Abstract
Elderly patients with dementia suffer from cognitive dysfunctions and neuropsychiatric symptoms (NPS) such as anxiety and depression. Alzheimer’s disease (AD) is a form of age-related dementia, and loss of cholinergic neurons is intimately associated with development of AD symptoms. We and others have reported that neural cell transplantation ameliorated cognitive dysfunction in AD model mice. It remains largely unclear whether neural cell transplantation ameliorates the NPS of AD. It would be interesting to determine whether NPS correlates with cognitive dysfunctions before and after neural cell transplantation in AD model mice. Based on the revalidation of our previous data from a Morris water maze test, we found that neural cell transplantation improved anxiety and depression significantly and marginally affected locomotion activity in AD mice. A correlation analysis revealed that the spatial learning function of AD mice was correlated with their NPS scores both before and after cell transplantation in a similar manner. In contrast, in the mice subjected to cell transplantation, spatial reference memory function was not correlated with NPS scores. These results suggested the neural cell transplantation in the AD model mice significantly improved NPS to the same degree as cognitive dysfunctions, possibly via distinct mechanisms, such as the cholinergic and GABAergic systems.
Keywords: Alzheimer’s disease, neural transplantation, neuropsychiatric symptoms, PDAPP Tg mice
Introduction
Alzheimer’s disease (AD) is a progressive neurodegenerative disorder characterized by cognitive decline and a number of neuropsychiatric symptoms (NPS), such as apathy, hyperactivity, and psychosis) [1,2,3,4]. AD severity is correlated with the degree of neural cell loss, particularly in the hippocampus [5,6,7].
The hippocampus plays critical roles in cognitive functions such as a spatial learning function and spatial reference memory function [8, 9]. Indeed, structural changes of the hippocampus were suggested to be a biomarker for cognitive dysfunction [10]. Typical pathologic features of AD include senile plaques consisting of aggregated amyloid beta and intracellular neurofibrillary tangles consisting of hyperphosphorylated tau proteins. Therapeutic approaches targeting amyloid beta and tau have been explored with the aim of eliminating these apparent causes of AD [11].
Acetylcholine (ACh) and gamma-aminobutyric acid (GABA) are released from cholinergic neurons and GABAergic neurons, respectively. These neurotransmitters are implicated in the development of AD. ACh acts via two classes of receptors: ionotropic nicotinic receptors (nAChRs) and metabotropic muscarinic receptors (mAChRs). The major subtypes of nAChRs, nAChRα7 and nAChRα4β2 regulate synaptic plasticity [12]. The activity of cholinergic neurons and ACh production by cholinergic neurons decrease in AD patients, and administration of anticholinergic drugs is associated with the development of AD [13, 14]. In fact, inhibition of ACh esterase improves cognitive functions and NPS severity in AD patients [15, 16]. GABA acts via distinct receptor subtypes in the central nervous system: the ionotropic GABAA and GABAC receptors (GABAAR and GABACR, respectively) and the metabotropic GABABR [17, 18]. There are also subtypes of GABAAR. GABAAα1, α2, α5, and γ2 mediate anti-anxiety effects. GABAAα1, α2, α4, α5, and δ are involved in learning and memory function in the hippocampus [19]. GABABR mediates modulation of excitability and synaptic transmission in the dentate gyrus (DG) and relieves anxiety and depression [20,21,22]. GABACR in hippocampal CA1 neurons is involved in depression symptoms [23]. However, the role of GABAergic neurons in the development of AD remains controversial because many reports have shown that agonists and also antagonists of GABA receptors improve cognitive dysfunction in AD [24].
Cognitive dysfunction accompanies NPS in AD [2,3,4]. NPS, known collectively as behavioral and psychological symptoms of dementia (BPSD), are also characteristics of AD [25]. We found that the levels of cognitive functions were partially correlated with susceptibility to NPS in dementia model mice [26]. Medications for BPSD, such as antipsychotics, antidepressants, mood stabilizers, and hypnotic drugs, are clinically used in patients with dementia [27].
Neural transplantation is becoming a promising therapeutic option, as transplantation of stem cells, including human induced pluripotent stem (hiPS) cells, have been shown to promote reconstruction of degenerated neural networks in AD [28]. We previously showed that transplantation of neural cells derived from hiPS cells in the hippocampus, improved cognitive dysfunction in AD model mice [29, 30].
We here studied whether the neural transplantation, in addition to improving cognitive dysfunction [29, 30], ameliorates NPS in AD model mice. We found that the neural transplantation improved NPS sufficiently and NPS scores were well correlated with spatial learning function in AD model mice.
Materials and Methods
Ethics statement
All experiments were approved by the Animal Care and Use Committee in St. Marianna University Scholl of Medicine (SMU) and were conducted according the institutional ethical guidelines for animal experiments and safety guidelines for gene manipulation experiments.
Mice
As an AD model, PDGF promoter-driven amyloid precursor protein (PDAPP) transgenic (Tg) mice, strain B6.Cg-Tg (PDGFB-APPSwInd) 20Lms/2J, that overexpress mutated human APP (APPV717F) were obtained from The Jackson Laboratory (Bar Harbor, ME, USA; RRID: MMRRC_034836-JAX, https://www.jax.org/strain/006293) [31]. PDAPP Tg mouse lines were maintained by breeding PDAPP Tg males to littermate control females. Mice were kept in an environmentally controlled clean room at the animal center of SMU. PDAPP Tg mice showed impaired cognitive dysfunction in a Morris water maze (MWM) test [32], and neural cell transplantation improved cognitive dysfunction [29, 30]. However, the correlation between cognitive function and NPS severity under neural cell transplantation was largely unknown.
Morris water maze test and behavioral analysis
We revalidated our previous data using an MWM test, focusing on NPS [29, 30]. For assessment of the therapeutic role of neural transplantation in spatial cognitive functions and NPS, PDAPP Tg mice before (1st trial started at day −6) and after (2nd trial started at day 22) transplantation were subjected to an MWM test (Fig. 1A, n=28). The MWM test assessed cognitive functions and NPS scores based on multiple parameters during the test, as described [33,34,35,36,37,38,39].
