Abstract
This is a collaboration between the British Society of Gastroenterology (BSG) and the European Society of Gastrointestinal Endoscopy (ESGE), and is a scheduled update of their 2016 guideline on endoscopy in patients on antiplatelet or anticoagulant therapy. The guideline development committee included representatives from the British Society of Haematology, the British Cardiovascular Intervention Society, and two patient representatives from the charities Anticoagulation UK and Thrombosis UK, as well as gastroenterologists. The process conformed to AGREE II principles, and the quality of evidence and strength of recommendations were derived using GRADE methodology. Prior to submission for publication, consultation was made with all member societies of ESGE, including BSG. Evidence-based revisions have been made to the risk categories for endoscopic procedures, and to the categories for risks of thrombosis. In particular a more detailed risk analysis for atrial fibrillation has been employed, and the recommendations for direct oral anticoagulants have been strengthened in light of trial data published since the previous version. A section has been added on the management of patients presenting with acute GI haemorrhage. Important patient considerations are highlighted. Recommendations are based on the risk balance between thrombosis and haemorrhage in given situations.
Summary of recommendations
Recommendations for the management of patients on antiplatelet therapy or anticoagulants undergoing elective endoscopic procedures are summarised in Figs. 1 and 2 . Risk stratification for endoscopic procedures are detailed in Table 1 , for antiplatelet agents in Table 2 , and for heparin bridging in patients on warfarin Table 3 .
Fig. 1.
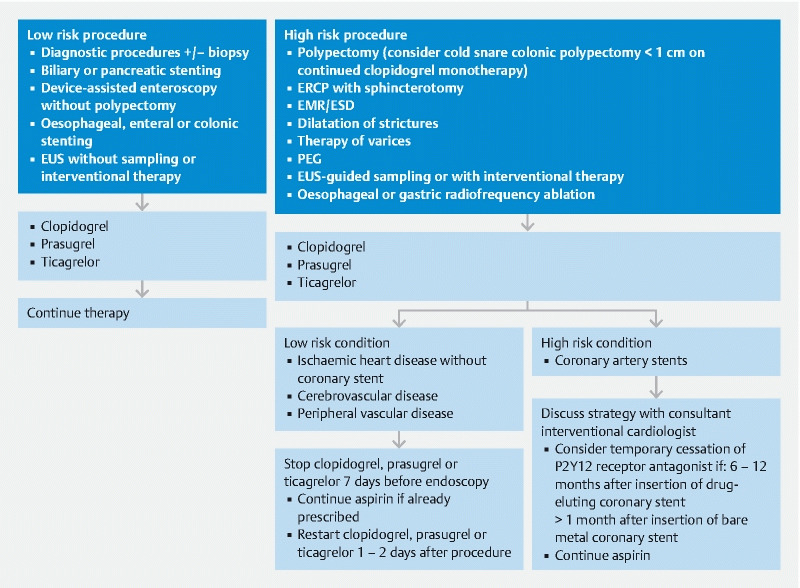
Guidelines for the management of patients on P2Y12 receptor antagonist antiplatelet agents undergoing endoscopic procedures: 2021 update. (EUS: endoscopic ultrasound, ERCP: endoscopic retrograde cholangiopancreatography, EMR: endoscopic mucosal resection, ESD: endoscopic submucosal dissection, PEG: percutaneous endoscopic gastroenterostomy)
Fig. 2.
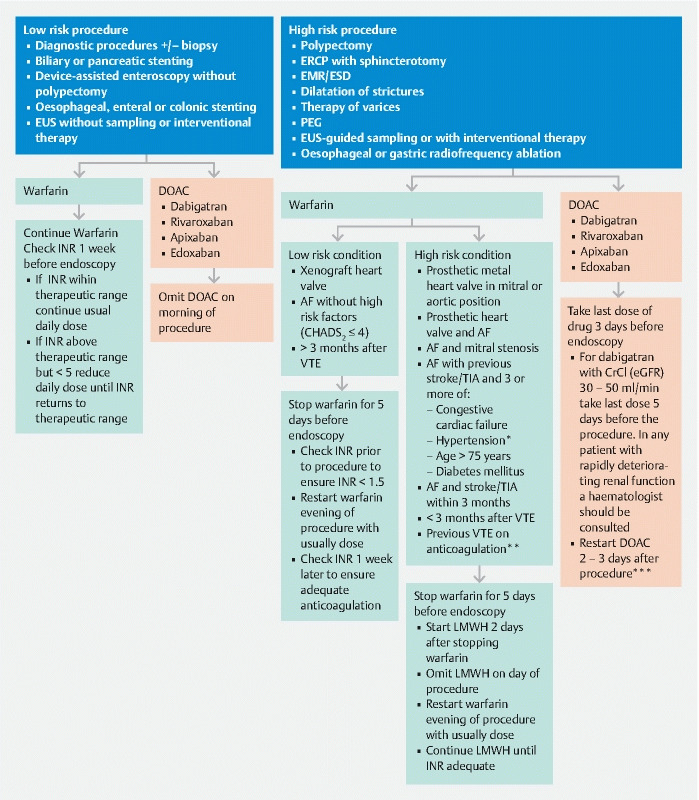
Guidelines for the management of patients on anticoagulants undergoing endoscopic procedures: 2021 update. *Blood pressure > 140 /90 mmHg or on antihypertensive medication **Previous VTE on anticoagulation and target INR now 3.5 ***depends on haemorrhagic and thrombotic risk, consider extending interval for ESD (EUS: endoscopic ultrasound, ERCP: endoscopic retrograde cholangiopancreatography, EMR: endoscopic mucosal resection, ESD: endoscopic submucosal dissection, PEG: percutaneous endoscopic gastroenterostomy, INR: international normalised ratio, AF: atrial fibrillation, VTE: venous thromboembolism, TIA: transient ischaemic attack, LMWH: low molecular weight heparin)
Table 1. Risk stratification of endoscopic procedures based on the risks of haemorrhage and of intervention required to treat a complication.
| High risk procedures | Low risk procedures |
| Endoscopic polypectomy * | Diagnostic procedures ± biopsy sampling |
| ERCP with sphincterotomy | Biliary or pancreatic stenting |
| Ampullectomy | Device-assisted enteroscopy without polypectomy |
| Endoscopic mucosal resection or endoscopic submucosal dissection | Oesophageal, enteral or colonic stenting |
| Endoscopic dilatation of strictures in the upper or lower gastrointestinal tract | Endoscopic ultrasound without sampling or interventional therapy |
| Endoscopic therapy of varices | |
| Percutaneous endoscopic gastrostomy | |
| Endoscopic ultrasound-guided sampling or with interventional therapy | |
| Oesophageal or gastric radiofrequency ablation |
ERCP, endoscopic retrograde cholngiopancreatography
consider cold snare resection of polyps < 1 cm on continued clopidogrel monotherapy
Table 2. Risk stratification for discontinuation of P2Y12 receptor antagonists clopidogrel, prasugrel or ticagrelor based on the risk of thrombosis.
| High risk of thrombosis | Low risk of thrombosis |
| Drug eluting coronary artery stents within 12 months of placement | Ischaemic heart disease without coronary stents |
| Bare metal coronary artery stents within 1 month of placement | Cerebrovascular disease |
| Peripheral vascular disease |
Table 3. Risk stratification for discontinuation of warfarin therapy with respect to the requirement for heparin bridging.
| High risk of thromboembolism | Low risk of thromboembolism |
| Prosthetic metal heart valve in mitral or aortic 1 position | Xenograft heart valve |
| Prosthetic heart valve and atrial fibrillation | |
| Atrial fibrillation and mitral stenosis | |
Atrial fibrillation with previous stroke or transient ischemic attack + 3 or more of:
|
Atrial fibrillation without high-risk factors (CHADS 2 ≤ 4) |
| Atrial fibrillation and previous stroke or transient ischaemic attack within 3 months | |
| < 3 months after venous thromboembolism 3 | > 3 months after venous thromboembolism |
| Previous venous thromboembolism on warfarin, and target INR now 3.5 |
Thrombophilia syndromes do not usually require heparin bridging, but individual cases should be discussed with a haematologist
heparin bridging for a metal aortic valve is recommended by European Society of Cardiology (ESC) and the European Association for Cardio-Thoracic Surgery (EACTS) guidelines 2017 33 , but this varies between international guidelines 31 32 and local guidance should be established in conjunction with cardiology or cardiothoracic services.
Blood pressure > 140 /90 mmHg or on antihypertensive medication
the majority of patients are now on DOACs for venous thromboembolism and bridging is not appropriate. Consider deferring a high risk procedure beyond 3 months therapy in this high risk group for thromboembolism
We recommend that all patients are advised of the thrombotic risks of discontinuing antiplatelets or anticoagulants, as well as the haemorrhagic risks of continuing therapy (strong recommendation, low quality evidence)
For all endoscopic procedures we recommend continuing aspirin (strong recommendation, low quality evidence), with the exception of ampullectomy (weak recommendation, low quality evidence). If considering aspirin discontinuation, this should be on an individual patient basis depending on the risks of thrombosis vs haemorrhage (weak recommendation, low quality evidence).
Low-risk endoscopic procedures
For low-risk endoscopic procedures we recommend continuing P2Y12 receptor antagonists as single or dual antiplatelet therapy (strong recommendation, low quality evidence).
For low-risk endoscopic procedures we suggest that warfarin therapy should be continued (weak recommendation, low quality evidence). It should be ensured that the INR does not exceed the therapeutic range in the week prior to the procedure (strong recommendation, low quality evidence).
For low-risk endoscopic procedures we suggest omitting the morning dose of DOACs on the day of the procedure (weak recommendation, low quality evidence).
High-risk endoscopic procedures
For high-risk endoscopic procedures in patients at low thrombotic risk, we recommend discontinuing P2Y12 receptor antagonists 7 days before the procedure (strong recommendation, moderate quality evidence). In patients on dual antiplatelet therapy, we recommend continuing aspirin (strong recommendation, low quality evidence).
For high-risk endoscopic procedures in patients at low thrombotic risk, we recommend discontinuing warfarin for 5 days before the procedure (strong recommendation, high quality evidence). Check INR prior to the procedure to ensure < 1.5 (strong recommendation, low quality evidence).
For high-risk endoscopic procedures in patients at high thrombotic risk, we recommend continuing aspirin and liaising with a consultant interventional cardiologist about the risk/benefit of discontinuing P2Y12 receptor antagonists (strong recommendation, high quality evidence).
For high-risk endoscopic procedures in patients at high thrombotic risk, we recommend that warfarin should be temporarily discontinued and substituted with low molecular weight heparin (strong recommendation, low quality evidence).
For high-risk endoscopic procedures in patients on DOACs, we recommend that the last dose of DOACs be taken 3 days before the procedure (strong recommendation, low quality evidence). For patients on dabigatran with a CrCl (or eGFR) of 30–50 mL/min we recommend that the last dose be taken 5 days prior to the procedure (strong recommendation, low quality evidence). In any patient with rapidly deteriorating renal function a haematologist should be consulted (strong recommendation, low quality evidence).
Post elective endoscopic procedures
If antiplatelet or anticoagulant therapy is discontinued, then we recommend this should be resumed up to 2 to 3 days after the procedure depending on the perceived haemorrhagic and thrombotic risks (strong recommendation, moderate quality evidence).
For all patients on antiplatelets or anticoagulants we recommend advising that there is an increased risk of post-procedure haemorrhage compared to patients not on these drugs (strong recommendation, low quality evidence).
Acute GI haemorrhage
We suggest that permanent discontinuation of aspirin for primary prophylaxis should be considered (weak recommendation, low quality evidence).
We suggest that aspirin for secondary prevention should not be routinely stopped. If it is stopped, it should be recommenced as soon as haemostasis is achieved, or there is no further evidence of haemorrhage (strong recommendation, moderate quality evidence).
We recommend that dual antiplatelet therapy is continued if possible in patients with coronary stents in situ and management should be in liaison with a consultant interventional cardiologist (strong recommendation, moderate quality evidence). In the case of major haemorrhage we recommend continuing aspirin if the P2Y12 receptor antagonist is interrupted (strong recommendation, moderate quality evidence). P2Y12 receptor antagonist therapy should be re-instated within 5 days, if still indicated (strong recommendation, moderate quality evidence).
We recommend withholding oral anticoagulant and correcting coagulopathy according to the severity of haemorrhage and the patient’s thrombotic risk in coordination with a consultant cardiologist/ haematologist. The correction of coagulopathy should not delay endoscopy or radiologic intervention (strong recommendation, low quality evidence).
In patients with haemodynamic instability who take vitamin K antagonists (VKAs), we recommend administering intravenous vitamin K and four-factor prothrombin complex concentrate (PCC) (strong recommendation, low quality evidence), or fresh frozen plasma if PCC is not available (weak recommendation, very low quality evidence)
In patients with haemodynamic instability who take DOACs, we suggest considering the use of reversal agents: idarucizumab in dabigatran treated patients, and andexanet in anti-factor Xa treated patients (weak recommendation, low quality evidence), or intravenous four-factor PCC if andexanet is not available (weak recommendation, very low quality evidence)
We recommend restarting anticoagulation following acute GI haemorrhage in patients with an indication for long-term anticoagulation (strong recommendation, low quality evidence). In patients at low thrombotic risk, we suggest restarting anticoagulation as soon as possible after seven days of anticoagulant interruption (weak recommendation, low quality evidence). In those at high-thrombotic risk, an earlier resumption of anticoagulation with heparin bridging, preferably within 3 days, is recommended (strong recommendation, low quality evidence).
Introduction and methods
The British Society of Gastroenterology (BSG) and the European Society of GI Endoscopy (ESGE) jointly published guidelines on endoscopy in patients on antiplatelet or anticoagulant therapy, including direct oral anticoagulants in 2016 1 2 . This is a scheduled five year update on the previous guidelines.
These guidelines were drafted by a working party comprising members of the BSG and ESGE, two haematologists representing the Thrombosis and Haemostasis Task Force of the British Society of Haematology, an interventional cardiologist representing the British Cardiovascular Intervention Society, and two patient representatives from the charities Anticoagulation UK and Thrombosis UK. Guidelines were prepared according to AGREE II principles 3 and comply with the requirements of the National Institute for Health and Care Excellence (NICE). Clinical questions were formulated using the PICO (Patients, Interventions, Controls, Outcomes) system. Search strategies were delegated to authors with responsibilities for specific sections. Literature searches were conducted using PubMed and OVID Medline, Embase and Cochrane Library. Additional searches were conducted using Google. Literature searches were run up to November 2020, and this time-point should be the starting point in the search for new evidence for future updates to these Guidelines. Quality of evidence and strength of recommendations were determined by the authors, and consensus achieved according to the GRADE system 4 . After agreement on a final version, the manuscript was subjected to internal peer review and revision by the BSG and the ESGE and sent to all individual ESGE members and member societies prior to publication. Conflict of interest statements were submitted by all authors and are listed at the end of this manuscript. This guideline was issued in 2021 and will be considered for review in 2026, or sooner if new evidence becomes available. This guideline has been co-published with permission in both Gut and Endoscopy.
Patient considerations
We recommend that all patients are advised of the thrombotic risks of discontinuing antiplatelets or anticoagulants, as well as the haemorrhagic risks of continuing therapy (strong recommendation, low quality evidence).
For all patients on antiplatelets or anticoagulants we recommend advising that there is an increased risk of post-procedure haemorrhage compared to patients not on these drugs (strong recommendation, low quality evidence).
As discussed, management of antithrombotic drugs is a balance of the risks of haemorrhage from the procedure versus the risks of thrombosis if antithrombotic medication is discontinued or modified. Haemorrhage secondary to high-risk endoscopic procedures can often be controlled by further endoscopic therapeutic measures, and is rarely fatal. A thrombotic stroke may result in lifelong disability, and a major cardiac event may result in death. Not only do the risks of thrombosis vs haemorrhage need to be assessed on an individual patient basis, but patients should be fully informed, and involved in this decision-making process. The risk of a potentially catastrophic thrombotic event such as a stroke may not be acceptable to a patient even if that risk is very low.
Once the decision to modify antithrombotic medication is made, then a personal plan for that patient should be made. This should include written as well as verbal information regarding the precise timing of any changes. This should include the time of last dose of a drug, and first dose when restarting. If multiple drugs are involved, or substitutions including bridging with heparin, then timing respective to each drug should be clearly communicated. Simple algorithms such as those illustrated in the PAUSE trial for DOACs 5 may be useful ( Fig. 3 ). Late haemorrhagic complications may occur one to two weeks after endoscopic therapy, and antithrombotic therapy will have often been reinstituted prior to this. Patients on antithrombotics should be advised that there is a possible increase in post procedure haemorrhage, and also advised how to seek urgent medical advice at any time of the day or night. We have produced a patient information sheet based on this guideline to assist with patient engagement (online supplemental file).
Fig. 3.
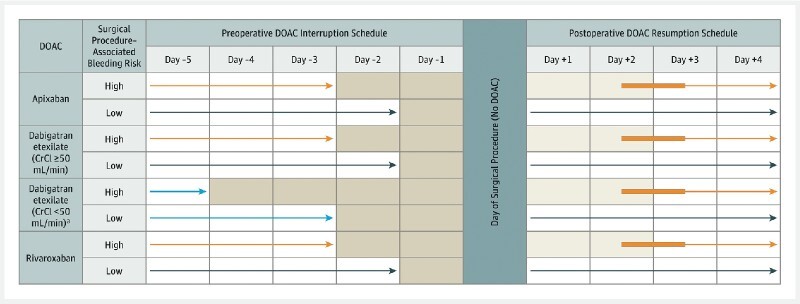
Perioperative direct oral anticoagulant (DOAC) management protocol. Reproduced with permission from JAMA Intern Med 2019:179(11);1469–78. Copyright (2019) American Medical Association. All rights reserved. [rerif] No DOAC was taken on certain days (shaded) and on the day of the elective surgery or procedure (including endoscopy). The light blue arrows refer to an exception to the basic management, a subgroup of patients taking dabigatran with a creatinine clearance(CrCl) less than 50 ng/ml. The orange arrows refer to patients having a high-bleed-risk procedure. Dark blue arrows refer to patients having a low-bleed-risk procedure. The thickened orange arrows refer to flexibility in timing of DOAC resumption after a procedure.
