Abstract
Retinal guanylate cyclases (RetGCs) promote the Ca2+-dependent synthesis of cGMP that coordinates the recovery phase of visual phototransduction in retinal rods and cones. The Ca2+-sensitive activation of RetGCs is controlled by a family of photoreceptor Ca2+ binding proteins known as guanylate cyclase activator proteins (GCAPs). The Mg2+-bound/Ca2+-free GCAPs bind to RetGCs and activate cGMP synthesis (cyclase activity) at low cytosolic Ca2+ levels in light-activated photoreceptors. By contrast, Ca2+-bound GCAPs bind to RetGCs and inactivate cyclase activity at high cytosolic Ca2+ levels found in dark-adapted photoreceptors. Mutations in both RetGCs and GCAPs that disrupt the Ca2+-dependent cyclase activity are genetically linked to various retinal diseases known as cone-rod dystrophies. In this review, I will provide an overview of the known atomic-level structures of various GCAP proteins to understand how protein dimerization and Ca2+-dependent conformational changes in GCAPs control the cyclase activity of RetGCs. This review will also summarize recent structural studies on a GCAP homolog from zebrafish (GCAP5) that binds to Fe2+ and may serve as a Fe2+ sensor in photoreceptors. The GCAP structures reveal an exposed hydrophobic surface that controls both GCAP1 dimerization and RetGC binding. This exposed site could be targeted by therapeutics designed to inhibit the GCAP1 disease mutants, which may serve to mitigate the onset of retinal cone-rod dystrophies.
Keywords: phototransduction, retinal guanylate cyclase, calcium, GCAP1, GCAP2, GCAP5
1. Introduction
1.1. Ca2+-Sensitive Regulation of RetGC Coordinates Visual Recovery
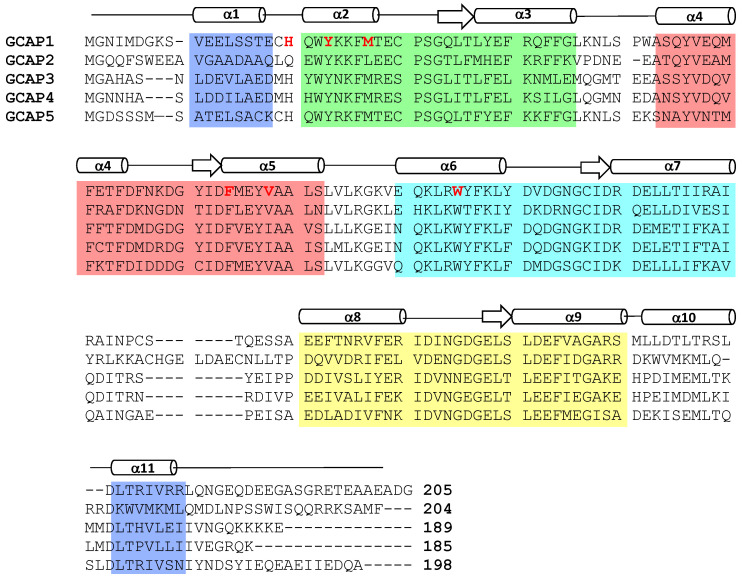
Visual excitation of retinal rod and cone photoreceptors is triggered by a phototransduction cascade in which light excitation activates a photoreceptor-specific phosphodiesterase that in turn hydrolyzes cGMP (see reviews by [1,2]). The light-induced lowering of cGMP levels in photoreceptor cells causes the closure of cGMP-gated cation channels in the plasma membrane, resulting in membrane hyperpolarization (see reviews by [3,4]). The light-induced membrane hyperpolarization rapidly recovers back to the resting potential of the dark state when the light stimulus is removed in a process known as visual recovery. The recovery phase of phototransduction involves replenishing the photoreceptor cGMP levels [5] by the Ca2+ sensitive activation [6,7] of retina-specific guanylate cyclases (RetGCs) [8,9]. The Ca2+-dependent activity of RetGC is controlled by intracellular domains [10,11] that interact with soluble EF-hand Ca2+ sensor proteins, called guanylate cyclase activator proteins (GCAP1-5, see Figure 1) [8,12,13,14,15,16].
Figure 1.
Amino acid sequence alignment of GCAP proteins (bovine GCAP1-2 and zebrafish GCAP3-5). Secondary structure elements (helices and strands) are depicted by cylinders and arrows. EF-hand residues are shaded in green, red, cyan, and yellow. The terminal residues that contact the myristoyl group are shaded purple. Exposed hydrophobic residues at the GCAP1 dimerization site are highlighted in bold and red.
Light-induced closure of cGMP-gated channels in vertebrate rod and cone photoreceptors causes a 10-fold decrease in the cytosolic free Ca2+ concentration [17,18]. RetGC catalysis is activated by Ca2+-free GCAPs in light-activated photoreceptors [8,12,13,19,20], whereas the cyclase activity is inhibited by Ca2+-bound GCAPs in dark-adapted photoreceptors [5,19,21]. During visual recovery, a photoreceptor cell exhibits a more than 10-fold increase in cGMP production due to the Ca2+-sensitive activation of RetGC by GCAPs [5,22] and is a critical step for controlling the recovery rate of a single-photon response [4,5] as well as the cone response to stronger light stimuli [23].
1.2. Ca2+/Mg2+ Binding to GCAPs Control Activation of RetGC
GCAP proteins bind to and activate RetGC in light-activated photoreceptors that contain low Ca2+ levels (less than 50 nM) and physiological Mg2+ levels (1 mM) [24,25,26,27]. Thus, GCAP proteins that exist in light-activated photoreceptors activate RetGC and are called the activator state. At least one Mg2+ binds to GCAP1 in the activator state [26], and NMR studies reveal that Mg2+ is bound to GCAP1 at the second EF-hand (EF2 in Figure 1) [28]. The apo-state of GCAP1 (Ca2+-free/Mg2+-free) contains a regular secondary structure [29] but does not adopt a stable three-dimensional fold [25,28]. The Ca2+-free/Mg2+-free GCAPs form a flexible molten-globule state, which could explain why GCAPs do not activate RetGC in the absence of Mg2+ [24]. Thus, Mg2+ binding to GCAP1 is required to stabilize its protein structure to promote activation of RetGC [8,25,30]. By contrast, Ca2+ binding to GCAP1 (in place of Mg2+ binding) stabilizes a distinct structure important for the inhibition of RetGC [21]. Ca2+ binds to GCAPs at the second, third, and fourth EF-hands (EF2, EF3, and EF4 in Figure 1) [31,32]. The apparent dissociation constant for Ca2+ binding to GCAPs is 100 nM [24,28], whereas Mg2+ binds to GCAPs in the micromolar range [25,27,28]. Dark-adapted rod cells have relatively high cytosolic Ca2+ levels ([Ca2+]free = 250–500 nM [18], which implies that GCAPs are nearly saturated with Ca2+ in dark-adapted rod cells. Light-activation of the rod cell causes a dramatic lowering of the cytosolic Ca2+ level ([Ca2+]free = 5–50 nM [17,18,33]) while the Mg2+ level remains fixed at [Mg2+]free ~ 1 mM [34]. Therefore, in light-adapted rods, GCAPs are bound to Mg2+ instead of Ca2+. In essence, the Mg2+-bound/Ca2+-free GCAPs in light-activated photoreceptors turn on the synthesis of cGMP to help restore the dark-adapted photoreceptor during visual recovery [8,12,13], whereas Ca2+-saturated GCAPs turn off the synthesis of cGMP in the resting dark state [19,21].
1.3. Mutations in GCAP1 Cause Retinal Disease
Mutations in GCAP1 that weaken or disable Ca2+ binding to the EF-hands cause GCAP1 to constitutively activate RetGC in rod and cones. Some of these mutations (Y99C, D100G, E111V, and E155G) are genetically linked to retinal diseases known as cone-rod dystrophies [29,35,36]. For example, the GCAP1 mutants (Y99C [21,37], D100G [38], E111V [39] and E155G [40,41]) each prevent Ca2+ binding to EF3 or EF4 under physiological conditions, which enables the Ca2+-free/Mg2+-bound GCAP1 activator state to persist in both light-activated and dark-adapted photoreceptors. In essence, these constitutively active GCAP1 mutants fail to turn off the cyclase activity in dark-adapted photoreceptors and cause persistent activation of RetGC [42,43]. This constitutive activation of RetGC causes elevated cGMP levels in photoreceptor cells that promote apoptosis and disease [42,44,45]. Future studies are needed to discover therapeutic agents that bind specifically to the constitutively active mutants of GCAP1 (Y99C, D100G, E111V, and E155G) to block or prevent their constitutive activation of RetGCs, which may diminish or slow down the onset of cone-rod dystrophies.
