Abstract
Vocal communication impairment and anxiety are co-occurring and interacting signs of Parkinson Disease (PD) that are common, poorly understood, and under-treated. Both vocal communication and anxiety are influenced by the noradrenergic system. In light of this shared neural substrate and considering that noradrenergic dysfunction is a defining characteristic of PD, tandem investigation of vocal impairment and anxiety in PD relative to noradrenergic mechanisms is likely to yield insights into the underlying disease-specific causes of these impairments. In order to address this gap in knowledge, we assessed vocal impairment and anxiety behavior relative to brainstem noradrenergic markers in a genetic rat model of early-onset PD (Pink1−/−) and wild type controls (WT). We hypothesized that 1) brainstem noradrenergic markers would be disrupted in Pink1−/−, and 2) brainstem noradrenergic markers would be associated with vocal acoustic changes and anxiety level. Rats underwent testing of ultrasonic vocalization and anxiety (elevated plus maze) at 4, 8, and 12 months of age. At 12 months, brainstem norepinephrine markers were quantified with immunohistochemistry. Results demonstrated that vocal impairment and anxiety were increased in Pink1−/− rats, and increased anxiety was associated with greater vocal deficit in this model of PD. Further, brainstem noradrenergic markers including TH and α1 adrenoreceptor immunoreactivity in the locus coeruleus, and β1 adrenoreceptor immunoreactivity in vagal nuclei differed by genotype, and were associated with vocalization and anxiety behavior. These findings demonstrate statistically significant relationships among vocal impairment, anxiety, and brainstem norepinephrine in the Pink1−/− rat model of PD.
Keywords: Parkinson Disease, Rat, Pink1, Ultrasonic Vocalization, Anxiety, Norepinephrine
1. Introduction
1.1. Vocal deficits and anxiety in Parkinson Disease are linked by lack of response to pharmacologic intervention, shared neural substrates, and onset and progression.
In addition to hallmark gross motor signs of Parkinson Disease (PD), 90% of individuals with PD exhibit vocal communication impairment that involves break-down of speech subsystems including respiration, phonation, and articulation [1–7]. The impact of vocal impairment on quality of life and disease burden is substantial [8–11]. Behavioral interventions for treating vocal impairment in PD, such as Lee Silverman Voice Treatment (LSVT), are time-intensive and result in incomplete and inconsistent improvement [12,13]. Pharmacologic interventions such as levodopa change some acoustic features of voice production (loudness) and speech production (lip movement), however, the functional impact of these changes is limited, with minimal improvement in speech intelligibility [14–16]. As a result, the vocal impairment in PD remains untreated. A deeper understanding of the disease-specific mechanisms that cause vocal impairment in PD is essential to develop much needed efficient and effective treatment.
Another consequence of PD that frequently co-occurs with vocal deficits is anxiety. Incidence of anxiety in patients with PD is estimated between 30 and 50%, and is associated with significant negative impact on quality of life and level of disability [17–20]. Some studies have reported reduced anxiety with behavioral treatment and medication. However, similar to vocal impairment, the benefits are incomplete and inconsistent [19,21,22]. As with vocal impairment, the mechanisms underlying anxiety in PD are not well-understood.
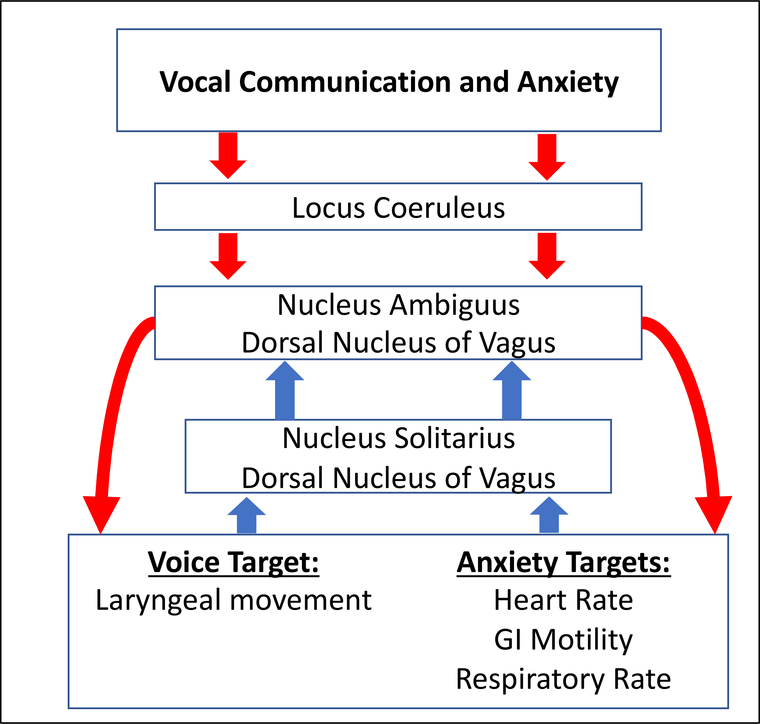
Relationships between affective state and behaviors mediated by cranial nerves, such as vocalization, have been identified in humans; however, the neural mechanisms that drive this relationship remain unclear [23–28]. A potential target mechanism is the locus coeruleus-vagal system. Vocal communication involves fine motor control of the larynx. Anxiety causes changes in degree of arousal of the autonomic nervous system. The Locus Coeruleus-Vagal system, largely driven by norepinephrine (NE), appears to be simultaneously responsible for modulation of vocalization and activation of autonomic responses to anxiety [29–33]. NE neurons in the locus coeruleus project to the dorsal motor nucleus of the vagus nerve(10N) and the nucleus ambiguus. The nucleus ambiguus houses motoneurons responsible for laryngeal movement, as well as neuron groups responsible for cardiopulmonary modulation, while the 10N houses preganglionic cells important for regulation of gastric digestive processes, and secretion of sweat (see Benarroch, 2018 for recent review) [34]. Sensory receptors in the periphery project to the nucleus of the solitary tract, which projects back to the nucleus ambiguus and the 10N in order to modulate laryngeal [35], gastric, and cardiopulmonary motor functions [36,37]. The fact that these neural substrates are linked to both vocal communication and anxiety suggests that the two phenomena may influence one another (Figure 1). Further, it suggests that they may both be susceptible to pathology of this shared substrate. In particular, concentrations of NE, NET, and density of both α1, β1 and α2 noradrenergic receptors in the locus coeruleus, dorsal motor nucleus of the vagus nerve, nucleus ambiguus, nucleus of the solitary tract, and the spinal sensory nucleus could potentially vary depending upon degree of vocal impairment and level of anxiety in PD. Neuroimaging studies in humans have demonstrated upregulation of α1 and β1 adrenoreceptors in neocortical structures in humans with PD compared to controls [38], and decreases in norepinephrine and number of noradrenergic cell bodies in the LC [39,40] have been demonstrated in the Pink1−/− rat model of PD. However, exploration of noradrenergic markers in lower brainstem structures, including those important for vocalization and anxiety, have been minimal.
Figure 1: Pathways of both vocal communication and anxiety.
Vocal communication and anxiety travel the same efferent (red) and afferent (blue) pathways, often with NE as a primary neurotransmitter. The disruption of NE these pathways could simultaneously explain both vocal impairment and anxiety in PD.
1.2: Brainstem noradrenergic disruption: a pathophysiological link between vocal deficits and anxiety in PD
PD is traditionally characterized by nigrostriatal dopaminergic cell death[41], leading to hallmark signs of tremor, bradykinesia, and postural instability [42]. While the physiologic mechanisms of vocal deficits in PD remain uncertain, it is becoming clear that they are at least partially independent of classical dopaminergic degeneration [43–45]. The likelihood of an extra-dopaminergic pathological process is further-supported by the fact that the most commonly prescribed medication, levodopa, improves motor impairments in the limbs [46], while non-hallmark signs, including vocal impairment and anxiety, have a minimal response to dopamine replacement therapies [14–16,19,21]. Aside from dopamine-mediated deficits, other neural substrates, including those governed by NE, are disrupted in PD [47–53]. It has thus been suggested that non-hallmark signs of PD, including vocal deficits and anxiety, may be related to NE processes [49,54,55].
An additional feature shared by vocal deficits and anxiety in PD is their manifestation in prodromal and early stages of the disease, compared to the later-appearing motor signs[56–60]. The possibility that vocal impairment and anxiety in PD are linked through NE mechanisms is further-supported by the fact that NE cells in the locus coeruleus die earlier in the disease process than dopaminergic cells in the nigrostriatal pathway [41,48]. Thus, the timelines of anxiety and vocal impairment and NE cell death are similar, and can be contrasted with the timelines of motor signs and dopaminergic cell death.
There is thus substantial overlap between vocal communication and anxiety in PD with regard to onset, lack of pharmacologic treatment response, and neuroanatomical and neurochemical substrates. As such, the tandem study of vocal communication and anxiety in PD at behavioral and histological levels is warranted. In order to address this gap in knowledge with increased experimental control and the ability to analyze neural tissue, we assessed vocal impairment and anxiety relative to brainstem noradrenergic markers in a translational model of PD in rats with a knockout of the Pink1 gene and wild type (WT) controls. The Pink1−/− rat is based on a genetic form of early and progressive PD (PARK6) that is nearly clinically identical to idiopathic PD [61]; the Pink1−/− rat has been well-validated as a model of vocal communication impairment in PD [39,40,62]. Additionally, noradrenergic differences in the LC have previously been identified in this model [39,40]and vocal acoustic outcomes have been correlated with norepinephrine in the LC [39]. Further, pharmacologic manipulation of norepinephrine in WT rats has been shown to modulate ultrasonic vocalization acoustics [63,64]. However, LC target nuclei in the brainstem have not been investigated relative to noradrenergic markers, vocalization, and anxiety. We hypothesized that 1) Pink1−/− rats would show increased anxiety compared to WT controls; 2) Anxiety level would be associated with vocal acoustic outcomes. 3): Number of cells labeled for tyrosine hydroxylase would be reduced in the locus coeruleus, and norepinephrine transporter and noradrenergic receptors would be altered in brainstem nuclei of Pink1−/− versus WT controls. 4) There would be a negative correlation between noradrenergic markers and both vocal impairment and anxiety.
2. Methods
2.1: Experimental Procedure
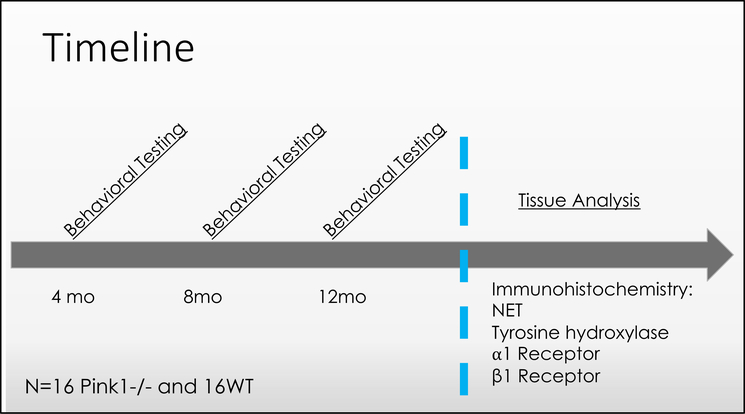
To assess the relationships among anxiety, vocalization, and NE in the Pink1−/− rat model of PD, Pink1−/− rats and WT control rats underwent anxiety testing by performing the elevated plus maze and were immediately transferred to their home cage for ultrasonic vocalization recording (behavioral assays described below). Data were collected at 4, 8, and 12 months of age (Figure 1). Following data collection at the 12-month time point, rats were euthanized, and neural tissue analyzed. (Figure 2)
Figure 2: Experimental timeline.
Behavioral testing includes analysis of ultrasonic vocalizations and measurement of anxiety behavior on the elevated plus maze. NET: norepinephrine transporter.
2.2: Animals
All procedures were approved by the University of Wisconsin-Madison School of Medicine and Public Health Institutional Animal Care and Use Committee (IACUC; protocol M005177-R01-A04) and were conducted in accordance with the NIH Guide for the Care and Use of Laboratory Animals, Eight Edition[65]. Thirty-two male, Long-Evans rats (16 Pink1−/− and 16 Wild Type) were used for this study. Power analysis determined that a sample size of 13 rats per group (Pink1−/− and WT) should detect differences in vocalization (based on differences in USV intensity reported by Grant et al, 2015 [40]) and brainstem tyrosine hydroxylase concentrations (based on Kelm-Nelson et al, 2018 [39]) with an α level of 0.05 and 90% power. A rate of 20% attrition was also accounted for. Thus, the total sample size for each group was n=16 rats. In addition, 12 female Long-Evans rats were used to elicit ultrasonic vocalizations. All animals were obtained from SAGE Labs (Envigo, Boyertown, PA). Rats were housed in pairs the Biomedical Research Model Services facilities of the UW School of Medicine and Public Health, were 12-hour light-cycle reversed and underwent behavioral testing under red light during the dark period when the rats were most active. Rats were handled and weighed weekly until testing and throughout the duration of the study. Standard husbandry and handling practices and procedures were used in accordance with institutional guidelines regarding animal experimentation. Animals were tested at 4, 8, and 12 months of age. These timepoints were chosen because they represent prodromal, early and middle stages of deficit progression in this model [40,66,67].