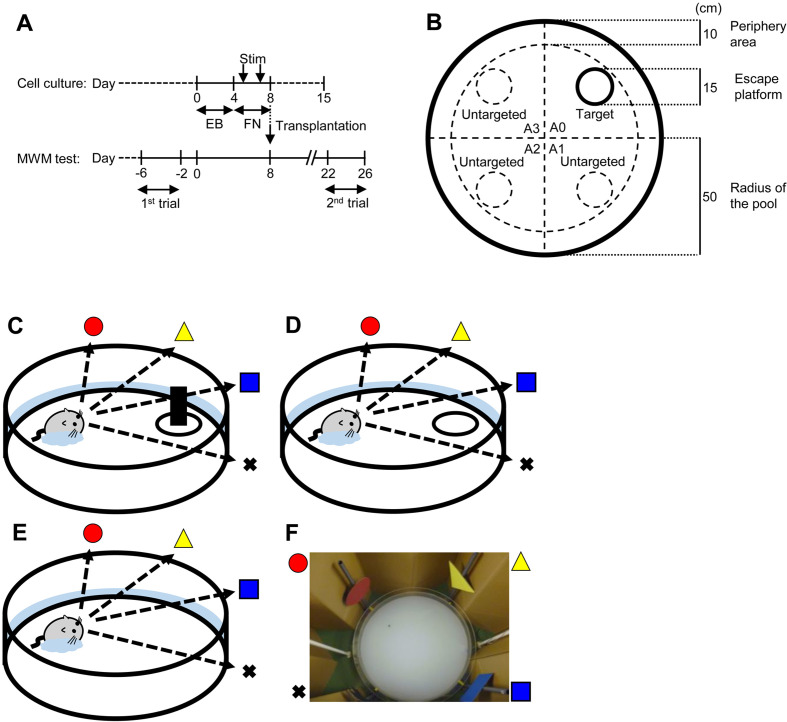
Fig. 1.
Schematic representations of human iPS cell-derived neural cell induction and functional assessment by Morris water maze test. (A) Neural induction and transplantation of of hiPS cells. Undifferentiated hiPS cells maintained with mouse embryonic fibroblasts were developed into EBs by 4-day floating culture. To differentiate neural cells, EBs were stimulated with RA, NOG, and SHH at days 5 and 7 (Stim) on a fibronectin (FN)-coated plate. The cells contained neural stem/progenitor cells (NSPCs), as previously reported. For measuring neurotransmitter secretion, culture supernatants of NSPCs were collected at days 5, 8, and 15 for applying ELISA. At day 8, NSPCs were transplanted into PDAPP Tg mice (n=28). Cognitive functions of the grafted PDAPP Tg mice were assessed by MWM test before (1st trial: from day −6 to day −2) and after (2nd trial: from day 22 to day 26) transplantation. (B) The MWM was conducted using a circular pool (diameter: 100 cm) with opaque water and a plastic escape platform (diameter: 15 cm). A 10-cm-wide banded zone along the wall was defined as the peripheral area for behavioral analysis. (C–E) Mice were subjected to the MWM test for 6 consecutive days as follows: (C) visible test on the first day using a platform visualized with a black bottle; (D) hidden test on 4 consecutive days using a platform without the black bottle submerged below the surface of the opaque water; and (E) probe test on the last day under the same conditions except that the platform was removed. The swimming trajectory was monitored by a CCD camera and recorded with a PC. (F) Picture of the experimental equipment for the MWM test.
Mice were subjected to the following tests on 6 consecutive days in each trial: visible test at the first day, hidden test on the next 4 consecutive days, and probe test on the final day. A circular pool (diameter: 100 cm) was filled with opaque water at 26 ± 0.5°C. The water basin was surrounded by a brown curtain. An escape platform (diameter: 15 cm) made of transparent acrylic resin was placed in one of four quadrants in the pool and set 1.5 cm below the water surface (Fig. 1B). At the start, mice were gently placed into the water basin facing the wall. The mice were allowed to search for the platform for 90 s; if they did not reach the platform in the defined time, they were manually placed onto the platform. The mice were allowed to stay on the platform for 30 s to learn and memorize the spatial position before the next attempt.
During the visual test, the platform was visualized by marking it with using a black bottle placed on top of it (Fig. 1C). The visible test was performed to examine visible acuity and motivation to escape from water. Mice that did not reach the platform 4 times in 8 attempts were excluded from this study. On the next 4 consecutive days, spatial learning function was examined by training the mice to find the hidden platform using spatial cues (hidden test: four attempts per day, Fig. 1D). We analyzed the data at day 4 in the hidden test. On the final day, spatial reference memory function was examined by placing the mice into the water basin after removal of the platform (probe test: 1 attempt, Fig. 1E). Mice allowed to search for the platform for 90 s; the amounts of time duration which each mouse stayed in the conditioned quadrant for the target platform and in the other quadrants were recorded. The times each performance of the test were summed and expressed as a percent. The number of crossings through the target platform area and untargeted platform areas were counted.
The time to reach the platform was defined as the platform escape latency (max 90 s). During the visible test, we assessed the motivation to escape the water using the escape latency. During the hidden test, we assessed spatial learning function using the escape latency. During the probe test, we assessed spatial reference memory function using the durations in the targeted and untargeted areas and the numbers of crossing through the targeted and untargeted areas. During each test, the locomotion activity and sensitivity to NPS were measured. The level of locomotion activity was assessed with the moving speed, sensitivity to anxiety was assessed with the level of thigmotaxis, and sensitivity to depression was assessed with the freezing time. Exploration in the periphery area (10-cm-wide banded zone along the wall) was defined as thigmotaxis (Fig. 1B). Animal movements were digitally recorded by an automated tracking system (O’Hara & Co., Ltd., Tokyo, Japan), which was implemented in a modified software based on the ImageJ software (NIH, Bethesda, ME, USA).
Transplantation
We transplanted neural precursor cells derived from hiPS cells into PDAPP mice as described previously [29, 30]. The hiPS cell line 253G1 was obtained from RIKEN BioResource Research Center (Ibaraki, Japan; RRID: CVCL_B518, http://cellbank.brc.riken.jp/cell_bank/CellInfo/?cellNo=HPS0002) and was maintained according to a standard protocol (Nakagawa et al., 2008). In brief, undifferentiated hiPS cells were floating cultured with a differentiation medium, DMEM/F-12 medium (D6421, Sigma, Ronkonkoma, NY, USA) containing 0.8× MEM Non-Essential Amino Acids Solution (Gibco, catalog no.1140-050; Thermo Fisher Scientific, Waltham, MA, USA), 0.8 × 2-mercaptoethanol (Gibco, catalog no. 21985-023; Thermo Fisher Scientific), 20 mM L-glutamine (Gibco, catalog no. 25030–081; Thermo Fisher Scientific), and 20% knockout serum replacement (Gibco, catalog no. 10828-028; Thermo Fisher Scientific), in a 100 mm Petri dish (product no. 351029; Corning, Corning, NY, USA) for 4 days to form embryoid bodies (EBs). Then the EBs were placed on a fibronectin-coated 6-well plate (product no. 354402; Corning) on day 4. From day 5, EB were cultured with DMEM/F-12 medium containing 1× N2 supplement (Invitrogen, catalog no. 17502-048; Thermo Fisher Scientific). On days 5 and 7, the medium was changed to DMEM/F-12 medium containing 1× N2 supplement, 1 µM retinoic acid (RA; product no. R2625; Sigma, St. Louis, MN, USA), 10 nM noggin-Fc (NOG; catalog no. 3344-NG; R&D Systems, Minneapolis, MN, USA), and 10 nM Sonic hedgehog (SHH; catalog no. 1845-SH; R&D Systems). For transplantation, the differentiated cells were harvested on day 8 as neural precursors and transplanted into mice (Fig. 1A). In brief, we perforated the bilateral parietal bones at 2.4 mm posterior and 2.0 mm lateral to the bregma under anesthesia. The cells (2.0 × 105 cells/2 µl) were then injected into the DG in the bilateral hippocampi through the center of the hole at a depth of 1.25 mm depth from the dura matter. Cyclosporine (3–8 mg/kg, Novartis Pharma, Rotkreuz, Switzerland) was administered daily.
