Abstract
Ensuring reliable supply of services from nature is key to the sustainable development and well-being of human societies. Varied and frequently complex relationships between biodiversity and ecosystem services have, however, frustrated our capacity to quantify and predict the vulnerability of those services to species extinctions. Here, we use a qualitative Boolean modelling framework to identify universal drivers of the robustness of ecosystem service supply to species loss. These drivers comprise simple features of the networks that link species to the functions they perform that, in turn, underpin a service. Together, they define what we call network fragility. Using data from >250 real ecological networks representing services such as pollination and seed-dispersal, we demonstrate that network fragility predicts remarkably well the robustness of empirical ecosystem services. We then show how to quantify contributions of individual species to ecosystem service robustness, enabling quantification of how vulnerability scales from species to services. Our findings provide general insights into the way species, functional traits, and the links between them together determine the vulnerability of ecosystem service supply to biodiversity loss.
Subject terms: Ecosystem ecology, Theoretical ecology, Ecosystem services
Responses of ecosystem services to species losses are highly context-dependent. Here, the authors develop a model to identify general rules in the robustness of ecosystem service supply to species losses, and demonstrate its applicability using real-world ecosystem service networks.
Introduction
Ecosystem services—ecological structures, functions or processes that directly or indirectly contribute to human wellbeing1—provide a plethora of benefits to humanity2,3. Through current global environmental change, humans are driving species to extinction at rates hundreds to thousands of times in excess of background4–6. Such extinctions compromise the capacity of ecosystems to reliably provide the goods and services upon which human societies depend7–13. The mechanisms through which ecosystem services collapse—that is, when they are either no longer being supplied or have declined to the point that they are no longer utilised by people (that is, are no longer in demand)—remain elusive, however, and vary with both ecological and social context14–20. Consequently, we have only a rudimentary understanding of the vulnerability of many, perhaps all, ecosystem services to species loss8,12,21–23. Certainly, different services vary in their vulnerability24, but we lack general rules that might explain these differences.
Here, we develop and test a simple model to measure and predict the robustness of ecosystem service supply to species loss. We take a purely qualitative perspective, where species are either present or absent and an ecosystem service is provided or not (Figs. 1a and 2a). We focus on the implications of species removal and aim to understand how functional redundancy and species richness combine to buffer ecological processes against biodiversity loss25–27. We limit our scope to the supply of ecosystem services and do not address here the extent or dynamics of human demand for those services. On the other hand, our approach could be applied to any emergent, high-level ecosystem process, not necessarily considered (or recognised) as a service.
Fig. 1. The principles of our approach to measuring the robustness of ecosystem services to species loss.
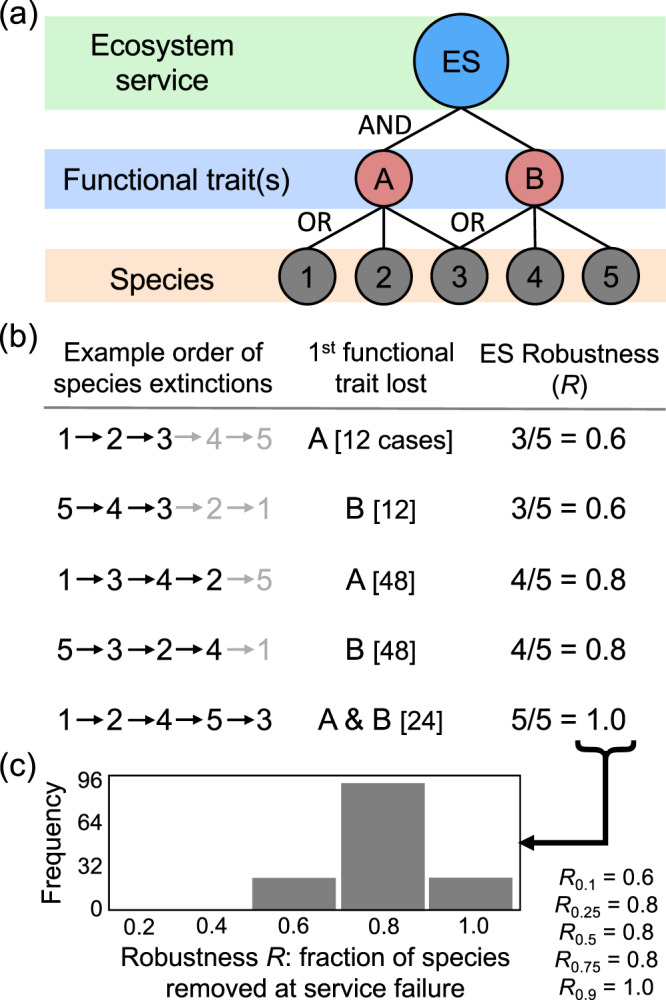
a We use a qualitative framework (see ‘Methods’ for a detailed description) whereby species [1–5 in this example] are linked to their functional traits [A and B] and are either present or absent from a community. An ecosystem service [ES] is supplied only when all underpinning functional traits are present in the community (the Boolean function AND; see ‘Methods’ and Supplementary Methods for justification). b As species are removed sequentially from the system, a functional trait is present until all species connected to that trait are lost. In this way, species losses are tolerated (the Boolean function OR) until a unique functional trait has been lost. ‘Example order of species extinctions’ presents illustrative examples of the sequential removal of the species in a, with sequences in grey indicating extinctions after service failure. ‘1st functional trait lost’ is the identity of the unique functional trait [A and/or B] first lost from the system—which, under our framework, necessarily causes service loss—with [n cases] indicating the number of extinction sequences for which this result is obtained [reflected also in the histogram (c)]. We measure ‘ES Robustness (R)’ as the fraction of species removals required to bring about loss of a service for each extinction sequence. c This results in a distribution of robustness R values, representing the fraction of species loss tolerated across all extinction scenarios. This distribution of R values can be described via its percentiles [denoted with subscript c] with, for example, the median robustness [R0.5] here equating to 0.8. That is, in 50% of extinction scenarios in this hypothetical example, 4/5 or fewer species extinctions are required to cause service failure.