Antiplatelet agents
For all endoscopic procedures we recommend continuing aspirin (strong recommendation, low quality evidence), with the exception of ampullectomy (weak recommendation, low quality evidence). If considering aspirin discontinuation, this should be on an individual patient basis depending on the risks of thrombosis vs haemorrhage (weak recommendation, low quality evidence).
For low-risk endoscopic procedures we recommend continuing P2Y12 receptor antagonists as single or dual antiplatelet therapy (strong recommendation, low quality evidence).
For high-risk endoscopic procedures in patients at low thrombotic risk, we recommend discontinuing P2Y12 receptor antagonists 7 days before the procedure (strong recommendation, moderate quality evidence). In patients on dual antiplatelet therapy, we recommend continuing aspirin (strong recommendation, low quality evidence).
For high-risk endoscopic procedures in patients at high thrombotic risk, we recommend continuing aspirin and liaising with a consultant interventional cardiologist about the risk/benefit of discontinuing P2Y12 receptor antagonists (strong recommendation, high quality evidence).
Aspirin
When given as long-term secondary prevention aspirin reduces vascular events by approximately one-third and vascular deaths by about one-sixth. In patients on long-term low-dose aspirin for secondary prevention, aspirin interruption was associated with a three-fold increased risk of cardiovascular or cerebrovascular events, and 70 % of these events occurred within 7–10 days after interruption 6 7 . In a randomized controlled trial (RCT) of 220 patients on low-dose aspirin for secondary prevention undergoing non-cardiac surgery, patients were randomized to continuation or temporary replacement of aspirin by placebo. 8 Major cardiac events occurred within 30 days in 1.8 % of the aspirin group compared to 9 % in the placebo group (p = 0.02). No difference in haemorrhagic complications was seen between the two groups. The risks related to continuation or discontinuation of aspirin for endoscopy are discussed in the sections on endoscopic procedures, and recommendations made accordingly.
With regard to patients on a DOAC and a single antiplatelet agent, such as aspirin, the AFIRE trial randomized patients with atrial fibrillation and stable coronary artery disease to rivaroxaban alone versus rivaroxaban and a single antiplatelet. They demonstrated that rivaroxaban alone was non inferior for the primary efficacy endpoint (a composite of thromboembolic events or death from any cause) and superior for the primary safety endpoint of major bleeding. However, this trial was not sufficiently powered to address the risk of stent thrombosis 9 . For patients with documented coronary artery disease and an indication for formal anticoagulation, e. g. atrial fibrillation, then a life-long single antiplatelet such as aspirin is no longer recommended, beyond the antiplatelet duration required post coronary stent insertion, in the latest ESC guidelines 10 . However, late stent thrombosis (1–12 months post stent implantation) and very late stent thrombosis (beyond 12 months post stent implantation) occur with a reported incidence of 0.5–1.0 % and 0.2–0.4 % respectively, and stent thrombosis is associated with a with a 4–45 % mortality 11 . Furthermore, technical aspects of the procedure, for example left main stents, may have an essential bearing on the subsequent risk of stent thrombosis. The trials addressing whether a long-term antiplatelet agent is needed in patients that have stents and require a DOAC, which suggest a DOAC alone may be safe, are not sufficiently powered to look at the important endpoint of stent thrombosis 9 12 . Many consultant interventional cardiologists in the UK recommend their patients remain on a single antiplatelet agent if they have stents and need a long term DOAC, although this will very much vary on an individual case-by-case basis. Therefore, in patients with coronary stents who are taking a DOAC the cessation of the single antiplatelet agent should always be discussed in advance with the consultant interventional cardiologist responsible for the patient’s care.
Following the publication of three RCTs in 2018 the use of aspirin in primary prevention in cardiovascular disease is no longer routinely recommended and should only be considered, on a case-by-case basis, in those with a very high individual risk of cardiovascular events 13 .
P2Y12 receptor antagonists
Antiplatelet drugs have a rapid onset of action, and irreversibly inhibit platelet activity. Clopidogrel, prasugrel and ticagrelor are antagonists of the P2Y12 receptor on platelets. Clopidogrel plus aspirin is more potent than aspirin alone 14 . For clopidogrel, platelet function returns to normal 5–7 days after withdrawal of the drug 15 . Prasugrel and ticagrelor are more rapidly acting and more potent platelet receptor antagonists than clopidogrel. Clopidogrel monotherapy may be used following a thromboembolic cerebrovascular accident, or for peripheral vascular disease. P2Y12 receptor antagonists are frequently used as dual antiplatelet therapy (DAPT) with aspirin in acute coronary syndrome (ACS) and after placement of coronary stents. High- and low-risk indications for P2Y12 receptor antagonists are listed in Table 2 .
Patients on DAPT, with aspirin and a P2Y12 receptor antagonist, particularly in the context of coronary artery stents, are at high risk of thrombosis if drug therapy is discontinued. Without antiplatelet therapy coronary stents are at high risk of occlusion due to thrombosis, and this confers an approximate 40 % risk of acute myocardial infarction or death. In one study, discontinuation of DAPT was associated with a hazard ratio (HR) of 161 for these events 16 . DAPT is generally prescribed for 12 months after drug eluting stents (DES) implantation, though there have been occasional reported instances of thrombosis after this duration. Prasugrel and ticagrelor are now the main drugs of choice used for ACS in the UK. Prasugrel has been shown in a large UK observational series to be associated with a lower mortality 17 This has been confirmed in a large randomised open-label trial 18 . The latest NICE guidelines for ACS now recommend prasugrel is used as the first line agent in ACS, unless the patient is on anticoagulant therapy, in which case clopidogrel is recommended. Clopidogrel is generally the first choice of P2Y12 inhibitor in patients undergoing elective percutaneous coronary intervention (PCI) and the current guidelines recommend a six-month course of DAPT post elective PCI 10 . Where temporary cessation of P2Y12 inhibitors in patients with stents has been agreed, after discussion with the responsible consultant interventional cardiologist, then stopping the P2Y12 inhibitors seven days pre-procedure will minimise the bleeding risk. However, it should be noted that, depending on the P2Y12 agent, this time can be shortened dependent on the P2Y12 agent in use. In patients with a very high risk of stent thrombosis there may also be a role for bridging either with i. v. cangrelor and/or GIIbIIIa agents. However, the role of such bridging agents is beyond the remit of this paper and this decision should be made along with the responsible consultant interventional cardiologist on a case-by-case basis 19 .
Bare metal stents (BMS), which only require one month of DAPT, are now much less frequently used and the national BCIS audit shows they are used in less than 15 % of cases in the UK, and many units no longer even stock them. Their main indication is for use in patients with a high bleeding risk or requiring urgent major surgery. Many UK units now use BioFreedom DES, these are polymer-free drug coated stents, which have a license of one month of DAPT and are superior to BMS with respect to major safety endpoints including bleeding and restenosis rates 20 . Finally there is increasing evidence that with a number of third generation DESs one month of DAPT can safely be used in patients at high bleeding risk without an increase in ischaemic events 21 22 23 . A single antiplatelet agent is continued in all cases after discontinuation of DAPT, this tends to be aspirin. In some cases, for example patients with previous stroke, the patients may have aspirin stopped at the end of the DAPT course and clopidogrel continued long-term. The important issue of a single antiplatelet agent in patients on long-term DOACs is discussed in the previous section.
Finally, in patients on a DOAC for atrial fibrillation and stroke prophylaxis who need DAPT after stent implantation, evidence from trials such as the RE-DUAL trial indicate that much shorter courses of triple therapy followed by a course of DOAC and clopidogrel alone are safer than previous practice of triple therapy up to a year 24 . The European Society of Cardiology and NICE guidelines, for both ACS and elective stenting, now support shorter durations of triple therapy followed by a DOAC and clopidogrel as the current standard of care 10 25 26 .
The duration of DAPT post PCI may also depend on a number of other factors, beyond the scope of this guideline. Therefore, in patients with coronary stents, we recommend that the endoscopist discusses the plan, with respect to potential DAPT cessation, with a consultant interventional cardiologist. Ideally this should be the consultant interventional cardiologist responsible for the patient’s care, who did the stent procedure.
Anticoagulants
Warfarin and heparin
For low-risk endoscopic procedures we suggest that warfarin therapy should be continued (low quality evidence, moderate recommendation). It should be ensured that the INR does not exceed the therapeutic range in the week prior to the procedure (low quality evidence, strong recommendation).
Tell the patient to continue warfarin and check the international normalized ratio (INR) during the week before the endoscopy;
If the INR result is within the therapeutic range then continue with the usual daily dose;
If the INR result is above the therapeutic range, but less than 5, then reduce the daily warfarin dose until the INR returns to within the therapeutic range;
If the INR is greater than 5 then defer the endoscopy and contact the anticoagulation clinic, or a medical practitioner, for advice.
For high-risk endoscopic procedures in patients at low thrombotic risk, we recommend discontinuing warfarin for 5 days before the procedure (strong recommendation, high quality evidence). Check INR prior to the procedure to ensure < 1.5 (strong recommendation, low quality evidence).
Stop warfarin for 5 days before the endoscopy;
Check the INR prior to the procedure to ensure its value is < 1.5;
On the day of the procedure restart warfarin with the usual daily dose that night;
Check INR one week later to ensure adequate anticoagulation.
For high-risk endoscopic procedures in patients at high thrombotic risk, we recommend that warfarin should be temporarily discontinued and substituted with low molecular weight heparin (LMWH) (moderate quality evidence, strong recommendation).
Warfarin should be stopped for 5 days before the procedure;
Two days after stopping warfarin commence daily therapeutic dose of LMWH;
Administer the last dose of LMWH at least 24 hours prior to the procedure;
Check the INR prior to the procedure to ensure its value is < 1.5;
Warfarin can be resumed on the day of the procedure with the usual dose that night;
Restart the daily therapeutic dose of LMWH on the day after the procedure;
Continue LMWH until a satisfactory INR is achieved.
Updated literature searches were conducted on the use of warfarin and heparin in patients undergoing endoscopy. There were no new data to indicate a change to the existing protocols above. Any new data relevant to interventional therapy are discussed in the sections on specific endoscopic procedures.
Direct oral anticoagulants
For low-risk endoscopic procedures we suggest omitting the morning dose of DOACs on the day of the procedure (weak recommendation, low quality evidence).
For high-risk endoscopic procedures in patients on DOACs, we recommend that the last dose of DOACs be taken 3 days before the procedure (strong recommendation. low quality evidence). For patients on dabigatran with a CrCl (or eGFR) of 30–50 mL/min we recommend that the last dose be taken 5 days prior to the procedure (strong recommendation, low quality evidence). In any patient with rapidly deteriorating renal function a haematologist should be consulted (strong recommendation, low quality evidence).
Direct oral anticoagulants have been a major advance in anticoagulant therapy. Dabigatran is an inhibitor of thrombin, and rivaroxaban, apixaban and edoxaban inhibit factor Xa. They do not need routine monitoring, and INR and activated partial thromboplastin time (aPTT) are unreliable indicators of anticoagulant activity. Unlike warfarin they have a rapid onset of action and full anticoagulant activity is established within 3 hours of the first dose. They have relatively short half-lives, but these may be prolonged in renal failure, particularly for dabigatran. In patients undergoing a high-risk procedure with a low thrombotic risk we recommend that the last dose of rivaroxaban, apixaban or edoxaban is taken 3 days before the procedure. Dabigatran may have to be stopped for longer than this, however, when renal function is significantly reduced 27 .
For patients on dabigatran with creatinine clearance (CrCl) of 30–50 mL/min we recommend that the last dose of the drug is 3–5 days before the procedure. Dabigatran therapy is contraindicated in patients with CrCl < 30 mL/min. Estimated glomerular filtration rate (eGFR) is a suitable alternative measurement of renal function, and the same numerical values apply for the purposes of these guidelines. If a patient on any DOAC is clinically deteriorating, his/her renal function should be checked before the procedure. These recommendations are supported by the findings of the PAUSE trial. 5 Three thousand and seven patients on apixaban, dabigatran or rivaroxaban due for elective interventional procedures had a standardised interruption of therapy ( Fig. 3 ): last dose of drug 2 days before low risk procedures (including diagnostic GI endoscopy) and 3 days before high risk procedures (including high risk therapeutic GI endoscopy; e. g., polypectomy). This was extended to last dose 5 days before a high risk procedure for dabigatran patients with CrCL < 50 mL/min. Resumption of therapy occurred at 1 day after low bleeding risk procedures and 2 to 3 days after high bleeding risk procedures. Low rates of major bleeding or arterial thromboembolism were observed. Low rates of venous thromboembolism were also observed although this was not a primary study outcome. We have maintained the recommendation to omit the morning dose of DOAC prior to low-risk endoscopic procedures, although the PAUSE trial demonstrates the safety of omitting the DOAC for 1 day before a low risk endoscopic procedure if desired
Bridging of anticoagulant therapy
Unfractionated heparin (UFH) and low molecular weight heparin (LMWH) have short half lives compared to warfarin and can be employed as an anticoagulation “bridge” while warfarin is temporarily discontinued for endoscopic procedures considered to have a significant risk of hemorrhage. UFH is administered by continuous intravenous infusion and LMWH by subcutaneous injection once or twice daily. The former requires an inpatient stay in hospital while warfarin is discontinued, and then re-introduced, and also requires frequent monitoring of APTT; the latter can often be managed in an outpatient setting without monitoring of anticoagulation levels. Some cardiologists have a preference for UFH for bridging warfarin in patients with metal heart valves. A meta-analysis demonstrated higher rates of bleeding in patients with mechanical heart valves bridged with LMWH vs UFH, but numbers were small in the LMWH studies 28 . A small multicentre registry study found no difference in adverse events between patients bridged with UFH or LMWH in this context 29 , and bridging with LMWH is now commonplace. Guidelines from the American Heart Association and American College of Cardiology do not recommend one strategy over the other 30 . Prosthetic metal heart valves in the mitral position are at particularly high risk of thrombosis if warfarin is temporarily discontinued for 5 to 7 days. Heparin bridging for patients with a bileaflet mechanical aortic valve replacement in the absence of AF is considered unnecessary in guidelines from the British Society of Haematology 31 and from the American College of Cardiology/American Heart Association 32 , but it is recommended in guidelines from the ESC and the European Association for Cardio-Thoracic Surgery 33 . There are no high quality studies to inform us, and consequently levels of evidence are low quality in all three guidelines. We have now placed mechanical aortic valve replacement in the high risk category requiring bridging, in line with European guidelines, but this should be decided in conjunction with local cardiology or cardiothoracic surgery services given the uncertainty. This should always be discussed with the consultant cardiologist responsible for the patient’s care.
The risk of thromboembolism with AF increases with additional cardiovascular factors such as hypertension, heart failure and diabetes and this risk has been quantified by the CHADS 2 score (annual risk of stroke increasing from 1.9 % with a score of 1 to 18.2 % with a score of 6). This has been updated with the CHA 2 DS 2 VASc scoring system in which the annual risk of stroke increases from 1.3 % with a score of 1 to 15.2 % with score of 9. A randomised, prospective, double-blind placebo-controlled trial was conducted in 1884 patients with AF undergoing a variety of operative procedures including approximately 50 % GI endoscopy 34 . Patients were randomised to LMWH or placebo, and risk factors were well matched. 38 % of the patients had CHADS 2 scores ≤ 3, ≤ 2 % had mitral stenosis and ≤ 3.4 % had CHADS 2 scores of 5 or 6. There was no significant difference in rates of thromboembolism between the LMWH and placebo groups, but there was a significant increase in major haemorrhagic events in the LMWH group vs placebo (3.2 % vs 1.3 %). Caution should be exercised when interpreting the results in the high-risk thromboembolic groups as the study was not designed or statistically powered to examine these categories. The previous BSG/ESGE guidelines 1 do not recommend bridging for non-valvular AF, ASGE guidelines 35 recommend bridging with LMWH for CHA 2 DS 2 VASc ≥ 2 and the APAGE/APSDE guidelines 36 recommend this for CHA 2 DS 2 VASc ≥ 5. CHADS 2 scores are unfortunately not directly equivalent to CHA 2 DS 2 VASc scores. Further research on the benefits of heparin bridging is required in high-risk non-valvular AF patients on warfarin in order to determine the optimum approach, but it would be reasonable to consider bridging patients with CHADS 2 scores ≥ 5 as recommended by the British Society of Haematology 31 . This applies to patients with AF and a previous stroke or transient ischaemic attack (TIA), and 3 of the following factors: congestive cardiac failure, hypertension (> 140 /90 mmHg or on antihypertensive medication), age > 75 years, or diabetes mellitus. Heparin bridging is also recommended for patients with AF who have had a stroke or TIA within 3 months. 31
Bridging with LMWH has also been studied in patients on DOACs. In a German registry, heparin bridging vs no bridging led to a higher rate of major haemorrhage (2.7 % vs 0.5 %, p = 0.01) with no reduction in thromboembolism 37 . In the RE-LY trial bridging of dabigatran with LMWH resulted in higher major haemorrhage rates compared to no bridging (6.5 % vs 1.8 %, p < 0.001) with no difference in thrombosis rates between the groups 38 .
In a Japanese study of 16,977 patients on warfarin or DOACs undergoing a variety of high risk endoscopy procedures a propensity matched analysis demonstrated a significant increase in post procedure GI bleeding and thromboembolism in patients who were bridged with heparin 39 . It should be noted that all patients were bridged with UFH rather than LMWH. For colonoscopy in patients on warfarin who were bridged with LMWH, several studies have demonstrated an increase in post polypectomy haemorrhage without a decrease in thromboembolic events 40 41 42 . Bridging with LMWH should, of course still be advocated for those patients on warfarin at high risk of thromboembolism ( Tab. 3 ), but patients should be advised of the increased risk of post-procedure haemorrhage. The safety of temporary cessation of DOAC therapy, without bridging, is supported by the findings of the PAUSE trial. 5
Patients with a history of venous thromboembolism within 3 months of commencing anticoagulant therapy are at high risk of recurrent thrombosis if anticoagulation is interrupted. Most of these patients are now commenced on a DOAC rather than warfarin, and bridging would not be recommended. Ideally we should not interrupt anticoagulation in this high risk group due to the risk of thrombosis; an elective low risk procedure could be performed without interrupting anticoagulation if necessary, but it may be preferable to defer a high risk procedure beyond three months anticoagulation if safe to do so.
