Abstract
We have determined the relationship between mRNA and protein expression levels for selected genes expressed in the yeast Saccharomyces cerevisiae growing at mid-log phase. The proteins contained in total yeast cell lysate were separated by high-resolution two-dimensional (2D) gel electrophoresis. Over 150 protein spots were excised and identified by capillary liquid chromatography-tandem mass spectrometry (LC-MS/MS). Protein spots were quantified by metabolic labeling and scintillation counting. Corresponding mRNA levels were calculated from serial analysis of gene expression (SAGE) frequency tables (V. E. Velculescu, L. Zhang, W. Zhou, J. Vogelstein, M. A. Basrai, D. E. Bassett, Jr., P. Hieter, B. Vogelstein, and K. W. Kinzler, Cell 88:243–251, 1997). We found that the correlation between mRNA and protein levels was insufficient to predict protein expression levels from quantitative mRNA data. Indeed, for some genes, while the mRNA levels were of the same value the protein levels varied by more than 20-fold. Conversely, invariant steady-state levels of certain proteins were observed with respective mRNA transcript levels that varied by as much as 30-fold. Another interesting observation is that codon bias is not a predictor of either protein or mRNA levels. Our results clearly delineate the technical boundaries of current approaches for quantitative analysis of protein expression and reveal that simple deduction from mRNA transcript analysis is insufficient.
The description of the state of a biological system by the quantitative measurement of the system constituents is an essential but largely unexplored area of biology. With recent technical advances including the development of differential display-PCR (21), of cDNA microarray and DNA chip technology (20, 27), and of serial analysis of gene expression (SAGE) (34, 35), it is now feasible to establish global and quantitative mRNA expression profiles of cells and tissues in species for which the sequence of all the genes is known. However, there is emerging evidence which suggests that mRNA expression patterns are necessary but are by themselves insufficient for the quantitative description of biological systems. This evidence includes discoveries of posttranscriptional mechanisms controlling the protein translation rate (15), the half-lives of specific proteins or mRNAs (33), and the intracellular location and molecular association of the protein products of expressed genes (32).
Proteome analysis, defined as the analysis of the protein complement expressed by a genome (26), has been suggested as an approach to the quantitative description of the state of a biological system by the quantitative analysis of protein expression profiles (36). Proteome analysis is conceptually attractive because of its potential to determine properties of biological systems that are not apparent by DNA or mRNA sequence analysis alone. Such properties include the quantity of protein expression, the subcellular location, the state of modification, and the association with ligands, as well as the rate of change with time of such properties. In contrast to the genomes of a number of microorganisms (for a review, see reference 11) and the transcriptome of Saccharomyces cerevisiae (35), which have been entirely determined, no proteome map has been completed to date.
The most common implementation of proteome analysis is the combination of two-dimensional gel electrophoresis (2DE) (isoelectric focusing-sodium dodecyl sulfate [SDS]-polyacrylamide gel electrophoresis) for the separation and quantitation of proteins with analytical methods for their identification. 2DE permits the separation, visualization, and quantitation of thousands of proteins reproducibly on a single gel (18, 24). By itself, 2DE is strictly a descriptive technique. The combination of 2DE with protein analytical techniques has added the possibility of establishing the identities of separated proteins (1, 2) and thus, in combination with quantitative mRNA analysis, of correlating quantitative protein and mRNA expression measurements of selected genes.
The recent introduction of mass spectrometric protein analysis techniques has dramatically enhanced the throughput and sensitivity of protein identification to a level which now permits the large-scale analysis of proteins separated by 2DE. The techniques have reached a level of sensitivity that permits the identification of essentially any protein that is detectable in the gels by conventional protein staining (9, 29). Current protein analytical technology is based on the mass spectrometric generation of peptide fragment patterns that are idiotypic for the sequence of a protein. Protein identity is established by correlating such fragment patterns with sequence databases (10, 22, 37). Sophisticated computer software (8) has automated the entire process such that proteins are routinely identified with no human interpretation of peptide fragment patterns.
In this study, we have analyzed the mRNA and protein levels of a group of genes expressed in exponentially growing cells of the yeast S. cerevisiae. Protein expression levels were quantified by metabolic labeling of the yeast proteins to a steady state, followed by 2DE and liquid scintillation counting of the selected, separated protein species. Separated proteins were identified by in-gel tryptic digestion of spots with subsequent analysis by microspray liquid chromatography-tandem mass spectrometry (LC-MS/MS) and sequence database searching. The corresponding mRNA transcript levels were calculated from SAGE frequency tables (35).
This study, for the first time, explores a quantitative comparison of mRNA transcript and protein expression levels for a relatively large number of genes expressed in the same metabolic state. The resultant correlation is insufficient for prediction of protein levels from mRNA transcript levels. We have also compared the relative amounts of protein and mRNA with the respective codon bias values for the corresponding genes. This comparison indicates that codon bias by itself is insufficient to accurately predict either the mRNA or the protein expression levels of a gene. In addition, the results demonstrate that only highly expressed proteins are detectable by 2DE separation of total cell lysates and that therefore the construction of complete proteome maps with current technology will be very challenging, irrespective of the type of organism.
MATERIALS AND METHODS
Yeast strain and growth conditions.
The source of protein and message transcripts for all experiments was YPH499 (MATa ura3-52 lys2-801 ade2-101 leu2-Δ1 his3-Δ200 trp1-Δ63) (30). Logarithmically growing cells were obtained by growing yeast cells to early log phase (3 × 106 cells/ml) in YPD rich medium (YPD supplemented with 6 mM uracil, 4.8 mM adenine, and 24 mM tryptophan) at 30°C (35). Metabolic labeling of protein was accomplished in YPD medium exactly as described elsewhere (4) with the exception that 1 ml of cells was labeled with 3 mCi to offset methionine present in YPD medium. Protein was harvested as described by Garrels and coworkers (12). Harvested protein was lyophilized, resuspended in isoelectric focusing gel rehydration solution, and stored at −80°C.
2DE.
Soluble proteins were run in the first dimension by using a commercial flatbed electrophoresis system (Multiphor II; Pharmacia Biotech). Immobilized polyacrylamide gel (IPG) dry strips with nonlinear pH 3.0 to 10.0 gradients (Amersham-Pharmacia Biotech) were used for the first-dimension separation. Forty micrograms of protein from whole-cell lysates was mixed with IPG strip rehydration buffer (8 M urea, 2% Nonidet P-40, 10 mM dithiothreitol), and 250 to 380 μl of solution was added to individual lanes of an IPG strip rehydration tray (Amersham-Pharmacia Biotech). The strips were allowed to rehydrate at room temperature for 1 h. The samples were run at 300 V–10 mA–5 W for 2 h, then ramped to 3,500 V–10 mA–5 W over a period of 3 h, and then kept at 3,500 V–10 mA–5 W for 15 to 19 h. At the end of the first-dimension run (60 to 70 kV · h), the IPG strips were reequilibrated for 8 min in 2% (wt/vol) dithiothreitol in 2% (wt/vol) SDS–6 M urea–30% (wt/vol) glycerol–0.05 M Tris HCl (pH 6.8) and for 4 min in 2.5% iodoacetamide in 2% (wt/vol) SDS–6 M urea–30% (wt/vol) glycerol–0.05 M Tris HCl (pH 6.8). Following reequilibration, the strips were transferred and apposed to 10% polyacrylamide second-dimension gels. Polyacrylamide gels were poured in a casting stand with 10% acrylamide–2.67% piperazine diacrylamide–0.375 M Tris base-HCl (pH 8.8)–0.1% (wt/vol) SDS–0.05% (wt/vol) ammonium persulfate–0.05% TEMED (N,N,N′,N′-tetramethylethylenediamine) in Milli-Q water. The apparatus used to run second-dimension gels was a noncommercial apparatus from Oxford Glycosciences, Inc. Once the IPG strips were apposed to the second-dimension gels, they were immediately run at 50 mA (constant)–500 V–85 W for 20 min, followed by 200 mA (constant)–500 V–85 W until the buffer front line was 10 to 15 mm from the bottom of the gel. Gels were removed and silver stained according to the procedure of Shevchenko et al. (29).
Protein identification.
Gels were exposed to X-ray film overnight, and then the silver staining and film were used to excise 156 spots of varying intensities, molecular weights, and isoelectric focusing points. In order to increase the detection limit by mass spectrometry, spots were cut out and pooled from up to four identical cold, silver-stained gels. In-gel tryptic digests of pooled spots were performed as described previously (29). Tryptic peptides were analyzed by microcapillary LC-MS with automated switching to MS/MS mode for peptide fragmentation. Spectra were searched against the composite OWL protein sequence database (version 30.2; 250,514 protein sequences) (24a) by using the computer program Sequest (8), which matches theoretical and acquired tandem mass spectra. A protein match was determined by comparing the number of peptides identified and their respective cross-correlation scores. All protein identifications were verified by comparison with theoretical molecular weights and isoelectric points.
mRNA quantitation.
