Abstract
Angiotensin II receptor blockers (telmisartan) prevent rodents from diet-induced obesity and improve their metabolic status. Hyperglycemia and obesity are associated with reduced cerebral blood flow and neurovascular uncoupling which may lead to behavioral deficits. We wanted to know whether a treatment with telmisartan prevents these changes in obesity.
We put young mice on high-fat diet and simultaneously treated them with telmisartan. At the end of treatment, we performed laser speckle imaging and magnetic resonance imaging to assess the effect on neurovascular coupling and cerebral blood flow. Different behavioral tests were used to investigate cognitive function.
Mice developed diet-induced obesity and after 16, not 8 weeks of high-fat diet, however, the response to whisker pad stimulation was about 30% lower in obese compared to lean mice. Simultaneous telmisartan treatment increased the response again by 10% compared to obese mice. Moreover, telmisartan treatment normalized high-fat diet-induced reduction of cerebral blood flow and prevented a diet-induced anxiety-like behavior. In addition to that, telmisartan affects cellular senescence and string vessel formation in obesity.
We conclude, that telmisartan protects against neurovascular unit impairments in a diet-induced obesity setting and may play a role in preventing obesity related cognitive deficits in Alzheimer’s disease.
Keywords: Cerebral blood flow, neurovascular coupling, obesity, renin angiotensin system, telmisartan
Introduction
Obesity is one of the most common preventable causes of death, but treatment is still not available or insufficient.1 Due to obesity, patients develop insulin and leptin resistance, high blood pressure, and have a higher risk for coronary diseases and ischemic stroke.2 Beside peripheral vascular changes, obesity leads to striking changes in the cerebral vasculature. Animal studies on obesity showed that cerebral arteries’ diameters are reduced, and wall thickness and stiffness of vessels are increased leading to an increased myogenic tone. Functional hyperemia depending on the metabolic demands of neurons and the capacity of vascular dilation is impaired in Zucker rats and rats fed with high-fat diet (HFD), a phenomenon that is known as neurovascular uncoupling.3,4 Furthermore, it has been described in rodent models of diabetes that cerebral blood flow (CBF) is reduced in these animals and autoregulation is impaired.5 Since the tightly controlled supply with oxygen and glucose is essential for normal brain function, deficiencies of this supply may also lead to cognitive decline. Upon HFD feeding, impairment in learning and memory performance and a decline in spatial memory have been shown in mice and rat models of obesity6 as well as psychiatric impairments such as anxiety-like behavior.7 A reduced CBF which is induced by obesity and insulin resistance was accused to contribute to the development of Alzheimer’s disease and dementia in humans.8
The renin angiotensin system (RAS) regulates body fluids and electrolyte balance and thereby critically influences blood pressure. Additionally, there is strong evidence that RAS is linked to obesity and glucose regulation. Angiotensin II type 1 (AT1) receptors are present in carbohydrate and lipid metabolism relevant tissues and mediate metabolic effects.9–11 AT1-receptor blocker (ARB) such as telmisartan (TEL) prevent rodents from diet-induced obesity (DIO),12–14 but are also effective in a therapeutic setting.13,15,16 These anti-obese effects occur only after high dosages and are independent of blood pressure reduction.13,17 The underlying mechanism remains a matter of debate although we found that brain related pathways are involved in DIO prevention. On the one hand, TEL sustains the penetration of leptin across the blood brain barrier and consequently prevents dysregulated leptin signaling in the brain of HFD-fed mice.10 On the other hand, for a cerebral function of TEL a full working RAS in the brain is crucial. In this regard, we found earlier that the weight gain preventing effect of TEL is blunted in mice with a brain specific angiotensinogen knockout.18
Considering all mentioned aspects of changes in obesity in terms of CBF and central effects of RAS on obesity, we here investigated effects of TEL on neurovascular coupling (NVC) and CBF in a DIO mouse model with different periods of feeding. We first hypothesize that NVC and CBF are impaired and reduced upon chronic HFD feeding and second that a treatment with TEL improves NVC and CBF in HFD-fed mice, which is the main hypothesis of this study. We addressed the hypothesis with laser speckle imaging (LSI) and magnetic resonance imaging (MRI) in mice fed with HFD for several weeks. In this context, MRI methods have been recently reviewed to be helpful for the characterization of the physio-pathological processes in the brain associated with obesity development.19 As a consequence of a potential reduction of CBF, we hypothesize further that HFD feeding leads to changes in behavior and TEL treatment can prevent these changes since there are studies which show that ARB have beneficial effects in Alzheimer’s disease.20,21 Therefore, we used different tests that address memory and anxiety-like behavior. We further investigated potential mechanisms contributing to HFD and TEL effects such as the formation of string vessels. String vessels are collapsed vessels consisting of basal membrane but no longer of endothelial cells and blood perfusion is not possible.22 We also tested for endothelial senescence with senescence-associated distension of satellites (SADS) as a marker, lipid accumulation and oxidative stress - processes that are known to be linked to obesity.7,23
Methods
Animals
All animal care and experimental procedures were conducted in accordance with the NIH guidelines for care and use of laboratory animals and were approved by the local animal ethics committee (Ministerium für Energiewende, Landwirtschaft, Umwelt, Natur und Digitalisierung des Landes Schleswig-Holstein, Germany) under the application number V241-60532/2017(126-10/17). The results of all studies involving animals are reported in accordance with the ARRIVE guidelines.24 The group sizes were assessed by a power analysis (corrected α = 0.008, power 80%) and is specified in study protocols 1–3. In total, 228 mice were used for this study.
5 weeks old wildtype C57BL/6N mice (Charles River, protocol 1, 3 A and 3B) and male 23 and 31 weeks old C57BL/6J DIO mice (protocol 2, #380050, Jackson Laboratory) were kept in standard housing in groups of 3-4 individuals randomized by weight. All mice were male because RAS is influenced by estrogen.25,26 Mice had ad libitum access to standard chow diet before start of each study and then received either high-fat diet (HFD, 60 kJ% fat (Lard), EF D12492 (I) mod., ssniff Spezialdiäten GmbH) or normal-fat diet (NFD, 10 kJ% fat, EF D12450B mod., ssniff Spezialdiäten GmbH).
For protocol 1, 3 A and 3B, mice additionally received TEL (8 mg/kgbw) or vehicle (CON) daily by oral gavage in a volume of 5 µl/gbw according to our previous studies.9,10,27
Protocol 1. Mice were treated for 8 weeks in total. On the last day of treatment, LSI was performed. Each of the four groups consisted of 13 animals. During surgery for LSI three mice died.
Protocol 2. The external breeder fed mice with NFD or HFD from week 7 of age on. When they reached an age of 21 and 29 weeks, they were shipped to our lab and further fed until they had received the diets for 16 or 24 weeks in total. During the last week of treatment Barnes maze behavior test and LSI were performed. Each of the four groups consisted of 13 animals. During surgery for LSI one mouse died.
Protocol 3. Mice were treated for 16 weeks in total. Here, we performed experiments in two cohorts. (A) During the last week of treatment open field, object place recognition tests and on the last day of treatment LSI measurements were performed (Figure S1(a)). Each group consisted of 18 mice. One mouse had to be killed during treatment period because of dysplastic teeth (B) During the last week of treatment, elevated plus maze test, body composition and blood pressure measurements were performed. On the last day of treatment, arterial spin labeling magnetic resonance imaging (ASL-MRI) and time-of-flight-(TOF) MR-angiography were performed (Figures, S1(b)). Each of the four groups consisted of 13 animals.