2.3: Behavioral Testing
2.3.1: Ultrasonic Vocalization Recording
Rats produce ultrasonic vocalizations (USVs) in the 50-kiloHertz (kHz) range to initiate and maintain conspecific contact. Increases in features of USVs, including mean peak frequency, bandwidth, and complexity result in increased approach behavior of conspecifics [68,69]. It is thus thought that these features are “preferred,” and can be considered to be at least partially goal directed [70–75]. Analysis of acoustic variables of rat USVs to study vocal impairment in PD and other neurologic diseases is well-established [40,62,64,76–79]. To assess vocal communication in the current study, USVs were elicited and recorded with the following mating paradigm, as reported in previous studies [40,78–80]. Test rats were placed in their home cage under a microphone attached to an ultrasonic recording system (Avisoft, Germany). A female conspecific in estrus was then placed in the male rat’s home cage. After the male rat showed interest in the female, the female was removed. The USVs from the male rat were then recorded for 90 seconds. USVs were analyzed offline using automated software (SASLab Pro, Avisoft, Germany). Waveforms generated by Avisoft were used to create spectrograms with the following parameters: Fast Fourier Transform (FFT) of 512 points, frame size of 100%, flat top window with temporal resolution set to display 75% overlap. Noise below 25 kilohertz(kHz) (including alarm calls) was removed via a high pass filter; calls above 25kHz were included in analysis. Dependent variables for the purposes of this study were the average bandwidth (kHz), mean peak frequency(kHz), duration(s), and intensity in decibels(dB) of calls.
2.3.2: Elevated Plus Maze:
The elevated plus maze was used to assess anxiety behavior [81–84]. The maze consists of a plus-shaped platform with 4 equally sized arms. Two arms, opposite one another, are open with no walls (50×10cm), and the remaining two arms have walls with open tops on 3 of 4 sides (50×10×50cm). Each arm is accessible from a square area in the center of the platform. The rats were placed in the center of the maze under red light and video-recorded for 5 minutes. Red light with sufficient intensity to allow tracking with video software was chosen to promote environmental validity. Movement was tracked and analyzed using EthoVision software (Noldus Ethovision XT (Wageningen, Netherlands)). Outcome variables were total entries into closed arms and total time spent in closed arms in seconds. Increased time and frequency of entry into closed arms represent increased anxiety. Because differences in overall movement between genotypes may be present, a closed arm ratio was also calculated using the formula:
A smaller ratio indicates increased preference for closed arms of the maze, indicating an increase in anxiety [81].
2.4: Neural Tissue Processing
After testing at the 12-month timepoint, rats were anesthetized under 5% isoflurane. Transcardial perfusion with cold saline was followed by 4% paraformaldehyde in 1% phosphate-buffered saline. Brains were post-fixed for 24 hours in 4% paraformaldehyde, then stored in 0.02% sodium azide at 4°C. Prior to tissue sectioning, brains were placed in a 30% sucrose for 48 hours. Brains were then mounted on a cryostat, sliced coronally at 50 microns from the cortex through the brainstem, placed in 30% sucrose cryoprotectant and stored at −20°C until they were stained for immunoreactivity over every 6th section across the entire brain (approximately Bregma 5.64 to −15.96).
2.5: Immunohistochemistry
Four separate immunohistochemistry assays were performed to compare noradrenergic markers between genotypes and assess the relationships between these markers and USVs and anxiety. Assays for noradrenergic markers included Tyrosine Hydroxylase(TH), α1 adrenoreceptors (α1AR), β1 adrenoreceptors (β1AR), and norepinephrine transporter (NET). Each assay was completed across three runs per assay, with each genotype equally represented in each run. For each assay, incubation in the absence of primary antibody was used for control sections, which resulted in absence of immunoreactivity. Confirmation of antibody specificity was demonstrated by manufactures. Western immunoblotting appropriately detected bands at molecular weights of 62kDA TH (EMD Millipore, Temecula, CA), 50kDA for β1AR (abcam, Cambridge, MA), 60kDA for α1AR (Thermo Fisher Scientific, Waltham, MA), and 80kDA for NET (Thermo Fisher Scientific, Waltham, MA). All antibodies stained appropriate patterns of distribution, as demonstrated previously [40,85–87].
For each assay, tissue sections were blocked in 20% normal goat serum and incubated overnight in a primary antibody solution (see Table 1 for primary antibody product information and concentrations), as recently described [40]. Samples were then incubated in conjugated biotinylated secondary solution at 1:500 (Millipore, BA-1000) for 2-hr and incubated in an avidin– biotin solution (Vector Laboratories, Burlingame, CA) for 1-hr, and the complex was visualized by using SIGMAFAST 3,3-diamino- benzidine with metal enhancer (DAB; Sigma Aldrich, St. Louis, MO, D0426). All sections were float mounted onto gelatin-coated slides, dehydrated in a graded series of alcohols and xylenes, and coverslipped with Cytosol 60 mounting medium (Richard-Allen Scientific, Kalamazoo, MI).
Table 1:
List of antibodies and quantification method for immunohistochemistry
| Primary Antibody | Immunogen Target | Manufacturer (product number); RRID; Lot Number | Host/Concentration: | Type |
Brain Region Quantification method |
|||
|---|---|---|---|---|---|---|---|---|
| LC | NTS | 10N | AMB | |||||
|
| ||||||||
| Anti-β1AR | Synthetic peptide corresponding to mouse β1AR aa 394–408 | abcam (ab3442); AB_10890808; GR3295387–4 | Rabbit/1:2000 | Polyclonal | US | ROD | US | US |
|
| ||||||||
| Anti-α1AR | Synthetic peptide corresponding to residues K(339) F S R E K K A A K T(349) of the 3rd intracellular loop of human α1AR | Thermo Fisher Scientific/Invitrogen (PA1–047); AB_2273801; UG277737 | Rabbit/1:2000 | Polyclonal | US | ROD | US | US |
|
| ||||||||
| Anti-NET | Peptide (C)KLLNASVLGDHTKYSK, corresponding to amino acid residues 189–204 of mouse NET | Thermo Fisher Scientific/Invitrogen (PA5–77494); AB_2736247; VB2931552 | Rabbit/1:5000 | Polyclonal | ROD | ROD | ROD | ROD |
|
| ||||||||
| Anti-TH | TH (NCBI gene ID: 25085 | EMD Millipore (AB152); AB_390204; 3328928 | Rabbit/1:2000 | Polyclonal | US | --- | --- | --- |
AR adrenoreceptor, NET norepinephrine transporter, TH tyrosine hydroxylase, LC locus coeruleus, NTS nucleus of the solitary tract, 10N dorsal motor nucleus of the vagus, AMB nucleus ambiguus, US unbiased stereology, ROD relative optical density, RRID research reference ID
2.6: Unbiased Stereology and Relative Optical Density Measurement
Unbiased cell count estimation was completed in the locus coeruleus (for TH, α1AR, β1AR), 10N ( for α1AR, β1AR), and nucleus ambiguus ( for α1AR, β1AR) using the optical fractionator method as described by West et al, 1991 and adapted from Kelm-Nelson et al, 2018 [88,89]. Cell number was estimated in the right or left hemisphere only for each rat. Stereological analyses were completed using Stereo Investigator® (MBF Bioscience, Williston, VT) and the Optical Fractionator Probe. An Olympus BX53 microscope was fitted with a QImaging Retiga 1300c monochrome camera and a Prior XYZ Proscan III motorized stage kit, with images displayed on a plasma screen monitor. Brain regions were outlined based on the Paxinos and Watson (2005) atlas of stereotaxic coordinates of the rat brain [90] using a 4x magnification. Three sections between bregma −9.48 and −10.2 were counted in the locus coeruleus. Eight to 9 sections were counted between bregma −12.36 and 14.76 in the 10N, and 7 sections between bregma −12.00 and −14.16 were counted in the nucleus ambiguus. For each nucleus, every 6th section was counted (250μm between sections). Random sampling of the outlined regions was completed at 40x magnification using a sampling grid of 100 × 100μm and a counting frame of 75 × 75μm with a dissector height of 12μm and guard zones of 2μm. Section thickness was measured at each counting site (average thickness of 20.4μm, 17.1μm and 19.5μm for locus coeruleus, 10N and nucleus ambiguus respectively). . This combination of sampling parameters was established to achieve a Gunderson coefficient of error (m=1) of less than 0.10 for each region, indicating that parameters used were accurate for stereological investigation. Cell bodies stained for TH, α1AR, and β1AR were counted if the top of the leading edge came into focus within the inclusion lines of the dissector and outside of the 2μm guard zones. Estimated cell counts were averaged for individual rats and combined to produce genotype averages.
Optical density measurements were taken in the locus coeruleus, 10N and nucleus ambiguus (for NET), and in the NTS (for NET, α1AR and β1AR). Sections containing nuclei of interest were outlined at 4x magnification using the slide-scanning workflow in Stereo Investigator®, and the equipment set-up described for stereology. Following background correction, images were obtained at 10x magnification to ensure similar focus between hemispheres. For locus coeruleus, 10N and nucleus ambiguus, the same number of sections were analyzed and at same bregma coordinates as above; in addition, 3 sections between bregma −12.96 and −13.8 were analyzed in the NTS (NET, α1AR, and β1AR). For each nucleus, both hemispheres of every 5th section were analyzed (250μm between sections). Analysis was completed using ImageJ (US National Institutes of Health, Bethesda, MD). ImageJ was then used to place uniformly-sized boxes inside of the nucleus of interest in each hemisphere (250 × 500 pixels in the locus coeruleus, 500 × 500 pixels in the NTS, 300 × 300 pixels in the nucleus ambiguus, and 250 × 500 pixels in the 10N), as well as a region devoid of staining (50 × 50 pixels region in the spinal tract of 5) on each slice. Images were converted from RGB color to 8-bit, and pixels in the images were calibrated on a provided gray scale (ImageJ). An optical density reading of the background and bilateral regions of interest were then taken. The relative optical density for each section was calculated as relative optical density = (average optical density of the nucleus of interest) – optical density of the background image. Relative optical density was then averaged for individual rats, and then combined to produce genotype averages.
2.7: Statistical Analysis
Mixed effect models were used to assess the influence of time point (3 levels), genotype (two levels), as well as interaction between time and genotype on USV and anxiety parameters. Multiple regressions were fitted separately to assess USV outcomes of call intensity, bandwidth, duration, and average peak frequency with anxiety, genotype, and the interaction between anxiety and genotype as independent variables at the 12-month timepoint. Student’s t-tests were used to compare brainstem NE markers between genotypes. Univariate linear regression analysis was conducted to assess relationships between brainstem NE and USV parameters, as well as between NE and anxiety at the 12-month timepoint. Sample sizes and degrees of freedom reflect incidental tissue loss (n=1 Pink1−/− rat for NET in the NTS and 10N), absent vocalization (n=1 WT rat at 4months, n=1 Pink1−/− rat at 8 months, and n=1 WT rat at 12 months), and a rat removing itself from the maze during anxiety testing (n=1 Pink1−/− rat at 4 months).
The outcome variables were USV measures of bandwidth, mean peak frequency, sound intensity, and call duration, as well as time spent in closed arms of the plus maze, and frequency of entries into closed arms of the plus maze. Independent variables included relative optical density of NET in the locus coeruleus, NTS, 10N, and nucleus ambiguus, relative optical density of α1AR and β1AR in the NTS, and estimated cell counts of cell bodies stained for TH in the locus coeruleus, and α1AR and β1AR separately in the locus coeruleus, 10N and nucleus ambiguus. For USV and plus maze measurements, inter-rater reliability was determined by calculating intra-class correlation coefficients (ICC) on 5% of data files. This methodology was chosen based on our hypothesis that observations from behavioral testing are the result of long-term changes to NE involving these brain regions. Statistical analyses were performed with a significance level of 0.05 using software R (version 3.6.0) and SAS (version 9.4). Due to the exploratory nature of this work and associated risk of type II statistical error, no corrections for multiple comparisons were made.
