Abstract
The gene expression program induced by NRF2 transcription factor plays a critical role in cell defense responses against a broad variety of cellular stresses, most importantly oxidative stress. NRF2 stability is fine-tuned regulated by KEAP1, which drives its degradation in the absence of oxidative stress. In the context of cancer, NRF2 cytoprotective functions were initially linked to anti-oncogenic properties. However, in the last few decades, growing evidence indicates that NRF2 acts as a tumor driver, inducing metastasis and resistance to chemotherapy. Constitutive activation of NRF2 has been found to be frequent in several tumors, including some lung cancer sub-types and it has been associated to the maintenance of a malignant cell phenotype. This apparently contradictory effect of the NRF2/KEAP1 signaling pathway in cancer (cell protection against cancer versus pro-tumoral properties) has generated a great controversy about its functions in this disease. In this review, we will describe the molecular mechanism regulating this signaling pathway in physiological conditions and summarize the most important findings related to the role of NRF2/KEAP1 in lung cancer. The focus will be placed on NRF2 activation mechanisms, the implication of those in lung cancer progression and current therapeutic strategies directed at blocking NRF2 action.
Keywords: NRF2 activation, NSCLC, therapeutic strategies
1. Introduction
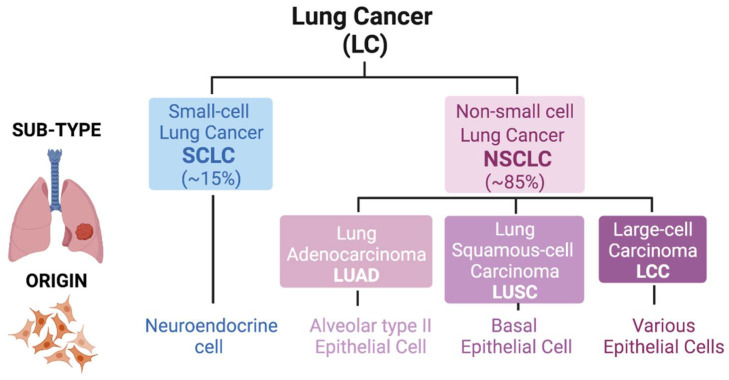
Lung cancer, one of the most commonly diagnosed malignancies, has some of the lowest 5-year survival rates and is responsible for around 20% of all cancer-related deaths worldwide [1,2]. Histologically, this malignancy is classified into two groups: small-cell lung cancer (SCLC) and non-small-cell lung cancer (NSCLC) (the latter being the most frequent, around 85%). In small-cell lung cancer, the tumor derives from cells of the neuroendocrine lineage upon loss of RB and TP53, whereas in non-small sub-types, the tumor originates from lung epithelia after distinct genetic events. NSCLC is further divided into three sub-types based mainly on the morphology of the transformed cells: adenocarcinoma (LUAD), squamous-cell carcinoma (LUSC) and large-cell carcinoma (LCC) [3]. The observation that LUAD typically arises in the distal lung, whereas LUSC arises centrally, probably reflects different cells-of-origin for these two lung cancer sub-types [4]. It is widely accepted that LUAD develops from alveolar type II (AT2) epithelial cells or cells within bronchioalveolar duct junctions, whereas LUSC develops from basal epithelial cells in airways [5]. LUSC differentiates into a stratified squamous epithelium that is not found in non-keratinizing epithelia. The third NSCLC sub-type, LCC, also originates from lung epithelial cells and represents a heterogeneous group of malignant neoplasms that lack the cytological and architectural characteristics of small cell and glandular carcinoma (LUAD) or squamous (LUSC) sub-types. All forms of lung cancer have poor prognosis, particularly SCLC and LUSC, which are typically observed in tobacco smokers [6]. LCC has a relatively better prognosis (depends on sub-type). While LUAD already has several available targeted therapies, SCLC has some therapies currently under study [7]. In the case of LUSC, there is an urgent need for development of targeted therapies (Figure 1).
Figure 1.
Classification of lung cancer. Lung tumors are divided into two main groups: Small-cell Lung Cancer (SCLC; ≈15% of cases) and Non-small cell Lung Cancer (NSCLC; ≈85% of cases). An SCLC tumor derives from neuroendocrine cell linage. However, in NSCLC, the tumor origin cell is different, being classified into three different sub-types: Adenocarcinoma (LUAD; alveolar type II epithelial cell), Squamous-cell Carcinoma (LUSC; basal epithelial cell) and Large-cell Carcinoma (LCC; various epithelial cells).
During the past half-century, different bioinformatic and next-generation sequencing analyses of data from large patient cohorts have permitted the identification of key genes involved in the generation and progression of many tumor types [8,9]. In this context, the Cancer Genome Atlas (TCGA) consortium and other projects have found that some genes related to antioxidant regulatory mechanisms, including the genes encoding for NRF2, NFE2L2 (nuclear factor erythroid 2-related factor 2) and its negative regulator, KEAP1 (Kelch-like ECH-associated Protein 1), are altered in several lung cancers [10,11,12]. In particular, gain-of-function (GOF) mutations of NFE2L2 or loss-of-function (LOF) mutations of KEAP1 are frequent in NSCLC tumors [13]. Indeed, KEAP1 mutations are frequently found in LUAD (17%) and LUSC (12%), whereas NFE2L2 mutations are more common in LUSC (15%) compared to LUAD (3%) [10]. Moreover, 26% of NSCLC tumors present high expression of nuclear NRF2 [14], with a higher incidence in the LUSC sub-type [10]. In LCC, this mutational phenomenon in NFE2L2/KEAP1 is less common [15,16,17].
Based on initial studies, NRF2 was first considered a tumor suppressor gene, since a number of studies in other cancer types (i.e., colon, melanoma) and in mice showed that NFE2L2 deficiency increases the susceptibility to cancer [18,19,20]. More recently, the linkage of GOF alterations in NFE2L2 with cancer progression and an inefficient response to classical cancer treatments (radiotherapy and chemotherapy) in tumors with active NRF2 [21,22,23] support that NRF2 might act as a tumor driver in several cancer sub-types (particularly in LUAD and LUSC). This dual role of NRF2 as tumor suppressor or driver, depending on the tumor type or stage, has given rise to a substantial debate about its role in cancer [13].
In this review, we will describe how this signaling pathway works in physiological conditions and summarize the most important findings related to the role of NRF2/KEAP1 in LUAD and LUSC, focusing on their activation mechanisms and their implications in the progression of cancer. In addition, current therapeutic strategies developed to target NRF2 in lung cancer will be considered.
2. NRF2/KEAP1 System in Physiological Conditions
Aerobic organisms require oxygen for survival; nonetheless, these organisms generate oxygen byproducts named reactive oxygen species (ROS) [24]. These oxygen byproducts together with reactive nitrogen species (RNS) regulate a variety of cellular responses [25]. However, these oxidized species have to be tightly regulated as an excess of ROS/RNS introduces oxidative damage to proteins, lipids and DNA and in turn to genetic and/or epigenetic alterations, which might lead to cell death [24]. As an adaptation to this oxidant environment, cells have developed a variety of antioxidant systems to keep cellular ROS levels under certain limits. NRF2 acts as a master regulator of these antioxidant mechanisms since it is responsible for the activation of several transcriptional programs in response to cellular oxidative stress [26].
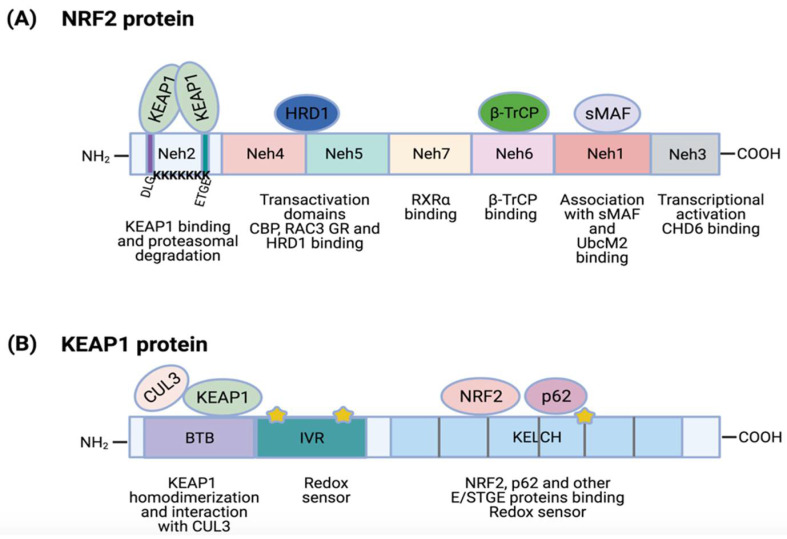
NRF2, encoded by NFE2L2, is a transcription factor that belongs to a protein family characterized by the presence of a cap ‘n’ collar (CNC) homology domain [27]. NRF2 is a 605 amino acid protein with a molecular weight of 68 kDa that contains seven highly conserved NRF2-ECH (erythroid-derived cap ´n´ collar homology, Neh) domains [28] (Figure 2A).
Figure 2.
NRF2 and KEAP1 protein structures. (A) NRF2 contains 7 highly conserved domains called Neh domains. Neh1 is required for complex formation with transcription factor sMAF, DNA binding and for binding to UbcM2. Neh2 contains ETGE and DLG sequences that are required for KEAP1 binding and 7 ubiquitin-lysine residues for targeting NRF2 for proteasomal degradation. Neh3 is needed for transcriptional activation (CHD6 binding). Neh4 and Neh5 are transactivation domains that bind activators (CBP, RAC3) or repressors (GR, HRD1). Neh6 regulates NRF2 stability by binding to β-TrCP. Finally, Neh7 can interact with RXRα, an NRF2 repressor; (B) KEAP1 protein contains 5 conserved regions: N-terminal region, BTB, IVR region, DGR domain and C-terminal region; DGR and C-terminal regions form a Kelch motif. The BTB domain facilitates KEAP1 homodimerization and CUL3 binding. The IVR region possesses a cysteine-rich domain that acts as a direct redox sensor. DGR/Kelch regions contains 6 repeats of a Kelch motif that mediate their interaction with NRF2 and other proteins with E/STGE conserved motifs, such as p62. This region also contains additional cysteine residues for stress sensing. Stars represent several cysteine residues located in IVR and Kelch domains.
The Neh1 is a basic region leucine-zipper motif required for DNA binding at antioxidant response elements (ARE; also named EpRE, electrophile response elements) of gene promoters and for NRF2 dimerization with other transcription factors [13,28]. The Neh1 region also regulates NRF2 stability by its interaction with the ubiquitin-conjugating enzyme, UbcM2 [29]. The Neh2 domain is located at the N-terminal region and it contains the ETGE (from aspartic acid, threonine, etc.) and DLG amino acid motifs, which are essential for the binding to KEAP1 [30]. In addition, the Neh2 domain contains seven ubiquitin-accepting lysine residues that mediate NRF2 proteasomal degradation [31]. In the C-terminal region is the Neh3 domain, needed for transcriptional activation. In fact, chromo-ATPase/helicase DNA binding protein family member (CHD6) has been identified to directly associate with the Neh3 domain via a VFLVPK motif [32]. Both Neh4 and Neh5 domains are two independent transactivation domains [33]. Co-activators such as CREB-binding protein (CBP) or receptor-associated co-activator 3 (RAC3) are able to bind to Neh5 to promote an increase in NRF2 expression [33,34]. Nevertheless, Neh4 and Neh5 can also bind other transcriptional regulators, such as glucocorticoid receptor (GR). Alam and colleagues showed that binding of GR to Neh4 and/or Neh5 domains can displace Histone acetyltransferase CBP and thus suppress H3K27 acetylation in the promoter of NFE2L2 target genes to produce a reduction of NFE2L2 transcriptional program [35]. The Neh6 domain is a serine rich region that is involved in the regulation of NRF2 stability [13]. It contains two amino acid motifs (DSGIS and DSAPGS), which are binding sites for the β-transducin repeat-containing protein (β-TrCP) that promote NRF2 poly-ubiquitination [36,37]. Finally, the Neh7 domain interacts with an NRF2 repressor, the retinoic X receptor α (RXRα), for the inhibition of NFE2L2 target gene transcription [38].
As mentioned earlier, the activity of NRF2 is negatively regulated by KEAP1, a substrate adapter protein for the E3 ubiquitin ligase complex CUL3/RBX1 (complex of human cullin-3 with human RING box protein 1) [24,39,40,41]. This regulatory role of KEAP1 was confirmed in animal models as Keap1 knockout mice showed constitutive activation of Nrf2 and sustained expression of Nrf2 target genes [42]. KEAP1 protein is widely expressed in different cell types and tissues and localizes in the cytoplasm perinuclear region, endoplasmic reticulum and to a lesser extent in the nucleus [43].
KEAP1 belongs to the BTB-Kelch protein family, which contains two canonical domains in common: a BTB domain (Broad-Complex, Tramtrack and Bric a brac) and a Kelch domain (domain present in Kelch proteins). The KEAP1 primary structure comprises 5 regions (depicted in Figure 2B): an N-terminal region (NTR), the BTB domain, an intervening region (IVR), a double-glycine repeat (DGR) domain and the C-terminal region (CTR); DGR and C-terminal domains form a Kelch domain (321–624) [44]. The BTB domain is needed for KEAP1 homodimerization and for the interaction with CUL3 [44,45]. The IVR region is a cysteine (Cys)-rich region containing direct redox sensors. The positively charged environment of basic amino acids K131, R135, K150 and H154 near to the Cys-rich region of the IVR region is responsible for the high reactivity of these Cys [46]. Next to it, there is the DGR/Kelch domain containing up to six repeats of the Kelch motif forming a six-bladed β-propeller structure that mediates its interaction with other proteins. The DGR/Kelch domain binds to the ETGE (or STGE) and DLG amino acid motifs of different partners including the NRF2 Neh2 domain [41,47,48] p62, B-cell lymphoma extra-large (Bcl-xL), dipeptidyl Peptidase 3 (DPP3), splicing factor, arginine/serine-rich 10 (SFRS10), D-site of albumin promoter binding protein (DBP), etc. [44]. The Kelch domain also contains several Cys residues involved in ROS stress sensing [13]. Indeed, human KEAP1 contains up to 27 Cys, around twice more than an average human protein [44]. Under oxidative stress, these Cys are oxidized, resulting in an ideal stress sensor for oxidants and/or electrophiles [49].
2.1. Canonical Pathway of NRF2/KEAP1
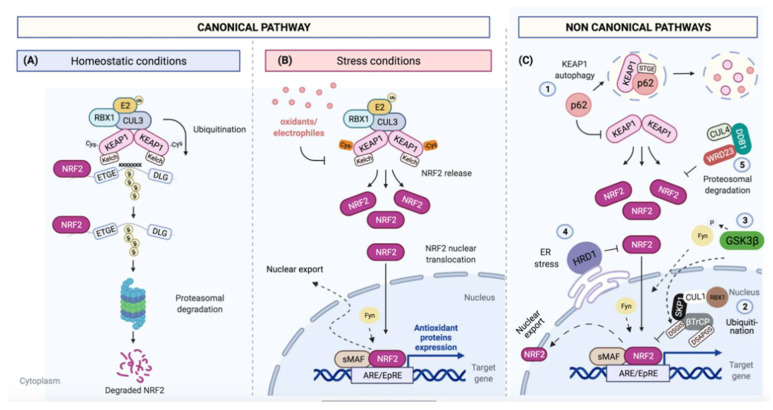
NRF2 protein has a short half-life [50]. In fact, under normal (unstressed) conditions, protein levels of NRF2 are usually low as KEAP1 binds to NRF2 for its CUL3/RBX1 E3 ubiquitin-dependent degradation [51]. This KEAP1/NRF2 interaction is explained in the ´hinge and latch´ model: each Kelch domain of KEAP1 binds to the NRF2 protein by a strong-binding ETGE motif (hinge) and a weak-binding DLG motif (latch), the first binding affinity being around 100-fold higher than the second one [31,52]. In this binding, NRF2/KEAP1 complex adopts two different conformations: in the open conformation, newly synthesized NRF2 binds by ETGE motif to one KEAP1 molecule, but it is not until the binding of a second KEAP1 molecule by DLG motif that a closed conformation is acquired, predisposing NRF2 to its poly-ubiquitination and degradation by the 26S proteasome [53] (Figure 3A).
Figure 3.
Canonical and non-canonical NRF2 pathways. (A) Under basal conditions, NRF2 ETGE/DLG motifs bind to KEAP1 Kelch domains. Binding to KEAP1 brings the CUL3/RBX1 E3 ubiquitin ligase into the complex and targets NRF2 for poly-ubiquitination and degradation by 26S proteasome; (B) Several oxidative and electrophilic stressors can modify critical KEAP1 cysteine residues, disrupting the KEAP1-NRF2 complex. As a consequence, NRF2 protein levels increase, causing its translocation into the nucleus where it forms a heterodimer with sMAF transcriptional factors to act on ARE/EpRE enhancer sequences for the control of its transcriptional program. Afterwards, NRF2 is exported to cytoplasm upon phosphorylation by different Src family kinases, such as Fyn; (C) NRF2 can be also regulated by KEAP1-independent mechanisms: (1) by p62/SQSTM1, which promotes KEAP1 autophagic degradation via its STGE binding motif; (2) by β-TrCP that can form a complex with CUL1/SKP1, promoting NRF2 ubiquitination and degradation; (3) by GSK-3β, which can phosphorylate β-TrCP, increasing NRF2 ubiquitination; (4) by HRD1, which can interact under reticulum stress conditions with Neh4 and 5 domains and trigger NRF2. Recently, (5) CUL4/DDB1/WDR23 was discovered as another E3 ubiquitin ligase to regulate NRF2, but its mechanism is still unclear.
Several oxidative and electrophilic cellular stressors can directly modify critical KEAP1 Cys residues (Cys257, Cys273, Cys288, Cys297 and Cys151) by oxido-reduction and/or alkylation reactions [54,55]. Their redox modifications cause a conformational change that disrupts Kelch<>DLG binding, avoiding the NRF2 degradation by the proteasome. Electrophilic modifications of KEAP1 Cys are accompanied by KEAP1 degradation by autophagy mechanisms [50]. The binding between KEAP1 and CUL3 can be also disrupted by inhibition of CUL3 neddylation [56]. CUL3 acts as a scaffold protein that binds to BTB domain of KEAP1, allowing the formation of a complex with an E2 ubiquitin-conjugating enzyme [26]. Indeed, the complete inhibition of CUL3 neddylation leads to the cellular accumulation of NRF2 [56].
Under ROS stress conditions, KEAP1 Cys are oxidized and KEAP1 is released from NRF2 causing an increase in NRF2 levels and its translocation to the nucleus where it forms a heterodimer with sMAF transcriptional factors (v-Maf avian musculoaponeurotic fibrosarcoma oncogene homolog) or Jun proteins (c-Jun, Jun-B and Jun-D) [13,57]. NRF2 binds to ARE/EpRE sequences [28]. The ARE/EpRE are cis-acting DNA enhancer sequences with the consensus sequence: 5′-RTGABnnnGCR-3′ (“n”, any nucleotide) [58].
