Abstract
Episodic simulation – the mental construction of a possible future event – has been consistently associated with enhanced activity in a set of neural regions referred to as the core network. In the current functional neuroimaging study, we assessed whether members of the core network are differentially associated with the subjective experience of future events (i.e., vividness) versus the objective content comprising those events (i.e., the amount of episodic details). During scanning, participants imagined future events in response to object cues. On each trial, participants rated the subjective vividness associated with each future event. Participants completed a post-scan interview where they viewed each object cue from the scanner and verbally reported whatever they had thought about. For imagined events, we quantified the number of episodic or internal details in accordance with the Autobiographical Interview (i.e., who, what, when, and where details of each central event). To test whether core network regions are differentially associated with subjective experience or objective episodic content, imagined future events were sorted as a function of their rated vividness or the amount of episodic detail. Univariate analyses revealed that some regions of the core network were uniquely sensitive to the vividness of imagined future events, including the hippocampus (i.e., high > low vividness), whereas other regions, such as the lateral parietal cortex, were sensitive to the amount of episodic detail in the event (i.e., high > low episodic details). The present results indicate that members of the core network support distinct episodic simulation-related processes.
Keywords: recall, imagination, recollection, hippocampus, vividness, parietal cortex, Autobiographical Interview
1. Introduction
Tulving’s (1972, 1983) concept of episodic memory has had a profound influence on many aspects of memory research. One of his most impactful ideas is that episodic memory supports mental time travel not only into the past, but also into the future (Tulving, 1985, 2002, 2005). Tulving’s ideas provided a foundation for the subsequent development of the concept of episodic simulation, or the ability to mentally represent future and other hypothetical episodes (Schacter et al., 2008; Szpunar et al., 2014). Episodic simulation is considered an adaptive cognitive function because it allows us to mentally try out a variety of possible future scenarios without engaging in actual behavior, which helps to prepare us for an actual future experience (Ingvar, 1979; Schacter, 2012; Jing et al., 2017). A growing number of behavioral and neural findings have indicated that the cognitive and neural processes that support episodic simulation overlap with those that support episodic memory (for reviews, see Schacter et al., 2012, 2017a).
These findings provide support for Tulving’s (1985, 2002) ideas about the role of episodic memory in future thinking, as well as for the related constructive episodic simulation hypothesis (Schacter and Addis, 2007, in press), which links the role of episodic memory in simulation of future experiences with constructive aspects of memory. By this view, 1) episodic retrieval plays a key role in accessing and flexibly recombining episodic details from past experiences (such as the who, what, when, and where of a prior episode) into simulations of novel future events, and 2) the flexible nature of episodic retrieval, though useful for constructing future simulations, is also a source of memory errors that are attributable to miscombining elements of past experiences. In support of the constructive episodic simulation hypothesis, age-related reductions in the retrieval of episodic details from remembered past episodes are accompanied by parallel reductions in episodic details for imagined future episodes (for a review, see Schacter et al., 2018; for analogous data in patients with medial temporal lobe lesions, see Kwan et al., 2010; Race et al., 2011; Kurczek et al., 2015; but see Squire et al., 2010 and Dede et al., 2016 for evidence of relatively preserved future imagining following medial temporal lobe damage). In addition, some studies have employed an episodic specificity induction (ESI; for a review, see Schacter and Madore, 2016). These studies have shown that an ESI, compared with various control conditions, similarly increases the number of episodic details people subsequently provide when they recall past events and simulate novel future events. Recent evidence has also directly linked flexible episodic retrieval processes with memory errors that result from miscombining episodic details from distinct but related events (Carpenter and Schacter, 2017, 2018).
Of direct relevance to the present experiment, studies employing functional magnetic resonance imaging (fMRI) have revealed recruitment of a common ‘core network’ of neural regions recruited during both episodic memory and simulation (Schacter et al., 2007; Benoit and Schacter, 2015). This set of regions, which largely overlaps with the default network (Spreng et al., 2009; Andrews-Hanna et al., 2010; Raichle, 2015), includes the lateral parietal cortex, medial temporal lobe including the hippocampus, medial prefrontal cortex, medial parietal cortex, and lateral temporal cortex. This set of regions has been assumed to reflect the common reliance on constructive processes that support episodic memory and simulation (for similar perspectives, see Buckner and Carroll, 2007; Hassabis and Maguire, 2007).
A relatively unexplored yet important area of research is to identify the specific contributions of the individual regions comprising the core network to memory and simulation. In the current fMRI study, we assessed whether members of the core network are differentially associated with two common indices of episodic processing: subjective episodic experience (i.e., vividness) versus objective amount of episodic content comprising those events (i.e., the number of details). We chose these two indices of episodic processing given their frequent use in investigations of the cognitive and neural substrates associated with autobiographical past and/or future thinking (for reviews, see Svodoba et al., 2006, Cabeza and St. Jacques, 2007; Moscovitch et al., 2016; Sheldon and Levine, 2016). In addition, there is reason to think that the two indices might be influenced by different cognitive processes. For example, events comprising greater objective detail may require greater executive demands such as the controlled selection and attention to appropriate details (cf., Benoit and Schacter, 2015; see also, Duarte et al., 2010). In contrast, subjective assessments of episodic content such as vividness may reflect the fidelity or salience of episodic details retrieved (Cooper et al., 2019) or act as an index of the availability of episodic details (D’Angiulli et al., 2019).
A number of studies have examined what core regions vary with the subjective experience of episodic content during episodic memory and/or simulation (e.g., Gilboa et al., 2004; Addis and Schacter, 2008; Rabin et al., 2010; Sheldon and Levine, 2013). In the study of Addis and Schacter (2008), for instance, participants were asked to either retrieve a past episode or imagine a future episode using a cue word. For each event, participants rated the subjective vividness of each event on a 5-point scale from ‘vague with no or few details’ to ‘vivid and highly detailed’. It was shown that neural activity in the hippocampus, among other regions, was greater for remembered and imagined episodes associated with high relative to low subjective vividness (i.e., vivid and highly detailed to vague/with no or few details, respectively). Addis and Schacter (2008) interpreted this effect as reflecting the role of the hippocampus in relational processing that is critical for both past and future events (see, Addis and Schacter, 2012; Eichenbaum and Cohen, 2014), with increasing levels of hippocampal activity reflecting the integration of increasing amounts of subjectively rated detail (i.e., level of vividness).
Other studies have examined what neural regions track the objective amount of episodic detail comprising past and future episodes (e.g., Addis et al., 2007a, 2011; Hach et al., 2014; Palombo et al., 2017; Thakral et al., 2017a, 2017b; Bonnici et al., 2018). In one study, we developed a paradigm to manipulate the number of details within an episode on a trial-to-trial basis (Thakral et al., 2017a). In this study, participants imagined future events in response to familiar place, person, and object cues. We manipulated the amount of episodic details by varying the number of event components that participants were cued to include in their simulated event (3, 4, or 5). Activity in the left prefrontal cortex (the superior frontal sulcus and middle frontal gyrus), left lateral parietal and temporal cortex (left angular gyrus and superior temporal sulcus, respectively), and the medial superior parietal lobe modulated as a function of the amount of simulated details (i.e., greater activity for simulations with five relative to three details).
Another way to quantify the amount of episodic detail is through the Autobiographical Interview (AI; Levine et al., 2002). In the AI, participants recall prior episodes or imagine future episodes. The details that they produce are categorized as either ‘internal’ or ‘external’ details. Internal details reflect the episodic details (e.g., what happened, who was there, and when and where the episode occurred, etc.). External details mainly reflect semantic or off-topic information (e.g., related facts, reflections on and inferences about the meaning of what happened, references to other episodes, or generic commentary). To identify what neural regions track the amount of episodic details (i.e., internal details), some neuroimaging studies have compared groups of participants who produce significantly different amounts of internal/episodic details, such as younger relative to older adults (Addis et al., 2011; see also, Addis et al., 2007a). In Addis et al. (2011), age-related reductions in internal detail production during remembering and imagining were linked to reduced activity in core regions such as bilateral precuneus, hippocampus, middle temporal, lateral parietal cortex, including the left angular gyrus, and regions of the prefrontal cortex such as the middle frontal gyri. Recent studies using transcranial magnetic stimulation (TMS) to disrupt neural activity in core network regions have also utilized the AI (Thakral et al., 2017b; Bonnici et al., 2018). These studies have shown that after TMS to core network regions such as the left lateral parietal cortex (left angular gyrus), relative to a control site, such as the vertex, participants generate fewer internal details for remembered and imagined episodes. Taken together, these findings suggest that certain core regions such as the lateral parietal and prefrontal cortex track the objective amount of episodic detail comprising remembered and imagined episodes.
The preceding evidence has linked certain core regions such as the hippocampus and lateral parietal cortex to the subjective episodic experience and/or the amount of objective episodic detail, and therefore provides convergent evidence that core network regions support these aspects of episodic processing. Although it is generally recognized that the vividness of recalled and/or imagined autobiographical episodes are correlated with the amount of internal details (e.g., Moscovitch et al., 2016), there are specific instances when these indices diverge (e.g., Kirwan et al., 2008; Levine et al., 2009; Addis et al., 2010, 2011). For example, patient M.L. generated a statistically equivalent amount of internal details for specific past episodes relative to age-matched controls but did so with a reduced subjective experience of this detail information (Levine et al., 2009). Additional data come from older adults (Addis et al., 2010, 2011) who generate significantly fewer internal details for remembered and imagined episodes relative to young adults but do so with equivalent levels of subjective vividness (for related evidence in patients with hippocampal damage during autobiographical memory, see Kirwan et al., 2008). There are also fMRI studies of different patient groups who, akin to older adults, generate fewer internal details for past and future events (e.g., patients diagnosed with depression; Hach et al., 2014). In the study of Hach et al. (2014), neural changes in the core network were observed relative to control groups even when past and future events were matched for subjective experience. Additional evidence comes from an fMRI study employing the ESI with the AI (Madore et al., 2016). In this study, participants were scanned while performing an episodic simulation task. After receiving an ESI versus a control induction, several core network regions, including the left hippocampus and right inferior parietal lobule, showed increased activity. These neural increases were linked to behavioral increases in episodic detail comprising the imagined future episodes following the ESI relative to the control induction as operationalized with the AI, despite no differences in subjective ratings in vividness as a function of induction. Taken together, these data suggest that separable neural substrates may support the retrieval of objective detail and the subjective experience of autobiographical content during episodic memory and simulation. Given that the neural correlates of these measures during memory and simulation have not been formally compared in healthy and neurologically intact individuals (but see Spaniol et al. (2009) for an early meta-analysis of non-autobiographical studies of episodic memory), it is unknown whether certain core network regions independently support subjective and objective measures of episodic processing. For example, although the findings of Madore et al. (2016) suggest that the hippocampus, among other regions, tracked the amount of objective episodic detail during episodic simulation, despite a non-significant difference across participants in subjective vividness (see above), the amount of objective detail and subjective vividness may still have co-varied from trial to trial. These findings therefore highlight that understanding the link between objective amount of episodic detail and select core network regions (e.g., the hippocampus) requires further investigations that include a direct comparison of objective episodic detail and subjective vividness.