Laser speckle imaging
Procedures were performed according to Wenzel et al.28 with small adjustments described below. Initially, mice were i.p. anesthetized with ketamine (70 µg/gbw, bela-pharm) plus xylazine (16 µg/gbw, Bayer AG) and 1–1.5% isoflurane, which was turned off during the measurements. Throughout the measurement, body temperature was maintained at 37 °C using a feedback-regulated warming system (TCAT-2LV, Physitemp Instruments). After tracheotomy, a small ventilatory tube was inserted into the trachea and connected to a ventilation device (MiniVent, Harvard Apparatus). Ventilation volume was constant, and frequency was adapted to a physiological expiratory CO2 concentration of 37–45 mmHg. This was controlled during the measurement with a capnometer (microCapstar, CWE Inc.). The skull stayed intact and only the skin above was removed. LSI (FLPI, Moor instruments) was performed using a recording rate of 0.25 Hz or 25 Hz (CO2 stimulation). For the electrical whisker pad stimulation (750 µA, 5 ms, 5 Hz for 5 s, 5 times with a delay of 1 min), three regions of interest (ROI) were set over the somatosensory cortex area and for the stimulation with CO2 (10% with 21% O2, rest N2) three ROI over big cortical vessels. Due to gas diffusion along the ventilation tube, the stimulation mix contained 7.8% CO2 when it reached the mice. Flux intensities were recorded throughout stimulations and normalized to baseline values for each ROI. Between whisker pad and CO2 stimulation a break of 15 min was performed. The ROI with the highest amplitude during stimulation was used for analysis and then merged with the values of the other animals. The analysis was done according to one of our previous studies.28 Representative images of measurement can be seen in Figures 2(a) and S10(a).
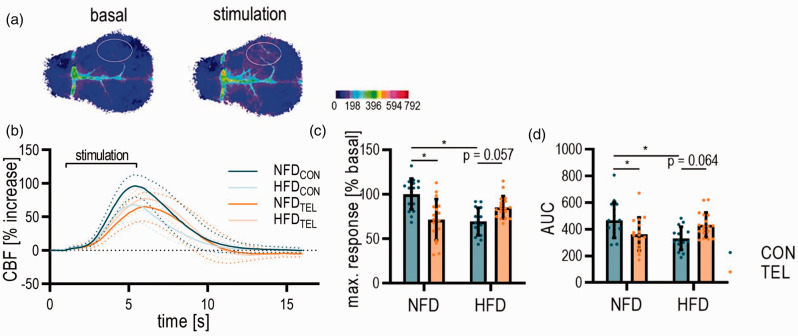
Figure 2.
TEL prevents neurovascular impairment upon HFD. Mice were fed with normal-fat (NFD) or high-fat diet (HFD) for 16 weeks and simultaneously treated with vehicle (CON) or telmisartan (TEL). (a) Representative images of LSI taken before and during whisker stimulation; dotted lines indicate the errors and solid lines the mean of the data; color scale indicates arbitrary units of LSI; Capped line above the graph indicates the onset and stop of stimulation. (b) Quantification of LSI upon whisker stimulation with (c) maximum response to whisker stimulation and (d) area under the curves (AUC). 2-way ANOVA results see table S3. *P < 0.05 in Bonferroni multiple comparison posttest; n = 17–18 per group. Data are meansSD.
Arterial spin labeling MRI
Procedures were performed according to Wenzel et al.28 with small adaptions. Mice were i.p. anesthetized with ketamine (80 µg/gbw, Ketaset, Zoetis Deutschland GmbH) plus medetomidin (0.5 µg/gbw, Dorbene, Zoetis Deutschland GmbH). Respiratory rate and body temperature were monitored and kept at 37 °C throughout measurements. Mice were scanned using a 7 Tesla small animal MRI scanner (ClinScan, Bruker) and a T2-weighted turbo spin-echo sequence. The parameters of measurements can be found in the supplement. First, time-of-flight (TOF) angiography was performed with the sequence parameters shown in the supplemental methods. A representative TOF-image can be seen in Figure 4(a). CBF was measured by arterial spin labeling (ASL) with Q2TIPS (quantitative imaging of perfusion using a single subtraction with interleaved thin-slice TI1 periodic saturation) with TI1/TI2/SS = 900/1400/1375 ms. Further ASL scanning parameters can be found in the supplemental methods. Representative ASL-images can be seen in Figure 3(a). All images were analyzed with Fiji ImageJ. The diameters of vessels were measured directly after the vessel branch of the bigger vessel with the measurement tool of ImageJ.
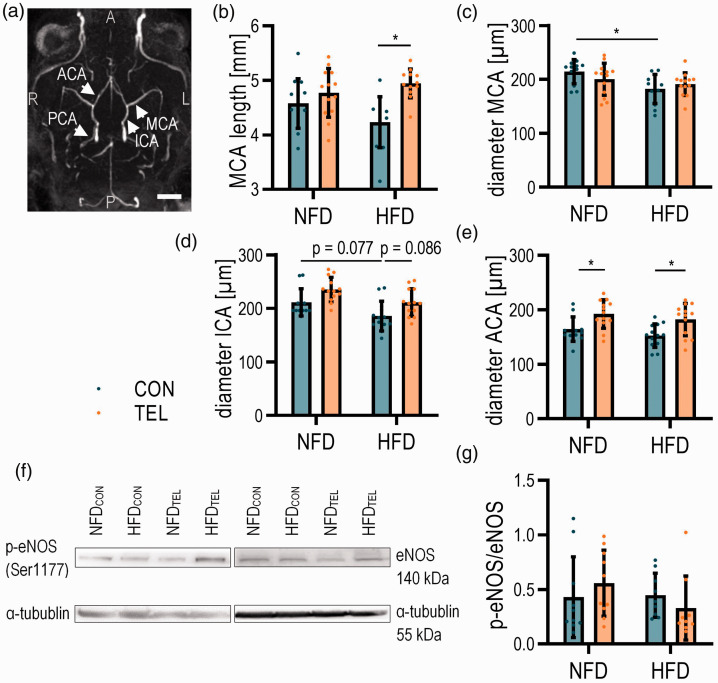
Figure 4.
TEL affects cerebral vessel diameter upon HFD. Mice were fed with normal-fat (NFD) or high-fat diet (HFD) for 16 weeks and simultaneously treated with vehicle (CON) or telmisartan (TEL). (a) Representative image of TOF-angiography and analyzed vessels (indicated by arrows; ACA anterior cerebral artery; ICA internal carotid artery; MCA middle cerebral artery; PCA posterior cerebral artery; A anterior; P posterior; L left; R right; scale bar 2 mm) with (b) length of MCA, diameter of (c) MCA, (d) ICA and (e) ACA are shown. (f) Representative western blot images and (g) quantification of p-eNOS/eNOS ratio. 2-way ANOVA results see table S5. *P < 0.05 in Bonferroni multiple comparison posttest; (a)–(e) n = 12–13 per group, (f)–(g) n = 10 per group; Data are meansSD.
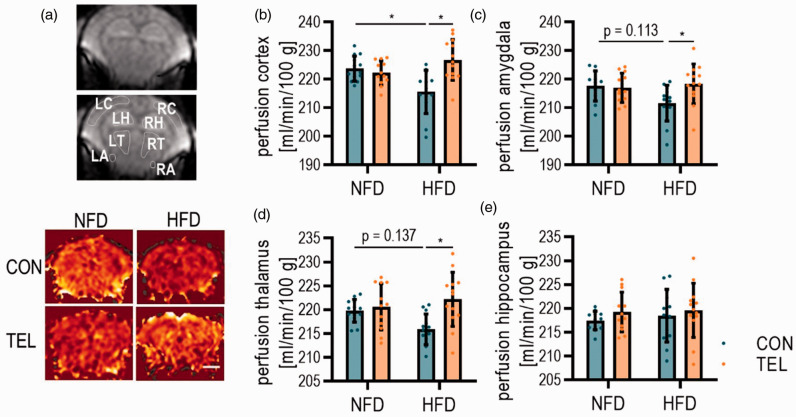
Figure 3.
TEL normalizes CBF upon HFD. Mice were fed with normal-fat (NFD) or high-fat diet (HFD) for 16 weeks and simultaneously treated with vehicle (CON) or telmisartan (TEL). (a) Representative images taken during ASL-MRI and indicated areas of interest for perfusion calculation (upper panel); LC left cortex; LH left hippocampus; LT left thalamus; LA left amygdala; RC right cortex; RH right hippocampus; RT right thalamus; RA right amygdala and representative images for each group during ASL-MRI (lower panel); scale bar 2 mm. Quantification of tissue perfusion in (b) cortex, (c) amygdala, (d) thalamus and (e) hippocampus. 2-way ANOVA results see table S4. n = 13 per group; *P < 0.05 in multiple comparison test; Data are means SD.