Velculescu and coworkers have previously generated frequency tables for yeast mRNA transcripts from the same strain grown under the same stated conditions as described herein (35). The SAGE technology is based on two main principles. First, a short sequence tag (15 bp) that contains sufficient information uniquely to identify a transcript is generated. A single tag is usually generated from each mRNA transcript in the cell which corresponds to 15 bp at the 3′-most cutting site for NlaIII. Second, many transcript tags can be concatenated into a single molecule and then sequenced, revealing the identity of multiple tags simultaneously. Over 20,000 transcripts were sequenced from yeast strain YPH499 growing at mid-log phase on glucose. Assuming the previously derived estimate of 15,000 mRNA molecules per cell (16), this would represent a 1.3-fold coverage even for mRNA molecules present at a single copy per cell and would provide a 72% probability of detecting such transcripts. Computer software which took for input the gene detected, examined the nucleotide sequence, and performed the calculation as described by Velculescu and coworkers (35) was written. In practice, we found that for 21 of 128 (16%) genes examined viable mRNA levels from SAGE data could not be calculated. This was because (i) no CATG site was found in the open reading frame (ORF), (ii) a CATG site was found but the corresponding 10-bp putative SAGE tag was not found in the frequency tables, or (iii) identical putative SAGE tags were present for multiple genes (e.g., TDH2_YEAST and TDH3_YEAST).
Protein quantitation.
[35S]methionine-labeled gels were exposed to X-ray film overnight, and then the silver stain and film were used to excise 156 spots of varying intensities, molecular weights, and pIs. The excised spots were placed in 0.6-ml microcentrifuge tubes, and scintillation cocktail (100 μl) was added. The samples were vortexed and counted. In addition, two parallel gels were electroblotted to polyvinylidene difluoride membranes. The membranes were exposed to X-ray film, and four intense single spots were excised from each membrane and subjected to amino acid analysis. For these four spots, a mean of 209 ± 4 cpm/pmol of protein/methionine was found. This number was used to quantitate all remaining spots in conjunction with the number of methionines present in the protein.
To ensure that proteins were labeled to equilibrium, parallel 2D gels were prepared and run on yeast metabolically labeled for 1, 2, 6, or 18 h. The corresponding 156 spots were excised from each gel, and radioactivity was measured by liquid scintillation counting for each spot. Calculated protein levels were highly reproducible for all time points measured after 1 h.
Calculation of codon bias and predicted half-life.
Codon bias values were extracted from the YPD spreadsheet (17). Protein half-lives were calculated based on the N-end rule (33). When the N-terminal processing was not known experimentally, it was predicted based on the affinity of methionine aminopeptidase (31).
RESULTS
Characteristics of proteome approach.
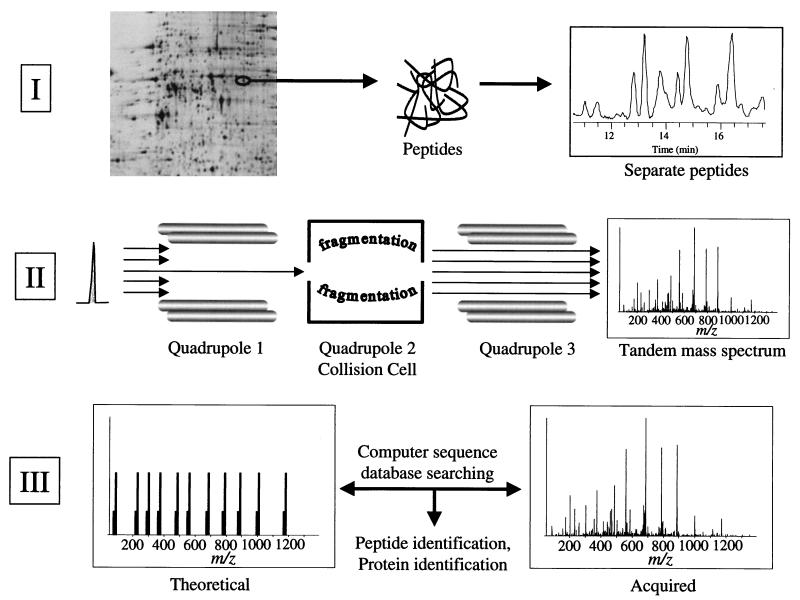
Nearly every facet of proteome analysis hinges on the unambiguous identification of large numbers of expressed proteins in cells. Several techniques have been described previously for the identification of proteins separated by 2DE, including N-terminal and internal sequencing (1, 2), amino acid analysis (38), and more recently mass spectrometry (25). We utilized techniques based on mass spectrometry because they afford the highest levels of sensitivity and provide unambiguous identification. The specific procedure used is schematically illustrated in Fig. 1 and is based on three principles. First, proteins are removed from the gel by proteolytic in-gel digestion, and the resulting peptides are separated by on-line capillary high-performance liquid chromatography. Second, the eluting peptides are ionized and detected, and the specific peptide ions are selected and fragmented by the mass spectrometer. To achieve this, the mass spectrometer switches between the MS mode (for peptide mass identification) and the MS/MS mode (for peptide characterization and sequencing). Selected peptides are fragmented by a process called collision-induced dissociation (CID) to generate a tandem mass spectrum (MS/MS spectrum) that contains the peptide sequence information. Third, individual CID mass spectra are then compared by computer algorithms to predicted spectra from a sequence database. This results in the identification of the peptide and, by association, the protein(s) in the spot. Unambiguous protein identification is attained in a single analysis by the detection of multiple peptides derived from the same protein.
FIG. 1.
Schematic illustration of proteome analysis by 2DE and mass spectrometry. In part I, proteins are separated by 2DE, stained spots are excised and subjected to in-gel digestion with trypsin, and the resulting peptides are separated by on-line capillary high-performance liquid chromatography. In part II, a peptide is shown eluting from the column in part I. The peptide is ionized by electrospray ionization and enters the mass spectrometer. The mass of the ionized peptide is detected, and the first quadrupole mass filter allows only the specific mass-to-charge ratio of the selected peptide ion to pass into the collision cell. In the collision cell, the energized, ionized peptides collide with neutral argon gas molecules. Fragmentation of the peptide is essentially random but occurs mainly at the peptide bonds, resulting in smaller peptides of differing lengths (masses). These peptide fragments are detected as a tandem mass (MS/MS) spectrum in the third quadrupole mass filter where two ion series are recorded simultaneously, one each from sequencing inward from the N and C termini of the peptide, respectively. In part III, the MS/MS spectrum from the selected, ionized peptide is compared to predicted tandem mass spectra computer generated from a sequence database. Provided that the peptide sequence exists in the database, the peptide and, by association, the protein from which the peptide was derived can be identified. Unambiguous protein identification is attained in a single analysis because multiple peptides are identified as being derived from the same protein.
Protein identification.
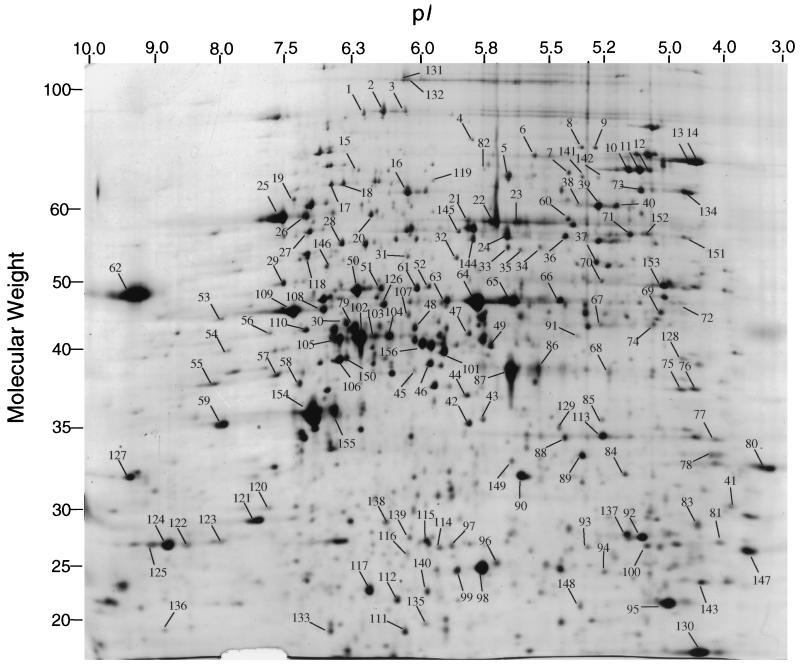
Yeast total cell protein lysate (40 μg), metabolically labeled with [35S]methionine, was electrophoretically separated by isoelectric focusing in the first dimension and by SDS–10% polyacrylamide gel electrophoresis in the second dimension. Proteins were visualized by silver staining and by autoradiography. Of the more than 1,000 proteins visible by silver staining, 156 spots were excised from the gel and subjected to in-gel tryptic digestion, and the resulting peptides were analyzed and identified by microspray LC-MS/MS techniques as described above. The proteins in this study were all identified automatically by computer software with no human interpretation of mass spectra. They are indicated in Fig. 2 and detailed in Table 1.
