Abstract
Mammalian embryogenesis depends on maternal factors accumulated in eggs prior to fertilization and on placental transfers later in gestation. In this review, we focus on initial events when the organism has insufficient newly synthesized embryonic factors to sustain development. These maternal factors regulate preimplantation embryogenesis both uniquely in pronuclear formation, genome reprogramming and cell fate determination and more universally in regulating cell division, transcription and RNA metabolism. Depletion, disruption or inappropriate persistence of maternal factors can result in developmental defects in early embryos. To better understand the origins of these maternal effects, we include oocyte maturation processes that are responsible for their production. We focus on recent publications and reference comprehensive reviews that include earlier scientific literature of early mouse development.
1. Introduction
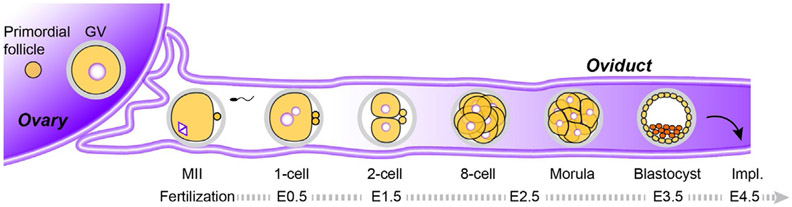
Preimplantation development begins with fertilization in which two haploid gametes fuse to form one-cell, diploid zygotes that divide and eventually form mature blastocysts. Although implantation is unique to mammals, the window of preimplantation development shares many similarities with non-mammalian animals and takes ~4–5 days in mice. The one-cell zygote remodels parental chromatin, activates embryonic transcription of its genome, defines early polarity and establishes different cell lineages. After implantation on the uterine wall, specified lineages— epiblast, primitive endoderm and trophectoderm—further differentiate and define the three germ layers of the developing embryo (Fig. 1).
Fig. 1.
An outline of mouse oogenesis and preimplantation development. Oocytes grow and undergo meiotic maturation within the ovary prior to ovulation into the oviduct where they are fertilized by capacitated sperm to form a zygote (embryonic day 1, E1). The 1-cell zygote divides into two totipotent blastomeres and continues to cleave (~E1.5). At the 8-cell stage (~E2.5), the embryo compacts to form the morula. After 2–3 additional rounds of cell division (~E3.5), a blastocoel forms, transforming the embryo into a blastocyst that implants on the wall of the uterus ~E4.5. Impl., Implantation.
Although many maternal mutants arrest at the 2-cell stage of development, others have defects later in preimplantation embryogenesis. Maternal factor deficiency leads to embryonic arrest either directly or indirectly. Directly, a maternal factor can regulate specific activities at a particular developmental stage, the lack of which immediately blocks development. Indirectly, a maternal factor can cause non-lethal defects that accumulate to create cellular stress that impairs development at a later stage. We will dissect the direct and indirect roles of maternal factors during mouse preimplantation by following the sequence of events in early development.
2. Initiation of embryonic development
The life of mammals begins at fertilization when sperm and egg meet in the oviductal ampulla of the female reproductive tract. Gametic fusion begins with membrane binding and can be visualized by sperm internalization that happens within minutes to hours of gamete mixing. However, interdigitating nuclear syngamy of the two haploid gametes does not occur prior to a sequence of key events that take place following sperm entry. In the absence of zygotic gene transcription, maternal factors actively participate in sperm protamine-to-histone transition, pronuclear formation, epigenetic remodeling, genomic imprinting protection and establishment of higher-order chromatin structures, the consequence of which collectively prepare the zygote for nuclear union.
Sperm chromatin undergoes dramatic remodeling after penetrating into the egg. Although protamine packaging of genomic DNA is critical for spermiogenesis, after fertilization, protamines must be replaced with somatic histones during the decondensation and recondensation phases of the sperm-to-paternal pronucleus transition (Balhorn, 2007). Decondensation happens right after sperm enter the egg cortical zone and requires reduction of intra- and inter-protamine disulfide bonds (Caglar et al., 2005). In mice, maternal NPM2 (nucleoplasmin 2) can induce sperm chromatin decondensation by regulating levels of histone acetylation (Burns et al., 2003; Inoue, Ogushi, Saitou, Suzuki, & Aoki, 2011), which is consistent with the documented role of Xenopus oocyte-supplied NPM in removing sperm protamine and facilitating nucleosome assembly (Philpott & Leno, 1992). Purified mouse NPM2 protein interacts with mouse P1/P2 protamines and displaces them from DNA (Ellard, Serpa, Petrotchenko, Borchers, & Ausio, 2016; Philpott & Leno, 1992). Genetic ablation of mouse Npm2 results in reduced female fertility due to disorganized chromatin, including the absence of nucleoli and heterochromatin (Burns et al., 2003; Ogushi et al., 2017). In the subsequent recondensation phase, oocyte-supplied histone 3.3 (H3.3), but not H3.1 or H3.2, can access and reorganize sperm DNA (van der Heijden et al., 2005). This H3.3 preference may differentiate paternal from maternal pronuclei and account for early transcription activation of the paternal genome (Kong et al., 2018) (Fig. 2A).
Fig. 2.
Maternal factors function in chromatin remodeling post-fertilization. (A) Paternal pronucleus formation by maternal NPM2 that mediates protamine removal and H3.3 that mediates recondensation. Maternal chromatin is occupied by H3.3 and H3.1/3.2. (B) Paternal DNA demethylation is mediated by maternal TET proteins while maternal DNA methylation persists due to the masking by DPPA3. (C) DNA methylation differs in paternal and maternal alleles during preimplantation development. Paternal DNA is actively and rapidly demethylated at the 1-cell stage whereas maternal DNA methylation decreases passively through cell division. Both paternal and maternal DNA then undergo remethylation by DNMT3A/3B beginning in blastocysts. Imprinted genes remain unaffected by DNMT1 during preimplantation. (D) Establishment of topologically associating domains (TADs) during preimplantation. While there are no clearly defined TADs at the 1-cell stage, they are gradually established during preimplantation development.
After sperm chromatin decondensation and recondensation, both sperm and egg haploid genomes form pronuclei and move toward each other whereupon their membranes interdigitate and disperse to facilitate nuclear union. During this process, active DNA demethylation (removal of 5mC) in paternal chromatin is mediated by maternal TET1/2/3 through a series of oxidative transformation to 5hmC (5-hydroxymethylcytosine), 5fC (5-formylcytosine) and 5caC (5-carboxylcytosine) (Wu & Zhang, 2014). All intermediate products can be converted to cytosine by base excision DNA repair or by 5caC decarboxylation (Wu & Zhang, 2010). In contrast, maternal DNA is largely spared from active demethylation by DPPA3 (developmental pluripotency associated 3) masking, which is recruited by dimethylation of Lys9 on maternal H3 (H3K9me2) (Nakamura et al., 2012). Maternal DNA demethylation occurs passively through replication-dependent dilution during subsequent embryonic cell cleavage (Mayer, Niveleau, Walter, Fundele, & Haaf, 2000). The three maternal TETs are partially redundant (Dawlaty et al., 2011; Gu et al., 2011; Kang et al., 2015; Li et al., 2011, 2016; Shen et al., 2014). Depletion of maternal TET3 impairs mouse paternal DNA demethylation in the 5mC to 5hmC conversion, which results in repression of paternal derived embryonic stem cell genes Oct4 and Nanog and increases embryonic lethality (Gu et al., 2011). However, TET3 depletion does not affect zygotic gene activation or preimplantation development (Shen et al., 2014). Maternal TET1 or TET2 depletion is associated with normal or subfertility (Dawlaty et al., 2011; Li et al., 2011). Double knockouts of maternal Tet1; Tet3 results in delayed or aborted preimplantation development due to increased transcriptome variability (Kang et al., 2015); and triple maternal knockout of Tet1–3 does not further worsen the phenotype (Li et al., 2016). The minor defects associated with Tet gene ablation may reflect the greater role exerted by passive DNA demethylation that occurs concurrently by dilution due to cell cleavage (Messerschmidt, Knowles, & Solter, 2014). Moreover, defects in zygotic transcription may not necessarily lead to an immediate phenotype because of maternal compensation (Fig. 2B).
