Abstract
Endophytic microorganisms present inside the host plant play an essential role in host fitness, nutrient supply and stress tolerance. Endophytes are often used in sustainable agriculture as biofertilizers, biopesticides and as inoculants to mitigate abiotic stresses including salinity, drought, cold and pH variation in the soil. In changing climatic conditions, abiotic stresses create global challenges to achieve optimum crop yields in agricultural production. Plants experience stress conditions that involve endogenous boosting of their immune system or the overexpression of their defensive redox regulatory systems with increased reactive oxygen species (ROS). However, rising stress factors overwhelm the natural redox protection systems of plants, which leads to massive internal oxidative damage and death. Endophytes are an integral internal partner of hosts and have been shown to mitigate abiotic stresses via modulating local or systemic mechanisms and producing antioxidants to counteract ROS in plants. Advancements in omics and other technologies have been made, but potential application of endophytes remains largely unrealized. In this review article, we will discuss the diversity, population and interaction of endophytes with crop plants as well as potential applications in abiotic stress management.
Keywords: abiotic stress, endophytes, reactive oxygen species (ROS), stress genes, plant defense system
1. Introduction
Global agricultural productivity is largely influenced by various abiotic factors including drought, salinity, cold, heat and variations in soil pH that hamper optimum agricultural yields. Changing climatic conditions and rising anthropogenic activity of growing populations accelerates the challenges of abiotic stresses [1]. However, uncertainty of climatic condition, irregularity in rainfall, heat waves and rise in the global temperatures directly affect the optimum growth and yield of crop plants because of their direct effect on reducing water availability, decreasing photosynthetic rates and creating drought conditions [2,3]. Under severe drought the level of water in the soil falls, while the salt content is increased, leading to osmotic stress and higher concentrations of salinity result in ionic toxicity and osmotic stress in roots [4]. Osmotic stress helps plants to absorb water but in saline soil, the osmotic pressure of soil solution surpasses the plant osmotic pressure and thereby reduces the uptake of water into the plant. In such circumstances, not water but ions such as Na+ and Cl− move into the plant [5]. In the current era, drought and salinity are the two most severe abiotic stress factors that affect growth and productivity of plants globally [4,6].
Drought is one of the most severe and emerging abiotic stresses that affect growth and productivity of plants via affecting several physiological and metabolic processes in crop plants [7]. Drought stress has drastic impacts on root physiology, leaf structure, nutrient uptake, photosynthetic activity and seedling germination, resulting in overall decreased growth of agricultural crops [8,9]. However, effects of drought on the plant system depends on the intensity and duration of exposure. Under short term drought, plants systems increase water use efficiency by reducing stomatal aperture and transpiration rate [10], while long term exposure of drought disrupts chloroplasts and starch granules, which directly affect photochemical activities and decrease transpiration rate of the plant [11,12].
Similarly, salinity is another challenging abiotic stress factor that severely affects physiological and metabolic processes of plants through reduce seedling growth, decreased photosynthetic activity, water stress, ion toxicity and decreased rates of protein synthesis and lipid metabolism [13,14]. Currently it has been estimated that approx. 20% of the total cultivable land faces saline stress globally and this will reach to 30% by 2050 [15]. Additionally, low rainfall and high temperatures both play crucial roles in increasing soil salinity, mainly in the arid and semi-arid regions of the world [16]. The severity of salinity on plant cells depends upon salt concentration and exposure time [17]. The onset of salinity can be seen as water stress that results in reduced leaf expansion, which further turns into complete inhibition of cell division and stomatal closure, while long-time exposure leads to premature leaf senescence resulting in decreased photosynthetic activity and ultimately death of crop plants [18,19].
Under stress, plants evoke a series of reactions in terms of signal transductions, stress responsive genes, activation or inactivation of functional proteins and responses in particular cell organelles, mainly chloroplasts, mitochondria and peroxisomes, to develop stress tolerance [20]. Plant systems also elevate their molecular behavior under stress conditions by secreting stress hormones and ROS, which functionally regulate cellular physiology to maintain normal functioning of plants [21,22].
However, to mitigate the challenges of abiotic stress and their impact on growth yield and productivity of plants, utilization of beneficial microbial strains is the most feasible, reliable and sustainable option [23]. It is known that plant microbial communities play an integral role in maintaining or enhancing growth and fitness of plants under various biotic and abiotic stress conditions [24]. In this review paper, we summarize research regarding endophytic microbial strains that are an integral part of a beneficial and sustainable approach in control of abiotic stresses including drought and salinity stresses.
2. Microbial Endophytes
Plants are host to microbial communities that include bacteria, archaea and fungi as epiphytes or endophytes. Even though, plant compartments, including phyllosphere (leaf surfaces), carpophore (fruit surfaces) and rhizosphere (root surfaces) harbor large numbers of microbes some of the microbes reside as endophytes inside the plant tissues without showing any external or apparent signs of infection [25,26]. De Bary [27] first introduced the term “endophyte” for microbes living inside plant tissue without causing any signs of infection. Petrini [28] defined endophytes as microorganisms that reside for some part of their life cycles inside host tissues. The term endophyte was first used for fungal species and later for bacterial species within plant tissues [25,29], With an advancement of omics and similar techniques, exploration of endophytic microbial communities has advanced and it has been recognized that all plants have endophytic microbes at all stages of their life cycles [30]. The dominant phyla of prokaryotic endophytes reported in the main databases (96%) include those 16S rRNA gene sequences belonging to Proteobacteria (54%), Actinobacteria (20%), Firmicutes (16%) and Bacteroidetes (6%). Members of the genus Pseudomonas, Enterobacter, Pantoea, Stenotrophomonas, Acinetobacter and Serratia are part of the main Gammaproteobacteria found as endophytes of various plant host species, and therefore, knowledge of them is deeper with respect to other less explored bacterial endophytes [25]. In the case eukaryotic endophytes, Hardoim and colleagues [25] report that there are in databases internal transcribed spacer (ITS) sequences assigned to the main phyla Glomeromycota (40%), Ascomycota (31%), Basidiomycota (20%), unidentified phyla (8%) and Zygomycota (0.1%). The phylum Glomeromycota only includes arbuscular mycorrhizal fungi (AMF), whose species have been reported as restorers of degraded ecosystems and facilitators of plant growth under diverse stress conditions [8].
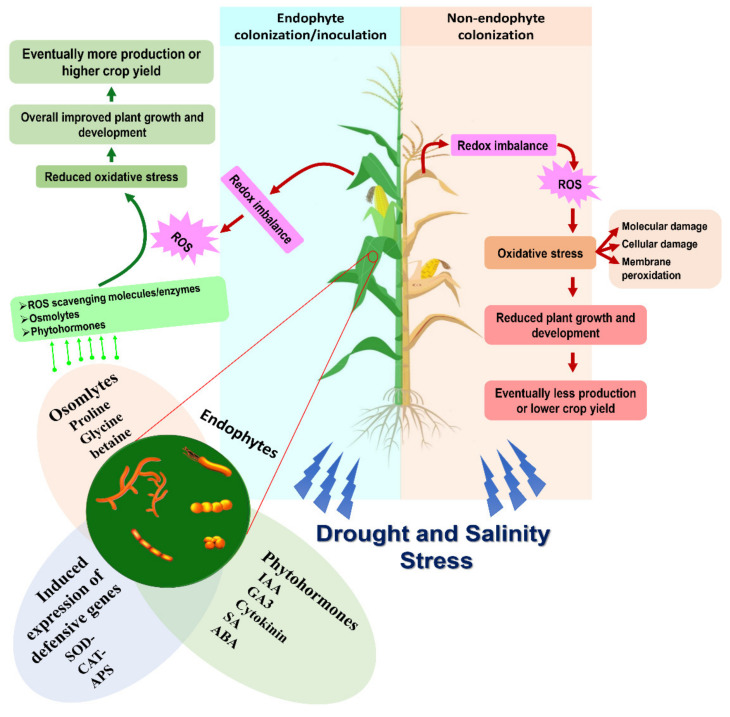
Endophytes provide support in acclimatizing crop plants under abiotic stress conditions, growth promotion and management of phytopathogens, and they help in activating stress responsive/induced genes of plants that are not usually activated under stress conditions. An overview of endophyte-mediated mechanisms for drought and salinity stress management in crop plants is provided in Figure 1.
Figure 1.
Overview of endophyte-mediated mechanisms for drought and salinity stress management in crop plants.
3. Entry and Colonization of Plants by Microbial Endophytes
During initial colonization to the host surface, endophytic microbes are confronted with immune response of the host. This may be overcome depending on the endophytic microbial strains and the particular host colonized. Successful colonization of the endophytic microbial strains is mediated by a series of reactions that are completed in several steps and regulated by genetic, metabolic or growth regulator factors [31]. In the initial step of colonization, microbes are attracted to the host surface (e.g., roots), which is facilitated through chemical exudates, including polysaccharides, amino acids, flavonoids, organic acids, etc. that act as chemo-attractants and nutrients for the microbes. Microbes move towards the host surface with the help of flagella, pilli or fimbri appendages [29], and may secrete biochemical compounds such as exopolysaccharides (EPS), lipopolysaccharides or biofilms that help in attachment of microbes to the plant surface to begin colonization [32].
The exopolysaccharides secreted by the bacterial cell facilitate endophyte colonization through attachment of bacterial cell to the host surface. Meneses et al. [33] reported, EPS secreted by Gluconacetobacter diazotrophicus Pal5 play an essential role in attachment and colonization of endophytic strain to the root surface of rice. Moreover, bacterial endophyte enters through the cell wall of the host via secreting cell wall degrading enzymes such as cellulases, endoglucanases and pectinases, that facilitate entry and colonization in the host tissue [33]. Reinhold-Hurek et al. [34] reported about endoglucanase mutant strain Azoarcus sp. BH72, having lower entry frequency of mutant strain in comparison to the wild strain. Additionally, the mutant strain is not able to spread at the aerial plant parts. However, colonization of endophytic fungi might be initiated through attachment of strain to the host surface and forming appressorium-like structures. Subsequently, fungal endophyte colonizes and migrates to the internal tissue via penetrating outer surface of the host plant [35]. Even though, cell wall remains intact during early colonization of Trichoderma to the tomato roots as observed in the microscopic study. However, in certain cases higher number of extracellular enzymes was reported in the host tissue during endophytic fungal colonization [36].