FIG. 2.
2D silver-stained gel of the proteins in yeast total cell lysate. Proteins were separated in the first dimension (horizontal) by isoelectric focusing and then in the second dimension (vertical) by molecular weight sieving. Protein spots (156) were chosen to include the entire range of molecular weights, isoelectric focusing points, and staining intensities. Spots were excised, and the corresponding protein was identified by mass spectrometry and database searching. The spots are labeled on the gel and correspond to the data presented in Table 1. Molecular weights are given in thousands.
TABLE 1.
Expressed genes identified from 2D gel in Fig. 2
| Mol wt | pI | Spot no. | YPD gene namea | Protein abundance (103 copies/cell) | mRNA abundance (copies/cell) | Codon bias |
|---|---|---|---|---|---|---|
| 17,259 | 6.75 | 133 | CPR1 | 15.2 | 61.7 | 0.769 |
| 18,702 | 4.80 | 83 | EGD2 | 20.1 | 5.2 | 0.724 |
| 18,726 | 4.44 | 147 | YKL056C | 61.2 | 88.4 | 0.831 |
| 18,978 | 5.95 | 135 | YER067W | 3.7 | 6.7 | 0.118 |
| 19,108 | 5.04 | 130 | YLR109W | 94.4 | 9.7 | 0.680 |
| 19,681 | 9.08 | 136 | ATP7 | 11.0 | NAbc | 0.246 |
| 20,505 | 6.07 | 111 | GUK1 | 16.5 | 3.7 | 0.422 |
| 21,444 | 5.25 | 148 | SAR1 | 5.4 | 10.4 | 0.455 |
| 21,583 | 4.98 | 95 | TSA1 | 110.6 | 40.1 | 0.845 |
| 22,602 | 4.30 | 80 | EFB1 | 66.1 | 23.8 | 0.875 |
| 23,079 | 6.29 | 112 | SOD2 | 12.6 | 2.2 | 0.351 |
| 23,743 | 5.44 | 137 | HSP26 | NAd | 0.7 | 0.434 |
| 24,033 | 5.97 | 96 | ADK1 | 17.4 | 16.4 | 0.656 |
| 24,058 | 4.43 | 143 | YKL117W | 29.2 | 10.4 | 0.339 |
| 24,353 | 6.30 | 140 | TFS1 | 8.1 | 0.7 | 0.146 |
| 24,662 | 5.85 | 99 | URA5 | 25.4 | 6.0 | 0.359 |
| 24,808 | 6.33 | 97 | GSP1 | 26.3 | 5.2 | 0.735 |
| 24,908 | 8.73 | 122 | RPS5 | 18.6 | NAc | 0.899 |
| 25,081 | 4.65 | 81 | MRP8 | 9.3 | NAc | 0.241 |
| 25,960 | 6.06 | 116 | RPE1 | 5.8 | 0.7 | 0.372 |
| 26,378 | 9.55 | 127 | RPS3 | 96.8 | NAc | 0.863 |
| 26,467 | 5.18 | 100 | VMA4 | 10.5 | 3.7 | 0.427 |
| 26,661 | 5.84 | 98 | TPI1 | NAd | NAc | 0.900 |
| 27,156 | 5.56 | 93 | PRE8 | 6.9 | 0.7 | 0.129 |
| 27,334 | 6.13 | 115 | YHR049W | 18.4 | 2.2 | 0.520 |
| 27,472 | 5.33 | 92 | YNL010W | 31.6 | 3.7 | 0.421 |
| 27,480 | 8.95 | 123 | GPM1 | 10.0 | 169.4 | 0.902 |
| 27,480 | 8.95 | 124 | GPM1 | 231.4 | 169.4 | 0.902 |
| 27,480 | 8.95 | 125 | GPM1 | 7.5 | 169.4 | 0.902 |
| 27,809 | 5.97 | 139 | HOR2 | 5.7 | 0.7 | 0.381 |
| 27,874 | 4.46 | 78 | YST1 | 13.6 | 52.8 | 0.805 |
| 28,595 | 4.51 | 41 | PUP2 | 4.4 | 0.7 | 0.147 |
| 29,156 | 6.59 | 114 | YMR226C | 14.5 | 2.2 | 0.283 |
| 29,244 | 8.40 | 120 | DPM1 | 5.0 | 11.2 | 0.362 |
| 29,443 | 5.91 | 48 | PRE4 | 3.4 | 3.7 | 0.162 |
| 30,012 | 6.39 | 138 | PRB1 | 21.2 | 1.5 | 0.449 |
| 30,073 | 4.63 | 77 | BMH1 | 14.7 | 28.2 | 0.454 |
| 30,296 | 7.94 | 121 | OMP2 | 67.4 | 41.6 | 0.499 |
| 30,435 | 6.34 | 89 | GPP1 | 70.2 | 11.2 | 0.703 |
| 31,332 | 5.57 | 88 | ILV6 | 13.9 | 3.0 | 0.402 |
| 32,159 | 5.46 | 113 | IPP1 | 63.1 | 3.7 | 0.752 |
| 32,263 | 6.00 | 149 | HIS1 | 22.4 | 4.5 | 0.232 |
| 33,311 | 5.35 | 84 | SPE3 | 15.1 | 6.7 | 0.468 |
| 34,465 | 5.60 | 129 | ADE1 | 8.7 | 5.2 | 0.305 |
| 34,762 | 5.32 | 85 | SEC14 | 10.9 | 6.0 | 0.373 |
| 34,797 | 5.85 | 42 | URA1 | 49.5 | 8.9 | 0.237 |
| 34,799 | 6.04 | 90 | BEL1 | 103.2 | 81.0 | 0.875 |
| 35,556 | 5.97 | 43 | YDL124W | 6.4 | 4.5 | 0.206 |
| 35,619 | 8.41 | 59 | TDH1 | 69.8 | 32.7c | 0.940 |
| 35,650 | 5.49 | 68 | CAR1 | 5.2 | 3.0 | 0.339 |
| 35,712 | 6.72 | 117 | TDH2 | 49.6 | 473.0c | 0.982 |
| 35,712 | 6.72 | 154 | TDH2 | 863.5 | 473.0c | 0.982 |
| 35,712 | 6.72 | 155 | TDH2 | 79.4 | 473.0c | 0.982 |
| 36,272 | 4.85 | 128 | APA1 | 8.7 | 0.7 | 0.425 |
| 36,358 | 5.05 | 75 | YJR105W | 17.6 | 17.1 | 0.522 |
| 36,358 | 5.05 | 76 | YJR105W | 27.5 | 17.1 | 0.522 |
| 36,596 | 6.37 | 79 | ADH2 | 58.9 | 260.0c | 0.711 |
| 36,714 | 6.30 | 102 | ADH1 | 746.1 | 260.0 | 0.913 |
| 36,714 | 6.30 | 103 | ADH1 | 17.6 | 260.0 | 0.913 |
| 36,714 | 6.30 | 104 | ADH1 | 61.4 | 260.0 | 0.913 |
| 36,714 | 6.30 | 105 | ADH1 | 52.7 | 260.0 | 0.913 |
| 37,033 | 6.23 | 44 | TAL1 | 44.8 | 3.7 | 0.701 |
| 37,796 | 7.36 | 57 | IDH2 | 29.4 | 6.7 | 0.330 |
| 37,886 | 6.49 | 106 | ILV5 | 76.0 | 4.5 | 0.892 |
| 38,700 | 7.83 | 55 | BAT1 | 30.9 | 11.2 | 0.469 |
| 38,702 | 6.24 | 46 | QCR2 | NAd | 2.2 | 0.326 |
| 39,477 | 5.58 | 86 | FBA1 | 17.8 | 183.6 | 0.935 |
| 39,477 | 5.58 | 87 | FBA1 | 427.2 | 183.6 | 0.935 |
| 39,540 | 6.50 | 150 | HOM2 | 60.3 | 4.5 | 0.592 |
| 39,561 | 6.12 | 156 | PSA1 | 96.4 | 27.5 | 0.718 |
| 41,158 | 6.01 | 49 | YNL134C | 14.9 | 1.5 | 0.316 |
| 41,623 | 7.18 | 58 | BAT2 | 19.0 | 8.9 | 0.250 |
