Abstract
Entamoeba histolytica is a microaerophilic protozoan parasite in which neither mitochondria nor mitochondrion-derived organelles have been previously observed. Recently, a segment of an E. histolytica gene was identified that encoded a protein similar to the mitochondrial 60-kDa heat shock protein (Hsp60 or chaperonin 60), which refolds nuclear-encoded proteins after passage through organellar membranes. The possible function and localization of the amebic Hsp60 were explored here. Like Hsp60 of mitochondria, amebic Hsp60 RNA and protein were both strongly induced by incubating parasites at 42°C. 5′ and 3′ rapid amplifications of cDNA ends were used to obtain the entire E. histolytica hsp60 coding region, which predicted a 536-amino-acid Hsp60. The E. histolytica hsp60 gene protected from heat shock Escherichia coli groEL mutants, demonstrating the chaperonin function of the amebic Hsp60. The E. histolytica Hsp60, which lacked characteristic carboxy-terminal Gly-Met repeats, had a 21-amino-acid amino-terminal, organelle-targeting presequence that was cleaved in vivo. This presequence was necessary to target Hsp60 to one (and occasionally two or three) short, cylindrical organelle(s). In contrast, amebic alcohol dehydrogenase 1 and ferredoxin, which are bacteria-like enzymes, were diffusely distributed throughout the cytosol. We suggest that the Hsp60-associated, mitochondrion-derived organelle identified here be named “crypton,” as its structure was previously hidden and its function is still cryptic.
Entamoeba histolytica is a protozoan parasite which causes amebic dysentery and liver abscess in individuals in developing countries that cannot prevent its fecal-oral spread (34). Amebae are obligate fermenters which lack enzymes of oxidative phosphorylation, Krebs cycle enzymes, and pyruvate dehydrogenase (36). Indeed, amebae for a long time have been considered to be amitochondriate (28). Instead amebae have fermentation proteins (pyruvate:ferredoxin oxidoreductase [POR], ferredoxin, and alcohol dehydrogenases [ADH1, ADHE, and ADH3]) which are absent from most other eukaryotes and resemble those found in anaerobic bacteria (21, 25, 41, 55). Metronidazole, the best anti-amebic drug, is reduced and activated when it receives an electron from ferredoxin reduced by POR (22). Phylogenetic analyses of E. histolytica POR, ferredoxin, and ADHE strongly suggest that the genes encoding these fermentation enzymes derive from an anaerobic bacterium, although the route of entry of these genes into the cell and the identity of the ancestor are not clear (41, 47). Whether E. histolytica fermentation enzymes are cytosolic or are compartmentalized in organelles such as hydrogenosomes (described below) remains to be determined (30).
It is likely that E. histolytica also had a mitochondrial endosymbiont, as a putative amebic 60-kDa heat shock protein (Hsp60) aligns in phylogenetic trees with mitochondrial Hsp60 (7, 13, 15, 54). Hsp60 peptides, also known as chaperonin 60, form cylindrical structures within the mitochondrial matrix. In association with 10-kDa heat shock proteins (Hsp10), Hsp60 peptides are involved in the refolding of mitochondrial proteins after they have passed through two organellar membranes (16, 18, 42). Eubacterial GroEL proteins are homologous to Hsp60, while GroES proteins are homologues to Hsp10. Numerous hsp60 genes have been identified from protozoan parasites, which have mitochondria (4, 37, 49, 50, 56). Further, mitochondrion-like Hsp60, Hsp10, and 70-kDa heat shock protein (Hsp70) are present in hydrogenosomes (anaerobic mitochondria) of the amitochondriate protozoan parasite Trichomonas vaginalis, which colonizes the vagina (5, 19, 30, 39). Like mitochondrial proteins, hydrogenosomal proteins, which also include POR, ferredoxin, and hydrogenase, are encoded by nuclear genes and contain organelle-targeting presequences at their amino termini (3, 8, 20, 42, 44, 45). Unlike mitochondria, hydrogenosomes lack circular DNAs containing rRNA and protein-encoding genes (30, 54).
A mitochondrion-like Hsp60, which is not induced by heat shock, is apparently located in the cystosol of Giardia lamblia, the amitochondriate protozoan parasite that colonizes the small intestine and causes diarrhea (40, 48). The presence of mitochondrial Hsp60 in early branching eukaryotes previously considered amitochondriate (E. histolytica, G. lamblia, and T. vaginalis) suggests that the common ancestor of all eukaryotes had the mitochondrial endosymbiont (5, 13, 15, 19, 26, 39, 40, 54). As well, genes encoding valyl-tRNA synthetase, which are believed to be of mitochondrial origin, have been cloned from G. lamblia and T. vaginalis (17). As amebae have no previously described organelles to which its Hsp60 might be targeted (28), it is not clear whether the amebic hsp60 gene is expressed, where Hsp60 might be located, and what function the amebic Hsp60 might have.
MATERIALS AND METHODS
Heat-induced expression of E. histolytica hsp60 mRNA and Hsp60 protein.
Axenically grown E. histolytica HM-1 in log phase was heat shocked by incubation for 1 h at 42°C. Heat-shocked and control amebae were lysed in guanidinium, and RNA was isolated by centrifugation through a cesium chloride cushion. Reverse transcription (RT)-PCR was performed with antisense (A1, ACTCCTCCCGTAAGTCTAGC) and sense (S1, GGAGATGGGACAACAACAGC) primers specific for the amebic hsp60 gene (7). As a positive control, RT-PCR was performed with antisense (CCGGTACCTTAGCAAGCATGAATCTTAG) and sense (TAATACGACTCACTATAGGATCCATGAAAG) primers specific for the amebapore A gene (Fig. 1) (27).
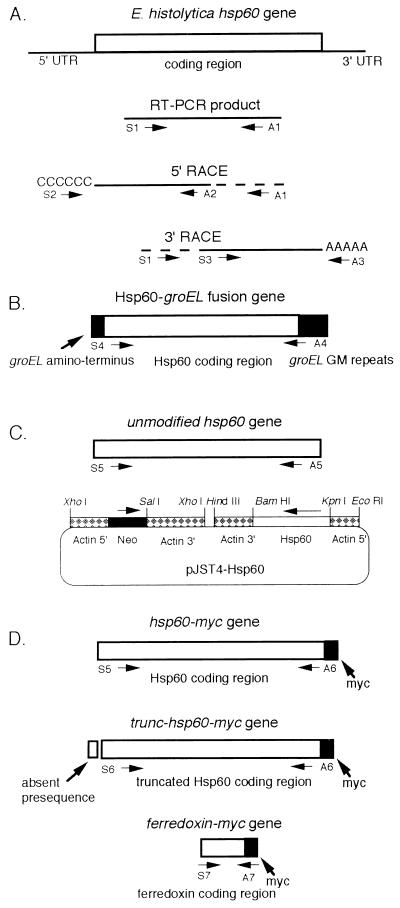
FIG. 1.
