Abstract
Throughout the 20th and 21st centuries, the incidence of non-communicable diseases (NCDs), also known as chronic diseases, has been increasing worldwide. Changes in dietary and physical activity patterns, along with genetic conditions, are the main factors that modulate the metabolism of individuals, leading to the development of NCDs. Obesity, diabetes, metabolic associated fatty liver disease (MAFLD), and cardiovascular diseases (CVDs) are classified in this group of chronic diseases. Therefore, understanding the underlying molecular mechanisms of these diseases leads us to develop more accurate and effective treatments to reduce or mitigate their prevalence in the population. Given the global relevance of NCDs and ongoing research progress, this article reviews the current understanding about NCDs and their related risk factors, with a focus on obesity, diabetes, MAFLD, and CVDs, summarizing the knowledge about their pathophysiology and highlighting the currently available and emerging therapeutic strategies, especially pharmacological interventions. All of these diseases play an important role in the contamination by the SARS-CoV-2 virus, as well as in the progression and severity of the symptoms of the coronavirus disease 2019 (COVID-19). Therefore, we briefly explore the relationship between NCDs and COVID-19.
Keywords: NCDs, obesity, diabetes, MAFLD, cardiovascular diseases, metabolism
1. Introduction
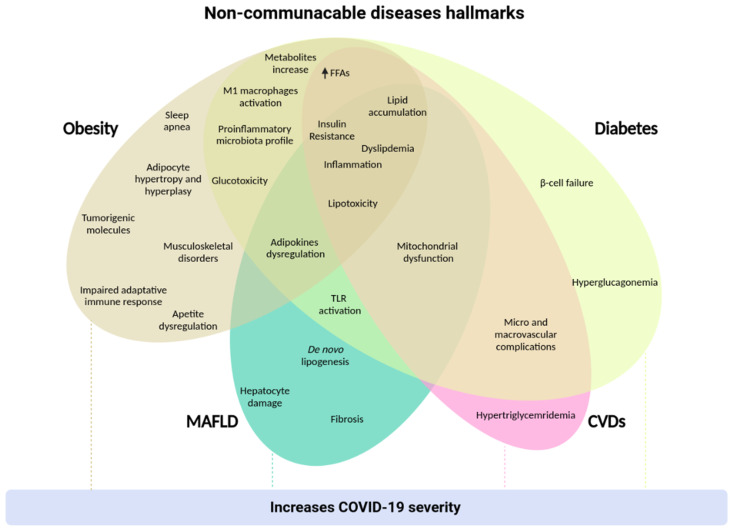
Non-communicable diseases (NCDs), also known as chronic diseases, are not directly transmissible from one person to another, and are the combination of genetic, physiological, environmental, and behavioral factors. The main NCDs are diabetes, cardiovascular diseases, cancers, and chronic respiratory diseases. The first three are associated with metabolic changes that increase the risk of suffering them. These changes are hypertension, overweight/obesity, hyperglycemia, and hyperlipidemia (WHO). The importance of these diseases was highlighted in the report of the World Health Organization (WHO) [1], in which it was reported that over 50% of the 57 million deaths worldwide, in 2016, occurred from diabetes (1.6 million people), cancer (9 million), and cardiovascular diseases (17.9 million) [2], posing a significant global health challenge. As well as genetics, unhealthy habits such as smoking, harmful use of alcohol, physical inactivity, and a calorie-rich diet are determinants for developing metabolism-related diseases; such behavioral factors lead to metabolic disorders such as hypertension, hyperglycemia, hyperlipidemia, and obesity, which comprise the major NCDs risk factors [3]. Furthermore, over the past two decades, the understanding of the association between metabolic disorders and metabolic associated fatty liver disease (MAFLD)—a less commonly discussed NCD—has placed it as an emerging risk factor for diabetes, cancer, and cardiovascular diseases (CVDs) [4].
In view of NCDs’ global threat, the WHO has adopted priority targets to reduce NCDs mortality and risk factor prevalence until 2025 [1]. However, the global prevalence of risk factors is still concerning. In 2015, one in four men and one in five women had hypertension, corresponding to 22% of the adults aged 18 years and over [5]. In recent decades, hypertension prevalence in high-income countries has declined; on the other hand, many low- and middle-income countries had stable or increasing levels. The contrast among income groups was slight regarding blood glucose levels in 2014. Most countries had between 7% and 9% of the population with hyperglycemia—except for the Eastern Mediterranean Region, which showed the highest levels (14%) [6]. Globally, the adult obesity prevalence in 2016 was 13% (650 million people); it is almost three times higher than in 1975 [5]. Although adult obesity rates distinguish between low- (7% of the population) and high-income countries (25%), the numbers keep rising in all income groups [2]. The prevalence of childhood obesity has also increased at higher rates in recent decades. From 1975 to 2016, the number of obese children and adolescents worldwide increased approximately eight-fold, reaching 124 million in 2016 [5]. Among obese and diabetic individuals, about 70–80% have MAFLD; this is the leading chronic liver disease worldwide, with a prevalence of 20–30%, affecting 1.8 billion people [7].
Countries’ ability to deal with NCDs proved to be even more critical during the coronavirus disease 2019 (COVID-19) pandemic, since the association between NCDs and COVID-19 severity have been reported. Hypertension, ischemic heart disease, type 2 diabetes (T2D), and cancer were among the most prevalent NCDs in Italian COVID-19 victims [8]. This association has also been observed in Spain, China, and the USA [9,10,11]. Additionally, a Chinese study showed that severe patients and non-survivors were overweight or obese, suggesting an association between body mass index (BMI) and COVID-19 severity [12]. In this scenario, given COVID-19′s restrictive measures, economic instability, and health crisis, NCDs’ prevention and management became even more challenging [13].
Following the multifactorial nature of metabolism-related diseases, their prevention and treatment consist of multidisciplinary strategies to tackle the physiological and metabolic impairments. Lifestyle interventions are the primary recommendations; however, some cases also require surgical or pharmacotherapeutic approaches [14]. Although some NCD medicines are well established—such as metformin and insulin for diabetes [15] and antihypertensive agents to control some CVDs [16]—so far, no agent has been approved for MAFLD [17]. Furthermore, the discovery of additional metabolic mechanisms of NCDs pathogenesis stimulates the search for new metabolic modulators. Since pharmacotherapy’s efficacy and safety rely on the agent’s mechanism of action, drug design and development are constantly advancing. In addition to traditional combination therapies, this field advances towards the evaluation of multitarget ligands and emerging therapeutic strategies [18].
Considering the global relevance of NCDs and the constant research progress, this article reviews the current understanding about NCDs and their related risk factors, with a focus on obesity, diabetes, MAFLD, and CVDs, summarizing the knowledge about their pathophysiology and highlighting available and emerging therapeutic strategies. In addition, we briefly discuss the relationship between these conditions and their related risk factors and COVID-19. A better understanding of this critical health issue and potential therapeutic approaches can help mitigate NCDs’ global impact.
2. Obesity
Obesity is a multifactorial and preventable disease, defined as an excessive accumulation of body fat [19]. In recent decades, obesity has been a major global health issue, with a considerable impact on morbidity, mortality, and healthcare expenditure [20,21]. This issue has increased rapidly, reaching epidemic proportions. About 39% of the world’s adult population is overweight and, among this, 13% was obese in 2016. In 2017, more than 4 million deaths worldwide were due to obesity and its associated comorbidities [1]. This epidemic includes childhood obesity, that has also raised dramatically in recent years [5]. Thus, it increases the risk of early-onset chronic consequences, such as elevated blood pressure, CVDs occurrence, and impaired glucose metabolism, that usually evolves to T2D [22,23,24]. Moreover, childhood obesity increases the risk of obesity in adults more than five-fold compared to non-obese children [25]. In addition, morbidity and mortality are also elevated later in life [26].
Obesity is essentially a long-term imbalance between energy consumption and expenditure, which creates an oversupply of energy, resulting in excess fat storage. The complexity of this pathogenesis relies on its multiple causes, such as environmental, sociocultural, physiological, genetic, epigenetic, and various other factors that act together to contribute to the origin, as well as the persistence, of this condition. In the last century, the world’s social and economic changes favored a positive energy balance. The industrialization process allows the population to increase the consumption of energy-rich and often highly-palatable foods, but poor in nutrients [20]. At the same time, this process of urbanization decreases the levels of daily physical activity and increases a sedentary lifestyle [27,28].
Beyond the global factors, our individual socioeconomic and cultural environment also affects the obesity incidence. Together with abundant tasty food and low physical activity, contemporary elements, such as medications with weight gain side effects, reduced sleep time, endocrine disruptors, and epigenetic effects are components that favor the obesity epidemic [29]. Hereditary factors also play a role in this condition. In this sphere, genetics, family history, and ethnic/racial variants can increase the susceptibility to obesity. The variability of population predisposition is predicted to range from 40% to 70% due to genetic differences [30,31]. There are more than 100 genes identified as obesity-related at different contribution scales. The fat mass and obesity-associated (FTO) gene is known to predispose obesity through an effect on BMI [32,33]. Another harmful variant would be defective leptin receptor or leptin production and abnormalities in the proopiomelanocortin (POMC) gene [34,35].
Genes work together with the environment in a complex network that combines metabolic processes and body weight adjustment to regulate energy balance [36,37]. Feeding behavior is regulated by neurons that are excited or suppressed by neuropeptide hormones that act as signals for food intake and energy expenditure. Among them, ghrelin is an orexigenic hormone secreted in the gastrointestinal (GI) tract in a fasted state, involving hunger perceptions [38]. Moreover, hunger is associated with food palatability, such as visual, olfactory, emotional eating, and increased reward-responses to food stimuli [39].
In short-term energy regulation, nutrient-derived signals from GI tract adjust appetite through amino acids, gut-brain peptides, and various neurotransmitters. Food intake induces a reduction in circulating ghrelin levels while increasing secretion of the anorectic hormones cholecystokinin (CCK), peptide YY3-36 (PYY), glucagon-like peptide-1 (GLP-1), and oxyntomodulin [40]. Long-term energy balance involves several central and peripheral mechanisms that act in a finely tuned regulation network to maintain metabolic homeostasis. Insulin and leptin secretion signal feedback information in response to food intake, regulating, in addition the appetite, the thermogenesis process, fat deposition, and cognitive processes involved in food consumption [36,39].
Excess adiposity causes alterations in whole-body homeostasis, leading to functional impairments in various metabolic functions [41] and considerably increases the risk of metabolic diseases. The pathophysiology of obesity culminates in distinct homeostatic mechanisms that hinder weight loss and benefit further weight gain. The storage of energy excess leads to an increase in the number (hyperplasia) and size (hypertrophy) of the adipocytes, as well as the ectopic distribution of lipid deposits in regions, such as blood vessels, visceral fat, cardiac fat, and muscles, in a process called dyslipidemia [42]. The enlargement of fat cells increases the number of pro-inflammatory factors, including leptin, interleukin-6 (IL-6), monocyte chemotactic protein 1 (MCP-1), and lipid metabolism metabolites, such as lactate and free fatty acids (FFA). Simultaneously, they lessen the release of adiponectin, an adipokine related to insulin sensitivity, and interleukin-10 (IL-10), an anti-inflammatory cytokine [43]. Together, adipocyte products can affect the brain and peripheral nervous system, modifying metabolism, and inflammatory processes.
Hypertrophic adipocytes work together with the microbiome to increase the inflammatory environment [44]. The gut microbiota is an essential environmental factor in energy balance, acting directly in food digestion to increase energy absorption. This process produces metabolites, such as lipopolysaccharides (LPS), short-chain fatty acids (FAs), and secondary bile acids, which act as signaling molecules, modulating hunger, nutrient absorption, gut motility, and energy balance [44]. In obesity, microbiota imbalance induced by high energy dense diet increase microbial products, such as LPS, that activate innate immunity, contributing to low-grade inflammation via increased expression of inflammatory mediators (e.g., TLR family, NOD-like receptor family and cytokines) and macrophage infiltration [45].
Increased circulating FFA and adipokines cause peripheral-tissue and nervous system dysfunction. Leptin is one of the multiple factors excessively secreted by hypertrophic fat cells. This adipokine acts directly on lipid accumulation by inhibiting hunger, signaling the cessation of adipocyte fat storage [46]. Plasma leptin levels are positively correlated with adiposity. With an abundance of food, secretion of leptin suppresses energy intake, while stimulating energy expenditure. However, in obesity, a prolonged increase in plasma leptin levels leads to decreased detection of the peripheral energy status, which culminates in ineffective satiety detection despite high energy storage and leptin levels [46,47]. This damaged mechanism leads to gradual weight gains due to a continuous positive energy balance, feeding the continuous cycle of hunger.
Excessive food intake, lipotoxicity, and elevated lipid accumulation induce the expression of cytokines and activation of cells involved in innate immunity [48]. As obesity progresses, adipose tissue macrophage infiltration increases in number and changes the gene expression profile to a greater inflammatory environment [49,50]. The increased inflammatory response includes proinflammatory M1 macrophages shift, NK cells activation, interferon γ (INF-γ) and chemokines production, accumulation of CD8+ T-cells and TH1-polarized lymphocytes [50,51,52].
Metabolic inflammation caused by circulating FFA also induces alterations in insulin release. Obesity and overweight are the main predictors to T2D development, a metabolic disease that relies on defective insulin signaling. Insulin sensitivity, as well as insulin secretion, can be reduced by obesity influence. The chronic abundance of energy maintains constantly high levels of plasma glucose, which lessen the β-cells response to incretins, decreasing insulin sensitivity and leading to insulin resistance (IR), a process that is also mediated by tumor necrosis factor α (TNFα), IL-1β, extracellular signal-regulated protein kinases 1 and 2 (ERK1/2), and c-Jun N-terminal kinases (JNKs) signaling [53,54,55]. At the same time, factors such as lipotoxicity, incretin resistance and glucotoxicity decrease β-cell mass, which, in turn, decreases insulin secretion. This impaired insulin signaling and lipotoxicity are also crucial factors to the development of MAFLD [56].
Many other diseases are also associated with obesity. The dyslipidemia process can induce CVDs, such as hypertension, myocardial infarction, and stroke [57,58]. The mechanical stress caused by over-weight leads to musculoskeletal disorders, such as osteoarthritis, as well as sleep apnea [59,60]. Increased levels of tumorigenic molecules, such as insulin-like growth factor 1 (IGF-1), are associated with several types of cancer, e.g., mammary, ovarian, prostate, gastrointestinal, liver, and renal cancer [61,62]. However, some obese patients do not have associated risk factors, a phenomenon described as ‘healthy obese’ [63]. Obesity is also a major cause of Alzheimer’s disease [64,65], decreased life expectancy [66,67], reduced quality of life, lower productivity, social disadvantages, and early retirement [67,68]. Furthermore, obesity is closely related to some mental illnesses, such as clinical depression [69], anxiety [70], and other brain disorders [71,72].
2.1. Management of Obesity
Obesity management demands a multidisciplinary approach with individualized programs. The development of a management strategy may consider the factors that contribute to obesity, as well as the overweight degree, the pre-existence of one or more associated diseases, and individual limitations. Currently, interventions are mainly based on controlling food intake and energy expenditure with changes in dietary and physical activity. The goal of treatment is the initial loss of at least 5% of the patient’s total weight. The greater the initial weight loss leads to better and faster health recovery. However, in some cases, behavioral changes alone are not enough, so pharmacotherapeutic or surgical interventions can also be part of the treatment [73].
2.1.1. Lifestyle Interventions
Comprehensive lifestyle intervention is the cornerstone of obesity management, and adjunctive treatment may be required for individuals with more compromised health, or for those who do not achieve the required weight loss [74]. Assisted behavioral changes help patients to understand and monitor their feeding behavior, creating a more conscious lifestyle [75]. However, despite the essential role of these programs in initial lifestyle changes, they fail in long-term attendance. The initial recommendations alone are not enough for re-education, without psychological assistance and adequate physical training, initial adherence to lifestyle changes is often abandoned, thus weight regain is frequent after the end of re-education programs [76].
The so-called “westernization” of lifestyle in recent decades facilitates the increase in drivers of obesity. Technologies are progressively evolving to make our lives more comfortable, as a result, the general population tends to be less active, increasing the odds of consuming more than expending. Automations and computer-based work are the majority of occupations, thus lowering the daily expenditure. This Western lifestyle also contributes to an increase in convenience foods (e.g., frozen, canned, and pre-cooked), greater fast-food availability, more effective food marketing, and larger food portions, which also corresponds with consumption of fewer home cooked meals. These factors contribute to an obesogenic environment that leads to rising levels of obesity worldwide [77,78].
Dietary interventions are essential for weight loss. Different dietary approaches with caloric restriction provide this effect, maintaining a negative energy balance. Usually, the guidelines recommend a 30% restriction on daily energy consumption, which is equivalent to 300 to 500 Kcal per day, added to an improvement in the nutritional quality of foods [79]. The choice of the calorie-restrict diet should be individualized according to the patient’s condition (i.e., gender, age, physical activity status) and preferences, in order to maximize program adherence [80].
Typically, different diets have variations in macronutrient composition; however, these differences do not imply a more effective approach. The key to effective weight loss is the long-term patient adherence to the diet, so it is important that diet choice can be matched to individual preferences [81].
Several dietary strategies can be used to induce weight loss by prioritizing one of several healthy dietary patterns. However, many of them are not nutritionally advisable or not properly considered healthy. Among those considered nutritionally recommended are dietary approach to stop hypertension (DASH), metabolic syndrome reduction in navarra (RESMENA), and Mediterranean diets, combined with low-fat (Fat: 10%–19%) and low-carbohydrate (Carbohydrate: 20%) content. These diets are commonly prescribed for weight loss and are equally effective with patient commitment [82].
DASH is an eating plan with positive effects on weight control and the cardiovascular system [83]. In this diet, the priority should be a high intake of fruits, vegetables, whole grains, and nuts. Fat-free and low-fat dairy products, as well as fish, poultry, and some legumes, such as beans, are also included. Vegetable oils are allowed, but tropical oils (such as coconut and palm oils), sugar-sweetened, refined sugar, and foods rich in saturated fats should be restricted [80].
The Mediterranean diet also emphasizes plant-based foods, such as fruits and vegetables, whole grains, legumes, and nuts. However, it also includes high intake of olive oil, moderate intake of fish and poultry, red wine in moderation, and low intake of red meat and sweets [80]. It is a very popular diet due to its role in lowering the risk of developing obesity, T2D, and CVDs. This diet also has positive effects during pregnancy, with a lower risk of fetal deficiency and promoting fetal development [84].
The RESMENA diet is a variant of the Mediterranean diet that reduces calorie intake by 30% and requires 30% of energy intake from protein. This diet also emphasizes consumption of anti-oxidant-rich fruits and vegetables and higher meal frequency (seven meals a day) [85]. In addition to weight loss, this diet reduces android fat mass (a region associated with hepatic steatosis) and waist circumference. Moreover, several biochemical parameters are improved, such as reduced transaminase levels, and maintenance of uric acid and serum glucose, indicating this diet as a good treatment option for obesity [85,86,87].
Intermittent fasting (IF) is another strategy that can induce weight loss. This eating plan has various arrangements within the premise of carrying out periods with little or no food consumption, interspersed with normal food intake on a recurrent basis [88]. During the ‘feeding window’, calorie intake is low and balanced, while during the ‘fasting window’, individuals ingest non-caloric drinks, such as water, coffee, and teas without any kind of sugar. [89]. In the most common IF, time-restricted feeding (TRF), the daily caloric intake must be consumed within a defined time window, followed by a fasting window that can range from 12 to 24 h [90]. Other protocols include fasting for up to 24 h twice a week and eating without restriction in the remaining days [91]; and alternate-day fasting (ADF), with no food restriction on eating days and no caloric intake on fasting days [91]. In addition to weight loss promoted by the caloric reduction that occurs naturally in IF, it promotes a metabolic shift that positively affects lipid and glucose metabolism, also improving diabetes, cardiovascular system (e.g., stroke), cancers, and neurological disorders, such as Alzheimer’s disease and Parkinson’s disease [88].
Another critical aspect is the inclusion of physical activity in the patient’s routine [92], which offers several benefits in addition to weight loss. Enhanced metabolic rates contribute to lowering the risk of CVDs and T2D. Additionally, the increase in muscle tissue improves bone health and joint stabilization. Moreover, physical activity promotes endorphin release and can contribute to overcome depression [93,94].
The initial recommendation is at least 150 min of moderate exercise or 75 min of vigorous physical activity per week [95]. Both aerobic and resistance training are recommended for weight loss. Although aerobic training improves cardiovascular function, resistance training promotes strength and muscle growth, which in turn increases the basal metabolic rate and, therefore, daily energy expenditure. The combination of both types of training demonstrated greater improvement in physical function and reduction in frailty compared to the isolated interventions [96].
Together, physical activity combined with dietary modification can promote optimal outcomes in overweight and obese patients when considering lifestyle interventions; however, in some cases, a pharmacological approach should be included [97].
2.1.2. Pharmacotherapy
According to the Endocrine Society guidelines [70,73,76], weight loss drugs should be considered in cases of BMI >30 or BMI from 27 to 29 with at least one comorbidity. Still, pharmacotherapy should be prescribed as adjunctive therapy and does not exclude dietetic and physical activity improvements [98]. Obesity pharmacotherapy treatment reinforces dietary intervention that results in caloric deficit. Weight-loss medications act by decreasing appetite, helping to resist binge eating or decreasing caloric absorption. Appetite suppressants may act on leptin or anorexigenic pathways. The combination of these strategies improves the efficiency of initial lifestyle changes, as well as maintenance of lost weight.
Currently, there is no single optimal medication to treat the whole spectrum of obesity. The effectiveness of a particular medication is proven with the loss of at least 5% of total weight occurring after three months of treatment. Other efficiency criteria are the improvement in current comorbidities, prevention of new associated diseases, and maintenance of weight loss [74,76,98]. Still, weight loss must be realistic and aim for long-term adherence. In most cases, 5%–10% of total weight loss in six months is achievable and sustainable over the long term.
Orlistat is the only anti-obesity medication that acts directly on the GI tract, inhibiting long-chain FAs absorption by blocking pancreatic lipase action. It is a naturally occurring lipstatin derivative that acts by binding to and inhibiting pancreatic and gastric lipases. Inactivated enzymes are unable to hydrolyze the triglycerides (TG) of dietary fat to absorbable FFA, thus decreasing dietary fat absorption by 30% of the recommended therapeutic dose [73,99]. Orlistat side effects include GI problems, such as abdominal pain, fecal urgency, flatulence, and oily stool. However, these symptoms can be ameliorated by following a low-fat diet with no more than 30% of total calories from fat and with the addition of a fiber supplement. As Orlistat reduces the absorption of fat-soluble vitamins (A, D, E, and K), multivitamin supplementation is also recommended to guarantee adequate nutritional balance [99].
Phentermine is the most frequently prescribed anti-obesity drug. It is an adrenergic agonist that acts on the central nervous system (CNS) and increases norepinephrine release, reducing appetite, and increasing the basal metabolic rate [99,100]. Phentermine causes mild increase in heart rate and blood pressure. Therefore, its monotherapy is only approved for short-term use (three months) and in younger patients without coronary disease or hypertension history. However, this medication is contraindicated for patients suffering from insomnia and anxiety disorders [101,102].
Topiramate is a gamma-aminobutyric acid (GABA) receptor modulator initially approved for seizures and migraine treatment. Topiramate administration in epilepsy treatment promoted significant weight loss, persuading the interest in this drug for obesity treatment [103]. The mechanism of action of Topiramate on weight loss is not yet totally understood; however, it is known as an appetite suppressant and satiety enhancer, acting as a neurostabilizer and enhancing thermogenesis [104].
The association of phentermine/topiramate extended-release was the first combination drug approved by the US Food and Drug Administration (FDA) in 2012 for long-term obesity treatment [105,106]. The combination of these two drugs induces additive and dose-dependent weight loss by targeting different pathways at the same time, being, therefore, more effective than monotherapy with these medications. Weight loss induced by phentermine/topiramate extended-release use is associated with improvement in various comorbid risk factors, such as improved glycemic control, lower blood pressure and TGs, and increased high-density lipoprotein (HDL)-cholesterol, reducing also T2D progression, even in the reduced use of complementary medications [106]. This medication should not be prescribed to individuals with CVD or a history of anxiety or insomnia due to the phentermine component.
A naltrexone/bupropion combination controls appetite and improves energy utilization [107,108]. Naltrexone is an opioid antagonist prescribed for alcohol and opioid dependence [109,110]. Bupropion inhibits serotonin, dopamine, and norepinephrine reabsorption, which regulates central reward pathways triggered by food stimuli. Its monotherapy is approved as an antidepressant and smoking cessation treatment [111]. Collectively, they activate POMC neurons, promoting the release of alpha melanocyte-stimulating hormone (α-MSH), a neuropeptide involved in body energy regulation. At the same time, naltrexone is also important in antagonizing an inhibitory feedback loop that limits anorectic ability of bupropion [112,113].
Liraglutide is a glucagon-like peptide 1 (GLP-1) receptor agonist, which acts directly on satiety signals, delaying gastric emptying, leading to reduced food intake. This is the only anti-obesity drug administered in the form of subcutaneous injection. The peptide binds to the GLP-1 receptor augmenting insulin secretion. Insulin release increases glucose uptake, lowering the glucose plasma level. Liraglutide also retards gastric emptying and decreases appetite [114,115]. Simultaneous use of liraglutide with insulin/insulin secretagogues may increase the hypoglycemic risk. Liraglutide mechanism of action does not involve neurotransmitters, therefore, it is indicated for patients who are also taking psychiatric medications [114,115].
2.1.3. Bariatric Surgery
Due to the high associated risks, bariatric surgery is recommended only in severe obesity, when BMI >40 or BMI >35 and there is at least one associated disease [116]. Currently, there are various types of intervention that result in different weight loss degrees. Each approach has different levels of associated benefits and risks that must be considered in conjunction with individual comorbidities and the patient’s history [117]. The three major surgical interventions used are: (I) Laparoscopic adjustable gastric band (LAGB)—the least invasive of the procedures, a band is placed around the stomach in a way that decreases in size; (II) Roux-en-Y gastric bypass (RYGB)—the removal of a large part of the stomach and the remaining portion is connected to the intestine, reducing the space available for food; (III) Laparoscopic sleeve gastrectomy (LSG), in which a large part of the stomach is also removed, but maintains the natural connection with the intestine [116,117].
2.1.4. New Drugs and Strategies
Recent discoveries in the modulation of the complex system that underlies energy homeostasis and obesity pathways unveils new perspectives in obesity drug discovery. Leptin is a central target in energy homeostasis that acts as a nutrient sensor, interrupting hunger signals. Obese patients are usually leptin-resistant and have higher levels of leptin, so manipulating leptin signaling to induce its sensitivity is one of the strategies currently explored [118]. Metreleptin is a recombinant human leptin analogue used in lipodystrophic disorders treatment, lowering hepatic steatosis and improving insulin sensitivity, hyperglycemia, and hypertriglyceridemia [119,120]. Its use in obesity treatment has been considered to help normalize decreased leptin levels caused by weight loss [121].
Another class of leptin signal modulation is the use of leptin sensitizers. Pramlintide is a synthetic amylin analogue that acts on short-term satiety signaling, delaying gastric emptying, thus reducing food intake [122]. Davalintide is another amylin mimetic peptide that has a greater affinity to amylin, calcitonin and calcitonin gene-related peptide receptors, which causes enhanced pharmacological actions on satiety signals [123].
Semaglutide is a novel GLP-1 agonist with an extended half-life that allows subcutaneous administration once a week. This peptide also has increased affinity for GLP-1 receptor and demonstrates superior efficacy in weight loss when compared to liraglutide [124,125]. Oral GLP-1 agonists are being tested as alternatives to injectable agents. In addition to the semaglutide in oral form [126], TTP-054 and ZYOGI have demonstrated promising results in effective weight loss with minimal side effects [127].
ZP4165 is a gastric inhibitory peptide analogue that acts by inducing insulin release and decreases hemoglobin A1c (HbA1c) levels in animal studies. Its action also involves the GLP-1 pathway, enhancing GLP-1 induced weight loss, suggesting that administration in combination with GLP-1 analogues may be a promising treatment for obesity [128]. Another mechanism studied to control the GLP-1 pathway is the use of dual agonists, such as the oxyntomodulin, a peptide co-secreted with GLP1 L-cells, in response to nutritional stimuli. Oxyntomodulin is a glucagon receptor (GcgR)/GLP-1 receptor agonist that has been demonstrated to suppress appetite and increase energy expenditure, thereby decreasing food intake [129]. Nonetheless, it has only short-term effects, which leads to studies with synthetic dual agonists with increased half-life, such as MEDI0382 [130], and tirzepatide [131]. A triple agonist for GLP-1, glucagon and GIP receptors, the triagonist 1706 is also in trial phase, demonstrating effectiveness in weight loss [132].
Cannabinoid receptor type 1 (CB1) neutral antagonists stimulate anorexigenic signaling, leading to weight loss by reducing food intake [133]. AM-6545 is a novel peripheral CB1 antagonist that has limited penetration in CNS and has demonstrated promising effects on weight loss, without the central side effects of the formerly commercialized CB1 antagonist rimonabant. AM-6545 presented high affinity and selectivity for the CB1 receptor, with dose-dependent reduction in food intake and food-reinforced behavior [134,135].
Cetilistat is a novel lipase inhibitor, similar to orlistat. Cetilistat treatment has demonstrated significant weight loss and improvement in glycemic control and lipid profiles, with a lower potential for GI side effects, such as diarrhea, flatulence, and oily spotting attributed to orlistat [136].
The utilization of vaccines to prevent or treat is a novel therapeutic approach to obesity management. Anti-obesity vaccines use the immune response logic to restrain appetite-stimulating hormones and decrease nutrient absorption. Ghrelin, an orexigenic hormone secreted by stomach cells, is one of these anti-obesity strategies. Anti-ghrelin vaccine lessens food intake and orexigenic signals while increasing energy expenditure in pigs. However, in human clinical trials, this vaccine did not show an additional weight loss, even with a strong antibody response to ghrelin [137]. Another anti-obesity vaccine under development is the anti-somatostatin, which promises to remove the inhibitory effects of somatostatin on growth hormone (GH) and IGF-1 secretion, thus inhibiting the increase in adiposity associated with low levels of these hormones [138,139]. Adenovirus 36 (ad36), known to enhance the obesity risk in humans by causing inflammation and adiposity, is a possible target for prophylactic anti-obesity vaccination [140,141].
The discovery of the brite adipocyte type unveils it as a promising therapeutic target for obesity treatment. Induction of brown-like white adipose tissue adipocytes (beige cells) can counteract obesity-induced metabolic processes and increase weight loss through high levels of thermogenic gene expression [142,143]. Cold exposure is a promising non-pharmacological approach to shift the thermogenic program in beige adipocytes by activating β adrenergic receptor (ADRB) expression [144]. Among dietary compounds, capsaicin, found in red pepper, is the most studied browning activator [145,146]; however, several nutritional components are now known to play a role in browning induction. Together with capsaicin, cucumin [147], and n-3 Polyunsaturated fatty acids (PUFAs), particularly the eicosapentaenoic acid (EPA), found in fish oil, also activate beige cells by activating ADRB3. EPA [148], green tea catechins [149], and resveratrol [150] also function as epigenetic modulators, inducing activation of peroxisome proliferator-activated receptor γ (PPARy) and PRDM16 transcription factors. Resveratrol, EPA, curcumin, berberine [151], and all-trans retinoic acid [148] act directly in mitochondrial biogenesis by activating AMP-activated protein kinase (AMPK) pathway. Pharmacological activators of beige cells under studies include β3-adrenergic receptor agonist [152], PPARy and PPARα activators [153,154,155], PGC-1α stabilizer [156], and metformin as an AMPK activator [157,158].
The different obesity treatments existing or under study are summarized in Table 1.
Table 1.
