Graphic Abstract
Dendrobium Sw. is one of the largest genera in the orchidaceous family and includes 900–2000 species. Among them, more than 80 Dendrobium species have been reported in China. However, there are only six Dendrobium species, namely, D. bigibbum var. superbum (syn. D. phalaenopsis), D. chrysanthum, D. fimbriatum, D. loddigesii, D. nobile, and D. officinale (syn. D. candidum), listed in the New Inventory of Existing Cosmetic Ingredients in China Launched. Artificial planting of Dendrobium species has been a great success in China. To better utilize Dendrobium resources for medicinal and cosmetic purposes, we summarize their traditional uses and pharmacologically active compounds for treating dermatological disorders in this review. “Orchidaceae”, “Dendrobium”, “traditional use”, “ethnobotany”, “dermatological disorder”, and “skin disease” were used as search terms to screen the literature. Cited references were collected between 1970 and 2020 from the Web of Science, China National Knowledge Internet (CNKI), SciFinder, Google Scholar, and Chinese books. From the search, it was found that there are 22 Dendrobium species with traditional uses in dermatological disorders, and 131 compounds from Dendrobium plants have been reported to possess anti-inflammatory, antimicrobial, antioxidant, antiaging, anti-psoriasis, and tyrosinase-inhibitory activities, implying that Dendrobium plants are important resources for the discovery of active compounds and the development of new drugs and cosmetics. D. crepidatum, D. denneanum, D. loddigesii, D. nobile, and D. officinale have been extensively studied. More research on other Dendrobium species is needed. The major active compounds found in Dendrobium species are phenanthrenes, alkaloids, flavonoids, phenylpropanoids, and lignans. Several compounds, such as loddigesiinol A, (S)-5-methoxy-2,4,7,9-tetrahydroxy-9,10-dihydrophenanthrene, (S)-4-methoxy-2,5,7,9-tetrahydroxy-9,10-dihydrophenanthrene, 2,5-dihydroxy-4-methoxy-phenanthrene 2-O-β-d-glucopyranoside, (9R)-1,2,5,9-tetrahydroxy-9,10-dihydrophenanthrene 5-O-β-d-glucopyranoside, (+)-homocrepidine A, and vicenin 2, have significant anti-inflammatory activities and inhibit nitric oxide (NO) production with IC50 values less than 5 μM, and these compounds are worthy of further study.

Keywords: Orchidaceae, Dendrobium, Traditional uses, Dermatological disorders, Anti-inflammatory
Introduction
Even though human skin is not the largest organ by weight or functional surface area [1], it is the main interface of the human body with the external environment. The skin meets the environment directly and thus is vulnerable to various types of damage. For this function, the skin possesses remarkable barrier qualities that protect humans from external pathogens and prevent the uncontrolled loss of water from the body. Although mortality rates for skin diseases are generally relatively low, they are often persistent, difficult to treat, can significantly impact quality of life and have a major psychological impact [2].
Dendrobium Sw. is one of the most important genera of Orchidaceae. The genus is one of the largest members of the orchidaceous family and includes 900–2000 species [3]. Dendrobium plants are mainly distributed in the tropics and subtropics in southern Asia, Oceanica, and elsewhere [4]. According to Plants of the World Online, there are 1,556 accepted Dendrobium species at present [5]. Among them, more than 80 Dendrobium species have been reported in China [4]. However, there are only six Dendrobium species, namely, D. bigibbum var. superbum Rchb.f. (syn. D. phalaenopsis Fitzg.), D. chrysanthum Wall. ex Lindl., D. fimbriatum Hook., D. loddigesii Rolfe, D. nobile Lindl, and D. officinale Kimura et Migo (syn. D. candidum Wall. ex Lindl.), listed in the New Inventory of Existing Cosmetic Ingredients in China Launched (IECIC 2015, Final Version) [6].
Artificial planting of Dendrobium species has been a great success in China. The total mass of annual cultivated Dendrobium plants in China now exceeds 19 million kg [7]. To better utilize Dendrobium resources for medicinal and cosmetic purposes, we summarize their traditional uses and pharmacologically active constituents for treating dermatological disorders. “Orchidaceae”, “Dendrobium”, “traditional use”, “ethnobotany”, “dermatological disorder”, and “skin disease” were used as search terms to screen the literature. Cited references were collected between 1970 and 2020 from the Web of Science, China National Knowledge Internet (CNKI), SciFinder, Google Scholar, and Chinese books. For pharmacological activities, only extracts or compounds with IC50, EC50, or MIC values less than 100 μg/mL were cited.
Traditional Uses of Dendrobium Species for Treating Dermatological Disorders
Traditional uses of 22 Dendrobium species for treating dermatological disorders by local people in Australia, Bangladesh, China, India, Indonesia, Liberia, Malaysia, and Nepal are found in the literature (Table 1).
Table 1.
Traditional uses of Dendrobium plants for treating dermatological disorders in different countries
| Latin name | Country | Local name | Part used | Traditional use | References |
|---|---|---|---|---|---|
| Dendrobium affine Steudel | Australia | Marndaja, tjalamarinj | Pseudobulbs | The sap from the pseudobulbs is directly squeezed onto sores to relieve itchy skin | [85] |
| Australia | – | Stems, bulbs | Fluid from the stem or bulb is used on skin to treat itching, cuts, sores and minor burns | [86] | |
| Dendrobium alpestre Royle | India | Jiwanti | Bulbs | For treating pimples, boils and other skin eruptions | [87] |
| Dendrobium amoenum Wall. ex Lindl | India | – | Leaves | Leaf paste is used to treat skin diseases | [88] |
| India | Mitha alu | Leaves | Leaves of D. amoenum pounded with Hedychium wardii C.E.C. Fisch. rhizomes are made into a paste, which is used to treat wounds and various skin diseases | [89] | |
| Nepal | Thuur | Pseudobulbs | A fresh paste is applied topically on burnt skin | [90] | |
| Dendrobium aphyllum (Roxb.) C.E.C. Fisch | Bangladesh | – | Leaves | A paste or juice of the leaves is used to treat wounds | [91] |
| China | Dou chun shi hu | Whole plants, stems | Whole plants are used to treat burns and scalds. Fresh stems are externally used to tread burns and scalds | [92, 93] | |
| India | – | Leaves | Fresh leaf juice is used to treat skin infections | [94] | |
| Dendrobium denneanum Kerr [syn. Dendrobium aurantiacum var. denneanum (Kerr) Z.H.Tsi] | China | Die qiao shi hu | Stems, leaves | Stems are used to treat impetigo. Dry leaves are externally used to treat impetigo | [92, 95] |
| Dendrobium canaliculatum R. Br | Australia | Marndaja | Pseudobulbs | Pseudobulbs are squeezed, and the sap is applied directly to sores to help heal them | [85] |
| Dendrobium chrysanthum Wall. ex Lindl | India | Nauawimu | Stems | Stem juice is applied on wounds and sores | [96] |
| Dendrobium crumenatum Sw | Malaysia | Daun sepuleh tulang | Leaves | A poultice made from leaves is used to treat boils and pimples | [7] |
| Dendrobium densiflorum Lindl | Nepal | Sungava | Pseudobulbs | Fresh pulp is applied to boils and pimples | [90] |
| Dendrobium discolor Lindl | Australia | – | Stems | A poultice is prepared from young canes to draw a boil. A liniment made from mature canes is used to treat ringworm | [97] |
| Dendrobium fimbriatum Hook | India | – | Leaves | A paste of fresh leaves is used to treat boils and pimples | [94] |
| Indiaa | Mokya tu | Leaves | Approximately 10 g of leaves are ground, made into a paste and applied twice a day for 10 days to heal cuts and wounds | [98] | |
| Dendrobium hancockii Rolfe | China | Xi ye shi hu | Stems | To treat ulcers | [99] |
| Dendrobium herbaceum Lindl | India | Agai | Roots | Fresh roots are burnt, and 10 g of the resultant ash is mixed with 10 ml mustard oil and applied on affected skin 2 to 3 times daily for several days until symptoms disappear | [100] |
| Dendrobium macraei Lindl. [syn. Flickingeria macraei (Lindl.) Seidenf.] | India | Sakar | Roots | One spoonful of a root paste along with 1 g of a seed powder of black pepper is administered orally on an empty stomach twice a day for 21 days to cure diseases, including skin allergies, and is applied on the affected part of skin to cure eczema | [101] |
| Dendrobium macrostachyum Lindl | India | Yanaimiratti | Aerial parts | Aerial parts are used for skin allergies | [102] |
| Dendrobium monticola P.F. Hunt & Summerh | Nepal | Jiwanti | Bulbs | For treating pimples, boils, and other skin eruptions | [103] |
| Dendrobium nobile Lindl | Bangladesh | – | Leaves, seeds | A leaf extract is made and is very effective for treating freshly cut wounds. A seed powder is used to cure cuts and wounds | [104] |
| India | – | Pseudobulbs | A pseudobulb extract is used to soothe burns | [105] | |
| India | – | Roots, seeds | Powdery seeds and root powder are used to heal wounds | [106] | |
| India | – | Seeds | Powdery seeds are applied to fresh wounds for quick healing | [107] | |
| India | – | Whole plants | Whole plant parts are used in the treatment of cuts and wounds | [108] | |
| Dendrobium ovatum (L.) Kraenzl | India | Unnesh chedi | Pseudostems | The plant is an emollient | [109] |
| Dendrobium planibulbe Lindl | Malaysia | Miga | – | A poultice is made by pounding the plant to treat dermatological lesions affecting the back of the neck | [7] |
| Dendrobium polyanthum Wall. ex Lindl. [syn. Dendrobium primulinum Lindl.] | China | Bao chun shi hu | Pseudobulbs | To treat burns and scalds, skin itching caused by a red rash, and eczema. Fresh pseudobulbs are ground with water to yield a juice, or fresh pseudobulbs are pounded and externally used to treat scalds. A decoction of dry pseudobulbs (15 g) is taken orally to treat skin itching caused by red rash | [110] |
| Dendrobium purpureum Roxb | Indonesia | – | Leaves, stems | To treat infected nails | [111] |
| Dendrobium sp. | Liberia | Gulubalama boblogie | Leaves | Crushed leaf extracts are applied on boils for fast relief | [112] |
aIn Ref. [98], the synonym Dendrobium fimbriatum Hook. var. occulatum Hook.f. of the plant was used
As shown in Table 1, the uses of Dendrobium plants include treatments of boils [Dendrobium alpestre Royle, D. crumenatum Sw., D. densiflorum Lindl., D. discolor Lindl., D. fimbriatum, D. monticola P.F. Hunt & Summerh., and Dendrobium sp. (local name: gulubalama boblogie)], cuts (Dendrobium affine Steudel, D. fimbriatum, and D. nobile), burns [D. affine, D. amoenum Wall. ex Lindl., D. aphyllum (Roxb.) C.E.C. Fisch., D. nobile, and D. polyanthum Wall. ex Lindl.], eczema (D. macraei Lindl. and D. polyanthum), impetigo (D. denneanum Kerr), infected nails (Dendrobium purpureum Roxb.), itchy skin (Dendrobium affine Steudel), pimples (D. alpestre, D. crumenatum, D. densiflorum, D. fimbriatum, and D. monticola), scalds (D. aphyllum and D. polyanthum), skin allergies (D. macraei and D. macrostachyum Lindl.), sores (D. affine, D. canaliculatum R. Br., and D. chrysanthum), ringworm (D. discolor), ulcer (D. hancockii Rolfe), and wounds (D. amoenum, D. aphyllum, D. chrysanthum, D. fimbriatum, and D. nobile). The treatments of boils (seven species), burns (five species), pimples (five species), and wounds (five species) are the most common uses (Table 1).
Plant parts used of Dendrobium species for treating dermatological disorders include aerial parts (one species), bulbs (three species), leaves (eight species), pseudobulbs (six species), pseudostems (one species), roots (three species), seeds (one species), stems (seven species), and whole plants (two species). Leaves, pseudobulbs, and stems are the most common parts used.
Pharmacological Activities of Extracts, Preparations, and Chemical Constituents from Dendrobium Plants for Treating Dermatological Disorders
Some extracts, preparations, and chemical constituents from Dendrobium plants exhibit pharmacological activities related to dermatological disorders, such as anti-inflammatory, antimicrobial, antioxidant, antiaging, anti-psoriasis, hair growth promoting, skin-moisturizing, and tyrosinase-inhibitory activities. A part of pharmacological activities is related to the traditional uses of Dendrobium plants. For example, anti-inflammatory activities are associated with treatments of eczema, itchy skin, and skin allergies, while antimicrobial activities are associated with treatments of boils, impetigo, and pimples.
