Abstract
Testosterone enanthate (TE) administration attenuates bone loss in orchiectomized (ORX) rats. However, testosterone administration may increase risk for prostate/lower urinary tract related adverse events and polycythemia in humans. Trenbolone enanthate (TREN) is a synthetic testosterone analogue that preserves bone mineral density (BMD) and results in less prostate enlargement than testosterone in young ORX rodents. The purpose of this experiment was to determine if intramuscular TREN administration attenuates bone loss and maintains bone strength, without increasing prostate mass or hemoglobin concentrations in skeletally mature ORX rodents. Forty, 10 month old male F344/Brown Norway rats were randomized into SHAM, ORX, ORX+TE (7.0 mg/week), and ORX+TREN (1.0 mg/week) groups. Following surgery, animals recovered for 1 week and then received weekly: vehicle, TE, or TREN intramuscularly for 5 weeks. ORX reduced total and trabecular (t) BMD at the distal femoral metaphysis compared with SHAMs, while both TREN and TE completely prevented these reductions. TREN treatment also increased femoral neck strength by 28% compared with ORX animals (p<0.05), while TE did not alter femoral neck strength. In addition, TE nearly doubled prostate mass, compared with SHAMs (p<0.05). Conversely, TREN induced a nonsignificant 20% reduction in prostate mass compared with SHAMs, ultimately producing a prostate mass that was 64% below that found in ORX+TE animals (p<0.01). Hemoglobin concentrations and levator ani/bulbocavernosus (LABC) muscle mass were elevated in ORX+TE and ORX+TREN animals to a similar degree above both SHAM and ORX conditions (p<0.01). In skeletally mature rodents, both high-dose TE and low-dose TREN completely prevented the ORX-induced loss of tBMD at the distal femoral metaphysis and increased LABC mass. TREN also augmented femoral neck strength and maintained prostate mass at SHAM levels. These findings indicate that TREN may be an advantageous agent for future clinical trials evaluating agents capable of preventing bone loss resulting from androgen deficiency.
Keywords: Androgen, 5-Alpha reductase, Anabolic steroid, Testosterone, Bone
Introduction
Hypogonadism (i.e., low circulating testosterone) is one factor underlying the loss of bone in older men [1]. Osteoporotic fractures carry a 25% lifetime risk in men following their fifth decade [2]. Men account for nearly 30% of hip fractures worldwide [3]. Importantly, following hip fracture the mortality risk is nearly doubled in men compared with women within 1 year of fracture [4,5]. Pharmacotherapy and testosterone replacement therapy (TRT) have been recommended for men with osteoporosis and sarcopenia; however, these therapies have several side effects [5]. Identifying agents which maintain both bone mineral density (BMD) and bone strength while displaying a more desirable side effect profile is of clinical importance. TRT is a common treatment in older hypogonadal men. However, replacement doses of testosterone only modestly improve BMD [6], and insufficient clinical evidence is currently available to evaluate fracture risk with this treatment [7]. Conversely, supraphysiologic testosterone administration fully protects against bone loss and successfully augments midshaft bone strength in young orchiectomized (ORX) rodent models [8–11]. However, a number of side effects preclude implementation of supraphysiologic testosterone administration as a viable therapy, of which increased risk for prostate growth/lower urinary tract symptoms and polycythemia occur most frequently [12]. As such, recent preclinical and clinical research has focused on evaluating the effects of various steroidal and non-steroidal selective androgen receptor modulators (SARMs), which exert effects in selective androgen-sensitive tissues (e.g., bone and muscle), without inducing unwanted side effects in others (e.g., prostate) [13,14].
17β-Hydroxyestra-4,9,11-trien-3-one (trenbolone) is a non-5 alpha reducible, non-aromatizable synthetic testosterone analogue. Trenbolone enanthate (TREN) partially preserves BMD and augments skeletal muscle mass in young ORX rodents without inducing prostate growth or hemoglobin elevations, at least when administered at lower doses (i.e., ≤ 1.0 mg/week) [15]. The ability of TREN to augment androgen-sensitive tissues is purported to derive from its ability to bind with both human and rodent androgen receptors (ARs) with an affinity three times greater than testosterone [15,16]. However, the mechanism(s) underlying the selective tissue specific effects of TREN requires further elucidation, but are likely to be androgen dependent because trenbolone is non-aromatizable [17] and induces feedback inhibition that potentially suppresses endogenous testosterone and estradiol production [15]. Trenbolone has been extensively studied for its ability to enhance skeletal muscle mass in several mammalian species [15,18–24]; however, only limited data exists on its potential bone protective effects, with no published reports evaluating the effects of TREN on bone in skeletally mature rodents.
The primary purpose of this study was to evaluate the effects of low-dose TREN administration on bone mineral characteristics and bone strength in skeletally mature male rodents. Secondary purposes were to determine the effects of TREN on (1) hemoglobin concentrations, (2) prostate mass, and (3) several androgen dependent tissue masses, including the levator ani/bulbocavernosus (LABC) muscle, retroperotineal fat pad, and kidney within a skeletally mature rodent model. We hypothesized that TREN will produce similar bone protective effects and a similar elevation in androgen-sensitive tissue mass as high-dose testosterone, without increasing prostate mass or elevating hemoglobin concentrations.
Methods
Animal care
Barrier-raised and viral pathogen-free Fischer F344/Brown Norway male rats aged 10 months were obtained from Charles River Laboratories (Wilmington, MA). Animals were individually housed in a temperature- and light-controlled room on a 12-h light, 12-h dark cycle. All rats underwent a 1 week acclimatization period prior to beginning experimental interventions. Rats were fed an ad libitum diet of Harlan rodent chow containing 3.1 kcal/g, distributed as 44.2% carbohydrate, 6.2% fat and 18.6% protein; 1.0% calcium, 0.7% phosphorus, 0.4% non-phytate phosphorus (no. 2018, Harlan Teklad Diets Madison, WI, USA) and tap water. All experimental procedures conformed to the ILAR Guide to the Care and Use of Experimental Animals and were approved by the Institutional Animal Care and Use Committee at the Gainesville VA Medical Center and in accordance with EU Directive 2010/63/EU.
Experimental design
The rats were divided into 4 groups (n=10/group), including sham surgery plus vehicle (SHAM), orchiectomy plus vehicle (ORX), ORX plus 1.0 mg trenbolone enanthate/week (ORX+TREN), ORX plus 7.0 mg testosterone enanthate/week (ORX+TE). With the exception of SHAM, all treatment groups underwent bilateral ORX, involving removal of the testes, epididymis, and epididymal fat pad under isoflurane anesthesia. Following surgery, a nutritional supplement (NIH protocol diet) [containing 1 packet Raspberry Jello® (Kraft Foods Global, Inc., Northfield, IL), 30 mL of STAT-VME® (PRN Pharmacal, Pensacola, FL), 10 mL vanilla Pediasure® (Abbott Laboratories, Columbus, OH), and 1 tablespoon Challenge™ soy protein (General Nutrition Corporation, Pittsburgh, PA)] was dissolved in 480 ml of water and refrigerated in ice cube trays. The postsurgery diet was provided for 1 week in order to minimize weight loss resulting from surgery. Following the week of recovery, rodents underwent weekly injections of drug or vehicle in alternating quadriceps for 5 weeks and blood was sampled at bi-weekly intervals from the tail tip for hemoglobin (Hb). At day 42, rats were euthanized, via intraperitoneal pentobarbital (120 mg/kg) injection, and blood was collected via cardiac puncture for analysis of bone formation and resorption markers, and circulating hormones. The left and right femurs and tibiae and prostate were also removed and weighed, and bone length was measured using a digital caliper (Mitutoyo, Aurora, IL). Serum, tibiae, and prostate were snap frozen in liquid nitrogen and stored at −80 ° C until further analysis. Femurs were wrapped in saline-soaked gauze, and stored at −20 °C in order to maintain the mechanical properties of the bone [25].