Pharmacological treatments for obesity. The compounds are divided into class, mechanism of action, drugs examples, current state of application, and its side effects.
| Class | Mecanism of Action | Drugs | Current State | Side Effects | Reference |
|---|---|---|---|---|---|
| Lipase inhibitors | Inhibit long-chain FAs absorption | Orlistat | Commercial | Abdominal pain Fecal urgency Flatulence Oil stool |
[73,99] |
| Induce weight loss Improve glycemic control Improve lipid profile |
Cetilistat | Under studies | Diarrhea Flatulence Oily spotting |
[136] | |
| Adrenergic agonists | Increase norepinephrine release in CNS | Phentermine | Commercial | Increase heart rate and blood pressure | [99,100,101,102] |
| GABA receptor modulators | Neuroestabilizer Enhance thermogenesis |
Topiramate | Commercial | Alteration of taste GI upset Nausea |
[103,104] |
| POMC neurons activators | Induce α-MSH release | Naltrexone/Bupropion | Commercial | Nausea | [107,108,109,110,111,112,113] |
| Delay gastric emptying Enhance insulin secretin |
Liraglutide * | Commercial | Transient nausea Vomiting |
[114,115] | |
| GLP-1 receptor agonists | Reduce blood glucose levels Induce weight loss |
Semaglutide TTP-054 ZYOGI |
Commercial | Nausea Vomiting Diarrhea |
[124,125,126,127] |
| Induce insulin release Decrease HbA1c levels |
ZP4165 | Under studies | Unknown | [128] | |
| Leptin analogues | Lower hepatic steatosis Improve insulin sensitivity |
Metreleptin | Commercial | Nausea | [119,120,121] |
| Amylin analogues | Delay gastric emptying | Pramlintide * Davalintide * |
Commercial | Nausea | [122,123] |
| Glucagon and GLP-1 receptors agonists | Supresses appetite Increase energy expenditure |
Oxyntomodulin | Under studies | Unknown | [129] |
| GLP-1, glucagon and GIP receptors agonists | Induce weight loss | Triagonist 1706 | Under studies | Unknown | [132] |
| CB1 antagonists | Stimulate anorexigenic signaling | AM-6545 | Under studies | Unknown | [134,135] |
| Vaccines | Restrain appetite-stimulating hormones Decrease nutrient absorption |
Anti-ghrelin Anti-somastatin Anti-ad36 |
Under studies | Unknown | [137,138,139,140,141] |
| Induction of beige-cells | Increase thermogenic gene expression Epigenetic modulators Activation of AMPK pathway |
Capsaicin Curcumin PUFAs |
Under studies | Unknown | [152,153,154,155,156,157,158] |
* Similar compounds for more than one NCD.
2.1.5. COVID-19 and Obesity
The recent pandemic of coronavirus disease, COVID-19, has been worsened by high levels of obesity and overweight in the world. The pathophysiological changes present in obesity, such as impaired immunity, chronic inflammation, and high blood pressure increase the risk of hospitalization in 113% and mortality by 48% in young individuals [159].
In obesity, abdominal fat compresses the diaphragm, restricting the airflow and decreasing lung capacity. Obstructive sleep apnea and other breathing disorders are common in obese individuals, which predisposes to hypoventilation-associated pneumonia, pulmonary hypertension, and cardiac stress. The large body mass also causes difficulties in intubation and mask ventilation [160].
Hormone and nutrient imbalance that are typical of obesity can impair adaptive and immune responses. Hyperglycemia can impair immune response, producing oxidants and glycation molecules [161]. Insulin and leptin signaling are crucial for T-cell activation, therefore, impairment of these pathways can lead to T-cell dysfunction [162,163]. Additionally, the chronic low-grade inflammation caused by constant high levels of leptin and other proinflammatory cytokines can decrease the immunity period covered by vaccines, as occurred with influenza vaccination.
3. Diabetes Mellitus
Diabetes mellitus is characterized by chronic hyperglycemia that impairs food metabolism. Causes of prolonged high levels of plasma glucose may be immune-mediated (type 1 diabetes), insulin resistance (type 2 diabetes), gestational diabetes, or others (neonatal, insipidus, brittle, LADA). Increased blood glucose leads to the classic diabetes symptoms: frequent urination (polyuria), increased thirst (polydipsia), and increased hunger (polyphagia), and can lead to the development of micro and macrovascular complications, resulting in nerves, heart, kidney, skin, and retina diseases [164]. Diabetes is a major global health issue. In 2019, about 463 million adults (from 20 to 79 years old) were living with diabetes and this number could increase to 700 million by 2045, causing 4.2 million deaths worldwide and being considered the fastest growing global health emergency [165].
Under normal metabolic conditions, food ingestion triggers insulin secretion by pancreatic β-cells, which induces glucose uptake in peripheral tissues and suppresses endogenous glucose production. Insulin acts directly on skeletal muscle, liver, and adipocytes via specific signaling pathways to induce various processes involved in glucose homeostasis [166]. In muscle, insulin improves glucose utilization by increasing the glucose transporter, GLUT4, and storage, promoting glycogen synthesis [167]. In the liver, the hormone activates glycogen synthesis and regulates lipogenic and gluconeogenic gene expression programs [168]. In adipocytes, it stimulates glucose uptake and lipogenesis, while decreasing lipolysis [169]. All of these integrated processes work simultaneously to keep blood glucose levels constant. To maintain the homeostasis, the blood glucose level must be sustained within a small interval despite the oscillations in supply and demand that occur in fasting/feeding cycles. Failures in insulin signaling block glucose uptake, leading to a prolonged hyperglycemic state [166].
Diabetes is characterized by β-cell failure, which can be auto-immune due to β-cell destruction, or by a progressive impairment of β-cells function that leads to insufficient insulin secretion. If insulin secretion is insufficient to regulate glucose uptake in peripheral tissues, β-cells need to increase the amount of secreted insulin in order to lower plasma glucose, a process called IR. The stress caused by constant overproduction of insulin can lead to β-cell failure followed by cell death [170].
3.1. Type 1 Diabetes Mellitus
In type 1 diabetes mellitus (T1D), insulin deficiency results from loss of pancreatic β-cells due to autoimmune-mediated destruction. This pathogenesis is a continuum disease that initiates with an early asymptomatic stage with auto-antibodies detection, this stage occurs years before the development of symptoms. Gradually, a decline in β-cell mass and dysglycemia that evolves to symptomatic T1D, which presents typical symptoms of hyperglycemia, such as weight loss, hyperphagia, and polyuria [171]. T1D is one of the most common metabolic diseases occurring in childhood, with more than 1.1 million children and adolescents affected in 2019 [165].
T1D is determined by genetic susceptibility, ineffective immune system, and environmental factors. A genome-wide association study and meta-analysis found 40 genetic loci associated with this disease [172]. Particularly, the HLA region on chromosome 6 has been identified as a T1D predisposition locus. This region provides half the susceptibility that leads to T1D risk; however, most loci associated with disease development are thought to involve immune responses, supporting the idea that genetic influences involve mechanisms that contribute to aberrant immune responsiveness [173].
The T1D autoimmune process begins with the activation of CD4+ T-lymphocytes, responsible for the secretion of IFNγ, macrophages and antigen presenting cells (APCs), such as dendritic cells (DCs). These cells generate antibodies to β-cell, which lead to chronic immunological responses, such as the secretion of cytokines (e.g., TNFα and IL-1) and activation of lymphocytes and NK cells. These activated cells work together to destroy pancreatic β-cells, inducing structural changes that suppress their ability to release insulin, leading to the development of T1D (127). This process produces various specific pancreatic islet auto-antibodies that are involved in the further development of the disease, including (I) islet cell autoantibodies (ICA), (II) glutamic acid decarboxylase auto-antibodies (GADA), (III) insulinoma associated 2 auto-antibodies (IA-2A), (IV) insulin auto-antibodies (IAA), and (V) recently described zinc transporter auto-antibodies (ZnT8A). These molecules are extremely important, mainly for patients with a non-canonical T1D phenotype, and are used to predict and confirm autoimmunity [174].
There is also the involvement of environmental factors in T1D development, such as viral infections, timing of the food introduction and gestational events. The contribution of exposure to these events on the development of T1D is believed to be small, but a combination of events can trigger the onset of a first β-cell auto-antibody [175].
3.2. Type 2 Diabetes Mellitus
Type 2 diabetes mellitus (T2D), also known as non-insulin dependent diabetes mellitus, is the most common form of diabetes, accounting for about 90% of all diabetes cases worldwide, according to the International Diabetes Federation [165]. This type of diabetes is characterized as an endocrine and metabolic disorder that associates environmental factors, such as energy-dense ‘Western’ nutrition, sedentary lifestyle, stress, aging, and obesity, with genetic factors, resulting in β-cell dysfunction and IR [176]. Although the genetic factor plays a significant role, the major cases of T2D are potentially preventable with a healthy diet and active lifestyle [177].
Prior to achieving the hyperglycemia that characterizes T2D, individuals manifest a stage of prediabetes. At this stage, the individual may present high fasting glucose levels, impaired glucose tolerance, and increased glycated HbA1c levels. Other biomarkers are high blood concentrations of proinflammatory cytokines, such as IL-6 and TNFα [178], gut microbiota profiles [179], and decreased sex hormone-binding globulin [180]. Prediabetes can be reversed through behavioral management, such as diet and sedentary lifestyle improvement. Increasing intake of whole grains and green leafy vegetables and lowering intake of highly processed and sugar-sweetened foods, and alcohol, combined with regular physical activity, can decrease the disease conversion to diabetes [177].
The causes, symptoms, and progression of this disease can vary substantially among individuals, but the main mechanism is the progressively impaired insulin secretion by pancreatic β-cells. IR decreases the efficiency of tissue glucose uptake by multiple abnormalities. The main tissues affected by IR are liver, muscle, and adipose tissue. However, this deficiency also affects pancreatic β-cells [181], intestinal metabolism [182], kidney [183], brain [184], and vasculature [185].
In the liver, additionally to IR, deficiency in insulin production and excessive production of glucagon (hyperglucagonemia) increase glucagon sensitivity and delivery of metabolic substrates, such as FAs, lactate, and glycerol. This leads to an increase in gluconeogenesis, despite the presence of fasting hyperinsulinemia and causes impaired suppression of insulin-responsive hepatic glucose production [186]. In muscle, IR affects glucose transport and phosphorylation, mitochondrial activity, glycogen synthesis, and pyruvate dehydrogenase complex activity [187]. The elevated glucose caused by dysfunctional uptake and gluconeogenesis lead to glucotoxicity in these tissues.
In adipose tissue, IR impairs the suppression of lipolysis and the release of FFA that normally occurs in high levels of insulin. Defective insulin signaling leads to glucose intolerance and triggers the efflux of FFA into circulation, thereby inducing a proinflammatory state [188]. Altered lipid metabolism can activate toll-like receptors (TLRs), affecting inflammation. In T2D, adipose tissue presents a high rate of macrophage infiltration and increased levels of proinflammatory cytokines and adipokines, such as leptin. These proinflammatory cytokines and high FFA levels can activate downstream kinases, such as TNF, IκB kinase-β (IKKβ), JUN amino-terminal kinase 1 (JNK1), and p38 MAPK, which induce phosphorylation on serine residues of the insulin receptor substrate (IRS) proteins. Moreover, these kinases may enhance the production of protein suppressors of cytokine signaling (SOCS) that block IRS action [189]. On the other hand, increased levels of IL-6 can stimulate hepatic gluconeogenesis, also inducing IR. Macrophage infiltration into adipose tissue increases proinflammatory M1 macrophages and T helper cells, while decreasing M2 macrophages and regulatory T cells, stimulating lipolysis itself [190].
T2D is associated with increased morbidity and mortality due to the development of complications that affect several organs. The life span of diabetic individuals is shortened by an average of 6 years, and the loss in life expectancy can reach 12 years in young onset development of T2D [191]. Diseases associated with T2D are divided in two categories: (I) macrovascular complications, such as CVD, that encompass coronary heart disease, peripheral vascular disease and cerebrovascular disease, which is a major motive of death and disability [192]; and (II) microvascular complications, due to severity and duration of hyperglycemia [193]. This includes retinopathy, neuropathy, and chronic kidney disease, which accounts for about 10% of deaths among diabetics [194]. The molecular mechanisms that contribute to the macro and microvascular complications are the same: reactive oxygen species (ROS) activate several proinflammatory pathways resulting in epigenetic changes. Thus, the expression of proinflammatory genes continues even after the normalization of glycemia [195].
3.3. Gestational Diabetes Mellitus
Gestational diabetes mellitus (GDM) occurs with the spontaneous development of hyperglycemia during pregnancy. Advanced maternal age, family history, poor eating habits, and obesity are the main risk factors for the development of this disease, which affects about 14% of pregnancies worldwide [165]. As pregnancy demands more energy, in early stages, there is an increase in insulin sensitivity, allowing greater glucose uptake in the adipose tissue. However, as pregnancy progresses, local and placental hormones such as estrogen, progesterone, leptin, cortisol, placental lactogen, and placental growth hormone, promote a state of IR [196]. GDM increases the risk of preterm birth and preeclampsia in children, which can result in overgrowth, since there is an increase in the placental transport of glucose, amino acids and FAs, stimulating the production of insulin and IGF-1. Moreover, this abnormal insulin production can cause pancreatic β-cell dysfunction and IR, even prenatally [197]. Usually, GDM resolves at the end of the gestation period. However, it can have lasting consequences, such as increased risk of development of T2D, a CVD in the mother and predisposition to obesity and T2D in children.
3.4. Maturity Onset Diabetes of the Young
This type of diabetes belongs to the subgroup defined as early diagnosis, typically before age of 25, and is not insulin dependent. It is characterized as an autosomal dominant disease with heterozygous mutations in various transcription factors that act in the development and maturation of pancreatic β-cells [198]. Despite its genetic origin, maturity onset diabetes of the young (MODY) is a heterogeneous disease, with different medical conditions and treatments associated with each subtype. To date, 14 different genetic mutations have been reported to be related to MODY, each of them corresponds to a MODY subtype. The six major MODY-causing genes encodes hepatocyte nuclear factor 4α (HNF4α), HNF1α, glucokinase (GCK), pancreatic and duodenal homeobox 1 (PDX1), HNF1β, and neurogenic differentiation 1 (NEUROD1) [199]. Due to its heterogeneity, early diagnosis based on next-generation sequencing has been essential to set individualized treatments, preventing long-term diabetes complications [200].
3.5. Other Types of Diabetes
There are several other types of diabetes, which occur less frequently in the population. Diabetes insipidus is characterized by the excretion of large volumes of dilute urine due to vasopressin deficiency, arginine vasopressin (AVP) resistance, or excessive water intake. It is mainly caused by a decrease in AVP secretion or action, which may be partial or complete, which can be acquired, or a genetic defect in the neurohypophysis [201]. Brittle diabetes occurs in a small group of patients with T1D, mainly women, with severe glycemic instability, poor metabolic control, and a compromised quality of life due to very common acute complications, hospital recoveries, and appearance of chronic problems [202]. Diabetes can also be developed due to diseases of the exocrine pancreas, such as acute pancreatitis [203] or cystic fibrosis [204]. Some hormones, such as GH, glucagon, and catecholamines, can antagonize insulin action. This mechanism can be exacerbated in tumors that produce excess of these hormones, inducing IR. In this case, diabetes may disappear or ameliorates with tumor removal [205]. Drug- or chemical-induced diabetes can arise over the use of compounds toxic to β-cells (160). In addition, certain infections, such as congenital rubella and cytomegalovirus, are also associated with autoimmune destruction of β-cells [206].
3.6. Management of Diabetes Mellitus
In diabetes, glycemic control is achieved by administration of antidiabetic medications that reverse the effects of its pathophysiological damaged insulin signaling. There are different classes of antidiabetic treatments and their choice varies according to several factors, such as the nature of diabetes, age, and the progression of the disease. Effective treatment requires multiple actions to circumvent the various pathophysiological defects. The strategy must be based on all the known pathogenic abnormalities and many individual factors called “ABCDE” of diabetes, that are: body weight, complications, duration, education and expense, and etiology [164]. Early diagnosis and implementation of therapeutic strategies are the most efficient to prevent progression of diabetes mellitus. Due to the lipotoxicity caused by obesity and physical inactivity, lifestyle interventions are a part of all intervention strategies, with or without drug treatment, depending on the factors mentioned above [207].
3.6.1. Dual Therapies
Insulin
Insulin has been widely used in patients with diabetes. Therapy is based on the patient’s weight and typical doses range from 0.4 to 1.0 units/kg/day, depending on the glycemia (always self-monitored), meal size, and tissue glucose demand. There are several types of insulin, which are categorized from fast-acting to long-acting, from insulin analogues to human insulins, primarily based on how it works and how quickly it acts [208]. Long-acting insulin analogues are thought to result in fewer hypoglycemic episodes and are given 1–3 times a day according to the patient’s pharmacokinetic properties to control glucose levels between meals and fasting. Postprandial insulin treatments comprise fast-acting analogues or regular short-acting insulin, which are given before each meal and each time a correction of high blood glucose is required, occurring mainly 3 times a day [209].
Insulin can be administered by two routes: injection or infusion. The injection can be done with syringes, which are injected into the fat layer just under the skin, or with insulin pens, which can be reusable or disposable. As an infusion, it can occur via a vein in the hospital, with constant supervision by specialists, or via insulin pumps, which are computerized devices programmed to transport insulin under the skin, considered more durable [208]. In T2D, insulin therapy is usually used after failure of other treatment strategies to control blood glucose and requires larger doses than T1D treatment. Insulin therapy is often combined with other antidiabetic drugs, and the most common combinations are with metformin or thiazolidinediones (TZD), but the combination with GLP-1, sodium/glucose co-transporter 2 (SGLT2) are also effective in lowering HbA1c blood levels [210].
Metformin
The most commonly prescribed antidiabetic drug is metformin, a biguanide that lowers hyperglycemia by reducing hepatic glucose production, which leads to decreased HbA1c and fasting plasma glucose. The mechanism of action of metformin is still unclear, but it is known to be related to mitochondrial dysfunction by inhibiting mitochondrial glycerophosphate dehydrogenase, mitochondrial complex I, and activation of AMPK, and has no effect on pancreatic β-cells function. Metformin is usually combined with other drugs that increase insulin secretion, such as sulfonylureas. Metformin alone does not improve muscle insulin sensitivity, and HbA1c progressively increases after the initial decrease [164,211]. Other examples of biguanides are phenformin and buformin.
SGLT2 Inhibitors
SGLT2 is responsible for about 90% of glucose reabsorption. SGLT2 inhibitors, such as canagliflozin, dapagliflozin, and empagliflozin, are used to lower glucose blood levels by preventing renal reabsorption of glucose, thereby increasing its excretion. This increased glucose excretion via glycosuria reduces blood glucose, which ameliorates glucotoxicity, improving β-cell function, and increasing insulin sensitivity [212,213].
Targeting GLP-1
GLP-1 is a peptide produced by the GI system in response to food intake and stimulates glucose-dependent insulin secretion while inhibiting glucagon secretion. However, the GLP-1 half-life lasts only a few minutes. It can be targeted to the diabetes treatment in two different forms: (I) targeting the GLP-1 receptor itself, with an incretin mimetic with an extended half-life; or (II) targeting dipeptidyl peptidase 4 (DPP4) enzyme that acts by inactivating GLP-1. DPP4 inhibitors, such as sitagliptin, vildagliptin, saxagliptin, linagliptin, and alogliptin, can prolong the half-life of GLP-1, thus improving the glycemic control in T2D [214]. GLP-1 receptor agonists, such as exenatide, liraglutide, lixisenatide, and dulaglutide, promote insulin secretion from pancreatic β-cells, inhibiting inappropriate glucagon secretion by pancreatic α-cells, delaying gastric emptying and controlling appetite. Moreover, this molecule can reduce pancreatic β-cell apoptosis, stimulate their proliferation and improve their survival rate, with a concomitant reduction in body weight, which is a positive effect, since diabetes is directly related to obesity [215].
3.6.2. T1D Therapies
Cyclosporin
To circumvent the autoimmune destruction of pancreatic β-cells, treatment with the immunosuppressive agent cyclosporin was the first immunotherapy tried. Cyclosporin is a calcineurin inhibitor that acts directly on T cells and was first tested in the 1980s in patients on insulin therapy for less than 2 months after diagnosis. A successful remission rate of diabetes throughout the treatment was observed; however when it was stopped, the disease progressed and resulted in the destruction of the residual β-cell mass, since the treatment could not be prolonged due to its effects, such as nephrotoxicity and an increased risk of cancer [216]. These results instigated researchers to investigate therapies that promote immune-tolerance, rather than immunosuppression, as well as short-term strategies to re-educate of these patient’s immune systems. Since then, various therapies have been tested, targeting T-cells, β-cells, antigen specific, among others, but many unanswered questions remain, especially regarding the mechanisms behind the development of this autoimmune disease [217].
Pramlintide
Pramlintide is administered adjunct with insulin treatment, which consists of injectable and oral glucose lowering drugs. Its active compound in the pramlintide acetate (SYMLIN) injection, is an amylin analogue and the first non-insulin T1D treatment. It reduces postprandial glucose concentrations, improves overall glycemic control and promotes a significant weight reduction [217]. Amylin is a 37 amino acid neurohormone co-secreted with insulin by pancreatic β-cells after a meal and its levels are reduced in T1D, and pramlintide is a synthetic analogue of amylin that was approved in April 2004 by the FDA [218].
Surgical Interventions
In some cases, pancreatic transplantation is an option for the patient with T1D. The procedure consists of a surgical operation with the normal pancreas of a decreased person inserted into the patient. After this procedure, the new pancreas produces insulin and this hormone therapy is not necessary anymore, but a special care is necessary with rejection of the new organ, being required the use of anti-rejection drugs or immunosuppressants for the rest of life. In addition to this, there is also pancreatic islet transplantation, being called allotransplantation, which consists of the purification and transfer of islets from a dead donor to the patient, resulting in the reestablishment of insulin secretion and is performed in patients with uncontrollable T1D levels [208]. However, both transplants carry the risk of sensitization against the same autoimmune antigen that led to prior β-cells collapse [219].
3.6.3. T2D Therapies
Sulfonylureas and Glinides
Sulfonylureas are oral hypoglycemic medications that lower glucose levels in the blood plasma by increasing insulin secretion. The high insulin level overcomes IR and lowers the HbA1c levels in the blood. However, as with metformin action, sulfonylureas had no long-term effect on blood glucose and HbA1c levels increased progressively after the initial decline. They trigger insulin release by directly binding and closing the ATP-sensitive K+-channels of β-cell plasma membrane, which provokes membrane depolarization, opening the voltage-sensitive Ca2+ channels, leading to the release of mature insulin granules. Despite their wide use in diabetes treatment, sulfonylureas are known to be associated with hypoglycemia, weight gain, increased risk of cardiovascular events and may even accelerate β-cells failure [220]. Another class of anti-diabetics that have similar mechanisms of action are the glinides. Drugs such as repaglinide and nateglinide are short-action insulin secretagogues, which are highly effective in lowering HbA1c blood levels. However, due to the short-term action, they require administration before each meal [221].
Thiazolidinediones (TZDs)
TZDs, such as pioglitazone and rosiglitazone, are insulin-sensitizing drugs that enhance insulin sensitivity in skeletal and cardiac muscle, liver, and adipocytes. Their mechanism of action occurs through activation of PPARγ, a nuclear receptor that regulates the transcription of several genes involved in glucose and lipid metabolism and energy balance, such as GLUT4, glycogen synthase, and pyruvate dehydrogenase. PPARγ activation increases fat oxidation, proliferation of adipocytes, lipogenesis, fat redistribution, and adiponectin levels, and reduces plasma FFA levels and pro-inflammatory cytokines [222]. Despite their anti-diabetic effects, the use of TZDs presents several adverse effects, such as fluid retention, weight gain, and trauma-related fractures. Hence, compounds with similar anti-diabetic effects, but with attenuated secondary effects are targets in the search for new anti-diabetics [223].
Alpha Glucosidase Inhibitors (AGIs)
AGIs have no effect on insulin secretion or sensitivity, they slow the carbohydrate absorption by averting alpha-glucosidases from converting polysaccharide carbohydrates to monosaccharides in the GI system, thus lowering post prandial blood glucose levels. Some GI adverse effects, such as diarrhea, nausea, and abdominal pain, are related to the use of AGIs [224].
As a summary, current approaches to the diabetes treatment are shown in Table 2.
Table 2.
Pharmacological treatments for diabetes mellitus. The compounds are divided into class, mechanism of action, drugs examples, current state of application and its side effects.
| Class | Mecanism of Action | Drugs | Type | Current State | Side Effects | Reference |
|---|---|---|---|---|---|---|
| Hormone | Reduce blood glucose levels | Insulin | 1 and 2 | Commercial | Hypoglicemia | [208,209] |
| Biguanides | Reduce hepatic glucose production | Metformin * Phenformin * Buformin * |
1 and 2 | Commercial | Abdominal discomfort | [169,211] |
| SGLT2 inhibitors | Prevent glucose reabsorption | Canagliflozin * Dapagliflozin * Empagliflozin * |
1 and 2 | Commercial | Urinary tract and genital infections Decrease in blood pressure Weight gain |
[193,212,213] |
| DPP4 inhibitors | Improve glycemic control | Sitagliptin * Vildagliptin * Saxagliptin * Linagliptin * Alogliptin * |
1 and 2 | Commercial | Hypoglicemia Loss of consciousness Gastrointestinal side effects |
[214] |
| GLP-1 receptor agonist | Promote insulin secretion | Exenatide * Liraglutide * Lixisenatide * Dulaglutide * |
1 and 2 | Commercial | Transient nausea Vomiting |
[215] |
| Calcineurin inhibitor | Inhibit T cell activation | Cyclosporin | 1 | Commercial | Nephrotoxicity Increase risk of cancer |
[216,217] |
| Amylin analogues | Reduce blood glucose levels Induce weight loss |
Pramlintide * | 1 | Commercial | Nausea | [217,219] |
| Sulfonylureas and glinides | Increase insulin secretion | Tolbutamide Glibenclamide Glipizide |
2 | Commercial | Hypoglicemia Weight gain |
[220,221] |
| PPARγ agonists | Increase tissues sensibility to insulin action | Rosiglitazone * Pioglitazone * |
2 | Commercial | Fluid retention Weight gain Trauma-related fractures |
[222,223] |
| Alpha glucosidase inhibitors | Slow the carbohydrate absorption | Acarbose | 2 | Commercial | Diarrhea Nausea Abdominal pain |
[224] |
* Similar compounds for more than one NCD.
3.7. COVID-19 and Diabetes
Data recorded during 2019 in China (179) showed that patients with severe disease had a higher prevalence of diabetes (16.2%) compared to those with non-severe disease (5.7%), and COVID-patients with diabetes had higher mortality, 7.3% versus 2.3% overall [225]. However, it should be noted that diabetes has been associated with a poor prognosis in other viral infections.
Many hypotheses are emerging to explain the relationship between diabetes and COVID-19. The first is that diabetic patients have an exaggerated proinflammatory response in the absence of appropriate immunostimulation by increasing the cytokines IL-1, IL-6, and TNFα, and this response could be more exaggerated with SARS-CoV-2 infection [226]. COVID-19 positive individuals with diabetes have been shown to have significantly increased levels of IL-6 and C-reactive protein compared to COVID-19 patients without diabetes [227]. Thus, diabetic patients may have a potential organ damage after SARS-CoV-2 infection by the exacerbate cytokine response, increasing mortality rates [228]. On the other hand, the overexpression of angiotensin-converting enzyme 2 (ACE2) in diabetic patients due to the use of ACE inhibitors (ACEi) or angiotensin-receptor blockers (ARBs), favors the entry of the virus in the host [226]. It should be noted that ACE2 is expressed in the pancreas, so the entry of SARS-CoV-2 into pancreatic islets may produce a β-cell dysfunction, and, consequently, a hyperglycemic state [229].
4. Metabolic Associated Fatty Liver Disease (MAFLD)
MAFLD, formerly known as non-alcoholic fatty liver disease (NAFLD), is a spectrum of diseases ranging from steatosis, characterized by an abnormal hepatic lipid accumulation; including non-alcoholic steatohepatitis (NASH), defined by liver inflammation and steatosis; that, when aggravated, it can lead to fibrosis, and, ultimately, evolve to cirrhosis and even hepatocellular carcinoma (HCC) [230]. MAFLD is estimated to affect about 1.8 billion people worldwide, comprising about 25% of the world’s population [231]. Although silent in many cases, MAFLD is the most prevalent liver disease among the population and its burden is expected to increase in the coming decades [232]. Despite these surprising facts, the MAFLD is still absent from global public health policies and there are still no approved pharmacological treatments for it.
The definition of MAFLD comprises the evidence of fat accumulation in the liver in addition to at least one of the following comorbidities: overweight/obesity, T2D, or evidence of metabolic dysregulation (e.g., larger waist circumference; elevated blood pressure, high TG or plasma HDL levels, prediabetes, homeostasis model assessment of insulin resistance (HOMA-IR) scores ≥2.5, and high plasma high-sensitivity C-reactive protein levels) [233].
Well-known hallmarks for the development and progression of MAFLD are IR, mitochondrial dysfunction, lipotoxicity, and inflammation. Dietary intake, such as excessive fructose and fat consumption, environmental factors, and genetic predispositions contribute to disease progression [230]. MAFLD is a multifactorial pathogenesis that evolves from abnormal TGs accumulation, comprising more than 5% of hepatocytes volume [234]. Several molecular dysregulations are associated with this event: elevated FA uptake, white adipose tissue (WAT) lipolysis, enhanced de novo lipogenesis (DNL), and defects in insulin signaling [235].
In MAFLD, the major source of lipids in hepatocyte accumulation is TG derived from WAT lipolysis (up to 60%), induced by irregularities in the insulin pathway. Impaired insulin signaling, caused by poor eating habits and by sedentary lifestyle, results in increased lipolysis in WAT [236]. This generates an influx of TG into the liver, leading to substrate overload and, consequently, the development of hepatic IR that leads to intensified accumulation of DNL and TG [237]. Under IR condition, the phosphodiesterase 3B enzyme (PDE-3B) is not active, inhibiting the protein kinase A (PKA) and the hormone sensitive lipase (HSL). Consequently, lipolysis is not suppressed, increasing levels of circulating FAs [238], which are harvested by CD36, FATP, FABP, and caveolin-1 transporters [239]. Once inside, these FAs are esterified to TGs by diglyceride acyltransferase (DGAT) 1/2, which are stored in lipid droplets or exported via VLDL, very low-density lipoprotein (LDL) (194). MAFLD patients have higher VLDL production, suggesting that even this greater export of TGs is not able to compensate for the increased uptake of FAs [240].
Hepatic DNL accounts for 25% of TG accumulation in MAFLD [236]. Excess fructose and impaired insulin signaling stimulate DNL through the action of different transcriptional factors, such as carbohydrate response element binding protein (ChREBP), PPARγ, and sterol response element binding protein 1c (SREBP-1c), which are responsible for increasing gene transcription of glycolysis, and DNL, such as hepatic pyruvate kinase in the former, and ACLY, FASN, and SCD1 in the latter [241].
Dietary TGs are transported into the circulation as chylomicrons [242] that are captured by the liver via LDL receptor (LDLR) and LDLR-related protein 1 (LRP1) [243]. After metabolizing, FA are exported from the liver packed in VLDL particles, accompanying cholesterol, phospholipids, and apolipoproteins [244]. In MAFLD, this mechanism is impaired by hepatic IR, which stimulates DNL without inhibiting VLDL production [245].
Fructose metabolism is another important pathway for hepatic lipid accumulation that stimulates hepatic DNL, which ultimately contributes to lipid accumulation in hepatocytes [246]. In healthy individuals, this pathway contributes up to 5% of total hepatic TGs; however, in individuals with MAFLD, this contribution can reach 23%. Thus, this considerable increase suggests that upregulation of fructose metabolism is associated with MAFLD progression [247].
One major hallmark that differentiates NASH from steatosis is the occurrence of hepatocyte damage, that is mainly associated with oxidative and endoplasmic reticulum (ER) stress caused by lipotoxicity and necroinflammation. Elevated levels of non-esterified fatty acids (NEFA) are extremely toxic to hepatic cells, a phenomenon called lipotoxicity [248], which increases hepatic gluconeogenesis [249]. The greater amount of acetyl-CoA, produced from FA oxidation, inhibits the pyruvate dehydrogenase complex (PDH), which redirects pyruvate to glucose production [190]. However, the malonyl-CoA produced in DNL initial stages inhibits carnitine palmitoyltransferase 1A (CPT1A), that, ultimately, leads to downregulation of mitochondrial oxidation [250].