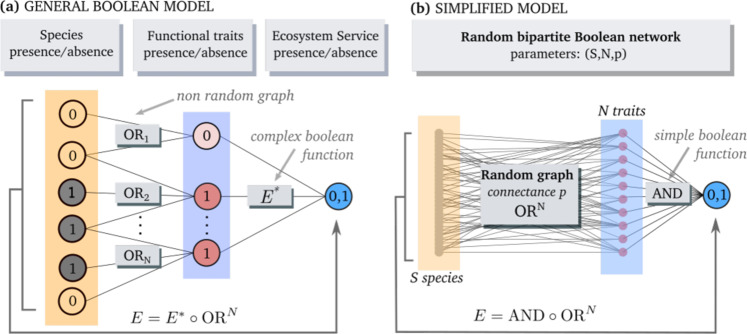
Fig. 2. A Boolean model for ecosystem services.
a We view an ecosystem service as a Boolean function E over the presence/absence configurations of S species (leftmost column). We distinguish species from their N functional traits (middle column). The configuration of any trait n is defined by the logical OR function over the Sn species that share that trait. This amounts to writing E as the composition , which should aim to remove as much functional redundancy as possible from the auxiliary function over trait configurations. b We simplify our model further by (i) considering random species-to-trait associations with connectance p and (ii) choosing the logical AND function for [that is, the least redundant function, which we show (Supplementary Methods) is representative of a random choice across the space of all Boolean functions]. In this model (see ‘Methods’ for full description), we can deduce analytical expressions for the percentiles of the distribution (considering all possible extinction sequences) of robustness of ecosystem service supply R: the fraction of species loss leading to loss of service supply.
We use Boolean functions to model a robust–vulnerable continuum28 of ecosystem services (Fig. 2). At one extreme is the logical AND function, where every species is essential to the supply of an ecosystem service. In this case, even the loss of a single species will result in service failure, owing to a complete lack of functional redundancy29. At the other extreme, all species are fully substitutable and only a single species is then needed to supply a service. In this case, the ecosystem service equates to the logical OR function, where full redundancy among species promotes robust service supply even in the face of multiple species extinctions25,29,30.
For a given ecosystem service, rather than consider a particular model of extinction scenarios, we instead consider all possible extinction sequences simultaneously (Fig. 1b). We study the entire distribution of robustness, defined as the fraction of extinctions—along all extinction scenarios—sufficient to bring about the loss of supply of the ecosystem service (Fig. 1c). Services that can, along most extinction sequences, tolerate a high number of extinctions before they collapse are the most robust. We assume that the supply of a service requires species that have particular traits—phenotypic attributes that direct niche exploitation—whose presence drive all of the various ecological processes that are necessary to underpin provision of the service17,31. Though many traits, such as body mass, occur along a continuous spectrum31–33, for simplicity we focus here only on whether or not a given species possesses a particular trait34. We consider traits purely in a functional sense29, understood here as the capacity of a species to perform a particular functional role required for the ecosystem service to be provided34. As such, traits are functional features that can be shared among species, as can be visualised by drawing, for a given service, a bipartite network linking species to traits19 (Figs. 1a and 2, but note that we could, in principle, refine the notion of traits by decomposing species into populations and differentiating traits within species).
Under these simplifying assumptions35, we reveal the universal drivers of the robustness distribution of any ecosystem service to species loss (see ‘Methods’). We show that any percentile of the robustness distribution is driven by a synthetic parameter that we call network fragility. Network fragility combines simple features of the species-to-traits bipartite network—the numbers of species, functional traits and links between them—that together determine the fraction of species loss that can be tolerated before ecosystem service failure. We then go on to show how our results, based on properties of random bipartite networks, can be applied to non-random ecological networks representing real-world ecosystem services, such as plant pollination and seed dispersal.