ELISA
To determine the expression levels of GABA and ACh in neural cells, culture supernatants were harvested on days 5, 8 and 15. Then GABA or ACh were measured using ELISA kits (Cloud-Clone Corp., Houston, TX, USA, for GABA; BioVision, Milpitas, CA, USA, for ACh). These data were expressed as the average ± SEM. Statistical analysis was performed using the paired t test or Wilcoxon signed-rank test for samples with equal or unequal variances, respectively. Differences were considered the statistically significant when P<0.05.
Experimental design and statistical analysis
The values for the behavioral and psychological parameters in the MWM test were obtained from our previous studies [29, 30]. Neural precursor cells derived from hiPS cells were transplanted into PDAPP Tg mice (n=28). All statistical analyses were performed using Statcel3 software (OMS Publishing, Tokyo, Japan). Statistical differences in parameters of the MWM test before and after transplantation were calculated by paired t test or Wilcoxon signed-rank test for samples with equal or unequal variances, respectively. All data were expressed as the average ± SEM. Differences were considered the statistically significant when P<0.05. Correlation analysis was performed using Pearson’s correlation to examine linear association. We were interested in “strong” correlation, with r≥0.6 or r≤−0.6 for the strength of correlation [40].
Results
Neural cell transplantation ameliorated neuropsychiatric symptoms of PDAPP Tg mice in the visible test
We previously reported that PDAPP Tg mice exhibited impaired spatial learning function and that neural transplantation improved the dysfunction [29, 30]. In this study, we assessed whether neural cell transplantation improved NPS in AD model mice and whether the cognitive functions were associated with the NPS of the mice with neural grafts.
At 14 days after neural transplantation in the PDAPP Tg mice, we conducted the 2nd MWM test (Fig. 1). In the visible test, we found that neural cell transplantation improved NPS, such as motivation to escape the water (escape latency, Fig. 2A), anxiety (thigmotaxis, Fig. 2B), and depression (freezing time, Fig. 2C), but did not affect locomotion activity (moving speed, Fig. 2D).
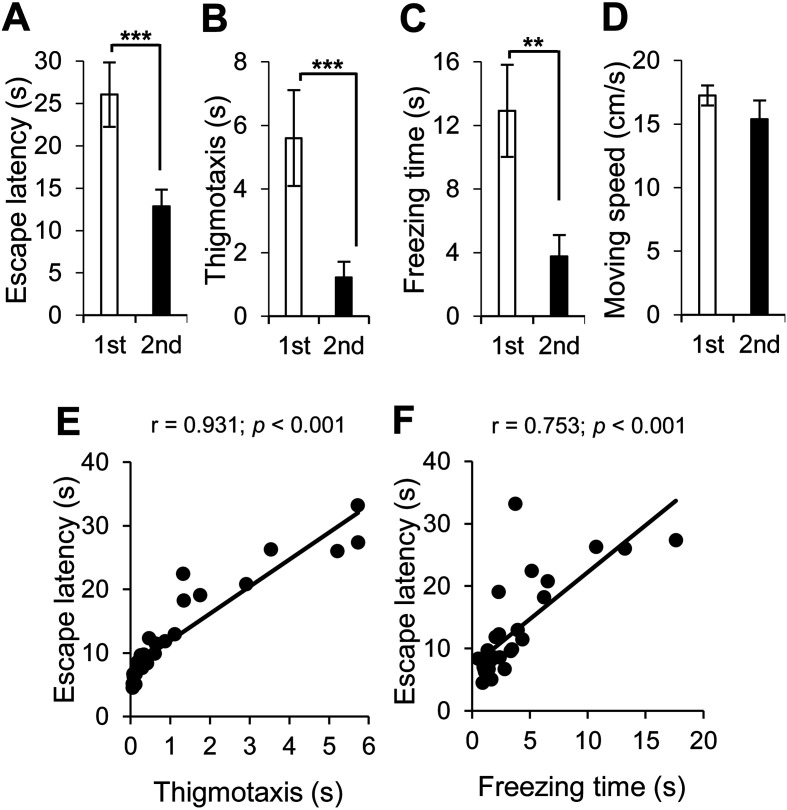
Fig. 2.
Neural cell transplantation ameliorated behavioral and neuropsychiatric symptoms of PDAPP Tg mice in the visible test. Behavioral and psychological symptoms in PDAPP Tg mice before (1st, in white) and after (2nd, in red) neural cell transplantation were assessed by the visible test with the following parameters: (A) escape latency for motivation to escape the water, (B) thigmotaxis for anxiety, (C) freezing time for depression, and (D) moving speed for locomotion activity. Average and SEM values are shown. **P<0.01; ***P<0.001. (E, F) A correlation analysis was conducted to examine the correlation between the following parameters in the PDAPP Tg mice with neural cell transplantation: (E) escape latency and thigmotaxis and (F) escape latency and freezing time. Motivation to escape the water was correlated with anxiety and depression. Correlation coefficients are indicated as r value. A correlation coefficient of r≥0.6 with a P value <0.001 was considered to indicate strong correlation and was indicated in red.
It was reported that the ventral hippocampus regulates motivation, anxiety, and depressive behaviors [41]. The motivation to escape the water was strongly correlated with anxiety (thigmotaxis, r=0.931, Fig. 2E) and depression (freezing time, r=0.753, Fig. 2F).
Spatial learning function was correlated with NPS scores in the hidden test
PDAPP Tg mice have abnormal spatial cognitive function. Neural cell transplantation ameliorated spatial learning function in the hidden test (Fig. 3A). It also improved anxiety (Fig. 3B) and depression (Fig. 3C), but it did not affect locomotion activity in the hidden test (Fig. 3D).