Despite global DNA demethylation, gene imprinting established in the parental germline remains unchanged by the maternal DNA methyltransferase DNMT1 (Hirasawa et al., 2008). DNMT1 targets hemi-methylated DNA to maintain the methylation pattern during replication (Jeltsch & Jurkowska, 2014). Mouse maternal DNMT1 depletion results in partial reduction of methylation at parental differentially methylated regions (DMRs), including paternal H19 and Rasgrf1 DMRs, and maternal Peg3 and Snrpn DMRs (Howell et al., 2001). In addition to DNMT1, the oocyte-specific isoform DNMT1o also contributes to maintaining allele-specific DNA methylation (Howell et al., 2001). Unlike DNMT1, DNMT3A/3B performs de novo DNA methylation (Okano, Bell, Haber, & Li, 1999). Oocyte-specific knockout of Dnmt3 causes hypomethylation of maternal DNA and recurrent post-implantation embryonic loss (Kaneda et al., 2010) (Fig. 2C). Similar to TET deficiency, even though dysregulated DNA methylation directly perturbs zygotic gene transcription, the physiological defect is manifest after implantation, which suggests proper maternal support can bypass certain zygotic deficiencies in this developmental window.
Recent studies have identified higher-order chromatin structure in mouse embryos and documented establishment of topologically associating domains (TADs) during preimplantation (Ke et al., 2017). In mouse oocytes or 1-cell zygotes, clearly defined TADs are not present; in sperm, however, somatic cell-like TADs have been identified, but are quickly lost at fertilization and the higher-order chromatin structures become obscure as in unfertilized eggs (Ke et al., 2017). TADs are reestablished at the 2-cell stage and maternal CTCF (CCCTC-binding factor) appears involved in this process: CTCF occupancy is enriched at the TAD boundaries together with chromatin structural proteins, active histone modifications including H3K4me3, H3K27Ac, H3K9Ac, H3K4me1 and SMC1 (Ke et al., 2017). The interactions between CTCF and other transcription factors persist during preimplantation development to maintain this chromatin configuration (Jung et al., 2019) (Fig. 2D). However, TAD formation relies on DNA replication and not zygotic gene transcription as previously documented in Drosophila (Hug, Grimaldi, Kruse, & Vaquerizas, 2017; Ke et al., 2017). Thus, as yet unknown maternal proteins/RNAs must be required to establish TADs during preimplantation development. In summary, most of the basic cellular activities and specific events in the oocyte-to-embryo transition are performed by oocyte loaded executers.
3. Transition to independence: Zygotic genome activation
Zygotic genome activation (ZGA) provides a blueprint for the independent future of embryos. As a highly conserved process from sea urchin to humans, ZGA refers to a huge number of genes transitioning from quiescence to transcription in a short time window after fertilization. In mice, the first evidence of ZGA comes from transcription of the paternal genome and emergence of transcripts unavailable in the egg. Increased mRNA levels have been described both for the entire transcriptome and for single gene loci at the 2-cell stage of development (Schultz, 1993). Later, the ZGA splits into two phases: minor and major. Minor ZGA happens specifically in the male pronucleus, both earlier and to a lesser extent than major ZGA (Aoki, Worrad, & Schultz, 1997) (Fig. 3A). The increasingly consensus is that ZGA contains many coordinated waves of gene transcription that form a cascade which differs from the old “burst” view of zygote transcription (Jukam, Shariati, & Skotheim, 2017). During ZGA, maternal factors regulate transcription, both directly and indirectly.
Fig. 3.
Maternal factors affect zygotic gene activation (ZGA). (A) Minor and major waves of ZGA in the 1- and 2-cell embryos. (B) Maternal factors can directly regulate ZGA by affecting transcription (red) or chromatin structure (blue), or indirectly regulate ZGA by affecting other cellular activities including RNA metabolism, DNA repair and cytoskeleton (black).
3.1. Maternal transcription factors prime ZGA
Many maternal transcription factors are loaded into the zygote as RNA which requires active translation to produce functional proteins including E2F transcription factor 1, RNA polymerase 1–3, MYST histone acetyltransferase 1 and SWI/SNF-related proteins (Potireddy, Vassena, Patel, & Latham, 2006). Most of the identified factors are not ZGA-specific, but are ubiquitously required for transcription and their zygotic ablation causes defects later in development (Maslon et al., 2019; Tsai et al., 2008).
Loss-of-function studies of maternal transcription factors have been achieved by gene ablation in late oocytes or in 1-cell embryos, which most immediately affects ZGA. Oocyte-specific knockout of Hsf1 (heat shock factor 1), which binds and controls expression of the minor ZGA gene Hspa1b, results in extensive defects and developmental arrest at the 2-cell stage (Bierkamp et al., 2010; Wilkerson, Murphy, & Sarge, 2008). Maternal-zygotic knockout of Cdx2, a well-known trophectoderm marker, leads to an earlier embryonic lethality compared to zygotic knockout, suggesting a unique role of maternal Cdx2 (Blij, Frum, Akyol, Fearon, & Ralston, 2012; Jedrusik, Cox, Wicher, Glover, & Zernicka-Goetz, 2015). Genetic depletion of maternal YAP (yes-associated protein) in oocytes prolongs the 2-cell stage, delays progression to 4-cells and causes concomitant transcriptional disruption of zygotic genes Rpl13 and Rrm2 (Yu, Ji, Dang, et al., 2016; Yu, Ji, Sha, et al., 2016). DUX (double-homeodomain proteins) is known to activate ZGA genes, and when depleted zygotically results in preimplantation arrest in ex vivo cultured embryos (De Iaco et al., 2017). Zygotic or maternal-zygotic knockout of Dux can have subtle defects in ZGA (Chen & Zhang, 2019) which raises the possibility that occupancy of DUX at actively transcribed genes could be compensated by other factors. PLAG1 (Pleomorphic adenoma gene 1) has been found to directly promote the transcription of a few zygotic genes having PLAG1 binding motifs (Madissoon et al., 2019). Heterozygous embryos lacking maternal PLAG1 delay the 2-cell stage development (Madissoon et al., 2019). Maternal transcription factors thus activate zygotic gene transcription both cooperatively and independently.
In addition to genetic ablation, siRNA or morpholinos have been used to degrade targeted transcripts or block translation for transient loss-of-function in early embryonic stages (Falco et al., 2007; Ma, Zeng, Schultz, & Tseng, 2006; Park et al., 2015; Wu & Scholer, 2014). An advantage of these approaches is that they target both maternal and zygotic RNA which can lead to a more severe phenotype than genetic perturbation of the maternal gene. Lack of BASONUCLIN in transgenic RNAi mouse models blocks embryonic development at the 2-cell stage by perturbing both RNA polymerase I and II mediated transcription (Ma et al., 2006). ZSCAN4 (zinc finger and SCAN domain-containing protein 4), a potential transcription factor possessing zinc finger domains, is expressed ubiquitously at the 2–4-cell stage. siRNA mediated depletion of ZSCAN4 in 1-cell zygotes causes arrest at the 2-cell stage (Falco et al., 2007). Although an oocyte-specific knockout of maternal Oct4 has little effect, injection of Oct4 morpholinos can disrupt embryogenesis at the morula stage, raising the possibility that zygotic OCT4 can quickly compensate for the loss of maternal OCT4 (Wu & Scholer, 2014). Eliminating maternal SEBOX (skin-embryo-brain-oocyte homeobox) by pronuclear injection of double-stranded RNA results in embryonic arrest at the 2–8-cell stage and the absence of several known zygotic transcripts including Mt1a, Rpl23, Ube2a and Wee1 (Park et al., 2015). In summary, oocyte-provided transcription factors can activate early embryonic genes by directly participating in the zygotic gene transcription network (Fig. 3B).
3.2. Preparing chromatin for transcription by maternal protein modifiers
In addition to directly controlling transcription, maternal factors can prepare chromatin for transcription. As discussed below, maternal factors can modify chromatin activity, mediate transcription factor binding to the genome, dissociate histones from the genome and demethylate genomic DNA (Bultman et al., 2006; Jimenez et al., 2015; Lu et al., 2016; Torres-Padilla & Zernicka-Goetz, 2006).