3. Results
3.1: Ultrasonic Vocalizations
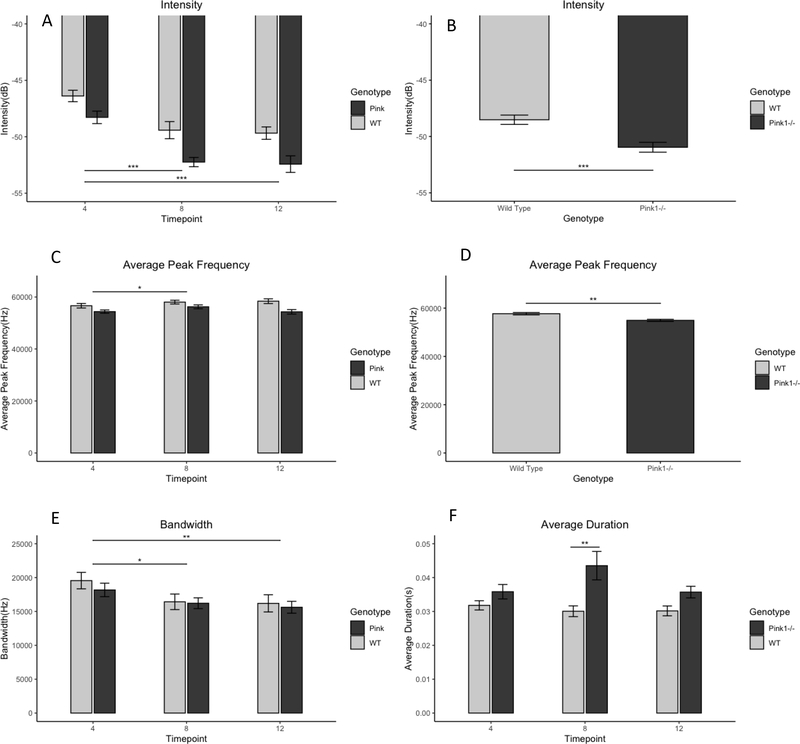
Inter-rater reliability was greater than 0.90 for USV measurements. There was no interaction between genotype and timepoint on bandwidth (F(2,57)=0.21, p>0.05). There was no main effect of genotype (F(1,30)=0.31, p=0.5796). There was a significant main effect of timepoint (F(2,57)=7.01,p=0.0019) on call bandwidth. Post-hoc analysis comparing least squares means demonstrated that bandwidth decreased after the 4-month timepoint. At 4 months (18803Hz), bandwidth was greater than 8months (16322Hz) and 12 months (15843hz) (t(57)=2.91, p=0.014 and t(57)=3.49, p=0.0027, respectively), but 8- and 12-month timepoints were not significantly different from one another(t(57)=0.56, p=0.89).
There was no interaction between genotype and timepoint on call intensity (F(2,57)=0.63, p=0.54). There was a significant main effect of genotype on call intensity (F(1,30)=15.79, p=0.0004). In post-hoc analysis, the Pink1−/− rat group (−50.02dB) produced USVs of lower sound intensity than the WT rat group (−48.51dB) (t(30)=3.97, p=0.004). There was also a significant main effect of timepoint on call intensity (F(2,57)=34.99, p<0.0001) (4mo=−47.35, 8mo=−50.87, 12mo=−51.07). USV sound intensity was lower at 4mo than 8mo (t(57)=7.03,p<0.0001) and 12mo (t(57)=7.43, p<0.0001). There was no difference in intensity between 8mo and 12mo (t(57)=0.37, p=0.93).
There was no interaction between genotype and timepoint on average peak frequency (F(2,57)=2.14, p=0.13). There were significant main effects of timepoint (F(2,57)=4.08, p=0.02) and genotype(F(1,30)=9.11,p=0.005) on average peak frequency of calls. The Pink1−/− rat group (54988hz) had a lower average peak frequency that the WT rat group (57653hz) (t(30)=3.02, p=0.005). Additionally, Average peak frequency at 4mo (55483hz) was lower than 8mo (57168hz) (t(57)=2.85,p=0.02), but was not significantly different from 12mo (56312) (t(57)=0.16, p=0.34). Average peak frequency did not differ from 8 to 12 months (t(57)=0.15, p=0.32).
There was a significant interaction between genotype and timepoint call duration (F2,57)=3.38, p=0.41). The Pink1−/− rat group and WT rat group did not differ in call duration at 4 months (Pink1−/− mean=0.036s, WT mean= 0.0321s, t(57)=1.18, p=0.85). By 8 months, duration was longer for The Pink1−/− group than for WT group (mean pink= 0.043s, mean WT= 0.030s, (t(57)=4.12, p=0.002 ). By 12 months, however, call duration was no longer different between genotypes (mean Pink1−/−=0.36s, mean WT= 0.030 (t(57)=1.65, p=0.57).
3.2: Anxiety
Inter-rater reliability was greater than 0.90 for plus maze measurements. Because a number of rats of both genotypes (n=8 to 17 per timepoint) did not enter the open arms of the maze at some timepoints, entries into and duration in open arms were not used as outcome measures (an average of 6 WT rats and 5 Pink1−/− rats per timepoint did not enter open arms).
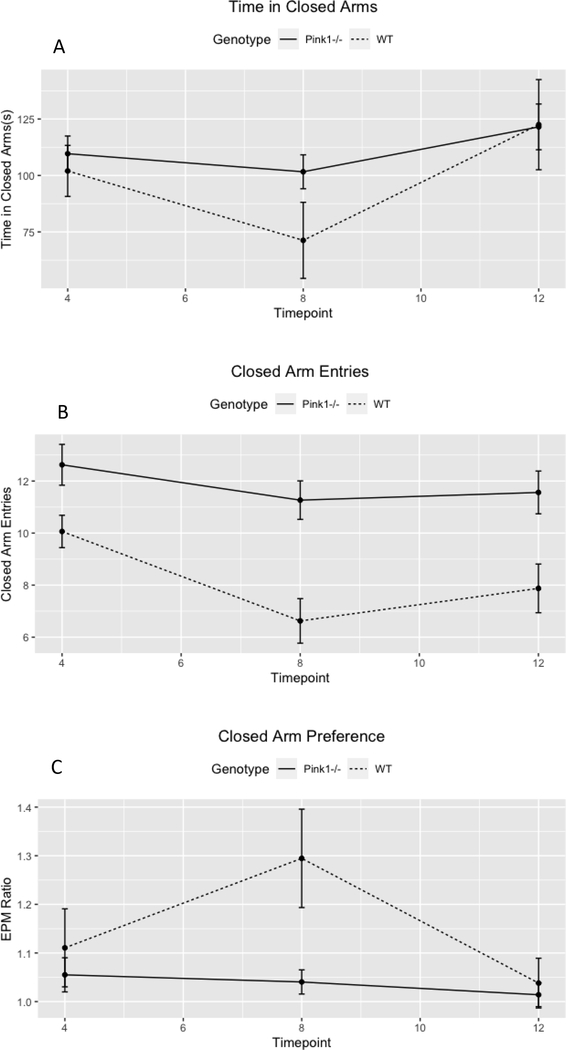
There was no interaction effect between genotype and timepoint on duration in closed arms of the maze (F(2,59)=1.02,p=0.37). There was a significant main effect of timepoint (F(2,59)=5.02,p=0.01) on duration in closed arms of the maze. Rats spent the least amount of time in closed arms of the maze at 8 months (mean 4=105.83, mean 8=86.36, mean 12=122.01). Rats spent less time in closed arms at 8 months than 4 months, but this did not reach statistical significance in post-hoc testing (t(59)=1.73, p=0.08). Rats spent more time in closed arms at 12 months than 8 months (t(59=−3.16, p=0.003). Four-month and 12-month timepoints did not differ in this measure (t(59)=−1.45,p=0.15). There was no main effect of genotype (f(1,30)=0.84,p=0.37).
There was no interaction between genotype and timepoint on number of closed arm entries (F(2,59)=1.26, p=0.29). There were significant main effects of Timepoint (F2,59)=7.18, p=0.0016) and Genotype (F(1,30)=18.29, p=0.0002) on number of entries into closed arms of the maze. Pink1−/− rats entered closed arms more often than WT rats (mean of 11.8 vs 8.17, respectively) (t(30)=4.28, p=0.0002). Averaged across genotypes (mean 4mo= 11.3 entries, mean 8mo= 8.9 entries, mean 12mo=9.7 entries), there were significant differences between 4 and 8 months (t(59)=3.7,p=0.001), and 4 and 12 months (t(59)=2.53, p=0.03), but not between 8 and 12 months (t(59)=−1.2, p=0.46).
Because overall differences in movement could potentially influence performance on the elevated plus maze, a ratio of the number of closed arm entries divided by closed arm time to total number of entries into any arm divided by total time in any arm was calculated. Lower numbers indicate increased preference for closed arms, and are thus though to be indicative of anxiety. There was no interaction between timepoint and genotype (F(2,59)=2.32, p=0.11) on this ratio. There was a main effect of genotype (F(1,30)=4.24, p=0.048). The closed ratio for Pink rats (mean=1.03) was smaller than for WT rats (mean=1.15) (t(59)=−2.06, p=0.048), indicating greater preference for closed arms. There was no main effect of timepoint (F(2,59)=3.0, p=0.058).
3.3: Relationships Among Anxiety, Ultrasonic Vocalization, and Genotype
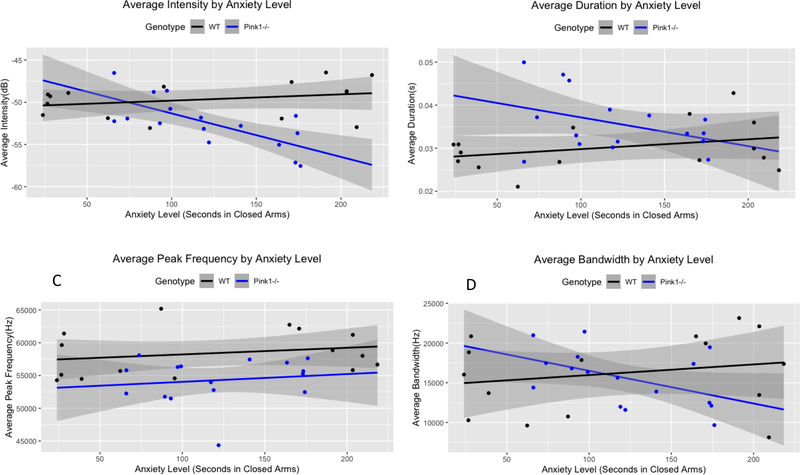
Four multiple linear regressions were fitted separately to assess USV outcomes of call intensity, bandwidth, duration, and average peak frequency with anxiety, genotype, and the interaction between anxiety and genotype as independent variables at the 12-month. timepoint. A significant regression model was found for call intensity (F(3,27) =9.41, p=0.0002), with an R2 of 0.51. There was an interaction between genotype and time in the closed arms of the maze. For every one-second increase in closed arm time, USV intensity decreased by 0.052dB in Pink1 −/− rats (β=−0.052, t(30)=−3.79, p=0.0008). Additionally, USV intensity was 4.39dB softer on average for WT−/− rats than for Pink1−/−s (β=−4.39, t(30)= −2.19, p=0.0007) when time in the closed arms of the maze was 0 seconds.
A significant regression was found for call duration (F(3,27) =3.57, p=0.027), with an R2 of 0.28. There was a non-significant interaction between genotype and time in the closed arms of the maze. For every one-second increase in closed arm time, USV duration decreased by 0.000069 seconds in Pink1 −/− rats (β=−0.000069, t(30)=−1.73, p=0.095). Additionally, USV duration was 0.016 seconds shorter on average for WT rats than for Pink1−/−s (β=−0.016, t(30)= −2.86, p=0.008) when time in the closed arms of the maze was 0 seconds.
A significant regression was also found for average peak frequency (F(3,27) =3.78, p=0.022), with an R2of 0.30. However, individual β-estimates were not able to significantly determine average peak frequency independently in this model, likely due to a high degree of collinearity.
A regression model to assess call bandwidth based on anxiety, genotype, and the interaction between anxiety and genotype was not significant (F(3,27) =1.16, p=0.34), with an R2 of 0.11.
3.4: Immunohistochemistry
3.4.1: Tyrosine Hydroxylase
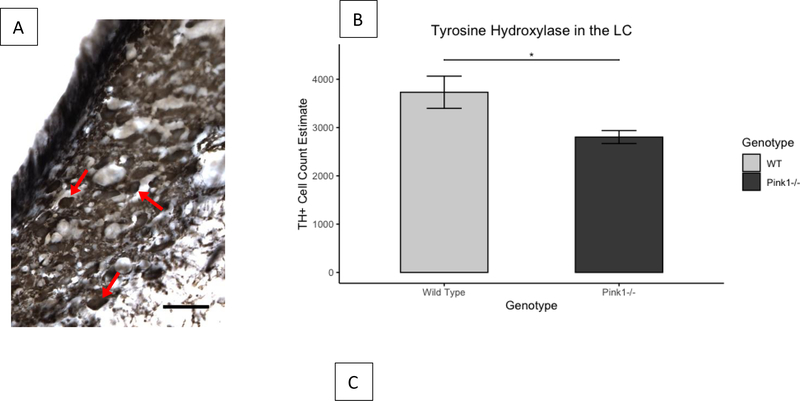
There were significantly fewer cells labeled for TH in the locus coeruleus for Pink1−/− rats (mean=2802, SD=537) than for WT rats (mean=3730, SD=1334) (t(19.75)=−2.58, p=0.018).
3.4.2: Norepinephrine Transporter
There were no differences between genotypes in relative optical density of NET in the locus coeruleus (t(28.51)=−0.517, p=0.61), the NTS, (t(28.56)=−1.4, p=0.17), the 10N (t(28.65)=−1.45, p=0.16), or the nucleus ambiguus (t(28.41)=−1.54, p=0.14).