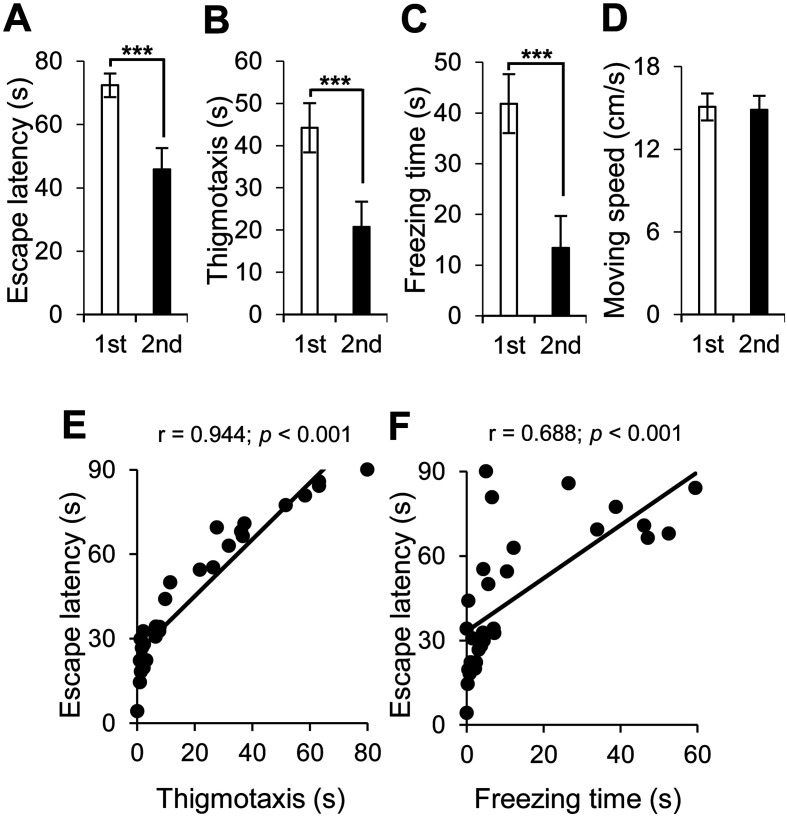
Fig. 3.
Neural cell transplantation ameliorated spatial learning function in PDAPP Tg mice, which was correlated with neuropsychiatric symptoms in the hidden test. Spatial learning function in PDAPP Tg mice before (1st, in white) and after (2nd, in red) neural cell transplantation was assessed by the hidden test with the following parameters: (A) escape latency for spatial learning function, (B) thigmotaxis for anxiety, (C) freezing time for depression, and (D) moving speed for locomotion activity. Average and SEM values are shown. ***P<0.001. (E, F) A correlation analysis was conducted to examine the correlation between the following parameters in the PDAPP Tg mice with neural cell transplantation: (E) escape latency and thigmotaxis and (F) escape latency and freezing time. Spatial learning function after neural cell transplantation was correlated with anxiety and depression. Correlation coefficients are indicated as r value. A correlation coefficient of r≥0.6 with a P value <0.001 was considered to indicate strong correlation and was indicated in red.
The correlation analysis for the hidden test showed that spatial learning function was correlated with anxiety (thigmotaxis, r=0.944, Fig. 3E) and depression (freezing time, r=0.688, Fig. 3F).
Spatial reference memory function was not correlated with NPS scores in the probe test
Neural cell transplantation increased the time spent in the target quadrant and decreased time spent in the untargeted quadrants in AD mice, suggesting enhancement of spatial reference memory function (Fig. 4A). Transplantation also improved anxiety (Fig. 4B) and depression (Fig. 4C), but it did not affect locomotion activity (Fig. 4D).
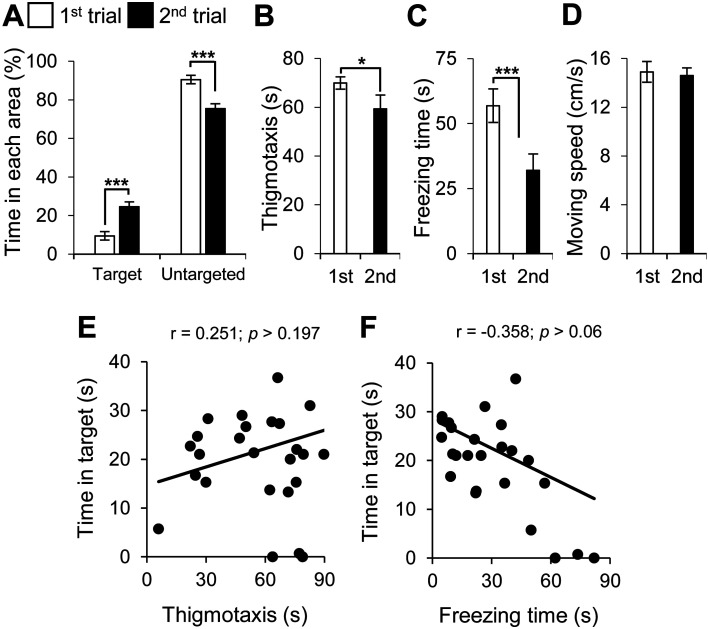
Fig. 4.
Neural transplantation ameliorated spatial memory function in PDAPP Tg mice, which was not correlated with neuropsychiatric symptoms in the probe test. Spatial memory function in PDAPP Tg mice before (1st, in white) and after (2nd, in red) neural transplantation was assessed by the probe test with the following parameters: (A) amounts of time in the targeted quadrant and untargeted quadrants for spatial reference memory function, (B) thigmotaxis for anxiety, (C) freezing time for depression, and (D) moving speed for locomotion activity. Average and SEM values are shown. *P<0.05; ***P<0.001. (E, F) A correlation analysis was conducted to examine the correlation between the following parameters in the PDAPP Tg mice with neural cell transplantation: (E) time in targeted quadrant and thigmotaxis and (F) time in targeted quadrant and freezing time. Spatial reference memory function was not correlated anxiety and depression after neural cell transplantation. Correlation coefficients are indicated as r value. A correlation coefficient of r≤−0.6 or r≥0.6 with a P value <0.001 was considered to indicate strong correlation, and no strong correlation was found among the parameters.
The correlation analysis for the probe test revealed that spatial reference memory function was not correlated with anxiety and depression (Figs. 4E and F).
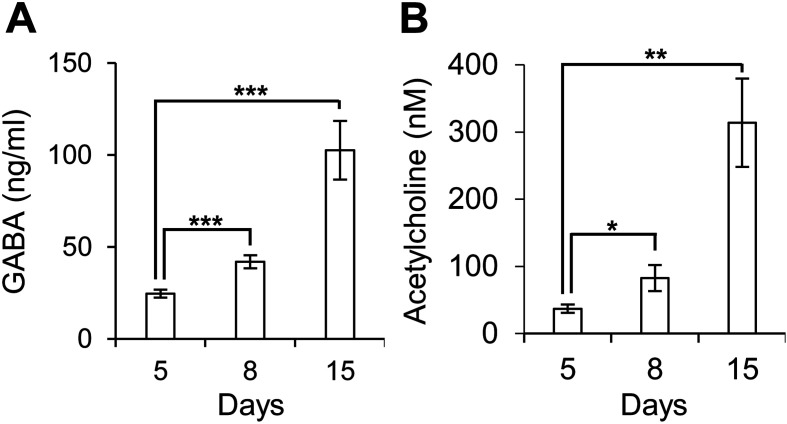
Neural cells derived from human iPS cells secreted GABA and acetylcholine
Previously, we showed that grafted neural cells expressed GABAA receptor, vesicular GABA transporter (VGAT), alpha 7 nicotinic acetylcholine receptors (α7nAChRs) and choline acetyltransferase (ChAT) in the host brain [30]. Whether GABA and Ach are present in culture supernatants during neural differentiation remained to be determined, and positive results were obtained in the present study (Fig. 5). These results suggested that transplanted neural cells functionally matured into GABAergic neurons and cholinergic neurons.