Over time, excess FA causes mitochondrial stress, leading to mitochondrial uncoupling, ROS production, and JNK activation [251]. In addition, other lipids in hepatocytes, such as lysophosphatidylcholine, ceramides, cholesterols, and diglycerides, can trigger hepatic IR and cell death [248]. These lipids are associated with increased ER stress, oxidative damage, and activation of NLRP3 inflammasome, which can damage hepatocytes and cause cell death by apoptosis, pyroptosis, and necropoptosis [252].
In addition to dysregulation of hepatic metabolic pathways, excess NEFA together with pathogen-associated molecular patterns induce inflammation by activating TLRs [253]. TLRs activate the pro-inflammation transcription factor NF-kB in hepatocytes, Kupffer cells (KC) and hepatic stellate cells (HSC) [254,255]. During increased inflammation, there is also an increment in the activity of NADPH oxidase, an enzyme responsible for ROS production in KC, which contributes to increased oxidative stress [256]. Through the production of ROS, KCs stimulate inflammatory signaling, mainly through the chemoattraction of other leukocytes [257]. Meanwhile, infiltrated pro-inflammatory macrophages stimulate the inflammatory process, which contributes to a vicious cycle of inflammation. Increased inflammation is strongly related to hepatic IR. The signaling of NF-kB and JNK, through TNFR1, RANKL, and ILR receptors, promotes the action of IKKb, a protein associated with increased IR due to phosphorylation of IRS-1/2 in hepatocytes [258].
Excess FAs and Ca2+ also induce mitochondrial adaptations, increasing ROS production and oxidative stress [259,260]. Obesity, even without MAFLD, increases mitochondrial respiration to its maximum [261]. However, obese individuals with NASH show an approximately 40% decrease in maximal respiration compared to healthy individuals, which may be associated with hepatic IR, mitochondrial uncoupling, and leaking activity [251]. In this pathway, chronic excess of mitochondrial acetyl-CoA leads to increased ROS production, decreased antioxidant capacity, and ATP depletion [262,263]. Increased ROS production also leads to oxidation of mitochondrial DNA, depolarization of the membrane, and translocation of cardiolipins to the cytosol, inducing cellular death [264].
In addition to these two hepatic apoptosis pathways, adipose tissue also contributes to fibrogenesis in MAFLD, increasing the secretion of proinflammatory cytokines and unbalancing secretion of leptin and adiponectin [265]. Both KC and HSC cells respond to leptin [265,266,267]. In KC cells, leptin upregulates TGF-β, inducing activation of HSC cells. In HSC cells, leptin induces the matrix metalloproteinase-1 inhibitor, TIMP-1, and collagen 1 production, while repressing matrix metalloproteinase 1. Leptin also upregulates microRNA 21, inducing the profibrogenic TGF-β/Smad pathway. In addition, leptin also upregulates a hedgehog pathway that keeps the activated phenotype of HSCs [266]. After NASH establishment, the aggravation of necroinflammation and fibrogenesis, immune cell infiltration, and activation of hepatic progenitor cells contribute to the disease progression from NASH to cirrhosis, and even to HCC [235].
In addition to the aforementioned environmental and metabolic factors, there are also genetic factors associated with MAFLD development. The best-known mutations are in patatin-like phospholipase 3 (PNPLA3), a gene that encodes a lipase [268]. Individuals with PNPLA3 mutations have lower DNL and lower expression of SREBP-1c [269]; however, they have increased levels of hepatic TG and decreased secretion of VLDL [270]. This genetic mutation is more common in the Latino population than in any other ethnicity [271] and increases the risk of NASH and HCC [272]. The molecular mechanism associated with PNPLA3 mutation is still poorly understood, but it is known that HSC cells have high expression of these genes [273]. Therefore, PNPLA3 mutations may be associated with greater activation of these cells, which increases the inflammation and fibrinogenesis. In the last few years, two other genes have gained attention as genetic risks of MAFLD. They are missense variants at the TM6SF2 and GCK receptor (GCKR) loci, associated with the disease severity and progression. In particular, TM6SF2 is associated with an increased CVD risk by increasing circulating LDL-cholesterol, and GCKR mutation is associated with MODY individuals [274].
The high prevalence of childhood obesity also increased the incidence of pediatric MAFLD which, in turn, is associated with increased overall mortality compared to the general population [275,276]. Additionally, MAFLD is recognized as a risk factor for CVDs in obese adult population, but this relationship is still discussed in children [277,278,279]. Recently, MAFLD criteria were evaluated in obese children, finding that diagnosis based on more than one MAFLD criterion is more accurate in this selected population, providing better identification of individuals with higher cardiometabolic risk and prediabetes. Thus, revealing the lack of more accurate description of MAFLD criteria in the context of childhood obesity [280].
4.1. Management of MAFLD
Currently, there is no definitive pharmacotherapy to treat the MAFLD spectrum. However, due to their metabolic dysfunctions interrelated with obesity and T2D, several treatments for the latter diseases are interchangeable. As with all other metabolic diseases, effective lifestyle changes, such as healthier dietary and increased physical activity, are beneficial strategies. Overall weight loss can reduce levels of intrahepatocellular lipids that damage liver cells [281]. Moreover, daily exercise training, as well as high fiber and protein intake, combined with a shift in major calorie intake for the morning meal are beneficial in MAFLD treatment [282]. In addition, pharmacological treatment can be used as an adjunct to these lifestyle modifications as bariatric surgery in morbidly obese patients, driving to gradual weight loss over time [283].
4.2. Anti-Diabetic Drugs
IR is the central factor behind toxic fat accumulation in the liver, as well as in steatohepatitis and fibrosis progression [284]. Thus, therapies focused on IR are also efficient in MAFLD treatment. Insulin sensitizers are a group of antidiabetic medications that have been proven to be effective in MAFLD treatment. Metformin treatment demonstrated improvement in aminotransferase levels and IR in diabetic and non-diabetic patients. However, it did not affect liver histology such as steatosis, inflammation, ballooning hepatocellular injury, and fibrosis pattern [285].
Treatment with TZDs increases FFA uptake by adipose tissue, lowering fat deposition in the liver, increasing hepatic lipogenesis and insulin sensitivity. Moreover, TZDs can also upregulate adiponectin, an anti-steatogenic adipokine [286]. Pioglitazone treatment was also able to reduce inflammation and fibrosis. Nevertheless, the use of TZDs have been restricted due to its increased risk of CVD development, congestive heart failure, bladder cancer, and bone loss [283].
Another class of antidiabetic medication used to treat MAFDL are the GLP-1 analogues. Liraglutide or exenatide treatments increase pancreatic insulin release and stimulate β-cell growth [287]. Liraglutide treatment demonstrates promising results in delaying fibrosis progression [288]. DPP-4 inhibitors, molecules that target GLP-1 receptors, are also used in MAFLD treatment [289].
SGLT2 inhibitors, such as canagliflozin, dapagliflozin, and empagliflozin, are glucose-lowering agents that have cardiovascular and renal protective action. T2D patients who also have MAFLD treated with SGLT2 inhibitors presented reduced liver fat content and achieved better biological markers of MAFLD, such as serum liver enzymes [290].
Obeticholic acid (OCA) is a synthetic bile acid with improved affinity for the farnesoid X receptor (FXR) [291]. Activation of FXR results in improved glucose metabolism and insulin sensitivity [292], reduced lipogenesis, and enhanced β-oxidation [293]. Bile acids also presented anti-inflammatory [294] and antifibrotic action [295].
4.3. Antilipidemic Agents
Dyslipidemia drugs are another approach to MAFLD treatment. Statins have been demonstrated to decrease hepatic FFA, steatosis, hepatic fibrosis, and the expression of inflammatory markers TNF-α and IL-6 [296,297]. PUFA use was able to improve overall symptoms and decrease TG and alanine transaminase (ALT) levels [298]. Fenofibrate and niacin were also promising in MAFLD treatment, but their use is not recommended due to possible hepatotoxicity and increased mortality risk [299].
Ezetimibe is a dyslipidemic medicament that acts by inhibiting cholesterol absorption. In studies with MAFLD, it showed promising results, as in histological observations, it was able to improve NASH and steatosis profiles. It also improves MAFLD biomarkers, such as aminotransferase, alanine aminotransferase, gamma-glutamyl transpeptidase, and LDL-cholesterol levels [300,301].
The stearoyl-CoA desaturase (SCD) inhibitor, Aramchol, initially used in gallstone treatment, has been shown to improve hepatic lipid accumulation in animal experiments, as well as in humans [302,303].
Acetyl-CoA carboxylase (ACC) inhibitors act by reducing DNL and inducing FA oxidation, thus decreasing hepatic FA content [304]. GS0976 is an ACC inhibitor that is under investigation in MAFLD treatment, which has shown improvements in hepatic lipid content, and biomarkers of fibrosis and apoptosis after 12 weeks of treatment (NCT02856555) [305,306].
4.4. Antioxidant Agents
Vitamin E is an antioxidant molecule used in NASH treatment to counteract the oxidative stress that leads to hepatocellular injury and disease progression [307]. Studies have shown that vitamin E has no impact on hepatic fibrosis. However, it was able to decrease aminotransferases levels, improve inflammation, steatosis, ballooning, and steatohepatitis in NASH subjects [308,309]. Still, vitamin E treatment is controversial due to its association with all-cause mortality [310], and increased risk of prostate cancer [311].
N-acetylcysteine (NAC) is another antioxidant agent that acts by increasing glutathione in hepatocytes. This mechanism reduces the amount of reactive oxygen species, thus limiting hepatocellular injury progression [312]. Betaine are nutritional antioxidants, also used in fatty liver treatment due to its anti-inflammatory, cytoprotective, antiapoptotic, and anti-steatogenic action, thus increasing insulin sensitivity [313].
4.5. Others
Elafibranor is a PPAR-α/δ agonist that has shown promising results in NASH treatment, with anti-inflammatory and antifibrotic effects, improving insulin sensitivity and liver function [314,315]. Another study demonstrates that elafibranor was able to resolve NASH without effects on fibrosis [316].
Pentoxifylline is a methylxanthine by-product that has been demonstrated to improve ALT levels, as well as steatosis, inflammation, and fibrosis inhibition TNF-α [317].
Probiotic therapy is an alternative to treat dysbiosis changes that are observed in MAFLD. Studies indicate that gut microbiome improvement by probiotics may ameliorate hepatic histology, inflammation, and biochemical markers [318,319]. Gut microbiome is also a target of IMM-124E, a hyperimmune bovine colostrum, which acts by reducing liver exposure to LPS and gut bacterial byproducts. This increases GLP-1, adiponectin, and regulatory T-cells, thus improving glycemic control [320].
Table 3 shows the different compounds under study used for the MAFLD treatment.
Table 3.
Pharmacological treatments for metabolic associated fatty liver disease. The compounds are divided into class, mechanism of action, drugs examples, current state of application, and its side effects.
| Class | Mecanism of Action | Drugs | Current State | Side Effects | Reference |
|---|---|---|---|---|---|
| Biguanides | Reduce aminotransferase levels Increase insulin sensitivity |
Metformin * | Under studies | Abdominal discomfort Diarrhea Nausea |
[285] |
| PPARy agonist | Increase FFA uptake, hepatic lipogenesis and insulin sensitivity | Rosiglitazone * Pioglitazone * |
Under studies | Increase risk of CVD development, congestive heart failure, bladder cancer and bone loss |
[283,286] |
| GLP-1 receptor agonists | Delay fibrosis progression | Liraglutide * Exenatide * |
Under studies | Transient nausea Vomiting |
[287,288,289] |
| SGLT2 inhibitors | Reduce fatty liver content Improve levels of serum liver enzymes |
Canagliflozin * Dapagliflozin * Empagliflozin * |
Under studies | Urinary tract and genital infections Decrease in blood pressure Weight gain |
[290] |
| FXR receptor agonist | Regulate hepatic metabolism of bile and cholesterol | Obeticholic acid | Under studies | Pruritus Dyslipidemia Fatigue Headache Gastrointestinal side effect |
[291,292,293,294,295] |
| Statins | Decrease hepatic FFA, steatosis, hepatic fibrosis and inflammatory markers | Atorvastatin Fluvastatin Lovastatin Pitavastatin Pravastatin Rosuvastatin |
Under studies | Muscle pain (myalgia) Creatine phosphokinase elevation |
[296,297] |
| Polyunsaturated fatty acids | Improve overall symptoms Decrease TG and alanine transaminase levels |
Eicosapentaenoic acid Docosahexaenoic acid |
Under studies | Unknown | [298] |
| Lipid lowering | Inhibit cholesterol and phytosterol absorption | Ezetimibe | Under studies | Hepatotoxicity Severe cholestatic hepatitis Acute autoimmune hepatitis |
[300,301] |
| Stearoyl-CoA desaturase inhibitors | Improve hepatic lipid accumulation | Aramchol | Under studies | Unknown | [302,303] |
| Acetyl-CoA carboxylase inhibitors | Reduce DNL and FA content | GS0976 | Under studies | Unknown | [305,306] |
| Antioxidants | Decrease aminotransferases levels Improve inflammation, steatosis, ballooning and steatohepatitis |
Vitamin E | Under studies | Increase blood pressure and heart failure risk | [307,308,309,310,311] |
| Increase glutathione in hepatocytes | N-acetylcysteine | Under studies | Nausea Vomiting Diarrhea Transient skin rash Flushing Epigastric pain Constipation |
[312] | |
| Induce anti-inflammatory, cytoprotective, antiapoptotic and anti-steatogenic actions | Betaine | Under studies | Gastrointestinal side effects | [313] | |
| PPAR-α/δ agonists | Induce anti-inflammatory and antifibrotic effects Improve insulin sensitivity and hepatic function |
Elafibranor | Under studies | Congestive heart failure Peripheral edema Bone fractures Weight gain |
[314,315,316] |
| Xanthine derivatives | Improve ALT levels, steatosis, inflammation and fibrosis | Pentoxifylline | Under studies | Unknown | [317] |
| Probiotic therapy | Improve hepatic histology, inflammation and biochemical markers | - | Under studies | Unknown | [318,319] |
| Hyperimmune bovine colostrum | Improve glycemic control Reduce liver exposure to LPS and gut bacterial bioproducts |
IMM-124E | Under studies | Unknown | [320] |
* Similar compounds for more than one NCD.
4.6. COVID-19 and MAFLD
Underlying liver diseases such as MAFLD [321,322] may also increase the risk of hospitalization and severity of COVID-19 [323]. Recent publications suggest that hepatic pro-inflammatory profile and increased ROS, characteristic of fatty liver patients, may worsen COVID-19 infection by intensifying the virus-induced inflammation [324,325]. Moreover, patients with liver disease had longer viral shedding time and increased rates of liver failure [326].
Liver fibrosis is another risk factor for COVID-19 severity. One study correlated the fibrosis-4 (FIB-4) index and the NAFLD fibrosis score (NFS) with COVID-19 severity [327]. Accordingly, intermediate and high FIB-4 and NFS scores were correlated with a higher risk of severe COVID-19. Additionally, patients with FIB-4 rate greater than 2.67 had higher risk of developing severe COVID-19, even in the absence of metabolic comorbidities. This state induces the release of hepatic pro-inflammatory cytokines that may also contribute to the exacerbation of virus-induced cytokine “storm” during the immune response to infection [327]. In this state, overproduction of proinflammatory cytokines disturbs coagulation pathways, creating imbalanced procoagulant and anticoagulant rates, thereby increasing the predisposition to microthrombosis, disseminated intravascular coagulation and multiple organ failure [328,329].
5. Cardiovascular Diseases (CVDs)
CVDs are still the main cause of mortality and morbidity worldwide [330], which is increasing globally [331], along with cardiovascular risk factors, such as obesity [332], T2D [333] and metabolic syndrome (MetS) [334]. The underlying cause of almost all CVDs, such as coronary vascular disease, cerebrovascular disease, venous thromboembolism, and peripheral vascular disease, is commonly preclinical atherosclerosis, which ultimately leads to myocardial infarction, cardiac arrhythmia, and stroke [330]. Although CVDs studies have been traditionally focused on the clinical aspects of the disease, numerous studies have shifted their focus on the metabolic basis of such conditions [331].
Cardiovascular function is heavily dependent on ATP availability, consequently demanding a constant supply of nutrients, i.e., fats, glucose, lactate, and ketones, to be used as fuel by the myofibrils. Cardiac cells use a wide range of metabolic pathways to obtain ATP, which include glycolysis, β-oxidation, tricarboxylic acid cycle or Krebs cycle, and oxidative phosphorylation [335]. Still, cardiovascular function is altered by MetS-associated metabolic alterations.
These MetS-associated cardiovascular risk factors, especially obesity, IR, and atherogenic dyslipidemia, lead to a myriad of vascular and cardiac diseases [336], including coronary atherosclerosis and calcification [337], cardiac dysfunction, myocardial infarction, and heart failure [338]. However, it is not completely understood how these risk factors contribute to the development of such a spectrum of cardiovascular conditions.
Evidence associates obesity and IR with an increased risk of CVDs [339]. Thus, obesity is especially related to two cardiovascular conditions: heart failure (also known as obesity cardiomyopathy) and cardiac atherosclerosis [340]. The major physical consequence of obesity is developing atherosclerotic CVDs, which increases the risk through risk factors brought by obesity, such as hypercholesterolemia, hypertension, hyperglycemia, atherogenic dyslipidemia, IR, proinflammatory state, and prothrombotic state [341]. However, obesity leads to alterations in the hemodynamic phenotype such as increased left ventricular mass [342]. In addition to that, although not fully understood, underlying molecular mechanisms, such as myocardial Ca2+ handling, are also deregulated, which is caused by changes in the expression of SERCA2A and ryanodine receptors, responsible for calcium transportation and, ultimately, leading to myocellular dysfunction in obesity and MetS [343].
A consequence of obesity to the cardiovascular system is high level of circulating FFA that results from enhanced glucose use and decreased FA oxidation. Thus, the heart adapts to this unbalanced metabolic profile by favoring increased FA regarding other metabolic fuels. This change in cardiac substrate availability, resulting in loss of myocardium fuel flexibility, has been associated with impaired cardiac function [344]. Further, the high levels of FA impair β-cell function and contribute to IR, this latter being directly linked to hypertriglyceridemia and considered a driver to CVDs [336]. Furthermore, evidence also suggests overexpression of FA transporters in Zucker rats [345].
As already mentioned, IR is another threat factor linked to cardiac dysfunction, increasing the risk of heart failure and atherosclerosis, especially in diabetic individuals [346]. A meta-analysis study with 516,000 participants argues that IR is the single most important cause of coronary artery disease and its prevention would avoid about 42% of myocardial infarctions [347]. Under IR condition, the switch between FA oxidation and glycolysis becomes impaired, which makes FAs the only source of energy in the heart. Consequently, the heart increases lipid uptake and accumulation and, ultimately, inflicts lipotoxicity [348]. As the switch between substrates depends on their availability through CD36 (for FA) and GLUT4 (for glucose) transporters, regulation of FA and glucose uptake and modulation of these transporters are possible therapeutic targets [349].
Evidence correlates IR with several cardiovascular events, namely, hypertension, fatal and nonfatal myocardial infarction, and sudden death [350,351], mainly through the development of dyslipidemia [346]. Such abnormal lipoprotein profiles have been associated with vascular inflammation and endothelial dysfunction [352], resulting in several CVDs, especially atherosclerosis [352,353]. Although not fully understood, evidence suggests that small dense LDL (sdLDL) and TG-rich lipoproteins (remnant lipoproteins) are atherogenic. HDL, on the other hand, is antiatherogenic, being characterized by enhanced reverse cholesterol transport, anti-inflammatory properties, protective capacity against LDL modification, among others [341]. Additionally, hypertriglyceridemia has been shown to increase the incidence of CVDs by about 76% in women and about 32% in men [354]. Moreover, the major drivers of dyslipidemia-induced CVDs are related to alterations in lipoprotein metabolism and increased release of FFA, causing lipotoxicity on endothelial cells [348].
Another well-known metabolic dysfunction related to CVDs is mitochondrial dysfunction, which includes structural changes in mitochondria [355], usually through formation of a giant mitochondria by enlargement of the mitochondria or the fusion of adjacent organelles by the action of fusion proteins, i.e., mitofusins 1/2 [356]. Other alterations in mitochondria include changes in the cristae and intramitochondrial creatine kinase crystals [357]. These abnormalities play a major role in various metabolic aberrations, i.e., enhanced oxidative stress, lower ATP production and energy supply, increased cell apoptosis, dysregulation of autophagy and ER stress, that ultimately contributes to CVDs pathogenesis [355]. Accordingly, restoring these cellular perturbations may be a significant therapeutic target that goes beyond the energetic impairment [355,358].
Therefore, each covered risk factor contributes through its own alterations in cardiovascular metabolism, which may overlap. Hence, taken together, it has been shown that CVDs have a complex etiology, in which multiple risk factors and pathological mechanisms contribute to their development.
5.1. Management of CVDs
5.1.1. Primary and Secondary Prevention Strategies
Prevention strategies for CVDs are required globally to reduce the risk of major cardiovascular events and, ultimately, related deaths [359,360]. There is consistent evidence to support adequate risk reduction strategies in individuals who have not developed any CVD (primary prevention) and in individuals with established diseases (secondary prevention) [359]. Thus, the modification risk factors related to pathogenesis of CVDs and implementation of therapeutic strategies have been able to mitigate major risk of cardiovascular events [360]. Based on this, prevention guidelines include several recommendations, e.g., healthy eating, regular physical activity, avoiding of tobacco and alcohol, and achieving a healthy weight. Further, drug-based prevention strategies are based on controlling blood pressure, cholesterol, hypercholesterolemia, and platelet aggregation to prevent major cardiovascular events [359,360,361].
Since T2D increases the risk of CVDs, glycemic control is an important target to prevent coronary-related diseases. Since then, sulphonylureas, metformin, and insulin have been used to control glycemia [362]. Still, SGLT2 inhibitor drugs, e.g., empagliflozin, have been administered to reduce the risk and mortality of CVDs during T2D treatment [213]. Moreover, recent studies have introduced new glucose-lowering drugs, such as semaglutide, liraglutide, and empagliflozin, which can decrease CVD incidence [213,363,364].
Blood pressure-lowering drugs are another prevention strategy, effective in preventing strokes, coronary heart disease, and heart failure [365,366]. Some antihypertensive agents, such as β-blockers, ACE inhibitors, angiotensin receptor blockers, calcium channel blockers and diuretics, prevent the aforementioned CVDs, affecting metabolism, inflammation and oxidative states [16]. Furthermore, statins have also been used to prevent stroke, coronary-related diseases, and sudden cardiac death by decreasing levels of cholesterol and lipoprotein in the blood. However, more recently, ezetimibe and antibodies that inhibit proprotein convertase subtilisin-kexin type 9 (PCSK9) have been employed to reduce LDL levels [367,368]. Nevertheless, acetylsalicylic acid (ASA) is recommended for all patients with atherosclerosis, patients after a stroke or atrial fibrillation, and patients with acute coronary syndrome (ACS). Still, patients with ACS were medicated with P2Y12 inhibitors, i.e., clopidogrel, ticagrelor, and prasugrel, along with ASA, to inhibit thromboxane A2 (TXA2) [369,370]. In addition to those, anticoagulants and vitamin K agonists have been shown to be effective against ACS recurrence [360]. Furthermore, combinations of hypertensive agents (lisinopril and atenolol or hydrochlorothiazide), ASA, and statin improved blood pressure and cholesterol concentrations [371].
5.1.2. Potential Therapeutic Targets
Recently, new therapeutic strategies have been investigated to reduce and mitigate CVD events, such as agents to modulate glucose and lipid metabolisms, mitochondrial targets, RNA-based therapies, and the endocannabinoid system.
Glucose Metabolism
Potential therapies to modulate the glucose metabolism in patients with CVDs risk have been investigated, with GLP-1 receptor agonists, DPP-4 inhibitors, SGLT2 inhibitors, and AMPK activators.
GLP-1 is a peptide hormone that mainly stimulates insulin secretion in β-cells, and its receptor is endogenously expressed in myocardial tissue and vascular endothelium. Evidence has demonstrated the protective role of GLP-1 receptor agonists in the cardiovascular system, decreasing abdominal visceral fat and systolic blood pressure, and improving endothelial and myocardial function [372], which ultimately reduces non-fatal stroke and the incidence of stroke and myocardial infarction [363,373]. However, GLP-1 is immediately subject to rapid degradation by DPP-4 [372] and, therefore, administration of DPP-4 inhibitors may reduce risk of major CVDs events [374,375]. In addition to those, SGLT2 plays an important role in glucose reabsorption in the kidney, and its inhibition decreases the blood glucose concentration, improving insulin sensitivity, reducing glucose toxicity and blood pressure, and inducing nephroprotection [376]. A study has shown that empagliflozin, an SGLT2 inhibitor, together with lipid-lowering therapy and antihypertensive medications reduce the incidence of risk factors, hospitalization, and death [213].
AMPK, considered the master metabolic regulator, has also been investigated as a therapeutic target for obesity, diabetes, and CVDs. Activation of AMPK directly phosphorylates several downstream targets and effectors, which are related to lipid metabolism (FA oxidation and DNL), glucose metabolism (glycolysis and glucose uptake), and mitochondrial integrity [377,378]. Furthermore, evidence suggests that AMPK activation yields cardioprotection, e.g., protects against hypertrophic cardiomyocyte growth and cardiac ischemia reperfusion injury [379,380]. As a result, many therapeutic agents currently used to treat diabetes can activate AMPK, including metformin and TZDs. The former has been shown to normalize the endothelial response via improved AMPK-induced nitric oxide (NO) production in animal models [381,382] and further analysis has demonstrated that metformin-induced AMPK activation suppresses 26S mediated GTP-cyclohydrolase degradation, which is the main mediator of endothelial dysfunction [383]. For the latter, there is evidence to support its cardioprotective properties, e.g., reducing ischemia, reperfusion, and myocardial infarction in mouse models [384].
Lipid metabolism
Elevated plasma lipoprotein(a) (Lp(a)) concentration has been associated with increased risk of CVDs [385]. Well-known agents, such as inhibitors of proprotein convertase subtilisin/kexin type 9 (PCSK9), nicotinic acid (niacin), statins and ASA, and novel molecules, such as antisense oligonucleotides (ASOs) and inhibitors of lipoprotein lipase (LPL) and its receptors, modulate Lp(a) levels. However, in clinical practice, no medication directly lowers Lp(a) levels, so the primary goal with patients with elevated Lp(a) levels is to reduce LDL-C levels [386].
Niacin administration studies have suggested that it reduces Lp(a), LDL-C, apolipoprotein (apo) B-100, sdLDL, and TG levels and raises HDL levels; however, clinical trials have reported that the role of niacin in lowering CVDs risk is questionable [387,388,389]. Furthermore, evidence indicates that ASA decreases serum Lp(a) concentrations, possibly achieved by reducing apo(a) gene transcription, which causes a reduction in LPA gene transcription [390]. Recently, PCSK9 inhibitors, e.g., evolocumab and alirocumab, have been combined with statins to reduce LDL-C, but this new class of medication has not yet been approved for the treatment of elevated Lp(a) levels [386,391]. However, the PCSK9-induced mechanism that lowers Lp(a) levels is unclear; hence, it has been hypothesized that a reduction in LDL-C and LDL-R may be involved in the lowering of Lp(a) levels [391].
Novel therapies have been evaluated to modulate Lp(a) synthesis. ASOs targeting apo(a) have been demonstrated to inhibit apo(a) synthesis and, consequently, Lp(a) secretion [392,393]. However, administration of an apo(a)-specific ASO, IONIS-APO(a)LRx, has been indicated to reduce plasma LDL-C levels and monocytes inflammatory effects, and, ultimately, Lp(a) levels [394]. Angiopoietin-like proteins (ANGPTLs), LPL inhibitors that hydrolyze circulating TG to FFA, have also been evaluated [395]. ANGPTL3 suppresses LPL activity, which reduces plasma levels of TG and LDL-C, while ANGPTL4 reduces plasma TG and increases HDL-C levels [395,396,397]. Both also play a role in glucose homeostasis [398,399,400]. Dewey et al. [401] showed that ASOs and monoclonal antibody-based inactivation of ANGPTL3 reduce plasma TG and LDL-C levels. In addition, a study demonstrated that metformin inhibits ANGPTL3 expression in the liver, modulating LPL activity and lowering plasma lipids [402]. Furthermore, LRP6 impairment exhibits elevated LDL, TG and fasting glucose levels, which also deregulate Wnt/β-catenin signaling and lipoprotein endocytosis [403]. With that, targeting LRP6 with small molecules, such as GNF-6231 inhibits the canonical and non-canonical effects of the Wnt ligand, slowing the progression of myocardial fibrosis and inflammation [404]. Another LRP6 antagonist is the Dickkof-related protein 1 (DKK1), which is an endogenous inhibitor that regulates blood pressure [405].
Mitochondrial Therapies
Mitochondrial dysfunctions play an important role in CVDs pathogenesis [355]; however, there are no medications to modulate mitochondrial functions available in clinical practice [406]. However, therapeutic agents have been investigated to target mitochondrial, including the mitochondria-targeted antioxidant MitoQ1, that decreases ROS production and has shown protective effect in hypertensive rat models [407], and carvedilol or antidiabetic drugs that prevent cardiac mitochondrial oxidative damage [408]. Therapies targeting endothelial NO synthase (eNOS) activity and function, using statins, ACE inhibitors, and AT1-receptor blockers, have been shown to improve cardiovascular prognosis by decreasing the level of oxidative stress, inflammation, and mediators of vascular dysfunctions [409,410]. In addition, third-generation β-blockers stimulate endothelial NO formation and improve oxidative stress in animal models [411,412].
5.1.3. Emerging Strategies
RNA-Based Therapies
Over the past decades, RNA-based therapies have emerged as an important strategy for the diagnosis and treatment of many diseases [413]. However, insights into the role of non-coding RNAs (ncRNAs) in health and disease have characterized them as valuable therapeutic targets [414]. Although ncRNAs do not encode a protein, ncRNAs play an important role in several pathways through post-transcriptional regulation of gene expression [413]; thus, affecting different biological processes, such as cell survival, differentiation, and proliferation [415]. Due to their broad influence on biological processes, the role of ncRNAs, particularly microRNAs (miRNAs), have been explored in CVDs development [415,416,417,418], as well as their potential therapeutic applications [413,419,420,421].
Several studies have explored miRNA-based treatment in CVDs, such as myocardial infarction, cardiac fibrosis, and atherosclerosis. Upregulation of miR-146a in a myocardial ischemia/reperfusion injury in mice showed a 55% reduction in myocardial infarct size and an improvement in cardiac function after myocardial infarction [422]. Likewise, overexpression of miR-99a in C57/BL6 mice subjected to myocardial infarction attenuated cardiac remodeling by preventing cardiomyocyte apoptosis and promoting autophagy, cardiac function gain and increasing survival ratio [423]. Conversely, downregulation of miR-433 in mice ameliorates cardiac fibrosis and ventricular dysfunction after myocardial infarction [424]. Similarly, the administration of miRNA mimetics is also explored for CVDs treatment, which act on mRNA degradation and translation inhibition [425]. Thereby, systemic administration of a miR-100 mimic in an LDLR-deficient atherosclerotic mouse model decreased 55% of the plaque area, attenuating atherosclerosis [426]. In addition, intracardiac administration of miR-199a-3p and miR-590-3p mimetics immediately after myocardial infarction in mice led to cardiac repair, reducing infarct size and preserving cardiac function [427].
Since the discovery of miRNA in Caenorhabditis elegans, a deeper understanding of miRNAs functionality is still needed to translate it into clinical practice. With that, many ncRNAs have not yet been characterized, leaving a broad horizon of potential targets for the development of RNA-based treatments for CVDs with improved efficacy. Therefore, RNA-based therapies are a promising field of research for the treatment of several diseases, including CVDs, but their application remains a challenge for the scientific community.