Cartoon of RT-PCR products and constructs for testing function and localization of amebic Hsp60. (A) Amebic hsp60 RT-PCR products. Primers are defined in Materials and Methods. (B) Construct used to test amebic Hsp60 in groEL bacteria. GroEL protein sequences are in black. (C) pJST4-Hst60 plasmid used for stable transfection of amebae. The coding regions of the amebic hsp60 gene (striped box) and bacterial neomycin phosphotransferase (black box) were expressed under amebic actin 1 gene promoters. (D) hsp60-myc, trunc-hsp60-myc, and ferredoxin-myc constructs.
Proteins from heat-shocked and control amebae were solubilized in lysis buffer (540 mg of urea/ml, 2% Triton X-100, 2% 2-mercaptoethanol, 2% ampholines [pH 3 to 10], 100 μg of E-64/ml) and electrophoresed on two-dimensional gels (31). Precast gels (Pharmacia Biotech AB, Uppsala, Sweden) contained ampholines from pH 3 to 10 in the first (isoelectric focusing) dimension and a gradient of acrylamide from 5 to 20% in the second (sodium dodecyl sulfate-polyacrylamide gel electrophoresis [SDS-PAGE]) dimension. Gels were stained with Coomassie blue, and a putative Hsp60 was identified as an ∼56-kDa spot, which increased after heat shock. This Hsp60 spot was confirmed by running two-dimensional gels of transfected parasites overexpressing Hsp60 under an actin promoter, identifying the Hsp60 spot, excising it, and determining the amino-terminal sequence (see below).
5′ and 3′ RACE to determine the predicted amino and carboxy termini of the amebic hsp60 gene.
5′ random amplification of cDNA ends (RACE) was performed with RNA from heat-shocked amebae to obtain the 5′ end of the hsp60 coding region and a portion of the 5′ untranslated region (UTR) (11). Briefly, a first-strand cDNA was made with RT and the Hsp60-specific antisense primer A1 (described above). Terminal transferase and dCTP were used to add a poly(C) tail to the 3′ end of this hsp60 cDNA. PCR was performed with this cDNA, a nested antisense primer (A2, GGAACTACACTTTGTGATGAGC) specific for the amebic hsp60 gene, and a sense primer (S2, CCACGCGTCGACTAGTACGGGGGGGGGGG) to poly(C) (Fig. 1). 3′ RACE was also performed with RNA from heat-shocked amebae. A first-strand cDNA was made with RT and an antisense primer (A3, CCACGCGTCGACTAGTACTTTTTTTTTTTTTTTTT) to the poly(A) tail present on all amebic mRNAs. A first round of PCR was performed with this cDNA, antisense primer A3, and the Hsp60-specific sense primer S1 (described above). A second round of PCR was performed with A3 and a nested primer (S3, GCTGCAGTAAGAGCTCCAGG) specific for the amebic hsp60 gene. The 5′ UTR of the Hsp60 mRNA was 5-bp long (ATTTA), while the 3′ UTR was 23-bp long (ATTTTACTTTTAAAAAAAAAAAA), including a 12-bp poly(A) tail. The sequence of the predicted amebic Hsp60 was identical to that recently deposited in GenBank (accession no. AF02966) with the exception of a substitution of Thr for Ser at position 61 (7, 40). PCR of genomic DNA with primers from the start and stop codons of the hsp60 gene (see below) produced the expected 1,611-bp product, demonstrating that introns are absent from the amebic hsp60 gene. Like other amebic genes, 90% of the third codon positions in the hsp60 coding region were A or T (9, 21, 25, 27, 33, 41, 55, 58). Protein sequences similar to the E. histolytica Hsp60 were obtained from GenBank by using BLAST (2).
Functional test of the amebic Hsp60 in the Escherichia coli groEL mutant.
A novel gene (hsp60-groEL) was made encoding an E. histolytica Hsp60-E. coli GroEL fusion protein in which the amino and carboxy termini of the amebic Hsp60 were replaced by those of E. coli (Fig. 1) (18, 29). An hsp60-groEL gene was constructed by PCR. The sense primer (S4, ACCATGGCAGCTAAAGACGTAAAATTCGGTAACGATTGTAGAGAAAATG) contained an NcoI site (italics) and encoded the first 10 amino acids of the bacterial GroEL protein (MAAKDVKFGN, underlined) and Asp11 to Asn15 of the amebic Hsp60 (double underlined). The antisense primer (A4, GGATCCTTACATCATGCCGCCCATGCCACCCATGCCGCCCATACCGCCAGCAGCGC C TAAG TCAGC TGCATCGTTTTTTGGTTCATCAGTTAT) contained a BamHI site (italics) and encoded Ile526 to Pro530 of the amebic Hsp60 (underlined) and the carboxyl-terminal repeats of the E. coli GroEL protein (KNDAADLGAAGGMGGMGGMGGMM, double underlined). The pSE380-Hsp60-groEL construct was made by cloning the Hsp60-groEL gene into the pSE380 vector. This construct was transformed into an E. coli groEL mutant which was grown on plates containing tetracycline and ampicillin (18). Transformed and nontransformed (control) E. coli groEL mutants were streaked onto agar plates and incubated overnight at 37°C (permissive temperature at which groEL mutants and wild-type E. coli grow) and 44°C (the temperature at which groEL mutants die and wild-type E. coli grow).
Overexpression of native amebic Hsp60, epitope-tagged Hsp60, and epitope-tagged ferredoxin in transfected parasites.
The coding region of the amebic hsp60 gene was isolated from genomic DNA by PCR. The sense primer (S5, GCGGTACCATGCTTTCATCTTCAAGTCAT) contained a KpnI site (italics) and encoded the first six amino acids at the amino terminus of the parasite’s Hsp60 protein (MLSSSSH, underlined). The antisense primer (A5, GCGGATCCATTAATTTCCTTTTTTATTGG) contained a BamHI site (italics) and encoded six amino acids at the carboxyl terminus of the organism’s Hsp60 protein (IKKEIN, underlined) (Fig. 1). The pJST4-Hsp60 plasmid was made by cloning the hsp60 coding region into the KpnI and BamHI sites of the pJST4 amebic transformation vector, which contains the neomycin phosphotransferase gene and the gene to be overexpressed (here hsp60) under amebic actin 1 gene promoters (Fig. 1) (12). The pJST4-Hsp60 plasmid was electroporated into E. histolytica HM-1 amebae, which weakly express the endogenous Hsp60 in axenic culture at 37°C, and selected with G418 to a final concentration of 50 μg/ml.
The location of the Hsp60 protein was determined on two-dimensional protein gels by identifying the ∼56-kDa, Coomassie blue-stained spot, the expression of which was increased in transfected but not nontransfected parasites. This was the same spot that was increased in heat-shocked parasites (see above). In addition, two-dimensional gels were transferred to polyvinylidene difluoride filters and stained with Ponceau; the Hsp60 spot was excised, and its amino-terminal sequence was determined by William Lane at the microchemistry facility in the biological laboratories of Harvard University.