One of the best studied maternal chromatin modifiers is BRG1, a catalytic subunit of a chromatin remodeling complex (Kim, Bresnick, & Bultman, 2009). Zygotic null homozygotes of Brg1 are embryonic lethal at the blastocyst stage while oocyte-depletion by Cre-loxP or 1-cell depletion by RNAi results in 2-cell arrest with reduced dimethyl-H3K4 (Bultman et al., 2006). BRG1 chromosomal localization during minor ZGA seems to be controlled by another transcription intermediary factor TIF1α, and ablation of TIF1α through RNAi or antibody blocking leads to mis-localization of BRG1 and RNA polymerase (Torres-Padilla & Zernicka-Goetz, 2006). Due to limited biological material, BRG1-binding sites in preimplantation embryos have not been identified. Knowing target genes of maternal BRG1 at the 2-cell stage would add greatly to our understanding of ZGA. Another factor, SIN3A, is a member of the HDAC1/2 family that functions during the oocyte to embryo transition (Yang & Seto, 2008). Disruption of oocyte SIN3A causes 2-cell arrest with dysregulated ZGA (Jimenez et al., 2015). Maternal NFY binds to open chromatin structures and can contribute to DNase I-hypersensitive sites formation (Lu et al., 2016). NFY depletion at the 2-cell stage disrupts zygotic gene expression (Lu et al., 2016).
In addition to local remodeling of chromatin structure for transcription, establishing higher-order structure can also affect ZGA (Wan et al., 2008). CTCF can facilitate or repress interactions of different genomic domains (Ong & Corces, 2014). Depletion of oocyte CTCF by transgenic RNAi causes defects in meiosis and mitosis and adversely affects ZGA which leads to delayed and failed development (Wan et al., 2008). Though TADs formation is independent of ZGA, genomic CTCF footprints remain constant from oocytes to preimplantation embryos which suggests that these sites require additional factors to regulate developmentally appropriate transcription (Jung et al., 2019). It may be that CTCF can recruit different interacting factors to achieve different regulatory roles, which would separate activation from repression, and TAD establishment from ZGA. Along with the development of low-input sequencing technology and single-cell omics, we anticipate further molecular clarity of CTCF binding dynamics and its relationship with other chromatin modifications in ZGA regulation (Fig. 3B).
3.3. Other maternal factors governing ZGA
In addition to specific transcription factors or chromatin modifiers, maternal factors can affect ZGA through multiple indirect pathways, such as controlled RNA splicing, translation and transcript stability. Zar1, one of the first maternal effect genes identified by mouse genetics, has been studied for more than 10 years (Wu et al., 2003). Zar1-depleted oocytes appear normal, but embryos derived from null females arrest at the 1-cell stage with marked reduction of ZGA (Wu et al., 2003). ZAR1-like (ZAR1L) has a similar protein sequence to ZAR1, and also induces 2-cell arrest by ectopic expression of its C terminus, with concomitant defects of histone modification, RNA synthesis and RNA polymerase II (Hu et al., 2010). The co-localization of ZAR1L and RNA granules indicate a role in RNA regulation (Hu et al., 2010). Meanwhile, the similarity between the carboxyl-termini of ZAR1 and ZAR1L suggests that ZAR1 may also regulate RNA stability to indirectly affect ZGA (Hu et al., 2010). ZFP36L2, known to bind the 3′ UTR of mRNA and participate in the deadenylation complex to trigger mRNA decay, leads to 2-cell arrest when the maternal ZFP36L2 protein is truncated at its amino-terminus (Ramos et al., 2004). Oocyte-specific depletion of ZFP36L2 stabilizes a group of histone modification genes normally degraded during oocyte maturation, which results in insufficient transcription termination and poor oocyte quality (Dumdie et al., 2018). It is possible that ZFP36L2 could adopt a similar strategy in targeting chromatin modifiers during ZGA. pSer473-Akt (phosphorylated Ser473-Akt), present in 1–2 cell nuclei, leads to impaired mRNA levels of some zygotic genes by its enzymatic inhibition (Chen et al., 2016). BTG4, a scaffold protein of the deadenylation-translation complex, can regulate RNA stability and translation across the oocyte-to-embryo transition (Wu & Dean, 2016). Btg4 knockout females produce morphologically normal eggs, but are infertile because embryos arrest after fertilization at 2-cell stage with perturbed transcriptomes (Liu et al., 2016; Yu, Ji, Dang, et al., 2016; Yu, Ji, Sha, et al., 2016). YTHDF2, an RNA N6-methyladenosine (m6A)-binding and stabilization enzyme, is essential to ZGA since its maternal disruption impairs degradation of its target genes and is also associated with reduced female fecundity (Ivanova et al., 2017).
In addition to post-transcription modification, the DNA repair system also indirectly affects ZGA. Maternal BCAS2 (breast carcinoma amplified sequence 2) deficiency compromises the DNA damage response and leads to 2–4-cell stage arrest accompanied by accumulation of damaged DNA, micronuclei and disrupted zygotic gene expression (Xu et al., 2015). Oocytes, depleted of HR6A, a ubiquitin-conjugating DNA repair enzyme homologous to yeast RAD6, have normal oogenesis; but the derived embryos fail to develop beyond the 2-cell stage (Roest et al., 2004). The ZGA defects described above could be caused indirectly by either cellular abnormalities or mis-regulated transcription factors. Some of the defects, especially those involved in maternal RNA metabolism, may generate deleterious deposits that impair oocyte quality prior to preimplantation development (discussed below).
The mammalian subcortical maternal complex (SCMC) plays essential roles in early embryonic development. The SCMC contains a group of proteins localizing in the cortical region of eggs and embryos and its components include MATER (official name, NLRP5), FLOPED (OOEP), TLE6, FILIA (KHDC3), PADI6, NLRP2 and ZBED3 (Gao et al., 2018; Li, Baibakov, & Dean, 2008; Lim & Knowles, 2015; Mahadevan et al., 2017; Zheng & Dean, 2009). The function of the SCMC begins as early as oocyte maturation by positioning the meiotic spindle and localizing cortical vesicles for exocytosis (Vogt et al., 2019). After fertilization, the SCMC continues to regulate cytoplasmic F-actin meshwork, RNA translation, epigenetic reprogramming and organelle redistribution (Bebbere, Masala, Albertini, & Ledda, 2016; Lu, Gao, Qin, & Li, 2017; Vogt et al., 2019). Depletion of SCMC components results in cleavage-stage embryonic arrest and maternal infertility (Li, Zheng, & Dean, 2010) (Fig. 3B). The concomitant ZGA failure in SCMC-deficient embryos is more likely to be a secondary effect of cellular stress accumulated through extensive dysregulation.
4. Other preimplantation events regulated by maternal factors
During preimplantation development, both maternal and zygotic factors regulate multiple cellular activities, including cell cleavage, embryonic polarity and cell fate specification, which are both highly conserved across the animal kingdom and have unique order-specific characteristics.
4.1. Cell cleavage
Cell cleavage during preimplantation development gradually molds embryonic polarity that guides further cell fate determination. The first cleavage of the mouse embryo generates two totipotent blastomeres (Tarkowski, 1959). Their totipotency has been documented by the two embryos that develop from the physically separated blastomeres, and by each compensating for the loss of the other (Tarkowski, 1959). Before fertilization, the egg develops asymmetry with its meiosis II spindle close to the cortical area as well as concomitant asymmetry of maternal PAR proteins and an actin meshwork. The first division cleaves the zygote longitudinally by passing through the site of the previous meiotic division and along the axis of animal-vegetal pole (Plusa, Grabarek, Piotrowska, Glover, & Zernicka-Goetz, 2002). Unique animal-pole maternal components are suggested to transmit meiotic pole localization information to the first cleavage plane, a model that has been confirmed in an animal-pole transplantation assay (Plusa et al., 2002). However, the molecular basis of these observations remains to be determined. The asymmetric localization of PAR proteins in the egg disappears during cleavage-stage development and only re-emerges after the 8-cell stage (Vinot et al., 2005). Thus, it is unlikely that PAR proteins have direct association with the regulation of the first mitotic division. Moreover, the newly transcribed zygotic genes during the minor ZGA may play an important role in building the mitotic machinery for the first cleavage.