Body composition
Body composition was measured with the Minispec BCA analyzer (LF-110, Bruker). Prior to measurement, mice were transferred to the room to acclimatize to the environment. For the measurement, mice were put into the restrainer, which was then placed into the analyzer.
Plethysmography blood pressure measurement
Blood pressure was measured according to Schuster et al.10 with a tail-cuff blood pressure analysis system (SC-1000, Hatteras Instruments) in dual operation mode. Mice were immobilized under the restrainer on the heated platform (100 F/37 °C). The settings of the device were used based on the experience for which we got the most consistent results10 and can be found in more detail in the supplemental methods.
Behavioral tests
For open field testing, locomotor activity of mice in an open chamber (35 × 35 cm), illuminated with 19 lux was observed and video recorded for 10 min.
In a 50 cm above floor elevated plus maze apparatus, anxiety-related exploration of mice was observed, and video recorded for 5 min. The maze had two open arms (5 × 35 cm) without walls and two closed arms (5 × 35 cm) with sidewalls. The distance covered in open arms relative to the distance in closed arms area was taken as an indicator for anxiety-like behavior.
For object place recognition test, mice were habituated to the open field (35 × 35 cm, illuminated with 19 lux) for 10 min daily on three consecutive days. On the day of testing, mice could explore the open field for 10 min, in which two identical objects were placed into the corners. After a delay of 1 h in their home cage, they could explore the field again for 5 min, in which one of the objects was relocated into another corner. After 24 h delay, the one object was relocated again, and mice could explore the field for 5 min. All tests were video recorded.
Barnes maze procedures were performed according to Binder et al.29 and details can be found in the supplement. The test was divided in an acquisition phase (day 1–5) and a probe trial (day 6). During acquisition, mice learned where on the brightly lighted maze the dark escape box is located. For spatial orientation, visual cues around the maze were placed. For five consecutive days, four acquisition trials for maximum 3 min were performed with an intertrial interval of 15 min. On day 6, the probe trial was performed for 90 s in which the escape box was replaced by a blind and the maze was rotated by 180° to exclude a potential orientation of the mice during the acquisition phase by unwished intramaze cues on the field, for example by cleaning marks. All trials of the Barnes maze were video recorded and analyzed with ANY-maze.
Immunohistochemistry
Mice were perfused with ringer solution containing heparin (10 IU/ml). Methanol-fixed cryosections (20 µm thick) were stained with primary antibodies as described in table S1 diluted in blocking solution (1% BSA/PBS) overnight at 4 °C and incubated with secondary antibodies in DAPI-containing (1:2000) blocking solution for 1 h the next day. Four pictures of cortical and amygdaloid staining per mouse were taken (10x objective) and analyzed. The length of Col IV-positive but CD31-negative vessels was measured manually. The quantification of total vessel length was performed in a partially automated manner using a custom macro implemented into to the image analysis software Fiji Image J, as described before.30
For lipid and SADS staining, we post-fixed brains in 4% paraformaldehyde (PFA) for 24 hours prior processing with 15% and 30% sucrose each overnight and paraffin embedding. After deparaffinization and hydration of the brain sections (3 µm thick), antigen retrieval was performed. Then slides were placed in blocking solution (1:60 normal goat serum [S-100, Vector Laboratories] in 0.1% BSA/PBS) for 60 min at room temperature. Primary antibodies (Table S1) were diluted in blocking solution and applied overnight at 4 °C. Slides were incubated with secondary antibody for 30 min, which was followed by fluorescence in situ hybridization for SADS detection. Therefore, tissues were cross linked with PFA and dehydrated in graded ethanol. Sections were denatured in hybridization buffer containing 2.5 mg/ml FAM-labelled CENPB-specific (centromere) (ATTCGTTGGAAACGGGA) peptide nucleic acid probe (Panagene), followed by hybridization. Slides were washed twice with 70% formamide in 2 × SSC for 15 min, followed by washes in 2 × SSC and PBS for 10 min. Sections were mounted in Vectashield DAPI-containing mounting media and imaged. In depth Z stacking (separated by 0.4 µm with 63 × objective) was used for quantification followed by Fiji Image J analysis. 10–11 images per cortex were taken containing on average 52 endothelial cells per mice. A more detailed protocol can be seen in the supplement.
Biochemical analysis
Procedures were performed according to Schuster et al.10 For blood glucose measurement, we used a commercial glucose sensor (Ascensia Elite XL and Elite Sensor, Bayer Vital GmbH, Germany) and took blood from the tail tip. For measurement of plasma protein levels of insulin, c-peptide, resistin, leptin, GIP and PYY, we used Milliplex mouse metabolic magnetic bead panel kit MMHMAG-44K and Luminex xMAP equipment (EMD Millipore, USA) according to manufacturer’s instructions.
Western blot
Whole brain samples were lysed in 4x SDS and incubated for 5 min at 95 °C. After protein determination with the Lowry procedure, 120 µg protein were loaded on SDS-PAGE gels. Proteins were transferred to nitrocellulose membranes, which were then incubated with primary antibodies (eNOS 1:2500: BD Transduction #610296; phospho-eNOS (Ser1177) 1:500: Cell Signaling Technology #9571S) at 4 °C overnight. With HRP-conjugated secondary antibodies (mouse IgG-HRP 1:5000: DakoCytomation #P0161; rabbit IgG-HRP 1:5000: DakoCytomation #0448) were used and incubated at room temperature for 1 h. For detection, we applied enhanced chemiluminescence (SuperSignal West Femto Substrate, Thermo Scientific) and a digital detection system (FUSIO SOLO S, Vilber).
Statistics
For all analyses, the experimenter was blinded. GraphPad Prism 8.0 and 9.0 was used for statistical analysis. All imaged data is presented as meansSD. We checked all data for outliers with Grubbs’s test and tested for Gaussian distribution with D'Agostino & Pearson test. For the statistical comparison of two groups, we used two-tailed Student’s t test. In case of unequal variances, we applied Welch’s correction. For more than two groups and data including more than one variable we used 2-way ANOVA followed by Bonferroni post-hoc test. A p-value <0.05 was considered statistically significant.
Results
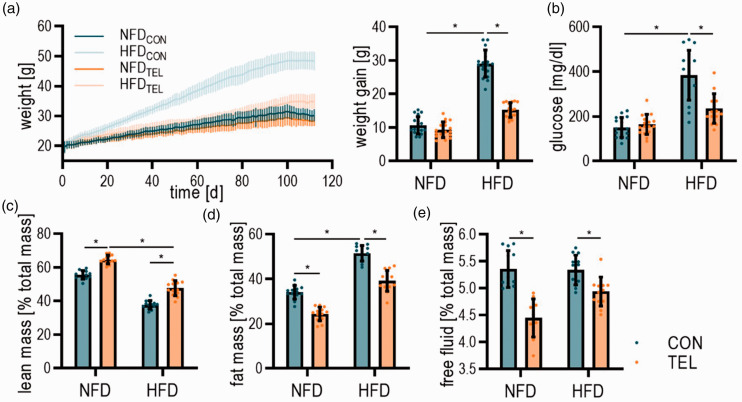
5- to 7-week-old mice were fed with NFD or HFD for 8, 16 and 24 weeks and simultaneously treated with vehicle (CON) or telmisartan (TEL). As repeatedly demonstrated in our previous studies,9,10,27 HFDCON animals developed DIO with higher glucose, insulin, leptin and resistin levels than NFDCON (Figures 1(a) and (b), S2–3). Additionally, HFDCON mice had an increased fat mass, whereas lean mass was reduced compared to NFDCON (Figure 1(c), (d) and (e)). Confirming prior results, TEL had beneficial effects on the metabolism in HFD-fed mice: It prevented mice from DIO, reduced glucose and leptin levels as well as fat mass, whereas lean mass was increased (Figures 1 and S2). Insulin levels were increased by TEL in NFD-fed mice but not further in HFD-fed mice (Figure S2(b)). After 16 weeks of HFD blood pressure did not increase but we observed a blood pressure reduction by TEL in mice that received NFD and HFD (Figure S4).