A novel gene (hsp60-myc tag) encoding the amebic Hsp60 labeled with a myc epitope at its carboxy terminus was constructed by PCR and the sense primer S5 (described above). The antisense primer (A6, GCGGATCCTTATAAATCTTCTTCTGAAATTAATTTTTGTTCATTAATTTCCTTTTTTATTGG) contained a BamHI site (italics) and encoded six amino acids at the carboxyl terminus of the organism’s Hsp60 protein (IKKEIN, underlined) and a myc epitope (EQKLISEEDL, double underlined) (Fig. 1) (10). The hsp60-myc tag gene was cloned into the pJST4 amebic transformation vector to make a construct called pJST4-Hsp60-Myc, which was electroporated into E. histolytica HM-1 amebae. A second novel gene (trunc-Hsp60-myc tag), encoding a myc-tagged Hsp60 that was missing the amino-terminal presequence, was constructed with the antisense primer A6 and a sense primer (S6, GAGGTACCATGTTATCAGGAATAAAG) containing a KpnI site (italics) and encoding Met and Hsp60 beginning at Leu15 (underlined). This construct was cloned into the pJST4 vector and transfected into amebae as described above.
To localize ferredoxin, amebae were transfected with pJST4 containing a novel gene (ferredoxin-myc) encoding the amebic ferredoxin labeled with a myc epitope at its carboxy terminus (Fig. 1). The coding region of the amebic ferredoxin gene was isolated from genomic DNA by PCR. The sense primer (S7, GCGGTACCATGGGAAAGATCACTATTGTT) contained a KpnI site (italics) and encoded the first seven amino acids at the amino terminus of the parasite’s ferredoxin (MGKITIV, underlined) (21). The antisense primer (A7, GCGGATCCTTATAAATCTTCTTCTGAAATTAATTTTTGTTCAACTCCTTG) contained a BamHI site and encoded three amino acids at the carboxyl terminus of the organism’s ferredoxin (QGV, underlined) and the myc epitope (EQKLISEEDL, double underlined) (Fig. 1).
Fluorescence confocal microscopy.
For indirect immunofluorescence and confocal microscopy, amebic trophozoites were fixed with 2% paraformaldehyde for 10 min at 4°C and permeabilized by incubation with 1% Nonidet P-40. To visualize Hsp60 in nontransfected parasites, a monospecific polyclonal rabbit antibody to amebic Hsp60 was made by immunizing animals with a multi-antigenic peptide (MAP) to 23 amino acids (SVGSLIATSEALITDEPIKKEIN) at the carboxy terminus of the amebic Hsp60. This rabbit anti-amebic Hsp60 antibody was purified on a column composed of the amebic Hsp60 MAP. This antibody was incubated with fixed and permeabilized amebae for 60 min at 37°C in phosphate-buffered saline and 2% bovine serum albumin (PBS-BSA). Parasites were washed four times and immunodecorated for 60 min at 37°C with fluorescein-labeled goat anti-rabbit antisera.
Amebae transfected with plasmids encoding Hsp60 or ferredoxin with myc-epitope tags were immunostained with a monoclonal anti-myc antibody (10 μg/ml) in PBS-BSA (10). Organisms were washed four times and immunodecorated for 60 min at 37°C with a fluorescein isothiocyanate (FITC)-conjugated goat anti-mouse sera. Negative controls include nontransfected parasites and nonimmune mouse sera.
To visualize amebic alcohol dehydrogenase 1, amebae were fixed and immunostained with a polyclonal rabbit anti-alcohol dehydrogenase 1 antibody made to a glutathione S-transferase fusion protein and immunodecorated with a fluorescein-labeled goat anti-rabbit antisera (25, 46). Fluorescently labeled parasites were observed with a Leica NT-TCS confocal microscope fitted with argon and krypton lasers. Images of amebae were recorded in 512 image size format with a 40× Planapo objective.
To identify pinocytotic vesicles, amebae were incubated with 1-mg/ml FITC-dextran for 30 min at 37°C, washed four times in PBS, and fixed with paraformaldehyde. To determine whether the amebae contained an organelle with an electrochemical gradient, parasites were incubated with 10 μg of rhodamine 123/ml or 10 μg of JC-1 dye/ml and observed with the fluorescence microscope (6, 35). As a positive control, Leishmania enriettii parasites, which contain a mitochondrion with a strong electrochemical gradient, were incubated with both dyes. To look for extranuclear DNA, parasites were fixed with 1% formaldehyde to prevent pinocytosis, stained with 1-μg/ml Hoechst dye 33258 for 30 min at 37°C, and examined with the fluorescence microscope.
RESULTS AND DISCUSSION
Heat-shock-induced expression of amebic Hsp60.
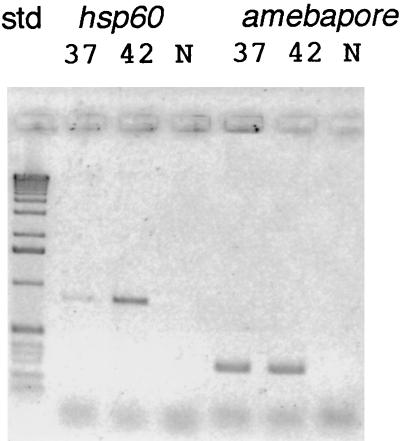
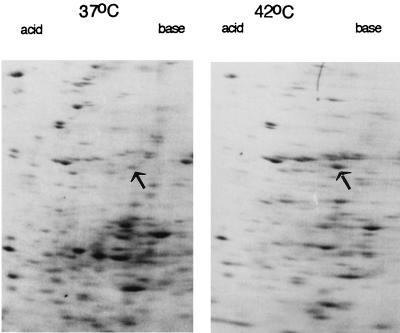
Amebic hsp60 mRNAs, detected by RT-PCR, were weakly present in control amebae incubated at 37°C and were increased in parasites incubated at 42°C (Fig. 2). In contrast, RT-PCR products of mRNAs for amebapore A, which is a lysosomal protein involved in killing bacteria and/or host cells, remained constant during the temperature shift from 37 to 42°C. By similar methods, we determined that amebic mRNAs encoding homologues of Hsp70 (also known as BiP), Hsc70, and inositol kinase were also increased with heat shock (unpublished data) (33). Two-dimensional protein gels of heat-shocked parasites versus controls without heat shock showed increased expression of a 56-kDa protein, which coincided with that of amebae transfected with their own hsp60 gene under an actin gene promoter (Fig. 3). These results, which are qualitative rather than quantitative, demonstrate that the amebic Hsp60 is appropriately named and may serve functions in the parasite comparable to those described for other eukaryotic Hsp60 proteins (16, 18, 42).
FIG. 2.
Results of RT-PCR with primers to the amebic Hsp60 by using total RNA from amebae cultured in the absence of heat shock (37°C) and after heat shock (1 h at 42°C). An ethidium-stained agarose gel of RT-PCR products was electronically reversed for ease of reproduction. Control RT-PCR with primers to the amebapore was performed with the same RNA targets. Lanes 3 and 6 contain negative controls (N) with no target RNA. These RT-PCR results are qualitative rather than quantitative.
FIG. 3.
High-power view of two-dimensional protein gels of amebae incubated at 37°C and after heat shock (42°C). Arrows mark putative Hsp60s, which were in the same location as recombinant Hsp60 expressed in amebae transfected with the pJST4-Hsp60 plasmid (data not shown). Note that the putative Hsp60 is not the only protein which changes in abundance with heat shock.
Chaperonin function of the amebic Hsp60 in an E. coli groEL mutant.
