkov A., Korotkov A., Korotkov E. MMsat—a database of potential micro- and minisatellites. Gene. 2008;409:53–60. doi: 10.1016/j.gene.2007.11.007. [DOI] [PubMed] [Google Scholar]
- 30.Yeh H.Y., Awad A. Genotyping of Campylobacter jejuni Isolates from Poultry by Clustered Regularly Interspaced Short Palindromic Repeats (CRISPR) Curr. Microbiol. 2020;77:1647–1652. doi: 10.1007/s00284-020-01965-w. [DOI] [PubMed] [Google Scholar]
- 31.Bartual S.G., Seifert H., Hippler C., Luzon M.A., Wisplinghoff H., Rodriguez-Valera F. Development of a multilocus sequence typing scheme for characterization of clinical isolates of Acinetobacter baumannii. J. Clin. Microbiol. 2005;43:4382–4390. doi: 10.1128/JCM.43.9.4382-4390.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Diancourt L., Passet V., Nemec A., Dijkshoorn L., Brisse S. The population structure of Acinetobacter baumannii: Expanding multiresistant clones from an ancestral susceptible genetic pool. PLoS ONE. 2010;5:e10034. doi: 10.1371/journal.pone.0010034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Shelenkov A., Mikhaylova Y., Yanushevich Y., Samoilov A., Petrova L., Fomina V., Gusarov V., Zamyatin M., Shagin D., Akimkin V. Molecular Typing, Characterization of Antimicrobial Resistance, Virulence Profiling and Analysis of Whole-Genome Sequence of Clinical Klebsiella pneumoniae Isolates. Antibiotics. 2020;9:261. doi: 10.3390/antibiotics9050261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Higgins P.G., Prior K., Harmsen D., Seifert H. Development and evaluation of a core genome multilocus typing scheme for whole-genome sequence-based typing of Acinetobacter baumannii. PLoS ONE. 2017;12:e0179228. doi: 10.1371/journal.pone.0179228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Venditti C., Vulcano A., D’Arezzo S., Gruber C.E.M., Selleri M., Antonini M., Lanini S., Marani A., Puro V., Nisii C., et al. Epidemiological investigation of an Acinetobacter baumannii outbreak using core genome multilocus sequence typing. J. Glob. Antimicrob. Resist. 2019;17:245–249. doi: 10.1016/j.jgar.2018.11.027. [DOI] [PubMed] [Google Scholar]
- 36.Evans B.A., Amyes S.G. OXA beta-lactamases. Clin. Microbiol. Rev. 2014;27:241–263. doi: 10.1128/CMR.00117-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Gaiarsa S., Batisti Biffignandi G., Esposito E.P., Castelli M., Jolley K.A., Brisse S., Sassera D., Zarrilli R. Comparative Analysis of the Two Acinetobacter baumannii Multilocus Sequence Typing (MLST) Schemes. Front. Microbiol. 2019;10:930. doi: 10.3389/fmicb.2019.00930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Cieslinski J.M., Arend L., Tuon F.F., Silva E.P., Ekermann R.G., Dalla-Costa L.M., Higgins P.G., Seifert H., Pilonetto M. Molecular epidemiology characterization of OXA-23 carbapenemase-producing Acinetobacter baumannii isolated from 8 Brazilian hospitals using repetitive sequence-based PCR. Diagn. Microbiol. Infect. Dis. 2013;77:337–340. doi: 10.1016/j.diagmicrobio.2013.07.018. [DOI] [PubMed] [Google Scholar]