| 41,728 | 7.29 | 110 | ERG10 | 24.1 | 4.5 | 0.543 |
| 41,900 | 5.42 | 74 | TOM40 | 22.3 | 2.2 | 0.375 |
| 42,402 | 6.29 | 45 | CYS3 | 6.7 | 8.9 | 0.621 |
| 42,883 | 5.63 | 67 | DYS1 | 15.8 | 5.2 | 0.526 |
| 43,409 | 6.31 | 107 | SER1 | 10.5 | 1.5 | 0.292 |
| 43,421 | 5.59 | 91 | ERG6 | 2.2 | 14.1 | 0.408 |
| 44,174 | 7.32 | 56 | YBR025C | 13.1 | 6.0 | 0.684 |
| 44,682 | 4.99 | 72 | TIF1 | 2.9 | 39.4 | 0.834 |
| 44,707 | 7.77 | 108 | PGK1 | 23.7 | 165.7 | 0.897 |
| 44,707 | 7.77 | 109 | PGK1 | 315.2 | 165.7 | 0.897 |
| 46,080 | 6.72 | 30 | CAR2 | 15.4 | NAc | 0.495 |
| 46,383 | 8.52 | 53 | IDP1 | 7.7 | 0.7 | 0.436 |
| 46,553 | 5.98 | 47 | IDP2 | 32.4 | NAc | 0.197 |
| 46,679 | 6.39 | 50 | ENO1 | 35.4 | 0.7 | 0.930 |
| 46,679 | 6.39 | 51 | ENO1 | 6.6 | 0.7 | 0.930 |
| 46,679 | 6.39 | 52 | ENO1 | 2.2 | 0.7 | 0.930 |
| 46,773 | 5.82 | 63 | ENO2 | 15.5 | 289.1 | 0.960 |
| 46,773 | 5.82 | 64 | ENO2 | 635.5 | 289.1 | 0.960 |
| 46,773 | 5.82 | 65 | ENO2 | 93.0 | 289.1 | 0.960 |
| 46,773 | 5.82 | 66 | ENO2 | 31.0 | 289.1 | 0.960 |
| 47,402 | 6.09 | 126 | COR1 | 2.5 | 0.7 | 0.422 |
| 47,666 | 8.98 | 54 | AAT2 | 11.7 | 6.0 | 0.338 |
| 48,364 | 5.25 | 73 | WTM1 | 74.5 | 13.4 | 0.365 |
| 48,530 | 6.20 | 61 | MET17 | 38.1 | 29.0 | 0.576 |
| 48,904 | 5.18 | 69 | LYS9 | 16.2 | 3.7 | 0.463 |
| 48,987 | 4.90 | 153 | SUP45 | 29.6 | 11.9 | 0.377 |
| 49,727 | 5.47 | 70 | PRO2 | 13.6 | 5.2 | 0.297 |
| 49,912 | 9.27 | 62 | TEF2 | 558.5 | 282.0 | 0.932 |
| 50,444 | 5.67 | 35 | YDR190C | 4.8 | 2.2 | 0.228 |
| 50,837 | 6.11 | 32 | YEL047C | 3.8 | 1.5 | 0.387 |
| 50,891 | 4.59 | 151 | TUB2 | 11.2 | 7.4 | 0.404 |
| 51,547 | 6.80 | 27 | LPD1 | 18.9 | 2.2 | 0.351 |
| 52,216 | 7.25 | 29 | SHM2 | 19.7 | 7.4 | 0.722 |
| 52,859 | 5.54 | 37 | YFR044C | 30.2 | 6.7 | 0.442 |
| 53,798 | 5.19 | 71 | HXK2 | 26.5 | 7.4 | 0.756 |
| 53,803 | 6.05 | 145 | GYP6 | 4.4 | 0.7 | 0.147 |
| 54,403 | 5.29 | 39 | ALD6 | 37.7 | 2.2 | 0.664 |
| 54,403 | 5.29 | 40 | ALD6 | 6.6 | 2.2 | 0.664 |
| 54,502 | 6.20 | 31 | ADE13 | 6.3 | 1.5 | 0.417 |
| 54,543 | 7.75 | 25 | PYK1 | 225.3 | 101.8 | 0.965 |
| 54,543 | 7.75 | 26 | PYK1 | 39.8 | 101.8 | 0.965 |
| 55,221 | 6.66 | 146 | YEL071W | 16.3 | 3.0 | 0.244 |
| 55,295 | 4.35 | 134 | PDI1 | 66.2 | 14.1 | 0.589 |
| 55,364 | 5.98 | 24 | GLK1 | 22.6 | 6.0 | 0.237 |
| 55,481 | 7.97 | 118 | ATP1 | 21.6 | 2.2 | 0.637 |
| 55,886 | 6.47 | 28 | CYS4 | 22.2 | NAc | 0.444 |
| 56,167 | 5.83 | 33 | ARO8 | 14.3 | 3.0 | 0.324 |
| 56,167 | 5.83 | 34 | ARO8 | 9.1 | 3.0 | 0.324 |
| 56,584 | 6.36 | 20 | CYB2 | 18.9 | NAc | 0.259 |
| 57,366 | 5.53 | 60 | FRS2 | 2.3 | 0.7 | 0.451 |
| 57,383 | 5.98 | 144 | ZWF1 | 5.6 | 0.7 | 0.215 |
| 57,464 | 5.49 | 36 | THR4 | 21.4 | 3.7 | 0.508 |
| 57,512 | 5.50 | 7 | SRV2 | 6.5 | NAc | 0.260 |
| 57,727 | 4.92 | 152 | VMA2 | 33.7 | 8.9 | 0.546 |
| 58,573 | 6.47 | 17 | ACH1 | 4.4 | 1.5 | 0.327 |
| 58,573 | 6.47 | 18 | ACH1 | 5.4 | 1.5 | 0.327 |
| 61,353 | 5.87 | 21 | PDC1 | 6.5 | 200.7 | 0.962 |
| 61,353 | 5.87 | 22 | PDC1 | 303.2 | 200.7 | 0.962 |
| 61,353 | 5.87 | 23 | PDC1 | 16.3 | 200.7 | 0.962 |
| 61,649 | 5.54 | 38 | CCT8 | 2.2 | 1.5 | 0.271 |
| 61,902 | 6.21 | 101 | PDC5 | 4.3 | NAc | 0.828 |
| 62,266 | 6.19 | 16 | ICL1 | 20.1 | NAc | 0.327 |
| 62,862 | 8.02 | 19 | ILV3 | 5.3 | 4.5 | 0.548 |
| 63,082 | 6.40 | 119 | PGM2 | 2.2 | 3.0 | 0.402 |
| 64,335 | 5.77 | 5 | PAB1 | 30.4 | 1.5 | 0.616 |
| 66,120 | 5.42 | 8 | STI1 | 6.7 | 0.7 | 0.313 |
| 66,120 | 5.42 | 9 | STI1 | 6.4 | 0.7 | 0.313 |
| 66,450 | 5.29 | 141 | SSB2 | 7.0 | NAc | 0.880 |
| 66,450 | 5.29 | 142 | SSB2 | 2.3 | NAc | 0.880 |
| 66,456 | 5.23 | 10 | SSB1 | 64.5 | 79.5 | 0.907 |
| 66,456 | 5.23 | 11 | SSB1 | 59.0 | 79.5 | 0.907 |
| 66,456 | 5.23 | 12 | SSB1 | 13.7 | 79.5 | 0.907 |
| 68,397 | 5.82 | 82 | LEU4 | 3.1 | 3.0 | 0.407 |
| 69,313 | 4.90 | 13 | SSA2 | 24.3 | 18.6 | 0.892 |
| 69,313 | 4.90 | 14 | SSA2 | 77.1 | 18.6 | 0.892 |
| 74,378 | 8.46 | 15 | YKL029C | 2.8 | 3.7 | 0.353 |
| 75,396 | 5.82 | 6 | GRS1 | 5.5 | 7.4 | 0.500 |
| 85,720 | 6.25 | 1 | MET6 | 2.0 | NAc | 0.772 |
| 85,720 | 6.25 | 2 | MET6 | 10.9 | NAc | 0.772 |
| 85,720 | 6.25 | 3 | MET6 | 1.4 | NAc | 0.772 |
| 93,276 | 6.11 | 131 | EFT1 | 17.9 | 41.6 | 0.890 |
| 93,276 | 6.11 | 132 | EFT1 | 5.7 | 41.6 | 0.890 |
| 102,064e | 6.61e | 94 | ADE3 | 4.8 | 5.2 | 0.423 |
| 107,482e | 5.33e | 4 | MCM3 | 2.7 | NAc | 0.240 |
YPD gene names are available from the YPD website (39).
NA, calculation could not be performed or was not available.
mRNA data inconclusive or NA.
No methionines in predicted ORF; therefore, protein concentration was not determined.
Measured molecular weight or pI did not match theoretical molecular weight or pI.