However, successful colonization of endophytes into plants involves compatible plant-microbe interactions, signaling molecules between microbes and host tissue [32,37] and depends upon various factor such as nature of microbe, host genotypes, plant exudates, nutrient availability, stress factors, as well as the surrounding environment [25]. The nature of a microbe is specific for the host plant or related group of hosts and may be mutualistic, neutral or pathogenic. The colonization patterns and efficacy of microbial strains are unique depending on the plant and microbe. For instance, pathogenic strains secrete higher amounts of cell wall degrading enzymes in comparison to symbiotic endophytes, and the entry of pathogenic strains causes increased hypersensitivity in host plants; however, symbiotic strains do not show this effect in host plants [29]. Using histochemical analysis, Chang et al. [38] proposed that secretion of the growth regulator ethylene by root cell intracellular endophytes was a first key communication in the microbe to plant interaction that triggered host root hairs to grow and release nutrients (exudates) and superoxide. To protect themselves from superoxide, endophytes produced antioxidants, including nitric oxide, to denature superoxide. These two chemical interactions between endophytes and plant cells represent key nutrient exchanges of carbon and nitrogen between the symbionts. Endophytes have also been shown to produce phytohormones, and these too may play roles in the interaction between microbe and plant [25]. Once in host tissues, endophytes move within plants via the conductive tissues, xylem and phloem [39,40]. Microbes enter into host tissues at plant meristems (root and shoot meristems) and through natural openings such as stomata, wounds, aerial parts of the plants, cotyledons or through root zone aerial parts of the plants [38,41]. The composition and diversity of endophytic microbes depends upon several factors including host genotype, plant age, plant organs, seasons and surrounding biotic and abiotic stress factors [42,43]. The physiology and metabolism of plants strongly depends on and is influenced by the associated microbiome under natural conditions [44]. Plant associated endophytic microbiomes regulate adaptive behavior against biotic and abiotic stress factors. In addition to these factors, climatic conditions, environmental stress, temperature, moisture content also influence the endophytic microbial diversity of the host plant [29]. Drought conditions affect root morphology of plants, leading to secretion of root exudates and changes that affect composition and chemical compounds in exudates, thus affecting diversity and abundance of microbes. Similarly, changing seasonal conditions also affect the microbial composition because of variation in the concentration of amino acids, proteins, sugar and organic acids [45].
Recent hypothesis proposed by Oono et al. [46], regarding endophyte species richness in the plant surviving under stress conditions. The lower nutrients and higher concentration of toxic compounds can limit the growth of fungi that may increase their diversity and predict species richness of endophyte, due to suppression of otherwise dominating species. Even though, differences in the endophytic diversity depends upon several factors such as host specificity, microclimatic conditions, seasonality. For instance, strong dry seasons can act as physiological filter for horizontally transmitted fungi, that present outside of leaves for parts of their life cycle that potentially led to lower richness of the local species pool of endophytes [47,48].
4. Reactive Oxygen Species and Abiotic Stress Factors
Reactive oxygen species (ROS) may be considered endogenously produced signal molecules or regulators produced by several plant organelles, including mitochondria, chloroplast or peroxisomes under stresses. ROS consist of a group of chemically reactive oxygen molecules such as hydrogen peroxide (H2O2), superoxide radical (O2•-), hydroxyl radical (OH•) and singlet oxygen (1O2) and are produced in plants under stress conditions [49,50].
Abiotic stress leads to overproduction of ROS that must be managed in a homeostatic pool; however, excess concentrations of ROS cause oxidative stress, which results in denaturation of protein structure, lipid peroxidation, nucleotide disruption and may affect plant physiology which ultimately leads to the death of plants [51].
In the plant system, mitigation of ROS excess concentrations generally leads to activation of either enzymatic or non-enzymatic antioxidant systems. Plants secrete several enzymes, including catalase (CAT), ascorbate peroxidase (APX), superoxide dismutase (SOD), glutathione reductase (GR), dehydroascorbate reductases (DHAR) and monodehydroascorbate reductases (MDHAR,); the nonenzymatic system involves quenching of ROS via synthesis of ascorbic acid (AsA), glutathione (GSH), carotenoids which quench free radicals and protect the plant cell from oxidative stress [52,53].
5. ROS and Signaling Molecules under Abiotic Stress
The plant system shows adaptive response under stress conditions at certain levels, via activating stress tolerance genes. However, crossing the tolerance limits, the sensor presents in the plant system, for instance gene COLD1, responsible for detecting cold stress in rice, senses the stress signal and responds [54]. Under homeostatic conditions, plants maintain a fine balance between production and quenching of ROS, while overproduction of ROS at the cellular level under stress conditions hampers the natural physiological or metabolic state of plants. ROS, however, play significant role in various functions including development via oxidizing polysaccharides in cell walls [55] or programmed cell death [56]. Moreover, the ROS produced in the various cellular compartments alter transcriptional or transcriptome levels [57]. The production, temporal and spatial distributions of ROS under different environmental conditions act as signal molecules [58]. Under stress conditions, NADPH bound by cytosolic membranes produces hydrogen peroxide (H2O2) that acts as a signaling molecule. Even though, production of moderate levels of H2O2 and O2 in peroxisomes act as signaling molecules [59]. In addition to ROS, several other signaling molecules such as phytohormones especially ABA, ethylene Ca2+, NO2, inositol phosphates and systemin, also serve as signaling molecules. The signaling molecules actively involved in regulating various biological functions of the plants system including modulation of gene expression, homeostasis under stress conditions [60,61].
6. Endophyte Mediated Drought and Salinity Stress Management
The endophytic microbiome shows mutualistic relations with the host plant in maintaining health or vigor [25,62,63]. Moreover, also essentially involved directly or indirectly in the growth and development of host plants via secreting various growth promoting attributes viz. phytohormone synthesis, nutrient acquisition and siderophore production, antibiotic phosphate solubilization, and by mitigating various biotic and abiotic conditions [64,65].
However, this has been explained in previous studies on the impacts of drought and salinity stress on the effect of growth, productivity or survivability of plants [63]. In a study Zhou et al. [66] reported improved seedling growth of Pinus tabulaeformis after inoculation of endophytic strain Phoma sp under draught condition. Wu et al. [67] reported decreased leaf area, photosynthetic pigments and photosynthetic efficiency under drought stress. Higher salinity in soil affects the survivability of plants by altering chemical, morphological and physiological processes [15].
In this context, microbial endophytes appear to be a suitable alternative for drought and salinity stress management. In the recent past, various microbial strains have been successfully utilized to increase drought tolerance. Inoculation of microbial endophytes or exogenous supply of phytohormones, significantly enhanced adaptive behavior of plants via improving photosynthetic activity, chlorophyll contents, root growth, water status, antioxidant enzymes, phytohormone signaling and nutrient uptake under drought conditions [68,69,70,71,72].
The latest published reports reinforce the utilization of endophytic strains in abiotic stress management. Naveed et al. [71] reported improved growth, water availability, as well as photosynthetic activity in maize cultivars under drought after inoculation of endophytic bacterial strains Burkholderia phytofirmans strain PsJN and Enterobacter sp. FD17. The endophytes inoculation improved seedling growth, shoot and root biomass and photochemical efficiency of PSII. Yandigeri et al. [71] demonstrated potential of endophytic bacterial strains Streptomyces coelicolor DE07, S. olivaceus DE10 and S. geysiriensis DE27, isolated from arid and drought affected regions, to increase tolerance of plants to intrinsic water stress and showed plant growth promotion after application to wheat seedlings. Additionally, the combined application of S. olivaceus DE10 + S. geysiriensis DE27 strains showed synergistic effects and showed improved response in terms of stress mitigation and growth promotion.
Jayakumar et al. [73] reported that several endophytic bacterial strains, including Bacillus sp., Providencia sp. and Staphylococcus spp., isolated from Ananas comosus, enhanced drought tolerance, and promoted growth as well as pathogen resistance. Similarly, Sandhya et al. [74] reported that several endophytic bacterial strains isolated from various crops in which most of the strains conferred drought tolerance up to (−1.02) matric potential also had growth promotion potential. Chen et al. [75] reported endophytic strain Pantoea alhagi isolated from Alhagi sparsifolia, after inoculation, enhanced the growth of wheat seedlings under drought conditions, additionally the endophyte-treated plant showed enhanced accumulation of soluble sugars and decreased concentrations of malondialdehyde. In the grass Brachypodium distachyon, drought stress was mitigated with the help of an endophytic bacterium Bacillus subtilis B26, which also upregulated the stress responsive genes [76]. Morsy et al. [77] reported that the endophytic fungal strains Ampelomyces sp. and Penicillium sp., isolated from stress inducing soil (drought and high salinity), enhanced drought tolerance (Ampelomyces sp.) and salinity tolerance (Penicillium sp.) in tomato. Table 1 summarizes some of the works reviewed here.
Table 1.
Examples of studies reporting beneficial activities between microbial endophytes and their plant host under drought and salinity stress conditions.
| Endophytic Strain | Type | Type of Stress | Mechanism of Stress Amelioration and/or Beneficial Activity |
Plant Host | Ref. |
|---|---|---|---|---|---|
| Phoma species | Fungi | Drought | Increased Proline Peroxidase (POD), Catalase (CAT), Superoxide dismutase (SOD) |
Pinus tabulaeformis | [66] |
|
Glomus mosseae, G. versiforme and G. diaphanum |
Fungi | Drought | Increment of peroxidase activity and beneficial effects on soil structure | Poncirus trifoliata | [67] |
| Endophyte consortia (Rhodotorula graminis, Burkholderia vietnamiensis, Rhizobium tropici, Acinetobacter calcoaceticus, Rahnella sp., Burkholderia sp., Enterobacter asburiae, Sphingomonas yanoikuyae, Pseudomonas sp., Curtobacterium sp.) |
Fungi + bacteria |
Drought | Reduced damage by reactive oxygen species (ROS), Increment of IAA | Populus sp. | [68] |
| Bacillus, Achromobacter, Klebsiella and Citrobacter | Bacteria | Drought | Production of 1-aminocyclopropane-1- carboxylate (ACC) deaminase |
Capsicum annuum L. | [69] |
| Burkholderia phytofirmans PsJN and Enterobacter sp. FD17 | Drought | Reduced H2O2 induced damage | Zea mays L. | [71] | |
|
Streptomyces coelicolor DE07, S. olivaceus DE10 and Streptomyces geysiriensis DE27 |
Bacteria | Drought | Phytohormone (IAA) synthesis and increment in water stress tolerance |
Triticum aestivum | [72] |
| Bacillus sp. Acb9, Providencia sp. Acb11, Staphylococcus sp. Acb12, Staphylococcus sp. Acb13 and Staphylococcus sp. Acb14 | Bacteria | Drought | Production of indole acetic acid, ACC deaminase and promotion of plant growth |
Ananas comosus, Vigna radiata | [73] |
|
Pseudomonas spp., Acitenobacter brumalii strain MZ30V92, Enterobacter asburiae strain MRC12, Sinorhizobium meliloti strain MRC31 |
Bacteria | Drought | Multiple plant growth-promoting traits | Not evaluated | [74] |
| Pantoea alhagi strain LTYR-11ZT | Bacteria | Drought | Increment on accumulation of soluble sugars, decreased accumulation of proline and malondialdehyde, and decreased degradation of chlorophyll in leaves of drought-stressed wheat plants |
Arabidopsis and wheat | [75] |
| Bacillus subtilis B26 | Bacteria | Drought | Upregulation of the drought-response genes, such as DREB2B-like, DHN3-like and LEA-14-A-like and modulation of the DNA methylation genes, such as MET1B-like, CMT3-like and DRM2-like, that regulate the process |
Brachypodium
distachyon |
[76] |
| Ampelomyces sp. and Penicillium sp. | Fungi | Drought and salinity | Enhanced plant growth, stress tolerance, recovery and fruit yield |
Tomato plants | [77] |
| Bacillus subtilis BERA 71 | Bacteria | Salinity | Enhanced level of ROS scavenging antioxidant enzymes (superoxide dismutase, peroxidase, catalase) |
Cicer arietinum seedling |
[78] |
| Streptomyces sp. | Bacteria | Salinity | Increased proline, K+, Ca+ and water contents and decreased ethylene, ROS, Na+ and Na+/K+ ratio | Oryza sativa seedling | [79] |
| Epichloë bromicola | Fungi | Salinity | Increased photosynthesis, chlorophyll content, antioxidant capacity and glycine betaine content |
Hordeum brevisubulatum Seedling |
[80] |
| Curvularia sp. | Fungi | Salinity | Elevates antioxidant enzymes (SOD and APX) |
Poplar plant | [81] |
| Piriformospora indica | Fungi | Salinity | Modulation of the expression levels of the major Na+ and K+ ion channels and balanced ion homeostasis of Na+/K+ |
Arabidopsis thaliana | [82] |
| Brachybacterium paraconglomeratum | Bacteria | Salinity | Enhanced level of proline, MDA, IAA in the inoculated plants | Chlorophytum borivilianum | [83] |
| Trichoderma harzianum | Fungi | Salinity | Reduces lipid peroxidation |
Lycopersicum esculentum seed |
[84] |
| Piriformospora indica | Fungi | Salinity | Enhanced plant growth and attenuated the NaCl-induced lipid peroxidation, metabolic heat efflux and fatty acid desaturation in leaves. In addition, significantly elevated the amount of ascorbic acid and increased the activities of antioxidant enzymes catalase, ascorbate peroxidase, dehydroascorbate reductase, monodehydroascorbate reductase and glutathione reductase |
Hordeaum Vulgare
Seedling |
[85] |
7. Phytohormone Modulation of Oxidative Stress Tolerance
It is well established that phytohormones play an essential role in maintaining the normal physiological and metabolic behavior of plants under stress conditions via adaptive responses [86,87]. Auxin (IAA), cytokinin (CK), gibberellin (GA); ethylene and abscisic acid (ABA) are the most common. Salicylic acid (SA), nitric oxide (NO), nitrogen dioxide (NO2), strigolactone (SL) and brassinosteroids (BR) are additional phytohormones that may also regulate plant growth under normal or stress conditions [88]. The coordinated synergistic and antagonistic effects of phytohormones essentially play an active role in stress management. The hormones auxin and cytokinin promote stomatal opening, ABA and ethylene regulation lead to stomatal closure under drought stress conditions [87].