Among NRF2 target genes, there are drug metabolizing enzymes (phase I-III), redox response transcription factors, anti-apoptotic proteins, carbohydrate and lipid metabolizing enzymes, cell cycle regulators, proliferation regulators, proteostasis machineries (regulators of autophagy and proteasomal degradation), heme and iron metabolizing proteins and xenobiotic transporters [58,59]. Therefore, the NRF2 activation protects cells from a broad variety of cellular stresses, most importantly oxidative stress [50]. It is important to highlight that the promoter region of NFE2L2 gene also contains an ARE sequence, providing the possibility of a positive feedback regulation, increasing the transcription of its target genes and promoting a fast response to any cellular stress [60].
After its transcriptional activation, NRF2 is exported from the nucleus to the cytoplasm; this transport is regulated by tyrosine phosphorylation on several residues. Members of Src subfamily A, like Fyn, are able to phosphorylate NRF2 in the nucleus for its nuclear export and degradation [61] (Figure 3B).
2.2. Non-Canonical Pathways
NRF2 can be also regulated by KEAP1-independent mechanisms. The ubiquitin binding autophagy receptor p62/sequestosome-1 (p62/SQSTM1) was identified as a regulator of ARE-element gene expression, independent of the cellular redox state [40]. p62/SQSTM1 binds to KEAP1 via its STGE motif and promotes its degradation, in turn increasing NRF2 protein levels [37]. Additionally, the E3 ubiquitin ligase complex formed by Cullin 1/S-phase kinase-associated protein 1/β-transducin repeat-containing protein (CUL1/SKP1/β-TrCP) can also regulate NRF2 levels [62]. β-TrCP serve as substrate recognition subunits for the SCFβ-TrCP (Skp1-Cullin1-F-Box protein) E3 ubiquitin ligases, resulting in NRF2 ubiquitination and degradation [36].
Another regulator of NRF2 is glycogen synthase kinase 3 beta (GSK-3β). This protein can phosphorylate β-TrCP, increasing the ability of β-TrCP to ubiquitinate NRF2 [36,50]. GSK-3β also regulates the phosphorylation and nuclear translocation of the tyrosine kinase Fyn, which in turn phosphorylates NRF2, promoting its return to the cytosol and degradation [63]. The phosphoinositide 3-kinase (PI3K) / protein kinase B (PKB) pathway is also involved in this regulatory mechanism due to its capacity to inhibit GSK-3β. Indeed, in keap1 knockout mice, additional loss of the PI3K negative regulator Pten promotes an increase in Nrf2 levels through the inactivation of Gsk-3β [64]. In addition, protein kinase B (PKB) also phosphorylates Ser-40 in the Neh2 domain, dissociating NRF2 from KEAP1 and increasing NRF2 protein levels [65].
Another NRF2E3 ubiquitin ligase is the HMG-CoA reductase degradation protein 1 (HRD1), which interacts with NRF2 Neh4 and 5 domains and triggers NRF2 degradation under endoplasmic reticulum stress [66]. Recently, the cullin4/damaged DNA binding protein-1/WD Repeat Domain 23 (CUL4/DDB1/WDR23) was discovered as yet another E3 ligase of NRF2 that acts independently of CUL3/KEAP1 and competes for NRF2, suppressing NRF2 activity. WRD23 binds near the NRF2 DLG motif, but its role in NRF2 stability is still unclear [67] (Figure 3C).
Finally, IkB kinase β (IKKβ) is able to interact with KEAP1 promoting IKKβ degradation [68]. In basal conditions, KEAP1-Cul3-E3 ligase complex leads to IKKβ ubiquitination and degradation by the proteasome. In response to oxidative stress, however, IKKβ/KEAP1 binding is disrupted, promoting IKKβ stabilization and the phosphorylation of IkBα. Phosphorylated IkBα in turn binds and activates NF-kB. The consequence is the activation of a variety of NF-kB target genes involved in important processes, such as inflammation, tumor invasion and angiogenesis [69,70,71].
3. Functions of NRF2 in Lung Cancer. The Dual Role of NRF2
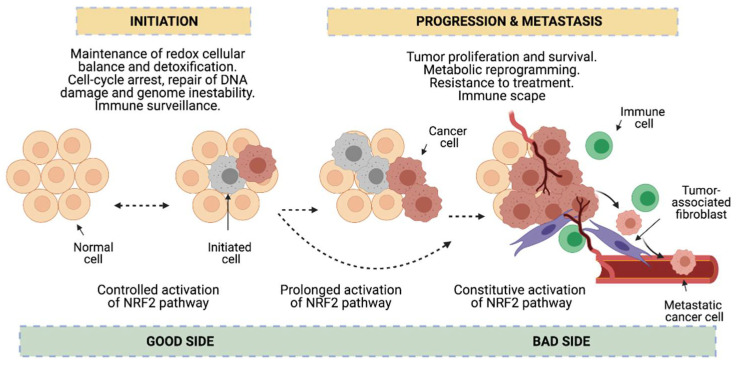
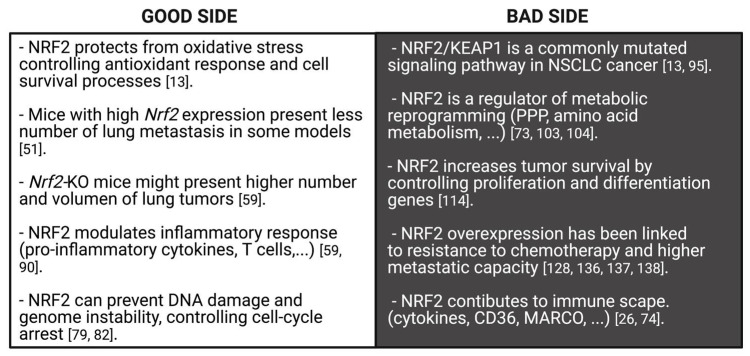
Redox status imbalance commonly appears in cancer [72]. Tumor cells exhibit permanent high ROS levels due to the oncogene activation, increased metabolic rates, hypoxia, mitochondrial and/or peroxisomal dysfunction as well as anchorage-independent growth [51]. In this context, NRF2 plays a key role acting as a major regulator of the antioxidant response. Nonetheless, its functions can be beneficial or prejudicial for tumorigenesis depending on the cancer-stage in lung cancer cells. In early stages of tumorigenesis, NRF2 activity seems to be important for avoiding premalignant carcinogenesis, DNA damage and initial cancer mutations [25,73]. However, at advanced stages, some actions of NRF2 can promote carcinogenesis [25]. Activation of the NRF2 pathway could be advantageous to protect tumor cells from oxidative stress [73] (Figure 4). Lung cancer cells seem to acquire a high dependency on the NRF2 pathway for the maintenance of its malignant phenotype, a process called NRF2 addiction [74].
Figure 4.
Dual role of NRF2 in cancer. NRF2 functions seem to be beneficial or prejudicial for tumorigenesis depending on the cancer stage. In early stages, NRF2 is protective in premalignant carcinogenesis and maintains redox cellular balance which assists in the detoxification process, DNA damage, cell-arrest, genome instability and immune surveillance (good side of NRF2 against cancer). However, prolonged or constitutive activation of NRF2 contributes to cancer progression and metastasis, since NRF2 favors tumor proliferation and survival, metabolic reprogramming, resistance to treatment and immune escape (bad side of NRF2).
This dual role of NRF2 in cancer has been investigated in several animal studies. Tao et al. described in models of LUAD that the NRF2 pre-activation by sulforaphane prevents tumor initiation. Nonetheless, once the tumor is initiated, NRF2 activation promotes the growth of the pre-existing tumors, giving rise to larger tumors compared to non-treated tumors [75]. Satoh et al. found that after exposure to the carcinogen urethan, Nrf2-deficient mice have a relative increase in the number of tumor foci after 8 weeks of treatment, but by 16 weeks of treatment, these same animals show less advanced malignancy [76]. The same group also demonstrated that in Keap1 knockdown mice, the growth of urethane-induced lung tumors is mitigated, thus preventing carcinogenesis. However, after transplantation, Keap1-knockdown mouse-derived cancer cells exhibited higher tumorigenicity compared to wild-type transplanted cells from control mice [77]. Thus, it is evident that NRF2 exhibits a dual role in cancer; however, there is still some ambiguity about its functions in this disease. In the following sections, the most important findings from NRF2’s beneficial (good side) or prejudicial (bad side) roles in NSCLC cancer will be summarized.
3.1. Good Side of NRF2 against NSCLC
NRF2 is able to protect cells from the oxidative stress generated during the transformation process by controlling several target genes which are mainly implicated in antioxidant defense and cell survival processes [13]. In this sense, NRF2 maintains an appropriate ratio of specific intracellular key antioxidants, such as reduced glutathione (GSH)/oxidized glutathione (GSSG), by controlling the expression of glutamate-cysteine ligase catalytic subunits (GCLC), glutamate cysteine ligase (GCL) or glutathione reductase 1 (GSR1) [78]. NRF2 also modulates the expression of several detoxification enzymes, such as glutathione peroxidase 2 (GPX2), thioredoxin 1 (TXN1), thioredoxin reductase 1 (TXNRD1), sulfiredoxin 1 (SRXN 1) and glutathione S-transferases (GSTs) [28]. All of them play important roles in the maintenance of cellular redox balance, which works against cancer progression.
In addition, NRF2 prevents DNA damage by regulating the expression of NAD (P) H Quinone Dehydrogenase 1 (NQO1) [79] required for the control of breast cancer 1 (BRCA1) and RAD51 recombinase (RAD51) mRNA levels; both proteins regulate homologous recombination (HR) during the repair of double-strand breaks (DSB) [80]. Accordingly, Nrf2-null mice are more susceptible to acute DNA damage [81] and in normal human lung fibroblasts, irradiation activates NRF2, which in turn reduces DSB levels [82]. These defense mechanisms against tumorigenesis managed by NRF2 have also been observed in additional animal studies [77,83,84]. For instance, Satoh et al. described that urethane-initiated LUAD have a smaller size when generated in keap1-knockdown mice compared to wild-type mice [77]. The protective function of NRF2 was corroborated in a Lewis lung carcinoma (3LL) mouse metastasis model, where the loss of Nfe2l2 was linked to high metastasis capacity [85]. Recently, it was observed that cysteine dioxygenase type 1 (Cdo1) accumulation in LUAD generated in Keap1R554Q/R554Q mutant mice correlated with reduced tumor formation [86]. Finally, it was shown that some NRF2 activators, such as oltipraz, are able to block B(a)P-initiated LUAD in mice [87]. Similarly, several clinical studies done in patients with other cancer types (melanoma, prostate, colorectal and renal carcinomas) have demonstrated that the intervention by small molecules or phytochemicals, such as glucoraphanin and bardoxolone methyl, are able to activate NRF2 signaling, suppressing the risk of cancer progression [88,89].
NRF2 is also able to modulate the inflammatory response at the tumor site. NRF2 decreases the expression of pro-inflammatory cytokines such as tumor necrosis factor alpha (TNF-α), interleukin (IL)-1β (IL-1β), or IL-6 [51]. In the case of cellular immunity, NRF2 is also able to recruit natural killer (NK) cells that secrete the isoform D of IL-17, promoting tumor rejection [90]. Itoh and colleagues found that NRF2 up-regulation in the surrounding microenvironment or in hematopoietic cells suppresses lung tumor progression in Kras-driven LUAD tumors [91]. This finding prompted the idea that NRF2 activation in the tumor microenvironment could reduce cancer progression by enhancing the immune response against cancer [92]. In a Keap1-wt xenograft model of lung cancer, using the Nrf2 inducer bardoxolone triggered an increase in NRF2 expression and reduced the number of lung metastases [51]. Along the same line, Zhang et al. demonstrated that Nrf2-deficient mice show a higher number and volume of lung LUAD tumors compared to WT mice, a lower number of T cells (CD8 cytotoxic T cells and CD4 helper T cells) and increased amounts of some cytokines (CSF-1), chemokines (CCL9, CXCL12, CXCL1) and peptide antigens [59].
Finally, some additional functions of NRF2 have been related to cancer prevention. NRF2 is involved in the control of cell cycle-arrest by regulating cyclin-dependent kinase inhibitors (CDKi) p15 (Cdkn2a) and p21 (Cdkn1a), in the prevention of genome instability [51], modulation of transcriptional initiation [49] and facilitation of aggresome formation during proteasomal stress [73]. Some in vitro studies show that NRF2 activation reduces LUAD cells’ capacity for migration [93,94]. Therefore, the implication from all the above-mentioned studies is that NRF2 may regulate a defense mechanism against tumor initiation, and also protect cells from cancer progression (Figure 5).
Figure 5.
Good and bad sides of NRF2 against lung cancer. NRF2 exhibits a dual role in lung cancer. On one hand, NRF2 protects from oxidative stress generated in cancerous development by controlling genes implicated in antioxidant and cell survival processes, which maintain an appropriate level of intracellular antioxidants. Indeed, NRF2 prevents DNA damage, genome instability and cell cycle-arrest. Moreover, NRF2 modulates the inflammatory response through an increase of T cells. As a consequence, Nrf2-knockout mice show high number and volume of lung tumors and Nrf2-high mice present a small number of lung metastasis. On the other hand, the NRF2/KEAP1 pathway is a commonly mutated signaling pathway in human lung cancer and some pro-oncogenic functions of NRF2 provide an advantageous condition for the progression of cancer cells. NRF2 is an important regulator of metabolic reprogramming and cancer proliferation. Both processes are important for cancer progression. Concurrently, NRF2 overexpression has been correlated with lower sensitivity to chemotherapeutic drugs and with high metastatic capacity. Finally, NRF2 contributes to immune escape by inducing a decrease of pro-inflammatory cytokines.
3.2. Bad Side of NRF2 in NSCLC Progression
As summarized in the above section, the NRF2/KEAP1 pathway has been considered as a tumor suppressor-signaling pathway due to its role in several defense mechanisms against tumor development. However, in the last decade, there has been growing evidence that this transcription factor has some pro-oncogenic properties. Activating mutations accumulate in some tumor types, suggesting that NRF2/KEAP1 may support an advantageous condition for cancer progression [13]. Although the first involvement of NRF2 in cancer was discovered in hepatocellular carcinoma [83], further experiments found elevated NRF2 protein levels in other malignancies, such as lung cancer [73,74]. NFE2L2/KEAP1 is a commonly mutated signaling pathway in NSCLC sub-types [95] and GOF mutations of NFE2L2 or LOF mutations of KEAP1 have been often seen in this kind of malignancy [13]. Whereas KEAP1 mutations were found in 17 % of LUAD and 12% of LUSC tumors, NFE2L2 mutations are more frequent in LUSC (15 % of LUSC) than LUAD (3 % of LUAD) [10]. Globally, 26% of NSCLC tumors exhibit an increase in nuclear NRF2 expression [14]. Regarding other lung cancer sub-types, Keap1 mutations have been found in an LCC tumor in mice [15], in SCLC (NCI-H1184, a human cell line) [16] and in some pulmonary large-cell neuroendocrine carcinoma (LCNECs) [17], although at a lower frequency than in LUSC or LUAD.
Interestingly, genetic analyses revealed that the NFE2L2 mutational profile found in LUAD is significantly different from LUSC [96]. In fact, while KEAP1 is mostly mutated in LUAD, NFE2L2 is mainly affected in LUSC [97,98]; in the latter, CUL3 is also significantly mutated [12,26]. Other genetic alterations in this signaling cascade also found in LUSC patients include single nucleotide polymorphisms (SNPs) in KEAP1 and CUL3 [99], an increased number of NFE2L2 copies [100], KEAP1 loss of heterozygosity (LOH) and KEAP1 promoter methylations [101]. LUSC also shows additionally NRF2-complexed hypomorph (ANCHOR) mutations. While most mutations found in KEAP1 show a reduced binding to NRF2, some, such as KEAP1R320Q and KEAP1R470C, exhibit an increased binding but fail to suppress NRF2 by forming a p62-dependent biomolecular complex. As a consequence, cells with these KEAP1 mutations present moderately elevated levels of NFE2L2-dependent transcription [102].
This “bad side” action of NRF2 in NSCLC involves the regulation of enzymes related to metabolic reprogramming, where dividing cells conduct aerobic glycolysis (Warburg effect), an important process in cancer progression [74,103]. NRF2 can up-regulate pyruvate kinase (PK), a key enzyme in glycolysis [103]. Indeed, NRF2 is able to increase glucose uptake and redirect it to the pentose phosphate pathway (PPP), which is highly connected to the glycolysis route. This is made possible due to NRF2-mediated transcription of glucose-6 phosphate dehydrogenase (G6PD), 6-phosphogluconate dehydrogenase (PGD), transketolase (TKT) and trans-aldolase 1 (TALDO1) [73,104]. Furthermore, Best et al. found that Keap1 mutant mice develop LUAD tumors with high levels of Taldo1, an enzyme that provides ribose-5-phosphate for nucleic acid synthesis and NADPH for lipid biosynthesis [105]. Simultaneous inactivation of Keap1 and Pten genes in mice promotes the formation of LUAD and re-programming of metabolism to the PPP [106]. Correspondingly, reduced tumor growth has been reported in Keap1 mutant NSCLC xenograft after G6pd and Tkt silencing [104].
NRF2 also regulates the expression of NADPH-producing enzymes (i.e., malic enzyme 1 (ME1), isocitrate dehydrogenase 1 (IDH1)) [73]. Surprisingly, NAPDH oxidase 2 (NOX2) and 4 (NOX4) are overexpressed in NSCLC [107,108,109] and generate superoxide and hydrogen peroxide, which in turn trigger NRF2 activation [109].
Another process clearly affected by NRF2 overexpression in cancer is amino acid metabolism, which facilitates the survival and proliferation of cancer cells under different stresses [73,74]. In this sense, studies using patient-derived xenografts (PDXs) and LUAD tumors from Keap1-deficient mice have shown an increased sensitivity to glutaminase inhibition (the enzyme that generates glutamate from glutamine), sensitizing KEAP1/NRF2 mutant NSCLC cells to radiotherapy [110,111]. Indeed, liver kinase B1 (LKB1)-deficient cells combined with NRF2 activation promotes glutamine-addictive metabolism in K-RAS mutant LUAD [112]. Similarly, NRF2 activation in KEAP1 mutant NSCLC lines promotes serine biosynthesis, required for the synthesis of key antioxidants, such as GSH. In addition, the NRF2 regulation of xCT, TXN and TXNRD1 promotes cysteine accumulation, a feature that correlates with poor prognosis in NSCLC cell lines and tumors [113]. This cysteine accumulation has been proposed as a metabolic liability in NSCLC cells, mainly in KEAP1 mutant LUAD, by increased stabilization of cysteine dioxygenase 1 (CDO1) [86].