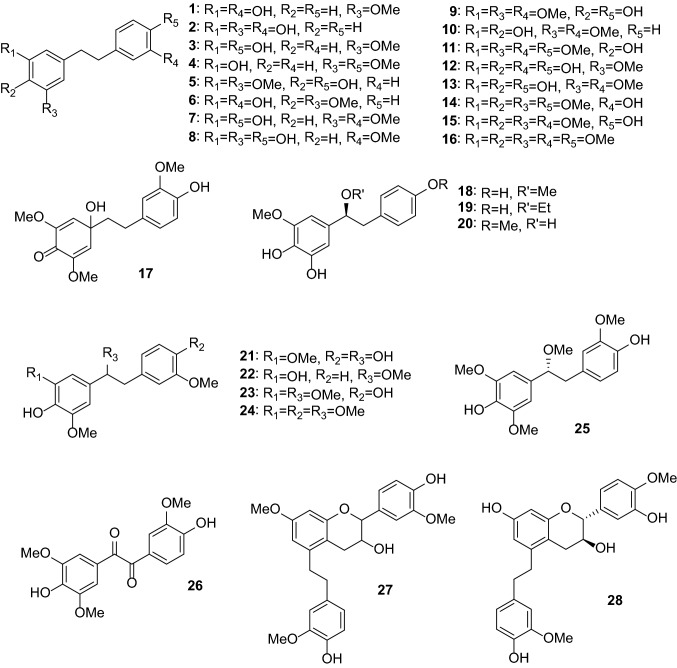
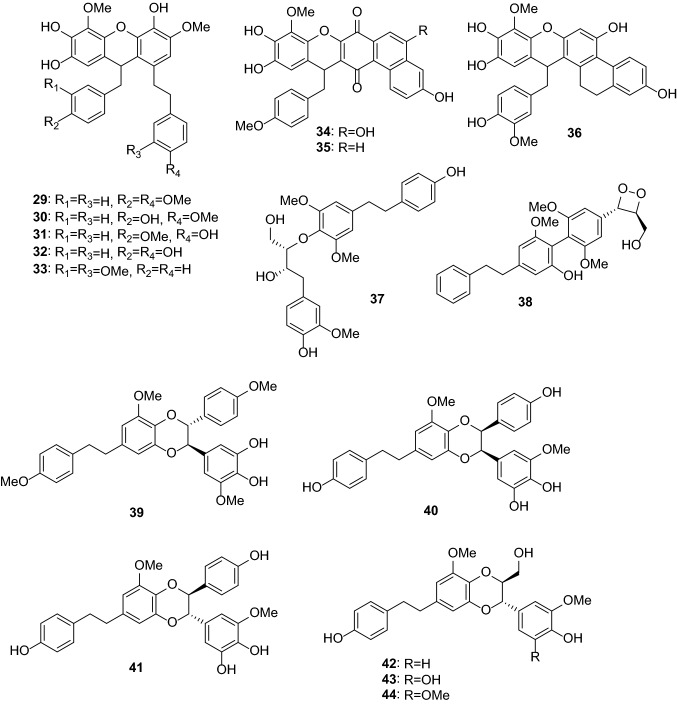
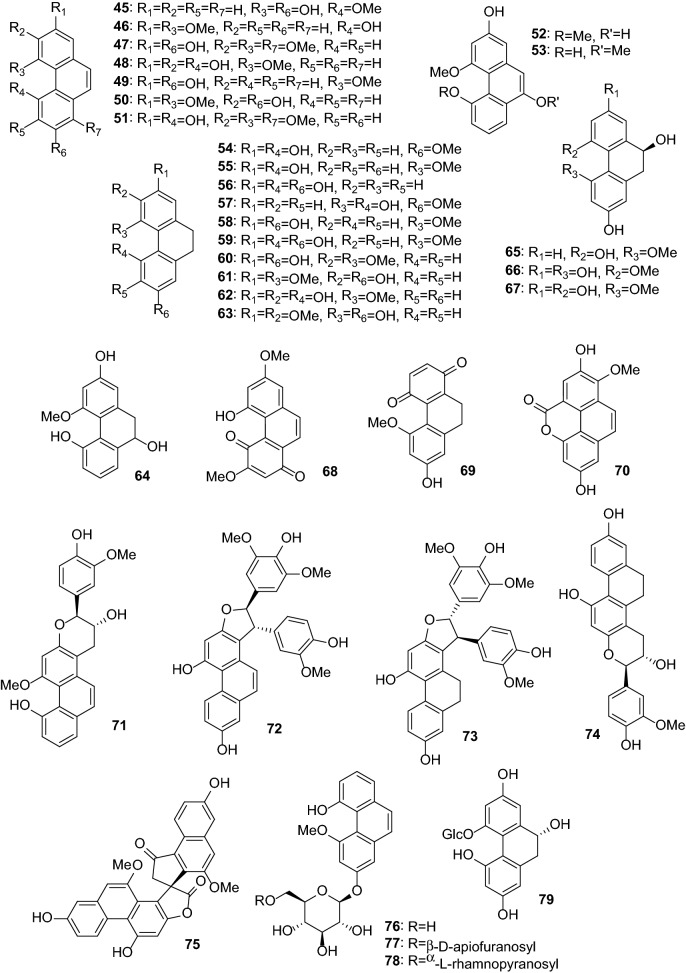
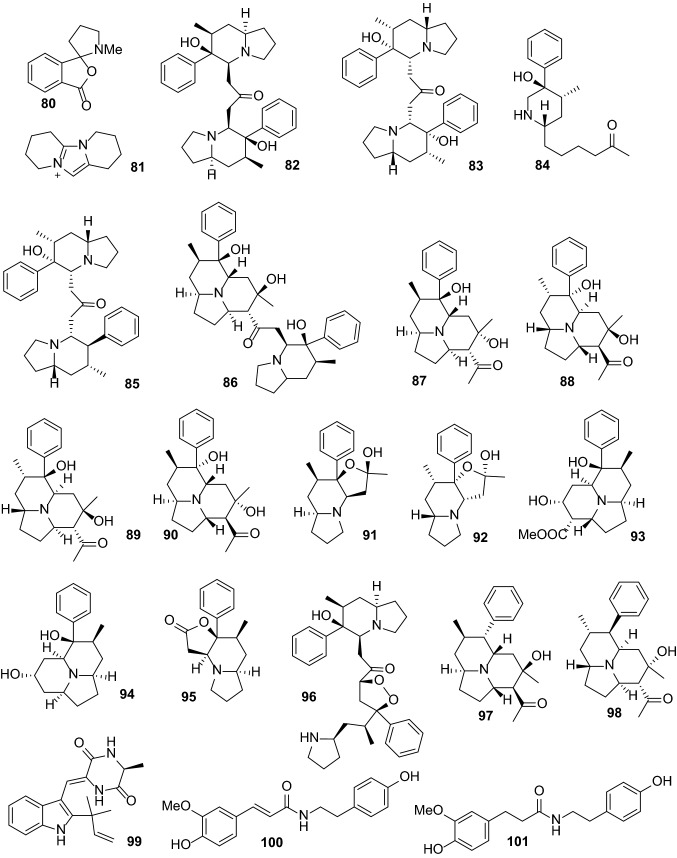
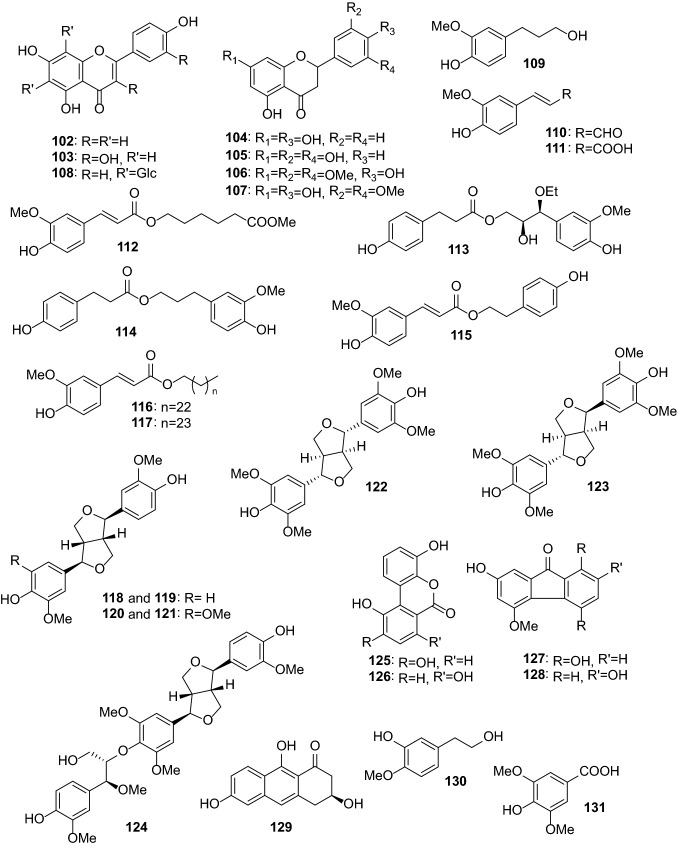
Anti-inflammatory and antioxidant activities are the most common activities of Dendrobium extracts and compounds. One hundred thirty-one compounds from Dendrobium plants have been reported to possess anti-inflammatory, antimicrobial, antioxidant, antiaging, anti-psoriasis, and tyrosinase-inhibitory activities (Table 2). These compounds include bibenzyls (1–44, Figs. 1 and 2), phenanthrenes (45–79, Fig. 3), alkaloids (80–101, Fig. 4), flavonoids (102–108), phenylpropanoids (109–117), lignans (118–124), and others (125–131, Fig. 5).
Table 2.
Pharmacologically active compounds (1–131) from Dendrobium plants
| No. | Name | Type | Source | Pharmacological activitiesa | References |
|---|---|---|---|---|---|
| 1 | Batatasin III | Bibenzyls | D. loddigesii | Antiaging (collagen production: EC50 3.2 μg/mL), anti-inflammatory (NO: IC50 21.9 μM), and antioxidant (DPPH: IC50 50.1 μg/mL) | [20, 49] |
| 2 | 3,3ʹ,5-Trihydroxybibenzyl | Bibenzyls | D. loddigesii | Anti-inflammatory (NO: IC50 13.1 μM) and antioxidant (DPPH: 85.8 μM) | [20] |
| Tyrosinase-inhibitory (IC50 37.9 μg/mL) | [49] | ||||
| 3 | 3,4ʹ-Dihydroxy-5-methoxybibenzyl | Bibenzyls | D. officinale | Antioxidant (ABTS: 5.3 μM) | [66] |
| 4 | 3-Hydroxy-4ʹ,5-dimethoxybibenzyl | Bibenzyls | D. heterocarpum | Anti-inflammatory | [15] |
| 5 | 4,4ʹ-Dihydroxy-3,5-dimethoxybibenzyl | Bibenzyls | D. loddigesii | Anti-inflammatory (NO: IC50 49.3 μM) and antioxidant (DPPH: IC50 94.5 μM) | [20] |
| 6 | 3,3ʹ-Dihydroxy-4,5-dimethoxybibenzyl | Bibenzyls | D. williamsonii | Antioxidant (DPPH: IC50 19.5 μM) | [74] |
| 7 | Gigantol | Bibenzyls | D. draconis | Antioxidant (DPPH: IC50 17.7 μM) | [47] |
| D. heterocarpum | Anti-inflammatory | [15] | |||
| D. loddigesii | Antioxidant | [49] | |||
| D. nobile | Anti-inflammatory (NO: IC50 32.9 μM) and antioxidant (DPPH: IC50 56.4 μM) | [23] | |||
| Dendrobium species | Antibacterial (Staphylococcus aureus: MIC 82.2 μg/mL) | [32, 33] | |||
| 8 | Tristin | Bibenzyls | D. loddigesii | Antioxidant | [49] |
| D. officinale | Antioxidant (ABTS: IC50 9.0 μΜ; DPPH: IC50 34.5 μM) | [66] | |||
| 9 | Moscatilin | Bibenzyls | D. loddigesii | Antioxidant | [49] |
| D. nobile | Anti-inflammatory (NO: IC50 27.6 and 36.8 μM) and antioxidant (DPPH: IC50 14.5 μM) | [22, 23] | |||
| D. secundum | Antioxidant (DPPH: IC50 5.1 μM) | [70] | |||
| D. williamsonii | Antioxidant (DPPH: IC50 8.5 μM) | [74] | |||
| 10 | Dendrobin A | Bibenzyls | D. nobile | Antioxidant (DPPH: IC50 40.3 μM) | [23] |
| 11 | Chrysotoxine | Bibenzyls | D. nobile | Antioxidant (DPPH: IC50 14.0 μM) | [23] |
| 12 | Dendrocandin E | Bibenzyls | D. officinale | Antioxidant (DPPH: IC50 15.6 μM) | [61] |
| 13 | 4,5,4ʹ-Trihydroxy-3,3ʹ-dimethoxybibenzyl | Bibenzyls | D. loddigesii | Antioxidant | [49] |
| D. secundum | Antioxidant (DPPH: IC50 15.9 μM) | [70] | |||
| 14 | Erianin | Bibenzyls | D. chrysotoxum | Antibacterial (srtA: IC50 20.9 μg/mL) | [34, 35] |
| Anti-psoriasis | [75] | ||||
| 15 | Crepidatin | Bibenzyls | D. loddigesii | Antioxidant | [49] |
| D. nobile | Antioxidant (DPPH: IC50 21.8 μM) | [23] | |||
| 16 | Chrysotobibenzyl | Bibenzyls | D. nobile | Anti-inflammatory (NO: IC50 48.2 μM) | [23] |
| 17 | Aphyllone B | Bibenzyls | D. aphyllum | Antioxidant | [42] |
| 18 | Dendrocandin C | Bibenzyls | D. officinale | Antioxidant (DPPH: IC50 34.2 μM) | [61] |
| 19 | Dendrocandin D | Bibenzyls | D. officinale | Antioxidant (DPPH: IC50 34.5 μM) | [61] |
| 20 | (S)-3,4,α-trihydroxy-5,4ʹ-dimethoxybibenzyl | Bibenzyls | D. officinale | Antioxidant (DPPH: IC50 32.3 μM) | [64] |
| 21 | Nobilin D | Bibenzyls | D. nobile | Anti-inflammatory (NO: IC50 15.3 μM) and antioxidant (DPPH: IC50 19.9 μM) | [23] |
| 22 | Nobilin A | Bibenzyls | D. nobile | Antioxidant (DPPH: IC50 87.1 μM) | [56] |
| 23 | Nobilin B | Bibenzyls | D. nobile | Antioxidant (DPPH: IC50 32.2 μM) | [56] |
| 24 | Nobilin C | Bibenzyls | D. nobile | Antioxidant (DPPH: IC50 47.4 μg/mL) | [56] |
| 25 | Loddigesiinol C | Bibenzyls | D. loddigesii | Antioxidant (DPPH: IC50 23.7 μM) | [19] |
| 26 | Loddigesiinol D | Bibenzyls | D. loddigesii | Anti-inflammatory (NO: IC50 69.7 μM) | [19] |
| 27 | Crepidatuol B | Bibenzyls | D. loddigesii | Antioxidant | [50] |
| 28 | Trigonopol B | Bibenzyls | D. loddigesii | Anti-inflammatory (NO: IC50 26.3 μM) and antioxidant (DPPH: IC50 60.1 μM) | [20] |
| 29 | Dendrocandin F | Bibenzyls | D. officinale | Antioxidant (DPPH: IC50 55.8 μM) | [62] |
| 30 | Dendrocandin G | Bibenzyls | D. officinale | Antioxidant (DPPH: IC50 32.4 μM) | [62] |
| 31 | Dendrocandin J | Bibenzyls | D. officinale | Aantioxidant (DPPH: IC50 36.8 μM) | [63] |
| 32 | Dendrocandin K | Bibenzyls | D. officinale | Antioxidant (DPPH: IC50 70.2 μM) | [63] |
| 33 | Nobilin E | Bibenzyls | D. nobile | Anti-inflammatory (NO: IC50 19.2 μM) and antioxidant (DPPH: IC50 21.0 μM) | [23] |
| 34 | Dendrocandin H | Bibenzyls | D. officinale | Antioxidant (DPPH: IC50 32.4 μM) | [62] |
| 35 | Dendrocandin L | Bibenzyls | D. officinale | Antioxidant (DPPH: IC50 45.0 μM) | [63] |
| 36 | (−)-Dendroparishiol | Bibenzyls | D. parishii | Anti-inflammatory | [28] |
| 37 | Dendrocandin M | Bibenzyls | D. officinale | Antioxidant (DPPH: IC50 60.5 μM) | [63] |
| 38 | 6ʺ-De-O-methyldendrofindlaphenol A | Bibenzyls | D. findlayanum | Anti-inflammatory (NO: IC50 21.4 μM) | [14] |
| 39 | Dendrocandin I | Bibenzyls | D. heterocarpum | Anti-inflammatory | [15] |
| D. officinale | Antioxidant (DPPH: IC50 21.3 μM) | [62] | |||
| 40 | Dendrocandin P | Bibenzyls | D. officinale | Antioxidant (DPPH: IC50 22.3 μM) | [63] |
| 41 | Dendrocandin Q | Bibenzyls | D. officinale | Antioxidant (DPPH: IC50 30.3 μM) | [63] |
| 42 | Dendrocandin N | Bibenzyls | D. officinale | Antioxidant (DPPH: IC50 87.6 μM) | [63] |
| 43 | Dendrocandin O | Bibenzyls | D. officinale | Antioxidant (DPPH: IC50 50.4 μM) | [63] |
| 44 | Dendrocandin U | Bibenzyls | D. officinale | Antioxidant (ABTS: IC50 10.0 μM) | [66] |
| 45 | Moscatin (plicatol B) | Phenanthrenes | D. denneanum | Anti-inflammatory (NO: IC50 6.3 μM) | [13] |
| D. loddigesii | Anti-inflammatory (NO: IC50 6.4 μM) and antioxidant (DPPH: IC50 59.8 μM) | [19, 50] | |||
| 46 | 5-Hydroxy-2,4-dimethoxyphenanthrene | Phenanthrenes | D. loddigesii | Anti-inflammatory (NO: IC50 5.3 μM) | [19] |
| 47 | Confusarin | Phenanthrenes | D. nobile | Antioxidant (DPPH: IC50 12.9 μM) | [57] |
| 48 | Fimbriol B | Phenanthrenes | D. nobile | Anti-inflammatory (NO: IC50 28.9 μM) | [22] |
| 49 | Flavanthrinin | Phenanthrenes | D. nobile | Antioxidant (DPPH: IC50 35.7 μM) | [57] |
| 50 | 5,7-Dimethoxyphenanthrene-2,6-diol | Phenanthrenes | D. nobile | Anti-inflammatory (NO: IC50 37.7 μM) | [22] |
| Antioxidant (DPPH: IC50 29.7 μM) | [57] | ||||
| 51 | 3,4,8-Trimethoxyphenanthrene-2,5-diol | Phenanthrenes | D. nobile | Anti-inflammatory (NO: IC50 20.4 μM) | [22] |
| 52 | Loddigesiinol A | Phenanthrenes | D. loddigesii | Anti-inflammatory (NO: IC50 2.6 μM) and antioxidant (DPPH: IC50 26.1 μM) | [19] |
| 53 | 2,5-Dihydroxy-4,9-dimethoxyphenanthrene | Phenanthrenes | D. nobile | Antioxidant (DPPH: IC50 34.8 μM) | [57] |
| 54 | Lusianthridin | Phenanthrenes | D. loddigesii | Anti-inflammatory (NO: IC50 4.6 μM) and antioxidant (IC50 62.2 μM) | [19] |
| D. nobile | Anti-inflammatory (NO: IC50 9.6 μM) | [22] | |||
| 55 | Hircinol | Phenanthrenes | D. draconis | Antioxidant (DPPH: IC50 22.3 μM) | [47] |
| D. loddigesii | Anti-inflammatory (NO: IC50 29.2 μM) | [19] | |||
| D. nobile | Anti-inflammatory (NO: IC50 26.4 μM) | [22] | |||
