Abstract
A slow rate of new drug discovery and higher costs of new drug development attracted the attention of scientists and physicians for the repurposing and repositioning of old medications. Experimental studies and off-label use of drugs have helped drive data for further studies of approving these medications. A deeper understanding of the pathogenesis of depression encourages novel discoveries through drug repurposing and drug repositioning to treat depression. In addition to reducing neurotransmitters like epinephrine and serotonin, other mechanisms such as inflammation, insufficient blood supply, and neurotoxicants are now considered as the possible involved mechanisms. Considering the mentioned mechanisms has resulted in repurposed medications to treat treatment-resistant depression (TRD) as alternative approaches. This review aims to discuss the available treatments and their progress way during repositioning. Neurotransmitters’ antagonists, atypical antipsychotics, and CNS stimulants have been studied for the repurposing aims. However, they need proper studies in terms of formulation, matching with regulatory standards, and efficacy.
Keywords: clinical trials, depression, major depressive disorder, new drugs, repurposing, repositioning, strategies
1. Introduction
Despite the high rate of technological progress and improvements in knowledge of different diseases, the discovery of new medications demonstrated a lower speed [1].
As the regulatory requirements for bringing a new medication to the market is becoming more challenging to meet, medication cost increases globally [2].
Drug repositioning, also called drug repurposing or re-tasking, is a promising strategy to introduce new indications for other therapeutic goals for an available drug in the market [3]. Since the safety profile of these medications was studied thoroughly before, the development of their formulation has been analyzed, and the medicines successfully passed the preclinical and clinical steps, the risk of failure decreases significantly [4]. To apply a drug repositioning strategy, three main steps are needed to be concluded; first, a molecule/substance should be suggested for the mentioned indication; second, preclinical models, including animal and computational models, should be assessed and last the efficacy of medication should be analyzed [5].
As a significant mental disorder, major depressive disorder (MDD) affects approximately 264 million globally [6]. Due to the insufficient therapeutic response of patients to the available medications, the need for new medicines has attracted scientists worldwide [7]. Various mechanisms have been associated with the prevalence of MDD, and these mechanisms directly influence medication selection. Changes in inflammatory biomarkers, neurotransmitters, age-related, and genetic factors are among the mechanisms constituting the indications of medications [8]. Moreover, new antidepressant medications usually act on multiple intra- and extra-cellular markers, indicating their poly-pharmacology indications [9]. This review aims to provide a complete insight into the drug repositioning strategy, especially the candidates that could be beneficial in managing MDD.
2. Drug Repurposing
2.1. History
Drug repurposing is the procedure of finding new indications for approved or investigational medications [10,11]. Investigational medicines may have had a desirable safety profile in phase I/II clinical trials [12] but never reached the market [11,12] due to reasons unrelated to safety [12], such as lack of efficacy [11]. Drug repurposing can emerge in different forms like repositioning, reformulation, and combination [13,14]. Drug repurposing was serendipitous and accidental in the past; whenever a medication had shown an off-target or a new on-target effect, it was investigated for commercial exploitation. [10]. So far, the most successful repurposed drugs have been found accidentally, and no systematic approach has been involved in the process [10,15]. Sildenafil citrate was an antihypertensive medication that got repurposed as retrospective clinical data analysis showed its positive effect on erectile dysfunction [10,16]. The year sildenafil got marketed by Pfizer for erectile dysfunction under the name Viagra® [17], it held a 47% share of this problem’s market, and the total sales were USD 2.05 billion worldwide [10]. Thalidomide is another well-known instance causing many severe skeletal birth defections in children whose mothers had taken this medication during the first trimester of pregnancy [1,10]. In consequence, thalidomide was withdrawn for four years [10]. In 1964, thalidomide was fortuitously recognized to be effective in erythema nodosum leprosum (ENL) [18]. Decades later, in 1999, thalidomide was discovered to be effective in multiple myeloma [19]. The positive outcome in multiple myeloma led to further derivative developments like lenalidomide [10]. Bupropion, an antidepressant medication got approved by the United States Food and Drug Administration (US-FDA) for smoking cessation [13], botulinum toxin A, the compound used for eye muscle disorders with cosmetic impacts [18] and minoxidil, the antihypertensive medication, became established for pattern hair loss in male and female [17,20] are some instances of well-known repurposed drugs [12]. Iproniazid, an antitubercular compound, was the first medication got reported for its antidepressant effect. This compound showed euphoria, psychostimulation, increased appetite, and improved sleep as the side effects [21]. The story of finding D-lysergic acid diethylamide (LSD) psychedelic effects is another exciting example of serendipitous discovery in the field of psychiatry. LSD was first synthesized in 1938, but it did not show considerable physiological effects in animal testing. LSD’s strong and extraordinary influences on the mind were accidentally discovered for the first time in 1943 [22]. Thirteen medications have been repurposed for depression or bipolar depression treatment by 2017 [13].
2.2. Different Types of Drug Repurposing
Drug reformulation, repositioning, and combination are counted as different drug repurposing/repositioning [13,14].
2.2.1. Drug Repositioning
It finds new indications for a medication that already has other therapeutic indications [13,14]. For instance, mifepristone, an anti-progesterone drug with an initial indication for abortion, was experimentally effective in psychotic depression [3,23].
2.2.2. Drug Reformulation
It is about using a medication in a new dosage form [13,14]. The new formulation can both be taken via the same old route or a different route of administration. An example of drug reformulation is formulating ketamine for intranasal and sublingual routes to treat MDD [13].
2.2.3. Drug Combination
It refers to using two or more medications together [13,14] to improve efficacy and safety [13]. For example, using quetiapine and antidepressants in combination leads to the increased effect of antidepressant medications in the elderly suffering from MDD and cerebrovascular deterioration. In addition, taking anti-inflammatory medicines with antidepressants enhances responses to first-line antidepressants [13].
2.3. Common Approaches
Before the development stage in drug repositioning, three levels should be considered. The first level is discovering appropriate molecules for the desired indication, wherein most drug repurposing approaches are related to it. The second one is evaluating effects in preclinical experiments. The last is the appraisal of efficacy in phase II clinical trials, given that safety has been approved in phase I clinical trials for the original use [10].
Repositioning approaches are divided into two major groups: computational and experimental strategies [10,12,16]. These are growingly being used together [10,16] and will be separately discussed as follows. Explanations, pros, and cons of the drug repurposing approaches are summarized in Table 1. In addition, examples of each separate approach are indicated in Table 2.
Table 1.
Explanations, advantages and disadvantages of approaches commonly used for drug repurposing.
| Explanation | Pros and/or Cons/Ref | |||||
|---|---|---|---|---|---|---|
| Common Approaches | Computational Approaches | Signature Matching | Transcriptomic, Metabolomics, and Proteomics | Transcriptomic | Comparison of Gene Expression in Healthy, Disease-Associated and Medication-Using State | Pros: finding new targets or off-target effects for existing medications [10], finding the new mechanism of action for drugs, involving more genetic level mechanisms in comparison to knowledge-based methods [25,32], low costs, public access to databases [23] Cons: medication-target genes not getting expressed in altered patterns and not being detectable [33] |
| Metabolomics | Recognizing potentially druggable targets in different diseases | Pros: involving more molecular level mechanisms in comparison to knowledge-based methods [25] | ||||
| Proteomics | Applying interaction between medication and proteome | Pros: gaining information about safety, probable medication toxicity, mode of action of small molecule medications [12], involving more genetic and/or molecular level mechanisms in comparison to knowledge-based methods, finding new mechanisms of action [25,32] | ||||
| Chemical structure | Developing networks based on shared chemical features | Cons: the difference between actual results and expectations, variety of physiological effects despite structure resemblance [10], possibility of happening alterations in structure due to biological activity [16] | ||||
| Adverse event profile | Finding shared targets, proteins, or pathways affected by different medications showing the same adverse effects | Pros: unlike animal models, both therapeutic and adverse effects are observable in humans [15] Cons: problems in extracting information on medication package inserts, lack of proper adverse event profile and causality assessment [10] | ||||
| Computational molecular | Testing different medications on a known target (conventional docking) or checking different targets | Pros: the ability to test all compounds with recognized structure [25] |
||||
| Docking | Match a particular medication (inverse docking) based on complementarity between ligand on target | Cons: difficulty in providing a 3D image of G-protein receptors, lack of eligible database providing appropriate information about targets’ structures, the difference between actual affinity between ligand and target and virtual results, different outcomes of different software packages [10], impossibility of identifying unknown mechanism beyond the known target in conventional docking [25] | ||||
| Genome-wide associated data (GWAS) | Finding genetic variants associated with common diseases and understanding the biology of disease | Pros: advances in technology, the accomplishment of the HGP, reduction in genotyping costs Cons: need for studies to assure in which direction gene variants affect disease, difficulty in identification responsible gene or gene variants due to linkage disequilibrium in gene-rich loci, not providing a comprehensive insight into disease pathophysiology, lack of definite knowledge of the human genome and the possibility of new human genes discovery [10], disease’s complicated genetic basis due to polygenic nature of illness [25] |
||||
| Pathway or network mapping | Building networks for disease or medication based on signature matching data, gene expression pattern, disease pathology, GWAS data, or protein interactions | Pros: narrowing the range of molecules from a large number to few targets [12] | ||||
| Experimental approaches | Retrospective clinical analysis | Electronic health records (EHRs) |
Reviewing and extracting data from structured (diagnosis and pathophysiology data, laboratory test results, and medication prescriptions) and unstructured (of patients’ symptoms reports and imaging data) | Pros: easy access to different resources such as WHO or FAERS database and ability to analyze these data [10], discovering adverse events that were not noticed during drug development by applying Natural Language to EHRs [2] Cons: ethical and legal obstacles, challenges in extracting unstructured data [1] |
||
| Post-marketing surveillance and clinical data | Analyzing post-marketing surveillance and clinical data to extract information | Cons: restriction in accessing to these data due to commercial and ethical reasons [1] | ||||
| Novel sources | Using immortalized human CCLs for screening different compounds, linking EHR data to DNA bank to identify an association between patients’ genome and patients’ illness, using patients’ online self-reported data while taking medicine | Pros: development of sequencing technologies, which helps ones collect more thorough information on each patient’s genetics (2), acceleration in the drug discovery process, reduction in research costs, increase in patient involvement, ability to assess the effectiveness of the in-use medication (3) Cons: Happening alterations that make in vitro results better (1), challenges in using big data and technology for analysis (2), bias in collecting data, threat in patients’ safety in case of self-prescription (3) [10] |
||||
| Binding assays | Realizing interactions between target and ligand by using different methods as chromatography and mass spectroscopy | - | ||||
| Phenotypic screening | Identifying compounds showing disease consequences related effects in model systems, without any prior information about targets | Pros: testing many medications for a therapeutic effect over a complete range of concentration [34], high flexibility for administration to numerous drugs or diseases [32] Cons: not reaching a complete picture by in vitro assays [33] |
||||
CCLs: Cancer cell lines: FAERS: Food and Drug Administration adverse event reporting system; HGP: Human genome project; Ref: Reference; WHO: World Health Organization.
Table 2.
Examples of repurposed/suggested repurposing medications for different types of depression with their repurposing approach.
| Medication Name | Repurposing Approach/Ref | New Indication Suggested by Article, or Investigational/FDA Approved/Ref |
|---|---|---|
| Atypical antipsychotics Quetiapine Aripiprazole Brexpiprazole |
Retrospective clinical analysis (PM surveillances and CD) [13] | MDD, BP1 depressive episodes (FA) [8] |
| NA | MDD (FA) [9] | |
| NA | MDD (FA) [35] | |
| Mecamylamine | Computational molecular docking [23] | Depression (SA) [23], MDD (INV) [17,19,20,36,37] |
| Cyproheptadine | Computational molecular docking [26] | NA |
| Dextromethorphan | Computational molecular docking [13] | MDD (INV) [38,39,40,41] |
| Pregabalin | GWAS [23] | MDD (SA) [23] |
| Gabapentin | GWAS [23] | MDD (SA) [23] |
| Cycloserine | GWAS [23] | BP depression (INV) [42], MDD (SA, INV) [23,36] |
| Risperidone | GWAS [23] | Extrapyramidal symptoms, suicidal ideation (INV) [43] |
| Cannabidiol | Pathway or network mapping [21] | MDD (SA) [21] |
| N-acetyl-l-cysteine | Pathway or network mapping [21] | MDD (SA) [21] |
| Sho-saiko-to | Pathway or network mapping [21] | NA |
| Spermine | Pathway or network mapping [21] | TRD (SA) [21] |
| Nimodipine | Pathway or network mapping [21] | Vascular depression in old patients (SA) [13] |
| Scopolamine | GWAS, Pathway or network mapping [13] | MDD (INV) [44,45] |
| Anti-inflammatory medications | Retrospective clinical analysis (PM surveillances and CD) [13] |
NA |
| Valproic acid | Retrospective clinical analysis (PM surveillances and CD) [13] |
Resistant depression (SA) [13] |
| Zinc | Retrospective clinical analysis (PM surveillances and CD) [21] |
MDD (INV) [46] |
| Pramipexole | Retrospective clinical analysis (EHRs) [21] | MDD (SA, INV) [21,47] |
| Telmisartan | Retrospective clinical analysis (EHRs) [21] | MDD (SA) [21] |
| Metformin | Retrospective clinical analysis (EHRs) [26] | MDD (INV) [48] |
| Phenothiazines | Retrospective clinical analysis (PM surveillances and CD) [26] | NA |
| Cabergoline | Signature matching (adverse event profile) [15] | NA |
| Modafinil | Signature matching (adverse event profile) [15] | MDD (INV) [49], depressive episode in BP1 disorder (INV) [50], major depressive episode in BP1 disorder (INV) [51] |
| Pergolide | Signature matching (adverse event profile) [15] | NA |
| Phenytoin | Signature matching (adverse event profile) [15] | NA |
| Chlorcyclizine | Signature matching (chemical structure) [26] | NA |
| Vorinostat | Signature matching (transcriptomic) [26] | Depression, anxiety, schizophrenia (SA) [26] |
| Statins | Signature matching (transcriptomic) [21] | NA |
| Ketamine (esketamine) | Signature matching (transcriptomic) [13] | MDD and bipolar depression, Depressive symptoms in adults with MDD with acute suicidal ideation or behavior (FA) [52] |
| Pioglitazone | Signature matching (transcriptomic) [13] | MDD, a major depressive episode in bipolar disorder (SA) [13] |
BP: Bipolar; CD: Clinical data; FA: FDA approved; GWAS: Genome-wide associated studies; INV: Investigational; MDD: Major depressive disorder; NA: Not available; PM: Post-marketing; Ref: Reference; SA: Suggested by articles; TRD: Treatment-resistant depression.
2.3.1. Computational Approaches
Computational approaches consist of data analysis. These data can be obtained from different resources. For example, gene expression, chemical structure [10,12], or electronic health records (EHRs) can all be kinds of data resources [10]. In comparison with experimental approaches, computational approaches have lower expenses and fewer barriers [3].