Hormone delivery
The enanthate esters of testosterone [11] and trenbolone [15] result in elevated circulating concentrations of each respective androgen for at least 7 days following intramuscular injection. Testosterone enanthate (Savient Pharmaceutical, East Brunswick, NJ) and TREN (Steraloids, Newport, RI) were dissolved in sesame oil (vehicle) with 5 mg/mL of chlorobutanol added as a preservative. Drugs were administered (0.1 mL) once every 7 days, under isoflurane anesthesia, into the quadriceps musculature. Injections were alternated between legs in order to reduce possible discomfort of repeated injections. The drug doses were chosen based on previous studies from our laboratory which reported that once-weekly (7.0 mg/week) TE administration fully prevents bone loss in young ORX animals, but results in significant prostate enlargement, and that low-dose (1.0 mg/week) TREN partially prevents bone loss in young ORX rodents (aged 3 months) without inducing prostate enlargement [15]. The older hypogonadal model selected for the experiment had masses nearly double our previous experiments, to ensure supraphysiologic serum testosterone levels and accounting for the increased body mass the dosages were kept at previous levels.
Hemoglobin and serum hormone analyses
Whole blood samples were assayed in duplicate using the Hgb Pro (ITC, Edison, NJ) photometer, which has an intra-assay CV of less than 2.41%. The remaining blood samples were centrifuged at 3000g for 12 min and serum aliquots were separated and stored at − 80 °C for later analysis. All serum hormone measurements were determined in duplicate within the same plate. Testosterone was determined by EIA which has a sensitivity of 0.04 ng/ml with an intra-assay CV of 5.3% (Alpco Diagnostics, Windham, NH). Trenbolone was determined by modifying a qualitative EIA (Neogen Corporation, Lexington, KY) with a sensitivity of 0.1 ng/ml and an intra-assay CV of 3.76%, according to methods previously devised in our laboratory [15]. Specifically, trenbolone (Sigma-Aldrich, St. Louis, MO) was dissolved in 100% ethanol (1 mg/ml) and subsequently serially diluted in hormone-free EIA buffer (Neogen Corporation, Lexington, KY) to produce a quantitative standard curve. Tartrate resistant acid phosphatase isoform 5b (Trap5b) was analyzed by a quantitative commercial immunofixed enzyme activity assay (Immunodiagnostic Systems, Fountain Hills, AZ) with a minimum detection of 0.1U/L with an intra-assay CV of <6%. Osteocalcin was determined by ELISA (Rat-Mid™ Osteocalcin, Immunodiagnostic systems, Fountain Hills, AZ) with a sensitivity of 50 ng/mL and an intra-assay CV of <6%.
Bone morphometry and mechanical strength (load to failure)
Prior to peripheral quantitative computerized tomography (pQCT), the left and right femurs were thawed to room temperature and remained wrapped in saline-soaked gauze except when undergoing analysis. The left femoral diaphysis and distal femoral metaphysis were scanned with a Stratec XCT Research M Instrument (Norland Medical Systems, Fort Atkinson, WI). Scans were performed at a distance of 5 mm (metaphysis) and 18 mm (diaphysis) proximal to the distal end of the femur for total, trabecular (t), and cortical (c) bone area (mm2), bone mineral content (mg/mm), bone mineral density (mg/cm3), cortical thickness (mm), and endocortical and periosteal circumference (mm).
Subsequent to pQCT, the right femoral neck was subjected to an axial load mechanical test. The femoral shaft was mounted vertically on the base of the testing machine and an axial load was applied to the femoral head using a steel rod. An initial preload of 10 N was applied and then the specimen was loaded at 1.0 mm/s until failure. Maximum load is reported for the femoral neck, expressed as force (measured in Newtons) as previously described by Yarrow and colleagues [11,15]. The midshaft of the left femur was subjected to a medial/lateral three point bending test which was performed using methods previously described by Leppanen and others [26]. Two 6 mm support rollers were positioned 50 mm apart, while a 6 mm loading roller was applied at a rate of 1 mm/s at the midpoint of the supports using a computer controlled mechanical testing machine (MTS Systems CO., Eden Prarie, MN) until failure. The data from these tests are reported as load to failure.
Statistical analysis
Results are reported as means±SEM, and p≤0.05 was the criterion for statistical significance. Dependent variables were separately analyzed using the Kruskal–Wallis test, which is robust to outliers. When indicated, pair wise comparisons were further evaluated with the Mann–Whitney U test. Pearson product moment correlations were performed on the SHAM, ORX, and ORX+TE groups in order to identify associations for the following variables of interest: (1) serum androgen concentrations, (2) bone mechanical strength, (3) markers of bone formation and resorption, and (4) hemoglobin concentrations. Serum hormone values that were below the lowest detectable standard are reported as such and were assigned a value equal to the sensitivity of each individual assay for the purposes of statistical analysis. Data were analyzed with the SPSS v15.0.0 statistical software package.
Results
Bone measurements, bone mineral density and bone mechanical strength Body mass
Final body weights were: SHAM 444±17 g; ORX 420±13 g; ORX+TE 405±11 g; ORX+TREN 413+8 g with no significant differences between groups. SHAMs, ORX, and ORX+TE animals experienced a non-significant 3% loss in body weight from post-surgery to sacrifice (p>0.05). ORX+TREN animals body weight decreased by 5% over the course of the intervention (p>0.05). There was no significant difference between the groups (p>0.05).
Femoral metaphysis characteristics
The bone mineral characteristics at the femoral diaphysis and distal femoral metaphysis are presented in Tables 1 and 2. In ORX animals, total BMC was reduced 9% (trend, p=0.053) at the femoral metaphysis, total BMD was reduced 5.5% (p<0.05), and tBMC and tBMD were reduced 21–24% (p<0.001) compared to SHAM. Both ORX+TE and ORX+TREN increased tBMC by 18–20% (p<0.01) and tBMD by 20–21% (p<0.01) compared with ORX treatment and ultimately maintained both tBMC and tBMD at the level of SHAMS. No differences in total, trabecular, or cortical bone area measurements were present among groups (Table 1).
Table 1.
Bone pQCT measures at the distal femoral metaphysis.
| SHAM (a) | ORX (b) | ORX+TE (c) | ORX+TREN (d) | |
|---|---|---|---|---|
| Total content (mg/mm) | 14.4±0.5 | 13.1±0.3a | 14.0±0.5 | 14.0±0.5 |
| Total density (mg/cm3) | 667.3±9.5 | 638.1±8.2a | 675.8±13.2b | 676.0±10.7b |
| Total area (mg/cm2) | 21.6±0.7 | 20.6±0.5 | 20.7±0.5 | 20.7±0.2 |
| Trabecular content (mg/mm) | 1.87±0.1 | 1.42±0.1 a† | 1.79±0.1b† | 1.79±0.1 b† |
| Trabecular density (mg/cm3) | 288.9±8.4 | 231.3±8.5 a† | 288.6±14.9 b† | 286.9±11.5 b† |
| Trabecular area (mg/cm2) | 6.5±0.2 | 6.2±0.1 | 6.2±0.2 | 6.2±0.2 |
| Cortical content (mg/mm) | 9.4±0.5 | 8.5±0.2 | 9.4±0.5 | 9.3±0.5 |
| Cortical density (mg/cm3) | 960.1±5.9 | 998.1±7.1 a† | 971.7±5.1 b | 982.0±8.6 |
| Cortical area (mg/cm2) | 9.8±0.6 | 8.6±0.3 | 9.7±0.5 | 9.5±0.5 |
Values represent means±SEM. Letters a–d indicate differences from respectively labeled groups
= vs. SHAM,
=vs. ORX,
=vs. ORX+TE,
=vs. ORX+TREN) at p<0.05 or
p<0.01.