Endophytic microorganisms produce hormones are stimulate indigenous levels of plant hormones; thus, endophytes modulate developmental or signaling processes in plants [89]. The phytohormones ethylene, IAA, GA and cytokinins are very commonly synthesized by endophytic microbial strains, which directly or indirectly modulate the growth of host plant cells and tissues [90]. The function of endophyte-produced phytohormones is very likely to stimulate plant cell growth in order to trigger release of nutrients to the endophytes [30]. There are numerous reports that show the effect of endophyte phytohormones to mitigate abiotic stress. Waqas et al. [91] reported improved macronutrient absorption in soyabean after inoculation with phytohormone secreting Galactomyces geotrichum endophytes. Zamioudis et al. [92] reported auxin transport potential of a strain of Pseudomonas that improved the architecture of the Arabidopsis root system. Verma et al. [90] reported auxin synthesis by strains of Pseudomonas sp. and Pantoea dispersa isolated from rice seeds, which after inoculation enhanced root and root hair growth of rice seedlings. Similarly, Shahzad et al. [93] reported that endophytic bacterial strain Bacillus amyloliquefaciens from rice seeds produced gibberellins (GAs) and their functional aspect of improving host physiology. The inoculation of B. amyloliquefaciens significantly enhanced SA production and decreased the concentration of endogenous abscisic acid and jasmonic acid in rice seedlings.
Further, in a study, Shahzad et al. [94] reported efficacy of the endophytic bacterial strain Bacillus amyloliquefaciens, which after inoculation significantly produced ABA with beneficial responses in the plant in mitigating salinity stress in the plant. Additionally, the rice inoculated with B. amyloliquefaciens significantly enhanced growth as well as enhanced levels of some essential antioxidant amino acids such as cysteine, aspartic acid, glutamic acid, phenylalanine and proline under stress conditions. Similarly, Bodhankar et al. [95] studied pre-treatment effects of maize seed with the endophytic strains Corynebacterium hansenii and Bacillus subtilis, which after inoculation improve growth and physiology of maize under drought stress. In addition, pre-treatment with C. hansenii improved relative water content, leaf proline and chlorophyll contents, whereas pre-treatment with B. subtilis enhanced fresh or dry weight of maize over the control plants under drought conditions. Rehman et al. [96] tested an endophytic Pseudomonas sp. strain, which after seed priming, improved growth and Zn status in the wheat. Even though, the author reported maximum yield enhancement after seed priming whereas soil and foliar application improved protein content, Zn concentration in the aleurone layer, endosperm and also in the overall grain.
In addition, ACC deaminase (1-aminocyclopropane-1-carboxylate) enzymes synthesized by endophytic microbial strains lower the ethylene levels in plants during stress conditions [97]. Jaemsaeng et al. [79] reported that the endophytic bacterial strain Streptomyces sp. imparted enhanced salt tolerance in rice through the action of 1-aminocyclopropane-1-carboxylate deaminase (ACCD) by converting a precursor of ethylene into ammonia and α-ketobutyrate, which consequently reduced ethylene levels in plants. Similarly, inoculation of Nicotiana attenuata with Sebacina vermifera improve fitness by altering ethylene signaling by the reduction of 1-aminocyclopropane-1-carboxylic acid (ACC) [98].
Further, in a study by Barnawal et al. [83], investigators reported improved salinity stress tolerance in Chlorophytum borivilianum after inoculation with endophyte Brachybacterium paraconglomeratum that decreased the concentration of ethylene through the deamination of ACC. In addition, improved growth and higher levels of antioxidant proline was observed in the endophyte treated plants. It is evident that ethylene is a key hormone that is impacted by endophytes that increase root growth and confer increased stress tolerance [41]. Other compounds that include antioxidant nitric oxide and nitrogen dioxide, that may also be growth promotional, may play key roles and act synergistically with ethylene to modulate plant stress [41]. Numerous papers suggest that ACC deaminase is a mechanism that contributes to increased endophyte-mediated stress tolerance, however, other evidence indicates that ACC deaminase is an incomplete or incorrect mechanism to explain endophyte-mediated stress tolerance [25,41]. Future work is needed to identify the precise microbe-plant interactions that result in endophyte-mediated stress tolerance.
8. Endophyte-Mediated Oxidative Stress Management
ROS generation within plants naturally occurs as plants undergo normal metabolic activities [99,100]. Under normal conditions, ROS act as signaling molecules and serve to maintain symbiosis with intracellular symbiotic bacteria [30,41,101], however, plants maintain homeostasis conditions through use of ROS scavengers, including antioxidant amino acids, enzymes and other antioxidant systems. However, the state of loss of equilibrium between generation and scavenging of ROS leads to excess ROS and oxidative damage to nucleotides, proteins, lipids and ultimately cell death [96]. Moreover, each cell compartment has specific mechanisms of ROS homeostasis or signaling depending upon the type of cell, level of stress and ROS gene network [58,102,103].
Inoculation of endophytic microbes into plants significantly mitigates the damage of oxidative stress caused by abiotic stress agents. The mechanisms used by endophytic microbes against salinity stress are similar to those used for drought stress. Moreover, colonization by endophytic microorganisms enhances plant levels of antioxidant enzyme concentrations such as (catalase (CAT), superoxide dismutase (SOD), peroxidase (POD) ascorbate peroxidase (APX) [63,104] or non-enzymatic antioxidant molecules such as AsA, GSH and carotenoids [105,106].
Endophytes induce synthesis of antioxidants to balance an array of free radicals that maintain normal cellular functioning. In addition, production of osmolytes maintain sodium-potassium ratio, which overcome the osmotic effect caused by stress factors [83]. Published reports in the recently reinforce findings of effective endophyte-modulated tolerance to ROS in plants under salinity and drought stress conditions [107,108]. Redman et al. [107] observed that endophyte inoculations significantly decreased the accumulated ROS in the plant cell by activating antioxidant enzymes. Baltruschat et al. [85] reported reduced levels of CAT, APX, GR DHAR in root tissues of barley under saline conditions. However, root colonization by Piriformospora indica elevated the antioxidant enzyme and ascorbic acid in the barley roots. Additionally, inoculation by P. indica significantly enhanced plant growth and attenuated NaCl-induced lipid peroxidation. Similarly, Zhang et al. [109] evaluated colonization potential of Trichoderma longibrachiatum T6 in wheat seedlings under 150 mM NaCl saline concentration. However, endophyte inoculation significantly enhanced chlorophyll content, root activity and proline accumulation in leaves. The inoculation significantly enhanced the concentration of antioxidant enzymes, mainly SOD, POD, CAT in wheat seedlings. Azad and Kaminskyj [110] reported that endophytic fungal strains Alternaria spp. and Trichoderma harzianum inoculation into tomato seedlings under salinity and drought stress conditions resulted in maintenance of photosynthetic efficiency and effectively reduced ROS accumulation. Abd-Allah et al. [78] extensively studied the inoculation impact of Bacillus subtilis in chickpea plants under saline conditions and observed enhanced levels of ROS scavenging antioxidant enzymes superoxide dismutase, peroxidase, catalase and glutathione reductase as well as ascorbic acid and glutathione; B. subtilis also enhanced plant biomass and photosynthetic pigments. Ahmad et al. [111] evaluated inoculation impact of Trichoderma harzianum in mustard seedlings and found that it significantly enhanced shoot and root length, and plant dry weight compared to non-inoculated plants under salinity stress conditions. Moreover, endophyte inoculation significantly enhanced the oil content and chlorophyll ‘a’, which was negatively impacted by NaCl concentration, in addition proline concentration was also enhanced, showing modulation of osmolytes and antioxidants in mustard seedlings. Therefore, exclusion or accumulation of Na+ concentration in the cell sap or plant cell is necessary to avoid stress [13,112]. To avoid the oxidative stress caused by Na+ in plants, exclusion of Na+ from the leaf surface is the most common phenomenon and reported by various authors from cereal crops studies [113]. However, failure of Na+ exclusion affects a plant’s morphology and causes premature death of older leaves, and the effect of toxicity varies with plant species and duration of exposure [13].
In a pot experiment root colonization by Pseudomonas pseudoalcaligenes of the model plant Arabidopsis improved growth under salinity stress and the possible reason for that tolerance was modulation in the expression levels Na+ and K+ ion channels that maintain ionic homeostasis of Na+/K+ and expression levels of stress genes [81]. Similarly, Eida et al. [114] reported that endophytic strain treatment resulted in tissue-specific transcriptional changes of ion transporters and reduced Na+/K+ shoot ratios in Arabidopsis under salinity stress conditions.
According to the Habitat-Adapted Symbiosis Hypothesis, plants select endophytes from soils in order to increase tolerance to the specific stressors in that particular environment/habitat [115]. Endophytic colonization modulates gene expression levels to maintain stress tolerance. In a study, Piriformospora indica colonization into Brassica campestris subspecies chinensis confered salinity tolerance and higher expression levels of some specific salt tolerance genes, including SOS1 and SOS2, NHX-type [116]. The effective colonization of Piriformospora indica also elevated the antioxidant enzymes SOD; POD, CAT and elevated phytohormones, mainly SA, GA, that are directly involved in stress tolerance [117].