Results
In our framework, an ecosystem service E consists of N required functional traits shared between S species, with p characterising the connectance of the species-to-traits network (Fig. 2). We note R(E), the distribution of robustness, that is, the distribution of the fraction of species loss along all extinction sequences that lead to ecosystem service failure (Fig. 1c). For random species-to-traits networks, we find that the cth percentile Rc(E) of R—the value Rc such that a fraction c of extinction sequences leads to service failure when losing less than S × Rc species (Fig. 1c)—takes the analytic form (if q = 1 − p; see Supplementary Methods)
| 1 |
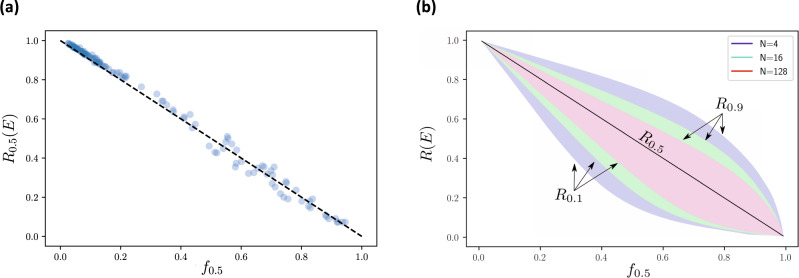
The statistics of R(E) are, therefore, driven entirely by a family of parameter values (fc), comprising measures of what we call network fragility. In particular, the median value for robustness of ecosystem service supply corresponds to f0.5 (Fig. 3a). The width of the distribution of R(E) is maximal at intermediate values of f0.5 and decreases as the number of traits N increases (Fig. 3b). This means that the robustness of ecosystem service supply to species loss is at its most predictable at both low and high values of network fragility and when the service is underpinned by many functional traits.
Fig. 3. Universal behaviour of the robustness of ecosystem service supply as a function of network fragility.
a For 125 random ecosystem services of the form shown in Fig. 2, we uniformly sampled 5 values for species richness S between 10 and 200, 20 for functional traits N between 10 and 100 and 10 for connectance p between 0 and 0.5 and for each considered 600 extinction scenarios leading to a loss of service, drawn uniformly over the set of all possible extinction sequences. We see that the median robustness of ecosystem service supply (that is, R0.5(E)) in our simplified Boolean model behaves as a simple decreasing linear function of median network fragility (f0.5). b For three values of number of traits (that is, N = 4, 16 and 128) and random values of number of species S and connectance p, we used the analytical formula in Eq. (1) to draw the contours of the bulk of the robustness distribution (that is, 10th–90th percentiles) as a function of f0.5, the value of fragility associated with median robustness. We see that, at fixed N, the percentiles can be expressed as functions of f0.5, with the largest spread at intermediate values of network fragility. At fixed fragility, increasing the number of traits N reduces the width of the distribution.
Next, we examine whether the one-to-one relationship between network fragility and the statistics of robustness of ecosystem service supply is universal, that is, valid beyond random species-to-traits association networks. To do this, we considered 251 real empirical bipartite networks from the Web of Life ecological networks database, representing a variety of ecosystem services, including, for example, pollination and seed dispersal (see ‘Methods’). We measured network fragility for each network directly from the numbers of species, traits and links it comprises. To measure the robustness of ecosystem service supply, we simulated random extinction sequences leading to the loss of at least one functional trait [for example, the loss of pollination of at least one plant species following pollinator species extinctions (see ‘Methods’ and Supplementary Methods)] and replicated this process 1000 times to give a numerical estimate of the distribution of R for each service represented by each empirical network. In addition, we computed the variance in the number of species per trait, (Sn), which we compared to a null expectation, 0(Sn), if the species-to-traits network were random [for a random network with connectance p, 0(Sn) = Spq]. We then defined dispersion d as
| 2 |
so that log10(d) gives a measure of deviation from randomness, vanishing if the observed variance is that of a random network, is negative if the variance is smaller and positive if it is larger.
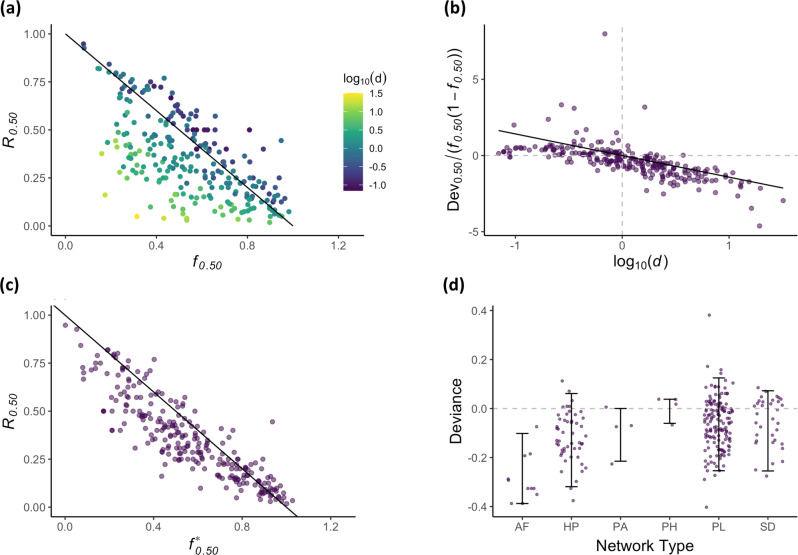
We found that network fragility explains well the robustness of ecosystem services in the empirical networks we examined (Spearman = −0.64 for c = 0.5; Fig. 4a). However, overdispersion [that is, log10(d) > 0] led systematically to lower robustness of ecosystem service supply than predicted, whereas underdispersion [log10(d) < 0] had the opposite effect. Correcting network fragility () to then account for the deviation from randomness of the species-to-trait network (Fig. 4b), where
| 3 |
Fig. 4. Robustness of ecosystem service supply and network fragility in empirical networks.