Table 2.
Bone pQCT measures at the femoral diaphysis.
| SHAM (a) | ORX (b) | ORX+TE (c) | ORX+TREN (d) | |
|---|---|---|---|---|
| Total content (mg/mm) | 11.8±0.2 | 11.2±0.2 | 11.4±0.3 | 11.0±0.2a |
| Total density (mg/cm3) | 985.9±11.5 | 987.6±11.5 | 1018.9±15.6 | 998.7±11.6 |
| Total area (mg/cm2) | 12.0±0.2 | 11.4±0.3 a | 11.2±0.3 a | 11.0±0.2 a |
| Cortical content (mg/mm) | 11.5±0.2 | 10.9±0.2 | 11.1±0.3 | 11.0±0.2 a |
| Cortical density (mg/cm3) | 1390.6±5.1 | 1388.7±4.8 | 1397.6±3.9 | 1397.2±5.0 |
| Cortical area (mg/cm2) | 8.3±0.1 | 7.9±0.2 | 8.0±0.2 | 7.7±0.2 a |
| Cortical thickness (mm) | 0.87±0.01 | 0.85±0.01 | 0.87±0.02 | 0.85±0.01 |
| Periosteal circumference (mm) | 12.3±0.1 | 12.0±0.1 | 11.8±0.2 a | 11.8±0.1 a |
| Endocortical circumference (mm) | 6.8±0.1 | 6.6±0.1 | 6.3±0.2 a | 6.5±0.1 |
Values represent means±SEM. Letters a–d indicate differences from respectively labeled groups
=vs. SHAM,
=vs. ORX,
=vs. ORX+TE,
=vs. ORX+TREN) at p<0.05 or
p<0.01.
Femoral diaphysis characteristics
In ORX animals, total bone area was reduced by 5% compared with SHAMs (p<0.05); however, no other differences were present compared with the other groups. ORX+TE did not prevent the ORX-induced reduction in total bone area and also resulted in 4–7% reductions in both periosteal and endocortical circumference respectively, compared with SHAMs (p<0.05). TREN administration was also unable to preserve total bone area at this skeletal site. Additionally, TREN administration resulted in 7% reductions in total BMC, 6% reduction in cBMC, 6% reduction in cortical bone area at the femoral diaphysis, compared with SHAMs (p<0.05). No differences were present for any other measure at this skeletal site.
Bone mechanical strength (load to failure)
Bone mechanical strength values measuring load to failure at the femoral neck were as follows: SHAM 206±10 N, ORX 175±12 N, ORX+TE 214±10 N, and ORX+TREN 225±5 N. ORX+TREN treatment increased femoral neck strength by 28% compared with ORX (p<0.05) and a trend was present indicating a 13.5% increase in femoral neck strength in ORX+TREN animals, compared with SHAMs (p=0.08, trend). Femoral midshaft strength values measuring load to failure were as follows: SHAM 173±9 N, ORX 161±10 N, ORX+TE 165±8 N, and ORX+TREN 164±7 N, with no differences present among groups.
Serum androgen concentrations
At sacrifice, serum testosterone concentrations were SHAM: 2.5±0.5 ng/mL, 0.11±0.1 ng/mL in ORX, 10.0±0.6 ng/mL in ORX+TE, and 0.20±0.1 ng/mL in ORX+TREN animals (Fig. 1, p<0.001). ORX resulted in a near complete reduction in serum testosterone concentrations (p<0.001) and ORX+TREN did not prevent this reduction. Conversely, ORX+TE treatment elevated serum testosterone concentrations nearly 5-fold above SHAM (p<0.001). Administration of TREN also resulted in elevation in serum trenbolone to 4.77±0.42 ng/mL (Fig. 2, p<0.001), whereas serum trenbolone concentrations were below the level of assay sensitivity in all other groups.
Fig. 1.
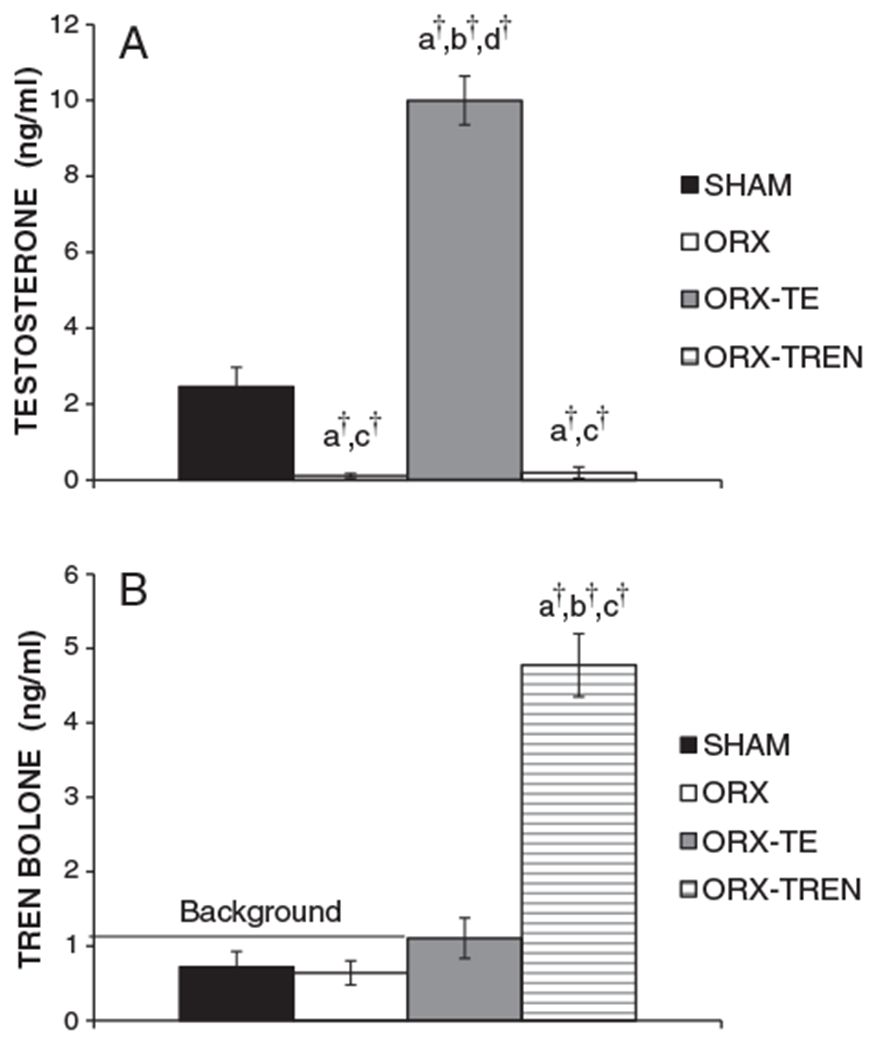
(A) Weekly intramuscular injections of testosterone enanthate (TE) result in elevated serum testosterone concentrations. (B) Weekly intramuscular injections of trenbolone enanthate (TREN) result in elevated serum trenbolone concentrations. Levels of TREN present in SHAM, ORX+V, and ORX+TE are from background noise of the assay. Values are means±SE, n=9–10/group. Letters a–d indicate differences from respectively labeled groups (a=vs. SHAM, b=vs. ORX, c=vs. ORX+TE, d=vs. ORX+TREN) at p<0.05 or †p<0.01.