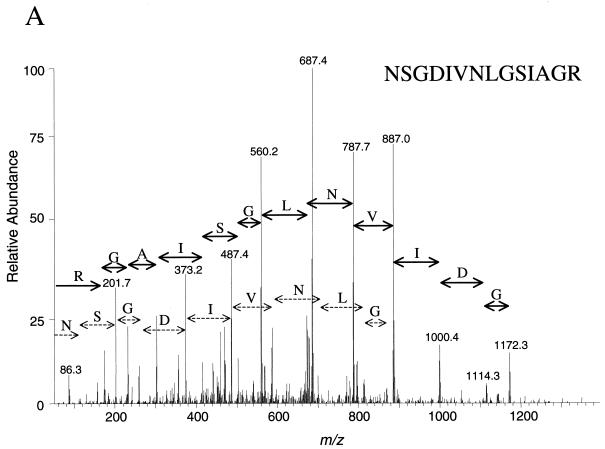
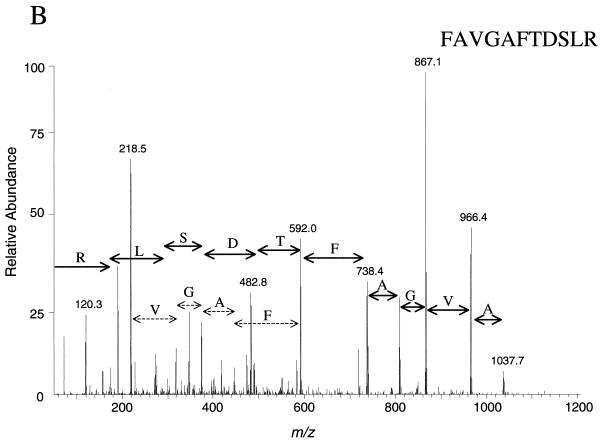
The CID spectra shown in Fig. 3 indicate that the quality of the identification data generated was suitable for unambiguous protein identification. The spectra represent the amino acid sequences of tryptic peptides NSGDIVNLGSIAGR (Fig. 3A) and FAVGAFTDSLR (Fig. 3B). Both peptides were derived from protein S57593 (hypothetical protein YMR226C), which migrated to spot 114 (molecular weight, 29,156; pI, 6.59) in the 2D gel in Fig. 2. Five other peptides from the same analysis were also computer matched to the same protein sequence.
FIG. 3.
Tandem mass (MS/MS) spectra resulting from analysis of a single spot on a 2D gel. The first quadrupole selected a single mass-to-charge ratio (m/z) of 687.2 (A) or 592.6 (B), while the collision cell was filled with argon gas, and a voltage which caused the peptide to undergo fragmentation by CID was applied. The third quadrupole scanned the mass range from 50 to 1,400 m/z. The computer program Sequest (8) was utilized to match MS/MS spectra to amino acid sequence by database searching. Both spectra matched peptides from the same protein, S57593 (yeast hypothetical protein YMR226C). Five other peptides from the same analysis were matched to the same protein.
Protein and mRNA quantitation.
For the 156 genes investigated, the protein expression levels ranged from 2,200 (PGM2) to 863,000 (TDH2/TDH3) copies/cell. The levels of mRNA for each of the genes identified were calculated from SAGE frequency tables (35). These tables contain the mRNA levels for 4,665 genes in yeast strain YPH499 grown to mid-log phase in YPD medium on glucose as a carbon source. In some instances, the mRNA levels could not be calculated for reasons stated in Materials and Methods. For the proteins analyzed in this study, mean transcript levels varied from 0.7 to 473 copies/cell.
Selection of the sample population for mRNA-protein expression level correlation.
The protein spots selected for identification were selected from spots visible by silver staining in the 2D gel. An attempt was made not to include spots where overlap with other spots was readily apparent. The number of proteins identified was 156 (Table 1). Some proteins migrated to more than one spot (presumably due to differential protein processing or modifications), and protein levels from these spots were calculated by integrating the intensities of the different spots. The 156 protein spots analyzed represented the products of 128 different genes. Genes were excluded from the correlation analysis only if part of the data set was missing; i.e., genes were excluded if (i) no mRNA expression data were available for the protein or putative SAGE tags were ambiguous, (ii) the amino acid sequence did not contain methionine, (iii) more than a single protein was conclusively identified as migrating to the same gel spot, or (iv) the theoretical and observed pIs and molecular weights could not be reconciled. After these criteria were applied, the number of genes used in the correlation analysis was 106.
Codon bias and predicted half-lives.
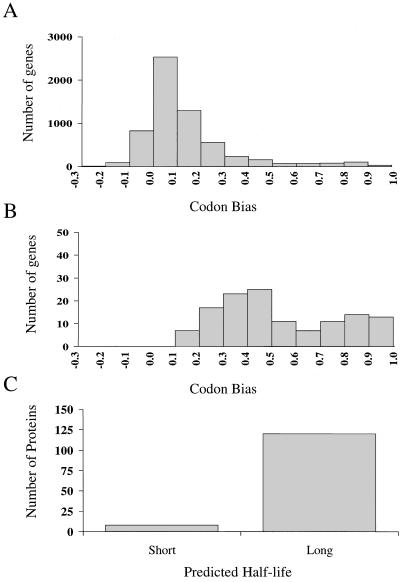
Codon bias is thought to be an indicator of protein expression, with highly expressed proteins having large codon bias values. The codon bias distribution for the entire set of more than 6,000 predicted yeast gene ORFs is presented in Fig. 4A. The interval with the largest frequency of genes is between the codon bias values of 0.0 and 0.1. This segment contains more than 2,500 genes. The distribution of the codon bias values of the 128 different genes found in this study (all protein spots from Fig. 2) is shown in Fig. 4B, and protein half-lives (predicted from applying the N-end rule [33] to the experimentally determined or predicted protein N termini) are shown in Fig. 4C. No genes were identified with codon bias values less than 0.1 even though thousands of genes exist in this category. In addition, nearly all of the proteins identified had long predicted half-lives (greater than 30 h).
FIG. 4.
Current proteome analysis technology utilizing 2DE without preenrichment samples mainly highly expressed and long-lived proteins. Genes encoding highly expressed proteins generally have large codon bias values. (A) Distribution of the yeast genome (more than 6,000 genes) based on codon bias. The interval with the largest frequency of genes is 0.0 to 0.1, with more than 2,500 genes. (B) Distribution of the genes from identified proteins in this study based on codon bias. No genes with codon bias values less than 0.1 were detected in this study. (C) Distribution of identified proteins in this study based on predicted half-life (estimated by N-end rule).
Correlation of mRNA and protein expression levels.
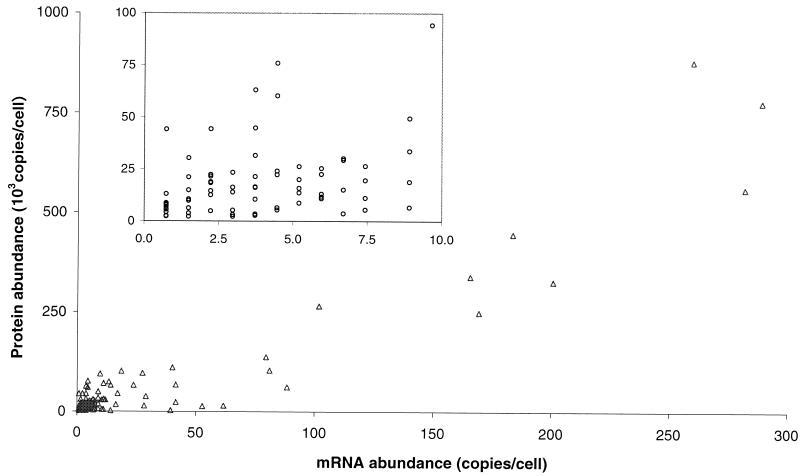
The correlation between mRNA and protein levels of the genes selected as described above is shown in Fig. 5. For the entire group (106 genes) for which a complete data set was generated, there was a general trend of increased protein levels resulting from increased mRNA levels. The Pearson product moment correlation coefficient for the whole data set (106 genes) was 0.935. This number is highly biased by a small number of genes with very large protein and message levels. A more representative subset of the data is shown in the inset of Fig. 5. It shows genes for which the message level was below 10 copies/cell and includes 69% (73 of 106 genes) of the data used in the study. The Pearson product moment correlation coefficient for this data set was only 0.356. We also found that levels of protein expression coded for by mRNA with comparable abundance varied by as much as 30-fold and that the mRNA levels coding for proteins with comparable expression levels varied by as much as 20-fold.
FIG. 5.
Correlation between protein and mRNA levels for 106 genes in yeast growing at log phase with glucose as a carbon source. mRNA and protein levels were calculated as described in Materials and Methods. The data represent a population of genes with protein expression levels visible by silver staining on a 2D gel chosen to include the entire range of molecular weights, isoelectric focusing points, and staining intensities. The inset shows the low-end portion of the main figure. It contains 69% of the original data set. The Pearson product moment correlation for the entire data set was 0.935. The correlation for the inset containing 73 proteins (69%) was only 0.356.