Fig. 5.
Neural cells derived from human iPS cells differentiated in GABA-secreting and acetylcholine-secreting neurons. Culture supernatants of neural cell induction cultures were collected on days 5, 8, and 15. GABA (A) and acetylcholine (B) levels were measured by ELISA (n=8). Average and SEM values are shown. *P<0.05; **P<0.01; ***P<0.001.
Discussion
In the present study, we here assessed individual neural dysfunctions related to dementia before and after neural transplantation and explored their correlations. We found that neural cell transplantation improved not only cognitive function but also NPS, such as anxiety and depression. Spatial reference memory function was not correlated with NPS scores. Nevertheless, spatial learning function was correlated with NPS scores. Thus, we demonstrated clear differences in the relationships between NPS and spatial reference memory function and between NPS and spatial learning function in AD mice after neural cell transplantation. Our findings were based on the revalidation of our previous data from an MWM test [29, 30]. Further study using other dedicated behavioral tests, such as the open-field test, elevated plus maze test, forced swimming test, may be needed to understand the relationships between improvement of cognitive functions and NPS after neural cell transplantation.
We found previously that transplantation of neural cells derived from hiPS cells improved cognitive dysfunctions in AD model mice [30]. Here, we suggest that the beneficial effects of neural cell transplantation on dementia-associated symptoms, including learning impairment, memory impairment, and NPS, may be the result of regeneration of multiple neural pathways/circuits in the mice.
The hippocampus is involved in cognitive function and emotional behavior [42,43,44,45]. It has been reported that the dorsal hippocampus is preferentially involved in cognitive function [46, 47]. The ventral hippocampus is related to NPS [48, 49]. Adult hippocampal neurogenesis occurs in the DG [50, 51], and it has been shown that neurogenesis in the DG is involved in improvement of NPS [52,53,54,55].
Our results suggest correlation between NPS and spatial learning function but not between NPS and spatial reference memory function. We interpret this data as suggesting that the neural pathways/circuits involved in spatial learning function may be different from those involved in spatial reference memory function in PDAPP Tg mice. Furthermore, it is possible that spatial learning dysfunction and NPS in mice with dementia share or are intimately associated with, at least in part, the pathological neural pathways/circuits where the neural transplantation exerted its positive effects.
In agreement with our current findings, previous reports implied that learning function was independent from memory function. Corticosterone-induced anxiety/depression model mice and mice with potassium ion channel deficiency in the hippocampi showed spatial learning dysfunction, whereas the spatial reference memory function was normal [56, 57].
It is known that the hippocampus plays an important role in locomotion activity [58]. It has been reported that physical activity is associated with a risk for cognitive decline and dementia [59, 60]. In the visible test, we found that the locomotion activity of the PDAPP Tg mice was lower than that of WT mice; however, neural cell transplantation had no effect on locomotion activity (Fig. 2). Thus, we interpreted this as suggesting that the improvement of cognitive functions and NPS caused by transplantation of neural cells into hippocampus was independent of locomotion activity.
Theta oscillations in the CA1 region of the hippocampus are important for locomotion activity [58]. Neural cell loss in the CA1 region of the hippocampus is a typical pathology in AD patients, and the neural cell loss in CA1 region occurs in the early stage of AD [61, 62]. Neural cell loss appeared in young PDAPP Tg mice, and the extent of neuron loss in the CA1 region increased with age [63]. Thus, these reports and our results suggested that loss of neural cells in the CA1 region may contribute to the impairment of locomotion activity.
We previously found that some neural cells grafted into the hilus of the hippocampus DG region migrated and accumulated around the cerebral cortex and hippocampus in conjunction with cholinergic cell differentiation, whereas other grafted neural cells remained around the hippocampus and had a GABAergic phenotype [30]. These two cell types may contribute to the broader effects of neural transplantation on AD-associated symptoms.
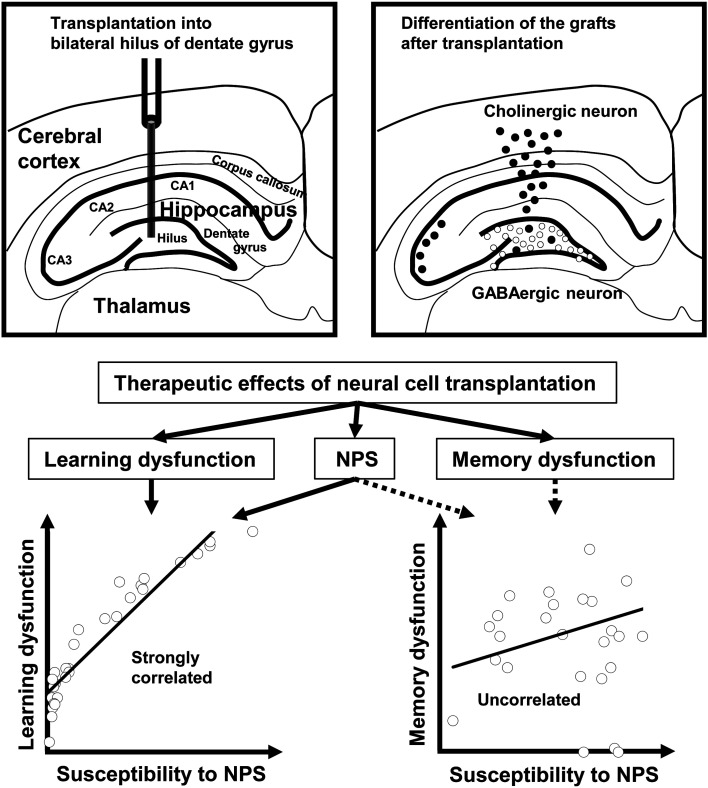
Indeed, GABAergic and cholinergic neurons are important for cognitive function and control of NPS, including anxiety and depression [64,65,66,67,68,69,70]. We previously reported that the majority of neural cells grafted into the DG differentiated into GABAergic and cholinergic neurons [71]. It was suggested that the former migrated to the hippocampus and that the latter migrated to the cerebral cortex. These two neural cell types may share multiple functional roles associated with memory functions in the brain. In the hippocampus, emerging functional neurons deriving from hiPS cell grafts may have some effects on NPS in mice with dementia (Fig. 6). Further studies are needed to elucidate the molecular and cellular mechanisms underlining the improvements in AD-associated symptoms.
Fig. 6.
The broader effects of human iPS cell-derived neural cell transplantation on dementia model mice. The grafted hiPS cell-derived neural cells differentiated into cholinergic (white circle) and GABAergic neurons (black circle). The cholinergic neurons migrated to the hippocampus, and the GABAergic neurons migrated to the cerebral cortex. These neural cells improved cognitive dysfunctions, such as spatial learning dysfunction and spatial reference memory dysfunction, and NPS, such as anxiety and depression. Spatial learning function was correlated with the NPS scores. Spatial reference memory function was not correlated with the NPS scores.