9. Studying the ‘Ome’ of Plant-Endophyte Interactions under Abiotic Stress
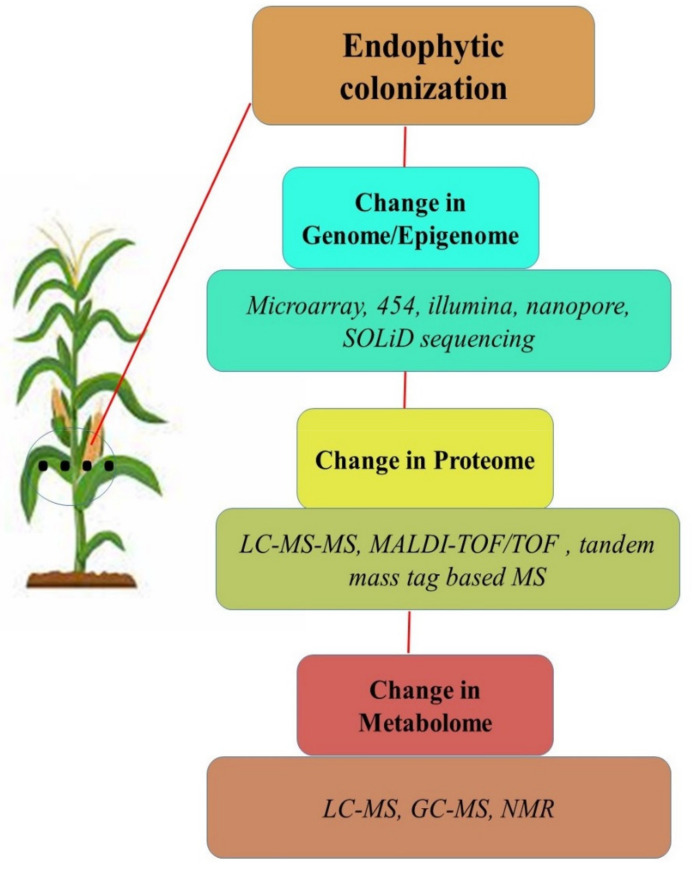
Endophytic microbes are known to modulate the genome, epigenome, proteome and metabolome of their hosts after inoculation to cope with abiotic stress. Plants with their modulated ‘ome’ after inoculation with endophytes bear better potential to ameliorate various abiotic stresses including drought and salinity. The molecular basis of endophytes in mitigating abiotic stress in crops is poorly understood. The recent developments in high-throughput technologies of sequencing and mass-spectroscopy based omics techniques have generated hopes for a detailed gene and protein study of molecular insights into the interaction of plant-endophytes during abiotic stress conditions (Figure 2).
Figure 2.
Overview of the ‘ome’ of plant-endophyte interactions under abiotic stress.
The whole genome sequencing of endophytic bacteria revealed the presence of biofilm associated and fusaric acid resistant genes, which can play a crucial role in amelioration of abiotic stress in their hosts [118]. Similarly, genome-sequencing analysis of abiotic stress tolerant endophytic fungus Pirifomospora indica, showed the presence of stress tolerant genes [119]. Proteomic studies of the same fungus showed accumulation of photosynthesis, energy related proteins under drought conditions. Whole genome sequencing of endophytic fungi Harpophora oryzae and Xylona heveae demonstrated the presence of genes required for nutrient acquisition, which can provide abiotic stress tolerance to crops [120]. Many plant-symbiotic fungi, bacteria, yeasts and actinomycetes have been sequenced for their transcriptome, proteome and metabolome, and this has confirmed the presence of multiple plant growth promoting and stress tolerant traits [121].
Culture-independent sequencing approaches including metagenomics, metatranscriptomics and metaproteomics have emerged as new tools for studying the unexplored wealth of endophytes for conferring abiotic stress tolerance in plants. Shotgun metagenome analysis of uncultured microbe communities of endophytic bacteria revealed the population of Proteobacteria and Actinobacteria which can play a role in plant-growth promotion and abiotic stress tolerance [122]. Change in endophytic bacterial communities of wheat, as assessed by 16S rRNA sequencing, was associated with change in drought stress conditions [123].
Not only the endophytes, but the ‘ome’ of plants is also modulated during their interactions with endophytes while coping with abiotic stress. The ‘omics’ of endophytes also may be modulated by ‘horizontal gene transfer’ and synergism while interacting with their host crop [124]. Coutinho et al. [125] reported the influence of host crop Oryza sativa on gene expression of endophytic Burkholderia kururiensis M130 was related to biofilm regulation and iron transport. Some of the endophytic Rhizobium and Xanthomonas sp. associated with crops have shown transfer of genes responsible for plant adaptation and survival [126]. Comparative transcriptomics and proteomics studies associated with Atractylodes lancea in response to endophytic fungus Gilmaniella sp. AL12 revealed regulated plant metabolites, with upregulation in terpene skeleton biosynthesis and upregulated genes annotated as β-farnesene synthase and β-caryophyllene synthase [127]. Similarly, to understand the interaction of endophytic Piriformospora indica and host Brassica napus, an LC-MS/MS based label-free quantitative proteome technique was used, revealing the change in metabolic pathways, stress response and increase in stress adapting metabolites after endophytic interactions [128].
Understanding the roles of endophytes-plant interactions at a molecular level is crucial to understanding crop coping mechanisms to abiotic stress and may lead to more sustainable agriculture. The uncultured microbiome of endophytes can also be exploited for coping the abiotic stress using next generation of sequencing technologies.
10. Hurdles and the Way Forward
Externally applied endophytes have shown to be promising for amelioration of abiotic stress as evident by multiple studies; although in some cases their high performance does not always hold under field conditions. To ensure their efficiency in large scale application and commercialization of the endophyte-based products for amelioration of abiotic stress, several factors should be optimized as indicated below.
10.1. Lack of Standard Protocol for Surface Sterilization and Endophyte Isolation
Isolation of endophytes is a primary step toward developing applications in crops using endophytes. However, there is still a lack of consensus for standard surface-sterilization techniques to remove the epiphytic microbiota from the plant surface. Currently for plant surface sterilization, several disinfectants such as ethanol (70%) and bleach (5%, 3%, 2.7%) for different time intervals have been used, but sometimes higher concentrations of the sterilizing agents may damage the plant tissue, which affects the endophytic microbial community obtained [27]. In addition, the latest Next Generation Sequencing (NGS) improves our understanding of both epiphytic and endophytic microbiomes. However, experiments using non-cultivable microbes are difficult, and thus NGS still leaves limitations for practical application of endophytes [29,33].
10.2. Endophytes Should Ameliorate Multiple Abiotic Stresses and Should Be Good Plant Colonizers with Broad Host Ranges
Under laboratory conditions, endophytes can be screened for a single abiotic stress such as drought or salinity, but in field conditions, the host plant may face multiple stresses simultaneously. To cope with field stresses, the endophytes should confer tolerance to multiple abiotic stresses. Endophytic microbes should be able to colonize diverse plants and crops so that their application is not limited to few crops [129]. Moghaddam et al. [130] proposed the isolation of endophytes from extreme habitats (e.g., deserts, tundra, high elevations, etc.) for better amelioration of multiple stresses.
10.3. Endophytes Should Be Good Soil and Plant Competitors to Compete with Native Soil and Plant Microbes for Entry into Plant Tissues
One major impediment to use of endophytes under field conditions is the presence of native soil microbes and endophytes that outcompete the applied biostimulant microbes for entry into plants. Microbes that are poor competitors with other plant or soil microbes may be excluded from entry into plants because of the presence of other microbes that are in much higher concentrations in soils and in root tissues, effectively blocking their entry. For application under field conditions, biostimulant microbes should be better at entering into plant tissues than many other soil microbes. The qualities that make an endophytic microbe a better competitor are not yet fully understood.
10.4. Endophytes Should Not Be Plant or Animal Pathogens
Before commercialization, endophytes should be screened in-planta for pathogenicity or production of toxins. Some fungal and bacterial endophytes may not produce toxins in culture, but in plants or with other microbes they may produce toxic metabolites [131]. Many stress-tolerance-conferring endophytes, including Colletotrichum sp., Alternaria sp., Fusarium sp. and Aspergillus sp., may also be producers of mycotoxins [132].
10.5. Exogenously Applied Endophytes Should Not Interfere with Functions of the Plant Microbiome
For sustainable agriculture, it is necessary that applied endophytes, or the metabolites isolated from the endophytes, should not affect the host plant microbiome negatively. White et al. [30] showed that some endophytes enter into plant roots and interfere with the rhizophagy process and oxidative extraction of nutrients from native microbes in root cells. The interference with oxidative nutrient extraction from microbes in root cells was termed ‘endobiome interference’ [133]. Endobiome interference may occur if the microbe is highly resistant to reactive oxygen (superoxide) that is used in plant root cells to control and extract nutrients from internalized microbes [41]. Microbes that cause endobiome interference will enhance stress and reduce fitness in plants, causing growth inhibition and reducing nutrient absorption into plants. Incompatible endophytes may thus further hamper a plants’ capacity for stress tolerance [133,134]. The goal thus is to add endophytes that synchronize with the native microbiome, improve plant development, enhance nutrient acquisition and enhance the ability of plants to tolerate abiotic stresses [41]. It is imperative to check interactions between the native plant microbiome (particularly in roots) and the exogenously applied endophytes.
10.6. Endophyte-Based Formulations Should Be Optimized for Economical and Sustained Release under Field Conditions
For application of endophytes or endophyte-derived metabolites, they may be formulated in liquid or powder form. Under field conditions, activity of the biostimulant microbes or metabolites depend upon the type of formulation, humidity and temperature of the environment and the type of active ingredients [129]. For successful application of the formulations, it is necessary to optimize every parameter, specifically for abiotic stress conditions. Further, for a successful product, it should be economical. Economy is achieved when a single application of the microbe product results in persistence in the field. Multiple applications increase the cost of the biostimulant product. Several carriers such as chitosan, milk protein and maltodextrins have been used in formulations to increase shelf life and support initial inoculum growth after application to plants [135].
10.7. Better Public Awareness, Biological Product-Friendly Government Policies and Streamlined Registration Processes Are Needed
The consumers of an endophyte-based product are commercial growers, gardeners and homeowners. The uncertainty regarding an unknown biological product may discourage the use of new biological technologies including those based on endophytes. Articles for general audiences, awareness programs, workshops and outreach activities should be conducted at grass root levels to educate potential consumers and local vendors regarding endophyte modes of action and benefits to the environment and human health. Government policies and the registration process for agricultural biostimulants differ from country to country [136]. Governments should support endophyte-based biostimulants by changing policies and laws and allowing easier registrations of biological products. Government and industry partnerships to fund research on endophyte-based technologies could help move endophytes from the lab to the market. The results of this effort would be a less contaminated environment and a more sustainable agricultural system that has increased resilience to confront future climate perturbations.