Signature Matching
This method compares exclusive features (signature) of medication with another medication or disease. Three different types of data could be used as resources for extracting medication characteristics: chemical structure, adverse event profiles, proteomics, transcriptomic, and metabolomics, which are explored aptly in the following [10]:
Transcriptomic: This technique compares gene expression in a healthy state, disease-associated state, and medication-using state. If a medication can reverse the expression pattern of the genes related to disease phenotype, it will probably also revert the disease phenotype itself [10,12,16,23,24,25].
An example of this approach is that ketamine improves mood by modulating miRNAs like miR-598-5p and miR-451 [13]. Histone deacetylase (HDAC) inhibitors like vorinostat are promising drug repositioning targets for depression, anxiety and schizophrenia treatment due to their role in affecting gene expression [26]. Peroxisome proliferator-activator receptor (PPAR-γ) agonists, especially pioglitazone, have significant antidepressant outcomes in MDD and major depressive episodes of bipolar disorder due to their role in adjusting responsible gene expressions [13]. HMG-CoA reductase inhibitors (statins) are PPAR-α ligands that increase the expression of some neuronal growth factors. Randomized controlled trials have suggested that they possess beneficial effects in combination with selective serotonin reuptake inhibitors (SSRIs) [21].
Metabolomics: Metabolomics is the study of all chemical procedures in the body [27]. A drug can be shared between two different disease treatments with similar pathophysiology [5]. This approach helps us to gain a comprehensive idea about the molecular processes involved in disease pathophysiology and finding how close our preclinical models to reality are [28]. Nuclear magnetic resonance and mass spectroscopy are two methods for analyzing the metabolome [29].
Proteomics: Most medications apply their therapeutic effects by interacting with protein targets, and it is crucial to understand these interactions for drug development [12].
Chemical Structure: In this method, networks are made based on the shared chemical features [10] as similarity in chemical structure may lead to the same biological activity [10,16]. As an example, chlorcyclizine belongs to the phenylpiperazine class. This class includes many antipsychotic and antidepressant medications and chemical structure similarities between these medications and chlorcyclizine make it likely to possess the same effects [26].
Adverse Event Profiles: A hypothesis suggests that two different medications showing the same adverse effects might affect a shared target, protein, or pathway [10,16]. As well, a medication’s adverse effect resembling a disease phenotype can imply a shared pathway or physiology between the drug and the illness [10,15,16]. In addition, if two treatments for one disease with different mechanisms demonstrate the same uncommon adverse effect, there may be a shared underlying mechanism that links adverse events and therapeutic effects [30]. Side effects are more helpful in predicting drug indications than chemical structure or protein targets. It is possible to extract adverse events data from chemical structures if a drug has not reached the clinical trial level [31].
Cabergoline, an ergot derivative dopamine agonist, showed delusion adverse events and got recognized to have antidepressant-like effects. Pergolide, another dopamine agonist, demonstrated antidepressant effects in Parkinson’s disease. Modafinil is a narcolepsy medication that may be effective in depression treatment in combination with fluoxetine. Phenytoin, the famous anticonvulsant medicine, can be efficient in depression. This effectiveness is a result of hyperacusis, the phenytoin adverse effect [15].
Computational Molecular Docking
The basis of this approach is complementarity between ligand and target [10,23]. In conventional docking, the target involved in the disease is already known, and different medications get tested. In inverse docking, a set of targets are studied to check if they match particular medicines [10]. Computational molecular docking indicates that dextromethorphan shows an antidepressant effect with rapid onset of action during the first administration days due to involving glutamatergic receptors [13]. Cyproheptadine was hypothesized to improve depression based on its potential ability to be a serotonin receptor (5-HT2) antagonist [26]. Computational molecular docking suggests that mecamylamine, a nicotinic receptor antagonist, might be effective in depression treatment [23].
Genome-Wide Associated Studies (GWAS)
This method is proceeded on finding genetic variants associated with common diseases and understanding the biology of disease. In addition, these data can result in recognition of shared targets between conditions [10].
GWAS suggest pregabalin, gabapentin, nitrendipine, alizapride, mesoridazine, levonorgestrel, diethylstilbestrol, papaverine, scopolamine, ketoconazole, arcaine sulfate, ifenprodil, cycloserine, risperidone, and sulpiride all can be repurposed for MDD [23].
Pathway or Network Mapping
This approach is building networks based on signature matching data, protein interactions [10,25,32], gene expression pattern [10,25], disease pathology, or GWAS data [10] to find similarity or relation between medication and disease [25]. Some disease-associated genes are not appropriate druggable targets. Therefore, constructing and analyzing such networks could be a way to find upstream or downstream genes which can be used for drug repurposing [10]. Pathway mapping based on disease pathogenesis showed that nimodipine, a calcium channel blocker, makes antidepressants effective in old patients suffering from vascular depression. Scopolamine, the muscarinic antagonist, possesses rapid antidepressant effects since the cholinergic system is responsible for the pathogenesis of mood disorders [13]. Cannabidiol is a propitious agent for MDD due to its effects on involved pathways in this disorder. Sho-saiko-to, a traditional Chinese medicine, showed antidepressant effects in mice upon its influence on the serotonergic system in the central nervous system. Medications that inhibit p38 mitogen-activated protein (p38-MAPK) signaling pathways such as neflamapimod may have desirable effects on depression as the p38-MAPK pathway is engaged in many cellular processes, especially neuro-inflammation. Spermine is useful in treatment-resistant depression (TRD), as it is a glutamatergic receptor modulator. N-acetyl-l-cysteine (NAC), the glutathione precursor, positively affects the different mechanisms involved in depression and is a beneficial nutraceutical for adding to antidepressant medications in MDD treatment [21].
2.3.2. Experimental Approaches
Retrospective Clinical Analysis
The main idea of this approach is reviewing and extracting valuable data from different resources such as EHRs, post-marketing surveillance, and clinical trials. This process would result in repositioning and using a medication for a dissimilar indication or finding an indication for a drug that had failed for its initial purpose [10].
EHRs: EHRs data are subdivided into structured (diagnosis and pathophysiology data, laboratory test results, and medication prescriptions) and unstructured (patients’ symptoms reports and imaging data) groups [10].
Analyzing data gained from the national health insurance of Taiwan research database showed metformin is a promising target for drug repositioning for depression and anxiety [26]. The results of observational or case-control studies indicated a lower risk of MDD in patients using angiotensin-converting enzyme inhibitors (ACEIs) like telmisartan in comparison with other antihypertensive medications. Case reports also demonstrated that pramipexole, a relatively new dopamine receptor agonist, has potential effects in treating MDD. An observational study on 82,643 women revealed the relation between higher flavonoid intake and lower risk of MDD. Last but not least, a meta-analysis of 17 observational studies suggested the association between depression and zinc deficiency [21].
Post-Marketing Surveillance and Clinical Data: Retrospective clinical data analysis showed that using anti-inflammatory medications, especially celecoxib, with antidepressants improves the responses to first-line antidepressants. Clinical data claims that valproic acid enhances the effects of antidepressants in resistant depression patients. Based on clinical data, quetiapine co-administered with antidepressants improves the outcomes in patients with MDD and cerebrovascular deterioration [13]. Reviewing prior literature has shown that phenothiazines have anti-depressive effects resembling tricyclic antidepressants. Atypical antipsychotics can also help treat depression as adjunctive or primary therapy based on a meta-analysis [28]. Taking antidepressants combined with zinc caused decreased depressive symptoms than antidepressants alone in randomized control trials [21].
Novel Sources
This approach consists of three methods. The first one is using immortalized human cancer cell lines (CCLs) for screening different compounds to examine if pharmacological and genetic data match. The second method links EHR data to DNA biobanks; thereby, identifying the association between the patient’s genome and the patient’s illness. The third novel resource is patients’ online self-reported data about their condition while taking medicine [10].
Binding Assays
Binding assessments help us realize target and ligand interactions. For example, affinity chromatography, mass spectroscopy, and cellular thermos ability assay (CETSA) techniques are three methods used in this approach [10].
Phenotypic Screening
This approach attempted to identify compounds showing effects of disease consequences in model systems without any earlier information about targets they affect [10].
2.4. Advantages
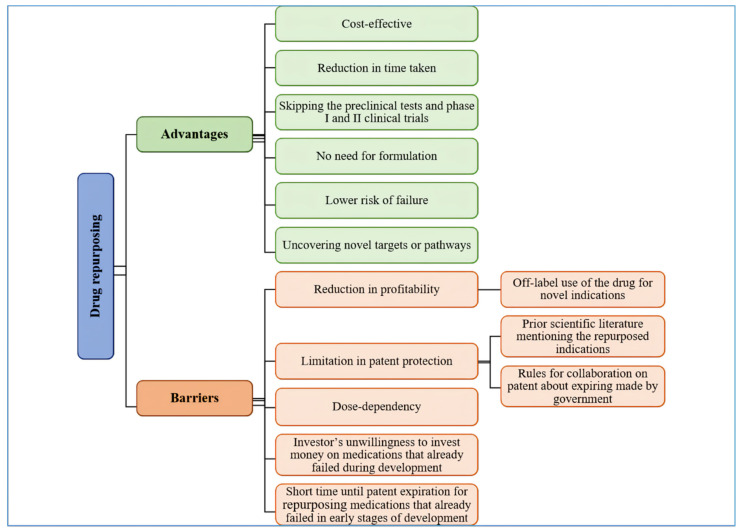
Drug discovery is a high-cost and lengthy process. Bringing a new medication to market takes 13–15 years and costs USD 2–3 billion [10,21,25]. Although the expenses are increasing, the number of approved medications has remained constant or even decreased through the past years [22,25]. Moreover, demands in therapeutic fields are growing, and traditional drug discovery cannot answer these needs [13]. In the case of psychiatric medications, it should be noted that this field has not been developed enough over time [26]. Drug repurposing is cost-effective and reduces the time taken to get a new medication to market [10,11,21] as it costs on average USD 300 million and takes about 6.5 years [10,21]. The preclinical tests [10,16] and phase I and II clinical trials can be skipped in drug repurposing if these steps have already passed for other indications and safety has been approved [10]. This is why drug repurposing may shorten the time needed and expenses as mentioned above [1,10].
Furthermore, if the formulation is appropriate for the new indication, there is no need for formulation development. This can also be another helping hand for reaching the stated aims. Another great pro of drug repurposing is the lowered risk of failure due to approved sufficient safety [10,16]. At last, drug repurposing may uncover novel targets or pathways in treating a disease, which can be exploited further [10].
2.5. Barriers
Although toxicity and safety are not obstacles in drug repurposing, some barriers lead to failures, such as patent consideration, regulatory issues, and organization hurdles. In brief, many of the repurposed uses are already mentioned in the prior scientific literature or clinical data leading to limitations in patent protection. In addition, when an available generic formulation gets repurposed for a new indication, profitability reduces. This reduction happens due to off-label using the medication for novel indications. Governments make some rules for collaboration on patents that are near expiring to save public benefit. Creating a new formulation or dosage forms, developing new derivatives with the same therapeutic effects, or presenting medication in a new geographic region market are strategies for making a profit from the repurposed drugs [10]. Another trouble is that the effect of the medication is dependent on its dose. Therefore, it is necessary to identify the appropriate dose for novel indications during clinical trials [16]. Investments might be another obstacle in repurposing medications that have already failed during the drug development process. This trouble happens due to investors’ unwillingness as they see medication’s failure. In addition, medicines that failed in later stages of drug development have less time until patent expiration for repurposed try. Designing parallel development processes for different indications can lower the risk of failure [1] (Figure 1).
Figure 1.
Advantages and barriers of drug repurposing.
3. Management of Depression
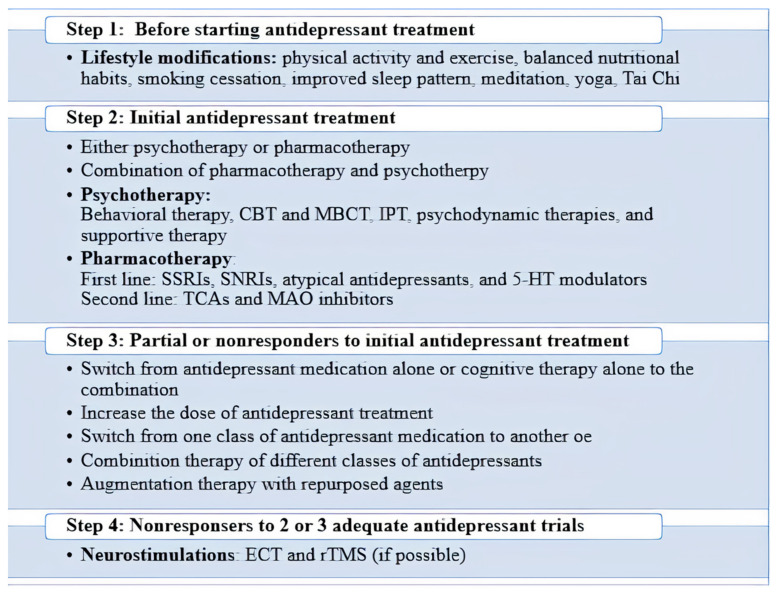
In order to manage a patient diagnosed with MDD, two or three main options, including psychotherapy, pharmacotherapy, and somatic interventions, exist. Some guidelines suggest that those with moderate to severe depression would benefit from both psychotherapy and pharmacotherapy. In a mildly depressed person, treatment could be initially based on psychotherapy, and if needed, switching to medication could be applied after weeks [17]. Physical activity and exercise, balanced nutritional habits, improved sleep patterns, etc., can impact mental health and might be beneficial towards depressive disorders. Training like meditation, yoga, Tai chi, or daily journaling events is another helpful way to reduce stress, leading to the improved mental condition [53] (Figure 2).
Figure 2.
MDD management steps. Abbreviations: CBT: Cognitive behavioral therapy; IPT: Interpersonal psychotherapy; MAO: Monoamine oxidase; MBCT: Mindfulness-based cognitive therapy; MDD: Major depressive disorder; SSRI: Selective serotonin reuptake inhibitors.
In mild-to-moderate depression, psychotherapy has proved to be adequate and comparable to pharmacological therapies. Many experts suggest different types of psychotherapy like cognitive-behavioral therapy, behavioral activation therapy, and interpersonal psychotherapy. However, for severe forms of depressive disorder, antidepressant drugs have appeared to be much more effective by possessing a more rapid onset of action. Furthermore, psychotherapy is often used in those who have shown a response to antidepressants to prevent its relapse. Overall, the combination of both therapies is suggested and has been demonstrated to be more effective to either alone [48]. It is shown that the beneficial effects of different psychotherapy methods can last for at least one year after the treatment process. However, many people refuse this choice due to its high costs, lack of time, and the recent issue of Coronavirus disease-2019 (COVID-19). As solutions, attending some group psychotherapies to reduce the costs, setting online or over the phone sessions are valued, although all patients still do not believe in such ways [17]. Somatic intervention is another non-pharmacological option for the treatment of MDD. Electroconvulsive therapy (ECT) and repetitive transcranial magnetic stimulus (rTMS) are some noninvasive examples suggested to be beneficial for patients who have already failed at least one antidepressant trial. Vagus nerve stimulation is a US-FDA-approved surgical and invasive procedure for the management of TRD, which of course, carries its risks [53].