The aim of the present study was to test for a functional-anatomic dissociation within the core network as a function of subjective and objective indices of episodic processing during episodic simulation. During scanning, participants imagined future events in response to object cues. On each trial, participants rated the subjective vividness associated with each future event on a 5-point scale (see also, Addis and Schacter, 2008). Participants later completed a post-scan interview where they viewed each object cue from the scanner and verbally reported whatever they had thought about. For imagined events, we quantified the number of episodic or internal details in accordance with the AI (see above). We first assessed the relationship of the processes under investigation (i.e., subjective vividness or amount of episodic detail) by asking whether those episodes associated with high subjective vividness are also associated with a greater amount of episodic details. Then, to test whether core network regions are differentially associated with subjective experience or objective episodic content, imagined future events were sorted as a function of their in-scan rated vividness or the post-scan amount of episodic detail. We then identified what regions tracked the level of subjective experience (e.g., regions recruited to a greater extent for future episodes high > low in subjective vividness) and what regions tracked the amount of episodic detail (e.g., regions recruited to a greater extent for future episodes high > low in episodic detail). In addition, we conducted a parametric modulation analysis to identify regions that tracked the level of detail or subjective experience on a trial-by-trial level while statistically controlling for the variance of the alternative index (e.g., after controlling for the individual trial vividness rating, what regions track in a continuous manner the level of episodic detail, and vice versa). To anticipate the results, we observed a dissociation where, among other core network regions, the hippocampus was uniquely sensitive to the subjective vividness of imagined future episodes and regions within the lateral parietal cortex tracked the amount of episodic detail comprising those episodes.
2. Method
Data from the experiment described below have been reported in two prior papers, one focused on the impact of an episodic specificity induction on future imagining (Madore et al., 2016) and the other on interactions between the hippocampus and ventromedial prefontal cortex during future imagining (Campbell et al., 2018). The outcomes of the principal analyses reported here have not been reported previously. The methods are described here in abbreviated form. See Madore et al. (2016) for full details.
2.1. Participants
Data from 31 participants were included in the analysis (mean (± 1 standard error) of 21 years ± 0.41; 20 females). One participant was excluded as they failed to provide a full set of post-scan data (i.e., only 5 of the six runs). The experimental protocol was approved by the Institutional Review Board of Harvard University and informed consent was obtained prior to participation. All participants self-reported to be right-handed, have normal or corrected-to-normal vision, and have no history of neurological or psychological impairment.
2.2. Experimental materials and procedure
Experimental materials comprised 72 object cue words taken from Clark and Paivio (2004). These cues, which have been employed in prior studies of remembering and imagining (e.g., Addis et al., 2007b), were rated on a 7-point scale as high in concreteness (mean (± 1 standard deviation) of 6.88 ± 0.02), imageability (mean of 5.84 ± 0.04), and Thorndike-Lorge frequency (reverse-scored mean of 1.65 ± 0.03). The lists were counterbalanced as a function of task (simulation and object comparison/non-episodic control task) and induction (specificity and control induction). Because our primary interest here was in dissociating two indices of episodic processing during simulation in general, rather than the effect of induction, we collapsed across the specificity and control inductions employed in Madore et al. (2016) to provide 6 runs of data per participant, thus maximizing statistical power.
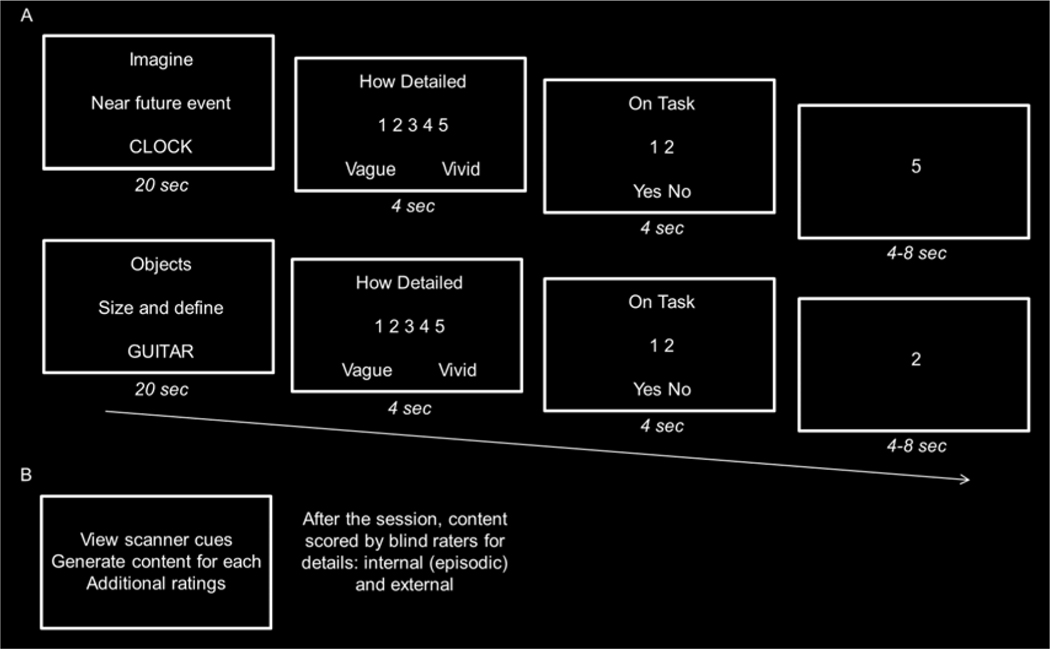
Participants completed six fMRI runs. Each run comprised 12 trials with a random presentation of 6 simulation trials and 6 object comparison/non-episodic control trials. Each run lasted 7 min and 34 sec with a 14 sec fixation period to begin and end each run. On each trial, participants were presented with the construction-elaboration paradigm for 20 sec (see Figure 1). Following each trial, participants responded to two ratings each presented for 4 sec (see below for details). Following the second rating, there was a rest period during which a basic odd/even number judgment task was performed (jittered at 4, 6, or 8 sec with a mean of 6 sec). Participants responded via a five-button response box in their right hand.
Figure 1.
Experimental paradigm. A. Scanning procedure. Participants completed six runs of functional neuroimaging in which they viewed object cues and generated an imagined event related to the cue (top) or a semantic object comparison and definitions related to the cue (bottom). Participants pressed a button when they had initially constructed an imagined event or size sentence for the object cue, and then elaborated on the contents of the imagined event or semantic definitions for the objects from the size sentence until the trial was over. Following each trial, participants rated their subjective vividness on a 5-point scale associated with each imagined event or associated objects following which they indicated whether they were on or off task for the associated trial. Trials ended with an odd/even jittered baseline task for 4, 6, or 8 s. B. Post-scan procedure. Participants viewed the cues they had seen for the tasks outside the scanner, and verbally stated what they had thought about for each one. Participant responses to the cues were audio-recorded, transcribed, and scored in accordance with the Autobiographical Interview for internal (i.e., episodic) and external details.
For simulation trials (Figure 1A, top), participants were instructed to silently imagine a novel, plausible, and future event related to the object cue within the next few years in as much detail as possible. Events had to be specific in time and place and not last for more than a few minutes to an hour. Participants were further instructed to imagine the event from a first-person perspective. With respect to the construction-elaboration component of the simulation task, participants were instructed to press their thumb using the button box in their right hand when they had constructed the event (i.e., when the event had come to mind), and after the button press, to elaborate and fill in all the details of the event until the trial was over. Details included but were not limited to people, actions, and emotions associated with the event. At the end of each simulation trial, participants first rated the subjective vividness of the event they had imagined on a 5-point scale ranging from 1 indicating least vivid with no/few details to 5 indicating many details and most vivid. The second rating involved participants indicating whether they were on or off task for the associated trial (i.e., 1 indicating yes, 2 indicating no).
For the object comparison/non-episodic control task (Figure 1A, middle), participants were instructed to silently generate two associated objects that were related to the object cue, and then to put them in a sentence sorting the three objects by their physical size (e.g., ‘violin is smaller than guitar which is smaller than piano’). With respect to the construction-elaboration component of the control task, participants were instructed to press their thumb when they had constructed the size sentence and then to elaborate on a semantic definition for each of the three objects until the trial was over. As in the simulation task, participants were instructed to generate everything they could for the definitions (i.e., to be as detailed as possible, which included generating typical functions, attributes, and characteristics of the objects). At the end of each control trial and akin to the simulation task, participants rated the amount of detail they thought their definitions contained on a 5-point scale and also rated whether they were engaged on task for the associated trial. As in our prior studies, we reasoned that the object-comparison task served as an appropriate control for the episodic simulation task because it required the search, retrieval, and integration of information related to the object cue, but did not involve the generation of a coherent episodic event (see also, Addis et al., 2007b, 2011; Thakral et al., 2017a). The current choice of control task is empirically validated by the fact that it yielded very similar results to other fMRI studies that have identified activity in the core network using the same as well as different control tasks (see results; for a review, see Benoit and Schacter, 2015).