Endocannabinoids
There is mounting evidence that the endocannabinoid system (ECS) influences the regulation of CVDs risk factors, such as hypertension and atherosclerosis [428]. Studies have explored ECS modulation, such as through endocannabinoids (e.g., anandamide (AEA), 2-arachidonoylglycerol), cannabinoid receptors antagonists (e.g., AM251), synthetic cannabinoids (e.g., WIN55212-2), or even related pathways (e.g., FA amide hydrolase inhibitors), to promote hypotensive effects in different types of hypertension, e.g., spontaneous, acute, and salt-induced hypertensions [429,430,431,432,433,434]. This evidence suggests that ECS-mediated hypotensive effects depend on the endocannabinoid or receptor involved and the type of hypertension being treated. However, further investigations are still required to elucidate the mechanisms behind the hypotensive effect and what are the appropriate therapeutic targets to be explored in clinical practice.
NO is an important cardiovascular signaling molecule [435]. In addition to playing a significant role in cardiovascular homeostasis [435], atherosclerosis [436], and renal damage [437], NO is also deeply involved in endocannabinoid-induced cardiovascular effects. AEA treatment promoted a notable relaxation of the thoracic aortas dependent on CB1 and CB2 activations [438]; however, when eNOS inhibitor, L-NAME, was administered, no AEA-evoked relaxation was observed, indicating that the AEA-induced vasodilation effect is also NO-dependent. Similarly, AEA-induced vasorelaxation was also reversed by L-NAME treatment in human mesenteric [439] and pulmonary [440] arteries, highlighting the role of NO in ECS-mediated vasodilation. Together, this evidence supports a central role for NO in cardiovascular homeostasis, as well as highlighting the NO contribution to the beneficial vascular effects induced by ECS.
Despite investigation of ECS strategies in hypertension treatment, the ECS have been shown to also play a role in CVDs development, especially by endothelial damage [441]. Endothelial CB1 signaling has been associated with proatherosclerotic effects by increasing oxidative stress and promoting immune cell recruitment into the arterial wall in atherosclerotic mice, apoE (−/−) [442]. Interestingly, a different study, also conducted with apoE (−/−) mice, presented that CB2 signaling promotes anti-inflammatory and anti-atherosclerotic effects [443]. Similar studies have shown that CB1 receptor activation in human primary coronary artery endothelial cells promoted cell-death and increased ROS levels [444]. However, CB1 activation promotes antioxidant effects in the digestive system [445] and CNS [446], highlighting that CB1 and CB2 receptors may exert different functions depending on their location.
Therefore, ECS is an exciting target for novel therapeutic strategies in CVD, especially those associated with hypertension and atherosclerosis. The broad distribution of the ECS throughout the organism makes it a versatile tool for targeting different diseases, given its wide range of modulation possibilities, such as receptor agonists and antagonists, metabolites, and enzymatic inhibitors and activators. However, CB1 and CB2 receptors can evoke opposite effects depending on their localization, making pharmacological vectorization strategies, such as nanotechnology, extremely important for accurate drug delivery.
The different compounds under study used to the CVDs treatment are shown as a summary in Table 4.
Table 4.
Pharmacological treatments for cardiovascular diseases. The compounds are divided into class, mechanism of action, drugs examples, current state of application, and its side effects.
| Class | Mecanism of Action | Drugs | Current State | Side Effects | Reference |
|---|---|---|---|---|---|
| GLP-1 receptor agonists | Reduce abdominal visceral fat and systolic blood pressure Improve endothelial and myocardial function |
Liraglutide * Exenatide * |
Under studies | Transient nausea Vomiting |
[363,372,373] |
| DPP4 inhibitors | Degrade GLP-1 | Sitagliptin * Vildagliptin * Saxagliptin * Linagliptin * Alogliptin * |
Under studies | Hypoglicemia Loss of consciousness Gastrointestinal side effects |
[374,375] |
| SGLT2 inhibitors | Promote glucose reabsorption Decrease blood glucose concentrationImprove insulin sensitivity Reduce glucose toxicity and blood pressure Induce nephroprotection |
Empagliflozin * | Under studies | Urinary tract and genital infections Decrease in blood pressure Weight gain |
[213,376] |
| AMPK activators | Induce NO production Suppress 26S mediated GTP-cyclohydrolase degradation |
Metformin * | Under studies | Abdominal discomfort Diarrhea Nausea |
[381,382,383] |
| Reduce isquemia, reperfusion and myocardial infarction | TZDs | Under studies | Increase risk of CVD development, congestive heart failure, bladder cancer and bone loss |
[384] | |
| Lp(a)and LPL-C modulators | Reduce Lp(a), LDL-c, apo B-100, sdLDL and TG levels Raise HDL levels |
Niacin | Under studies | Hepatic toxicity Myopathy Blurred vision Nausea Vomiting |
[387,388,389] |
| Decrease Lp(a) levels | ASA | Under studies | GI upset Nausea |
[390] | |
| Reduce LDL-c levels | Evolocumab Alirocumab |
Under studies | Nasopharyngitis Injection site pain Arthralgia Back pain |
[386,391] | |
| Inhibit apo(a) synthesis and LP(a) secretion | ASOs | Under studies | Unknown | [392,393] | |
| LPL inhibitors | Suppress LPL activityReduce TG level s Increase HDL-C levels |
ANGPTLs | Under studies | Unknown | [395,396,397,398,399,400,401,402] |
| Mitochondrial therapies | Decrease ROS production | MitoQ1 | Under studies | Unknown | [407] |
| Reduce oxidative stress and inflammation | Statins ACE inhibitors AT-1 receptor blocker |
Under studies | Unknown | [409,410,411,412] | |
| RNA-based therapies | Reduce myocardial infarct size Improve cardiac function |
miR-146a | Under studies | Unknown | [422] |
| Prevent cardiomyocyte apoptosis Promote autophagy |
miR-99a | Under studies | Unknown | [423] | |
| Ameliorate cardiac fibrosis and ventricular dysfunction | miR-433 | Under studies | Unknown | [424] | |
| Attenuate atherosclerosis | miR-100 | Under studies | Unknown | [426] | |
| Promote cardiac repair Reduce infarct size Preserve cardiac function |
miR-199a-3p miR-590-3p |
Under studies | Unknown | [427] | |
| Endocannabinoids | Induce hypotensive and vascular effects | Anandamide 2-arachidonoylglycerol |
Under studies | Neuropsychiatric side effects | [429,430,431,432,433,434] |
* Similar compounds for more than one NCD.
5.2. COVID-19 and CVDs
ACE2 is located in the cell membrane or circulating in the bloodstream. This enzyme is responsible for transforming angiotensin I into II, which is a potent vasoconstrictor agent. Thus, ACE2 is a blood pressure modulator. The SARS-CoV-2 entry into the host cell through the binding of the viral S protein to ACE2. Therefore, the interaction between S protein and ACE2 has been considered a promising therapeutic target [447]. CVDs and their risk factors, such as hypertension, were common pre-existing conditions in COVID-19 patients, with a prevalence of 15% of hypertension and 15% for other CVDs [448].
The use of ACEi and angiotensin-receptor blocker (ARB) therapy is a standard practice in hypertension treatment as we have discussed in the management of CVDs section. Recently, it has been questioned whether the use of antihypertensive medications would have a favorable or deleterious impact on people infected with SARS-CoV-2, since they can modulate ACE2 expression. Thus, possibly turning the users of ACEis and ARBs into susceptible individuals to increased entry and propagation of the viral host cells [449]. On the other hand, the treatment of COVID-19 patients with ACEi or ARB is not harmful [450,451], and the ACE2 modulation may be beneficial in patients with lung injury because of its anti-inflammatory effects [449,452].
6. Conclusions
Taking all these metabolic alterations together, we observe that human metabolism is a finely tuned network of biomolecular interactions, from simple alterations, such as leptin and insulin resistance, to complex ones, such as modifications in metabolic enzymes and redox metabolism. These deregulations can lead to a wide range of diseases, including hormonal disorders, obesity, diabetes, MAFLD, CVDs, and cancer. With each one of these diseases having its own characteristics and complications, but of common origin that consists of an imbalance in the human metabolism (Figure 1). Each metabolism-related disease has its specific target tissues, e.g., liver cells in MAFLD, adipose tissues in obesity and diabetes, and a set of disruptions in the finely tuned metabolic network that characterizes it. However, all these alterations mainly came from the 21th century way of life, with high calorie diet, with sugary and fatty foods, and low physical activity. These factors are directly responsible for the increase in such diseases in recent decades; however, we must not ignore the genetic and epigenetic contribution to the development and progression of such diseases.
Figure 1.
Hallmarks of non-communicable diseases. Scheme with the main characteristics and complications of the NCDs represented in 5-way Veen diagram and their relationship with COVID-19. Obesity is shown in the brown, metabolic associated fatty liver disease—MAFLD—in the blue, cardiovascular diseases—CVDs—in the pink, and diabetes mellitus in the yellow. The relationship between the diseases are shown in the intersections.
Treatments for these NCDs should integrate lifestyle changes and pharmacological or surgical approaches, when necessary, to successfully cure them or at least mitigate their burdens. Comprehensive lifestyle interventions, such as adequate physical training, psychological assistance, and dietary re-education, are the core step to combat the metabolic conditions covered in this review. The major barriers to successful lifestyle changes are long-term adherence to the proposed interventions and complete reeducation of patients’ lifestyle towards a more conscious one. Hence, in some situations, individuals fail to achieve the required weight loss or have compromised health; thus, adjunctive treatments, i.e., pharmacological or surgical interventions, may be required. Interestingly, pharmacological treatments tend to share targets and medications among NCDs, mainly due to shared or complementary metabolic changes. Thus, we showed some medications that are employed to treat more than one NCD, such as metformin, liraglutide, empagliflozin, and even treat more than one simultaneously. Therefore, treatments should be designed in an individualized manner to address the patient’s condition.
With this silent pandemic of NCDs that we are currently facing globally, its burdens are pronounced with the COVID-19. The clearest relationship between both pandemics are the increased risk of hospitalization, severity, and mortality, sharing molecular mechanisms, mainly related to inflammation and cytokine storm. However, further investigation is still required by the scientific community to fully understand the underlying relationship between these pandemics.
Therefore, reducing the burden of such metabolism-related diseases demands multidisciplinary approaches, which combine individual interventions with environmental and social changes. A better comprehension of notable regional specificities that contribute to the prevalence and trends of such diseases can help identify environmental and social causes and provide guidance on developing intervention strategies. In general, our comprehension of metabolism has become increasingly thorough in the past few decades. With these advances, our knowledge of underlying the mechanisms, progression and prognosis of diseases related to metabolic alterations are also deepening. Despite advances in recent years, more extensive research is still required to further improve diagnosis, therapy, and minimize the chance of chronic complications development. Additionally, accessing and reducing cost for high quality and powered genetic techniques will provide a wealth of information and opportunities for enhanced targeted treatment. Therefore, continuous investment in this field of research is essential to effectively target and mitigate the global pandemic of metabolic diseases-related we face.
Acknowledgments
We thank the Brazilian Biosciences National Laboratory (LNBio), part of the Brazilian Center for Research in Energy and Materials (CNPEM). Figures were created with BioRender.com (accessed on 5 August 2021).
Author Contributions
J.V.S.G. and M.M.G.D. helped to outline and figure design and wrote the paper. A.J.V.C.B. wrote the paper. M.F.T. designed figures and tables. A.C.M.F. conceptualized, proposed the outline and edited the paper and figures. M.G.-A. proposed the outline, conceptualized figures and wrote and edited the paper. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by the “Fundação de Amparo à Pesquisa do Estado de São Paulo” (FAPESP) [grant number 19/14465-1; 19/10274-7]; “Conselho Nacional de Desenvolvimento Científico e Tecnológico” (CNPq) [grant number 304514/2018-7]; “Coordenação de Aperfeiçoamento de Pessoal de Nível Superior” (CAPES) [#8887.373113/2019-00], CNPEM and Graduate Program in Functional and Molecular Biology (IB-Unicamp).
Informed Consent Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.World Health Organization. [(accessed on 10 October 2020)]; Available online: www.who.int/news-room/fact-sheets/
- 2.World Health Organization Global Health Estimates 2016, Deaths by Cause, Age, Sex, by Country and by Region, 2000–2016. [(accessed on 12 August 2020)]; Available online: https://www.who.int/healthinfo/global_burden_disease/GHE2016_Deaths_WBInc_2000_2016.xls.
- 3.Sohail M.U., Yassine H.M., Sohail A., Thani A.A. Impact of Physical Exercise on Gut Microbiome Inflammation the Pathobiology of Metabolic, Disorders. Rev. Diabet Stud. 2019;15:35–48. doi: 10.1900/RDS.2019.15.35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kim K.H., Lee M.S. Pathogenesis of Nonalcoholic Steatohepatitis and Hormone-Based Therapeutic Approaches. Front. Endocrinol. 2018;9:485. doi: 10.3389/fendo.2018.00485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.NCD Risk Factor Collaboration (NCD-RisC) Worldwide trends in body-mass index, underweight, overweight, and obesity from 1975 to 2016, a pooled analysis of 2416 population-based measurement studies in 128.9 million children, adolescents, and adults. Lancet. 2017;390:2627–2642. doi: 10.1016/S0140-6736(17)32129-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Collaboration NCDRF: Worldwide trends in diabetes since 1980, a pooled analysis of 751 population-based studies with 4.4 million participants. Lancet. 2016;387:1513–1530. doi: 10.1016/S0140-6736(16)00618-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Younossi Z., Anstee Q.M., Marietti M., Hardy T., Henry L., Eslam M., George J., Bugianesi E. Global burden of NAFLD and NASH: Trends, predictions, risk factors and prevention. Nat. Rev. Gastroenterol. Hepatol. 2018;15:11–20. doi: 10.1038/nrgastro.2017.109. [DOI] [PubMed] [Google Scholar]
- 8.Characteristics of COVID-19 Patients Dying in Italy. [(accessed on 28 January 2021)]; Available online: https://www.epicentro.iss.it/en/coronavirus/bollettino/ReportCOVID-2019_29_april_2020.pdf.
- 9.III IdSC: Informe COVID-19 nº 28. 04 de Mayo de 2020. Informe Sobre la Situación de COVID-19 en España. [(accessed on 28 January 2021)];2020 Available online: https://www.isciii.es/QueHacemos/Servicios/VigilanciaSaludPublicaRENAVE/EnfermedadesTransmisibles/Paginas/-COVID-19.-Informes-previos.aspx.
- 10.Epidemiology Working Group for NCIP Epidemic Response, Chinese Center for Disease Control and Prevention The epidemiological characteristics of an outbreak of 2019 novel coronavirus diseases (COVID-19) in China. Zhonghua Liu Xing Bing Xue Za Zhi. 2020;41:145–151. doi: 10.3760/cma.j.issn.0254-6450.2020.02.003. [DOI] [PubMed] [Google Scholar]
- 11.Richardson S., Hirsch J.S., Narasimhan M., Crawford J.M., McGinn T., Davidson K.W., the Northwell COVID-19 Research Consortium. Barnaby D.P., Becker L.B., Chelico J.D., et al. Presenting Characteristics, Comorbidities, and Outcomes Among 5700 Patients Hospitalized With COVID-19 in the New York City Area. JAMA. 2020;323:2052–2059. doi: 10.1001/jama.2020.6775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Peng Y.D., Meng K., Guan H.Q., Leng L., Zhu R.R., Wang B.Y., He M.A., Cheng L.X., Huang K., Zeng Q.T. [Clinical characteristics outcomes of 112 cardiovascular disease patients infected by 2019-nCoV] Zhonghua Xin Xue Guan Bing Za Zhi. 2020;48:450–545. doi: 10.3760/cma.j.cn112148-20200220-00105. [DOI] [PubMed] [Google Scholar]
- 13.Kluge H.H.P., Wickramasinghe K., Rippin H.L., Mendes R., Peters D.H., Kontsevaya A., Breda J. Prevention control of non-communicable diseases in the COVID-19, response. Lancet. 2020;395:1678–1680. doi: 10.1016/S0140-6736(20)31067-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kushner R.F. Weight Loss Strategies for Treatment of Obesity: Lifestyle Management and Pharmacotherapy. Prog. Cardiovasc. Dis. 2018;61:246–252. doi: 10.1016/j.pcad.2018.06.001. [DOI] [PubMed] [Google Scholar]
- 15.Nathan D.M., Buse J.B., Davidson M.B., Ferrannini E., Holman R.R., Sherwin R., Zinman B., American Diabetes Association and the European Association for the Study of Diabetes Medical management of hyperglycemia in type 2 diabetes: A consensus algorithm for the initiation and adjustment of therapy: A consensus statement of the American Diabetes Association and the European Association for the Study of Diabetes. Diabetes Care. 2009;32:193–203. doi: 10.2337/dc08-9025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Suliburska J., Skrypnik K., Szulinska M., Kupsz J., Markuszewski L., Bogdanski P. Diuretics, Ca-Antagonists, and Angiotensin-Converting Enzyme Inhibitors Affect Zinc Status in Hypertensive Patients on Monotherapy: A Randomized Trial. Nutrients. 2018;10:1284. doi: 10.3390/nu10091284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Friedman S.L., Neuschwander-Tetri B.A., Rinella M., Sanyal A.J. Mechanisms of NAFLD development and therapeutic strategies. Nat. Med. 2018;24:908–922. doi: 10.1038/s41591-018-0104-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Scotti L., Monteiro A.F.M., de Oliveira Viana J., Mendonca Junior F.J.B., Ishiki H.M., Tchouboun E.N., Santos R., Scotti M.T. Multi-Target Drugs Against Metabolic Disorders. Endocr. Metab. Immune Disord. Drug. Targets. 2019;19:402–418. doi: 10.2174/1871530319666181217123357. [DOI] [PubMed] [Google Scholar]
- 19.De Lorenzo A., Romano L., Di Renzo L., Di Lorenzo N., Cenname G., Gualtieri P. Obesity: A preventable, treatable, but relapsing disease. Nutrition. 2020;71:110615. doi: 10.1016/j.nut.2019.110615. [DOI] [PubMed] [Google Scholar]
- 20.Chooi Y.C., Ding C., Magkos F. The epidemiology of obesity. Metabolism. 2019;92:6–10. doi: 10.1016/j.metabol.2018.09.005. [DOI] [PubMed] [Google Scholar]
- 21.Hu F.B. Obesity and mortality: Watch your waist, not just your weight. Arch. Intern. Med. 2007;167:875–876. doi: 10.1001/archinte.167.9.875. [DOI] [PubMed] [Google Scholar]
- 22.Di Bonito P., Pacifico L., Licenziati M.R., Maffeis C., Morandi A., Manco M., Del Giudice E.M., Di Sessa A., Campana G., Moio N., et al. Elevated blood pressure, cardiometabolic risk and target organ damage in youth with overweight and obesity. Nutr. Metab. Cardiovasc. Dis. 2020;30:1840–1847. doi: 10.1016/j.numecd.2020.05.024. [DOI] [PubMed] [Google Scholar]
- 23.Di Bonito P., Pacifico L., Chiesa C., Valerio G., Miraglia Del Giudice E., Maffeis C., Morandi A., Invitti C., Licenziati M.R., Loche S., et al. Impaired fasting glucose and impaired glucose tolerance in children and adolescents with overweight/obesity. J. Endocrinol. Investig. 2017;40:409–416. doi: 10.1007/s40618-016-0576-8. [DOI] [PubMed] [Google Scholar]
- 24.Di Bonito P., Valerio G., Licenziati M.R., Campana G., Del Giudice E.M., Di Sessa A., Morandi A., Maffeis C., Chiesa C., Pacifico L., et al. Uric acid, impaired fasting glucose and impaired glucose tolerance in youth with overweight and obesity. Nutr. Metab. Cardiovasc. Dis. 2021;31:675–680. doi: 10.1016/j.numecd.2020.10.007. [DOI] [PubMed] [Google Scholar]
- 25.Simmonds M., Burch J., Llewellyn A., Griffiths C., Yang H., Owen C., Duffy S., Woolacott N. The use of measures of obesity in childhood for predicting obesity and the development of obesity-related diseases in adulthood: A systematic review and meta-analysis. Health Technol. Assess. 2015;19:1–336. doi: 10.3310/hta19430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Twig G., Yaniv G., Levine H., Leiba A., Goldberger N., Derazne E., Ben-Ami Shor D., Tzur D., Afek A., Shamiss A., et al. Body-Mass Index in 2.3 Million Adolescents and Cardiovascular Death in Adulthood. N. Engl. J. Med. 2016;374:2430–2440. doi: 10.1056/NEJMoa1503840. [DOI] [PubMed] [Google Scholar]
- 27.Church T.S., Thomas D.M., Tudor-Locke C., Katzmarzyk P.T., Earnest C.P., Rodarte R.Q., Martin C.K., Blair S.N., Bouchard C. Trends over 5 decades in U.S. occupation-related physical activity and their associations with obesity. PLoS ONE. 2011;6:e19657. doi: 10.1371/journal.pone.0019657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Swinburn B.A., Sacks G., Hall K.D., McPherson K., Finegood D.T., Moodie M.L., Gortmaker S.L. The global obesity pandemic: Shaped by global drivers and local environments. Lancet. 2011;378:804–814. doi: 10.1016/S0140-6736(11)60813-1. [DOI] [PubMed] [Google Scholar]
- 29.Janesick A.S., Shioda T., Blumberg B. Transgenerational inheritance of prenatal obesogen exposure. Mol. Cell Endocrinol. 2014;398:31–35. doi: 10.1016/j.mce.2014.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bergin J.E., Neale M.C., Eaves L.J., Martin N.G., Heath A.C., Maes H.H. Genetic and environmental transmission of body mass index fluctuation. Behav. Genet. 2012;42:867–874. doi: 10.1007/s10519-012-9567-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Elks C.E., den Hoed M., Zhao J.H., Sharp S.J., Wareham N.J., Loos R.J., Ong K.K. Variability in the heritability of body mass index: A systematic review and meta-regression. Front. Endocrinol. 2012;3:29. doi: 10.3389/fendo.2012.00029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Locke A.E., Kahali B., Berndt S.I., Justice A.E., Pers T.H., Day F.R., Powell C., Vedantam S., Buchkovich M.L., Yang J., et al. Genetic studies of body mass index yield new insights for obesity biology. Nature. 2015;518:197–206. doi: 10.1038/nature14177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Shungin D., Winkler T.W., Croteau-Chonka D.C., Ferreira T., Locke A.E., Magi R., Strawbridge R.J., Pers T.H., Fischer K., Justice A.E., et al. New genetic loci link adipose and insulin biology to body fat distribution. Nature. 2015;518:187–196. doi: 10.1038/nature14132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Farooqi I.S., Drop S., Clements A., Keogh J.M., Biernacka J., Lowenbein S., Challis B.G., O’Rahilly S. Heterozygosity for a POMC-null mutation and increased obesity risk in humans. Diabetes. 2006;55:2549–2553. doi: 10.2337/db06-0214. [DOI] [PubMed] [Google Scholar]
- 35.Parton L.E., Ye C.P., Coppari R., Enriori P.J., Choi B., Zhang C.Y., Xu C., Vianna C.R., Balthasar N., Lee C.E., et al. Glucose sensing by POMC neurons regulates glucose homeostasis and is impaired in obesity. Nature. 2007;449:228–232. doi: 10.1038/nature06098. [DOI] [PubMed] [Google Scholar]
- 36.Camilleri M. Peripheral mechanisms in appetite regulation. Gastroenterology. 2015;148:1219–1233. doi: 10.1053/j.gastro.2014.09.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.van der Klaauw A.A., Farooqi I.S. The hunger genes: Pathways to obesity. Cell. 2015;161:119–132. doi: 10.1016/j.cell.2015.03.008. [DOI] [PubMed] [Google Scholar]
- 38.Muller T.D., Nogueiras R., Andermann M.L., Andrews Z.B., Anker S.D., Argente J., Batterham R.L., Benoit S.C., Bowers C.Y., Broglio F., et al. Ghrelin. Mol. Metab. 2015;4:437–460. doi: 10.1016/j.molmet.2015.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Makaronidis J.M., Batterham R.L. Obesity body weight regulation the brain: Insights from, fMRI. Br. J. Radiol. 2018;91:20170910. doi: 10.1259/bjr.20170910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Manning S., Batterham R.L. The role of gut hormone peptide YY in energy and glucose homeostasis: Twelve years on. Annu. Rev. Physiol. 2014;76:585–608. doi: 10.1146/annurev-physiol-021113-170404. [DOI] [PubMed] [Google Scholar]
- 41.Bays H.E., Gonzalez-Campoy J.M., Henry R.R., Bergman D.A., Kitabchi A.E., Schorr A.B., Rodbard H.W., Adiposopathy Working G. Is adiposopathy (sick fat) an endocrine disease? Int. J. Clin. Pract. 2008;62:1474–1483. doi: 10.1111/j.1742-1241.2008.01848.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Muir L.A., Neeley C.K., Meyer K.A., Baker N.A., Brosius A.M., Washabaugh A.R., Varban O.A., Finks J.F., Zamarron B.F., Flesher C.G., et al. Adipose tissue fibrosis, hypertrophy, and hyperplasia: Correlations with diabetes in human obesity. Obesity. 2016;24:597–605. doi: 10.1002/oby.21377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hammarstedt A., Gogg S., Hedjazifar S., Nerstedt A., Smith U. Impaired Adipogenesis and Dysfunctional Adipose Tissue in Human Hypertrophic Obesity. Physiol. Rev. 2018;98:1911–1941. doi: 10.1152/physrev.00034.2017. [DOI] [PubMed] [Google Scholar]
- 44.Heiss C.N., Olofsson L.E. Gut Microbiota-Dependent Modulation of Energy Metabolism. J. Innate. Immun. 2018;10:163–171. doi: 10.1159/000481519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Sanz Y., Moya-Perez A. Microbiota, inflammation and obesity. Adv. Exp. Med. Biol. 2014;817:291–317. doi: 10.1007/978-1-4939-0897-4_14. [DOI] [PubMed] [Google Scholar]
- 46.Friedman J.M., Halaas J.L. Leptin and the regulation of body weight in mammals. Nature. 1998;395:763–770. doi: 10.1038/27376. [DOI] [PubMed] [Google Scholar]
- 47.Myers M.G., Jr Leibel R.L., Seeley R.J., Schwartz M.W. Obesity and leptin resistance: Distinguishing cause from effect. Trends. Endocrinol. Metab. 2010;21:643–651. doi: 10.1016/j.tem.2010.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Cani P.D., Amar J., Iglesias M.A., Poggi M., Knauf C., Bastelica D., Neyrinck A.M., Fava F., Tuohy K.M., Chabo C., et al. Metabolic endotoxemia initiates obesity and insulin resistance. Diabetes. 2007;56:1761–1772. doi: 10.2337/db06-1491. [DOI] [PubMed] [Google Scholar]
- 49.Strissel K.J., Stancheva Z., Miyoshi H., Perfield J.W., DeFuria J., 2nd, Jick Z., Greenberg A.S., Obin M.S. Adipocyte death, adipose tissue remodeling, and obesity complications. Diabetes. 2007;56:2910–2918. doi: 10.2337/db07-0767. [DOI] [PubMed] [Google Scholar]
- 50.Nishimura S., Manabe I., Nagasaki M., Eto K., Yamashita H., Ohsugi M., Otsu M., Hara K., Ueki K., Sugiura S., et al. CD8+ effector T cells contribute to macrophage recruitment and adipose tissue inflammation in obesity. Nat. Med. 2009;15:914–920. doi: 10.1038/nm.1964. [DOI] [PubMed] [Google Scholar]
- 51.Sell H., Habich C., Eckel J. Adaptive immunity in obesity and insulin resistance. Nat. Rev. Endocrinol. 2012;8:709–716. doi: 10.1038/nrendo.2012.114. [DOI] [PubMed] [Google Scholar]
- 52.Ohmura K., Ishimori N., Ohmura Y., Tokuhara S., Nozawa A., Horii S., Andoh Y., Fujii S., Iwabuchi K., Onoe K., et al. Natural killer T cells are involved in adipose tissues inflammation and glucose intolerance in diet-induced obese mice. Arterioscler. Thromb. Vasc. Biol. 2010;30:193–199. doi: 10.1161/ATVBAHA.109.198614. [DOI] [PubMed] [Google Scholar]
- 53.Nieto-Vazquez I., Fernandez-Veledo S., Kramer D.K., Vila-Bedmar R., Garcia-Guerra L., Lorenzo M. Insulin resistance associated to obesity: The link TNF-alpha. Arch. Physiol. Biochem. 2008;114:183–194. doi: 10.1080/13813450802181047. [DOI] [PubMed] [Google Scholar]
- 54.Tanti J.F., Ceppo F., Jager J., Berthou F. Implication of inflammatory signaling pathways in obesity-induced insulin resistance. Front. Endocrinol. 2012;3:181. doi: 10.3389/fendo.2012.00181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Tack C.J., Stienstra R., Joosten L.A., Netea M.G. Inflammation links excess fat to insulin resistance: The role of the interleukin-1 family. Immunol. Rev. 2012;249:239–252. doi: 10.1111/j.1600-065X.2012.01145.x. [DOI] [PubMed] [Google Scholar]