Altered lipid metabolism is another important NRF2-regulated metabolic feature in cancer. NRF2 reduces the expression of several fatty acid synthesis enzymes, such as fatty acid synthase (FASN), stearoyl CoA desaturase 1 (SCD1) and lipases, including in this last enzyme the phospholipase A2 Group VII (PLA2G7) [103].
The constitutive activation of NRF2 in cancer is also correlated with increased survival of tumor cells under unfavorable conditions because of the control by NRF2 of critical regulators of cell proliferation and differentiation [13,114]. NRF2 is able to control cell proliferation by increasing insulin-like growth factor-1 (IGF1), platelet derived growth factor C (PDGFC), and vascular endothelial growth factor C (VEGFC) levels [51]. Takahashi et al. found that the high activity of NRF2 in some NSCLC cell lines is key for efficient spheroid formation [115]. A new mechanism related to NRF2 contribution to increase proliferation was recently proposed: the activation of Notch Receptor 3 (NOTCH3). Surprisingly, this mechanism only was seen in NFE2L2-overexpressed NSCLC, raising the idea that the genes induced by NRF2 in cancer cells might be different from those induced in NFE2L2-WT background [116]. In addition, some authors found a correlation between NRF2 pathway alterations and poor survival. In fact, nuclear NRF2 expression has been associated with worse progress-free survival in NSCLC patients [14]. Lung tumors with high expression levels of NQO1, a NFE2L2 target gene, have worse prognosis than those with wild-type NQO1 [117]. Furthermore, overexpression of CUL3 in LUAD patients (resulting in NRF2 degradation) has been related to a reduction of tumor growth in vivo and a better overall survival illustrating the involvement of NRF2 in LUAD progression [118]. Tong et al. also found that NRF2–negative and NQO1–negative NSCLC patient samples were correlated with better prognosis and disease-free survival [119].
Another family of NRF2 effectors are the aldo-keto reductases (AKRs, AKR1B, AKR1C1/2 and AKR1C3) which are upregulated in many LUSC and some LUAD tumor biopsies with somatic mutations in the NFE2L2 gene [120]. AKR1C1 has been proposed as a target for the anti-tumor compound wentilactone A in SCLC cells [121]; indeed, the aldo-keto reductases are considered as biomarkers for NRF2 status in human tumors [120]. In this regard, AKR1B10 gene overexpression has been also considered as a prognostic factor for poor recurrence-free survival in resected LUAD patients [122].
NRF2 also regulates proteins required for stemness, such as Aldehyde de-hydrogenases (ALDH), Sirtuin 1 (SIRT1) and others [123,124]. Li et al. described that EZH2 (enhancer of zeste homolog 2) inhibits lung cancer growth both in vitro and in vivo, and it does so by binding and repressing the NFE2L2 promoter [125]. Additionally, tyrosine kinase receptors, such as insulin-like growth factor 1 receptor (IGF1R) and erb-B2 receptor tyrosine kinase 3 (ERBB3), were recently discovered to be also important for KEAP1-mutant lung cell growth [126]. Krall et al. showed that KEAP1 loss is involved in the resistance of NSCLC cell lines with mutations in EGFR, ALK, B-RAF and K/N-RAS to selective inhibitors of these kinases [127].
The most frequent cause of mortality in lung cancer patients is metastasis. In this regard, several studies have related constitutive activation of NRF2 with this cancer stage [73]. Cells with NFE2L2 overexpression have the ability to grow in an anchorage-independent manner, presenting a high metastatic capacity [128]. Aljohani et al. described that NFE2L2E63Q and KEAP1R601L mutations, both presenting constitutive activation of NRF2, are highly enriched in NSCLC metastasis [129]. This relation seems to be due to the regulation that NRF2 exerts on the expression of heme oxygenase 1 (encoded by HMOX-1 gene). NRF2 can increase HMOX-1 expression, and in turn that of the BTB Domain and CNC Homolog 1 (BACH1) gene. By enhancing BACH1 levels, NRF2 provokes the expression of pro-metastasis genes, such as those related to matrix metallopeptidases and CXCR4 in LUAD [130].
NRF2 can also promote lung cancer progression by regulating genes involved in angiogenesis [74,131], hypoxia [28,132], epithelial-mesenchymal transition (EMT) [74] and focal adhesion [133]. NRF2 is also able to regulate the cancer immune microenvironment. NRF2 can contribute to immune escape by controlling the expression of several cytokines [74], the scavenger receptors cluster of differentiation 36 (CD36) and macrophage receptors with a collagenous structure (MARCO) [26]. In agreement, KEAP1 mutations in LUAD have been associated with reduced leukocyte infiltration [134] and NFE2L2 mutant tumors exhibit low expression of different markers of immune response [135] (Figure 5).
3.3. Another Bad Side of NRF2 Activation: Resistance to Chemotherapy
NRF2 overexpression has been also linked to decreased sensitivity to chemotherapeutic drugs. Nfe2l2 silencing in xenografts produces higher susceptibility to platinum-based chemotherapy compared to control xenografts [136]. Several reports have demonstrated that mutations in the NRF2/KEAP1 pathway are linked to worse outcomes after platinum-based chemotherapy [135,137,138]. The inhibition of tumor growth of A549 LUAD xenografts with paclitaxel was improved by inhibiting NRF2 pathway (through the administration of diosmetin) [139]. Frank et al. found that NSCLC patients with KEAP1/NRF2 activating mutations do not respond to second/third line chemotherapy [97]; moreover, Ceston et al. found that LUSC patients with NRF2 active do not benefit from adjuvant chemotherapy comparing to the ones with the NRF2 unaltered [140]. All of these studies suggest that KEAP1/NFE2L2 mutations might be used as a local recurrence risk predictor for chemotherapy.
The chemoresistance promoted by this transcription factor is due, at least in part, to NRF2 control of expression of drug transport genes, such as the cysteine/glutamate antiporter system Xc-subunit gene (SLC7A11) [28]. SLC7A11 is overexpressed in different NSCLC cell lines and its silencing with shRNAs causes cell growth inhibition [141]. Similarly, another NRF2 target involved in chemoresistance is the multidrug resistance protein 3 (MDR3). In a previous study with the NSCLC cell lines A549 and H460 (both contain a KEAP1 mutation), cells presented constitutively high levels of MDR3 [142]. Furthermore, in response to chemotherapy, NRF2 is able to inhibit apoptosis as it induces B-cell lymphoma 2 (Bcl-2) and Bcl-xL expression, which in turn reduces caspase 3/7 activation [143,144]. Furthermore, Chakrabarti et al. found that glutamate-oxaloacetate transaminase 1 (GOT1) and malic enzyme 1 (ME1) expression, both gene targets of NRF2, could be used as predictive markers in the treatment response to radiation therapy in NSCLC patients [145]. Finally, the homeodomain-interacting protein kinase 2 (HIPK2) has been recently reported as a new NRF2 target with anti-apoptotic functions [146].
The importance of NRF2 in drug resistance is also closely linked to the control that this transcription factor exerts on iron metabolism. In fact, NRF2 has a key role in the protection of lung cancer cells from ferroptosis, a cell death mechanism involving iron-dependent lipid peroxidation [51]. The involvement of NRF2 in this kind of cell death is caused by NRF2 control of the expression of iron pool-related genes, i.e., ferritin heavy chain 1 (FTH1) [103], glutathione peroxidase 4 (GPX4) and genes involved in glutathione and NADPH syntheses [147].
4. Mechanisms Conferring NRF2 Activation
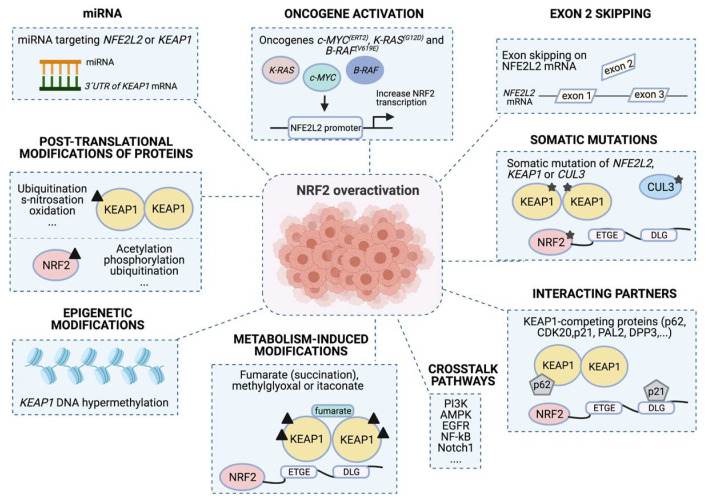
The persistent activation of NRF2 in lung cancer cells is caused by different molecular changes, such as genomic alterations (genetic, epigenetic or oncogene signaling), transcription and translation abnormalities, post-translation modifications and/or altered interactions with other proteins [28,58,74]. All these molecular changes promote, in the end, the constitutive activation of NRF2 in lung cancer cells [58] and in turn NRF2 addiction (see Section 3) (Figure 6).
Figure 6.
Molecular mechanisms confer NRF2 addiction. Different mechanisms can contribute to promote NRF2 over-activation. First, somatic mutations on NFE2L2/KEAP1/CUL3; second, differential slicing and loss of exon 2 during mRNA processing; third, mutations in other oncogenes, such as c-MYC (ERT2), K-RAS (G12D) and B-RAF (V619E) that increase NFE2L2 transcriptional levels. NRF2 can also be activated by: fourth, expression of different miRNAs that can regulate NRF2 and KEAP1 expression; fifth, post-translational modifications of NRF2 and KEAP1 that promote or suppress NRF2 activity; or sixth, epigenetic modifications, such as histone post-translation modifications in KEAP1 and NFE2L2 promoter regions that increase NRF2 accumulation. Finally, seventh: metabolites that can modify several residues in KEAP1, provoke NRF2 activation (metabolism-induced modifications); and eighth, NRF2 and KEAP1 interactions with other proteins (interacting partners) or pathways (crosstalk pathways) can also result in NRF2 activation.
4.1. Somatic Mutations of NFE2L2/KEAP1/CUL3
The most common mechanisms promoting constitutive NRF2 activation are LOF mutations of KEAP1 and GOF mutations of NFE2L2 [74], which are frequently found in NSCLC sub-types [10,95] (see Section 3.2). In the case of NFE2L2, GOF mutations are mainly seen in the DLG and ETGE motifs [26,28]. In contrast, although KEAP1 LOF mutations are mostly located in Kelch domain, they are also seen throughout the gene [58,134]. Some KEAP1 mutations decrease NRF2 proteasomal degradation and hence cause NRF2 accumulation in the nucleus [6,26]. Other mutations, such as those causing KEAP1 hypomorphic function (partial loss of gene function) [28] as well as “ANCHOR” or super-binder mutations, also induce constitutive NRF2 activation [44]. In fact, the Keap1 deletion LUSC-like mouse model presents Nrf2 activation [21]. Concurrently, NSCLC cell lines with KEAP1/NFE2L2 mutations present increased expression of some NFE2L2 target genes, such as HMOX1, GCLCM, TXN, GCLC, NQO1 or GSR [98]. In the case of CUL3 mutations, they have been identified in the lung as well as hereditary type-2 papillary renal cell carcinoma [26,58].
For NRF2 activation, it is important to consider that other mutated genes that contribute to NSCLC development (EGFR, TP53, KRAS, PTEN and PIK3CA) [135,148] correlate with particular NFE2L2/KEAP1 mutations. Mutations in NFE2L2 usually co-occur with PI3KCA and TP53 mutations [149,150]; meanwhile, KEAP1 mutations co-occur more frequently with mutations in K-RAS or STK11 [105,138]. EGFR mutations can coexist with NFE2L2 mutations [148,151].
4.2. Exon 2 Skipping on NFE2L2 mRNA
Another important event that can occur during NFE2L2 mRNA processing is the loss of its exon 2. The consequence of this differential splicing is the formation of NFE2L2 aberrant transcripts that lack DLG or ETGE motifs, leading to a permanent nuclear location, and therefore promoting constitutive NRF2 activation [152]. This altered mRNA processing has been observed in NSCLC and head-neck cancer [153].
4.3. Oncogene Activation
Although the NRF2 pathway is mostly regulated at the protein level (by ubiquitination; see Section 1.2) [13], several molecular mechanisms and transcription factors exert control on its transcription levels. However, these processes have been less characterized [37].
Different mutations in several oncogenes, such as c-MYC(ERT2), K-RAS(G12D) and B-RAF(V619E), can affect NFE2L2 transcriptional levels and/or activity [13,74]. Part of the pro-tumorigenic action of these oncogene mutations seems to be NRF2 activation [25,51,74]. Tao and DeNicola et al. identified enhanced NFE2L2 mRNA levels with constitutive expression of K-RAS(G12D) [154,155] and showed that K-RAS binds to NFE2L2 exon 1 and up-regulates its expression, conferring chemoresistance on NSCLC cells [154]. Moreover, around 30% of lung carcinoma cases with aggressive proliferation show KEAP1 mutations, K-RAS/H-RAS mutations and Tp53 LOF mutations [28]. Some studies suggest that K-RAS(G12D) and B-RAF(V619E) may increase NFE2L2 transcription levels through Jun and Myc [155].
Other transcription factors that increase NRF2 transcription include Aryl hydrocarbon receptor/Aryl hydrocarbon receptor nuclear translocator (AHR/ARNT) [156] or NRF2 itself by its ARE element, the latter leading to a positive feedback mechanism [157].
4.4. miRNA
MicroRNAs (miRNAs) are short, single-stranded and non-coding RNAs that bind to the 3′-untranslated region of a gene transcript, promoting its mRNA degradation or inhibition of translation [13,74]. The first miRNA found to regulate NFE2L2 expression levels was miR-144, whose expression decreases NRF2 protein levels [158]. Other miRNAs that suppress NRF2 activation in different cancers are miR-507, miR-634, miR450a, miR129-5p, miR-340, miR-146b, miR-28, miR-153, miR142-5p, mi-R27a, miR-144, miR34a and miR-93 [28,58,156].
Other miRNAs have the ability to increase the expression of NFE2L2 and/or its target genes. This is the case with miR-155, which increases the expression of some NRF2 target genes, such as NQO1 and HMOX1 genes, leading to resistance to arsenic trioxide (ATO) stress [159]. Increased expression of NFE2L2 was observed by targeting the 3′UTR of KEAP1 mRNA with miR-24-3p, miR-7, miR-200a, miR-421, miR-141, miR-626 and miR-873 [44,156].
Certain long non-coding RNAs are also involved in NRF2 activation: i.e., Urothelial Cancer Associated 1 (UCA1), HOX Transcript Antisense RNA (HOTAIR), Metastasis Associated Lung Adenocarcinoma Transcript 1 (MALAT1) or Taurine Up Regulated 1 (TUG1) [156].
4.5. Post-Translational Modifications
The post-translational modifications that can regulate NRF2 levels are acetylation, phosphorylation and ubiquitination [74]. In fact, NRF2 can be acetylated by CBP [160], acetyl transferase p300 [161] and hMOF (human males absent on the first), all of which result in increased NRF2 protein levels in the nucleus [162]. Correspondingly, NRF2 can be deacetylated by Sirtuin 1 (SIRT1) [160] or SIRT2, resulting in reduced NRF2 levels in the nucleus [74]. In addition, NRF2 can be stabilized by deubiquitination by the deubiquitinating enzyme 3 (DUB3) [163]. Recently, it was found that NRF2 could also be glycated, reducing its protein stability and binding to MAF [73].
KEAP1 can also undergo several post-translational modifications including ubiquitination, s-nitrosation, alkylation, glycosylation, oxidation, carbonylation, S-glutathionylation, succination and sulfhydrylation, which in turn affect NRF2 protein levels. In this sense, a recent report has described that deubiquitinating enzyme Ubiquitin Specific Peptidase 15 (USP15) inhibits the NRF2 activation through deubiquitination of KEAP1. In contrast, s-nitrosylation and oxidative KEAP1 modification induces NRF2 activation [44]. Indeed, it has been identified that KEAP1 can be glycosylated, although the consequences of this modification remain unclear [73]. Other modifications that can affect KEAP1 are malonylation, acetylation or palmitoylation, although their consequences are also still unknown [44].
4.6. Epigenetic Modifications
Histone post-translational modifications by EZH are another mechanism through which expression levels of NFE2L2/KEAP1 can be regulated [125]. Epigenetic modifications have been described in KEAP1 and NFE2L2 promoter regions [28]. Whereas EZH inhibits NFE2L2 expression and decreases NSCLC growth in vivo and in vitro [125], hypermethylation of KEAP1 promoter inhibits its expression and results in increased NRF2 levels [164]. The latter modification has been related to poor clinical prognosis in NSCLC [165]. Indeed, Sparaneo et al., studying lung carcinoids (derived from the neuroendocrine system) (n = 47), found that whereas 50% exhibit KEAP1 promoter methylation, 60% present KEAP1 LOH; thus, in some tumors, both copies of KEAP1 may be inactivated [101].
4.7. NRF2/KEAP1 Interacting Partners
Another way of affecting NRF2 function is though interactions with different proteins. Some proteins are able to bind to KEAP1 and disrupt its binding with NRF2, resulting in NRF2 activation. This is the case of p62/SQSTM1, which promotes KEAP1 degradation by autophagy and hence increases NRF2 activity [37]. Surprisingly, in this molecular axis, NRF2 is able to bind to the promoter region of p62, favoring KEAP1 degradation and in turn its own activation [166].
Other examples of interacting partners are those related to cell cycle regulators, such as cyclin dependent kinase 20 (CDK20). This cyclin has an ETGE motif which permits its binding to KEAP1 and results in an accumulation of NRF2 protein. In fact, overexpression of CDK20 gene has been found in NSCLC. This overexpression was critical for the chemoresistance response in this malignancy [167]. Similarly, p21 is capable of interacting with NRF2 through its KRR motif. This binding disrupts NRF2 binding to KEAP1, promoting an activation of the NRF2 signaling pathway [168]. Similarly, other KEAP1 interacting partners bind to the ETGE motif and trigger NRF2 stabilization. These include the Wilms tumor gene on the X chromosome (WTX) or the partner and localizer of BRCA2 (PALB2) or DPP3 [28]. Ji et al. identified p53-induced protein with a death domain (PIDD) as a KEAP1-interacting partner, which promotes NRF2 stabilization and increases chemoresistance in H1299 NSCLC cells both in vitro and in vivo [169]. KEAP1 also interacts with Actin via its DGR domain, resulting in NRF2 nuclear translocation [44]. KEAP1 is also able to bind to Nestin via its Kelch domain-ESGE motif in NSCLC cells [170] and to the inhibitor of the apoptosis stimulating protein of p53 (iASPP) via the Kelch domain-DLT motif [171]. Other authors, such as Cheng et al., identified that the family with sequence similarity 129, member B or Niban-like protein 1 (FAM129B) competes for KEAP1 binding via both DLG and ETGE motifs, being that this process is linked to poor prognosis in breast (BRCA) and lung cancer (LUSC) [172]. Wang et al. showed that mitogen-activated protein kinase phosphatase 1 (MKP-1) binds to NRF2 Neh2 domain to inhibit its ubiquitination in NSCLC cells [173]. Recently, up to 46 new NRF2 interacting partners have been identified, which activate or repress NRF2 [174]. Further research is needed to understand their molecular mechanisms as well as consequences on NRF2/KEAP1 signaling pathways.