- 39.Wareth G., Brandt C., Sprague L.D., Neubauer H., Pletz M.W. Spatio-Temporal Distribution of Acinetobacter baumannii in Germany-A Comprehensive Systematic Review of Studies on Resistance Development in Humans (2000–2018) Microorganisms. 2020;8:375. doi: 10.3390/microorganisms8030375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Rafei R., Dabboussi F., Hamze M., Eveillard M., Lemarie C., Gaultier M.P., Mallat H., Moghnieh R., Husni-Samaha R., Joly-Guillou M.L., et al. Molecular analysis of Acinetobacter baumannii strains isolated in Lebanon using four different typing methods. PLoS ONE. 2014;9:e115969. doi: 10.1371/journal.pone.0115969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Caldart R.V., Fonseca E.L., Freitas F., Rocha L., Vicente A.C. Acinetobacter baumannii infections in Amazon Region driven by extensively drug resistant international clones, 2016–2018. Mem. Inst. Oswaldo. Cruz. 2019;114:e190232. doi: 10.1590/0074-02760190232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Jia H., Sun Q., Ruan Z., Xie X. Characterization of a small plasmid carrying the carbapenem resistance gene bla OXA-72 from community-acquired Acinetobacter baumannii sequence type 880 in China. Infect. Drug Resist. 2019;12:1545–1553. doi: 10.2147/IDR.S202803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Tomaras A.P., Dorsey C.W., Edelmann R.E., Actis L.A. Attachment to and biofilm formation on abiotic surfaces by Acinetobacter baumannii: Involvement of a novel chaperone-usher pili assembly system. Microbiology. 2003;149:3473–3484. doi: 10.1099/mic.0.26541-0. [DOI] [PubMed] [Google Scholar]
- 44.Choi A.H., Slamti L., Avci F.Y., Pier G.B., Maira-Litran T. The pgaABCD locus of Acinetobacter baumannii encodes the production of poly-beta-1-6-N-acetylglucosamine, which is critical for biofilm formation. J. Bacteriol. 2009;191:5953–5963. doi: 10.1128/JB.00647-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Sarshar M., Behzadi P., Scribano D., Palamara A.T., Ambrosi C. Acinetobacter baumannii: An Ancient Commensal with Weapons of a Pathogen. Pathogens. 2021;10:387. doi: 10.3390/pathogens10040387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Liu C., Chang Y., Xu Y., Luo Y., Wu L., Mei Z., Li S., Wang R., Jia X. Distribution of virulence-associated genes and antimicrobial susceptibility in clinical Acinetobacter baumannii isolates. Oncotarget. 2018;9:21663–21673. doi: 10.18632/oncotarget.24651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.He X., Lu F., Yuan F., Jiang D., Zhao P., Zhu J., Cheng H., Cao J., Lu G. Biofilm Formation Caused by Clinical Acinetobacter baumannii Isolates Is Associated with Overexpression of the AdeFGH Efflux Pump. Antimicrob. Agents Chemother. 2015;59:4817–4825. doi: 10.1128/AAC.00877-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Vazquez-Lopez R., Solano-Galvez S.G., Juarez Vignon-Whaley J.J., Abello Vaamonde J.A., Padro Alonzo L.A., Rivera Resendiz A., Muleiro Alvarez M., Vega Lopez E.N., Franyuti-Kelly G., Alvarez-Hernandez D.A., et al. Acinetobacter baumannii Resistance: A Real Challenge for Clinicians. Antibiotics. 2020;9:205. doi: 10.3390/antibiotics9040205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Tang J., Chen Y., Wang X., Ding Y., Sun X., Ni Z. Contribution of the AbaI/AbaR Quorum Sensing System to Resistance and Virulence of Acinetobacter baumannii Clinical Strains. Infect. Drug Resist. 2020;13:4273–4281. doi: 10.2147/IDR.S276970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Schneidewind L., Kranz J., Tandogdu Z. Rising significance of antibiotic stewardship in urology and urinary tract infections—a rapid review. Curr. Opin. Urol. 2021;31:285–290. doi: 10.1097/MOU.0000000000000897. [DOI] [PubMed] [Google Scholar]
- 51.Provenzani A., Hospodar A.R., Meyer A.L., Leonardi Vinci D., Hwang E.Y., Butrus C.M., Polidori P. Multidrug-resistant gram-negative organisms: A review of recently approved antibiotics and novel pipeline agents. Int. J. Clin. Pharm. 2020;42:1016–1025. doi: 10.1007/s11096-020-01089-y. [DOI] [PubMed] [Google Scholar]