a The relationship between simulated robustness of ecosystem service supply (Rc = R0.5) and analytically estimated network fragility (fc = f0.5) for 251 empirical networks from the Web of Life follows the linear approximation Rc = 1 − fc. Colour indicates network dispersion [log10(d)]. b The residuals off the linear prediction Rc = 1 − fc correlate with log10(d), which we model as (Rc − 1 + fc)/(fc(1 − fc)) = log10(d), where, for the case of R0.5, = −1.42. c Correcting network fragility for dispersion according to log10(d) yields an even closer relationship with robustness of ecosystem service supply. d Residuals from R0.5 − by network type show that our model captures well the universal properties, where AF = anemone–fish (n = 10), HP = host–parasite (n = 51), PA = plant–ant (n = 4), PH = plant–herbivore (n = 4), PL = pollination (n = 148), and SD = seed dispersal (n = 34). Error bars show the 5th and 95th quantiles of the data.
improved the relationship with robustness of ecosystem service supply considerably (Spearman = –0.89 for c = 0.5; Fig. 4c) and captures well its universal properties (Fig. 4c, d and Supplementary Fig. 1). Moreover, we confirmed that uncertainty in ecosystem service robustness in the empirical networks was greatest at intermediate values of network fragility (Supplementary Fig. 2), as predicted by our analytical model (Fig. 3b). Variance among the different types of empirical networks in our analysis (Fig. 4d) suggests that there may be further nuances in the structure of those networks that contribute to their robustness to species loss beyond the universal characteristics we describe. However, these unknown drivers play a minor role in comparison to network fragility.
Whereas the distribution of robustness reflects the complex topology of species presence–absence configurations over which a given ecosystem service is supplied, network fragility can be evaluated directly from the most basic features of species-to-traits associations—the numbers of species, functional traits that underpin the service and links between them. This local nature of network fragility therefore allows us to study its behaviour under any specific species extinction scenario. This crucial property allows us to quantify the contribution of any given species or combination of species to the robustness of ecosystem service supply. We can, for example, ask how much network fragility would increase if species i were lost? The loss of a species with Li traits will obviously reduce richness , but it will also affect connectance as
| 4 |
Naturally, the species with the most links will reduce connectance, and therefore increase network fragility, the most. Species loss may, however, also affect dispersion d via its effect on the variance in the number of species per trait (Sn). If we define , the mean number of species that have the same traits as species i, and = L/N the mean number of species per trait, we get that (approximately)
| 5 |
Thus, dispersion (and, therefore, network fragility) increases the most if is small compared to , that is, if the traits of species i are uncommon. It is, therefore, those species that are generalist providers of multiple uncommon traits that contribute the most to the robustness of ecosystem service supply. This is an intuitive statement, but our theory makes it a quantitative measure, integrating precisely the relevant features of the service to which a given species contributes.
Discussion
Our model reveals how species richness and trait generality combine to determine the robustness of ecosystem service supply to the loss of species. Moreover, by also enabling quantification of the contribution of individual species to the robustness of ecosystem service supply, the model comprises a general tool for predicting and managing the vulnerability of ecosystem service provision (or, indeed, any ecological process) in the face of rapid and ongoing global environmental change.
Our theory predicts that extinction scenarios targeting species that possess multiple uncommon traits will likely lead to more rapid collapse of ecosystem service supply. That is, a species with unique links in a bipartite species-to-traits network is more likely to contribute substantially to robustness36. As a network transitions via sequential extinctions from a starting point of high robustness, it will, however, pass through a window at intermediate network fragility where uncertainty in robustness is maximal. Thus, increasing network fragility via loss of species (or loss of their functional roles) not only reduces the robustness of service supply but also increases the uncertainty surrounding predictions of ecosystem service loss in the face of uncertain species extinction scenarios.
The theory we present comprises a highly simplified representation of nature—our model assumes that ecosystem services are either provided or not and that the loss of a single unique functional trait causes ecosystem service provision to cease. The latter assumption may seem to be overly conservative, yet we show (Supplementary Methods) that it captures well the robustness of randomly generated services. In addition, we did not consider either species abundances—which did not stabilise crop pollination services in a previous study30—or intraspecific trait variation, which often supports ecosystem service supply37. Such detailed knowledge could, however, easily be incorporated in our model by replacing species with populations or even individuals. The resulting network representation of the service would then become highly complex19. Importantly, however, our notion of network fragility would still apply and, because of its simplicity, would remain tractable. All in all, our abstractions of natural systems enabled us to gain general insights into biodiversity–ecosystem services relationships, taking a significant step forward in our understanding of the stability of ecosystem service provision in the face of widespread biodiversity loss.