Author Contributions
MAM, AN, and NS designed the research. MAM analyzed the data and wrote the paper. MAM and NS edited the paper. NF and KT transplanted neural cells. All authors performed the MWM tests.
Funding
This work was supported by JSPS KAKENHI (Grant Nos. JP17K01378, JP17K14978, JP19K20681, and JP19K12766) and the SRF Foundation (No. 2019Y005).
Conflict of Interest
The authors declare no competing financial interest.
References
- 1.Edwards ER, Spira AP, Barnes DE, Yaffe K. Neuropsychiatric symptoms in mild cognitive impairment: differences by subtype and progression to dementia. Int J Geriatr Psychiatry. 2009; 24: 716–722. doi: 10.1002/gps.2187 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kalueff AV. Neurobiology of memory and anxiety: from genes to behavior. Neural Plast. 2007; 2007: 78171. doi: 10.1155/2007/78171 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Szu JI, Binder DK. The Role of Astrocytic Aquaporin-4 in Synaptic Plasticity and Learning and Memory. Front Integr Nuerosci. 2016; 10: 8. doi: 10.3389/fnint.2016.00008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Justice NJ. The relationship between stress and Alzheimer’s disease. Neurobiol Stress. 2018; 8: 127–133. doi: 10.1016/j.ynstr.2018.04.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mu Y, Gage FH. Adult hippocampal neurogenesis and its role in Alzheimer’s disease. Mol Neurodegener. 2011; 6: 85. doi: 10.1186/1750-1326-6-85 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Karlawish J, Jack CR, Jr, Rocca WA, Snyder HM, Carrillo MC. Alzheimer’s disease: The next frontier-Special Report 2017. Alzheimers Dement. 2017; 13: 374–380. doi: 10.1016/j.jalz.2017.02.006 [DOI] [PubMed] [Google Scholar]
- 7.Atri A. The Alzheimer’s Disease Clinical Spectrum: Diagnosis and Management. Med Clin North Am. 2019; 103: 263–293. doi: 10.1016/j.mcna.2018.10.009 [DOI] [PubMed] [Google Scholar]
- 8.Squire LR. Memory and the hippocampus: a synthesis from findings with rats, monkeys, and humans. Psychol Rev. 1992; 99: 195–231. doi: 10.1037/0033-295X.99.2.195 [DOI] [PubMed] [Google Scholar]
- 9.Deng W, Aimone JB, Gage FH. New neurons and new memories: how does adult hippocampal neurogenesis affect learning and memory? Nat Rev Neurosci. 2010; 11: 339–350. doi: 10.1038/nrn2822 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ott CV, Johnson CB, Macoveanu J, Miskowiak K. Structural changes in the hippocampus as a biomarker for cognitive improvements in neuropsychiatric disorders: A systematic review. Eur Neuropsychopharmacol. 2019; 29: 319–329. doi: 10.1016/j.euroneuro.2019.01.105 [DOI] [PubMed] [Google Scholar]
- 11.Madav Y, Wairkar S, Prabhakar B. Recent therapeutic strategies targeting beta amyloid and tauopathies in Alzheimer’s disease. Brain Res Bull. 2019; 146: 171–184. doi: 10.1016/j.brainresbull.2019.01.004 [DOI] [PubMed] [Google Scholar]
- 12.Roberts JP, Stokoe SA, Sathler MF, Nichols RA, Kim S. Selective co-activation of α7- and α4β2-nicotinic acetylcholine receptors reverses beta-amyloid-induced synaptic dysfunction. J Biol Chem. 2021; 296: 100402. doi: 10.1016/j.jbc.2021.100402 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hampel H, Mesulam MM, Cuello AC, Farlow MR, Giacobini E, Grossberg GT, et al. The cholinergic system in the pathophysiology and treatment of Alzheimer’s disease. Brain. 2018; 141: 1917–1933. doi: 10.1093/brain/awy132 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Richardson K, Fox C, Maidment I, Steel N, Loke YK, Arthur A, et al. Anticholinergic drugs and risk of dementia: case-control study. BMJ. 2018; 361: k1315. doi: 10.1136/bmj.k1315 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sharma K. Cholinesterase inhibitors as Alzheimer’s therapeutics (Review). Mol Med Rep. 2019; 20: 1479–1487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Parnetti L, Amici S, Lanari A, Gallai V. Pharmacological treatment of non-cognitive disturbances in dementia disorders. Mech Ageing Dev. 2001; 122: 2063–2069. doi: 10.1016/S0047-6374(01)00316-5 [DOI] [PubMed] [Google Scholar]
- 17.Bowery NG, Hudson AL, Price GW. GABAA and GABAB receptor site distribution in the rat central nervous system. Neuroscience. 1987; 20: 365–383. doi: 10.1016/0306-4522(87)90098-4 [DOI] [PubMed] [Google Scholar]
- 18.Bormann J, Feigenspan A. GABAC receptors. Trends Neurosci. 1995; 18: 515–519. doi: 10.1016/0166-2236(95)98370-E [DOI] [PubMed] [Google Scholar]
- 19.Engin E, Benham RS, Rudolph U. An Emerging Circuit Pharmacology of GABAA Receptors. Trends Pharmacol Sci. 2018; 39: 710–732. doi: 10.1016/j.tips.2018.04.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Martín-Belmonte A, Aguado C, Alfaro-Ruíz R, Moreno-Martínez AE, de la Ossa L, Martínez-Hernández J, et al. Density of GABAB Receptors Is Reduced in Granule Cells of the Hippocampus in a Mouse Model of Alzheimer’s Disease. Int J Mol Sci. 2020; 21: 2459. doi: 10.3390/ijms21072459 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Li ZL, Wang Y, Zou HW, Jing XY, Liu YJ, Li LF. GABA(B) receptors within the lateral habenula modulate stress resilience and vulnerability in mice. Physiol Behav. 2021; 230: 113311. doi: 10.1016/j.physbeh.2021.113311 [DOI] [PubMed] [Google Scholar]
- 22.Kumar K, Sharma S, Kumar P, Deshmukh R. Therapeutic potential of GABA(B) receptor ligands in drug addiction, anxiety, depression and other CNS disorders. Pharmacol Biochem Behav. 2013; 110: 174–184. doi: 10.1016/j.pbb.2013.07.003 [DOI] [PubMed] [Google Scholar]
- 23.Xu JY, Yang B, Sastry BR. The involvement of GABA-C receptors in paired-pulse depression of inhibitory postsynaptic currents in rat hippocampal CA1 pyramidal neurons. Exp Neurol. 2009; 216: 243–246. doi: 10.1016/j.expneurol.2008.11.013 [DOI] [PubMed] [Google Scholar]
- 24.Calvo-Flores Guzmán B, Vinnakota C, Govindpani K, Waldvogel HJ, Faull RLM, Kwakowsky A. The GABAergic system as a therapeutic target for Alzheimer’s disease. J Neurochem. 2018; 146: 649–669. doi: 10.1111/jnc.14345 [DOI] [PubMed] [Google Scholar]