Perturbation of many maternal factors essential for ZGA could potentially affect cell cleavage and result in 2-cell arrest (as discussed in the previous section). Additionally, several maternal proteins regulate cell cleavage independent of ZGA and are more associated with the mitotic apparatus. Maternal CENPC (centromere protein C) contributes to the first several mitotic divisions in mice and its depletion results in morula stage lethality (Kalitsis, Fowler, Earle, Hill, & Choo, 1998). Activation of maternal PLK1 (Polo-like kinase 1) by phosphorylation precedes pronuclear fusion in 1-cell zygotes, and, when inhibited, perturbs microtubule assembly and prevents completion of the first mitotic division (Baran, Solc, Kovarikova, Rehak, & Sutovsky, 2013). Oocyte-specific depletion of ERK1/2 can result in embryonic arrest at the 1–2-cell stage (Sha et al., 2017) due to insufficient translation activation in oocytes. Maternal SETDB1 deficient embryos arrest during preimplantation due to impaired chromosome segregation (Eymery, Liu, Ozonov, Stadler, & Peters, 2016).
4.2. Cell fate specification
Although each of the two blastomeres formed by the first mitotic cleavage is totipotent, thereafter they gradually gain different fates directing them toward distinct cell lineages. The first blastomere to divide will contribute to the embryonic portion of the blastocyst whereas the later dividing blastomere will contribute to the abembryo (Piotrowska, Wianny, Pedersen, & Zernicka-Goetz, 2001). The loss of totipotency is supported by the bimodal gene expression pattern at the late 2-cell and early 4-cell stages (Biase, Cao, & Zhong, 2014). The bimodal distribution of gene expression is reproducible among embryos and overlaps with differential expression of ICM (inner cell mass) and TE (trophectoderm) genes at the blastocyst stage (Biase et al., 2014). A recent report suggests that the earliest polarity may form within 6h after the first mitotic division (Casser et al., 2017). In these experiments, early 2-cell embryos were separated and cultured to blastocysts.
Consistently, the investigators observed different abilities of the pairs to generate the epiblast (Casser et al., 2017). The loss of totipotency is likely due to coordinated regulation of both maternal and zygotic factors. However, maternal proteins are most likely involved in this process since parthenogenetic embryos can develop up to embryonic day 10 (E10) which is well-past the time of implantation (Niwa et al., 2004). Moreover, the differences of the transcriptomes in the two initial blastomeres are likely to be further modulated by localized translational regulation that can be traced back to the polarity of the egg (Plusa et al., 2002).
The next major differentiation happens at the morula-to-blastocyst transition during which an inner vs. outer polarization underlies the future ICM and TE lineages, respectively, and contributes to the dichotomy of the embryo and placenta. The earliest marker for inner cells is Sox2, while the earliest marker for outer cells is Cdx2, and the separation of SOX2- and CDX2-expressing cells serves as the foundation of inner and outer lineages (Avilion et al., 2003; Strumpf et al., 2005). Both Sox2 and Cdx2 have maternal and zygotic contributions, and the two genes are regulated dynamically in parallel (Avilion et al., 2003; Wicklow et al., 2014). Maternal-zygotic HIPPO signaling pathway is central for establishing SOX2-CDX2 polarity: HIPPO intracellular mediators YAP1/TAZ/TEAD4 directly activate Cdx2 expression in outer cells where they moderately inhibit Sox2 expression (Wicklow et al., 2014). The outer HIPPO activity is further modulated by Rho-ROCK pathway to limit expansion of outer fate (Shi et al., 2017). Notch signaling can also function synergistically with HIPPO in activating Cdx2 in the outer cells (Rayon et al., 2014). On the other hand, HIPPO in the inner cells is inhibited by LATS1/2 though YAP phosphorylation which is further modulated by NF2/MERLIN (Cockburn, Biechele, Garner, & Rossant, 2013). Phosphorylated YAP loses its nuclear localization and normal regulatory roles. Maternal Sox2 knockouts seem to have little effect on preimplantation development, but its overexpression can cause 2-cell arrest (Campolo et al., 2013; Pan & Schultz, 2011) (Fig. 4). In summary, the allocation of different signaling pathways breaks the balance of SOX2 and CDX2 to initiate inner-outer embryo polarity (Frum, Murphy, & Ralston, 2018).
Fig. 4.
Gene network in cell fate specification and differentiation in morula-to-blastocyst transition. (A) SOX2 and CDX2 determine inner-outer polarity at the morula stage. HIPPO pathway modulates the SOX2 and CDX2 balance through interacting with Notch and Rho pathways. The inner-outer polarity is maintained by a SOX2-NANOG-OCT4 network in the inner cell mass (ICM), which is inhibited by CDX2 in the trophectoderm (TE). (B) The established inner-outer polarity persists and the ICM differentiates by priming NANOG and GATA6 to form the epiblast and primitive endoderm which are regulated by zygotic FGF signaling.
The inner-outer polarity is further stabilized by gene networks. SOX2 can form a regulatory core with OCT4 and NANOG, all of which are stemness markers that dominate the ICM to activate self-renewal genes (Niwa, 2007). Meanwhile, CDX2 efficiently represses Oct4 and Nanog expression in the TE (Strumpf et al., 2005). During further lineage differentiation, NANOG and GATA6 prime the epiblast and primitive endoderm formation of the ICM which are modulated by FGF-ERK signaling (Frankenberg et al., 2011). Maternal Fgf4 mutant embryos exhibit no obvious defects and maternal/zygotic Fgf4 mutants have defects similar to the zygotic Fgf4 mutant alone, suggesting that maternal FGF4 is not essential for later lineage specification at implantation (Kang, Piliszek, Artus, & Hadjantonakis, 2013). Taken together, these observations suggest that maternal signaling pathways interact with each other to initiate and advance cell fate specification but may not be important for further lineage differentiation.
5. Maternal factors require timely clearance
Not only the presence but also the timely degradation of maternal factors is essential for early development. Over-expression of cRNA encoding several maternal genes leads to embryonic defects (Gazdag et al., 2009; Jimenez et al., 2015; Pan & Schultz, 2011; Peng et al., 2012; Zeng et al., 2018). Both maternal RNA and proteins are down regulated in early embryos by degradation at the transcript level, but there are also mechanisms to promote maternal protein clearance independent of the stability of cognate RNA.
Cellular RNA level decreases during oocyte maturation (from GV to MII) and from the 1- to 2-cell stage due to reduced transcription and selective RNA degradation (Yu, Ji, Dang, et al., 2016; Yu, Ji, Sha, et al., 2016). Maternal RNA degradation refers to all RNA degradation happening before and after fertilization (Dumdie et al., 2018; Ma, Flemr, Strnad, Svoboda, & Schultz, 2013; Ma, Fukuda, & Schultz, 2015; Piko & Clegg, 1982; Yu, Ji, Dang, et al., 2016; Yu, Ji, Sha, et al., 2016). To better specify the function of maternal RNA degradation, we separate the term by the time point of fertilization into maternal transcriptome shaping (before gamete fusion) and maternal RNA clearance (after gamete fusion). The goal of maternal transcriptome shaping is to produce a fertilizable egg with the proper complement of RNA, and the consequence of maternal RNA clearance is to transfer the control of development from maternal to embryonic programs.
Impaired maternal transcriptome shaping results in poor oocyte quality. Several known oocyte-specific knockouts of genes that regulate RNA metabolism exhibit coordinated dysregulation in RNA degradation, translation and deadenylation (Yu, Ji, Dang, et al., 2016; Yu, Ji, Sha, et al., 2016; Zeng et al., 2018). Special cytoplasmic polyadenylation elements (CPEs) in the 3′ UTR of RNA molecules can switch the RNA translational activity by interactions with either inactivating or activating CPE-binding proteins; thus the number and distance between several CPEs, CPE-like elements and poly(A) signal sequences in one mRNA can define a combinatorial code to control translation and stability (Groisman et al., 2006; Pique, Lopez, Foissac, Guigo, & Mendez, 2008; Sha et al., 2017; Yang et al., 2017). The CCR4-NOT complex, bridging deadenylation and translation of mRNA, is also critical in maternal transcriptome shaping (Collart & Panasenko, 2017). Mutations disrupting members of this complex result in meiotic defects and reduced fertility (Sha et al., 2018; Yu, Ji, Dang, et al., 2016; Yu, Ji, Sha, et al., 2016). A unique characteristic of oocytes is the discordance of RNA deadenylation and degradation—several known deadenylated RNA are not degraded but are protected in RNA-containing granules (Paynton, Rempel, & Bachvarova, 1988) (Fig. 5A). RNA-containing granules in oocytes control access of translation/degradation machinery to certain RNA molecules, i.e., DDX1-association with Aog2, Zar1, Tle6, Floped and Tif1α RNAs protects them from degradation (Hildebrandt et al., 2019). In the absence of DDX1, the embryo arrests at the 2–4-cell stage of development (Hildebrandt et al., 2019). However, the molecular mechanisms that determine whether RNAs will be selectively degraded or protected during oocyte maturation remains unclear. Thus, protein coding RNAs can be either polyadenylated for translation or deadenylated during oocyte maturation. The deadenylated RNAs can be degraded further or protected in cytoplasmic granules. Once delivered into the zygotes, the RNAs can be released from granule protection for polyadenylation, translation or degradation (Fig. 5B).