3.4.3: α1 Adrenoreceptors
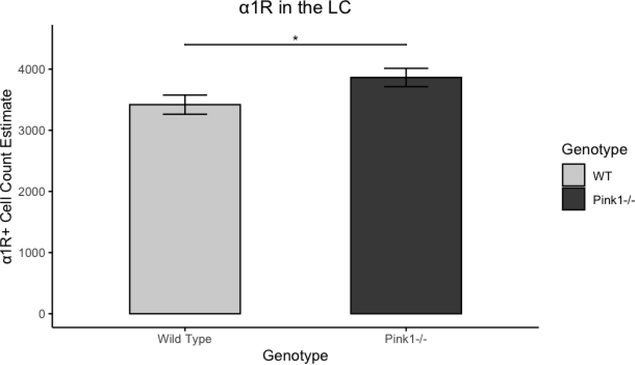
There were more cells labeled for α1R in the in the locus coeruleus for Pink1−/− rats (mean=3863, SD=598) than for WT rats (mean=3418, SD=628) (t(29.93)=2.05, p=0.0495). There were no differences between genotypes in the relative optical density of α1R in the NTS (t(29.48)=0.53, p=0.6). The number of cells labeled for α1R did not differ between genotypes in the 10N (t(29.82)=0.89, p=0.38) or in the nucleus ambiguus (t(29.71)=0.69, p=0.50)
3.4.4: β1 Adrenoreceptors
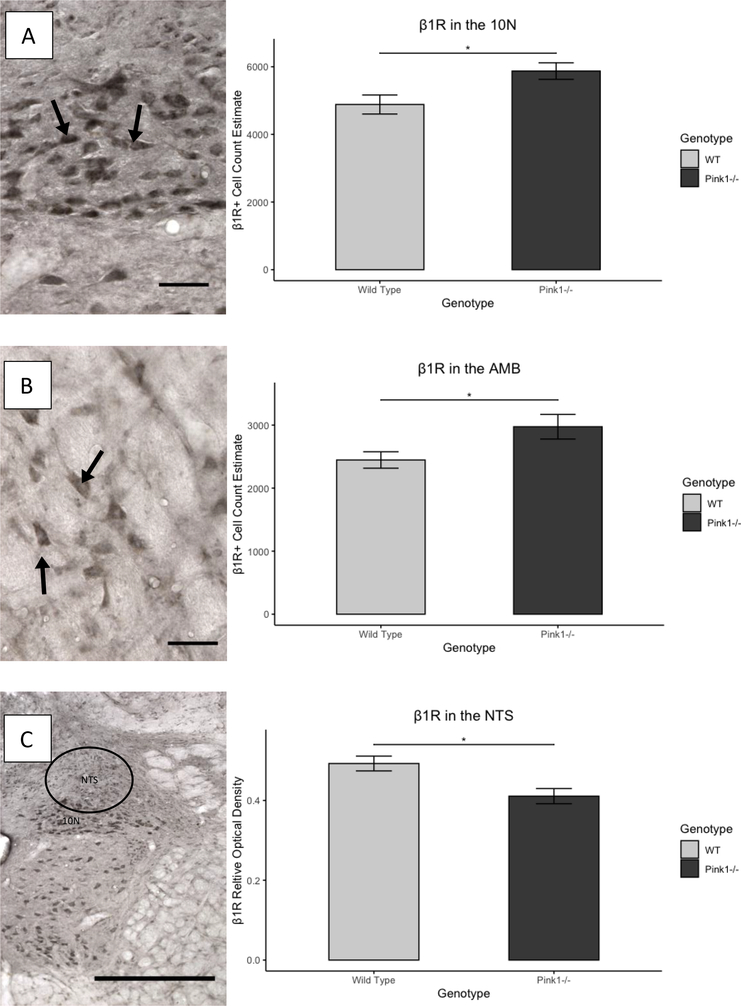
There was no difference in number of cells labeled for β1R in the locus coeruleus between genotypes (t(27.12)=1.07, p=0.29). The relative optical density of β1R in the NTS was significantly lower for Pink1−/− rats (mean=0.41, SD=0.076) than WT rats (mean=0.49, SD=0.075) (t(29.98)= −3.08, p=0.0044). There were more cells labeled for β1R in the in the 10N for Pink1−/− rats (mean=5872, SD=977) than for WT rats (mean=4883, SD=1124) (t(29.43)=2.65, p=0.013). In the nucleus ambiguus, Pink1−/− rats also had a greater number of cells labeled for β1R. (mean=2974, SD=780) than WT rats (mean=2446,SD=521) (t(26.16)=2.25, p=0.033).
3.5: Relationships Between Ultrasonic Vocalization and Noradrenergic Markers
3.5.1: Tyrosine Hydroxylase
Linear regressions were fitted to determine associations between USV outcomes and the number of cells labeled for TH in the locus coeruleus. Number of cells labeled for TH in the locus coeruleus did not determine USV bandwidth (F(1,29)=0.20, p=0.66, R^2 of 0.01), intensity (F(1,29)=2.56, p=0.12, R^2 of 0.08), average peak frequency (F(1,29)=0.7, p=0.41, R^2 of 0.02), or duration (F(1,29)=1.14, p=0.29, R^2 of 0.04).
3.5.2: Norepinephrine Transporter
Linear regressions were fitted to determine associations between USV outcomes and the relative optical density of NET in the locus coeruleus, NTS, 10N and nucleus ambiguus. No significant regressions were found (Table 2).
Table 2.
Linear regression result assessing relationships between brainstem noradrenergic markers and vocal acoustic outcomes. US: unbiased stereology. ROD: relative optical density. LC: locus coeruleus. NTS: nucleus of the solitary tract. 10N: dorsal motor nucleus of the vagus. AMB: nucleus ambiguus.
| Noradrenergic Marker | Outcome Measure(s) | Independent Variables | Results |
|---|---|---|---|
| Tyrosine Hydroxylase | Average Bandwidth | US in the LC | F(1,29)=0.20, p=0.66), R2=0.01. |
| Average Intensity | US in the LC | F(1,29)=2.56, p=0.12), R2=0.08. | |
| Average Peak Frequency | US in the LC | F(1,29)=0.7, p=0.41), R2=0.02. | |
| Average Duration | US in the LC | F(1,29)=1.14, p=0.29), R2=0.04. | |
| Norepinephrine Transporter | Average Bandwidth | ROD in the LC | F(1,29)=1.47, p=0.24, R2=0.05. |
| ROD in the NTS | F(1,28)=0.28, p=0.6, R2=0.01. | ||
| ROD in the 10N | F(1,28)=0.32, p=0.58, R2=0.01. | ||
| ROD in the AMB | F(1,29)=0.05, p=0.82, R2=0.002. | ||
| Average Intensity | ROD in the LC | F(1,29)=0.29, p=0.6, R2=0.01. | |
| ROD in the NTS | F(1,28)=0.69, p=0.41, R2=0.02. | ||
| ROD in the 10N | F(1,28)=0.62, p=0.44, R2=0.02. | ||
| ROD in the AMB | F(1,29)=0.17, p=0.68, R2=0.01. | ||
| Average Peak Frequency | ROD in the LC | F(1,29)=0.31, p=0.58), R2=0.01. | |
| ROD in the NTS | F(1,28)=2.46, p=0.13 R2=0.08. | ||
| ROD in the 10N | F(1,28)=1.25, p=0.27, R2=0.04. | ||
| ROD in the AMB | F(1,29)=0.96, p=0.34, R2=0.03. | ||
| Average Duration | ROD in the LC | F(1,29)=0.61, p=0.44), R2=0.02. | |
| ROD in the NTS | F(1,28)=0.18, p=0.67), R2=0.006. | ||
| ROD in the 10N | F(1,28)=0.1, p=0.76), R2=0.003. | ||
| ROD in the AMB | F(1,29)=0.03, p=0.86), R2=0.001. | ||
| β1 Receptor | Average Bandwidth | US in the LC | F(1,29)=0.36, p=0.55), R2=0.01. |
| ROD in the NTS | F(1,29)=1.19, p=0.28), R2=0.04. | ||
| US in the 10N | F(1,29)=1.92, p=0.17), R2=0.06. | ||
| US in the AMB | F(1,29)=1.65, p=0.21), R2=0.05. | ||
| Average Intensity | US in the LC | F(1,29)=0.84, p=0.37), R2=0.03. | |
| ROD in the NTS | F(1,29) =4.74, p=0.04), R20.14. | ||
| US in the 10N | F(1,29) =13.57, p=0.0 0,), R2=0.32. | ||
| US in the AMB | F(1,29) =9.43, p=0.005), R2=0.25. | ||
| Average Peak Frequency | US in the LC | F(1,29)=0.15, p=0.70), R2=0.01. | |
| ROD in the NTS | F(1,29)=3.73, p=0.06), R2=0.11 | ||
| US in the 10N | F(1,29)=1.77, p=0.19), R2=0.06. | ||
| US in the AMB | F(1,29) =6.3, p=0.02), R2=0.18. | ||
| Average Duration | US in the LC | F(1,29)=0.57, p=0.45), R2=0.001. | |
| ROD in the NTS | F(1,29)=0.42, p=0.52), R2=0.01. | ||
| US in the 10N | F(1,29)=0.46, p=0.51), R2=0.02. | ||
| US in the AMB | F(1,29)=0.08, p=0.78), R2=0.003. | ||
| α 1 Receptor | Average Bandwidth | Us n the LC | F(1,29)=0.03, p=0.87), R2=0.001. |
| ROD in the NTS | F(1,29)=0.17, p=0.69), R2=0.006. | ||
| US in the 10N | F(1,29)=0.63, p=0.44), R2=0.02. | ||
| US in the AMB | F(1,29)=0.35, p=0.56), R2=0.01. | ||
| Average intensity | US in the LC | F(1,29)=0.49, p=0.48), R2=0.02. | |
| ROD in the NTS | F(1,29)=0.23, p=0.64), R2=0.01. | ||
| US in the 10N | F(1,29)=2.96, p=0.10), R2=0.09. | ||
| US in the AMB | F(1,29)=1.04, p=0.32), R2=0.04. | ||
| Average Peak Frequency | US in the LC | F(1,29)=0.24, p=0.63), R2=0.008. | |
| ROD in the NTS | F(1,29)=0.69, p=0.41), R2=0.02. | ||
| US in the 10N | F(1,29)=0.12, p=0.73), R2=0.004. | ||
| US in the AMB | F(1,29)=0.28, p=0.60), R2=0.009. | ||
| Average Duration | |||
| US in the LC | F(1,29)=0.67, p=0.42), R2=0.02. | ||
| ROD in the NTS | F(1,29)=1.16, p=0.29), R2=0.04. | ||
| US in the 10N | F(1,29)=0.31, p=0.58), R2=0.01. | ||
| US in the AMB | F(1,29)=2.57, p=0.12), R2=0.08. | ||
3.5.3: α1 Adrenoreceptors
Linear regressions were fitted to determine associations between USV outcomes and number of cells labeled for α1 receptors in the locus coeruleus, nucleus ambiguus, and 10N, and relative optical density of α1 receptors in the NTS. No significant regressions were found (Table 2).
3.5.4: β1 Adrenoreceptors
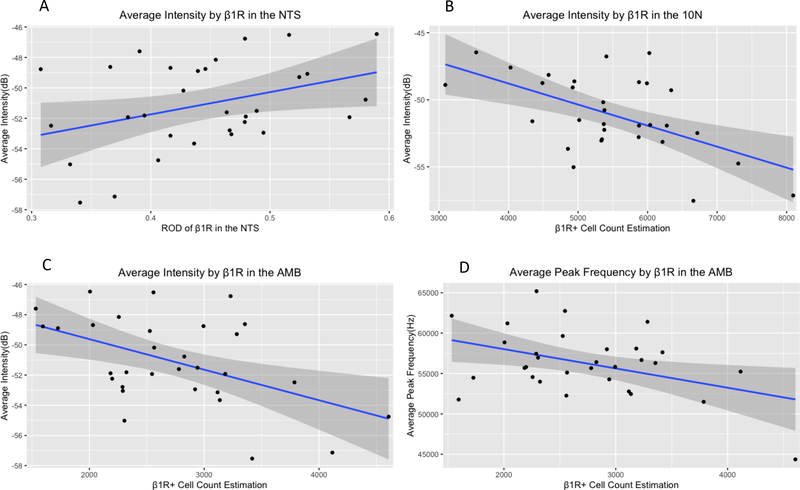
Linear regressions were fitted to determine associations between USV outcomes and number of cells labeled for β1 receptors in the locus coeruleus, nucleus ambiguus, and 10N, and relative optical density of β1 receptors in the NTS. No significant correlations were found for duration or bandwidth. Three independent variables were found to be significantly associated with intensity. In the NTS, (F(1,29) =4.74, β=14.59, 95% confidence interval=1.46 to 27.73 p=0.04), R2=0.14) for every unit increase in relative optical density, intensity increased by 14.59dB. In the 10n (F(1,29) =13.57, β=−0.002, 95%CI= −0.002 to −0.0007 p=0.001, R2=0.320), for every additional cell labeled for β1R, intensity decreased by 0.002dB. In the nucleus ambiguus (F(1,29) =9.43, β=−0.002, 95%CI=−0.003 to −0.0006. p=0.005, R2=0.25), for every additional cell labeled for β1R, intensity decree sed, 0.002dB.
In addition, cell count estimations for β1R-labeled cells in the nucleus ambiguus were significantly associated with average peak frequency (F(1,29) =6.3, β=−2.39, 95%CI=−4.26 to −0.52, p=0.02), with an R2 of 0.18). For every additional cell labeled for β1R in the nucleus ambiguus, average peak frequency decreased by 2.39Hz.