Immediately after scanning, participants completed a post-scan interview (Figure 1B). Participants viewed each object cue from the scanner and in a self-paced manner were instructed to verbally report whatever they had thought about with the specific instruction, and not to add anything they had not thought about. Each trial was self-paced and participants completed additional ratings for each trial (for full details, see Madore et al., 2016). Participants spoke without any input or probing from the experimenter. After participants had finished speaking, they hit the space bar to move on to the next trial (for similar procedures, see Addis et al., 2007; Madore et al., 2015, 2019). Before the study was conducted, a pilot study showed that participants could describe what they had silently generated (for full results, see Madore et al., 2016 and Madore and Schacter, 2016).
Participants’ verbal reports were audio-recorded and later transcribed. Each simulation was scored in accordance with the Autobiographical interview (Levine et al., 2002) to segment the descriptions of future events into internal (i.e., episodic) and external (e.g., semantic) information. Internal details included the who, what, where, and when elements of the central event specific in time and place, whereas external details included factual information, off-topic and repetitive information, and commentary (for examples of this scoring approach, see Gaesser et al., 2011; Madore et al., 2014; Madore and Schacter, 2016). Two independent raters blind to the hypotheses of interest scored the transcriptions. Inter-rater reliability was high for internal and external details (Cronbach’s α ≥ 0.90). Time spent generating each response to simulation trials (mean (± 1 standard deviation) 25.39 ± 15.03s) and object comparison/control trials (27.76 ± 10.08s) did not significantly differ (p > 0.20). Total word count per response for simulation trials (53.29 ± 31.52) and object comparison/control trials (49.11 ± 19.32) also did not significantly differ (p > 0.20).
2.3. Image acquisition and analysis
Imaging data were acquired on a 3 Tesla Siemens Prisma scanner equipped with a 32-channel head coil. Anatomic data were acquired with a magnetization-prepared rapid gradient echo sequence (TR = 2.53 s, TE = 1.64, 3.50, 5.36 and 7.22 ms, 176 slices, 1 mm3 resolution). Functional data were acquired with a multiband echo-planar imaging sequence (TR = 2 s, TE = 30 ms, SMS = 3, 69 slices, multiband factor of 3, 2mm3 resolution).
Analyses were conducted using Statistical Parametric Mapping (SPM12, Wellcome Department of Cognitive Neurology, London, UK). The first four volumes were excluded to account for T1-saturation. Functional data preprocessing included slice-time correction, two-pass spatial realignment, and normalization into Montreal Neurological Institute (MNI) space (no resampling). Functional data were smoothed with a 8 mm full-width-half-maximum (FWHM) Gaussian kernel. Anatomic images were normalized into MNI space.
Univariate analysis was conducted in a two stage general linear model (GLM). In the first stage, the blood oxygen level-dependent response for the construction and elaboration periods were modeled separately for each simulation and non-episodic control trial using the canonical hemodynamic response function in SPM12 (for similar approaches, see Addis et al., 2007b; Addis and Schacter, 2008; Madore et al., 2016). Specifically, the construction period was modeled with a delta function 2 s after cue onset and the elaboration period was modeled with a delta function 2 s after the participant made a button press (mean elaboration 8.65 s across tasks).
Two first-level models were created to separately identify regions during episodic simulation associated with objective content (i.e., the number of episodic details comprising each imagined future event) and subjective vividness (i.e., the rated vividness associated with each imagined future event). In the first-level model employed to identify regions associated with subjective vividness, we separated episodic simulation trials as a function of the in-scan vividness rating. To ensure sufficient trials in each vividness bin, the 5-point rating scale was split into two bins of roughly equivalent numbers of trials (‘high vividness’ consisting of ratings 4 and 5 (18.35 ± 1.51 trials), and ‘low vividness’ consisting of ratings 1, 2, and 3 (12.03 ± 1.38 trials)). In the first-level model employed to identify regions associated with objective content, we separated episodic simulation trials as a function of the episodic details quantified based on the post-scan interview. In order to segment trials as a function of objective content, on an individual participant basis, we conducted a median split across trials (15.96 ± 1.32 and 9.79 ± 0.87 high and low details, respectively). The number of trials associated with the high and low episodic detail bin was 13.48 ± 0.66 and 16.94 ± 0.87 trials, respectively. Note that the high trial bin included trials that equaled the median (i.e., high internals included trials larger than and equal to the median, and the low internal detail bin included only those trials smaller than the median). A similar pattern of results was obtained when for each participant the number of trials was matched.
Each of the two first-level models contained 6 events of interest modeling: 1) construction-related activity for high vividness or high episodic details during episodic simulation, 2) construction-related activity for low vividness or low episodic details during episodic simulation, 3) construction-related activity for non-episodic control trials, 4) elaboration-related activity for high vividness or high episodic details during episodic simulation, 5) elaboration-related activity for low vividness or low episodic details during episodic simulation, and 6) elaboration-related activity for non-episodic control trials. Two additional events of no interest were included in each model: trials where participants were ‘off-task’ (see above; these trials were also excluded for the behavioral analysis, see Results) and the rating period (i.e., a delta function for the simulation and object ratings). Six regressors representing movement-related variance (three for rotation and three for rigid-body translation) and regressors modeling each scan session were also entered into the design matrix. An AR(1) model was used to estimate and correct for nonsphericity of the error covariance (Friston et al., 2002). Data across the six runs were concatenated (see also, Thakral et al., 2017a). Temporal smoothing was conducted before estimation of the parameter estimates (i.e., the default high-pass filter of 128 sec in SPM12). Although we modeled the elaboration period, here we focus on construction-related activity because this portion of the trial places the greatest demands on retrieval and recombination, as indicated by prior work showing that effects of an episodic specificity induction are observed only during construction (Madore et al., 2016; see also, Campbell et al., 2018)1.
The participant-specific parameter estimates for each of the two first-level models described above were carried forward to a second analysis stage where they were entered into a respective repeated measures ANOVA with participants modeled as a random effect. The ANOVA model employed factors of trial phase (construction or elaboration) and amount of episodic detail or level of vividness. Each ANOVA model was then used to conduct planned comparisons to identify regions associated with the amount of episodic detail (i.e., high > low episodic detail) and subjective vividness (i.e., high > low vividness).
An individual voxel threshold of p < 0.005 was employed. To correct for multiple comparisons, a cluster extent threshold of 105 voxels was employed (corrected threshold of p < 0.05; Slotnick et al., 2003; Slotnick, 2017). This cluster extent was computed using a Monte Carlo simulation with 10,000 iterations. The Monte Carlo simulation modeled activity in each voxel using a normally distributed random number (mean of zero and unit variance) and Type-I error was assumed to be equal to the individual voxel threshold p-value (p < 0.005) in a volume defined by the functional acquisition dimensions. Spatial correlation was simulated by smoothing with a 10 mm FWHM Gaussian, which was estimated using the residual mean-square image of the participant-specific first level models. The probability of observing successively larger cluster sizes was computed based on the Monte Carlo cluster size distribution, and the cluster extent threshold was selected such that the probability of observing that or larger clusters was less than p < 0.05. This procedure yielded a cluster extent threshold of 105 voxels. The outcome of the core network contrast (i.e., episodic simulation > non-episodic control) was interrogated via inclusive masking with each of the two contrasts to identify core regions associated with subjective vividness (i.e., high > low vividness) and objective content (i.e., high > low episodic details).The latter orthogonal contrasts were thresholded at p < 0.01 and combined with the statistically-independent core network contrast (at p < 0.005) to give a conjoint threshold of p < 0.0005 (Fisher, 1950; Lazar et al., 2002; for further details on computing the joint probability, see Slotnick & Schacter, 2004). Critically, this conjoint threshold was used along with the cluster extent correction of 105 voxels. Thus any clusters associated with the high > low vividness contrast and the high > low episodic detail contrast are only considered significant if they exceeded a size of 105 voxels. We have employed this method of inclusive masking between independent contrasts with similar thresholds in our prior and recent studies of episodic memory and simulation (e.g., Thakral, Yu, & Rugg, 2015; Thakral, Wang, & Rugg, 2015; Thakral et al., 2017a).
One limitation of the analyses described above is that each of the two first-level models utilized the same simulation trials (i.e., they were sorted either as a function of subjective vividness or amount of episodic details), and therefore the parameter estimates from each respective first-level model cannot be directly compared to provide evidence of a significant interaction effect. To provide a direct test of a dissociation across subjective and objective indices of episodic processing, we conducted a parametric modulation analysis to identify regions that varied with each index of episodic processing (e.g., amount of detail) and at the same time account for the variance associated with the other index (e.g., level of vividness). To identify regions that modulated uniquely as a function of episodic detail, at the first-level we entered, trial-by-trial, a detail score for each imagined event obtained in the post-scan interview and as a covariate of no-interest the rated level of in-scan vividness. The detail score covariate was modeled linearly, represented the orthogonal contribution of detail in the absence of any other covariates, and was mean-centered according to SPM algorithms (Mumford et al., 2015; for similar procedures, see Madore et al., 2016). At the second level, we entered the first-level images corresponding to the detail score covariate into a random-effects one-sample t-test. The analogous procedure was employed to identify regions that modulate uniquely as a function of subjective vividness (i.e., the in-scan vividness rating was entered as the covariate of interest and the post-scan detail score was entered as a covariate of no interest).
3. Results
3.1. Behavioral results
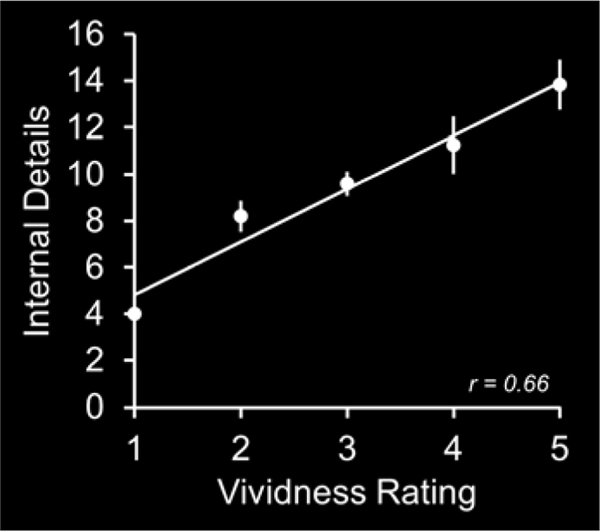
We conducted a behavioral analysis to examine whether subjective vividness of imagined future events covaries with the number of episodic details comprising those events as operationalized with the AI. In this analysis, on an individual participant basis, we correlated the number of episodic details with the associated vividness rating across imagined future events (see Figure 2 for an individual participant correlation plot that represents the analytical approach). These correlations were significantly greater than 0 across participants (mean (± 1 standard error) Spearman correlation of r = 0.10 ± 0.04, mean beta of 0.71 ± 0.21; correlations significantly greater than 0; p < 0.01). These findings indicate that imagined future events comprising high amounts of episodic detail are those that are also associated with a high subjective vividness.