Fig. 2.
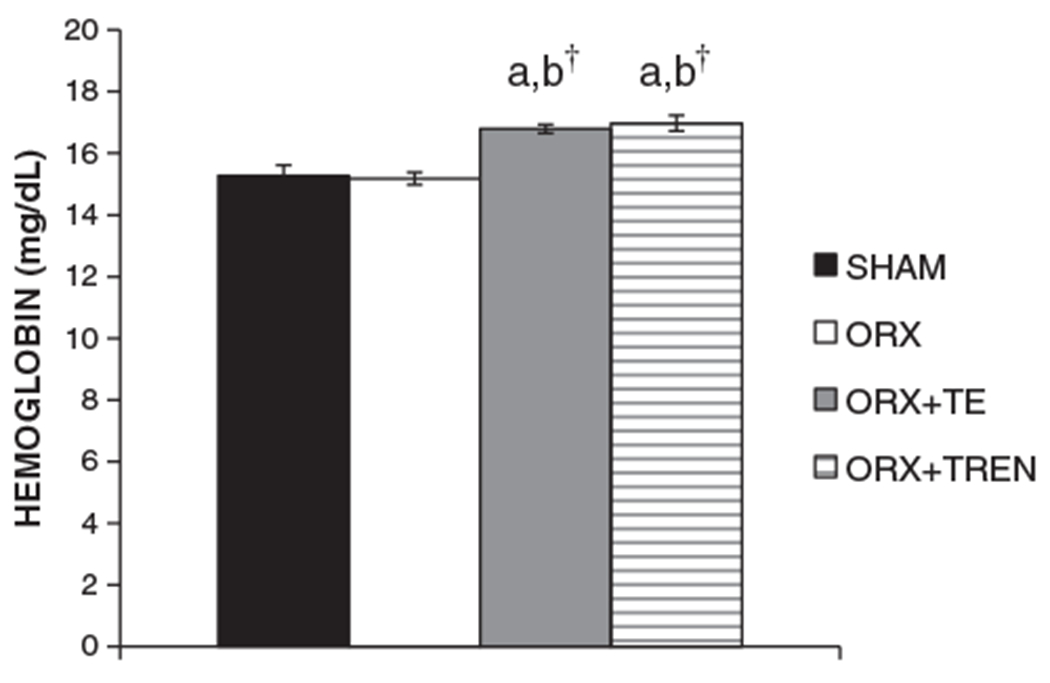
Hemoglobin levels at sacrifice. Values are means±SE, n=9–10/group. Letters a–d indicate differences from respectively labeled groups (a=vs. SHAM, b=vs. ORX, c=vs. ORX+TE, d=vs. ORX+TREN) at p<0.05 or †p<0.01.
Hemoglobin concentration
At sacrifice, whole blood Hgb concentrations were SHAM: 15.3±0.4 mg/dL, ORX: 15.2±0.3 mg/dL, ORX+TE: 16.8±0.2 mg/dL, ORX+TREN: 17.0±0.3 mg/dL (Fig. 3). ORX treatment did not alter Hgb concentrations, whereas TE and TREN treatments elevated whole blood Hgb concentrations 10–11% above both SHAM and ORX conditions (p<0.01). No differences in Hgb concentrations were present between TE and TREN treatments.
Fig. 3.
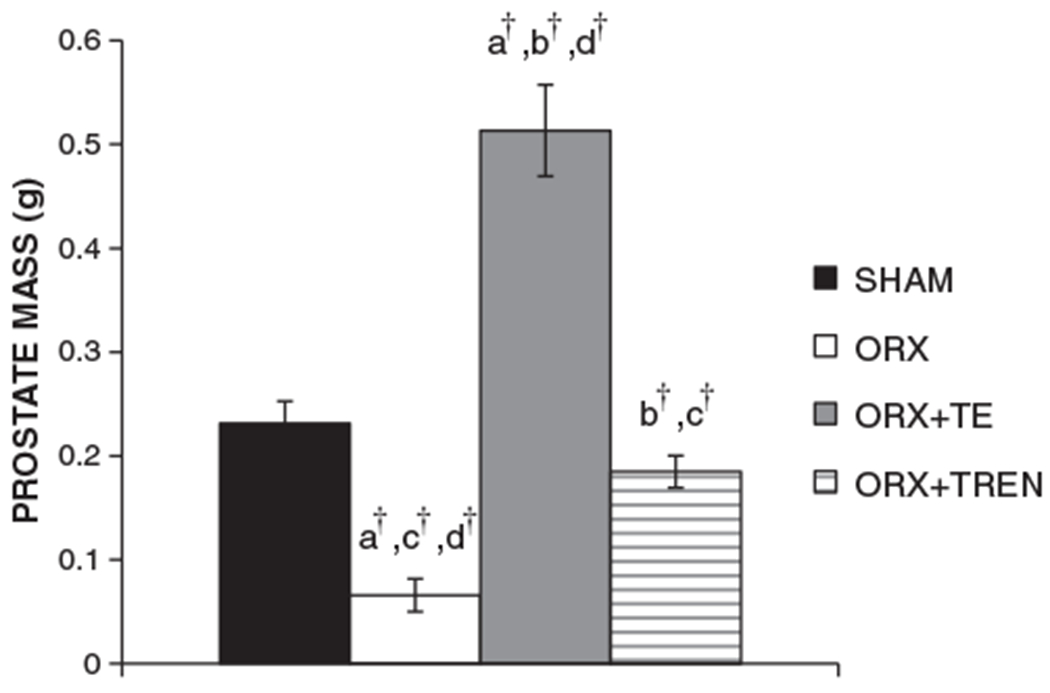
Prostate masses at sacrifice. Values are means±SE, n=9–10/group. Letters a–d indicate differences from respectively labeled groups (a=vs. SHAM, b=vs. ORX, c=vs. ORX+TE, d=vs. ORX+TREN) at p<0.05 or †p<0.01.
Prostate
Prostate masses were: 0.23±0.02 g SHAM, 0.07±0.02 g ORX, 0.51±0.04 g ORX+TE, and 0.19±0.02 g ORX+TREN. ORX treatment reduced prostate mass by 72% compared to SHAM (Fig. 4, p<0.001), while ORX+TE treatment completely prevented this decline and resulted in a prostate mass that was 2-fold greater than SHAMs and 6-fold greater than ORX (p<0.001). ORX+TREN treatment partially prevented the ORX-induced reduction in prostate mass, ultimately resulting in a non-significant 20% reduction in prostate mass compared to SHAMs (p=0.12) and a 64% smaller prostate mass than in ORX+TE animals (p<0.001).
Fig. 4.