- 25.Finkel S. Introduction to behavioural and psychological symptoms of dementia (BPSD). Int J Geriatr Psychiatry. 2000; 15:(Suppl 1): S2–S4. doi: [DOI] [PubMed] [Google Scholar]
- 26.Murayama MA, Arimitsu N, Shimizu J, Fujiwara N, Takai K, Ikeda Y, et al. Female dominance of both spatial cognitive dysfunction and neuropsychiatric symptoms in a mouse model of Alzheimer’s disease. Exp Anim. 2021; 70: 398–405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wang F, Feng TY, Yang S, Preter M, Zhou JN, Wang XP. Drug Therapy for Behavioral and Psychological Symptoms of Dementia. Curr Neuropharmacol. 2016; 14: 307–313. doi: 10.2174/1570159X14666151208114232 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Duncan T, Valenzuela M. Alzheimer’s disease, dementia, and stem cell therapy. Stem Cell Res Ther. 2017; 8: 111. doi: 10.1186/s13287-017-0567-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Fujiwara N, Shimizu J, Takai K, Arimitsu N, Saito A, Kono T, et al. Restoration of spatial memory dysfunction of human APP transgenic mice by transplantation of neuronal precursors derived from human iPS cells. Neurosci Lett. 2013; 557:(Pt B): 129–134. doi: 10.1016/j.neulet.2013.10.043 [DOI] [PubMed] [Google Scholar]
- 30.Fujiwara N, Shimizu J, Takai K, Arimitsu N, Ueda Y, Wakisaka S, et al. Cellular and molecular mechanisms of the restoration of human APP transgenic mouse cognitive dysfunction after transplant of human iPS cell-derived neural cells. Exp Neurol. 2015; 271: 423–431. doi: 10.1016/j.expneurol.2015.07.008 [DOI] [PubMed] [Google Scholar]
- 31.Games D, Adams D, Alessandrini R, Barbour R, Berthelette P, Blackwell C, et al. Alzheimer-type neuropathology in transgenic mice overexpressing V717F beta-amyloid precursor protein. Nature. 1995; 373: 523–527. doi: 10.1038/373523a0 [DOI] [PubMed] [Google Scholar]
- 32.Hartman RE, Izumi Y, Bales KR, Paul SM, Wozniak DF, Holtzman DM. Treatment with an amyloid-beta antibody ameliorates plaque load, learning deficits, and hippocampal long-term potentiation in a mouse model of Alzheimer’s disease. J Neurosci. 2005; 25: 6213–6220. doi: 10.1523/JNEUROSCI.0664-05.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Morris R. Developments of a water-maze procedure for studying spatial learning in the rat. J Neurosci Methods. 1984; 11: 47–60. doi: 10.1016/0165-0270(84)90007-4 [DOI] [PubMed] [Google Scholar]
- 34.Chen G, Chen KS, Knox J, Inglis J, Bernard A, Martin SJ, et al. A learning deficit related to age and beta-amyloid plaques in a mouse model of Alzheimer’s disease. Nature. 2000; 408: 975–979. doi: 10.1038/35050103 [DOI] [PubMed] [Google Scholar]
- 35.Vorhees CV, Williams MT. Morris water maze: procedures for assessing spatial and related forms of learning and memory. Nat Protoc. 2006; 1: 848–858. doi: 10.1038/nprot.2006.116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Simon P, Dupuis R, Costentin J. Thigmotaxis as an index of anxiety in mice. Influence of dopaminergic transmissions. Behav Brain Res. 1994; 61: 59–64. doi: 10.1016/0166-4328(94)90008-6 [DOI] [PubMed] [Google Scholar]
- 37.España J, Giménez-Llort L, Valero J, Miñano A, Rábano A, Rodriguez-Alvarez J, et al. Intraneuronal beta-amyloid accumulation in the amygdala enhances fear and anxiety in Alzheimer’s disease transgenic mice. Biol Psychiatry. 2010; 67: 513–521. doi: 10.1016/j.biopsych.2009.06.015 [DOI] [PubMed] [Google Scholar]
- 38.Huang Y, Zhou W, Zhang Y. Bright lighting conditions during testing increase thigmotaxis and impair water maze performance in BALB/c mice. Behav Brain Res. 2012; 226: 26–31. doi: 10.1016/j.bbr.2011.08.043 [DOI] [PubMed] [Google Scholar]
- 39.Karabeg MM, Grauthoff S, Kollert SY, Weidner M, Heiming RS, Jansen F, et al. 5-HTT deficiency affects neuroplasticity and increases stress sensitivity resulting in altered spatial learning performance in the Morris water maze but not in the Barnes maze. PLoS One. 2013; 8: e78238. doi: 10.1371/journal.pone.0078238 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Evans JD. Straightforward Statistics for the Behavioral Sciences. Brooks/Cole Publishing, Pacific Grove. 1996. [Google Scholar]
- 41.Kheirbek MA, Hen R. Dorsal vs ventral hippocampal neurogenesis: implications for cognition and mood. Neuropsychopharmacology. 2011; 36: 373–374. doi: 10.1038/npp.2010.148 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Fanselow MS, Dong HW. Are the dorsal and ventral hippocampus functionally distinct structures? Neuron. 2010; 65: 7–19. doi: 10.1016/j.neuron.2009.11.031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Morris RG, Schenk F, Tweedie F, Jarrard LE. Ibotenate Lesions of Hippocampus and/or Subiculum: Dissociating Components of Allocentric Spatial Learning. Eur J Neurosci. 1990; 2: 1016–1028. doi: 10.1111/j.1460-9568.1990.tb00014.x [DOI] [PubMed] [Google Scholar]
- 44.Hock BJ, Jr, Bunsey MD. Differential effects of dorsal and ventral hippocampal lesions. J Neurosci. 1998; 18: 7027–7032. doi: 10.1523/JNEUROSCI.18-17-07027.1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Morris RG, Garrud P, Rawlins JN, O’Keefe J. Place navigation impaired in rats with hippocampal lesions. Nature. 1982; 297: 681–683. doi: 10.1038/297681a0 [DOI] [PubMed] [Google Scholar]
- 46.Bannerman DM, Deacon RM, Offen S, Friswell J, Grubb M, Rawlins JN. Double dissociation of function within the hippocampus: spatial memory and hyponeophagia. Behav Neurosci. 2002; 116: 884–901. doi: 10.1037/0735-7044.116.5.884 [DOI] [PubMed] [Google Scholar]
- 47.Pothuizen HH, Zhang WN, Jongen-Rêlo AL, Feldon J, Yee BK. Dissociation of function between the dorsal and the ventral hippocampus in spatial learning abilities of the rat: a within-subject, within-task comparison of reference and working spatial memory. Eur J Neurosci. 2004; 19: 705–712. doi: 10.1111/j.0953-816X.2004.03170.x [DOI] [PubMed] [Google Scholar]
- 48.Bannerman DM, Grubb M, Deacon RM, Yee BK, Feldon J, Rawlins JN. Ventral hippocampal lesions affect anxiety but not spatial learning. Behav Brain Res. 2003; 139: 197–213. doi: 10.1016/S0166-4328(02)00268-1 [DOI] [PubMed] [Google Scholar]