- 52.Shelenkov A., Slavokhotova A., Odintsova T. Predicting Antimicrobial and Other Cysteine-Rich Peptides in 1267 Plant Transcriptomes. Antibiotics. 2020;9:60. doi: 10.3390/antibiotics9020060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Ghosh C., Sarkar P., Issa R., Haldar J. Alternatives to Conventional Antibiotics in the Era of Antimicrobial Resistance. Trends Microbiol. 2019;27:323–338. doi: 10.1016/j.tim.2018.12.010. [DOI] [PubMed] [Google Scholar]
- 54.Bebbington C., Yarranton G. Antibodies for the treatment of bacterial infections: Current experience and future prospects. Curr. Opin. Biotechnol. 2008;19:613–619. doi: 10.1016/j.copbio.2008.10.002. [DOI] [PubMed] [Google Scholar]
- 55.Uppu D.S., Manjunath G.B., Yarlagadda V., Kaviyil J.E., Ravikumar R., Paramanandham K., Shome B.R., Haldar J. Membrane-active macromolecules resensitize NDM-1 gram-negative clinical isolates to tetracycline antibiotics. PLoS ONE. 2015;10:e0119422. doi: 10.1371/journal.pone.0119422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Tyumentseva M., Mikhaylova Y., Prelovskaya A., Tyumentsev A., Petrova L., Fomina V., Zamyatin M., Shelenkov A., Akimkin V. Genomic and Phenotypic Analysis of Multidrug-Resistant Acinetobacter baumannii Clinical Isolates Carrying Different Types of CRISPR/Cas Systems. Pathogens. 2021;10:205. doi: 10.3390/pathogens10020205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Wick R.R., Judd L.M., Gorrie C.L., Holt K.E. Unicycler: Resolving bacterial genome assemblies from short and long sequencing reads. PLoS Comput. Biol. 2017;13:e1005595. doi: 10.1371/journal.pcbi.1005595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Liu B., Zheng D., Jin Q., Chen L., Yang J. VFDB 2019: A comparative pathogenomic platform with an interactive web interface. Nucleic Acids Res. 2019;47:D687–D692. doi: 10.1093/nar/gky1080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Arbatsky N.P., Shneider M.M., Dmitrenok A.S., Popova A.V., Shagin D.A., Shelenkov A.A., Mikhailova Y.V., Edelstein M.V., Knirel Y.A. Structure and gene cluster of the K125 capsular polysaccharide from Acinetobacter baumannii MAR13-1452. Int. J. Biol. Macromol. 2018;117:1195–1199. doi: 10.1016/j.ijbiomac.2018.06.029. [DOI] [PubMed] [Google Scholar]
- 60.Wyres K.L., Cahill S.M., Holt K.E., Hall R.M., Kenyon J.J. Identification of Acinetobacter baumannii loci for capsular polysaccharide (KL) and lipooligosaccharide outer core (OCL) synthesis in genome assemblies using curated reference databases compatible with Kaptive. Microb. Genom. 2020;6:e000339. doi: 10.1099/mgen.0.000339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Couvin D., Bernheim A., Toffano-Nioche C., Touchon M., Michalik J., Neron B., Rocha E.P.C., Vergnaud G., Gautheret D., Pourcel C. CRISPRCasFinder, an update of CRISRFinder, includes a portable version, enhanced performance and integrates search for Cas proteins. Nucleic Acids Res. 2018;46:W246–W251. doi: 10.1093/nar/gky425. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The bacterial genomes presented in this study are openly available in NCBI Genbank under the following accession numbers: JAEPWJ000000000 (CriePir33), JAEPWI000000000 (CriePir87), JAEPWG000000000 (CriePir168), JAHHIS000000000 (CriePir254), JAEPWB000000000 (CriePir298), JAEPVY000000000 (CriePir306), JAEPVX000000000 (CriePir307), JAEPVW000000000 (CriePir308), and JAEPVV000000000 (CriePir309).