Patients with thrombophilia syndromes should be discussed with a haematologist. Factor V Leiden and the common prothrombin mutation F2 G20210A are low-risk thrombophilias and bridging is not required. Patients with deficiencies of antithrombin, protein C or protein S are at higher risk of thrombosis, but in most of these patients bridging therapy will not be required.
Endoscopic procedures
Minor haemorrhage is not uncommon during therapeutic endoscopic procedures, but we have considered it to be clinically significant when haemoglobin value falls by more than 20 g/L, necessitates blood transfusion, or causes an unplanned hospital admission. Haemorrhage may be apparent at the time of endoscopy, or delayed up to two weeks following the procedure. For those in whom antithrombotic therapy is interrupted, the latter situation presents a higher risk, as therapy will usually have been recommenced within that period. There are relatively few studies of the risks of haemorrhage for endoscopic procedures in patients who continue antithrombotic therapy, therefore much of the data underlying the risk levels for these procedures applies to the baseline risk of haemorrhage without antithrombotic therapy ( Table 3 ).
Diagnostic endoscopy and mucosal biopsy
Diagnostic endoscopies, including mucosal biopsy sampling, confer a minimal risk of haemorrhage, and no severe haemorrhage has been reported in studies involving thousands of patients in total 43 44 45 46 47 . No increased risk of haemorrhage from biopsy has been found in studies of patients on aspirin, clopidogrel or warfarin 48 49 . A prospective case control study of patients taking antiplatelet or anticoagulant drugs, including DOACs, found no incidence of significant bleeding in either the antithrombotic or control group who had upper GI biopsies taken 50 . Only 19 of the 277 patients who continued antithrombotics were on DOACs. The approximate mean number of biopsies per patient was only two in either group in this study, and in all the above studies only small numbers of biopsies were taken. In a prospective registry study 119 patients were identified who had continuation of DOAC for endoscopy, and 29 patients had biopsies on continued DOAC 51 . There were 2 cases of non-major clinically significant haemorrhage and no cases of major haemorrhage. There is one case report of life-threatening bleeding following multiple biopsies for Barretts oesophagus in a patient on aspirin who was later found to have high grade dysplasia 52 . A prospective study of endoscopic practice in Italy 53 regarding adherence to the previous BSG/ESGE antithrombotic guidelines 1 found an increased incidence of haemorrhage in patients undergoing mucosal biopsies in whom DOAC therapy was continued compared to those in whom the morning dose was withheld as recommended in the guidelines (2/38 5.2 % vs 2/114 1.7 %, respectively). However, numbers of cases were small, the study was not adequately powered to determine this issue, and the difference did not reach statistical significance.
The pharmacokinetic profile, and hence pharmacodynamic effect, of DOACs varies such that some individuals will have higher peak levels 2 to 6 hours after oral administration 54 . Consequently, at the time of an endoscopic biopsy the anticoagulant effect due to a DOAC is not accurately predictable. Due to this uncertainty regarding the level of anticoagulation on DOACs at the time of endoscopy, and the absence of reliable test of anticoagulation on these drugs, we continue to suggest omitting the dose of DOAC on the morning of the procedure to allow an adequate safety margin. This applies to both once daily and twice daily regimens.
Resection of GI polyps
Polypectomy including endoscopic mucosal resection (EMR)
Studies of colonoscopic polypectomy have identified a risk of post polypectomy bleeding (PPB) of 0.07–1.7 % 47 55 56 57 58 . It is important to differentiate between immediate and delayed PPB, and also to identify the severity of bleeding; these factors are not always clear in the literature. Delayed PPB is of particular importance in patients in whom antithrombotic therapy has been interrupted, as bleeding will often occur once antithrombotics have been restarted. In a BSG audit of 20,085 colonoscopies in the UK, 52 (0.26 %) haemorrhages were reported, but only 3 (0.01 %) were major 59 . Data from the English National Bowel Cancer Screening Programme found an overall PPB rate of 1.14 %, with a rate of severe bleeding requiring transfusion of 0.08 %. 60
Risk factors for PPB include polyp size 61 use of pure cutting current 62 and use of a non microprocessor-controlled current for endoscopic mucosal resection (EMR) 63 . Use of endoscopic clips or submucosal injection of diluted adrenaline may also reduce the risk of PPB 64 65 , Caution is advised, however, when using clips prior to excision for pedunculated polyps as one RCT was closed prematurely due to complications: one perforation (1.5 %) and 3 mucosal burns (4.5 %) 66 . An RCT of cold- vs hot-snare polypectomy (341 vs 346 polyps respectively) of polyps 4–9 mm demonstrated no significant haemorrhage in the cold-snare group 67 . In all of these studies, patients on antiplatelet therapy or anticoagulation were excluded.
A number of studies have examined the risks of resection of small colonic polyps on continued antitcoagulant therapy. A single centre case series of 1,177 cold snare polypectomies compared PPB in those on antiplatelets or anticoagulants compared to those not on these drugs 68 . There was an increase in immediate bleeding, mostly in warfarin patients, but no significant difference in delayed bleeding (up to 2 weeks) between the groups. A retrospective study of 223 polypectomies (< 1 cm, with or without diathermy) in 123 patients on continued warfarin therapy found a rate of haemorrhage requiring transfusion of 0.8 %. This was despite routine prophylactic clipping of polypectomies 69 . In a RCT (159 polyps < 1 cm in 70 patients) examining hot vs cold snaring of polyps in anticoagulated patients, the rate of immediate haemorrhage in the hot snare vs. the cold snare group was 23.0 % vs 5.7 %, respectively, and that of delayed haemorrhage requiring intervention 14 % vs 0 %, respectively 70 . A RCT compared cold polypectomy < 1 cm in patients on DOAC or warfarin bridged with UFH compared to cold polypectomy on continued anticoagulant. 71 The incidence of polypectomy-related major bleeding was high at 12.0 % in the former group and 4.7 % in the latter. The majority of patients in the study would not have not have been considered for bridging by British Society of Haematology guidelines 31 , and LMWH would have been recommended for any that did qualify for bridging.
The risk of polypectomy on continued antiplatelet therapy has also been studied. Aspirin monotherapy has been found to be safe in colonoscopic polypectomy 72 73 74 . A double blind RCT included patients on clopidogrel therapy who required colonoscopy 75 . They were randomised at 7 days before procedure into either continuing with Clopidogrel 75 mg daily or placebo until the morning of colonoscopy. If discontinued, clopidogrel was restarted after colonoscopy when oral intake was allowed. More than 90 % of patients had polyps les than 1 cm. There was no significant difference in immediate or delayed PPB, or in cardiothrombotic events, between the two groups. These conclusions, however, need to be treated with some caution. Although the study aim was to examine the effect of clopidogrel, a high proportion of patients were on DAPT. The groups were well matched but numbers were relatively small to examine differences in an infrequent event, and the rate of PPB in the placebo group was higher than expected when compared with other studies of patients undergoing polypectomy not previously on antiplatelet agents. A meta-analysis 76 assessing the risks of immediate and PPB associated with continued clopidogrel use at time of polypectomy included the above trial. A total of 5 studies were identified which included 655 patients in the ‘continuing clopidogrel’ group and 6620 controls with interrupted clopidogrel therapy. There was an increased risk of PPB in the continuing clopidogrel group (Risk ratio –1.96 CI 1.36–2.83; p = 0.0003). There was no significant difference in serious cardio thrombotic events occurring within 30 days of the procedure. In the above studies the great majority of polypectomies were < 1 cm. Further research is required, particularly with respect to polypectomy on DAPT, but based on the above data, it may be safe to undertake polypectomy for polyps < 1 cm in size on clopidogrel monotherapy. To minimise the risk of PPB cold snare polypectomy may be advisable. Alternatively, temporarily substituting aspirin for clopidogrel may be desirable 7 days prior to colonoscopy.
In large case series of EMR, the incidence of immediate and delayed bleeding ranged between 3.7–11.3 % and 0.6–6.2 %, respectively 63 77 78 . For EMR of small lesions (< 1 cm), however, PPB rates were similar to those reported following conventional polypectomy 78 . A retrospective cohort study did not find aspirin (continued till day of procedure) or P2Y12 receptor antagonists (stopped 5–7 days before EMR) to be significant factors associated with bleeding post EMR of colon neoplasia 79 . However only a small minority (aspirin 20 % and clopidogrel 2 %) of patients were taking these medications. The rate of delayed post-EMR bleeding in the oesophagus is low (0.6 to 0.9 %), even in studies that include a high proportion of patients with a temporary cessation of antiplatelet therapy 80 81 . In two retrospective observational studies of duodenal EMR, delayed bleeding was reported in 14/113 (12.3 %) 82 and 7/111 patients (6.3 %) 80 despite the prophylactic use of endoclips in 82 % of cases in the latter. There are conflicting studies on the use of prophylactic endoclips for EMR 83 84 85 . A cost-efficacy analysis concluded that prophylactic placement of endoscopic clips after polypectomy was a cost-effective strategy for patients receiving antiplatelet or anticoagulation therapy. 86 Prophylactic use of endoclips may therefore be advisable for EMR in patients on antithrombotics due to the increased risk of PPB.
Endoscopic submucosal dissection
There have been several studies of ESD on continuous low dose aspirin since the previous version of this guideline. One retrospective multicentre study 87 for colonic ESD, and 6 retrospective studies of gastric ESD 88 89 90 91 92 93 , comparing continuation with interruption of aspirin, found no significant differences in delayed bleeding the two groups. This was confirmed by two meta-analyses which also reported that continuation of low dose aspirin does not increase the post procedure bleeding after ESD 94 95 . This was also observed in the group of patients under dual antiplatelet therapy in which aspirin was continued alone 88 . Furthermore, inappropriate discontinuation of anti-platelet agents was significantly associated with increased risk of thrombosis 90 . A meta-analysis found a thrombosis rate of 2.1 % in the aspirin-interrupted vs. none in the aspirin-continued group of patients 95 .
Continued thienopyridine (clopidogrel or prasugrel) or aspirin use did not increase delayed haemorrhage for gastric ESD in one retrospective study 92 . A non-randomised retrospective comparative study found, however, that continuation of any antithrombotic, or heparin bridging, increased the risk of haemorrage with gastric ESD 93 . The risk of haemorrhage following gastric ESD is increased with the number of antiplatelet agents taken or when antiplatelet drugs are combined with anticoagulation 96 . For colorectal ESD, antiplatelet agents except for aspirin alone were independent risk factors of delayed bleeding (OR 4.04, 95 % CI 1.44–11.30, p = 0.008) in a retrospective multicentre study including 180/1189 patients on antiplatelets 87 .
In a large national database including 16,977 patients who underwent high-risk endoscopic procedures under oral anticoagulation, upper and lower GI ESD were found to be significantly associated with post procedure haemorrhage 39 . The delayed bleeding rate after ESD at any location has been found to be 16 % in patients who have had warfarin or DOACs, including those with heparin bridging (UFH) 97 . A meta-analysis of ESD studies found a significant increase in delayed haemorrhage and an increase in thrombosis with heparin bridging compared to those who discontinued anticoagulation without bridging 94 . A meta-analysis focused on heparin bridging therapy (UFH) for gastric ESD patients on warfarin or DOACs found an increased risk of haemorrhage without any benefit in thrombosis 95 . Studies of colonic ESD on anticoagulants have included small numbers and retrospective analysis, and conflicting conclusions have been derived 87 98 99 . Few data are available for oesophageal or duodenal ESD on antithrombotics. The risk of bleeding after esophageal ESD is lower at compared with other locations 100 101 , but one retrospective study found a significant higher readmission rate after oesophageal ESD for patients with history of antiplatelet or anticoagulant use (56.4 % vs 34.1 %; P = 0.01) 102 .
Several methods have been proposed to reduce the risk of haemorrhage after ESD including pharmacological (PPI), mechanical (clips, endoscopic hand sutures) and local topical (fibrin glue and polyglycolic acid sheets, hemostatic powder) techniques, and these should be particularly considered in patients on antithrombotic therapy.
Endoscopic Retrograde Cholangiopancreatography (ERCP)
In a meta-analysis (21 prospective studies; 16855 patients), the overall haemorrhage rate following endoscopic retrograde cholangiopancreatography (ERCP) was 1.3 %, with 71 % of these being graded as moderate and 29 % as severe; the mortality rate was 0.05 % overall 103 . Post-ERCP haemorrhage is most frequently seen after endoscopic biliary sphincterotomy. An ESGE guideline on ERCP-related adverse events has suggested that patients should be considered to be at increased risk for post-sphincterotomy haemorrhage if at least one of the following factors is present: anticoagulant intake, platelet count < 50,000 /mm 3 , cirrhosis, dialysis for end-stage renal disease, intraprocedural bleeding and low endoscopist experience 104 . There are a number of measures which can reduce the risk of haemorrhage at ERCP including avoidance of sphincterotomy prior to biliary stenting 105 , and use of a blended current rather than pure-cutting current 106 107 108 . These, and other measures to reduce the risk of haemorrhage are discussed in previous ESGE guidelines 104 105 .
Two uncontrolled retrospective studies reported post-endoscopic sphincterotomy (ES) bleeding in 8 (19 %) of 43 patients under antiplatelet monotherapy or dual therapy, including only one significant episode of haemorrhage 109 110 . Controlled studies of bleeding following ES performed under antiplatelet therapy have failed to demonstrate an increase in patients taking antiplatelets at the time of ES vs controls, but studies were underpowered to show a difference 110 111 112 . A single retrospective study (762 patients) has compared post-ES bleeding rates in patients who discontinued antiplatelets for > 7 days (n = 29), < 7 days (n = 83) or did not discontinue antiplatelets (n = 49) 111 . The incidence of bleeding (defined as clinical evidence of bleeding or a drop in Hb > 2 g/dL) was respectively 10.3 % (delayed, 6.9 %), 6.0 % (delayed, 2.4 %) and 16.3 % (delayed, 14.3 %). The only significant association was between sustained antiplatelet use and the delayed type of post-ES bleeding. Of note, a majority of antiplatelet users were taking aspirin only. A retrospective study in which 50/191 patients undergoing ES were on aspirin, showed no statistically significant increase in haemorrhage in the aspirin group 113 . Haemorrhage following endoscopic sphincteroplasty has been reported in 0 % to 8.6 % of patients 114 . One small case series suggested that continued aspirin therapy may be safe 115 , but there are no data on other antithrombotics. There are no data for biliary mechanical lithotripsy, cholangioscopy or electrohydraulic lithotripsy therapy in patients taking antiplatelets or anticoagulants.
Ampullectomy
A meta-analysis (29 studies, 1751 patients) reported that haemorrhage occurs in 10.6 % of ampullectomies with approximately 25 % and 5 % requiring blood transfusion and non-conservative management, respectively 116 . Various measures to prevent haemorrhage have been studied, but more research is required to make definitive conclusions. A nonrandomized pilot study (n = 80) suggested that routinely closing the mucosal defect with clips following ampullectomy is effective to prevent delayed bleeding (5 % vs. 22.5 %, P = 0.026) 117 . A RCT has suggested that, compared with the Autocut mode, the Endocut mode may prevent immediate but not delayed bleeding in cases with tumors > 14 mm; it causes more frequent crush artifacts 118 . Prophylactic argon plasma coagulation has been reported as effective in a retrospective study (n = 82) and ineffective in a RCT (n = 54) to prevent post-ampullectomy bleeding 119 120 . Submucosal injection prior to ampullectomy did not prevent bleeding in a RCT (n = 50) and a retrospective matched cohort analysis (n = 50) 121 122 ; furthermore it was associated with higher tumor recurrence rate and a shorter recurrence-free survival in the retrospective study 121 . There are no data on ampullectomy on anticoagulants or P2Y12 receptor antagonists as these are usually withdrawn. Given the high haemorrhage rate, withdrawal of aspirin should be considered on an individual basis depending on the risks of thrombosis.
Endoscopic ultrasound-guided with fine-needle aspiration (EUS-FNA), and other interventions
The incidence of haemorrhage following EUS-guided sampling has been analysed in several systematic reviews; the figure per thousand was 1.28 for EUS-FNA (51 studies, 10,941 patients) 123 , 5.81 for EUS-fine needle biopsy (FNB) (51 studies, 5,330 patients) 124 and 6.63 for EUS-FNA of pancreatic cystic lesions (40 studies, 5,124 patients) 125 . Four studies reported on haemorrhage following EUS-guided sampling in patients prescribed antithrombotic agents 126 127 128 129 . The only significant differences were between patients on prophylactic low molecular weight heparin and controls 128 . In the largest study, however, no severe haemorrhage was found in patients who continued or discontinued antithrombotic therapy, and only one thromboembolic event occurred 129 .
EUS-guided biliary drainage, an alternative to ERCP-guided biliary stenting, has been suggested to be safely feasible in patients with sustained use of antiplatelets and/or anticoagulants. In a retrospective study that included 41 patients on antiplatelets and/or anticoagulants (23 patients under DAPT, anticoagulant or a combination of antiplatelet and anticoagulant) and 154 controls, bleeding rates were not significantly different in the antiplatelet/anticoagulants and control groups (7.3 % and 2.6 %, respectively) 130 . The safety of EUS-guided biliary drainage should however be confirmed in prospective studies, adequately powered to evidence a significant difference, before a recommendation can be made. Invasive therapeutic procedures such as EUS and cystgastrostomy or necrosectomy have a significant risk of major haemorrhage and should be considered as high risk for P2Y12 receptor antagonists or anticoagulants. The risk on aspirin is unknown.
Endoscopic dilatation
Review of studies which included greater than 100 patients with benign upper GI strictures, either anastomotic, achalasia, post-ESD, eosinophilic, gastric outlet obstruction or mixed etiology, reveals a risk of haemorrhage well below 1 % 131 132 133 134 135 136 137 138 139 140 . A systematic review and meta-analysis of studies of endoscopic dilatation of gastroduodenal Crohn’s disease strictures revealed a 2.1 % per procedure risk of haemorrhage 141 . In a prospective study of dilatation in 55 patients with oesophageal carcinoma no clinically relevant haemorrhage was observed 142 . Dilatation for lower GI strictures, either iatrogenic or related to inflammatory bowel disease, revealed no significant haemorrhage in prospective studies or larger retrospective case series (over 100 patients) 143 144 145 146 147 . No data were found regarding dilatation in malignant colonic strictures.