Though biodiversity is foundational to the sustained supply of ecosystem services, ecological factors alone do not determine their overall vulnerability38. Ecosystem services are linked intrinsically to human valuation1,2,35, and shifts in their value can play an overarching and even dominant role39,40. The overall vulnerability of ecosystem services may, therefore, depend on the stability of human values to an even greater extent than on biodiversity. Models linking the ecological and social aspects of ecosystem services, and the interactions between them, are, therefore, fundamental to understanding how ecosystem services will respond to future changes7,19,20,40,41. This delineates a challenge for future research: how can we best integrate the robustness of ecosystem service supply with the various drivers of human valuation and demand? Our modelling framework serves as a stepping stone in that direction.
By enabling identification of the key species that contribute to the robustness of ecosystem service supply, our theory provides a focus for management efforts that require consideration of the conservation value of individual species8,10,32,34,36,42–44. Focussing on the contribution of individual species to robustness and the vulnerability of those species to extinction through their life histories, interactions and functional traits24,31,45,46 will enable empirical quantification of how vulnerability scales from species through to ecosystem services11,12,15,18,43. Ultimately, such a shift will provide a far richer and mechanistic understanding of the sustainability of nature’s contributions to people under global environmental change.
Methods
A Boolean model for the robustness of ecosystem service supply
We view the supply of an ecosystem service as a Boolean function E over the presence/absence configurations of S species
| 6 |
We consider here only the presence and absence of species, though one could replace species with individuals to explore population-level contexts. Ecosystem service provision is also considered as binary—a service is either provided or it is not. Though these assumptions clearly simplify reality35, they nonetheless provide the foundation from which general insights applicable to real ecosystems can be developed, as we will show.
We are interested in the robustness R(E) of supply of a given ecosystem service E. That is, the distribution—considering all possible extinction sequences—of the fraction of species extinctions that leads to loss of ecosystem service supply. Robustness is maximal and reduced to a single number if the service is lost only when all species are extinct [R(E) ≡ 1], and minimal and reduced to a single number if any species extinction is sufficient to cause service failure [R(E) ≡ 1/S]. More formally, if we consider all extinction scenarios, R is a distribution over the range . This distribution encodes the complex topology of species presence–absence configurations over which an ecosystem service is supplied. Considering the proportion of extinctions (rather than the absolute number) that result in service failure allows us to compare across systems of different species richness. It also leads to a notion of ecosystem service robustness that relates directly to the expected time to service loss, given a mean species extinction rate (Supplementary Methods).
Next, we distinguish species from their N potential functional traits that relate to the ecosystem service of interest. We model functional redundancy by determining the configuration of trait n with the Boolean function OR over the configuration space of the Sn species that share this trait (Fig. 2a). Thus, a trait is present until all species that have this trait are extinct. If we denote ORn as the extension of the OR function over the Sn species that share a given trait to the whole species configuration space (that is, a partial OR function), we have a mapping from S species configurations to N traits configurations:
| 7 |
The state of the ecosystem service E is then determined by an auxiliary Boolean function over trait configurations. In other words, we factorise E as the composition
| 8 |
This factorisation is always possible, since by choosing N = S we could always make ORN into a trivial mapping where each species has a unique trait and take . To be useful, however, this factorisation should aim at having the lowest number of distinct functional traits relative to the service, thus removing as much functional redundancy as possible from the auxiliary function .
To derive generic drivers of the robustness of ecosystem service supply, we need to define a universe of ecosystem services rich enough to be representative of real ecosystem services, yet simple enough to permit an analytical treatment of their robustness. Based on our factorisation [Eq. (8)], there are two parts to the problem, and we make simplifying assumptions for both. We start by defining a universe of species-to-traits mapping. A simplifying choice is to assume the species-to-traits network to be a random graph with connectance p. We then need a model for the auxiliary function . A natural choice, given our treatment of the species-to-trait association, would be to pick at random and uniformly over the set of all possible Boolean functions over trait configurations. However, we can make a much simpler choice at a low cost in behavioural complexity. In terms of robustness of ecosystem service supply, we find (by drawing the auxiliary function at random uniformly over the set of all Boolean functions of {0,1}N; Supplementary Methods) that assuming the AND function (that is, the least redundant function) for is representative of the outcome of a random choice. Thus, we now have, for any choice of S, N and p, a random set (the random component is in the species-to-trait graph, Fig. 2b) of ecosystem services of the form
| 9 |
This then allows us to derive an analytic form for the robustness of ecosystem service supply (Supplementary Methods).
Analysis of empirical networks
For our analysis of empirical networks, we downloaded all 258 available bipartite webs from the Web of Life ecological networks data set (http://www.web-of-life.es), which comprised anemone–fish (n = 17), host–parasite (n = 51), plant–ant (n = 4), plant–pollinator (n = 148) and seed–disperser (n = 34) networks. We removed three networks that comprised only a single trait (that is, where all species connected to the same single interaction partner) and four that showed no variation in the number of species per trait (of which the latter were all anemone–fish webs), leaving 251 networks for analysis. We converted the count-based association matrix to binary so that connections between species and traits were either present or absent. In these networks, the ‘traits‘ are also species. For example, we view pollinators as the species that provide for the presence of a plant13, which is a web-level trait. These web-level traits then underpin the provision of the overall pollination service, which we consider here to be the pollination of all plants in the network. That is, in order for there to be an arbitrarily defined pollination service for this ecosystem47, we demand that all plants (traits) are present so that, if one plant is lost, then the pollination service is lost. In contrast, the pollinators themselves are compensable for a given plant (trait) and so species loss is tolerated if there remains another species to pollinate that plant48. Similarly, we have chosen to view the networks as trait–species such that seed–disperser refers to plant species as the trait and disperser as the species that provides for that trait. That these networks really are or are not ecosystem services, or whether some species would go extinct together due to interactions15, is not relevant for our purpose. We simply use these empirical networks as a rich set of non-random example networks on which to showcase the relevance of our theory beyond random networks.