3.6: Relationships Between Anxiety and Noradrenergic Markers
3.6.1: Tyrosine Hydroxylase
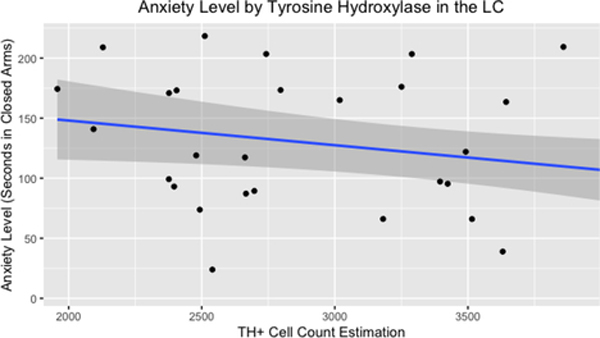
A linear regression was fitted to determine associations between duration in closed arms of the plus maze and the number of cells labeled for TH in the locus coeruleus. A significant association was found (F(1,30 =4.6, β=−0.02, 95%CI= −0.04to −0.001 p=0.04), with an R2 of 0.13. For every additional cell labeled for TH in the locus coeruleus, duration in closed arms of the plus maze decreased by 0.02 seconds.
3.6.2: Norepinephrine Transporter
Linear regressions were fitted to determine associations between duration in closed arms of the plus maze and OD of NET in the locus coeruleus, NTS, 10N and nucleus ambiguus. Regressions using the locus coeruleus (F(1,30)=0.26, p=0.61, R^2=0.009), NTS (F(1,29)=0.55, p=0.47, R^2=0.02), 10N (F(1,29)=0.35, p=0.56, R^2=0.01), and nucleus ambiguus (F(1,30)=0.61, p=0.44, R^2=0.02) were not significant.
3.6.3: α1 Adrenoreceptors
Linear regressions were fitted to determine associations between duration in closed arms of the plus maze and the number of cells labeled for α1R in the locus coeruleus, 10N and nucleus ambiguus, and on the relative optical density of α1R in the NTS. Regressions using the locus coeruleus (F(1,30)=1.62, p=0.21, R^2=0.05), NTS (F(1,30)=0.06, p=0.8, R^2=0.002), 10N (F(1,30)=0.03, p=0.87, R^2=0.001) and nucleus ambiguus (F(1,30)=.69, p=0.41, R^2=0.02) were not significant.
3.6.4: β1 Adrenoreceptors
Linear regressions were fitted to determine associations between duration in closed arms of the plus maze and the number of cells labeled for β1R in the locus coeruleus, 10N and nucleus ambiguus, and on the relative optical density of β1R in the NTS. Regressions using the locus coeruleus (F(1,30)=3.51, p=0.07, R^2=0.10), NTS (F(1,30)=0.1,p=0.76, R^2=0.003), 10N (F(1,30)=0.43, p=0.51, R^2=0.01) and nucleus ambiguus (F(1,30)=0.1, p=0.75, R^2=0.003) were not significant.
4: Discussion
The current study demonstrates that several aspects of the noradrenergic system are disrupted in the brainstem of the Pink1−/− rat model of PD. Further, these neural differences are not only associated with anxiety and vocal impairment, but anxiety and genotype interact to influence changes to vocalization that mimic some aspects of hypokinetic dysarthria in humans with PD. These findings are relevant to a growing body of research that suggests noradrenergic dysfunction is a primary contributing factor to early and non-hallmark signs of PD [47–52,54,55].
Anxiety-Like Behavior
The current findings are relevant to future translational work assessing anxiety in PD. Consistent with previous investigations of anxiety in the Pink1−/− rat model of PD, [66,67,91], we identified an increase in anxiety-like behavior in Pink1−/− rats compared to WT controls. The current study followed rats further (12 months vs 8 months and earlier) than in previous work with this progressive model, allowing for a more-nuanced interpretation. This longitudinal data revealed a U-shaped curve in anxiety-like behaviors for WT rats, with an increase in exploratory activity (e.g., decreased anxiety-like behavior) at 8 months compared to 4 and 12 months. This U-shaped curve is expected in WT rodents [92], but was absent in the Pink1−/− rat (Figure 4). While Pink1−/− rats demonstrated increased anxiety-like behavior across timepoints, the spike at the 8 month timepoint is important in that it is thought to represent early-to-mid -stage progression in human PD, a time when prevalence of anxiety also increases [93]. While the exact etiology of anxiety in PD is not yet well understood, it is likely a combination of disease-specific biological factors and psychological responses to disease progression and motor manifestation [94]. Future work that longitudinally controls for increased stressors in the Pink1−/− rat [91] may help to disentangle biological and experiential contributors to anxiety in PD.
Figure 4.
Anxiety Behavior. A: Time spent in closed arms. B: Entries into closed arms. C: Ratio of closed arm entries/time in closed arms to total entries into any arm/total time spent in any arm. Pink1−/− rats spent more time in closed arms than WT rats (B), and demonstrated a greater preference for closed arms(C). Number of entries into closed arms did not differ by genotype. Across genotypes, rats made more entries into closed arms at 4 months than 8 or 12 months, and spent the least amount of time in closed arms at 8 months. Error bars indicate +/− SEM.
Relationships between anxiety and vocalization
Consistent with previous studies [39,40,62,64,95], vocalizations of Pink1−/− rats were reduced in intensity and had a lower average peak frequency compared to WT controls. A novel finding of the study was the genotype-dependent relationship between anxiety and vocalization. Specifically, the vocalizations of Pink1−/− rats were associated with anxiety level, whereas those of WT controls were not. Relationships between voice and speech, and changes to cognitive/emotional state or anxiety level in humans have been well-documented [24,26,28,96–98]. This is intuitively understood when observing degradation of voice quality and speaking performance in the setting of anxiety during public speaking. Interestingly, this phenomenon of degraded voice quality and speaking performance during anxiety can be reduced through various methods of cognitive-behavioral training [99–101]. Our understanding of the neurobiological underpinnings of the phenomena are generally limited to physical measurements of autonomic arousal [99]. Interestingly, normalization of these measures of arousal occurs during targeted cognitive-behavioral intervention, and has been simultaneously correlated with improved performance and with activation of higher cortical structures [101], suggesting that the process by which degradation of voice and speech performance in the setting of anxiety can be overcome is “top-down” in nature. In this context, a possible explanation for the influence of genotype on the interaction between anxiety level and vocal acoustic outcomes is an impairment of “top-down,” higher cortical processes in Pink1−/− model. This might explain our observation that anxiety-based disruption to vocalization is present for Pink1−/− rats but not for WT controls.
To explore these findings further, future work would benefit from using alternative means of comparing vocalization to anxiety-like behavior, such as analyzing USVs produced while on the elevated plus maze. These correlational findings should also be further-explored through experimental control of the “dose” of anxiety given to each rat, assessing how anxiety “dose” influences vocal outcomes, and then assessing how genotype interacts with the relationship between anxiety “dose” and vocal outcomes. Because continuous assessment of anxiety state and controlled modulation of anxiety dose is challenging in animal models, such a design may be more feasible in humans with PD than in rats.
Brainstem Noradrenergic Markers
It has been well-established that noradrenergic degeneration in the locus coeruleus precedes and is more extensive than dopaminergic degeneration in the nigro-striatal pathway in humans with PD [41,102]. The reduced number of cell bodies immunoreactive for TH in the locus coeruleus in Pink1−/− rats compared to WT controls in the current study mirrors human findings, and replicates previous work in our laboratory [40]. Additionally, the number of cell bodies reactive for α1 receptors was increased in the locus coeruleus of Pink1−/− rats, compared to WT controls. Increased expression of α1 receptors has been demonstrated in humans with PD [38,103], and over-expression of the α1B receptor subtype in murine models has resulted in central nervous system apoptosis and was associated with Parkinson-like hind-limb disorders.[104] While the mechanism by which this increased expression occurs is not understood, it is thought to be a compensatory response to reduced bioavailability of norepinephrine.
Differences in noradrenergic markers in the other brainstem structures were minimal with the exception of β1 receptor, with an increase in number of positively-labeled cell bodies in the nucleus ambiguus and 10N, and a decrease in the relative optical density in the NTS. While the roles of the nucleus ambiguus and NTS in vocal communication are clear in that they house laryngeal motoneurons and are the primary target for laryngeal sensory information, respectively, the 10N is also important for laryngeal function in that, in addition to being a preganglionic sensory nucleus, it contains targets for sensory information carried by the superior laryngeal nerve, and thus could be implicated in regulation of laryngeal movement [105]. Reduced vocal intensity, an important translational aspect of this rat model of vocal deficit in PD, was significantly associated with number of cell bodies labeled for β1 receptor in the nucleus ambiguus and 10N and by the relative optical density of β1 receptor in the NTS. This finding is consistent with previous studies that have correlated vocalization with noradrenergic disruption in the central nervous system [39,40]. In light of previous work that has shown increased vocal intensity in WT rats who were intraperitoneally injected with the β1 receptor antagonist, propranolol [64], our finding of a negative correlation between β1 immunoreactive cell bodies in laryngeal motor nuclei suggests that β1 receptors may be an important pharmacologic target in the treatment of vocal impairment in PD. Contrary to findings reported by Grant et al, 2015, number of TH immunoreactive cell bodies in the locus coeruleus was not significantly associated with any USV parameters. A possible explanation for this discrepancy is methodological differences implemented when conducting cell count estimation and the timepoint when data was collected (in the current study, rats were 12 months versus 8 months). Interestingly, the number of TH immunoreactive cell bodies in the locus coeruleus was weakly correlated with anxiety level. Thus, while anxiety and vocalization are associated with one another and are associated with some brainstem noradrenergic factors, the brainstem noradrenergic factors investigated do not appear to modulate the relationship between anxiety and vocalization in this model of PD. Future studies should investigate vocalization, anxiety, and noradrenergic factors at higher cortical levels, which may provide greater insight into the relationships among anxiety, vocalization, and noradrenergic deficits. Finally, it is likely that more-complex interactions involving multiple other neurotransmitter systems including serotonergic, glutamatergic, and adenosinergic systems, could explain the relationships between vocalization and anxiety in PD more-completely. Compelling evidence for such interactions comes from research outside of rat models of PD, in which the number of USVs produced has been shown to be influenced by complex interactions among multiple neurotransmitter systems across the brain [106–110]. Investigation into the influence of these neurotransmitter systems on both number and acoustic features of USVs in PD models is likely to yield a more nuanced understanding of how disease mechanisms influence behavioral deficits.
4.1. Limitations
In the study of anxiety, researchers and clinicians often differentiate between trait anxiety and state anxiety. Trait anxiety reflects an aspect of personality that predisposes one to increases in state anxiety, which has been defined as a transient emotional state associated with physiological arousal and conscious awareness of perceived negative feelings about future events [111,112]. While these two aspects of anxiety, by definition, interact with one another, they are distinct and are represented by different functional connectivity profiles in the human brain [113]. Assessment of state and trait anxiety in humans with PD demonstrates that both are increased relative to controls [114], reiterating the relationship between these aspects of anxiety. In animal models of anxiety, correlations between trait and state anxiety are less clear, and performance on measures that reflect trait anxiety, such as free exploration paradigms, are not consistently associated with measures that reflect state anxiety, such as the elevated plus maze [115]. While pink1−/− rats demonstrated greater anxiety-like behavior on the elevated plus maze than WT controls at each timepoint in the current study, a limitation is that these are simply multiple and repeated measures of state anxiety. Nevertheless, a consistency between anxiety-like behavior in Pink1−/− rats and humans with PD was observed.
It should also be acknowledged that performance on the elevated plus maze only reflects some aspects of anxiety-like behavior. Replication of the current study using alternative lighting conditions, such as bright white light, or alternative means of measuring anxiety-like behavior, such as time spent in the light portion of a light-dark box apparatus, or time spent exploring the center of an open-field apparatus could provide a more-complete understanding of anxiety-like behavior in this model of PD. Further, sub-analyses of different types of USV calls (for example, flat versus frequency-modulated) may be important when assessing relationships between vocalization and anxiety, as measured with these alternative methods.
An additional limitation of the study is the assessment of neural tissue at the 12-month timepoint only. It is possible that genotypic differences in brainstem noradrenergic markers may be evident at different timepoints relative either to further disease progression or to neuroplastic compensation in response to earlier neurobiological changes. It must also be acknowledged that, while unbiased stereology and measurement of relative optical density allow for a high degree of spatial resolution in assessment of neural tissue differences, they may be less sensitive to genotypic differences than more-direct methods of measurement such as high-performance liquid chromatography or western blot analysis. Future studies would benefit from additional quantification methods to solidify understanding of brainstem NE differences.
4.2. Future Directions and Translational Research Implications
The relationship between vocal outcomes and anxiety in humans is also present in animal models of PD, and appears to be influenced by central noradrenergic systems. Tandem assessment of voice and anxiety in both health and disease, especially in PD, is likely to improve management of these signs/symptoms and to increase understanding of their disease-specific etiologies. Given the relationships among anxiety, vocalization, and central noradrenergic systems demonstrated in this study, future research with the Pink1−/− model of PD should investigate vocalization and anxiety during pharmacologic manipulation of norepinephrine. In an effort to guide translation to human populations, assessment of voice and anxiety in convenience samples of patients with PD who are being treated with medications that modify the noradrenergic system should be conducted.