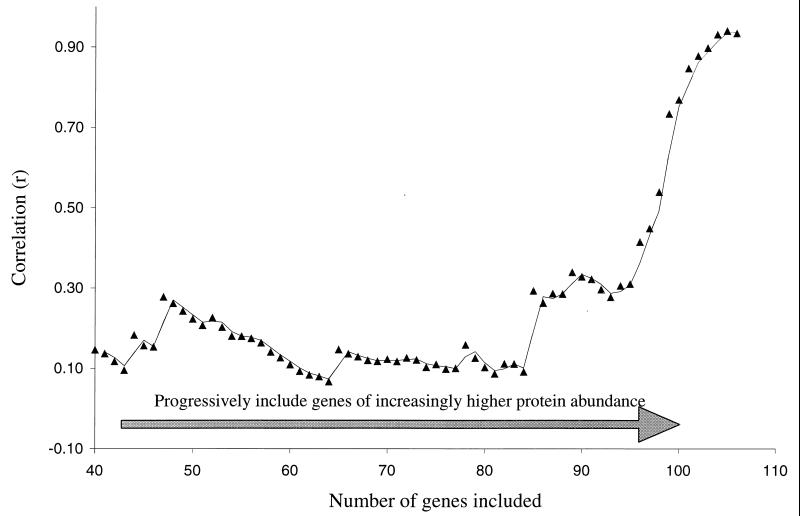
The distortion of the correlation value induced by the uneven distribution of the data points along the x axis is further demonstrated by the analysis in Fig. 6. The 106 samples included in the study were ranked by protein abundance, and the Pearson product moment correlation coefficient was repeatedly calculated after including progressively more, and higher-abundance, proteins in each calculation. The correlation values remained relatively stable in the range of 0.1 to 0.4 if the lowest-expressed 40 to 95 proteins used in this study were included. However, the correlation value steadily climbed by the inclusion of each of the 11 very highly expressed proteins.
FIG. 6.
Effect of highly abundant proteins on Pearson product moment correlation coefficient for mRNA and protein abundance in yeast. The set of 106 genes was ranked according to protein abundance, and the correlation value was calculated by including the 40 lowest-abundance genes and then progressively including the remaining 66 genes in order of abundance. The correlation value climbs as the final 11 highly abundant proteins are included.
Correlation of protein and mRNA expression levels with codon bias.
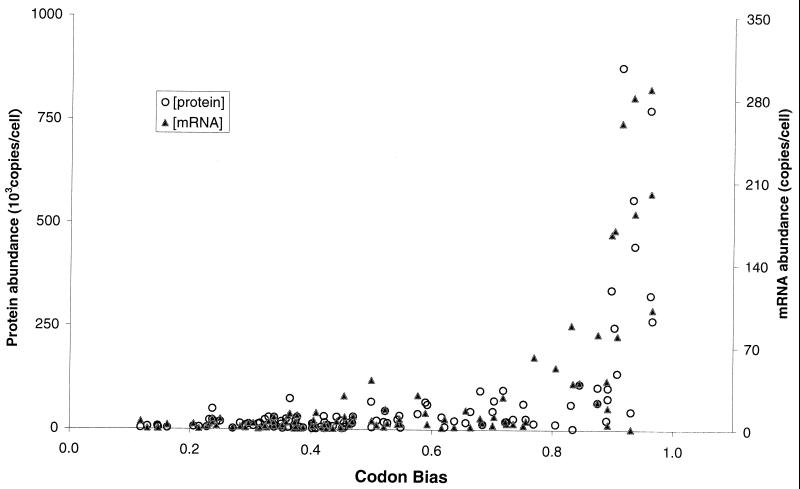
Codon bias is the propensity for a gene to utilize the same codon to encode an amino acid even though other codons would insert the identical amino acid in the growing polypeptide sequence. It is further thought that highly expressed proteins have large codon biases (3). To assess the value of codon bias for predicting mRNA and protein levels in exponentially growing yeast cells, we plotted the two experimental sets of data versus the codon bias (Fig. 7). The distribution patterns for both mRNA and protein levels with respect to codon bias were highly similar. There was high variability in the data within the codon bias range of 0.8 to 1.0. Although a large codon bias generally resulted in higher protein and message expression levels, codon bias did not appear to be predictive of either protein levels or mRNA levels in the cell.
FIG. 7.
Relationship between codon bias and protein and mRNA levels in this study. Yeast mRNA and protein expression levels were calculated as described in Materials and Methods. The data represent the same 106 genes as in Fig. 5.
DISCUSSION
The desired end point for the description of a biological system is not the analysis of mRNA transcript levels alone but also the accurate measurement of protein expression levels and their respective activities. Quantitative analysis of global mRNA levels currently is a preferred method for the analysis of the state of cells and tissues (11). Several methods which either provide absolute mRNA abundance (34, 35) or relative mRNA levels in comparative analyses (20, 27) have been described elsewhere. The techniques are fast and exquisitely sensitive and can provide mRNA abundance for potentially any expressed gene. Measured mRNA levels are often implicitly or explicitly extrapolated to indicate the levels of activity of the corresponding protein in the cell. Quantitative analysis of protein expression levels (proteome analysis) is much more time-consuming because proteins are analyzed sequentially one by one and is not general because analyses are limited to the relatively highly expressed proteins. Proteome analysis does, however, provide types of data that are of critical importance for the description of the state of a biological system and that are not readily apparent from the sequence and the level of expression of the mRNA transcript. This study attempts to examine the relationship between mRNA and protein expression levels for a large number of expressed genes in cells representing the same state.
Limits in the sensitivity of current protein analysis technology precluded a completely random sampling of yeast proteins. We therefore based the study on those proteins visible by silver staining on a 2D gel. Of the more than 1,000 visible spots, 156 were chosen to include the entire range of molecular weights, isoelectric focusing points, and staining intensities displayed on the 2D protein pattern. The genes identified in this study shared a number of properties. First, all of the proteins in this study had a codon bias of greater than 0.1 and 93% were greater than 0.2 (Fig. 4B). Second, with few exceptions, the proteins in this study had long predicted half-lives according to the N-end rule (Fig. 4C). Third, low-abundance proteins with regulatory functions such as transcription factors or protein kinases were not identified.
Because the population of proteins used in this study appears to be fairly homogeneous with respect to predicted half-life and codon bias, it might be expected that the correlation of the mRNA and protein expression levels would be stronger for this population than for a random sample of yeast proteins. We tested this assumption by evaluating the correlation value if different subsets of the available data were included in the calculation. The 106 proteins were ranked from lowest to highest protein expression level, and the trend in the correlation value was evaluated by progressively including more of the higher-abundance proteins in the calculation (Fig. 6). The correlation value when only the lower-abundance 40 to 93 proteins were examined was consistently between 0.1 and 0.4. If the 11 most abundant proteins were included, the correlation steadily increased to 0.94. We therefore expect that the correlation for all yeast proteins or for a random selection would be less than 0.4. The observed level of correlation between mRNA and protein expression levels suggests the importance of posttranslational mechanisms controlling gene expression. Such mechanisms include translational control (15) and control of protein half-life (33). Since these mechanisms are also active in higher eukaryotic cells, we speculate that there is no predictive correlation between steady-state levels of mRNA and those of protein in mammalian cells.
Like other large-scale analyses, the present study has several potential sources of error related to the methods used to determine mRNA and protein expression levels. The mRNA levels were calculated from frequency tables of SAGE data. This method is highly quantitative because it is based on actual sequencing of unique tags from each gene, and the number of times that a tag is represented is proportional to the number of mRNA molecules for a specific gene. This method has some limitations including the following: (i) the magnitude of the error in the measurement of mRNA levels is inversely proportional to the mRNA levels, (ii) SAGE tags from highly similar genes may not be distinguished and therefore are summed, (iii) some SAGE tags are from sequences in the 3′ untranslated region of the transcript, (iv) incomplete cleavage at the SAGE tag site by the restriction enzyme can result in two tags representing one mRNA, and (v) some transcripts actually do not generate a SAGE tag (34, 35).
For the SAGE method, the error associated with a value increases with a decreasing number of transcripts per cell. The conclusions drawn from this study are dependent on the quality of the mRNA levels from previously published data (35). Since more than 65% of the mRNA levels included in this study were calculated to 10 copies/cell or less (40% were less than 4 copies/cell), the error associated with these values may be quite large. The mRNA levels were calculated from more than 20,000 transcripts. Assuming that the estimate of 15,000 mRNA molecules per cell is correct (16), this would mean that mRNA transcripts present at only a single copy per cell would be detected 72% of the time (35). The mRNA levels for each gene were carefully scrutinized, and only mRNA levels for which a high degree of confidence existed were included in the correlation value.