2. Results and Discussion
2.1. Structural Architecture of GCAPs
Mammalian photoreceptors have two different GCAP isoforms (GCAP1 and GCAP2 in Figure 1) that are more than 65% identical to GCAP homologs found in zebrafish photoreceptors (GCAP3-5 in Figure 1). All of the GCAPs contain ~200 residues, 4 EF-hand motifs (highlighted in color in Figure 1), a myristoyl group covalently attached to the N-terminal glycine, and non-conserved residues at the N- and C-termini (α1 and α11 in Figure 1). The second, third, and fourth EF-hands each bind to Ca2+ or Mg2+ as described above. The first EF-hand (EF1) does not bind to Ca2+ or Mg2+ because of unfavorable residues in the EF-hand binding loop (Cys29 in GCAP1 or Arg25 in GCAP3, see Figure 1). The lack of metal binding to the first EF-hand allows it to adopt an unusual structure that interacts with the N-terminal myristoyl group [32,46,47]. Outside of the core EF-hand region, the non-conserved helices (α1 and α11, highlighted purple in Figure 1) both form contacts with the myristoyl group [32]. Atomic-level structures are known for Ca2+-bound forms of GCAP1 [32] and GCAP2 [31], and Mg2+-bound/Ca2+-free GCAP1 [48] as described below.
2.1.1. NMR Structure of GCAP2
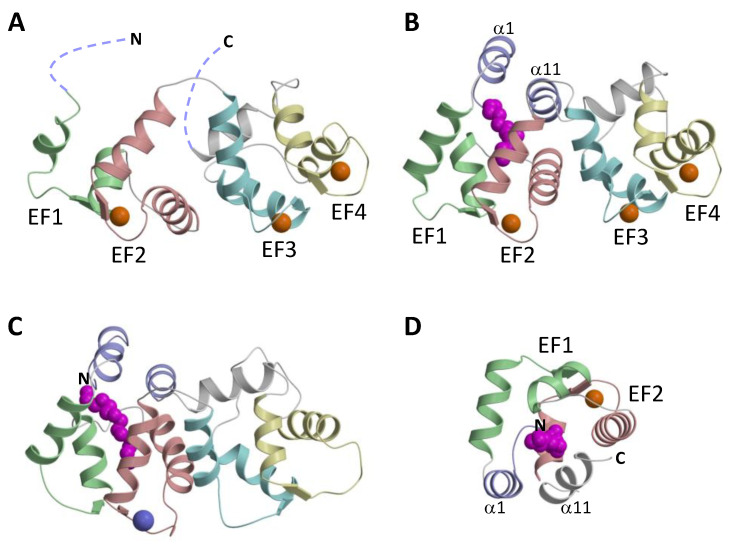
The NMR structure of the Ca2+-saturated and unmyristoylated GCAP2 (Figure 2A) was the first atomic-resolution structure of a GCAP protein [31]. The first 20 amino acids from the N-terminus and the last 19 residues from the C-terminus in unmyristoylated GCAP2 could not be resolved by NMR (see dotted lines in Figure 2A). The core region of GCAP2 (residues 23–185) contains 4 EF-hands that are structurally similar to the EF-hands in Ca2+-bound recoverin [49,50]. An important structural difference is that Ca2+ is bound at EF2, EF3, and EF4 in GCAP2, in contrast to recoverin where Ca2+ is bound only at EF2 and EF3 [51]. The lack of N-terminal myristoylation in the NMR structure of GCAP2 may contribute to the structural disorder at the N- and C-termini (dotted lines in Figure 2A). This could account for why the N-terminal myristoyl group in GCAP2 is exposed to the exterior in the presence of lipid bilayer membranes [52,53], which could enable the myristoyl group to anchor GCAP2 to membranes [54,55]. The exposed and unstructured N-terminal region in the GCAP2 NMR structure may explain why GCAP2 can exhibit Ca2+-dependent membrane binding, whereas GCAP1 does not [56].
Figure 2.
Atomic-level structures of unmyristoylated GCAP2 (A), myristoylated GCAP1 (B), Mg2+-bound GCAP1V77E (C), and myristoyl group binding site in Ca2+-bound GCAP1 (D). The color scheme is the same as in Figure 1. The EF-hands are shaded green, red, cyan, and yellow. The terminal helices (α1 and α11) that contact the myristoyl group are colored purple. Bound Mg2+ and Ca2+ are colored purple and orange, respectively. The N-terminal myristoyl group is colored magenta.
2.1.2. Crystal Structure of GCAP1
The x-ray crystal structure of myristoylated GCAP1 (Figure 2B) showed the N-terminal myristoyl group to be sequestered inside the protein [32]. The four EF-hands in GCAP1 (Figure 1 and Figure 2B) are grouped into two globular domains: the N-domain is comprised of EF1 and EF2 and the C-domain is comprised of EF3 and EF4. Ca2+ is bound to GCAP1 at EF2, EF3, and EF4, and the structure of each Ca2+-bound EF-hand in GCAP1 (Figure 2B) adopts the familiar open conformation as seen in calmodulin and other Ca2+-bound EF-hand proteins [57]. Indeed, the interhelical angles for each Ca2+-bound EF-hand in GCAP1 are nearly identical to those of GCAP2 (Figure 2A). A unique structural feature of GCAP1 is that the N-terminal α-helix (α1 in Figure 1) and C-terminal helix (α11) are held closely together by their mutual interaction with the N-terminal myristoyl group (Figure 2D). Thus, the covalently attached myristoyl group in GCAP1 is sequestered within a unique environment inside the Ca2+-bound protein and prevents GCAP1 from having a Ca2+-myristoyl switch [28,56]. In essence, the myristoyl group serves to bridge both the N-terminal and C-terminal ends of the protein, which explains how Ca2+-induced conformational changes in the C-terminal domain (particularly in EF4) might be transmitted to a possible target binding site in EF1. A Ca2+-myristoyl tug mechanism [58,59] has been proposed to explain how Ca2+-induced conformational changes in EF4 serve to “tug” on the adjacent C-terminal helix that connects structurally to the myristoyl group and EF1. This tug mechanism helps explain how Ca2+-induced structural changes in EF4 might be relayed to the cyclase binding region in EF1 [60]. The Ca2+-induced structural changes involving the C-terminal helix might also be related to Ca2+-dependent phosphorylation of S201 in GCAP2 [25].