4.8. Metabolism-Induced Modifications
An example of a metabolism-induced modification is succination, which can affect KEAP1 function. In this reaction, fumarate modifies Cys residues in KEAP1 (possibly Cys151 and Cys288), impairing its ability to degrade NRF2 and leading to NRF2 activation [175]. Similarly, methylglyoxal (MGx), which is generated in glycolytic metabolism, induces the crosslink at Cys and Arg residues in KEAP1, triggering NFE2L2 transcriptional program [176]. Finally, itaconate, a product of the tricarboxylic acid cycle (TCA) cycle, promotes alkylation of KEAP1 Cys residues (Cys151, Cys257, Cys288, Cys273 and Cyst 297) leading to NRF2 activation [73,177].
4.9. Crosstalk Pathways
Different pathways can modulate the activation or inhibition state of NRF2. Mitsuishi et al. demonstrated that activation of the PI3K/protein kinase B (AKT) pathway increases NFE2L2 mRNA levels as well as its translocation to the nucleus [104]. The same effect has been described for AMP-activated protein kinase (AMPK), which is able to disrupt GSK-3β-NRF2 complex, allowing NRF2 nuclear translocation [178]. Similarly, EGFR induces the phosphorylation and ubiquitination of KEAP1, which in turn releases NRF2 [179]. Other pathways with the ability to promote NRF2 activation are the nuclear factor kappa light chain enhancer of activated B cells (NF-κB) [180] and NOTCH1, both increasing NFE2L2 transcription. Additionally, NOTCH1 gene contains, in its promoter, an ARE element, allowing for its transcriptional regulation by NRF2 [181,182].
Of particular interest is the puzzling effect of mutant TP53 on NRF2 regulation. On one hand, when cells have high basal levels of NFE2L2 target genes and present GOF TP53 mutations, p53 is able to further increase NFE2L2 transcriptional program. However, in cells with normal NFE2L2 target levels, mutant TP53 decreases the expression of NFE2L2 target genes after oxidative stress [183]. In agreement, Tung et al. found NFE2L2 increased expression levels in NSCLC cells with TP53 mutations [150]. Another protein with the ability to inhibit the NRF2 pathway is the transcriptional repressor BACH1. BACH1 competes with NRF2 for ARE binding site of HMOX1 gene, in turn decreasing HO-1 levels [184].
5. Therapeutic Strategies for NRF2 Addiction
Classical NSCLC and SCLC treatment involves surgery (whenever possible), chemotherapy and radiotherapy. In recent years, targeted therapy (inhibitors of EGFR, ALK or ROS1 for NSCLC and inhibitors of PARP or DLL3 for SCLC) and immunotherapy (pembrolizumab, nivolumab, durvalumab, etc.) have been incorporated as therapeutic strategies for NSCLC and SCLC [185,186,187]. Many of these therapies have been applied to LUAD with optimal results; however, they are usually ineffective for LUSC. Since approximately 20% of lung cancers are LUSC and the survival rates for these patients remain unacceptably low, the development of targeted therapies for this tumor sub-type is critical [12]. Towards this end, NRF2 could prove to be a promising candidate for LUSC targeted therapy.
The emergence of drug resistance is frequent and it unmasks the need to find new compounds capable of avoiding these side effects [188]. In this regard, numerous studies are focused on the mechanism of resistance to each particular treatment. Recent studies point at the NRF2/KEAP1 pathway as a molecular mechanism linked to the emergence of resistance to chemotherapy treatment resistance, making this transcription factor an ideal target to recover drug sensitivity [97,135,137,138,140]. For this reason, the development of new therapeutic inhibitory strategies for NRF2-addicted lung cancer cells has attracted significant interest. Among them, the use of NRF2 inhibitors, immunotherapies for patients with active NRF2-bearing tumors or inhibitors of downstream NRF2 effectors are being widely studied [74].
5.1. Direct NRF2 Inhibitors
Several small molecules have been shown to inhibit NRF2 activity in tumors harboring KEAP1 or NFE2L2 mutations [26]. Although some of these direct NRF2 inhibitors have been widely used in pre-clinical studies, many of them are of poor efficacy in the clinic, showing low specificity and bioactivity [74]. Moreover, the molecular mechanism of some of these is still not well understood [37].
One of these direct NRF2 inhibitors is brusatol, which was identified as an NRF2 inhibitor in 2011 [189]. This compound is able to increase the response to irradiation combined with chemotherapeutic drugs (such as cisplatin) [190], thus reducing cancer cell proliferation in A549 LUAD cells in vitro and in vivo. Although brusatol enhances the poly-ubiquitination of NRF2, the molecular mechanism of its action is still unknown [189]. Later studies showed that brusatol has low specificity for NRF2 as this compound decreases the expression level of many other short half-life proteins, acting as a general inhibitor of the translation machinery [191]. Another inhibitor used in NSCLC, both for in vitro and in vivo studies, is the flavonoid luteolin [192,193]. Luteolin accelerates NFE2L2 mRNA turnover and sensitizes cells, such as A549, to chemotherapeutic drugs (oxaliplatin, bleomycin and doxorubicin) [192]. Moreover, when combined with cisplatin, it is more effective in reducing tumor growth in A549 LUAD xenografts than cisplatin alone [193]. This compound has been also used in combination with ascorbic acid for the treatment of patients with NFE2L2 mutations [194]. Other studies have shown that luteolin, in low doses, can activate NFE2L2 and HO-1 genes in HepG2 liver hepatocellular cells [195]. Further studies are needed to determine the specific mechanism of luteolin action [74].
Several nuclear factors, such as RXRα [38], retinoic acid receptor alpha (RARα) [196], estrogen receptor alpha (ERα) [197], peroxisome proliferator-activated receptor-γ (PPARγ) [198] or GR [35] have been shown to inhibit the NRF2 pathway. RARα, in the presence of the ligand all-trans retinoic acid (ATRA), forms a complex with the Neh7 domain of NRF2, blocking its ARE binding [38,196]. Similarly, bexarotene is another agonist described for RXRα [199]. Clobetasol propionate (CB) is also an interesting glucocorticoid candidate that was identified from a clinical compound library. CB interferes with NRF2 nuclear translocation via GSK-3β. CB promotes the poly-ubiquitination of NRF2 by β-TrCP, which in turn suppresses the growth of KEAP1 mutant NSCLC lung cancer xenografts [200].
Other groups have carried out alternative chemical screening approaches to find small molecule inhibitors for NRF2 [26]. ML385, ARE expression modulator 1 (AEM1) and 4-(2-Cyclohexylethoxy) aniline (IM3829) were identified as synthetic NRF2 inhibitors in NSCLC lines [201,202,203]. ML385 binds to the Neh1 domain of NRF2 and blocks the ARE binding of NRF2, increasing the efficacy of some chemotherapeutic drugs (carboplatin, doxorubicin and paclitaxel). However, its selectivity for NRF2 has not yet been evaluated [201]. In the case of AEM1, it reduces NRF2 activity without altering NRF2 or KEAP1 protein levels and its combination with doxorubicin sensitizes A549 LUAD cells to etoposide and 5-fluorouracil [202]. Finally, IM3829 reduces NFE2L2 mRNA levels and in combination with radiation, it inhibits the growth of NSCLC xenografts [203].
Some other NRF2 pathway inhibitors used in in vitro studies are trigonelline [204], chrysin (5,7-digydroxyflavone) [205], apigenin (4′,5,7-trihydroxyflavone) [206], halofuginone [207], cordycepin [208] or PHA-767491 and AZ-628 [209].
More recently, newly identified NRF2 inhibitors have been tested in NSCLC lung cells. This is the case for RNA-binding motif protein 47 (RBM47) [210], 3′,4′,5′,5,7-pentamethoxyflavone (PMF) [211], 4-methoxychalcone (4-MC) [212], triptolide [213], homoharringtonine [214], convallatoxin [215], diosmetin [139], flumethasone [216], coroglaucigenin (CGN) [217] and kaempferol [185]. Other studies have focused on disrupting NRF2/KEAP1 binding. This is the case for some potent phytochemicals, such as 3-(Dimethylamino)-3-imino-N-(4-methylphenyl) propanamide or phlorizin [218] or K67 (N-[2-acetonyl-4-(4-ethoxybenzenesulfonyla-mino) naphthalene-1-yl]-4-ethoxybenzenesulfonamide), that disrupt KEAP1-p62 binding [219]. Although most of them are effective in reducing NRF2 expression, more studies are needed to clarify their specificity and action in in vivo models. Moreover, it is still early to know the real possibilities of these inhibitors for cancer treatment due to the lack of clinical studies.
Finally, computational methods are being used to identify new chemical compounds capable of activating (2-nitrofluorene, resorcinol, etc.) or repressing (dexamethasone, sulfisoxazole, etc.) the NRF2 expression in human cells [220]. Further specificity and efficacy tests in lung cancer clinical trials are needed to validate the beneficial effects of NRF2 inhibition, either by itself or on sensitizing lung cancer to chemotherapy [134].
5.2. Immunotherapy for Active-NRF2 Tumors
Tumors escape surveillance and detection expressing PD-L1, which interacts with T cells and suppress the antitumor T cell response [221]. PD-L1 is directly controlled by NRF2 [222] and the tumor microenvironment of NRF2-active lung cancer cells present high levels of immunosuppressive proteins such as PD-L1 [106]. Interestingly, increased PD-L1 expression is associated with NFE2L2 mutations in LUSC and KEAP1 mutations in LUAD. These findings suggest that NFE2L2/KEAP1 mutations/activation could be of benefit for LUSC and LUAD treatment by immunotherapy [223,224]. Furthermore, CTLA-4, which acts as a negative regulator of T cell response, has been a topic of research in NSCLC [221]. Indeed, sensitivity to immunotherapy was detected in NRF2-active Keap1 and Pten LUAD-like mice models that present high levels of PD-L1. When LUAD in these mice were treated with cycles of anti-PD-L1/anti-Ctla-4 compounds, they achieved a surprising reduction in tumor burden. Further research is needed to verify the correlation between immunotherapy and NRF2 pathway activation in clinical trials, although this study suggests that high NRF2 activity tumors, particularly LUAD, could benefit from this treatment [106]. Therefore, activation of NRF2 could help to increase efficacy of immunomodulatory compounds [74].
5.3. Inhibitors of NRF2 Downstream Effectors
Inhibition of NRF2 downstream effectors combined with conventional therapy may result in an increase in patients’ progression-free survival rate. For instance, serine biosynthesis inhibition (CBR-5884) [225]; glutaminolysis inhibition (CB-839) [226]; PPP inhibition (polydatin) [227]; IL11 inhibition; or glutathione synthesis inhibition using, e.g., BSO (buthionine sulfoximine), 2-AAPA, sulfasalazine, or erastin [228,229,230,231,232].
In vivo studies in K-RAS-driven human LUAD xenografts and PDXs exhibiting KEAP1 mutations show a reduction in tumor growth after treatment with glutaminase inhibitor CB-839 [110]. Most likely, targeting some metabolic pathways downstream of NRF2 could be a good strategy for NRF2-active lung cancer cells [134]. In a library screen, Mattheus et al. identified new possible inhibitors for NRF2-targets (lyngbyabellin A, grassypeptolide A and dolastatin 12) that reduce NFE2L2-target gene expression in MDA-MB-231 breast cell line and A549 cell line [233]. At the same time, for pathways that crosstalk with NRF2, pre-existing compounds for these pathways can be used in combination with NRF2–directed compounds [13]. In fact, a phase I trial combining MLN0128 (sapanisertib), an mTOR inhibitor, and CB-839, a glutaminolysis inhibitor, is ongoing in patients with advanced NSCLC (KRAS-mutant LUAD and LUSC) having NFE2L2/KEAP1 mutations [234].
6. Conclusions
Overall, it is noteworthy that while NRF2 exerts cytoprotective effects in the prevention of malignant transformation in healthy tissues, once some tumor types are generated, NRF2 is also important in maintaining the cancerous state by protecting cancer cells from environmental ROS and limiting the damage induced by chemotherapy. This dual role of NRF2 discussed here in the context of NSCLC appears to be dependent on the stage of the tumor. NRF2 functions as a tumor suppressor generally at tumor initiation stages, while NRF2 pro-oncogenic functions are usually found at advanced stages of the tumor. Several reports have determined that lung tumor cells acquire an NRF2 overexpression dependency for maintenance of its malignant phenotype, a process labeled NRF2 addiction. The tumor protection exerted by NRF2 supports the cancer growth via multiple molecular mechanisms. Further research on this dual role of NRF2 is needed to clarify its functions in each cancer stage. The use of human samples, such as human biopsies or 3D cultures (organoids), could be helpful in this effort.
Therefore, it is reasonable to assume that blocking NRF2 activity in fully malignant lung tumors with constitutively active NRF2 may be an important strategy for the treatment of this disease. While previous studies have tried to provide direct NRF2 inhibitors that regulate its levels or action, currently, none have yielded strong and efficient results. Moreover, immunotherapy directed by NRF2 activation status has to be considered, since NRF2-addicted lung cancer cells exhibit high levels of immunosuppressive proteins, such as PD-L1. Nevertheless, it is still early for its use in patients due to the absence of clinical studies. Other therapeutic strategies not focused on direct NRF2 inhibition may be considered; i.e., the exploration of indirect methods of inhibition, such as upstream and/or downstream protein kinases, can be an open new therapeutic field for lung cancer patients. In this regard, screening methods involving gene-editing technologies, such as the CRISPR-CAS system, can be of great help to define new NRF2 regulators. Furthermore, the blockade of other genetic regulators, such as microRNAs, long non-coding RNAs or interference with interacting partners could also be considered as viable targets for NRF2-addicted lung cancer cells.
Acknowledgments
We thank A. González-García for the critical review and NidhiGupta Williams for the editorial assistance.
Author Contributions
Conceptualization, M.S.-O., A.C.C. and A.G.; writing—original draft preparation, M.S.-O. and A.G.; writing—review and editing, A.C.C. and A.G.; supervision, A.C.C. and A.G.; funding acquisition, A.C.C. All authors have read and agreed to the published version of the manuscript.