Fig. 5.
Maternal transcriptome shaping and maternal RNA clearance. (A) Maternal transcriptome shaping happens during oocyte maturation from GV to MII stages. The protein coding transcripts can be either polyadenylated for translation (red), or deadenylated. The deadenylated RNAs can be degraded (blue) or protected in RNA-containing granules (magenta). (B) Maternal RNA clearance happens post-fertilization to ensure zygotic control of subsequent developmental programs. The egg-deposited RNAs can be either polyadenylated for translation, or deadenylated for degradation.
Maternal RNA clearance in mice is even more poorly understood. Unlike zebrafish in which micro-RNA 430 dramatically mediates RNA decay during a brief mid-blastula window (Giraldez et al., 2006), 1-cell mouse embryos take a much longer time to divide and micro-RNAs are not required for transcript degradation (Suh et al., 2010). Maternal-zygotic depletion of DGCR8 (required for miRNA processing) had no effect on preimplantation development and maternal mRNAs targeted by the miRNAs do not degrade more significantly compared to non-miRNA targeted mRNAs (Suh et al., 2010; Yang et al., 2016). On the other hand, DICER (required for the processing of both miRNA and siRNA) is essential for oocyte maturation (Murchison et al., 2007). Thus, it is possible that endo-siRNA (endogenous small interfering RNA) plays more important roles in mouse maternal RNA metabolism. At this moment, many of the known mutants identified with maternal RNA clearance defects reflect poor oocyte quality due to impaired maternal transcriptome shaping (Wu et al., 2003; Yu, Ji, Dang, et al., 2016; Yu, Ji, Sha, et al., 2016). Improved strategies for depleting maternal components after fertilization will be required to more conclusively investigate the role of maternal factors in early embryogenesis.
Other than clearing proteins by getting rid of their cognate mRNA, maternal proteins also can be enzymatically degraded. Maternal Cullin-ring finger ligase-4 (CRL4) complexes can target proteins for polyubiquitination and proteasomal degradation. This facilitates oocyte development and zygotic genome reprogramming and when disrupted results in female infertility (Yu et al., 2013; Zhang et al., 2018). Oocytes deficient in SENP7, a SUMOylation enzyme, experience meiotic arrest with cyclin downregulation and spindle defects (Huang et al., 2017). In summary, maternal factors clearance, both active through selective targeting and passive through insufficient transcription, is an essential process for preimplantation development.
6. Concluding remarks
Maternal factors selectively participate in preimplantation development. Their metabolism, function and clearance are dynamically regulated to guarantee successful embryogenesis. For mechanistic investigations, both genetics and transient assays have advantages and disadvantages. Genetic perturbation produces more consistent and reliable phenotypes necessary for drawing firm conclusions. However, genetic manipulations can adversely affect oocyte quality and inadvertently perturb post-fertilization embryonic development. Although transient assays performed at the 1-cell stage provide a clean background to study loss-of-function, there remain concerns of specificity, toxicity and artifacts introduced by in vitro culture systems. In addition, transient assays often fail to separate maternal and zygotic functions of a gene product. For future investigations, approaches of rapid protein clearance, including Trim-Away and auxin-inducible degron systems, should provide an ideal way to study the function of maternal factors in early embryogenesis (Clift et al., 2017; Holland, Fachinetti, Han, & Cleveland, 2012). Meanwhile, the application of low-input, high throughput sequencing technology will inevitably benefit our understanding of maternal regulation of embryonic development.
Acknowledgments
We apologize to the authors whose work could not be cited because of space constraints. This research was supported by the Intramural Research Program of the NIH, National Institute of Diabetes and Digestive and Kidney Disease.
References
- Aoki F, Worrad DM, & Schultz RM (1997). Regulation of transcriptional activity during the first and second cell cycles in the preimplantation mouse embryo. Developmental Biology, 181, 296–307. [DOI] [PubMed] [Google Scholar]
- Avilion AA, Nicolis SK, Pevny LH, Perez L, Vivian N, & Lovell-Badge R (2003). Multipotent cell lineages in early mouse development depend on SOX2 function. Genes & Development, 17, 126–140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balhorn R (2007). The protamine family of sperm nuclear proteins. Genome Biology, 8, 227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baran V, Solc P, Kovarikova V, Rehak P, & Sutovsky P (2013). Polo-like kinase 1 is essential for the first mitotic division in the mouse embryo. Molecular Reproduction and Development, 80, 522–534. [DOI] [PubMed] [Google Scholar]
- Bebbere D, Masala L, Albertini DF, & Ledda S (2016). The subcortical maternal complex: Multiple functions for one biological structure? Journal of Assisted Reproduction and Genetics, 33, 1431–1438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biase FH, Cao X, & Zhong S (2014). Cell fate inclination within 2-cell and 4-cell mouse embryos revealed by single-cell RNA sequencing. Genome Research, 24, 1787–1796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bierkamp C, Luxey M, Metchat A, Audouard C, Dumollard R, & Christians E (2010). Lack of maternal heat shock factor 1 results in multiple cellular and developmental defects, including mitochondrial damage and altered redox homeostasis, and leads to reduced survival of mammalian oocytes and embryos. Developmental Biology, 339, 338–353. [DOI] [PubMed] [Google Scholar]
- Blij S, Frum T, Akyol A, Fearon E, & Ralston A (2012). Maternal cdx2 is dispensable for mouse development. Development, 139, 3969–3972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bultman SJ, Gebuhr TC, Pan H, Svoboda P, Schultz RM, & Magnuson T (2006). Maternal BRG1 regulates zygotic genome activation in the mouse. Genes & Development, 20, 1744–1754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burns KH, Viveiros MM, Ren Y, Wang P, DeMayo FJ, Frail DE, et al. (2003). Roles of npm2 in chromatin and nucleolar organization in oocytes and embryos. Science, 300, 633–636. [DOI] [PubMed] [Google Scholar]
- Caglar GS, Hammadeh M, Asimakopoulos B, Nikolettos N, Diedrich K, & Al-Hassani S (2005). In vivo and in vitro decondensation of human sperm and assisted reproduction technologies. In Vivo, 19, 623–630. [PubMed] [Google Scholar]
- Campolo F, Gori M, Favaro R, Nicolis S, Pellegrini M, Botti F, et al. (2013). Essential role of sox2 for the establishment and maintenance of the germ cell line. Stem Cells, 31, 1408–1421. [DOI] [PubMed] [Google Scholar]
- Casser E, Israel S, Witten A, Schulte K, Schlatt S, Nordhoff V, et al. (2017). Totipotency segregates between the sister blastomeres of two-cell stage mouse embryos. Scientific Reports, 7, 8299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen J, Lian X, Du J, Xu S, Wei J, Pang L, et al. (2016). Inhibition of phosphorylated ser473-akt from translocating into the nucleus contributes to 2-cell arrest and defective zygotic genome activation in mouse preimplantation embryogenesis. Development, Growth & Differentiation, 58, 280–292. [DOI] [PubMed] [Google Scholar]
- Chen Z, & Zhang Y (2019). Loss of dux causes minor defects in zygotic genome activation and is compatible with mouse development. Nature Genetics, 51, 947–951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clift D, McEwan WA, Labzin LI, Konieczny V, Mogessie B, James LC, et al. (2017). A method for the acute and rapid degradation of endogenous proteins. Cell, 171, 1692–1706.e1618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cockburn K, Biechele S, Garner J, & Rossant J (2013). The hippo pathway member Nf2 is required for inner cell mass specification. Current Biology, 23, 1195–1201. [DOI] [PubMed] [Google Scholar]