Figure 2.
Participant correlation plot between the amount of internal details and subjective vividness rating that represents the analytical approach.
3.2. fMRI results
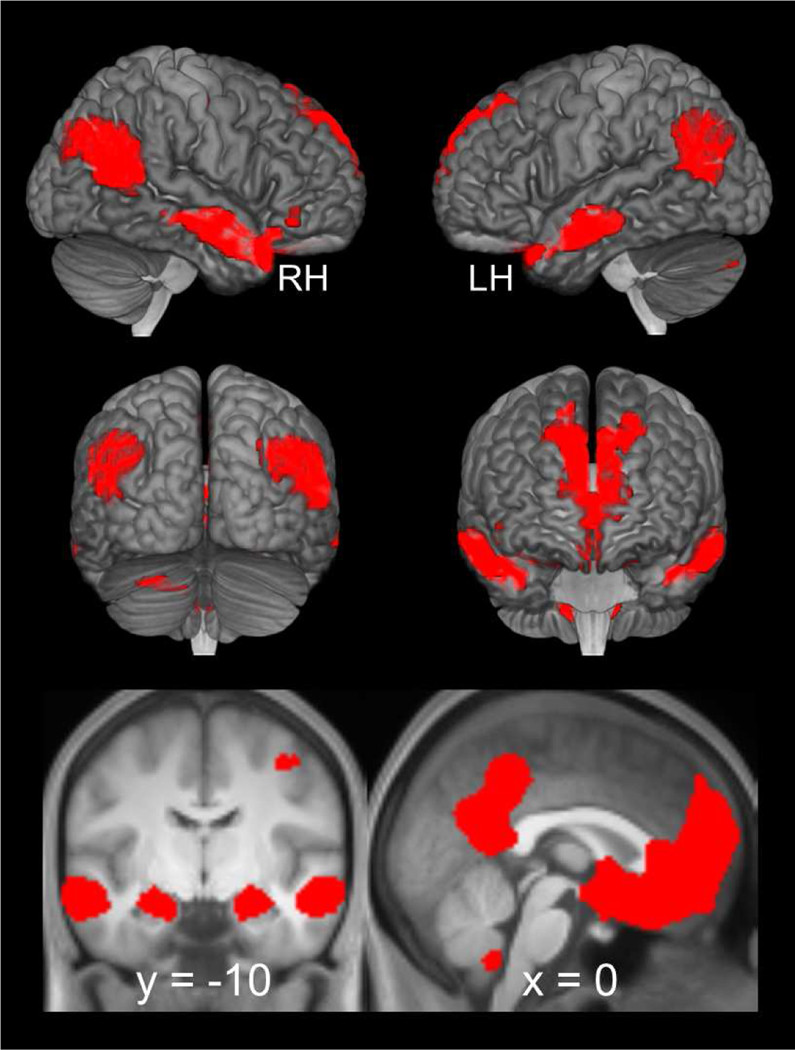
We first replicated prior reports of simulation-related neural activity with the contrast of episodic simulation > non-episodic control (i.e., collapsing across high and low subjective vividness and number of episodic details). As detailed in Figure 3 and Table 1, and replicating prior studies of episodic simulation (for a review, see Benoit and Schacter, 2015), simulation-related effects were observed in every region of the core network including lateral parietal cortex, lateral temporal cortex, medial prefrontal cortex, and the medial temporal lobe, including the hippocampus.
Figure 3.
Simulation-related neural activity identified with the contrast of episodic simulation > non-episodic control (i.e., collapsing across high and low subjective vividness and number of episodic details). Results are also projected onto a cortical surface using the skull-stripped template of MRIcroGL (see, Rorden, Karnath, & Bonilha, 2007) and overlaid onto the coronal and sagittal sections of the across-participants mean T1-weighted anatomical image.
Table 1.
Loci of episodic simulation effects
| MNI Coordinates | Peak Z | Number of above-threshold voxels | Region | ||
|---|---|---|---|---|---|
|
| |||||
| X | y | z | |||
| Episodic simulation > Non-episodic control | |||||
| 0 | 52 | −10 | Inf | 18834 | Left medial prefrontal cortex |
| 4 | −54 | 20 | Inf | Right precuneus/posterior cingulate/retrosplenial cortex | |
| −2 | −56 | 22 | Inf | Left precuneus/posterior cingulate/retrosplenial cortex | |
| −2 | 6 | −10 | Inf | Left ventral striatum | |
| 24 | −30 | −16 | 6.79 | Right parahippocampal cortex | |
| −24 | −38 | −12 | 6.40 | Left parahippocampal cortex | |
| 24 | −16 | −18 | 6.66 | Right hippocampus | |
| −24 | −16 | −18 | 6.12 | Left hippocampus | |
| 20 | 28 | 38 | 4.89 | Right superior frontal sulcus | |
| −20 | 32 | 44 | 4.62 | Left superior frontal sulcus | |
| 54 | −6 | −14 | Inf | 2363 | Right superior temporal sulcus |
| 46 | 22 | −28 | 6.86 | Right anterior temporal lobe | |
| 26 | 16 | −20 | 3.84 | Right orbitofrontal gyrus | |
| −60 | −8 | −14 | 7.08 | 1470 | Left superior temporal sulcus |
| −66 | −18 | −12 | 5.89 | Left middle temporal gyrus | |
| −50 | 18 | −30 | 3.89 | Left anterior temporal lobe | |
| 46 | −58 | 20 | 6.90 | 2915 | Right superior temporal sulcus |
| 48 | −72 | 36 | 5.29 | Right angular gyrus | |
| −44 | −76 | 34 | 6.42 | 2013 | Left angular gyrus |
| −42 | −60 | 16 | 3.79 | Left superior temporal sulcus | |
| 10 | −46 | −46 | 5.51 | 603 | Right cerebellum |
| −8 | −50 | −46 | 5.41 | Left cerebellum | |
| −24 | −80 | −34 | 4.00 | 248 | Left cerebellum |
| 38 | −14 | 50 | 3.67 | 145 | Right precentral sulcus |
| Episodic simulation > Non-episodic control inclusively masked with High > Low vividness | |||||
| 58 | −6 | −16 | 7.14 | 164 | Right superior temporal |
| −24 | −18 | −16 | 4.57 | 128 | Left hippocampus |
| −18 | −6 | −20 | 3.01 | Left amygdala | |
| Episodic simulation > Non-episodic control inclusively masked with High > Low internal details | |||||
| 44 | −58 | 20 | 6.47 | 337 | Right superior temporal sulcus |
| 46 | −74 | 28 | Right angular gyrus | ||
| −6 | 50 | 38 | 5.84 | 271 | Left precuneus/posterior cingulate cortex |
| −4 | −54 | 50 | Left medial superior parietal lobule | ||
| −50 | −72 | 20 | 5.24 | 562 | Left superior temporal sulcus |
| −44 | −68 | 24 | Left angular gyrus | ||
| −22 | 36 | 46 | 4.31 | 106 | Left superior frontal sulcus |
Coordinates for cluster sub-peaks which lay in distinct cortical regions are listed directly below relevant peak cluster.
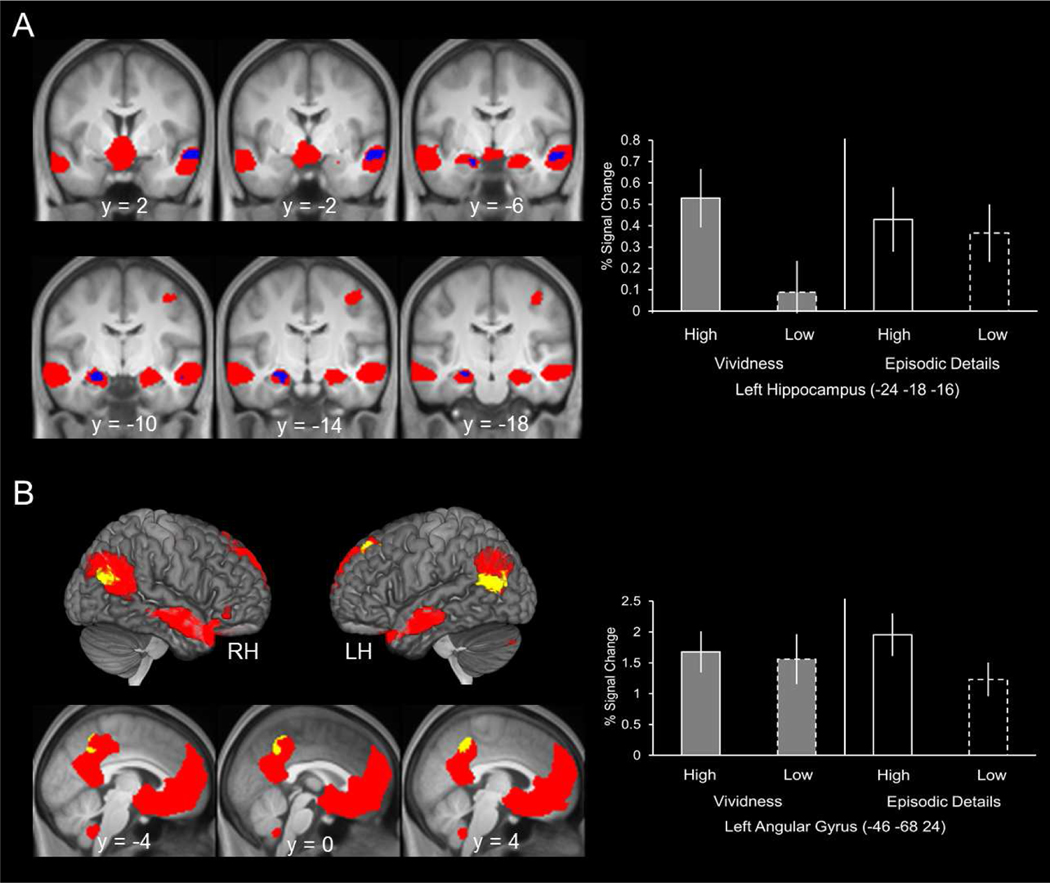
Critically, we then went on to identify which of the above episodic simulation effects were associated with subjective vividness and the amount of episodic detail. To achieve this aim, we employed inclusive masking. First, to identify simulation effects that modulated as a function subjective vividness, we inclusively masked the outcome of the episodic simulation > non-episodic control contrast (threshold of p < 0.005, see above) with the high > low subjective vividness contrast (mask threshold of p < 0.01;see Methods). . Figure 4A illustrates the simulation effects that varied as a function of subjective vividness. As detailed in Table 1, vividness-sensitive effects were observed in two regions, the left hippocampus (extending slightly into the amygdala; spatial extent of y = −6 to y = 28) and the right superior temporal sulcus. In accordance with how these effects were identified, the extracted parameter estimates demonstrate that the simulation effects within the hippocampus, for example, were modulated by subjective vividness (high > low; see bars 1 and 2). We also illustrate the parameter estimates extracted from the alternative model where the same trials were sorted as a function of the amount of episodic detail (see bars 3 and 4). As is apparent from the parameter estimates, the left hippocampus was not sensitive to amount of episodic detail (a follow-up t-test revealed that this difference was not significant (p > 0.20; note that this test is independent of the procedure used to identify the activity).