For each of the binary bipartite webs, we calculated network fragility and dispersion-corrected network fragility using, respectively, Eqs. (1) and (3). Connectance p was estimated as L(S × N), where L is the number of links. The distribution of robustness of ecosystem service supply R was then determined by simulation, whereby species were selected at random and removed sequentially until a trait (and hence the service; Supplementary Methods), was lost and R was then quantified as the proportion of species lost until service collapse (Fig. 1). By simulating sequential extinctions and measuring robustness as a distribution in this way, our model encompasses all possible combinations of extinction orders, which is critical given the non-random nature of species extinctions in real ecosystems49–51.
Reporting summary
Further information on research design is available in the Nature Research Reporting Summary linked to this article.
Supplementary information
Acknowledgements
We thank Owen Petchey, Andy Purvis and various members of the School of Natural Sciences at Trinity College Dublin, particularly Ruth Kelly and Yvonne Buckley, for discussions and their input during the development of the project. We also thank Jordi Bascompte for developing and maintaining the Web of Life Database. S.R.P.-J.R. was supported by a Trinity College Dublin Ussher Scholarship and an Irish Research Council Postgraduate Scholarship (GOIPG/2018/3023). A.L.J. and J.-F.A. were funded by Irish Research Council Laureate Award IRCLA/2017/186 to A.L.J. M.L. and J.-F.A. were supported by the TULIP Laboratory of Excellence (ANR-10-LABX-41). C.D.W. was supported by a Postgraduate Award from Trinity College Dublin.
Author contributions
S.R.P.-J.R. and I.D. led the project; J.-F.A. and A.L.J. developed the models; S.R.P.-J.R., J.-F.A., M.L., C.D.W., J.C.R., A.L.J. and I.D. developed the ideas, wrote the manuscript and contributed critically to drafts.
Data availability
The species–traits network data generated in this study have been deposited in Zenodo under accession code 10.5281/zenodo.4749405 (ref. 52). The empirical network data are publicly available from the Web of Life ecological networks database (http://www.web-of-life.es).
Code availability
Simulations and data analyses were performed in R. All code necessary to reproduce the results are publicly accessible from Zenodo: 10.5281/zenodo.4749405 (ref. 52).
Competing interests
The authors declare no competing interests.
Footnotes
Peer review informationNature Communications thanks Anna Eklöf and the other anonymous reviewer(s) for their contribution to the peer review of this work.
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
These authors contributed equally: Samuel R. P.-J. Ross, Jean-François Arnoldi.
Supplementary information
The online version contains supplementary material available at 10.1038/s41467-021-25507-5.
References
- 1.Costanza R, et al. Twenty years of ecosystem services: how far have we come and how far do we still need to go? Ecosyst. Serv. 2017;28:1–16. doi: 10.1016/j.ecoser.2017.09.008. [DOI] [Google Scholar]
- 2.Costanza R, et al. The value of the world’s ecosystem services and natural capital. Nature. 1997;387:253–260. doi: 10.1038/387253a0. [DOI] [Google Scholar]
- 3.Mace GM, Norris K, Fitter AH. Biodiversity and ecosystem services: a multilayered relationship. Trends Ecol. Evol. 2012;27:19–26. doi: 10.1016/j.tree.2011.08.006. [DOI] [PubMed] [Google Scholar]
- 4.Pimm SL, et al. The biodiversity of species and their rates of extinction, distribution, and protection. Science. 2014;344:1246752. doi: 10.1126/science.1246752. [DOI] [PubMed] [Google Scholar]
- 5.De Vos JM, Joppa LN, Gittleman JL, Stephens PR, Pimm SL. Estimating the normal background rate of species extinction. Conserv. Biol. 2015;29:452–462. doi: 10.1111/cobi.12380. [DOI] [PubMed] [Google Scholar]
- 6.Humphreys AM, Govaerts R, Ficinski SZ, Nic Lughadha E, Vorontsova MS. Global dataset shows geography and life form predict modern plant extinction and rediscovery. Nat. Ecol. Evol. 2019;3:1043–1047. doi: 10.1038/s41559-019-0906-2. [DOI] [PubMed] [Google Scholar]
- 7.Hooper DU, et al. Effects of biodiversity on ecosystem functioning: a consensus of current knowledge. Ecol. Monogr. 2005;75:3–35. doi: 10.1890/04-0922. [DOI] [Google Scholar]