11. Conclusions and Future Perspectives
Microbial endophyte biology is a growing field of research. The increasing output of research articles over the past two and half decades show that there has been an increasingly growing interest among researchers in the study of endophytic microbes. A significant body of knowledge has been accumulated over these years with regard to endophytic microbes and their effects on plants. We now know that communities of microbes [30] colonize plants in their shoots and roots. In many cases, microbes actually enter into plant cells themselves [30,41,137,138]. Studies on intracellular microbes involved in the rhizophagy cycle suggest that the interaction between endophytic microbe and the plant may be very intimate to the extent of a direct protoplast interaction within plant cells [30,41]. What is currently lacking is knowledge of the intimate microbe cell to plant cell interactions or ‘cross talk’ that results in all the beneficial effects in plants. What are the ‘words’ (or ‘signals’) uttered between endophytes and host plant cells that result in oxidative stress tolerance in plants? What is known is that plants respond to this interaction with intracellular endophytes by secretion of ROS [30,41]. The jury is not in yet, but it may be the host response to the endophytes with ROS (superoxide) that results in plant expression of increased oxidative stress coping systems. The signal sent to host cells that triggers the oxidative response may be the key to understanding the endophyte-host interaction. It is in that cross talk between endophyte and host that determines if the plant recognizes the microbes as friendly endophytic microbe or pathogen. We look toward the future when we may learn more about this intimate conversation between endophyte and host cells. Current and future research must focus on microbial endophytes to improve plant/crop productivity and create a more sustainable agricultural system where environmental degradation due to excessive agrochemicals is minimized.
Acknowledgments
Authors are thankful to the Agriculture Research Organization for providing lab facilities.
Author Contributions
Conceptualization, A.K., S.D. and J.F.W.; methodology, A.K.; investigation, J.F.W. and A.K.; writing—original draft preparation, H.V., D.K., M.K., V.K.S., V.K. and S.K.S.; writing—review and editing, G.S., J.F.W. and S.D. All authors have read and agreed to the published version of the manuscript.
Funding
Funding support was provided to J.W. from USDA-NIFA Multistate Project W4147 and the New Jersey Agricultural Experiment Station.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
No authors have conflict of interest.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Singh D., Singh S.K., Singh V.K., Verma H., Mishra M., Rashmi K., Kumar A. Plant growth-promoting bacteria: Application in bioremediation of salinity and heavy metal–contaminated soils. In: Kumar A., Singh V.K., Singh P., Mishra V.K., editors. Microbe Mediated Remediation of Environmental Contaminants. Woodhead Publishing; Sawston, UK: 2021. pp. 73–78. [DOI] [Google Scholar]
- 2.Wang Y., Frei M. Stressed food—The impact of abiotic environmental stresses on crop quality. Agric. Ecosyst. Environ. 2011;141:271–286. doi: 10.1016/j.agee.2011.03.017. [DOI] [Google Scholar]
- 3.Hu H., Xiong L. Genetic engineering and breeding of drought-resistant crops. Annu. Rev. Plant Biol. 2014;65:715–741. doi: 10.1146/annurev-arplant-050213-040000. [DOI] [PubMed] [Google Scholar]
- 4.Ma Y., Dias M.C., Freitas H. Drought and salinity stress responses and microbe-induced tolerance in plants. Front. Plant Sci. 2020;11:1750. doi: 10.3389/fpls.2020.591911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Munns R., Passioura J.B., Colmer T.D., Byrt C.S. Osmotic adjustment and energy limitations to plant growth in saline soil. New Phytol. 2020;225:1091–1096. doi: 10.1111/nph.15862. [DOI] [PubMed] [Google Scholar]
- 6.Kumar A., Singh V.K., Tripathi V., Singh P.P., Singh A.K. Plant Growth-Promoting Rhizobacteria (PGPR): Perspective in Agriculture under Biotic and Abiotic Stress; Crop Improv. Through Microb. Biotechnol. 2018:333–342. doi: 10.1016/B978-0-444-63987-5.00016-5. [DOI] [Google Scholar]
- 7.Chaves M.M., Flexas J., Pinheiro C. Photosynthesis under drought and salt stress: Regulation mechanisms from whole plant to cell. Ann. Bot. 2009;103:551–560. doi: 10.1093/aob/mcn125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zhang Z., Zhang J., Xu G., Zhou L., Li Y. Arbuscular mycorrhizal fungi improve the growth and drought tolerance of Zenia insignis seedlings under drought stress. New For. 2019;50:593–604. doi: 10.1007/s11056-018-9681-1. [DOI] [Google Scholar]
- 9.Li J., Cang Z., Jiao F., Bai X., Zhang D., Zhai R. Influence of drought stress on photosynthetic characteristics and protective enzymes of potato at seedling stage. J. Saudi Soc. Agric. Sci. 2017;16:82–88. doi: 10.1016/j.jssas.2015.03.001. [DOI] [Google Scholar]
- 10.Li P., Yang H., Wang L., Liu H., Huo H., Zhang C., Liu A., Zhu A., Hu J., Lin Y., et al. Physiological and transcriptome analyses reveal short-term responses and formation of memory under drought stress in rice. Front. Genet. 2019;10:55. doi: 10.3389/fgene.2019.00055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zhou X., Zhou L., Nie Y., Fu Y., Du Z., Shao J., Zheng Z., Wang X. Similar responses of soil carbon storage to drought and irrigation in terrestrial ecosystems but with contrasting mechanisms: A meta-analysis. Agric. Ecosyst. Environ. 2016;228:70–81. doi: 10.1016/j.agee.2016.04.030. [DOI] [Google Scholar]
- 12.Shao R.X., Xin L.F., Zheng H.F., Li L.L., Ran W.L., Mao J., Yang Q.H. Changes in chloroplast ultrastructure in leaves of drought-stressed maize inbred lines. Photosynthetica. 2016;54:74–80. doi: 10.1007/s11099-015-0158-6. [DOI] [Google Scholar]
- 13.Munns R., Tester M. Mechanisms of salinity tolerance. Annu. Rev. Plant Biol. 2008;59:651–681. doi: 10.1146/annurev.arplant.59.032607.092911. [DOI] [PubMed] [Google Scholar]
- 14.Gupta A., Singh S.K., Singh M.K., Singh V.K., Modi A., Singh P.K., Kumar A. Abatement of Environmental Pollutants: Trends and Strategies. Elsevier; Cambridge, MA, USA: 2019. Plant growth-promoting rhizobacteria and their functional role in salinity stress management; pp. 151–160. [DOI] [Google Scholar]
- 15.Otlewska A., Migliore M., Dybka-Stępień K., Manfredini A., Struszczyk-Świta K., Napoli R., Białkowska A., Canfora L., Pinzari F. When salt meddles between plant, soil, and microorganisms. Front. Plant Sci. 2020;11:1429. doi: 10.3389/fpls.2020.553087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Etesami H., Maheshwari D.K. Use of plant growth promoting rhizobacteria (PGPRs) with multiple plant growth promoting traits in stress agriculture: Action mechanisms and future prospects. Ecotoxicol. Environ. Saf. 2018;156:225–246. doi: 10.1016/j.ecoenv.2018.03.013. [DOI] [PubMed] [Google Scholar]
- 17.Munns R. Salinity: Environment—Plants—Molecules. Springer; Dordrecht, The Netherlands: 2006. Salinity, Growth and phytohormones; pp. 271–290. [DOI] [Google Scholar]
- 18.Cramer G.R., Nowak R.S. Supplemental manganese improves the relative growth, net assimilation and photosynthetic rates of salt-stressed barley. Physiol. Plant. 1992;84:600–605. doi: 10.1111/j.1399-3054.1992.tb04710.x. [DOI] [Google Scholar]
- 19.Parida A.K., Das A.B. Salt tolerance and salinity effects on plants: A review. Ecotoxicol. Environ. Saf. 2005;60:324–349. doi: 10.1016/j.ecoenv.2004.06.010. [DOI] [PubMed] [Google Scholar]
- 20.Hirayama T., Shinozaki K. Research on plant abiotic stress responses in the post-genome era: Past, present and future. Plant J. 2010;61:1041–1052. doi: 10.1111/j.1365-313X.2010.04124.x. [DOI] [PubMed] [Google Scholar]
- 21.Choudhury S., Panda P., Sahoo L., Panda S.K. Reactive oxygen species signaling in plants under abiotic stress. Plant Signal. Behav. 2013;8:e23681. doi: 10.4161/psb.23681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Huang H., Ullah F., Zhou D.X., Yi M., Zhao Y. Mechanisms of ROS regulation of plant development and stress responses. Front. Plant Sci. 2019;10:800. doi: 10.3389/fpls.2019.00800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kaushal M., Wani S.P. Rhizobacterial-plant interactions: Strategies ensuring plant growth promotion under drought and salinity stress. Agric. Ecosyst. Environ. 2016;231:68–78. doi: 10.1016/j.agee.2016.06.031. [DOI] [Google Scholar]
- 24.Kumar A., Verma J.P. Does plant—Microbe interaction confer stress tolerance in plants: A review? Microbiol. Res. 2018;207:41–52. doi: 10.1016/j.micres.2017.11.004. [DOI] [PubMed] [Google Scholar]
- 25.Hardoim P.R., van Overbeek L.S., Berg G., Pirttilä A.M., Compant S., Campisano A., Döring M., Sessitsch A. The hidden world within plants: Ecological and evolutionary considerations for defining functioning of microbial endophytes. Microbiol. Mol. Biol. Rev. 2015;79:293–320. doi: 10.1128/MMBR.00050-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.White J.F., Kingsley K.L., Zhang Q., Verma R., Obi N., Dvinskikh S., Elmore M.T., Verma S.K., Gond S.K., Kowalski K.P. Review: Endophytic microbes and their potential applications in crop management. Pest Manag. Sci. 2019;75:2558–2565. doi: 10.1002/ps.5527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.De Bary A. Morphologie und Physiologie der Pilze, Flechten und Myxomyceten. W. Engelmann; Leipzig, Germany: 1866. [Google Scholar]
- 28.Petrini O. Fungal Endophytes of Tree Leaves. In: Andrews J.H., Hirano S.S., editors. Microbial Ecology of Leaves. Springer; New York, NY, USA: 1991. pp. 179–197. Brock/Springer Series in Contemporary Bioscience. [DOI] [Google Scholar]
- 29.Kumar A., Droby S., Singh V.K., Singh S.K., White J.F. Microbial Endophytes. Elsevier; Cambridge, MA, USA: 2020. Entry, colonization, and distribution of endophytic microorganisms in plants; pp. 1–33. [DOI] [Google Scholar]