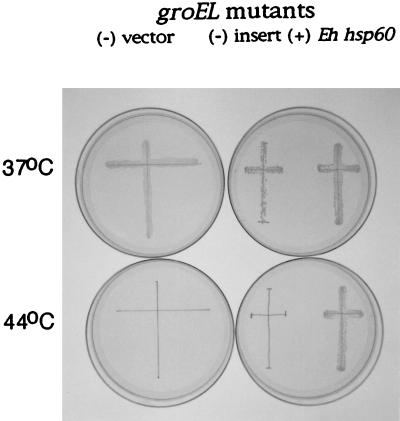
The entire 1,608-bp amebic hsp60 coding region, encoding a putative 536-amino-acid (Mr, 56,788) Hsp60, was obtained by 5′ and 3′ RACE, with RNA from heat-shocked parasites (Fig. 1) (11). To test the function of the amebic Hsp60, the E. histolytica hsp60 gene was expressed in an E. coli groEL mutant. This mutant lacks an Hsp60-like chaperonin and so is killed when incubated at 44°C (18). To increase the likelihood of success, the E. histolytica hsp60 gene was modified at its 5′ and 3′ ends to encode an amebic Hsp60 protein with amino and carboxy termini like those of the E. coli GroEL protein. The groEL mutant transfected with a plasmid containing the modified E. histolytica hsp60 gene survived incubation at 44°C (Fig. 4). Control groEL mutants, which were not transfected or were transfected with a plasmid containing no foreign gene, died at 44°C. These results demonstrate that the amebic Hsp60 can act as a chaperonin in E. coli and suggest a similar function for Hsp60 in the parasite. It remains to be determined whether a wild-type amebic hsp60 gene, rather than the hsp60-groEL fusion gene used here, is able to complement the groEL mutant (29).
FIG. 4.
Plate showing groEL mutants incubated at 37°C (permissive temperature) or 44°C (nonpermissive temperature). Each groEL mutant was streaked as a cross, which was marked with a pen. A nontransformed groEL mutant was maintained on plates containing tetracycline (left), while groEL mutants transformed with the pSE380 vectors were maintained on plates containing tetracycline and ampicillin (right). A groEL mutant complemented with the amebic Hsp60-E. coli groEL fusion gene (Eh hsp60) grew at both temperatures. In contrast, the control groEL mutant [(−) vector] failed to grow at 44°C. Similarly, a groEL mutant which was transformed with the pSE380 vector without an insert [(−) insert] failed to grow at 44°C.
Absence of Gly and Met repeats at the carboxy terminus of the predicted amebic Hsp60.
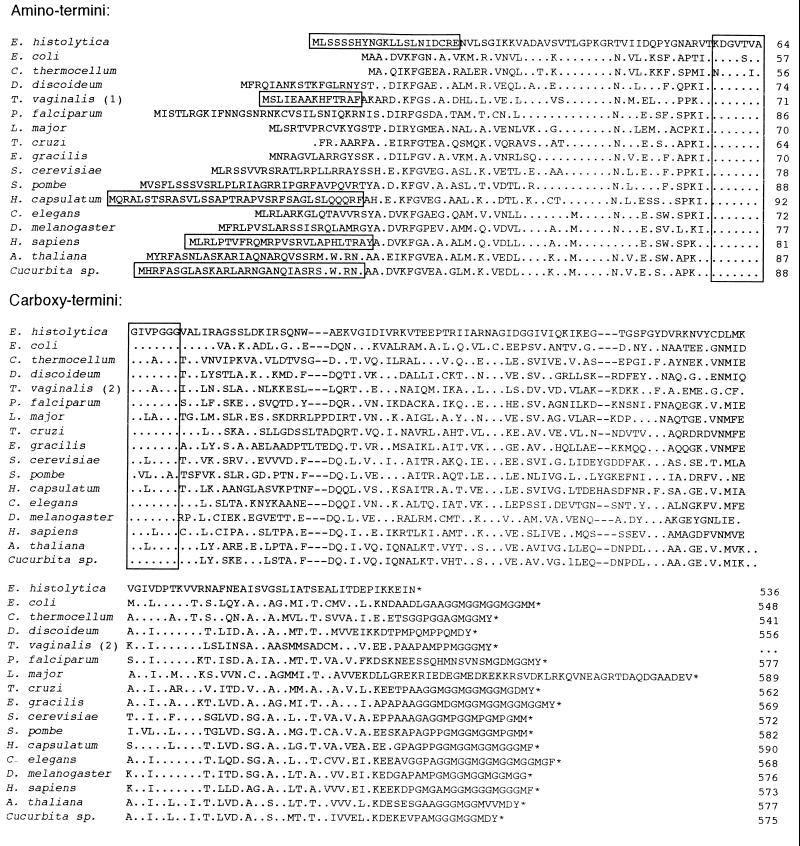
The carboxyl terminus of the E. histolytica Hsp60 lacked Gly and Met residues, which are repeated at the carboxy termini of all other Hsp60 and GroEL proteins except that of Leishmania major (Fig. 5) (2, 4, 5, 18, 19, 23, 37, 39, 45, 49–51, 56, 57). The absence of the carboxy terminal repeats and the somewhat short amebic amino terminus of the E. histolytica Hsp60 (see below) made it 20 to 53 amino acids shorter than other eukaryotic Hsp60 proteins. The functional significance of the absence of carboxy-terminal repeats of the amebic Hsp60 is unclear. A truncated E. coli GroEL protein lacking the Gly and Met repeats behaves like the wild-type GroEL protein in vitro but has numerous subtle deficiencies compared with the wild-type GroEL protein in vivo (29).
FIG. 5.
Alignment of the amino and carboxyl termini of the predicted E. histolytica Hsp60 in single-letter code with GroEL proteins of E. coli (X07850) (18) and Clostridium thermocellum (Z68137) and Hsp60s of Dictyostalium discoideum (U72247), T. vaginalis (1, U26966 [5] and 2, U57000 [39]), Plasmodium falciparum (U38963) (50), L. major (U59320) (37), Trypanosoma cruzi (X67473) (49), Euglena gracilis (U49053) (56), Saccharomyces cerevisiae (M33301) (23), Schizosaccharomyces pombe (D50609) (57), Histoplasma capsulatum (L11390), Caenorhabditis elegans (L36035), Drosophila melanogaster (X99341), Homo sapiens (M22382) (45), Arabidopsis thaliana (Z11547), and Cucurbita sp. (pumpkin, X70868) (51). Periods indicate amino acids identical to those of the amebic Hsp60, dashes indicate gaps, and asterisks indicate stop codons. Vertical boxes indicate location of primers used previously to identify a segment of the amebic Hsp60 (7). Horizontal boxes indicate proven organelle-targeting presequences of amebic, trichomonad, Histoplasma, human, and pumpkin Hsp60. The T. vaginalis Hsp60 sequence is a composite of two published sequences, each of which is incomplete, so the total length could not be determined.
Hydrogenosome-like, organelle-targeting presequence at the amino terminus of the E. histolytica Hsp60.