3.1. Pharmacotherapy
After a patient is diagnosed with MDD, a psychiatrist can start pharmacologic treatment to symptom remission. Antidepressants take approximately 3–4 weeks to exert their effects, although there is always the risk of relapse or recurrence of mood episodes even after the therapy. Choosing the first-line treatment for a patient depends on multiple factors, including age, concurrent medical conditions or psychiatric state, adverse effect profiles of the drug and its interactions, ease of access, cost, convenience and patient’s preference, safety in overdose, etc. Another essential issue is the patient’s initial responses to antidepressants (if taken) and the family history [53].
3.1.1. Current Antidepressant Medications
Today, according to Katzung Basic and Clinical Pharmacology’s last edition, five (or six) main antidepressant categories exist and exert their effects through various molecular targets and mechanisms of action. These categories include selective serotonin reuptake inhibitors (SSRIs), serotonin-norepinephrine reuptake inhibitors (SNRIs) with two subgroups of selective serotonin-norepinephrine reuptake inhibitors, and tricyclic antidepressants (TCAs), 5-HT2 receptor modulators, tetracyclic and unicyclic antidepressants, monoamine oxidase inhibitors (MAOIs) [54]. The sixth category consists of some various antidepressant medications such as tianeptine (alpha-amino-3-hydroxy-5-methyl-4-isoxazole propionic acid (AMPA) and N-methyl-D-aspartate (NMDA) receptors modulator) [55], reboxetine (a selective inhibitor of norepinephrine reuptake), agomelatine (an agonist for melatonin (MT1 and MT2) and serotonin (5-HT2C) receptors) [19], ademetionine (a major methyl donor, required for the synthesis of several neurotransmitters) [56] and agmatine (an NMDA receptor inhibitor) [57], etc.
3.1.2. Repurposed Drugs for MDD
Plenty of repurposed agents for depression are studied or have gotten US-FDA approval for use in the clinic from different pharmacological categories. These categories vary from some central nervous system (CNS)-related medications like the second generation (atypical) antipsychotics, NMDA receptor antagonists and anesthetics, GABA receptor modulators, dopamine agonists, anticholinergic agents, CNS stimulants, anticonvulsant agents, histamine antagonists and ergot derivatives to even some unrelated ones such as thyroid products, antidiabetic agents, anti-inflammatory agents, antibiotics, HMG-CoA reductase inhibitors, calcium channel blockers, angiotensin-converting enzyme inhibitors, antineoplastic agents and some nutritional supplements. These agents exert their antidepressant effects through various pathways due to their pharmacological category. The complete list of repurposed drugs for MDD with a particular focus on their mechanism of action, significant adverse effects, contraindications, and dosages (if available) are provided in Table 3. Moreover, related clinical trials studying their effects on MDD in real-world settings are summarized in Table 4.
Table 3.
Pharmacological and clinical profile of repurposed medications for MDD.
| Medication/Bioactive Compound Brand Name/ Ref |
US-FDA Approval | Pharmacological Category/Mechanism of Action | Dosage | Significant Adverse Effects | Contraindications |
|---|---|---|---|---|---|
|
Aripiprazole Abilify® [58] |
Bipolar disorder, irritability associated with autistic disorder, MDD, TRD, schizophrenia, Tourette disorder | Partial agonist at the D2 and 5-HT1A receptors, an antagonist at the 5-HT2A/Second-generation (atypical) antipsychotic | MDD and TRD as an adjunctive treatment. Oral: 2 to 5 mg/day | >10%: decreased HDL-C, increased LDL-C, increased serum cholesterol, increased serum TG, weight gain, akathisia, headache, increased serum glucose, constipation, nausea, and vomiting | Hypersensitivity to aripiprazole or any component of the formulation |
| Increase dose based on response in 5 mg increments up to a maximum of 15–20 mg/day | |||||
|
Brexanolone Zulresso® [59] |
Postpartum depression (PPD) in adults |
The mechanism of action is not fully understood, but is thought to be related to its positive allosteric modulation of GABAA receptors/GABAA receptor-positive modulator |
Postpartum depression. IV: 0 to 4 h: 30 mcg/kg/h 4 to 24 h: 60 mcg/kg/h 24 to 52 h: 90 (or 60) mcg/kg/h 52 to 56 h: 60 mcg/kg/h 56 to 60 h: 30 mcg/kg/hour |
>5%: sedation/somnolence, dry mouth, loss of consciousness, and flushing/hot flush | No contraindications were listed in the US-FDA monograph |
|
Brexpiprazole Rexulti® [60] |
MDD, schizophrenia | Partial agonist activity for 5-HT1A and D2 receptors and antagonist activity for 5-HT2A receptors/Second-generation (atypical) antipsychotic | MDD as an adjunct therapy to antidepressants. Oral: 0.5 mg or 1 mg once daily; titrate to 1 mg once daily, followed by 2 mg once daily; maximum daily dose: 3 mg | >10%: increased serum TG, weight gain, akathisia | Hypersensitivity (e.g., anaphylaxis, facial swelling, rash, urticarial) to brexpiprazole or any component of the formulation |
|
Cabergoline Dostinex® [61] |
Hyperprolactinemic disorders | Long-acting dopamine receptor agonist with a high affinity for D2 receptors, a potent 5-HT2B-receptor agonist/Ergot derivative | NA | Headache, dizziness, nausea | Known hypersensitivity to cabergoline, ergot derivatives, or any component of the formulation, uncontrolled hypertension; history of cardiac valvular disorders, history of pulmonary, pericardial, or retroperitoneal fibrotic disorders |
|
Cannabidiol Epidiolex® [62] |
Treatment of seizures associated with Lennox-Gastaut syndrome or Dravet syndrome in patients with 2 years of age and older | The precise mechanisms of its anticonvulsant effect in humans are unknown; however, it does not appear to exert its effects through interaction with cannabinoid receptors/Anticonvulsant, cannabinoid | NA | >10%: somnolence, decreased appetite, diarrhea, transaminase elevations, fatigue, malaise and asthenia, rash, insomnia, sleep disorder and poor quality sleep, infections | Hypersensitivity to cannabidiol or any of the ingredients in Epidiolex® |
|
Celecoxib CeleBREX® [63] |
Acute pain, ankylosing spondylitis, juvenile idiopathic arthritis, osteoarthritis, primary dysmenorrhea, rheumatoid arthritis | Inhibits prostaglandin synthesis by decreasing COX-2 enzyme/Analgesic, nonopioid, NSAID, COX-2 selective | NA | Serious cardiovascular thrombotic events, myocardial infarction, and stroke increased the risk of serious gastrointestinal adverse events, including bleeding, ulceration, and perforation of the stomach or intestines | Hypersensitivity to celecoxib, sulfonamides, aspirin, other NSAIDs, or any component of the formulation |
|
Cycloserine Seromycin® [64] |
Tuberculosis, UTI | Inhibits bacterial cell wall synthesis by competing with amino acid (D-alanine) for incorporation into the bacterial cell wall/Antibiotic, antitubercular agent | NA | Cardiac arrhythmia, cardiac failure, coma, confusion, dizziness, drowsiness, dysarthria, headache, hyperreflexia, paresis, paresthesia, psychosis, restlessness, seizure, vertigo, skin rash, cyanocobalamin deficiency, folate deficiency, increased liver enzymes, hypersensitivity reaction, tremor | Hypersensitivity to cycloserine or any component of the formulation, epilepsy, depression, severe anxiety, or psychosis, severe renal insufficiency, excessive concurrent use of alcohol |
|
Cyproheptadine Euro-Cyproheptadine®, PMS-Cyproheptadine® [65] |
Allergic conditions | Potent antihistamine and serotonin antagonist with anticholinergic effects competes with histamine H1 receptors/First, generation histamine H1 antagonist, a piperidine derivative | NA | Extrasystole, hypotension, palpitations, tachycardia, ataxia, chills, confusion, dizziness, drowsiness, euphoria, excitement, fatigue, hallucination, headache, hysteria, insomnia, irritability, nervousness, neuritis, paresthesia, restlessness, sedation, seizure, vertigo, etc. | MAO inhibitor therapy, close angle glaucoma, stenosing peptic ulcer, symptomatic prostatic hypertrophy, bladder neck obstruction, pyloroduodenal obstruction, elderly, debilitated patients |
|
Dextromethorphan Buckleys cough®, Creomulsion adult®, Cough DM®, Delsym®, etc. [66] |
Cough suppressant | Decreases the sensitivity of cough receptors and interrupts cough impulse transmission by depressing the medullary cough center through sigma receptor stimulation/Antitussive, NMDA receptor antagonist | NA | Dizziness, drowsiness, nervousness, restlessness, gastrointestinal distress, nausea, stomach pain, vomiting | Concurrent administration with or within two weeks of discontinuing MAO inhibitor |
|
Esketamine Spravato® [52] |
TRD, MDD with suicidality |
Nonselective, noncompetitive NMDA receptor antagonist/NMDA receptor antagonist | TRD. Intranasal: induction: 56 mg (may increase to 84 mg) twice weekly for 4 weeks Maintenance: on week 5, the previously established dose (56 or 84 mg) once weekly—on week 9 and onward continue effective dose (56 to 84 mg) once weekly or every 2 weeks MDD with suicidality. Intranasal: 84 mg twice weekly for 4 weeks; may reduce dosage to 56 mg twice weekly |
>5%: dissociation, dizziness, nausea and vomiting, sedation, vertigo, hypoesthesia, anxiety, lethargy, increased blood pressure, feeling drunk | Hypersensitivity to esketamine, ketamine, or any of the excipients, Aneurysmal vascular disease or arteriovenous malformation, intracerebral hemorrhage |
|
Etanercept Enbrel®, Erelzi®, Brenzys® [67] |
Ankylosing spondylitis, plaque psoriasis, polyarticular juvenile idiopathic arthritis, psoriatic arthritis, rheumatoid arthritis | Binds TNF-α and blocks its interaction with cell surface receptors/Antirheumatic, disease-modifying, TNF-α blocking agent | NA | >10%: skin rash, diarrhea, positive ANA titer, antibody development, infection, injection site reaction, respiratory tract infections | Sepsis, hypersensitivity to etanercept, or any component of the formulation |
|
Gabapentin Fanatrex FusePaq®, Gralise®, Gralise Starter®, Neuraptine®, Neurontin® [68] |
Postherpetic neuralgia, seizures | Modulates the release of excitatory neurotransmitters which participate in epileptogenesis and nociception/Anticonvulsant, GABA analog | NA | >10%: dizziness, drowsiness, ataxia, fatigue, viral infections | Hypersensitivity to gabapentin or any component of the formulation |
|
Infliximab Avsola®, Inflectra®, Remicade®, Renflexis® [69] |
Ankylosing spondylitis, Crohn disease, plaque psoriasis, psoriatic arthritis, rheumatoid arthritis, ulcerative colitis | Binds to human TNF-α and interfere with endogenous TNF-α activity/Antirheumatic, disease-modifying, gastrointestinal agent, immunosuppressant agent, monoclonal antibody, TNF-α blocking agent | NA | >10%: abdominal pain, nausea, anemia, increased ALT, antibody development, increased ANA titer, abscess, infection, headache, cough, pharyngitis, sinusitis | Hypersensitivity to infliximab, murine proteins, or any component of the formulation |
|
Ketamine Ketalar® [70] |
Induction and maintenance of general anesthesia | Noncompetitive NMDA receptor antagonist (blocks glutamate)/General anesthetic | Depressive episodes and MDD. IV: 0.5 mg/kg administered over 40 min; 1 to 3 times weekly; may increase dose to 0.75 to 1 mg/kg. Treatment up to 6 weeks (optimal duration of therapy is unknown) | >10%: prolonged emergence from anesthesia (includes confusion, delirium, dreamlike state, excitement, hallucinations, irrational behavior, vivid imagery) | Hypersensitivity to ketamine or any component of the formulation, conditions in which an increase in blood pressure would be hazardous |
|
Liothyronine Cytomel®, Triostat® [71] |
Thyroid disorders, a myxedema coma | Manufactured form of the thyroid hormone triiodothyronine (T3) and exerts its many metabolic effects through control of DNA transcription and protein synthesis/Thyroid product | Antidepressant augmentation. Oral: 25 mcg/day; may be increased to 50 mcg/day after ~1 week. Dose ranges of 20 to 62.5 mcg/day have been studied in clinical trials. |
1% to 10%: cardiac arrhythmia, tachycardia, hypotension, myocardial infraction | Hypersensitivity to liothyronine sodium or any component of the formulation, uncorrected adrenal insufficiency, untreated thyrotoxicosis, concurrent use with artificial rewarming of the patient |
|
Lisdexamfetamine Vyvanse® [72] |
ADHD, binge eating disorder | Converts to the active component dextroamphetamine, a noncatecholamine, sympathomimetic amines that cause a release of dopamine and NE from their storage sites/Central nervous system stimulant | NA | >10%: insomnia, decreased appetite, xerostomia, upper abdominal pain | Hypersensitivity to amphetamine products or any component of the formulation, concurrent use of MAO inhibitor, or within 14 days of the last MAO inhibitor dose |
|
Lithium Lithobid® [73] |
