Abstract
Background
Beneficial effects of napping on cognition have been suggested in cross-sectional studies. This study aimed to clarify longitudinal associations between cognitive decline and sleep characteristics, particularly daytime napping, over a 5-year period in older adults.
Methods
Study participants were 389 community-dwelling individuals aged ≥65 years living in Ojiya City, Niigata, Japan. Baseline and follow-up examinations were conducted in 2011–2013 and 2016–2018, respectively. Trained nurses visited and interviewed participants to collect the following information at baseline and follow-up: demographic characteristics, disease history, lifestyle habits including bedtime, sleeping hours, and daytime nap duration, and cognitive function. The assessment of cognitive function was performed using the revised Hasegawa’s dementia scale (HDS-R), with cognitive decline defined as a change in the HDS-R of ≤ − 3 over 5 years. Odds ratios (ORs) for cognitive decline were calculated using multiple logistic regression analysis.
Results
Mean age of participants was 74.6 years (SD 6.4), and the cumulative incidence of cognitive decline was 106/389 (27.3%). The adjusted OR for 1–29 min daytime napping was significantly lower compared to that for no napping (OR = 0.47, 95%CI: 0.23–0.96). Earlier bedtime was associated with cognitive decline (adjusted P for trend = 0.0480).
Conclusion
Short daytime napping (< 30 min) reduces the risk of cognitive decline over 5 years for community-dwelling older people. A future study will be necessary to confirm the effect of short napping on the reduction of risk for clinically diagnosed dementia.
Supplementary Information
The online version contains supplementary material available at 10.1186/s12877-021-02418-0.
Keywords: Cohort studies, Napping, Sleep, Cognitive decline, Dementia, Epidemiology, Preventive medicine
Background
Dementia places a tremendous burden on society worldwide. The total number of people with dementia in the world was estimated to be 35.6 million in 2010, and this number is projected to increase to 115.4 million in 2050 [1]. The total cost of dementia is also enormous, estimated at US$ 604 billion in 2010 [1]. Under these circumstances, the prevention of dementia and dementia-related disorders is of high priority.
The role of sleep in cognitive function and dementia has drawn attention, although evidence is still insufficient [2]. According to recent reviews and meta-analyses, sleep duration and sleep disturbance are determinants of cognitive decline and dementia [3–7]. Moreover, daytime napping is reportedly associated with cognitive function in older adults [8–11]. However, findings from previous studies have been somewhat inconsistent; some reported possible adverse effects of napping, especially long napping, on cognitive function [9, 11], whereas others reported possible beneficial effects of napping, especially short napping [8, 10]. Furthermore, except for one longitudinal study [8], only cross-sectional studies [9–11] have been conducted.
We previously conducted an epidemiologic study to investigate associations between cognitive impairment and lifestyle factors, including sleep characteristics and daytime napping, in community-dwelling older adults [12]. The present study aimed to clarify longitudinal associations between cognitive decline and sleep characteristics, in particular whether daytime napping is beneficial or harmful for cognitive function, based on 5-year follow-up data from participants of the study mentioned above.
Methods
Design and participants
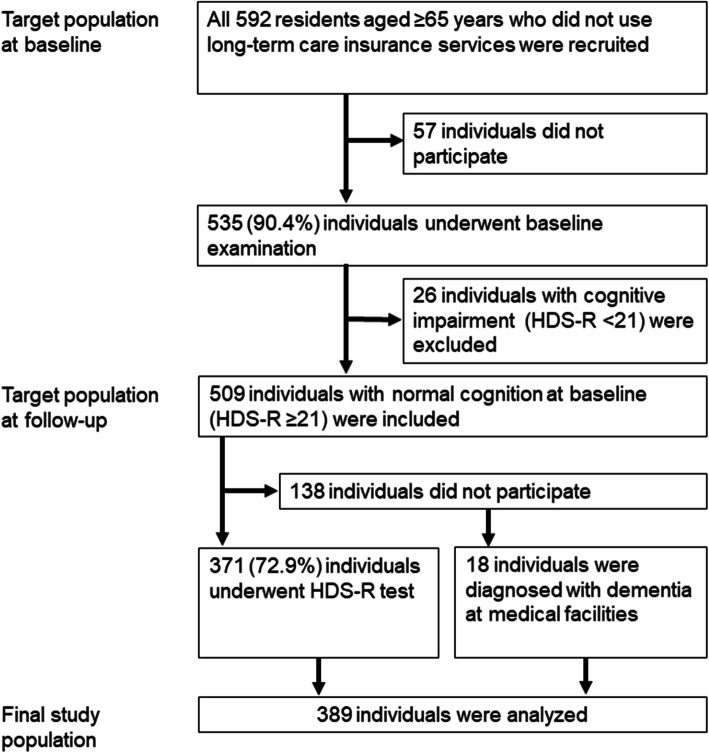
This study was a 5-year follow-up cohort study. Participants of the baseline study were included in the study, and those with cognitive impairment diagnosed by the revised Hasegawa’s dementia scale (HDS-R) at baseline were excluded. Participants at baseline were community-dwelling older adults living in the following three areas of Ojiya City, Niigata, which were set by the city government as model areas: Heiseicho (an urban area), Matto (a rural, farming area), and central Katakai (an urban area) [12]. Among all 592 residents aged ≥65 years who were not receiving long-term care insurance services and who were invited to participate in the study, 535 (90.4%) underwent the baseline examination. A high participation rate (90.4%) was obtained due to efforts of public health nurses in charge of each area. Of these 535 residents, 509 (95.1%) who were considered cognitively normal were invited to participate in the present 5-year follow-up study, and 371 (72.9%) underwent the follow-up examination. We also included 18 individuals who had normal cognitive function at baseline and did not participate in the follow-up examination, but were diagnosed with dementia at medical facilities during the follow-up period, because these individuals met our diagnostic criteria of cognitive decline described below. The final study cohort thus comprised 389 individuals. Figure 1 shows the flow of participant enrollment. Informed consent was obtained from all participants who underwent the follow-up examination. The consent was verbal because, according to the Ethical Guidelines for Medical and Health Research Involving Human Subjects in Japan [13], investigators are not required to obtain informed consent in writing for human studies which are not invasive or do not involve interventions. The protocol of this study was approved by the Ethics Committee of Niigata University.