Funding
This work was funded by grants from the Spanish Ministry of Science and Innovation (SAF2016-79195-R and PID2019-106937RB-I00 to A.C.C.), the Madrid Regional Government (BMD-3804 to A.C.C.) and an AECC (16035-Spanish Association Against Cancer) grant to A.C.C.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Bray F., Ferlay J., Soerjomataram I., Siegel R.L., Torre L.A., Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2018;68:394–424. doi: 10.3322/caac.21492. [DOI] [PubMed] [Google Scholar]
- 2.Cardona A.F., Ricaurte L., Zatarain-Barrón Z.L., Arrieta O. Squamous cell lung cancer: Genomic evolution and personalized therapy. Salud Publica Mex. 2019;61:329–338. doi: 10.21149/10115. [DOI] [PubMed] [Google Scholar]
- 3.Oser M.G., Niederst M.J., Sequist L.V., Engelman J.A. Transformation from non-small-cell lung cancer to small-cell lung cancer: Molecular drivers and cells of origin. Lancet Oncol. 2015;16:e165–e172. doi: 10.1016/S1470-2045(14)71180-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chen Z., Fillmore C.M., Hammerman P.S., Kim C.F., Wong K.-K. Non-small-cell lung cancers: A heterogeneous set of diseases. Nat. Rev. Cancer. 2014;14:535–546. doi: 10.1038/nrc3775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hynds R.E., Frese K.K., Pearce D.R., Grönroos E., Dive C., Swanton C. Progress towards non-small-cell lung cancer models that represent clinical evolutionary trajectories. Open Biol. 2021;11:200247. doi: 10.1098/rsob.200247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zeng Z., Wang Z.-Y., Li Y.-K., Ye D.-M., Zeng J., Hu J.-L., Chen P.-F., Xiao J., Zou J., Li Z.-H. Nuclear factor erythroid 2 (NF-E2)-related factor 2 (Nrf2) in non-small cell lung cancer. Life Sci. 2020;254:117325. doi: 10.1016/j.lfs.2020.117325. [DOI] [PubMed] [Google Scholar]
- 7.Taniguchi H., Sen T., Rudin C.M. Targeted Therapies and Biomarkers in Small Cell Lung Cancer. Front. Oncol. 2020;10:741. doi: 10.3389/fonc.2020.00741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Blanpain C. Tracing the cellular origin of cancer. Nat. Cell Biol. 2013;15:126–134. doi: 10.1038/ncb2657. [DOI] [PubMed] [Google Scholar]
- 9.Kobayashi A., Waku T. New addiction to the NRF2-related factor NRF3 in cancer cells: Ubiquitin-independent proteolysis through the 20S proteasome. Cancer Sci. 2020;111:6–14. doi: 10.1111/cas.14244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kandoth C., McLellan M.D., Vandin F., Ye K., Niu B., Lu C., Xie M., Zhang Q., McMichael J.F., Wyczalkowski M.A., et al. Mutational landscape and significance across 12 major cancer types. Nature. 2013;502:333–339. doi: 10.1038/nature12634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Singh A., Misra V., Thimmulappa R.K., Lee H., Ames S., Hoque M.O., Herman J.G., Baylin S.B., Sidransky D., Grabielson E., et al. Dysfunctional KEAP1-NRF2 interaction in non-small-cell lung cancer. PLoS Med. 2006;3:e420. doi: 10.1371/journal.pmed.0030420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cancer Genome Atlas Research Network Comprehensive genomic characterization of squamous cell lung cancers. Nature. 2012;489:519–525. doi: 10.1038/nature11404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Menegon S., Columbano A., Giordano S. The Dual Roles of NRF2 in Cancer. Trends Mol. Med. 2016;22:578–593. doi: 10.1016/j.molmed.2016.05.002. [DOI] [PubMed] [Google Scholar]
- 14.Solis L.M., Behrens C., Dong W., Suraokar M., Ozburn N.C., Moran C.A., Corvalan A.H., Biswal S., Swisher S.G., Bekele B.N., et al. Nrf2 and Keap1 abnormalities in non-small cell lung carcinoma and association with clinicopathologic features. Clin. Cancer Res. 2010;16:3743–3753. doi: 10.1158/1078-0432.CCR-09-3352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ohta T., Iijima K., Miyamoto M., Nakahara I., Tanaka H., Ohtsuji M., Suzuki T., Kobayashi A., Yokota J., Sakiyama T., et al. Loss of Keap1 function activates Nrf2 and provides advantages for lung cancer cell growth. Cancer Res. 2008;68:1303–1309. doi: 10.1158/0008-5472.CAN-07-5003. [DOI] [PubMed] [Google Scholar]
- 16.Padmanabhan B., Tong K.I., Ohta T., Nakamura Y., Scharlock M., Ohtsuji M., Kang M.I., Kobayashi A., Yokoyama S., Yamamoto M. Structural basis for defects of Keap1 activity provoked by its point mutations in lung cancer. Mol. Cell. 2006;21:689–700. doi: 10.1016/j.molcel.2006.01.013. [DOI] [PubMed] [Google Scholar]
- 17.George J., Walter V., Peifer M., Alexandrov L.B., Seidel D., Leenders F., Maas L., Müller C., Dahmen I., Delhomme T.M., et al. Integrative genomic profiling of large-cell neuroendocrine carcinomas reveals distinct subtypes of high-grade neuroendocrine lung tumors. Nat. Commun. 2018;9:1048. doi: 10.1038/s41467-018-03099-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zhu H., Jia Z., Trush M.A., Li Y.R. Nrf2 Deficiency Promotes Melanoma Growth and Lung Metastasis. React. Oxyg. Species. 2016;2:308–314. doi: 10.20455/ros.2016.853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hammad A., Zheng Z.-H., Gao Y., Namani A., Shi H.-F., Tang X. Identification of novel Nrf2 target genes as prognostic biomarkers in colitis-associated colorectal cancer in Nrf2-deficient mice. Life Sci. 2019;238:116968. doi: 10.1016/j.lfs.2019.116968. [DOI] [PubMed] [Google Scholar]
- 20.Cheung K.L., Lee J.H., Khor T.O., Wu T.-Y., Li G.X., Chan J., Yang C.S., Kong A.-N.T. Nrf2 knockout enhances intestinal tumorigenesis in Apc(min/+) mice due to attenuation of anti-oxidative stress pathway while potentiates inflammation. Mol. Carcinog. 2014;53:77–84. doi: 10.1002/mc.21950. [DOI] [PubMed] [Google Scholar]
- 21.Jeong Y., Hoang N.T., Lovejoy A., Stehr H., Newman A.M., Gentles A.J., Kong W., Truong D., Martin S., Chaudhuri A., et al. Role of KEAP1/NRF2 and TP53 Mutations in Lung Squamous Cell Carcinoma Development and Radiation Resistance. Cancer Discov. 2017;7:86–101. doi: 10.1158/2159-8290.CD-16-0127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Xia D., Zhang X.-R., Ma Y.-L., Zhao Z.-J., Zhao R., Wang Y.-Y. Nrf2 promotes esophageal squamous cell carcinoma (ESCC) resistance to radiotherapy through the CaMKIIα-associated activation of autophagy. Cell Biosci. 2020;10:90. doi: 10.1186/s13578-020-00456-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chen J., Solomides C., Simpkins F., Simpkins H. The role of Nrf2 and ATF2 in resistance to platinum-based chemotherapy. Cancer Chemother. Pharmacol. 2017;79:369–380. doi: 10.1007/s00280-016-3225-1. [DOI] [PubMed] [Google Scholar]
- 24.Bauer A.K., Hill T., Alexander C.-M. The involvement of NRF2 in lung cancer. Oxid. Med. Cell. Longev. 2013;2013:746432. doi: 10.1155/2013/746432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sporn M.B., Liby K.T. NRF2 and cancer: The good, the bad and the importance of context. Nat. Rev. Cancer. 2012;12:564–571. doi: 10.1038/nrc3278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Robertson H., Dinkova-Kostova A.T., Hayes J.D. NRF2 and the Ambiguous Consequences of Its Activation during Initiation and the Subsequent Stages of Tumourigenesis. Cancers. 2020;12:3609. doi: 10.3390/cancers12123609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Baird L., Yamamoto M. The Molecular Mechanisms Regulating the KEAP1-NRF2 Pathway. Mol. Cell. Biol. 2020;40:e00099-20. doi: 10.1128/MCB.00099-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Smolková K., Mikó E., Kovács T., Leguina-Ruzzi A., Sipos A., Bai P. Nuclear Factor Erythroid 2-Related Factor 2 in Regulating Cancer Metabolism. Antioxid. Redox. Signal. 2020;33:966–997. doi: 10.1089/ars.2020.8024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Plafker K.S., Nguyen L., Barneche M., Mirza S., Crawford D., Plafker S.M. The ubiquitin-conjugating enzyme UbcM2 can regulate the stability and activity of the antioxidant transcription factor Nrf2. J. Biol. Chem. 2010;285:23064–23074. doi: 10.1074/jbc.M110.121913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tong K.I., Katoh Y., Kusunoki H., Itoh K., Tanaka T., Yamamoto M. Keap1 recruits Neh2 through binding to ETGE and DLG motifs: Characterization of the two-site molecular recognition model. Mol. Cell. Biol. 2006;26:2887–2900. doi: 10.1128/MCB.26.8.2887-2900.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tong K.I., Padmanabhan B., Kobayashi A., Shang C., Hirotsu Y., Yokoyama S., Yamamoto M. Different electrostatic potentials define ETGE and DLG motifs as hinge and latch in oxidative stress response. Mol. Cell. Biol. 2007;27:7511–7521. doi: 10.1128/MCB.00753-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Nioi P., Nguyen T., Sherratt P.J., Pickett C.B. The carboxy-terminal Neh3 domain of Nrf2 is required for transcriptional activation. Mol. Cell. Biol. 2005;25:10895–10906. doi: 10.1128/MCB.25.24.10895-10906.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zhang J., Hosoya T., Maruyama A., Nishikawa K., Maher J.M., Ohta T., Motohashi H., Fukamizu A., Shibahara S., Itoh K., et al. Nrf2 Neh5 domain is differentially utilized in the transactivation of cytoprotective genes. Biochem. J. 2007;404:459–466. doi: 10.1042/BJ20061611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kim J.-H., Yu S., Chen J.D., Kong A.N. The nuclear cofactor RAC3/AIB1/SRC-3 enhances Nrf2 signaling by interacting with transactivation domains. Oncogene. 2013;32:514–527. doi: 10.1038/onc.2012.59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Alam M.M., Okazaki K., Nguyen L.T.T., Ota N., Kitamura H., Murakami S., Shima H., Igarashi K., Sekine H., Motohashi H. Glucocorticoid receptor signaling represses the antioxidant response by inhibiting histone acetylation mediated by the transcriptional activator NRF2. J. Biol. Chem. 2017;292:7519–7530. doi: 10.1074/jbc.M116.773960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Chowdhry S., Zhang Y., McMahon M., Sutherland C., Cuadrado A., Hayes J.D. Nrf2 is controlled by two distinct β-TrCP recognition motifs in its Neh6 domain, one of which can be modulated by GSK-3 activity. Oncogene. 2013;32:3765–3781. doi: 10.1038/onc.2012.388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kang J.-S., Nam L.B., Yoo O.-K., Keum Y.-S. Molecular mechanisms and systemic targeting of NRF2 dysregulation in cancer. Biochem. Pharmacol. 2020;177:114002. doi: 10.1016/j.bcp.2020.114002. [DOI] [PubMed] [Google Scholar]
- 38.Wang H., Liu K., Geng M., Gao P., Wu X., Hai Y., Li Y., Li Y., Luo L., Hayes J. D., et al. RXRα inhibits the NRF2-ARE signaling pathway through a direct interaction with the Neh7 domain of NRF2. Cancer Res. 2013;73:3097–3108. doi: 10.1158/0008-5472.CAN-12-3386. [DOI] [PubMed] [Google Scholar]
- 39.Kobayashi A., Kang M.-I., Okawa H., Ohtsuji M., Zenke Y., Chiba T., Igarashi K., Yamamoto M. Oxidative stress sensor Keap1 functions as an adaptor for Cul3-based E3 ligase to regulate proteasomal degradation of Nrf2. Mol. Cell. Biol. 2004;24:7130–7139. doi: 10.1128/MCB.24.16.7130-7139.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Liu Y., Kern J.T., Walker J.R., Johnson J.A., Schultz P.G., Luesch H. A genomic screen for activators of the antioxidant response element. Proc. Natl. Acad. Sci. USA. 2007;104:5205–5210. doi: 10.1073/pnas.0700898104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.McMahon M., Itoh K., Yamamoto M., Hayes J.D. Keap1-dependent proteasomal degradation of transcription factor Nrf2 contributes to the negative regulation of antioxidant response element-driven gene expression. J. Biol. Chem. 2003;278:21592–21600. doi: 10.1074/jbc.M300931200. [DOI] [PubMed] [Google Scholar]
- 42.Wakabayashi N., Itoh K., Wakabayashi J., Motohashi H., Noda S., Takahashi S., Imakado S., Kotsuji T., Otsuka F., Roop D.R., et al. Keap1-null mutation leads to postnatal lethality due to constitutive Nrf2 activation. Nat. Genet. 2003;35:238–245. doi: 10.1038/ng1248. [DOI] [PubMed] [Google Scholar]
- 43.Watai Y., Kobayashi A., Nagase H., Mizukami M., McEvoy J., Singer J.D., Itoh K., Yamamoto M. Subcellular localization and cytoplasmic complex status of endogenous Keap1. Genes Cells. 2007;12:1163–1178. doi: 10.1111/j.1365-2443.2007.01118.x. [DOI] [PubMed] [Google Scholar]
- 44.Kopacz A., Kloska D., Forman H.J., Jozkowicz A., Grochot-Przeczek A. Beyond repression of Nrf2: An update on Keap1. Free Radic. Biol. Med. 2020;157:63–74. doi: 10.1016/j.freeradbiomed.2020.03.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Zipper L.M., Mulcahy R.T. The Keap1 BTB/POZ dimerization function is required to sequester Nrf2 in cytoplasm. J. Biol. Chem. 2002;277:36544–36552. doi: 10.1074/jbc.M206530200. [DOI] [PubMed] [Google Scholar]
- 46.McMahon M., Lamont D.J., Beattie K.A., Hayes J.D. Keap1 perceives stress via three sensors for the endogenous signaling molecules nitric oxide, zinc, and alkenals. Proc. Natl. Acad. Sci. USA. 2010;107:18838–18843. doi: 10.1073/pnas.1007387107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Li X., Zhang D., Hannink M., Beamer L.J. Crystal structure of the Kelch domain of human Keap1. J. Biol. Chem. 2004;279:54750–54758. doi: 10.1074/jbc.M410073200. [DOI] [PubMed] [Google Scholar]
- 48.Katoh Y., Iida K., Kang M.-I., Kobayashi A., Mizukami M., Tong K.I., McMahon M., Hayes J.D., Itoh K., Yamamoto M. Evolutionary conserved N-terminal domain of Nrf2 is essential for the Keap1-mediated degradation of the protein by proteasome. Arch. Biochem. Biophys. 2005;433:342–350. doi: 10.1016/j.abb.2004.10.012. [DOI] [PubMed] [Google Scholar]
- 49.Nam L.B., Keum Y.-S. Binding partners of NRF2: Functions and regulatory mechanisms. Arch. Biochem. Biophys. 2019;678:108184. doi: 10.1016/j.abb.2019.108184. [DOI] [PubMed] [Google Scholar]
- 50.Taguchi K., Yamamoto M. The KEAP1-NRF2 System as a Molecular Target of Cancer Treatment. Cancers. 2020;13:46. doi: 10.3390/cancers13010046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Rojo de la Vega M., Chapman E., Zhang D.D. NRF2 and the Hallmarks of Cancer. Cancer Cell. 2018;34:21–43. doi: 10.1016/j.ccell.2018.03.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.McMahon M., Thomas N., Itoh K., Yamamoto M., Hayes J.D. Dimerization of substrate adaptors can facilitate cullin-mediated ubiquitylation of proteins by a “tethering” mechanism: A two-site interaction model for the Nrf2-Keap1 complex. J. Biol. Chem. 2006;281:24756–24768. doi: 10.1074/jbc.M601119200. [DOI] [PubMed] [Google Scholar]
- 53.Baird L., Llères D., Swift S., Dinkova-Kostova A.T. Regulatory flexibility in the Nrf2-mediated stress response is conferred by conformational cycling of the Keap1-Nrf2 protein complex. Proc. Natl. Acad. Sci. USA. 2013;110:15259–15264. doi: 10.1073/pnas.1305687110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Dinkova-Kostova A.T., Holtzclaw W.D., Cole R.N., Itoh K., Wakabayashi N., Katoh Y., Yamamoto M., Talalay P. Direct evidence that sulfhydryl groups of Keap1 are the sensors regulating induction of phase 2 enzymes that protect against carcinogens and oxidants. Proc. Natl. Acad. Sci. USA. 2002;99:11908–11913. doi: 10.1073/pnas.172398899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Luo Y., Eggler A.L., Liu D., Liu G., Mesecar A.D., van Breemen R.B. Sites of alkylation of human Keap1 by natural chemoprevention agents. J. Am. Soc. Mass Spectrom. 2007;18:2226–2232. doi: 10.1016/j.jasms.2007.09.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Soucy T.A., Smith P.G., Milhollen M.A., Berger A.J., Gavin J.M., Adhikari S., Brownell J.E., Burker K.E., Cardin D.P., Critchley S., et al. An inhibitor of NEDD8-activating enzyme as a new approach to treat cancer. Nature. 2009;458:732–736. doi: 10.1038/nature07884. [DOI] [PubMed] [Google Scholar]
- 57.Venugopal R., Jaiswal A.K. Nrf2 and Nrf1 in association with Jun proteins regulate antioxidant response element-mediated expression and coordinated induction of genes encoding detoxifying enzymes. Oncogene. 1998;17:3145–3156. doi: 10.1038/sj.onc.1202237. [DOI] [PubMed] [Google Scholar]
- 58.Jaramillo M.C., Zhang D.D. The emerging role of the Nrf2-Keap1 signaling pathway in cancer. Genes Dev. 2013;27:2179–2191. doi: 10.1101/gad.225680.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Zhang D., Rennhack J., Andrechek E.R., Rockwell C.E., Liby K.T. Identification of an Unfavorable Immune Signature in Advanced Lung Tumors from Nrf2-Deficient Mice. Antioxid. Redox. Signal. 2018;29:1535–1552. doi: 10.1089/ars.2017.7201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Raghunath A., Sundarraj K., Nagarajan R., Arfuso F., Bian J., Kumar A.P., Sethi G., Perumal E. Antioxidant response elements: Discovery, classes, regulation and potential applications. Redox. Biol. 2018;17:297–314. doi: 10.1016/j.redox.2018.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Kaspar J.W., Jaiswal A.K. Tyrosine phosphorylation controls nuclear export of Fyn, allowing Nrf2 activation of cytoprotective gene expression. FASEB J. 2011;25:1076–1087. doi: 10.1096/fj.10-171553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Cuadrado A. Structural and functional characterization of Nrf2 degradation by glycogen synthase kinase 3/β-TrCP. Free Radic. Biol. Med. 2015;88:147–157. doi: 10.1016/j.freeradbiomed.2015.04.029. [DOI] [PubMed] [Google Scholar]