Figure 4.
A. Simulation-related neural activity that modulated as a function of subjective vividness. These effects were identified by inclusively masking the outcome of the episodic simulation > non-episodic control contrast (shown in red) with the high > low subjective vividness contrast. The results of this inclusive mask are shown in blue. Illustrated on the right are the parameter estimates extracted from the peak voxel within the left hippocampus cluster for each of two first-level models employed to model each of the four events of interest (i.e., imagined events associated with high vividness, imagined events associated with low vividness, imagined events associated with high episodic details, and imagined events associated with low episodic details). B. Simulation-related neural activity that modulated as a function of the amount of episodic details. These effects were identified by inclusively masking the outcome of the episodic simulation > non-episodic control contrast (shown in red) with the high > low episodic details contrast. The results of this inclusive mask are shown in yellow. Illustrated on the right are the parameter estimates extracted from the peak voxel within the left angular gyrus cluster for each of two first-level models employed to model each of the four events of interest. Results are also projected onto a cortical surface using the skull-stripped template of MRIcroGL (see, Rorden, Karnath, & Bonilha, 2007) and overlaid onto the coronal and sagittal sections of the across-participants mean T1-weighted anatomical image.
An analogous procedure to that described above was employed to identify simulation effects that modulated as a function of the amount of episodic detail (i.e., the outcome of the episodic simulation > non-episodic control contrast (threshold of p < 0.005) was inclusively masked with the high > low episodic detail contrast (mask threshold of p < 0.01). Figure 4B illustrates the simulation effects that varied as a function of the amount of episodic detail. As detailed in Table 1, amount-sensitive effects were observed in, among other regions, the bilateral angular gyrus (extending into the superior temporal sulcus), medial superior parietal lobule, and left superior frontal cortex. In accordance with how these effects were identified, the extracted parameter estimates demonstrate that the simulation effects within the left angular gyrus, for example, were modulated by the amount of episodic detail vividness (high > low; see bars 3 and 4), but not by level of subjective vividness (see bars 1 and 2; p > 0.20).
Although the above analyses provide evidence that regions previously shown to support episodic simulation are differentially sensitive to two indices of episodic processing (i.e., subjective vividness and objective episodic detail), the analyses do not allow a direct comparison of the two indices. To provide a direct test of a dissociation across the two indices, we conducted a parametric modulation analysis to identify regions that uniquely varied with each index of episodic processing.
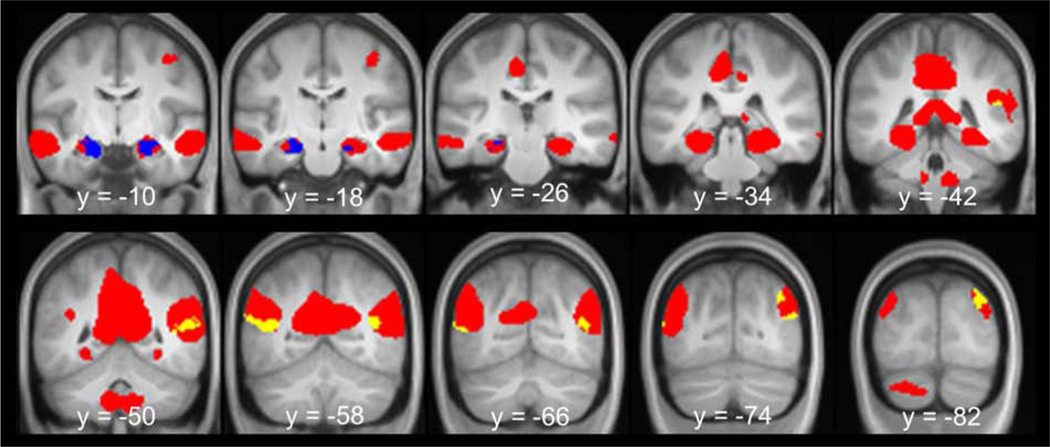
The results of the parametric modulation analyses are shown in Figure 5 and Table 2. As in the analyses reported above, we inclusively masked the contrast of episodic simulation > non-episodic control (threshold of p < 0.005) with the result of each parametric modulation analysis (mask threshold of p < 0.01) to identify which ‘core’ regions modulated as a function of each index of episodic processing. As detailed in Table 2, parametric modulation effects associated with vividness were evident in the bilateral hippocampus (Figure 5, blue). In contrast, parametric modulation effects associated with the amount of episodic details were evident in the bilateral lateral parietal cortex, including the angular gyrus extending into the superior temporal sulcus (Figure 5, yellow).
Figure 5.
Results of the parametric modulation analyses. Parametric modulation effects associated with vividness are shown in blue and parametric modulation effects associated with the amount of episodic details are shown in yellow. Shown in red are simulation effects identified with the contrast episodic simulation > non-episodic control contrast (collapsed across vividness and amount of episodic detail). Results are overlaid onto the coronal sections of the across-participants mean T1-weighted anatomical image.
Table 2.
Loci of episodic simulation effects - Parametric modulation effects
| MNI Coordinates | Peak Z | Number of above-threshold voxels | Region | ||
|---|---|---|---|---|---|
|
| |||||
| X | y | z | |||
| Level of vividness | |||||
| −18 | −14 | −24 | 4.52 | 259 | Left hippocampus |
| 26 | −6 | −18 | 3.57 | 163 | Right hippocampus |
| Amount of episodic detail | |||||
| −46 | −56 | 12 | 3.51 | 200 | Left superior temporal sulcus |
| −46 | −70 | 8 | 2.97 | Left angular gyrus | |
| 38 | −80 | 38 | 3.27 | 139 | Right angular gyrus |
| 38 | −60 | 14 | 3.05 | 331 | Right superior temporal sulcus |
| 44 | −72 | 18 | 3.01 | Right angular gyrus | |
Coordinates for cluster sub-peaks which lay in distinct cortical regions are listed directly below relevant peak cluster.
4. Discussion
In the current fMRI study, neural activity commonly associated with episodic simulation (i.e., the core network; Benoit and Schacter, 2015) was dissociated across two indices of episodic processing: subjective vividness and objective amount of episodic detail. We operationalized the objective amount of episodic detail as the number of internal details in the AI (Levine et al., 2002). Our behavioral analysis revealed that these two indices of episodic processing were correlated. These results demonstrate that the processes under investigation are confounded such that imagined future events comprising high amounts of episodic detail are those that are also associated with a high subjective vividness. Although correlated, our fMRI analysis revealed that certain core network regions are differentially associated with subjective experience and objective episodic content. Specifically, the fMRI analysis revealed that regions that tracked the level of objective detail included bilateral lateral parietal cortex (angular gyrus), bilateral lateral temporal cortex (superior temporal sulcus), bilateral medial superior parietal lobe, and regions of prefrontal cortex (superior frontal sulcus). Only two regions were found to vary with the subjective experience/vividness of simulated episodic content, the hippocampus and right superior temporal sulcus extending into the anterior temporal lobe. We discuss the implications of these findings below.
The finding that the hippocampus was uniquely associated with the subjective vividness of imagined future episodes replicates prior reports also linking the hippocampus to the subjective experience of episodic content during autobiographical past and future thinking (e.g., Gilboa et al., 2004; Addis and Schacter, 2008; Rabin et al., 2010). The present data extend these findings by dissociating subjective episodic content (i.e., self-rated vividness) from correlated increases in objective content (i.e., the number of episodic details quantified by the AI). The current findings are consistent with prior theoretical perspectives on vividness-related processes. According to multiple-trace memory theory (e.g., Moscovitch et al., 2005), vividness ratings reflect the strength or availability of detail information, with higher vividness intensity associated with greater access to episodic detail information (see, D’Angiulli et al., 2013). This model is consistent with our prior proposals of hippocampal function during episodic simulation (e.g., Addis et al., 2007b; Schacter and Addis, 2009; Addis and Schacter, 2012; Schacter et al., 2017b). According to such proposals, one role for the hippocampus during simulation is to act as a pointer or index to relevant memory traces, resulting in the reinstatement and retrieval of specific episodic details (Moscovitch et al., 2005). Thus, the vividness rating may reflect the level of access of specific episodic details employed to construct future episodes. Alternatively, it may be the case that the increase in hippocampal activity as a function of vividness may reflect the amount of relational binding between details and/or the encoding of the simulated event into memory, two other hallmark hippocampal processes (for a review, see Addis and Schacter, 2012; Schacter et al., 2017a). If the number of episodic details as indexed by the AI reflects the engagement of binding and/or encoding processes, then hippocampal activity would have been observed for the contrast of high > low episodic details. Because hippocampal activity was observed with the contrast high > low vividness and was not sensitive to the amount of episodic details (at least as operationalized with the AI), we believe that a parsimonious account of the present hippocampal effects is a retrieval-based account. Nevertheless, it will be important for future studies to not only replicate the current pattern of effects, but to also measure the extent to which the hippocampal effects are dissociable from those that reflect encoding (e.g., Thakral et al., 2017c).