Fig. 1.
Flow chart of participant enrollment
Baseline examination
The baseline examination was conducted in three areas of Ojiya city in October–December 2011 for Heisei-cho residents, August–November 2012 for Matto residents, and July–September 2013 for Katakai residents. Trained nurses visited and interviewed participants to collect the following information: demographic characteristics, health status (including cognitive function) and lifestyle, family environment (living with family or alone), current occupational status (unemployed/retired or employed), and histories of hypertension, cerebrovascular disease, and diabetes. The interviewer’s guide of this study is shown in Additional Information 1. Cognitive function was assessed using the HDS-R [14]. We also collected information regarding alcohol consumption (classified into five categories: 1) non-drinker, 2) < 7 gou (1 gou is equivalent to 180 mL of Japanese sake), 3) 7–13 gou, 4) 14–20 gou, and 5) ≥21 gou per week) and smoking status (classified into three categories: 1) non-smoker, 2) past smoker, and 3) current smoker). Usual bedtime and sleeping hours were asked and recorded, and the duration of daytime napping was recorded as 1) none, 2) < 30 min, 3) 30–59 min, and 4) ≥60 min. We did not consider the number of daytime naps because this information was not obtained during the interviews. Participants were also asked about current sleep disturbances and the use of sleeping pills. Details of the baseline examination have been described previously [12].
Five-year follow-up examination
The follow-up examination, including the HDS-R test, was conducted 5 years after, and in the same manner as, the baseline examination, i.e., in October–December 2016 for Heisei-cho residents, August–November 2017 for Matto residents, and July–August 2018 for Katakai residents.
Assessment of cognitive function
The HDS-R, a 30-point test, was used to assess general cognitive function, with a score ≤ 20 defined as cognitive impairment at baseline. The HDS-R was developed to screen for dementia (sensitivity: 0.90, specificity: 0.82) with a cutoff of 20/21 [14]. The HDS-R has been used in East Asian populations [15, 16] and demonstrated to have a diagnostic accuracy similar to that of the MMSE [17]. One advantage of using the HDS-R over the MMSE is its diagnostic accuracy regardless of education level [17]. The HDS-R was administered during baseline and follow-up examinations, and change in HDS-R (ΔHDS-R = [score at follow-up] – [score at the baseline]) was calculated. We used a cutoff of ΔHDS-R ≤ -3 to detect the presence of cognitive decline, referring to the cutoff of ΔMMSE ≤-3 (30-point test) used in previous studies [18, 19], based on the fact that longitudinal scores of HDS-R and MMSE change in the same direction in community-dwelling individuals [20]. We also included 18 individuals who had normal cognitive function at baseline and did not participate in the follow-up examination, but were diagnosed with dementia at medical facilities, as having cognitive decline.
Statistical methods
The χ2 test was used to test for independence of categorical data for participant characteristics. Cumulative incidence of cognitive decline was calculated and compared according to levels of potential predictor variables by odds ratios (ORs) computed using simple and multiple logistic regression analyses. Dose-dependent associations were assessed by simple and multiple logistic regression analyses and reported as P for trend values. First, unadjusted ORs for cognitive decline according to potential predictors were calculated. Second, ORs were adjusted for age and baseline HDS-R. Third, we calculated multivariable-adjusted ORs (hereafter referred to as “fully-adjusted ORs”) of bedtime, duration of sleep, and duration of nap for cognitive decline using the forced entry method to adjust for the confounding effects of all potential predictor variables. For example, the OR for duration of sleep was calculated and adjusted for age, baseline HDS-R, sex, region (dummy variables), family environment, job status, histories of hypertension, cerebrovascular diseases, and diabetes, alcohol consumption, smoking status, bedtime, duration of nap, presence of sleep disturbance, and use of sleeping pills. Duration of nap was transformed into a dummy variable (0, 1–29 min; 1, others) because the results suggest that a 1–29 min nap is associated with a decreased risk of cognitive decline. Although a cutoff of ΔHDS-R ≤ -3 was used to detect cognitive decline, we used cutoffs of ≤ − 4 and ≤ − 2 for the sensitivity analyses. Outliers exceeding 3SD were not found among continuous variables. SAS statistical software (release 9.1.3, SAS Institute Inc., Cary, NC, USA) was used for data analysis. P < 0.05 was considered statistically significant.