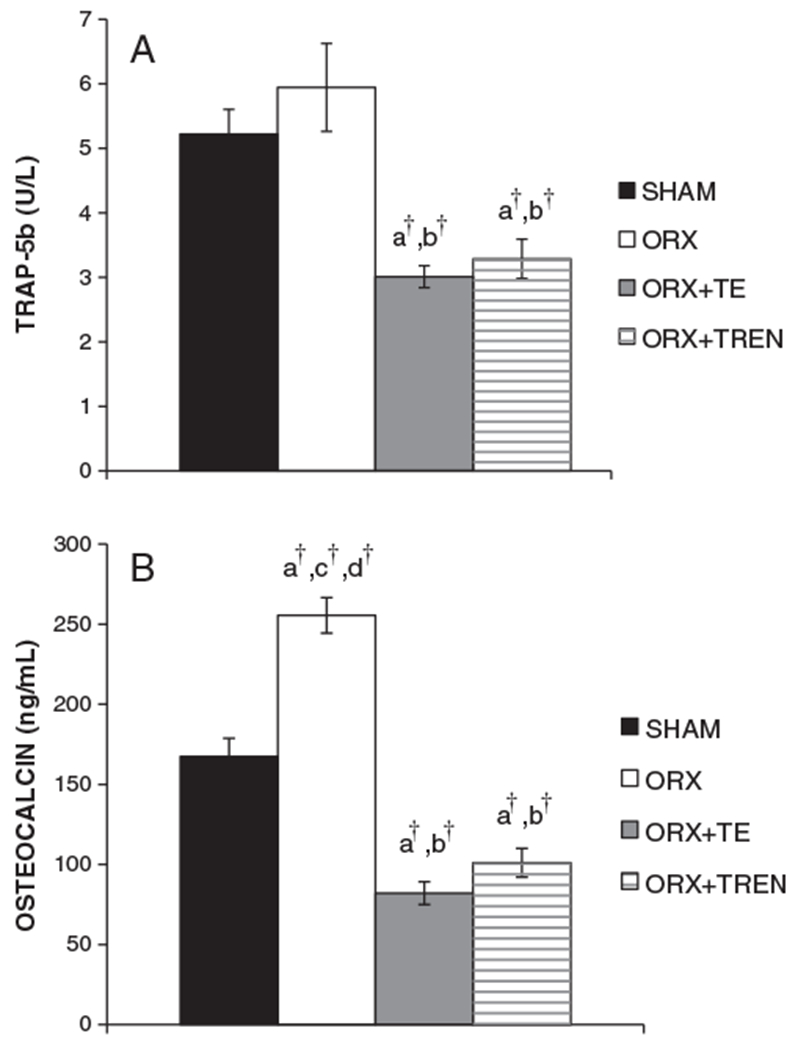
Serum markers of bone turnover. (A) Serum Trap5b at sacrifice. (B) Serum osteocalcin at sacrifice. Values are means±SE, n=9–10/group. Letters a–d indicate differences from respectively labeled groups (a=vs. SHAM, b=vs. ORX, c=vs. ORX+TE, d=vs. ORX+TREN) at p<0.05 or †p<0.01.
Systemic markers of bone turnover
At sacrifice, tartrate resistant acid phosphatase (TRAP5b), a marker of bone resorption, was 5.2±0.4 U/L SHAM, 5.9±0.7 U/L ORX, 3.0±0.2 U/L ORX+TE, and 3.3±0.3 U/L ORX+TREN. Both androgen treatments lowered serum TRAP5b concentrations to a similar magnitude compared to SHAM (p<0.01) and ORX (p<0.01). At sacrifice, serum osteocalcin, a marker of bone formation was 167.4±11.4 ng/mL SHAM, 255.5±11.1 ng/mL ORX, 82.1±7.1 ng/mL ORX+TE, and 101.1±9.0 ng/mL ORX+TREN. ORX elevated serum osteocalcin by 53% compared to SHAMs (p<0.001). TE treatment reduced serum osteocalcin by 51% compared to SHAMs (p<0.001) and by 3-fold compared to ORX (p<0.001). TREN administration also reduced serum osteocalcin by 40% compared to SHAMs and by more than 2-fold compared to ORX (p<0.001). There was no difference in serum osteocalcin between ORX+TE and ORX+TREN animals. Serum osteocalcin concentrations were moderately and positively correlated with serum Trap5b (r=0.620, p<0.01). Serum testosterone was strongly and inversely associated with serum osteocalcin (r=−0.871, p<0.01) and Trap5b (r=−0.672, p<0.01) for SHAM, ORX, and ORX+TE groups.
Levator ani bulbocavernosus muscle
The androgen-sensitive LABC muscle masses were 1.07±0.04 g SHAM, 0.68±0.03 g ORX, 1.54±0.03 g ORX+TE, and 1.54±0.04 g ORX+TREN. ORX treatment reduced LABC mass by 36% compared to SHAM (p<0.01). Administration of TE and TREN resulted in a 44% increase in the LABC compared to SHAM (p<0.01). Moreover, ORX+TE and ORX+TREN animals experienced a 126% increase in LABC mass compared to ORX animals (p<0.01). There was no significant difference between ORX+TE and ORX+TREN groups for LABC mass (p>0.05).
Retroperitoneal fat pad mass
Following the intervention, retroperitoneal fat pad masses were 3.26±0.33 g SHAM, 4.06±0.20 g ORX, 2.25±0.08 g ORX+TE, and 2.84±0.12 g ORX+TREN. ORX animals tended to increase retroperitoneal fat pad mass ~25% (p=0.052, trend) compared to SHAM. Conversely, a respective 13% and 31% reduction in retroperitoneal fat pad mass were evident for ORX+TREN and ORX+TE compared to SHAM (p<0.05). In a similar fashion, ORX+TREN decreased retroperitoneal fat pad mass by 30% and ORX+TE treatment significantly reduced retroperitoneal fat pad mass by 45% (p<0.01), compared with the ORX group. ORX+TE treatment also resulted in a reduced retroperitoneal fat pad mass compared to ORX+TREN animals (p<0.01).
Kidney
Kidney masses were the following: 1.12±0.04 g SHAM, 0.96±0.02 g ORX, 1.20±0.04 g ORX+TE, and 1.15±0.02 g ORX+TREN. After 6 weeks, kidney mass in ORX animals was reduced by 13% compared to SHAM (p<0.05). Androgen treatments significantly increased kidney mass between 18% and 23% compared to ORX (p<0.01). However there was no significant differences between androgen treatments, and neither androgen treatment resulted in kidney masses significantly larger than SHAM.
Discussion
Testosterone is known to protect against androgen deficiency induced bone loss in males, either through direct androgen-mediated mechanisms and/or indirectly, through estrogen-mediated mechanism following aromatization to estradiol (E2) [11,26–29]. The principal new observation from this study is that trenbolone enanthate (TREN), a non-5 alpha reducible and non-aromatizable androgen, fully protected against trabecular bone loss at the distal femoral metaphysis in a skeletally mature, sex-hormone deficient rodent model and also resulted in increased femoral neck strength compared with ORX animals. TREN appeared to mitigate one of the primary side effects associated with high-dose TE administration, as evidenced by its ability to maintain prostate mass at the level of SHAMs, while TE resulted in an approximate doubling of prostate mass. However, both TREN and high-dose TE resulted in similar elevations in hemoglobin concentrations. Additionally, both TREN and high-dose TE increased LABC muscle mass above that of SHAM animals and reduced retroperitoneal fat pad mass below Sham levels.