2.1.3. Ca2+-Induced Conformational Changes in GCAP1
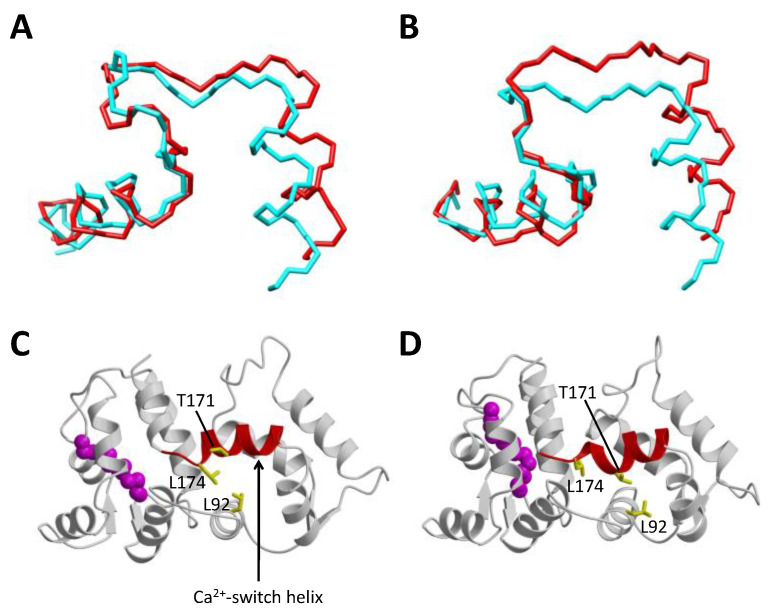
The atomic-level structure of Ca2+-free/Mg2+-bound activator form of wild-type GCAPs is currently not known. A GCAP1 mutant, V77E (called GCAP1V77E) was shown previously to abolish dimerization of GCAP1 that significantly sharpened its NMR spectrum, and GCAP1V77E was used to solve the NMR structure of Ca2+-free/Mg2+-bound GCAP1V77E [48]. The NMR structure of Ca2+-free/Mg2+-bound GCAP1V77E is shown in Figure 2C. The overall structure of Ca2+-free/Mg2+-bound GCAP1V77E is similar to the crystal structure of Ca2+-bound GCAP1 (root mean squared deviation of main-chain atoms is 2.4 Ǻ when comparing the two structures). The overall structural similarity between Ca2+-free and Ca2+-bound GCAP1 may explain why GCAP1 has nearly a 100-fold higher Ca2+-binding affinity compared to the Ca2+ sensor proteins like recoverin and calmodulin that undergo large and unfavorable conformational changes coupled to Ca2+ binding [49,57]. In a sense, the GCAP proteins are more like the Ca2+ buffer proteins (calbindins and parvalbumin) that adopt pre-formed EF-hand open structures in the absence of Ca2+, which allows the buffer proteins to have maximal Ca2+ binding affinity [57]. However, small Ca2+-dependent structural changes are detected within the EF-hands: Ca2+ binding to EF2 reveals a small change in the helix packing angle (Figure 3A). The interhelical angle of the Mg2+-bound EF2 (114°, highlighted red in Figure 3A) is slightly more closed than the interhelical angle of Ca2+-bound EF2 (110°, highlighted cyan in Figure 3A). A similar Ca2+-induced opening of the interhelical angle is also apparent in EF3 (Figure 3B). Thus, the small Ca2+-dependent conformational changes in EF2 and EF3 might be functionally important for regulating RetGC. The largest Ca2+-induced structural change in GCAP1 is observed in the Ca2+ switch helix (residues 169–174 highlighted red in Figure 3C,D). Residues in the Ca2+ switch helix (T171 and L174) exhibit Ca2+-dependent solvent accessibility. T171 is exposed in the Ca2+-free structure, whereas it becomes buried and makes contact with L92 in the Ca2+-bound structure. Conversely, L174 is buried and makes contact with L92 in the Ca2+-free structure, in contrast to its solvent-exposed environment in the Ca2+-bound structure. These Ca2+-dependent contacts to the Ca2+ switch helix may be important for switching GCAP1 from the Ca2+-free activator to the Ca2+-bound inhibitor states. The Ca2+-induced shortening of the Ca2+ switch helix may also serve a role in modulating Ca2+-dependent contacts with RetGC.
Figure 3.
Ca2+-induced conformational changes in GCAP1. Main chain structures of EF2 (A), EF3 (B), Mg2+-bound/Ca2+-free GCAP1V77E (C), and Ca2+-bound GCAP1 (D). The Ca2+-free structures of EF2 and EF3 (red in panels (A,B)) are overlaid on top of the Ca2+-bound structures (cyan). EF2 and EF3 exhibit a Ca2+-induced decrease in interhelical angle. The Ca2+-switch helix (residues 169–174) undergoes a Ca2+-induced shortening (highlighted red in panels (C,D)).
2.2. Dimeric Structures of GCAP1 and GCAP2
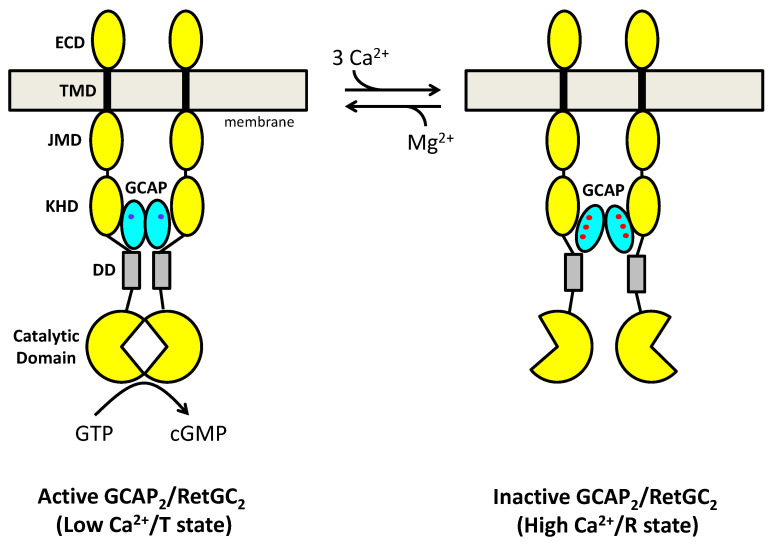
The GCAP proteins have a propensity to self-associate as dimers at protein concentrations in the micromolar range or higher [48,60,61,62]. The relatively high dissociation constant of GCAP dimerization may shift into the physiological range if a pre-formed GCAP dimer binds with nanomolar affinity to a RetGC dimer [63] to form a 2:2 complex (GCAP2/RetGC2) [64]. In essence, the high-affinity binding of GCAP1 to RetGC should shift the apparent dissociation constant of the GCAP1 dimer (bound to RetGC) into the sub-micromolar range. Ca2+-induced structural changes to the quaternary structure of a GCAP2/RetGC2 complex (Figure 4) are proposed here to amplify the relatively small Ca2+-induced change in the GCAP1 tertiary structure (Figure 3). Indeed, the binding of Ca2+ to GCAP1 was shown previously to cause a 6-fold decrease in the dissociation constant for GCAP1 dimerization [61]. Missense mutations affecting Ca2+ binding to GCAP1 also lead to cone-rod dystrophies by altering protein dimerization and functional properties [65]. Thus, Ca2+-dependent quaternary structural changes in the GCAP2/RetGC2 complex may allosterically regulate the RetGC cyclase activity (Figure 4), similar to the allosteric regulation of O2 binding to hemoglobin [66]. Recall for hemoglobin, the O2-induced change in the tertiary structure of hemoglobin is quite small, but O2 binding causes a much larger change in the quaternary structure of the hemoglobin tetramer, known as the T → R transition. A similar allosteric transition may take place in the GCAP2/RetGC2 complex (Figure 4) and therefore explain how Ca2+ binding can modulate cyclase activity with positive cooperativity [8,14]. Atomic-level structures of dimeric forms of GCAP1 [67] and GCAP2 [68] have been reported and were described in a recent review [69]. I will provide an updated overview of the dimeric GCAP structures below.
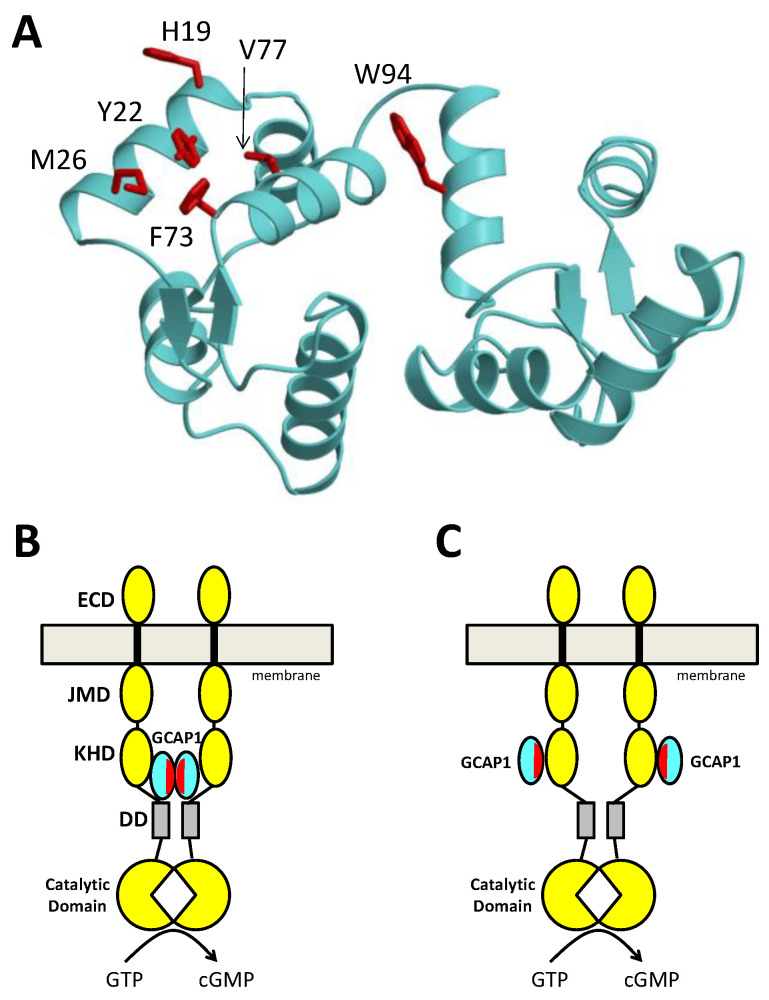
Figure 4.