Results
The mean age of participants was 74.6 years (SD 6.4). Table 1 shows the baseline characteristics of participants by sex. Significant sex-based differences were observed in family environment, job status, and alcohol consumption. Specifically, significantly higher proportions of women lived alone, were unemployed, had lower alcohol consumption, and had a later bedtime than men. Moreover, women took significantly shorter naps than men, and had a greater proportion of those who do not nap at all.
Table 1.
Participant characteristics at baseline
| Characteristics | Men (n = 159) | Women (n = 230) | P value |
|---|---|---|---|
| Age (years) | |||
| 65–69 | 39 (24.5%) | 58 (25.2%) | 0.9955 |
| 70–79 | 83 (52.2%) | 117 (50.9%) | |
| 80–89 | 35 (22.0%) | 52 (22.6%) | |
| 90–99 | 2 (1.3%) | 3 (1.3%) | |
| Baseline HDS-R score | |||
| ≥ 26 | 126 (79.3%) | 195 (84.8%) | 0.1575 |
| 21–25 | 33 (20.8%) | 35 (15.2%) | |
| Area of residence | |||
| Heisei-cho | 40 (25.2%) | 63 (27.4%) | 0.8174 |
| Matto | 67 (42.1%) | 98 (42.6%) | |
| Katakai | 52 (32.7%) | 69 (30.0%) | |
| Family environment | |||
| Living with family | 153 (96.2%) | 206 (89.6%) | 0.0155 |
| Living alone | 6 (3.8%) | 24 (10.4%) | |
| Job status | |||
| Employed | 84 (52.8%) | 52 (22.6%) | < 0.0001 |
| Unemployed/retired | 75 (47.2%) | 178 (77.4%) | |
| History of hypertension | |||
| Absent | 82 (51.6%) | 116 (50.4%) | 0.8254 |
| Present | 77 (48.4%) | 114 (49.6%) | |
| History of cerebrovascular disease | |||
| Absent | 150 (94.3%) | 224 (97.4%) | 0.1244 |
| Present | 9 (5.7%) | 6 (2.6%) | |
| History of diabetes | |||
| Absent | 146 (91.8%) | 210 (91.3%) | 0.8565 |
| Present | 13 (8.2%) | 20 (8.7%) | |
| Alcohol consumptiona(gou/wk) | |||
| Non-drinker | 41 (25.8%) | 167 (72.6%) | < 0.0001 |
| < 7 | 21 (13.2%) | 37 (16.1%) | |
| 7–13 | 36 (22.6%) | 22 (9.6%) | |
| 14–20 | 41 (25.8%) | 3 (1.3%) | |
| ≥ 21 | 20 (12.6%) | 1 (0.4%) | |
| Smoking | |||
| Non-smoker | 53 (33.3%) | 224 (97.4%) | < 0.0001 |
| Past smoker | 60 (37.7%) | 2 (0.9%) | |
| Current smoker | 46 (28.9%) | 4 (1.7%) | |
| Bedtime | |||
| -8:59 p.m. | 27 (17.0%) | 18 (7.8%) | < 0.0001 |
| 9:00–9:59 p.m. | 62 (39.0%) | 54 (23.5%) | |
| 10:00–10:59 p.m. | 39 (24.5%) | 79 (34.3%) | |
| 11:00 p.m.- | 31 (19.5%) | 79 (34.3%) | |
| Duration of nighttime sleep (hr) | |||
| < 6 | 23 (14.5%) | 48 (20.9%) | 0.1437 |
| 6–6.9 | 38 (23.9%) | 65 (28.3%) | |
| 7–7.9 | 52 (32.7%) | 73 (31.7%) | |
| 8–8.9 | 35 (22.0%) | 32 (13.9%) | |
| ≥ 9 | 11 (6.9%) | 12 (5.2%) | |
| Duration of daytime nap (min) | |||
| 0 | 52 (32.7%) | 101 (43.9%) | 0.0098 |
| 1–29 | 35 (22.0%) | 63 (27.4%) | |
| 30–59 | 39 (24.5%) | 35 (15.2%) | |
| ≥ 60 | 33 (20.8%) | 31 (13.5%) | |
aEquivalent to Japanese sake (1 gou of sake is equivalent to 180 mg sake or 27 g ethanol)
The overall cumulative incidence of cognitive decline was 106/389 (27.3%). Table 2 shows the cumulative incidence, unadjusted ORs, and age- and baseline-HDS-R-adjusted ORs for cognitive decline according to levels of predictor variables. Age was robustly associated with unadjusted ORs for cognitive decline. The age- and baseline-HDS-R-adjusted OR was significantly lower for “1–29 (min)” daytime napping relative to no napping (reference).
Table 2.