- Collart MA, & Panasenko OO (2017). The ccr4-not complex: Architecture and structural insights. Sub-Cellular Biochemistry, 83, 349–379. [DOI] [PubMed] [Google Scholar]
- Dawlaty MM, Ganz K, Powell BE, Hu YC, Markoulaki S, Cheng AW, et al. (2011). Tet1 is dispensable for maintaining pluripotency and its loss is compatible with embryonic and postnatal development. Cell Stem Cell, 9, 166–175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Iaco A, Planet E, Coluccio A, Verp S, Duc J, & Trono D (2017). Dux-family transcription factors regulate zygotic genome activation in placental mammals. Nature Genetics, 49, 941–945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dumdie JN, Cho K, Ramaiah M, Skarbrevik D, Mora-Castilla S, Stumpo DJ, et al. (2018). Chromatin modification and global transcriptional silencing in the oocyte mediated by the mRNA decay activator ZFP36L2. Developmental Cell, 44, 392–402. e397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellard K, Serpa JJ, Petrotchenko EV, Borchers CH, & Ausio J (2016). Expression and purification of the full murine NPM2 and study of its interaction with protamines and histones. Biochemistry and Biophysics Reports, 6, 165–171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eymery A, Liu Z, Ozonov EA, Stadler MB, & Peters AH (2016). The methyl-transferase setdb1 is essential for meiosis and mitosis in mouse oocytes and early embryos. Development, 143, 2767–2779. [DOI] [PubMed] [Google Scholar]
- Falco G, Lee SL, Stanghellini I, Bassey UC, Hamatani T, & Ko MS (2007). Zscan4: A novel gene expressed exclusively in late 2-cell embryos and embryonic stem cells. Developmental Biology, 307, 539–550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frankenberg S, Gerbe F, Bessonnard S, Belville C, Pouchin P, Bardot O, et al. (2011). Primitive endoderm differentiates via a three-step mechanism involving Nanog and RTK signaling. Developmental Cell, 21, 1005–1013. [DOI] [PubMed] [Google Scholar]
- Frum T, Murphy TM, & Ralston A (2018). Hippo signaling resolves embryonic cell fate conflicts during establishment of pluripotency in vivo. eLife, 7, e42298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao Z, Zhang X, Yu X, Qin D, Xiao Y, Yu Y, et al. (2018). Zbed3 participates in the subcortical maternal complex and regulates the distribution of organelles. Journal of Molecular Cell Biology, 10, 74–88. [DOI] [PubMed] [Google Scholar]
- Gazdag E, Santenard A, Ziegler-Birling C, Altobelli G, Poch O, Tora L, et al. (2009). TBP2 is essential for germ cell development by regulating transcription and chromatin condensation in the oocyte. Genes & Development, 23, 2210–2223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giraldez AJ, Mishima Y, Rihel J, Grocock RJ, Van Dongen S, Inoue K, et al. (2006). Zebrafish MiR-430 promotes deadenylation and clearance of maternal mRNAs. Science, 312, 75–79. [DOI] [PubMed] [Google Scholar]
- Groisman I, Ivshina M, Marin V, Kennedy NJ, Davis RJ, & Richter JD (2006). Control of cellular senescence by CPEB. Genes & Development, 20, 2701–2712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gu TP, Guo F, Yang H, Wu HP, Xu GF, Liu W, et al. (2011). The role of tet3 DNA dioxygenase in epigenetic reprogramming by oocytes. Nature, 477, 606–610. [DOI] [PubMed] [Google Scholar]
- Hildebrandt MR, Wang Y, Li L, Yasmin L, Glubrecht DD, & Godbout R (2019). Cytoplasmic aggregation of DDX1 in developing embryos: Early embryonic lethality associated with DDX1 knockout. Developmental Biology. 10.1016/j.ydbio.2019.07.014 [Epub ahead of print]. [DOI] [PubMed] [Google Scholar]
- Hirasawa R, Chiba H, Kaneda M, Tajima S, Li E, Jaenisch R, et al. (2008). Maternal and zygotic Dnmt1 are necessary and sufficient for the maintenance of DNA methylation imprints during preimplantation development. Genes & Development, 22, 1607–1616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holland AJ, Fachinetti D, Han JS, & Cleveland DW (2012). Inducible, reversible system for the rapid and complete degradation of proteins in mammalian cells. Proceedings of the National Academy of Sciences of the United States of America, 109, E3350–E3357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howell CY, Bestor TH, Ding F, Latham KE, Mertineit C, Trasler JM, et al. (2001). Genomic imprinting disrupted by a maternal effect mutation in the Dnmt1 gene. Cell, 104, 829–838. [DOI] [PubMed] [Google Scholar]
- Hu J, Wang F, Zhu X, Yuan Y, Ding M, & Gao S (2010). Mouse ZAR1-like (XM_359149) colocalizes with mRNA processing components and its dominant-negative mutant caused two-cell-stage embryonic arrest. Developmental Dynamics, 239, 407–424. [DOI] [PubMed] [Google Scholar]
- Huang CJ, Wu D, Jiao XF, Khan FA, Xiong CL, Liu XM, et al. (2017). Maternal SENP7 programs meiosis architecture and embryo survival in mouse. Biochimica et Biophysica Acta. Molecular Cell Research, 1864, 1195–1206. [DOI] [PubMed] [Google Scholar]
- Hug CB, Grimaldi AG, Kruse K, & Vaquerizas JM (2017). Chromatin architecture emerges during zygotic genome activation independent of transcription. Cell, 169, 216–228.e219. [DOI] [PubMed] [Google Scholar]
- Inoue A, Ogushi S, Saitou M, Suzuki MG, & Aoki F (2011). Involvement of mouse nucleoplasmin 2 in the decondensation of sperm chromatin after fertilization. Biology of Reproduction, 85, 70–77. [DOI] [PubMed] [Google Scholar]
- Ivanova I, Much C, Di Giacomo M, Azzi C, Morgan M, Moreira PN, et al. (2017). The RNA m(6)a reader YTHDF2 is essential for the post-transcriptional regulation of the maternal transcriptome and oocyte competence. Molecular Cell, 67, 1059–1067. e1054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jedrusik A, Cox A, Wicher KB, Glover DM, & Zernicka-Goetz M (2015). Maternal-zygotic knockout reveals a critical role of Cdx2 in the morula to blastocyst transition. Developmental Biology, 398, 147–152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeltsch A, & Jurkowska RZ (2014). New concepts in DNA methylation. Trends in Biochemical Sciences, 39, 310–318. [DOI] [PubMed] [Google Scholar]
- Jimenez R, Melo EO, Davydenko O, Ma J, Mainigi M, Franke V, et al. (2015). Maternal SIN3A regulates reprogramming of gene expression during mouse preimplantation development. Biology of Reproduction, 93, 89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jukam D, Shariati SAM, & Skotheim JM (2017). Zygotic genome activation in vertebrates. Developmental Cell, 42, 316–332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jung YH, Kremsky I, Gold HB, Rowley MJ, Punyawai K, Buonanotte A, et al. (2019). Maintenance of CTCF- and transcription factor-mediated interactions from the gametes to the early mouse embryo. Molecular Cell, 75, 154–171.e155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalitsis P, Fowler KJ, Earle E, Hill J, & Choo KH (1998). Targeted disruption of mouse centromere protein c gene leads to mitotic disarray and early embryo death. Proceedings of the National Academy of Sciences of the United States of America, 95, 1136–1141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaneda M, Hirasawa R, Chiba H, Okano M, Li E, & Sasaki H (2010). Genetic evidence for Dnmt3a-dependent imprinting during oocyte growth obtained by conditional knockout with Zp3-cre and complete exclusion of Dnmt3b by chimera formation. Genes to Cells, 15, 169–179. [DOI] [PubMed] [Google Scholar]
- Kang J, Lienhard M, Pastor WA, Chawla A, Novotny M, Tsagaratou A, et al. (2015). Simultaneous deletion of the methylcytosine oxidases Tet1 and Tet3 increases transcriptome variability in early embryogenesis. Proceedings of the National Academy of Sciences of the United States of America, 112, E4236–E4245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang M, Piliszek A, Artus J, & Hadjantonakis AK (2013). FGF4 is required for lineage restriction and salt-and-pepper distribution of primitive endoderm factors but not their initial expression in the mouse. Development, 140, 267–279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ke Y, Xu Y, Chen X, Feng S, Liu Z, Sun Y, et al. (2017). 3D chromatin structures of mature gametes and structural reprogramming during mammalian embryogenesis. Cell, 170, 367–381.e320. [DOI] [PubMed] [Google Scholar]