Allosteric regulation of a GCAP2/RetGC2 complex. Cyclase activity (synthesis of cGMP) is modulated by a Ca2+-dependent change in the quaternary structure of the GCAP2/RetGC2 complex. The Ca2+-free/Mg2+-bound GCAP1 dimer (cyan ovals with bound Mg2+ in blue) binds to the RetGC dimer (yellow) and activates cyclase activity (left panel). The Ca2+- bound GCAP1 dimer (cyan ovals with three bound Ca2+ in red) binds to the RetGC dimer (yellow) and inactivates cyclase activity (right panel). Thus, the binding of 3 Ca2+ to the GCAPs promotes the T → R transition (turns off cyclase activity), whereas the dissociation of Ca2+ and binding of Mg2+ promotes the R → T transition (turns on cyclase activity). Each RetGC dimer subunit is composed of an extracellular domain (ECD), transmembrane domain (TMD in black), juxtamembrane domain (JMD), kinase homology domain (KHD), dimerization domain (DD, gray), and catalytic cyclase domain (notched circles).
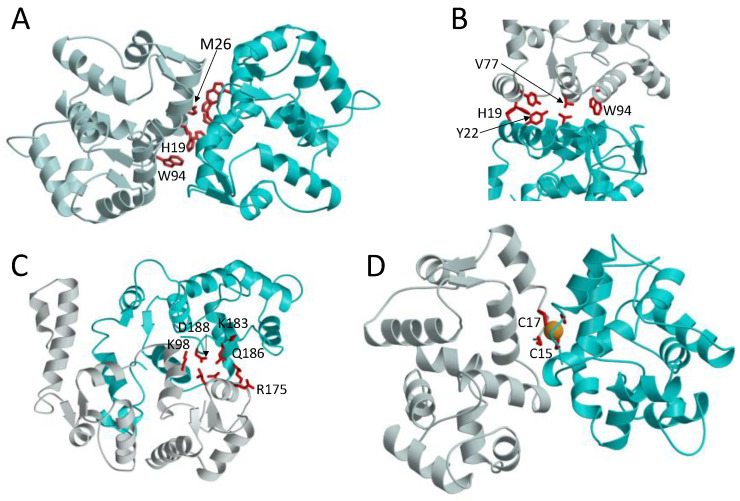
The atomic-level structure of a GCAP1 dimer (Figure 5A) was modeled previously by a molecular docking approach that used intermolecular distance restraints experimentally measured by EPR-DEER [67] and a separate dimerization model was calculated from small X-ray scattering (SAXS) measurements [61]. The GCAP1 dimer is comprised of mostly hydrophobic intermolecular contacts at the dimer interface (Figure 5B). The most apparent intermolecular contacts involve exposed hydrophobic residues: H19, Y22, M26, V77, and W94 (Figure 5B). A key linchpin contact is formed by the methyl side-chain atoms of V77 that each contact one another at the dimer interface and perhaps explain why the V77E mutation disrupts GCAP1 dimerization [48]. The GCAP1 dimerization site is further stabilized by intermolecular contacts formed by exposed aromatic side chains of H19, Y22, F73, and W94. The point mutation p.H19Y in human GCAP1 that is located in the dimer interface was identified in patients diagnosed with retinitis pigmentosa, and the H19Y GCAP1 mutant protein disrupts RetGC regulation and dimer formation [61,70]. Single point mutations of the hydrophobic residues at the GCAP1 dimer interface (H19A, Y22A, F73A, V77E, and W94A) also each weaken the dimerization dissociation constant and abolish the activation of RetGC by GCAP1 [67]. Thus, the hydrophobic contacts at the GCAP1 dimer interface (Figure 5B) are essential for both its dimerization and activation of RetGCs. This implies that GCAP1 dimerization may be important for activating RetGC and therefore supports the idea of a pre-formed GCAP1 dimer that binds to the dimeric RetGC to stabilize a high affinity 2:2 target complex as discussed above (Figure 4 and Figure 6B). Alternatively, the pre-formed GCAP1 dimer in solution may not exist in the presence of RetGC, because residues in the GCAP1 dimer interface (Figure 5B) appear to overlap with residues that interact with RetGC [71]. Thus, the residues at the GCAP1 dimerization site may prefer to interact with RetGC in the presence of saturating RetGC (Figure 6B), and the binding of RetGC to GCAP1, in this case, would be expected to prevent GCAP1 dimerization. Future studies are needed to probe whether the structure of the GCAP1 dimer (Figure 5A) will remain intact upon its binding to RetGC. In particular, future cryoEM studies are needed to determine the atomic-level structure of RetGC bound to GCAP1.
Figure 5.
Dimeric structures of GCAP1 (A), GCAP2 (C), and GCAP5 (D). A close-up view of the GCAP1 dimerization site (B) reveals intermolecular contacts between aromatic residues (red). The GCAP2 dimerization site is stabilized by intermolecular salt bridges and hydrogen bonds (highlighted by red residues in panel (C)). The GCAP5 dimerization site is stabilized by a bound Fe2+ (orange sphere) that is chelated by C15 and C17 (D).
Figure 6.
Druggable Hotspot on GCAP1 (A) and RetGC activation by dimeric (B) or monomeric (C) GCAPs. The structure of GCAP1 (cyan) contains exposed hotspot residues (red) that can mediate GCAP dimerization (B) or RetGC binding (C). RetGC (yellow) is proposed here to be activated by either a preformed GCAP1 dimer (B) or by monomeric GCAP1 (C). Small molecule drugs or peptides that bind to the hotspot are expected to prevent cyclase activation by constitutively active GCAP1 mutants and therefore may serve as therapeutics for cone-rod dystrophies. Each RetGC dimer subunit (yellow) is composed of an extracellular domain (ECD), transmembrane domain (black), juxtamembrane domain (JMD), kinase homology domain (KHD), dimerization domain (gray), and catalytic cyclase domain.
A structure of the GCAP2 dimer (Figure 5C) was reported previously based on a mass spectrometry analysis [68,72]. The overall quaternary structure of the GCAP2 dimer (Figure 5C) is very different from that of GCAP1 (Figure 5A). In contrast to the dimerization site in GCAP1, the GCAP2 dimerization site is comprised of mostly polar and charged amino acid residues (K98, L167, V171, R175, K183, Q186, D188 highlighted red in Figure 5C). The GCAP2 interface is therefore stabilized primarily by intermolecular salt bridges and hydrogen bonds. The side-chain atoms of R175 in GCAP2 form intermolecular hydrogen bonds with the polar side-chain atoms of Q186 (Figure 5C), and the side-chain atoms of K98 form an intermolecular salt bridge with the side chain carboxylate atoms of D188 (Figure 5C). These intermolecular polar contacts in the GCAP2 dimer are not conserved in the other GCAPs and may explain why the GCAP2 dimer structure (Figure 5C) is quite different from that of GCAP1 (Figure 5A). The different quaternary structures for the GCAP1 and GCAP2 dimers might help to understand their different targeting of RetGC [73,74]. GCAP1 has been shown previously to bind to the kinase homology domain in RetGC [74,75], in contrast to GCAP2 that has been suggested to bind to RetGC residues (Y1016–S1103) at the C-terminus [73].