- 63.Jain A.K., Jaiswal A.K. GSK-3beta acts upstream of Fyn kinase in regulation of nuclear export and degradation of NF-E2 related factor 2. J. Biol. Chem. 2007;282:16502–16510. doi: 10.1074/jbc.M611336200. [DOI] [PubMed] [Google Scholar]
- 64.Taguchi K., Hirano I., Itoh T., Tanaka M., Miyajima A., Suzuki A., Motohashi H., Yamamoto M. Nrf2 enhances cholangiocyte expansion in Pten-deficient livers. Mol. Cell. Biol. 2014;34:900–913. doi: 10.1128/MCB.01384-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Huang H.-C., Nguyen T., Pickett C.B. Phosphorylation of Nrf2 at Ser-40 by protein kinase C regulates antioxidant response element-mediated transcription. J. Biol. Chem. 2002;277:42769–42774. doi: 10.1074/jbc.M206911200. [DOI] [PubMed] [Google Scholar]
- 66.Wu T., Zhao F., Gao B., Tan C., Yagishita N., Nakajima T., Wong P.K., Chapman E., Fang D., Zhang D.D. Hrd1 suppresses Nrf2-mediated cellular protection during liver cirrhosis. Genes Dev. 2014;28:708–722. doi: 10.1101/gad.238246.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Lo J.Y., Spatola B.N., Curran S.P. WDR23 regulates NRF2 independently of KEAP1. PLoS Genet. 2017;13:e1006762. doi: 10.1371/journal.pgen.1006762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Lee D.F., Kuo H.P., Liu M., Chou C.K., Xia W., Du Y., Shen J., Chen C.-T., Huo L., Hsu M.-C., et al. KEAP1 E3 ligase-mediated downregulation of NF-kappaB signaling by targeting IKKbeta. Mol. Cell. 2009;36:131–140. doi: 10.1016/j.molcel.2009.07.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Thu K.L., Pikor L.A., Chari R., Wilson I.M., Macaulay C.E., English J.C., Tsao M.-S., Gazdar A.F., Lam S., Lam W.L., et al. Genetic disruption of KEAP1/CUL3 E3 ubiquitin ligase complex components is a key mechanism of NF-KappaB pathway activation in lung cancer. J. Thorac. Oncol. 2011;6:1521–1529. doi: 10.1097/JTO.0b013e3182289479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Tian H., Zhang B., Di J., Jiang G., Chen F., Li H., Li L., Pei D., Zheng J. Keap1: One Stone kills three birds Nrf2, IKKb and Bcl-2/Bcl-xL. Cancer Lett. 2012;325:25–34. doi: 10.1016/j.canlet.2012.06.007. [DOI] [PubMed] [Google Scholar]
- 71.Wardyn J.D., Ponsford A.H., Sanderson C.M. Dissecting molecular cross-talk between NRF2 and NF-kB response pathways. Biochem. Soc. Trans. 2015;43:621–626. doi: 10.1042/BST20150014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Yang Y., Karakhanova S., Werner J., Bazhin A.V. Reactive oxygen species in cancer biology and anticancer therapy. Curr. Med. Chem. 2013;20:3677–3692. doi: 10.2174/0929867311320999165. [DOI] [PubMed] [Google Scholar]
- 73.DeBlasi J.M., DeNicola G.M. Dissecting the Crosstalk between NRF2 Signaling and Metabolic Processes in Cancer. Cancers. 2020;12:3023. doi: 10.3390/cancers12103023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Hammad A., Namani A., Elshaer M., Wang X.J., Tang X. “NRF2 addiction” in lung cancer cells and its impact on cancer therapy. Cancer Lett. 2019;467:40–49. doi: 10.1016/j.canlet.2019.09.016. [DOI] [PubMed] [Google Scholar]
- 75.Tao S., Rojo de la Vega M., Chapman E., Ooi A., Zhang D.D. The effects of NRF2 modulation on the initiation and progression of chemically and genetically induced lung cancer. Mol. Carcinog. 2018;57:182–192. doi: 10.1002/mc.22745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Satoh H., Moriguchi T., Takai J., Ebina M., Yamamoto M. Nrf2 prevents initiation but accelerates progression through the Kras signaling pathway during lung carcinogenesis. Cancer Res. 2013;73:4158–4168. doi: 10.1158/0008-5472.CAN-12-4499. [DOI] [PubMed] [Google Scholar]
- 77.Satoh H., Moriguchi T., Saigusa D., Baird L., Yu L., Rokutan H., Igarashi K., Ebina M., Shibata T., Yamamoto M. NRF2 Intensifies Host Defense Systems to Prevent Lung Carcinogenesis, but After Tumor Initiation Accelerates Malignant Cell Growth. Cancer Res. 2016;76:3088–3096. doi: 10.1158/0008-5472.CAN-15-1584. [DOI] [PubMed] [Google Scholar]
- 78.Higgins L.G., Kelleher M.O., Eggleston I.M., Itoh K., Yamamoto M., Hayes J.D. Transcription factor Nrf2 mediates an adaptive response to sulforaphane that protects fibroblasts in vitro against the cytotoxic effects of electrophiles, peroxides and redox-cycling agents. Toxicol. Appl. Pharmacol. 2009;237:267–280. doi: 10.1016/j.taap.2009.03.005. [DOI] [PubMed] [Google Scholar]
- 79.Singh B., Bhat N.K., Bhat H.K. Induction of NAD(P)H-quinone oxidoreductase 1 by antioxidants in female ACI rats is associated with decrease in oxidative DNA damage and inhibition of estrogen-induced breast cancer. Carcinogenesis. 2012;33:156–163. doi: 10.1093/carcin/bgr237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Sun X., Wang Y., Ji K., Liu Y., Kong Y., Nie S., Li N., Hao J., Xie Y., Xu C., et al. NRF2 preserves genomic integrity by facilitating ATR activation and G2 cell cycle arrest. Nucleic Acids Res. 2020;48:9109–9123. doi: 10.1093/nar/gkaa631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Liu F., Ichihara S., Valentine W.M., Itoh K., Yamamoto M., Sheik Mohideen S., Kitoh J., Ichihara G. Increased susceptibility of Nrf2-null mice to 1-bromopropane-induced hepatotoxicity. Toxicol. Sci. 2010;115:596–606. doi: 10.1093/toxsci/kfq075. [DOI] [PubMed] [Google Scholar]
- 82.Wang J., Konishi T. Nuclear factor (erythroid-derived 2)-like 2 antioxidative response mitigates cytoplasmic radiation-induced DNA double-strand breaks. Cancer Sci. 2019;110:686–696. doi: 10.1111/cas.13916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Ikeda H., Nishi S., Sakai M. Transcription factor Nrf2/MafK regulates rat placental glutathione S-transferase gene during hepatocarcinogenesis. Biochem. J. 2004;380:515–521. doi: 10.1042/bj20031948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Ramos-Gomez M., Dolan P.M., Itoh K., Yamamoto M., Kensler T.W. Interactive effects of nrf2 genotype and oltipraz on benzo[a]pyrene-DNA adducts and tumor yield in mice. Carcinogenesis. 2003;24:461–467. doi: 10.1093/carcin/24.3.461. [DOI] [PubMed] [Google Scholar]
- 85.Satoh H., Moriguchi T., Taguchi K., Takai J., Maher J.M., Suzuki T., Winnard P.T., Raman V., Ebina M., Nukiwa T., et al. Nrf2-deficiency creates a responsive microenvironment for metastasis to the lung. Carcinogenesis. 2010;31:1833–1843. doi: 10.1093/carcin/bgq105. [DOI] [PubMed] [Google Scholar]
- 86.Kang Y.P., Torrente L., Falzone A., Elkins C.M., Liu M., Asara J.M., Dibble C.C., DeNicola G.M. Cysteine dioxygenase 1 is a metabolic liability for non-small cell lung cancer. eLife. 2019;8:e45572. doi: 10.7554/eLife.45572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Sharma S., Gao P., Steele V.E. The chemopreventive efficacy of inhaled oltipraz particulates in the B[a]P-induced A/J mouse lung adenoma model. Carcinogenesis. 2006;27:1721–1727. doi: 10.1093/carcin/bgl052. [DOI] [PubMed] [Google Scholar]
- 88.Traka M.H., Melchini A., Coode-Bate J., Al Kadhi O., Saha S., Defernez M., Troncoso-Rey P., Kibblewhite H., O’Neill C.M., Bernuzzi F., et al. Transcriptional changes in prostate of men on active surveillance after a 12-mo glucoraphanin-rich broccoli intervention-results from the Effect of Sulforaphane on prostate CAncer PrEvention (ESCAPE) randomized controlled trial. Am. J. Clin. Nutr. 2019;109:1133–1144. doi: 10.1093/ajcn/nqz012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Hong D.S., Kurzrock R., Supko J.G., He X., Naing A., Wheler J., Lawrence D., Eder J.P., Meyer C.J., Ferguson D.A., et al. A phase I first-in-Human trial of Bardoxolone methyl in patients with advanced solid tumors and lymphomas. Clin. Cancer Res. 2012;18:3396–3406. doi: 10.1158/1078-0432.CCR-11-2703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Saddawi-Konefka R., Seelige R., Gross E.T.E., Levy E., Searles S.C., Washington A., Santosa E.K., Liu B., O’Sullivan T.E., Harismendy O., et al. Nrf2 Induces IL-17D to Mediate Tumor and Virus Surveillance. Cell Rep. 2016;16:2348–2358. doi: 10.1016/j.celrep.2016.07.075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Itoh K., Chiba T., Takahashi S., Ishii T., Igarashi K., Katoh Y., Oyake T., Hayashi N., Satoh K., Hatayama I., et al. An Nrf2/small Maf heterodimer mediates the induction of phase II detoxifying enzyme genes through antioxidant response elements. Biochem. Biophys. Res. Commun. 1997;236:313–322. doi: 10.1006/bbrc.1997.6943. [DOI] [PubMed] [Google Scholar]
- 92.Hayashi M., Kuga A., Suzuki M., Panda H., Kitamura H., Motohashi H., Yamamoto M. Microenvironmental Activation of Nrf2 Restricts the Progression of Nrf2-Activated Malignant Tumors. Cancer Res. 2020;80:3331–3344. doi: 10.1158/0008-5472.CAN-19-2888. [DOI] [PubMed] [Google Scholar]
- 93.Chen L.-H., Liao C.-Y., Lai L.-C., Tsai M.-H., Chuang E.Y. Semaphorin 6A Attenuates the Migration Capability of Lung Cancer Cells via the NRF2/HMOX1 Axis. Sci. Rep. 2019;9:13302. doi: 10.1038/s41598-019-49874-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Ryu D., Lee J.-H., Kwak M.-K. NRF2 level is negatively correlated with TGF-β1-induced lung cancer motility and migration via NOX4-ROS signaling. Arch. Pharm. Res. 2020;43:1297–1310. doi: 10.1007/s12272-020-01298-z. [DOI] [PubMed] [Google Scholar]
- 95.Singh A., Daemen A., Nickles D., Jeon S.-M., Foreman O., Sudini K., Gnad F., Lajoie S., Gour N., Mitzner W., et al. NRF2 Activation Promotes Aggressive Lung Cancer and Associates with Poor Clinical Outcomes. Clin. Cancer Res. 2021;27:877–888. doi: 10.1158/1078-0432.CCR-20-1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Gandara D.R., Hammerman P.S., Sos M.L., Lara P.N., Hirsch F.R. Squamous Cell Lung Cancer: From Tumor Genomics to Cancer Therapeutics. Clin. Cancer Res. 2015;21:2236–2243. doi: 10.1158/1078-0432.CCR-14-3039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Frank R., Scheffler M., Merkelbach-Bruse S., Ihle M.A., Kron A., Rauer M., Ueckeroth F., König K., Michels S., Fischer R., et al. Clinical and Pathological Characteristics of KEAP1- and NFE2L2-Mutated Non-Small Cell Lung Carcinoma (NSCLC) Clin. Cancer Res. 2018;24:3087–3096. doi: 10.1158/1078-0432.CCR-17-3416. [DOI] [PubMed] [Google Scholar]
- 98.Zhang M., Zhang L., Li Y., Sun F., Fang Y., Zhang R., Wu J., Zhou G., Song H., Xue L., et al. Exome sequencing identifies somatic mutations in novel driver genes in non-small cell lung cancer. Aging. 2020;12:13701–13715. doi: 10.18632/aging.103500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Zhang Y., Fan H., Fang S., Wang L., Chen L., Jin Y., Jiang W., Lin Z., Shi Y., Zhan C., et al. Mutations and expression of the NFE2L2/KEAP1/CUL3 pathway in Chinese patients with lung squamous cell carcinoma. J. Thorac. Dis. 2016;8:1639–1644. doi: 10.21037/jtd.2016.06.08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Sasaki H., Shitara M., Yokota K., Hikosaka Y., Moriyama S., Yano M., Fujii Y. Increased NRF2 gene (NFE2L2) copy number correlates with mutations in lung squamous cell carcinomas. Mol. Med. Rep. 2012;6:391–394. doi: 10.3892/mmr.2012.921. [DOI] [PubMed] [Google Scholar]
- 101.Sparaneo A., Fabrizio F.P., la Torre A., Graziano P., Di Maio M., Fontana A., Bisceglia M., Rossi A., Pizzolitto S., De Maglio G., et al. Effects of KEAP1 Silencing on the Regulation of NRF2 Activity in Neuroendocrine Lung Tumors. Int. J. Mol. Sci. 2019;20:2531. doi: 10.3390/ijms20102531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Cloer E.W., Siesser P.F., Cousins E.M., Goldfarb D., Mowrey D.D., Harrison J.S., Weir S.J., Dokholyan N.V., Major M.B. p62-Dependent Phase Separation of Patient-Derived KEAP1 Mutations and NRF2. Mol. Cell. Biol. 2018;38:e00644-17. doi: 10.1128/MCB.00644-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Zhao J., Lin X., Meng D., Zeng L., Zhuang R., Huang S., Lv W., Hu J. Nrf2 Mediates Metabolic Reprogramming in Non-Small Cell Lung Cancer. Front. Oncol. 2020;10:578315. doi: 10.3389/fonc.2020.578315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Mitsuishi Y., Taguchi K., Kawatani Y., Shibata T., Nukiwa T., Aburatani H., Yamamoto M., Motohashi H. Nrf2 redirects glucose and glutamine into anabolic pathways in metabolic reprogramming. Cancer Cell. 2012;22:66–79. doi: 10.1016/j.ccr.2012.05.016. [DOI] [PubMed] [Google Scholar]
- 105.Best S.A., Ding S., Kersbergen A., Dong X., Song J.-Y., Xie Y., Reljic B., Kaiming L., Vince J.E., Rathi V., et al. Distinct initiating events underpin the immune and metabolic heterogeneity of KRAS-mutant lung adenocarcinoma. Nat. Commun. 2019;10:4190. doi: 10.1038/s41467-019-12164-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Best S.A., De Souza D.P., Kersbergen A., Policheni A.N., Dayalan S., Tull D., Rathi V., Gray D.H., Ritchie M.E., McConville M.J., et al. Synergy between the KEAP1/NRF2 and PI3K Pathways Drives Non-Small-Cell Lung Cancer with an Altered Immune Microenvironment. Cell Metab. 2018;27:935–943. doi: 10.1016/j.cmet.2018.02.006. [DOI] [PubMed] [Google Scholar]
- 107.Leung E.L.-H., Fan X.-X., Wong M.P., Jiang Z.-H., Liu Z.-Q., Yao X.-J., Lu L.-L., Zhou Y.-L., Tin V.P.-C., Liu L., et al. Targeting Tyrosine Kinase Inhibitor-Resistant Non-Small Cell Lung Cancer by Inducing Epidermal Growth Factor Receptor Degradation via Methionine 790 Oxidation. Antioxid. Redox. Signal. 2016;24:263–279. doi: 10.1089/ars.2015.6420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Zeng C., Wu Q., Wang J., Yao B., Ma L., Yang Z., Li J., Liu B. NOX4 supports glycolysis and promotes glutamine metabolism in non-small cell lung cancer cells. Free Radic. Biol. Med. 2016;101:236–248. doi: 10.1016/j.freeradbiomed.2016.10.500. [DOI] [PubMed] [Google Scholar]
- 109.Wu Q., Yao B., Li N., Ma L., Deng Y., Yang Y., Zeng C., Yang Z., Liu B. Nrf2 mediates redox adaptation in NOX4-overexpressed non-small cell lung cancer cells. Exp. Cell Res. 2017;352:245–254. doi: 10.1016/j.yexcr.2017.02.014. [DOI] [PubMed] [Google Scholar]
- 110.Romero R., Sayin V.I., Davidson S.M., Bauer M.R., Singh S.X., LeBoeuf S.E., Karakousi T.R., Ellis D.C., Bhutkar A., Sánchez-Rivera F.J., et al. Keap1 loss promotes Kras-driven lung cancer and results in dependence on glutaminolysis. Nat. Med. 2017;23:1362–1368. doi: 10.1038/nm.4407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Binkley M.S., Jeon Y.-J., Nesselbush M., Moding E.J., Nabet B.Y., Almanza D., Kunder C., Stehr H., Yoo C.H., Rhee S., et al. KEAP1/NFE2L2 Mutations Predict Lung Cancer Radiation Resistance That Can Be Targeted by Glutaminase Inhibition. Cancer Discov. 2020;10:1826–1841. doi: 10.1158/2159-8290.CD-20-0282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Galan-Cobo A., Sitthideatphaiboon P., Qu X., Poteete A., Pisegna M.A., Tong P., Chen P.-H., Boroughs L.K., Rodriguez M.L.M., Zhang W., et al. LKB1 and KEAP1/NRF2 Pathways Cooperatively Promote Metabolic Reprogramming with Enhanced Glutamine Dependence in KRAS-Mutant Lung Adenocarcinoma. Cancer Res. 2019;79:3251–3267. doi: 10.1158/0008-5472.CAN-18-3527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.DeNicola G.M., Chen P.-H., Mullarky E., Sudderth J.A., Hu Z., Wu D., Tang H., Xie Y., Asara J.M., Huffman K.E., et al. NRF2 regulates serine biosynthesis in non-small cell lung cancer. Nat. Genet. 2015;47:1475–1481. doi: 10.1038/ng.3421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Singh A., Bodas M., Wakabayashi N., Bunz F., Biswal S. Gain of Nrf2 function in non-small-cell lung cancer cells confers radioresistance. Antioxid. Redox. Signal. 2010;13:1627–1637. doi: 10.1089/ars.2010.3219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Takahashi N., Cho P., Selfors L.M., Kuiken H.J., Kaul R., Fujiwara T., Harris I.S., Zhang T., Gygi S.P., Brugge J.S., et al. 3D Culture Models with CRISPR Screens Reveal Hyperactive NRF2 as a Prerequisite for Spheroid Formation via Regulation of Proliferation and Ferroptosis. Mol. Cell. 2020;80:828–844. doi: 10.1016/j.molcel.2020.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Okazaki K., Anzawa H., Liu Z., Ota N., Kitamura H., Onodera Y., Alam M.M., Matsumaru D., Suzuki T., Katsuoka F., et al. Enhancer remodeling promotes tumor-initiating activity in NRF2-activated non-small cell lung cancers. Nat. Commun. 2020;11:5911. doi: 10.1038/s41467-020-19593-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Li Z., Zhang Y., Jin T., Men J., Lin Z., Qi P., Piao Y., Yan G. NQO1 protein expression predicts poor prognosis of non-small cell lung cancers. BMC Cancer. 2015;15:207. doi: 10.1186/s12885-015-1227-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Zhou J., Zhang S., Xu Y., Ye W., Li Z., Chen Z., He Z. Cullin 3 overexpression inhibits lung cancer metastasis and is associated with survival of lung adenocarcinoma. Clin. Exp. Metastasis. 2020;37:115–124. doi: 10.1007/s10585-019-09988-9. [DOI] [PubMed] [Google Scholar]