Figure 1.
TEL improves the phenotype of the metabolic syndrome parameters upon HFD. Mice were fed with normal fat (NFD) or high fat diet (HFD) for 16 weeks and simultaneously treated with vehicle (CON) or telmisartan (TEL). (a) Development of weight over time and total weight gain at the end of treatment; (b) glucose levels; (C-E) Body composition measuring with (c) lean mass; (d) fat mass; and (e) free fluid. 2-way ANOVA results see table S2. *P < 0.05 in multiple comparison test; (a–b) n = 17–18 per group; (c–e) n = 13 per group; Data are meansSD.
TEL improves NVC and normalizes CBF in HFD-fed mice after 16 weeks of treatment
To examine the HFD and TEL effects on NVC, we measured the perfusion response to whisker stimulation by LSI (Figure 2(a)). After 8 weeks of HFD, the perfusion at the area of somatosensory cortex increased by 50–60% compared to the baseline upon electrical whisker stimulation, but HFD did not influence the maximal response (Figure S5(a)). After extending the feeding period from 8 to 16 and 24 weeks, the response to whisker stimulation was impaired in mice with DIO (Figure S6).
A simultaneous treatment with TEL together with 16 weeks of feeding restores the HFD-induced reduction of the response to whisker stimulation and the reduction of the area under the curve (AUC, Figure 2). However, TEL also influenced the response to stimulation in NFD-fed mice (Figure 2(b) to (d)), whereas we did not see any TEL effects after 8 weeks of treatment (Figure S5(a)). Upon the stimulation with CO2, the perfusion response was not affected by HFD or TEL treatment for 8, 16 or 24 weeks (Figures S5 and S7-8). Since the NVC reduction was not higher after 24 compared to 16 weeks of HFD feeding, we decided to perform all further experiments together with TEL treatment for 16 weeks.
We further investigated whether HFD or TEL had any effects on baseline cerebral blood flow. Since LSI is not suitable for measurements of absolute perfusion in the brain, we performed arterial spin labeling (ASL) MRI and calculated the absolute tissue perfusion in different brain areas (Figure 3(a)). Here, we observed a reduction of CBF in HFDCON mice by about 8 ml/min/100 g tissue in the cortical area, which then was normalized by TEL to NFDCON level (Figure 3(b)). In the amygdala and thalamus of HFDCON mice, CBF reduction was not significant, but additional TEL treatment increased CBF to a significantly higher level, whereas CBF in the hippocampal area was not affected by HFD or TEL (Figure 3(c) to (e)).
Since the middle cerebral artery (MCA) next to the other pial vessels contributes to the regulation of CBF and may influence the perfusion of the smaller penetrating vessel in the brain,31 we further investigated HFD and TEL effects on MCA structure. We revealed by TOF angiography a reduced MCA diameter and by trend a reduction of MCA length in HFDCON mice (Figure 4(b) and (c)), whereas the diameters of internal carotid artery (ICA) and anterior cerebral artery (ACA) were not clearly affected by HFD (Figure 4(d) and (e)). TEL increased MCA length despite HFD feeding back to NFDCON levels but did not have a clear effect on the diameters of MCA and ICA (Figure 4(b) to (d)). ACA diameter was increased in TEL-treated mice, even if they were fed with NFD (Figure 4(e)). However, we could not observe an increase of activation of endothelial nitric oxide (NO) synthase (eNOS) as one of the key players in vasodilation32 by TEL treatment (Figure 4(g)). There was only a tendency of increase in NFDTEL mice compared to NFDCON, but no difference between lean and obese mice.
TEL has beneficial effects on anxiety-like behavior in HFD-fed mice, but no effect on memory
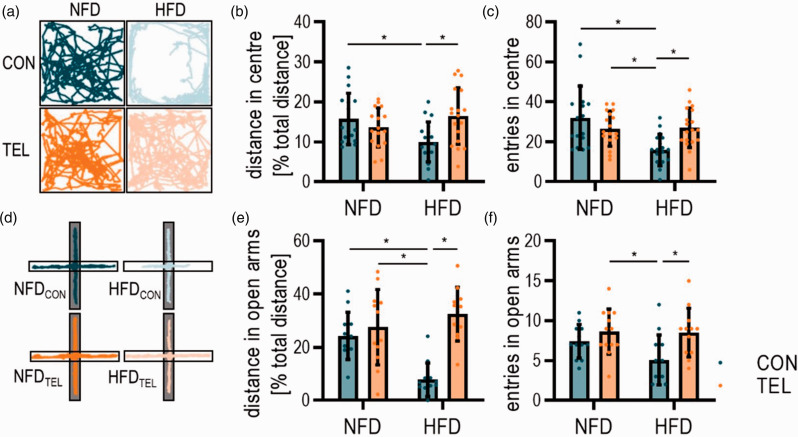
Next, we hypothesized that HFD-induced CBF reduction leads to changes in behavior. To investigate anxiety-like behavior, we performed open field testing (Figure 5(a) to (c)). Here, we observed a more severe anxiety-like behavior in HFDCON compared to NFDCON mice. NFDCON mice covered higher distances in the central zone of the arena and entered the center more often than HFDCON mice (Figure 5(b) and (c)). TEL decreased the anxiety-like phenotype of HFD-fed mice by increasing the covered distance as well as number of entries into the central zone, whereas TEL in NFD-fed mice had no effect (Figure 5(b) and (c)). These results were confirmed with a second test for anxiety-like behavior, the elevated plus maze (Figure 5(d) to (f)). HFDCON mice covered a shorter distance in open arms of the maze than NFDCON mice and preferred to stay in the closed arms, meaning that the anxiety level of mice with DIO is higher. The anxiety level in HFDTEL mice was significantly reduced (Figure 5(e) and (f)). However, in both tests the total distance mice covered did not differ between groups, suggesting that the observed behavioral patterns are not driven by changes in activity or mobility of treated mice (Figure S9(a) and (b)).
Figure 5.
TEL normalizes HFD-induced increase of anxiety-like behavior. Mice were fed with normal-fat (NFD) or high-fat diet (HFD) for 16 weeks and simultaneously treated with vehicle (CON) or telmisartan (TEL). (a) Representative track reports during open field, grey part of the plus indicates closed arms and white open arms; (b) quantification of distance covered in inner zone of the arena and (c) frequency of entries into inner zone. (d) Representative track reports during elevated plus maze, (e) quantification of the distance covered in open arms of the arena and (f) frequency of entries into open arms are shown. 2-way ANOVA results see table S6. *P < 0.05 in Bonferroni multiple comparison posttest. (a)–(c) n = 17–18 per group; (d)–(f) n = 12–13 per group; Data are meansSD.
Since studies have shown that obesity and insulin resistance lead to memory decline,6 we tested for memory performance in our DIO model (Figures S9(c) to (k), S10). Surprisingly, our assessment did not show any differences in object place recognition test performance between NFDCON and HFDCON or between HFDCON and HFDTEL (Figure S9(d) and (h)). We also performed another memory test for mice that only got HFD, but no TEL treatment. Barnes maze memory test (Figure S10) did not reveal a clear difference between lean and obese mice after 16 and 24 weeks except for a slight tendency in numbers of primary errors on the probe trial after 24 weeks of feeding (Figure S10(b)). Thus, we conclude that mice with DIO show a stronger anxiety-like phenotype that improves with TEL treatment, but neither HFD nor TEL significantly affects memory in our setting.