- 56.Fabbrini E., Sullivan S., Klein S. Obesity and nonalcoholic fatty liver disease: Biochemical, metabolic, and clinical implications. Hepatology. 2010;51:679–689. doi: 10.1002/hep.23280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Singh G.M., Danaei G., Farzadfar F., Stevens G.A., Woodward M., Wormser D., Kaptoge S., Whitlock G., Qiao Q., Lewington S., et al. The age-specific quantitative effects of metabolic risk factors on cardiovascular diseases and diabetes: A pooled analysis. PLoS ONE. 2013;8:e65174. doi: 10.1371/journal.pone.0065174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Van Gaal L.F., Mertens I.L., De Block C.E. Mechanisms linking obesity with cardiovascular disease. Nature. 2006;444:875–880. doi: 10.1038/nature05487. [DOI] [PubMed] [Google Scholar]
- 59.Anandacoomarasamy A., Caterson I., Sambrook P., Fransen M., March L. The impact of obesity on the musculoskeletal system. Int. J. Obes. 2008;32:211–222. doi: 10.1038/sj.ijo.0803715. [DOI] [PubMed] [Google Scholar]
- 60.Wearing S.C., Hennig E.M., Byrne N.M., Steele J.R., Hills A.P. Musculoskeletal disorders associated with obesity: A biomechanical perspective. Obes. Rev. 2006;7:239–250. doi: 10.1111/j.1467-789X.2006.00251.x. [DOI] [PubMed] [Google Scholar]
- 61.Doerstling S.S., O’Flanagan C.H., Hursting S.D. Obesity and Cancer Metabolism: A Perspective on Interacting Tumor-Intrinsic and Extrinsic Factors. Front. Oncol. 2017;7:216. doi: 10.3389/fonc.2017.00216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Park J., Morley T.S., Kim M., Clegg D.J., Scherer P.E. Obesity and cancer--mechanisms underlying tumour progression and recurrence. Nat. Rev. Endocrinol. 2014;10:455–465. doi: 10.1038/nrendo.2014.94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Wildman R.P., Muntner P., Reynolds K., McGinn A.P., Rajpathak S., Wylie-Rosett J., Sowers M.R. The obese without cardiometabolic risk factor clustering and the normal weight with cardiometabolic risk factor clustering: Prevalence and correlates of 2 phenotypes among the US population (NHANES 1999–2004) Arch. Intern. Med. 2008;168:1617–1624. doi: 10.1001/archinte.168.15.1617. [DOI] [PubMed] [Google Scholar]
- 64.Luchsinger J.A., Cheng D., Tang M.X., Schupf N., Mayeux R. Central obesity in the elderly is related to late-onset Alzheimer disease. Alzheimer Dis. Assoc. Disord. 2012;26:101–105. doi: 10.1097/WAD.0b013e318222f0d4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Razay G., Vreugdenhil A., Wilcock G. Obesity, abdominal obesity and Alzheimer disease. Dement. Geriatr. Cogn. Disord. 2006;22:173–176. doi: 10.1159/000094586. [DOI] [PubMed] [Google Scholar]
- 66.Bray G.A., Kim K.K., Wilding J.P.H., World Obesity F. Obesity: A chronic relapsing progressive disease process. A position statement of the World Obesity Federation. Obes. Rev. 2017;18:715–723. doi: 10.1111/obr.12551. [DOI] [PubMed] [Google Scholar]
- 67.Fontaine K.R., Redden D.T., Wang C., Westfall A.O., Allison D.B. Years of life lost due to obesity. JAMA. 2003;289:187–193. doi: 10.1001/jama.289.2.187. [DOI] [PubMed] [Google Scholar]
- 68.Woolf A.D., Pfleger B. Burden of major musculoskeletal conditions. Bull. World Health Organ. 2003;81:646–656. [PMC free article] [PubMed] [Google Scholar]
- 69.Jantaratnotai N., Mosikanon K., Lee Y., McIntyre R.S. The interface of depression and obesity. Obes. Res. Clin. Pract. 2017;11:1–10. doi: 10.1016/j.orcp.2016.07.003. [DOI] [PubMed] [Google Scholar]
- 70.Luppino F.S., de Wit L.M., Bouvy P.F., Stijnen T., Cuijpers P., Penninx B.W., Zitman F.G. Overweight, obesity, and depression: A systematic review and meta-analysis of longitudinal studies. Arch. Gen. Psychiatry. 2010;67:220–229. doi: 10.1001/archgenpsychiatry.2010.2. [DOI] [PubMed] [Google Scholar]
- 71.Monda V., La Marra M., Perrella R., Caviglia G., Iavarone A., Chieffi S., Messina G., Carotenuto M., Monda M., Messina A. Obesity and brain illness: From cognitive and psychological evidences to obesity paradox. Diabetes Metab. Syndr. Obes. 2017;10:473–479. doi: 10.2147/DMSO.S148392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Martins L.B., Monteze N.M., Calarge C., Ferreira A.V.M., Teixeira A.L. Pathways linking obesity to neuropsychiatric disorders. Nutrition. 2019;66:16–21. doi: 10.1016/j.nut.2019.03.017. [DOI] [PubMed] [Google Scholar]
- 73.Tchang B.G., Saunders K.H., Igel L.I. Best Practices in the Management of Overweight and Obesity. Med. Clin. N. Am. 2021;105:149–174. doi: 10.1016/j.mcna.2020.08.018. [DOI] [PubMed] [Google Scholar]
- 74.Ryan D.H., Kahan S. Guideline Recommendations for Obesity Management. Med. Clin. N. Am. 2018;102:49–63. doi: 10.1016/j.mcna.2017.08.006. [DOI] [PubMed] [Google Scholar]
- 75.Stewart T., Han H., Allen R.H., Bathalon G., Ryan D.H., Newton R.L., Jr., Williamson D.A. HEALTH: Efficacy of an internet/population-based behavioral weight management program for the U.S. Army. J. Diabetes Sci. Technol. 2011;5:178–187. doi: 10.1177/193229681100500125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Apovian C.M., Aronne L.J., Bessesen D.H., McDonnell M.E., Murad M.H., Pagotto U., Ryan D.H., Still C.D. Pharmacological management of obesity: An endocrine Society clinical practice guideline. J. Clin. Endocrinol. Metab. 2015;100:342–362. doi: 10.1210/jc.2014-3415. [DOI] [PubMed] [Google Scholar]
- 77.Bluher M. Obesity: Global epidemiology and pathogenesis. Nat. Rev. Endocrinol. 2019;15:288–298. doi: 10.1038/s41574-019-0176-8. [DOI] [PubMed] [Google Scholar]
- 78.Loffler A., Luck T., Then F.S., Luck-Sikorski C., Pabst A., Kovacs P., Bottcher Y., Breitfeld J., Tonjes A., Horstmann A., et al. Effects of psychological eating behaviour domains on the association between socio-economic status and BMI. Public Health Nutr. 2017;20:2706–2712. doi: 10.1017/S1368980017001653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Garvey W.T., Mechanick J.I., Brett E.M., Garber A.J., Hurley D.L., Jastreboff A.M., Nadolsky K., Pessah-Pollack R., Plodkowski R. Reviewers of the AACEOCPG: American Association of Clinical Endocrinologists and American College of Endocrinology Comprehensive Clinical Practice Guidelines for Medical Care of Patients with Obesity. Endocr Pract. 2016;3:1–203. doi: 10.4158/EP161365.GL. [DOI] [PubMed] [Google Scholar]
- 80.Gonzalez-Muniesa P., Martinez-Gonzalez M.A., Hu F.B., Despres J.P., Matsuzawa Y., Loos R.J.F., Moreno L.A., Bray G.A., Martinez J.A. Obesity. Nat. Rev. Dis. Primers. 2017;3:17034. doi: 10.1038/nrdp.2017.34. [DOI] [PubMed] [Google Scholar]
- 81.American College of Cardiology/American Heart Association Task Force on Practice Guidelines OEP: Executive summary: Guidelines (2013) for the management of overweight and obesity in adults: A report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines and the Obesity Society published by the Obesity Society and American College of Cardiology/American Heart Association Task Force on Practice Guidelines. Based on a systematic review from the The Obesity Expert Panel, 2013. Obesity. 2014;22:S5–S39. doi: 10.1002/oby.20821. [DOI] [PubMed] [Google Scholar]
- 82.Bray G.A., Siri-Tarino P.W. The Role of Macronutrient Content in the Diet for Weight Management. Endocrinol. Metab. Clin. N. Am. 2016;45:581–604. doi: 10.1016/j.ecl.2016.04.009. [DOI] [PubMed] [Google Scholar]
- 83.Golpour-Hamedani S., Mohammadifard N., Khosravi A., Feizi A., Safavi S.M. Dietary approaches to stop hypertension diet and obesity: A cross-sectional study of Iranian children and adolescents. ARYA Atheroscler. 2017;13:7–13. [PMC free article] [PubMed] [Google Scholar]
- 84.D’Innocenzo S., Biagi C., Lanari M. Obesity and the Mediterranean Diet: A Review of Evidence of the Role and Sustainability of the Mediterranean Diet. Nutrients. 2019;11:1306. doi: 10.3390/nu11061306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.de la Iglesia R., Lopez-Legarrea P., Abete I., Bondia-Pons I., Navas-Carretero S., Forga L., Martinez J.A., Zulet M.A. A new dietary strategy for long-term treatment of the metabolic syndrome is compared with the American Heart Association (AHA) guidelines: The MEtabolic Syndrome REduction in NAvarra (RESMENA) project. Br. J. Nutr. 2014;111:643–652. doi: 10.1017/S0007114513002778. [DOI] [PubMed] [Google Scholar]
- 86.Cantero I., Abete I., Monreal J.I., Martinez J.A., Zulet M.A. Fruit Fiber Consumption Specifically Improves Liver Health Status in Obese Subjects under Energy Restriction. Nutrients. 2017;9:667. doi: 10.3390/nu9070667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.de la Iglesia R., Lopez-Legarrea P., Celada P., Sanchez-Muniz F.J., Martinez J.A., Zulet M.A. Beneficial effects of the RESMENA dietary pattern on oxidative stress in patients suffering from metabolic syndrome with hyperglycemia are associated to dietary TAC and fruit consumption. Int. J. Mol. Sci. 2013;14:6903–6919. doi: 10.3390/ijms14046903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Mattson M.P., Longo V.D., Harvie M. Impact of intermittent fasting on health and disease processes. Ageing. Res. Rev. 2017;39:46–58. doi: 10.1016/j.arr.2016.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Stockman M.C., Thomas D., Burke J., Apovian C.M. Intermittent Fasting: Is the Wait Worth the Weight? Curr. Obes. Rep. 2018;7:172–185. doi: 10.1007/s13679-018-0308-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Varady K.A., Bhutani S., Church E.C., Klempel M.C. Short-term modified alternate-day fasting: A novel dietary strategy for weight loss and cardioprotection in obese adults. Am. J. Clin. Nutr. 2009;90:1138–1143. doi: 10.3945/ajcn.2009.28380. [DOI] [PubMed] [Google Scholar]
- 91.St-Onge M.P., Ard J., Baskin M.L., Chiuve S.E., Johnson H.M., Kris-Etherton P., Varady K., on behalf of the American Heart Association Obesity Committee of the Council on Lifestyle and Cardiometabolic Health. Council on Cardiovascular Disease in the Young. Council on Clinical Cardiology et al. Meal Timing and Frequency: Implications for Cardiovascular Disease Prevention: A Scientific Statement from the American Heart Association. Circulation. 2017;135:e96–e121. doi: 10.1161/CIR.0000000000000476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Jensen M.D., Ryan D.H., Apovian C.M., Ard J.D., Comuzzie A.G., Donato K.A., Hu F.B., Hubbard V.S., Jakicic J.M., Kushner R.F., et al. 2013 AHA/ACC/TOS guideline for the management of overweight and obesity in adults: A report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines and The Obesity Society. J. Am. Coll. Cardiol. 2014;63:2985–3023. doi: 10.1016/j.jacc.2013.11.004. [DOI] [PubMed] [Google Scholar]
- 93.Donnelly J.E., Blair S.N., Jakicic J.M., Manore M.M., Rankin J.W., Smith B.K. Appropriate physical activity intervention strategies for weight loss and prevention of weight regain for adults. Med. Sci. Sports Exerc. 2009;41:459–471. doi: 10.1249/MSS.0b013e3181949333. [DOI] [PubMed] [Google Scholar]
- 94.Hill J.O., Wyatt H.R. Role of physical activity in preventing and treating obesity. J. Appl. Physiol. 2005;99:765–770. doi: 10.1152/japplphysiol.00137.2005. [DOI] [PubMed] [Google Scholar]
- 95.Swift D.L., McGee J.E., Earnest C.P., Carlisle E., Nygard M., Johannsen N.M. The Effects of Exercise and Physical Activity on Weight Loss and Maintenance. Prog. Cardiovasc. Dis. 2018;61:206–213. doi: 10.1016/j.pcad.2018.07.014. [DOI] [PubMed] [Google Scholar]
- 96.Villareal D.T., Aguirre L., Gurney A.B., Waters D.L., Sinacore D.R., Colombo E., Armamento-Villareal R., Qualls C. Aerobic or Resistance Exercise, or Both, in Dieting Obese Older Adults. N. Engl. J. Med. 2017;376:1943–1955. doi: 10.1056/NEJMoa1616338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Jakicic J.M., Rogers R.J., Davis K.K., Collins K.A. Role of Physical Activity and Exercise in Treating Patients with Overweight and Obesity. Clin. Chem. 2018;64:99–107. doi: 10.1373/clinchem.2017.272443. [DOI] [PubMed] [Google Scholar]
- 98.Toplak H., Woodward E., Yumuk V., Oppert J.M., Halford J.C., Fruhbeck G. 2014 EASO Position Statement on the Use of Anti-Obesity Drugs. Obes. Facts. 2015;8:166–174. doi: 10.1159/000430801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Igel L.I., Kumar R.B., Saunders K.H., Aronne L.J. Practical Use of Pharmacotherapy for Obesity. Gastroenterology. 2017;152:1765–1779. doi: 10.1053/j.gastro.2016.12.049. [DOI] [PubMed] [Google Scholar]
- 100.Rothman R.B., Baumann M.H., Dersch C.M., Romero D.V., Rice K.C., Carroll F.I., Partilla J.S. Amphetamine-type central nervous system stimulants release norepinephrine more potently than they release dopamine and serotonin. Synapse. 2001;39:32–41. doi: 10.1002/1098-2396(20010101)39:1<32::AID-SYN5>3.0.CO;2-3. [DOI] [PubMed] [Google Scholar]
- 101.Richard D., Ferland J., Lalonde J., Samson P., Deshaies Y. Influence of topiramate in the regulation of energy balance. Nutrition. 2000;16:961–966. doi: 10.1016/S0899-9007(00)00452-4. [DOI] [PubMed] [Google Scholar]
- 102.Wilding J., Van Gaal L., Rissanen A., Vercruysse F., Fitchet M., Group O.-S. A randomized double-blind placebo-controlled study of the long-term efficacy and safety of topiramate in the treatment of obese subjects. Int. J. Obes. Relat. Metab. Disord. 2004;28:1399–1410. doi: 10.1038/sj.ijo.0802783. [DOI] [PubMed] [Google Scholar]
- 103.Fleming J.W., McClendon K.S., Riche D.M. New obesity agents: Lorcaserin and phentermine/topiramate. Ann. Pharmacother. 2013;47:1007–1016. doi: 10.1345/aph.1R779. [DOI] [PubMed] [Google Scholar]
- 104.Singh J., Kumar R. Phentermine-topiramate: First combination drug for obesity. Int. J. Appl. Basic Med. Res. 2015;5:157–158. doi: 10.4103/2229-516X.157177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Gadde K.M., Allison D.B., Ryan D.H., Peterson C.A., Troupin B., Schwiers M.L., Day W.W. Effects of low-dose, controlled-release, phentermine plus topiramate combination on weight and associated comorbidities in overweight and obese adults (CONQUER): A randomised, placebo-controlled, phase 3 trial. Lancet. 2011;377:1341–1352. doi: 10.1016/S0140-6736(11)60205-5. [DOI] [PubMed] [Google Scholar]
- 106.Garvey W.T., Ryan D.H., Look M., Gadde K.M., Allison D.B., Peterson C.A., Schwiers M., Day W.W., Bowden C.H. Two-year sustained weight loss and metabolic benefits with controlled-release phentermine/topiramate in obese and overweight adults (SEQUEL): A randomized, placebo-controlled, phase 3 extension study. Am. J. Clin. Nutr. 2012;95:297–308. doi: 10.3945/ajcn.111.024927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Bouza C., Angeles M., Munoz A., Amate J.M. Efficacy and safety of naltrexone and acamprosate in the treatment of alcohol dependence: A systematic review. Addiction. 2004;99:811–828. doi: 10.1111/j.1360-0443.2004.00763.x. [DOI] [PubMed] [Google Scholar]
- 108.Greenway F.L., Whitehouse M.J., Guttadauria M., Anderson J.W., Atkinson R.L., Fujioka K., Gadde K.M., Gupta A.K., O’Neil P., Schumacher D., et al. Rational design of a combination medication for the treatment of obesity. Obesity. 2009;17:30–39. doi: 10.1038/oby.2008.461. [DOI] [PubMed] [Google Scholar]
- 109.Foley K.F., DeSanty K.P., Kast R.E. Bupropion: Pharmacology and therapeutic applications. Expert Rev. Neurother. 2006;6:1249–1265. doi: 10.1586/14737175.6.9.1249. [DOI] [PubMed] [Google Scholar]
- 110.Hasegawa H., Meeusen R., Sarre S., Diltoer M., Piacentini M.F., Michotte Y. Acute dopamine/norepinephrine reuptake inhibition increases brain and core temperature in rats. J. Appl. Physiol. 2005;99:1397–1401. doi: 10.1152/japplphysiol.00435.2005. [DOI] [PubMed] [Google Scholar]
- 111.Greenway F.L., Fujioka K., Plodkowski R.A., Mudaliar S., Guttadauria M., Erickson J., Kim D.D., Dunayevich E., Group C-IS Effect of naltrexone plus bupropion on weight loss in overweight and obese adults (COR-I): A multicentre, randomised, double-blind, placebo-controlled, phase 3 trial. Lancet. 2010;376:595–605. doi: 10.1016/S0140-6736(10)60888-4. [DOI] [PubMed] [Google Scholar]
- 112.Farr O.M., Tsoukas M.A., Triantafyllou G., Dincer F., Filippaios A., Ko B.J., Mantzoros C.S. Short-term administration of the GLP-1 analog liraglutide decreases circulating leptin and increases GIP levels and these changes are associated with alterations in CNS responses to food cues: A randomized, placebo-controlled, crossover study. Metabolism. 2016;65:945–953. doi: 10.1016/j.metabol.2016.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Farr O.M., Upadhyay J., Rutagengwa C., DiPrisco B., Ranta Z., Adra A., Bapatla N., Douglas V.P., Douglas K.A.A., Nolen-Doerr E., et al. Longer-term liraglutide administration at the highest dose approved for obesity increases reward-related orbitofrontal cortex activation in response to food cues: Implications for plateauing weight loss in response to anti-obesity therapies. Diabetes Obes. Metab. 2019;21:2459–2464. doi: 10.1111/dom.13827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Pi-Sunyer X., Astrup A., Fujioka K., Greenway F., Halpern A., Krempf M., Lau D.C., le Roux C.W., Violante Ortiz R., Jensen C.B., et al. A Randomized, Controlled Trial of 3.0 mg of Liraglutide in Weight Management. N. Engl. J. Med. 2015;373:11–22. doi: 10.1056/NEJMoa1411892. [DOI] [PubMed] [Google Scholar]
- 115.Van Can J., Sloth B., Jensen C.B., Flint A., Blaak E.E., Saris W.H. Effects of the once-daily GLP-1 analog liraglutide on gastric emptying, glycemic parameters, appetite and energy metabolism in obese, non-diabetic adults. Int. J. Obes. 2014;38:784–793. doi: 10.1038/ijo.2013.162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Le Roux C.W., Heneghan H.M. Bariatric Surgery for Obesity. Med. Clin. N. Am. 2018;102:165–182. doi: 10.1016/j.mcna.2017.08.011. [DOI] [PubMed] [Google Scholar]
- 117.Saunders K.H., Igel L.I., Saumoy M., Sharaiha R.Z., Aronne L.J. Devices and Endoscopic Bariatric Therapies for Obesity. Curr. Obes. Rep. 2018;7:162–171. doi: 10.1007/s13679-018-0307-x. [DOI] [PubMed] [Google Scholar]
- 118.Wada N., Hirako S., Takenoya F., Kageyama H., Okabe M., Shioda S. Leptin and its receptors. J. Chem. Neuroanat. 2014;61–62:191–199. doi: 10.1016/j.jchemneu.2014.09.002. [DOI] [PubMed] [Google Scholar]
- 119.Chou K., Perry C.M. Metreleptin: First global approval. Drugs. 2013;73:989–997. doi: 10.1007/s40265-013-0074-7. [DOI] [PubMed] [Google Scholar]
- 120.Tchang B.G., Shukla A.P., Aronne L.J. Metreleptin and generalized lipodystrophy and evolving therapeutic perspectives. Expert Opin. Biol. Ther. 2015;15:1061–1075. doi: 10.1517/14712598.2015.1052789. [DOI] [PubMed] [Google Scholar]
- 121.Kissileff H.R., Thornton J.C., Torres M.I., Pavlovich K., Mayer L.S., Kalari V., Leibel R.L., Rosenbaum M. Leptin reverses declines in satiation in weight-reduced obese humans. Am. J. Clin. Nutr. 2012;95:309–317. doi: 10.3945/ajcn.111.012385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Samsom M., Szarka L.A., Camilleri M., Vella A., Zinsmeister A.R., Rizza R.A. Pramlintide, an amylin analog, selectively delays gastric emptying: Potential role of vagal inhibition. Am. J. Physiol. Gastrointest Liver Physiol. 2000;278:G946–G951. doi: 10.1152/ajpgi.2000.278.6.G946. [DOI] [PubMed] [Google Scholar]
- 123.Mack C.M., Soares C.J., Wilson J.K., Athanacio J.R., Turek V.F., Trevaskis J.L., Roth J.D., Smith P.A., Gedulin B., Jodka C.M., et al. Davalintide (AC2307), a novel amylin-mimetic peptide: Enhanced pharmacological properties over native amylin to reduce food intake and body weight. Int. J. Obes. 2010;34:385–395. doi: 10.1038/ijo.2009.238. [DOI] [PubMed] [Google Scholar]
- 124.Christou G.A., Katsiki N., Blundell J., Fruhbeck G., Kiortsis D.N. Semaglutide as a promising antiobesity drug. Obes. Rev. 2019;20:805–815. doi: 10.1111/obr.12839. [DOI] [PubMed] [Google Scholar]
- 125.Lau J., Bloch P., Schaffer L., Pettersson I., Spetzler J., Kofoed J., Madsen K., Knudsen L.B., McGuire J., Steensgaard D.B., et al. Discovery of the Once-Weekly Glucagon-Like Peptide-1 (GLP-1) Analogue Semaglutide. J. Med. Chem. 2015;58:7370–7380. doi: 10.1021/acs.jmedchem.5b00726. [DOI] [PubMed] [Google Scholar]
- 126.Avgerinos I., Michailidis T., Liakos A., Karagiannis T., Matthews D.R., Tsapas A., Bekiari E. Oral semaglutide for type 2 diabetes: A systematic review and meta-analysis. Diabetes Obes. Metab. 2020;22:335–345. doi: 10.1111/dom.13899. [DOI] [PubMed] [Google Scholar]
- 127.Fosgerau K., Hoffmann T. Peptide therapeutics: Current status and future directions. Drug. Discov. Today. 2015;20:122–128. doi: 10.1016/j.drudis.2014.10.003. [DOI] [PubMed] [Google Scholar]
- 128.Norregaard P.K., Deryabina M.A., Tofteng Shelton P., Fog J.U., Daugaard J.R., Eriksson P.O., Larsen L.F., Jessen L. A novel GIP analogue, ZP4165, enhances glucagon-like peptide-1-induced body weight loss and improves glycaemic control in rodents. Diabetes Obes Metab. 2018;20:60–68. doi: 10.1111/dom.13034. [DOI] [PubMed] [Google Scholar]
- 129.Pocai A. Action and therapeutic potential of oxyntomodulin. Mol. Metab. 2014;3:241–251. doi: 10.1016/j.molmet.2013.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Henderson S.J., Konkar A., Hornigold D.C., Trevaskis J.L., Jackson R., Fritsch Fredin M., Jansson-Lofmark R., Naylor J., Rossi A., Bednarek M.A., et al. Robust anti-obesity and metabolic effects of a dual GLP-1/glucagon receptor peptide agonist in rodents and non-human primates. Diabetes Obes. Metab. 2016;18:1176–1190. doi: 10.1111/dom.12735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Thomas M.K., Nikooienejad A., Bray R., Cui X., Wilson J., Duffin K., Milicevic Z., Haupt A., Robins D.A. Dual GIP and GLP-1 Receptor Agonist Tirzepatide Improves Beta-cell Function and Insulin Sensitivity in Type 2 Diabetes. J. Clin. Endocrinol. Metab. 2021;106:388–396. doi: 10.1210/clinem/dgaa863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Dragano N.R.V., Ferno J., Dieguez C., Lopez M., Milbank E. Recent Updates on Obesity Treatments: Available Drugs and Future Directions. Neuroscience. 2020;437:215–239. doi: 10.1016/j.neuroscience.2020.04.034. [DOI] [PubMed] [Google Scholar]
- 133.Salamone J.D., McLaughlin P.J., Sink K., Makriyannis A., Parker L.A. Cannabinoid CB1 receptor inverse agonists and neutral antagonists: Effects on food intake, food-reinforced behavior and food aversions. Physiol. Behav. 2007;91:383–388. doi: 10.1016/j.physbeh.2007.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Cluny N.L., Vemuri V.K., Chambers A.P., Limebeer C.L., Bedard H., Wood J.T., Lutz B., Zimmer A., Parker L.A., Makriyannis A., et al. A novel peripherally restricted cannabinoid receptor antagonist, AM6545, reduces food intake and body weight, but does not cause malaise, in rodents. Br. J. Pharmacol. 2010;161:629–642. doi: 10.1111/j.1476-5381.2010.00908.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Randall P.A., Vemuri V.K., Segovia K.N., Torres E.F., Hosmer S., Nunes E.J., Santerre J.L., Makriyannis A., Salamone J.D. The novel cannabinoid CB1 antagonist AM6545 suppresses food intake and food-reinforced behavior. Pharmacol. Biochem. Behav. 2010;97:179–184. doi: 10.1016/j.pbb.2010.07.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Yamada Y., Kato T., Ogino H., Ashina S., Kato K. Cetilistat (ATL-962), a novel pancreatic lipase inhibitor, ameliorates body weight gain and improves lipid profiles in rats. Horm. Metab. Res. 2008;40:539–543. doi: 10.1055/s-2008-1076699. [DOI] [PubMed] [Google Scholar]
- 137.Altabas V., Zjacic-Rotkvic V. Anti-ghrelin antibodies in appetite suppression: Recent advances in obesity pharmacotherapy. Immunotargets Ther. 2015;4:123–130. doi: 10.2147/ITT.S60398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Arimura A., Smith W.D., Schally A.V. Blockade of the stress-induced decrease in blood GH by anti-somatostatin serum in rats. Endocrinology. 1976;98:540–543. doi: 10.1210/endo-98-2-540. [DOI] [PubMed] [Google Scholar]
- 139.Haffer K.N. Effects of novel vaccines on weight loss in diet-induced-obese (DIO) mice. J. Anim. Sci. Biotechnol. 2012;3:21. doi: 10.1186/2049-1891-3-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Na H.N., Kim H., Nam J.H. Prophylactic and therapeutic vaccines for obesity. Clin. Exp. Vaccine Res. 2014;3:37–41. doi: 10.7774/cevr.2014.3.1.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Na H.N., Nam J.H. Proof-of-concept for a virus-induced obesity vaccine; vaccination against the obesity agent adenovirus 36. Int. J. Obes. 2014;38:1470–1474. doi: 10.1038/ijo.2014.41. [DOI] [PubMed] [Google Scholar]
- 142.Srivastava S., Veech R.L. Brown and Brite: The Fat Soldiers in the Anti-obesity Fight. Front. Physiol. 2019;10:38. doi: 10.3389/fphys.2019.00038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Wang S., Pan M.H., Hung W.L., Tung Y.C., Ho C.T. From white to beige adipocytes: Therapeutic potential of dietary molecules against obesity and their molecular mechanisms. Food Funct. 2019;10:1263–1279. doi: 10.1039/C8FO02154F. [DOI] [PubMed] [Google Scholar]
- 144.Rosell M., Kaforou M., Frontini A., Okolo A., Chan Y.W., Nikolopoulou E., Millership S., Fenech M.E., MacIntyre D., Turner J.O., et al. Brown and white adipose tissues: Intrinsic differences in gene expression and response to cold exposure in mice. Am. J. Physiol. Endocrinol. Metab. 2014;306:E945–E964. doi: 10.1152/ajpendo.00473.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Kawada T., Watanabe T., Takaishi T., Tanaka T., Iwai K. Capsaicin-induced beta-adrenergic action on energy metabolism in rats: Influence of capsaicin on oxygen consumption, the respiratory quotient, and substrate utilization. Proc. Soc. Exp. Biol. Med. 1986;183:250–256. doi: 10.3181/00379727-183-42414. [DOI] [PubMed] [Google Scholar]
- 146.Baskaran P., Krishnan V., Ren J., Thyagarajan B. Capsaicin induces browning of white adipose tissue and counters obesity by activating TRPV1 channel-dependent mechanisms. Br. J. Pharmacol. 2016;173:2369–2389. doi: 10.1111/bph.13514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Di Pierro F., Bressan A., Ranaldi D., Rapacioli G., Giacomelli L., Bertuccioli A. Potential role of bioavailable curcumin in weight loss and omental adipose tissue decrease: Preliminary data of a randomized, controlled trial in overweight people with metabolic syndrome. Preliminary study. Eur. Rev. Med Pharmacol. Sci. 2015;19:4195–4202. [PubMed] [Google Scholar]
- 148.Laiglesia L.M., Lorente-Cebrian S., Prieto-Hontoria P.L., Fernandez-Galilea M., Ribeiro S.M., Sainz N., Martinez J.A., Moreno-Aliaga M.J. Eicosapentaenoic acid promotes mitochondrial biogenesis and beige-like features in subcutaneous adipocytes from overweight subjects. J. Nutr. Biochem. 2016;37:76–82. doi: 10.1016/j.jnutbio.2016.07.019. [DOI] [PubMed] [Google Scholar]
- 149.Yan J., Zhao Y., Zhao B. Green tea catechins prevent obesity through modulation of peroxisome proliferator-activated receptors. Sci. China. Life. Sci. 2013;56:804–810. doi: 10.1007/s11427-013-4512-2. [DOI] [PubMed] [Google Scholar]
- 150.Wang S., Liang X., Yang Q., Fu X., Rogers C.J., Zhu M., Rodgers B.D., Jiang Q., Dodson M.V., Du M. Resveratrol induces brown-like adipocyte formation in white fat through activation of AMP-activated protein kinase (AMPK) alpha1. Int. J. Obes. 2015;39:967–976. doi: 10.1038/ijo.2015.23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Zhang Z., Zhang H., Li B., Meng X., Wang J., Zhang Y., Yao S., Ma Q., Jin L., Yang J., et al. Berberine activates thermogenesis in white and brown adipose tissue. Nat. Commun. 2014;5:5493. doi: 10.1038/ncomms6493. [DOI] [PubMed] [Google Scholar]
- 152.Cypess A.M., Weiner L.S., Roberts-Toler C., Franquet Elia E., Kessler S.H., Kahn P.A., English J., Chatman K., Trauger S.A., Doria A., et al. Activation of human brown adipose tissue by a beta3-adrenergic receptor agonist. Cell Metab. 2015;21:33–38. doi: 10.1016/j.cmet.2014.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Merlin J., Sato M., Nowell C., Pakzad M., Fahey R., Gao J., Dehvari N., Summers R.J., Bengtsson T., Evans B.A., et al. The PPARgamma agonist rosiglitazone promotes the induction of brite adipocytes, increasing beta-adrenoceptor-mediated mitochondrial function and glucose uptake. Cell Signal. 2018;42:54–66. doi: 10.1016/j.cellsig.2017.09.023. [DOI] [PubMed] [Google Scholar]