- 30.Gupta A., Verma H., Singh P.P.P., Singh P.P.P., Singh M., Mishra V., Kumar A. Seed Endophytes: Biology and Biotechnology. Springer International Publishing; Heidelberg, Germany: 2019. Rhizome endophytes: Roles and applications in sustainable agriculture; pp. 405–421. [Google Scholar]
- 31.Kumar A., Zhimo Y., Biasi A., Salim S., Feygenberg O., Wisniewski M., Droby S. Endophytic Microbiome in the Carposphere and Its Importance in Fruit Physiology and Pathology. In: Spadaro D., Droby S., Gullino M.L., editors. Postharvest Pathology: Plant Pathology in the 21st Century. Volume 11. Springer; Cham, Schwitzerland: 2021. pp. 73–88. [DOI] [Google Scholar]
- 32.Compant S., Clément C., Sessitsch A. Plant growth-promoting bacteria in the rhizo- and endosphere of plants: Their role, colonization, mechanisms involved and prospects for utilization. Soil Biol. Biochem. 2010;42:669–678. doi: 10.1016/j.soilbio.2009.11.024. [DOI] [Google Scholar]
- 33.Meneses C.H.S.G., Rouws L.F.M., Simoes-Araujo J.L., Vidal M.S., Baldani J.I. Exopolysaccharide production is required for biofilm formation and plant colonization by the nitrogen-fixing endophyte Gluconacetobacter diazotrophicus. Mol. Plant Microbe Interact. 2011;24:1448–1514. doi: 10.1094/MPMI-05-11-0127. [DOI] [PubMed] [Google Scholar]
- 34.Reinhold-Hurek B., Maes T., Gemmer S., Van Montagu M., Hurek T. An endoglucanase is involved in infection of rice roots by the not-cellulose-metabolizing endophyte Azoarcus sp. strain BH72. Mol. Plant Microbe Interact. 2006;19:181–188. doi: 10.1094/MPMI-19-0181. [DOI] [PubMed] [Google Scholar]
- 35.Nogueira-Lopez G., Greenwood D.R., Middleditch M., Winefield C., Eaton C., Steyaert J.M., Mendoza-Mendoza A. The apoplastic secretome of Trichoderma virens during interaction with maize roots shows an inhibition of plant defence and scavenging oxidative stress secreted proteins. Front. Plant Sci. 2018;9:40. doi: 10.3389/fpls.2018.00409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Yan L., Zhu J., Zhao X., Shi J., Jiang C., Shao D. Beneficial effects of endophytic fungi colonization on plants. Appl. Microbiol. Biotechnol. 2019;103:3327–3340. doi: 10.1007/s00253-019-09713-2. [DOI] [PubMed] [Google Scholar]
- 37.Brader G., Compant S., Mitter B., Trognitz F., Sessitsch A. Metabolic potential of endophytic bacteria. Curr. Opin. Biotecnol. 2014;27:30–37. doi: 10.1016/j.copbio.2013.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Chang X., Kingsley K.L., White J.F. Chemical interactions at the interface of plant root hair cells and intracellular bacteria. Microorganisms. 2021;9:1041. doi: 10.3390/microorganisms9051041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Compant S., Kaplan H., Sessitsch A., Nowak J., Ait Barka E., Clément C. Endophytic colonization of Vitis vinifera L. by Burkholderia phytofirmans strain PsJN: From the rhizosphere to inflorescence tissues. FEMS Microbiol. Ecol. 2008;63:84–93. doi: 10.1111/j.1574-6941.2007.00410.x. [DOI] [PubMed] [Google Scholar]
- 40.Gasser I., Cardinale M., Müller H., Heller S., Eberl L., Lindenkamp N., Kaddor C., Steinbüchel A., Berg G. Analysis of the endophytic lifestyle and plant growth promotion of Burkholderia terricola ZR2-12. Plant Soil. 2011;347:125–136. doi: 10.1007/s11104-011-0833-8. [DOI] [Google Scholar]
- 41.Zinniel D.K., Lambrecht P., Harris N.B., Feng Z., Kuczmarski D., Higley P., Ishimaru C.A., Arunakumari A., Barletta R.G., Vidaver A.K. Isolation and characterization of endophytic colonizing bacteria from agronomic crops and prairie plants. Appl. Environ. Microbiol. 2002;68:2198–2208. doi: 10.1128/AEM.68.5.2198-2208.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Pacifico D., Squartini A., Crucitti D., Barizza E., Lo Schiavo F., Muresu R., Carimi F., Zottini M. The role of the endophytic microbiome in the grapevine response to environmental triggers. Front. Plant Sci. 2019;10:1256. doi: 10.3389/fpls.2019.01256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Jones P., Garcia B.J., Furches A., Tuskan G.A., Jacobson D. Plant host-associated mechanisms for microbial selection. Front. Plant Sci. 2019;10:862. doi: 10.3389/fpls.2019.00862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Agler M.T., Ruhe J., Kroll S., Morhenn C., Kim S.T., Weigel D., Kemen E.M. Microbial hub taxa link host and abiotic factors to plant microbiome variation. PLoS Biol. 2016;14:e1002352. doi: 10.1371/journal.pbio.1002352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kuffner M., Hai B., Rattei T., Melodelima C., Schloter M., Zechmeister-Boltenstern S., Jandl R., Schindlbacher A., Sessitsch A. Effects of season and experimental warming on the bacterial community in a temperate mountain forest soil assessed by 16S rRNA gene pyrosequencing. FEMS Microbiol. Ecol. 2012;82:551–562. doi: 10.1111/j.1574-6941.2012.01420.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Oono R., Black D., Slessarev E., Sickler B., Strom A., Apigo A. Species diversity of fungal endophytes across a stress gradient for plants. New Phytol. 2020;228:210–225. doi: 10.1111/nph.16709. [DOI] [PubMed] [Google Scholar]
- 47.Arnold A.E., Herre E.A. Canopy cover and leaf age affect colonization by tropical fungal endophytes: Ecological pattern and process in Theobroma cacao (Malvaceae) Mycologia. 2003;95:388–398. doi: 10.1080/15572536.2004.11833083. [DOI] [PubMed] [Google Scholar]
- 48.Oita S., Ibáñez A., Lutzoni F., Miadlikowska J., Geml J., Lewis L.A., Hom E.F., Carbone I., U’Ren J.M., Arnold A.E. Climate and seasonality drive the richness and composition of tropical fungal endophytes at a landscape scale. Commun. Biol. 2021;4:313. doi: 10.1038/s42003-021-01826-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Peleg Z., Blumwald E. Hormone balance and abiotic stress tolerance in crop plants. Curr. Opin. Plant Biol. 2011;14:290–295. doi: 10.1016/j.pbi.2011.02.001. [DOI] [PubMed] [Google Scholar]
- 50.Gill S.S., Tuteja N. Reactive oxygen species and antioxidant machinery in abiotic stress tolerance in crop plants. Plant Physiol. Biochem. 2010;48:909–930. doi: 10.1016/j.plaphy.2010.08.016. [DOI] [PubMed] [Google Scholar]
- 51.Demidchik V. Mechanisms of oxidative stress in plants: From classical chemistry to cell biology. Environ. Exp. Bot. 2015;109:212–228. doi: 10.1016/j.envexpbot.2014.06.021. [DOI] [Google Scholar]
- 52.Hasanuzzaman M., Bhuyan M.H.M.B., Zulfiqar F., Raza A., Mohsin S.M., Al Mahmud J., Fujita M., Fotopoulos V. Reactive oxygen species and antioxidant defense in plants under abiotic stress: Revisiting the crucial role of a universal defense regulator. Antioxidants. 2020;9:681. doi: 10.3390/antiox9080681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Kumari M., Pandey S., Mishra S.K., Giri V.P., Agarwal L., Dwivedi S., Pandey A.K., Nautiyal C.S., Mishra A. Omics-based mechanistic insight into the role of bioengineered nanoparticles for biotic stress amelioration by modulating plant metabolic pathways. Front. Bioeng. Biotechnol. 2020;17:242. doi: 10.3389/fbioe.2020.00242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Ma C., Burd S., Lers A. MiR408 is involved in abiotic stress responses in Arabidopsis. Plant J. 2015;84:169–187. doi: 10.1111/tpj.12999. [DOI] [PubMed] [Google Scholar]
- 55.Müller M., Munné-Bosch S. Ethylene response factors: A key regulatory hub in hormone and stress signaling. Plant Physiol. 2015;169:32–41. doi: 10.1104/pp.15.00677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Torres M.A., Dangl J.L. Functions of the respiratory burst oxidase in biotic interactions, abiotic stress and development. Curr. Opin. Plant Biol. 2005;8:397–403. doi: 10.1016/j.pbi.2005.05.014. [DOI] [PubMed] [Google Scholar]
- 57.Apel K., Hirt H. Reactive oxygen species: Metabolism, oxidative stress, and signal transduction. Annu. Rev. Plant Biol. 2004;55:373–399. doi: 10.1146/annurev.arplant.55.031903.141701. [DOI] [PubMed] [Google Scholar]
- 58.Dietz K.J., Mittler R., Noctor G. Recent progress in understanding the role of reactive oxygen species in plant cell signaling. Plant Physiol. 2016;171:1535–1539. doi: 10.1104/pp.16.00938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Grant J.J., Loake G.J. Role of reactive oxygen intermediates and cognate redox signaling in disease resistance. Plant Physiol. 2000;124:21–29. doi: 10.1104/pp.124.1.21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Laloi C., Apel K., Danon A. Reactive oxygen signalling: The latest news. Curr. Opin. Plant Biol. 2004;7:323–328. doi: 10.1016/j.pbi.2004.03.005. [DOI] [PubMed] [Google Scholar]
- 61.Neill S.J., Desikan R., Clarke A., Hurst R.D., Hancock J.T. Hydrogen peroxide and nitric oxide as signalling molecules in plants. J. Exp. Bot. 2002;53:1237–1247. doi: 10.1093/jexbot/53.372.1237. [DOI] [PubMed] [Google Scholar]
- 62.Rudgers J.A., Afkhami M.E., Rúa M.A., Davitt A.J., Hammer S., Huguet V.M. A fungus among us: Broad patterns of endophyte distribution in the grasses. Ecology. 2009;90:1531–1539. doi: 10.1890/08-0116.1. [DOI] [PubMed] [Google Scholar]
- 63.White J.F., Torres M.S. Is plant endophyte-mediated defensive mutualism the result of oxidative stress protection? Physiol. Plant. 2010;138:440–446. doi: 10.1111/j.1399-3054.2009.01332.x. [DOI] [PubMed] [Google Scholar]
- 64.Clay K. The ecology and evolution of endophytes. Agric. Ecosyst. Environ. 1993;44:39–64. doi: 10.1016/0167-8809(93)90038-Q. [DOI] [Google Scholar]
- 65.Shade A., Jacques M.A., Barret M. Ecological patterns of seed microbiome diversity, transmission, and assembly. Curr. Opin. Microbiol. 2017;37:15–22. doi: 10.1016/j.mib.2017.03.010. [DOI] [PubMed] [Google Scholar]
- 66.Zhou X.R., Dai L., Xu G.F., Wang H.S. A strain of Phoma species improves drought tolerance of Pinus tabulaeformis. Sci. Rep. 2021;11:7637. doi: 10.1038/s41598-021-87105-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Wu Q.S., Xia R.X., Zou Y.N. Improved soil structure and citrus growth after inoculation with three arbuscular mycorrhizal fungi under drought stress. Eur. J. Soil Biol. 2008;44:122–128. doi: 10.1016/j.ejsobi.2007.10.001. [DOI] [Google Scholar]