Bipolar disorder | Influence the reuptake of serotonin and/or NE and inhibit 2nd messenger systems involving phosphatidylinositol cycle, increasing glutamate clearance, enhancing the expression of neurotrophic factors (BDNF)/Antimanic agent | MDD and TRD as an adjunctive treatment. Oral: 300 to 600 mg/day in 1 to 2 divided doses; may increase every 1 to 5 days to a target dose of 600 mg to 1.2 g/day in divided doses. Clinical improvement may take up to 6 weeks. | The most significant adverse reaction is lithium toxicity; signs: diarrhea, vomiting, drowsiness, muscular weakness, and lack of coordination |
Hypersensitivity to lithium or any component of the formulation |
|
Metformin D-Care DM2®, Fortamet®, Glucophage®, Glumetza®, Riomet® [74] |
Type 2 diabetes mellitus | Decreases hepatic glucose production, reduces intestinal absorption of glucose and improve insulin sensitivity, increases peripheral glucose uptake and utilization/Antidiabetic agent, biguanide | NA | >10%: diarrhea, flatulence, nausea and vomiting, dyspepsia, abdominal pain, lactic acidosis, vitamin B12 deficiency | Hypersensitivity to metformin or any component of the formulation, severe renal dysfunction, metabolic acidosis |
|
Mecamylamine Vecamyl® [75] |
Hypertension | Inhibits acetylcholine at the autonomic ganglia/Ganglionic blocking agent | NA | Orthostatic hypotension, syncope altered mental status, convulsions, fatigue, paresthesia, sedation decreased libido, anorexia, constipation, glossitis, intestinal obstruction, nausea, vomiting, xerostomia, urinary retention, tremor, weakness, blurred vision, mydriasis, pulmonary edema, pulmonary fibrosis |
Hypersensitivity to mecamylamine or any component of the formulation; mild, moderate, labile hypertension, coronary insufficiency or recent myocardial infarction, uremia, glaucoma, organic pyloric stenosis, coadministration with antibiotics or sulfonamides |
|
Minocycline CoreMino®, Minocin®, Minolira®, Solodyn®, Ximino® [76] |
Different types of infections, acne, etc. | Inhibits bacterial protein synthesis by binding with the 30S and possibly the 50S ribosomal subunit/Antibiotic, tetracycline derivative | NA | 1% to 10%: pruritus, urticaria, dizziness, fatigue, malaise, drowsiness, arthralgia, tinnitus | Hypersensitivity to minocycline, other tetracyclines, or any component of the formulation |
|
Modafinil Provigil®, Alertec® [77,78] |
Narcolepsy, obstructive sleep apnea, shift work sleep disorder | Increase dopamine in the brain by blocking dopamine transporters/Central nervous system stimulant | Antidepressant augmentation for MDD. Oral: 100 mg/day for 3 to 7 days, then increase to 200 mg/day; further, adjust dose based on response and tolerability up to 400 mg/day. | Headache, nausea, nervousness, rhinitis, diarrhea, back pain, anxiety, insomnia, dizziness, dyspepsia |
Contraindicated in patients with known hypersensitivity to modafinil, armodafinil or its inactive ingredients |
|
N-acetyl cysteine Acetadote®, Cetylev®, Parvolex® [79] |
Acetaminophen overdose, mucolytic | Restoring hepatic glutathione, serving as a glutathione substitute, exerts mucolytic effects through its free sulfhydryl group, which opens up the disulfide bonds in the mucoproteins/Antidote, mucolytic agent | NA | >10%: autoimmune disease, anaphylactoid reaction | Hypersensitivity to acetylcysteine or any component of the formulation |
|
Nimodipine Nymalize®, Nimotop® [80] |
Subarachnoid hemorrhage | Inhibits calcium ion from entering the slow channels/Calcium channel blocker, dihydropyridine | NA | 1% to 10%: decrease blood pressure, bradycardia, headache, nausea | Concomitant use with potent CYP3A4 inhibitors |
|
Olanzapine ZyPREXA® [81] |
Acute agitation/aggression associated with psychiatric disorders, bipolar disorder, unipolar MDD, TRD, schizophrenia | Combination of dopamine and serotonin type 2 receptor antagonism/Antimanic agent, second-generation (atypical) antipsychotic | MDD and psychotic depression as an adjunctive therapy. Oral: 5 mg once daily; may increase the dose in increments of 5 mg up to 20 mg/day. TRD as an adjunctive therapy. Oral: 5 mg once daily; may increase dose up to 20 mg/day. |
Postural hypotension, constipation, weight gain, dizziness, personality disorder, akathisia, sedation, headache, increased appetite, abdominal pain, pain in extremity, fatigue, dry mouth, asthenia, drowsiness, tremor | No contraindication with ZyPREXA® monotherapy, caution in combination therapy with fluoxetine, lithium or valproate |
| A fixed-dose of olanzapine/fluoxetine combination may be used instead of separate components. | |||||
|
Pergolide Permax®, Prascend® [82] |
Adjunctive treatment to levodopa/carbidopa in the management of the signs and symptoms of Parkinson’s disease |
Potent dopamine receptor agonist/Ergot derivative | NA | Dyskinesia, hallucinations, somnolence, insomnia, nausea, constipation, diarrhea, dyspepsia, rhinitis |
Hypersensitive to pergolide mesylate or other ergot derivatives |
|
Pioglitazone Actos® [83] |
Type 2 diabetes mellitus | Improving target cell response to insulin, a potent and selective agonist for PPARγ/Antidiabetic agent, thiazolidinedione | NA | >10%: edema, hypoglycemia, upper respiratory tract infection | Hypersensitivity to pioglitazone or any component of the formulation, NYHA class III/IV heart failure |
|
Phenytoin Dilantin®, Phenytek® [84] |
Seizures (non-emergent use), status epilepticus | Stabilizes neuronal membranes and decreases seizure activity by modulating efflux or influx of sodium, shortens action potential in the heart/Anticonvulsant, hydantoin | NA | Nystagmus, ataxia, slurred speech, decreased coordination, somnolence, mental confusion |
Hypersensitivity to phenytoin, other hydantoins, or any component of the formulation, concurrent use of delavirdine, history of prior acute hepatotoxicity attributable to phenytoin |
|
Pramipexole Mirapex® [85] |
Parkinson disease, restless legs syndrome | Nonergot dopamine agonist with specificity for the D2 subfamily dopamine receptor. Additionally, it binds to D3 and D4 receptors/Anti-parkinson agent, dopamine agonist | NA | >10%: orthostatic hypotension, drowsiness, extrapyramidal reaction, insomnia, dizziness, hallucination, headache, restless leg syndrome, abnormal dreams, nausea, constipation, dyskinesia, asthenia, accidental injury | Parkinson disease, restless legs syndrome |
|
Pregabalin Lyrica®, Lyrica CR® [86] |
Fibromyalgia, neuropathic pain associated with diabetic peripheral neuropathy or spinal cord injury, partial-onset seizures, postherpetic neuralgia | modulates calcium influx at the nerve terminals, thereby inhibits excitatory neurotransmitter release, may also affect descending noradrenergic and serotonergic pain transmission pathways from the brainstem to the spinal cord/Anticonvulsant, GABA analog | NA | Peripheral edema, Dizziness, drowsiness, headache, fatigue, weight gain, xerostomia, visual field loss, blurred vision | Hypersensitivity (e.g., angioedema) to pregabalin or any component of the formulation |
|
Quetiapine SEROquel® [87] |
Bipolar disorder, unipolar MDD schizophrenia | Antipsychotic activity through a combination of dopamine type 2 (D2) and serotonin type 2 (5-HT2) antagonism/Second-generation (atypical) antipsychotic | MDD or nonpsychotic depression as an adjunctive therapy. Oral: 50 mg/day on days 1 and 2; increase to 150 mg/day on day 3. Usual dosage range: 150 to 300 mg/day. |
>10%: increased blood pressure, orthostatic hypotension, tachycardia, decreased HDL-C, increased serum cholesterol, increased serum TG, weight gain | Hypersensitivity to quetiapine or any component of the formulation |
| Doses up to 600 mg/day may be needed in psychotic depression. Nonpsychotic depression, monotherapy. Oral: 20 mg once daily; may be gradually increased up to 300 mg/day. | |||||
|
Risperidone Perseris®, RisperDAL®, RisperiDONE® [88] |
Bipolar disorder, schizophrenia, bipolar mania, irritability associated with autistic disorder | High 5-HT2 and dopamine-D2 receptor antagonist activity/Antimanic agent, second-generation (atypical) antipsychotic | MDD and TRD, as an adjunctive therapy. Oral: 0.25 to 0.5 mg/day; may increase dose in increments of 0.25 to 1 mg/day every 3 to 7 days up to 3 mg/day. Usual effective dose: 1 to 1.5 mg/day. |
Activating/sedating effects, angioedema, dyslipidemia, extrapyramidal symptoms, hematologic abnormalities, hyperglycemia, weight gain, hyperprolactinemia, neuroleptic malignant syndrome, orthostatic hypotension, QT prolongation, sexual dysfunction, temperature dysregulation | Hypersensitivity to risperidone, paliperidone, or any component of the formulation |
|
Rosiglitazone Avandia® [89] |
Type 2 diabetes mellitus | Improving target cell response to insulin, a potent and selective agonist for PPARγ/Antidiabetic agent, thiazolidinedione | NA | >10%: increased HDL-C, increased LDL-C, increased total serum cholesterol, weight gain | Hypersensitivity to rosiglitazone or any component of the formulation; NYHA class III/IV heart failure |
|
Scopolamine (hyoscine) Transderm Scop®, Buscopan® [90] |
Prevention of nausea and vomiting associated with motion sickness, recovery from anesthesia, and surgery | Blocks the action of acetylcholine; increases cardiac output, dries secretions, antagonizes histamine and serotonin/anticholinergic agent | NA | >10%: drowsiness, dizziness, xerostomia | Hypersensitivity to scopolamine, other belladonna alkaloids, or any component of the formulation, narrow-angle glaucoma |
|
Statins, for example,Lovastatin Altoprev®, Mevacor® [91] |
Adjunctive therapy to diet to reduce elevated total cholesterol, LDL cholesterol, Apo B and TG and to increase HDL-C in patients with primary hypercholesterolemia | competitively blocking the active site of HMG-CoA reductase/Antilipemic agent, HMG-CoA reductase inhibitor | NA | >10%: Increased creatine phosphokinase | Hypersensitivity to lovastatin or any component of the formulation, active liver disease or unexplained persistent elevations of serum transaminases, concomitant use of potent CYP3A4 inhibitors |
|
Telmisartan Micardis® [92] |
Cardiovascular risk reduction, hypertension | Nonpeptide AT1 angiotensin II receptor antagonist/Angiotensin II receptor blocker, antihypertensive | NA | 1% to 10%: intermittent claudication, chest pain, hypertension, peripheral edema, dizziness, fatigue, headache, pain, dermal ulcer, diarrhea, abdominal pain, dyspepsia, nausea, UTI, back pain, myalgia, upper respiratory tract infection, sinusitis, cough, flu-like symptoms, pharyngitis | Known hypersensitivity (e.g., anaphylaxis, angioedema) to telmisartan or any component of the formulation, concurrent use of aliskiren in patients with diabetes |
|
Valproic acid [93] |
Bipolar disorder, focal (partial) onset and generalized onset seizures, migraine prophylaxis | Increased availability and enhance the action of GABA, blocks voltage-dependent Na channels/Anticonvulsant, antimanic agent, histone deacetylase inhibitor | NA | >10: headache, drowsiness, dizziness, insomnia, pain, nervousness, alopecia, nausea and vomiting, abdominal pain, diarrhea, dyspepsia, anorexia, thrombocytopenia, infections, tremor, weakness, diplopia, visual disturbance, flu-like symptoms, accidental injury | Hypersensitivity to valproic acid, divalproex, derivatives, or any component of the formulation, hepatic disease, urea cycle disorders, mitochondrial disorders caused by mutations in mitochondrial DNA |
|
Vorinostat Zolinza® [94,95] |
Cutaneous T-cell lymphoma | Inhibits HDAC enzymes, HCAC1, HDAC2, HDAC3, and HDAC6, which catalyze acetyl group removal from protein lysine residues/Antineoplastic agent, histone deacetylase inhibitor | NA | >10%: peripheral edema, fatigue, chills, dizziness, headache, alopecia, pruritus, hyperglycemia, weight loss, dehydration, diarrhea, nausea, dysgeusia, anorexia, xerostomia, constipation, vomiting, decreased appetite, proteinuria, thrombocytopenia, anemia, muscle spasm, increased serum creatinine, cough, fever, upper respiratory tract infection | Severe hepatic impairment |
|
Zinc Galzin®, Wilzin® [96] |
Wilson disease | Induces production of the copper-binding protein metallothionein in enterocytes/Chelating agent | NA | Gastric irritation increased serum amylase, increased serum lipase, increased serum ALP | Hypersensitivity to zinc acetate or any component of the formulation |
ADHD: Attention-deficit/hyperactivity disorder; ALP: Alkaline phosphatase; ALT: Alanine aminotransferase; ANA: Antinuclear antibodies; BDNF: Brain-derived neurotrophic factor; COX-2: Cyclooxygenase-2; CYP3A4: Cytochrome P450 3A4; GABA: Gamma-aminobutyric acid; HDAC: Histone deacetylase; HDL-C: High-density lipoprotein-cholesterol; HMG-CoA: 3-Hydroxy-3-Methyl-Glutaryl-CoA; LDL-C: Low-density lipoprotein-Cholesterol; MAO: Monoamine oxidase; MDD: Major depressive disorder; NA: Not available; NE: Norepinephrine; NMDA: N-Methyl-D-aspartate; NSAIDs: Nonsteroidal anti-inflammatory drugs; NYHA: New York Heart Association; PPAR: Peroxisome proliferator-activated receptor; TG: Triglyceride; TNF-α: Tumor necrosis factor-alpha; TRD: Treatment-resistant depression; US-FDA: The United States food and drug administration; UTI: Urinary tract infection.
Table 4.
Clinical trials which study the effects of repurposed medications on MDD since 2015.