- 154.Loh R.K.C., Formosa M.F., Eikelis N., Bertovic D.A., Anderson M.J., Barwood S.A., Nanayakkara S., Cohen N.D., La Gerche A., Reutens A.T., et al. Pioglitazone reduces cold-induced brown fat glucose uptake despite induction of browning in cultured human adipocytes: A randomised, controlled trial in humans. Diabetologia. 2018;61:220–230. doi: 10.1007/s00125-017-4479-9. [DOI] [PubMed] [Google Scholar]
- 155.Rachid T.L., Silva-Veiga F.M., Graus-Nunes F., Bringhenti I., Mandarim-de-Lacerda C.A., Souza-Mello V. Differential actions of PPAR-alpha and PPAR-beta/delta on beige adipocyte formation: A study in the subcutaneous white adipose tissue of obese male mice. PLoS ONE. 2018;13:e0191365. doi: 10.1371/journal.pone.0191365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Pettersson-Klein A.T., Izadi M., Ferreira D.M.S., Cervenka I., Correia J.C., Martinez-Redondo V., Southern M., Cameron M., Kamenecka T., Agudelo L.Z., et al. Small molecule PGC-1alpha1 protein stabilizers induce adipocyte Ucp1 expression and uncoupled mitochondrial respiration. Mol. Metab. 2018;9:28–42. doi: 10.1016/j.molmet.2018.01.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Kim E.K., Lee S.H., Lee S.Y., Kim J.K., Jhun J.Y., Na H.S., Kim S.Y., Choi J.Y., Yang C.W., Park S.H., et al. Metformin ameliorates experimental-obesity-associated autoimmune arthritis by inducing FGF21 expression and brown adipocyte differentiation. Exp. Mol. Med. 2018;50:e432. doi: 10.1038/emm.2017.245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Tokubuchi I., Tajiri Y., Iwata S., Hara K., Wada N., Hashinaga T., Nakayama H., Mifune H., Yamada K. Beneficial effects of metformin on energy metabolism and visceral fat volume through a possible mechanism of fatty acid oxidation in human subjects and rats. PLoS ONE. 2017;12:e0171293. doi: 10.1371/journal.pone.0171293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Popkin B.M., Du S., Green W.D., Beck M.A., Algaith T., Herbst C.H., Alsukait R.F., Alluhidan M., Alazemi N., Shekar M. Individuals with obesity and COVID-19, A global perspective on the epidemiology and biological relationships. Obes. Rev. 2020;21:e13128. doi: 10.1111/obr.13128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Stefan N., Birkenfeld A.L., Schulze M.B., Ludwig D.S. Obesity and impaired metabolic health in patients with COVID-19. Nat. Rev. Endocrinol. 2020;16:341–342. doi: 10.1038/s41574-020-0364-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Sheetz M.J., King G.L. Molecular understanding of hyperglycemia’s adverse effects for diabetic complications. JAMA. 2002;288:2579–2588. doi: 10.1001/jama.288.20.2579. [DOI] [PubMed] [Google Scholar]
- 162.Saucillo D.C., Gerriets V.A., Sheng J., Rathmell J.C., Maciver N.J. Leptin metabolically licenses T cells for activation to link nutrition and immunity. J. Immunol. 2014;192:136–144. doi: 10.4049/jimmunol.1301158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Tsai S., Clemente-Casares X., Zhou A.C., Lei H., Ahn J.J., Chan Y.T., Choi O., Luck H., Woo M., Dunn S.E., et al. Insulin Receptor-Mediated Stimulation Boosts T Cell Immunity during Inflammation and Infection. Cell Metab. 2018;28:922–934.e924. doi: 10.1016/j.cmet.2018.08.003. [DOI] [PubMed] [Google Scholar]
- 164.American Diabetes A: 2. Classification and Diagnosis of Diabetes. Diabetes Care. 2017;40:S11–S24. doi: 10.2337/dc17-S005. [DOI] [PubMed] [Google Scholar]
- 165.IDF Diabetes Atlas. [(accessed on 10 October 2020)]; Available online: https://www.diabetesatlas.org/en/
- 166.White M.F. Insulin signaling in health and disease. Science. 2003;302:1710–1711. doi: 10.1126/science.1092952. [DOI] [PubMed] [Google Scholar]
- 167.Karlsson H.K., Zierath J.R. Insulin signaling and glucose transport in insulin resistant human skeletal muscle. Cell Biochem. Biophys. 2007;48:103–113. doi: 10.1007/s12013-007-0030-9. [DOI] [PubMed] [Google Scholar]
- 168.Michael M.D., Kulkarni R.N., Postic C., Previs S.F., Shulman G.I., Magnuson M.A., Kahn C.R. Loss of insulin signaling in hepatocytes leads to severe insulin resistance and progressive hepatic dysfunction. Mol. Cell. 2000;6:87–97. doi: 10.1016/S1097-2765(05)00015-8. [DOI] [PubMed] [Google Scholar]
- 169.Laviola L., Perrini S., Cignarelli A., Natalicchio A., Leonardini A., De Stefano F., Cuscito M., De Fazio M., Memeo V., Neri V., et al. Insulin signaling in human visceral and subcutaneous adipose tissue in vivo. Diabetes. 2006;55:952–961. doi: 10.2337/diabetes.55.04.06.db05-1414. [DOI] [PubMed] [Google Scholar]
- 170.Petersen M.C., Shulman G.I. Mechanisms of Insulin Action and Insulin Resistance. Physiol. Rev. 2018;98:2133–2223. doi: 10.1152/physrev.00063.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Insel R.A., Dunne J.L., Atkinson M.A., Chiang J.L., Dabelea D., Gottlieb P.A., Greenbaum C.J., Herold K.C., Krischer J.P., Lernmark A., et al. Staging presymptomatic type 1 diabetes: A scientific statement of JDRF, the Endocrine Society, and the American Diabetes Association. Diabetes Care. 2015;38:1964–1974. doi: 10.2337/dc15-1419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Barrett J.C., Clayton D.G., Concannon P., Akolkar B., Cooper J.D., Erlich H.A., Julier C., Morahan G., Nerup J., Nierras C., et al. Genome-wide association study and meta-analysis find that over 40 loci affect risk of type 1 diabetes. Nat. Genet. 2009;41:703–707. doi: 10.1038/ng.381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Noble J.A., Valdes A.M., Varney M.D., Carlson J.A., Moonsamy P., Fear A.L., Lane J.A., Lavant E., Rappner R., Louey A., et al. HLA class I and genetic susceptibility to type 1 diabetes: Results from the Type 1 Diabetes Genetics Consortium. Diabetes. 2010;59:2972–2979. doi: 10.2337/db10-0699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Saberzadeh-Ardestani B., Karamzadeh R., Basiri M., Hajizadeh-Saffar E., Farhadi A., Shapiro A.M.J., Tahamtani Y., Baharvand H. Type 1 Diabetes Mellitus: Cellular and Molecular Pathophysiology at A Glance. Cell J. 2018;20:294–301. doi: 10.22074/cellj.2018.5513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Katsarou A., Gudbjornsdottir S., Rawshani A., Dabelea D., Bonifacio E., Anderson B.J., Jacobsen L.M., Schatz D.A., Lernmark A. Type 1 diabetes mellitus. Nat. Rev. Dis. Primers. 2017;3:17016. doi: 10.1038/nrdp.2017.16. [DOI] [PubMed] [Google Scholar]
- 176.Ozougwu J.C., Obimba K.C., Belonwu C.D., Unakalamba C. The pathogenesis and pathophysiology of type 1 and type 2 diabetes mellitus. J. Physiol. Pathophysiol. 2013;4:46–57. doi: 10.5897/JPAP2013.0001. [DOI] [Google Scholar]
- 177.Schellenberg E.S., Dryden D.M., Vandermeer B., Ha C., Korownyk C. Lifestyle interventions for patients with and at risk for type 2 diabetes: A systematic review and meta-analysis. Ann. Intern. Med. 2013;159:543–551. doi: 10.7326/0003-4819-159-8-201310150-00007. [DOI] [PubMed] [Google Scholar]
- 178.Wang X., Bao W., Liu J., Ouyang Y.Y., Wang D., Rong S., Xiao X., Shan Z.L., Zhang Y., Yao P., et al. Inflammatory markers and risk of type 2 diabetes: A systematic review and meta-analysis. Diabetes Care. 2013;36:166–175. doi: 10.2337/dc12-0702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Esteve E., Ricart W., Fernandez-Real J.M. Gut microbiota interactions with obesity, insulin resistance and type 2 diabetes: Did gut microbiote co-evolve with insulin resistance? Curr. Opin. Clin. Nutr. Metab. Care. 2011;14:483–490. doi: 10.1097/MCO.0b013e328348c06d. [DOI] [PubMed] [Google Scholar]
- 180.Ding E.L., Song Y., Manson J.E., Hunter D.J., Lee C.C., Rifai N., Buring J.E., Gaziano J.M., Liu S. Sex hormone-binding globulin and risk of type 2 diabetes in women and men. N. Engl. J. Med. 2009;361:1152–1163. doi: 10.1056/NEJMoa0804381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Kulkarni R.N., Bruning J.C., Winnay J.N., Postic C., Magnuson M.A., Kahn C.R. Tissue-specific knockout of the insulin receptor in pancreatic beta cells creates an insulin secretory defect similar to that in type 2 diabetes. Cell. 1999;96:329–339. doi: 10.1016/S0092-8674(00)80546-2. [DOI] [PubMed] [Google Scholar]
- 182.Honka H., Makinen J., Hannukainen J.C., Tarkia M., Oikonen V., Teras M., Fagerholm V., Ishizu T., Saraste A., Stark C., et al. Validation of [18F] fluorodeoxyglucose and positron emission tomography (PET) for the measurement of intestinal metabolism in pigs, and evidence of intestinal insulin resistance in patients with morbid obesity. Diabetologia. 2013;56:893–900. doi: 10.1007/s00125-012-2825-5. [DOI] [PubMed] [Google Scholar]
- 183.Artunc F., Schleicher E., Weigert C., Fritsche A., Stefan N., Haring H.U. The impact of insulin resistance on the kidney and vasculature. Nat. Rev. Nephrol. 2016;12:721–737. doi: 10.1038/nrneph.2016.145. [DOI] [PubMed] [Google Scholar]
- 184.Blazquez E., Velazquez E., Hurtado-Carneiro V., Ruiz-Albusac J.M. Insulin in the brain: Its pathophysiological implications for States related with central insulin resistance, type 2 diabetes and Alzheimer’s disease. Front. Endocrinol. 2014;5:161. doi: 10.3389/fendo.2014.00161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Meijer R.I., De Boer M.P., Groen M.R., Eringa E.C., Rattigan S., Barrett E.J., Smulders Y.M., Serne E.H. Insulin-induced microvascular recruitment in skin and muscle are related and both are associated with whole-body glucose uptake. Microcirculation. 2012;19:494–500. doi: 10.1111/j.1549-8719.2012.00174.x. [DOI] [PubMed] [Google Scholar]
- 186.Loria P., Lonardo A., Anania F. Liver and diabetes. A vicious circle. Hepatol. Res. 2013;43:51–64. doi: 10.1111/j.1872-034X.2012.01031.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.DeFronzo R.A. Insulin resistance, lipotoxicity, type 2 diabetes and atherosclerosis: The missing links. The Claude Bernard Lecture 2009. Diabetologia. 2010;53:1270–1287. doi: 10.1007/s00125-010-1684-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Gastaldelli A., Gaggini M., DeFronzo R.A. Role of Adipose Tissue Insulin Resistance in the Natural History of Type 2 Diabetes: Results from the San Antonio Metabolism Study. Diabetes. 2017;66:815–822. doi: 10.2337/db16-1167. [DOI] [PubMed] [Google Scholar]
- 189.Romeo G.R., Lee J., Shoelson S.E. Metabolic syndrome, insulin resistance, and roles of inflammation--mechanisms and therapeutic targets. Arterioscler. Thromb. Vasc. Biol. 2012;32:1771–1776. doi: 10.1161/ATVBAHA.111.241869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Perry R.J., Camporez J.G., Kursawe R., Titchenell P.M., Zhang D., Perry C.J., Jurczak M.J., Abudukadier A., Han M.S., Zhang X.M., et al. Hepatic acetyl CoA links adipose tissue inflammation to hepatic insulin resistance and type 2 diabetes. Cell. 2015;160:745–758. doi: 10.1016/j.cell.2015.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Harding J.L., Pavkov M.E., Magliano D.J., Shaw J.E., Gregg E.W. Global trends in diabetes complications: A review of current evidence. Diabetologia. 2019;62:3–16. doi: 10.1007/s00125-018-4711-2. [DOI] [PubMed] [Google Scholar]
- 192.Zheng Y., Ley S.H., Hu F.B. Global aetiology and epidemiology of type 2 diabetes mellitus and its complications. Nat. Rev. Endocrinol. 2018;14:88–98. doi: 10.1038/nrendo.2017.151. [DOI] [PubMed] [Google Scholar]
- 193.Duckworth W., Abraira C., Moritz T., Reda D., Emanuele N., Reaven P.D., Zieve F.J., Marks J., Davis S.N., Hayward R., et al. Glucose control and vascular complications in veterans with type 2 diabetes. N. Engl. J. Med. 2009;360:129–139. doi: 10.1056/NEJMoa0808431. [DOI] [PubMed] [Google Scholar]
- 194.van Dieren S., Beulens J.W., van der Schouw Y.T., Grobbee D.E., Neal B. The global burden of diabetes and its complications: An emerging pandemic. Eur. J. Cardiovasc. Prev. Rehabil. 2010;17:S3–S8. doi: 10.1097/01.hjr.0000368191.86614.5a. [DOI] [PubMed] [Google Scholar]
- 195.Brownlee M. The pathobiology of diabetic complications: A unifying mechanism. Diabetes. 2005;54:1615–1625. doi: 10.2337/diabetes.54.6.1615. [DOI] [PubMed] [Google Scholar]
- 196.Di Cianni G., Miccoli R., Volpe L., Lencioni C., Del Prato S. Intermediate metabolism in normal pregnancy and in gestational diabetes. Diabetes Metab. Res. Rev. 2003;19:259–270. doi: 10.1002/dmrr.390. [DOI] [PubMed] [Google Scholar]
- 197.Plows J.F., Stanley J.L., Baker P.N., Reynolds C.M., Vickers M.H. The Pathophysiology of Gestational Diabetes Mellitus. Int. J. Mol. Sci. 2018;19:3342. doi: 10.3390/ijms19113342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Hattersley A.T., Patel K.A. Precision diabetes: Learning from monogenic diabetes. Diabetologia. 2017;60:769–777. doi: 10.1007/s00125-017-4226-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Urakami T. Maturity-onset diabetes of the young (MODY): Current perspectives on diagnosis and treatment. Diabetes Metab. Syndr. Obes. 2019;12:1047–1056. doi: 10.2147/DMSO.S179793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Vaxillaire M., Froguel P., Bonnefond A. How Recent Advances in Genomics Improve Precision Diagnosis and Personalized Care of Maturity-Onset Diabetes of the Young. Curr. Diab. Rep. 2019;19:79. doi: 10.1007/s11892-019-1202-x. [DOI] [PubMed] [Google Scholar]
- 201.Christ-Crain M., Bichet D.G., Fenske W.K., Goldman M.B., Rittig S., Verbalis J.G., Verkman A.S. Diabetes insipidus. Nat. Rev. Dis. Primers. 2019;5:54. doi: 10.1038/s41572-019-0103-2. [DOI] [PubMed] [Google Scholar]
- 202.Chaudhary N., Tyagi N. Diabetes mellitus: An Overview. Int. J. Res. Dev. Pharm. Life Sci. 2018;7:3030–3033. doi: 10.21276/IJRDPL.2278-0238.2018.7(4).3030-3033. [DOI] [Google Scholar]
- 203.Gullo L., Pezzilli R., Morselli-Labate A.M., Italian Pancreatic Cancer Study Group Diabetes and the risk of pancreatic cancer. N. Engl. J. Med. 1994;331:81–84. doi: 10.1056/NEJM199407143310203. [DOI] [PubMed] [Google Scholar]
- 204.Yi Y., Norris A.W., Wang K., Sun X., Uc A., Moran A., Engelhardt J.F., Ode K.L. Abnormal Glucose Tolerance in Infants and Young Children with Cystic Fibrosis. Am. J. Respir. Crit. Care. Med. 2016;194:974–980. doi: 10.1164/rccm.201512-2518OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Kirti K., Tarr J.M., Ahmad S.I., Eva M.K., Rakesh C. Diabetes, An Old Disease, a New Insigh. 1st ed. Springer; New York, NY, USA: 2013. [Google Scholar]
- 206.Pandit M.K., Burke J., Gustafson A.B., Minocha A., Peiris A.N. Drug-induced disorders of glucose tolerance. Ann. Intern. Med. 1993;118:529–539. doi: 10.7326/0003-4819-118-7-199304010-00008. [DOI] [PubMed] [Google Scholar]
- 207.Campbell M.D., Sathish T., Zimmet P.Z., Thankappan K.R., Oldenburg B., Owens D.R., Shaw J.E., Tapp R.J. Benefit of lifestyle-based T2DM prevention is influenced by prediabetes phenotype. Nat. Rev. Endocrinol. 2020;16:395–400. doi: 10.1038/s41574-019-0316-1. [DOI] [PubMed] [Google Scholar]
- 208.Veni D.K., Gupta N.V. Diabetes mellitus treatment: A rapid review on innovative therapies. Asian J. Pharm. Clin. Res. 2019;12:46–53. doi: 10.22159/ajpcr.2019.v12i1.28285. [DOI] [Google Scholar]
- 209.Janez A., Guja C., Mitrakou A., Lalic N., Tankova T., Czupryniak L., Tabak A.G., Prazny M., Martinka E., Smircic-Duvnjak L. Insulin Therapy in Adults with Type 1 Diabetes Mellitus: A Narrative Review. Diabetes Ther. 2020;11:387–409. doi: 10.1007/s13300-019-00743-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Gough S.C., Bode B., Woo V., Rodbard H.W., Linjawi S., Poulsen P., Damgaard L.H., Buse J.B., Investigators NNt Efficacy and safety of a fixed-ratio combination of insulin degludec and liraglutide (IDegLira) compared with its components given alone: Results of a phase 3, open-label, randomised, 26-week, treat-to-target trial in insulin-naive patients with type 2 diabetes. Lancet Diabetes Endocrinol. 2014;2:885–893. doi: 10.1016/S2213-8587(14)70174-3. [DOI] [PubMed] [Google Scholar]
- 211.Foretz M., Guigas B., Viollet B. Understanding the glucoregulatory mechanisms of metformin in type 2 diabetes mellitus. Nat. Rev. Endocrinol. 2019;15:569–589. doi: 10.1038/s41574-019-0242-2. [DOI] [PubMed] [Google Scholar]
- 212.Wiviott S.D., Raz I., Bonaca M.P., Mosenzon O., Kato E.T., Cahn A., Silverman M.G., Zelniker T.A., Kuder J.F., Murphy S.A., et al. Dapagliflozin and Cardiovascular Outcomes in Type 2 Diabetes. N. Engl. J. Med. 2019;380:347–357. doi: 10.1056/NEJMoa1812389. [DOI] [PubMed] [Google Scholar]
- 213.Zinman B., Wanner C., Lachin J.M., Fitchett D., Bluhmki E., Hantel S., Mattheus M., Devins T., Johansen O.E., Woerle H.J., et al. Empagliflozin, Cardiovascular Outcomes, and Mortality in Type 2 Diabetes. N. Engl. J. Med. 2015;373:2117–2128. doi: 10.1056/NEJMoa1504720. [DOI] [PubMed] [Google Scholar]
- 214.Aroda V.R., Henry R.R., Han J., Huang W., DeYoung M.B., Darsow T., Hoogwerf B.J. Efficacy of GLP-1 receptor agonists and DPP-4 inhibitors: Meta-analysis and systematic review. Clin. Ther. 2012;34:1247–1258.e1222. doi: 10.1016/j.clinthera.2012.04.013. [DOI] [PubMed] [Google Scholar]
- 215.Wang W., Liu H., Xiao S., Liu S., Li X., Yu P. Effects of Insulin Plus Glucagon-Like Peptide-1 Receptor Agonists (GLP-1RAs) in Treating Type 1 Diabetes Mellitus: A Systematic Review and Meta-Analysis. Diabetes Ther. 2017;8:727–738. doi: 10.1007/s13300-017-0282-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Feutren G., Papoz L., Assan R., Vialettes B., Karsenty G., Vexiau P., Du Rostu H., Rodier M., Sirmai J., Lallemand A., et al. Cyclosporin increases the rate and length of remissions in insulin-dependent diabetes of recent onset. Results of a multicentre double-blind trial. Lancet. 1986;2:119–124. doi: 10.1016/S0140-6736(86)91943-4. [DOI] [PubMed] [Google Scholar]
- 217.Warshauer J.T., Bluestone J.A., Anderson M.S. New Frontiers in the Treatment of Type 1 Diabetes. Cell Metab. 2020;31:46–61. doi: 10.1016/j.cmet.2019.11.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.Edelman S., Maier H., Wilhelm K. Pramlintide in the treatment of diabetes mellitus. BioDrugs. 2008;22:375–386. doi: 10.2165/0063030-200822060-00004. [DOI] [PubMed] [Google Scholar]
- 219.Aref A., Zayan T., Pararajasingam R., Sharma A., Halawa A. Pancreatic transplantation: Brief review of the current evidence. World J. Transplant. 2019;9:81–93. doi: 10.5500/wjt.v9.i4.81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.Proks P., Reimann F., Green N., Gribble F., Ashcroft F. Sulfonylurea stimulation of insulin secretion. Diabetes. 2002;51:368–376. doi: 10.2337/diabetes.51.2007.S368. [DOI] [PubMed] [Google Scholar]
- 221.Kalra S., Aamir A.H., Raza A., Das A.K., Azad Khan A.K., Shrestha D., Qureshi M.F., Md F., Pathan M.F., Jawad F., et al. Place of sulfonylureas in the management of type 2 diabetes mellitus in South Asia: A consensus statement. Indian J. Endocrinol. Metab. 2015;19:577–596. doi: 10.4103/2230-8210.163171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222.Hauner H. The mode of action of thiazolidinediones. Diabetes Metab. Res. Rev. 2002;2:S10–S15. doi: 10.1002/dmrr.249. [DOI] [PubMed] [Google Scholar]
- 223.Eldor R., DeFronzo R.A., Abdul-Ghani M. In vivo actions of peroxisome proliferator-activated receptors: Glycemic control, insulin sensitivity, and insulin secretion. Diabetes Care. 2013;36:162–174. doi: 10.2337/dcS13-2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.Van de Laar F.A., Lucassen P.L., Akkermans R.P., Van de Lisdonk E.H., Rutten G.E., Van Weel C. Alpha-glucosidase inhibitors for type 2 diabetes mellitus. Cochrane Database Syst. Rev. 2005;CD003639:1–176. doi: 10.1002/14651858.CD003639.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225.Wu Z., McGoogan J.M. Characteristics of and Important Lessons from the Coronavirus Disease 2019 (COVID-19) Outbreak in China: Summary of a Report of 72314 Cases from the Chinese Center for Disease Control and Prevention. JAMA. 2020;323:1239–1242. doi: 10.1001/jama.2020.2648. [DOI] [PubMed] [Google Scholar]
- 226.Pal R., Bhansali A. COVID-19, diabetes mellitus and ACE2: The conundrum. Diabetes Res. Clin. Pract. 2020;162:108132. doi: 10.1016/j.diabres.2020.108132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227.Guo W., Li M., Dong Y., Zhou H., Zhang Z., Tian C., Qin R., Wang H., Shen Y., Du K., et al. Diabetes is a risk factor for the progression and prognosis of COVID-19. Diabetes Metab. Res. Rev. 2020;e3319:1–9. doi: 10.1002/dmrr.3319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228.Cuschieri S., Grech S. COVID-19 and diabetes: The why, the what and the how. J. Diabetes Complicat. 2020;34:107637. doi: 10.1016/j.jdiacomp.2020.107637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229.Yang J.K., Lin S.S., Ji X.J., Guo L.M. Binding of SARS coronavirus to its receptor damages islets and causes acute diabetes. Acta Diabetol. 2010;47:193–199. doi: 10.1007/s00592-009-0109-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230.Brunt E.M., Wong V.W., Nobili V., Day C.P., Sookoian S., Maher J.J., Bugianesi E., Sirlin C.B., Neuschwander-Tetri B.A., Rinella M.E. Nonalcoholic fatty liver disease. Nat. Rev. Dis. Primers. 2015;1:15080. doi: 10.1038/nrdp.2015.80. [DOI] [PubMed] [Google Scholar]
- 231.Younossi Z.M., Koenig A.B., Abdelatif D., Fazel Y., Henry L., Wymer M. Global epidemiology of nonalcoholic fatty liver disease-Meta-analytic assessment of prevalence, incidence, and outcomes. Hepatology. 2016;64:73–84. doi: 10.1002/hep.28431. [DOI] [PubMed] [Google Scholar]
- 232.Lazarus J.V., Colombo M., Cortez-Pinto H., Huang T.T., Miller V., Ninburg M., Schattenberg J.M., Seim L., Wong V.W.S., Zelber-Sagi S. NAFLD—Sounding the alarm on a silent epidemic. Nat. Rev. Gastroenterol. Hepatol. 2020;17:377–379. doi: 10.1038/s41575-020-0315-7. [DOI] [PubMed] [Google Scholar]
- 233.Eslam M., Newsome P.N., Sarin S.K., Anstee Q.M., Targher G., Romero-Gomez M., Zelber-Sagi S., Wai-Sun Wong V., Dufour J.F., Schattenberg J.M., et al. A new definition for metabolic dysfunction-associated fatty liver disease: An international expert consensus statement. J. Hepatol. 2020;73:202–209. doi: 10.1016/j.jhep.2020.03.039. [DOI] [PubMed] [Google Scholar]
- 234.Ress C., Kaser S. Mechanisms of intrahepatic triglyceride accumulation. World J. Gastroenterol. 2016;22:1664–1673. doi: 10.3748/wjg.v22.i4.1664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 235.Anstee Q.M., Reeves H.L., Kotsiliti E., Govaere O., Heikenwalder M. From NASH to HCC: Current concepts and future challenges. Nat. Rev. Gastroenterol. Hepatol. 2019;16:411–428. doi: 10.1038/s41575-019-0145-7. [DOI] [PubMed] [Google Scholar]
- 236.Trauner M., Arrese M., Wagner M. Fatty liver and lipotoxicity. Biochim. Biophys. Acta. 2010;1801:299–310. doi: 10.1016/j.bbalip.2009.10.007. [DOI] [PubMed] [Google Scholar]
- 237.Anderson N., Borlak J. Molecular mechanisms and therapeutic targets in steatosis and steatohepatitis. Pharmacol. Rev. 2008;60:311–357. doi: 10.1124/pr.108.00001. [DOI] [PubMed] [Google Scholar]
- 238.Morigny P., Houssier M., Mouisel E., Langin D. Adipocyte lipolysis and insulin resistance. Biochimie. 2016;125:259–266. doi: 10.1016/j.biochi.2015.10.024. [DOI] [PubMed] [Google Scholar]
- 239.Berk P.D. Regulatable fatty acid transport mechanisms are central to the pathophysiology of obesity, fatty liver, and metabolic syndrome. Hepatology. 2008;48:1362–1376. doi: 10.1002/hep.22632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 240.Diraison F., Moulin P., Beylot M. Contribution of hepatic de novo lipogenesis and reesterification of plasma non esterified fatty acids to plasma triglyceride synthesis during non-alcoholic fatty liver disease. Diabetes Metab. 2003;29:478–485. doi: 10.1016/S1262-3636(07)70061-7. [DOI] [PubMed] [Google Scholar]
- 241.Wang Y., Viscarra J., Kim S.J., Sul H.S. Transcriptional regulation of hepatic lipogenesis. Nat. Rev. Mol. Cell Biol. 2015;16:678–689. doi: 10.1038/nrm4074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 242.Hodson L., Gunn P.J. Publisher Correction: The regulation of hepatic fatty acid synthesis and partitioning: The effect of nutritional state. Nat. Rev. Endocrinol. 2020;16:340. doi: 10.1038/s41574-020-0345-9. [DOI] [PubMed] [Google Scholar]
- 243.Cooper A.D. Hepatic uptake of chylomicron remnants. J. Lipid Res. 1997;38:2173–2192. doi: 10.1016/S0022-2275(20)34932-4. [DOI] [PubMed] [Google Scholar]
- 244.Tessari P., Coracina A., Cosma A., Tiengo A. Hepatic lipid metabolism and non-alcoholic fatty liver disease. Nutr. Metab. Cardiovasc. Dis. 2009;19:291–302. doi: 10.1016/j.numecd.2008.12.015. [DOI] [PubMed] [Google Scholar]
- 245.Fabbrini E., Mohammed B.S., Magkos F., Korenblat K.M., Patterson B.W., Klein S. Alterations in adipose tissue and hepatic lipid kinetics in obese men and women with nonalcoholic fatty liver disease. Gastroenterology. 2008;134:424–431. doi: 10.1053/j.gastro.2007.11.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 246.Hannou S.A., Haslam D.E., McKeown N.M., Herman M.A. Fructose metabolism and metabolic disease. J. Clin. Investig. 2018;128:545–555. doi: 10.1172/JCI96702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 247.Softic S., Cohen D.E., Kahn C.R. Role of Dietary Fructose and Hepatic De Novo Lipogenesis in Fatty Liver Disease. Dig. Dis. Sci. 2016;61:1282–1293. doi: 10.1007/s10620-016-4054-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 248.Marra F., Svegliati-Baroni G. Lipotoxicity and the gut-liver axis in NASH pathogenesis. J. Hepatol. 2018;68:280–295. doi: 10.1016/j.jhep.2017.11.014. [DOI] [PubMed] [Google Scholar]
- 249.Petersen M.C., Vatner D.F., Shulman G.I. Regulation of hepatic glucose metabolism in health and disease. Nat. Rev. Endocrinol. 2017;13:572–587. doi: 10.1038/nrendo.2017.80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 250.Foster D.W. Malonyl-CoA: The regulator of fatty acid synthesis and oxidation. J. Clin. Investig. 2012;122:1958–1959. doi: 10.1172/JCI63967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 251.Koliaki C., Szendroedi J., Kaul K., Jelenik T., Nowotny P., Jankowiak F., Herder C., Carstensen M., Krausch M., Knoefel W.T., et al. Adaptation of hepatic mitochondrial function in humans with non-alcoholic fatty liver is lost in steatohepatitis. Cell. Metab. 2015;21:739–746. doi: 10.1016/j.cmet.2015.04.004. [DOI] [PubMed] [Google Scholar]
- 252.Schuster S., Cabrera D., Arrese M., Feldstein A.E. Triggering resolution of inflammation in, NASH. Nat. Rev. Gastroenterol. Hepatol. 2018;15:349–364. doi: 10.1038/s41575-018-0009-6. [DOI] [PubMed] [Google Scholar]
- 253.Kesar V., Odin J.A. Toll-like receptors and liver disease. Liver Int. 2014;34:184–196. doi: 10.1111/liv.12315. [DOI] [PubMed] [Google Scholar]
- 254.Arrese M., Cabrera D., Kalergis A.M., Feldstein A.E. Innate Immunity Inflammation in, NAFLD/NASH. Dig Dis. Sci. 2016;61:1294–1303. doi: 10.1007/s10620-016-4049-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 255.Petrasek J., Dolganiuc A., Csak T., Kurt-Jones E.A., Szabo G. Type I interferons protect from Toll-like receptor 9-associated liver injury and regulate IL-1 receptor antagonist in mice. Gastroenterology. 2011;140:697–708.e694. doi: 10.1053/j.gastro.2010.08.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 256.Chatterjee S., Rana R., Corbett J., Kadiiska M.B., Goldstein J., Mason R.P. P2X7 receptor-NADPH oxidase axis mediates protein radical formation and Kupffer cell activation in carbon tetrachloride-mediated steatohepatitis in obese mice. Free Radic. Biol. Med. 2012;52:1666–1679. doi: 10.1016/j.freeradbiomed.2012.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 257.Morinaga H., Mayoral R., Heinrichsdorff J., Osborn O., Franck N., Hah N., Walenta E., Bandyopadhyay G., Pessentheiner A.R., Chi T.J., et al. Characterization of distinct subpopulations of hepatic macrophages in HFD/obese mice. Diabetes. 2015;64:1120–1130. doi: 10.2337/db14-1238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 258.Kiechl S., Wittmann J., Giaccari A., Knoflach M., Willeit P., Bozec A., Moschen A.R., Muscogiuri G., Sorice G.P., Kireva T., et al. Blockade of receptor activator of nuclear factor-kappaB (RANKL) signaling improves hepatic insulin resistance and prevents development of diabetes mellitus. Nat. Med. 2013;19:358–363. doi: 10.1038/nm.3084. [DOI] [PubMed] [Google Scholar]
- 259.Han M.S., Park S.Y., Shinzawa K., Kim S., Chung K.W., Lee J.H., Kwon C.H., Lee K.W., Lee J.H., Park C.K., et al. Lysophosphatidylcholine as a death effector in the lipoapoptosis of hepatocytes. J. Lipid Res. 2008;49:84–97. doi: 10.1194/jlr.M700184-JLR200. [DOI] [PubMed] [Google Scholar]