2.3. GCAP5 Is a Fe2+ Sensor in Zebrafish Photoreceptors
GCAP homologs are conserved in all vertebrate photoreceptors, and zebrafish photoreceptors contain particular GCAP homologs (GCAP3–5 in Figure 1) [16,76] that do not exist in mammals. The zebrafish homolog called GCAP5 has an amino acid sequence that is perhaps the most divergent of all GCAPs (Figure 1). The first 20 amino acids from the amino-terminus in GCAP5 are particularly unique and contain non-conserved Cys residues (Cys15 and Cys17) that were shown previously to bind Fe2+ [62]. One Fe2+ binds with nanomolar affinity to two molecules of GCAP5 at the dimer interface and at least two other Fe2+ molecules bind to GCAP5 with a dissociation constant in the micromolar range [62]. The nanomolar Fe2+ binding to GCAP5 is abolished by the GCAP5 mutations (C15A and C17A), implying that the high-affinity Fe2+ is chelated by the sulfhydryl side chains of Cys15 and Cys17. By contrast, the lower affinity Fe2+ binding was not affected by the Cys mutations (C15A and C17A). A detailed NMR titration revealed that the lower affinity Fe2+ ions are likely binding to the second and third EF-hands in the absence of Ca2+ because the micromolar Fe2+ binding is abolished in the presence of saturating Ca2+ levels. The Ca2+-free/Fe2+-free/Mg2+-bound GCAP5 causes ~10-fold activation of RetGC activity, which is somewhat lower than the cyclase activation promoted by Ca2+-free/Mg2+-bound GCAP1 [62]. Unlike GCAP1 and GCAP2, both the Ca2+-free and Ca2+-bound forms of GCAP5 can each activate RetGC. Interestingly, the Fe2+-bound GCAP5 is unable to activate RetGC even at low Ca2+ levels in light-adapted photoreceptors. The Fe2+-induced cyclase inhibition by GCAP5 suggests that Fe2+ binding to GCAP5 may serve to modulate cyclase activity and therefore GCAP5 could act as a Fe2+ sensor for phototransduction in zebrafish photoreceptors [62].
A structural model of Fe2+-bound GCAP5 was determined by an NMR-guided homology modeling approach [62] (Figure 5D). GCAP5 was measured by size-exclusion chromatography to form a protein dimer at micromolar protein concentrations [62] and was accordingly modeled to form a dimer in the structure. The GCAP5 dimer structure (Figure 5D) is somewhat similar to the structure of the GCAP1 dimer (Figure 5A). The GCAP5 dimerization site contains exposed hydrophobic residues (H18, Y21, M25, F72, V76, and W93) that are also present in the GCAP1 dimer (Figure 5B). A single Fe2+ is bound to the GCAP5 dimer in which the bound Fe2+ is chelated by the side chains of Cys15 and Cys17. The bound Fe2+ bridges two GCAP5 molecules into a [Fe(SCys)4] dimeric complex [62] like that observed previously in two-iron superoxide reductases [77,78]. The four cysteinyl thiolate groups that ligate the bound Fe2+ are similar in structure to the four Cys residues found in the Cys4 zinc finger motif that binds to Zn2+ [79]. The structural similarity to the Cys4 zinc finger suggests that GCAP5 may also bind to Zn2+ in place of Fe2+. High levels of Zn2+ are found in retinal photoreceptor cells, and Zn2+ may play a role in phototransduction [80]. Future studies are needed to test whether Zn2+ can bind to GCAP5 and test whether Zn2+ binding to GCAP5 can regulate RetGCs in zebrafish photoreceptors.
2.4. Druggable Hot Spot on the Structure of GCAP1
The structure of GCAP1 reveals exposed hydrophobic residues (H19, Y22, M26, F73, V77, and W94) that are clustered on the surface of the protein and form a potential hot spot for drug targeting (Figure 6A). These exposed hydrophobic residues are located at the GCAP1 dimerization site (Figure 5B), which explains why single mutations to these residues (H19A, Y22A, M26A, F73A, V77E, and W94E) both weaken dimerization and abolish cyclase activation [67]. A schematic model of a preformed GCAP1 dimer bound to RetGC suggests how GCAP1 dimerization might promote cyclase activation (Figure 6B). Alternatively, a monomeric form of GCAP1 bound to RetGC could also promote cyclase activation (Figure 6C), if the exposed hotspot on GCAP1 were to bind directly to RetGC as suggested by [71]. Regardless of whether the exposed hotspot facilitates GCAP1 dimerization (Figure 6B) or binds to RetGC (Figure 6C), this hotspot (highlighted red in Figure 6) could be targeted for drug design. Small molecules or peptides that bind specifically to the hotspot region are expected to block GCAP1 dimerization and/or RetGC binding and should therefore prevent cyclase activation by GCAP1. Small molecule inhibitors that bind to the hotspot region within constitutively active GCAP1 mutants (Y99C, D100G, E111V, and E155G) should block their activation of RetGCs, and therefore diminish the onset of cone-rod dystrophies. Future studies are needed to first screen for drug molecules that bind to the GCAP1 hot spot and then determine whether these drugs can serve as therapeutics for cone-rod dystrophies.
Funding
This research was funded by the National Eye Institute (R01 EY012347).
Institutional Review Board Statement
Not Applicable.
Informed Consent Statement
Not Applicable.
Data Availability Statement
Not Applicable.
Conflicts of Interest
The author declares no conflict of interest.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Stryer L. Visual excitation and recovery. J. Biol. Chem. 1991;266:10711–10714. doi: 10.1016/S0021-9258(18)99072-1. [DOI] [PubMed] [Google Scholar]
- 2.Baylor D. How photons start vision. Proc. Natl. Acad. Sci. USA. 1996;93:560–565. doi: 10.1073/pnas.93.2.560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Pugh E.N., Duda T., Sitaramayya A., Sharma R.K., Pugh J.E.N. Photoreceptor Guanylate Cyclases: A Review. Biosci. Rep. 1997;17:429–473. doi: 10.1023/A:1027365520442. [DOI] [PubMed] [Google Scholar]
- 4.Pugh E., Nikonov S., Lamb T. Molecular mechanisms of vertebrate photoreceptor light adaptation. Curr. Opin. Neurobiol. 1999;9:410–418. doi: 10.1016/S0959-4388(99)80062-2. [DOI] [PubMed] [Google Scholar]
- 5.Burns E.M., A Baylor D. Activation, Deactivation, and Adaptation in Vertebrate Photoreceptor Cells. Annu. Rev. Neurosci. 2001;24:779–805. doi: 10.1146/annurev.neuro.24.1.779. [DOI] [PubMed] [Google Scholar]
- 6.Koch K.-W., Stryer L. Highly cooperative feedback control of retinal rod guanylate cyclase by calcium ions. Nat. Cell Biol. 1988;334:64–66. doi: 10.1038/334064a0. [DOI] [PubMed] [Google Scholar]
- 7.Koutalos Y., Yau K.W. Regulation of sensitivity in vertebrate rod photoreceptors by calcium. Trends Neurosci. 1996;19:73–81. doi: 10.1016/0166-2236(96)89624-X. [DOI] [PubMed] [Google Scholar]
- 8.Dizhoor A. The human photoreceptor membrane guanylyl cyclase, RetGC, is present in outer segments and is regulated by calcium and a soluble activator. Neuron. 1994;12:1345–1352. doi: 10.1016/0896-6273(94)90449-9. [DOI] [PubMed] [Google Scholar]