- 68.Khan Z., Rho H., Firrincieli A., Hung S.H., Luna V., Masciarelli O., Kim S.H., Doty S.L. Growth enhancement and drought tolerance of hybrid poplar upon inoculation with endophyte consortia. Curr. Plant Biol. 2016;6:38–47. doi: 10.1016/j.cpb.2016.08.001. [DOI] [Google Scholar]
- 69.Marasco R., Rolli E., Ettoumi B., Vigani G., Mapelli F., Borin S., Abou-Hadid A.F., El-Behairy U.A., Sorlini C., Cherif A., et al. A drought resistance-promoting microbiome is selected by root system under desert farming. PLoS ONE. 2012;7:e48479. doi: 10.1371/journal.pone.0048479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Singh V.K., Singh A.K., Singh P.P., Kumar A. Interaction of plant growth promoting bacteria with tomato under abiotic stress: A review. Agric. Ecosyst. Environ. 2018;267:129–140. doi: 10.1016/j.agee.2018.08.020. [DOI] [Google Scholar]
- 71.Naveed M., Mitter B., Reichenauer T.G., Wieczorek K., Sessitsch A. Increased drought stress resilience of maize through endophytic colonization by Burkholderia phytofirmans PsJN and Enterobacter sp. FD17. Environ. Exp. Bot. 2014;97:30–39. doi: 10.1016/j.envexpbot.2013.09.014. [DOI] [Google Scholar]
- 72.Yandigeri M.S., Meena K.K., Singh D., Malviya N., Singh D.P., Solanki M.K., Yadav A.K., Arora D.K. Drought-tolerant endophytic actinobacteria promote growth of wheat (Triticum aestivum) under water stress conditions. Plant Growth Regul. 2012;68:411–420. doi: 10.1007/s10725-012-9730-2. [DOI] [Google Scholar]
- 73.Jayakumar A., Padmakumar P., Nair I.C., Radhakrishnan E.K. Drought tolerant bacterial endophytes with potential plant probiotic effects from Ananas comosus. Biologia. 2020;75:1769–1778. doi: 10.2478/s11756-020-00483-1. [DOI] [Google Scholar]
- 74.Sandhya V., Shrivastava M., Ali S.Z., Sai Shiva Krishna Prasad V. Endophytes from maize with plant growth promotion and biocontrol activity under drought stress. Russ. Agric. Sci. 2017;43:22–34. doi: 10.3103/S1068367417010165. [DOI] [Google Scholar]
- 75.Chen C., Xin K., Liu H., Cheng J., Shen X., Wang Y., Zhang L. Pantoea alhagi, a novel endophytic bacterium with ability to improve growth and drought tolerance in wheat. Sci. Rep. 2017;7:1–14. doi: 10.1038/srep41564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Gagné-Bourque F., Mayer B.F., Charron J.B., Vali H., Bertrand A., Jabaji S. Accelerated growth rate and increased drought stress resilience of the model grass Brachypodium distachyon colonized by Bacillus subtilis B26. PLoS ONE. 2015;10:e0130456. doi: 10.1371/journal.pone.0130456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Morsy M., Cleckler B., Armuelles-Millican H. Fungal endophytes promote tomato growth and enhance drought and salt tolerance. Plants. 2020;9:877. doi: 10.3390/plants9070877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Abd-Allah E.F., Alqarawi A.A., Hashem A., Radhakrishnan R., Al-Huqail A.A., Al-Otibi F.O.N., Malik J.A., Alharbi R.I., Egamberdieva D. Endophytic bacterium Bacillus subtilis (BERA 71) improves salt tolerance in chickpea plants by regulating the plant defense mechanisms. J. Plant Interact. 2018;13:37–44. doi: 10.1080/17429145.2017.1414321. [DOI] [Google Scholar]
- 79.Jaemsaeng R., Jantasuriyarat C., Thamchaipenet A. Molecular interaction of 1-aminocyclopropane-1-carboxylate deaminase (ACCD)-producing endophytic Streptomyces sp. GMKU 336 towards salt-stress resistance of Oryza sativa L. cv. KDML105. Sci. Rep. 2018;8:1950. doi: 10.1038/s41598-018-19799-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Chen T., Johnson R., Chen S., Lv H., Zhou J., Li C. Infection by the fungal endophyte Epichloë bromicola enhances the tolerance of wild barley (Hordeum brevisubulatum) to salt and alkali stresses. Plant Soil. 2018;428:353–370. doi: 10.1007/s11104-018-3643-4. [DOI] [Google Scholar]
- 81.Pan X., Qin Y., Yuan Z. Potential of a halophyte-associated endophytic fungus for sustaining Chinese white poplar growth under salinity. Symbiosis. 2018;76:109–116. doi: 10.1007/s13199-018-0541-8. [DOI] [Google Scholar]
- 82.Abdelaziz M.E., Kim D., Ali S., Fedoroff N.V., Al-Babili S. The endophytic fungus Piriformospora indica enhances Ara-bidopsis thaliana growth and modulates Na+/K+ homeostasis under salt stress conditions. Plant Sci. 2017;263:107–115. doi: 10.1016/j.plantsci.2017.07.006. [DOI] [PubMed] [Google Scholar]
- 83.Barnawal D., Bharti N., Tripathi A., Pandey S.S., Chanotiya C.S., Kalra A. ACC-deaminase-producing endophyte Brachbacterium paraconglomeratum strain SMR20 ameliorates chlorophytum salinity stress via altering phytohormone generation. J. Plant Growth Regul. 2016;35:553–564. doi: 10.1007/s00344-015-9560-3. [DOI] [Google Scholar]
- 84.Matsouri F., Björkman T., Harman G.E. Seed treatment with Trichoderma harzianum alleviates biotic, abiotic and physiological stresses in germinating seeds and seedlings. Phytopathol. 2010;100:1213–1221. doi: 10.1094/PHYTO-03-10-0091. [DOI] [PubMed] [Google Scholar]
- 85.Baltruschat H., Fodor J., Harrach B.D., Niemczyk E., Barn B., Gullner G., Janeczko A., Kogel K.H., Schäfer P., Schwarczinger I., et al. Salt tolerance of barley induced by the root endophyte Piriformospora indica is associated with a strong increase in antioxidants. New Phytol. 2008;180:501–510. doi: 10.1111/j.1469-8137.2008.02583.x. [DOI] [PubMed] [Google Scholar]
- 86.Weyers J.D.B., Paterson N.W. Plant hormones and the control of physiological processes. New Phytol. 2001;152:375–407. doi: 10.1046/j.0028-646X.2001.00281.x. [DOI] [PubMed] [Google Scholar]
- 87.Khan N., Bano A., Ali S., Babar M.A. Crosstalk amongst phytohormones from planta and PGPR under biotic and abiotic stresses. Plant Growth Regul. 2020;90:189–203. doi: 10.1007/s10725-020-00571-x. [DOI] [Google Scholar]
- 88.Bedini A., Mercy L., Schneider C., Franken P., Lucic-Mercy E. Unraveling the initial plant hormone signaling, metabolic mechanisms and plant defense triggering the endomycorrhizal symbiosis behavior. Front. Plant Sci. 2018;9:871. doi: 10.3389/fpls.2018.01800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Pinski A., Betekhtin A., Hupert-Kocurek K., Mur L.A.J., Hasterok R. Defining the genetic basis of plant–endophytic bacteria interactions. Int. J. Mol. Sci. 2019;20:1947. doi: 10.3390/ijms20081947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Verma S.K., Kingsley K., Irizarry I., Bergen M., Kharwar R.N., White J.F. Seed-vectored endophytic bacteria modulate development of rice seedlings. J. Appl. Microbiol. 2017;122:1680–1691. doi: 10.1111/jam.13463. [DOI] [PubMed] [Google Scholar]
- 91.Waqas M., Kim Y.H., Khan A.L., Shahzad R., Asaf S., Hamayun M., Kang S.M., Khan M.A., Lee I.J. Additive effects due to biochar and endophyte application enable soybean to enhance nutrient uptake and modulate nutritional parameters. J. Zhejiang Univ. Sci. B. 2017;18:109–124. doi: 10.1631/jzus.B1500262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Zamioudis C., Korteland J., Van Pelt J.A., Van Hamersveld M., Dombrowski N., Bai Y., Hanson J., Van Verk M.C., Ling H.Q., Schulze-Lefert P., et al. Rhizobacterial volatiles and photosynthesis-related signals coordinate MYB72 expression in Arabidopsis roots during onset of induced systemic resistance and iron-deficiency responses. Plant J. 2015;84:309–322. doi: 10.1111/tpj.12995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Shahzad R., Waqas M., Khan A.L., Asaf S., Khan M.A., Kang S.M., Yun B.W., Lee I.J. Seed-borne endophytic Bacillus amyloliquefaciens RWL-1 produces gibberellins and regulates endogenous phytohormones of Oryza sativa. Plant Physiol. Biochem. 2016;106:236–243. doi: 10.1016/j.plaphy.2016.05.006. [DOI] [PubMed] [Google Scholar]
- 94.Shahzad R., Khan A.L., Bilal S., Waqas M., Kang S.M., Lee I.J. Inoculation of abscisic acid-producing endophytic bacteria enhances salinity stress tolerance in Oryza sativa. Environ. Exp. Bot. 2017;136:68–77. doi: 10.1016/j.envexpbot.2017.01.010. [DOI] [Google Scholar]
- 95.Bodhankar S., Grover M., Reddy G. In planta screening of maize seed endophytic bacteria for potential applications under dryland conditions. Indian J. Dryl. Agric. Res. Dev. 2019;34:53. doi: 10.5958/2231-6701.2019.00009.5. [DOI] [Google Scholar]
- 96.Rehman A., Farooq M., Naveed M., Nawaz A., Shahzad B. Seed priming of Zn with endophytic bacteria improves the productivity and grain biofortification of bread wheat. Eur. J. Agron. 2018;94:98–107. doi: 10.1016/j.eja.2018.01.017. [DOI] [Google Scholar]
- 97.Doty S.L., Oakley B., Xin G., Kang J.W., Singleton G., Khan Z., Vajzovic A., Staley J.T. Diazotrophic endophytes of native black cottonwood and willow. Symbiosis. 2009;47:23–33. doi: 10.1007/BF03179967. [DOI] [Google Scholar]
- 98.Barazani O., Von Dahl C.C., Baldwin I.T. Sebacina vermifera promotes the growth and fitness of Nicotiana attenuata by inhibiting ethylene signaling. Plant Physiol. 2007;144:1223–1232. doi: 10.1104/pp.107.097543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Foyer C.H., Noctor G. Stress-triggered redox signalling: What’s in pROSpect? Plant Cell Environ. 2016;39:951–964. doi: 10.1111/pce.12621. [DOI] [PubMed] [Google Scholar]
- 100.Mignolet-Spruyt L., Xu E., Idänheimo N., Hoeberichts F.A., Mühlenbock P., Brosche M., Van Breusegem F., Kangasjärvi J. Spreading the news: Subcellular and organellar reactive oxygen species production and signalling. J. Exp. Bot. 2016;67:3831–3844. doi: 10.1093/jxb/erw080. [DOI] [PubMed] [Google Scholar]
- 101.White J.F., Kingsley K.I., Kowalski K.P., Irizarry I., Micci A., Soares M.A., Bergen M.S. Disease protection and allelopathic interactions of seed-transmitted endophytic pseudomonads of invasive reed grass (Phragmites australis) Plant Soil. 2018;422:195–208. doi: 10.1007/s11104-016-3169-6. [DOI] [Google Scholar]