Cumulative incidence and odds ratios (ORs) for cognitive declinea according to levels of predictor variables
| Predictors | Cumulative incidence | Unadjusted OR (95% CI) | Adjusted ORb (95% CI) | Fully-adjustedc OR (95% CI) |
|---|---|---|---|---|
| Age (years) | P for trend< 0.0001 | |||
| 65–69 | 12/97 (12.4%) | 1 (Ref) | ||
| 70–79 | 46/200 (23.0%) | 2.12 (1.06–4.21) | ||
| 80–89 | 45/87 (51.7%) | 7.59 (3.63–15.85) | ||
| 90–99 | 3/5 (60.0%) | 10.62 (1.61–70.22) | ||
| Sex | ||||
| Men | 46/159 (28.9%) | 1 (Ref) | 1 (Ref) | |
| Women | 60/230 (26.1%) | 0.87 (0.55–1.36) | 0.80 (0.49–1.29) | |
| Area of residence | ||||
| Heisei-cho | 30/103 (29.1%) | 1 (Ref) | 1 (Ref) | |
| Matto | 37/165 (22.4%) | 0.70 (0.40–1.23) | 0.70 (0.39–1.27) | |
| Katakai | 39/121 (32.2%) | 1.16 (0.65–2.05) | 1.14 (0.62–2.09) | |
| Family environment | ||||
| Living with family | 94/359 (26.2%) | 1 (Ref) | 1 (Ref) | |
| Living alone | 12/30 (40.0%) | 1.88 (0.87–4.05) | 1.81 (0.81–4.03) | |
| Job status | ||||
| Employed | 23/136 (16.9%) | 1 (Ref) | 1 (Ref) | |
| Unemployed/retired | 83/253 (32.8%) | 2.40 (1.43–4.03) | 1.78 (1.03–3.07) | |
| History of hypertension | ||||
| Absent | 44/198 (22.2%) | 1 (Ref) | 1 (Ref) | |
| Present | 62/191 (32.5%) | 1.68 (1.07–2.64) | 1.23 (0.76–1.99) | |
| History of cerebrovascular disease | ||||
| Absent | 101/374 (27.0%) | 1 (Ref) | 1 (Ref) | |
| Present | 5/15 (33.3%) | 1.35 (0.45–4.05) | 1.23 (0.40–3.80) | |
| History of diabetes | ||||
| Absent | 99/356 (27.8%) | 1 (Ref) | 1 (Ref) | |
| Present | 7/33 (21.2%) | 0.70 (0.29–1.66) | 0.72 (0.29–1.77) | |
| Alcohol consumptiond(gou/wk) | P for trend = 0.3590 | P for trend = 0.7898 | ||
| Non-drinker | 64/208 (30.8%) | 1 (Ref) | 1 (Ref) | |
| < 7 | 13/58 (22.4%) | 0.65 (0.33–1.29) | 0.71 (0.34–1.45) | |
| 7–13 | 11/58 (19.0%) | 0.53 (0.26–1.08) | 0.70 (0.33–1.48) | |
| 14–20 | 11/44 (25.0%) | 0.75 (0.36–1.58) | 0.95 (0.43–2.09) | |
| ≥ 21 | 7/21 (33.3%) | 1.13 (0.43–2.92) | 1.88 (0.68–5.18) | |
| Smoking | P for trend = 0.2706 | P for trend = 0.0563 | ||
| Non-smoker | 72/277 (26.0%) | 1 (Ref) | 1 (Ref) | |
| Past smoker | 17/62 (27.4%) | 1.08 (0.58–2.00) | 1.31 (0.67–2.56) | |
| Current smoker | 17/50 (34.0%) | 1.47 (0.77–2.79) | 1.94 (0.97–3.87) | |
| Bedtime | P for trend = 0.0698 | P for trend = 0.1472 | P for trend = 0.0480 | |
| -8:59 p.m. | 13/45 (28.9%) | 1 (Ref) | 1 (Ref) | 1 (Ref) |
| 9:00–9:59 p.m. | 38/116 (32.8%) | 1.20 (0.57–2.54) | 1.28 (0.58–2.85) | 1.29 (0.55–3.04) |
| 10:00–10:59 p.m. | 33/118 (28.0%) | 0.96 (0.45–2.04) | 0.93 (0.42–2.09) | 0.80 (0.32–2.00) |
| 11:00 p.m.- | 22/110 (20.0%) | 0.62 (0.28–1.36) | 0.71 (0.30–1.65) | 0.50 (0.18–1.39) |
| Duration of nighttime sleep (hr) | P for trend = 0.0284 | P for trend = 0.2737 | P for trend = 0.7540 | |
| < 6 | 17/71 (23.9%) | 1.00 (0.50–1.97) | 1.16 (0.57–2.36) | 1.33 (0.61–2.88) |
| 6–6.9 | 24/103 (23.3%) | 0.96 (0.52–1.78) | 1.03 (0.54–1.94) | 1.13 (0.57–2.24) |
| 7–7.9 | 30/125 (24.0%) | 1 (Ref) | 1 (Ref) | 1 (Ref) |
| 8–8.9 | 26/67 (38.8%) | 2.01 (1.06–3.81) | 1.67 (0.84–3.32) | 1.55 (0.74–3.26) |
| ≥ 9 | 9/23 (39.1%) | 2.04 (0.80–5.17) | 1.73 (0.63–4.76) | 1.47 (0.50–4.35) |
| Duration of daytime nap (min) | P for trend = 0.1085 | P for trend = 0.8387 | P for trend = 0.9923 | |
| None | 44/153 (28.8%) | 1 (Ref) | 1 (Ref) | 1 (Ref) |
| 1–29 | 15/98 (15.3%) | 0.45 (0.23–0.86) | 0.46 (0.23–0.90) | 0.47 (0.23–0.96) |
| 30–59 | 21/74 (28.4%) | 0.98 (0.53–1.82) | 0.83 (0.43–1.59) | 0.79 (0.40–1.58) |
| ≥ 60 | 26/64 (40.6%) | 1.70 (0.92–3.12) | 1.11 (0.57–2.15) | 1.05 (0.51–2.13) |
| Sleep disturbance | ||||