- 9.Lowe D.G., Dizhoor A., Liu K., Gu Q., Spencer M., Laura R., Lu L., Hurley J.B. Cloning and expression of a second photoreceptor-specific membrane retina guanylyl cyclase (RetGC), RetGC-2. Proc. Natl. Acad. Sci. USA. 1995;92:5535–5539. doi: 10.1073/pnas.92.12.5535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Duda T., Fik-Rymarkiewicz E., Venkataraman V., Krishnan R., Koch K.-W., Sharma R.K. The Calcium-Sensor Guanylate Cyclase Activating Protein Type 2 Specific Site in Rod Outer Segment Membrane Guanylate Cyclase Type 1. Biochemistry. 2005;44:7336–7345. doi: 10.1021/bi050068x. [DOI] [PubMed] [Google Scholar]
- 11.Laura R.P., Dizhoor A.M., Hurley J.B. The membrane guanylyl cyclase, retinal guanylyl cyclase-1, is activated through its intracellular domain. J. Biol. Chem. 1996;271:11646–11651. doi: 10.1074/jbc.271.20.11646. [DOI] [PubMed] [Google Scholar]
- 12.Dizhoor A.M., Olshevskaya E.V., Henzel W., Wong S.C., Stults J.T., Ankoudinova I., Hurley J.B. Cloning, Sequencing, and Expression of a 24-kDa Ca2+-binding Protein Activating Photoreceptor Guanylyl Cyclase. J. Biol. Chem. 1995;270:25200–25206. doi: 10.1074/jbc.270.42.25200. [DOI] [PubMed] [Google Scholar]
- 13.Gorczyca W., Polans A.S., Surgucheva I.G., Subbaraya I., Baehr W., Palczewski K. Guanylyl cyclase activating protein. A calcium-sensitive regulator of phototransduction. J. Biol. Chem. 1995;270:22029–22036. doi: 10.1074/jbc.270.37.22029. [DOI] [PubMed] [Google Scholar]
- 14.Palczewski K., Subbaraya I., Gorczyca W.A., Helekar B.S., Ruiz C.C., Ohguro H., Huang J., Zhao X., Crabb J.W., Johnson R.S., et al. Molecular cloning and characterization of retinal photoreceptor guanylyl cyclase-activating protein. Neuron. 1994;13:395–404. doi: 10.1016/0896-6273(94)90355-7. [DOI] [PubMed] [Google Scholar]
- 15.Scholten A., Koch K.-W. Differential Calcium Signaling by Cone Specific Guanylate Cyclase-Activating Proteins from the Zebrafish Retina. PLoS ONE. 2011;6:e23117. doi: 10.1371/journal.pone.0023117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rätscho N., Scholten A., Koch K.-W. Expression profiles of three novel sensory guanylate cyclases and guanylate cyclase-activating proteins in the zebrafish retina. Biochim. Biophys. Acta (BBA) Bioenerg. 2009;1793:1110–1114. doi: 10.1016/j.bbamcr.2008.12.021. [DOI] [PubMed] [Google Scholar]
- 17.Gray-Keller M.P., Detwiler P. The calcium feedback signal in the phototransduction cascade of vertebrate rods. Neuron. 1994;13:849–861. doi: 10.1016/0896-6273(94)90251-8. [DOI] [PubMed] [Google Scholar]
- 18.Woodruff M.L., Sampath A.P., Matthews H.R., Krasnoperova N.V., Lem J., Fain G.L. Measurement of cytoplasmic calcium concentration in the rods of wild-type and transducin knock-out mice. J. Physiol. 2002;542:843–854. doi: 10.1113/jphysiol.2001.013987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dizhoor A., Hurley J.B. Inactivation of EF-hands Makes GCAP-2 (p24) a Constitutive Activator of Photoreceptor Guanylyl Cyclase by Preventing a Ca2+-induced “Activator-to-Inhibitor” Transition. J. Biol. Chem. 1996;271:19346–19350. doi: 10.1074/jbc.271.32.19346. [DOI] [PubMed] [Google Scholar]
- 20.Méndez A., Burns M.E., Sokal I., Dizhoor A., Baehr W., Palczewski K., Baylor D.A., Chen J. Role of guanylate cyclase-activating proteins (GCAPs) in setting the flash sensitivity of rod photoreceptors. Proc. Natl. Acad. Sci. USA. 2001;98:9948–9953. doi: 10.1073/pnas.171308998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dizhoor A.M., Boikov S.G., Olshevskaya E.V. Constitutive Activation of Photoreceptor Guanylate Cyclase by Y99C Mutant of GCAP-1. Possible role in causing human autosomal dominant cone degeneration. J. Biol. Chem. 1998;273:17311–17314. doi: 10.1074/jbc.273.28.17311. [DOI] [PubMed] [Google Scholar]
- 22.Hodgkin A.L., Nunn B.J. Control of light-sensitive current in salamander rods. J. Physiol. 1988;403:439–471. doi: 10.1113/jphysiol.1988.sp017258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sakurai K., Chen J., Kefalov V.J. Role of Guanylyl Cyclase Modulation in Mouse Cone Phototransduction. J. Neurosci. 2011;31:7991–8000. doi: 10.1523/JNEUROSCI.6650-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Dizhoor A.M., Olshevskaya E.V., Peshenko I.V. Mg2+/Ca2+ cation binding cycle of guanylyl cyclase activating proteins (GCAPs): Role in regulation of photoreceptor guanylyl cyclase. Mol. Cell. Biochem. 2009;334:117–124. doi: 10.1007/s11010-009-0328-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Peshenko I.V., Dizhoor A.M. Guanylyl cyclase-activating proteins (GCAPs) are Ca2+/Mg2+ sensors: Implications for photoreceptor guanylyl cyclase (RetGC) regulation in mammalian photoreceptors. J. Biol. Chem. 2004;279:16903–16906. doi: 10.1074/jbc.C400065200. [DOI] [PubMed] [Google Scholar]
- 26.Peshenko I.V., Dizhoor A.M. Ca2+ and Mg2+ binding properties of GCAP-1. Evidence that Mg2+-bound form is the physiological activator of photoreceptor guanylyl cyclase. J. Biol. Chem. 2006;281:23830–23841. doi: 10.1074/jbc.M600257200. [DOI] [PubMed] [Google Scholar]
- 27.Peshenko I.V., Dizhoor A.M. Activation and Inhibition of Photoreceptor Guanylyl Cyclase by Guanylyl Cyclase Activating Protein 1 (GCAP-1): THE FUNCTIONAL ROLE OF Mg2+/Ca2+ EXCHANGE IN EF-HAND DOMAINS. J. Biol. Chem. 2007;282:21645–21652. doi: 10.1074/jbc.M702368200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lim S., Peshenko I., Dizhoor A., Ames J.B. Effects of Ca2+, Mg2+, and Myristoylation on Guanylyl Cyclase Activating Protein 1 Structure and Stability. Biochemistry. 2009;48:850–862. doi: 10.1021/bi801897p. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Dell’Orco D., Behnen P., Linse S., Koch K.-W. Calcium binding, structural stability and guanylate cyclase activation in GCAP1 variants associated with human cone dystrophy. Cell. Mol. Life Sci. 2010;67:973–984. doi: 10.1007/s00018-009-0243-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Marino V., Sulmann S., Koch K.-W., Dell’Orco D. Structural effects of Mg2+ on the regulatory states of three neuronal calcium sensors operating in vertebrate phototransduction. Biochim. Biophys. Acta (BBA) Bioenerg. 2015;1853:2055–2065. doi: 10.1016/j.bbamcr.2014.10.026. [DOI] [PubMed] [Google Scholar]
- 31.Ames J.B., Dizhoor A., Ikura M., Palczewski K., Stryer L. Three-dimensional Structure of Guanylyl Cyclase Activating Protein-2, a Calcium-sensitive Modulator of Photoreceptor Guanylyl Cyclases. J. Biol. Chem. 1999;274:19329–19337. doi: 10.1074/jbc.274.27.19329. [DOI] [PubMed] [Google Scholar]