4.3. Conclusions
Vocal deficits and anxiety are related in the Pink1−/− model of PD, and are both influenced by norepinephrine. Future investigations with increased control of anxiety level and noradrenergic manipulation will help to further clarify the disease-specific nature of these relationships.
Figure 3.
Ultrasonic Vocalization. A: Average intensity of calls by genotype and timepoint; B: Average intensity with data collapsed across time to show main effect for genotype. Less-negative value indicates louder call. C: Average peak frequency; D: Average peak frequency data collapsed across time to show main effect for genotype. Pink1−/− calls were significantly lower than WT across timepoints; average peak frequency at 8 months was greater than at 4 months, regardless of genotype. E: Average bandwidth. Bandwidth was significantly smaller at 8 and 12 months than 4 months for both genotypes. F: Duration. Pink1−/− calls were significantly longer than WT calls at the 8-month timepoint only. dB: decibels. Hz: Hertz. * p<0.05. **p<0.01. ***p<0.001. Error bars indicate +/− SEM.
Figure 5.
Relationships between anxiety behavior and ultrasonic vocalization. A: Average intensity by time spent in closed arms. B: Average duration by time spent in closed arms. C: Average peak frequency by time spent in closed arms. D: Average bandwidth by time spent in closed arms. Intensity and duration of Pink1−/− calls were associated with anxiety level, with increases in anxiety correlating to decreases in duration and intensity. Calls of WT rats were less strongly associated with anxiety. dB: decibels. Hz: Hertz. Gray shading indicates 95% confidence interval.
Figure 6.
Cell count estimates of tyrosine hydroxylase and α1R immunoreactivity in the locus coeruleus. A: Representative photomicrograph of TH immunoreactivity in the LC. 40x magnification, scale bar is 50μm, arrow points to immunoreactive cell. B: Cell count estimates for TH+ immunoreactivity. C: Cell count estimates for α1R immunoreactivity. *p<0.05. Light and dark gray bars indicate WT and Pink1−/−, respectively. TH+: positive staining for tyrosine hydroxylase immunoreactivity. α1R: α1 adrenoreceptor immunoreactivity. LC: locus coeruleus. Error bars indicate +/− SEM.
Figure 7:
β1R immunoreactivity. A: Left: Representative photomicrograph of β1R immunoreactivity in the. Right: Cell count estimates in the 10N. B: Left: Representative photomicrograph of β1R in the AMB. Right: Cell count estimates in the AMB. C: Representative photomicrograph of β1R in the NTS. Relative optical density in the NTS. β1R cell count estimates in the 10N and AMB were greater for Pink1−/− rats than for WTs. Relative optical density in the NTS was lower for Pink1−/− rats than for WTs. A and B images at 40x magnification, scale bars are 50μm; C image taken at 10x magnification, scale bar is 500μm. Arrows in A and B point to immunoreactive cells. β1R: β1 adrenoreceptor immunoreactivity. 10N: dorsal motor nucleus of the vagus. NTS: Nucleus of the solitary tract. *p<0.05. Light and dark gray bars indicate WT and Pink1−/−, respectively. Error bars indicate +/− SEM.
Figure 8:
Relationships between β1R immunoreactivity and ultrasonic vocalization intensity and average peak frequency. A: Average intensity by ROD of β1R in the NTS. B: Average intensity by β1R+ cell count estimates in the 10N C: Average intensity by β1R cell count estimates in the AMB. D: Average peak frequency by β1R cell count estimates in the AMB. Increases in cell count estimates in the AMB and 10N were associated with decreases in average intensity, and decreases in ROD in the NTS were associated with increases in intensity. Decreases in cell count estimation in the AMB were associated with decreases in Average peak Frequency. Β1R: β1 adrenoreceptor immunoreactivity. 10N: dorsal motor nucleus of the vagus. AMB: nucleus ambiguus. NTS: Nucleus of the solitary tract. dB: decibels. Hz: Hertz. ROD: relative optical density. Gray shading indicates 95% confidence interval.
Figure 9:
Relationship between anxiety behavior and cell count estimation for TH immunoreactivity in the LC. Greater estimate of TH+ cells in the LC was associated with decreased number of seconds in closed arms of the plus maze, indicating decreased anxiety. TH+: tyrosine hydroxylase immunoreactivity. LC: locus coeruleus. Gray shading indicates 95% confidence interval.
Highlights:
Pink1−/− rats demonstrate more anxiety behaviors than wild type controls, mirroring human Parkinson disease.
Anxiety is correlated with vocal deficits in Pink1−/− rats.
Brainstem noradrenergic markers differ between Pink1−/− rats and wild type controls.
Brainstem noradrenergic markers are correlated with vocal deficits and anxiety.
Funding:
This work was supported by the National Institutes of Health [NIDCD T32 DC009401; NIDCD, R01 DC014358, R01 DC018584-01A1, R21 DC016135-03]
Abbreviations:
- 10N
Dorsal Motor Nucleus of the Vagus
- AMB
nucleus ambiguous
- CI
Confidence interval
- LC
locus coeruleus
- NET
norepinephrine transporter
- NTS
Nucleus of the solitary tract
- PD
Parkinson Disease
- Pink1−/−
PTEN induced putative kinase 1 gene knockout
- ROD
relative optical density
- US
unbiased stereology
- USV
ultrasonic vocalization
- WT
wild type
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- [1].Logemann JA, Fisher HB, Boshes B, Blonsky ER, Frequency and Coocurrence of Vocal Tract Dysfunctions in the Speech of a Large Sample of parkinson Patients, J. Speech Hear. Disord. (1978) 47–57. [DOI] [PubMed] [Google Scholar]
- [2].Sapir S, Ramig L, Fox C, Speech and swallowing disorders in Parkinson disease, Curr. Opin. Otolaryngol. Head Neck Surg. 16 (2008) 205–210. [DOI] [PubMed] [Google Scholar]
- [3].Huber JE, Darling M, Effect of Parkinson’s Disease on the Production of Structured and Unstructured Speaking Tasks: Respiratory Physiologic and Linguistic Considerations, J. Speech, Lang. Hear. Res. 54 (2011) 33–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Fox CM, Ramig LO, Vocal Sound Pressure Level and Self-Perception of Speech and Voice in Men and Women With Idiopathic Parkinson Disease, Am. J. Speech-Language Pathol. 6 (1997) 85–94. [Google Scholar]
- [5].Anand S, Stepp CE, Listener Perception of Monopitch, Naturalness, and Intelligibility for Speakers With Parkinson ‘ s Disease, J. Speech, Lang. Hear. Res. 58 (2015) 1134–1144. 10.1044/2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Matheron D, Stathopoulos ET, Huber JE, Sussman JE, Laryngeal Aerodynamics in Healthy Older Adults and Adults With Parkinson ‘ s Disease, J. Speech, Lang. Hear. Res. 60 (2017) 507–524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Stepp CE, Relative fundamental frequency during vocal onset and offset in older speakers with and without Parkinson’s disease, J. Acoust. Soc. Am. 133 (2013) 1637–1643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Miller N, Noble E, Jones D, Burn D, Life with communication changes in Parkinson ‘ s, Age Ageing. 35 (2006) 235–239. 10.1093/ageing/afj053. [DOI] [PubMed] [Google Scholar]
- [9].Barone P, Antonini A, Colosimo C, Marconi R, Morgante L, Avarello TP, Bottacchi E, Cannas A, Ceravolo G, Ceravolo R, Cicarelli G, Gaglio RM, Giglia RM, Iemolo F, Manfredi M, Meco G, Nicoletti A, Pederzoli M, Petrone A, Pisani A, The Priamo Study: A Multicenter Assessment of Nonmotor Symptoms and Their Impact on Quality of Life in Parkinson’s Disease, Mov. Disord. 24 (2009) 1641–1649. 10.1002/mds.22643. [DOI] [PubMed] [Google Scholar]
- [10].Martinez-Martin P, Rodriguez-blazquez C, Kurtis MM, Chaudhuri KR, The Impact of Non-Motor Symptoms on Health-Related Quality of Life of Patients with Parkinson’s Disease, Mov. Disord. 26 (2011) 399–406. 10.1002/mds.23462. [DOI] [PubMed] [Google Scholar]
- [11].Lirani-Silva C, Mourao LF, Gobbi LTB, Dysarthria and Quality of Life in neurologically healthy elderly and patients with Parkinson’s disease, Commun. Disord. Audiol. Swallowing. 27 (2015) 248–254. 10.1590/2317-1782/20152014083. [DOI] [PubMed] [Google Scholar]
- [12].Ramig L, Halpern A, Spielman J, Fox C, Freeman K, Speech Treatment in Parkinson’s Disease: Randomized Controlled Trial (RCT), Mov. Disord. 33 (2018) 1777–1791. 10.1002/mds.27460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Mahler LA, Ramig LO, Fox C, Evidence-based treatment of voice and speech disorders in Parkinson disease, Curent Opin. Otolaryngol. Head Neck Surg. 23 (2015) 209–215. 10.1097/MOO.0000000000000151. [DOI] [PubMed] [Google Scholar]
- [14].Pinho P, Monteiro L, Soares FMDP, Tourinho L, Melo A, Nobrega AC, Impact of levodopa treatment in the voice pattern of Parkinson ‘ s disease patients : a systematic review and meta-analysis O impacto do tratamento com levodopa na, CoDAS. 30 (2018) 1–7. 10.1590/2317-1782/20182017200. [DOI] [PubMed] [Google Scholar]
- [15].Sanabria J, Ruiz PG, Gutierrez R, Marquez F, Escobar P, Gentil M, Cenjor C, The Effect of Levodopa on Vocal Function in Parkinson’s Disease, Clin. Neuropharmacol. 24 (2001) 99–102. [DOI] [PubMed] [Google Scholar]
- [16].Wolfe VI, Garvin JS, Bacon M, Waldrop W, SPEECH CHANGES IN PARKINSON’S DISEASE DURING TREATMENT WITH L-DOPA, J. Commun. Disord. 8 (1975) 271–279. [DOI] [PubMed] [Google Scholar]
- [17].Yamanishi T, Tachibana H, Oguru M, Matsui K, Toda K, Okuda B, Oka N, Anxiety and Depression in Patients with Parkinson’s Disease, Intern. Med. 52 (2013) 539–545. 10.2169/internalmedicine.52.8617. [DOI] [PubMed] [Google Scholar]
- [18].Broen MPG, Narayen NE, Kuijf ML, Dissanayaka NNW, Leentjens AFG, Prevalence of Anxiety in Parkinson’s Disease : A Systematic Review and Meta-Analysis, Mov. Disord. 31 (2016) 1125–1133. 10.1002/mds.26643. [DOI] [PubMed] [Google Scholar]
- [19].Renfroe JB, Turner TH, Hinson VK, Prevalence, impact, and management of depression and anxiety in patients with Parkinson ‘ s disease, J. park. Restless Legs Syndr. 6 (2016) 15–22. [Google Scholar]
- [20].Carod-artal FJ, Ziomkowski S, Mourao Mesquita H, Martinez-Martin P, Anxiety and depression: Main determinants of health-related quality of life in Brazilian patients with Parkinson’s disease, Park. Relat. Disord. 14 (2008) 102–108. 10.1016/j.parkreldis.2007.06.011. [DOI] [PubMed] [Google Scholar]
- [21].Calleo JS, Amspoker AB, Sarwar AI, Kunik ME, Jankovic J, Marsh L, York M, Stanley MA, A Pilot Study of a Cognitive–Behavioral Treatment for Anxiety and Depression in Patients With Parkinson Disease, J. Geriatr. Psychiatry Neurol. 28 (2015) 210–217. 10.1177/0891988715588831. [DOI] [PubMed] [Google Scholar]
- [22].Weintraub D, Mavandadi S, Mamikonyan E, Siderowf AD, Duda JE, Hurtig HI, Colcher A, Horn SS, Navem S, Ten Have TR, Stern MB, Atomoxetine for depression and other neuropsychiatric symptoms in Parkinson disease, Neurology. 75 (2010) 448–455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Misono S, Peterson CB, Meredith L, Banks K, Bandyopadhyay D, Yueh B, Frazier PA, Psychosocial Distress in Patients Presenting With Voice Concerns, J. Voice. 28 (2014) 753–761. 10.1016/j.jvoice.2014.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].van Mersbergen M, Lyons P, Riegler D , Vocal Responses in Heighted States of Arousal, J. Voice. (2015). 10.1016/j.jvoice.2015.12.011. [DOI] [PubMed] [Google Scholar]
- [25].Manor Y, Balas M, Giladi N, Mootanah R, Cohen JT, Anxiety, depression and swallowing disorders in patients with Parkinson’s disease, Park. Relat. Disord. 15 (2009) 453–456. 10.1016/j.parkreldis.2008.11.005. [DOI] [PubMed] [Google Scholar]