| Absent | 91/338 (26.9%) | 1 (Ref) | 1 (Ref) | |
| Present | 14/50 (28.0%) | 1.06 (0.54–2.05) | 1.20 (0.60–2.41) | |
| Use of sleeping pills | ||||
| No | 81/308 (26.3%) | 1 (Ref) | 1 (Ref) | |
| Yes | 25/81 (30.9%) | 1.25 (0.73–2.14) | 1.13 (0.64–2.00) | |
aΔHDS-R ≤ 3
bAdjusted for age and baseline HDS-R score
cAdjusted for age, baseline HDS-R score, sex, region (dummy variables), family environment, job status, histories of hypertension, cerebrovascular diseases, and diabetes, alcohol consumption, smoking status, bedtime, duration of sleep, duration of nap (0, 1–29 min; 1, others), presence of sleep disturbance, and use of sleeping pills
dEquivalent to Japanese sake (1 gou of sake is equivalent to 180 ml sake or 27 g ethanol)
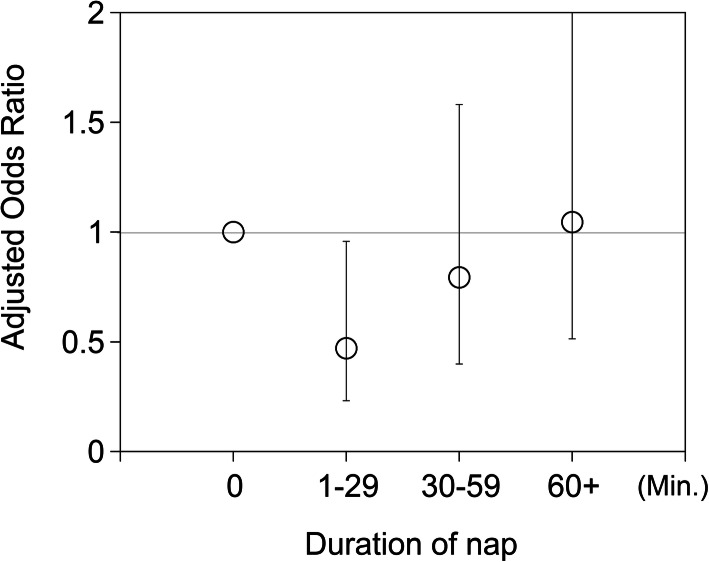
The fully-adjusted OR was significantly lower for “1–29 (min)” daytime napping relative to no napping (OR = 0.47, 95%CI: 0.23–0.96) (Table 2, Fig. 2). Earlier bedtime was dose-dependently associated with cognitive decline (fully-adjusted P for trend = 0.0480) (Table 2). However, the duration of nighttime sleep was not associated with cognitive decline, sleep disturbance, or the use of sleeping pills. Napping duration and nighttime sleep duration were not significantly correlated with each other (Spearman’s correlation coefficient r = 0.082, P = 0.1036).
Fig. 2.
Odds ratios (ORs) for cognitive decline (ΔHDS-R ≤ -3) over 5 years according to daytime nap duration. Participants taking 1–29 min naps had a significantly lower risk of cognitive decline
Sensitivity analyses were conducted to confirm the significant findings above. When a cutoff of ΔHDS-R ≤ -4 was used to detect cognitive decline, the fully-adjusted OR for cognitive decline was significantly lower for “1–29 (min)” daytime napping relative to no napping (fully-adjusted OR = 0.39, 95%CI: 0.16–0.91; Additional Figure 1), and the fully-adjusted P for trend of bedtime was 0.1612. When a cutoff of ΔHDS-R ≤ -2 was used, the fully-adjusted OR was marginally lower for “1–29 (min)” daytime napping relative to no napping (fully-adjusted OR = 0.58, 95%CI: 0.32–1.05; Additional Figure 2), and earlier bedtime was associated with cognitive decline (fully-adjusted P for trend = 0.0025), with a fully-adjusted OR of 0.28 (95%CI: 0.11–0.71) for “11:00 p.m.-” relative to “-8:59 p.m.” (reference).
Discussion
This study is the first to report a significant decrease in cognitive decline in older adults who habitually take short daytime naps (< 30 min). Cross-sectional studies previously showed associations between daytime napping and cognitive impairment. Cross et al. [9] reported that longer napping is significantly correlated with increased levels of cognitive deficits in 133 adults aged > 50 years. Similarly, Owusu et al. [11] showed that unintentional, longer naps were correlated with poorer performance on cognitive tests in 2549 community-dwelling adults aged ≥65 years. While these studies suggested the unfavorable effect of longer napping, shorter napping reportedly had a favorable effect on cognitive performance [21]. In a cross-sectional study in clinical settings, Asada et al. [21] found that napping for up to 60 min, but not more than 60 min, was protective against the development of Alzheimer’s disease. Lin et al. [10] conducted a large-scale cross-sectional study in 10,740 Chinese older adults (≥60 years) and found that short nappers (< 30 min) had a significantly lower prevalence of cognitive impairment as assessed by the MMSE compared to no nappers and long nappers (≥30 min). Our findings are consistent with this report.