The amino portion of the amebic Hsp60, which includes 55 amino acids not previously identified, aligned without gaps with other Hsp60 or GroEL proteins beginning at Lys11 (Fig. 5) (2, 4, 5, 16, 18, 19, 23, 37, 39, 44, 45, 49–51, 56, 57). However, the amebic Hsp60 sequence contained numerous unique amino acids from Lys12 to Val29 and from Thr44 to Val56, further demonstrating its difference from other mitochondrial Hsp60s.
Eukaryotic Hsp60s have mitochondrion-targeting presequences at their amino-termini, while bacterial GroEL proteins, which are cytosolic, lack such presequences (Fig. 5) (8, 18, 42, 44, 51). Mitochondrion-targeting presequences, which are proven for the Hsp60s of humans, Leishmania major, and Cucurbita sp. (pumpkin) and are putative for the rest of the Hsp60, are 20 to 30 amino acids long, are enriched in Ser and Arg, lack negatively charged amino acids, and contain an endopeptidase cleavage site. The amebic Hsp60 contained an amino-terminal decapeptide (Met-Leu-Ser-Ser-Ser-Ser-His-Tyr-Asn-Gly), which was distinct from those of other eukaryotic Hsp60s but included multiple amino acids (underlined) present at the amino-terminus of most T. vaginalis proteins targeted to hydrogenosomes (Fig. 6) (3, 20). Similarly, the amino terminus of the amebic nicotinamide nucleotide transhydrogenase (NNT) (MSTSSSIEEEVFNYMKITNNFVSVGNIIIS), a homologue of the mitochondrial NNT, contains numerous Ser residues (7, 58). In vitro experiments with T. vaginalis ferredoxin demonstrated the importance of Leu2 (also present in the amebic Hsp60) for targeting ferredoxin to hydrogenosomes (3).
FIG. 6.
Alignment of the amino-terminal organelle-targeting presequence of E. histolytica Hsp60 with those of T. vaginalis hydrogenosomal proteins (3, 20). Dashes indicate amino acids identical to those of the amebic Hsp60. To identify the amebic presequence, an Hsp60 spot was excised from a two-dimensional gel of amebic proteins and sequenced by Edman degradation.
The amebic amino-terminal Hsp60 decapeptide is apparently part of an organelle-targeting presequence, because it and 11 other amino acids were removed from Hsp60 in vivo. The amebic Hsp60, which was overexpressed in cultured trophozoites and isolated on two-dimensional gels similar to that shown in Fig. 3, had an amino terminus that began at Asn22 (Fig. 6). The 21-amino-acid amebic Hsp60 presequence is similar in length to mitochondrial presequences and somewhat longer than hydrogenosomal presequences. The amebic Hsp60 presequence has an Arg residue at −2 relative to the cleavage site, as is the case for most mitochondrial and hydrogenosomal presequences (Fig. 5 and 6) (3, 8, 20, 42, 44). These results suggest that the organelles to which amebic Hsp60 is targeted (see below) have receptors and endopeptidases which are similar to those of mitochondria and hydrogenosomes (42).
Organellar location of the amebic Hsp60 and cytosolic location of alcohol dehydrogenase 1 and ferredoxin.
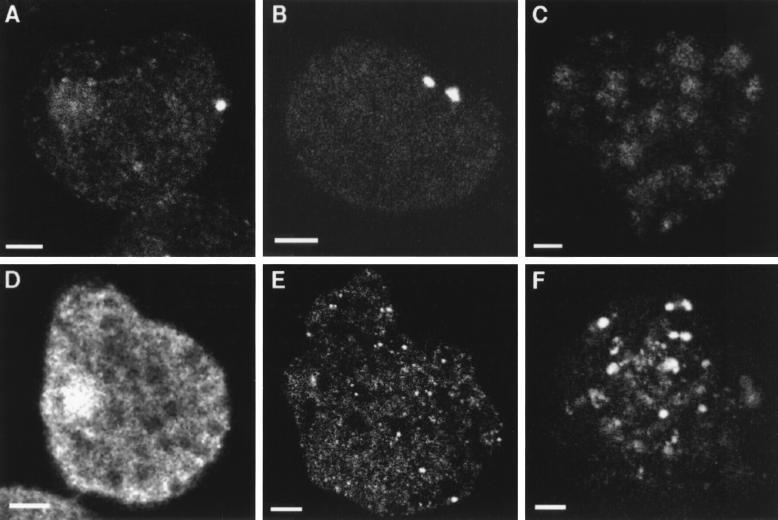
Amebic Hsp60 was localized to a short cylindrical organelle in nontransfected parasites by using antibodies to a peptide at the carboxy terminus of amebic Hsp60 (Fig. 7A). Although many cells contained one Hsp60-associated organelle, some cells contained two or three organelles. Nearly identical results were obtained with transfected parasites overexpressing Hsp60 with a carboxy-terminal myc tag by using anti-myc antibodies (Fig. 1 and 7B) (10). A truncated myc-tagged Hsp60, which lacked 13 amino acids at the amino terminus, went to the cytosol of transfected parasites rather than to the organelle (Fig. 7C). This result demonstrates that targeting of Hsp60 to the organelle is dependent upon the presence of multiple Ser and/or positively charged residues at its amino terminus, as has been shown for hydrogenosomal ferredoxin or mitochondrial proteins (3, 8, 42).
FIG. 7.
Fluorescence confocal micrographs of E. histolytica trophozoites localizing an Hsp60-associated organelle and contrasting it with the amebic cytosol or pinosomes. Antibodies to Hsp60 (A) identified a short, cylindrical organelle in a nontransfected parasite. Antibodies to a myc identified two similar organelles in transfected amebae overexpressing Hsp60 with a C-terminal myc tag (B). However, most parasites labeled with anti-Hsp60 or anti-myc antibodies contained a single organelle. In contrast, anti-myc antibodies had a cytosolic distribution when parasites were transfected with a truncated hsp60 gene encoding an Hsp60 lacking Ser and positively charged residues in its presequence (C). Antibodies to ADH1 in nontransfected parasites (D) gave a cytosolic distribution, with some labeling of the nucleus. Similarly, anti-myc antibodies had a cytosolic, if somewhat granular, distribution when parasites were transfected with a ferredoxin gene (E) encoding a peptide with a myc tag at its carboxy terminus. Pinocytosed FITC-dextran by nontransformed amebae fills hundreds of vesicles (F), most of which were small.
Absent from the Hsp60-containing organelles was the fermentation enzyme alcohol dehydrogenase 1, which was located in the parasite cytosol by polyclonal rabbit antibodies (Fig. 7D) (25). Also absent from the Hsp60-containing organelles was ferredoxin, which was overexpressed with a myc tag in transfected parasites and detected with anti-myc antibodies (Fig. 7E) (10, 21). Hsp60-associated organelles were distinct from spherical vacuoles containing pinocytosed dextran (Fig. 7F). Organelles containing Hsp60 were also distinct from spherical secretory vesicles where the amebic chitinase, which has a secretory signal sequence at its amino terminus that obeys the -3, -1 rule, goes when expressed in transfected parasites under an actin promoter (data not shown) (9, 12, 53).