Protein abundance was determined by metabolic radiolabeling with [35S]methionine. The calculation required knowledge of three variables: the number of methionines in the mature protein, the radioactivity contained in the protein, and the specific activity of the radiolabel normalized per methionine. The number of methionines per protein was determined from the amino acid sequence of the proteins identified by tandem mass spectrometry. For some proteins, it was not known whether the methionine of the nascent polypeptide was processed away. The N termini of those proteins were predicted based on the specificity of methionine aminopeptidase (31). If the N-terminal processing did not conform to the predicted specificity of processing enzymes, the calculation of the number of methionines would be affected. This discrepancy would affect most the quantitation of a protein with a very low number of methionines. The average number of calculated methionines per protein in this study was 7.2. We therefore expect the potential for erroneous protein quantitation due to unusual N-terminal processing to be small.
The amount of radioactivity contained in a single spot might be the sum of the radioactivity of comigrating proteins. Because protein identification was based on tandem mass spectrometric techniques, comigrating proteins could be identified. However, comigrating proteins were rarely detected in this study, most likely because relatively small amounts of total protein (40 μg) were initially loaded onto the gels, which resulted in highly focused spots containing generally 1 to 25 ng of protein. Because of the relatively small amount loaded, the concentrations of any potentially comigrating protein would likely be below the limit of detection of the mass spectrometry technique used in this study (1 to 5 ng) and below the limit of visualization by silver staining (1 to 5 ng). In the overwhelming majority of the samples analyzed, numerous peptides from a single protein were detected. It is assumed that any comigrating proteins were at levels too low to be detected and that their influence in the calculation would be small.
The specific activity of the radiolabel was determined by relating the precise amount of protein present in selected spots of a parallel gel, as determined by quantitative amino acid composition analysis, to the number of methionines present in the sequence of those proteins and the radioactivity determined by liquid scintillation counting. It is possible that the resulting number might be influenced by unavoidable losses inherent in the amino acid analysis procedure applied. Because four different proteins were utilized in the calculation and the experiment was done in duplicate, the specific activity calculated is thought to be highly accurate. Indeed, the specific activities calculated for each of the four proteins varied by less than 10%. Any inconsistencies in the calculation of the specific activity would result in differences in the absolute levels calculated but not in the relative numbers and would therefore not influence the correlation value determined.
The protein quantitative method used eliminates a number of potential errors inherent in previous methods for the quantitation of proteins separated by 2DE, such as preferential protein staining and bias caused by inequalities in the number of radiolabeled residues per protein. Any 2D gel-based method of quantitation is complicated by the fact that in some cases the translation products of the same mRNA migrated to different spots. One major reason is posttranslational modification or processing of the protein. Also, artifactual proteolysis during cell lysis and sample preparation can lead to multiple resolved forms of the protein. In such cases, the protein levels of spots coded for by the same mRNA were pooled. In addition, the existence of other spots coded for by the same mRNA that were not analyzed by mass spectrometry or that were below the limit of detection for silver staining cannot be ruled out. However, since this study is based on a class of highly expressed proteins, the presence of undetected minor spots below silver staining sensitivity corresponding to a protein analyzed in the study would generally cause a relatively small error in protein quantitation.
Codon bias is a measure of the propensity of an organism to selectively utilize certain codons which result in the incorporation of the same amino acid residue in a growing polypeptide chain. There are 61 possible codons that code for 20 amino acids. The larger the codon bias value, the smaller the number of codons that are used to encode the protein (19). It is thought that codon bias is a measure of protein abundance because highly expressed proteins generally have large codon bias values (3, 13).
Nearly all of the most highly expressed proteins had codon bias values of greater than 0.8. However, we detected a number of genes with high codon bias and relative low protein abundance (Fig. 7). For example, the expressed gene with both the second largest protein and mRNA levels in the study was ENO2_YEAST (775,000 and 289.1 copies/cell, respectively). ENO1_YEAST was also present in the gel at much lower protein and mRNA levels (44,200 and 0.7 copies/cell, respectively). The codon bias values for ENO2 and ENO1 are similar (0.96 and 0.93, respectively), but the expression of the two genes is differentially regulated. Specifically, ENO1_YEAST is glucose repressed (6) and was therefore present in low abundance under the conditions used. Other genes with large codon bias values that were not of high protein abundance in the gel include EFT1, TIF1, HXK2, GSP1, EGD2, SHM2, and TAL1. We conclude that merely determining the codon bias of a gene is not sufficient to predict its protein expression level.
Interestingly, codon bias appears to be an excellent indicator of the boundaries of current 2D gel proteome analysis technology. There are thousands of genes with expressed mRNA and likely expressed protein with codon bias values less than 0.1 (Fig. 4A). In this study, we detected none of them, and only a very small percentage of the genes detected in this study had codon bias values between 0.1 and 0.2 (Fig. 4B). Indeed, in every examined yeast proteome study (5, 7, 13, 28) where the combined total number of identified proteins is 300 to 400, this same observation is true. It is expected that for the more complex cells of higher eukaryotic organisms the detection of low-abundance proteins would be even more challenging than for yeast. This indicates that highly abundant, long-lived proteins are overwhelmingly detected in proteome studies. If proteome analysis is to provide truly meaningful information about cellular processes, it must be able to penetrate to the level of regulatory proteins, including transcription factors and protein kinases. A promising approach is the use of narrow-range focusing gels with immobilized pH gradients (IPG) (23). This would allow for the loading of significantly more protein per pH unit covered and also provide increased resolution of proteins with similar electrophoretic mobilities. A standard pH gradient in an isoelectric focusing gel covers a 7-pH-unit range (pH 3 to 10) over 18 cm. A narrow-range focusing gel might expand the range to 0.5 pH units over 18 cm or more. This could potentially increase by more than 10-fold the number of proteins that can be detected. Clearly, current proteome technology is incapable of analyzing low-abundance regulatory proteins without employing an enrichment method for relatively low-abundance proteins. In conclusion, this study examined the relationship between yeast protein and message levels and revealed that transcript levels provide little predictive value with respect to the extent of protein expression.
ACKNOWLEDGMENTS
This work was supported by the National Science Foundation Science and Technology Center for Molecular Biotechnology, NIH grant T32HG00035-3, and a grant from Oxford Glycosciences.
We thank Jimmy Eng for expert computer programming, Garry Corthals and John R. Yates III for critical discussion, and Siavash Mohandesi for expert technical help.