To date, only one longitudinal study of up to 10 years has been conducted [8], which reported that the risk of MMSE-assessed cognitive impairment was significantly lower in those who habitually take naps, regardless of duration, in a UK cohort (median age, 75 years). The discrepancy between their findings and ours may be due to the different definitions of cognitive impairment. The present study used a decrease in HDS-R score of ≤ − 3 points to define cognitive impairment, whereas Keage et al. [8] used newly diagnosed cognitive impairment according to MMSE scores. It is also possible that factors such as the difference in ethnicity might have played a role.
A number of studies have reported on the physiologically beneficial effects of napping on cognitive performance in adults [22, 23]. One study even suggested that napping leads to improved cognitive performance in older adults [24]. However, the specific effects of short naps are not fully understood. Short naps (< 30 min) reportedly improved cognitive performance and alertness and were associated with less sleep inertia [22, 24]. Moreover, an epidemiologic study found that short naps, but not long naps, had a protective effect against cardiovascular risk [23], suggesting that short naps have stress-releasing effects. These findings suggest that short naps might also be beneficial for cognitive function, since cognitive decline and dementia are considered stress-related conditions/diseases [25, 26].
The underlying mechanism by which short napping confers a beneficial effect is unclear. However, there is growing evidence that brain β-amyloid is cleared during sleep through the glymphatic system [27], and that low sleep quality is associated with brain β-amyloid burden in older adults [27]. Thus, short napping may be beneficial against brain β-amyloid by improving sleep quality. Short napping may also have a direct, favorable effect on β-amyloid clearance, although this will need to be investigated in future studies.
Recent meta-analyses have found an association between longer sleep duration and increased risk of cognitive decline [3, 4]. However, we did not find a significant association between the duration of nighttime sleep and the risk of cognitive decline, although longer sleep groups (“8–8.9-h” and “≥9-h” groups) tended to have a higher risk of cognitive decline (OR = 1.55, 95%CI: 0.74–3.26, N = 67, and OR = 1.47, 95%CI: 0.50–4.35, N = 23; respectively). The non-significant ORs may partly be due to insufficient sample sizes of the longer sleep groups. Nonetheless, our findings are consistent with the findings of the meta-analyses mentioned above [3, 4].
In the present study, an earlier bedtime was associated with a higher risk of cognitive decline (fully-adjusted P for trend = 0.0480). While evidence is scarce, a large cohort study [28] found no association between bedtime and the risk of dementia, although that study only classified bedtime as earlier or later than 11 PM. Older people living with dementia reportedly go to bed early [29]. Thus, the effect of bedtime on cognition warrants further examination.
Some baseline characteristics differed by sex, including the duration of daytime nap, in our study population. Moreover, a significantly greater proportion of women took no daytime nap compared to men, suggesting that this, as well as other female-specific characteristics (e.g., family environment, job status, alcohol consumption), may account for the higher risk of dementia in women relative to men.
The present study has several strengths. First, we used a cohort design, which is preferable for detecting causal associations. Second, this study had a high participation rate at baseline (90.4%) and an acceptable follow-up rate (72.9%). Finally, information regarding participant lifestyle was obtained and confirmed through interviews during home visits by experienced nurses.
This study also has some limitations. First, we obtained sleep-related information through interviews by trained nurses, but the information was based on self-report. Therefore, there is a possibility of misclassification bias, which might have led to an underestimation of associations between predictors and outcomes. Second, we did not collect information on naptime, although the timing of naps is an important factor related to cognitive function [24]. We did not systematically obtain information on the number of naps either. These two aspects, as well as nap duration, could influence sleep quality at night, which in turn could affect cognitive function. Finally, we did not evaluate conditions which could affect sleep, such as depression and sleep apnea.
Conclusion
Short daytime napping (< 30 min) reduces the risk of cognitive decline over 5 years for community-dwelling older adults. Further studies will be needed to determine if short naps decrease the risk of clinically-diagnosed dementia.
Supplementary Information
Additional file 1: Figure 1. Odds ratios (ORs) for cognitive decline (ΔHDS-R ≤ -4) over 5 years according to daytime nap duration.
Additional file 2: Figure 2. Odds ratios (ORs) for cognitive decline (ΔHDS-R ≤ -2) over 5 years according to daytime nap duration.
Additional file 3. The interviewer’s guide.
Acknowledgements
We thank the staff of Ojiya City Office who helped us with data collection. This work was supported in part by the Niigata Institute for Traumatic Stress (Mental Health and Welfare Association in Niigata Prefecture). We used the supercomputer at ACCMS, Kyoto University.
Abbreviations
- HDS-R
Hasegawa’s dementia scale
- ORs
Odds ratios
- SD
Standard deviation
- CI
Confidence interval
Authors’ contributions
All authors contributed to the study conception and design. KK contributed to design and conceptualization, analyzing data, drafting the manuscript, and revising the manuscript for intellectual content. KN contributed to design and conceptualization, analyzing data, and revising the manuscript for intellectual content. YW and TS contributed to revising the manuscript for intellectual content. CT, NH, and HS contributed to design and conceptualization and data collection. All authors read and approved the final manuscript.
Funding
Initial of author who received the award: KK.
Grant number awarded to the author: Grant of the Kimura Foundation for Nursing Education (2018).
URL of the funder’s website: http://www.nurseed.jp/projects/research/index.shtml.