- Kim SI, Bresnick EH, & Bultman SJ (2009). BRG1 directly regulates nucleosome structure and chromatin looping of the alpha globin locus to activate transcription. Nucleic Acids Research, 37, 6019–6027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kong Q, Banaszynski LA, Geng F, Zhang X, Zhang J, Zhang H, et al. (2018). Histone variant H3.3-mediated chromatin remodeling is essential for paternal genome activation in mouse preimplantation embryos. The Journal of Biological Chemistry, 293, 3829–3838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li L, Baibakov B, & Dean J (2008). A subcortical maternal complex essential for preimplantation mouse embryogenesis. Developmental Cell, 15, 416–425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Z, Cai X, Cai CL, Wang J, Zhang W, Petersen BE, et al. (2011). Deletion of Tet2 in mice leads to dysregulated hematopoietic stem cells and subsequent development of myeloid malignancies. Blood, 118, 4509–4518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li X, Yue X, Pastor WA, Lin L, Georges R, Chavez L, et al. (2016). Tet proteins influence the balance between neuroectodermal and mesodermal fate choice by inhibiting wnt signaling. Proceedings of the National Academy of Sciences of the United States of America, 113, E8267–E8276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li L, Zheng P, & Dean J (2010). Maternal control of early mouse development. Development, 137, 859–870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim AK, & Knowles BB (2015). Controlling endogenous retroviruses and their chimeric transcripts during natural reprogramming in the oocyte. The Journal of Infectious Diseases, 212(Suppl. 1), S47–S51. [DOI] [PubMed] [Google Scholar]
- Liu Y, Lu X, Shi J, Yu X, Zhang X, Zhu K, et al. (2016). BTG4 is a key regulator for maternal mrna clearance during mouse early embryogenesis. Journal of Molecular Cell Biology, 8, 366–368. [DOI] [PubMed] [Google Scholar]
- Lu X, Gao Z, Qin D, & Li L (2017). A maternal functional module in the mammalian oocyte-to-embryo transition. Trends in Molecular Medicine, 23, 1014–1023. [DOI] [PubMed] [Google Scholar]
- Lu F, Liu Y, Inoue A, Suzuki T, Zhao K, & Zhang Y (2016). Establishing chromatin regulatory landscape during mouse preimplantation development. Cell, 165, 1375–1388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma J, Flemr M, Strnad H, Svoboda P, & Schultz RM (2013). Maternally recruited DCP1A and DCP2 contribute to messenger RNA degradation during oocyte maturation and genome activation in mouse. Biology of Reproduction, 88, 11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma J, Fukuda Y, & Schultz RM (2015). Mobilization of dormant Cnot7 mRNA promotes deadenylation of maternal transcripts during mouse oocyte maturation. Biology of Reproduction, 93, 48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma J, Zeng F, Schultz RM, & Tseng H (2006). Basonuclin: A novel mammalian maternal-effect gene. Development, 133, 2053–2062. [DOI] [PubMed] [Google Scholar]
- Madissoon E, Damdimopoulos A, Katayama S, Krjutskov K, Einarsdottir E, Mamia K, et al. (2019). Pleomorphic adenoma gene 1 is needed for timely zygotic genome activation and early embryo development. Scientific Reports, 9, 8411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahadevan S, Sathappan V, Utama B, Lorenzo I, Kaskar K, & Van den Veyver IB (2017). Maternally expressed NLRP2 links the subcortical maternal complex (SCMC) to fertility, embryogenesis and epigenetic reprogramming. Scientific Reports, 7, 44667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maslon MM, Braunschweig U, Aitken S, Mann AR, Kilanowski F, Hunter CJ, et al. (2019). A slow transcription rate causes embryonic lethality and perturbs kinetic coupling of neuronal genes. The EMBO Journal, 38, e101244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayer W, Niveleau A, Walter J, Fundele R, & Haaf T (2000). Demethylation of the zygotic paternal genome. Nature, 403, 501–502. [DOI] [PubMed] [Google Scholar]
- Messerschmidt DM, Knowles BB, & Solter D (2014). DNA methylation dynamics during epigenetic reprogramming in the germline and preimplantation embryos. Genes & Development, 28, 812–828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murchison EP, Stein P, Xuan Z, Pan H, Zhang MQ, Schultz RM, et al. (2007). Critical roles for dicer in the female germline. Genes & Development, 21, 682–693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakamura T, Liu YJ, Nakashima H, Umehara H, Inoue K, Matoba S, et al. (2012). PGC7 binds histone H3K9me2 to protect against conversion of 5mC to 5hmC in early embryos. Nature, 486, 415–419. [DOI] [PubMed] [Google Scholar]
- Niwa H (2007). How is pluripotency determined and maintained? Development, 134, 635–646. [DOI] [PubMed] [Google Scholar]
- Niwa K, Takano R, Obata Y, Hiura H, Komiyama J, Ogawa H, et al. (2004). Nuclei of oocytes derived from mouse parthenogenetic embryos are competent to support development to term. Biology of Reproduction, 71, 1560–1567. [DOI] [PubMed] [Google Scholar]
- Ogushi S, Yamagata K, Obuse C, Furuta K, Wakayama T, Matzuk MM, et al. (2017). Reconstitution of the oocyte nucleolus in mice through a single nucleolar protein, NPM2. Journal of Cell Science, 130, 2416–2429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okano M, Bell DW, Haber DA, & Li E (1999). DNA methyltransferases Dnmt3a and Dnmt3b are essential for de novo methylation and mammalian development. Cell, 99, 247–257. [DOI] [PubMed] [Google Scholar]
- Ong CT, & Corces VG (2014). CTCF: An architectural protein bridging genome topology and function. Nature Reviews. Genetics, 15, 234–246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan H, & Schultz RM (2011). SOX2 modulates reprogramming of gene expression in two-cell mouse embryos. Biology of Reproduction, 85, 409–416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park MW, Kim KH, Kim EY, Lee SY, Ko JJ, & Lee KA (2015). Associations among sebox and other MEGs and its effects on early embryogenesis. PLoS One, 10, e0115050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paynton BV, Rempel R, & Bachvarova R (1988). Changes in state of adenylation and time course of degradation of maternal mRNAs during oocyte maturation and early embryonic development in the mouse. Developmental Biology, 129, 304–314. [DOI] [PubMed] [Google Scholar]
- Peng H, Chang B, Lu C, Su J, Wu Y, Lv P, et al. (2012). NLRP2, a maternal effect gene required for early embryonic development in the mouse. PLoS One, 7, e30344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Philpott A, & Leno GH (1992). Nucleoplasmin remodels sperm chromatin in xenopus egg extracts. Cell, 69, 759–767. [DOI] [PubMed] [Google Scholar]
- Piko L, & Clegg KB (1982). Quantitative changes in total RNA, total poly(a), and ribosomes in early mouse embryos. Developmental Biology, 89, 362–378. [DOI] [PubMed] [Google Scholar]
- Piotrowska K, Wianny F, Pedersen RA, & Zernicka-Goetz M (2001). Blastomeres arising from the first cleavage division have distinguishable fates in normal mouse development. Development, 128, 3739–3748. [DOI] [PubMed] [Google Scholar]
- Pique M, Lopez JM, Foissac S, Guigo R, & Mendez R (2008). A combinatorial code for CPE-mediated translational control. Cell, 132, 434–448. [DOI] [PubMed] [Google Scholar]
- Plusa B, Grabarek JB, Piotrowska K, Glover DM, & Zernicka-Goetz M (2002). Site of the previous meiotic division defines cleavage orientation in the mouse embryo. Nature Cell Biology, 4, 811–815. [DOI] [PubMed] [Google Scholar]