REFERENCES
- 1.Aebersold R H, Leavitt J, Saavedra R A, Hood L E, Kent S B. Internal amino acid sequence analysis of proteins separated by one- or two-dimensional gel electrophoresis after in situ protease digestion on nitrocellulose. Proc Natl Acad Sci USA. 1987;84:6970–6974. doi: 10.1073/pnas.84.20.6970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Aebersold R H, Teplow D B, Hood L E, Kent S B. Electroblotting onto activated glass. High efficiency preparation of proteins from analytical sodium dodecyl sulfate-polyacrylamide gels for direct sequence analysis. Eur J Biochem. 1986;261:4229–4238. [PubMed] [Google Scholar]
- 3.Bennetzen J L, Hall B D. Codon selection in yeast. J Biol Chem. 1982;257:3026–3031. [PubMed] [Google Scholar]
- 4.Boucherie H, Dujardin G, Kermorgant M, Monribot C, Slonimski P, Perrot M. Two-dimensional protein map of Saccharomyces cerevisiae: construction of a gene-protein index. Yeast. 1995;11:601–613. doi: 10.1002/yea.320110702. [DOI] [PubMed] [Google Scholar]
- 5.Boucherie H, Sagliocco F, Joubert R, Maillet I, Labarre J, Perrot M. Two-dimensional gel protein database of Saccharomyces cerevisiae. Electrophoresis. 1996;17:1683–1699. doi: 10.1002/elps.1150171106. [DOI] [PubMed] [Google Scholar]
- 6.Carmen A A, Brindle P K, Park C S, Holland M J. Transcriptional regulation by an upstream repression sequence from the yeast enolase gene ENO1. Yeast. 1995;11:1031–1043. doi: 10.1002/yea.320111105. [DOI] [PubMed] [Google Scholar]
- 7.Ducret A, VanOostveen I, Eng J K, Yates J R, Aebersold R. High throughput protein characterization by automated reverse-phase chromatography/electrospray tandem mass spectrometry. Protein Sci. 1998;7:706–719. doi: 10.1002/pro.5560070320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Eng J, McCormack A, Yates J R. An approach to correlate tandem mass spectral data of peptides with amino acid sequences in a protein database. J Am Soc Mass Spectrom. 1994;5:976–989. doi: 10.1016/1044-0305(94)80016-2. [DOI] [PubMed] [Google Scholar]
- 9.Figeys D, Ducret A, Yates J R, Aebersold R. Protein identification by solid phase microextraction-capillary zone electrophoresis-microelectrospray-tandem mass spectrometry. Nat Biotechnol. 1996;14:1579–1583. doi: 10.1038/nbt1196-1579. [DOI] [PubMed] [Google Scholar]
- 10.Figeys D, VanOostveen I, Ducret A, Aebersold R. Protein identification by capillary zone electrophoresis/microelectrospray ionization-tandem mass spectrometry at the subfemtomole level. Anal Chem. 1996;68:1822–1828. doi: 10.1021/ac960191h. [DOI] [PubMed] [Google Scholar]
- 11.Fraser C M, Fleischmann R D. Strategies for whole microbial genome sequencing and analysis. Electrophoresis. 1997;18:1207–1216. doi: 10.1002/elps.1150180803. [DOI] [PubMed] [Google Scholar]
- 12.Garrels J I, Futcher B, Kobayashi R, Latter G I, Schwender B, Volpe T, Warner J R, McLaughlin C S. Protein identifications for a Saccharomyces cerevisiae protein database. Electrophoresis. 1994;15:1466–1486. doi: 10.1002/elps.11501501210. [DOI] [PubMed] [Google Scholar]
- 13.Garrels J I, McLaughlin C S, Warner J R, Futcher B, Latter G I, Kobayashi R, Schwender B, Volpe T, Anderson D S, Mesquita-Fuentes F, Payne W E. Proteome studies of Saccharomyces cerevisiae: identification and characterization of abundant proteins. Electrophoresis. 1997;18:1347–1360. doi: 10.1002/elps.1150180810. [DOI] [PubMed] [Google Scholar]
- 14.Gygi S P, Aebersold R. Absolute quantitation of 2-DE protein spots. In: Link A J, editor. 2-D protocols for proteome analysis. Totowa, N.J: Humana Press; 1998. pp. 417–421. [DOI] [PubMed] [Google Scholar]
- 15.Harford J B, Morris D R. Post-transcriptional gene regulation. New York, N.Y: Wiley-Liss, Inc.; 1997. [Google Scholar]
- 16.Hereford L M, Rosbash M. Number and distribution of polyadenylated RNA sequences in yeast. Cell. 1977;10:453–462. doi: 10.1016/0092-8674(77)90032-0. [DOI] [PubMed] [Google Scholar]
- 17.Hodges P E, Payne W E, Garrels J I. The Yeast Protein Database (YPD): a curated proteome database for Saccharomyces cerevisiae. Nucleic Acids Res. 1998;26:68–72. doi: 10.1093/nar/26.1.68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Klose J, Kobalz U. Two-dimensional electrophoresis of proteins: an updated protocol and implications for a functional analysis of the genome. Electrophoresis. 1995;16:1034–1059. doi: 10.1002/elps.11501601175. [DOI] [PubMed] [Google Scholar]
- 19.Kurland C G. Codon bias and gene expression. FEBS Lett. 1991;285:165–169. doi: 10.1016/0014-5793(91)80797-7. [DOI] [PubMed] [Google Scholar]
- 20.Lashkari D A, DeRisi J L, McCusker J H, Namath A F, Gentile C, Hwang S Y, Brown P O, Davis R W. Yeast microarrays for genome wide parallel genetic and gene expression analysis. Proc Natl Acad Sci USA. 1997;94:13057–13062. doi: 10.1073/pnas.94.24.13057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Liang P, Pardee A B. Differential display of eukaryotic messenger RNA by means of the polymerase chain reaction. Science. 1992;257:967–971. doi: 10.1126/science.1354393. [DOI] [PubMed] [Google Scholar]
- 22.Link A J, Hays L G, Carmack E B, Yates J R., III Identifying the major proteome components of Haemophilus influenzae type-strain NCTC 8143. Electrophoresis. 1997;18:1314–1334. doi: 10.1002/elps.1150180808. [DOI] [PubMed] [Google Scholar]
- 23.Nawrocki A, Larsen M R, Podtelejnikov A V, Jensen O N, Mann M, Roepstorff P, Gorg A, Fey S J, Larsen P M. Correlation of acidic and basic carrier ampholyte and immobilized pH gradient two-dimensional gel electrophoresis patterns based on mass spectrometric protein identification. Electrophoresis. 1998;19:1024–1035. doi: 10.1002/elps.1150190618. [DOI] [PubMed] [Google Scholar]
- 24.O’Farrell P H. High resolution two-dimensional electrophoresis of proteins. J Biol Chem. 1975;250:4007–4021. [PMC free article] [PubMed] [Google Scholar]
- 24a.OWL Protein Sequence Database. 2 August 1998, posting date. [Online.] http://bmbsgi11.leeds.ac.uk/bmb5dp/owl.html. [8 January 1999, last date accessed.]
- 25.Patterson S D, Aebersold R. Mass spectrometric approaches for the identification of gel-separated proteins. Electrophoresis. 1995;16:1791–1814. doi: 10.1002/elps.11501601299. [DOI] [PubMed] [Google Scholar]
- 26.Pennington S R, Wilkins M R, Hochstrasser D F, Dunn M J. Proteome analysis: from protein characterization to biological function. Trends Cell Biol. 1997;7:168–173. doi: 10.1016/S0962-8924(97)01033-7. [DOI] [PubMed] [Google Scholar]
- 27.Shalon D, Smith S J, Brown P O. A DNA microarray system for analyzing complex DNA samples using two-color fluorescent probe hybridization. Genome Res. 1996;6:639–645. doi: 10.1101/gr.6.7.639. [DOI] [PubMed] [Google Scholar]
- 28.Shevchenko A, Jensen O N, Podtelejnikov A V, Sagliocco F, Wilm M, Vorm O, Mortensen P, Boucherie H, Mann M. Linking genome and proteome by mass spectrometry: large-scale identification of yeast proteins from two dimensional gels. Proc Natl Acad Sci USA. 1996;93:14440–14445. doi: 10.1073/pnas.93.25.14440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Shevchenko A, Wilm M, Vorm O, Mann M. Mass spectrometric sequencing of proteins from silver-stained polyacrylamide gels. Anal Chem. 1996;68:850–858. doi: 10.1021/ac950914h. [DOI] [PubMed] [Google Scholar]
- 30.Sikorski R S, Hieter P. A system of shuttle vectors and yeast host strains designed for efficient manipulation of DNA in Saccharomyces cerevisiae. Genetics. 1989;122:19–27. doi: 10.1093/genetics/122.1.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tsunasawa S, Stewart J W, Sherman F. Amino-terminal processing of mutant forms of yeast iso-1-cytochrome c. The specificities of methionine aminopeptidase and acetyltransferase. J Biol Chem. 1985;260:5382–5391. [PubMed] [Google Scholar]
- 32.Urlinger S, Kuchler K, Meyer T H, Uebel S, Tamp’e R. Intracellular location, complex formation, and function of the transporter associated with antigen processing in yeast. Eur J Biochem. 1997;245:266–272. doi: 10.1111/j.1432-1033.1997.00266.x. [DOI] [PubMed] [Google Scholar]
- 33.Varshavsky A. The N-end rule: functions, mysteries, uses. Proc Natl Acad Sci USA. 1996;93:12142–12149. doi: 10.1073/pnas.93.22.12142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Velculescu V E, Zhang L, Vogelstein B, Kinzler K W. Serial analysis of gene expression. Science. 1995;270:484–487. doi: 10.1126/science.270.5235.484. [DOI] [PubMed] [Google Scholar]
- 35.Velculescu V E, Zhang L, Zhou W, Vogelstein J, Basrai M A, Bassett D E, Jr, Hieter P, Vogelstein B, Kinzler K W. Characterization of the yeast transcriptome. Cell. 1997;88:243–251. doi: 10.1016/s0092-8674(00)81845-0. [DOI] [PubMed] [Google Scholar]
- 36.Wilkins M R, Williams K L, Appel R D, Hochstrasser D F. Proteome research: new frontiers in functional genomics. Berlin, Germany: Springer-Verlag; 1997. [Google Scholar]
- 37.Wilm M, Shevchenko A, Houthaeve T, Breit S, Schweigerer L, Fotsis T, Mann M. Femtomole sequencing of proteins from polyacrylamide gels by nano-electrospray mass spectrometry. Nature. 1996;379:466–469. doi: 10.1038/379466a0. [DOI] [PubMed] [Google Scholar]
- 38.Yan J X, Wilkins M R, Ou K, Gooley A A, Williams K L, Sanchez J C, Golaz O, Pasquali C, Hochstrasser D F. Large-scale amino-acid analysis for proteome studies. J Chromatogr A. 1996;736:291–302. doi: 10.1016/0021-9673(95)01285-0. [DOI] [PubMed] [Google Scholar]
- 39.YPD Website. 6 March 1998, revision date. [Online.] Proteome, Inc. http://www.proteome.com/YPDhome.html. [8 January 1999, last date accessed.]