- [26].Helou LB, Wang W, Ashmore RC, Rosen CA, Abbott KV, Intrinsic Laryngeal Muscle Activity in Response to Autonomic Nervous System Activation, Laryngoscope. 123 (2013) 2756–2765. 10.1002/lary.24109. [DOI] [PubMed] [Google Scholar]
- [27].Macpherson MK, Abur D, Stepp CE, Acoustic Measures of Voice and Physiologic Measures of Autonomic Arousal during Speech as a Function of Cognitive Load, J. Voice. 31 (2017) 504.e1–504.e9. 10.1016/j.jvoice.2016.10.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].van Mersbergen M, Patrick C, Glaze L, Functional Dysphonia During Mental Imagery: Testing the Trait Theory, J. Speech Lang. Hear. Res. 51 (2008) 1405–1423. [DOI] [PubMed] [Google Scholar]
- [29].Kalia M, Fuxe K, Goldstein M, Harfstrand A, Agnati L, Coyle J, Evidence for the existence of a putative dopamine-,adrenaline- and noradrenaline-containing vagal motor neurons in the brainstem of the rat, Neurosci. Lett. 50 (1984) 57–62. [DOI] [PubMed] [Google Scholar]
- [30].Samuels ER, Szabadi E, Functional Neuroanatomy of the Noradrenergic Locus Coeruleus: Its Roles in the Regulation of Arousal and Autonomic Function Part I: Principles of Functional Organisation, Curr. Neuropharmacol. 6 (2008) 235–253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].George MS, Ward HE, Ninan PT, Pollack M, Nahas Z, Anderson B, Kose S, Howland RH, Goodman WK, Ballenger JC, A pilot study of vagus nerve stimulation (VNS) for treatment-resistant anxiety disorders, Brain Stimul. 1 (2008) 112–121. 10.1016/j.brs.2008.02.001. [DOI] [PubMed] [Google Scholar]
- [32].Wang X, Byrne P, Mendelowitz D, Optogenetic Stimulation of Locus Ceruleus Neurons Augments Inhibitory Transmission to Parasympathetic Cardiac Vagal Neurons via Activation of Brainstem alpha1 and beta1 Receptors, J. Neurosci. 34 (2014) 6182–6189. 10.1523/JNEUROSCI.5093-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Mather M, Yoo HJ, V Clewett D, Lee T, Greening SG, Ponzio A, Min J, Thayer JF, Higher locus coeruleus MRI contrast is associated with lower parasympathetic influence over heart rate variability, Neuroimage. 150 (2017) 329–335. 10.1016/j.neuroimage.2017.02.025.Higher. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Benarroch EE, Locus coeruleus, Cell Tissue Res. 373 (2018) 221–232. 10.1007/s00441-017-2649-1. [DOI] [PubMed] [Google Scholar]
- [35].Ludlow CL, Central Nervous System Control of Voice and Swallowing, J. Clin. Neurophysiol. 32 (2015) 294–303. 10.1097/WNP.0000000000000186.Central. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Petko B, Tadi P, Neuroanatomy, Nucleus Ambiguus, StatPearls, Treasure Island, Florida, 2020. https://www.ncbi.nlm.nih.gov/books/NBK547744/ (accessed January 21, 2021). [Google Scholar]
- [37].Travagli RA, Hermann GE, Browning KN, Rogers RC, Brainstem Circuits Regulating Gastric Function, Annu. Rev. Physiol. 68 (2008) 279–305. 10.1146/annurev.physiol.68.040504.094635.Brainstem. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Cash R, Ruberg M, Raisman R, Agid Y, Adrenergic receptors in Parkinson’s disease, Brain Res. 322 (1984) 269–275. 10.1016/0006-8993(84)90117-3. [DOI] [PubMed] [Google Scholar]
- [39].Kelm-Nelson CA, Trevino A, Ciucci MR, Quantitative Analysis of Catecholamines in the Pink1 −/− Rat Model of Early-onset Parkinson’s Disease, Neuroscience. 379 (2018) 126–141. 10.1016/j.neuroscience.2018.02.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Grant LM, Kelm-Nelson CA, Hilby BL, V Blue K, Rajamanickam ESP, Pultorak JD, Fleming SM, Ciucci MR, Evidence for Early and Progressive Ultrasonic Vocalization and Oromotor Deficits in a PINK1 Gene Knockout Rat Model of Parkinson’s Disease, J. Neurosci. Res. 93 (2015) 1713–1727. 10.1002/jnr.23625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Braak RH, Ghebremedhin E, Rüb U, Bratzke H, Del Tredici K, Stages in the development of Parkinson’s disease-related pathology, Cell Tissue Res. 318 (2004) 121–134. 10.1007/s00441-004-0956-9. [DOI] [PubMed] [Google Scholar]
- [42].Bernheimer H, Birkmayer W, Hornykiewicz O, Jellinger K, Seitelberger F, Brain Dopamine and the Syndromes of Parkinson and Huntington: Clinical, Morphological and Neurochemical Correlations, J. Neurol. Sci. 20 (1973) 415–455. [DOI] [PubMed] [Google Scholar]
- [43].Brabenec L, Mekyska J, Galaz Z, Rektorova I, Speech disorders in Parkinson’s disease: early diagnostics and effects of medication and brain stimulation, J. Neural Transm. 124 (2017) 303–334. 10.1007/s00702-017-1676-0. [DOI] [PubMed] [Google Scholar]
- [44].Plowman-Prine EK, Okun MS, Sapienza CM, Shrivastav R, Fernandez HH, Foote KD, Ellis C, Rodriguez AD, Burkhead LM, Rosenbek JC, Perceptual characteristics of Parkinsonian speech: a comparison of the pharmacological effects of levodopa across speech and non-speech motor systems., NeuroRehabilitation. 24 (2009) 131–44. 10.3233/NRE-2009-0462. [DOI] [PubMed] [Google Scholar]
- [45].Schulz GM, Grant MK, Effects of speech therapy and pharmacologic and surgical treatments on voice and speech in Parkinson’s disease: a review of the literature., J. Commun. Disord. 33 (2000) 59–88. [DOI] [PubMed] [Google Scholar]
- [46].The Parkinson Study Group, Levodopa and the Progression of Parkinson’s Disease, N. Engl. J. Med. 351 (2004) 2498–2508. [DOI] [PubMed] [Google Scholar]
- [47].Rommelfanger KS, Weinshenker D, Norepinephrine : The redheaded stepchild of Parkinson’s disease, Biochem. Pharmacol. 74 (2007) 177–190. 10.1016/j.bcp.2007.01.036. [DOI] [PubMed] [Google Scholar]
- [48].Del Tredici K, Braak H, Dysfunction of the locus coeruleus – norepinephrine system and related circuitry in Parkinson’s disease-related dementia, J. Neurol. Neurosurg. Psychiatry. 84 (2013) 774–783. 10.1136/jnnp-2011-301817. [DOI] [PubMed] [Google Scholar]
- [49].Espay AJ, Lewitt PA, Kaufmann H, Norepinephrine Deficiency in Parkinson’s Disease: The Case for Noradrenergic Enhancement Norepinephrine, Mov. Disord. 29 (2014) 1710–1719. 10.1002/mds.26048. [DOI] [PubMed] [Google Scholar]
- [50].Vazey EM, Aston-Jones G, The emerging role of norepinephrine in cognitive dysfunctions of Parkinson’s disease, Front. Behav. Neurosci. 6 (2012) 1–6. 10.3389/fnbeh.2012.00048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Lewitt PA, Norepinephrine: the next therapeutics frontier for Parkinson’s disease, Transl. Neurodegener. 1 (2012) 1–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].Marien MR, Colpaert FC, Rosenquist AC, Noradrenergic mechanisms in neurodegenerative diseases: a theory, Brain Res. Rev. 45 (2004) 38–78. 10.1016/j.brainresrev.2004.02.002. [DOI] [PubMed] [Google Scholar]
- [53].Buddhala C, Loftin SK, Kuley BM, Cairns NJ, Meghan C, Perlmutter JS, Kotzbauer PT, Dopaminergic, serotonergic, and noradrenergic deficits in Parkinson disease, Ann. Clin. Transl. Neurol. 2 (2015) 949–959. 10.1002/acn3.246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54].Kreiner G, Rafa-za K, Barut J, Chmielarz P, Kot M, Bagin M, Parlato R, Daniel WA, Nalepa I, Stimulation of noradrenergic transmission by reboxetine is beneficial for a mouse model of progressive parkinsonism, Sci. Rep. 9 (2019) 1–9. 10.1038/s41598-019-41756-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [55].Cash R, Dennis T, L’Heureux R, Raisman R, Javoy-Agid F, Scatton B, Parkinson’s disease and dementia: norepinephrine and dopamine in locus ceruleus., Neurology. 37 (1987) 42–44. [DOI] [PubMed] [Google Scholar]
- [56].Kano O, Ikeda K, Cridebring D, Takazawa T, Yoshii Y, Iwasaki Y, Neurobiology of Depression and Anxiety in Parkinson’s Disease, Parkinsons. Dis. 2011 (2011) 1–5. 10.4061/2011/143547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [57].Faivre F, Joshi A, Bezard E, Barrot M, The hidden side of Parkinson’s disease: Studying pain, anxiety and depression in animal models, Neurosci. Biobehav. Rev. 96 (2019) 335–352. 10.1016/j.neubiorev.2018.10.004. [DOI] [PubMed] [Google Scholar]
- [58].Pont-sunyer C, Hotter A, Gaig C, Seppi K, The Onset of Nonmotor Symptoms in Parkinson’s Disease (The ONSET PD Study), Mov. Disord. 30 (2015) 229–237. 10.1002/mds.26077. [DOI] [PubMed] [Google Scholar]
- [59].Shiba M, Bower JH, Maraganore DM, Mcdonnell SK, Peterson BJ, Ahlskog JE, Schaid DJ, Rocca WA, Anxiety Disorders and Depressive Disorders Preceding Parkinson ‘ s Disease : A Case-Control Study, Mov. Disord. 15 (2000) 669–677. [DOI] [PubMed] [Google Scholar]
- [60].Harel BT, Cannizzaro MS, Cohen H, Reilly N, Snyder PJ, Acoustic characteristics of Parkinsonian speech: a potential biomarker of early disease progression and treatment, J. Neurolinguistics. 17 (2004) 439–453. 10.1016/j.jneuroling.2004.06.001. [DOI] [Google Scholar]
- [61].Dehay B, Bezard E, New Animal Models of Parkinson’s Disease, Mov. Disord. 26 (2011) 1198–1205. 10.1002/mds.23546. [DOI] [PubMed] [Google Scholar]
- [62].Kelm-Nelson CA, Yang KM, Ciucci MR, Exercise Effects on Early Vocal Ultrasonic Communication Dysfunction in a PINK1 Knockout MOdel of Parkinson’s Disease, J. Park. Dis. 5 (2015) 749–763. 10.3233/JPD-150688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [63].Wright JM, Dobosiewicz MRS, Clarke PBS, Alpha- and Beta- Adrenergic Receptors Differentially Modulate the Emission of Spontaneous and Amphetamine-Induced 50-kHz Ultrasonic Vocalizations in Adult Rats, Neuropsychopharmacology. 37 (2012) 808–821. 10.1038/npp.2011.258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [64].Grant LM, Barth KJ, Muslu C, Kelm-nelson CA, Bakshi VP, Ciucci MR, Noradrenergic Receptor Modulation Influences the Acoustic Parameters of Pro-Social Rat Ultrasonic Vocalizations, Behav. Neurosci. 132 (2018) 269–283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [65].National A Research Council Committee for the Update of the Guide for the Care and Use of Laboratory, Guide for the care and use of laboratory animals, 2011. [Google Scholar]
- [66].Marquis JM, Lettenberger SE, Kelm-Nelson CA, Early-onset Parkinsonian behaviors in female Pink1−/− rats, Behav. Brain Res. 377 (2020) 1–15. 10.1016/j.bbr.2019.112175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [67].Dave KD, De Silva S, Sheth NP, Ramboz S, Beck MJ, Quang C, Switzer RC, Ahmad SO, Sunkin SM, Walker D, Cui X, Fisher DA, McCoy AM, Gamber K, Ding X, Goldberg MS, Benkovic SA, Haupt M, Baptista MAS, Fiske BK, Sherer TB, Frasier MA, Phenotypic characterization of recessive gene knockout rat models of Parkinson’s disease, Neurobiol. Dis. 70 (2014) 190–203. 10.1016/j.nbd.2014.06.009. [DOI] [PubMed] [Google Scholar]
- [68].Willadsen M, seffer D, Schwarting RKW, Wöhr M, Rodent ultrasonic communication: Male prosocial 50-khz ultrasonic vocalizations elicit social approach behavior in female rats (rattus norvegicus), J. Comp. Psychol. 128 (2014) 56–64. 10.1037/a0034778. [DOI] [PubMed] [Google Scholar]
- [69].Pultorak JD, Kelm-Nelson CA, Holt LR, V Blue K, Ciucci MR, Johnson AM, Decreased approach behavior and nucleus accumbens immediate early gene expression in response to Parkinsonian ultrasonic vocalizations in rats, Soc. Neurosci. 11 (2016) 365–379. 10.1080/17470919.2015.1086434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [70].Bialy M, Rydz M, Kaczmarek L, Precontact 50-kHz vocalizations in male rats during acquisition of sexual experience., Behav. Neurosci. 114 (2000) 983–990. 10.1037/0735-7044.114.5.983. [DOI] [PubMed] [Google Scholar]