- 260.Rocha M., Apostolova N., Diaz-Rua R., Muntane J., Victor V.M. Mitochondria and T2D: Role of Autophagy, ER Stress, and Inflammasome. Trends Endocrinol. Metab. 2020;31:725–741. doi: 10.1016/j.tem.2020.03.004. [DOI] [PubMed] [Google Scholar]
- 261.Begriche K., Massart J., Robin M.A., Bonnet F., Fromenty B. Mitochondrial adaptations and dysfunctions in nonalcoholic fatty liver disease. Hepatology. 2013;58:1497–1507. doi: 10.1002/hep.26226. [DOI] [PubMed] [Google Scholar]
- 262.Morris E.M., Rector R.S., Thyfault J.P., Ibdah J.A. Mitochondria and redox signaling in steatohepatitis. Antioxid. Redox Signal. 2011;15:485–504. doi: 10.1089/ars.2010.3795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 263.Schmid A.I., Szendroedi J., Chmelik M., Krssak M., Moser E., Roden M. Liver ATP synthesis is lower and relates to insulin sensitivity in patients with type 2 diabetes. Diabetes Care. 2011;34:448–453. doi: 10.2337/dc10-1076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 264.Scorrano L., Oakes S.A., Opferman J.T., Cheng E.H., Sorcinelli M.D., Pozzan T., Korsmeyer S.J. BAX and BAK regulation of endoplasmic reticulum Ca2+: A control point for apoptosis. Science. 2003;300:135–139. doi: 10.1126/science.1081208. [DOI] [PubMed] [Google Scholar]
- 265.Nazal L., Riquelme A., Solis N., Pizarro M., Escalona A., Burotto M., Mendez J.I., Saint-Jean C., Concha M.J., Giovanni S., et al. Hypoadiponectinemia and its association with liver fibrosis in morbidly obese patients. Obes. Surg. 2010;20:1400–1407. doi: 10.1007/s11695-009-0051-0. [DOI] [PubMed] [Google Scholar]
- 266.Arab J.P., Arrese M., Trauner M. Recent Insights into the Pathogenesis of Nonalcoholic Fatty Liver Disease. Annu. Rev. Pathol. 2018;13:321–350. doi: 10.1146/annurev-pathol-020117-043617. [DOI] [PubMed] [Google Scholar]
- 267.Ikejima K., Okumura K., Lang T., Honda H., Abe W., Yamashina S., Enomoto N., Takei Y., Sato N. The role of leptin in progression of non-alcoholic fatty liver disease. Hepatol. Res. 2005;33:151–154. doi: 10.1016/j.hepres.2005.09.024. [DOI] [PubMed] [Google Scholar]
- 268.Romeo S., Kozlitina J., Xing C., Pertsemlidis A., Cox D., Pennacchio L.A., Boerwinkle E., Cohen J.C., Hobbs H.H. Genetic variation in PNPLA3 confers susceptibility to nonalcoholic fatty liver disease. Nat. Genet. 2008;40:1461–1465. doi: 10.1038/ng.257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 269.Mancina R.M., Matikainen N., Maglio C., Soderlund S., Lundbom N., Hakkarainen A., Rametta R., Mozzi E., Fargion S., Valenti L., et al. Paradoxical dissociation between hepatic fat content and de novo lipogenesis due to PNPLA3 sequence variant. J. Clin. Endocrinol. Metab. 2015;100:E821–E825. doi: 10.1210/jc.2014-4464. [DOI] [PubMed] [Google Scholar]
- 270.Pirazzi C., Adiels M., Burza M.A., Mancina R.M., Levin M., Stahlman M., Taskinen M.R., Orho-Melander M., Perman J., Pujia A., et al. Patatin-like phospholipase domain-containing 3 (PNPLA3) I148M (rs738409) affects hepatic VLDL secretion in humans and in vitro. J. Hepatol. 2012;57:1276–1282. doi: 10.1016/j.jhep.2012.07.030. [DOI] [PubMed] [Google Scholar]
- 271.Kalia H.S., Gaglio P.J. The Prevalence and Pathobiology of Nonalcoholic Fatty Liver Disease in Patients of Different Races or Ethnicities. Clin. Liver. Dis. 2016;20:215–224. doi: 10.1016/j.cld.2015.10.005. [DOI] [PubMed] [Google Scholar]
- 272.Liu Y.L., Patman G.L., Leathart J.B., Piguet A.C., Burt A.D., Dufour J.F., Day C.P., Daly A.K., Reeves H.L., Anstee Q.M. Carriage of the PNPLA3 rs738409 C >G polymorphism confers an increased risk of non-alcoholic fatty liver disease associated hepatocellular carcinoma. J. Hepatol. 2014;61:75–81. doi: 10.1016/j.jhep.2014.02.030. [DOI] [PubMed] [Google Scholar]
- 273.Bruschi F.V., Claudel T., Tardelli M., Caligiuri A., Stulnig T.M., Marra F., Trauner M. The PNPLA3 I148M variant modulates the fibrogenic phenotype of human hepatic stellate cells. Hepatology. 2017;65:1875–1890. doi: 10.1002/hep.29041. [DOI] [PubMed] [Google Scholar]
- 274.Sookoian S., Pirola C.J. Genetic predisposition in nonalcoholic fatty liver disease. Clin. Mol. Hepatol. 2017;23:1–12. doi: 10.3350/cmh.2016.0109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 275.Marzuillo P., Grandone A., Perrone L., Miraglia Del Giudice E. Understanding the pathophysiological mechanisms in the pediatric non-alcoholic fatty liver disease: The role of genetics. World J. Hepatol. 2015;7:1439–1443. doi: 10.4254/wjh.v7.i11.1439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 276.Feldstein A.E., Charatcharoenwitthaya P., Treeprasertsuk S., Benson J.T., Enders F.B., Angulo P. The natural history of non-alcoholic fatty liver disease in children: A follow-up study for up to 20 years. Gut. 2009;58:1538–1544. doi: 10.1136/gut.2008.171280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 277.Di Sessa A., Umano G.R., Miraglia Del Giudice E. The Association between Non-Alcoholic Fatty Liver Disease and Cardiovascular Risk in Children. Children. 2017;4:57. doi: 10.3390/children4070057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 278.Di Sessa A., Umano G.R., Miraglia Del Giudice E., Santoro N. From the liver to the heart: Cardiac dysfunction in obese children with non-alcoholic fatty liver disease. World J. Hepatol. 2017;9:69–73. doi: 10.4254/wjh.v9.i2.69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 279.Koot B.G., de Groot E., van der Baan-Slootweg O.H., Bohte A.E., Nederveen A.J., Jansen P.L., Stoker J., Benninga M.A. Nonalcoholic fatty liver disease and cardiovascular risk in children with obesity. Obesity. 2015;23:1239–1243. doi: 10.1002/oby.21076. [DOI] [PubMed] [Google Scholar]
- 280.Di Sessa A., Guarino S., Umano G.R., Arenella M., Alfiero S., Quaranta G., Miraglia Del Giudice E., Marzuillo P. MAFLD in Obese Children: A Challenging Definition. Children. 2021;8:247. doi: 10.3390/children8030247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 281.Barrera F., George J. The role of diet nutritional intervention for the management of patients with, NAFLD. Clin. Liver Dis. 2014;18:91–112. doi: 10.1016/j.cld.2013.09.009. [DOI] [PubMed] [Google Scholar]
- 282.El-Agroudy N.N., Kurzbach A., Rodionov R.N., O’Sullivan J., Roden M., Birkenfeld A.L., Pesta D.H. Are Lifestyle Therapies Effective for NAFLD Treatment? Trends. Endocrinol. Metab. 2019;30:701–709. doi: 10.1016/j.tem.2019.07.013. [DOI] [PubMed] [Google Scholar]
- 283.Chalasani N., Younossi Z., Lavine J.E., Diehl A.M., Brunt E.M., Cusi K., Charlton M., Sanyal A.J. The diagnosis and management of non-alcoholic fatty liver disease: Practice Guideline by the American Association for the Study of Liver Diseases, American College of Gastroenterology, and the American Gastroenterological Association. Hepatology. 2012;55:2005–2023. doi: 10.1002/hep.25762. [DOI] [PubMed] [Google Scholar]
- 284.Neuschwander-Tetri B.A. Hepatic lipotoxicity and the pathogenesis of nonalcoholic steatohepatitis: The central role of nontriglyceride fatty acid metabolites. Hepatology. 2010;52:774–788. doi: 10.1002/hep.23719. [DOI] [PubMed] [Google Scholar]
- 285.Li Y., Liu L., Wang B., Wang J., Chen D. Metformin in non-alcoholic fatty liver disease: A systematic review and meta-analysis. Biomed. Rep. 2013;1:57–64. doi: 10.3892/br.2012.18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 286.Yu J.G., Javorschi S., Hevener A.L., Kruszynska Y.T., Norman R.A., Sinha M., Olefsky J.M. The effect of thiazolidinediones on plasma adiponectin levels in normal, obese, and type 2 diabetic subjects. Diabetes. 2002;51:2968–2974. doi: 10.2337/diabetes.51.10.2968. [DOI] [PubMed] [Google Scholar]
- 287.Cuthbertson D.J., Irwin A., Gardner C.J., Daousi C., Purewal T., Furlong N., Goenka N., Thomas E.L., Adams V.L., Pushpakom S.P., et al. Improved glycaemia correlates with liver fat reduction in obese, type 2 diabetes, patients given glucagon-like peptide-1 (GLP-1) receptor agonists. PLoS ONE. 2012;7:e50117. doi: 10.1371/journal.pone.0050117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 288.Armstrong M., Gaunt P., Aithal G., Barton D., Hull D., Parker R., Hazlehurst J.M., Guo K., Abouda G., Aldersley M.A., et al. Liraglutide safety and efficacy in patients with non-alcoholic steatohepatitis (LEAN): A multicentre, double-blind, randomised, placebo-controlled phase 2 study. Lancet. 2016;387:679–690. doi: 10.1016/S0140-6736(15)00803-X. [DOI] [PubMed] [Google Scholar]
- 289.Miyazaki M., Kato M., Tanaka K., Tanaka M., Kohjima M., Nakamura K., Enjoji M., Nakamuta M., Kotoh K., Takayanagi R. Increased hepatic expression of dipeptidyl peptidase-4 in non-alcoholic fatty liver disease and its association with insulin resistance and glucose metabolism. Mol. Med. Rep. 2012;5:729–733. doi: 10.3892/mmr.2011.707. [DOI] [PubMed] [Google Scholar]
- 290.Scheen A.J. Beneficial effects of SGLT2 inhibitors on fatty liver in type 2 diabetes: A common comorbidity associated with severe complications. Diabetes Metab. 2019;45:213–223. doi: 10.1016/j.diabet.2019.01.008. [DOI] [PubMed] [Google Scholar]
- 291.Mudaliar S., Henry R.R., Sanyal A.J., Morrow L., Marschall H.U., Kipnes M., Adorini L., Sciacca C.I., Clopton P., Castelloe E., et al. Efficacy and safety of the farnesoid X receptor agonist obeticholic acid in patients with type 2 diabetes and nonalcoholic fatty liver disease. Gastroenterology. 2013;145:574–582.e571. doi: 10.1053/j.gastro.2013.05.042. [DOI] [PubMed] [Google Scholar]
- 292.Ma K., Saha P.K., Chan L., Moore D.D. Farnesoid X receptor is essential for normal glucose homeostasis. J. Clin. Investig. 2006;116:1102–1109. doi: 10.1172/JCI25604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 293.Watanabe M., Houten S.M., Wang L., Moschetta A., Mangelsdorf D.J., Heyman R.A., Moore D.D., Auwerx J. Bile acids lower triglyceride levels via a pathway involving FXR, SHP, and SREBP-1c. J. Clin. Investig. 2004;113:1408–1418. doi: 10.1172/JCI21025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 294.Wagner M., Zollner G., Trauner M. Nuclear bile acid receptor farnesoid X receptor meets nuclear factor-kappaB: New insights into hepatic inflammation. Hepatology. 2008;48:1383–1386. doi: 10.1002/hep.22668. [DOI] [PubMed] [Google Scholar]
- 295.Zhang S., Wang J., Liu Q., Harnish D.C. Farnesoid X receptor agonist WAY-362450 attenuates liver inflammation and fibrosis in murine model of non-alcoholic steatohepatitis. J. Hepatol. 2009;51:380–388. doi: 10.1016/j.jhep.2009.03.025. [DOI] [PubMed] [Google Scholar]
- 296.Gomez-Dominguez E., Gisbert J.P., Moreno-Monteagudo J.A., Garcia-Buey L., Moreno-Otero R. A pilot study of atorvastatin treatment in dyslipemid, non-alcoholic fatty liver patients. Aliment Pharmacol. Ther. 2006;23:1643–1647. doi: 10.1111/j.1365-2036.2006.02926.x. [DOI] [PubMed] [Google Scholar]
- 297.Okada Y., Yamaguchi K., Nakajima T., Nishikawa T., Jo M., Mitsumoto Y., Kimura H., Nishimura T., Tochiki N., Yasui K., et al. Rosuvastatin ameliorates high-fat and high-cholesterol diet-induced nonalcoholic steatohepatitis in rats. Liver Int. 2013;33:301–311. doi: 10.1111/liv.12033. [DOI] [PubMed] [Google Scholar]
- 298.Zhu F.S., Liu S., Chen X.M., Huang Z.G., Zhang D.W. Effects of n-3 polyunsaturated fatty acids from seal oils on nonalcoholic fatty liver disease associated with hyperlipidemia. World J. Gastroenterol. 2008;14:6395–6400. doi: 10.3748/wjg.14.6395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 299.Hossain N., Kanwar P., Mohanty S.R. AComprehensive Updated Review of Pharmaceutical Nonpharmaceutical Treatment for NAFLD. Gastroenterol. Res. Pract. 2016;2016:7109270. doi: 10.1155/2016/7109270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 300.Nakade Y., Murotani K., Inoue T., Kobayashi Y., Yamamoto T., Ishii N., Ohashi T., Ito K., Fukuzawa Y., Yoneda M. Ezetimibe for the treatment of non-alcoholic fatty liver disease: A meta-analysis. Hepatol. Res. 2017;47:1417–1428. doi: 10.1111/hepr.12887. [DOI] [PubMed] [Google Scholar]
- 301.Yoneda M., Fujita K., Nozaki Y., Endo H., Takahashi H., Hosono K., Suzuki K., Mawatari H., Kirikoshi H., Inamori M., et al. Efficacy of ezetimibe for the treatment of non-alcoholic steatohepatitis: An open-label, pilot study. Hepatol. Res. 2010;40:566–573. doi: 10.1111/j.1872-034X.2010.00644.x. [DOI] [PubMed] [Google Scholar]
- 302.Gilat T., Leikin-Frenkel A., Goldiner L., Laufer H., Halpern Z., Konikoff F.M. Arachidyl amido cholanoic acid (Aramchol) is a cholesterol solubilizer and prevents the formation of cholesterol gallstones in inbred mice. Lipids. 2001;36:1135–1140. doi: 10.1007/s11745-001-0824-3. [DOI] [PubMed] [Google Scholar]
- 303.Safadi R., Konikoff F.M., Mahamid M., Zelber-Sagi S., Halpern M., Gilat T., Oren R., Group F. The fatty acid-bile acid conjugate Aramchol reduces liver fat content in patients with nonalcoholic fatty liver disease. Clin. Gastroenterol. Hepatol. 2014;12:2085–2091.e2081. doi: 10.1016/j.cgh.2014.04.038. [DOI] [PubMed] [Google Scholar]
- 304.Imai N., Cohen D.E. Trimming the Fat: Acetyl-CoACarboxylase Inhibition for the Management of NAFLD. Hepatology. 2018;68:2062–2065. doi: 10.1002/hep.30206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 305.Goedeke L., Bates J., Vatner D.F., Perry R.J., Wang T., Ramirez R., Li L., Ellis M.W., Zhang D., Wong K.E., et al. Acetyl-CoA Carboxylase Inhibition Reverses NAFLD and Hepatic Insulin Resistance but Promotes Hypertriglyceridemia in Rodents. Hepatology. 2018;68:2197–2211. doi: 10.1002/hep.30097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 306.Sumida Y., Yoneda M. Current future pharmacological therapies for, NAFLD/NASH. J. Gastroenterol. 2018;53:362–376. doi: 10.1007/s00535-017-1415-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 307.Harrison S.A., Torgerson S., Hayashi P., Ward J., Schenker S. Vitamin E and vitamin C treatment improves fibrosis in patients with nonalcoholic steatohepatitis. Am. J. Gastroenterol. 2003;98:2485–2490. doi: 10.1111/j.1572-0241.2003.08699.x. [DOI] [PubMed] [Google Scholar]
- 308.Dufour J.F., Oneta C.M., Gonvers J.J., Bihl F., Cerny A., Cereda J.M., Zala J.F., Helbling B., Steuerwald M., Zimmermann A., et al. Randomized placebo-controlled trial of ursodeoxycholic acid with vitamin e in nonalcoholic steatohepatitis. Clin. Gastroenterol. Hepatol. 2006;4:1537–1543. doi: 10.1016/j.cgh.2006.09.025. [DOI] [PubMed] [Google Scholar]
- 309.Hasegawa T., Yoneda M., Nakamura K., Makino I., Terano A. Plasma transforming growth factor-beta1 level and efficacy of alpha-tocopherol in patients with non-alcoholic steatohepatitis: A pilot study. Aliment Pharmacol. Ther. 2001;15:1667–1672. doi: 10.1046/j.1365-2036.2001.01083.x. [DOI] [PubMed] [Google Scholar]
- 310.Miller E.R., 3rd, Pastor-Barriuso R., Dalal D., Riemersma R.A., Appel L.J., Guallar E. Meta-analysis: High-dosage vitamin E supplementation may increase all-cause, mortality. Ann. Intern. Med. 2005;142:37–46. doi: 10.7326/0003-4819-142-1-200501040-00110. [DOI] [PubMed] [Google Scholar]
- 311.Klein E.A., Thompson I.M., Jr Tangen C.M., Crowley J.J., Lucia M.S., Goodman P.J., Minasian L.M., Ford L.G., Parnes H.L., Gaziano J.M., et al. Vitamin E and the risk of prostate cancer: The Selenium and Vitamin E Cancer Prevention Trial (SELECT) JAMA. 2011;306:1549–1556. doi: 10.1001/jama.2011.1437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 312.Khoshbaten M., Aliasgarzadeh A., Masnadi K., Tarzamani M.K., Farhang S., Babaei H., Kiani J., Zaare M., Najafipoor F. N-acetylcysteine improves liver function in patients with non-alcoholic Fatty liver disease. Hepat. Mon. 2010;10:12–16. [PMC free article] [PubMed] [Google Scholar]
- 313.Kathirvel E., Morgan K., Nandgiri G., Sandoval B.C., Caudill M.A., Bottiglieri T., French S.W., Morgan T.R. Betaine improves nonalcoholic fatty liver and associated hepatic insulin resistance: A potential mechanism for hepatoprotection by betaine. Am. J. Physiol. Gastrointest. Liver. Physiol. 2010;299:G1068–G1077. doi: 10.1152/ajpgi.00249.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 314.Cariou B., Hanf R., Lambert-Porcheron S., Zair Y., Sauvinet V., Noel B., Flet L., Vidal H., Staels B., Laville M. Dual peroxisome proliferator-activated receptor alpha/delta agonist GFT505 improves hepatic and peripheral insulin sensitivity in abdominally obese subjects. Diabetes Care. 2013;36:2923–2930. doi: 10.2337/dc12-2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 315.Staels B., Rubenstrunk A., Noel B., Rigou G., Delataille P., Millatt L.J., Baron M., Lucas A., Tailleux A., Hum D.W., et al. Hepatoprotective effects of the dual peroxisome proliferator-activated receptor alpha/delta agonist, GFT505, in rodent models of nonalcoholic fatty liver disease/nonalcoholic steatohepatitis. Hepatology. 2013;58:1941–1952. doi: 10.1002/hep.26461. [DOI] [PubMed] [Google Scholar]
- 316.Ratziu V., Harrison S.A., Francque S., Bedossa P., Lehert P., Serfaty L., Romero-Gomez M., Boursier J., Abdelmalek M., Caldwell S., et al. Elafibranor, an Agonist of the Peroxisome Proliferator-Activated Receptor-alpha and -delta, Induces Resolution of Nonalcoholic Steatohepatitis Without Fibrosis Worsening. Gastroenterology. 2016;150:1147–1159.e1145. doi: 10.1053/j.gastro.2016.01.038. [DOI] [PubMed] [Google Scholar]
- 317.Zein C.O., Yerian L.M., Gogate P., Lopez R., Kirwan J.P., Feldstein A.E., McCullough A.J. Pentoxifylline improves nonalcoholic steatohepatitis: A randomized placebo-controlled trial. Hepatology. 2011;54:1610–1619. doi: 10.1002/hep.24544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 318.Iacono A., Raso G.M., Canani R.B., Calignano A., Meli R. Probiotics as an emerging therapeutic strategy to treat NAFLD: Focus on molecular and biochemical mechanisms. J. Nutr. Biochem. 2011;22:699–711. doi: 10.1016/j.jnutbio.2010.10.002. [DOI] [PubMed] [Google Scholar]
- 319.Perumpail B.J., Li A.A., John N., Sallam S., Shah N.D., Kwong W., Cholankeril G., Kim D., Ahmed A. The Therapeutic Implications of the Gut Microbiome Probiotics in Patients with, NAFLD. Diseases. 2019;7:27. doi: 10.3390/diseases7010027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 320.Mizrahi M., Shabat Y., Ben Ya’acov A., Lalazar G., Adar T., Wong V., Muller B., Rawlin G., Ilan Y. Alleviation of insulin resistance liver damage by oral administration of Imm124-E is mediated by increased Tregs associated with increased serum, G.L.P.-1.; adiponectin: Results of a phase I/II clinical trial in, NASH. J. Inflamm. Res. 2012;5:141–150. doi: 10.2147/JIR.S35227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 321.Bramante C., Tignanelli C.J., Dutta N., Jones E., Tamariz L., Clark J.M., Usher M., Metlon-Meaux G., Ikramuddin S. Non-alcoholic fatty liver disease (NAFLD) and risk of hospitalization for Covid-19. MedRxiv. 2020:1–7. doi: 10.1101/2020.09.01.20185850. [DOI] [Google Scholar]
- 322.Eslam M., Sanyal A.J., George J., International Consensus Panel MAFLD: A Consensus-Driven Proposed Nomenclature for Metabolic Associated Fatty Liver Disease. Gastroenterology. 2020;158:1999–2014.e1991. doi: 10.1053/j.gastro.2019.11.312. [DOI] [PubMed] [Google Scholar]
- 323.Zhou Y.J., Zheng K.I., Wang X.B., Sun Q.F., Pan K.H., Wang T.Y., Ma H.L., Chen Y.P., George J., Zheng M.H. Metabolic-associated fatty liver disease is associated with severity of COVID-19. Liver. Int. 2020;40:2160–2163. doi: 10.1111/liv.14575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 324.Sharma P., Kumar A. Metabolic dysfunction associated fatty liver disease increases risk of severe Covid-19. Diabetes Metab. Syndr. 2020;14:825–827. doi: 10.1016/j.dsx.2020.06.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 325.Targher G., Mantovani A., Byrne C.D., Wang X.B., Yan H.D., Sun Q.F., Pan K.H., Zheng K.I., Chen Y.P., Eslam M., et al. Risk of severe illness from COVID-19 in patients with metabolic dysfunction-associated fatty liver disease and increased fibrosis scores. Gut. 2020;69:1545–1547. doi: 10.1136/gutjnl-2020-321611. [DOI] [PubMed] [Google Scholar]
- 326.Ji D., Qin E., Xu J., Zhang D., Cheng G., Wang Y., Lau G. Non-alcoholic fatty liver diseases in patients with COVID-19, A retrospective study. J. Hepatol. 2020;73:451–453. doi: 10.1016/j.jhep.2020.03.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 327.Hegyi P.J., Váncsa S., Ocskay K., Dembrovszky F., Kiss S., Farkas N., Erőss B., Szakács Z., Hegyi P., Pár G. Metabolic Associated Fatty Liver Disease Is Associated with an Increased Risk of Severe COVID-19, A Systematic Review with Meta-Analysis. Front. Med. 2021;8:277. doi: 10.3389/fmed.2021.626425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 328.Jose R.J. Manuel A: COVID-19 cytokine storm: The interplay between inflammation and coagulation. Lancet Respir. Med. 2020;8:E46–E47. doi: 10.1016/S2213-2600(20)30216-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 329.Tang N., Li D., Wang X., Sun Z. Abnormal coagulation parameters are associated with poor prognosis in patients with novel coronavirus pneumonia. J. Thromb. Haemost. 2020;18:844–847. doi: 10.1111/jth.14768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 330.Flora G.D., Nayak M.K. A Brief Review of Cardiovascular Diseases, Associated Risk Factors and Current Treatment Regimes. Curr. Pharm. Des. 2019;25:4063–4084. doi: 10.2174/1381612825666190925163827. [DOI] [PubMed] [Google Scholar]
- 331.O’Rourke K., VanderZanden A., Shepard D., Leach-Kemon K. Cardiovascular Disease Worldwide, 1990–2013. JAMA. 2015;314:1905. doi: 10.1001/jama.2015.14994. [DOI] [Google Scholar]
- 332.Friedrich M.J. Global Obesity Epidemic Worsening. JAMA. 2017;318:603. doi: 10.1001/jama.2017.10693. [DOI] [PubMed] [Google Scholar]
- 333.Chen L., Magliano D.J., Zimmet P.Z. The worldwide epidemiology of type 2 diabetes mellitus--present and future perspectives. Nat. Rev. Endocrinol. 2011;8:228–236. doi: 10.1038/nrendo.2011.183. [DOI] [PubMed] [Google Scholar]
- 334.Saklayen M.G. The Global Epidemic of the Metabolic Syndrome. Curr. Hypertens. Rep. 2018;20:12. doi: 10.1007/s11906-018-0812-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 335.Yaniv Y., Juhaszova M., Nuss H.B., Wang S., Zorov D.B., Lakatta E.G., Sollott S.J. Matching ATP supply and demand in mammalian heart: In vivo, in vitro, and in silico perspectives. Ann. N. Y. Acad. Sci. 2010;1188:133–142. doi: 10.1111/j.1749-6632.2009.05093.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 336.O’Neill S., O’Driscoll L. Metabolic syndrome: A closer look at the growing epidemic and its associated pathologies. Obes. Rev. 2015;16:1–12. doi: 10.1111/obr.12229. [DOI] [PubMed] [Google Scholar]
- 337.Yamazoe M., Hisamatsu T., Miura K., Kadowaki S., Zaid M., Kadota A., Torii S., Miyazawa I., Fujiyoshi A., Arima H., et al. Relationship of Insulin Resistance to Prevalence and Progression of Coronary Artery Calcification Beyond Metabolic Syndrome Components: Shiga Epidemiological Study of Subclinical Atherosclerosis. Arterioscler. Thromb. Vasc. Biol. 2016;36:1703–1708. doi: 10.1161/ATVBAHA.116.307612. [DOI] [PubMed] [Google Scholar]
- 338.Perrone-Filardi P., Paolillo S., Costanzo P., Savarese G., Trimarco B., Bonow R.O. The role of metabolic syndrome in heart failure. Eur. Heart J. 2015;36:2630–2634. doi: 10.1093/eurheartj/ehv350. [DOI] [PubMed] [Google Scholar]
- 339.Flegal K.M., Graubard B.I., Williamson D.F., Gail M.H. Cause-specific excess deaths associated with underweight, overweight, and obesity. JAMA. 2007;298:2028–2037. doi: 10.1001/jama.298.17.2028. [DOI] [PubMed] [Google Scholar]
- 340.Alpert M.A. Obesity cardiomyopathy: Pathophysiology and evolution of the clinical syndrome. Am. J. Med. Sci. 2001;321:225–236. doi: 10.1097/00000441-200104000-00003. [DOI] [PubMed] [Google Scholar]
- 341.Grundy S.M. Obesity, metabolic syndrome, and cardiovascular disease. J. Clin. Endocrinol. Metab. 2004;89:2595–2600. doi: 10.1210/jc.2004-0372. [DOI] [PubMed] [Google Scholar]
- 342.Turkbey E.B., McClelland R.L., Kronmal R.A., Burke G.L., Bild D.E., Tracy R.P., Arai A.E., Lima J.A., Bluemke D.A. The impact of obesity on the left ventricle: The Multi-Ethnic Study of Atherosclerosis (MESA) JACC Cardiovasc. Imaging. 2010;3:266–274. doi: 10.1016/j.jcmg.2009.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 343.Sassoon D.J., Goodwill A.G., Noblet J.N., Conteh A.M., Herring B.P., McClintick J.N., Tune J.D., Mather K.J. Obesity alters molecular and functional cardiac responses to ischemia/reperfusion and glucagon-like peptide-1 receptor agonism. Basic Res. Cardiol. 2016;111:43. doi: 10.1007/s00395-016-0563-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 344.Pascual F., Coleman R.A. Fuel availability and fate in cardiac metabolism: A tale of two substrates. Biochim. Biophys. Acta. 2016;1861:1425–1433. doi: 10.1016/j.bbalip.2016.03.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 345.Coort S.L., Luiken J.J., van der Vusse G.J., Bonen A., Glatz J.F. Increased FAT (fatty acid translocase)/CD36-mediated long-chain fatty acid uptake in cardiac myocytes from obese Zucker rats. Biochem. Soc. Trans. 2004;32:83–85. doi: 10.1042/bst0320083. [DOI] [PubMed] [Google Scholar]
- 346.Ormazabal V., Nair S., Elfeky O., Aguayo C., Salomon C., Zuniga F.A. Association between insulin resistance and the development of cardiovascular disease. Cardiovasc. Diabetol. 2018;17:122. doi: 10.1186/s12933-018-0762-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 347.Eddy D., Schlessinger L., Kahn R., Peskin B., Schiebinger R. Relationship of insulin resistance and related metabolic variables to coronary artery disease: A mathematical analysis. Diabetes Care. 2009;32:361–366. doi: 10.2337/dc08-0854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 348.Zhou Y.T., Grayburn P., Karim A., Shimabukuro M., Higa M., Baetens D., Orci L., Unger R.H. Lipotoxic heart disease in obese rats: Implications for human obesity. Proc. Natl. Acad. Sci. USA. 2000;97:1784–1789. doi: 10.1073/pnas.97.4.1784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 349.Ramirez E., Picatoste B., Gonzalez-Bris A., Oteo M., Cruz F., Caro-Vadillo A., Egido J., Tunon J., Morcillo M.A., Lorenzo O. Sitagliptin improved glucose assimilation in detriment of fatty-acid utilization in experimental type-II diabetes: Role of GLP-1 isoforms in Glut4 receptor trafficking. Cardiovasc. Diabetol. 2018;17:12. doi: 10.1186/s12933-017-0643-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 350.Bonora E., Kiechl S., Willeit J., Oberhollenzer F., Egger G., Targher G., Alberiche M., Bonadonna R.C., Muggeo M. Prevalence of insulin resistance in metabolic disorders: The Bruneck Study. Diabetes. 1998;47:1643–1649. doi: 10.2337/diabetes.47.10.1643. [DOI] [PubMed] [Google Scholar]
- 351.Tenenbaum A., Adler Y., Boyko V., Tenenbaum H., Fisman E.Z., Tanne D., Lapidot M., Schwammenthal E., Feinberg M.S., Matas Z., et al. Insulin resistance is associated with increased risk of major cardiovascular events in patients with preexisting coronary artery disease. Am. Heart J. 2007;153:559–565. doi: 10.1016/j.ahj.2007.01.008. [DOI] [PubMed] [Google Scholar]
- 352.Yadav R., Hama S., Liu Y., Siahmansur T., Schofield J., Syed A.A., France M., Pemberton P., Adam S., Ho J.H., et al. Effect of Roux-en-Y Bariatric Surgery on Lipoproteins, Insulin Resistance, and Systemic and Vascular Inflammation in Obesity and Diabetes. Front. Immunol. 2017;8:1512. doi: 10.3389/fimmu.2017.01512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 353.De Luca C., Olefsky J.M. Inflammation and insulin resistance. FEBS Lett. 2008;582:97–105. doi: 10.1016/j.febslet.2007.11.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 354.Austin M.A., Hokanson J.E., Edwards K.L. Hypertriglyceridemia as a cardiovascular risk factor. Am. J. Cardiol. 1998;81:7B–12B. doi: 10.1016/S0002-9149(98)00031-9. [DOI] [PubMed] [Google Scholar]
- 355.Chistiakov D.A., Shkurat T.P., Melnichenko A.A., Grechko A.V., Orekhov A.N. The role of mitochondrial dysfunction in cardiovascular disease: A brief review. Ann. Med. 2018;50:121–127. doi: 10.1080/07853890.2017.1417631. [DOI] [PubMed] [Google Scholar]