| Treatment (Tₓ) Phase/Year First, Posted Ref |
Dosage (Duration of Therapy) |
Subjects/F/M Condition Groups |
Study Design | Results | Non-Serious AEs (Treatment-Related) |
Serious AEs (Treatment-Related) |
|---|---|---|---|---|---|---|
| General anesthetics | ||||||
| Ketamine NA/2016 [97] |
Experimental: 1 mg/kg IV ketamine for the duration of their ECT index course over 2–3 weeks Active comparator: 1 mg/kg of IV methohexital for the duration of their ECT index course over 2–3 weeks |
52/NA/NA MDD |
R, PG, DB | NA | NA | NA |
| Ketamine Early phase I/2015 [98] |
Experimental: ketamine + TAU Active comparator: midazolam + TAU |
9/NA/NA MDD, BP1 disorder, BP2 disorder, BP depression, suicidal ideation |
R, PG, DB | NA | NA | NA |
| Ketamine Phase I/2017 [99] |
Experimental: 4 ketamine infusions at 0.05 mg/kg—once weekly Active comparator: 4 infusions at 0.045 mg/kg—once weekly |
25/NA/NA A major depressive episode, unipolar depression, BP depression |
R, PG, QB | NA | NA | NA |
| Ketamine Phase IV/2016 [100] |
Placebo: saline 0.9%, IV administration of 0.2 mg/kg or 50 mg Medication: ketamine (1st phase) IV administration of 0.2 mg/kg or 50 mg Medication: ketamine (2nd phase) additional 4 sessions (twice a week, 2 weeks) of 0.5 mg/kg over 40 min |
45/NA/NA MDD |
R, PG, QB | NA | NA | NA |
| Ketamine Phase IV/2015 [101] |
Medication: ketamine 0.5 mg/kg over 40 min IV Other: MRI technology will be used before and after ketamine for patients with depression |
16/8/8 MDD, anxious depression, depression |
SG, OL | NA | None | Not reported |
| Ketamine NA/2021 [102] |
Received IV ketamine in 2014–15 and will be evaluated in 5 years | 11/NA/NA/ MDD, medication abuse, medications, relapse |
Retrospective | NA | NA | NA |
| Ketamine NA/2017 [103] |
Experimental: ketamine and 16 CBT sessions over 14 weeks Active comparator: ketamine and psychoeducation all sessions over 14 weeks |
28/NA/NA MDD |
R, PG, SB | NA | NA | NA |
| Ketamine Phase II/2018 [104] |
Experimental: different dosages and regimens for MIJ821 Active comparator: ketamine infusion 0.5 mg/kg weekly Placebo Comparator: placebo infusion |
70/35/35 TRD |
R, PG, DB | NA | None | Hyperacusis, photophobia, vision blurred, dry mouth, nausea, fatigue, feeling of relaxation, gait disturbance, increased systolic blood pressure, decreased platelet count, dizziness, dysgeusia, headache, paresthesia, sciatica, somnolence, anxiety, depersonalization/derealization disorder, disinhibition, irritability, alopecia, pruritus |
| Ketamine Phase II/2016 [105] |
Experimental: NRX-101 oral capsule + ketamine IV infusion + saline solution IV infusion Active comparator: lurasidone oral capsule + ketamine IV infusion + saline solution IV infusion |
22/16/6 BP depression, suicidal ideation, suicide attempts |
R, SG, QB | NA | None | Angina pectoris, tinnitus, vision blurred, diarrhea, dry mouth, coordination abnormal, dizziness, dysmetropsia, hypoesthesia, sedation, restlessness, depression, suicidal ideation, acute kidney injury, dry skin, hypertension |
| Antimanic agents | ||||||
| Lithium Phase III/2015 [106] |
Experimental: 40 mg ITI-007 administered orally as capsules once daily for 6 weeks Experimental: 60 mg ITI-007 administered orally as capsules once daily for 6 weeks Placebo Comparator: placebo administered orally as visually-matched capsules once daily for 6 weeks |
529/NA/NA BD |
R, PG, QB | NA | NA | NA |
| Risperidone 2011 [68,107] |
Groups: olanzapine users, quetiapine users, risperidone users, all other antipsychotic users | 17743/9692/8051 SCH = 475 MDD = 798 BP disorder = 270 Generalized anxiety disorder = 17 Other mental health disorders = 637 Unknown indication = 15546 Quetiapine = 4658 Olanzapine = 5856 Risperidone = 7229 |
Observational, cohort, retrospective | Comparison between quetiapine and olanzapine: quetiapine is associated with lower extrapyramidal symptoms and diabetes mellitus Comparison between quetiapine and risperidone: quetiapine is associated with lower extrapyramidal symptoms, but higher failed suicide attempt rates |
NA | NA |
| Atypical antipsychotics | ||||||
| Aripiprazole Phase II/2016 [108] |
Oral tablet (2,5,10,15,20, or 30 mg) with an IEM QD (8 weeks) |
49/31/18 BP1 (n = 22) SCH (n = 15) MDD (n = 12) Tx = 49 |
SG, OL, multi-center | NA | Rash, erythema, pruritus skin irritation, upper respiratory tract infection, sinusitis, headache, syncope, meniscus injury, sunburn, peripheral swelling, pain in extremity | Not reported |
| Quetiapine Phase III/2016 [109] |
Active comparator: levomilnacipran ER 20–120 mg/d starting at 20 mg/d on days 1–2, 40 mg/d on days 3–7 in week 1, then between 40–120 mg/d during weeks 2–8 Active comparator: 1uetiapine XR 50 mg/d on day 1–2, 150 mg/d on days 3–7 in week 1, then between 150–300 mg/d during weeks 1–8 along with their current antidepressant |
60/NA/NA MDD |
R, PG, TB | NA | NA | NA |
| Quetiapine Phase II/2017 [110] |
Experimental: 1 capsule of 20 mg JNJ-42847922 and 1 capsule of placebo once daily for 14 days. Then, JNJ-42847922 dose can be increased to 40 mg (2 capsules) until day 167 Active comparator: 1 capsule of quetiapine XR 50 mg along with 1 capsule of placebo once daily for 2 days, followed by 1 capsule of 150 mg along with 1 capsule of placebo once daily from day 3–14. Then, the dose can be increased to 300 mg (2 capsules) until day 167. |
107/NA/NA MDD |
R, PG, DB | NA | NA | NA |
| Patients should continue to take their baseline SSRI/SNRI. | ||||||
| Brexpiprazole Phase III/2018 [35] |
Experimental: brexpiprazole, 2–3 mg/day, once daily for 6 weeks, oral administration Placebo Comparator: placebo, 2–3 mg/day, once daily for 6 weeks, oral administration |
65/NA/NA MDD |
R, PG, TB | NA | NA | NA |
| Dopamine agonists | ||||||
| Pramipexole Phase IV/2014 [47,111] |
Started at 0.125 mg BD PO and increased by 0.25 mg/day every 3–4 days to a target range of 1.0–2.5 mg/day (6 weeks) |
51/25/26 MDD (n = 26) Healthy (n = 25) Healthy control patients did not receive study medication and only have baseline measures |
Non-randomized, PG, OL | Symptom’s improvement | Nausea, heartburn, vomiting, ↑appetite, ↓appetite, diarrhea, constipation, somnolence, restlessness, insomnia, forgetfulness, sleep attacks, compulsive behaviors, impaired concentration, headache, lightheadedness, dizziness, dry mouth, fatigue, ↓ libido, sexual dysfunction, skin problems, sweating, impaired coordination, tremor, blurry vision, bruising | Not reported |
| Armodafinil Phase III/2011 [50,112] |
Tablet, PO, QD in the morning, started at 50 mg/day; the dosage was increased by 50 mg/day on days 2 and 4, up to 150 mg/day. (8 weeks) |
399/241/158 depressive episode despite maintenance therapy for BP1 disorder P = 199 Tx = 200 |
R, PG, DB, PC, multi-center | ↓Severity of depression, ↓depressive symptoms, Improved functioning |
Headache, nausea | Anxiety, BP1 disorder, depression, insomnia, suicide attempt, cholecystitis chronic, social stay hospitalization |
| Armodafinil Phase III/2010 [51,113] |
Tablet, PO, QD in the morning, started at 50 mg/kg and titrated up in the first week to 150 or 200 mg/kg. Treatment with 200 mg/kg dose was discontinued via a protocol amendment. (8 weeks) |
492/273/219 major depressive episode despite BP1 disorder maintenance therapy P = 230 Tx (150 mg/kg) = 232 Tx (200 mg/kg) = 30 |
R, PG, DB, PC, multi-center | ↓ Depressive symptoms | Nausea, diarrhea, dry mouth, toothache, dyspepsia, headache, dizziness, insomnia, anxiety, suicidal ideation, nasopharyngitis, cough | Mania, psychotic disorder, suicidal ideation, pulmonary embolism, abortion spontaneous, accidental overdose, non-cardiac chest pain, coronary artery disease |
| Armodafinil Phase III/2010 [49,114] |
Tablet, PO, QD in the morning, started at 50 mg/kg and titrated up in the first week to 150 or 200 mg/kg. Treatment with 200 mg/kg dose was discontinued via a protocol amendment. (8 weeks) |
433/288/145 major depressive episode while taking at least 4 weeks of conventional maintenance medication P = 199 Tx (150 mg/kg) =201 Tx (200 mg/kg) =33 |
R, PG, DB, PC, multi-center | Improvement in depressive symptoms | Diarrhea, nausea, headache, migraine, insomnia, feeling jittery | Psychotic disorder, suicidal ideation, depressive symptom, aggression, acute hepatic failure, hepatitis acute |
| Modafinil with D-cycloserine (DCS) Phase III/2015 [7] |
250 mg DCS before two weekly sessions, 100 mg modafinil before two weekly sessions | 36/20/14/2 (transgender) MDD P = 11 Tx (modafinil) = 12 Tx (DCS) = 13 |
R, PG, DB, PC | NA | ↑Energy/concentration, fatigue/low motivation | Not reported |
| Supplementation | ||||||
| Omega-3 PUFA Not applicable/2018 [115] |
One capsule (EPA 300 mg and 200 mg DHA) QD were given antidepressant (citalopram, escitalopram, paroxetine 1 tablet at night time). (12 weeks) |
70/NA/NA Taking antidepressants P = NA Tx = NA |
R, PG, SB, PC | NA | NA | NA |
| n-3 Polyunsaturated fatty acid Not applicable/2017 [116] |
2 g of EPA and 1 g of DHA (12 weeks) |
60/NA/NA MDD and cardiovascular disease P = NA Tx = NA |
R, PG, DB, PC | NA | NA | NA |
| Zinc Not applicable/2020 [46] |
Oral 30 mg zinc sulfate QD with SSRIs (8 weeks) |
100/NA/NA MDD P = 50 Tx = 50 |
R, PG, DB, PC | NA | NA | NA |
| NMDA receptor antagonist | ||||||
| Dextromethorphan hydrobromide Phase I/2016 [38] |
Two 75 mg capsules PO, separated by 4 h | 4/NA/NA MDD P = NA Tx = NA |
R, PG, DB, PC | NA | NA | NA |
| Esketamine Phase III/2017 [117,118] |
84 mg intranasal, twice a week (on days 1,4,8,11,15,18,22 and 25) along with the standard of care antidepressant treatment initiated on day 1 (4 weeks) |
225/139/86 MDD and have suicidal ideation with intent but without psychotic features P = 112 Tx = 113 |
R, PG, DB, PC, multi-center | ↓ MADRS total score (improved), improvement in the severity of suicidality measured by CGI-SS-r | Dizziness, headache, somnolence, dysgeusia, hypoesthesia, sedation, dizziness postural, nausea, constipation, vomiting, dissociation, insomnia, anxiety, ↑ blood pressure, blurred vision, vertigo | Suicidal depression, depression, suicide attempt, diabetic ketoacidosis |
| Esketamine Phase III/2017 [119,120] |
84 mg intranasal, twice a week (on days 1,4,8,11,15,18,22 and 25) along with the standard of care antidepressant treatment initiated on day 1 (4 weeks) |
227/136/91 MDD and having suicidal ideation with intent, but without psychotic features P = 113 Tx = 114 |
R, PG, DB, PC, multi-center | ↓MADRS total score (improved), ↓ in the severity of suicidality measured by CGI-SS-r | Dizziness, dysgeusia, somnolence, headache, paresthesia, sedation, hypoesthesia, dizziness postural, nausea, vomiting, paresthesia oral, dry mouth, constipation, hypoesthesia oral, dissociation, anxiety, euphoric mood, depersonalization/derealization disorder, insomnia, vision blurred, diplopia, nasal discomfort, oropharyngeal pain, throat irritation, vertigo, ↑ blood pressure, hyperhidrosis, feeling drunk | Suicide attempt, suicidal ideation, depersonalization/derealization disorder |
| Esketamine Phase II/2019 [121] |
Four low, medium, or high doses (three different groups) on days 1,4,8 and 11 via dry powder inhaler (2 weeks) |
88/NA/NA TRD in the course of MDD P = NA Tx = NA |
R, PG, DB, PC, multi-center | NA | NA | NA |
| Esketamine Phase I/2016 [122,123] |
Treatment A: intranasal placebo on day 1 and oral placebo on day 2 Treatment B: intranasal placebo on day 1 and oral alcohol on day 2 Treatment C: 84 mg of intranasal esketamine on day 1 and oral placebo on day 2 Participants will receive one of the ABC, BCA, CAB, CBA, ACB, or BAC treatments in part A and intranasal placebo on day 1 and 84 mg of intranasal esketamine on days 4,8,11,15,18,22 and 25 in part B |
23/16/7 MDD P = 20 Tx = 23 |
Part A: R, cross-over assignment, SB, PC, active-controlled, double-dummy, 3-period Part B: OL, PC, fixed sequence, single period |
Improvement in overall depression scores in MADRS | Dissociation, dizziness, dysgeusia, paresthesia, fatigue, paresthesia oral, nausea, headache, feeling abnormal, nasal discomfort, vision blurred, somnolence, euphoric mood, tinnitus, diplopia, ↑ blood pressure, feeling drunk, dysarthria, feeling of relaxation, illusion, hypoesthesia, altered time perception | Not reported |
| Combined medications | ||||||
| AXS-05 Phase III/2019 [40,124] |
Oral tablets of 45 mg dextromethorphan and 105 mg bupropion, BID (6 weeks) |
327/215/112 Moderate or severe MDD P = 164 Tx = 163 |
R, PG, DB, PC, multi-center | ↓ MADRS total score, improvement in daily functioning, improvement in quality of life | Dizziness, nausea, headache, diarrhea, somnolence, dry mouth | Not reported |
| AXS-05 Phase II/2018 [39,125] |
45 mg dextromethorphan and 105 mg bupropion BID and Bupropion as AC (6 weeks) |
80/51/29 Moderate or severe MDD AC = 37 Tx = 43 |
R, PG, DB, active-controlled, multi-center | ↓ MADRS total score, improvement in core symptoms of depression | Nausea, dizziness, dry mouth, ↓ appetite, anxiety | Not reported |
| AXS-05 Phase III/2019 [41,126] |
Oral tablets of 45 mg dextromethorphan and 105 mg bupropion, BID (12 months) |
876/380/496 MDD including TRD Tx = 876 |