- [71].Blanchard RJ, Agullana R, McGee L, Weiss S, Blanchard DC, Sex differences in the incidence and sonographic characteristics of antipredator ultrasonic cries in the laboratory rat (Rattus norvegicus)., J. Comp. Psychol. 106 (1992) 270–277. 10.1037/0735-7036.106.3.270. [DOI] [PubMed] [Google Scholar]
- [72].Brudzynski SM, Pniak A, Social contacts and production of 50-kHz short ultrasonic calls in adult rats., J. Comp. Psychol. 116 (2002) 73–82. [DOI] [PubMed] [Google Scholar]
- [73].Riede T, Rat ultrasonic vocalization shows features of a modular behavior, J. Neurosci. 34 (2014) 6874–6878. 10.1523/JNEUROSCI.0262-14.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [74].McGinnis MY, Vakulenko M, Characterization of 50-kHz ultrasonic vocalizations in male and female rats, Physiol. Behav. 80 (2003) 81–88. 10.1016/S0031-9384(03)00227-0. [DOI] [PubMed] [Google Scholar]
- [75].Wöhr M, Houx B, Schwarting RKW, Spruijt B, Effects of experience and context on 50-kHz vocalizations in rats, Physiol. Behav. 93 (2008) 766–776. 10.1016/J.PHYSBEH.2007.11.031. [DOI] [PubMed] [Google Scholar]
- [76].Ciucci M, Ma TS, Fox C, Kane J, Ramig L, Schallert T, Qualitative changes in ultrasonic vocalization in rats after unilateral dopamine depletion or haloperidol: A preliminary study, Behav. Brain Res. 182 (2007) 284–289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [77].Ahrens A, Ma S, Maier E, Duvauchelle C, Schallert T, Robinson T, Repeated Intravenous Amphetamine Exposure: Rapid and Persistent Sensitization of 50-kHz Ultrasonic Trill Calls in Rats, Behav. Brain Res. 197 (2009) 205–209. 10.1016/j.bbr.2008.08.037.Repeated. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [78].Johnson A, Doll E, Grant L, Ringle L, Shier J, Ciucci M, Targeted Training of Ultrasonic Vocalizations in Aged and Parkinsonian Rats., J. Vis. Experments. 54 (2011). 10.3791/2835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [79].Kelm-Nelson CA, Brauer AFL, Ciucci MR, Vocal training, levodopa, and environment effects on ultrasonic vocalizations in a rat neurotoxin model of Parkinson disease, Behav. Brain Res. 307 (2016) 54–64. 10.1016/j.bbr.2016.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [80].Johnson AM, Ciucci MR, Connor NP, Vocal training mitigates age-related changes within the vocal mechanism in old rats, Journals Gerontol. - Ser. A Biol. Sci. Med. Sci. 68 (2013) 1458–1468. 10.1093/gerona/glt044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [81].Walf AA, Frye CA, The use of the elevated plus maze as an assay of anxiety-related behavior in rodents, Nat. Protoc. 2 (2007) 322–328. 10.1038/nprot.2007.44.The. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [82].Pellow S, Chopin P, File SE, Briley M, Validation of open : closed arm entries in an elevated plus-maze as a measure of anxiety in the rat, J. Neurosci. Methods. 14 (1985) 149–167. 10.1016/0165-0270(85)90031-7. [DOI] [PubMed] [Google Scholar]
- [83].Hogg S, A review of the validity and variability of the elevated plus- maze as an animal model of anxiety, Pharmacol. Biochem. Behav. 54 (1996) 21–30. [DOI] [PubMed] [Google Scholar]
- [84].Hopkins ME, Bucci DJ, Interpreting the effects of exercise on fear conditioning: The influence of time of day., Behav. Neurosci. 124 (2010) 868–872. 10.1037/a0021200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [85].Smith HR, Beveridge TJR, Porrino LJ, Distribution of norepinephrine transporters in the non-human primate brain, Neuroscience. 138 (2006) 703–714. 10.1016/j.neuroscience.2005.11.033. [DOI] [PubMed] [Google Scholar]
- [86].Ghosh A, Torraville SE, Mukherjee B, Walling SG, Martin GM, Harley CW, Yuan Q, An experimental model of Braak’s pretangle proposal for the origin of Alzheimer’s disease: The role of locus coeruleus in early symptom development, Alzheimer’s Res. Ther. 11 (2019) 1–17. 10.1186/s13195-019-0511-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [87].Wee S, Mandyam CD, Lekic DM, Koob GF, α1-Noradrenergic system role in increased motivation for cocaine intake in rats with prolonged access, Eur. Neuropsychopharmacol. 18 (2008) 303–311. 10.1016/j.euroneuro.2007.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [88].West MJ, Slomianka L, Gundersen HJG, Unbiased stereological estimation of the total number of neurons in the subdivisions of the rat hippocampus using the optical fractionator, Anat. Rec. 231 (1991) 482–497. 10.1002/ar.1092310411. [DOI] [PubMed] [Google Scholar]
- [89].Kelm-Nelson CA, Brauer AFL, Barth KJ, Lake JM, Sinnen MLK, Stehula FJ, Muslu C, Marongiu R, Kaplitt MG, Ciucci MR, Characterization of early-onset motor deficits in the Pink1−/− mouse model of Parkinson disease, Brain Res. 1680 (2018) 1–12. 10.1016/j.brainres.2017.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [90].Paxinos G, Watson C, The rat brain in stereotaxic coordinates, Burlington, MA, 2005. [DOI] [PubMed] [Google Scholar]
- [91].Grigoruta M, Martinez-Martinez A, Dagda RY, D.R. K, Molecular Neurodegeneration Psychological stress phenocopies brain mitochondrial dysfunction and motor deficits as observed in a Parkinsonian rat model, Mol. Neurobiol. (2019) 1–18. 10.1007/s12035-019-01838-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [92].Lafaille M, Féron C, U-shaped relationship between ageing and risk-taking behaviour in a wild-type rodent, Anim. Behav. 97 (2014) 45–52. 10.1016/j.anbehav.2014.08.018. [DOI] [Google Scholar]
- [93].Pontone GM, Williams JR, Anderson KE, Chase G, Goldstein SA, Grill S, Hirsch ES, Lehmann S, Little JT, Margolis RL, Rabins PV, Weiss HD, Marsh L, Prevalence of anxiety disorders and anxiety subtypes in patients with Parkinson’s disease, Mov. Disord. 24 (2009) 1333–1338. 10.1002/mds.22611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [94].Dissanayaka NNW, Sellbach A, Matheson S, Sullivan JDO, Silburn PA, Byrne GJ, Marsh R, Mellick GD, Anxiety Disorders in Parkinson’s Disease: Prevalence and Risk Factors, Mov. Disord. 25 (2010) 838–845. 10.1002/mds.22833. [DOI] [PubMed] [Google Scholar]
- [95].Johnson RA, Kelm-Nelson CA, Ciucci MR, Changes to Ventilation, Vocalization, and Thermal Nociception in the Pink1−/−Rat Model of Parkinson’s Disease. (Preprint), 1–16., J. Parkinsons. Dis. 10 (2020) 489–504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [96].van Mersbergen M, Lanza E, Modulation of Relative Fundamental Frequency During Transient Emotional States, J. Voice. (2018). 10.1016/j.jvoice.2018.07.020. [DOI] [PubMed] [Google Scholar]
- [97].van Mersbergen M, Delany M, Vocal responses to emotional picture viewing, Logop. Phoniatr. Vocology. 39 (2014) 99–107. 10.3109/14015439.2013.777108. [DOI] [PubMed] [Google Scholar]
- [98].Dietrich M, Andreatta RD, Jiang Y, Stemple JC, Limbic and cortical control of phonation for speech in response to a public speech preparation stressor, Brain Imaging Behav. Online Ahe (2019). 10.1007/s11682-019-00102-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [99].Bodie GD, A racing heart, rattling knees, and ruminative thoughts: Defining, explaining, and treating public speaking anxiety, Commun. Educ. 59 (2010) 70–105. 10.1080/03634520903443849. [DOI] [Google Scholar]
- [100].Shaw TA, Juncos DG, Winter D, Piloting a New Model for Treating Music Performance Anxiety: Training a Singing Teacher to Use Acceptance and Commitment Coaching With a Student, Front. Psychol. 11 (2020) 1–14. 10.3389/fpsyg.2020.00882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [101].Glassman LH, Forman EM, Herbert JD, Bradley LE, Foster EE, Izzetoglu M, Ruocco AC, The Effects of a Brief Acceptance-Based Behavioral Treatment Versus Traditional Cognitive-Behavioral Treatment for Public Speaking Anxiety: An Exploratory Trial Examining Differential Effects on Performance and Neurophysiology, Behav. Modif. 40 (2016) 748–776. 10.1177/0145445516629939. [DOI] [PubMed] [Google Scholar]
- [102].Braak H, Del Tredici K, Rub U, de Vos RAI, Jansen Steur ENH, Braak E, Staging of brain pathology related to sporadic Parkinson’s disease., Neurobiol. Aging. 24 (2003) 197–211. [DOI] [PubMed] [Google Scholar]
- [103].Sharp SI, Ballard CG, Chen CPLH, Francis PT, Aggressive behavior and neuroleptic medication are associated with increased number of alpha1-adrenoceptors in patients with alzheimer disease, Am. J. Geriatr. Psychiatry. 15 (2007) 435–437. 10.1097/01.JGP.0000237065.78966.1b. [DOI] [PubMed] [Google Scholar]
- [104].Zuscik MJ, Sands S, Ross SA, Waugh DJJ, Gaivin RJ, Morilak D, Perez DM, Overexpression of the α1B-adrenergic receptor causes apoptotic neurodegeneration: Multiple system atrophy, Nat. Med. 6 (2000) 1388–1394. 10.1038/82207. [DOI] [PubMed] [Google Scholar]
- [105].Kobler JB, Datta S, Goyal RK, Benecchi EJ, Innervation of the larynx, pharynx, and upper esophageal sphincter of the rat, J. Comp. Neurol. 349 (1994) 129–147. 10.1002/cne.903490109. [DOI] [PubMed] [Google Scholar]
- [106].Hamed A, Kursa MB, Inter-individual differences in serotonin and glutamate co-transmission reflect differentiation in context-induced conditioned 50-kHz USVs response after morphine withdrawal, Brain Struct. Funct. 233 (2018) 3149–3167. 10.1007/s00429-018-1683-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [107].Hamed A, Kursa MB, Social deprivation substantially changes multi-structural neurotransmitter signature of social interaction: Glutamate concentration in amygdala and VTA as a key factor in social encounter-induced 50-kHz ultrasonic vocalization, Eur. Neuropsychopharmacol. 37 (2020) 82–99. 10.1016/j.euroneuro.2020.06.010. [DOI] [PubMed] [Google Scholar]
- [108].Costa G, Morelli M, Simola N, Involvement of glutamate NMDA receptors in the acute, long-term, and conditioned effects of amphetamine on rat 50 kHz ultrasonic vocalizations, Int. J. Neuropsychopharmacol. 18 (2015) 1–12. 10.1093/ijnp/pyv057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [109].Simola N, Costa G, Morelli M, Activation of adenosine A2A receptors suppresses the emission of pro-social and drug-stimulated 50-kHz ultrasonic vocalizations in rats: Possible relevance to reward and motivation, Psychopharmacology (Berl). 233 (2016) 507–519. 10.1007/s00213-015-4130-8. [DOI] [PubMed] [Google Scholar]
- [110].Wöhr M, Van Gaalen MM, Schwarting RKW, Affective communication in rodents: Serotonin and its modulating role in ultrasonic vocalizations, Behav. Pharmacol. 26 (2015) 506–521. 10.1097/FBP.0000000000000172. [DOI] [PubMed] [Google Scholar]
- [111].Spielberger CD, The effects of anxiety on complex learning and academic achievement, in: Spielberge CD (Ed.), Anxiety Behav, Academic Press, New York, New York, 1966: pp. 361–398. [Google Scholar]
- [112].Endler NS, Kocovski NL, State and trait anxiety revisited, J. Anxiety Disord. 15 (2001) 231–245. 10.1016/S0887-6185(01)00060-3. [DOI] [PubMed] [Google Scholar]
- [113].Saviola F, Pappaianni E, Monti A, Grecucci A, Jovicich J, De Pisapia N, Trait and state anxiety are mapped differently in the human brain, Sci. Rep. 10 (2020) 1–11. 10.1038/s41598-020-68008-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [114].Mondolo F, Jahanshahi M, Grana A, Biasutti E, Cacciatori E, Benedetto P, Evaluation of anxiety in Parkinson’s disease with some commonly used rating scales, Neurol. Sci. 28 (2007) 270–275. 10.1007/s10072-007-0834-9. [DOI] [PubMed] [Google Scholar]
- [115].Goes TC, Antunes FD, Teixeira-Silva F, Trait and state anxiety in animal models: Is there correlation?, Neurosci. Lett. 450 (2009) 266–269. 10.1016/j.neulet.2008.11.037. [DOI] [PubMed] [Google Scholar]