A study of the complications arising from 504 balloon dilations in 237 patients with achalasia revealed 4 (1.7 %) asymptomatic haematomas, but no clinically significant haemorrhage 148 . There were, however, 7 (3 %) perforations. In a RCT of pneumatic dilatation vs. laparoscopic myotomy for achalasia there were no reported haemorrhages but 8/108 (9.5 %) patients experienced perforation during the treatment course 149 .
Dilatation of GI strictures in the upper or lower GI tract appears to be a low risk procedure, with the exception of Crohn’s disease-related ileal strictures, and balloon dilatation for achalasia. There are no data, however, on dilatation of strictures on antiplatelets or anticoagulants. This, together with the inaccessibility of the site of haemorrhage for endoscopic haemostasis, has led us to continue to consider endoscopic dilatation as a high risk procedure on P2Y12 receptor antagonists or anticoagulants.
Endoscopic stenting
There are no studies on endoscopic stenting at any site in the GI tract in patients taking antiplatelets or anticoagulants. Available data regarding haemorrhage risk, from RCTs, prospective, and mostly retrospective studies, are heterogeneous regarding details of the time interval from stent placement until clinically relevant haemorrhage occurred, In deciding whether stent insertion is a high or low risk procedure we considered haemorrhage within 7 days of insertion.
Previous reviews of risks associated with endoscopic stent insertion have been confounded by the variety of stents used and the evolution of stent design with time. With respect to esophageal self-expanding metal stents (SEMS), 8 recent studies of demonstrated a risk of 0 % significant haemorrhage within 7 days of insertion 150 151 152 153 154 155 156 157 . However, studies including delayed haemorrhage showed a risk of 9 % in an RCT comparing small-diameter stents with large-diameter stents and an 8 % risk in a large retrospective series of 997 patients 158 159 . Causes of haemorrhage included aorta-esophageal fistula formation, and continued oozing from tumours 154 160 .
With respect to duodenal SEMS, large (> 100 patients) and recent retrospective and prospective studies showed a haemorrhage risk within 7 days of less than 1 % 161 162 163 . The one exception was a retrospective study including 152 patients, whereby the safety and benefits of SEMS combined with chemotherapy were investigated: in total 4 patients (2.6 %) suffered from haemorrhage that requiring blood transfusion. Though none of these patients had concomitant chemotherapy, two thirds had coexisting biliary obstruction for which an intervention was simultaneously performed 164 .
With respect to colonic SEMS, a large prospective series of 513 patients and 6 retrospective studies found the risk of clinically relevant haemorrhage within 7 days to be 0 % 165 166 167 168 169 170 171 172 173 174 . A systematic review identifying 40 studies on SEMS for the management of emergency malignant large bowel obstruction identified 9 studies reporting on clinical relevant bleeding which occurred in 0.5 % (8 out of 1474 patients) 175 .
We have considered endoscopic stenting at all sites in the GI tract to be low risk for haemorrhage within 7 days. Patients on antithrombotics may be at increased risk of delayed haemorrhage, however.
Percutaneous endoscopic gastrostomy (PEG)
Minor haemorrhage around the wound site at PEG placement is usually short-lived. Severe haemorrhage is rare, and may be secondary to PEG tract bleeding, injuries to the gastric artery, splenic or mesenteric vein (massive retroperitoneal bleeding), or rectus sheath hematoma 176 177 178 . Rarely, laparotomy may be required as a result of puncturing of the gastric artery at the time of insertion 177 .
The overall risk of haemorrhage for PEG placement was 1.5 % in a case series of 950 patients 179 . There are few data on continued administration of antithrombotics at the time of PEG insertion. A meta-analysis showed continuing anti-platelet therapy such as clopidogrel may be safe. 180 A German retrospective study of PEG insertion with patients on UFH, phenprocoumon, LMWH, aspirin, clopidogrel and combinations showed no evidence of increased haemorrhage. 181 A further study concluded that giving aspirin or clopidogrel either 72 hours before or 48 hours after the procedure did not increase bleeding risk 182 . The above studies are, however, of inadequate quality to definitively demonstrate an effect due to the drugs studied. A large retrospective study of patients undergoing endoscopic procedures on anticoagulants included 2322 PEG performed on warfarin and 1484 on DOACs, with a risk of post-endoscopy bleeding of 2.0 % and 1.2 %, respectively 39 .
Device-assisted enteroscopy
Single-balloon, double-balloon and spiral enteroscopy devices are available. The risk of haemorrhage from enteroscopy has been reported at 0.2 %- 0.3 % 183 184 . Spiral enteroscopy was not associated with a risk of clinically significant haemorrhage in a small case series. 185 Double balloon enteroscopy is associated with a perforation rate of 0.1–0.4 % 183 186 and this rises to 1.5 % if polypectomy is performed 186 . In a retrospective single centre study of 420 patients undergoing double-balloon enteroscopy, it was noted that 13 % were on anticoagulation 187 . Although the risk of bleeding may be low for diagnostic procedures, therapeutic procedure such as polypectomy would confer a high risk of haemorrhage on antithrombotics. Endoscopic therapy was performed in 47.1 % of 257 double-balloon procedures in a UK case series 188 .
Oesophageal variceal banding
Generally, variceal band ligation (VBL) is undertaken when there is recent or active bleeding. However, elective variceal banding may be necessary at surveillance. In a case-control study of bleeding following VBL 3.4 % of patients had haemorrhage secondary to banding induced ulcerations 189 . In a study of 605 patients undergoing VBL, 21 (3.5 %) patients had spontaneous bleeding due to band slippages confirmed at endoscopy, and 11 died 189 . Multivariate analysis found no increased risk of bleeding in those on aspirin, although this applied to only 8/605 patients
A study of planned VBL in cirrhotic patients, including 265 patients on LMWH, showed no increase in post-procedural haemorrhage or reduction in survival compared to those not on LMWH 190 . An uncontrolled case series of 5 patients undergoing elective VBL on continued warfarin observed no post-banding haemorrhage 191 . Placing no more than 6 bands per session may help reduce the risk of post-banding ulcer haemorrhage 192 . In a large retrospective series, the risk of haemorrhage in patients undergoing variceal banding on DOAC or warfarin was high for both groups at 19.2 % and 25.9 % respectively p = 0.49 39 . There are no studies of variceal band ligation in patients on P2Y12 receptor antagonists.
Ablative therapies
Radiofrequency ablation (RFA) in the oesophagus is a well established treatment for dysplasia within Barretts oesophagus. A meta-analysis of studies of oesophageal RFA has found a rate of haemorrhage of 1 %. 193 RFA has been used to treat gastric antral vascular ectasia, but we have only small case studies to inform us, as demonstrated in a systematic review 194 . In one case series of 21 patients there was a 10 % ulceration rate necessitating discontinuation of RFA therapy. We have therefore classified oesophageal and gastric RFA as high risk for hemorrhage with respect to P2Y12 receptor antagonists and anticoagulants. There are no data on aspirin, but we advise continuation in ither procedures with similar rates of haemorrhage.
Argon plasma coagulation is used as an ablative therapy for a wide variety of indications throughout the GI tract, including ablation of vascular abnormalities including angiodysplasia and radiation proctitis, ablation of tumours and margins of resected polyps, ablation of dysplastic Barretts oesophagus, and occasionally as a haemostatic measure. With different indications there is variation in size of the ablated mucosal field, energy delivered, and consequences including rarely perforation. There are no data on continued use of antithrombotics with respect to the risk of haemorrhage from APC, and we are therefore unable to provide specific guidance in this regard.
Restarting antithrombotic therapy for elective procedures
There are few data to inform us on the optimal time to restart antithrombotic therapy, if discontinued, after elective endoscopic therapy. Decisions in all cases will be based on the perceived risk of haemorrhage following the procedure versus the risk of thrombosis to an individual patient. It should be remembered that DOACs exert an anticoagulant effect within hours, compared to days for warfarin. Data from the PAUSE study indicates that restarting a DOAC 2–3 days after a high risk procedure has a low risk of thromboembolic events 5 . Data on restarting therapy after acute GI haemorrhage are discussed in that section. A prospective cohort study in Italy 53 evaluated the risks of haemorrhage and thrombosis in relation to compliance with the previous version of this BSG/ESGE guideline. For cases who underwent polypectomy there was a trend to more intraprocedural bleeding if DOAC was not stopped as per the guideline for high-risk procedures. Stopping longer than the guidelines did not reduce the intraprocedural bleeding risk. Also, restarting the DOAC immediately after polypectomy, rather than a delay of 24–48hrs (as suggested by the guidelines) in high-risk patients led to an almost doubling of the delayed bleeding risk without a reduction in thrombosis, although these measures did not reach statistical significance.
For procedures with a very high risk of haemorrhage such as ESD, it may be desirable to delay restarting antithrombotics beyond the intervals recommended in these guidelines. In a meta-analysis, post-ESD bleeding risk was not significantly increased if antithrombotic therapy was resumed at least 1 week after ESD whereas resumption of antithrombotic therapy immediately or within 1 week after ESD was significantly associated with post-ESD bleeding 94 . The incidence of thrombosis was not analysed, however, and this needs to be considered on an individual patient basis.
Risk stratification for elective endoscopic procedures
Endoscopic procedures carry a higher risk of haemorrhage, and certain clinical situations will result in a high risk of thromboembolic complications should antiplatelets or anticoagulants be withdrawn. Procedures have been classified as high-risk or low-risk for haemorrhage primarily based on the risks of haemorrhage in patients not taking antiplatelets or anticoagulants, as well as some limited data available regarding endoscopy in patients in whom these drugs were continued ( Table 1 ). Tables 2 and 3 stratify risk for discontinuation of P2Y12 receptor antagonists or warfarin respectively according to clinical scenario, and the risks of thromboembolic sequelae on discontinuation of therapy.
Diagnostic endoscopic procedures, with or without biopsy, are classified as low-risk for haemorrhage, though there is concern regarding biopsy on DOACs. The likelihood of having to undertake therapy with a risk of haemorrhage should also be considered, for example colonoscopy when polyps have been found in 22.5–34.2 % of patients in large studies 47 55 . Endoscopists may therefore choose to manage colonoscopies as high-risk procedures with respect to P2Y12 receptor antagonists and anticoagulants. Similar considerations apply to ERCP if there is uncertainty as to whether sphincterotomy will be required.
Acute GI haemorrhage on antiplatelets and anticoagulants
Antiplatelets
We suggest that permanent discontinuation of aspirin for primary prophylaxis should be considered (weak recommendation, low quality evidence).
We suggest that aspirin for secondary prevention should not be routinely stopped. If it is stopped, it should be recommenced as soon as haemostasis is achieved, or there is no further evidence of bleeding (strong recommendation, moderate quality evidence).
We recommend that dual antiplatelet therapy is continued if possible in patients with coronary stents in situ and management should be in liaison with a consultant interventional cardiologist (strong recommendation, moderate quality evidence). In the case of major haemorrhage we recommend continuing aspirin if the P2Y12 receptor antagonist is interrupted (strong recommendation, moderate quality evidence). P2Y12 receptor antagonist therapy should be re-instated within 5 days, if still indicated (strong recommendation, moderate quality evidence).
Antiplatelets confer an increased risk of spontaneous haemorrhage, but also present an increased risk to the patient when this occurs. In the case of life-threatening haemorrhage, this is of urgent concern, but withdrawal of antiplatelet therapy confers a risk of thrombosis. A meta-analysis of studies of patients on aspirin for secondary prophylaxis found that discontinuation of aspirin was associated with a 3-fold increased risk of major adverse cardiac events, increasing to an odds ratio of 89 for major cardiac events in patients with coronary stents 6 . If GI haemorrhage occurs in a patient with a recently placed coronary stent on DAPT, then life-threatening thrombosis could occur if therapy is interrupted. The imperative, after adequate resuscitation, is to achieve haemostasis within the GI tract and urgent facilities should be available to provide this. Liaison with a consultant interventional cardiologist should occur in the emergency setting. If it is deemed necessary to temporarily discontinue antiplatelet therapy, this should be limited to the P2Y12 receptor antagonist, and aspirin continued 195 . The timing of restarting antithrombotic therapy after acute GI haemorrhage will be determined by the risk of re-bleeding and the risk of acute thrombosis without antithrombotic therapy 196 . P2Y12 receptor antagonists in patients with coronary stents should be restarted within a maximum of 5 days due to the high risk of stent thrombosis after this time. This timeframe represents an optimal balance between haemorrhage and thrombosis 195 , though it has not been tested prospectively.
For patients on aspirin monotherapy for secondary prophylaxis, there is a benefit to continued therapy. A prospective placebo-controlled RCT of 156 patients following endoscopically controlled upper GI haemorrhage, demonstrated a reduction in all-cause mortality in the group receiving low-dose aspirin (1.3 % vs 12.9 %) 197 . There was an excess of bleeds in the aspirin group (10.3 % vs 5.4 %) but none were fatal. 5 patients in the placebo arm died of thromboembolic events. If aspirin is stopped then it should be reintroduced as soon as haemostasis has been achieved.
A retrospective study of 118 patients on antiplatelets or anticoagulants presenting with acute GI haemorrhage compared outcomes in those who had antithrombotic therapy permanently discontinued compared to those in whom it was restarted 198 . In those in whom antithrombotic therapy was discontinued the hazard ratio for thrombotic events was 5.77 (95 %CI 1.26–26.35) and for mortality 3.32 (1.07–10.31) compared to those in whom it was restarted. It is therefore important to have a plan for consideration of restarting antithrombotic therapy in all such patients presenting with GI haemorrhage.
Anticoagulants
We recommend withholding oral anticoagulant and correcting coagulopathy according to the severity of haemorrhage and the patient’s thrombotic risk in coordination with a consultant cardiologist/haematologist. The correction of coagulopathy should not delay endoscopy or radiologic intervention (strong recommendation, low quality evidence).
In patients with haemodynamic instability who take vitamin K antagonists (VKAs), we recommend administering intravenous vitamin K and four-factor prothrombin complex concentrate (PCC) (strong recommendation, low quality evidence), or fresh frozen plasma if PCC is not available (weak recommendation, very low quality evidence)
In patients with haemodynamic instability who take DOACs, we suggest considering the use of reversal agents: idarucizumab in dabigatran patients, and andexanet in anti-factor Xa treated patients (weak recommendation, low quality evidence), or intravenous four-factor PCC if andexanet is not available (weak recommendation, very low quality evidence)
We recommend restarting anticoagulation following acute GI haemorrhage in patients with an indication for long-term anticoagulation (strong recommendation, low quality evidence). In patients at low thrombotic risk, we suggest restarting anticoagulation as soon as possible after seven days of anticoagulant interruption (weak recommendation, low quality evidence). In those at high-thrombotic risk, an earlier resumption of anticoagulation with heparin bridging, preferably within 3 days, is recommended (strong recommendation, low quality evidence).
Anticoagulant use is reported in up to 15 % and 25 % of patients presenting with acute upper and lower GI bleeding, respectively 199 200 201 . GI bleeding-related mortality in these patients is high (up to 8–12 %), but mainly related to patients’ comorbidities. Anticoagulants are independent predictors of neither mortality nor in-hospital rebleeding 202 203 204 205 , provided they are managed appropriately. Temporary discontinuation of anticoagulation is the “standard of care” in patients with clinically significant GI bleeding 206 207 .
The anticoagulant effect of Vitamin K antagonists (VKA) such as warfarin can persist for 3–5 days. The need for a rapid correction of VKA-related coagulopathy with reversal agents mainly depends on the severity of haemorrhage, but their use requires caution in patients at high thrombotic risk (e. g. mechanical heart valve) since their use has been associated with thromboembolism 208 . Vitamin K takes several hours to correct anticoagulation, so the use of 4-factor Prothrombin Complex Concentrate (PCC) may be necessary to rapidly reverse anticoagulation in patients with active bleeding and haemodynamic instability. PCC is preferred over FFP, for a lower volume load, faster onset of action, and no need to check the patient’s blood group. Concomitant low-dose vitamin K is recommended in this situation to prevent an “INR rebound”.
Unlike VKAs, DOACs are characterized by a relatively short half-lives, so that their anticoagulant activity usually rapidly wanes-off over 12–24 hours. Consequently, most cases of major GI haemorrhage can be managed by withholding the drug and waiting for the anticoagulant effects to resolve. In hemodynamically unstable patients, acute reversal of anticoagulation may be required 209 210 . Vitamin K, FFP, and protamine administration are ineffective. Idarucizumab, a humanized antibody fragment that neutralizes the effect of dabigatran, is now currently available as first-line reversal agent in dabigatran patients presenting with life-threatening/ uncontrolled bleeding 211 212 . Idarucizumab reverses dabigatran-related coagulopathy rapidly (within a few minutes) and persistently (for about 24 hours) in more than 98 % of the patients, with a low thrombotic complication rate (6 % at 90 days). Andexanet alfa, an inactive form of factor-Xa (FXa) that neutralizes circulating FXa inhibitors, has recently been approved as an antidote to apixaban and rivaroxaban in patients with life threatening haemorrhage, but its clinical use is hindered by its limited availability, the prohibitive cost, and safety concern about possible procoagulant effect 213 . 4-factor PCC at fixed dose (2000 IU) may represent an alternative to andexanet alpha, with lower thromboembolic risk, but uncertain efficacy 214 215 216 .
Correction of coagulopathy, when required, should not delay urgent endoscopic or radiological interventions when indicated, as these procedures can be safely and effectively performed at therapeutic levels of anticoagulation in the context of acute haemorrhage 217 218 .
After bleeding cessation, observational studies 219 220 221 222 and two meta-analyses 223 224 consistently indicate there is a net clinical benefit of restarting anticoagulation, due to a reduced risk of thromboembolism and death, despite an increased risk of rebleeding. However, evidence on the optimal timing for restarting anticoagulation is limited. A single retrospective study indicates an excess risk of GI bleeding and of thromboembolism for an interval of warfarin interruption shorter than 7 days and longer than 30 days, respectively 219 . Since there is a trend toward a reduced incidence of thromboembolic events the earlier warfarin is introduced, it is reasonable to restart warfarin as soon as possible by day seven following its interruption. Data on the optimal timing of DOAC resumption are lacking. In analogy with warfarin, restarting DOACs as soon as possible by day seven after their interruption seems reasonable in most cases. The DOAC rapid onset of action, resulting in a full anticoagulation within 2–4 hours, warrants some caution with an earlier resumption.