The funder had no role in the study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Availability of data and materials
Data are available to researchers who meet the criteria for access to confidential data. We cannot provide individual data because informed consent to provide data to anyone outside the research group was not obtained from participants. Please contact the corresponding author (principal investigator: Dr. K Nakamura) regarding any requests for access to confidential data.
Declarations
Ethics approval and consent to participate
This study was approved by the Ethics Committee of Niigata University. Informed consent was obtained from all participants. The consent was verbal because, according to the Ethical Guidelines for Medical and Health Research Involving Human Subjects in Japan, investigators are not required to obtain informed consent in writing for human studies which are not invasive or do not involve interventions. We maintain records of the methods for providing and content of the information and a list of the participants.
Consent for publication
Not applicable.
Competing interests
The authors have no conflicts of interest to report.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.International WHOaAsD . Dementia: a public health priority. Geneva: World Health Organization; 2012. [Google Scholar]
- 2.Livingston G, Sommerlad A, Orgeta V, Costafreda SG, Huntley J, Ames D, Ballard C, Banerjee S, Burns A, Cohen-Mansfield J, Cooper C, Fox N, Gitlin LN, Howard R, Kales HC, Larson EB, Ritchie K, Rockwood K, Sampson EL, Samus Q, Schneider LS, Selbæk G, Teri L, Mukadam N. Dementia prevention, intervention, and care. Lancet. 2017;390(10113):2673–2734. doi: 10.1016/S0140-6736(17)31363-6. [DOI] [PubMed] [Google Scholar]
- 3.Kim HB, Myung SK, Lee SM, Park YC. Longer duration of sleep and risk of cognitive decline: a Meta-analysis of observational studies. Neuroepidemiology. 2016;47(3–4):171–180. doi: 10.1159/000454737. [DOI] [PubMed] [Google Scholar]
- 4.Wu L, Sun D, Tan Y. A systematic review and dose-response meta-analysis of sleep duration and the occurrence of cognitive disorders. Sleep Breath. 2018;22(3):805–814. doi: 10.1007/s11325-017-1527-0. [DOI] [PubMed] [Google Scholar]
- 5.Liang Y, Qu LB, Liu H. Non-linear associations between sleep duration and the risks of mild cognitive impairment/dementia and cognitive decline: a dose-response meta-analysis of observational studies. Aging Clin Exp Res. 2019;31(3):309–320. doi: 10.1007/s40520-018-1005-y. [DOI] [PubMed] [Google Scholar]
- 6.Wennberg AMV, Wu MN, Rosenberg PB, Spira AP. Sleep disturbance, cognitive decline, and dementia: a review. Semin Neurol. 2017;37(4):395–406. doi: 10.1055/s-0037-1604351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Shi L, Chen SJ, Ma MY, Bao YP, Han Y, Wang YM, Shi J, Vitiello MV, Lu L. Sleep disturbances increase the risk of dementia: a systematic review and meta-analysis. Sleep Med Rev. 2018;40:4–16. doi: 10.1016/j.smrv.2017.06.010. [DOI] [PubMed] [Google Scholar]
- 8.Keage HA, Banks S, Yang KL, Morgan K, Brayne C, Matthews FE. What sleep characteristics predict cognitive decline in the elderly? Sleep Med. 2012;13(7):886–892. doi: 10.1016/j.sleep.2012.02.003. [DOI] [PubMed] [Google Scholar]
- 9.Cross N, Terpening Z, Rogers NL, Duffy SL, Hickie IB, Lewis SJ, Naismith SL. Napping in older people 'at risk' of dementia: relationships with depression, cognition, medical burden and sleep quality. J Sleep Res. 2015;24(5):494–502. doi: 10.1111/jsr.12313. [DOI] [PubMed] [Google Scholar]
- 10.Lin JF, Li FD, Chen XG, He F, Zhai YJ, Pan XQ, Wang XY, Zhang T, Yu M. Association of postlunch napping duration and night-time sleep duration with cognitive impairment in Chinese elderly: a cross-sectional study. BMJ Open. 2018;8(12):e023188. doi: 10.1136/bmjopen-2018-023188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Owusu JT, Wennberg AMV, Holingue CB, Tzuang M, Abeson KD, Spira AP. Napping characteristics and cognitive performance in older adults. Int J Geriatr Psychiatry. 2019;34(1):87–96. doi: 10.1002/gps.4991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nakamura K, Kitamura K, Watanabe Y, Shinoda H, Sato H, Someya T. Rural-urban differences in the prevalence of cognitive impairment in independent community-dwelling elderly residents of Ojiya city, Niigata prefecture, Japan. Environ Health Prev Med. 2016;21(6):422–429. doi: 10.1007/s12199-016-0542-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mnistry of Health, Labour, and Welfare. Ethical Guidelines for Medical and Health Research Involving Human Subjects. 2015. https://www.mhlw.go.jp/file/06-Seisakujouhou-10600000-Daijinkanboukouseikagakuka/0000080278.pdf.