- 32.Stephen R., Bereta G., Golczak M., Palczewski K., Sousa M.C. Stabilizing Function for Myristoyl Group Revealed by the Crystal Structure of a Neuronal Calcium Sensor, Guanylate Cyclase-Activating Protein 1. Structure. 2007;15:1392–1402. doi: 10.1016/j.str.2007.09.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sampath A., Matthews H., Cornwall M., Fain G. Bleached Pigment Produces a Maintained Decrease in Outer Segment Ca2+ in Salamander Rods. J. Gen. Physiol. 1998;111:53–64. doi: 10.1085/jgp.111.1.53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Chen C., Nakatani K., Koutalos Y. Free Magnesium Concentration in Salamander Photoreceptor Outer Segments. J. Physiol. 2003;553:125–135. doi: 10.1113/jphysiol.2003.053280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Jiang L., Baehr W. GCAP1 Mutations Associated with Autosomal Dominant Cone Dystrophy. Adv. Exp. Med. Biol. 2009;664:273–282. doi: 10.1007/978-1-4419-1399-9_31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Behnen P., Dell’Orco D., Koch K.-W. Involvement of the calcium sensor GCAP1 in hereditary cone dystrophies. Biol. Chem. 2010;391:631–637. doi: 10.1515/bc.2010.063. [DOI] [PubMed] [Google Scholar]
- 37.Payne A., Downes S.M., Bessant D.A., Taylor R., Holder G.E., Warren M., Bird A.C., Bhattacharya S.S. A mutation in guanylate cyclase activator 1A (GUCA1A) in an autosomal dominant cone dystrophy pedigree mapping to a new locus on chromosome 6p21.1. Hum. Mol. Genet. 1998;7:273–277. doi: 10.1093/hmg/7.2.273. [DOI] [PubMed] [Google Scholar]
- 38.Nong E., Lee W., Merriam J.E., Allikmets R., Tsang S.H. Disease progression in autosomal dominant cone-rod dystrophy caused by a novel mutation (D100G) in the GUCA1A gene. Doc. Ophthalmol. 2013;128:59–67. doi: 10.1007/s10633-013-9420-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Marino V., Cortivo G.D., Oppici E., Maltese P.E., D’Esposito F., Manara E., Ziccardi L., Falsini B., Magli A., Bertelli M., et al. A novel p. (Glu111Val) missense mutation in GUCA1A associated with cone-rod dystrophy leads to impaired calcium sensing and perturbed second messenger homeostasis in photoreceptors. Hum. Mol. Genet. 2018;27:4204–4217. doi: 10.1093/hmg/ddy311. [DOI] [PubMed] [Google Scholar]
- 40.Wilkie S.E., Li Y., Deery E.C., Newbold R.J., Garibaldi D., Bateman J.B., Zhang H., Lin W., Zack D., Bhattacharya S.S., et al. Identification and Functional Consequences of a New Mutation (E155G) in the Gene for GCAP1 That Causes Autosomal Dominant Cone Dystrophy. Am. J. Hum. Genet. 2001;69:471–480. doi: 10.1086/323265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wilkie S.E., Newbold R.J., Deery E., Walker C., Stinton I., Ramamurthy V., Hurley J.B., Bhattacharya S.S., Warren M.J., Hunt D.M. Functional characterization of missense mutations at codon 838 in retinal guanylate cyclase correlates with disease severity in patients with autosomal dominant cone-rod dystrophy. Hum. Mol. Genet. 2000;9:3065–3073. doi: 10.1093/hmg/9.20.3065. [DOI] [PubMed] [Google Scholar]
- 42.Olshevskaya E.V., Calvert P.D., Woodruff M.L., Peshenko I.V., Savchenko A.B., Makino C.L., Ho Y.-S., Fain G.L., Dizhoor A.M. The Y99C Mutation in Guanylyl Cyclase-Activating Protein 1 Increases Intracellular Ca2+ and Causes Photoreceptor Degeneration in Transgenic Mice. J. Neurosci. 2004;24:6078–6085. doi: 10.1523/JNEUROSCI.0963-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Sokal I., Li N., Surgucheva I., Warren M., Payne A., Bhattacharya S.S., Baehr W., Palczewski K. GCAP1(Y99C) Mutant Is Constitutively Active in Autosomal Dominant Cone Dystrophy. Mol. Cell. 1998;2:129–133. doi: 10.1016/S1097-2765(00)80121-5. [DOI] [PubMed] [Google Scholar]
- 44.Olshevskaya E.V., Peshenko I.V., Savchenko A.B., Dizhoor A.M. Retinal Guanylyl Cyclase Isozyme 1 Is the Preferential In Vivo Target for Constitutively Active GCAP1 Mutants Causing Congenital Degeneration of Photoreceptors. J. Neurosci. 2012;32:7208–7217. doi: 10.1523/JNEUROSCI.0976-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Woodruff M.L., Olshevskaya E.V., Savchenko A.B., Peshenko I.V., Barrett R., Bush R.A., Sieving P.A., Fain G.L., Dizhoor A.M. Constitutive Excitation by Gly90Asp Rhodopsin Rescues Rods from Degeneration Caused by Elevated Production of cGMP in the Dark. J. Neurosci. 2007;27:8805–8815. doi: 10.1523/JNEUROSCI.2751-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Peshenko I.V., Olshevskaya E.V., Dizhoor A.M. Binding of guanylyl cyclase activating protein 1 (GCAP1) to retinal guanylyl cyclase (RetGC1): The role of individual EF-hands. J. Biol. Chem. 2008;283:21747–21757. doi: 10.1074/jbc.M801899200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ermilov A.N., Olshevskaya E.V., Dizhoor A. Instead of Binding Calcium, One of the EF-hand Structures in Guanylyl Cyclase Activating Protein-2 Is Required for Targeting Photoreceptor Guanylyl Cyclase. J. Biol. Chem. 2001;276:48143–48148. doi: 10.1074/jbc.M107539200. [DOI] [PubMed] [Google Scholar]
- 48.Lim S., Peshenko I.V., Olshevskaya E.V., Dizhoor A., Ames J.B. Structure of Guanylyl Cyclase Activator Protein 1 (GCAP1) Mutant V77E in a Ca2+-free/Mg2+-bound Activator State. J. Biol. Chem. 2016;291:4429–4441. doi: 10.1074/jbc.M115.696161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ames J.B., Ishima R., Tanaka T., Gordon J.I., Stryer L., Ikura M. Molecular mechanics of calcium–myristoyl switches. Nat. Cell Biol. 1997;389:198–202. doi: 10.1038/38310. [DOI] [PubMed] [Google Scholar]
- 50.Flaherty K.M., Zozulya S., Stryer L., McKay D.B. Three-dimensional structure of recoverin, a calcium sensor in vision. Cell. 1993;75:709–716. doi: 10.1016/0092-8674(93)90491-8. [DOI] [PubMed] [Google Scholar]
- 51.Ames J.B., Porumb T., Tanaka T., Ikura M., Stryer L. Amino-terminal Myristoylation Induces Cooperative Calcium Binding to Recoverin. J. Biol. Chem. 1995;270:4526–4533. doi: 10.1074/jbc.270.9.4526. [DOI] [PubMed] [Google Scholar]
- 52.Theisgen S., Scheidt H.A., Magalhães A., Bonagamba T.J., Huster D. A solid-state NMR study of the structure and dynamics of the myristoylated N-terminus of the guanylate cyclase-activating protein-2. Biochim. Biophys. Acta (BBA) Biomembr. 2010;1798:266–274. doi: 10.1016/j.bbamem.2009.06.028. [DOI] [PubMed] [Google Scholar]
- 53.Vogel A., Schröder T., Lange C., Huster D. Characterization of the myristoyl lipid modification of membrane-bound GCAP-2 by 2H solid-state NMR spectroscopy. Biochim. Biophys. Acta (BBA) Biomembr. 2007;1768:3171–3181. doi: 10.1016/j.bbamem.2007.08.024. [DOI] [PubMed] [Google Scholar]
- 54.Hwang J.-Y., Koch K.-W. Calcium- and Myristoyl-Dependent Properties of Guanylate Cyclase-Activating Protein-1 and Protein-2. Biochemistry. 2002;41:13021–13028. doi: 10.1021/bi026618y. [DOI] [PubMed] [Google Scholar]
- 55.Margetić A., Nannemann D., Meiler J., Huster D., Theisgen S. Guanylate Cyclase-Activating Protein-2 Undergoes Structural Changes upon Binding to Detergent Micelles and Bicelles. Biochim. Biophys. Acta (BBA) Biomembr. 2014;1838:2767–2777. doi: 10.1016/j.bbamem.2014.07.012. [DOI] [PubMed] [Google Scholar]