TEL can protect from cerebrovascular remodeling in HFD feeding depending on the area
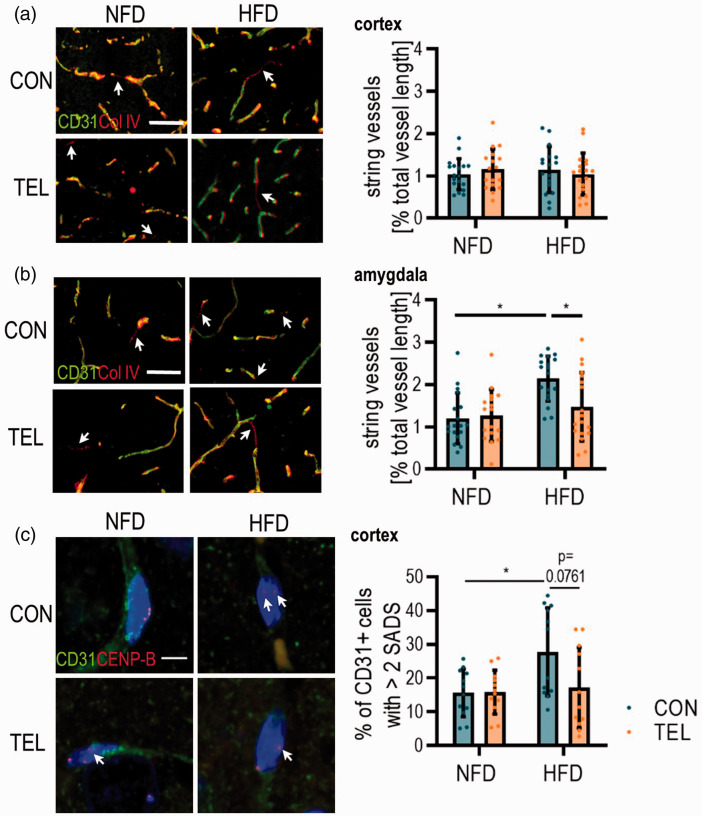
We further investigated potential mechanisms contributing to lower CBF and neurovascular reactivity in DIO mice. To address vasoregression, we first tested for string vessel formation in cortical and amygdaloid area (Figure 6(a) and (b)). Therefore, we stained for collagen IV (Col IV) as part of the basement membrane and CD31 as a marker for endothelial cells and calculated the length of vessels that are CD31− but Col IV+. The length of string vessels in the cortical area was the same in all four groups (Figure 6(a)). In the amygdala, however, we observed double the amount of string vessels in HFDCON compared to lean NFDCON mice. In HFDTEL mice, string vessel length was similar to NFDCON (Figure 6(b)) even though total vessel length and the number of vessel branches did not differ (Figure S11). Thus, HFD-induced higher string vessel formation seems to occur only in the amygdala.
Figure 6.
TEL has protective effects on cerebral vasculature morphology. Mice were fed with normal-fat (NFD) or high-fat diet (HFD) for 16 weeks and simultaneously treated with vehicle (CON) or telmisartan (TEL). Representative images of immunohistochemistry with antibodies for a marker of endothelial cells (CD31) and basal membrane (Col IV) and quantification of string vessel length (CD31−/Col IV+, indicated by arrows) in (a) cortex and (b) amygdala; scale bar 50 µm. (c) Representative images showing peri/centromeric satellite DNA signals in the cortical area (SADS, indicated by arrows); Micrographs showing single CD31+ (green) cell nuclei with centromere protein-B (CENP-B, red); scale bar 5 µm and percentage of CD31+ cells with >2 SADS are shown (right). 2-way ANOVA results see table S7. *P < 0.05 Bonferroni multiple comparison posttest. (a)–(b) n = 17–18 per group (4 images per n) and (c) n = 6 per group (in average 52 CD31+ cells per n); Data are meansSD.
Next, we investigated HFD and TEL effects on cellular senescence, a process mainly characterized by irreversible cell cycle arrest and known to be linked to obesity.7 We used SADS as an already described marker for senescence33 to identify senescent CD31+ cells in the cortex (Figure 6(c)), which means that these cells show an increase in heterochromatin decondensation at the peri/centromere satellite region. For HFDCON mice, we observed more CD31+ cells with more than two SADS events compared to NFDCON. Close to significance, this effect decreased back to NFDCON level by TEL in HFD-fed mice (Figure 6(c)).
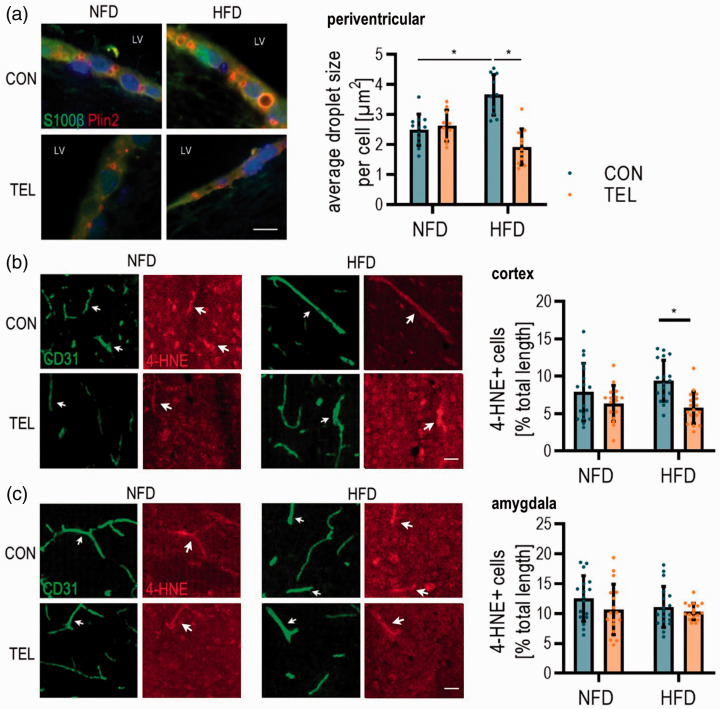
To investigate further potential mechanisms underlying CBF reduction, we tested for factors of oxidative stress. Since oxidative stress is linked to lipid accumulation,23 we first stained for lipid droplets in the ependymal cells of the lateral ventricle to get an idea of the oxidative state in the brain. Here, we clearly observed an increase of droplet size in HFDCON compared to NFDCON mice and a reduction back to control level in HFDTEL mice (Figure 7(a)). This led to the hypothesis that cerebral oxidative stress is increased in obese mice. Since there is a known link between obesity and oxidative stress-induced changes in blood vessels,5 we stained for 4-hydroxynonenal (4-HNE) as a marker for oxidative stress in endothelial cells. Here, we observed higher numbers of vessels that are positive for 4-HNE in the cortex of HFDCON compared to HFDTEL mice (Figure 7(b)), even though there was no difference between NFDCON and HFDCON littermates. Amygdaloid CD31+ cells were not affected in terms of higher levels of 4-HNE (Figure 7(c)). In total, we conclude that the processes possibly contributing to CBF reduction are dependent on the brain area.
Figure 7.
HFD and TEL effects on oxidative stress depend on brain area. Mice were fed with normal-fat (NFD) or high-fat diet (HFD) for 16 weeks and simultaneously treated with vehicle (CON) or telmisartan (TEL). (a) Representative images of immunohistochemistry with antibodies for a marker of lipid droplets (Perilipin 2, Plin2) and ependymal cells (S100β) along the lateral ventricle (left) quantification of lipid droplet size per cell (right); scale bar 10 µm. Representative images of immunohistochemistry of endothelial marker CD31 and oxidative stress marker 4-hydroxynonenal (4-HNE) (left) and quantification of CD31+/4-HNE+ cell length (right) in (b) cortex and (c) amygdala; scale bar 30 µm. 2-way ANOVA results see table S8. *P < 0.05 in Bonferroni multiple comparison posttest. (a) n = 12 per group; (b–c) n = 17–18 per group (4 images per n); Data are meansSD.