- 49.Bagot RC, Parise EM, Peña CJ, Zhang HX, Maze I, Chaudhury D, et al. Ventral hippocampal afferents to the nucleus accumbens regulate susceptibility to depression. Nat Commun. 2015; 6: 7062. doi: 10.1038/ncomms8062 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kempermann G, Song H, Gage FH. Neurogenesis in the Adult Hippocampus. Cold Spring Harb Perspect Biol. 2015; 7: a018812. doi: 10.1101/cshperspect.a018812 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Nicola Z, Fabel K, Kempermann G. Development of the adult neurogenic niche in the hippocampus of mice. Front Neuroanat. 2015; 9: 53. doi: 10.3389/fnana.2015.00053 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Santarelli L, Saxe M, Gross C, Surget A, Battaglia F, Dulawa S, et al. Requirement of hippocampal neurogenesis for the behavioral effects of antidepressants. Science. 2003; 301: 805–809. doi: 10.1126/science.1083328 [DOI] [PubMed] [Google Scholar]
- 53.Boldrini M, Underwood MD, Hen R, Rosoklija GB, Dwork AJ, John Mann J, et al. Antidepressants increase neural progenitor cells in the human hippocampus. Neuropsychopharmacology. 2009; 34: 2376–2389. doi: 10.1038/npp.2009.75 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Tanti A, Belzung C. Neurogenesis along the septo-temporal axis of the hippocampus: are depression and the action of antidepressants region-specific? Neuroscience. 2013; 252: 234–252. doi: 10.1016/j.neuroscience.2013.08.017 [DOI] [PubMed] [Google Scholar]
- 55.Tanti A, Belzung C. Hippocampal neurogenesis: a biomarker for depression or antidepressant effects? Methodological considerations and perspectives for future research. Cell Tissue Res. 2013; 354: 203–219. doi: 10.1007/s00441-013-1612-z [DOI] [PubMed] [Google Scholar]
- 56.Darcet F, Mendez-David I, Tritschler L, Gardier AM, Guilloux JP, David DJ. Learning and memory impairments in a neuroendocrine mouse model of anxiety/depression. Front Behav Neurosci. 2014; 8: 136. doi: 10.3389/fnbeh.2014.00136 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Typlt M, Mirkowski M, Azzopardi E, Ruettiger L, Ruth P, Schmid S. Mice with deficient BK channel function show impaired prepulse inhibition and spatial learning, but normal working and spatial reference memory. PLoS One. 2013; 8: e81270. doi: 10.1371/journal.pone.0081270 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.López Ruiz JR, Osuna Carrasco LP, López Valenzuela CL, Franco Rodríguez NE, de la Torre Valdovinos B, Jiménez Estrada I, et al. The hippocampus participates in the control of locomotion speed. Neuroscience. 2015; 311: 207–215. doi: 10.1016/j.neuroscience.2015.10.034 [DOI] [PubMed] [Google Scholar]
- 59.Laurin D, Verreault R, Lindsay J, MacPherson K, Rockwood K. Physical activity and risk of cognitive impairment and dementia in elderly persons. Arch Neurol. 2001; 58: 498–504. doi: 10.1001/archneur.58.3.498 [DOI] [PubMed] [Google Scholar]
- 60.Najar J, Östling S, Gudmundsson P, Sundh V, Johansson L, Kern S, et al. Cognitive and physical activity and dementia: A 44-year longitudinal population study of women. Neurology. 2019; 92: e1322–e1330. doi: 10.1212/WNL.0000000000007021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.West MJ, Coleman PD, Flood DG, Troncoso JC. Differences in the pattern of hippocampal neuronal loss in normal ageing and Alzheimer’s disease. Lancet. 1994; 344: 769–772. doi: 10.1016/S0140-6736(94)92338-8 [DOI] [PubMed] [Google Scholar]
- 62.Scheff SW, Price DA, Schmitt FA, DeKosky ST, Mufson EJ. Synaptic alterations in CA1 in mild Alzheimer disease and mild cognitive impairment. Neurology. 2007; 68: 1501–1508. doi: 10.1212/01.wnl.0000260698.46517.8f [DOI] [PubMed] [Google Scholar]
- 63.Wright AL, Zinn R, Hohensinn B, Konen LM, Beynon SB, Tan RP, et al. Neuroinflammation and neuronal loss precede Aβ plaque deposition in the hAPP-J20 mouse model of Alzheimer’s disease. PLoS One. 2013; 8: e59586. doi: 10.1371/journal.pone.0059586 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Froemke RC. Plasticity of cortical excitatory-inhibitory balance. Annu Rev Neurosci. 2015; 38: 195–219. doi: 10.1146/annurev-neuro-071714-034002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Lowery-Gionta EG, DiBerto J, Mazzone CM, Kash TL. GABA neurons of the ventral periaqueductal gray area modulate behaviors associated with anxiety and conditioned fear. Brain Struct Funct. 2018; 223: 3787–3799. doi: 10.1007/s00429-018-1724-z [DOI] [PubMed] [Google Scholar]
- 66.Mann EO, Kohl MM, Paulsen O. Distinct roles of GABA(A) and GABA(B) receptors in balancing and terminating persistent cortical activity. J Neurosci. 2009; 29: 7513–7518. doi: 10.1523/JNEUROSCI.6162-08.2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Möhler H. The GABA system in anxiety and depression and its therapeutic potential. Neuropharmacology. 2012; 62: 42–53. doi: 10.1016/j.neuropharm.2011.08.040 [DOI] [PubMed] [Google Scholar]
- 68.Ferreira-Vieira TH, Guimaraes IM, Silva FR, Ribeiro FM. Alzheimer’s disease: Targeting the Cholinergic System. Curr Neuropharmacol. 2016; 14: 101–115. doi: 10.2174/1570159X13666150716165726 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Mineur YS, Obayemi A, Wigestrand MB, Fote GM, Calarco CA, Li AM, et al. Cholinergic signaling in the hippocampus regulates social stress resilience and anxiety- and depression-like behavior. Proc Natl Acad Sci USA. 2013; 110: 3573–3578. doi: 10.1073/pnas.1219731110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Picciotto MR, Higley MJ, Mineur YS. Acetylcholine as a neuromodulator: cholinergic signaling shapes nervous system function and behavior. Neuron. 2012; 76: 116–129. doi: 10.1016/j.neuron.2012.08.036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Noboru Suzuki JS, Takai K. Nagisa Arimitsu, Tomoko Suzuki, Naruyoshi Fujiwara. Cellular and Molecular Mechanisms Governing Functional Recovery of Dementia Mice after Neuronal cell Transplantation. J Neurosci Neurosurg. 2017; 1: 1–8. [Google Scholar]