SG, OL, multi-center | ↓ Depression symptoms, improvement in functioning | Dizziness, nausea, headache, dry mouth, ↓ appetite | Not reported |
| Anticholinergic agents | ||||||
| Scopolamine Phase IV/2017 [45] |
Experimental: scopolamine (0.3 mg/1 mL, IM) BID; escitalopram (10 mg/d PO) QD Experimental: scopolamine (0.3 mg/1 mL, IM) QD; placebo (1 mL saline, IM) QD; escitalopram (10 mg/d PO) QD Placebo comparator: placebo (1 mL saline, IM) BID; escitalopram (10 mg/d PO) QD |
66/NA/NA MDD |
R, PG, QB | NA | NA | NA |
| Scopolamine Phase IV/2017 [44] |
Experimental: participants will receive active medications scopolamine and naltrexone Placebo comparator: participants will receive placebo medication |
14/NA/NA Depression |
R, PG, DB | NA | NA | NA |
| Mucolytic agents | ||||||
| N-acetyl cysteine Early phase I/2014 [127] |
Experimental: sertraline and N-acetyl cysteine for 7 weeks Experimental: citalopram and N-acetyl cysteine for 7 weeks Experimental: existing depression medication treatment and N-acetyl cysteine for 7 weeks |
10/NA/NA MDD |
Non-randomized, SG, OL | NA | NA | NA |
| Antibiotics | ||||||
| Minocycline Early phase I/2015 [128] |
Experimental: minocycline 50 mg/day on week 1, 50 mg/BID on week 2, and 100 mg/BID weeks 3–8. For tapering, the dose will be reduced to 50 mg BID for a week and then stopped Placebo comparator: the number and appearance of the pills would be identical to those in the minocycline arm |
115/NA/NA MDD |
R, SG, QB | NA | NA | NA |
| Minocycline Phase II/2015 [129] |
Experimental: minocycline and standard antidepressant treatment Placebo comparator: placebo and standard antidepressant treatment |
168/NA/NA MDD |
R, PG, TB | NA | NA | NA |
| Cycloserine Not applicable/2017 [130,131] |
Group A: placebo + placebo: placebo 8 mm pill, single dose Group B: fludrocortisone + placebo: fludrocortisone Astonin H 0, 1 gm, single-dose + placebo 8 mm pill, single dose Group C: D-cycloserine + placebo: cycloserine 250 mg capsule, single-dose + placebo 8 mm pill, single dose Group D: fludrocortisone + D-Cycloserine: fludrocortisone Astonin H 0, 1 gm, single-dose + cycloserine 250 mg capsule, single dose |
232/182/50 MDD = 116 Healthy = 116 P = 58 Group B = 58 Group C = 58 Group D = 58 |
R, PG, DB, PC | ↑ Cognitive empathy in the group with stimulated mineralocorticoid receptor, ↓ cognitive empathy only for positive emotions in MDD patients with NMDA-R stimulation, | NA | NA |
| D-cycloserine Phase IIb/III/2016 [42,105] |
Experimental: NRX-101 (D-cycloserine + lurasidone) oral capsule with fixed-dose (administered to subjects who respond to an intravenous infusion of ketamine—0.5 mg/kg administered over 40 min; on Day 0)ketamine intravenous infusion (Randomized administration of Ketamine or Placebo in a 3 to 1 ratio), saline solution intravenous infusion (Randomized administration of Ketamine or Placebo in a 3 to 1 ratio) Active comparator: Lusaridone in the same dosage as lurasidone in NRX-101 ketamine intravenous infusion (Randomized administration of Ketamine or Placebo in a 3 to 1 ratio), saline solution intravenous infusion (Randomized administration of Ketamine or Placebo in a 3 to 1 ratio) (6 weeks) |
22/16/6 BP depression, suicidal ideas, suicidal ideation, attempted suicide Ketamine Followed by NRX-101 = 12 Ketamine Followed by Lurasidone = 5 Saline Followed by NRX-101 = 4 Saline Followed by Lurasidone = 1 |
R, SA, QB, multicenter | NA | Angina pectoris, palpitation, tinnitus, diplopia, vision blurred, diarrhea, vomiting, dyspepsia, abdominal distention, dry mouth, nausea, gastroesophageal reflux disease, fatigue, vulvovaginal candidiasis, thermal burn, wound, ↑weight, muscle spasm, coordination abnormal, dizziness, dysmetropsia, headache, hypoesthesia, akathisia, hypersomnia, lethargy, somnolence, sedation, dissociation, restlessness, euphoric mood, anorgasmia, depressed mood, depression, suicidal ideation, acute kidney injury, ejaculation delayed, dry skin, rash, hypertension | Not reported |
| Antidiabetic agents | ||||||
| Metformin Phase I and II/2019 [132] |
Experimental: fluoxetine 20 mg capsule once daily for 12 weeks + metformin 1000 mg XR tablet once daily for 12 weeks Placebo comparator: fluoxetine 20 mg capsule once daily for 12 weeks + placebo tablet once daily for 12 weeks |
80/NA/NA MDD |
R, PG, DB | NA | NA | NA |
| Ganglionic blocking agents | ||||||
| Mecamylamine (TC-5214) Phase III/2012 [132,133] |
Experimental: SSRI/SNRI + TC-5214, 0.1 mg BID SSRI/SNRI + TC-5214, 1 mg BID SSRI/SNRI + TC-5214, 4 mg BID Placebo comparator: SSRI/SNRI + placebo BID (8 weeks) |
696/498/198 MDD with inadequate response to no more than one antidepressant P = 174 0.1 mg = 174 1 mg = 174 4 mg = 174 |
R, PG, DB, PC, multi-center | No significant difference between the two groups | Headache, dizziness, somnolence, dysgeusia, constipation, dry mouth, nausea, abdominal pain upper, diarrhea, vomiting, insomnia, agitation, nightmare, nervousness, fatigue, hyperhidrosis, influenza, UTI, ↑ alanine aminotransferase, nasopharyngitis, orthostatic hypotension, hypertension, hypotension, vertigo, ↑ appetite | Suicide attempt, suicidal ideation, depression, pneumonia, UTI, toxicity to various agents, food poisoning |
| Mecamylamine (TC-5214) Phase III/2010 [37,134] |
Experimental: SSRI/SNRI + oral tablet TC-5214, 1–4 mg BID Placebo comparator: SSRI/SNRI + oral tablet placebo BID (8 weeks) |
319/200/119 MDD or depression with inadequate response to no more than one antidepressant P = 160 Tx = 159 |
R, PG, DB, PC, multi-center | No significant difference between the two groups | Constipation, diarrhea, nausea, dry mouth, vomiting, headache, dizziness, somnolence, dizziness postural, abnormal dreams, insomnia, anxiety, agitation, nightmare, upper respiratory tract infection, nasopharyngitis, fatigue, ↑ weight, orthostatic hypotension, hyperhidrosis, musculoskeletal stiffness | headache |
| Mecamylamine (TC-5214) Phase III/2010 [132,134] |
Experimental: SSRI/SNRI + oral tablet TC-5214, 1–4 mg BID Placebo comparator: SSRI/SNRI + oral tablet placebo BID (8 weeks) |
295/189/106 MDD or depression with inadequate response to no more than one antidepressant P = 148 Tx = 147 |
R, PG, DB, PC, multi-center | No significant difference between the two groups | Constipation, nausea, dry mouth, diarrhea, abdominal distention, abdominal pain upper, headache, dizziness, somnolence, dizziness postural, sedation, tremor, orthostatic hypotension, hypertension, nasopharyngitis, sinusitis, influenza, fatigue, asthenia, insomnia, anxiety, back pain, vertigo, hyperhidrosis, vision blurred, ↑ appetite, ↑ aspartate aminotransferase | Uterine cancer |
| Mecamylamine (TC-5214) Phase III/2010 [132,133] |
Experimental: SSRI/SNRI + TC-5214, 0.5 mg BID SSRI/SNRI + TC-5214, 2 mg BID SSRI/SNRI + TC-5214, 4 mg BID Placebo comparator: SSRI/SNRI + placebo BID (8 weeks) |
641/355/276 MDD or depression with inadequate response to no more than one antidepressant P = 161 0.5 mg = 160 2 mg = 160 4 mg = 160 |
R, PG, DB, PC, multi-center | No significant difference between the two groups | Blurred vision, constipation, nausea, dry mouth, diarrhea, abdominal distention, vomiting, dyspepsia, flatulence, fatigue, pyrexia, asthenia, pain, nasopharyngitis, bronchitis, upper respiratory tract infection, sinusitis, ↑ aspartate aminotransferase, ↑ appetite, back pain, muscle spasms, muscle tightness, myalgia, headache, dizziness, somnolence, dizziness postural, akathisia, insomnia, nightmare, agitation, abnormal dreams, pollakiuria, orthostatic hypotension, hypertension | Major depression, suicidal ideation, clavicle fracture, rib fracture, scapula fracture, obstructive uropathy, renal failure acute, upper respiratory tract infection, benign prostatic hyperplasia, pulmonary fibrosis, convulsion |
| Mecamylamine (TC-5214) Phase III/2010 [135,136] |
Experimental: SSRI/SNRI + oral tablet TC-5214, 1–4 mg BID Placebo comparator: SSRI/SNRI + oral tablet placebo BID (52 weeks) |
813/566/247 MDD or depression with inadequate response to no more than one antidepressant P = 203 Tx = 610 |
R, PG, DB, PC, multi-center | No significant difference between the two groups | Constipation, nausea, dry mouth, diarrhea, abdominal pain, vomiting, abdominal pain upper, flatulence, abdominal distension, fatigue, seasonal allergy, nasopharyngitis, bronchitis, upper respiratory tract infection, sinusitis, influenza, UTI, gastroenteritis, gastroenteritis viral, contusion, muscle strain, ↑ weight, ↑ blood pressure, ↑ appetite, arthralgia, back pain, muscle spasms, musculoskeletal pain, neck pain, pain in extremities, myalgia, headache, dizziness, somnolence, dizziness postural, sedation, memory impairment, migraine, paresthesia, tremor, insomnia, abnormal dreams, agitation, anxiety, bruxism, cough, nasal congestion, oropharyngeal pain, wheezing, hyperhidrosis, rash, hypertension, orthostatic hypotension | Bradycardia, abdominal hernia, diverticulum, hemorrhoids, small intestinal obstruction, cellulitis, oral infection, pneumonia, brain contusion, cerebral vertebral fracture, contusion, facial bones fracture, fibula fracture, tibia fracture, toxicity to various agents, musculoskeletal chest pain, abortion spontaneous, alcohol withdrawal syndrome, psychotic disorder, suicidal ideation, suicide attempt, ovarian torsion, hypertensive crisis |
AC: Active comparator; ADT: antidepressant therapy; AEs: Adverse events; BD: bipolar depression; BID: twice a day; BP: Bipolar; CBT: Cognitive behavioral therapy; CGI-SS-r: Clinical Global Impression–Severity of Suicidality—revised; DB: Double blind; DHA: Docosahexaenoic acid; EPA: Eicosatetraenoic acid; F: Female; FA: factorial assignment; IEM: Ingestible event marker; IM: intramuscular; ITI-007:Lumateperone LE: Leukocyte esterase; M: Male; MADRS: Montgomery-Asberg depression rating scale; MDD: Major depressive disorder; NA: Not available; NMDA: N-methyl-D-aspartate receptor; OL: Open label; P: Placebo; PC: Placebo-controlled; PG: Parallel-group; PO: by mouth; PUFA: Polyunsaturated fatty acid; QD: once a day; QB: Quadruple blind R: Randomized; SA: Sequential Assignment; SB: single blind; SCH: Schizophrenia; SG: Single group; SNRI: serotonin norepinephrine reuptake inhibitor; SSRI: Selective serotonin reuptake inhibitor; TAU: Treatment as usual; TB: Triple blind; TRD: Treatment-resistant depression; Tx: Treatment; WBC: White blood cell; UTI: Urinary tract infection.
4. Conclusions
MDD, as a severe mental disorder declining the quality of life of the patient, requires on-time screening and management. Psychotherapy, pharmacotherapy, and somatic interventions are among the suggested managements. However, due to the incomplete response of the patients to the approved medications, physicians tend to prescribe medications on their off-label use. Hence, a great need for a drug repositioning method took place for the MDD management medications. Drug repositioning is a cost-effective method that decreases the required time to introduce medicine to the market.
Moreover, as there are available data on the safety profile of the medications, the risk of failure decreases significantly, and this method is capable of uncovering novel targets to treat a disease. Various pharmacological categories, including neurotransmitters’ antagonists, atypical antipsychotics, and CNS stimulants, have been studied for the repurposing aims. However, proper studies on the formulation, regulatory, and efficacy of the medication are required to better this approach.
Author Contributions
Conceptualization, M.A. and S.N.; methodology, H.M.S. and I.A.; software, H.M.S. and I.A.; validation, T.M.; formal analysis, M.D.; data curation, H.M.S. and I.A.; writing—original draft preparation, H.M.S. and I.A.; writing—review and editing, T.M. and M.D.; supervision, M.A. and S.N. All authors have read and agreed to the published version of the manuscript.
Funding
This research was not funded.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Novac N. Challenges and opportunities of drug repositioning. Trends Pharmacol. Sci. 2013;34:267–272. doi: 10.1016/j.tips.2013.03.004. [DOI] [PubMed] [Google Scholar]
- 2.Ashburn T.T., Thor K.B. Drug repositioning: Identifying and developing new uses for existing drugs. Nat. Rev. Drug Discov. 2004;3:673–683. doi: 10.1038/nrd1468. [DOI] [PubMed] [Google Scholar]
- 3.Xue H., Li J., Xie H., Wang Y. Review of Drug Repositioning Approaches and Resources. Int. J. Biol. Sci. 2018;14:1232–1244. doi: 10.7150/ijbs.24612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pillaiyar T., Meenakshisundaram S., Manickam M., Sankaranarayanan M. A medicinal chemistry perspective of drug repositioning: Recent advances and challenges in drug discovery. Eur. J. Med. Chem. 2020;195:112275. doi: 10.1016/j.ejmech.2020.112275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dudley J.T., Deshpande T., Butte A.J. Exploiting drug-disease relationships for computational drug repositioning. Brief. Bioinform. 2011;12:303–311. doi: 10.1093/bib/bbr013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Depression. [(accessed on 22 June 2021)]; Available online: https://www.who.int/health-topics/depression#tab=tab_1.