The current study extends previous work from our laboratory examining the ability of various androgens to safely prevent the ORX-induced loss of musculoskeletal tissue. We have established that both 3 month old ORX F344 and 3 month old ORX Brown Norway rats mimic many of the catabolic changes associated with hypogonadism, including bone loss [8–10]. Using these models, bone and muscle loss are evident as early as 2 weeks following ORX [8] and high-dose TE treatment is capable of completely preventing this loss and also improving bone strength in young gonadectomized rodents [8–11,15]. Animals in the current study differed from our previous model in several ways: (1) animals were crossbred F344/Brown Norway rats, (2) animals underwent ORX at 10 months of age, in order to evaluate the bone protective ability of TE and TREN in a skeletally mature model, and (3) all animals underwent 1-week hormone deprivation, prior to drug administration, in order to mimic a short-duration loss of testosterone that occurs in older hypogonadal men prior to TRT. In addition, we selected a low-dose of TREN (1.0 mg/week) that we have previously shown to augment skeletal muscle mass and partially prevent ORX-induced bone loss in young male rodents, without inducing prostate enlargement [15]. In the current study, pQCT analysis revealed a significant loss of trabecular bone at the distal femoral metaphysis 6 weeks following ORX. Interestingly, low-dose TREN fully protected against ORX-induced bone loss at this skeletal site in our current study, which is in contrast to the partial prevention of bone loss we previously observed in young ORX rodents [15]. Administration of 7.0 mg/week of TE and 1.0 mg/week of trenbolone resulted in significant depression of circulating osteocalcin and Trap5b levels that underlie the preserved bone mass. As evidence, ORX slightly, yet non-significantly, increased serum Trap5b and concomitantly increased serum osteocalcin, indicating increased bone turnover. Conversely, both TE and TREN reduced bone resorption (Trap5b) and bone formation (osteocalcin) markers to below the level of SHAM animals, similar to what occurs in young animals following androgen administration [11,15].
In elderly men, hypogonadism is associated with a loss of BMD, increased risk of bone fractures [30,31], and a near doubling of the 1 year mortality rate following hip fracture, as compared to women [4]. These results demonstrate the severity of bone fractures in older men, especially at the hip. As such, it remains important to evaluate bone strength, along with BMD, when determining the effectiveness of bone protective agents. An important finding from our current study is that TREN, but not high-dose TE treatment, increased bone strength at the femoral neck, a common fracture site in hypogonadal elderly men [32,33], when compared to ORX animals. It remains unclear why femoral neck strength was influenced by TREN treatment, but not TE. Trenbolone potentially exerts part of its influence on bone through the reduction in circulating corticosterone [34], and through its antiglucocorticoid activity [17]. Corticosterone has been shown by Henneicke and colleagues [35] to suppress serum osteocalcin levels and increase Trap5b levels in mice. Additionally, trenbolone has agonist action at the progesterone receptor [17], which may play a minor role in bone remodeling in skeletally mature male rats. In this regard, norethisterone, a progestagen, has shown an ability to increase bone density in castrated male mice [36]. However, the direct effects of progesterone receptor binding in the male rat model remains to be determined.
Testosterone is known to exert bone protective effects directly, via interaction with ARs and/or indirectly [37–39], following 5-alpha reduction or aromatization to its potent metabolites dihydrotestosterone (DHT) and estradiol (E2) [1], respectively. In fact, a small degree of localized skeletal-specific aromatization of testosterone to E2 is thought to be essential for bone protection in males [40]. We did not evaluate E2 concentrations in this study because currently available commercial assays lack the sensitivity to detect E2 in ORX male rodents. However, our current results suggest that the highly potent synthetic testosterone analogue TREN is capable of providing bone protection despite its apparent inability to undergo aromatization to E2 [17]. Interestingly, TREN completely suppresses endogenous testosterone production, the only known source of E2 in male rodents [15]. Additionally, neither trenbolone nor its primary metabolites are estrogenic [41], and in some oviparous species trenbolone may even exert anti-estrogenic effects [24]. Dihydrotestosterone, the 5α reduced metabolite of testosterone, also preserves BMD to a similar degree as testosterone, despite the inability of DHT to undergo aromatization to E2 [42,43]. As such, it appears that TREN (and certain other non-aromatizable androgens) are capable of exerting bone protection directly, through androgen-mediated pathways, and in the apparent absence of estrogenic influence because trenbolone is metabolized only to less potent androgens in vivo.
Testosterone provides clear benefit on the preservation of muscle and bone in androgen deficient men [44,45], but also increases risk for a number of adverse conditions. Of these, prostate enlargement and prostate/lower urinary tract symptoms are commonly reported side effects of TRT [12,44–47]. Our current results confirm previous findings from our laboratory which demonstrate that high-dose TE administration increases prostate mass in a rather dramatic manner [9–11,15]. In contrast, TREN maintained prostate mass at the level of SHAMs, similar to results we have previously reported in young intact and young ORX animals [15]. Interestingly, TE and TREN equally influenced changes in other androgen responsive tissues, including the kidney and LABC muscle in the rat. However, TE treatment resulted in a more robust reduction in retroperitoneal fat pad mass than TREN. In the clinical setting, it has long been assumed that TRT is associated with prostate cancer. However, there is no direct evidence indicating that TRT increases risk for cancer in tissues which highly express 5α reductase, such as prostate [12,48]. In this regard, TREN may be a beneficial alternative to high-dose TE, considering that trenbolone and its primary metabolites are non-estrogenic [41], and cannot undergo 5 alpha reduction or aromatization to more potent steroids [24]; however, further studies are necessary to confirm this hypothesis and to evaluate other potential side effects associated with TREN administration. Some side effects of trenbolone are known, for example trenbolone has been shown to reduce testicular weight and size in intact male pigs [49] potentially through feedback inhibition, similar to the effects of high-dose testosterone [49]. High-dose trenbolone has also been shown to negatively impact immune function in castrated CD-1 type mice [50] coupled with the potential genotoxicity impact on vertebrate organisms of trenbolone [24]. Additionally, as with any androgen, it remains important to evaluate the cancer inducing potential of TREN prior to advancing this agent to clinical testing.
In humans, polycythemia is the most prevalent adverse event associated with TRT [12,51]. In our current study, administration of TREN resulted in similarly elevated Hgb concentrations to that of high-dose TE administration. Elevated Hgb concentrations are often considered a negative finding, considering the health risks associated with polycythemia [52,53]. However, the ability of androgens to boost circulating Hgb concentrations may also have positive clinical ramifications for a certain proportion of hypogonadal patients with concomitant anemia, which is non-responsive to iron repletion [54], or erythropoietin therapy [55]. Recent estimates place the overall prevalence of anemia in those aged 65 and older at 10% [56]. A recent study, in a cohort of 175 community-dwelling aged individuals aged 65 and older reported that 44% of all anemia cases presented with anemia of unknown etiology [57]. Clearly, future research examining the etiology of polycythemia in patients undergoing androgen replacement therapy is needed in order to minimize the potential risks of this treatment, while maintaining its benefits of on musculoskeletal tissue.
Conclusions
Low-dose TREN maintains trabecular BMD and LABC muscle mass to the same degree as high-dose TE in skeletally mature male ORX rodents. Additionally, in contrast to TE, low-dose TREN maintained prostate mass at the level of intact animals and elevated femoral neck strength compared to ORX animals. However, the clinical ramifications of the increased Hgb concentrations associated with both high-dose TE and TREN administration remain to be determined. In conclusion, TREN appears to possess androgen-sensitive tissue-specificity which may render it advantageous for future preclinical and clinical trials examining the safety and efficacy of various pharmacological agents which may prevent androgen deficiency associated bone and muscle loss.
Acknowledgments
This work was supported by VA Merit Award to SEB and a VA Career Development Award-2 to JFY.
Sources of Support:
This work was supported by VA Merit Award to SEB and a VA Career Development Award-2 to JFY. The funding agency had no role in the study design, collection, analysis, preparation or submission of this manuscript.