The neural regions identified to be uniquely associated with the objective amount of episodic detail replicate a prior fMRI study that directly manipulated the number of details during episodic simulation (Thakral et al., 2017a). In that study as in the present one, amount-dependent effects were observed in the left prefrontal cortex (the superior frontal sulcus and middle frontal gyrus), left lateral parietal and temporal cortex (left angular gyrus and superior temporal sulcus, respectively), and the medial superior parietal lobe. As in our prior study, we interpret these effects as reflecting processes that support the amount of episodic detail comprising future episodes. For example, the medial superior parietal lobe and prefrontal cortex effects likely reflect control-related processes that scale with the amount of episodic detail comprising future events (e.g., the shifting of attention between details and controlled selection of disparate episodic details, respectively; see Thakral et al., 2017a, see also, Benoit and Schacter, 2015). With respect to the lateral parietal effects in the left angular gyrus, in our prior study, we interpreted the effects as reflecting the representation of mnemonic content (e.g., Vilberg and Rugg, 2008; Rugg and King, 2018). The current lateral parietal effects are also consistent with recent TMS data indicating that inhibitory TMS to the left angular gyrus leads to a reduction in the internal details generated for both remembered past and imagined future episodes (Thakral et al., 2017b; Bonnici et al., 2018). Bonnici et al. (2018) further showed that after TMS to the left angular gyrus relative to TMS to a control site (vertex), participants experienced remembered episodes more from a third-person perspective relative to a first-person perspective. Interestingly, and consistent with the present data, Bonnici et al. (2018) failed to find a TMS effect of vividness (i.e., after TMS to the left angular gyrus relative to the control site, remembered episodes were subjectively experienced as equally vivid). These findings raise the possibility that the left angular gyrus may be sensitive to some subjective characteristics of the episodic experience (such as perspective) but not all (such as vividness).
The present hippocampal and parietal dissociation is very similar to a recent study from our group employing a multi-voxel pattern similarity analysis (MVPA) examining the relationship across episodic memory and simulation at the level of individual event details (Thakral et al., in press). In this study, participants recalled past episodes each comprising two event details, a personally familiar location and person. Participants also simulated novel future episodes using recombined pairs of person and location details taken from different recalled episodes. Participants rated the vividness of each location and person in their memory and simulation. Employing MVPA, we interrogated the similarity between neural patterns during memory and simulation at the level of individual event details. Within the hippocampus, pattern similarity was not only specific to the matching of individual event details (i.e., similarity was greatest for past and future episodes when those episodes shared an event detail), but modulated as a function of the vividness with which participants experienced those details during later simulation (i.e., pattern similarity was greatest for details associated with high relative to low vividness during episodic simulation). In contrast to the hippocampus, pattern similarity within the lateral parietal cortex, specifically the left angular gyrus, was not sensitive to the vividness of simulated information but instead was sensitive only to the matching of individual detail information. Given that the vividness of details during memory was behaviorally correlated with the vividness of those same details during later simulation, we reasoned that the hippocampal pattern similarity effects reflected the role of this region in the retrieval and reinstatement of episodic information from specific prior episodes. In contrast, we conjectured that the pattern similarity effects within the left angular gyrus reflect the role of this region in representing mnemonic content not specific to a prior episode (i.e., information that does not depend on the reinstatement of a specific memory captured by the vividness rating; cf., Wing et al., 2015). The current hippocampal and lateral parietal effects parallel those observed in our MVPA study. The convergence of the present results with our prior MVPA findings indicating that the hippocampus is also sensitive to vividness suggest that vividness ratings may reflect the sensitivity of the hippocampus to specific information during episodic simulation (e.g., information that is tied to a specific memorial context). In contrast, the sensitivity to internal details in the left angular gyrus may reflect the role of this region in supporting the complex and high-level mnemonic information that comprise future episodes (for related perspectives, see Rugg and King, 2018; Ramanan et al., 2018, 2019; see also, Binder and Desai, 2011). Alternatively, the left angular gyrus activity may reflect the automatic generation of personal semantics (i.e., the generalized facts that define personally relevant stimuli; Renoult et al., 2012) associated with the internal details that comprise future events. Although the current data cannot disambiguate between these possibilities, one important avenue for future research will be to collect a measure of internal/episodic details as in the AI in conjunction with a measure of personal semantics to specify the neural substrates associated with the types of information known to comprise past and future events (e.g., Renoult et al., 2016).
An important point to remember about the current findings, specifically those pertaining to the hippocampus and angular gyrus, is that the selectivity of these regions to subjective and objective indices of episodic processing, respectively, should not be overgeneralized. As detailed above, there are certain instances where the hippocampus supports the retrieval of specific episodic details during simulation (i.e., when those details are associated with high vividness; Thakral et al., in press). Relevant to issues concerning the link between episodic detail during past and future thinking and the hippocampus, AI data from patients with hippocampal amnesia have been inconclusive, with some studies finding reduction in episodic details and others failing to find such differences (cf., Kirwan et al., 2008; Kwan et al., 2010; Squire et al., 2010; Race et al., 2011; Kurczek et al., 2015; Dede et al., 2016). One possibility is that episodic details as operationalized by the AI alone may reflect more generic episodic processing, which may explain why the neural regions currently shown to be associated with internal/objective details overlapped with those previously theorized to be associated with personal semantics (Renoult et al., 2012). In addition, there are a number of factors that may mediate the relationship between objective and subjective indices of episodic processing and associated neural correlates. For example, this relationship may depend on the type of episodic details that comprise future episodes (e.g., those comprising more visuospatial details may be more vivid, while those more generic and/or less personal may be less vivid yet still comprise objective details). We also highlight that the current results are limited to autobiographical forms of future episodic thinking. Relevant to this limitation are data from studies of episodic memory that have used lab-based materials (e.g., words, pictures) to link the hippocampus with objective memory accuracy (i.e., source memory accuracy) and the lateral parietal cortex to subjective memory (i.e., remembering relative to knowing; Slotnick, 2010; see also, Kuhl & Chun, 2014; Bonnici et al., 2016; Richter et al., 2016; for a meta-analysis, see Spaniol et al., 2009; for a review, see Moscovitch et al., 2016). Understanding the relation between these findings and the current results is an important topic for future research.
In addition to the hippocampus, the only other region that was found to be sensitive to the vividness of episodic simulation was the right superior temporal sulcus. A more posterior region present in the same sulcus and contiguous with the cluster identified in the right inferior parietal lobule (encompassing the angular gyrus) was found to be sensitive to the objective amount of episodic detail. These findings suggest the presence of an anterior-posterior gradient in the superior temporal sulcus, with more anterior regions sensitive to subjective indices of episodic processing and more posterior aspects sensitive to objective indices of episodic processing. This distinction is reminiscent of prior evidence for an anterior-posterior functional gradient within the right superior temporal sulcus during social cognition (Deen et al., 2015), with posterior regions sensitive to mental state understanding (i.e., theory of mind) and more anterior aspects sensitive to more perceptual functions of social cognition (e.g., vocal sounds and language). One speculative possibility is that the posterior superior temporal activity reflects the inference and imagining of the thoughts and feelings of the other people comprising the future episodes (i.e., the internal details). In contrast, the more anterior superior temporal activity may reflect the perceptual aspects of the future episode that track subjective vividness. We emphasize that this account is ad hoc and requires testing in future research.
There are a number of limitations of the present experiment that deserve mention. One is that we only observed a dissociation between subjective and objective indices of episodic processing during simulation. Although the neural regions that support episodic simulation strongly overlap those engaged during memory, there are a set of regions that are engaged to a greater extent during episodic simulation relative to memory, which include core regions such as the hippocampus, as well as non-core regions like those that fall within the frontoparietal control network (Benoit and Schacter, 2015). Of direct relevance to this point, we did observe an effect of high > low internal details in the left superior frontal sulcus. The peak coordinate of this cluster (−22 36 46) was in very close spatial proximity to the peak coordinate within the left dorsolateral prefrontal cluster reported in Benoit and Schacter (2015) for the contrast of episodic simulation > memory (−24 30 46). These findings provide support for the possibility that detailed simulations and detailed episodic memories might benefit from somewhat different cognitive processes. We note however that the contrast of high > low internal details did identify activity in regions commonly engaged during episodic memory and simulation (e.g., bilateral angular gyrus). These findings highlight the need for future work that aims to assess whether the present dissociation between episodic detail and vividness extends to paradigms that include episodic memory. Moreover, it will be important for future studies to employ seed-based connectivity analyses to examine how control regions, such as those in the prefrontal cortex, interact with core regions, such as the angular gyrus, and to assess whether such interactions differ as a function of episodic simulation and memory (for a similar approach with connectivity between core regions, see Campbell et al., 2018). An additional limitation comes from the fact that we were unable to examine the different internal detail sub-categories of the AI (Levine et al., 2002). This limitation stems from the short trial length needed for the fMRI paradigm. Future work is necessary to examine whether the hippocampus, although insensitive to internal details as a whole, might be sensitive to certain sub-categories (e.g., event details relative to the thoughts/emotions sub-category; see, Race et al., 2011). Of relevance to this point, the current study is a reanalysis of a data set from an experiment that examined the effects of an ESI on core network activity (see Introduction). However, due to limited trial numbers, an examination of differential impacts on internal details vs. subjective vividness as a function of induction was not possible. The current reanalysis addressed a different question by using both median-split and continuous analytic approaches (vs. a continuous approach in the original study), a statistical threshold of p < 0.005 and 105 voxels (vs. p < 0.005 and 10 voxels in the original study), and collapsed across induction for adequate power (relative to comparing induction-related outcomes), and found no relationship between internal details and hippocampal activity. A link between internal details and the hippocampus in one study and not in another could be due to analytic decisions, task manipulations that change brain-behavior relationships, or other factors not examined in the current study. In the original study, we explicitly indicated that this finding should be treated as preliminary, and note that future work should explore if there are other task-state manipulations like ESI that impact the relationship between internal details and the hippocampus.