In patients at very high thrombotic risk (e. g. prosthetic metal heart valve in mitral position), cardiology societies recommend an earlier resumption of anticoagulation with UFH in those at highest risk, rapid titration of prophylactic doses of LMWH to therapeutic doses within 48–72 hours 209 210 . The choice of strategy should be discussed with the patient’s cardiologist. If the risk of restarting anticoagulation is estimated to outweigh the benefits, a consultation with the referral specialist (hematologist, neurologist, and/or cardiologist) is advisable, and alternatives to anticoagulation, such as a left atrial appendage occlusion in AF, or inferior vena cava filter for acute deep venous thrombosis (DVT), may be considered 209 210 .
Disclaimer
These joint BSG and ESGE guidelines represent a consensus of best practice based on the available evidence at the time of preparation. They may not apply in all situations and should be interpreted in the light of specific clinical situations and resource availability. Further controlled clinical studies may be needed to clarify aspects of these statements, and revision may be necessary as new data appear. Clinical consideration may justify a course of action at variance to these recommendations, but we suggest that reasons for this are documented in the medical record. BSG and ESGE guidelines are intended to be an educational device to provide information that may assist endoscopists in providing care to patients. They are not rules and should not be construed as establishing a legal standard of care or as encouraging, advocating, requiring, or discouraging any particular treatment.
Acknowledgements
We gratefully acknowledge the contribution of Dr Sanne Hoogenboom from the Academic Medical Centre, Amsterdam, who undertook the literature searches for risks associated with endoscopic dilatation and endoscopic stenting. We are indebted to Professor Tony Gershlick, an interventional cardiologist who made a major contribution to the antiplatelet advice in the 2008 and 2016 versions of this guideline, and which underpin the recommendations in this update. Professor Gershlick sadly died due to Covid-19 before being able to contribute to this updated document.
Footnotes
Competing interests Dr Radaelli has received speaker fees from Bristol-Meyers Squibb, Pfizer and Boehringer Ingelheim. Dr Alikhan has received fees from Alexion, Bayer, Boehringer Ingelheim, Bristol-Meyers Squibb, Daiichi, Pfizer and Portola. Dr Lester has received speaker fees from Sanofi Aventis and Leo Pharma, speaker fees and advisory board fees from Bayer, Daichii Sankyo, Pfizer, and Boehringer Ingelheim, and support to attend a scientific meeting from Boehringer Ingelheim. Prof Van- Hooft has received departmental research grant support from Cook Medical and Abbott, lecture fees from Medtronics and Cook Medical, and consultancy fees from Boston Scientific and Olympus. None of the remaining authors have competing interests to declare.
Publication information.
This article is published simultaneously in the journals Endoscopy and Gut. © 2021. European Society of Gastrointestinal Endoscopy, British Society of Gastroenterology, the Author(s) or their employer(s). This article is published by Thieme.
Supplementary material :
References
- 1.Veitch A M, Vanbiervliet G, Gershlick A H et al. Endoscopy in patients on antiplatelet or anticoagulant therapy, including direct oral anticoagulants: British Society of Gastroenterology (BSG) and European Society of Gastrointestinal Endoscopy (ESGE) guidelines. Gut. 2016;65:374–389. doi: 10.1136/gutjnl-2015-311110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Veitch A M, Vanbiervliet G, Gershlick A H et al. Endoscopy in patients on antiplatelet or anticoagulant therapy, including direct oral anticoagulants: British Society of Gastroenterology (BSG) and European Society of Gastrointestinal Endoscopy (ESGE) guidelines. Endoscopy. 2016;48:385–402. doi: 10.1055/s-0042-102652. [DOI] [PubMed] [Google Scholar]
- 3.AGREE Next Steps Consortium The AGREE II Instrument (Electronic version) 2009. Available at (Accessed May 2021):http://www.agreetrust.org
- 4.Guyatt G H, Oxman A D, Vist G E et al. GRADE: an emerging consensus on rating quality of evidence and strength of recommendations. Bmj. 2008;336:924–926. doi: 10.1136/bmj.39489.470347.AD. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Douketis J D, Spyropoulos A C, Duncan J et al. Perioperative Management of Patients With Atrial Fibrillation Receiving a Direct Oral Anticoagulant. JAMA internal medicine. 2019;179:1469–1478. doi: 10.1001/jamainternmed.2019.2431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Biondi-Zoccai G G, Lotrionte M, Agostoni P et al. A systematic review and meta-analysis on the hazards of discontinuing or not adhering to aspirin among 50,279 patients at risk for coronary artery disease. European heart journal. 2006;27:2667–2674. doi: 10.1093/eurheartj/ehl334. [DOI] [PubMed] [Google Scholar]
- 7.Maulaz A B, Bezerra D C, Michel P et al. Effect of discontinuing aspirin therapy on the risk of brain ischemic stroke. Archives of neurology. 2005;62:1217–1220. doi: 10.1001/archneur.62.8.1217. [DOI] [PubMed] [Google Scholar]
- 8.Oscarsson A, Gupta A, Fredrikson M et al. To continue or discontinue aspirin in the perioperative period: a randomized, controlled clinical trial. British journal of anaesthesia. 2010;104:305–312. doi: 10.1093/bja/aeq003. [DOI] [PubMed] [Google Scholar]
- 9.Yasuda S, Kaikita K, Akao M et al. Antithrombotic Therapy for Atrial Fibrillation with Stable Coronary Disease. The New England journal of medicine. 2019;381:1103–1113. doi: 10.1056/NEJMoa1904143. [DOI] [PubMed] [Google Scholar]
- 10.Knuuti J, Wijns W, Saraste A et al. 2019 ESC Guidelines for the diagnosis and management of chronic coronary syndromes. European heart journal. 2020;41:407–477. doi: 10.1093/eurheartj/ehz425. [DOI] [PubMed] [Google Scholar]
- 11.Gori T, Polimeni A, Indolfi C et al. Predictors of stent thrombosis and their implications for clinical practice. Nature reviews Cardiology. 2019;16:243–256. doi: 10.1038/s41569-018-0118-5. [DOI] [PubMed] [Google Scholar]
- 12.Lopes R D, Heizer G, Aronson R et al. Antithrombotic Therapy after Acute Coronary Syndrome or PCI in Atrial Fibrillation. The New England journal of medicine. 2019;380:1509–1524. doi: 10.1056/NEJMoa1817083. [DOI] [PubMed] [Google Scholar]
- 13.Dasa O, Pepine C J, Pearson T A. Aspirin in Primary Prevention: What Changed? A Critical Appraisal of Current Evidence. The American journal of cardiology. 2021;141:38–48. doi: 10.1016/j.amjcard.2020.11.014. [DOI] [PubMed] [Google Scholar]
- 14.Budaj A, Yusuf S, Mehta S R et al. Benefit of clopidogrel in patients with acute coronary syndromes without ST-segment elevation in various risk groups. Circulation. 2002;106:1622–1666. doi: 10.1161/01.cir.0000029926.71825.e2. [DOI] [PubMed] [Google Scholar]
- 15.Korte W, Cattaneo M, Chassot P G et al. Peri-operative management of antiplatelet therapy in patients with coronary artery disease: joint position paper by members of the working group on Perioperative Haemostasis of the Society on Thrombosis and Haemostasis Research (GTH), the working group on Perioperative Coagulation of the Austrian Society for Anesthesiology, Resuscitation and Intensive Care (OGARI) and the Working Group Thrombosis of the European Society for Cardiology (ESC) Thrombosis and haemostasis. 2011;105:743–749. doi: 10.1160/TH10-04-0217. [DOI] [PubMed] [Google Scholar]
- 16.Iakovou I, Schmidt T, Bonizzoni E et al. Incidence, predictors, and outcome of thrombosis after successful implantation of drug-eluting stents. Jama. 2005;293:2126–2130. doi: 10.1001/jama.293.17.2126. [DOI] [PubMed] [Google Scholar]
- 17.Olier I, Sirker A, Hildick-Smith D JR et al. Association of different antiplatelet therapies with mortality after primary percutaneous coronary intervention. Heart. 2018;104:1683–1690. doi: 10.1136/heartjnl-2017-312366. [DOI] [PubMed] [Google Scholar]
- 18.Schupke S, Neumann F J, Menichelli M et al. Ticagrelor or Prasugrel in Patients with Acute Coronary Syndromes. The New England journal of medicine. 2019;381:1524–1534. doi: 10.1056/NEJMoa1908973. [DOI] [PubMed] [Google Scholar]
- 19.Valgimigli M, Bueno H, Byrne R A et al. 2017 ESC focused update on dual antiplatelet therapy in coronary artery disease developed in collaboration with EACTS: The Task Force for dual antiplatelet therapy in coronary artery disease of the European Society of Cardiology (ESC) and of the European Association for Cardio-Thoracic Surgery (EACTS) European heart journal. 2018;39:213–260. doi: 10.1093/eurheartj/ehx419. [DOI] [PubMed] [Google Scholar]
- 20.Urban P, Meredith I T, Abizaid A et al. Polymer-free Drug-Coated Coronary Stents in Patients at High Bleeding Risk. The New England journal of medicine. 2015;373:2038–2047. doi: 10.1056/NEJMoa1503943. [DOI] [PubMed] [Google Scholar]
- 21.Kandzari D E, Kirtane A J, Windecker S et al. One-Month Dual Antiplatelet Therapy Following Percutaneous Coronary Intervention With Zotarolimus-Eluting Stents in High-Bleeding-Risk Patients. Circulation Cardiovascular interventions. 2020;13:e009565. doi: 10.1161/CIRCINTERVENTIONS.120.009565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Varenne O, Cook S, Sideris G et al. Drug-eluting stents in elderly patients with coronary artery disease (SENIOR): a randomised single-blind trial. Lancet. 2018;391:41–50. doi: 10.1016/S0140-6736(17)32713-7. [DOI] [PubMed] [Google Scholar]
- 23.Watanabe H, Domei T, Morimoto T et al. Effect of 1-Month Dual Antiplatelet Therapy Followed by Clopidogrel vs 12-Month Dual Antiplatelet Therapy on Cardiovascular and Bleeding Events in Patients Receiving PCI: The STOPDAPT-2 Randomized Clinical Trial. Jama. 2019;321:2414–2427. doi: 10.1001/jama.2019.8145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cannon C P, Bhatt D L, Oldgren J et al. Dual Antithrombotic Therapy with Dabigatran after PCI in Atrial Fibrillation. The New England journal of medicine. 2017;377:1513–1524. doi: 10.1056/NEJMoa1708454. [DOI] [PubMed] [Google Scholar]
- 25.Collet J P, Thiele H, Barbato E et al. 2020 ESC Guidelines for the management of acute coronary syndromes in patients presenting without persistent ST-segment elevation. European heart journal. 2021;42:1289–1367. doi: 10.1093/eurheartj/ehaa575. [DOI] [PubMed] [Google Scholar]
- 26.Ibanez B, James S, Agewall S et al. 2017 ESC Guidelines for the management of acute myocardial infarction in patients presenting with ST-segment elevation: The Task Force for the management of acute myocardial infarction in patients presenting with ST-segment elevation of the European Society of Cardiology (ESC) European heart journal. 2018;39:119–177. doi: 10.1093/eurheartj/ehx393. [DOI] [PubMed] [Google Scholar]
- 27.Schulman S, Carrier M, Lee A Y et al. Perioperative Management of Dabigatran: A Prospective Cohort Study. Circulation. 2015;132:167–173. doi: 10.1161/CIRCULATIONAHA.115.015688. [DOI] [PubMed] [Google Scholar]
- 28.Passaglia L G, de Barros G M, de Sousa M R. Early postoperative bridging anticoagulation after mechanical heart valve replacement: a systematic review and meta-analysis. Journal of thrombosis and haemostasis : JTH. 2015;13:1557–1567. doi: 10.1111/jth.13047. [DOI] [PubMed] [Google Scholar]
- 29.Spyropoulos A C, Turpie A G, Dunn A S et al. Perioperative bridging therapy with unfractionated heparin or low-molecular-weight heparin in patients with mechanical prosthetic heart valves on long-term oral anticoagulants (from the REGIMEN Registry) The American journal of cardiology. 2008;102:883–889. doi: 10.1016/j.amjcard.2008.05.042. [DOI] [PubMed] [Google Scholar]
- 30.Otto C M, Nishimura R A, Bonow R O et al. 2020 ACC/AHA Guideline for the Management of Patients With Valvular Heart Disease: A Report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice Guidelines. Circulation. 2021;143:e72–e227. doi: 10.1161/CIR.0000000000000923. [DOI] [PubMed] [Google Scholar]
- 31.Keeling D, Tait R C, Watson H et al. Peri-operative management of anticoagulation and antiplatelet therapy. British journal of haematology. 2016;175:602–613. doi: 10.1111/bjh.14344. [DOI] [PubMed] [Google Scholar]
- 32.Nishimura R A, Otto C M, Bonow R O et al. 2017 AHA/ACC Focused Update of the 2014 AHA/ACC Guideline for the Management of Patients With Valvular Heart Disease: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Journal of the American College of Cardiology. 2017;70:252–289. doi: 10.1016/j.jacc.2017.03.011. [DOI] [PubMed] [Google Scholar]
- 33.Baumgartner H, Falk V, Bax J J et al. 2017 ESC/EACTS Guidelines for the management of valvular heart disease. European heart journal. 2017;38:2739–2791. doi: 10.1093/eurheartj/ehx391. [DOI] [PubMed] [Google Scholar]
- 34.Douketis J D, Spyropoulos A C, Kaatz S et al. Perioperative Bridging Anticoagulation in Patients with Atrial Fibrillation. The New England journal of medicine. 2015;373:823–833. doi: 10.1056/NEJMoa1501035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Acosta R D, Abraham N S, Chandrasekhara V et al. The management of antithrombotic agents for patients undergoing GI endoscopy. Gastrointestinal endoscopy. 2016;83:3–16. doi: 10.1016/j.gie.2015.09.035. [DOI] [PubMed] [Google Scholar]
- 36.Chan F KL, Goh K L, Reddy N et al. Management of patients on antithrombotic agents undergoing emergency and elective endoscopy: joint Asian Pacific Association of Gastroenterology (APAGE) and Asian Pacific Society for Digestive Endoscopy (APSDE) practice guidelines. Gut. 2018;67:405–417. doi: 10.1136/gutjnl-2017-315131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Beyer-Westendorf J, Gelbricht V, Forster K et al. Peri-interventional management of novel oral anticoagulants in daily care: results from the prospective Dresden NOAC registry. European heart journal. 2014;35:1888–1896. doi: 10.1093/eurheartj/eht557. [DOI] [PubMed] [Google Scholar]
- 38.Douketis J D, Healey J S, Brueckmann M et al. Perioperative bridging anticoagulation during dabigatran or warfarin interruption among patients who had an elective surgery or procedure. Substudy of the RE-LY trial. Thrombosis and haemostasis. 2015;113:625–632. doi: 10.1160/TH14-04-0305. [DOI] [PubMed] [Google Scholar]
- 39.Nagata N, Yasunaga H, Matsui H et al. Therapeutic endoscopy-related GI bleeding and thromboembolic events in patients using warfarin or direct oral anticoagulants: results from a large nationwide database analysis. Gut. 2018;67:1805–1812. doi: 10.1136/gutjnl-2017-313999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ishigami H, Arai M, Matsumura T et al. Heparin-bridging therapy is associated with a high risk of post-polypectomy bleeding regardless of polyp size. Digestive endoscopy : official journal of the Japan Gastroenterological Endoscopy Society. 2017;29:65–72. doi: 10.1111/den.12692. [DOI] [PubMed] [Google Scholar]
- 41.Lin D, Soetikno R M, McQuaid K et al. Risk factors for postpolypectomy bleeding in patients receiving anticoagulation or antiplatelet medications. Gastrointestinal endoscopy. 2018;87:1106–1113. doi: 10.1016/j.gie.2017.11.024. [DOI] [PubMed] [Google Scholar]
- 42.Ono S, Ishikawa M, Matsuda K et al. Clinical impact of the perioperative management of oral anticoagulants in bleeding after colonic endoscopic mucosal resection. BMC gastroenterology. 2019;19:206. doi: 10.1186/s12876-019-1124-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Macrae F A, Tan K G, Williams C B. Towards safer colonoscopy: a report on the complications of 5000 diagnostic or therapeutic colonoscopies. Gut. 1983;24:376–383. doi: 10.1136/gut.24.5.376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Reiertsen O, Skjoto J, Jacobsen C D et al. Complications of fiberoptic gastrointestinal endoscopy--five yearsʼ experience in a central hospital. Endoscopy. 1987;19:1–6. doi: 10.1055/s-2007-1013011. [DOI] [PubMed] [Google Scholar]
- 45.Rogers B H, Silvis S E, Nebel O T et al. Complications of flexible fiberoptic colonoscopy and polypectomy. Gastrointestinal endoscopy. 1975;22:73–77. doi: 10.1016/s0016-5107(75)73705-7. [DOI] [PubMed] [Google Scholar]
- 46.Vu C K, Korman M G, Bejer I et al. Gastrointestinal bleeding after cold biopsy. The American journal of gastroenterology. 1998;93:1141–1143. doi: 10.1111/j.1572-0241.1998.346_e.x. [DOI] [PubMed] [Google Scholar]
- 47.Wexner S D, Garbus J E, Singh J J et al. A prospective analysis of 13,580 colonoscopies. Reevaluation of credentialing guidelines. Surgical endoscopy. 2001;15:251–261. doi: 10.1007/s004640080147. [DOI] [PubMed] [Google Scholar]