- 356.Hoppel C.L., Tandler B., Fujioka H., Riva A. Dynamic organization of mitochondria in human heart and in myocardial disease. Int. J. Biochem. Cell Biol. 2009;41:1949–1956. doi: 10.1016/j.biocel.2009.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 357.Stadhouders A.M., Jap P.H., Winkler H.P., Eppenberger H.M., Wallimann T. Mitochondrial creatine kinase: A major constituent of pathological inclusions seen in mitochondrial myopathies. Proc. Natl. Acad. Sci. USA. 1994;91:5089–5093. doi: 10.1073/pnas.91.11.5089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 358.Doenst T., Nguyen T.D., Abel E.D. Cardiac metabolism in heart failure: Implications beyond ATP production. Circ. Res. 2013;113:709–724. doi: 10.1161/CIRCRESAHA.113.300376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 359.Hobbs F.D. Cardiovascular disease: Different strategies for primary and secondary prevention? Heart. 2004;90:1217–1223. doi: 10.1136/hrt.2003.027680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 360.Leong D.P., Joseph P.G., McKee M., Anand S.S., Teo K.K., Schwalm J.D., Yusuf S. Reducing the Global Burden of Cardiovascular Disease, Part 2: Prevention and Treatment of Cardiovascular Disease. Circ. Res. 2017;121:695–710. doi: 10.1161/CIRCRESAHA.117.311849. [DOI] [PubMed] [Google Scholar]
- 361.Pietrzak E., Cotea C., Pullman S. Primary and secondary prevention of cardiovascular disease: Is there a place for Internet-based interventions? J. Cardiopulm. Rehabil. Prev. 2014;34:303–317. doi: 10.1097/HCR.0000000000000063. [DOI] [PubMed] [Google Scholar]
- 362.Gaede P., Vedel P., Larsen N., Jensen G.V., Parving H.H., Pedersen O. Multifactorial intervention and cardiovascular disease in patients with type 2 diabetes. N. Engl. J. Med. 2003;348:383–393. doi: 10.1056/NEJMoa021778. [DOI] [PubMed] [Google Scholar]
- 363.Marso S.P., Bain S.C., Consoli A., Eliaschewitz F.G., Jodar E., Leiter L.A., Lingvay I., Rosenstock J., Seufert J., Warren M.L., et al. Semaglutide and Cardiovascular Outcomes in Patients with Type 2 Diabetes. N. Engl. J. Med. 2016;375:1834–1844. doi: 10.1056/NEJMoa1607141. [DOI] [PubMed] [Google Scholar]
- 364.Marso S.P., Daniels G.H., Brown-Frandsen K., Kristensen P., Mann J.F., Nauck M.A., Nissen S.E., Pocock S., Poulter N.R., Ravn L.S., et al. Liraglutide and Cardiovascular Outcomes in Type 2 Diabetes. N. Engl. J. Med. 2016;375:311–322. doi: 10.1056/NEJMoa1603827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 365.Czernichow S., Ninomiya T., Huxley R., Kengne A.P., Batty G.D., Grobbee D.E., Woodward M., Neal B., Chalmers J. Impact of blood pressure lowering on cardiovascular outcomes in normal weight, overweight, and obese individuals: The Perindopril Protection Against Recurrent Stroke Study trial. Hypertension. 2010;55:1193–1198. doi: 10.1161/HYPERTENSIONAHA.109.140624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 366.Law M.R., Morris J.K., Wald N.J. Use of blood pressure lowering drugs in the prevention of cardiovascular disease: Meta-analysis of 147 randomised trials in the context of expectations from prospective epidemiological studies. BMJ. 2009;338:b1665. doi: 10.1136/bmj.b1665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 367.Cannon C.P., Blazing M.A., Giugliano R.P., McCagg A., White J.A., Theroux P., Darius H., Lewis B.S., Ophuis T.O., Jukema J.W., et al. Ezetimibe Added to Statin Therapy after Acute Coronary Syndromes. N. Engl. J. Med. 2015;372:2387–2397. doi: 10.1056/NEJMoa1410489. [DOI] [PubMed] [Google Scholar]
- 368.Sabatine M.S., Leiter L.A., Wiviott S.D., Giugliano R.P., Deedwania P., De Ferrari G.M., Murphy S.A., Kuder J.F., Gouni-Berthold I., Lewis B.S., et al. Cardiovascular safety and efficacy of the PCSK9 inhibitor evolocumab in patients with and without diabetes and the effect of evolocumab on glycaemia and risk of new-onset diabetes: A prespecified analysis of the FOURIER randomised controlled trial. Lancet Diabetes Endocrinol. 2017;5:941–950. doi: 10.1016/S2213-8587(17)30313-3. [DOI] [PubMed] [Google Scholar]
- 369.Schupke S., Neumann F.J., Menichelli M., Mayer K., Bernlochner I., Wohrle J., Richardt G., Liebetrau C., Witzenbichler B., Antoniucci D., et al. Ticagrelor or Prasugrel in Patients with Acute Coronary Syndromes. N. Engl. J. Med. 2019;381:1524–1534. doi: 10.1056/NEJMoa1908973. [DOI] [PubMed] [Google Scholar]
- 370.Yusuf S., Zhao F., Mehta S.R., Chrolavicius S., Tognoni G., Fox K.K. Clopidogrel in Unstable Angina to Prevent Recurrent Events Trial I: Effects of clopidogrel in addition to aspirin in patients with acute coronary syndromes without ST-segment elevation. N. Engl. J. Med. 2001;345:494–502. doi: 10.1056/NEJMoa010746. [DOI] [PubMed] [Google Scholar]
- 371.Thom S., Poulter N., Field J., Patel A., Prabhakaran D., Stanton A., Grobbee D.E., Bots M.L., Reddy K.S., Cidambi R., et al. Effects of a fixed-dose combination strategy on adherence and risk factors in patients with or at high risk of CVD: The UMPIRE randomized clinical trial. JAMA. 2013;310:918–929. doi: 10.1001/jama.2013.277064. [DOI] [PubMed] [Google Scholar]
- 372.Koliaki C., Doupis J. Incretin-based therapy: A powerful and promising weapon in the treatment of type 2 diabetes mellitus. Diabetes Ther. 2011;2:101–121. doi: 10.1007/s13300-011-0002-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 373.Husain M., Birkenfeld A.L., Donsmark M., Dungan K., Eliaschewitz F.G., Franco D.R., Jeppesen O.K., Lingvay I., Mosenzon O., Pedersen S.D., et al. Oral Semaglutide and Cardiovascular Outcomes in Patients with Type 2 Diabetes. N. Engl. J. Med. 2019;381:841–851. doi: 10.1056/NEJMoa1901118. [DOI] [PubMed] [Google Scholar]
- 374.Ou H.T., Chang K.C., Li C.Y., Wu J.S. Risks of cardiovascular diseases associated with dipeptidyl peptidase-4 inhibitors and other antidiabetic drugs in patients with type 2 diabetes: A nation-wide longitudinal study. Cardiovasc. Diabetol. 2016;15:41. doi: 10.1186/s12933-016-0350-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 375.Ou H.T., Chang K.C., Li C.Y., Wu J.S. Comparative cardiovascular risks of dipeptidyl peptidase 4 inhibitors with other second- and third-line antidiabetic drugs in patients with type 2 diabetes. Br. J. Clin. Pharmacol. 2017;83:1556–1570. doi: 10.1111/bcp.13241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 376.Heerspink H.J., Perkins B.A., Fitchett D.H., Husain M., Cherney D.Z. Sodium Glucose Cotransporter 2 Inhibitors in the Treatment of Diabetes Mellitus: Cardiovascular and Kidney Effects, Potential Mechanisms, and Clinical Applications. Circulation. 2016;134:752–772. doi: 10.1161/CIRCULATIONAHA.116.021887. [DOI] [PubMed] [Google Scholar]
- 377.Kemp B.E., Stapleton D., Campbell D.J., Chen Z.P., Murthy S., Walter M., Gupta A., Adams J.J., Katsis F., van Denderen B., et al. AMP-activated protein kinase, super metabolic regulator. Biochem. Soc. Trans. 2003;31:162–168. doi: 10.1042/bst0310162. [DOI] [PubMed] [Google Scholar]
- 378.Smith B.K., Steinberg G.R. AMP-activated protein kinase, fatty acid metabolism, and insulin sensitivity. Curr. Opin. Clin. Nutr. Metab. Care. 2017;20:248–253. doi: 10.1097/MCO.0000000000000380. [DOI] [PubMed] [Google Scholar]
- 379.Paiva M.A., Goncalves L.M., Providencia L.A., Davidson S.M., Yellon D.M., Mocanu M.M. Transitory activation of AMPK at reperfusion protects the ischaemic-reperfused rat myocardium against infarction. Cardiovasc. Drugs Ther. 2010;24:25–32. doi: 10.1007/s10557-010-6222-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 380.Shibata R., Ouchi N., Ito M., Kihara S., Shiojima I., Pimentel D.R., Kumada M., Sato K., Schiekofer S., Ohashi K., et al. Adiponectin-mediated modulation of hypertrophic signals in the heart. Nat. Med. 2004;10:1384–1389. doi: 10.1038/nm1137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 381.Davis B.J., Xie Z., Viollet B., Zou M.H. Activation of the AMP-activated kinase by antidiabetes drug metformin stimulates nitric oxide synthesis in vivo by promoting the association of heat shock protein 90 and endothelial nitric oxide synthase. Diabetes. 2006;55:496–505. doi: 10.2337/diabetes.55.02.06.db05-1064. [DOI] [PubMed] [Google Scholar]
- 382.Fujita Y., Hosokawa M., Fujimoto S., Mukai E., Abudukadier A., Obara A., Ogura M., Nakamura Y., Toyoda K., Nagashima K., et al. Metformin suppresses hepatic gluconeogenesis and lowers fasting blood glucose levels through reactive nitrogen species in mice. Diabetologia. 2010;53:1472–1481. doi: 10.1007/s00125-010-1729-5. [DOI] [PubMed] [Google Scholar]
- 383.Wang S., Xu J., Song P., Viollet B., Zou M.H. In vivo activation of AMP-activated protein kinase attenuates diabetes-enhanced degradation of GTP cyclohydrolase, I. Diabetes. 2009;58:1893–1901. doi: 10.2337/db09-0267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 384.Morrison A., Yan X., Tong C., Li J. Acute rosiglitazone treatment is cardioprotective against ischemia-reperfusion injury by modulating AMPK, Akt, and JNK signaling in nondiabetic mice. Am. J. Physiol. Heart. Circ. Physiol. 2011;301:H895–H902. doi: 10.1152/ajpheart.00137.2011. [DOI] [PubMed] [Google Scholar]
- 385.Tsimikas S., Hall J.L. Lipoprotein(a) as a potential causal genetic risk factor of cardiovascular disease: A rationale for increased efforts to understand its pathophysiology and develop targeted therapies. J. Am. Coll. Cardiol. 2012;60:716–721. doi: 10.1016/j.jacc.2012.04.038. [DOI] [PubMed] [Google Scholar]
- 386.Saeed A., Virani S.S. Lipoprotein(a) and cardiovascular disease: Current state and future directions for an enigmatic lipoprotein. Front. Biosci. 2018;23:1099–1112. doi: 10.2741/4635. [DOI] [PubMed] [Google Scholar]
- 387.Chapman M.J., Redfern J.S., McGovern M.E., Giral P. Niacin and fibrates in atherogenic dyslipidemia: Pharmacotherapy to reduce cardiovascular risk. Pharmacol. Ther. 2010;126:314–345. doi: 10.1016/j.pharmthera.2010.01.008. [DOI] [PubMed] [Google Scholar]
- 388.Yeang C., Clopton P.C., Tsimikas S. Lipoprotein(a)-cholesterol levels estimated by vertical auto profile correlate poorly with Lp(a) mass in hyperlipidemic subjects: Implications for clinical practice interpretation of Lp(a)-mediated risk. J. Clin. Lipidol. 2016;10:1389–1396. doi: 10.1016/j.jacl.2016.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 389.Yeang C., Hung M.Y., Byun Y.S., Clopton P., Yang X., Witztum J.L., Tsimikas S. Effect of therapeutic interventions on oxidized phospholipids on apolipoprotein B100 and lipoprotein(a) J. Clin. Lipidol. 2016;10:594–603. doi: 10.1016/j.jacl.2016.01.005. [DOI] [PubMed] [Google Scholar]
- 390.Akaike M., Azuma H., Kagawa A., Matsumoto K., Hayashi I., Tamura K., Nishiuchi T., Iuchi T., Takamori N., Aihara K., et al. Effect of aspirin treatment on serum concentrations of lipoprotein(a) in patients with atherosclerotic diseases. Clin. Chem. 2002;48:1454–1459. doi: 10.1093/clinchem/48.9.1454. [DOI] [PubMed] [Google Scholar]
- 391.Raal F.J., Giugliano R.P., Sabatine M.S., Koren M.J., Langslet G., Bays H., Blom D., Eriksson M., Dent R., Wasserman S.M., et al. Reduction in lipoprotein(a) with PCSK9 monoclonal antibody evolocumab (AMG 145): A pooled analysis of more than 1,300 patients in 4 phase II trials. J. Am. Coll. Cardiol. 2014;63:1278–1288. doi: 10.1016/j.jacc.2014.01.006. [DOI] [PubMed] [Google Scholar]
- 392.Graham M.J., Viney N., Crooke R.M., Tsimikas S. Antisense inhibition of apolipoprotein (a) to lower plasma lipoprotein (a) levels in humans. J. Lipid Res. 2016;57:340–351. doi: 10.1194/jlr.R052258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 393.Merki E., Graham M., Taleb A., Leibundgut G., Yang X., Miller E.R., Fu W., Mullick A.E., Lee R., Willeit P., et al. Antisense oligonucleotide lowers plasma levels of apolipoprotein (a) and lipoprotein (a) in transgenic mice. J. Am. Coll. Cardiol. 2011;57:1611–1621. doi: 10.1016/j.jacc.2010.10.052. [DOI] [PubMed] [Google Scholar]
- 394.Viney N.J., van Capelleveen J.C., Geary R.S., Xia S., Tami J.A., Yu R.Z., Marcovina S.M., Hughes S.G., Graham M.J., Crooke R.M., et al. Antisense oligonucleotides targeting apolipoprotein(a) in people with raised lipoprotein(a): Two randomised, double-blind, placebo-controlled, dose-ranging trials. Lancet. 2016;388:2239–2253. doi: 10.1016/S0140-6736(16)31009-1. [DOI] [PubMed] [Google Scholar]
- 395.Zhang R. The ANGPTL3-4-8 model, a molecular mechanism for triglyceride trafficking. Open Biol. 2016;6:150272. doi: 10.1098/rsob.150272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 396.Dijk W., Kersten S. Regulation of lipid metabolism by angiopoietin-like proteins. Curr. Opin. Lipidol. 2016;27:249–256. doi: 10.1097/MOL.0000000000000290. [DOI] [PubMed] [Google Scholar]
- 397.Shan L., Yu X.C., Liu Z., Hu Y., Sturgis L.T., Miranda M.L., Liu Q. The angiopoietin-like proteins ANGPTL3 and ANGPTL4 inhibit lipoprotein lipase activity through distinct mechanisms. J. Biol. Chem. 2009;284:1419–1424. doi: 10.1074/jbc.M808477200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 398.Aryal B., Singh A.K., Zhang X., Varela L., Rotllan N., Goedeke L., Chaube B., Camporez J.P., Vatner D.F., Horvath T.L., et al. Absence of ANGPTL4 in adipose tissue improves glucose tolerance and attenuates atherogenesis. JCI Insight. 2018;3:e97918. doi: 10.1172/jci.insight.97918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 399.Gusarova V., O’Dushlaine C., Teslovich T.M., Benotti P.N., Mirshahi T., Gottesman O., Van Hout C.V., Murray M.F., Mahajan A., Nielsen J.B., et al. Genetic inactivation of ANGPTL4 improves glucose homeostasis and is associated with reduced risk of diabetes. Nat. Commun. 2018;9:2252. doi: 10.1038/s41467-018-04611-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 400.Wang Y., McNutt M.C., Banfi S., Levin M.G., Holland W.L., Gusarova V., Gromada J., Cohen J.C., Hobbs H.H. Hepatic ANGPTL3 regulates adipose tissue energy homeostasis. Proc. Natl. Acad. Sci. USA. 2015;112:11630–11635. doi: 10.1073/pnas.1515374112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 401.Dewey F.E., Gusarova V., Dunbar R.L., O’Dushlaine C., Schurmann C., Gottesman O., McCarthy S., Van Hout C.V., Bruse S., Dansky H.M., et al. Genetic and Pharmacologic Inactivation of ANGPTL3 and Cardiovascular Disease. N. Engl. J. Med. 2017;377:211–221. doi: 10.1056/NEJMoa1612790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 402.Lin L., Burke J., Venkatesh S., Sadana P. AMPK-SIRT1-independent inhibition of ANGPTL3 gene expression is a potential lipid-lowering mechanism of metformin. J. Pharm. Pharmacol. 2019;71:1421–1428. doi: 10.1111/jphp.13138. [DOI] [PubMed] [Google Scholar]
- 403.Go G.W. Low-Density Lipoprotein Receptor-Related Protein 6 (LRP6) Is a Novel Nutritional Therapeutic Target for Hyperlipidemia, Non-Alcoholic Fatty Liver Disease, and Atherosclerosis. Nutrients. 2015;7:4453–4464. doi: 10.3390/nu7064453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 404.Bastakoty D., Saraswati S., Joshi P., Atkinson J., Feoktistov I., Liu J., Harris J.L., Young P.P. Temporary, Systemic Inhibition of the WNT/beta-Catenin Pathway promotes Regenerative Cardiac Repair following Myocardial Infarct. Cell Stem. Cells Regen. Med. 2016;2:2. doi: 10.16966/2472-6990.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 405.Cheng P.W., Chen Y.Y., Cheng W.H., Lu P.J., Chen H.H., Chen B.R., Yeh T.C., Sun G.C., Hsiao M., Tseng C.J. Wnt Signaling Regulates Blood Pressure by Downregulating a GSK-3beta-Mediated Pathway to Enhance Insulin Signaling in the Central Nervous System. Diabetes. 2015;64:3413–3424. doi: 10.2337/db14-1439. [DOI] [PubMed] [Google Scholar]
- 406.Bonora M., Wieckowski M.R., Sinclair D.A., Kroemer G., Pinton P., Galluzzi L. Targeting mitochondria for cardiovascular disorders: Therapeutic potential and obstacles. Nat. Rev. Cardiol. 2019;16:33–55. doi: 10.1038/s41569-018-0074-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 407.McLachlan J., Beattie E., Murphy M.P., Koh-Tan C.H., Olson E., Beattie W., Dominiczak A.F., Nicklin S.A., Graham D. Combined therapeutic benefit of mitochondria-targeted antioxidant, MitoQ10, and angiotensin receptor blocker, losartan, on cardiovascular function. J. Hypertens. 2014;32:555–564. doi: 10.1097/HJH.0000000000000054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 408.Toyoda S., Haruyama A., Inami S., Arikawa T., Saito F., Watanabe R., Sakuma M., Abe S., Nakajima T., Tanaka A., et al. Effects of carvedilol vs bisoprolol on inflammation and oxidative stress in patients with chronic heart failure. J. Cardiol. 2020;75:140–147. doi: 10.1016/j.jjcc.2019.07.011. [DOI] [PubMed] [Google Scholar]
- 409.Daiber A., Xia N., Steven S., Oelze M., Hanf A., Kroller-Schon S., Munzel T., Li H. New Therapeutic Implications of Endothelial Nitric Oxide Synthase (eNOS) Function/Dysfunction in Cardiovascular Disease. Int. J. Mol. Sci. 2019;20:187. doi: 10.3390/ijms20010187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 410.Wenzel P., Schulz E., Oelze M., Muller J., Schuhmacher S., Alhamdani M.S., Debrezion J., Hortmann M., Reifenberg K., Fleming I., et al. AT1-receptor blockade by telmisartan upregulates GTP-cyclohydrolase I and protects eNOS in diabetic rats. Free Radic. Biol. Med. 2008;45:619–626. doi: 10.1016/j.freeradbiomed.2008.05.009. [DOI] [PubMed] [Google Scholar]
- 411.Broeders M.A., Doevendans P.A., Bekkers B.C., Bronsaer R., van Gorsel E., Heemskerk J.W., Egbrink M.G., van Breda E., Reneman R.S., van Der Zee R. Nebivolol: A third-generation beta-blocker that augments vascular nitric oxide release: Endothelial beta(2)-adrenergic receptor-mediated nitric oxide production. Circulation. 2000;102:677–684. doi: 10.1161/01.CIR.102.6.677. [DOI] [PubMed] [Google Scholar]
- 412.Oelze M., Daiber A., Brandes R.P., Hortmann M., Wenzel P., Hink U., Schulz E., Mollnau H., von Sandersleben A., Kleschyov A.L., et al. Nebivolol inhibits superoxide formation by NADPH oxidase and endothelial dysfunction in angiotensin II-treated rats. Hypertension. 2006;48:677–684. doi: 10.1161/01.HYP.0000239207.82326.29. [DOI] [PubMed] [Google Scholar]
- 413.Lu D., Thum T. RNA-based diagnostic and therapeutic strategies for cardiovascular disease. Nat. Rev. Cardiol. 2019;16:661–674. doi: 10.1038/s41569-019-0218-x. [DOI] [PubMed] [Google Scholar]
- 414.Esteller M. Non-coding RNAs in human disease. Nat. Rev. Genet. 2011;12:861–874. doi: 10.1038/nrg3074. [DOI] [PubMed] [Google Scholar]
- 415.Small E.M., Olson E.N. Pervasive roles of microRNAs in cardiovascular biology. Nature. 2011;469:336–342. doi: 10.1038/nature09783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 416.Latronico M.V., Condorelli G. MicroRNAs and cardiac pathology. Nat. Rev. Cardiol. 2009;6:419–429. doi: 10.1038/nrcardio.2009.56. [DOI] [PubMed] [Google Scholar]
- 417.Schober A., Weber C. Mechanisms of MicroRNAs in Atherosclerosis. Annu. Rev. Pathol. 2016;11:583–616. doi: 10.1146/annurev-pathol-012615-044135. [DOI] [PubMed] [Google Scholar]
- 418.Thum T. Noncoding RNAs and myocardial fibrosis. Nat. Rev. Cardiol. 2014;11:655–663. doi: 10.1038/nrcardio.2014.125. [DOI] [PubMed] [Google Scholar]
- 419.Lucas T., Bonauer A., Dimmeler S. RNA Therapeutics in Cardiovascular Disease. Circ. Res. 2018;123:205–220. doi: 10.1161/CIRCRESAHA.117.311311. [DOI] [PubMed] [Google Scholar]
- 420.Mellis D., Caporali A. MicroRNA-based therapeutics in cardiovascular disease: Screening and delivery to the target. Biochem. Soc. Trans. 2018;46:11–21. doi: 10.1042/BST20170037. [DOI] [PubMed] [Google Scholar]
- 421.van Rooij E., Olson E.N. MicroRNA therapeutics for cardiovascular disease: Opportunities and obstacles. Nat. Rev. Drug. Discov. 2012;11:860–872. doi: 10.1038/nrd3864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 422.Wang X., Ha T., Liu L., Zou J., Zhang X., Kalbfleisch J., Gao X., Williams D., Li C. Increased expression of microRNA-146a decreases myocardial ischaemia/reperfusion injury. Cardiovasc. Res. 2013;97:432–442. doi: 10.1093/cvr/cvs356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 423.Li Q., Xie J., Li R., Shi J., Sun J., Gu R., Ding L., Wang L., Xu B. Overexpression of microRNA-99a attenuates heart remodelling and improves cardiac performance after myocardial infarction. J. Cell Mol. Med. 2014;18:919–928. doi: 10.1111/jcmm.12242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 424.Tao L., Bei Y., Chen P., Lei Z., Fu S., Zhang H., Xu J., Che L., Chen X., Sluijter J.P., et al. Crucial Role of miR-433 in Regulating Cardiac Fibrosis. Theranostics. 2016;6:2068–2083. doi: 10.7150/thno.15007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 425.Beermann J., Piccoli M.T., Viereck J., Thum T. Non-coding RNAs in Development and Disease: Background, Mechanisms, and Therapeutic Approaches. Physiol. Rev. 2016;96:1297–1325. doi: 10.1152/physrev.00041.2015. [DOI] [PubMed] [Google Scholar]
- 426.Pankratz F., Hohnloser C., Bemtgen X., Jaenich C., Kreuzaler S., Hoefer I., Pasterkamp G., Mastroianni J., Zeiser R., Smolka C., et al. MicroRNA-100 Suppresses Chronic Vascular Inflammation by Stimulation of Endothelial Autophagy. Circ/ Res. 2018;122:417–432. doi: 10.1161/CIRCRESAHA.117.311428. [DOI] [PubMed] [Google Scholar]
- 427.Lesizza P., Prosdocimo G., Martinelli V., Sinagra G., Zacchigna S., Giacca M. Single-Dose Intracardiac Injection of Pro-Regenerative MicroRNAs Improves Cardiac Function After Myocardial Infarction. Circ. Res. 2017;120:1298–1304. doi: 10.1161/CIRCRESAHA.116.309589. [DOI] [PubMed] [Google Scholar]
- 428.Cunha P., Romao A.M., Mascarenhas-Melo F., Teixeira H.M., Reis F. Endocannabinoid system in cardiovascular disorders—New pharmacotherapeutic opportunities. J. Pharm. Bioallied. Sci. 2011;3:350–360. doi: 10.4103/0975-7406.84435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 429.Godlewski G., Alapafuja S.O., Batkai S., Nikas S.P., Cinar R., Offertaler L., Osei-Hyiaman D., Liu J., Mukhopadhyay B., Harvey-White J., et al. Inhibitor of fatty acid amide hydrolase normalizes cardiovascular function in hypertension without adverse metabolic effects. Chem. Biol. 2010;17:1256–1266. doi: 10.1016/j.chembiol.2010.08.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 430.Ho W.S., Gardiner S.M. Acute hypertension reveals depressor and vasodilator effects of cannabinoids in conscious rats. Br. J. Pharmacol. 2009;156:94–104. doi: 10.1111/j.1476-5381.2008.00034.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 431.Jarai Z., Wagner J.A., Goparaju S.K., Wang L., Razdan R.K., Sugiura T., Zimmer A.M., Bonner T.I., Zimmer A., Kunos G. Cardiovascular effects of 2-arachidonoyl glycerol in anesthetized mice. Hypertension. 2000;35:679–684. doi: 10.1161/01.HYP.35.2.679. [DOI] [PubMed] [Google Scholar]
- 432.Polak A., Harasim-Symbor E., Malinowska B., Kasacka I., Pedzinska-Betiuk A., Weresa J., Chabowski A. The effects of chronic FAAH inhibition on myocardial lipid metabolism in normotensive and DOCA-salt hypertensive rats. Life Sci. 2017;183:1–10. doi: 10.1016/j.lfs.2017.06.019. [DOI] [PubMed] [Google Scholar]
- 433.Wang Y., Kaminski N.E., Wang D.H. Endocannabinoid regulates blood pressure via activation of the transient receptor potential vanilloid type 1 in Wistar rats fed a high-salt diet. J. Pharmacol. Exp. Ther. 2007;321:763–769. doi: 10.1124/jpet.106.112904. [DOI] [PubMed] [Google Scholar]
- 434.Wheal A.J., Bennett T., Randall M.D., Gardiner S.M. Cardiovascular effects of cannabinoids in conscious spontaneously hypertensive rats. Br. J. Pharmacol. 2007;152:717–724. doi: 10.1038/sj.bjp.0707410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 435.Farah C., Michel L.Y.M., Balligand J.L. Nitric oxide signalling in cardiovascular health and disease. Nat. Rev. Cardiol. 2018;15:292–316. doi: 10.1038/nrcardio.2017.224. [DOI] [PubMed] [Google Scholar]
- 436.Camargo A.B., Manucha W. [Potential protective role of nitric oxide and Hsp70 linked to functional foods in the atherosclerosis] Clin. Investig. Arterioscler. 2017;29:36–45. doi: 10.1016/j.artere.2016.05.002. [DOI] [PubMed] [Google Scholar]
- 437.Mazzei L., Docherty N.G., Manucha W. Mediators and mechanisms of heat shock protein 70 based cytoprotection in obstructive nephropathy. Cell Stress Chaperones. 2015;20:893–906. doi: 10.1007/s12192-015-0622-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 438.Guo Z., Liu Y.X., Yuan F., Ma H.J., Maslov L., Zhang Y. Enhanced vasorelaxation effect of endogenous anandamide on thoracic aorta in renal vascular hypertension rats. Clin. Exp. Pharmacol. Physiol. 2015;42:950–955. doi: 10.1111/1440-1681.12450. [DOI] [PubMed] [Google Scholar]
- 439.Stanley C.P., Hind W.H., Tufarelli C., O’Sullivan S.E. The endocannabinoid anandamide causes endothelium-dependent vasorelaxation in human mesenteric arteries. Pharmacol. Res. 2016;113:356–363. doi: 10.1016/j.phrs.2016.08.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 440.Baranowska-Kuczko M., Kozlowska H., Kozlowski M., Schlicker E., Kloza M., Surazynski A., Grzęda E., Malinowska B. Mechanisms of endothelium-dependent relaxation evoked by anandamide in isolated human pulmonary arteries. Naunyn Schmiedeberg’s Arch. Pharmacol. 2014;387:477–486. doi: 10.1007/s00210-014-0961-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 441.Quercioli A., Pataky Z., Vincenti G., Makoundou V., Di Marzo V., Montecucco F., Carballo S., Thomas A., Staub C., Steffens S., et al. Elevated endocannabinoid plasma levels are associated with coronary circulatory dysfunction in obesity. Eur. Heart J. 2011;32:1369–1378. doi: 10.1093/eurheartj/ehr029. [DOI] [PubMed] [Google Scholar]
- 442.Montecucco F., Matias I., Lenglet S., Petrosino S., Burger F., Pelli G., Braunersreuther V., Mach F., Steffens S., Di Marzo V. Regulation and possible role of endocannabinoids and related mediators in hypercholesterolemic mice with atherosclerosis. Atherosclerosis. 2009;205:433–441. doi: 10.1016/j.atherosclerosis.2008.12.040. [DOI] [PubMed] [Google Scholar]
- 443.Steffens S., Veillard N.R., Arnaud C., Pelli G., Burger F., Staub C., Karsak M., Zimmer A., Frossard J.L., Mach F. Low dose oral cannabinoid therapy reduces progression of atherosclerosis in mice. Nature. 2005;434:782–786. doi: 10.1038/nature03389. [DOI] [PubMed] [Google Scholar]
- 444.Rajesh M., Mukhopadhyay P., Hasko G., Liaudet L., Mackie K., Pacher P. Cannabinoid-1 receptor activation induces reactive oxygen species-dependent and -independent mitogen-activated protein kinase activation and cell death in human coronary artery endothelial cells. Br. J. Pharmacol. 2010;160:688–700. doi: 10.1111/j.1476-5381.2010.00712.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 445.Abdel-Salam O. Gastric acid inhibitory and gastric protective effects of Cannabis and cannabinoids. Asian Pac. J. Trop. Med. 2016;9:413–419. doi: 10.1016/j.apjtm.2016.04.021. [DOI] [PubMed] [Google Scholar]
- 446.Palomba L., Silvestri C., Imperatore R., Morello G., Piscitelli F., Martella A., Cristino L., Di Marzo V. Negative Regulation of Leptin-induced Reactive Oxygen Species (ROS) Formation by Cannabinoid CB1 Receptor Activation in Hypothalamic Neurons. J. Biol. Chem. 2015;290:13669–13677. doi: 10.1074/jbc.M115.646885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 447.Nishiga M., Wang D.W., Han Y., Lewis D.B., Wu J.C. COVID-19 and cardiovascular disease: From basic mechanisms to clinical perspectives. Nat. Rev. Cardiol. 2020;17:543–558. doi: 10.1038/s41569-020-0413-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 448.Huang C., Wang Y., Li X., Ren L., Zhao J., Hu Y., Zhang L., Fan G., Xu J., Gu X., et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395:497–506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 449.Bosso M., Thanaraj T.A., Abu-Farha M., Alanbaei M., Abubaker J., Al-Mulla F. The Two Faces of ACE2: The Role of ACE2 Receptor and Its Polymorphisms in Hypertension and COVID-19. Mol. Ther. Methods. Clin. Dev. 2020;18:321–327. doi: 10.1016/j.omtm.2020.06.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 450.Li J., Wang X., Chen J., Zhang H., Deng A. Association of Renin-Angiotensin System Inhibitors with Severity or Risk of Death in Patients with Hypertension Hospitalized for Coronavirus Disease 2019 (COVID-19) Infection in Wuhan, China. JAMA Cardiol. 2020;5:825–830. doi: 10.1001/jamacardio.2020.1624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 451.Reynolds H.R., Adhikari S., Pulgarin C., Troxel A.B., Iturrate E., Johnson S.B., Hausvater A., Newman J.D., Berger J.S., Bangalore S., et al. Renin-Angiotensin-Aldosterone System Inhibitors and Risk of Covid-19. N. Engl. J. Med. 2020;382:2441–2448. doi: 10.1056/NEJMoa2008975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 452.Vaduganathan M., Vardeny O., Michel T., McMurray J.J.V., Pfeffer M.A., Solomon S.D. Renin-Angiotensin-Aldosterone System Inhibitors in Patients with Covid-19. N. Engl. J. Med. 2020;382:1653–1659. doi: 10.1056/NEJMsr2005760. [DOI] [PMC free article] [PubMed] [Google Scholar]