| 56 | 9,10-Dihydrophenanthrene-2,4,7-triol | Phenanthrenes | D. denneanum | Anti-inflammatory (NO: IC50 32.7 μM) | [13] |
| D. loddigesii | Anti-inflammatory (NO: IC50 8.6 μM) and antioxidant (DPPH: IC50 14.1 μM) | [20, 50] | |||
| 57 | 2-Methoxy-9,10-dihydrophenanthrene-4,5-diol | Phenanthrenes | D. denneanum | Anti-inflammatory (NO: IC50 7.6 μM) | [13] |
| 58 | Coelonin | Phenanthrenes | D. nobile | Anti-inflammatory (NO: IC50 10.2 μM) | [22] |
| 59 | 7-Methoxy-9,10-dihydrophenanthrene-2,4,5-triol | Phenanthrenes | D. draconis | Antioxidant (DPPH: IC50 10.2 μM) | [47] |
| 60 | Erianthridin | Phenanthrenes | D. nobile | Anti-inflammatory (NO: IC50 19.5 μM) | [22] |
| 61 | Flavanthridin | Phenanthrenes | D. nobile | Anti-inflammatory (NO: IC50 34.1 μM) | [22] |
| 62 | Epheneranthol C | Phenanthrenes | D. nobile | Anti-inflammatory (NO: IC50 17.6 μM) | [22] |
| 63 | Ephemeranthol A | Phenanthrenes | D. nobile | Anti-inflammatory (NO: IC50 12.0 μM) | [22] |
| 64 | Rotundatin (plicatol C) | Phenanthrenes | D. loddigesii | Anti-inflammatory (NO: IC50 29.1 μM) | [19] |
| 65 | (S)-4-Methoxy-9,10-dihydrophenanthrene-2,5,9-triol | Phenanthrenes | D. denneanum | Anti-inflammatory (NO: IC50 27.4 μM) | [13] |
| 66 | (S)-5-Methoxy-2,4,7,9-tetrahydroxy-9,10-dihydrophenanthrene | Phenanthrenes | D. denneanum | Anti-inflammatory (NO: IC50 3.1 μM) | [13] |
| 67 | (S)-4-Methoxy-2,5,7,9-tetrahydroxy-9,10-dihydrophenanthrene | Phenanthrenes | D. denneanum | Anti-inflammatory (NO: IC50 4.2 μM) | [13] |
| 68 | Denbinobin | Phenanthrenes | D. moniliforme | Anti-inflammatory | [21] |
| 69 | 5-Methoxy-7-hydroxy-9,10-dihydro-1,4-phenanthrenequinone | Phenanthrenes | D. draconis | Antioxidant (DPPH: IC50 72.6 μg/mL) | [47] |
| 70 | Fimbriatone | Phenanthrenes | D. nobile | Antioxidant (DPPH: IC50 40.8 μg/mL) | [57] |
| 71 | Loddigesiinol B | Phenanthrenes | D. loddigesii | Anti-inflammatory (NO: IC50 9.9 and 10.9 μM) | [19, 20] |
| 72 | Loddigesiinol I | phenanthrenes | D. loddigesii | Anti-inflammatory (NO: IC50 7.5 μM) | [20] |
| 73 | Loddigesiinol J | Phenanthrenes | D. loddigesii | Anti-inflammatory (NO: IC50 14.6 μM) | [20] |
| 74 | Chrysotoxol A | Phenanthrenes | D. loddigesii | Anti-inflammatory (NO: IC50 10.9 μM) and antioxidant (DPPH: IC50 23.2 μM) | [20] |
| 75 | Dendrochrysanene | Phenanthrenes | D. chrysanthum | Anti-inflammatory | [9] |
| 76 | 2,5-Dihydroxy-4-methoxy-phenanthrene 2-O-β-d-glucopyranoside | Phenanthrenes | D. denneanum | Anti-inflammatory (NO: IC50 4.6 μM) | [13] |
| 77 | 2,5-Dihydroxy-4-methoxy-phenanthrene 2-O-β-d-apiofuranosyl-(1 → 6)-β-d-glucopyranoside | Phenanthrenes | D. denneanum | Anti-inflammatory (NO: IC50 16.9 μM) | [13] |
| 78 | 2,5-Dihydroxy-4-methoxy-phenanthrene 2-O-α-l-rhamnopyranosyl-(1 → 6)-β-d-glucopyranoside | Phenanthrenes | D. denneanum | Anti-inflammatory (NO: IC50 41.5 μM) | [13] |
| 79 | (9R)-1,2,5,9-Tetrahydroxy-9,10-dihydrophenanthrene 5-O-β-d-glucopyranoside | Phenanthrenes | D. denneanum | Anti-inflammatory (NO: IC50 0.7 μM) | [13] |
| 80 | Shihunine | Alkaloids | D. loddigesii | Anti-inflammatory (NO: IC50 11.5 μg/mL) | [18] |
| 81 | Anosmine | Alkaloids | D. nobile | Anti-inflammatory (NO: IC50 16.1 μg/mL) | [18] |
| 82 | (+)-Homocrepidine A | Alkaloids | D. crepidatum | Anti-inflammatory (NO: IC50 3.6 μM) | [11] |
| 83 | (−)-Homocrepidine A | Alkaloids | D. crepidatum | Anti-inflammatory (NO: IC50 22.8 μM) | [11] |
| 84 | Homocrepidine B | Alkaloids | D. crepidatum | Anti-inflammatory (NO: IC50 27.6 μM) | [11] |
| 85 | (+)-Dendrocrepidamine A | Alkaloids | D. crepidatum | Anti-inflammatory (NO: IC50 16.1 μM) | [12] |
| 86 | Dendrocrepidamine B | Alkaloids | D. crepidatum | Anti-inflammatory (NO: IC50 14.3 μM) | [12] |
| 87 | (−)-Crepidine | Alkaloids | D. crepidatum | Anti-inflammatory (NO: IC50 29.9 μM) | [12] |
| 88 | (+)-Crepidine | Alkaloids | D. crepidatum | Anti-inflammatory (NO: IC50 81.9 μM) | [12] |
| 89 | (−)-Dendrocrepidine A | Alkaloids | D. crepidatum | Anti-inflammatory (NO: IC50 18.5 μM) | [12] |
| 90 | (+)-Dendrocrepidine A | Alkaloids | D. crepidatum | Anti-inflammatory (NO: IC50 30.2 μM) | [12] |
| 91 | (−)-Isocrepidamine | Alkaloids | D. crepidatum | Anti-inflammatory (NO: IC50 16.3 μM) | [12] |
| 92 | (+)-Isocrepidamine | Alkaloids | D. crepidatum | Anti-inflammatory (NO: IC50 73.0 μM) | [12] |
| 93 | Dendrocrepidine B | Alkaloids | D. crepidatum | Anti-inflammatory (NO: IC50 51.8 μM) | [10] |
| 94 | Dendrocrepidine C | Alkaloids | D. crepidatum | Anti-inflammatory (NO: IC50 29.7 μM) | [10] |
| 95 | Dendrocrepidine D | Alkaloids | D. crepidatum | Anti-inflammatory (NO: IC50 40.1 μM) | [10] |
| 96 | Dendrocrepidine E | Alkaloids | D. crepidatum | Anti-inflammatory (NO: IC50 35.2 μM) | [10] |
| 97 | (−)-Dendrocrepidine F | Alkaloids | D. crepidatum | Anti-inflammatory (NO: IC50 13.3 μM) | [10] |
| 98 | (+)-Dendrocrepidine F | Alkaloids | D. crepidatum | Anti-inflammatory (NO: IC50 42.7 μM) | [10] |
| 99 | Neoechinulin A | Alkaloids | D. loddigesii | Anti-inflammatory (NO: IC50 50.0 μM) | [20] |
| 100 | Moupinamide | Alkaloids | D. officinale | Antioxidant (DPPH: IC50 53.8 μM) | [65] |
| 101 | Dihydroferuloyltyramine | Alkaloids | D. officinale | Antioxidant (DPPH: IC50 35.8 μg/mL) | [65] |
| 102 | Apigenin | Flavonoids | D. williamsonii | Antioxidant (DPPH: IC50 19.3 μM) | [74] |
| 103 | Quercetin | Flavonoids | D. tosaense | Antioxidant | [54] |
| 104 | Naringenin | Flavonoids | D. loddigesii | Anti-inflammatory (NO: IC50 26.9 μM) | [20] |
| 105 | 3′,5,5′,7-Tetrahydroxyflavanone | Flavonoids | D. officinale | Antioxidant (DPPH: IC50 29.8 μM) | [65] |
| 106 | 5,4ʹ-Dihydroxy-7,3ʹ,5ʹ-trimethoxyflavanone | Flavonoids | D. loddigesii | Anti-inflammatory (NO: IC50 24.9 μM) and antioxidant (DPPH: IC50 78.9 μg/mL) | [20] |
| 107 | 5,7,4ʹ-Trihydroxy-3ʹ,5ʹ-dimethoxyflavanone | Flavonoids | D. loddigesii | Anti-inflammatory (NO: IC50 19.1 μM) | [20] |
| 108 | Vicenin 2 (vicenin II) | Flavonoids | D. officinale | Anti-inflammatory (TNF-α: IC50 6.8 μM; NO: IC50 3.9 μM) | [25, 26] |
| 109 | Dihydroconiferyl alcohol | Phenylpropanoids | D. nobile | Antioxidant (DPPH: IC50 50.9 μM) | [56] |
| 110 | Coniferylaldehyde | Phenylpropanoids | D. nobile | Antioxidant (IC50 22.8 μg/mL) | [56] |
| 111 | Ferulic acid | Phenylpropanoids | D. officinale | Antioxidant (DPPH: IC50 64.9 μM) | [65] |
| D. secundum | Antioxidant (DPPH: IC50 37.5 μM) | [70] | |||
| 112 | 6-Feruloyloxyhexanoic ester | Phenylpropanoids | Dendrobium cv. Sonia | Anti-inflammatory (NO: IC50 29.6 μM) | [29] |
| 113 | Threo-7-O-ethyl-9-O-(4-hydroxyphenyl)propionyl-guaiacylglycerol | Phenylpropanoids | D. loddigesii | Antioxidant | [49] |
| 114 | Dihydroconiferyl dihydro-p-coumarate | Phenylpropanoids | D. loddigesii | Antioxidant | [49] |
| D. officinale | Antioxidant (DPPH: IC50 78.2 μM) | [65] | |||
| 115 | p-Hydroxyphenethyl trans-ferulate | Phenylpropanoids | D. loddigesii | Antioxidant | [49] |
| 116 | n-Tetracosyl trans-ferulate | Phenylpropanoids | D. moniliforme | Antioxidant | [54] |
| 117 | n-Pentacosyl trans-ferulate | Phenylpropanoids | D. moniliforme | Antioxidant | [54] |
| 118 | (−)-Pinoresinol | Lignans | D. loddigesii | Anti-inflammatory (NO: IC50 89.5 μM) | [19] |
| 119 | Pinoresinol | Lignans | D. nobile | Antioxidant (DPPH: IC50 60.6 μM) | [57] |
| Dendrobium cv. Sonia | Anti-inflammatory (NO: IC50 26.3 μM) | [29] | |||
| 120 | (−)-Medioresinol | Lignans | D. loddigesii | Anti-inflammatory (NO: IC50 5.0 μM) | [19] |
| 121 | Medioresinol | Lignans | D. nobile | Antioxidant (DPPH: IC50 27.9 μM) | [57] |
| 122 | Syringaresinol | Lignans | D. loddigesii | Anti-inflammatory (NO: IC50 1.9 μM) and antioxidant (IC50 31.1 μM) | [20] |
| D. nobile | Antioxidant (DPPH: IC50 9.8 μM) | [57] | |||