Discussion
The main hypothesis of this study was that TEL prevents HFD-induced reduction of NVC, CBF and behavioral changes. With this study, we initially demonstrated that HFD effects on NVC are strongly time dependent: 8 weeks of HFD feeding is not sufficient for the development of neurovascular impairments and a reduction of CBF. In contrast, Li et al. showed reduced NVC and CBF after 8 weeks of HFD. The authors observed a blunted change in CBF upon whisker stimulation, indicating impairment of NVC without changes in blood pressure, glucose, or insulin levels.3 Similarly, Boitard et al. fed mice with HFD for a period of 11 weeks, but they stated that exposure to HFD leads to cerebral effects only when started in juvenile but not in adult age.34 Therefore, we started HFD feeding in our facility as early as possible which was at 5 weeks of age. Still, we showed that only 16 and 24 weeks of HFD lead to neurovascular uncoupling and an area dependent CBF reduction, which suggests that starting HFD in juvenile age is not the only parameter that needs to be considered. In addition to the age at HFD onset, we conclude that the development of neurovascular impairments is strongly dependent on the length of HFD feeding. Considering the TEL effect, it can additionally be dependent on the dosage of TEL. In former studies we showed that the prevention of weight gain by ARBs occurs only with high dosages.13,17 Therefore, we used the same high dosage that we have used previously for investigating beneficial effects of TEL in HFD mice.9,10 We assume that the beneficial effects of TEL on NVC and CBF only occurs with these high dosages in HFD similar to the prevention of weight gain, but with this study we cannot exclude a TEL effect on NVC or CBF with a lower dosage. Moreover, for a cerebral activity a bioavailability of TEL is crucial. Only at high doses have ARBs been shown to be able to penetrate the central nervous system significantly.35,36
Surprisingly, we observed that TEL in NFD-fed mice influenced the perfusion response to whisker stimulation. We assume that this effect correlates with the impact of TEL on blood pressure in NFD-fed mice. A reduced blood pressure could have led to a decreased perfusion thereby to a smaller amplitude during stimulation. Since we could not see a lower perfusion in the specific brain regions this aspect cannot be resolved with this study. Further experiments with other anti-hypertension drugs could be helpful. However, it should be mentioned that the anti-hypertensive drugs amlodipine or ramipril did not show an effect on weight gain reduction if given in anti-hypertensive dosages whereas TEL did.13,37
Next, we asked what mechanisms contributes to HFD-induced neurovascular impairment and CBF reduction in our DIO model. It has been shown that cerebral vessel structure is altered by hypertension leading to reduction of CBF.38 However, this was not the case in our DIO model, since we did not observe any effect of HFD on blood pressure, which has also been seen before.10 Several studies (also in humans) have shown that changes in cerebral vasculature are a consequence of hyperglycemia and diabetes (type 1 and 2).39,40 Rats with type 2 diabetes had compromised NVC upon whisker stimulation and a decrease in CBF in somatosensory cortex.40 Our model relates to the phenotype of type-2 diabetes. It is likely that the milieu of high glucose, insulin and lipid levels contributes to NVC and CBF reduction in our DIO model since we observed a severe increase of glucose and lipid levels after 16 weeks of treatment. Other studies have shown before that the chronic consumption of high fat diets leads to obesity and negatively affects brain function in terms of a worsen outcome after ischemic stroke41 and that short-term high-fat and high-sucrose feeding impairs hypothalamic neuronal circuits, particularly in the arcuate, the ventromedial and the dorsomedial nucleus.42 Visa versa, short-term exercise training effectively decreases insulin-stimulated brain glucose uptake in sedentary subjects with insulin resistance.43
Moreover, insulin-induced CBF reduction may be an important determinant of cognitive decline observed in obesity.44,45 McNeilly et al. showed cognitive impairment in rats with an adult onset of obesity but no link between obesity and impaired hippocampal function has been found.46 With our study we could not find HFD effects on memory. We clearly observed neurovascular impairments in the cortical area, but hippocampal CBF was not affected upon HFD, suggesting that memory is not impaired by HFD and that CBF reduction is brain area dependent. The discrepancies to other studies can be explained by the age of treated mice as the onset of obesity seems to be crucial for the development of hippocampal function deficits.34 We hypothesize that HFD starting at 5 weeks of age requires an even longer feeding period until the mice are aged to observe effects on memory since we noticed a tendency to memory deficits after 24 weeks of feeding. Our results suggest that the most affected phenotype at the given age of HFD mice is anxiety-like behavior which has been described earlier.7 Corresponding to the present results, we have previously shown that impairments of the neurovascular unit affect anxiety.28 Thus, we conclude, that the CBF reduction and neurovascular uncoupling, especially in the cortical area but also in the amygdala, contribute to the HFD-induced anxiety. The normalization/increase of CBF in the cortex and the amygdala together with a reduced anxiety level by TEL treatment supports this connection.
Encouraged by the discoveries of positive effects of TEL on metabolism and central effects of TEL and RAS,9,10,12,14,18,47 we decided to investigate beneficial TEL effects on NVC and CBF in HFD-fed mice. Here, we showed that TEL prevents HFD-fed mice from developing neurovascular deficits such as neurovascular uncoupling and CBF reduction. TEL effects were also time dependent as our results indicate that 8 weeks of TEL treatment is not sufficient to reveal any significant effects. Metabolic diseases induce cerebrovascular remodeling and alteration of the structure of cerebral vasculature as in reduced diameter of the MCA as well as increased wall thickness.48 We showed that the AT1-receptor blockage by TEL treatment normalizes HFD-induced reduction of cerebral vessel diameter. These HFD and TEL effects on big cerebral vessel may influence the supply of the penetrating vessels. One of the major generators of dilatory response in the cerebral circulation is the production of NO by eNOS, which is suggested to be dysregulated in the context of type 2 diabetes.49 Here, we did not observe any significant effects of HFD or TEL effects on eNOS activation, which led us conclude that vasodilation by eNOS activation does not play a significant role in HFD-induced CBF reduction and neurovascular coupling which has been shown before.50 Regarding this, however, several studies have shown that NO plays a role in neurovascular coupling especially in the cortical area.51–53 We used whole brain samples for eNOS detection. This is a limitation of our study since mechanisms of dilation differ along the cerebrovascular tree and in different brain regions.28,54 Next to eNOS the cyclooxygenase-2 could be involved in regulation of vasodilation in our DIO model since obesity is strongly linked to inflammatory responses.23 We showed in one of our earlier studies that inflammatory changes like an increase of astrogliosis, TNFα and IL-1α level were prevented by TEL treatment in the hypothalamus of HFD-fed mice.9
Other parameters like endothelial death, oxidative stress, endothelial dysfunction and hyperglycemia, contributing to vasoregression have been observed in metabolic disorders.48 Consequently, we further investigated whether TEL preserves HFD-induced changes in morphology, senescence, and oxidative stress. We found an area dependency for these parameters similarly to the abovementioned neurovascular deficits. For the cortex we observed neither an HFD nor a TEL effect on string vessel formation, but we saw that TEL normalizes an HFD-induced increase of senescence of endothelial cells. SADS have been described to be an early event of cellular senescence,33 a process that has also been shown to be involved in anxiety-like behavior in obese mice.7 Moreover, there is a known link between obesity and lipid accumulation and an altered redox state leading to increased oxidative stress.23 4-HNE is found in higher amounts during oxidative stress due to increased lipid peroxidation.55 Here, TEL reduced oxidative stress in endothelial cells in the cortex despite HFD feeding. In the amygdala, however, we observed a higher number of non-perfused string vessels suggesting that a decrease of functional vessels contributes to a reduced CBF. The HFD-induced increase of string vessel formation was prevented by TEL.
We conclude from our study that TEL prevents mice from HFD-induced neurovascular uncoupling and CBF reduction in an area and time dependent way. Also, the deficits driving mechanisms are dependent on the affected brain area. In the cortex, endothelial senescence may be driven by increased oxidative stress contributing to neurovascular deficits, whereas in the amygdala a decline of functional endothelial cells plays a role. It has been shown in many studies that obesity, hyperglycemia, and diabetes affect cerebral vasculature in different ways. Additionally, studies highlight a physiological role of AT1-receptors and their blockage to CBF regulation, which consequently affects behavior. That these results were predominantly shown in Alzheimer’s disease models,20 emphasizes the novelty and importance of our study since we showed the neurovascular protective role of TEL in a classical DIO model depending on a time parameter. Apart from the capacity of TEL for hypertension treatment, we suggest a powerful prevention and treatment strategy for complications that come along with obesity, such as Alzheimer’s disease. Further investigations with even longer feeding and treatment periods are necessary to see the effect of juvenile onset of obesity on cerebral vasculature of aged mice. We expect the observed effects to be even stronger in aged mice that got HFD from a young age on. To further address a potential treatment capacity of TEL against obesity related Alzheimer’s disease, a TEL effect on markers of Alzheimer’s disease, such as amyloid beta and hyperphosphorylated tau proteins, in our DIO model with aged mice needs to be investigated.