- 8.Worm B, et al. Impacts of biodiversity loss on ocean ecosystem services. Science. 2006;314:787–790. doi: 10.1126/science.1132294. [DOI] [PubMed] [Google Scholar]
- 9.Cardinale BJ, et al. Biodiversity loss and its impact on humanity. Nature. 2012;486:59–67. doi: 10.1038/nature11148. [DOI] [PubMed] [Google Scholar]
- 10.Isbell F, Tilman D, Polasky S, Loreau M. The biodiversity-dependent ecosystem service debt. Ecol. Lett. 2015;18:119–134. doi: 10.1111/ele.12393. [DOI] [PubMed] [Google Scholar]
- 11.Oliver TH, et al. Declining resilience of ecosystem functions under biodiversity loss. Nat. Commun. 2015;6:10122. doi: 10.1038/ncomms10122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Smale DA, et al. Marine heatwaves threaten global biodiversity and the provision of ecosystem services. Nat. Clim. Change. 2019;9:306–312. doi: 10.1038/s41558-019-0412-1. [DOI] [Google Scholar]
- 13.Reilly JR, et al. Crop production in the USA is frequently limited by a lack of pollinators. Proc. R. Soc. B. 2020;287:20200922. doi: 10.1098/rspb.2020.0922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tscharntke T, Klein AM, Kruess A, Steffan-Dewenter I, Thies C. Landscape perspectives on agricultural intensification and biodiversity-ecosystem service management. Ecol. Lett. 2005;8:857–874. doi: 10.1111/j.1461-0248.2005.00782.x. [DOI] [Google Scholar]
- 15.Kaiser‐Bunbury CN, Muff S, Memmott J, Müller CB, Caflisch A. The robustness of pollination networks to the loss of species and interactions: a quantitative approach incorporating pollinator behaviour. Ecol. Lett. 2010;13:442–452. doi: 10.1111/j.1461-0248.2009.01437.x. [DOI] [PubMed] [Google Scholar]
- 16.Hautier Y, et al. Eutrophication weakens stabilizing effects of diversity in natural grasslands. Nature. 2014;508:521–525. doi: 10.1038/nature13014. [DOI] [PubMed] [Google Scholar]
- 17.Duncan C, Thompson JR, Pettorelli N. The quest for a mechanistic understanding of biodiversity–ecosystem services relationships. Proc. R. Soc. B. 2015;282:20151348. doi: 10.1098/rspb.2015.1348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Oliver TH, et al. Biodiversity and resilience of ecosystem functions. Trends Ecol. Evol. 2015;30:673–684. doi: 10.1016/j.tree.2015.08.009. [DOI] [PubMed] [Google Scholar]
- 19.Dee LE, et al. Operationalizing network theory for ecosystem service assessments. Trends Ecol. Evol. 2017;32:118–130. doi: 10.1016/j.tree.2016.10.011. [DOI] [PubMed] [Google Scholar]
- 20.Mastrángelo ME, et al. Key knowledge gaps to achieve global sustainability goals. Nat. Sustain. 2019;2:1115–1121. doi: 10.1038/s41893-019-0412-1. [DOI] [Google Scholar]
- 21.Isbell F, et al. Biodiversity increases the resistance of ecosystem productivity to climate extremes. Nature. 2015;526:574–577. doi: 10.1038/nature15374. [DOI] [PubMed] [Google Scholar]
- 22.Mace GM, Hails RS, Cryle P, Harlow J, Clarke SJ. Towards a risk register for natural capital. J. Appl. Ecol. 2015;52:641–653. doi: 10.1111/1365-2664.12431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Donohue I, et al. Navigating the complexity of ecological stability. Ecol. Lett. 2016;19:1072–1085. doi: 10.1111/ele.12648. [DOI] [PubMed] [Google Scholar]
- 24.Keyes AA, McLaughlin JP, Barner AK, Dee LE. An ecological network approach to predict ecosystem service vulnerability to species losses. Nat. Commun. 2021;12:1586. doi: 10.1038/s41467-021-21824-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yachi S, Loreau M. Biodiversity and ecosystem productivity in a fluctuating environment: the insurance hypothesis. Proc. Natl Acad. Sci. USA. 1999;96:1463–1468. doi: 10.1073/pnas.96.4.1463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pillar VD, et al. Functional redundancy and stability in plant communities. J. Veg. Sci. 2013;24:963–974. doi: 10.1111/jvs.12047. [DOI] [Google Scholar]
- 27.Feit B, Blüthgen N, Traugott M, Jonsson M. Resilience of ecosystem processes: a new approach shows that functional redundancy of biological control services is reduced by landscape simplification. Ecol. Lett. 2019;22:1568–1577. doi: 10.1111/ele.13347. [DOI] [PubMed] [Google Scholar]
- 28.Salski, A. Ecological applications of fuzzy logic. Pages 3–14 in Ecological Informatics (ed. Recknagel, F.) (Springer, 2003).