| D. secundum | Antioxidant (DPPH: IC50 11.4 μM) | [70] | |||
| Dendrobium cv. Sonia | Anti-inflammatory (NO: IC50 27.7 μM) | [29] | |||
| 123 | Lirioresinol A | Lignans | D. nobile | Antioxidant (DPPH: IC50 30.9 μM) | [57] |
| 124 | Sesqui-illisimonan A | Lignans | Dendrobium cv. Sonia | Anti-inflammatory (NO: IC50 31.6 μM) | [29] |
| 125 | Dendrocoumarin | Benzocoumarins | D. nobile | Antibacterial (Staphylococcus aureus: MIC 2.5 μg/mL; Micrococcus tetragenus: MIC 5.0 μg/mL) | [36] |
| 126 | Itolide A | Benzocoumarins | D. nobile | Antibacterial (Staphylococcus aureus: MIC 2.5 μg/mL; Micrococcus tetragenus: MIC 5.0 μg/mL) | [36] |
| 127 | Dendroflorin | Fluorenones | D. nobile | Anti-inflammatory (NO: IC50 13.4 μg/mL) and antioxidant (DPPH: IC50 16.2 μM) | [23] |
| D. palpebrae | Antioxidant | [69] | |||
| 128 | Nobilone | Fluorenones | D. nobile | Anti-inflammatory (NO: IC50 38.1 μg/mL) | [23] |
| 129 | 3,6,9-Trihydroxy-3,4-dihydroanthracen-1-(2H)-one | Anthracenes | D. loddigesii | Anti-inflammatory (NO: IC50 43.8 μM) and antioxidant (DPPH: IC50 22.8 μM) | [20] |
| 130 | 3-Hydroxy-4-methoxyphenylethanol | Phenylethanoids | D. nobile | Antioxidant (DPPH: IC50 64.5 μM) | [56] |
| 131 | Syringic acid | Benzoic acid derivatives | D. nobile | Antioxidant (DPPH: IC50 8.1 μM) | [56] |
| D. officinale | Antioxidant (DPPH: IC50 36.5 μM) | [65] |
aPharmacological data with EC50, IC50, or MIC values are presented herein. Other data can be found in the text
Fig. 1.
Chemical structures of pharmacologically active bibenzyls (1–28) from Dendrobium plants (I)
Fig. 2.
Chemical structures of pharmacologically active bibenzyls (29–44) from Dendrobium plants (II)
Fig. 3.
Chemical structures of pharmacologically active phenanthrenes (45–79) from Dendrobium plants
Fig. 4.
Chemical structures of pharmacologically active alkaloids (80–101) from Dendrobium plants
Fig. 5.
Chemical structures of pharmacologically active flavonoids (102–108), phenylpropanoids (109–117), lignans (118–124) and other active compounds (125–131) from Dendrobium plants
Anti-Inflammatory Activity
Skin inflammation is the most common complaint of those suffering from dermatological diseases. Inflammatory skin diseases are divided into acute and chronic conditions. Acute skin inflammation is associated with occasional rashes, itching and skin redness and may be caused by ultraviolet or ionizing radiation, allergens or chemical irritants. Chronic inflammatory skin diseases include atopic dermatitis (such as eczema), seborrheic dermatitis, psoriasis, and rosacea. Chronic inflammatory skin diseases may lead to significant and serious disruption of skin immunity [8].
Dendrobium chrysanthum Wall. ex Lindl.
A phenanthrene, dendrochrysanene (75), was isolated from the stems of D. chrysanthum collected from Yunnan, China. This compound significantly suppressed the mRNA levels of TNF-α, IL-8, IL10, and iNOS in murine peritoneal macrophages at a concentration of 11.2 μg/mL. The compound may be a potentially useful new anti-inflammatory agent [9].
Dendrobium crepidatum Lindl. & Paxton
A research was conducted on D. crepidatum stems collected from Yunnan, China. Total alkaloids (yield, 2.3%) were obtained from D. crepidadum stems, which exhibited inhibitory effects on nitric oxide (NO) production in lipopolysaccharide (LPS)-activated mouse peritoneal macrophages, with an IC50 value of 18.7 μg/mL. The active alkaloids were found to be dendrocrepidine A (85; IC50, 39.8 μM), dendrocrepidine B (93; IC50, 51.8 μM), dendrocrepidine C (94; IC50, 29.7 μM), dendrocrepidine D (95; IC50, 40.1 μM), dendrocrepidine E (96; IC50, 35.2 μM), (±)-dendrocrepidine F (IC50, 38.4 μM), (−)-dendrocrepidine F (97; IC50, 13.3 μM), and (+)-dendrocrepidine F (98; IC50, 42.7 μM) [10]. In another study, the racemic mixture of (±)-homocrepidine A was separated into a pair of enantiomers, (+)-homocrepidine A (82) and (−)-homocrepidine A (83). (±)-Homocrepidine A, (+)-homocrepidine A, (−)-homocrepidine A, and homocrepidine B (84) also inhibited NO production with IC50 values of 14.7, 3.6, 22.8, and 27.6 μM, respectively, as compared with the positive control indomethacin (IC50, 42.2 μM) [11]. In a very recent study, (±)-dendrocrepidine A (IC50, 63.8 μM), (+)-dendrocrepidine A (85; IC50, 16.1 μM), dendrocrepidine B (86; IC50, 14.3 μM), (±)-crepidine (IC50, 51.1 μM), (−)-crepidine (87; IC50, 29.9 μM), (+)-crepidine (88; IC50, 81.9 μM), (±)-dendrocrepidine A (IC50, 27.5 μM), (−)-dendrocrepidine A (89; IC50, 18.5 μM), (+)-dendrocrepidine A (90; IC50, 30.2 μM), (±)-isocrepidamine (IC50, 27.4 μM), (−)-isocrepidamine (91; IC50, 16.3 μM), and (+)-isocrepidamine (92; IC50, 73.0 μM) also exhibited inhibitory effects on NO production. Dexamethasone (IC50, 47.0 μM) was used as a positive control [12].
Dendrobium denneanum Kerr.
A series of phenanthrene derivatives were isolated from D. denneanum stems collected from Sichuan, China, and 2,5-dihydroxy-4-methoxy-phenanthrene 2-O-β-d-glucopyranoside (76; IC50, 4.6 μM), 2,5-dihydroxy-4-methoxy-phenanthrene 2-O-β-d-apiofuranosyl-(1 → 6)-β-d-glucopyranoside (77; IC50, 16.9 μM), 2,5-dihydroxy-4-methoxy-phenanthrene 2-O-α-l-rhamnopyranosyl-(1 → 6)-β-d-glucopyranoside (78; IC50, 41.5 μM), (S)-5-methoxy-2,4,7,9-tetrahydroxy-9,10-dihydrophenanthrene (66; IC50, 3.1 μM), (S)-4-methoxy-2,5,7,9-tetrahydroxy-9,10-dihydrophenanthrene (67; IC50, 4.2 μM), (9R)-1,2,5,9-tetrahydroxy-9,10-dihydrophenanthrene 5-O-β-d-glucopyranoside (79; IC50, 0.7 μM), 4-methoxyphenanthrene-2,5-diol (moscatin, 45; IC50, 6.3 μM), (S)-4-methoxy-9,10-dihydrophenanthrene-2,5,9-triol (65; IC50, 27.4 μM), 2-methoxy-9,10-dihydrophenanthrene-4,5-diol (57; IC50, 7.6 μM), and 9,10-dihydrophenanthrene-2,4,7-triol (56; IC50, 32.7 μM) inhibited NO production in LPS-activated mouse macrophage RAW264.7 cells, as compared with the positive control curcumin (IC50, 6.2 μM) [13].
Dendrobium findlayanum C.S.P. Parish & Rchb.f.
Seco-dendrobine-type alkaloids and phenolics were isolated from D. findlayanum stems collected from Yunnan, China. Bibenzyl 6ʺ-de-O-methyldendrofindlaphenol A (38) inhibited NO production in RAW 264.7 cells with an IC50 value of 21.4 μM. MG-132 (IC50, 0.2 μM) was used as a positive control [14].
Dendrobium heterocarpum Lindl.
3-Hydroxy-4ʹ,5-dimethoxybibenzyl (4), gigantol (7), and dendrocandin I (39) were isolated from whole plants of D. heterocarpum collected from Yunnan, China, and these compounds reduced nitric oxide (NO) production in LPS-activated mouse macrophage RAW264.7 cells. At a concentration of 25 μM, the inhibition percentages of these three compounds were 51, 52, and 59%, respectively. NG-Monomethyl-L-arginine (L-NMMA) was used as a positive control (IC50, 34 μM) [15].
Dendrobium huoshanense Z.Z. Tang & S.J. Cheng
Dendrobium huoshanense Z.Z. Tang & S.J. Cheng was not accepted by “Plants of the World online.” However, because this name is used in the Chinese Pharmacopoeia (2020 edition) [16], it is cited in this review.
A pilot study was conducted to evaluate the clinical and immunomodulatory effects of an orally administered extract of D. huoshanense leaves and stems in children with moderate to severe recalcitrant atopic dermatitis (AD). AD is a common inflammatory skin disorder for which few safe and effective systemic treatments are available. Twenty-seven patients aged 4–18 years with AD who did not respond to topical therapy were treated with polysaccharides derived from D. huoshanense for 4 weeks and were followed-up for another 4 weeks. The results showed that the polysaccharide from D. huoshanense reduced the levels of some cytokines associated with AD and had beneficial effects on symptoms. No serious adverse effects occurred when the polysaccharide was administered orally for 4 weeks [17].