- Potireddy S, Vassena R, Patel BG, & Latham KE (2006). Analysis of polysomal mRNA populations of mouse oocytes and zygotes: Dynamic changes in maternal mRNA utilization and function. Developmental Biology, 298, 155–166. [DOI] [PubMed] [Google Scholar]
- Ramos SB, Stumpo DJ, Kennington EA, Phillips RS, Bock CB, Ribeiro-Neto F, et al. (2004). The CCCH tandem zinc-finger protein Zfp36l2 is crucial for female fertility and early embryonic development. Development, 131, 4883–4893. [DOI] [PubMed] [Google Scholar]
- Rayon T, Menchero S, Nieto A, Xenopoulos P, Crespo M, Cockburn K, et al. (2014). Notch and hippo converge on Cdx2 to specify the trophectoderm lineage in the mouse blastocyst. Developmental Cell, 30, 410–422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roest HP, Baarends WM, de Wit J, van Klaveren JW, Wassenaar E, Hoogerbrugge JW, et al. (2004). The ubiquitin-conjugating DNA repair enzyme HR6A is a maternal factor essential for early embryonic development in mice. Molecular and Cellular Biology, 24, 5485–5495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schultz RM (1993). Regulation of zygotic gene activation in the mouse. BioEssays, 15, 531–538. [DOI] [PubMed] [Google Scholar]
- Sha QQ, Dai XX, Dang Y, Tang F, Liu J, Zhang YL, et al. (2017). A MAPK cascade couples maternal mRNA translation and degradation to meiotic cell cycle progression in mouse oocytes. Development, 144, 452–463. [DOI] [PubMed] [Google Scholar]
- Sha QQ, Yu JL, Guo JX, Dai XX, Jiang JC, Zhang YL, et al. (2018). CNOT6L couples the selective degradation of maternal transcripts to meiotic cell cycle progression in Mouse oocyte. The EMBO Journal, 37, e99333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen L, Inoue A, He J, Liu Y, Lu F, & Zhang Y (2014). Tet3 and DNA replication mediate demethylation of both the maternal and paternal genomes in mouse zygotes. Cell Stem Cell, 15, 459–471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi X, Yin Z, Ling B, Wang L, Liu C, Ruan X, et al. (2017). Rho differentially regulates the hippo pathway by modulating the interaction between Amot and Nf2 in the blastocyst. Development, 144, 3957–3967. [DOI] [PubMed] [Google Scholar]
- Strumpf D, Mao CA, Yamanaka Y, Ralston A, Chawengsaksophak K, Beck F, et al. (2005). Cdx2 is required for correct cell fate specification and differentiation of trophectoderm in the mouse blastocyst. Development, 132, 2093–2102. [DOI] [PubMed] [Google Scholar]
- Suh N, Baehner L, Moltzahn F, Melton C, Shenoy A, Chen J, et al. (2010). Microrna function is globally suppressed in mouse oocytes and early embryos. Current Biology, 20, 271–277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tarkowski AK (1959). Experiments on the development of isolated blastomers of mouse eggs. Nature, 184, 1286–1287. [DOI] [PubMed] [Google Scholar]
- Torres-Padilla ME, & Zernicka-Goetz M (2006). Role of TIF1alpha as a modulator of embryonic transcription in the mouse zygote. The Journal of Cell Biology, 174, 329–338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsai SY, Opavsky R, Sharma N, Wu L, Naidu S, Nolan E, et al. (2008). Mouse development with a single E2F activator. Nature, 454, 1137–1141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Heijden GW, Dieker JW, Derijck AA, Muller S, Berden JH, Braat DD, et al. (2005). Asymmetry in histone H3 variants and lysine methylation between paternal and maternal chromatin of the early mouse zygote. Mechanisms of Development, 122, 1008–1022. [DOI] [PubMed] [Google Scholar]
- Vinot S, Le T, Ohno S, Pawson T, Maro B, & Louvet-Vallee S (2005). Asymmetric distribution of par proteins in the mouse embryo begins at the 8-cell stage during compaction. Developmental Biology, 282, 307–319. [DOI] [PubMed] [Google Scholar]
- Vogt EJ, Tokuhiro K, Guo M, Dale R, Yang G, Shin SW, et al. (2019). Anchoring cortical granules in the cortex ensures trafficking to the plasma membrane for postfertilization exocytosis. Nature Communications, 10, 2271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wan LB, Pan H, Hannenhalli S, Cheng Y, Ma J, Fedoriw A, et al. (2008). Maternal depletion of CTCF reveals multiple functions during oocyte and preimplantation embryo development. Development, 135, 2729–2738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wicklow E, Blij S, Frum T, Hirate Y, Lang RA, Sasaki H, et al. (2014). Hippo pathway members restrict SOX2 to the inner cell mass where it promotes ICM fates in the mouse blastocyst. PLoS Genetics, 10, e1004618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilkerson DC, Murphy LA, & Sarge KD (2008). Interaction of HSF1 and HSF2 with the Hspa1b promoter in mouse epididymal spermatozoa. Biology of Reproduction, 79, 283–288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu D, & Dean J (2016). BTG4, a maternal mRNA cleaner. Journal of Molecular Cell Biology, 8, 369–370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu G, & Scholer HR (2014). Role of Oct4 in the early embryo development. Cell Regeneration (London), 3, 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu X, Viveiros MM, Eppig JJ, Bai Y, Fitzpatrick SL, & Matzuk MM (2003). Zygote arrest 1 (Zar1) is a novel maternal-effect gene critical for the oocyte-to-embryo transition. Nature Genetics, 33, 187–191. [DOI] [PubMed] [Google Scholar]
- Wu SC, & Zhang Y (2010). Active DNA demethylation: Many roads lead to Rome. Nature Reviews. Molecular Cell Biology, 11, 607–620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu H, & Zhang Y (2014). Reversing DNA methylation: Mechanisms, genomics, and biological functions. Cell, 156, 45–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu Q, Wang F, Xiang Y, Zhang X, Zhao ZA, Gao Z, et al. (2015). Maternal BCAS2 protects genomic integrity in mouse early embryonic development. Development, 142, 3943–3953. [DOI] [PubMed] [Google Scholar]
- Yang Q, Lin J, Liu M, Li R, Tian B, Zhang X, et al. (2016). Highly sensitive sequencing reveals dynamic modifications and activities of small RNAs in mouse oocytes and early embryos. Science Advances, 2, e1501482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang XJ, & Seto E (2008). The Rpd3/Hda1 family of lysine deacetylases: From bacteria and yeast to mice and men. Nature Reviews Molecular Cell Biology, 9, 206–218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Y, Yang CR, Han SJ, Daldello EM, Cho A, Martins JPS, et al. (2017). Maternal mRNAs with distinct 3′ UTRs define the temporal pattern of Ccnb1 synthesis during mouse oocyte meiotic maturation. Genes & Development, 31, 1302–1307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu C, Ji SY, Dang YJ, Sha QQ, Yuan YF, Zhou JJ, et al. (2016). Oocyte-expressed yes-associated protein is a key activator of the early zygotic genome in mouse. Cell Research, 26, 275–287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu C, Ji SY, Sha QQ, Dang Y, Zhou JJ, Zhang YL, et al. (2016). BTG4 is a meiotic cell cycle-coupled maternal-zygotic-transition licensing factor in oocytes. Nature Structural & Molecular Biology, 23, 387–394. [DOI] [PubMed] [Google Scholar]
- Yu C, Zhang YL, Pan WW, Li XM, Wang ZW, Ge ZJ, et al. (2013). CRL4 complex regulates mammalian oocyte survival and reprogramming by activation of TET proteins. Science, 342, 1518–1521. [DOI] [PubMed] [Google Scholar]
- Zeng J, Jiang M, Wu X, Diao F, Qiu D, Hou X, et al. (2018). SIRT4 is essential for metabolic control and meiotic structure during mouse oocyte maturation. Aging Cell, 17, e12789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang YL, Zhao LW, Zhang J, Le R, Ji SY, Chen C, et al. (2018). DCAF13 promotes pluripotency by negatively regulating SUV39H1 stability during early embryonic development. The EMBO Journal, 37, e98981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng P, & Dean J (2009). Role of Filia, a maternal effect gene, in maintaining euploidy during cleavage-stage mouse embryogenesis. Proceedings of the National Academy of Sciences of the United States of America, 106, 7473–7478. [DOI] [PMC free article] [PubMed] [Google Scholar]