Consistent with their cytosolic localization, amebic ADH1 and ferredoxin lack either signal sequences or organelle-targeting presequences (8, 21, 25, 53). Amebic POR and ADHE, which also lack signal sequences or organelle-targeting presequences, have been found on the surface of amebae and in one report in organelles (POR) (8, 38, 41, 43, 55). How amebic POR and ADHE get to these locations is not clear. In contrast, hydrogenosomal proteins of T. vaginalis, which include POR, ferredoxin, and succinate dehydrogenases, have organellar-targeting presequences (Fig. 6) (3, 20). Finally, a putative G. lamblia Hsp60 has been localized with heterologous antibodies to the parasite cytosol (39, 48).
Apparent lack of DNA or electrochemical gradient in the Hsp60-associated organelle.
In order to further characterize the Hsp60-associated organelle, amebae were incubated with rhodamine 123 or JC-1, which are cationic and hydrophobic dyes that target organelles with a strong electrochemical gradient (6, 35). Although mitochondria of L. enriettii were strongly labeled with each dye, neither dye labeled any structures within amebae. These results suggest that there is no strong electrochemical gradient within the Hsp60-associated organelles of amebae. A weak electrochemical gradient within the organelle may be made with ATP produced in the cytosol by substrate-level phosphorylation (36). Further, the Hsp60-associated organelles failed to stain with Hoechst dye, which stained the kinetoplastid of L. enriettii. Although the possibility of a small genome remaining within the Hsp60-associated organelle cannot be ruled out, these results suggest that DNA from these organelles has shifted to the nucleus, as has been shown for hydrogenosomes of trichomonads (30). We were unable to confirm recent observations of large cytoplasmic accumulations of DNA within cultured amebae (32).
Likelihood that the E. histolytica Hsp60 functions as an organellar chaperonin.
Strong circumstantial evidence suggests that the E. histolytica Hsp60 functions as a chaperonin within a mitochondrion-derived organelle. First, Hsp60 is expressed after heat shock. Second, the amebic hsp60 gene complements an E. coli groEL mutant. Third, the primary structure of amebic Hsp60 is similar to those of other Hsp60 and GroEL proteins (7, 39). Fourth, an amino-terminal presequence is cleaved from amebic Hsp60 in vivo and Hsp60 is located within an organelle. In addition, E. histolytica contains genes encoding proteins homologous to cytosolic Hsp70 and 14-3-3 (also known as mitochondrial stimulation factor or MSF) (1, 12a, 33). Cytosolic Hsp70s maintain proteins targeted to the mitochondria in a translocation-competent conformation, while MSFs recognize mitochondrial import signals on mitochondrial precursor proteins and target them to receptors on mitochondria (1, 42).
Unknown function of the amebic mitochondrion-derived organelle, tentatively named here “crypton.”
The Hsp60-associated organelles identified here are small and rare (often one per cell), so they may have been overlooked in electron microscopic studies of amebae (28). These organelles may have been seen with anti-POR antibodies, although our results with ferredoxin are contradictory (38). They may also have been seen by H. N. Ray and coworkers with histological stains in the pre-electron microscopy era (reference 7 and references therein). The amebic organelle is unique among mitochondrion-derived organelles because it contains neither enzymes of oxidative phosphorylation (like mitochondria) nor fermentation enzymes (like hydrogenosomes of T. vaginalis) (3, 5, 19, 20, 30, 39).
The paucity of the amebic Hsp60-associated organelles and their small size are reminiscent of petite mitochondria of anaerobically grown yeast or the atrophic mitochondria of bloodstream trypanosomes, which use glycolysis rather than oxidative phosphorylation (14, 52). The difference between the amebic organelles and the mitochondria of petite yeast or bloodstream trypanosomes is that the latter change back to operative mitochondria when yeast are exposed to oxygen or trypanosomes are transferred to the insect vector. We suggest that the amebic Hsp60-associated, mitochondrion-derived organelle identified here be named crypton, as its structure was previously hidden and its function is still cryptic. Recently, apicomplexa have been shown to have a plastid organelle bound by four membranes, the function of which is not yet certain (24).
ACKNOWLEDGMENTS
This work was supported in part by National Institutes of Health grants AI-33492 (to J.S.) and HL-330099 and HL-43510 (to R.R.).
We thank Graham Clark and Andrew Roger for the identification of a segment of the amebic Hsp60 gene, without which these experiments could not have been performed. We acknowledge the expert technical support of Juliet Mervis for confocal microscopy and Jean Lai for image analysis.
REFERENCES
- 1.Alam R, Hachiya N, Sakaguchi M, Kawabata S, Iwanaga S, Kitajima M, Mihara K, Omura T. cDNA cloning and characterization of mitochondrial import stimulation factor (MSF) purified from rat liver cytosol. J Biochem. 1994;116:416–425. doi: 10.1093/oxfordjournals.jbchem.a124541. [DOI] [PubMed] [Google Scholar]
- 2.Altschul S F, Madden T L, Schaffer A A, Zhang J, Zhang Z, Miller W, Lipman D J. Gapped BLAST and PSI-BLAST: a new generation of protein database search programs. Nucleic Acids Res. 1997;25:3389–3402. doi: 10.1093/nar/25.17.3389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bradley P J, Lahti C J, Plumper E, Johnson P J. Targeting and translocation of proteins into the hydrogenosome of the protist Trichomonas: similarities with mitochondrial protein import. EMBO J. 1997;16:3484–3493. doi: 10.1093/emboj/16.12.3484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bringaud F, Peyrucaud S, Baltz D, Giroud C, Simpson L, Baltz T. Molecular characterization of the mitochondrial heat shock protein 60 gene from Trypanosoma brucei. Mol Biochem Parasitol. 1995;74:119–123. doi: 10.1016/0166-6851(95)02486-7. [DOI] [PubMed] [Google Scholar]
- 5.Bui E T N, Bradley P J, Johnson P J. A common evolutionary origin for mitochondria and hydrogenosomes. Proc Natl Acad Sci USA. 1996;93:9651–9656. doi: 10.1073/pnas.93.18.9651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chen L B. Fluorescent labeling of mitochondria. Methods Cell Biol. 1989;29:103–123. doi: 10.1016/s0091-679x(08)60190-9. [DOI] [PubMed] [Google Scholar]
- 7.Clark C G, Roger A J. Direct evidence for secondary loss of mitochondria in Entamoeba histolytica. Proc Natl Acad Sci USA. 1995;92:6518–6521. doi: 10.1073/pnas.92.14.6518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Claros M G, Vincens P. Computational method to predict mitochondrially imported proteins and their targeting sequences. Eur J Biochem. 1996;241:779–786. doi: 10.1111/j.1432-1033.1996.00779.x. [DOI] [PubMed] [Google Scholar]
- 9.De la Vega H, Specht C A, Semino C E, Robbins P W, Eichinger D, Caplivski D, Ghosh S, Samuelson J. Cloning and expression of chitinases of Entamoebae. Mol Biochem Parasitol. 1996;85:139–147. doi: 10.1016/s0166-6851(96)02817-4. [DOI] [PubMed] [Google Scholar]
- 10.Evan G I, Lewis G K, Ramsay G, Bishop J M. Isolation of monoclonal antibodies specific for human c-myc proto-oncogene product. Mol Cell Biol. 1985;5:3610–3616. doi: 10.1128/mcb.5.12.3610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Frohman M A, Dush M K, Martin G R. Rapid production of full-length cDNAs from rare transcripts: amplification using a single gene-specific oligonucleotide primer. Proc Natl Acad Sci USA. 1988;85:8998–9002. doi: 10.1073/pnas.85.23.8998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ghosh S K, Lohia A, Kumar A, Samuelson J. Overexpression of P-glycoprotein gene 1 by transfected Entamoeba histolytica confers emetine-resistance. Mol Biochem Parasitol. 1996;82:257–260. doi: 10.1016/0166-6851(96)02733-8. [DOI] [PubMed] [Google Scholar]
- 12a.Ghosh, S. K., J. Samuelson, and A. Lohia. Unpublished data.