- 48.Ono S, Fujishiro M, Kodashima S et al. Evaluation of safety of endoscopic biopsy without cessation of antithrombotic agents in Japan. Journal of gastroenterology. 2012;47:770–774. doi: 10.1007/s00535-012-0538-7. [DOI] [PubMed] [Google Scholar]
- 49.Whitson M J, Dikman A E, von Althann C et al. Is gastroduodenal biopsy safe in patients receiving aspirin and clopidogrel? a prospective, randomized study involving 630 biopsies. Journal of clinical gastroenterology. 2011;45:228–233. doi: 10.1097/MCG.0b013e3181eb5efd. [DOI] [PubMed] [Google Scholar]
- 50.Yuki T, Ishihara S, Yashima K et al. Bleeding Risk Related to Upper Gastrointestinal Endoscopic Biopsy in Patients Receiving Antithrombotic Therapy: A Multicenter Prospective Observational Study. Curr Ther Res Clin Exp. 2017;84:32–36. doi: 10.1016/j.curtheres.2017.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Heublein V, Pannach S, Daschkow K et al. Gastrointestinal endoscopy in patients receiving novel direct oral anticoagulants: results from the prospective Dresden NOAC registry. Journal of gastroenterology. 2018;53:236–246. doi: 10.1007/s00535-017-1346-x. [DOI] [PubMed] [Google Scholar]
- 52.Mannath J, Subramanian V, Kaye P V et al. Life-threatening bleeding following Barrett's surveillance biopsies. Endoscopy. 2010;42 02:E211–212. doi: 10.1055/s-0030-1255717. [DOI] [PubMed] [Google Scholar]
- 53.Radaelli F, Fuccio L, Paggi S et al. Periendoscopic management of direct oral anticoagulants: a prospective cohort study. Gut. 2019;68:969–976. doi: 10.1136/gutjnl-2018-316385. [DOI] [PubMed] [Google Scholar]
- 54.Baglin T. Clinical use of new oral anticoagulant drugs: dabigatran and rivaroxaban. British journal of haematology. 2013;163:160–167. doi: 10.1111/bjh.12502. [DOI] [PubMed] [Google Scholar]
- 55.Bowles C J, Leicester R, Romaya C et al. A prospective study of colonoscopy practice in the UK today: are we adequately prepared for national colorectal cancer screening tomorrow? Gut. 2004;53:277–283. doi: 10.1136/gut.2003.016436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Gibbs D H, Opelka F G, Beck D E et al. Postpolypectomy colonic hemorrhage. Diseases of the colon and rectum. 1996;39:806–810. doi: 10.1007/BF02054448. [DOI] [PubMed] [Google Scholar]
- 57.Rosen L, Bub D S, Reed J F et al. Hemorrhage following colonoscopic polypectomy. Diseases of the colon and rectum. 1993;36:1126–1131. doi: 10.1007/BF02052261. [DOI] [PubMed] [Google Scholar]
- 58.Sieg A, Hachmoeller-Eisenbach U, Eisenbach T. Prospective evaluation of complications in outpatient GI endoscopy: a survey among German gastroenterologists. Gastrointestinal endoscopy. 2001;53:620–627. doi: 10.1067/mge.2001.114422. [DOI] [PubMed] [Google Scholar]
- 59.Gavin D R, Valori R M, Anderson J T et al. The national colonoscopy audit: a nationwide assessment of the quality and safety of colonoscopy in the UK. Gut. 2013;62:242–249. doi: 10.1136/gutjnl-2011-301848. [DOI] [PubMed] [Google Scholar]
- 60.Rutter M D, Nickerson C, Rees C J et al. Risk factors for adverse events related to polypectomy in the English Bowel Cancer Screening Programme. Endoscopy. 2014;46:90–97. doi: 10.1055/s-0033-1344987. [DOI] [PubMed] [Google Scholar]
- 61.Sawhney M S, Salfiti N, Nelson D B et al. Risk factors for severe delayed postpolypectomy bleeding. Endoscopy. 2008;40:115–119. doi: 10.1055/s-2007-966959. [DOI] [PubMed] [Google Scholar]
- 62.Kim H S, Kim T I, Kim W H et al. Risk factors for immediate postpolypectomy bleeding of the colon: a multicenter study. The American journal of gastroenterology. 2006;101:1333–1341. doi: 10.1111/j.1572-0241.2006.00638.x. [DOI] [PubMed] [Google Scholar]
- 63.Burgess N G, Metz A J, Williams S J et al. Risk factors for intraprocedural and clinically significant delayed bleeding after wide-field endoscopic mucosal resection of large colonic lesions. Clin Gastroenterol Hepatol. 2014;12:651–661 e1-3. doi: 10.1016/j.cgh.2013.09.049. [DOI] [PubMed] [Google Scholar]
- 64.Corte C J, Burger D C, Horgan G et al. Postpolypectomy haemorrhage following removal of large polyps using mechanical haemostasis or epinephrine: a meta-analysis. United European gastroenterology journal. 2014;2:123–130. doi: 10.1177/2050640614522619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Li L Y, Liu Q S, Li L et al. A meta-analysis and systematic review of prophylactic endoscopic treatments for postpolypectomy bleeding. International journal of colorectal disease. 2011;26:709–719. doi: 10.1007/s00384-011-1141-8. [DOI] [PubMed] [Google Scholar]
- 66.Quintanilla E, Castro J L, Rabago L R et al. Is the use of prophylactic hemoclips in the endoscopic resection of large pedunculated polyps useful? A prospective and randomized study. Journal of interventional gastroenterology. 2012;2:183–188. doi: 10.4161/jig.23741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Kawamura T, Takeuchi Y, Asai S et al. A comparison of the resection rate for cold and hot snare polypectomy for 4-9 mm colorectal polyps: a multicentre randomised controlled trial (CRESCENT study) Gut. 2018;67:1950–1957. doi: 10.1136/gutjnl-2017-314215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Arimoto J, Chiba H, Ashikari K et al. Safety of Cold Snare Polypectomy in Patients Receiving Treatment with Antithrombotic Agents. Digestive diseases and sciences. 2019;64:3247–3255. doi: 10.1007/s10620-019-5469-1. [DOI] [PubMed] [Google Scholar]
- 69.Friedland S, Sedehi D, Soetikno R. Colonoscopic polypectomy in anticoagulated patients. World journal of gastroenterology: WJG. 2009;15:1973–1976. doi: 10.3748/wjg.15.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Horiuchi A, Nakayama Y, Kajiyama M et al. Removal of small colorectal polyps in anticoagulated patients: a prospective randomized comparison of cold snare and conventional polypectomy. Gastrointestinal endoscopy. 2014;79:417–423. doi: 10.1016/j.gie.2013.08.040. [DOI] [PubMed] [Google Scholar]
- 71.Takeuchi Y, Mabe K, Shimodate Y et al. Continuous Anticoagulation and Cold Snare Polypectomy Versus Heparin Bridging and Hot Snare Polypectomy in Patients on Anticoagulants With Subcentimeter Polyps: A Randomized Controlled Trial. Annals of internal medicine. 2019;171:229–237. doi: 10.7326/M19-0026. [DOI] [PubMed] [Google Scholar]
- 72.Hui C K, Lai K C, Yuen M F et al. Does withholding aspirin for one week reduce the risk of post-sphincterotomy bleeding? Alimentary pharmacology & therapeutics. 2002;16:929–936. doi: 10.1046/j.1365-2036.2002.01251.x. [DOI] [PubMed] [Google Scholar]
- 73.Shiffman M L, Farrel M T, Yee Y S. Risk of bleeding after endoscopic biopsy or polypectomy in patients taking aspirin or other NSAIDS. Gastrointestinal endoscopy. 1994;40:458–462. doi: 10.1016/s0016-5107(94)70210-1. [DOI] [PubMed] [Google Scholar]
- 74.Yousfi M, Gostout C J, Baron T H et al. Postpolypectomy lower gastrointestinal bleeding: potential role of aspirin. The American journal of gastroenterology. 2004;99:1785–1789. doi: 10.1111/j.1572-0241.2004.30368.x. [DOI] [PubMed] [Google Scholar]
- 75.Chan F KL, Kyaw M H, Hsiang J C et al. Risk of Postpolypectomy Bleeding With Uninterrupted Clopidogrel Therapy in an Industry-Independent, Double-Blind, Randomized Trial. Gastroenterology. 2019;156:918–925 e1. doi: 10.1053/j.gastro.2018.10.036. [DOI] [PubMed] [Google Scholar]
- 76.Li D F, Chang X, Fang X et al. Colonoscopic post-polypectomy bleeding in patients on uninterruptedclopidogrel therapy: A systematic review and meta-analysis. Experimental and therapeutic medicine. 2020;19:3211–3218. doi: 10.3892/etm.2020.8597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Cipolletta L, Rotondano G, Bianco M A et al. Endoscopic resection for superficial colorectal neoplasia in Italy: a prospective multicentre study. Digestive and liver disease : official journal of the Italian Society of Gastroenterology and the Italian Association for the Study of the Liver. 2014;46:146–151. doi: 10.1016/j.dld.2013.09.019. [DOI] [PubMed] [Google Scholar]
- 78.Heresbach D, Kornhauser R, Seyrig J A et al. A national survey of endoscopic mucosal resection for superficial gastrointestinal neoplasia. Endoscopy. 2010;42:806–813. doi: 10.1055/s-0030-1255715. [DOI] [PubMed] [Google Scholar]
- 79.Elliott T R, Tsiamoulos Z P, Thomas-Gibson S et al. Factors associated with delayed bleeding after resection of large nonpedunculated colorectal polyps. Endoscopy. 2018;50:790–799. doi: 10.1055/a-0577-3206. [DOI] [PubMed] [Google Scholar]
- 80.Qumseya B J, Wolfsen C, Wang Y et al. Factors associated with increased bleeding post-endoscopic mucosal resection. Journal of digestive diseases. 2013;14:140–146. doi: 10.1111/1751-2980.12002. [DOI] [PubMed] [Google Scholar]
- 81.Namasivayam V, Prasad G A, Lutzke L S et al. The risk of endoscopic mucosal resection in the setting of clopidogrel use. ISRN gastroenterology. 2014;2014:494157. doi: 10.1155/2014/494157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Nonaka S, Oda I, Tada K et al. Clinical outcome of endoscopic resection for nonampullary duodenal tumors. Endoscopy. 2015;47:129–135. doi: 10.1055/s-0034-1390774. [DOI] [PubMed] [Google Scholar]
- 83.Liaquat H, Rohn E, Rex D K. Prophylactic clip closure reduced the risk of delayed postpolypectomy hemorrhage: experience in 277 clipped large sessile or flat colorectal lesions and 247 control lesions. Gastrointestinal endoscopy. 2013;77:401–407. doi: 10.1016/j.gie.2012.10.024. [DOI] [PubMed] [Google Scholar]
- 84.Feagins L A, Nguyen A D, Iqbal R et al. The prophylactic placement of hemoclips to prevent delayed post-polypectomy bleeding: an unnecessary practice? A case control study. Digestive diseases and sciences. 2014;59:823–828. doi: 10.1007/s10620-014-3055-0. [DOI] [PubMed] [Google Scholar]
- 85.Shioji K, Suzuki Y, Kobayashi M et al. Prophylactic clip application does not decrease delayed bleeding after colonoscopic polypectomy. Gastrointestinal endoscopy. 2003;57:691–694. doi: 10.1067/mge.2003.193. [DOI] [PubMed] [Google Scholar]
- 86.Parikh N D, Zanocco K, Keswani R N et al. A cost-efficacy decision analysis of prophylactic clip placement after endoscopic removal of large polyps. Clinical gastroenterology and hepatology : the official clinical practice journal of the American Gastroenterological Association. 2013;11:1319–1324. doi: 10.1016/j.cgh.2012.12.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Seo M, Song E M, Cho J W et al. A risk-scoring model for the prediction of delayed bleeding after colorectal endoscopic submucosal dissection. Gastrointestinal endoscopy. 2019;89:990–998 e2. doi: 10.1016/j.gie.2018.11.029. [DOI] [PubMed] [Google Scholar]
- 88.Harada H, Suehiro S, Murakami D et al. Feasibility of gastric endoscopic submucosal dissection with continuous low-dose aspirin for patients receiving dual antiplatelet therapy. World journal of gastroenterology : WJG. 2019;25:457–468. doi: 10.3748/wjg.v25.i4.457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Horikawa Y, Mizutamari H, Mimori N et al. Effect of Continued Administration of Low-dose Aspirin for Intraoperative Bleeding Control in Gastric Endoscopic Submucosal Dissection. Digestion. 2019;100:139–146. doi: 10.1159/000494250. [DOI] [PubMed] [Google Scholar]
- 90.Igarashi K, Takizawa K, Kakushima N et al. Should antithrombotic therapy be stopped in patients undergoing gastric endoscopic submucosal dissection? Surgical endoscopy. 2017;31:1746–1753. doi: 10.1007/s00464-016-5167-4. [DOI] [PubMed] [Google Scholar]
- 91.Kono Y, Obayashi Y, Baba Y et al. Postoperative bleeding risk after gastric endoscopic submucosal dissection during antithrombotic drug therapy. Journal of gastroenterology and hepatology. 2018;33:453–460. doi: 10.1111/jgh.13872. [DOI] [PubMed] [Google Scholar]
- 92.Oh S, Kim S G, Kim J et al. Continuous Use of Thienopyridine May Be as Safe as Low-Dose Aspirin in Endoscopic Resection of Gastric Tumors. Gut and liver. 2018;12:393–401. doi: 10.5009/gnl17384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.So S, Ahn J Y, Kim N et al. Comparison of the effects of antithrombotic therapy on delayed bleeding after gastric endoscopic resection: a propensity score-matched case-control study. Gastrointestinal endoscopy. 2019;89:277–285 e2. doi: 10.1016/j.gie.2018.08.028. [DOI] [PubMed] [Google Scholar]
- 94.Dong J, Wei K, Deng J et al. Effects of antithrombotic therapy on bleeding after endoscopic submucosal dissection. Gastrointestinal endoscopy. 2017;86:807–816. doi: 10.1016/j.gie.2017.07.017. [DOI] [PubMed] [Google Scholar]
- 95.Jaruvongvanich V, Sempokuya T, Wijarnpreecha K et al. Continued versus interrupted aspirin use and bleeding risk after endoscopic submucosal dissection of gastric neoplasms: a meta-analysis. Annals of gastroenterology : quarterly publication of the Hellenic Society of Gastroenterology. 2018;31:344–349. doi: 10.20524/aog.2018.0251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Tomida H, Yoshio T, Igarashi K et al. Influence of anticoagulants on the risk of delayed bleeding after gastric endoscopic submucosal dissection: a multicenter retrospective study. Gastric cancer : official journal of the International Gastric Cancer Association and the Japanese Gastric Cancer Association. 2021;24:179–189. doi: 10.1007/s10120-020-01105-0. [DOI] [PubMed] [Google Scholar]
- 97.Kubo K, Kato M, Mabe K. Risk Factors for Delayed Bleeding after Therapeutic Gastrointestinal Endoscopy in Patients Receiving Oral Anticoagulants: A Multicenter Retrospective Study. Digestion. 2021;102:161–169. doi: 10.1159/000502952. [DOI] [PubMed] [Google Scholar]
- 98.Harada H, Nakahara R, Murakami D et al. The effect of anticoagulants on delayed bleeding after colorectal endoscopic submucosal dissection. Surgical endoscopy. 2020;34:3330–3337. doi: 10.1007/s00464-019-07101-5. [DOI] [PubMed] [Google Scholar]
- 99.Yamashita K, Oka S, Tanaka S et al. Use of anticoagulants increases risk of bleeding after colorectal endoscopic submucosal dissection. Endosc Int Open. 2018;6:E857–E864. doi: 10.1055/a-0593-5788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Li P, Ma B, Gong S et al. Endoscopic submucosal tunnel dissection for superficial esophageal neoplastic lesions: a meta-analysis. Surgical endoscopy. 2020;34:1214–1223. doi: 10.1007/s00464-019-06875-y. [DOI] [PubMed] [Google Scholar]
- 101.Yang D, Zou F, Xiong S et al. Endoscopic submucosal dissection for early Barrett's neoplasia: a meta-analysis. Gastrointestinal endoscopy. 2018;87:1383–1393. doi: 10.1016/j.gie.2017.09.038. [DOI] [PubMed] [Google Scholar]
- 102.Hanada Y, Wang K K. Safety and feasibility of same-day discharge after esophageal endoscopic submucosal dissection. Gastrointestinal endoscopy. 2021;93:853–860. doi: 10.1016/j.gie.2020.07.037. [DOI] [PubMed] [Google Scholar]
- 103.Andriulli A, Loperfido S, Napolitano G et al. Incidence rates of post-ERCP complications: a systematic survey of prospective studies. The American journal of gastroenterology. 2007;102:1781–1788. doi: 10.1111/j.1572-0241.2007.01279.x. [DOI] [PubMed] [Google Scholar]
- 104.Dumonceau J M, Kapral C, Aabakken L et al. ERCP-related adverse events: European Society of Gastrointestinal Endoscopy (ESGE) Guideline. Endoscopy. 2020;52:127–149. doi: 10.1055/a-1075-4080. [DOI] [PubMed] [Google Scholar]
- 105.Manes G, Paspatis G, Aabakken L et al. Endoscopic management of common bile duct stones: European Society of Gastrointestinal Endoscopy (ESGE) guideline. Endoscopy. 2019;51:472–491. doi: 10.1055/a-0862-0346. [DOI] [PubMed] [Google Scholar]
- 106.Li D F, Yang M F, Chang X et al. Endocut Versus Conventional Blended Electrosurgical Current for Endoscopic Biliary Sphincterotomy: A Meta-Analysis of Complications. Digestive diseases and sciences. 2019;64:2088–2094. doi: 10.1007/s10620-019-05513-w. [DOI] [PubMed] [Google Scholar]
- 107.Rey J F, Beilenhoff U, Neumann C S et al. European Society of Gastrointestinal Endoscopy (ESGE) guideline: the use of electrosurgical units. Endoscopy. 2010;42:764–772. doi: 10.1055/s-0030-1255594. [DOI] [PubMed] [Google Scholar]
- 108.Verma D, Kapadia A, Adler D G. Pure versus mixed electrosurgical current for endoscopic biliary sphincterotomy: a meta-analysis of adverse outcomes. Gastrointestinal endoscopy. 2007;66:283–290. doi: 10.1016/j.gie.2007.01.018. [DOI] [PubMed] [Google Scholar]
- 109.Abdel Samie A A, Sun R, Vohringer U et al. Safety of endoscopic sphincterotomy in patients under dual antiplatelet therapy. Hepato-gastroenterology. 2013;60:659–661. doi: 10.5754/hge12356. [DOI] [PubMed] [Google Scholar]