Supplemental Material
Supplemental material, sj-pdf-1-jcb-10.1177_0271678X211003497 for Telmisartan prevents high-fat diet-induced neurovascular impairments and reduces anxiety-like behavior by Gianna Huber, Mikolaj Ogrodnik, Jan Wenzel, Ines Stölting, Lukas Huber, Olga Will, Eva Peschke, Urte Matschl, Jan-Bernd Hövener, Markus Schwaninger, Diana Jurk and Walter Raasch in Journal of Cerebral Blood Flow & Metabolism
Acknowledgments
We thank Sonja Binder (Institute for Experimental and Clinical Pharmacology and Toxicology, University of Lübeck) for providing her expertise in animal behavior experiments, Jens Mittag (Medical Clinic 1, UKSH, Lübeck) for providing the blood pressure measurement system, and Jan Sedlacik (Biomedical Engineering & Imaging Sciences, King’s College London) for providing advice for MRI performance and analysis. Kiel University and the Medical Faculty are acknowledged for supporting the Molecular Imaging North Competence Center (MOIN CC) as a core facility for imaging in vivo and telmisartan was a kind gift from Böhringer Ingelheim, Germany.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was supported by research grants from the German Research Foundation to the GRK 1957 “Adipocyte-Brain Crosstalk”, University of Lübeck and GRK 2154 “Materials for Brain”, University of Kiel, a grant of the German Centre for Cardiovascular Research (DZHK) and by the Cluster of Excellence PMI 1267.
Declaration of conflicting interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Authors’ contributions: G.H., M.O., J.W., I.S., L.H., O.W., E.P. and U.M. performed the experiments; G.H., J.W., J.-B.H., M.S., D.J., and W.R. designed study and protocols; G.H. and W.R. analyzed data, and G.H. and W.R. wrote the manuscript.
Data availability: The data that support the findings of this study are available from the corresponding author upon reasonable request. Some data may not be made available because of privacy or ethical restrictions.
ORCID iD: Jan Wenzel https://orcid.org/0000-0001-6313-2439
Supplemental material: Supplemental Material is provided on the JCBM website.
References
- 1.Jaacks LM, Vandevijvere S, Pan A, et al. The obesity transition: stages of the global epidemic. Lancet Diabetes Endocrinol 2019; 7: 231–240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wang HH, Lee DK, Liu M, et al. Novel insights into the pathogenesis and management of the metabolic syndrome. Pediatr Gastroenterol Hepatol Nutr 2020; 23: 189–230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Li W, Prakash R, Chawla D, et al. Early effects of high-fat diet on neurovascular function and focal ischemic brain injury. Am J Physiol Regul Integr Comp Physiol 2013; 304: R1001–R1008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Osmond JM, Mintz JD, Dalton B, et al. Obesity increases blood pressure, cerebral vascular remodeling, and severity of stroke in the Zucker rat. Hypertension 2009; 53: 381–386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Coucha M, Abdelsaid M, Ward R, et al. Impact of metabolic diseases on cerebral circulation: structural and functional consequences. Compr Physiol 2018; 8: 773–799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Valladolid-Acebes I, Fole A, Martin M, et al. Spatial memory impairment and changes in hippocampal morphology are triggered by high-fat diets in adolescent mice. Is there a role of leptin? Neurobiol Learn Mem 2013; 106: 18–25. [DOI] [PubMed] [Google Scholar]
- 7.Ogrodnik M, Zhu Y, Langhi LGP, et al. Obesity-induced cellular senescence drives anxiety and impairs neurogenesis. Cell Metab 2019; 29: 1061–1077.e8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pedditzi E, Peters R, Beckett N.The risk of overweight/obesity in mid-life and late life for the development of dementia: a systematic review and meta-analysis of longitudinal studies. Age Ageing 2016; 45: 14–21. [DOI] [PubMed] [Google Scholar]
- 9.Rawish E, Nickel L, Schuster F, et al. Telmisartan prevents development of obesity and normalizes hypothalamic lipid droplets. J Endocrinol 2020; 244: 95–110. [DOI] [PubMed] [Google Scholar]
- 10.Schuster F, Huber G, Stölting I, et al. Telmisartan prevents diet-induced obesity and preserves leptin transport across the blood-brain barrier in high-fat diet-fed mice. Pflugers Arch 2018; 470: 1673–1689. [DOI] [PubMed] [Google Scholar]
- 11.Michel MC, Brunner HR, Foster C, et al. Angiotensin II type 1 receptor antagonists in animal models of vascular, cardiac, metabolic and renal disease. Pharmacol Ther 2016; 164: 1–81. [DOI] [PubMed] [Google Scholar]
- 12.Müller-Fielitz H, Lau M, Geißler C, et al. Preventing leptin resistance by blocking angiotensin II AT1 receptors in diet-induced obese rats. Br J Pharmacol 2015; 172: 857–868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Müller-Fielitz H, Hübel N, Mildner M, et al. Chronic blockade of angiotensin AT1 receptors improves cardinal symptoms of metabolic syndrome in diet-induced obesity in rats. Br J Pharmacol 2014; 171: 746–760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Müller-Fielitz H, Landolt J, Heidbreder M, et al. Improved insulin sensitivity after long-term treatment with AT1 blockers is not associated with PPARγ target gene regulation. Endocrinology 2012; 153: 1103–1115. [DOI] [PubMed] [Google Scholar]
- 15.Fujisaka S, Usui I, Kanatani Y, et al. Telmisartan improves insulin resistance and modulates adipose tissue macrophage polarization in high-fat-fed mice. Endocrinology 2011; 152: 1789–1799. [DOI] [PubMed] [Google Scholar]
- 16.Souza-Mello V, Gregório Bianca M, Cardoso-de-Lemos Fernando S, et al. Comparative effects of telmisartan, sitagliptin and metformin alone or in combination on obesity, insulin resistance, and liver and pancreas remodelling in C57BL/6 mice fed on a very high-fat diet. Clin Sci 2010; 119: 239–250. [DOI] [PubMed] [Google Scholar]
- 17.Müller-Fielitz H, Markert A, Wittmershaus C, et al. Weight loss and hypophagia after high-dose AT1-blockade is only observed after high dosing and depends on regular leptin signalling but not blood pressure. Naunyn Schmiedebergs Arch Pharmacol 2011; 383: 373–384. [DOI] [PubMed] [Google Scholar]
- 18.Winkler M, Schuchard J, Stölting I, et al. The brain renin-angiotensin system plays a crucial role in regulating body weight in diet-induced obesity in rats. Br J Pharmacol 2016; 173: 1602–1617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lizarbe B, Campillo B, Guadilla I, et al. Magnetic resonance assessment of the cerebral alterations associated with obesity development. J Cereb Blood Flow Metab 2020; 40: 2135–2151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Saavedra JM.Evidence to consider angiotensin II receptor blockers for the treatment of early alzheimer's disease. Cell Mol Neurobiol 2016; 36: 259–279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Takeda S, Sato N, Takeuchi D, et al. Angiotensin receptor blocker prevented beta-amyloid-induced cognitive impairment associated with recovery of neurovascular coupling. Hypertension 2009; 54: 1345–1352. [DOI] [PubMed] [Google Scholar]