- 13.Gray M W. The evolutionary origins of organelles. Trends Genet. 1989;5:294–299. doi: 10.1016/0168-9525(89)90111-x. [DOI] [PubMed] [Google Scholar]
- 14.Grivell L A. Nucleo-mitochondrial interactions in mitochondrial gene expression. Crit Rev Biochem Mol Biol. 1995;30:121–164. doi: 10.3109/10409239509085141. [DOI] [PubMed] [Google Scholar]
- 15.Gupta R S. Evolution of the chaperonin families (Hsp60, Hsp10 and Tcp-1) of proteins and the origin of eukaryotic cells. Mol Microbiol. 1995;15:1–11. doi: 10.1111/j.1365-2958.1995.tb02216.x. [DOI] [PubMed] [Google Scholar]
- 16.Hartl F U. Molecular chaperones in cellular protein folding. Nature. 1996;381:571–580. doi: 10.1038/381571a0. [DOI] [PubMed] [Google Scholar]
- 17.Hashimoto T, Sanchez L B, Shirakura T, Muller M, Hasegawa M. Secondary loss of mitochondria in Giardia lamblia and Trichomonas vaginalis revealed by valyl-tRNA synthetase phylogeny. Proc Natl Acad Sci USA. 1998;95:6860–6865. doi: 10.1073/pnas.95.12.6860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hemmingsen S M, Woolford C, van der Vies S M, Tilly K, Dennis D T, Georgopoulos C P, Hendrix R W, Ellis R J. Homologous plant and bacterial proteins chaperone oligomeric protein assembly. Nature. 1988;333:330–334. doi: 10.1038/333330a0. [DOI] [PubMed] [Google Scholar]
- 19.Horner D S, Hirt R P, Kilvington S, Lloyd D, Embley T M. Molecular data suggest an early acquisition of the mitochondrion endosymbiont. Proc R Soc Lond Ser B. 1996;263:1053–1059. doi: 10.1098/rspb.1996.0155. [DOI] [PubMed] [Google Scholar]
- 20.Hrdy I, Muller M. Primary structure and eubacterial relationship of the pyruvate:ferredoxin oxidoreductase of the amitochondriate eukaryote, Trichomonas vaginalis. J Mol Evol. 1995;41:388–396. [PubMed] [Google Scholar]
- 21.Huber M, Garfinkel L, Gitler C, Mirelman D, Revel M, Rozenblatt S. Nucleotide sequence analysis of an Entamoeba histolytica ferredoxin gene. Mol Biochem Parasitol. 1988;31:27–34. doi: 10.1016/0166-6851(88)90142-9. [DOI] [PubMed] [Google Scholar]
- 22.Johnson P J. Metronidazole and drug resistance. Parasitol Today. 1993;9:183–186. doi: 10.1016/0169-4758(93)90143-4. [DOI] [PubMed] [Google Scholar]
- 23.Johnson R B, Fearon K, Mason T, Jindal S. Cloning and characterization of the yeast chaperonin HSP60 gene. Gene. 1989;84:295–302. doi: 10.1016/0378-1119(89)90503-9. [DOI] [PubMed] [Google Scholar]
- 24.Kohler S, Delwiche C F, Denny P W, Tilney L G, Webster P, Wilson R J, Palmer J D, Roos D S. A plastid of probable green algal origin in Apicomplexan parasites. Science. 1997;275:1485–1489. doi: 10.1126/science.275.5305.1485. [DOI] [PubMed] [Google Scholar]
- 25.Kumar A, Shen P-S, Descoteaux S, Pohl J, Bailey G, Samuelson J. Cloning and expression of an NADP+-dependent alcohol dehydrogenase gene of Entamoeba histolytica. Proc Natl Acad Sci USA. 1992;85:1782–1786. doi: 10.1073/pnas.89.21.10188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Leipe D D, Gunderson J H, Nerad T A, Sogin M L. Small subunit ribosomal RNA of Hexamita inflata and the quest for the first branch in the eukaryotic tree. Mol Biochem Parasitol. 1993;59:41–48. doi: 10.1016/0166-6851(93)90005-i. [DOI] [PubMed] [Google Scholar]
- 27.Leippe M, Tannich E, Nickel R, Horstmann R D, Muller-Eberhard H J. Primary structure of the pore-forming peptide of pathogenic Entamoeba histolytica. EMBO J. 1992;11:3501–3506. doi: 10.1002/j.1460-2075.1992.tb05432.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Martinez-Palomo A. Cell biology. In: Brown K N, editor. The biology of Entamoeba histolytica. New York, N.Y: John Wiley, Research Studies Press; 1982. pp. 5–59. [Google Scholar]
- 29.McLennan N F, Girshovich A S, Lissin N M, Charters Y, Masters M. The strongly conserved carboxyl-terminus glycine-methionine motif of the Escherichia coli GroEL chaperonin is dispensable. Mol Microbiol. 1993;71:49–58. doi: 10.1111/j.1365-2958.1993.tb01096.x. [DOI] [PubMed] [Google Scholar]
- 30.Muller M. The hydrogenosome. J Gen Microbiol. 1993;139:2879–2889. doi: 10.1099/00221287-139-12-2879. [DOI] [PubMed] [Google Scholar]
- 31.O’Farrell P Z, Goodman H M, O’Farrell P H. High resolution two-dimensional electrophoresis of basic as well as acidic proteins. Cell. 1977;12:1133–1141. doi: 10.1016/0092-8674(77)90176-3. [DOI] [PubMed] [Google Scholar]
- 32.Orozco E, Gharaibeh R, Riveron A M, Delgadillo D M, Mercado M, Sanchez T, Gomez Conde E, Vargas M A, Lopez-Revilla R. A novel cytoplasmic structure containing DNA networks in Entamoeba histolytica trophozoites. Mol Gen Genet. 1997;254:250–257. doi: 10.1007/s004380050413. [DOI] [PubMed] [Google Scholar]
- 33.Ortner S, Plaimauer B, Binder M, Wiedermann G, Scheiner O, Duchene M. Humoral immune response against a 70-kilodalton heat shock protein of Entamoeba histolytica in a group of patients with invasive amoebiasis. Mol Biochem Parasitol. 1992;54:175–183. doi: 10.1016/0166-6851(92)90110-6. [DOI] [PubMed] [Google Scholar]
- 34.Ravdin J I. Amebiasis. State-of-the-art clinical article. Clin Infect Dis. 1995;20:1453–1464. doi: 10.1093/clinids/20.6.1453. [DOI] [PubMed] [Google Scholar]
- 35.Reers M, Smiley S T, Mottola-Hartshorn C, Chen A, Lin M, Chen L B. Mitochondrial membrane potential monitored by JC-1 dye. Methods Enzymol. 1995;260:406–417. doi: 10.1016/0076-6879(95)60154-6. [DOI] [PubMed] [Google Scholar]
- 36.Reeves R E. Metabolism of Entamoeba histolytica Schaudinn, 1903. Adv Parasitol. 1984;23:105–142. doi: 10.1016/s0065-308x(08)60286-9. [DOI] [PubMed] [Google Scholar]