- 7.Little A. Treatment-resistant depression. Am. Fam. Physician. 2009;80:167–172. [PubMed] [Google Scholar]
- 8.Baune B.T., Smith E., Reppermund S., Air T., Samaras K., Lux O., Brodaty H., Sachdev P., Trollor J.N. Inflammatory biomarkers predict depressive, but not anxiety symptoms during aging: The prospective Sydney Memory and Aging Study. Psychoneuroendocrinology. 2012;37:1521–1530. doi: 10.1016/j.psyneuen.2012.02.006. [DOI] [PubMed] [Google Scholar]
- 9.Taylor C., Fricker A.D., Devi L.A., Gomes I. Mechanisms of action of antidepressants: From neurotransmitter systems to signaling pathways. Cell. Signal. 2005;17:549–557. doi: 10.1016/j.cellsig.2004.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pushpakom S., Iorio F., Eyers P.A., Escott K.J., Hopper S., Wells A., Doig A., Guilliams T., Latimer J., McNamee C., et al. Drug repurposing: Progress, challenges and recommendations. Nat. Rev. Drug Discov. 2019;18:41–58. doi: 10.1038/nrd.2018.168. [DOI] [PubMed] [Google Scholar]
- 11.Henriksen K., Christiansen C., Karsdal M.A. Serological biochemical markers of surrogate efficacy and safety as a novel approach to drug repositioning. Drug Discov. Today. 2011;16:967–975. doi: 10.1016/j.drudis.2011.06.010. [DOI] [PubMed] [Google Scholar]
- 12.Pulley J.M., Rhoads J.P., Jerome R.N., Challa A.P., Erreger K.B., Joly M.M., Lavieri R.R., Perry K.E., Zaleski N.M., Shirey-Rice J.K., et al. Using What We Already Have: Uncovering New Drug Repurposing Strategies in Existing Omics Data. Annu. Rev. Pharmacol. Toxicol. 2020;60:333–352. doi: 10.1146/annurev-pharmtox-010919-023537. [DOI] [PubMed] [Google Scholar]
- 13.Ebada M.E. Drug repurposing may generate novel approaches to treating depression. J. Pharm. Pharmacol. 2017;69:1428–1436. doi: 10.1111/jphp.12815. [DOI] [PubMed] [Google Scholar]
- 14.Caban A., Pisarczyk K., Kopacz K., Kapuśniak A., Toumi M., Rémuzat C., Kornfeld A. Filling the gap in CNS drug development: Evaluation of the role of drug repurposing. J. Mark. Access Health Policy. 2017;5:1299833. doi: 10.1080/20016689.2017.1299833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yang L., Agarwal P. Systematic drug repositioning based on clinical side-effects. PLoS ONE. 2011;6:e28025. doi: 10.1371/journal.pone.0028025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ko Y. Computational Drug Repositioning: Current Progress and Challenges. Appl. Sci. 2020;10:5076. doi: 10.3390/app10155076. [DOI] [Google Scholar]
- 17.Otte C., Gold S.M., Penninx B.W., Pariante C.M., Etkin A., Fava M., Mohr D.C., Schatzberg A.F. Major depressive disorder. Nat. Rev. Dis. Primers. 2016;2:1–20. doi: 10.1038/nrdp.2016.65. [DOI] [PubMed] [Google Scholar]
- 18.Koda-Kimble M.A., Young L.Y., editors. Applied Therapeutics: The Clinical Use of Drugs. 7th ed. Lippincott Williams & Wilkins; Philadelphia, PA, USA: 2001. [Google Scholar]
- 19.Block S.G., Nemeroff C.B. Emerging antidepressants to treat major depressive disorder. Asian J. Psychiatry. 2014;12:7–16. doi: 10.1016/j.ajp.2014.09.001. [DOI] [PubMed] [Google Scholar]
- 20.Molero P., Ramos-Quiroga J., Martin-Santos R., Calvo-Sánchez E., Gutiérrez-Rojas L., Meana J. Antidepressant efficacy and tolerability of ketamine and esketamine: A critical review. CNS Drugs. 2018;32:411–420. doi: 10.1007/s40263-018-0519-3. [DOI] [PubMed] [Google Scholar]
- 21.Fabbri C., Kasper S., Zohar J., Souery D., Montgomery S., Albani D., Forloni G., Ferentinos P., Rujescu D., Mendlewicz J., et al. Drug repositioning for treatment-resistant depression: Hypotheses from a pharmacogenomic study. Prog. Neuro-Psychopharmacol. Biol. Psychiatry. 2021;104:110050. doi: 10.1016/j.pnpbp.2020.110050. [DOI] [PubMed] [Google Scholar]
- 22.Kraus C., Wasserman D., Henter I.D., Acevedo-Diaz E., Kadriu B., Zarate C.A., Jr. The influence of ketamine on drug discovery in depression. Drug Discov. Today. 2019;24:2033–2043. doi: 10.1016/j.drudis.2019.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Shukla R., Henkel N.D., Alganem K., Hamoud A.-r., Reigle J., Alnafisah R.S., Eby H.M., Imami A.S., Creeden J.F., Miruzzi S.A., et al. Signature-based approaches for informed drug repurposing: Targeting CNS disorders. Neuropsychopharmacology. 2021;46:116–130. doi: 10.1038/s41386-020-0752-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Arakelyan A., Nersisyan L., Nikoghosyan M., Hakobyan S., Simonyan A., Hopp L., Loeffler-Wirth H., Binder H. Transcriptome-Guided Drug Repositioning. Pharmaceutics. 2019;11:677. doi: 10.3390/pharmaceutics11120677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Park K. A review of computational drug repurposing. Transl. Clin. Pharm. 2019;27:59–63. doi: 10.12793/tcp.2019.27.2.59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zhao K., So H.C. Drug Repositioning for Schizophrenia and Depression/Anxiety Disorders: A Machine Learning Approach Leveraging Expression Data. IEEE J. Biomed. Health Inform. 2019;23:1304–1315. doi: 10.1109/JBHI.2018.2856535. [DOI] [PubMed] [Google Scholar]
- 27.Troisi J., Cavallo P., Colucci A., Pierri L., Scala G., Symes S., Jones C., Richards S. Chapter Three-Metabolomics in genetic testing. In: Makowski G.S., editor. Advances in Clinical Chemistry. Volume 94. Elsevier; Amsterdam, The Netherlands: 2020. pp. 85–153. [DOI] [PubMed] [Google Scholar]
- 28.Robertson D.G., Frevert U. Metabolomics in drug discovery and development. Clin. Pharmacol. Ther. 2013;94:559–561. doi: 10.1038/clpt.2013.120. [DOI] [PubMed] [Google Scholar]
- 29.Gligorijević V., Malod-Dognin N., Pržulj N. Integrative methods for analyzing big data in precision medicine. Proteomics. 2016;16:741–758. doi: 10.1002/pmic.201500396. [DOI] [PubMed] [Google Scholar]
- 30.Hurle M.R., Yang L., Xie Q., Rajpal D.K., Sanseau P., Agarwal P. Computational drug repositioning: From data to therapeutics. Clin. Pharmacol. Ther. 2013;93:335–341. doi: 10.1038/clpt.2013.1. [DOI] [PubMed] [Google Scholar]
- 31.Hodos R.A., Kidd B.A., Shameer K., Readhead B.P., Dudley J.T. In silico methods for drug repurposing and pharmacology. Wiley Interdiscip. Rev. Syst. Biol. Med. 2016;8:186–210. doi: 10.1002/wsbm.1337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Jin G., Wong S.T. Toward better drug repositioning: Prioritizing and integrating existing methods into efficient pipelines. Drug Discov. Today. 2014;19:637–644. doi: 10.1016/j.drudis.2013.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Alaimo S., Pulvirenti A. Network-Based Drug Repositioning: Approaches, Resources, and Research Directions. Methods Mol. Biol. 2019;1903:97–113. doi: 10.1007/978-1-4939-8955-3_6. [DOI] [PubMed] [Google Scholar]
- 34.Parvathaneni V., Kulkarni N.S., Muth A., Gupta V. Drug repurposing: A promising tool to accelerate the drug discovery process. Drug Discov. Today. 2019;24:2076–2085. doi: 10.1016/j.drudis.2019.06.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Otsuka Beijing Research Institute The Safety and Efficacy of Brexpiprazole as Adjunctive Therapy in the Treatment of Major Depressive Disorder. [(accessed on 3 June 2021)]; Available online: https://ClinicalTrials.gov/show/NCT03487198.
- 36.Salvadore G., Singh J.B. Ketamine as a fast-acting antidepressant: Current knowledge and open questions. CNS Neurosci. Ther. 2013;19:428–436. doi: 10.1111/cns.12103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.A Study to Assess the Efficacy and Safety of TC-5214 as an Adjunct Therapy in Patients With Major Depressive Disorder (MDD) [(accessed on 22 June 2021)]; Available online: https://ClinicalTrials.gov/show/NCT01157078.
- 38.Medical University of South Carolina A Trial of Dextromethorphan for Treatment of Major Depressive Disorder. [(accessed on 3 June 2021)]; Available online: https://ClinicalTrials.gov/show/NCT02860962.
- 39.Axsome Therapeutics, Inc Assessing Symptomatic Clinical Episodes in Depression. [(accessed on 3 June 2021)]; Available online: https://ClinicalTrials.gov/show/NCT03595579.
- 40.Axsome Therapeutics, Inc A Trial of AXS-05 in Patients With Major Depressive Disorder. [(accessed on 3 June 2021)]; Available online: https://ClinicalTrials.gov/show/NCT04019704.
- 41.Axsome Therapeutics, Inc Open-Label Safety Study of AXS-05 in Subjects With Depression. [(accessed on 3 June 2021)]; Available online: https://ClinicalTrials.gov/show/NCT04039022.
- 42.Drevets W.C., Zarate C.A., Jr., Furey M.L. Antidepressant effects of the muscarinic cholinergic receptor antagonist scopolamine: A review. Biol. Psychiatry. 2013;73:1156–1163. doi: 10.1016/j.biopsych.2012.09.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.O’Connor R.M., Grenham S., Dinan T.G., Cryan J.F. microRNAs as novel antidepressant targets: Converging effects of ketamine and electroconvulsive shock therapy in the rat hippocampus. Int. J. Neuropsychopharmacol. 2013;16:1885–1892. doi: 10.1017/S1461145713000448. [DOI] [PubMed] [Google Scholar]
- 44.The Taub Group The Safety and Efficacy of Naltrexone and Scopolamine Utilized in the Treatment of Major Depression. [(accessed on 3 June 2021)]; Available online: https://ClinicalTrials.gov/show/NCT03386448.
- 45.Capital Medical University Effectiveness Study of Scopolamine Combined with Escitalopram in Patients With MDD. [(accessed on 3 June 2021)]; Available online: https://ClinicalTrials.gov/show/NCT03131050.
- 46.Unipolar Major Depression in Adults: Choosing Initial Treatment. [(accessed on 20 June 2021)]; Available online: https://www.uptodate.com/contents/unipolar-major-depression-in-adults-choosing-initial-treatment#H51.
- 47.New York State Psychiatric Institute. Columbia University. Research Foundation for Mental Hygiene, Inc. Mclean Hospital. Icahn School of Medicine at Mount Sinai. National Institute of Mental Health (NIMH) Imaging Dopamine Release in Depression. [(accessed on 3 June 2021)]; Available online: https://ClinicalTrials.gov/show/NCT02033369.
- 48.Sadat City University The Antidiabetic Metformin as a Novel Adjunct to Antidepressants in Major Depressive Disorder Patients. [(accessed on 3 June 2021)]; Available online: https://ClinicalTrials.gov/show/NCT04088448.
- 49.De Assis Lima I.V., Almeida-Santos A.F., Ferreira-Vieira T.H., Aguiar D.C., Ribeiro F.M., Campos A.C., de Oliveira A.C.P. Antidepressant-like effect of valproic acid—Possible involvement of PI3K/Akt/mTOR pathway. Behav. Brain Res. 2017;329:166–171. doi: 10.1016/j.bbr.2017.04.015. [DOI] [PubMed] [Google Scholar]
- 50.Guo M., Mi J., Jiang Q.M., Xu J.M., Tang Y.Y., Tian G., Wang B. Metformin may produce antidepressant effects through improvement of cognitive function among depressed patients with diabetes mellitus. Clin. Exp. Pharmacol. Physiol. 2014;41:650–656. doi: 10.1111/1440-1681.12265. [DOI] [PubMed] [Google Scholar]
- 51.Study to Evaluate the Efficacy and Safety of Armodafinil Treatment as Adjunctive Therapy in Adults With Major Depression Associated With Bipolar I Disorder. [(accessed on 22 June 2021)]; Available online: https://ClinicalTrials.gov/show/NCT01072630.
- 52.US Food and Drug Administration Esketamine Nasal Spray. [(accessed on 5 June 2021)]; Available online: https://www.accessdata.fda.gov/drugsatfda_docs/label/2019/211243lbl.pdf.
- 53.Zeind C.S., Carvalho M.G. Applied Therapeutics: The Clinical Use of Drugs. Wolters Kluwer Health; Philadelphia, PA, USA: 2017. [Google Scholar]
- 54.Katzung B.G. Basic and Clinical Pharmacology. 14th ed. McGraw-Hill Education; New York, NY, USA: 2017. [Google Scholar]
- 55.Kole M.H., Swan L., Fuchs E. The antidepressant tianeptine persistently modulates glutamate receptor currents of the hippocampal CA3 commissural associational synapse in chronically stressed rats. Eur. J. Neurosci. 2002;16:807–816. doi: 10.1046/j.1460-9568.2002.02136.x. [DOI] [PubMed] [Google Scholar]
- 56.Bortolato B., Miskowiak K.W., Köhler C.A., Maes M., Fernandes B.S., Berk M., Carvalho A.F. Cognitive remission: A novel objective for the treatment of major depression? BMC Med. 2016;14:9. doi: 10.1186/s12916-016-0560-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Camargo A., Rodrigues A.L.S. Novel targets for fast antidepressant responses: Possible role of endogenous neuromodulators. Chronic Stress. 2019;3:2470547019858083. doi: 10.1177/2470547019858083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Aripiprazole (Short-Acting Oral and Injectable and Long-Acting Injectable [Abilify Maintena]): Drug Information. [(accessed on 4 June 2021)]; Available online: https://uptodatefree.ir/topic.htm?path=aripiprazole-short-acting-oral-and-injectable-and-long-acting-injectable-abilify-maintena-drug-information.
- 59.US Food and Drug Administration Brexanolone. [(accessed on 5 June 2021)]; Available online: https://www.accessdata.fda.gov/drugsatfda_docs/label/2019/211371lbl.pdf.
- 60.Brexpiprazole: Drug Information. [(accessed on 4 June 2021)]; Available online: https://uptodatefree.ir/topic.htm?path=brexpiprazole-drug-information.
- 61.Cabergoline: Drug Information. [(accessed on 10 June 2021)]; Available online: https://uptodatefree.ir/topic.htm?path=cabergoline-drug-information.
- 62.US Food and Drug Administration Epidiolex. [(accessed on 5 June 2021)]; Available online: https://www.accessdata.fda.gov/drugsatfda_docs/label/2018/210365lbl.pdf.
- 63.Celecoxib: Drug Information. [(accessed on 4 June 2021)]; Available online: https://uptodatefree.ir/topic.htm?path=celecoxib-drug-information.
- 64.Cycloserine: Drug Information. [(accessed on 22 June 2021)]; Available online: https://uptodatefree.ir/topic.htm?path=cycloserine-drug-information#F155547.
- 65.Cyproheptadine: Drug Information. [(accessed on 4 June 2021)]; Available online: https://uptodatefree.ir/topic.htm?path=cyproheptadine-drug-information.
- 66.Dextromethorphan: Drug Information. [(accessed on 4 June 2021)]; Available online: https://uptodatefree.ir/topic.htm?path=dextromethorphan-drug-information.
- 67.Etanercept (Including Biosimilars of Etanercept): Drug Information. [(accessed on 4 June 2021)]; Available online: https://uptodatefree.ir/topic.htm?path=etanercept-drug-information.
- 68.Gabapentin: Drug Information. [(accessed on 22 June 2021)]; Available online: https://uptodatefree.ir/topic.htm?path=gabapentin-drug-information.
- 69.Infliximab (Including Biosimilars of Infliximab): Drug Information. [(accessed on 4 June 2021)]; Available online: https://uptodatefree.ir/topic.htm?path=infliximab-including-biosimilars-of-infliximab-drug-information.
- 70.Ketamine: Drug Information. [(accessed on 4 June 2021)]; Available online: https://uptodatefree.ir/topic.htm?path=ketamine-drug-information.
- 71.Liothyronine: Drug Information. [(accessed on 4 June 2021)]; Available online: https://uptodatefree.ir/topic.htm?path=liothyronine-drug-information.
- 72.Lisdexamfetamine: Drug Information. [(accessed on 4 June 2021)]; Available online: https://uptodatefree.ir/topic.htm?path=lisdexamfetamine-drug-information.
- 73.Lithium: Drug Information. [(accessed on 4 June 2021)]; Available online: https://uptodatefree.ir/topic.htm?path=lithium-drug-information.
- 74.Metformin: Drug Information. [(accessed on 4 June 2021)]; Available online: https://uptodatefree.ir/topic.htm?path=metformin-drug-information.
- 75.Mecamylamine: Drug Information. [(accessed on 22 June 2021)]; Available online: https://uptodatefree.ir/topic.htm?path=mecamylamine-drug-information.
- 76.Minocycline: Drug Information. [(accessed on 4 June 2021)]; Available online: https://uptodatefree.ir/topic.htm?path=minocycline-drug-information.
- 77.Modafinil: Drug Information. [(accessed on 10 June 2021)]; Available online: https://uptodatefree.ir/topic.htm?path=modafinil-drug-information.
- 78.US Food and Drug Administration PROVIGIL® (Modafinil) [(accessed on 10 June 2021)]; Available online: https://www.accessdata.fda.gov/drugsatfda_docs/label/2007/020717s020s013s018lbl.pdf.
- 79.Acetylcysteine: Drug Information. [(accessed on 4 June 2021)]; Available online: https://uptodatefree.ir/topic.htm?path=acetylcysteine-drug-information.
- 80.Nimodipine: Drug Information. [(accessed on 4 June 2021)]; Available online: https://uptodatefree.ir/topic.htm?path=nimodipine-drug-information.
- 81.Olanzapine: Drug Information. [(accessed on 4 June 2021)]; Available online: https://uptodatefree.ir/topic.htm?path=olanzapine-drug-information.
- 82.US Food and Drug Administration Permax® Pergolide Mesylate. [(accessed on 10 June 2021)]; Available online: https://www.accessdata.fda.gov/drugsatfda_docs/label/2003/19385slr030,031,035_permax_lbl.pdf.
- 83.Pioglitazone: Drug Information. [(accessed on 4 June 2021)]; Available online: https://uptodatefree.ir/topic.htm?path=pioglitazone-drug-information.
- 84.Phenytoin: Drug Information. [(accessed on 10 June 2021)]; Available online: https://uptodatefree.ir/topic.htm?path=phenytoin-drug-information.