- 119.Tong Y.-H., Zhang B., Yan Y.-Y., Fan Y., Yu J.-W., Kong S.-S., Zhang D., Fuang L., Su D., Lin N.-M. Dual-negative expression of Nrf2 and NQO1 predicts superior outcomes in patients with non-small cell lung cancer. Oncotarget. 2017;8:45750–45758. doi: 10.18632/oncotarget.17403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.MacLeod A.K., Acosta-Jiménez L., Coates P.J., McMahon M., Carey F.A., Honda T., Hayes J.D., Henderson C.J., Wolf C.R. Aldo-keto reductases are biomarkers of NRF2 activity and are co-ordinately overexpressed in non-small cell lung cancer. Br. J. Cancer. 2017;117:e1. doi: 10.1038/bjc.2017.80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Jiang W., Meng L., Xu G., Lv C., Wang H., Tian H., Chen R., Jiao B., Wang B., Huang C. Wentilactone A induces cell apoptosis by targeting AKR1C1 gene via the IGF-1R/IRS1/PI3K/AKT/Nrf2/FLIP/Caspase-3 signaling pathway in small cell lung cancer. Oncol. Lett. 2018;16:6445–6457. doi: 10.3892/ol.2018.9486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Hung J.-J., Yeh Y.-C., Hsu W.-H. Prognostic significance of AKR1B10 in patients with resected lung adenocarcinoma. Thorac. Cancer. 2018;9:1492–1499. doi: 10.1111/1759-7714.12863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Alnouti Y., Klaassen C.D. Tissue distribution, ontogeny, and regulation of aldehyde dehydrogenase (Aldh) enzymes mRNA by prototypical microsomal enzyme inducers in mice. Toxicol. Sci. 2008;101:51–64. doi: 10.1093/toxsci/kfm280. [DOI] [PubMed] [Google Scholar]
- 124.Yoon D.S., Choi Y., Lee J.W. Cellular localization of NRF2 determines the self-renewal and osteogenic differentiation potential of human MSCs via the P53-SIRT1 axis. Cell Death Dis. 2016;7:e2093. doi: 10.1038/cddis.2016.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Li Z., Xu L., Tang N., Xu Y., Ye X., Shen S., Niu X., Lu S., Chen Z. The polycomb group protein EZH2 inhibits lung cancer cell growth by repressing the transcription factor Nrf2. FEBS Lett. 2014;588:3000–3007. doi: 10.1016/j.febslet.2014.05.057. [DOI] [PubMed] [Google Scholar]
- 126.Vartanian S., Lee J., Klijn C., Gnad F., Bagniewska M., Schaefer G., Zhang D., Tan J., Watson S.A., Liu L., et al. ERBB3 and IGF1R Signaling Are Required for Nrf2-Dependent Growth in KEAP1-Mutant Lung Cancer. Cancer Res. 2019;79:4828–4839. doi: 10.1158/0008-5472.CAN-18-2086. [DOI] [PubMed] [Google Scholar]
- 127.Krall E.B., Wang B., Munoz D.M., Ilic N., Raghavan S., Niederst M.J., Yu K., Ruddy D.A., Aguirre A.J., Kim J.W., et al. KEAP1 loss modulates sensitivity to kinase targeted therapy in lung cancer. eLife. 2017;6:e18870. doi: 10.7554/eLife.18970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Shibata T., Saito S., Kokubu A., Suzuki T., Yamamoto M., Hirohashi S. Global downstream pathway analysis reveals a dependence of oncogenic NF-E2-related factor 2 mutation on the mTOR growth signaling pathway. Cancer Res. 2010;70:9095–9105. doi: 10.1158/0008-5472.CAN-10-0384. [DOI] [PubMed] [Google Scholar]
- 129.Aljohani H.M., Aittaleb M., Furgason J.M., Amaya P., Deeb A., Chalmers J.J., Bahassi E.M. Genetic mutations associated with lung cancer metastasis to the brain. Mutagenesis. 2018;33:137–145. doi: 10.1093/mutage/gey003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Lignitto L., LeBoeuf S.E., Homer H., Jiang S., Askenazi M., Karakousi T.R., Pass H.I., Bhutkar A.J., Tsirigos A., Ueberheide B., et al. Nrf2 Activation Promotes Lung Cancer Metastasis by Inhibiting the Degradation of Bach1. Cell. 2019;178:316–329. doi: 10.1016/j.cell.2019.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Cai M.-C., Chen M., Ma P., Wu J., Lu H., Zhang S., Liu J., Zhao X., Zhuang G., Yu Z., et al. Clinicopathological, microenvironmental and genetic determinants of molecular subtypes in KEAP1/NRF2-mutant lung cancer. Int. J. Cancer. 2019;144:788–801. doi: 10.1002/ijc.31975. [DOI] [PubMed] [Google Scholar]
- 132.Tian Y., Liu Q., He X., Yuan X., Chen Y., Chu Q., Wu K. Emerging roles of Nrf2 signal in non-small cell lung cancer. J. Hematol. Oncol. 2016;9:14. doi: 10.1186/s13045-016-0246-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Namani A., Liu K., Wang S., Zhou X., Liao Y., Wang H., Wang X.J., Tang X. Genome-wide global identification of NRF2 binding sites in A549 non-small cell lung cancer cells by ChIP-Seq reveals NRF2 regulation of genes involved in focal adhesion pathways. Aging. 2019;11:12600–12623. doi: 10.18632/aging.102590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Best S.A., Sutherland K.D. “Keaping” a lid on lung cancer: The Keap1-Nrf2 pathway. Cell Cycle. 2018;17:1696–1707. doi: 10.1080/15384101.2018.1496756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Choi M., Kadara H., Zhang J., Parra E.R., Rodríguez-Canales J., Gaffney S.G., Zhao Z., Behrens C., Fujimoto J., Chow C., et al. Mutation profiles in early-stage lung squamous cell carcinoma with clinical follow-up and correlation with markers of immune function. Ann. Oncol. 2017;28:83–89. doi: 10.1093/annonc/mdw437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Singh A., Boldin-Adamsky S., Thimmulappa R.K., Rath S.K., Ashush H., Coulter J., Blackford A., Goodman S.N., Bunz F., Watson W.H., et al. RNAi-mediated silencing of nuclear factor erythroid-2-related factor 2 gene expression in non-small cell lung cancer inhibits tumor growth and increases efficacy of chemotherapy. Cancer Res. 2008;68:7975–7984. doi: 10.1158/0008-5472.CAN-08-1401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Jeong Y., Hellyer J.A., Stehr H., Hoang N.T., Niu X., Das M., Padda S.K., Ramchandran K., Neal J.W., Wakelee H., et al. Role of KEAP1/NFE2L2 Mutations in the Chemotherapeutic Response of Patients with Non-Small Cell Lung Cancer. Clin. Cancer Res. 2020;26:274–281. doi: 10.1158/1078-0432.CCR-19-1237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Arbour K.C., Jordan E., Kim H.R., Dienstag J., Yu H.A., Sánchez-Vega F., Lito P., Berger M., Solit D.B., Hellmann M., et al. Effects of Co-occurring Genomic Alterations on Outcomes in Patients with KRAS-Mutant Non-Small Cell Lung Cancer. Clin. Cancer Res. 2018;24:334–340. doi: 10.1158/1078-0432.CCR-17-1841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Chen X., Wu Q., Chen Y., Zhang J., Li H., Yang Z., Yang Y., Deng Y., Zhang L., Liu B. Diosmetin induces apoptosis and enhances the chemotherapeutic efficacy of paclitaxel in non-small cell lung cancer cells via Nrf2 inhibition. Br. J. Pharmacol. 2019;176:2079–2094. doi: 10.1111/bph.14652. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 140.Cescon D.W., She D., Sakashita S., Zhu C.-Q., Pintilie M., Shepherd F.A., Tsao M.-S. NRF2 Pathway Activation and Adjuvant Chemotherapy Benefit in Lung Squamous Cell Carcinoma. Clin. Cancer Res. 2015;21:2499–2505. doi: 10.1158/1078-0432.CCR-14-2206. [DOI] [PubMed] [Google Scholar]
- 141.Ji X., Qian J., Rahman S.M.J., Siska P.J., Zou Y., Harris B.K., Hoeksema M.D., Trenary I.A., Heidi C., Eisenberg R., et al. xCT (SLC7A11)-mediated metabolic reprogramming promotes non-small cell lung cancer progression. Oncogene. 2018;37:5007–5019. doi: 10.1038/s41388-018-0307-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Mahaffey C.M., Zhang H., Rinna A., Holland W., Mack P.C., Forman H.J. Multidrug-resistant protein-3 gene regulation by the transcription factor Nrf2 in human bronchial epithelial and non-small-cell lung carcinoma. Free Radic. Biol. Med. 2009;46:1650–1657. doi: 10.1016/j.freeradbiomed.2009.03.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Niture S.K., Jaiswal A.K. Nrf2 protein up-regulates antiapoptotic protein Bcl-2 and prevents cellular apoptosis. J. Biol. Chem. 2012;287:9873–9886. doi: 10.1074/jbc.M111.312694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Niture S.K., Jaiswal A.K. Nrf2-induced antiapoptotic Bcl-xL protein enhances cell survival and drug resistance. Free Radic. Biol. Med. 2013;57:119–131. doi: 10.1016/j.freeradbiomed.2012.12.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Chakrabarti G. Mutant KRAS associated malic enzyme 1 expression is a predictive marker for radiation therapy response in non-small cell lung cancer. Radiat. Oncol. 2015;10:145. doi: 10.1186/s13014-015-0457-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Torrente L., Sánchez C., Moreno R., Chowdhry S., Cabello P., Isono K., Koseki H., Honda T., Hayes J.D., Dinkova-Kostova A.T., et al. Crosstalk between NRF2 and HIPK2 shapes cytoprotective responses. Oncogene. 2017;36:6204–6212. doi: 10.1038/onc.2017.221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Wu K.C., Cui J.Y., Klaassen C.D. Beneficial role of Nrf2 in regulating NADPH generation and consumption. Toxicol. Sci. 2011;123:590–600. doi: 10.1093/toxsci/kfr183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Jiang R., Zhang B., Teng X., Hu P., Xu S., Zheng Z., Liu R., Tang T., Ye F. Validating a targeted next-generation sequencing assay and profiling somatic variants in Chinese non-small cell lung cancer patients. Sci. Rep. 2020;10:2070. doi: 10.1038/s41598-020-58819-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Scheffler M., Bos M., Gardizi M., König K., Michels S., Fassunke J., Heydt C., Künstlinger H., Ihle M., Ueckeroth F., et al. PIK3CA mutations in non-small cell lung cancer (NSCLC): Genetic heterogeneity, prognostic impact and incidence of prior malignancies. Oncotarget. 2015;6:1315–1326. doi: 10.18632/oncotarget.2834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Tung M.-C., Lin P.-L., Wang Y.-C., He T.-Y., Lee M.-C., Yeh S.D., Chen C.-Y., Lee H. Mutant p53 confers chemoresistance in non-small cell lung cancer by upregulating Nrf2. Oncotarget. 2015;6:41692–41705. doi: 10.18632/oncotarget.6150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Hellyer J.A., Stehr H., Das M., Padda S.K., Ramchandran K., Neal J.W., Diehn M., Wakelee H.A. Impact of KEAP1/NFE2L2/CUL3 mutations on duration of response to EGFR tyrosine kinase inhibitors in EGFR mutated non-small cell lung cancer. Lung Cancer. 2019;134:42–45. doi: 10.1016/j.lungcan.2019.05.002. [DOI] [PubMed] [Google Scholar]
- 152.Barrera-Rodríguez R. Importance of the Keap1-Nrf2 pathway in NSCLC: Is it a possible biomarker? Biomed. Rep. 2018;9:375–382. doi: 10.3892/br.2018.1143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Goldstein L.D., Lee J., Gnad F., Klijn C., Schaub A., Reeder J., Daemen A., Bakalarski C.E., Holcomb T., Shames D.S., et al. Recurrent Loss of NFE2L2 Exon 2 Is a Mechanism for Nrf2 Pathway Activation in Human Cancers. Cell Rep. 2016;16:2605–2617. doi: 10.1016/j.celrep.2016.08.010. [DOI] [PubMed] [Google Scholar]
- 154.Tao S., Wang S., Moghaddam S.J., Ooi A., Chapman E., Wong P.K., Zhang D.D. Oncogenic KRAS confers chemoresistance by upregulating NRF2. Cancer Res. 2014;74:7430–7441. doi: 10.1158/0008-5472.CAN-14-1439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.DeNicola G.M., Karreth F.A., Humpton T.J., Gopinathan A., Wei C., Frese K., Mangal D., Yu K.H., Yeo C.J., Calhoun E.S., et al. Oncogene-induced Nrf2 transcription promotes ROS detoxification and tumorigenesis. Nature. 2011;475:106–109. doi: 10.1038/nature10189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Bhattacharjee S., Dashwood R.H. Epigenetic Regulation of NRF2/KEAP1 by Phytochemicals. Antioxidants. 2020;9:865. doi: 10.3390/antiox9090865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Kwak M.-K., Itoh K., Yamamoto M., Kensler T.W. Enhanced expression of the transcription factor Nrf2 by cancer chemopreventive agents: Role of antioxidant response element-like sequences in the nrf2 promoter. Mol. Cell. Biol. 2002;22:2883–2892. doi: 10.1128/MCB.22.9.2883-2892.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Sangokoya C., Telen M.J., Chi J.-T. microRNA miR-144 modulates oxidative stress tolerance and associates with anemia severity in sickle cell disease. Blood. 2010;116:4338–4348. doi: 10.1182/blood-2009-04-214817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Gu S., Lai Y., Chen H., Liu Y., Zhang Z. miR-155 mediates arsenic trioxide resistance by activating Nrf2 and suppressing apoptosis in lung cancer cells. Sci. Rep. 2017;7:12155. doi: 10.1038/s41598-017-06061-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Kawai Y., Garduño L., Theodore M., Yang J., Arinze I.J. Acetylation-deacetylation of the transcription factor Nrf2 (nuclear factor erythroid 2-related factor 2) regulates its transcriptional activity and nucleocytoplasmic localization. J. Biol. Chem. 2011;286:7629–7640. doi: 10.1074/jbc.M110.208173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Ganner A., Pfeiffer Z.-C., Wingendorf L., Kreis S., Klein M., Walz G., Neumann-Haefelin E. The acetyltransferase p300 regulates NRF2 stability and localization. Biochem. Biophys. Res. Commun. 2020;524:895–902. doi: 10.1016/j.bbrc.2020.02.006. [DOI] [PubMed] [Google Scholar]
- 162.Chen Z., Ye X., Tang N., Shen S., Li Z., Niu X., Lu S., Xu L. The histone acetylranseferase hMOF acetylates Nrf2 and regulates anti-drug responses in human non-small cell lung cancer. Br. J. Pharmacol. 2014;171:3196–3211. doi: 10.1111/bph.12661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Zhang Q., Zhang Z.-Y., Du H., Li S.-Z., Tu R., Jia Y.-F., Zheng Z., Song X.-M., Du R.-L., Zhang X.D. DUB3 deubiquitinates and stabilizes NRF2 in chemotherapy resistance of colorectal cancer. Cell Death Differ. 2019;26:2300–2313. doi: 10.1038/s41418-019-0303-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Guo D., Wu B., Yan J., Li X., Sun H., Zhou D. A possible gene silencing mechanism: Hypermethylation of the Keap1 promoter abrogates binding of the transcription factor Sp1 in lung cancer cells. Biochem. Biophys. Res. Commun. 2012;428:80–85. doi: 10.1016/j.bbrc.2012.10.010. [DOI] [PubMed] [Google Scholar]
- 165.Muscarella L.A., Parrella P., D’Alessandro V., la Torre A., Barbano R., Fontana A., Tancredi A., Guarnieri V., Balssamo T., Coco M., et al. Frequent epigenetics inactivation of KEAP1 gene in non-small cell lung cancer. Epigenetics. 2011;6:710–719. doi: 10.4161/epi.6.6.15773. [DOI] [PubMed] [Google Scholar]
- 166.Jain A., Lamark T., Sjøttem E., Larsen K.B., Awuh J.A., Øvervatn A., McMahon M., Hayes J.D., Johansen T. p62/SQSTM1 is a target gene for transcription factor NRF2 and creates a positive feedback loop by inducing antioxidant response element-driven gene transcription. J. Biol. Chem. 2010;285:22576–22591. doi: 10.1074/jbc.M110.118976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Wang Q., Ma J., Lu Y., Zhang S., Huang J., Chen J., Bei J.-X., Yang K., Wu G., Huang K., et al. CDK20 interacts with KEAP1 to activate NRF2 and promotes radiochemoresistance in lung cancer cells. Oncogene. 2017;36:5321–5330. doi: 10.1038/onc.2017.161. [DOI] [PubMed] [Google Scholar]
- 168.Chen W., Sun Z., Wang X.-J., Jiang T., Huang Z., Fang D., Zhang D.D. Direct interaction between Nrf2 and p21(Cip1/WAF1) upregulates the Nrf2-mediated antioxidant response. Mol. Cell. 2009;34:663–673. doi: 10.1016/j.molcel.2009.04.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Ji L., Zhang R., Chen J., Xue Q., Moghal N., Tsao M.-S. PIDD interaction with KEAP1 as a new mutation-independent mechanism to promote NRF2 stabilization and chemoresistance in NSCLC. Sci. Rep. 2019;9:12437. doi: 10.1038/s41598-019-48763-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Wang J., Lu Q., Cai J., Wang Y., Lai X., Qiu Y., Huang Y., Ke Q., Zhang Y., Guan Y., et al. Nestin regulates cellular redox homeostasis in lung cancer through the Keap1-Nrf2 feedback loop. Nat. Commun. 2019;10:5043. doi: 10.1038/s41467-019-12925-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Hayes J.D., Dinkova-Kostova A.T. Oncogene-Stimulated Congestion at the KEAP1 Stress Signaling Hub Allows Bypass of NRF2 and Induction of NRF2-Target Genes that Promote Tumor Survival. Cancer Cell. 2017;32:539–541. doi: 10.1016/j.ccell.2017.10.009. [DOI] [PubMed] [Google Scholar]
- 172.Cheng K.-C., Lin R.-J., Cheng J.-Y., Wang S.-H., Yu J.-C., Wu J.-C., Liang Y.-J., Hsu H.-M., Yu J., Yu A.L. FAM129B, an antioxidative protein, reduces chemosensitivity by competing with Nrf2 for Keap1 binding. EBioMedicine. 2019;45:25–38. doi: 10.1016/j.ebiom.2019.06.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Wang H., Liu K., Chi Z., Zhou X., Ren G., Zhou R., Li Y., Tang X., Wuang X.J. Interplay of MKP-1 and Nrf2 drives tumor growth and drug resistance in non-small cell lung cancer. Aging. 2019;11:11329–11346. doi: 10.18632/aging.102531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Poh J., Ponsford A.H., Boyd J., Woodsmith J., Stelzl U., Wanker E., Harper N., MacEwan D., Sanderson C.M. A functionally defined high-density NRF2 interactome reveals new conditional regulators of ARE transactivation. Redox Biol. 2020;37:101686. doi: 10.1016/j.redox.2020.101686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Adam J., Hatipoglu E., O’Flaherty L., Ternette N., Sahgal N., Lockstone H., Baban D., Nye E., Stamp G.W., Wolhuter K., et al. Renal cyst formation in Fh1-deficient mice is independent of the Hif/Phd pathway: Roles for fumarate in KEAP1 succination and Nrf2 signaling. Cancer Cell. 2011;20:524–537. doi: 10.1016/j.ccr.2011.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Bollong M.J., Lee G., Coukos J.S., Yun H., Zambaldo C., Chang J.W., Chin E.N., Ahmad I., Chatterjee A.K., Lairson L.L., et al. A metabolite-derived protein modification integrates glycolysis with KEAP1-NRF2 signalling. Nature. 2018;562:600–604. doi: 10.1038/s41586-018-0622-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Mills E.L., Ryan D.G., Prag H.A., Dikovskaya D., Menon D., Zaslona Z., Jedrychowski M.P., Costa A.S.H., Higgins M., Hams E., et al. Itaconate is an anti-inflammatory metabolite that activates Nrf2 via alkylation of KEAP1. Nature. 2018;556:113–117. doi: 10.1038/nature25986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Joo M.S., Kim W.D., Lee K.Y., Kim J.H., Koo J.H., Kim S.G. AMPK Facilitates Nuclear Accumulation of Nrf2 by Phosphorylating at Serine 550. Mol. Cell. Biol. 2016;36:1931–1942. doi: 10.1128/MCB.00118-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Huo L., Li C.-W., Huang T.-H., Lam Y.C., Xia W., Tu C., Chang W.-C., Hsu J.L., Lee D.-F., Nie L., et al. Activation of Keap1/Nrf2 signaling pathway by nuclear epidermal growth factor receptor in cancer cells. Am. J. Transl. Res. 2014;6:649–663. [PMC free article] [PubMed] [Google Scholar]