- 22.Brown WR.A review of string vessels or collapsed, empty basement membrane tubes. J Alzheimers Dis 2010; 21: 725–739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fernández-Sánchez A, Madrigal-Santillán E, Bautista M, et al. Inflammation, oxidative stress, and obesity. Int J Mol Sci 2011; 12: 3117–3132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Percie Du Sert N, Hurst V, Ahluwalia A, et al. The ARRIVE guidelines 2.0: updated guidelines for reporting animal research. J Cereb Blood Flow Metab 2020; 40: 1769–1777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Fischer M, Baessler A, Schunkert H.Renin angiotensin system and gender differences in the cardiovascular system. Cardiovasc Res 2002; 53: 672–677. [DOI] [PubMed] [Google Scholar]
- 26.Oelkers WKH.Effects of estrogens and progestogens on the renin-aldosterone system and blood pressure. Steroids 1996; 61: 166–171. [DOI] [PubMed] [Google Scholar]
- 27.Dapper C, Schuster F, Stölting I, et al. The antiobese effect of AT1 receptor blockade is augmented in mice lacking mas. Naunyn Schmiedebergs Arch Pharmacol 2019; 392: 865–877. [DOI] [PubMed] [Google Scholar]
- 28.Wenzel J, Hansen CE, Bettoni C, et al. Impaired endothelium-mediated cerebrovascular reactivity promotes anxiety and respiration disorders in mice. Proc Natl Acad Sci U S A 2020; 117: 1753–1761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Binder S, Mölle M, Lippert M, et al. Monosynaptic Hippocampal-Prefrontal Projections Contribute to Spatial Memory Consolidation in Mice. J Neurosci 2019; 39: 6978–6991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ridder DA, Wenzel J, Müller K, et al. Brain endothelial TAK1 and NEMO safeguard the neurovascular unit. J Exp Med 2015; 212: 1529–1549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Faraci FM, Heistad DD.Regulation of large cerebral arteries and cerebral microvascular pressure. Circ Res 1990; 66: 8–17. [DOI] [PubMed] [Google Scholar]
- 32.Moncada S, Higgs A.The L-arginine-nitric oxide pathway. N Engl J Med 1993; 329: 2002–2012. [DOI] [PubMed] [Google Scholar]
- 33.Swanson EC, Manning B, Zhang H, et al. Higher-order unfolding of satellite heterochromatin is a consistent and early event in cell senescence. J Cell Biol 2013; 203: 929–942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Boitard C, Etchamendy N, Sauvant J, et al. Juvenile, but not adult exposure to high-fat diet impairs relational memory and hippocampal neurogenesis in mice. Hippocampus 2012; 22: 2095–2100. [DOI] [PubMed] [Google Scholar]
- 35.Culman J, von Heyer C, Piepenburg B, et al. Effects of systemic treatment with irbesartan and losartan on Central responses to angiotensin II in conscious, normotensive rats. Eur J Pharmacol 1999; 367: 255–265. [DOI] [PubMed] [Google Scholar]
- 36.Seltzer A, Bregonzio C, Armando I, et al. Oral administration of an AT1 receptor antagonist prevents the central effects of angiotensin II in spontaneously hypertensive rats. Brain Res 2004; 1028: 9–18. [DOI] [PubMed] [Google Scholar]
- 37.Miesel A, Müller-Fielitz H, Jöhren O, et al. Double blockade of angiotensin II (at(1))-receptors and ACE does not improve weight gain and glucose homeostasis better than single-drug treatments in obese rats. Br J Pharmacol 2012; 165: 2721–2735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Rigsby CS, Pollock DM, Dorrance AM.Spironolactone improves structure and increases tone in the cerebral vasculature of male spontaneously hypertensive stroke-prone rats. Microvasc Res 2007; 73: 198–205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Vetri F, Qi M, Xu H, et al. Impairment of neurovascular coupling in type 1 diabetes mellitus in rats is prevented by pancreatic islet transplantation and reversed by a semi-selective PKC inhibitor. Brain Res 2017; 1655: 48–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kelly-Cobbs AI, Prakash R, Coucha M, et al. Cerebral myogenic reactivity and blood flow in type 2 diabetic rats: role of peroxynitrite in hypoxia-mediated loss of myogenic tone. J Pharmacol Exp Ther 2012; 342: 407–415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Haley MJ, Krishnan S, Burrows D, et al. Acute high-fat feeding leads to disruptions in glucose homeostasis and worsens stroke outcome. J Cereb Blood Flow Metab 2019; 39: 1026–1037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Mohr AA, Garcia-Serrano AM, Vieira JP, et al. A glucose-stimulated BOLD fMRI study of hypothalamic dysfunction in mice fed a high-fat and high-sucrose diet. J Cereb Blood Flow Metab. Epub ahead of print 5 August 2020. DOI: 10.1177/0271678X20942397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Honkala SM, Johansson J, Motiani KK, et al. Short-term interval training alters brain glucose metabolism in subjects with insulin resistance. J Cereb Blood Flow Metab 2018; 38: 1828–1838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Greenwood CE, Winocur G.High-fat diets, insulin resistance and declining cognitive function. Neurobiol Aging 2005; 26Suppl 1: 42–45. [DOI] [PubMed] [Google Scholar]
- 45.Winocur G, Greenwood CE.Studies of the effects of high fat diets on cognitive function in a rat model. Neurobiol Aging 2005; 26: 46–49. [DOI] [PubMed] [Google Scholar]
- 46.McNeilly AD, Williamson R, Sutherland C, et al. High fat feeding promotes simultaneous decline in insulin sensitivity and cognitive performance in a delayed matching and non-matching to position task. Behav Brain Res 2011; 217: 134–141. [DOI] [PubMed] [Google Scholar]
- 47.Huber G, Schuster F, Raasch W.Brain renin-angiotensin system in the pathophysiology of cardiovascular diseases. Pharmacol Res 2017; 125: 72–90. [DOI] [PubMed] [Google Scholar]
- 48.Ergul A, Abdelsaid M, Fouda AY, et al. Cerebral neovascularization in diabetes: implications for stroke recovery and beyond. J Cereb Blood Flow Metab 2014; 34: 553–563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Schwaninger RM, Sun H, Mayhan WG.Impaired nitric oxide synthase-dependent dilatation of cerebral arterioles in type II diabetic rats. Life Sci 2003; 73: 3415–3425. [DOI] [PubMed] [Google Scholar]
- 50.Wang Q, Kjaer T, Jørgensen MB, et al. Nitric oxide does not act as a mediator coupling cerebral blood flow to neural activity following somatosensory stimuli in rats. Neurol Res 1993; 15: 33–36. [DOI] [PubMed] [Google Scholar]
- 51.Kitaura H, Uozumi N, Tohmi M, et al. Roles of nitric oxide as a vasodilator in neurovascular coupling of mouse somatosensory cortex. Neurosci Res 2007; 59: 160–171. [DOI] [PubMed] [Google Scholar]
- 52.Dirnagl U, Lindauer U, Villringer A.Role of nitric oxide in the coupling of cerebral blood flow to neuronal activation in rats. Neurosci Lett 1993; 149: 43–46. [DOI] [PubMed] [Google Scholar]
- 53.Krimer LS, Muly EC, Williams GV, et al. Dopaminergic regulation of cerebral cortical microcirculation. Nat Neurosci 1998; 1: 286–289. [DOI] [PubMed] [Google Scholar]
- 54.Hayashi T, Katsumi Y, Mukai T, et al. Neuronal nitric oxide has a role as a perfusion regulator and a synaptic modulator in cerebellum but not in neocortex during somatosensory stimulation – an animal PET study. Neurosci Res 2002; 44: 155–165. [DOI] [PubMed] [Google Scholar]
- 55.Singhal SS, Singh SP, Singhal P, Horne D, et al. Antioxidant role of glutathione S-transferases: 4-Hydroxynonenal, a key molecule in stress-mediated signaling. Toxicol Appl Pharmacol 2015; 289: 361–370. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, sj-pdf-1-jcb-10.1177_0271678X211003497 for Telmisartan prevents high-fat diet-induced neurovascular impairments and reduces anxiety-like behavior by Gianna Huber, Mikolaj Ogrodnik, Jan Wenzel, Ines Stölting, Lukas Huber, Olga Will, Eva Peschke, Urte Matschl, Jan-Bernd Hövener, Markus Schwaninger, Diana Jurk and Walter Raasch in Journal of Cerebral Blood Flow & Metabolism