Dendrobium loddigesii Rolfe
Shihunine (80) was isolated from D. loddigesii stems collected from Yunnan, China. The alkaloid showed anti-inflammatory activity using the method of NO production the polysaccharide in RAW 264.7 cells activated by LPS with an IC50 value of 11.5 μg/mL. L-NG-monomethyl arginine citrate (IC50 7.2 μg/mL) was used as a positive control [18].
Phenanthrenes and bibenzyls were isolated from D. loddigesii stems collected from Guangdong, China. These compounds were evaluated for their inhibitory activities against NO production. Loddigesiinol A (52; IC50, 2.6 μM), moscatin (plicatol B, 45; IC50, 6.4 μM), 5-hydroxy-2,4-dimethoxyphenanthrene (46; IC50, 5.3 μM), lusianthridin (54; IC50, 4.6 μM), rotundatin (64, plicatol C; IC50, 29.1 μM), hircinol (55; IC50, 29.2 μM), loddigesiinol B (71; IC50, 10.9 μM), loddigesiinol D (26; IC50, 69.7 μM), (−)-pinoresinol (118; IC50, 89.5 μM), and (−)-medioresinol (120; IC50, 5.0 μM) inhibited NO production, as compared with the positive controls aminoguanidine (IC50, 17.5 μM) and resveratrol (IC50, 22.0 μM) [19].
Fifteen compounds were isolated from a preparation of D. loddigesii originating in Yunnan, China. Chrysotoxol A (74; IC50, 10.9 μM), neoechinulin A (99; IC50, 50.0 μM), 3,6,9-trihydroxy-3,4-dihydroanthracen-1-(2H)-one (129; IC50, 43.8 μM), 4,4ʹ-dihydroxy-3,5-dimethoxybibenzyl (5; IC50, 49.3 μM), naringenin (104; IC50, 26.9 μM), 5,4ʹ-dihydroxy-7,3ʹ,5ʹ-trimethoxyflavanone (106; IC50, 24.9 μM), 5,7,4ʹ-trihydroxy-3ʹ,5ʹ-dimethoxyflavanone (IC50, 19.1 μM), batatasin III (1; IC50, 21.9 μM), 3,3ʹ,5-trihydroxybibenzyl (2; IC50, 13.1 μM), trigonopol B (28; IC50, 26.3 μM), syringaresinol (122; IC50, 1.9 μM), 9,10-dihydrophenanthrene-2,4,7-triol (56; IC50, 8.6 μM), loddigesiinol B (71; IC50, 9.9 μM), loddigesiinol I (72; IC50, 7.5 μM), and loddigesiinol J (73; IC50, 14.6 μM) inhibited NO production in RAW 264.7 cells activated by LPS when compared with the positive control L-NMMA (IC50, 29.0 μM) [20].
Dendrobium moniliforme (L.) Sw.
Denbinobin (68) was isolated from D. moniliforme stems. At 1 μM, this compound significantly inhibited the formation of TNF-α and prostaglandin E2 (PGE2) (about 62 and 43% inhibition, respectively) in RAW264.7 cells stimulated with 1 μg/mL of LPS. In N9 cells (murine microglial cell line) stimulated with 10 ng/mL of LPS plus 10 unit/mL of interferon-γ (IFN-γ), denbinobin (3 μM) reduced TNF-α and nitrite formation (about 70 and 44% inhibition, respectively) [21].
Dendrobium nobile Lindl.
An alkaloid, anosmine (81), was isolated from D. nobile, which was purchased from Guangzhou, China. The compound exhibited anti-inflammatory activity using the method of NO production inhibition in RAW 264.7 cells activated by LPS with an IC50 value of 16.1 μg/mL, which was compared with L-NG-monomethyl arginine citrate (IC50, 7.2 μg/mL) as a positive control [18].
Phenanthrenes from the methanolic extract of D. nobile stems were evaluated for their potential to inhibit LPS-induced production of NO in murine macrophage RAW 264.7 cells. 3,4,8-Trimethoxyphenanthrene-2,5-diol (51; IC50, 20.4 μM), hircinol (55; IC50, 26.4 μM), erianthridin (60; IC50, 19.5 μM), ephemeranthol A (63; IC50, 12.0 μM), 5,7-dimethoxyphenanthrene-2,6-diol (50; IC50, 35.7 μM), moscatilin (9; IC50, 27.6 μM), coelonin (58; IC50, 10.2 μM), flavanthridin (61; IC50, 34.1 μM), epheneranthol C (62; IC50, 17.6 μM), lusianthridin (54; IC50, 9.6 μM), and fimbriol B (48; IC50, 28.9 μM) inhibited NO production. Aminoguanidine (IC50, 17.5 μM) was used as a positive control [22].
Nobilin D (21; IC50, 15.3 μM), nobilin E (33; IC50, 19.2 μM), nobilone (128; IC50, 38.1 μM), chrysotobibenzyl (16; IC50, 48.2 μM), moscatilin (9; IC50, 36.8 μM), gigantol (7; IC50, 32.9 μM), and dendroflorin (127; IC50, 13.4 μM) from D. nobile stems collected from Yunnan, China, exhibited inhibitory effects on NO production in the murine macrophage-like cell line RAW264.7 activated by LPS and IFN-γ. Resveratrol (IC50, 23.5 μM) was used as a positive control [23].
Ephemeranthol A (63) was isolated from D. nobile stems, and its anti-inflammatory activity was evaluated in Raw 264.7 cells. This compound reduced NO production in a dose-dependent manner. Notably, with pretreatment with 12.5 μg/mL ephemeranthol A, NO production decreased to the level of the cell-only control [24].
Dendrobium officinale Kimura & Migo
Vicenin 2 (vicenin II, 108) was suggested to be a common component in D. officinale leaves of different origins [25]. This di-C-glucosylflavone was synthesized, and it inhibited TNF-α expression and NO production with IC50 values of 6.8 and 3.9 μM, respectively, as compared with the positive control apigenin (IC50 = 18.5 and 19.0 μM, respectively) [26].
Two types of polysaccharides in D. officinale leaves, DLP-1 and DLP-2, were obtained by hot water extraction. DLP-1 (5 μg/mL) and DLP-2 (50 μg/mL) were shown to be effective in protecting THP-1 cells, a human leukemia monocytic cell line, from LPS-stimulated cytotoxicity and inhibited reactive oxygen species formation. In addition, both DLP-1 (5 μg/mL) and DLP-2 (50 μg/mL) significantly suppressed TLR4, myeloid differentiation factor (MyD88), and tumor necrosis factor receptor-associated factor-6 (TRAF-6) mRNA and protein expression in LPS-stimulated THP-1 cells [27].
Dendrobium parishii H. Low
(−)-Dendroparishiol (36) from the whole plant of D. parishii collected from Thailand was evaluated for its anti-inflammatory effects in LPS-stimulated RAW264.7 murine macrophage cells. At 12.5, 25 and 50 μg/mL, (−)-dendroparishiol reduced the expression of iNOS and COX-2 in LPS-treated RAW264.7 cells [28].
Dendrobium cv. Sonia
Ten compounds were isolated from Dendrobium cv. Sonia stems collected from Nanjing, China, and their anti-inflammatory activities were evaluated. 6-Feruloyloxyhexanoic ester (112; IC50, 29.6 μM), pinoresinol (119; IC50, 26.3 μM), syringaresinol (122; IC50, 27.7 μM), and sesqui-illisimonan A (124; IC50, 31.6 μM) exhibited inhibitory activities on NO production. Aminoguanidine (IC50, 27.3 μM) was used as a positive control [29].
Dendrobium tosaense Makino
Via oral administration at dosages of 30, 100, and 300 mg/kg for one week in an atopic dermatitis murine model, the standardized ethyl acetate extract of cultured D. tosaense stems protected mice from OVA/TNCB-induced skin lesions of atopic dermatitis [30].
Antimicrobial Activity
Microorganisms can cause skin infections, such as carbuncles, furuncles, cellulitis, impetigo, boils (Staphylococcus aureus), folliculitis (S. aureus, Pseudomonas aeruginosa), ringworm (Microsporum spp., Epidermophyton spp., and Trichophyton spp.), acne (Propionibacterium acnes), and foot odor (Brevibacterium spp.) [31].
Gigantol (7) has been found in 33 Dendrobium species [32]. This compound showed inhibitory activity against Staphylococcus aureus with an MIC value of 82.2 μg/mL [33].
Sortase A (srtA), a transpeptidase in gram-positive bacteria, can anchor surface proteins that play a vital role in the pathogenesis of these bacteria. SrtA is known as a potential antivirulent drug target to treat bacterial infections. Erianin (14) was isolated from D. chrysotoxum stems [34]. This compound could inhibit the activity of srtA in vitro with an IC50 value of 20.9 μg/mL [35].
Dendrocoumarin (125) and itolide A (126) from D. nobile stems collected from Hainan, China, showed antibacterial activities against Staphylococcus aureus with the same MIC value of 2.5 μg/mL and Micrococcus tetragenus with the same MIC value of 5.0 μg/mL. The positive control was ciprofloxacin, with MIC values of 0.6 and 0.3 μg/mL for the two bacteria, respectively [36].
Antioxidant and Antiaging Effects
The skin shows obvious signs of aging due to age, ultraviolet radiation exposure, and chemical pollution [37]. The changes in the skin are among the most visible signs of aging, including wrinkles, sagging skin, age spots dryness, and the loss of fat, which cause the skin to lose its natural smoothness [38]. The sum of the deleterious free radical reactions is a major contributor to the aging process [39]. In intrinsically aged skin, the quantity of dermal collagen decreases, and elastin accumulates structural abnormalities [40].
Dendrobium amoenum Wall. Ex Lindl.
Chloroform and acetone extracts of D. amoenum stems collected from Nepal showed 2,2-diphenyl-1-picrylhydrazyl (DPPH) free radical scavenging activities with IC50 values of 36.5 and 53.2 μg/mL, respectively [41].
Dendrobium aphyllum (Roxb.) C.E.C. Fisch.
Aphyllone B (17) from the stems of D. aphyllum collected from Yunnan, China, possessed DPPH radical scavenging activity with a scavenging percentage of 88% at a concentration of 100 μg/mL [42].
Dendrobium crepidatum Lindl. & Paxton
Ethanol and acetone extracts of D. crepidatum stems collected from Nepal showed DPPH free radical scavenging activities with IC50 values of 73.9 and 99.4 μg/mL, respectively, as compared with the positive control ascorbic acid (IC50, 38.2 μg/mL) [43].
Dendrobium denneanum Kerr
The ethanolic extract of D. denneanum [syn. D. aurantiacum var. denneanum (Kerr) Z.H. Tsi] stems collected from Yunnan, China, exhibited DPPH radical scavenging activity with an IC50 value of 92.6 μg/mL and was compared with α-tocopherol (IC50, 25 μg/mL) as a positive control. Three compounds were obtained by bioguided isolation. Unfortunately, the activities of these compounds were weaker than those of the crude extract [44].
A bibenzyl-rich fraction from D. denneanum stems collected from Sichuan, China, exhibited DPPH scavenging activity with an EC50 of 62.8 μg/mL. Vitamin C (EC50, 3.4 μg/mL) was used as a positive control [45].
Dendrobium denudans D. Don
The methanol extract of D. denudans stems collected from India showed in vitro antioxidant activity by a reducing power assay with an IC50 value of 10.1 μg/mL. Ascorbic acid (IC50, 3.9 μg/mL) was used as a positive control [46].
Dendrobium draconis Rchb.f.
5-Methoxy-7-hydroxy-9,10-dihydro-1,4-phenanthrenequinone (69; IC50, 283.3 μM or 72.6 μg/mL), hircinol (55; IC50, 22.3 μM), gigantol (7; IC50, 17.7 μM), and 7-methoxy-9,10-dihydrophenanthrene-2,4,5-triol (59; IC50, 10.2 μM) were isolated from D. draconis stems collected from Thailand and exhibited DPPH radical scavenging activities. Quercetin (IC50, 2.4 μM) and Trolox (IC50, 11.7 μM) were used as positive controls [47].
Dendrobium huoshanense Z.Z. Tang & S.J. Cheng
A polyphenol extract was obtained from D. huoshanense collected from Anhui, China, and the extract exhibited DPPH and 2,2ʹ-azino-bis(3-ethylbenzothiazoline-6-sulfonic acid) diammonium salt (ABTS) radical scavenging activities with IC50 values of 57 and 27 μg/mL, respectively, as compared with the positive control vitamin C [48].
Dendrobium loddigesii Rolfe
A series of phenolic compounds were found in D. loddigesii collected from Yunnan, China. From its stems, threo-7-O-ethyl-9-O-(4-hydroxyphenyl)propionyl-guaiacylglycerol (113), crepidatin (15), moscatilin (9), 4,5,4′-trihydroxy-3,3′-dimethoxybibenzyl (13), 4′,5-dihydroxy-3,3′-dimethoxybibenzyl (gigantol, 7), tristin (8), dihydroconiferyl dihydro-p-coumarate (114), and p-hydroxyphenethyl trans-ferulate (115) were obtained, and these compounds exhibited significant activities, with DPPH scavenging capacities ranging from 89 to 94% at 100 μg/mL, as compared with the positive control Trolox, which led to an inhibition of 96% at a concentration of 25 μg/mL. Batatasin III (1) significantly stimulated the collagen production activity of human dermal fibroblasts-adult (HDFa) (EC50 3.2 μg/mL) and was compared with TGF-β as a positive control, with an inhibition of 66% at a concentration of 0.01 μg/mL [49]. From complete plants, moscatin (45), 9,10-dihydrophenanthrene-2,4,7-triol (56), and crepidatuol B (27) were obtained, and these compounds showed significant activities, with DPPH scavenging capacities ranging from 84 to 95% at 100 μg/mL, as compared with the positive control Trolox, which led to an inhibition of 96% at a concentration of 25 μg/mL [50].