- 110.Koh H R, Park C H, Chung M W et al. Endoscopic retrograde cholangiopancreatography in patients with previous acute coronary syndrome. Gut and liver. 2014;8:674–679. doi: 10.5009/gnl13301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Lee M G, Kim J, Lee S H et al. Effect of sustained use of platelet aggregation inhibitors on post-endoscopic sphincterotomy bleeding. Digestive endoscopy : official journal of the Japan Gastroenterological Endoscopy Society. 2014;26:737–744. doi: 10.1111/den.12271. [DOI] [PubMed] [Google Scholar]
- 112.Onal I K, Parlak E, Akdogan M et al. Do aspirin and non-steroidal anti-inflammatory drugs increase the risk of post-sphincterotomy hemorrhage--a case-control study. Clinics and research in hepatology and gastroenterology. 2013;37:171–176. doi: 10.1016/j.clinre.2012.04.010. [DOI] [PubMed] [Google Scholar]
- 113.Nelson D B, Freeman M L. Major hemorrhage from endoscopic sphincterotomy: risk factor analysis. Journal of clinical gastroenterology. 1994;19:283–287. doi: 10.1097/00004836-199412000-00004. [DOI] [PubMed] [Google Scholar]
- 114.Rouquette O, Bommelaer G, Abergel A et al. Large balloon dilation post endoscopic sphincterotomy in removal of difficult common bile duct stones: a literature review. World journal of gastroenterology: WJG. 2014;20:7760–7766. doi: 10.3748/wjg.v20.i24.7760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Poincloux L, Rouquette O, Privat J et al. Large-balloon dilation of the sphincter of Oddi after sphincterotomy or infundibulotomy to extract large calculi or multiple common bile duct stones without using mechanical lithotripsy. Scandinavian journal of gastroenterology. 2013;48:246–251. doi: 10.3109/00365521.2011.647064. [DOI] [PubMed] [Google Scholar]
- 116.Spadaccini M, Fugazza A, Frazzoni L et al. Endoscopic papillectomy for neoplastic ampullary lesions: A systematic review with pooled analysis. United European gastroenterology journal. 2020;8:44–51. doi: 10.1177/2050640619868367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Kagawa K, Kubota K, Kurita Y et al. Effect of preventive closure of the frenulum after endoscopic papillectomy: A prospective pilot study. Journal of gastroenterology and hepatology. 2020;35:374–349. doi: 10.1111/jgh.14922. [DOI] [PubMed] [Google Scholar]
- 118.Iwasaki E, Minami K, Itoi T et al. Impact of electrical pulse cut mode during endoscopic papillectomy: Pilot randomized clinical trial. Digestive endoscopy : official journal of the Japan Gastroenterological Endoscopy Society. 2020;32:127–135. doi: 10.1111/den.13468. [DOI] [PubMed] [Google Scholar]
- 119.Nam K, Song T J, Kim R E et al. Usefulness of argon plasma coagulation ablation subsequent to endoscopic snare papillectomy for ampullary adenoma. Digestive endoscopy : official journal of the Japan Gastroenterological Endoscopy Society. 2018;30:485–492. doi: 10.1111/den.13008. [DOI] [PubMed] [Google Scholar]
- 120.Yang J K, Hyun J J, Lee T H. Can prophylactic argon plasma coagulation reduce delayed post-papillectomy bleeding? A prospective multicenter trial. Gastroenterol Hepatol. 2021;36:467–473. doi: 10.1111/jgh.15186. [DOI] [PubMed] [Google Scholar]
- 121.Chung K H, Lee S H, Choi J H et al. Effect of submucosal injection in endoscopic papillectomy of ampullary tumor: Propensity-score matching analysis. United European gastroenterology journal. 2018;6:576–585. doi: 10.1177/2050640617745459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Hyun J J, Lee T H, Park J S et al. A prospective multicenter study of submucosal injection to improve endoscopic snare papillectomy for ampullary adenoma. Gastrointestinal endoscopy. 2017;85:746–755. doi: 10.1016/j.gie.2016.08.013. [DOI] [PubMed] [Google Scholar]
- 123.Wang K X, Ben Q W, Jin Z D et al. Assessment of morbidity and mortality associated with EUS-guided FNA: a systematic review. Gastrointestinal endoscopy. 2011;73:283–290. doi: 10.1016/j.gie.2010.10.045. [DOI] [PubMed] [Google Scholar]
- 124.Li D F, Wang J Y, Yang M F et al. Factors associated with diagnostic accuracy, technical success and adverse events of endoscopic ultrasound-guided fine-needle biopsy: A systematic review and meta-analysis. Journal of gastroenterology and hepatology. 2020;35:1264–1276. doi: 10.1111/jgh.14999. [DOI] [PubMed] [Google Scholar]
- 125.Zhu H, Jiang F, Zhu J et al. Assessment of morbidity and mortality associated with endoscopic ultrasound-guided fine-needle aspiration for pancreatic cystic lesions: A systematic review and meta-analysis. Digestive endoscopy : official journal of the Japan Gastroenterological Endoscopy Society. 2017;29:667–675. doi: 10.1111/den.12851. [DOI] [PubMed] [Google Scholar]
- 126.Inoue T, Okumura F, Sano H et al. Bleeding risk of endoscopic ultrasound-guided fine-needle aspiration in patients undergoing antithrombotic therapy. Digestive endoscopy : official journal of the Japan Gastroenterological Endoscopy Society. 2017;29:91–96. doi: 10.1111/den.12687. [DOI] [PubMed] [Google Scholar]
- 127.Kawakubo K, Yane K, Eto K et al. A Prospective Multicenter Study Evaluating Bleeding Risk after Endoscopic Ultrasound-Guided Fine Needle Aspiration in Patients Prescribed Antithrombotic Agents. Gut and liver. 2018;12:353–359. doi: 10.5009/gnl17293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Kien-Fong Vu C, Chang F, Doig L et al. A prospective control study of the safety and cellular yield of EUS-guided FNA or Trucut biopsy in patients taking aspirin, nonsteroidal anti-inflammatory drugs, or prophylactic low molecular weight heparin. Gastrointestinal endoscopy. 2006;63:808–813. doi: 10.1016/j.gie.2005.09.033. [DOI] [PubMed] [Google Scholar]
- 129.Polmanee P, Hara K, Mizuno N et al. Outcomes of EUS-FNA in patients receiving antithrombotic therapy. Endosc Int Open. 2019;7:E15–E25. doi: 10.1055/a-0735-9107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Ogura T, Nishioka N, Ueno S et al. Antiplatelet and/or anticoagulant treatment does not increase hemorrhagic adverse events during EUS-guided biliary drainage. Gastrointestinal endoscopy. 2020;92:659–666. doi: 10.1016/j.gie.2020.04.038. [DOI] [PubMed] [Google Scholar]
- 131.Harvey P R, Coupland B, Mytton J et al. Outcomes of pneumatic dilatation and Heller's myotomy for achalasia in England between 2005 and 2016. Gut. 2019;68:1146–1151. doi: 10.1136/gutjnl-2018-316544. [DOI] [PubMed] [Google Scholar]
- 132.Qi L, He W, Yang J et al. Endoscopic balloon dilation and submucosal injection of triamcinolone acetonide in the treatment of esophageal stricture: A single-center retrospective study. Exp Ther Med. 2018;16:5248–5252. doi: 10.3892/etm.2018.6858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Vermeulen B D, de Zwart M, Sijben J et al. Risk factors and clinical outcomes of endoscopic dilation in benign esophageal strictures: a long-term follow-up study. Gastrointestinal endoscopy. 2020;91:1058–1066. doi: 10.1016/j.gie.2019.12.040. [DOI] [PubMed] [Google Scholar]
- 134.Pope E, Mansour M, Berseneva M et al. Outcomes and Predictors for Re-stenosis of Esophageal Stricture in Epidermolysis Bullosa: A Multicenter Cohort Study. J Pediatr Gastroenterol Nutr. 2020;71:310–314. doi: 10.1097/MPG.0000000000002820. [DOI] [PubMed] [Google Scholar]
- 135.Dougherty M, Runge T M, Eluri S et al. Esophageal dilation with either bougie or balloon technique as a treatment for eosinophilic esophagitis: a systematic review and meta-analysis. Gastrointest Endosc. 2017;86:581–591 e3. doi: 10.1016/j.gie.2017.04.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Runge T M, Eluri S, Cotton C C et al. Outcomes of Esophageal Dilation in Eosinophilic Esophagitis: Safety, Efficacy, and Persistence of the Fibrostenotic Phenotype. Am J Gastroenterol. 2016;111:206–213. doi: 10.1038/ajg.2015.399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Kochhar R, Malik S, Gupta P et al. Etiological spectrum and response to endoscopic balloon dilation in patients with benign gastric outlet obstruction. Gastrointest Endosc. 2018;88:899–908. doi: 10.1016/j.gie.2018.06.037. [DOI] [PubMed] [Google Scholar]
- 138.Baumann A J, Mramba L K, Hawkins R B et al. Endoscopic Dilation of Bariatric RNY Anastomotic Strictures: a Systematic Review and Meta-analysis. Obes Surg. 2018;28:4053–4063. doi: 10.1007/s11695-018-3491-6. [DOI] [PubMed] [Google Scholar]
- 139.Kishida Y, Kakushima N, Kawata N et al. Complications of endoscopic dilation for esophageal stenosis after endoscopic submucosal dissection of superficial esophageal cancer. Surg Endosc. 2014;29:2953–2959. doi: 10.1007/s00464-014-4028-2. [DOI] [PubMed] [Google Scholar]
- 140.van Halsema E E, Noordzij I C, van Berge Henegouwen MI et al. Endoscopic dilation of benign esophageal anastomotic strictures over 16 mm has a longer lasting effect. Surg Endosc. 2017;31:1871–1881. doi: 10.1007/s00464-016-5187-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Bettenworth D, Gustavsson A, Atreja A et al. A Pooled Analysis of Efficacy, Safety, and Long-term Outcome of Endoscopic Balloon Dilation Therapy for Patients with Stricturing Crohnʼs Disease. Inflamm Bowel Dis. 2017;23:133–142. doi: 10.1097/MIB.0000000000000988. [DOI] [PubMed] [Google Scholar]
- 142.Molina J C, Goudie E, Pollock C et al. Balloon Dilation for Endosonographic Staging in Esophageal Cancer - A Phase 1 Clinical Trial. Ann Thorac Surg. 2020;28:28. doi: 10.1016/j.athoracsur.2020.06.063. [DOI] [PubMed] [Google Scholar]
- 143.Lan N, Wu J J, Wu X R et al. Endoscopic treatment of pouch inlet and afferent limb strictures: stricturotomy vs. balloon dilation. Surg Endosc. 2020;35:1722–1733. doi: 10.1007/s00464-020-07562-z. [DOI] [PubMed] [Google Scholar]
- 144.Andujar X, Loras C, Gonzalez B et al. Efficacy and safety of endoscopic balloon dilation in inflammatory bowel disease: results of the large multicenter study of the ENEIDA registry. Surgical Endoscopy. 2020;34:1112–1122. doi: 10.1007/s00464-019-06858-z. [DOI] [PubMed] [Google Scholar]
- 145.Lan N, Shen B. Endoscopic Stricturotomy Versus Balloon Dilation in the Treatment of Anastomotic Strictures in Crohn's Disease. Inflamm Bowel Dis. 2018;24:897–907. doi: 10.1093/ibd/izx085. [DOI] [PubMed] [Google Scholar]
- 146.Lan N, Stocchi L, Ashburn J H et al. Outcomes of Endoscopic Balloon Dilation vs Surgical Resection for Primary Ileocolic Strictures in Patients With Crohn's Disease. Clin Gastroenterol Hepatol. 2018;16:1260–1267. doi: 10.1016/j.cgh.2018.02.035. [DOI] [PubMed] [Google Scholar]
- 147.Biraima M, Adamina M, Jost R et al. Long-term results of endoscopic balloon dilation for treatment of colorectal anastomotic stenosis. Surg Endosc. 2016;30:4432–4437. doi: 10.1007/s00464-016-4762-8. [DOI] [PubMed] [Google Scholar]
- 148.Metman E H, Lagasse J P, d'Alteroche L et al. Risk factors for immediate complications after progressive pneumatic dilation for achalasia. The American journal of gastroenterology. 1999;94:1179–1185. doi: 10.1111/j.1572-0241.1999.01062.x. [DOI] [PubMed] [Google Scholar]
- 149.Boeckxstaens G E, Annese V, des Varannes SB et al. Pneumatic dilation versus laparoscopic Hellerʼs myotomy for idiopathic achalasia. The New England journal of medicine. 2011;364:1807–1816. doi: 10.1056/NEJMoa1010502. [DOI] [PubMed] [Google Scholar]
- 150.Didden P, Reijm A N, Erler N S et al. Fully vs. partially covered selfexpandable metal stent for palliation of malignant esophageal strictures: a randomized trial (the COPAC study) Endoscopy. 2018;50:961–971. doi: 10.1055/a-0620-8135. [DOI] [PubMed] [Google Scholar]
- 151.Kim J Y, Kim S G, Lim J H et al. Clinical outcomes of esophageal stents in patients with malignant esophageal obstruction according to palliative additional treatment. J Dig Dis. 2015;16:575–584. doi: 10.1111/1751-2980.12280. [DOI] [PubMed] [Google Scholar]
- 152.Mao-de-Ferro S, Serrano M, Ferreira S et al. Stents in patients with esophageal cancer before chemoradiotherapy: high risk of complications and no impact on the nutritional status. European Journal of Clinical Nutrition. 2016;70:409–410. doi: 10.1038/ejcn.2015.206. [DOI] [PubMed] [Google Scholar]
- 153.Padhan R K, Nongthombam S K, Venuthurimilli A et al. Assessment of safety and efficacy of an indigenous self-expandable fully covered esophageal metal stent for palliation of esophageal cancer. Indian J Cancer. 2016;53:534–537. doi: 10.4103/0019-509X.204760. [DOI] [PubMed] [Google Scholar]
- 154.So H, Ahn J Y, Han S et al. Efficacy and Safety of Fully Covered Self-Expanding Metal Stents for Malignant Esophageal Obstruction. Dig Dis Sci. 2018;63:234–241. doi: 10.1007/s10620-017-4839-9. [DOI] [PubMed] [Google Scholar]
- 155.Vermeulen B D, Reijm A N, van der Bogt R D et al. Through-the-scope placement of a fully covered metal stent for palliation of malignant dysphagia: a prospective cohort study (with video) Gastrointest Endosc. 2019;90:972–979. doi: 10.1016/j.gie.2019.06.030. [DOI] [PubMed] [Google Scholar]
- 156.Bechtler M, Wagner F, Fuchs E S et al. Biliary metal stents for proximal esophageal or hypopharyngeal strictures. Surg Endosc. 2015;29:3205–3208. doi: 10.1007/s00464-014-4061-1. [DOI] [PubMed] [Google Scholar]
- 157.McCain S, McCain S, Quinn B et al. The role of biodegradable stents in the management of benign and malignant oesophageal strictures: A cohort study. Surgeon. 2016;14:322–326. doi: 10.1016/j.surge.2015.01.002. [DOI] [PubMed] [Google Scholar]
- 158.White R E, Chepkwony R, Mwachiro M et al. Randomized Trial of Small-diameter Versus Large-diameter Esophageal Stents for Palliation of Malignant Esophageal Obstruction. J Clin Gastroenterol. 2015;49:660–665. doi: 10.1097/MCG.0000000000000333. [DOI] [PubMed] [Google Scholar]
- 159.Reijm A N, Didden P, Schelling S JC et al. Self-expandable metal stent placement for malignant esophageal strictures – changes in clinical outcomes over time. Endoscopy. 2019;51:18–29. doi: 10.1055/a-0644-2495. [DOI] [PubMed] [Google Scholar]
- 160.Iwasaki H, Mizushima T, Suzuki Y et al. Factors That Affect Stent-Related Complications in Patients with Malignant Obstruction of the Esophagus or Gastric Cardia. Gut Liver. 2017;11:47–54. doi: 10.5009/gnl16172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Choi Y K, Ahn J Y, Na H K et al. Winged Partially Covered Self-Expandable Metal Stent to Prevent Distal Migration in Malignant Gastric Outlet Obstruction. Dig Dis Sci. 2018;63:3409–3416. doi: 10.1007/s10620-018-5284-0. [DOI] [PubMed] [Google Scholar]
- 162.Kumar V, Ghoshal U C, Mohindra S et al. Palliation of malignant gastroduodenal obstruction with self-expandable metal stent using side- and forward-viewing endoscope: Feasibility and outcome. Jgh Open. 2019;3:65–70. doi: 10.1002/jgh3.12110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Lee H, Min B H, Lee J H et al. Covered metallic stents with an anti-migration design vs. uncovered stents for the palliation of malignant gastric outlet obstruction: a multicenter, randomized trial. Am J Gastroenterol. 2015;110:1440–1449. doi: 10.1038/ajg.2015.286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Miyabe K, Hayashi K, Nakazawa T et al. Safety and benefits of self-expandable metallic stents with chemotherapy for malignant gastric outlet obstruction. Dig Endosc. 2015;27:572–581. doi: 10.1111/den.12424. [DOI] [PubMed] [Google Scholar]
- 165.Bayraktar B, Ozemir I A, Kefeli U et al. Colorectal stenting for palliation and as a bridge to surgery: A 5-year follow-up study. World J Gastroenterol. 2015;21:9373–9379. doi: 10.3748/wjg.v21.i31.9373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Consolo P, Giacobbe G, Cintolo M et al. Colonic acute malignant obstructions: effectiveness of self-expanding metallic stent as bridge to surgery. Turkish Journal of Gastroenterology. 2017;28:40–45. doi: 10.5152/tjg.2016.0249. [DOI] [PubMed] [Google Scholar]
- 167.Feng Y D, Yu Q, Li M.Endoscopic Self-Expandable Metallic Stent Insertion without Fluoroscopic Guidance Is Feasible and Safe for Acute Colonic Obstruction Caused by Colorectal Cancer Gastroenterol Res Pract 2020 10.1155/2020/6810164[Published online 10 Jan 2020] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Gurbulak B, Gurbulak E K, Akgun I E et al. Endoscopic stent placement in the management of malignant colonic obstruction: Experiences from two centers. Ulus Cerrahi Derg. 2015;31:132–137. doi: 10.5152/UCD.2015.2828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Cheng Y, Zhu Y. 03: 54 PM Abstract No. 312 Comparison of through-the-scope stent insertion with standard stent insertion for the management of malignant colorectal obstruction: a prospective study. Journal of vascular and interventional radiology. 2019;30:S138‐S9. doi: 10.1007/s10151-016-1527-2. [DOI] [PubMed] [Google Scholar]