- 56.Olshevskaya E.V., Hughes R.E., Hurley J.B., Dizhoor A. Calcium Binding, but Not a Calcium-Myristoyl Switch, Controls the Ability of Guanylyl Cyclase-activating Protein GCAP-2 to Regulate Photoreceptor Guanylyl Cyclase. J. Biol. Chem. 1997;272:14327–14333. doi: 10.1074/jbc.272.22.14327. [DOI] [PubMed] [Google Scholar]
- 57.Ikura M. Calcium binding and conformational response in EF-hand proteins. Trends Biochem. Sci. 1996;21:14–17. doi: 10.1016/S0968-0004(06)80021-6. [DOI] [PubMed] [Google Scholar]
- 58.Peshenko I.V. Calcium-myristoyl Tug. J. Biol. Chem. 2012;287:13972–13984. doi: 10.1074/jbc.M112.341883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Marino V., Dell’Orco D. Allosteric communication pathways routed by Ca2+/Mg2+ exchange in GCAP1 selectively switch target regulation modes. Sci. Rep. 2016;6:34277. doi: 10.1038/srep34277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Lim S., Peshenko I.V., Dizhoor A., Ames J.B. Structural Insights for Activation of Retinal Guanylate Cyclase by GCAP1. PLoS ONE. 2013;8:e81822. doi: 10.1371/journal.pone.0081822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Bonì F., Marino V., Bidoia C., Mastrangelo E., Barbiroli A., Dell’Orco D., Milani M. Modulation of Guanylate Cyclase Activating Protein 1 (GCAP1) Dimeric Assembly by Ca2+ or Mg2+: Hints to Understand Protein Activity. Biomolecules. 2020;10:1408. doi: 10.3390/biom10101408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Lim S., Scholten A., Manchala G., Cudia D., Zlomke-Sell S.-K., Koch K.-W., Ames J.B. Structural Characterization of Ferrous Ion Binding to Retinal Guanylate Cyclase Activator Protein 5 from Zebrafish Photoreceptors. Biochemistry. 2017;56:6652–6661. doi: 10.1021/acs.biochem.7b01029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Olshevskaya E.V., Ermilov A.N., Dizhoor A. Dimerization of Guanylyl Cyclase-activating Protein and a Mechanism of Photoreceptor Guanylyl Cyclase Activation. J. Biol. Chem. 1999;274:25583–25587. doi: 10.1074/jbc.274.36.25583. [DOI] [PubMed] [Google Scholar]
- 64.Peshenko I.V., Olshevskaya E.V., Yao S., Ezzeldin H.H., Pittler S., Dizhoor A.M. Activation of Retinal Guanylyl Cyclase RetGC1 by GCAP1: Stoichiometry of Binding and Effect of New LCA-Related Mutations. Biochemistry. 2010;49:709–717. doi: 10.1021/bi901495y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Cortivo G.D., Marino V., Bonì F., Milani M., Dell’Orco D. Missense mutations affecting Ca2+-coordination in GCAP1 lead to cone-rod dystrophies by altering protein structural and functional properties. Biochim. Biophys. Acta (BBA) Bioenerg. 2020;1867:118794. doi: 10.1016/j.bbamcr.2020.118794. [DOI] [PubMed] [Google Scholar]
- 66.Monod J., Wyman J., Changeux J.-P. On the nature of allosteric transitions: A plausible model. J. Mol. Biol. 1965;12:88–118. doi: 10.1016/S0022-2836(65)80285-6. [DOI] [PubMed] [Google Scholar]
- 67.Lim S., Roseman G., Peshenko I., Manchala G., Cudia D., Dizhoor A., Millhauser G., Ames J.B. Retinal guanylyl cyclase activating protein 1 forms a functional dimer. PLoS ONE. 2018;13:e0193947. doi: 10.1371/journal.pone.0193947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Pettelkau J., Schröder T., Ihling C.H., Olausson B.E.S., Kölbel K., Lange C., Sinz A. Structural Insights into Retinal Guanylylcyclase–GCAP-2 Interaction Determined by Cross-Linking and Mass Spectrometry. Biochemistry. 2012;51:4932–4949. doi: 10.1021/bi300064v. [DOI] [PubMed] [Google Scholar]
- 69.Ames J.B. Dimerization of Neuronal Calcium Sensor Proteins. Front. Mol. Neurosci. 2018;11:397. doi: 10.3389/fnmol.2018.00397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Abbas S., Marino V., Weisschuh N., Kieninger S., Solaki M., Dell’Orco D., Koch K.-W. Neuronal Calcium Sensor GCAP1 Encoded by GUCA1A Exhibits Heterogeneous Functional Properties in Two Cases of Retinitis Pigmentosa. ACS Chem. Neurosci. 2020;11:1458–1470. doi: 10.1021/acschemneuro.0c00111. [DOI] [PubMed] [Google Scholar]
- 71.Peshenko I.V., Olshevskaya E.V., Lim S., Ames J.B., Dizhoor A.M. Identification of Target Binding Site in Photoreceptor Guanylyl Cyclase-activating Protein 1 (GCAP1) J. Biol. Chem. 2014;289:10140–10154. doi: 10.1074/jbc.M113.540716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Pettelkau J., Thondorf I., Theisgen S., Lilie H., Schröder T., Arlt C., Ihling C.H., Sinz A. Structural Analysis of Guanylyl Cyclase-Activating Protein-2 (GCAP-2) Homodimer by Stable Isotope-Labeling, Chemical Cross-Linking, and Mass Spectrometry. J. Am. Soc. Mass Spectrom. 2013;24:1969–1979. doi: 10.1007/s13361-013-0734-6. [DOI] [PubMed] [Google Scholar]
- 73.Duda T., Pertzev A., Sharma R.K. Differential Ca2+ Sensor Guanylate Cyclase Activating Protein Modes of Photoreceptor Rod Outer Segment Membrane Guanylate Cyclase Signaling. Biochemistry. 2012;51:4650–4657. doi: 10.1021/bi300572w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Peshenko I.V., Olshevskaya E.V., Dizhoor A.M. Evaluating the Role of Retinal Membrane Guanylyl Cyclase 1 (RetGC1) Domains in Binding Guanylyl Cyclase-activating Proteins (GCAPs) J. Biol. Chem. 2015;290:6913–6924. doi: 10.1074/jbc.M114.629642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Laura R.P., Hurley J.B. The Kinase Homology Domain of Retinal Guanylyl Cyclases 1 and 2 Specifies the Affinity and Cooperativity of Interaction with Guanylyl Cyclase Activating Protein-2. Biochemistry. 1998;37:11264–11271. doi: 10.1021/bi9809674. [DOI] [PubMed] [Google Scholar]
- 76.Imanishi Y., Yang L., Sokal I., Filipek S., Palczewski K., Baehr W. Diversity of Guanylate Cyclase-Activating Proteins (GCAPs) in Teleost Fish: Characterization of Three Novel GCAPs (GCAP4, GCAP5, GCAP7) from Zebrafish (Danio rerio) and Prediction of Eight GCAPs (GCAP1-8) in Pufferfish (Fugu rubripes) J. Mol. Evol. 2004;59:204–217. doi: 10.1007/s00239-004-2614-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Demaré F., Kurtz D.M., Nordlund P. The structure of Desulfovibrio vulgaris rubrerythrin reveals a unique combination of rubredoxin-like FeS4 and ferritin-like diiron domains. Nat. Struct. Mol. Biol. 1996;3:539–546. doi: 10.1038/nsb0696-539. [DOI] [PubMed] [Google Scholar]
- 78.Emerson J., Cabelli D.E., Kurtz D.M. An engineered two-iron superoxide reductase lacking the [Fe(SCys)4] site retains its catalytic properties in vitro and in vivo. Proc. Natl. Acad. Sci. USA. 2003;100:3802–3807. doi: 10.1073/pnas.0537177100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Tang Q., Liu Y.-P., Yan X.-X., Liang D.-C. Structural and functional characterization of Cys4 zinc finger motif in the recombination mediator protein RecR. DNA Repair. 2014;24:10–14. doi: 10.1016/j.dnarep.2014.09.012. [DOI] [PubMed] [Google Scholar]
- 80.Redenti S., Ripps H., Chappell R.L. Zinc release at the synaptic terminals of rod photoreceptors. Exp. Eye Res. 2007;85:580–584. doi: 10.1016/j.exer.2007.07.017. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not Applicable.