Phenanthrenes and bibenzyls were isolated from D. loddigesii stems collected from Guangdong, China. Loddigesiinol A (52; IC50, 26.1 μM), moscatin (45; IC50, 59.8 μM), lusianthridin (54; IC50, 62.2 μM), and loddigesiinol C (25; IC50, 23.7 μM) showed activities using the DPPH-scavenging assay, as compared to the positive controls resveratrol (IC50, 28.7 μM) and aminoguanidine (IC50, 21.7 μM) [19].
Fifteen compounds were isolated from a preparation of D. loddigesii stems collected from Yunnan, China. Chrysotoxol A (74; IC50, 23.2 μM), 3,6,9-trihydroxy-3,4-dihydroanthracen-1-(2H)-one (129; IC50, 22.8 μM), 4,4ʹ-dihydroxy-3,5-dimethoxybibenzyl (5; IC50, 94.5 μM), 5,4ʹ-dihydroxy-7,3ʹ,5ʹ-trimethoxyflavanone (106; IC50, 227.7 μM or 78.9 μg/mL), batatasin III (1; IC50, 204.9 μM or 50.1 μg/mL), 3,3ʹ,5-trihydroxybibenzyl (2; IC50, 85.8 μM), trigonopol B (28; IC50, 60.1 μM), syringaresinol (122; IC50, 31.1 μM), and 9,10-dihydrophenanthrene-2,4,7-triol (56; IC50, 14.1 μM) showed DPPH radical scavenging activities. Vitamin C (IC50, 29.8 μM) was used as a positive control [20].
Dendrobium longicornu Lindl.
The acetonic extract of D. longicornu stems collected from Nepal showed DPPH radical scavenging activity with an IC50 value of less than 100 μg/mL and was compared with ascorbic acid (IC50, < 50 μg/mL) as a positive control [51].
Dendrobium macrostachyum Lindl.
Ethanolic extracts of D. macrostachyum stems and leaves collected from India exhibited DPPH radical scavenging activities with IC50 values of 10.2 and 31.5 μg/mL, respectively, as compared with the positive control ascorbic acid (IC50, 18.4 μg/mL); these extracts also had ABTS radical scavenging activities with IC50 values of 31.5 and 49.1 μg/mL, respectively, as compared with the positive control ascorbic acid (IC50, 34.9 μg/mL). The activities of the ethanol extracts were better than those of petroleum ether, methanol, or water extracts [52].
Dendrobium moniliforme (L.) Sw.
The DPPH radical scavenging activities of hexane, chloroform, acetone, and ethanol extracts of D. moniliforme stems collected from Nepal were measured, showing IC50 values of 52.7, 42.4, 49.6, and 58.8 μg/mL, respectively, as compared with the positive control ascorbic acid (IC50, 38.2 μg/mL) [53].
Based on bioguided fractionation and isolation, a mixture of n-pentacosyl trans-ferulate (117) and n-tetracosyl trans-ferulate (116) (1:4) was obtained from tissue culture-raised plants of D. moniliforme. At 100 μg/mL, the mixture of the alkyl ferulates exhibited DPPH radical scavenging activity (inhibition ˃ 50%) [54].
Dendrobium nobile Lindl.
Flavonoids have been detected in ethyl acetate, n-butanol, and aqueous extracts of D. nobile leaves collected from Guangdong, China. These three extracts showed DPPH free radical scavenging activities with IC50 values of 21, 11, and 13 μg/mL, respectively, with Trolox used as a reference compound (IC50, 7 μg/mL) [55].
Bibenzyls and other phenolic compounds were isolated from D. nobile stems collected from Yunnan, China. Nobilin A (22; IC50, 87.1 μM), nobilin B (23; IC50, 32.2 μM), nobilin C (24; IC50, 136.0 μM or 47.4 μg/mL), dihydroconiferyl alcohol (109; IC50, 50.9 μM), coniferylaldehyde (110; IC50, 127.9 μM or 22.8 μg/mL), 3-hydroxy-4-methoxyphenylethanol (130; IC50, 64.5 μM), and syringic acid (131; IC50, 8.1 μM) exhibited DPPH radical scavenging activities. Vitamin C (IC50, 18.0 μM) and BHT (IC50, 90.9 μM) were used as positive controls [56].
Nobilin D (21; IC50, 19.9 μM), nobilin E (33; IC50, 21.0 μM), crepidatin (15; IC50, 21.8 μM), dendrobin A (10; IC50, 40.3 μM), chrysotoxine (11; IC50, 14.0 μM), moscatilin (9; IC50,14.5 μM), gigantol (7; IC50 56.4 μM), and dendroflorin (127; IC50, 16.2 μM) from D. nobile stems exhibited DPPH radical scavenging activities. Vitamin C (IC50, 18.0 μM) was used as a positive control [23].
Phenanthrenes and lignans were isolated from D. nobile stems in Yunnan, China. Fimbriatone (70; IC50, 144.5 μM or 40.8 μg/mL), confusarin (47; IC50, 12.9 μM), flavanthrinin (49; IIC50, 35.7 μM), 2,5-dihydroxy-4,9-dimethoxyphenanthrene (53; IC50, 34.8 μM), 5,7-dimethoxyphenanthrene-2,6-diol (50; IC50, 29.7 μM), syringaresinol (122; IC50, 9.8 μM), pinoresinol (119; IC50, 60.6 μM), medioresinol (121; IC50, 27.9 μM), and lirioresinol A (123; IC50, 30.9 μM) exhibited DPPH radical scavenging activities. Vitamin C (IC50, 18.0 μM) and BHT (IC50, 90.9 μM) were used as positive controls. For all phenanthrenes and lignans, an electron-donating methoxy group in the ortho position that donates to the phenolic hydroxy group exhibits enhanced antioxidant activity [57].
Dendrobium officinale Kimura & Migo
Ethanolic extracts of D. officinale flowers, leaves, and stems collected from Zhejiang, China, in which the total flavonoid contents measured were 1.8, 0.25, and 0.052%, respectively, were prepared. These extracts showed DPPH scavenging activities with IC50 values of 0.2 μg/mL, 17.4 μg/mL, and 10.4 μg/mL, respectively, as compared with the positive control vitamin C (IC50, 7.5 μg/mL) [58].
The chloroform extract of D. officinale stems collected from Yunnan, China, exhibited ABTS radical scavenging activity with an IC50 value of 88.8 μg/mL [59].
The antioxidant activities of D. officinale (Syn. D. candidum) collected from different areas of Hainan, China, were compared. Ethanolic extracts of plant samples collected from Sanya, Qiongzhong, and Baoting exhibited the best DPPH radical scavenging activities, with IC50 values of 15.9, 20.2, and 78.7 μg/mL, respectively [60].
A series of bibenzyls were isolated from D. officinale (syn. D. candidum) stems collected from Zhejiang, China. Dendrocandin C (18; IC50, 34.2 μM), dendrocandin D (19; IC50, 34.5 μM), dendrocandin E (12; IC50, 15.6 μM) [61], dendrocandin F (29; IC50, 55.8 μM), dendrocandin G (30; IC50, 32.4 μM), dendrocandin H (34; IC50, 19.8 μM), dendrocandin I (39; IC50, 21.3 μM) [62], dendrocandin J (31; IC50, 36.8 μM), dendrocandin K (32; IC50, 70.2 μM), dendrocandin L (35; IC50, 45.0 μM), dendrocandin M (37; IC50, 60.5 μM), dendrocandin N (42; IC50, 87.6 μM), dendrocandin O (43; IC50, 50.4 μM), dendrocandin P (40; IC50, 22.3 μM), dendrocandin Q (41; IC50, 30.3 μM) [63], and (S)-3,4,α-trihydroxy-5,4ʹ-dimethoxybibenzyl (20; IC50,IC50, 32.3 μM) exhibited DPPH radical scavenging activities [64]. Vitamin C (IC50, 23.2 μM) was used as a positive control [61–64].
Moupinamide (100; IC50, 53.8 μM), dihydroconiferyl dihydro-p-cumarate (114; IC50, 78.2 μM), dihydroferuloyltyramine (101; IC50,113.5 μM or 35.8 μg/mL), syringic acid (131; IC50, 36.5 μM), ferulic acid (111; IC50, 64.9 μM), and 3′,5,5′,7-tetrahydroxyflavanone (105; IC50, 29.8 μM) from D. officinale (syn. D. catenatum) stems collected from Zhejiang, China, showed DPPH free radical scavenging activities. Vitamin C (IC50, 23.2 μM) was used as a positive control [65].
Eight bibenzyls were isolated from D. officinale (syn. D. catenatum) stems collected from Shenzhen, China. Dendrocandin U (44), 3,4ʹ-dihydroxy-5-methoxybibenzyl (3), and 3,4ʹ,5-trihydroxy-3ʹ-methoxybibenzyl (tristin, 8) exhibited significant ABTS radical scavenging activities with IC50 values of 10.0, 5.3, and 9.0 μM, respectively, as compared with the positive control vitamin C (IC50, 6.5 μM). 3,4ʹ,5-Trihydroxy-3ʹ-methoxybibenzyl also exhibited DPPH scavenging activity with an IC50 value of 34.5 μM. Vitamin C (IC50, 14.9 μM) was used as a positive control [66].
D. officinale protocorm powder in deionized water (10, 25 and 50 mg/mL, external administration) significantly reduced erythema and protected the skin from dryness in a hairless mouse model with UV irradiation-induced skin damage using matrixyl (10 mg/mL) as a positive control. This study demonstrated that D. officinale protocorms can inhibit photodamage in the skin of hairless mice [67].
An in vivo experiment using photoaged model mice was conducted. The results showed that the ultrafine powder and fine powder of D. officinale (syn. D. candidum) stems possess a certain preventive effect on photoaging, and the effect of ultrafine powder is better than that of fine powder [68].
Dendrobium palpebrae Lindl.
A fluorenone, dendroflorin (127), was isolated from D. palpebrae whole plants collected from Thailand. This compound significantly decreased ROS in H2O2-stimulated RAW264.7 cells in a dose-dependent manner (12.5–50 μg/mL) [69].
Dendrobium secundum (Blume) Lindl. ex Wall.
Five compounds were isolated from D. secundum stems collected from Thailand. 4,5,4ʹ-Trihydroxy-3,3ʹ-dimethoxybibenzyl (13; IC50 15.9 μM), moscatilin (9; IC50 5.1 μM), syringaresinol (122; IC50 11.4 μM), and ferulic acid (111; IC50 37.5 μM) exhibited DPPH free radical scavenging activities, which were compared with the positive controls quercetin and Trolox (IC50, 2.5 and 11.7 μM, respectively) [70].
Dendrobium signatum Rchb.f.
D. signatum leaves collected from Thailand were extracted with ethanol by sonication-maceration for 30 min. The extract showed DPPH radical scavenging activity, with a measured IC50 value of 97.2 μg/mL, with ascorbic acid used as a reference compound (IC50, 21.7 μg/mL) [71].
Dendrobium speciosum Sm.
Methanolic extract of D. speciosum leaves collected from Australia containing polyphenols (1.2%) and flavonoids (0.2%) showed DPPH scavenging activity, with a measured IC50 value of 26 μg/mL. Trolox was used as a reference compound (IC50, 20 μg/mL) [72].
Dendrobium tosaense Makino
The effects of methanolic extracts obtained from three Dendrobium species propagated in vitro on DPPH scavenging were investigated. The D. tosaense extract was the most active extract, with an IC50 value of 79.9 μg/mL, as compared with the positive control α-tocopherol (IC50, 58.2 μM) [73].
Based on bioguided fractionation and isolation, quercetin (103) was obtained from tissue culture-raised plants of D. tosaense. At 100 μg/mL, quercetin exhibited DPPH radical scavenging activity (inhibition ˃ 50%) [54].
Dendrobium williamsonii Day & Rchb.f.
Six compounds were isolated from D. williamsonii whole plants collected from Thailand and evaluated these isolates for their DPPH radical scavenging activities. 3,3ʹ-Dihydroxy-4,5-dimethoxybibenzyl (6), moscatilin (9), and apigenin (102) were active, with IC50 values of 19.5, 8.5, and 19.3 μM, respectively, as compared with the positive controls, quercetin (IC50, 8.3 μM) and vitamin C (IC50 42.4 μM) [74].
Anti-Psoriasis Activity
Psoriasis is a recurrent skin disease described as keratinocyte hyperproliferation and aberrant differentiation. At concentrations ranging from 12.5 nM to 50 nM, erianin (14) inhibited proliferation and induced apoptosis in a human keratinocyte cell line (HaCaT). Erianin could be recognized as a potential anti-psoriasis drug [75]. This compound was previously isolated from D. chrysotoxum [34].
Hair Growth Promoting Effects
Alopecia is a skin disease characterized by reduced hair [76]. The condition has a strong influence on the mental and psychological health of patients [77].