- 14.Imai YH, K. The revised Hasegawa's dementia scale (HDS-R)-evaluation of its usefulness as a screening test for dementia. Hong Kong J Psychiatry. 1994;4(Suppl 2):20–24. [Google Scholar]
- 15.Jeong JW, Kim KW, Lee DY, Lee SB, Park JH, Choi EA, Choe JY, Do YJ, Ryang JS, Roh HA, Park YS, Choi Y, Woo JI. A normative study of the revised Hasegawa dementia scale: comparison of demographic influences between the revised Hasegawa dementia scale and the Mini-mental status examination. Dement Geriatr Cogn Disord. 2007;24(4):288–293. doi: 10.1159/000107592. [DOI] [PubMed] [Google Scholar]
- 16.Rosli R, Tan MP, Gray WK, Subramanian P, Chin AV. Cognitive assessment tools in Asia: a systematic review. Int Psychogeriatr. 2016;28(2):189–210. doi: 10.1017/S1041610215001635. [DOI] [PubMed] [Google Scholar]
- 17.Kim KW, Lee DY, Jhoo JH, Youn JC, Suh YJ, Jun YH, Seo EH, Woo JI. Diagnostic accuracy of mini-mental status examination and revised hasegawa dementia scale for Alzheimer's disease. Dement Geriatr Cogn Disord. 2005;19(5–6):324–330. doi: 10.1159/000084558. [DOI] [PubMed] [Google Scholar]
- 18.Hensel A, Angermeyer MC, Riedel-Heller SG. Measuring cognitive change in older adults: reliable change indices for the Mini-mental state examination. J Neurol Neurosurg Psychiatry. 2007;78(12):1298–1303. doi: 10.1136/jnnp.2006.109074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Taniguchi Y, Yoshida H, Fujiwara Y, Motohashi Y, Shinkai S. A prospective study of gait performance and subsequent cognitive decline in a general population of older Japanese. J Gerontol A Biol Sci Med Sci. 2012;67(7):796–803. doi: 10.1093/gerona/glr243. [DOI] [PubMed] [Google Scholar]
- 20.Yamamoto N, Yamanaka G, Ishikawa M, Takasugi E, Murakami S, Yamanaka T, Ishine M, Matsubayashi K, Hanafusa T, Otsuka K. Cardio-ankle vascular index as a predictor of cognitive impairment in community-dwelling elderly people: four-year follow-up. Dement Geriatr Cogn Disord. 2009;28(2):153–158. doi: 10.1159/000235642. [DOI] [PubMed] [Google Scholar]
- 21.Asada T, Motonaga T, Yamagata Z, Uno M, Takahashi K. Associations between retrospectively recalled napping behavior and later development of Alzheimer's disease: association with APOE genotypes. Sleep. 2000;23(5):629–634. [PubMed] [Google Scholar]
- 22.Lovato N, Lack L. The effects of napping on cognitive functioning. Prog Brain Res. 2010;185:155–166. doi: 10.1016/B978-0-444-53702-7.00009-9. [DOI] [PubMed] [Google Scholar]
- 23.Faraut B, Andrillon T, Vecchierini MF, Leger D. Napping: a public health issue. From epidemiological to laboratory studies. Sleep Med Rev. 2017;35:85–100. doi: 10.1016/j.smrv.2016.09.002. [DOI] [PubMed] [Google Scholar]
- 24.Milner CE, Cote KA. Benefits of napping in healthy adults: impact of nap length, time of day, age, and experience with napping. J Sleep Res. 2009;18(2):272–281. doi: 10.1111/j.1365-2869.2008.00718.x. [DOI] [PubMed] [Google Scholar]
- 25.Machado A, Herrera AJ, de Pablos RM, Espinosa-Oliva AM, Sarmiento M, Ayala A, Venero JL, Santiago M, Villarán RF, Delgado-Cortés MJ, Argüelles S, Cano J. Chronic stress as a risk factor for Alzheimer's disease. Rev Neurosci. 2014;25(6):785–804. doi: 10.1515/revneuro-2014-0035. [DOI] [PubMed] [Google Scholar]
- 26.Greenberg MS, Tanev K, Marin MF, Pitman RK. Stress, PTSD, and dementia. Alzheimers Dement. 2014;10(3 Suppl):S155–S165. doi: 10.1016/j.jalz.2014.04.008. [DOI] [PubMed] [Google Scholar]
- 27.Cordone S, Annarumma L, Rossini PM, De Gennaro L. Sleep and β-amyloid deposition in Alzheimer disease: insights on mechanisms and possible innovative treatments. Front Pharmacol. 2019;10:695. doi: 10.3389/fphar.2019.00695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bokenberger K, Strom P, Dahl Aslan AK, Johansson AL, Gatz M, Pedersen NL, Akerstedt T. Association between sleep characteristics and incident dementia accounting for baseline cognitive status: a prospective population-based study. J Gerontol A Biol Sci Med Sci. 2017;72(1):134–139. doi: 10.1093/gerona/glw127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Harris M, Grando V. When is nighttime? A description of bedtime in persons with dementia in the nursing home. Geriatr Nurs. 2014;35(6):474–478. doi: 10.1016/j.gerinurse.2014.06.012. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional file 1: Figure 1. Odds ratios (ORs) for cognitive decline (ΔHDS-R ≤ -4) over 5 years according to daytime nap duration.
Additional file 2: Figure 2. Odds ratios (ORs) for cognitive decline (ΔHDS-R ≤ -2) over 5 years according to daytime nap duration.
Additional file 3. The interviewer’s guide.
Data Availability Statement
Data are available to researchers who meet the criteria for access to confidential data. We cannot provide individual data because informed consent to provide data to anyone outside the research group was not obtained from participants. Please contact the corresponding author (principal investigator: Dr. K Nakamura) regarding any requests for access to confidential data.