References
- [1].Compston JE. Sex steroids and bone. Physiol Rev 2001;81(1):419–47. [DOI] [PubMed] [Google Scholar]
- [2].Shuler FD, Conjeski J. Defining bone health and fracture risk in West Virginia: the World Health Organization FRAX assessment tool. W V Med J 2011;107(5):12–7. [PubMed] [Google Scholar]
- [3].Johnell O, Kanis JA. An estimate of the worldwide prevalence and disability associated with osteoporotic fractures. Osteoporos Int 2006;17(12):1726–33. [DOI] [PubMed] [Google Scholar]
- [4].Bass E, French DD, Bradham DD, Rubenstein LZ. Risk-adjusted mortality rates of elderly veterans with hip fractures. Ann Epidemiol 2007;17(7):514–9. [DOI] [PubMed] [Google Scholar]
- [5].Rao SS, Budhwar N, Ashfaque A. Osteoporosis in men. Am Fam Physician 2010;82(5):503–8. [PubMed] [Google Scholar]
- [6].Leifke E, Korner HC, Link TM, Behre HM, Peters PE, Nieschlag E. Effects of testosterone replacement therapy on cortical and trabecular bone mineral density, vertebral body area and paraspinal muscle area in hypogonadal men. Eur J Endocrinol 1998;138(1):51–8. [DOI] [PubMed] [Google Scholar]
- [7].Martin AC. Osteoporosis in men: a review of endogenous sex hormones and testosterone replacement therapy. J Pharm Pract 2011;24(3):307–15. [DOI] [PubMed] [Google Scholar]
- [8].Borst SE, Conover CF. Orchiectomized Fischer 344 male rat models body composition in hypogonadal state. Life Sci 2006;79(4):411–5. [DOI] [PubMed] [Google Scholar]
- [9].Borst SE, Conover CF, Carter CS, Gregory CM, Marzetti E, Leeuwenburgh C, et al. Anabolic effects of testosterone are preserved during inhibition of 5alpha-reductase. Am J Physiol Endocrinol Metab 2007;293(2):E507–14. [DOI] [PubMed] [Google Scholar]
- [10].Borst SE, Lee JH, Conover CF. Inhibition of 5alpha-reductase blocks prostate effects of testosterone without blocking anabolic effects. Am J Physiol Endocrinol Metab 2005;288(1):E222–7. [DOI] [PubMed] [Google Scholar]
- [11].Yarrow JF, Conover CF, Purandare AV, Bhakta AM, Zheng N, Conrad B, et al. Supraphysiological testosterone enanthate administration prevents bone loss and augments bone strength in gonadectomized male and female rats. Am J Physiol Endocrinol Metab 2008;295(5):E1213–22. [DOI] [PubMed] [Google Scholar]
- [12].Calof OM, Singh AB, Lee ML, Kenny AM, Urban RJ, Tenover JL, et al. Adverse events associated with testosterone replacement in middle-aged and older men: a metaanalysis of randomized, placebo-controlled trials. J Gerontol A Biol Sci Med Sci 2005;60(11):1451–7. [DOI] [PubMed] [Google Scholar]
- [13].Mohler ML, Bohl CE, Jones A, Coss CC, Narayanan R, He Y, et al. Nonsteroidal selective androgen receptor modulators (SARMs): dissociating the anabolic and androgenic activities of the androgen receptor for therapeutic benefit. J Med Chem 2009;52(12):3597–617. [DOI] [PubMed] [Google Scholar]
- [14].Rosen J, Negro-Vilar A. Novel, non-steroidal, selective androgen receptor modulators (SARMs) with anabolic activity in bone and muscle and improved safety profile. J Musculoskelet Neuronal Interact 2002;2(3):222–4. [PubMed] [Google Scholar]
- [15].Yarrow JF, Conover CF, McCoy SC, Lipinska JA, Santillana CA, Hance JM, et al. 17beta-Hydroxyestra-4,9,11-trien-3-one (trenbolone) exhibits tissue selective anabolic activity: effects on muscle, bone, adiposity, hemoglobin, and prostate. Am J Physiol Endocrinol Metab 2011;300(4):E650–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Bauer ER, Daxenberger A, Petri T, Sauerwein H, Meyer HH. Characterisation of the affinity of different anabolics and synthetic hormones to the human androgen receptor, human sex hormone binding globulin and to the bovine progestin receptor. APMIS 2000;108(12):838–46. [DOI] [PubMed] [Google Scholar]
- [17].Wilson VS, Lambright C, Ostby J, Gray LE Jr. In vitro and in vivo effects of 17beta-trenbolone: a feedlot effluent contaminant. Toxicol Sci 2002;70(2):202–11. [DOI] [PubMed] [Google Scholar]
- [18].Gonzalez JM, Carter JN, Johnson DD, Ouellette SE, Johnson SE. Effect of ractopamine-hydrochloride and trenbolone acetate on longissimus muscle fiber area, diameter, and satellite cell numbers in cull beef cows.J Anim Sci 2007;85(8):1893–901. [DOI] [PubMed] [Google Scholar]
- [19].Parr SL, Chung KY, Galyean ML, Hutcheson JP, DiLorenzo N, Hales KE, et al. Performance of finishing beef steers in response to anabolic implant and zilpaterol hydrochloride supplementation. J Anim Sci 2011;89(2):560–70. [DOI] [PubMed] [Google Scholar]
- [20].Parr SL, Chung KY, Hutcheson JP, Nichols WT, Yates DA, Streeter MN, et al. Dose and release pattern of anabolic implants affects growth of finishing beef steers across days on feed. J Anim Sci 2011;89(3):863–73. [DOI] [PubMed] [Google Scholar]
- [21].Thompson SH, Boxhorn LK, Kong WY, Allen RE. Trenbolone alters the responsiveness of skeletal muscle satellite cells to fibroblast growth factor and insulin-like growth factor I. Endocrinology 1989;124(5):2110–7. [DOI] [PubMed] [Google Scholar]
- [22].Vernon BG, Buttery PJ. Protein turnover in rats treated with trienbolone acetate. Br J Nutr 1976;36(3):575–9. [DOI] [PubMed] [Google Scholar]
- [23].Vernon BG, Buttery PJ. The effect of trenbolone acetate with time on the various responses of protein synthesis of the rat. Br J Nutr 1978;40(3):563–72. [DOI] [PubMed] [Google Scholar]
- [24].Yarrow JF, McCoy SC, Borst SE. Tissue selectivity and potential clinical applications of trenbolone (17beta-hydroxyestra-4,9,11-trien-3-one): A potent anabolic steroid with reduced androgenic and estrogenic activity. Steroids 2010;75(6):377–89. [DOI] [PubMed] [Google Scholar]
- [25].Pelker RR, Friedlaender GE, Markham TC, Panjabi MM, Moen CJ. Effects of freezing and freeze-drying on the biomechanical properties of rat bone. J Orthop Res 1984;1(4):405–11. [DOI] [PubMed] [Google Scholar]
- [26].Page ST, Amory JK, Bowman FD, Anawalt BD, Matsumoto AM, Bremner WJ, et al. Exogenous testosterone (T) alone or with finasteride increases physical performance, grip strength, and lean body mass in older men with low serum T. J Clin Endocrinol Metab 2005;90(3):1502–10. [DOI] [PubMed] [Google Scholar]
- [27].Turner RT, Colvard DS, Spelsberg TC. Estrogen inhibition of periosteal bone formation in rat long bones: down-regulation of gene expression for bone matrix proteins. Endocrinology 1990;127(3):1346–51. [DOI] [PubMed] [Google Scholar]