- 37.Rey-Ladino J A, Joshi P B, Singh B, Gupta R, Reiner N E. Leishmania major: molecular cloning, sequencing, and expression of the heat shock protein 60 gene reveals unique carboxyl terminal peptide sequences. Exp Parasitol. 1997;85:249–263. doi: 10.1006/expr.1996.4137. [DOI] [PubMed] [Google Scholar]
- 38.Rodriguez M A, Garcia-Perez R M, Mendoza L, Sanchez T, Guillen N, Orozco E. The pyruvate:ferredoxin oxidoreductase is located in the plasma membrane and in a cytoplasmic structure in Entamoeba. Microb Pathog. 1998;25:1–10. doi: 10.1006/mpat.1998.0202. [DOI] [PubMed] [Google Scholar]
- 39.Roger A J, Clark C G, Doolittle W F. A possible mitochondrial gene in the early-branching amitochondriate protist Trichomonas vaginalis. Proc Natl Acad Sci USA. 1996;93:14618–14622. doi: 10.1073/pnas.93.25.14618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Roger A J, Svard S G, Tovar J, Clark C G, Smith M W, Gillen F D, Sogin M L. A mitochondrial-like chaperonin 60 gene in Giardia lamblia: evidence that diplomonads once harbored an endosymbiont related to the progenitor of mitochondria. Proc Natl Acad Sci USA. 1998;95:229–234. doi: 10.1073/pnas.95.1.229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Rosenthal B, Mai Z, Caplivski D, Ghosh S, de la Vega H, Graf T, Samuelson J. Evidence for the bacterial origin of genes encoding fermentation enzymes of the amitochondriate protozoan parasite Entamoeba histolytica. J Bacteriol. 1997;179:3736–3745. doi: 10.1128/jb.179.11.3736-3745.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ryan K R, Jensen R E. Protein translocation across mitochondrial membranes: what a long strange trip it is. Cell. 1995;83:517–519. doi: 10.1016/0092-8674(95)90089-6. [DOI] [PubMed] [Google Scholar]
- 43.Samarawickrema N A, Brown D M, Upcroft J A, Thammapalerd N, Upcroft P. Involvement of superoxide dismutase and pyruvate:ferredoxin oxidoreductase in mechanisms of metronidazole resistance in Entamoeba histolytica. J Antimicrob Chemother. 1997;40:833–840. doi: 10.1093/jac/40.6.833. [DOI] [PubMed] [Google Scholar]
- 44.Schatz G. Signals guiding proteins to their correct locations in mitochondria. Eur J Biochem. 1987;165:1–6. doi: 10.1111/j.1432-1033.1987.tb11186.x. [DOI] [PubMed] [Google Scholar]
- 45.Singh B, Patel H V, Ridley R G, Freeman K B, Gupta R S. Mitochondrial import of the human chaperonin (HSP60) protein. Biochem Biophys Res Commun. 1990;169:391–396. doi: 10.1016/0006-291x(90)90344-m. [DOI] [PubMed] [Google Scholar]
- 46.Smith D B, Johnson K S. Single-step purification of polypeptides expressed in Escherichia coli as fusions with glutathione S-transferase. Gene. 1988;67:31–40. doi: 10.1016/0378-1119(88)90005-4. [DOI] [PubMed] [Google Scholar]
- 47.Smith M W, Feng D-F, Doolittle R F. Evolution by acquisition: the case for horizontal gene transfers. Trends Biochem Sci. 1992;17:489–493. doi: 10.1016/0968-0004(92)90335-7. [DOI] [PubMed] [Google Scholar]
- 48.Soltys B J, Gupta R S. Presence and cellular distribution of a 60-kDa protein related to mitochondrial HSP60 in Giardia lamblia. J Parasitol. 1994;80:580–590. [PubMed] [Google Scholar]
- 49.Sullivan M A, Olson C L, Winquist A G, Engman D M. Expression and localization of Trypanosoma cruzi hsp60. Mol Biochem Parasitol. 1994;68:197–208. doi: 10.1016/0166-6851(94)90165-1. [DOI] [PubMed] [Google Scholar]
- 50.Syin C, Goldman N D. Cloning of a Plasmodium falciparum gene related to the human 60-kDa heat shock protein. Mol Biochem Parasitol. 1996;79:13–19. doi: 10.1016/0166-6851(96)02633-3. [DOI] [PubMed] [Google Scholar]
- 51.Tsugeki R, Mori H, Nishimura M. Purification, cDNA cloning and Northern-blot analysis of mitochondrial chaperonin 60 from pumpkin cotyledons. Eur J Biochem. 1992;209:453–458. doi: 10.1111/j.1432-1033.1992.tb17309.x. [DOI] [PubMed] [Google Scholar]
- 52.Tyler K M, Matthews K R, Gull K. The bloodstream differentiation-division of Trypanosoma brucei studied using mitochondrial markers. Proc R Soc Lond Ser B. 1997;264:1481–1490. doi: 10.1098/rspb.1997.0205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.von Heijne G. Signal sequences. The limits of variation. J Mol Biol. 1985;184:99–105. doi: 10.1016/0022-2836(85)90046-4. [DOI] [PubMed] [Google Scholar]
- 54.Yang D, Oyaizu Y, Oyaizu H, Olsen G J, Woese C R. Mitochondrial origins. Proc Natl Acad Sci USA. 1985;82:4443–4447. doi: 10.1073/pnas.82.13.4443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Yang W, Li E, Kairong T, Stanley S L., Jr Entamoeba histolytica has an alcohol dehydrogenase homologous to the multifunctional adhE gene product of Escherichia coli. Mol Biochem Parasitol. 1994;64:253–260. doi: 10.1016/0166-6851(93)00020-a. [DOI] [PubMed] [Google Scholar]
- 56.Yasuhira S, Simpson L. Phylogenetic affinity of mitochondria of Euglena gracilis and kinetoplastids using cytochrome oxidase 1 and hsp60. J Mol Evol. 1990;44:341–347. doi: 10.1007/pl00006152. [DOI] [PubMed] [Google Scholar]
- 57.Yoshida H, Yanagi H, Yura T. Cloning and characterization of the mitochondrial HSP60-encoding gene of Schizosaccharomyces pombe. Gene. 1995;167:163–166. doi: 10.1016/0378-1119(96)82966-0. [DOI] [PubMed] [Google Scholar]
- 58.Yu Y, Samuelson J. Primary structure of an Entamoeba histolytica nicotinamide nucleotide transhydrogenase. Mol Biochem Parasitol. 1994;68:323–328. doi: 10.1016/0166-6851(94)90178-3. [DOI] [PubMed] [Google Scholar]