- 102.Nath M., Bhatt D., Prasad R., Gill S.S., Anjum N.A., Tuteja N. Reactive oxygen species generation-scavenging and signaling during Plant-Arbuscular mycorrhizal and Piriformospora indica interaction under stress condition. Front. Plant Sci. 2016;7:1574. doi: 10.3389/fpls.2016.01574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Mittler R., Vanderauwera S., Gollery M., Van Breusegem F. Reactive oxygen gene network of plants. Trends Plant Sci. 2004;9:490–498. doi: 10.1016/j.tplants.2004.08.009. [DOI] [PubMed] [Google Scholar]
- 104.Lata R., Chowdhury S., Gond S.K., White J.F. Induction of abiotic stress tolerance in plants by endophytic microbes. Lett. Appl. Microbiol. 2018;66:268–276, doiorg/101111/lam12855. doi: 10.1111/lam.12855. [DOI] [PubMed] [Google Scholar]
- 105.Waller F., Achatz B., Baltruschat H., Fodor J., Becker K., Fischer M., Heier T., Hückelhoven R., Neumann C., Von Wettstein D., et al. The endophytic fungus Piriformospora indica reprograms barley to salt-stress tolerance, disease resistance, and higher yield. Proc. Natl. Acad. Sci. USA. 2005;102:13386–13391. doi: 10.1073/pnas.0504423102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Prasad R., Kamal S., Sharma P.K., Oelmüller R., Varma A. Root endophyte Piriformospora indica DSM 11827 alters plant morphology, enhances biomass and antioxidant activity of medicinal plant Bacopa monniera. J. Basic Microbiol. 2013;53:1016–1024. doi: 10.1002/jobm.201200367. [DOI] [PubMed] [Google Scholar]
- 107.Redman R.S., Kim Y.O., Woodward C.J.D.A., Greer C., Espino L., Doty S.L., Rodriguez R.J. Increased fitness of rice plants to abiotic stress via habitat adapted symbiosis: A strategy for mitigating impacts of climate change. PLoS ONE. 2011;6:e14823. doi: 10.1371/journal.pone.0014823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Singh L.P., Gill S.S., Tuteja N. Unraveling the role of fungal symbionts in plant abiotic stress tolerance. Plant Signal. Behav. 2011;6:175–191. doi: 10.4161/psb.6.2.14146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Zhang S., Gan Y., Xu B. Application of plant-growth-promoting fungi Trichoderma longibrachiatum T6 enhances tolerance of wheat to salt stress through improvement of antioxidative defense system and gene expression. Front. Plant Sci. 2016;7:1405. doi: 10.3389/fpls.2016.01405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Azad K., Kaminskyj S. A fungal endophyte strategy for mitigating the effect of salt and drought stress on plant growth. Symbiosis. 2016;68:73–78. doi: 10.1007/s13199-015-0370-y. [DOI] [Google Scholar]
- 111.Ahmad P., Hashem A., Abd-Allah E.F., Alqarawi A.A., John R., Egamberdieva D., Gucel S. Role of Trichoderma harzianum in mitigating NaCl stress in Indian mustard (Brassica juncea L) through antioxidative defense system. Front. Plant Sci. 2015;14:868. doi: 10.3389/fpls.2015.00868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Rajendran K., Tester M., Roy S.J. Quantifying the three main components of salinity tolerance in cereals. Plant Cell Environ. 2009;32:237–249. doi: 10.1111/j.1365-3040.2008.01916.x. [DOI] [PubMed] [Google Scholar]
- 113.Carillo P., Mastrolonardo G., Nacca F., Parisi D., Verlotta A., Fuggi A. Nitrogen metabolism in durum wheat under salinity: Accumulation of proline and glycine betaine. Funct. Plant Biol. 2008;35:412–426. doi: 10.1071/FP08108. [DOI] [PubMed] [Google Scholar]
- 114.Eida A.A., Alzubaidy H.S., de Zélicourt A., Synek L., Alsharif W., Lafi F.F., Hirt H., Saad M.M. Phylogenetically diverse endophytic bacteria from desert plants induce transcriptional changes of tissue-specific ion transporters and salinity stress in Arabidopsis thaliana. Plant Sci. 2019;280:228–240. doi: 10.1016/j.plantsci.2018.12.002. [DOI] [PubMed] [Google Scholar]
- 115.Rodriguez R.J., Henson J., Van Volkenburgh E., Hoy M., Wright L., Beckwith F., Kim Y.O., Redman R.S. Stress tolerance in plants via habitat-adapted symbiosis. ISME J. 2008;2:404–416. doi: 10.1038/ismej.2007.106. [DOI] [PubMed] [Google Scholar]
- 116.Khalid M., Hassani D., Liao J., Xiong X., Bilal M., Huang D. An endosymbiont Piriformospora indica reduces adverse effects of salinity by regulating cation transporter genes, phytohormones, and antioxidants in Brassica campestris ssp. Chinensis. Environ. Exp. Bot. 2018;153:89–99. doi: 10.1016/j.envexpbot.2018.05.007. [DOI] [Google Scholar]
- 117.Jha Y., Subramanian R.B. Potassium Solubilizing Microorganisms for Sustainable Agriculture. Springer; New Delhi, India: 2016. Regulation of plant physiology and antioxidant enzymes for alleviating salinity stress by potassium-mobilizing bacteria; pp. 149–162. [DOI] [Google Scholar]
- 118.Megías E., Megías M., Ollero F.J., Hungria M. Draft genome sequence of Pantoea ananatis strain AMG521, a rice plant growth-promoting bacterial endophyte isolated from the Guadalquivir marshes in southern Spain. Genome Announc. 2016;4:e01681-15. doi: 10.1128/genomeA.01681-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Qiang X., Weiss M., Kogel K.H., Schäfer P. Piriformospora indica-a mutualistic basidiomycete with an exceptionally large plant host range. Mol. Plant Pathol. 2012;13:508–518. doi: 10.1111/j.1364-3703.2011.00764.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Chetia H., Kabiraj D., Bharali B., Ojha S., Barkataki M.P., Saikia D., Singh T., Mosahari P.V., Sharma P., Bora U. Advances in Endophytic Fungal Research, Fungal Biology. Springer; Cham, Switzerland: 2019. Exploring the benefits of endophytic fungi via omics; pp. 51–81. [DOI] [Google Scholar]
- 121.Kaul S., Sharma T., Dhar M.K. “Omics” tools for better understanding the plant–endophyte interactions. Front. Plant Sci. 2016;7:955. doi: 10.3389/fpls.2016.00955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Hong C.E., Kim J.U., Lee J.W., Bang K.H., Jo I.H. Metagenomic analysis of bacterial endophyte community structure and functions in Panax ginseng at different ages. 3 Biotech. 2019;9:1–8. doi: 10.1007/s13205-019-1838-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Žiarovská J., Medo J., Kyseľ M., Zamiešková L., Kačániová M. Endophytic bacterial microbiome diversity in early developmental stage plant tissues of wheat varieties. Plants. 2020;9:266. doi: 10.3390/plants9020266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Tiwari P., Bae H. Horizontal gene transfer and endophytes: An implication for the acquisition of novel traits. Plants. 2020;9:305. doi: 10.3390/plants9030305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Coutinho B.G., Licastro D., Mendonça-Previato L., Cámara M., Venturi V. Plant-Influenced gene expression in the rice endophyte Burkholderia kururiensis M130. Mol. Plant-Microbe Interact. 2015;28:10–21. doi: 10.1094/MPMI-07-14-0225-R. [DOI] [PubMed] [Google Scholar]
- 126.Van Elsas J.D., Turner S., Bailey M.J. Horizontal gene transfer in the phytosphere. New Phytol. 2003;157:525–537. doi: 10.1046/j.1469-8137.2003.00697.x. [DOI] [PubMed] [Google Scholar]
- 127.Yuan J., Zhang W., Sun K., Tang M.J., Chen P.X., Li X., Dai C.C. Comparative transcriptomics and proteomics of atractylodes lancea in response to endophytic fungus gilmaniella sp. AL12 reveals regulation in plant metabolism. Front. Microbiol. 2019;10:1208. doi: 10.3389/fmicb.2019.01208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Shrivastava N., Jiang L., Li P., Sharma A.K., Luo X., Wu S., Pandey R., Gao Q., Lou B. Proteomic approach to understand the molecular physiology of symbiotic interaction between Piriformospora indica and Brassica napus. Sci. Rep. 2018;8:5773. doi: 10.1038/s41598-018-23994-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Kumari M., Kamat S., Dixit R., Pandey S., Giri V.P., Mishra A. In: Microbial Formulation Approaches in Post-Harvest Disease Management. Kumar A., Dobry S., editors. Elsevier Press; Cambridge, MA, USA: 2020. pp. 279–305. [DOI] [Google Scholar]
- 130.Moghaddam M.S.H., Safaie N., Soltani J., Hagh-Doust N. Desert-adapted fungal endophytes induce salinity and drought stress resistance in model crops. Plant Physiol. Biochem. 2021;160:225–238. doi: 10.1016/j.plaphy.2021.01.022. [DOI] [PubMed] [Google Scholar]
- 131.Schulz B., Haas S., Junker C., Andrée N., Schobert M. Fungal endophytes are involved in multiple balanced antagonisms. [(accessed on 1 August 2021)];Curr. Sci. 2015 109:39–44. Available online: wwwjstororg/stable/24905689. [Google Scholar]
- 132.Thirumalai E., Venkatachalam A., Suryanarayanan T.S. Fungal endophytes of betel leaves: The need to study mycotoxin-producing endophytes in leafy vegetables. Sydowia. 2020;66:2. doi: 10.12905/0380.sydowia73-2020-0083. [DOI] [Google Scholar]
- 133.White J.F., Chang X., Kingsley K.L., Zhang Q., Chiaranunt P., Micci A., Velazquez F., Elmore M., Crane S., Li S., et al. Endophytic bacteria in grass crop growth promotion and biostimulation. Grass Res. 2021;1:1–9. doi: 10.48130/GR-2021-0005. [DOI] [Google Scholar]
- 134.Furtado B.U., Gołębiewski M., Skorupa M., Hulisz P., Hrynkiewicz K. Bacterial and fungal endophytic microbiomes of Salicornia europaea. Appl. Environ. Microbiol. 2019;85:e00305-19. doi: 10.1128/AEM.00305-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Martin A., Atares L., Chiralt A. Improving function of biocontrol agents incorporated in antifungal fruit coatings: A review. Biocon. Sci. Technol. 2017;27:10. doi: 10.1080/09583157.2017.1390068. [DOI] [Google Scholar]
- 136.Chitnis V.R., Suryanarayanan T.S., Nataraja K.N., Prasad S.R., Oelmüller R., Shaanker R.U. fungal endophyte-mediated crop improvement: The way ahead. Front. Plant Sci. 2020;11:561007. doi: 10.3389/fpls.2020.561007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Paungfoo-Lonhienne C., Rentsch D., Robatzek S., Webb R.I., Sagulenko E., Näsholm T., Schmidt S., Lonhienne T.G.A. Turning the table: Plants consume microbes as a source of nutrients. PLoS ONE. 2010;5:e11915. doi: 10.1371/journal.pone.0011915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Thomas P., Agrawal M., Bharathkumar C.B. Diverse cellular colonizing endophytic bacteria in field shoots and in vitro cultured papaya with physiological and functional implications. Physiol. Plant. 2019;166:729–747. doi: 10.1111/ppl.12825. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.