- 85.Pramipexole: Drug Information. [(accessed on 4 June 2021)]; Available online: https://uptodatefree.ir/topic.htm?path=pramipexole-drug-information.
- 86.Pregabalin: Drug Information. [(accessed on 22 June 2021)]; Available online: https://uptodatefree.ir/topic.htm?path=pregabalin-drug-information.
- 87.Quetiapine: Drug Information. [(accessed on 4 June 2021)]; Available online: https://uptodatefree.ir/topic.htm?path=quetiapine-drug-information.
- 88.Risperidone: Drug Information. [(accessed on 4 June 2021)]; Available online: https://uptodatefree.ir/topic.htm?path=risperidone-drug-information.
- 89.Rosiglitazone: Drug Information. [(accessed on 4 June 2021)]; Available online: https://uptodatefree.ir/topic.htm?path=rosiglitazone-drug-information.
- 90.Scopolamine (Hyoscine) (Systemic): Drug Information. [(accessed on 4 June 2021)]; Available online: https://uptodatefree.ir/topic.htm?path=scopolamine-hyoscine-systemic-drug-information.
- 91.Lovastatin: Drug Information. [(accessed on 4 June 2021)]; Available online: https://uptodatefree.ir/topic.htm?path=lovastatin-drug-information.
- 92.Telmisartan: Drug Information. [(accessed on 4 June 2021)]; Available online: https://uptodatefree.ir/topic.htm?path=telmisartan-drug-information.
- 93.Valproate: Drug Information. [(accessed on 4 June 2021)]; Available online: https://uptodatefree.ir/topic.htm?path=valproate-drug-information.
- 94.Vorinostat: Drug Information. [(accessed on 4 June 2021)]; Available online: https://uptodatefree.ir/topic.htm?path=vorinostat-drug-information.
- 95.US Food and Drug Administration Zolinza (Vorinostat) Capsules Label. [(accessed on 8 June 2021)]; Available online: https://www.accessdata.fda.gov/drugsatfda_docs/label/2011/021991s002lbl.pdf.
- 96.Zinc Acetate: Drug Information. [(accessed on 4 June 2021)]; Available online: https://uptodatefree.ir/topic.htm?path=zinc-acetate-drug-information.
- 97.VA Puget Sound Health Care System. Ketamine Anesthesia for Improvement of Depression in ECT. [(accessed on 3 June 2021)]; Available online: https://ClinicalTrials.gov/show/NCT02752724.
- 98.Sunnybrook Health Sciences Centre Effect of Ketamine vs. Active Placebo on Suicidal Ideation in Depressed Inpatients with Major Depressive Disorder or Bipolar Depression. [(accessed on 3 June 2021)]; Available online: https://ClinicalTrials.gov/show/NCT02593643.
- 99.St Patrick’s Hospital. Ketamine as an Adjunctive Therapy for Major Depression. [(accessed on 3 June 2021)]; Available online: https://ClinicalTrials.gov/show/NCT03256162.
- 100.Shalvata Mental Health Center Intra-Nasal vs. Intra-Venous Ketamine Administration. [(accessed on 3 June 2021)]; Available online: https://ClinicalTrials.gov/show/NCT02644629.
- 101.Massachusetts General Hospital. Brain & Behavior Research Foundation. National Institutes of Health Ketamine for Depression: An MRI Study. [(accessed on 3 June 2021)]; Available online: https://ClinicalTrials.gov/show/NCT02544607.
- 102.Sheba Medical Center Ketamine IV Classic Protocol: Five Years Follow Up. [(accessed on 3 June 2021)]; Available online: https://ClinicalTrials.gov/show/NCT04824157.
- 103.Yale University Cognitive Therapy to Sustain the Antidepressant Effects of Intravenous Ketamine in Treatment-Resistant Depression. [(accessed on 3 June 2021)]; Available online: https://ClinicalTrials.gov/show/NCT03027362.
- 104.Novartis Pharmaceuticals. Novartis Proof of Concept Study Evaluating the Efficacy and Safety of MIJ821 in Patients with Treatment-Resistant Depression. [(accessed on 3 June 2021)]; Available online: https://ClinicalTrials.gov/show/NCT03756129.
- 105.NeuroRx, Inc. Massachusetts General Hospital. Target Health Inc Sequential Therapy for the Treatment of Severe Bipolar Depression. [(accessed on 3 June 2021)]; Available online: https://ClinicalTrials.gov/show/NCT02974010.
- 106.Intra-Cellular Therapies, Inc Clinical Trial Evaluating ITI-007 as an Adjunctive Therapy to Lithium or Valproate for the Treatment of Bipolar Depression. [(accessed on 3 June 2021)]; Available online: https://ClinicalTrials.gov/show/NCT02600507.
- 107.Heintjes E.M., Overbeek J.A., Penning-van Beest F.J., Brobert G., Herings R.M. Post authorization safety study comparing quetiapine to risperidone and olanzapine. Hum. Psychopharmacol. 2016;31:304–312. doi: 10.1002/hup.2539. [DOI] [PubMed] [Google Scholar]
- 108.Otsuka Pharmaceutical Development & Commercialization, Inc Exploratory Trial to Assess the Functionality of an Integrated Call Center for the Digital Medicine System. [(accessed on 3 June 2021)]; Available online: https://ClinicalTrials.gov/show/NCT02722967.
- 109.Duke University. Forest Laboratories. Institute for Advanced Medical Research Levomilnacipran ER vs. Adjunctive Quetiapine for Adults with Inadequate Relief with SSRIs in MDD. [(accessed on 3 June 2021)]; Available online: https://ClinicalTrials.gov/show/NCT02720198.
- 110.Janssen Research & Development, LLC A Study to Compare the Efficacy, Safety, and Tolerability of JNJ-42847922 Versus Quetiapine Extended-Release as Adjunctive Therapy to Antidepressants in Adult Participants With Major Depressive Disorder Who Have Responded Inadequately to Antidepressant Therapy. [(accessed on 3 June 2021)]; Available online: https://ClinicalTrials.gov/show/NCT03321526.
- 111.Whitton A.E., Reinen J.M., Slifstein M., Ang Y.S., McGrath P.J., Iosifescu D.V., Abi-Dargham A., Pizzagalli D.A., Schneier F.R. Baseline reward processing and ventrostriatal dopamine function are associated with pramipexole response in depression. Brain J. Neurol. 2020;143:701–710. doi: 10.1093/brain/awaa002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Frye M.A., Amchin J., Bauer M., Adler C., Yang R., Ketter T.A. Randomized, placebo-controlled, adjunctive study of armodafinil for bipolar I depression: Implications of novel drug design and heterogeneity of concurrent bipolar maintenance treatments. Int. J. Bipolar Disord. 2015;3:34. doi: 10.1186/s40345-015-0034-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Ketter T.A., Yang R., Frye M.A. Adjunctive armodafinil for major depressive episodes associated with bipolar I disorder. J. Affect. Disord. 2015;181:87–91. doi: 10.1016/j.jad.2015.04.012. [DOI] [PubMed] [Google Scholar]
- 114.Calabrese J.R., Frye M.A., Yang R., Ketter T.A. Efficacy and safety of adjunctive armodafinil in adults with major depressive episodes associated with bipolar I disorder: A randomized, double-blind, placebo-controlled, multicenter trial. J. Clin. Psychiatry. 2014;75:1054–1061. doi: 10.4088/JCP.13m08951. [DOI] [PubMed] [Google Scholar]
- 115.Allama Iqbal Open University Islamabad. King Edward Medical University Role of Omega-3 Polyunsaturated Fatty Acid in the Management of Major Depressive Disorder. [(accessed on 3 June 2021)]; Available online: https://ClinicalTrials.gov/show/NCT03732378.
- 116.China Medical University Hospital. National Science Council of Taiwan Omega-3 Polyunsaturated Fatty Acids on Major Depressive Disorder in Patients With Cardiovascular Diseases. [(accessed on 3 June 2021)]; Available online: https://ClinicalTrials.gov/show/NCT03072823.
- 117.Janssen Research & Development, LLC 54135419SUI3001: A Study to Evaluate the Efficacy and Safety of Intranasal Esketamine in Addition to Comprehensive Standard of Care for the Rapid Reduction of the Symptoms of Major Depressive Disorder, Including Suicidal Ideation, in Adult Participants Assessed to be at Imminent Risk for Suicide. [(accessed on 3 June 2021)]; Available online: https://clinicaltrials.gov/ct2/show/NCT03039192.
- 118.Fu D.J., Ionescu D.F., Li X., Lane R., Lim P., Sanacora G., Hough D., Manji H., Drevets W.C., Canuso C.M. Esketamine Nasal Spray for Rapid Reduction of Major Depressive Disorder Symptoms in Patients Who Have Active Suicidal Ideation With Intent: Double-Blind, Randomized Study (ASPIRE I) J. Clin. Psychiatry. 2020;81 doi: 10.4088/JCP.19m13191. [DOI] [PubMed] [Google Scholar]
- 119.Janssen Research & Development, LLC 54135419SUI3002: A Study to Evaluate the Efficacy and Safety of Intranasal Esketamine in Addition to Comprehensive Standard of Care for the Rapid Reduction of the Symptoms of Major Depressive Disorder, Including Suicidal Ideation, in Adult Participants Assessed to be at Imminent Risk for Suicide. [(accessed on 3 June 2021)]; Available online: https://ClinicalTrials.gov/show/NCT03097133.
- 120.Ionescu D.F., Fu D.J., Qiu X., Lane R., Lim P., Kasper S., Hough D., Drevets W.C., Manji H., Canuso C.M. Esketamine Nasal Spray for Rapid Reduction of Depressive Symptoms in Patients With Major Depressive Disorder Who Have Active Suicide Ideation With Intent: Results of a Phase 3, Double-Blind, Randomized Study (ASPIRE II) Int. J. Neuropsychopharmacol. 2021;24:22–31. doi: 10.1093/ijnp/pyaa068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Celon Pharma SA. National Center for Research and Development Efficacy, Safety and Pharmacokinetic Study of Inhaled Esketamine in Treatment-resistant Depression. [(accessed on 3 June 2021)]; Available online: https://ClinicalTrials.gov/show/NCT03965858.
- 122.A Study to Evaluate the Effects of a Single-Dose and Repeat-Administration of Intranasal Esketamine on On-Road Driving in Participants With Major Depressive Disorder. [(accessed on 3 June 2021)]; Available online: https://ClinicalTrials.gov/show/NCT02919579.
- 123.JNJ-54135419 (Esketamine) [(accessed on 3 June 2021)]; Available online: https://s3.amazonaws.com/ctr-jnj-7051/CR108228/d2a59202-2675-439e-a1f3-fe2d4c870cd2/f0a86e38-1c8c-495d-8394-7a6a46291e9c/CR108228_CSR_Synopsis-v1.pdf.
- 124.Efficacy and Safety of AXS-05, an Oral NMDA Receptor Antagonist with Multimodal Activity, in Major Depressive Disorder: Results from the GEMINI Trial. [(accessed on 3 June 2021)]; Available online: https://d3dyybxyjb4kyh.cloudfront.net/pdfs/APA+2021+GEMINI+poster+3_31_21+FINAL.pdf.
- 125.Efficacy and Safety of AXS-05, an Oral NMDA Receptor Antagonist with Multimodal Activity, in Major Depressive Disorder: Results from the ASCEND Trial. [(accessed on 3 June 2021)]; Available online: https://d3dyybxyjb4kyh.cloudfront.net/pdfs/APA+2021+ASCEND+poster+3_31_21+FINAL.pdf.
- 126.Sustained Efficacy and Long-Term Safety of AXS-05, an Oral NMDA Receptor Antagonist, in Major Depressive Disorder: COMET Study Results. [(accessed on 3 June 2021)]; Available online: https://d3dyybxyjb4kyh.cloudfront.net/pdfs/APA+2021+COMET+poster+3_31_21+FINAL.pdf.
- 127.Meyer J., Centre for Addiction and Mental Health New Treatment Approach for Major Depressive Disorder Based Upon Targeting Monoamine Oxidase A (MAO-A) [(accessed on 3 June 2021)]; Available online: https://ClinicalTrials.gov/show/NCT02269540.
- 128.Meyer J., Centre for Addiction and Mental Health Biomarkers of Neuroinflammation and Anti-Inflammatory Treatments in Major Depressive Disorder. [(accessed on 3 June 2021)]; Available online: https://ClinicalTrials.gov/show/NCT02362529.
- 129.Charite University Adjunct Minocycline in Treatment-Resistant Depression. [(accessed on 3 June 2021)]; Available online: https://ClinicalTrials.gov/show/NCT02456948.
- 130.Mineralocorticoid Receptor, NMDA Receptor and Cognitive Function in Depression. [(accessed on 22 June 2021)]; Available online: https://ClinicalTrials.gov/show/NCT03062150.
- 131.Nowacki J., Wingenfeld K., Kaczmarczyk M., Chae W.R., Abu-Tir I., Deuter C.E., Piber D., Hellmann-Regen J., Otte C. Cognitive and emotional empathy after stimulation of brain mineralocorticoid and NMDA receptors in patients with major depression and healthy controls. Neuropsychopharmacology. 2020;45:2155–2161. doi: 10.1038/s41386-020-0777-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.A Study to Assess the Efficacy and Safety of TC-5214 as an Adjunct Therapy in Patients With Major Depressive Disorder. [(accessed on 22 June 2021)]; Available online: https://ClinicalTrials.gov/show/NCT01197508.
- 133.Möller H.J., Demyttenaere K., Olausson B., Szamosi J., Wilson E., Hosford D., Dunbar G., Tummala R., Eriksson H. Two Phase III randomised double-blind studies of fixed-dose TC-5214 (dexmecamylamine) adjunct to ongoing antidepressant therapy in patients with major depressive disorder and an inadequate response to prior antidepressant therapy. World. J. Biol. Psychiatry. 2015;16:483–501. doi: 10.3109/15622975.2014.989261. [DOI] [PubMed] [Google Scholar]
- 134.Vieta E., Thase M.E., Naber D., D’Souza B., Rancans E., Lepola U., Olausson B., Szamosi J., Wilson E., Hosford D., et al. Efficacy and tolerability of flexibly-dosed adjunct TC-5214 (dexmecamylamine) in patients with major depressive disorder and inadequate response to prior antidepressant. Eur. Neuropsychopharmacol. 2014;24:564–574. doi: 10.1016/j.euroneuro.2013.12.008. [DOI] [PubMed] [Google Scholar]
- 135.A Study to Assess the Long-Term Safety of TC-5214 as an Adjunct Therapy in Patients With Major Depressive Disorder. [(accessed on 22 June 2021)]; Available online: https://ClinicalTrials.gov/show/NCT01152554.
- 136.Tummala R., Desai D., Szamosi J., Wilson E., Hosford D., Dunbar G., Eriksson H. Safety and tolerability of dexmecamylamine (TC-5214) adjunct to ongoing antidepressant therapy in patients with major depressive disorder and an inadequate response to antidepressant therapy: Results of a long-term study. J. Clin. Psychopharmacol. 2015;35:77–81. doi: 10.1097/JCP.0000000000000269. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.