- 180.Rushworth S.A., Zaitseva L., Murray M.Y., Shah N.M., Bowles K.M., MacEwan D.J. The high Nrf2 expression in human acute myeloid leukemia is driven by NF-κB and underlies its chemo-resistance. Blood. 2012;120:5188–5198. doi: 10.1182/blood-2012-04-422121. [DOI] [PubMed] [Google Scholar]
- 181.Wakabayashi N., Skoko J.J., Chartoumpekis D.V., Kimura S., Slocum S.L., Noda K., Palliyaguru D.L., Fujimuro M., Boley P.A., Tanaka Y., et al. Notch-Nrf2 axis: Regulation of Nrf2 gene expression and cytoprotection by notch signaling. Mol. Cell. Biol. 2014;34:653–663. doi: 10.1128/MCB.01408-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Paul M.K., Bisht B., Darmawan D.O., Chiou R., Ha V.L., Wallace W.D., Chon A.T., Hegab A.E., Grogan T., Elashoff D.A., et al. Dynamic changes in intracellular ROS levels regulate airway basal stem cell homeostasis through Nrf2-dependent Notch signaling. Cell Stem Cell. 2014;15:199–214. doi: 10.1016/j.stem.2014.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Lisek K., Campaner E., Ciani Y., Walerych D., Del Sal G. Mutant p53 tunes the NRF2-dependent antioxidant response to support survival of cancer cells. Oncotarget. 2018;9:20508–20523. doi: 10.18632/oncotarget.24974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Reichard J.F., Motz G.T., Puga A. Heme oxygenase-1 induction by NRF2 requires inactivation of the transcriptional repressor BACH1. Nucleic Acids Res. 2007;35:7074–7086. doi: 10.1093/nar/gkm638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Fouzder C., Mukhuty A., Kundu R. Kaempferol inhibits Nrf2 signalling pathway via downregulation of Nrf2 mRNA and induces apoptosis in NSCLC cells. Arch. Biochem. Biophys. 2021;697:108700. doi: 10.1016/j.abb.2020.108700. [DOI] [PubMed] [Google Scholar]
- 186.Patil P.D., Shepherd F., Johnson D.H. A Career in Lung Cancer: Pushing Beyond Chemotherapy. Am. Soc. Clin. Oncol. Educ. Book. 2019;39:583–589. doi: 10.1200/EDBK_239397. [DOI] [PubMed] [Google Scholar]
- 187.Yang S., Zhang Z., Wang Q. Emerging therapies for small cell lung cancer. J. Hematol. Oncol. 2019;12:47. doi: 10.1186/s13045-019-0736-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Yin Y., Liu H., Xu J., Shi D., Zhai L., Liu B., Wang L., Liu G., Qin J. miR-144-3p regulates the resistance of lung cancer to cisplatin by targeting Nrf2. Oncol. Rep. 2018;40:3479–3488. doi: 10.3892/or.2018.6772. [DOI] [PubMed] [Google Scholar]
- 189.Ren D., Villeneuve N.F., Jiang T., Wu T., Lau A., Toppin H.A., Zhang D.D. Brusatol enhances the efficacy of chemotherapy by inhibiting the Nrf2-mediated defense mechanism. Proc. Natl. Acad. Sci. USA. 2011;108:1433–1438. doi: 10.1073/pnas.1014275108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Sun X., Wang Q., Wang Y., Du L., Xu C., Liu Q. Brusatol Enhances the Radiosensitivity of A549 Cells by Promoting ROS Production and Enhancing DNA Damage. Int. J. Mol. Sci. 2016;17:997. doi: 10.3390/ijms17070997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Vartanian S., Ma T.P., Lee J., Haverty P.M., Kirkpatrick D.S., Yu K., Stokoe D. Application of Mass Spectrometry Profiling to Establish Brusatol as an Inhibitor of Global Protein Synthesis. Mol. Cell. Proteom. 2016;15:1220–1231. doi: 10.1074/mcp.M115.055509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Tang X., Wang H., Fan L., Wu X., Xin A., Ren H., Wang X.J. Luteolin inhibits Nrf2 leading to negative regulation of the Nrf2/ARE pathway and sensitization of human lung carcinoma A549 cells to therapeutic drugs. Free Radic. Biol. Med. 2011;50:1599–1609. doi: 10.1016/j.freeradbiomed.2011.03.008. [DOI] [PubMed] [Google Scholar]
- 193.Chian S., Thapa R., Chi Z., Wang X.J., Tang X. Luteolin inhibits the Nrf2 signaling pathway and tumor growth in vivo. Biochem. Biophys. Res. Commun. 2014;447:602–608. doi: 10.1016/j.bbrc.2014.04.039. [DOI] [PubMed] [Google Scholar]
- 194.Huppke P., Weissbach S., Church J.A., Schnur R., Krusen M., Dreha-Kulaczewski S., Kühn-Velten W.N., Wolf A., Huppke B., Millan F., et al. Activating de novo mutations in NFE2L2 encoding NRF2 cause a multisystem disorder. Nat. Commun. 2017;8:818. doi: 10.1038/s41467-017-00932-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Paredes-Gonzalez X., Fuentes F., Jeffery S., Saw C.L.-L., Shu L., Su Z.-Y., Kong A.-N.T. Induction of NRF2-mediated gene expression by dietary phytochemical flavones apigenin and luteolin. Biopharm. Drug Dispos. 2015;36:440–451. doi: 10.1002/bdd.1956. [DOI] [PubMed] [Google Scholar]
- 196.Wang X.J., Hayes J.D., Henderson C.J., Wolf C.R. Identification of retinoic acid as an inhibitor of transcription factor Nrf2 through activation of retinoic acid receptor alpha. Proc. Natl. Acad. Sci. USA. 2007;104:19589–19594. doi: 10.1073/pnas.0709483104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Lo R., Matthews J. The aryl hydrocarbon receptor and estrogen receptor alpha differentially modulate nuclear factor erythroid-2-related factor 2 transactivation in MCF-7 breast cancer cells. Toxicol. Appl. Pharmacol. 2013;270:139–148. doi: 10.1016/j.taap.2013.03.029. [DOI] [PubMed] [Google Scholar]
- 198.Ikeda Y., Sugawara A., Taniyama Y., Uruno A., Igarashi K., Arima S., Ito S., Takeuchi K. Suppression of rat thromboxane synthase gene transcription by peroxisome proliferator-activated receptor gamma in macrophages via an interaction with NRF2. J. Biol. Chem. 2000;275:33142–33150. doi: 10.1074/jbc.M002319200. [DOI] [PubMed] [Google Scholar]
- 199.Wu J., Wang H., Tang X. Rexinoid inhibits Nrf2-mediated transcription through retinoid X receptor alpha. Biochem. Biophys. Res. Commun. 2014;452:554–559. doi: 10.1016/j.bbrc.2014.08.111. [DOI] [PubMed] [Google Scholar]
- 200.Choi E.-J., Jung B.-J., Lee S.-H., Yoo H.-S., Shin E.-A., Ko H.-J., Chang S., Kim S.-Y., Jeon S.-M. A clinical drug library screen identifies clobetasol propionate as an NRF2 inhibitor with potential therapeutic efficacy in KEAP1 mutant lung cancer. Oncogene. 2017;36:5285–5295. doi: 10.1038/onc.2017.153. [DOI] [PubMed] [Google Scholar]
- 201.Singh A., Venkannagari S., Oh K.H., Zhang Y.-Q., Rohde J.M., Liu L., Nimmagadda S., Sudini K., Brimacombe K.R., Gajghate S., et al. Small Molecule Inhibitor of NRF2 Selectively Intervenes Therapeutic Resistance in KEAP1-Deficient NSCLC Tumors. ACS Chem. Biol. 2016;11:3214–3225. doi: 10.1021/acschembio.6b00651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Bollong M.J., Yun H., Sherwood L., Woods A.K., Lairson L.L., Schultz P.G. A Small Molecule Inhibits Deregulated NRF2 Transcriptional Activity in Cancer. ACS Chem. Biol. 2015;10:2193–2198. doi: 10.1021/acschembio.5b00448. [DOI] [PubMed] [Google Scholar]
- 203.Lee S., Lim M.-J., Kim M.-H., Yu C.-H., Yun Y.-S., Ahn J., Song J.-Y. An effective strategy for increasing the radiosensitivity of Human lung Cancer cells by blocking Nrf2-dependent antioxidant responses. Free Radic. Biol. Med. 2012;53:807–816. doi: 10.1016/j.freeradbiomed.2012.05.038. [DOI] [PubMed] [Google Scholar]
- 204.Roh J.-L., Kim E.H., Jang H., Shin D. Nrf2 inhibition reverses the resistance of cisplatin-resistant head and neck cancer cells to artesunate-induced ferroptosis. Redox Biol. 2017;11:254–262. doi: 10.1016/j.redox.2016.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Gao A.-M., Ke Z.P., Shi F., Sun G.-C., Chen H. Chrysin enhances sensitivity of BEL-7402/ADM cells to doxorubicin by suppressing PI3K/Akt/Nrf2 and ERK/Nrf2 pathway. Chem. Biol. Interact. 2013;206:100–108. doi: 10.1016/j.cbi.2013.08.008. [DOI] [PubMed] [Google Scholar]
- 206.Gao A.-M., Zhang X.-Y., Ke Z.-P. Apigenin sensitizes BEL-7402/ADM cells to doxorubicin through inhibiting miR-101/Nrf2 pathway. Oncotarget. 2017;8:82085–82091. doi: 10.18632/oncotarget.18294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Tsuchida K., Tsujita T., Hayashi M., Ojima A., Keleku-Lukwete N., Katsuoka F., Otsuki A., Kikuchi H., Oshima Y., Suzuki M., et al. Halofuginone enhances the chemo-sensitivity of cancer cells by suppressing NRF2 accumulation. Free Radic. Biol. Med. 2017;103:236–247. doi: 10.1016/j.freeradbiomed.2016.12.041. [DOI] [PubMed] [Google Scholar]
- 208.Dong J., Li Y., Xiao H., Luo D., Zhang S., Zhu C., Jiang M., Cui M., Lu L., Fan S. Cordycepin sensitizes breast cancer cells toward irradiation through elevating ROS production involving Nrf2. Toxicol. Appl. Pharmacol. 2019;364:12–21. doi: 10.1016/j.taap.2018.12.006. [DOI] [PubMed] [Google Scholar]
- 209.Liu H.Y., Tuckett A., Fennell M., Garippa R., Zakrzewski J.L. Repurposing of the CDK inhibitor PHA-767491 as a NRF2 inhibitor drug candidate for cancer therapy via redox modulation. Investig. New Drugs. 2018;36:590–600. doi: 10.1007/s10637-017-0557-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Sakurai T., Isogaya K., Sakai S., Morikawa M., Morishita Y., Ehata S., Miyazono K., Koinuma D. RNA-binding motif protein 47 inhibits Nrf2 activity to suppress tumor growth in lung adenocarcinoma. Oncogene. 2017;36:5083. doi: 10.1038/onc.2017.191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Hou X., Bai X., Gou X., Zeng H., Xia C., Zhuang W., Chen X., Zhao Z., Huang M., Jin J. 3′,4′,5′,5,7-pentamethoxyflavone sensitizes Cisplatin-resistant A549 cells to Cisplatin by inhibition of Nrf2 pathway. Mol. Cells. 2015;38:396–401. doi: 10.14348/molcells.2015.2183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Lim J., Lee S.H., Cho S., Lee I.-S., Kang B.Y., Choi H.J. 4-methoxychalcone enhances cisplatin-induced oxidative stress and cytotoxicity by inhibiting the Nrf2/ARE-mediated defense mechanism in A549 lung cancer cells. Mol. Cells. 2013;36:340–346. doi: 10.1007/s10059-013-0123-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Zhu J., Wang H., Chen F., Lv H., Xu Z., Fu J., Hou Y., Xu Y., Pi J. Triptolide enhances chemotherapeutic efficacy of antitumor drugs in non-small-cell lung cancer cells by inhibiting Nrf2-ARE activity. Toxicol. Appl. Pharmacol. 2018;358:1–9. doi: 10.1016/j.taap.2018.09.004. [DOI] [PubMed] [Google Scholar]
- 214.Kang J.-S., Lee J., Nam L.B., Yoo O.-K., Pham K.-T., Duong T.-H.-M., Keum Y.-S. Homoharringtonine stabilizes secondary structure of guanine-rich sequence existing in the 5′-untranslated region of Nrf2. Bioorg. Med. Chem. Lett. 2019;29:2189–2196. doi: 10.1016/j.bmcl.2019.06.049. [DOI] [PubMed] [Google Scholar]
- 215.Lee J., Kang J.-S., Nam L.B., Yoo O.-K., Keum Y.-S. Suppression of NRF2/ARE by convallatoxin sensitises A549 cells to 5-FU-mediated apoptosis. Free Radic. Res. 2018;52:1416–1423. doi: 10.1080/10715762.2018.1489132. [DOI] [PubMed] [Google Scholar]
- 216.Zhou Y., Zhou Y., Wang K., Li T., Yang M., Wang R., Chen Y., Cao M., Hu R. Flumethasone enhances the efficacy of chemotherapeutic drugs in lung cancer by inhibiting Nrf2 signaling pathway. Cancer Lett. 2020;474:94–105. doi: 10.1016/j.canlet.2020.01.010. [DOI] [PubMed] [Google Scholar]
- 217.Sun M., Pan D., Chen Y., Li Y., Gao K., Hu B. Coroglaucigenin enhances the radiosensitivity of human lung cancer cells through Nrf2/ROS pathway. Oncotarget. 2017;8:32807–32820. doi: 10.18632/oncotarget.16454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.Akmal A., Javaid A., Hussain R., Kanwal A., Zubair M., Ashfaq U.A. Screening of phytochemicals against Keap1- NRF2 interaction to reactivate NRF2 Functioning: Pharmacoinformatics based approach. Pak. J. Pharm. Sci. 2019;32(Suppl. S6):2823–2828. [PubMed] [Google Scholar]
- 219.Saito T., Ichimura Y., Taguchi K., Suzuki T., Mizushima T., Takagi K., Hirose Y., Nagahashi M., Iso T., Fukutomi T., et al. p62/Sqstm1 promotes malignancy of HCV-positive hepatocellular carcinoma through Nrf2-dependent metabolic reprogramming. Nat. Commun. 2016;7:12030. doi: 10.1038/ncomms12030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.Rooney J.P., Chorley B., Hiemstra S., Wink S., Wang X., Bell D.A., Walter B., Corton J.C. Mining a human transcriptome database for chemical modulators of NRF2. PLoS ONE. 2020;15:e0239367. doi: 10.1371/journal.pone.0239367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221.Derman B.A., Mileham K.F., Bonomi P.D., Batus M., Fidler M.J. Treatment of advanced squamous cell carcinoma of the lung: A review. Transl. Lung Cancer Res. 2015;4:524–532. doi: 10.3978/j.issn.2218-6751.2015.06.07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222.Zhu B., Tang L., Chen S., Yin C., Peng S., Li X., Liu T., Liu W., Han C., Stawski L., et al. Targeting the upstream transcriptional activator of PD-L1 as an alternative strategy in melanoma therapy. Oncogene. 2018;37:4941–4954. doi: 10.1038/s41388-018-0314-0. [DOI] [PubMed] [Google Scholar]
- 223.Xu X., Yang Y., Liu X., Cao N., Zhang P., Zhao S., Chen D., Li L., He Y., Dong X., et al. NFE2L2/KEAP1 Mutations Correlate with Higher Tumor Mutational Burden Value/PD-L1 Expression and Potentiate Improved Clinical Outcome with Immunotherapy. Oncologist. 2020;25:e955–e963. doi: 10.1634/theoncologist.2019-0885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.Gandhi L., Rodríguez-Abreu D., Gadgeel S., Esteban E., Felip E., De Angelis F., Domine M., Clingan P., Hochmair M.J., Powell S.F., et al. Pembrolizumab plus Chemotherapy in Metastatic Non-Small-Cell Lung Cancer. N. Engl. J. Med. 2018;378:2078–2092. doi: 10.1056/NEJMoa1801005. [DOI] [PubMed] [Google Scholar]
- 225.Mullarky E., Lucki N.C., Beheshti Zavareh R., Anglin J.L., Gomes A.P., Nicolay B.N., Wong J.C.Y., Christen S., Takahashi H., Singh P.K., et al. Identification of a small molecule inhibitor of 3-phosphoglycerate dehydrogenase to target serine biosynthesis in cancers. Proc. Natl. Acad. Sci. USA. 2016;113:1778–1783. doi: 10.1073/pnas.1521548113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226.Boysen G., Jamshidi-Parsian A., Davis M.A., Siegel E.R., Simecka C.M., Kore R.A., Dings R.P.M., Griffin R.J. Glutaminase inhibitor CB-839 increases radiation sensitivity of lung tumor cells and human lung tumor xenografts in mice. Int. J. Radiat. Biol. 2019;95:436–442. doi: 10.1080/09553002.2018.1558299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227.Mele L., Paino F., Papaccio F., Regad T., Boocock D., Stiuso P., Lombardi A., Liccardo D., Aquino G., Barbieri A., et al. A new inhibitor of glucose-6-phosphate dehydrogenase blocks pentose phosphate pathway and suppresses malignant proliferation and metastasis in vivo. Cell Death Dis. 2018;9:572. doi: 10.1038/s41419-018-0635-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228.Lee H.R., Cho J.M., Shin D.h., Yong C.S., Choi H.G., Wakabayashi N., Kwak M.-K. Adaptive response to GSH depletion and resistance to L-buthionine-(S,R)-sulfoximine: Involvement of Nrf2 activation. Mol. Cell. Biochem. 2008;31:23–31. doi: 10.1007/s11010-008-9853-y. [DOI] [PubMed] [Google Scholar]
- 229.Zhao Y., Seefeldt T., Chen W., Carlson L., Stoebner A., Hanson S., Foll R., Matthees D.P., Palakurthi S., Guan X. Increase in thiol oxidative stress via glutathione reductase inhibition as a novel approach to enhance cancer sensitivity to X-ray irradiation. Free Radic. Biol. Med. 2009;47:176–183. doi: 10.1016/j.freeradbiomed.2009.04.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230.Sleire L., Skeie B.S., Netland I.A., Førde H.E., Dodoo E., Selheim F., Leiss L., Heggdal J.I., Pedersen P.-H., Wang J., et al. Drug repurposing: Sulfasalazine sensitizes gliomas to gamma knife radiosurgery by blocking cystine uptake through system Xc-, leading to glutathione depletion. Oncogene. 2015;34:5951–5959. doi: 10.1038/onc.2015.60. [DOI] [PubMed] [Google Scholar]
- 231.Dixon S.J., Patel D.N., Welsch M., Skouta R., Lee E.D., Hayano M., Thomas A.G., Gleason C.E., Tatonetti N.P., Slusher B.S., et al. Pharmacological inhibition of cystine-glutamate exchange induces endoplasmic reticulum stress and ferroptosis. eLife. 2014;3:e02523. doi: 10.7554/eLife.02523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 232.Kitamura H., Onodera Y., Murakami S., Suzuki T., Motohashi H. IL-11 contribution to tumorigenesis in an NRF2 addiction cancer model. Oncogene. 2017;36:6315–6324. doi: 10.1038/onc.2017.236. [DOI] [PubMed] [Google Scholar]
- 233.Matthews J.H., Liang X., Paul V.J., Luesch H. A Complementary Chemical and Genomic Screening Approach for Druggable Targets in the Nrf2 Pathway and Small Molecule Inhibitors to Overcome Cancer Cell Drug Resistance. ACS Chem. Biol. 2018;13:1189–1199. doi: 10.1021/acschembio.7b01025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 234.Riess J.W., Frankel P., Shackelford D., Dunphy M., Badawi R.D., Nardo L., Cherry S.R., Lanza I., Reid J., Gonsalves W.I., et al. Phase 1 Trial of MLN0128 (Sapanisertib) and CB-839 HCl (Telaglenastat) in Patients with Advanced NSCLC (NCI 10327): Rationale and Study Design. Clin. Lung Cancer. 2021;22:67–70. doi: 10.1016/j.cllc.2020.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.