- 29.Ehrlich PR, Mooney HA. Extinction, substitution, and ecosystem services. Bioscience. 1983;33:248–254. doi: 10.2307/1309037. [DOI] [Google Scholar]
- 30.Winfree R, Kremen C. Are ecosystem services stabilized by differences among species? A test using crop pollination. Proc. R. Soc. B. 2009;276:229–237. doi: 10.1098/rspb.2008.0709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Díaz S, et al. Incorporating plant functional diversity effects in ecosystem service assessments. Proc. Natl Acad. Sci. USA. 2007;104:20684. doi: 10.1073/pnas.0704716104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Petchey OL, Gaston KJ. Extinction and the loss of functional diversity. Proc. R. Soc. B. 2002;269:1721–1727. doi: 10.1098/rspb.2002.2073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Petchey OL, Hector A, Gaston KJ. How do different measures of functional diversity perform? Ecology. 2004;85:847–857. doi: 10.1890/03-0226. [DOI] [Google Scholar]
- 34.Maseyk FJF, Demeter L, Csergő AM, Buckley YM. Effect of management on natural capital stocks underlying ecosystem service provision: a ‘provider group’ approach. Biodivers. Conserv. 2017;26:3289–3305. doi: 10.1007/s10531-017-1406-9. [DOI] [Google Scholar]
- 35.Schröter M, et al. Assumptions in ecosystem service assessments: increasing transparency for conservation. Ambio. 2021;50:289–300. doi: 10.1007/s13280-020-01379-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Dee LE, et al. When do ecosystem services depend on rare species? Trends Ecol. Evol. 2019;34:746–758. doi: 10.1016/j.tree.2019.03.010. [DOI] [PubMed] [Google Scholar]
- 37.Des Roches S, Pendleton LH, Shapiro B, Palkovacs EP. Conserving intraspecific variation for nature’s contributions to people. Nat. Ecol. Evol. 2021;5:574–582. doi: 10.1038/s41559-021-01403-5. [DOI] [PubMed] [Google Scholar]
- 38.Lafuite A-S, de Mazancourt C, Loreau M. Delayed behavioural shifts undermine the sustainability of social–ecological systems. Proc. R. Soc. B. 2017;284:20171192. doi: 10.1098/rspb.2017.1192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Costanza R, et al. Changes in the global value of ecosystem services. Glob. Environ. Change. 2014;26:152–158. doi: 10.1016/j.gloenvcha.2014.04.002. [DOI] [Google Scholar]
- 40.Wyborn C, et al. Imagining transformative biodiversity futures. Nat. Sustain. 2020;3:670–672. doi: 10.1038/s41893-020-0587-5. [DOI] [Google Scholar]
- 41.Bodin Ö, et al. Improving network approaches to the study of complex social–ecological interdependencies. Nat. Sustain. 2019;2:551–559. doi: 10.1038/s41893-019-0308-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Palumbi SR, et al. Managing for ocean biodiversity to sustain marine ecosystem services. Front. Ecol. Environ. 2009;7:204–211. doi: 10.1890/070135. [DOI] [Google Scholar]
- 43.Fanin N, et al. Consistent effects of biodiversity loss on multifunctionality across contrasting ecosystems. Nat. Ecol. Evol. 2018;2:269–278. doi: 10.1038/s41559-017-0415-0. [DOI] [PubMed] [Google Scholar]
- 44.White L, O’Connor NE, Yang Q, Emmerson MC, Donohue I. Individual species provide multifaceted contributions to the stability of ecosystems. Nat. Ecol. Evol. 2020;4:1594–1601. doi: 10.1038/s41559-020-01315-w. [DOI] [PubMed] [Google Scholar]
- 45.Mace GM, et al. Quantification of extinction risk: IUCN’s system for classifying threatened species. Conserv. Biol. 2008;22:1424–1442. doi: 10.1111/j.1523-1739.2008.01044.x. [DOI] [PubMed] [Google Scholar]
- 46.Moreno-Mateos D, et al. The long-term restoration of ecosystem complexity. Nat. Ecol. Evol. 2020;4:676–685. doi: 10.1038/s41559-020-1154-1. [DOI] [PubMed] [Google Scholar]
- 47.Winfree R, et al. Species turnover promotes the importance of bee diversity for crop pollination at regional scales. Science. 2018;359:791–793. doi: 10.1126/science.aao2117. [DOI] [PubMed] [Google Scholar]
- 48.Memmott J, Waser NM, Price MV. Tolerance of pollination networks to species extinctions. Proc. R. Soc. B. 2004;271:2605–2611. doi: 10.1098/rspb.2004.2909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Purvis A, Agapow PM, Gittleman JL, Mace GM. Nonrandom extinction and the loss of evolutionary history. Science. 2000;288:328–330. doi: 10.1126/science.288.5464.328. [DOI] [PubMed] [Google Scholar]
- 50.Gross K, Cardinale BJ. The functional consequences of random vs. ordered species extinctions. Ecol. Lett. 2005;8:409–418. doi: 10.1111/j.1461-0248.2005.00733.x. [DOI] [Google Scholar]
- 51.Estes JA, et al. Trophic downgrading of planet Earth. Science. 2011;333:301–306. doi: 10.1126/science.1205106. [DOI] [PubMed] [Google Scholar]
- 52.Ross, S. R. P.-J. et al. Code from: Universal scaling of robustness of ecosystem services to species loss (Version V0.4.2-beta). zenodo10.5281/zenodo.4749405 (2021). [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The species–traits network data generated in this study have been deposited in Zenodo under accession code 10.5281/zenodo.4749405 (ref. 52). The empirical network data are publicly available from the Web of Life ecological networks database (http://www.web-of-life.es).
Simulations and data analyses were performed in R. All code necessary to reproduce the results are publicly accessible from Zenodo: 10.5281/zenodo.4749405 (ref. 52).