- 170.Han L J, Song X J, Yu B et al. Safety evaluation of preoperative stent insertion and clinical analysis on comparison of outcomes between preoperative stent insertion and emergency surgery in the treatment of obstructive left-sided colorectal cancer. Pakistan Journal of Medical Sciences. 2020;36:376–381. doi: 10.12669/pjms.36.3.1707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Parodi A, De Ceglie A, De Luca L et al. Endoscopic stenting as bridge-to-surgery (BTS) in left-sided obstructing colorectal cancer: Experience with conformable stents. Clinics and Research in Hepatology and Gastroenterology. 2016;40:638–644. doi: 10.1016/j.clinre.2016.03.007. [DOI] [PubMed] [Google Scholar]
- 172.Saeed K M, Zafar W, Masood M A et al. Self-Expanding Metallic Stents (SEMS) in Left-Sided Colonic Cancer--a Cancer Center Experience. J Gastrointest Cancer. 2016;47:69–74. doi: 10.1007/s12029-015-9789-x. [DOI] [PubMed] [Google Scholar]
- 173.Faraz S, Salem S B, Schattner M et al. Predictors of clinical outcome of colonic stents in patients with malignant large-bowel obstruction because of extracolonic malignancy. Gastrointestinal Endoscopy. 2018;87:1310–1317. doi: 10.1016/j.gie.2017.12.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Miyasako Y, Kuwai T, Ishaq S et al. Newly developed self-expandable Niti-S MD colonic metal stent for malignant colonic obstruction. World J Gastrointest Surg. 2020;12:138–148. doi: 10.4240/wjgs.v12.i4.138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Atukorale Y N, Church J L, Hoggan B L et al. Self-Expanding Metallic Stents for the Management of Emergency Malignant Large Bowel Obstruction: a Systematic Review. J Gastrointest Surg. 2016;20:455–462. doi: 10.1007/s11605-015-2997-7. [DOI] [PubMed] [Google Scholar]
- 176.Rahnemai-Azar A A, Rahnemaiazar A A, Naghshizadian R et al. Percutaneous endoscopic gastrostomy: indications, technique, complications and management. World journal of gastroenterology: WJG. 2014;20:7739–7751. doi: 10.3748/wjg.v20.i24.7739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Schurink C A, Tuynman H, Scholten P et al. Percutaneous endoscopic gastrostomy: complications and suggestions to avoid them. European journal of gastroenterology & hepatology. 2001;13:819–823. doi: 10.1097/00042737-200107000-00010. [DOI] [PubMed] [Google Scholar]
- 178.Ubogu E E, Zaidat O O. Rectus sheath hematoma complicating percutaneous endoscopic gastrostomy. Surgical laparoscopy, endoscopy & percutaneous techniques. 2002;12:430–432. doi: 10.1097/00129689-200212000-00008. [DOI] [PubMed] [Google Scholar]
- 179.Anderloni A, Di Leo M, Barzaghi F et al. Complications and early mortality in percutaneous endoscopic gastrostomy placement in lombardy: A multicenter prospective cohort study. Digestive and liver disease: official journal of the Italian Society of Gastroenterology and the Italian Association for the Study of the Liver. 2019;51:1380–1387. doi: 10.1016/j.dld.2019.03.024. [DOI] [PubMed] [Google Scholar]
- 180.Lucendo A J, Sanchez-Casanueva T, Redondo O et al. Risk of bleeding in patients undergoing percutaneous endoscopic gastrotrostomy (PEG) tube insertion under antiplatelet therapy: a systematic review with a meta-analysis. Revista espanola de enfermedades digestivas : organo oficial de la Sociedad Espanola de Patologia Digestiva. 2015;107:128–136. [PubMed] [Google Scholar]
- 181.Richter-Schrag H J, Richter S, Ruthmann O et al. Risk factors and complications following percutaneous endoscopic gastrostomy: a case series of 1041 patients. Canadian journal of gastroenterology = Journal canadien de gastroenterologie. 2011;25:201–206. doi: 10.1155/2011/609601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Richter J A, Patrie J T, Richter R P et al. Bleeding after percutaneous endoscopic gastrostomy is linked to serotonin reuptake inhibitors, not aspirin or clopidogrel. Gastrointestinal endoscopy. 2011;74:22–34 e1. doi: 10.1016/j.gie.2011.03.1258. [DOI] [PubMed] [Google Scholar]
- 183.Gerson L B, Tokar J, Chiorean M et al. Complications associated with double balloon enteroscopy at nine US centers. Clinical gastroenterology and hepatology : the official clinical practice journal of the American Gastroenterological Association. 2009;7:1177–1182, 82 e1-3. doi: 10.1016/j.cgh.2009.07.005. [DOI] [PubMed] [Google Scholar]
- 184.Noujaim M G, Parish A, Raines D.Use, Yield, and Risk of Device-assisted Enteroscopy in the United States: Results From a Large Retrospective Multicenter Cohort J Clin Gastroenterol 2020 10.1097/MCG.0000000000001426[Epub ahead of print: 17 Sep 2020] [DOI] [PubMed] [Google Scholar]
- 185.Buscaglia J M, Dunbar K B, Okolo P I et al. The spiral enteroscopy training initiative: results of a prospective study evaluating the Discovery SB overtube device during small bowel enteroscopy (with video) Endoscopy. 2009;41:194–199. doi: 10.1055/s-0028-1119602. [DOI] [PubMed] [Google Scholar]
- 186.Moschler O, May A, Muller M K et al. Complications in and performance of double-balloon enteroscopy (DBE): results from a large prospective DBE database in Germany. Endoscopy. 2011;43:484–489. doi: 10.1055/s-0030-1256249. [DOI] [PubMed] [Google Scholar]
- 187.Shelnut D J, Sims O T, Zaibaq J N et al. Predictors for outcomes and readmission rates following double balloon enteroscopy: a tertiary care experience. Endosc Int Open. 2018;6:E751–E757. doi: 10.1055/a-0602-3967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Pattni V, Tate D J, Terlevich A et al. Device-assisted enteroscopy in the UK: description of a large tertiary case series under conscious sedation. Frontline gastroenterology. 2018;9:122–128. doi: 10.1136/flgastro-2017-100842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Vanbiervliet G, Giudicelli-Bornard S, Piche T et al. Predictive factors of bleeding related to post-banding ulcer following endoscopic variceal ligation in cirrhotic patients: a case-control study. Alimentary pharmacology & therapeutics. 2010;32:225–232. doi: 10.1111/j.1365-2036.2010.04331.x. [DOI] [PubMed] [Google Scholar]
- 190.Bianchini M, Cavani G, Bonaccorso A et al. Low molecular weight heparin does not increase bleeding and mortality post-endoscopic variceal band ligation in cirrhotic patients. Liver international: official journal of the International Association for the Study of the Liver. 2018;38:1253–1262. doi: 10.1111/liv.13728. [DOI] [PubMed] [Google Scholar]
- 191.Bajaj J S, Franco J. Endoscopic band ligation of esophageal varices in patients on anticoagulation. Journal of clinical gastroenterology. 2008;42:782–785. doi: 10.1097/mcg.0b013e31804bb98b. [DOI] [PubMed] [Google Scholar]
- 192.Duenas E, Cachero A, Amador A et al. Ulcer bleeding after band ligation of esophageal varices: Risk factors and prognosis. Digestive and liver disease : official journal of the Italian Society of Gastroenterology and the Italian Association for the Study of the Liver. 2020;52:79–83. doi: 10.1016/j.dld.2019.06.019. [DOI] [PubMed] [Google Scholar]
- 193.Qumseya B J, Wani S, Desai M et al. Adverse Events After Radiofrequency Ablation in Patients With Barrett's Esophagus: A Systematic Review and Meta-analysis. Clinical gastroenterology and hepatology : the official clinical practice journal of the American Gastroenterological Association. 2016;14:1086–1095 e6. doi: 10.1016/j.cgh.2016.04.001. [DOI] [PubMed] [Google Scholar]
- 194.Maida M, Camilleri S, Manganaro M et al. Radiofrequency Ablation for Treatment of Refractory Gastric Antral Vascular Ectasia: A Systematic Review of the Literature. Gastroenterology research and practice. 2017;2017:5.609647E6. doi: 10.1155/2017/5609647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Eisenberg M J, Richard P R, Libersan D et al. Safety of short-term discontinuation of antiplatelet therapy in patients with drug-eluting stents. Circulation. 2009;119:1634–1642. doi: 10.1161/CIRCULATIONAHA.108.813667. [DOI] [PubMed] [Google Scholar]
- 196.Scott M J, Veitch A, Thachil J. Reintroduction of anti-thrombotic therapy after a gastrointestinal haemorrhage: if and when? British journal of haematology. 2017;177:185–197. doi: 10.1111/bjh.14599. [DOI] [PubMed] [Google Scholar]
- 197.Sung J J, Lau J Y, Ching J Y et al. Continuation of low-dose aspirin therapy in peptic ulcer bleeding: a randomized trial. Annals of internal medicine. 2010;152:1–9. doi: 10.7326/0003-4819-152-1-201001050-00179. [DOI] [PubMed] [Google Scholar]
- 198.Siau K, Hannah J L, Hodson J et al. Stopping antithrombotic therapy after acute upper gastrointestinal bleeding is associated with reduced survival. Postgraduate medical journal. 2018;94:137–142. doi: 10.1136/postgradmedj-2017-135276. [DOI] [PubMed] [Google Scholar]
- 199.Hearnshaw S A, Logan R F, Lowe D et al. Acute upper gastrointestinal bleeding in the UK: patient characteristics, diagnoses and outcomes in the 2007 UK audit. Gut. 2011;60:1327–1335. doi: 10.1136/gut.2010.228437. [DOI] [PubMed] [Google Scholar]
- 200.Marmo R, Koch M, Cipolletta L et al. Predicting mortality in non-variceal upper gastrointestinal bleeders: validation of the Italian PNED Score and Prospective Comparison with the Rockall Score. The American journal of gastroenterology. 2010;105:1284–1291. doi: 10.1038/ajg.2009.687. [DOI] [PubMed] [Google Scholar]
- 201.Oakland K, Guy R, Uberoi R et al. Acute lower GI bleeding in the UK: patient characteristics, interventions and outcomes in the first nationwide audit. Gut. 2018;67:654–662. doi: 10.1136/gutjnl-2016-313428. [DOI] [PubMed] [Google Scholar]
- 202.Oakland K, Kothiwale S, Forehand T et al. External Validation of the Oakland Score to Assess Safe Hospital Discharge Among Adult Patients With Acute Lower Gastrointestinal Bleeding in the US. JAMA Netw Open. 2020;3:e209630. doi: 10.1001/jamanetworkopen.2020.9630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Rubin T A, Murdoch M, Nelson D B. Acute GI bleeding in the setting of supratherapeutic international normalized ratio in patients taking warfarin: endoscopic diagnosis, clinical management, and outcomes. Gastrointestinal endoscopy. 2003;58:369–373. [PubMed] [Google Scholar]
- 204.Shingina A, Barkun A N, Razzaghi A et al. Systematic review: the presenting international normalised ratio (INR) as a predictor of outcome in patients with upper nonvariceal gastrointestinal bleeding. Alimentary pharmacology & therapeutics. 2011;33:1010–1018. doi: 10.1111/j.1365-2036.2011.04618.x. [DOI] [PubMed] [Google Scholar]
- 205.Strate L L, Orav E J, Syngal S. Early predictors of severity in acute lower intestinal tract bleeding. Archives of internal medicine. 2003;163:838–843. doi: 10.1001/archinte.163.7.838. [DOI] [PubMed] [Google Scholar]
- 206.Ansell J, Hirsh J, Hylek E et al. Pharmacology and management of the vitamin K antagonists: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines (8th Edition) Chest. 2008;133:160S–198S. doi: 10.1378/chest.08-0670. [DOI] [PubMed] [Google Scholar]
- 207.Radaelli F, Dentali F, Repici A et al. Management of anticoagulation in patients with acute gastrointestinal bleeding. Digestive and liver disease : official journal of the Italian Society of Gastroenterology and the Italian Association for the Study of the Liver. 2015;47:621–627. doi: 10.1016/j.dld.2015.03.029. [DOI] [PubMed] [Google Scholar]
- 208.Nagata N, Sakurai T, Moriyasu S et al. Impact of INR monitoring, reversal agent use, heparin bridging, and anticoagulant interruption on rebleeding and thromboembolism in acute gastrointestinal bleeding. PloS one. 2017;12:e0183423. doi: 10.1371/journal.pone.0183423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Steffel J, Verhamme P, Potpara T S et al. The 2018 European Heart Rhythm Association Practical Guide on the use of non-vitamin K antagonist oral anticoagulants in patients with atrial fibrillation. European heart journal. 2018;39:1330–1393. doi: 10.1093/eurheartj/ehy136. [DOI] [PubMed] [Google Scholar]
- 210.Tomaselli G F, Mahaffey K W, Cuker A et al. 2020 ACC Expert Consensus Decision Pathway on Management of Bleeding in Patients on Oral Anticoagulants: A Report of the American College of Cardiology Solution Set Oversight Committee. Journal of the American College of Cardiology. 2020;76:594–622. doi: 10.1016/j.jacc.2020.04.053. [DOI] [PubMed] [Google Scholar]
- 211.Cuker A, Burnett A, Triller D et al. Reversal of direct oral anticoagulants: Guidance from the Anticoagulation Forum. American journal of hematology. 2019;94:697–709. doi: 10.1002/ajh.25475. [DOI] [PubMed] [Google Scholar]
- 212.Pollack C V, Jr, Reilly P A, van Ryn J et al. Idarucizumab for Dabigatran Reversal - Full Cohort Analysis. The New England journal of medicine. 2017;377:431–441. doi: 10.1056/NEJMoa1707278. [DOI] [PubMed] [Google Scholar]
- 213.Connolly S J, Crowther M, Eikelboom J W et al. Full Study Report of Andexanet Alfa for Bleeding Associated with Factor Xa Inhibitors. The New England journal of medicine. 2019;380:1326–1335. doi: 10.1056/NEJMoa1814051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Majeed A, Agren A, Holmstrom M et al. Management of rivaroxaban- or apixaban-associated major bleeding with prothrombin complex concentrates: a cohort study. Blood. 2017;130:1706–1712. doi: 10.1182/blood-2017-05-782060. [DOI] [PubMed] [Google Scholar]
- 215.Piran S, Khatib R, Schulman S et al. Management of direct factor Xa inhibitor-related major bleeding with prothrombin complex concentrate: a meta-analysis. Blood Adv. 2019;3:158–167. doi: 10.1182/bloodadvances.2018024133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Smith M N, Deloney L, Carter C et al. Safety, efficacy, and cost of four-factor prothrombin complex concentrate (4F-PCC) in patients with factor Xa inhibitor-related bleeding: a retrospective study. Journal of thrombosis and thrombolysis. 2019;48:250–255. doi: 10.1007/s11239-019-01846-5. [DOI] [PubMed] [Google Scholar]
- 217.Choudari C P, Rajgopal C, Palmer K R. Acute gastrointestinal haemorrhage in anticoagulated patients: diagnoses and response to endoscopic treatment. Gut. 1994;35:464–466. doi: 10.1136/gut.35.4.464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.Jamula E, Lloyd N S, Schwalm J D et al. Safety of uninterrupted anticoagulation in patients requiring elective coronary angiography with or without percutaneous coronary intervention: a systematic review and metaanalysis. Chest. 2010;138:840–847. doi: 10.1378/chest.09-2603. [DOI] [PubMed] [Google Scholar]
- 219.Qureshi W, Mittal C, Patsias I et al. Restarting anticoagulation and outcomes after major gastrointestinal bleeding in atrial fibrillation. The American journal of cardiology. 2014;113:662–668. doi: 10.1016/j.amjcard.2013.10.044. [DOI] [PubMed] [Google Scholar]
- 220.Sengupta N, Feuerstein J D, Patwardhan V R et al. The risks of thromboembolism vs. recurrent gastrointestinal bleeding after interruption of systemic anticoagulation in hospitalized inpatients with gastrointestinal bleeding: a prospective study. The American journal of gastroenterology. 2015;110:328–335. doi: 10.1038/ajg.2014.398. [DOI] [PubMed] [Google Scholar]
- 221.Staerk L, Lip G Y, Olesen J B et al. Stroke and recurrent haemorrhage associated with antithrombotic treatment after gastrointestinal bleeding in patients with atrial fibrillation: nationwide cohort study. Bmj. 2015;351:h5876. doi: 10.1136/bmj.h5876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222.Witt D M, Delate T, Garcia D A et al. Risk of thromboembolism, recurrent hemorrhage, and death after warfarin therapy interruption for gastrointestinal tract bleeding. Archives of internal medicine. 2012;172:1484–1491. doi: 10.1001/archinternmed.2012.4261. [DOI] [PubMed] [Google Scholar]
- 223.Little D, Chai-Adisaksopha C, Hillis C et al. Resumption of anticoagulant therapy after anticoagulant-related gastrointestinal bleeding: A systematic review and meta-analysis. Thrombosis research. 2019;175:102–109. doi: 10.1016/j.thromres.2019.01.020. [DOI] [PubMed] [Google Scholar]
- 224.Tapaskar N, Pang A, Werner D A et al. Resuming Anticoagulation Following Hospitalization for Gastrointestinal Bleeding Is Associated with Reduced Thromboembolic Events and Improved Mortality: Results from a Systematic Review and Meta-Analysis. Digestive diseases and sciences. 2021;66:554–566. doi: 10.1007/s10620-020-06248-9. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