In an in vivo experiment, C57BL/6 J mice were externally administered D. officinale (Guangdong, China) polysaccharides (DOP, 5.0 g/L) for 21 days. The average hair growth score and average quality of C57BL/6 J mice in the DOP group were significantly better than those in the control groups [78].
Skin-Moisturizing Effects
When the water content in the stratum corneum drops to less than 10%, the skin appears dry, loses elasticity, and wrinkles and skin aging accelerates [77].
The moisture retention rate of D. huoshanense (Anhui, China) polysaccharide on human skin is significantly higher than that of glycerol after external administration for 6 h, 8 h and 12 h. The stimulus value of normal skin and damaged skin of rabbits is less than 0.5, indicating that D. huoshanense polysaccharides do not cause skin irritation [79].
Polysaccharides from white orchids (Dendrobium cv. Khao Sanan) flowers cultivated in Thailand were evaluated in vivo for their skin hydration efficacy in human volunteers. The efficacy of white orchid polysaccharides at 0.3% was noted to be superior in terms of skin hydration efficacy than sea weed polysaccharides at 0.2% [80].
An in vivo experiment was conducted to evaluate the moisturizing effects of a D. nobile stem extract on human skin. The moisturizing abilities after 30 min and 2 h were greater than 1 for the D. nobile stem extract (2.0%) [81].
An in vivo experiment showed that 20 μg/mL ethanolic extract of D. officinale collected from Yunnan, China, exhibited a skin moisturizing effect. After 2.5 h of use, skin hydrature increased by 16% compared with that before use (P < 0.05) [82].
Tyrosinase-Inhibitory Activity
Tyrosinase inhibitors are used for hyperpigmentation and developing skin whitening agents.
3,3ʹ,5-Trihydroxybibenzyl (2) from D. loddigesii stems collected from Yunnan, China, revealed significant inhibitory activity against tyrosinase with an IC50 value of 37.9 μg/mL, as compared with the positive control kojic acid (IC50, 8.0 μg/mL) [49].
At concentrations of 12.5, 25 and 50 μg/mL, the CH2Cl2 extract of D. moniliforme leaves collected from South Korea inhibited melanogenesis in murine melanoma cells (B16F10), implying that D. moniliforme is effective against hyperpigmentation disorders and that it is considered a possible antimelanogenic agent in topical application [83].
Ethanolic extracts of Dendrobium cv. Sonia flowers and Dendrobium cv. Sonia pink flowers collected from Thailand showed tyrosinase inhibitory activities using l-tyrosine as a substrate. The IC50 values were 57.4 μg/mL and 83.2 μg/mL, respectively, as compared with the positive control kojic acid (IC50,151.7 μg/mL) [84].
Conclusion
There are 22 Dendrobium species with traditional uses for treating dermatological disorders by local people in eight countries, and there are 131 compounds from Dendrobium plants reported to possess anti-inflammatory, antimicrobial, antioxidant, antiaging, anti-psoriasis, and tyrosinase-inhibitory activities, which implies that Dendrobium plants are important resources for the discovery of active compounds and the development of new drugs and cosmetics. However, only D. crepidatum, D. denneanum, D. loddigesii, D. nobile, and D. officinale have been extensively studied. More research on other Dendrobium species is needed.
The major active compounds found in Dendrobium species are phenanthrenes, alkaloids, flavonoids, phenylpropanoids, and lignans. Several compounds, such as loddigesiinol A, (S)-5-methoxy-2,4,7,9-tetrahydroxy-9,10-dihydrophenanthrene, (S)-4-methoxy-2,5,7,9-tetrahydroxy-9,10-dihydrophenanthrene, 2,5-dihydroxy-4-methoxy-phenanthrene 2-O-β-d-glucopyranoside, (9R)-1,2,5,9-tetrahydroxy-9,10-dihydrophenanthrene 5-O-β-d-glucopyranoside, (+)-homocrepidine A, and vicenin 2, have significant anti-inflammatory activities and inhibit NO production with IC50 values less than 5 μM, and these compounds are worthy of further study.
Acknowledgments
This research was funded by the Beijing DR PLANT Biotechnology Co., Ltd. and the Second Tibetan Plateau Scientific Expedition and Research (STEP) Program of Ministry of Science and Technology of the People’s Republic of China (2019QZKK0502).
Declarations
Conflict of interest
The author declares that there are no conflicts of interest associated with this work.
References
- 1.Sontheimer RD. J. Investig. Dermatol. 2014;134:581–582. doi: 10.1038/jid.2013.335. [DOI] [PubMed] [Google Scholar]
- 2.Wagh VV, Jain AK. J. Herb. Med. 2020;19:100234. [Google Scholar]
- 3.Bhattacharyya P, Paul P, Kumaria S, Tandon P. Acta Physiol. Plant. 2018;40:137. [Google Scholar]
- 4.Zheng S-G, Hu Y-D, Zhao R-X, Yan S, Zhang X-Q, Zhao T-M, Chun Z. Planta. 2018;248:769–784. doi: 10.1007/s00425-018-2960-4. [DOI] [PubMed] [Google Scholar]
- 5.Plants of the World online, http://www.plantsoftheworldonline.org/taxon/urn:lsid:ipni.org:names:325886-2. Accessed on March 4, 2021.
- 6.CIRS, Inventory of Existing Chemical Ingredient in China. http://www.cirs-reach.com/news-and-articles/new-inventory-of-existing-cosmetic-ingredients-in-china-launched-(iecic-2015,-final-version).html. Accessed on March 4, 2021.
- 7.Teoh ES. Orchids as Aphrodisiac, Medicine Or Food. Singapore: Springer; 2019. pp. 79–282. [Google Scholar]
- 8.Lim HS, Yo SR, Lee MY, Seo CS, Shin HK, Jeong SJ. Mol. Med. Rep. 2018;17:2515–2522. doi: 10.3892/mmr.2017.8172. [DOI] [PubMed] [Google Scholar]
- 9.Yang L, Qin LH, Bligh SA, Bashall A, Zhang CF, Zhang M, Wang ZT, Xu LS. Bioorg. Med. Chem. 2006;14:3496–3501. doi: 10.1016/j.bmc.2006.01.004. [DOI] [PubMed] [Google Scholar]
- 10.Hu Y, Ren J, Wang L, Zhao X, Zhang M, Shimizu K, Zhang C. Phytochemistry. 2018;149:12–23. doi: 10.1016/j.phytochem.2018.02.006. [DOI] [PubMed] [Google Scholar]
- 11.Hu Y, Zhang C, Zhao X, Wang Y, Feng D, Zhang M, Xie H. J. Nat. Prod. 2016;79:252–256. doi: 10.1021/acs.jnatprod.5b00801. [DOI] [PubMed] [Google Scholar]
- 12.Hu Y, Yang H, Ding X, Liu J, Wang X, Hu L, Liu M, Zhang C. Bioorg. Chem. 2020;100:103809. doi: 10.1016/j.bioorg.2020.103809. [DOI] [PubMed] [Google Scholar]
- 13.Lin Y, Wang F, Yang LJ, Chun Z, Bao JK, Zhang GL. Phytochemistry. 2013;95:242–251. doi: 10.1016/j.phytochem.2013.08.008. [DOI] [PubMed] [Google Scholar]
- 14.Yang D, Cheng ZQ, Yang L, Hou B, Yang J, Li XN, Zi CT, Dong FW, Liu ZH, Zhou J, Ding ZT, Hu JM. J. Nat. Prod. 2018;81:227–235. doi: 10.1021/acs.jnatprod.7b00150. [DOI] [PubMed] [Google Scholar]
- 15.Yang X-B, Yan S, Hu J-M, Zhou J. Nat. Prod. Res. Dev. 2019;31:1745–1752. [Google Scholar]
- 16.Editorial Board of Chinese Pharmacopoeia . Chinese Pharmacopoeia. Beijing: China Medical Science Press; 2020. pp. 94–97. [Google Scholar]
- 17.Wu KG, Li TH, Chen CJ, Cheng HI, Wang TY. Int. J. Immunopathol. Pharmacol. 2011;24:367–375. doi: 10.1177/039463201102400210. [DOI] [PubMed] [Google Scholar]
- 18.Chen H, Li X, Xu Y, Lo K, Zheng H, Hu H, Wang J, Lin Y. Molecules. 2018;23:1185. doi: 10.3390/molecules23051185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ito M, Matsuzaki K, Wang J, Daikonya A, Wang N-L, Yao X-S, Kitanaka S. Chem. Pharm. Bull. 2010;58:628–633. doi: 10.1248/cpb.58.628. [DOI] [PubMed] [Google Scholar]
- 20.Li X, Chen H, He W, Yang W, Ni F, Huang Z, Hu H, Wang J. Acta Sci. Nat. Univ. Sunyatseni. 2019;58:96–102. [Google Scholar]
- 21.Lin TH, Chang SJ, Chen CC, Wang JP, Tsao LT. J. Nat. Prod. 2001;64:1084–1086. doi: 10.1021/np010016i. [DOI] [PubMed] [Google Scholar]
- 22.Hwang JS, Lee SA, Hong SS, Han XH, Lee C, Kang SJ, Lee D, Kim Y, Hong JT, Lee MK, Hwang BY. Bioorg. Med. Chem. Lett. 2010;20:3785–3787. doi: 10.1016/j.bmcl.2010.04.054. [DOI] [PubMed] [Google Scholar]
- 23.Zhang X, Xu JK, Wang J, Wang NL, Kurihara H, Kitanaka S, Yao XS. J. Nat. Prod. 2007;70:24–28. doi: 10.1021/np060449r. [DOI] [PubMed] [Google Scholar]
- 24.Kim JH, Oh S-Y, Han S-B, Uddin GM, Kim CY, Lee JK. Arch. Pharm. Res. 2015;38:1117–1126. doi: 10.1007/s12272-014-0511-5. [DOI] [PubMed] [Google Scholar]
- 25.Zhang XF, Zhou CH, Zhang LK, Jiang M, Xie ZS, Yuan Y, Huang YC, Luo YY, Wei G. Chin. J. Exp. Tradit. Med. Formulae. 2019;25:29–34. [Google Scholar]
- 26.Shie JJ, Chen CA, Lin CC, Ku AF, Cheng TJR, Fang JM, Wong CH. Org. Biomol. Chem. 2010;8:4451–4462. doi: 10.1039/c0ob00011f. [DOI] [PubMed] [Google Scholar]
- 27.Zhang M, Wu J, Han J, Shu H, Liu K. Chem. Cent. J. 2018;12:109. doi: 10.1186/s13065-018-0480-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kongkatitham V, Muangnoi C, Kyokong N, Thaweesest W, Likhitwitayawuid K, Rojsitthisak P, Sritularak B. Phytochem. Lett. 2018;24:31–38. doi: 10.1080/10286020.2018.1429416. [DOI] [PubMed] [Google Scholar]
- 29.Cai BX, Song LX, Hu HJ, Han ZZ, Zhou Y, Wang ZT, Yang L. Nat. Prod. Res. 2020 doi: 10.1080/14786419.2020.1782404. [DOI] [PubMed] [Google Scholar]
- 30.Wu CT, Huang KS, Yang CH, Chen YC, Liao JW, Kuo CL, Chen CL, Lo SF, Hsieh CC, Tsay HS. Int. J. Pharm. 2014;463:193–200. doi: 10.1016/j.ijpharm.2013.08.015. [DOI] [PubMed] [Google Scholar]
- 31.Orchard A, van Vuuren S. Evid. Based Complement. Alternat. Med. 2017;2017:4517971. doi: 10.1155/2017/4517971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.He L, Su Q, Bai L, Li M, Liu J, Liu X, Zhang C, Jiang Z, He J, Shi J, Huang S, Guo L. Eur. J. Med. Chem. 2020;204:112530. doi: 10.1016/j.ejmech.2020.112530. [DOI] [PubMed] [Google Scholar]
- 33.Ren J, Qian XP, Guo YG, Li T, Yan SK, Jin HZ, Zhang WD. Phytochem. Lett. 2016;18:64–67. [Google Scholar]
- 34.Dong FW, Luo HR, Wan QL, Xu FQ, Fan WW, Wang KJ, Li N, Hu JM. Bull. Korean Chem. Soc. 2012;33:2247–2250. [Google Scholar]
- 35.Ouyang P, He X, Yuan ZW, Yin ZQ, Fu H, Lin J, He C, Liang X, Lv C, Shu G, Yuan ZX, Song X, Li L, Yin L. Toxins. 2018;10:385. doi: 10.3390/toxins10100385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zhou XM, Zhang B, Chen GY, Han CR, Jiang KC, Luo MY, Meng BZ, Li WX, Lin SD. Nat. Prod. Res. 2018;32:2464–2467. doi: 10.1080/14786419.2017.1419241. [DOI] [PubMed] [Google Scholar]