One final limitation of the present study is that we focused on identifying dissociations across subjective and objective indices of episodic processing during simulation. We also conducted an analysis aimed at identifying regions commonly associated with both subjective vividness and the objective amount of episodic details (full details are available from the first author). The results of this analysis were however null. Although the significant behavioral correlation may seem at odds with these null fMRI results, it is important to keep in mind that any number or pattern of neural effects may contribute to any given behavioral response (i.e., a neural dissociation does not imply a behavioral or cognitive dissociation; cf., Slotnick , 2013). To illustrate, the present results indicate that the hippocampus and parietal cortex are associated with subjective vividness and internal details, respectively. How can a dissociation in the brain give rise to a behavioral association? Many brain regions mediate episodic processing during simulation, not only the hippocampus and parietal cortex, but also the parahippocampal cortex and the prefrontal cortex, among other regions. Although the current results are limited to the identification of a dissociation across only two indices of episodic processing, additional studies along the same lines as the present will be required to evaluate the nature of episodic processing in other neural regions during simulation.
In conclusion, the present findings have important implications for studies examining episodic processing during either memory or simulation because they suggest that two common indices of episodic processing (i.e., self-rated vividness and amount of episodic details) operate via distinct neural mechanisms. Prior work has shown that a variety of phenomenological characteristics are correlated with episodic forms of thinking (e.g., D’Argembeau and Van der Linden, 2004, 2012). The current experiment highlights that future studies aimed at identifying the neural correlates of autobiographical past and future thinking should not assume that any given measure of episodic processing reflects a single cognitive function.
Highlights.
Episodic simulation is the ability to pre-experience hypothetical future episodes
Episodic simulation associated with neural activity in the ‘core network’
We identified core network regions associated with vividness
We identified core network regions associated with the amount of episodic details
The core network dissociates across subjective and objective episodic processing
Acknowledgements
This research was supported by NIMH Grant R01MH60941 (to DLS) and National Institute on Aging grant F32AG059341 (to KPM). We thank Aleea Devitt for insightful comments.
Footnotes
A reviewer requested an analysis of the elaboration phase data. No significant results were obtained from these analyses (i.e., no effects of vividness or internal details were observed during the elaboration period of the trial).
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Addis DR, Cheng T, P. Roberts R, Schacter DL. 2011. Hippocampal contributions to the episodic simulation of specific and general future events. Hippocampus 21:1045–1052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Addis DR, Moscovitch M, McAndrews MP. 2007a. Consequences of hippocampal damage across the autobiographical memory network in left temporal lobe epilepsy. Brain 130:2327–2342. [DOI] [PubMed] [Google Scholar]
- Addis DR, Roberts RP, Schacter DL. 2011. Age-related neural changes in autobiographical remembering and imagining. Neuropsychologia 49:3656–3669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Addis DR, Schacter DL. 2012. The hippocampus and imagining the future: Where do we stand? Front. Hum. Neurosci. 5:173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Addis DR, Wong AT, Schacter DL. 2007b. Remembering the past and imagining the future: Common and distinct neural substrates during event construction and elaboration. Neuropsychologia 45:1363–1377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andrews-Hanna JR, Reidler JS, Sepulcre J, Poulin R, Buckner RL. 2010. Functional-anatomic fractionation of the brain’s default network. Neuron 65:550–562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benoit RG, Schacter DL. 2015. Specifying the core network supporting episodic simulation and episodic memory by activation likelihood estimation. Neuropsychologia 75:450–457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Binder JR, Desai RH. 2011. The neurobiology of semantic memory. Trends Cogn. Sci. 15:527–536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonnici HM, Cheke LG, Green DAE, Fitzgerald THMB, Simons JS. 2018. Specifying a causal role for angular gyrus in autobiographical memory. J. Neurosci. 38:10438–10443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonnici HM, Richter FR, Yazar Y, Simons JS. 2016. Multimodal feature integration in the angular gyrus during episodic and semantic retrieval. J. Neurosci. 36:5462–5471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buckner RL, Carroll DC. 2007. Self-projection and the brain. Trends Cogn. Sci. 11:49–57. [DOI] [PubMed] [Google Scholar]
- Cabeza R, St Jacques P. 2007. Functional neuroimaging of autobiographical memory. Trends Cogn. Sci. 11:219–27. [DOI] [PubMed] [Google Scholar]
- Campbell KL, Madore KP, Benoit RG, Thakral PP, Schacter DL. 2018. Increased hippocampus to ventromedial prefrontal connectivity during the construction of episodic future events. Hippocampus 28:76–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carpenter AC, Schacter DL. 2017. Flexible retrieval: When true inferences produce false memories. J. Exp. Psychol. Learn. Mem. Cogn. 43: 335–349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carpenter AC, Schacter DL. 2018. False memories, false preferences: Flexible retrieval mechanisms supporting successful inference bias novel decisions. J. Exp. Psychol. Gen. 147: 988–1004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper RA, Kensinger EA, Ritchey M. 2019. Memories fade: The relationship between memory vividness and remembered visual salience. Psychol. Sci. 30:657–668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D’Angiulli A, Runge M, Faulkner A, Zakizadeh J, Chan A, Morcos S. 2013. Vividness of visual imagery and incidental recall of verbal cues, when phenomenological availability reflects long-term memory accessibility. Front. Psychol. 4:1–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D’Argembeau A, Van der Linden M. 2012. Predicting the phenomenology of episodic future thoughts. Conscious. Cogn. 21:1198–1206. [DOI] [PubMed] [Google Scholar]
- D’Argembeau A, Van Der Linden M. 2004. Phenomenal characteristics associated with projecting oneself back into the past and forward into the future: Influence of valence and temporal distance. Conscious. Cogn. 13:844–858. [DOI] [PubMed] [Google Scholar]
- Dede AJO, Wixted JT, Hopkins RO, Squire LR. 2016. Autobiographical memory, future imagining, and the medial temporal lobe. Proc. Natl. Acad. Sci. 113:13474–13479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deen B, Koldewyn K, Kanwisher N, Saxe R. 2015. Functional organization of social perception and cognition in the superior temporal sulcus. 25:4596–4609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duarte A, Henson RN, Graham KS. 2008. The effects of aging on the neural correlates of subjective and objective recollection. Cereb. Cortex 18:2169–2180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eichenbaum H, Cohen NJ. 2014. Can we reconcile the declarative memory and spatial navigation views on hippocampal function? Neuron 83:764–770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fisher RA. 1950. Statistical methods for research workers. London: Oliver and Boyd. [Google Scholar]
- Friston KJ, Glaser DE, Henson RN a, Kiebel S, Phillips C, Ashburner J. 2002. Classical and Bayesian inference in neuroimaging: Applications. Neuroimage 16:484–512. [DOI] [PubMed] [Google Scholar]
- Gaesser B, Sacchetti DC, Addis DR, Schacter DL. 2011. Characterizing age-related changes in remembering the past and imagining the future. Psychol. Aging 26:80–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilboa A, Winocur G, Grady CL, Hevenor SJ, Moscovitch M. 2004. Remembering our past: Functional neuroanatomy of recollection of recent and very remote personal events. Cereb. Cortex 14:1214–1225. [DOI] [PubMed] [Google Scholar]
- Hach S, Tippett LJ, Addis DR. 2014. Neural changes associated with the generation of specific past and future events in depression. Neuropsychologia 65:41–55. [DOI] [PubMed] [Google Scholar]
- Hassabis D, Maguire EA. 2007. Deconstructing episodic memory with construction. Trends Cogn. Sci. 11:299–306. [DOI] [PubMed] [Google Scholar]
- Ingvar DH. 1979. Hyperfrontal distribution of the cerebral grey matter flow in resting wakefulness: On the functional anatomy of the conscious state. Acta Neurol. Scand. 60: 12–25. [DOI] [PubMed] [Google Scholar]
- Jing HG, Madore KP, Schacter DL. 2017. Preparing for what might happen: An episodic specificity induction impacts the generation of alternative future events. Cognition 169: 118–128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirwan CB, Bayley PJ, Galvan VV., Squire LR. 2008. Detailed recollection of remote autobiographical memory after damage to the medial temporal lobe. Proc. Natl. Acad. Sci. USA 105:2676–2680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuhl BA, Chun MM. 2014. Successful remembering elicits event-specific activity patterns in lateral parietal cortex. J. Neurosci. 34:8051–8060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurczek J, Wechsler E, Ahuja S, Jensen U, Cohen NJ, Tranel D, Duff MC 2015. Differential contributions of the hippocampus and medial prefrontal cortex to self-projection and self-referential processing. Neuropsychol. 73:116–126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwan D, Carson N, Addis DR, Rosenbaum RS. 2010. Deficits in past remembering extend to future imagining in a case of developmental amnesia. Neuropsychol. 48:3179–3186. [DOI] [PubMed] [Google Scholar]
- Lazar NA, Luna B, Sweeney JA, Eddy WF 2002. Combining brains: a survey of methods for statistical pooling of information. Neuroimage 16:538–550. [DOI] [PubMed] [Google Scholar]
- Levine B, Svoboda E, Hay JF, Winocur G, Moscovitch M. 2002. Aging and autobiographical memory: Dissociating episodic from semantic retrieval. Psychol. Aging 17:677–689. [PubMed] [Google Scholar]
- Levine B, Svoboda E, Turner GR, Mandic M, Mackey A. 2009. Behavioral and functional neuroanatomical correlates of anterograde autobiographical memory in isolated retrograde amnesic patient M.L. Neuropsychologia 47:2188–2196. [DOI] [PubMed] [Google Scholar]