- [28].Turner RT, Lifrak ET, Beckner M, Wakley GK, Hannon KS, Parker LN. Dehydroepi-androsterone reduces cancellous bone osteopenia in ovariectomized rats. Am J Physiol 1990;258(4 Pt 1):E673–7. [DOI] [PubMed] [Google Scholar]
- [29].Turner RT, Wakley GK, Hannon KS. Differential effects of androgens on cortical bone histomorphometry in gonadectomized male and female rats. J Orthop Res 1990;8(4):612–7. [DOI] [PubMed] [Google Scholar]
- [30].Anderson FH, Francis RM, Selby PL, Cooper C. Sex hormones and osteoporosis in men. Calcif Tissue Int 1998;62(3):185–8. [DOI] [PubMed] [Google Scholar]
- [31].Burge R, Dawson-Hughes B, Solomon DH, Wong JB, King A, Tosteson A. Incidence and economic burden of osteoporosis-related fractures in the United States, 2005-2025. J Bone Miner Res 2007;22(3):465–75. [DOI] [PubMed] [Google Scholar]
- [32].Higano CS. Androgen-deprivation-therapy-induced fractures in men with nonmetastatic prostate cancer: what do we really know? Nat Clin Pract Urol 2008;5(1):24–34. [DOI] [PubMed] [Google Scholar]
- [33].Warriner AH, Patkar NM, Curtis JR, Delzell E, Gary L, Kilgore M, et al. Which fractures are most attributable to osteoporosis? J Clin Epidemiol 2011;64(1):46–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Sillence MN, Rodway RG. Effects of trenbolone acetate and testosterone on growth and on plasma concentrations of corticosterone and ACTH in rats. J Endocrinol 1990;126(3):461–6. [DOI] [PubMed] [Google Scholar]
- [35].Henneicke H, Herrmann M, Kalak R, Brennan-Speranza TC, Heinevetter U, Bertollo N, et al. Corticosterone selectively targets endo-cortical surfaces by an osteoblast-dependent mechanism. Bone 2011;49(4):733–42. [DOI] [PubMed] [Google Scholar]
- [36].Broulik PD, Broulikova K, Necas E. Progestagens androgenic action on the bone of male castrated mice. Prague Med Rep 2006;107(4):401–8. [PubMed] [Google Scholar]
- [37].Vandenput L, Swinnen JV, Boonen S, Van Herck E, Erben RG, Bouillon R, et al. Role of the androgen receptor in skeletal homeostasis: the androgen-resistant testicular feminized male mouse model. J Bone Miner Res 2004;19(9):1462–70. [DOI] [PubMed] [Google Scholar]
- [38].Vanderschueren D, Vandenput L. Androgens and osteoporosis. Andrologia 2000;32(3):125–30. [DOI] [PubMed] [Google Scholar]
- [39].Vanderschueren D, Vandenput L, Boonen S, Lindberg MK, Bouillon R, Ohlsson C. Androgens and bone. Endocr Rev 2004;25(3):389–425. [DOI] [PubMed] [Google Scholar]
- [40].Vandenput L, Ohlsson C. Estrogens as regulators of bone health in men. Nat Rev Endocrinol 2009;5(8):437–43. [DOI] [PubMed] [Google Scholar]
- [41].Le Guevel R, Pakdel F. Assessment of oestrogenic potency of chemicals used as growth promoter by in-vitro methods. Hum Reprod 2001;16(5):1030–6. [DOI] [PubMed] [Google Scholar]
- [42].Kasperk CH, Wakley GK, Hierl T, Ziegler R. Gonadal and adrenal androgens are potent regulators of human bone cell metabolism in vitro. J Bone Miner Res 1997;12(3):464–71. [DOI] [PubMed] [Google Scholar]
- [43].Wakley GK, Schutte HD Jr., Hannon KS, Turner RT. Androgen treatment prevents loss of cancellous bone in the orchidectomized rat. J Bone Miner Res 1991;6(4):325–30. [DOI] [PubMed] [Google Scholar]
- [44].Bhasin S, Cunningham GR, Hayes FJ, Matsumoto AM, Snyder PJ, Swerdloff RS, et al. Testosterone therapy in adult men with androgen deficiency syndromes: an endocrine society clinical practice guideline. J Clin Endocrinol Metab 2006;91(6): 1995–2010. [DOI] [PubMed] [Google Scholar]
- [45].Bhasin S, Tenover JS. Age-associated sarcopenia-issues in the use of testosterone as an anabolic agent in older men. J Clin Endocrinol Metab 1997;82(6):1659–60. [DOI] [PubMed] [Google Scholar]
- [46].Bhasin S, Woodhouse L, Casaburi R, Singh AB, Mac RP, Lee M, et al. Older men are as responsive as young men to the anabolic effects of graded doses of testosterone on the skeletal muscle. J Clin Endocrinol Metab 2005;90(2):678–88. [DOI] [PubMed] [Google Scholar]
- [47].Borst SE, Mulligan T. Testosterone replacement therapy for older men. Clin Interv Aging 2007;2(4):561–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Shabsigh R, Crawford ED, Nehra A, Slawin KM. Testosterone therapy in hypogonadal men and potential prostate cancer risk: a systematic review. Int J Impot Res 2009;21(1):9–23. [DOI] [PubMed] [Google Scholar]
- [49].Lopez-Bote C, Sancho G, Martinez M, Ventanas J, Gazquez A, Roncero V. Trenbolone acetate induced changes in the genital tract of male pigs. Zentralbl Veterinarmed B 1994;41(1):42–8. [DOI] [PubMed] [Google Scholar]
- [50].Hotchkiss AK, Nelson RJ. An environmental androgen, 17beta-trenbolone, affects delayed-type hypersensitivity and reproductive tissues in male mice. J Toxicol Environ Health A 2007;70(2):138–40. [DOI] [PubMed] [Google Scholar]
- [51].Fernandez-Balsells MM, Murad MH, Lane M, Lampropulos JF, Albuquerque F, Mullan RJ, et al. Clinical review 1: Adverse effects of testosterone therapy in adult men: a systematic review and meta-analysis. J Clin Endocrinol Metab 2010;95(6):2560–75. [DOI] [PubMed] [Google Scholar]
- [52].Jaillard AS, Hommel M, Mazetti P. Prevalence of stroke at high altitude (3380 m) in Cuzco, a town of Peru. A population-based study. Stroke 1995;26(4):562–8. [DOI] [PubMed] [Google Scholar]
- [53].Kunnas T, Solakivi T, Huuskonen K, Kalela A, Renko J, Nikkari ST. Hematocrit and the risk of coronary heart disease mortality in the TAMRISK study, a 28-year follow-up. Prev Med 2009;49(1):45–7. [DOI] [PubMed] [Google Scholar]
- [54].Price EA, Mehra R, Holmes TH, Schrier SL. Anemia in older persons: etiology and evaluation. Blood Cells Mol Dis 2011;46(2):159–65. [DOI] [PubMed] [Google Scholar]
- [55].Costa E, Pereira BJ, Rocha-Pereira P, Rocha S, Reis F, Castro E, et al. Role of prohepcidin, inflammatory markers and iron status in resistance to rhEPO therapy in hemodialysis patients. Am J Nephrol 2008;28(4):677–83. [DOI] [PubMed] [Google Scholar]
- [56].Guralnik JM, Eisenstaedt RS, Ferrucci L, Klein HG, Woodman RC. Prevalence of anemia in persons 65 years and older in the United States: evidence for a high rate of unexplained anemia. Blood 2004;104(8):2263–8. [DOI] [PubMed] [Google Scholar]
- [57].Artz AS, Thirman MJ. Unexplained anemia predominates despite an intensive evaluation in a racially diverse cohort of older adults from a referral anemia clinic. J Gerontol A Biol Sci Med Sci 2011;66(8):925–32. [DOI] [PubMed] [Google Scholar]