- 37.Cao C, Xiao Z, Wu Y, Ge C. Nutrients. 2020;12:870. doi: 10.3390/nu12030870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Shanbhag S, Nayak A, Narayan R, Nayak UY. Adv. Pharm. Bull. 2019;9:348–359. doi: 10.15171/apb.2019.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Harman D. Proc. Natl. Acad. Sci. USA. 1981;78:7124–7128. doi: 10.1073/pnas.78.11.7124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Russell-Goldman E, Murphy GF. Am. J. Pathol. 2020;190:1356–1369. doi: 10.1016/j.ajpath.2020.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Poudel MR, Chand MB, Karki N, Pant B. Bot. Orient. J. Plant Sci. 2015;9:20–26. [Google Scholar]
- 42.Yang D, Liu LY, Cheng ZQ, Xu FQ, Fan WW, Zi CT, Dong FW, Zhou J, Ding ZT, Hu JM. Fitoterapia. 2015;100:11–18. doi: 10.1016/j.fitote.2014.11.004. [DOI] [PubMed] [Google Scholar]
- 43.Paudel MR, Chand MB, Pant B, Pant B. Biomolecules. 2019;9:478. doi: 10.3390/biom9090478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Yang L, Han H, Nakamura N, Hattori M, Wang Z, Xu L. Phytother. Res. 2007;21:696–698. doi: 10.1002/ptr.2133. [DOI] [PubMed] [Google Scholar]
- 45.Jia F, Xia HL, Ning ZJ, Huang P, Huang XY, Tang JJ, Zhang TM. Sci. Technol. Food Ind. 2014;35:62–66. [Google Scholar]
- 46.Singh CB, Devi MC, Thokchom DS, Sengupta M, Singh AK. J. Pharmacogn. Phytochem. 2015;4:6–11. [Google Scholar]
- 47.Sritularak B, Anuwat M, Likhitwitayawuid K. J. Asian Nat. Prod. Res. 2011;13:251–255. doi: 10.1080/10286020.2010.546354. [DOI] [PubMed] [Google Scholar]
- 48.Wei M, Liu YY, Cai WR, Qian SH, Zhang K. Food Mach. 2016;32:136–140. [Google Scholar]
- 49.Ma RJ, Yang L, Bai X, Li JY, Yuan MY, Wang YQ, Xie Y, Hu JM, Zhou J. Nat. Prod. Bioprospec. 2019;9:329–336. doi: 10.1007/s13659-019-00219-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Yan S, Ma RJ, Yang L, Li JY, Yang XB, Hu JM. Nat. Prod. Res. Dev. 2019;31:615–620. [Google Scholar]
- 51.Paudel MR, Chand MB, Pant B, Pant B. Pharmacogn. J. 2017;9:499–503. [Google Scholar]
- 52.Sukumaran NP, Yadav RH. Anc. Sci. Life. 2016;35:240–244. doi: 10.4103/0257-7941.188181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Paudel MR, Chand MB, Pant B, Pant B, Complement BMC. Altern. Med. 2018;18:134. doi: 10.1186/s12906-018-2197-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Lo SF, Mulabagal V, Chen CL, Kuo CL, Tsay HS. J. Agric. Food Chem. 2004;52:6916–6919. doi: 10.1021/jf040017r. [DOI] [PubMed] [Google Scholar]
- 55.Li Y, Li H, Ji X, Cen Z, Yan J, Wu J. China Pharm. 2018;29:330–333. [Google Scholar]
- 56.Zhang X, Xu JK, Wang NL, Kurihara H, Yao XS, Wang Z. Chin. Pharm. J. 2008;43:829–832. [Google Scholar]
- 57.Zhang X, Xu JK, Wang NL, Kurihara H, Yao XS. J. Chin. Pharm. Sci. 2008;17:314–318. [Google Scholar]
- 58.Li F, Wei Y, Chen Y. Acta Chin. Med. 2019;34:1020–1023. [Google Scholar]
- 59.Huang Q, Shen Y, Zhang C, Luo A, Fan Y. Chin. J. Appl. Environ. Biol. 2014;20:438–442. [Google Scholar]
- 60.Wang J, Chen HX, Xing LS, Wang YW, Huang YH, Liu HQ. Nat. Prod. Res. Dev. 2015;27:768–773. [Google Scholar]
- 61.Li Y, Wang CL, Wang YJ, Guo SX, Yang JS, Chen XM, Xiao PG. Chem. Pharm. Bull. 2009;57:218–219. doi: 10.1248/cpb.57.218. [DOI] [PubMed] [Google Scholar]
- 62.Li Y, Wang CL, Wang YJ, Wang FF, Guo SX, Yang JS, Xiao PG. Chem. Pharm. Bull. 2009;57:997–999. doi: 10.1248/cpb.57.997. [DOI] [PubMed] [Google Scholar]
- 63.Li Y, Wang CL, Zhao HJ, Guo SX. J. Asian Nat. Prod. Res. 2014;16:1035–1043. doi: 10.1080/10286020.2014.967230. [DOI] [PubMed] [Google Scholar]
- 64.Li Y, Wang CL, Zhao HJ, Guo SX. Chem. Nat. Compd. 2015;51:1052–1054. [Google Scholar]
- 65.Li Y, Wang CL, Wang FF, Dong HL, Guo SX, Yang JS, Xiao PG. Chin. Pharm. J. 2010;45:975–979. [Google Scholar]
- 66.Zhu LJ, Wang MQ, Qin Y, Wang MN, Zhang GQ, Niu LT, Chen JB, Zhang X, Yao XS. J. Asian Nat. Prod. Res. 2020 doi: 10.1080/10286020.2020.1826937. [DOI] [PubMed] [Google Scholar]
- 67.Mai Y, Niu Z, He W, Lai X, Huang S, Zheng X. Biol. Pharm. Bull. 2019;42:728–735. doi: 10.1248/bpb.b18-00901. [DOI] [PubMed] [Google Scholar]
- 68.Li H, Qian LQ, Chen X, Zhang NY, Lei SS, Li B, Lyu GY, Chen SH. China J. Chin. Mater. Med. 2019;44:4677–4684. doi: 10.19540/j.cnki.cjcmm.20190510.401. [DOI] [PubMed] [Google Scholar]
- 69.Kyokong N, Muangnoi C, Thaweesest W, Kongkatitham V, Likhitwitayawuid K, Rojsitthisak P, Sritularak B. J. Asian Nat. Prod. Res. 2019;21:391–397. doi: 10.1080/10286020.2018.1429416. [DOI] [PubMed] [Google Scholar]
- 70.Sritularak B, Duangrak N, Likhitwitayawuid K. Z. Naturforsch. 2011;66:205–208. doi: 10.1515/znc-2011-5-602. [DOI] [PubMed] [Google Scholar]
- 71.T. Chimsook, In Phytochemical screening, total phenolic content, antioxidant activities and cytotoxicity of Dendrobium signatum leaves (MATEC Web of Conferences, EDP Sciences, 2016), p. 03005
- 72.Moretti M, Cossignani L, Messina F, Dominici L, Villarini M, Curini M, Marcotullio MC. Food Chem. 2013;140:660–665. doi: 10.1016/j.foodchem.2012.10.022. [DOI] [PubMed] [Google Scholar]
- 73.Lo SF, Nalawade SM, Mulabagal V, Matthew S, Chen CL, Kuo CL, Tsay HS. Biol. Pharm. Bull. 2004;27:731–735. doi: 10.1248/bpb.27.731. [DOI] [PubMed] [Google Scholar]
- 74.Rungwichaniwat P, Sritularak B, Likhitwitayawuid K. Pharmacogn. J. 2014;6:36–41. [Google Scholar]
- 75.Mo C, Shetti D, Wei K. Molecules. 2019;24:2727. doi: 10.3390/molecules24152727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Mingsan M, Mengfan P, Dandan L, Zhengwang Z. J. King Saud Univ. Sci. 2020;32:2669–2674. [Google Scholar]
- 77.Guo L, Qi J, Du D, Liu Y, Jiang X. Pharm. Biol. 2020;58:664–673. doi: 10.1080/13880209.2020.1787470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Chen J, Qi H, Li JB, Yi YQ, Chen D, Hu XH, Wang ML, Sun XL, Wei XY. China J. Chin. Mater. Med. 2014;39:291–295. [PubMed] [Google Scholar]
- 79.Gu FL, Jiang XP, Chen YJ, Han BX, Chen NF, Wei CB. Nat. Prod. Res. Dev. 2018;30:1701–1705. [Google Scholar]
- 80.Kanlayavattanakul M, Pawakongbun T, Lourith N. Chin. Herb. Med. 2019;11:400–405. [Google Scholar]
- 81.Gui YH, Meng X, Liang QM, Gong SZ. Deterg. Cosmet. 2017;40(22–24):26. [Google Scholar]
- 82.Chen M, Sun Y, Zhao Y. Acta Univ. Tradit. Med. Sin. Pharmacol. Shanghai. 2015;29:70–73. [Google Scholar]
- 83.Ko YJ, Yang SK, Song SM, Yoon WJ, Bae KH. J. Biol. Act. Prod. Nat. 2015;5:12–17. [Google Scholar]
- 84.Athipornchai A, Jullapo N, Afr S. J. Bot. 2018;119:188–192. [Google Scholar]
- 85.Smith NM. J. Adelaide Bot. Gard. 1991;14:1–65. [Google Scholar]
- 86.Tucci J, Wilkens S. Aust. J. Rural Health. 2016;24:156–169. doi: 10.1111/ajr.12256. [DOI] [PubMed] [Google Scholar]
- 87.Behera D, Rath CC, Mohapatra U. Floric Ornam. Biotechnol. 2013;7:53–59. [Google Scholar]
- 88.Barua U, Hore DK, Rathi RS, Das G. Environ. Ecol. 2006;24:736–742. [Google Scholar]
- 89.Maikhuri RK, Ramakrishnan PS. J. Econ. Tax. Bot. 1992;10:61–78. [Google Scholar]
- 90.Subedi A, Kunwar B, Choi Y, Dai Y, van Andel T, Chaudhary RP, de Boer HJ, Gravendeel B. J. Ethnobiol. Ethnomed. 2013;9:64. doi: 10.1186/1746-4269-9-64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Akhter M, Hoque MM, Rahman M, Huda MK. J. Med. Plants Stud. 2017;5:265–268. [Google Scholar]
- 92.Li WH. Records of Yunnan Province. Kunming: Yunnan People’s Publishing House; 1995. Medical records, In Compilation Committee of Local Records; pp. 960–962. [Google Scholar]
- 93.Zhang HY, Zhang ZY. Handbook of Traditional Chinese Medicine Resources in China. Beijing: Science Press; 1994. pp. 1531–1536. [Google Scholar]
- 94.Rahamtulla M, Pradhan UC, Roy AK, Rampilla V, Khasim SM. Ethnomedicinal aspects of some orchids from Darjeeling Himalaya, India. In: Khasim SM, Hegde SN, González-Arnao MT, Thammasiri K, editors. Orchid Biology: Recent Trends & Challenges. Singapore: Springer; 2020. pp. 451–472. [Google Scholar]
- 95.Li H. Orchidaceae. In: Wu Z, editor. Flora Yunnanica. Beijing: Science Press; 2003. pp. 100–819. [Google Scholar]
- 96.Laha R, Lalremruata PC, Vanlalpeka R. Int. J. Basic Appl. Res. 2018;8:371–384. [Google Scholar]
- 97.Lawler LJ, Slaytor M. Med. J. Australia. 1970;2:1259–1261. doi: 10.5694/j.1326-5377.1970.tb63459.x. [DOI] [PubMed] [Google Scholar]
- 98.Chowlu K, Mahar KS, Das AK. Indian J. Nat. Prod. Resour. 2017;8:89–93. [Google Scholar]
- 99.Yunnan Medicinal Materials Company Limited . List of Traditional Chinese Medicine Resources in Yunnan. Beijing, China: Science Press; 1993. pp. 669–672. [Google Scholar]
- 100.Tiwari AP, Joshi B, Ansari AA. Nat. Sci. 2012;10:33–37. [Google Scholar]
- 101.Dash PK, Sahoo S, Bal S. Ethnobot. Leaflets. 2008;12:70–78. [Google Scholar]
- 102.Jagathes Kumar S, Ashok Kumar R, Uma G, Subbaiyan B, Aravindhan V, Balasubramaniam V. Int. J. Recent Adv. Multidiscip. Res. 2015;2:1047–1055. [Google Scholar]
- 103.B. Vaidya, M. Shrestha, N. Joshee, In Report on Nepalese orchid species with medicinal properties, In Proceedings of Nepal-Japan Joint Symposium-2000, Kathmandu, Nepal (The Himalayan plants, can they save us? Proceeding of Nepal-Japan joint symposium on conservation and utilization of Himalayan medicinal resources, 2000), p. 146–152
- 104.Hossain MM. Med. Aromat. Plant Sci. Biotechnol. 2009;3:100–106. [Google Scholar]
- 105.Roy AR, Patel RS, Patel VV, Yadav DS. J. Orchid Soc. India. 2007;21:15–17. [Google Scholar]
- 106.Deb CR, Deb MS, Jamir NS, Imchen T. Pleione. 2009;3:209–211. [Google Scholar]
- 107.Medhi RP, Chakrabarti S. Indian J. Tradit. Knowl. 2009;8:11–16. [Google Scholar]
- 108.Yonzone R, Lama D, Bhujel RB, Rai S. Indian Forester. 2014;140:413–418. [Google Scholar]
- 109.Shanavaskhan AE, Sivadasan M, Alfarhan AH, Thomas J. Indian J. Tradit. Knowl. 2012;11:250–258. [Google Scholar]
- 110.Zhu ZY, Wei QH, Gao L. The Annals of National Medicine in Yunnan. Kunming: The Nationalities Publishing House of Yunnan; 2012. pp. 186–187. [Google Scholar]
- 111.Nurfadilah S. IOP Conf. Ser. Earth Environ. Sci. 2020;473:012063. doi: 10.1088/1755-1315/474/7/072047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Kpadehyea JT, Fernando ES, Tinio CE, Buot IE. Electron. J. Biol. 2015;11:165–175. [Google Scholar]