- Madore KP, Schacter DL. 2016. Remembering the past and imagining the future: Selective effects of an episodic specificity induction on detail generation. Q. J. Exp. Psychol. 69:285–298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madore KP, Szpunar KK, Addis DR, Schacter DL. 2016. Episodic specificity induction impacts activity in a core brain network during construction of imagined future experiences. Proc. Natl. Acad. Sci. USA 113:10696–10701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madore KP, Gaesser B, Schacter DL. 2014. Constructive episodic simulation: Dissociable effects of a specificity induction on remembering, imagining, and describing in young and older adults. J. Exp. Psychol. Learn. Mem. Cogn. 40:609–622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madore KP, Thakral PP, Beaty RE, Addis DR, Schacter DL. 2019. Neural mechanisms of episodic retrieval support divergent creative thinking. Cereb. Cortex 29:150–166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madore KP, Schacter DL. 2016. Remembering the past and imagining the future: Selective effects of an episodic specificity induction on detail generation. Q. J. Exp. Psychol. 69:285–298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moscovitch M, Cabeza R, Winocur G, Nadel L. 2016. Episodic memory and beyond: The hippocampus and neocortex in transformation. Annu. Rev. Psychol. 67:105–134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moscovitch M, Rosenbaum RS, Gilboa A, Addis DR, Westmacott R, Grady C, McAndrews MP, Levine B, Black S, Winocur G, et al. 2005. Functional neuroanatomy of remote episodic, semantic and spatial memory: A unified account based on multiple trace theory. J. Anat. 207:35–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mumford JA, Poline JB, Poldrack RA. 2015. Orthogonalization of regressors in fMRI models. PLoS One 10:1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palombo DJ, Bacopulos A, Amaral RSC, Olsen RK, Todd RM, Anderson AK, Levine B. 2018. Episodic autobiographical memory is associated with variation in the size of hippocampal subregions. Hippocampus 28:69–75. [DOI] [PubMed] [Google Scholar]
- Rabin JS, Gilboa A, Stuss DT, Mar RA, Rosenbaum RS. 2010. Common and unique neural correlates of autobiographical memory and theory of mind. J. Cogn. Neurosci. 22:1095–1111. [DOI] [PubMed] [Google Scholar]
- Race E, Keane MM, Verfaellie M. 2011. Medial temporal lobe damage causes deficits in episodic memory and episodic future thinking not attributable to deficits in narrative construction. J. Neurosci. 31:10262–10269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raichle ME. 2015. The brain’s default mode network. Annu. Rev. Neurosci. 38:433–47. [DOI] [PubMed] [Google Scholar]
- Ramanan S, Bellana B. 2019. A domain-general role for the angular gyrus in retrieving internal representations of the external world. J. Neurosci. 39:2978–2980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramanan S, Piguet O, Irish M. 2018. Rethinking the role of the angular gyrus in remembering the past and imagining the future: The contextual integration model. Neurosci. 24:342–352. [DOI] [PubMed] [Google Scholar]
- Renoult L, Tanguay A, Beaudry M, Tavakoli P, Rabipour S, Campbell K, Moscovitch M, Levine B, Davidson PSR. 2016. Personal semantics: Is it distinct from episodic and semantic memory? An electrophysiological study of memory for autobiographical facts and repeated events in honor of Shlomo Bentin. Neuropsychologia 83:242–256. [DOI] [PubMed] [Google Scholar]
- Renoult L, Davidson PSR, Palombo DJ, Moscovitch M, Levine B. 2012. Personal semantics: At the crossroads of semantic and episodic memory. Trends Cogn. Sci. 16:550–558. [DOI] [PubMed] [Google Scholar]
- Richter FR, Cooper RA, Bays PM, Simons JS. 2016. Distinct neural mechanisms underlie the success, precision, and vividness of episodic memory. Elife 5:1–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rorden C, Karnath H, Bonilha L. 2007. Improving lesion-symptom mapping. J. Cogn. Neurosci. 19:1081–1088. [DOI] [PubMed] [Google Scholar]
- Rugg MD, King DR. 2018. Ventral lateral parietal cortex and episodic memory retrieval. Cortex 107:238–250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schacter DL. 2012. Adaptive constructive processes and the future of memory. American Psychologist 67: 603–613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schacter DL, Addis DR. (in press). Memory and imagination: Perspectives on constructive episodic simulation. In Abraham A. editor. The Cambridge Handbook of the Imagination. Cambridge (England): Cambridge University Press. [Google Scholar]
- Schacter DL, Addis DR, Buckner RL. 2007. Remembering the past to imagine the future: The prospective brain. Nat. Rev. Neurosci. 8: 657–661. [DOI] [PubMed] [Google Scholar]
- Schacter DL, Addis DR, Buckner RL. 2008. Episodic simulation of future events: Concepts, data, and applications. Ann. N. Y. Acad. Sci. 1124:39–60. [DOI] [PubMed] [Google Scholar]
- Schacter DL, Addis DR, Hassabis D, Martin VC, Spreng RN, Szpunar KK. 2012. The future of memory: Remembering, imagining, and the brain. Neuron 76:677–694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schacter DL, Addis DR. 2007. The cognitive neuroscience of constructive memory: Remembering the past and imagining the future. Philos. Trans. R. Soc. B Biol. Sci. 362:773–786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schacter DL, Addis DR. 2009. On the nature of medial temporal lobe contributions to the constructive simulation of future events. Philos. Trans. R. Soc. B Biol. Sci. 364:1245–1253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schacter DL, Addis DR, Szpunar KK. 2017b. Escaping the past: Contributions of the hippocampus to future thinking and imagination. In Hannula DE, Duff MC (Eds.) The hippocampus from cells to systems: Structure, connectivity, and functional contributions to memory and flexible cognition (pp. 439–465). New York: Springer. [Google Scholar]
- Schacter DL, Benoit RG, Szpunar KK. 2017a. Episodic future thinking: Mechanisms and functions. Curr. Opin. Behav. Sci. 17:41–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schacter DL, Devitt AL, & Addis DR. (2018). Episodic future thinking and cognitive aging. In Oxford Research Encyclopedia of Psychology. Oxford University Press. [Google Scholar]
- Sheldon S, Levine B. 2013. Same as it ever was: Vividness modulates the similarities and differences between the neural networks that support retrieving remote and recent autobiographical memories. Neuroimage 83:880–891. [DOI] [PubMed] [Google Scholar]
- Sheldon S, Levine B. 2016. The role of the hippocampus in memory and mental construction. Ann. N. Y. Acad. Sci. 1369:76–92. [DOI] [PubMed] [Google Scholar]
- Slotnick SD. 2010. Does the hippocampus mediate objective binding or subjective remembering? Neuroimage 49:1769–76. [DOI] [PubMed] [Google Scholar]
- Slotnick SD. 2013. The nature of recollection in behavior and the brain. Neuroreport 24:663–670. [DOI] [PubMed] [Google Scholar]
- Slotnick SD. 2017. Cluster success: fMRI inferences for spatial extent have acceptable false-positive rates. Cogn. Neurosci. 8:150–155. [DOI] [PubMed] [Google Scholar]
- Slotnick SD, Moo LR, Segal JB, Hart J. 2003. Distinct prefrontal cortex activity associated with item memory and source memory for visual shapes. Cogn. Brain Res. 17:75–82. [DOI] [PubMed] [Google Scholar]
- Slotnick SD, Schacter DL. 2004. A sensory signature that distinguishes true from false memories. Nat. Neurosci. 7:664–672. [DOI] [PubMed] [Google Scholar]
- Spaniol J, Davidson PSR, Kim ASN, Han H, Moscovitch M, Grady CL. 2009. Event-related fMRI studies of episodic encoding and retrieval: Meta-analyses using activation likelihood estimation. Neuropsychologia 47:1765–1779. [DOI] [PubMed] [Google Scholar]
- Spreng RN, Mar RA, Kim ASN. 2008. The common neural basis of autobiographical memory, prospection, navigation, theory of mind, and the default mode: A quantitative meta-analysis. J. Cogn. Neurosci. 21:489–510. [DOI] [PubMed] [Google Scholar]
- Squire LR, van der Horst AS, McDuff SG, Frascino JC, Hopkins RO, Mauldin KN. (2010) Role of the hippocampus in remembering the past and imagining the future. Proc Natl Acad Sci U S A 107:19044 –19048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Svoboda E, McKinnon MC, Levine B. 2006. The functional neuroanatomy of autobiographical memory: A meta-analysis. Neuropsychologia 44:2189–2208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szpunar KK, Spreng RN, Schacter DL. 2014. A taxonomy of prospection: Introducing an organizational framework for future-oriented cognition. Proc. Nat. Acad. Sci. USA 111: 18414–18421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thakral PP, Benoit RG, Schacter DL. 2017a. Imagining the future: The core episodic simulation network dissociates as a function of timecourse and the amount of simulated information. Cortex 90:12–30 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thakral PP, Benoit RG, Schacter DL. 2017c. Characterizing the role of the hippocampus during episodic simulation and encoding. Hippocampus 27:1275–1284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thakral PP, Madore KP, Addis DR, Schacter DL. In press. Reinstatement of event details during episodic simulation in the hippocampus. Cereb. Cortex. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thakral PP, Madore KP, Schacter DL. 2017b. A role for the left angular gyrus in episodic simulation and memory. J. Neurosci. 37:8142–8149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thakral PP, Wang TH, Rugg MD. 2015. Cortical reinstatement and the confidence and accuracy of source memory. NeuroImage 109:118–129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thakral PP, Yu SS, Rugg MD. 2015. The hippocampus is sensitive to the mismatch in novelty between items and their contexts. Brain Res, 1602:144–152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tulving E. 1972. Episodic and semantic memory. In Tulving E, Donaldson W. (Eds.) Organization of memory (pp. 381–403). New York: Academic Press. [Google Scholar]
- Tulving E. 1983. Elements of episodic memory. Oxford: Clarendon Press. [Google Scholar]
- Tulving E. 1985. Memory and consciousness. Canad. Psychol. 26: 1–12. [Google Scholar]
- Tulving E. 2002. Episodic memory: From mind to brain. Annu. Rev. Psychol. 53: 1–25. [DOI] [PubMed] [Google Scholar]
- Tulving E. 2005. Episodic memory and autonoesis: Uniquely human? In Terrace HS & Metcalfe J (Eds.) The missing link in cognition: Origins of self reflective consciousness (pp.3–56). Oxford: Oxford University Press. [Google Scholar]
- Vilberg KL, Rugg MD. 2008. Memory retrieval and the parietal cortex: A review of evidence from a dual-process perspective. Neuropsychologia 46:1787–1799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wing EA, Ritchey M, Cabeza R. 2015. Reinstatement of individual past events revealed by the similarity of distributed activation patterns during encoding and retrieval. J. Cogn. Neurosci. 27:679–691. [DOI] [PMC free article] [PubMed] [Google Scholar]